Abstract
Cannabinoid CB1 receptors (CB1Rs) have been shown to be a promising target in medication development for the treatment of addiction. However, clinical trials with SR141716A (rimonabant, a selective CB1R antagonist/inverse agonist) for the treatment of obesity and smoking cessation failed due to unwanted side effects, such as depression, anxiety, and suicidal tendencies. Recent preclinical studies suggest that the neutral CB1R antagonist AM4113 may retain the therapeutic anti-addictive effects of SR141716A in nicotine self-administration models and possibly has fewer unwanted side effects. However, little is known about whether AM4113 is also effective for other drugs of abuse, such as opioids and psychostimulants, and whether it produces depressive side effects similar to SR141716A in experimental animals. In this study, we demonstrated that systemic administration of AM4113 (3 and 10 mg/kg) dose-dependently inhibited the self-administration of intravenous heroin but not cocaine or methamphetamine, whereas SR141716A (3 and 10 mg/kg) dose-dependently inhibited the self-administration of heroin and methamphetamine but not cocaine. In the electrical brain-stimulation reward (BSR) paradigm, SR141716A (3 and 10 mg/kg) dose-dependently increased the BSR stimulation threshold (i.e., decreased the stimulation reward), but AM4113 had no effect on BSR at the same doses, suggesting that SR141716A may produce aversive effects while AM4113 may not. Together, these findings show that neutral CB1R antagonists such as AM4113 deserve further research as a new class of CB1R-based medications for the treatment of opioid addiction without SR141716A-like aversive effects.
Keywords: CB1 receptors, AM4113, SR141716A, CB1 neutral antagonist, CB1 inverse agonist, drug reward, aversion, depression, self-administration, brain-stimulation reward
Introduction
Drug addiction is characterized by persistent drug-taking and drug-seeking behaviors, despite serious negative physiological, medical, or social consequences [1, 2]. Although great progress has been made in understanding the neural mechanisms underlying drug addiction, treatments remain limited in their ability to successfully reduce addiction-related behaviors [2, 3]. Accumulating evidence suggests that the cannabinoid system plays a vital modulatory role in diverse functions that may contribute to drug abuse [4].
Two types of cannabinoid receptor subtypes, cannabinoid CB1 receptor (CB1R) and cannabinoid CB2 receptor, have been cloned and identified in the brain [5–8]. Since the CB1Rs are found in the mesocorticolimbic system [8, 9] and activation of CB1Rs has been shown to excite midbrain dopamine neurons and increase dopamine release in the nucleus accumbens [10–13], it has been proposed that CB1R antagonists may have therapeutic effects in the treatment of drug abuse and addiction [14, 15]. In support of this hypothesis, CB1R antagonists have been shown to attenuate addiction-related behaviors for several classes of abused drugs [16], such as cocaine [17], heroin [18–21], methamphetamine [22], and nicotine [23], as assessed in intravenous drug self-administration [19, 22, 24], conditioned place preferences [25], and reinstatement of drug-seeking behavior [17, 22, 26, 27] models.
SR141716A (rimonabant) was the first selective CB1R antagonist to be developed, and it has been shown to be promising in the treatment of obesity, smoking cessation, and other drug addictions [28–32]. However, clinical studies suggest that SR141716A has significant adverse effects such as nausea and emesis and, more seriously, depression and suicidal tendencies [33, 34]. As a consequence, SR141716A has been withdrawn from clinical trials worldwide. As other CB1R antagonists/inverse agonists, such as AM251 and taranabant, have similar adverse side effects [35, 36], almost all CB1R inverse agonist-related research projects in major pharmaceutical companies worldwide have been terminated [14, 15].
The reasons underlying the adverse psychiatric effects observed with SR141716A treatment are unclear, but they may be related to its inverse agonist profile [14, 15]. Therefore, it was proposed that neutral CB1R antagonists without inverse agonist profiles should retain the therapeutic anti-addictive effects without the unwanted psychiatric effects. Accordingly, several neutral CB1R antagonists have been developed, including AM4113, AM6257, NESS0327, LH-21, and PIMSR [37–41]. AM4113 is a pyrazole-3-carboxamide analog of SR141716A [37]. Unlike SR141716A and AM251, AM4113 does not alter forskolin-stimulated cAMP formation in vitro [35], suggesting a lack of an inverse agonist profile. In experimental animals, AM4113 does not produce significant side effects, such as nausea, malaise, or anxiety-like effects [35, 37, 42], suggesting an improved safety profile over inverse CB1R agonists [43, 44]. Strikingly, recent studies in rodents and non-human primates indicated that AM4113 significantly inhibits nicotine and Δ9-THC self-administration, ethanol consumption, and nicotine-induced, cue-induced, or yohimbine-induced reinstatement of nicotine-seeking behavior [45–47], suggesting the possible utility of AM4113 in the treatment of nicotine and cannabis addiction as well as alcoholism.
Although evidence supporting the efficacy of AM4113 for addiction is accumulating, little is known regarding whether the therapeutic effects produced by AM4113 can be generalized to other drugs of abuse, such as opioids or illicit psychostimulants such as cocaine and methamphetamine. Given the currently global epidemic in opioid abuse and overdose deaths [48] and the lack of approved medications for the treatment of psychostimulant addiction [49], the development of novel pharmacotherapies for opioid and psychostimulant abuse is urgently and desperately needed. In addition, although some evidence has suggested that AM4113 may have fewer unwanted effects than SR141716A [46, 50], there is a paucity of key evidence indicating whether AM4113 produces similar aversive or depressive effects as SR141716A, which caused clinical trial termination. Therefore, in the present study, we first explored the potential utility of AM4113 in the treatment of opioid or psychostimulant abuse and addiction by using the “gold standard” intravenous drug self-administration paradigm. We then used the electrical brain-stimulation reward (BSR), the most commonly used paradigm to evaluate drug reward vs. aversion [51], to examine, and compare the effects of AM4113 and SR141716A on brain-reward function.
Materials and methods
Animals
Male Long–Evans rats (Charles River Laboratories, Raleigh, NC) weighing 280–320 g were used for all experiments. All animals were housed individually in a climate-controlled room on a reversed light–dark cycle with free access to food and water. Experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the United States National Academy of Sciences and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse (NIDA).
Drugs and chemicals
Methamphetamine HCl, cocaine HCl, and heroin HCl were obtained from the NIDA and were dissolved in sterile 0.9% physiological saline for all treatments. AM4113 and SR141716A were obtained from the Center of Drug Discovery at Northeastern University, Boston, and were dissolved in 5% cremophor.
Experiment 1: intravenous drug self-administration
Intravenous catheter surgery
Under standard aseptic surgical techniques, all animals were prepared for experimentation by surgical catheterization of the right external jugular vein, as described previously [17, 52]. Briefly, animals were anesthetized by an intraperitoneal (i.p.) injection of sodium pentobarbital (65 mg/kg), and catheters constructed of microrenathane (Braintree Scientific Inc., Braintree, MA, USA) were inserted into the right jugular vein. After being sutured into place, the catheter was passed subcutaneously to the top of the skull and excited to a connector (a modified 24-g cannula; Plastics One, Roanoke, VA, USA), which was then mounted to the skull with jeweler’s stainless-steel screws and dental acrylic. To prevent clogging, the catheters were flushed daily with a heparin–saline solution (30 IU/mL heparin; ICN Biochemicals, Cleveland, OH, USA).
Apparatus
Intravenous drug self-administration experiments were conducted in operant chambers (32 × 25 × 33 cm) from Med Associates Inc. (Georgia, VT, USA). Each chamber included two levers, one designated as active and one designated as inactive, which were located 6.5 cm above the floor. A cue light and a speaker were located 12 cm above the active lever. A house light was turned on during each 3-h test session. Depression of the active lever activated an infusion pump located outside the chamber; depression of the inactive lever was recorded but had no scheduled consequence. To promote acquisition and maintenance of drug self-administration behaviors, each drug infusion was paired with a conditioned cue light and sound (tone).
General self-administration procedure
After recovery from surgery, animals were placed into standard operant chambers for drug self-administration under a fixed-ratio 1 (FR1) reinforcement schedule. Animals were allowed to respond with the active lever to receive one of the following drugs: heroin (1 mg/kg/infusion), methamphetamine (0.05 mg/kg/infusion), or cocaine (1 mg/kg/infusion). All drugs were delivered in 0.08 mL over 4.6 s. During the 4.6-s infusion time, additional responses were recorded but did not lead to additional infusions. Inactive lever presses were counted but had no consequence. After stable FR1-reinforced responding was established, animals were then switched to a fixed-ratio 2 (FR2) reinforcement schedule to promote higher levels of drug-seeking behaviors. Self-administration training continued with heroin (0.5 mg/kg/infusion), methamphetamine (0.05 mg/kg/infusion), or cocaine (0.5 mg/kg/infusion). To avoid drug overdose during the self-administration period, each animal was limited to a maximum of 50 injections per 3-h session. The following criteria were used to determine when stable drug-maintained responding had been established: less than 10% variability in infusions earned and active lever presses and an active:inactive lever response ratio of at least 2:1 for a minimum of 3 consecutive days.
Effect of AM4113 or SR141716A pretreatment on drug self-administration
After stable heroin, methamphetamine, or cocaine self-administration under FR2 reinforcement was established as described above (requiring ~21–33 sessions), each rat randomly received one of two doses of AM4113 (3 and 10 mg/kg, i.p.), one of two doses of SR141716A (3 and 10 mg/kg), or vehicle (equal volume of 5% cremophor solution) 30 min prior to self-administration test sessions. After each drug test, animals were given an additional 5–7 days of self-administration until baseline responding was reestablished prior to testing the next dose of drug. The order of testing for the various doses of AM4113 or SR141716A was arranged according to a Latin square design.
Experiment 2: intracranial electrical Brain-Stimulation Reward
Surgery
Intracranial electrical BSR was performed as described previously [17]. Briefly, animals were anesthetized by an i.p. injection of sodium pentobarbital (65 mg/kg). Under standard aseptic surgical techniques, animals were stereotaxically implanted with unilateral monopolar stainless-steel stimulating electrodes (Plastics One, Roanoke, VA, USA) into the medial forebrain bundle at the anterior–posterior level of the lateral hypothalamus (from Bregma, AP-2.56, ML ± 1.9, and DV –8.6; Paxinos and Watson 1998), and the electrodes were mounted to the skull with jeweler’s screws and dental acrylic. A wire leading from the electrode was wrapped around a skull screw to serve as a current return.
Apparatus and general procedure
All training and testing were conducted in standard operant chambers (Med Associates, Inc., Georgia, VT, USA), which were enclosed in ventilated, sound-attenuating cabinets.
The general procedures for electrical BSR were identical to those described previously [17]. Briefly, after 7 days of recovery from surgery, rats were allowed to self-train (autoshape) to lever-press for rewarding BSR. Each press on the lever resulted in a 500-ms train with 0.1-ms rectangular cathodal pulses through the electrode implanted into the rat’s medial forebrain bundle, followed by a 500-ms “timeout” in which further presses did not produce brain stimulation. Initial stimulation parameters were set at 72 Hz and 200 μA. If an animal did not learn to lever-press, the stimulation intensity was increased daily by 50 μA until the animal acquired responding (45–60 responses/30 s) or a maximum of 800 μA was reached. Animals that did not lever-press at 800 μA or that exhibited unwanted effects from the stimulation (e.g., head or body movements or vocalization) were removed from the experiment.
Rate–frequency BSR procedure
After the establishment of lever-pressing for BSR, animals were presented with a series of 16 different pulse frequencies, ranging from 141 to 25 Hz, in descending order. At each pulse frequency, animals were allowed to respond for two 30-s time periods (bins), after which the pulse frequency decreased by 0.05 log units. After each 30-s bin, the lever retracted for 5 s. Throughout the experiment, the animals underwent three sessions a day. The response rate for each frequency was defined as the mean number of lever responses during two 30-s bins. Because lever-pressing behavior tended to be variable during the first session (the “warm-up” session) but was stable during the second and third sessions, data from the first session were discarded, and the data from the second and third sessions were designated as the baseline-session data and test-session data, respectively. The BSR threshold (θ0) was defined as the minimum frequency at which the animal responded for rewarding stimulation. The Ymax was defined as the maximal operant response (lever presses/30 s) for BSR. The BSR θ0 and Ymax were mathematically derived for each “baseline” run and each “drug” test run by analyzing each rate–frequency BSR function generated by a given animal over a given descending series of pulse frequencies using “best-fit” mathematical algorithms [17].
Effects of AM4113 or SR141716A on BSR
After a stable θ0 value or Ymax value was established (<10% variation over 5 continuous days), the effects of AM4113 or SR141716A on BSR were assessed. On each test day, the animals were randomly assigned to treatment groups. Either vehicle or a dose of AM4113 (3 and 10 mg/kg, i.p.) or SR141716A (3 and 10 mg/kg, i.p.) was administered. Following each test session, animals received an additional 5–7 days of BSR restabilization until a new baseline θ0 value or Ymax value was established. The order of testing for various doses of AM4113 or SR141716A was counterbalanced according to a Latin square design. The effect of AM4113 or SR141716A on BSR was evaluated by comparing alterations in θ0 or Ymax values in the presence or absence of each dose of drug pretreatment.
Data analyses
All data are presented as the mean ± SEM. One-way repeated-measures analysis of variance (ANOVA) was used to analyze the effects of different doses of AM4113 or SR141716A on drug self-administration and BSR. Post hoc individual group comparisons were carried out using the Student–Newman–Keuls (SNK) test. The minimally acceptable statistical significance was set at a probability level of P < 0.05 for all tests.
Results
AM4113 and SR141716A inhibit heroin self-administration
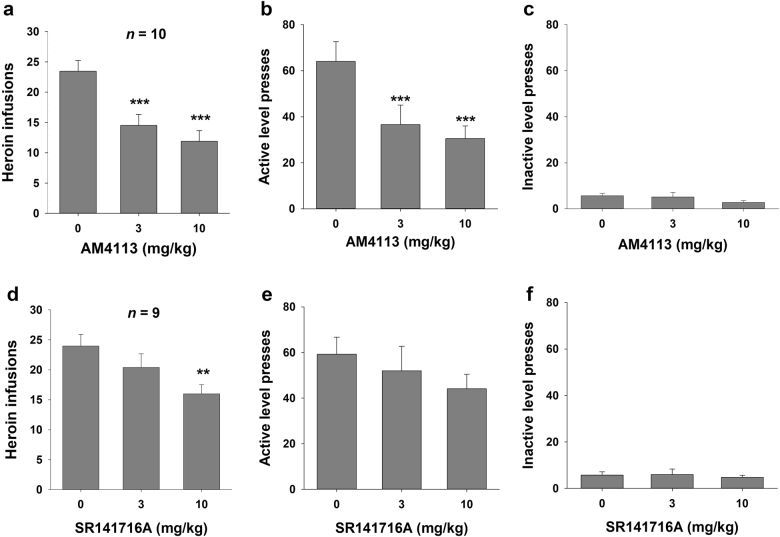
Figure 1 shows the effects of AM4113 (3 or 10 mg/kg, i.p.) or SR141716A (3 or 10 mg/kg, i.p.) on heroin self-administration under an FR2 reinforcement schedule. Pretreatment with AM4113 or SR141716A significantly and dose-dependently inhibited heroin self-administration. For AM4113, one-way repeated-measures ANOVA indicated statistically significant effects for heroin infusions and active lever presses (F2, 18 = 20.64, P < 0.001 and F2, 18 = 19.60, P < 0.001, respectively). Post hoc testing further revealed statistically significant reductions in infusions and active lever presses for heroin after 3 mg/kg (q = 8.66, P < 0.001 or q = 6.80, P < 0.001) and 10 mg/kg AM4113 (q = 6.71, P < 0.001 or q = 8.31, P < 0.001) compared with the vehicle treatment group.
Fig. 1.
Effects of AM4113 or SR141716A on heroin self-administration under a fixed-ratio 2 (FR2) reinforcement schedule. Administration of AM4113 (3 and 10 mg/kg, i.p.) significantly decreased the number of heroin infusions (a) and active lever presses (b), but had no effect on inactive lever presses (c). Administration of SR141716A (3 and 10 mg/kg, i.p.) dose-dependently decreased the number of heroin infusions (d), but did not alter active lever presses (e) or inactive lever presses (f). Data are presented as the mean ± SEM. **P < 0.01 and ***P < 0.001 vs. vehicle (0 mg/kg, i.p.)
For SR141716A, one-way repeated-measures ANOVA indicated statistically significant effects on heroin infusions (F2, 16 = 7.43, P < 0.01). Post hoc individual group comparisons revealed a statistically significant reduction in heroin infusions after 10 mg/kg AM4113 (q = 5.44, P < 0.01) but not after 3 mg/kg SR141716A (q = 2.44, P = NS) compared with the vehicle treatment group. No statistically significant effects were observed in active lever presses following SR141716A (F2, 16 = 2.61, P = NS). Moreover, neither AM4113 nor SR141716A affected inactive lever responding (F2, 18 = 1.43, P = NS and F2, 16 = 0.27, P = NS, respectively).
AM4113, but not SR141716A, inhibits methamphetamine self-administration
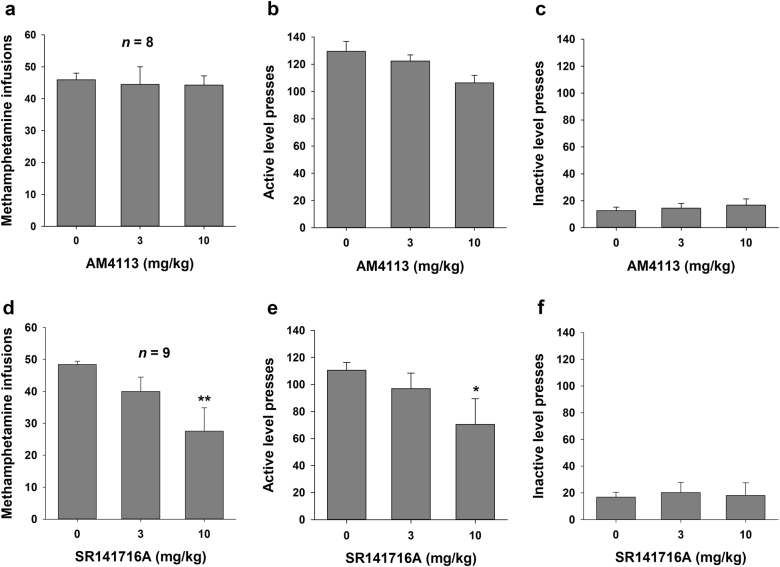
Figure 2 shows the effects of AM4113 (3 or 10 mg/kg, i.p.) and SR141716A (3 or 10 mg/kg, i.p.) on methamphetamine self-administration under an FR2 reinforcement schedule. Pretreatment with AM4113 (3 or 10 mg/kg, i.p.) had no significant effects on methamphetamine self-administration (infusions: F2, 14 = 0.07, P = NS; active lever presses: F2, 14 = 3.63, P = NS). In contrast, pretreatment with SR141716A dose-dependently decreased responding maintained by methamphetamine. One-way repeated-measures ANOVA indicated statistically significant effects of SR141716A on infusions and active lever presses for methamphetamine (F2, 16 = 5.84, P < 0.01 and F2, 16 = 4.14, P < 0.05, respectively). Post hoc individual group comparisons revealed statistically significant reductions in infusions and active lever presses for methamphetamine after 10 mg/kg AM4113 (q = 4.80, P < 0.01 and q = 4.00, P < 0.05) but not after 3 mg/kg SR141716A (q = 1.94, P = NS and q = 1.36, P = NS) compared with the vehicle treatment group. Neither AM4113 nor SR141716A pretreatment altered inactive lever presses (F2, 14 = 0.73, P = NS and F2, 16 = 0.13, P = NS, respectively).
Fig. 2.
Effects of AM4113 or SR141716A on methamphetamine self-administration under a FR2 reinforcement schedule. Administration of AM4113 did not alter the number of methamphetamine infusions (a), active lever presses (b), or inactive lever presses (c). Administration of SR141716A (10 mg/kg, i.p.) significantly decreased the number of methamphetamine infusions (d) and active lever presses (e), but had no effect on inactive lever presses (f). Data are presented as the mean ± SEM. *P < 0.05 and **P < 0.01 vs. vehicle (0 mg/kg, i.p.)
AM4113 and SR141716A have no effect on cocaine self-administration
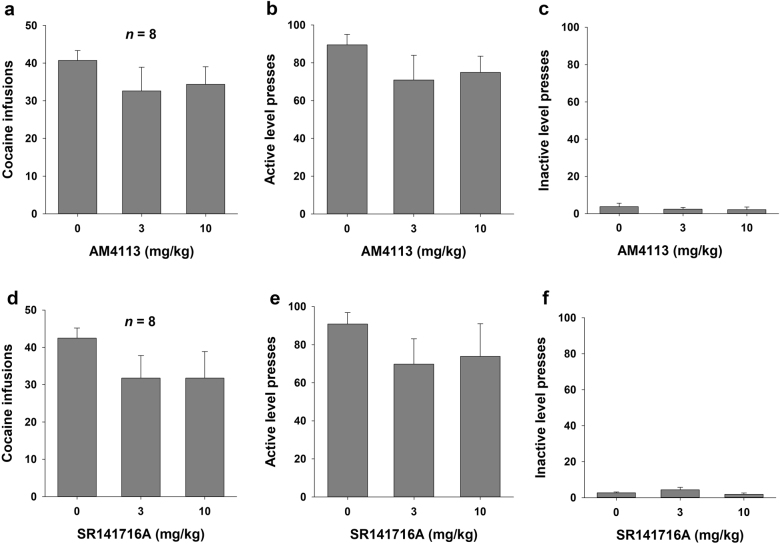
Figure 3 shows the effects of AM4113 (3 or 10 mg/kg, i.p.) or SR141716A (3 or 10 mg/kg, i.p.) on cocaine self-administration under a FR2 reinforcement schedule. One-way repeated-measures ANOVA indicated that neither AM4113 (3 or 10 mg/kg, i.p.) nor SR141716A (3 or 10 mg/kg, i.p.) pretreatment significantly altered cocaine self-administration (F2, 14 = 3.22, P = NS and F2, 14 = 2.41, P = NS, respectively). Similarly, neither AM4113 nor SR141716A had significant effects on active lever pressing (F2, 14 = 3.55, P = NS and F2, 14 = 2.14, P = NS, respectively) or inactive lever pressing (F2, 14 = 1.13, P = NS or F2, 14 = 1.89, P = NS, respectively) for cocaine reward.
Fig. 3.
Effects of AM4113 or SR141716A on cocaine self-administration under a FR2 reinforcement schedule. Administration of AM4113 did not alter the number of cocaine infusions (a), active lever presses (b), or inactive lever presses (c). Administration of SR141716A did not alter the number of cocaine infusions (d), active lever presses (e), or inactive lever presses (f). Data are presented as the mean ± SEM
SR141716A, but not AM4113, inhibits electrical BSR
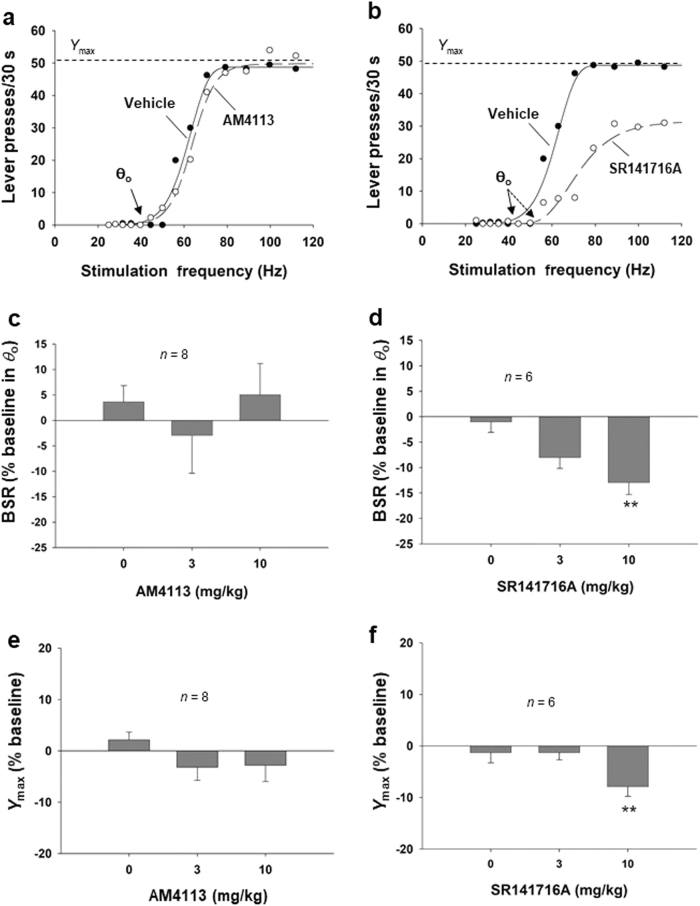
Figure 4a and b shows representative stimulation–response rate curves from individual animals after each dose of AM4113 or SR141716A, indicating BSR thresholds (θ0) and maximal operant response (Ymax level). AM4113 had no significant effect on these measures, while SR141716A significantly and dose-dependently shifted the stimulation–response rate curve to the right and increased the BSR stimulation threshold (θ0). These results suggest that there is a reduction in BSR in the presence of SR141716A, such that higher stimulation intensity (or frequency in this study) is required to achieve a given level of reward. Figure 4c and d shows the mean effects of AM4113 and SR141716A on BSR. One-way repeated-measures ANOVA indicated that pretreatment with AM4113 (Fig. 4c, F3, 14 = 0.30, P = NS) had no significant effect on θ0 values or the Ymax level (Fig. 4e, F3, 14 = 0.62, P = NS).
Fig. 4.
Effects of AM4113 and SR141716A on brain-stimulation reward (BSR). a, b Representative stimulation–response rate curves indicating that AM4113 had no effect on BSR, while SR141716A dose-dependently shifted the curve to the right and increased the BSR threshold (θ0). c AM4113 pretreatment did not affect the BSR threshold (θ0) at either dose tested. d SR141716A (10 mg/kg, i.p.) significantly decreased BSR, as assessed by the increased stimulation threshold (θ0) value. e AM4113 did not alter maximal operant responses (Ymax level). f SR141716A dose-dependently decreased the maximal operant responses (Ymax level). Data are presented as the mean ± SEM. **P < 0.01 vs. vehicle (0 mg/kg, i.p.)
In contrast to AM4113, one-way repeated-measures ANOVA for SR141716A data indicated that pretreatment with SR141716A significantly decreased BSR, as assessed by increased θ0 values (Fig. 4d, F3, 16 = 5.84, P < 0.01). Post hoc individual group comparisons using the SNK revealed statistically significant reductions in θ0 values after 10 mg/kg SR141716A (q = 4.80, P < 0.01) but not after 3 mg/kg AM4113 (q = 1.94, P = NS) compared with the vehicle treatment group. In addition, administration of SR141716A significantly altered Ymax levels (Fig. 4f, F3, 16 = 5.84, P < 0.01). Post hoc individual group comparisons using the SNK revealed a statistically significant reduction in the Ymax level after 10 mg/kg SR141716A (q = 4.80, P < 0.01) but not after 3 mg/kg AM4113 (q = 1.94, P = NS) compared with the vehicle treatment group.
Discussion
The present study is the first to demonstrate that the neutral CB1R antagonist AM4113 (3 and 10 mg/kg) dose-dependently inhibits opioid self-administration in the drug self-administration paradigm. This finding is consistent with previous reports, indicating that AM4113 also reduced nicotine self-administration and reinstatement of nicotine-seeking behavior [46, 47]. However, at the same drug doses (3–10 mg/kg) that were effective in suppressing heroin self-administration, AM4113 altered neither methamphetamine nor cocaine self-administration. In contrast, SR141716A significantly inhibited both heroin and methamphetamine intake at the higher dose tested (10 mg/kg). These results suggest that AM4113 retains the significant therapeutic anti-addictive effects of SR141716A. Importantly, SR141716A also significantly inhibited electrical BSR (as assessed by increased BSR stimulation threshold), suggesting that this drug has aversive or depressive effects, while AM4113 did not, indicating that AM4113 may not have SR141716A-like adverse psychiatric effects. Compared to SR141716A, AM4113 appeared to be more effective at reducing heroin self-administration at a lower dose, suggesting that AM4113 may have an efficacy as a pharmacotherapy for opioid addictions without the risk of depressive side effects.
Intravenous drug self-administration is one of the most commonly used animal models to study drug reward and relapse [53]. Under this model, accumulating evidence has indicated that CB1R antagonists/inverse agonists (such as SR141716A and AM251) are effective in reducing heroin-seeking and heroin-taking behaviors [18, 20, 54–56], as well as nicotine-seeking and nicotine-taking behaviors [57–60]. Consistent with these findings, in the present study, we reported that the neutral CB1R antagonist AM4113 was also effective in suppressing heroin self-administration in rats, suggesting that this neutral CB1R antagonist could act as an alternative to SR141716A in the development of a medication for the treatment of opioid abuse and addiction.
The decrease in heroin self-administration produced by AM4113 was unlikely due to non-specific sedation or locomotor impairment because AM4113 neither altered inactive lever responses during the self-administration experiments nor altered active lever responses in electrical BSR (as assessed by Ymax). It also failed to alter cocaine or methamphetamine self-administration at the same drug doses used in the heroin self-administration testing. Therefore, we exclude the possibility that AM4113 inhibits heroin self-administration by non-specific inhibition of locomotor activity.
There are some conflicting findings regarding CB1 involvement in a psychostimulant reward. In the present study, we found that neither AM4113 nor SR141716A altered cocaine self-administration under the FR2 schedule at the same drug doses. This finding is consistent with previous reports that CB1Rs were not critically involved in cocaine reward and dependence [6] and that AM4113 significantly inhibited nicotine, but not cocaine, self-administration in non-human primates [47]. Similarly, other studies have indicated that CB1R antagonists/inverse agonists (SR141716A and AM251) also failed to alter cocaine self-administration under FR reinforcement in rodents [26, 52, 61, 62] and non-human primates [47].
With respect to methamphetamine, our data indicated that AM4113 (3 and 10 mg/kg) did not inhibit methamphetamine self-administration under FR2 reinforcement conditions, while SR141716A, at 10 mg/kg, significantly inhibited methamphetamine intake but not active lever responding. This finding is consistent with previous reports that SR141716A and AM251 attenuated methamphetamine self-administration [22, 63] and reinstatement of methamphetamine-seeking behavior [64, 65]. However, at least one group has reported negative findings of AM251 on methamphetamine-induced reinstatement [66]. These observations suggest that the brain CB1Rs may be differentially involved in cocaine vs. methamphetamine reward processes. The reduction in methamphetamine self-administration may also be related to the inverse agonist effects of SR141716A or AM251 at the CB1Rs.
Electrical BSR is a reliable and sensitive method for studying the effects of drugs directly on the neural circuitry that underlies brain reward [67]. In BSR, the lowering of thresholds by a test compound is interpreted as the potentiation of the mesolimbic activity subjectively perceived as rewarding. Conversely, raising BSR thresholds is interpreted as aversion or depreciation of the rewarding value of electrical stimulation [68]. Within this framework, our data indicate that systemic administration of AM4113 had no significant effect on BSR compared to vehicle, suggesting that AM4113 has neither rewarding nor aversive effects on its own. These findings are consistent with earlier reports that showed chronic treatment with AM4113 did not alter performance on the elevated plus maze or forced swim test, which are preclinical assays of anxiety and depression, respectively [46]. In contrast, we found that SR141716A produced significant inhibition of BSR at the higher dose tested (10 mg/kg), which is in accordance with clinical findings that SR141716A produces mood-depressant side effects.
In conclusion, the present study demonstrates that the neutral CB1R antagonist AM4113 produced significant inhibitory effects on heroin self-administration but not on methamphetamine or cocaine self-administration. Unlike the CB1R inverse agonist/antagonist SR141716A, AM4113 alone has no significant effect on the BSR threshold, suggesting that it will have fewer mood-depressant-like side effects. These results suggest that AM4113 or other more potent neutral CB1R antagonists may be effective in the treatment of opioid abuse and addiction without adverse psychiatric effects.
Acknowledgements
This research was supported by the Intramural Research Program (IRP) of the National Institute on Drug Abuse (NIDA) (Z1A DA000389), National Institutes of Health (NIH).
Author contributions
ELG, AM, Y-lW, and Z-xX designed the experiments. X-hH, KV, G-hB, and JZ performed the experiments. X-hH, CJJ, and Z-xX analyzed the data and wrote the manuscript with help from all other co-authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yan-lin Wang, Email: wyl0342@sina.com.
Zheng-xiong Xi, Email: zxi@mail.nih.gov.
References
- 1.Brady JE, Li G. Prevalence of alcohol and other drugs in fatally injured drivers. Addiction. 2013;108:104–14. doi: 10.1111/j.1360-0443.2012.03993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naim-Feil J, Zangen A. Addiction. Handb Clin Neurol. 2013;116:613–30. doi: 10.1016/B978-0-444-53497-2.00049-8. [DOI] [PubMed] [Google Scholar]
- 3.Jing L, Qiu Y, Zhang Y, Li JX. Effects of the cannabinoid CB(1) receptor allosteric modulator ORG 27569 on reinstatement of cocaine- and methamphetamine-seeking behavior in rats. Drug Alcohol Depend. 2014;143:251–6. doi: 10.1016/j.drugalcdep.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robson PJ. Therapeutic potential of cannabinoid medicines. Drug Test Anal. 2014;6:24–30. doi: 10.1002/dta.1529. [DOI] [PubMed] [Google Scholar]
- 5.Xi ZX, et al. Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14:1160–6. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiskerke J, Pattij T, Schoffelmeer AN, De Vries TJ. The role of CB1 receptors in psychostimulant addiction. Addict Biol. 2008;13:225–38. doi: 10.1111/j.1369-1600.2008.00109.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang HY, et al. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci USA. 2014;111:E5007–15. doi: 10.1073/pnas.1413210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herkenham M, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–6. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–68. doi: 10.1016/0306-4522(92)90409-U. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Paredes W, Lowinson JH, Gardner EL. Delta 9-tetrahydrocannabinol enhances presynaptic dopamine efflux in medial prefrontal cortex. Eur J Pharmacol. 1990;190:259–62. doi: 10.1016/0014-2999(90)94136-L. [DOI] [PubMed] [Google Scholar]
- 11.Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–50. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 12.Oleson EB, Cheer JF. A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harb Perspect Med. 2012;2: pii:a012229. doi: 10.1101/cshperspect.a012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lupica CR, Riegel AC. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48:1105–16. doi: 10.1016/j.neuropharm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology. 2009;205:171–4. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamaleddin IH, et al. Role of the endogenous cannabinoid system in nicotine addiction: novel insights. Front Psychiatry. 2015;6:41. doi: 10.3389/fpsyt.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312:875–83. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- 17.Xi ZX, et al. Cannabinoid CB1 receptor antagonists attenuate cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology. 2008;33:1735–45. doi: 10.1038/sj.npp.1301552. [DOI] [PubMed] [Google Scholar]
- 18.Navarro M, et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–50. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caille S, Parsons LH. Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology. 2006;31:804–13. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- 20.Caille S, Parsons LH. SR141716A reduces the reinforcing properties of heroin but not heroin-induced increases in nucleus accumbens dopamine in rats. Eur J Neurosci. 2003;18:3145–9. doi: 10.1111/j.1460-9568.2003.02961.x. [DOI] [PubMed] [Google Scholar]
- 21.Navarro M, et al. Cannabinoid receptor antagonist reduces heroin self-administration only in dependent rats. Eur J Pharmacol. 2004;501:235–7. doi: 10.1016/j.ejphar.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Schindler CW, et al. Effects of cannabinoid receptor antagonists on maintenance and reinstatement of methamphetamine self-administration in rhesus monkeys. Eur J Pharmacol. 2010;633:44–9. doi: 10.1016/j.ejphar.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cahill K, Ussher MH. Cannabinoid type 1 receptor antagonists for smoking cessation. Cochrane Database Syst Rev. 2011;16:CD005353. [DOI] [PMC free article] [PubMed]
- 24.De Vries TJ, Homberg JR, Binnekade R, Raaso H, Schoffelmeer AN. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology. 2003;168:164–9. doi: 10.1007/s00213-003-1422-1. [DOI] [PubMed] [Google Scholar]
- 25.Chaperon F, Soubrie P, Puech AJ, Thiebot MH. Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology. 1998;135:324–32. doi: 10.1007/s002130050518. [DOI] [PubMed] [Google Scholar]
- 26.De Vries TJ, et al. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–4. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- 27.Ward SJ, Rosenberg M, Dykstra LA, Walker EA. The CB1 antagonist rimonabant (SR141716) blocks cue-induced reinstatement of cocaine seeking and other context and extinction phenomena predictive of relapse. Drug Alcohol Depend. 2009;105:248–55. doi: 10.1016/j.drugalcdep.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carai MA, Colombo G, Gessa GL. Rimonabant: the first therapeutically relevant cannabinoid antagonist. Life Sci. 2005;77:2339–50. doi: 10.1016/j.lfs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Pacher P, et al. Hemodynamic profile, responsiveness to anandamide, and baroreflex sensitivity of mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol. 2005;289:H533–41. doi: 10.1152/ajpheart.00107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rigotti NA, et al. A randomized controlled trial of adding the nicotine patch to rimonabant for smoking cessation: efficacy, safety and weight gain. Addiction. 2009;104:266–76. doi: 10.1111/j.1360-0443.2008.02454.x. [DOI] [PubMed] [Google Scholar]
- 31.Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369:71–7. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 32.Leite CE, Mocelin CA, Petersen GO, Leal MB, Thiesen FV. Rimonabant: an antagonist drug of the endocannabinoid system for the treatment of obesity. Pharmacol Rep. 2009;61:217–24. doi: 10.1016/S1734-1140(09)70025-8. [DOI] [PubMed] [Google Scholar]
- 33.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–97. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 34.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. J Am Med Assoc. 2006;295:761–75. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 35.Chambers AP, et al. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2185–93. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- 36.Proietto J, et al. A clinical trial assessing the safety and efficacy of the CB1R inverse agonist taranabant in obese and overweight patients: low-dose study. Int J Obes. 2010;34:1243–54. doi: 10.1038/ijo.2010.38. [DOI] [PubMed] [Google Scholar]
- 37.Sink KS, et al. The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008;33:946–55. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sink KS, Vemuri VK, Wood J, Makriyannis A, Salamone JD. Oral bioavailability of the novel cannabinoid CB1 antagonist AM6527: effects on food-reinforced behavior and comparisons with AM4113. Pharmacol Biochem Behav. 2009;91:303–6. doi: 10.1016/j.pbb.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiu S, et al. Synthesis and characterization of NESS 0327: a novel putative antagonist of the CB1 cannabinoid receptor. J Pharmacol Exp Ther. 2003;306:363–70. doi: 10.1124/jpet.103.049924. [DOI] [PubMed] [Google Scholar]
- 40.Seltzman HH, et al. Metabolic profiling of CB1 neutral antagonists. Methods Enzymol. 2017;593:199–215. doi: 10.1016/bs.mie.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavon FJ, et al. Antiobesity effects of the novel in vivo neutral cannabinoid receptor antagonist 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1H-1,2,4-triazole--LH 21. Neuropharmacology. 2006;51:358–66. doi: 10.1016/j.neuropharm.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 42.Jarbe TU, et al. Intrinsic effects of AM4113, a putative neutral CB1 receptor selective antagonist, on open-field behaviors in rats. Pharmacol Biochem Behav. 2008;91:84–90. doi: 10.1016/j.pbb.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kangas BD, et al. Cannabinoid discrimination and antagonism by CB(1) neutral and inverse agonist antagonists. J Pharmacol Exp Ther. 2013;344:561–7. doi: 10.1124/jpet.112.201962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wills KL, et al. CB1 antagonism: interference with affective properties of acute naloxone-precipitated morphine withdrawal in rats. Psychopharmacology. 2014;231:4291–300. doi: 10.1007/s00213-014-3575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balla A, et al. Cannabinoid-1 receptor neutral antagonist reduces binge-like alcohol consumption and alcohol-induced accumbal dopaminergic signaling. Neuropharmacology. 2017;131:200–8. doi: 10.1016/j.neuropharm.2017.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gueye AB, et al. The CB1 neutral antagonist AM4113 retains the therapeutic efficacy of the inverse agonist rimonabant for nicotine dependence and weight loss with better psychiatric tolerability. Int J Neuropsychopharmacol. 2016;19:pii:pyw068. doi: 10.1093/ijnp/pyw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schindler CW, et al. Blockade of nicotine and cannabinoid reinforcement and relapse by a cannabinoid CB1-receptor neutral antagonist AM4113 and inverse agonist rimonabant in squirrel monkeys. Neuropsychopharmacology. 2016;41:2283–93. doi: 10.1038/npp.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolodny A, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–74. doi: 10.1146/annurev-publhealth-031914-122957. [DOI] [PubMed] [Google Scholar]
- 49.Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–86. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Storr MA, et al. Differential effects of CB(1) neutral antagonists and inverse agonists on gastrointestinal motility in mice. Neurogastroenterol Motil. 2010;22:787–96. doi: 10.1111/j.1365-2982.2010.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–84. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 52.Xi ZX, et al. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–6. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 54.Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl) -4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther. 2003;306:93–102. doi: 10.1124/jpet.102.047928. [DOI] [PubMed] [Google Scholar]
- 55.Fattore L, et al. Cannabinoid CB(1) antagonist SR 141716A attenuates reinstatement of heroin self-administration in heroin-abstinent rats. Neuropharmacology. 2005;48:1097–104. doi: 10.1016/j.neuropharm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez-Jaimes L, Polis I, Parsons LH. Attenuation of cue-induced heroin-seeking behavior by cannabinoid CB1 antagonist infusions into the nucleus accumbens core and prefrontal cortex, but not basolateral amygdala. Neuropsychopharmacology. 2008;33:2483–93. doi: 10.1038/sj.npp.1301630. [DOI] [PubMed] [Google Scholar]
- 57.Cohen C, Kodas E, Griebel G. CB1 receptor antagonists for the treatment of nicotine addiction. Pharmacol Biochem Behav. 2005;81:387–95. doi: 10.1016/j.pbb.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 58.Shoaib M. The cannabinoid antagonist AM251 attenuates nicotine self-administration and nicotine-seeking behaviour in rats. Neuropharmacology. 2008;54:438–44. doi: 10.1016/j.neuropharm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 59.Budzynska B, Kruk M, Biala G. Effects of the cannabinoid CB1 receptor antagonist AM 251 on the reinstatement of nicotine-conditioned place preference by drug priming in rats. Pharmacol Rep. 2009;61:304–10. doi: 10.1016/S1734-1140(09)70036-2. [DOI] [PubMed] [Google Scholar]
- 60.Simonnet A, Cador M, Caille S. Nicotine reinforcement is reduced by cannabinoid CB1 receptor blockade in the ventral tegmental area. Addict Biol. 2013;18:930–6. doi: 10.1111/j.1369-1600.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 61.Filip M, et al. Involvement of cannabinoid CB1 receptors in drug addiction: effects of rimonabant on behavioral responses induced by cocaine. Pharmacol Rep. 2006;58:806–19. [PubMed] [Google Scholar]
- 62.Lesscher HM, Hoogveld E, Burbach JP, van Ree JM, Gerrits MA. Endogenous cannabinoids are not involved in cocaine reinforcement and development of cocaine-induced behavioural sensitization. Eur Neuropsychopharmacol. 2005;15:31–7. doi: 10.1016/j.euroneuro.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Vinklerova J, Novakova J, Sulcova A. Inhibition of methamphetamine self-administration in rats by cannabinoid receptor antagonist AM 251. J Psychopharmacol. 2002;16:139–43. doi: 10.1177/026988110201600204. [DOI] [PubMed] [Google Scholar]
- 64.Anggadiredja K, et al. Endocannabinoid system modulates relapse to methamphetamine seeking: possible mediation by the arachidonic acid cascade. Neuropsychopharmacology. 2004;29:1470–8. doi: 10.1038/sj.npp.1300454. [DOI] [PubMed] [Google Scholar]
- 65.Hiranita T, Nawata Y, Sakimura K, Yamamoto T. Methamphetamine-seeking behavior is due to inhibition of nicotinic cholinergic transmission by activation of cannabinoid CB1 receptors. Neuropharmacology. 2008;55:1300–6. doi: 10.1016/j.neuropharm.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Boctor SY, Martinez JL, Jr., Koek W, France CP. The cannabinoid CB1 receptor antagonist AM251 does not modify methamphetamine reinstatement of responding. Eur J Pharmacol. 2007;571:39–43. doi: 10.1016/j.ejphar.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–40. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- 68.Fish EW, et al. Alcohol, cocaine, and brain stimulation-reward in C57Bl6/J and DBA2/J mice. Alcohol Clin Exp Res. 2010;34:81–9. doi: 10.1111/j.1530-0277.2009.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paxinos G & Watson C (1998) The Rat Brain in Stereotaxic Coordinates. Academic Press.