Abstract
Conjunctival melanoma is a rare primary ocular tumor. So far, no standard treatment exists for metastatic disease. Similarly to cutaneous melanoma, up to 50% of conjunctival melanomas harbor BRAF mutations. The most common is represented by V600E. Combined therapy with BRAF and MEK inhibitors is approved for BRAF mutant cutaneous metastatic melanomas. Herein, we report a case of a 70-years old patient with a metastatic conjunctival melanoma harboring V600E BRAF mutation successfully treated with dabrafenib and trametinib.
Keywords: BRAF, MEK, target therapy, conjunctival, melanoma
Introduction
Conjunctival malignant melanoma is a rare primary ocular tumor with an incidence of 0.2–0.7 per million in Europe and the US (1, 2). Metastases in conjunctival melanoma spread through lymphatic and hematic way, reaching lungs, liver, skin, and brain (3–5). Ten-year mortality rate of conjunctival melanoma is ~30% (5–7). So far, no standard treatment is approved for metastatic conjunctival melanoma.
The Ras/Raf/Mitogen-activated protein kinase (MAPK) pathway has been described as one of the most important regulatory pathways involved in melanoma development (8, 9). MAPK is a signaling cascade starting from receptor-linked tyrosine kinases that activate Ras, then B-raf, MEK1 and MEK2 and finally ERK, that regulates transcriptional factors involved in proliferation, differentiation, apoptosis, and senescence. Around 40–50% of cutaneous melanomas harbor BRAF mutations, that appear at an early stage of tumor development. In fact, activating BRAF mutations have a pro-oncogenic effect. The most common mutation is represented by the substitution of a valine with glutamic acid in the codon 600 (V600E), that increases the catalytic activity of B-raf (10, 11).
Conjunctival melanoma has not been completely characterized at the level of genetics, however, BRAF mutations have been reported in up to 50%, with V600E as the most frequent one (around 80–90%), followed by V600K (2, 8, 12–15).
Treatment with the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib is approved for V600-BRAF mutant patients with cutaneous but not conjunctival melanomas (16–18). In fact, due to its rarity, conjunctival melanoma patients are not included in most clinical trials with BRAF or MEK inhibitors.
Herein, we present a case of a patient with metastatic conjunctival melanoma harboring BRAF V600E mutation who was effectively treated with dabrafenib and trametinib. To the best of our knowledge, there is no previously described treatment response to dabrafenib and trametinib in metastatic conjunctival melanoma.
Case Description
A 70-years-old Caucasian man presented in April 2016 with a pigmented lesion of about 1 cm located in the temporal limbus of the left bulbar conjunctiva (Figure 1). Patient was in good clinical conditions; the Eastern Cooperative Oncology Group Performance Status (ECOG PS) was 0. No relevant comorbidities or concomitant medications existed. The patient was an officer employee and had no familiarity for neoplasms nor environmental risk factor exposure. The patient underwent a complete escissional biopsy of the bulbar conjunctiva. The histological analysis demonstrated a conjunctival melanoma with a thickness of 0.3 cm. The extracted DNA was amplified with specific primers for exon 15 of BRAF gene and then sequenced. Codon 600 was also analyzed through Idylla™ BRAF mutation test. The heterozygote mutation of codon V600 was detected, but the specific amminoacidic substitution was not identified due to the scarcity of histological tissue.
Figure 1.
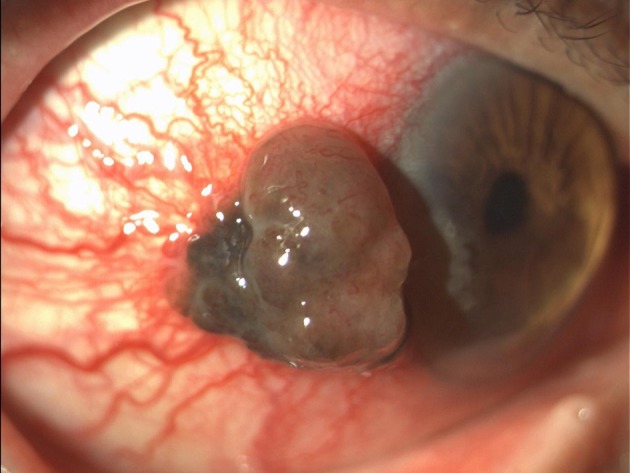
Conjunctival melanoma at presentation.
In July 2017, the patient noticed a tumefaction of the right parotid region. The 18fluoro-desossi-glucose (FDG) positron emission tomography (PET)/computerized tomography (CT) scan showed an uptake in a nodule of the right parotid gland (ø 23 mm) and latero-cervical lymph node metastases with a maximal standard uptake value (SUV) of 4.4. The fine needle aspiration cytology (FNAC) revealed melanoma cells. Therefore, the patient underwent a right parotidectomy with latero-cervical lymph node dissection. The histologic exam confirmed the parotid metastasis from conjunctival melanoma. Metastasis of melanoma was also detected in 1/13 lymph nodes. There was no evidence of extra nodal invasion. The extracted DNA was amplified with specific primers for exon 15 of BRAF gene and then sequenced. V600E mutation (T1799A) was detected.
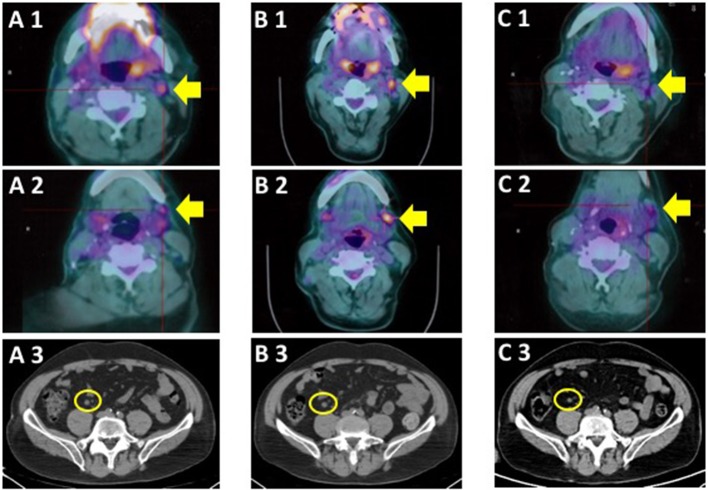
The 18FDG PET scan after intervention (October 2017), revealed a residual tumor in the lymph nodes located in left retro-mandibular and latero-cervical areas (maximal SUV 3.9) and a nodule in the mesenteric adipose tissue in right hypogastrium (Figure 2A). LDH was 700 IU/L (ULN 450 IU/L). Other cases of BRAF mutant conjunctival melanoma experienced a disease control with BRAF inhibition therapy (Table 1). In metastatic cutaneous melanoma, the combination of anti-BRAF and anti-MEK demonstrated better survival results vs. anti-BRAF alone. Indeed, we hypothesized that the target combination therapy could be the best option also for this BRAF mutant conjunctival melanoma patient. The combination of these drugs is not approved for conjunctival melanoma, thus we requested the off-label use in accordance with our institutional policy. After the institutional permission, the written consent was obtained by the patient. Then we started therapy with Dabrafenib (150 mg twice daily) and Trametinib (2 mg daily), choosing the standard dosage approved for metastatic cutaneous melanoma (24).
Figure 2.
Comparison of 18FDG-PET/CT scan at baseline (A), first (B) and second (C) assessment during the treatment. 1: left retro-mandibular lymph node, indicated by yellow arrows; 2: left latero-cervical lymph node, indicated by yellow arrows; 3: nodule in the mesenteric adipose tissue in right hypogastrium, circled.
Table 1.
Previous reports of treatment with BRAF and/or MEK inhibitors in conjunctival melanoma patients.
| References | Disease sites | Drug | Duration | Side effects | Outcome |
|---|---|---|---|---|---|
| Pahlitzsch et al. (19) | Local disease | Vemurafenib (not specified dose) | 16 months | Weight loss, nausea and vomiting, headaches | Local control |
| Pinto Torres et al. (20) | Local recurrent disease | Vemurafenib (960 mg twice a day, reduced to 480 mg twice a day due to toxicity) | 34 months | Grade 2 diarrhea, grade 2 arthralgia, grade 1 skin rash, needing a dose reduction | Complete response |
| Maleka et al. (21) | Pre-treated metastatic disease | Vemurafenib (960 mg twice a day, reduced to 720 mg twice a day due to toxicity) | 4 months | Grade 2 maculopapular rash, needing a dose reduction | Partial response |
| Griewank et al. (22) | Pre-treated metastatic disease | Dabrafenib (150 mg twice a day) | 6 months | [Not reported] | Partial response |
| Dagi Glass et al. (23) | Locally advanced disease | Dabrafenib and Trametinib interrupted for toxicity | 1.5 months | Nausea and vomiting, leading to therapy discontinuation | Patient followed for over 23 mo without distant spread |
| Vemurafenib | 3.5 months | [Not reported] | |||
| Pembrolizumab | 2 months | ||||
| Vemurafenib and Cobimetinib (added after 4 months). | Not reported | [Not reported] |
Vital signs, dermatological exam and biochemistry analysis had been performed monthly. The therapy was complicated by fever with body temperature between 37.5 and 38°C. No signs of infections were detected. Patient took paracetamol for the fever and the adverse event resolved without squeal after 2 months. No dose reduction or interruption was needed.
The first 18FDG-PET/CT scan after 4 months of therapy revealed the persistence of lymph node disease (with increased maximal SUV to 4.4), while the mesenteric nodule was stable (Figure 2B). The cranial CT scan with contrast enhancement did not reveal any sign of tumor recurrence. The combination of Dabrabenib/Trametinib was continued.
The following 18FDG-PET/CT scan was performed after further 4 months of therapy. It showed the reduction of the metabolic activity of the lymph node metastases of the neck (maximal SUV 3). The mesenteric nodule was not modified (Figure 2C). The magnetic resonance imaging (MRI) of the brain did not reveal ocular recurrence of melanoma nor encephalic metastases.
Ophthalmic examinations have been performed every 6 months, and they detected no signs of uveitis or iridocyclitis. The patient never complained of photophobia, ocular pain, or other visual alterations.
The blood analysis performed in August 2018 revealed a grade 1 hypertransaminasemia.
On September 2018, the treatment with dabrafenib and trametinib is ongoing at the initial dose. No dose reduction was necessary.
Discussion
Over the last years, therapy for metastatic cutaneous melanoma has been strengthened by target therapies, including treatments against the MAPK pathway. Dabrafenib is a highly selective inhibitor of mutated BRAF and trametinib inhibits MEK1 and MEK2. The combination of BRAF and MEK inhibitors shows response rates up to 70% with an improved survival in metastatic cutaneous melanoma harboring BRAF mutations of the codon 600 (16–18, 25). NCT01584648 registration trial showed a median progression free survival for V600-BRAF mutant melanoma patients treated with dabrafenib plus trametinib of 9.3 months, and an overall survival of 25 months (17, 24).
It has been shown that activating BRAF mutations are detectable in up to 50% of conjunctival melanomas, of which about 80% are V600E, 15–20% V600K (15, 26). In vitro, Vemurafenib and Dabrafenib inhibit the growth of BRAF-mutated conjunctival melanoma cell lines; MEK inhibitors also promote apoptosis of cell lines from conjunctival melanoma (27). However, neither BRAF inhibitors nor MEK inhibitors are yet approved for patients with conjunctival melanomas.
Previous attempts of treating metastatic conjunctival melanomas with BRAF inhibitors have been reported (Table 1). A patient with a BRAF-mutant conjunctival melanoma that refused the exenteration of the orbit was treated with BRAF inhibitor Vemurafenib for 16 months, obtaining a local control that permitted a more conservative surgery. The patient developed weight loss, nausea, and vomiting (19).
A second patient with a recurrent BRAF mutant conjunctival melanoma was effectively treated with Vemurafenib, experiencing a complete remission for over 3 years. Dose reduction was needed because of toxicity, represented by grade 2 diarrhea, grade 2 arthralgia, and grade 1 skin rash (20).
A third patient with a BRAF mutant pre-treated metastatic conjunctival melanoma benefitted from Vemurafenib with a partial response for about 4 months. Grade 2 maculopapular rash was reported, needing a dose reduction (21).
Another patient with pre-treated metastatic conjunctival melanoma expressing BRAF mutation developed a partial response for over 6 months of therapy with the BRAF inhibitor Dabrafenib (22).
Another report described a patient with a locally advanced conjunctival melanoma that was initially treated with combined BRAF/MEK inhibitors (Dabrafenib and Trametinib). After 1.5 months, the treatment was interrupted for toxicity represented by nausea and vomiting, and Vemurafenib alone was continued. Therapy was interrupted 3.5 months later for progressive disease to start Pembrolizumab. After progression, firstly the BRAF inhibitor alone (Vemurafenib) was restarted and then a MEK inhibitor (Cobimetinib) was added. The patient was followed for over 23 months without evidence of distant spread (23).
In advanced cutaneous melanoma, the combined inhibition of BRAF/MEK in case of BRAF mutation is effective. The lack of clinical trial does not allow to consider this mutation as a therapeutic target in conjunctival melanoma. However, the patient described in this case report achieved a clinical benefit from this treatment. Thus, it is conceivable that patients with BRAF-mutant conjunctival melanoma could benefit from treatment with BRAF/MEK inhibitors.
Conclusions
No standard treatment for metastatic conjunctival melanoma has been established. To our knowledge, we present the first patient with a BRAF-mutant metastatic conjunctival melanoma who has been effectively treated with a combined use of BRAF/MEK inhibitor. BRAF mutations need to be investigated in conjunctival melanomas. Further studies are needed to assess the efficacy of BRAF and MEK inhibitors in this subtype of ocular melanoma.
Ethics Statement
Written informed consent, according to the Declaration of Helsinki, for treatment and publication of this case report, including the images, was obtained from the patient.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of non-cutaneous melanomas in the US. Cancer. (2005) 103:1000–7. 10.1002/cncr.20866 [DOI] [PubMed] [Google Scholar]
- 2.Kastelan S, Gverović Antunica A, Orešković LB, Rabatic JS, Kasun B, Bakija I. Conjunctival melanoma - epidemiological trends and features. Pathol Oncol Res. (2018) 24:787–96. 10.1007/s12253-018-0419-3 [DOI] [PubMed] [Google Scholar]
- 3.Shields CL, Shields JA, Gündüz K, Cater J, Mercado GV, Gross N, et al. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Arch Ophthalmol. (2000) 118:1497–507. 10.1001/archopht.118.11.1497 [DOI] [PubMed] [Google Scholar]
- 4.Esmaeli B, Wang X, Youssef A, Gershenwald JE. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer over four decades. Ophthalmol. (2001) 108:2101–5. 10.1016/S0161-6420(01)00782-5 [DOI] [PubMed] [Google Scholar]
- 5.Missotten GS, Keijser S, De Keizer RJ, De Wolff-Rouendaal D. Conjunctival melanoma in the Netherlands: a nationwide study. Invest Ophthalmol Vis Sci. (2005) 46:75–82. 10.1167/iovs.04-0344 [DOI] [PubMed] [Google Scholar]
- 6.Triay E, Bergman L, Nilsson B, All-Ericsson C, Seregard S. Time trends in the incidence of conjunctival melanoma in Sweden. Br J Ophthalmol. (2009) 93:1524–8. 10.1136/bjo.2009.157933 [DOI] [PubMed] [Google Scholar]
- 7.Shildkrot Y, Wilson MW. Conjunctival melanoma: pitfalls and dilemmas in management. Curr Opin Ophthalmol. (2010) 21:380–6. 10.1097/ICU.0b013e32833b7aab [DOI] [PubMed] [Google Scholar]
- 8.Spendlove HE, Damato BE, Humphreys J, Barker KT, Hiscott PS, Houlston RS. BRAF mutations are detectable in conjunctival but not uveal melanomas. Melanoma Res. (2004) 14:449–52. 10.1097/00008390-200412000-00003 [DOI] [PubMed] [Google Scholar]
- 9.Dahl C, Guldberg P. The genome and epigenome of malignant melanoma. APMIS. (2007) 115:1161–76. 10.1111/j.1600-0463.2007.apm_855.xml.x [DOI] [PubMed] [Google Scholar]
- 10.Goel VK, Lazar AJ, Warneke CL, Redston MS, Haluska FG. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J Invest Dermatol. (2006) 126:154–60. 10.1038/sj.jid.5700026 [DOI] [PubMed] [Google Scholar]
- 11.Omholt K, Platz A, Kanter L, Ringborg U, Hansson J. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. (2003) 9:6483–8. [PubMed] [Google Scholar]
- 12.Seregard S. Conjunctival melanoma. Surv Ophthalmol. (1998) 42:321–50. [DOI] [PubMed] [Google Scholar]
- 13.Gear H, Williams H, Kemp EG, Roberts F. BRAF mutations in conjunctival melanoma. Invest Ophthalmol Vis Sci. (2004) 45:2484–8. 10.1167/iovs.04-0093 [DOI] [PubMed] [Google Scholar]
- 14.Lake SL, Jmor F, Dopierala J, Taktak AF, Coupland SE, Damato BE. Multiplex ligation-dependent probe amplification of conjunctival melanoma reveals common BRAF V600E gene mutation and gene copy number changes. Invest Ophthalmol Vis Sci. (2011) 52:5598–604. 10.1167/iovs.10-6934 [DOI] [PubMed] [Google Scholar]
- 15.Griewank KG, Westekemper H, Murali R, Mach M, Schilling B, Wiesner T, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. (2013) 19:3143–52. 10.1158/1078-0432.CCR-13-0163 [DOI] [PubMed] [Google Scholar]
- 16.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. (2012) 367:1694–703. 10.1056/NEJMoa1210093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. (2015) 386:444–51. 10.1016/S0140-6736(15)60898-4 [DOI] [PubMed] [Google Scholar]
- 18.Grob JJ, Amonkar MM, Karaszewska B, Schachter J, Dummer R, Mackiewicz A, et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): results of a phase 3, open-label, randomised trial. Lancet Oncol. (2015) 16:1389–98. 10.1016/S1470-2045(15)00087-X [DOI] [PubMed] [Google Scholar]
- 19.Pahlitzsch M, Bertelmann E, Mai C. Conjunctival melanoma and BRAF inhibitor therapy. J Clin Exp Ophthalmol. (2014) 5:322 10.4172/2155-9570.1000322 [DOI] [Google Scholar]
- 20.Pinto Torres S, André T, Gouveia E, Costa L, Passos MJ. Systemic treatment of metastatic conjunctival melanoma. Case Rep Oncol Med. (2017) 2017:4623964. 10.1155/2017/4623964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maleka A, Astrom G, Byström P, Ullenhag GJ. A case report of a patient with metastatic ocular melanoma who experienced a response to treatment with the BRAF inhibitor vemurafenib. BMC Cancer. (2016) 16:634. 10.1186/s12885-016-2657-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griewank KG, Westekemper H, Schilling B, Livingstone E, Schimming T, Sucker A, et al. Conjunctival melanomas harbor BRAF and NRAS mutations–letter. Clin Cancer Res. (2013) 19:6331–2. 10.1158/1078-0432.CCR-13-2368 [DOI] [PubMed] [Google Scholar]
- 23.Dagi Glass LR, Lawrence DP, Jakobiec FA, Freitag SK. Conjunctival melanoma responsive to combined systemic BRAF/MEK inhibitors. Ophthalmic Plast Reconstr Surg. (2017) 33:e114–6. 10.1097/IOP.0000000000000833 [DOI] [PubMed] [Google Scholar]
- 24.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. (2014) 371:1877–88. 10.1056/NEJMoa1406037 [DOI] [PubMed] [Google Scholar]
- 25.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. (2015) 372:30–9. 10.1056/NEJMoa1412690 [DOI] [PubMed] [Google Scholar]
- 26.Larsen AC, Dahmcke CM, Dahl C, Siersma VD, Toft PB, Coupland SE, et al. A retrospective review of conjunctival melanoma presentation, treatment, and outcome and an investigation of features associated with BRAF mutations. JAMA Ophthalmol. (2015) 133:1295–303. 10.1001/jamaophthalmol.2015.3200 [DOI] [PubMed] [Google Scholar]
- 27.Cao J, Heijkants RC, Jochemsen AG, Dogrusöz M, de Braud MJ, van der Velden PA, et al. Targeting of the MAPK and AKT pathways in conjunctival melanoma shows potential synergy. Oncotarget. (2016) 8:58021–36. 10.18632/oncotarget.10770 [DOI] [PMC free article] [PubMed] [Google Scholar]