Abstract
A cheap and simple sample preparation method, consisting of a dispersive solid-phase method and an adsorbent, activated carbon decorated PAN nanofibers, was employed and used for the extraction of antibiotics (ciprofloxacin, danofloxacin, and enrofloxacin) in wastewater. Electrospun PAN nanofibers that were decorated with activated carbon produced from waste tires were used as the solid phase and the antibiotics analyzed by using high-performance liquid chromatography. Parameters such as pH, mass of adsorbent (MA), extraction volume (EV), and extraction time (ET) were optimized owing to their potential effect on the extraction of antibiotics from water. The recovery of all antibiotics was satisfactory, in the range of 90%–99%. The limits of detection and quantification were 0.05, 0.11, 0.20, and 0.53, 1.21, 2.17 µg/L, respectively. The precision was determined from the repeatability and reproducibility and expressed as the intra-day (n = 20) and inter-day (n = 5) precision. The intra-day and inter-day precision was reported in terms of the percentage relative standard deviation, which was 3% and 4%, respectively. The adsorption capacity of the activated carbon-decorated PAN nanofibers was satisfactory, and the reusability of the adsorbent was impressive when reused ten times. The accuracy of the dispersive solid phase extraction (DSPE) was validated by spike recovery tests; the results proved the reliability and efficiency of adsorbing antibiotics from wastewater. Finally, the proposed method was applied to wastewater samples collected from a wastewater treatment plant, which included influent, secondary, and effluent wastewater.
Keywords: Wastewater, Nanofibers, Activated carbon, Antibiotics, Polyacrylonitrile
1. Introduction
Pharmaceutical drugs are essential for animal and human health and are used on a large scale as growth promoters and for therapeutic purposes [1]. These drugs are classified as emerging pollutants owing to their persistence and biologically active behavior in the environment [1]. Relatively little information is known about their reactivity and toxicity as they are not regulated in terms of threshold or accepted environmental levels [2]. There are numerous pharmaceutical drugs that are used frequently to combat sickness; of these, antibiotics are one type of drugs that pose serious threats to humans, animals, and the environment [3]. Antibiotics are useful to humans as they prevent bacterial infections, but their release into the environment is a problem that needs to be resolved to allow water bodies to be free of such toxic pollutants [3]. Antibiotics such as fluoroquinolones (FQs), a type of quinolone antibiotic, have been extensively used as veterinary medicines [4]. Fluorinated quinolones, such as ciprofloxacin, danofloxacin, and enrofloxacin, have been in use for the past 20 years and they are of particular concern to the environment [4]. These antibiotics are known to treat gram-positive and gram-negative bacteria and their purpose is to inhibit DNA gyrase [5], [6]. The consumption of FQs is a problem as they are partially metabolized and then excreted from the body, in urine and feces, in their active forms [5], [7]. The prevalence of FQs in the environment is related to the reuse of wastewater for irrigation, groundwater replenishment, and discharge into water bodies [7]. However, the continual accumulation of FQs in soil and sediments poses a serious threat to plants and water systems owing to their adsorption onto organic matter and minerals [7]. The presence of FQs in water bodies is attributable to wastewater treatment plants (WWTPs), which are a major route for the existence of toxic chemicals or, indeed, a source of drug-resistant human pathogens [6]. In addition, the insufficient treatment of wastewater by WWTPs aggravates the spread of FQs in the environment owing to the discharge of untreated effluents, which will ultimately contaminate the soil, the food chain, and drinking water [1]. The indirect introduction of FQs in water has been linked to the validation of analytical methods and a deficiency in appropriate information about the effects of such compounds, together with their metabolites, in aquatic systems [7]. As these pollutants are present in water bodies in low (trace) levels, this necessitates sample preparation that incorporates preconcentration techniques to isolate these drugs and permit their accurate quantification in wastewater [8].
Analytical techniques that are often employed for the determination of organic pollutants include gas chromatography mass spectrometry (GC-MS) [9], ultraviolet-visible spectroscopy (UV–Vis) [10], liquid chromatography-mass spectroscopy (LC-MS) [11], and high-performance liquid chromatography (HPLC) [12]. Among the readily available detection techniques, HPLC is of specific interest for the analysis of antibiotics because of its sensitivity and user-friendliness. It is a challenge to determine these trace contaminants directly as they exist in complex matrices, which necessitates the use of preconcentration methods [13]. To eliminate the matrix effect, various preconcentration methods have been routinely employed, including liquid-liquid extraction (LLE) [14], dispersive liquid-liquid extraction (DLLME) [15], dispersive solid-phase extraction (DSPE) [16], and the most commonly used, solid-phase extraction (SPE). The DSPE method, which is a combination of a solid-phase extraction and the dispersion of a reliable solid-phase material, is uniquely flexible [17]. In addition, DSPE offers countless advantages, including the ability to retain the analytes in the solid-phase material, good percentage recoveries, excellent extraction efficiencies, and the ability to tailor the solid-phase material for the adsorption of emerging pollutants [18].
The adsorption of antibiotics is one of the preferred approaches to confiscate organic pollutants from water owing to the desirable qualities: ease of operation, low energy cost, and environmental friendliness [19]. Recently, the preparation of polymeric adsorbents for the adsorption of organics has been performed by using the electrospinning method, which is advantageous because of its low cost and simplicity [20]. Electrospinning has become popular for the preparation of nanofibers with attractive electronic properties and large surface-to-volume ratios [20]. Furthermore, the use of electrospun nanofibers has been employed in different fields, including the removal of inorganic and organic pollutants in water [21]. Of the numerous polymers that are usually used to prepare a spinning solution, polyacrylonitrile (PAN) is a favorite owing to its spinnability in solution, high carbon yield, and stability in the environment [22], [23]. Electrospun PAN nanofibers are attractive candidates because of their high loading capacity and ability to act as a support for the incorporation of effective sorbents, such as nanoparticles [24], metal oxides [25], and activated carbon [26].
Activated carbon has been used widely for the removal of pollutants in the environment [27], [28]. This adsorbent exhibits properties that are dependent on the starting material (precursor) and the preparation process [29]. Precursors such as biomaterials (e.g., walnut shell, coconut shell, orange peel, or almond shell) and waste materials, such as waste tires, have been investigated for the production of activated carbon [30], [31], [32]. Waste tires are a significant source of carbon as they are a waste material that is frequently dumped in landfills and become a problem to the environment [33]. The advantages of activated carbon as an adsorbent material include large surface area, controllable pore structures, modifiable surface chemistry, and low acid/base reactivity [34]. To the best of our knowledge, the incorporation of activated carbon into nanofibers has not yet been explored and their influence as combined adsorbents for the removal of antibiotics has not been evaluated.
Therefore, the purpose of study was to extract fluorinated quinolones shown in Fig. 1, such as ciprofloxacin (CIPRO), danofloxacin (DANO), and enrofloxacin (ENRO), from wastewater. The preparation of activated carbon was achieved from waste tires by chemically activating carbon with hydrogen peroxide. The electrospun PAN nanofibers were prepared by using the electrospinning method and were used as a support material for the activated carbon. Thereafter, the loading/decoration of PAN electrospun nanofibers with activated carbon was investigated to assess their effectiveness in the adsorbance of fluorinated quinolones. The prepared activated carbon-decorated PAN nanofibers were evaluated for their use as a solid-phase material. The potential applicability of the proposed solid-phase material was investigated through use as a dispersive solid-phase material for the preconcentration of CIPRO, DANO, and ENRO antibiotics.
Fig. 1.
The structures of (A) ciprofloxacin (pKa: 6.09 and 8.62), (B) danofloxacin (pKa: 6.22 and 9.43), and (C) enrofloxacin (pKa: 6.19 and 7.59) [35].
2. Experimental
2.1. Instrumentation
The prepared adsorbent was examined by using various characterization techniques to ensure whether the structural properties of the material were suitable for the adsorption of antibiotics (CIPRO, DANO, and ENRO). A scanning electron microscope (TESCAN Model Vega 3LMH) was used to assess the surface morphology of the adsorbent. A transmission electron microscope (Jeol JEM-2100F, JEOL Inc, Akishima, Japan) equipped with a LaB6 source was used to examine the incorporation of activated carbon on PAN nanofibers. Samples for transmission electron microscopy (TEM) were prepared by placing a drop of sample onto a carbon-coated copper (Cu) grid. The chemical changes of the functional groups of raw carbon, chemically activated carbon, plain PAN nanofibers, and PAN nanofibers decorated with activated carbon were monitored by using a Fourier transform infrared (FT-IR) spectrometer (Spectrum 100, PerkinElmer, USA) equipped with a universal attenuated total reflectance (ATR) accessory. X-ray diffraction (XRD) patterns were characterized for all prepared materials by using a Philips X-ray generator model PW 3710/31. The surface properties of the activated carbon-decorated PAN nanofibers and activated carbon were determined by the Brunauer-Emmett-Teller (BET) multipoint method using a Surface Area and Porosity Analyzer (ASAP2020 V3. 00H, Micromeritics Instrument Corporation, Norcross, USA). The analysis of antibiotics was conducted by using HPLC (Agilent, 1200 Infinity series, equipped with a photo diode array detector), equipped with ChemStation software; a reverse phase (C-18) column was used for the separation of antibiotics in a simultaneous analysis.
2.2. Materials
The fluorinated quinolones were obtained from Sigma Aldrich (South Africa) with a minimum purity of 99.5%. Polyacrylonitrile and N,N-dimethylformamide (DMF) were purchased from Sigma Aldrich (South Africa). All HPLC-grade solvents (methanol and acetonitrile) and acids (phosphoric acid (H3PO4)), and hydrogen peroxide (H2O2; 30%) used in this study were obtained from Sigma Aldrich (South Africa). HPLC-grade methanol (99.9%) and acetonitrile (98%), which were used as solvents for the elution of antibiotics, were obtained from Sigma-Aldrich (Fluka, St. Louis, MO, USA). Ammonium hydroxide and acetic acid were used to adjust the pH to obtain model solutions. Ultrapure water (Milli-Q water) was used throughout the study.
2.3. Sampling
Samples were collected from the Daspoort WWTP in Pretoria (South Africa). The Daspoort WWTP is a wastewater plant that treats primarily domestic wastewater (95% of treated material) and industrial wastewater (5% of treated material). The technology that is used to treat wastewater at this plant is the activated sludge technology. The influent, secondary wastewater, and effluent wastewater were collected and stored in glass bottles. Samples were passed through a 0.45 µm PVDF Acrodisc syringe filter prior to analysis.
2.4. Preparation of activated carbon
Activated carbon was prepared from waste tires by using a pyrolysis process under nitrogen purge (inert conditions). The waste tire was collected from a landfill site near Lenasia, South Africa; upon arrival in the lab, the tire was washed to remove debris, cut into pieces, and pyrolyzed at 900 °C. Thereafter, carbon black (the product) was chemically activated for 3 h at 200 °C by using hydrogen peroxide (H2O2). The activated carbon was cooled and washed with 5% HCl to eradicate inorganic impurities. Subsequently, the activated carbon was dried and finally calcined at 500 °C for 3 h.
2.5. Preparation of activated carbon-decorated PAN nanofibers
Nanofibers were prepared by using the electrospinning process. The spinning solution comprised PAN (Mw = 150,000) and DMF (the solvent), which were mixed together and stirred at 50 °C for 12 h until a homogeneous solution was obtained that was suitable for electrospinning. Before the introduction of activated carbon, different weight percentages of PAN solutions were prepared: 8 wt%, 10 wt%, 12 wt%, and 14 wt%. Thereafter, activated carbon (0.05 g, 0.1 g, 0.2 g, and 0.4 g) was incorporated into the polymer solution. The electrospinning parameters, such as flow rate, tip-to-collector distance, and voltage power, were optimized to obtain optimum parameters. The spinning solution containing activated carbon was filled in a syringe with a metal needle, and the metal needle and the collector (aluminum foil) were connected to the voltage power supply. This produced the electrospun PAN mat decorated with activated carbon, which was left in the oven to evaporate the remaining solvent from the polymer solution.
2.6. Sample preparation method
The sample preparation method of choice for this work was DSPE owing to its effectiveness as a preconcentration method for the isolation of antibiotics from water. A sample volume of 50 mL was used for the optimization and application experiments and the mass of the solid-phase material (activated carbon decorated nanofibers) was 50–100 mg. The concentration of the model solution was 200 µg/L for all the analytes, which were extracted simultaneously. The DSPE method was performed on a mechanical shaker with different extraction times (5–20 min). After the adsorption of antibiotics, the adsorbed analytes were eluted with different solvents (methanol, acetonitrile, and phosphoric acid/acetonitrile) to evaluate their effectiveness for desorption of the isolated antibiotics. The elution of analytes was assisted by shaking the sample solution and the sample was prepared for analysis by a final filtration step through a 0.45 µm filter.
2.7. Optimization of the DSPE method
The optimization of the experimental parameters was used as the initial screening process to determine the most influential parameters that affect the performance of the DSPE method. Analysis of the optimization was performed by using a multivariate statistical tool (central composite design) such as response surface methodology. In this study, the optimization process was conducted by using a two-level (24–1) fractional factorial design and the factors/parameters and levels involved in the experimental design are shown in Table 1. The electron domain (minimum, central point, and maximum) was selected as shown in the design matrix in Table 1. The parameters, such as mass of adsorbent (MA), extraction time (ET), extraction volume (EV), and pH, were the selected factors that might influence the preconcentration of CIPRO, DANO, and ENRO. The optimization of the DSPE method was conducted to determine the optimum parameters and to assess parameter interactions to reveal those with the greatest effect.
Table 1.
Factors and levels used in the factorial design.
| Variables | Minimum | Central point | Maximum |
|---|---|---|---|
| pH | 4 | 5 | 9 |
| Mass of adsorbent (mg) | 20 | 60 | 100 |
| Extraction time (min) | 2 | 6 | 10 |
| Extraction volume (mL) | 1.5 | 2.5 | 3.5 |
2.8. Adsorption studies
The relationship between the adsorbent and the adsorbate was evaluated by studying the adsorption capacity of the solid-phase material for the extraction of CIPRO, DANO, and ENRO from wastewater using the DSPE method. The adsorption of CIPRO, DANO, and ENRO by activated carbon-decorated PAN nanofibers was performed by using a mechanical shaker at 80 rpm at 25 °C. This study was performed with the optimized conditions of the chosen parameters. The capacity of adsorption of nanofibers was evaluated from a 50 mL solution of antibiotics in a 100 mL glass bottle containing 75 mg of the solid-phase material. The solutions of known concentration of antibiotics were prepared in the range of 10–100 mg/L and the extraction time was conducted for 11 min at pH 6.5. The analytes were then eluted with 3.3 mL of a mixture 85% phosphoric acid (H3PO4) and 15% acetonitrile. The amount of analytes that were adsorbed onto the activated carbon-decorated PAN nanofibers was calculated using Eqs. (1), (2):
| (1) |
where qe (mg/g) is the amount of CIPRO, DANO, or ENRO adsorbed per gram of the adsorbent, C0 and Ce (mg/L) are the initial and equilibrium concentration of the bulk solution, V (L) is the volume of the aqueous solution, and M (g) is the mass of activated carbon-decorated PAN nanofibers used.
| (2) |
where qmax (mg/g) is the maximum amount of adsorption and KL is the adsorption constant; these were treated as constants that could be obtained from the slopes and intercepts of linear plot of Ce/qe vs Ce. As an alternative, the Freundlich isotherm model was also explored, which assumes that the adsorption takes place on heterogeneous surfaces. The linear equation that represents the Freundlich isotherm model is expressed in Eq. (3):
| (3) |
where qe is the solid phase adsorbate concentration at equilibrium liquid-phase concentration, Ce is the equilibrium liquid phase concentration, KF is the Freundlich constant and 1/n is the heterogeneity factor. Usually, the KF constant and 1/n are determined from a plot of ln qe vs ln Ce.
3. Results and discussion
3.1. Surface characterization of solid-phase material
The surface morphology was studied by using SEM, which provides information about the structural characteristics of the activated carbon-decorated PAN nanofibers. For the preparation of the PAN nanofibers, tire-derived activated carbon that was chemically activated with H2O2 was incorporated in PAN nanofibers. The raw activated carbon is shown in Fig. 2 and Fig. 3 illustrates the plain PAN nanofibers. Different magnifications of the chemically activated carbon are present in Fig. 2 and it is evident that cavities, or rather depressions, developed on the surface of the activated carbon. This morphology was attributed to the fact that the final product was calcinated in a furnace.
Fig. 2.
SEM images for activated carbon obtained from waste tires.
Fig. 3.
SEM images of plain PAN electrospun nanofibers.
The plain PAN nanofibers prepared from a 10 wt% polymer solution are shown in Fig. 3. Different magnifications were shown to contain a uniform dispersion of nanofibers. Of all the polymer solutions, a decision was made on which PAN solution presented the best properties with regard to the combination of the solvent and the polymer. The size of the nanofibers was uniform and evenly distributed, which proved that the optimized parameters (flow rate, distance between collector and tip, and electric voltage) were satisfactory for electrospinning.
TEM analysis was performed to assess the incorporation of activated carbon onto the PAN nanofibers. The TEM images shown in Fig. 4 show that the activated carbon sat on the PAN nanofibers. As shown in Fig. 4A, the activated carbon was incorporated inside the PAN nanofibers, indicating that they may have a hollow shape. Activated carbon clusters were observed on top of the PAN nanofibers (Figs. 4B and C, whereas Fig. 4F shows the distribution of activated carbon within the PAN nanofibers).
Fig. 4.
TEM images of activated carbon-decorated PAN electrospun nanofibers.
The Brunauer-Emmett-Teller (BET) technique was used to verify the difference in all materials that were characterized in terms of surface area and pore sizes. The BET results were tabulated for the raw carbon and all intermediates up to the final product (activated carbon-decorated PAN nanofibers), which are shown in Table 2. The surface area results increased from the raw carbon black material to the activated carbon that was obtained by high-temperature calcination. A significant decrease in both the surface area and the pores occurred in plain PAN nanofibers and the activated carbon-decorated PAN nanofibers. The depletion of surface properties was observed owing to the lower, or indeed minimum, amount of activated carbon that was incorporated into the 10 wt% polymer solution.
Table 2.
BET results of activated carbon, plain PAN, and activated carbon-decorated PAN nanofibers (AC-NFs).
| Sample | Surface area (m2/g) | Pore volume (cm3/g) | Pore diameter (nm) |
|---|---|---|---|
| Raw | 11 | 0.1 | 2.2 |
| Activated carbon | 1075 | 2.6 | 4.8 |
| Plain PAN | 46 | 0.05 | 1.6 |
| AC-NFs | 75 | 0.2 | 2.8 |
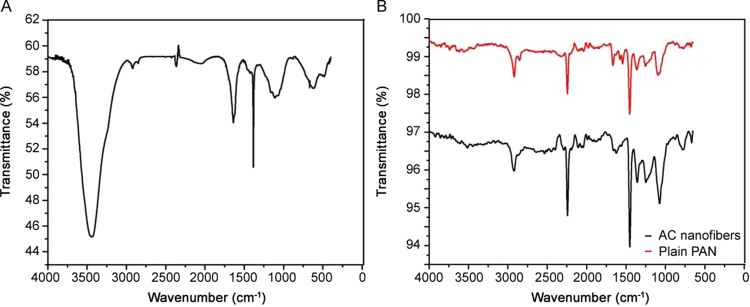
The FT-IR spectra presented in Fig. 5 explain the functional groups that have developed on the surface of the activated carbon (Fig. 5A), plain PAN nanofibers, and activated carbon-decorated PAN nanofibers (Fig. 5B). The spectra of the activated carbon that was chemically activated with H2O2 contain functional groups that are necessary for the uptake of organic pollutants. Oxygen functional groups were successfully incorporated into the surface of the AC. The spectra of both the materials contained major bands at 3400 cm−1, 1720 cm−1, 1627 cm−1, and 1580 cm−1 that were assigned to the O–H group of phenols and C–O bonds [35]. The broadest band, at 3400 cm−1, is known to be the stretching O–H vibration of the hydroxyl group, which is commonly seen when water is used in the preparation process. The peak at 1720 cm−1 was assigned to the stretching vibrations of the carboxyl groups (C–O) in the acidic surface oxygen. The band at 1627 cm−1 was characteristic of the sp2 hybridized C–C aromatic skeletal stretching of graphite [36] and the band observed at 1580 cm−1 was also attributed to the aromatic ring-stretching vibrations. The spectrum of the plain electrospun PAN was also illustrated in Fig. 5B; there were clear differences compared with the spectrum of activated carbon. The absorption frequency of 3435 cm−1 was assigned to OH stretching, whereas 2939 cm−1 and 2871 cm−1 were attributed to C–H stretching in the CH, CH2, and CH3 groups. The stretching frequency of C N was observed at 2242 cm−1 and the peak at 1734 cm−1 was attributed to C O stretching [22]. At 1073 cm−1, a peak was attributable to the C-O stretching in acetate ester. The spectrum of the activated carbon-decorated PAN nanofibers was obtained as shown in Fig. 5B. A change was expected when blending the activated carbon with the PAN nanofibers; however, the spectrum remained unchanged. Owing to the similarities that were observed in the plain PAN nanofibers and those decorated with activated carbon, the logical explanation was that the incorporation of activated carbon was spectrally insignificant as the polymer covering the activated carbon blocked them from absorbing light.
Fig. 5.
FT-IR spectra of (A) activated carbon and (B) plain PAN nanofibers and activated-carbon nanofibers.
The zeta potential was determined for both activated carbon and the activated carbon-decorated PAN nanofibers at pH values between 2 and 11. The surface charge of the activated carbon was found to be slightly negative at pH 5.7, whereas after blending activated carbon with PAN nanofibers, the pH at the point of zero charge (pHzpc) was found to be 6.7. The sudden shift in pH was attributed to the introduction of certain functional groups, such as the amine and carboxylic groups, onto the surface of the adsorbent. The change in pH was explained as a result of the protonation of the surface of the activated carbon-decorated PAN nanofibers.
3.2. Effect of adsorbent and eluent selection
The PAN electrospun nanofibers were decorated with the tire based-activated carbon using different masses of activated carbon (0.05 g, 0.1 g, 0.2 g, and 0.4 g). A comparative study of the effect of the mass of activated carbon on the adsorption of CIPRO, DANO, and ENRO from water was conducted. Parameters such as pH (6), MA (100 mg), ET (10 min), and EV (3 mL) were selected to determine which adsorbent was efficient for the adsorption of antibiotics and the concentration used for this test was 5 mg/L. The results of this study are shown in Fig. 6; it was observed that 0.4 g adsorbent was impressive for the adsorption of antibiotics, with acetonitrile used as the eluent. In Fig. 6, plain PAN nanofibers and activated carbon alone were examined with regard to their potential for the adsorption of CIPRO, DANO, and ENRO. The results obtained confirmed the efficiencies and functions of materials tested; plain PAN nanofibers were not as effective as activated carbon, which makes sense as the purpose of this study was to use PAN nanofibers as support for the tire-based activated carbon. Therefore, these results made good scientific sense in this study as it was difficult to recover the activated carbon in solution and by supporting activated carbon, the application of such materials is attractive owing to their simple and easy removal from in solution. In Fig. 6, it is shown that the adsorption of activated carbon (0.4 g, raw) and activated carbon-decorated PAN nanofibers (0.4 g) was similar to each other in terms of the amount of antibiotics adsorbed.
Fig. 6.
The effect of activated carbon with different masses incorporated in PAN nanofibers, AC, and plain PAN on adsorption.
The selection of eluent was performed to evaluate the most effective solvent/acid mixture for the desorption of CIPRO, DANO, and ENRO from activated carbon decorated PAN electrospun nanofibers. Acetonitrile, methanol, and phosphoric acid (85%) mixed with acetonitrile were chosen as the eluents for the extraction of antibiotics and a comparative study was conducted. This selection process was performed by using the DSPE method and the parameters were fixed at pH 6, 3 mL extraction volume, 100 g of adsorbent, and 10 min extraction time. The concentration of the model solution was 5 mg/L and the sample volume used was 50 mL. As shown in Fig. 7, after applying the DSPE method, the desorption of antibiotics by H3PO4/ACN mixture was effective as compared with other solvents (e.g., methanol and ACN). The HPLC analysis was performed in triplicate. It was evident that the H3PO4/ACN mixture obtained relatively good recoveries, between 80% and 85% for CIPRO, DANO, and ENRO, whereas methanol and acetonitrile gave poor results, with recoveries below 40%.
Fig. 7.
The selection of eluent for the extraction of antibiotics (MeOH, methanol and ACN, acetonitrile).
3.3. Optimization of the DSPE method
Optimization of the DSPE was conducted by using a two-level full factorial (24–1) design to evaluate the most influential parameters in the optimization. Central composite design (surface response methodology) was employed as the statistical method. The parameters that were used in this study were pH, mass of adsorbent, extraction volume, and extraction time, as shown in Table 2. The outcome of the results was performed in accordance with the experimental design matrix shown in Table 3, comprising 18 experiments, and the results were reported as the percentage recovery. Analysis of variance (ANOVA) was used for further analysis and the data were presented as a Pareto chart. ANOVA was used to generate the Pareto chart to communicate how the individual parameters affected the extraction of CIPRO, DANO, and ENRO from model solutions. In addition, the interaction of the parameters was assessed with regard to their effect in the extraction of antibiotics. The Pareto chart is presented in Fig. 8; the reference line signifies the absolute value, which is proportional to the value of the estimated effects and is helpful for the comparison of the relative importance of effects [37].
Table 3.
The design matrix and the results of the two-level fractional factorial design.
| Experimental run | MA (mg) | pH | ET (min-1) | EV (mL) | CIPRO (%R) | DANO (%R) | ENRO (%R) |
|---|---|---|---|---|---|---|---|
| 1. | 100 | 9 | 20 | 1.5 | 44 | 39 | 42 |
| 2. | 100 | 9 | 5 | 1.5 | 43 | 37 | 42 |
| 3. | 100 | 3 | 20 | 3 | 43 | 37 | 41 |
| 4. | 50 | 9 | 5 | 3 | 49 | 39 | 45 |
| 5. | 100 | 3 | 5 | 3 | 45 | 35 | 38 |
| 6. | 50 | 3 | 20 | 1.5 | 42 | 37 | 40 |
| 7. | 50 | 9 | 20 | 3 | 41 | 35 | 39 |
| 8. | 50 | 3 | 5 | 1.5 | 56 | 48 | 54 |
| 9. | 40 | 6 | 11 | 2.25 | 49 | 40 | 48 |
| 10. | 110 | 6 | 11 | 2.25 | 45 | 37 | 42 |
| 11. | 75 | 1.7 | 11 | 2.25 | 41 | 34 | 36 |
| 12. | 75 | 10 | 11 | 2.25 | 49 | 43 | 46 |
| 13. | 75 | 6 | 0.4 | 2.25 | 47 | 40 | 44 |
| 14. | 75 | 6 | 22 | 2.25 | 55 | 48 | 51 |
| 15. | 75 | 6 | 11 | 1.19 | 91 | 85 | 85 |
| 16. | 75 | 6 | 11 | 3.31 | 94 | 88 | 88 |
| 17. | 75 | 6 | 11 | 2.25 | 87 | 79 | 82 |
| 18. | 75 | 6 | 11 | 2.25 | 90 | 88 | 90 |
Fig. 8.
Pareto chart of effects on optimization.
According to the Pareto chart, the bars of all chosen parameters (EV, pH, MA, and ET) exceeded the reference line; this is indicative of significance at the 95% confidence level. Only one Pareto chart is shown, as all others were the same. The parameters pH, MA, and ET had a negative effect on the removal of antibiotics in water; conversely, EV was the only parameter that exerted a positive effect on the extraction of antibiotics. However, most of the interactions amongst parameters were insignificant compared with the promising recoveries of antibiotics. The interaction between pH and MA was significant as the bar crossed the reference line, indicating a positive effect on the analytical response. Owing to the parameters that influenced the extraction procedure, using surface response methodology, 3D surface plots were generated to examine the main effects, quadratic effects, and interactive effects of significant factors. The 3D surface plots together with the interactions are presented in Fig. 9. The surface response method was further explained by the help of a quadratic equation to confirm the optimum conditions that were obtained. Each 3D plot was determined as a function of two parameters between pH, MA, EV, and ET. The ultimate optimum conditions were found to be 6.5, 75 mg, 12 min, and 3.5 mL for pH, MA, ET, and EV, respectively.
Fig. 9.
The 3D surface response plots describing the interaction of parameters.
3.4. Analytical performance of the DSPE method
The analytical performance of the preconcentration (DSPE) method was evaluated in terms of the limit of detection (LOD), limit of quantification (LOQ), percentage relative standard deviation (precision), and correlation coefficient. HPLC analysis of CIPRO, DANO, and ENRO was used to examine the adsorption performance at the selected parameters. The simultaneous detection of CIPRO, DANO, and ENRO in water was observed at the wavelength of 280 nm. The retention times of CIPRO, DANO, and ENRO were 6.12 min, 7.02 min, and 8.24 min, respectively. Calibration standards were prepared between 10 and 100 µg/L from a stock solution of 5 mg/L and the correlation coefficient was determined to be 0.9998 for the standards used. The LOD and LOQ were calculated by using and , respectively, where the SD is the standard deviation of the analytical responses of the lowest calibration standard solutions (n = 10) and b is the slope of the calibration curve. Therefore, the LOD and LOQ of the preconcentration method for the detection of CIPRO, DANO, and ENRO were 0.05, 0.11, and 0.20 µg/L and 0.53, 1.21, and 2.17 µg/L, respectively. The precision was determined in terms of repeatability and reproducibility and it was expressed as the intra-day (n = 20) and inter-day (n = 5) precision. The intra-day and inter-day precision was reported in terms of the percentage relative standard deviation, which was 3% and 4%, respectively. The preconcentration factor was determined to be 15.
3.5. Comparison of the analytical performance with other reported methods
The DSPE preconcentration method was compared with the extraction methods that have been documented in the literature to assess their analytical performance. The comparison was narrowed down to only the analytes (CIPRO, DANO and ENRO) of interest. The tabulated results were compared to analytical figures such as LODs, LOQs, %RSD, and the preconcentration factor. The comparison of the current work with other documented work [38], [39], [40], [41], [42], [43] is presented in Table 4. It should be noted that the comparison was not limited to water samples, and various matrices were investigated. The analytical performance of the proposed method was superior to that of other methods in terms of the LOD, LOQ, and RSD. This study also presented a preconcentration factor that was comparable with other methods.
Table 4.
Comparison of various extraction methods with the proposed method.
| Extraction method | Analytes | Matrix | LOD (µg/L) | LOQ (µg/L) | %RSD | PF | Detection | Ref. |
|---|---|---|---|---|---|---|---|---|
| US-IL-DLLME | CIPRO, DANO and ENRO | Groundwater | 0.8–10 | 3–33 | 6–8.6 | 122–205 | HPLC-FD | [38] |
| DLLME | CIPRO, DANO and ENRO | Chicken liver | 5–16 | 23–51 | 4.3–6.6 | HPLC-DAD | [39] | |
| MIP-SPE | CIPRO, DANO and ENRO | Water | 0.01–0.30 | 0.190 | 4–9 | HPLC | [40] | |
| HF-SLM | CIPRO, DANO and ENRO | Surface water | 0.01–0.02 | 8 | 99–155 | HPLC | [41] | |
| MIP-SPE | CIPRO, DANO and ENRO | Milk | 1.76–12.4 | 5.8–41.4 | 1.6–14.9 | HPLC-UV | [42] | |
| SPE | CIPRO | Water | 2–5 | 4.1–6.3 | 514 | HPLC | [43] | |
| DSPE | CIPRO, DANO and ENRO | Wastewater | 0.05–0.20 | 0.53–2.17 | 4 | 15 | HPLC-AD | Current method |
3.6. Adsorption studies
The ability of the activated carbon-decorated PAN nanofibers to adsorb CIPRO, DANO, and ENRO was evaluated in this study. The relationship between the adsorbent (solid) and the adsorbate (liquid) was investigated by employing the Langmuir and Freundlich isotherm models. Model solutions were prepared between 10 and 300 mg/L and the ability of each adsorbent to remove antibiotics from water was tested. This study was performed using the conditions obtained from the optimization procedure for the DSPE method. The experimental adsorption data were fitted to the linearized equations of the Langmuir and Freundlich isotherm models. The R2 values of the fitted data were 0.998 for the Langmuir model and 0.988 for the Freundlich model. Therefore, Langmuir isotherm model was better than the Freundlich in terms of the correlation coefficient. The Langmuir model explains monolayer absorption, whereby the sorption of the adsorbate molecules at one active site does not influence the adsorption of other molecules at neighboring sites. The intermolecular attraction forces between the adsorbate molecules in the monolayer and molecules in solution decreased immensely with distance [19]. For CIPRO, DANO, and ENRO, the pKa values ranged between 6.09 and 9.43, indicating that these antibiotics can exist as cations, zwitterions, or as anions under different pH conditions. Thus, the interaction between the adsorbent and the adsorbate was electrostatic; moreover, based on the experimental observations, activated carbon-decorated PAN nanofibers were able to efficiently extract CIPRO, DANO, and ENRO. Furthermore, the adsorption selectivity and efficiency were improved by modifying the pore size of the adsorbent, which could be tailored to the analyte of interest [19]. Therefore, maximum adsorption capacity (qmax) values for CIPRO, DANO, and ENRO were 93, 99, and 112, respectively.
3.7. The reusability studies
The reusability of the activated carbon-decorated PAN nanofibers was assessed through the adsorption and desorption of CIPRO, DANO, and ENRO. This study was conducted to evaluate the reliability of the adsorbent when used several times. A model solution was used to conduct this study in conjunction with the DSPE method. It was observed that the adsorbent was reusable ten times, as proven by the obtained satisfactory recoveries of between 96% and 98%. After ten cycles, a noticeable decline was observed; the adsorption/desorption of antibiotics was between approximately 64% and 70%.
3.8. Validation and application of activated carbon-decorated PAN nanofibers
The validation of the proposed DSPE method was conducted to determine the accuracy of the sample preparation method for the extraction of antibiotics. Owing to the unavailability of certified reference materials for CIPRO, DANO, and ENRO, the spike recovery test was a reliable method to test for accuracy. This study was performed by the employing optimum conditions and the analytical response was reported as a percentage recovery that ranged between 98% and 102%. The relative standard deviation was calculated as less than 3.6%, as the experiments were repeated three times and the results are presented in Table 5.
Table 5.
Validation of the DSPE method.
| Analytes | Added (µg/L) | Found (µg/L) | Recovery (%) |
|---|---|---|---|
| CIPRO | 0 | 0.04 ± 3.3 | |
| 50 | 49.8 ± 2.6 | 99.6 | |
| DANO | 0 | 0.09 ± 3.6 | |
| 50 | 50.9 ± 1.1 | 102 | |
| ENRO | 0 | 0.11 ± 3.2 | |
| 50 | 49.4 ± 2.8 | 98 |
Finally, the developed DSPE method was applied to real wastewater samples, including influent, secondary, and effluent wastewater. Amongst the three sampling points, CIPRO, DANO, and ENRO were only detected in influent wastewater in the analysis of raw samples. This step was crucial to obtain insight into whether the analytes of interest were contained in the sample. In the other samples, which were the secondary and effluent wastewater samples, antibiotics were not detected, which confirmed the efficiency of the treatment system of the wastewater plant. As shown in the results in Table 6, DANO and ENRO were not detected, which implied that the biological treatment stage was partially effective when using natural bacteria to consume these antibiotics. The concentration of antibiotics in the influent wastewater is an indication of the pharmaceutical drugs that enter the treatment plant at trace levels.
Table 6.
Application of the activated carbon-decorated PAN nanofibers in real samples.
| Samples | CIPRO |
DANO |
ENRO |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Added (µg/L) | Found (µg/L) | Recovery (%) | Added (µg/L) | Found (µg/L) | Recovery (%) | Added (µg/L) | Found (µg/L) | Recovery (%) | |
| Influent wastewater | 0 | 0.04 ± 3.3 | 99 | 0 | 0.09 ± 3.6 | 98 | 0 | 0.11 ± 3.2 | 99 |
| 10 | 9.87 ± 2.8 | 10 | 9.77 ± 3.3 | 10 | 9.99 ± 3.5 | ||||
| Secondary wastewater | 0 | 0.11 ± 3.2 | 98 | 0 | 0.11 ± 3.2 | 101 | 0 | N/D | 102 |
| 10 | 9.75 ± 2.3 | 10 | 10.1 ± 3.9 | 10 | 10.2 ± 4.0 | ||||
| Effluent wastewater | 0 | 0.01 ± 4.1 | 102 | 0 | N/D | 99 | 0 | N/D | 103 |
| 10 | 10.2 ± 3.7 | 10 | 9.89 ± 4.0 | 10 | 10.3 ± 4.0 | ||||
4. Conclusion
Unique activated carbon-decorated PAN electrospun nanofibers were successfully prepared and applied to the extraction of CIPRO, DANO, and ENRO from model solutions and real wastewater samples. Dispersive SPE was chosen as the preconcentration technique to assist the extract antibiotics from water. The attractiveness of the surface properties of the prepared adsorbent was evidenced through the analysis of SEM, TEM, FT-IR, BET, and zeta potential. Before the sample preparation method and the adsorbent were applied, optimization of the parameters pH, MA, ET, and EV were optimized for the extraction of antibiotics from water. The optimum conditions obtained were satisfactory considering the performance of the DSPE method. The analytical performance of the DSPE method was examined in terms of LODs and the LOQ, which were 0.05–0.20 µg/L and 0.53–2.17 µg/L, respectively. Furthermore, a comparative study was performed and the method used in this study showed to be superior to other methods. The adsorption capacity of the prepared adsorbent was investigated and it was found that the maximum adsorption capacity was 93–112. Out of the two isotherm models (Langmuir and Freundlich), the Langmuir model was chosen as the R2 value was better than that obtained from the Freundlich model. The activated carbon-decorated PAN nanofibers were observed to be highly reusable, with satisfactory recovery for more than 10 uses. Ultimately, the adsorbent was applied in real samples collected from a domestic WWTP. The potential applicability of the adsorbent was impressive for the extraction antibiotics; this may might a solution for wastewater treatment systems that are facing the challenge of organic pollutants.
Acknowledgments
We would like to thank National Research Foundation (NRF, grant no. SFH14073184214) for providing financial support, and the University of Johannesburg for enabling this study possible by making laboratory facilities available.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Sotelo J.L., Rodríguez A.R., Mateos M.M., et al. Adsorption of pharmaceutical compounds and an endocrine disruptor from aqueous solutions by carbon materials. J. Environ. Sci. Health Part B. 2012;47:640–652. doi: 10.1080/03601234.2012.668462. [DOI] [PubMed] [Google Scholar]
- 2.Chen B., Han J., Wang Y., et al. Separation, enrichment and determination of ciprofloxacin using thermoseparating polymer aqueous two-phase system combined with high performance liquid chromatography in milk, egg, and shrimp samples. Food Chem. 2014;148:105–111. doi: 10.1016/j.foodchem.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Petrie B., Barden R., Kasprzyk-Hordern B. A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015;72:3–27. doi: 10.1016/j.watres.2014.08.053. [DOI] [PubMed] [Google Scholar]
- 4.Nakata H., Kannan K., Jones P.D., et al. Determination of fluoroquinolone antibiotics in wastewater effluents by liquid chromatography–mass spectrometry and fluorescence detection. Chemosphere. 2005;58:759–766. doi: 10.1016/j.chemosphere.2004.08.097. [DOI] [PubMed] [Google Scholar]
- 5.Hubicka U., Žmudzki P., Žuromska-Witek B., et al. Determination and characterization of selected flouroquinolones oxidation products under potassium permanganate treatment. Acta Pol. Pharm. 2015;2:1101–1114. [Google Scholar]
- 6.Espinosa-Mansilla A., Jiménez Girón A., La Peña D., et al. Simultaneous determination of the residues of fourteen quinolones and fluoroquinolones in fish samples using liquid chromatography with photometric and fluorescence detection. Czech J. Food Sci. 2012;30:1. [Google Scholar]
- 7.Frade V.M.F., Dias M., Teixeira A.C.S.C., et al. Environmental contamination by fluoroquinolones. Braz. J. Pharm. Sci. 2014;50:41–54. [Google Scholar]
- 8.Dimpe K.M., Nomngongo P.N. Current sample preparation methodologies for analysis of emerging pollutants in different environmental matrices. TrAC. 2016;82:199–207. [Google Scholar]
- 9.Cai J., Chen G.S., Qiu J.L., et al. Hollow fiber based liquid phase microextraction for the determination of organochlorine pesticides in ecological textiles by gas chromatography-mass spectrometry. Talanta. 2016;146:375–380. doi: 10.1016/j.talanta.2015.08.069. [DOI] [PubMed] [Google Scholar]
- 10.Suárez R., Clavijo S., Avivar J., et al. On-line in-syringe magnetic stirring assisted dispersive liquid–liquid microextraction HPLC–UV method for UV filters determination using 1-hexyl-3-methylimidazolium hexafluorophosphate as extractant. Talanta. 2016;148:589–595. doi: 10.1016/j.talanta.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Unceta N., Sampedro M.C., Bakar N.K.A., et al. Multi-residue analysis of pharmaceutical compounds in wastewaters by dual solid-phase microextraction coupled to liquid chromatography electrospray ionization ion trap mass spectrometry. J. Chromatogr. A. 2010;1217:3392–3399. doi: 10.1016/j.chroma.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Dong S., Hu S., Yang Z., et al. An ionic liquid-based ultrasound assisted dispersive liquid–liquid microextraction procedure followed by HPLC for the determination of low concentration of phytocides in soil. Microchem. J. 2013;110:221–226. [Google Scholar]
- 13.Kuban P., Bocek P. Direct coupling of supported liquid membranes to capillary electrophoresis for analysis of complex samples: a tutorial. Anal. Chim. Acta. 2013;787:10–23. doi: 10.1016/j.aca.2013.04.065. [DOI] [PubMed] [Google Scholar]
- 14.Chen T., Tzing S., Ding W. Rapid screening of haloacetamides in water using salt-assisted liquid–liquid extraction coupled injection-port silylation gas chromatography–mass spectrometry. J. Chromatogr. A. 2015;1422:340–344. doi: 10.1016/j.chroma.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 15.Yao C., Li T., Twu P., et al. Selective extraction of emerging contaminants from water samples by dispersive liquid–liquid microextraction using functionalized ionic liquids. J. Chromatogr. A. 2011;1218:1556–1566. doi: 10.1016/j.chroma.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 16.Duan Y., Dai C., Zhang Y., et al. Selective trace enrichment of acidic pharmaceuticals in real water and sediment samples based on solid-phase extraction using multi-templates molecularly imprinted polymers. Anal. Chim. Acta. 2013;758:93–100. doi: 10.1016/j.aca.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Speltini A., Sturini M., Maraschi F., et al. Graphene-derivatized silica as an efficient solid-phase extraction sorbent for pre-concentration of fluoroquinolones from water followed by liquid-chromatography fluorescence detection. J. Chromatogr. A. 2015;1379:9–15. doi: 10.1016/j.chroma.2014.12.047. [DOI] [PubMed] [Google Scholar]
- 18.Wen Y., Chen L., Li J., et al. Recent advances in solid-phase sorbents for sample preparation prior to chromatographic analysis. TrAC. 2014;59:26–41. [Google Scholar]
- 19.Li X., Chen S., Fan X., et al. Adsorption of ciprofloxacin, bisphenol and 2-chlorophenol on electrospun carbon nanofibers: in comparison with powder activated carbon. J. Colloid Interface Sci. 2015;447:120–127. doi: 10.1016/j.jcis.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 20.Maddah B., Soltaninezhad M., Adib K., et al. Activated carbon nanofiber produced from electrospun PAN nanofiber as a solid phase extraction sorbent for the preconcentration of organophosphorus pesticides. Sep. Sci. Technol. 2017;52:700–711. [Google Scholar]
- 21.Liu J., Wang S., Yang J., et al. ZnCl2 activated electrospun carbon nanofiber for capacitive desalination. Desalination. 2014;344:446–453. [Google Scholar]
- 22.E.F. Chaúque, L.N. Dlamini, A.A. Adelodun, et al., Electrospun polyacrylonitrile nanofibers functionalized with EDTA for adsorption of ionic dyes, J. Phys. Chem. Earth (Parts A/B/C, 2016)
- 23.Ali A.A., Eltabey M.M., Farouk W.M., et al. Electrospun precursor carbon nanofibers optimization by using response surface methodology. J. Electrost. 2014;72:462–469. [Google Scholar]
- 24.Álvarez P.M., Jaramillo J., López-Pinero F., et al. Preparation and characterization of magnetic TiO2 nanoparticles and their utilization for the degradation of emerging pollutants in water. Appl. Catal. B: Environ. 2010;100:338–345. [Google Scholar]
- 25.Wu N., Wei H., Zhang L. Efficient removal of heavy metal ions with biopolymer template synthesized mesoporous titania beads of hundreds of micrometers size. Environ. Sci. Technol. 2011;46:419–425. doi: 10.1021/es202043u. [DOI] [PubMed] [Google Scholar]
- 26.Gupta V.K., Agarwal S., Saleh T.A. Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J. Hazard. Mater. 2011;185:17–23. doi: 10.1016/j.jhazmat.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 27.Mui E.L.K., Cheung W.H., Valix M., et al. Mesoporous activated carbon from waste tyre rubber for dye removal from effluents. Micropor. Mesopor. Mater. 2010;130:287–294. [Google Scholar]
- 28.Ghaedi M., Ahmadi F., Tavakoli Z., et al. Three modified activated carbons by different ligands for the solid phase extraction of copper and lead. J. Hazard. Mater. 2008;152:1248–1255. doi: 10.1016/j.jhazmat.2007.07.108. [DOI] [PubMed] [Google Scholar]
- 29.Tanthapanichakoon W., Ariyadejwanich P., Japthong P. Desorption characteristics of phenol and reactive dyes from aqueous solution on mesoporous activated carbon prepared from waste tires. Water Res. 2005;39:1347–1353. doi: 10.1016/j.watres.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 30.Gupta V.K., Gupta B., Rastogi A., et al. A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye—Acid Blue 113. J. Hazard. Mater. 2011;186:891–901. doi: 10.1016/j.jhazmat.2010.11.091. [DOI] [PubMed] [Google Scholar]
- 31.Soylak M. Determination of trace amounts of copper in high-purity aluminum samples after preconcentration on an activated carbon column. Fresen Environ. Bull. 1998;7:383–387. [Google Scholar]
- 32.Gundogdu A., Duran C., Senturk H.B., et al. Adsorption of phenol from aqueous solution on a low-cost activated carbon produced from tea industry waste: equilibrium, kinetic, and thermodynamic study. J. Chem. Eng. Data. 2012;57(10):2733–2743. [Google Scholar]
- 33.Gupta V.K., Nayak A., Agarwal S., et al. Potential of activated carbon from waste rubber tire for the adsorption of phenolics: effect of pre-treatment conditions. J. Colloid Interface Sci. 2014;417:420–430. doi: 10.1016/j.jcis.2013.11.067. [DOI] [PubMed] [Google Scholar]
- 34.Martınez J.D., Puy N., Murillo R., et al. Waste tyre pyrolysis–a review. Renew. Sust. Energ. Rev. 2013;23:179–213. [Google Scholar]
- 35.Moema D., Nindi M.M., Dube S. Development of a dispersive liquid–liquid microextraction method for the determination of fluoroquinolones in chicken liver by high performance liquid chromatography. Anal. Chim. Acta. 2012;730:80–86. doi: 10.1016/j.aca.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L., Chang X., Li Z., et al. Selective solid-phase extraction using oxidized activated carbon modified with triethylenetetramine for preconcentration of metal ions. J. Mol. Struct. 2010;964:58–62. [Google Scholar]
- 37.Al-Saadi A.A., Saleh T.A., Gupta V.K. Spectroscopic and computational evaluation of cadmium adsorption using activated carbon produced from rubber tires. J. Mol. Liq. 2013;188:136–142. [Google Scholar]
- 38.Nomngongo P.N., Ngila J.C. Functionalized nanometer-sized alumina supported micro-solid phase extraction coupled to inductively coupled plasma mass spectrometry for preconcentration and determination of trace metal ions in gasoline samples. RSC Adv. 2014;4:46257–46264. [Google Scholar]
- 39.Vázquez M.M.P., Vázquez P.P., Galera M., et al. Determination of eight fluoroquinolones in groundwater samples with ultrasound-assisted ionic liquid dispersive liquid–liquid microextraction prior to high-performance liquid chromatography and fluorescence detection. Anal. Chim. Acta. 2012;748:20–27. doi: 10.1016/j.aca.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 40.Benito-Pena E., Urraca J.L., Sellergren B., et al. Solid-phase extraction of fluoroquinolones from aqueous samples using a water-compatible stochiometrically imprinted polymer. J. Chromatogr. A. 2018;1208:62–70. doi: 10.1016/j.chroma.2008.08.109. [DOI] [PubMed] [Google Scholar]
- 41.Poliwoda A., Krzyzak M., Wieczorek P.P. Supported liquid membrane extraction with single hollow fiber for the analysis of fluoroquinolones from environmental surface water samples. J. Chromatogr. A. 2010;1217:3590–3597. doi: 10.1016/j.chroma.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 42.He H., Dong C., Li B., et al. Fabrication of enrofloxacin imprinted organic–inorganic hybrid mesoporous sorbent from nanomagnetic polyhedral oligomeric silsesquioxanes for the selective extraction of fluoroquinolones in milk samples. J. Chromatogr. A. 2014;1361:23–33. doi: 10.1016/j.chroma.2014.07.089. [DOI] [PubMed] [Google Scholar]
- 43.Chen B., Wang W., Huang Y. Cigarette filters as adsorbents of solid-phase extraction for determination of fluoroquinolone antibiotics in environmental water samples coupled with high-performance liquid chromatography. Talanta. 2012;88:237–243. doi: 10.1016/j.talanta.2011.09.066. [DOI] [PubMed] [Google Scholar]