Short abstract
Background
Cognitive impairment can complicate minor stroke, but there is limited information on risk factors including peak cognitive ability earlier in life.
Methods
We recruited patients with clinically-evident lacunar or minor non-lacunar ischaemic stroke, recorded clinical features, vascular risk factors, magnetic resonance imaging-detected stroke sub-type and small vessel disease burden. At 1–3 and 12 months after stroke, we assessed educational attainment (years of education), current cognition (Addenbrooke’s Cognitive Examination–Revised), pre-morbid intelligence (National Adult Reading Test) and dependency (modified Rankin Scale).
Results
We recruited 157 patients (87 lacunar, 64 non-lacunar ischaemic strokes), median age 66 (inter-quartile range 56–74) years, 36/157 (23%) patients had a Addenbrooke’s Cognitive Examination–Revised score < 82 at one to three months, 29/151 (19%) had a Addenbrooke’s Cognitive Examination–Revised < 82 at one year. Lower National Adult Reading Test score (cognitive impairment per point on National Adult Reading Test odds ratio 0.91, 95% confidence interval 0.87, 0.95) and older age (per year of age odds ratio 1.04 (95% confidence interval 1.01, 1.08) predicted one-year cognitive impairment more than stroke severity (per point on National Institute of Health Stroke Scale odds ratio 0.96 (95% confidence interval 0.0.68, 1.31)) or vascular risk factors e.g. hypertension (odds ratio for diagnosis of hypertension 0.52 (95% confidence interval 0.24, 1.15). Cognitive impairment was associated with having more white matter hyper-intensities (odds ratio per point increase in Fazekas score 1.42, 95% confidence interval 1.11, 1.83).
Discussion
This observational study provides evidence that pre-morbid intelligence quotient and education predict cognition after stroke, and confirms the association between cognitive impairment and small vessel disease.
Conclusion
Pre-morbid intelligence should be considered in future studies of post-stroke cognition.
Keywords: Stroke, post-stroke dementia, intelligence quotient, National Adult Reading Test, lacunar stroke
Introduction
Up to 20% of patients have dementia after stroke1 and cognitive impairment is now considered an important outcome after stroke in clinical trials: e.g. in the Secondary Prevention of Small Subcortical Stroke (SPS3) trial of 1636 patients with lacunar stroke, mild cognitive impairment (MCI) was present in 41% of those with minimal physical dependency (modified Rankin score (mRS) of 0–1) at a median of 63 days post-stroke.2 However, while severe stroke increases cognitive impairment after stroke, prediction of post-stroke cognitive impairment in general remains suboptimal, particularly in patients with less severe stroke subtypes such as lacunar stroke, which is surprising since these strokes are typically small, located in subcortical tissues and the clinical features do not include higher cortical dysfunction.3 In a previous systematic review focusing on lacunar versus non-lacunar ischaemic stroke, it was unclear whether there were differences between stroke subtypes, there were few studies that assessed cognitive impairment at later times (a year or more) after stroke, and little data on factors that predicted cognitive impairment after stroke.3
Cognitive decline in the years prior to stroke is a well-established risk factor for post-stroke cognitive impairment and dementia.4 Pre-stroke cognition is often measured using tests such as the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE)5 to capture decline in the patient’s cognitive ability in the 10 years leading up to the stroke.1,3 However, cognitive function immediately prior to a stroke is not the same as ‘pre-morbid intelligence quotient (IQ)’ which refers to an individual's ‘best-ever’ IQ as measured in young adulthood, since the stroke may have occurred in old age when pre-stroke cognition may be lower than peak cognition in youth.4 Whilst it is rare to have direct measurements of pre-morbid IQ (e.g. from preserved records from childhood IQ tests), the National Adult Reading Test (NART) measures ‘crystallised cognition’, which uses the ability to pronounce irregular words as a proxy measure of peak IQ, correlates well with childhood IQ,6 plus is robust to cognitive declines in early dementia.7
There are few data on pre-morbid IQ and post-stroke cognitive impairment, yet pre-morbid IQ is likely to influence cognition after stroke, in part through its association with educational attainment (which is also negatively associated with post stroke cognitive impairment)1 and possibly because lower IQ in youth increases the risk of stroke in later life.8 Therefore pre-morbid IQ would appear to be an important factor to measure in studies of cognition after stroke yet has not been assessed in any studies to date.1,3
We determined the risk of cognitive impairment after lacunar and minor non-lacunar ischaemic stroke (i.e. expected not to result in long-term physical dependency), assessed the effect of pre-morbid (best-ever) IQ versus educational attainment as well as other easily-obtained potential clinical and imaging predictors and updated our previous meta-analysis to set the present study in the context of the existing literature.
Materials and methods
Recruitment and clinical assessment
The recruitment and clinical assessment processes have been described previously.9 Briefly, we recruited consecutive patients presenting with a minor stroke to the Lothian regional stroke service to which all patients presenting with a stroke in our catchment area are referred. All patients gave written informed consent and Lothian Ethics of Medical Research Committee (REC 09/81,101/54) and NHS Lothian R + D Office (2009/W/NEU/14) approved the study. We defined a ‘minor stroke' as one with an National Institute of Health Stroke Scale (NIHSS) less than 7, which was not expected to cause impairment of basic activities of daily living as defined by Barthel, such as to leave the patient dependent,10 though it may cause impairment of instrumental activities of daily living. We selected this population as we wished to include patients who were likely to be well enough to return for testing at one month and one year post-stroke.
We included consecutive adult patients (over 18 years), able to have Magnetic Resonance Imaging (MRI) to confirm stroke subtype, with the capacity to give consent themselves, and with no other life-limiting condition likely to preclude follow-up at one year. We excluded patients who could not consent, for whatever reason including cognitive impairment or aphasia. An experienced stroke clinician carefully assessed all patients for eligibility, recorded clinical information and determined the clinical stroke subtype according to the Bamford classification11 into ‘lacunar’ or ‘cortical’, the former as representative of a small vessel stroke subtype and the latter to provide an alternative subtype of stroke of similar severity to the lacunar group, who are prescribed similar secondary prevention drugs, as a relevant comparison group against which to judge the cognitive status of the lacunar stroke group.
We performed MRI including diffusion-weighted imaging (DWI) at presentation, using the same 1.5 Tesla GE Signa HDxt scanner throughout. We defined a ‘lacunar’ infarct on DWI as a small focal hyperintense signal in the deep grey or white matter of the cerebral hemispheres or brainstem, not involving the cerebral cortex, and ≤20 mm in maximum diameter. Other lesions were classed as non-lacunar. If the clinical stroke syndrome was not consistent with the DWI lesion, we used the DWI lesion classification. If the MRI did not show an acute lesion or any other explanation for the stroke symptoms, but the patient had a definite clinical diagnosis of stroke, a panel of experts classed the stroke based on clinical findings.
An experienced neuroradiologist performed a structured quantification of the MRI findings, blind to clinical and cognitive data. We defined all imaging features according to the STandards for ReportIng Vascular changes on nEuroimaging (STRIVE Criteria).12 We rated white matter hyperintensities (WMH) using the Fazekas score.13 We rated small vessel disease (SVD) features according to the STRIVE criteria14 and then created a ‘total SVD score’ of combined SVD features using the validated scale15 which awards one point each for one or more microbleeds, moderate-severe enlarged perivascular spaces in the basal ganglia, one or more lacuneas, and periventricular WMH > 2 on the Fazekas scale.
Cognitive testing
Cognitive testing was performed at 1–3 and 12 months post-stroke. We initially planned to test all patients at one month post-stroke, but many were still not well enough to return to hospital for cognitive testing so were tested later. The primary outcome measure was the Addenbrookes Cognitive Examination, Revised (ACE-R), which is of equivalent sensitivity to the MoCA in detecting MCI.16 In order to ensure we were identifying cognitive impairment which was of clinical significance, we defined cognitive impairment as an ACE-R score <82 (sensitivity of 84%, specificity of 100% for dementia).17 We repeated the analysis using the ACE-R cut off of <88, as a sensitivity analysis. A trained clinician (SM) carried out the ACE-R, and other cognitive tests. We measured depression using the Beck Depression Index (BDI),18 and pre-morbid IQ using the NART.7
The NART has been validated in the seventh and eighth decades against actual cognitive ability at age 11 in local subjects,7 and test results remain constant in early to moderate dementia, whilst other cognitive tests deteriorate.19 We also recorded the number of years spent in full-time education as a measure of educational attainment. All tests were carried out in the hospital, as we did not have resources for home visits. The NART took 5–10 minutes to administer as part of a cognitive battery of tests, and the research received training from an experienced research psychologist at the Centre for Cognitive Aging and Cognitive Epidemiology at Edinburgh University.
Follow-up
We invited patients for a follow-up appointment at one-year post-stroke. At this appointment, we repeated the cognitive tests, assessed disability using the modified Rankin Scale (mRS),20 ascertained if they had developed additional vascular risk factors or experienced a further stroke or transient ischaemic attack (TIA), and if so, whether they had presented to stroke services. If patients could not attend for follow-up, we obtained information on recurrent stroke or TIA and mRS by telephone, postal questionnaire, or from their GP (General Practitioner).
Statistical analysis
We performed statistical analysis using R statistical software R Core Team (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org). We used logistic regression to calculate the odds ratio (OR) and 95% confidence interval (CI) of an ACE-R <82 for patients with and without test features, both unadjusted and adjusted for age and NART/education. The number of exploratory variables was restricted to one variable per 10 subjects in the smallest group to guard against over-fitting. We used the root mean square error (MSE) to examine if NART predicted ACE-R more strongly than years of education. We compared the cognitive findings at one to three months and one year with MRI features of SVD burden and stroke sub-type at presentation. We performed an additional analysis using linear regression modelling and interaction terms to explore the relationship between ACE-R and NART at different levels of education.
Results
Patient characteristics
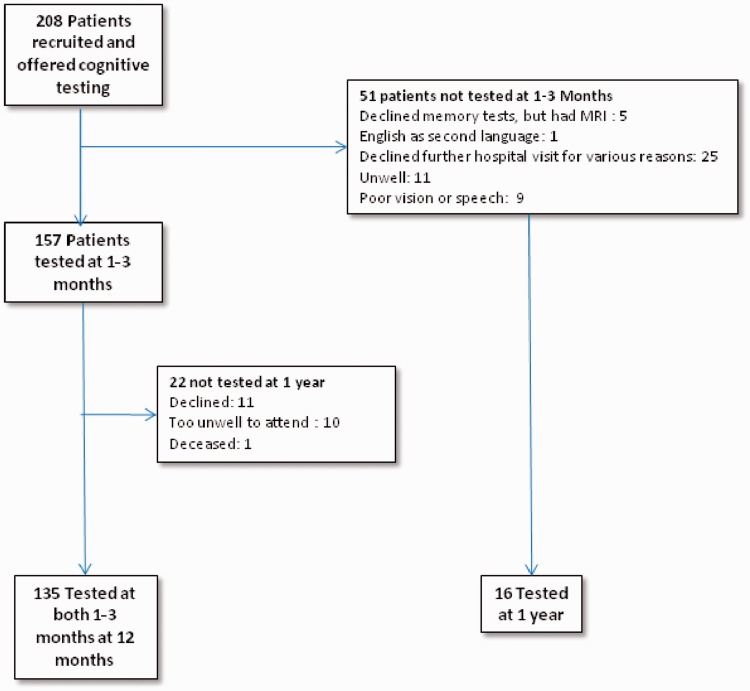
We recruited 208 patients (88 lacunar, 120 cortical strokes), of whom 157 returned for formal cognitive testing at one to three months post-stroke (Figure 1) and 151 (63 lacunar, 88 non-lacunar) at one year (see Figure 1 and Table 1 for reasons for non-testing). Tested patients were younger and less likely to be dependent at one year (Table 1). There was no difference between those tested and not tested with respect to baseline or one year NIHSS score or recurrent stroke. No patients had a diagnosis of pre-stroke dementia or MCI. In the period between onset of symptoms and cognitive testing, two patients had recurrent vascular events (one stroke, one TIA), and another patient who had an early recurrent stroke was not well enough to attend for cognitive testing.
Figure 1.
Recruitment and follow-up of patients.
Table 1.
Characteristics of patients who had cognitive testing, compared to those who were recruited but were not able to be tested.
| Patients tested | Patients not tested | ||
|---|---|---|---|
| 1–3 Months | n = 157 | n = 51 | P |
| Age at index stroke (IQR) | 66 (56–75) | 71 (63-80) | <0.01 |
| Female gender | 64 (41%) | 24 (47%) | 0.51 |
| Previous stroke (prior to index event) | 19 (12%) | 4 (10%) | 0.80 |
| NIHSS at worst point (IQR) | 2 (1–3) | 2 (1–3) | 0.81 |
| 1 Year | N = 151 | N = 57 | |
| Age at stroke (IQR) | 66 (56–74) | 73 (61–82) | <0.01 |
| Female gender | 58 (39%) | 30 (52%) | 0.51 |
| Previous stroke (prior to index event) | 19 (13%) | 5 (7%) | 0.58 |
| NIHSS at worst point (IQR) | 2 (1–3) | 2 (1–3) | 0.51 |
| Modified Rankin score at one year (IQR) | 1 (0–2) | 2 (1–3) | <0.01 |
| Stroke during follow-up | 12 (8%) | 5 (9%) | 0.78 |
| Tested at 1–3 months, but not 1 year n =22 | |||
| Age at stroke (IQR) | 65 (56–72.5) | ||
| Female gender | 12 (55%) | ||
| NIHSS at worst point (IQR) | 2 (1.5–3) | ||
| Modified Rankin score at 1 year (IQR) | 1 (0–2) | ||
| Stroke during follow-up | 2 (9%) | ||
| ACE-R < 82 at 1-3 months | 9 (41%) | ||
| Reasons not tested at 1 year | Declined repeat test 11, too unwell 10, deceased 1 | ||
| Not tested at 1-3 months, but tested at 1 year n = 16 | |||
| Age at stroke(IQR) | 72 (66–79.25) | ||
| Female gender | 6 (38%) | ||
| NIHSS at worst point (IQR) | 1 (1–2) | ||
| Modified Rankin score at 1 year (IQR) | 1 (0–2) | ||
| Stroke during follow-up | 1 (6%) | ||
| ACE-R<82 at 1 year | 6 (38%) | ||
| Reasons not tested at 1–3 months | Dysphasia which improved 1, forgot reading glasses 2, unable to attend due to work 1, too unwell 1, declined 11 | ||
| Tested at both 1–3 months and 1 year n=135 | |||
| Age at stroke (IQR) | 65 (56–72.5) | ||
| Female gender | 52 (39%) | ||
| NIHSS at worst point (IQR) | 2 (1.5–3) | ||
| Modified Rankin score at 1 year (IQR) | 1 (0–2) | ||
| Stroke during follow-up | 11 (8%) | ||
| ACE-R < 82 at 1–3 months | 27 (20%) | ||
| ACE-R < 82 at 1 year | 23 (17%) | ||
ACE-R: Addenbrookes Cognitive Examination, Revised; IQR: inter-quartile range; NIHSS: National Institute of Health Stroke Scale;
Influence of premorbid intelligence (NART)
A higher pre-morbid intelligence as estimated using the NART (Table 2) was associated with male sex (median NART scores 40.5 in men, 36 in women p = 0.02), the absence of atrial fibrillation (AF, median 41.5 in patients without AF, 36 in patients with AF, p = 0.049), and alcohol use over the recommended limit (median 42 in patients who used excess alcohol, 36 in patients who did not). There were strong associations between NART and ACE-R at one to thee months (r = 0.47, p < 0.01), and between NART and the number of years of full-time education (r = 0.45, p < 0.01). Patients who were older when recruited to the study with stroke tended to have a slightly higher NART score, although this did not reach statistical significance (correlation of NART and older age, r = 0.10, p = 0.21). Patients with a previous stroke in our study did not have a significantly lower NART than patients without a previous stroke, the median NART for patients with a previous stroke was 39 (inter-quartile range (IQR) 26.5–43) whilst the median NART for patients without a previous stroke was 41 (IQR 26.5–43) a difference that was not statistically significant (p = 0.17, calculated using Wilcox Mann–Whitney test) in this sample size. The difference might be significant in a much larger data set.
Table 2.
Median National Adult Reading Test (NART) score at one to three months post-stroke of patients with and without different variables.
| No. of patients with variable (%) | Median NART with variable (IQR) | Median NART without variable (IQR) | p | Logistic regression Unadjusted OR per 10 point higher NART with parameter |
Logistic regression Age and sex adjusted OR per 10 point higher NART with parameter | |
|---|---|---|---|---|---|---|
| Sex (F) | 62 (41%) | 36 (28–40) | 40.5 (28–40) | 0.02 | 1.27 (95% CI 0.95, 1.73) | NA |
| Prior to index stroke, the patient had a past medical history of: | ||||||
| TIA | 17 (11%) | 34 (25–42) | 38 (25-42) | 0.26 | 0.77 (95% CI 0.49,1.21) | 0.72 (95% CI 0.46, 1.13) |
| Stroke | 19 (12%) | 36 (30–42) | 38 (30-42) | 0.65 | 0.9 (95% CI 0.59,1.41) | 0.86 (95% CI 0.56, 1.34) |
| Stroke or TIA | 30 (20%) | 34.5 (26–41.75) | 39 (26-41.75) | 0.15 | 0.74 (95% CI 0.52,1.07) | 0.7 ( 95% CI 0.48, 1.01) |
| Ischaemic heart disease | 32 (21%) | 37.5 (24.5–44) | 38 (24.5-44) | 0.62 | 0.8 (95% CI 0.56,1.14) | 0.71 (95% CI 0.49, 1.03) |
| Peripheral vascular disease | 10 (6.5%) | 38 (31.25–43.75) | 38 (31.25-43.75) | 0.76 | 1.11 (95% CI 0.62,2.16) | 1.01 (95% CI 0.56, 1.97) |
| Diabetes | 20 (13%) | 41 (28–45.5) | 37 (28-45.5) | 0.48 | 1.06 (95% CI 0.69,1.69) | 1 (95% CI 0.65, 1.59) |
| Hypertension | 108 (71%) | 38 (30–44) | 34 (30-44) | 0.06 | 1.43 (95% CI 1.04,1.98) | 1.43 (95% CI 1.03, 2) |
| AF0 | 13 (8.5%) | 41.5 (39.5–44.75) | 36 (39.5-44.75) | 0.05 | 1.56 (95% CI 0.88,3.05) | 1.47 (95% CI 0.82, 2.95) |
| Hyperlipidaemia | 100 (65%) | 37 (29–44) | 39 (29-44) | 0.76 | 1.05 (95% CI 0.77,1.43) | 1.04 (95% CI 0.76, 1.42) |
| Smoker | 46 (30%) | 35.5 (27.75–42) | 39 (27.75-42) | 0.24 | 1.06 (95% CI 0.76,1.45) | 1.09 (95% CI 0.78, 1.52) |
| Any alcohol use | 97 (63%) | 39 (31–45) | 33 (31-45) | <0.01 | 1.06 (95% CI 0.76,1.45) | 1.09 (95% CI 0.78, 1.52) |
| Previous or current excess alcohol use | 15 (9.8%) | 37 (31–42.5) | 38 (31-42.5) | 0.68 | 1.15 (95% CI 0.71,2) | 1.06I (95% CI 0.65, 1.82) |
| Current alcohol use over the recommended limit (14 units for women, 21 for men) | 24 (16%) | 42 (38.25–47) | 36 (38.25-47) | <0.01 | 1.53 (95% CI 0.99,2.5) | 1.5 (95% CI 0.97, 2.48) |
| Final diagnosis of lacunar stroke | 118 (45%) | 41 (33.5–45) | 41 (33.5-45) | 0.91 | 0.9 (95% CI 0.67,1.22) | 0.93 (95% CI 0.69, 1.26) |
ACE-R: Addenbrookes Cognitive Examination, Revised; BDI: Beck Depression Index; CI: confidence interval; IQR: inter-quartile range; OR: odds ratio; NIHSS: National Institute of Health Stroke Scale; TIA, transient ischaemic attack.
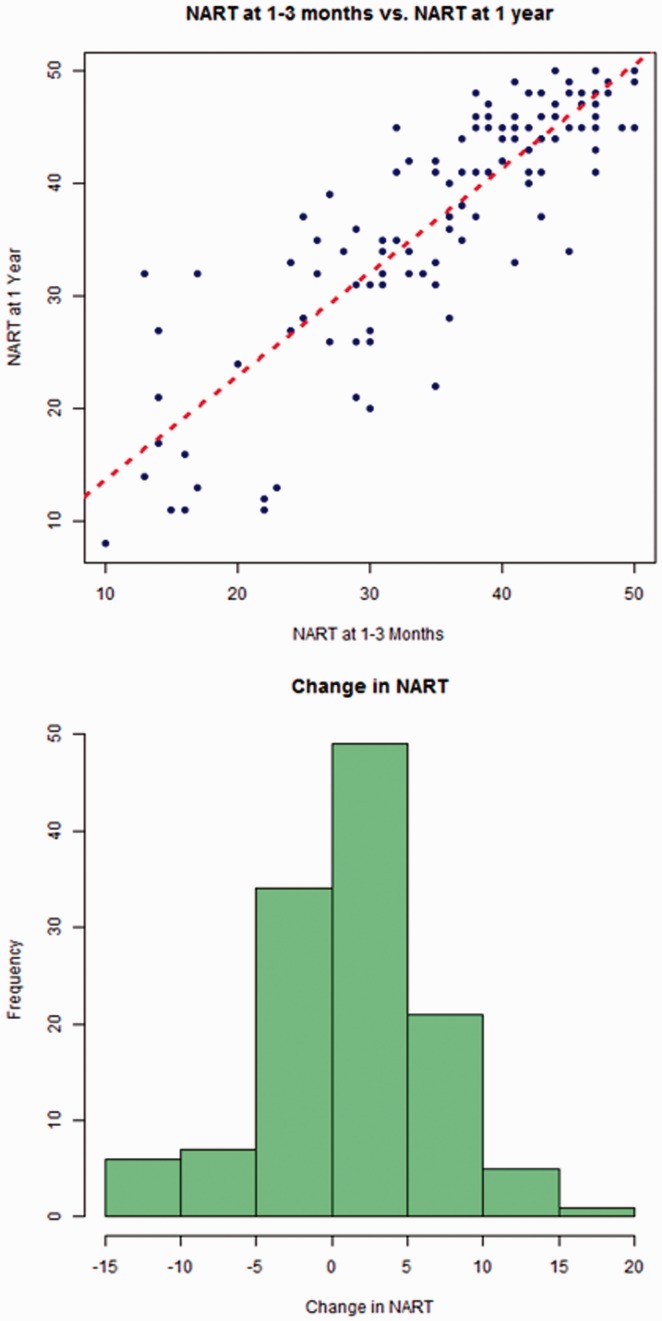
There was little change in NART between one and three months and one-year post-stroke, and NART at one to three months was strongly predictive of NART at one year (r = 0.86, p < 0.001, Figure 2).
Figure 2.
NART at one to three months and one year post stroke.
Cognition after stroke (ACE-R scores) at 1–3 and 12 months and predictors
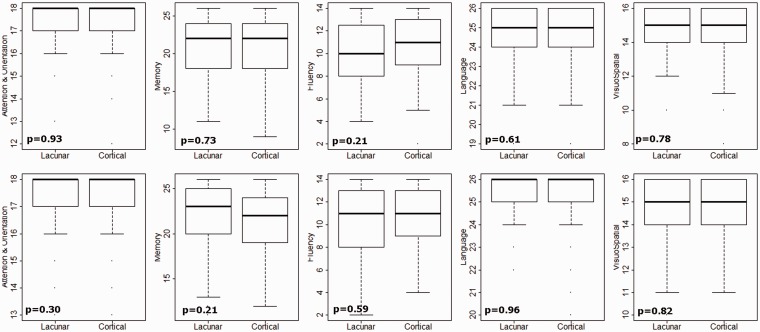
We found that 36/157 (23%) patients had an ACE-R score < 82 at one to three months and 29/151 (19%) had an ACE-R score <82 at one year. A higher ACE-R was associated with younger age, higher NART and more years of education (Table 3). There was no difference in cognitive impairments between lacunar and non-lacunar ischaemic stroke subtypes. Patients with non-lacunar stroke were not more likely to be impaired in the domains associated with higher cortical dysfunction (language or visuospatial function, Figure 3) than those with lacunar stroke. Both unadjusted (Table 3) and adjusted (Supplementary Table) analyses show that ACE-R <82 was predicted by older age (OR per year of age 1.04, 95% CI 1.00, 1.08), lower NART (age adjusted OR per point on NART 0.92, 95% CI 0.88, 0.96), fewer years of education, (age adjusted OR per year of education 0.68, 95% CI 0.48, 0.87), higher Fazekas Score (OR per point increase in Fazekas Score 1.42, 95% CI 1.11, 1.83) and higher total SVD score (OR per point increase in SVD score 1.68, 95% CI 1.05, 2.7).
Table 3.
ACE-R at one to three months and one year post-stroke, associated variables and odds ratios on univariate analysis.
| 1–3 months post-index stroke |
1 year post-index stroke |
|||||||
|---|---|---|---|---|---|---|---|---|
| ACE-R < 82 | ACE-R ≥ 82 | P | Un-adjusted | ACE-R < 82 | ACE-R ≥ 82 | P | Un-adjusted | |
| N (%) or median (IQR) | N (%) or median (IQR) | N (%) or median (IQR) | N (%) or median (IQR) | |||||
| Total: | n = 36 | n = 121 | OR (95% CI)a | n = 29 | n = 122 | OR (95% CI)a | ||
| Age (per year) | 72 (63–80) | 64 (56–74) | 0.01 | 1.04 (1.01, 1.08) | 71 (63-76) | 65 (56–73) | 0.049 | 1.04 (1.00, 1.08) |
| Male sex | 13 (36%) | 51 (42%) | 0.52 | 1.29 (0.6, 2.84) | 12 (41%) | 46 (38%) | 0.72 | 0.86 (0.38, 1.99) |
| No of years of education | 10 (9–10) | 11 (10–13) | 0.004 | 0.63 (0.43, 0.82) | 10 (9–10) | 11 (10–14) | <0.01 | 0.28 (0.13, 0.52) |
| Previous stroke | 4 (11%) | 15 (12%) | 0.84 | 0.88 (0.24, 2.64) | 3 (10%) | 16 (13%) | 0.68 | 0.76 (0.17, 2.51) |
| IHD prior to index stroke | 10 (28%) | 23 (19%) | 0.26 | 1.64 (0.67, 3.81) | 5 (17%) | 23 (19%) | 0.84 | 0.9 (0.28, 2.45) |
| PVD prior to index stroke | 4 (11%) | 6 (5%) | 0.20 | 2.4 (0.58, 8.91) | 2 (7%) | 5 (4%) | 0.52 | 1.73(0.24, 8.53) |
| Diabetes | 5 (14%) | 15 (12%) | 0.81 | 1.14 (0.35, 3.21) | 5 (17%) | 13 (11%) | 0.33 | 1.75 (0.52, 5.13) |
| Hypertension | 21 (58%) | 88 (73%) | 0.10 | 0.52 (0.24, 1.15) | 17 (59%) | 92 (75%) | 0.074 | 0.46 (0.2, 1.09) |
| AF | 3 (8%) | 10 (8%) | 0.99 | 1.01 (0.22, 3.53) | 4 (14%) | 10 (8%) | 0.36 | 1.79 (0.46, 5.85) |
| Smoker | 10 (28%) | 38 (31%) | 0.50 | 1.15 (0.97, 0.45) | 8 (28%) | 34 (28%) | 0.54 | 0.71 (0.92, 0.25) |
| Alcohol over recommended limit | 3 (8%) | 22 (18%) | 0.17 | 0.41 (0.16, 1.46) | 2 (7%) | 24 (20%) | 0.11 | 0.30 (0.67, 1.36) |
| NIHSS | 2 (1–3) | 2 (1–3) | 0.46 | 1.11 (0.83, 1.47) | 2 (1–3) | 2 (1–3) | 0.81 | 0.96 (0.68, 1.31) |
| NART at 1-3 months | 31 (19–37) | 40 (31–45) | <0.001 | 0.92 (0.89, 0.96) | 30 (17–33) | 40 (33–45) | <0.01 | 0.91 (0.86, 0.95) |
| BDI at 1-3 months | 11 (8–19) | 8.5 (4–14) | 0.069 | 1.04 (1, 1.08) | 11 (6–17) | 8 (4–13) | 0.34 | 1.02 (0.97, 1.07) |
| Cortical stroke (versus lacunar) | 19 (53%) | 70 (58%) | 0.59 | 0.81 (0.39–1.72) | 15 (52%) | 73 (60%) | 0.43 | 0.72 (0.32–1.62) |
| Recurrent TIA | Na | Na | Na | 0 (0%) | 4 (3%) | 0.99 | NA (NA, NA) | |
| Recurrent stroke | Na | Na | Na | 5 (17%) | 7 (6%) | 0.05 | 3.4 (0.94, 11.65) | |
| Any recurrent vascular event | Na | Na | Na | 5 (17%) | 11 (9%) | 0.20 | 2.1 (0.62, 6.37) | |
| NART at 1 year† | Na | Na | Na | 31 (23–35) | 43 (34–47) | <0.01 | 0.92 (0.88, 0.96) | |
| mRS score | Na | Na | Na | 2 (1–2) | 1 (0–2) | 0.13 | 1.4 (0.91, 2.18) | |
| Total Fazekas score | 2 (2–4) | 2 (2–4) | 0.49 | 1.08 (0.87, 1.34) | 4 (2–6) | 2 (2–4) | 0.006 | 1.42 (1.11, 1.83) |
| Total number of microbleeds | 0 (0–0) | 0 (0–0) | 0.80 | 1.01 (0.91, 1.1) | 0 (0–0) | 0 (0–0) | 0.65 | 1.02 (0.92, 1.1) |
| Total atrophy score | 4 (2–6) | 4(2–6) | 0.42 | 1.07 (0.91, 1.26) | 6 (3–6) | 4 (2–6) | 0.13 | 1.15 (0.96, 1.37) |
| Periventricular space score | 4 (3–6) | 5 (4–6) | 0.46 | 0.94 (0.8, 1.1) | 5 (5–7) | 5 (4–6) | 0.22 | 1.12 (0.93, 1.34) |
| SVD score | 1 (0–2) | 1 (0–2) | 0.39 | 1.14 (0.84, 1.54) | 2 (1–2) | 1 (0–2) | 0.02 | 1.46 (1.06, 2.04) |
ACE-R: Addenbrookes Cognitive Examination, Revised; CI: confidence interval; IQR: inter-quartile range; OR: odds ratio.
aFor dichotomous variables, the odds of patients with variable having cognitive impairment versus odds in those without the variable, for continuous odds per year (for age/years of education) or per point for rating scales.
Figure 3.
Cognitive impairment in different domains in lacunar and cortical stroke at one month (top) and one year (bottom) index stroke with p values for the different between lacunar and cortical stroke.
To determine if the NART predicted ACE-R at one year more strongly than years of education, we constructed two unadjusted linear regression models and compared the root MSEs: the mean error between the ACE-R predicted by the NART or the number of years of education and the actual ACER. Total years of education had an root MSE of 7.34 ACE-R points, whereas NART at one to three months had an MSE of 7.33 indicating little difference between NART and the number of years of education in the ability to predict ACE-R.
As a further analysis we repeated the analysis using the cut off of ACER < 88 and again the strongest predictor of ACE-R was older age, lower NART/years of education (supporting information).
ACE-R largely remained static in the first year after stroke. Of 27 patients who were impaired at one to three months, 19 (70%) were still impaired at one year and 8 (30%) had improved; of 108 patients who were not impaired at one to three months, four had developed cognitive impairment by one year. ACE-R at one to three months was highly correlated with ACE-R at one year 0.85, p < 0.001 with no significant difference between the two (median ACE-R at one to three months 90, median ACE-R at one year 91, p = 0.34).
Post-stroke cognition (ACE-R) and dependence (mRS)
Patients who were more dependent at one year post-stroke had lower ACE-R scores: median ACE-R for patients with an mRS of ≥ 2 was 89 (IQR 81.75–91.25), whereas the median ACE-R for patients with an mRS of < 2 was 92 (IQR 86–95, p = 0.02). On multivariable logistic regression using the proportional odds model,21 the relationship between functional outcome and cognitive impairment remained significant after correction for age, NIHSS score, NART and BDI: the adjusted OR was 0.93 (95% CI 0.88–0.98).
Relationship between NART, ACE-R and education
To further explore the relationship between NART, ACE-R and education, we conducted a further, post-hoc analysis, which indicated that education may weaken the connection between NART and ACE-R in that the relationship between ACE-R and NART fell more steeply in those with fewer years of education (Supplementary Figure 2). We constructed a further multivariable model to include an interaction term (Supplementary Table 2), the coefficient was small it is probable that our sample was lacking the power to explore this relationship.
Results of the updated systematic review
To establish if our patients were comparable to other studies of cognition after lacunar versus non-lacunar stroke, we updated our systematic review with data from our study and the only other relevant study published since our review.22 The details of the studies are given in supplementary table. Thus, a total of 18 studies and 7814 patients were included (12.5% more studies and 20% more patients since 2013). The updated OR of cognitive impairment in lacunar compared to non-lacunar stroke did not change significantly: updated OR 0.75 (91% CI 0.47–1.20) compared to 0.72 (95% 0.42–1.20) previously (Supplementary Figure 1).
Discussion
We found that: the NART (which estimates pre-morbid IQ) was a stronger predictor of post-stroke cognition than vascular risk factors or stroke severity and suggest that it should be accounted for in studies of post-stroke cognition; additionally cognitive impairment was common and associated with greater likelihood of being dependent (higher mRS) at one year post-stroke. We confirmed that patients with lacunar stroke were as likely to have cognitive impairment one year after stroke as patients with a similarly mild non-lacunar stroke. Post-stroke cognitive impairment was also associated with fewer years of education. While there was no definite difference between pre-morbid IQ and education in terms of prediction of ACE-R in this single study, the NART is a more direct measure of pre-morbid IQ than education so future larger studies should examine whether pre-morbid IQ or education is the stronger predictor and whether education can modify the effect of pre-morbid IQ, as assessed by the NART or equivalent test, on post-stroke cognitive impairment. Future confirmation that education can reduce the adverse effect of pre-morbid IQ on post stroke cognitive impairment as suggested in Supplementary Figure 2 may help identify additional ways in which public health strategies could, in the long term, improve outcomes after stroke.
The strengths of this study include accurate stroke subtyping, adjustment for confounding from pre-existing SVD, depression, and pre-morbid IQ, and a comparator group with a different subtype of stroke, but similar severity and prescribed similar secondary prevention, against whom to compare the results for the lacunar stroke patients. Weaknesses include the sample size, which is smaller than multi-centre studies but among the larger studies in our systematic review, and complete cognitive data in 73% a year, which is, however, comparable to other studies. Pendlebury et al. estimated that loss of untestable patients may lead to a sevenfold underestimation of cognitive impairment.23 Since we started this work, there is more evidence that some assessments can provide a good agreement between telephone and face-to-face assessment.24 Another weakness is that we did not formally test for pre-stroke cognitive impairment: we excluded patients who lacked the capacity to consent but did not use a formal test of pre-stroke cognitive impairment falling short of incapacity, as use of the IQ-CODE5 requires an informant and has been discontinued by other studies.23 Whilst we did include patients who did not have an acute ischaemic lesion visible on diffusion imaging on MRI, our previous analysis9 demonstrated that at one year there was little clinical difference between patients who did or did not have a visible DWI lesion when presentation with stroke with respect to cognitive impairment, recurrent stroke/TIA or disability and no alternative diagnosis apart from stroke. Therefore, we did not feel it would be appropriate to exclude patients without a DWI-visible lesion at presentation from the study. Our used dichotomised data may have reduced the power slightly but may produce results which are more clinically meaningful. Our exploration of the relationship between education, NART and post-stroke cognitive impairment is interesting, and worthy of further research, however the sample size was limited.
Our updated meta-analysis demonstrates that our study was consistent with other studies identified in our, 2013 systematic review, indicating our sample is relevant to other studies of cognition after lacunar versus non-lacunar stroke. It is notable that our results were similar to other studies in the review, despite many of these being of a hospital population including patients with more severe stroke. It is likely that the included patients, who were able to complete testing were actually those with a milder impairment: Lees et al.25 found that only 22% of patients on a rehabilitation ward with disabling stroke were able to complete cognitive screening tests.
Our findings are similar to studies that found an association between cognition and education 26 and little association between domain-specific cognitive impairments and stroke subtype.27 The SPS 3 study found a higher rate of MCI than our rate of cognitive impairment but was not included in our meta-analysis because it lacked a non-lacunar control group.
This work has implications for clinicians and researchers. Firstly it underlines that it is important to consider that pre-morbid IQ may influence results of research into the aetiology of, and outcomes after stroke, and therefore should be measured in studies examining post-stroke cognition. Measurement of years of education is an alternative, particularly in a population where availability of education is universally consistent.
We demonstrate that it is feasible to use the NART to test pre-morbid intelligence in patients with minor stroke; the test took 5–10 minutes to administer, was completed by the majority of patients, and researchers could be trained in one session.
Disadvantages of the use of the NART are that it is an additional test for the patient leading to an increased burden of testing, and validated versions are not available in all languages. Whilst recording the years of education is an attractive alternative, and should certainly be considered in settings where NART is not feasible, it should be noted that although pre-morbid cognitive ability and education are inter-related, they are not the same, since pre-morbid cognitive ability reflects factors (e.g. processing speed) which are not affected by education as well as factors (e.g. reasoning ability and visuospatial function) that are affected by education.28 However, the NART is a direct measure of pre-morbid intelligence and also parametric which enables more sophisticated statistical analysis.
Whether measured directly by NART or indirectly through years of education, pre-morbid IQ is an important factor in post-stroke cognition, and should be accounted for in research into post-stroke cognition.
Supplementary Material
Acknowledgements
We thank the patients and their families, the Radiographers at the Brain Research Imaging Centre and the Stroke Research Network.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Wellcome Trust (grant 088134/Z/09/A); Scottish Funding Council and the Chief Scientist Office of Scotland for funding the Scottish Imaging Network: A Platform for Scientific Excellence (‘SINAPSE’). FD holds a NHS Research Scotland and Stroke Association-Garfield Weston Foundation Senior Lectureship.
Informed consent
All patients gave written informed consent.
Ethical approval
All patients gave written informed consent and Lothian Ethics of Medical Research Committee (REC 09/81,101/54) and NHS Lothian R + D Office (2009/W/NEU/14) approved the study.
Guarantor
JMW.
Contributorship
SDM conceived, designed and carried out the cognitive study, analysed the data, and wrote the manuscript. FND advised on the design of the study, identified suitable patients and commented on the manuscript. KS assisted with data collection and checking. JS assessed MRI data and SVD scores, and critical revision of the manuscript. MSD advised on the design of the study, suitable cognitive tests, identified suitable patients, advised on the diagnosis of stroke, and subtype of stroke, and served on the expert panel. FMC carried out the statistical analysis relating to the interaction between NART, ACE-R and Education. JMW conceived, obtained funding for and oversaw the study, including data management, assessment of the MRI data, the data analysis and interpretation, critical revisions of the manuscript and takes full responsibility for the study.
All authors reviewed and edited the manuscript and approved the final version of the manuscript
References
- 1.Pendlebury ST andRothwell PM.. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009; 8: 1006–1018. [DOI] [PubMed] [Google Scholar]
- 2.Jacova C, Pearce LA, Costello R, et al. Cognitive impairment in lacunar strokes: the SPS3 trial. Ann Neurol 2012; 72: 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makin SDJ, Turpin S, Dennis MS, et al. Cognitive impairment after lacunar stroke: systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J Neurol Neurosurg Psychiat 2013; 84: 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henon H. Pre-stroke dementia: prevalence and associated factors. Cerebrovasc Dis 2000; 10(supplement 4): 45–48. [DOI] [PubMed] [Google Scholar]
- 5.Jorm AF andJacomb PA.. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med 1989; 19: 1015–1022. [DOI] [PubMed] [Google Scholar]
- 6.Dykiert D andDeary IJ.. Retrospective validation of WTAR and NART scores as estimators of prior cognitive ability using the Lothian Birth Cohort 1936. Psychol Assess 2013; 25: 1361–1366. [DOI] [PubMed] [Google Scholar]
- 7.McGurn B, Starr JM, Topfer JA, et al. Pronunciation of irregular words is preserved in dementia, validating premorbid IQ estimation. Neurology 2004; 62: 1184–1186. [DOI] [PubMed] [Google Scholar]
- 8.McHutchison CAB, Cvoro V, Shenkin SD, et al. Education, socioeconomic status and intelligence in childhood as risk factors for stroke in later life: a meta-analysis. Epidemology 2017; 28(4): 608–618. [DOI] [PubMed] [Google Scholar]
- 9.Makin SD, Doubal FN, Dennis MS, et al. Clinically confirmed stroke with negative diffusion-weighted imaging magnetic resonance imaging: longitudinal study of clinical outcomes, stroke recurrence, and systematic review. Stroke 2015; 46: 3142–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahoney FI andBarthel DW.. Functional evaluation: the barthel index. Md State Med J 1965; 14: 61–65. [PubMed] [Google Scholar]
- 11.Bamford J, Sandercock P, Dennis M, et al. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991; 337: 1521–1526. [DOI] [PubMed] [Google Scholar]
- 12.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987; 149: 351–356. [DOI] [PubMed] [Google Scholar]
- 14.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration: a united approach. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staals J, Makin SD, Doubal FN, et al. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014; 83: 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pendlebury ST, Mariz J, Bull L, et al. MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards Neuropsychological Battery after TIA and stroke. Stroke 2012; 43: 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mioshi E, Dawson K, Mitchell J, et al. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriat Psychiat 2006; 21: 1078–1085. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571. [DOI] [PubMed] [Google Scholar]
- 19.O’Carroll RE Baikie EM andWhittick JE.. Does the National Adult Reading Test hold in dementia? Br J Clin Psychol 1987; 26: 315–316. [DOI] [PubMed] [Google Scholar]
- 20.Dennis M, Mead G, Doubal F, et al. Determining the modified Rankin score after stroke by postal and telephone questionnaires. Stroke 2012; 43: 851. [DOI] [PubMed] [Google Scholar]
- 21.Katz MH. Multivariable analysis: a practical guide for clinicians and public health researchers. 3rd ed. Cambridge; New York: Cambridge University Press, 2011, pp. xv, 233. [Google Scholar]
- 22.Wong A, Nyenhuis D, Black SE, et al. Montreal Cognitive Assessment 5-minute protocol is a brief, valid, reliable, and feasible cognitive screen for telephone administration. Stroke 2015; 46: 1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pendlebury ST, Chen PJ, Welch SJ, et al. Methodological factors in determining risk of dementia after transient ischemic attack and stroke: (II) effect of attrition on follow-up. Stroke 2015; 46: 1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pendlebury ST, Welch SJ, Cuthbertson FC, et al. Telephone assessment of cognition after transient ischemic attack and stroke: modified telephone interview of cognitive status and telephone Montreal Cognitive Assessment versus face-to-face Montreal Cognitive Assessment and neuropsychological battery. Stroke 2013; 44: 227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lees RA, Hendry Ba K, Broomfield N, et al. Cognitive assessment in stroke: feasibility and test properties using differing approaches to scoring of incomplete items. Int J Geriatr Psychiatry 2017; 32: 1072–1078. [DOI] [PubMed] [Google Scholar]
- 26.Pearce LA, McClure LA, Anderson DC, et al. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: a secondary analysis from the SPS3 randomised trial. Lancet Neurol 2014; 13: 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards JD, Jacova C, Sepehry AA, et al. A quantitative systematic review of domain-specific cognitive impairment in lacunar stroke. [Review]. Neurology 2013; 80: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritchie SJ Bates TC andDeary IJ.. Is education associated with improvements in general cognitive ability, or in specific skills? Dev Psychol 2015; 51: 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.