Short abstract
Introduction
We aimed to assess alterations in glucose, blood pressure and temperature in acute ischaemic stroke and investigate their association with early all-cause mortality and functional outcome.
Patients and methods
We studied all consecutive acute ischaemic stroke patients admitted in 2001–2010 to the Acute Stroke Unit, at Alexandra University Hospital, in Athens. Serial measurements were performed in the first seven days post-stroke and different parameters have been estimated: mean daily values, variability, subject-specific baseline levels and rate of change in serial measurements. Cox-proportional-hazards-model analysis and logistic-regression analysis were applied to investigate the association between these parameters and all-cause mortality and functional outcome after adjustment for known confounders of stroke outcome.
Results
In 1271 patients (mean age 72.3 ± 11.2 years), after adjusting for confounders, baseline glucose levels (HR: 1.005, 95%CI: 1.001–1.01; p = 0.017), variability of systolic BP (SBP) as estimated by standard deviation (HR: 1.028, 95%CI: 1.01–1.048; p = 0.005), the baseline temperature (HR: 2.758, 95%CI: 2.067–3.68; p < 0.001) and the rate of temperature change (HR: 1.841, 95%CI: 1.616–2.908; p < 0.001) were independently associated with all-cause mortality within three months. Poor functional outcome was associated with subject-specific baseline values of temperature (OR: 1.743; 95%CI: 1.076–2.825; p = 0.024), the rate of SBP (OR: 1.159; 95% CI: 1.047–1.280; p = 0.004) and temperature change (OR: 1.402; 95% CI: 1.061–1.853; p = 0.018).
Discussion
The main strength of our study is that we analysed simultaneously three parameters and we used four different variables for each parameter of interest.
Conclusion
Baseline glucose levels, variability of SBP and baseline temperature and its rate of change are independent predictors of all-cause mortality. Baseline values of temperature and the rate of changes in SBP and temperature are independent predictors of poor functional outcome.
Keywords: Acute stroke, hyperglycaemia, temperature, blood pressure, mortality
Introduction
Acute stroke unit (ASU) care has been associated with reduction in stroke mortality, dependency and need for institutionalisation.1 Patients treated in ASU are closely monitored for physiological and biochemical variables such as glucose, blood pressure (BP) and temperature, among others. Several observational studies have reported associations of these variables with stroke prognosis, the majority finding them to be positively correlated with poor outcome.2–11 Moreover, some studies of BP and glucose found this association to follow a U- or J-shaped curve.12–14 Such findings led to randomised controlled studies of stroke interventions aimed at reducing or normalising these physiological and biochemical parameters, mainly targeting admission values.15–18 Unfortunately no benefit from interventions was observed and the reasons for this are still widely discussed.19
Most observational studies have focused on one parameter (BP or hyperglycaemia) without taking into consideration the values of the other parameters during the acute and sub-acute phase of stroke.12–14 Furthermore, recent studies have found that the actual variability of these parameters and not their absolute values are more significant factors in outcome.20,21
In this context, we aimed to simultaneously investigate the values of BP, glucose and temperature during the acute and sub-acute phase of acute ischaemic stroke (AIS) in a series of patients who were treated in an ASU, in relation to all-cause mortality and poor functional outcome.
Patients and methods
Study population
We included all consecutive patients with first-ever AIS admitted within 24 h after symptom onset to the ASU of Alexandra Hospital (Athens, Greece) between, 2001 and 2010. Patients with transient ischaemic attacks (TIAs), intracerebral haemorrhage or subarachnoid haemorrhage were excluded. The institutional Review Board of the hospital approved the study; written informed consent was not obtained from patients as waived by the board.
Definitions and outcomes
The methodology used for the data collection has been described before.22 All parameters were prospectively collected using pre-specified definitions. Stroke severity on admission was estimated by the National Institutes of Health Stroke Scale (NIHSS). Estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in renal disease (MDRD) formula (for creatinine in µmol/L).23 Stroke aetiology was classified by the TOAST criteria.24 Hypertension was defined as systolic blood pressure (SBP) >140 mm Hg or diastolic blood pressure (DBP) >90 mm Hg measured at least twice before stroke or if the patient was already receiving antihypertensive medications. Diabetes mellitus was defined as a fasting blood glucose level >7.0 mmol/L before stroke or if the patient was already receiving antidiabetic drugs or insulin. Dyslipidaemia was defined as a cholesterol concentration >6.5 mmol/L the day after admission, or if the patient had a previous diagnosis of dyslipidaemia. A subject was considered as a smoker when they had smoked daily prior to the stroke. Coronary artery disease (CAD) was assessed by questionnaire and relevant medical confirmation. SBP and DBP were measured manually and or automated on admission and at 3-hourly intervals during the first week. Serum glucose was measured on admission and thereafter three times a day, before main meals. Glucose levels were measured either via bedside capillary finger prick or laboratory serum glucose testing. Body temperature was measured on admission and at 3-hourly intervals via tympanic thermometer.
The primary outcomes of our study were death from any cause within three months from the AIS and poor functional outcome at three months; the zero time from which the outcome was assessed was the time of stroke onset. Death was confirmed by the evaluation of all available information from hospital records, death certificates, necropsy findings and primary physician’s notes. Poor functional outcome was defined as a modified Rankin Scale Score of >2. Follow-up was regularly performed at the outpatient clinic or by medical personnel at the patient’s residence in cases not able to attend the outpatient clinic.
Statistical analysis
Daily mean values and standard deviation (SD) over the first seven days of hospitalisation were calculated for glucose, SBP, DBP and temperature. Continuous variables are summarised as mean ± SD or median (interquartile range) when deviation from normal distribution was observed. Nominal variables were summarised as absolute and percentage values. Histograms and distribution plots (percentile–percentile and quantile–quantile plots) were used to evaluate the normality of the parameters of interest. Correlations between quantitative variables (i.e. modified Rankin Scale (mRS) at three months and continuous baseline predictors or between different indices of the same parameter of interest) were evaluated using Pearson’s or Spearman's correlation coefficient. Differences between groups of interest (subjects that died within three months versus survivors) were assessed by independent samples t test or Mann-Whitney test for continuous variables and chi-square test for nominal variables. Due to the within-subjects design of our study that assessed glucose, BP and temperature measurements on multiple occasions (day 1 through day 7), we used linear mixed models including random intercepts and slopes with an unstructured covariance matrix. Then, for each individual, we obtained the subject-specific intercepts and slopes of glucose, SBP, DBP and temperature by using the best linear unbiased estimates (BLUPs). Regression assumptions for the linear mixed models analysis were checked by visual inspection of the residuals in each model.
Two outcomes of interest were assessed in this study: (a) all-cause death up to three months after acute stroke (primary endpoint) and (b) poor functional outcome at three months after acute stroke (secondary endpoint). In terms of the main outcome, time to event (i.e. all-cause death) was of primary interest and survival analysis was implemented. Cox proportional hazards models were used to examine the association between baseline predictors and death within three months; we used three different models for each parameter of interest to capture more accurately the variability across the first seven days of hospitalisation: (a) mean value of glucose, SBP, DBP and temperature for each patient; (b) SD of glucose, SBP, DBP and temperature data for each patient; and (c) subject-specific intercepts and rate of changes of glucose, SBP, DBP and temperature. The proportional hazard assumption of Cox models was assessed using the appropriate graph and statistical test (Schoenfeld residuals). Associations are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). For the secondary outcome (i.e. poor functional outcome at 3 months), we performed multivariable logistic regression analysis and used identical models to the survival analysis. Odds ratio (OR) with 95% CIs are reported for the secondary outcome analysis.
For both survival and logistic regression analysis, final multivariable models were adjusted for all available major confounders of biological plausibility including traditional risk factors, stroke severity, renal function and in hospital treatment medication and no selection strategy was implemented. In detail, covariates in the final models were age, gender, NIHSS score, eGFR, stroke subtype, history of hypertension, diabetes, smoking, dyslipidaemia, CAD, TIAs, atrial fibrillation, aspirin and/or anticoagulation administration and thrombolysis performance. In multivariable analyses, variable inflation factor (VIF) was used to assess co-linearity among covariates. A VIF value greater than 4 was considered indicative of collinearity.25 In the final multivariable models, mean values for glucose, BP indices and temperature were not simultaneously assessed with the random intercept and slope model since (a) both were highly correlated (Spearman's rho > 0.89 and p value < 0.001 for both) and (b) the mean values were not of interest if slope had an impact on the outcome.
In addition, we sought to assess the incremental value of the multiple measurements of the parameters of interest over established prognostic factors in terms of discrimination and reclassification. The incremental predictive value was assessed by Harrell’s C-index for censored time-to-event data.26 The integrated discrimination index (IDI), which is equivalent to the difference in discrimination slopes, was also calculated. Finally, we calculated the continuous Net Reclassification Index (NRI).27 Our sample size of 1271 subjects with survival follow-up data and 241 deaths provided over 90% power to establish an alteration in HR (two-sided) ≥1.5 for Cox models for the all-cause mortality endpoint. Type I error was predefined at 0.05. Statistical analysis was performed with STATA, version 12 (StataCorp, College Station, TX). Statistical significance was set at α = 0.05. For multiple comparisons in parameters of interest (i.e. mean values of glucose across the 7 days of hospitalisation), a Bonferroni correction was applied and the level of the statistical significance was set at p/n = 0.0063.
Results
Of the 1357 patients with AIS who were admitted during the study period, 86 (6.3%) were excluded: 32 had a previous mRS score ≥2, 25 had missing creatinine values, 10 patients were lost to three month follow-up and 19 patients had missing values for the main study parameters (BP, glucose, temperature). The mean age of the 1271 patients (722 women, 56.8%) who were included in the analysis was 72.3 ± 11.2 years. Across a three-month period, 241 deaths (18.96%) were recorded. The baseline patient characteristics are summarised in Tables 1 and 2 stratified by mortality status at three months.
Table 1.
Baseline patient characteristics stratified by mortality status up to three months.
| Characteristic | Alive(n = 1030) | Dead(n = 241) | p value |
|---|---|---|---|
| Women | 601 (58.4) | 121 (50.2) | 0.022 |
| Age | 71.1 (11.0) | 77.5 (10.4) | 0.001 |
| History of | |||
| Hypertension | 750 (72.8) | 158 (65.6) | 0.025 |
| Diabetes | 319 (31.0) | 68 (28.2) | 0.403 |
| Dyslipidaemia | 351 (34.1) | 47 (19.5) | 0.001 |
| Smoking | 341 (33.1) | 56 (23.2) | 0.003 |
| Transient ischaemic attack | 130 (12.6) | 20 (8.30) | 0.061 |
| Coronary artery disease | 226 (21.9) | 58 (24.1) | 0.476 |
| Atrial fibrillation | 415 (40.3) | 134 (55.6) | 0.001 |
| Admission values | |||
| NIHSSb | 6 (13) | 22 (10) | 0.001 |
| eGFRb | 75 (40) | 60 (46) | 0.001 |
| Stroke classification (TOAST) | |||
| Lacunar | 189 (18.3) | 2 (0.8) | 0.001 |
| Large vessel atherosclerosis | 199 (19.3) | 27 (11.2) | 0.001 |
| Cardioembolism | 429 (41.7) | 148 (61.4) | 0.001 |
| Undetermined aetiology | 196 (19.0) | 63 (26.1) | 0.016 |
| Other aetiology | 17 (1.7) | 1 (0.4) | 0.117 |
| In-hospital treatment | |||
| Thrombolysis | 27 (2.6) | 5 (2.1) | 0.626 |
| Aspirin | 837 (81.3) | 182 (75.5) | 0.044 |
| LMWH | 711 (69.0) | 136 (56.4) | 0.001 |
NIHSS: National Institute of Health Stroke Scale; eGFR: estimated glomerular filtration rate (mL/min/1.73 m2); LMWH: low molecular weight heparin (for deep venous thrombosis prophylaxis).
aNumbers in parentheses for nominal data indicate percentages. Continuous variables are expressed as mean ± standard deviation except than non-normally distributed variables.
bDescribed as median (inter-quartile range).
Table 2.
Time course for values of physiological parameters during the acute and sub-acute phase of ischaemic stroke.a
| Death at 3 months |
||||||||
|---|---|---|---|---|---|---|---|---|
| Systolic blood pressure (mm Hg) |
Diastolic blood pressure (mm Hg) |
Glucose (mg/dL) |
Temperature (°C) |
|||||
| Time point | Yes | No | Yes | No | Yes | No | Yes | No |
| Day 1 | 149 (27.0) | 147 (23.4) | 83.2 (10.7) | 83.8 (11.7) | 158* (68.7) | 142 (60.9) | 37.0* (0.9) | 36.7 (0.6) |
| Day 2 | 147 (26.3) | 146 (22.9) | 82.2 (11.6) | 82.1 (11.8) | 147* (69.0) | 124 (52.3) | 37.5* (1.3) | 36.6 (0.8) |
| Day 3 | 147 (25.1) | 144 (23.0) | 81.7 (12.0) | 81.7 (10.7) | 156* (80.7) | 130 (55.8) | 37.6* (1.5) | 36.7 (0.6) |
| Day 4 | 145 (26.7) | 145 (22.3) | 80.3 (13.3) | 81.6 (10.9) | 150* (71.0) | 129 (56.0) | 37.6* (1.4) | 36.7 (0.5) |
| Day 5 | 145 (23.3) | 144 (22.0) | 79.8 (12.1) | 81.0 (11.0) | 153 (69.5) | 135 (59.1) | 37.5* (1.5) | 36.6 (0.4) |
| Day 6 | 143 (25.0) | 142 (21.9) | 79.3 (11.4) | 80.8 (10.4) | 148 (69.3) | 136 (57.4) | 37.4* (1.4) | 36.6 (0.4) |
| Day 7 | 141 (23.0) | 141 (21.0) | 78.8 (11.6) | 80.5 (10.5) | 158 (69.0) | 138 (58.4) | 37.3* (1.4) | 36.6 (0.3) |
aData are means and SD for normally distributed variables (systolic/diastolic blood pressure, glucose) and median (interquartile range) for temperature. Glucose values can be converted from mg/dL to mmol/L by dividing by 18.
*Denotes significant differences in parameters on interest between subjects that died up to three months and survivors, after Bonferroni correction for multiple measurements (level of statistical significance = 0.0063).
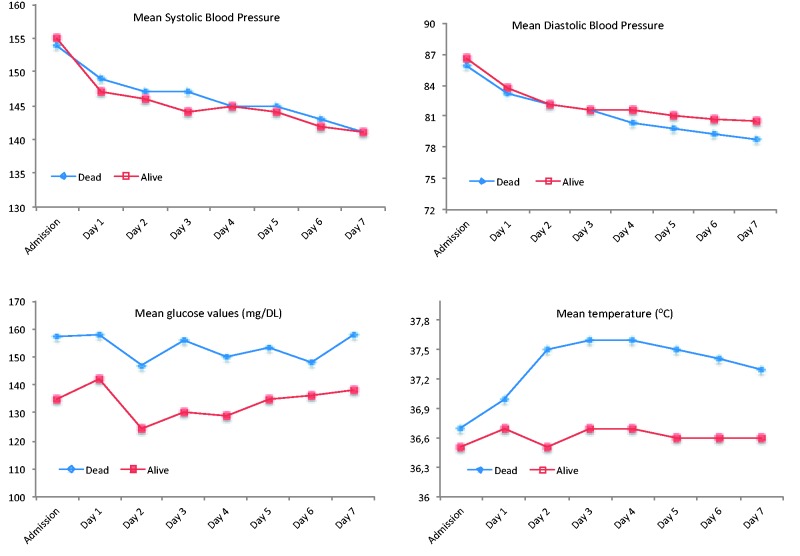
Compared to patients who were alive at three months, patients who died within three months were more often men (p = 0.022), older (p = 0.001) and had higher admission NIHSS (p = 0.001) and increased prevalence of traditional risk factors (Table 1). In addition, non-survivors presented with increased mean glucose levels up to the fourth day of hospitalisation (p < 0.006 for all comparisons) and increased mean temperature across the entire hospital stay (p < 0.006 for all comparisons) (Table 2 and Figure 1). On the contrary, there were no significant differences between the two groups in mean SBP or DBP throughout the observational period (Table 2).
Figure 1.
Course of physiological parameters during the first week after acute ischaemic stroke according to patient mortality status at three months.
Death within three months from acute stroke
In Cox regression multivariable analysis, mean glucose (HR: 1.010; 95% CI: 1.007–1.013; p < 0.001) and mean temperature (HR: 1.045; 95% CI: 1.001–1.091; p = 0.045) over the first seven days of hospitalisation were predictors of three-month all-cause mortality after adjustment for age, sex, NIHSS, eGFR, stroke risk factors (history of hypertension, diabetes, smoking, dyslipidaemia, CAD, TIAs, atrial fibrillation), stroke subtype and in-hospital treatment (aspirin, anticoagulation, thrombolysis) (Table 3, Model 1). The mean SBP and DBP indices during the acute phase of stroke were not associated with three-month all-cause mortality (p > 0.05). Furthermore, in multivariable Cox regression model 2, we tested the association of the magnitude of changes (SD) in the parameters of interest (glucose, SBP, DBP and temperature) with all-cause mortality at three months. As shown in Table 3, only the SD of SBP across the first seven days of hospitalisation independently predicted three-month all-cause mortality after adjustment for all baseline confounders (HR: 1.042; 95% CI: 1.020–1.063; p < 0.001). Finally, in model 3 we assessed simultaneously the impact of the subject-specific baseline value and the subject-specific rate of change of SBP, glucose and temperature across the hospitalisation. Interestingly, increased baseline values of glucose and temperature (p < 0.001 for both) as well as increased rates of change in time for both parameters (HR: 1.043; 95% CI: 1.008–1.080; p = 0.017 for glucose and HR: 1.914; 95% CI: 1.712–2.141, p < 0.001 for temperature, respectively) were significantly associated with all-cause mortality independently of all baseline covariates (Table 3, Model 3).
Table 3.
Multivariable Cox proportional-hazard models (upper panel) and logistic regression analysis (lower panel) for the association of variables of interest and short-term (up to 3 months) death and poor functional outcome.
| Model 1 |
Model 2 |
Model 3 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mean of values) |
(SD of values) |
(Subject-specific baseline values) |
(Subject specific rate of changes) |
|||||||||
| Variable | HR/OR | 95% CI | p value | HR/OR | 95% CI | p value | HR/OR | 95% CI | p value | HR/OR | 95% CI | p value |
| Death up to 3 months (n = 241) | ||||||||||||
| Glucose (mg/dL) (N = 1269) | 1.010 | 1.007–1.013 | <0.001 | 1.005 | 0.999–1.011 | 0.100 | 1.011 | 1.008–1.015 | <0.001 | 1.043 | 1.008–1.080 | 0.017 |
| SBP (mm Hg) (N = 1214) | 1.009 | 0.998–1.021 | 0.111 | 1.042 | 1.020–1.063 | <0.001 | 1.004 | 0.994–1.015 | 0.380 | 0.944 | 0.872–1.023 | 0.158 |
| DBP (mm Hg) (N = 1212) | 0.987 | 0.964–1.011 | 0.289 | 1.027 | 0.990–1.065 | 0.151 | 0.993 | 0.972–1.015 | 0.548 | 0.842 | 0.695–1.021 | 0.081 |
| Temperature (°C) (N = 1246) | 1.045 | 1.001–1.091 | 0.045 | 0.987 | 0.938–1.038 | 0.611 | 3.387 | 2.597–4.418 | <0.001 | 1.914* | 1.712–2.141 | <0.001 |
| Poor functional outcome at 3 months (n = 668) | ||||||||||||
| Glucose (mg/dL) (N = 1269) | 1.003 | 0.998–1.008 | 0.219 | 1.006 | 0.997–1.016 | 0.196 | 1.004 | 0.998–1.010 | 0.190 | 1.046 | 0.982–1.115 | 0.161 |
| SBP (mm Hg) (N = 1214) | 1.003 | 0.992–1.014 | 0.573 | 1.013 | 0.987–1.040 | 0.322 | 1.007 | 0.995–1.020 | 0.259 | 1.156 | 1.047–1.277 | 0.004 |
| DBP (mm Hg) (N = 1212) | 1.004 | 0.981–1.027 | 0.750 | 0.978 | 0.935–1.024 | 0.345 | 1.011 | 0.984–1.040 | 0.424 | 1.515 | 1.168–1.965 | 0.002 |
| Temperature (oC) (N = 1246) | 1.728 | 1.188–2.51 | 0.004 | 1.349 | 0.578–3.15 | 0.489 | 1.775 | 1.137–2.772 | 0.012 | 1.336* | 1.036–1.722 | 0.026 |
SD: standard deviation.
Adjusted for baseline parameters: age, gender, NIHSS, eGFR, stroke subtype, history of hypertension, diabetes, smoking, dyslipidaemia, coronary artery diseases, TIAs, atrial fibrillation and in-hospital treatment (aspirin, anticoagulation, thrombolysis). Poor functional outcome, mRS > 2..
*Per 0.1°C change.
In a further analysis, all significant predictors derived from the previous analyses (baseline glucose and changes in glucose levels, baseline temperature and changes in temperature and SD of SBP) were entered into the fully adjusted Cox model (Table 4). Based on this analysis, baseline glucose levels, SD of SBP and the baseline temperature and slope of temperature change predicted all-cause mortality up to three-months independently of each other and of all baseline covariates. After adjustment for all the other parameters of interest and all potential confounders, a 1-SD increase in glucose levels increased the risk of all-cause mortality within three months by 35.3%; a 1-SD increase in SBP variability increased the all-cause mortality by 21.2%; a 1-SD increase in subject-specific temperature values doubled the odds (HR = 2.02) for all-cause mortality within three months; an increase of 0.2°C in the rate of temperature change per day (e.g. beginning with a mean temperature of 36.6°C at day 1 and presenting a mean temperature of 38°C at day 7) increased more than 3-fold (HR = 3.39) the all-cause mortality.
Table 4.
Multivariable Cox proportional-hazards models (upper panel) and logistic regression analysis (lower panel) for the association of significant variables of interest and short-term (up to 3 months) death and poor functional outcome.
| Variable | HR | 95% CI | p value |
|---|---|---|---|
| Death up to 3 months (n = 241) | |||
| Subject-specific baseline values of glucose | 1.005 | 1.001–1.01 | 0.017 |
| Subject-specific rate in changes of glucose values | 1.01 | 0.969–1.052 | 0.651 |
| SD of systolic BP | 1.028 | 1.01–1.048 | 0.005 |
| Subject-specific baseline values of temperature | 2.758 | 2.067–3.68 | <0.001 |
| Subject-specific rate in changes of temperature | 1.841* | 1.616–2.098 | <0.001 |
| Poor functional outcome at 3 months (n = 668) | |||
| Subject-specific baseline values of SBP | 1.007 | 0.994–1.019 | 0.303 |
| Subject-specific rate in changes of SBP values | 1.159 | 1.047–1.280 | 0.004 |
| Subject-specific baseline values of temperature | 1.743 | 1.076–2.825 | 0.024 |
| Subject-specific rate in changes of temperature | 1.402* | 1.061–1.853 | 0.018 |
SD: standard deviation.
Adjusted for baseline parameters: age, gender, NIHSS, eGFR, stroke subtype, history of hypertension, diabetes, smoking, dyslipidaemia, coronary artery diseases, TIAs, atrial fibrillation and in-hospital treatment (aspirin, anticoagulation, thrombolysis). Poor functional outcome, mRS > 2.
*Per 0.1°C change.
Taking into account the fact that indices of all three variables were associated with all-cause mortality within three months, we calculated measures of discrimination and reclassification in order to identify the parameter with incremental value over established risk factors for death after stroke. All three parameters correctly reclassified patients into risk categories for death at three months after stroke (mean glucose: NRI = 27.7%, p < 0.001; baseline intercept and rate of glucose changes: NRI = 14.6%, p = 0.041, SD of SBP: NRI = 21.6%, p = 0.044; baseline intercept and rate of temperature change: NRI = 57.8%, p < 0.001) (Table 5). The IDI was also significant for all parameters (Table 5). In contrast, only glucose and temperature parameters (mean values and random intercept and rate of changes) level provided incremental value for correctly discriminating the patients who died within three months (Harrell's C, p < 0.05 for all) over the best predictive model of all established risk factors (Table 5).
Table 5.
Improvement in model discrimination and reclassification of multiple measurements of modifiable physiological factors over the best predictive model for short-term death (up to 3 months).a
| Discrimination |
Reclassification |
||||||
|---|---|---|---|---|---|---|---|
| Continuous NRI |
|||||||
| Model | Harrell’s C | p value | IDI coefficient(SE) | Among event subjects | Amongnon-event subjects | Overall (SE) | p value |
| Model 1 | 0.84 (0.82–0.87) | ||||||
| Model 1 + glucose (mean) | 0.85 (0.83–0.87) | 0.023 | 2.2 (0.59)** | 3.74 | 24.00 | 27.7 (7.2) | <0.001 |
| Model 1 + glucose (subject-specific baseline values and rate in changes) | 0.85 (0.83–0.87) | 0.024 | 0.1 (0.03)* | −8.72 | 23.34 | 14.6 (7.2) | 0.041 |
| Model 1 + SBP (SD) | 0.85 (0.82–0.87) | 0.138 | 1.38 (0.51)* | −5.66 | 27.28 | 21.6 (7.6) | 0.044 |
| Model 1 + temperature (mean) | 0.87 (0.84–0.89) | <0.001 | 0.48 (0.2)* | 0.6 | 40.8 | 41.4 (5.7) | <0.001 |
| Model 1 + temperature (subject-specific baseline values and rate in changes)b | 0.87 (0.85–0.89) | <0.001 | 0.79 (0.1)** | 19.84 | 37.96 | 57.8 (7.2) | <0.001 |
IDI: integrated discrimination index; NRI: Net Reclassification Index; SBP: systolic blood pressure; SE: standard error.
aModel 1: Age, gender, NIHSS, eGFR, stroke subtype, history of hypertension, diabetes, smoking, dyslipidaemia, coronary artery diseases, TIAs, atrial fibrillation and in-hospital treatment (aspirin, anticoagulation, thrombolysis).
bPer 0.1°C change.
*Indicates level of statistical significance <0.05.
**Indicates level of statistical significance <0.001.
Poor functional outcome at three months after acute stroke
At three months after the AIS, all study's patients had an evaluation of functional status. Six hundred sixty-eight subjects (52.56%) presented a poor three-month functional outcome.
In logistic regression multivariable analysis for the secondary outcome, mean values (OR: 1.728; 95% CI: 1.188–2.51; p = 0.004), baseline values (OR: 1.775; 95% CI: 1.137–2.772; p = 0.012) and the slope of temperature changes (OR: 1.336; 95% CI: 1.036–1.722; p = 0.026) over the first seven days of hospitalisation were predictors of poor functional outcome at three months after adjustment for age, sex, NIHSS, eGFR, stroke risk factors (history of hypertension, diabetes, smoking, dyslipidaemia, CAD, TIAs, atrial fibrillation), stroke subtype and in-hospital treatment (aspirin anticoagulation, thrombolysis) (Table 3, Model 1 and 3). Respectively, the slope of alterations in SBP and DBP during the acute phase of stroke were independent determinants for unfavourable functional outcome at three months (p < 0.005 for both) (Table 3, Model 3). Conversely, mean values and variability indices of glucose across the first seven days of hospitalisation were not associated with the secondary outcome (Table 3). In the fully adjusted logistic regression model incorporating all significant predictors (SBP and DBP indices were not assessed simultaneously due to severe collinearity), baseline values of temperature (OR: 1.743; 95% CI: 1.076–2.825; p = 0.024) at admission as well as the rate of SBP (OR: 1.159; 95% CI: 1.047–1.280; p = 0.004) and temperature change (OR: 1.402; 95% CI: 1.061–1.853; p = 0.018) per day were independently associated with impaired functional status at three months (Table 4).
Discussion
The present study investigated three important physiological factors during the acute and sub-acute phase of AIS. Subject-specific baseline glucose and temperature levels, variability of SBP and the rate of temperature changes are independent predictors of three-month all-cause mortality. Baseline subject specific values of temperature as well as the rate of SBP and temperature changes are independent predictors of poor functional outcome.
The novelty of our study was that we simultaneously examined different parameters of three important modifiable physiological factors in a large series of AIS patients treated in an ASU. Firstly, we performed serial measurements throughout the hyperacute and sub-acute phase of stroke. AIS is a dynamic fluctuating condition continuously changing during the first few days, where processes such as death of the ischaemic penumbra, brain oedema and haemorrhagic transformation may develop and affect outcome.28–30 During this frequently unstable period, even though abnormalities in the aforementioned parameters may normalise by themselves within hours, in many patients these persist or even worsen.2,14 In this context, serial measurements are more representative than single values. Secondly, in order to capture more accurately the fluctuations of the aforementioned parameters, we included in our multivariate models four different characteristics for each physiological factor: mean daily values, variability, subject-specific baseline values and the rate of changes during the first seven days. Most studies in the literature have studied single admission values, mean levels or variability of one factor using dichotomisation and arbitrary cut-offs for the statistical analysis. Thirdly, we studied a large series of stroke patients and carried out an extensive statistical analysis to minimise the risk of a type II error. The use of continuous classification analyses27 enhanced our findings and suggests that the three calculated parameters are not simply associated but are in fact strong prognostic biomarkers in AIS.
In most research studies, a rise in blood sugar level in the setting of AIS has been associated with greater infarct size, symptomatic intracerebral haemorrhage and worse clinical outcome,2–4,28,30,31 especially for patients without pre-existing diabetes.4 A J-shaped association between serum glucose and functional outcome has been described in a recent study.13 Interestingly, the development of hyperglycaemia as shown by serial glucose evaluations has been suggested to be of higher prognostic value compared to single glucose measurement2,3,21,28 and our findings are consistent with this. However, the clinical benefit of intervention to reduce plasma glucose in acute stroke patients remains unproven.16,32,33 In our study, baseline glucose values predicted three-month mortality but not functional outcome. The effect of hyperglycaemia on mortality could be explained by considering hyperglycaemia as a surrogate index for critical illness, nosocomial infections and severe stroke. Ntaios et al. have been previously shown that persistent hyperglycaemia at 24–48 h in AIS patients is not associated with a worse functional outcome at three months. We extended the period of measurements up to seven days and no relation was found to the three months functional outcome. Furthermore, it is still unclear whether hyperglycaemia in AIS is an epiphenomenon of the severity of the underlying stroke or if it is in itself directly harmful to the ischaemic brain.
Regarding BP, our study revealed that BP variability and rate of changes in AIS are independent predictors of all-cause mortality and functional outcome respectively, whereas mean BP values are not. Our results are in agreement with previous studies demonstrating that measures of BP variability may be better indicators of outcome than absolute BP values.20,34–36 During the acute and sub-acute post-stroke phases, BP fluctuations seem to have an important influence on cerebral perfusion because of impaired cerebral autoregulation. This hypothesis may help to explain the ongoing uncertainty surrounding BP management in acute stroke and the lack of benefit from interventions, which actively alter BP in AIS.15,37 Our findings add to a number of previous reports showing variable and sometimes contradictory results with both higher and lower blood pressure levels being associated with stroke mortality and poor functional outcome (U-shaped or J-shaped relationship).5,7,12,38–40 Sample size, different cut-off thresholds, dichotomisation of BP in statistical analysis, and endpoint selection using death and dependency as one variable may have influenced these results. Furthermore, important confounders including glucose and fever have been disregarded in previous studies, and most studies are based on sole assessment of hyperacute BP levels.
Most studies on the association of temperature with post-stroke mortality have focused on hyperthermia on admission8 or maximum temperature within the first 72 h.29,41 However some studies conducted up to seven days post-stroke demonstrated that hyperthermia beyond the hyperacute phase could be a stronger predictor of poor outcomes.9 In consistence with previous studies, our results point out temperature as a strong predictor of outcome in AIS. Baseline temperature values and temperature rise – expressed as the rate of changes across the seven days following stroke – predicted three-month all-cause mortality and functional outcome. The effect of temperature on mortality could be explained by considering fever as a surrogate index for nosocomial infections, which could expectedly increase mortality, whereas the association between temperature and poor functional outcome may imply a strong association with the survival of penumbra in the acute and sub-acute phase of ischaemic stroke. Our results add to our understanding of the effect of temperature dynamics over time in relation to outcome. This effect is still not completely understood, especially in view of the fact that variable results have so far been obtained from studies assessing stroke outcome with admission temperature,8 early or delayed hyperthermia,29,41 peak fever,9 fever duration,9 fever events9 and temperature dynamics.41 A wide range of definitions has been used for hyperthermia and different statistical methods have been applied in these analyses, examining body temperature either as a dichotomised (e.g. febrile versus afebrile), or as a continuous variable.42 These variations make the interpretation of these results difficult.
The main strengths of this analysis are the large size of the study population of consecutive patients and the assessment of serial values of several physiological parameters beyond the hyperacute phase; in addition, important confounders were adjusted for: basic clinical data, major risk factors and in-hospital treatment, allowing for a more holistic investigation of the complex and multifactorial nature of acute stroke pathophysiology. Even though the best method for analysing serial data is still unclear, we used three different variables for each parameter of interest (mean, SD, subject-specific baseline values and rate of changes) in order to assess more accurately their course during the first seven days of hospitalisation; moreover, we avoided stratification, dichotomisation and arbitrary cut-off thresholds for measurement of physiological parameters that may result in loss of information and considerable bias.
On the other hand, we need to keep in mind that this was a single-centre study, which may have introduced selection bias. Furthermore, glucose, BP and temperature-lowering therapy were not standardised and it was not possible to control for antipyretic, antihypertensive and anti-diabetic drug use in the multivariate analysis. In addition, these results show only associations between these parameters, without trying to distinguish cause from effect of changes in measured parameters. Moreover, data on the aetiology of fever events were not analysed and it is possible that fever was a marker of significant infection, which could be a confounder of our results. Also, other physiological parameters like blood oxygen levels were not sequentially recorded in the registry and therefore, no data were available to be included in the analysis. Finally, our results cannot guide recommendations for treatment decision in clinical practice, as they do not offer a threshold level for initiating active treatment.
In summary, all main modifiable physiological variables (glucose, BP, temperature) throughout the acute and sub-acute stroke phase seem to be independent predictors of three-month all-cause mortality, whereas temperature and BP were independent predictors of poor functional outcome. Nevertheless, different parameters of each variable seem to be associated with prognosis, a finding that underscores the multifactorial and complex nature of acute stroke physiology. Our study adds to the understanding of the complex physiology in AIS in that the variability and slope of change of physiological parameters may have an effect in outcome and may inform the design of future intervention trials18 targeting multiple physiological parameters throughout the acute and sub-acute post-stroke period.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
The ethics committee of the Alexandra Hospital approved this study.
Informed consent
Written informed consent was obtained from the patient(s).
Guarantor
KV.
Contributorship
Dr Skafida: acquisition of data, study concept, preparation of manuscript. Dr Mitrakou: critical revision of manuscript, study supervision. Dr Alevizaki: critical revision of manuscript. Dr Spengos: critical revision of manuscript. Dr Georgiopoulos: statistical analysis and interpretation. Dr Takis: critical revision and interpretation. Dr. Ntaios: critical revision of manuscript. Dr Vemmos: acquisition of data, study concept, statistical analysis and interpretation, preparation of manuscript, study supervision.
References
- 1.Stroke Unit Trialists C. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2002; CD000197. [DOI] [PubMed] [Google Scholar]
- 2.Yong M andKaste M.. Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS-ii trial. Stroke 2008; 39: 2749–2755. [DOI] [PubMed] [Google Scholar]
- 3.Fuentes B, Castillo J, San Jose B, et al. The prognostic value of capillary glucose levels in acute stroke: the glycemia in acute stroke (glias) study. Stroke 2009; 40: 562–568. [DOI] [PubMed] [Google Scholar]
- 4.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001; 32: 2426–2432. [DOI] [PubMed] [Google Scholar]
- 5.Carlberg B Asplund K andHagg E.. The prognostic value of admission blood pressure in patients with acute stroke. Stroke 1993; 24: 1372–1375. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen HS, Nakayama H, Raaschou HO, et al. Effect of blood pressure and diabetes on stroke in progression. Lancet 1994; 344: 156–159. [DOI] [PubMed] [Google Scholar]
- 7.Willmot M Leonardi-Bee J andBath PM.. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension 2004; 43: 18–24. [DOI] [PubMed] [Google Scholar]
- 8.Reith J, Jorgensen HS, Pedersen PM, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet 1996; 347: 422–425. [DOI] [PubMed] [Google Scholar]
- 9.Phipps MS, Desai RA, Wira C, et al. Epidemiology and outcomes of fever burden among patients with acute ischemic stroke. Stroke 2011; 42: 3357–3362. [DOI] [PubMed] [Google Scholar]
- 10.Hajat C Hajat S andSharma P.. Effects of poststroke pyrexia on stroke outcome: a meta-analysis of studies in patients. Stroke 2000; 31: 410–414. [DOI] [PubMed] [Google Scholar]
- 11.Fuentes B, Ntaios G, Putaala J, et al. European Stroke Organisation (ESO) guidelines on glycaemia management in acute stroke. Eur Stroke J. 2018; 3: 5–21. [DOI] [PMC free article] [PubMed]
- 12.Leonardi-Bee J, Bath PM, Phillips SJ, et al. Blood pressure and clinical outcomes in the international stroke trial. Stroke 2002; 33: 1315–1320. [DOI] [PubMed] [Google Scholar]
- 13.Ntaios G, Egli M, Faouzi M, et al. J-shaped association between serum glucose and functional outcome in acute ischemic stroke. Stroke 2010; 41: 2366–2370. [DOI] [PubMed] [Google Scholar]
- 14.Allport L, Baird T, Butcher K, et al. Frequency and temporal profile of poststroke hyperglycemia using continuous glucose monitoring. Diabetes Care 2006; 29: 1839–1844. [DOI] [PubMed] [Google Scholar]
- 15.Lee M, Ovbiagele B, Hong KS, et al. Effect of blood pressure lowering in early ischemic stroke: meta-analysis. Stroke 2015; 46: 1883–1889. [DOI] [PubMed] [Google Scholar]
- 16.Bellolio MF Gilmore RM andGanti L.. Insulin for glycaemic control in acute ischaemic stroke. Cochrane Database Syst Rev 2014; CD005346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.den Hertog HM, van der Worp HB, van Gemert HM, et al. The paracetamol (acetaminophen) in stroke (PAIS) trial: a multicentre, randomised, placebo-controlled, phase iii trial. Lancet Neurol 2009; 8: 434–440. [DOI] [PubMed] [Google Scholar]
- 18.Middleton S, McElduff P, Ward J, et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): a cluster randomised controlled trial. The Lancet 2011; 378: 1699–1706. [DOI] [PubMed] [Google Scholar]
- 19.Ntaios G, Papavasileiou V, Bargiota A, et al. Intravenous insulin treatment in acute stroke: a systematic review and meta-analysis of randomized controlled trials. Int J Stroke 2014; 9: 489–493. [DOI] [PubMed] [Google Scholar]
- 20.Manning LS, Rothwell PM, Potter JF, et al. Prognostic significance of short-term blood pressure variability in acute stroke: systematic review. Stroke 2015; 46: 2482–2490. [DOI] [PubMed] [Google Scholar]
- 21.Yoo DS, Chang J, Kim JT, et al. Various blood glucose parameters that indicate hyperglycemia after intravenous thrombolysis in acute ischemic stroke could predict worse outcome. PloS One 2014; 9: e94364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vemmos KN, Takis CE, Georgilis K, et al. The athens stroke registry: results of a five-year hospital-based study. Cerebrovasc Dis 2000; 10: 133–141. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254. [DOI] [PubMed] [Google Scholar]
- 24.Adams HP, Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 25.Belsley DA Kuh E andWelsch RE.. Regression diagnostics: Identifying influential data and sources of collinearity. New York, NY: Wiley, 1980. [Google Scholar]
- 26.Harrell FE Jr. Lee KL andMark DB.. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 27.Pencina MJ D'Agostino RB Sr. andSteyerberg EW.. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011; 30: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baird TA, Parsons MW, Phan T, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke 2003; 34: 2208–2214. [DOI] [PubMed] [Google Scholar]
- 29.Castillo J, Davalos A, Marrugat J, et al. Timing for fever-related brain damage in acute ischemic stroke. Stroke 1998; 29: 2455–2460. [DOI] [PubMed] [Google Scholar]
- 30.Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke 2008; 39: 2249–2256. [DOI] [PubMed] [Google Scholar]
- 31.Ntaios G, Abatzi C, Alexandrou M, et al. Persistent hyperglycemia at 24–48 h in acute hyperglycemic stroke patients is not associated with a worse functional outcome. Cerebrovasc Dis 2011; 32: 561–566. [DOI] [PubMed] [Google Scholar]
- 32.Gray CS, Hildreth AJ, Sandercock PA, et al. Glucose–potassium–insulin infusions in the management of post-stroke hyperglycaemia: the UK glucose insulin in stroke trial (GIST-UK). Lancet Neurol 2007; 6: 397–406. [DOI] [PubMed] [Google Scholar]
- 33.Ntaios G, Egli M, Arsovska A, et al. An intravenous insulin protocol for strict glycemic control in acute ischaemic stroke. Eur J Neurol 2012; 19: 443–451. [DOI] [PubMed] [Google Scholar]
- 34.Chung JW, Kim N, Kang J, et al. Blood pressure variability and the development of early neurological deterioration following acute ischemic stroke. J Hypertens 2015; 33: 2099–2106. [DOI] [PubMed] [Google Scholar]
- 35.Stead LG, Gilmore RM, Vedula KC, et al. Impact of acute blood pressure variability on ischemic stroke outcome. Neurology 2006; 66: 1878–1881. [DOI] [PubMed] [Google Scholar]
- 36.Kakaletsis N, Ntaios G, Milionis H, et al. Prognostic value of 24-h abpm in acute ischemic stroke for short-, medium-, and long-term outcome: a systematic review and meta-analysis. Int J Stroke 2015; 10: 1000–1007. [DOI] [PubMed] [Google Scholar]
- 37.Ntaios G Bath P andMichel P.. Blood pressure treatment in acute ischemic stroke: a review of studies and recommendations. Curr Opin Neurol 2010; 23: 46–52. [DOI] [PubMed] [Google Scholar]
- 38.Ntaios G Lambrou D andMichel P.. Blood pressure change and outcome in acute ischemic stroke: the impact of baseline values, previous hypertensive disease and previous antihypertensive treatment. J Hypertens 2011; 29: 1583–1589. [DOI] [PubMed] [Google Scholar]
- 39.Ntaios G Lambrou D andMichel P.. Blood pressure changes in acute ischemic stroke and outcome with respect to stroke etiology. Neurology 2012; 79: 1440–1448. [DOI] [PubMed] [Google Scholar]
- 40.Tsivgoulis G andNtaios G.. Blood pressure variability in subacute ischemic stroke: a neglected potential therapeutic target. Neurology 2012; 79: 2014–2015. [DOI] [PubMed] [Google Scholar]
- 41.den Hertog HM, van der Worp HB, van Gemert HM, et al. An early rise in body temperature is related to unfavorable outcome after stroke: data from the PAIS study. J Neurol 2011; 258: 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ntaios G, Dziedzic T, Michel P, et al. European Stroke Organisation (ESO) guidelines for the management of temperature in patients with acute ischemic stroke. Int J Stroke 2015; 10: 941–949. [DOI] [PubMed] [Google Scholar]