Short abstract
Introduction
In ischaemic stroke care, fast reperfusion is essential for disability free survival. It is unknown if bypassing thrombolysis centres in favour of endovascular thrombectomy (mothership) outweighs transport to the nearest thrombolysis centre for alteplase and then transfer for endovascular thrombectomy (drip-and-ship). We use conditional probability modelling to determine the impact of treatment times on transport decision-making for acute ischaemic stroke.
Materials and methods
Probability of good outcome was modelled using a previously published framework, data from the Irish National Stroke Register, and an endovascular thrombectomy registry at a tertiary referral centre in Ireland. Ireland was divided into 139 regions, transport times between each region and hospital were estimated using Google’s Distance Matrix Application Program Interface. Results were mapped using ArcGIS 10.3.
Results
Using current treatment times, drip-and-ship rarely predicts best outcomes. However, if door to needle times are reduced to 30 min, drip-and-ship becomes more favourable; even more so if turnaround time (time from thrombolysis to departure for the endovascular thrombectomy centre) is also reduced. Reducing door to groin puncture times predicts better outcomes with the mothership model.
Discussion
This is the first case study modelling pre-hospital transport for ischaemic stroke utilising real treatment times in a defined geographic area. A moderate improvement in treatment times results in significant predicted changes to the optimisation of a national acute stroke patient transport strategy.
Conclusions
Modelling patient transport for system-level planning is sensitive to treatment times at both thrombolysis and thrombectomy centres and has important implications for the future planning of thrombectomy services.
Keywords: Ischaemic stroke, endovascular therapy, thrombolysis, health services research
Introduction
In ischaemic stroke care, fast reperfusion is essential to improve disability free survival.1–5 Endovascular thrombectomy (EVT) is an extremely effective therapy with early reperfusion rates of ∼75% and a number needed to treat of only seven to prevent one poor outcome (Modified Rankin Scale (mRS) 2–6 at 90 days).6 However, this therapy is only applicable to ischaemic stroke patients with large vessel occlusions (LVOs). Medical thrombolysis with alteplase is more widely available but is less effective in patients with LVOs; only achieving early reperfusion rates of 8–35%.7–9
For both treatments, time is critical. The probability of achieving good outcome with EVT decreases with time. For every nine-min delay in onset to reperfusion, one out of every 100 patients will have greater functional disability at 90 days.10 The equipment and expertise needed restrict this procedure to large tertiary hospitals, typically situated in urban areas. For patients outside the catchment area of EVT centres, it is unknown if delayed, but more effective, reperfusion with EVT outweighs the benefits of early thrombolysis. In the pre-hospital scenario, this manifests as an option between bypassing a thrombolysis centre in favour of EVT at an EVT centre (mothership) and direct transport to the thrombolysis centre for thrombolysis followed by transfer for EVT (drip-and-ship).
The natural extension of more widely available thrombolysis usage and the introduction of EVT has led to a re-evaluation of the drip-and-ship model.11–13 Adding complexity, only a small proportion of suspected stroke patients have LVOs and are eligible for EVT. Thus, adopting a mothership method for all stroke patients may result in unnecessary and long transports for patients that will never undergo EVT. However, for the patients with LVO, only 8–35% achieve recanalisation with thrombolysis meaning that transport to a thrombolysis centre may be futile.7,14 A retrospective analysis of drip-and-ship versus mothership in Korea found no difference in recanalisation or 90-day outcome between the two transportation strategies. However, this was performed prior to publication of positive trials of EVT, the number of patients was small (N = 105, of whom 88 received EVT), and the difference in onset to arterial access between the groups was relatively short (219 vs 300 min) suggesting that the thrombolysis and EVT centres were likely close in proximity.15 Bypassing thrombolysis centres (mothership) for patients with suspected LVO led to improved outcomes in a recent Danish study.16
Recently, using conditional probability modelling techniques, the drip-and-ship and mothership transport options (Figure 1) have been explored to help plan future EVT services.17,18 In this analysis, we apply one of these frameworks17 in conjunction with both real and optimised transport and treatment times to the Irish Healthcare system to explore patient transport in a geographically defined area and assess how changes in treatment times impact transport decisions.
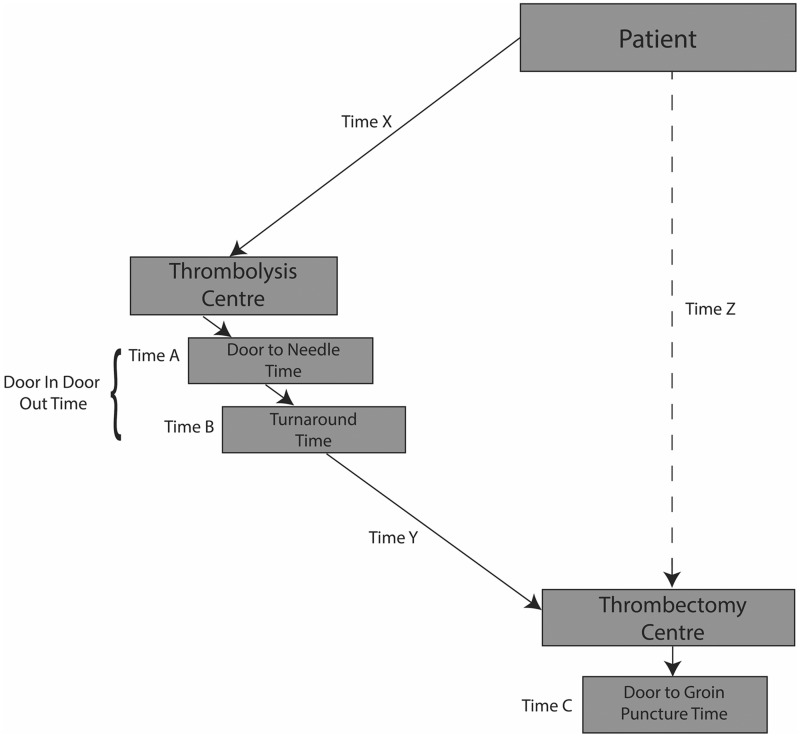
Figure 1.
Drip-and-ship and mothership transport components. The dashed line represents the mothership model; the solid lines represent the drip-and-ship model. Times X, Y and Z are transportation times. Times A, B and C are treatment times. Adapted from Holodinsky et al. Drip-and-ship versus Direct to Comprehensive Stroke Centre: Conditional Probability Modelling.17
Methods
Setting
In Ireland (population 4.5 M, ∼70,000 km2), there are approximately 4500 ischaemic strokes per annum. There are 25 hospitals that provide 24/7 thrombolysis services; one of which (Beaumont Hospital, herein referred to as EVT Centre 1) is a tertiary neurosurgical centre in Dublin equipped to provide EVT, a second EVT centre is currently in development (Cork University Hospital, herein referred to as EVT Centre 2).
Patients
Data from 699 ischaemic stroke patients (from 22 hospitals) treated with thrombolysis between 1 January 2014 and 31 December 2015 were obtained from the Irish National Stroke Register. The National Stroke Register contains clinical data including stroke onset time, hospital arrival time and time of alteplase administration. Among the 8969 ischaemic strokes in 2014 and 2015, 6026 were captured in the register (67%). Only hospitals submitting complete data for >80% of stroke patients are included in the register. Data from 312 ischaemic stroke patients who had EVT at EVT Centre 1 over the same time period were obtained from a local registry.19 Among these patients, 53% received thrombolysis beforehand – the primary reasons for not receiving thrombolysis were unknown time of onset, outside of treatment time window, elevated international normalized ratio (INR) elevated blood pressure or recent trauma/surgery.
Analyses
Probability of good outcome for patients with LVO was modelled for each transport scenario using a pre-defined strategy.17 These models approach this problem physiologically and are based on the probability of achieving reperfusion, the time from onset to reperfusion, and the probability of achieving a good outcome (mRS 0–2 at 90 days) given reperfusion at a certain time from symptom onset. See Supplemental Data File for complete modelling methods. There are several simplifying assumptions in this model including the following: (1) stroke onset is witnessed, (2) the probability of good outcome given reperfusion for both EVT and alteplase decays with time, the probability of achieving reperfusion with EVT is time invariant and the probability of reperfusion with alteplase decays linearly, (3) all LVOs are treatment eligible and (4) reperfusion is only achieved through treatment.17
Time intervals used for modelling were obtained from two data sources. The median door to needle (DTN) times for each centre were obtained from the Irish National Stroke Register. For hospitals not included in the registry, the DTN was imputed as the national median. Door to puncture (DTP) times were obtained from an EVT registry at EVT Centre 1. Patients were stratified into two groups: those who presented directly to EVT Centre 1 (mothership) and those who were referred from a thrombolysis centre (drip-and-ship). Median DTP for each group was calculated; it was assumed these times would be similar at EVT Centre 2 once it is fully operational. A detailed list of model variables, definitions, values and data sources is listed in Table 1 in the Supplemental Data File.
Ireland was divided into 139 regions using postal codes. The postal code shape files were imported into ArcGIS 10.3 (Esri, Redlands, CA). Using the Feature To Point tool, the geographic centre of each region was identified and mapped. Each thrombolysis and EVT centre was mapped using global positioning system coordinates. Using Google’s Distance Matrix Application Programming Interface (Mountain View, CA), the ground transportation time from each region’s centre to the nearest thrombolysis and EVT centres was estimated, as well as the time between each thrombolysis and EVT centre.
These variables were entered into the drip-and-ship and mothership models and the model with the greatest probability of good outcome for each region was determined. If these probabilities were within 0.02 of one another, the two transport methods were classified as equivalent. As EVT Centre 2 does not yet perform EVT on a 24/7 basis, scenarios were created both with and without this EVT centre. The impact of faster treatment times was also explored.
The output of the models is shown graphically using colour-coded maps to illustrate whether the drip-and-ship model or mothership model of stroke patient transportation predicted superior, inferior or similar final stroke outcome.
Results
Data summary
Over the two-year study period, there were 699 patients treated with alteplase and 312 treated with EVT (258 using the drip-and-ship method), with a median DTN time of 75 min and a median DTP time of 100 min (mothership) and 10 min (drip-and-ship). Sixteen different scenarios were generated using combinations of EVT availability and treatment times (Table 2 in the Supplemental Data File.)
Model application
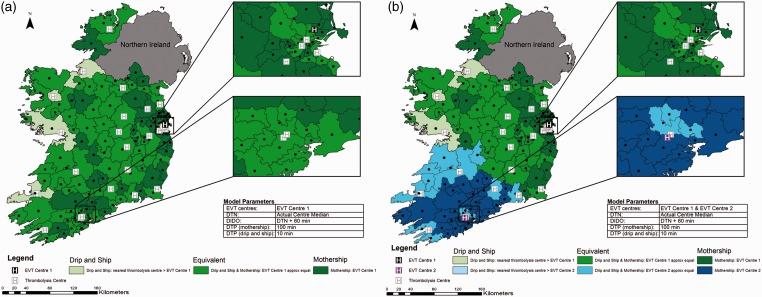
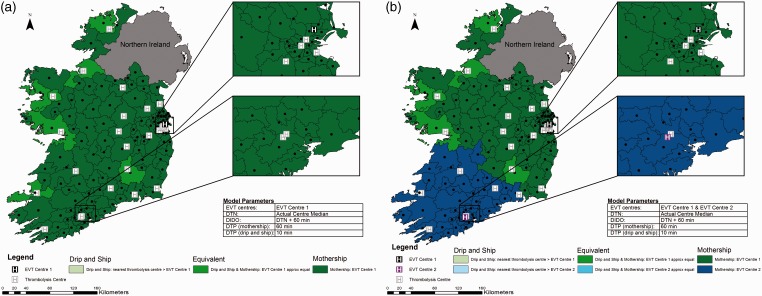
Utilising current treatment times and one EVT centre, for most regions, the two strategies are approximately equivalent. There are only a few regions where the drip-and-ship strategy is the best choice (Figure 2 Panel A). In areas where the patient’s closest thrombolysis centre is in the opposite direction of the EVT centre, mothership is the best option, because the drip-and-ship model involves driving the same road twice and extra drive time is therefore avoided.
Figure 2.
Current State of Ireland. Map shows Ireland broken into 139 regions with geographic centres plotted. Actual median door to needle time for each centre is used, time from alteplase administration to departure for the endovascular thrombectomy (EVT) centre is 60 min, door to groin puncture time is 100 min in the mothership scenario and 10 min in the drip-and-ship scenario. Panel A displays one EVT centre; panel B shows two EVT centres. Regions are colour coded to show the method predicting the best probability of good outcome.
DIDO: door-in-door-out; DTN: door to needle; DTP: door to puncture.
Figure 2 Panel B shows the same model with the addition of EVT Centre 2 in the southwest corner of the country. The six thrombolysis centres closest to this centre have been designated as its referral sites which serve approximately 27% of Ireland’s population.20 In this region, with current treatment times over a relatively short distance, the drip-and-ship approach never provides the best probability of good outcome and for the majority of regions, the mothership approach is the preferred transport strategy.
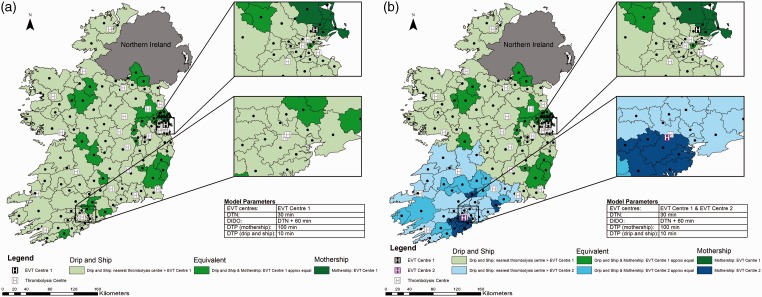
Impact of reducing DTN
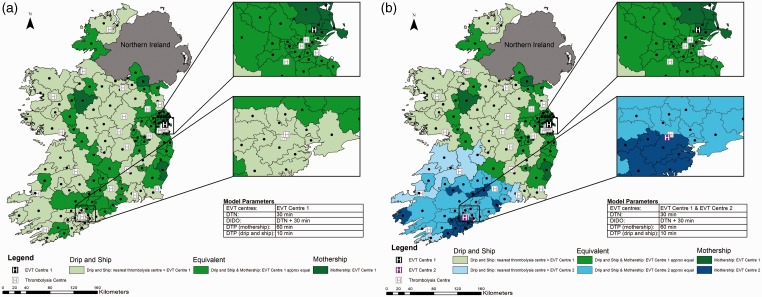
Holding other treatment times at baseline values, if DTN time is reduced to a median of 30 min at all hospitals, the drip-and-ship model almost always provides the greatest probability of good outcome (Figure 3 Panel A). The mothership model is predicted to be better only in regions close to an EVT centre. In a few regions, further away from the EVT centre in which travel to the closest thrombolysis centre would require moving away from the EVT centre, both transport approaches result in equivalent outcomes.
Figure 3.
Impact of reducing door to needle time. Map shows Ireland broken into 139 regions with geographic centres plotted. Door to needle time is reduced to 30 min, time from alteplase administration to departure for the endovascular thrombectomy (EVT) centre is 60 min, door to groin puncture time is 100 min in the mothership scenario and 10 minutes in the drip-and-ship scenario. Panel A displays one EVT centre; panel B shows two EVT centres. Regions are colour coded to show the method predicting the best probability of good outcome.
DIDO: door-in-door-out; DTN: door to needle; DTP: door to puncture.
With the addition of EVT Centre 2, the mothership model results in better predicted outcomes in regions close to this centre and in two regions where the closest thrombolysis centre requires travel away from EVT Centre 2 (Figure 3 Panel B). For the majority of regions, the drip-and-ship approach predicts the best patient outcomes.
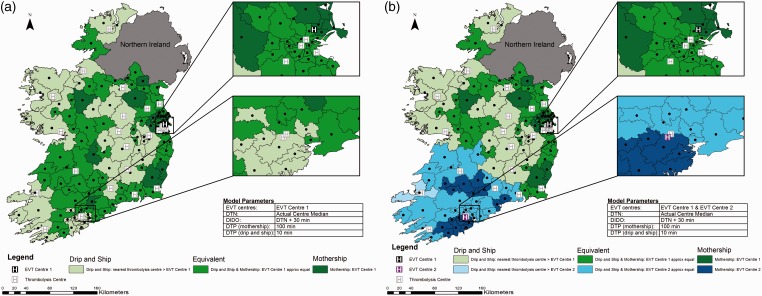
Impact of reducing door-in-door-out time
Door-in-door-out (DIDO) time is comprised of both DTN time and the time from thrombolysis administration to departure for the EVT centre (turnaround time). Reducing DIDO time by reducing the turnaround time to 30 min, while holding other times at baseline values, has a significant impact on transport decisions. The area where the drip-and-ship model provides the best probability of good outcome for the patient increases (Figure 4 Panel A). This impact is also seen when both EVT centres are operational (Figure 4 Panel B).
Figure 4.
Impact of reducing door-in-door-out time. Map shows Ireland broken into 139 regions with geographic centres plotted. Actual median door to needle time for each centre is used, door-in-door-out time is reduced by reducing time from alteplase administration to departure for the endovascular thrombectomy (EVT) centre to 30 min, door to groin puncture time is 100 min in the mothership scenario and 10 min in the drip-and-ship scenario. Panel A displays one EVT centre; panel B shows two EVT centres. Regions are colour coded to show the method predicting the best probability of good outcome.
DIDO: door-in-door-out; DTN: door to needle; DTP: door to puncture.
Figure 1 in the Supplemental Data File shows the impact of reducing DIDO time to 60 min overall (while holding DTP times at baseline values) by both reducing DTN to 30 min and reducing the turnaround time to 30 min. This has a profound impact on transport decision-making and the drip-and-ship model predicts the best outcome for the patient in almost all regions of the country.
Impact of reducing door to groin puncture time
Holding all other treatment times at baseline values, transport decision-making is highly sensitive to changes in DTP time at the EVT centre. Door to groin puncture times at EVT Centre 1 in the drip-and-ship scenario are very fast (median = 10 min). This can be attributed to the ability to pre-alert the neuroradiology team while the patient is being transported, the decision to forgo re-imaging on arrival (the size and population distribution of Ireland make most transfers times very short thus baseline imaging at the referring hospital is still medically appropriate), and the patient being brought directly to the neuro-angiography suite by EMS on arrival. However, for patients who present directly to EVT Centre 1, DTP time was substantially longer (median = 100 min). Figure 5 illustrates the impact of decreasing DTP time to 60 min in the mothership scenario (DTP time in drip-and-ship remains unchanged) holding other times at baseline values. Here, the drip-and-ship scenario never provides the best probability of good outcome for the patient, even when there is only one fully operational EVT centre serving the entire country.
Figure 5.
Impact of reducing door to groin puncture time. Map shows Ireland broken into 139 regions with geographic centres plotted. Actual median door to needle time for each centre is used, time from alteplase administration to departure for the endovascular thrombectomy (EVT) centre is 60 min, door to groin puncture time is reduced to 60 min in the mothership scenario and remains at 10 min in the drip-and-ship scenario. Panel A displays one EVT centre; panel B shows two EVT centres. Regions are colour coded to show the method predicting the best probability of good outcome.
DIDO: door-in-door-out; DTN: door to needle; DTP: door to puncture.
Figure 2 in the Supplemental Data File shows the impact of simultaneously reducing DTN and DTP times, while holding the turnaround time at baseline value. In contrast to the model showing only reduced DTN time, here, the drip-and-ship model is rarely favourable when there is only one EVT referral centre and is never favourable in the catchment area of EVT Centre 2. A similar result is seen when simultaneously reducing DTP time and turnaround time, while holding DTN time at baseline values. Again, the drip-and-ship model is rarely favourable (Figure 3 in the Supplemental Data File).
Impact of having optimal treatment times
If all treatment times are optimised; 60-min DIDO time (comprised of 30-min DTN time and 30-min turnaround time) and 60-min DTP time in the mothership scenario, the drip-and-ship model remains relevant in the majority of the country (Figure 6). The mothership model prevails in areas very close to EVT centres and in a few further regions where the drip-and-ship method would require initial transport away from the EVT centre.
Figure 6.
Impact of an optimised system. Map shows Ireland broken into 139 regions with geographic centres plotted. Door to needle time is reduced to 30 min, time from alteplase administration to departure for the endovascular thrombectomy (EVT) centre is reduced to 30 min, door to groin puncture time is reduced to 60 min in the mothership scenario and remains at 10 min in the drip-and-ship scenario. Panel A displays one EVT centre; panel B shows two EVT centres. Regions are colour coded to show the method predicting the best probability of good outcome.
DIDO: door-in-door-out; DTN: door to needle; DTP: door to puncture.
Discussion
We have presented the first case study of modelling pre-hospital transport for ischaemic stroke patients with LVO utilising real treatment times in a defined geographic area. While prior analyses have shown modelling results using optimised treatment times,17,18 few jurisdictions are currently achieving such optimised times21,22 and therefore modelling utilising current treatment times is of benefit to system planners. Modelling patient transport for system-level planning is highly sensitive to treatment times at thrombolysis and EVT centres. A moderate improvement in treatment times results in significant predicted changes to the optimisation of a national acute stroke patient transport strategy.
The two single changes that had the largest impact on transport decisions were reducing DTN time and DTP time. Reducing DTN time highlights the benefits of early thrombolysis treatment if direct access to EVT is not efficient. Alternatively, if the time to EVT can be reduced, the predicted benefit of early thrombolysis is negated in all scenarios. The impact of minimising the time between thrombolysis and EVT is also important. Even when DTN and DTP times are optimised, if DIDO time remains long (due to delay in departing for the EVT centre), the predicted benefit of early thrombolysis rarely supersedes direct transport to EVT services (mothership). For system-level planning, it is crucial that all stroke centres are operating as efficiently as possible and variability in treatment times is kept to a minimum as changes in treatment times at any point have large predicted impacts on the transport decision.
The effect these changes have on workload at the EVT centre must also be considered. If DTP is reduced in isolation, there will be substantial increase in volume at the EVT centres as the mothership model predicts the best outcome for all potential strokes. While our model is based on patients with known LVO, a proportion of these patients may not be EVT candidates and this unnecessary increase in volume may adversely affect workflow for EVT candidates. Conversely, in an optimised system, the drip-and-ship scenario predicts the best outcome for most regions. This scenario also has the benefit of early imaging and specialist consults (either in house or via telehealth) ensuring that only appropriate EVT candidates are transferred to the EVT centre, thereby reducing unnecessary workload at the EVT centre.
The potential impact of having a second fully operational EVT centre is also illustrated. With current treatment times, there are several regions in the immediate vicinity of EVT Centre 2 which would still benefit most from direct transport to EVT Centre 1 in Dublin, a ∼300 km drive, even when there is the potential to have EVT services in their own region. Apart from improved patient outcomes, having two fully operational EVT centres will allow patients to remain closer to home and reduce the caseload at the current single EVT centre. Additionally, this will lessen the burden on ambulance services. Taking a patient from the southwest of Ireland to EVT Centre 1 is not only costly but it also removes an ambulance from this region for an entire day, which is potentially detrimental to the remaining population in these areas. This scenario is entirely preventable by having EVT services available 24/7 in southwest Ireland. A recent health technology assessment of providing EVT services on a national basis in Ireland, which included two fully operational EVT centres, found providing this service to be cost-effective under typical willingness-to-pay thresholds.23
These models have limitations. Our model includes a simplifying assumption that LVO can be identified in the field and currently that is only partly true. Screening tools have incomplete sensitivity and specificity for identifying LVO. There are several clinical screening tools which can be used by paramedics to identify ischaemic stroke with reasonable but imperfect accuracy. For example, the Los Angeles Motor Scale (LAMS) score has been shown to identify LVOs with high sensitivity and specificity, especially in patients with severe middle cerebral artery syndrome at presentation.24,25 Patients who present as false positives on a LAMS screen include minor ischaemic stroke, haemorrhagic stroke and stroke mimics. Those with minor ischaemic stroke could have better outcomes if first transported to their nearest stroke centre for thrombolysis, rather than incurring a delay to treatment with transport to a more distant comprehensive centre. Including minor stroke patients in the transport model may bias the model towards the drip-and-ship strategy. This analysis considers ground transport only as it is the primary mode of pre-hospital transport in Ireland. Ground transport times can be variable due to traffic, weather and ambulance availability. The addition of air transport in the model may impact transport decisions. Future research should also include the impact that mobile stroke units may have on transport decisions in metropolitan areas.
The data source is not without limitation. The Irish National Stroke Register does not capture all ischaemic stroke patients (67% captured in 2014–2015) as it covers all major stroke centres but not all hospitals in Ireland where stroke patients are treated. However, the majority of LVO patients, which this study applies to, would have been treated at major stroke centres and are therefore captured in the register. This analysis was performed in a small country (∼70,000 km2) similar in size to the state of West Virginia, USA, making the results relevant to other small countries, various states, health care regions or even large metropolitan areas.
We have used this modelling and mapping technique as a case study to show how real treatment data entered into a pre-specified conditional probability model can be used to visualise and inform policy around acute stroke transport and treatment. Using this methodology, the impact of system-wide changes such as the addition of a new EVT centre and the reduction of treatment times can be determined. This case study can be expanded to other health jurisdictions using local treatment times for similar purposes.
In conclusion, modelling patient transport for system-level planning is sensitive to treatment times at both thrombolysis and EVT centres. Optimising all aspects of major acute ischaemic stroke care, including decreasing DTN and DIDO at thrombolysis centres and DTP at EVT centres will result in more favourable patient outcomes for patients with stroke due to LVO and will favourr a continued drip-and-ship strategy for patients requiring EVT.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Carmel Brennan and Emer Shelley (National Stroke Register); Siobhan Jennings (earlier similar work); Sinead Duff (EVT registry at Beaumont Hospital); Professor Anne Hickey (providing critical feedback); Alan Moore, Alan Martin, Linda Brewer and Ciaran Donegan (consult geriatricians at Beaumont Hospital); Paul O’Brien (thrombectomy services) and Patricia Daly (data collection).
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JT reports personal fees from Neuravi, outside the submitted work. MDH reports grants from Medtronic Inc, grants from Stryker Inc, grants from Alberta Innovates, during the conduct of the study; personal fees from Merck, non-financial support from Hoffmann-La Roche Canada Ltd, grants from Covidien (Medtronic), grants from Boehringer-Ingleheim, grants from NoNO Inc, outside the submitted work; In addition, MDH has a patent Systems and Methods for Assisting in Decision-Making and Triaging for Acute Stroke Patients pending to US Patent office Number: 62/086,077 and owns stock in Calgary Scientific Incorporated, a company that focuses on medical imaging software, is a director of the Canadian Federation of Neurological Sciences, a not-for-profit group and has received grant support from Alberta Innovates Health Solutions, CIHR, Heart & Stroke Foundation of Canada, National Institutes of Neurological Disorders and Stroke. DW reports personal fees from Boehringer Ingelheim, personal fees from Daiichi Sankyo, personal fees from Bayer, personal fees from Bristol Myers Squibb, outside the submitted work. All other authors report no conflicts of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JKH reports funding from Alberta Innovates (formerly Alberta Innovates – Health Solutions) and the Ireland Canada University Foundation.
Informed consent and ethical approval
This study was approved with waiver of consent by the Conjoint Health Research Ethics Board at the University of Calgary, Canada and the Health Service Executive, Dublin, Ireland.
Guarantor
JKH
Contributorship
Study conception and design: JKH, MDH, DW. Data analysis and acquisition: JKH, ABP, LRJ, JM, PM. Drafted manuscript: JKH, MDH, DW. Revised manuscript for important intellectual content and centre specific insight: JT, NK, PJK, SM, RC, TW, SC, SP, PB, AO, DJHM, BM, SL, GW, JH. All authors approved final manuscript.
References
- 1.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 12372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 2.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 3.Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 4.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 6.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 7.del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992; 32: 78–86. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 2010; 41: 2254–2258. [DOI] [PubMed] [Google Scholar]
- 9.Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012; 379: 2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA Am Med Assoc 2016; 316: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi AI, Chaudhry SA, Rodriguez GJ, et al. Outcome of the “Drip-and-Ship” paradigm among patients with acute ischemic stroke: results of a statewide study. Cerebrovasc Dis Extra 2012; 2: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tekle WG, Chaudhry SA, Hassan AE, et al. Drip-and-ship thrombolytic treatment paradigm among acute ischemic stroke patients in the United States. Stroke 2012; 43: 1971–1974. [DOI] [PubMed] [Google Scholar]
- 13.Sheth KN, Smith EE, Grau-Sepulveda MV, et al. Drip and ship thrombolytic therapy for acute ischemic stroke: use, temporal trends, and outcomes. Stroke 2015; 46: 732–739. [DOI] [PubMed]
- 14.Southerland AM, Johnston KC, Molina CA, et al. Suspected large vessel occlusion: should emergency medical services transport to the nearest primary stroke center or bypass to a comprehensive stroke center with endovascular capabilities? Stroke 2016; 47: 1965–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park MS, Yoon W, Kim JT, et al. Drip, ship, and on-demand endovascular therapy for acute ischemic stroke. Quinn TJ, editor. PLoS One 2016; 11: e0150668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohamad NF Hastrup S andRasmussen M.. Bypassing primary stroke centre reduces delay and improves outcomes for patients with large vessel occlusion. Eur Stroke J 2016; 1: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holodinsky JK, Williamson TS, Kamal N, et al. Versus direct to comprehensive stroke center: conditional probability modeling. Stroke 2017; 48: 233–238. [DOI] [PubMed] [Google Scholar]
- 18.Milne MSW, Holodinsky JK, Hill MD, et al. Drip 'n ship versus mothership for endovascular treatment: modeling the best transportation options for optimal outcomes. Stroke 2017; 48: 791–794. Mar [DOI] [PubMed] [Google Scholar]
- 19.McCusker MW, Robinson S, Looby S, et al. Endovascular treatment for acute ischaemic stroke with large vessel occlusion: the experience of a regional stroke service. Clin Radiol 2015; 70: 1408–1413. [DOI] [PubMed] [Google Scholar]
- 20.Central Statistics Office. Census of Population 2016. – Preliminary Results [Internet]. Central Statistics Office, http://cso.ie/en/releasesandpublications/ep/p-cpr/censusofpopulation2016-preliminaryresults/geochan/ (accessed 7 February 2017)
- 21.Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA 2014; 311: 1632–1640. [DOI] [PubMed] [Google Scholar]
- 22.Strbian D, Ahmed N, Wahlgren N, et al. Trends in door-to-thrombolysis time in the safe implementation of stroke thrombolysis registry: effect of center volume and duration of registry membership. Stroke 2015; 46: 1275–1280. [DOI] [PubMed] [Google Scholar]
- 23.Health Information and Quality Authority. Health technology assessment of a national emergency endovascular service for mechanical thrombectomy in the management of acute ischaemic stroke. Mahon, Cork: HIQA, 2017, pp.1–197. [Google Scholar]
- 24.Nazliel B, Starkman S, Liebeskind DS, et al. A brief prehospital stroke severity scale identifies ischemic stroke patients harboring persisting large arterial occlusions. Stroke 2008; 39: 2264–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao H, Coote S, Pesavento L, et al. Large vessel occlusion scales increase delivery to endovascular centers without excessive harm from misclassifications. Stroke 2017; 48: 568–573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.