Short abstract
Introduction
The sources of emboli in those with embolic stroke of undetermined source may differ in old and young. We assessed the frequency, features and potential embolic sources of younger vs. older embolic stroke of undetermined source patients in the embolic stroke of undetermined source Global Registry.
Patients and methods
Cross-sectional study of consecutive patients over age 18 years, with recent ischaemic strokes at 19 centres conducted in 2013–2014. Characteristics of embolic stroke of undetermined source patients who aged ≤50 years were analysed and compared with embolic stroke of undetermined source patients who aged >50 years.
Results
Among 2144 patients with ischaemic stroke, 323 (15.1%, 95% confidence interval: 13.6–16.7%) were ≤50 years old and, 1821 >50 years. 24% (n = 78) of young vs. 15% (n = 273) of older patients met embolic stroke of undetermined source criteria. The mean age of young embolic stroke of undetermined source patients was 40 years (standard deviation +/−9), 33% were women and the most prevalent vascular risk factor was hypertension (38%). Conventional vascular risk factors were less frequent in younger embolic stroke of undetermined source patients. Fewer young embolic stroke of undetermined source patients (63%) had potential minor risk embolic sources identified vs. older embolic stroke of undetermined source patients (77%) (p = 0.02). Stroke severity on admission was similar in younger vs. older patients (National Institute of Health Stroke Scale (NIHSS) 3 vs. 4, p = 0.06).
Discussion
Young embolic stroke of undetermined source patients comprise an important subset of ischaemic stroke patients around the world. Severity of stroke on admission and 30-day mortality rates are similar among young and older patients. However, there are important differences between younger vs. older embolic stroke of undetermined source patients with respect to risk factors, and potential embolic sources that could affect response to anticoagulants vs. antiplatelet therapies.
Conclusion
This study provides a benchmark for the global frequency and characteristics of young embolic stroke of undetermined source patients and shows consistent high frequency of embolic stroke of undetermined source in young adults.
Keywords: Embolic stroke of undetermined source, stroke in young
Introduction
In 2014, the clinical construct of embolic stroke of undetermined source (ESUS) was introduced to identify patients with non-lacunar cryptogenic ischaemic strokes in whom embolism was the likely mechanism.1 The underlying embolic sources in ESUS patients are heterogeneous. As it is hypothesized that most emboli are thrombotic, and that anticoagulants might be more efficacious than antiplatelet agents for secondary prevention of stroke in ESUS patients, the ESUS construct is the basis for three ongoing randomized controlled trials (RCTs) comparing non-vitamin K antagonist direct-acting oral anticoagulants with aspirin for secondary stroke prevention.2–4 In total, these trials will recruit about 13,500 ESUS patients. However, there will be a limited number of ESUS patients under the age of 50 years enrolled in these studies because younger patients were excluded based on an assumption that they are at lower risk of outcome events and may thus compromise the statistical power of the studies. Although these trials are likely to provide valuable information about older ESUS patients, there will likely be a relative gap in the knowledge concerning the clinical characteristics of young ESUS patients even at their conclusion.
We sought to determine the clinical characteristics of young ESUS patients and to compare them with older ESUS patients in order to elucidate potential differences in embolic sources.
Patients and methods
The methods of the ESUS Global Registry Project have been previously reported.5 Briefly, data from consecutive patients with recent ischaemic stroke were sought at 19 stroke research centres in 19 different countries, in 2013–2014. This retrospective registry aimed to review 100 patients with acute ischaemic stroke at each site or until 25 patients meeting ESUS criteria (Table 1) were identified. Sites retrospectively identified consecutive inpatients evaluated for recent stroke either from hospital discharge diagnosis codes or from databases associated with acute stroke units. The study was compliant with local institutional research board regulations.
Table 1.
Criteria for diagnosis of embolic stroke of undetermined source (ESUS).a
| 1. Ischaemic stroke detected by CT or MRI that is not lacunar.b |
| 2. Absence of extracranial or intracranial atherosclerosis causing >50% luminal stenosis in arteries supplying the area of ischaemia. |
| 3. No major-risk cardioembolic source of embolism.c |
| 4. No other specific cause of stroke identified (e.g. arteritis, dissection, migraine/vasospasm, drug abuse). |
aRequires minimum diagnostic evaluation that includes cardiac rhythm monitoring for >24 h with automated rhythm detection.1
bLacunar defined as a subcortical infarct <1.5 cm (<2.0 cm on MRI diffusion images) in largest dimension, including on MRI diffusion-weighted images, and in the distribution of the small, penetrating cerebral arteries of the cerebral hemispheres and pons.
cPermanent or paroxysmal atrial fibrillation, sustained atrial flutter, intracardiac thrombus, prosthetic cardiac valve, atrial myxoma or other cardiac tumours, mitral stenosis, recent (<4 weeks) myocardial infarction, left ventricular ejection fraction <30%, valvular vegetations or infective endocarditis.
CT: computed tomography; MRI: magnetic resonance imaging.
Data were analysed according to five global regions: Europe, North America, Latin America, East Asia and Pacific. Descriptive analyses regarding the frequency of key features were done using t test and Chi-square; and data were compared for patients aged ≤50 (termed young ESUS) and over 50 years.
Results
Among 2144 patients with recent ischaemic stroke from 19 stroke research centres in 19 countries, 323 (15.1%, 95% confidence interval (CI): 13.6–16.7%) were ≤50 years old and 1821 were older than 50 years.
Frequency of ESUS by country and global region
Of the 323 patients aged ≤ 50 years, 78 (24%, 95% CI 20–29%) met ESUS criteria (Table 2).
Table 2.
Frequency of young ESUS patients in 19 centres of different countries.
| Site | No. of ischaemic stroke patients age ≤50 yrs | ESUSan (%) |
|---|---|---|
| Buenos Aires, Argentina | 7 | 3 (43) |
| Perth, Australia | 21 | 7 (33) |
| Brussels, Belgium | 5 | 1 (20) |
| Sao Paulo, Brazil | 23 | 1 (4) |
| Hamilton, Canada | 14 | 5 (36) |
| Beijing, China | 20 | 9 (45) |
| Paris, France | 8 | 5 (63) |
| Heidelberg, Germany | 3 | 0 (0) |
| Galway, Ireland | 8 | 3 (38) |
| Rome, Italy | 10 | 2 (20) |
| Tokyo, Japan | 12 | 5 (42) |
| Mexico City, Mexico | 86 | 11 (13) |
| Amsterdam, Netherlands | 11 | 0 (0) |
| Manila, Philippines | 32 | 4 (13) |
| Coimbra, Portugal | 10 | 3 (30) |
| Moscow, Russia | 11 | 5 (45) |
| Seoul, South Korea | 13 | 8 (62) |
| Glasgow, United Kingdom | 12 | 2 (17) |
| Philadelphia, United States | 17 | 4 (24) |
| Total | 323 | 78 (24) |
Yrs: years.
aESUS: embolic stroke of undetermined source per criteria in Table 1.
The highest frequency was found in East Asia (49%) and the lowest in Latin America (13%) (Table 3).
Table 3.
Frequency of young ESUS patients by global region.a
| Region | No. of ischaemic stroke patients ≤50 yrs | ESUSn (%) |
|---|---|---|
| Europe (9 sites) | 78 | 21 (27) |
| North America (2 sites) | 31 | 9 (29) |
| Latin America (3 sites) | 116 | 15 (13) |
| East Asia (3 sites) | 45 | 22 (49) |
aOne site each in Australia and the Philippines are not considered.
Reasons for not ESUS
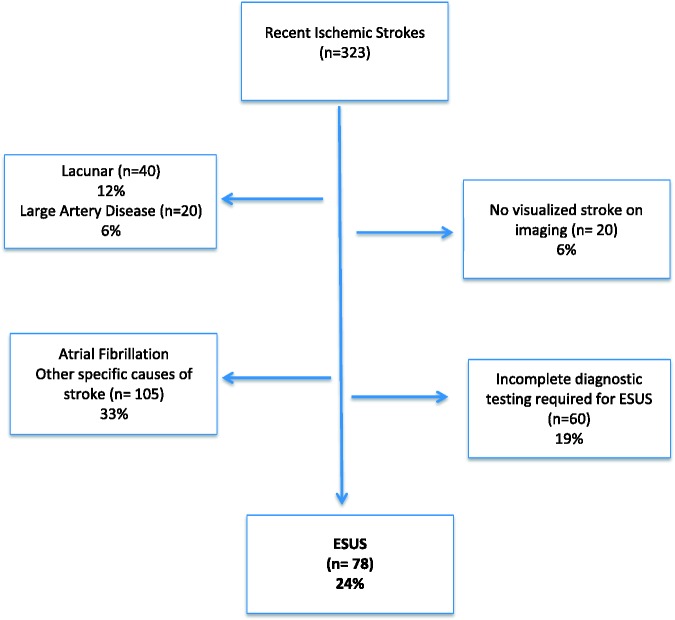
One of the frequent reasons that young patients with ischaemic stroke did not meet the criteria for ESUS was lack of diagnostic tests done to meet the ESUS criteria. Of note, 24-h cardiac rhythm monitoring (required for ESUS diagnosis) was not routinely carried out in 48%, and echocardiography (required for ESUS diagnosis) was not routinely done in 28% of young ischaemic stroke patients. Other reasons that ischaemic stroke patients did not meet the ESUS criteria were: other stroke causes such as dissection, arteritis and major risk cardioembolic sources (except Atrial Fibrillation (AF)) 27%, lacunar strokes (12%), non-visualized strokes 6% (stroke visualization required for ESUS diagnosis) and carotid artery stenosis (6%). Atrial fibrillation only accounted for 5% of ischaemic stroke in the young (Figure 1). All young ESUS patients in the registry had undergone intracranial vessel imaging either by Computed Tomograpyhy Angiogram (CTA), Magnetic Resonance Angiogram (MRA) or transcranial Doppler and was deemed not to have ≥ 50% stenosis of the intracranial arteries.
Figure 1.
Frequency of embolic stroke of undetermined source (ESUS) in patients under 50 years as a proportion of all ischaemic strokes under 50 years.
Demographic risk factors and clinical features of ESUS patients
The mean age of the young ESUS patients was 40 years and 33% were women.
Among the 78 young ESUS patients, hypertension, diabetes and coronary artery disease were present in 36, 21 and 7%, respectively; 11% were taking antiplatelet therapy at stroke onset. A history of stroke or TIA prior to the index stroke was present in 5% of the patients. Median (interquartile range) NIHSS score near hospital admission was 3 (1–7); 9% received intravenous tissue plasminogen activator at the time of the index stroke (Table 4).
Table 4.
Features of ESUS patients ≤ 50 years vs. >50 years.
|
ESUS patients |
|||||
|---|---|---|---|---|---|
|
Age ≤50 yrs(N=78) |
Age >50 yrs(N=273) |
||||
| Na | % or Mean | Na | % or Mean | P valueb | |
| Mean age (yrs) | 78 | 40 (9) | 273 | 68 (10) | <0.0001 |
| Men (%) | 78 | 67 | 272 | 54 | 0.0688 |
| Diabetes (%) | 78 | 21 | 273 | 26 | 0.3755 |
| Hypertension (%) | 78 | 36 | 270 | 73 | <0.0001 |
| Current tobacco use (%) | 78 | 28 | 273 | 21 | 0.1707 |
| Coronary artery disease (%) | 74 | 7 | 258 | 11 | 0.3812 |
| History of stroke or TIA prior to index stroke (%) | 78 | 5 | 270 | 21 | 0.0006 |
| Heart failure (%) | 73 | 0 | 258 | 2 | 0.3452 |
| Hyperlipidemia (%) | 72 | 28 | 254 | 43 | 0.0285 |
| Peripheral vascular disease (%) | 71 | 0 | 236 | 4 | 0.1240 |
| Antiplatelet therapy at the time of index stroke (%) | 76 | 11 | 270 | 36 | <0.0001 |
| Anticoagulant therapy at the time of index stroke (%) | 72 | 3 | 255 | 1 | 0.2117 |
| Median NIHSS score near admission (IQR) | 58 | 3 (1, 7) | 228 | 4 (2, 9) | 0.0553 |
| IV TPA for index stroke (%) | 78 | 9 | 269 | 19 | 0.0551 |
| Death within 30 days (%) | 67 | 1 | 208 | 2 | >0.90 |
| Antithrombotic therapy at discharge | |||||
| ASA (%) | 78 | 59 | 263 | 53 | 0.3669 |
| Clopidogrel (%) | 78 | 18 | 263 | 19 | >0.90 |
| Dual antiplatelet (%) | 78 | 13 | 263 | 20 | 0.1835 |
| Warfarin/vitamin K antagonist only (%) | 78 | 8 | 263 | 3 | 0.0980 |
| Rivaroxaban, dabigatran or apixaban only (%) | 78 | 1 | 263 | 2 | >0.90 |
| None only (%) | 78 | 0 | 263 | 2 | 0.5776 |
ASA: aspirin; ESUS: embolic strokes of undetermined source; IQR: interquartile range; TIA: Transient Ischeamic Attack; IV: Intra venous; TPA: Tissue plasminogen activator; yrs: years.
aOnly complete cases used to calculate the percentages.
bP values for categorical comparison – Fisher's exact test; P values for continuous comparison – Wilcoxon rank-sum test.
Magnetic resonance imaging (MRI) scans of the brain were done in 94%, with a previous stroke (i.e. prior to index stroke) present on MRI in 30%. Transoesophageal echocardiography (TEE) was undertaken in 49% of young ESUS patients, with patent foramen ovale (PFO) present in 32% and complex aortic arch plaque reported in 3% of those undergoing TEE.
At hospital discharge, 77% of young ESUS patients received antiplatelet monotherapy, 9% anticoagulant therapy and the remainder (13%) were discharged on dual antiplatelet therapy (Table 4). The vascular risk factor profile of young ESUS patients was significantly different from older ESUS patients. In contrast, there was no statistically significant difference in the presenting NIHSS score or the 30-day mortality rate among younger and older ESUS patients (1% in young ESUS patients compared with 2% in the older ESUS patients) (Table 4).
Frequency of minor-risk potential embolic sources in ESUS patients
Of young ESUS patients with anterior circulation stroke, 69% had non-stenotic cervical carotid artery plaque either of ipsilateral or contralateral carotid arteries; this was less than in patients over the age of 50 years (82%) and was statistically significant (p = 0.0393). Among those who underwent transthoracic (precordial) echocardiography, mitral annular calcification or myxomatous changes (0%), and aortic valve stenosis or calcification (1%), were not commonly seen (Table 5). The prevalence of PFO among this young ESUS population who had TEE was 32%. At least one minor-risk potential embolic source was identified in 63% of young ESUS patients compared to 77% of older ESUS patients (p = 0.0197), 14% of young ESUS patients had two or more potential minor risk embolic sources compared to 26% of older ESUS patients (Table 5).
Table 5.
Frequency of potential minor risk embolic sources among ESUS patients ≤50 years vs. >50 years.
| Minor risk potential embolic sources | Age ≤50N (%) | Age > 50N (%) | P valuea |
|---|---|---|---|
| Carotid artery non-stenotic plaquesb | 37 (69) | 171 (82) | 0.0393 |
| Mitral annular calcification and thickening with myxomatous changesc | 0 (0) | 17 (7) | 0.0296 |
| Aortic valve stenosis and calcificationc | 1 (1) | 23 (9) | 0.0367 |
| Hypokinetic/akinetic left ventriclec | 3 (4) | 10 (4) | >0.90 |
| Moderate-to-severely dilated left atriumc | 6 (8) | 13 (5) | 0.3894 |
| Aortic arch atherosclerotic plaquesd | 1 (3) | 34 (40) | <0.0001 |
| Patent foramen ovalec | 7 (10) | 12 (5) | 0.1487 |
| Patent foramen ovaled | 12 (32) | 19 (22) | 0.2605 |
| Any minor risk potential embolic sources | 49 (63) | 209 (77) | 0.0197 |
| ≥2 minor-risk embolic sources | 11 (14) | 72 (26) | 0.0240 |
ESUS: embolic strokes of undetermined source; TTE: Transthoracic Echocardiogram; POCS: Posterior Circulation Stroke; CTA: Computed Tomography Angiogram; MRA: Magnetic Resonance Angiogram.
aP values for categorical comparison – Fisher's exact test.
bIn all ESUS patients excluding POCS either on CTA, MRA or carotid ultrasonography.
cESUS patients who had TTE.
dESUS patients who had TEE.
Discussion
Following the completion of the large randomized trials testing anticoagulants in ESUS patients in 2018, clinical interest in the ESUS construct is likely to increase exponentially. There will be intense interest to know whether the results of these trials are generalizable to young adults with ESUS: are these patients similar to those enrolled in the trials (i.e. older patients) or are they quite different in their clinical characteristics and embolic sources. Approximately 10% of ischaemic strokes occur in people under the age of 50 years, the age threshold most often used to define ‘young-onset stroke’.6 While the age cut off is biologically arbitrary, it is based on the differing clinical characteristics, risk factors and stroke aetiologies between younger and older stroke patients. By available estimates, 15–20% of ESUS patients will be under 50 years of age and not included in the RCTs. To our knowledge, there is only one study published that reports the clinical characteristics of young ESUS patients (age <55years) and this study is limited by the small sample size.7 Our multicentre study found that young ESUS patients comprise about one in four ischaemic strokes in patients aged 50 years and under with a varying proportion between centres. The most likely reason for the varying proportion of young ESUS patients between centres is the extent of diagnostic tests performed at each centre and this reflects the existing variations in global practice in diagnostic testing at major stoke research centres. For example, one in five young ischaemic stroke patients was not considered to be ESUS due to lack of diagnostic testing to meet the ESUS criteria. 24-holter monitoring and echocardiography required for the diagnosis of ESUS was not routinely done in young ischaemic stroke patients at many centres, so only a small fraction could be categorized as ESUS. Therefore, this rate is likely to be higher at centres that routinely undertake the complete diagnostic evaluation required to meet ESUS criteria. In contrast, the frequency of ESUS patients might be lower in centres that perform more extensive examinations for stroke aetiology (e.g. 30-day Electrocradiogram (ECG) monitoring, cardiac MRI, advanced intracranial vessel imaging, advanced carotid plaque imaging and routine TEE). The frequency seen in our study is consistent with the frequency of ESUS noted in the most recent systematic review of ESUS patients.8 In our study, when those patients who did not undergo the complete diagnostic investigation required for ESUS diagnosis were excluded (n = 60, most often lack of cardiac investigations), the frequency of ESUS was 30%. Considering an estimated 40–45% frequency of cryptogenic ischaemic strokes in young adults based on the recent literature,6,8 this frequency of ESUS supports the notion that most (but not all) non-lacunar cryptogenic ischaemic strokes are likely due to embolism.
Young ESUS patients on average had relatively minor strokes (median NIHSS 3) with a low 30-day mortality rate (1%). Their mean age (40 years) was similar to non-ESUS ischaemic stroke patients without AF, but significantly lower than, that of non-ESUS stroke patients with atrial fibrillation (43 years). The mild severity of stroke in ESUS patients is consistent with smaller emboli, e.g. originating from cardiac valves and arterial sources, in contrast to larger emboli that typically originate in the cardiac chambers (e.g. left atrial thrombus in patients with atrial fibrillation.9 Our observations regarding stroke severity are consistent with those regarding ESUS patients from the Athens Stroke Registry.10 The substantially younger age and milder strokes of ESUS patients compared with patients with AF-associated stroke support different embolic origins in the majority of patients (i.e. that undiagnosed paroxysmal atrial fibrillation is unlikely to underlie most ESUS).
Of interest, AF was identified only in 5% of consecutive young ischaemic stroke patients compared to 20–30% of older ischaemic stroke patients.5 Recent studies suggest that prolonged (>1 month) cardiac rhythm monitoring identifies additional patients with cryptogenic stroke who have episodes of paroxysmal atrial fibrillation,11,12 often brief and of uncertain aetiologic relevance.13 More recent studies have shown a strong and significant correlation between the mean age of the stroke cohort and the frequency of AF.14 These possibly could be the reasons for the lack of routine prolonged cardiac monitoring seen in these patients.
Interestingly, there was a relatively high frequency of conventional vascular risk factors and prior brain infarction in ESUS patients with a mean age of 40 years. Since ours is the first study assessing these factors, it is difficult to know whether this potentially represents patient selection or accurate characterization of young ESUS patients. Further studies are needed to clarify this. The relatively high frequency of prior brain infarction on MRI hints at a high stroke recurrence risk in young ESUS patients. It is known from the ongoing large randomized trials that the stroke recurrence risk among older ESUS patients is higher than expected.
However, the frequency of conventional vascular risk factors was less frequent among young ESUS patients than older ESUS patients. Most (63%) young ESUS patients had at least one minor-risk embolic source identified that could have been the cause of stroke, and nearly one in six (14%) had two or more potential embolic sources identified. However, the majority of the minor embolic sources were carotid artery non-stenotic plaques, a condition that is often thought to be only a marker of atherosclerotic disease but not the cause of ischaemic stroke. Interestingly, the frequency of PFO among young ESUS patients undergoing TEE (32%) was not significantly increased over that expected in the general population. This may be explained by the small sample size plus the fact that TEE is not mandatory according to ESUS criteria and was used selectively, as only 38% of the young stroke patients had a TEE. The prevalence of PFO has been described in cryptogenic stroke patients but has not yet been described in an ESUS population and the significance of PFO in a younger ESUS population is yet to be determined.
Antiplatelet therapy was used for secondary stroke prevention in two-thirds of young ESUS patients, in line with recent guideline recommendations.15–17 Interestingly, close to one in six patients were discharged on dual antiplatelet therapy even though there is no evidence to suggest a clear benefit of dual antiplatelet therapy over monotherapy in this situation. This may reflect the clinical equipoise that exit among physicians about best management of these patients. Although emboli vary in composition, almost all include thrombus, and it has been proposed that anticoagulants could offer more protection against recurrent stroke for ESUS patients than antiplatelet agents.1 Ongoing trials will show whether novel anticoagulant drugs will be superior to aspirin in ESUS patients.2–4
Limitations of this study include participation of selected, high-volume stroke centres. A single site in each country may not accurately reflect the spectrum of stroke. However, the study sites represented many global regions allowing characterization of ESUS patients around the world. To ensure data quality in this retrospective study, standardized data collection forms were used along with specific definitions. The cross-sectional design of the study is another limitation, which did not allow us to gather data on prognosis and treatment effects of young ESUS patients. This limits our ability in this study to assess whether the younger patients with ESUS are at a lower risk of future stroke than older patients and the treatment responses.
Conclusion
In summary, this study provides a benchmark for the global frequency and clinical features of young patients with ESUS. Young ESUS patients comprise an important subset of ischaemic stroke patients around the world. Severity of stroke on admission and 30-day mortality rates are similar among young and older patients. However, there are important differences between younger vs. older ESUS patients with respect to risk factors, and potential embolic sources that could affect response to anticoagulants vs. antiplatelet therapies.
Acknowledgements
RV is an investigator at NIHR Imperial Biomedical Research Centre.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: ESUS Global Registry was funded by Bayer Health Care, Hamilton Health Sciences Strategic Initiatives Program and the Canadian Stroke Prevention Intervention Network.
Informed consent
Informed consent was waived by all involved Ethics Boards for this retrospective chart review.
Ethical approval
The study was approved by an institutional review committee at each participating centre.
Guarantor
KSP.
Contributorship
KSP and RH researched literature and conceived the study. BS was involved in data analysis. KSP wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
References
- 1.Hart RG, Diener HC, Coutts SB, et al. on behalf of the Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014; 13: 429–438. [DOI] [PubMed] [Google Scholar]
- 2.Hart RG, Sharma M, Mundl H, et al. for the NAVIGATE ESUS Steering Committee. Rivaroxaban for secondary stroke prevention in patients with embolic strokes of undetermined source: design of the NAVIGATE ESUS randomized trial. Eur Stroke J 2016; 1: 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diener HC, Easton JD, Granger CB, et al. Design of randomized, double-blind, evaluation in secondary stroke prevention comparing the efficacy and safety of the oral thrombin inhibitor dabigatran etexilate vs. acetylsalicyclic acid in patient with embolic stroke of undetermined source (RE-SPEcT ESUS). Int J Stroke 2015; 10: 1309–1312. [DOI] [PubMed] [Google Scholar]
- 4.ATTICUS. https//clinicaltrials.gov/ct2/show/nct024271 26. (accessed 6 July 2016).
- 5.Perera KS, Vanassche T, Bosch J, et al. Embolic strokes of undetermined source: prevalence and patient features in the ESUS Global Registry. Int J Stroke 2016; 11: 526–533. [DOI] [PubMed] [Google Scholar]
- 6.Putaala J, Metso AJ, Metso TM, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke. The Helsinki Young Stroke Registry. Stroke 2009; 40: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 7.Ladeira F, Barbosa R, Caetano A, et al. Embolic stroke of unknown source (ESUS) in young patients. Int J Stroke 2015; 10: 165. [DOI] [PubMed] [Google Scholar]
- 8.Hart RG, Catanese L, Perera KS, et al. Embolic stroke of undetermined source: systematic review and clinical update. Stroke 2017; 48: 867–872. [DOI] [PubMed] [Google Scholar]
- 9.Hart RG. Cardiogenic embolism to the brain. Lancet 1992; 339: 589–594. [DOI] [PubMed] [Google Scholar]
- 10.Ntaios G, Papavasileiou V, Milionis H, et al. Embolic strokes of undetermined source in the Athens Stroke Registry: a descriptive analysis. Stroke 2015; 46: 176–181. [DOI] [PubMed] [Google Scholar]
- 11.Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370: 2467–2477. [DOI] [PubMed] [Google Scholar]
- 12.Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014; 370: 2478–2486. [DOI] [PubMed] [Google Scholar]
- 13.Arsava EM, Base DF, Atalar E, et al. Ischemic stroke phenotype in patients with non-sustained atrial fibrillation. Stroke 2015; 46: 634–640. [DOI] [PubMed] [Google Scholar]
- 14.Perera KS, Vanassche T, Bosch J, et al. Global survey of the frequency of atrial fibrillation–associated stroke: embolic stroke of undetermined source Global Registry. Stroke 2016; 47: 2197–2202. [DOI] [PubMed] [Google Scholar]
- 15.European Stroke Organization (ESO) Executive Committee and ESO Writing Committee . Guidelines for the management of ischemic stroke and transient ischemic attack 2008. Cerebrovas Dis 2008; 25: 457–505. [DOI] [PubMed] [Google Scholar]
- 16.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 45: 2160–2236. [DOI] [PubMed] [Google Scholar]
- 17.Lansberg MG, O’donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e601S–e636S. [DOI] [PMC free article] [PubMed] [Google Scholar]