TO THE EDITOR:
Langerhans cell histiocytosis (LCH) is characterized by granulomatous lesions with pathologic CD207+ dendritic cells.1 More than 40% of children with high-risk LCH (involving bone marrow, liver, and/or spleen) are not cured by frontline chemotherapy, with the highest risk of morbidity and mortality in those with poor initial response.2 Uncontrolled LCH is associated with increased morbidity,3 including progressive LCH-associated neurodegeneration (LCH-ND).4 Activating somatic mutations in MAPK pathway genes occur in most cases of LCH, with ∼60% attributable to BRAF V600E5-8; otherwise, the mutation burden is low.6,7 Recently, BRAF V600E mutations were identified in mononuclear cells from peripheral blood and in brain biopsies of patients with LCH-ND, supporting LCH-ND as a tissue-specific process driven by ERK activation.9,10
High-dose nucleoside analogs and hematopoietic cell transplantation are effective salvage strategies, although they are associated with high treatment-related morbidity and mortality.11-13 Given the central role of MAPK pathway activation in pathogenesis, targeted inhibition of the MAPK pathway may be an effective therapeutic strategy for LCH.14,15 Phase 1 to 2 trials and series of adults with LCH and the related disorder of Erdheim-Chester disease (ECD) treated with the BRAF V600E inhibitor vemurafenib reported universal metabolic response (objective response rate of 43% when using RECIST criteria; additional studies using metabolic response criteria by positron emission tomography demonstrated objective response rates of 100%).16-18 Discontinuation of therapy frequently resulted in relapse, with 75% of adults with ECD progressing after discontinuation of therapy in the LOVE study.19 Although early reports of MAPK pathway inhibition in adults with LCH and/or ECD have demonstrated promising response rates, the efficacy and safety of MAPK pathway inhibition in children with LCH remains uncertain. There are few reports in pediatric LCH, although a few cases have suggested potential for responses to MAPK inhibition.20-22 The optimal therapy for this age group is not well established, and improved strategies are urgently needed for children with refractory/relapsed high-risk LCH and LCH-ND.
NACHO-LIBRE (North American Consortium for Histiocytosis–Registry Study of LCH and Related Disorders: Inhibition of MAPK Pathway Activation [RAS, BRAF, MEK, and ERK]) was designed to systematically evaluate the efficacy and toxicity of MAPK inhibitors in a retrospective cohort of children with LCH. Institutional review board approval was obtained from Baylor College of Medicine, and NACHO member and partner institutions contributed outcomes for children with LCH (systemic and/or LCH-ND) treated with MAPK pathway inhibitors; none of the patients were enrolled in a clinical trial for these drugs. Early data from patients 1, 4, 12, and 21 have been reported previously, with extended treatment course and toxicity information reported here.9,23
Medical records from 21 pediatric patients with LCH (systemic and/or LCH-ND) from 14 institutions were systematically reviewed (supplemental Table 1, available on the Blood Web site). All patients had experienced failure of at least 1 prior therapy and had a proven MAPK pathway somatic mutation (BRAF V600E, n = 20; MAP2K1_c293_310del, n = 1; supplemental Table 1). Response assessments were based on best response using applicable criteria for each individual, according to modified RECIST 1.1 criteria specific for LCH (supplemental Methods),24 including positron emission tomography (metabolic) criteria, bone marrow evaluation, serial brain magnetic resonance imaging, and ataxia rating score using the Scale for Assessment and Rating of Ataxia.25 All patients were age <21 years (median age at start of therapy, 6.9 years; range, 0.4-20.7 years), with a median disease duration of 4 years before start of MAPK inhibitor (range, 0.07-18.4 years) and median of 3 prior treatments (range, 1-9 treatments). At the start of MAPK inhibition, 13 patients had LCH-ND (clinical and radiographic evidence of disease, n = 11; radiographic evidence only, n = 2); the remaining 8 patients had systemic disease without LCH-ND (7 with high-risk organ involvement). Patients were treated for a median of 12.4 months (range, 0.6-44.6 months), with a median follow-up time of 13.3 months (range, 0.6-45.8 months) from start of MAPK inhibitor therapy. Reasons for treatment discontinuation are noted in supplemental Table 1.
Overall response rate was 86% for the entire cohort based on best response using RECIST criteria modified for LCH (supplemental Table 1; supplemental Methods). Four (19%) of 21 patients achieved a complete response (CR), and 14 patients (67%) achieved a partial response (PR), whereas 2 patients (10%) achieved stable disease (SD), and 1 patient (4%) died early during therapy as a result of progressive disease complicated by secondary macrophage activation syndrome (supplemental Table 1; Figure 1). Of the 13 patients who had any LCH-ND, none achieved a CR, but 12 (92%) achieved a PR and 1 patient (8%) maintained SD by either clinical or radiographic assessment (supplemental Table 1). Of the 10 patients who had any other LCH disease at start of therapy, 4 (40%) achieved a best response of CR, 3 (30%) achieved a PR, 1 (10%) had SD, and 1 (10%) had progressive disease.
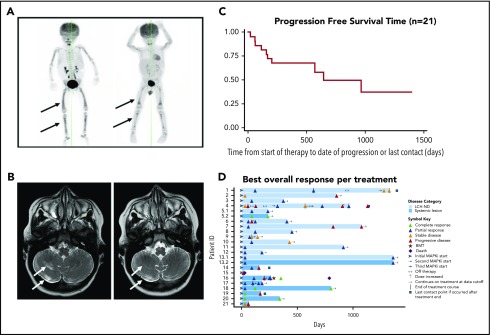
Figure 1.
Outcomes of pediatric LCH patients treated with MAPK pathway inhibitors. (A) [18F]fluorodeoxyglucose–positron emission tomography scan from patient 17 with disseminated high-risk LCH before (left) and 2 months after (right) treatment with vemurafenib, scored as a partial metabolic response. (B) Magnetic resonance imaging brain (axial T2 fluid-attenuated inversion recovery) from patient 6 with radiologic LCH-ND before (right) and 7 months after (left) treatment with vemurafenib, scored as a partial radiologic response. (C) Graphic representation of progression-free survival (PFS) time (37%) for all patients treated with MAPK inhibition (MAPKi; n = 21). (D) Individual swimmer plots for each patient (n = 21), depicting PFS with MAPKi. Responses for patients (5 and 13) who had both LCH-ND and systemic LCH at start of MAPKi are subdivided as follows: 5.1, swimmer plot for LCH-ND; 5.2, swimmer plot for parietal bone lesion; 13.1, swimmer plot for LCH-ND; 13.2, swimmer plot for LCH liver involvement. BMT, bone marrow transplantation.
Patients who had a shorter duration of LCH-ND or were clinically asymptomatic before start of MAPK inhibitor tended to have the best response to MAPK inhibition (supplemental Table 1). Median progression-free survival time after start of therapy was 14.2 months (range, 4.6-44.7 months). Among those who experienced an event, median time to disease progression or recurrence was 2.8 months (range, 0.6-21.2 months). Kaplan-Meier progression-free survival estimate from start of therapy to last contact was 37% (median follow-up time, 14 months; range, 0.6-46.5 months), and overall survival was 90% (1 death resulting from transplantation-related mortality; Figure 1). Four (19%) of the 21 patients experienced grade 3 or 4 toxicity, and 2 patients required dose modification. Five patients (24%) received concurrent therapies (supplemental Table 1).
Ten patients had measurable BRAF V600E–mutated peripheral blood mononuclear cells (PBMCs) or bone marrow cells before the start of MAPK inhibition, with subsequent molecular assessments obtained after start of MAPK inhibitor. In contrast to patients treated with chemotherapy, in whom the presence of BRAF V600E+ PBMCs reflects disease burden,11 the percentage of BRAF V600E+ PBMCs in patients treated with MAPK inhibition in this series did not reliably correlate with disease activity or clinical response (supplemental Figure 1).
In this multisite retrospective review of patients with multiple previous treatment failures, MAPK pathway inhibition was associated with an overall response rate of 86% and no treatment-related mortality, which compares favorably to alternative chemotherapy salvage strategies.12,13 These data are valuable as a collection of experiences from a challenging cohort of LCH patients for whom previous therapies failed. Patients with relapsed/refractory high-risk systemic LCH generally experienced early benefit from this strategy, although few achieved a sustained CR. Patients with relatively early onset of LCH-ND had better radiologic and clinical responses compared with patients with longstanding LCH-ND.
We hypothesize that inhibition of the MAPK pathway may confer clinical benefit by blocking differentiation and proliferation of precursor cells with hyperactive MAPK signaling.15 However, MAPK inhibition may have limited cytotoxic potential, as supported by persistence of BRAF V600E+ mononuclear cells in blood and bone marrow, even in patients with impressive clinical responses, and relapse after discontinuation of therapy. If MAPK inhibition arrests pathogenic mechanisms but does not kill LCH precursor cells, alternative approaches may therefore be necessary for cure. For example, combination of MAPK inhibition with conventional cytotoxic chemotherapies may be a consideration for future trials to test potential to achieve sustained clinical improvement and eradicate the underlying precursor cells. Acknowledging intrinsic limitations of multicenter retrospective series, this study demonstrates potential for patients with refractory or relapsed LCH or LCH-ND to respond to MAPK inhibition. Future prospective trials are required to determine optimal patient population, timing and duration of MAPK inhibition, mutation-specific responses to specific inhibitors,26 and potential for improved outcomes with strategies that combine MAPK inhibitors with other targeted agents and/or chemotherapeutic agents for children with LCH.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by St. Baldrick’s Foundation, which supports the North American Consortium for Histiocytosis (Consortium Award; C.E.A., C.R.-G.). Additional grant support was provided by the Leukemia/Lymphoma Foundation (Translational Research Program Award; C.E.A., C.R.-G.), the HistioCure Foundation (Texas Children’s Cancer Center Histiocytosis Program), the American Society of Hematology (Scholar Award in Clinical Research), and the National Cancer Institute, National Institutes of Health, K12 grant (K12CA090433; O.S.E.).
Appendix
The members of the NACHO-LIBRE Study Group are: Daniel J. Zinn, Texas Children’s Cancer and Hematology Centers, Texas Children’s Hospital, Houston, TX, and Division of Pediatric Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX; Erin C. Peckham-Gregory, Texas Children’s Cancer and Hematology Centers, Texas Children’s Hospital, Houston, TX, and Division of Pediatric Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX; Howard Lin, Texas Children’s Cancer and Hematology Centers, Texas Children’s Hospital, Houston, TX, and Division of Pediatric Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX; Michael M. Henry, Center for Cancer and Blood Disorders, Phoenix Children’s Hospital, Phoenix, AZ; Bruce Morland, Oncology Department, Birmingham Children’s Hospital National Health Service (NHS) Foundation Trust, Birmingham, United Kingdom; Carolyn Fein Levy, Steven and Alexandra Cohen Children’s Medical Center, New Hyde Park, NY; Ashish Kumar, Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Deborah Schiff, Department of Pediatrics, University of California–San Diego, La Jolla, CA; Nicole Karras, Department of Pediatrics, City of Hope National Medical Center, Duarte, CA; Roland L. Chu, Division of Hematology/Oncology, Department of Pediatrics, Children’s Hospital of Michigan, Wayne State University, Detroit, MI; Oussama Abla, Division of Haematology/Oncology, Department of Paediatrics, The Hospital for Sick Children, Toronto, ON, Canada; Nobuko Hijiya, Division of Pediatric Hematology Oncology, Ann & Robert H. Lurie Children’s Hospital of Chicago, Northwestern University Feinberg School of Medicine, Chicago, IL; Sun Young Kim, Division of Pediatric Hematology Oncology, Ann & Robert H. Lurie Children’s Hospital of Chicago, Northwestern University Feinberg School of Medicine, Chicago, IL; Patrick K. Campbell, Departments of Oncology and Global Pediatric Medicine, St Jude Children’s Research Hospital, Memphis, TN; Wendy Woods-Swafford, Blank Children’s Cancer and Blood Disorders Center, Des Moines, IA; Lorimar P. Ramirez, Texas Children’s Cancer and Hematology Centers, Texas Children’s Hospital, Houston, TX, and Division of Pediatric Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX; Cristina Fernandes, Bi-Lo Charities Children’s Cancer Center, Children’s Hospital of Greenville Health System, Greenville, SC; Matthew J. Murray, Department of Pathology, University of Cambridge, Cambridge, UK, and Department of Paediatric Haematology and Oncology, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK; Harshal Abhyankar, Texas Children’s Cancer and Hematology Centers, Texas Children’s Hospital, Houston, TX, and Division of Pediatric Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX; Rikhia Chakraborty, Texas Children’s Cancer and Hematology Centers, Texas Children’s Hospital, Houston, TX, and Division of Pediatric Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX; Brooks Scull, Texas Children’s Cancer and Hematology Centers, Texas Children’s Hospital, Houston, TX, and Division of Pediatric Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX; Andrew C. Sher, Division of Pediatric Radiology, Department of Pediatrics, Baylor College of Medicine, Houston, TX; Michelle L. Hermiston, Department of Pediatrics, University of California San Francisco (UCSF), UCSF Benioff Children’s Hospital, San Francisco, CA; Michael D. Hogarty, Division of Oncology, The Children’s Hospital of Philadelphia, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA; Michael B. Jordan, Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Miriam Merad, Department of Oncological Science, Icahn School of Medicine at Mount Sinai, New York, NY; Barrett J. Rollins, Division of Medical Oncology, Dana-Farber Cancer Institute, Brigham & Women’s Hospital, Harvard Medical School, Boston, MA; Kenneth L. McClain, Texas Children’s Cancer and Hematology Centers, Texas Children’s Hospital, Houston, TX, and Division of Pediatric Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX; and Tsz-Kwong Man, Texas Children’s Cancer and Hematology Centers, Texas Children’s Hospital, Houston, TX, and Division of Pediatric Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX.
Contributor Information
Collaborators: Daniel J. Zinn, Erin C. Peckham-Gregory, Howard Lin, Michael M. Henry, Bruce Morland, Carolyn Fein Levy, Ashish Kumar, Deborah Schiff, Nicole Karras, Roland L. Chu, Oussama Abla, Nobuko Hijiya, Sun Young Kim, Patrick K. Campbell, Wendy Woods-Swafford, Lorimar P. Ramirez, Cristina Fernandes, Matthew J. Murray, Harshal Abhyankar, Rikhia Chakraborty, Brooks Scull, Andrew C. Sher, Michelle L. Hermiston, Michael D. Hogarty, Michael B. Jordan, Miriam Merad, Barrett J. Rollins, Kenneth L. Yoshimi, and Tsz-Kwong Man
Authorship
Contribution: C.E.A. and C.R.-G. conceived of the study and managed study design, oversight, and analysis; O.S.E. implemented the study, analyzed data, and drafted the manuscript; J.V. contributed patient data and reviewed the manuscript; and all authors reviewed and approved the final manuscript. Members of the NACHO-LIBRE Study Group participated in study design and analysis, and reviewed and approved the final manuscript.
Conflict-of-interest disclosure: N.H. has served as a consultant for Novartis. The remaining authors declare no competing financial interests.
A complete list of the members of the NACHO-LIBRE Study Group appears in “Appendix.”
Correspondence: Olive S. Eckstein, 1102 Bates St, Ste 1025.21, Houston, TX 77030; e-mail: eckstein@bcm.edu; and Carl E. Allen, 1102 Bates St, Ste 1025.20, Houston, TX 77030; e-mail: ceallen@txch.org.
REFERENCES
- 1.Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379(9):856-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gadner H, Minkov M, Grois N, et al. ; Histiocyte Society. Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood. 2013;121(25):5006-5014. [DOI] [PubMed] [Google Scholar]
- 3.Haupt R, Nanduri V, Calevo MG, et al. Permanent consequences in Langerhans cell histiocytosis patients: a pilot study from the Histiocyte Society-Late Effects Study Group. Pediatr Blood Cancer. 2004;42(5):438-444. [DOI] [PubMed] [Google Scholar]
- 4.Yeh EA, Greenberg J, Abla O, et al. Evaluation and treatment of Langerhans cell histiocytosis patients with central nervous system abnormalities: Current views and new vistas. Pediatr Blood Cancer. 2018;65(1):26784. [DOI] [PubMed] [Google Scholar]
- 5.Badalian-Very G, Vergilio JA, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116(11):1919-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty R, Hampton OA, Shen X, et al. Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis. Blood. 2014;124(19):3007-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond EL, Durham BH, Haroche J, et al. Diverse and targetable kinase alterations drive histiocytic neoplasms. Cancer Discov. 2016;6(2):154-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown NA, Furtado LV, Betz BL, et al. High prevalence of somatic MAP2K1 mutations in BRAF V600E-negative Langerhans cell histiocytosis. Blood. 2014;124(10):1655-1658. [DOI] [PubMed] [Google Scholar]
- 9.McClain KL, Picarsic J, Chakraborty R, et al. CNS Langerhans cell histiocytosis: common hematopoietic origin for LCH-associated neurodegeneration and mass lesions. Cancer. 2018;124(12):2607-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mass E, Jacome-Galarza CE, Blank T, et al. A somatic mutation in erythro-myeloid progenitors causes neurodegenerative disease. Nature. 2017;549(7672):389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veys PA, Nanduri V, Baker KS, et al. Haematopoietic stem cell transplantation for refractory Langerhans cell histiocytosis: outcome by intensity of conditioning. Br J Haematol. 2015;169(5):711-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernard F, Thomas C, Bertrand Y, et al. Multi-centre pilot study of 2-chlorodeoxyadenosine and cytosine arabinoside combined chemotherapy in refractory Langerhans cell histiocytosis with haematological dysfunction. Eur J Cancer. 2005;41(17):2682-2689. [DOI] [PubMed] [Google Scholar]
- 13.Donadieu J, Bernard F, van Noesel M, et al. ; Salvage Group of the Histiocyte Society. Cladribine and cytarabine in refractory multisystem Langerhans cell histiocytosis: results of an international phase 2 study. Blood. 2015;126(12):1415-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berres ML, Lim KP, Peters T, et al. BRAF-V600E expression in precursor versus differentiated dendritic cells defines clinically distinct LCH risk groups [published correction appears in J Exp Med. 2015;212(2):281]. J Exp Med. 2014;211(4):669-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogstad B, Berres ML, Chakraborty R, et al. RAF/MEK/extracellular signal-related kinase pathway suppresses dendritic cell migration and traps dendritic cells in Langerhans cell histiocytosis lesions. J Exp Med. 2018;215(1):319-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haroche J, Cohen-Aubart F, Emile JF, et al. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester disease. J Clin Oncol. 2015;33(5):411-418. [DOI] [PubMed] [Google Scholar]
- 18.Diamond EL, Subbiah V, Lockhart AC, et al. Vemurafenib for BRAF V600-mutant Erdheim-Chester disease and Langerhans cell histiocytosis: analysis of data from the histology-independent, phase 2, open-label VE-BASKET study. JAMA Oncol. 2018;4(3):384-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen Aubart F, Emile JF, Carrat F, et al. Targeted therapies in 54 patients with Erdheim-Chester disease, including follow-up after interruption (the LOVE study). Blood. 2017;130(11):1377-1380. [DOI] [PubMed] [Google Scholar]
- 20.Héritier S, Jehanne M, Leverger G, et al. Vemurafenib use in an infant for high-risk Langerhans cell histiocytosis. JAMA Oncol. 2015;1(6):836-838. [DOI] [PubMed] [Google Scholar]
- 21.Váradi Z, Bánusz R, Csomor J, et al. Effective BRAF inhibitor vemurafenib therapy in a 2-year-old patient with sequentially diagnosed Langerhans cell histiocytosis and Erdheim-Chester disease. OncoTargets Ther. 2017;10:521-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolenová A, Schwentner R, Jug G, et al. Targeted inhibition of the MAPK pathway: emerging salvage option for progressive life-threatening multisystem LCH. Blood Adv. 2017;1(6):352-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azorsa DO, Lee DW, Wai DH, et al. Clinical resistance associated with a novel MAP2K1 mutation in a patient with Langerhans cell histiocytosis. Pediatr Blood Cancer. 2018;65(9):e27237. [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205-216. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz-Hübsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale [published correction appears in Neurology. 2006;67(2):299]. Neurology. 2006;66(11):1717-1720. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Chang MT, McKay D, et al. Allele-specific mechanisms of activation of MEK1 mutants determine their properties. Cancer Discov. 2018;8(5):648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.