Abstract
Nowadays, the use of nanostructures in various medical and biological fields such as drug delivery in cancer treatment is increasing. Among the nanostructures, graphene oxide (GO) is an excellent candidate for drug delivery application because of its unique properties. For more stability, GO can bind with various polymers by its carboxyl, hydroxyl and epoxy functional groups. In this study, firstly GO synthesized by the improved Hummers chemical method and then polyethylene glycol polymer was conjugated to it by using EDC/NHS catalyst. Finally, curcumin (Cur) as anti-cancer drug has been loaded onto the PEGylated graphene oxide (GO-PEG). Next, curcumin loaded onto PEGylated graphene oxide (GO-PEG-Cur) were evaluated by using ultraviolet, Fourier transform infrared spectroscopy, differential scanning calorimeter, atomic microscopic force and dynamic light scattering. The amount of loaded drug was calculated about 4.5% with the help of the standard curcumin curve and UV/Vis spectrometer. Also, the result of release shows that maximum drug release rate for this nanocarrier in pH 5.5 and 7.4 was measured 50% and 60%, respectively, after 96 hours. The results showed that the zeta-potential analysis of GO-PEG-Cur was about -13.9 mV that expresses a negative surface charge for produced nanocarrier.
Keyword: Pharmaceutical chemistry
1. Introduction
Cancer is one of the most serious diseases and responsible for millions mortality every year [1]. Among current cancer treatments, chemotherapy plays a significant role in the curing cancer [2]. However, this method has merits and limitation for treatment by using chemical anticancer drugs which applies side effects to normal cells. To address this problem, some researchers have used nanoparticles such as, liposomes [3], polymeric nanoparticles [4], magnetic nanoparticles [5] and biological nanoparticles [6] as a drug carrier for decreasing side effects.
Among the drug nanocarriers, graphene, a single layer of sp2-hybridized honeycomb carbon atoms with two-dimensional (2-D) sheets, is a new candidate for drug delivery applications [7]. It has some unique properties such as an electron mobility exceeding (15000 cm2 V−1S−1) at room temperature, thermal conductivity about (5000 Wm−1K−1) and a high surface area (2630 m2 g−1) which this surface is higher than other surfaces of nanomaterials used for drug delivery applications [8]. The GO, which is called functional graphene, has functional groups such as the carboxyl, hydroxyl and the epoxy group on the its structure which causes graphene to be stable suspension in water, and be attached to other anti-cancer drugs and biocompatible polymers. To prevent phagocytosis effect of GO by the immune system in body, biocompatible materials should be used. Polyethylene glycol (PEG) due to reduced uptake by reticuloendothelial system and its high aqueous solubility is one of biocompatible polymer used widely in anticancer drug delivery [9]. Curcumin (diferuloylmethane) (Cur) is one of the main components of the spice of turmeric (2–5%) which has antioxidant, anti-inflammatory and anti-cancer properties. It inhibits lipid peroxidation and neutralizes lipid radicals by Diels-Alder reaction as a chain-breaking antioxidant [10].
By changing surface morphology in GO, GO-PEG, GO-Cur-PEG, Palmieri and et al showed PEG and Cur coating on GO by AFM analysis. Besides, Cur release kinetics from graphene oxide coatings was investigated at different time points. lowest release related to GO-Cur with the amount of 1.3 μg/ml after 1 hour and 40 min. while, release of Cur from GO-Cur-PEG was faster than GO-Cur and is about 114 μg/ml after 3 min [11].
Bugli1 and et al at in 2018 showed Cur loaded on graphene oxide is effective antibacterial for methicillin-resistant Staphylococcus aureus (MRSA) which mostly roots skin and soft tissue infections. They showed by measuring the optical density every 15 min Cur bonding has proper ability to GO. While the Cur concentrations were 90, 183 and 273 mg ml–1 the loading efficiency (%) was 91, 91 and 90 respectively [12].
Zhiyuan and et al attached magnetic Iron Oxide nanoparticles (Fe3O4) and paclitaxel (strong chemotherapy drug anti-cancer), to reduced GO (rGO) and showed that this sample had not any toxic effects on normal cells and could inhibit increase the growth of breast cancer cells (MCF-7) [13]. The antibacterial potential of the PEGylated-GOAg nanoparticleCur composite was evaluated by Dubey and Gopinath. They showed an enhanced antibacterial effect compared to various nanoformulations to serve as a perfect wound dressing material. Additionally, they also have shown that PEGylated GO increases the physiological stability of GO. In 2015, Hatamie and et al. coupled curcumin to rGO by π − π interaction. They reported when concentrations are <70 μg/mL in the cell culture medium, there is not significant toxicity and it also led to some cell growths (25% after 48 h incubation time) [14]. In 2010, Yang et al. conjugated polyethylene glycol (PEG) to GO sheets and then injected into mice with intravascular way. They showed that graphene has no toxicity during 40 days by fluorescence imaging from the mice body and it also reduces the effects of the reticuloendothelial system [15].
In this work, GO was synthesized by the Hummers method and then PEG was conjugated to the GO by Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) catalyst to produce strong ester interaction between PEG and GO which is called PEGylated graphene oxide (GO-PEG). Then, to obtain Cur-loaded GO-PEG (GO-PEG-Cur), Cur was loaded onto GO-PEG via π-stacking and was analyzed by fourier transforms infrared (FTIR), differential scanning calorimetry (DSC), atomic microscopic force (AFM) and dynamic light scattering (DLS). Also, amount of loaded drug was calculated about 4.5% by using curcumin standard curve. Finally, maximum drug release rate of PEG-GO-Cur after 96 hours was found to be about 50 and 60 in pH 5.5 and 7.4, respectively. Magnetic Resonance Spectroscopy (MRS) have shown that human tumors were a little alkaline in compare to the normal tissues. Vaupel et al. support the pHMRS findings that most tumors are near neutrality or slightly alkaline like normal tissues [16]. As we know that is first reported about controlled release nanosheet for curcumin delivery around the neutrality and this is proper for human tumors.
2. Results and discussion
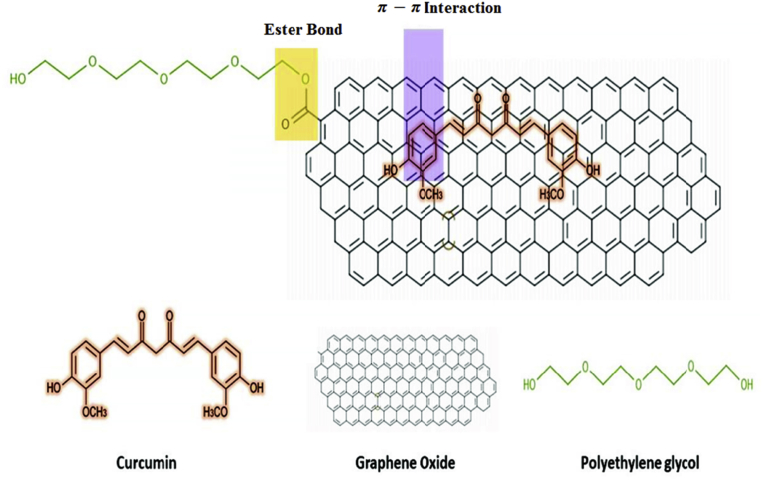
In this section, the achievements of the experiments are illustrated. Characterization of GO, GO-PEG and GO-PEG-Cur were evaluated performed by UV-Vis, FTIR, DSC and AFM. Finally, after analyzing GO-PEG-Cur sample by DLS, the Cur release from drug delivery system prepared was investigated in vitro. Fig. 1 illustrates a simple schematic of GO-PEG-Cur bonding and structure of materials was used in this paper.
Fig. 1.
Schematic structure of GO-PEG-Cur.
2.1. UV-Vis analysis
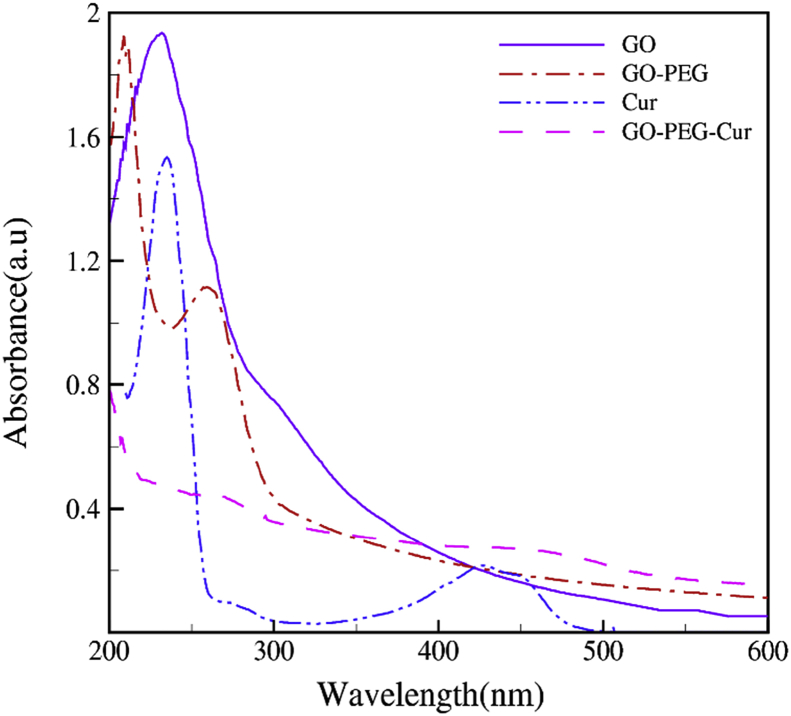
UV-vis spectra of GO, GO-PEG, Cur and GO-PEG-Cur aqueous suspensions dispersions are shown in Fig. 2. The absorptions bands in 231 nm and 307 nm are related to electronic transition π − π∗ for C-C aromatic rings and transition n−π∗ for C=O in GO [17]. The GO-PEG shows a red shift of the plasma peak to 260 nm which further confirms synthesis of PEGylated GO. There are two peaks in 236 nm and 430 nm in Cur spectra. As we know maximum absorption peak for Cur is 420–430 nm in UV-Vis spectra [18]. Consequently, an absorption peak appears at 445 nm reign, characteristic of Cur in the UV-Vis spectrum, which suggest binding of Cur to GO-PEG. Compared to the Cur suspension, the absorption peak for Cur adsorbed on GO-PEG is red-shifted almost 10 nm, most likely causes by π − π∗ interaction between Cur and GO-PEG.
Fig. 2.
UV-vis spectrum for GO, GO-PEG, Cur and GO-PEG-Cur in aqueous dispersions.
2.2. FTIR analysis
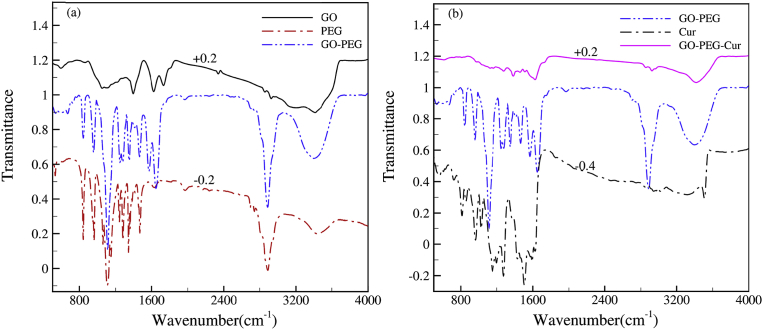
The FTIR spectrum of GO, PEG and GO-PEG has been shown in Fig. 3a. In the FTIR spectrum of GO the broad peak in ∼3417 cm−1 and ∼1735 cm−1 correspond to the stretching vibrations of the O-H, C=O group in carbonyl and carboxyl. In addition, peak in ∼1624 cm−1 is related to C=C and the peaks in ∼1395 cm−1 and ∼1056 cm−1 are attributed to C-O bonds [19]. Besides, the sharp peak in 2889 cm−1, 1630 cm−1 and 1111 cm−1 assign to the stretching vibrations of the C-H, C=O and C-O in PEG FTIR spectrum. Also, peaks in the 1465 cm−1 and 1340 cm−1 region represent the C-H deformation vibrations. In addition, peaks in the 1284 cm−1 and 1242 cm−1 region correspond to the O-H bending vibrations which represents a pure PEG. As it is shown in Fig. 3a, the main functional groups of each substance are almost kept in GO-PEG. The results of FTIR suggest that there are C=O, O-H groups in GO and PEG [20]. Fig. 3b shows the FTIR spectrum of GO-PEG, Cur and GO-PEG-Cur. In curcumin spectrum, the peaks 3507 cm−1 and 3016 cm−1 are attributed to the O-H and C-H stretching vibrations. Peaks 1602 cm−1 and 1505 cm−1 correspond to the C=C symmetric aromatic ring and C=O, respectively. In addition, the absorption peak 1275 cm−1 and 962 cm−1 are related to C-O for enol and benzoate trans-C-H structure of curcumin. By comparing the Cur, GO-PEG and GO-PEG-Cur spectra, it is clear main functional group in every sample exist in final sample GO-PEG-Cur again by UV-Vis spectrum.
Fig. 3.
FTIR spectrum for (a) GO-PEG and (b) GO-PEG-Cur.
2.3. DSC analysis
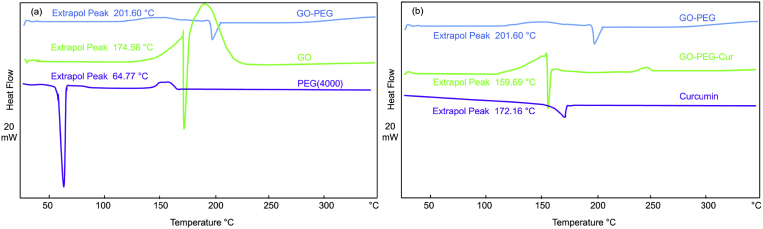
DSC measurements have been further shown to study the thermal properties of the our composites. As shown in the Fig. 4a, The peak of the exotherm at 174.5 °C is related to reduction of GO and The peak 64.7 °C is correspond the melting point for PEG [21, 22]. After PEGylation of GO, a peak appears in 201.6 °C which shows increasing stability of GO by PEG and reduction GO in this term shifted to 201.6 °C.
Fig. 4.
DSC thermograms of (a) GO-PEG and (b) GO-PEG-Cur.
As shown in the Fig. 4b the melting points of curcumin and GO-PEG-Cur are at 172.16 °C and 160 °C, respectively. Curcumin due to the π − π∗ interaction with GO likely acts as an impurity for PEGylated GO and reduced the final temperature point in GO-PEG-Cur sample.
2.4. AFM analysis
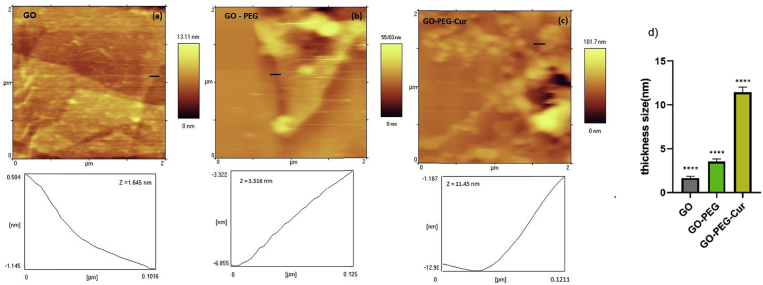
AFM images are a proper choice to determine the morphology of GO, GO-PEG and GO-PEG-Cur surface. AFM height profile in Fig. 5a clearly shows that GO sheets have the thickness of around 1.65 nm that confirms the monolayer GO sheets were prepared. After pegylation of GO that inhibits immediate aggregation of GO suspension in salt and biological solutions, the analysis AFM of pegylated GO was performed. As shown in Fig. 5b the thickness of GO-PEG sample measured by AFM image is the around 3.52 nm. Generally, thickness of PEGylated GO is less than 10 nm [23], so the resulting morphology from both samples confirms the conjugation of PEG onto GO sheets. A noticeable geometry deformation in Fig. 5c also shows that Cur has strongly affect in topography of GO-PEG surface. The AFM analysis of the final sample (GO-PEG-Cur) exhibits a value of Z = 11.45 nm for thickness. It is clear that GO-PEG-Cur surface is too roughness apparently and is not as flat as GO and GO-PEG surface. Maximum thickness reported for nano disk curcumin is 7.22 nm [24]. Then, by regarding to thickness for AFM image results can be concluded that Cur has been immobilized on GO-PEG. As shown in Fig. 5d showed significant difference between GO, GO-PEG, GO-PEG-Cur.
Fig. 5.
Topography surface by AFM for (a) GO, (b) GO-PEG and (c) GO-PEG- Cur, (d) Bars marked with **** (p < .0001) showed significant difference between GO, GO-PEG, GO-PEG-Cur.
2.5. Zeta potential analysis
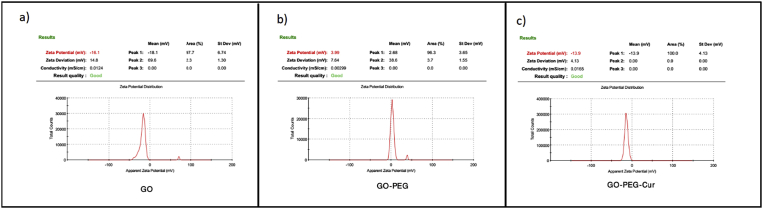
Surface charge can have significant role in the determination of the protein adsorption and cellular interactions in the physiological system. The phagocytes such as macrophages react more strongly to positive charged NPs than to negative charged NPs. The zeta potential of NPs was determined by DLS by using Smoluchowski equation. Zeta potential for GO and pegylated GO are −16.1 and 3.99, respectively as shown in the Fig. 6 the zeta potential for GO-PEG-Cur is about −13.9, which represents our nanocarrier has a highly negative surface charge. Therefore, it seems our formulation has properly potential for using in drug delivery due to delaying phagocyte process in blood circulation.
Fig. 6.
Zeta potential analysis for the a) GO = −16.1, b) GO-PEG = 3.99, c) GO-PEG-Cur = −13.9.
2.6. Investigation of drug release in neutral and acidic environments
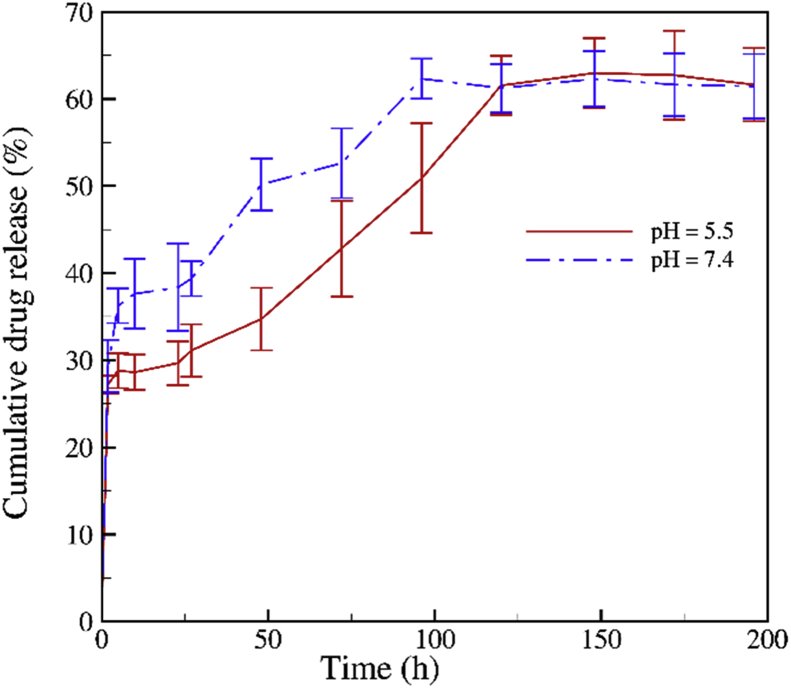
The release behavior of curcumin drug from pegylated GO was investigated in two buffering environments pH 5.5 and 7.4. We found that ∼50% of Cur loaded on nanocarrier was released after 96 hours in an acidic solution of pH 5.5 (see Fig. 7). As shown in Fig. 7, the release rate reduces when pH was adjusted to 7.4 and begin 60% after 96 hours. Then, our results show curcumin has tendency be released in base environments than acidic environments. This allows us to freely control the drug in both pH and have properly time for bringing the drugs to destination. Magnetic Resonance Spectroscopy (MRS) have shown that human tumors were a little alkaline in compare to the normal tissues. Vaupel et al. supported the pHMRS findings that most tumors are near neutrality or slightly alkaline like normal tissues [25]. Therefore, the pH-dependent anti-cancer drug release from pegylated GO can be exploited for drug delivery applications. By comparing the release rate of curcumin with other anti-cancer drugs such as doxorubicin (DOX) [26] and also owing to very low citoxity of curumin and GO at low concentrations, it is expected that designed nano drug (GO-PEG-Cur) can be candidated in treatment of human cancer cells.
Fig. 7.
Cumulative amount of curcumin released from GO-PEG sample at two different pH (acidic and base environments).
3. Conclusion
In this research, the modified Hummer method was synthesized for producing GO sheets and characterized. PEGylation of GO was performed using EDC/NHS catalyst and then curcumin was immobilized onto PEGylated GO sheets. Subsequently, our samples were evaluated by FTIR, Uv-Vis AFM spectroscopies and DSC and DLS analyzers. The results of analyses suggest that PEG polymers are conjugated to GO by esterification bonding. Also, curcumin as anti-cancer drug was loaded onto PEGylated GO sheets by interaction and the amount of its loading was about 4.5%. Moreover, the measurements of surface charge by zeta potential analyzer for GO-PEG-Cur sample indicates the value of −13.9%. Owing to the value and sign of surface charges phagocytic activity in blood circulation will be delayed. This suggests that our designed nanocarrier is a biocompatible material and can be employed in drug delivery systems, both in vitro and in vivo. Finally, the results of release confirmed that drug released depends on pH and the amount of curcumin released form PEGylated GO is more in base environment. It was found that 50% of curcumin immobilized on nanocarrier was released after 96 hours in pH 5.5, while for pH 7.4 is 60%.
4. Experimental
4.1. Materials and methods
Graphite was purchased from Asbury Graphite Mills in US and Ethyl-3-(3- dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS), PEG (Mn:4000) and curcumin (Cur) were purchased from Sigma Chemical (St, Louis, MO). PBS was provided in our laboratory. Other chemicals were analytical grade and directly used without further purification.
4.2. Synthesis of GO
GO was synthesized using modified hummers methods [27]. Briefly, 0.3 g graphite (Asbury Graphite Mills, US) powder was added into 35 ml sulfuric acid (H2SO4) (Merck 97%). Then, this mixture was stirred (1200 rpm, 25 °C) for 51 min. In following, 0.09 g permanganate potassium (KMnO4) was added gradually into mixture which was placed in ice bath. The mixture was then stirred (1200 rpm, 40 °C) for 2 hours to turn its color to green. The mixture was placed into ice bath again and then was diluted with 900 ml deionized (DI) water to change its color to brown. After that, 4.5 ml hydrogen peroxide (H2O2) was slowly added to the mixture for releasing hydrogen gas. The obtained suspension was centrifuged (4000 rpm, 25 °C) and washed with deionized water to reach pH 5. Finally, the obtained graphite oxide suspension was sonicated (EURANDA model 4D, ITALY) for 30 minutes at 40 kHz and dried in oven (40 °C,24 h) to produce GO.
4.3. Synthesis of PEG-functionalized GO (PEGylated GO)
For PEGylation of GO, esterification reaction between carboxylic acid group of GO and hydroxyl group of PEG should be done. For this purpose, carboxyl group should be increased in GO sheets. Thus, 20 mL of sodium hydroxide (120 mg/mL) was added to 20 ml GO suspension (2 mg/ml). After sonication for 4 hours, 3 ml hydrochloric acid (HCl) was added to reduce the pH of the suspension. Then, it was centrifuged (Eppendorf AG 22331 Hamburg, Germany) twice (8000 rpm, 25 °C) for 15 minutes to produce GO carboxylic acid (GO-COOH). In order to activate the carboxylic acid group in GO, catalysts, including 400 mg EDC and 240 mg NHS were added to suspension and stirred for 24 hours. For PEGylation of GO, 1500 mg PEG4000 added to the above suspension and stirring continued for 24 hours. Finally, the suspension was washed with DI water by centrifugation (8000 rpm, 25 °C) for 30 minutes. In this way, PEG was conjugated to GO and the resulting sample (GO-PEG) was evaluated by FT-IR, DSC and AFM.
4.4. Loading of curcumin on PEGylated GO
For loading Cur on PEGylated GO, 2.250 mL of the Cur solution (3.33 mg/ml) was added to aqueous 7 ml GO-PEG suspension (4.28 mg/mL) and stirred for overnight. Cur rate in compare to PEGylated GO was about 1:4. The suspension was centrifuged (12000 rpm, 25 °C) for 75 minutes and dried for 48 hours at 40 °C in the oven to obtain the final sample which is called curcumin-bonded PEGylated GO (GO-PEG-Cur). The GO-PEG-Cur was evaluated by FT-IR, DSC and AFM. Fig. 1 illustrates a simple schematic of GO-PEG-Cur bonding and structure of materials was used in this paper. After preparation of final sample, 2.39 mg (GO-PEG-Cur) was added to 1.5 ml acetone and shacked in incubator for 46 h and then centrifuged (30 min, 1200 rpm). after that the amount of supernatant Cur release was measured by UV spectrophotometer at 428 nm, and then by Cur standard curve and Eq. (1), Cur loading was achieved 4.5%.
| (1) |
4.5. In vitro release of curcumin anti-cancer drug
After the loading of Cur onto GO-PEG, drug release was evaluated in Phosphate-buffered saline (PBS), at pH 5.5 and 7.4 which represent cancer cells pH and blood pH, respectively. To prepare PBS solution, 8 g sodium chloride (NaCl), 0.2 g potassium chloride (KCl), 1.44 g disodium hydrogen phosphate (Na2HPO4), 24 g potassium dihydrogen phosphate (KH2PO4) and 20 mL tween (2%) were added in 980 mL DI water. HCl was used for decrease pH of PBS to 5.5, and then, 2 mg/mL of the GO-PEG-Cur were transported to a dialysis tube with 12 kDa molecular weight cut off. The dialysis tube was filled with 20 mL of the release medium and incubated with a shaker incubator (37 °C, 100 rpm) gently, and the drug molecular diffusing out of the tube are sampled for analysis. At predetermined time intervals, 2 mL of the release medium was kept and replaced with the same amount of fresh release medium to maintain sink conditions.
5. Instrumentation
5.1. UV-visible analysis
The analysis of GO, GO-PEG, Cur and GO-PEG-Cur aqueous were carried out by using Uv-Visible (Thermo electron corporation model NO Gensys 10.s) spectrum in the wavelength range 200–600 nm. Every sample was diluted by 1.5 mL DI water and kept in quarts' cell.
5.2. FTIR analysis
The chemical structure of samples was characterized by fourier transform infrared spectroscopy (FT-IR) (Bruker, Tensor 27, Biotage, Germany). Transparent pills were made by mixing and grinding 2 mg of selected samples with 200 mg of white powder KBr and then compressing the powder (pressure, 12 Ton). The FTIR spectra of the KBr pill can record broad range between 400 to 4000 cm−1. Each spectrum was obtained using 16 scans.
5.3. Differential scanning calorimetry (DSC)
DSC analysis is one of the methods for the determination of thermal variations. The thermal properties of GO, PEG and the freeze-dried powder of GO-PEG, GO-PEG- Cur were investigated utilizing a DSC apparatus (Mettler Toledo, model Star SW 9.30, Schwerzenbach, Switzerland). Our samples were evaluated with a heating rate of 15 °C/min at temperature 30–400 °C.
5.4. Morphology study by atomic force microscopy (AFM)
The morphology of the nanosheets was observed by using atomic force microscopy (AFM) (Ara research Co, Nano Expert, model No. 0101/A, Iran). One droplet of GO suspension (5 μL with the concentration of 0.1 mg/mL) was dropped on a freshly cleaved mica substrate (1 cm2) and air-dried (drop-casting). AFM analysis was performed in contact AFM mode.
5.5. Zeta potential evaluation by DLS
Surface charge of nanoparticles (NPs) was carried out by DLS. We used Malvern Instruments model NO. Zen 3600 Zetasizer. GO-PEG-Cur aqua was diluted with DI water and put it in zeta cell. Each spectrum was obtained using 25 scans. This analysis 3 times is repeated.
5.6. Statistical analysis
One way anvoa was used for statistical analysis of data.
Declarations
Author contribution statement
Jalil Charmi: Conceived and designed the experiments; Performed the experiments.
Hamed Nosrati: Performed the experiments; Analyzed and interpreted the data.
Jafar M. Amjad: Contributed reagents, materials, analysis tools or data.
Ramin Mohammadkhani: Conceived and designed the experiments.
Hosein Danafar: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Hamed Nosrati, Email: hnosratti@gmail.com, nosrati.hamed@zums.ac.ir.
Ramin Mohammadkhani, Email: rmkhani@znu.ac.ir, ramin6072@gmail.com.
Hosein Danafar, Email: danafar@zums.ac.ir.
References
- 1.Islami F., Goding Sauer A., Miller K.D., Siegel R.L., Fedewa S.A., Jacobs E.J., McCullough M.L., Patel A.V., Ma J., Soerjomataram I. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA: Cancer J. Clin. 2018;68(1):31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 2.Bansal R., Acharya P.C. Man-made cytotoxic steroids: exemplary agents for cancer therapy. Chem. Rev. 2014;114(14):6986–7005. doi: 10.1021/cr4002935. [DOI] [PubMed] [Google Scholar]
- 3.Pattni B.S., Chupin V.V., Torchilin V.P. New developments in liposomal drug delivery. Chem. Rev. 2015;115(19):10938–10966. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 4.Roque L., Castro P., Molpeceres J., Viana A.S., Roberto A., Reis C., Rijo P., Tho I., Sarmento B., Reis C. Bioadhesive polymeric nanoparticles as strategy to improve the treatment of yeast infections in oral cavity: in-vitro and ex-vivo studies. Eur. Polym. J. 2018;104:19–31. [Google Scholar]
- 5.Manjili, Kheiri Hamidreza, Ma’mani Leila, Tavaddod Sharareh, Mashhadikhan Maedeh, Shafiee Abbas, Naderi-Manesh Hossein. D, L-sulforaphane loaded Fe3O4@ gold core shell nanoparticles: a potential sulforaphane delivery system. PloS one 11, no. 2016;3:e0151344. doi: 10.1371/journal.pone.0151344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jahanban-Esfahlan Ali, Dastmalchi Siavoush, Davaran Soodabeh. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int. J. Biol. Macromol. 2016;91:703–709. doi: 10.1016/j.ijbiomac.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 7.Kakran M., Sahoo N.G., Bao H., Pan Y., Li L. Functionalized graphene oxide as nanocarrier for loading and delivery of ellagic acid. Curr. Med. Chem. 2011;18(29):4503–4512. doi: 10.2174/092986711797287548. [DOI] [PubMed] [Google Scholar]
- 8.Chyada F.A., Jabur A.R., Alwan H.A. Effect addition of graphene on electrical conductivity and tensile strength for Recycled electric power transmission wires. Energy Procedia. 2017;119:121–130. [Google Scholar]
- 9.Banerjee S.S., Aher N., Patil R., Khandare J. Poly (ethylene glycol)-prodrug conjugates: concept, design, and applications. J. Drug Deliv. 2012:2012. doi: 10.1155/2012/103973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilken R., Veena M.S., Wang M.B., Srivatsan E.S. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Canc. 2011;10(1):12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmieri V., Bugli F., Cacaci M., Perini G., Maio F.D., Delogu G., Torelli R., Conti C., Sanguinetti M., Spirito M.D. Graphene oxide coatings prevent Candida albicans biofilm formation with a controlled release of curcumin-loaded nanocomposites. Nanomedicine. 2018;13(22):2867–2879. doi: 10.2217/nnm-2018-0183. [DOI] [PubMed] [Google Scholar]
- 12.Bugli F., Cacaci M., Palmieri V., Di Santo R., Torelli R., Ciasca G., Di Vito M., Vitali A., Conti C., Sanguinetti M. Curcumin-loaded graphene oxide flakes as an effective antibacterial system against methicillin-resistant Staphylococcus aureus. Interface Focus. 2018;8(3):20170059. doi: 10.1098/rsfs.2017.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao M.-h., Ma M., Chen Y., Jia X.-q., Xu G., Xu H.-x., Chen H.-r., Wu R. Multifunctional Bi2S3/PLGA nanocapsule for combined HIFU/radiation therapy. Biomaterials. 2014;35(28):8197–8205. doi: 10.1016/j.biomaterials.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Hatamie S., Akhavan O., Sadrnezhaad S.K., Ahadian M.M., Shirolkar M.M., Wang H.Q. Curcumin-reduced graphene oxide sheets and their effects on human breast cancer cells. Mater. Sci. Eng. C. 2015;55:482–489. doi: 10.1016/j.msec.2015.05.077. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y., Asiri A.M., Tang Z., Du D., Lin Y. Graphene based materials for biomedical applications. Mater. Today. 2013;16(10):365–373. [Google Scholar]
- 16.Griffiths J.R. Are cancer cells acidic? Br. J. Canc. 1991;64:425–427. doi: 10.1038/bjc.1991.326. PMID:1911181, PMCID: PMC1977628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai Q., Zhu S., Luo X., Zou M., Huang S. Ultraviolet-visible spectroscopy of graphene oxides. AIP Adv. 2012;2(3) [Google Scholar]
- 18.da Silva-Buzanello R.A., Ferro A.C., Bona E., Cardozo-Filho L., de Araújo P.H.H., Leimann F.V., Gonçalves O.H. Validation of an Ultraviolet–visible (UV–Vis) technique for the quantitative determination of curcumin in poly (l-lactic acid) nanoparticles. Food Chem. 2015;172:99–104. doi: 10.1016/j.foodchem.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Kumar N., Das S., Bernhard C., Varma G. Effect of graphene oxide doping on superconducting properties of bulk MgB2. Supercond. Sci. Technol. 2013;26(9) [Google Scholar]
- 20.Wang C., Feng L., Yang H., Xin G., Li W., Zheng J., Tian W., Li X. Graphene oxide stabilized polyethylene glycol for heat storage. Phys. Chem. Chem. Phys. 2012;14(38):13233–13238. doi: 10.1039/c2cp41988b. [DOI] [PubMed] [Google Scholar]
- 21.Glover A.J., Cai M., Overdeep K.R., Kranbuehl D.E., Schniepp H.C. In situ reduction of graphene oxide in polymers. Macromolecules. 2011;44(24):9821–9829. [Google Scholar]
- 22.Dabbagh A., Mahmoodian R., Abdullah B.J.J., Abdullah H., Hamdi M., Abu Kasim N.H. Low-melting-point polymeric nanoshells for thermal-triggered drug release under hyperthermia condition. Int. J. Hyperth. 2015;31(8):920–929. doi: 10.3109/02656736.2015.1094147. [DOI] [PubMed] [Google Scholar]
- 23.Chen M.-L., Shen L.-M., Chen S., Wang H., Chen X.-W., Wang J.-H. In situ growth of β-FeOOH nanorods on graphene oxide with ultra-high relaxivity for in vivo magnetic resonance imaging and cancer therapy. J. Mater. Chem. B. 2013;1(20):2582–2589. doi: 10.1039/c3tb20234h. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh M., Singh A.T., Xu W., Sulchek T., Gordon L.I., Ryan R.O. Curcumin nanodisks: formulation and characterization. Nanomed. Nanotechnol. Biol. Med. 2011;7(2):162–167. doi: 10.1016/j.nano.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 26.Sun X., Liu Z., Welsher K., Robinson J.T., Goodwin A., Zaric S., Dai H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008;1(3):203–212. doi: 10.1007/s12274-008-8021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcano D.C., Kosynkin D.V., Berlin J.M., Sinitskii A., Sun Z., Slesarev A., Alemany L.B., Lu W., Tour J.M. Improved synthesis of graphene oxide. ACS Nano. 2010;4(8):4806–4814. doi: 10.1021/nn1006368. [DOI] [PubMed] [Google Scholar]