Abstract
Background
Although general guidelines are available for established silicone gel breast implants, the unique characteristics of the latest Motiva implants warrant specific guidelines.
Objectives
This study aimed to generate consensus recommendations and summarize expert-based advice to better understand current surgical practices and to establish guidelines for surgeons transitioning from other implant devices to the Motiva implants.
Methods
A survey was compiled by 12 plastic surgeon experts in aesthetic and reconstructive breast surgery and 1 biotechnology scientist, and distributed to 36 plastic surgeons to establish a consensus on the use of these devices. Surgical techniques, complication rates, and implant selection were among the topics discussed.
Results
The experts agreed on 3 core principles regarding the use of Motiva Round and Ergonomix implants. Firstly, the dissected pocket needs to be close fitting and steps must be taken to prevent expansion of the pocket. Secondly, implant selection must be individualized. Finally, surgical planning and technique must be carefully considered. When questioned about problems they had ecountered, 84.6% of the experts agreed that they experienced fewer overall complications and 76.9% confirmed reduced capsular contracture rates with these devices. Overall, 84.6% of the experts favored selecting Motiva Ergonomix implants over Round implants to achieve a more natural look. In addition, 92.3% of the experts agreed that Motiva implants, due to their innovative technology, reduce the risk of anaplastic large-cell lymphoma.
Conclusions
This international consensus of leading practitioners will assist plastic surgeons with patient selection, preoperative planning, and surgical technique. These recommendations are designed to optimize surgical outcomes, resulting in lower overall complication rates, more natural-looking breasts, and highly satisfied patients.
Scientific consensus is defined as the collective judgment, position, and opinion of a cohort of specialists considered experts in their respective fields by their peers. This definition clearly implies general agreements that might not necessarily be unanimous.
When a group of experts reach a consensus, they can issue scientific position statements intended to communicate a summary of the science from the "inside" to the "outside" of the scientific community. These lead to a situation in which those within the discipline can often recognize such a consensus where it exists. In cases where there is little controversy regarding the subject under study, establishing what the consensus is can be quite straightforward.
Notwithstanding, it is clearly recognized that most models of scientific change rely on new data produced by scientific experiments. However, since Karl Popper proposed that because no amount of experiments could ever entirely and unequivocally prove a scientific theory, but a single experiment can disprove one, science should be based on falsifiability—in other words any theory can be falsifiable if it is contradicted by a basic statement, which, in a successful or failed falsification, must correspond to an observation. This concept would make it almost impossible for science to advance as it presents severe obstacles to new concepts. Although Popper’s falsifiability approach constitutes a logical theory for the practice of science, it does not necessarily reflect a view on how science should actually progress over time. This is exactly where expert consensuses are fundamental as they serve as guidance to new theories and help to move science forward. The mere perception of whether a scientific consensus exists on a given issue, and how strong that conception is, has been described as a "gateway belief" upon which other beliefs and then actions are based. Surgeons will rely on experts and their vast experience until they learn from their own observations and prove their own theories inside their practices.
In 2011, the first of what the authors consider to be the sixth generation of breast implants, incorporating Motiva’s bioengineered SmoothSilk/SilkSurface biocompatible implant shell surfaces, was released globally by Establishment Labs (Coyol Free Zone, Alajuela, Costa Rica). Motiva uses three-dimensional (3D) surface imprinting to produce a controlled, nanoscale-structured, cell-friendly “smooth surface.”1,2 The surface topography is engineered via a 3D inverted imprinting technology to create a uniform and controlled surface with a very low roughness (average 3200 ± 600 nm) that is designed to influence cell behavior to minimize the chronic inflammatory foreign body response.3 Inflammatory cells can recognize the topographic landscape but the surface is not rough enough to cause friction with the surrounding tissues. The evolution of silicone gel–filled breast implants, as noted by Maxwell and Gabriel,4 has been based on stringent analysis of their mechanical properties and safety led by the American Society for Testing and Materials (ASTM) and the Food and Drug Administration (FDA). Technologic advances around gel cohesiveness and various aspects of the shell have played an important role in the gradual improvement of breast protheses over the past decades.
Compared with their fifth-generation predecessors, the surfaces of Motiva breast implants offer a more biocompatible topography. These implants also combine enhanced rheologic properties with a 100% gel filling, which confers distinctive properties, mimicking the natural dynamics of breast tissue and thereby diminishing complications linked to the performance of previous-generation implants (eg, chronic inflammation, stiffness).
Moreover, these implants bring a series of innovations that differentiate them from implants produced by other manufacturers: a visible barrier layer, a directly produced nanoscale smooth surface, and an optional radiofrequency identification device to ensure full traceability. Safety is, therefore, also enhanced.
On a clinical level, this surface reduces complications such as capsular formation and contracture, and consequently reoperation rates, to <1%.1 In fact, a single-center study on 5813 Motiva breast augmentation cases found no Baker grade III/IV contracture.1 Minimizing the chronic inflammation that precedes breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) may also, theoretically, reduce the risk of developing this uncommon disease.5,6
The Motiva Round implant is filled with a proprietary gel, ProgressiveGel PLUS, which is a soft gel with moderate rigidity designed to balance the relationship between viscoelastic and elastic deformation, and thereby maintain the round breast upper pole fullness shape.
The Ergonomix breast implant is a “dynamic” anatomic implant. It is filled with ProgressiveGel Ultima, a highly elastic gel possessing low rigidity and superior adaptability that in the authors’ opinion gives a more natural look and feel to the breast. Furthermore, the gel and shell binding have the same elastic properties, enabling them to act as a single body structure.
This paper summarizes the findings of a consensus survey by renowned experts that aimed to understand current surgical practices in order to establish guidelines for surgeons who wish to transition from other implant types to the Motiva implants.
METHODS
On September 16, 2017, Establishment Labs convened a meeting of 12 plastic surgeons (“Experts”) and 1 biotechnology scientist at Lake Garda, Italy, to discuss the use of Motiva Round and Ergonomix implants. The panel included plastic surgeons practicing in Australia, Brazil, the United Kingdom, the United States, Spain, Sweden, and Italy. These Experts had significant experience in breast augmentation surgery, with at least 10 years of practice and over 2 years of experience involving the implantation of at least 50 Motiva devices.
The survey was performed in 2 stages. First, the Experts led a discussion on the various surgical techniques and issues relevant to these implants. Based on this discussion, a questionnaire was designed, refined by N.C., and electronically delivered to 36 plastic surgeons (also “Experts”) via Survey Monkey (SurveyMonkey Inc., San Mateo, CA; www.surveymonkey.com). Surgeons who did not have at least 2 years of experience with the implants were ineligible for inclusion, even if they were regarded as “experts.” The questions are shown in Appendix A (available online as Supplemental Material at www.aestheticsurgeryjournal.com) and required a multiple-choice response; none required commentary. Six main topics were addressed: physician experience, Motiva implant complications, Ergonomix selection factors and clinicians’ experiences, Ergonomix clinical outcomes, Ergonomix infection prevention, and Ergonomix overall clinical experience. The survey was disseminated to recipients on March 30, 2018; reminders were sent approximately every 2 to 4 weeks and the survey finally closed on July 2, 2018. Recipients were asked for their identities although this was not mandatory, and they could opt out of the survey. The results were compiled using SurveyMonkey’s native spreadsheet and charting functions. A consensus was reached when >75% of respondents agreed with a response, a strong consensus was reached if >90% agreed, and absolute consensus was reached when all respondents agreed.
RESULTS
In the first part, the Experts agreed on 3 core principles on the use of Motiva Round and Ergonomix implants.
The dissected pocket needs to be close fitting and must be prevented from expanding. Because very thin capsules are formed, undesirable expansion of the pocket may occur. Precise dissection of this pocket is a critical surgical step. Patients must minimize implant movement during the early postoperative period to maintain the dissected pocket boundaries. Three months of breast support should be stipulated, and patients must be educated about the importance of this element of the process.
Implant selection. Selection of the appropriate implant is based on the anatomy and desires of each patient. A “round” breast appearance with a fuller upper pole is best achieved with a Round implant with ProgressiveGel PLUS. A more anatomically natural look requires an Ergonomix implant with ProgressiveGel Ultima.
The differences between surgical planning and techniques when using anatomic or smooth implants must be understood. Surgeons trained to use only anatomic implants must apply some of the principles used with smooth, round implants when using an Ergonomix.
In the second part, 19 surgeons responded to survey invitations; 13 completed all questions in full. Overall, the surgeons who responded were highly experienced (Figure 1)— over 84% had performed breast augmentations for at least 15 years. In general, but not related exclusively to Motiva implants, half of the Experts stated that they follow their patients regularly for an average of >5 years, while a third do so for 3 to 5 years. More than half of the surgeons had used Motiva implants for >3 years, and over a third for 1 to 2 years. This group therefore had both depth and range of clinical knowledge, experience, and history with these implants.
Figure 1.
How many years have you been performing breast augmentations?
Overall, 76.9% (consensus) of the surgeons agreed that using Motiva implants reduced capsular contracture rates (Figure 2), whereas 84.6% agreed that it led to many fewer overall complications. Over half (53.9%) agreed that the rupture rates of Motiva implants were lower.
Figure 2.
In your experience, are your capsular contracture rates reduced with the use of Motiva implants?
Consensus was reached on the choice of an Ergonomix over a Round implant to achieve a more natural anatomic look (84.6%; Figure 3), and the majority (69.2%) also would do this to achieve a softer breast. Most surgeons (69.2%) would place the Ergonomix at a slightly higher position than would be planned for a smooth Round device, whereas the same proportion would also consider placing the Ergonomix slightly higher than planned for a textured Round device; 61.5% would place an Ergonomix a little higher than planned for an anatomic device. Over half (53.9%) would fix the inframammary fold (IMF) with sutures, whereas only 23% would use a flap. A closed and tight pocket would be dissected by most surgeons (69.2%).
Figure 3.
For what indications would an Ergonomix be chosen over a Round implant?
There was an absolute consensus (100%) that the Ergonomix produces softer breasts, and 84.6% agreed that it leads to less capsular contracture. Nearly all surgeons (92.3%, strong consensus) found they were able to prevent any displacement problems through accurate implant pocket dissection.
With respect to their overall clinical experience, 92.3% (strong consensus) transitioned to Motiva implants due to the technology of these devices (Figure 4). A majority (76.9%, consensus) cited softness and safety (61.5%) as reasons for their switch, and a few were influenced by marketing.
Figure 4.
Why did you transition to Motiva implants?
Fewer than half of the experts (46.2%) actually implement the 14-point plan to reduce biofilm (Figure 5). Over one-third (38.5%) do not routinely irrigate the tissue pocket with Betadine (povidone-iodine) or antibiotic solutions, whereas a further third (30.8%) use Betadine, and far fewer (15.4%) surgeons use either triple antibiotics or both triple antibiotics and Betadine.
Figure 5.
Do you implement the 14-point plan to reduce biofilm when using these implants?
Over half (53.9%) of the surgeons reported positive experiences when placing the Ergonomix via a transaxillary approach or periareolar approach. Over three-fourths (76.9%, consensus) noted positive experiences when placing the Ergonomix in the subpectoral plane, and over half (53.9%) reported positive experiences when using the subglandular plane.
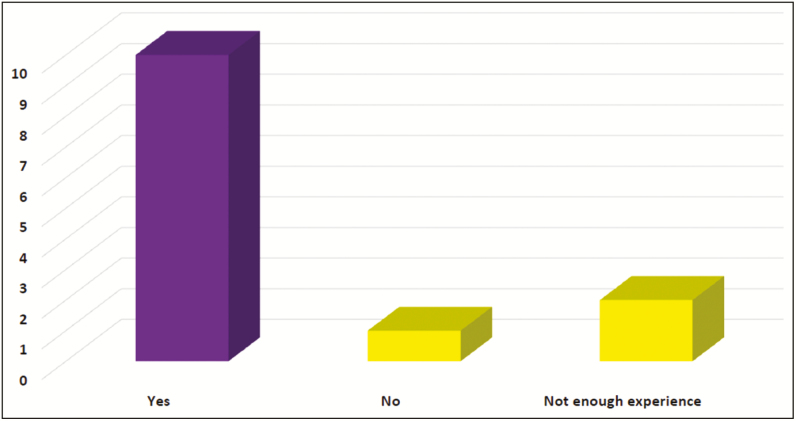
There was a strong consensus, 92.3%, among the experts that these Motiva implants may reduce the risk of BIA-ALCL6 (Figure 6).
Figure 6.
Do you think these implants may reduce the risk of ALCL?
All areas of consensus are summarized in Table 1.
Table 1.
Summary of Consensus Statements
| Statements of consensus | Consensus level |
|---|---|
| Motiva implants reduced capsular contracture rates | 77%—agreement |
| Motiva implants led to many fewer overall complications | 85%—agreement |
| With regards to double capsules, the implant should be replaced | 100%—absolute |
| An Ergonomix implant, rather than a Round implant, should be chosen to achieve a more natural look | 84.6%—agreement |
| The Ergonomix implant settles into the tissue pocket | 84.6%—agreement |
| The Ergonomix could be placed in the submuscular plane | 100—absolute |
| The Ergonomix implant produced softer breasts | 100%—absolute |
| The Ergonomix implant produced less capsular contracture | 84.6%—agreement |
| Pocket stretching and implant displacement was a disadvantage | 76.9%—agreement |
| Ergonomix reduced the risk of ALCL | 92.3%—strong |
| Nearly all surgeons would prevent these problems by accurately dissecting the implant pocket | 92.3%—strong |
| Technology was the main reason for transitioning to Motiva implants | 92.3%—strong |
| Softness was the main reason for transitioning to Motiva implants | 76.9%—agreement |
| The Ergonomix implant led to positive experiences in breast augmentation or mastopexy procedures | 76.9%—agreement |
| The Ergonomix implant led to positive experiences when placing it in the subpectoral plane | 76.9%—agreement |
| Surgeons are not more likely to do a dual-plane release with an Ergonomix implant than they would with any other implant | 84.6%—agreement |
ALCL, anaplastic large-cell lymphoma.
Levels of consensus were: agreement with statement, 75%-84%; strong agreement with statement, 85%-99%; absolute agreement, 100%.
DISCUSSION
To reach a consensus among plastic surgery experts on the best clinical and surgical practices for Motiva Round and Ergonomix implants, a formal discussion was convened, and a survey was conducted. Consensus recommendations were compiled by extracting expert opinions from the survey and collecting personal experiences and opinions via discussions. The authors understand that the final cohort of Experts was not large, and this poses a limitation to their conclusions, although this is compensated by the stature these Experts have acquired in the course of their professional and academic careers. The statements and recommendations in this document therefore pertain only to surgeons who currently implant Motiva Round and Ergonomix devices. Surgeons implanting other devices may find this content to be useful to their practice but should note that these recommendations only reflect the preferences of this current group. Alternative approaches with these devices may exist but were not captured due to the precise nature of the responses required.
Planning and Implant Selection
Lower Pole Expansion
The surgeons felt that the high-elasticity Ergonomix often stretches the lower pole. However, because this can give a very natural aesthetic appearance, this expansion may be desirable. There was strong agreement among the Experts that the Ergonomix “settles” and descends from its initial implanted position, but the time at which this occurs varied between a period of at least 6 weeks and 6 to 12 months. Slightly higher implant placement should be considered, to account for tissue elasticity facilitating implant settling and to manage the problem of descent due to gravity. This descent may be beneficial to patients with a constricted lower pole as it allows expansion into a surgically released constriction. Conversely, physicians must proceed carefully when performing vertical closure, or when there is tissue laxity after breastfeeding or massive weight loss.
IMF
A conservative approach is critical here—the IMF should be left untouched and/or completely fixed. If required, lowering of the IMF is best done conservatively and the implant should be precisely positioned at the fold. Regardless, proper patient assessment, understanding of the desired upper pole appearance, and counseling on implant volumes will enable the selection of a device that does not require IMF lowering.
Surgical Technique Considerations
Incision
The Ultima gel and Monobloc technology make insertion through a small incision easier. The Experts agreed that these implants can be readily placed via an axillary or periareolar approach.
Biofilm Amelioration
Fewer than half of the surgeons use the 14-point plan (46%), whereas one-third do not use it at all, and the rest use a modified version (23%). There was no consensus on using antibiotic and/or Betadine in the pocket or for implant dipping, with surgeons split between using Betadine only, triple antibiotics only, or both or none of these. Good surgical technique is recommended at all times; however, the group recommended that the rigid 14-point plan should be used as per an individual surgeon’s discretion and as a personal preference only. These conclusions were supported by the work of James et al7 which demonstrated that the surfaces of Motiva implants aggregate less biofilm than a textured device, or smooth implants for that matter.
IMF Fixation
There was consensus that the IMF should be fixed after implant placement, either with sutures or a local flap depending on the surgeon’s preference. Educational materials decrypting IMF fixation techniques will be freely given to physicians. The Sforza technique is an example of a simple and efficient technique that has been used to treat >5000 patients (Video 1).1
Video 1.
Watch now at https://academic.oup.com/asj/article-lookup/doi/10.1093/asj/sjz054
Sequence of Procedures With Mastopexy
With mastopexy augmentation, placing the implant before mastopexy gives good outcomes.
Postoperative Care
When using aggressively textured implants, many surgeons recommend avoiding exercise for 3 months to allow tissue attachment to the implant and reduce the risk of seromas and double capsules. However, with Motiva’s SmoothSilk surface, patients can fully mobilize earlier, around 6 weeks postprocedure. Most patients are allowed to resume work and physical activities after 2 weeks, starting with lower-body exercises and slowly building to full-body muscular activity. This allows the modern active women to feel more confident with the benefits of her surgical procedure. The patient should wear a support garment during this time, to limit movement and maintain the dissected pocket boundaries. Three months of good breast support is needed until the thin capsule is formed. Patients must be educated on the importance of this. Likewise, massage should be avoided to minimize tissue stretching and implant displacement.
Complications With Motiva Implants
Overall, there was consensus that Motiva implants caused fewer complications than other implants, including contractures and ruptures. It was felt by 60% that lateral displacement and bottoming out were potentially problematic if unanticipated but avoidable with good surgical technique and tight pocket dissection. The authors recognize that other factors, including patient selection, implant size, lifestyle, etc, may also influence complication rates. However, the Experts can only confirm that so far in their experience, the lower complication rate published by Sforza et al1 is reproducible in their practices.
The Motiva® implant is a sixth-generation silicone breast implant. It has a unique surface that it is bioengineered to be a cell-friendly smooth surface. Clinically, this minimizes capsule formation and reduces complications.8 This group’s consensus now provides surgical insight and usage guidelines from expert surgeons with experience in using this device.
From the perspective of the Experts, the most significant issues are related to the minimal capsule formation. Tight and precise pocket dissection can prevent malposition problems. Surgeons must also understand the consequence of using 100% gel-filled implants, and that their volumes and projections would be higher than expected when considering base measurements. The IMF must be respected as much as possible and surgically supported with deep sutures or a local flap to prevent it stretching and causing bottoming out. Nevertheless, this group reported lower capsule contracture rates and fewer complications overall. They felt that Motiva implants are likely to have a lower risk of causing BIA-ALCL because of their unique ability to reduce the chronic inflammation associated with implant surfaces.9–11 Moreover, according to the latest surface categorization published by Jones et al,11 Motiva implants are in the same risk group as smooth implants. Therefore, the statistical probability of developing BIA-ALCL with a Motiva implant must be considered at least the same as with a smooth implant. It should be noted that there are no reported BIA-ALCL cases with exclusive smooth implant procedures to date.12 A summary of the expert opinion on the differences in the use of textured devices and Motiva implants is reported in Table 2.
Table 2.
Summary of Conclusions About the Differences in Using Textured and Motiva Implants
| Any traditional textures including macrosurfaces and anatomic devices | Motiva Round and Ergonomix | |
|---|---|---|
| Incision location | IMF preferable | Suitable for any approach: IMF, transaxillary, or periareolar |
| Incision size | Usually starting with 4-5 cm and increasing according to implant size | Allows minimally invasive starting with 2 cm start, increasing according to implant size |
| Pocket size | Precise pocket, but usually larger because of the implant design (especially anatomic) and rough surface | Fit to the device, must be tight |
| Type of pocket (dissection plane) |
Preferable submuscular exclusively | Suitable for any pocket: subglandular, subfascial, or subpectoral |
| Insertion of the implants | More difficult even with sleeves due to harder less elastic gels | Easier especially with sleeves due to softer more elastic gels |
| IMF dissection | Needed to allow implant fitting | Must be conservative and IMF must be fixed when disrupted to avoid bottoming out |
| Muscle dissection | More pectoralis release is needed to accommodate especially with anatomic devices | Minimal pectoral disruptions due to implant softness |
| Exercise | Avoid from 8 weeks to 3 months (anatomic implants) | Allowed after 4 weeks as per surgeons’ discretion |
| Inflammation | More inflammation due to surface roughness | Less inflammation due to a bioengineered cell-friendly surface |
| Inflammation-related complications | Frequently reported double capsules and late seromas | No reports of double capsules or late seromas to date |
| Malposition | Rotation of anatomic devices, less common with round devices | Lateral displacement in case of large pocket displacement with poor laxity tissue |
IMF, inframammary fold.
CONCLUSIONS
The absence of innovation in breast implant devices for over quarter of a century has hindered surgical progress, although surgeons now have the possibility to migrate to these new bioengineered devices.13,14 However, beyond scientific papers that can be presented or published, there is a need for further reassurance from peer experts within the same field regarding Motiva’s innovative technology, safety, and reliability. Knowledge of clinical experience is, in fact, the only way we can confirm that patients with Ergonomix implants are able obtain a more natural appearance, a softer breast, and higher satisfaction with fewer complications. Although the consensus discussions solely focused on breast augmentation, the overall experience was positive and encouraging. Further papers with long-term data and experience with this implant for other indications, including revision cases and breast reconstruction, will be necessary to reassure plastic surgeons worldwide.
Disclosures
Dr. Sforza serves as coordinator of the Medical Advisory Board (MAB), has a consulting agreement with Establishment Labs Holdings, Inc., is a US clinical trial investigator, has received an option grant in September 2016 for 36,953 Class A Ordinary Shares and a restricted share grant in April 2018 for 68,233 Class A Ordinary Shares in Establishment Labs Holdings, and the author's institution on April 17, 2014 entered into a Supply Agreement with Establishment Labs Holdings, Inc. Dr. Hammond has consulting agreements with Mentor Corporation, the Musculoskeletal Transplant Foundation, Establishment Labs Holdings, Inc., and Nova Plasma Ltd; receives book royalties from Elsevier; is a member of the MAB at Establishment Labs Holdings, Inc; has a development agreement, including royalties for future products, has shares in Establishment Labs Holdings, Inc., and is also an investigator in Establishment Labs Holdings, Inc.’s US clinical trial. Dr. Botti declared no potential conflicts of interest with respect to the research, authorship, and publication of this article. Dr. Hedén has consulting agreements with Establishment Labs Holdings, Inc., Allergan, and Mentor, has shares in Establishment Labs Holdings, Inc., and is also an investigator in Establishment Labs Holdings, Inc.’s US clinical trial. Dr. Chacón-Quirós is a member of the MAB and consultant for Establishment Labs Holdings, Inc., and has shares in Establishment Labs Holdings, Inc. Dr. Munhoz is a member of the MAB at Establishment Labs Holdings, Inc., and has shares in Establishment Labs Holdings, Inc. Dr. Kinney is a member of the Scientific Advisory Board at Establishment Labs Holdings, Inc., and has shares in Establishment Labs Holdings, Inc., and is also an investigator in Establishment Labs Holdings, Inc.’s US clinical trial. Dr. Corduff has a consulting agreement with Establishment Labs Holdings, Inc., is a member of the MAB and has shares in Establishment Labs Holdings, Inc.
Funding
This article was supported by Establishment Labs (Alajuela, Costa Rica), who co-funded the development of this supplement.
Supplementary Material
REFERENCES
- 1. Sforza M, Zaccheddu R, Alleruzzo A, et al. Preliminary 3-year evaluation of experience with SilkSurface and VelvetSurface Motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases. Aesthet Surg J. 2018;38(suppl_2):S62-S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mendonça Munhoz A, Santanelli di Pompeo F, De Mezerville R. Nanotechnology, nanosurfaces and silicone gel breast implants: current aspects. Case Reports Plast Surg Hand Surg. 2017;4(1):99-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolfram D, Dolores W, Rainer C, et al. Cellular and molecular composition of fibrous capsules formed around silicone breast implants with special focus on local immune reactions. J Autoimmun. 2004;23(1):81-91. [DOI] [PubMed] [Google Scholar]
- 4. Maxwell GP, Gabriel A. Breast implant design. Gland Surg. 2017;6(2):148-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kadin ME, Epstein AL, Adams W, et al. Evidence that some breast implant associated anaplastic large cell lymphomas arise in the context of allergic inflammation. Blood. 2017;130(Suppl 1):4030. [Google Scholar]
- 6. Leberfinger AN, Behar BJ, Williams NC, et al. Breast implant-associated anaplastic large cell lymphoma: a systematic review. JAMA Surg. 2017;152(12): 1161-1168. [DOI] [PubMed] [Google Scholar]
- 7. James GA, Boegli L, Hancock J, Kinney BM. Bacterial adhesion and biofilm formation on textured breast implant shell materials. Aesthetic Plast Surg. 2018. doi: 10.1007/s00266-018-1234-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quirós MC, Bolaños MC, Fassero JJ. Six-year prospective outcomes of primary breast augmentation with nano surface implants. Aesthet Surg J. 2018. doi: 10.1093/asj/sjy196. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loch-Wilkinson A, Beath KJ, Knight RJW, et al. Breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand: high-surface-area textured implants are associated with increased risk. Plast Reconstr Surg. 2017;140(4):645-654. [DOI] [PubMed] [Google Scholar]
- 10. ASPS/ASAPS Update. Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) quick facts and FAQs https://www.surgery.org/downloads/blasts/BIA-ALCL. Accessed December 19, 2018.
- 11. Jones P, Mempin M, Hu H, et al. The functional influence of breast implant outer shell morphology on bacterial attachment and growth. Plast Reconstr Surg. 2018;142(4):837-849. [DOI] [PubMed] [Google Scholar]
- 12. Sforza M. Breast implants associated ALCL (BIA-ALCL): a personal overview for patients. J Surg Open Access. 2018;4(4). doi: 10.16966/2470-0991.176. [DOI] [Google Scholar]
- 13. Sforza M, Spear S, Hammond D. The 21st century silicone breast implant. J Surg Open Access. 2016;2(4). doi: 10.16966/2470-0991.e107. [DOI] [Google Scholar]
- 14. Sforza M, Andjelkov K, Zaccheddu R, Husein R, Atkinson C. A preliminary assessment of the predictability of fat grafting to correct silicone breast implant-related complications. Aesthet Surg J. 2016;36(8):886-894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.