Abstract
Background
Assessing quality by considering input, process and output level quality variables is important to ensure improved quality services. Designing and execution of an effective quality management system are aimed for the purpose of quality improvement, error reduction and associated risks. Therefore, this review is designed to assess the value of accreditation on the performance of healthcare institutions in ensuring quality improvement interventions. Moreover, this review presents important concepts of accreditation and the aspects of quality.
Methods
Published articles were downloaded using EndNote® application software program from PubMed (NML) database, Web of Sciences (TS) and Google Scholar. From a total of 883 downloaded full-text published materials, only 28 journals and 1 report issued from 2010 to 2017 were used for the development of this review.
Result
The overall quality of healthcare services in developing countries was error-prone and suffered from limitations. These could be associated with wrong interventions and increased risks. Accreditation schemes have been implemented to provide quality care and ensure safety.
Conclusion
Evaluation feedback induces interventions aimed at quality improvement and ensures better management systems, good process design, wise resource utilization, meeting patients' need and increased satisfaction. Hence, stakeholders must be engaged in the provision of improve quality patient care and reduce associated risks. Hence, giving special quality improvement attention helps to improve quality healthcare services.
Keywords: Quality, accreditation, quality assessment
Introduction
Implementing effective quality systems has long-term benefits of ensuring accurate diagnosis for improved clinical outcome (1). According to Donabedian, the ultimate aim of quality inspection is system improvement (2). It is possible to technically use standardized and harmonized procedures as important factors for effective and improved quality patient care. However, defining and measuring quality service is subjective, complex and has multidimensions (3).
Healthcare services provided among developing countries have long been neglected, underfinanced, received little attention, and as the result, poor quality services have been provided (4–6). Establishing good process design enables us o achieve or exceed customers' expectations at a reduced cost (7). Quality assessment indicator tools evaluate the entire processes and assess performance, ensure continuous quality improvement and increase customer satisfaction and develop confidence (4). They measure patient safety and associated risks factors (4,5). Inappropriately designed process could be the reasons for the delay and/or wrong decisions (6,8). Hence, effective quality assessment tools and measurement systems are important to assess performance, find gaps and initiate quality improvement interventions (7).
Healthcare institutions communicate with (9) and report to (10) recognized international accreditation bodies for getting feedback and/or accreditation on input, process, and the output level quality variables. The process by itself is increasingly subjected to legislation and regulations demanding competence and commitment of qualified quality assessors (5). Accreditation scheme improves accuracy and safety and customer satisfaction (11). It also ensures reliable, competent and safe service for better service outcome and supports continuous quality improvement, boosts morale, and at a reduced cost (12). Therefore, the government, payers and accreditation bodies need to work together to achieve universal quality coverage (13).
Developing, maintaining, improving and sustaining quality patient care requires the involvement and commitment of every worker from higher principals to operational workers. The extent of accreditation covers entire phases of service generation processes (14). Therefore, standardizing the entire processes and continuously inspecting the quality of the processes after revising the quality and assessment feedback ensures improved quality services.
Method and Materials
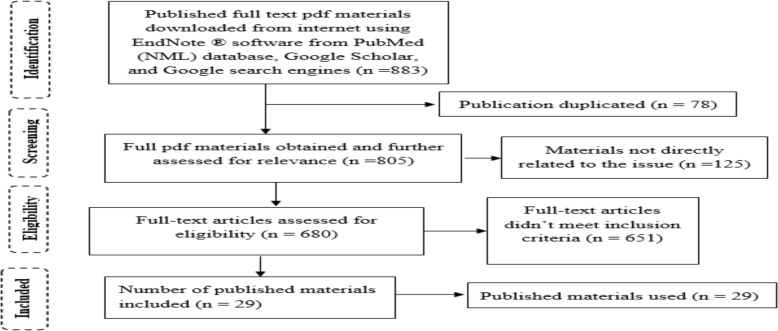
A qualitative research design was applied to develop this review article. After conceptualizing ideas, keywords were identified as a quality laboratory, laboratory accreditation, quality assessment and quality. Published materials issued from 2010 to 2017 were searched from the electronic databases of PubMed (NML), Web of Science (TS) and Google scholar using EndNote ® application software. From the total of 883 downloaded full-text pdf materials, 29 published materials were eligible for the development of this review (Figure 1).
Figure 1.
PRISMA flow diagram of article selection processes
Results
Accreditation success factors: Committed managers, excellent leadership policy and enabling environment are crucial for improved quality services provision. National accreditation policy designs must consider affordable and accessible healthcare systems (15). In addition to this, effective leadership (16) and good governance (17) are essential for the successful accomplishment of tasks, improvement of quality and achievement of targeted goals. Moreover, high-level medical knowledge plus technical and non-technical skills are important factors for safe, effective and patient-centered quality care (18).
Setting basic quality standards and efficient accreditation implementation programs are fundamental drivers of quality improvement (19). These activities are important to ensure the ethical and efficient use of resources and increase the value of the quality requirements of modern healthcare intervention (9).
Proper implementation of Quality System Essentials guarantees the production of quality laboratory results (20). This principally reduces errors (4), turnaround time, supports continuous quality improvement, boosts morale, ensure cost efficiency (21), and improves quality (5) in laboratory medicine. Therefore, accreditation increases the healthcare-seeking behavior of patients.
Accreditation contributing factors for ISO 15189, laboratory accreditation: Most supervisory positions were offered support from technical experts having little management skills. They were unable to effectively manage, meet service goals, motivate staff, initiate changes, and sustain physician relations. These were the main reasons for failing to meet standard quality laboratory results (4–6) which could be attributable to wrong decisions and associated risks. Failure to stick identification labels on equipment and/or updating complex training records was the most common reasons for ISO17025 non-compliance (9). Assessing quality at all phases of testing is an important issue for accreditation. Using inappropriate specimen containers, requesting activities, reporting and result in interpreting and fail to property file were common for the occurrences of errors and failure factors contributing to ISO 15189 accreditation (6).
Accreditation process: According to Ernest Amory Codman and Avedis Donabedian, the principal aim of accreditation is to improve quality and ensure patient safety (5). Legitimate, ethical and cost-effective accreditation is important in the healthcare industry (9). Hence, accredited laboratories should explicitly specify which tests are performed under accreditation condition as a mandatory ISO 15189 quality requirement (14). This creates a clear understanding among stakeholders on the actual performance of a particular test done in accredited laboratories.
Accreditation requires sufficient finance for initial development and sustaining, ongoing management, operations and surveyors training to provider organizations for the desired improvement (13). Moreover, according to the report of the Australian Council on Healthcare Standards, the average annual accreditation cost of a given healthcare institution varies from 0.03% to 0.60% of the total operating costs for a small rural hospital (22). Therefore, implementing accreditation enables the institution to retain annual estimated revenue of $40,000 (23). Hence, this ensures cost-effective quality healthcare services.
Accreditation witnesses competency and ensures quality improvement opportunities towards local or international standards. However, the experience of Danish Public Hospitals did not show significant quality improvement performances (24). Hence, it is important to continuously inspect the service quality after accreditation for quality improvement (25). Post-accreditation quality assessment using hand hygiene and Staphylococcus aureus bacteremia incidence rate as quality indicators were used to demonstrate the process and outcome performance of laboratories, respectively (25). Quality demands continuous improvement process in rendering services so as to offer the best possible quality services within the context of limited resources.
Accreditation experience in healthcare settings: Historically, a century ago, errors were considered to be restricted only to the analytical performance which had been overlooking nearly half the pre-analytical and over a quarter of the post-analytical errors (4).
The first formal accreditation was started in the United States of America in 1917 by “American College of Surgeons” to define suitability of surgical training program for meeting “Minimum Standard for Hospitals” (26). The first healthcare “quality foundations movement” was made in 1965 (2). However, a remarkable transformation was made in the 1990s to develop, maintain and sustain the right principle which helps to generate reliable laboratory result (4). Since the 1990s, voluntary or mandatory (21) regulations were enacted and by now Finland, Ireland, the Netherlands, Sweden, Switzerland and the UK almost implemented ISO 15189 and ISO 22870 (27). However, countries have differences in quality achievement, assessment practices and implementation strategies (27).
The accreditation bodies for performance requirements (9) grant verifications for meeting the minimum standard requirement of ISO 15189 (14). Nowadays, accreditation is increasingly applied globally (21). On June 9 in 2015, representatives from over 90 countries celebrated World Accreditation Day as a global initiative jointly established by the International Accreditation Forum and the International Laboratory Accreditation Cooperation with the intention of raising awareness on the importance of accreditation thematically focusing on how it supports the delivery of health and social care (21).
The performance of African laboratories assessed with the tough ISO15189 quality requirement is poor. WHO Regional Office for Africa (WHO/AFRO) strategic direction priorities for 2010–2015 highlighted the important laboratory quality services through partnerships and harmonization of technical support to countries so as to accelerate actions on HIV/AIDS, malaria and tuberculosis. Participants of the WHO Expert who met to finalize the stepwise laboratory accreditation process were convened by in Nairobi, Kenya, July 2011 (28).
Hence, the WHO-AFRO recommends an interim accreditation using flexible standards. The scheme enables developing countries accreditation based on the step-wise rating scale towards ISO 15189 free of charge which is better than the tough ISO 15189 accreditation requirement (19) which gives points based on either pass or fail options.
The Ethiopian Standard Agency (ESA) adopted ISO15189:2012 as ES ISO15189:2013 Ethiopian standard document, and Ethiopian national accreditation office uses the guideline to evaluate laboratories for meeting the minimum requirement of quality services. Bethzatha was accredited in May 2015 by meeting the requirement of ISO 15189:2013 because of glucose, creatinine, cholesterol, alanine aminotransferase and aspartate aminotransferase (20). High-tech laboratory equipment, highly qualified laboratory professionals, well-organized laboratory, training given for both technical and non-technical staffs, smooth work relations and efficient management reviews processes were reasons for success (20). From 30 laboratories assessed for meeting the requirements of WHO Afro in Addis Ababa only 1 (3.3%), private laboratories scored 155(62.0%) which is star 1 (29).
In conclusion, developing, implementing and sustaining accreditation is a cost incurring, time demanding, though endeavor. Developing countries gain more privileges from flexible accreditation scope than fixed scopes at affordable prices and promising success in meeting international standards. Accreditation has the advantage of significant quality improvement during the pre-accreditation phase than the performance outcome during the postaccreditation phase. On the other hand, in the absence of research evidence in developing country contexts favors the government and private healthcare facilities to design interventions so as to standardize processes and improve the quality of laboratories. However; wrong interventions or delayed healthcare services are associated with poor quality results.
The majority of laboratories in Africa, particularly in Ethiopia, are not accredited. Of the 340 accredited laboratories in Africa, only 28(8.2%) are in sub-Saharan Africa; the other 312 primarily private laboratories are located in South Africa. Sub-Saharan Africa has a population of more than 800 million, the majority of whom rely on government services for healthcare (28).
Therefore, accurate diagnosis and evidencebased decisions heavily depend on the laboratory result, clinical finding and/or a combination of these results. These error-prone, unreliable, inaccessible services demand major mandatory immediate action for more improved quality laboratory services and subsequent evidence-based decision for sooner recovery and improved quality of life of the patients.
Acknowledgements
The authors would like to acknowledge the copyright holders of the materials used in this review.
References
- 1.Sciacovelli L, Lippi G, Sumarac Z, West J, Garcia D, Furtado V, et al. Quality Indicators in Laboratory Medicine: the status of the progress of IFCC Working Group “Laboratory Errors and Patient Safety” project. Clin Chem Lab Med. 2017;55(3):348–357. doi: 10.1515/cclm-2016-0929. [DOI] [PubMed] [Google Scholar]
- 2.John Z, Howard M. Donabedian's Lasting Framework for Health Care Quality. N Engl J Med. 2016;375(3):205–207. doi: 10.1056/NEJMp1605101. [DOI] [PubMed] [Google Scholar]
- 3.Ali M. Factors Affecting Medical Service Quality. Iranian J Publ Health. 2014;43(2):210–220. [PMC free article] [PubMed] [Google Scholar]
- 4.Plebani M. The Journey Toward Quality and Patient Safety in Laboratory Medicine Continues. North American Journal of Medical Sciences. 2014;6(5):229–230. doi: 10.4103/1947-2714.132942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plebani M, Sciacovelli L, Chiozza M, Panteghini M. Once upon a time: a tale of ISO 15189 accreditation. Clin Chem Lab Med. 2015;53(8):1127–1129. doi: 10.1515/cclm-2015-0355. [DOI] [PubMed] [Google Scholar]
- 6.Plebani M. Diagnostic error and laboratory medicine-causes and strategies. The Journal of the International Federation of Clinical Chemistry and Laboratory Medicine. 2015;26(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- 7.Devkaran S, O'Farrell P. The impact of hospital accreditation on quality measures: an interrupted time series analysis. BMC health services research. 2015;15:137. doi: 10.1186/s12913-015-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh H, Schiff G, Graber M, Onakpoya I, Thompson M. The global burden of diagnostic errors in primary care. BMJ quality & safety. 2016 doi: 10.1136/bmjqs-2016-005401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson I, Smye M, Wallace I. Meta-audit of laboratory ISO accreditation inspections: measuring the old emperor's clothes. Microbiology Open. 2016;5(1):95–105. doi: 10.1002/mbo3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adirim T, Meade K, Mistry K. A New Era in Quality Measurement: The Development and Application of Quality Measures. Pediatrics. 2017;139(1):e20163442. doi: 10.1542/peds.2016-3442. [DOI] [PubMed] [Google Scholar]
- 11.Masanza M, Nqobile N, Mukanga D, Gitta N. Laboratory capacity building for the International Health Regulations (IHR[2005]) in resource-poor countries: the experience of the African Field Epidemiology Network (AFENET) BMC Public Health. 2010;10(1):1–7. doi: 10.1186/1471-2458-10-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsen Elva Forum IA, editor. Accreditation: Supporting the Delivery of Health and Social Care. Canada: 2015. [Google Scholar]
- 13.Kedar S, Anne L, Anuwat S, Girdhar G. Accreditation as a path to achieving universal quality health coverage. Globalization and Health. 2014;10(68):1–8. doi: 10.1186/s12992-014-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thelen M, Vanstapel F, Kroupis C, Vukasovic I, Boursier G, Barrett E, et al. Flexible scope for ISO 15189 accreditation: a guidance prepared by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Working Group Accreditation and ISO/CEN standards (WG-A/ISO) Clin Chem Lab Med. 2015;53(8):1173–1180. doi: 10.1515/cclm-2015-0257. [DOI] [PubMed] [Google Scholar]
- 15.Ashigbie P, Azameti D, Wirtz V. Challenges of medicines management in the public and private sector under Ghana's National Health Insurance Scheme - A qualitative study. Journal of pharmaceutical policy and practice. 2016;9:6. doi: 10.1186/s40545-016-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadideen H, Weldon S, Saadeddin M, Loon M, Kneebone R. A Video Analysis of Intraand Interprofessional Leadership Behaviors Within “The Burns Suite”: Identifying Key Leadership Models. Journal of surgical education. 2016;73(1):31–39. doi: 10.1016/j.jsurg.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Bismark M, Studdert D. Governance of quality of care: a qualitative study of health service boards in Victoria, Australia. BMJ quality & safety. 2014;23(6):474–482. doi: 10.1136/bmjqs-2013-002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott J, Revera M, McRitchie A, Riviello R, Smink D, Yule S. Non-technical skills and health care provision in low- and middleincome countries: a systematic review. Medical education. 2016;50(4):441–455. doi: 10.1111/medu.12939. [DOI] [PubMed] [Google Scholar]
- 19.Aryankhesal A. Strategic Faults in Implementation of Hospital Accreditation Programs in Developing Countries: Reflections on the Iranian Experience. International journal of health policy and management. 2016;5(9):515–517. doi: 10.15171/ijhpm.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abebe N. Successful ISO 15189 Accreditation in the Bethzatha Advanced Medical Laboratory in Ethiopia. Ejifcc. 2016;27(4):351–353. [PMC free article] [PubMed] [Google Scholar]
- 21.Merethe K, Line B, Volkert S, Flemming B, Susanne R, Jens S, et al. Accreditation in general practice in Denmark: study protocol for a cluster-randomized controlled trial. Trials. 2017;18(69):1–9. doi: 10.1186/s13063-017-1818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mumford V, Greenfield D, Hogden A, Forde K, Westbrook J, Braithwaite J. Counting the costs of accreditation in acute care: an activity-based costing approach. BMJ Open. 2015;5(9):e008850. doi: 10.1136/bmjopen-2015-008850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kibet E, Moloo Z, Ojwang P, Sayed S, Mbuthia A, Adam R. Measurement of improvement achieved by participation in international laboratory accreditation in sub-Saharan Africa: the Aga Khan University Hospital Nairobi experience. American journal of clinical pathology. 2014;141(2):188–195. doi: 10.1309/AJCPV8A9MRWHGXEF. [DOI] [PubMed] [Google Scholar]
- 24.Bogh S, Falstie-Jensen A, Bartels P, Hollnagel E, Johnsen S. Accreditation and improvement in process quality of care: a nation wide study. International journal for quality in health care. 2015;27(5):336–343. doi: 10.1093/intqhc/mzv053. [DOI] [PubMed] [Google Scholar]
- 25.Ke SW, Lin WC, Tsai CF. Research performance of AACSB accredited institutions in Taiwan: before versus after accreditation. SpringerPlus. 2016;5(1):1285. doi: 10.1186/s40064-016-2934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes R, Shea K, Watson P. The Canadian Tissue Repository Network Biobank Certification and the College of American Pathologists Biorepository Accreditation Programs: Two Strategies for Knowledge Dissemination in Biobanking. Biopreservation and biobanking. 2016 doi: 10.1089/bio.2016.0021. [DOI] [PubMed] [Google Scholar]
- 27.Boursier G, Vukasovic I, Brguljan P, Lohmander M, Ghita I, Bernabeu A, et al. Accreditation process in European countries - an EFLM survey. Clin Chem Lab Med. 2016;54(4):545–551. doi: 10.1515/cclm-2015-0780. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization regional office for Africa, author. WHO Guide for the Stepwise Laboratory Improvement Process Towards Accreditation in the African Region. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abera E, Taye B, Belay G, Ashenafi A. The status of medical laboratory towards of AFRO-WHO accreditation process in government and private health facilities in Addis Ababa, Ethiopia. Pan African Medical Journal. 2015;22:136. doi: 10.11604/pamj.2015.22.136.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]