Abstract
Portopulmonary hypertension (PoPH) is a form of pulmonary arterial hypertension (PAH) that can develop as complication of portal hypertension. Treatment of PoPH includes PAH-specific therapies and in certain cases, such therapies are necessary to facilitate a successful liver transplantation. A significant number of barriers may limit the adequate treatment of patients with PoPH and explain the poorer survival of these patients when compared to other types of PAH. Until recently, only one randomized controlled trial has included PoPH patients and the majority of treatment data is derived from relatively small observational studies. In the present manuscript we review some of the barriers in the treatment of patients with PoPH and implications for liver transplantation.
Keywords: portopulmonary hypertension, cirrhosis, treatment, side effects, liver transplantation
Introduction:
Portopulmonary hypertension (PoPH) is defined as pulmonary arterial hypertension (PAH) associated with portal hypertension of intra or extrahepatic origin (1, 2). Pulmonary arterial hypertension requires a specific hemodynamic profile that includes a resting mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg, a pulmonary artery wedge pressure (PAWP) ≤ 15 mmHg and a pulmonary vascular resistance (PVR) > 3 Wood units (3, 4). Meanwhile, portal hypertension is defined as a portal venous gradient of ≥ 6 mmHg (5). The prevalence of PoPH is 1 – 6% in patients with portal hypertension (6–8) and 5% in candidates for liver transplantation (9).
Patients with PoPH have worse survival than individuals with other types of PAH (10, 11). In fact, the Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL registry) showed a 5-year survival of 40% for patients with PoPH (n=174) compared with 64% for individuals with idiopathic or heritable PAH, even when the pulmonary hemodynamic profile appeared more favorable in PoPH subjects (10). Interestingly, at the time of enrollment in this registry, patients with PoPH were less likely to be treated for PAH (10). In the United Kingdom National PAH registry, treatment-naïve patients with a recent diagnosis of PoPH (n=110) had a 5-year survival of 35%; worse than patients with idiopathic PAH.
PAH-specific treatment is indicated for patients with PoPH (2, 12); however, the long-term impact of this approach remains vastly unexplored (13, 14) with some studies questioning its efficacy (11, 15). Importantly, the liver transplant mortality increases in patients with PoPH, particularly in subjects with a mPAP ≥ 35 mmHg and PVR > 3 Wood units (16, 17). In patients with this unfavorable hemodynamic profile, PAH-specific therapies are used to improve pulmonary hemodynamics and right heart function, with the expectation of decreasing the perioperative mortality of liver transplantation (18, 19).
All but one of the randomized studies that led to the FDA approval of current PAH-specific medications excluded patients with PoPH (20). Relatively small observational studies reported on the use of PAH-specific medications to treat patients with PoPH (21, 22). These data remain insufficient to adequately guide evidence-based treatment decisions (Table 1). In addition, patients with PoPH appear to have frequent side effects with recommended doses of PAH medications, particularly prostacyclin analogues. In this manuscript, we examine the available literature and question whether a) the presence of advanced liver disease increases the incidence of side effects in patients treated with PAH-specific therapies and b) the worse outcomes observed in patients with PoPH are in part related to barriers in receiving an adequate PAH treatment.
Table 1:
Studies that included ≥ 3 patients and described the effect of PAH-specific therapies in PoPH.
| First author, year, reference |
n | WH O funct ional class |
PVR (dyn*s*cm −5) |
Medication | MELD / Child-Pugh score |
Outcomes |
|---|---|---|---|---|---|---|
| Phosphodiesterase-5 inhibitors | ||||||
| Hemmes et al, 2009(87) | 10 | I- IV | Mean ±SD 664 ± 336 |
Sildenafil 20–50mg three times daily. | MELD:mean ±SD 14±3.3 | ✓Improvement in functional class, exercise tolerance and cardiopulmonary hemodynamics. Three patients were listed for liver transplantation and one was successfully transplanted. |
| Reichenberger et al, 2006(48) | 13 | III- IV | Mean ±SD 759± 338 |
Sildenafil 50mg three times daily. | C-P: A,B,C | ✓Improvement in functional class, exercise tolerance and cardiopulmonary hemodynamics. |
| Fisher et al, 2015(88) | 20 | III- IV | Mean ±SD 683± 259 |
Sildenafil 20–25 mg three times daily (n=19), tadalafil 40 mg daily (n=1) | C-P: A,B,C MELD: median,(range) 15 (13–18) |
✓Improvement in functional class and cardiopulmonary hemodynamics. No change in exercise tolerance. |
| Gough et al, 2009(89) | 11 | I -III | Mean: 575 | Sildenafil 25–50mg three times daily. | C-P: B,C MELD:mean ±SD 14±4.6 |
✓Improvement in cardiopulmonary hemodynamics. One patient had a successful liver transplant. |
| Endothelin receptor antagonists | ||||||
| Hoeper et al (2005)(41) | 11 | II-IV | Mean ±SD 944 ±519 |
Bosentan 62.5 mg twice daily for 4–8 weeks, increased to 125 mg twice daily | C-P: A | ✓Improvement in functional class, exercise tolerance and cardiopulmonary hemodynamics. One patient had worsening ascites. No evidence of liver toxicity. |
| Savale et al (2013) (52) | 34 | II-IV | Mean ±SD 696 ± 264 |
Bosentan 62.5 mg twice daily for 4 weeks, increased to 125mg twice daily | C-P: A, B | ✓Improvement in functional class, exercise tolerance and cardiopulmonary hemodynamics. Three patients died of right heart failure. Elevation of liver enzymes was noted in several patients. |
| Cartin-Ceba et al (2011)(42) | 13 | II-III | Median (IQR) 445 (329–834) |
Ambrisentan 5mg daily for 4 weeks, increased to 10mg daily | C-P: A,B,C MELD: median of 10 (IQR,8.5–15) |
✓Improvement in cardiopulmonary hemodynamics and BNP levels. One patient underwent successful liver transplantation. One patient had periorbital bleeding, peripheral edema and 8 pounds weight gain. No evidence of liver toxicity |
| Prostacyclin analogues | ||||||
| Krowka et al (1999)(90) | 15 | II-IV | Mean ±SD: Acute phase: 525±286 Long-term phase: 373±191 |
Acute phase: IV epoprostenol 4–10 ng/kg/min over 60 min (n=14). Long-term phase: IV epoprostenol up to 48 ng/kg/min (n=10) |
C-P: B,C | ✓Acute phase: improvement in cardiopulmonary hemodynamics. Hypotension, headache and nausea were noted. ✓Long-term phase: no improvement in cardiopulmonary hemodynamics. One patient died of worsening heart failure and another had sudden death after successful liver transplantation. |
| Awdish et al, 2013(91) | 21 | I-III | Mean ±SD 537± 160 |
IV epoprostenol 20.8 ± 13.9 ng/kg/min |
C-P: A,B,C MELD: mean ±SD: 12.5 ±5.1 |
✓Improvement in exercise tolerance and cardiopulmonary hemodynamics. Seven patients were transplanted successfully, and four patients were listed for liver transplantation. |
| Kuo et al, 1997(92) | 4 | II-IV | N/A | IV epoprostenol up to 28 ng/kg/min | C-P: B | ✓Improvement in cardiopulmonary hemodynamics. |
| Melgosa et al, 2010(47) | 21 | I- IV |
Acute phase: 564±282 Long-term phase: 802±313 |
Acute-phase: 21 patients were given 2.8 µg of inh iloprost. Long-term phase: inh iloprost 5 µg six times daily for 1 year (3 patients also received bosentan 125 mg twice daily) |
MELD: mean ± SD Acute-phase 15.0±2.5 Long-term phase 11.1±5.3 |
✓Acute-phase: improvement in cardiopulmonary hemodynamics. ✓Long-term phase: improvement in exercise tolerance and functional class but no change in cardiopulmonary hemodynamics. Two patients worsened their pulmonary hypertension. |
| Sakai et al, 2009 (43) | 3 | N/A | 249,304, 718 | IV treprostinil : 45, 36 and 106 ng/kg/min |
MELD: 22, 33, N/A | ✓Improvement in cardiopulmonary hemodynamics in two patients who underwent successful liver transplantation. |
| Ashfaq et al,2006(30) | 16 | II-IV | Mean ±SD: Moderate PoPH (n=6) 402±87 Severe PoPH (n=10) 551±92 |
IV epoprostenol (n=15, 2 patients also received bosentan). One patient was treated with diltiazem. | C-P: B,C MELD: mean ±SD: Moderate 11.9 ± 4.5 Severe 15.2 ± 4.6 |
✓Improvement in cardiopulmonary hemodynamics. Eleven patients were successfully transplanted. |
| Sussman et al, 2006(93) | 8 | N/A | Mean 410 | IV epoprostenol at 2–8 ng/kg/min | MELD: mean ±SD 17±6.4 | ✓Improved cardiopulmonary hemodynamics. Six patients were listed for liver transplantation (four were successfully transplanted) |
| Hoeper et al, 2007 (46) | 31 | II-III | 812±337(iloprost) and 866±422 (bosentan) | Iloprost 5ug inh six times daily (n=13) or bosentan 125mg twice daily (n=18). | C-P: A,B MELD:mean ±SD 12±3 and 10±3 |
✓Bosentan was a safe. Compared with iloprost, patients treated with bosentan had better effects on exercise capacity, hemodynamics and higher survival and event-free survival. |
| Fix et al,2007(94) | 19 | II-IV | Mean 670 (95%CI: 556–784) |
Epoprostenol (n=19). In 7 patients sildenafil was added. | C-P: A,B,C MELD: median, (range) 14 (7–26) |
✓Improved cardiopulmonary hemodynamics. Two patients underwent liver transplantation. Epoprostenol was discontinued in 2 and sildenafil in 4 patients given side effects. |
WHO: World Health Organization
N/A: not available
MELD: Model for End-stage Liver Disease
C-P: Child-Pugh score
SD: standard deviation
IQR: interquartile range
CI: confidence interval
mPAP: mean pulmonary artery pressure
PVR: pulmonary vascular resistance
IV: intravenous
Inh: inhaled
a). Establishing the diagnosis of PoPH
An accurate diagnosis of PoPH is essential since patients with advanced liver disease have other reasons for pulmonary hypertension (PH) such as volume overload and hyperdynamic state; conditions that may not necessarily impact liver transplant outcomes (23). A right heart catheterization is required, both to confirm the diagnosis of PH (mPAP ≥ 25 mmHg) and establish the origin. In cases of volume overload and/or hyperdynamic state the PAWP (> 15 mmHg) and/or cardiac index (≥ 4 L/min/m2) are elevated while the PVR remains below 3 Wood units (or 240 dynes.s.cm−5) (24). Volume overload is treated with diuresis with careful attention to renal function. A hyperdynamic state is inherent to the liver disease given splanchnic vasodilation and intrahepatic and/or mesenteric arteriovenous shunts (25); hence, there are no specific treatments for this condition apart from for liver transplantation (26). Importantly, other comorbidities (anemia, obesity, arteriovenous connections, Beriberi and hyperthyroidism) that increase the cardiac index need to be recognized (27).
b). Effect of PAH-specific therapies in PoPH
The goals of treating patients with PoPH are to alleviate symptoms, facilitate liver transplantation and ultimately, improve outcomes. A meta-analysis of 12 studies that included patients with PoPH showed that PAH-specific therapies improved pulmonary hemodynamics and functional capacity (28). Observational studies suggest that PAH-specific treatment may improve outcomes when compared to historical data (12) and potentially increase the eligibility and reduce the risks associated with liver transplantation (29, 30). However, it remains unclear whether PAH-specific treatment impacts the transplant free survival; particularly since any potential survival advantage may be curtailed by the advanced liver disease and associated comorbidities (11, 15).
Recent analyses on three PH registries suggest that PAH patients who sustain or achieve a low-risk category (31) during follow-up have better prognosis (32–34). However, one registry (33) excluded patients with PoPH, and the other two (32, 34) included a limited number of patients with this condition (2.6% and 5.6% of the entire cohort). It remains unclear whether an aggressive PAH-specific treatment, aimed at achieving low risk criteria is beneficial in PoPH patients; particularly when the hemodynamic goals for liver transplant are achieved. Moreover, the parameters used to define these risk criteria; i.e. World Health Organization (WHO) functional class, six-minute walk distance (35), N-terminal prohormone of brain natriuretic peptide (36), and hemodynamic determinations (37, 38), are inherently affected in patients with liver cirrhosis and therefore may not change with PAH treatment.
c). PAH-specific therapies used in PoPH:
1. Phosphodiesterase-5 inhibitors
Sildenafil was found to be effective in improving functional class, exercise tolerance and hemodynamics in PoPH (Table 1). Limited information exists on the use of once-a-day tadalafil in PoPH (39).
2. Soluble guanylate cyclase stimulator
The PATENT-1 study randomized patients with PAH to the soluble guanylate cyclase stimulator riociguat or placebo and included a limited number of PoPH patients (n=13, 11 subjects received riociguat and 2 placebo) (20). Riociguat appeared to improve functional and hemodynamic determinations in patients with PoPH; however, some patients experienced side effects (headaches (n=3) and peripheral edema (n=3)). In addition, one patient died of sepsis related to bronchopneumonia, an event not ascribed to the study drug (40).
3. Endothelin receptor antagonists
Hoeper et al. (41) reported improvements in symptoms, exercise capacity and hemodynamics in PoPH patients treated with bosentan, a medication that was well tolerated. Similar results were reported by other investigators (Table 1). Bosentan causes an elevation of liver transaminases ≥ three-fold the upper limit of normal in ~11% of the patients; however, ambrisentan and macitentan, rarely cause hepatotoxicity. Ambrisentan has been associated with dramatic improvements in hemodynamics and WHO functional class in PoPH patients (42). A randomized, double-blind clinical study (PORTICO, NCT02382016), testing macitentan in patients with PoPH has recently finished enrollment. The primary outcome of the study is change in PVR at 12 weeks.
4. Prostacyclin analogues and prostacyclin receptor (IP) agonist
Intravenous epoprostenol has been used in PoPH patients as a mean to improve their hemodynamic profile to facilitate liver transplant (Table 1). Treprostinil is a chemically stable analogue of prostacyclin and its intravenous formulation has been used in PoPH patients with success (43). Continuous intravenous infusions of epoprostenol and treprostinil require active participation of the patient and/or caregiver. This fact is important since 30–45% of the patients with advanced liver disease develop hepatic encephalopathy, a condition associated with difficulties in performing activities of daily living (44, 45), which can make difficult the treatment with parenteral medications. For instance, subjects may accidentally disconnect their intravenous access or may not be able replace medication cassette reservoirs given agitation, confusion and/or somnolence. Furthermore, inadequate manipulation of vascular catheters can lead to catheter malfunction, bleeding and/or bloodstream infections.
Inhaled iloprost has been associated with long-term improvements in symptoms and exercise tolerance in PoPH (46, 47). Limited data exit on the use of inhaled treprostinil in patients with PoPH (48). Oral treprostinil has not been studied in patients with PoPH. Selexipag, an oral prostacyclin receptor (IP) agonist, has not been examined in patients with PoPH. Beraprost, an oral prostacyclin analog not available in the US, was used in a patient with POPH who exhibited improvements in symptoms, functional capacity and hemodynamic determinations (49). We use inhaled treprostinil or oral treprostnil or selexipag very carefully, paying particular attention to side effects and considering longer dosing intervals, lower doses and slower titration.
Interestingly, a retrospective study in PoPH patients compared treatment with inhaled iloprost (n=13) versus oral bosentan (n=18) and showed that both treatments were safe; however, patients treated with bosentan had a distinct improvement in functional capacity and pulmonary hemodynamics, with better overall and event free survival at 1-, 2- and 3-year (46).
d). Influence of liver disease on the metabolism of PAH medications:
Except for epoprostenol, the liver is the predominant metabolic site for PAH-specific medications (Table 2); therefore, PAH medications are expected to have a longer half-life and higher serum concentration in patients with liver disease; factors associated with more frequent medication side effects (50). In addition, the capacity of the liver to metabolize drugs depends on its blood flow and enzyme activity, both of which can be affected in patients with liver disease (51). In support of this, Savale et al. noted that the plasma concentration of bosentan in patients with PoPH (Child-Pugh class B cirrhosis) was higher than individuals with idiopathic PAH, possibly due to a decrease in the liver uptake of bosentan, given lower efficiency of the organic anion transporter peptide (52). Frey et al. demonstrated a higher riociguat exposure (after a single oral dose) in individuals with Child-Pugh class B cirrhosis than healthy controls (53).
Table 2:
Pharmacokinetics, gastrointestinal side effects and dosing of PAH-specific medications in liver disease
| Medication | Metabolism | Excretion^ | GI side effects > 1% |
Dosing of PHA medications by degree of liver impairment |
|---|---|---|---|---|
| Sildenafil PO | Hepatic via CYP3A4 (major) and CYP2C9 (minor route). |
Feces: 80% Urine: 13% |
Dyspepsia, diarrhea, gastritis, nausea, increased liver enzymes |
C-P A, B: No adjustment C-P C: Not studied |
| Tadalafil PO | Hepatic, via CYP3A4 |
Feces: 61% Urine: 36% |
Dyspepsia, nausea, GERD, abdominal pain, diarrhea, gastroenteritis, dysphagia, abnormal liver function tests. |
C-P A, B: Use with caution; consider initial dose of 20 mg once daily C-P C: Not studied |
| Riociguat PO | Hepatic via CYP1A1, CYP3A, CYP2C8 and CYP2J2. |
Feces: 53% Urine: 40% |
Dyspepsia, nausea, diarrhea, vomiting, gastritis, constipation, GERD |
C-P A, B: No adjustments CP C: Not studied |
| Bosentan PO | Hepatic via CYP2C9 and CYP3A4 to three primary metabolites. |
Feces: mainly Urine: <3% |
Increased in AST and ALT |
C-P A: No adjustment C-P B,C: Avoid use |
| Ambrisentan PO | Hepatic via CYP3A4, CYP2C19, and UGT 1A9S, 2B7S, and 1A3S | Feces: mainly | Dyspepsia |
C-P A: No adjustment C-P B, C: Use not recommended. |
| Macitentan PO | Hepatic via CYP3A4 (major) and CYP2C19 |
Feces: 24% Urine: 50% |
Increased liver enzymes. | No dosage adjustments provided. |
| Epoprostenol IV | Rapidly hydrolyzed |
Feces: 4% Urine: 84% |
Nausea, vomiting, anorexia, diarrhea. | No dosage adjustments provided. |
| Treprostinil SQ / IV | Hepatic via CYP2C8 |
Feces: 13% Urine: 79% |
Diarrhea, nausea. |
C-P A, B: Use with caution and titrate slowly. CP C: No dosage adjustments provided. Use with caution and titrate slowly. |
| Treprostinil inh | Hepatic via CYP2C8 |
Feces: 13% Urine: 79% |
Diarrhea, nausea. | No dosage adjustments provided. Use with caution and titrate slowly. |
| Treprostinil PO | Hepatic via CYP2C8 |
Feces: 13% Urine: 79% |
Diarrhea, nausea. |
C-P A: Use with caution and titrate slowly. C-P B: Avoid use. C-P C: Use is contraindicated. |
| Selexipag PO | Hepatic via CYP3A4, CYP2C8, UGT1A3 and UGT2B7. |
Feces: 93% Urine: - |
Diarrhea, nausea, vomiting, decreased appetite |
C-P A: No dosage adjustment necessary. C-P B: Once daily. C-P C: Avoid use |
| Iloprost inh | Hepatic via beta oxidation of the carboxyl side chain |
Feces: 12% Urine: 68% |
Nausea, vomiting, glossalgia |
CP A: No dosage adjustment necessary. C-P B, C: Consider increasing dosing interval |
Data was obtained from Lexicomp Online (http://online.lexi.com/lco/action/home), Wolters Kluwer, accessed in October 2017.
Excretion percentages are approximate.
Abbreviations: ALT: alanine aminotransferase, AST: aspartate aminotransferase, C-P: Child-Pugh, CYP: cytochrome P, IV: intravenous, PO: orally, SQ: subcutaneous, UGT: uridine 5’-diphosphate glucuronosyltransferases.
Phosphodiestearase-5 inhibitors are metabolized by the cytochrome P450 system (CYP3A4) and rarely cause liver injury (54). Endothelin receptor blockers are also metabolized by the cytochrome P450 system (CYP3A4, CYP2C9 and CYP2C19). A dose-dependent rise in liver function tests was observed in individuals receiving bosentan in its landmark trial (BREATHE-1) (55), possibly due to accumulation of cytotoxic bile that leads to liver cell damage (56). A relatively small study in patients with POPH treated with ambrisentan (n=13) showed no changes in hepatic transaminases (42). McGoon et al. (57) found that ambrisentan, at lower than the FDA approved dose, was well tolerated (without significant increases in liver function tests) in patients (n=36) who had experienced liver function test abnormalities while receiving bosentan or sitaxsentan.
Epoprostenol is metabolized by rapid hydrolysis and causes limited (< 1%) hepatic side effects. Treprostinil and iloprost are metabolized by the liver (CYP2C8 and beta oxidation, respectively). Some studies suggest that prostacyclin analogues exert a cytoprotective action on liver cells, an effect that might be beneficial in patients with PoPH (58, 59). Peterson et al. showed that the clearance of a single dose of oral treprostinil decreased with the severity of the hepatic impairment, resulting in higher plasma levels (~8-fold higher in patients with severe hepatic impairment) and more side effects (60).
e). The side effects of PAH-specific medications overlap with the clinical manifestations of liver disease
Patients with advanced liver disease have characteristic clinical manifestations, inherent to their disease and treatments, including nausea, vomiting, diarrhea, abdominal distension, anorexia, fatigue, malaise, weight loss, and fluid retention (61, 62). Patients with liver disease who develop PoPH might present with fatigue, dyspnea, dizziness, ascites and peripheral edema, especially as right heart failure ensues (63). These clinical manifestations may overlap with common side effects of PAH-specific medications such as nausea, vomiting, anorexia, and edema (Table 3); making it difficult to correctly attribute the origin of certain signs and symptoms to underlying medical conditions or side effects of PAH medications.
Table 3:
Barriers to treat PoPH patients with PAH-specific medications.
| 1. Accurate diagnosis |
| a. Need to rule out volume overload and high flow state as reasons for high mPAP |
| b. Measure portal venous gradient or adequately establish the presence of portal hypertension |
| 2. Limited evidence-based information |
| a. Scarce information supporting efficacy of PAH-specific therapies |
| b. Lack of clinical trials “proof” in PoPH |
| c. Unclear whether combination or triple PAH-specific therapy results in better outcomes in patients with PoPH. |
| d. Lack of a national PoPH registry |
| 3. Advanced liver disease and its complications have an impact on drug metabolism and treatment adherence |
| a. Overlap of liver signs/symptoms with medications side effects |
| b. Thrombocytopenia related to PAH, prostacyclin use and liver disease |
| c. Impact of hepatic encephalopathy on PAH treatment (e.g. parenteral administration, compliance with twice or thrice daily dosing, etc) |
| 4. Liver transplantation |
| a. Variable criteria to offer / deny liver transplantation among liver transplant teams and regional review boards |
| b. Restrictive criteria to obtain Model for End-stage Liver Disease.(MELD) exception points based on PoPH (95) |
| c. Not appreciating / recognizing the positive treatment effect on PVR, CO, and RV function when minimal changes in mPAP occur (23). |
| d. Limited studies documenting dose reduction/ discontinuation of PAH-specific therapies post-liver transplant |
In order to prevent or reduce side effects, PAH medications can be started at lower doses or titrated slowly; in addition, combination therapy can be initiated sequentially instead of concurrently to be able to ascribe side effects to a particular PAH medication. However, a gentle initiation and titration of PAH-specific medications may extend the time needed to achieve the pulmonary hemodynamic goals for liver transplantation; potentially delaying listing and increasing the risk of complications due to the underlying liver disease.
f). Effect of PAH-specific medications on portal hypertension:
There are limited data on the effect of PAH-specific medications on portal hypertension. The hepatic sinusoidal resistance is in part regulated by the nitric oxide-cyclic guanosine monophosphate system (64). Portal pressure may increase when there is a decrease in nitric oxide release by the liver sinusoidal endothelial cells (65, 66); an effect that could be mitigated by phosphodiesterase-5 inhibitors (67). Phosphodiesterase-5 inhibitors decrease the hepatic sinusoidal resistance (68) but also increase the splanchnic blood flow (69); accounting for the variable effects on the hepatic venous pressure gradient (39, 70, 71). Riociguat reduced the portal pressure in an animal model of biliary cirrhosis (72) but no data are available in humans.
Endothelin increases the intrahepatic vascular resistance, leading to portal hypertension (73, 74). In rats with biliary cirrhosis, bosentan decreased the portal pressure by reducing the hepatocollateral vascular resistance (75). In a mice model of cirrhosis, the chronic administration of endothelin receptor antagonists caused a reduction in liver fibrosis and portal pressure (76). Prostacyclin analogues increase the hepatic blood flow (77, 78). In an animal model of biliary cirrhosis, prostacyclin administration did not affect portal pressure (77). In patients with PoPH (n=8), Melgosa et al. showed that the hepatic venous pressure gradient and hepatic blood flow did not change at 30 and 60 minutes after the inhalation of iloprost (47).
g). Factors to consider in the selection of PAH-specific therapies:
When prescribing PAH-specific medications to patients with PoPH it is important to consider the presence of certain signs and symptoms as well as underlying medical conditions (e.g. renal failure). Patients with severe fluid retention may not be good candidates for endothelin receptor antagonists and patients with pronounced nausea and dyspepsia may not tolerate prostacyclin analogues. In addition, certain interactions are important to consider: ethanol may enhance the hypotensive effects of phosphodiesterase-5 inhibitors and increase the absorption of oral treprostinil, organic nitrates may increase the vasodilatory effect of phosphodiesterase-5 inhibitors, and cyclosporine may increase the serum concentrations of bosentan and ambrisentan.
Before making treatment decisions, it is essential to assess the living conditions, social support, adherence to other treatments, patient’s capacity to be educated on the use of different PAH-specific medications, insurance drug coverage, copays and eligibility for medication assistance programs.
h). Factors affecting the treatment of PoPH:
Certain factors germane to patients with PoPH (Table 3) may affect the intensity (1–3) and hence the effectiveness of PAH-specific therapies (79). Importantly, studies have shown that appropriate dosing as well as the use of combination therapy improve outcomes in PAH patients (80–82). The REVEAL registry included 118 patients with PoPH of whom 56% were treated with phosphodiesterase-5 inhibitors, 29 % with IV or SQ prostacyclin analogues, 14% with inhaled or PO prostacyclin analogues, 7% with endothelin receptor antagonists and 16% received no PAH-specific therapy at the time of inclusion. The proportion of treatment naïve patients decreased at 90 (11%) and 365 (5%) days from enrollment. Interestingly, patients with PoPH were less likely to receive PAH-specific treatment both at enrollment and 90 days compared to subjects with idiopathic or heritable PAH (10). In our experience, side effects of PAH medications appear to be more pronounced in patients with PoPH that other types of PAH.
Swanson et al. proposed to divide PoPH patients in two groups based on PAH severity. Patients with stable liver disease and mild to moderate PAH could be treated with oral therapies, with drug escalation based on response. Meanwhile, patients with moderate to severe PAH, particularly those with unstable liver disease, need to be treated more aggressively with parenteral prostacyclin therapy (83). The intensity of treatment depends on the severity of PAH and the degree of hemodynamic improvement required for liver transplantation. We particularly focus on decreasing the PVR since the mPAP may remain elevated due to a high PAWP in the setting of volume overload, or high cardiac output from the inherent hyperdynamic state– a key point to remember especially in liver transplant candidates.
i). Impact of PAH-specific therapies on liver transplantation eligibility:
There is no standardized approach for the management of PoPH, particularly in patients that are considered for liver transplantation. The general goals of treatment are as guidelines would recommend for other PAH types (84, 85). The hemodynamic treatment goal for safe liver transplantation (mPAP < 35 mmHg and PVR < 5 Wood units or PVR < 3 Wood units irrespective of mPAP with satisfactory right ventricular function by echocardiogram (22)) espoused by ILTS guidelines, can be attained via numerous medication options (17). This hemodynamic target for liver transplantation fluctuates among the institutions based on their multidisciplinary evaluation, comorbidities, inclusion of PVR in the hemodynamic evaluation and prior experiences with similar patients. Although predictors of waitlist mortality exist, i.e. PVR and MELD score (96), there are no clear predictors of treatment response. Until prospective studies address this issue, it may be prudent to use intravenous prostacyclin analogues and or combination therapies in the most severe cases of PoPH, especially if liver transplantation is to be considered (86). For example, in a liver transplant candidate with normal right ventricular function, a mPAP of 40 mmHg, cardiac index of 4.3 L/min and PVR of 3.5 Wood units, treatment with an oral PAH-specific therapy might be sufficient to achieve the goal of a mPAP < 35 mmHg or PVR < 3 Wood units. In contrast, in a liver transplant candidate with dilated and dysfunctional right ventricle with mean PAP of 50 mmHg, cardiac index of 2.2 L/min and PVR of 9 Wood units, parenteral prostacyclin analogues, sometimes in combination with oral agents, offer the best chance be able to meet the hemodynamic goals and list the patient for liver transplantation. Importantly, regardless of therapies, transplant in the setting of POPH remains higher risk and resolution of PoPH post-transplant is unpredictable. In addition, attaining an improvement and ideally a normalization of right ventricular function is of great importance, especially in transplant candidates.
Conclusions:
Treatment of PoPH patients is challenging and needs to be individualized. In comparison with treatment in other types of PAH, patients with PoPH have frequent side effects that limited the use of certain medications and the dose achieved. Furthermore, in patients with PoPH it is critical to optimize their clinical condition and hemodynamic status to minimize the perioperative risk associated with liver transplantation.
Figure 1:
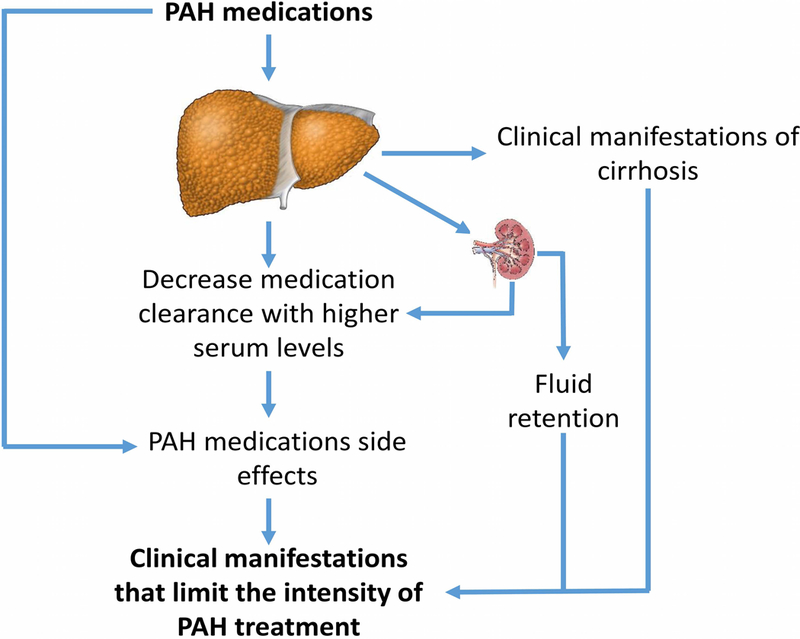
Barriers that limit the effectiveness of PAH therapy
Acknowledgments
Funding sources: A.R.T. is supported by NIH grant # R01HL130307.
Abbreviations:
- mPAP
mean pulmonary artery pressure
- MELD
Model for End-stage Liver Disease
- PAH
pulmonary arterial hypertension
- PAWP
pulmonary artery wedge pressure
- PH
pulmonary hypertension
- PoPH
portopulmonary hypertension
- PVR
pulmonary vascular resistance
- REVEAL
Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management
- WHO
World Health Organization
Footnotes
Conflict of interest statements:
Batool AbuHalimeh MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Michael J Krowka MD: Steering Committee member for PORTICO (Actelion-sponsored multicenter international study of macitentan in portopulmonary hypertension).
Adriano R. Tonelli MD MSc: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Contributor Information
Batool AbuHalimeh, Pathobiology Division, Lerner Research Institute. Cleveland Clinic, OH, USA. abuhalb@ccf.org.
Michael J Krowka, Department of Gastroenterology and Hepatology and Department of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, Minnesota, USA. krowka.michael@mayo.edu.
Adriano R. Tonelli, Department of Pulmonary, Allergy and Critical Care Medicine. Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA. tonella@ccf.org.
References:
- 1.Krowka MJ. Portopulmonary hypertension: diagnostic advances and caveats. Liver Transpl 2003;9:1336–1337. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MB, Committee ETFP-HVDPS. Pulmonary-Hepatic vascular Disorders (PHD). Eur Respir J 2004;24:861–880. [DOI] [PubMed] [Google Scholar]
- 3.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004;43:5S–12S. [DOI] [PubMed] [Google Scholar]
- 4.Galiè N, Simonneau G. [The Fifth World Symposium on Pulmonary Hypertension]. Turk Kardiyol Dern Ars 2014;42 Suppl 1:1–4. [PubMed] [Google Scholar]
- 5.Kumar A, Sharma P, Sarin SK. Hepatic venous pressure gradient measurement: time to learn! Indian J Gastroenterol 2008;27:74–80. [PubMed] [Google Scholar]
- 6.Hadengue A, Benhayoun MK, Lebrec D, Benhamou JP. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology 1991;100:520–528. [DOI] [PubMed] [Google Scholar]
- 7.Kawut SM, Taichman DB, Ahya VN, Kaplan S, Archer-Chicko CL, Kimmel SE, Palevsky HI. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transpl 2005;11:1107–1111. [DOI] [PubMed] [Google Scholar]
- 8.Colle IO, Moreau R, Godinho E, Belghiti J, Ettori F, Cohen-Solal A, Mal H, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology 2003;37:401–409. [DOI] [PubMed] [Google Scholar]
- 9.Krowka MJ, Swanson KL, Frantz RP, McGoon MD, Wiesner RH. Portopulmonary hypertension: Results from a 10-year screening algorithm. Hepatology 2006;44:1502–1510. [DOI] [PubMed] [Google Scholar]
- 10.Krowka MJ, Miller DP, Barst RJ, Taichman D, Dweik RA, Badesch DB, McGoon MD. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest 2012;141:906–915. [DOI] [PubMed] [Google Scholar]
- 11.Sithamparanathan S, Nair A, Thirugnanasothy L, Coghlan JG, Condliffe R, Dimopoulos K, Elliot CA, et al. Survival in portopulmonary hypertension: Outcomes of the United Kingdom National Pulmonary Arterial Hypertension Registry. J Heart Lung Transplant 2017;36:770–779. [DOI] [PubMed] [Google Scholar]
- 12.Swanson KL, Wiesner RH, Nyberg SL, Rosen CB, Krowka MJ. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant 2008;8:2445–2453. [DOI] [PubMed] [Google Scholar]
- 13.Chua R, Keogh A, Miyashita M. Novel use of sildenafil in the treatment of portopulmonary hypertension. J Heart Lung Transplant 2005;24:498–500. [DOI] [PubMed] [Google Scholar]
- 14.Zardi EM, Zardi DM, Giorgi C, Chin D, Dobrina A. Portopulmonary hypertension and hepatorenal syndrome. Two faces of the same coin. Eur J Intern Med 2017. [DOI] [PubMed] [Google Scholar]
- 15.Le Pavec J, Souza R, Herve P, Lebrec D, Savale L, Tcherakian C, Jais X, et al. Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med 2008;178:637–643. [DOI] [PubMed] [Google Scholar]
- 16.Krowka MJ, Mandell MS, Ramsay MA, Kawut SM, Fallon MB, Manzarbeitia C, Pardo M Jr., et al. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transpl 2004;10:174–182. [DOI] [PubMed] [Google Scholar]
- 17.Krowka MJ, Fallon MB, Kawut SM, Fuhrmann V, Heimbach JK, Ramsay MA, Sitbon O, et al. International Liver Transplant Society Practice Guidelines: Diagnosis and Management of Hepatopulmonary Syndrome and Portopulmonary Hypertension. Transplantation 2016;100:1440–1452. [DOI] [PubMed] [Google Scholar]
- 18.Freeman RB, Gish RG, Harper A, Davis GL, Vierling J, Lieblein L, Klintmalm G, et al. Model for end-stage liver disease (MELD) exception guidelines: results and recommendations from the MELD Exception Study Group and Conference (MESSAGE) for the approval of patients who need liver transplantation with diseases not considered by the standard MELD formula. Liver Transpl 2006;12:S128–136. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg DS, Fallon MB. Model for end-stage liver disease-based organ allocation: managing the exceptions to the rules. Clin Gastroenterol Hepatol 2013;11:452–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghofrani HA, Galie N, Grimminger F, Grunig E, Humbert M, Jing ZC, Keogh AM, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013;369:330–340. [DOI] [PubMed] [Google Scholar]
- 21.Stauber RE, Olschewski H. Portopulmonary hypertension: short review. Eur J Gastroenterol Hepatol 2010;22:385–390. [DOI] [PubMed] [Google Scholar]
- 22.Krowka MJ. Portopulmonary hypertension. Semin Respir Crit Care Med 2012;33:17–25. [DOI] [PubMed] [Google Scholar]
- 23.DeMartino ES, Cartin-Ceba R, Findlay JY, Heimbach JK, Krowka MJ. Frequency and Outcomes of Patients With Increased Mean Pulmonary Artery Pressure at the Time of Liver Transplantation. Transplantation 2017;101:101–106. [DOI] [PubMed] [Google Scholar]
- 24.Castro M, Krowka MJ, Schroeder DR, Beck KC, Plevak DJ, Rettke SR, Cortese DA, et al. Frequency and clinical implications of increased pulmonary artery pressures in liver transplant patients. Mayo Clin Proc 1996;71:543–551. [DOI] [PubMed] [Google Scholar]
- 25.Milani A, Zaccaria R, Bombardieri G, Gasbarrini A, Pola P. Cirrhotic cardiomyopathy. Dig Liver Dis 2007;39:507–515. [DOI] [PubMed] [Google Scholar]
- 26.Park SC, Beerman LB, Gartner JC, Zitelli BJ, Malatack JJ, Fricker FJ, Fischer DR, et al. Echocardiographic findings before and after liver transplantation. Am J Cardiol 1985;55:1373–1378. [DOI] [PubMed] [Google Scholar]
- 27.Reddy YN, Melenovsky V, Redfield MM, Nishimura RA, Borlaug BA. High-Output Heart Failure: A 15-Year Experience. J Am Coll Cardiol 2016;68:473–482. [DOI] [PubMed] [Google Scholar]
- 28.Faisal M, Siddiqi F, Alkaddour A, Bajwa AA, Shujaat A. Effect of PAH specific therapy on pulmonary hemodynamics and six-minute walk distance in portopulmonary hypertension: a systematic review and meta-analysis. Pulm Med 2014;2014:528783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fritz JS, Fallon MB, Kawut SM. Pulmonary vascular complications of liver disease. Am J Respir Crit Care Med 2013;187:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashfaq M, Chinnakotla S, Rogers L, Ausloos K, Saadeh S, Klintmalm GB, Ramsay M, et al. The impact of treatment of portopulmonary hypertension on survival following liver transplantation. Am J Transplant 2007;7:1258–1264. [DOI] [PubMed] [Google Scholar]
- 31.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, et al. 2015. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 32.Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, Olsson KM, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017;50. [DOI] [PubMed] [Google Scholar]
- 33.Boucly A, Weatherald J, Savale L, Jais X, Cottin V, Prevot G, Picard F, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017;50. [DOI] [PubMed] [Google Scholar]
- 34.Kylhammar D, Kjellstrom B, Hjalmarsson C, Jansson K, Nisell M, Soderberg S, Wikstrom G, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2017. [DOI] [PubMed] [Google Scholar]
- 35.Alameri HF, Sanai FM, Al Dukhayil M, Azzam NA, Al-Swat KA, Hersi AS, Abdo AA. Six Minute Walk Test to assess functional capacity in chronic liver disease patients. World J Gastroenterol 2007;13:3996–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Licata A, Corrao S, Petta S, Genco C, Cardillo M, Calvaruso V, Cabibbo G, et al. NT pro BNP plasma level and atrial volume are linked to the severity of liver cirrhosis. PLoS One 2013;8:e68364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gould L, Shariff M, Zahir M, Di Lieto M. Cardiac hemodynamics in alcoholic patients with chronic liver disease and a presystolic gallop. J Clin Invest 1969;48:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blei AT, Mazhar S, Davidson CJ, Flamm SL, Abecassis M, Gheorghiade M. Hemodynamic evaluation before liver transplantation: insights into the portal hypertensive syndrome. J Clin Gastroenterol 2007;41 Suppl 3:S323–329. [DOI] [PubMed] [Google Scholar]
- 39.Deibert P, Bremer H, Roessle M, Kurz-Schmieg AK, Kreisel W. PDE-5 inhibitors lower portal and pulmonary pressure in portopulmonary hypertension. Eur Respir J 2007;29:220–221. [DOI] [PubMed] [Google Scholar]
- 40.Cartin-Ceba R, Halank M, Ghofrani HA, Humbert M, Mattson J, Fritsch A, Krowka MJ. Riociguat treament for porto-pulmonary hypertension: a subgroup analysis from the Patent-1 study. Am J Respir Crit Care Med 2016;193:A7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoeper MM, Halank M, Marx C, Hoeffken G, Seyfarth HJ, Schauer J, Niedermeyer J, et al. Bosentan therapy for portopulmonary hypertension. Eur Respir J 2005;25:502–508. [DOI] [PubMed] [Google Scholar]
- 42.Cartin-Ceba R, Swanson K, Iyer V, Wiesner RH, Krowka MJ. Safety and efficacy of ambrisentan for the treatment of portopulmonary hypertension. Chest 2011;139:109–114. [DOI] [PubMed] [Google Scholar]
- 43.Sakai T, Planinsic RM, Mathier MA, de Vera ME, Venkataramanan R. Initial experience using continuous intravenous treprostinil to manage pulmonary arterial hypertension in patients with end-stage liver disease. Transpl Int 2009;22:554–561. [DOI] [PubMed] [Google Scholar]
- 44.Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci 2003;48:1622–1626. [DOI] [PubMed] [Google Scholar]
- 45.Schomerus H, Hamster W. Quality of life in cirrhotics with minimal hepatic encephalopathy. Metab Brain Dis 2001;16:37–41. [DOI] [PubMed] [Google Scholar]
- 46.Hoeper MM, Seyfarth HJ, Hoeffken G, Wirtz H, Spiekerkoetter E, Pletz MW, Welte T, et al. Experience with inhaled iloprost and bosentan in portopulmonary hypertension. Eur Respir J 2007;30:1096–1102. [DOI] [PubMed] [Google Scholar]
- 47.Melgosa MT, Ricci GL, García-Pagan JC, Blanco I, Escribano P, Abraldes JG, Roca J, et al. Acute and long-term effects of inhaled iloprost in portopulmonary hypertension. Liver Transpl 2010;16:348–356. [DOI] [PubMed] [Google Scholar]
- 48.Reichenberger F, Voswinckel R, Steveling E, Enke B, Kreckel A, Olschewski H, Grimminger F, et al. Sildenafil treatment for portopulmonary hypertension. Eur Respir J 2006;28:563–567. [DOI] [PubMed] [Google Scholar]
- 49.Barst RJ, McGoon M, McLaughlin V, Tapson V, Rich S, Rubin L, Wasserman K, et al. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol 2003;41:2119–2125. [DOI] [PubMed] [Google Scholar]
- 50.McLean AJ, Morgan DJ. Clinical pharmacokinetics in patients with liver disease. Clin Pharmacokinet 1991;21:42–69. [DOI] [PubMed] [Google Scholar]
- 51.Rodighiero V Effects of liver disease on pharmacokinetics. An update. Clin Pharmacokinet 1999;37:399–431. [DOI] [PubMed] [Google Scholar]
- 52.Savale L, Magnier R, Le Pavec J, Jaïs X, Montani D, O’Callaghan DS, Humbert M, et al. Efficacy, safety and pharmacokinetics of bosentan in portopulmonary hypertension. Eur Respir J 2013;41:96–103. [DOI] [PubMed] [Google Scholar]
- 53.Frey R, Becker C, Unger S, Schmidt A, Wensing G, Muck W. Assessment of the effects of hepatic impairment and smoking on the pharmacokinetics of a single oral dose of the soluble guanylate cyclase stimulator riociguat (BAY 63–2521). Pulm Circ 2016;6:S5–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolfhagen FH, Vermeulen HG, de Man RA, Lesterhuis W. Initially obscure hepatotoxicity attributed to sildenafil. Eur J Gastroenterol Hepatol 2008;20:710–712. [DOI] [PubMed] [Google Scholar]
- 55.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002;346:896–903. [DOI] [PubMed] [Google Scholar]
- 56.Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, Meier PJ. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 2001;69:223–231. [DOI] [PubMed] [Google Scholar]
- 57.McGoon MD, Frost AE, Oudiz RJ, Badesch DB, Galie N, Olschewski H, McLaughlin VV, et al. Ambrisentan therapy in patients with pulmonary arterial hypertension who discontinued bosentan or sitaxsentan due to liver function test abnormalities. Chest 2009;135:122–129. [DOI] [PubMed] [Google Scholar]
- 58.Bozkurt S, Ersoy E, Tekýn HE, Bayram O, Anadol Z, Onuk E, Ercan ZS. The cytoprotective effect of iloprost against carbon tetrachloride induced necrosis in rat liver. Res Commun Mol Pathol Pharmacol 1997;95:343–346. [PubMed] [Google Scholar]
- 59.Davies DS, Fawthrop DJ, Nasseri-Sina P, Wilson JW, Hardwick SJ, Boobis AR. Paracetamol toxicity and its prevention by cytoprotection with iloprost. Toxicol Lett 1992;64–65 Spec No:575–580. [DOI] [PubMed] [Google Scholar]
- 60.Peterson L, Marbury T, Marier J, Laliberte K. An evaluation of the pharmacokinetics of treprostinil diolamine in subjects with hepatic impairment. J Clin Pharm Ther 2013;38:518–523. [DOI] [PubMed] [Google Scholar]
- 61.Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol 2013;7:141–155. [DOI] [PubMed] [Google Scholar]
- 62.García-Pagán JC, Gracia-Sancho J, Bosch J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol 2012;57:458–461. [DOI] [PubMed] [Google Scholar]
- 63.Medarov BI, Chopra A, Judson MA. Clinical aspects of portopulmonary hypertension. Respir Med 2014;108:943–954. [DOI] [PubMed] [Google Scholar]
- 64.Wiest R, Groszmann RJ. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology 2002;35:478–491. [DOI] [PubMed] [Google Scholar]
- 65.Sarela AI, Mihaimeed FM, Batten JJ, Davidson BR, Mathie RT. Hepatic and splanchnic nitric oxide activity in patients with cirrhosis. Gut 1999;44:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shah V, Haddad FG, Garcia-Cardena G, Frangos JA, Mennone A, Groszmann RJ, Sessa WC. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J Clin Invest 1997;100:2923–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee KC, Yang YY, Huang YT, Lee FY, Hou MC, Lin HC, Lee SD. Administration of a low dose of sildenafil for 1 week decreases intrahepatic resistance in rats with biliary cirrhosis: the role of NO bioavailability. Clin Sci (Lond) 2010;119:45–55. [DOI] [PubMed] [Google Scholar]
- 68.Lee KC, Yang YY, Wang YW, Hou MC, Lee FY, Lin HC, Lee SD. Acute administration of sildenafil enhances hepatic cyclic guanosine monophosphate production and reduces hepatic sinusoid resistance in cirrhotic patients. Hepatol Res 2008;38:1186–1193. [DOI] [PubMed] [Google Scholar]
- 69.Deibert P, Schumacher YO, Ruecker G, Opitz OG, Blum HE, Rössle M, Kreisel W. Effect of vardenafil, an inhibitor of phosphodiesterase-5, on portal haemodynamics in normal and cirrhotic liver -- results of a pilot study. Aliment Pharmacol Ther 2006;23:121–128. [DOI] [PubMed] [Google Scholar]
- 70.Tzathas C, Christidou A, Ladas SD. Sildenafil (viagra) is a risk factor for acute variceal bleeding. Am J Gastroenterol 2002;97:1856. [DOI] [PubMed] [Google Scholar]
- 71.Clemmesen JO, Giraldi A, Ott P, Dalhoff K, Hansen BA, Larsen FS. Sildenafil does not influence hepatic venous pressure gradient in patients with cirrhosis. World J Gastroenterol 2008;14:6208–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwabl P, Brusilovskaya K, Riedl F, Bauer D, Strobel B, Fida S, Supper P, et al. The soluble guanylyl cyclase stimulator riociguat reduces liver fibrosis and portal pressure in cirrhotic rats. Z Gastroenterol 2015;53:P101. [Google Scholar]
- 73.Neuhofer W, Gülberg V, Gerbes AL. Endothelin and endothelin receptor antagonism in portopulmonary hypertension. Eur J Clin Invest 2006;36 Suppl 3:54–61. [DOI] [PubMed] [Google Scholar]
- 74.Pitts KR. Endothelin receptor antagonism in portal hypertension. Expert Opin Investig Drugs 2009;18:135–142. [DOI] [PubMed] [Google Scholar]
- 75.Sogni P, Moreau R, Gomola A, Gadano A, Cailmail S, Calmus Y, Clozel M, et al. Beneficial hemodynamic effects of bosentan, a mixed ET(A) and ET(B) receptor antagonist, in portal hypertensive rats. Hepatology 1998;28:655–659. [DOI] [PubMed] [Google Scholar]
- 76.Feng HQ, Weymouth ND, Rockey DC. Endothelin antagonism in portal hypertensive mice: implications for endothelin receptor-specific signaling in liver disease. Am J Physiol Gastrointest Liver Physiol 2009;297:G27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oberti F, Sogni P, Cailmail S, Moreau R, Pipy B, Lebrec D. Role of prostacyclin in hemodynamic alterations in conscious rats with extrahepatic or intrahepatic portal hypertension. Hepatology 1993;18:621–627. [PubMed] [Google Scholar]
- 78.Guarner F, Guarner C, Prieto J, Colina I, Quiroga J, Casas J, Freixa R, et al. Increased synthesis of systemic prostacyclin in cirrhotic patients. Gastroenterology 1986;90:687–694. [DOI] [PubMed] [Google Scholar]
- 79.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34–41. [DOI] [PubMed] [Google Scholar]
- 80.Galie N, Barbera JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, Peacock AJ, et al. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N Engl J Med 2015;373:834–844. [DOI] [PubMed] [Google Scholar]
- 81.Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galie N, Ghofrani HA, et al. Selexipag for the Treatment of Pulmonary Arterial Hypertension. N Engl J Med 2015;373:2522–2533. [DOI] [PubMed] [Google Scholar]
- 82.Pulido T, Adzerikho I, Channick RN, Delcroix M, Galie N, Ghofrani HA, Jansa P, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013;369:809–818. [DOI] [PubMed] [Google Scholar]
- 83.Swanson KL. Treatment of portopulmonary hypertension--isn’t it time to move forward? Liver Transpl 2008;14:270–271. [DOI] [PubMed] [Google Scholar]
- 84.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 85.Galie N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 2013;62:D60–72. [DOI] [PubMed] [Google Scholar]
- 86.Legros L, Chabanne C, Camus C, Fournet M, Houssel-Debry P, Latournerie M, Jezequel C, et al. Oral pulmonary vasoactive drugs achieve hemodynamic eligibility for liver transplantation in portopulmonary hypertension. Dig Liver Dis 2017;49:301–307. [DOI] [PubMed] [Google Scholar]
- 87.Hemnes AR, Robbins IM. Sildenafil monotherapy in portopulmonary hypertension can facilitate liver transplantation. Liver Transpl 2009;15:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fisher JH, Johnson SR, Chau C, Kron AT, Granton JT. Effectiveness of phosphodiesterase-5 inhibitor therapy for portopulmonary hypertension. Can Respir J 2015;22:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gough MS, White RJ. Sildenafil therapy is associated with improved hemodynamics in liver transplantation candidates with pulmonary arterial hypertension. Liver Transpl 2009;15:30–36. [DOI] [PubMed] [Google Scholar]
- 90.Krowka MJ, Frantz RP, McGoon MD, Severson C, Plevak DJ, Wiesner RH. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): A study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology 1999;30:641–648. [DOI] [PubMed] [Google Scholar]
- 91.Awdish RL, Cajigas HR. Early initiation of prostacyclin in portopulmonary hypertension: 10 years of a transplant center’s experience. Lung 2013;191:593–600. [DOI] [PubMed] [Google Scholar]
- 92.Kuo PC, Johnson LB, Plotkin JS, Howell CD, Bartlett ST, Rubin LJ. Continuous intravenous infusion of epoprostenol for the treatment of portopulmonary hypertension. Transplantation 1997;63:604–606. [DOI] [PubMed] [Google Scholar]
- 93.Sussman N, Kaza V, Barshes N, Stribling R, Goss J, O’Mahony C, Zhang E, et al. Successful liver transplantation following medical management of portopulmonary hypertension: a single-center series. Am J Transplant 2006;6:2177–2182. [DOI] [PubMed] [Google Scholar]
- 94.Fix OK, Bass NM, De Marco T, Merriman RB. Long-term follow-up of portopulmonary hypertension: effect of treatment with epoprostenol. Liver Transpl 2007;13:875–885. [DOI] [PubMed] [Google Scholar]
- 95.DuBrock HM, Goldberg DS, Sussman NL, Bartolome SD, Kadry Z, Salgia RJ, Mulligan DC, et al. Predictors of Waitlist Mortality in Portopulmonary Hypertension. Transplantation 2017;101:1609–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]


