Abstract
BACKGROUND & AIMS:
The American Consortium of Early Liver Transplantation for Alcoholic Hepatitis comprises 12 centers from 8 United Network for Organ Sharing regions studying early liver transplantation (LT) (without mandated period of sobriety) for patients with severe alcoholic hepatitis (AH). We analyzed the outcomes of these patients.
METHODS:
We performed a retrospective study of consecutive patients with a diagnosis of severe AH and no prior diagnosis of liver disease or episodes of AH, who underwent LT before 6 months of abstinence from 2006 through 2017 at 12 centers. We collected data on baseline characteristics, psychosocial profiles, level of alcohol consumption before LT, disease course and treatment, and outcomes of LT. The interval of alcohol abstinence was defined as the time between last drink and the date of LT. The primary outcomes were survival and alcohol use after LT, defined as slip or sustained.
RESULTS:
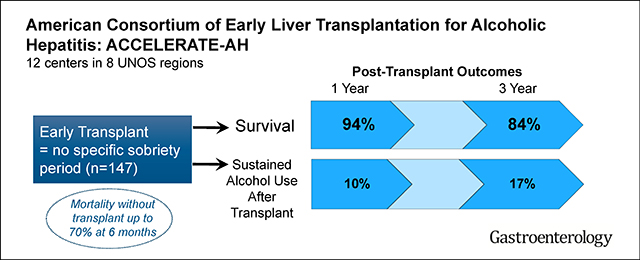
Among 147 patients with AH who received liver transplants, the median duration of abstinence before LT was 55 days; 54% received corticosteroids for AH and the patients had a median Lille score of 0.82 and a median Sodium Model for End-Stage Liver Disease score of 39. Cumulative patient survival percentages after LT were 94% at 1 year (95% confidence interval [CI], 89%–97%) and 84% at 3 years (95% CI, 75%–90%). Following hospital discharge after LT, 72% were abstinent, 18% had slips, and 11% had sustained alcohol use. The cumulative incidence of any alcohol use was 25% at 1 year (95% CI, 18%–34%) and 34% at 3 years (95% CI, 25%–44%) after LT. The cumulative incidence of sustained alcohol use was 10% at 1 year (95% CI, 6%–18%) and 17% at 3 years (95% CI, 10%–27%) after LT. In multivariable analysis, only younger age was associated with alcohol following LT (P = .01). Sustained alcohol use after LT was associated with increased risk of death (hazard ratio, 4.59; P = .01).
CONCLUSIONS:
In a retrospective analysis of 147 patients who underwent early LT (before 6 months of abstinence) for severe AH, we found that most patients survive for 1 year (94%) and 3 years (84%), similar to patients receiving liver transplants for other indications. Sustained alcohol use after LT was infrequent but associated with increased mortality. Our findings support the selective use of LT as a treatment for severe AH. Prospective studies are needed to optimize selection criteria, management of patients after LT, and long-term outcomes.
Keywords: Recidivism, Relapse, UNOS, 6 Months, ACCELERATE-AH
Graphical Abstract
This article has an accompanying continuing medical education activity, also eligible for MOC credit, on page e21. Learning Objective: Upon completion of this CME activity, successful learners will be able to determine if a patient with severe alcoholic hepatitis is appropriate for consideration of early liver transplantation and identify expected outcomes after transplantation
Alcoholic hepatitis (AH) is characterized by acute onset of jaundice due to excessive alcohol, accounting for approximately 4 of every 100,000 US hospital admissions and 10% of deaths from alcohol-related liver disease (ALD).1,2 True incidence is thought to be higher and is histologically present in 10%–35% of patients with alcohol use disorder.3 Severe AH, defined as Maddrey’s discriminant function ≥32, portends a 6-month mortality up to 70% if refractory to corticosteroids, as determined by Lille score >0.45.4,5 Few effective medical therapies exist6,7 and despite high mortality, liver transplantation (LT) as rescue therapy remains controversial.8,9
Most US centers require 6 months of abstinence pre-LT for ALD.1,10 The rationale was to provide time to determine whether liver function could improve to avoid LT. Critics argue this time period is not well supported by evidence and is an unreliable predictor of alcohol use post-LT.11–14 Most importantly, in severe AH, this requirement presents an unrealistic barrier—by definition, a patient with AH has recently consumed alcohol and 75%–90% of deaths occur within 2 months.5,11,15 Given the high mortality and lack of effective therapies, selected patients with severe AH may benefit from early LT (ie, before achieving 6 months of sobriety).
Despite significant controversy,8,9 US data to inform policy are sparse. US studies with LT for AH are limited to 2 small single-center experiences,16,17 and a 2004–2010 United Network Organ Sharing (UNOS) review,18 and although encouraging, suffers from small sample size or, in the UNOS cohort, imprecise case definition and lack of information regarding post-LT alcohol use.
Given that early LT offers potentially lifesaving therapy for severe AH, there is a need to better understand the post-LT outcomes that are achievable, both short and long term, as well as key factors influencing post-LT survival and alcohol use.8,19 The American Consortium of Early Liver Transplantation for Alcoholic Hepatitis (ACCELERATE-AH) is a multi-center observational study defining LT and alcohol-related outcomes in early LT for severe AH.
Methods
Study Population
Twelve LT centers provided detailed retrospective data on all patients receiving transplants for severe acute AH. Sites were queried about specific selection criteria, the process of selection, and post-transplantation care (Supplementary Table 1). Inclusion criteria were age older than 18 years, presentation with clinically diagnosed severe acute AH (ie, jaundice, prolonged international normalized ratio, chronic and recent alcohol use), no prior diagnosis of chronic liver disease or episodes of AH, and LT before 6 months of alcohol abstinence. Severity of the AH episode was based on Maddrey’s discriminant function, with severe defined as a score of 32 or higher. All patients included in this study had severe disease by this criterion. All patients had strong social support by family and friends, did not have severe comorbid medical disorders, and were expected to adhere to lifelong alcohol abstinence. All patients were evaluated by a transplantation social worker with detailed substance abuse evaluation before LT and, in all but 1 center, this evaluation is routinely supplemented by a separate evaluation by an addiction specialist. Exclusion criteria were other liver diseases (eg, viral hepatitis), human immunodeficiency virus, and other contraindications to LT. These inclusion and exclusion criteria are similar to those used in a European study published previously.15 Ten of 12 centers required full consensus agreement by the LT committee in the decision to list for early LT—2 centers did not utilize this as an inclusion criteria. Five centers had a designated provider leading the selection process. No center strictly excluded patients with recent gastrointestinal bleeding or recent infection, and only 2 centers strictly excluded patients with history of comorbid psychiatric disease, even if well controlled.
Investigators from each center retrospectively collected data about baseline characteristics, psychosocial profiles (eg, illicit substance abuse history, family history of alcohol use disorder, history of alcohol-related legal issues, and history of rehabilitation attempts), quantification of pre-LT alcohol use, clinical course (eg, medical therapy for AH, Lille score, changes in the Sodium Model for End-Stage Liver Disease [MELD-Na]), and post-LT outcomes (ie, graft failure, survival, and post-LT alcohol use). The interval of alcohol abstinence pre-LT was defined as the time between last drink and transplantation date. There was no mandated or prescribed abstinence period for any patient included in this study. Laboratory values were recorded at initial hospitalization for severe AH, transplant listing, and transplantation date. We assessed the concordance of clinician-diagnosed severe acute AH. We examined the concordance between clinician-diagnosed severe AH and the inclusion criteria for clinical trials in AH recently published by the National Institute on Alcohol Abuse and Alcoholism Alcoholic (NIAAA) Hepatitis Consortia. Their inclusion criteria are valuable, as they allow for a diagnosis of probable and possible AH using clinical and laboratory criteria alone and without a requirement for biopsy unless confounding factors are present.20 However, it is important to note that the NIAAA Alcoholic Hepatitis Consortia definitions exclude patients with very severe AH, defined as a Maddrey’s score >60 or MELD >30, from clinical trials.20 For the concordance assessment, we used inclusion criteria but not exclusion criteria, and for patients with confounding factors, such as sepsis or drug toxicity, we used explant histology to confirm AH.20 Lille scores were calculated for all patients; if they received corticosteroid therapy, the Lille score was calculated at day 7 from initiation of corticosteroids. If corticosteroids were not given, the Lille score was calculated at day 7 from initial hospitalization for AH (for interhospital transfers, if complete day-7 outside hospital records could not be obtained, this variable was coded as missing). Psychosocial profiles and quantification of pre-LT alcohol use were drawn from LT evaluation records.
All patients underwent LT between November 2006 and March 2017. Records of explant histology were evaluated for steatohepatitis (eg, ballooning hepatocytes, Mallory bodies, and neutrophil predominance) and fibrosis.
Assessment of Alcohol Use Post–Liver Transplantation
Centers queried LT recipients regarding any alcohol use and documented responses at every post-LT visit. Seven of 12 centers supplemented direct questioning with routine urinary ethyl glucuronide or blood phosphatidylethanol testing and 3 additional centers selectively performed ethyl glucuronide or phosphatidylethanol testing at the discretion of the managing provider.21,22 The remaining 2 centers monitored for alcohol use post-LT by direct patient questioning at every post-LT visit.
There is no standard definition of alcohol relapse.23 Any alcohol use post-LT was captured with the average daily alcohol consumption based on patient report. To distinguish between a “slip” and sustained alcohol use, patients were assessed as to whether they were still drinking at most recent follow-up.23 Thus, alcohol use post-LT was categorized as none, alcohol use with recovered sobriety (“slip”), or sustained alcohol use. To distinguish between sustained alcohol use and a recent slip, sustained use was a minimum duration of 100 days. Additionally, we defined binge drinking as >6 U/d for men or 4 U/d for women13 and frequent drinking as alcohol use in ≥4 d/ wk, regardless of quantity per day.13
Statistical Analysis
Demographic, clinical characteristics, and pre-LT disease management were described using frequency (percentage) and median (interquartile range [IQR]). Characteristics were compared by explant histology and by alcohol use post-LT using χ2 and Wilcoxon rank-sum tests. Post-LT alcohol use and survival rates were estimated separately using Kaplan-Meier method, with follow-up time starting at LT date and ending at date of first alcohol use and death, respectively. Patients without the event of interest were censored at last follow-up. Log-rank test compared survival by post-LT alcohol use. The proportion of LT recipients with post-LT alcohol use by differing post-LT center practices was compared using Fisher’s exact test.
We used logistic regression to determine which factors were associated with post-LT alcohol use. Factors with univariate P < .1 were included in the multivariable model. To evaluate risk of post-LT death, Cox proportional hazards regression estimated hazard ratios (HRs) and 95% CIs for factors of interest. We performed a sensitivity analysis by excluding the 8 patients who underwent LT before 2011, including probability of alcohol use post-LT, probability of post-LT death, univariate and multivariable analysis of predictors of alcohol use post-LT, and univariate and bivariate analysis of predictors of post-LT death. All analyses were completed using SAS, version 9.4 (SAS Institute Inc, Cary, NC).
This study was approved by the Institutional Review Board at each participating center. University of California at San Francisco is the designated coordinating center.
Results
Cohort Selection
A total of 147 patients from 12 LT centers across 8 UNOS regions met inclusion and exclusion criteria; 26 of 147 patients were described in prior publications,16,17 with updated follow-up data included in the current study. Nine of 12 centers, contributing 126 of 147 (86%) patients reliably obtained selection committee data. Of 432 patients with severe AH, 155 (35.9%) were listed and 126 (29.1%) underwent LT (20 died on waitlist, and 9 were delisted due to clinical improvement). The range of acceptance for listing among the 9 centers was 13%–100%. Primary reasons not to list were psychosocial (73%), medical contraindications (12%), clinical improvement during evaluation (8%), and financial/insurance (7%).
Cohort Characteristics
All patients received transplants between November 2006 and March 2017, with 94% of LTs occurring in 2011 and beyond. A total of 79% (116 of 147) would have met the NIAAA inclusion criteria for AH clinical trials.20 Median post-LT follow-up was 1.6 years (IQR, 0.8–3.0 years). Patients were 71% male, 83% were Caucasian, 66% were privately insured, and median pre-LT abstinence time was 55 days (IQR, 36–91 days). Baseline characteristics are summarized in Table 1. Complete records on pre-LT assessment of alcohol use disorder by an addiction specialist or medical social worker were available in all patients.
Table 1.
Baseline Characteristics (n = 147)
| Characteristic | Data |
|---|---|
| Age, y, median (IQR) | 43 (34–51) |
| Male, n (%) | 105 (73) |
| Race/ethnicity, n (%) | 122 (83) |
| Caucasian | |
| African American | 8 (5) |
| Hispanic | 8 (5) |
| Asian | 5 (3) |
| Other | 4 (3) |
| Employed, n (%) | 82 (56) |
| Private medical insurance, n (%) | 97 (66) |
| Married/stable companion, n (%) | 91 (62) |
| History of comorbid psychiatric disease, n (%) | 45 (31) |
| Substance abuse history, n (%) | 25 (17) |
| Active smoker | |
| Marijuana | 13 (9) |
| Non-marijuana illicit substance | 18 (12) |
| History of failed rehabilitation attempt, n (%) | 44 (30) |
| Family history of alcohol use disordera, n (%) | 56 (39) |
| History of alcohol-related legal issues, n (%) | 43 (29) |
| Alcohol consumption immediately before hospitalization,b U/d, median (IQR) | 10 (6–15) |
| Years of heavy drinking,c median (IQR) | 15 (8–25) |
| Received corticosteroids for AH, n (%) | 80 (54) |
| Hepatitis B surface antigen, n (%) | 0 (0) |
| Anti-hepatitis C antibody, n (%) | 1d (0) |
Three missing values.
Four missing values.
Nine missing values.
One patient with positive anti–hepatitis C virus (HCV) antibody with negative HCV RNA.
At initial hospitalization, median discriminant function was 78 (IQR, 58–103) and MELD-Na was 35 (IQR, 29–39). Median day-7 Lille score was 0.82 (IQR, 0.56–0.97). Median MELD-Na at listing was 38 (IQR, 34–40). Median interval between listing and LT was 7 days (IQR, 3–12 days).
Pretransplantation Management
Eighty of 147 (54%) patients received corticosteroid treatment for AH either alone or in combination with pentoxifylline. Corticosteroid therapy for AH was not given to 67 patients due to confirmed or presumed infection (n = 29), gastrointestinal bleeding (n = 10), renal injury (n = 8), other non–liver-related complications (n = 2), and per managing provider’s decision (n = 18). Thirteen patients excluded from corticosteroid therapy received pentoxifylline.
The median interval between initial hospitalization and listing date was 13 days (IQR, 6 to 31 days). Forty of 147 (27%) patients had improvement in MELD-Na from hospitalization to LT listing, with median improvement of 2 points (range, −1 to −11). Median MELD-Na among these patients at LT listing was 37 (range, 25 to 42); among patients with available Lille scores (14 missing values), all except 3 had day-7 Lille score >0.45. Clinical courses are detailed in Table 2.
Table 2.
Severity of Disease Characteristics (n = 147)
| Characteristic | Data |
|---|---|
| At initial hospitalization | |
| Sodium, mg/dL, median (IQR) | 133 (129 to 137) |
| INR, median (IQR) | 2.1 (1.7 to 2.5) |
| Bilirubin, mg/dL, median (IQR) | 26.3 (16.9 to 34.1) |
| Creatinine, mg/dL, median (IQR) | 1.86 (1.00 to 3.46) |
| White blood cell,a cells/mm3, median (IQR) | 16.4 (11.2 to 23.1) |
| Aspartate aminotransferase,a U/L, median (IQR) | 122 (80 to 182) |
| Alanine aminotransferase,a U/L, median (IQR) | 52 (34 to 84) |
| MELD-Na score,c median (IQR) | 35 (29 to 39) |
| Maddrey’s score,d median (IQR) | 78 (58 to 102) |
| During hospitalization | |
| Day-7 Lille score (all patients),b median (IQR) | 0.82 (0.56 to 0.97) |
| MELD-Na score at listing, median (IQR) | 38 (34 to 40) |
| MELD-Na score at LT, median (IQR) | 39 (35 to 40) |
| ΔMELD-Na from hospitalization to listing, median (IQR) | 1 (−1 to 6) |
| Increase, n (%) | 83 (56) |
| No change, n (%) | 24 (16) |
| Decrease, n (%) | 40 (27) |
| ΔMELD-Na from hospitalization to LT, median (IQR) | 2 (0 to 6.5) |
| Increase, n (%) | 96 (65) |
| No change, n (%) | 18 (11) |
| Decrease, n (%) | 33 (22) |
| Time between initial hospitalization and listing, d, median (IQR) | 13 (6 to 31) |
| Time between listing and LT, d, median (IQR) | 7 (3 to 12) |
INR, international normalized ratio.
Eleven missing values.
Thirty-four missing values. Seven patients underwent LT before 7 d from initial hospitalization.
The MELD-Na score measures the severity of end-stage liver disease and correlates with 90-d mortality, with a range from 4 to 40, calculated as follows: MELD = 3.78*log(bilirubin) + 11.2*log(INR) + 9.57*log(creatinine) + 6.43; MELD-Na = MELD + 1.32*(137-Na) – [0.033*MELD*(137-Na)].
The Maddrey’s discriminant function measures the severity of AH, with scores of 32 and higher indicating a severe episode, calculated as follows: 4.6 × (patient’s prothrombin time in seconds − control’s prothrombin time in seconds) + patient’s serum bilirubin level in milligrams per deciliter.
Post-Transplantation Survival
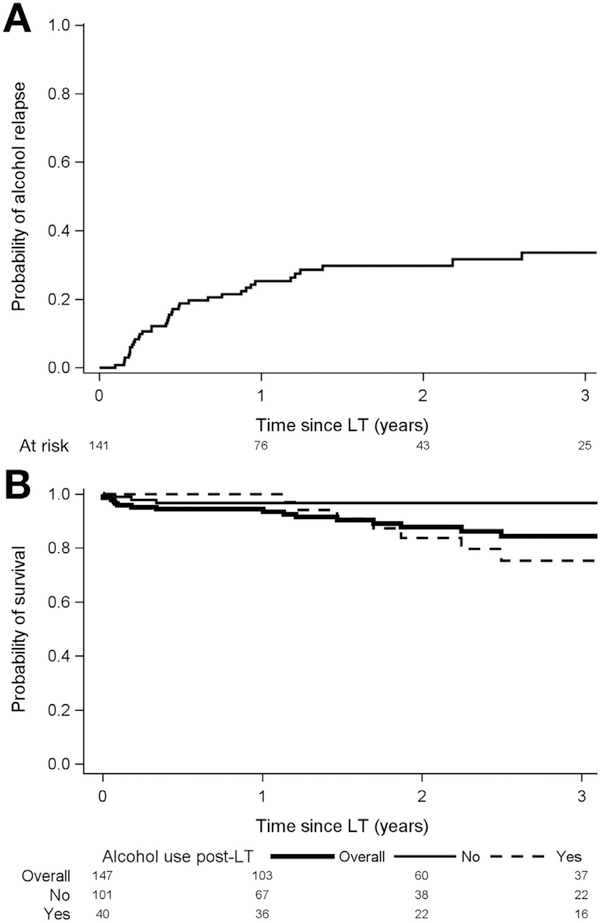
Cumulative post-LT survival at 1 and 3 years was 94% (95% CI, 89%–97%) and 84% (95% CI, 75%–90%). Predictors of death were more than 10 drinks per day at presentation (HR, 3.17; 95% CI, 1.04–9.67; P = .04) any alcohol use post-LT (HR, 3.54; 95% CI, 1.06–11.85; P = .04), and sustained alcohol use post-LT (HR, 4.59; 95% CI, 1.45–14.54; P = .01) (Table 3). Bivariate analysis of pre-LT factors associated with death showed the association of more than 10 drinks per day at presentation and post-LT death was modestly attenuated (HR, 2.77; P = .07) by use of corticosteroids (HR, 2.10; P = .17). Of 18 deaths, 9 occurred within 3 months of LT: 8 of these 9 received corticosteroid therapy for AH pre-LT (P = .04 vs no corticosteroids) and 5 died of sepsis. The other 9 deaths occurred more than 1 year post-LT (range, 1.1–6.0 years); 7 were alcohol-related (6 were due to graft failure secondary to recurrent ALD, and 1 due to alcohol overdose). In 141 patients surviving to home discharge, cumulative 3-year survival was 97% (95% CI, 90%–99%) in those with no alcohol use vs 75% (95% CI, 55%–88%) in those with post-LT alcohol use (P = .03) (Figure 1B).
Table 3.
Univariate Risk of Overall Post–Liver Transplantation Death
| Characteristic | HR (95% CI) | P value |
|---|---|---|
| Demographic | ||
| Sex, female | 1.16 (0.43–3.08) | .77 |
| Race, non-Caucasian | 1.16 (0.33–4.09) | .81 |
| Age (per year) | 1.01 (0.97–1.05) | .71 |
| Non–private insurance | 1.97 (0.78–4.97) | .15 |
| Single/divorced/widowed | 1.77 (0.66–4.73) | .25 |
| History of comorbid psychiatric disease | 0.79 (0.28–2.23) | .66 |
| History of illicit substance abuse | 0.37 (0.05–2.79) | .34 |
| History of failed rehabilitation attempt | 0.46 (0.13–1.59) | .22 |
| Family history of alcohol use disorder | 0.70 (0.26–1.93) | .50 |
| Unemployed immediately before hospitalization | 1.00 (0.39–2.54) | 1.00 |
| History of alcohol-related legal issues | 1.26 (0.47–3.38) | .64 |
| More than 10 drinks/d at presentation | 3.17 (1.04–9.67) | .04 |
| Years of heavy drinking | 1.01 (0.96–1.06) | .61 |
| Days of pretransplantation abstinence | 0.99 (0.98–1.00) | .14 |
| Clinical | ||
| Pre-LT corticosteroid use for AH | 2.52 (0.90–7.09) | .08 |
| Renal Replacement therapy at LT | 0.47 (0.19–1.20) | .12 |
| MELD-Na score at hospitalization | 0.97 (0.91–1.04) | .39 |
| MELD-Na score at listing | 0.97 (0.88–1.06) | .47 |
| MELD-Na score at LT | 0.95 (0.87–1.05) | .32 |
| ΔMELD-Na from hospitalization to LT | 1.01 (0.93–1.09) | .85 |
| Maddrey’s score | 1.00 (0.98–1.01) | .46 |
| Day-7 Lille score | 7.89 (0.45–139.00) | .16 |
| Post-LT alcohol use | ||
| Any alcohol use post-LT | 3.54 (1.06–11.85) | .04 |
| Sustained alcohol use post-LTa | 4.59 (1.45–14.54) | .01 |
NOTE. Bold type indicates variables significantly associated with increased risk of post-liver transplantation death, with P<.05.
Sustained alcohol use post-LT was defined as a patient who had any evidence of alcohol use post-LT, and was still drinking at last follow-up.
Figure 1.
(A) Kaplan-Meier curve for probability of alcohol use post-LT in 141 patients surviving to home discharge. The cumulative rate of alcohol use was 25%, 30%, and 34% at 1, 2, and 3 years, respectively. (B) Kaplan-Meier post-LT survival curve in 141 patients surviving to home discharge, stratified by no alcohol use post-LT (non-bolded solid line) vs alcohol use post-LT (dashed line).The cumulative survival at 1 and 3 years post-LT was 97% and 97% in those with no alcohol use compared to 100% and 75% in those with post-LT alcohol use (P = .03). The cumulative overall survival of the entire cohort (n = 147 bolded solid line) at 1 and 3 years. post-LT was 94% and 84%.
Post-Transplantation Alcohol Use
With a median follow-up of 1.6 years, among 141 LT recipients who were discharged home, 101 (72%) had no evidence of alcohol use post-LT, 25 (18%) had slips only, and 15 (11%) had sustained use (Table 4). Of the 40 patients with any alcohol use, first recognition by the transplantation team was by clinical interview in 25 (62%), positive urine ethyl glucuronide or phosphatidylethanol testing in 11 (28%), and liver biopsy in 4 (10%). Cumulative probability of any alcohol use was 25% (95% CI, 18%–34%) and 34% (95% CI, 25%–44%) at 1 and 3 years post-LT (Figure 1A). Median time to first alcohol use was 160 days (IQR, 79–346 days) post-LT. Cumulative probability of sustained alcohol use was 10% (95% CI, 6%–18%) and 17% (95% CI, 10%–27%) at 1 and 3 years post-LT. Of the 40 patients with any alcohol use, 33 (82%) were reported to have binge or frequent drinking.
Table 4.
Patterns of Alcohol Use Post–Liver Transplantation (n = 40)a
| Characteristics | Data |
|---|---|
| Any alcohol use post-LT, n (%) | |
| Slip onlyb | 25 (62) |
| Sustained alcohol usec | 15 (38) |
| Binge drinkingd | 29 (72) |
| Frequent drinkinge | 21 (52) |
| Both binge and frequent drinking | 17 (42) |
| Method of first detection of alcohol use post-LT, n (%) | |
| Clinical interview | 25 (62) |
| Urine ethyl glucuronide or phosphatidylethanol | 11 (28) |
| Liver biopsy | 4 (10) |
| Median time to first drink post-LT, d, median (IQR) | 160 (79–346) |
| Slip onlyb | 153 (69–331) |
| Sustained alcohol usec | 201 (93–386) |
Forty of 141 (28%) patients surviving to home discharge had evidence of any alcohol use post liver transplantation.
Slips were defined as any evidence of alcohol use, followed by sobriety at last follow-up.
Sustained alcohol use was defined as any alcohol use, and still drinking at last follow-up.
Binge drinking was defined as more than 6 units of alcohol in 1 d for men or 4 units for women.
Frequent drinking was defined as alcohol use in 4 d or more in a week, regardless of quantity per day.
In univariate analysis of pre-LT variables, younger age was associated with any alcohol use post-LT (Table 5). In multivariable analysis, only younger age was associated with alcohol use post-LT (odds ratio, 0.95; 95% CI, 0.92– 0.99; P = .01). The only predictor for sustained alcohol use (n = 15) was more than 10 drinks per day at presentation (odds ratio, 6.5; 95% CI, 1.4–30.4; P = .02).
Table 5.
Factors Associated With Alcohol Use Post Liver Transplantation
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| Characteristic | OR (95% CI) | P value | OR (95% CI) | P value |
| Sex, female | 1.32 (0.60–2.92) | .49 | — | — |
| Race, non-Caucasian | 1.66 (0.66–4.19) | .28 | — | — |
| Age (per year) | 0.95 (0.92–0.99) | .01 | 0.95 (0.91–0.99) | .01 |
| Non-private insurance | 1.29 (0.60–2.78) | .51 | — | — |
| Single/divorced/widowed | 1.54 (0.73–3.25) | .25 | — | — |
| History of comorbid psychiatric disease | 1.22 (0.56–2.64) | .62 | — | — |
| History of illicit substance abuse | 1.31 (0.45–3.76) | .62 | — | — |
| History of failed rehabilitation attempt | 1.56 (0.72–3.39) | .26 | — | — |
| Family history of alcohol use disorder | 1.33 (0.63–2.82) | .46 | — | — |
| Unemployed immediately before hospitalization | 1.22 (0.58–2.55) | .60 | — | — |
| History of alcohol-related legal issues | 2.03 (0.93–4.41) | .08 | 2.03 (0.91–4.52) | .08 |
| More than 10 drinks/d at presentation | 1.28 (0.60–2.72) | .53 | — | — |
| Years of heavy drinking | 0.98 (0.94–1.01) | .21 | — | — |
| Days of pretransplantation abstinence | 1.00 (0.99–1.01) | .74 | — | — |
NOTE. Bold type indicates variables significantly associated with alcohol use post liver transplantation, with P<.05. OR, odds ratio.
Of note, post-transplantation follow-up for alcohol relapse was not uniform across centers. The minority of centers offered pharmacologic therapy, had an on-site substance abuse counselor in the follow-up clinic, in-house psychosocial interventions (eg, motivational enhancement therapy), or a designated hepatologist or transplantation surgeon to follow patients undergoing LT for this indication. No center had a dedicated addiction unit officially incorporated into the transplant program. Two centers prescribed pharmacotherapy on a case-by-case basis post-LT and another 2 centers offered in-house psychosocial interventions (eg, motivational enhancement therapy). There was no difference in the overall proportion of patients with alcohol use post-LT in centers offering pharmacotherapy vs no pharmacotherapy (32% vs 27%; P = .68) or those offering in-house psychosocial interventions vs not (45% vs 25%; P = .07).
Liver Transplant Recipients Before 2011
In a sensitivity analysis excluding 8 patients who underwent LT before 2011 (n = 139), cumulative probability of alcohol use post-LT at 1 and 3 years was 26% (95% CI, 19%–35%) and 34% (95% CI, 25%–45%). Cumulative probability of post-LT death at 1 and 3 years was 95% (95% CI, 90%–98%) and 85% (95% CI, 75%–91%). Younger age remained the only predictor of alcohol use post-LT in multivariable analysis (odds ratio, 0.96; 95% CI, 0.92–0.99; P = .02). In bivariate analysis adjusting for corticosteroid use, alcohol use post-LT was a significant predictor of post-LT death (HR, 4.2; 95% CI, 1.05–17.0; P = .04).
Explant Histology
Of 146 patients with available explant histology records, 96% had cirrhosis; 59% had steatohepatitis, with the remainder having cirrhosis alone without concurrent steatohepatitis. Median time of abstinence pre-LT was lower among those with histologic steatohepatitis vs those without (48 vs 72 days; P = .01). Cumulative 1-year and 3-year post-LT survival was 92% (95% CI, 84%–96%) and 86% (95%-CI, 76%–92%) in those with histologic steatohepatitis vs 97% (95% CI, 87%–99%), and 83% (95% CI, 65%–92%) in those without (P = .69). In univariate analysis, histologic steatohepatitis was not associated with death (HR, 1.50; 95% CI, 0.56–4.00; P = .42).
Discussion
The ACCELERATE-AH study includes 12 LT programs in 8 UNOS regions, providing a comprehensive description of the US LT landscape for severe AH. Prior studies were from single centers, thus not necessarily representative of general experience, and lacked sample size to evaluate factors associated with post-LT outcomes. In severe AH, where 6-month mortality can reach 70%, our study shows that early LT is used across the United States as rescue therapy. Indeed, it seems incontrovertible that this indication saved patient lives: with median MELD-Na and Lille scores of 39 and 0.82, respectively, our cohort was predicted to have <20% median 6-month survival without LT,17,24 instead, survival was 94% and 84% at 1 and 3 years post-LT. These 3-year survival rates are excellent, comparable with LT survival rates reported for alcoholic cirrhosis, and higher than other well-accepted indications for LT (eg, hepatocellular carcinoma).25
There remains concern regarding alcohol use post-LT and its potential to cause graft loss and death. Studies show 1- and 5-year survival rates post-LT for ALD are similar to other indications,3,23,26 despite alcohol use rates ranging 8%–22% and 30%–40% at 1 and 5 years post-LT, respectively.13,27,28 In our cohort, albeit with relatively short follow-up, probability of any alcohol use was 25% (95% CI, 18%–34%) and 34% (95% CI, 25%–44%) at 1 and 3 years post-LT and sustained drinking was present in 11%. Although drinking patterns and likelihood of reestablishing sobriety might be different among those transplanted for AH vs non-acute ALD, the reported rates of alcohol use post-LT were not strikingly different. Nevertheless, achieving the lowest rates of harmful alcohol use post-LT possible is an important goal for all LT recipients.
This study highlights the importance of preventing harmful alcohol use, as sustained alcohol use post-LT was the strongest predictor of death. The majority of deaths beyond the perioperative period were alcohol-related, highlighting the need for tools to predict alcohol use post-LT and improved post-LT management of alcohol use disorder. This finding aligns with studies showing an association between return to heavy drinking post-LT and rapid allograft fibrosis and death.29,30 In contrast to prior studies,29,30 we observed fairly rapid allograft loss in a small number of patients with harmful patterns of alcohol use post-LT, with deaths secondary to recurrent ALD within 2 years of LT. Whether this is a reflection of earlier or heavier alcohol use post-LT than seen in other studies, or differences in sensitivity to alcohol (genetic or other factors) of individual patients with history of severe AH requires additional study. Early recognition of alcohol use post-LT and timely interventions would be important means of avoiding alcohol-related deaths. Younger age was identified as a potential predictor of alcohol use post-LT. Younger age has been found to be an association in cohorts examining alcohol use post-LT for ALD.29,30 These findings highlight the importance of careful patient selection, and characteristics (age, quantity of pre-LT alcohol use) that might help to refine selection to improve post-LT alcohol use prognostication and outcomes.
Although all patients underwent LT for the indication of clinically diagnosed severe AH, only 59% had histologic steatohepatitis on explant, with the remainder having cirrhosis alone without features of steatohepatitis, indicating not all met histologic definition of AH. This differs from studies where the clinical diagnosis of acute AH was discordant to histology in only approximately 25% of patients.31 However, we found the interval of alcohol abstinence pre-LT was significantly longer in those without histologic steatohepatitis (72 vs 48 days; P = .01), suggesting patients might lack histologic AH due to the time interval from active drinking to histologic evaluation. Our cohort may be more similar to real-world US practice, where liver biopsy to diagnose AH is uncommon.32,33 The proportion of patients (96%) with incidental cirrhosis on histology is consistent with other studies.7 The NIAAA recently published inclusion criteria for clinical studies in AH.20 Retrospectively, we determined that most (79%) of the patients in ACCELERATE-AH would have met inclusion criteria for AH clinical trials if explant histology is viewed as equivalent of pre-LT liver biopsy to confirm AH in patients with confounding factors. However, the NIAAA definition specifically excludes patients with very severe disease, defined as a Maddrey’s discriminant function >60 or MELD >30, which encompasses the majority of our study population. At this time, there are no standardized criteria to diagnose very severe disease, which highlights the need for additional study in this subpopulation at highest risk for mortality. We also acknowledge that despite a clinical diagnosis of AH, some might have undergone LT for acute on chronic liver failure due to alcohol-related cirrhosis rather than true AH, and prognostic scores in AH have generally been studied in biopsy-proven AH. However, with median MELD-Na of 39 among those without histologic steatohepatitis on explant, they would be unlikely to survive 6 months without LT.
Our experience differs from the 2011 European study by Mathurin et al.15 First, patients in the European study followed a strict prospective protocol, where only those with medically refractory (92% received corticosteroids for treatment of AH) and liver-biopsy proven AH underwent evaluation for early LT; our cohort is more representative of US practice in that almost half (46%) did not receive corticosteroids, and AH was clinically diagnosed rather than biopsy-proven.15 Additionally, in the European study, 6-month survival was 77%, whereas in our cohort, 1-year survival was 94%. This improved survival may be related to less use of pre-LT corticosteroids for AH in our cohort: 5 of 6 deaths in the European cohort were secondary to infection within 2 weeks post-LT, with a trend of longer duration of pre-LT corticosteroids in deceased patients.15 Similarly in our cohort, pre-LT corticosteroids were associated with early death, with infection as the main cause. Although additional study is needed to determine whether this association is causal, it would be prudent to ensure prompt discontinuation of corticosteroids as determined by Lille score in medical nonresponders. Our decreased pre-LT corticosteroid use reflects real-world practice, where many with severe AH present with contraindications to corticosteroids and data showing long-term survival benefit are lacking.
The previous European study suggested only 7.7% of referred patients were appropriate candidates for LT using a rigorous selection process.15 In our study, the rate of approval for LT by multidisciplinary transplantation committees for the indication of AH was 35.9%, but with significant variability among sites. Although this appears high, the denominator in our study included only patients formally presented to the LT committee, as it was not possible to reliably capture all of those patients with severe AH who were referred for consideration of LT but were incompletely evaluated. The variability among centers might reflect the degree of provider screening before formal LT evaluation and heterogeneity in selection practice among centers, with those with highest acceptance rates possibly reflecting a significant threshold of screening before referral or differences in selectivity by the LT committee. Our results highlight the need to generate prospective data, with protocolled selection and post-LT management of this patient population, to best inform national policy and US practice.
Our study has limitations. First, the study is retrospective and data are limited to those collected by the sites. While the relative lack of missing data attests to rigorous follow-up and documentation by participating centers, likely due to the exceptional circumstances by which the patients underwent LT, there is clear need for prospective studies in this field and higher-quality data to best inform US policy. Second is the lack of uniform assessment of alcohol use across sites. Alcohol use post-LT was generally assessed without use of standardized questionnaires, which likely underestimates true prevalence, with minor drinking more likely to be missed. However, all patients have been followed closely, and queried of any post-LT alcohol use at every visit. In addition, most centers practice routine ethyl glucuronide or phosphatidylethanol testing, which are sensitive assays for recent alcohol use. Although not ideal, our reporting is unlikely to miss “harmful” drinking per World Health Organization International Classification of Diseases, 10 revision guidelines, which manifests with organ damage or other physical, social, or psychiatric injury.34 Finally, there was incomplete information and a lack of standardization regarding prevention and management of alcohol use post-LT. Given the consequences of alcohol use post-LT, post-LT interventions to prevent alcohol use, or to prevent a slip from becoming sustained alcohol use, are needed, and best suited for a prospective study with protocolled alcohol use monitoring.
Despite these challenges, the study has several strengths. First, inclusion of multiple sites minimizes center-specific biases. Second, we captured data on both quantity and quality of alcohol use post-LT. While complete alcohol abstinence is desired, preventing slips from becoming harmful drinking may be an alternative strategy, especially applicable to AH patients.23 Indeed, our cohort shows a significant number with alcohol use post-LT able to regain sobriety: of 40 patients with reported alcohol use post-LT, 25 (62%) were no longer drinking at last follow-up. Third, our sample size and heterogeneity of patients has allowed identification of possibly important factors influencing outcomes (eg, pre-LT corticosteroid use and younger age).
In conclusion, the ACCELERATE-AH study found that early LT for severe AH achieves excellent 1- and 3-year survival rates. Sustained alcohol use, present in 11% post-LT, impacts survival. These results support the use of early LT as a rescue therapy for acute AH and highlight the need for prospective studies to assess strategies used to prevent and treat alcohol use post-LT.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
Severe alcoholic hepatitis portends high up to 70% 6-month mortality, and no medical therapies have been shown to benefit long-term survival. These patients are typically not liver transplant candidates.
NEW FINDINGS
3-year survival after liver transplant was high (84%) among patients with a pre-transplant diagnosis of severe alcoholic hepatitis. Sustained alcohol use after transplant was present in 11% of patients.
LIMITATIONS
This study was retrospective, did not have a control group for comparison, and did not address long-term outcomes.
IMPACT
The selective use of liver transplantation can be lifesaving for medically refractory alcoholic hepatitis, and the 3-year survival rate and frequency of alcohol use after transplant appear to be acceptable.
Acknowledgments
Funding
Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases: UCSF Liver Center P30 DK026743 and T32 DK060414 to Brian P. Lee.
Abbreviations used in this paper:
- AH
alcoholic hepatitis
- ALD
alcohol-related liver disease
- HR
hazard ratio
- IQR
interquartile range
- LT
liver transplantation
- MELD-Na
Sodium Model of End-Stage Liver Disease
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
- UNOS
United Network Organ Sharing
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2018.04.009.
References
- 1.Yang AL, Vadhavkar S, Singh G, et al. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med 2008;168:649–656. [DOI] [PubMed] [Google Scholar]
- 2.Paula H, Asrani SK, Boetticher NC, et al. Alcoholic Liver disease-related mortality in the United States: 1980– 2003. Am J Gastroenterol 2010;105:1782–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol 2010;105:14–32. [DOI] [PubMed] [Google Scholar]
- 4.Mathurin P Corticosteroids for alcoholic hepatitis— what’s next? J Hepatol 2005;43:526–533. [DOI] [PubMed] [Google Scholar]
- 5.Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007;45:1348–1354. [DOI] [PubMed] [Google Scholar]
- 6.Thursz MR, Richardson P, Allison M, et al. Prednisolone or Pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;372:1619–1628. [DOI] [PubMed] [Google Scholar]
- 7.Ramond M, Poynard T, Rueff B. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med 1992;326:507–512. [DOI] [PubMed] [Google Scholar]
- 8.Lucey MR. Liver transplantation for severe alcoholic hepatitis—the PRO view. Liver Int 2017;37:343–344. [DOI] [PubMed] [Google Scholar]
- 9.Fung JYY. Liver transplantation for severe alcoholic hepatitis—the CON view. Liver Int 2017;37:340–342. [DOI] [PubMed] [Google Scholar]
- 10.Lucey M, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009;360:2758–2769. [DOI] [PubMed] [Google Scholar]
- 11.Donckier V, Lucidi V, Gustot T, Moreno C. Ethical considerations regarding early liver transplantation in patients with severe alcoholic hepatitis not responding to medical therapy. J Hepatol 2014;60:866–871. [DOI] [PubMed] [Google Scholar]
- 12.Dureja P, Lucey MR. The place of liver transplantation in the treatment of severe alcoholic hepatitis. J Hepatol 2010;52:759–764. [DOI] [PubMed] [Google Scholar]
- 13.Dimartini A, Day N, Dew MA, et al. Alcohol consumption patterns and predictors of use following liver transplantation for alcoholic liver disease. Liver Transpl 2006;412:813–820. [DOI] [PubMed] [Google Scholar]
- 14.Artru F, Louvet A, Mathurin P. Liver transplantation for patients with alcoholic hepatitis. Liver Int 2017; 37:337–339. [DOI] [PubMed] [Google Scholar]
- 15.Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011;365:1790–1800. [DOI] [PubMed] [Google Scholar]
- 16.Im GY, Kim-Schluger L, Shenoy A, et al. Early liver transplantation for severe alcoholic hepatitis in the United States—a single-center experience. Am J Transplant 2016;16:841–849. [DOI] [PubMed] [Google Scholar]
- 17.Lee BP, Chen P-H, Haugen C, et al. Three-year results of a pilot program in early liver transplantation for severe alcoholic hepatitis. Ann Surg 2017;265:20–29. [DOI] [PubMed] [Google Scholar]
- 18.Singal AK, Bashar H, Anand BS, et al. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: Exploratory analysis from the UNOS database. Hepatology 2012;55:1398–1405. [DOI] [PubMed] [Google Scholar]
- 19.Ursic-Bedoya J, Faure S, Donnadieu-rigole H, Pageaux G. Liver transplantation for alcoholic liver disease : lessons learned and unresolved issues. World J Gastroenterol 2015;21:10994–11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crabb DW, Bataller R, Chalasani NP, et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016;150:785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piano S, Marchioro L, Gola E, et al. Assessment of alcohol consumption in liver transplant candidates and recipients: the best combination of the tools available. Liver Transplant 2014;20:815–822. [DOI] [PubMed] [Google Scholar]
- 22.Staufer K, Andresen H, Vettorazzi E, et al. Urinary ethyl glucuronide as a novel screening tool in patients pre- and post-liver transplantation improves detection of alcohol consumption. Hepatology 2011; 54:1640–1649. [DOI] [PubMed] [Google Scholar]
- 23.Lucey MR. Liver transplantation for alcoholic liver disease. Nat Rev Gastroenterol Hepatol 2014;11:300–307. [DOI] [PubMed] [Google Scholar]
- 24.Louvet A, Labreuche J, Artru F, et al. Combining data from liver disease scoring systems better predicts outcomes of patients with alcoholic hepatitis. Gastroenterology 2015;149:398–406. [DOI] [PubMed] [Google Scholar]
- 25.Kim WR, Smith JM, Skeans A, et al. OPTN/SRTR 2012 annual data report: liver. Am J Transplant 2012; 14(Suppl 1):69–96. [DOI] [PubMed] [Google Scholar]
- 26.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2015 annual data report: liver. Am J Transplant 2017;17 (Suppl 1):174–251. [DOI] [PubMed] [Google Scholar]
- 27.Dimartini A, Dew MA, Day N, et al. Trajectories of alcohol consumption following liver transplantation. Am J Transplant 2010;10:2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleming MF, Smith MJ, Oslakovic E, et al. Phosphatidylethanol detects moderate-to-heavy alcohol use in liver transplant recipients. Alcohol Clin Exp Res 2017; 41:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice JP, Eickhoff J, Agni R, et al. Abusive drinking after liver transplantation is associated with allograft loss and advanced allograft fibrosis. Liver Transpl 2013;19: 1377–1386. [DOI] [PubMed] [Google Scholar]
- 30.Dumortier J, Dharancy S, Cannesson A, et al. Recurrent alcoholic cirrhosis in severe alcoholic relapse after liver transplantation: a frequent and serious complication. Am J Gastroenterol 2015;110:1160–1166. [DOI] [PubMed] [Google Scholar]
- 31.Hardy T, Wells C, Kendrick S, et al. White cell count and platelet count associate with histological alcoholic hepatitis in jaundiced harmful drinkers. BMC Gastroenterol 2013;13:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieber SR, Rice JP, Lucey MR, Bataller R. Controversies in clinical trials for alcoholic hepatitis. J Hepatol 2018; 68:586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen TA, DeShazo JP, Thacker LR, et al. The worsening profile of alcoholic hepatitis in the United States. Alcohol Clin Exp Res 2016;40:1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders. Int Classif 1992;10: 1–267. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.