Abstract
The effect of a poly herbal drug Majun Baladur (MB) was studied on the transgenic Drosophila melanogaster expressing human alpha synuclein in the neurons (PD flies). The equivalents of recommended dose for human were established for 20 g of fly food i.e. 0.0014, 0.0028, 0.0042 and 0.0056 g per 20 g of diet. The PD flies were allowed to feed on it for 24 days before performing the assays. The exposure to MB increased the life span and improves the activity of PD flies. The PD flies exposed to 0.0014, 0.0028, 0.042 and 0.0056 g of MB showed a dose dependent significant delay of 1.47, 1.88, 2.52 and 3.05 folds in the climbing ability compared to unexposed PD flies. A dose dependent significant decrease of 1.38, 1.45, 1.48 and 1.65 folds in TBARS; 1.08, 1.11, 1.17 and 1.20 folds in the GST activity; 1.20, 1.28, 1.39 and 1.52 folds in the PC content; 1.43, 1.53, 1.65 and 1.79 folds in the Caspase-9 activity; 1.21, 1.31, 1.53 and 1.64 folds in the activity of Caspase-3 and 1.24, 1.42, 1.50 and 1.79 folds in the activity of catalase; 1.50, 1.63, 1.88 and 2.06 folds in the activity of SOD in PD flies exposed to 0.0014, 0.0028, 0.042 and 0.0056 g of MB, respectively. A significant dose dependent increase of 1.20, 1.29, 1.33 and 1.44 folds in as NPSH content was observed in PD flies exposed to 0.0014, 0.0028, 0.042 and 0.0056 g of MB, respectively. The exposure to MB protects the loss of dopaminergic neurons as is evident by immunohistochemistry. It is concluded that MB is potent in reducing the PD symptoms being mimicked in the transgenic flies.
Keyword: Neuroscience
1. Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder. Recent reports suggest that PD affects about 1% of the population [1]. The selective loss of dopaminergic neurons in the substantia nigra pars compacta is considered to be one of the pathological hallmark of PD [2]. The loss of dopaminergic neurons in brain leads to the deficiency of dopamine and results in behavioral abnormality [3]. The abnormal protein aggregation (α-synuclein) and oxidative stress is considered to be the main cause for the damage of dopaminergic neurons [4]. The over-expression of either wild or mutant type of α synuclein leads to the formation of Lewy bodies [3]. The formation of Lewy bodies not only results in the death of dopaminergic neurons but also increase the oxidative stress in the brain (as the degenerating neurons will also produce reactive oxygen species) [5, 6]. Due to the involvement of ethical issues it has become difficult to study the effects of naturally occurring or synthetic compounds on PD patients. The screening process has been enhanced by the development of different models primarily based on α-synuclein (αS) either transgenic, mutant or wild forms in mice, flies and C. elegans [7]. By using the yeast-based UAS (Upstream Activation Sequence)-Gal-4 systems for the activation of gene expression in Drosophila the transgenic Drosophila models have been developed for studying various human diseases, including the neurodegenerative disorders [8]. The transgenic Drosophila expressing either wild type or familial PD linked mutants (A53T and A30P) of human α-synuclein is widely accepted and is currently being used to study the various aspects associated with the disease [9]. These flies replicate several features of human PD such as locomotor dysfunction, Lewy body formation and age dependent loss of dopaminergic neurons [3]. As there is no permanent cure for the disease and the efficacy of the synthetic drugs also decline after some period of the treatment [10], the current research is now directed towards finding naturally occurring antioxidants of plant origin [11]. In this context, the unani system of traditional medicine is widely accepted in Indian sub continent and is practiced in India [12]. Majun Baladur (MB) is a polyherbal formulation, prescribed to patients having neurodegenerative ailments, under unani system of traditional medicine. Till date, no one has attempted to study the effect of MB on PD patients or in any in vitro/in vivo models expressing PD/PD like symptoms. Hence, we decided to study the effect of MB on the transgenic Drosophila model of PD. The traditional medicines have been proved therapeutically/prophylactically fruitful against manifold diseases [13]. In the present study the effect of MB was studied on the transgenic Drosophila model of PD.
2. Material and methods
2.1. Preparation of MB and establishment of doses
The doses of MB were established as per the method described by Jansen et al. [14]. The equivalents of recommended dose for human were established per 20g of fly food, and we studied the effect of 0.0014, 0.0028, 0.042 and 0.0056g per 20 g of diet. The PD flies were allowed to feed on it for 24 days. MB was prepared by using the useful parts of the medicinal plants as listed in Table 1, according to the National Formulary of Unani Medicine Part-I, Convention of Research in Unani medicine, Ministry of health and Family Welfare (Department of AYUSH), Government of India. After preparation, the MB was tested for the presence of Heavy metals (Lead, Mercury, Arsenic, Cadmium), Aflatoxins (Aflatoxin B1, Aflatoxin G1, Aflatoxin B2, Aflatoxin G2) and Pesticides (Alachlor, Aldarin & Dieldrin, Azinophos-methyl, Bromopropylate, Chlordane, Chlorfenvinphos, Chlorpyrifos, Chlorpyrifos-methyl, Cypermethrin, DDT, Deltamethrin, Diazinon, Dichlorvos, Diathiocarbamates, Endosulfan, Endrin, Ethion, Fenitrothion, Fenvalerate, Fonofos, Heptachlor, Hexachlorobenzene, Hexachlorocyclohexene isomer, Lindane, Malathion, Methidathion, Parathion, Parathion Methyl, Permethrin, Phosalone, Piperonyl butaoxide, Primiphos Methyl, Pyrethrins, Quintozen). Test for Bacterial/Yeast and moulds were also performed. The test for specific pathogens such as E. coli, Salmonella, S. aureus, P. aeruginosa were also carried out.
Table 1.
Medicinal plant ingredients of Majun Baladur (a Unani polyherbal druga).
| Unani names | Botanical names/Zoological name | Family name | Part used | Weight (g) |
|---|---|---|---|---|
| Kunjad | Sesamum indicum | Pedaliaceae | Seed | 30 |
| Maghz-e-Tukhm-e-Baladur | Semicarpus anacardium | Anacardiaceae | Kernel | 30 |
| Maghz-e-Badam | Prunus amygdalus | Rosaceae | Kernel | 30 |
| Maghz-e-Chilghoza | Pinus gerardina | Pinaceae | Kernel | 30 |
| Asgand | Withania somnifera | Solanaceae | Root | 30 |
| Aqarqarha | Anacyclus pyrethrum | Compositae | Root | 30 |
| Khulajan | Alpinia galango | Zingiberaceae | Root | 30 |
| Bisbasa | Myristica fragrans | Myristicaceae | Aril | 30 |
| Jauzbuwa | Myristica fragrans | Myristicaceae | Nutmeg | 20 |
| Zanjabeel | Zingiber officinale | Zingiberaceae | Rhizome | 20 |
| Salab Misri | Orchis latifolia | Orchidaceae | Tuberous Root | 20 |
| Filfil Daraz | Piper longum | Piperaceae | Seed | 15 |
| Mastagi | Pistacia lentiscus | Anacardiaceae | Resin | 15 |
| Tukhm-e-Halyun | Asparagus officinalis | Liliaceae | Seed | 15 |
| Tukhm-e-Gazar | Daucus carota | Umbelliferae | Seed | 10 |
| Tukhm-e-Anjara | Ficus carica | Moraceae | Seed | 10 |
| Tukhm-e-Konch | Macuna prurita | Leguminosae | Seed | 10 |
| Zafran | Crocus sativus | Iridaceae | Style and stigma | 10 |
| Samundar Sokh | Salvia plebeia | Lamiaceae | Seed | 5 |
| Qand Safaid (Granular sugar) | A product made from sugarcane | 375 | ||
| Asal (Honey) | Apis mellifera | 1000 |
Ref. National Formulary of Unani medicine, Part-I, page 122-23, Central council for Research in Unani Medicine, Govt. of INDIA, Ministry of health and Family Welfare (Department of AYUSH), New Delhi.
2.2. GC-MS analysis
The extract of MB was prepared in acetone as per the method described in our earlier published work [15]. The extract was subjected to GC-MS analysis and content were analysed using Mass meter GC/MS acquisition B.07.06.270418-18-July-2017, Agilent Technologies, Inc.
2.3. Evaluation of antioxidant properties of MB
2.3.1. Superoxide anion scavenging assay
The inhibition of Nitroblue tetrazolium (NBT) reduction by phenazin methosulphate (PMS) generated O2·− was used to determine the superoxide anion scavenging activity of the MB [16]. The reaction mixture consist of 75 μl of each concentration of MB, 750 μl of Tris HCl (100 mM; pH 7.4); 187 μl of NBT (300 μM), 187 μl of NADH (936 μM). The reaction was initiated by adding phenazin methosulphate (PMS) (120 μM). The reaction mixture was incubated at 25 °C for 5 min and the OD was read at 560 nm and the degree of scavenging was calculated by the following equation:
2.3.2. Diphenyl-picrylhydrazyl (DPPH) free radical scavenging
For estimating free radical scavenging potential of the MB, DPPH method as described by Wongsawatkul et al [17] was used in the present study. When DPPH (a stable purple color) react with an antioxidant, it is reduced to yield a light yellow coloured diphenyl picrylhydrazine. Color change was spectrophotometrically measured. The reaction mixture consists of 500μl of each concentration of MB and 250 μl of DPPH (0.3 mM). The reaction mixture was shaken vigorously and allowed to stand at room temperature in the dark for 25 min. The OD was read at 518 nm and the radical scavenging activity was calculated by the following equation:
| % Radical scavenging = (1 − Absorbance of sample/Absorbance of control) × 100 |
2.4. Drosophila stocks
Transgenic fly lines that express wild-type human synuclein (h-αS) under UAS control in neurons “[w[*]; P{w[+mC] = UAS–Hsap/SNCA.F}” 5B and GAL4 “w[*]; P{w[+mC] = GAL4- elavL}”3] were obtained from Bloomington Drosophila Stock Centre (Indiana University, Bloomington, IN). When the males of UAS (Upstream Activation Sequence)-Hsap/SNCA.F strains are crossed with the females of GAL4-elav. L (vice-versa), the progeny will express human αS in the neurons [3].
2.5. Drosophila culture and crosses
The flies were cultured on standard Drosophila food containing agar, corn meal, sugar and yeast at 25 °C (24 ± 1) [18] . Crosses were set up as described in our earlier published work [19]. The PD flies were allowed to feed separately on different doses of MB mixed in the diet for 24 days. The PD flies were also exposed separately to 10−3 M of L-dopamine. The UASHsap/SNC.F act as a control. The control flies were allowed separately to feed on the selected doses of MB.
2.6. Drosophila activity pattern analysis
From the 12th day the activity of flies (males) in all treated groups were analyzed by using Drosophila Activity Monitor (TriTek, USA). The activity was recorded every hour for a total of 311 h and the data was analyzed by Actogram J software. The results were presented as chi-square periodogram [20, 21].
2.7. Drosophila climbing assay
The climbing assay was performed as described by Pendleton et al. [22]. Ten flies were placed in an empty glass vial (10.5 cm × 2.5 cm). A horizontal line was drawn 8 cm above the bottom of the vial. After the flies had acclimated for 10 min at room temperature, both controls and treated groups were assayed at random, to a total of 10 trials for each. The mean values were calculated and then averaged and a group mean and standard error were obtained.
2.8. Drosophila life span determination
Newly enclosed flies ( non-PD control and PD flies) were placed in the culture tubes (10 flies per tube) containing desired concentration of the MB. The flies were transferred to a new diet at every 3rd day containing desired concentration of the MB till the last one died [23].
2.9. Preparation of homogenate for biochemical assays
Fly heads from each group were isolated (50 heads/group; five replicates/group) and the homogenate was prepared in 0.1 M phosphate buffer for the biochemical assays.
2.10. Estimation of thiobarbituric acid reactive species (TBARS)
TBARS were measured according to the method described by Ohkawa et al [24]. The reaction mixture consisted of 5μl of 10 mM butyl-hydroxy toluene (BHT), 200μl of 0.67% thiobarbituric acid, 600μl of 1% O-phosphoric acid, 105μl of distilled water and 90 μl of supernatant. The resultant mixture was incubated at 90 °C for 45 min and the OD was measured at 535 nm. The results were expressed as μ moles of TBARS formed/h/gram tissue.
2.11. Estimation of glutathione-S-transferase (GST) activity
The glutathione-S-transferase activity was determined by the method of Habig et al [25]. The reaction mixture consist of 500μl of 0.1 M phosphate buffer, 150 μl of 10 mM CDNB, 200 μl of 10 mM reduced glutathione and 50 μl of supernatant. The OD was taken at 340 nm and the enzyme activity was expressed as μ moles of CDNB conjugates/min/mg protein.
2.12. Estimation of non-protein thiol (NPSH) content
The Non-protein thiol (NPSH) content was estimated colorimetrically using Ellman's reagent (DTNB) according to the procedure described by Jollow et al. [26]. The supernatant was precipitated with 4% sulphosalicyclic acid in the ratio of 1:1. The samples were kept at 4 °C for 1 hr and then subjected to centrifugation at 5000 rpm for 10 min at 4 °C. The assay mixture consisted of 550 μl of 0.1M phosphate buffer, 100 μl of supernatant and 100 μl of DTNB. The OD was read at 412 nm and the results were expressed as μ moles of NPSH/gram tissue.
2.13. Estimation of protein carbonyl content (PCC)
The PC content was estimated according to the protocol described by Hawkins et al [27]. The head homogenate was diluted to a protein concentration of approx 1 mg/ml. About 250 μl of each diluted homogenate was taken in eppendorf centrifuge tubes separately. To it 250 μl of 10 mM 2,4-dinitrophenyl hydrazine (dissolved in 2.5M HCl) was added, vortexed and kept in dark for 20 min. About 125 μl of 50% (w/v) trichloroacetic acid (TCA) was added, mixed thoroughly and incubated at −20 °C for 15 min. The tubes were then centrifuged at 4 °C for 10 min at 9000 rpm. The supernatant was discarded and the pellet obtained was washed twice by ice cold ethanol: ethyl acetate (1:1). Finally, the pellets were re-dissolved in 1 ml of 6M guanidine hydrochloride and the absorbance was read at 370 nm.
2.14. Assay for caspase-9 (Dronc) and caspase-3 (Drice) activities
The assay was performed according to the manufacturer protocol with some modification (Bio-Vision, CA, USA). The assay was based on spectrophotometric detection of the chromophore p-nitroanilide (pNA) obtained after specific action of caspase-3 and caspase-9 on tetrapeptide substrates, DEVD-pNA and LEHD-pNA, respectively. The assay mixture consisted of 50 μl of cell suspension and 50 μl of chilled cell lysis buffer incubated on ice for 10 min. After incubation, 50 μl of 2X reaction buffer (containing 10mM DTT) with 200 μM substrate (DEVD-pNA for Drice, and IETD-pNA for Dronc) was added and incubated at 37 °C for 1.5 hr. The reaction was quantified at 405 nm.
2.15. Determination of catalase (CAT) activity
The activity of catalase was determined by kinetic method described by Beers and Sizer [28] where rate of dismutation of H2O2 to water and molecular oxygen is proportional to the concentration of catalase in the sample. The reaction mixture contained 650 μL of 0.1M phosphate buffer, 333 μL of H2O2 (0.05M) and 17 μL of sample. A decrease in OD was measured for 2 minutes, every 30 seconds at 240 nm. Thereafter the activity of catalase was calculated and expressed as μ moles of H2O2 consumed/min/mg of protein.
2.16. Determination of superoxide dismutase activity (SOD) activity
The method described by Marklund and Marklund [29] was used for the estimation of SOD activity. Reaction mixture was made adding 17 μL of sample to 950 μL of 0.1 M phosphate buffer. Finally, the reaction was initiated by adding pyrogallol. An increase in OD was noted at 420 nm for 3 min after every 30 seconds. The results were expressed as units/mg protein.
2.17. Immunohistochemistry
The fly heads were isolated and the paraffin sections were prepared according to the procedure described by Palladino et al [30]. The sections were deparaffinized and rehydrated. The slides were blocked in 8% Bovine Serum Albumin (BSA) for 2.5 hrs. Then the slides were washed with phosphate buffer saline (pH 7.2) containing 2% BSA for 5 minutes. After washing the slides were incubated with primary antibody (anti-tyrosine hydroxylase, Merck) in a humidified chamber for 12 hrs at 4 °C. The slides were then washed with PBS containing 2% BSA for 5 min and incubated with secondary antibody (Goat anti-Rabbit alkaline phosphatase, Santacruz, Biotechnology, USA) at room temperature for 2 hrs. The final wash was given by PBS containing 2% BSA for 5 min. BCIP-NBT was used as a chromogenic substrate which interacts with secondary antibody to produce blue coloured product. The slides were then mounted in DPX and observed under the microscope. The activity of dopaminergic neurons was quantified in terms of TH-positive cells using Image-J software.
2.18. Statistical analysis
The data was subjected to statistical analysis through one way analysis of variance (ANOVA) posthocTukey test by SPSS 16 taking significant at 5% level of probability. The survival analysis was done by Kalpan-Meier analysis.
3. Results
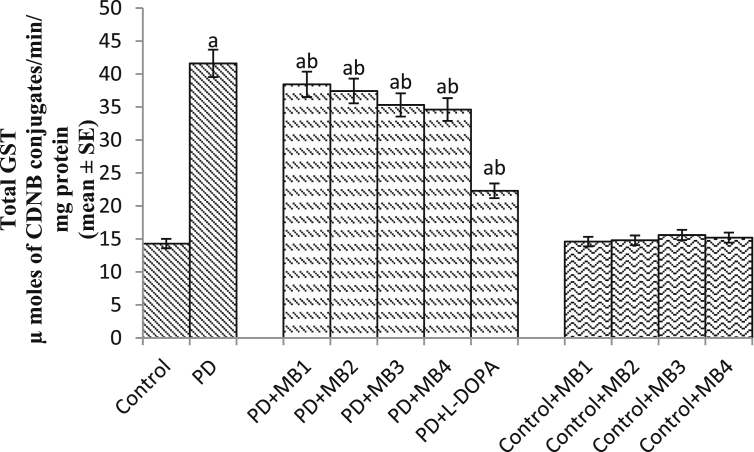
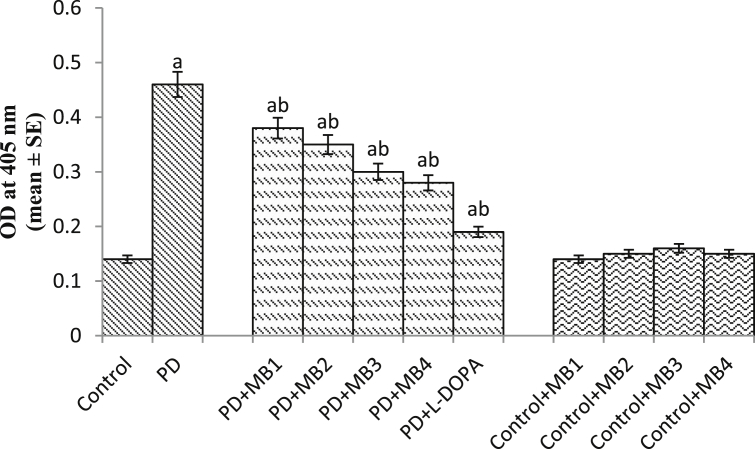
Table 2 shows the details of the tests performed on MB. No heavy metals, aflatoxins and pesticides were reported in the prepared MB. All the tests were performed at Delhi Drug Test House (An ISO recognized test house). MB was also found to be negative for any microbial and other specific pathogens (Table 2). The results obtained by GC-MS analysis are shown in Fig. 1S and Table 3. Fig. 1S represent the chromatogram for the polyherbal drug. Fig. 2S describes the properties of the major compounds present in the extract. The results showed the presence of terpenoids and other organic compounds of biological significance. The result obtained for DPPH scavenging assay are shown in Table 4. A dose dependent significant increase in the free radical scavenging potential was observed compared to ascorbic acid and the IC50 was found to be 550 μg/ml (Table 4). Table 5 shows the potential of scavenging superoxide anion of MB. A dose dependent significant increase in the potential of scavenging superoxide anion was observed compared to ascorbic acid with an IC50 value of 538.64 μg/ml (Table 5). To establish the duration of treatment the climbing assay was performed every 3rd day after the emergence of PD as well as control flies (Fig. 1). From the 9th day onwards a significant loss of climbing ability was observed between the PD and control flies (Fig. 1; p < 0.05). Based on the results obtained the duration of exposure was set for 24 days and the PD flies were exposed to the selected doses of MB for 24 days. The PD flies exposed to 0.0014, 0.0028, 0.042 and 0.0056 g of MB showed a dose dependent significant delay of 1.47, 1.88, 2.52 and 3.05 folds in the climbing ability, compared to unexposed PD flies (Fig. 2; p < 0.05). The PD flies exposed to 10−3 M of dopamine showed a significant delay of 3.76 fold in the loss of climbing ability compared to unexposed PD flies (Fig. 2; p < 0.05). The activity of the control flies, treated PD flies and PD flies were recorded for 311 hrs and expressed as chi-square periodogram Figs. 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 13(a&b). The PD flies shows a decline the average activity [Fig. 4(a&b)] compared to control flies [Fig. 3(a &b)]. The PD flies exposed to different doses of MB showed a dose dependent improvement/delay in the loss of average activity [Figs. 5, 6, 7 and 8(a&b)]. The flies exposed to 10−3 M of dopamine also showed a delay in the loss of activity pattern [Fig. 9(a&b)]. The control flies exposed to selected doses of MB did not show any change in the average activity pattern [Figs. 10, 11, 12 and 13(a&b)]. The results obtained for survival assay is shown in Fig. 14. A significant dose dependent increase in the Km values of the PD flies was observed upon the exposure to various doses of MB compared to unexposed PD flies (Fig. 14; p < 0.05). The PD flies exposed to 10−3 M of dopamine also showed a significant increase in Km values compared to unexposed PD flies (Fig. 14; p < 0.05). The PD flies showed a significant increase of 1.79 folds in TBARS compared to control flies (Fig. 15; p < 0.05). The PD flies exposed to 0.0014, 0.0028, 0.042 and 0.0056g of MB showed a dose dependent significant decrease of 1.38, 1.45, 1.48 and 1.65 folds in TBARS compared to unexposed PD flies (Fig. 15; p < 0.05). The PD flies exposed to 10−3 M of dopamine showed a significant decrease of 1.48 fold in the TBARS compared to unexposed PD flies (Fig. 15; p < 0.05). The PD flies showed a significant increase of 2.90 folds in the GST activity compared to control flies (Fig. 16; p < 0.05). The PD flies exposed to 0.0014, 0.0028, 0.042 and 0.0056g of MB showed a dose dependent significant decrease of 1.08, 1.11, 1.17 and 1.20 folds in the GST activity compared to unexposed PD flies (Fig. 16; p < 0.05). The PD flies exposed to 10−3 M of dopamine showed a significant decrease of 1.86 fold in the GST activity compared to unexposed PD flies (Fig. 16; p < 0.05). The results obtained of NPSH content showed a significant decrease of 1.88 folds in the PD flies compared to control flies (Fig. 17; p < 0.05). The PD flies exposed to 0.0014, 0.0028, 0.042 and 0.0056g of MB showed a significant dose dependent increase of 1.20, 1.29, 1.33 and 1.44 folds in the NPSH content compared to unexposed PD flies (Fig. 17; p < 0.05). The PD flies exposed to 10−3 M of dopamine showed a significant increase of 1.48 fold in the NPSH content compared to unexposed PD flies (Fig. 17; p < 0.05). The PD flies showed a significant increase of 4.57 folds in the PC content compared to control flies (Fig. 18; p < 0.05). The PD flies exposed to 0.0014, 0.0028, 0.042 and 0.0056g of MB showed a dose dependent significant decrease of 1.20, 1.28, 1.39 and 1.52 folds in the PC content compared to unexposed PD flies (Fig. 18; p < 0.05). The PD flies exposed to 10−3 M of dopamine showed a significant decrease of 2.20 fold compared to unexposed PD flies (Fig. 18; p < 0.05). The PD flies showed a significant increase of 3.58 folds in the Caspase-9 activity compared to control flies (Fig. 19; p < 0.05). The PD flies exposed to 0.0014, 0.0028, 0.042 and 0.0056g of MB showed a significant dose dependent decrease of 1.43, 1.53, 1.65 and 1.79 folds in the Caspase-9 activity compared to unexposed PD flies (Fig. 19; p < 0.05). The PD flies exposed to 10−3 M of dopamine showed a significant decrease of 2.04 fold in the Caspase-9 activity compared to unexposed PD flies (Fig. 19; p < 0.05). The PD flies showed a significant increase of 3.28 folds in the activity of Caspase-3 compared to control flies (Fig. 20; p < 0.05). The PD flies exposed to 0.0014, 0.0028, 0.042 and 0.0056g of MB showed a dose dependent significant decrease of 1.21, 1.31, 1.53 and 1.64 folds in the activity of Caspase-3 compared to unexposed PD flies (Fig. 20; p < 0.05). The PD flies exposed to 10−3 M of dopamine showed a significant decrease of 2.42 fold compared to unexposed PD flies (Fig. 20; p < 0.05). The PD flies showed a significant increase of 2.99 folds in the activity of catalase compared to control flies (Fig. 21; p < 0.05). The PD flies exposed to various doses of MB showed a significant decrease of 1.24, 1.42, 1.50 and 1.79 folds in the activity of catalase compared to unexposed PD flies (Fig. 21; p < 0.05). The PD flies exposed to 10−3 M of dopamine showed a significant decrease of 1.93 fold in the catalase activity compared to unexposed PD flies (Fig. 21; p < 0.05). The PD flies showed a significant increase of 2.66 folds in the activity of SOD (Fig. 22; p < 0.05). The PD flies exposed to 0.0014, 0.0028, 0.042 and 0.0056g of MB showed a significant dose dependent decrease of 1.50, 1.63, 1.88 and 2.06 folds in the activity of SOD compared to unexposed PD flies (Fig. 22; p < 0.05). The PD flies exposed to 10−3 M of dopamine showed a significant decrease of 2.25 fold compared to unexposed PD flies (Fig. 22; p < 0.05). The results of tyrosine hydroxylase immunostaining are shown in Fig. 23(a–g). The PD flies showed a marked age dependent reduction in the activity of tyrosine hydroxylase compared to control. The exposure of PD flies to different doses of MB showed an increased immunoreactivity in comparison to untreated PD flies.
Table 2.
Polyherbal drug Majun Baladur tested for the presence of following parameters.
| S.No. | Test parameters | Test results | Limit of quantification | Permissible limits as per API | Method |
|---|---|---|---|---|---|
| 1. | Test for heavy metals | ||||
| 1.1 | Lead as Pb (mg/kg) | Not detected | 2.50 | Not more than 10 | AAS |
| 1.2 | Mercury as Hg (mg/kg) | Not detected | 0.5 | Not more than 1 | AAS |
| 1.3 | Arsenic as As (mg/kg) | Not detected | 1.25 | Not more than 3 | AAS |
| 1.4 | Cadmium as Cd (mg/kg) | Not detected | 0.25 | Not more than 0.3 | AAS |
| 2. | Test for aflatoxins | ||||
| 2.1 | Aflatoxin B1 (mg/kg) | Not detected | 0.001 | Not more than 0.5 | LCMSMS |
| 2.2 | Aflatoxin G1 (mg/kg) | Not detected | 0.001 | Not more than 0.5 | LCMSMS |
| 2.3 | Aflatoxin B2 (mg/kg) | Not detected | 0.001 | Not more than 0.1 | LCMSMS |
| 2.4 | Aflatoxin G2 (mg/kg) | Not detected | 0.001 | Not more than 0.1 | LCMSMS |
| 3. | Pesticides residue | ||||
| 3.1 | Alachlor (mg/kg) | Not detected | 0.02 | 0.02 | GCMSMS |
| 3.2 | Aldrin & dieldrin (Sum of) (mg/kg) | Not detected | 0.04 | 0.05 | GCMSMS |
| 3.3 | Azinophos-methyl (mg/kg) | Not detected | 0.04 | 1.0 | GCMSMS |
| 3.4 | Bromopropylate (mg/kg) | Not detected | 0.08 | 3.0 | GCMSMS |
| 3.5 | Chlordane (Sun of cis, trans and oxychlordane) (mg/kg) | Not detected | 0.04 | 0.05 | GCMSMS |
| 3.6 | Chlorfenvinphos (mg/kg) | Not detected | 0.04 | 0.5 | GCMSMS |
| 3.7 | Chlorpyrifos (mg/kg) | Not detected | 0.04 | 0.2 | GCMSMS |
| 3.8 | Chlorpyrifos-methyl (mg/kg) | Not detected | 0.04 | 0.1 | GCMSMS |
| 3.9 | Cypermethrin (and isomers) (mg/kg) | Not detected | 0.10 | 1.0 | GCMSMS |
| 3.10 | DDT (Sum of p,p-DDT,p,p-DDE and p,p-TDE) (mg/kg) | Not detected | 0.04 | 1.0 | GCMSMS |
| 3.11 | Deltamethrin (mg/kg) | Not detected | 0.10 | 0.5 | GCMSMS |
| 3.12 | Diazinon (mg/kg) | Not detected | 0.04 | 0.5 | GCMSMS |
| 3.13 | Dichlorvos (mg/kg) | Not detected | 0.04 | 1.0 | GCMSMS |
| 3.14 | Diathiocarbamates (as CS2) | Not detected | 0.01 | 2.0 | UV-VIS Spectrophotometry |
| 3.15 | Endosulfan (Sum of isomer & endosulfan sulphate) (mg/kg) | Not detected | 0.04 | 3.0 | GCMSMS |
| 3.16 | Endrin (mg/kg) | Not detected | 0.04 | 0.05 | GCMSMS |
| 3.17 | Ethion (mg/kg) | Not detected | 0.04 | 2.0 | GCMSMS |
| 3.18 | Fenitrothion (mg/kg) | Not detected | 0.04 | 0.5 | GCMSMS |
| 3.19 | Fenvalerate (mg/kg) | Not detected | 0.10 | 1.5 | GCMSMS |
| 3.20 | Fonofos (mg/kg) | Not detected | 0.04 | 0.05 | GCMSMS |
| 3.21 | Heptachlor (Sum of heptachlor & heptachlor epoxide) (mg/kg) | Not detected | 0.04 | 0.05 | GCMSMS |
| 3.22 | Hexachlorobenzene (mg/kg) | Not detected | 0.04 | 0.1 | GCMSMS |
| 3.23 | Hexachlorocyclohexene isomer (other than γ) (mg/kg) | Not detected | 0.04 | 0.3 | GCMSMS |
| 3.24 | Lindane (γ- Hexachlorocyclohexene) (mg/kg) | Not detected | 0.04 | 0.6 | GCMSMS |
| 3.25 | Malathion (mg/kg) | Not detected | 0.04 | 1.0 | GCMSMS |
| 3.26 | Methidathion (mg/kg) | Not detected | 0.04 | 0.2 | GCMSMS |
| 3.27 | Parathion (mg/kg) | Not detected | 0.04 | 0.5 | GCMSMS |
| 3.28 | Parathion methyl (mg/kg) | Not detected | 0.04 | 0.2 | GCMSMS |
| 3.29 | Permethrin (mg/kg) | Not detected | 0.04 | 1.0 | GCMSMS |
| 3.30 | Phosalone (mg/kg) | Not detected | 0.04 | 0.1 | LCMSMS |
| 3.31 | Piperonyl butoxide (mg/kg) | Not detected | 0.04 | 3.0 | LCMSMS |
| 3.32 | Primiphos methyl (mg/kg) | Not detected | 0.04 | 4.0 | LCMSMS |
| 3.33 | Pyrethrins (Sum of isomer) (mg/kg) | Not detected | 0.10 | 3.0 | GCMSMS |
| 3.34 | Quintozen (sum of Quintozene, pentachloroaniline and methyl pentachlorophenyl sulphide) (mg/kg) | Not detected | 0.10 | 1.0 | LCMSMS |
| 4. | Test for microbiology | ||||
| Total bacterial count (cfu/gm) | 60 | – | Not more than 1 × 10−5 cfu/gm | – | |
| Total yeast & mould (cfu/gm) | <10 | – | Not more than 1 × 10−5 cfu/gm | – | |
| 5. | Any specific pathogens | ||||
| E. coli/gm | Absent | – | Absent | – | |
| Salmonella/gm | Absent | – | Absent | – | |
| S. aureus/gm | Absent | – | Absent | – | |
| P. aeruginosa/gm | Absent | – | Absent | – | |
Table 3.
Integration peak list of polyherbal drug Baladur.
| Peak | Start | RT | End | Height | Area | Area % | Area sum percent | Compound name | Nature |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 13.32 | 13.39 | 13.49 | 20773.22 | 86304.18 | 10.37 | 1.53 | Benzene, (1-methylethyl)- | Organic compound |
| 2. | 27.94 | 28.01 | 28.21 | 9530.58 | 79366.46 | 9.54 | 1.41 | Tetraacetyl-d-xylonic nitrile | Organic compound |
| 3. | 29.58 | 30.18 | 30.41 | 49220.54 | 832012.17 | 100 | 14.77 | (+)-Dibenzoy-L-tartaric acid anhydride | Organic compound |
| 4. | 30.6 | 30.67 | 30.82 | 16331.9 | 85652.31 | 10.29 | 1.52 | Azulene | Terpenoids |
| 5. | 31.36 | 31.43 | 31.56 | 13149.75 | 72115.77 | 8.67 | 1.28 | 1,7 Octanediol, 3,7, dimethyl- | Polyurethanes |
| 6. | 34.02 | 34.11 | 34.55 | 26535.16 | 243506.69 | 29.27 | 4.32 | 6-Acetyl-beta-D-mannose | Organic compound |
| 7. | 38.03 | 38.15 | 38.28 | 15136.78 | 91282.15 | 10.97 | 1.62 | 1,3-benzodioxole, 5-(1-propenyl)- | Organic compound |
| 8. | 39.01 | 39.11 | 39.39 | 10239.04 | 86312.46 | 10.37 | 1.53 | 2-Myristynoyl pantetheine | Acetyl group |
| 9. | 45.49 | 45.58 | 45.7 | 55224 | 272163.26 | 32.71 | 4.83 | Tetradecane | Alkane hydrocarbon |
| 10. | 45.77 | 45.86 | 46 | 13855.35 | 71722.57 | 8.62 | 1.27 | Benzene 1,2-dimethoxy-4-(1-propenyl)- | Organic compound |
| 11. | 51.61 | 51.72 | 51.87 | 46256.47 | 235720.53 | 28.33 | 4.19 | Tetradecane 2,6,10-trimethyl- | Organic compound |
| 12. | 52.8 | 52.9 | 53.16 | 73094.84 | 426918.64 | 51.31 | 7.58 | 1,3-Benzodioxole 4-methoxy-6-(2-propenyl)- | Anticholinergic |
| 13. | 54.99 | 55.09 | 55.24 | 122177.97 | 616988.88 | 74.16 | 10.95 | Benzene, 1,2,3 trimethoxy-5-(2-propenyl)- | Organic compound |
| 14. | 57.04 | 57.16 | 57.31 | 17054.04 | 113804.03 | 13.68 | 2.02 | Paromomycin | Antimicrobial |
| 15. | 57.43 | 57.55 | 57.82 | 122894.86 | 681940.65 | 81.96 | 12.11 | Hexadecane | Organic compound |
| 16. | 60.07 | 60.13 | 60.35 | 9669.5 | 56179.96 | 6.75 | 1 | Tetraacetyl-d-xylonic nitrile | Organic compound |
| 17. | 60.38 | 60.43 | 60.56 | 9674.07 | 51536.76 | 6.19 | 0.92 | 2-Myristnoyl pantetheine | Organic compound |
| 18. | 62.95 | 63.05 | 63.18 | 25511.09 | 124225.82 | 14.93 | 2.21 | 2-Myristnoyl pantetheine | Organic compound |
| 19. | 65.75 | 65.82 | 66.09 | 12503.87 | 96017.79 | 11.54 | 1.7 | N,N′-Bis (Carbobenzyloxy)-lysine methyl(ester) | Organic compound |
| 20. | 67.32 | 67.4 | 67.5 | 75343.35 | 299693.36 | 36.02 | 5.32 | Octadecane | Alkane hydrocarbon |
| 21. | 69.73 | 69.81 | 69.93 | 13907.63 | 61356.88 | 7.37 | 1.09 | 1,2-Benzenedicarboxylic acid butyl octyl ester | Phthalic anhydride |
| 22. | 72.6 | 72.75 | 72.94 | 63484.21 | 351634.69 | 42.26 | 6.24 | 1,2-Benzenedicarboxylic acid butyl octyl ester | Phthalic anhydride |
| 23. | 73.75 | 73.82 | 73.93 | 27072.57 | 105978.37 | 12.74 | 1.88 | octadecane 3-ethyl-5-(2-ethylbutyl)- | Organic compound |
| 24. | 77.3 | 77.37 | 77.62 | 21879.87 | 133442.48 | 16.04 | 2.37 | octadecanal 2 bromo | Stearyl aldehyde |
| 25. | 83.29 | 83.36 | 83.44 | 10983.07 | 50289.01 | 6.04 | 0.89 | 1H-2,8a Methanocyclopenta[a] cyclopropa [e]cyclodecen-11-one | Terpenoids |
| 26. | 85.46 | 85.55 | 86.17 | 17143.09 | 192703.75 | 23.16 | 3.42 | 1H-2,8a Methanocyclopenta[a] cyclopropa [e]cyclodecen-11-one | Terpenoids |
Table 4.
DPPH scavenging activity at various concentrations of polyherbal drug Baladur.
| Concentration (μg/ml) | DPPH inhibition (%) |
|
|---|---|---|
| Majun Baladur | Ascorbic acid | |
| 100 | 10.25 ± 0.82* | 62.43 ± 1.3* |
| 200 | 16.73 ± 0.75* | 71.22 ± 1.9* |
| 300 | 25.63 ± 1.20* | 76.23 ± 0.96* |
| 400 | 36.41 ± 0.94* | 81.39 ± 0.84* |
| 500 | 45.69 ± 0.56* | 87.22 ± 0.92* |
| IC50 (μg/ml) | 550 ± 1.079 | 130.78 ± 0.51 |
Values are mean ± S.E.M of triplicate determination.
*P ≤ 0.05 compared to control.
Table 5.
Superoxide anion scavenging activity at various concentrations of polyherbal drug Baladur.
| Concentration (μg/ml) | Superoxide anion scavenging activity (%) |
|
|---|---|---|
| Majoon Baladur | Ascorbic acid | |
| 100 | 16.24 ± 1.2* | 51.23 ± 0.92* |
| 200 | 21.24 ± 1.9* | 62.42 ± 0.52* |
| 300 | 30.21 ± 0.92* | 66.93 ± 0.42* |
| 400 | 39.42 ± 0.67* | 72.13 ± 0.93* |
| 500 | 47.23 ± 0.52* | 80.43 ± 0.81* |
| IC50 (μg/ml) | 538.65 ± 1.51 | 54.39 ± 0.55 |
Values are mean ± S.E.M of triplicate determination.
*P ≤ 0.05 compared to control.
Fig. 1.
Climbing ability in Parkinson's disease (PD) flies and control for a period of 24 days. The values are mean of five assays.
Fig. 2.
Effect of Majun Baladur (MB) on the climbing ability activity of flies. [MB1 = 0.0014 g; MB2 = 0.0028 g; MB3 = 0.0042 g; MB4 = 0.0056 g; PD = PD flies; Dopamine = 10−3 M; N = 50]. The flies were allowed to feed on the diet supplemented with Majun Baladur for 24 days and then assayed for climbing ability activity. [The doses were established per 20g of diet; asignificant difference with respect to control, p < 0.05; bsignificant difference with respect to PD flies p < 0.05].
Fig. 3.
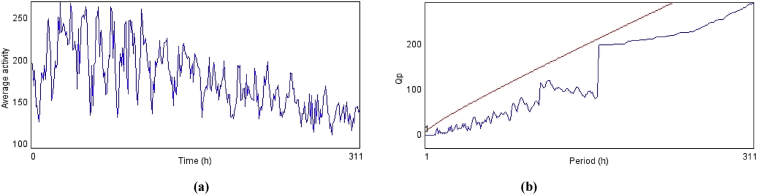
(a) and (b) shows the average activity pattern and chi-square periodogram, respectively for control flies (N = 20).
Fig. 4.
(a) and (b) shows the average activity pattern and chi-square periodogram, respectively for PD flies (N = 20).
Fig. 5.
(a) and (b) shows the average activity pattern and chi-square periodogram, respectively for PD flies exposed to polyherbal drug Baladur (0.0014 g). [The doses were established per 20 g of diet].
Fig. 6.
(a) and (b) shows the average activity pattern and chi-square periodogram, respectively for PD flies exposed to polyherbal drug Baladur (0.0028 g). [The doses were established per 20 g of diet].
Fig. 7.
(a) and (b) shows the average activity pattern and chi-square periodogram, respectively for PD flies exposed to polyherbal drug Baladur (0.0042 g). [The doses were established per 20 g of diet].
Fig. 8.
(a) and (b) shows the average activity pattern and chi-square periodogram, respectively for PD flies exposed to polyherbal drug Baladur (0.0056 g). [The doses were established per 20 g of diet].
Fig. 9.
(a) and (b) shows the average activity pattern and chi-square periodogram, respectively for PD flies exposed to dopamine (10−3 M).
Fig. 10.
(a) and (b) shows the average activity pattern and chi-square periodogram, respectively for control flies exposed to polyherbal drug Baladur (0.0014 g).[The doses were established per 20 g of diet].
Fig. 11.
(a) and (b) shows the average activity pattern and chi-square periodogram, respectively for control flies exposed to polyherbal drug Baladur (0.0028 g).[The doses were established per 20 g of diet].
Fig. 12.
(a) and (b) shows the average activity pattern and chi-square periodogram, respectively for control flies exposed to polyherbal drug Baladur (0.0042 g).[The doses were established per 20 g of diet].
Fig. 13.
(a) and (b) shows the average activity pattern and chi-square periodogram, respectively for control flies exposed to polyherbal drug Baladur (0.0056 g).[The doses were established per 20 g of diet].
Fig. 14.
Effect of Majun Baladur (MB) on the survival rate. [MB1 = 0.0014 g; MB2 = 0.0028 g; B3 = 0.0042 g; B4 = 0.0056 g; PD = PD flies; Dopamine = 10−3 M; N = 50]. The values are the mean of 5 assays.
Fig. 15.
Effect of Majun Baladur (MB) on the thiobarbituric acid reactive species (TBARS) in the brains of flies. [MB1 = 0.0014 g; MB2 = 0.0028 g; MB3 = 0.0042 g; MB4 = 0.0056 g; PD = PD flies; Dopamine = 10−3 M; N = 50]. The flies were allowed to feed on the diet supplemented with Majun Baladur for 24 days and then assayed for lipid peroxidation. [The doses were established per 20 g of diet; asignificant difference with respect to control, p < 0.05; bsignificant difference with respect to PD flies p < 0.05].
Fig. 16.
Effect of Majun Baladur (MB) on the glutathione-S-transferase (GST) activity in the brains of flies. [MB1 = 0.0014 g; MB2 = 0.0028 g; MB3 = 0.0042 g; MB4 = 0.0056 g; PD = PD flies; Dopamine = 10−3 M; N = 50]. The flies were allowed to feed on the diet supplemented with Majun Baladur for 24 days and then assayed for GST activity. [The doses were established per 20 g of diet; asignificant difference with respect to control, p < 0.05; bsignificant difference with respect to PD flies p < 0.05].
Fig. 17.
Effect of Majun Baladur (MB) on the Non-protein thiol (NPSH) content in the brains of flies. [MB1 = 0.0014 g; MB2 = 0.0028 g; MB3 = 0.0042 g; MB4 = 0.0056 g; PD = PD flies; Dopamine = 10−3 M; N = 50]. The flies were allowed to feed on the diet supplemented with Majun Baladur for 24 days and then assayed for NPSH content. [The doses were established per 20 g of diet; asignificant difference with respect to control, p < 0.05; bsignificant difference with respect to PD flies p < 0.05].
Fig. 18.
Effect of Majun Baladur (MB) on the protein carbonyl content in the brains of flies. [MB1 = 0.0014 g; MB2 = 0.0028 g; MB3 = 0.0042 g; MB4 = 0.0056 g; PD = PD flies; Dopamine = 10−3 M; N = 50]. The flies were allowed to feed on the diet supplemented with Majun Baladur for 24 days and then assayed for protein carbonyl content. [The doses were established per 20 g of diet; asignificant difference with respect to control, p < 0.05; bsignificant difference with respect to PD flies p < 0.05].
Fig. 19.
Effect of Majun Baladur (MB) on the Caspase-9 activity in the brains of flies. [MB1 = 0.0014 g; MB2 = 0.0028 g; MB3 = 0.0042 g; MB4 = 0.0056 g; PD = PD flies; Dopamine = 10−3 M; N = 50]. The flies were allowed to feed on the diet supplemented with Majun Baladur for 24 days and then assayed for Caspase-9. [The doses were established per 20 g of diet; asignificant difference with respect to control, p < 0.05; bsignificant difference with respect to PD flies p < 0.05].
Fig. 20.
Effect of Majun Baladur (MB) on the Caspase-3 activity in the brains of flies. [MB1 = 0.0014 g; MB2 = 0.0028 g; MB3 = 0.0042 g; MB4 = 0.0056 g; PD = PD flies; Dopamine = 10−3 M; N = 50]. The flies were allowed to feed on the diet supplemented with Majun Baladur for 24 days and then assayed for Caspase-3. [The doses were established per 20 g of diet; asignificant difference with respect to control, p < 0.05; bsignificant difference with respect to PD flies p < 0.05].
Fig. 21.
Effect of Majun Baladur (MB) on the catalase in the brains of flies. [MB1 = 0.0014 g; MB2 = 0.0028 g; MB3 = 0.0042 g; MB4 = 0.0056 g; PD = PD flies; Dopamine = 10−3 M; N = 50]. The flies were allowed to feed on the diet supplemented with Majun Baladur for 24 days and then assayed for catalase. [The doses were established per 20 g of diet; asignificant difference with respect to control, p < 0.05; bsignificant difference with respect to PD flies p < 0.05].
Fig. 22.
Effect of Majun Baladur (MB) on the Superoxide dismutase (SOD) content in the brains of flies. [MB1 = 0.0014 g; MB2 = 0.0028 g; MB3 = 0.0042 g; MB4 = 0.0056 g; PD = PD flies; Dopamine = 10−3 M; N = 50]. The flies were allowed to feed on the diet supplemented with Majun Baladur for 24 days and then assayed for Superoxide dismutase (SOD). [The doses were established per 20 g of diet; asignificant difference with respect to control, p < 0.05; bsignificant difference with respect to PD flies p < 0.05].
Fig. 23.
Tyrosine hydroxylase (TH) immunostaining performed on the brain sections of flies after 24 days of the exposure; a-Control, b-PD fly, c-PD fly exposed to MB1 = 0.0014 g, d-PD fly exposed to MB2 = 0.0028 g, e-PD fly exposed to MB3 = 0.0042 g, f-PD fly exposed to MB4 = 0.0056 g, g-Dopaminergic neurons were quantified in terms of TH-positive cells from the total area of the brain by using Image J software (N = 5). [MB1 = 0.0014 g; MB2 = 0.0028 g; MB3 = 0.0042 g; MB4 = 0.0056 g; PD = PD flies.
4. Discussion
The results of the present study reveal that MB is potent in reducing the PD symptoms in the transgenic Drosophila expressing human alpha synuclein in the neurons. The supplementation of MB for 24 days significantly reduced the oxidative stress and delayed the loss of behavioral parameters. Oxidative stress has been reported to play a central role in the progression of neurodegenerative diseases although its relationship with α-synuclein toxicity has not been well elucidated [9]. α-synuclein is one of several proteins associated with neurodegenerative disease that have high propensity to aggregate [31]. The aggregation of synuclein is followed by the formation of Lewy bodies and increase in the oxidative stress leading to the damage of dopaminergic neurons [32]. In our present study the exposure of PD flies to MB showed a dose dependent significant increase in the life span of PD flies. Reduced life span of fly has been linked with various neurodegenerative disorders [33]. Earlier studies on natural plant products/extract have also shown an increase in the life span of fruit flies expressing the symptoms of neurodegenerative disorder [15, 23, 33, 34, 35]. The exposure of PD flies to MB also prevents the loss in the activity of flies as is evident from the average activity pattern recorded by Drosophila Activity Monitor (DAM). Oxidative stress plays a main role in the neurodegenerative diseases [36] and leads to the damage of lipid, protein and DNA [37]. TBARS, PCC, NPSH, GST, SOD and CAT are the reliable markers of oxidative stress. The increase in TBARS and PC content in our study indicated the generation of free radicals that damage the lipid and protein. A significant dose dependent decrease in the TBARS and PC content in the PD flies exposed to various doses of MB showed that it has a potential of scavenging ROS. Our results obtained for DPPH and superoxide anions scavenging assays also support the antioxidant potential of MB that could contribute towards the protection against the oxidative stress. NPSH is a tripeptide, involved in many biological actions, including enzymatic reactions, molecular transport, protein and nucleic acid biosynthesis, microtubule formation, signal transduction, gene expression and protection of cells against oxidative damage [38]. The low level of NPSH and increased activity of GST has been reported during oxidative stress [39]. Not only the formation of Lewy bodies but also the degenerating neurons are also responsible for producing endogenous toxins (hydrogen peroxide) and other ROS that may further damage the normal neurons [5]. The depletion in the NPSH content and high GST activity have been reported in the brains of humans suffering from PD as well as in experimental models of PD [40, 41]. In our present study decrease in NPSH levels and increase in the GST activity was observed in the brains of PD flies. ROS may lead to increase in the activity of SOD as it is a free radical scavenger and prevents the damage caused by peroxidase reactions. The increase in the activity of SOD and CAT also supports the generation of ROS in the neurons. The exposure of PD flies to MB showed a dose dependent decrease in the activities of SOD as well as CAT. The results support that phytoconstituents present in the MB are potent enough to reduced the symptoms of PD being expressed in the transgenic Drosophila model of PD. Earlier the effect of Khamira Aresham and Majun Baladur against cerebral ischemia induced oxidative damage was found to be protective [13]. MB was found to be neuroprotective by potentiating the antioxidant defense system of the brain during cerebral ischemia damage in rats [42]. Depleted cellular enzymatic activities are used as a marker of oxidative stress [43, 44]. Neuronal and cognitive impairments were reported to be improved by the restoration of NPSH content and lowering the lipidperoxidase during ischemic damage after the exposure to decocted of herbs (Gagamjungjihwa) and (Frustus Euodiae) and the Chinese herbal formula (FBD) [2, 4]. In our present study the exposure of PD flies to MB prevents the loss of climbing ability, overall activity, increased the life span of the PD flies in a dose dependent manner. Tyrosine hydroxylase (TH) is the rate limiting enzyme in dopamine synthesis. It is present in dopaminergic neurons where it catalyzes the formation of L-DOPA from L-tyrosine [38]. The deficiency in TH is considered to be an important hallmark in PD [45]. Studies have shown that the interaction of TH with α-synuclein plays a pivotal role in the functioning of dopaminergic neurons and in the pathophysiology of the disease [46, 47, 48]. The interaction of TH and α-synuclein was further proven by the immunoprecipitation studies that confirmed the co-precipitation of two in the brain homogenate. Moreover, their co-localization was also confirmed using immune electron microscopy technique. The aggregation of α-synuclein disrupts the dopamine homeostasis resulting in the death of dopaminergic neurons. The over expression of α-synuclein in the dopaminergic cell lines have been shown to cause reduction in the TH activity and phosphorylation [49]. The results in our present study also showed a reduction in TH reactivity with age in PD flies which may be due to the accumulation of α-synuclein in PD flies. Due to the similarity between the dopaminergic network, mode of action, behaviour and gene response in D. melanogaster and mammalian system has made the fly a very attractive model for anti-parkinsonism drug discovery [50, 51]. The PD flies exposed to MB showed a dose dependent increase in the TH reactivity, hence suggesting the neuroprotective role of MB. Our earlier studies with geraniol and genistein showed that they were not potent in inhibiting the expression of α-synuclein and formation of Lewy bodies but delay the PD symptoms. The antioxidant potential of the natural plant products was suggested to be the cause of their protective effect [34, 52, 53].
5. Conclusion
The disruption of the enzymatic antioxidant system is followed by mitochondrial damage via depletion of NPSH and in turn enhances the production of free radicals [54]. Proteotoxicity and the dopaminergic neuronal death in Drosophila due to the expression of alpha synuclein and lewy bodies are well established [55, 56, 57, 58]. We found that PD flies exposed to MB showed decreased TBARS levels which were accompanied by decreased SOD and CAT activity followed by increased NPSH levels. Hence, the decrease in the –SH levels could be due to an increase in GST activity in the head of PD flies, MB provides a neuro protection against the PD symptoms (being mimicked in PD flies) due to the synergistic antioxidant effects of the constituents present in it. The data obtained in our study provides a scientific explanation for the use of MB for the treatment of PD. However, the characterization of the various antioxidants present in the formulation and the elucidation of their possible mode of action will be the part of our future study.
Declarations
Author contribution statement
Yasir Hasan Siddique: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Falaq Naz, Rahul: Performed the experiments; Analyzed and interpreted the data.
Mohammad Rashid: Performed the experiments; Wrote the paper.
Tajuddin: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Dr. Yasir Hasan Siddique was supported by the Ministry of AYUSH New Delhi, India (No. Z.28015/87/2016-HPC (EMR)-AYUSH-C).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix. ASupplementary data
The following is the supplementary data related to this article:
References
- 1.Muñoz-Soriano V., Paricio N. Drosophila models of Parkinson's disease: discovering relevant pathways and novel therapeutic strategies. Parkinsons Dis. 2011;2011:1–14. doi: 10.4061/2011/520640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee B., Choi E.J., Lee E.J., Han S.M., Hahm D.H. The neuroprotective effect of methanol extract of gagamjungjihwan and fructus euodiae on ischemia-induced neuronal and cognitive impairment in the rat. Evid. Based Complement Alternat. Med. 2011;2011:685254. doi: 10.1093/ecam/nep028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feany M.B., Bender W.W. A Drosophila model of Parkinson's disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 4.Lin Z., Zhu D., Yan Y., Yu B., Wang Q., Shen P P. An antioxidant phytotherapy to rescue neuronal oxidative stress. Evid. Based Complement Alternat. Med. 2011;2011:519517. doi: 10.1093/ecam/nen053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giasson B.I., Duda J.E., Murray I.V., Chen Q., Souza J.M., Hurtig H.I. Oxidative damage linked to neurodegeneration by selective a-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 6.Conway A.R.A., Cowan N., Bunting M.F. The cocktail party phenomenon revisited: the importance of working memory capacity. Psychon. Bull. Rev. 2001;8:331–335. doi: 10.3758/bf03196169. [DOI] [PubMed] [Google Scholar]
- 7.Giráldez-Pérez R., Antolín-Vallespín M., Muñoz M., Sánchez-Capelo A. Models of α-synuclein aggregation in Parkinson's disease. Acta Neuropathol. Commun. 2014;2:176. doi: 10.1186/s40478-014-0176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaltiel-Karyo R., Davidi D., Menuchin Y., Frenkel-Pinter M., Marcus-Kalish M., Ringo J. A novel, sensitive assay for behavioral defects in Parkinson's disease model Drosophila. Parkinsons Dis. 2012;2012:697564. doi: 10.1155/2012/697564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizuno H., Fujikake N., Wada K., Nagai Y. α-Synuclein transgenic Drosophila as a model of Parkinson's disease and related synucleinopathies. Parkinsons Dis. 2010;2011:212706. doi: 10.4061/2011/212706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanam S., Siddique Y.H. Dopamine: agonists and neurodegenerative disorders. Curr Drug Tar. 2017 doi: 10.2174/1389450118666171117124340. [DOI] [PubMed] [Google Scholar]
- 11.Sundaram R., Mitra S.K. Antioxidant activity of ethyl acetate soluble fraction of Acacia arabica bark in rats. Indian J. Pharmacol. 2007;39:33–38. [Google Scholar]
- 12.Shakya B., Siddique Y.H. Evaluation of toxic potential of arecoline on the third instar larvae of transgenic Drosophila melanogaster (hsp70-lacZ) Bg9. Toxicol. Res. 2018;7:432–443. doi: 10.1039/c7tx00305f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yousuf S., Salim S., Ahmad M., Ahmed A.S., Ansari M.A., Islam F. Protective effect of Khamira Abresham Uood Mastagiwala against free radical induced damage in focal cerebral ischemia. J. Ethnopharmacol. 2005;99:179–184. doi: 10.1016/j.jep.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 14.Janssen C.I., Kiliaan A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 2014;53:1–17. doi: 10.1016/j.plipres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Siddique Y.H., Mustajab S.F., Jyoti S., Naz F. GC-MS analysis of Eucalyptus citriodora leaf extract and its role on the dietary supplementation in transgenic Drosophila model of Parkinson's disease. Food Chem. Toxicol. 2013;55:29–35. doi: 10.1016/j.fct.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Sghaier M.B., Bhouri W., Neffati A., Boubaker J., Skandrani I., Bouhlel I. Chemical investigation of different crude extracts from Teucrium ramosissimum leaves. Correlation with their antigenotoxic and antioxidant properties. Food Chem. Toxicol. 2011;49:191–201. doi: 10.1016/j.fct.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Wongsawatkul O., Prachayasittikul S., Isarankura-Na-Ayudhya C., Satayavivad J., Ruchirawat S., Prachayasittikul V. Vasorelaxant and antioxidant activities of Spilanthes acmella Murr. Int. J. Mol. Sci. 2008;9:2724–2744. doi: 10.3390/ijms9122724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddique Y.H., Naz F., Jyoti S. Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of Parkinson's disease. BioMed Res. Intern. 2014;2014 doi: 10.1155/2014/606928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddique Y.H., Ara G., Jyoti S., Afzal M. Protective effect of curcumin in transgenic Drosophila melanogaster model of Parkinson's disease. Alter. Med. Stud. 2002;2:3. [Google Scholar]
- 20.Chiu J.C., Low K.H., Pike D.H., Yildirim E., Edery I. Assaying locomotor activity to study circadian rhythms and sleep parameters in Drosophila. J. Vis. Exp. 2010;43:e2157. doi: 10.3791/2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosato E., Kyriacou C.P. Analysis of locomotor activity rhythms in Drosophila. Nat. Protoc. 2006;1:559–568. doi: 10.1038/nprot.2006.79. [DOI] [PubMed] [Google Scholar]
- 22.Pendleton R.G., Parvez F., Sayed M., Hillman R. Effects of pharmacological agents upon a transgenic model of Parkinson's disease in Drosophila melanogaster. J. Pharmacol. Exp. Ther. 2002;300:91–96. doi: 10.1124/jpet.300.1.91. [DOI] [PubMed] [Google Scholar]
- 23.Long J., Gao H., Sun L., Liu J., Zhao-Wilson X. Grape extract protects mitochondria from oxidative damage and improves locomotor dysfunction and extends lifespan in a Drosophila Parkinson's disease model. Rejuvenation Res. 2009;12:321–331. doi: 10.1089/rej.2009.0877. [DOI] [PubMed] [Google Scholar]
- 24.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 25.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 26.Jollow D.J., Mitchell J.R., Zamppaglione Z., Gillette J.R. Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolites. Pharmacology. 1974;11:151–157. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 27.Hawkins C.L., Morgan P.E., Davies M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009;46:965–988. doi: 10.1016/j.freeradbiomed.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Beers R.G., Sizer I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952:133–140. PMID: 14938361. [PubMed] [Google Scholar]
- 29.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 30.Palladino M.J., Keegan L.P., O'connell M.A., Reenan R.A. A-to-I pre-mRNA editing in Drosophila is primarily involved in adult nervous system function and integrity. Cell. 2000;102:437–449. doi: 10.1016/s0092-8674(00)00049-0. [DOI] [PubMed] [Google Scholar]
- 31.Cookson M.R. The biochemistry of Parkinson's disease. Annu. Rev. Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- 32.Periquet M., Fulga T., Myllykangas L., Schlossmacher M.G., Feany M.B. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J. Neurosci. 2007;27:3338–3346. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S.I., Jung J.W., Ahn Y.J., Restifo L.L., Kwon H.W. Drosophila as a model system for studying lifespan and neuroprotective activities of plant-derived compounds. J. Asia Pacif. Entomol. 2011;14:509–517. [Google Scholar]
- 34.Siddique Y.H., Naz F., Jyoti S., Fatima A., Khanam S., Rahul Effect of Centella asiatica leaf extract on the dietary supplementation in transgenic Drosophila model of Parkinson's disease. Parkinsons Dis. 2014;2014 doi: 10.1155/2014/262058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siddique Y.H., Jyoti S. Alteration in biochemical parameters in the brain of transgenic Drosophila melanogaster model of Parkinson's disease exposed to Apigenin. Integ. Med. Res. 2017;6:245–253. doi: 10.1016/j.imr.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simonian N.A., Coyle J.T. Oxidative stress in neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- 37.Ryter S.W., Kim H.P., Hoetzel A., Park J.W., Nakahira K., Wang X. Mechanisms of cell death in oxidative stress. Antioxidants Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 38.Tabrez S., Jabir N.R., Shakil S., Greig N.H., Alam Q., Abuzenadah M.A. A synopsis on the role of tyrosine hydroxylase in Parkinson's disease. CNS Neurol. Disord. Drug Targets. 2012;11:395–409. doi: 10.2174/187152712800792785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shakya A.K., Sharma N., Saxena M., Shrivastava S., Shukla S. Evaluation of the antioxidant and hepatoprotective effect of Majoon-e-Dabeed-ul-ward against carbon tetrachloride induced liver injury. Exp. Toxicol. Pathol. 2012;64:767–773. doi: 10.1016/j.etp.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Johnson W.M., Wilson-Delfosse A.L., Mieyal J.J. Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients. 2012;4:1399–1440. doi: 10.3390/nu4101399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y., Carvey P.M., Ling Z. Altered glutathione homeostasis in animals prenatally exposed to lipopolysaccharide. Neurochem. Int. 2007;50:671–680. doi: 10.1016/j.neuint.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yousuf S., Atif F., Ahmad M., Ishrat T., Khan B., Islam F. Neuroprotection offered by Majun Khadar, a traditional unani medicine, during cerebral ischemic damage in rats. Evid. Based Complement Alternat. Med. 2011;2011 doi: 10.1093/ecam/nep224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan M.B., Hoda M.N., Yousuf S., Ishrat T., Ahmad M., Ahmad A.S. Prevention of cognitive impairments and neurodegeneration by Khamira Abresham Hakim Arshad Wala. J. Ethnopharmacol. 2006;108:68–73. doi: 10.1016/j.jep.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Ishra T., Khan M.B., Hoda M.N., Yousuf S., Ahmad M., Ansari M.A. Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav. Brain Res. 2006;171:9–16. doi: 10.1016/j.bbr.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Adams J., Jr., Chang M.L., Klaidman L. Parkinsons Disease-redox mechanisms. Curr. Med. Chem. 2001;8:809–814. doi: 10.2174/0929867013372995. PMID: 11375751. [DOI] [PubMed] [Google Scholar]
- 46.Calvo A.C. 2010. Function and Regulation of Phenylalanine and Tyrosine Hydroxylases from Human and Caenorhabditis elegans. With Focus in Evolutionary Aspects and Development of New Therapies. [Google Scholar]
- 47.Gao N., Li Y.H., Li X., Yu S., Fu G.L. Effect of α-synuclein on the promoter activity of tyrosine hydroxylase gene. Neurosci. Bull. 2007;23:53–57. doi: 10.1007/s12264-007-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu D., Jin L., Wang H., Zhao H., Zhao C., Duan C. Silencing α-synuclein gene expression enhances tyrosine hydroxylase activity in MN9D cells. Neurochem. Res. 2008;33:1401–1409. doi: 10.1007/s11064-008-9599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez R.G., Waymire J.C., Lin E., Liu J.J., Guo F., Zigmond M.J. A role for α-synuclein in the regulation of dopamine biosynthesis. J. Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jimenez-Del-Rio M., Guzman-Martinez C., Velez-Pardo C. The effects of polyphenols on survival and locomotor activity in Drosophila melanogaster exposed to iron and paraquat. Neurochem. Res. 2010;35:227–238. doi: 10.1007/s11064-009-0046-1. [DOI] [PubMed] [Google Scholar]
- 51.Nichols C.D. Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol. Ther. 2006;112:677–700. doi: 10.1016/j.pharmthera.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 52.Siddique Y.H., Naz F., Jyoti S., Ali F., Fatima A., Rahul Protective effect of Geraniol on the transgenic Drosophila model of Parkinson's disease. Environ. Toxicol. Pharmacol. 2016;43:225–231. doi: 10.1016/j.etap.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Ara G., Afzal M., Jyoti S., Naz F., Rahul, Siddique Y.H. Effect of myricetin on the loss of dopaminergic neurons in the transgenic Drosophila model of Parkinson's disease. Curr. Drug Ther. 2019;14:58–64. [Google Scholar]
- 54.Bianchini M.C., Gularte C.O.A., Nogara P.A., Krum B.N., Gayer M.C., Bridi J.C., Ávila D.S. Thimerosal inhibits Drosophila melanogaster tyrosine hydroxylase (Dm TyrH) leading to changes in dopamine levels and impaired motor behaviour: implications for neurotoxicity. Metallomics. 2019 doi: 10.1039/c8mt00268a. [DOI] [PubMed] [Google Scholar]
- 55.Khair A., Salema B., Dhanushkodi N.R., Ardah M.T., Chen W., Yang Y., Haque M.E. Silencing of glucocerebrosidase gene in Drosophila enhances the aggregation of Parkinson's disease associated α-synuclein mutant A53T and affects locomotor activity. Front. Neurosci. 2018;12:81. doi: 10.3389/fnins.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohite G.M., Dwivedi S., Das S., Kumar R., Paluri S., Mehra S., Ruhela N., Jha N.N., Maji S.K. Parkinson's disease associated α-synuclein familial mutants promote dopaminergic neuronal death in Drosophila melanogaster. ACS Chem. Neurosci. 2018;9(11):2628–2638. doi: 10.1021/acschemneuro.8b00107. [DOI] [PubMed] [Google Scholar]
- 57.Szabo A., Tofaris G.K. Alpha-Synuclein. Humana Press; New York, NY: 2019. Monitoring α-synuclein proteotoxicity in Drosophila models; pp. 199–208. [DOI] [PubMed] [Google Scholar]
- 58.Jeng J.H., Tsai C.L., Hahn L.J., Yang P.J., Kuo Y.S., MYP Kuo. Arecoline cytotoxicity on human oral mucosal fibroblasts related to cellular thiol and esterase activities. Food Chem. Toxicol. 1993;377:751–756. doi: 10.1016/s0278-6915(99)00050-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.