Abstract
T-cells critically contribute to protection against Mycobacterium tuberculosis infection, and impaired T-cell responses can lead to disease progression. Pro-inflammatory and immunosuppressive cytokines affect T-cells, and fine-tuned regulation of cytokine signaling via the Jak/STAT signaling pathways is crucial for appropriate T-cell function. Constitutive STAT3 phosphorylation as a consequence of aberrant cytokine signaling has been described to occur in pathognomonic T-cell responses in inflammatory and autoimmune diseases. We characterized blood samples from tuberculosis patients (n=28) and healthy contacts (n=28) from Ghana for M. tuberculosis-specific T-cell responses, constitutive cytokine production, and SOCS3 and pSTAT3 expression. Lentiviral modulation of primary CD4+ T-cells was performed to determine the effects of SOCS3 on T-cell functions. T-cells from tuberculosis patients expressed higher levels of IL-10 and IL-6 and lower levels of T helper type (TH)17 cytokines after M. tuberculosis-specific stimulation compared to healthy contacts. In addition, tuberculosis patients had higher IL-10 and IL-6 levels in the supernatants of non-stimulated immune cells and plasma samples compared to healthy contacts. Notably, aberrant cytokine expression was accompanied by high constitutive pSTAT3 levels and SOCS3 expression in T-cells. Multivariate analysis identified an IL-6/IL-10 co-expression-based principal component in tuberculosis patients that correlated with high pSTAT3 levels. SOCS3 contributed to a regulatory component, and tuberculosis patients with high SOCS3 expression showed decreased TH1 cytokine expression and impaired IL-2-induced STAT5 phosphorylation. SOCS3 over-expression in primary CD4+ T-cells confirmed the SOCS3 inhibitory function on IL-2-induced STAT5 phosphorylation. We conclude that constitutive pSTAT3 and high SOCS3 expression are influential factors that indicate impaired T-cell functions in tuberculosis patients.
Keywords: tuberculosis, Interleukin-6, Interleukin-10, STAT3, SOCS3
Introduction
Tuberculosis is a chronic infectious disease caused by M. tuberculosis. M. tuberculosis is transmitted via aerosols exhaled by tuberculosis patients and eventually inhaled by close contacts. Immune surveillance protects the vast majority of infected individuals, and only a minority of M. tuberculosis-infected individuals develops tuberculosis disease. T-cells are crucial for protection against M. tuberculosis, and impaired TH1 immunity is specifically associated with an increased risk of developing tuberculosis disease.1 The key TH1 cytokine IFN-γ produced by M. tuberculosis-specific T-cells is a well-established marker for detection of M. tuberculosis infection. However, quantification of IFN-γ expression of T-cells from M. tuberculosis-infected healthy donors is neither sufficient to predict the risk of developing tuberculosis nor reliable as a biomarker indicating acute disease.2 Additional candidate biomarkers have been identified that may indicate disease mechanisms and T-cell failure to protect against tuberculosis more adequately.2 Several studies have reported higher IL-6 expression by T-cells and increased IL-6 serum concentrations in tuberculosis patients.3,4,5,6,7,8 Most of these studies concomitantly detected higher IL-10 serum concentrations in tuberculosis patients.3,4,5,6,7 Both IL-6 and IL-10 are produced by T-cells (as well as by other immune cell subsets) and directly affect T-cell functions via receptor signaling.9 IL-6 is a mediator of inflammation, and its crucial role in inflammatory and auto-immune diseases is well-established.10 Modulation of IL-6 availability and signaling of immune cells has been proven to be an efficient therapeutic approach, such as for rheumatoid arthritis.11 During T-cell polarization, IL-6 is of central importance for the generation of IL-17-producing TH17 cells, which are major immune drivers of inflammation.12 Furthermore, IL-6 inhibits the generation of regulatory T-cells, the main immunosuppressive T-cell population.13 IL-10 is a central effector molecule of immune regulation and characterizes type 1 regulatory T-cells in particular.14 Originally described as a TH2-related cytokine, IL-10 plays an important role in balancing immunity and limiting inflammatory responses. Consistent with this role, IL-10-deficient mice develop spontaneous inflammatory bowel disease.15 Many sources of IL-10 have been described, including TH1 and TH17 effector cells, which can produce IL-10 in an IL-6- or IL-27-dependent manner.16 In chronic viral infections, IL-10 expression by TH1 cells has been associated with antigen persistence and self-limiting immune responses.17,18
IL-6 and IL-10 signal via cellular receptor complexes that lack inherent signaling activity. Therefore, recruitment of subsidiary cofactors is required, and signal transduction function is mainly exerted by Jak/STAT pathway molecules for IL-6 and IL-10 receptors.19 Both IL-6 and IL-10 receptors specifically interact with Jak1, leading to recruitment, phosphorylation, and dimerization of the transcription factor STAT3.19 Similar transcription factor usage by both cytokines led to the obvious question of how IL-6 and IL-10 promote opposite cellular functions. Differences between immune cell subpopulations, including distinct IL-6/IL-10 receptor expression, may partially explain this phenomenon.9,20 Another important difference between IL-6 and IL-10 receptor signaling is exerted at the level of feedback regulation. IL-6 and IL-10 both induce expression of the inhibitory molecule SOCS3; however, whereas SOCS3 inhibits IL-6 signaling (by interacting with the gp130 receptor chain21), IL-10 receptor signaling is not affected by SOCS3 expression.22 Consistently, IL-10 induces prolonged STAT3 phosphorylation compared to IL-6.23 SOCS3 plays a role in multiple processes affecting T-cell functions.24 In addition to regulation of T-cell polarization, SOCS3 also inhibits T-cell receptor activation and proliferation.25,26 These SOCS3 effects are exerted via inhibition of the T-cell receptor, the CD28 co-receptor, and IL-2R signaling.25,26,27,28 SOCS3 is strongly implicated in host immune responses against M. tuberculosis infection.29 Previous studies have also reported increased levels of SOCS3 RNA in T-cells from tuberculosis patients,8,30,31 and effects of SOCS3 in resistance against tuberculosis have been identified in animal models.20
The goal of this study was to elucidate possible roles of aberrant cytokine expression and cytokine receptor signaling events on T-cell functions during tuberculosis pathogenesis in humans. To this end, we determined T-cell and plasma cytokine production levels in vitro, as well as STAT phosphorylation and SOCS3 protein expression levels in T-cells from tuberculosis patients and healthy contacts. Principal component analysis was performed to identify underlying complex profiles that distinguished tuberculosis patients and healthy contacts. Functional T-cell assays and modulation of SOCS3 expression were performed to elucidate impaired effector T-cell functions in human tuberculosis patients.
Materials and methods
Donor recruitment
We recruited adult tuberculosis patients (n=28) and exposed household contacts without symptoms of tuberculosis (hereafter referred to as ‘healthy contacts’ throughout the manuscript) (n=28) in an observational hospital-based study. Tuberculosis patients (n=5) and healthy contacts (n=2) with known HIV infection, as well as individuals with incomplete experimental data sets (i.e., tuberculosis patients (n=4); healthy contacts (n=3)), were excluded from this study. The study group characteristics of the ultimately included tuberculosis patients (n=19) and healthy contacts (n=23) are shown in Table 1. Tuberculosis patients were recruited at the Komfo Anokye Teaching Hospital (KATH), the Kwame Nkrumah University of Science and Technology (KNUST) Hospital, the Suntreso Government Hospital (SGH), and the Manhyia Hospital, all in Ghana, between 2015 and 2016. Healthy contacts had lived in the same household as tuberculosis index patients for over four months prior to diagnosis. Diagnosis of tuberculosis was based on patient history, chest X-ray, and sputum smear test. Chemotherapy according to the Ghanaian guidelines was initiated immediately after blood samples (approximately 10 ml) were taken. Individual donor samples from these study groups have been included for functional T-cell assays and analyses of IL-6 plasma levels published previously.31
Table 1.
Study group characteristics
| TB | Contacts | |
|---|---|---|
| Number of Persons (N) | 19 | 23 |
| Median age, years (range) | 39.5 (20–77)a | 40.0 (21–65) |
| Mean age, years | 40.7 | 39.8 |
| Male/Female (% male) | 13/6 (68) | 9/14 (39) |
aone value missing.
Ethical statement
The study was approved by the Committee on Human Research, Publication and Ethics (CHRPE/221/14, CHPRE/AP/328/15) at the School of Medical Sciences (SMS) at the Kwame Nkrumah University of Science and Technology (KNUST) in Kumasi, Ghana. All study participants provided written informed consent.
Whole blood in vitro cultures for measurement of supernatant cytokines
Heparinized whole blood (100 μl) diluted 1:1 in RPMI 1640 medium supplemented with L-Glutamine (2 mM) and Penicillin/Streptomycin (50 U/ml)) was cultured for 72 h in 96-well U-bottom plates at 37 °C and 5% CO2. Cells were stimulated with M. tuberculosis purified protein derivative (PPD) (10 μg/ml, Statens Serum Institute, Copenhagen, Denmark) or left unstimulated (medium only). After the culture period, plates were centrifuged, and supernatants were harvested and immediately frozen at −80 °C. Samples were then simultaneously thawed and measured using a flow cytometry-based LEGENDplex kit (BioLegend, San Diego, CA, USA) according to the manufacturer’s instructions. Briefly, culture supernatants (12.5 μl) were diluted 1:2 in assay buffer and incubated for 2 h at room temperature with specific antibody-labelled beads for cytokine analyses. Streptavidin-PE was added, and samples were incubated for an additional 30 min. Samples were then washed and analyzed with a BD LSR Fortessa flow cytometer (BD Biosciences, San Jose, CA, USA). Concentrations of each cytokine were determined by LEGENDplex Data Analysis Software v7 (VigeneTech, Carlisle, MA, USA) according to the manufacturer's instructions.
PBMC in vitro cultures for measurement of phosphorylated STAT molecules
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood by density centrifugation (Ficoll, Biochrom, Berlin, Germany) according to the manufacturer’s instructions. Anti-human CD4 antibodies (AlexaFluor488-labelled, clone RPTA-4, BioLegend) were added to each sample, which were then incubated for 15 min at 37 °C and 5% CO2 with pre-warmed (37 °C) X-vivo15 medium (100 μl, Lonza, Basel, Switzerland) supplemented with L-Glutamine (2 mM), and Penicillin/Streptomycin (50 U/ml) with or without human recombinant IL-2 (25 U/ml) or IL-6 (25 ng/ml) (both from BioLegend). Then, cells were fixed using True-Nuclear Transcription Factor buffer (BioLegend) and permeabilized with methanol as described previously.31 Samples were then centrifuged, washed in PBS containing FCS (10%) and stained for pSTAT3 (APC-conjugated, clone LUVNKLA, eBioscience, Waltham, MA, USA) or pSTAT5 Y694 (PE-conjugated, clone SRBCZX, eBioscience). Sample measurement was performed on a BD Accuri C6 flow cytometer (BD Biosciences). Flow cytometry-based STAT phosphorylation analyses were performed to characterize pSTAT levels in T-cell subpopulations. Similar approaches for quantification of constitutive STAT3 phosphorylation have been published previously.32,33 Alternative methods (e.g., Western blot) were not used because of the restricted donor sample volumes.
Measurement of plasma cytokine and soluble IL-6R levels
Blood plasma was harvested from EDTA BD vacutainer tubes (BD Biosciences) according to manufacturer’s instructions and immediately stored at −80 °C. After simultaneous thawing, the levels of a subset of cytokines, including IL-10, IL-21, IL-23, IL-27 and soluble IL-6R, were measured by ELISA Ready-SET-Go! (eBioscience) and Human sIL-6R INSTANT ELISA kits (ThermoFisher Scientific, Waltham, MA, USA) according to the manufacturers’ instructions. All samples were analyzed in duplicate. Samples were measured using an Infinite M200 ELISA reader (Tecan, Männedorf, Switzerland). Concentrations were calculated from respective standard curves by applying 4-parametric logistic regression. Samples outside the detection range were set to the corresponding lower or upper range value.
SOCS3 analysis in CD4+ T-cells in whole blood
Whole blood was stained with PE-conjugated anti-human CD4 (RPTA-4; BioLegend) and FITC-conjugated anti-human CD45RA (clone HI100; BioLegend) for 30 min on ice in the dark. After incubation, red blood cell lysis was performed (Red Blood Cell lysis buffer, Roche, Basel, Switzerland) according to the manufacturer’s instructions, followed by washing with PBS supplemented with 10% FCS (Sigma Aldrich, St Louis, MO, USA). PBMCs were then fixed with Fixation Buffer (BioLegend) and permeabilized using permeabilization/washing buffer (BioLegend). Subsequently, permeabilized cells were stained with an anti-human SOCS3 antibody (Abcam, Cambridge, UK) conjugated with Dy650 (DyLight™ 650 microscale antibody labelling kit; ThermoFisher) and analyzed using a BD Accuri C6 flow cytometer (BD Biosciences). For data analysis, we used FlowJo software (Version 10, FlowJo LLC, Ashland, OR, USA).
Whole blood in vitro stimulation for measurement of T-cell activation and intracellular cytokine levels
Heparinized blood was diluted (1:1) in RPMI 1640 supplemented with L-glutamine (2 mM) and Penicillin/Streptomycin (50 U/ml). Cells were then stimulated with PPD (10 μg/ml) or left unstimulated at 37 °C, 5% CO2 for 16 h. After 2.5 h, Brefeldin A (Sigma Aldrich) was added at a concentration of 3.75 μg/ml. Thereafter, erythrocytes were lysed (Red Blood Cell Lysis Buffer, Roche) according to the manufacturer’s instructions. Then, cells were fixed and permeabilized (BioLegend) and stained with an antibody against CD4 (AlexaFluor488-labelled, clone RPTA-4, BioLegend), IFN-γ (PE, clone 25723.11, BD Biosciences) or IL-2 (PerCP-Cy5.5, clone MQ1-17H12) and CD40L/CD154 (APC, clone 24.31, BioLegend). Cells were measured using a BD Accuri C6 flow cytometer (BD Biosciences) and analyzed with FlowJo software Version 10, FlowJo LLC). To avoid bias for PPD-specific cytokine production and T-cell activation, spontaneous cytokine expression was subtracted from PPD-induced cytokine production. Limited fluorescence channels restricted our analyses to four markers in Kumasi/Ghana. Therefore, we included the CD4 T-cell marker in addition to effector cytokine and activation markers and omitted the often-used T-cell marker CD3 as previous studies have shown a predominant CD3+/CD4+ phenotype of cytokine-producing T cells. In addition, cytokine-expressing CD3−CD4+ cells were rarely detected by the applied in vitro assay.34,35
Lentiviral transduction of SOCS3 and small hairpin RNA targeting SOCS3
Lentiviral transduction using the Lentiviral Gene Ontology (LeGO) assay was performed as described previously.36 Briefly, enriched CD4+ T-cells from healthy donors were activated by in vitro culture (X-vivo15 medium as described above) with anti-CD3/CD28-coated beads (0.2 μl, ThermoFisher) and IL-7 (10 ng/ml) for two days. Thereafter, T-cells were transduced using supernatants containing viral particles carrying SOCS3 cDNA,36 anti-SOCS3 shRNA (TRCN0000057075, Sigma-Aldrich), a vector control, and eBFP for detection of transduced T-cells by flow cytometry. After transduction, cells were washed and incubated for four days. Then, flow cytometry analyses for SOCS3 expression were performed as described previously.36 Briefly, cells were incubated with Fixable Viability Dye eFluor 780 (eBioscience) at room temperature for 15 min prior to fixation and permeabilization. Cells were stained with PE-Cy7-conjugated anti-human CD4 (OKT4, BioLegend) and an anti-human SOCS3 antibody (Abcam) conjugated with Dy650 (DyLight™ 650 microscale antibody labelling kit; ThermoFisher) for 30 min on ice in the dark and analyzed using a BD LSRFortessa flow cytometer (BD Biosciences). For quantitative analyses, mean fluorescence intensities were compared between vector control, shRNA, and SOCS3 cDNA-treated cells. However, absolute differences could not be directly deduced from these analyses because of background cellular fluorescence.36 Therefore, these results confirm differential expression, but the exact level of SOCS3 differences could not be deduced.
pSTAT5 analysis after IL-2 stimulation was performed three days after transduction as described above, with the following modifications: after methanol fixation, samples were stored at −20 °C for up to one week in methanol. An anti-human CD4 antibody (PE-Cy7, clone RPTA-4, BioLegend) was stained along with the pSTAT5 antibody (PE-conjugated, clone SRBCZX, eBioscience) and analyzed using a BD LSRFortessa flow cytometer (BD Biosciences).
Statistical analyses
Statistical analyses were performed using SPSS software version 24 (IBM Corp., Armonk, NY, USA) and GraphPad prism version 7 (GraphPad Software, La Jolla, CA, USA). The respective tests used are indicated in the Figure legends.
To reduce the complexity of measured immunological parameters into a smaller number of variables, a principal component-based multivariate analysis (PCA) was performed as described previously.37 Briefly, variables were log10-transformed to factor in the skewness of data (e.g., non-normal distributions and low proportions of positive producers for several cytokines) and to minimize the effects of outlier values. PCA was performed using SPSS v24. Only principal components (PC) accounting for a sufficient amount of variance (i.e., an eigenvalue>2, chosen based on the scree plot curve) were considered, and regression factor scores were extracted for subsequent analysis. Within a PC, variables with a loading factor≥0.5 or≤−0.5 were considered to be reflected by the PCA.37
Results
Lower M. tuberculosis-specific IL-17/IL-22 and higher IL-6/IL-10 expression in tuberculosis patients
M. tuberculosis PPD-specific cytokine expression was compared between tuberculosis patients and healthy contacts. High IFN-γ concentrations were detected in supernatants of PPD stimulated whole blood samples, but there was no significant difference between the study groups (Figure 1a). TNF-α concentrations were generally low, with several values below the limit of detection. TH17 cytokine levels, e.g., IL-17F and IL-22, were lower in tuberculosis patients compared to healthy contacts (IL-17F: P=0.015, IL-22: P=0.038, respectively) (Figure 1a). As expected, IL-17F and IL-22 expression correlated strongly in individual donors from both study groups (for all donors: r=0.78, P<0.0001; Figure 1b). Notably, IL-6 and IL-10 levels were higher in tuberculosis patients after PPD-specific stimulation compared to healthy contacts (Figure 1a). In summary, PPD-induced cytokines showed lower TH17 responses and higher IL-6/IL-10 production, whereas TH1 responses were similar between tuberculosis patients and healthy contacts.
Figure 1.
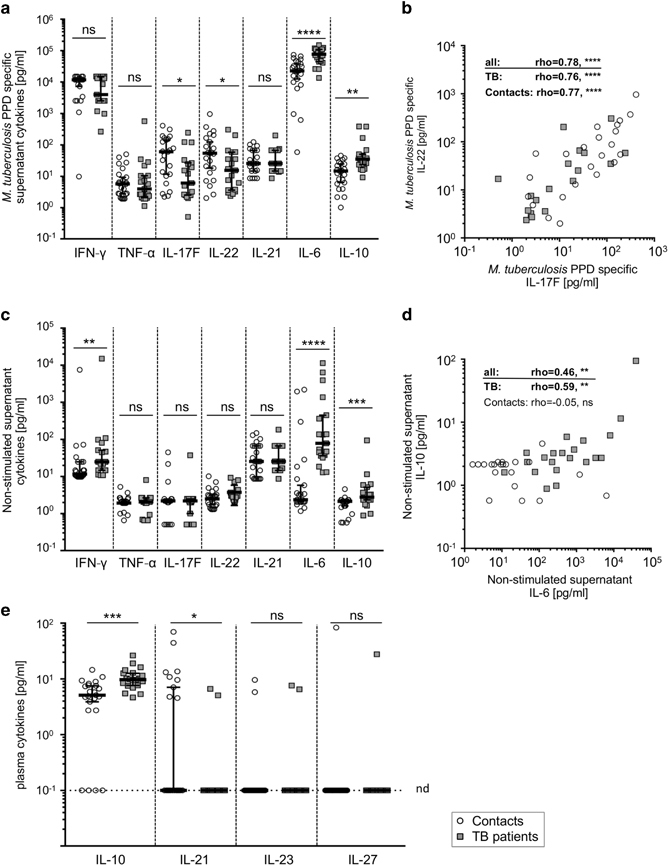
M. tuberculosis PPD-specific and constitutive cytokine expression and plasma cytokine concentrations from tuberculosis patients and healthy contacts. Cytokine concentrations of IFN-γ, TNF-α, IL-17F, IL-22, IL-21, IL-6, and IL-10 of supernatants from diluted whole blood after 72 h in vitro culture with (a and b) or without (c and d) M. tuberculosis PPD are depicted. Association of differentially expressed cytokines are shown in (b) for PPD-specific IL-17F and IL-22, and (d) for constitutive IL-6 and IL-10 values. (e) Plasma concentrations of IL-10, IL-21, IL-23, IL-27 are shown for healthy contacts and tuberculosis patients. The dotted line indicates the detection limit for the respective cytokines. Symbols placed on this line indicate values below the detection limit. All samples were measured in duplicate, and mean values are indicated as open circles for healthy contacts and grey squares for tuberculosis patients. Study group medians and percentiles (25, 75) are shown. Significant differences are indicated by asterisks. Nominal P-values for the Mann-Whitney U-test (two-tailed) were calculated and shown as * for P<0.05, ** for P<0.01, *** for P<0.001, and **** for P<0.0001. The Spearman Rank test was used to determine significant correlations for all donors and both study groups separately. Correlation coefficients (rho) and nominal P-values are given. ns: not significant, nd: not detectable.
Higher spontaneous IL-6 and IL-10 expression levels in tuberculosis patients
Marked variability was observed for cytokine concentrations of non-stimulated whole blood cultures from individual donors. Hence, we next compared cytokine levels in non-stimulated supernatants in the study groups. TNF-α, IL-17, and IL-22 concentrations were generally low and comparable between the study groups. In contrast, IL-10, IL-6, and IFN-γ concentrations were higher in tuberculosis patients compared to healthy contacts (for IL-6, P<0.0001; for IL-10, P=0.006; and for IFN-γ, P=0.007; Figure 1c). For tuberculosis patients, IL-6 and IL-10 levels correlated positively (r=0.59, P=0.0019; Figure 1d), whereas no correlation was found for healthy contacts (r=−0.05, P=0.83; Figure 1d). Since spontaneous cytokine expression may affect the interpretation of PPD-specific cytokines, we calculated ΔPPD values for individual donors (Supplementary Figure 1). Similar results, including higher IL-6/IL-10 and comparable IFN-γ concentrations, were found compared to non-subtracted PPD comparisons (Supplementary Figure 1). Therefore, tuberculosis patients had higher M. tuberculosis-specific IL-6/IL-10 concentrations, as well as higher spontaneous IL-6, IL-10, and IFN-γ expression in cultured whole blood. We speculated that spontaneous in vitro IL-6/IL-10 expression could be due to aberrantly high plasma concentrations of causative cytokines.
Higher IL-6 and IL-10 plasma levels may trigger constitutive T-cell cytokine expression in tuberculosis patients
Several cytokines have been reported to induce IL-6 and IL-10 expression in T-cells. To identify possible triggers of spontaneous IL-6 and IL-10 expression, we evaluated several plasma cytokine candidates (i.e., IL-6, IL-10, IL-21 IL-23, IL-27). IL-23 and IL-27 concentrations were below the detection limit for the vast majority of donors, and no differences were detected between the study groups (Figure 1e). IL-21 was detected in a minority of donors, primarily in healthy contacts (P=0.015; Figure 1e). In contrast, IL-10 levels were significantly higher in tuberculosis patients compared to healthy contacts (P=0.0002; Figure 1e). The IL-6 plasma levels from these study groups have already been published, with significantly higher IL-6 levels in tuberculosis patients.31 Since IL-6 signaling partly depends on the presence of the soluble IL-6 receptor (sIL-6R), we also determined sIL-6R plasma concentrations; however, we found no differences between the study groups (Supplementary Figure 2). These findings raised the question of whether IL-6/IL-10-dependent receptor signaling processes are affected in tuberculosis patients.
Ex vivo aberrantly high STAT3 phosphorylation and impaired IL-6 responses of T-cells from tuberculosis patients
IL-6 and IL-10 signal transduction is largely dependent on STAT3.19 Therefore, we analyzed STAT3 phosphorylation in T-cells from tuberculosis patients and healthy contacts by intracellular flow cytometry analysis. Representative histograms for a tuberculosis patient and a healthy contact are shown in Figure 2a. In the absence of in vitro re-stimulation, we found significantly higher pSTAT3 levels in CD4+ T-cells of tuberculosis patients compared to healthy contacts (P<0.0001; Figure 2b). Whereas the addition of IL-6 led to increased pSTAT3 levels in healthy contacts (P=0.0007; Figure 2b), no significant IL-6-induced STAT3 phosphorylation was detected in tuberculosis patients (P=0.51; Figure 2b). However, pSTAT3 levels in the presence of IL-6 were higher in tuberculosis patients as compared to healthy contacts (P=0.0003; Figure 2b). To avoid a possible bias from serial mean fluorescence analyses, we included non-stimulated pSTAT5 levels as an internal control for individual donor pSTAT3 measures. No differences for pSTAT5 expression were detected between tuberculosis patients and healthy contacts (Supplementary Figure 3). However, comparison of individual donor pSTAT3/pSTAT5 ratios confirmed higher constitutive pSTAT3 levels in tuberculosis patients (P<0.0001; Figure 2c). Since IL-6-induced STAT3 phosphorylation is inhibited by SOCS3, we next evaluated SOCS3 protein expression in T-cells.
Figure 2.
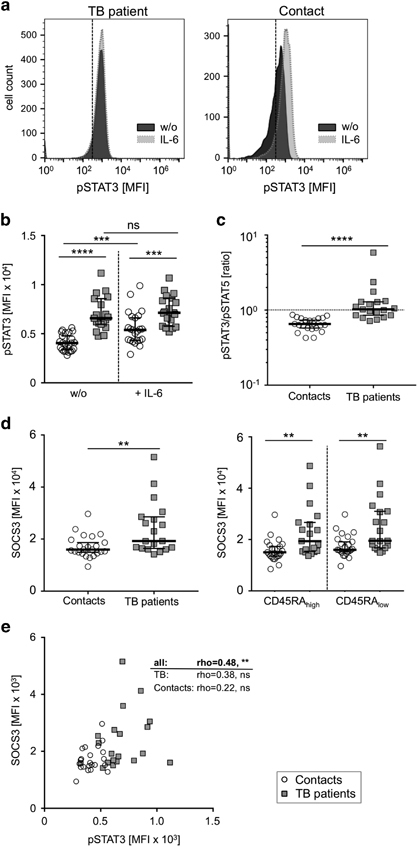
Constitutive and IL-6-induced STAT3 phosphorylation and SOCS3 expression in CD4+ T-cells from tuberculosis patients and healthy contacts. (a–c) Phosphorylation of STAT3 with or without IL-6 in vitro stimulation and (d) SOCS3 expression of CD4+ T-cells from tuberculosis patients and healthy contacts. (a) Representative histograms indicating non-stimulated (w/o) and IL-6-induced pSTAT3 expression of samples from a tuberculosis patient (left graph) and a healthy contact (right graph) are shown. Dotted lines indicate similar mean fluorescence intensities (MFI) of non-stained control samples for both respective study groups. (b) Study group comparisons of non-stimulated (w/o) and IL-6-induced pSTAT3 levels are shown. (c) The ratios between non-stimulated pSTAT3 and pSTAT5 values calculated for each individual donor. (d) SOCS3 expression for all CD4+ T-cells (left graph) as well as for CD45RAhigh and CD45RAlow (right graph) CD4+ T-cell subpopulations are shown. Tuberculosis patients are represented by grey squares, healthy contacts are depicted as open circles. Study group medians and percentiles (25, 75) are given. Significant differences are indicated by asterisks. Nominal P-values for the Mann-Whitney U-test (two-tailed) were calculated and shown as * for P<0.05, ** for P<0.01, *** for P<0.001, and **** for P<0.0001. ns: not significant. (e) Correlation plots for constitutive pSTAT3 and SOCS3 expression are shown for CD4+ T-cells. The Spearman Rank test was used to determine significant correlations for all donors and both study groups separately. Correlation coefficients (rho) and nominal P-values are given.
Higher SOCS3 expression in CD4+ T-cells from tuberculosis patients correlates with pSTAT3 levels
Previously, we and others described increased SOCS3 mRNA expression levels in T-cells from acute tuberculosis patients.8,30,38 Here, we determined SOCS3 protein expression levels by intracellular flow cytometry to characterize CD4+ T-cells and additional subpopulations. CD4+ T-cells of tuberculosis patients had higher SOCS3 protein expression compared to healthy contacts (P=0.006; Figure 2d, left graph). Similarly, we found higher SOCS3 levels in naïve (CD45RAhigh) and memory (CD45RAlow) CD4+ T-cells in tuberculosis patients compared to healthy contacts (Figure 2d, right graph). Since pSTAT3 induces SOCS3 transcription, we next determined the association between SOCS3 and STAT3 expression in T-cells. A significant correlation (for all donors; rho=0.48, P=0.001) was detected for CD4+ T-cells (Figure 2e) and was also found for CD4+ T-cell subpopulations (Supplementary Figure 4). Therefore, constitutive STAT3 phosphorylation is accompanied by higher SOCS3 protein levels in tuberculosis patients.
SOCS3 is negatively correlated with T-cell activation and IFN-γ expression
SOCS3 has previously been shown to regulate T-cell receptor activation.25,26,27,28 In addition, we and others have reported SOCS3 inhibitory effects on TH1 cells.36,39,40 We therefore measured the expression of T-cell activation marker CD40L in combination with intracellular IFN-γ and IL-2 after PPD-specific in vitro stimulation (Figure 3a). Tuberculosis patients and healthy contacts had similar proportions of M. tuberculosis PPD-induced CD40L-positive T-cells co-expressing IL-2 (Figure 3b). The results in CD40L/IFN-γ co-expressing T-cells were comparable to CD40L/IL-2 and have been published previously.31 Notably, CD40L/IFN-γ- and CD40L/IL-2-positive T-cell proportions were negatively correlated with SOCS3 expression exclusively in tuberculosis patients (for CD40L/IFN-γ: rho=−0.59, P=0.007; for CD40L/IL-2: rho=−0.64, P=0.004; Figure 3c)), whereas no correlation was detected for healthy contacts or for all donors collectively (for CD40L/IFN-γ: rho=−0.09, P=0.54; for CD40L/IL-2: rho=−0.16, P=0.29; Figure 3c). Negative correlations between SOCS3 and T-cell activation/cytokine expression were also found for CD45RAhigh and for CD45RAlow T-cell subpopulations (Supplementary Figure 5). This suggested potential inhibitory effects of high SOCS3 levels on activated TH1 cytokine-expressing CD4+ T-cells from tuberculosis patients.
Figure 3.
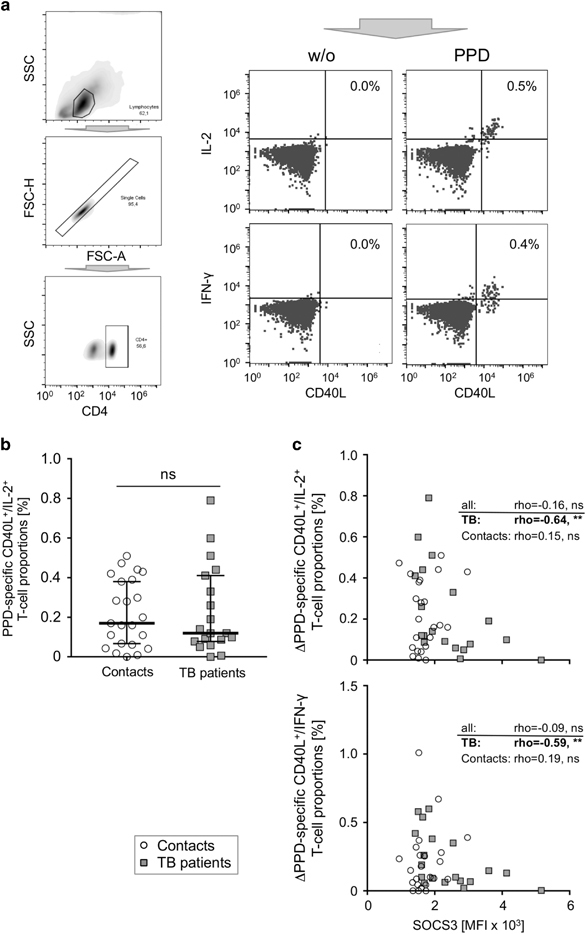
M.tuberculosis-specific activation and intracellular cytokine expression in association with SOCS3 expression in both study groups. (a) Representative graphs depicting the gating procedure for intracellular cytokine analysis are shown. (b) Proportions of CD40L/IL-2-positive CD4+ T-cells are shown for tuberculosis patients (grey squares) and healthy contacts (open circles). (c) Correlation plots between SOCS3 expression and CD40L/IL-2 (upper graph) as well as CD40L/IFN-γ (lower graph) are shown for CD4+ T-cells. The Spearman Rank test was used to determine significant correlations for all donors and both study groups separately. Correlation coefficients (rho) and nominal P-values are given. ns: not significant.
High SOCS3 expression in CD4+ T-cells from tuberculosis patients inhibits IL-2-induced STAT5 phosphorylation
The negative correlation of T-cell cytokine expression with high SOCS3 expression in tuberculosis patients may be due to reported SOCS3 inhibitory effects on IL-2-dependent T-cell proliferation.26,36 Therefore, we measured levels of STAT5 phosphorylation, which is the main mediator of IL-2 receptor signaling. After stimulation with IL-2, we found lower pSTAT5 levels in CD4+ T-cells from tuberculosis patients compared to healthy contacts (P=0.031; Figure 4a). A moderate but significant correlation of lower pSTAT5 levels with higher SOCS3 expression was detected for individual donors (rho=−0.31, P=0.046; Figure 4b), which was mainly due to a tendency towards negative correlation in tuberculosis patients (rho=−0.38, P=0.1; Figure 4b). To characterize SOCS3-mediated effects on IL-2 receptor signaling, we modulated SOCS3 expression in CD4+ T-cells by lentiviral transduction. Representative graphs of eBFP expression are shown in Figure 4c. Flow cytometry mean fluorescence analyses confirmed ectopic SOCS3 over-expression (P=0.021) and shRNA-mediated inhibition of SOCS3 expression (P=0.004) in CD4+ T-cells (Figure 4d). Analysis of IL-2-induced STAT5 phosphorylation confirmed decreased pSTAT5 levels in CD4+ T-cells over-expressing SOCS3 (mean: 0.12, equivalent to an 88% reduction compared to vector control, P=0.0004; Figure 4e), whereas shRNAs targeting SOCS3 had only minor effects on SOCS3 after short-term IL-2 stimulation (P=0.8; Figure 4e). These results suggested that high SOCS3 levels in T-cells from tuberculosis patients impaired IL-2 receptor signaling.
Figure 4.

IL-2-induced pSTAT5 levels in T-cells from both study groups, and effects of lentiviral SOCS3 modulation on IL-2-induced pSTAT5. (a and b) IL-2-induced STAT5 phosphorylation of CD4+ T-cells is shown for tuberculosis patients (grey squares) and healthy contacts (open circles). (b) Correlation of IL-2-induced pSTAT5 with SOCS3 expression. The Spearman Rank test was used to determine significant correlations for all donors and both study groups separately. Correlation coefficients (rho) and nominal P-values are given. (c) Representative flow cytometry histogram indicating fluorescence (eBFP) expression of non-transduced, vector control-, SOCS3cDNA-, and SOCS3shRNA-transduced CD4+ T-cells are shown. (d) Flow cytometry-based quantification of CD4+ T-cells with SOCS3cDNA (dark grey) and SOCS3shRNA (black line, open) compared to vector control (bright grey). The results from seven experiments are shown. (e) STAT5 phosphorylation in CD4+ T-cells from three experiments with increased or reduced SOCS3 expression. Nominal P-values for the paired t Test (two-tailed) were shown. MFI, mean fluorecence intensity.
Multivariate analyses identified a principal component of T-cells correlating with constitutive pSTAT3 levels that further classifies tuberculosis patients
Multiple factors determined in this study may contribute to failed immunity in human tuberculosis. To decipher the complex associated immunological factors and to identify patterns, we performed principal component multivariate analysis. We included data from supernatants of non-stimulated and PPD-stimulated whole blood, plasma cytokines, and CD4+ T-cells with PPD-specific intracellular cytokines and SOCS3 expression, as described above. Overall, four principal components (PC) were extracted based on an eigenvalue>2, which together accounted for 56.9% of the total variance (Table 2). Within each PC, variables with a loading factor of>0.5 were considered strong influencing factors. PC-1 accounted for the largest amount of the total data variance (20.5%) and was primarily characterized by constitutive and PPD-induced TH17-related cytokines (e.g., IL-17F and IL-22) from supernatants, as well as activated T-cell populations expressing IFN-γ or IL-2. PC-2 (accounting for 15.7% of the total data variance) was characterized by SOCS3 expression in T-cells, plasma IL-6 and IL-10 concentrations, constitutive IL-6/IL-10 production and PPD-induced IL-6/IL-10 expression (Table 2). PC-3 was characterized by IL-21 expression (in the presence or absence of PPD), and PC-4 included only constitutive IL-17F expression (negatively associated). To determine the relationship of PCs to constitutive pSTAT3 levels, we correlated the regression factor scores of each PC with pSTAT3 levels. Whereas PC-1 was not correlated with pSTAT3 (rho=−0.01, P=0.95; Figure 5a, left graph), a strong positive correlation of PC-2 (rho=0.75, P<0.0001) with constitutive pSTAT3 levels (Figure 5a, right graph) was observed. Neither PC-3 nor PC-4 significantly correlated with pSTAT3 levels. Furthermore, regression scores were compared between TB patients and healthy contacts. Notably, the median regression score of PC-1 was comparable between tuberculosis patients and healthy contacts (Figure 5b, left graph), whereas the regression score for PC-2 was significantly different between tuberculosis patients and healthy contacts (Figure 5b, right graph). We concluded that constitutive pSTAT3 expression is associated with simultaneously elevated levels of SOCS3, spontaneous IL-6/IL-10 expression, and PPD-specific IL-6/IL-10 expression in CD4+ T-cells in tuberculosis patients.
Table 2.
Principal Component analyses
| Parameter | Principal component | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Inhibitory | SOCS3 on CD4 | −0.065 | 0.674 | −0.152 | 0.146 |
| Plasma cytokines | plasma IL-6 | −0.046 | 0.758 | 0.203 | 0.122 |
| plasma IL-6R | 0.565 | 0.041 | 0.211 | −0.250 | |
| plasma IL-10 | 0.226 | 0.537 | 0.044 | 0.014 | |
| plasma IL-21 | 0.377 | −0.260 | −0.176 | 0.186 | |
| plasma IL-23 | 0.489 | −0.018 | −0.068 | −0.115 | |
| plasma IL-27 | −0.303 | −0.206 | −0.276 | −0.259 | |
| Restim cytokines | IL-6 unstim | 0.514 | 0.595 | −0.056 | 0.137 |
| IL-6 PPD | 0.396 | 0.543 | 0.153 | 0.483 | |
| IL-10 unstim | 0.393 | 0.528 | −0.228 | −0.379 | |
| IL-10 PPD | 0.499 | 0.602 | 0.148 | 0.015 | |
| IFNγ unstim | 0.574 | 0.129 | −0.132 | −0.518 | |
| IFNγ PPD | 0.380 | −0.124 | −0.338 | 0.344 | |
| TNFα unstim | 0.123 | −0.341 | 0.694 | −0.148 | |
| TNFα PPD | 0.571 | −0.191 | 0.088 | 0.163 | |
| IL-17f unstim | 0.571 | −0.211 | 0.054 | −0.596 | |
| IL-17f PPD | 0.620 | −0.418 | −0.383 | −0.020 | |
| IL-21 unstim | 0.330 | −0.200 | 0.847 | 0.111 | |
| IL-21 PPD | 0.250 | −0.161 | 0.886 | 0.079 | |
| IL-22 unstim | 0.528 | 0.245 | −0.023 | −0.468 | |
| IL-22 PPD | 0.587 | −0.464 | −0.250 | 0.056 | |
| ICS cytokines | PPD_CD40LhiIFNγhi | 0.597 | −0.160 | −0.194 | 0.505 |
| PPD_CD40LhiIL2hi | 0.601 | −0.336 | −0.133 | 0.402 | |
| Eigenvaluea | 4.714 | 3.602 | 2.715 | 2.06 | |
| Amount of variance (%) | 20.496 | 15.661 | 11.803 | 8.957 | |
| Cumulative variance (%) | 20.496 | 36.157 | 47.96 | 56.917 | |
aextracted if eigenvalue >2.
Variables with a loading factor ≥0.5 or ≤−0.5 are indicated in bold.
Figure 5.
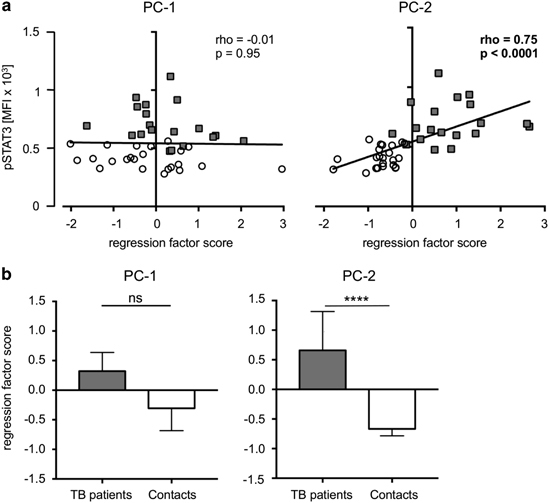
Multivariate analyses of influential factors and correlation with constitutive pSTAT3 in tuberculosis patients and healthy contacts. (a) Correlation of principal component (PC)-1 (left graph) and PC-2 (right graph) with constitutive pSTAT3 levels. The Spearman Rank test was used to determine significant correlations for all donors and both study groups separately. Correlation coefficients (rho) and nominal P-values are given. (b) Regression scores for PC-1 (left graph) and PC-2 (right graph) are compared between tuberculosis patients and healthy contacts. Data are presented as medians, and nominal P-values for the Mann-Whitney U-test (two-tailed) are shown.
Discussion
This study investigated the role of cytokines and their associated T-cell functions in human tuberculosis patients. To our knowledge, this is the first report identifying constitutive STAT3 phosphorylation in association with high SOCS3 protein expression as a feature of CD4+ T-cells in tuberculosis patients. Spontaneous in vitro and M. tuberculosis-specific IL-6 and IL-10 expression, as well as higher IL-6/IL-10 plasma levels, likely cause aberrant cytokine signaling events in tuberculosis patients. Multivariate principal component analysis revealed that spontaneously produced IL-6/IL-10, PPD-specific IL-6/IL-10 production, and SOCS3 expression in CD4+ T-cells clustered together in a single component that strongly correlated with high constitutive pSTAT3 levels. High SOCS3 levels correlated with weak TH1 responses in tuberculosis patients, likely due to inhibited IL-2 signaling and T-cell proliferation.
Constitutive STAT3 phosphorylation has been described for malignant diseases, and several functions during tumorigenesis have been attributed to STAT3 activation.41 In T-cell lymphoma, aberrantly high pSTAT3 levels are accompanied by high SOCS3 expression and contribute to tumor survival.42,43 High pSTAT3 levels have also been described for inflammatory diseases.32,33,44,45,46 Lovato et al. described constitutive STAT3 phosphorylation of intestinal and peripheral blood T-cells from patients with Crohn’s disease.44 Additional studies have further confirmed the important role of STAT3 in Crohn’s disease,46,47,48 and identified the IL-6/STAT3 cytokine signaling pathway as a therapeutic target against chronic inflammation and cancer development in inflammatory bowel disease.49 More recently, constitutive STAT3 phosphorylation was described in T-cells from rheumatoid arthritis patients.32,33,45 These studies reported a dependence between pSTAT3 levels—mainly in CD4+ T-cells and monocytes—and high IL-6 plasma concentrations in rheumatoid arthritis patients.32,33,45 The central role of IL-6 in constitutive STAT3 phosphorylation in rheumatoid arthritis was strengthened by transcriptomic and functional T-cell analyses.45 Furthermore, the levels of other potential causative cytokines of pSTAT3 induction (i.e., IL-10, IL-21, IL-23, and IL-27) were not increased in plasma samples from rheumatoid arthritis patients.32,33,45,50 Of note, whereas constitutive STAT3 phosphorylation in inflammatory and autoimmune disease studies could be increased by the addition of IL-6 in vitro, pSTAT3 levels in CD4+ T cells from tuberculosis patients were not increased in the presence of exogenous IL-6, and IL-6-induced pSTAT3 expression in healthy contacts did not reach the levels observed in tuberculosis patients. This indicates aberrantly high pSTAT3 expression and potentially impaired IL-6-mediated T-cell responses in tuberculosis patients. We strengthened the finding of increased constitutive pSTAT3 expression by including concomitant measures of pSTAT5 levels for individual donors. Both study groups had similar median pSTAT5 levels and, more importantly, individual donor pSTAT3/pSTAT5 ratios confirmed differential pSTAT3 levels and excluded possible effects of asynchronous flow cytometry measurements.
IL-6 is a well-known key factor in the development of TH17 cells, which are centrally involved in the development of inflammatory diseases.12 We detected lower proportions of IL-17F- and IL-22-producing TH17 cells in tuberculosis patients, which was consistent with previous studies.6,51 Different roles of STAT3 activation between inflammatory diseases and chronic infectious tuberculosis may reflect the complexity of STAT3 induction and regulation in different cell populations.29 The important role of STAT3 and its regulator, SOCS3, in the immune response against tuberculosis is well-established.24 Several studies have demonstrated the increased expression of SOCS3 mRNA expression in T-cells and PBMCs during tuberculosis infection.8,30,31,36,38 Analysis of protein expression by flow cytometry enabled us to characterize SOCS3 expression in several T-cell subpopulations in the present study. We identified increased SOCS3 protein expression in naïve (CD45RAhigh) and memory (CD45RAlow) CD4+ T-cells from tuberculosis patients. We also detected a correlation between constitutive STAT3 phosphorylation and SOCS3 expression in different T-cell subpopulations. In contrast to previous studies concerning pSTAT3/SOCS3 expression in inflammatory diseases, we detected higher IL-10 plasma levels, as well as higher spontaneous IL-10 production, in PBMCs from tuberculosis patients compared to healthy contacts. Higher IL-10 plasma concentrations have been described for tuberculosis patients,3,4,5,6,7 and spontaneous IL-10 production in non-activated PBMCs in vitro from tuberculosis patients has also been reported previously.4,5 Higher IL-10 expression was accompanied by higher IL-6 plasma levels in the present and previous studies.3,4,5,6,7 In addition, M. tuberculosis-infected macrophages were shown to have increased pSTAT3 levels. In macrophages, ESAT-6-induced IL-6 or IL-10 expression have been identified as possible causes.52,53 In addition to spontaneous IL-10 and IL-6 production, T-cells from tuberculosis patients were strongly activated in the presence of M. tuberculosis PPD to produce IL-10 and IL-6. These results suggested a dominant immunosuppressive T-cell phenotype in tuberculosis patients characterized by IL-10-positive M. tuberculosis-specific T-cells. Type 1 regulatory T-cells, as well as IL-10-producing effector T-cells, may account for the phenomenon. Induction of IL-10 expression in effector T-cells is well-established in chronic viral infections17,54 and has also been described in tuberculosis.55,56,57,58 In general, IL-10 co-expression in effector T-cells is hypothesized to play a role in down-regulating immune responses after pathogen eradication to minimize harmful side effects of immune responses.59,60 Interestingly, the induction of IL-10 in effector T-cells is dependent on IL-6 (or IL-27)-mediated STAT3 activation.16 These seemingly opposed functions of STAT3 are at least partially explained by its regulator, SOCS3.61 Whereas transient SOCS3 expression has been shown to be important for inflammatory T-cell and monocyte functions, the absence of SOCS3—and also permanent SOCS3 expression—was found to promote immunosuppressive or modulatory immune responses.61 This is reflected in IL-6 and IL-10 functions, as well as in their receptors. IL-6 induces SOCS3 via STAT3 phosphorylation, and IL-6 receptor signaling is suppressed by SOCS3 binding to the gp130 chain.61 In contrast, IL-10 induces SOCS3 expression, but no inhibitory functions of SOCS3 on IL-10R signaling have been described.22 Against this background, we hypothesized that the concomitant IL-6 and IL-10 expression measured in T-cells and plasma of tuberculosis patients promotes IL-10-mediated immune suppression and not the inflammatory arms of immune responses. Consistently, we found a correlation between high SOCS3 expression and low TH1 responses in tuberculosis patients and contacts. Although TH1 responses were comparable between tuberculosis patients and contacts, we conclude that TH1 responses may be stronger during acute tuberculosis in the absence of SOCS3. SOCS3 was found to suppress T-cell activation and proliferation via inhibition of T-cell co-stimulation by IL-2.36 To determine the role of SOCS3 in this context we performed lentiviral modulation of SOCS3 expression and detected inhibition of IL-2-mediated STAT5 phosphorylation when over-expressing SOCS3.
Overall, our data confirm previous findings of higher IL-10/IL-6 cytokine levels in tuberculosis patients and add novel data concerning constitutive pSTAT3/SOCS3 expression, which likely drives an immunosuppressive/anti-proliferation T-cell response in tuberculosis patients. This impaired T-cell function may contribute to the inefficient immune response in active tuberculosis disease that renders long-term multi-drug chemotherapy necessary. Against the background of ongoing immunomodulatory treatment trials for chronic infectious diseases, this study identifies novel candidate biomarkers for evaluating the efficacy of T-cell recovery.
Supplementary information
Acknowledgments
We would like to thank all study participants, study nurses, and physicians who made this study possible. This study was supported by the German Research Foundation (DFG, JA 1479/5-1) to N Nausch, E. Owusu-Dabo, and M. Jacobsen. K. Harling were supported by the ‘Hedwig und Waldemar Hort Stipendienstiftung’. E. Adankwah and M. Jacobsen were supported by the Manchot graduate school ‘Molecules of Infection (MOI)-3’.
Electronic supplementary material
Supplementary Figures for this article can be found on the Cellular & Molecular Immunology website 10.1038/cmi.2018.5
References
- 1.Cooper AM, Flynn JL. The protective immune response to Mycobacterium tuberculosis. Curr Opin Immunol. 1995;7:512–516. doi: 10.1016/0952-7915(95)80096-4. [DOI] [PubMed] [Google Scholar]
- 2.Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nature reviews Immunology. 2011;11:343–354. doi: 10.1038/nri2960. [DOI] [PubMed] [Google Scholar]
- 3.Chowdhury IH, Ahmed AM, Choudhuri S, Sen A, Hazra A, Pal NK, et al. Alteration of serum inflammatory cytokines in active pulmonary tuberculosis following anti-tuberculosis drug therapy. Molecular immunology. 2014;62:159–168. doi: 10.1016/j.molimm.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Masood KI, Rottenberg ME, Salahuddin N, Irfan M, Rao N, Carow B, et al. Expression of M. tuberculosis-induced suppressor of cytokine signaling (SOCS) 1, SOCS3, FoxP3 and secretion of IL-6 associates with differing clinical severity of tuberculosis. BMC infectious diseases. 2013;13:13. doi: 10.1186/1471-2334-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masood KI, Rottenberg ME, Carow B, Rao N, Ashraf M, Hussain R, et al. SOCS1 gene expression is increased in severe pulmonary tuberculosis. Scand J Immunol. 2012;76:398–404. doi: 10.1111/j.1365-3083.2012.02731.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Zhang M, Liao M, Graner MW, Wu C, Yang Q, et al. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am J Respir Crit Care Med. 2010;181:734–742. doi: 10.1164/rccm.200909-1463OC. [DOI] [PubMed] [Google Scholar]
- 7.Verbon A, Juffermans N, Van Deventer SJ, Speelman P, Van Deutekom H, Van Der Poll T. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin Exp Immunol. 1999;115:110–113. doi: 10.1046/j.1365-2249.1999.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashenafi S, Aderaye G, Bekele A, Zewdie M, Aseffa G, Hoang AT, et al. Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOCS3. Clin Immunol. 2014;151:84–99. doi: 10.1016/j.clim.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Jones LL, Alli R, Li B, Geiger TL. Differential T Cell Cytokine Receptivity and Not Signal Quality Distinguishes IL-6 and IL-10 Signaling during Th17 Differentiation. J Immunol. 2016;196:2973–2985. doi: 10.4049/jimmunol.1402953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirano T, Kishimoto T. Interleukin-6: possible implications in human diseases. Ric Clin Lab. 1989;19:1–10. doi: 10.1007/BF02871787. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka T, Narazaki M, Kishimoto T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb Perspect Biol 2017. [DOI] [PMC free article] [PubMed]
- 12.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romagnani S. Regulation of the T cell response. Clin Exp Allergy. 2006;36:1357–1366. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- 14.Wu K, Bi Y, Sun K, Wang C. IL-10-producing type 1 regulatory T cells and allergy. Cell Mol Immunol. 2007;4:269–275. [PubMed] [Google Scholar]
- 15.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-P. [DOI] [PubMed] [Google Scholar]
- 16.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 17.Parish IA, Marshall HD, Staron MM, Lang PA, Brustle A, Chen JH, et al. Chronic viral infection promotes sustained Th1-derived immunoregulatory IL-10 via BLIMP-1. J Clin Invest. 2014;124:3455–3468. doi: 10.1172/JCI66108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 20.Carow B, Reuschl AK, Gavier-Widen D, Jenkins BJ, Ernst M, Yoshimura A, et al. Critical and independent role for SOCS3 in either myeloid or T cells in resistance to Mycobacterium tuberculosis. PLoS pathogens. 2013;9:e1003442. doi: 10.1371/journal.ppat.1003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN, et al. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol. 2003;4:546–550. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- 22.Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, et al. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol. 2003;170:3263–3272. doi: 10.4049/jimmunol.170.6.3263. [DOI] [PubMed] [Google Scholar]
- 23.Braun DA, Fribourg M, Sealfon SC. Cytokine response is determined by duration of receptor and signal transducers and activators of transcription 3 (STAT3) activation. J Biol Chem. 2013;288:2986–2993. doi: 10.1074/jbc.M112.386573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carow B, Rottenberg ME. SOCS3, a Major Regulator of Infection and Inflammation. Frontiers in Immunology. 2014;5:58. doi: 10.3389/fimmu.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee A, Banks AS, Nawijn MC, Chen XP, Rothman PB. Cutting edge: Suppressor of cytokine signaling 3 inhibits activation of NFATp. J Immunol. 2002;168:4277–4281. doi: 10.4049/jimmunol.168.9.4277. [DOI] [PubMed] [Google Scholar]
- 26.Yu CR, Mahdi RM, Ebong S, Vistica BP, Gery I, Egwuagu CE. Suppressor of cytokine signaling 3 regulates proliferation and activation of T-helper cells. J Biol Chem. 2003;278:29752–29759. doi: 10.1074/jbc.M300489200. [DOI] [PubMed] [Google Scholar]
- 27.Cohney SJ, Sanden D, Cacalano NA, Yoshimura A, Mui A, Migone TS, et al. SOCS-3 is tyrosine phosphorylated in response to interleukin-2 and suppresses STAT5 phosphorylation and lymphocyte proliferation. Molecular and Cellular Biology. 1999;19:4980–4988. doi: 10.1128/MCB.19.7.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto A, Seki Y, Watanabe R, Hayashi K, Johnston JA, Harada Y, et al. A role of suppressor of cytokine signaling 3 (SOCS3/CIS3/SSI3) in CD28-mediated interleukin 2 production. The Journal of experimental medicine. 2003;197:425–436. doi: 10.1084/jem.20020939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rottenberg ME, Carow B. SOCS3 and STAT3, major controllers of the outcome of infection with Mycobacterium tuberculosis. Semin Immunol. 2014;26:518–532. doi: 10.1016/j.smim.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Jacobsen M, Repsilber D, Kleinsteuber K, Gutschmidt A, Schommer-Leitner S, Black G, et al. Suppressor of cytokine signaling-3 is affected in T-cells from tuberculosisTB patients. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2011;17:1323–1331. doi: 10.1111/j.1469-0691.2010.03326.x. [DOI] [PubMed] [Google Scholar]
- 31.Lundtoft C, Afum-Adjei Awuah A, Rimpler J, Harling K, Nausch N, Kohns M, et al. Aberrant plasma IL-7 and soluble IL-7 receptor levels indicate impaired T-cell response to IL-7 in human tuberculosis. PLoS pathogens. 2017;13:e1006425. doi: 10.1371/journal.ppat.1006425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isomaki P, Junttila I, Vidqvist KL, Korpela M, Silvennoinen O. The activity of JAK-STAT pathways in rheumatoid arthritis: constitutive activation of STAT3 correlates with interleukin 6 levels. Rheumatology (Oxford) 2015;54:1103–1113. doi: 10.1093/rheumatology/keu430. [DOI] [PubMed] [Google Scholar]
- 33.Kuuliala K, Kuuliala A, Koivuniemi R, Oksanen S, Hamalainen M, Moilanen E, et al. Constitutive STAT3 Phosphorylation in Circulating CD4+ T Lymphocytes Associates with Disease Activity and Treatment Response in Recent-Onset Rheumatoid Arthritis. PLoS One. 2015;10:e0137385. doi: 10.1371/journal.pone.0137385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller H, Detjen AK, Schuck SD, Gutschmidt A, Wahn U, Magdorf K, et al. Mycobacterium tuberculosis-specific CD4+, IFNgamma+, and TNFalpha+ multifunctional memory T cells coexpress GM-CSF. Cytokine. 2008;43:143–148. doi: 10.1016/j.cyto.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Schuck SD, Mueller H, Kunitz F, Neher A, Hoffmann H, Franken KL, et al. Identification of T-cell antigens specific for latent mycobacterium tuberculosis infection. PLoS One. 2009;4:e5590. doi: 10.1371/journal.pone.0005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleinsteuber K, Heesch K, Schattling S, Sander-Juelch C, Mock U, Riecken K, et al. SOCS3 promotes interleukin-17 expression of human T cells. Blood. 2012;120:4374–4382. doi: 10.1182/blood-2011-11-392738. [DOI] [PubMed] [Google Scholar]
- 37.Sokal RR, RF. Biometry: the principles and practice of statistics in biological research. New York: W.H. Freeman & Co Ltd. 1981.
- 38.Mistry R, Cliff JM, Clayton CL, Beyers N, Mohamed YS, Wilson PA, et al. Gene-expression patterns in whole blood identify subjects at risk for recurrent tuberculosis. The Journal of infectious diseases. 2007;195:357–365. doi: 10.1086/510397. [DOI] [PubMed] [Google Scholar]
- 39.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 40.Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168:3181–3187. doi: 10.4049/jimmunol.168.7.3181. [DOI] [PubMed] [Google Scholar]
- 41.Yuan J, Zhang F, Niu R. Multiple regulation pathways and pivotal biological functions of STAT3 in cancer. Sci Rep. 2015;5:17663. doi: 10.1038/srep17663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brender C, Lovato P, Sommer VH, Woetmann A, Mathiesen AM, Geisler C, et al. Constitutive SOCS-3 expression protects T-cell lymphoma against growth inhibition by IFNalpha. Leukemia. 2005;19:209–213. doi: 10.1038/sj.leu.2403610. [DOI] [PubMed] [Google Scholar]
- 43.Brender C, Nielsen M, Kaltoft K, Mikkelsen G, Zhang Q, Wasik M, et al. STAT3-mediated constitutive expression of SOCS-3 in cutaneous T-cell lymphoma. Blood. 2001;97:1056–1062. doi: 10.1182/blood.V97.4.1056. [DOI] [PubMed] [Google Scholar]
- 44.Lovato P, Brender C, Agnholt J, Kelsen J, Kaltoft K, Svejgaard A, et al. Constitutive STAT3 activation in intestinal T cells from patients with Crohn's disease. J Biol Chem. 2003;278:16777–16781. doi: 10.1074/jbc.M207999200. [DOI] [PubMed] [Google Scholar]
- 45.Anderson AE, Pratt AG, Sedhom MA, Doran JP, Routledge C, Hargreaves B, et al. IL-6-driven STAT signalling in circulating CD4+ lymphocytes is a marker for early anticitrullinated peptide antibody-negative rheumatoid arthritis. Ann Rheum Dis. 2016;75:466–473. doi: 10.1136/annrheumdis-2014-205850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mudter J, Weigmann B, Bartsch B, Kiesslich R, Strand D, Galle PR, et al. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am J Gastroenterol. 2005;100:64–72. doi: 10.1111/j.1572-0241.2005.40615.x. [DOI] [PubMed] [Google Scholar]
- 47.Nieminen JK, Niemi M, Sipponen T, Salo HM, Klemetti P, Farkkila M, et al. Dendritic cells from Crohn's disease patients show aberrant STAT1 and STAT3 signaling. PLoS One. 2013;8:e70738. doi: 10.1371/journal.pone.0070738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li C, Iness A, Yoon J, Grider JR, Murthy KS, Kellum JM, et al. Noncanonical STAT3 activation regulates excess TGF-beta1 and collagen I expression in muscle of stricturing Crohn's disease. J Immunol. 2015;194:3422–3431. doi: 10.4049/jimmunol.1401779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atreya R, Neurath MF. Signaling molecules: the pathogenic role of the IL-6/STAT-3 trans signaling pathway in intestinal inflammation and in colonic cancer. Curr Drug Targets. 2008;9:369–374. doi: 10.2174/138945008784221116. [DOI] [PubMed] [Google Scholar]
- 50.Pratt AG, Swan DC, Richardson S, Wilson G, Hilkens CM, Young DA, et al. A CD4 T cell gene signature for early rheumatoid arthritis implicates interleukin 6-mediated STAT3 signalling, particularly in anti-citrullinated peptide antibody-negative disease. Ann Rheum Dis. 2012;71:1374–1381. doi: 10.1136/annrheumdis-2011-200968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bandaru A, Devalraju KP, Paidipally P, Dhiman R, Venkatasubramanian S, Barnes PF, et al. Phosphorylated STAT3 and PD-1 regulate IL-17 production and IL-23 receptor expression in Mycobacterium tuberculosis infection. European Journal of Immunology. 2014;44:2013–2024. doi: 10.1002/eji.201343680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jung BG, Wang X, Yi N, Ma J, Turner J, Samten B. Early Secreted Antigenic Target of 6-kDa of Mycobacterium tuberculosis Stimulates IL-6 Production by Macrophages through Activation of STAT3. Sci Rep. 2017;7:40984. doi: 10.1038/srep40984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Queval CJ, Song OR, Deboosere N, Delorme V, Debrie AS, Iantomasi R, et al. STAT3 Represses Nitric Oxide Synthesis in Human Macrophages upon Mycobacterium tuberculosis Infection. Sci Rep. 2016;6:29297. doi: 10.1038/srep29297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rojas JM, Avia M, Martin V, Sevilla N. IL-10: A Multifunctional Cytokine in Viral Infections. J. Immunol Res. 2017;2017:6104054. doi: 10.1155/2017/6104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerosa F, Nisii C, Righetti S, Micciolo R, Marchesini M, Cazzadori A, et al. CD4(+) T cell clones producing both interferon-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin Immunol. 1999;92:224–234. doi: 10.1006/clim.1999.4752. [DOI] [PubMed] [Google Scholar]
- 56.Cyktor JC, Carruthers B, Beamer GL, Turner J. Clonal expansions of CD8+ T cells with IL-10 secreting capacity occur during chronic Mycobacterium tuberculosis infection. PLoS One. 2013;8:e58612. doi: 10.1371/journal.pone.0058612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, et al. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest. 2000;105:1317–1325. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinheiro RO, de Oliveira EB, Dos Santos G, Sperandio da Silva GM, de Andrade Silva BJ, Teles RM, et al. Different immunosuppressive mechanisms in multi-drug-resistant tuberculosis and non-tuberculous mycobacteria patients. Clin Exp Immunol. 2013;171:210–219. doi: 10.1111/cei.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 60.Haringer B, Lozza L, Steckel B, Geginat J. Identification and characterization of IL-10/IFN-gamma-producing effector-like T cells with regulatory function in human blood. The Journal of experimental medicine. 2009;206:1009–1017. doi: 10.1084/jem.20082238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


