Abstract
Efficient immune responses against invading pathogens often involve coordination between cells from both the innate and adaptive immune systems. For multiple decades, it has been believed that CD8+ memory T cells and natural killer (NK) cells constantly and uniformly recirculate. Only recently was the existence of noncirculating memory T and NK cells that remain resident in the peripheral tissues, termed tissue-resident memory T (TRM) cells and tissue-resident NK (trNK) cells, observed in various organs owing to improved techniques. TRM cells populate a wide range of peripheral organs, including the skin, sensory ganglia, gut, lungs, brain, salivary glands, female reproductive tract, and others. Recent findings have demonstrated the existence of TRM in the secondary lymphoid organs (SLOs) as well, leading to revision of the classic theory that they exist only in peripheral organs. trNK cells have been identified in the uterus, skin, kidney, adipose tissue, and salivary glands. These tissue-resident lymphocytes do not recirculate in the blood or lymphatic system and often adopt a unique phenotype that is distinct from those of circulating immune cells. In this review, we will discuss the recent findings on the tissue residency of both innate and adaptive lymphocytes, with a particular focus on CD8+ memory T cells, and describe some advances regarding unconventional T cells (invariant NKT cells, mucosal-associated invariant T cells (MAIT), and γδ T cells) and the emerging family of trNK cells. Specifically, we will focus on the phenotypes and functions of these subsets and discuss their implications in anti-viral and anti-tumor immunity.
Keywords: Tissue-resident memory T cell, Tissue-resident NK cell, Viral infection, Cancer
Subject terms: Innate lymphoid cells, CD8-positive T cells, NK cells
Introduction
The bridge between the innate and adaptive immune systems is critical for efficient immune responses against invading pathogens, and proper coordination and balance between various innate and adaptive cell types are necessary in the process. In the classic view, naive T cells are stimulated by their cognate antigens presented by antigen-presenting cells (APCs) and undergo clonal expansion and differentiate into effector T cells that migrate to the site of infection.1 After elimination of the infection, a minority of these effector T cells remain alive and differentiate into memory T cells, which can be found in the blood, secondary lymphoid organs (SLOs), and tissues throughout the rest of the body.2 Natural killer (NK) cells, on the other hand, are widely distributed throughout the body. These NK cells are now termed conventional NK (cNK) cells; they originate in the bone marrow and migrate through the circulation to different tissues.3 For many decades, it has been believed that CD8+ memory T cells and NK cells constantly and uniformly recirculate throughout the body.
In contrast to the classic view of lymphocyte recirculation, studies in the past decade have led to the characterization of lymphocyte populations permanently residing in nonlymphoid organs. These populations include tissue-resident memory T (TRM) cells, unconventional T cells (invariant NKT (iNKT) cells, mucosal-associated invariant T (MAIT) cells, and γδ T cells), and the emerging family of tissue-resident NK (trNK) cells. Although these cells are different, they share a number of features pertaining to their tissue-resident functions.4 The existence of these populations has been proved in various settings due to improvements in technologies. The use of parabiosis, in which the circulatory systems of two animals are conjoined for an extended period of time, is the most direct way to demonstrate tissue residency. Recirculating cells will migrate between conjoined animals and establish equilibrium between the two, while tissue-resident cells will not, demonstrating a lack of recirculation.5 Other techniques used to prove tissue residency include tissue transplantation and the blockade of cell recirculation.
TRM cells have been found in the skin, sensory ganglia, gut, lungs, brain, salivary glands, female reproductive tract, and other sites, while trNK cells have been identified in the uterus, skin, kidney, adipose tissue and salivary glands. These tissue-resident lymphocytes do not recirculate in the blood or lymphatic system and often adopt unique phenotypes and functions distinct from those of circulating lymphocytes.6 In this review, we will discuss the recent findings on the tissue residency of both innate and adaptive lymphocytes, with a particular focus on TRM cells, and provide some description of advances regarding unconventional T cells (iNKT cells, MAIT cells, and γδ T cells) and the emerging family of trNK cells. As most findings on TRM cells have been made regarding CD8+ TRM cells, we use the term “TRM cells” to refer to CD8+ TRM cells throughout the review.
Tissue-resident memory CD8+ T cells
Naive T cells migrate between blood and SLOs such as the spleen, lymph nodes (LNs), and mucosal-immune system (MIS) to search for their cognate antigens. They activate and proliferate extensively once they encounter cognate antigens presented by APCs. These activated cells differentiate into effector T cells capable of cytokine production and cytolytic activity, and they then migrate to the site of infection.1 When the infection is successfully eliminated, the majority of effector T cells die through apoptosis during the contraction phase, and the remaining cells differentiate into memory T cells, which can be found in the blood, SLOs, and tissues throughout the rest of the body.2 These memory T cells rapidly upregulate their effector functions and respond quickly upon rechallenge, thereby protecting against secondary infections outside of SLOs due to their broad distribution and high clonal frequency.7
Memory T cells play an important role in adaptive immunity. Researchers have defined the existence of two subsets of memory T cells based on their homing molecules and recirculation patterns.8 One of these subsets is the central memory T cells (TCM), which express the SLO homing molecules CCR7 and CD62L, primarily recirculate between SLOs and blood and rapidly proliferate upon antigen restimulation.9,10 The other subset is the effector memory T cells (TEM), which, unlike TCM, lack expression of SLO homing molecules and instead express higher levels of integrins and chemokine receptors that indicate the potential to migrate between nonlymphoid tissues.9 TEM express high levels of effector molecules and provide immediate effector functions when monitoring inflamed peripheral sites.
Due to technical shortcomings, the classic theory that divides memory T cells into two subsets has been assumed correct for a very long time, and it has always been believed that memory T cells constantly and uniformly recirculate between peripheral tissues and blood. However, significant revisions of this long-standing concept have been made thanks to improved techniques such as parabiosis, tissue transplantation, and blockade of T cell recirculation. The existence of noncirculating memory T cells that remain resident in the peripheral tissues, termed TRM cells, was defined in the skin and sensory ganglia after acute infection with herpes simplex virus (HSV), in the small intestine epithelium after lymphocytic choriomeningitis virus (LCMV) infection, and in the skin after vaccinia virus (VacV) infection.11–13 The work of these two laboratories has transformed the classic knowledge of memory T cell subsets in tissues and led to the discovery of TRM cells in many different organs.14 The TRM subset has become the cutting-edge population and the spotlight of research on T cells.
Signature phenotypes of CD8+ TRM cells in various tissues
TRM cells are both phenotypically and transcriptionally unique. They populate a wide range of peripheral organs, including the skin, sensory ganglia, gut, lungs, brain, salivary glands, female reproductive tract, and other tissues. Although migration is the main determinant of TRM cells, they are also identified by elevated expression of CD69 and the integrin CD103.7 Coexpression of CD69 and CD103 is observed on the majority of TRM cells, however, some fall into other phenotypic categories.14 For example, although CD103+ TRM cells are generally found in the small intestine and gut,15–20 oral infection with Yersinia pseudotuberculosis results in the development of both CD103+ and CD103− TRM cells in the lamia propria.21,22 Brain TRM cells express CD103 in models of intranasal (i.n.) infection with vesicular stomatitis virus (VSV),23 intracranial infection with Theiler’s murine encephalomyelitis virus (TMEV),24 and toxoplasma infection,25 but lack CD103 expression when elicited by LCMV infection. Both CD103+ and CD103− TRM cells can be found in the kidney.17,26
Previous findings have suggested a lack of CD103 expression in several organs such as the spleen,16,17,23,27 LNs16,23,27, and liver.28–30 However, contrary recent findings have identified TRM cells within SLOs and suggested that restimulation of nonlymphoid memory CD8+ T cells within the skin or mucosa can give rise to bona fide TRM cells specifically within draining LNs.31 In addition, populations of CD69+CD103+CXCR6+ TRM cells expressing low levels of sphingosine-1-phosphate receptor 1 (S1PR1) and KLF2, and CD69+CD103+CXCR6+CXCR3+ TRM cells with a T-betloEomesloBlimp-1hiHobitlo phenotype have been identified in the liver.32,33 CD103+ TRM cells are often present in the sensory ganglia,11 skin,11,13,15,16,34 lungs,15,35–37 salivary gland,16,17,38 reproductive tract,17,39 thymus40 and pancreas,17,41 and a minimal number of CD103+ TRM cells can also be found in the heart.17
Of note, intravascular staining has enabled the discovery that 95% of CD69+CD103+ TRM cells isolated from the mouse lung via standard methods are confined to the pulmonary vasculature instead of the lung tissue, suggesting an overestimate of the TRM population within the lung.28 On the other hand, parabiosis and quantitative immunofluorescence microscopy studies have revealed that conventional isolation methods and identification of TRM cells by phenotypic markers underestimate the size of the TRM population.42 In addition, different phenotypic markers and subsets have been identified in different organs. For example, CCR8 has been proposed as a marker for long-lived memory T cells in human skin, and CCR8+ T cells bear all the hallmarks of TRM cells, including surface expression of both CD103 and CD69.43 CD49a has recently been identified as a marker to differentiate CD8+ TRM cells into CD49a+ TRM cells that produce IFN-γ and CD49a− TRM cells that produce IL-17 in human skin epithelia.44 CD8+CD28− TRM cells have been identified in the skin lesions of patients in the early stages of systemic sclerosis.45 CD69+S1PR1− TRM cells were found in the nasal polyps of patients with chronic rhinosinusitis.46 CD103+CD39+ tumor-infiltrating CD8+ T cells have been found for six different malignancies including melanoma, lung cancer, head and neck squamous cell carcinoma (HNSCC), ovarian cancer, and rectal cancer,47 and high numbers of perforin-expressing TRM cells are found in patients with urothelial urinary bladder cancer (UBC).48
These findings indicate that (1) there may be substantial phenotypic heterogeneity among TRM cell subsets; (2) the tissue microenvironment and infection route can contribute to the regulation of phenotype and function of these cells; (3) phenotypic markers of TRM cells may differ between humans and mice; and (4) limitations in experimental techniques may confound our knowledge of TRM populations; cytometric identification based on phenotypic markers sometimes cannot represent a whole population. Therefore, identification of TRM cells based solely on the coexpression of CD69 and CD103 may not reliably identify all TRM cells. Distinct phenotypic markers should be used in studying TRM cells of different tissues, and lack of recirculation and using imaging techniques should be helpful methods in defining the TRM population.
Differentiation and maintenance of CD8+ TRM cells
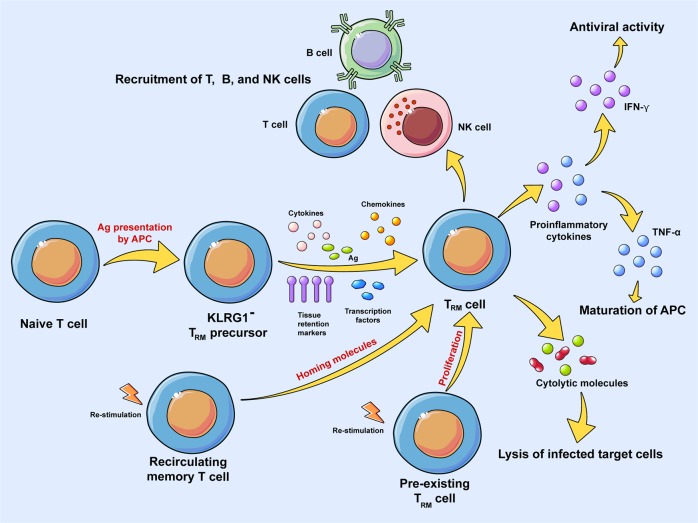
A large number of effector T cells enter the site of infection to clear pathogens during the acute phase of an immune response. Killer cell lectin-like receptor G1 (KLRG1) is induced on antigen-stimulated effector CD8+ T cells and has been considered as a marker of senescence. KLRG1+ cells receiving intermediate amounts of activating and inflammatory signals downregulate KLRG1 during the contraction phase in a Bach2-dependent manner,49 and the KLRG1− effector population contains TRM precursor cells. KLRG1− TRM precursor cells stop migrating immediately after pathogen clearance and begin to differentiate into TRM cells with phenotypic markers of residence, often as a result of the differential effects of stimulation by transcription factors, cytokines, chemokines and cognate antigen.12,15,16,50
The majority of TRM cells express CD69 and CD103. The integrin CD103 binds to E-cadherin, and this interaction promotes the retention of TRM cells within certain epithelial tissues.34 Deficiency in CD103 expression results in impaired tissue retention of TRM cells in a variety of peripheral tissues.15,17,18,23,51,52 However, the necessity of CD103 for T cell residence in many organs remains unclear.15,17,51,53 Notably, CD103− TRM cells do not rely on CD103 for their retention in specific tissues. CD69 is also important for TRM differentiation; it blocks the functional activity of S1PR1 by interfering with its cell surface expression and therefore resists S1P-mediated signals that enable tissue egress.16,38,54 Other molecules also participate in the differentiation and accumulation of TRM cells. For example, lung antigen-specific CD8+ T cells express both the very late antigen-1 (VLA-1) and CD103 after respiratory mucosal immunization55; the tumor necrosis factor (TNF) superfamily molecule LIGHT can promote the generation of lung-resident CD8+ T cells after an acute respiratory virus infection56; and the immune checkpoint ligand B7-H1 is essential for the maintenance and accumulation of virus-specific TRM cells in the central nervous system (CNS): Long-term maintenance of TRM cells is diminished in B7-H1 knockout mice.24
Transcriptional factors involved in the regulation of TRM differentiation include Hobit, Blimp-1, KLF2, Eomes and T-bet.15,57,58 In addition, the transcription factor Runx3 was recently identified as a key regulator of TRM cell differentiation that supports the expression of tissue-residency genes and suppresses genes associated with tissue egress and recirculation.59
The cytokine TGF-β promotes the expression of CD103 in barrier tissues,17,60 and a deficiency in TGF-βR on CD8+ T cells consequently leads to the failure of T cells to differentiate into TRM cells.34,38,61 Interestingly, TGF-β induces liver-adapted TRM cells and controls the formation of CD69+CD103− TRM cells in the kidney.26,33 Additionally, IL-15 drives the differentiation of TRM in some but not all tissues; the absence of TGF-β and IL-15 signaling results in impaired differentiation of CD103+ TRM cells and the loss of this population from the skin over time.15,58,62,63 Sequential signaling by IL-15 followed by TGF-β also induces liver-adapted TRM cells.33 New findings have also suggested the importance of pro-inflammatory cytokines, such as IFN-γ and IL-12, on the differentiation of TRM populations; impaired IFN-γ or IL-12 signaling results in defective differentiation of CD103−CD69+ TRM cells and reduces TRM persistence in the intestine.64
In addition to cytokines, chemokines are also important in the trafficking and localization of TRM cells. For example, the chemokine receptors CXCR6 and CCR10 are required for optimal formation of a TRM population in the skin; lack of either CXCL10 or CXCR3 compromises the mobilization of TRM cells within latently infected trigeminal ganglia and results in the absence of signals required for differentiation15,65,66; CXCR3 is also critical for T cell accumulation in uninfected salivary glands,67 and CXCL17 is required for the mobilization of TRM cells in the vaginal mucosa (VM).68
Differentiation of recirculating TEM cells depends on prolonged cognate antigen stimulation. However, antigen stimulation is not always required in the context of TRM cells, and whether it is required for TRM cell differentiation depends on the tissue of residence. For example, antigen recognition is required for brain TRM cells to differentiate23; is critical for TRM formation in the skin after infections with VacV-expressing model antigens69; and is essential for TRM development in the lungs and airways after infection with respiratory syncytial virus (RSV).70 On the contrary, long-lived intraepithelial CD103+ TRM cells can be generated in the absence of antigen recognition in the skin and mucosa in HSV and vaginal challenge models16; prolonged cognate antigen stimulation is dispensable for intestinal TRM ontogeny17; and noninflammatory vaccination enables the establishment of TRM cells in the female genital tract.61 Therefore, differentiation and maintenance of TRM cells differ from classic models of those processes for TEM cells, and the local microenvironment drives the phenotypic diversity of TRM cells.
Interestingly, although TRM cells have not been identified in SLOs in past studies, a new study using parabiosis of “dirty” mice has demonstrated that restimulation of nonlymphoid memory CD8+ T cells within the skin or mucosa results in the accumulation of TRM cells within draining LNs, indicating that nonlymphoid cells can give rise to SLO TRM cells.31 Secondary skin TRM cells can also be formed from pre-existing TRM cells, as well as from precursors recruited from the circulation, without displacement of the pre-existing TRM cell pool.71 Reactivation of mucosal TRM cells in the reproductive tracts triggers the recruitment of recirculating memory T cells that differentiate independently of antigen stimulation and contribute substantially to the boosted secondary TRM cell population.72
The length of TRM persistence varies between different tissues. For example, CD103+CD69+ TRM cells in nasal tissues are relatively stable for at least three months after a total respiratory tract (TRT) infection, while the number significantly declines in the lung.73 In contrast, skin TRM cells displace epidermal niches and can stay there for up to a year after HSV infection.74 Therefore, the persistence of TRM cells in different microenvironments is dependent on multiple tissue-specific factors.75 In addition, survival signals are essential for TRM cells to repopulate and maintain their niche. For example, TRM cells require IL-15 to support their maintenance after HSV-1 infection,15 while they rely on the aryl hydrocarbon receptor (AhR) for long-term persistence in the epidermis.74 Loss of these signals results in the loss of TRM cells. On the other hand, a recent study by Takamura et al. has revealed that the progressive loss of temporarily created “spaces” (RAMDs) over time may be the reason for the shorter lifespan of lung TRM cells.76
In conclusion, the differentiation and maintenance of TRM cells are tissue-specific and depend on a variety of factors including but not limited to: (1) certain molecules that are essential for the differentiation of TRM cells; (2) transcription factors that regulate differentiation of TRM cells; (3) tissue-specific environmental cues such as cytokines, chemokines, antigen stimulation, etc. that are involved in the differentiation of TRM cells; (4) nonlymphoid cells that contribute to the accumulation of TRM cells; and (5) pre-existing TRM cells or recirculating memory T cells that give rise to secondary TRM cells (Fig. 1). The fates of TRM cells (whether they become exhausted, migrate to other regions, transform into other cells (lose phenotypic markers, etc.), or are reactivated) and the reasons they undergo a specific fate merit further research.
Fig. 1.
Differentiation, maintenance, and function of TRM cells. Naïve T cells are activated and transform into KLRG1−TRM precursor cells. KLRG1− TRM precursor cells stop migrating immediately after pathogen clearance and begin to differentiate into TRM cells with phenotypic markers of residence, which often results from the differential effects of transcription factors, cytokines, chemokines, and cognate antigen stimulation. Recirculating memory T cells and pre-existing TRM cells give rise to secondary TRM cells upon restimulation. Activated TRM cells are involved in a variety of effector functions, including cytolytic activity, the secretion of proinflammatory cytokines such as IFN-γ and TNF-α, and the recruitment of other adaptive and innate immune cells
CD8+ TRM cells in anti-viral immunity
TRM cells, as a subset of memory T cells that reside permanently in the peripheral tissues, are believed to provide stronger protective immune responses than circulating memory T cells do.7 TRM cells provide frontline defense against invading pathogens due to their massive presence in the barrier tissues. The function and role of TRM cells in the control of viral infections have been demonstrated in numerous animal infection models and human patients. Here, we review the most recent evidence of TRM-mediated viral control in different tissues.
Due to their tissue-specific locations, TRM cells provide local protection immediately upon antigen re-encounter and promote rapid immune responses.77 This property of TRM cells has been demonstrated in various animal infection models and even in human patients.11,38,67,68,78–81 An increased number of CXCR8+ TRM cells has been detected in the VM of mice after intravaginal HSV-1 infection, and these cells contribute to the clearance of viral infection.68 Defective accumulation of TRM cells in the VM results in more virus replication and a reduced number of functional CD8+ T cells in the local tissue.68 Significant increases in both the number and function of HSV-specific CXCR3+ TRM cells have been detected in the trigeminal ganglia of mice following UV-B-induced HSV-1 reactivation, which protects the host from recurrent HSV infection, and a lack of TRM is again associated with recurrent ocular HSV infection.65 In addition, accumulation of TRM cells in the skin provides enhanced control against viral infection with HSV-1.11,62,82 TRM cells generated by the “prime and pull” protocol may reduce the spread of infectious HSV-2 into the sensory neurons and prevent development of clinical disease.61
Accumulation of TRM cells has been established in the brain of newborn mice after intraperitoneal mouse cytomegalovirus (MCMV) infection, which provides protection against primary MCMV infection and reduce brain pathology.83 Depletion of these cells results in virus reactivation and enhanced inflammation in the brain.83 Models using LCMV have revealed the protective role of brain-resident TRM cells in restricting viral infection in the CNS.84 TRM cells are generated and maintained in the CNS tissues after intracranial infection with TMEV, and a lack of this cell population results in compromised control of heterologous virus rechallenge.24 In addition, TRM cells accumulate within the brain after chronic Toxoplasma gondii infection and contribute to parasite control within the CNS.25
In the respiratory tract, TRM cells may confer protective immunity against viruses such as RSV,80,85 Sendai virus,86 and influenza.36,73,87,88 High levels of lung-resident TRM cells are induced by administration of the mucosal Sendai virus-engineered recombinant anti-TB vaccine (SeV85AB), which leads to a rapid and strong recall response against Mtb challenge infection.89 Generation of TRM cells by intranasal vaccination of RSV antigen-expressing MCMV results in earlier T cell responses and viral clearance after RSV challenge.90
Human immunodeficiency virus (HIV)-infected women display a high frequency of CD103−CD8+ TRM cells residing close to the epithelial basal membrane; accumulation of this subset is associated with HIV viral load.91 HIV-specific TRM cells are established in the VM and are capable of initiating a tissue-wide immune response after combined intranasal and intravaginal mucosal immunization with recombinant influenza-HIV vectors.88
Upon activation of TRM cells, they can rapidly secrete a number of proinflammatory cytokines, such as IFN-γ, TNF-α and IL-12 (Fig. 1).81,86,92,93 TRM cells generated by intranasal administration of RSV antigen-expressing MCMV secrete IFN-γ after RSV challenge.90 In human skin, CD8+CD49a+ TRM cells produce IFN-γ, whereas CD8+CD49a− TRM cells produce IL-17, which promotes local inflammation in the skin.44 CD103+ TRM cells produce both IFN-γ and TNF-α after chronic Toxoplasma gondii infection.25 In addition, allergen-induced epidermal accumulation of IL-17A-producing and IFN-γ-producing skin-resident TRM cells correlates with the magnitude of the challenge response.94
These mediators may drive the local activation and recruitment of both innate and adaptive immune cells (Fig. 1). For example, secretion of IFN-γ by TRM cells in the female mouse reproductive tract leads to the recruitment of immune cells including T cells, B cells and NK cells, while secretion of TNF-α by TRM cells leads to the maturation of APCs.93 Alternatively, activated TRM cells in the mouse skin can alter the local tissue environment by secreting cytokines, which leads to the induction of an IFN-γ-dependent antiviral program.92 The activation of HIV-specific CD8+ TRM cells results in the recruitment of both adaptive and innate immune cells in the VM.88 On the other hand, TRM cells can lyse infected target cells directly (Fig. 1). For example, CD8+CD49a+ TRM cells in human skin may induce the expression of perforin and granzyme B upon stimulation with IL-15, which in turn promotes a strong cytotoxic response.44 In general, TRM cells play a protective role in viral immunity, in which they provide stronger and faster immune responses against invading pathogens. Defective accumulation of TRM cells in local tissues often results in impaired immune responses, elevated viral load and recurrent infection. TRM cells confer their effector functions through the secretion of proinflammatory cytokines, the recruitment of other immune cells, and the enhancement of cytotoxic responses against target cells (Fig. 1).
CD8+ TRM cells in anti-tumor immunity
CD8+ T cells are extremely important in protection against tumor cells. However, in order for CD8+ T cells to fight against tumor cells, they must first migrate into the local tumor microenvironment to respond to tumor antigens. Although the role of TRM cells in anti-viral immune responses has been extensively studied over the last decade, the role of TRM cells in anti-tumor immune responses has yet to be fully discerned. Recent findings have revealed an accumulation of CD103+ TRM cells in several human solid tumors including ovarian,95–97 breast,98 lung,99–102 liver,103 and urinary bladder48 cancers. The abundance of these TRM cells has been associated with prolonged survival and better prognosis in patients with pulmonary squamous cell carcinoma (pSCC),101 hepatocellular carcinoma (HCC),103 triple-negative breast cancer (TNBC),104 recurrent laryngeal squamous cell carcinoma (LSCC),105 or HNSCC.47 In addition, a high number of TRM cells infiltrating the tumors is associated with lower tumor stage in patients with UBC.48 In contrast, the accumulation of TRM cells in melanoma tumors has not been associated with prolonged survival or a better prognosis, suggesting that TRM cells are associated with a better prognosis in some cancers but not in others106,107; TRM cells may have different local anti-tumor responses in different malignancies.
The functions of CD103+ TRM cells remain to be explored. Several studies have proposed a protective role of these TRM cells in the tumor microenvironment. CD103+CD39+ CD8+ tumor-infiltrating lymphocytes (TILs) from HNSCC patients kill autologous tumor cells in an MHC class I-dependent manner.47 Binding of the adhesion-associated protein paxillin (Pxn) to the subunit tail of CD103 expressed on tumor-specific cytotoxic T lymphocyte (CTL) clones may alter both adhesion and spreading of freshly isolated CD8+CD103+ lung TILs and CD103+ tumor-specific CTL clones and severely compromise the functions of these CTL clones against autologous tumor cells.100 Skin TRM cells generated as a result of autoimmune vitiligo produce IFN-γ and are critical for protection against melanoma rechallenge.52 TRM cells generated by OVA-encoding VacV may also provide effective anti-tumor immunity against OVA-expressing melanoma.108 In addition, CTLs from CD103hi tumors in lung cancer display features of enhanced cytotoxicity,99 and CD103+CD8+ TILs from nonsmall-cell lung carcinoma (NSCLC) patients display increased activation-induced cell death and specific cytolytic activity toward autologous tumor cells.102
One of the major functions of TRM cells is the recruitment of other immune cells from the circulation, the cooperation of TRM and recirculating TCM cells has been identified in a mouse model of vitiligo in which they work together to maintain disease.109 Thus, it is reasonable that TRM cells may confer protective functions against tumor cells through the recruitment of circulating tumor-specific T cells. Of note, circulating CD8+ T cells and CD8+ TRM cells cooperate in anti-tumor immunity, and circulating CD8+ T cells may adopt a TRM phenotype within the tumor and reside in the local tissue after tumor elimination.108
Two recent studies have pointed out that local proliferation of pre-existing TRM cells gives rise to secondary TRM cells upon rechallenge, and these newly recruited antigen-specific or bystander TRM cells are generated without replacement of the pre-existing TRM cells.71,72 A study of breast cancer also demonstrated that newly arrived TRM cells in the tumor are functional, while TRM cells established previously are dysfunctional; the persistence of these tumor-active TRM cells in the tumor site does not depend on antigen stimulation but is sustained by tumor-associated macrophage (TAM)-derived IL-15.110 These findings suggest that irrelevant cells may be present in the tumor and can compete with functional newly arrived TRM cells for cytokine resources.
On the other hand, immune checkpoint molecules are particularly enriched within T cells with phenotypic and genomic features of TRM cells in tumors.107 Some CD103+CD8+ TILs isolated from NSCLC patients have transcriptomic and phenotypic signatures of TRM cells and frequently express PD-1 and Tim-3.102 CD103+ TILs within the tumor epithelium in ovarian cancer also express PD-1, LAG-3 and Tim-3.97 PD-1 is additionally overexpressed in patients with urinary bladder cancer, HBV-related liver cancer, head and neck cancer, or breast cancer.47,48,103,104 These findings suggest that the TRM subset of TILs may adopt an exhausted phenotype and may be a major target of immune checkpoint blockade. Furthermore, TILs secrete less IL-2, IFN-γ and TNF-α compared with circulating counterparts.106 A study by Gabriely et al. has suggested that tumor-associated CD103+ CD8 T cells have regulatory properties, demonstrated by increased expression of CTLA-4 and IL-10, and they also have protumorigenic properties: adoptive transfer of CD103+ CD8 T cells promotes tumor growth.111 Therefore, the role of TRM cells in anti-tumor immunity is still debated, as they are protective in certain cancers but not in others. Further knowledge on the generation of appropriate TRM cells to act against tumor cells is essential for improved tumor immunotherapy.
Tissue-resident unconventional T cells
In contrast to the “conventional” CD8+ TRM cells, “unconventional” or “innate-like” T cells expressing T cell receptors (TCRs) with limited diversity, such as αβTCR-expressing iNKT cells, MAIT cells, and γδ T cells, have also been shown to be tissue-resident.4 These lymphocytes often recognize nonclassical and nonpolymorphic major histocompatibility complex (MHC)-like molecules, or MHC-unrelated presenting molecules.4
iNKT cells are lipid-sensing innate T cells expressing a semi-invariant αβTCR that only recognizes glycolipid antigens presented by the MHC class I-like molecule CD1d.112,113 These cells often express the transcription factor promyelocytic leukemia zinc finger (PLZF) and play a role in tumor surveillance and control of certain viral and bacterial infections.5,114 Three subsets of iNKT cells, termed NKT1, NKT2 and NKT17, have been described. These subsets express distinct transcriptional factors and corresponding cytokines and have been shown to localize to different tissues.115–121 The long-lived thymus-resident population of mature NKT cells is capable of rapid and prolonged production of IFN-γ and IL-4.122 Liver-resident NKT cells are retained locally through constitutive LFA-1-intercellular adhesion molecule (ICAM)-1 interaction induced by PLZF.123 However, iNKT cells accumulating in adipose tissue lack PLZF but express the transcriptional factor E4BP4,124 they produce IL-2 and IL-10 and control the homeostasis of Treg cells and macrophages in this tissue.124 Of note, iNKT cells also express CD69, a hallmark of TRM cells.5
MAIT cells are very abundant in humans and exhibit innate-like functions similar to those described for iNKT cells. Like iNKT cells, they also express a semi-invariant αβTCR that only recognizes bacterial metabolites derived from the synthesis of vitamin B presented by the MHC-related protein 1 (MR1).4,125,126 In addition, MAIT cells also respond quite sensitively to non-TCR signals, such as inflammatory cytokines including IL-7, IL-12, IL-15, IL-18, and IFN-α/β.127 Interestingly, MAIT cells confer a robust IFN-γ and granzyme B response to inflammatory signals but have limited responsiveness when stimulated directly by their TCR.128 MAIT cells are often marked by high expression of the C-type lectin CD161129,130 and expression of the transcription factors PLZF and RORγt.131,132 They are especially enriched in mucosal tissues, including the lung, liver and intestinal tract133 and are also abundant in peripheral blood where they coexpress CD161 and CD26.134 MAIT cells exhibit tissue homing properties and produce inflammatory cytokines.135 Human MAIT cells display a chemokine receptor expression pattern (CCR9intCCR7−CCR5hiCXCR6hiCCR6hi) that indicates preferential homing to the tissues, such as the intestine and liver. They produce IFN-γ and granzyme B as well as high levels of IL-17 after phorbol myristate acetate (PMA) and ionomycin stimulation.136 IL-12 and IL-18 activate liver-resident MAIT cells to produce a substantial amount of IFN-γ.137 MAIT cells have been found within primary and metastatic tumors. However, whether they play an aggravating role in malignancies or contribute to anti-cancer immunity is still unclear.127
T cells expressing the γδTCR represent another innate-like T cell subset that recognizes conserved nonpeptide antigens.138 γδ T cells are greatly enriched in mucosal and epithelial sites, such as the skin, respiratory, digestive and reproductive tracts. They also comprise a small proportion (1–5%) of the circulating lymphocytes in the peripheral blood. γδ T cells derive from thymic precursors and migrate into tissues early during development where they then persist as tissue-resident cells.139,140 These cells frequently express tissue-specific TCRs, which are invariant or closely related, resulting in distinguished roles for γδ T cells in different tissues.140,141 Similar to MAIT cells, IFN-γ and IL-17 are produced by different γδ T cell subsets.142,143 Dermal γδ T cells express the tissue-resident T cell markers CD69 and CD103 and additionally bear skin homing receptors and produce IL-17 and IL-22.144 CD69+CD103+ tissue-resident γδ T cells are expanded in the lungs of mice reinfected with B. pertussis and produce significantly more IL-17 than γδ T cells from infected unprimed mice.145 Resident memory γδ T cells in the LNs also produce IL-17A.146 γδ T cells contribute to the protective immunity against pathogens, tumor surveillance, and regulation of the innate and adaptive immune responses.147 They are involved in various diseases, including viral and microbial infections, autoimmune diseases and cancer.140,148
The emerging family of Tissue-Resident NK cells
Tissue residency is a hallmark of the innate lymphoid cell (ILC) family. ILCs can be subdivided into three groups based on differential expression of phenotypic markers and transcription factors and production of different cytokines. Group 1 ILC (ILC1) cells, including trNK cells, are directed by the transcription factor T-bet and produce IFN-γ; group 2 ILC (ILC2) cells are directed by the transcription factor GATA-3 and produce IL-15 and IL-13; group 3 ILC (ILC3) cells are directed by the transcription factor RORγt and produce IL-17. Interestingly, trNK cells have been distinguished from the cNK cells and are considered the “innate counterparts” of TRM cells.5,149,150
The new era of trNK cell biology began with the discovery of CD49a+ liver-resident NK cells in a contact hypersensitivity (CHS) model through a parabiosis study.3,151,152 Two distinct subsets of murine NK cells were identified in this study: CD49a−DX5+ cNK cells that circulate in the blood, and CD49a+DX5− trNK cells that remain resident in the liver.151,153 CD49a+ trNK cells reside in the liver sinusoidal blood, they possess memory potential and confer hapten-specific CHS responses upon hapten challenge.151 In addition to CD49a, liver-resident NK cells express higher levels of CXCR6, CXCR3, CD69 and the TNF-related apoptosis-inducing ligand (TRAIL),154 and they strictly require the transcription factors T-bet, Hobit and PLZF for their development but are independent of Eomes.154 A recent study also demonstrated the requirement for AhR in the maintenance of liver-resident CD49a+TRAIL+CXCR6+DX5− NK cells and their hapten memory function.155 Hepatic CD49a+ NK cells are induced by culturing cells with IL-2, IL-12, IL-15, IL-18, or the cytokine cocktail (IL-2/IL-12/IL-15/IL-18) and may produce high quantities of IFN-γ and TNF-α.156 These cells degranulate less efficiently than cNK cells, however, due to their higher expression of TRAIL, they are capable of inducing cell death in TRAIL-sensitive target cells.3
Findings on liver trNK cells provide new insights into the discovery of CD49a+DX5− NK cells in the uterus, skin, kidney, lung, and adipose tissues.157–160 trNK cells in the skin are T-bet-dependent and lack Eomes expression.157 However, unlike liver and skin trNK cells, uterus and kidney trNK cells are T-bet-independent.158,160,161 Eomes+CD49a+ NK cells are most abundant during early pregnancy, while Eomes−CD49a+ NK cells dominate before puberty.162 Interestingly, a recent study using an immune-competent NK cell-specific reporter mouse showed that although both cNK and trNK cells accumulate in the mouse uterus, only trNK cells proliferate, and these proliferating trNK cells are the source of uterus NK cells during endometrial decidualization.163 trNK cells in salivary glands are positive for both CD49a and DX5149 and are present in normal numbers in T-bet-, Eomes-, and NFIL3-deficient mice. However, lack of TGF-β signaling significantly decreases salivary gland NK cell numbers.149,164 Unlike liver NK cells, lung NK cells control viral proliferation after primary influenza virus infection but do not protect mice against secondary influenza virus infection,165 suggesting the absence of a memory phenotype and function in lung NK cells. An in vitro cytokine-based feeder-free system has been developed as an approach to generate CD49a+Eomes−/+ NK cells using IL-15 and IL-4; IL-15 is essential for the development and maintenance of CD49a+ NK cells, while IL-4 induces the expression of Eomes and converts Eomes−CD49b− NK cells into CD49a+Eomes+ NK cells.166
Inspired by these findings, several groups have attempted to define trNK cells in humans. A T-bet+Eomes−CD49a+ NK cell subset with a phenotype homologous to that of CD49a+ trNK cells in the murine liver has been identified in the human liver but not in afferent or efferent blood of the liver.167 This trNK cell subset is CD56bright and expresses killer cell Ig-like receptor (KIR), NKG2C, and low levels of CD16, CD57 and perforin.167 This subset expresses high levels of inflammatory cytokines such as IFN-γ, TNF and GM-CSF and degranulates poorly upon stimulation.167 Although trNK cells are believed to arise from precursors distinct from cNK cells and lack Eomes expression,168,169 a recent study has identified an Eomeshi population of NK cells in the human liver that is completely absent in the blood.170 Eomeslo NK cells circulate freely, whereas Eomeshi NK cells are unable to leave the liver.170 This population accounts for more than 50% of human liver NK cells and largely overlaps with CD56brightCXCR6+ NK cells. However, these cells do not overlap with CD49a+ liver-resident NK cells.171 Thus, two nonoverlapping NK cell populations have been identified in the human liver: CD49a+ NK cells and Eomeshi (largely CD56brightCXCR6+) NK cells (Table 1). It was found that immature CD16− NK cells can differentiate into both hepatic-specific CD49a+ and CXCR6+ NK cells.172 Interestingly, CyTOF analysis has revealed CD49e as a discriminating marker in human hepatic NK cells and suggested that CD49e− NK cells are the human liver-resident NK cells instead of CD49a+ NK cells.173 Unlike murine liver-resident NK cells, both CD49e− and CXCR6+ NK cell populations in humans express Eomes rather than T-bet (Table 1). Furthermore, a CD49a+Eomes+ subset of NK cells has been identified at the maternal-fetal interface in both humans and mice.174 A decrease in this NK cell subset impairs fetal development and results in fetal growth restriction.174
Table 1.
Unique features of human liver-resident NK (lrNK) cells
| CD49a+ lrNK cell | Eomeshi lrNK cell | CD49e- lrNK cell | |
|---|---|---|---|
| % of total intrahepatic NK | ~0–13% | ~50–60% | ~60% |
| Phenotypic marker | CD56brightCD16− | CD56brightCD16− | CD56brightCD16− |
| CD49a | + | − | ND |
| CD49e | − | − | − |
| CD69 | + | ++ | ++ |
| CD103 | + | − | ND |
| CD127 | − | − | ND |
| CXCR6 | − | + | + |
| NKG2A | − | + | ++ |
| NKG2C | ++ | − | ND |
| NKG2D | + | + | + |
| KIRs | + | + | ND |
| NKp30 | + | ND | ND |
| NKp44 | − | + | + |
| NKp46 | + | ++ | ND |
| CD226 | + | + | ND |
| Transcription factor | |||
| Eomes | + | ++ | ++ |
| T-bet | ++ | + | + |
| Cytokine secretion | |||
| IFN-γ | + | ++ | +++ |
| TNF-α | +++ | + | + |
| GM-CSF | + | + | ND |
| Cytolytic molecule | |||
| CD107a | + | + | + |
| Perforin | + | + | ND |
| Granzyme A | + | ND | ND |
| Granzyme B | ++ | + | ND |
| Granzyme K | ND | ++ | ND |
| References | a,b | b–d | e |
Although CD103 is one of the signature markers of TRM cells, there is little evidence regarding expression of CD103 on trNK cells. Human intrahepatic CD3−CD49a+CD56+ NK cells only express low levels of CD103,167 while neither Eomeshi nor CD49e− NK cells express CD103 (Table 1). In addition, human Eomes+CD49a+ decidual NK (dNK) cells,175 CD56+ NK cells in nasal lavage,176 and CD56brightCD49a+ NK cells in the lung177 express CD103.
trNK cells perform functions that are significantly different than those of cNK cells. Liver-resident trNK cells have a more potent and faster response to haptens and viruses through cytokine production or clonal-like expansion.151,178,179 Furthermore, they also contribute to immunosurveillance; the absence of CD49a+CD103+ NK cells results in accelerated tumor growth.180,181 In addition, a greater proportion of lung-resident CD56brightCD49a+ NK cells express surface CD107a compared with CD56brightCD49a− NK cells in lung explants infected ex vivo with Influenza A Virus; the former population provides early and important control of viral infection177 and plays a dominant role in controlling metastatic tumor growth in the lung182.
Concluding remarks
The existence of lymphocytes remaining resident in the peripheral tissues has challenged the long-standing concepts of memory T cells and NK cells, and the newly defined tissue-resident subsets have been the subject of numerous cutting-edge studies.
TRM cells are located in a wide range of peripheral organs including the skin, sensory ganglia, gut, lungs, brain, salivary glands, female reproductive tract, and other sites. However, a recent study by Beura et al. demonstrated the existence of TRM cells in the draining LN as well,31 which further enhances our knowledge of TRM cells. Although coexpression of CD69 and CD103 is observed on the majority of TRM cells, CD103− TRM cells also exist, and other phenotypic markers such as CCR8, CXCR3, CXCR6, and CD49a have been used to identify and classify TRM cells. CD49a has recently been identified as a marker to differentiate TRM cells into CD49a+ TRM cells, which produce IFN-γ, and CD49a− TRM cells, which produce IL-17, in human skin epithelia,44 suggesting the presence of different subsets within TRM cells. CD49a is also a typical marker of liver-resident NK cells in mice. CD49a−DX5+ NK cells are cNK cells that circulate in the blood, while CD49a+DX5− NK cells are liver-resident NK cells that exhibit very different transcriptional and phenotypic features than those of the cNK cells.151 CD49a+DX5− NK cells have also been found in the uterus, skin, kidney, and adipose tissue. Unlike in the murine liver, where only one population of liver-resident NK cells has been identified, two nonoverlapping NK cell populations have been identified in the human liver: CD49a+ NK cells and Eomeshi (largely CD56brightCXCR6+) NK cells.167 A recent finding has also suggested that CD49e− NK cells may be human liver-resident NK cells,173 implying a new possibility in the identification of human liver-resident NK cells. Although tissue-resident lymphocytes are identified through the expression of the surface receptors discussed above, it is still not clear whether additional phenotypic markers and subsets are involved. The tissue microenvironment and infection route can both contribute to the regulation of phenotype and function of these cells, and phenotypic markers may differ between humans and mice.
Of note, studies done using intravascular staining have been suggested to result in an overestimate of TRM populations within lung tissues,28 while parabiosis and quantitative immunofluorescence microscopy studies have revealed that conventional isolation methods and identification through phenotypic markers underestimate the size of TRM population.42 These findings suggest that limitations in experimental techniques can hamper our knowledge; cytometric identification through phenotypic markers sometimes cannot represent an entire population. Lack of recirculation and histology and/or other imaging techniques will fully reveal tissue-resident cell population and their functions.
The differentiation and maintenance of tissue-resident populations are crucial to long-term host survival. It is still unclear which populations of cells give rise to the TRM and trNK subsets. TRM cells may be derived from KLRG1− TRM precursor cells, from nonlymphoid or recirculating memory CD8+ T cells, or from pre-existing TRM cells. The factors that determine the fate of TRM cells in different tissues are not well defined. Additionally, it remains questionable whether a common developmental pathway exists. Of note, the possibility of peripheral tissue-derived precursor cells that give rise to TRM cells should not be ruled out, as such precursors have been identified for trNK cells.151
TRM cells provide stronger anti-viral immune responses to a variety of viruses upon rechallenge compared to circulating memory T cells, while trNK cells provide more potent and faster responses to haptens, viruses, or viral-like particles. TRM cells are now considered to be promising mediators of long-lived peripheral immunity to be elicited by future vaccines; however, effective responses in vaccine settings still merit further research. The role of TRM cells in anti-tumor immune responses is yet to be fully discerned; their accumulation has been confirmed in several human solid tumors and has been associated with prolonged survival and a favorable prognosis. However, some of the TRM cells in the tumor microenvironment tend to adopt an exhausted phenotype, suggesting that the role of TRM cells in anti-tumor immunity is still unclear; they are protective in certain cancers but not in others. trNK cells also contribute to immunosurveillance, the absence of which results in accelerated tumor growth. Few have suggested using TRM cells as the targets in checkpoint immunotherapy due to their higher expression of co-inhibitory receptors. However, targeting TRM cells in this setting should be approached carefully, as they sometimes protect the host against tumor cells. Generally, a deeper and more comprehensive understanding of the immune responses at sites of infection is necessary for tissue-resident cell-based immunotherapies to develop. The underlying mechanisms and molecular regulators involved in the activation, persistence and effector functions of these tissue-resident cells could be used in order to enhance the immune responses in local tissues.
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFA0507403), the National Natural Science Foundation of China (81788101, 81701631, 31390433, and 31670908) and the Chinese Academy of Sciences (XDB29030000).
Conflict of Interest
No potential conflicts of interest were disclosed.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bevan MJ. Memory T cells as an occupying force. Eur. J. Immunol. 2011;41:1192–1195. doi: 10.1002/eji.201041377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosato PC, Beura LK, Masopust D. Tissue resident memory T cells and viral immunity. Curr. Opin. Virol. 2017;22:44–50. doi: 10.1016/j.coviro.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng, H. & Sun, R. Liver-resident N. K. cells and their potential functions. Cell Mol. Immunol.14, 890–894 (2017). [DOI] [PMC free article] [PubMed]
- 4.Fan X, Rudensky AY. Hallmarks of tissue-resident lymphocytes. Cell. 2016;164:1198–1211. doi: 10.1016/j.cell.2016.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackay LK, Kallies A. Transcriptional regulation of tissue-resident lymphocytes. Trends Immunol. 2017;38:94–103. doi: 10.1016/j.it.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Lugli E, Hudspeth K, Roberto A, Mavilio D. Tissue-resident and memory properties of human T-cell and NK-cell subsets. Eur. J. Immunol. 2016;46:1809–1817. doi: 10.1002/eji.201545702. [DOI] [PubMed] [Google Scholar]
- 7.Shin H. Formation and function of tissue-resident memory T cells during viral infection. Curr. Opin. Virol. 2018;28:61–67. doi: 10.1016/j.coviro.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 9.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 10.Thom JT, Oxenius A. Tissue-resident memory T cells in cytomegalovirus infection. Curr. Opin. Virol. 2016;16:63–69. doi: 10.1016/j.coviro.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 12.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klonowski KD, et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/S1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 14.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 15.Mackay LK, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 16.Mackay LK, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl Acad. Sci. USA. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey KA, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheridan BS, et al. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity. 2014;40:747–757. doi: 10.1016/j.immuni.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, Bevan MJ. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013;39:687–696. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 21.Bergsbaken T, Bevan MJ. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8(+) T cells responding to infection. Nat. Immunol. 2015;16:406–414. doi: 10.1038/ni.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Z, Molloy MJ, Usherwood EJ. CD4(+) T-cell dependence of primary CD8(+) T-cell response against vaccinia virus depends upon route of infection and viral dose. Cell Mol. Immunol. 2016;13:82–93. doi: 10.1038/cmi.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl Acad. Sci. USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavelko KD, Bell MP, Harrington SM, Dong H. B7-H1 Influences the accumulation of virus-specific tissue resident memory T cells in the central nervous system. Front Immunol. 2017;8:1532. doi: 10.3389/fimmu.2017.01532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landrith TA, et al. CD103(+) CD8 T cells in the Toxoplasma-infected brain exhibit a tissue-resident memory transcriptional profile. Front Immunol. 2017;8:335. doi: 10.3389/fimmu.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma C, Mishra S, Demel EL, Liu Y, Zhang N. TGF-beta controls the formation of kidney-resident T cells via promoting effector T cell extravasation. J. Immunol. 2017;198:749–756. doi: 10.4049/jimmunol.1601500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenkel JM, Fraser KA, Masopust D. Cutting edge: resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J. Immunol. 2014;192:2961–2964. doi: 10.4049/jimmunol.1400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson KG, et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J. Immunol. 2012;189:2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tse SW, Cockburn IA, Zhang H, Scott AL, Zavala F. Unique transcriptional profile of liver-resident memory CD8+ T cells induced by immunization with malaria sporozoites. Genes Immun. 2013;14:302–309. doi: 10.1038/gene.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shuai Z, et al. Adaptive immunity in the liver. Cell Mol. Immunol. 2016;13:354–368. doi: 10.1038/cmi.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beura LK, et al. T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity. 2018;48:327–338 e325. doi: 10.1016/j.immuni.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stelma F, et al. Human intrahepatic CD69 + CD8+ T cells have a tissue resident memory T cell phenotype with reduced cytolytic capacity. Sci. Rep. 2017;7:6172. doi: 10.1038/s41598-017-06352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pallett LJ, et al. IL-2(high) tissue-resident T cells in the human liver: sentinels for hepatotropic infection. J. Exp. Med. 2017;214:1567–1580. doi: 10.1084/jem.20162115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ariotti S, et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc. Natl Acad. Sci. USA. 2012;109:19739–19744. doi: 10.1073/pnas.1208927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakim LM, Gupta N, Mintern JD, Villadangos JA. Enhanced survival of lung tissue-resident memory CD8(+) T cells during infection with influenza virus due to selective expression of IFITM3. Nat. Immunol. 2013;14:238–245. doi: 10.1038/ni.2525. [DOI] [PubMed] [Google Scholar]
- 36.Wu T, et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 2014;95:215–224. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takamura S. Persistence in temporary lung niches: a survival strategy of lung-resident memory CD8(+) T cells. Viral Immunol. 2017;30:438–450. doi: 10.1089/vim.2017.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc. Natl Acad. Sci. USA. 2011;108:16741–16746. doi: 10.1073/pnas.1107200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuburu N, et al. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J. Clin. Invest. 2012;122:4606–4620. doi: 10.1172/JCI63287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmann M, Oschowitzer A, Kurzhals SR, Kruger CC, Pircher H. Thymus-resident memory CD8+ T cells mediate local immunity. Eur. J. Immunol. 2013;43:2295–2304. doi: 10.1002/eji.201343519. [DOI] [PubMed] [Google Scholar]
- 41.Radenkovic M, et al. Characterization of resident lymphocytes in human pancreatic islets. Clin. Exp. Immunol. 2017;187:418–427. doi: 10.1111/cei.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinert EM, et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell. 2015;161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCully ML, et al. CCR8 expression defines tissue-resident memory T cells in human skin. J. Immunol. 2018;200:1639–1650. doi: 10.4049/jimmunol.1701377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheuk S, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287–300. doi: 10.1016/j.immuni.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li G, et al. Skin-resident effector memory CD8(+)CD28(-) T cells exhibit a profibrotic phenotype in patients with systemic sclerosis. J. Invest Dermatol. 2017;137:1042–1050. doi: 10.1016/j.jid.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ickrath P, et al. Accumulation of CD69+ tissueresident memory T cells in the nasal polyps of patients with chronic rhinosinusitis. Int J. Mol. Med. 2018;42:1116–1124. doi: 10.3892/ijmm.2018.3653. [DOI] [PubMed] [Google Scholar]
- 47.Duhen T, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 2018;9:2724. doi: 10.1038/s41467-018-05072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartana CA, et al. Tissue-resident memory T cells are epigenetically cytotoxic with signs of exhaustion in human urinary bladder cancer. Clin. Exp. Immunol. 2018;194:39–53. doi: 10.1111/cei.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herndler-Brandstetter D, et al. KLRG1(+) effector CD8(+) T cells lose KLRG1, differentiate into all memory T cell lineages, and convey enhanced protective immunity. Immunity. 2018;48:716–729 e718. doi: 10.1016/j.immuni.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee YT, et al. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J. Virol. 2011;85:4085–4094. doi: 10.1128/JVI.02493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malik, B. T. et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci. Immunol.2, eaam6346 (2017). [DOI] [PMC free article] [PubMed]
- 53.Cepek KL, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 54.Kim SK, Reed DS, Heath WR, Carbone F, Lefrancois L. Activation and migration of CD8 T cells in the intestinal mucosa. J. Immunol. 1997;159:4295–4306. [PubMed] [Google Scholar]
- 55.Haddadi S, et al. Expression and role of VLA-1 in resident memory CD8 T cell responses to respiratory mucosal viral-vectored immunization against tuberculosis. Sci. Rep. 2017;7:9525. doi: 10.1038/s41598-017-09909-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desai P, et al. The TNF superfamily molecule LIGHT promotes the generation of circulating and lung-resident memory CD8 T cells following an acute respiratory virus infection. J. Immunol. 2018;200:2894–2904. doi: 10.4049/jimmunol.1701499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mackay LK, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352:459–463. doi: 10.1126/science.aad2035. [DOI] [PubMed] [Google Scholar]
- 58.Skon CN, et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milner JJ, et al. Runx3 programs CD8(+) T cell residency in non-lymphoid tissues and tumours. Nature. 2017;552:253–257. doi: 10.1038/nature24993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohammed J, et al. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-beta. Nat. Immunol. 2016;17:414–421. doi: 10.1038/ni.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mackay LK, et al. T-box transcription factors combine with the cytokines TGF-beta and IL-15 to control tissue-resident memory T cell fate. Immunity. 2015;43:1101–1111. doi: 10.1016/j.immuni.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 63.Schenkel JM, et al. IL-15-independent maintenance of tissue-resident and boosted effector memory CD8 T cells. J. Immunol. 2016;196:3920–3926. doi: 10.4049/jimmunol.1502337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergsbaken T, Bevan MJ, Fink PJ. Local inflammatory cues regulate differentiation and persistence of CD8(+) tissue-resident memory T cells. Cell Rep. 2017;19:114–124. doi: 10.1016/j.celrep.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Srivastava, R. et al. CXCL10/CXCR3-dependent mobilization of herpes simplex virus-specific CD8(+) TEM and CD8(+) TRM cells within infected tissues allows efficient protection against recurrent herpesvirus infection and disease. J. Virol. 91, e00278–17 (2017). [DOI] [PMC free article] [PubMed]
- 66.Zaid A, et al. Chemokine receptor-dependent control of skin tissue-resident memory T cell formation. J. Immunol. 2017;199:2451–2459. doi: 10.4049/jimmunol.1700571. [DOI] [PubMed] [Google Scholar]
- 67.Caldeira-Dantas S, et al. The chemokine receptor CXCR3 promotes CD8(+) T cell accumulation in uninfected salivary glands but is not necessary after murine cytomegalovirus infection. J. Immunol. 2018;200:1133–1145. doi: 10.4049/jimmunol.1701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srivastava R, et al. CXCL17 chemokine-dependent mobilization of CXCR8(+)CD8(+) effector memory and tissue-resident memory T cells in the vaginal mucosa is associated with protection against genital herpes. J. Immunol. 2018;200:2915–2926. doi: 10.4049/jimmunol.1701474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khan TN, Mooster JL, Kilgore AM, Osborn JF, Nolz JC. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J. Exp. Med. 2016;213:951–966. doi: 10.1084/jem.20151855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kinnear E, et al. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol. 2018;11:249–256. doi: 10.1038/mi.2017.46. [DOI] [PubMed] [Google Scholar]
- 71.Park SL, et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol. 2018;19:183–191. doi: 10.1038/s41590-017-0027-5. [DOI] [PubMed] [Google Scholar]
- 72.Beura LK, et al. Intravital mucosal imaging of CD8(+) resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat. Immunol. 2018;19:173–182. doi: 10.1038/s41590-017-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pizzolla, A, et al. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci. Immunol. 2, eaam6970 (2017). [DOI] [PubMed]
- 74.Zaid A, et al. Persistence of skin-resident memory T cells within an epidermal niche. Proc. Natl Acad. Sci. USA. 2014;111:5307–5312. doi: 10.1073/pnas.1322292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takamura S. Niches for the long-term maintenance of tissue-resident memory T cells. Front Immunol. 2018;9:1214. doi: 10.3389/fimmu.2018.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takamura S, et al. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J. Exp. Med. 2016;213:3057–3073. doi: 10.1084/jem.20160938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gebhardt T, Mackay LK. Local immunity by tissue-resident CD8(+) memory T cells. Front Immunol. 2012;3:340. doi: 10.3389/fimmu.2012.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gebhardt T, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 79.Jiang X, et al. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kinnear E, et al. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol. 2018;11:290. doi: 10.1038/mi.2017.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schenkel JM, et al. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davies B, et al. Cutting edge: tissue-resident memory T cells generated by multiple immunizations or localized deposition provide enhanced immunity. J. Immunol. 2017;198:2233–2237. doi: 10.4049/jimmunol.1601367. [DOI] [PubMed] [Google Scholar]
- 83.Brizic, I. et al. Brain-resident memory CD8(+) T cells induced by congenital CMV infection prevent brain pathology and virus reactivation. Eur. J. Immunol.48, 950–964 (2018). [DOI] [PMC free article] [PubMed]
- 84.Steinbach K, et al. Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. J. Exp. Med. 2016;213:1571–1587. doi: 10.1084/jem.20151916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jozwik A, et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat. Commun. 2015;6:10224. doi: 10.1038/ncomms10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. Airway-resident memory CD8 T cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-gamma production. J. Immunol. 2015;195:203–209. doi: 10.4049/jimmunol.1402975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Teijaro JR, et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tan, H. X. et al. Induction of vaginal-resident HIV-specific CD8 T cells with mucosal prime-boost immunization. Mucosal. Immunol. 11, 994–1007 (2017). [DOI] [PubMed]
- 89.Hu Z, et al. Sendai virus mucosal vaccination establishes lung-resident memory CD8 T cell immunity and boosts BCG-primed protection against TB in mice. Mol. Ther. 2017;25:1222–1233. doi: 10.1016/j.ymthe.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morabito KM, et al. Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol. 2017;10:545–554. doi: 10.1038/mi.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gibbs, A. et al. HIV-infected women have high numbers of CD103-CD8+ T cells residing close to the basal membrane of the ectocervical epithelium. J. Infect. Dis.218, 453–465 (2017). [DOI] [PubMed]
- 92.Ariotti S, et al. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–105. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- 93.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat. Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmidt JD, et al. Rapid allergen-induced interleukin-17 and interferon-gamma secretion by skin-resident memory CD8(+) T cells. Contact Dermat. 2017;76:218–227. doi: 10.1111/cod.12715. [DOI] [PubMed] [Google Scholar]
- 95.Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin. Cancer Res. 2014;20:434–444. doi: 10.1158/1078-0432.CCR-13-1877. [DOI] [PubMed] [Google Scholar]
- 96.Webb JR, et al. Profound elevation of CD8+ T cells expressing the intraepithelial lymphocyte marker CD103 (alphaE/beta7 Integrin) in high-grade serous ovarian cancer. Gynecol. Oncol. 2010;118:228–236. doi: 10.1016/j.ygyno.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 97.Webb JR, Milne K, Nelson BH. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol. Res. 2015;3:926–935. doi: 10.1158/2326-6066.CIR-14-0239. [DOI] [PubMed] [Google Scholar]
- 98.Wang ZQ, et al. CD103 and intratumoral immune response in breast cancer. Clin. Cancer Res. 2016;22:6290–6297. doi: 10.1158/1078-0432.CCR-16-0732. [DOI] [PubMed] [Google Scholar]
- 99.Ganesan AP, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat. Immunol. 2017;18:940–950. doi: 10.1038/ni.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gauthier L, et al. Paxillin binding to the cytoplasmic domain of CD103 promotes cell adhesion and effector functions for CD8(+) resident memory T cells in tumors. Cancer Res. 2017;77:7072–7082. doi: 10.1158/0008-5472.CAN-17-1487. [DOI] [PubMed] [Google Scholar]
- 101.Koh J, et al. Prognostic implications of intratumoral CD103+ tumor-infiltrating lymphocytes in pulmonary squamous cell carcinoma. Oncotarget. 2017;8:13762–13769. doi: 10.18632/oncotarget.14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Djenidi F, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J. Immunol. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 103.Lim, C. J. et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut Epub ahead of print. 10.1136/gutjnl-2018-316510 (2018). [DOI] [PubMed]
- 104.Savas P, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 2018;24:986–993. doi: 10.1038/s41591-018-0078-7. [DOI] [PubMed] [Google Scholar]
- 105.Mann, J. E. et al. Analysis of tumor-infiltrating CD103 resident memory T-cell content in recurrent laryngeal squamous cell carcinoma. Cancer Immunol. Immunother. Epub ahead of print. 10.1007/s00262-018-2256-3 (2018). [DOI] [PMC free article] [PubMed]
- 106.Boddupalli CS, et al. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight. 2016;1:e88955. doi: 10.1172/jci.insight.88955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reading JL, et al. The function and dysfunction of memory CD8(+) T cells in tumor immunity. Immunol. Rev. 2018;283:194–212. doi: 10.1111/imr.12657. [DOI] [PubMed] [Google Scholar]
- 108.Enamorado M, et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8(+) T cells. Nat. Commun. 2017;8:16073. doi: 10.1038/ncomms16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Richmond, J. M. et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J. Invest. Dermatol. Epub ahead of print (2018). [DOI] [PMC free article] [PubMed]
- 110.Boldajipour B, Nelson A, Krummel MF. Tumor-infiltrating lymphocytes are dynamically desensitized to antigen but are maintained by homeostatic cytokine. JCI Insight. 2016;1:e89289. doi: 10.1172/jci.insight.89289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gabriely, G. et al. Targeting latency-associated peptide promotes antitumor immunity. Sci. Immunol. 2, eaaj1738 (2017). [DOI] [PMC free article] [PubMed]
- 112.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J. Exp. Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bendelac A, et al. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 114.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat. Rev. Immunol. 2012;12:845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Constantinides MG, Bendelac A. Transcriptional regulation of the NKT cell lineage. Curr. Opin. Immunol. 2013;25:161–167. doi: 10.1016/j.coi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coquet JM, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc. Natl Acad. Sci. USA. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee YJ, et al. Tissue-specific distribution of iNKT cells impacts their cytokine response. Immunity. 2015;43:566–578. doi: 10.1016/j.immuni.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Watarai H, et al. Development and function of invariant natural killer T cells producing T(h)2-and T(h)17-cytokines. PLoS Biol. 2012;10:e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Horst AK, Neumann K, Diehl L, Tiegs G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol. Immunol. 2016;13:277–292. doi: 10.1038/cmi.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bandyopadhyay K, Marrero I, Kumar V. NKT cell subsets as key participants in liver physiology and pathology. Cell Mol. Immunol. 2016;13:337–346. doi: 10.1038/cmi.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berzins SP, McNab FW, Jones CM, Smyth MJ, Godfrey DI. Long-term retention of mature NK1.1+ NKT cells in the thymus. J. Immunol. 2006;176:4059–4065. doi: 10.4049/jimmunol.176.7.4059. [DOI] [PubMed] [Google Scholar]
- 123.Thomas SY, et al. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J. Exp. Med. 2011;208:1179–1188. doi: 10.1084/jem.20102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lynch L, et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nat. Immunol. 2015;16:85–95. doi: 10.1038/ni.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Le Bourhis L, et al. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol. 2011;32:212–218. doi: 10.1016/j.it.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 126.Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1- and MR1-restricted T cells. Annu Rev. Immunol. 2014;32:323–366. doi: 10.1146/annurev-immunol-032713-120243. [DOI] [PubMed] [Google Scholar]
- 127.Haeryfar, S. M. M., Shaler, C. R. & Rudak, P. T. Mucosa-associated invariant T cells in malignancies: a faithful friend or formidable foe? Cancer Immunol. Immunother.67, 1885–1896 (2018). [DOI] [PMC free article] [PubMed]
- 128.Slichter, C. K. et al. Distinct activation thresholds of human conventional and innate-like memory T cells. JCI Insight1, e86292 (2016). [DOI] [PMC free article] [PubMed]
- 129.Billerbeck E, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc. Natl Acad. Sci. USA. 2010;107:3006–3011. doi: 10.1073/pnas.0914839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Turtle CJ, Swanson HM, Fujii N, Estey EH, Riddell SR. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity. 2009;31:834–844. doi: 10.1016/j.immuni.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Koay HF, et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat. Immunol. 2016;17:1300–1311. doi: 10.1038/ni.3565. [DOI] [PubMed] [Google Scholar]
- 132.Franciszkiewicz K, et al. MHC class I-related molecule, MR1, and mucosal-associated invariant T cells. Immunol. Rev. 2016;272:120–138. doi: 10.1111/imr.12423. [DOI] [PubMed] [Google Scholar]
- 133.Meermeier, E. W., Harriff, M. J., Karamooz, E. & Lewinsohn, D. M. MAIT cells and microbial immunity. Immunol. Cell Biol. 96, 607–617 (2018). [DOI] [PMC free article] [PubMed]
- 134.Sharma PK, et al. High expression of CD26 accurately identifies human bacteria-reactive MR1-restricted MAIT cells. Immunology. 2015;145:443–453. doi: 10.1111/imm.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rouxel, O. & Lehuen, A. Mucosal-associated invariant T cells in autoimmune and immune-mediated diseases. Immunol. Cell Biol.96, 618–629 (2018). [DOI] [PubMed]
- 136.Dusseaux M, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 137.Jo J, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog. 2014;10:e1004210. doi: 10.1371/journal.ppat.1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hovav AH. Human gammadelta T cells: rapid, stable and clonally reactive. Cell Mol. Immunol. 2017;14:646–648. doi: 10.1038/cmi.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nielsen MM, Witherden DA, Havran WL. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 2017;17:733–745. doi: 10.1038/nri.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheng M, Hu S. Lung-resident gammadelta T cells and their roles in lung diseases. Immunology. 2017;151:375–384. doi: 10.1111/imm.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chien YH, Meyer C, Bonneville M. gammadelta T cells: first line of defense and beyond. Annu Rev. Immunol. 2014;32:121–155. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
- 142.Jensen KD, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bai H, et al. Respective IL-17A production by gammadelta T and Th17 cells and its implication in host defense against chlamydial lung infection. Cell Mol. Immunol. 2017;14:850–861. doi: 10.1038/cmi.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jiang X, et al. Dermal gammadelta T cells do not freely re-circulate out of skin and produce IL-17 to promote neutrophil infiltration during primary contact hypersensitivity. PLoS ONE. 2017;12:e0169397. doi: 10.1371/journal.pone.0169397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Misiak A, Wilk MM, Raverdeau M, Mills KH. IL-17-producing innate and pathogen-specific tissue resident memory gammadelta T cells expand in the lungs of Bordetella pertussis-infected mice. J. Immunol. 2017;198:363–374. doi: 10.4049/jimmunol.1601024. [DOI] [PubMed] [Google Scholar]
- 146.Romagnoli PA, Sheridan BS, Pham QM, Lefrancois L, Khanna KM. IL-17A-producing resident memory gammadelta T cells orchestrate the innate immune response to secondary oral Listeria monocytogenes infection. Proc. Natl Acad. Sci. USA. 2016;113:8502–8507. doi: 10.1073/pnas.1600713113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 148.Wu D, Wu P, Qiu F, Wei Q, Huang J. Human gammadeltaT-cell subsets and their involvement in tumor immunity. Cell Mol. Immunol. 2017;14:245–253. doi: 10.1038/cmi.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cortez VS, et al. Transforming growth factor-beta signaling guides the differentiation of innate lymphoid cells in salivary glands. Immunity. 2016;44:1127–1139. doi: 10.1016/j.immuni.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fuchs A, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Peng H, et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Invest. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Peng H, Wisse E, Tian Z. Liver natural killer cells: subsets and roles in liver immunity. Cell Mol. Immunol. 2016;13:328–336. doi: 10.1038/cmi.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Peng H, Tian Z. Tissue-resident natural killer cells in the livers. Sci. China Life Sci. 2016;59:1218–1223. doi: 10.1007/s11427-016-0334-2. [DOI] [PubMed] [Google Scholar]
- 154.Peng H, Tian Z. Diversity of tissue-resident NK cells. Semin Immunol. 2017;31:3–10. doi: 10.1016/j.smim.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 155.Zhang LH, Shin JH, Haggadone MD, Sunwoo JB. The aryl hydrocarbon receptor is required for the maintenance of liver-resident natural killer cells. J. Exp. Med. 2016;213:2249–2257. doi: 10.1084/jem.20151998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hydes T, et al. IL-12 and IL-15 induce the expression of CXCR6 and CD49a on peripheral natural killer cells. Immun. Inflamm. Dis. 2018;6:34–46. doi: 10.1002/iid3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sojka DK, et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife. 2014;3:e01659. doi: 10.7554/eLife.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Victorino F, et al. Tissue-resident NK cells mediate ischemic kidney injury and are not depleted by anti-Asialo-GM1 antibody. J. Immunol. 2015;195:4973–4985. doi: 10.4049/jimmunol.1500651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Richter M, et al. Collagen distribution and expression of collagen-binding alpha1beta1 (VLA-1) and alpha2beta1 (VLA-2) integrins on CD4 and CD8 T cells during influenza infection. J. Immunol. 2007;178:4506–4516. doi: 10.4049/jimmunol.178.7.4506. [DOI] [PubMed] [Google Scholar]
- 160.Sojka DK, Tian Z, Yokoyama WM. Tissue-resident natural killer cells and their potential diversity. Semin. Immunol. 2014;26:127–131. doi: 10.1016/j.smim.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tayade C, et al. Differential transcription of Eomes and T-bet during maturation of mouse uterine natural killer cells. J. Leukoc. Biol. 2005;78:1347–1355. doi: 10.1189/jlb.0305142. [DOI] [PubMed] [Google Scholar]
- 162.Filipovic I, et al. Molecular definition of group 1 innate lymphoid cells in the mouse uterus. Nat. Commun. 2018;9:4492. doi: 10.1038/s41467-018-06918-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sojka DK, et al. Cutting edge: local proliferation of uterine tissue-resident NK cells during decidualization in mice. J. Immunol. 2018;201:2551–2556. doi: 10.4049/jimmunol.1800651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cortez VS, Fuchs A, Cella M, Gilfillan S, Colonna M. Cutting edge: salivary gland NK cells develop independently of Nfil3 in steady-state. J. Immunol. 2014;192:4487–4491. doi: 10.4049/jimmunol.1303469. [DOI] [PubMed] [Google Scholar]
- 165.Li T, et al. Respiratory influenza virus infection induces memory-like liver NK cells in mice. J. Immunol. 2017;198:1242–1252. doi: 10.4049/jimmunol.1502186. [DOI] [PubMed] [Google Scholar]
- 166.Ni X, et al. Cytokine-based generation of CD49a(+)Eomes(-/+) natural killer cell subsets. Front Immunol. 2018;9:2126. doi: 10.3389/fimmu.2018.02126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Marquardt N, et al. Cutting edge: identification and characterization of human intrahepatic CD49a+ NK cells. J. Immunol. 2015;194:2467–2471. doi: 10.4049/jimmunol.1402756. [DOI] [PubMed] [Google Scholar]
- 168.Tang L, et al. Differential phenotypic and functional properties of liver-resident NK cells and mucosal ILC1s. J. Autoimmun. 2016;67:29–35. doi: 10.1016/j.jaut.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 169.Robinette ML, et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat. Immunol. 2015;16:306–317. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cuff AO, et al. Eomeshi NK cells in human liver are long-lived and do not recirculate but can be replenished from the circulation. J. Immunol. 2016;197:4283–4291. doi: 10.4049/jimmunol.1601424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Male V. Liver-resident NK cells: the human factor. Trends Immunol. 2017;38:307–309. doi: 10.1016/j.it.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 172.Hydes T, et al. Natural killer cell maturation markers in the human liver and expansion of an NKG2C+KIR+ population. Lancet. 2015;385(Suppl 1):S45. doi: 10.1016/S0140-6736(15)60360-9. [DOI] [PubMed] [Google Scholar]
- 173.Aw Yeang HX, et al. Cutting edge: human CD49e- NK cells are tissue resident in the liver. J. Immunol. 2017;198:1417–1422. doi: 10.4049/jimmunol.1601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fu B, et al. Natural killer cells promote fetal development through the secretion of growth-promoting factors. Immunity. 2017;47:1100–1113 e1106. doi: 10.1016/j.immuni.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 175.Montaldo E, et al. Unique Eomes(+) NK cell subsets are present in uterus and decidua during early pregnancy. Front Immunol. 2015;6:646. doi: 10.3389/fimmu.2015.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rebuli ME, Pawlak EA, Walsh D, Martin EM, Jaspers I. Distinguishing human peripheral blood NK cells from CD56(dim)CD16(dim)CD69(+)CD103(+) resident nasal mucosal lavage fluid cells. Sci. Rep. 2018;8:3394. doi: 10.1038/s41598-018-21443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cooper GE, Ostridge K, Khakoo SI, Wilkinson TMA, Staples KJ. Human CD49a(+) lung natural killer cell cytotoxicity in response to influenza A virus. Front. Immunol. 2018;9:1671. doi: 10.3389/fimmu.2018.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 180.Dadi S, et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell. 2016;164:365–377. doi: 10.1016/j.cell.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 2016;13:267–276. doi: 10.1038/cmi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yamamoto Y, et al. Lung-resident natural killer cells control pulmonary tumor growth in mice. Cancer Sci. 2018;109:2670–2676. doi: 10.1111/cas.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stegmann KA, et al. CXCR6 marks a novel subset of T-bet(lo)Eomes(hi) natural killer cells residing in human liver. Sci. Rep. 2016;6:26157. doi: 10.1038/srep26157. [DOI] [PMC free article] [PubMed] [Google Scholar]