Abstract
Although lymphocytes are known to circulate throughout lymphoid tissues and blood, they also establish residency in nonlymphoid organs, most prominently in barrier tissues, such as the intestines. The adaptation of T lymphocytes to intestinal environments requires constant discrimination between natural stimulation from commensal flora and food and pathogens that need to be cleared. Genetic variations that cause a defective defense or a break in tolerance along with environmental cues, such as infection or imbalances in the gut microbiota known as dysbiosis, can trigger several immune disorders via the activation of T lymphocytes in the intestines. Elucidation of the immune mechanisms that distinguish between commensal flora and pathogenic organisms may reveal therapeutic targets for the prevention or modulation of inflammatory diseases and boost the efficacy of cancer immunotherapy. In this review, we discuss the development and adaptation of T lymphocytes in the intestine, how these cells protect the host against pathogenic infections while tolerating food antigens and commensal microbiota, and the potential implications of targeting these cells for disease management and therapeutics.
Keywords: Intestinal T cells, Pathogenic infection, Gut microbiota, T-cell therapy
Subject terms: Mucosal immunology, Cellular immunity
Development and maturation of intestinal T cells
Intestinal lymphocytes are continuously exposed to food and microbial antigens. These lymphocytes have evolved uniquely precise strategies to help maintain the integrity of the intestinal barrier and immune homeostasis. The intestinal epithelium separates the body from the outside environment as an impermeable barrier. The lymphocytes located at this barrier between enterocytes are referred to as intraepithelial lymphocytes (IELs). Due to this specific location, IELs directly contact enterocytes and are in immediate proximity to antigens in the gut lumen. Thus, IELs have a wide range of regulatory and effector capabilities, including the prevention of pathogenic invasion and maintenance of tolerance to prevent extensive tissue damage. IELs are almost exclusively T cells, and their numbers even exceed those in the spleen.1 According to their different pathways of development and maturation (Table 1), IELs can be divided into two major subsets. The “conventional” (or “type a”) intestinal T cells express TCRαβ along with CD4 or CD8αβ as coreceptors (Fig. 1). The other major subset, i.e., “nonconventional” (or “type b”) intestinal T cells, expresses either TCRαβ or TCRγδ and typically CD8αα homodimers (Fig. 1). In addition to IELs, intestinal T cells can also be found in the lamina propria (LP), which is the layer of connective tissue that lies beneath the epithelium. In contrast to IELs, T cells serving as lamina propria lymphocytes (LPLs) are derived from conventional T cells that undergo conventional thymic development, are primed in secondary lymphoid organs, and migrate to the LP with an effector memory phenotype.2
Table 1.
Two types of Intestinal T cells
| Conventional (type a) | Nonconventional (type b) | |
|---|---|---|
| Characteristic expression markers | CD4+ TCRαβ+ or CD8αβ+ TCRαβ+ | CD8αα+ or CD8αα− and CD4−CD8αβ−TCRαβ+ or TCRγδ+ |
| Locations | IELs and LPLs | Mostly in IELs |
| Functions | Defense against infections (cytotoxic T cells, Th1, Th2, and Th17), proinflammation (Th17); tolerance of commensal bacteria and food antigens (pTregs) | Immune regulation, defense against infections, maintenance of intestinal homeostasis, tolerance of intestinal antigens, enrichment for self-antigen specificity |
| Development and maturation | Develop in thymus, circulate and migrate into GALTs, activated by antigens to become effector memory cells, and home to the gut | Selected in thymus, guided directly to the gut |
IELs intraepithelial lymphocytes, LPLs lamina propria lymphocytes, GALTs gut-associated lymphoid tissues
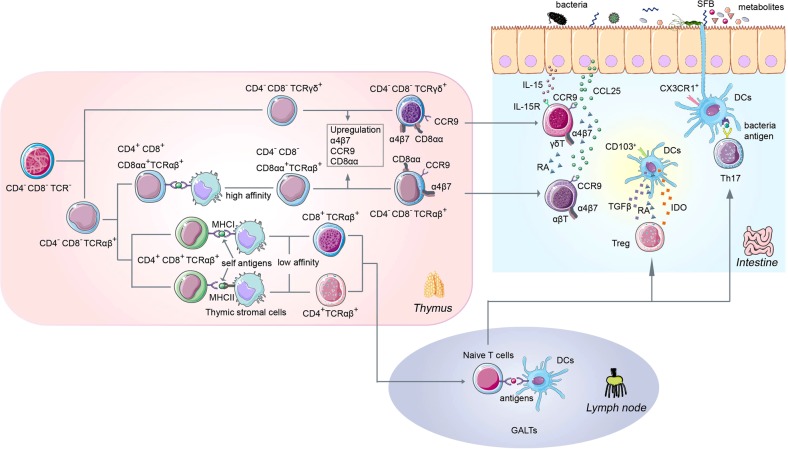
Fig. 1.
Development and maturation of intestinal T lymphocytes. Intestinal T cells can be classified as induced “conventional” (or “type a”) intestinal T cells or “nonconventional” (or “type b”) intestinal T cells. Conventional intestinal T cells express TCRαβ and CD4 or CD8αβ and serve as TCR coreceptors. Nonconventional intestinal T cells express either TCRαβ or TCRγδ and typically also express CD8αα homodimers. Conventional T cells are derived from CD4−CD8−(DN) progenitors in the thymus and develop into SP CD4+T (MHC I) cells or CD8+ T cells (MHC II). These cells subsequently migrate to peripheral lymphoid organs, such as the lymph nodes, where they encounter antigens and acquire an activated effector phenotype that drives their migration to the gut. Alternatively, immature triple-negative thymocytes (CD4−CD8−TCR−) in the thymus differentiate into double-negative (CD4−CD8−), TCRγδ-positive or TCRαβ-positive intestinal T-cell precursors. TCRαβ-positive T-cell precursors partially acquire their antigen-experienced phenotype during selection by self-antigens presented by thymic stromal cells. The upregulation of gut-homing-associated molecules, including the integrin α4β7, the chemokine receptor CCR9, and CD8αα homodimers, guide these TCRγδ-positive or TCRαβ-positive T-cell precursors to the intestine. For instance, T cells are attracted by the chemokine CCL25 (ligand of CCR9) secreted by the intestinal epithelial cells. In the gut, an environment abundant in microbial and food antigens presented by dendritic cells (DCs) can shape diverse functionally specialized T-cell populations with remarkable plasticity to trans-differentiate into T cells bearing other features, even with opposing functions. Factors secreted by epithelial or other intestinal cells, such as IL-15 and retinal acid (RA), promote the retention of T cells in the intestine
These intestinal T cells have different phenotypes and functions due to their origin in the thymus and the effects of the intestinal environment (Table 1). Thus, we discuss the pathways of the thymic development and maturation of intestinal T cells to clearly explain the roles of T lymphocytes in the intestinal mucosa.
Thymic development
Conventional T cells develop in the thymus from CD4−CD8− (double-negative, DN) progenitors (Fig. 1). The selection and lineage commitment of conventional T cells have been extensively reviewed elsewhere.3 In brief, following TCRβ expression, DN progenitors enter a CD4+ CD8+ double-positive (DP) stage. Strongly self-reactive DP cells are purged by major histocompatibility complex (MHC)-peptide engagement, whereas DP cells with a low affinity to the MHC-peptide are positively selected by MHC-I and MHC-II interactions and subsequently develop into SP CD4+T (MHC II) cells or CD8+ T cells (MHC I). In contrast to conventional T cells, which undergo positive selection in the thymus, some CD4 and CD8αβ double-negative progenitors express either TCRγδ or TCRαβ without positive selection in the thymus. Most of these cells express CD8αα homodimers and lack the conventional T-cell coreceptors CD4 and CD8αβ (Fig. 1).4
The difference between conventional T and unconventional T-cell development in the thymus can be attributed to an alternative process of selection for self-reactivity (Fig. 1). Among conventional T cells, the high affinity of the T-cell receptors (TCRs) to self-antigens and MHC could lead to clonal depletion.5 This process, which has been defined as negative selection, aims to induce self-tolerance.6 However, a small group of thymocytes with TCRs that have a high affinity to self-antigens are not eliminated and develop into unconventional T-cell lineages.7 CD4 and CD8 double-negative TCRαβ T cells, CD8αα TCRαβ T cells, and thymic regulatory T cells (tTregs) are considered unconventional T cells and develop via this alternative selection pathway. These cells usually display an antigen-experienced phenotype and frequently exert immune regulatory functions.
Maturation in the intestine
Most intestinal T cells mature in peripheral lymphoid organs. These cells gain the expression of intestinal homing receptors to migrate into the intestine.
After leaving the thymus, naive T cells migrate into gut-associated lymphoid tissues (GALTs) through the circulation. In GALTs, such as Peyer’s patches and mesenteric lymph nodes (MLNs),8 naive CD4+T and CD8αβ+ T cells are primed by antigen-presenting cells (APCs) and acquire the ability to migrate to intestinal tissues by upregulating gut-homing molecules, such as integrin α4β7, chemokine receptor CCR9, activation marker CD44, adhesion molecule LFA-1, and very late antigen-4 (VLA-4, also known as α4β1) (Fig. 1).9,10 Then, such T cells are attracted by chemokines to enter the intestine via interactions with parallel ligands secreted by intestinal cells. The chemokine receptors on the T cells determine their distinct locations in the intestine.11 For example, CCL2512 and CCL2813 are constitutively secreted by small intestinal epithelial and colonic cells, respectively. The T cells expressing the corresponding chemokine receptors, i.e., CCR9 (receptor for CCL25) or CCR10 (receptor for CCL28), become attracted to and migrate through the vascular endothelium to enter the intestine (Fig. 1). In humans and mice, almost all T cells in the small intestine express CCR9.14 While chemokines guide the migration of T cells, the integrins expressed on the T-cell surface, such as α4β7, interact with adhesion molecules expressed on endothelial and epithelial cells, such as mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1), to initiate attachment to and diffusion into the tissue.15 Interestingly, more than 90% of lymphocytes in the small intestine are α4β7-integrin positive, and a deficiency in α4β7 leads to the disruption of GALT formation.16 Furthermore, CD4+CD8+ DP T cells have been described in several species, including humans. These cells are proliferating activated memory cells that display a relatively high expression of CCR5; thus, these cells are the preferred targets for the treatment of simian immunodeficiency virus/human immunodeficiency virus (HIV) infection.17,18
Environmental factors might induce the adaptation of T cells to the microenvironment in the intestine through antigen presentation. Conventional CD4+ T cells differentiate into different subsets of T helper cells (Th1, Th2, Th17, and iTreg) by sensing specific environmental cues in the intestine. The APCs, such as dendritic cells (DCs) and intestinal epithelial cells (IECs), in the intestinal barrier can regulate T-cell differentiation in response to various triggers from the intestinal lumen.19,20 For example, short-chain fatty acids (SCFAs) reportedly stimulate CD103+ DCs within the epithelium to secrete TGF-β, retinoic acid, and indoleamine 2,3-dioxygenase (IDO) to induce pTreg differentiation (Fig. 1).21–27 While epithelium-adhering bacteria are sensed by CX3CR1+CD103−DCs, microbial antigens are presented to T cells to initiate the polarized differentiation of Th17, which could induce an immune response to protect the host.28,29 Retinoic acid, which is a vitamin A metabolite derived from the diet, is reportedly an important inducer of gut-homing receptors in intestinal T cells.30 Retinoic acid promotes the expression of α4β7 integrin and CCR9 on T-cell surfaces to promote the retention of these cells in the small intestine.31 Furthermore, the intestinal microbial and food antigens presented by DCs can shape diverse functional specialized T-cell populations, with remarkable plasticity to trans-differentiate into T cells bearing other features and even opposing functions, e.g., inflammatory Th17 cells can become regulatory Tr1 cells.32
The unique pattern of gut-homing molecules derived from the thymic development stage also helps these T cells adapt to the intestinal environment. For example, most nonconventional T cells in the thymus are CD8αα+ T cells.4 The CD8αα homodimers can bind classical or nonclassical MHC-I on epithelial cells. Due to its exclusion from the TCR activation complex, CD8αα acts as a TCR repressor that reduces the antigen sensitivity of the TCR and, thus, negatively regulates T-cell activation. As a result, these T cells are normally immunologically quiescent in intestinal locales. Both conventional and nonconventional intestinal T cells upregulate CD8αα in the periphery to assist in the adaptation to the microenvironment of the intestine.33–35
Although lumen-derived signals may not be involved in the generation and migration of unconventional intestinal T cells, these signals are required for their maintenance and differentiation in the gut.36 The trans-presentation of IL-15 by IECs reportedly induces the transcription factor T-bet and drives the development of CD8αα+ T cells.37–39 In addition, metabolites derived from the diet, such as aryl hydrocarbon receptor (AhR) ligands, regulate the maintenance of TCRγδ+ T cells in the gut epithelium.40
Both nonconventional intestinal T cells and conventional intestinal T cells provide immuno-defense against pathogens while maintaining immune tolerance to food and commensals.
Intestinal T cells mediate protective immune responses during infection
As the intestine is a dominant site for exposure to potential microbial pathogens, the recognition of foreign antigens allows the intestinal mucosa to rapidly generate a robust adaptive immune response. T lymphocytes can protect the host by clearing infected cells through the production of cytokines that strengthen the barrier function or recruitment of other immune-protective and immune regulatory cells. HIV infection causes the depletion and dysfunction of gut resident immune populations, such as CD4+ T cells and Th17 cells.41,42 As a result, up to 90% of HIV-infected patients develop infectious diarrhea during the progression to AIDS (acquired immunodeficiency syndrome). AIDS-associated gastrointestinal symptoms are driven by viral or bacterial infections, including infections by Cytomegalovirus, Escherichia coli, Salmonella, and Shigella,43 highlighting the critical role of intestinal T cells in the control of and protection against pathogenic infections (Fig. 2).
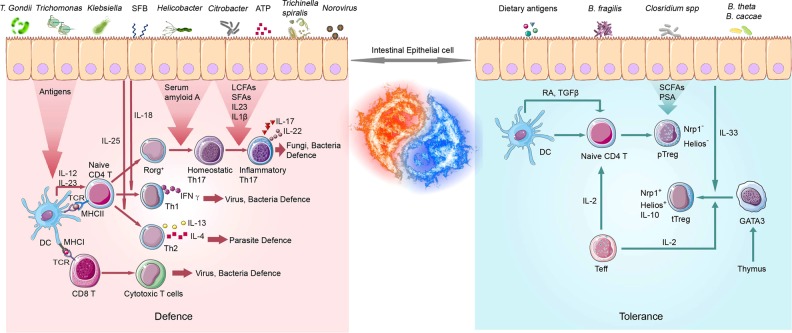
Fig. 2.
Regulation of T lymphocytes by microbial signals in the intestine. Due to frequent exposure to large numbers of foreign antigens, a robust but regulated immune response is key to maintaining intestinal homeostasis. CD4-positive T helper cells express cytokines with diverse functions and are central responders to microbial signals by either initiating the inflammatory response to fight against pathogens or shaping an environment tolerable of commensals and food antigens. CD8+ T cells are activated by DC-presented antigens and kill infected or damaged cells. RAR-related orphan receptor (ROR)γt-positive Th17 cells, which account for 30–40% of differentiated memory CD4+ T cells in the lamina propria (LP), are key effector T cells. Under homeostatic conditions, Th17 cells produce cytokines, such as IL-17A, IL-17F, and IL-22, which protect the host from bacterial invasion by stimulating the production of antimicrobial proteins and contribution to the formation of tight junctions between intestinal epithelial cells. However, exposure to dietary antigens or the overgrowth of inflammatory bacteria initiates the differentiation of naive CD4+ T cells into pathogenic effector T cells. In cases of dysbiosis, Th17 cells become pathogenic and produce IFN-γ and GM-CSF when stimulated by IL-23 and IL-1β, thus promoting inflammatory or autoimmune diseases. Another important T-cell population, i.e., regulatory T cells (Tregs), exists in the intestine to maintain homeostasis by balancing inflammatory effects or inducing the tolerant response to antigens from commensals or food. One subset of Helios and receptor neuropilin 1 (Nrp1)-positive Tregs are thymus-derived (tTregs). Another subset of Helios and Nrp1-negative Tregs are peripherally differentiated Treg (pTregs). Colonic pTregs induced by antigens and metabolites are produced by commensal bacteria, whereas pTregs in the small intestine are mainly maintained by food antigens. GM-CSF granulocyte-macrophage colony-stimulating factor
Viral infection
Several viruses, including rotavirus, norovirus, coxsackievirus, enterovirus 71, hepatitis A virus, and hepatitis E virus, spread through the fecal−oral route. Norovirus, which is the most common cause of foodborne illness worldwide, infects both children and adults and causes 685 million cases of acute gastroenteritis (inflammation of the stomach or intestines) annually. Rotavirus is most severe in infants and young children. Most children in the United States have had at least one bout of rotavirus infection by the age of 5 years. Adults infected with rotavirus may not have symptoms but can still spread the illness.
IELs serve as the first line of defense against viral infection via the cytolysis of dysregulated IECs and cytokine-mediated re-growth of healthy IECs. Almost three decades ago, IELs were reported to proliferate during oral infection by reovirus and rotavirus.44 Unsurprisingly, neonates are more sensitive to enteroviral (coxsackievirus, rotavirus, and norovirus) infections, whereas disease in adults is much milder,45 which could be at least partially explained by the relative immaturity of the immune compartment of the neonatal gut as the number of IELs is low at birth and during weaning but increases substantially thereafter.46,47 The activation of IELs in vivo rapidly promotes the type I/III interferon (IFN) receptor-dependent upregulation of IFN-responsive genes in the villus epithelium. In turn, activated IEL mediators protect cells against viral infection in vitro. In addition, the preactivation of IELs in vivo profoundly inhibits norovirus infection.48 Specifically, the transfer of mouse norovirus (MNV)-specific CD8 T cells to persistently infected Rag1−/− mice, which do not produce mature T cells or B cells, is capable of reducing the viral load.49 Furthermore, the immunization of mouse strains lacking one or more lymphocyte populations shows that CD4+ T cells are effectors of the protection against rotavirus as observed after intranasal immunization in mice with chimeric VP6 protein and adjuvant LT(R192G).50 However, viruses have evolved to evade adaptive immunity. Tomov et al. revealed a strategy of immune evasion by MNV via the induction of a CD8+ T-cell program normally reserved for latent pathogens and persistence in an immune-privileged enteric niche.51
Bacterial infection
Intestinal T cells protect the host from bacterial invasion either by direct killing or the production of numerous cytokines.52 Enteropathogenic E. coli (EPEC) and enterohemorrhagic E. coli (EHEC) are two clinically important human gastrointestinal pathogens. Citrobacter rodentium (C. rod), which infects mice and shares several pathogenic mechanisms with EPEC and EHEC, has become the principal rodent model in studies investigating infections by those enteropathogens. In cases of extracellular C. rod infection, the cytokines secreted by Th17 cells, such as IL17A, IL17F, and IL22, exert protective effects.53,54 The IL-22 receptor is highly expressed on nonhematopoietic (mainly stromal and epithelial) intestinal cells and promotes the elaboration of antimicrobial peptides, including RegIIIb, RegIIIc, and mucins, via the activation of STAT3.55 Oral infection by the intracellular bacterial pathogen Listeria monocytogenes (Lm) induces a robust intestinal CD8+T-cell response. Studies blocking effector T-cell migration have revealed that intestinal tissue-resident memory T (Trm) cells are critical for secondary protection.56 Infection by Lm also induces a robust endogenous listeriolysin O (LLO)-specific CD4+ T-cell response with distinct intestinal phenotypic and functional characteristics. The depletion of CD4+ T cells in immunized mice leads to the persistence of an elevated bacterial burden after a challenge infection, highlighting the critical role of memory CD4+ T cells in the control of intestinal intracellular pathogens.57
In contrast, commensal bacteria can induce an adaptive immune response that protects against the invasion of pathogens. For example, intestinal segmented filamentous bacterial (SFB) colonization induces a response by IL-17-producing RORγt+ helper T (Th17) cells, thus protecting mice against infection by the enteric rodent pathogen.58 Similarly, a microbiota community comprising three strains of Lactobacillus, four strains of S24-7, two strains of Bacteroides, one strain of Clostridia, and a Prevotella species was found to promote IFN-γ production and suppress Salmonella enterica serovar tissue invasion and disease.59
T-lymphocyte-mediated tolerance to food and commensals
Given the large number of commensal microorganisms in existence and the considerable amount of foreign protein antigen uptake in the daily diet, unnecessary reactions to harmless environmental antigens must be suppressed; otherwise, excessive intestinal inflammation and tissue damage could ensue. Tolerance is mainly mediated by forkhead box P3 (FOXP3)-expressing CD4+ Treg cells. FOXP3+ Treg cells include the following two developmentally different subsets: thymus-derived Helios and receptor neuropilin 1 (Nrp1)-positive Treg (tTreg) cells and peripherally derived Helios and Nrp1-negative Treg (pTreg) cells that develop as conventionally naive CD4+ T cells in the thymus but become FOXP3-expressing cells in peripheral tissues.60 More than 30% of CD4+ T cells in the colonic LP and approximately 20% of cells in the small intestinal LP are FOXP3+ Treg cells. These values are much higher than those of the average FOXP3+ proportion throughout the body, which is approximately 10% of the total CD4+ T-cell population in peripheral lymphoid tissue.61 The numbers of Treg cells in the colonic LP are reduced in germ-free mice, indicating that the accumulation and functional maturation of colonic pTreg cells are affected by the intestinal flora. However, compared with wild-type mice, the numbers of pTreg cells in the small intestines remain either unchanged or even increase in germ-free mice, indicating that a microbiota-independent induction of pTregs occurs in the small intestine.62 Kim et al. showed that the number of small intestinal, but not colonic, pTreg cells is severely reduced in germ-free mice fed an antigen-free diet.62 These findings suggest that a substantial part of the Treg cell population in the small intestines, but not in the colon, is induced by dietary antigens.
Induction of Treg cells by commensal bacteria
Antigen-specific pTreg cells in the gut LP further proliferate after the generation of food-specific (or commensal antigen-specific) Foxp3+ Treg cells in MLNs, indicating that the gut microbiota affects the number, function, and TCR repertoire of colonic Treg cells (Fig. 2).63,64 Colonization with the Clostridia and Bacteroides species, which are two prominent members of the mammalian gut microbiota, leads to the induction and maintenance of colonic Treg cells. Honda and colleagues reported that the oral administration of a mixture of 46 strains of conventional mice-derived Clostridia in germ-free mice leads to the strong induction of colonic Treg cells.65 Similarly, 17 strains of Clostridia isolated from a healthy adult Japanese volunteer have a strong capability to induce Treg cells in the colons of mice and rats.66 Colonization with Clostridium ramosum (cluster XVIII) has also been shown to induce an increase in the number of RORγt+ Treg cells. Monocolonization with Bacteroides fragilis boosts IL-10 production by colonic Treg cells, and this activity is mediated by bacterial polysaccharide A (PSA).64 Monocolonization with Bacteroides thetaiotaomicron produces SCFAs that induce the accumulation of FOXP3+ cells, particularly NRP1−RORγt+ p Treg cells, in the mouse colon.21,22 In mice, a treatment regimen involving VSL#3 (a mixture of eight strains of Bifidobacterium, Lactobacillus, and Streptococcus species) and Lactobacillus reuteri or Lactobacillus murinus was found to increase the percentage of Treg cells in the intestines.67 As this list continues to expand, the development of novel treatments to control autoimmune disease based on a combination of probiotic bacteria could be possible.
Induction of Treg cells by food antigens
The development of intestinal Treg cells is also affected by antigens and metabolites derived from the diet (Fig. 2). The antigen specificity of Treg cells has been shown to be important for food tolerance in humans. After their generation in lymph nodes, pTreg cells need to be homed to the gut, where they undergo local expansion and establish oral tolerance.63 Potentially immunogenic proteins are first subjected to denaturation and degradation by digestion in the gut. The proteins and peptides that survive denaturation and digestion pass through the epithelial barrier and are sampled by the luminal processes of CX3CR1+ cells. IECs may also directly present antigens to T cells by expressing MHC class II on basolateral surfaces.68 Retinoic acid metabolized from dietary vitamin A by DCs in the LP plays a crucial role in the differentiation and accumulation of Treg cells.69,70 Tryptophan, which is an essential amino acid present in various foods, is metabolized to kynurenine by IDO activity in IECs and DCs and contributes to Treg cell development via the aryl hydrocarbon receptor.64 Despite research progress, the mechanism by which food antigens induce tolerant Treg cells is still poorly understood.
Overreactions to food or substances in food disrupt tolerance, leading to food allergies. It is estimated that 4−8% of people in developed countries have at least one type of food allergy, and no effective treatment is currently available.71 Celiac disease is a serious autoimmune disorder that occurs in genetically predisposed people in whom the ingestion of gluten leads to damage to the small intestine.72 Continuous progress in the understanding of the correlation between Treg-cell-mediated tolerance and food allergy could lead to better treatments for these diseases.
Implications for diseases and therapeutics
The interactions between intestinal T cells and the gut environment are crucial for maintaining dynamic immune homeostasis. By secreting soluble factors or directly interacting with other cells in the intestine, T cells help maintain the integrity of the epithelial layer, clear infected cells, help B cells produce IgA, and even produce various cytokines to create an inflammatory or tolerant environment in response to pathogens or commensals. The dysregulation of T-cell-mediated processes, including the abnormal development and differentiation of T cells, caused by genetic polymorphisms or mutations and inappropriate recognition or response to antigens derived from pathogens, commensals, and food leads to a disruption in homeostasis and eventually causes various diseases, including intestinal inflammation, systemic autoimmune diseases, and even cancer.
Intestinal inflammation
Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, represents chronic relapsing disorders affecting the gastrointestinal tract.73,74 Dysregulated T cells are considered major pathogenic cells.
An enhanced T-cell-mediated response or a break in tolerance can cause uncontrolled inflammation and consequent tissue damage in the intestine. International collaborative genetic studies have identified dozens of genes enriched in the adaptive immune pathway that contribute to IBD susceptibility, highlighting the critical role of T cells in the inflammatory response.75,76 The overlap in the susceptibility loci of IBD and mycobacteria infection, including IL12B, IRF8, IFNGR1, IFNGR2, STAT1, TYK2, and STAT3, highlight the proinflammatory role of pathogenic bacteria. Consistently, numerous bacteria reportedly promote the inflammatory response under specific conditions. In the context of a dextran sulfate sodium (DSS)-induced IBD mouse model, Proteus mirabilis was found to promote intestinal inflammation by inducing IL-1β production via NLRP3 inflammasome activation in recruited inflammatory monocytes.77 It is well established that IL-1β can induce the polarization of Th17 cells. Similarly, SFB colonize the terminal ileum in mice and induce effector T cells, particularly Th17 cells.58 In these cases, the elevated T-cell inflammatory activation by bacteria contributes to the pathogenesis of IBD. Interestingly, helminth infections inhibit the colonization of inflammatory Bacteroides species by promoting the establishment of a protective microbiota enriched in Clostridiales via type 2 immunity. Consequently, helminth infections could be protective against the development of Crohn’s disease in a Nod2-deficient mouse model.78
However, in the DSS-induced mouse colitis model system, γδ T cells help preserve the integrity of damaged epithelial surfaces by providing the localized delivery of an epithelial cell growth factor. Thus, γδ T cells play a protective role in colitis by enhancing the intestinal defense and maintaining intestinal homeostasis.
Deficiencies in the number and functions of Treg cells and elevated Th1- and Th17-associated cytokines have been observed in both IBD patients and mouse models.79–81 A spontaneous gene-targeted model, i.e., IL-10-deficient mice, exhibited spontaneous pancolitis and cecal inflammation by 8–16 weeks of age.82 Another well-characterized mouse model of chronic colitis is also induced by the disruption of T-cell homeostasis. After transferring CD45RBhi T cells (naive T cells without Tregs) into immunodeficient mice lacking T and B cells, pancolitis and small bowel inflammation were observed at 5–8 weeks.83
Recently, in humans, antibodies to several novel targets, including distinct Th cell-associated cytokines, have been considered effective treatment for IBD. The IL-12-mediated mucosal Th1 response, which includes the secretion of IFNγ, tumor necrosis factor (TNF), and IL-6, contributes to the pathogenesis of Crohn’s disease.84,85 Th17 cells have been shown to play a role in the development of both Crohn’s disease and ulcerative colitis.86,87 Furthermore, IL-12 (p35-p40) is a key cytokine involved in the differentiation of Th1 cells, whereas IL-23 (p19-p40) is an activator of Th17 cells. Moreover, IL-12 (p35-p40) and IL-23 (p19-p40) share the same subunit, i.e., p40. These findings provide a rational basis for targeting Th1 and Th17 cytokines for the treatment of IBD. Recently, various antibodies targeting IL-12/IL-23 p40 and IL-23 p19 have been developed for preclinical and clinical studies. The blockade of IL-23 p19 and p40 could effectively suppress intestinal inflammation in mouse models of colitis,88–90 further proving the pathogenic role of Th17 cells in intestinal inflammation. Therapies targeting T-cell cytokines might serve as promising clinical therapeutic options for patients with IBD.
Several cytokine blockers still fail to effectively treat IBD. Treatment with anti-IFNγ, which is also known as fontolizumab, shows low efficacy in patients with active Crohn’s disease.91 Secukinumab, which is a human anti-IL17A monoclonal antibody, represents another example that aggravates Crohn’s disease in many patients.92 The role of IL-17A in IBD is very controversial. The treatment failure with anti-IL17A in IBD may be due to the protective effects of IL17A on gut epithelial cells,93,94 which is consistent with findings showing that IL17A inactivation does not ameliorate experimental colitis in mice.80,95 In addition to IL-17A, Th17 cells produce many other potential proinflammatory cytokines, such as IL-17F, IL-22, TNF, and granulocyte-macrophage colony-stimulating factor (GM-CSF). Neutralizing IL-17A has no significant therapeutic effects on Th17-mediated diseases, indicating that additional factors secreted by Th17 may play pathogenic roles. Similarly, the blockade of IL-17F, IL-22, and TNF has different effects depending on the disease model. GM-CSF produced by Th17 cells is reportedly essential for the ability of these cells to drive inflammation in an experimental autoimmune encephalomyelitis (EAE) model.96,97 Considering the role of Th17 cells and the secreted factors involved in intestinal inflammation, finding the precise pathogenic factor secreted by Th17 cells could lead to more specific targeting strategies by reducing the side effects that eliminate Th17 cells.
Celiac disease is another serious inflammatory disorder that occurs in genetically predisposed people in whom the ingestion of gluten leads to damage to the small intestine.72 Immune tolerance mediated by T helper cells is critical for the prevention of celiac disease. Genetically susceptible people who express human leukocyte antigen (HLA) DQ2 or DQ8 molecules display an inflammatory T helper 1 (TH1) immune response against dietary gluten present in wheat.98,99 Genetically modified mouse models with an altered T-cell response have been used to study celiac disease. In one transgenic mouse model, endogenous MHC class II genes are replaced with the disease-susceptible HLA class II alleles DQ2 or DQ8, leading to an abnormal antigen presentation to T cells.100,101 In addition, transgenic mice overexpressing interleukin-15 (IL-15), resulting in an accumulation of IELs in the intestine, have also been used to generate a model of chronic inflammation.102 Furthermore, environmental factors, such as intestinal microbes, also contribute to the pathogenesis of celiac disease. Reovirus infection can suppress peripheral regulatory T-cell (pTreg) conversion and promote Th1 immunity to the dietary antigen gluten, eliciting the pathological processes of celiac disease.103
Enteropathy-associated T-cell lymphoma is a severe complication of celiac disease.104 Enterocytes in patients with celiac disease reportedly overexpress IL-15, which transmits potent antiapoptotic signals to intraepithelial T cells,105 leading to the accumulation of these cells.106,107 Malamut et al. reported that IL-15 initiates the survival pathway in human intraepithelial T cells.36 Thus, the use of an anti-IL-15 antibody was considered an effective way to treat celiac disease. Interestingly, a fully humanized IL-15-specific antibody effectively blocks the phosphorylation of Jak3 and STAT5, both of which are downstream of the survival pathway, which, in turn, induces apoptosis in intraepithelial T cells and eliminates their massive accumulation.36 These findings prove that targeting IL-15 and its downstream effectors is promising for the treatment of celiac disease.
Systemic autoimmune diseases
In addition to intestinal inflammation, the abnormal activation of T cells in the intestine can also lead to autoimmune diseases that occur outside of the gut, such as primary sclerosing cholangitis (PSC), multiple sclerosis (MS), autoimmune arthritis, type 1 diabetes, and graft-versus-host disease (GVHD).
For example, PSC is an autoimmune liver disease that often presents with IBD. Nearly 20% of hepatic infiltrates are α4β7-integrin+CCR9+T cells that are recruited from the gut by the aberrant expression of the chemokine CCL25 in PSC.108,109 As these mucosal T cells are recruited to the liver by the aberrant expression of the gut-specific chemokine CCL25, which binds CCR9 expressed on T cells and activates α4/β7 binding to MAdCAM-1 in the hepatic endothelium, targeting this axis could have therapeutic benefit in PSC.110 Vedolizumab, which is a monoclonal antibody against α4/β7, has shown a beneficial effect in ulcerative colitis and may have therapeutic benefit in patients with PSC.111
An additional source of liver T cells is evident in GVHD in which donor T cells mediate an immune attack against host tissues. Recipients of donor T cells who are deficient in α4β7 show significantly alleviated disease progression in the intestine and liver.112,113 Thus, T cells normally restricted to the gut can be recruited to the liver in response to aberrantly expressed endothelial cell adhesion molecules and chemokines, altering the hepatic immune balance and leading to chronic inflammatory liver disease.
Multiple sclerosis is a chronic inflammatory disease characterized by the infiltration of lymphocytes into neuronal tissue and demyelination due to immune attack. As a widely used animal model of MS, EAE closely mimics the clinical symptoms of MS and can be induced by active immunization or autoreactive T cell transfer. Mice maintained under germ-free conditions or treated with antibiotics develop significantly attenuated EAE. Such findings reveal that the gut microbiota is involved in the progression of MS. Intestinal SFB colonization induces IL-17A-producing Th17 cells, thus promoting neuroinflammation.114 Dietary metabolites also affect EAE pathogenesis by shaping distinct T helper cell responses. For example, long-chain fatty acids (LCFAs) promote the differentiation and proliferation of Th1 and/or Th17 cells, which worsen EAE. However, SCFAs support Treg cell differentiation. Treatment with SCFAs could significantly ameliorate EAE and reduce axonal damage via long-lasting imprinting on lamina-propria-derived Treg cells. Therefore, this option might be a promising treatment for neuronal inflammation.23
Type 1 diabetes is a chronic metabolic disease mediated by inflammation. A dysregulated gut microbiota could promote this autoimmune disease through various effects on pathogenic and regulatory T lymphocytes. In addition, studies have suggested that the aberrant migration of intestinal T cells into islets contributes to the pathogenesis of type 1 diabetes. In both animal models of and human patients with type I diabetes, α4β7 integrin-expressing T cells have been found to infiltrate islets. Moreover, treatment with antibodies targeting α4β7 integrin or the endothelial marker MADCAM1 significantly alleviates the development of type 1 diabetes in non-obese diabetic (NOD) mice.115–117 This finding suggests that autoreactive T cells in islets may be induced and migrate from the gut. However, another study provided evidence that induced T cells in the intestines also have the ability to prevent the development of type 1 diabetes. Gut-induced type 1 regulatory T (Tr1) cells migrate to islets and control disease progression by secreting the immunosuppressive cytokine IL-10.118 Moreover, studies investigating microbial metabolites have shown that acetate and butyrate provide protection against diabetes. Specifically, acetate significantly reduces the frequency of autoreactive T cells in NOD mice by limiting the ability of B cells to expand populations of autoreactive T cells. Butyrate enhances the number and function of regulatory T cells, contributing to the protection against inflammation. Consequently, these metabolites control inflammation in the pancreas. Thus, dietary metabolites could serve as promising nonpharmaceutical approaches for the treatment or prevention of type 1 diabetes.119 A study conducted by Hebbandi et al. demonstrated that a close relationship exists between diabetogenic T cells and the intestinal microenvironment.120 These authors observed that diabetogenic IGRP206–214-reactive CD8+ T cells could cross-react with gut microbial antigens encoded by an integrase expressed by Bacteroides, thereby leading to the accumulation of these cells in the intestine to protect against colitis.120
Cancer and cancer immunotherapy
T cells play a fundamental role in cancer initiation, progression, and immune-therapies. An over-activated T-cell-mediated inflammatory response promotes cancer progression, especially on the mucosal surface. Various Th-cell-mediated immune responses have been implicated in the pathogenesis of colitis-associated cancer. Bacteroides fragilis, which is an enterotoxigenic commensal bacterium found in the human colon, promotes colonic tumorigenesis via the activation of Th17 T-cell responses.121 Tregs have been demonstrated to exert anti-inflammatory effects and protect against colitis-associated cancer depending on the gastrointestinal flora and levels of IL-10 secretion.122 These findings suggest that strategies targeting distinct mucosal Th cells in vivo could be used as a therapeutic approach in colon cancer.
Nevertheless, exhausted T cells fail to clear cancer cells. Cancer immunotherapy, which activates the immune system of the host to destroy cancer cells, can provide long-term benefits or even a cure. The most successful strategies affecting T cells to date include the use of antibodies against immune-checkpoint molecules, such as cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD1). Other strategies, including cancer vaccines and oncolytic viruses, are under active development and have great potential to enhance immunotherapy efficiency. In line with its capability to modify the immune response of the host, gut microbiota is crucial for the efficacy of checkpoint blockade immunotherapy. Patients undergoing antibiotic treatment respond poorly to anti-PD1 treatment.123 Different research groups have found that various bacterial species, such as Akkermansia, are enriched in antibiotic-free organisms.123,124 Another study has shown that Akkermansia can significantly promote the response rate of anti-PD1 therapy likely through a T-cell-mediated response.125 Tumors in germ-free mice receiving fecal microbiota transplantation (FMT) from responsive patients display a higher density of CD8+ T cells than tumors in mice receiving nonresponder FMT.124 An understanding of the precise role and underlying mechanism by which environmental cues or intestinal T cells promote the efficacy of tumor immunotherapy could lead to better treatment or eventually a cure for cancer.
Summary
One of the most abundant immune cell populations in the intestine comprise T cells. These cells develop and mature either in the thymus or the intestine and are stimulated by antigens in GALTs, MLNs, and the LP. Both thymic and peripherally induced intestinal T cells play a role in sustaining the barrier function and maintaining intestinal homeostasis via interactions with microbes and metabolites in the lumen along with other cell types, such as epithelial cells and APCs, in the intestinal microenvironment. As inflammatory mediators, intestinal T cells initiate protective immune responses against various pathogens, such as viruses, bacteria, and parasites. However, these cells are trained to tolerate food antigens and commensals and even work as regulators preventing uncontrolled inflammation and tissue damage. Under normal circumstances, the balance between T-cell-mediated defense and tolerance is key to maintain intestinal homeostasis. These T cells are dynamic and flexible, are capable of adapting to instantaneous environmental changes in the intestine, and are constantly contacting with microbes, metabolites, infiltrating cells, and cytokines. Genetic susceptibility causing abnormal T-cell-mediated immune responses and inflammatory triggers in the intestine or lumen likely to initiate uncontrolled inflammation and a break in tolerance can, in turn, promote and exacerbate detrimental diseases. Thus, an understanding of the development of intestinal T cells and their adaptation to tissues and intercellular communication with microbes and metabolites in the intestine may shed light for the identification of effective interventions for intestinal inflammation or even cancer immunotherapy.
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2018YFA0508000) (S.Z.); the National Natural Science Foundation of China (81822021, 91842105, 31770990, 81788101 and 81821001) (S.Z.), and (81871284) (H.M.); and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB29030101) (S.Z.).
Competing interests
The authors declare no competing interests.
References
- 1.Darlington D, Rogers AW. Epithelial lymphocytes in the small intestine of the mouse. J. Anat. 1966;100:813–830. [PMC free article] [PubMed] [Google Scholar]
- 2.Zeitz M, et al. Phenotype and function of lamina propria T lymphocytes. Immunol. Res. 1991;10:199–206. doi: 10.1007/BF02919693. [DOI] [PubMed] [Google Scholar]
- 3.Hayday A, Gibbons D. Brokering the peace: the origin of intestinal T cells. Mucosal Immunol. 2008;1:172–174. doi: 10.1038/mi.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai L, Peng H. Generating CD8alphaalpha IELs from two sources of thymic precursors. Cell Mol. Immunol. 2018;15:640–641. doi: 10.1038/cmi.2017.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 6.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat. Rev. Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin TA, Hogquist KA, Jameson SC. The fourth way? Harnessing aggressive tendencies in the thymus. J. Immunol. 2004;173:6515–6520. doi: 10.4049/jimmunol.173.11.6515. [DOI] [PubMed] [Google Scholar]
- 8.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agace WW. T-cell recruitment to the intestinal mucosa. Trends Immunol. 2008;29:514–522. doi: 10.1016/j.it.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iijima N, Iwasaki A. Tissue instruction for migration and retention of TRM cells. Trends Immunol. 2015;36:556–564. doi: 10.1016/j.it.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svensson M, et al. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J. Clin. Invest. 2002;110:1113–1121. doi: 10.1172/JCI0215988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hieshima K, et al. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J. Immunol. 2003;170:1452–1461. doi: 10.4049/jimmunol.170.3.1452. [DOI] [PubMed] [Google Scholar]
- 14.Kunkel EJ, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Mu L. An expanding stage for commensal microbes in host immune regulation. Cell Mol. Immunol. 2017;14:339–348. doi: 10.1038/cmi.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner N, et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 17.Pahar B, Lackner AA, Veazey RS. Intestinal double-positive CD4+ CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur. J. Immunol. 2006;36:583–592. doi: 10.1002/eji.200535520. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Das A, Lackner AA, Veazey RS, Pahar B. Intestinal double-positive CD4+ CD8+ T cells of neonatal rhesus macaques are proliferating, activated memory cells and primary targets for SIVMAC251 infection. Blood. 2008;112:4981–4990. doi: 10.1182/blood-2008-05-160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiberio L, et al. Chemokine and chemotactic signals in dendritic cell migration. Cell Mol. Immunol. 2018;15:346–352. doi: 10.1038/s41423-018-0005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikulic J, Longet S, Favre L, Benyacoub J, Corthesy B. Secretory IgA in complex with Lactobacillus rhamnosus potentiates mucosal dendritic cell-mediated Treg cell differentiation via TLR regulatory proteins, RALDH2 and secretion of IL-10 and TGF-beta. Cell Mol. Immunol. 2017;14:546–556. doi: 10.1038/cmi.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furusawa Y, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 22.Arpaia N, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haghikia A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015;43:817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Farache J, et al. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity. 2013;38:581–595. doi: 10.1016/j.immuni.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDole JR, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shan M, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welty NE, et al. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. J. Exp. Med. 2013;210:2011–2024. doi: 10.1084/jem.20130728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 30.Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 31.Iwata M, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Gagliani N, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheroutre H, Lambolez F. Doubting the TCR coreceptor function of CD8alphaalpha. Immunity. 2008;28:149–159. doi: 10.1016/j.immuni.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 2011;11:445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y, et al. Mucosal memory CD8(+) T cells are selected in the periphery by an MHC class I molecule. Nat. Immunol. 2011;12:1086–1095. doi: 10.1038/ni.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malamut G, et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J. Clin. Invest. 2010;120:2131–2143. doi: 10.1172/JCI41344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma LJ, Acero LF, Zal T, Schluns KS. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8alphaalpha IELs. J. Immunol. 2009;183:1044–1054. doi: 10.4049/jimmunol.0900420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klose CS, et al. The transcription factor T-bet is induced by IL-15 and thymic agonist selection and controls CD8alphaalpha(+) intraepithelial lymphocyte development. Immunity. 2014;41:230–243. doi: 10.1016/j.immuni.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Reis BS, Hoytema van Konijnenburg DP, Grivennikov SI, Mucida D. Transcription factor T-bet regulates intraepithelial lymphocyte functional maturation. Immunity. 2014;41:244–256. doi: 10.1016/j.immuni.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 41.Lu XF, et al. Preferential loss of gut-homing alpha 4 beta 7 CD4(+) T cells and their circulating functional subsets in acute HIV-1 infection. Cell. Mol. Immunol. 2016;13:776–784. doi: 10.1038/cmi.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhaijee F, Subramony C, Tang SJ, Pepper DJ. Human immunodeficiency virus-associated gastrointestinal disease: common endoscopic biopsy diagnoses. Pathol. Res. Int. 2011;2011:247923. doi: 10.4061/2011/247923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dharakul T, Rott L, Greenberg HB. Recovery from chronic rotavirus infection in mice with severe combined immunodeficiency—virus clearance mediated by adoptive transfer of immune Cd8+ lymphocytes-T. J. Virol. 1990;64:4375–4382. doi: 10.1128/jvi.64.9.4375-4382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 46.Kuo S, El Guindy A, Panwala CM, Hagan PM, Camerini V. Differential appearance of T cell subsets in the large and small intestine of neonatal mice. Pediatr. Res. 2001;49:543–551. doi: 10.1203/00006450-200104000-00017. [DOI] [PubMed] [Google Scholar]
- 47.Williams AM, et al. Effects of microflora on the neonatal development of gut mucosal T cells and myeloid cells in the mouse. Immunology. 2006;119:470–478. doi: 10.1111/j.1365-2567.2006.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swamy M, et al. Intestinal intraepithelial lymphocyte activation promotes innate antiviral resistance. Nat. Commun. 2015;6:7090. doi: 10.1038/ncomms8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomov VT, et al. Persistent enteric murine norovirus infection is associated with functionally suboptimal virus-specific CD8 T cell responses. J. Virol. 2013;87:7015–7031. doi: 10.1128/JVI.03389-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNeal MM, et al. CD4 T cells are the only lymphocytes needed to protect mice against rotavirus shedding after intranasal immunization with a chimeric VP6 protein and the adjuvant LT(R192G) J. Virol. 2002;76:560–568. doi: 10.1128/JVI.76.2.560-568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomov VT, et al. Differentiation and protective capacity of virus-specific CD8(+) T cells suggest murine norovirus persistence in an immune-privileged enteric niche. Immunity. 2017;47:723–738 e725. doi: 10.1016/j.immuni.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen HB, Chen ZW. The crucial roles of Th17-related cytokines/signal pathways in M. tuberculosis infection. Cell. Mol. Immunol. 2018;15:216–225. doi: 10.1038/cmi.2017.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishigame H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Zenewicz LA, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 56.Sheridan BS, et al. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity. 2014;40:747–757. doi: 10.1016/j.immuni.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romagnoli PA, et al. Differentiation of distinct long-lived memory CD4 T cells in intestinal tissues after oral Listeria monocytogenes infection. Mucosal Immunol. 2017;10:520–530. doi: 10.1038/mi.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thiemann S, et al. Enhancement of IFNgamma production by distinct commensals ameliorates salmonella-induced disease. Cell Host Microbe. 2017;21:682–694 e685. doi: 10.1016/j.chom.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Bilate AM, Lafaille JJ. Induced CD4(+)Foxp3(+) regulatory T cells in immune tolerance. Annu. Rev. Immunol. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 61.Boll G, Reimann J. Lamina propria T-cell subsets in the small and large-intestine of euthymic and athymic mice. Scand. J. Immunol. 1995;42:191–201. doi: 10.1111/j.1365-3083.1995.tb03645.x. [DOI] [PubMed] [Google Scholar]
- 62.Kim KS, et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016;351:858–863. doi: 10.1126/science.aac5560. [DOI] [PubMed] [Google Scholar]
- 63.Hadis U, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 64.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Atarashi K, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atarashi K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 67.Dolpady J, et al. Oral probiotic VSL#3 prevents autoimmune diabetes by modulating microbiota and promoting Indoleamine 2,3-dioxygenase-enriched tolerogenic intestinal environment. J. Diabetes Res. 2016;2016:7569431. doi: 10.1155/2016/7569431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buning J, et al. Multivesicular bodies in intestinal epithelial cells: responsible for MHC class II-restricted antigen processing and origin of exosomes. Immunology. 2008;125:510–521. doi: 10.1111/j.1365-2567.2008.02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 70.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014;133:291–307. doi: 10.1016/j.jaci.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 72.Meresse B, Ripoche J, Heyman M, Cerf-Bensussan N. Celiac disease: from oral tolerance to intestinal inflammation, autoimmunity and lymphomagenesis. Mucosal Immunol. 2009;2:8–23. doi: 10.1038/mi.2008.75. [DOI] [PubMed] [Google Scholar]
- 73.Podolsky DK. Inflammatory bowel disease (2) N. Engl. J. Med. 1991;325:1008–1016. doi: 10.1056/NEJM199110033251406. [DOI] [PubMed] [Google Scholar]
- 74.Neurath MF. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017;14:269–278. doi: 10.1038/nrgastro.2016.208. [DOI] [PubMed] [Google Scholar]
- 75.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu TC, Stappenbeck TS. Genetics and pathogenesis of inflammatory bowel disease. Annu. Rev. Pathol.: Mech. Dis., Vol. 11. 2016;11:127–148. doi: 10.1146/annurev-pathol-012615-044152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seo SU, et al. Distinct commensals induce interleukin-1beta via NLRP3 inflammasome in inflammatory monocytes to promote intestinal inflammation in response to injury. Immunity. 2015;42:744–755. doi: 10.1016/j.immuni.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramanan D, et al. Helminth infection promotes colonization resistance via type 2 immunity. Science. 2016;352:608–612. doi: 10.1126/science.aaf3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Powrie F, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 80.Leppkes M, et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 81.Wedebye Schmidt EG, et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm. Bowel Dis. 2013;19:1567–1576. doi: 10.1097/MIB.0b013e318286fa1c. [DOI] [PubMed] [Google Scholar]
- 82.Berg DJ, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J. Clin. Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ostanin DV, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G135–G146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fuss IJ, et al. Both IL-12p70 and IL-23 are synthesized during active Crohn’s disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal antibody. Inflamm. Bowel Dis. 2006;12:9–15. doi: 10.1097/01.MIB.0000194183.92671.b6. [DOI] [PubMed] [Google Scholar]
- 85.Monteleone G, et al. Interleukin 12 is expressed and actively released by Crohn’s disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169–1178. doi: 10.1016/S0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- 86.Neurath MF. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 87.Monteleone I, Pallone F, Monteleone G. Th17-related cytokines: new players in the control of chronic intestinal inflammation. BMC Med. 2011;9:122. doi: 10.1186/1741-7015-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neurath MF. IL-23: a master regulator in Crohn disease. Nat. Med. 2007;13:26–28. doi: 10.1038/nm0107-26. [DOI] [PubMed] [Google Scholar]
- 89.De Nitto D, Sarra M, Cupi ML, Pallone F, Monteleone G. Targeting IL-23 and Th17-cytokines in inflammatory bowel diseases. Curr. Pharm. Des. 2010;16:3656–3660. doi: 10.2174/138161210794079164. [DOI] [PubMed] [Google Scholar]
- 90.Yen D, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reinisch W, et al. A dose escalating, placebo controlled, double blind, single dose and multidose, safety and tolerability study of fontolizumab, a humanised anti-interferon gamma antibody, in patients with moderate to severe Crohn’s disease. Gut. 2006;55:1138–1144. doi: 10.1136/gut.2005.079434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hueber W, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee JS, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43:727–738. doi: 10.1016/j.immuni.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maxwell JR, et al. Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity. 2015;43:739–750. doi: 10.1016/j.immuni.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 95.Awasthi A, Kuchroo VK. IL-17A directly inhibits TH1 cells and thereby suppresses development of intestinal inflammation. Nat. Immunol. 2009;10:568–570. doi: 10.1038/ni0609-568. [DOI] [PubMed] [Google Scholar]
- 96.El-Behi M, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Codarri L, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 98.Tjon JM, van Bergen J, Koning F. Celiac disease: how complicated can it get? Immunogenetics. 2010;62:641–651. doi: 10.1007/s00251-010-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Keuning JJ, Pena AS, van Leeuwen A, van Hooff JP, va Rood JJ. HLA-DW3 associated with coeliac disease. Lancet. 1976;1:506–508. doi: 10.1016/S0140-6736(76)90294-4. [DOI] [PubMed] [Google Scholar]
- 100.Stepniak D, Koning F. Celiac disease—sandwiched between innate and adaptive immunity. Hum. Immunol. 2006;67:460–468. doi: 10.1016/j.humimm.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 101.Ju JM, Marietta EV, Murray JA. Generating transgenic mouse models for studying Celiac disease. Methods Mol. Biol. 2015;1326:23–33. doi: 10.1007/978-1-4939-2839-2_3. [DOI] [PubMed] [Google Scholar]
- 102.Yokoyama S, Takada K, Hirasawa M, Perera LP, Hiroi T. Transgenic mice that overexpress human IL-15 in enterocytes recapitulate both B and T cell-mediated pathologic manifestations of celiac disease. J. Clin. Immunol. 2011;31:1038–1044. doi: 10.1007/s10875-011-9586-7. [DOI] [PubMed] [Google Scholar]
- 103.Bouziat R, et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science. 2017;356:44–50. doi: 10.1126/science.aah5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cellier C, et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356:203–208. doi: 10.1016/S0140-6736(00)02481-8. [DOI] [PubMed] [Google Scholar]
- 105.Mention JJ, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730–745. doi: 10.1016/S0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 106.Di Sabatino A, et al. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut. 2006;55:469–477. doi: 10.1136/gut.2005.068684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maiuri L, et al. Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology. 2000;119:996–1006. doi: 10.1053/gast.2000.18149. [DOI] [PubMed] [Google Scholar]
- 108.Eksteen B, et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9 + gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J. Exp. Med. 2004;200:1511–1517. doi: 10.1084/jem.20041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seidel D, et al. CD8 T cells primed in the gut-associated lymphoid tissue induce immune-mediated cholangitis in mice. Hepatology. 2014;59:601–611. doi: 10.1002/hep.26702. [DOI] [PubMed] [Google Scholar]
- 110.Ali AH, Carey EJ, Lindor KD. Current research on the treatment of primary sclerosing cholangitis. Intractable Rare Dis. Res. 2015;4:1–6. doi: 10.5582/irdr.2014.01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feagan BG, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 112.Petrovic A, et al. LPAM (alpha 4 beta 7 integrin) is an important homing integrin on alloreactive T cells in the development of intestinal graft-versus-host disease. Blood. 2004;103:1542–1547. doi: 10.1182/blood-2003-03-0957. [DOI] [PubMed] [Google Scholar]
- 113.Murai M, et al. Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat. Immunol. 2003;4:154–160. doi: 10.1038/ni879. [DOI] [PubMed] [Google Scholar]
- 114.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang XD, et al. A predominant role of integrin alpha 4 in the spontaneous development of autoimmune diabetes in nonobese diabetic mice. Proc. Natl. Acad. Sci. USA. 1994;91:12604–12608. doi: 10.1073/pnas.91.26.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hanninen A, Salmi M, Simell O, Jalkanen S. Mucosa-associated (beta 7-integrinhigh) lymphocytes accumulate early in the pancreas of NOD mice and show aberrant recirculation behavior. Diabetes. 1996;45:1173–1180. doi: 10.2337/diab.45.9.1173. [DOI] [PubMed] [Google Scholar]
- 117.Yang XD, Sytwu HK, McDevitt HO, Michie SA. Involvement of beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the development of diabetes in obese diabetic mice. Diabetes. 1997;46:1542–1547. doi: 10.2337/diacare.46.10.1542. [DOI] [PubMed] [Google Scholar]
- 118.Yu H, et al. Intestinal type 1 regulatory T cells migrate to periphery to suppress diabetogenic T cells and prevent diabetes development. Proc. Natl. Acad. Sci. USA. 2017;114:10443–10448. doi: 10.1073/pnas.1705599114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marino E, et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 2017;18:552–562. doi: 10.1038/ni.3713. [DOI] [PubMed] [Google Scholar]
- 120.Hebbandi Nanjundappa R, et al. A gut microbial mimic that hijacks diabetogenic autoreactivity to suppress colitis. Cell. 2017;171:655–667 e617. doi: 10.1016/j.cell.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 121.Wu S, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Erdman SE, Poutahidis T. Roles for inflammation and regulatory T cells in colon cancer. Toxicol. Pathol. 2010;38:76–87. doi: 10.1177/0192623309354110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Routy B, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 124.Gopalakrishnan V, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zitvogel L, Ma Y, Raoult D, Kroemer G, Gajewski TF. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science. 2018;359:1366–1370. doi: 10.1126/science.aar6918. [DOI] [PubMed] [Google Scholar]