Short abstract
Objective
Irisin is a myokine that greatly affects energy expenditure and systemic metabolism. While thyroid hormone is likely associated with irisin, a direct relationship remains to be fully elucidated. This study aimed to investigate plasma irisin levels in Chinese patients with hypothyroidism.
Methods
A total of 155 subjects were divided into the hypothyroidism group or the control group. Fifty-seven patients in the hypothyroidism group received levothyroxine treatment. Baseline irisin levels were measured in the two groups and post-treatment levels were measured in the hypothyroidism group.
Results
Irisin levels were significantly lower in the hypothyroidism group than in the control group. In the hypothyroidism group, irisin levels were positively associated with free triiodothyronine and free thyroxine levels, and negatively associated with thyrotropin levels. In the hypothyroidism group, irisin levels were significantly increased after levothyroxine treatment. Multiple linear regression models showed that total cholesterol and free thyroxine levels were the only significant predictors of serum irisin levels.
Conclusions
Irisin levels are decreased in patients with hypothyroidism. Our results suggest that decreased irisin levels are directly associated with reduced thyroid hormone levels. These values may be restored after levothyroxine treatment in Chinese patients with hypothyroidism.
Keywords: Irisin, hypothyroidism, thyroid hormone, creatine kinase, myokine, levothyroxine
Introduction
Irisin is a specific myokine that is significantly increased after exercise training and may play a role in regulating whole-body energy expenditure.1,2 Researchers have proposed several physiological functions of irisin. Irisin can promote brown adipocyte recruitment in white fat by increasing energy expenditure.1–3 Irisin can also affect lipid metabolism4–5 and modulate glucose and glucose-related effects in several metabolic diseases.6–7 Hypothyroidism is a disorder of the endocrine system that results from low production of thyroid hormone from the thyroid gland. This leads to metabolic dysfunction because thyroid hormone is an essential regulator of glucose–lipid metabolism and energy homeostasis.8–10
Our previous study showed a relationship between expression of fibroblast growth factor 21 and metabolic disorders,11 which indicated that patients with hypothyroidism had insulin resistance and abnormal blood lipid levels.12,13 Lin et al.14 found that thyroid hormone could induce browning of white fat through thyroid hormone receptors (TRs), and this was accompanied by increased expression of uncoupling protein 1 (UCP1). Furthermore, several researchers have reported that irisin significantly affects metabolism by inducing browning of subcutaneous white adipocytes through increased UCP1 expression and subsequent stimulation of oxygen consumption and thermogenesis.1–3 Irisin and thyroid hormone are involved in adipogenesis of white adipose tissue and brown adipose tissue.15–17 While a few studies have investigated the relationship between circulating irisin and decreased thyroid hormone levels in patients with hypothyroidism, the results have been varied with no clear findings.18–22
Therefore, the present study aimed to investigate the relationship between irisin and thyroid hormone levels in Chinese patients with hypothyroidism.
Materials and methods
Subjects
Initially, 103 patients with primary overt hypothyroidism were recruited from the Department of Endocrinology at Beijing Chaoyang Hospital from January 2013 to December 2014. To diagnose primary hypothyroidism, blood tests were performed for each patient to assess their levels of free triiodothyronine (FT3), free thyroxine (FT4), and thyrotropin (TSH). Those patients with a preexisting cardiovascular disease, diabetes mellitus, renal disease, or other endocrine diseases were excluded from this study. After 26 patients were excluded from the study on the basis of these criteria, 77 patients were enrolled in the hypothyroidism group. The control group included 78 healthy subjects who were volunteers from the Physical Examination Center at Beijing Chaoyang Hospital. Subjects in the hypothyroidism and control groups were office workers or retirees, all of who experienced light manual labor. The study protocol was designed according to the Declaration of Helsinki guidelines and approved by the Medical Ethics Committee of Beijing Chaoyang Hospital. Before the study, written informed consent was obtained from all patients, and those individuals in the hypothyroidism group were informed about the potential effects associated with levothyroxine treatment.
Sample collection
General demographic information and physical data were collected from all patients. The patients were asked to only wear underwear for the height and weight measurements, which were measured to the nearest 0.5 cm and 0.1 kg, respectively. After fasting overnight, baseline and post-treatment blood samples were collected from a peripheral vein of each patient. The samples were subjected to routine analyses, which included measurement of irisin, total cholesterol (CHOL), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), creatine kinase (CK), fasting blood (plasma) glucose (FPG), FT3, FT4, and TSH.
The patients with hypothyroidism received levothyroxine treatment. After treatment, normal thyroid function was restored in 57 of the patients during the following 12 months. The measurements were repeated after the euthyroid state was achieved.
Data collection and laboratory tests
The height and weight of patients were measured by the same trained group. The body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared. In our previous study, serum irisin levels were accurately measured using a highly quantitative enzyme-linked immunosorbent assay (ELISA) kit from Phoenix Pharmaceuticals Science, Inc. (Burlingame, CA, USA). An automated ELISA reader was used to quantify the results (Varioskan Flash Spectral Scanning Multimode Reader; Thermo Fisher Scientific, Waltham, MA, USA). Irisin levels in the two measurements were consistent.23 Therefore, in the present study, serum irisin levels were measured using an ELISA kit. CHOL, HDL-C, LDL-C, TG, CK, and FPG levels were measured using the Dade-Behring Dimension RXL Autoanalyzer (Siemens Healthcare Diagnostics, Marburg, Germany). The reference intervals for CHOL, HDL-C, LDL-C, TG, CK, and FPG were 3.62 to 5.70 mmol/L, 1.03 to 1.55 mmol/L, 1.81 to 3.36 mmol/L, 0.56 to 2.26 mmol/L, 38 to 174 U/L, and 3.3 to 6.1 mmol/L, respectively. FT3, FT4, and TSH levels were measured by an electrochemiluminescence immunoassay technique using the Abbott Architect i2000 (Abbott Diagnostics, Abbott Park, IL, USA). The reference intervals for FT3, FT4, and TSH were 1.71 to 3.71 pg/mL, 0.70 to 1.48 ng/dL, and 0.35 to 4.94 µIU/mL, respectively.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences software package (Version 17.0, SPSS Inc., Chicago, IL, USA). Data are expressed as the mean ± standard deviation, except for TG and CK, which did not follow a normal distribution and are expressed as medians (upper and lower quartiles). Comparisons between the control group and the hypothyroidism group were carried out using the independent samples test or the Mann–Whitney U test. Associations between baseline levels of irisin and the other baseline parameters were examined using Pearson’s or Spearman’s rank correlation analyses and multiple linear regression analysis. Changes between baseline and post-treatment values were evaluated using the paired samples test or the two-related-samples tests. Different irisin levels among the control group and the hypothyroidism group at baseline and post-treatment were analyzed using analysis of variance. Multiple linear regression analysis was used to show the factors associated with changes in irisin levels in patients with hypothyroidism after levothyroxine treatment. All tests were two-tailed and P values < 0.05 were considered statistically significant.
Results
Clinical characteristics of the study participants
The characteristics and laboratory examination values of the participants are shown in Table 1. CHOL, HDL-C, LDL-C, TG, CK, and TSH levels in the control group were significantly lower than those in the hypothyroidism group (CHOL, LDL-C, TG, CK, and TSH, all P < 0.01; HDL-C, P < 0.05). FT3 and FT4 levels were significantly higher in the control group than in the hypothyroidism group (both P < 0.001). Irisin levels were significantly lower in the hypothyroidism group than in the control group (P = 0.011). There were no significant differences in the sex, age, height, weight, BMI, or FBG between the two groups.
Table 1.
General information and clinical characteristics of the subjects.
| Control group(n = 78) | Hypothyroidism group(n = 77) | P value | |
|---|---|---|---|
| Sex (M/F) | 12/66 | 14/63 | 0.641 |
| Age, years | 44.74 ± 13.92 | 41.97 ± 12.70 | 0.198 |
| Height, m | 1.63 ± 0.06 | 1.63 ± 0.07 | 0.925 |
| Weight, kg | 62.68 ± 10.06 | 65.41 ± 9.25 | 0.081 |
| BMI, kg/m2 | 23.76 ± 3.81 | 24.74 ± 3.20 | 0.085 |
| CHOL, mmol/L | 4.94 ± 0.98 | 6.32 ± 1.81 | <0.001 |
| HDL-C, mmol/L | 1.54 ± 0.36 | 1.68 ± 0.47 | 0.033 |
| LDL-C, mmol/L | 2.84 ± 0.72 | 3.58 ± 1.19 | <0.001 |
| TG, mmol/L | 1.18 (0.73–1.56) | 1.49 (0.97–2.29) | 0.006 |
| CK, U/L | 84.00 (68.75–100.00) | 261.00 (125.00–542.50) | <0.001 |
| FBG, mmol/L | 4.90 ± 0.70 | 4.90 ± 0.72 | 0.968 |
| FT3, pg/mL | 2.91 ± 0.42 | 1.73 ± 0.62 | <0.001 |
| FT4, ng/dL | 1.10 ± 0.11 | 0.47 ± 0.11 | <0.001 |
| TSH, uIU/mL | 2.37 ± 1.16 | 81.22 ± 22.96 | <0.001 |
| Irisin, ng/mL | 54.08 ± 25.79 | 44.09 ± 22.24 | 0.011 |
Data are expressed as mean ± standard deviation unless indicated otherwise. TG and CK are shown as the median (upper and lower quartiles). M: male; F: female; BMI: body mass index; CHOL: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TG: triglycerides; CK: creatine kinase; FBG: fasting blood glucose; FT3: free triiodothyronine; FT4: free thyroxine; TSH: thyrotropin.
Correlations between irisin and other variables
No correlations were found between irisin and other variables in the control group (Table 2). In the hypothyroidism group, plasma irisin levels were positively associated with FT3 (r = 0.239, P = 0.036) and FT4 levels (r = 0.456, P < 0.001), and negatively associated with CHOL (r = −0.429, P < 0.001), LDL-C (r = −0.383, P = 0.001), TG (r = −0.284, P = 0.012), and TSH levels (r = −0.408, P < 0.001). There was no detectable relationship between irisin and CK in either group. Multiple linear regression analysis was performed to determine the parameters that were independently associated with irisin levels in the hypothyroidism group. We found that CHOL and FT4 levels were the only significant predictors of serum irisin levels (CHOL: β = −0.311, P = 0.004; FT4: β = 0.352, P = 0.001).
Table 2.
Correlations between irisin levels and patients’ characteristics, blood lipids, and thyroid function indices in the control group and hypothyroidism group.
| Parameters | Control group (n = 78) |
Hypothyroidism group (n = 77)Multiple regression |
||||
|---|---|---|---|---|---|---|
| r | P value | r | P value | β | P value | |
| Age, years | 0.165 | 0.150 | 0.208 | 0.069 | ||
| BMI, kg/m2 | 0.019 | 0.867 | −0.129 | 0.264 | ||
| CHOL, mmol/L | −0.003 | 0.978 | −0.429 | <0.001 | −0.311 | 0.004 |
| HDL-C, mmol/L | −0.009 | 0.938 | −0.196 | 0.088 | ||
| LDL-C, mmol/L | 0.001 | 0.988 | −0.383 | 0.001 | ||
| TG, mmol/L | 0.043 | 0.709 | −0.284 | 0.012 | ||
| CK, U/L | −0.100 | 0.385 | 0.037 | 0.748 | ||
| FBG, mmol/L | −0.182 | 0.110 | −0.136 | 0.238 | ||
| FT3, pg/mL | 0.087 | 0.447 | 0.239 | 0.036 | ||
| FT4, ng/dL | −0.122 | 0.286 | 0.456 | <0.001 | 0.352 | 0.001 |
| TSH, µIU/mL | 0.054 | 0.636 | −0.408 | <0.001 | ||
Pearson rank correlation was used to assess the correlations between irisin levels and the patients’ characteristics, blood lipid levels, and thyroid function indices in the two groups. TG and CK levels were analyzed by Spearman’s rank correlation to assess their correlation with irisin levels. The parameters were included in multiple linear regression analysis. M: male; F: female; BMI: body mass index; CHOL: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TG: triglycerides; CK: creatine kinase; FBG: fasting blood glucose; FT3: free triiodothyronine; FT4: free thyroxine; TSH: thyrotropin.
Changes in metabolic parameters and irisin levels after levothyroxine treatment
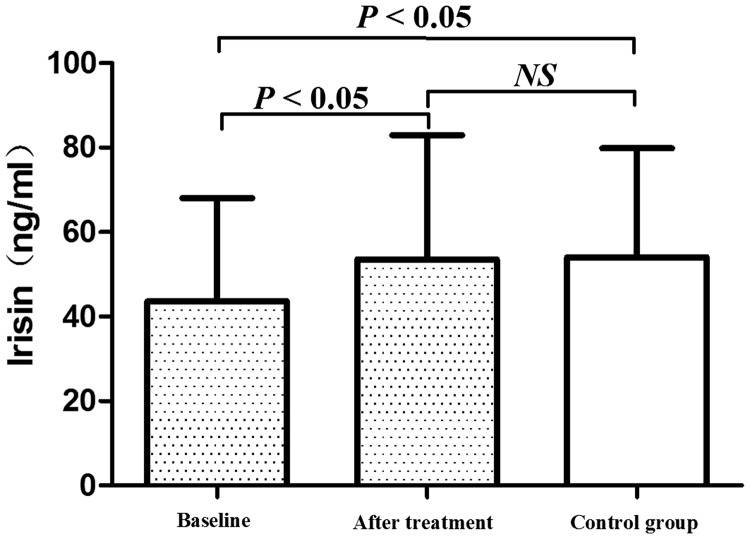
During the 12 months of levothyroxine treatment, 57 patients with hypothyroidism completed the study. These patients received an average dose of 101.75 µg/day levothyroxine to achieve a euthyroid state. BMI and levels of CHOL, HDL-C, LDL-C, TG, CK, and TSH were decreased after levothyroxine treatment, while FT3 and FT4 levels were increased after treatment compared with baseline values (all P < 0.05, Table 3). Plasma irisin levels were also significantly increased in hypothyroid patients after levothyroxine treatment compared with baseline values (P = 0.001, Table 3, Figure 1). Different irisin levels in the control and hypothyroidism groups (baseline and post-treatment) were analyzed using analysis of variance. Baseline irisin levels were lower in the control group than baseline levels in the hypothyroidism group (P < 0.05). However, there was no significant difference in irisin levels between the control group and post-treatment hypothyroidism group (53.56 ± 29.38 vs. 54.08 ± 25.79 ng/mL, Figure 1).
Table 3.
Comparison of clinical parameters after levothyroxine treatment in patients with hypothyroidism.
| Hypothyroidism group (n = 57) |
P value | ||
|---|---|---|---|
| Baseline | After treatment | ||
| Sex (M/F) | 11/46 | ||
| Age, years | 42.25 ± 13.24 | ||
| Weight, kg | 66.06 ± 8.94 | 63.84 ± 8.36 | <0.001 |
| BMI, kg/m2 | 24.88 ± 3.19 | 24.04 ± 2.92 | <0.001 |
| CHOL, mmol/L | 6.50 ± 1.92 | 4.65 ± 0.91 | <0.001 |
| HDL-C, mmol/L | 1.74 ± 0.49 | 1.43 ± 0.32 | 0.015 |
| LDL-C, mmol/L | 3.75 ± 1.21 | 2.68 ± 0.89 | <0.001 |
| TG, mmol/L | 1.43 (0.96–2.03) | 1.09 (0.79–1.34) | <0.001 |
| CK, U/L | 242.00 (122.50–604.50) | 87.00 (63.50–158.00) | <0.001 |
| FBG, mmol/L | 4.92 ± 0.67 | 4.83 ± 0.52 | 0.297 |
| FT3, pg/mL | 1.78 ± 0.66 | 2.97 ± 0.53 | <0.001 |
| FT4, ng/dL | 0.48 ± 0.11 | 1.20 ± 0.19 | <0.001 |
| TSH, uIU/ml | 79.88 ± 24.30 | 2.88 ± 1.50 | <0.001 |
| Irisin, ng/mL | 43.63 ± 24.44 | 53.56 ± 29.38 | 0.001 |
BMI: body mass index; CHOL: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TG: triglycerides; CK: creatine kinase; FBG: fasting blood glucose; FT3: free triiodothyronine; FT4: free thyroxine; TSH: thyrotropin.
Figure 1.
Graph showing plasma irisin levels at baseline, after levothyroxine treatment, and in the control group. Plasma irisin levels were significantly lower at baseline than in the control group or after treatment. There was no significant difference in plasma irisin levels between the control group and after treatment. Values are expressed as mean ± standard deviation.
Linear regression analysis of factors associated with the change in irisin levels in patients with hypothyroidism
After adjusting for changes in BMI, and CHOL, HDL-C, LDL-C, TG, CK, FPG, FT3, FT4, and TSH levels, a change in TSH levels was the only significant predictor of increased irisin levels in patients with hypothyroidism after levothyroxine treatment (β = −0.361, P = 0.006).
Discussion
Muscle tissue has recently been recognized as an important regulator of lipid and glucose metabolism. Irisin is a type of myokine that is cleaved and secreted from fibronectin type III domain-containing protein 5. Irisin is regulated by the peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC1-α), which upregulates UCP1 expression and induces brown fat-like development.1 This then promotes thermogenesis, increases total energy expenditure, decreases obesity, increases lipid metabolism, and modulates glucose metabolism.1–7 Hypothyroidism, one of the most common endocrine diseases worldwide, may be associated with several metabolic disorders. Consistent with other studies, our results showed that hypothyroidism may be associated with metabolic imbalance, abnormal energy homeostasis, oxidative stress, and insulin resistance.11–13,24–26 Thyroid hormones regulate energy expenditure and glucose–lipid metabolism, both of which are also regulated by irisin. The relationship between irisin and thyroid disorders is thought to be highly complex and multifaceted because findings from previous studies have often been inconclusive.18–22,27–29 To the best of our knowledge, this is the first report to describe the association between decreased plasma irisin levels and decreased thyroid hormone levels, which is not related to CK levels. Irisin levels could be restored to normal levels after levothyroxine treatment in Chinese hypothyroid patients.
We found that irisin levels were significantly lower in patients with hypothyroidism than in healthy participants. Additionally, irisin levels were positively associated with FT3 and FT4 levels, and negatively associated with TSH levels. FT4 was a significant predictor of serum irisin levels. After thyroid hormone levels were restored to normal levels by levothyroxine treatment, irisin levels also increased to the normal range. Our findings are in agreement with several previous studies.18,19 However, increased irisin levels have only been observed in short-term studies using patients with mild hypothyroidism or subclinical hypothyroidism.20–22 A study by Panagiotou et al.20 followed 34 patients who were under replacement therapy with levothyroxine. However, these patients stopped levothyroxine treatment and replaced it with T3 for 3 weeks before completely stopping treatment for 2 weeks. In this manner, iatrogenic hypothyroidism was induced and higher irisin levels were observed in the patients. Elevated irisin levels have been detected in patients with mild hypothyroidism (average TSH level: 13.1 µIU/mL) and subclinical hypothyroidism.21,22 Therefore, our results cannot be directly compared with previous findings because patients from this study had primary overt hypothyroidism. Atici et al.27,28 found that irisin levels were increased in rats with hypothyroidism and hyperthyroidism. Short-term iatrogenic hypothyroidism was induced by daily intraperitoneal administration of 6-N-propyl-2-thiouracil (10 mg/kg/day) for 3 weeks. Irisin levels in the hypothyroidism group were lower than those in the hyperthyroidism group, which suggested that irisin levels were strongly correlated with thyroid hormone levels. In our study, correlation analyses showed that irisin levels were positively associated with FT3 and FT4 levels, and negatively associated with TSH levels.27,28 This finding is consistent with that by Şahin et al.’s study29 of patients with overt hypothyroidism.
Thyroid hormone acts through nuclear thyroid TRs to regulate expression of several genes, including PGC-1α.30 PGC-1α is a strong coactivator of TRs,31 and it is likely an important factor that mediates some of the effects of thyroid hormone. There is evidence suggesting that thyroid hormone induces PGC-1α expression in several organs.32–34 Consistent with these reports, our study suggests that decreased thyroid hormone levels lead to reduced plasma irisin levels through regulation of PGC-1α.
In our study, CK levels were significantly increased in the hypothyroidism group. While several studies have suggested that irisin is closely associated with CK,18,19 we did not find any relationship between irisin and CK levels. Anastasilakis et al.35 found that decreased irisin levels in patients with myocardial infarction were independently associated with CK and CK-myocardial band isoenzyme. This finding suggested that irisin was not a passive product, but an energetically secreted molecule. In another study, Zybek-Kocik et al.18 showed that irisin levels were not associated with other markers of muscular dysfunction, such as dystrophin and titin.36,37 Recent research also showed no correlation between irisin and CK levels in patients with hyperthyroidism.29 Therefore, irisin and CK might be regulated independently, and decreased irisin levels are not an indicator of muscle damage in patients with hypothyroidism.
Consistent with other studies, our previous work showed that most patients with hypothyroidism have abnormal blood lipid profiles.8–10,13 In the present study, we also found elevated blood lipid levels in patients with hypothyroidism. We also observed that circulating irisin levels were negatively associated with CHOL, LDL-C, and TG levels. Additionally, CHOL was a significant predictor of serum irisin levels. These findings are in agreement with previous studies that demonstrated a relationship between irisin and lipid metabolism in other disease models.38,39 The mechanism by which irisin improves lipid metabolism may be its suppression of CHOL and TG synthesis in hepatocytes.40,41 These findings suggest that irisin is one of the factors of dyslipidemia in patients with hypothyroidism.
This study has some limitations. First, we were unable to evaluate PGC-1α and fibronectin type III domain-containing protein 5 expression in patients with hypothyroidism before and after levothyroxine treatment. These data could have strongly supported our conclusions. Second, the relationship between irisin and lipid metabolism is complex and more evidence is required to explain our conclusions in patients with hypothyroidism.
In summary, we found that decreased irisin levels are associated with decreased thyroid hormone levels in Chinese patients with hypothyroidism. Thyroid hormone levels are restored to normal after levothyroxine treatment. Additional research is required to better understand the physiology and pathophysiology of irisin, especially to investigate its role in lipid metabolism.
Author contributions
Ning Yang and Guang Wang conceived and designed the experiments; Heng Zhang performed the experiments; Xia Gao, Li Miao, Zhi Yao, and Yuan Xu analyzed the data; Ning Yang and Guang Wang contributed reagents/materials/analysis tools; and Ning Yang and Guang Wang wrote the paper.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by a grant from the Chinese National Natural Science Foundation (No. 81770792). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Boström P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno-Navarrete JM, Ortega F, Serrano M, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab 2013; 98: E769–E778. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Li R, Meng Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014; 63: 514–525. [DOI] [PubMed] [Google Scholar]
- 4.Gouni-Berthold I, Berthold HK, Huh JY, et al. Effects of lipid-lowering drugs on irisin in human subjects in vivo and in human skeletal muscle cells ex vivo. PLoS One 2013; 8: e72858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang HJ, Zhang XF, Ma ZM, et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol 2013; 59: 557–562. [DOI] [PubMed] [Google Scholar]
- 6.Park KH, Zaichenko L, Brinkoetter M, et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab 2013; 98: 4899–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Daghri NM, Alkharfy KM, Rahman S, et al. Irisin as a predictor of glucose metabolism in children: sexually dimorphic effects. Eur J Clin Invest 2014; 44: 119–124. [DOI] [PubMed] [Google Scholar]
- 8.Santos-Palacios S, Brugos-Larumbe A, Guillén-Grima F, et al. A cross-sectional study of the association between circulating TSH level and lipid profile in a large Spanish population. Clin Endocrinol (Oxf) 2013; 79: 874–881. [DOI] [PubMed] [Google Scholar]
- 9.Garduno-Garcia Jde J, Alvirde-Garcia U, Lopez-Carrasco G, et al. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol 2010; 163: 273–278. [DOI] [PubMed] [Google Scholar]
- 10.Song Y, Yao X, Ying H. Thyroid hormone action in metabolic regulation. Protein Cell 2011; 2: 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G, Liu J, Yang N, et al. Levothyroxine treatment restored the decreased circulating fibroblast growth factor 21 levels in patients with hypothyroidism. Eur J Intern Med 2016; 31: 94–98. [DOI] [PubMed] [Google Scholar]
- 12.Yang N, Yao Z, Miao L, et al. Novel clinical evidence of an association between homocysteine and insulin resistance in patients with hypothyroidism or subclinical hypothyroidism. PLoS One 2015; 10: e0125922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang N, Yao Z, Miao L, et al. Homocysteine diminishes Apolipoprotein A-I function and expression in patients with hypothyroidism: a cross-sectional study. Lipids Health Dis 2016; 15: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JZ, Martagon AJ, Cimini SL, et al. Pharmacological activation of thyroid hormone receptors elicits a functional conversion of white to brown fat. Cell Rep 2015; 13: 1528–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianco AC, McAninch EA. The role of thyroid hormone and brown adipose tissue in energy homoeostasis. Lancet Diabetes Endocrinol 2013; 1: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draman MS, Stechman M, Scott-Coombes D, et al. The role of thyrotropin receptor activation in adipogenesis and modulation of fat phenotype. Front Endocrinol(Lausanne) 2017; 8: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lapa C, Maya Y, Wagner M, et al. Activation of brown adipose tissue in hypothyroidism. Ann Med 2015; 47: 538–545. [DOI] [PubMed] [Google Scholar]
- 18.Zybek-Kocik A, Sawicka-Gutaj N, Szczepanek-Parulska E, et al. The association between irisin and muscle metabolism in different thyroid disorders. Clin Endocrinol (Oxf) 2018; 88: 460–467. [DOI] [PubMed] [Google Scholar]
- 19.Zybek-Kocik A, Sawicka-Gutaj N, Wrotkowska E, et al. Time-dependent irisin concentration changes in patients affected by overt hypothyroidism. Endokrynol Pol 2016; 67: 476–480. [DOI] [PubMed] [Google Scholar]
- 20.Panagiotou G, Pazaitou-Panayiotou K, Paschou SA, et al. Changes in thyroid hormone levels within the normal and/or subclinical hyper- or hypothyroid range do not affect circulating irisin levels in humans. Thyroid 2016; 26: 1039–1045. [DOI] [PubMed] [Google Scholar]
- 21.Ateş İ, Altay M, Topçuoğlu C, et al. Circulating levels of irisin is elevated in hypothyroidism, a case-control study. Arch Endocrinol Metab 2016; 60: 95–100. [DOI] [PubMed] [Google Scholar]
- 22.Stratigou T, Dalamaga M, Antonakos G, et al. Hyperirisinemia is independently associated with subclinical hypothyroidism: correlations with cardiometabolic biomarkers and risk factors. Endocrine 2018; 61: 83–93. [DOI] [PubMed] [Google Scholar]
- 23.Feng X, Gao X, Jia Y, et al. PPAR-α agonist fenofibrate decreased serum irisin levels in type 2 diabetes patients with hypertriglyceridemia. PPAR Res 2015; 2015: 924131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 2008; 88: 1379–1406. [DOI] [PubMed] [Google Scholar]
- 25.Brenta G. Why can insulin resistance be a natural consequence of thyroid dysfunction? J Thyroid Res 2011; 2011: 152850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duntas LH. Thyroid disease and lipids. Thyroid 2002; 12: 287–293. [DOI] [PubMed] [Google Scholar]
- 27.Atici E, Menevse E, Baltaci AK, et al. Both experimental hypothyroidism and hyperthyroidism increase cardiac irisin levels in rats. Bratisl Lek Listy 2018; 119: 32–35. [DOI] [PubMed] [Google Scholar]
- 28.Atici E, Mogulkoc R, Baltaci AK, et al. Both hypothyroidism and hyperthyroidism increase plasma irisin levels in rats. Horm Mol Biol Clin Investig 2017; 33. pii:/j/hmbci.2018.33.issue-3/hmbci-2017-0054/hmbci-2017-0054.xml. doi: 10.1515/hmbci-2017-0054. [DOI] [PubMed] [Google Scholar]
- 29.Şahin M, Canpolat AG, Çorapçioğlu D, et al. Association between circulating irisin levels and epicardial fat in patients with treatment-naïve overt hyperthyroidism. Biomarkers 2018; 23: 742–747. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 30.Yuan C, Nguyen P, Baxter JD, et al. Distinct ligand-dependent and independent modes of thyroid hormone receptor (TR)/PGC-1α interaction. J Steroid Biochem Mol Biol 2013; 133: 58–65. [DOI] [PubMed] [Google Scholar]
- 31.Puigserver P, Wu Z, Park CW, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998; 92: 829–839. [DOI] [PubMed] [Google Scholar]
- 32.Weitzel JM, Radtke C, Seitz HJ. Two thyroid hormone-mediated gene expression patterns in vivo identified by cDNA expression arrays in rat. Nucleic Acids Res 2001; 29: 5148–5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatsukano T, Kurisu J, Fukumitsu K, et al. Thyroid hormone induces PGC-1α during dendritic outgrowth in mouse cerebellar purkinje cells. Front Cell Neurosci 2017; 11: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irrcher I, Adhihetty P, Sheehan T, et al. PPARγ coactivator-1α expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am J Physiol Cell Physiol 2003; 284: C1669–C1677. [DOI] [PubMed] [Google Scholar]
- 35.Anastasilakis AD, Koulaxis D, Kefala N, et al. Circulating irisin levels are lower in patients with either stable coronary artery disease (CAD) or myocardial infarction (MI) versus healthy controls, whereas follistatin and activin A levels are higher and can discriminate MI from CAD with similar to CK-MB accuracy. Metabolism 2017; 73: 1–8. [DOI] [PubMed] [Google Scholar]
- 36.Ohlendieck K, Campbell KP. Dystrophin constitutes 5% of membrane cytoskeleton in skeletal muscle. FEBS Lett 1991; 283: 230–234. [DOI] [PubMed] [Google Scholar]
- 37.Kruger M, Sachse C, Zimmermann WH, et al. Thyroid hormone regulates developmental titin isoform transitions via the phosphatidylinositol-3-kinase/AKT pathway. Circ Res 2008; 102: 439–447. [DOI] [PubMed] [Google Scholar]
- 38.Duran ID, Gülçelik NE, Ünal M, et al. Irisin levels in the progression of diabetes in sedentary women. Clin Biochem 2015; 48: 1268–1272. [DOI] [PubMed] [Google Scholar]
- 39.Ebert T, Kralisch S, Wurst U, et al. Association of metabolic parameters and rs726344 in FNDC5 with serum irisin concentrations. Int J Obes (Lond) 2016; 40: 260–265. [DOI] [PubMed] [Google Scholar]
- 40.Park MJ, Kim DI, Choi JH, et al. New role of irisin in hepatocytes: the protective effect of hepatic steatosis in vitro. Cell Signal 2015; 27: 1831–1839. [DOI] [PubMed] [Google Scholar]
- 41.Tang H, Yu R, Liu S, et al. Irisin inhibits hepatic cholesterol synthesis via AMPK-SREBP2 signaling. EBioMedicine 2016; 6: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]