Short abstract
Gastric varices are found in approximately 20% of patients with portal hypertension. Endoscopic procedures involving the injection of cyanoacrylate (CYA) have proven to be the therapies of choice for primary treatment of gastric varices and have resulted in higher hemostasis rates and lower recurrent bleeding rates compared with band ligation and sclerotherapy. Nevertheless, serious adverse events associated with CYA injection, including glue embolization, have been reported in numerous articles and have occasionally led to fatal adverse events. Gastric fundal varices with abnormal shunts are higher-risk than those without abnormal shunts, and their treatment is more challenging. Endoscopic ultrasound (EUS)-guided puncture is an important technique in the field of digestive endoscopy. EUS has advantages that include improved therapeutic targeting, enhanced variceal detection, the ability to confirm varix obliteration with Doppler examination, and the ability to perform accurate observations of gastric varices that are not affected by blood in the stomach. The coils currently used for intravascular embolization can be precisely delivered into a varix through fine-needle puncture under EUS guidance, and this technique has provided a new approach for varix obliteration. We herein describe two patients with severe gastric fundal varices who were treated with EUS-guided coil injection and CYA embolization.
Keywords: Endoscopic ultrasound, coils, cyanoacrylate, gastric varices, portal hypertension, intravascular embolization
Introduction
Gastric varices (GVs) are found in approximately 20% of patients with portal hypertension.1 Although gastric variceal hemorrhage is a serious event accompanied by high rates of recurrent bleeding and mortality, there is no global consensus regarding the best treatment options. Endoscopic procedures involving the injection of cyanoacrylate (CYA) have been suggested as the therapies of choice for primary treatment of GVs2–4 and have resulted in higher hemostasis rates and lower recurrent bleeding rates than band ligation and sclerotherapy.5,6 Nevertheless, serious adverse events associated with CYA injection, including glue embolization, have been reported in numerous articles and have occasionally led to fatal adverse events.7 Treatment of gastric fundal varices (GFVs) with abnormal shunts is riskier and more challenging.
Endoscopic ultrasound (EUS)-guided puncture is an important technique in the field of digestive endoscopy. EUS has advantages that include improved therapeutic targeting, enhanced variceal detection, the ability to confirm variceal obliteration with Doppler examination, and the ability to accurately observe GVs that are not obscured by hemorrhage in the stomach.8 The coils currently used for intravascular embolization can be precisely delivered into a varix through fine-needle puncture under EUS guidance, and this technique has provided a new approach for variceal obliteration.9 We herein describe two patients with severe GFVs who were treated with EUS-guided coil injection and CYA embolization. These cases are being reported to illustrate that EUS-guided injection of coils and CYA glue may be the optimal treatment choice in patients with GFVs with abnormal shunts. This article is a case report. No information about the patients was revealed, and ethics approval was therefore unnecessary. We obtained consent from both patients described in this report.
Case reports
Case 1
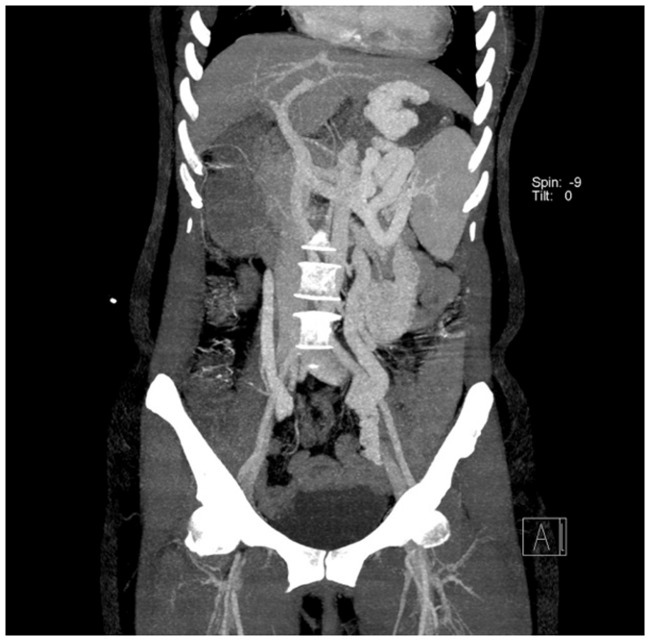
A 45-year-old man was hospitalized for hematemesis for 2 hours on 11 December 2017. He had no history of hematemesis or melena; however, he had a 20-year history of heavy drinking (500 g/day) and a history of hepatitis B. After admission to the hospital, his Child–Pugh class was B. Endoscopic examination revealed severe GFVs (Figure 1). Computed tomography (CT) imaging of the abdomen suggested cirrhosis and GFVs with a splenorenal shunt (Figure 2).
Figure 1.
Endoscopic examination revealing severe isolated gastric varices.
Figure 2.
Computed tomography imaging of the abdomen showing cirrhosis and gastric fundal varices with a splenorenal shunt.
Case 2
A 46-year-old woman was hospitalized on 7 December 2017 for a 5-year history of liver cirrhosis and a 2-year history of repeated hematemesis and black stools. One month before admission, the patient had experienced hematemesis and black stools. She was treated at a local hospital and subsequently discharged. The patient then presented to our hospital for endoscopic treatment. She had a history of hepatitis B, but no history of heavy drinking. After admission to the hospital, her Child–Pugh class was A. Cirrhosis, splenomegaly, and GFVs were found on abdominal ultrasound. Gastroduodenoscopy revealed severe GFVs (Figure 3). CT imaging of the abdomen suggested cirrhosis, splenomegaly, and GFVs with splenorenal and gastrorenal shunts (Figure 4).
Figure 3.
Gastroduodenoscopy showing severe isolated gastric varices.
Figure 4.
Computed tomography imaging of the portal vein suggesting cirrhosis, splenomegaly, and gastric fundal varices with splenorenal and gastrorenal shunts.
Procedures
EUS-guided coil injection and CYA therapy were performed as follows (Figures 5–9). The gastric fundus was distended with water to improve acoustic coupling and visualization of the GVs. A curvilinear array echoendoscope (EU-ME1; Olympus, Tokyo, Japan) that allowed for color Doppler imaging was placed in the distal esophagus to visualize the vascular anatomy in an anterograde manner. The largest varix was identified in the target vein. Intravascular punctures of the GFVs were performed using a saline solution-primed 19-G fine-needle aspiration needle (Boston Scientific, Marlborough, MA, USA) under EUS guidance via a transesophageal–transcrural approach. Synthetic fiber embolization coils (Tornado or Nester; Cook Medical Inc., Bloomington, IN, USA) were deployed into each varix through the fine-needle aspiration needle using the stiff end of a guidewire. The size of the coil was selected based on the diameter of the varix to be punctured. The “sandwich method” and a sclerosing agent [lauromacrogol (Shaanxi Tianyu Pharmaceutical, Shaanxi, China) + fibrin glue (N-butyl-2-cyanoacrylate; Compont, Beijing, China) + a sclerosing agent] were used for the EUS-guided glue injection, which was performed after coil deployment through the same needle. The absence of blood flow in the GFV after therapy was confirmed by color Doppler imaging. The operative duration for both patients was within 15 minutes.
Figure 5.
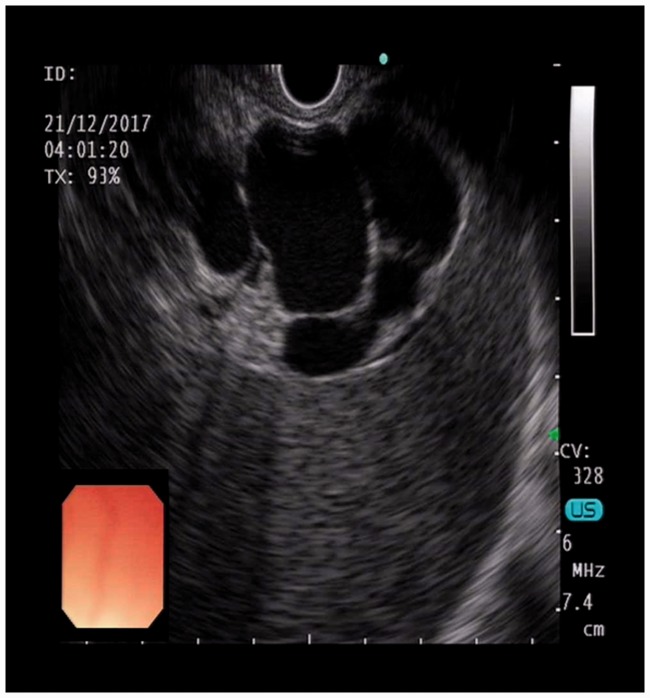
Endoscopic ultrasound view of the gastric fundal varix.
Figure 6.
Endoscopic ultrasound color Doppler blood flow image of the inner vascular pattern of the gastric fundal varix.
Figure 7.
Endoscopic ultrasound-guided puncture of a varix with a 19-G needle and coil deployment.
Figure 8.
Endoscopic ultrasound-guided injection of a sclerosing agent and cyanoacrylate.
Figure 9.
Endoscopic ultrasound color Doppler image showing the absence of blood flow in the gastric fundal varix after therapy.
Results
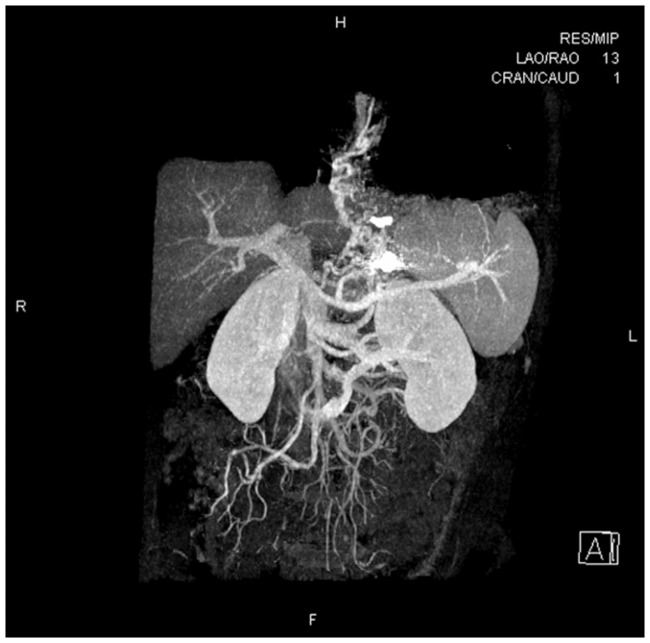
The first patient’s GVs were 39 × 30 mm in size. He required one coil (diameter, 0.035 inches; coil diameter, 10 mm; straight length, 120 mm) and 5 mL of sclerosing agent + 6 mL of CYA + 5 mL of sclerosing agent. The second patient’s GVs were 56 × 45 mm in size. She required three coils (diameter, 0.035 inches; coil diameters, 6, 7, and 8 mm; straight lengths, 58, 80, and 82 mm) and 8 mL of sclerosing agent + 6 mL: of CYA + 5 mL of sclerosing agent. There was no blood flow from the GFVs after treatment in either patient based on color Doppler assessments. To determine the effects of therapy, vascular CT examinations of the portal vein system were performed after both procedures (Figures 10 and 11). There were no adverse reactions after the procedure in either patient.
Figure 10.
Portal vein system computed tomography showing a gastric fundal varix that was significantly smaller after treatment.
Figure 11.
Portal vein system computed tomography showing a gastric fundal varix that was significantly smaller after treatment.
Discussion
Many morphological types of GVs are associated with portal hypertension. According to the Sarin classification,10 GVs are divided into isolated GVs (IGVs) and gastroesophageal varices (GOVs) and into localized (type I) and diffuse (type II) types. In type I GVs, the so-called feeding vein is a single large vessel that emerges from the supplying vein, penetrates the muscle layer of the gastric wall, and flows into the left renal vein to form a splenorenal shunt. Type II GVs consist of a network of connective intramural blood vessels. GOVs are extensions of esophageal varices to different areas of the stomach, including the greater curvature (type II GOVs) and the lesser curvature (type I GOVs). Studies have demonstrated that spontaneous splenorenal shunts occur in 14% to 21% of patients with hepatic cirrhosis.11 Splenorenal shunts can share partial portal pressure, but the risk of ectopic embolism is increased after endoscopic treatment with variceal bleeding in patients with splenorenal shunts.
GOV hemorrhage is one of the most common complications of portal hypertension, with an incidence rate of 35% to 80% and a high mortality rate that can reach 50%.12 Currently available therapeutic regimens include vasoactive drugs (nonselective beta blockers), balloon tamponade,13 endoscopic therapies (i.e., band ligation, sclerotherapy, and histoacryl glue embolization), transjugular intrahepatic portosystemic shunt placement, balloon-occluded retrograde transvenous obliteration, and surgical treatments (e.g., shunting, disconnection, and splenectomy).14,15 Endoscopic tissue adhesive injection is recommended as the first-choice treatment for GOV hemorrhage, and the Baveno V Consensus Workshop recommended CYA injection as the first-line therapy for bleeding GVs.5 However, glue embolization is associated with some of the most serious adverse events in endoscopic therapy.
Coils with attached synthetic fibers act as a “scaffold” within the vessels. This scaffold allows the tissue glue to be better contained to the varices, thus reducing or preventing the risk of glue embolization due to the decreased volume of the CYA injection. In 2011, Binmoeller et al.16 first combined coil placement with tissue glue injection under EUS guidance for the treatment of GFVs, and the results indicated that this method was feasible. In 2013, Romero-Castro et al.17 described 11 patients with GVs who were treated with EUS-guided coil application; the GV obliteration rate was >90%, and the incidence of complications (glue embolism) was lower than that in the CYA injection group. In 2016, Bhat et al.18 reported a 6-year study of combined coil and CYA injection under EUS guidance for the treatment of GFVs and suggested that this therapeutic method was safe and effective for inducing hemostasis during active bleeding and for the primary prevention of high-risk GVs. These authors also suggested that combined therapy might be safer than CYA treatment alone and could reduce the risk of glue embolization.18
EUS-guided injection of CYA into the perforating vessel can be performed to minimize the amount of CYA used and reduce the risk of embolization. However, this technique can be time-consuming, and it can be very difficult to identify and target the feeding vein. Therefore, we selected the entire GV complex as the target of the EUS-guided coil placement and CYA injection treatment. Fujii-Lau et al.19 indicated that the diameter of the coils used should be approximately 1.25 to 1.5 times the diameter of the targeted vessel. Bhat et al.18 suggested that the coil diameter should be selected according to the short-axis diameter of the varix. For the first patient in the present report, we chose a coil with a diameter of 10 mm. The diameter of the varicose vein mass was larger in the second than first patient. A larger coil, such as a coil with a diameter of 15 mm, should theoretically be chosen. However, Levy and Wong Kee Song20 recommended smaller and shorter coils because of their ease of use and potentially lower risk of acute bleeding. Therefore, we chose a smaller and shorter coil for treatment of the second patient. In addition, to ensure that the three coils were placed in the proper order, each coil that was chosen was smaller than the previously injected coil. Additionally, we had hypothesized that under the premise of the same volume of the coil, the surface area produced by using a number of small-diameter coils would be larger, which would be more favorable for the adsorption of CYA to block the blood flow. Therefore, we used three small coils for the second patient. This hypothesis still needs further study. During rupture of a dead space without varicose veins, irritation from gastric juices may increase the risk of bleeding. To completely block the blood flow and prevent the formation of a dead space without blood flow, the volume of CYA we used was the same as the size of the varicose veins. Six milliliters of CYA was injected per varix in both patients. The procedures were successfully performed in both cases, and no blood flow signal from the GVs was detected by EUS. Binmoeller et al.16 reported the treatment of GFVs with coils and CYA injections under EUS guidance. Most (93%) patients in their study received only one coil, and the mean CYA volume was 1.4 mL. Bhat et al.18 also described this technique and used an average of 1.4 coils and 2 mL of glue per patient. Therefore, methods to reduce the number of coils and the volume of tissue glue used are worth discussing. The risk of glue embolization increases with the amount of tissue glue used. Therefore, to avoid the risk of CYA embolization, some researchers have suggested that GVs should be treated by the deployment of coils alone. Clinical experience with GV embolization using only coil deployment under EUS guidance is very limited to date. Romero-Castro et al.17 reported the use of a mean of 5.8 ± 1.2 coils (range, 2–13 coils) per patient.
Under EUS guidance, the position of coil placement can be accurately controlled, the risk of glue embolization can be reduced, and the naturally formed splenorenal shunt can be preserved. EUS-guided glue injection does not require multidisciplinary cooperation and does not require the use of X-rays. Additionally, EUS-guided coil placement and CYA injection is less expensive than endoscopic balloon-occluded retrograde transvenous obliteration. Further research is needed to determine whether the hemostasis rate can be increased, the rebleeding rate can be reduced, and adverse reactions can be minimized via the use of EUS guidance compared with traditional endoscopic glue injection. Chest and abdominal CT scans should be performed after procedures to evaluate whether heterotopic embolization with CYA has occurred. In our cases, surveillance upper endoscopy and EUS were planned at 1, 3, 9, and 15 months after the index treatment. Additional studies are needed to prove whether EUS-guided coil injection with or without glue injection is a technically feasible and safe treatment and whether this treatment is more advantageous than traditional endoscopic therapy.
Declaration of conflicting interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Norton ID, Andrews JC, Kamath PS. Management of ectopic varices. Hepatology 1998; 28: 1154–1158. [DOI] [PubMed] [Google Scholar]
- 2.de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and the therapy in portal hypertension. J Hepatol 2005; 43: 167–176. [DOI] [PubMed] [Google Scholar]
- 3.Imazu H, Seewald S, Omar S, et al. Endoscopic treatment for portal hypertension: what’s new in the last 12 months? Endoscopy 2005; 37: 116–121. [DOI] [PubMed] [Google Scholar]
- 4.Irani S, Kowdley K, Kozarek R. Gastric varices. An updated review of management. J Clin Gastroenterol 2011; 45: 133–148. [DOI] [PubMed] [Google Scholar]
- 5.de Franchis R, Baveno VF. . Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010; 53: 762–768. [DOI] [PubMed] [Google Scholar]
- 6.ASGE Technology Committee, Bhat YM, Banerjee S, Barth BA, et al. Tissue adhesives: cyanoacrylate glue and fibrin sealant. Gastrointest Endosc 2013; 78: 209–215. [DOI] [PubMed] [Google Scholar]
- 7.Seewald S, Leong T, Imazu H, et al. A standardized injection technique and regimen ensures success and safety of N-butyl-2-cyanoacrylate injection for the treatment of gastric fundal varices. Gastointest Endosc 2008; 68: 447–454. [DOI] [PubMed] [Google Scholar]
- 8.Romero-Castro R, Pellicer-Bautista FJ, Jimenez-Saenz M, et al. EUS-guided injection of cyanoacrylate in perforating feeding veins in gastric varices: results in 5 cases. Gastrointest Endosc 2007; 66: 402–407. [DOI] [PubMed] [Google Scholar]
- 9.Rose SC. Mechanical devices for arterial occlusion and therapeutic vascular occlusion utilizing steel coil technique: clinical applications. AJR Am J Roentgenol 2009; 192: 321–324. [DOI] [PubMed] [Google Scholar]
- 10.Sarin SK, Kumar A. Gastric varices: profile, classification and management. Am J Gastroenterol 1989; 84: 1244–1249. [PubMed] [Google Scholar]
- 11.Tarantino G, Citro V, Conca P, et al. What are the implications of the spontaneous splenorenal shunts in liver cirrhosis? BMC Gastroenterol 2009; 9: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Yu J, Zhang B. Endoscopic diagnosis and treatment of portal hypertension complicated with portal hypertension in patients with cirrhosis. Chinese Clinician 2013; 41: 3–5. [Google Scholar]
- 13.Panes J, Teres J, Bosch J, et al. Efficacy of balloon tamponade in the treatment of bleeding gastric and esophageal varices. Results in 151 consecutive episodes. Dig Dis Sci 1988; 33: 454–459. [DOI] [PubMed] [Google Scholar]
- 14.Tripathi D, Therapondos G, Jackson E, et al. The role of the transjugular intrahepatic portosystemic stent shunt (TIPSS) in the management of bleeding gastric varices: clinical and haemodynamic correlations. Gut 2002; 51: 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanagawa H, Mima S, Kouyama H, et al. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol 1996; 11: 51–58. [DOI] [PubMed] [Google Scholar]
- 16.Binmoeller KF, Weilert F, Shah JN, et al. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection (with videos). Gastrointest Endosc 2011; 74: 1019–1025. [DOI] [PubMed] [Google Scholar]
- 17.Romero-Castro R, Ellrichmann M, Ortiz-Moyano C, et al. EUS-guided coil versus cyanoacrylate therapy for the treatment of gastric varices: a multicenter study (with videos). Gastrointest Endosc 2013; 78: 711–721. [DOI] [PubMed] [Google Scholar]
- 18.Bhat YM, Weilert F, Fredrick RT, et al. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: a large U.S. experience over 6 years (with video). Gastrointest Endosc 2016; 83: 1164–1172. [DOI] [PubMed] [Google Scholar]
- 19.Fujii-Lau LL, Law R, Wong Kee Song LM, et al. Endoscopic ultrasound (EUS)-guided coil injection therapy of esophagogastric and ectopic varices. Surg Endosc 2016; 30: 1396–1404. [DOI] [PubMed] [Google Scholar]
- 20.Levy MJ, Wong Kee Song LM. EUS-guided angiotherapy for gastric varices: coil, glue, and sticky issues. Gastrointest Endosc 2013; 78: 722–725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.