Short abstract
Background/Aims
Vonoprazan is a new a potassium-competitive acid blocker (P-CAB) that was recently developed in Japan. However, vonoprazan’s efficacy in healing gastric ulcers after endoscopic submucosal dissection (ESD) remains controversial. This study aimed to compare the efficacy of P-CABs and proton pump inhibitors (PPIs) in healing post-ESD ulcers.
Materials and Methods
This prospective randomized controlled trial (UMIN000017386) enrolled 40 patients with gastric neoplasia, who underwent ESD at our hospital from April 2015 to January 2016. Before ESD, patients were randomly divided into the following two groups: group V, vonoprazan 20 mg/day; or group R, rabeprazole 10 mg/day. Medications were taken 1 day before to 4 weeks after ESD. The ESD-induced artificial ulcer size was measured just after ESD and 4 weeks after ESD to calculate the reduction rate as follows: (ulcer area 4 weeks after ESD)/(ulcer area just after ESD) × 100.
Results
Eighteen patients in group V and 15 patients in group R were analyzed. The mean reduction rate was significantly different in groups V and R (93.3% vs 96.6%, respectively). Post-ESD bleeding was observed in two patients in group R and drug-induced hepatic injury in one patient in group R.
Conclusion
Rabeprazole facilitated the healing process post-ESD.
Keywords: Endoscopic submucosal dissection, artificial gastric ulcer, gastric neoplasia, vonoprazan, rabeprazole, reduction rate
Introduction
Endoscopic submucosal dissection (ESD) is an established treatment for early-stage gastric neoplasm in Japan.1–3 ESD provides a more accurate histopathological diagnosis relative to that obtained via a piecemeal endoscopic resection, and it may also improve the patient’s subsequent quality of life compared with surgery.4–6 However, in 4%–6% of patients, delayed bleeding occurs within 24 hours or more after ESD and can have serious consequences.7 The frequency of delayed bleeding varies with the tumor location and size.8,9 Post-ESD ulcers are considered to be the most significant predictor of post-ESD bleeding.10,11 However, optimal treatment for the prevention of delayed bleeding has not been established because the effect of acid suppression is not entirely understood.12
Proton-pump inhibitors (PPIs) have been reported to be an effective treatment for peptic ulcer disease.13 The duration of PPI treatment in post-ESD ulcers is usually 4–8 weeks, which is similar to the regimen for non-iatrogenic gastric ulcers. Moreover, a 4-week course of lansoprazole is reportedly as effective as an 8-week course.14 Among the many available PPIs, rabeprazole is commonly used in Asian countries because it is less affected by the CYP2C19 genetic polymorphism, which is more prevalent in Japan, China, and Korea than in western countries.15–17 The optimal dose of rabeprazole for the resolution of post-ESD ulcers and symptom resolution is reportedly 10 mg once daily, with no apparent advantage to using higher doses.18 Based on these reports, 10 mg of rabeprazole daily for 4 weeks may be sufficient to heal post-ESD ulcers. However, there is no consensus on the optimal treatment of these ulcers, although many reports describe the healing of post-ESD ulcers by PPIs.
Vonoprazan is a novel oral potassium-competitive acid blocker (P-CAB) that was discovered and developed by the Takeda Pharmaceutical Company in Japan.19 Vonoprazan competitively inhibits binding of the potassium ion to H+, K+-ATPase (proton pump) in the final step of acid secretion in gastric parietal cells. It is administered under the Japanese health insurance system at 20 mg once daily for the treatment of gastroduodenal ulcers. In preclinical studies, vonoprazan produced more potent and more sustained suppression of gastric acid secretion compared with lansoprazole.20,21 These effects appear to be related to greater accumulation of vonoprazan into, and its subsequent slower clearance from, gastric glands.22 Based on these reports, P-CABs, a new class of acid-suppressing agents, are a potential alternative to PPIs for the treatment of acid-related diseases, and are not affected by the acid secretory state, mealtimes, and CYP2C19 polymorphism.19,23–26
Thus, our objective was to measure the healing efficacy of the P-CAB vonoprazan and the PPI rabeprazole post-ESD. We hypothesized that a 4-week course of vonoprazan would be more effective than a 4-week course of rabeprazole to treat artificial gastric ulcer in patients undergoing endoscopic submucosal resection for gastric neoplasia.
Materials and methods
Study design and patients
This study was designed as a prospective randomized controlled trial and aimed to measure the comparative efficacy of a P-CAB and a PPI in healing post-ESD ulcers. We compared the reduction rate between the P-CAB vonoprazan and the PPI rabeprazole.
Before ESD treatment, patients who were scheduled to undergo treatment for gastric neoplasia (gastric cancer and adenoma) at Juntendo University School of Medicine from April 2015 to January 2016 were recruited. Informed consent was obtained from all patients and the study was approved by the Ethics Committee of Juntendo University (Registration number UMIN000017386).
In this study, all patients were interviewed, and baseline characteristics such as age, sex, medication history, underlying disease, initial diagnosis, and previous endoscopic findings were prospectively investigated. All patients were also monitored for the occurrence of complications, including bleeding, throughout the 4-week observation period. Delayed bleeding was recorded when hematemesis or melena was observed or when the hemoglobin concentration decreased by more than 2 g/dL. Patients using antithrombotic agents, non-steroidal anti-inflammatory drug and steroids, and those with a tendency to bleed, with severe cardiopulmonary complications, or who were on dialysis were excluded. Patients taking PPIs, histamine-2 receptor antagonists (H2RAs), or gastrointestinal motility-improving drugs, stopped taking all medications on the day before entering this study and then started taking only the test drugs.
Study protocol
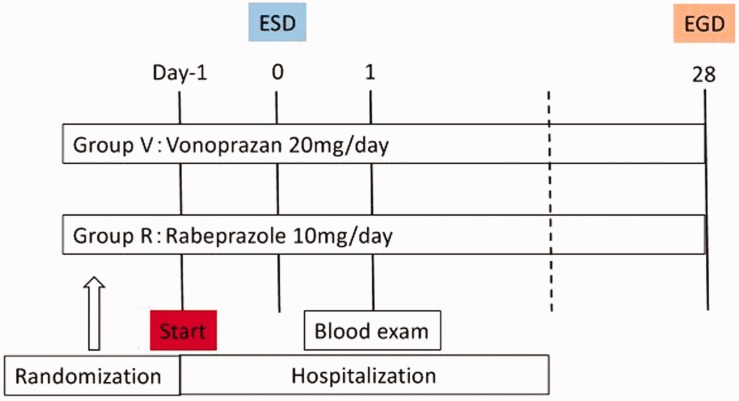
Patients were admitted to our hospital 2 days before ESD. Thereafter, they were randomly assigned to one of two groups using a computer-generated random number table. The study groups were group V (vonoprazan, 20 mg) or group R (rabeprazole, 10 mg). Study medication was taken beginning on the evening of the day before ESD until 4 weeks after ESD. Patients underwent esophagogastroduodenoscopy (EGD) 4 weeks after ESD, and we measured the size of ulcers and calculated the reduction rate by comparing the ulcer area just after ESD to that 4 weeks after ESD (Figure 1).
Figure 1.
Study protocol.
Measurement of ESD-induced ulcer area and ulcer stages
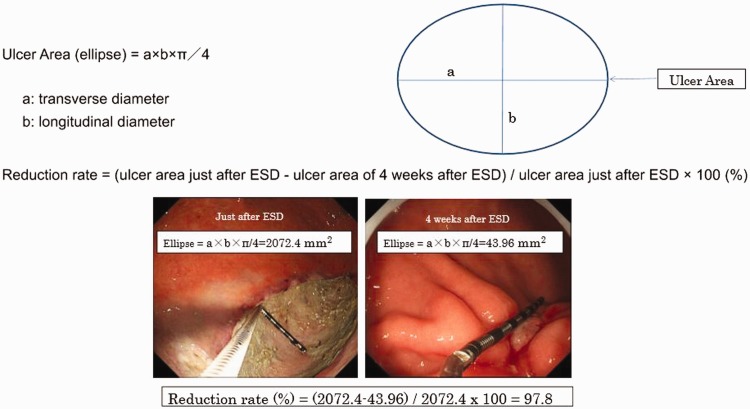
The size of the ulcers was defined using the long and short diameter of the gastric ulcers, and measured using measuring forceps (M2-3U, M2-4K; Olympus Medical Systems, Tokyo, Japan). Ulcer areas were calculated using the following formula: (π × longer diagonal line × shorter diagonal line)/4. Figure 2 shows a sample calculation of ellipsoidal ulcer area. The reduction rate was calculated as follows: (ulcer area 4 weeks after ESD)/(ulcer area just after ESD) × 100 (%) (Figure 2).
Figure 2.
Measurement of ESD-induced ulcer area.
In addition, the gastric ulcer stage was classified using a six-stage Sakita–Miwa classification as follows: active (A1, A2), healing (H1, H2), and scarring (S1, S2) (Table 1).
Table 1.
Sakita–Miwa classification.
| Stages | Findings |
|---|---|
| Active stage | |
| A1 | Ulcer that contains mucus coating, with marginal elevation because of edema |
| A2 | Mucus-coated ulcer with discrete margin and less edema than active stage 1 |
| Healing stage | |
| H1 | Unhealed ulcer covered by regenerating epithelium <50%, with or without converging folds |
| H2 | Ulcer with a mucosal break but almost covered with regenerative epithelium |
| Scaring stage | |
| S1 | Red scar with rough epithelialization without mucosal break |
| S2 | White scar with complete re-epithelialization |
ESD procedure
ESD was performed using a single-channel gastroscope (GIF-Q260J; Olympus Medical Systems) and an electrosurgical unit (VAIO-300D; ERBE, Tübingen, Germany). The electrosurgical knives, Dual knife (KD-650L; Olympus Medical Systems) and IT knife-2 (KD-611L; Olympus Medical Systems), were used. Coagraspers (FD-410LR; Olympus Medical Systems) were used as electrosurgical hemostatic forceps. To create a submucosal fluid cushion, 0.9% saline solution containing 0.5% indigo carmine and 0.001% epinephrine were used. Thereafter, the resected specimens were stretched, pinned flat on a cork board, and measured.
Statistical analysis
Statistical analyses were performed using the paired t-test, chi-square test, and Fisher’s exact test, as appropriate. The statistical analyses were performed using the SAS statistical package, version 9.4 (SAS Institute, Cary, NC, USA). A p value of less than 0.05 was considered statistically significant.
Results
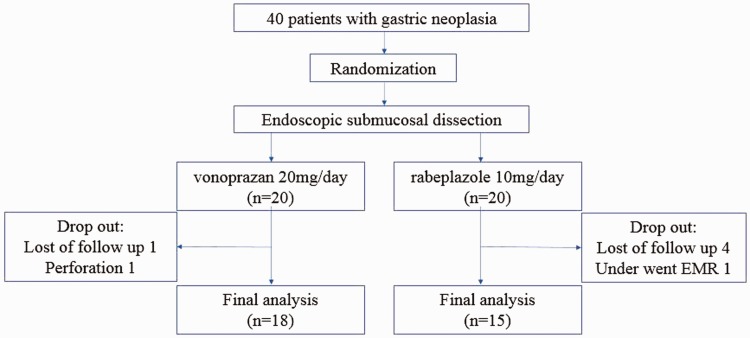
Forty patients were enrolled and randomly assigned in a 1:1 ratio between the two groups from April 2015 to January 2016. Seven patients (two patients in group V and five patients in group R) were excluded from the final analysis (Figure 3). In group V, one patient did not undergo EGD 4 weeks after ESD, and another experienced perforation during ESD. In group R, four patients did not undergo EGD 4 weeks after ESD, and one patient underwent endoscopic mucosal resection (EMR) because the tumor size was small. There were 18 patients in the vonoprazan group (group V) and 15 patients in the rabeprazole group (group R) who completed the study and were included in the analysis.
Figure 3.
Final analysis.
Baseline patient characteristics
The baseline characteristics of the 33 patients are shown in Table 2. No significant differences were observed in sex, age, body mass index, alcohol use, smoking, diabetes mellitus, hypertension, and Helicobacter pylori infection.
Table 2.
Baseline characteristics of patients.
| Characteristics | VonoprazanGroup V(n=18) | RabeprazoleGroup R(n=15) | p value |
|---|---|---|---|
| Sex: Male/Female | 13/5 | 11/4 | 0.75a |
| Age (y): Mean ± SD | 69 ± 9.3 | 70.9 ± 8.8 | 0.57b |
| Body mass index (kg/m2) | 22.7 ± 2.8 | 23.4 ± 2.9 | 0.53b |
| Alcohol: yes/no | 13/5 | 6/9 | 0.13a |
| Smoke: yes/no | 5/13 | 5/10 | 0.97a |
| Diabetes mellitus: +/− | 3/15 | 0/15 | 0.23a |
| Hypertension (%) | 9 (50) | 6 (46) | 0.82a |
| H. pylori infection (%) | 6/15 (40) | 2/13 (15.3) | 0.21a |
Data are expressed as mean ± SD or number of patients (%).
achi-square test, bt-test.
Group V, patient who took vonoprazan; Group R, patient who took rabeprazole.
Clinicopathological characteristics of lesions and ESD results
The clinicopathological data of lesions and ESD results are shown in Table 3. No significant differences (group V vs group R) were observed in the tumor size (148.6 ± 324.5 mm2 vs 145.7 ± 158.5 mm2), location of tumors (U/M/L: 1/4/13 vs 2/8/5), macro scopic findings (0-I/0-IIa/0-IIb/0-IIc: 0/3/4/11 vs 1/5/2/7), histology (adenoma/papillary adenocarcinoma/well differentiated adenocarcinoma/moderately differentiated adenocarcinoma/signet-ring cell carcinoma, poorly differentiated adenocarcinoma/others: 3/0/12/1/1/1 vs 1/0/10/1/2/1), depth of invasion (mucosal layer/submucosal layer: 17/1 vs 14/1), submucosal fibrosis (+/−: 5/18 vs 1/15), and lymphatic invasion (+/−: 1/18 vs 0/15). Venous invasion was not observed in either group. For ESD results, no significant differences (group V vs group R) were observed in the procedure time (minutes, 68.3 vs 44.9) or in the complete resection rate (16/18 [88.9%] vs 15/15 [100%]).
Table 3.
Clinicopathological characteristics of lesions and ESD results.
| Characteristics | Group V(n=18) | Group R(n=15) | p value |
|---|---|---|---|
| Tumor size (mm2) | 148.6 ± 324.5 | 145.7 ± 158.5 | 0.97a |
| Location of tumor (U/M/L) | 1/4/13 | 2/8/5 | 0.21b |
| Macroscopic findings (%) | 0.91b | ||
| 0-I | 0/18 (0) | 1/15 (6.7) | |
| 0-IIa | 3/18 (16.7) | 5/15 (33.3) | |
| 0-IIb | 4/18 (22.2) | 2/15 (13.3) | |
| 0-IIc | 11/18 (61.1) | 7/15 (46.7) | |
| Histopathological findings | 0.97b | ||
| Adenoma | 3 | 1 | |
| Pap | 0 | 0 | |
| Well diff | 12 | 10 | |
| Moderate diff | 1 | 1 | |
| Sig, por | 1 | 2 | |
| Others | 1 | 1 | |
| Depth of invasion (mucosal/submucosal) | 17/1 | 14/1 | 1.0b |
| Submucosal fibrosis (%) | 5/18 (27.7) | 1/15 (6.6) | 0.37b |
| Lymphatic invasion (%) | 1/18 (5.5) | 0/15 (0) | 1.0b |
| Venous invasion (%) | 0/18 (0) | 0/15 (0) | 1.0b |
| Procedure time (minutes) | 68.3 ± 70.1 | 44.9 ± 21.0 | 0.17a |
| Delayed bleeding | 0/18 (0) | 2/15 (13.3) | 0.19b |
| Curative resection rate | 16/18 (88.9) | 15/15 (100) | 0.48b |
Data are expressed as the mean ± SD or number of cases (%).
at-test, bchi-square test.
Group V, patient who took vonoprazan; Group R, patient who took rabeprazole.
U, upper third of the stomach; M, middle third of the stomach; L, lower third of the stomach; Pap, papillary adenocarcinoma; Well diff, well differentiated adenocarcinoma; Moderate diff, moderately differentiated adenocarcinoma; Sig, signet-ring cell carcinoma; por, poorly differentiated adenocarcinoma.
Ulcer size, reduction rate, and ulcer stage after ESD treatment
The ulcer size and reduction rate after ESD treatment are shown in Table 4. The ulcer mean area at just after ESD was 741.3 ± 666.8 mm2 in group V and 1022.4 ± 640.5 mm2 in group R, which was not significantly different. The mean ulcer area at 4 weeks after ESD was 48.0 ± 52.0 mm2 in group V and 31.0 ± 19.1 mm2 in group R, which was also not significantly different. The ulcer reduction rate at 4 weeks after ESD was 93.3% in group V and 96.6% in group R, which was a significant difference (p = 0.009). All ulcers at 4 weeks after ESD in group R healed to more than 90%, whereas four of 18 (22.2%) patients in group V had delayed ulcer healing. All gastric ulcer stages at 4 weeks after ESD were classified as stage H1 or H2 (H1/H2; Group V: 9/9 vs Group R: 6/9), and there was no significant difference in the gastric ulcer stage between the two groups (Table 5).
Table 4.
Ulcer size and reduction rate after ESD treatment.
| Group V (n=18) | Group R (n=15) | p value | |
|---|---|---|---|
| Ulcer size (mm2) | |||
| 0 | 741.3 ± 666.8 | 1022.4 ± 640.5 | 0.24a |
| 28 | 48.0 ± 52.0 | 31.0 ± 19.1 | 0.22a |
| Reduction rate (%) | 93.3 | 96.6 | 0.009a |
at-test.
Group V, patient who took vonoprazan; Group R, patient who took rabeprazole.
Table 5.
Ulcer stages 4 weeks after ESD.
| Ulcer stage H1/H2 (Sakita-Miwa classification) | Group V (n=18) | Group R (n=15) | p value |
|---|---|---|---|
| A1/A2 | 0 | 0 | |
| H1/H2 | 9/9 | 6/9 | 0.82a |
| S1/S2 | 0 | 0 |
achi-square test.
Group V, patient who took vonoprazan; Group R, patient who took rabeprazole.
Preventive effects of vonoprazan and rabeprazole on bleeding from post-ESD ulcers and adverse events
Delayed bleeding was observed in two patients in group R (13.3%), and drug-induced hepatic injury was observed in one patient in group R (6.7%). There were no significant differences in the preventive effects and adverse events between the two groups.
Discussion
In this study, the ulcer reduction rate at 4 weeks after ESD was significantly different between vonoprazan 20 mg (group V) and rabeprazole 10 mg (group R) (p = 0.009). The ulcer reduction rate was higher in group R compared with group V, indicating that rabeprazole facilitated the healing process more quickly than vonoprazan in post-ESD ulcers.
Vonoprazan produces a more potent and more sustained suppression of gastric acid secretion compared with PPIs. The pH ≥ 4 and pH ≥ 5 holding time ratios of vonoprazan 20 mg daily over 24 hours increased to 95% and 91%, respectively.27 However, the pH ≥ 4 holding time ratio for rabeprazole 10 mg daily over 24 hours increased to about 20%–25%.28 Generally, the optimal treatment for peptic ulcers should aim to increase the intragastric pH to >3 for a period of 18–20 hours per day to allow healing to take place within 3–4 weeks.29 Therefore, vonoprazan is thought to be more effective than rabeprazole, which showed a significantly better ulcer reduction rate and improved ulcer healing post-ESD ulcers. This result was similar to the effect shown when PPIs and H2Ras were compared.13
There are few reports about the efficacy of vonoprazan or the healing effect of vonoprazan in artificial gastric ulcers after ESD. To the best of our knowledge, the following three reports were published. Muraoka et al.30 reported a historical control study, where the ulcer contraction rate at 4 weeks after ESD in the group taking vonoprazan was significantly greater than that in the esomeprazole group. However, Kagawa et al.31 reported a historical control study, in which the ulcer size reduction rates were not significantly different between the group taking vonoprazan for 5 weeks and the group taking PPIs for 8 weeks. Takahashi et al.32 reported a prospective randomized controlled study, which showed that the ulcer size reduction rates were not significantly different between the group taking vonoprazan for 4 weeks and the group taking lansoprazole for 4 weeks. Therefore, the efficacy of vonoprazan in reducing the size of post-ESD ulcers remains controversial. However, two of these three studies were historical control studies. Only one prospective randomized controlled study was performed, and it showed that the ulcer size reduction rates were not significantly different between in the vonoprazan and lansoprazole groups. Therefore, this prospective randomized controlled study is the first study to suggest that rabeprazole was significantly more effective than vonoprazan in healing post-ESD ulcers.
Although vonoprazan is theoretically a more potent acid suppressor, ulcer healing was delayed in four of 18 (22.2%) patients in group V. There were no risk factors for delaying ulcer healing, such as comorbidities or taking a steroid, in these patients. Park et al.18 reported that 10 mg of rabeprazole has an equal efficacy in the healing ESD-induced ulcers after 4 weeks compared with the standard dose of rabeprazole (20 mg/day). These results could be explained by the different properties of peptic and artificial ulcers. Peptic ulcers are thought to develop in vulnerable sites with hyperacidity. These ulcers extend deeper and laterally because there is a breakdown of gastric mucosal defense mechanisms. Conversely, artificial ulcers, which do not extend deeper and laterally, occur iatrogenically in hypoacidic or normal environments where mucosal defense mechanisms are functioning.18 Vonoprazan and lansoprazole were also reported to have equivalent therapeutic effects on peptic ulcer after 8 weeks.33 Analogous with these differences, artificial ulcers may require lower-than-standard doses of PPIs for healing because of the depth of ulcers and the normal mucosal defense mechanisms. Therefore, the mean reduction rate after ESD treatment may not be significantly different between vonoprazan and PPIs. However, in this study rabeprazole was significantly more effective than vonoprazan in reducing post-ESD ulcers. Arakawa et al.34 have reported that acid induces the cytoprotective prostaglandins (PGs) and enhances mucosal integrity, which indicates that ulcer healing might be delayed because of PG suppression when intra-gastric pH is high. Therefore, we speculated that the ulcer healing might be delayed when the intragastric pH is too high. However, further study is needed to elucidate the reason for the delay in ulcer healing when the intragastric pH is too high.
No ulcer left a scar at 4 weeks after ESD. Therefore, we could not evaluate the probability of scar stage in both groups. Kim et al.35 reported that vonoprazan was superior to PPI for ulcer healing at 8 weeks after ESD. Therefore, evaluating the reduction rate at 8 weeks after ESD may show clear results for the healing rate of ulcers.
Post-ESD ulcers are considered to be the most significant predictor of delayed bleeding after ESD.10,11 Tsuji et al.36 have reported that multivariate logistic regression analysis showed that tumors located in the lower third of the stomach, inexperienced operators coagulating the ulcer floor, and daily use of medication potentially related to gastric injury/bleeding were significantly associated with bleeding after ESD. In our study, although there were no significant differences, delayed bleeding occurred in two patients in group R (p = 0.19). Although these two patients had an ulceration scar under the lesions and experienced a longer-than-median procedure time, these conditions were not associated with the risk factors of delayed bleeding that were reported previously. However, such conditions might have led to delayed bleeding in these two patients. Delayed bleeding tends to occur within 24–48 h after ESD.37 The most important factor in preventing delayed bleeding is a rapid rise in intragastric pH, and vonoprazan raises intragastric pH more rapidly than PPIs.22,38 Green et al.39 have reported that platelet aggregation and plasma coagulation were both virtually abolished at intragastric pH 5.4. Therefore, vonoprazan might be more effective than PPIs for preventing delayed bleeding. However, a larger study is needed to clarify whether vonoprazan is more effective than PPIs for preventing delayed bleeding.
Although our study was a prospective randomized controlled trial, it had some limitations. The sample size was relatively small, and there remains the potential for other confounding factors. The study protocol had a relatively short duration, so all ulcers did not reach the scarring stage. Therefore, we could not compare the scarring rate in the rabeprazole and vonoprazan groups. Moreover, the CYP2C19 genetic polymorphism in patients was not investigated.
In conclusion, the effectiveness of vonoprazan is not superior to rabeprazole in healing post-ESD ulcers. Therefore, our study suggested that 10 mg of rabeprazole daily for 4 weeks is sufficient for the management of post-ESD ulcers and to heal a stomach ulcer after ESD.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Gotoda T, Kondo H, Ono H, et al. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc 1999; 50: 560–563. [DOI] [PubMed] [Google Scholar]
- 2.Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6: 119–121. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe K, Ogata S, Kawazoe S, et al. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc 2006; 63: 776–782. [DOI] [PubMed] [Google Scholar]
- 4.Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001; 48: 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujishiro M, Yahagi N, Nakamura M, et al. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc 2006; 63: 243–249. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto H, Kawata H, Sunada K, et al. Success rate of curative endoscopic mucosal resection with circumferential mucosal incision assisted by submucosal injection of sodium hyaluronate. Gastrointest Endosc 2002; 56: 507–512. [DOI] [PubMed] [Google Scholar]
- 7.Okada K, Yamamoto Y, Kasuga A, et al. Risk factors for delayed bleeding after endoscopic submucosal dissection for gastric neoplasm. Surg Endosc 2011; 25: 98–107. [DOI] [PubMed] [Google Scholar]
- 8.Shiba M, Higuchi K, Kadouchi K, et al. Risk factors for bleeding after endoscopic mucosal resection. World J Gastroenterol 2005; 11: 7335–7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc 2005; 17: 54–58. [Google Scholar]
- 10.Mukai S, Cho S, Kotachi T, et al. Analysis of delayed bleeding after endoscopic submucosal dissection for gastric epithelial neoplasms. Gastroenterol Res Pract 2012; 2012: 875323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto O, Fujishiro M, Kodashima S, et al. Short-term healing process of artificial ulcers after gastric endoscopic submucosal dissection. Gut Liver 2011; 5: 293–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramakrishnan K, Salinas RC. Peptic ulcer disease. Am Fam Physician 2007; 76: 1005–1012. [PubMed] [Google Scholar]
- 13.Gisbert JP, González L, Calvet Xet al. Proton pump inhibitors versus H2-antagonists: a meta-analysis of their efficacy in treating bleeding peptic ulcer. Aliment Pharmacol Ther 2001; 15: 917–926. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Baek EK, Choi CH, et al. Comparison of the efficacy of 4- and 8-week lansoprazole treatment for ESD-induced gastric ulcers: a randomized, prospective, controlled study. Surg Endosc 2014; 28: 235–241. [DOI] [PubMed] [Google Scholar]
- 15.Horai Y, Kimura M, Furuie H, et al. Pharmacodynamic effects and kinetic disposition of rabeprazole in relation to CYP2C19 genotypes. Aliment Pharmacol Ther 2001; 15: 793–803. [DOI] [PubMed] [Google Scholar]
- 16.Ji S, Kim HS, Kim JW, et al. Comparison of the efficacy of rabeprazole 10 mg and omeprazole 20 mg for the healing rapidity of peptic ulcer diseases. J Gastroenterol Hepatol 2006; 21: 1381–1387. [DOI] [PubMed] [Google Scholar]
- 17.Stedman CA, Barclay ML. Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther 2000; 14: 963–978. [DOI] [PubMed] [Google Scholar]
- 18.Park HJ, Kim HS, Kim BRet al. Half-dose rabeprazole has an equal efficacy to standard-dose rabeprazole on endoscopic submucosal dissection-induced ulcer. Dig Dis Sci 2013; 58: 1054–1061. [DOI] [PubMed] [Google Scholar]
- 19.Shin JM, Inatomi N, Munson K, et al. Characterization of a novel potassium‐competitive acid blocker of the gastric H,K‐ATPase, 1‐[5‐(2‐fluorophenyl)‐1‐(pyridin‐3‐ylsulfonyl)‐1H‐pyrrol‐3‐yl]‐N‐methylmethanamine monofumarate (TAK‐438). J Pharmacol Exp Ther 2011; 339: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hori Y, Imanishi A, Matsukawa J, et al. 1‐[5‐(2‐fluorophenyl)‐1‐(pyridin‐3‐ylsulfonyl)‐1H‐pyrrol‐3‐yl]‐N‐methylmethanamine monofumarate (TAK‐438), a novel and potent potassium‐competitive acid blocker for the treatment of acid‐related diseases. J Pharmacol Exp Ther 2010; 335: 231–238. [DOI] [PubMed] [Google Scholar]
- 21.Hori Y, Matsukawa J, Takeuchi T, et al. A study comparing the antisecretory effect of TAK‐438, a novel potassium‐competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther 2011; 337: 797–804. [DOI] [PubMed] [Google Scholar]
- 22.Matsukawa J, Hori Y, Nishida H, et al. A comparative study on the modes of action of TAK‐438, a novel potassium‐competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. Biochem Pharmacol 2011; 81: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 23.Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects–a randomised open-label cross-over study. Aliment Pharmacol Ther 2015; 42: 719–730. [DOI] [PubMed] [Google Scholar]
- 24.Garnock-Jones KP. Vonoprazan: first global approval. Drugs 2015; 75: 439–443. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins H, Sakurai Y, Nishimura Aet al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 2015; 41: 636–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashida K, Sakurai Y, Hori T, et al. Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther 2016; 43: 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kagami T, Sahara S, Ichikawa H, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther 2016; 43: 1048–1059. [DOI] [PubMed] [Google Scholar]
- 28.Furuta K, Kohata Y, Fujiwara Y, et al. Intra-gastric pH following single oral administrations of rabeprazole and esomeprazole: double-blind cross-over comparison. J Clin Biochem Nutr 2014; 55: 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burget DW, Chiverton SG, Hunt RH. Is there an optimal degree of acid suppression for healing of duodenal ulcers? A model of the relationship between ulcer healing and acid suppression. Gastroenterology 1990; 99: 345–351. [DOI] [PubMed] [Google Scholar]
- 30.Muraoka D, Arai M, Kasamatsu S, et al. Vonoprazan is superior to proton pump inhibitors in healing artificial ulcers of the stomach post-endoscopic submucosal dissection. Dig Endosc 2017; 29: 57–64. [DOI] [PubMed] [Google Scholar]
- 31.Kagawa T, Iwamuro M, Ishikawa S, et al. Vonoprazan prevents bleeding from endoscopic submucosal dissection-induced gastric ulcers. Aliment Pharmacol Ther 2016; 44: 583–591. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, Sato Y, Kohisa J, et al. Vonoprazan 20mg vs lansoprazole 30mg for endoscopic submucosal dissection-induced gastric ulcers. World J Gastrointest Endosc 2016; 8: 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeda. Takecab® (vonoprazan tablets): Japanese prescribing information. 2014. Accessed 5 Feb 2015.
- 34.Arakawa T, et al. Effect of acid on gastric mucosal prostaglandins and gastric protection in rat. Japanese J of inflammation 1984; 4: 17–20. [Google Scholar]
- 35.Kim EH, Park SW, Nam E, et al. Comparative efficacy of various anti-ulcer medications after gastric endoscopic submucosal dissection: a systematic review and network meta-analysis. Surg Endosc 2018. doi: 10.1007/s00464-018-6409-4 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Tsuji Y, Ohata K, Ito T, et al. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol 2010; 16: 2913–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SJ, Choi CW, Kang DHet al. Second-look endoscopy and factors associated with delayed bleeding after endoscopic submucosal dissection. World J Gastrointest Endosc 2016; 8: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hikichi T, Sato M, Watanabe K, et al. Oral rabeprazole administration on a procedure day suppresses bleeding after endoscopic submucosal dissection for gastric neoplasms. Fukushima J Med Sci 2014; 60: 68–74. [DOI] [PubMed] [Google Scholar]
- 39.Green FW, Jr, Kaplan MM, Curtis LE, et al. Effect of acid and pepsin on blood coagulation and platelet aggregation. Gastroenterology 1978; 74: 38–43. [PubMed] [Google Scholar]