Short abstract
Objectives
This study sought to describe events related to the degradation/resorption of a device composed of polylactic acid (PLA) after implantation into Wistar rats.
Methods
Five-millimeter-diameter PLA rigid scaffolds and flexible analogs were elaborated, bioactivated through culture with osteoblasts, and implanted into the parietal bones of adult Wistar rats after 15 days. After 3 months, the samples were recovered and analyzed via optical microscopy (histochemical techniques) and scanning electron microscopy. This research was approved by the animal ethics review committee of Universidad of Valle in Cali, Colombia, according to the endorsement of the ethics committee CEAS 001-016.
Results
Initially, there was surface erosion and fragmentation of the device, inducing an inflammatory response compatible with the foreign body reaction, in addition to the presence of a pseudocapsule and a mixed inflammatory infiltrate that was responsible for phagocytosis of the material. Regeneration of the defect via the apposition of new bone occurred simultaneously with resorption of the material.
Conclusions
The results illustrated that the degradation/resorption of PLA occurs in a centripetal pattern.
Keywords: Foreign body reaction, phagocytosis, extracellular matrix, osteoblast, polylactic acid, resorption, hydrolytic degradation
Introduction
Polylactic acid (PLA) is widely used in the fabrication of biomedical devices because of its chemical and physical properties, which allow the elaboration of several types of materials such as resorbable osteosynthetic screws and drug delivery gels. Additionally, PLA can be combined with other materials to improve its properties and comply with the requirements of the desired application.1,2
PLA is a biodegradable aliphatic polyester3 existing as two isomeric forms, namely poly-l-lactic acid (PLLA) and poly-d-lactic acid, and mixtures of both forms are also used for some applications. PLLA is most commonly used in biomedical applications because of its biocompatibility, biodegradability, and physical and chemical characteristics.4 PLLA confers mechanical resistance to the scaffold during cell colonization, and it is ultimately replaced by new tissue.5
Chitosan (CHIT) is a biodegradable natural polymer obtained via the deacetylation of chitin, leading to glucosamine units mixed with N-acetylglucosamine.6,7 CHIT has excellent biological properties, including high hydrophilicity and biocompatibility as well as appropriate biodegradability.6,8
The biological properties of CHIT are attributable to its cationic nature under slightly acidic conditions, allowing electrostatic interactions among aminoglycans, proteoglycans, and other negatively charged molecules present in the extracellular matrix.5 The combination of PLLA and CHIT is extremely attractive because of their synergic effects.9 One of the applications of PLLA-CHIT is the fabrication of scaffolds for tissue engineering.
In the development of bone-regenerating structures, PLLA provides mechanical resistance, and other materials such as polyglycolic acid or polycaprolactone alter degradability or other physical characteristics. Meanwhile, collagen and CHIT improve the biological qualities.8,10 The possibility of mixing PLLA with others materials would confer great versatility to biomedical device development, specifically scaffold fabrication for tissue engineering, with applications in cardiovascular tissue,11 osseous tissue,12 and cartilage.13 Materials featuring incorporated plasticizers such as polyethylene glycol can improve the printing parameters of porous scaffolds,14 which in turn can be used to carry bone-forming cells to the site of interest.15 Similarly flexible scaffolds have been manufactured through electrospinning techniques to transport bioactive substances.16
Depending on the application, resorption of the biomedical device over time according to its function is expected. In the case of scaffolds, it is ideal that resorption of the device is progressive and simultaneous with the apposition of bone tissue. This resorption must be complete, and components harmful to cells should not be produced.
The processes by which PLLA is degraded after implantation and in contact with cells have long been discussed. It is accepted that PLLA is degraded via a hydrolytic mechanism with cellular participation. According to Middleton and Tipton,17 it occurs in two stages. First, water goes through the amorphous phase and attacks the chemical bonds, turning long chains into shorter ones and causing losses of mass, after which the crystalline areas are subsequently affected. In the second stage, enzymatic degradation occurs.
Device degradation occurs both on the surface and internally. Because of initial contact with corporal fluids, ester links are attacked, producing lactic acid and oligomers.18 This superficial erosion allows the creation of cracks and pores that facilitate the diffusion of fluids toward the interior, forming internal cavities in which the hydrolytic degradation process will continue. The degradation process is the same in vitro and in vivo until the release of the oligomers. Under laboratory conditions, the device is eroded and fractioned until it is reduced to lactic acid.
Under in vivo conditions, the presence of PLLA fragments may induce inflammatory responses once the device is implanted. The response can become more severe,19 or the degradation process continues simultaneously with the foreign body reaction. In the foreign body reaction, immune cells attempt to phagocyte the foreign body (device or PLLA fragment) and limit damage by surrounding it with a fibrous capsule.18 For absorbable biomedical devices, the inflammatory response occurs simultaneously with hydrolytic degradation, dividing the structure into smaller particles that are phagocytosed by inflammatory cells, in which elimination of the waste occurs.
Materials and methods
Scaffold preparation, culture, and implantation
Rigid and flexible scaffolds were prepared using PLLA (NatureWorks, Minnetonka, MN, USA) with a weight of 85,000 g/mol. Rigid scaffolds consisted of 5-mm-diameter, 1-mm-thick PLA porous structures mixed with NaCl (74–177 µm) that were subjected to fusion molding under a pressure of 1000 psi and temperature of 170°C. The NaCl particles were subsequently removed via lixiviation to achieve interconnected porosity exceeding 75%. Once the porous conformation was obtained, 0.5% CHIT (Sigma-Aldrich) was incorporated via pressure injection. Flexible scaffolds were obtained via coaxial electrospinning to incorporate CHIT (2% w/w in 1% acetic acid) as a coating for the PLLA fibers.
Rigid and flexible scaffolds were cultured with osteoblasts for 15 days in an estrogenic culture medium under an atmosphere of 5% CO2, with the medium changed every 3 days, to obtain a preliminary deposit of bone extracellular matrix. Once the formation of extracellular matrix was verified by scanning electron microscopy (SEM), the synthetic extracellular matrices were implanted for 3 months in the parietal bones of 40 Wistar rats, in which critical size defects (5 mm in diameter) were created using an empty defect as a control. The size of the sample followed the ISO standard 10993-6 (test animals and implantation sites) After 3 months, the samples were recovered, fixed in a 2% PBS-glutaraldehyde solution, and processed to evaluate the degradation/resorption of the polymeric matrices and bone tissue neoformation via SEM and histochemistry.
This research was approved by the animal ethics review committee of Universidad of Valle in Cali, Colombia, according to the endorsement of the ethics committee CEAS 001-016.
Hydrolytic degradation test
To determine mass loss, a gravimetric test was conducted in which the weight of the samples before immersion in saline solution (Wo) and the wet weight (Wtp) were considered. Each sample was dipped into a flask containing a saline solution and then introduced into a degradation camera at 37°C. The mass loss was calculated using the following equation:
Results
Preliminary characterization of polymeric scaffolds
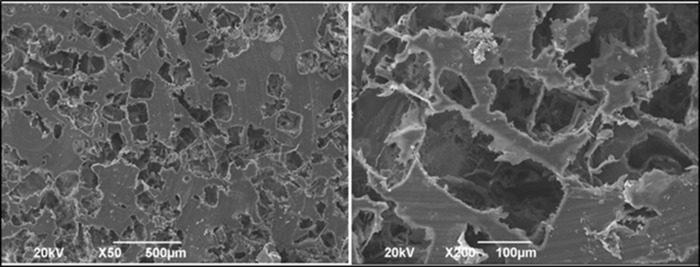
Through SEM analysis (JEOL, model JSM 6490LV), the device morphology was observed. Figures 1 and 2 present the morphological characteristics of both types of scaffolds.
Figure 1.
Rigid scaffolds.
The morphology of rigid scaffolds was assessed using scanning electron microscopy. The porous distribution is appreciated at ×50 magnification, and at greater magnification, the surface irregularity and porous interconnectivity are observed.
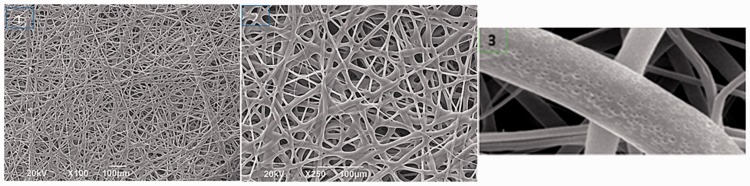
Figure 2.
Flexible scaffolds.
The morphology of flexible scaffolds was assessed using scanning electron microscopy. Interfibrillary spaces are appreciated, and in the foreground, the surface texture is characterized by its nanoporosity. Image 1: ×100; Image 2: ×250; Image 3: ×400.
Hydrolytic degradation
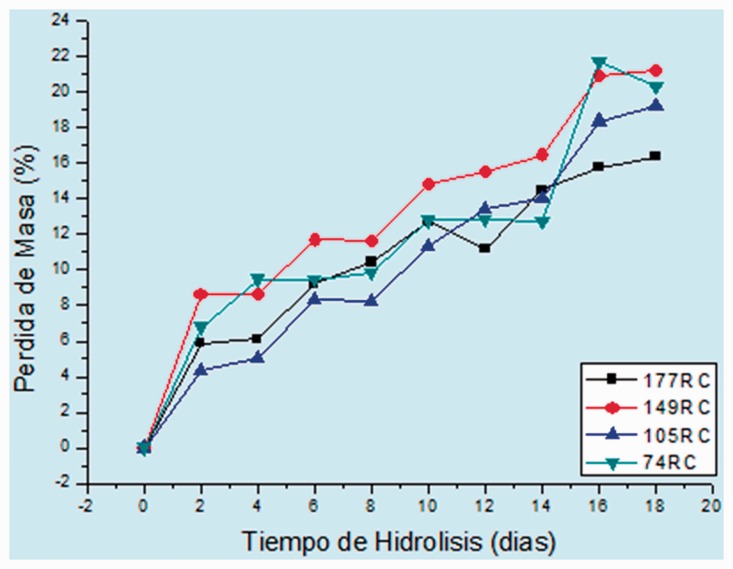
The degradation behavior was similar in all cases, and it was characterized by an extremely large loss of mass in the first days, which is explained by the initial attack on the amorphous portion (Figures 3 and 4).
Figure 3.
Hydrolytic degradation of rigid scaffolds.
Hydrolytic degradation curves for rigid scaffolds.
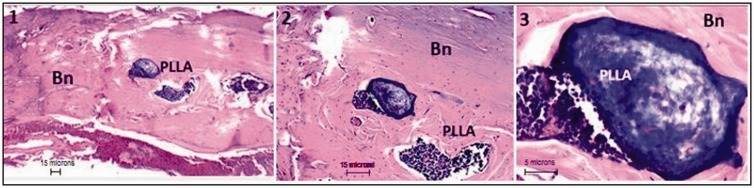
Figure 4.
Degradation/apposition of rigid scaffolds.
PLLA-CHIT rigid scaffold 3 months after implantation. Bn: neoformed bone; PLLA: poly-l-lactic acid; CHIT: chitosan. H&E technique at ×4 (image 1), ×10 (image 2), and ×40 (image 3).
In vivo degradation
After 3 months of implantation in intra-bone preparations, the samples exhibited reabsorption of the material and 65% defect regeneration. Most of the material had been replaced by newly formed bone, as shown in Figure 4. The resorption/apposition process appeared to occur in a centripetal pattern, with some scaffold fragments remaining at the center of the defect. Strong cellular activity was observed at higher magnification.
At higher magnification, it was observed that the scaffold fragments were surrounded by a pseudocapsule delimited by mixed cells (mono- and multinucleated) via phagocytosis of smaller portions of material as well as neoformed bone replacing the material that had been completely reabsorbed (Figure 5). When flexible scaffolds were used, the degradation/resorption had a similar behavior featuring almost complete resorption of the polymeric material with persistence of fragments in the center of the preparation and at the surface in contact with skin.
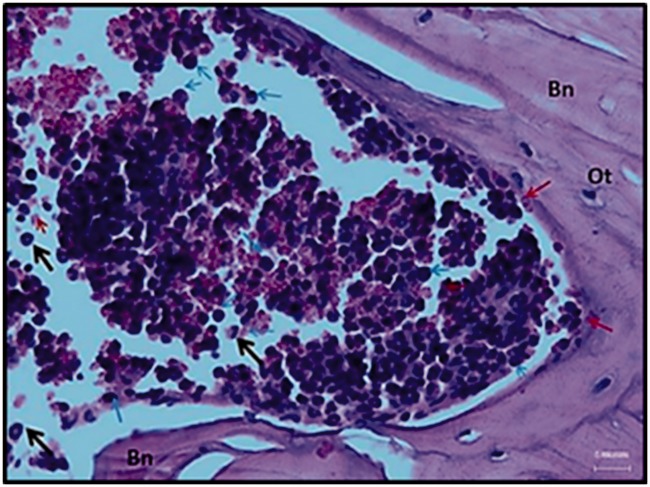
Figure 5.
Degradation zone/resorption of a rigid poly-l-lactic acid-chitosan scaffold.
Rigid scaffold 3 months after implantation. The remaining fragments are in the degradation/resorption process. Bn: Neoformed bone; neutrophils (black arrow); monocytes (blue arrow); megakaryocytes (red arrow). Abundant erythrocytes are observed (pink). Hematoxylin and eosin technique at ×40.
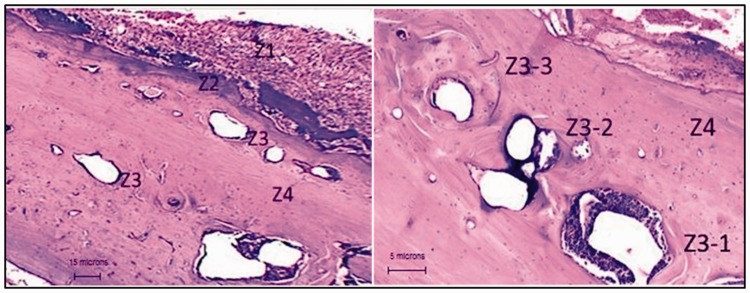
As shown in Figure 6, four different zones could be identified in the implant site. Z1 displays a large number of polymeric fibers (area close to skin). Z3-1 corresponds to a large PLLA-CHIT fragment with a pseudocapsule and large inflammatory infiltrate. Z2 corresponds to the interface between the neoformed bone and the persistent fibers. Z3 and Z3-3 reveal the manner by which some PLLA-CHIT portions are degraded and replaced by neoformed bone tissue, and Z4 presents the complete resorption of the material and its replacement by neoformed tissue with normal characteristics.
Figure 6.
Resorption zone/flexible scaffold neoformation.
Grafted area with a flexible scaffold of poly-l-lactic acid-chitosan. Hematoxylin and eosin technique. The different zones described in the text are appreciated. The materials on the left at ×10 and on the right at ×40 are in different stages of degradation.
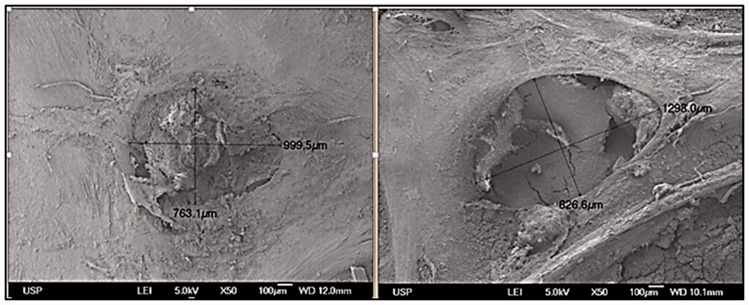
Figure 7 shows a preparation grafted with a rigid scaffold. An extremely small zone of the preparation that had not been regenerated is appreciated, and the rest of the surface did not differ from normal bone tissue. In the center of the defect, some spaces in which the material was partially reabsorbed were noted. At higher magnification (Figure 8), osteoblasts were observed forming extracellular matrix in areas in which the material had been resorbed.
Figure 7.
Resorption zone of the rigid scaffolds.
Rigid poly-l-lactic acid-chitosan scaffold implanted into the parietal bone of a Wistar rat. Scanning electron microscopy. Remnant material in the center of the preparation is observed.
Figure 8.
Information zone of the rigid scaffold.
Rigid poly-l-lactic acid-chitosan scaffold implanted in the parietal bone of a Wistar rat. Scanning electron microscopy. Osteoblasts (Ob) are depositing extracellular matrix (Mx) in an area in which the polymeric material was completely reabsorbed. Bn: normal bone. Corresponds to a magnification of Figure 7.
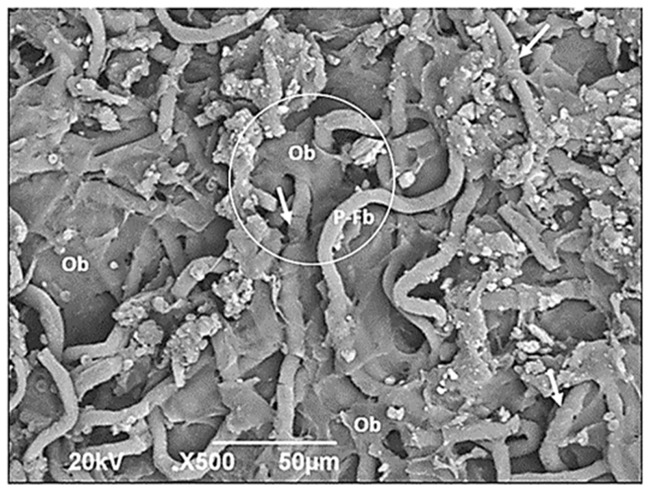
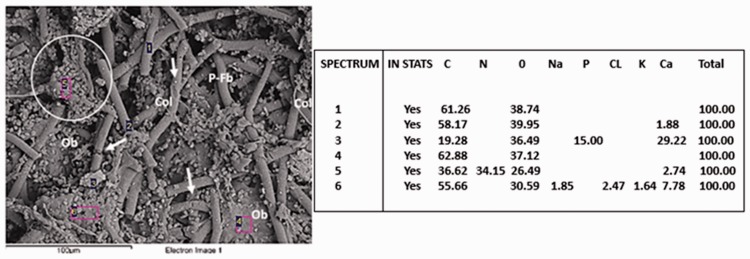
Figure 9 shows osteoblasts using the polymeric fibers as a provisional matrix and filling the interfibrillary spaces. PLLA-CHIT fibers can be observed in the degradation process. Meanwhile, pH variations did not appear to affect the biocompatibility; in fact, the images obtained via SEM revealed osteoblasts located on the PLLA fibers, some of them exhibiting clear degradation (Figures 9 and 10, circle areas). Nevertheless, they continued to produce abundant extracellular matrix with the presence of calcium, phosphorus, and nitrogen, as shown by the elementary analysis (Figure 10).
Figure 9.
Neoformation zone in flexible scaffolds.
Flexible poly-l-lactic acid (PLLA)-chitosan scaffold implanted into the parietal bone of a Wistar rat (scanning electron microscopy ×500). Ob: osteoblast; P-Fb: PLLA fibers; Arrows: degrading PLLA fibers. The circle shows an osteoblast on a PLLA fiber.
Figure 10.
Deposit of extracellular matrix in flexible scaffolds.
Flexible poly-l-lactic acid (PLLA)-chitosan scaffold implanted into the parietal bone of a Wistar rat. Ob: osteoblast; P-Fb: PLLA fibers; Arrows: Degrading PLLA fibers. The circle shows an osteoblast on a PLLA fiber with abundant calcium content, in addition to phosphorus and nitrogen. Scanning electron microscopy/energy dispersive spectroscopy ×500.
Discussion
Hydrolytic in vitro degradation tests represent a good starting point, but it should be remembered that once the device is implanted, the immune response of the organism attempts to reabsorb the material through cellular mechanisms. The preliminary characterization revealed an important loss of mass in the initial days after implantation, reflecting normal behavior given that the first stage of hydrolysis occurs in amorphous zones, which by their disorganized nature facilitate liquid diffusion, whereas the second stage occurring in crystalline areas is slower.20
In porous scaffolds, the characteristics of the surface make the initial attack at the amorphous zone easier, thereby increasing the porosity, followed by fractionation of the polymeric structure. Other processes occur simultaneously. There is a decrease in pH as the bonds in the polymeric chain are broken and the more crystalline portions are subjected to cellular activity in an attempt to degrade them, accompanied by the presence of blood vessels and bone cells.21
The centripetal pattern of resorption/neoformation was previously described by Valencia et al,22,23 who also reported that bone formation occurs simultaneously with the resorption of PLLA. Whereas the degradation/resorption of the material in rigid scaffolds appears to occur simultaneously with bone neoformation, in flexible scaffolds, the process displays small differences. First, extracellular matrix is deposited into the interfibrillary spaces, and then resorption of the polymeric fibers occurs while maintaining the centripetal pattern. A layer of non-degraded fibers was observed on the surface next to skin (Figure 6), which is understandable because this is a relatively avascular zone.
The initial mechanism of polymer degradation appears to be hydrolytic. In both rigid and flexible scaffolds, erosion on the surface occurs first, and as access to the smaller portions is achieved, particles that will be phagocytosed by inflammatory cells in a similar process as described for foreign bodies are released.24
The first part of the process has been studied using hydrolytic degradation assays. According to Elsawy et al.,25 the degradation of PLLA solid structures can occur through two mechanisms: heterogeneous surface reactions and erosion in block or homogeneous. Via either mechanism, once the initial attack of the ester groups occurs, the number of carboxyl terminal groups tends to increase, autocatalyzing hydrolysis of the ester unions.
The autocatalysis process is responsible for the escape of soluble oligomers from the polymeric matrix, inducing the foreign body inflammatory response observed in this study. Conversely, non-soluble oligomers or those that fail to escape from the interior of the matrix would cause a decline in pH and therefore acidification of the medium,25 thereby affecting the biocompatibility through lactic acid accumulation and inducing an extremely strong inflammatory response.19
In relation to pH-related cytotoxicity, different authors have suggested the influence of other factors. According to Solís et al.,20 the decrease in pH is smaller when a high-molecular-weight product is used because it has a more chemically stable structure, thus slowing the degradation process. According to Stankwvich et al.,26 the cytotoxicity attributed to the increase in acidity occurs only if the organism cannot eliminate the acid accumulated via rapid degradation of the material. Ramot et al.27 reported that the organism can reverse the decline in pH that can reach a value of 4–5, and additionally, the complications reported for PLLA orthopedic devices are scarce (less than 10% according to Stankevich et al.)26
Proposed model
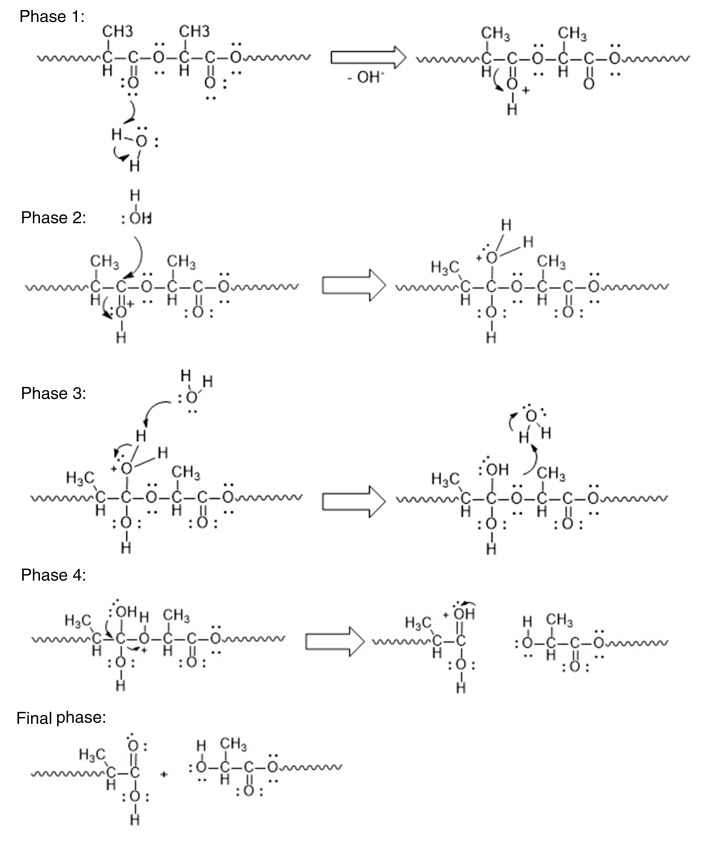
This model integrates the events that occur once the PLLA device is implanted in a living organism. Initially, exposure to water present in the extracellular fluid triggers the hydrolytic degradation described in the diagrams of phases in Figure 11, in which the polymeric chain is attacked on its ester linkages and fragmented into oligomers of variable size, and this process is repeated until (oligomers of reduced size or lactic acid are obtained (Phase 1–4).
Figure 11.
Hydrolytic degradation of polylactic acid.
The attack on the material occurs in a random manner, initially on its surface, producing erosion of the material with detachment of small fragments, but also inside the material, taking advantage of the presence of pores and the formation of cracks because of erosion of the surface. The hydrolytic degradation process induces a decrease in pH, which changes the inflammatory response from acute to chronic, with phagocytic cells surrounding the material in a pseudocapsule in an attempt to limit the damage.
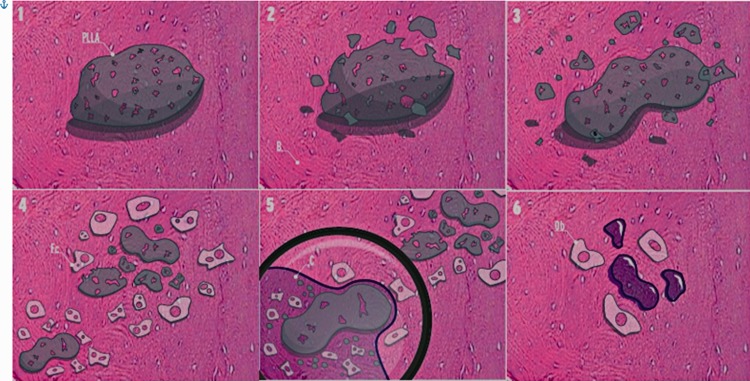
In Figure 12, image 1 represents a PLLA device implanted in bone tissue. In images 2 and 3, superficial erosion occurs, and fragmentation is caused by the hydrolytic degradation process. In image 4, the need to reabsorb the fragments and the decrease in pH attributed to hydrolytic degradation induce an inflammatory response. Image 5 corresponds to a magnification of the lower left corner of image 4 with the formation of a pseudocapsule surrounding a large PLLA fragment and the presence of a mixed inflammatory infiltrate. In image 6, most of the material has been degraded and phagocytosed by inflammatory cells, and some persisting small fragments correspond to the central portions of the device and the newly formed bone tissue.
Figure 12.
Reabsorption of a polylactic acid device.
The degradation sequence of PLLA and the phagocytosis process are presented, as described in the text. PLLA: Elaborated poly-l-lactic acid device; B: Bone; Fc: Phagocytic cells; C: Capsule; b: Osteoblasts.
Recommendations
Within the limitations of the research, a mechanism was proposed through which degradation and reabsorption of the PLA device implanted in intraosseous preparations occur. Additional investigations are recommended to characterize the inflammatory response at different implantation times.
Conclusion
Devices fabricated using PLA were reabsorbed through a mechanism of hydrolytic degradation with a phagocytic process for both rigid and flexible scaffolds. The resorption pattern was a centripetal type from erosion and initial fragmentation on its surface. The decrease in pH caused by the hydrolytic degradation did not affect cellular viability, and apposition of new bone tissue was simultaneously observed with resorption of the material.
Acknowledgments
The author acknowledges Professor Hector Fabio Zuluaga of the University of Valle for reviewing this paper.
Declaration of conflicting interest
The author declares that there is no conflict of interest.
Funding
The author acknowledges the Vice-rectory for Research of the University of Valle for funding this research.
References
- 1.Lasprilla AJ Martinez GA Lunelli BH et al.. Poly-lactic acid synthesis for application in biomedical devices - A review. Biotechnol Adv 2012; 30: 321–328. Available from: 10.1016/j.biotechadv.2011.06.019 [DOI] [PubMed] [Google Scholar]
- 2.Tyler B Gullotti D Mangraviti A et al.. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv Drug Deliv Rev 2016; 107: 163–175. Available from: 10.1016/j.addr.2016.06.018 [DOI] [PubMed] [Google Scholar]
- 3.Dorati R Colonna C Tomasi C et al.. Design of 3D scaffolds for tissue engineering testing a tough polylactide-based graft copolymer. Mater Sci Eng C Mater Biol Appl 2014; 34: 130–139. Available from: 10.1016/j.msec.2013.08.037 [DOI] [PubMed] [Google Scholar]
- 4.Balakrishnan H Hassan A Wahit M et al.. Novel toughened polylactic acid nanocomposite: mechanical, thermal and morphological properties. Mater Des 2010; 31: 3289–3298. Available from: 10.1016/j.matdes.2010.02.008 [DOI] [Google Scholar]
- 5.Mano JF, Hungerford G, Ribelles JLG. Bioactive poly(L-lactic acid)-chitosan hybrid scaffolds. Mater Sci Eng C 2008; 28: 1356–1365. [Google Scholar]
- 6.Liu C, Xia Z, Czernuszka JT. Review paper three-dimensional scaffolds for tisse engineering. Trans IChemE, Part A, Chem Eng Res Des 2001; 85: 1051–1064. [Google Scholar]
- 7.Mourya VK, Inamdar NN. Chitosan-modifications and applications : opportunities galore. React Funct Polym. 2008; 68: 1013–1051. [Google Scholar]
- 8.Serra IR Fradique R Vallejo MC et al.. Production and characterization of chitosan/gelatin/β-TCP scaffolds for improved bone tissue regeneration. Mater Sci Eng C Mater Biol Appl 2015; 55: 592–604. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0928493115301247 [DOI] [PubMed] [Google Scholar]
- 9.Elsawy MA Kim KH Park JW et al.. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew Sustain Energy Rev 2017; 79: 1346–1352. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1364032117307876 [Google Scholar]
- 10.Wade R, Burdick J. Advances in nanofibrous scaffolds for biomedical applications: from electrospinning to self-assembly. Nano Today 2014; 9: 722–742. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1748013214001364 [Google Scholar]
- 11.Wang L Wu Y Hu T et al.. Acta Biomaterialia Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomater 2017; 59: 68–81. Available from: 10.1016/j.actbio.2017.06.036 [DOI] [PubMed] [Google Scholar]
- 12.Riches P, Jia L, Turnbull G, et al. Bioactive Materials 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018; 3: 278–314. Available from: doi: 10.1016/j.bioactmat.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armiento AR Stoddart MJ Alini M et al.. Acta Biomaterialia Biomaterials for articular cartilage tissue engineering : learning from biology. Acta Biomater 2018; 65: 1–20. Available from: 10.1016/j.actbio.2017.11.021 [DOI] [PubMed] [Google Scholar]
- 14.Serra T Ortiz-hernandez M Engel E et al.. Relevance of PEG in PLA-based blends for tissue engineering 3D-printed scaffolds. Mater Sci Eng C Mater Biol Appl 2014; 38: 55–62. Available from: 10.1016/j.msec.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 15.Diomede F, Gugliandolo A, Cardelli P, et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles : a new tool for bone defect repair. Stem Cell Res Ther. 2018; 9: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cianci E, Trubiani O, Diomede F, et al. Immobilization and delivery of biologically active Lipoxin A 4 using electrospinning technology. Int J Pharm 2016; 515: 254–261. Available from: 10.1016/j.ijpharm.2016.09.077 [DOI] [PubMed] [Google Scholar]
- 17.Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000; 21: 2335–2346. [DOI] [PubMed] [Google Scholar]
- 18.Silva D da, Kaduri M, Poley M, et al. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem Eng J 2018; 340: 9–14. Available from: http://linkinghub.elsevier.com/retrieve/pii/S138589471830010X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.X. Wang. Overview on biocompatibilities of implantable biomaterials. In: Pignatello R, editor. Advances in biomaterials science and applications in biomedicine, London, IntechOpen, 2013. p. 112–54.
- 20.Solis Y, Betancourth C, Valencia C, et al. Implantación de un relleno de ácido poliláctico para regeneración ósea. Inf Técnico 2009; 73: 6–15. Available from: http://biblioteca.sena.edu.co/exlibris/aleph/u21_1/alephe/www_f_spa/icon/45896/Informador73/paginas/implantacion/pagina2.html [Google Scholar]
- 21.Valencia C. Descripción de cambios histologicos en respuesta a una matriz de ácido poliláctico implantada en tibias de conejo. Rev Odontos. 2008; 30: 4–6. [Google Scholar]
- 22.Valencia C, Pastrana E, Arroyave E, et al. “Determinación del tiempo de reabsorción de una matriz de ácido poliláctico utilizada como substituto óseo en cavidades preparadas en tibias de conejo”. Rev Odontos. 2008; 30: 29. [Google Scholar]
- 23.Valencia C, Pastrana E, Arroyave E, et al. “Determinación del tiempo de reabsorción de una matriz de ácido poliláctico utilizada como substituto óseo en cavidades preparadas en tibias de conejo”. Rev Odontos. 2008; 31: 29. [Google Scholar]
- 24.Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin 2015; 33: 497–523. Available from: 10.1016/j.det.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 25.Elsawy MA Kim KH Park JW et al.. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew Sustain Energy Rev 2017; 79: 1346–1352. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1364032117307876 [Google Scholar]
- 26.Stankevich KS, Gudima A, Filimonov VD, et al. Surface modification of biomaterials based on high-molecular polylactic acid and their effect on inflammatory reactions of primary human monocyte-derived macrophages: perspective for personalized therapy. Mater Sci Eng C Mater Biol Appl 2015; 51: 117–126. Available from: 10.1016/j.msec.2015.02.047 [DOI] [PubMed] [Google Scholar]
- 27.Ramot Y, Haim-Zada M, Domb AJ, et al. Biocompatibility and safety of PLA and its copolymers. Adv Drug Deliv Rev 2016; 107: 153–162. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0169409X16300989 [DOI] [PubMed] [Google Scholar]