Short abstract
Objective
To investigate the role of alteplase, a widely-used thrombolytic drug, in platelet function.
Methods
Human platelets were incubated with different concentrations of alteplase followed by analysis of platelet aggregation in response to adenosine diphosphate (ADP), collagen, ristocetin, arachidonic acid or epinephrine using light transmittance aggregometry. Platelet activation and surface levels of platelet receptors GPIbα, GPVI and αIIbβ3 were analysed using flow cytometry. The effect of alteplase on clot retraction was also examined.
Results
This study demonstrated that alteplase significantly inhibited platelet aggregation in response to ADP, collagen and epinephrine in a dose-dependent manner, but it did not affect ristocetin- or arachidonic acid-induced platelet aggregation. Alteplase did not affect platelet activation as demonstrated by no differences in P-selectin levels and PAC-1 binding being observed in collagen-stimulated platelets after alteplase treatment compared with vehicle. There were no changes in the surface levels of the platelet receptors GPIbα, GPVI and αIIbβ3 in alteplase-treated platelets. Alteplase treatment reduced thrombin-mediated clot retraction.
Conclusions
Alteplase inhibits platelet aggregation and clot retraction without affecting platelet activation and surface receptor levels.
Keywords: Alteplase, platelet aggregation, activation, surface receptors, clot retraction
Introduction
Platelets are enucleate cells that play an important role in the regulation of thrombosis and haemostasis.1,2 At the site of vascular injury, circulating platelets adhere and firmly attach to the subendothelial matrix through a membrane glycoprotein (GP) receptor complex known as GPIb-IX-V, which binds to von Willebrand factor (vWF); and another, GPVI, which binds to collagen.3 After binding to their respective ligand, platelet intracellular signalling pathways are activated, leading to a change in platelet shape, granule release and finally integrin αIIbβ3 activation, which binds to fibrinogen, fibronectin or vWF and mediates platelet aggregation (known as αIIbβ3 inside-out signalling).4–6 Engagement of fibrinogen or other ligands also triggers αIIbβ3 outside-in signalling transduction and subsequent platelet spreading and clot retraction as well as thrombus stabilization.7,8 This process is critical for preventing blood loss (i.e. haemostasis). Abnormal platelet function is closely associated with bleeding or thrombosis.9,10
Alteplase is a thrombolytic drug widely used for the treatment of acute myocardial infarction with ST-elevation,11 massive pulmonary embolism,12 occluded central venous access devices13 or other severe blood clotting disorders.14,15 The main mechanism by which alteplase exerts its thrombolytic effect is thought to be the catalytic conversion of plasminogen to plasmin after binding to fibrin-rich clots and plasmin in turn degrades the fibrin matrix of the formed thrombi, resulting in thrombolysis.16 Given that platelets play an important role in the formation of both arterial and venous thrombosis,1,2 whether alteplase affects platelet function remains poorly understood. This present study aimed to investigate the role of alteplase in platelet function by incubating human platelets with different concentrations of alteplase.
Materials and methods
Reagents
Alteplase was purchased from Boehringer Ingelheim (Ingelheim am Rhein, Germany). Adenosine diphosphate (ADP), collagen, ristocetin, arachidonic acid and epinephrine were purchased from Helena Laboratories (Beaumont, TX, USA). Collagen-related peptide (CRP) was prepared as previously described.17 Fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD41a and FITC-conjugated mouse anti-human PAC-1 antibodies were purchased from BD Biosciences (San Jose, CA, USA) and Becton Dickinson (San Jose, CA, USA), respectively. Phycoerythrin (PE)-conjugated mouse anti-human CD62p (P-selectin) and purified mouse anti-human glycoprotein (GP) VI antibody were purchased from eBioscience (San Diego, CA, USA). FITC-conjugated mouse anti-human CD42b antibody was purchased from Abcam (Cambridge, MA, USA). FITC-conjugated goat anti-mouse IgG was purchased from ZSGB-BIO (Beijing, China). Thrombin was purchase from Sigma-Aldrich (St Louis, MO, USA).
Platelet preparation
Human platelets were isolated as previously described.17,18 Briefly, venous blood from a healthy donor, who had not ingested aspirin or other medications in the preceding 10 days, was collected into 3.2% (w/v) trisodium citrate using a 19-gauge winged infusion kit and centrifuged for 20 min at 120 g at room temperature using an Allegra® X-15R benchtop centrifuge (Beckman Coulter Life Sciences, Indianapolis, IN, USA) to obtain platelet-rich plasma (PRP). Platelet-poor plasma (PPP) was obtained by centrifuging PRP at 1350 g using an Allegra® X-15R benchtop centrifuge (Beckman Coulter Life Sciences) for 15 min at room temperature. All procedures involving collection of human blood were approved by the Medical Ethics Committee of the Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu Province, China. Written informed consent had been obtained and all clinical investigations were conducted in accordance with the ethical standards and according to the principles expressed in the Declaration of Helsinki.
Platelet aggregation
Platelet aggregation was performed in human citrated PRP. After treatment with different concentrations of alteplase (1, 10, 100 and 200 μg/ml) for 60 min at 37°C, human platelet aggregation (5 × 107) in response to ADP (5 μM), collagen; 2.5 μg/ml), ristocetin (1.5 mg/ml), arachidonic acid (500 μg/ml) or epinephrine (75 μM) was determined by light transmittance aggregometry (AggRAM™ System; Helena Laboratories) as previously described.17,18 PPP was used as a negative control. Platelet aggregation was defined as the percentage of maximum platelet aggregation (monitored for 5 min).
Platelet activation
Platelet activation was assessed by detecting the surface levels of the α-granule glycoprotein P-selectin and by the activation-dependent binding of PAC-1 to platelet αIIbβ3 as described previously.17 Human citrated PRP (5 × 105) was incubated with different concentrations of alteplase (1, 10, 100 or 200 μg/ml) for 60 min at 37°C followed by CRP (10 μg/ml) stimulation for 15 min. After that, PE-conjugated mouse anti-human P-selectin antibody (2.5 μg/ml) or FITC-conjugated mouse anti-human PAC-1 antibody (1 μg/ml) was added and incubated for 15 min followed by analysis of platelet activation by flow cytometry (BD LSRFortessa™; BD Biosciences).
Levels of platelet surface receptors
The levels of platelet surface receptors, GPIbα, GPVI and αIIbβ3 were measured using flow cytometry. After treatment with different concentrations of alteplase (1, 10, 100 or 200 μg/ml) for 60 min at 37°C, human citrated PRP (5 × 105) was incubated with FITC-conjugated mouse anti-human CD42b antibody (GPIbα; 1 μg/ml), FITC-conjugated mouse anti-human CD41a antibody (αIIb; 10 μl/test) or mouse anti-human GPVI antibody (2.5 μg/ml) for 30 min at 37°C followed by analysis of the levels of these receptors using flow cytometry (BD LSRFortessa™; BD Biosciences). Detection of GPVI was achieved using FITC-conjugated goat anti-mouse IgG as described previously.17,18 As platelet agonists such as CRP have been reported to induce the ectodomain shedding of platelet receptor GPIbα and GPVI, leading to reduced surface levels of platelet receptors,19 stimulation was not performed for measuring the surface levels of platelet receptors.
Clot retraction
After treatment with different concentrations of alteplase (1 or 10 μg/ml) for 60 min at 37°C, human citrated PRP (3 × 108/ml) was supplemented with 2 mM Ca2+ and 0.5 mg/ml fibrinogen (Sigma-Aldrich) and clot retraction was initiated by thrombin (1 U/ml) (Sigma-Aldrich) stimulation at 37°C. Images were captured every 15 min.
Statistical analyses
Data were analysed using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA) and are presented as mean ± SD. One-way analysis of variance with Newman–Keuls multiple comparison post-hoc analysis was performed to compare the differences among multiple groups. A P < 0.05 was considered statistically significant.
Results
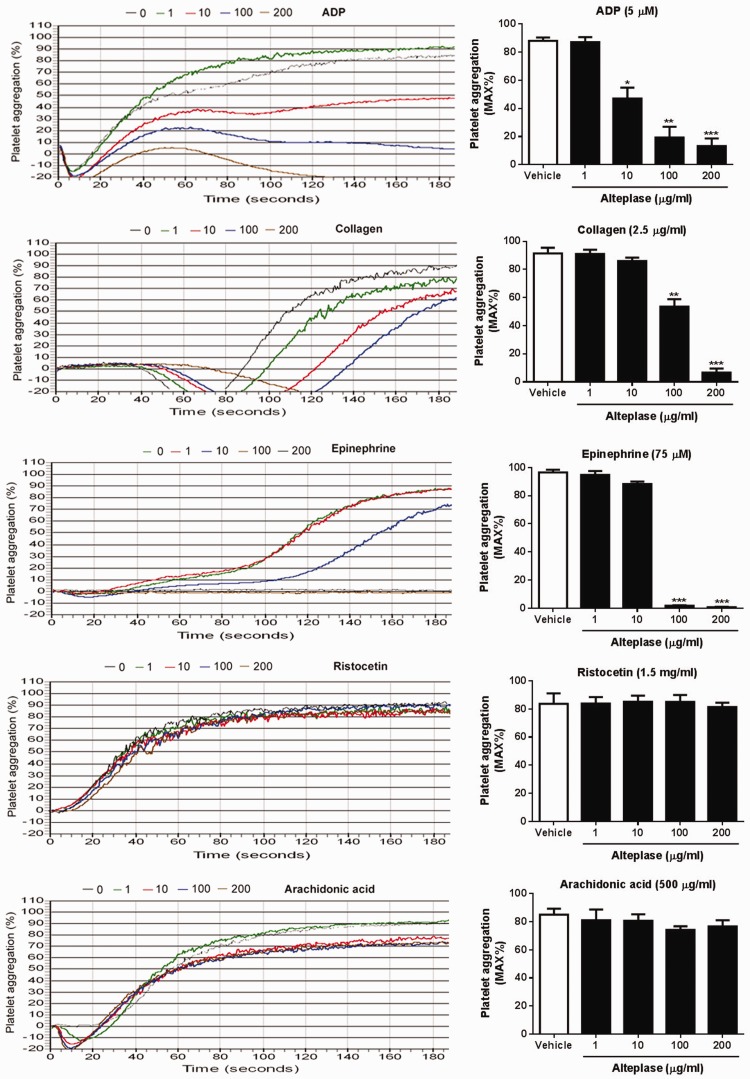
To evaluate whether alteplase plays a role in platelet aggregation, PRP was incubated with different concentrations of alteplase and a platelet aggregation assay was performed using light transmittance aggregometry. As seen in Figure 1, alteplase significantly inhibited platelet aggregation in response to ADP (5 μM), collagen (2.5 μg/ml) and epinephrine (75 μM) in a dose-dependent manner (P < 0.05 for some concentrations of alteplase). However, alteplase did not affect ristocetin- or arachidonic acid-induced platelet aggregation even at a higher dose (200 μg/ml).
Figure 1.
Effect of alteplase treatment on platelet aggregation. Platelet aggregation was performed in human citrated platelet rich plasma. After treatment with different concentrations of alteplase (1, 10, 100 and 200 μg/ml) for 60 min at 37°C, human platelet aggregation (5 × 107) in response to adenosine diphosphate (ADP; 5 μM), collagen (2.5 μg/ml), ristocetin (1.5 mg/ml), arachidonic acid (500 μg/ml) or epinephrine (75 μM) was determined by light transmittance aggregometry. Data presented as mean ± SD; *P < 0.05; **P < 0.01; ***P < 0.001; compared with vehicle; one-way analysis of variance with Newman–Keuls multiple comparison post-hoc analysis. The colour version of this figure is available at: http://imr.sagepub.com.
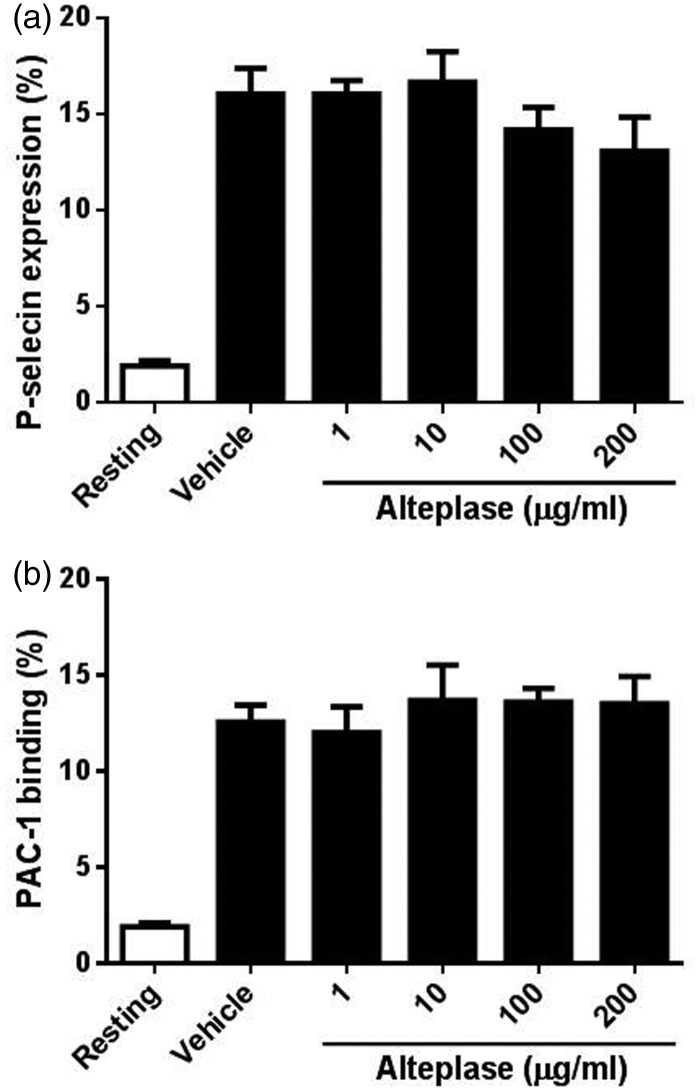
As platelet activation occurs upstream of platelet aggregation, the study assessed whether the reduced platelet aggregation induced by alteplase was due to insufficient platelet activation by measuring P-selectin levels and αIIbβ3 activation using flow cytometry. As seen in Figure 2, no significant changes of P-selectin levels (Figure 2a) and αIIbβ3 activation (PAC-1 binding) (Figure 2b) were found in collagen-stimulated platelets after alteplase treatment, indicating that alteplase does not affect platelet activation.
Figure 2.
Effect of alteplase treatment on platelet activation. Platelet activation was assessed by detecting the surface levels of the α-granule glycoprotein P-selectin and by the activation-dependent binding of PAC-1 to platelet αIIbβ3. Human platelets (5 × 105) were incubated with different concentrations of alteplase (1, 10, 100 or 200 μg/ml) for 60 min at 37°C followed by stimulation with collagen-related peptide (10 μg/ml) for 15 min. Platelet activation was analysed by flow cytometry using phycoerythrin-conjugated mouse anti-human P-selectin antibody (a) or fluorescein isothiocyanate-conjugated mouse anti-human PAC-1 antibody (b). Data presented as mean ± SD.
Platelet surface glycoprotein receptors, GPIbα, GPVI and αIIbβ3, play an important role in the regulation of aggregation at the site of vascular injury.20,21 The study evaluated whether alteplase affects the levels of platelet surface receptors using flow cytometry and showed that alteplase-treated platelets displayed no significant changes of the surface levels of platelet receptors GPIbα (Figure 3a), GPVI (Figure 3b) and αIIbβ3 (Figure 3c) even at a higher concentration. These findings were consistent with the lack of any effect of alteplase on platelet P-selectin levels and αIIbβ3 activation, suggesting that the alteplase-induced decrease of platelet aggregation was not due to the levels of platelet surface receptors.
Figure 3.
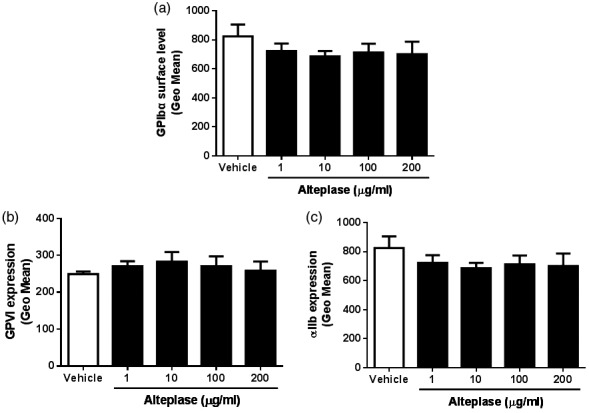
Effect of alteplase treatment on the levels of platelet surface receptors GPIbα, GPVI and αIIbβ3. Following treatment of platelets with different concentrations of alteplase (1, 10, 100 or 200 μg/ml) for 60 min at 37°C, the surface levels of GPIbα, GPVI and αIIbβ3 were measured using flow cytometry and fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD42b antibody (GPIbα; a), mouse anti-human GPVI antibody (detected using FITC-conjugated goat anti-mouse IgG; b) or FITC-conjugated mouse anti-human CD41a antibody (αIIb) (c). Data presented as mean ± SD.
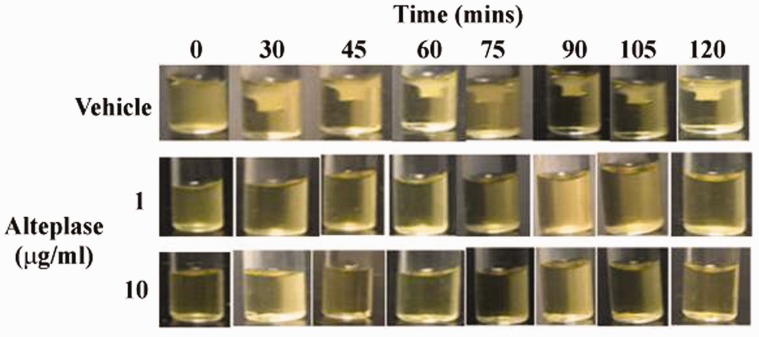
Clot retraction plays an important role in the stabilization of thrombus formation.7 The study also measured the effect of alteplase on clot retraction. As shown in Figure 4, clot retraction was observed in vehicle-treated human platelets over time and alteplase treatment impaired clot retraction.
Figure 4.
Effect of alteplase treatment on clot retraction. Platelets (3 × 108/ml) were supplemented with 2 mM Ca2+ and 0.5 mg/ml fibrinogen. After alteplase treatment (1 or 10 µg/ml) for 60 min at 37°C, clot retraction in was initiated by thrombin (1 U/ml) stimulation at 37°C. Images were captured every 15 min.
Discussion
As a thrombolytic drug, alteplase is widely used for the treatment of acute myocardial infarction with ST-elevation,11 massive pulmonary embolism,12 occluded central venous access devices13 or other severe blood clotting disorders.14,15 Considering the critical role played by platelets in the development and pathogenesis of several blood disorders, whether alteplase affects platelet function remains poorly understood. In this current study, by incubating human platelets with different concentrations of alteplase, it was demonstrated that alteplase treatment significantly reduced platelet aggregation in response to stimulation without affecting platelet activation and the surface levels of key platelet receptors.
Platelets orchestrate thrombosis and haemostasis. In response to vascular injury, circulating platelets adhere to exposed subendothelial matrix through membrane receptors, leading to initiation of intraplatelet signalling transduction and subsequent integrin αIIbβ3 activation, which binds to fibrinogen and mediates platelet aggregation.4 This current study demonstrated that alteplase treatment significantly inhibited platelet aggregation in response to collagen, ADP and epinephrine in a dose-dependent manner, consistent with a previous study that observed reduced platelet aggregation in patients treated with alteplase compared with baseline.22 Interestingly, alteplase treatment did not affect ristocetin-induced platelet aggregation even at a higher dose, consistent with a previous study that demonstrated no significant change of ristocetin-induced platelet aggregation during the first 24 h after alteplase treatment in patients.23 This might be due to ristocetin-induced agglutination of platelets, which is independent of fibrinogen binding.24 In addition, alteplase did not affect arachidonic acid-induced platelet aggregation, which was consistent with a previous study that showed that plasmin treatment of platelets did not inhibit arachidonate-induced platelet aggregation.25
Platelet surface receptors GPIbα, GPVI and αIIbβ3 are critical for platelet adhesion, activation and aggregation as a consequence of them binding to their respective ligands.20 Engagement of platelet receptors GPIbα and GPVI after binding to vWF and collagen, respectively, causes platelet shape change, α-granule release (P-selectin production) and finally results in activation of integrin αIIbβ3, which mediates platelet aggregation.5 As platelet activation also plays an important role in platelet function, this current study also measured whether alteplase affects platelet activation by measuring P-selectin levels and αIIbβ3 activation using flow cytometry. Alteplase treatment does not affect platelet activation in response to collagen stimulation as demonstrated by no significant changes in P-selectin levels and PAC-1 binding. Consistent with these findings, the current study also observed that alteplase treatment did not affect the surface levels of platelet surface receptors GPIbα, GPVI and αIIbβ3.
Ligand binding to αIIbβ3 also triggers outside-in signalling transduction, resulting in phosphorylation of intracellular signalling molecules, such as c-Src, Syk and primary phospholipase Cɣ2, which mediates platelet spreading, clot retraction and thrombus stabilization.7 Clot retraction refers to the process in which activated platelets transduce contractile forces onto the fibrin network of a thrombus, which over time increases clot density and decreases clot size.26 This process has been demonstrated to be important for the promotion of clot stability and maintenance of blood vessel patency.26 In this current study, alteplase treatment significantly inhibited clot retraction, consistent with the role of alteplase in degrading the fibrin matrix of the formed thrombi through processing plasminogen to plasmin.
In conclusion, this current study demonstrated that alteplase inhibits platelet aggregation and clot retraction without affecting platelet activation or the surface levels of platelet surface receptors GPIbα, GPVI and αIIbβ3.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Wang Y, Andrews M, Yang Y, et al. Platelets in thrombosis and hemostasis: old topic with new mechanisms. Cardiovasc Hematol Disord Drug Targets 2012; 12: 126–132. [DOI] [PubMed] [Google Scholar]
- 2.Brass LF, Diamond SL, Stalker TJ. Platelets and hemostasis: a new perspective on an old subject. Blood Adv 2016; 1: 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiao J, Arthur JF, Gardiner EE, et al. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol 2018; 14: 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li ZY, Delaney MK, O'Brien KA, et al. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol 2010; 30: 2341–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nieswandt B, Varga-Szabo D, Elvers M. Integrins in platelet activation. J Thromb Haemost 2009; 7: 206–209. [DOI] [PubMed] [Google Scholar]
- 6.Estevez B, Du X. New concepts and mechanisms of platelet activation signaling. Physiology (Bethesda) 2017; 32: 162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durrant TN, van den Bosch MT, Hers I. Integrin αIIbβ3 outside-in signaling. Blood 2017; 130: 1607–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plow EF, Ma YQ. Inside-out, outside-in: what's the difference? Blood 2007; 109: 3128–3129. [Google Scholar]
- 9.Rao AK, Songdej N. Platelet disorders: the next generation is in. Blood 2016; 127: 2781–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattaneo M. Inherited platelet-based bleeding disorders. J Thromb Haemost 2003; 1: 1628–1636. [DOI] [PubMed] [Google Scholar]
- 11.Sinnaeve P, Alexander J, Belmans A, et al. One-year follow-up of the ASSENT-2 trial: a double-blind, randomized comparison of single-bolus tenecteplase and front-loaded alteplase in 16,949 patients with ST-elevation acute myocardial infarction. Am Heart J 2003; 146: 27–32. [DOI] [PubMed] [Google Scholar]
- 12.Smithburger PL, Campbell S, Kane-Gill SL. Alteplase treatment of acute pulmonary embolism in the intensive care unit. Crit Care Nurse 2013; 33: 17–27. [DOI] [PubMed] [Google Scholar]
- 13.Smith LH. Alteplase for the management of occluded central venous access devices: safety considerations. Clin J Oncol Nurs 2008; 12: 155–157. [DOI] [PubMed] [Google Scholar]
- 14.Desilles JP, Loyau S, Syvannarath V, et al. Alteplase reduces downstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke 2015; 46: 3241–3248. [DOI] [PubMed] [Google Scholar]
- 15.Chang R, Horne MK, 3rd, Shawker TH, et al. Low-dose, once-daily, intraclot injections of alteplase for treatment of acute deep venous thrombosis. J Vasc Interv Radiol 2011; 22: 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adivitiya, Khasa YP. The evolution of recombinant thrombolytics: Current status and future directions. Bioengineered 2017; 8: 331–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiao J, Wu Y, Wu X, et al. An absence of platelet activation following thalidomide treatment in vitro or in vivo. Oncotarget 2017; 8: 35776–35782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao J, Wu Y, Liu Y, et al. Busulfan triggers intrinsic mitochondrial-dependent platelet apoptosis independent of platelet activation. Biol Blood Marrow Transplant 2016; 22: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 19.Qiao JL, Shen Y, Gardiner EE, et al. Proteolysis of platelet receptors in humans and other species. Biol Chem 2010; 391: 893–900. [DOI] [PubMed] [Google Scholar]
- 20.Rivera J, Lozano ML, Navarro-Nunez L, et al. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica 2009; 94: 700–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sangkuhl K, Shuldiner AR, Klein TE, et al. Platelet aggregation pathway. Pharmacogenet Genomics 2011; 21: 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moser M, Nordt T, Peter K, et al. Platelet function during and after thrombolytic therapy for acute myocardial infarction with reteplase, alteplase, or streptokinase. Circulation 1999; 100: 1858–1864. [DOI] [PubMed] [Google Scholar]
- 23.Gurbel PA, Serebruany VL, Shustov AR, et al. Effects of reteplase and alteplase on platelet aggregation and major receptor expression during the first 24 hours of acute myocardial infarction treatment. J Am Coll Cardiol 1998; 31: 1466–1473. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins CS, Clemetson KJ, Luscher EF. Studies on the mechanism of ristocetin-induced platelet agglutination: binding of ristocetin to platelets. J Lab Clin Med 1979; 93: 220–231. [PubMed] [Google Scholar]
- 25.Schafer AI, Adelman B. Plasmin inhibition of platelet function and of arachidonic acid metabolism. J Clin Invest 1985; 75: 456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samson AL, Alwis I, Maclean JAA, et al. Endogenous fibrinolysis facilitates clot retraction in vivo. Blood 2017; 130: 2453–2462. [DOI] [PubMed] [Google Scholar]