Short abstract
Nonalcoholic fatty liver disease (NAFLD) and its pathologically more severe form, nonalcoholic steatohepatitis (NASH), have become prevalent worldwide and carry an increased risk of developing hepatocellular carcinoma and other metabolic diseases. Diverse animal models have been proposed to replicate particular characteristics of NAFLD and NASH and have provided significant clues to the critical molecular targets of NASH treatment. In this review, we summarize the histopathology, pathogenesis, and molecular basis of NAFLD progression and discuss the benchmark animal models of NAFLD/NASH.
Keywords: Nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, histopathology, pathogenesis, dietary model, genetic model
Introduction
Nonalcoholic fatty liver disease (NAFLD) represents a progressive liver disorder ranging from simple liver steatosis to nonalcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and ultimately hepatocellular carcinoma, in the absence of excessive alcohol intake.1,2 NAFLD is becoming one of the most alarming chronic liver diseases because it is one of the fastest growing indicators for adult liver transplantation and a major cause of hepatocellular carcinoma.3–6 Development of NAFLD has a strong association with metabolic abnormalities such as obesity, insulin resistance (IR), and type 2 diabetes, and NAFLD itself is a risk factor for cardiovascular disease. Patients with NAFLD are at high risk of dying from cardiovascular disease and other metabolic diseases.7–9 NASH is the pathologically more severe form of NAFLD and is characterized by hepatocellular ballooning, active hepatocellular necrosis, and liver inflammation with the presence of steatosis; moreover, it is associated with more rapid progression of fibrosis and cirrhosis.10,11 Given the rapid growth in NAFLD prevalence, further research on the exact pathogenic mechanisms and potential drug treatments for NASH is imperative. Established animal models have vividly highlighted the important aspects of each stage of NAFLD and provide significant clues to the critical molecular events during NAFLD development, which opens up new opportunities for treatment of NAFLD in humans. This review will summarize the pathogenesis and molecular basis of NAFLD and discuss the benchmark animal models that recapitulate the histopathology and pathophysiology associated with human NAFLD.
Histopathology of NAFLD and NASH
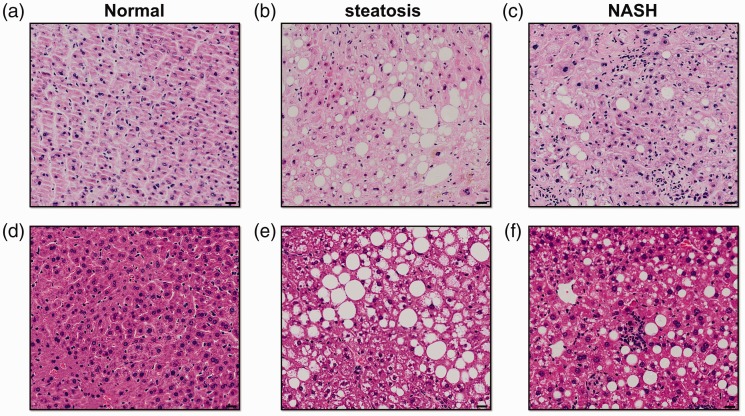
Intracytoplasmic lipid accumulation in the form of triglycerides is an iconic feature of NAFLD. Liver biopsy followed by histological analysis is the gold standard for confirming the presence and activity of NAFLD, which is histologically diagnosed when hepatic triglyceride accumulation occurs in more than 5% of hepatocytes.12 Grading and staging systems for NAFLD consider a wide spectrum of histopathology features. In particular, semiquantitative scoring assesses 4 major histological features: steatosis (0–3), hepatocellular ballooning (0–2), inflammation (0–3), and fibrosis (0–4).13,14 The size of fat droplets can differ; macrovesicular steatosis is the predominant pattern seen in NAFLD and is characterized by large vacuoles that occupy the whole cytoplasm and push the nucleus to one side of the cell. Some NAFLD patients, however, present with multiple small lipid vacuoles in the cytoplasm and the nucleus remains unmoved, which is termed “microvesicular steatosis.”15 Hepatocellular ballooning, which refers to cells with swollen and rarefied cytoplasm, is a distinguishing feature of progression to NASH.16 Hepatocellular ballooning is often associated with Mallory-Denk bodies, which result from the clumping of cytokeratins and subsequent ubiquitination.17 Inflammation is another remarkable feature of NASH development. Lobular inflammation and portal inflammation can both present in NASH. Lobular inflammation, which is characterized by the presence of small clusters of inflammatory cells near ballooned hepatocytes, reflects the dysregulation of cytokine and chemokine expression in the fatty liver.10,14,18 Portal inflammation is common and usually mild in NASH patients. Increased portal inflammation is associated with many clinical and pathologic features of progressive NASH and may be considered a marker of aggravation and advanced disease.19 NASH patients often develop a typical “chicken-wire” fibrosis surrounding individual or groups of hepatocytes that is termed “pericellular fibrosis.” This finding reflects progression of NASH and it can further spread to the portal areas and subsequently lead to septal fibrosis and even cirrhosis.20 Representative haematoxylin and eosin (H&E)-stained sections of human NASH and murine steatohepatitis are shown in Figure 1.
Figure 1.
Histopathological features of human and murine nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) as determined by haematoxylin and eosin (H&E) staining. (a) Healthy human liver; (b) simple liver steatosis in human; (c) human NASH with hepatocellular ballooning and inflammation; (d) healthy murine liver (C57BL/6 mice fed a normal diet); (e) liver of obese ob/ob mice fed a normal diet that spontaneously developed liver steatosis; and (f) liver of C57BL/6 mice fed a methionine- and choline-deficient (MCD) diet for 4 weeks; mice developed steatosis with notable inflammation. Scale bars, 20 µm
Pathogenesis and molecular basis of NAFLD and NASH
The pathogenesis of NAFLD and the factors that promote progression from simple steatosis to NASH are complex. Lipid accumulation in hepatocytes and its interplay with inflammatory responses, cellular stress, and cell death are believed to be the major factors contributing to NAFLD development.21–24 Genetic factors and intestinal dysbiosis are also crucial.25
Steatosis occurs whenever the rate of import or synthesis of lipid by hepatocytes exceeds the rate of export or degradation.26,27 Triglyceride is the most conspicuous type of lipid in the livers of NAFLD patients, so steatosis can be graded according to the extent of triglyceride accumulation. However, the triglycerides are not hepatotoxic compared with the other types of lipids that accumulate in the fat liver (including fatty acids, diacylglycerol, oxysterols, cholesterol, and phospholipids), so steatosis grade or severity does not predict hepatic injury, inflammation, or fibrosis.28,29 Overnutrition and particularly IR are closely associated with the etiology of steatosis and provide the initiating and propagating damage for liver injury and resultant inflammation.30,31 Fatty acid accumulation, in turn, exacerbates IR and hyperinsulinemia, leading to further steatosis and inflammation.32–34 Overnutrition increases free acid influx from diets and consequent uptake by the liver, resulting in increases in de novo lipogenesis in the liver. Overnutrition also induces chronic inflammation and promotes IR.35,36 IR is tightly associated with lipid accumulation in the liver and subsequent steatosis. IR promotes increased efflux of free fatty acids (FFA) from adipose tissues and overwhelms FFA uptake by the liver because insulin cannot suppress adipose tissue lipolysis via hormone-sensitive lipase when IR occurs.36 IR promotes lipid accumulation in the liver primary by mediating uptake of FFA via the scavenger receptor CD36 and uptake of free cholesterol (FC) via CD36 and oxidised low-density lipoprotein (ox-LDL).37 IR-associated hyperinsulinaemia and hyperglycaemia promote hepatic de novo lipogenesis by upregulating the key lipid synthesis regulator sterol regulatory element-binding protein isoform 1c (SREBP-1c) and the glucose metabolism regulator carbohydrate response element-binding protein (ChREBP), respectively.38 In addition, hyperinsulinaemia can directly suppress β-oxidation of FFA.39 More importantly, IR-associated hyperinsulinaemia is implicated in driving the accumulation of cytotoxic lipid species such as FC in the liver and activating the c-Jun N-terminal kinase (JNK) signaling pathway, resulting in mitochondrial damage and hepatocyte injury in a process called “lipotoxicity.”25,40 Molecules released from damaged hepatocytes further promote changes in signaling pathways that regulate cellular stress (such as oxidative stress and endoplasmic reticulum stress) and inflammatory responses, thus perpetuating hepatocellular injury and subsequent cell death and promoting NAFLD development.41–43
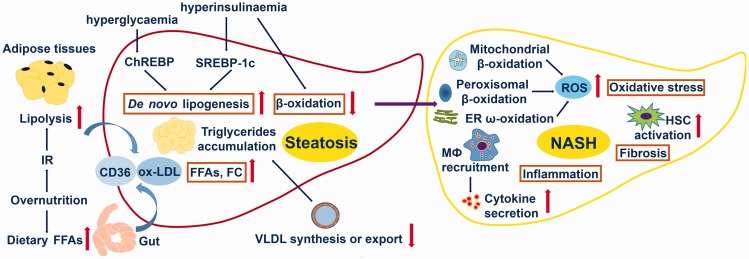
An increase in the production of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, and activation of toll-like receptors (TLR) and the NLRP3 inflammasome are critically involved in pathophysiology of various aspects of NASH.44,45 TLR4 links to the activation of nuclear factor (NF)-ĸB and macrophage recruitment in steatohepatitis.46 The NLRP3 inflammasome, which is highly expressed in liver, is associated with IL-1β release.47 These elements and their interaction perpetuate liver damage, inflammation, and fibrosis, resulting in progression of NASH (Figure 2).
Figure 2.
Major processes in pathogenesis of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). NAFLD is mainly associated with increased hepatic de novo lipogenesis, increased adipose tissue lipolysis, increased efflux of dietary free fatty acids (FFAs) impaired β-oxidation and impaired synthesis or export of very-low-density lipoprotein (vLDL). NASH is mainly associated with increased oxidative stress, activated inflammatory responses, and increased hepatic fibrosis. IR = insulin resistance; ChREBP = carbohydrate response element-binding protein; SREBP-1c = sterol regulatory element-binding protein isoform 1c; FC = free cholesterol; ox-LDL = oxidised low-density lipoprotein; CD36 = cluster of differentiation 36; ER = endoplasmic reticulum; MΦ = macrophage; ROS = reactive oxygen species; HSC = hepatic stellate cells
Dietary and genetic animal models of NAFLD and NASH
High-fat diet
The high-fat diet (HFD) model is a good simulation of the modern Western diet. The main calorie intake (energy) of HFD is derived from fat (45% to 75%). Animals fed with HFD can replicate the major histopathology and pathogenesis seen in human NAFLD. With long-term HFD feeding, animals develop obesity, IR, and hepatic damage.
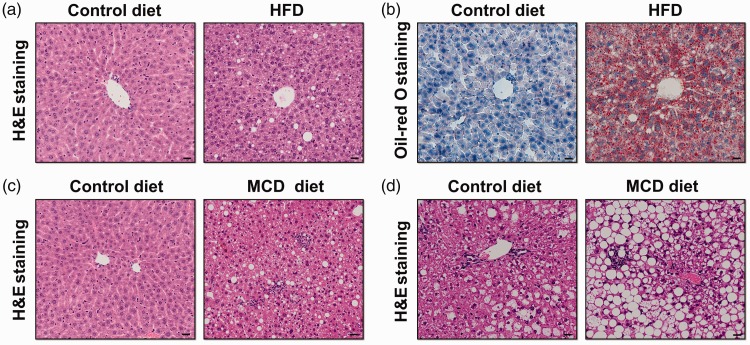
The classic HFD model was established in male rats and involved feeding a diet with 71% fat, 11% carbohydrates, and 18% protein for 3 weeks. A standard diet containing 35% fat, 47% carbohydrates, and 18% protein was used as the control; this diet has the same fat content as the average US diet. Rats fed HFD developed steatosis, IR, mitochondrial dysfunction, and mononuclear inflammation, accompanied by increased hepatic TNF-α and cytochrome P4502E1 (CYP2E1) induction.48 Another frequently used HFD animal model was established in mice. Male mice (C57BL/6 strain) that received the same HFD for up to 16 weeks became obese and showed steatosis, hepatocyte ballooning, increased serum glucose, and decreased adiponectin, indicating hyperglycaemia and IR.49 Similarly, our group found that male C57BL/6 mice fed a HFD (60% fat, 20% carbohydrates, and 20% protein) for 12 weeks developed steatosis (Figure 3a and 3b).
Figure 3.
Histopathological features of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in different murine models as determined by haematoxylin and eosin (H&E) and Oil-red O staining. Haematoxylin and eosin (H&E) (a) and Oil-red O (b) stained sections of C57BL/6 mice fed a control diet or high-fat diet (HFD) for 12 weeks; (c) H&E stained sections of C57BL/6 mice fed methionine- and choline-deficient (MCD) diets for 4 weeks showed steatosis and inflammation compared with those fed a control diet; (d) ob/ob mice fed a control diet spontaneously developed liver steatosis, and those fed MCD diets for 4 weeks developed steatosis with inflammation. Scale bars, 20 µm
HFD diets can replicate the hallmark features of altered metabolic parameters seen in human NAFLD but the degree of hepatic pathology is not as severe. Increasingly, studies use additional elements in the HFD to more closely mimic human NAFLD, such as diets supplemented with fructose, cholesterol, or both.50,51
High-fat, high-fructose diet
A significantly increased consumption of calories from fructose-rich foods has been confirmed to be closely associated with development of human NAFLD and severity of fibrosis.52,53
Male mice (C57BL/6 strain) that were fed a high-fat, high-fructose (HFHF) diet—that is, a HFD (58 kcal% fat) supplemented with 42 g/L of carbohydrates (mixed at a ratio of 55% fructose and 45% sucrose by weight) in drinking water—for 16 weeks developed more severe hepatic oxidative stress, increased hepatic macrophage aggregation, and exacerbated liver fibrosis compared with mice fed HFD without carbohydrate supplementation. However, both groups showed gains in body weight and body fat mass and increased steatosis, fasting glucose, and IR, indicating that fructose consumption is required for NAFLD progression.54
Fructose can promote de novo lipogenesis in liver, inhibits β-oxidation, and induces hepatic insulin, which result in rapid development of intrahepatic lipid accumulation. Excessive consumption of fructose also promotes intestinal bacterial overgrowth and leads to hepatocellular damage, thus triggering NAFLD progression.55
High-fat, high-cholesterol diet
High cholesterol intake can induce dyslipidemia and IR, and it has been recognized as a critical factor associated with hepatic inflammation and NAFLD progression in both animal models and humans.56–58
Male mice (C57BL/6 strain) fed a high-fat, high-cholesterol (HFHC) diet (15% fat and 1% cholesterol) for 30 weeks became obese and developed more profound hepatic steatosis and inflammation, as well as typical perisinusoidal fibrosis compared with mice fed a single HFD or high-cholesterol diet, both of which resulted in increased hepatic steatosis with little inflammation and no signs of fibrosis. Mice fed with HFHC diets also showed hypercholesterolaemia and a significant reduction in serum adiponectin levels.57 Obese foz/foz mice (deficient in the Alms1 gene) fed a HFD containing different percentages of cholesterol (0.0%, 0.2%, or 2.0%) for 24 weeks showed different outcomes. Mice fed with 2.0% cholesterol had higher hepatic cholesterol content and much higher alanine aminotransferase (ALT) levels than other groups, suggesting that increased accumulation of free cholesterol is associated with NAFLD progression.59
Cholesterol increases hepatic oxidative stress and promotes hepatic apoptosis, macrophage recruitment, and fibrogenesis, thus triggering NAFLD progression.
Methionine- and choline-deficient diet
Feeding animals a methionine- and choline-deficient (MCD) diet is a commonly used nutritional model for NASH. MCD diets are usually highly enriched in sucrose (40%) and moderately enriched with fat (10%) but deficient in methionine and choline, which are essential for hepatic β-oxidation and production of very-low-density lipoprotein (vLDL).60 Depriving animals of methionine and choline causes notable steatosis, inflammation, hepatic ballooning, reactive oxygen species (ROS)-mediated liver damage, and fibrosis.2
Hepatic steatosis can be seen within 1 to 2 weeks of MCD induction.60,61 Moreover, mice fed with MCD diets developed extensive necro-inflammation as early as 2 weeks, and the typical chicken-wire fibrosis can be seen as early as 6 weeks after MCD induction, similar to the presentation in human NASH.62,63 MCD feeding also increases serum ALT levels and induces ballooning degeneration of hepatocytes in mice.64 Similarly, our group found that male C57BL/6 mice fed with MCD diets developed steatosis and inflammation at 4 weeks (Figure 3c). The severity of steatohepatitis in MCD-fed mice is associated with impaired hepatic adiponectin action and adipogenic transformation of hepatocytes.64 The responsiveness that develops in mice fed the MCD diet depends on sex and strain. For example, C57BL/6 mice exhibited more pronounced release of transaminases than did DBA/2J mice, whereas long-term MCD induction caused more severe liver injury, even hepatocarcinogenesis, in DBA/2J mice, but did not result in carcinogenesis in C57BL/6 mice.61,65 The notable inflammation observed in MCD-induced steatohepatitis is associated with increased macrophage infiltration in the liver, activation of the NF-ĸB signaling pathway, and concomitant increases in downstream pro-inflammatory cytokines, such as TNF-α, monocyte chemoattractant protein (MCP)-1, transforming growth factor (TGF)-β, and IL-6.66–68 MCD diets also promote induction of adhesion molecules, such as intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1, which are essential for polymorph recruitment.68–70
Animal models using MCD diets can replicate the hallmark pathological features of severe human NASH more closely than other dietary-based animal models. The steatosis, inflammation, and fibrosis induced by MCD diets develop more quickly than with HFD and other Western diet models. Cellular stress, such as endoplasmic reticulum (ER) stress, oxidative stress, and auto-phagocytic stress, is more pronounced in the MCD model than in other dietary-based NAFLD models.71
However, the MCD diet model has obvious disadvantages. Mice fed MCD diets always exhibit significant loss of body weight and the liver decreases proportionally in size, which go against effects seen in overweight and obese individuals with NAFLD.2,72 In addition, the metabolic profile in the MCD model is opposite to that seen in NAFLD patients: serum levels of triglyceride, insulin, leptin, and fasting glucose are dampened, whereas serum adiponectin is not decreased.72 Therefore, db/db (deficient in leptin receptor activity) or ob/ob (deficient in leptin) mice are often used in the MCD model to better imitate human NASH. Findings suggest that db/db mice fed with MCD diet for 4 weeks show remarkable hepatic inflammation and fibrosis.73
ob/ob and db/db mice
Leptin-deficient (ob/ob) mice, which carry an autosomal recessive mutation in the leptin gene, develop spontaneous liver steatosis under normal chow feeding. ob/ob mice are grossly overweight and show the altered metabolic parameters seen in human NAFLD, such as hyperinsulinaemia, hyperglycaemia, and IR.60,74,75 However, ob/ob mice are resistant to hepatic fibrosis, given that leptin is essential for the hepatic fibrogenic response to liver injury.76 In addition, the ob/ob mouse model is limited to spontaneous steatohepatitis unless secondary insults are added (such as a HFD or MCD diet or administration of small doses of lipopolysaccharide endotoxin).77 Our group found that male ob/ob mice (C57BL/6 strain) fed MCD diets developed steatosis and inflammation at 4 weeks (Figure 3d).db/db mice are homozygous for the autosomal recessive diabetic gene (db), which encodes a point mutation in the leptin receptor and leads to defective leptin signaling.63 db/db mice are obese and diabetic and develop macrovesicular hepatic steatosis accompanied by hyperglycaemia and hyperinsulinaemia.74,75 Unlike ob/ob mice, db/db mice exhibit normal or elevated levels of leptin but are resistant to its effects. Similarly, db/db mice do not spontaneously develop inflammation or show features of NASH without further insult.The ob/ob and db/db mice are good genetic models of NAFLD because they develop pronounced hepatic steatosis and show the significant altered metabolic characteristics seen in human NAFLD. db/db mice can also be used to study the progression of steatosis to NASH in the presence of secondary insults such as a MCD diet. However, congenital leptin deficiency or leptin resistance caused by gene mutations is not prevalent in obese humans or NASH patients, so the ob/ob and db/db mice models are limited in their ability to reflect the genesis of human obesity or NASH.63,78
foz/foz mice
Obese foz/foz mice, which carry a mutated Alms1 gene, spontaneously develop hepatic steatosis, obesity, diabetes, and IR, and show significant upregulation of cholesterol levels. HFD feeding can accentuate transition of simple steatosis to steatohepatitis by aggravating metabolic abnormalities, resulting in severe hepatocyte ballooning, inflammation, and fibrosis, accompanied by significant decreases in adiponectin levels and increases in cholesterol levels. However, despite upregulation of hepatic triglyceride content, serum triglyceride levels remain unchanged in foz/foz mice, even those fed with HFD.79,80 All foz/foz mice are obese but the severity of NASH is strain dependent. Serum ALT levels and NAFLD activity score were higher (worse) in foz/foz C57BL6/J mice than in foz/foz BALB/c mice fed with HFD. Moreover, HFD-induced fibrosis was severe in foz/foz C57BL6/J mice but absent in foz/foz BALB/c mice.81
To date, diverse animal models have been proposed to mimic particular characteristics of human NAFLD, such as the American lifestyle–induced obesity syndrome (ALIOS) model, the diet-induced animal model of nonalcoholic fatty liver disease (DIAMOND) model, and the ldlr−/− mice model.82–84 Recently, a murine NASH model was proposed that showed rapid progression of extensive fibrosis and hepatocellular carcinoma. The model used a Western diet, which contained high fat, high fructose, and high cholesterol, combined with a low weekly dose of intraperitoneal carbon tetrachloride (CCl4). This model captures the progressive stages of human fatty liver disease, from simple steatosis to inflammation, fibrosis, and cancer.85 It is important to choose the appropriate animal model to meet the research purpose (Table 1).
Table 1.
Commonly used animal models of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH)
| Model | Diet formula (kcal%) | Obesity | Insulin resistance | Steatosis | Steatohepatitis | Fibrosis |
|---|---|---|---|---|---|---|
| Methionine- and choline-deficient diet (MCD) | 40% sucrose, 10% fat, methionine (−), choline (−) | No | Hepatic insulin resistance | Yes | Yes | Yes |
| High-fat diet (HFD) | 45% to 75% fat. Classic model is 71% fat, 18% protein, and 11% carbohydrates | Yes | Yes | Yes | Yes, but mild | Yes, but mild |
| High-fat, high-fructose diet (HFHF) | HFD supplemented with fructose (usually 23 g/L in drinking water) | Yes | Yes | Yes | Yes, but mild | Yes, but mild |
| High-fat, high-cholesterol diet (HFHC) | Approximately 1% cholesterol fed in conjunction with HFD (usually 15% to 40% fat) | Yes | Yes | Yes | Yes | Yes |
| ob/ob mice | — | Yes | Yes | Yes | No1 | No1 |
| db/db mice | — | Yes | Yes | Yes | No1 | No1 |
| foz/foz mice | — | Yes | Yes | Yes | No1 | No1 |
1Although these mice do not develop steatohepatitis and fibrosis spontaneously, additionally feeding with MCD diets or HFD can promote development of steatohepatitis and fibrosis (not in ob/ob mice)
Conclusion
NAFLD is becoming a worldwide issue because of changes in lifestyle and resultant overnutrition. Lipid accumulation in the liver and its interplay with inflammation, oxidative stress, cell death, and autophagy is considered a major process of NAFLD progression. However, the exact mechanisms of NAFLD progression remain largely unknown. The use of animal models to replicate the important aspects of NAFLD progression provides significant clues to the critical molecular events that occur during NAFLD development and suggests a number of therapeutic targets for future treatment of NAFLD. Nevertheless, none of established animal models are perfect and it is important to choose the appropriate animal model to meet the research purpose.
Acknowledgments
All authors contributed to writing the manuscript and approved the final version.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006; 43: S99–S112. doi:10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 2.Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2011; 8: 35–44. doi:10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- 3.Brunt EM, Wong VW, Nobili V, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers 2015; 1: 15080, doi:10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 4.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148: 547–555. doi:10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011; 365: 1118–1127. doi:10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 6.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014; 59: 2188–2195. doi:10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 7.Ma J, Hwang SJ, Pedley A, et al. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol 2017; 66: 390–397. doi:10.1016/j.jhep.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology 1990; 12: 1106–1110. [DOI] [PubMed] [Google Scholar]
- 9.Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med 1999; 107: 450–455. [DOI] [PubMed] [Google Scholar]
- 10.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol 2010; 5: 145–171. doi:10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 11.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM 2010; 103: 71–83. doi:10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tandra S, Yeh MM, Brunt EM, et al. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J Hepatol 2011; 55: 654–659. doi:10.1016/j.jhep.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999; 94: 2467–2474. doi:10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 14.Bedossa P, Patel K. Biopsy and noninvasive methods to assess progression of nonalcoholic fatty liver disease. Gastroenterology 2016; 150: 1811–1822.e1814, doi:10.1053/j.gastro.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Lefkowitch JH. Morphology of alcoholic liver disease. Clin Liver Dis 2005; 9: 37–53. doi:10.1016/j.cld.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999; 116: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 17.Stumptner C, Fuchsbichler A, Heid H, et al. Mallory body—a disease-associated type of sequestosome. Hepatology 2002; 35: 1053–1062. doi:10.1053/jhep.2002.32674. [DOI] [PubMed] [Google Scholar]
- 18.Ganz M, Szabo G. Immune and inflammatory pathways in NASH. Hepatol Int 2013; 7: 771–781. doi:10.1007/s12072-013-9468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunt EM, Kleiner DE, Wilson LA, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD—clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology 2009; 49: 809–820. doi:10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313–1321. doi:10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 21.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science 2011; 332: 1519–1523. doi:10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010; 140: 900–917. doi:10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gual P, Gilgenkrantz H, Lotersztajn S. Autophagy in chronic liver diseases: the two faces of Janus. Am J Physiol Cell Physiol 2017; 312: C263–C273. doi:10.1152/ajpcell.00295.2016. [DOI] [PubMed] [Google Scholar]
- 24.Farrell GC, van Rooyen D, Gan L, et al. NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver 2012; 6: 149–171. doi:10.5009/gnl.2012.6.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong VW, Chitturi S, Wong GL, et al. Pathogenesis and novel treatment options for non-alcoholic steatohepatitis. Lancet Gastroenterol Hepatol 2016; 1: 56–67. doi:10.1016/s2468-1253(16)30011-5. [DOI] [PubMed] [Google Scholar]
- 26.Bradbury MW, Berk PD. Lipid metabolism in hepatic steatosis. Clin Liver Dis 2004; 8: 639–671, xi, doi:10.1016/j.cld.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Machado MV, Cortez-Pinto H. Non-alcoholic fatty liver disease: what the clinician needs to know. World J Gastroenterol 2014; 20: 12956–12980. doi:10.3748/wjg.v20.i36.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puri P, Baillie RA, Wiest MM, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007; 46: 1081–1090. doi:10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 2007; 45: 1366–1374. doi:10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 30.Kitade H, Chen G, Ni Y, et al. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients 2017; 9: pii: E387. doi:10.3390/nu9040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol 2002; 16: 663–678. [DOI] [PubMed] [Google Scholar]
- 32.Gruben N, Shiri-Sverdlov R, Koonen DP, et al. Nonalcoholic fatty liver disease: A main driver of insulin resistance or a dangerous liaison? Biochim Biophys Acta 2014; 1842: 2329–2343. doi:10.1016/j.bbadis.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol 2014; 220: T1–T23. doi:10.1530/joe-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miao H, Zhang Y, Lu Z, et al. FOXO1 involvement in insulin resistance-related pro-inflammatory cytokine production in hepatocytes. Inflamm Res 2012; 61: 349–358. doi:10.1007/s00011-011-0417-3. [DOI] [PubMed] [Google Scholar]
- 35.Yu J, Shen J, Sun TT, et al. Obesity, insulin resistance, NASH and hepatocellular carcinoma. Semin Cancer Biol 2013; 23: 483–491. doi:10.1016/j.semcancer.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Nieto-Vazquez I, Fernández-Veledo S, Krämer DK, et al. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem 2008; 114: 183–194. doi:10.1080/13813450802181047. [DOI] [PubMed] [Google Scholar]
- 37.Kashyap SR, Ioachimescu AG, Gornik HL, et al. Lipid-induced insulin resistance is associated with increased monocyte expression of scavenger receptor CD36 and internalization of oxidized LDL. Obesity (Silver Spring) 2009; 17: 2142–2148. doi:10.1038/oby.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araya J, Rodrigo R, Videla LA, et al. Increase in long-chain polyunsaturated fatty acid n–6/n–3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 2004; 106: 635–643. doi:10.1042/cs20030326. [DOI] [PubMed] [Google Scholar]
- 39.Hamel FG, Bennett RG, Upward JL, et al. Insulin inhibits peroxisomal fatty acid oxidation in isolated rat hepatocytes. Endocrinology 2001; 142: 2702–2706. doi:10.1210/endo.142.6.8178. [DOI] [PubMed] [Google Scholar]
- 40.Machado MV, Diehl AM. Pathogenesis of nonalcoholic steatohepatitis. Gastroenterology 2016; 150: 1769–1777. doi:10.1053/j.gastro.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Shen J, Man K, et al. CXCL10 plays a key role as an inflammatory mediator and a non-invasive biomarker of non-alcoholic steatohepatitis. J Hepatol 2014; 61: 1365–1375. doi:10.1016/j.jhep.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Han J, Man K, et al. CXC chemokine receptor 3 promotes steatohepatitis in mice through mediating inflammatory cytokines, macrophages and autophagy. J Hepatol 2016; 64: 160–170. doi:10.1016/j.jhep.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Yu J, Ip E, Dela Peña A, et al. COX-2 induction in mice with experimental nutritional steatohepatitis: Role as pro-inflammatory mediator. Hepatology 2006; 43: 826–836. doi:10.1002/hep.21108. [DOI] [PubMed] [Google Scholar]
- 44.Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Dig Dis 2010; 28: 179–185. doi:10.1159/000282083. [DOI] [PubMed] [Google Scholar]
- 45.Tilg H, Moschen AR, Szabo G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2016; 64: 955–965. doi:10.1002/hep.28456. [DOI] [PubMed] [Google Scholar]
- 46.Wu R, Nakatsu G, Zhang X, et al. Pathophysiological mechanisms and therapeutic potentials of macrophages in non-alcoholic steatohepatitis. Expert Opin Ther Targets 2016; 20: 615–626. doi:10.1517/14728222.2016.1125883. [DOI] [PubMed] [Google Scholar]
- 47.Mridha AR, Wree A, Robertson AAB, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 2017; 66: 1037–1046. doi:10.1016/j.jhep.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lieber CS, Leo MA, Mak KM, et al. Model of nonalcoholic steatohepatitis. Am J Clin Nutr 2004; 79: 502–509. [DOI] [PubMed] [Google Scholar]
- 49.Eccleston HB, Andringa KK, Betancourt AM, et al. Chronic exposure to a high-fat diet induces hepatic steatosis, impairs nitric oxide bioavailability, and modifies the mitochondrial proteome in mice. Antioxid Redox Signal 2011; 15: 447–459. doi:10.1089/ars.2010.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen T, Abdelmalek MF, Sullivan S, et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J Hepatol 2018; 68: 1063–1075. doi:10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ioannou GN. The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol Metab 2016; 27: 84–95. doi:10.1016/j.tem.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Abdelmalek MF, Suzuki A, Guy C, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 2010; 51: 1961–1971. doi:10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ouyang X, Cirillo P, Sautin Y, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 2008; 48: 993–999. doi:10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohli R, Kirby M, Xanthakos SA, et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology 2010; 52: 934–944. doi:10.1002/hep.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim JS, Mietus-Snyder M, Valente A, et al. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol 2010; 7: 251–264. doi:10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 56.Subramanian S, Goodspeed L, Wang S, et al. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J Lipid Res 2011; 52: 1626–1635. doi:10.1194/jlr.M016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savard C, Tartaglione EV, Kuver R, et al. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology 2013; 57: 81–92. doi:10.1002/hep.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wouters K, van Gorp PJ, Bieghs V, et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 2008; 48: 474–486. doi:10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- 59.Van Rooyen DM, Larter CZ, Haigh WG, et al. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology 2011; 141: 1393–1403, 1403.e1-5. doi:10.1053/j.gastro.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol 2006; 87: 1–16. doi:10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanuri G, Bergheim I. In vitro and in vivo models of non-alcoholic fatty liver disease (NAFLD). Int J Mol Sci 2013; 14: 11963–11980. doi:10.3390/ijms140611963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamada T, Obata A, Kashiwagi Y, et al. Gd-EOB-DTPA-enhanced-MR imaging in the inflammation stage of nonalcoholic steatohepatitis (NASH) in mice. Magn Reson Imaging 2016; 34: 724–729. doi:10.1016/j.mri.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 63.Lau JK, Zhang X, Yu J. Animal models of non-alcoholic fatty liver disease: current perspectives and recent advances. J Pathol 2017; 241: 36–44. doi:10.1002/path.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larter CZ, Yeh MM, Williams J, et al. MCD-induced steatohepatitis is associated with hepatic adiponectin resistance and adipogenic transformation of hepatocytes. J Hepatol 2008; 49: 407–416. doi:10.1016/j.jhep.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 65.Kirsch R, Clarkson V, Shephard EG, et al. Rodent nutritional model of non-alcoholic steatohepatitis: species, strain and sex difference studies. J Gastroenterol Hepatol 2003; 18: 1272–1282. [DOI] [PubMed] [Google Scholar]
- 66.Leclercq IA, Farrell GC, Sempoux C, et al. Curcumin inhibits NF-kappaB activation and reduces the severity of experimental steatohepatitis in mice. J Hepatol 2004; 41: 926–934. doi:10.1016/j.jhep.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 67.Dela Pena A, Leclercq I, Field J, et al. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology 2005; 129, 1663–1674. doi:10.1053/j.gastro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 68.McCuskey RS, Ito Y, Robertson GR, et al. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology 2004; 40: 386–393. doi:10.1002/hep.20302. [DOI] [PubMed] [Google Scholar]
- 69.Ip E, Farrell G, Hall P, et al. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology 2004; 39: 1286–1296. doi:10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- 70.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol 1999; 66: 876–888. [DOI] [PubMed] [Google Scholar]
- 71.Machado MV, Michelotti GA, Xie G, et al. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PloS One 2015; 10: e0127991, doi:10.1371/journal.pone.0127991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol 2004; 40: 47–51. [DOI] [PubMed] [Google Scholar]
- 73.Sahai A, Malladi P, Pan X, et al. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol 2004; 287: G1035–G1043. doi:10.1152/ajpgi.00199.2004. [DOI] [PubMed] [Google Scholar]
- 74.Sanches SC, Ramalho LN, Augusto MJ, et al. Nonalcoholic steatohepatitis: a search for factual animal models. Biomed Res Int 2015; 2015: 574832, doi:10.1155/2015/574832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trak-Smayra V, Paradis V, Massart J, et al. Pathology of the liver in obese and diabetic ob/ob and db/db mice fed a standard or high-calorie diet. Int J Exp Pathol 2011; 92: 413–421. doi:10.1111/j.1365-2613.2011.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leclercq IA, Farrell GC, Schriemer R, et al. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol 2002; 37: 206–213. [DOI] [PubMed] [Google Scholar]
- 77.Yang SQ, Lin HZ, Lane MD, et al. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A 1997; 94: 2557–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paz-Filho G, Mastronardi C, Delibasi T, et al. Congenital leptin deficiency: diagnosis and effects of leptin replacement therapy. Arq Bras Endocrinol Metabol 2010; 54: 690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arsov T, Silva DG, O'Bryan MK, et al. Fat Aussie—a new Alström syndrome mouse showing a critical role for ALMS1 in obesity, diabetes, and spermatogenesis. Mol Endocrinol 2006; 20: 1610–1622. doi:10.1210/me.2005-0494. [DOI] [PubMed] [Google Scholar]
- 80.Arsov T, Larter CZ, Nolan CJ, et al. Adaptive failure to high-fat diet characterizes steatohepatitis in Alms1 mutant mice. Biochem Biophys Res Commun 2006; 342: 1152–1159. doi:10.1016/j.bbrc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 81.Farrell GC, Mridha AR, Yeh MM, et al. Strain dependence of diet-induced NASH and liver fibrosis in obese mice is linked to diabetes and inflammatory phenotype. Liver Int 2014; 34: 1084–1093. doi:10.1111/liv.12335. [DOI] [PubMed] [Google Scholar]
- 82.Larner DP, Morgan SA, Gathercole LL, et al. Male 11beta-HSD1 knockout mice fed trans-fats and fructose are not protected from metabolic syndrome or nonalcoholic fatty liver disease. Endocrinology 2016; 157: 3493–3504. doi:10.1210/en.2016-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Asgharpour A, Cazanave SC, Pacana T, et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol 2016; 65: 579–588. doi:10.1016/j.jhep.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morrison MC, Verschuren L, Salic K, et al. Obeticholic acid modulates serum metabolites and gene signatures characteristic of human NASH and attenuates inflammation and fibrosis progression in Ldlr-/-.Leiden mice. Hepatol Commun 2018; 2: 1513–1532. doi:10.1002/hep4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsuchida T, Lee YA, Fujiwara N, et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J Hepatol 2018; 69: 385–395. doi:10.1016/j.jhep.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]