Short abstract
Hepatic splenosis is an uncommon condition that occurs following traumatic splenic rupture or splenectomy. The case of a 41-year-old male patient with multiple isolated liver masses indistinguishable from primary and metastatic liver tumours is reported. Following laparotomy, the liver lesions were resected and histopathology confirmed a diagnosis of hepatic splenosis. At an 18-month follow-up examination, no abnormalities in routine blood test, liver function, and liver computed tomography (CT) scanning were observed. After review of the literature, the following diagnostic criteria for hepatic splenosis are proposed: (1) a history of splenic trauma or splenectomy; (2) lesion(s) with a surrounding rim, particularly near the liver capsule identified by CT scanning; (3) findings on superparamagnetic iron oxide-enhanced magnetic resonance imaging or technetium-99m heat-damaged red cell scanning; and (4) histopathological findings (needle biopsy or surgical pathology). The following diagnostic process is also proposed: suspect diagnosis when criteria 1 and 2 are met; make diagnosis when criterion 3 is met; confirm diagnosis when criterion 4 is met. Laparotomy is recommended for either diagnosis or treatment when invasive procedures are necessary.
Keywords: Hepatic splenosis, diagnosis, treatment
Introduction
Splenosis is a benign condition commonly resulting from traumatic splenic rupture or splenectomy.1,2 Hepatic splenosis is rare and is usually diagnosed accidentally.3 Due to its low prevalence, hepatic splenosis is difficult to diagnose by non-invasive methods, particularly when the mass presents as a malignant disease on imaging or the patient has a risk of liver tumour or a history of cancer. Thus, the diagnosis of hepatic splenosis remains elusive and requires further investigation. Here, the case of a patient with hepatic splenosis mimicking liver metastases is reported. In addition, the PubMed database was searched between January 1993 and December 2016 for literature relating to splenosis, and the resultant literature was analysed in order to provide information on the diagnosis and treatment of this disease.
Case report
This work was conducted following approval by the ethics committees at Chongqing University Cancer Hospital. The patient provided written informed consent.
The patient was a 41-year-old male who was referred to Chongqing Cancer Hospital in December 2015 with liver tumours of unknown origin. He had no history of weight loss, abdominal pain or jaundice. His medical history included an urgent splenectomy due to traumatic rupture of the spleen as the result of a traffic accident in July 1994. There was no self-reported history of smoking or alcohol misuse. There was no positive sign on physical examination, except for a previous surgical scar. Routine blood analysis showed a platelet count of 521 × 109/l and haemoglobin of 76 g/l. His liver function was normal and graded as A (score, 5) according to the Child-Turcotte-Pugh classification.4 His α-fetoprotein (AFP) level was 1.3 ng/ml (normal range, 0–8.1 ng/ml), carcinoembryonic antigen was 1.27 ng/ml (normal range, 1–5 ng/ml), and carbohydrate antigen 19-9 was 10.76 U/ml (normal range, 0–30.9 U/ml). Chest radiography was normal. Ultrasonography revealed a mass in the right liver region and an abdominal computed tomography (CT) scan showed multiple lesions in the liver, abdominal wall and splenectomy bed (Figure 1). A metastatic process was initially suspected due to anaemia and multiple lesions. No tumours were found by oesophagogastroduodenoscopy and colonoscopy, however, a duodenal ulcer was found. Since the liver lesions had an unknown aetiology, a biopsy was recommended. The patient refused a transcutaneous biopsy, and thus, an exploratory laparoscopy was indicated following a multidisciplinary consultation.
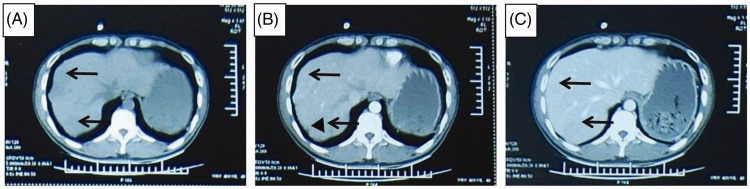
Figure 1.
Abdominal computed tomography scan showing lesions in the liver located near the liver capsule (arrows) with a rim surrounding the lesion (triangle): (A) low density lesions located near the capsule in unenhanced phase; (B) lesions washed-in and enhanced during hepatic arterial phase revealing a rim surrounding the lesion (triangle); and (C) lesions washed-out in portal venous phase
The patient underwent an exploratory laparoscopy. General anaesthesia was induced using sufentanil (15 µg total dose), midazolam (2 mg total dose), propofol (100 mg total dose) and rocuronium bromide (50 mg total dose), and anaesthesia was maintained using remifentanil (2 mg total dose), propofol (1400 mg total dose) and sevoflurane (50 ml total dose). Sufentanil (45 µg) and atracurium (15 mg) were administered according the patient’s condition during surgery. A 1.3-cm incision was made along the superior umbilical fold. A pneumoperitoneum was created with a Veress needle, and a 10-mm trocar was placed as a camera port. Two 12-mm trocars and two 5-mm trocars were then introduced with direct visual observation at the right upper quadrant, subxiphoid, right midclavicular, and anterior axillary lines, respectively. Operative findings showed an intrahepatic lesion of 4 × 2 cm in size located on the surface of liver segment VIII and a nodule of 1.5 × 1.3 cm on the abdominal wall. A lesion in segment VII adhered to the diaphragm and dented the surface of the liver. All lesions had clear boundaries with the liver, and were rapidly removed without hepatic resection. Subsequent histopathological results confirmed that the lesions consisted of spleen tissue (Figure 2).
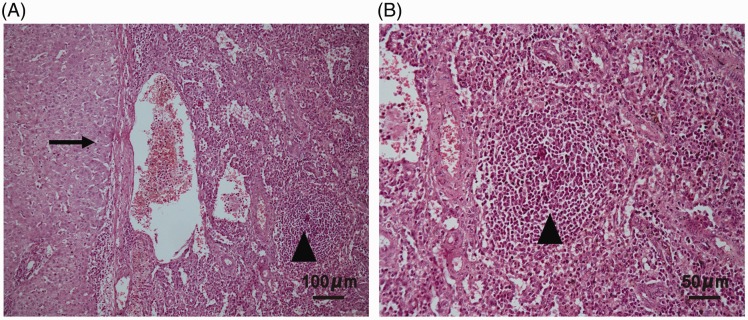
Figure 2.
Representative photomicrographs of a liver lesion tissue section stained with haematoxylin and eosin showing hepatic splenosis and normal liver tissue: (A) fibrous tissue isolates the liver and spleen parenchyma (arrow) with liver tissue on the left and spleen tissue on the right including lymphoid follicular tissue (triangle), original magnification × 100; and (B) lymphoid follicular tissue (triangle), original magnification × 200.
The diagnosis of hepatic splenosis was thus established. Follow-up examination including routine blood analysis, liver function, and liver CT scanning at 18 months did not show any abnormalities. No adverse or unanticipated events occurred.
Discussion
The first case of splenosis was described by Albrecht in 18965 and the condition was named by Buchbinder and Lipkoff in 1939.6 Splenosis is mainly found in patients who have undergone splenectomy1 and although is considered a rare event, its incidence can be up to 67% in cases of traumatic splenic rupture.2 Splenosis can be found in any part of the body, but common sites include the serosal surface of the small bowel, greater omentum, and parietal peritoneum, and the serosal surface of the large intestine and mesentery.3
Hepatic splenosis is frequently found in the left lobe of the liver and is usually located near the liver capsule. The most likely mechanism underlying splenosis is the seeding of splenic fragments onto serosal surfaces during splenic trauma or splenectomy7 and cell proliferation, promoted by local hypoxia of the liver.8 Hepatic splenosis is rarely identified in the clinic, with no more than 41 cases published between 1993 and 2016 (Table 1),1,8–44 and due to its low prevalence, knowledge of the clinical features and diagnosis of splenosis remains limited. Hepatic splenosis has no specific symptoms, although abdominal pain may arise due to heterotopic splenic infarction or compression, resulting in missed diagnosis.10,11 Hepatic splenosis is difficult to distinguish from adenoma and hepatocellular carcinoma, and can occur as single or multiple lesions at the surface or within the liver,1,12,13 which may be misinterpreted as metastatic lesions in patients with malignant tumours.
Table 1.
Results summary from a literature review of hepatic splenosis showing 38 articles detailing 41 cases of hepatic splenosis published between 1993 and 2016.
| Author, year | Age, years/sex | Reason for splenectomy | AFP level | Lesion location/size, cm | Number | Primary diagnosis | Method to confirm diagnosis |
|---|---|---|---|---|---|---|---|
| Yoshimitsu, 19939 | 51/F | Banti syndrome | N | S3/2.5 | 1 | HCC | Surgery |
| Gruen, 199610 | 38/F | Trauma | NM | S3.4/3.9 | 1 | Adenoma,FNH | Surgery |
| D’Angelica, 199711 | 38/F | Trauma | NM | S3/4 | 1 | Adenoma,FNH | Surgery |
| Davidson, 199712 | 54/M | Surgicaltrauma | NM | LL/2 | 1 | NA | Necropsy |
| DeVuysere, 199913 | 50/M | Trauma | N | S2/6.0 | Multiple | Splenosis | Biopsy |
| Foroudi, 199914 | 59/F | Trauma | NM | S6 | Multiple | Metastasis | Tc-99mHDRS |
| Pekkafali, 200215 | 21/M | Trauma | NM | LL/3.4 | 1 | Splenosis | Tc-99mHDRS |
| Gamulin, 200216 | 49/M | Trauma | NM | LL/6.6 | 1 | Lymphoma | Surgery |
| Kim, 200317 | 43/M | Trauma | N | S6 | 1 | HCC | Surgery |
| Costanzo, 200418 | 58/M | Trauma | 412 | LL/4.8 | 1 | HCC | Biopsy Tc-99mHDRS |
| Costanzo, 200418 | 48/F | Trauma | 327 | S3/3.1 | 1 | HCC | Biopsy |
| Kondo, 200419 | 55/M | Trauma | NM | S7 | 1 | Tumour | Biopsy |
| Ferraioli, 200520 | 40/M | Trauma | N | S7/6.0 | 1 | Hepatic Splenosis | Biopsy |
| Yeh, 200821 | 64/M | Trauma | N | S6/2.5 | 1 | HCC | Surgery |
| Lu, 200822 | 59/M | Trauma | N | S7 | Multiple | Splenosis | Tc-99mHDRS |
| Choi, 20087 | 32/M | Trauma | 17.3 | S4,S6 | Multiple | HCC | Surgery |
| Grande, 200823 | 41/M | Trauma | N | S7 | Multiple | Splenosis | Tc-99mHDRS |
| Nakajima, 200824 | 41/M | Trauma | NM | S6 | 1 | Splenosis | Biopsy |
| Yu, 200925 | 54/M | Trauma | N | S2/4 | 1 | Hepatoma | Surgery |
| Abu Hilal, 200926 | 60/M | Trauma | Mild rise | S7/2.5 | Multiple | HCC | Surgery |
| Kashgari, 200927 | 52/M | Trauma | N | S7/2.1 | 1 | HCC | Biopsy |
| Menth, 200928 | 43/M | Trauma | 6.4 | S2 | Multiple | HCC | Tc-99mHDRS |
| Mescoli, 201029 | 68/F | NH | NM | S3,5,7 | Multiple | FNH | Biopsy |
| Mescoli, 201029 | 54/M | Trauma | NM | LL/3 | 1 | Metastasis | Surgery |
| Tsitouridis, 201030 | 63/M | Trauma | NM | LL/8 | 1 | Splenosis | Surgery |
| Tsitouridis, 201030 | 64/M | Gastric leiomyosarcoma | NM | S4/5 | 1 | Peritoneal implantation | Biopsy |
| Kang, 201131 | 54/M | Trauma | NM | S2/3.5 | Multiple | Metastasis | Surgery |
| Liu, 201232 | 49/F | Trauma | NM | LL | Multiple | Metastasis | Surgery |
| Li, 201233 | 61/M | Trauma | NM | S4,S7 | Multiple | Splenosis | Biopsy |
| Liu, 201234 | 38/M | Trauma | N | S2/3.3 | 1 | Hepatic tumour | Laparoscopy |
| Inchingolo, 201335 | 53/M | Trauma | N | S3/3.5 | 1 | HCC, Adenoma | Surgery |
| Krawczyk, 201336 | 39/F | Trauma | NM | S2/3.2 | Multiple | Splenosis | Tc-99mHDRS |
| Röther, 201337 | 62/M | Trauma | N | S5/1.8 | Multiple | HCC | Laparoscopy |
| Kandil, 201438 | 45/F | Haemolyticanaemia | N | S2/5.0 | 1 | HCC | Surgery |
| Wu, 20151 | 33/M | Trauma | N | S2/3.5 | 1 | HCC | Surgery |
| Liu, 201539 | 33/M | Trauma | N | S3/4.2 | Multiple | Hepatic tumour | Biopsy |
| Aramoana, 201540 | 58/M | Trauma | NM | S6/4.6 | 1 | Splenosis | Surgery |
| Grambow, 201541 | 53/M | Trauma | N | 3.5 | 1 | HCC | Surgery |
| Fung, 201642 | 55/M | Trauma | N | S6/4.7 | Multiple | Hepatic tumour | Surgery |
| He, 201643 | 51/M | Trauma | NM | S2,S6 | Multiple | Metastasis | Biopsy |
| Jereb, 201644 | 22/M | Trauma | N | S6,S2 | Multiple | Metastasis | Biopsy |
AFP, α-fetoprotein; F, female; FNH, focal nodular hyperplasia; HCC, hepatocellular carcinoma; LL, left lobe; M, male; N, normal; NA, not associated with hepatic splenosis; NM, not mentioned; NH, no history of splenectomy; S2, 3, 4, 5, 6, 7, hepatic segments II, III, IV, V, VI, VII; Tc-99m HDRS, technetium-99m heat-damaged red cell scanning.
In the present case, secondary liver cancer was initially suspected due to ultrasonography and CT findings of multiple lesions in the liver, abdominal wall and splenectomy bed. Furthermore, low AFP levels and negative results for hepatitis increased the possibility of metastatic lesions. Due to negative findings on endoscopy, and patient refusal to undergo a transcutaneous liver biopsy, laparoscopic exploration was performed. The intrahepatic lesions were removed and diagnosed as hepatic splenosis by pathology.
False diagnoses not only have the potential to increase medical costs and the risk of adverse effects due to an invasive procedure, but also result in incorrect treatment. In the published case studies found in the present study, only 10 patients were appropriately diagnosed13,15,20,22–24,30,33,36,40 and the remaining patients were incorrectly diagnosed with liver tumours. Misdiagnosis of liver cancer due to chronic hepatitis and abnormal AFP usually results in patients receiving unnecessary transcatheter arterial chemoembolization7 or surgeries,26 or losing the opportunity of liver transplantation.18 Such incorrect treatments have resulted in secondary damage in these patients.17,18 With the increased prevalence of abdominal trauma,45,46 hepatic splenosis may occur more often. Therefore, standard and effective diagnostic criteria need to be established for this disease.
In addition to a history of splenic trauma and splenectomy, alternative techniques have been used to distinguish hepatic splenosis from other hepatic masses, such as CT scans,15 technetium-99m heat-damaged red cell scanning (Tc-99m HDRS) and superparamagnetic iron oxide-enhanced magnetic resonance imaging (SPIO-MRI).13,23,28 Low AFP level has been suggested as an index for diagnosing hepatic splenosis, however, in patients who also have hepatocellular carcinoma (HCC) or chronic hepatitis, the splenosis could be misinterpreted as a liver tumour, resulting in incorrect disease staging and loss of opportunity for optimal surgery/transplant or conservative treatments. Thus, pathological evidence was also required to confirm diagnosis according to the results of surgery or biopsy in 35 of the reported patients.1,7,9–11,13,16–21,24–27,29,35,37–44 Based on current knowledge, the present authors propose the following criteria and process for diagnosing hepatic splenosis. Criteria: (1) a history of splenic trauma or splenectomy; (2) lesion(s) with a surrounding rim, particularly near the liver capsule identified by CT scanning; (3) findings on SPIO-MRI or Tc-99m HDRS; and (4) histopathological findings (needle biopsy or surgical pathology). Diagnostic process: suspect diagnosis of hepatic splenosis when criteria 1 and 2 are met; make diagnosis when criterion 3 is met; confirm diagnosis when criterion 4 is met.
Splenosis treatment relies on the reason for splenectomy and complications of the lesion. For patients who have undergone splenectomy for splenic trauma, conservative treatment may be given in most cases without compressive symptoms. For patients who have undergone splenectomy for haematologic disorders, removing the splenosis may prevent disease recurrence.47 If invasive procedures are necessary (for either diagnosis or treatment), laparotomy is recommended.
The present results may be limited by the retrospective nature of the case study and literature review. Thus, data on more cases should be continuously collected to further assess and verify the proposed diagnostic criteria, and a future observational study remains necessary to elucidate the specificity and sensitivity of each of the criteria.
In conclusion, due to the rareness and asymptomatic nature of hepatic splenosis, it remains difficult to diagnose, leading to missed and false diagnoses. Following a case study and review of the literature, the present authors have proposed criteria and a process for diagnosing hepatic splenosis. Laparotomy is recommended for either diagnosis or treatment when invasive procedures are necessary.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by grants to NQ Gong from the Clinical Research Physician Program of Tongji Medical college, HUST and to XF Sun from the Major State Basic Research Development Program of China (No. 2013CB530803, 973).
References
- 1.Wu C, Zhang B, Chen L, et al. Solitary perihepatic splenosis mimicking liver lesion: a case report and literature review. Medicine (Baltimore) 2015; 94: e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingston CD, Levine BA, Lecklitner ML, et al. Incidence and function of residual splenic tissue following splenectomy for trauma in adults. Arch Surg 1983; 118: 617–620. [DOI] [PubMed] [Google Scholar]
- 3.Ksiadzyna D, Peña AS. Abdominal splenosis. Rev Esp Enferm Dig 2011; 103: 421–426. [PubMed] [Google Scholar]
- 4.Hong SH, Kim JE, Cho ML, et al. Comparison of the Child-Turcotte-Pugh classification and the model for end-stage liver disease score as predictors of the severity of the systemic inflammatory response in patients undergoing living-donor liver transplantation. J Korean Med Sci 2011; 26: 1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albrecht H. A case of very numerous, over the whole peritoneum, scattered accessory spleens. Beitr z Path Anat 1896; 20: 513–527 [In German]. [Google Scholar]
- 6.Buchbinder JH, Lipkoff CJ. Splenosis: multiple peritoneal splenic implants following abdominal injury: A report of a case and review of the literature. Surgery 1939; 6: 927–934. [Google Scholar]
- 7.Choi GH, Ju MK, Kim JY, et al. Hepatic splenosis preoperatively diagnosed as hepatocellular carcinoma in a patient with chronic hepatitis B: a case report. J Korean Med Sci 2008; 23: 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwok CM, Chen YT, Lin HT, et al. Portal vein entrance of splenic erythrocytic progenitor cells and local hypoxia of liver, two events cause intrahepatic splenosis. Med Hypotheses 2006; 67: 1330–1332. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimitsu K, Aibe H, Nobe T, et al. Intrahepatic splenosis mimicking a liver tumor. Abdom Imaging 1993; 18: 156–158. [DOI] [PubMed] [Google Scholar]
- 10.Gruen DR, Gollub MJ. Intrahepatic splenosis mimicking hepatic adenoma. AJR Am J Roentgenol 1997; 168: 725–726. [DOI] [PubMed] [Google Scholar]
- 11.D'Angelica M, Fong Y, Blumgart LH. Isolated hepatic splenosis: first reported case. HPB Surg 1998; 11: 39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson LA, Reid IN. Intrahepatic splenic tissue. J Clin Pathol 1997; 50: 532–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vuysere S, Van Steenbergen W, Aerts R, et al. Intrahepatic splenosis: imaging features. Abdom Imaging 2000; 25: 187–189. [DOI] [PubMed] [Google Scholar]
- 14.Foroudi F, Ahern V, Peduto A. Splenosis mimicking metastases from breast carcinoma. Clin Oncol (R Coll Radiol) 1999; 11: 190–192. [DOI] [PubMed] [Google Scholar]
- 15.Pekkafali Z, Karsli AF, Silit E, et al. Intrahepatic splenosis: a case report. Eur Radiol 2002; 12(Suppl 3): S62–S65. [DOI] [PubMed] [Google Scholar]
- 16.Gamulin A, Oberholzer J, Rubbia-Brandt L, et al. An unusual, presumably hepatic mass. Lancet 2002; 360: 2066. [DOI] [PubMed] [Google Scholar]
- 17.Kim KA, Park CM, Kim CH, et al. An interesting hepatic mass: splenosis mimicking a hepatocellular carcinoma (2003:9b) . Eur Radiol 2003; 13: 2713–2715. [DOI] [PubMed] [Google Scholar]
- 18.Di Costanzo GG, Picciotto FP, Marsilia GM, et al. Hepatic splenosis misinterpreted as hepatocellular carcinoma in cirrhotic patients referred for liver transplantation: report of two cases. Liver Transpl 2004; 10: 706–709. [DOI] [PubMed] [Google Scholar]
- 19.Kondo M, Okazaki H, Takai K, et al. Intrahepatic splenosis in a patient with chronic hepatitis C. J Gastroenterol 2004; 39: 1013–1015. [DOI] [PubMed] [Google Scholar]
- 20.Ferraioli G, Di Sarno A, Coppola C, et al. Contrast-enhanced low-mechanical-index ultrasonography in hepatic splenosis. J Ultrasound Med 2006; 25: 133–136. [DOI] [PubMed] [Google Scholar]
- 21.Yeh ML, Wang LY, Huang CI, et al. Abdominal splenosis mimicking hepatic tumor: a case report. Kaohsiung J Med Sci 2008; 24: 602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu HC, Su CW, Lu CL, et al. Hepatic splenosis diagnosed by spleen scintigraphy. Am J Gastroenterol 2008; 103: 1842–1844. [DOI] [PubMed] [Google Scholar]
- 23.Grande M, Lapecorella M, Ianora AA, et al. Intrahepatic and widely distributed intraabdominal splenosis: multidetector CT, US and scintigraphic findings. Intern Emerg Med 2008; 3: 265–267. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T, Fujiwara A, Yamaguchi M, et al. Intrahepatic splenosis with severe iron deposition presenting with atypical magnetic resonance images. Intern Med 2008; 47: 743–746. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Xia L, Li T, et al. Intrahepatic splenosis mimicking hepatoma. BMJ Case Rep 2009; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu Hilal M, Harb A, Zeidan B, et al. Hepatic splenosis mimicking HCC in a patient with hepatitis C liver cirrhosis and mildly raised alpha feto protein; the important role of explorative laparoscopy. World J Surg Oncol 2009; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashgari AA, Al-Mana HM, Al-Kadhi YA. Intrahepatic splenosis mimicking hepatocellular carcinoma in a cirrhotic liver. Saudi Med J 2009; 30: 429–432. [PubMed] [Google Scholar]
- 28.Menth M, Herrmann K, Haug A, et al. Intra-hepatic splenosis as an unexpected cause of a focal liver lesion in a patient with hepatitis C and liver cirrhosis: a case report. Cases J 2009; 2: 8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mescoli C, Castoro C, Sergio A, et al. Hepatic spleen nodules (HSN). Scand J Gastroenterol 2010; 45: 628–632. [DOI] [PubMed] [Google Scholar]
- 30.Tsitouridis I, Michaelides M, Sotiriadis C, et al. CT and MRI of intraperitoneal splenosis. Diagn Interv Radiol 2010; 16: 145–149. [DOI] [PubMed] [Google Scholar]
- 31.Kang KC, Cho GS, Chung GA, et al. Intrahepatic splenosis mimicking liver metastasis in a patient with gastric cancer. J Gastric Cancer 2011; 11: 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Ji B, Wang G, et al. Abdominal multiple splenosis mimicking liver and colon tumors: a case report and review of the literature. Int J Med Sci 2012; 9: 174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Snow-Lisy D, Klein EA. Hepatic splenosis diagnosed after inappropriate metastatic evaluation in patient with low-risk prostate cancer. Urology 2012; 79: e73–e74. [DOI] [PubMed] [Google Scholar]
- 34.Liu K, Liang Y, Liang X, et al. Laparoscopic resection of isolated hepatic splenosis mimicking liver tumors: case report with a literature review . Surg Laparosc Endosc Percutan Tech 2012; 22: e307–e311. [DOI] [PubMed] [Google Scholar]
- 35.Inchingolo R, Peddu P, Karani J. Hepatic splenosis presenting as arterialised liver lesion in a patient with NASH. Eur Rev Med Pharmacol Sci 2013; 17: 2853–2856. [PubMed] [Google Scholar]
- 36.Krawczyk M, Schneider G, Farmakis G, et al. Splenosis mimicking hepatic adenoma. J Clin Exp Hepatol 2013; 3: 351–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rother M, Dufour JF, Schnuriger B. An uncommon cause of a focal liver lesion. Post-traumatic splenosis. Gastroenterology 2013; 144: 510, 659. [DOI] [PubMed] [Google Scholar]
- 38.Kandil TS, El Sorogy M, Naiem Y, et al. Post-splenectomy splenosis presenting as hepatocellular carcinoma in the left lateral section of the liver: a case report. Int J Surg Case Rep 2014; 5: 877–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C, Liu J, Wang F. Intrahepatic splenosis mimicking liver cancer: report of a case and review of literature. Int J Clin Exp Pathol 2015; 8: 1031–1035. [PMC free article] [PubMed] [Google Scholar]
- 40.Aramoana J, Wylie N, Koea JB. Hepatic splenosis. ANZ J Surg 2018; 88: E359–E360. [DOI] [PubMed] [Google Scholar]
- 41.Grambow E, Weinrich M, Zimpfer A, et al. Ectopic spleen tissue - an underestimated differential diagnosis of a hypervascularised liver tumour. Viszeralmedizin 2015; 31: 445–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fung A, Chok K, Lo A, et al. Hepatobiliary and pancreatic: hepatic splenosis: a rare differential of a liver mass in an HBV endemic area. J Gastroenterol Hepatol 2016; 31: 1238. [DOI] [PubMed] [Google Scholar]
- 43.He ZL, Xu X, Ke QH, et al. Successful diagnosis of intrahepatic splenosis mimicking hepatic tumor. Kaohsiung J Med Sci 2016; 32: 224–225. [DOI] [PubMed] [Google Scholar]
- 44.Jereb S, Trotovsek B, Skrbinc B. Hepatic splenosis mimicking liver metastases in a patient with history of childhood immature teratoma. Radiol Oncol 2016; 50: 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCraig LF, Burt CW. National hospital ambulatory medical care survey: 2003 emergency department summary. Adv Data 2005; 358: 1–40. [PubMed] [Google Scholar]
- 46.Pitts SR, Niska RW, Xu J, et al. National hospital ambulatory medical care survey: 2006 emergency department summary National Health Statistics Reports. Report no. 7, 6 August 2008, www.cdc.gov/nchs/data/nhsr/nhsr007.pdf (2008, accessed 24 February 2010). [PubMed]
- 47.Wekerle T, Eichinger S, Maier A, et al. Intrahepatic splenic tissue in a patient with recurrent idiopathic thrombocytopenic purpura. Surgery 1998; 123: 596–599. [DOI] [PubMed] [Google Scholar]