Abstract
Background
Postoperative pulmonary complications (PPC) may result in longer duration of in-hospital stay and even mortality. Both thoracic surgery and intraoperative mechanical ventilation settings add considerably to the risk of PPC. It is unclear if one-lung ventilation (OLV) for thoracic surgery with a strategy of intraoperative high positive end-expiratory pressure (PEEP) and recruitment maneuvers (RM) reduces PPC, compared to low PEEP without RM.
Methods
PROTHOR is an international, multicenter, randomized, controlled, assessor-blinded, two-arm trial initiated by investigators of the PROtective VEntilation NETwork. In total, 2378 patients will be randomly assigned to one of two different intraoperative mechanical ventilation strategies. Investigators screen patients aged 18 years or older, scheduled for open thoracic or video-assisted thoracoscopic surgery under general anesthesia requiring OLV, with a maximal body mass index of 35 kg/m2, and a planned duration of surgery of more than 60 min. Further, the expected duration of OLV shall be longer than two-lung ventilation, and lung separation is planned with a double lumen tube. Patients will be randomly assigned to PEEP of 10 cmH2O with lung RM, or PEEP of 5 cmH2O without RM. During two-lung ventilation tidal volume is set at 7 mL/kg predicted body weight and, during OLV, it will be decreased to 5 mL/kg. The occurrence of PPC will be recorded as a collapsed composite of single adverse pulmonary events and represents the primary endpoint.
Discussion
PROTHOR is the first randomized controlled trial in patients undergoing thoracic surgery with OLV that is adequately powered to compare the effects of intraoperative high PEEP with RM versus low PEEP without RM on PPC. The results of the PROTHOR trial will support anesthesiologists in their decision to set intraoperative PEEP during protective ventilation for OLV in thoracic surgery.
Trial registration
The trial was registered in clinicaltrials.gov (NCT02963025) on 15 November 2016.
Electronic supplementary material
The online version of this article (10.1186/s13063-019-3208-8) contains supplementary material, which is available to authorized users.
Keywords: Mechanical ventilation, positive end-expiratory pressure, recruitment maneuver, one-lung ventilation, thoracic surgery, postoperative pulmonary complication
Background
Postoperative pulmonary complications (PPC) increase morbidity, resulting in longer duration of in-hospital stay and even increased mortality [1–3]. Several independent risk factors for the development of PPC have been identified [4], including patients’ health conditions, surgical approaches, and anesthetic management [5]. In addition, thoracic surgery [3] and intraoperative mechanical ventilation settings [2] add considerably to the risk of PPC.
Experimental [6–8] and clinical evidence [9–11] show that mechanical ventilation has the potential to aggravate or even initiate lung injury (so called ventilator-induced lung injury; VILI). Repetitive collapse/reopening of lung units (atelectrauma), overdistension of lung units (volutrauma), and increased airway pressures (barotrauma) are possible mechanisms underlying VILI [12–14]. While positive end-expiratory pressure (PEEP) can minimize atelectrauma and low tidal volumes (VT) reduce volutrauma, ventilation at low airway pressures may decrease barotrauma.
A metanalysis showed that use of low VT is associated with favorable outcomes in patients without injured lungs [15]. More recently, another meta-analysis showed a decrease in the incidence of lung injury, pulmonary infection, and atelectasis in patients receiving intraoperative mechanical ventilation with low VT and PEEP [16]. In patients undergoing abdominal surgery, an intraoperative ventilation strategy with low VT and PEEP improved postoperative lung function [17] and even outcome [16]. In contrast, when low VT is used, the use of high PEEP combined with recruitment maneuvers (RM), as compared to low PEEP without RM, does not add to protection against PPC [18]. To our knowledge, the potential of high PEEP and RM during one-lung ventilation (OLV) for thoracic surgery to reduce PPC has not been investigated in adequately powered trials [19, 20]. Due to mediastinal displacement, surgical manipulation, and chest immobilization, pressures in the dependent lung [21] and atelectasis formation are higher during thoracic surgery as compared with the other types of surgeries [22]. Thus, OLV might benefit from mechanical ventilation with high PEEP and RM.
In view of these facts, we designed the PROtective ventilation with high versus low PEEP during OLV for THORacic surgery (PROTHOR) trial. We hypothesized that intraoperative mechanical ventilation using high PEEP with periodic RM, as compared to low PEEP without RM, will prevent PPC in patients undergoing thoracic surgery with OLV.
Methods
Objectives and design
PROTHOR is an international, multicenter, randomized, controlled, assessor-blinded, two-arm trial initiated by investigators of the PROtective VEntilation NETwork (http://provenet.eu). In total, 2378 patients will be randomly assigned to one of two different intraoperative mechanical ventilation strategies (see CONSORT diagram, Fig. 1).
Fig. 1.
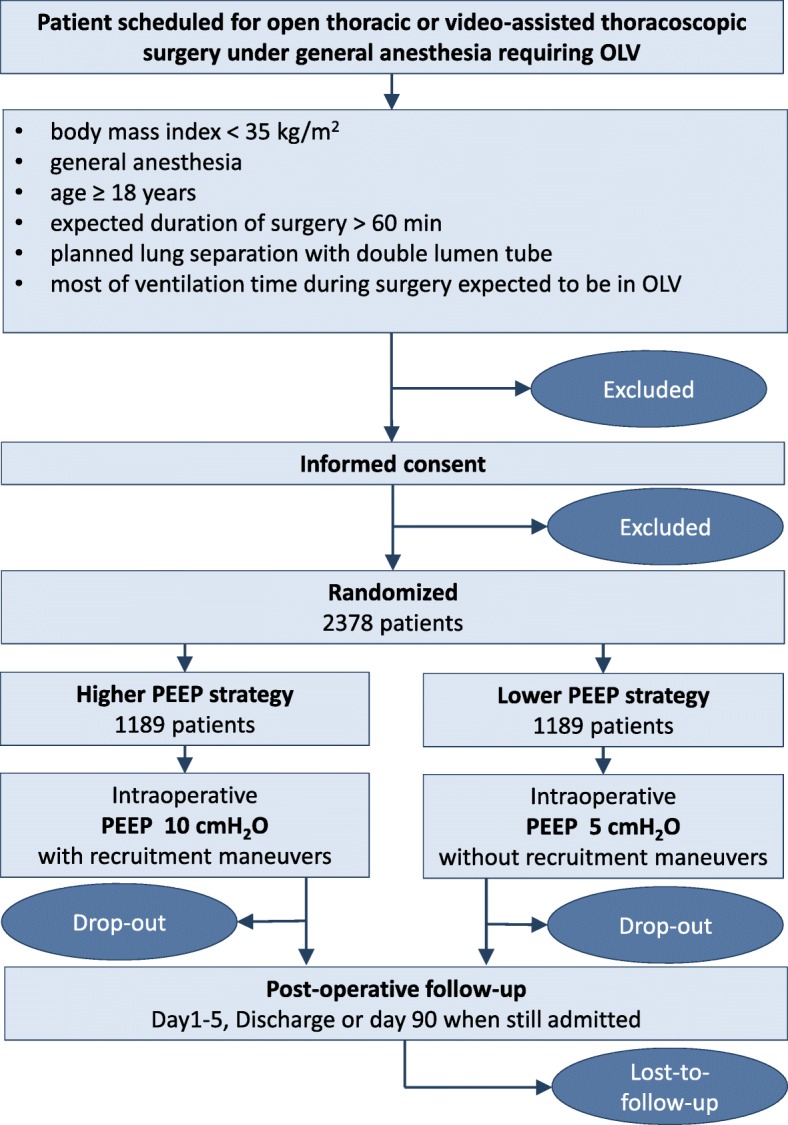
CONSORT Diagram for the PROTHOR trial. OLV one-lung ventilation, PEEP positive end-expiratory airway pressure
The PROTHOR trial tests the hypothesis that, in patients undergoing thoracic surgery under OLV, high levels of PEEP and RM, as compared with low levels of PEEP without RM, reduce PPC.
Study population
Investigators screen patients aged 18 years or above scheduled for open thoracic or video-assisted thoracoscopic surgery under general anesthesia requiring OLV, with a maximal body mass index of 35 kg/m2, and a planned duration of surgery of more than 60 min. Further, the expected duration of OLV shall be longer than two-lung ventilation (TLV), and lung separation is planned with a double lumen tube. The number of patients meeting these enrollment criteria will be recorded by means of a screening log file.
Patients are excluded if they have documented chronic obstructive pulmonary disease (COPD) GOLD grades III and IV, lung fibrosis, documented bullae, severe emphysema or pneumothorax; uncontrolled asthma; heart failure New York Heart Association grade 3 and 4 or coronary heart disease Canadian Cardiovascular Society grade 3 and 4; previous lung surgery; at-rest documented mean pulmonary arterial hypertension > 25 mmHg, or systolic pulmonary arterial pressure > 40 mmHg (as estimated by ultrasound); documented or suspected neuromuscular disease (e.g., thymoma, myasthenia, myopathies, muscular dystrophies); are planned for mechanical ventilation after surgery; are planned for bilateral procedures; undergo lung separation with a method other than double lumen tube; are operated in prone position; show persistent hemodynamic instability or intractable shock (as judged by the treating physician); have intracranial injury or tumor; are enrolled in other interventional studies or refuse informed consent; are pregnant (excluded by anamnesis and/or laboratory analysis); have documented preoperative hypercapnia > 45 mmHg (6 kPa, kPa); are planned for esophagectomy, pleural surgery only, sympathectomy surgery only, chest wall surgery only, mediastinal surgery only, and lung transplantation without surgical treatment of the lung tissue. Additionally, patients will be excluded if aspiration, moderate respiratory failure, infiltrates, pulmonary infection, atelectasis, cardiopulmonary edema, pleural effusion, pneumothorax, pulmonary embolism, purulent pleurisy, or lung hemorrhage are diagnosed before surgery.
Intervention
Mechanical ventilation
Mechanical ventilation is applied in volume-controlled mode. Following intubation, PEEP is set according to the randomization group, i.e., 5 cmH2O in the low PEEP level group and 10 cmH2O in the high PEEP level group. In both groups, the PEEP is maintained unchanged until extubation, unless rescue for hypoxemia mandates adjustments. If auto-PEEP is suspected, the respiratory rate or inspiratory to expiratory time (I:E) ratio may be changed at discretion of the treating physician.
In the high PEEP group, RM are performed at the following occasions:
after bronchoscopy or disconnection of the ventilated lung from the mechanical ventilator
at start of OLV
every 1 hour during OLV
after re-expansion of the non-dependent lung to resume TLV
end of surgery in supine position
During TLV, VT is set at 7 mL/kg predicted body weight (PBW). The PBW is calculated according to a predefined formula, as follows: 50 + 0.91 x (height in cm – 152.4) for males and 45.5 + 0.91 x (height in cm – 152.4) for females [23].
During OLV, VT will be decreased to 5 mL/kg PBW, while keeping other settings initially unchanged. If peak pressure > 40 cmH2O, or plateau pressure > 30 cmH2O, the I:E ratio is first changed to 1:1. Thereafter, VT can be decreased to 4 mL/kg PBW.
Further settings are fraction of inspiratory oxygen (FIO2) ≥ 0.4, I:E 1:1 to 1:2, and respiratory rate adjusted to normocapnia (partial arterial carbon dioxide pressure (PaCO2) between 35 and 45 mmHg).
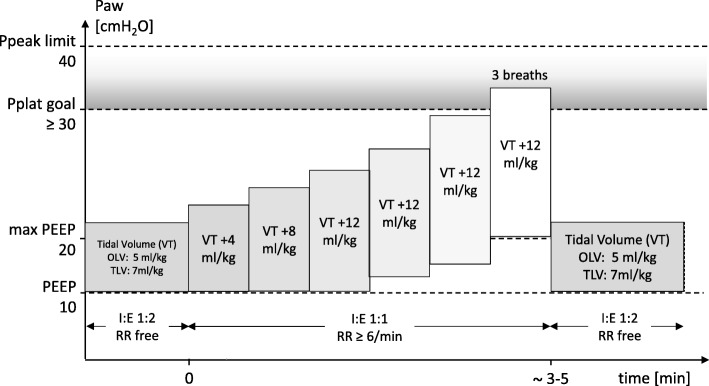
RM and lung expansion maneuvers
Standardized RM (Fig. 2) are performed with stepwise increase of VT in volume-controlled ventilation (Table 1).
Fig. 2.
Standardized lung recruitment maneuver in the high PEEP group. Ppeak peak airway pressure, Pplat plateau airway pressure, PEEP positive end-expiratory airway pressure, VT tidal volume normalized for predicted body weight, RR respiratory rate, I:E ratio between inspiratory and expiratory time
Table 1.
Recruitment and lung re-expansion maneuver steps
| Recruitment maneuver | 1. Increase FIO2 to 1.0 2. Set peak inspiratory pressure limit to 45 cmH2O 3. Set respiratory rate to six breaths/min 4. Set I:E ratio to 1:1 5. Increase VT in steps of approximately 2 mL/kg PBW until plateau pressure reaches 30–40 cmH2O 6. If the maximum VT allowed by the anesthesia ventilator is achieved and the plateau pressure is lower than 30 cmH2O, increase the PEEP as needed, to a maximum of 20 cmH2O 7. Allow three breaths while maintaining plateau pressure of 30–40 cmH2O 8. Set VT, PEEP, respiratory rate, and I:E ratio back to pre-recruitment values |
| Lung re-expansion maneuver | 1. Keep the non-ventilated under visual inspection, whenever possible 2. Connect the CPAP device with adequate oxygen flow (FIO2 1.0) to the non-ventilated lung 3. Set CPAP to 10 cmH2O during 20 s 4. Set CPAP to 15 cmH2O during 20 s 5. Set CPAP to 20 cmH2O during 20 s 6. If performed as part of a rescue therapy for hypoxemia and oxygenation has been restored, reduce CPAP as soon as possible to 10 cmH2O, or 5 cmH2O, or disconnect the CPAP device |
RM recruitment maneuver, FIO2 inspiratory fraction of oxygen, I:E ratio inspiratory to expiratory ratio, VT tidal volume, PBW predicted body weight, PEEP positive end-expiratory pressure, CPAP continuous positive airway pressure
A lung re-expansion maneuver of the non-ventilated lung may be necessary in both groups due to different reasons, including detection of air leaks by request of surgeons, as part of a rescue strategy due to hypoxemia, or before switching from OLV to TLV to re-expand the collapsed lung. Such a maneuver is performed in a hemodynamically stable patient (as judged by the anesthesiologist) and in agreement with the surgeon. To obtain standardization among centers, re-expansion maneuvers of non-ventilated lungs are performed with continuous positive airway pressure (Table 1).
Rescue strategies for intraoperative hypoxemia and intraoperative hypercapnia
If hypoxemia, defined as peripheral oxygen saturation (SpO2) < 90% for longer than 1 min occurs, rescue should be performed (Table 2). If hypercapnia (PaCO2 > 60 mmHg) with respiratory acidosis (pHa < 7.20) occurs during OLV, different steps are applied in the high and low PEEP groups (Table 2).
Table 2.
Rescue strategies for intraoperative hypoxemia and hypercapnia
| If hypoxemia occurs in the high PEEP group during TLV | 1. Apply RM 2. Increase PEEP to 12 cmH2O and apply RM 3. Increase FIO2 in steps of 0.1 until 1.0 4. Consider stepwise decrease of PEEP down to 8 cmH2O |
| If hypoxemia occurs in the low PEEP group during TLV | 1. Increase FIO2 in steps of 0.1 until 1.0 2. Apply RM 3. Increase PEEP to 6 cmH2O 4. Apply RM 5. Increase PEEP to 7 cmH2O 6. Apply RM |
| If hypoxemia occurs in the high PEEP group during OLV | 1. Apply RM 2. Increase PEEP to 12 cmH2O and apply RM 3. Increase FIO2 in steps of 0.1 up to 1.0 4. Apply oxygen to the non-ventilated lung, consider using CPAP (see lung re-expansion maneuver) up to a pressure of 20 cmH2O, or selective oxygen insufflation via fiberscope 5. Consider stepwise decrease of PEEP of the ventilated lung to 8 cmH2O 6. Consider surgical intervention (e.g., clamping of the pulmonary artery by surgeon) 7. Consider administration of inhaled nitric oxide or prostacyclin, or intravenous almitrine (provided the drug is approved in your country/institution) 8. Switch to TLV |
| If hypoxemia occurs in the low PEEP group during OLV | 1. Increase FIO2 in steps of 0.1 up to 1.0 2. Apply oxygen to the non-ventilated lung, consider CPAP therapy (re-expansion of the non-ventilated lung) up to a pressure of 20 cmH2O, or selective oxygen insufflation via fiberscope 3. Apply RM to the ventilated lung 4. Increase PEEP to 6 cmH2O 5. Apply RM to the ventilated lung 6. Increase PEEP to 7 cmH2O 7. Apply RM to the ventilated lung 8. Consider surgical intervention (e.g., clamping of the pulmonary artery by surgeon) 9. Consider administration of inhaled nitric oxide or prostacyclin, or intravenous almitrine (provided the drug is approved in your country/institution) 10. Switch to TLV |
| If hypercapnia (PaCO2 > 60 mmHg) with respiratory acidosis (pHa < 7.20) occurs during OLV, these steps are applied in the high and low PEEP groups | 1. Increase the respiratory rate (maximum 30/min, while minimizing intrinsic PEEP) 2. Increase VT stepwise up to 7 mL/kg PBW 3. Switch to TLV |
RM recruitment maneuver, PEEP positive end-expiratory pressure, TLV two lung ventilation, FIO2 inspiratory fraction of oxygen, OLV one lung ventilation, CPAP continuous positive airway pressure, PaCO2 arterial partial pressure of carbon dioxide, pHa arterial pH value, VT tidal volume, PBW predicted body weight
Standard procedures
To avoid interference with the trial intervention, routine elements of perioperative anesthesia care (including general anesthesia, postoperative pain management, physiotherapeutic procedures, and fluid management) are performed according to each center’s specific expertise and clinical routine. The following approaches are suggested (not mandatory) for anesthetic management:
Use of inhaled isoflurane, desflurane or sevoflurane, intravenous propofol, remifentanil or sufentanil, and cisatracurium, atracurium, vecuronium, or rocuronium (as required)
Use of sugammadex or a balanced solution of prostigmine, or neostigmine and atropine or glycopyrrolate for reversal of muscle relaxation, guided by neuromuscular function monitoring (for example, train-of-four stimulation)
For postoperative pain management to achieve a VAS pain score below 3 use regional anesthesia, including epidural, paravertebral, and intercostal blockade, and consideration of indications, contra-indications, and local preferences is encouraged, but not obligatory
Use of physiotherapy by early mobilization, deep breathing exercises with and without incentive spirometry, and stimulation of cough in the postoperative period
Avoid fluid underload and overload
Use of invasive measurement of arterial blood pressure whenever indicated
Use of appropriate prophylactic antibiotics whenever indicated
Use of gastric tubes, urinary bladder catheters, and more invasive monitoring according to individual needs, as well as local practice and/or guidelines
In addition, the study protocol stresses that routine intraoperative monitoring should include measurements of blood pressure, pulse oximetry, end-tidal carbon dioxide fraction, and electrocardiography. Every patient should receive at least one peripheral venous line to allow adequate fluid resuscitation during the study period. Other procedures should follow the Safe Surgery Checklist of the World Health Organization as published (www.who.int/patientsafety/safesurgery/en/index.html).
Minimization of bias
Allocation sequence is computer generated (nQuery Version 4.0) using permuted blocks with random sizes of 4, 6, and 8. Allocation is stratified per center with an allocation ratio of 1:1 for each group. The process of sequence generation and storage is managed by an independent database manager not involved in patient care. Randomization is then performed patient-by-patient using a web interface (REDcap™).
At each study site, at least two assessors are involved with the study. One assessor is involved with the intraoperative mechanical ventilation strategy and performs randomization as well as the interventions defined in the protocol. A second assessor, who is blinded to randomization, performs postoperative visits and assessment of primary and secondary endpoints.
Study endpoints
The primary endpoint is a collapsed composite of all PPC developing within the first 5 postoperative days. With this approach each complication has an equal weight. Patients who develop a least one complication are considered as meeting the primary endpoint.
PPC are defined as follows:
aspiration pneumonitis (defined as respiratory failure after the inhalation of regurgitated gastric contents)
moderate respiratory failure (SpO2 < 90% or PaO2 < 60 mmHg for 10 min in room air, responding to oxygen > 2 L/min)
severe respiratory failure (need for non-invasive or invasive mechanical ventilation due to poor oxygenation)
adult respiratory distress syndrome (mild, moderate, or severe according to the Berlin definition [24])
pulmonary infection (defined as new or progressive radiographic infiltrate plus at least two of the following: antibiotic treatment, tympanic temperature > 38 °C, leukocytosis or leucopenia (white blood cell (WBC) count < 4000 cells/mm3 or > 12,000 cells/mm3) and/or purulent secretions)
atelectasis (suggested by lung opacification with shift of the mediastinum, hilum, or hemidiaphragm towards the affected area, and compensatory over-inflation in the adjacent non-atelectatic lung)
cardiopulmonary edema (defined as clinical signs of congestion, including dyspnea, edema, rales, and jugular venous distention, with the chest x-ray demonstrating increase in vascular markings and diffuse alveolar interstitial infiltrates)
pleural effusion (chest x-ray demonstrating blunting of the costophrenic angle, loss of the sharp silhouette of the ipsilateral hemidiaphragm in upright position, evidence of displacement of adjacent anatomical structures, or (in supine position) a hazy opacity in one hemithorax with preserved vascular shadows)
pneumothorax (defined as air in the pleural space with no vascular bed surrounding the visceral pleura)
pulmonary infiltrates (chest x-ray demonstrating new monolateral or bilateral infiltrate without other clinical signs)
prolonged air leakage (air leak requiring at least 7 days of postoperative chest tube drainage)
purulent pleuritic (receiving antibiotics for a suspected infection, as far as not explained by the preoperative patient condition alone)
pulmonary embolism (as documented by pulmonary arteriogram or autopsy, or supported by ventilation/perfusion radioisotope scans, or documented by echocardiography and receiving specific therapy)
lung hemorrhage (bleeding through the chest tubes requiring reoperation, or three or more red blood cell packs)
Secondary clinical endpoints include:
extended PPC, including bronchospasm (defined as newly detected expiratory wheezing treated with bronchodilators) or mild respiratory failure (SpO2 < 90% or PaO2 < 60 mmHg for 10 min in room air, responding to oxygen ≤ 2 L/min)
intraoperative complications (use of continuous positive airway pressure for the non-ventilated lung, use of inhaled nitric oxide/prostacycline, use of selective fiberoscope insufflation, hypotension unresponsive to fluids and/or vasoactive drugs, new arrhythmias unresponsive to intervention, need for high dosage of vasoactive drugs (a dosage at the tolerance limit of the treating physician), need for massive transfusion, life-threatening surgical complication including major bleeding, tension pneumothorax, intracranial injury, hypoxemia and hypercapnia rescue maneuvers, deviation from prescribed PEEP or VT)
postoperative extrapulmonary complications
need for unexpected intensive care unit admission or readmission
number of hospital-free days at day 28
90-day survival
in-hospital survival
arterial blood gas analysis during surgery (PaO2, PaCO2, pHa)
any postoperative respiratory intervention (new requirement of non-invasive ventilation or mechanical ventilation)
Postoperative extrapulmonary complications include:
systemic inflammatory response syndrome (presence of two or more of the following findings: body temperature < 36 °C or > 38 °C, heart rate > 90 beats per minute, respiratory rate > 20 breaths per minute or, on blood gas, a PaCO2 < 32 mmHg (4.3 kPa), WBC count < 4000 cells/mm3 or > 12,000 cells/mm3, or > 10% band forms)
sepsis (systemic inflammatory response syndrome in response to a confirmed infectious process; infection can be suspected or proven (by culture, stain, or polymerase chain reaction), or a clinical syndrome pathognomonic for infection)
specific evidence for infection includes WBCs in normally sterile fluid (such as urine or cerebrospinal fluid, evidence of a perforated viscera (free air on abdominal x-ray or computer tomography scan, signs of acute peritonitis), abnormal chest x-ray consistent with pneumonia (with focal opacification), or petechiae, purpura, or purpura fulminans)
severe sepsis (sepsis with organ dysfunction, hypoperfusion, or hypotension), septic shock (sepsis with refractory arterial hypotension or hypoperfusion abnormalities in spite of adequate fluid resuscitation); signs of systemic hypoperfusion may be either end-organ dysfunction or serum lactate greater than 4 mmol/dL, other signs include oliguria and altered mental status
septic shock id defined as sepsis plus hypotension after aggressive fluid resuscitation, typically upwards of 6 L or 40 mL/kg of crystalloid
extra-pulmonary infection (wound infection + any other infection)
coma (Glasgow Coma Score < 8 in the absence of therapeutic coma or sedation)
acute myocardial infarction (detection of rise and/or fall of cardiac markers (preferably troponin) with at least one value above the 99th percentile of the upper reference limit, together with symptoms of ischemia, electrocardiography changes indicative of new ischemia, development of pathological Q-waves, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality or sudden unexpected cardiac death, involving cardiac arrest with symptoms suggestive of cardiac ischemia (but death occurring before the appearance of cardiac markers in blood))
acute renal failure (renal failure documented as follows: Risk: increased creatinine × 1.5 or glomerular filtration rate (GFR) decrease > 25% or urine output (UO) < 0.5 mL/kg/h × 6 h; Injury: increased creatinine × 2 or GFR decrease > 50% or UO < 0.5 mL/kg/h × 12 h; Failure: increased creatinine × 3 or GFR decrease > 75% or UO < 0.3 mL/kg/h × 24 h or anuria × 12 h; Loss: persistent acute renal failure = complete loss of kidney function > 4 weeks)
disseminated intravascular coagulation (score documented as follows: platelet count < 50 (2 points), < 100 (1 point), or ≥ 100 (0 points); D-dimer > 4 μg/mL (2 points), > 0.39 μg/mL (1 point) or ≤ 0.39 μg/mL (0 points); prothrombin time > 20.5 s (2 points), > 17.5 s (1 point), or ≤ 17.5 s (0 points), if ≥ 5 points: overt disseminated intravascular coagulation)
stroke (new clinical signs of stroke lasting longer than 24 h and corresponding findings in radiologic imaging)
hepatic failure (hepatic failure during short-term follow-up (5 postoperative days) is considered as follows: bilirubin serum level > 2 mg/dL + elevation of alanine amino transferase/aspartate amino transferase + lactate dehydrogenase × 2 above normal values; during long-term follow-up (until postoperative day 90) at new presence of hepatic encephalopathy and coagulopathy (international normalized ratio (INR) > 1.5) within 8 weeks after initial signs of liver injury (e.g., jaundice) without evidence for chronic liver disease)
gastrointestinal failure (any type of gastrointestinal bleeding or gastrointestinal failure score documented as follows: 0 = normal gastrointestinal function; 1 = enteral feeding with under 50% of calculated needs or no feeding 3 days after abdominal surgery; 2 = food intolerance or intra-abdominal hypertension; 3 = food intolerance and intra-abdominal hypertension; and 4 = abdominal compartment syndrome)
At the discretion of participating centers, blood and urine samples are collected preoperatively as well as directly postoperative and on the postoperative days 1–5. Samples will be analyzed centrally for systemic markers of inflammation and coagulation (including but not limited to interleukins 6 and 8, thrombin-antithrombin, protein C, and plasminogen activator inhibitor-1) as well as systemic markers of injury to the lungs (including but not limited to plasma E-cadherin, soluble receptor for advanced glycation end-products, surfactant proteins A and D, and distal organs, including renal injury (including but not limited to plasma/urine neutrophil gelatinase-associated lipocalin, and cystatin C). The standard operating procedure for collecting and processing plasma and urine is available in Additional file 1.
Study visits and data collection
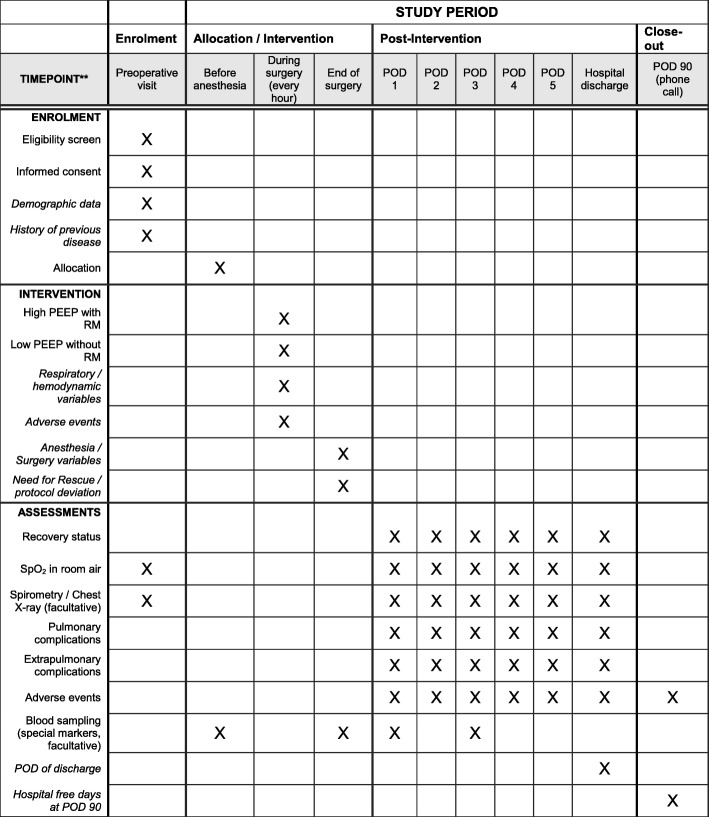
Patients are visited preoperatively, intraoperatively, daily between postoperative days 1 and 5, and on discharge. On postoperative day 90, patients are contacted by phone (Fig. 3).
Fig. 3.
Schedule of enrolment, interventions, and assessments. POD postoperative day, PEEP positive end-expiratory airway pressure, RM (lung) recruitment maneuver, SpO2 peripheral oxygen saturation
Patients are screened according to inclusion criteria. All patients meeting the inclusion criteria are registered in a screening log file by each center. Eligible patients meeting none of the exclusion criteria are asked by the physician for written informed consent (the consent form and information to study patients form are available in Additional file 1).
Baseline variables are collected, including gender, age, height, weight, ARISCAT Score, physical status according to the American Society of Anesthesiologists, functional status according to cumulated ambulation score, metabolic equivalents, cardiovascular status (heart failure according to the New York Heart Association, coronary heart disease according to Canadian Cardiovascular Society, atrial flutter/fibrillation, arterial hypertension), pulmonary status (chronic obstructive pulmonary disease, including steroids and/or inhalation therapy use, respiratory infection within the last month, use of non-invasive ventilatory support), history of obstructive sleep apnea (including Apnea and Hypopnea index or STOP-Bang score in patients without diagnosis of obstructive sleep apnea), metabolic status (diabetes mellitus, including data on treatment), history of active cancer, smoking status, alcohol status, gastroesophageal reflux, oral medication (e.g., use of antibiotics, statins, aspirin), preoperative organ function (SpO2 in supine position, upper body elevated 30–45 degrees breathing room air; if possible, respiratory rate, heart rate, mean arterial pressure, body temperature, airway secretion, including data on purulence, visual analogue scales (1–10) for dyspnea, thoracic rest pain and coughing pain).
Preoperative non-mandatory measurements include spirometry (arterial partial pressure of oxygen, carbon dioxide and pH value, forced vital capacity (FVC), forced expiratory volume in one second (FEV1), Tiffeneau value (FEV1/FVC), total lung capacity, diffusing capacity for carbon monoxide, and maximal oxygen consumption), predicted postoperative respiratory function (predicted postoperative FVC, FEV1, and diffusing capacity for carbon monoxide), chest x-ray (assessed for infiltrates, pleural effusion, atelectasis, pneumothorax, and cardiopulmonary edema) as well as routine laboratory tests (including hemoglobin, hematocrit, WBC count, platelet count, INR, partial thromboplastin time, creatinine, blood urea nitrogen, alanine amino transferase, aspartate amino transferase, bilirubin, c-reactive protein, and procalcitonin).
During the intraoperative visit, both surgery- as well as anesthesia-related data are recorded, including duration of anesthesia (from intubation to extubation or exit of operating room if on mechanical ventilation), duration of OLV and TLV, duration of surgery (from incision to closure), total blood loss, total urine output, side of OLV and side of surgery, method of lung separation (double lumen tube, endobronchial blocker, double lumen tube with embedded camera), way of placement confirmation (fiberoptic bronchoscopy, embedded camera), administration of antibiotics, use of regional anesthesia (epidural, paravertebral, other), use of non-invasive ventilation during induction, patient position during induction, patient temperature at the end of surgery, monitoring of neuromuscular function during anesthesia, use of neuromuscular blocker antagonists, priority and type of surgery, wound classification, type of surgical resection, patient position during surgery, estimated amount of lung resection, and drugs and fluids administered during anesthesia (e.g., anesthetics, vasoactive drugs, transfusion).
Ventilator settings, hemodynamics, need for rescue strategy, and adverse events (AEs) are recorded at anesthesia induction, with the patient in final surgical position and TLV, 10 min after OLV, hourly thereafter during OLV, and at the end of surgery with TLV in supine position. The routine measurements are documented first, then the gas probes are taken; thereafter, the RM is performed in the high PEEP group.
RM are documented during the plateau phase of the RM in the high PEEP group after bronchoscopy or disconnection of the ventilated lung from the mechanical ventilator, after the beginning of OLV, every 1 hour during OLV, after re-expansion of the non-dependent lung and resumption of TLV, and at the end of surgery in supine position.
Clinical data, including actual organ function and the presence of PPC, are scored during postoperative visits on a daily basis. Additionally, secondary endpoints, such as postoperative extrapulmonary complications, need for unexpected intensive care unit admission or readmission, and any type of postoperative respiratory intervention, are recorded. On day 1 after surgery, fluid and transfusion data are recorded in a detailed manner. Furthermore, the use of physiotherapy, breathing exercises, antibiotics as well as the cumulated ambulation score, status of wound healing, postoperative nausea, and vomiting are assessed.
Non-mandatory measures include chest x-ray, spirometry, and routine laboratory tests. Patients will be visited until discharge.
The number of hospital-free days at day 28 (including readmission since hospital discharge) and 90-day survival are calculated. Day 90 is defined as the last day of follow-up; accordingly, patients still admitted to hospital will be last visited on that day.
Study dropouts
Participation in the trial is voluntary. Patients have the right to withdraw consent to the study at any time for any reason without any consequence for further medical treatment. The reasons and circumstances for study discontinuation will be documented in the case report form (CRF). Primarily, all data will be analyzed according to the intention-to-treat principle. Secondarily, data will be analyzed per-protocol.
Handling of data
The objective of the clinical data management plan is to provide high-quality data by adopting standardized procedures to minimize the number of errors and missing data and, consequently, to generate an accurate database for analysis. Two members of the research team perform study monitoring. Remote monitoring is performed to signal early aberrant patterns, issues with consistency, credibility, and other anomalies. On-site assessment of protocol adherence and completeness of the research dossier will be conducted in up to 10 sites including the highest number of patients, and also neighbor sites to them.
Patient data are collected in pseudonymous form using a patient (identification) number composed of six digits, the first three of which correspond to the site ID and the remaining digits correspond to the patient inclusion number at the respective site. Study data are collected and managed using REDCap™ electronic data capture tools hosted at the Clinical Trial Coordination Center (KKS) of the University of Dresden, Germany. REDCap™ (Research Electronic Data Capture) is a Secure Sockets Layer encrypted, password-protected, web-based application designed to support data capture for research studies [25]. Full access to the final trial dataset will be granted to selected investigators only. If a sub-study is approved by the steering committee, access only to data related to the sub-study will be granted to the respective principal investigator.
Sample size calculations
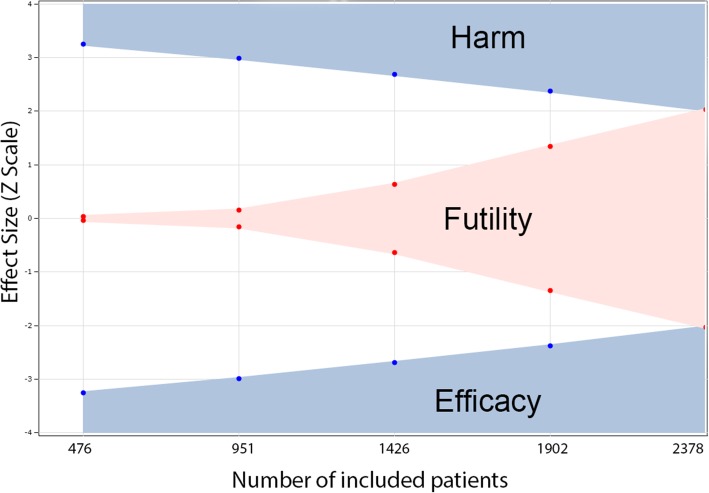
For this trial, we have planned to use an adaptive trial design, which accumulates data and uses external information to modify aspects of the design without undermining the validity and integrity of the trial. The group sequential methods design gives us the possibility for early stopping of the study if the experimental treatment shows a statistically significant therapeutic advantage at an interim assessment, but also allows early stopping for futility if the interim analysis reveals that, with high probability, the trial will be negative (Fig. 4).
Fig. 4.
Effect size (Z) according to enrollment of patients in the PROTHOR trial (including dropouts). Values of Z were obtained from an adaptive sequential design (see text) with stopping criteria for harm, futility, and efficacy of the intervention
Sample size calculation was based on our primary study endpoint, taking data collected from a subset of patients undergoing OLV for thoracic surgery in a prospective observational, multicenter, international study (LAS VEGAS) [26] into account. LAS VEGAS showed an incidence of approximately 23% for a PPC composite comparable to the present definition. Assuming a significance level of 0.05 and a power of 90% to detect the expected difference in postoperative pulmonary complications between the high PEEP group of 17.25% and the low PEEP group of 23% (risk ratio of 0.75), a sample size of 2259 has been calculated. Assuming a dropout rate of 5%, a total of 2378 patients have to be included in the study.
We used the software package East® for sample size calculations (East®, Version 6.3.1, Cytel Inc., USA). The Difference of Proportions test has been used to compare the independent samples from two populations (Group Sequential Design for a Binomial Superiority Trial, discrete endpoint two sample test, parallel design, difference of proportions, using the unpooled estimate of variance). The sample size calculation was done with the following parameters: Superiority Design, two-sided test; alpha 0.05; Power 0.9, allocation ratio 1; Proportion1 = 0.23; Proportion2 = 0.1725; Difference in Proportions = − 0.058.
We used an alpha-spending function to generate efficacy boundaries and a beta-spending function to generate futility boundaries (Fig. 4; gamma family spending function, type I error 0.05, type II error 0.1). By using a gamma of − 4 for the alpha and gamma of − 2 for the beta spending function we have a moderate hurdle for early stopping for efficacy and a reasonable chance to stop early due to futility (Table 3).
Table 3.
Z-statistic boundaries and boundary crossing probabilities
| Look | Information fraction | N | Cumulative alpha spent | Cumulative beta spent | Z-efficacy/harm | Z-futility | Boundary crossing probabilities under H1 | |
|---|---|---|---|---|---|---|---|---|
| Efficacy | Futility | |||||||
| 1 | 0.2 | 452 | 0.001 | 0.008 | ±3.252 | ±0.031 | 0.042 | 0.008 |
| 2 | 0.4 | 904 | 0.004 | 0.019 | ±2.986 | ±0.152 | 0.17 | 0.011 |
| 3 | 0.6 | 1355 | 0.009 | 0.036 | ±2.692 | ±0.631 | 0.281 | 0.017 |
| 4 | 0.8 | 1807 | 0.022 | 0.062 | ±2.374 | ±1.344 | 0.263 | 0.026 |
| 5 | 1.0 | 2259 | 0.05 | 0.1 | ±2.025 | ±2.025 | 0.143 | 0.038 |
Values were calculated using power = 0.90, alpha = 0.05, gamma spending function − 4 for the alpha and − 2 for the beta, expected incidence of postoperative pulmonary complications of 23% and 17.25% in the lower and higher positive end-expiratory pressure groups, respectively. Number of patients (N) is shown without correcting for dropouts. Look, interim analysis; H1, hypothesis 1 (group difference exists)
We constructed a non-binding futility boundary in such a way that it can be overruled if desired without inflating the type 1 error. This flexibility is important, since the data monitoring committee might well prefer to keep the trial going to gather additional information, despite crossing the futility boundary.
We planned to take five interim assessments at the data for evidence of efficacy, harm, and/or futility with the aim of possibly stopping the trial early. The planned number of assessments describes the number of time points, including the closing date of the study, at which the investigator plans to analyze the thus far collected data. The spacing of assessments will be equal. Therefore, interim analyses will be performed after 20% (476 patients), 40% (952 patients), 60% (1426 patients), 80% (1902 patients), and 100% of patients (2378 in total) included.
Patients will be randomly assigned to one of the two groups using a website-based data entry and randomization platform (REDcap™, Ver 6.6.2 Vanderbilt University, Tennessee, USA). Randomization will be conducted using blocks of 4, 6, and 8 patients, in aleatory fashion. Thereby, group sizes will be comparable at interim analyses, which will be conducted in a group-blinded manner.
Statistical analysis
Continuous distribution of the data will be assessed by visual inspection of histograms and D’Agostino–Pearson’s normality tests. For both arms, the baseline characteristics will be expressed as counts and percentages, means and standard deviations, or medians and interquartile ranges whenever appropriate.
Ventilatory parameters and vital signs over the surgery will be analyzed using a mixed-effect model with repeated measures and with patients and centers as a random-effect. No or minimal losses to follow-up for the primary and secondary outcomes are anticipated. Complete-case analysis will be carried out for all the outcomes. However, if more than 1% of missing data were found for the primary outcome, a sensitivity analysis using multiple imputations and estimating equation methods will be carried out.
Hypothesis tests will be two-sided with a significance level of 5% with exception of the primary outcome, due to the correction for the interim analyses. We will not adjust p values for multiple comparisons. Analyses will be performed using the R (R Core Team, 2016, Vienna, Austria) program.
Primary outcome
The effects of the intervention on incidence of PPC will be reported as numbers and percentages and estimated with risk ratios and 95% confidence intervals calculated with Wald’s likelihood ratio approximation test and with χ2 tests for hypothesis testing. For the analysis of the primary outcome, the result will be considered significant if the p value is less than 0.0428 (correspondent to the Z-value of 2.025 for efficacy or futility in the final analysis in Table 3). Kaplan–Meier curves will be used to report time to PPC. Curves will be compared with the log-rank tests, and hazard ratios with 95% confidence intervals will be calculated with Cox proportional hazard models without adjustment for covariates. The proportional hazard assumptions will be tested using scaled Schoenfeld residuals and alternative parametric survival models will be used if the proportionality assumption is not sustained.
Secondary outcomes
The effect of the intervention on secondary binary outcomes will be assessed with risk ratio and 95% confidence intervals calculated with Wald’s likelihood ratio approximation test and with χ2 tests for hypothesis testing. The effects of the intervention on hospital-free days at day 28 will be estimated with a Student t test and reported as the mean difference between the two groups. The consistency of the findings of the Student t-test for the hospital-free days at day 28 will be confirmed according to the mean ratio calculated by a generalized additive model considering a zero-inflated beta distribution.
Finally, 90-day mortality will be assessed using Kaplan–Meier curves, and hazard ratios with 95% confidence intervals will be calculated with Cox proportional hazard models without adjustment for covariates. The proportional hazard assumptions will be tested using scaled Schoenfeld residuals and alternative parametric survival models will be used if the proportionality assumption is not sustained.
Subgroup analyses
Treatment effects on incidence of PPC will be analyzed according to the following subgroups: (1) non-thoracoscopic versus thoracoscopic; (2) lateral decubitus versus supine position; (3) baseline SpO2 < 96% versus SpO2 ≥ 96%; and (4) COPD versus non-COPD. The effects on subgroups will be evaluated according to the interaction effects between each subgroup and the study arms by generalized linear models and presented in a forest plot.
Per-protocol analyses: The per-protocol population will consist of patients truly ventilated with the pre-specified protocol. Thus, patients will be excluded from this population if receiving PEEP < 10 cmH2O in the high PEEP group or PEEP > 5 cmH2O and FIO2 < 1.0 in the low PEEP group, in any measurement during the surgery.
Other exploratory analyses
As a sensitivity analysis, the effect of the intervention on the primary outcome will be re-estimated using a generalized linear mixed-effect model with stratification variables (center) as random effects. Since the primary outcome of the present study is a composite one, the choice of the statistical method is an important part of the design because various methods provide different power, depending on the situation. In addition to the standard analysis described above, the following analyses will be performed:
Count analysis – the number of positive component events (i.e., ‘count’) across the composite will be assessed. The groups will be compared on the count using a Mann–Whitney test, and the odds ratio with the 95% confidence interval will be assessed with a proportional odds logistic regression model
Individual component analysis – the effect of the intervention in each component will be analyzed using a generalized linear model using a Bonferroni correction for multiple comparisons; the 99.64% Bonferroni-corrected confidence intervals will be reported (1 – 0.05/14 = 0.9964)
Common effect test – a multivariate (i.e., multiple outcomes per subject) generalized estimating equations (GEE) model will be used to estimate a common effect odds ratio across the components
Average relative effect test – the average relative effect test will be assessed by averaging the component-specific treatment effect from the distinct effects model, and testing whether the average is equal to zero; in the GEE distinct effect model, a distinct treatment effect is estimated for each component
Heterogeneity of treatment effect – heterogeneity of treatment effect across components will be assessed by a treatment-by-component interaction test in the distinct effects GEE model
Clinical severity weight – each component will be weighted by a clinical severity weight determined a posteriori; a multivariate (i.e., multiple outcomes per subject) GEE model will be used to estimate a common effect odds ratio across the components while applying the severity weights
Cleaning and locking of the database
The database will be locked as soon as all data are entered and all discrepant or missing data are resolved – or if all efforts are employed and we consider that the remaining issues cannot be fixed. In this step, the data will be reviewed before database locking. After that, the study database will be locked and exported for statistical analysis. At this stage, permission for access to the database will be removed for all investigators, and the database will be archived.
Missing data
No or minimal losses to follow-up for the primary and secondary outcomes are anticipated. Complete-case analysis will be carried out for all the outcomes, that is, excluding patients with missing data in the outcome of interest. However, if more than 1% of missing data were found for the primary outcome, a sensitivity analysis using multiple imputations and estimating equation methods will be performed.
Sub-studies
Participating centers are allowed to conduct sub-studies provided that (1) no interference with the primary protocol occurs; (2) approval by the local institutional review board is obtained; and (3) the steering committee accepts the proposal according to its originality, feasibility, and importance. Publication of sub-studies, in any form, is strictly forbidden until the results of the primary study have been published.
Trial organization
The trial is managed by a team consisting of the chief investigator (Mert Sentürk), the trial coordinator (Thomas Kiss), the statisticians (A. Serpa Neto, K. Schubert and M. Kuhn), the informatics technician responsible for the web-based electronic data capture system (Marko Kaeppler), and independent monitors. A steering committee contributed to the design and revision of the study, and will be responsible for interpretation of data and compilation of a resulting manuscript.
Patient data and safety is closely monitored by a data safety and monitoring board (DSMB) that consists of a chairperson (Daniel Sessler) and four further members (Arthur Slutsky, Andreas Hoeft, Jean-Louis Vincent, Jennifer Hunter). All AEs entered into the electronic CRF within pre-specified time frames, including severe AEs and suspected unexpected severe adverse reactions, are monitored by an international AE manager (Ary Serpa Neto), who provides the DSMB with reports for review. The DSMB further monitors the overall status of the trial, e.g., progress of patient enrollment, general adherence to protocol, and completeness of data entry. Monitoring visits will be conducted as deemed necessary by the DSMB.
National coordinators are responsible for administration and communication with local principal investigators, as well as assistance during trial management and data collection.
When submitting the report on the results of the trial for possible publication, sites will be eligible to one collaborative co-authorship plus a further co-authorship for every 20 treated patients with complete datasets.
Discussion
The PROTHOR trial was designed to determine whether a high level of PEEP with RM, as compared to low PEEP without RM, during OLV for thoracic surgery, prevents PPC. We opted for testing the impact of two ventilation strategies at the same low VT in order to focus on the independent effects of different airway pressures, especially PEEP.
The decision to use a PEEP value of 5 cmH2O in the low PEEP group has been derived from a recent study on the practice of intraoperative mechanical ventilation and consensus agreement of the steering committee [26]. In order to allow generalizability of results and to impact on clinical practice, we opted for a pragmatic study, where a fixed level of high PEEP is used. The decision of using a PEEP of 10 cmH2O in the high PEEP group was based on the fact that this value, on average, resulted in maximal dynamic compliance of the respiratory system during OLV in a recent study, and was accompanied by minor variability only [27]. Additionally, this value is only 2 cmH2O higher than needed to effectively increase oxygenation and decrease physiological dead space [21, 28], while avoiding substantial hemodynamic impairment.
Even a PEEP titrated to a respiratory mechanics target, for example, the compliance of the respiratory system [27], represents a compromise in terms of regional overdistension and collapse-reopening of lung units. Depending on regional differences, even this optimal PEEP will not completely prevent atelectasis formation [29]. Thus, even an individualized PEEP titration in the high PEEP group would also result in a compromise between atelectrauma and volutrauma or barotrauma, and likely not differ importantly from the value selected a priori in the present trial.
The RM is based on a stepwise increase of VT and PEEP. This maneuver allows opening of lung units without interruption of mechanical ventilation and ensures standardization across different centers. Since it uses volume-controlled ventilation, virtually all anesthesia ventilators can perform this maneuver. The target airway pressure range for recruitment was based on the fact that a level of 30 cmH2O was proposed in a recent study [30], and that airway pressure exceeding 40 cmH2O does not importantly contribute to open lungs even in mild acute respiratory distress syndrome [31].
We decided for a combination of RM and PEEP in the high PEEP group. PEEP per se may not be enough to open atelectatic lung units. A CT study showed that, in patients at higher risk for development of intraoperative atelectasis, the combination of high PEEP and RM was able to revert lung collapse, whereas isolated high PEEP or RM did not achieve the same effect [32]. Furthermore, during OLV, RM followed by PEEP has been shown to be associated with a more homogenous distribution of ventilation [33].
The inspiratory time of approximately 5 s was chosen to allow enough pressure versus time product (over at least three consecutive cycles) to open atelectatic lung units. We opted for recruiting lungs not only after intubation, but also every hour thereafter, in order to revert possible progressive de-recruitment at PEEP of 10 cmH2O. For both the lower and higher PEEP groups, rescue protocols for the progression of intraoperative hypoxemia were defined in order to protect patients while allowing a standardized approach that minimizes the interference with the respective interventions. Importantly, deviations of the protocol, even rescue due to hypoxemia, are explicitly allowed, provided this in the best interest of patients.
It is worth noting that recommendations have been made also with regard to different phases and aspects of the anesthetic procedure, including monitoring, choice of anesthetics agents, muscle paralysis and its reversal, intravascular volume loading and maintenance, and postoperative analgesia. However, PROTHOR is a pragmatic study and influence on local practice of respective sites is kept at a minimum, focusing on factors that are more directly related with the hypothesis investigated.
Besides postoperative respiratory failure, several other adverse pulmonary events seem to add to the odds of mortality in the surgical population. In-hospital length of stay and mortality increase with the number of single pulmonary AEs in the postoperative period [3]. Therefore, in the PROTHOR trial we opted for a binary collapsed composite of single adverse pulmonary events as primary endpoint, despite the fact that single events may differ in terms of severity. Thus, the use of PPC as primary endpoint in the PROTHOR trial not only has clinical relevance for the practicing anesthetist, but increases the study power due to summation of incidences of single AEs. In spite of this, the study analysis will address not only the composite itself, but also the incidence of each element separately.
Not only the respiratory but also other organ systems may be impaired in the postoperative period in thoracic surgery patients. Thus, the analysis will also address the impact of intraoperative mechanical ventilation on single organs and a collapsed composite of non-pulmonary AEs, namely postoperative extrapulmonary complications. In addition, further relevant outcome measures that might be related to PPC and postoperative extrapulmonary complications, especially the hospital-free days at day 28, will be addressed. This outcome variable is not only a measure of morbidity, but also has direct impact on related health costs. Since we anticipate that, during surgery, both the lower and the higher PEEP groups will impact on intraoperative oxygenation, respiratory system mechanics, and arterial blood pressure, intraoperative respiratory function and hemodynamic variables will also be evaluated.
Much attention has been paid to safety in the PROTHOR trial. Accordingly, data and patient safety during the PROTHOR trial is closely monitored by a DSMB. Additionally, an AE manager has been designated. A web-based electronic data capture system (REDCap™) is used for building the database within a secure system, while allowing access to the eCRF and randomization of patients into groups.
We included complications that may be not directly related to VILI, more specifically pulmonary embolism and lung hemorrhage. However, the mechanical ventilation setting has been identified as an independent risk factor for venous thromboembolism [34]. Both mechanical ventilation and PEEP tend to decrease right and left ventricular preload, especially in the presence of hypovolemia and may increase venous thromboembolism risk by exacerbation of venous stasis. Recruitment maneuvers but also redistribution of lung perfusion during OLV and TLV may facilitate lung hemorrhage, which has been defined as bleeding through the chest tubes requiring reoperation or transfusion.
In summary, PROTHOR is the first randomized controlled trial in patients undergoing thoracic surgery that is adequately powered to compare the effects of intraoperative high PEEP with RM versus low PEEP without RM during OLV on PPC. The results of the PROTHOR trial will support anesthesiologists in their decision to set intraoperative PEEP during OLV with low VT for thoracic surgery.
Trial status
The PROTHOR trial is currently recruiting patients. Recruitment started January 2017. Estimated completion date 2021.
| Site name | Collaborator surname | Collaborator name | Email address |
| Military Medical Academy, Belgrade, Serbia | Neskovic | Vojislava | vojkan43@gmail.com |
| Radovic | Nevena | nevence1@yahoo.com | |
| Rondovic | Goran | grondovic@gmail.com | |
| Stamenkovic | Dusica | dusicastamenkovic@yahoo.com | |
| Vukovic | Rade | radvuk@gmail.com | |
| Zeba | Snjezana | snjezanazeba@hotmail.com | |
| Department of Anaesthesiology, University Hospital Aachen, Aachen, Germany | Rossaint | Rolf | rrossaint@ukaachen.de |
| Coburn | Mark | mcoburn@ukaachen.de | |
| Kowark | Ana | akowark@ukaachen.de | |
| Ziemann | Sebastian | sziemann@ukaachen.de | |
| van Waesberghe | Julia | jvanwaesberg@ukaachen.de | |
| Department of Anesthesiology, Academic Medical Center Amsterdam, Amsterdam, The Netherlands | Bauer | Wolfgang | w.o.bauer@amc.uva.nl |
| Terwindt | Lotte | l.e.terwindt@amc.uva.nl | |
| Attikon University Hospital, Athens, Greece | Kostopanagiotou | Kostas | kostop@hotmail.co.uk |
| Kostroglou | Andreas | andreaskostr@gmail.com | |
| Kyttari | Katerina | akyttari@gmail.com | |
| Sidiropoulou | Tatiana | tatianasid@gmail.com | |
| University Hospital Clínic de Barcelona, Spain | Jiménez Andújar | María-José | jjimenez@somclinic.cat |
| López-Baamonde | Manuel | lopez10@clinic.cat | |
| Navarro Ripoll | Ricard | rnavarr1@clinic.cat | |
| Rivera Vallejo | Lorena | lorivera@clinic.cat | |
| Weill Cornell Medicine, Department of Anesthesiology, New York, USA | Henry | Matthew | mah2065@med.cornell.edu |
| Jegarl | Anita | anj2024@med.cornell.edu | |
| Murrell | Matthew | mtm9006@med.cornell.edu | |
| O’Hara | Patrick | pao2011@med.cornell.edu | |
| Steinkamp | Michele | mls9004@med.cornell.edu | |
| Fachkrankenhaus Coswig GmbH Zentrum für Pneumologie, Allergologie, Beatmungsmedizin, Thoraxchirurgie |
Kraßler | Jens | krasslerj@fachkrankenhaus-coswig.de |
| Schäfer | Susanne | schaefers@fachkrankenhaus-coswig.de | |
| Department of Anesthesiology and Intensive Care Medicine, Pulmonary Engineering Group, University Hospital Carl Gustav Carus, Dresden, Germany | Becker | Charlotte | charlotte-becker@gmx.net |
| Birr | Katja | katjabirr@gmail.com | |
| Bluth | Thomas | thomas.bluth@uniklinikum-dresden.de | |
| Gama de Abreu | Marcelo | mgabreu@uniklinikum-dresden.de | |
| Hattenhauer | Sara | sara.hattenhauer@uniklinikum-dresden.de | |
| Kiss | Thomas | thomas.kiss@uniklinikum-dresden.de | |
| Scharffenberg | Martin | martin.scharffenberg@uniklinikum-dresden.de | |
| Teichmann | Robert | teichmannrobert@aol.com | |
| Wittenstein | Jakob | jakob.wittenstein@uniklinikum-dresden.de | |
| Department of Morpholo gy, Surgery and Experimental Medicine, University of Ferrara, Ferrara, Italy | Costanza | Vitali | costanza.vitali@student.unife.it |
| Savino | Spadaro | savinospadaro@gmail.com | |
| Volta | Carlo Alberto | vlc@unife.it | |
| Ragazzi | Riccardo | rgc@unife.it | |
| Calandra | Camilla | camilla.calandra@gmail.com | |
| Dept of Anesthesia and Intensive Care, University of Foggia, Italy, OO Riuniti Hospital | |||
| Mariano | Karim | karim_mariano@hotmail.it | |
| Mirabella | Lucia | lucia.mirabella@unifg.it | |
| Mollica | Giuseppina | giusymollica@virgilio.it | |
| Montrano | luigi | luigi.montrano@unifg.it | |
| Department of Anesthesiology and Intensive Care Medicine Clinic, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany | Loop | Torsten | torsten.loop@uniklinik-freiburg.de |
| Semmelmann | Axel | axel.semmelmann@uniklinik-freiburg.de | |
| Wirth | Steffen | steffen.wirth@uniklinik-freiburg.de | |
| Department of Anesthesiology, Fudan University Shanghai Cancer Center; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China | Miao | Changhong | miaochh@aliyun.com |
| Zhong | Jing | ziteng1934@163.com | |
| Lv | Hu | lvhu086@126.com | |
| Wang | Hui | 2502425738@qq.com | |
| Zhang | Xue | zx02190554@126.com | |
| Zhang | Yue | aileencheung0807@163.com | |
| IRCCS San Martino Policlinico Hospital, Genoa, Italy | Pelosi | Paolo | ppelosi@hotmail.com |
| Corsi | Laura | corsilaura@yahoo.it | |
| Partroniti | Nicolò | nicoloantonino.patroniti@unige.it | |
| Mandelli | Maura | maura.mandelli@gmail.com | |
| Bonatti | Giulia | giulia.bonatti@gmail.com | |
| Simonassi | Francesca | francesca.simonassi@gmail.com | |
| Gratarola | Angelo | a.gratarola@gmail.com | |
| Insular Hospital, Gran Canaria, Spain | Rodriguez Ruiz | Juan José | juanjo.rodriguezruiz@gmail.com |
| Socorro | Tania | austania@gmail.com | |
| University Hospital of Heraklion, Heraklion, Greece | Christofaki | Maria | mchristofaki@yahoo.gr |
| Nyktari | Vasileia | vnyktari@gmail.com | |
| Papaioannou | Alexandra | papaioaa@uoc.gr | |
| University Istanbul University, Istanbul Medical Faculty, Department of Anaesthesiology and Intensive Care, Istanbul, Turkey | Şentürk | Nüzhet Mert | senturkm@istanbul.edu.tr |
| Bingul | Emre | dremrebingul@gmail.com | |
| Orhan Sungur | Mukadder | mukadder.orhan@gmail.com | |
| Sungur | Zerrin | zerrin_sr@yahoo.com | |
| University Hospital of Munich, Munich, Germany | Heidegger | Manuel | manuel.heidegger@campus.lmu.de |
| Dossow | Vera | vera.dossow@med.uni-muenchen.de | |
| Jerichow | Wiebke | w.jerichow@web.de | |
| Kammerer | Tobias | tobias.kammerer@med.uni-muenchen.de | |
| Richter | Julia | julia.richter@richtersisters.de | |
| Schuba | Barbara | barbara.schuba@med.uni-muenchen.de | |
| Speck | Eike | eike.speck@med.uni-muenchen.de | |
| Stierle | Anna-Lisa | anna@stierle-mail.de | |
| University Hospital of Prague, Prague, Czech Republic | Bruthans | Jan | jan.bruthans@vfn.cz |
| Matek | Jan | jan.matek@vfn.cz | |
| Michálek | Pavel | pavel.michalek@vfn.cz | |
| Radboud University Medical Centre Nijmegen, The Netherlands | Didden | Loes | loes.didden@radboudumc.nl |
| Hofland | Jan | jan.hofland@radboudumc.nl | |
| Kuut | Marieke | marieke.kuut@radboudumc.nl | |
| Mourisse | Jo | jo.mourisse@radboudumc.nl | |
| Hospital Universitario de la Ribera, Alzira, Spain | Aragon | Sonsoles | aragon_son@gva.es |
| Esturi | Rafael | esturi_raf@gva.es | |
| Miñana | Encarna | minyana_enc@gva.es | |
| Sanchez | Fernando | sanchez_fergar@gva.es | |
| Department of Anaesthesia, Postoperative ICU, Pain Relief & Palliative Care Clinic, ‘Sotiria’ Chest Diseases Hospital, Athens, Greece | Sfikas | Elaine | elaisfikas@hotmail.com |
| Kapezanos | Athanasios | a.kapezanos@yahoo.com | |
| Papamichail | Konstantinos | kospapam@yahoo.gr | |
| Toufektzian | Levon | tlevon@gmail.com | |
| Voyagis | Gregorios | gsvgasman@yahoo.com | |
| Hospital General Universitario of Valencia, Valencia, Spain | Granell Gil | Manuel | mgranellg@hotmail.com |
| Vergara Sánchez | Asunción | asuncionvergara@gmail.com | |
| De Andres | Jose | deandres_jos@gva.es | |
| Morales Sarabia | Javier | jems.com@gmail.com | |
| Broseta Lleó | Ana | ana.broseta@gmail.com | |
| Hernández Laforet | Javier | jaherla@hotmail.com | |
| Murcia Anaya | Mercedes | merxemurcia@gmail.com | |
| Hospital Álvaro Cunqueiro, Vigo, Spain | Pereira Matalobos | Denis | denispema@gmail.com |
| Aguirre Puig | Pilar | pilaraguirrepuig@yahoo.es | |
| Division Anesthesiology and ICU, Department of Thoracic Surgery Jordanovac University Hospital Centre Zagreb, Zagreb,Croatia | Špiček Macan | Jasna | mspicekj@hotmail.com |
| Karadza | Vjekoslav | vkaradza@xnet.hr | |
| Kolaric | Nevenka | nevenkakolaric@yahoo.com | |
| University Medical Centre Ljubljana, Slovenia | Andjelković | Lea | lea.andjelkovic@gmail.com |
| Drnovšek Globokar | Mojca | mojca.drnovsek@gmail.com | |
| Gorjup | Kristina | kristinagorjup@gmail.com | |
| Mavko | Ana | ana.mavko@gmail.com | |
| Pirc | Dejan | pirc.dejan@gmail.com | |
| Institutul de Pneumoftiziologie, Bucharest, Romania | Genoveva | Cadar | genovevacadar@hotmail.com |
| Istrate | Raluca | raluca_crintea@yahoo.com | |
| Stoica | Radu | raduati1957@gmail.com | |
| Central Military Emergency University Hospital, Bucharest, Romania | Corneci | Dan | dcorneci@yahoo.com |
| Tanase | Narcis Valentin | tanasenv@yahoo.com | |
| Clinic for Anesthesia and Intensive Therapy, Clinical Center Nis, School of Medicine, University of Nis, Nis, Serbia | Radmilo | Jankovic | jankovic.radmilo@gmail.com |
| Cvetanovic | Vladan | vladan.cvetanovic@gmail.com | |
| Dinic | Vesna | vesnadinic1981@gmail.com | |
| Grbesa | Tijana | grbesatijana@gmail.com | |
| Jovic | Katarina | katarina.jovic76@gmail.com | |
| Nikolic | Aleksandar | draleksandarnikolic@hotmail.com | |
| Stojanovic | Milena | milenastojanoviclaci@gmail.com | |
| Veselinovic | Ines | inesveselinovic@gmail.com | |
| Vukovic | Anita | anita83ptr@gmail.com | |
| Merheim Hospital, Cologne, Germany | Wappler | Frank | wapplerf@kliniken-koeln.de |
| Defosse | Jerome Michel | defossej@kliniken-koeln.de | |
| Wehmeier | Stefanie | wehmeiers@kliniken-koeln.de | |
| University Hospital Münster, Department of Anesthesiology, Intensive Care and Pain Medicine, Münster, Germany | Ermert | Thomas | ermert@uni-muenster.de |
| Zarbock | Alexander | zarbock@uni-muenster.de | |
| Wenk | Manuel | manuelwenk@uni-muenster.de | |
| Hospital Marie Lannelongue, Le Plessis-Robinson, France | Ion | Daniela Iolanda | iolandaion@yahoo.com |
| Ionescu | Cristian | iraducristi@yahoo.com | |
| Department of Anesthesiology and Intensive Care Medicine, University Hospital Otto von Guericke, Magdeburg, Germany | Schilling | Thomas | thomas.schilling@med.ovgu.de |
| Macharadze | Tamar | tamrikomacharadze@hotmail.com | |
| Taichung Veterans General Hospital, Taichung City, Taiwan | Li | Pei-Ching | pei9502@gmail.com |
| Chang | Yi-Ting | kikicoco36@gmail.com | |
| Anestesia e Rianimazione, Policlinico Univ. G. Martino, Messina, Italy | Noto | Alberto | dralbert@unime.it |
| Calì | Placido | placidocali@alice.it | |
| Desalvo | Giovanni | giannidesalvo@gmail.com | |
| Deluca | Raffaele | delucaraffa@gmail.com | |
| Giofre’ | Nicola | ngiofre@hotmail.it |
Additional files
PROTHOR-Patient information. (DOCX 19 kb)
Acknowledgements
We thank Marko Kaeppler for building and maintenance of the electronic CRF. We are indebted to all PROTHOR investigators listed in the Appendix.
Funding
Funded in the United Kingdom by the AAGBI/NIAA (Association of Anaesthesists of Great Britain and Ireland/National Institute of Academic Anaesthesia). Additionally, each participating center accounts for the participants individual costs (institutional funding). The coordination center is supported by funding from the Department of Anesthesiology and Intensive Care Medicine, University Hospital Carl Gustav Carus, Technische Universität Dresden, Germany.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Appendix
PROTHOR Investigators
Authors’ contributions
TK conceived and designed the study, coordinates the overall study, and drafted the manuscript. JW, CB, and KB participated in the design of the study, act as local investigators in Dresden, and contributed to the final manuscript. GC participated in the design of the study and contributed to the final manuscript. EC participated in the design of the study, coordinates the study in the United States of America, and contributed to the final manuscript. MRET participated in the design of the study and contributed to the final manuscript. LFF participated in the design of the study, coordinates the study in Brazil, and contributed to the final manuscript. CG participated in the design of the study and contributed to the final manuscript. MG participated in the design of the study, coordinates the study in Spain, and contributed to the final manuscript. MGdA conceived and designed the study and drafted the manuscript, coordinates the study in Germany. TH participated in the design of the study and contributed to the final manuscript. MH participated in the design of the study, coordinates the study in the Netherlands, and contributed to the final manuscript. RJ participated in the design of the study, coordinates the study in Serbia, and contributed to the final manuscript. WK participated in the design of the study and contributed to the final manuscript. JK participated in the design of the study and contributed to the final manuscript. TL participated in the design of the study, coordinates the study in Germany, and contributed to the final manuscript. MJL participated in the design of the study, coordinates the study in Switzerland, and contributed to the final manuscript. NM participated in the design of the study and contributed to the final manuscript. GHM participated in the design of the study, coordinates the study in the United Kingdom, contributed to the final manuscript, and obtained funding to allow Portfolio inclusion by the NIHR in the United Kingdom. MTM participated in the design of the study, coordinates the study in the United States of America, and contributed to the final manuscript. VN participated in the design of the study and coordinates the study in Serbia. ZNS participated in the design of the study and contributed to the final manuscript. VN participated in the design of the study and coordinates the study in Serbia. PP conceived and designed the study, drafted the manuscript, and coordinates the study in Italy. RR participated in the design of the study and contributed to the final manuscript. MS conceived and designed the study and drafted the manuscript. ASN performed the sample size calculation, drafted the statistical analysis plan, and contributed to the final manuscript. PS participated in the design of the study and contributed to the final manuscript. LS participated in the design of the study, coordinates the study in Belgium, and contributed to the final manuscript. TV participated in the design of the study, coordinates the study in Hungary, and contributed to the final manuscript. GV participated in the design of the study, coordinates the study in Greece, and contributed to the final manuscript. JZ participated in the design of the study and contributed to the final manuscript. MS conceived and designed the study and drafted the manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Ethics approval and consent to participate
The Istanbul University, Medical Faculty, Ethical Committee of clinical research approved the study on 08.04.2016 (File number 2016/483, study protocol version 1.9). Additionally, the institutional review board at the University Hospital Dresden, Technische Universität Dresden, Dresden, Germany, approved the study on 23.09.2016 (reference no. EK 392092016, study protocol version 1.9). The respective review boards of participating sites also approved the study. PROTHOR is designed in accordance to the principles of the Declaration of Helsinki. Written informed consent was obtained from every enrolled patient upon request by the local institutional review board.
Consent for publication
Not applicable.
Competing interests
MRET received free airway device samples from Ambu in April 2014 and from Airtraq in March 2015 for use in another three published studies. He has no direct financial or other interests in Ambu or Airtraq (in the context of this and his studies).
All other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
T. Kiss, Email: thomas.kiss@uniklinikum-dresden.de
J. Wittenstein, Email: jakob.wittenstein@uniklinikum-dresden.de
C. Becker, Email: charlotte-becker@gmx.net
K. Birr, Email: katjabirr@gmail.com
G. Cinnella, Email: gilda.cinnella@unifg.it
E. Cohen, Email: edmond.cohen@mountsinai.org
M. R. El Tahan, Email: mohamedrefaateltahan@hotmail.com
L. F. Falcão, Email: luizfernandofalcao@gmail.com
C. Gregoretti, Email: c.gregoretti@gmail.com
M. Granell, Email: mgranellg@hotmail.com
T. Hachenberg, Email: thomas.hachenberg@med.ovgu.de
M. W. Hollmann, Email: m.w.hollmann@amc.uva.nl
R. Jankovic, Email: jankovic.radmilo@gmail.com
W. Karzai, Email: karzai@zentralklinik.de
J. Krassler, Email: KrasslerJ@fachkrankenhaus-coswig.de
T. Loop, Email: torsten.loop@uniklinik-freiburg.de
M. J. Licker, Email: marc-joseph.licker@hcuge.ch
N. Marczin, Email: n.marczin@imperial.ac.uk
G. H. Mills, Email: g.h.mills@sheffield.ac.uk
M. T. Murrell, Email: mtm9006@med.cornell.edu
V. Neskovic, Email: znisnevi@montefiore.org
Z. Nisnevitch-Savarese, Email: znisnevi@montefiore.org
P. Pelosi, Email: ppelosi@hotmail.com
R. Rossaint, Email: rrossaint@ukaachen.de
M. J. Schultz, Email: marcus.j.schultz@gmail.com
A. Serpa Neto, Email: aryserpa@terra.com.br
P. Severgnini, Email: paolo.severgnini@uninsubria.it
L. Szegedi, Email: laszlo.szegedi@skynet.be
T. Vegh, Email: veghdr@gmail.com
G. Voyagis, Email: gsvgasman@yahoo.com
J. Zhong, Email: ziteng1934@163.com
M. Gama de Abreu, Email: mgabreu@uniklinikum-dresden.de
M. Senturk, Email: senturkm@istanbul.edu.tr
for the PROTHOR investigators:
Vojislava Neskovic, Nevena Radovic, Goran Rondovic, Dusica Stamenkovic, Rade Vukovic, Snjezana Zeba, Rolf Rossaint, Mark Coburn, Ana Kowark, Sebastian Ziemann, Julia van Waesberghe, Wolfgang Bauer, Lotte Terwindt, Kostas Kostopanagiotou, Andreas Kostroglou, Katerina Kyttari, Tatiana Sidiropoulou, María-José Jiménez Andújar, Manuel López-Baamonde, Ricard Navarro Ripoll, Lorena Rivera Vallejo, Matthew Henry, Anita Jegarl, Matthew Murrell, Patrick O’Hara, Michele Steinkamp, Jens Kraßler, Susanne Schäfer, Charlotte Becker, Katja Birr, Thomas Bluth, Marcelo Gama de Abreu, Sara Hattenhauer, Thomas Kiss, Martin Scharffenberg, Robert Teichmann, Jakob Wittenstein, Vitali Costanza, Spadaro Savino, Carlo Alberto Volta, Riccardo Ragazzi, Karim Mariano, Lucia Mirabella, Giuseppina Mollica, Luigi Montrano, Torsten Loop, Axel Semmelmann, Steffen Wirth, Changhong Miao, Jing Zhong, Hu Lv, Hui Wang, Xue Zhang, Yue Zhang, Paolo Pelosi, Laura Corsi, Nicolò Partroniti, Maura Mandelli, Giulia Bonatti, Francesca Simonassi, Angelo Gratarola, Juan José Rodriguez Ruiz, Tania Socorro, Maria Christofaki, Vasileia Nyktari, Alexandra Papaioannou, Nüzhet Mert Şentürk, Emre Bingul, Mukadder Orhan Sungur, Zerrin Sungur, Manuel Heidegger, Vera Dossow, Wiebke Jerichow, Tobias Kammerer, Julia Richter, Barbara Schuba, Eike Speck, Anna-Lisa Stierle, Jan Bruthans, Jan Matek, Pavel Michálek, Loes Didden, Jan Hofland, Marieke Kuut, Jo Mourisse, Sonsoles Aragon, Rafael Esturi, Encarna Miñana, Fernando Sanchez, Elaine Sfikas, Athanasios Kapezanos, Konstantinos Papamichail, Levon Toufektzian, Gregorios Voyagis, Manuel Granell Gil, Asunción Vergara Sánchez, José De Andres, Javier Morales Sarabia, Ana Broseta Lleó, Javier Hernández Laforet, Mercedes Murcia Anaya, Denis Pereira Matalobos, Pilar Aguirre Puig, Jasna Špiček Macan, Vjekoslav Karadza, Nevenka Kolaric, Lea Andjelković, Mojca Drnovšek Globokar, Kristina Gorjup, Ana Mavko, Dejan Pirc, Cadar Genoveva, Raluca Istrate, Radu Stoica, Dan Corneci, Narcis Valentin Tanase, Jankovic Radmilo, Vladan Cvetanovic, Vesna Dinic, Tijana Grbesa, Katarina Jovic, Aleksandar Nikolic, Milena Stojanovic, Ines Veselinovic, Anita Vukovic, Frank Wappler, Jerome Michel Defosse, Stefanie Wehmeier, Thomas Ermert, Carola Wempe, Manuel Wenk, Daniela Iolanda Ion, Cristian Ionescu, Thomas Schilling, Tamar Macharadze, Pei-Ching Li, Yi-Ting Chang, Alberto Noto, Placido Calì, Giovanni Desalvo, Raffaele Deluca, and Nicola Giofre
References
- 1.Canet J, Sabate S, Mazo V, Gallart L, de Abreu MG, Belda J, et al. Development and validation of a score to predict postoperative respiratory failure in a multicentre European cohort: A prospective, observational study. Eur J Anaesthesiol. 2015;32(7):458–470. doi: 10.1097/EJA.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 2.Serpa Neto A, Hemmes SN, Barbas CS, Beiderlinden M, Fernandez-Bustamante A, Futier E, et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med. 2014;2(12):1007–1015. doi: 10.1016/S2213-2600(14)70228-0. [DOI] [PubMed] [Google Scholar]
- 3.Mazo V, Sabate S, Canet J, Gallart L, de Abreu MG, Belda J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121(2):219–231. doi: 10.1097/ALN.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 4.Neto AS, da Costa LGV, Hemmes SNT, Canet J, Hedenstierna G, Jaber S, et al. The LAS VEGAS risk score for prediction of postoperative pulmonary complications: An observational study. Eur J Anaesthesiol. 2018;35(9):691–701. doi: 10.1097/EJA.0000000000000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guldner A, Kiss T, Serpa Neto A, Hemmes SN, Canet J, Spieth PM, et al. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: a comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology. 2015;123(3):692–713. doi: 10.1097/ALN.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 6.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99(5):944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157(1):294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 8.Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289(16):2104–2112. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- 9.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282(1):54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 10.Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, et al. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111(4):826–835. doi: 10.1097/ALN.0b013e3181b764d2. [DOI] [PubMed] [Google Scholar]
- 11.Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175(2):160–166. doi: 10.1164/rccm.200607-915OC. [DOI] [PubMed] [Google Scholar]
- 12.Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol (1985) 2000;89(4):1645–1655. doi: 10.1152/jappl.2000.89.4.1645. [DOI] [PubMed] [Google Scholar]
- 13.Dos Santos CC, Slutsky AS. The contribution of biophysical lung injury to the development of biotrauma. Annu Rev Physiol. 2006;68:585–618. doi: 10.1146/annurev.physiol.68.072304.113443. [DOI] [PubMed] [Google Scholar]
- 14.Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology. 2005;102(4):838–854. doi: 10.1097/00000542-200504000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, Pasqualucci Mde O, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308(16):1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 16.Hemmes SN, Serpa Neto A, Schultz MJ. Intraoperative ventilatory strategies to prevent postoperative pulmonary complications: a meta-analysis. Curr Opin Anaesthesiol. 2013;26(2):126–133. doi: 10.1097/ACO.0b013e32835e1242. [DOI] [PubMed] [Google Scholar]
- 17.Severgnini P, Selmo G, Lanza C, Chiesa A, Frigerio A, Bacuzzi A, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology. 2013;118(6):1307–1321. doi: 10.1097/ALN.0b013e31829102de. [DOI] [PubMed] [Google Scholar]
- 18.PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology. Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet. 2014;384(9942):495–503. doi: 10.1016/S0140-6736(14)60416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen Y, Zhong M, Wu W, Wang H, Feng M, Tan L, et al. The impact of tidal volume on pulmonary complications following minimally invasive esophagectomy: a randomized and controlled study. J Thorac Cardiovasc Surg. 2013;146(5):1267–1273. doi: 10.1016/j.jtcvs.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 20.Yang M, Ahn HJ, Kim K, Kim JA, Yi CA, Kim MJ, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: A randomized controlled trial. Chest. 2011;139(3):530–537. doi: 10.1378/chest.09-2293. [DOI] [PubMed] [Google Scholar]
- 21.Tusman G, Bohm SH, Sipmann FS, Maisch S. Lung recruitment improves the efficiency of ventilation and gas exchange during one-lung ventilation anesthesia. Anesth Analg. 2004;98(6):1604–1609. doi: 10.1213/01.ANE.0000068484.67655.1A. [DOI] [PubMed] [Google Scholar]
- 22.Benumof J. Conventional and differential lung management of one-lung ventilation. Anesthesia for Thoracic Surgery. 2. Philadelphia: W.B. Saunders; 1995. [Google Scholar]
- 23.Devine B. Gentamicin therapy. Drug Intell Clin Pharm. 1974;8:650–655. doi: 10.1177/106002807400801104. [DOI] [Google Scholar]
- 24.ARDS Definition Task Force Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LAS VEGAS Investigators Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS - an observational study in 29 countries. Eur J Anaesthesiol. 2017;34(8):492–507. doi: 10.1097/EJA.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrando C, Mugarra A, Gutierrez A, Carbonell JA, Garcia M, Soro M, et al. Setting individualized positive end-expiratory pressure level with a positive end-expiratory pressure decrement trial after a recruitment maneuver improves oxygenation and lung mechanics during one-lung ventilation. Anesth Analg. 2014;118(3):657–665. doi: 10.1213/ANE.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 28.Unzueta C, Tusman G, Suarez-Sipmann F, Bohm S, Moral V. Alveolar recruitment improves ventilation during thoracic surgery: a randomized controlled trial. Br J Anaesth. 2012;108(3):517–524. doi: 10.1093/bja/aer415. [DOI] [PubMed] [Google Scholar]
- 29.Bluth T, Teichmann R, Kiss T, Bobek I, Canet J, Cinnella G, et al. Protective intraoperative ventilation with higher versus lower levels of positive end-expiratory pressure in obese patients (PROBESE): study protocol for a randomized controlled trial. Trials. 2017;18:202. doi: 10.1186/s13063-017-1929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 31.Cressoni M, Chiumello D, Algieri I, Brioni M, Chiurazzi C, Colombo A, et al. Opening pressures and atelectrauma in acute respiratory distress syndrome. Intensive Care Med. 2017;43(5):603–611. doi: 10.1007/s00134-017-4754-8. [DOI] [PubMed] [Google Scholar]
- 32.Reinius H, Jonsson L, Gustafsson S, Sundbom M, Duvernoy O, Pelosi P, et al. Prevention of atelectasis in morbidly obese patients during general anesthesia and paralysis: a computerized tomography study. Anesthesiology. 2009;111(5):979–987. doi: 10.1097/ALN.0b013e3181b87edb. [DOI] [PubMed] [Google Scholar]
- 33.Kozian A, Schilling T, Schutze H, Senturk M, Hachenberg T, Hedenstierna G. Ventilatory protective strategies during thoracic surgery: effects of alveolar recruitment maneuver and low-tidal volume ventilation on lung density distribution. Anesthesiology. 2011;114(5):1025–1035. doi: 10.1097/ALN.0b013e3182164356. [DOI] [PubMed] [Google Scholar]
- 34.Cook D, Attia J, Weaver B, McDonald E, Meade M, Crowther M. Venous thromboembolic disease: an observational study in medical-surgical intensive care unit patients. J Crit Care. 2000;15(4):127–132. doi: 10.1053/jcrc.2000.19224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PROTHOR-Patient information. (DOCX 19 kb)
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.