Abstract
Background:
In the CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) study, Cladribine Tablets significantly improved clinical and magnetic resonance imaging (MRI) outcomes (vs placebo) in patients with relapsing-remitting multiple sclerosis.
Objective:
Describe two clinically relevant definitions for patients with high disease activity (HDA) at baseline of the CLARITY study (utility verified in patients receiving placebo) and assess the treatment effects of Cladribine Tablets 3.5 mg/kg compared with the overall study population.
Methods:
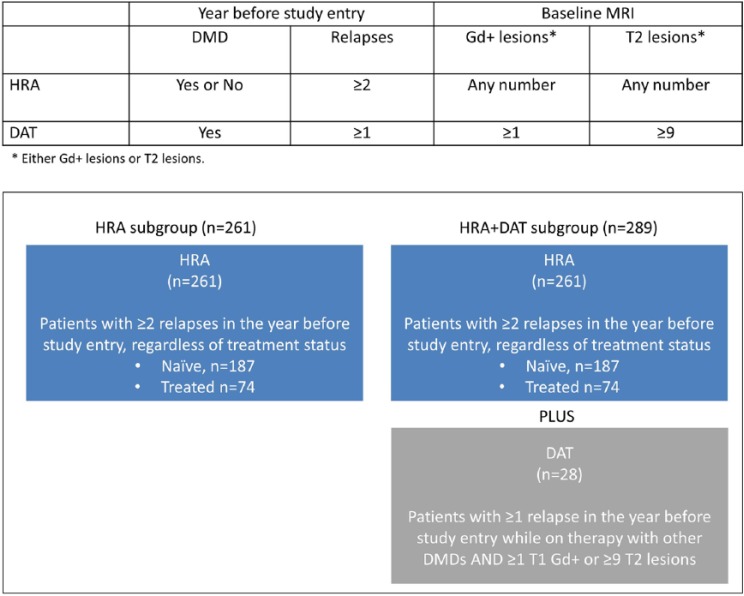
Outcomes of patients randomised to Cladribine Tablets 3.5 mg/kg or placebo were analysed for subgroups using HDA definitions based on high relapse activity (HRA; patients with ⩾2 relapses during the year prior to study entry, whether on DMD treatment or not) or HRA plus disease activity on treatment (HRA + DAT; patients with ⩾2 relapses during the year prior to study entry, whether on DMD treatment or not, PLUS patients with ⩾1 relapse during the year prior to study entry while on therapy with other DMDs and ⩾1 T1 Gd+ or ⩾9 T2 lesions).
Results:
In the overall population, Cladribine Tablets 3.5 mg/kg reduced the risk of 6-month-confirmed Expanded Disability Status Scale (EDSS) worsening by 47% vs placebo. A risk reduction of 82% vs placebo was seen in both the HRA and HRA + DAT subgroups (vs 19% for non-HRA and 18% for non-HRA + DAT), indicating greater responsiveness to Cladribine Tablets 3.5 mg/kg in patients with HDA. There were consistent results for other efficacy endpoints. The safety profile in HDA patients was consistent with the overall CLARITY population.
Conclusion:
Patients with HDA showed clinical and MRI responses to Cladribine Tablets 3.5 mg/kg that were generally better than, or at least comparable with, the outcomes seen in the overall CLARITY population.
Keywords: Cladribine Tablets, high disease activity, efficacy, safety, risk:benefit
Introduction
There is considerable variability among patients with multiple sclerosis (MS), particularly in their response to therapy with disease-modifying drugs (DMDs).1–3 Poorer prognosis for patients with MS can be predicted by relapses and magnetic resonance imaging (MRI) disease activity during treatment.4,5 The combination of clinical and MRI parameters has also been used to monitor the response of patients to therapy and predict the likelihood of future clinical events and worsening of disability.6–9 Assessing the benefit:risk balance is key to selecting the appropriate treatment for patients, with respect to the risk of future relapses or disability worsening.10
Efficacy of Cladribine Tablets (MAVENCLAD®; Merck Serono Europe Ltd, London, UK), in patients with relapsing MS, has been demonstrated in the CLARITY study on the basis of clinical and MRI measurements, or the composite endpoint of no evidence of disease activity (NEDA).11,12 Furthermore, the CLARITY Extension study showed that treatment with Cladribine Tablets administered as two short (4 or 5 days) weekly treatments at the start of months 1 and 2 in each treatment year, followed by no additional active treatment produced durable clinical benefits.13 Patients with relapsing MS who show an increased rate of relapse or disability progression can be described as having high disease activity (HDA). The current manuscript describes definitions of HDA used in new post hoc analyses of the CLARITY study. In the summary of product characteristics (SmPC) of fingolimod and natalizumab, the European Medicines Agency (EMA) has previously defined patients with HDA as those with ‘rapidly evolving severe relapsing remitting multiple sclerosis defined by 2 or more disabling relapses in one year, and with 1 or more Gadolinium-enhancing (Gd+) lesions on brain MRI or a significant increase in T2 lesion load as compared to a previous recent MRI’. The application of such criteria often depends on the availability of repeated clinical or imaging observations, which may unnecessarily delay treatment in precisely the patients who most need appropriate therapy. Therefore, due to the heterogeneity of MS and lack of agreed definitions or consensus for identifying patients with HDA, the current analyses were conducted using retrospective information on relapses in the year before the CLARITY study began and a single baseline MRI scan. Consequently, two definitions of HDA were used: one is based on relapse rate, which can identify patients with higher clinical disease activity, and the other is based on a combination of relapse rate and poor response to treatment as assessed by MRI activity, which can identify patients with higher overall levels of disease activity.
Therefore, the objectives of this manuscript are to describe the two clinically relevant definitions of patients with HDA and to verify the utility of the definitions by presenting assessments of clinical activity from patients treated with placebo in the CLARITY study. The treatment effects of Cladribine Tablets 3.5 mg/kg in subgroups of patients, meeting or not meeting the definitions of HDA in the CLARITY study, are also described. Treatment outcomes for the subgroups were compared with those for the overall study population (comprising both HDA and non-HDA patients).
Methods
The methods and outcomes of the CLARITY study have been published previously.11 Briefly, the study enrolled male and female patients aged 18–65 years with a definite diagnosis of relapsing-remitting multiple sclerosis (RRMS) according to the 2005 McDonald criteria,14 including at least one relapse in the last 12 months before study entry, but no relapses in the 28 days before entry, neurological lesions detectable by MRI consistent with MS, and an Expanded Disability Status Scale (EDSS) score of 0–5.5.
In CLARITY, patients were excluded if they had received immunosuppressive therapy at any time before study entry or cytokine-based therapy, intravenous immune globulin therapy, or plasmapheresis within 3 months before study entry, or if previous treatment with two or more DMDs had failed. Eligible patients were assigned (1:1:1) to either Cladribine Tablets 3.5 mg/kg, 5.25 mg/kg (cumulative dose over 96 weeks) or matching placebo.
Based on evidence that relapse activity and MRI lesions have an influence on disability worsening in clinical studies,4–9 the two HDA criteria developed were retrospectively applied in order to identify such patients and evaluate their response to Cladribine Tablets. Because of the better benefit:risk ratio seen with the lower dose in the CLARITY study, the current retrospective analysis presents data for patients randomised to Cladribine Tablets 3.5 mg/kg (N = 433) or placebo (N = 437) using the two different HDA definitions, based on relapse history, prior treatment and MRI characteristics.
Cladribine Tablets were granted marketing authorisation by the European Commission on 25 August 2017 for the treatment of highly active relapsing MS as defined by clinical or imaging features. The results presented in the manuscript correspond to definitions of HDA given in the EMA SmPC for the approved product that correspond to treatment-naïve patients with relapse activity or patients with evidence of disease activity while on treatment with a DMD.15
Subgroup analyses
Two overlapping sets of criteria were retrospectively applied in the analysis of baseline disease characteristics to subdivide patients into HDA groups (Figure 1). The first, which is referred to in this manuscript as the high relapse activity (HRA) subgroup, comprises patients with ⩾2 relapses during the year prior to study entry, whether on DMD treatment or not. The second, which is referred to as the HRA plus disease activity on treatment (HRA + DAT) subgroup, comprises patients with ⩾2 relapses during the year prior to study entry, whether on DMD treatment or not, and patients with ⩾1 relapse and during the year prior to study entry while on therapy with other DMDs and ⩾1 T1 Gd+ or ⩾9 T2 lesions.
Figure 1.
Definitions used for the HRA and HRA + DAT subgroups.
DMD: disease modifying drug; DAT: disease activity on treatment; HRA: high relapse activity; HRA+DAT: high relapse activity plus disease activity on treatment.
Disease activity determination in placebo-treated patients
The utility of these criteria to retrospectively identify patients with HDA among patients receiving placebo was assessed by examining annualised relapse rate (ARR), time to first qualifying relapse and time to 6-month-confirmed EDSS worsening in this arm of the study. If placebo-treated patients who fulfilled the criteria for either HDA definition departed from the clinical behaviour of the overall population (comprising both HDA and non-HDA patients) by manifesting evidence of higher disease activity during the first 2 years of the study, this was considered verification of the utility of the criteria used to define HDA.
Effect of treatment with Cladribine Tablets in subgroups of patients based on HDA definitions
Efficacy analyses from CLARITY assessed the overall population, the HRA and HRA + DAT subgroups and corresponding non-HRA and non-HRA + DAT subgroups, based on time to 6-month-confirmed EDSS worsening, ARR, time to first qualifying relapse and the proportion of patients achieving NEDA. Data on 3-month-confirmed EDSS worsening and MRI outcomes were also analysed. Safety data for applicable subgroups and comparisons with the overall population are included.
Statistics
All analyses were post hoc and not pre-specified; no multiplicity adjustments were done to the resulting p-values. All comparisons where the p-value was less than 0.05 by statistical testing should be regarded as nominally significant. The efficacy parameters were analysed by various models. Time to first qualifying relapse and time to EDSS worsening were analysed by Cox proportional hazards model, and Kaplan–Meier estimates are presented. Endpoints based on proportions of subjects (NEDA score) were analysed by a logistic regression model. Qualifying relapse rate was analysed by Poisson regression model with fixed effects for treatment and number of relapses in the previous year and the log of time on study as the offset variable. The cumulative number of lesions was analysed by a negative binomial regression model with fixed effects for treatment group and study, with baseline number of lesions as covariate and with the log of number of scans as the offset variable. Subgroup by treatment interaction was analysed by Cox proportional hazard model adjusted for treatment, subgroup, subgroup-by-treatment interaction and baseline values as covariates.
Note that the analyses presented for the overall CLARITY population are slightly different to those in the original publication.11 In the current report, data are derived from an integrated database which included other studies with Cladribine Tablets; imputation methods were aligned between studies resulting in these slight differences.
Results
Baseline demographics and disease characteristics for the intent-to-treat (ITT) population are shown in Supplementary Table 1 and for the safety population in Supplementary Table 2. Apart from characteristics used to define the subgroups (i.e. relapses in the 12 months before the study, prior use of DMDs and MRI activity), patient demographics and other disease characteristics at baseline were similar across the subgroups of patients who met or did not meet the HRA or HRA + DAT criteria and the overall population of patients. Patients in the HRA and HRA + DAT subgroups had shorter disease duration at study baseline than the non-HDA counterpart subgroups and the overall population.
The overall efficacy analysis based on the ITT population involved 870 patients randomised to placebo (N = 437) or Cladribine Tablets 3.5 mg/kg (N = 433). Of those who met the HRA criteria, 131 were randomised to placebo and 130 to Cladribine Tablets 3.5 mg/kg (Supplementary Table 1). Among the non-HRA patients, 306 were randomised to placebo and 303 to Cladribine Tablets 3.5 mg/kg. Of those who met the HRA + DAT criteria, 149 were randomised to placebo and 140 to Cladribine Tablets 3.5 mg/kg. Among the non-HRA + DAT patients, 288 were randomised to placebo and 293 to Cladribine Tablets 3.5 mg/kg.
Outcomes in subgroups of patients receiving placebo in CLARITY
ARR was higher in the placebo-treated HRA and HRA + DAT subgroups than in the overall placebo population and the placebo-treated groups who did not meet these criteria (Table 1). Both time to first qualifying relapse and time to 6-month-confirmed EDSS worsening were shorter for placebo-treated patients in the HRA and HRA + DAT subgroups, than in the overall placebo population and the placebo-treated groups who did not meet these criteria (Table 1). The increased ARR and shorter times to relapse and EDSS worsening in these placebo-treated patients, highlight the increased rates of disease activity in patients retrospectively identified using the HRA and HRA + DAT criteria and verify the utility of these criteria for assessing the clinical efficacy of Cladribine Tablets 3.5 mg/kg in patients with HDA.
Table 1.
Outcomes in patients treated with placebo in CLARITY (overall and in HRA and HRA + DAT subgroups).
| Overall N = 437 | HRA N = 131 | Non-HRA N = 306 | HRA + DAT N = 149 | Non-HRA + DAT N = 288 | |
|---|---|---|---|---|---|
| ARR (95% CI) | 0.35 (0.31–0.39) | 0.50 (0.41–0.60) | 0.29 (0.24–0.34) | 0.47 (0.40–0.57) | 0.29 (0.24–0.34) |
| Time to first qualifying relapse (20% patients), days (95% CI) | 225 (153–280) | 127 (84–236) | 260 (174–407) | 144 (90–237) | 260 (156–433) |
| Time to 6-month-confirmed EDSS worsening (10% patients), days (95% CI) | 245 (127–345) | 110 (85–245) | 330 (161–497) | 162 (85–247) | 329 (156–498) |
ARR: annualised relapse rate; CI: confidence interval; EDSS: Expanded Disability Status Scale; HRA: high relapse activity; HRA + DAT: high relapse activity plus disease activity on treatment.
Percentiles are estimated from a Kaplan–Meier survival curve.
Outcomes in subgroups of patients receiving Cladribine Tablets 3.5 mg/kg in CLARITY
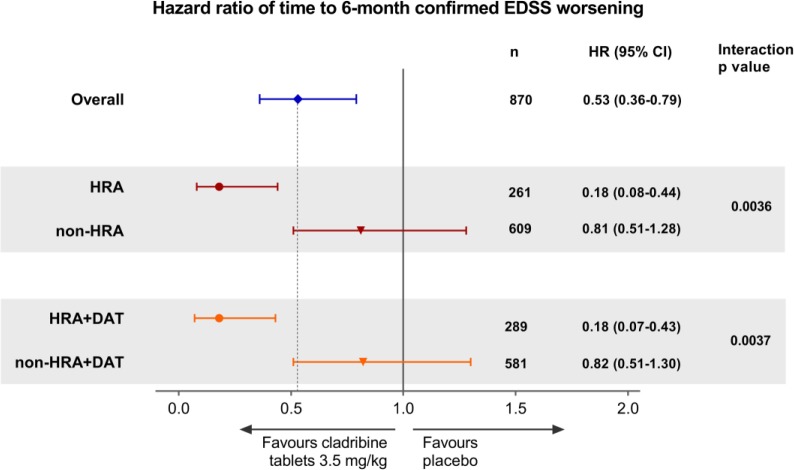
In the overall CLARITY population (N = 870), Cladribine Tablets 3.5 mg/kg reduced the risk of 6-month-confirmed EDSS worsening by 47% (hazard ratio (HR) = 0.53, 95% confidence interval (CI) = 0.36–0.79; p = 0.0016) vs placebo (Figure 2). A larger risk reduction in time to 6-month-confirmed EDSS worsening for Cladribine Tablets 3.5 mg/kg vs placebo of 82% was seen in HRA patients and in HRA + DAT patients. Interaction p-values indicated that this increase was significant (interaction p-values; p = 0.0036 for HRA vs non-HRA, and p = 0.0037 for HRA + DAT vs non-HRA + DAT).
Figure 2.
Forest plot of hazard ratio of time to 6-month-confirmed EDSS worsening by HDA subgroup for Cladribine Tablets 3.5 mg/kg vs placebo.
EDSS: Expanded Disability Status Scale; HDA: high disease activity; HRA: high relapse activity; HRA+DAT: high relapse activity plus disease activity on treatment.
The effects of Cladribine Tablets 3.5 mg/kg in patients meeting the criteria for HDA were also demonstrated for time to 3-month-confirmed EDSS worsening (72% risk reduction in both HDA subgroups vs 20% for both non-HDA subgroups; see Supplementary Figure 1 for details).
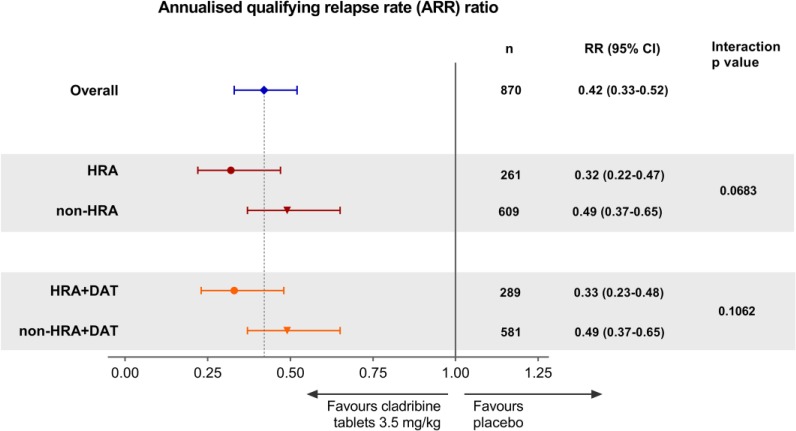
For ARR, the relative risk (RR) ratio favoured Cladribine Tablets 3.5 mg/kg in the overall population (RR = 0.42, 95% CI = 0.33–0.52; p < 0.0001; Figure 3). The RR ratios also favoured Cladribine Tablets 3.5 mg/kg in each HDA subgroup and indicated even larger reductions in RR for patients who met the criteria for HRA and HRA + DAT compared with the overall population. However, the interaction p-values did not reach significance (Figure 3). Time to first qualifying relapse in the subgroups of patients meeting criteria for HDA at baseline are shown in Supplementary Figure 2.
Figure 3.
Forest plot of relative risk of ARR by HDA subgroup for Cladribine Tablets 3.5 mg/kg vs placebo.
ARR: Annualised Relapse Rate; HDA: high disease activity; HRA: high relapse activity; HRA + DAT: high relapse activity plus disease activity on treatment.
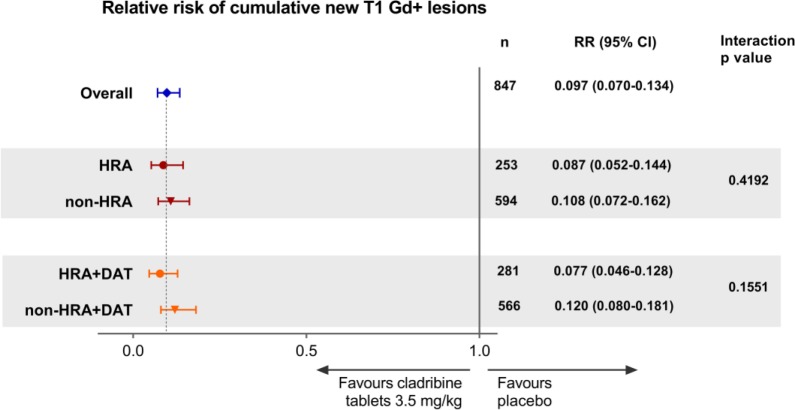
The RR of cumulative new T1 Gd+ lesions for patients in both HDA subgroups treated with Cladribine Tablets 3.5 mg/kg was low, with strong effects observed in each treatment subgroup (Figure 4). Similar results were seen for analyses performed for active T2 lesions and combined unique lesions (see Supplementary Figures 3 and 4).
Figure 4.
Forest plot of relative risk of cumulative number of new T1 Gd+ lesions by HDA subgroups for Cladribine Tablets 3.5 mg/kg vs placebo.
Gd+; gadolinium enhancing; HDA: high disease activity; HRA: high relapse activity; HRA+DAT: high relapse activity plus disease activity on treatment.
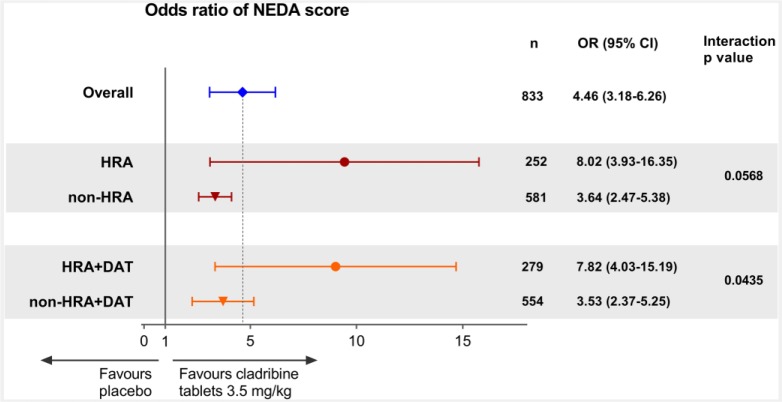
For assessment of disease-free status, NEDA was selected and defined as no relapses, no 3-month-confirmed EDSS worsening, no T1 Gd+ lesions and no active T2 lesions. Odds ratios (ORs) for the HRA and HRA + DAT subgroups were 8.02, 95% CI = 3.93–16.35; p < 0.0001 and 7.82, 95% CI = 4.03–15.19; p < 0.0001, respectively (Figure 5). Observed point estimates in HDA subgroups were systematically more favourable than the respective non-HDA subgroups, although not reaching significance except for HRA + DAT (p = 0.0435; Figure 5).
Figure 5.
Forest plot of odds ratio of NEDA score by HDA subgroups for Cladribine Tablets 3.5 mg/kg vs placebo.
Gd+; gadolinium enhancing; HDA: high disease activity; HRA: high relapse activity; HRA+DAT: high relapse activity plus disease activity on treatment; NEDA: no evidence of disease activity (defined as no relapses, no 3-month confirmed EDSS worsening, no T1 Gd+ lesions and no active T2 lesions).
Safety
Overall, review of the safety data for the HRA and HRA + DAT subgroups did not reveal evidence for new safety findings compared with those described for the overall CLARITY population (Table 2).16 Baseline demographics and disease characteristics for the safety population are shown in Supplementary Table 2. It should be noted that the HRA and HRA + DAT subgroups included a lower number of patients than the corresponding non-HRA and non-HRA + DAT subgroups; therefore, comparisons need to be considered with caution.
Table 2.
Summary of safety data for Cladribine Tablets 3.5 mg/kg by HDA subgroup (safety population).
| Characteristic | Placebo |
Cladribine Tablets 3.5 mg/kg |
||||
|---|---|---|---|---|---|---|
| Overall N = 433 | HRA N = 131 | HRA + DAT N = 148 | Overall N = 442 | HRA N = 131 | HRA + DAT N = 142 | |
| Any treatment-emergent AE, n (%) | 317 (73.2) | 100 (76.3) | 112 (75.7) | 359 (81.2) | 99 (75.6) | 109 (76.8) |
| 95% CI | 68.8–77.3 | 68.1–83.3 | 67.9–82.3 | 77.3–84.8 | 67.3–82.7 | 68.9–83.4 |
| Any treatment-related AE, n (%) | 167 (38.6) | 53 (40.5) | 61 (41.2) | 251 (56.8) | 68 (51.9) | 74 (52.1) |
| 95% CI | 34.0–43.3 | 32.0–49.4 | 33.2–49.6 | 52.0–61.5 | 43.0–60.7 | 43.6–60.6 |
| Any severe treatment-emergent AE, n (%) | 31 (7.2) | 12 (9.2) | 17 (11.5) | 37 (8.4) | 10 (7.6) | 10 (7.0) |
| 95% CI | 4.9–10.0 | 4.8–5.5 | 6.8–17.8 | 6.0–11.4 | 3.7–13.6 | 3.4–12.6 |
| Any serious treatment-emergent AE, n (%) | 32 (7.4) | 10 (7.6) | 11 (7.4) | 44 (10.0) | 17 (13.0) | 17 (12.0) |
| 95% CI | 5.1–10.3 | 3.7–13.6 | 3.8–12.9 | 7.3–13.1 | 7.7–20.0 | 7.1–18.5 |
AE: adverse event; CI: confidence interval; HRA: high relapse activity; HRA + DAT: high relapse activity plus disease activity on treatment.
Discussion
Disease activity, in both treated or treatment-naïve patients with relapsing MS, can be predictive of future disease worsening and overall poor prognosis.4,17–20 Consequently, it is important to study the effects of DMDs in patients with HDA who may be at risk for poor long-term clinical outcomes. Clinical and MRI markers of MS disease activity are of value in assessing the therapeutic effects of DMDs and have been used to predict patients at risk of disease worsening and disability worsening.4,17,18 Patients included in the CLARITY study had at least one relapse in the last 12 months before study entry, so had active disease, but the current analyses further explored the association of baseline characteristics and treatment outcome.11 The HDA subgroups presented in this analysis included patients with a wide variety of clinical histories, including both treatment-naïve and previously treated patients with relapsing MS.
Analysis of the placebo arm in the CLARITY study shows that the criteria for the HRA and HRA + DAT subgroups can identify patients who are more likely to experience both relapses and EDSS worsening. Specifically, patients meeting these criteria had higher ARR and shorter time to first qualifying relapse and time to 6-month-confirmed EDSS worsening while on placebo. Treatment with Cladribine Tablets 3.5 mg/kg showed clear benefit in the overall population and in patients with HDA. Risk of 6-month-confirmed EDSS worsening was reduced by up to 82% in HDA subgroups of patients at increased risk of disability worsening. Analysis of patients in these CLARITY subgroups also showed statistically significant reductions in the risk of relapses and in the time to first qualifying relapse. Importantly, the efficacy seen in patients with HDA was achieved with no additional safety concerns, supporting a positive and even better benefit:risk profile for Cladribine Tablets in this population compared with the overall population.
For patients with a high risk of future events, it is important to identify therapeutic options that can provide high levels of efficacy, preferably with an early onset of effect and without additional safety risk. Treatment with Cladribine Tablets has been previously shown to produce consistent reductions in ARR and provide an early onset of effect based on MRI assessments compared with placebo, across the spectrum of baseline demographics and disease characteristics represented in the CLARITY study.21,22 Previous publications from the CLARITY study have also included data on treatment outcomes with Cladribine Tablets in groups of patients using alternative definitions of HDA at baseline (⩾2 relapses in the previous year and either ⩾1 T1 Gd+ lesions or ⩾9 T2 lesions at baseline).12
The current definitions of HRA and HRA + DAT, with utility verified through examination of their ability to identify placebo-treated patients at increased risk of relapses or EDSS worsening at the study baseline show consistency with the previous observations. They also examined a greater range of key outcomes in HDA groups compared with the earlier publication. Importantly, the use of these definitions provides a structured and systematic assessment of the effects of Cladribine Tablets, in patients who may be at an increased risk of disability worsening due to HRA or DAT. In particular, both of these simple definitions are potentially useful for routine clinical practice.
Conclusion
In the CLARITY study, patients identified with two clinically relevant HDA criteria showed clinical and MRI responses to Cladribine Tablets 3.5 mg/kg that were generally better than, or at least comparable with, the outcomes seen in the overall CLARITY study population. There were statistically significant improvements in time to 6-month-confirmed disability favouring both HDA subgroups versus patients without HDA. The safety profile of Cladribine Tablets 3.5 mg/kg in HDA patients is consistent with that described for the overall CLARITY population, supporting a positive benefit:risk profile for Cladribine Tablets in this population of patients with HDA. Therefore, treatment with Cladribine Tablets 3.5 mg/kg demonstrates significant clinical effects regardless of which highly active disease population is analysed. Treatment with Cladribine Tablets 3.5 mg/kg also led to consistent results for ARR, time to first qualifying relapse, NEDA, time to 3-month-confirmed disability worsening and MRI endpoints for patients with HDA.
Supplementary Material
Supplementary Material, MSJ771875_supplementary_material for Efficacy of Cladribine Tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: A post hoc analysis of the CLARITY study by Gavin Giovannoni, Per Soelberg Sorensen, Stuart Cook, Kottil W Rammohan, Peter Rieckmann, Giancarlo Comi, Fernando Dangond, Christine Hicking and Patrick Vermersch in Multiple Sclerosis Journal
Acknowledgments
The authors would like to thank patients and their families, investigators, co-investigators and the study teams at each of the participating centres and at Merck KGaA, Darmstadt, Germany. Medical writing assistance was provided by Mark O’Connor of inScience Communications, Springer Healthcare, Chester, UK, and was funded by Merck KGaA, Darmstadt, Germany.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: G.G. has received speaker honoraria and consulting fees from Abbvie, Atara Bio, Almirall, Bayer Schering Pharma, Biogen Idec FivePrime, GlaxoSmithKline, GW Pharma, Merck, Pfizer Inc, Protein Discovery Laboratories, Teva Pharmaceutical Industries Ltd, Sanofi-Genzyme, UCB, Vertex Pharmaceuticals, Ironwood and Novartis and has received research support unrelated to this study from Biogen Idec, Merck, Novartis and Ironwood. P.S.S. has served on the advisory boards for Biogen, Merck, Novartis, Teva, MedDay Pharmaceuticals and GSK; on steering committees or independent data-monitoring boards in trials sponsored by Merck, Teva, GSK and Novartis and has received speaker honoraria from Biogen Idec, Merck, Teva, Sanofi-Aventis, Genzyme and Novartis. His department has received research support from Biogen, Merck, Teva, Novartis, Roche and Genzyme. S.C. has received honoraria for lectures/consultations from Merck, Bayer HealthCare, Sanofi-Aventis, Neurology Reviews, Biogen Idec, Teva Pharmaceuticals and Actinobac Biomed Inc; has served on the advisory boards for Bayer HealthCare, Merck, Actinobac Biomed, Teva Pharmaceuticals and Biogen Idec and received grant support from Bayer HealthCare. K.W.R. has received honoraria for lectures and steering committee meetings from EMD Serono, Biogen Idec, Sanofi-Aventis, Genzyme, Novartis, Teva Neurosciences, Acorda and Roche/Genentech. P.R. has received honoraria for lectures/steering committee meetings from Merck, Biogen Idec, Bayer Schering Pharma, Boehringer-Ingelheim, Sanofi-Aventis, Genzyme, Novartis, Teva Pharmaceutical Industries and Serono Symposia International Foundation. G.C. has received consulting fees from Novartis, Teva Pharmaceutical Industries Ltd, Sanofi-Aventis, Merck, Receptors, Biogen Idec, Genentech-Roche and Bayer Schering; lecture fees from Novartis, Teva Pharmaceutical Industries Ltd, Sanofi-Aventis, Merck, Biogen Dompè, Bayer Schering and Serono Symposia International Foundation and trial grant support from Novartis, Teva Pharmaceutical Industries Ltd, Sanofi-Aventis, Receptors, Biogen Idec, Genentech-Roche, Merck, Biogen Dompè and Bayer Schering. F.D. is an employee of EMD Serono, Inc, Billerica, USA, a business of Merck KGaA, Darmstadt, Germany. C.H. is an employee of Merck KGaA, Darmstadt, Germany. P.V. has received honoraria or consulting fees from Biogen, Sanofi-Genzyme, Bayer, Novartis, Merck, GSK, Roche, Servier and Almirall and research support from Biogen, Sanofi-Genzyme, Bayer and Merck.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was sponsored by EMD Serono, Inc, a business of Merck KGaA, Darmstadt, Germany (in the USA) and Merck Serono SA – Geneva, an affiliate of Merck KGaA Darmstadt, Germany (ROW).
Contributor Information
Gavin Giovannoni, Department of Neurology, Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
Per Soelberg Sorensen, Danish Multiple Sclerosis Center, Department of Neurology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Stuart Cook, Department of Neurology & Neurosciences, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, NJ, USA.
Kottil W Rammohan, MS Research Center, Department of Neurology, University of Miami Miller School of Medicine, Miami, FL, USA.
Peter Rieckmann, Department of Neurology, Hospital for Nervous Diseases, Medical Park Loipl, Bischofswiesen, Germany/University of Erlangen-Nürnberg, Erlangen, Germany.
Giancarlo Comi, Department of Neurology, Università Vita-Salute San Raffaele and Institute of Experimental Neurology, Ospedale San Raffaele, Milan, Italy.
Fernando Dangond, EMD Serono, Inc., Billerica, MA, USA.
Christine Hicking, Merck KGaA, Darmstadt, Germany.
Patrick Vermersch, University of Lille, CHU Lille, LIRIC-INSERM U995, FHU Imminent, Lille, France.
References
- 1. Chiu AW, Richert N, Ehrmantraut M, et al. Heterogeneity in response to interferon beta in patients with multiple sclerosis: A 3-year monthly imaging study. Arch Neurol 2009; 66: 39–43. [DOI] [PubMed] [Google Scholar]
- 2. Mahurkar S, Suppiah V, O’Doherty C. Pharmacogenomics of interferon beta and glatiramer acetate response: A review of the literature. Autoimmun Rev 2014; 13: 178–186. [DOI] [PubMed] [Google Scholar]
- 3. Shirani A, Zhao Y, Karim ME, et al. Investigation of heterogeneity in the association between interferon beta and disability progression in multiple sclerosis: An observational study. Eur J Neurol 2014; 21: 835–844. [DOI] [PubMed] [Google Scholar]
- 4. Bermel RA, You X, Foulds P, et al. Predictors of long-term outcome in multiple sclerosis patients treated with interferon beta. Ann Neurol 2013; 73: 95–103. [DOI] [PubMed] [Google Scholar]
- 5. Dobson R, Rudick RA, Turner B, et al. Assessing treatment response to interferon-beta: Is there a role for MRI? Neurology 2014; 82: 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rio J, Castillo J, Rovira A, et al. Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler 2009; 15: 848–853. [DOI] [PubMed] [Google Scholar]
- 7. Rio J, Rovira A, Tintore M, et al. Evaluating the response to glatiramer acetate in relapsing-remitting multiple sclerosis (RRMS) patients. Mult Scler 2014; 20: 1602–1608. [DOI] [PubMed] [Google Scholar]
- 8. Rio J, Ruiz-Pena JL. Short-term suboptimal response criteria for predicting long-term non-response to first-line disease modifying therapies in multiple sclerosis: A systematic review and meta-analysis. J Neurol Sci 2016; 361: 158–167. [DOI] [PubMed] [Google Scholar]
- 9. Prosperini L, Mancinelli CR, De Giglio L, et al. Interferon beta failure predicted by EMA criteria or isolated MRI activity in multiple sclerosis. Mult Scler 2014; 20: 566–576. [DOI] [PubMed] [Google Scholar]
- 10. Merkel B, Butzkueven H, Traboulsee AL, et al. Timing of high-efficacy therapy in relapsing-remitting multiple sclerosis: A systematic review. Autoimmun Rev 2017; 16(6): 658–665. [DOI] [PubMed] [Google Scholar]
- 11. Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362: 416–426. [DOI] [PubMed] [Google Scholar]
- 12. Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with Cladribine Tablets in the CLARITY study: A post-hoc and subgroup analysis. Lancet Neurol 2011; 10: 329–337. [DOI] [PubMed] [Google Scholar]
- 13. Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of Cladribine Tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult Scler J 2018; 24: 1594–1604. [DOI] [PubMed] [Google Scholar]
- 14. Polman CH, Reingold SC, Edan G, et al. Diagnostic Criteria for Multiple Sclerosis: 2005 Revisions to the “McDonald Criteria”. Ann Neurol 2005; 58: 840–846. [DOI] [PubMed] [Google Scholar]
- 15. Mavenclad EU. SmPC, https://www.medicines.org.uk/emc/medicine/34044 (accessed 12 January 2018).
- 16. Cook S, Vermersch P, Comi G, et al. Safety and tolerability of Cladribine Tablets in multiple sclerosis: The CLARITY (Cladribine Tablets treating multiple sclerosis orallY) study. Mult Scler 2011; 17: 578–593. [DOI] [PubMed] [Google Scholar]
- 17. Freedman MS, Selchen D, Arnold DL, et al. Treatment optimization in MS: Canadian MS Working Group updated recommendations. Can J Neurol Sci 2013; 40: 307–323. [DOI] [PubMed] [Google Scholar]
- 18. Rudick RA, Polman CH. Current approaches to the identification and management of breakthrough disease in patients with multiple sclerosis. Lancet Neurol 2009; 8: 545–559. [DOI] [PubMed] [Google Scholar]
- 19. Mowry EM. Natural history of multiple sclerosis: Early prognostic factors. Neurol Clin 2011; 29: 279–292. [DOI] [PubMed] [Google Scholar]
- 20. Swanton J, Fernando K, Miller D. Early prognosis of multiple sclerosis. Handb Clin Neurol 2014; 122: 371–391. [DOI] [PubMed] [Google Scholar]
- 21. Rammohan K, Giovannoni G, Comi G, et al. Cladribine Tablets for relapsing-remitting multiple sclerosis: Efficacy across patient subgroups from the phase III CLARITY study. Mult Scler Relat Disord 2012; 1: 49–54. [DOI] [PubMed] [Google Scholar]
- 22. Comi G, Cook SD, Giovannoni G, et al. MRI outcomes with Cladribine Tablets for multiple sclerosis in the CLARITY study. J Neurol 2013; 260: 1136–1146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material, MSJ771875_supplementary_material for Efficacy of Cladribine Tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: A post hoc analysis of the CLARITY study by Gavin Giovannoni, Per Soelberg Sorensen, Stuart Cook, Kottil W Rammohan, Peter Rieckmann, Giancarlo Comi, Fernando Dangond, Christine Hicking and Patrick Vermersch in Multiple Sclerosis Journal