Abstract
Aims
To assess the efficacy of Xen in reducing intraocular pressure (IOP) in varying glaucoma subtypes. To assess the effect of combined phacoemulsification. To determine the frequency of complications and explore further bleb management needed.
Methods
Retrospective case note review of all patients undergoing Xen implantation across four centres from August 2015 to May 2017.
Results
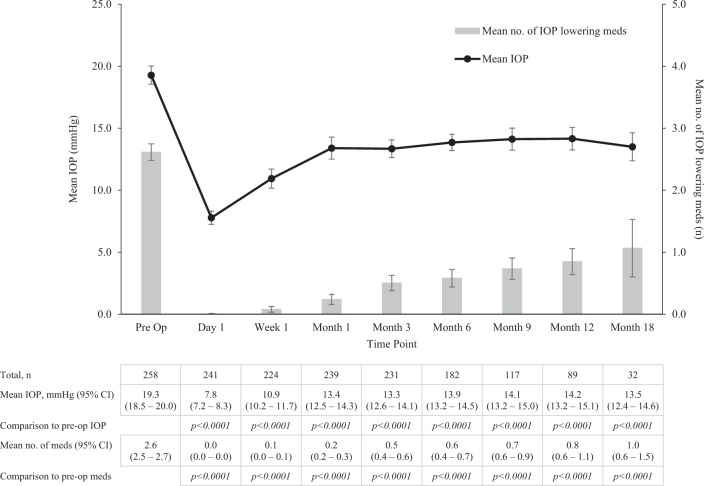
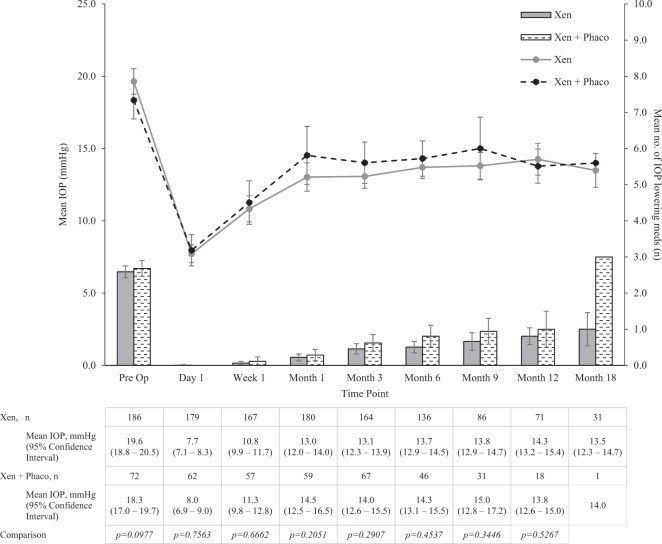
In total, 259 consecutive surgeries of 226 patients were reviewed. IOP reduced from 19.3 (SD ± 6.0) mmHg preoperatively to 14.2 (SD ± 4.4) at month 12 and 13.5 (SD ± 3.3) at month 18 (p < 0.0001). Medication usage reduced from 2.6 (±1.1) preoperatively to 0.8 (±1.0) at month 12 (p < 0.0001) and 1.1 (±1.3) medications at month 18 (p < 0.0001). Simultaneous phacoemulsification did not alter outcomes as Xen IOP was 14.3 (SD ± 4.7) mmHg and Phaco-Xen was 13.8 (SD ± 2.6) mmHg at month 12 (p = 0.5367). Xen appears to be effective in previous failed filtration surgery. Adverse events included: IOP spikes of ≥30 mmHg in 33 (12.7%) cases, secondary filtration surgery required in 24 (9.3%) cases; implant exposure in 6 (2.3%) cases; persistent hypotonous maculopathy in 5 (1.9%) cases; persistent choroidal effusions in 4 (1.5%) cases; a cyclodialysis cleft secondary to implant insertion in 1 (0.5%) case; and 1 (0.5%) case of endophthalmitis post-implant bleb resuturing. In all, 40.9% of cases required postoperative bleb needling or antimetabolite injection.
Conclusions
Xen reduces IOP and medications at 18 months. Adverse events are uncommon. Careful postoperative surveillance and low threshold for bleb management is needed. Xen is safe and effective in mild to moderate glaucoma.
Introduction
It has been 50 years since the introduction of the Molteno implant, the first contemporary glaucoma drainage device [1]. Today, a variety of tubes, valves and shunts are at the disposal of the glaucoma surgeon. The risks of traditional glaucoma surgery i.e. trabeculectomy are well-established; and the possibility of bleb-related infections, bleb leaks, dysaesthesia along with frequent follow-up, suture tension adjustment and bleb management prompts many surgeons to seek alternative approaches [2, 3]. In the quest to decrease surgical time and develop an easy-to-perform, reproducible, safe and effective technique to reduce intraocular pressure (IOP) and decrease dependence on drops, minimally invasive glaucoma surgical devices (MIGS) come to the fore.
One such MIGS device is the Xen gel implant (Allergan Inc.); indicated for the management of open-angle non-inflammatory glaucomas, it utilises the ab-interno approach [4] to create a flow pathway from the anterior chamber to the subconjunctival space, bypassing the resistance of the trabecular meshwork and collector channels. The 6 mm long, 45 µm wide lumen creates a conduit that is intended to maintain outflow at 2–2.5 µL/min as calculated by the Hagen−Poiseuille equation [5, 6]. The gelatin implant comes preloaded in a disposable injector with a 27-gauge double-bevelled needle and is designed to be implanted through a self-sealing corneal incision [7, 8]. The procedure is typically augmented with a 0.1 mL subconjunctival injection of mitomycin C (MMC) at 0.2 mg/mL [9]. The implant softens, minimally swells and becomes flexible after implantation [9], allowing it to conform to ocular tissue, thus theoretically minimising migration, erosion and corneal endothelial damage [10].
There are a few published studies using the earlier versions of the Xen implant with 63 µm and 140 µm wide lumens, where no antimetabolite was used [11, 12]; thus, these reports cannot be directly compared with the currently used 45 µm device (Xen45). We found studies assessing outcomes with the Xen45 implant with or without cataract surgery [13–17]. These studies describe the Xen implant in ≤70 eyes in a carefully selected group of patients with open-angle non-inflammatory glaucoma.
We aim to investigate the efficacy and safety of Xen in real-life glaucoma practice, including relatively contraindicated subtypes of glaucoma and eyes with previous failed filtration surgery.
Methods
We performed a retrospective case note review on all consecutive Xen surgeries performed by five surgeons across four centres between August 2015 and May 2017. Patients were identified through theatre records and surgery codes assigned by clinical coding. Data were collected on patient demographics, diagnoses, previous glaucoma treatments and surgical notes. Pre- and postoperative IOP, which was measured using a calibrated Goldmann applanation tonometer, and medications at each follow-up were recorded. Pre- and postoperative best corrected visual acuity (BCVA) was recorded using logMAR charts at 6 m, with appropriate conversion where an alternative visual acuity assessment tool was used [18, 19]. All complications and further procedures during the follow-up period were analysed.
Informed consent was obtained from patients undergoing surgery. We conducted the audit within an ethical framework, as outlined in the Declaration of Helsinki. All surgeries were performed by consultant ophthalmic surgeons with a subspecialty interest in glaucoma. Pilocarpine 2% was administered preoperatively to achieve pupil constriction. Implantation was augmented with 0.1 mL of MMC at 0.2 mg/mL, injected into the subconjunctival/sub-Tenon’s space and massaged into the superonasal quadrant. A self-sealing corneal incision was constructed, with subsequent viscoelastic insertion to deepen the anterior chamber. Using the preloaded injector, the Xen implant was inserted anterior to the trabecular meshwork to reduce the risk of haemorrhage from Schlemm’s canal, following which viscoelastic was aspirated. Intraoperative gonioscopy was performed to ensure correct insertion, position of the implant and subsequent bleb formation. All patients received standardised postoperative antibiotic and steroid medications, i.e. guttae chloramphenicol four times a day for 2 weeks and guttae dexamethasone 0.1% or prednisolone 1%, 2-hourly for 2 weeks, tapering down depending on postoperative progress. All IOP-lowering medications were stopped at the time of surgery.
Data were recorded on Microsoft Excel 2016 and statistical analysis was performed using SPSS (IBM, 2009). Means with standard deviations (SD) were calculated for IOPs and means with 95% confidence intervals were calculated for the non-parametric medication use at all time points. The chi-square goodness of fit test showed that IOP followed a normal distribution at all time points; therefore, the paired Student’s t test was used to compare baseline and postoperative mean IOP. We performed subgroup analysis for: surgeries with versus without combined phacoemulsification; eyes with previous filtration surgery, glaucoma-related laser procedures versus no previous procedures; type of anaesthetic used for surgery; and outcomes across the four centres. For subgroup analysis, the ANOVA Single factor test was used for comparison of three or more measures of mean IOP. For the non-parametric medication usage, the Wilcoxon signed-rank test was used to compare two variables and for frequency of bleb modulations, the Kruskal−Wallis test was used to compare three variables. A p < 0.05 was considered as statistically significant.
Results
We performed 259 surgeries on 226 patients (Table 1) with 91 patients reaching 12-month follow-up. During implant surgery, 72 cases had simultaneous phacoemulsification whereas 187 had stent implantation alone. In all, 208 (80.3%) eyes had open-angle glaucoma (OAG); other forms of glaucoma included 13 (5.0%) cases of pseudoexfoliation glaucoma (PXF), 6 (2.3%) eyes with pigment dispersion syndrome (PDS), 6 (2.3%) eyes with post-traumatic angle recession and 5 (1.9%) eyes with neovascular glaucoma (NVG). 18 (6.9%) eyes had previous filtration surgery including 11 trabeculectomies, 6 Xen stents and 1 Ahmed valve.
Table 1.
Patient demographics
| No. of surgeries | 259 |
| Age, years | |
| Mean (95% confidence interval) | 74.8 (73.4–76.2) |
| Range | 37–96 |
| Sex, n (%) | |
| Male | 144 (55.6) |
| Female | 115 (44.4) |
| Ethnicity, n (%) | |
| White | 254 (98.1) |
| Asian Indian | 3 (1.2) |
| Arab | 2 (0.7) |
| Operated eye, n (%) | |
| Right | 130 (50.2) |
| Left | 129 (49.8) |
| Pre-op IOP, mmHg | |
| Mean (95% confidence interval) | 19.3 (18.5–20.0) |
| Range | 5–40 |
| No. of IOP-lowering medications pre-op | |
| Mean (95% confidence interval) | 2.6 (2.5–2.7) |
| Range | 0–4 |
| Surgery type, n (%) | |
| Xen | 187 (72.2) |
| Xen + Phaco | 72 (27.8) |
| Previous glaucoma intervention, n (%) | |
| Laser trabeculoplasty | 72 (34.4) |
| Trabeculectomy | 11 (4.2) |
| Valve/Stent | 7 (3.3) |
| Diagnoses, n (%) | |
| OAG | 208 (80.3) |
| NTG | 17 (6.6) |
| PXF | 13 (5.0) |
| PDS | 6 (2.3) |
| Trauma | 6 (2.3) |
| NVG | 5 (1.9) |
| Iatrogenic | 1 (0.4) |
| Other | 3 (1.2) |
| Anaesthesia, n (%) | |
| Sub-Tenon’s | 108 (41.7) |
| Topical + Intracameral | 53 (20.5) |
| Peribulbar | 34 (13.1) |
| Unknown | 64 (24.7) |
OAG primary open angle glaucoma, NTG normal tension glaucoma, PXF pseudoexfoliation syndrome, PDS pigment dispersion syndrome, NVG neovascular glaucoma
IOP reduced from a preoperative mean of 19.3 (SD ± 6.0) to 7.8 (SD ± 4.2), 10.9 (SD ± 5.9), 13.4 (SD ± 7.0), 13.9 (SD ± 4.5), 14.1 (SD ± 4.9), 14.2 (SD ± 4.4), 13.5 (SD ± 3.3) mmHg at day 1, week 1, months 1, 3, 6, 9, 12 and 18 (Fig. 1) (p < 0.0001 at all time points). Patients were treated with a mean of 2.6 (±0.1) medications prior to implant surgery compared to 1.1 ( ± 0.5) medications at month 18 (p < 0.0001). 51.1 and 51.6% of patients were medication free at 12 and 18 months.
Fig. 1.
Mean IOP and mean number of IOP-lowering medications required pre- and post Xen implant at day 1, week 1, month 1, month 3, month 6, month 9, month 12 and month 18, shown with 95% confidence intervals. Comparison of the pre- and postoperative IOP and number of medications at each time point was performed with the paired Student’s t test and Wilcoxon signed rank test respectively (p < 0.05)
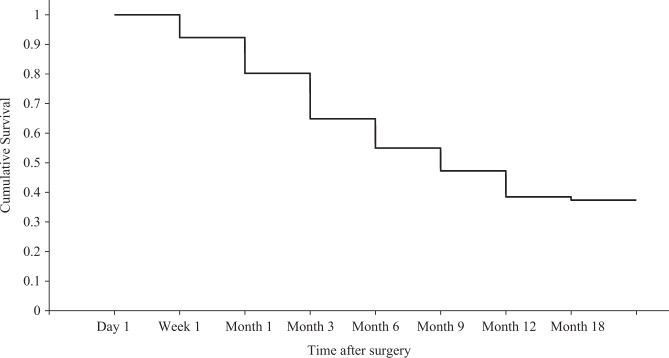
For surgeries followed up to 12 months, 37.4% (34 cases) showed complete success, i.e. postoperative IOP remained ≤21 mmHg and showed a 20% reduction from preoperative IOP without medications and without significant complications (secondary filtration surgery, endophthalmitis or unresolved hypotonous maculopathy) (Fig. 2). Partial success was defined as IOP ≤ 21 mmHg and a 20% reduction from preoperative IOP with glaucoma medications and without significant complications and was present in 24.2% (22 cases) at 12 months. Failure was evident in 38% (35 cases) and was defined as IOP > 21 mmHg in two consecutive visits or without a 20% reduction in IOP compared to preoperatively. We found a high rate of ‘failures’ with this classification, as some cases had preoperative IOPs of 22–26 mmHg on four glaucoma agents and following surgery IOPs maintained at ≤ 21 mmHg without medications, however, had not met the 20% reduction criterion, therefore adding to the failure rate.
Fig. 2.
Kaplan−Meier survival plots for all Xen surgeries that completed 12-month follow-up (n = 91). Complete surgical success was defined as IOP ≤ 21 mmHg and a 20% reduction from preoperative IOP without medications and without significant complications (secondary filtration surgery, endophthalmitis or unresolved hypotonous maculopathy)
There was no difference in mean IOP or number of medications used preoperatively in the Xen alone and Xen with phacoemulsification cohorts (p = 0.0977) and no difference was found in IOP or medication usage between the two groups at all time points postoperatively (Fig. 3). 42.1 and 53.4% of cases were medication free in the Xen and Xen-phaco groups at 12 months. There was also no difference found in rates of postoperative bleb management or complications between the Xen and Xen-phaco groups.
Fig. 3.
Mean IOP pre- and post-op at day 1, week 1, month 1, month 3, month 6, month 9, month 12 and month 18, for Xen with and without combined cataract surgery, shown with 95% confidence intervals. Comparison of mean IOP between the two groups at each time point was performed with the Student’s t test (p < 0.05)
We found a similar efficacy in IOP reduction in eyes with or without previous glaucoma filtration surgery: mean preoperative IOP reduced from 19.8 (SD ± 6.9) and 19.4 ( ± 13.3) mmHg to 13.9 (SD ± 4.0) and 13.5 (±6.3) mmHg at 12 months respectively. We also found no difference in number of IOP-lowering medications used, rates of bleb management or adverse events in either subgroups.
The most frequent complication (34.7%, n = 90) was numerical hypotony, i.e. an IOP < 6 mmHg (Table 2), and 72% of these cases resolved at week 1 without intervention. One case however required basic saline solution to reform the anterior chamber in week 1 and another case needed reformation with Healon GV at month 3 and was subsequently followed by secondary filtration surgery. Hypotonous maculopathy occurred in 5 (1.9%) cases: one resolved spontaneously by week 1; two resolved by month 1; one had a bleb leak that underwent resuturing twice and eventual resolution of maculopathy was at month 3; and one had a large diffuse bleb that underwent cryotherapy and remained unresolved at month 9, losing six lines of Snellen visual acuity. Four (1.5%) cases of choroidal effusions lasting >1 month extended posterior to the equator without threatening the macula. A postoperative IOP spike of ≥30 mmHg occurred in 33 cases (12.7%) most frequently by month 3, though we found that this could arise at any time from week 1 (due to a clot blocking the implant) to month 12 (due to an encysted bleb). Secondary filtration surgery or endoscopic cyclophotocoagulation (ECP) was needed in 29 cases (11.1%) and comprised 7 trabeculectomies, 5 Baerveldt tubes, 5 Ahmed valves, 5 ECPs, 4 further Xen implants, 1 Cypass stent and 2 cases under consideration. We found transient occlusion of the implant leading to an IOP of ≥20 mmHg in 10 (3.9%) cases, 9 caused by iris blocking the inlet to the implant and 1 due to an air bubble occlusion that resolved spontaneously. Persistent BCVA loss of ≥2 lines or ≥10 ETDRS letters lasting >1 month occurred in 9 (3.5%) eyes. Large, overhanging dysaesthetic blebs developed in 6 (3.9%) eyes, requiring postoperative lancing, bandage contact lenses, cryotherapy to the conjunctiva or subsequent bleb revision for hypotony and/or patient discomfort. 6 cases (2.3%) had implant exposure secondary to bleb leak, which required resuturing. There was one (0.4%) case of endophthalmitis following a bleb leak that required resuturing twice. Other complications we experienced were central retinal vein occlusion in one (0.5%) case and a cyclodialysis cleft secondary to implant insertion in one (0.5%) case.
Table 2.
Frequency of complications following Xen surgery (n = 259)
| Complication | Frequency, n (%) |
|---|---|
| Numerical hypotony (IOP <6mmHg) | 90 (34.7) |
| IOP spike (≥30mmHg) | 33 (12.7) |
| Transient Xen occlusion e.g. iris, blood (causing IOP ≥20mmHg) | 10 (3.9) |
| ≥2 Snellen lines vison loss lasting >1 month | 9 (3.5) |
| Large dyaesthetic bleb | 6 (2.3) |
| Xen exposure | 6 (2.3) |
| Hypotonous maculopathy | 5 (1.9) |
| Choroidal effusion lasting >1 month | 4 (1.5) |
| Endophthalmitis | 1 (0.4) |
| Central retinal vein occlusion | 1 (0.4) |
| Cyclodialysis cleft | 1 (0.4) |
Regarding further procedures, 16 cases (6.2%) required bleb or implant revision or repositioning. Apart from ECP, 2 further cases (0.7%) underwent other laser procedures to reduce IOP (Supplementary Table 1).
Bleb management
We suggest describing postoperative bleb management as two different forms:
physical bleb management involving needling or physical breakdown of adhesions or scarring in the bleb (without an antimetabolite injection);
pharmacological bleb management involving injection of an antimetabolite or steroid into the bleb to aid draining and prevent scarring. In this context, the term pharmacological management does not include re-introduction of IOP-lowering medications.
There was a mean of 1.6 postoperative bleb manipulations needed per case, where 4.6% (12 cases) required physical, 13.5% (35 cases) required pharmacological management and 22.4% (58 cases) required both. Most bleb managements took place between months 1 and 3 (Supplementary Figure 1) with the modal point being month 2. The time point where bleb manipulation was done however ranged from day 1 where a further injection of steroid was administered to the bleb to month 18 for blebs that were needled and given 5-fluorouracil (5-FU). Of the antimetabolites used postoperatively, 8.5% received 0.1 mL of MMC at 0.1 mg/mL and 91.5% received 0.1 mL of 5-FU at 25 mg/mL. Six of all cases developed corneal toxicity or filamentary keratitis secondary to repeated postoperative antimetabolite injections.
We analysed the effect that the anaesthetic under which the implant was inserted has on postoperative bleb management. Implants inserted under a peribulbar or sub-Tenon’s block required multiple needlings or antimetabolites (Supplementary Figure 2) compared to a less-invasive topical anaesthetic (p < 0.05). Up to 10 repeated antimetabolite injections were recorded for implants inserted under a sub-Tenon’s anaesthetic.
To assess whether subtle surgical technique modifications performed at different centres altered outcomes, we performed a variance analysis on IOP and number of medications pre- and postoperatively across the four centres. We found a persistent mean IOP at month 12 of between 13.0 and 15.5 mmHg and there was no difference in percentage IOP reduction across the centres. There was however a difference in the thresholds for restarting IOP-lowering medications.
Discussion
Augmented trabeculectomy continues to be the gold standard for the surgical management of glaucoma, though ophthalmologists continue to look for a safer, less invasive and more predictable method of reducing IOP [20]. The large number of MIGS devices developed in recent years is evidence that a safe and simple surgical procedure to treat mild to moderate glaucoma is sought after, as described previously [21].
In this study, we present a multi-centre case series of a heterogenous mix of glaucoma patients treated with the 45 µm Xen implant. We find a 26.4% reduction in IOP at 12 months from baseline, compared to 25.0% [13], 29.1% [15], 36.7% [10], 37.0% [16], 41.8% [9], 41.8% [17] and 46.6% [14] found previously. We consider our sample to be representative of everyday glaucoma practice, as our analysis included a diverse cohort of patients and ages, as well as incorporated eyes with neovascular glaucoma, previous trabeculectomy or glaucoma drainage device and other scenarios where prognosis may have been guarded. This is translatable to most surgeons’ patient cohorts. All cases included in the study were consecutive cases of all surgeons during the study period.
Our study has its limitations in view of its retrospective nature and only 91 of the 259 cases (34%) reached 12-month follow-up. IOP measurements were not corrected for individual central corneal thickness. Normal tension glaucoma was included in the study, which could lessen the postoperative IOP even if the stent was not functional. However, measurements were being compared for individuals and not between groups and therefore both these aspects should not have any significance in altering statistical outcomes. We had a range of baseline IOP and it should be noted that there will be a degree of selection bias as the decision to offer Xen implantation was determined by individual surgeons, without standardised inclusion or exclusion criteria. The lowest preoperative IOP of 5 mmHg was due to a Xen case complicated by a bleb leak and this was returned to theatre for suturing; the surgeon decided to remove the previous stent and a new stent was inserted, thereby classifying this as secondary filtration surgery and a new case with its own preoperative IOP. In addition to the maximum four topical IOP-lowering agents, 23 patients were also on preoperative acetazolamide of varying doses. Five patients required post-implant acetazolamide for less than 1 week each. Confounding factors were also present when comparing two variables, e.g. previous filtration surgery or glaucoma subtype could have been confounding factors when comparing outcomes with and without combined phacoemulsification. The aim of this study however was to assess the efficacy of Xen in real-world practice and heterogenicity of patient demographics and target eyes was desired.
Postoperative bleb management
Postoperative bleb management following Xen implantation has been discussed in some detail [9, 10, 13–15, 17] and bleb morphology following Xen was studied using anterior segment optical coherence tomography [22]. A high rate of postoperative bleb management for Xen was found in our cohort. In our series, all surgeons took a proactive approach to early bleb management, before IOP increased. A unique feature of Xen is ‘shrink wrapping’ of the implant by conjunctiva which needs to be prevented; therefore, any flattening of the bleb at the exit site or encapsulating of the bleb warrants further treatment. Theoretically, once the bleb has matured and subconjunctival fibrosis occurred, the resultant IOP may be limited by subconjunctival resistance—therefore close monitoring and a low threshold for early intervention of a hyperaemic or encapsulated bleb is likely to result in lesser fibrosis and better outcomes for the patient [9, 23].
We introduce describing bleb management as:
Pharmacological management of the bleb: involving injection of an antimetabolite or steroid into the bleb to prevent scarring. The authors found that this may reverse early fibrotic changes in the conjunctivo-tenon area and attempts to reduce further fibrosis. Repeated pharmacological management by means of a subconjunctival injection in the clinic is often required in encapsulated or erythematous blebs and may prevent the need for physical management.
Physical management of the bleb: involving ‘needling’ or a physical breakdown of fibrosis and adhesions obstructing aqueous flow. This can be performed in clinic or theatre by lancing the fibrotic bleb wall with a 30-gauge needle.
Separating these two management techniques allows standardisation and creates a greater understanding of the causes of poorer outcomes. We suggest that such distinction between physical and pharmacological bleb management is used in future studies.
Phacoemulsification effect
MIGS devices combined with cataract surgery show significant IOP reduction [24–28]. The IOP-lowering effect of cataract surgery is often vital in this combination [29]. On the other hand, trabeculectomy alone has been shown to reduce IOP to a large extent than combining trabeculectomy with cataract surgery [30–32]. We did not find any statistical difference in IOP, number of medications used, bleb management rates or complications, when Xen implantation was combined with phacoemulsification.
Does a topical and intracameral anaesthetic lead to better outcomes?
Though the study did not intend to answer this question, we found a trend where surgeries performed under a sub-Tenon’s or peribulbar block were more likely to require postoperative bleb management, compared to those that were performed under a topical and intracameral anaesthetic. The former two forms of anaesthesia lead to more subconjunctival haemorrhage, causing an adhesive effect between tissue layers and delivery of pro-inflammatory cells and cytokines to the area. With an intended outflow of 2–2.5 µL/min that the lumen is designed to provide, a small amount of haemorrhage or fibrosis could affect drainage. Of the 29 cases requiring secondary filtration surgery/ECP, 23 were following a sub-Tenon’s or peribulbar anaesthetic, suggesting a minimally invasive anaesthetic could possibly reduce the need for postoperative intervention and have better outcomes. Due to a heterogenous sample and confounding factors in this cohort, future studies would need to assess this in detail.
The trabeculectomy study of 429 patients found a mean IOP of 12.4 (±4) mmHg at 2 years [33]. In comparison, this series found a mean IOP of 13.5 (SD ± 3.3) mmHg at 18 months. For many patients, the circa-15 mmHg IOP may be effective at controlling rates of progression, if glaucoma is mild to moderate and target IOP is not 12 mmHg.
We find that bleb management for Xen is as important as for trabeculectomy. Flow rates are fixed and at a low rate with Xen and there is inability of titrating flow with releasable sutures like a trabeculectomy. In the trabeculectomy series, 43% of patients needed suture manipulation and a further 17% had bleb needling [33]. Our study found 41% of Xen cases required bleb management (physical or pharmacological) due to the proactive approach of the surgeons postoperatively, before IOP increased. We postulate that because aqueous flow exits the implant in a very focal location, conjunctival resistance greatly influences IOP. Trabeculectomy flaps produce a larger surface area of drainage and flow rates can be increased if conjunctival resistance alters. Xen therefore appears to need significant conjunctival assessment and bleb intervention for IOP outcomes of 15 mmHg to be achieved. In a comparative study of standalone trabeculectomy versus standalone Xen, 43 and 31% of cases required needling in the Xen and trabeculectomy cohorts respectively with an additional 50% of trabeculectomy eyes undergoing suture lysis [34]. Xen appears to have similar postoperative intervention rates to that for a trabeculectomy. We did not perform a cost-effectiveness analysis in the study, but the economic advantage of Xen is certainly tempered by the need for frequent follow-up and bleb management and this is further offset if the patient eventually requires further filtration surgery due to surgical failure. It would be beneficial to assess this aspect in future long-term studies.
Comparing other postoperative sequelae, Kirwan et al. [33] found 17% of cases had bleb leaks in the trabeculectomy cohort compared to our 2.3%—the higher rate is expected in trabeculectomy due to conjunctival peritomy performed, which is not the case for Xen; 7% required resuturing or revision compared to our 6.2%; 5.6% had a loss of >2 Snellen lines compared to our 3.5%; 0.9% developed blebitis or bleb-related endophthalmitis compared to 0.4% in our series. Xen shows an overall safety advantage and faster visual recovery compared with trabeculectomy.
The Tube versus Trabeculectomy study [35, 36] emphasises that tube shunts may hold an advantage in terms of efficacy and fewer complications when performed in cases of refractory glaucoma. Prior trabeculectomy or filtration surgery is a relative contraindication to Xen due to conjunctival fibrotic ‘priming’; however, these were included in the study provided that the proposed area for stent implantation was deemed healthy at preoperative assessment. We illustrate the success of inserting the implant in patients who had a previous failed trabeculectomy (Supplementary Table 2). With small numbers and limited duration of follow-up, it is not yet clear whether Xen truly represents a viable long-term solution in such cases. Hohberger et al. described successful implantation of Xen in a case of iridocorneal endothelial syndrome after Descemet membrane endothelial keratoplasty, also suggesting that the implant may be a promising option in difficult situations [37].
Conclusions
In this series of 259 eyes, the Xen stent reduced IOP and number of medications required at all time points up to month 18. Longer follow-up is needed to determine whether this trend persists. Simultaneous phacoemulsification does not appear to alter outcomes. The implant may be a viable option in patients who have had previous failed filtration surgery. Surgeries under a topical anaesthetic required less postoperative bleb management and were less likely to fail, an aspect that needs further study. Adverse events are uncommon but frequent proactive bleb management is required and so Xen should be reserved for those surgeons who are comfortable with early and late bleb management. Xen has a more favourable safety profile than trabeculectomy and can be considered if target IOP is 14–16 mmHg rather than 10–12 mmHg, making it a viable surgical option in managing mild to moderate glaucoma.
Summary
What was known before
Glaucoma surgeons continue to look for a safe, effective, minimally invasive surgery to reduce intraocular pressure and dependence on drops.
Xen gel implant reduces IOP and number of medications for open angle glaucoma in small-scale studies.
What this study adds
Provides a multi-centre large-scale data sought after by ophthalmologists globally due to current paucity of literature, including a variety of real-life glaucoma subtypes making the study translatable to most surgeons’ patient cohorts.
Provides recommendations for describing postoperative bleb management as either physical (needling) or pharmacological (antimetabolite) bleb management.
Reveals possible new relationship on how choice of anaesthetic can influence outcomes and presents outcomes in patients with previous failed filtration surgery.
Electronic supplementary material
Acknowledgements
We are greatly thankful to Virag Varga at UHS for performing surgeries that were included in the study.
Author contributions
AK designed the study, acquired the data at Royal Surrey County Hospital and Conquest Hospital, analysed and interpreted the data, drafted the manuscript and wrote the final version. DL conceived the study, analysed and interpreted the data and approved the final version. CD, BB and MR acquired the data at Essex County Hospital, revised the manuscript and approved the final version. PG acquired the data at Conquest Hospital, revised the manuscript and approved the final version. AT, AH, AJ and NA acquired the data at University Hospital Southampton, revised the manuscript and approved the final version.
Compliance with ethical standards
Conflict of interest
DL reports previously receiving honoraria from Allergan, Alcon, Endo Optiks, Thea, MSD, BVI Visitec and Santen outside of this work. CD reports receiving an educational travel grant from Allergan outside the work. PG reports previously receiving honoraria from Allergan prior to the work. NA reports previously receiving an educational travel grant from Nuffield Health and honoraria from Allergan and Thea outside this work. AJ reports receiving non-financial support from Aquesys prior to the study and a grant as well as non-financial support from Allergan outside the work. The remaining authors declare that they have no conflict of interest.
Electronic supplementary material
The online version of this article (10.1038/s41433-018-0243-8) contains supplementary material, which is available to authorized users.
References
- 1.Freedman J. What is new after 40 years of glaucoma implants. J Glaucoma. 2010;19:504–8. doi: 10.1097/IJG.0b013e3181ca7850. [DOI] [PubMed] [Google Scholar]
- 2.Vijaya L, Manish P, Ronnie G, Shantha B. Management of complications in glaucoma surgery. Indian J Ophthalmol. 2011;59:131. doi: 10.4103/0301-4738.73689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeBry P. Incidence of late-onset bleb-related complications following trabeculectomy with mitomycin. Arch Ophthalmol. 2002;120:297. doi: 10.1001/archopht.120.3.297. [DOI] [PubMed] [Google Scholar]
- 4.Lewis R. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J Cataract Refract Surg. 2014;40:1301–6. doi: 10.1016/j.jcrs.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Manasses D, Au L. The new era of glaucoma micro-stent surgery. Ophthalmol Ther. 2016;5:135–46. doi: 10.1007/s40123-016-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheybani A, Reitsamer H, Ahmed I. Fluid dynamics of a novel micro-fistula implant for the surgical treatment of glaucoma. Invest Ophthalmol Vis Sci. 2015;56:4789. doi: 10.1167/iovs.15-16625. [DOI] [PubMed] [Google Scholar]
- 7.Horvath C, Romoda LO. Methods for deploying an intraocular shunt from a deployment device and into an eye. Aquesys Inc. USA; Patent 8 663 303. 2014.
- 8.Allergan, Inc. Directions for use for the XEN® Glaucoma Treatment System—Model #5513-001: XEN® Glaucoma Treatment System. 2017. https://allergan-web-cdn-prod.azureedge.net/actavis/actavis/media/allergan-pdf-documents/labeling/xen/dfu_xen_glaucoma_treatment_system_us_feb2017.pdf.
- 9.Tan S, Walkden A, Au L. One-year result of XEN45 implant for glaucoma: efficacy, safety, and postoperative management. Eye. 2017 doi: 10.1038/eye.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grover D, Fellman R. Outcomes for ab interno bleb revision with a novel translimbal sclerostomy spatula. J Glaucoma. 2017;26:633–7. doi: 10.1097/IJG.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 11.Sheybani A, Dick HB, Ahmed II. Early clinical results of a novel ab interno gel implant for the surgical treatment of open-angle glaucoma. J Glaucoma. 2016;25:e691–696. doi: 10.1097/IJG.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 12.Sheybani A, Lenzhofer M, Hohensinn M, et al. Phacoemulsification combined with a new ab interno gel implant to treat open-angle glaucoma: pilot study. J Cataract Refract Surg. 2015;41:1905–9. doi: 10.1016/j.jcrs.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Galal A, Bilgic A, Eltanamly R, Osman A. XEN glaucoma implant with mitomycin C 1-year follow-up: result and complications. J Ophthalmol. 2017;2017:1–5. doi: 10.1155/2017/5457246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stalmans I, Fea A, Reitsamer H, Lavin CA. Minimally invasive approach to sub-conjunctival outflow: 1 year results of an ab-interno gelatin stent in combination with preoperative MMC injection for the treatment of primary open angle glaucoma. Acta Ophthalmol. 2015;93.
- 15.Pérez-Torregrosa VT, Olate-Pérez Aacute, Cerdà-Ibáñez M, Gargallo-Benedicto A, Osorio-Alayo V, Barreiro-Rego A, et al. Combined phacoemulsification and XEN45 surgery from a temporal approach and 2 incisions. Arch Soc Esp Oftalmol. 2016;91:415–21. doi: 10.1016/j.oftal.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Sheybani A, Ahmed I. Ab interno gelatin implant with mitomycin-C combined with cataract surgery for treatment of open-angle glaucoma: 1 year results. Paper presented at ASCRS, USA; 2015.
- 17.De Gregorio A, Pedrotti E, Russo L, Morselli S. Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent. Int Ophthalmol. 2017 doi: 10.1007/s10792-017-0571-x. [DOI] [PubMed] [Google Scholar]
- 18.Royal College of Ophthalmologists. Snellen and LogMAR acuity testing. https://www.rcophth.ac.uk/patients/snellen-and-logmar-acuity-testing. Accessed 10 August 2017.
- 19.Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the Freiburg Visual Acuity Test. Invest Ophthalmol Vis Sci. 2006;47:1236. doi: 10.1167/iovs.05-0981. [DOI] [PubMed] [Google Scholar]
- 20.Loewen NA, Schuman JS. There has to be a better way: evolution of internal filtration glaucoma surgeries. Br J Ophthalmol. 2013;97:1228–9. doi: 10.1136/bjophthalmol-2013-303237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manasses D, Au L. The new era of glaucoma micro-stent surgery. Ophthalmol Ther. 2016;5:135–46. doi: 10.1007/s40123-016-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa L, Cardigos J, Crisostomo S, Anjos R, Sa Cardoso M, Gomes T. Macroscopic analysis of filtering bleb functionality after XEN Gel Stent implantation with Anterior Segment Optical Coherence Tomography. Acta Ophthalmol. 2016;94.
- 23.Fernandez-Garcia A, Romero C, Garzon N. “Dry lake” technique for the treatment of hypertrophic bleb following Xen gel implant placement. Arch Soc Esp Oftalmol. 2015;90:536–8. doi: 10.1016/j.oftal.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Francis BA. Trabectome combined with phacoemulsification versus phacoemulsification alone: a prospective, nonrandomized controlled surgical trial. Clin Surg Ophthalmol. 2010;28:1–7. [Google Scholar]
- 25.Samuelson TW, Katz LJ, Wells JM, et al. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmol. 2011;118:459–67. doi: 10.1016/j.ophtha.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma: randomized double-masked clinical trial. J Cataract Refract Surg. 2010;36:407–12. doi: 10.1016/j.jcrs.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Hoeh H, Ahmed IK, Grisanti S, et al. Early postoperative safety and surgical outcomes after implantation of a suprachoroidal micro-stent for the treatment of open-angle glaucoma concomitant with cataract surgery. J Cataract Refract Surg. 2013;39:431–7. doi: 10.1016/j.jcrs.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 28.Craven ER, Katz LJ, Wells JM, Giamporcaro JE. iStent Study Group Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38:1339–45. doi: 10.1016/j.jcrs.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Poley B, Lindstrom R, Samuelson T, Schulze R. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes. J Cataract Refract Surg. 2009;35:1946–55. doi: 10.1016/j.jcrs.2009.05.061. [DOI] [PubMed] [Google Scholar]
- 30.Mathew R, Murdoch I. The silent enemy: a review of cataract in relation to glaucoma and trabeculectomy surgery. Brit J Ophthalmol. 2011;95:1350–4. doi: 10.1136/bjo.2010.194811. [DOI] [PubMed] [Google Scholar]
- 31.Belluci R, Perfetti S, Babighian S, Morselli S, Bonomi L. Filtration complications after trabeculectomy and after phaco-trabeculectomy. Acta Ophthalmol Scand Suppl. 1997;224:44–5. doi: 10.1111/j.1600-0420.1997.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 32.Caprioli J, Park HJ, Weitzman M. Temporal corneal phacoemulsification combined with superior trabeculectomy: a controlled study. Trans Am Ophthalmol Soc. 1996;94:451–68. [PMC free article] [PubMed] [Google Scholar]
- 33.Kirwan JF, Lockwood AJ, Shah P, Macleod A, Broadway DC, King AJ, et al. Trabeculectomy in the 21st century: a multicenter analysis. Ophthalmol. 2013;120:2532–9. doi: 10.1016/j.ophtha.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 34.Schlenker M, Gulamhusein H, Conrad-Hengerer I, Somers A, Lenzhofer M, Stalmans I, et al. Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmol. 2017 doi: 10.1016/j.ophtha.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL, et al. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153:789–803. doi: 10.1016/j.ajo.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–e801. doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hohberger B, Welge-Lüen UC, Lämmer R. ICE-syndrome: a case report of implantation of a microbypass Xen gel stent after DMEK transplantation. J Glaucoma. 2017;26:e103–104. doi: 10.1097/IJG.0000000000000584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.