Abstract
Background
The goal of the research reported here was to understand the patient experience of living with myelofibrosis (MF) and establish content validity of the Modified Myeloproliferative Neoplasm Symptom Assessment Diary (MPN-SD).
Methods
Qualitative interviews were performed in patients with MF, including both concept elicitation and cognitive debriefing. Patients with MF were asked to spontaneously report on their signs, symptoms, and impacts of MF, as well as their understanding of the MPN-SD content, and use of the tool on an electronic platform. A supplementary literature review and meetings with MF experts were also performed.
Results
Twenty-three patients with MF participated in qualitative interviews. Signs and symptoms most commonly reported by ruxolitinib-experienced patients (n = 16) were: fatigue and/or tiredness (n = 16, 100%), shortness of breath (n = 11, 69%), pain below the ribs on the left side and/or stomach pain and/or abdominal pain (n = 9, 56%), and enlarged spleen (n = 9, 56%) and for ruxolitinib-naïve patients (n = 7) were: fatigue and/or tiredness (n = 6, 86%), pain below the ribs on the left side (n = 6, 86%), enlarged spleen (n = 4, 57%), full quickly/filling up quickly (n = 4, 57%), night sweats and/or general sweats (n = 4, 57%), and itching (n = 4, 57%). Patients demonstrated that they were able to read, understand, and provide meaningful responses to the MPN-SD. The final version of the MPN-SD includes the 10 most commonly reported concepts from the MF patient interviews.
Conclusions
The findings demonstrate the comprehensiveness of the MPN-SD in assessing MF symptoms in both ruxolitinib-experienced and ruxolitinib-naïve patients, while remaining easy for patients to understand and complete.
Keywords: Primary myelofibrosis, Patient reported outcome measures, Symptom assessment, Product labeling, Janus kinase inhibitors, Myeloproliferative disorders
Background
Myelofibrosis (MF) is a malignant clonal disease characterized by progressive marrow failure, splenomegaly, and decreased longevity due to infections, bleeding, and leukemic transformation [1]. MF comprises numerous burdensome symptoms for patients, including fatigue, night sweats, upper left quadrant abdominal pain, bone pain, and, among others, unintentional weight loss. These symptoms, in turn, often have negative impacts on patients’ quality of life; patients with MF frequently complain of night sweats, severe itching, early fullness or satiety, and a variety of pains in their bones and muscles, under their ribs, and in their abdomen, which all contribute to a reduced health related quality of life (HRQoL) [2].
When assessing the full impact of disease and treatment on HRQoL, it is important to consider the patient’s perspective. Indeed, the European Medicines Agency (EMA) [3] and United States (US) Food and Drug Administration (FDA) [4] acknowledge the importance of the patient’s voice in clinical trials by outlining steps for good patient-reported outcome (PRO) instrument development. The only currently FDA-approved drug for first-line treatment of MF is Jakafi™ (ruxolitinib [RUX]) [5]. RUX is indicated for the treatment of patients with intermediate or high-risk MF, including primary MF (PMF), post-polycythemia vera (PPV-MF), and post-essential thrombocythemia (PET-MF), having been shown to reduce spleen volume and improve MF-associated symptoms in those populations.
The modified Myelofibrosis Symptom Assessment Form (MFSAF) v2.0 diary (a symptom assessment PRO tool) was used successfully to obtain PRO label claims from the FDA [6] and EMA in patients naïve to RUX inhibition (RUX-naïve). Fatigue, a hallmark symptom of MF, is missing from MFSAF v2.0, and it is unclear if this tool is appropriate for patients resistant or intolerant to RUX treatment (RUX-experienced). To overcome these issues, this study aimed to develop a new PRO instrument. Specifically, the goal of this study was to understand the patient perspective of living with MF and establish content validity of a new PRO instrument for administration in both RUX-experienced and RUX-naïve MF patients. Data was collected from three sources: patients, experts, and the published literature. The result of this work contributed to the development of the Myeloproliferative Neoplasm Symptom Assessment Diary (MPN-SD), a PRO instrument designed for the purpose for assessing key symptoms among RUX-experienced and RUX-naïve MF patients.
Methods
This study was conducted in accordance with the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) PRO Good Research Practices Task Force Report: Part 1 [7] and 2 [8] and FDA PRO Guidance [4], which each specify methods for development of a PRO tool.
Qualitative interviews with MF patients
Patient recruitment
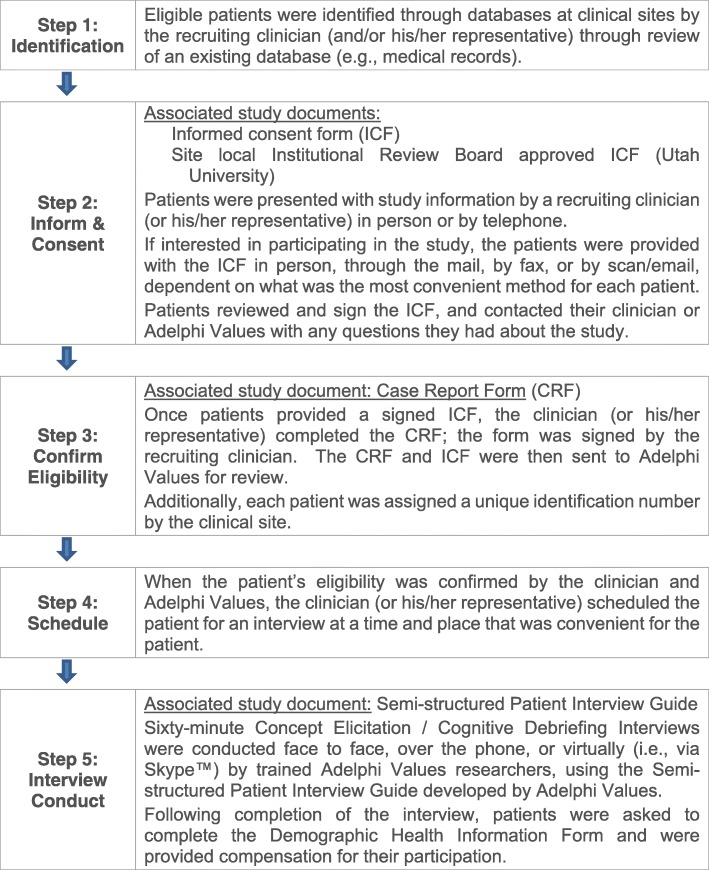
All study documents were institutional review board-approved prior to study initiation. Interviews were scheduled after a potential participant confirmed his or her interest in participating, signed informed consent and the Health Insurance Portability and Accountability Act (HIPAA) Authorization, and was deemed eligible (see Fig. 1 displaying the recruitment process).
Fig. 1.
Agency and academic research site patient recruitment process
Patient eligibility criteria
Eligible patients included those who were ≥ 18 years of age; fluent in US English; willing and able to participate and complete questionnaires; diagnosed with PMF or secondary MF (PET-MF and PPV-MF) as per World Health Organization 2008 criteria; had failed prior treatment with a RUX (RUX-experienced), or had no prior RUX treatment and Intermediate-1, Intermedate-2 or High-risk MF according to the Dynamic International Prognostic Scoring System (RUX-naïve); had a palpable spleen; and had reported current MF symptoms. A patient was not eligible if he or she met any of the following exclusion criteria: previous treatment with a licensed or experimental smoothened inhibitor; targeted anti-cancer therapy up to 14 days prior to enrollment; a history of drug or alcohol abuse within the last 6 months; cognitive impairment; the presence of a psychiatric condition; or any other clinically relevant concern that would interfere with the patient’s ability to provide written informed consent and/or participate.
Patient interview methods
A semi-structured interview guide was developed to capture open-ended and probing questions regarding symptoms and impacts of MF from the patients (concept elicitation interviews, CEIs); evaluate the MPN-SD for readability, comprehensibility, relevance, and comprehensiveness (cognitive debriefing interviews, CDIs); and assess the usability of the electronic patient-reported outcome device (ePRO usability testing). CEI, CDI, and ePRO usability testing were conducted during the same interview session. Interviews were audio recorded; conducted face to face, over the telephone, or virtually; and each lasted 60–90 min. Interviewers were trained via the National Institutes of Health Human Participant Protection.
Data collection
During CEIs, symptom expressions were tracked; participants ranked the top five symptoms they would like to have improve with treatment; and reported on the impact of MF symptoms. Concepts were tabulated for frequency (i.e., number and percent of spontaneous reports).
For CDIs, the MPN-SD was presented on an ePRO device and patients were asked to “think aloud” about the process they used to arrive at each answer. Data were collected on the words, terms, or concepts that were not understood or were interpreted differently than intended [9]. The MPN-SD was modified mid-way and a second wave of interviews was performed to evaluate the changes. The usability of the ePRO device was evaluated via questionnaire.
Audio-recordings of interviews were transcribed, anonymized, and entered into ATLAS.ti Version 7.0 (ATLAS.ti Scientific Software Development GmbH, Berlin) [10]. A code book was developed based on researchers identifying transcript text relevant to the research objectives and matching it to a code from the coding scheme that best characterized the data [11]. Analysis was performed separately for RUX-experienced and RUX-naïve patient groups. Saturation was evaluated; saturation characterizes the point at which no new information can be generated from additional interviews [7]. The demonstration of saturation is used as evidence for the adequacy of the sample size (if saturation is not observed, additional interviews may be considered).
Literature review
In order to provide further evidence of the relevance and importance of concepts identified during patient interviews, and to help guide revisions to the interview guide between interview waves, a conceptual literature review was conducted concurrent with the start of patient interviews. It was designed to capture key articles that identify, define, and substantiate the symptom-level concepts experienced by adults with MF. The search was conducted January 2015 via the OvidSP platform. Key words included: “myelofibrosis,” “osteomyelofibrosis,” “patient report,” “qol,” “quality,” “signs,” and “symptoms.” The search was limited to humans, US English, and journal publication type. No limits were placed on publication year. Concepts from the literature were categorized and prioritized (reported by ≥5 full text articles) according to symptoms, impacts, or MF treatment (see Fig. 2).
Fig. 2.
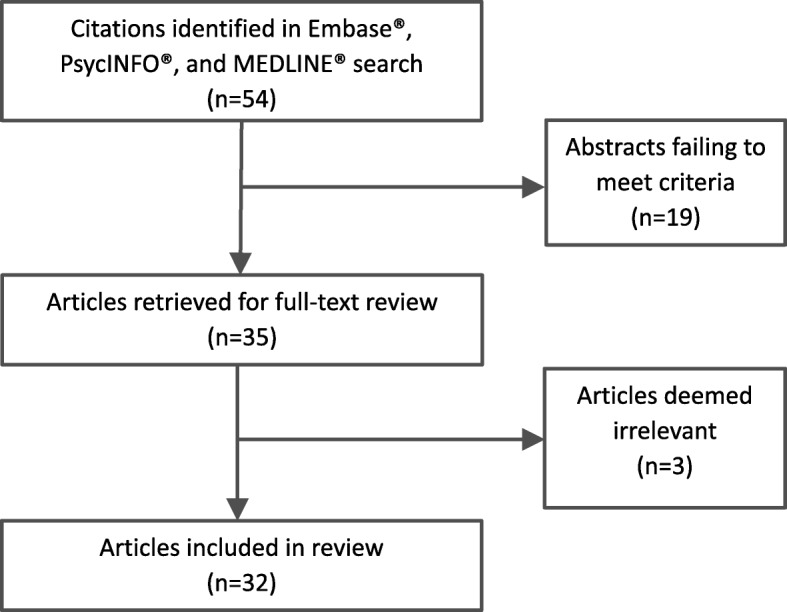
Literature review screening process and article flow
Expert advice meetings
Interviews with four experts in MF substantiated symptom and impact level concepts of measurement. Experts were identified from a list of clinicians who specialized in hematology. Interviews were conducted over the phone by trained interviewers, followed a semi-structured interview guide, and were each 60 min. The interviews were analyzed using semi-quantitative and qualitative data analytic methods via ATLAS.ti Version 7.0 (ATLAS.ti Scientific Software Development GmbH, Berlin) [10].
Results
Qualitative interviews with MF patients
Twenty-three MF patients participated in interviews (RUX-experienced = 16, RUX-naïve = 7). Eleven were recruited and enrolled from Global Market Research Group, three from Mayo Clinic, six from Utah University, and three from the MPN Research Foundation. Interviews occurred between October 2014 and September 2015.
Demographics
Mean age for RUX-experienced patients was 67 years (range of 30 to 85, standard deviation [SD] ±15.5). Most were white (n = 14, 88%), and male (n = 9, 56%). Thirty-one percent of patients reported “fair” general health (n = 5) and 69% rated the severity of their condition as moderate (n = 11). Mean age for RUX-naïve patients was 59 years (range of 46 to 71, SD ±8.8). All were white/Caucasian (n = 7, 100%) and most were male (n = 4, 57%). Seventy-one percent of RUX-naïve patients (n = 5) reported being in “good” general health and rated the severity of their condition as mild. See Table 1 for additional demographic characteristics.
Table 1.
Patient reported demographic and health information
| Total combined sample (N = 23) N (%) |
RUX- experienced total (n = 16) n (%) |
RUX-naïve total (n = 7) n (%) |
|
|---|---|---|---|
| Gender | |||
| Female | 10 (43.5%) | 7 (43.8%) | 3 (42.9%) |
| Male | 13 (56.5%) | 9 (56.3%) | 4 (57.1%) |
| Age | |||
| Range | 29.9–85.4 | 29.9–85.4 | 45.9–70.7 |
| Mean (SD) | 65.5 (12.2) | 67.2 (15.5) | 58.7 (8.8) |
| Ethnicity | |||
| Not Hispanic/Latino | 23 (100.0%) | 16 (100.0%) | 7 (100.0%) |
| Race | |||
| White/Caucasian | 21 (91.3%) | 14 (87.5%) | 7 (100.0%) |
| Othera | 2 (8.7%) | 2 (12.5%) | 0 (0.0%) |
| Education | |||
| High school diploma (or GED) or less | 5 (21.7%) | 3 (18.8%) | 2 (28.6%) |
| Some college or certificate program | 5 (21.7%) | 4 (25.0%) | 1 (14.3%) |
| College or university degree (2- or 4-year) | 7 (30.4%) | 3 (18.8%) | 4 (57.1%) |
| Graduate degree | 6 (26.1%) | 6 (37.5%) | 0 (0.0%) |
| Other | 1 (4.3%) | 1 (6.3%) | 0 (0.0%) |
| Living status | |||
| Living alone | 3 (13.0%) | 3 (18.8%) | 0 (0.0%) |
| Living with family or friends | 18 (78.3%) | 12 (75.0%) | 6 (85.7%) |
| Other | 2 (8.7%) | 1 (6.3%) | 1 (14.3%) |
| Annual household income | |||
| Under $25,000 | 2 (8.7%) | 1 (6.3%) | 1 (14.3%) |
| $25,000 to $49,999 | 1 (4.3%) | 1 (6.3%) | 0 (0.0%) |
| $50,000 to $74,999 | 6 (26.1%) | 4 (25.0%) | 2 (28.6%) |
| $75,000 to $99,999 | 6 (26.1%) | 3 (18.8%) | 3 (42.9%) |
| $100,000 and over | 4 (17.4%) | 4 (25.0%) | 0 (0.0%) |
| Not answered | 4 (17.4%) | 3 (18.8%) | 1 (14.3%) |
| Work status | |||
| Working full-time | 5 (21.7%) | 2 (12.5%) | 3 (42.9%) |
| Working part-time | 1 (4.3%) | 1 (6.3%) | 0 (0.0%) |
| Retired | 14 (60.9%) | 12 (75.0%) | 2 (28.6%) |
| Unemployed | 1 (4.3%) | 0 (0.0%) | 1 (14.3%) |
| On disability | 2 (8.7%) | 1 (6.3%) | 1 (14.3%) |
| Relationship status | |||
| Single | 1 (4.3%) | 0 (0.0%) | 1 (14.3%) |
| Have a significant other | 18 (78.3%) | 13 (81.3%) | 5 (71.4%) |
| Married | 1 (4.3%) | 0 (0.0%) | 1 (14.3%) |
| Divorced/Separated | 2 (8.7%) | 2 (12.5%) | 0 (0.0%) |
| Widowed | 1 (4.3%) | 1 (6.3%) | 0 (0.0%) |
| Health in general | |||
| Excellent | 1 (4.3%) | 1 (6.3%) | 0 (0.0%) |
| Very good | 4 (17.4%) | 3 (18.8%) | 1 (14.3%) |
| Good | 8 (34.8%) | 3 (18.8%) | 5 (71.4%) |
| Fair | 6 (26.1%) | 5 (31.3%) | 1 (14.3%) |
| Poor | 4 (17.4%) | 4 (25.0%) | 0 (0.0%) |
| Condition severity | |||
| Mild | 6 (26.1%) | 1 (6.3%) | 5 (71.4%) |
| Moderate | 12 (52.2%) | 11 (68.8%) | 1 (14.3%) |
| Severe | 5 (21.7%) | 4 (25.0%) | 1 (14.3%) |
a1 patient stated “some Native American / northern European”; 1 patient did not specify race
Concept elicitation interviews
Patient-reported symptoms of MF
A total of 29 symptoms were expressed by RUX-experienced patients, and 14 symptoms were reported by RUX-naïve patients. Eleven symptoms were reported by both groups of patients. Eighteen symptoms were reported by RUX-experienced patients only. Three symptoms (appetite loss, cold, and rashes) were reported by RUX-naïve patients only (see Table 2). Table 3 displays examples of MF patient quotes for the prioritized symptom expressions.
Table 2.
Myelofibrosis primary symptom concepts
| Concept | Frequency of RUX-experienced patient reports; n = 16 n (%) |
Frequency of RUX-naïve patient reports; n = 7 n (%) |
Total (N = 23) N (%) |
Concept reported by both RUX-experienced and RUX-naïve patients |
|---|---|---|---|---|
| Fatigue and/or tiredness | 16 (100.0%) | 6 (85.7%) | 22 (95.7%) | ✓ |
| Shortness of breath | 11 (68.8%) | 0 (0%) | 11 (47.8%) | |
| Pain below the ribs on the left side and/or stomach pain and/or abdominal pain | 9 (56.3%) | 6 (85.7%) | 15 (65.2%) | ✓ |
| Enlarged spleen | 9 (56.3%) | 4 (57.1%) | 13 (56.5%) | ✓ |
| Uncomfortable abdomen and/or abdominal discomfort and/or bloating and/or feeling full | 8 (50.0%) | 1 (14.3%) | 9 (39.1%) | ✓ |
| Full quickly and/or filling up quickly and/or eating less | 7 (43.8%) | 4 (57.1%) | 11 (47.8%) | ✓ |
| Night sweats and/or general sweats | 6 (37.5%) | 4 (57.1%) | 10 (43.5%) | ✓ |
| Bone pain | 6 (37.5%) | 1 (14.3%) | 7 (30.4%) | ✓ |
| Muscle pain and/or muscle cramps | 6 (37.5%) | 0 (0%) | 6 (26.1%) | |
| Itching | 5 (31.3%) | 4 (57.1%) | 9 (39.1%) | ✓ |
| Dizziness | 3 (18.8%) | 1 (14.3%) | 4 (17.4%) | ✓ |
| Bruise easily | 3 (18.8%) | 0 (0%) | 3 (13.0%) | |
| Balance | 2 (12.5%) | 0 (0%) | 2 (8.7%) | |
| Brain fog | 2 (12.5%) | 0 (0%) | 2 (8.7%) | |
| Frequent urination | 2 (12.5%) | 0 (0%) | 2 (8.7%) | |
| Hot flashes | 2 (12.5%) | 0 (0%) | 2 (8.7%) | |
| Muscle weakness | 2 (12.5%) | 0 (0%) | 2 (8.7%) | |
| Peripheral neuropathy and/or numbness | 2 (12.5%) | 0 (0%) | 2 (8.7%) | |
| Weight loss | 1 (6.3%) | 3 (42.8%) | 4 (17.4%) | ✓ |
| Appetite loss | 0 (0%) | 1 (14.3%) | 1 (4.3%) | |
| Cold | 0 (0%) | 1 (14.3%) | 1 (4.3%) | |
| Joint pain | 1 (6.3%) | 1 (14.3%) | 2 (8.7%) | ✓ |
| Rashes | 0 (0%) | 1 (14.3%) | 1 (4.3%) | |
| Achiness | 1 (6.3%) | 0 (0%) | 1 (4.3%) | |
| Back pain | 1 (6.3%) | 0 (0%) | 1 (4.3%) | |
| Burning sensation | 1 (6.3%) | 0 (0%) | 1 (4.3%) | |
| Fevers and/or chills | 1 (6.3%) | 0 (0%) | 1 (4.3%) | |
| Gout | 1 (6.3%) | 0 (0%) | 1 (4.3%) | |
| Hand pain | 1 (6.3%) | 0 (0%) | 1 (4.3%) | |
| Headaches | 1 (6.3%) | 0 (0%) | 1 (4.3%) | |
| Inflammation in arm | 1 (6.3%) | 0 (0%) | 1 (4.3%) | |
| Nausea | 1 (6.3%) | 0 (0%) | 1 (4.3%) |
Table 3.
Example patient expressions
| Concept | RUX-experienced example patient expressions | RUX-naïve example patient expressions |
|---|---|---|
| Fatigue and/or tiredness |
(05-02-M-74)
… all of the sudden I started getting more lethargic and, and more tired, more fatigued.
(01-05-F-55) I felt that I was very tired all the time. |
(01-04-M-50)
… when I was first diagnosed I’d get really fatigued during the day. Come home, take a three-four hour nap and, uh, never felt any different. Still fatigued …
(01-14-F-67) I was just tired.… But every day around three o’clock I laid down and went to bed and I couldn’t sit up very long.… my body just felt very uncomfortable. |
| Shortness of breath | (01-02-M-73) I can’t, sometimes, ah, I feel like I can’t even catch my breath. It’s, it’s getting worse. It’s not getting better at all.… | N/A |
| Pain below the ribs on the left side and/or stomach pain and/or abdominal pain | (03-09-F-29) I used to have a lot of spleen pain before I was on treatment.… … it’s upper left … right under where my boob is … all the way to the left. Like on the side. |
(01-17-M-70)
… it is definitely on the, uh, left side of the ribcage.… It’s continual.… But it’s not chronic in that the only time I’d really know I have it is if I press on it or I tighten my belt too tight. It’s not that it’s a throbbing continual pain.
(01-17-M-70) … it is definitely on the, uh, left side of the ribcage.… It’s continual.… But it’s not chronic in that the only time I’d really know I have it is if I press on it or I tighten my belt too tight. It’s not that it’s a throbbing continual pain. |
| Enlarged spleen |
(03-01-M-66)
A: Uh, yes.… The spleen pain was very – I knew when that
pain – I call it abdominal pain because my spleen was so big. It was bigger than a football and, and it went from one side of my body to the other. So whenever it hurt it would hurt in my entire abdomen area, but it was … definitely caused by the spleen. I, I could tell. |
(01-16-F-59)
I have, uh, an enlarged spleen, which I can feel.… Well I don’t feel it here when I’m sitting up, but when I lie down
(01-01-F-58) I felt that my spleen might be enlarged, but of course I’m not sure. I couldn’t – I don’t know … So when the spleen becomes enlarged sometimes it feels uncomfortable or you get some sort of pain down there |
| Uncomfortable abdomen and/or abdominal discomfort and/or bloating and/or feeling full | (05-01-F-69) Back in December of 2007 … I had been experiencing since the previous October a lot of pain on my left side … feeling very full all the time, very, very kind of bloated and uncomfortable. | (01-04-M-50-P) I get an almost a nausea feeling from it. Uh, and other than that it means you feel full. |
| Full quickly and/or filling up quickly and/or eating less |
(05-01-F-69)
… feeling very full all the time …
03-09-F-29) I had been noticing, up to that point, I’d been eating less.…I was having a really hard time eating … regular sized meals … |
(01-04-M-50-P) I do have that too.… the feeling of fullness is I would eat – like right now I would eat maybe a quarter of the portion I’m normally used to eating and it just – I can’t eat no more. I’m just full. |
| Night sweats and/or general sweats | (05-03-M-79) I was having terrible night sweats.… They were very, very bad sweats.… It’s almost like I was waddling on water and as soon as I went to bed, they started. And then they’d go on all night.… Every night. |
(01-01-F-58-P)
Well I wake up and I just feel like a flush of warmth like, um, kind of like hot flashes
(01-14-F-67) …I just can sometimes just sit there and have company and have 10 napkins besides me and just keep, um, just keep trying to wipe away the sweat. |
| Bone pain | (03-07-F-64) I would get a lot of long bone pain … particularly when I was lying on my couch.… I’ve only had it in my legs. I’ve never had it substantially in my arms, but … I still will get that in my legs. |
(01-01-F-58)
I have bone pain.…
(01-01-F-58) … every time I lie down it hurts for a little while. Um, when I get up it hurts for a little while and if I am say driving for a long time it hurts. |
| Muscle pain and/or muscle cramps | (03-09-F-29) … within the first year a few more symptoms kind of started showing up, like … more bone pain, joint pain and muscle pain. | N/A |
| Itching | (01-02-M-73-P) ...it was the worse thing you ever want to see. I mean, I was slapping myself, it was so bad.… I didn’t want to scratch it because I was leaving … red blotches, so I was slapping myself. |
(01-01-F-58)
I had no rash, nothing coming up on my body, but just a very severe itching, particularly after showers.
(01-01-F-58) … in 2013, last year, uh, I had been feeling very severe itching, |
| Dizziness | (01-05-F-55) … a little dizzy. | (01-04-M-50) … weight loss and dizziness.… Uh, it’s like, uh, getting on like one of them kids’ toys and getting spun around and then standing up and you just – it’s – sometimes it’s difficult to stand up. Usually I got to lean up against something for about 10 s for it to go away. |
| Bruise easily | 05-01-F-69) Well very easily bruising of course because of the lower platelet count.… if I get a cut or, you know, something like that it bleeds for quite a while.… I always, you know, of course have the bruising if I bump into anything.… | N/A |
| Balance | (01-09-F-73) The balance is when I get up and I tend to sometimes go to my left and hopefully there’s something there to grab on to. I’m actually considering a cane that – I feel I’m at that point where I should have something to … rely on.… You know, and I’ve learned to get up slowly and … | N/A |
| Brain fog | (03-09-F-29) It wasn’t that bad around the time that I was first diagnosed, then even … the first year or so … I didn’t really have it that bad. And I don’t know how much of it is because of the disease or it’s the Jakafi.… I don’t know which one … causes that. Or if it’s just the lack of sleep that I get.… Or … the fatigue … | N/A |
| Frequent urination | (01-09-F-73) It’s just … I have to go to the bathroom and when I have to go I better move quickly. Yeah … and it’s more so now because my bladder is being squished more … which I hate. I wear … the little panty liners when I came when I’m home. If I go out I wear a very thick one. And I’ve been looking at … those stupid diapers for adults, which I’m not looking forward to but … just to be on the safe side. ‘Cause if I have to go it’s going. It’s not gonna stop. | N/A |
| Hot flashes | (05-01-F-69) I’ve had somewhat of the … night sweats.… I think the only thing I experience at night has more to do with hot flashes than actual night sweats. | N/A |
| Muscle weakness | (01-09-F-73) … the legs not wanting to function.… And muscle weakness. I have no muscle whatsoever in my body.… Just no strength. | N/A |
| Peripheral neuropathy and/or numbness | (05-01-F-69) I do have some … peripheral neuropathy in my … lower legs … in my shin area and my feet. | N/A |
| Weight loss | (05-03-M-79) I went to my family doctor because I had lost a lot of weight.… I could barely stand up.… I found out the reason was that my blood count was so low … Of course I lost so much weight, I lost about 40 pounds in a month | (01-11-M-69) I just remember that I just started losing weight for no, no apparent reason.… I was losing weight and I didn’t really need to. |
| Appetite loss | N/A | (01-01-F-58) I used to eat, uh, more food in, in a meal and I used to have more meals and now I, I often, uh, skip a meal often and, uh, I, uh, I don’t have the appetite, uh, that I used to have. |
| Cold | N/A | (01-03-M-59) I notice I get a cold every year, uh, but I don’t know if that’s just [State] in general, you know. When I came back – I never got a cold when I was in [Country]. I got the flu, but not a cold every year. |
| Joint pain | (03-09-F-29) I would say within the first year a few more symptoms … started showing up, like … joint pain … | (01-11-M-69) I usually experience some type of joint pain.… |
| Rashes | N/A | (01-03-M-59) So, uh, besides that I get rashes. |
| Achiness | (05-05-M-85) I was hurting, aching. … they took me to the doctor to find out why I was weak and lightheaded – and aching.… | N/A |
| Back pain | (01-02-M-73) In my back. I have severe back pains since all this has come out, too … I’ve always had back pain but not, nothing like this here.… it would come and go but this one here has come and it stays. It doesn’t leave me. It’s constant. | N/A |
| Burning sensation | (05-01-F-69) … felt like you had kind of hot blood running through your … body, especially through your upper torso to where it was a burning sensation.… | N/A |
| Fevers and/or chills | (04-03-M-73) Once in a while when I have to go to bed early I need a couple Tylenols I get a fever or a chill, variable between those two extremes. That’s not very common … maybe once every two weeks or so, when I overexert. | N/A |
| Gout | (05-04-F-76) … it came on with the myelofibrosis … at one point they started me on the RUX2, and then they said, oh, we forgot to give you the gout medicine, so.… Then they gave me one, and that didn’t hold it, so they gave me a second one. So I was taking it twice a day. I’m only taking it once a day now.… | N/A |
| Hand pain | (03-07-F-64) It’s only in my hands. The first time it happened was before I was diagnosed with myelofibrosis … I was in my kitchen up north, walking across the room and got intense pain in the joint, the inner joint, the palm side of one finger, intense, 10. And I looked at it, and it was purple, like I called it a splinter hemorrhage which it’s not correct. It was like a star.… it was like a very specific black and blue point … | N/A |
| Headaches | (01-09-F-73) Terrible headaches. | N/A |
| Inflammation in arm | (01-09-F-73) It was a lot of fluid in my elbow and it’s, it’s come down here and I’m not quite sure what’s going on there.… it’s very red and sore.… It’s swollen … | N/A |
| Nausea |
(05-04-F-76-P)
I just don’t want to eat. It, it makes me feel sick to my stomach.… Makes me feel like I might vomit.
(05-04-F-76-P) Oh, lots.… every time I pull the food out.… And start – and start eating it. Usually I can get a couple bites before it hits. |
N/A |
All 16 (100%) RUX-experienced patients reported fatigue and/or tiredness (“…all of a sudden I started getting more lethargic and more tired, more fatigued” [05–02-M-74]), while 11 (69%) reported shortness of breath (“…I feel like I can’t even catch my breath. It’s, it’s getting worse. It’s not getting better at all.…” [01–02-M-73]), and nine (56%) reported pain below the ribs on the left side and/or stomach pain and/or abdominal pain (“I used to have a lot of spleen pain before I was on treatment.…it’s upper left … right under where my boob is … all the way to the left. Like on the side.” [03–09-F-29]), and enlarged spleen. The signs and symptoms most commonly reported by the RUX-naïve patients were fatigue and/or tiredness (reported by six patients, 86%; “I’d get really fatigued during the day. Come home, take a three-four-hour nap and, uh, never felt any different. Still fatigued …” [01–04-M-50]), pain below the ribs on the left side (reported by six patients, 86%; “…it is definitely on the, uh, left side of the ribcage.… It’s continual.…” [01–17-M-70]), and enlarged spleen, full quickly/filling up quickly, night sweats and/or general sweats, and itching (reported by four patients, 57%, each).
Patient-reported impacts of MF
Thirty-two impact concepts were expressed by RUX-experienced patients and 20 by RUX-naïve patients. The most frequently reported impacts for the RUX-experienced patients were impact of daily activities (n = 13, 81%), physical impact (n = 13, 81%), emotional and/or psychological impact (n = 11, 69%), social impact (n = 9, 56%), and impact on work and/or school (n = 8, 50%). The most commonly reported impacts for the RUX-naïve patients were social (n = 5, 71%), emotional and/or psychological (n = 4, 57%), and impact on work (n = 4, 57%).
Saturation
Concept saturation was achieved for both groups (see Table 4 for analysis of RUX-experienced patients, and Table 5 for analysis of RUX-naïve patients). Based on these saturation results, it was determined that an adequate number of interviews were performed.
Table 4.
Saturation grid for subjects with MF who are treatment-experienced (n = 16)
| Concepts | First four interviews vs. next foura | First eight interviews vs. next foura | First twelve interviews vs. next foura | Totalb | Saturation achievedc |
|---|---|---|---|---|---|
| Fatigue and/or tiredness | 3 vs. 4 | 7 vs. 3 | 10 vs. 3 | 13 | Yes |
| Enlarged spleen | 1 vs. 2 | 3 vs. 2 | 5 vs. 3 | 8 | Yes |
| Pain below the ribs on the left side and/or stomach pain and/or abdominal pain | 1 vs. 2 | 3 vs. 0 | 3 vs. 3 | 6 | Yes |
| Shortness of breath | 4 vs. 0 | 4 vs. 1 | 5 vs. 1 | 6 | Yes |
| Muscle pain and/or muscle cramps | 1 vs. 2 | 3 vs. 0 | 3 vs. 2 | 5 | Yes |
| Bone pain | 0 vs. 1 | 1 vs. 1 | 2 vs. 2 | 4 | Yes |
| Itching | 1 vs. 1 | 2 vs. 1 | 3 vs. 1 | 4 | Yes |
| Night sweats/general sweats | 1 vs. 2 | 3 vs. 1 | 4 vs. 0 | 4 | Yes |
| Uncomfortable abdomen and/or abdominal discomfort and/or bloating and/or feeling full | 0 vs. 2 | 2 vs. 0 | 2 vs. 2 | 4 | Yes |
| Bruise easily | 1 vs. 1 | 2 vs. 0 | 2 vs. 1 | 3 | Yes |
| Dizziness | 2 vs. 0 | 2 vs. 1 | 3 vs. 0 | 3 | Yes |
| Balance | 2 vs. 0 | 2 vs. 0 | 2 vs. 0 | 2 | Yes |
| Brain fog | 0 vs. 0 | 0 vs. 0 | 0 vs. 2 | 2 | Questionable |
| Frequent urination | 1 vs. 0 | 1 vs. 1 | 2 vs. 0 | 2 | Yes |
| Full quickly and/or filling up quickly and/or eating less | 0 vs. 1 | 1 vs. 0 | 1 vs. 1 | 2 | Yes |
| Muscle weakness | 1 vs. 0 | 1 vs. 1 | 2 vs. 0 | 2 | Yes |
| Peripheral neuropathy | 0 vs. 2 | 2 vs. 0 | 2 vs. 0 | 2 | Yes |
| Achiness | 0 vs. 0 | 0 vs. 1 | 1 vs. 0 | 1 | Yes |
| Back pain | 1 vs. 0 | 1 vs. 0 | 1 vs. 0 | 1 | Yes |
| Burning sensation | 0 vs. 1 | 1 vs. 0 | 1 vs. 0 | 1 | Yes |
| Fevers/chills | 0 vs. 0 | 0 vs. 1 | 1 vs. 0 | 1 | Yes |
| Gout | 0 vs. 0 | 0 vs. 1 | 1 vs. 0 | 1 | Yes |
| Hand pain | 0 vs. 0 | 0 vs. 0 | 0 vs. 1 | 1 | Questionable |
| Headaches | 0 vs. 0 | 0 vs. 0 | 0 vs. 1 | 1 | Questionable |
| Hot flashes | 0 vs. 1 | 1 vs. 0 | 1 vs. 0 | 1 | Yes |
| Inflammation in arm | 1 vs. 0 | 1 vs. 0 | 1 vs. 0 | 1 | Yes |
| Joint pain | 0 vs. 0 | 0 vs. 0 | 0 vs. 1 | 1 | Questionable |
| Weight loss | 0 vs. 0 | 0 vs. 0 | 0 vs. 1 | 1 | Questionable |
aSaturation is considered to be reached if a downward trend in the elicitation of new sign and symptom concepts is observed and no new concepts that are relevant (i.e., pertinent to the research question and/or not idiosyncratic to an individual subject) emerge in the final set of interviews. Saturation is considered questionable if new concepts emerge in the final quartile of interview. Subjects included in each group of interviews are as follows: Group 1 (01–05, 01–02, 01–09, and 01–07); Group 2 (03–01, 05–01, 05–02, and 04–01); Group 3 (05–04, 05–05, 05–03, and 04–03); and Group 4 (04–02, 05–07, 03–07, and 03–09)
bTotal number of subjects who reported the concept spontaneously
cQuestionable: concepts were considered not relevant to the research question and/or were idiosyncratic to the individual patient
Table 5.
Saturation grid for subjects with MF who are treatment naïve (n = 7)
| Root Concept | Interviews 1–3 vs. Interviews 4–5 (first three interviews vs. next two)a |
Interviews 1–5 vs. Interviews 6–7 (first five interviews vs. last two)a |
Totalb | Saturation achieved |
|---|---|---|---|---|
| Fatigue and/or tiredness | 2 vs. 2 | 4 vs. 2 | 6 | Yes |
| Pain below the ribs on the left side | 2 vs. 1 | 3 vs. 2 | 5 | |
| Enlarged spleen | 2 vs. 1 | 3 vs. 1 | 4 | |
| Night sweats/general sweats | 1 vs. 2 | 3 vs. 0 | 3 | |
| Weight loss | 2 vs. 1 | 3 vs. 0 | 3 | |
| Itching | 1 vs. 0 | 1 vs. 1 | 2 | |
| Appetite loss | 1 vs. 0 | 1 vs. 0 | 1 | |
| Bone pain | 1 vs. 0 | 1 vs. 0 | 1 | |
| Cold | 1 vs. 0 | 1 vs. 0 | 1 | |
| Dizziness | 1 vs. 0 | 1 vs. 0 | 1 | |
| Full quickly/filling up quickly | 1 vs. 0 | 1 vs. 0 | 1 | |
| Joint pain | 0 vs. 1 | 1 vs. 0 | 1 | |
| Rashes | 1 vs. 0 | 1 vs. 0 | 1 |
aSaturation is considered to be reached if a downward trend in the elicitation of new sign and symptom concepts is observed and no new concepts that are relevant (i.e., pertinent to the research question and/or not idiosyncratic to an individual subject) emerge in the final set of interviews. Saturation is considered questionable if new concepts emerge in the final quartile of interview. Subjects included in each group of interviews are as follows: Group 1 (01–01, 01–03, 01–04); Group 2 (01–11, 01–14); Group 3 (01–16, 01–17)
bTotal number of subjects who reported the concept spontaneously
Cognitive debriefing interview and ePRO usability
The final version of the 10-item MPN-SD includes items on filling up quickly, abdominal discomfort, inactivity, itching, night sweats, pain below the ribs on the left side, bone pain, fatigue (tiredness), shortness of breath, and appetite (loss); see Table 6 for the final version of the MPN-SD. Participants interpreted the MPN-SD instructions, items, and response options as the developers intended, and the concepts were relevant to both RUX-experienced and RUX-naïve patients.
Table 6.
MPN-SD
| INSTRUCTION TEXT: The following screens display questions about your myelofibrosis symptoms. Please rate each symptom at its WORST during the PAST 24 h | ||
|---|---|---|
| 1 | During the PAST 24 HOURS, at its WORST, how was your … Filling up quickly when you eat (feeling of fullness soon after you begin to eat) |
(Absent) 0 1 2 3 4 5 6 7 8 9 10 (Worst Imaginable) |
| 2 | During the PAST 24 HOURS, at its WORST, how was your … Abdominal discomfort (feeling uncomfortable, pressure or bloating) |
(Absent) 0 1 2 3 4 5 6 7 8 9 10 (Worst Imaginable) |
| 3 | During the PAST 24 HOURS, at its WORST, how was your … Inactivity (including work, home and social activities) |
(Absent) 0 1 2 3 4 5 6 7 8 9 10 (Worst Imaginable) |
| 4 | During the PAST 24 HOURS, at its WORST, how was your … Night sweats (excessive sweating during sleep) |
(Absent) 0 1 2 3 4 5 6 7 8 9 10 (Worst Imaginable) |
| 5 | During the PAST 24 HOURS, at its WORST, how was your … Itching |
(Absent) 0 1 2 3 4 5 6 7 8 9 10 (Worst Imaginable) |
| 6 | During the PAST 24 HOURS, at its WORST, how was your … Bone pain (widespread pain, not joint pain or arthritis) |
(Absent) 0 1 2 3 4 5 6 7 8 9 10 (Worst Imaginable) |
| 7 | During the PAST 24 HOURS, at its WORST, how was your … Pain below the ribs on the left side |
(Absent) 0 1 2 3 4 5 6 7 8 9 10 (Worst Imaginable) |
| 8 | During the PAST 24 HOURS, at its WORST, how was your … Fatigue (tiredness) |
(Absent) 0 1 2 3 4 5 6 7 8 9 10 (Worst Imaginable) |
| 9 | During the PAST 24 HOURS, at its WORST, how was your … Shortness of breath |
(Absent) 0 1 2 3 4 5 6 7 8 9 10 (Worst Imaginable) |
| 10 | During the PAST 24 HOURS, how was your … Appetite |
(Normal appetite) 0 1 2 3 4 5 6 7 8 9 10 (Complete loss of appetite) |
The MPN-SD instructions were modified based on two waves of interviews. Eight MF patients provided feedback on the original MPN-SD instructions (five RUX-experienced and three RUX-naïve patients), and five MF patients provided feedback on the alternate instructions (one RUX-experienced and four RUX-naïve patients).
The original instructions “During the PAST 24 HOURS, at its WORST, how was each of the following myelofibrosis symptoms? Please tap one number.” were modified to “The following screens display questions about your myelofibrosis symptoms. Please rate each symptom at its WORST during the PAST 24 HOURS.” More patients interpreted the alternate instructions correctly (92% of RUX experienced [n = 13] and 100% of RUX-naïve patients [n = 7]) as compared to the original instructions (92% of RUX-experienced and 43% of RUX-naïve patients).
“Bone or muscle pain” in the original MPN-SD was modified to “bone pain” based on feedback from five RUX-experienced and seven RUX-naïve patients. RUX-experienced patients (n = 5) indicated that bone pain was more relevant to their experience, expressing that (05–03-M-79) “Yes, I prefer the other one, the bone pain.… I’m not aware of any muscle pain associated with myelofibrosis.” Further, separate items for fatigue and tiredness in the original MPN-SD were combined into “fatigue (tiredness)” based on feedback from 12 RUX-experienced and seven RUX-naïve patients. Sixty-one percent of the RUX-experienced and 43% of RUX-naïve patients reported that fatigue and tiredness were the same. Patients expressed that (05–02-M-74) “…all of the sudden I started getting more lethargic and, and more tired, more fatigued.”
All RUX-experienced patients spontaneously reported shortness as breath as relevant and important to their experience of MF, five patients mentioned it spontaneously and six confirmed its relevance when probed. Experts also prioritized shortness of breath. Based on these two lines of evidence a shortness of breath item was added to the MPN-SD.
An item for ‘appetite’ was added to the MPN-SD. Appetite loss was relevant and important for treatment naïve patients (14%), by experts, and in the literature. The concept did not come up spontaneously in the treatment experienced patient interviews, although weight loss did.
No revisions were made to either the MPN-SD response options or to the ePRO device. The final MPN-SD includes 10 items, with response options ranging from 0 to 10 (0 = absent, 10 = worst imaginable for nine items, and 0 = normal appetite to 10 = complete loss of appetite for one item). It has a 24-h recall period and is administered via ePRO device. The MPN-SD has a total symptom scoring algorithm. See Table 6 for the final version of the MPN-SD.
Literature review
Thirty-two full text articles were included in the literature review [1, 2, 12–41]. A total of 32 symptoms were identified from the literature (see Table 7). The symptom concepts most frequently attested in the reviewed literature were: night sweats, fatigue, itching, bone pain, weight loss, fever, abdominal discomfort, early satiety, pain under the left ribs, dyspnea, and appetite loss. All of these, with the exception of weight loss and fever (which are clinically assessed), were also included in the symptom concepts assessed in the MPN-SD.
Table 7.
Concepts identified in the literature review
| Concept | Concept description |
|---|---|
| Night sweats [1, 2, 12, 13, 15, 17–26, 28–32, 34, 35, 37–39, 41] (including nocturnal sweats) | Episodes of nighttime sweating that soak your nightclothes or bedding and are related to some underlying cause |
| Fatigue [2, 13, 15, 17, 19–22, 24–30, 33–39, 41] | Feeling of tiredness that lasts a long time and doesn’t go away even after rest |
| Itching [1, 2, 12, 13, 15, 17–22, 24, 26, 28, 38, 39, 41] | Skin tingling or irritation that makes one want to scratch the itchy area |
| Bone pain [1, 2, 12, 13, 15, 17, 18, 20–22, 24–26, 28, 32, 34, 37, 39, 41] (including bone tenderness) | Aching or other discomfort in one or more bones |
| Weight loss [2, 12, 13, 15–17, 19, 21, 23–25, 28–30, 34, 37, 38] (Including undesired weight loss, weight loss attributed to gastric ulcer) | Decrease in body weight, when one did not try to lose the weight on their own |
| Fever [2, 12, 15, 16, 20, 21, 23–25, 28–30, 35, 37, 38] | Body temperature that is higher than normal |
| Abdominal discomfort/pain [1, 12, 13, 15, 17, 18, 21, 22, 24–26, 28, 34, 37–39, 41] | Pain/discomfort in the area from below one’s chest to groin |
| Early satiety [1, 12, 13, 15, 17, 18, 22, 24, 28, 33, 34, 39, 41] | Feeling full sooner than normal or after eating less than usual |
| Pain under left ribs [1, 2, 16, 18, 22, 26, 28, 39, 41] (including spleen pain, left upper quadrant/subcostal pain, occasional pain or discomfort from the enlarged spleen) | Pain or discomfort located under the ribs on the side of the abdomen |
| Dyspnea [19, 20, 26–28, 34, 41] | Feeling of not getting enough air (shortness of breath) |
| Appetite loss [19, 20, 26, 27, 34, 39, 41] | Reduced desire to eat |
| Pain/discomfort [20, 26, 27, 39, 41] | Feeling triggered in the nervous system. It may be sharp or dull, it may come and go, or it may be constant. It may be felt in one area of the body, such as the back, abdomen or chest or may be felt all over |
| Cough [21, 22, 25, 33, 34] | Reflex that keeps one’s throat and airways clear |
| Muscle pain [1, 18, 26, 39, 41] | Involves more than one muscle and can involve ligaments, tendons, and fascia, the soft tissues that connect muscles, bones, and organs |
| Headache [12, 21] (including chronic headache) | Pain in any region of the head |
| Numbness/tingling [12, 21, 32] (peripheral neuropathy) | Abnormal sensations that can occur anywhere in your body, but are often felt in your fingers, hands, feet, arms, or legs |
| Constipation [20, 27] | Having three or fewer bowel movements in a week. The stool can be hard and dry and sometimes it is painful to pass |
| Diarrhea [20, 27] | Having loose, watery stools more than three times in 1 day. One may also have cramps, bloating, nausea and an urgent need to have a bowel movement |
| Anorexia [20, 37] | Becoming too thin, but one doesn’t eat enough |
| Dizziness/vertigo [12, 21] | Dizziness is a feeling of being lightheaded or losing your balance. Vertigo is a feeling that the room is spinning |
| Nausea [20, 27] | An uneasy or unsettled feeling in the stomach together with an urge to vomit |
| Swelling [20, 25] (including swollen extremities) | Swelling of extremities (arms and legs) |
| Altered bowels [25] | Altered movement of food through the digestive tract. This could be in the form of diarrhea, constipation or bowel incontinence |
| Altered consciousness [28] | Altered awareness |
| Delirium [28] | Sudden severe confusion due to rapid changes in brain function that occur with physical or mental illness |
| Easy bruising [37] | Bleeding episodes |
| Palpitations [28] (including compensatory tachycardia) | Feelings or sensations that your heart is pounding or racing. They can be felt in your chest, throat, or neck |
| Photophobia [28] | Eye discomfort in bright light |
| Sweats [16] | Sweating is the release of liquid from the body’s sweat glands. Patients may experience excessive sweating due to MF |
| Abdominal swelling [14] | When the belly area is bigger than usual |
| Vomiting [20] | Forcing the contents of the stomach up through the esophagus and out of the mouth |
| Weakness [28] | Reduced strength in one or more muscles |
Expert advice meetings
Four experts in hematology/oncology, hematology, and internal medicine, who all worked academic (teaching) hospitals, participated in the expert advice meetings. Twenty-four symptoms and 31 impacts were identified by experts. All experts reported that patients with MF generally experience the same set of symptoms pre- and post-RUX treatment failure. See Table 8 for the list of symptoms identified by the experts.
Table 8.
Expert-reported symptoms of myelofibrosis
| Symptoms | Descriptiona | Experienced pre and/or post ruxolitinib treatment failure | Frequency of expert report (N = 4) n (%)b |
|---|---|---|---|
| Fatigue | Lack of energy, feeling of weakness and lethargy | Pre | 4 (100%) |
| Post | 4 (100%) | ||
| Night sweats | Excessive sweating at night requiring a change of clothing and/or beddings | Pre | 4 (100%) |
| Post | 4 (100%) | ||
| Weight loss | Unintentional loss of weight | Pre | 4 (100%) |
| Post | 4 (100%) | ||
| Abdominal pain | Pain associated with an enlarged spleen | Pre | 4 (100%) |
| Post | 3 (75%) | ||
| Fever | Low grade fever, feelings of hotness that may or may not be accompanied by sweating. A reoccurrence of fever after receiving ruxolitinib treatment could signal treatment failure or a true infection/progression of disease |
Pre | 3 (75%) |
| Post | 3 (75%) | ||
| Bone pain | Constant pain experienced all over the body. May respond to ruxolitinib treatment but not completely | Pre | 3 (75%) |
| Post | 2 (50%) | ||
| Constipation | Bowel movement once every 3 days characterized by hard stools or straining to go to the bathroom. It could be associated with the enlarged spleen (enlarged spleen blocking patients’ bowels). It is not a common MF symptom | Pre | 3 (75%) |
| Post | 2 (50%) | ||
| Poor appetite | Lack of feeling of hunger or not wanting to eat. It is typically associated with early satiety and enlarged spleen | Pre | 2 (50%) |
| Post | 2 (50%) | ||
| Early satiety | Feeling full fast after eating a meal. It could be associated with the enlarged spleen | Pre | 2 (50%) |
| Post | 2 (50%) | ||
| Abdominal discomfort | Associated with the enlarged spleen | Pre | 2 (50%) |
| Post | 1 (25%) | ||
| Diarrhea | Loose bowel movements or loose stools. | Pre | 2 (50%) |
| Post | 1 (25%) | ||
| Itching | It is also known as pruritus and can occur all over the body or certain parts of the body. It can occur when a patient comes in contact with a trigger (e.g., water) | Pre | 2 (50%) |
| Post | 1 (25%) | ||
| Shortness of breath | Needing to catch one’s breath. It can occur at rest or with exertion | Pre | 2 (50%) |
| Post | 1 (25%) | ||
| Abdominal fullness | Excess gas build up in the intestinesc | Pre | 1 (25%) |
| Post | 1 (25%) | ||
| Bleeding | Break in blood vessels such as gastrointestinal, mucosal, and hemorrhoidal bleeding, and nose bleeds. It typically occurs externally. | Pre | 1 (25%) |
| Post | 2 (50%) | ||
| Easy bruising | Bruising (blue-like appearance) under the skin due to minor trauma | Pre | 1 (25%) |
| Post | 1 (25%) | ||
| Lightheadedness | Feeling of dizziness | Pre | 1 (25%) |
| Post | 1 (25%) | ||
| Nausea | Feeling an urge to vomit.c It could be as a result of eating specific kinds of food or the enlarged spleen | Pre | 1 (25%) |
| Post | 1 (25%) | ||
| Reflux | Leaking of contents (food or liquid) backwards from the stomach into the esophagusc | Pre | 1 (25%) |
| Sweating | Release of liquid from the body’s sweat glands. Patients may experience excessive sweating due to MFc | Pre | 2 (50%) |
| Headaches | An ache or pain in the head | Pre | 1 (25%) |
| Impotent | Loss of sexual function | Pre | 1 (25%) |
| Vomiting | Forcing the contents of the stomach up through the esophagus and out of the mouthc | Pre | 1 (25%) |
aExpert’s description of the symptom except where noted
bNumber of experts who reported the symptom
cDefinition retrieved from the US National Library of Medicine
Discussion
This work demonstrates the comprehensiveness of the MPN-SD in assessing MF symptoms in RUX-experienced and RUX-naïve patients. The MPN-SD is simple and easy for patients to understand and complete and was developed following the US FDA regulatory guidance on PRO instruments. The patient CEI and CDI, expert interviews, literature review, and usability testing have shown that the MPN-SD has robust content validation.
Concepts identified in the CEIs, CDIs, and usability testing indicated that the MPN-SD is suitable for use in both MF patient groups. Of importance is the applicability of the MPN-SD in the clinical setting and ensuring that the instrument captures the real world experience of MF patients. Experts provided support for the use of the MPN-SD to assess many relevant symptoms of MF in both RUX-naïve and RUX-experienced patients.
A recent internet survey study [2] of 1179 patients with myeloproliferative disorders identified fatigue as one of the most frequently reported symptoms (80.7%) and main contributor to poor quality of life. Indeed, findings from this work indicate that fatigue is a relevant component of MF and if it were improved it would greatly benefit patients’ lives. Given that patients talked about both fatigue and tiredness as the same experience, the two concepts were included in one item on the final MPN-SD.
Several limitations of this study should be considered. Only three of the 16 RUX-experienced patients participated in the ePRO usability interviews; this is a very small sample size to draw conclusions from. The study sample is predominately white, which limits the generalizability of the findings to other racial groups. The CEIs and CDIs were combined in this study; one of the main criticisms of this approach is respondent bias. To attempt to reduce bias, the interview order was randomized. The study reported herein was completed in 2015; thus it does not reflect literature published after 2015, and more recent information could be available at the time of this publication.
The next step in development of the MPN-SD is psychometric evaluation. Demonstration of adequate psychometric performance (e.g., score reliability, construct-related validity, and sensitivity to change) and the creation of score interpretation guidelines (e.g., using anchor-based analyses) could facilitate the use of the MPN-SD to support the development, regulatory approval, and expedited availability of novel medicines for MF patients.
Conclusion
Improved understanding and development of an appropriate measurement tool to assess patients’ experiences of MF expands a perspective that was previously based primarily on tools developed for RUX-naïve patients. This work may help identify critical target areas for evaluation in clinical studies and guide investigators in selecting outcome variables suitable for intervention for MF patients.
Acknowledgements
Not applicable.
Funding
Funding for this study was provided by Pfizer, Inc.
Availability of data and materials
The data that support the findings of this study are available from Pfizer, Inc. but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Pfizer, Inc.
Abbreviations
- CDI
Cognitive debriefing interview
- CEI
Concept elicitation interview
- EMA
European Medicines Agency
- ePRO
Electronic patient-reported outcome
- FDA
United States Food and Drug Administration
- HIPAA
Health Insurance Portability and Accountability Act
- HRQoL
Health-related quality of life
- ISPOR
International Society for Pharamacoeconomics and Outcomes Research
- MF
Myelofibrosis
- MFSAF
Myelofibrosis Symptom Assessment Form
- MPN-SD
Myeloproliferative Neoplasm Symptom Assessment Diary
- PET-MF
Post-essential thrombocythemia myelofibrosis
- PMF
Primary myelofibrosis
- PPV-MF
Post-polycythemia vera myelofibrosis
- PRO
Patient-reported outcome
- RUX
Ruxolitinib
- US
United States
Authors’ contributions
RM: Conceptualization, data curation, formal analysis, supervision, visualization, writing review and editing. YS: Conceptualization, formal analysis, funding acquisition, methodology, project administration, resources, supervision, validation, visualization, writing review and editing. AW: Conceptualization, writing review and editing, methodology. JTP: Conceptualization, writing-review and editing. KT: Conceptualization, data curation, formal analysis, methodology, writing review. EJ: Writing review and editing. RS: Data curation, supervision, validation, draft preparation, reviewing and editing. ALS: Conceptualization, funding acquisition, methodology, project administration, formal analysis, supervision, writing – original draft, and writing review and editing. MK: Conceptualization, methodology, project administration, formal analysis, writing – original draft, and writing review and editing. FO: Data curation, formal analysis, methodology, and writing review and editing. FP: Conceptualization, methodology, data curation, formal analysis, writing review and editing. JCC: Methodology, writing review and editing. CH: Conceptualization, reading and editing. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Ethical review and approval of all documents related to both concept elicitation and cognitive debriefing patient interviews was provided by a centralized independent review board, Copernicus Group IRB. All patient participants provided signed informed consent and the Health Insurance Portability and Accountability Act Authorization prior to any data being collected.
Consent for publication
Not applicable.
Competing interests
AW, YS and JCC are employees and stockholders of Pfizer Inc., which funded the study. ALS, MK, FO, and FP are employees of Adelphi Values, paid consultants to Pfizer in connection with the development of this manuscript. RAM, JTP, KT, EJ, RS, and CH have no conflict of interest to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ruben A. Mesa, Email: mesa.ruben@mayo.edu
Yun Su, Email: yun_su@msn.com.
Adrien Woolfson, Email: adrianwoolfson@yahoo.com.
Josef T. Prchal, Email: Josef.Prchal@hsc.utah.edu
Kathleen Turnbull, Email: kathleen.turnbull@pfizer.com.
Elias Jabbour, Email: ejabbour@mdanderson.org.
Robyn Scherber, Email: Scherber@uthscsa.edu.
Alan L. Shields, Email: alan.shields@adelphivalues.com
Meaghan Krohe, Phone: 708 218 6017, Email: meaghan.krohe@adelphivalues.com.
Funke Ojo, Email: funke.olude@gmail.com.
Farrah Pompilus, Email: farrah.pompilus@gmail.com.
Joseph C. Cappelleri, Email: joseph.c.cappelleri@pfizer.com
Claire Harrison, Email: Claire.Harrison@gstt.nhs.uk.
References
- 1.Mesa RA, Shields A, Hare T, et al. Progressive burden of myelofibrosis in untreated patients: assessment of patient-reported outcomes in patients randomized to placebo in the COMFORT-I study. Leuk Res. 2013;37:911–916. doi: 10.1016/j.leukres.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international internet-based survey of 1179 MPD patients. Cancer. 2007;109:68–76. doi: 10.1002/cncr.22365. [DOI] [PubMed] [Google Scholar]
- 3.European Medicines Agency. Reflection paper on the use of patient reported outcome (PRO) measures in oncology studies (draft). 2014. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-reflection-paper-use-patient-reported-outcome-pro-measures-oncology-studies_en.pdf. Accessed 26 Mar 2019. [DOI] [PubMed]
- 4.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research et al. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. 2009. [Google Scholar]
- 5.Incyte. FDA Approves Incyte's Jakafi (TM) (ruxolitinib) for Patients with Myelofibrosis. 2011. https://investor.incyte.com/news-releases/news-release-details/fda-approves-incytes-jakafitm-ruxolitinib-patients-myelofibrosis. Accessed 26 Mar 2019.
- 6.US Food and Drug Administration: JAKAFI™ (ruxolitinib) - label and approval History. 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202192lbl.pdf. Accessed 26 Mar 2019.
- 7.Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity-establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1-eliciting concepts for a new PRO instrument. Value Health. 2011;14:967–977. doi: 10.1016/j.jval.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity-establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 2-assessing respondent understanding. Value Health. 2011;14:978–988. doi: 10.1016/j.jval.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Willis GB. Cognitive interviewing: a tool for improving questionnaire design. Thousand Oaks: Sage Publications; 2005. [Google Scholar]
- 10.Friese S. ATLAS.Ti 7 user guide and reference. Berlin: ATLAS.ti Scientific Software Development GmbH; 2013. [Google Scholar]
- 11.Miles MB, Huberman AM. Qualitative data analysis. London: Sage Publications; 1994. [Google Scholar]
- 12.Abelsson J, Andreasson B, Samuelsson J, et al. Patients with polycythemia vera have worst impairment of quality of life among patients with newly diagnosed myeloproliferative neoplasms. Leuk Lymphoma. 2013;54:2226–2230. doi: 10.3109/10428194.2013.766732. [DOI] [PubMed] [Google Scholar]
- 13.Atallah E, Verstovsek S. Emerging drugs for myelofibrosis. Expert Opin Emerg Drugs. 2012;17:555–570. doi: 10.1517/14728214.2012.748748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barugola G, Cavallini A, Lipari G, et al. The role of splenectomy in myelofibrosis with myeloid metaplasia. Minerva Chir. 2010;65:619–625. [PubMed] [Google Scholar]
- 15.Becker H, Engelhardt M, von Bubnoff M, et al. Ruxolitinib. Cancer Res. 2014;201:249–257. doi: 10.1007/978-3-642-54490-3_16. [DOI] [PubMed] [Google Scholar]
- 16.Bouabdallah R, Coso D, Gonzague-Casabianca L, et al. Safety and efficacy of splenic irradiation in the treatment of patients with idiopathic myelofibrosis: a report on 15 patients. Leuk Res. 2000;24:491–495. doi: 10.1016/S0145-2126(00)00018-7. [DOI] [PubMed] [Google Scholar]
- 17.Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30:4098–4103. doi: 10.1200/JCO.2012.42.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gimenez E, Besses C, Boque C, et al. Indirect and non-medical economic burden, quality-of-life, and disabilities of the myelofibrosis disease in Spain. J Med Econ. 2014;17:435–441. doi: 10.3111/13696998.2014.903257. [DOI] [PubMed] [Google Scholar]
- 19.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 20.Harrison CN, Mesa RA, Kiladjian JJ, et al. Health-related quality of life and symptoms in patients with myelofibrosis treated with ruxolitinib versus best available therapy. Br J Haematol. 2013;162:229–239. doi: 10.1111/bjh.12375. [DOI] [PubMed] [Google Scholar]
- 21.Johansson P, Mesa R, Scherber R, et al. Association between quality of life and clinical parameters in patients with myeloproliferative neoplasms. Leuk Lymphoma. 2012;53:441–444. doi: 10.3109/10428194.2011.619608. [DOI] [PubMed] [Google Scholar]
- 22.Komrokji R, Verstovsek S. Assessing efficacy in myelofibrosis treatment: a focus on JAK inhibition. Expert Rev Hematol. 2012;5:631–641. doi: 10.1586/ehm.12.50. [DOI] [PubMed] [Google Scholar]
- 23.Kremyanskaya M, Atallah EL, Hoffman R, et al. Clarifying the use of ruxolitinib in patients with myelofibrosis. Oncology (Williston Park) 2013;27:706–714. [PubMed] [Google Scholar]
- 24.Mesa RA, Schwager S, Radia D, et al. The myelofibrosis symptom assessment form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009;33:1199–1203. doi: 10.1016/j.leukres.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mesa RA, Kantarjian H, Tefferi A, et al. Evaluating the serial use of the myelofibrosis symptom assessment form for measuring symptomatic improvement: performance in 87 myelofibrosis patients on a JAK1 and JAK2 inhibitor (INCB018424) clinical trial. Cancer. 2011;117:4869–4877. doi: 10.1002/cncr.26129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mesa RA, Gotlib J, Gupta V, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2013;31:1285–1292. doi: 10.1200/JCO.2012.44.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mesa RA, Kiladjian JJ, Verstovsek S, et al. Comparison of placebo and best available therapy for the treatment of myelofibrosis in the phase 3 COMFORT studies. Haematologica. 2014;99:292–298. doi: 10.3324/haematol.2013.087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mughal TI, Vaddi K, Sarlis NJ, et al. Myelofibrosis-associated complications: pathogenesis, clinical manifestations, and effects on outcomes. Int J Gen Med. 2014;7:89–101. doi: 10.2147/IJGM.S51800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostojic A, Vrhovac R, Verstovsek S. Ruxolitinib: a new JAK1/2 inhibitor that offers promising options for treatment of myelofibrosis. Future Oncol. 2011;7:1035–1043. doi: 10.2217/fon.11.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostojic A, Vrhovac R, Verstovsek S. Ruxolitinib for the treatment of myelofibrosis: its clinical potential. Ther Clin Risk Manag. 2012;8:95–103. doi: 10.2147/TCRM.S23277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padrnos L, Mesa RA. A closer look at pacritinib: a JAK2/FLT3 inhibitor for the treatment of myelofibrosis. Expert Opinion on Orphan Drugs. 2014;2:725–733. doi: 10.1517/21678707.2014.927761. [DOI] [Google Scholar]
- 32.Quintas-Cardama A, Kantarjian H, Cortes J, et al. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov. 2011;10:127–140. doi: 10.1038/nrd3264. [DOI] [PubMed] [Google Scholar]
- 33.Santos FP, Verstovsek S. Breakthroughs in myeloproliferative neoplasms. Hematology. 2012;17(Suppl 1):S55–8. doi: 10.1179/102453312X13336169155574. [DOI] [PubMed] [Google Scholar]
- 34.Scherber R, Dueck AC, Johansson P. The myeloproliferative neoplasm symptom assessment form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118:401–408. doi: 10.1182/blood-2011-01-328955. [DOI] [PubMed] [Google Scholar]
- 35.Tefferi A, Barosi G, Mesa RA, et al. International working group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for myelofibrosis research and treatment (IWG-MRT) Blood. 2006;108:1497–1503. doi: 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- 36.Tefferi A, Hudgens S, Mesa R, et al. Use of the functional assessment of Cancer therapy--anemia in persons with myeloproliferative neoplasm-associated myelofibrosis and anemia. Clin Ther. 2014;36:560–566. doi: 10.1016/j.clinthera.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Thiele J, Kvasnicka HM, Werden C, et al. Idiopathic primary osteo-myelofibrosis: a clinico-pathological study on 208 patients with special emphasis on evolution of disease features, differentiation from essential thrombocythemia and variables of prognostic impact. Leuk Lymphoma. 1996;22:303–317. doi: 10.3109/10428199609051762. [DOI] [PubMed] [Google Scholar]
- 38.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verstovsek S. Ruxolitinib: an oral Janus kinase 1 and Janus kinase 2 inhibitor in the management of myelofibrosis. Postgrad Med. 2013;125:128–135. doi: 10.3810/pgm.2013.01.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wade R, Rose M, Neilson AR, et al. Ruxolitinib for the treatment of myelofibrosis: a NICE single technology appraisal. Pharmacoeconomics. 2013;31:841–852. doi: 10.1007/s40273-013-0083-0. [DOI] [PubMed] [Google Scholar]
- 41.Yang LPH, Keating GM. Ruxolitinib. Drugs. 2012;72:2117–2127. doi: 10.2165/11209340-000000000-00000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Pfizer, Inc. but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Pfizer, Inc.