Abstract
Background
Unexpected weight loss is a symptom of serious disease in primary care, for example between 1 in 200 and 1 in 30 patients with unexpected weight loss go on to develop cancer. However, it remains unclear how and when general practitioners (GPs) should investigate unexpected weight loss. Without clarification, GPs may wait too long before referring (choosing to watch and wait and potentially missing a diagnosis) or not long enough (overburdening hospital services and exposing patients to the risks of investigation). The overall aim of this study is to provide the evidence necessary to allow GPs to more effectively manage patients with unexpected weight loss.
Methods
A retrospective cohort analysis of UK Clinical Practice Research Datalink (CPRD) data to: (1) describe how often in UK primary care the symptom of reported weight loss is coded, when weight is measured, and how GPs respond to a patient attending with unexpected weight loss; (2) identify the predictive value of recorded weight loss for cancer and serious disease in primary care, using cumulative incidence plots to compare outcomes between subgroups and Cox regression to explore and adjust for covariates. Preliminary work in CPRD estimates that weight loss as a symptom is recorded for approximately 148,000 eligible patients > 18 years and is distributed evenly across decades of age, providing adequate statistical power and precision in relation to cancer overall and common cancers individually. Further stratification by cancer stage will be attempted but may not be possible as not all practices within CPRD are eligible for cancer registry linkage, and staging information is often incomplete. The feasibility of using multiple imputation to address missing covariate values will be explored.
Discussion
This will be the largest reported retrospective cohort of primary care patients with weight measurements and unexpected weight loss codes used to understand the association between weight measurement, unexpected weight loss, and serious disease including cancer. Our findings will directly inform international guidelines for the management of unexpected weight loss in primary care populations.
Keywords: Weight loss, Early detection of cancer, Serious disease, Primary care, Cohort study
Background
A 2014 systematic review suggests that the positive predictive value (PPV) for cancer is 33% in patients with an unexpected 10% loss of weight from baseline over 6–12 months. The same review reported a wide range of differential diagnoses for patients with unexpected weight loss, including advanced heart failure, chronic obstructive pulmonary disease, renal disease, pancreatic insufficiency, malabsorption, and endocrine disease, with up to 25% of patients without a diagnosis to explain their weight loss after extended follow-up [1]. However, these data mainly come from hospital inpatient populations or patients referred to the outpatient clinic where the prevalence of cancer and serious disease is much higher than in primary care as GPs have already filtered out many cases of weight loss that are more likely to be attributable to another cause. Given the absence of appropriate clinical guidelines or standardised practice, clinicians have been reported to take a wide range of action in response to patients with unexpected weight loss, from doing nothing through to ordering “extensive blind investigations” because of the fear of underlying cancer [2].
On the basis of primary care research, NICE (2015) has since suggested that unexpected weight loss is a sign of seven cancers, citing evidence from 14 studies reporting positive predictive values (PPVs) of 0.4–3% [3]. The problem for GPs is how to interpret and implement the term weight loss in these cancer guidelines: NICE do not define the degree of weight loss, or the time period of loss, that should prompt referral. Most cited studies referred to in the NICE guidelines define weight loss on the basis of a coded entry in the GP record, often based on a report of weight loss (volunteered by, or elicited from, the patient) rather than measured weight change [4–6]. Only one study referred to by NICE quantified the degree of weight loss that predicts colorectal cancer in primary care reporting odds ratios of 1.2 (95% CI 0.99–1.5) for 5–9.9% and 2.5 (2.1–3) for ≥ 10% weight loss [7]. However, in this study, weight loss was defined by comparing the last recorded weight with the highest recorded weight in the preceding 2 years [7], as weight is not routinely recorded in primary care and is considered a common missing variable in primary care databases [8].
There is an evidence gap for a comprehensive study to describe the use of weight measurement and coding for unexpected weight loss in primary care and for a study that determines the association between unexpected weight loss and cancer and serious disease that may lead to a comprehensive recommendation for the investigation of unexpected weight loss in primary care.
Objective
The overall objective is to provide the evidence necessary to allow GPs to more effectively manage unexpected weight loss.
Aims and rationale
Aim 1.1
To describe how often and when weight is measured, and the symptom of unexpected weight loss recorded as a code, in adults aged > 18 years, in NHS primary care.
Aim 1.2
To describe what action is taken in response to unexpected weight loss, in adults aged > 18 years, in NHS primary care.
Weight measurements and weight loss codes will be categorised using a rule-based search strategy developed as part of this project to identify the clinical purpose and clinical condition related to each weight entry in the primary care record, and the investigations requested, medications prescribed, and referrals made in response to the symptom of weight loss.
Aim 2.1
To identify the predictive value of unexpected weight loss recorded as a symptom for cancer in primary care in adults aged > 18 years.
Aim 2.2
If the symptom of unexpected weight loss predicts cancer, to explore if it is (i) independent of other symptoms, signs, and test results and (ii) restricted to late-stage disease.
Aim 2.3
To ascertain the predictive value of unexpected weight loss recorded as a symptom for serious disease in primary care.
The evidence regarding the predictive value of unexpected weight loss for cancer in primary care, which underpins the 2015 NICE guideline, does not cover all cancer types or take cancer stage at diagnosis into account. We will identify the predictive value of unexpected weight loss in primary care across all cancer types, explore the incremental predictive value of symptom combinations, and examine the association with cancer stage at diagnosis using a matched open cohort study design. In cases where cancer is excluded, an understanding of which alternative diagnoses are related to unexpected weight loss will inform subsequent management decisions in primary care. We will therefore identify the disease groups for which unexpected weight loss is also predictive to develop clinical guidance for the investigation of unexpected weight loss in primary care.
Study type
Aim 1: Descriptive
The descriptive epidemiology of weight measurement and weight loss coding in NHS primary care.
Aims 2.1 and 2.3: Hypothesis testing
A cohort study of weight loss as a sign of cancer and serious disease in NHS primary care.
Aim 2.2: Exploratory
Exploratory analysis to investigate the influence of covariates on the relationship between weight loss and the occurrence of cancer and serious disease.
Study design
The design of the study is an open cohort study.
Sample size
In preparing this ISAC application, a preliminary search of 20 GP practices from 2000 to 2013 was conducted. Of 127,024 patients > 40 years with acceptable records, 80,562 (63.4%) had at least one weight measurement recorded during that period, 30,728 (24.1%) had two weight measurements within 6 months of each other, and 40,436 (31.8%) within 1 year; 3079 (2.4%) of patients had a Read code for weight loss but only half of these had an accompanying weight measurement.
Two thousand one hundred eighty-four patients with weight loss are required to detect a hazard ratio of 2 (a change in incidence of 1.5 to 3%) at 99% power (0.05% alpha) using a ratio of one case to five controls. It is anticipated that the study will therefore have sufficient power for stratification by cancer type, cancer stage, and using symptom combinations even though linkage to cancer registry may only be possible in approximately 60% of cancer cases [9].
Preliminary work in Clinical Practice Research Datalink (CPRD) estimated that that unexpected weight loss is coded as a symptom for about approximately 148,000 patients > 18 years and is distributed evenly across decades of age providing adequate statistical power and precision for a comprehensive cohort study investigating cancer and serious disease in adults (> 18 years). For example, if 3% of patients with weight loss develop cancer the number of Events Per Variable will far exceed the minimum number required for robust statistical modelling.
Data linkage
NCDR Cancer Registry Data
Linkage to the cancer register is required as cancer is a major outcome variable in this cohort study. Cancer registry data will provide more accurate information on cancer site and stage than reliance on the primary care record.
Office of National Statistics (ONS) mortality data
Linkage is required to cross-validate cause of death for patients confirmed to have died of cancer using Cancer Registry Data linkage and to identify or confirm the cause of death in patients with and without serious disease as identified by the GP record.
Index of Multiple Deprivation (IMD) scores
They are required to provide a GP (and where possible patient) level proxy for socioeconomic status to be used when describing both the baseline characteristics in the descriptive analysis of Aim 1 and the cohort analysis of Aim 2. IMD score will also be used as a covariate in the multivariate cox regression analysis as part of Aim 2 (see below).
Study population
The study population is summarised in Fig. 1.
Fig. 1.
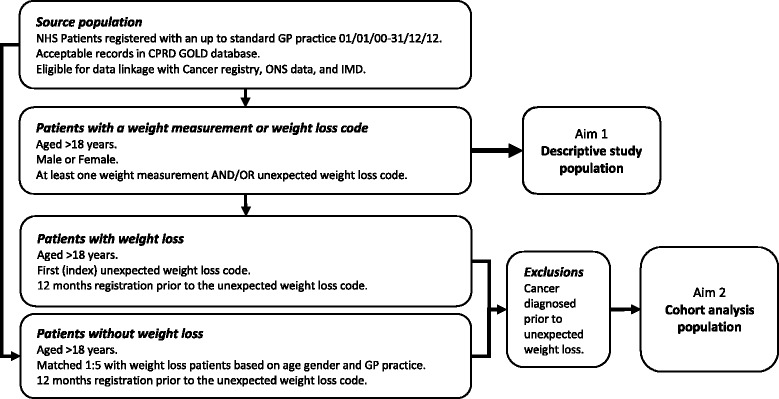
Flowchart of study populations
Aim 1: Descriptive study
NHS patients > 18 years
Registered with a GP practice 1 January 2000–31 December 2011
Eligible for data linkage with Cancer registry and ONS data
Aim 2: Cohort analysis
Inclusions:
NHS patients > 18 years
Registered with a GP practice 1 January 2000–31 December 2011
Eligible for data linkage with Cancer registry and ONS data.
Patients with one of the unexpected weight loss codes (defined in Table 1)
Table 1.
Weight measurement and unexpected weight loss codes
| Unexpected weight loss codes | ||
| Medcode | Readcode | Readterm |
| 126 | 22A6.00 | O/E—underweight |
| 654 | 1623 | Weight decreasing |
| 1581 | 162..00 | Weight symptom |
| 3647 | R032.00 | [D]Abnormal loss of weight |
| 4663 | 1625 | Abnormal weight loss |
| 5812 | 1625.11 | Abnormal weight loss—symptom |
| 12,398 | 1D1A.00 | Complaining of weight loss |
| 12,530 | R034800 | [D]Underweight |
| 14,764 | 162Z.00 | Weight symptom NOS |
| 22,005 | 2224 | O/E—cachexic |
| 24,068 | R2y4.00 | [D]Cachexia |
| 32,914 | 22K3.00 | Body Mass Index low K/M2 |
| 37,937 | 22A8.00 | Weight loss from baseline weight |
| 42,309 | 22A7.00 | Baseline weight |
| 53,801 | R2y4z00 | [D]Cachexia NOS |
| 102,563 | 1627 | Unintentional weight loss |
| Weight measurement codes | ||
| 2 | 22A..00 | O/E—weight |
| 8105 | 22K..00 | Body mass index |
| 9015 | 22K4.00 | Body mass index 25–29—overweight |
| 13,278 | 22K5.00 | Body mass index 30+—obesity |
| 21,520 | 22AZ.00 | O/E—weight NOS |
| 22,556 | 22K7.00 | Body mass index 40+—severely obese |
| 24,496 | 22K6.00 | Body mass index less than 20 |
| 28,937 | 22K2.00 | Body mass index high K/M2 |
| 28,946 | 22K1.00 | Body mass index normal K/M2 |
| 44,291 | 22K8.00 | Body mass index 20–24—normal |
| 101,047 | 22K9.00 | Body mass index centile |
| 105,791 | 22K9000 | Baseline body mass index centile |
| 105,800 | 22KB.00 | Baseline body mass index |
| 107,231 | 22KA.00 | Target body mass index |
Exclusions:
Patients with a diagnosis of cancer prior to the index symptom of weight loss.
Selection of comparison group(s) or controls
Aim 1: Descriptive study
-No comparison group is required.
Aim 2: Cohort analysis
A matched cohort of patients without weight loss—patients without a coded entry for weight loss will be matched for age and sex and selected from the population of patients registered with the same practice having consulted within ± 3 months of the index weight loss code.
Matching for age and sex will ensure there are sufficient patients without weight loss in each age and sex strata.
A 1:5 sampling ratio achieves the best balance between data cost and statistical power (see sample size).
Exposures, outcomes and covariates
Aim 1: Descriptive study
Outcome 1: Objective weight measurement—quantitative weight measurements.
Outcome 2: Weight loss code—Read Codes defined in Table 1.
Patients with objective weight measurements or the symptom of unexpected weight loss recorded using the following Medcodes and Read codes listed in Table 1.
Aim 2: Cohort analysis
Exposure—weight loss
Patients with the symptom of weight loss recorded using the unexpected weight loss Medcodes and Read Codes listed in Table 1. Weight loss codes will be independently categorised for clinical relavence by four co-investigators based on the results of the descriptive analysis, then consensus reached through discussion.
Outcome—cancer
A library of over 1600 Read Codes and ICD-10 codes (grouped by site—see Table 2) developed by Hamilton and colleagues will be reviewed, updated using Read Code searches, and validated through consensus amongst co-investigators. All new cancer diagnoses in the 24 months following the weight loss code will be identified in CPRD and linked cancer registry data. To inform this analysis, data will also be extracted on cancer stage, grade, tumour size, and histology at diagnosis.
Table 2.
Cancer codes
| Cancer | Read code | Description | Medcode | ICD 10 |
|---|---|---|---|---|
| Bladder | B490.00 | Malignant neoplasm of trigone of urinary bladder | 38,862 | C670 |
| B491.00 | Malignant neoplasm of dome of urinary bladder | 44,996 | C671 | |
| B492.00 | Malignant neoplasm of lateral wall of urinary bladder | 35,963 | C672 | |
| B493.00 | Malignant neoplasm of anterior wall of urinary bladder | 19,162 | C673 | |
| B494.00 | Malignant neoplasm of posterior wall of urinary bladder | 42,012 | C674 | |
| B495.00 | Malignant neoplasm of bladder neck | 41,571 | C675 | |
| B496.00 | Malignant neoplasm of ureteric orifice | 28,241 | C676 | |
| B497.00 | Malignant neoplasm of urachus | 42,023 | C677 | |
| B49y000 | Malignant neoplasm, overlapping lesion of bladder | 47,801 | C678 | |
| B49y.00 | Malignant neoplasm of other site of urinary bladder | 36,949 | C679 | |
| B49z.00 | Malignant neoplasm of urinary bladder NOS | 31,102 | C679 | |
| Breast | B335200 | Malignant neoplasm of skin of breast | 30,543 | C445 |
| B34..11 | CA female breast | 348 | C50 | |
| B34..00 | Malignant neoplasm of female breast | 3968 | C50 | |
| B340000 | Malignant neoplasm of nipple of female breast | 23,380 | C500 | |
| B340.00 | Malignant neoplasm of nipple and areola of female breast | 26,853 | C500 | |
| B340z00 | Malignant neoplasm of nipple or areola of female breast nos | 59,831 | C500 | |
| B340100 | Malignant neoplasm of areola of female breast | 64,686 | C500 | |
| B341.00 | Malignant neoplasm of central part of female breast | 31,546 | C501 | |
| B342.00 | Malignant neoplasm of upper-inner quadrant of female breast | 29,826 | C502 | |
| B343.00 | Malignant neoplasm of lower-inner quadrant of female breast | 45,222 | C503 | |
| B344.00 | Malignant neoplasm of upper-outer quadrant of female breast | 23,399 | C504 | |
| B345.00 | Malignant neoplasm of lower-outer quadrant of female breast | 42,070 | C505 | |
| B346.00 | Malignant neoplasm of axillary tail of female breast | 20,685 | C506 | |
| B34y000 | Malignant neoplasm of ectopic site of female breast | 95,057 | C508 | |
| B34yz00 | Malignant neoplasm of other site of female breast nos | 38,475 | C509 | |
| B34y.00 | Malignant neoplasm of other site of female breast | 56,715 | C509 | |
| B34z.00 | Malignant neoplasm of female breast nos | 9470 | C509 | |
| Cervix | B410z00 | Malignant neoplasm of endocervix nos | 50,285 | C530 |
| B410.00 | Malignant neoplasm of endocervix | 48,820 | C530 | |
| B410000 | Malignant neoplasm of endocervical canal | 57,235 | C530 | |
| B410100 | Malignant neoplasm of endocervical gland | 53,103 | C530 | |
| B411.00 | Malignant neoplasm of exocervix | 50,297 | C531 | |
| B412.00 | Malignant neoplasm, overlapping lesion of cervix uteri | 58,094 | C538 | |
| B41y100 | Malignant neoplasm of squamocolumnar junction of cervix | 57,719 | C538 | |
| B41y000 | Malignant neoplasm of cervical stump | 95,505 | C538 | |
| B41yz00 | Malignant neoplasm of other site of cervix nos | 43,435 | C539 | |
| B41z.00 | Malignant neoplasm of cervix uteri nos | 28,311 | C539 | |
| B41y.00 | Malignant neoplasm of other site of cervix | 32,955 | C539 | |
| Colorectal | B134.11 | Carcinoma of caecum | 22,163 | C180 |
| B134.00 | Malignant neoplasm of caecum | 3811 | C180 | |
| B136.00 | Malignant neoplasm of ascending colon | 10,946 | C182 | |
| B130.00 | Malignant neoplasm of hepatic flexure of colon | 9088 | C183 | |
| B131.00 | Malignant neoplasm of transverse colon | 6935 | C184 | |
| B137.00 | Malignant neoplasm of splenic flexure of colon | 18,619 | C185 | |
| B132.00 | Malignant neoplasm of descending colon | 10,864 | C186 | |
| B133.00 | Malignant neoplasm of sigmoid colon | 2815 | C187 | |
| B138.00 | Malignant neoplasm, overlapping lesion of colon | 93,478 | C188 | |
| B13y.00 | Malignant neoplasm of other specified sites of colon | 48,231 | C189 | |
| B13z.11 | Colonic cancer | 9118 | C189 | |
| B13z.00 | Malignant neoplasm of colon nos | 28,163 | C189 | |
| B140.00 | Malignant neoplasm of rectosigmoid junction | 27,855 | C19 | |
| B141.12 | Rectal carcinoma | 5901 | C20 | |
| B141.11 | Carcinoma of rectum | 7219 | C20 | |
| B141.00 | Malignant neoplasm of rectum | 1800 | C20 | |
| B14y.00 | Malig neop other site rectum, rectosigmoid junction and anus | 55,659 | C218 | |
| B14z.00 | Malignant neoplasm rectum,rectosigmoid junction and anus nos | 50,974 | C218 | |
| B1z0.11 | Cancer of bowel | 11,628 | C260 | |
| B18y200 | Malignant neoplasm of mesorectum | 30,165 | C481 | |
| Larynx | B214.00 | Malignant neoplasm, overlapping lesion of larynx | 50,579 | C328 |
| B21z.00 | Malignant neoplasm of larynx NOS | 9237 | C329 | |
| B21y.00 | Malignant neoplasm of larynx, other specified site | 26,813 | C329 | |
| B210.00 | Malignant neoplasm of glottis | 318 | C320 | |
| B215.00 | Malignant neoplasm of epiglottis NOS | 55,374 | C321 | |
| B211.00 | Malignant neoplasm of supraglottis | 26,165 | C321 | |
| B212.00 | Malignant neoplasm of subglottis | 22,441 | C322 | |
| B213z00 | Malignant neoplasm of laryngeal cartilage NOS | 97,332 | C323 | |
| B213000 | Malignant neoplasm of arytenoid cartilage | 63,460 | C323 | |
| B213.00 | Malignant neoplasm of laryngeal cartilage | 43,111 | C323 | |
| B213100 | Malignant neoplasm of cricoid cartilage | 37,805 | C323 | |
| Thyroid | B213300 | Malignant neoplasm of thyroid cartilage | 47,862 | C323 |
| B53..00 | Malignant neoplasm of thyroid gland | 5637 | C73 | |
| Sarcoma | B150200 | Primary angiosarcoma of liver | 68,410 | C223 |
| B1z1100 | Fibrosarcoma of spleen | 72,224 | C261 | |
| B30z000 | Osteosarcoma | 19,437 | C419 | |
| B339.00 | Dermatofibrosarcoma protuberans | 24,375 | C449 | |
| B33z000 | Kaposi’s sarcoma of skin | 27,931 | C460 | |
| B05z000 | Kaposi’s sarcoma of palate | 37,549 | C462 | |
| B6z0.00 | Kaposi’s sarcoma of lymph nodes | 50,290 | C463 | |
| B592X00 | Kaposi’s sarcoma of multiple organs | 65,466 | C468 | |
| Byu5300 | [X]kaposi’s sarcoma, unspecified | 93,665 | C469 | |
| B59zX00 | Kaposi’s sarcoma, unspecified | 49,525 | C469 | |
| B600000 | Reticulosarcoma of unspecified site | 60,242 | C833 | |
| B600100 | Reticulosarcoma of lymph nodes of head, face, and neck | 71,031 | C833 | |
| B600700 | Reticulosarcoma of spleen | 95,058 | C833 | |
| B600300 | Reticulosarcoma of intra-abdominal lymph nodes | 70,374 | C833 | |
| B600.00 | Reticulosarcoma | 1481 | C839 | |
| B601000 | Lymphosarcoma of unspecified site | 71,625 | C850 | |
| B601200 | Lymphosarcoma of intrathoracic lymph nodes | 62,380 | C850 | |
| B601.00 | Lymphosarcoma | 27,416 | C850 | |
| B601100 | Lymphosarcoma of lymph nodes of head, face and neck | 71,238 | C850 | |
| B601z00 | Lymphosarcoma nos | 63,723 | C850 | |
| B601300 | Lymphosarcoma of intra-abdominal lymph nodes | 64,670 | C850 | |
| B653.00 | Myeloid sarcoma | 70,724 | C923 | |
| B653100 | Granulocytic sarcoma | 39,629 | C923 | |
| B67y000 | Lymphosarcoma cell leukaemia | 72,197 | C947 | |
| B304200 | Malignant neoplasm of humerus | 61,741 | C400 | |
| B304000 | Malignant neoplasm of scapula | 49,054 | C400 | |
| B304300 | Malignant neoplasm of radius | 92,371 | C400 | |
| B304.00 | Malignant neoplasm of scapula and long bones of upper arm | 71,810 | C400 | |
| B304z00 | Malignant neoplasm of scapula and long bones of upper arm NOS | 65,880 | C400 | |
| B304400 | Malignant neoplasm of ulna | 64,848 | C400 | |
| B305.00 | Malignant neoplasm of hand bones | 73,530 | C401 | |
| B305.12 | Malignant neoplasm of metacarpal bones | 72,464 | C401 | |
| B305C00 | Malignant neoplasm of fifth metacarpal bone | 94,427 | C401 | |
| B305z00 | Malignant neoplasm of hand bones NOS | 73,556 | C401 | |
| B305100 | Malignant neoplasm of carpal bone—lunate | 69,104 | C401 | |
| B305000 | Malignant neoplasm of carpal bone—scaphoid | 57,988 | C401 | |
| B305D00 | Malignant neoplasm of phalanges of hand | 86,812 | C401 | |
| B307z00 | Malignant neoplasm of long bones of leg NOS | 62,630 | C402 | |
| B307.00 | Malignant neoplasm of long bones of leg | 68,055 | C402 | |
| B307200 | Malignant neoplasm of tibia | 40,814 | C402 | |
| B307100 | Malignant neoplasm of fibula | 50,402 | C402 | |
| B307000 | Malignant neoplasm of femur | 56,513 | C402 | |
| B308300 | Malignant neoplasm of medial cuneiform | 34,878 | C403 | |
| B308800 | Malignant neoplasm of first metatarsal bone | 69,927 | C403 | |
| B308B00 | Malignant neoplasm of fourth metatarsal bone | 92,382 | C403 | |
| B308100 | Malignant neoplasm of talus | 95,182 | C403 | |
| B308D00 | Malignant neoplasm of phalanges of foot | 58,949 | C403 | |
| B308200 | Malignant neoplasm of calcaneum | 72,212 | C403 | |
| B30X.00 | Malignant neoplasm/bones + articular cartilage/limb, unspecified | 43,614 | C409 | |
| Byu3100 | [X]Malignant neoplasm/bones + articular cartilage/limb, unspecified | 73,296 | C409 | |
| B300600 | Malignant neoplasm of parietal bone | 54,747 | C410 | |
| B300400 | Malignant neoplasm of occipital bone | 55,953 | C410 | |
| B300z00 | Malignant neoplasm of bones of skull and face NOS | 69,146 | C410 | |
| B300300 | Malignant neoplasm of nasal bone | 95,458 | C410 | |
| B300900 | Malignant neoplasm of zygomatic bone | 50,299 | C410 | |
| B300C00 | Malignant neoplasm of vomer | 44,452 | C410 | |
| B300500 | Malignant neoplasm of orbital bone | 50,298 | C410 | |
| B300700 | Malignant neoplasm of sphenoid bone | 55,595 | C410 | |
| B300200 | Malignant neoplasm of malar bone | 59,520 | C410 | |
| B300B00 | Malignant neoplasm of turbinate | 96,445 | C410 | |
| B300000 | Malignant neoplasm of ethmoid bone | 53,594 | C410 | |
| B300100 | Malignant neoplasm of frontal bone | 53,599 | C410 | |
| B300800 | Malignant neoplasm of temporal bone | 62,104 | C410 | |
| B300.00 | Malignant neoplasm of bones of skull and face | 59,036 | C410 | |
| B300A00 | Malignant neoplasm of maxilla | 17,475 | C410 | |
| B301.00 | Malignant neoplasm of mandible | 33,833 | C411 | |
| B302100 | Malignant neoplasm of thoracic vertebra | 32,372 | C412 | |
| B302.00 | Malignant neoplasm of vertebral column | 16,704 | C412 | |
| B302000 | Malignant neoplasm of cervical vertebra | 46,939 | C412 | |
| B302200 | Malignant neoplasm of lumbar vertebra | 54,691 | C412 | |
| B302z00 | Malignant neoplasm of vertebral column NOS | 49,701 | C412 | |
| B303000 | Malignant neoplasm of rib | 37,842 | C413 | |
| B303.00 | Malignant neoplasm of ribs, sternum and clavicle | 27,528 | C413 | |
| B303100 | Malignant neoplasm of sternum | 49,491 | C413 | |
| B303z00 | Malignant neoplasm of rib, sternum and clavicle NOS | 51,237 | C413 | |
| B303500 | Malignant neoplasm of xiphoid process | 54,493 | C413 | |
| B303300 | Malignant neoplasm of costal cartilage | 60,403 | C413 | |
| B303200 | Malignant neoplasm of clavicle | 66,639 | C413 | |
| B306.00 | Malignant neoplasm of pelvic bones, sacrum and coccyx | 54,631 | C414 | |
| B306100 | Malignant neoplasm of ischium | 59,223 | C414 | |
| B306400 | Malignant neoplasm of coccygeal vertebra | 66,908 | C414 | |
| B306z00 | Malignant neoplasm of pelvis, sacrum or coccyx NOS | 38,938 | C414 | |
| B306300 | Malignant neoplasm of sacral vertebra | 40,966 | C414 | |
| B306200 | Malignant neoplasm of pubis | 51,921 | C414 | |
| B306000 | Malignant neoplasm of ilium | 44,609 | C414 | |
| Byu3200 | [X]Malignant neoplasm/overlap lesion/bone + articular cartilage | 63,300 | C418 | |
| B30W.00 | Malignant neoplasm/overlap lesion/bone + articular cartilage | 67,451 | C418 | |
| B303400 | Malignant neoplasm of costo-vertebral joint | 67,763 | C418 | |
| B30z.00 | Malignant neoplasm of bone and articular cartilage NOS | 16,075 | C419 | |
| Byu3300 | [X]Malignant neoplasm/bone + articular cartilage, unspecified | 43,151 | C419 | |
| B310z00 | Malig neop connective and soft tissue head, face, neck NOS | 73,718 | C490 | |
| B310100 | Malignant neoplasm of soft tissue of face | 40,014 | C490 | |
| B310000 | Malignant neoplasm of soft tissue of head | 59,382 | C490 | |
| B310300 | Malignant neoplasm of cartilage of ear | 60,035 | C490 | |
| B310.00 | Malignant neoplasm of connective and soft tissue head, face and neck | 43,475 | C490 | |
| B310200 | Malignant neoplasm of soft tissue of neck | 48,517 | C490 | |
| B310400 | Malignant neoplasm of tarsus of eyelid | 49,463 | C490 | |
| B311500 | Malignant neoplasm of connective and soft tissue of thumb | 63,988 | C491 | |
| B311200 | Malignant neoplasm of connective and soft tissue of fore-arm | 57,482 | C491 | |
| B311100 | Malignant neoplasm of connective and soft tissue, upper arm | 64,345 | C491 | |
| B311000 | Malignant neoplasm of connective and soft tissue of shoulder | 50,222 | C491 | |
| B311400 | Malignant neoplasm of connective and soft tissue of finger | 91,586 | C491 | |
| B311300 | Malignant neoplasm of connective and soft tissue of hand | 19,321 | C491 | |
| B311.00 | Malignant neoplasm connective and soft tissue upper limb/shoulder | 53,989 | C491 | |
| B312300 | Malignant neoplasm of connective and soft tissue of lower leg | 30,542 | C492 | |
| B312400 | Malignant neoplasm of connective and soft tissue of foot | 54,222 | C492 | |
| B312.00 | Malignant neoplasm of connective and soft tissue of hip and leg | 66,088 | C492 | |
| B312z00 | Malignant neoplasm connective and soft tissue hip and leg NOS | 90,546 | C492 | |
| B312200 | Malignant neoplasm connective and soft tissue of popliteal space | 54,965 | C492 | |
| B312100 | Malignant neoplasm of connective and soft tissue thigh and upper leg | 44,805 | C492 | |
| B313100 | Malignant neoplasm of diaphragm | 54,186 | C493 | |
| B313.00 | Malignant neoplasm of connective and soft tissue of thorax | 22,290 | C493 | |
| B313000 | Malignant neoplasm of connective and soft tissue of axilla | 29,160 | C493 | |
| B313200 | Malignant neoplasm of great vessels | 72,522 | C493 | |
| B314.00 | Malignant neoplasm of connective and soft tissue of abdomen | 45,071 | C494 | |
| B314z00 | Malignant neoplasm of connective and soft tissue of abdomen NOS | 60,247 | C494 | |
| B314000 | Malignant neoplasm of connective and soft tissue of abdominal wall | 66,488 | C494 | |
| B315z00 | Malignant neoplasm of connective and soft tissue of pelvis NOS | 58,836 | C495 | |
| B315000 | Malignant neoplasm of connective and soft tissue of buttock | 70,463 | C495 | |
| B315200 | Malignant neoplasm of connective and soft tissue of perineum | 59,152 | C495 | |
| B315.00 | Malignant neoplasm of connective and soft tissue of pelvis | 51,965 | C495 | |
| B315100 | Malignant neoplasm of connective and soft tissue of inguinal region | 67,324 | C495 | |
| Byu5800 | [X]Mal neoplasm/connective + soft tissue of trunk, unspecified | 91,896 | C496 | |
| B314100 | Malig neoplasm of connective and soft tissues of lumb spine | 94,272 | C496 | |
| B316.00 | Malig neop of connective and soft tissue trunk unspecified | 57,471 | C496 | |
| B31z.00 | Malignant neoplasm of connective and soft tissue, site NOS | 15,182 | C499 | |
| Byu5900 | [X]Malignant neoplasm/connective + soft tissue, unspecified | 91,457 | C499 | |
| B31y.00 | Malignant neoplasm connective and soft tissue other specified site | 65,233 | C499 | |
| Kidney | B4A0.00 | Malignant neoplasm of kidney parenchyma | 1599 | C64 |
| B4A..11 | Renal malignant neoplasm | 18,712 | C64 | |
| B4A..00 | Malignant neoplasm of kidney and other unspecified urinary organs | 13,559 | C64 | |
| B4A0000 | Hypernephroma | 7978 | C64 | |
| B4A1000 | Malignant neoplasm of renal calyces | 27,540 | C65 | |
| B4A1z00 | Malignant neoplasm of renal pelvis NOS | 54,184 | C65 | |
| B4A1.00 | Malignant neoplasm of renal pelvis | 12,389 | C65 | |
| B4Az.00 | Malignant neoplasm of kidney or urinary organs NOS | 29,462 | C689 | |
| Lung | B221100 | Malignant neoplasm of hilus of lung | 33,444 | C340 |
| B221.00 | Malignant neoplasm of main bronchus | 12,870 | C340 | |
| B221z00 | Malignant neoplasm of main bronchus NOS | 21,698 | C340 | |
| B221000 | Malignant neoplasm of carina of bronchus | 17,391 | C340 | |
| B222.11 | Pancoast’s syndrome | 20,170 | C341 | |
| B222.00 | Malignant neoplasm of upper lobe, bronchus or lung | 10,358 | C341 | |
| B222000 | Malignant neoplasm of upper lobe bronchus | 31,700 | C341 | |
| B222100 | Malignant neoplasm of upper lobe of lung | 25,886 | C341 | |
| B222z00 | Malignant neoplasm of upper lobe, bronchus or lung NOS | 44,169 | C341 | |
| B223100 | Malignant neoplasm of middle lobe of lung | 39,923 | C342 | |
| B223z00 | Malignant neoplasm of middle lobe, bronchus or lung NOS | 54,134 | C342 | |
| B223.00 | Malignant neoplasm of middle lobe, bronchus or lung | 31,268 | C342 | |
| B223000 | Malignant neoplasm of middle lobe bronchus | 41,523 | C342 | |
| B224z00 | Malignant neoplasm of lower lobe, bronchus or lung NOS | 42,566 | C343 | |
| B224100 | Malignant neoplasm of lower lobe of lung | 12,582 | C343 | |
| B224000 | Malignant neoplasm of lower lobe bronchus | 18,678 | C343 | |
| B224.00 | Malignant neoplasm of lower lobe, bronchus or lung | 31,188 | C343 | |
| B225.00 | Malignant neoplasm of overlapping lesion of bronchus and lung | 36,371 | C348 | |
| B22z.00 | Malignant neoplasm of bronchus or lung NOS | 3903 | C349 | |
| Byu2000 | [X]malignant neoplasm of bronchus or lung, unspecified | 40,595 | C349 | |
| B22z.11 | Lung cancer | 2587 | C349 | |
| B22y.00 | Malignant neoplasm of other sites of bronchus or lung | 38,961 | C349 | |
| B26..00 | Malignant neoplasm, overlap lesion of resp and intrathor orgs | 66,646 | C398 | |
| B2zy.00 | Malignant neoplasm of other site of respiratory tract | 29,283 | C399 | |
| Hodgkins lymphoma | B613.00 | Hodgkin’s disease, lymphocytic-histiocytic predominance | 38,939 | C810 |
| B613600 | Hodgkin’s, lymphocytic-histiocytic pred intrapelvic nodes | 95,338 | C810 | |
| B613z00 | Hodgkin’s, lymphocytic-histiocytic predominance nos | 29,876 | C810 | |
| B613300 | Hodgkin’s, lymphocytic-histiocytic pred intra-abdominal node | 73,532 | C810 | |
| B613000 | Hodgkin’s, lymphocytic-histiocytic predominance unspec site | 71,142 | C810 | |
| B613200 | Hodgkin’s, lymphocytic-histiocytic pred intrathoracic nodes | 92,245 | C810 | |
| B613100 | Hodgkin’s, lymphocytic-histiocytic pred of head, face, neck | 68,330 | C810 | |
| B613500 | Hodgkin’s, lymphocytic-histiocytic pred inguinal and leg | 93,951 | C810 | |
| B614400 | Hodgkin’s nodular sclerosis of lymph nodes of axilla and arm | 65,483 | C811 | |
| B614300 | Hodgkin’s nodular sclerosis of intra-abdominal lymph nodes | 61,149 | C811 | |
| B614.00 | Hodgkin’s disease, nodular sclerosis | 29,178 | C811 | |
| B614100 | Hodgkin’s nodular sclerosis of head, face and neck | 55,303 | C811 | |
| B614z00 | Hodgkin’s disease, nodular sclerosis NOS | 63,054 | C811 | |
| B614200 | Hodgkin’s nodular sclerosis of intrathoracic lymph nodes | 67,506 | C811 | |
| B614000 | Hodgkin’s disease, nodular sclerosis of unspecified site | 57,225 | C811 | |
| B614800 | Hodgkin’s nodular sclerosis of lymph nodes of multiple sites | 19,140 | C811 | |
| B615200 | Hodgkin’s mixed cellularity of intrathoracic lymph nodes | 58,684 | C812 | |
| B615z00 | Hodgkin’s disease, mixed cellularity NOS | 94,005 | C812 | |
| B615.00 | Hodgkin’s disease, mixed cellularity | 49,605 | C812 | |
| B615100 | Hodgkin’s mixed cellularity of lymph nodes head, face, neck | 94,407 | C812 | |
| B615000 | Hodgkin’s disease, mixed cellularity of unspecified site | 97,863 | C812 | |
| B616.00 | Hodgkin’s disease, lymphocytic depletion | 67,703 | C813 | |
| B616400 | Hodgkin’s lymphocytic depletion lymph nodes axilla and arm | 63,625 | C813 | |
| B616000 | Hodgkin’s lymphocytic depletion of unspecified site | 95,049 | C813 | |
| ByuD000 | [X]other Hodgkin’s disease | 43,415 | C817 | |
| B610.00 | Hodgkin’s paragranuloma | 65,489 | C817 | |
| B611.00 | Hodgkin’s granuloma | 44,196 | C817 | |
| B61z100 | Hodgkin’s disease NOS of lymph nodes of head, face and neck | 59,778 | C819 | |
| B61..00 | Hodgkin’s disease | 2462 | C819 | |
| B61zz00 | Hodgkin’s disease NOS | 42,461 | C819 | |
| B61z800 | Hodgkin’s disease NOS of lymph nodes of multiple sites | 97,746 | C819 | |
| B61z200 | Hodgkin’s disease NOS of intrathoracic lymph nodes | 59,755 | C819 | |
| B61z.00 | Hodgkin’s disease NOS | 53,397 | C819 | |
| B61z000 | Hodgkin’s disease NOS, unspecified site | 61,662 | C819 | |
| B61z400 | Hodgkin’s disease NOS of lymph nodes of axilla and arm | 91,900 | C819 | |
| B61z700 | Hodgkin’s disease NOS of spleen | 94,279 | C819 | |
| B612.00 | Hodgkin’s sarcoma | 64,036 | C817 | |
| B612400 | Hodgkin’s sarcoma of lymph nodes of axilla and upper limb | 68,039 | C817 | |
| Non-Hodgkins lymphoma | B627000 | Follicular non-Hodgkin’s small cleaved cell lymphoma | 28,639 | C820 |
| B627100 | Follicular non-Hodgkin’s mixed sml cleavd & lge cell lymphoma | 70,842 | C821 | |
| B627200 | Follicular non-Hodgkin’s large cell lymphoma | 49,262 | C822 | |
| B627B00 | Other types of follicular non-Hodgkin’s lymphoma | 31,576 | C827 | |
| ByuD100 | [X]other types of follicular non-Hodgkin’s lymphoma | 67,518 | C827 | |
| B620500 | Nodular lymphoma of lymph nodes of inguinal region and leg | 94,995 | C829 | |
| B627C11 | Follicular lymphoma NOS | 17,182 | C829 | |
| B620000 | Nodular lymphoma of unspecified site | 66,327 | C829 | |
| B620100 | Nodular lymphoma of lymph nodes of head, face and neck | 45,264 | C829 | |
| B620z00 | Nodular lymphoma NOS | 65,701 | C829 | |
| B620.00 | Nodular lymphoma (brill - symmers disease) | 5179 | C829 | |
| B620300 | Nodular lymphoma of intra-abdominal lymph nodes | 92,068 | C829 | |
| B627C00 | Follicular non-Hodgkin’s lymphoma | 21,549 | C829 | |
| B620800 | Nodular lymphoma of lymph nodes of multiple sites | 58,082 | C829 | |
| B627300 | Diffuse non-Hodgkin’s small cell (diffuse) lymphoma | 50,668 | C830 | |
| B627500 | Diffuse non-Hodgkin mixed small & large cell (diffuse) lymphoma | 50,695 | C832 | |
| B627600 | Diffuse non-Hodgkin’s immunoblastic (diffuse) lymphoma | 53,551 | C834 | |
| B627700 | Diffuse non-Hodgkin’s lymphoblastic (diffuse) lymphoma | 17,460 | C835 | |
| B627800 | Diffuse non-Hodgkin’s lymphoma undifferentiated (diffuse) | 65,180 | C836 | |
| B602300 | Burkitt’s lymphoma of intra-abdominal lymph nodes | 97,577 | C837 | |
| B602z00 | Burkitt’s lymphoma NOS | 71,304 | C837 | |
| B602.00 | Burkitt’s lymphoma | 21,402 | C837 | |
| B602500 | Burkitt’s lymphoma of lymph nodes of inguinal region and leg | 92,380 | C837 | |
| B602100 | Burkitt’s lymphoma of lymph nodes of head, face and neck | 59,115 | C837 | |
| B627D00 | Diffuse non-Hodgkin’s centroblastic lymphoma | 70,509 | C838 | |
| ByuDC00 | [X]Diffuse non-Hodgkin’s lymphoma, unspecified | 64,515 | C839 | |
| B627X00 | Diffuse non-Hodgkin’s lymphoma, unspecified | 39,798 | C839 | |
| B622.00 | Sezary’s disease | 35,014 | C841 | |
| B62x000 | T-zone lymphoma | 90,201 | C842 | |
| B62x100 | Lymphoepithelioid lymphoma | 57,737 | C843 | |
| B62x200 | Peripheral t-cell lymphoma | 12,464 | C844 | |
| B62xX00 | Oth and unspecif peripheral and cutaneous t cell lymphomas | 44,318 | C845 | |
| B627W00 | Unspecified b-cell non-Hodgkin’s lymphoma | 31,794 | C851 | |
| ByuDE00 | [X]unspecified b-cell non-Hodgkin’s lymphoma | 63,375 | C851 | |
| ByuD300 | [X]Other specified types of non-Hodgkin’s lymphoma | 64,336 | C857 | |
| B62y100 | Malignant lymphoma NOS of lymph nodes of head, face and neck | 50,696 | C859 | |
| B62y500 | Malignant lymphoma NOS of lymph node inguinal region and leg | 63,105 | C859 | |
| B62y400 | Malignant lymphoma NOS of lymph nodes of axilla and arm | 34,089 | C859 | |
| B62y000 | Malignant lymphoma NOS of unspecified site | 57,427 | C859 | |
| B62y700 | Malignant lymphoma NOS of spleen | 60,092 | C859 | |
| ByuDF11 | [X]Non-Hodgkin’s lymphoma NOS | 7940 | C859 | |
| B62y600 | Malignant lymphoma NOS of intrapelvic lymph nodes | 71,262 | C859 | |
| B62y200 | Malignant lymphoma NOS of intrathoracic lymph nodes | 72,725 | C859 | |
| B62yz00 | Malignant lymphoma NOS | 15,027 | C859 | |
| ByuDF00 | [X]Non-Hodgkin’s lymphoma, unspecified type | 8649 | C859 | |
| B62y.00 | Malignant lymphoma NOS | 12,335 | C859 | |
| B62y300 | Malignant lymphoma NOS of intra-abdominal lymph nodes | 42,579 | C859 | |
| B62x600 | True histiocytic lymphoma | 95,630 | C963 | |
| B6z..00 | Malignant neoplasm lymphatic or haematopoietic tissue NOS | 49,301 | C969 | |
| B62y800 | Malignant lymphoma NOS of lymph nodes of multiple sites | 15,504 | C969 | |
| B621000 | Mycosis fungoides of unspecified site | 95,949 | C840 | |
| B621500 | Mycosis fungoides of lymph nodes of inguinal region and leg | 72,714 | C840 | |
| B621.00 | Mycosis fungoides | 12,006 | C840 | |
| B621800 | Mycosis fungoides of lymph nodes of multiple sites | 95,012 | C840 | |
| B621400 | Mycosis fungoides of lymph nodes of axilla and upper limb | 96,379 | C840 | |
| B621300 | Mycosis fungoides of intra-abdominal lymph nodes | 91,674 | C840 | |
| B621z00 | Mycosis fungoides NOS | 38,005 | C840 | |
| B62x400 | Malignant reticulosis | 62,437 | C857 | |
| Melanoma | B320.00 | Malignant melanoma of lip | 70,637 | C430 |
| B321.00 | Malignant melanoma of eyelid including canthus | 54,632 | C431 | |
| B322000 | Malignant melanoma of auricle (ear) | 59,061 | C432 | |
| B322.00 | Malignant melanoma of ear and external auricular canal | 57,260 | C432 | |
| B322z00 | Malignant melanoma of ear and external auricular canal NOS | 73,744 | C432 | |
| B323100 | Malignant melanoma of chin | 71,136 | C433 | |
| B323200 | Malignant melanoma of eyebrow | 47,094 | C433 | |
| B323500 | Malignant melanoma of temple | 58,958 | C433 | |
| B323z00 | Malignant melanoma of face NOS | 67,806 | C433 | |
| Byu4000 | [X]malignant melanoma of other + unspecified parts of face | 56,925 | C433 | |
| B323.00 | Malignant melanoma of other and unspecified parts of face | 47,252 | C433 | |
| B323300 | Malignant melanoma of forehead | 68,133 | C433 | |
| B323400 | Malignant melanoma of external surface of nose | 45,139 | C433 | |
| B323000 | Malignant melanoma of external surface of cheek | 41,278 | C433 | |
| B324000 | Malignant melanoma of scalp | 55,881 | C434 | |
| B324.00 | Malignant melanoma of scalp and neck | 65,625 | C434 | |
| B324100 | Malignant melanoma of neck | 45,306 | C434 | |
| B325700 | Malignant melanoma of back | 43,463 | C435 | |
| B325800 | Malignant melanoma of chest wall | 51,209 | C435 | |
| B325600 | Malignant melanoma of umbilicus | 43,715 | C435 | |
| B325100 | Malignant melanoma of breast | 32,768 | C435 | |
| B325300 | Malignant melanoma of groin | 34,259 | C435 | |
| B325200 | Malignant melanoma of buttock | 53,629 | C435 | |
| B325500 | Malignant melanoma of perineum | 95,629 | C435 | |
| B325.00 | Malignant melanoma of trunk (excluding scrotum) | 38,689 | C435 | |
| B325z00 | Malignant melanoma of trunk, excluding scrotum, NOS | 45,760 | C435 | |
| B325000 | Malignant melanoma of axilla | 49,814 | C435 | |
| B326200 | Malignant melanoma of fore-arm | 45,755 | C436 | |
| B326400 | Malignant melanoma of finger | 25,602 | C436 | |
| B326300 | Malignant melanoma of hand | 62,475 | C436 | |
| B326000 | Malignant melanoma of shoulder | 50,505 | C436 | |
| B326500 | Malignant melanoma of thumb | 63,997 | C436 | |
| B326z00 | Malignant melanoma of upper limb or shoulder NOS | 55,292 | C436 | |
| B326100 | Malignant melanoma of upper arm | 54,685 | C436 | |
| B326.00 | Malignant melanoma of upper limb and shoulder | 65,164 | C436 | |
| B327500 | Malignant melanoma of ankle | 42,714 | C437 | |
| B327700 | Malignant melanoma of foot | 41,490 | C437 | |
| B327000 | Malignant melanoma of hip | 73,536 | C437 | |
| B327100 | Malignant melanoma of thigh | 51,873 | C437 | |
| B327800 | Malignant melanoma of toe | 36,899 | C437 | |
| B327200 | Malignant melanoma of knee | 54,305 | C437 | |
| B327.00 | Malignant melanoma of lower limb and hip | 46,255 | C437 | |
| B327600 | Malignant melanoma of heel | 61,246 | C437 | |
| B327300 | Malignant melanoma of popliteal fossa area | 39,878 | C437 | |
| B327z00 | Malignant melanoma of lower limb or hip NOS | 64,327 | C437 | |
| B327900 | Malignant melanoma of great toe | 53,369 | C437 | |
| B327400 | Malignant melanoma of lower leg | 37,872 | C437 | |
| B32y000 | Overlapping malignant melanoma of skin | 96,585 | C438 | |
| B32z.00 | Malignant melanoma of skin NOS | 28,556 | C439 | |
| Byu4100 | [X]malignant melanoma of skin, unspecified | 19,444 | C439 | |
| B32..00 | Malignant melanoma of skin | 865 | C439 | |
| B32y.00 | Malignant melanoma of other specified skin site | 42,153 | C439 | |
| Myeloma | B63z.00 | Immunoproliferative neoplasm or myeloma NOS | 43,450 | C889 |
| B630.12 | Myelomatosis | 15,211 | C900 | |
| B630.00 | Multiple myeloma | 4944 | C900 | |
| B630300 | Lambda light chain myeloma | 46,042 | C900 | |
| B631.00 | Plasma cell leukaemia | 39,187 | C901 | |
| B630100 | Solitary myeloma | 19,028 | C902 | |
| B630200 | Plasmacytoma NOS | 21,329 | C902 | |
| B630000 | Malignant plasma cell neoplasm, extramedullary plasmacytoma | 22,158 | C902 | |
| Oesophagus | B100.00 | Malignant neoplasm of cervical oesophagus | 61,695 | C150 |
| B101.00 | Malignant neoplasm of thoracic oesophagus | 41,362 | C151 | |
| B102.00 | Malignant neoplasm of abdominal oesophagus | 63,470 | C152 | |
| B103.00 | Malignant neoplasm of upper third of oesophagus | 50,789 | C153 | |
| B104.00 | Malignant neoplasm of middle third of oesophagus | 54,171 | C154 | |
| B105.00 | Malignant neoplasm of lower third of oesophagus | 42,416 | C155 | |
| B106.00 | Malignant neoplasm, overlapping lesion of oesophagus | 67,497 | C158 | |
| B10y.00 | Malignant neoplasm of other specified part of oesophagus | 53,591 | C159 | |
| B10z.00 | Malignant neoplasm of oesophagus NOS | 30,700 | C159 | |
| B10z.11 | Oesophageal cancer | 4865 | C159 | |
| B110111 | Malignant neoplasm of gastro-oesophageal junction | 94,278 | C160 | |
| Ovary | B440.00 | Malignant neoplasm of ovary | 7805 | C56 |
| B440.11 | Cancer of ovary | 1986 | C56 | |
| B44..00 | Malignant neoplasm of ovary and other uterine adnexa | 19,141 | C578 | |
| Pancreas | B162.00 | Malignant neoplasm of ampulla of vater | 10,949 | C241 |
| B170.00 | Malignant neoplasm of head of pancreas | 8771 | C250 | |
| B171.00 | Malignant neoplasm of body of pancreas | 40,810 | C251 | |
| B172.00 | Malignant neoplasm of tail of pancreas | 39,870 | C252 | |
| B173.00 | Malignant neoplasm of pancreatic duct | 35,535 | C253 | |
| B174.00 | Malignant neoplasm of islets of langerhans | 35,795 | C254 | |
| B17y.00 | Malignant neoplasm of other specified sites of pancreas | 48,537 | C257 | |
| B17yz00 | Malignant neoplasm of specified site of pancreas NOS | 95,783 | C257 | |
| B175.00 | Malignant neoplasm, overlapping lesion of pancreas | 97,875 | C258 | |
| B17y000 | Malignant neoplasm of ectopic pancreatic tissue | 96,635 | C259 | |
| B17z.00 | Malignant neoplasm of pancreas NOS | 34,388 | C259 | |
| Prostate | B46..00 | Malignant neoplasm of prostate | 780 | C61 |
| Stomach | B110100 | Malignant neoplasm of cardio-oesophageal junction of stomach | 22,894 | C160 |
| B110z00 | Malignant neoplasm of cardia of stomach NOS | 37,859 | C160 | |
| B110.00 | Malignant neoplasm of cardia of stomach | 32,022 | C160 | |
| B113.00 | Malignant neoplasm of fundus of stomach | 32,362 | C161 | |
| B114.00 | Malignant neoplasm of body of stomach | 43,572 | C162 | |
| B112.00 | Malignant neoplasm of pyloric antrum of stomach | 19,318 | C163 | |
| B111z00 | Malignant neoplasm of pylorus of stomach NOS | 59,092 | C164 | |
| B111100 | Malignant neoplasm of pyloric canal of stomach | 41,215 | C164 | |
| B111000 | Malignant neoplasm of prepylorus of stomach | 48,237 | C164 | |
| B111.00 | Malignant neoplasm of pylorus of stomach | 21,620 | C164 | |
| B115.00 | Malignant neoplasm of lesser curve of stomach unspecified | 42,193 | C165 | |
| B116.00 | Malignant neoplasm of greater curve of stomach unspecified | 55,434 | C166 | |
| B11y000 | Malignant neoplasm of anterior wall of stomach nec | 65,312 | C168 | |
| B11y100 | Malignant neoplasm of posterior wall of stomach nec | 96,802 | C168 | |
| B117.00 | Malignant neoplasm, overlapping lesion of stomach | 51,690 | C168 | |
| B11yz00 | Malignant neoplasm of other specified site of stomach NOS | 65,372 | C169 | |
| B11y.00 | Malignant neoplasm of other specified site of stomach | 55,019 | C169 | |
| B11z.00 | Malignant neoplasm of stomach NOS | 14,800 | C169 | |
| Testis | B470200 | Seminoma of undescended testis | 7740 | C620 |
| B470.00 | Malignant neoplasm of undescended testis | 64,602 | C620 | |
| B470300 | Teratoma of undescended testis | 36,325 | C620 | |
| B470z00 | Malignant neoplasm of undescended testis NOS | 96,429 | C620 | |
| B471z00 | Malignant neoplasm of descended testis NOS | 91,509 | C621 | |
| B471000 | Seminoma of descended testis | 21,786 | C621 | |
| B471100 | Teratoma of descended testis | 9476 | C621 | |
| B471.00 | Malignant neoplasm of descended testis | 19,475 | C621 | |
| B47z.00 | Malignant neoplasm of testis NOS | 38,510 | C629 | |
| B47z.11 | Seminoma of testis | 2961 | C629 | |
| B47z.12 | Teratoma of testis | 15,989 | C629 | |
| B48y100 | Malignant neoplasm of tunica vaginalis | 47,668 | C637 | |
| Uterus | B431000 | Malignant neoplasm of lower uterine segment | 59,097 | C540 |
| B431z00 | Malignant neoplasm of isthmus of uterine body NOS | 70,729 | C540 | |
| B431.00 | Malignant neoplasm of isthmus of uterine body | 43,940 | C540 | |
| B430211 | Malignant neoplasm of endometrium | 49,400 | C541 | |
| B430200 | Malignant neoplasm of endometrium of corpus uteri | 2890 | C541 | |
| B430300 | Malignant neoplasm of myometrium of corpus uteri | 45,793 | C542 | |
| B430100 | Malignant neoplasm of fundus of corpus uteri | 68,155 | C543 | |
| B432.00 | Malignant neoplasm of overlapping lesion of corpus uteri | 16,967 | C548 | |
| B43z.00 | Malignant neoplasm of body of uterus NOS | 33,617 | C549 | |
| B43y.00 | Malignant neoplasm of other site of uterine body | 31,608 | C549 | |
| B430000 | Malignant neoplasm of cornu of corpus uteri | 72,723 | C549 | |
| B430z00 | Malignant neoplasm of corpus uteri NOS | 45,490 | C549 | |
| B43..00 | Malignant neoplasm of body of uterus | 7046 | C549 | |
| B40..00 | Malignant neoplasm of uterus, part unspecified | 2744 | C55 | |
| Vulval | B451.00 | Malignant neoplasm of labia majora | 43,761 | C510 |
| B453.00 | Malignant neoplasm of clitoris | 53,910 | C512 | |
| B45y000 | Malignant neoplasm of overlapping lesion of vulva | 27,617 | C518 | |
| B454.00 | Malignant neoplasm of vulva unspecified | 4554 | C519 | |
| B454.11 | Primary vulval cancer | 11,991 | C519 | |
| B451z00 | Malignant neoplasm of labia majora NOS | 59,362 | C510 | |
| B451000 | Malignant neoplasm of greater vestibular (Bartholin’s) gland | 47,899 | C510 | |
| B452.00 | Malignant neoplasm of labia minora | 58,061 | C511 | |
| Vaginal | B450.00 | Malignant neoplasm of vagina | 37,328 | C52 |
| B450100 | Malignant neoplasm of vaginal vault | 10,698 | C52 | |
| B450z00 | Malignant neoplasm of vagina NOS | 60,772 | C52 |
Outcome—serious disease
A library of candidate Read Codes for the most common serious diseases related to unexpected weight loss will be developed by combining two approaches: (i) review of the most frequent diagnostic codes entered in the clinical record within the period surrounding the unexpected weight loss code (descriptive study analysis section); (ii) review of the literature on causes of unexpected weight loss [1, 2]. A list of these candidate conditions will be reviewed independently by four co-investigators until consensus is reached on up to 20 serious diseases to be identified in the 24 months following the weight loss code.
Covariates
Data will also be extracted to explore the effect of the following factors which could independently impact the recording of weight and the occurrence of cancer:
Personal characteristics—age, gender, ethnicity, smoking history, alcohol intake, family history of cancer, and IMD score recorded before the date of the weight loss code (index date).
Co-morbidity—recorded before the index date (no time limit) or implied from the prescribing record at the index date.
Other cancer symptoms and signs—using Read Codes for symptoms shown to have an independent association with cancer as described by NICE [3]. These will be sought for 3 months before to 2 years after the index date.
Results of basic cancer investigations used routinely in primary care: CxR, FBC, LFTs (inc. alkaline phosphatase), calcium, PSA, CA125, and inflammatory markers. These will be sought for 3 months before to 2 years after the index date.
Data/statistical analysis
Aim 1: Descriptive study
To describe how often and when weight is recorded, we will request preliminary CPRD searches to identify all: (1) Read coded entries for weight loss and (2) quantitative weight measurements.
A subset of patients with weight measurements and unexpected weight loss codes will be used to develop a rule-based search strategy to categorise: (1) the clinical purpose (e.g. prevention, monitoring, diagnosis); (2) the related clinical condition (e.g. diabetes, heart failure, cancer). The GPs’ subsequent actions will be described in terms of (1) investigations requested, (2) medications prescribed, and (3) referrals made. The search strategy will then be applied to the entire cohort of weight measurements and weight loss codes.
The most effective method to identify the reason for the weight entry and the subsequent action will be investigated. For example, codelists will be developed to capture the clinical purpose of the consultation associated with each weight measurement or weight loss code: health check codes will be used to identify prevention activity; chronic disease review codes will be used to identify monitoring. For associated clinical conditions, symptom and diagnostic codes entered at the same time as each weight measurement or weight loss code will be ascertained and frequency ranked for the entire descriptive study population. Initially, searches will be performed on the day of the weight entry, then a sensitivity analysis will be performed increasing the time window to ± 1 day of the weight entry, then 1 week, 1 month, and so on. This strategy will be repeated to identify investigation and referral codes following entry of the weight loss code.
Aim 2: Cohort analysis
Cumulative incidence plots
Cumulative incidence plots will be used to describe the probability of cancer or serious disease over time for those with and without weight loss. These will be assessed in aggregate and stratified by disease type, cancer stage, grade, tumour size, histology, and covariates.
Differences between those with and without weight loss will be assessed using the log-rank test.
Multivariate Cox regression
Cox regression will be used to estimate the adjusted hazard ratios (HR) for cancer or serious disease associated with weight loss recorded as a symptom.
The impact of choosing to restrict the follow-up period on the predictive value of weight loss will be explored by limiting the analysis by time period (0–6, 6–12, 12–18, and 18–24 months) and by including weight loss as a time dependent variable.
Age at index date, sex, ethnicity, IMD score, co-morbidity, smoking, and alcohol intake will be included, and the predictive value of other symptoms and investigations will be explored for (1) all cancers in aggregate, (2) cancer type, (3) by cancer stage, (4) by tumour size, (5) by grade of cancer and (6) serious disease type.
Performance of diagnostic strategies
To allow clinical guidance to be developed on how to rule-in or rule-out cancer or serious disease in adult patients (> 18 years) with unexpected weight loss, diagnostic accuracy measures will be calculated for investigative strategies including those described in the literature including the subgroups of (1) gender and (2) age-group.
Plan for addressing confounding
Aim 1: Descriptive study
Not required.
Aim 2: Cohort analysis
Patients who have conditions which might explain the weight loss (e.g. co-morbidities at the time of entry to the cohort or planned dieting) will be included and the impact of their inclusion assessed in multivariate and sensitivity analyses.
Patients with coded weight loss will be matched with patients without a weight loss code based on GP practice to account for systematic biases in coding between practices.
Age at index date, sex, IMD score, co-morbidity, smoking, and alcohol intake will be adjusted for in the multivariate modelling.
Plan for addressing missing data
Aim 1: Descriptive study
Weight is cited as a missing variable in CPRD as GPs do not routinely measure weight in NHS primary care [8]. This descriptive analysis will add to our understanding of how often and when weight is recorded.
We will also describe the completeness of personal characteristics (as defined above) in relation to weight measurements and weight loss codes.
Aim 2: Cohort analysis
As measurements appear to be too infrequent to allow us to identify weight loss from serial weight measurement data, the cohort design will make best use of the coded weight loss information available in CPRD. For this reason, we do not intend to impute missing weight measurement values in the primary analysis, although the feasibility of using multiple imputation to address missing covariate values will be explored [10].
Discussion
Within this section, we expand on the protocol as submitted to ISAC to elucidate decisions made about study design and to report developments made since commencing the study. We have incorporated and expanded upon the “Limitations of the study design, data sources and analytical methods” section of the original ISAC protocol.
Reliance on weight loss coding
It appears from our preliminary searches that weight measurement is infrequent for the majority of patients in primary care, most likely initiated by a concern for underlying disease or existing chronic disease management. This is consistent with studies that acknowledge weight measurement as a source of missing data in NHS primary care records [8]. Consequently, the detection of weight loss from serial weight measurements cannot be relied on as a method of defining weight loss. Our descriptive analysis is designed to identify whether a group of patients exists who undergo weight measurements more frequently, in which a future analysis involving serial weight measurements may be feasible. However, any subgroup is unlikely to be representative of the NHS primary care population. We have therefore chosen to focus on weight loss coding.
As with previous primary care studies using routinely collected data, an assumption will be made that the absence of a symptom code represents the absence of the symptom [5, 11]. This assumption has two major limitations: firstly, a coded entry is reliant on the patient visiting the GP and reporting the symptom; and secondly, that the GP chooses to enter the code in the record. Lack of the former would lead to an underestimation of the associated HR, and for the latter, selective recording of symptoms only deemed severe by the GP could lead to overestimated HRs. The latter is likely to differ by GP but cluster by GP practice, as GPs within the same practice are likely to have more similar approaches to coding. One method to address these limitations would be to analyse free-text entries to identify reported but uncoded symptoms, but at present CPRD does not allow requests for free-text entries and we will cite this as a weakness of our study [12]. We decided to adjust for age and sex in multivariate analysis as the association between weight loss and cancer is not established for these variables.
Sample size for cohort analysis
Progress since the initial ISAC application has established that there are 148,000 patients eligible patients aged > 18 years with an unexpected weight loss code as described in Appendix 1 (preliminary pilot work had suggested there was at least 30,000). This will therefore be the largest primary care CPRD cohort study using unexpected weight loss coding as the exposure variable. We originally calculated that only 2184 patients with weight loss are required to detect a hazard ratio of 2 at 99% power (0.05% alpha) using an enrolment ratio of 1:5. That is, a change in a cancer risk from a PPV of 1.5% in patients without weight loss to 3% in patients with weight loss. An alternative approach to estimating sample size is the number of Events Per Varaible in multivariate modelling. If 3% of patients with weight loss develop cancer the number of Events Per Variable will far exceed the minimum number of ten required for robust multivariate modelling. It is anticipated that the study will therefore have sufficient power for stratification by cancer type.
We aim to understand the association between weight loss and cancer in as much detail as the data permits. However, we accept it may not be possible to stratify for cancer stage or for other covariates with sufficient numbers remaining in each stratum. Cancer stage information is unsatisfactory in CPRD, which is why we have requested data linkage to the cancer registry (which will also be incomplete, but less so). Lifestyle covariates are non-essential for our main aim (to determine the predictive value of weight loss for cancer), and we will only perform analysis on sub-strata when numbers permit. Multiple imputation will be explored for these (and all other relevant missing) variables.
Investigation and referral outcomes
There remains uncertainty over the completeness of investigation and referral data until the descriptive analysis has been conducted. Data for laboratory investigations are likely to be more complete than data on radiological and endoscopic investigations, as laboratory investigations are commonly transmitted directly into the electronic health record from the laboratory whereas results for the other tests are not. Further linkage to the Diagnostic Imaging Dataset (for radiology activity) and Hospital Event Statistics (for endoscopy activity) may be necessary if these data are judged to be incomplete following the descriptive analysis, which would allow a formal comparison of data completeness to be conducted between these datasets and CPRD.
Implications
A second cohort study using American primary care data is also in set-up to assess whether there is greater value in defining weight loss using serial weight measurements rather than a reliance on patient reported weight loss and a GP entered code. In particular, this study aims to establish whether weight loss detected using change in serial weight measurements leads to less advanced disease at diagnosis.
Together, these studies will provide the largest reported retrospective cohorts of primary care patients with unexpected weight loss used to understand the association between unexpected weight loss and serious disease including cancer. We hope our findings will directly inform international guidelines for the management of unexpected weight loss in primary care populations.
Acknowledgements
We thank Professor David Mant for his insight and expertise that greatly assisted the development of this protocol.
Funding
BDN is funded by NIHR DRF-2015-08-185. The NIHR peer-reviewed this protocol at an earlier stage as part of the application for funding. This article presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Availability of data and materials
Not applicable.
Abbreviations
- BMI
Body mass index
- CPRD
Clinical Practice Research Datalink
- IMD
Index of Multiple Deprivation
- ISAC
Independent Scientific Advisory Group (to the CPRD)
- Medcode
The CPRD unique code for the medical term selected by the GP
- NCDR
National Cancer Data Repository
- NICE
National Institute for Health and Care Excellence
- Read code
The standard clinical terminology system used in general practice in the UK
Authors’ contributions
BDN prepared the first draft of the protocol. All authors reviewed and edited the protocol. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
B. D. Nicholson, Email: brian.nicholson@phc.ox.ac.uk
P. Aveyard, Email: paul.aveyard@phc.ox.ac.uk
F. D. R. Hobbs, Email: richard.hobbs@phc.ox.ac.uk
M. Smith, Email: margaret.smith@phc.ox.ac.uk
A. Fuller, Email: alice.fuller@phc.ox.ac.uk
R. Perera, Email: rafael.perera@phc.ox.ac.uk
W. Hamilton, Email: W.hamilton@exeter.ac.uk
S. Stevens, Email: sarah.steven@phc.ox.ac.uk
C. R. Bankhead, Email: clare.bankhead@phc.ox.ac.uk
References
- 1.Wong CJ. Involuntary weight loss. The Medical clinics of North America. 2014;98(3):625–643. doi: 10.1016/j.mcna.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 2.McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. doi: 10.1136/bmj.d1732. [DOI] [PubMed] [Google Scholar]
- 3.NICE. Suspected cancer: recognition and referral (NG12). London: National Institute for Health and Care Excellence; 2015. [PubMed]
- 4.Stapley S, Peters TJ, Neal RD, Rose PW, Walter FM, Hamilton W. The risk of oesophago-gastric cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer. 2013;108(1):25–31. doi: 10.1038/bjc.2012.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton W. The CAPER studies: five case-control studies aimed at tpidentifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer. 2009;101(Suppl 2):S80–6. [DOI] [PMC free article] [PubMed]
- 6.Collins GS, Altman DG. Identifying patients with undetected pancreatic cancer in primary care: an independent and external validation of QCancer((R)) (Pancreas) The British journal of general practice : the journal of the Royal College of General Practitioners. 2013;63(614):e636–e642. doi: 10.3399/bjgp13X671623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton W, Lancashire R, Sharp D, Peters TJ, Cheng K, Marshall T. The risk of colorectal cancer with symptoms at different ages and between the sexes: a case-control study. BMC Med. 2009;7:17. doi: 10.1186/1741-7015-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marston L, Carpenter JR, Walters KR, Morris RW, Nazareth I, Petersen I. Issues in multiple imputation of missing data for large general practice clinical databases. Pharmacoepidemiol Drug Saf. 2010;19(6):618–626. doi: 10.1002/pds.1934. [DOI] [PubMed] [Google Scholar]
- 9.Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Int J Epidemiol. 2015;44(3):827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welch C, Bartlett J, Petersen I. Application of multiple imputation using the two-fold fully conditional specification algorithm in longitudinal clinical data. Stata J. 2014;14(2):418–431. [PMC free article] [PubMed] [Google Scholar]
- 11.Hippisley-Cox J, Coupland C. Symptoms and risk factors to identify men with suspected cancer in primary care: derivation and validation of an algorithm. The British journal of general practice : the journal of the Royal College of General Practitioners. 2013;63(606):e1–10. doi: 10.3399/bjgp13X660724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price SJ, Stapley SA, Shephard E, Barraclough K, Hamilton WT. Is omission of free text records a possible source of data loss and bias in Clinical Practice Research Datalink studies? A case-control study. BMJ Open. 2016;6(5):e011664. doi: 10.1136/bmjopen-2016-011664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


