Abstract
Aim:
This study aimed to identify the pathogenic bacteria responsible for the septicemic disease affecting white sea bream brooders and determining the sensitivity of the recovered isolates to different antibiotics followed by estimation of long-acting oxytetracycline (OTC) efficacy in controlling this disease, and finally, determining the proper dose regimen.
Materials and Methods:
Biolog microbial identification system was used for determination of the pathogens which are responsible for this disease. Agar disk diffusion test and minimum inhibitory concentration (MIC) were used to determine the antibiotic susceptibility of recovered isolates. Oxytetracycline (OTC) was used at a dose level of 100 mg/kg body weight for the treatment of diseased fish, and the OTC concentration in the serum samples was determined by high-performance liquid chromatography.
Results:
Fifteen Staphylococcus epidermidis and 11 Bacillus cereus isolates were recovered from the lesion of muscle, tail, eye, and heart blood. S. epidermidis isolates were sensitive to OTC, ciprofloxacin, enrofloxacin, spiramycin, erythromycin (E), and florfenicol. B. cereus isolates were sensitive to all mentioned antibiotics except E. Based on the MIC test, all B. cereus isolates were sensitive to OTC with MIC ranging between <0.125 and 4 µg/ml and 11 S. epidermidis isolates were sensitive with MIC ranging between <0.125 and 8 µg/ml, while four isolates were resistant. Different degrees of degenerative changes were present in the hepatopancreas, posterior kidney, eye, and skin tissues of diseased fish.
Conclusion:
Single intraperitoneal injection of long-acting OTC at a dose of 100 mg/kg body weight was effective in termination of S. epidermidis and B. cereus infection in white sea bream (D. sargus) broodstock.
Keywords: Bacillus cereus, histopathology, high-performance liquid chromatography, oxytetracycline, sensitivity, Staphylococcus epidermidis
Introduction
Aquaculture is one of the fastest growing animal production sectors worldwide, where farmed finfish production rate has increased from 27.6 to 52.3 million (nearly duplicated) over the past decade from 2005 to 2015 [1]. Aquaculture is considered as an imperative choice to provide cheap animal protein for the growing world population. The global fishery production for human consumption reached 76 million tons in 2015. The aquaculture production is expected to exceed the fishery production by 1.36% fold at 2025 [2].
Expansion in marine aquaculture is an urgent priority for the Egyptian government, so it launched the largest maricultural farm in the Middle East at Berket Ghalioun. It targeted to culture about 5460 hectares with different marine fish species, and so there is a need for diversification and introduction of new species. White sea bream (Diplodus sargus) is considered a good candidate for aquaculture as it has a high commercial value and good acceptability by consumers [3]. It is also an omnivorous fish and, therefore, needs fewer nutritional requirements in comparison to other Sparidae [4].
Bacterial diseases are the most dominant pathogens that are responsible for severe diseases and outbreaks in marine-cultured fish [5,6]. Most of the bacterial disease etiological agents are considered a part of the normal flora that is present in water, stress condition as bad water quality, inadequate diet, overcrowding, and frequent handling during artificial spawning are considered to be predisposing factors of lowering fish immunity and subsequently initiating disease conditions [7,8]. Streptococcus spp., Lactococcus garvieae, Staphylococcus epidermidis, and Micrococcus luteus are among the most common Gram-positive bacterial infection of cultured marine fish [9,10], while vibriosis is the most Gram-negative bacterial infection [11-13].
Route of drug administration is a limiting factor that plays a considerable role in the success or failure of a treatment process, as it greatly affects the drug pharmacokinetic parameters including absorption, distribution, biotransformation, and excretion [14]. Intraperitoneal and intravenous drug administration provides not only the maximum bioavailability but also allows the drug to reach the site of infection very rapidly achieving high serum level [15], as well. Drug formulation has a vital role in the dosage regimen (frequency of application), i.e., long-acting preparations provide sustainable drug release in treated animal serum, which allow long-lasting effect that may extend for several days [16].
Broodstocks are extremely valuable and expensive fish. It requires a long time and effort for preparation; loss of any brooder fish is equal to the loss of thousands of produced fry each season, and so control of broodstock diseases is considered a priority for sustainable aquaculture.
This study aimed to identify the pathogenic bacteria responsible for the septicemic disease affecting white sea bream brooders and determining the sensitivity of the recovered isolates to different antibiotics followed by estimation of long-acting oxytetracycline (OTC) efficacy in controlling this disease, and finally, determining the proper dose regimen.
Materials and Methods
Ethical approval
Fish used in the current research were handled, transported, examined, and treated following the guidelines of the National Advisory Committee for Laboratory Animal Research (NACLAR) [17] and CCAC [18] regarding the care and use of fish in research, teaching, and testing which were approved by the National Institute of Oceanography and Fisheries (NIOF) Ethical Committee, Egypt.
Study location
The present work was conducted in the marine hatchery, NIOF, Alexandria, located at longitude 31°12’44.8” N and latitude 29°53’05.1”E.
Fish specimens
Sixty white sea bream (D. sargus, Linnaeus 1758) broodstocks were examined through clinical examination. Of them, 23 were dead and moribund fish were used for postmortem, bacteriological, and histopathological examination. 20 clinically diseased fish were used in the treatment trial, and 10 healthy fish were used for the determination of antibiotic concentration in their serum. Fish ranged between 210 and 587 g in body weight and 16-28 cm in total length.
Antemortem and postmortem inspection
Antemortem and postmortem examinations were done as described by Stoskopf [19] for the detection of any changes in fish behavior or appearance also for the determination of any gross abnormalities in internal organs.
Isolation of bacterial etiological agent
It was performed as described by Aboyadak et al. [20], in which five samples were taken from each fish (tail lesions, muscle lesions, eye lesions if present, hepatopancreas, and heart blood). Each sample was individually inoculated to Tryptic Soy Broth for 12 h, then streaked on tryptic soy agar (Oxoid®), and incubated at 33°C for 18-24 h.
Identification of the isolated strains using biolog system
Few colonies from each recovered isolate were smeared on a glass slide and stained with Gram stain following the procedures mentioned by Black and Black [21].
Biolog microbial identification system (Biolog Inc., Hayward, CA, USA) is an automated, accurate, and rapid identification method that is based on carbon source utilization assay as each microorganism has a phenotypic fingerprint. GEN III MicroPlate has 12 columns each of eight rows: The first nine columns are for 71 carbon source utilization assays and the rest 3 columns for 23 chemical sensitivity assays. The utilization of carbon sources and resistance to the inhibitory chemicals were determined through tetrazolium redox dye.
A surface area of about 3 mm in diameter from the pure isolate that was grown on Tryptic Soy Agar was picked up using a cotton-tipped inoculator’s swab. The swap was transferred and suspended in a special clean tube containing inoculating fluid. The turbidity was measured and adjusted to 95% using turbidimeter. After that, the bacterial cell suspension was poured into Biolog GEN III Microplates using the multichannel pipette. The inoculated microplate was covered with its lid and placed for incubation, and the result of the monitoring was logged in the OmniLog system.
Histopathological examination
The histopathological examination was carried out according to Suvarna et al. [22]. Tissue specimens from the hepatopancreas, posterior kidney, skin, and eye were fixed in 10% buffered formalin, dehydrated in ascending grade ethyl alcohol and cleared in xylene, sectioned to 4-µm thickness, mounted over a glass slide, and then stained with hematoxylin and eosin (H and E). Stained tissue sections were examined and photographed using Optika Microscope with a digital camera (Optika, Italy).
Antibiotic susceptibility test
Agar disk diffusion test was done to determine the sensitivity of the recovered bacterial isolates to OTC (OTC 30 µg), ciprofloxacin (CIP 5 µg), enrofloxacin (ENR 5 µg), florfenicol (F 10 µg), spiramycin (SP 100 µg), and erythromycin (E 15 µg) according to CLSI [23].
Determination of the minimum inhibitory concentration (MIC) of OTC
It was determined for all recovered isolates using broth macrodilution test according to Hack et al. [24].
Preparation of OTC standard solution
About 10.66 µl was pipetted from Alamycin 300® and made up to 1000 µl with sterile distilled water to achieve a final concentration of 3.2 mg/1000 µl, and then, a double-fold serial dilution was done for 10 consecutive dilutions.
Procedures
The overnight growth of each bacterial isolate on tryptic soy broth was diluted and adjusted to 0.5 McFarland standard. After that, 0.5 ml was added to 99.5 ml sterile Mueller Hinton Broth, then 4.9 ml was added to each of 11 sterile screw-caped, numbered test tubes followed by addition of 100 µl from previously prepared OTC standard solution to the corresponding test tube to achieve a final concentration of 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, and 0.125 µg/ml, while the last tube was left without antibiotic as control. The prepared test tubes were incubated at 33°C for 24 h. MIC was determined as the lowest concentration at no visible bacterial growth.
Treatment experiment
Drug
Alamycin 300® (Norbrook Co., United Kingdom) long-acting solution contains OTC dihydrate 30 mg/ml.
Fish grouping
Twenty clinically diseased D. sargus were randomly divided into two equal groups, each containing 10 fish; Group 1 was treated with Alamycin 300 at a dose of 100 mg/kg body weight by intraperitoneal injection, and Group 2 remains as a control. Each group was kept in 2000-L fiberglass tank supplied with marine water 35‰ with continuous aeration. The temperature was thermostatically controlled at 25°C and fish were observed for 14 days from the first dose.
Determination of OTC in fish serum by high-performance liquid chromatography (HPLC)
Ten healthy D. sargus were used in this experiment; each fish was inoculated with Alamycin 300® at a dose of 100 mg/kg body weight. Fish were kept in a fiberglass tank at which water temperature was thermostatically adjusted at 25°C, and then 500 µl blood was collected through the caudal vessels from each fish at 24, 48, 72, 96, 120, 144, and 168-h post-injection. The serum was separated by centrifuge at 5000 rpm and then was stored at −23°C. OTC concentration was determined in serum samples according to the method described by Lei et al. [25] using Nexera X2 HPLC system, Shimadzu, Japan, and C18 reverse-phase column, Zorbax SB-C18 5.0 µm, 4.6 mm×250 mm, Agilent, USA. The mobile phase consisted of 0.01 mol/l oxalic acid, methanol, and acetonitrile with a volume ratio of 83:7:10, respectively. OTC level was detected at 355-nm wavelength.
Results
Antemortem and postmortem inspection
The inspections show hemorrhagic skin ulcers of different degrees (starting with scale desquamation, hyperemia, followed by deep hemorrhagic skin ulcers reaching the deep musculature), unilateral exophthalmia, fin erosions, and hemorrhagic tail with severe erosions (Figure-1a and b). Congested hepatopancreas and kidney were the most prominent PM lesion. The recorded mortality was estimated by 38.33% (23 of 60 fish).
Figure-1.
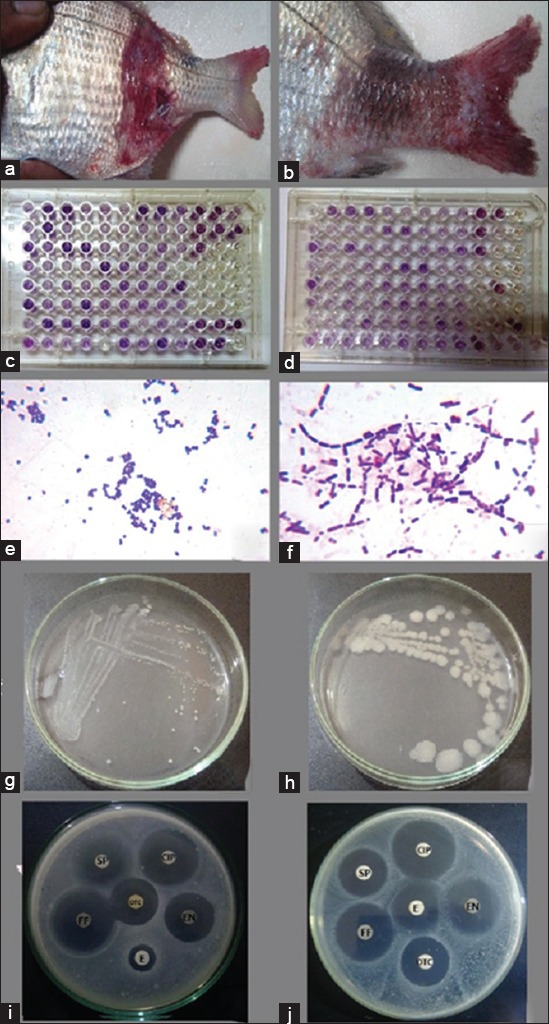
(a and b) Naturally infected white sea bream (Diplodus sargus) showing scale desquamation, skin ulceration even complete loss of skin, and appearance of musculature with congestion and hemorrhage in caudal peduncle together with tail erosion and hemorrhages. (c and d) Biolog GEN III microplate showing the biochemical profile of Staphylococcus epidermidis (c) and Bacillus cereus (d). (e) Gram-stained S. epidermidis appeared as Gram-positive cocci, 0.65-0.91 µm in diameter, present as single, pairs, and clusters. (f) Gram-stained B. cereus appeared as Gram-positive spore-forming long bacilli, 4.97-7.48 µm in length and 1.58-1.64 in width, arranged in short or long chains. (g) S. epidermidis colonies on Tryptic Soy Agar appeared as white pinpoint colonies about 0.2-1 mm in diameter.)h) B. cereus colonies on Tryptic Soy Agar appeared as large white granular colonies with irregular perimeters about 1.5-5 mm in diameter. (i) Antibiogram indicated the sensitivity of B. cereus to oxytetracycline (OTC), ciprofloxacin (CIP), enrofloxacin (ENR), florfenicol (F), and spiramycin (SP), while it resists erythromycin (E). (j) Antibiogram indicated the sensitivity of S. epidermidis to OTC, CIP, ENR, F, SP, and E.
Results of bacterial isolation and identification
Twenty-six Gram-positive isolates were recovered from nine moribund fish; 15 isolates were cocci, and 11 were bacilli (Table-1).
Table-1.
Number of Staphylococcus epidermidis and Bacillus cereus isolates from each organ of diseased white sea bream (Diplodus sargus) broodstock.
| Organ of isolation | Number of Staphylococcus epidermidis isolates | Number of Bacillus cereus isolates |
|---|---|---|
| Muscle | 5 | 5 |
| Eye | 1 | 1 |
| Heart | 4 | 0 |
| Tail | 5 | 5 |
| Total | 15 | 11 |
Biolog microbial identification system illustrates the presence of 15 S. epidermidis (Figure-1c) and 11 Bacillus cereus isolates (Figure-1d).
Culture characters of the recovered bacteria
S. epidermidis appeared as white pinpoint colonies about 0.2-1 mm in diameter (Figure-1g), while B. cereus appeared as large white granular colonies with irregular perimeters about 1.5-5 mm in diameter (Figure-1h).
Morphological characteristics of the recovered bacteria
S. epidermidis appeared as Gram-positive cocci ranged between 0.65 and 0.91 µm in diameter, and it was present as single, pairs, and clusters (Figure-1e), while B. cereus appeared as Gram-positive rods present in short or long chains, and each bacillus was 4.97-7.48 µm in length and 1.58-1.64 in width (Figure-1f).
S. epidermidis was isolated from the heart, tail, muscle, and eye, while B. cereus isolate was isolated from the tail, muscle, and eye as shown in Table-1.
Results of the histopathological examination
The histopathological study of naturally infected D. sargus tissues revealed the presence of different pathological alterations (Figure-2). Hepatopancreas tissue lost their normal architecture with the presence of inflammatory reaction manifested in mononuclear cell infiltration, necrotic areas were also present, hepatic tissue congestion expressed as congested hepatic sinusoids, and distended blood vessels engorged with erythrocytes (Figure-2a-c). Posterior kidney tissue was severely affected as glomerular hypertrophy with narrowed Bowman’s space was present. Some degenerated shrinkage glomerular tuft was also observed. Furthermore, renal tubules were involved in this degenerative changes in which detachment of tubular epithelium was observed. Inflammation of renal tissue was presented as interstitial mononuclear cell infiltration and melanomacrophage center activation (Figure-2d-f). Exophthalmic tissue examination indicated marked separation between retina layers, especially pigment epithelium and the photoreceptor layers, as these tissues appeared corrugated (Figure-2g). Ulcerated skin lesions show destruction of epidermis that was completely lost with exposure of dermis and presence of leukocytic infiltration, but, in less affected cases, the superficial layer of the stratified squamous epithelium was eroded (Figure-2h-j).
Figure-2.
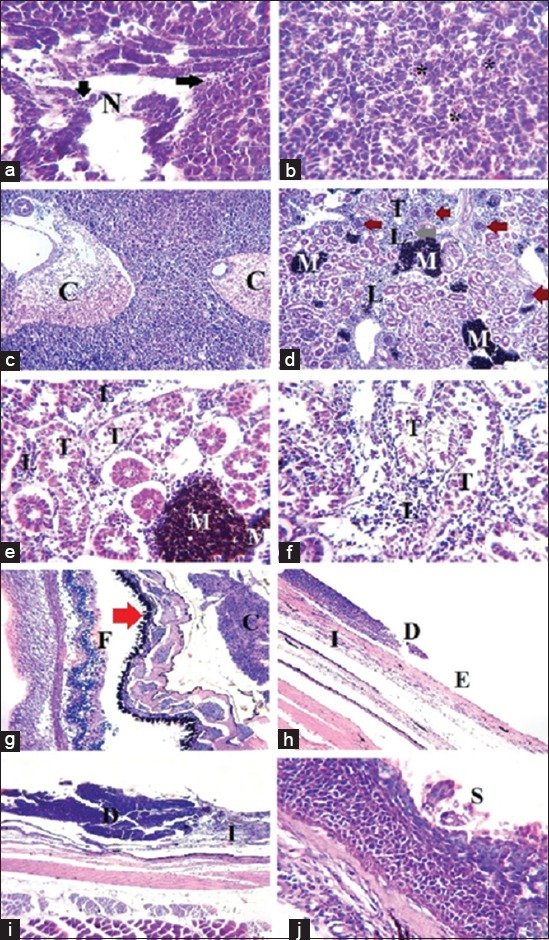
Histopathological lesions induced by Staphylococcus epidermidis and Bacillus cereus infection in affected white sea bream (Diplodus sargus). (a-c) Hepatopancreas showing loss of normal tissue architecture, presence of necrotic areas (N) with mononuclear cell infiltration (arrow), congested hepatic sinusoids (*) and congested distended blood vessels (C), hematoxylin and eosin (H and E), X=400 in (a and b) and 100 in (c). (d-f) Posterior kidney showing glomerular hypertrophy with narrow Bowman’s space (brown arrow), degenerated shrinkage glomerular taught (gray arrow), detached tubular epithelium (T), interstitial mononuclear cell infiltration (L), melanomacrophage centers activation (M), H and E, X=100 in (d) and 100 in (e and f). (g) Eye with marked separation between retina layers, especially, pigment epithelium layer (red arrow) which also is corrugated and the photoreceptor layer (F), (C) is the choroid body, H and E, X=100. (h-j) Skin showing destruction of epidermis even complete loss (D) with exposure of dermis (E) and presence of leukocytic infiltration (I), in less affected cases loss of the superficial layer of the stratified squamous epithelium (S), H and E, X=100 in (h and i) and 400 in (j).
Antibiotic sensitivity test results
S. epidermidis was sensitive to OTC, CIP, ENR, F, SP, and E (Figure-1j), while B. cereus was sensitive to all mentioned antibiotics except E (Figure-1i) as presented in Table-2.
Table-2.
Antibiotics susceptibility test results for Staphylococcus epidermidis and Bacillus cereus.
| Antibiotic | Staphylococcus epidermidis | Bacillus cereus | ||
|---|---|---|---|---|
| Inhibition zone mm | Interpretation | Inhibition zone mm | Interpretation | |
| OTC 30 μg | 22 | Susceptible | 21 | Susceptible |
| CIP 5 μg | 27 | Susceptible | 26 | Susceptible |
| ENR 5 μg | 24 | Susceptible | 22 | Susceptible |
| F 10 μg | 24 | Susceptible | 27 | Susceptible |
| SP 100 μg | 21 | Susceptible | 17 | Susceptible |
| E 15 μg | 19 | Susceptible | 11 | Resistant |
OTC=Oxytetracycline, CIP=Ciprofloxacin, ENR=Enrofloxacin, F=Florfenicol, SP=Spiramycin, E=Erythromycin
The MIC of OTC
Twenty out of 26 isolates resembling 9 S. epidermidis and all the 11 B. cereus were highly susceptible to OTC with MIC ranged between <0.12 and 4 µg/ml, while two S. epidermidis isolates were intermediately susceptible with MIC equal to 8 µg/ml, but four isolates were resistant with MIC equal to 16-32 µg/ml (Table-3).
Table-3.
MIC of oxytetracycline.
| Bacterial strain | Isolate Number | MIC of oxytetracycline μg/ml | Interpretation |
|---|---|---|---|
| Staphylococcus epidermidis | 1 | <0.125 | Susceptible |
| 2 | 16 | Resistant | |
| 3 | 32 | Resistant | |
| 4 | 8 | Intermediate | |
| 5 | 32 | Resistant | |
| 6 | 0.5 | Susceptible | |
| 7 | 32 | Resistant | |
| 8 | 8 | Intermediate | |
| 9 | 4 | Susceptible | |
| 10 | <0.125 | Susceptible | |
| 11 | 4 | Susceptible | |
| 12 | 0.125 | Susceptible | |
| 13 | 0.5 | Susceptible | |
| 14 | 0.125 | Susceptible | |
| 15 | 4 | Susceptible | |
| Bacillus cereus | 1 | <0.125 | Susceptible |
| 2 | <0.125 | Susceptible | |
| 3 | 2 | Susceptible | |
| 4 | <0.125 | Susceptible | |
| 5 | 1 | Susceptible | |
| 6 | 1 | Susceptible | |
| 7 | 1 | Susceptible | |
| 8 | 1 | Susceptible | |
| 9 | 4 | Susceptible | |
| 10 | 0.25 | Susceptible | |
| 11 | 0.25 | Susceptible |
Interpretation: Susceptible ≤4, Intermediate=8, Resistant ≥16 μg/ml. MIC=Minimum inhibitory concentration
Results of treatment trial
Treated fish showed gradual improvement starting with disappearance of hyperemia and hemorrhage from tail and skin ulcers at the 2nd-day post-treatment followed by partial healing of ulcers throughout the 1st week. The cumulative mortality during 2 weeks after treatment was 40% in infected non-treated group but was only 10% in the treated group as one severely ulcerated fish died at the 2nd day post-treatment.
OTC level in fish serum following intraperitoneal inoculation
OTC level in D. sargus serum reached 34.57±1.09, 25.98±0.96, 20.39±1.2, 13.10±0.32, 10.04±0.26, 7.68±0.42, and 5.14±0.23 (µg/ml) at 24, 48, 72, 96, 120, 144, and 168 h, respectively, following the intraperitoneal injection as presented in Table-4.
Table-4.
Oxytetracycline levels in the serum of white sea bream (Diplodus sargus) broodstock at different time points post-treatment.
| Sampling time (h postinjection) | Oxytetracycline level (μg/ml) |
|---|---|
| 24 | 34.57±1.09 |
| 48 | 25.98±0.96 |
| 72 | 20.39±1.2 |
| 96 | 13.10±0.32 |
| 120 | 10.04±0.26 |
| 144 | 7.68±0.42 |
| 168 | 5.14±0.23 |
n=6, values are mean±standard error
Discussion
Introduction of a new species is an essential element for expansion in marine culture; White Sea bream (D. sargus) is an excellent candidate for this purpose as it has good acceptability for consumers; and increased fry (seeds) production is an essential element for sustainable aquaculture.
Streptococci considered a potential cause of diseases in mariculture [26]. S. epidermidis is considered a potential fish pathogen as it can lead to localized, as well as systemic, infection in cultured fresh and marine fish species [27-29]. Infections with B. cereus were also recorded by Baya et al. [30] and Chandra et al. [31] in striped bass and stinging catfish, respectively.
In the current study, D. sargus broodstock suffered from increased mortality that was estimated by 38.33%. Diseased fish showed deep skin ulcers that reached the musculature, hemorrhages, fin and tail erosions, and exophthalmia with congested parenchymatic organs (hepatopancreas and posterior kidney), and similarly, anti- and post-mortem lesions were recorded by Golomazou et al. [3] in diseased caged white sea bream. Kubilay and Uluköy [32] isolated S. epidermidis from diseased gilthead sea bream (Sparus aurata) with hemorrhages on gills, skin, and fins, and Kusuda and Sugiyama [33] isolated S. epidermidis from diseased yellowtail (Seriola quinqueradiata) and red sea bream (Chrysophrys major) that showed exophthalmia, swelling, and congestion of caudal peduncle, these observations were identical to the present work findings. Chandra et al. [31] reported nearly similar signs in stinging catfish. Heteropneustes fossilis infected with B. cereus. Parallel to the current findings Austin [34] cited the presence of generalized necrotizing dermatitis in B. cereus-infected fish. Aboyadak et al. [7] recorded similar clinical signs and postmortem lesions in sea bass infected with B. cereus and S. epidermidis.
The histopathological examination indicated the presence of some pathological lesions involving hepatopancreas as inflammation, congestion, and necrosis. Posterior kidney showed degenerated glomeruli and renal tubules with inflammatory cell aggregation. These findings were supported by Austin [34], who reported the presence of focal necrosis and petechial hemorrhages in the liver and kidney of B. cereus-infected fish. Marked separation between retina layers especially pigment epithelium and the photoreceptor layer of affected eye which could have resulted from the increased intraocular pressure.
The ante- and post-mortem findings together with the histopathological lesions indicated the severity of the current infection affecting D. sargus broodstock and it is mainly induced by different virulence factors produced by the isolated causative agents, especially in stressed broodstock that suffered from frequent handling and hormonal treatment (injection) which is accompanied with scales detachment and skin abrasions that act as a porter of entry for pathogenic and opportunistic bacteria.
S. epidermidis is not considered a part of normal fish flora as it has many virulence factors responsible for its pathogenicity. They are mainly extracellular products as proved by Huang et al. [27], who found that inoculation of tilapia with filter-sterilized culture broth of S. epidermidis causes similar mortality as bacterial suspension contains 1.34×109 CFU/ml. Namvar et al. [35] mentioned some virulence factors of S. epidermidis as biofilm formation, polysaccharide intercellular adhesion, surface adhesion protein, poly-γ-glutamic acid (responsible for phagocytosis inhibition), toxins as staphylococcal enterotoxin-like toxin, and delta toxin. Pinheiro et al. [36] recorded that S. epidermidis exhibits high toxigenic potential as they identified eight enterotoxin genes from 85 clinical isolates.
B. cereus has many virulence factors, including hemolysin, which have a dermonecrotic and vascular permeability activity [37]. Prasad [38] isolated enterotoxigenic B. cereus from 10 marine fish guts. B. cereus is known to produce substances responsible for virulence including hemolysins (I, II, and III), enterotoxins, cytotoxin K, and phospholipase which in combination induce the disease condition [39]. B. cereus is famous as foodborne poisoning microorganism, but it is also considered the causative agent of many other serious diseases such as endocarditis, ocular infection, myonecrosis and cutaneous infections, nosocomial infections, meningitis, and urinary tract infection [40-44].
S. epidermidis grown as white pinpoint colonies (0.2-1 mm in diameter), morphologically present as Gram-positive cocci 0.65-0.91 µm in diameter which was identical to that described by Huang et al. [27] and Kusuda and Sugiyama [33] for S. epidermidis isolated from clinically diseased fish.
B. cereus colonies were large white granular with irregular perimeters, about 1.5-5 mm in diameter, B. cereus appeared as Gram-positive spore-forming long bacilli, 4.97-7.48 µm in length and 1.58-1.64 in width, arranged in short or long chains that are similar to the description reported by Bottone [45].
The recovered S. epidermidis isolates were sensitive to CIP, ENR, F, SP, and E, but only four isolates resisted OTC. These results are in complete harmony with Kusuda and Sugiyama [33] as they reported its sensitivity to OTC, chloramphenicol, and E, but it partially agrees with that of Kubilay and Uluköy [32] as they recorded S. epidermidis sensitivity to CIP, ENR, and chloramphenicol, while it was resistant to OTC and E. Sergelidis et al. [46] recorded the sensitivity of S. epidermidis isolated from fish to quinolones and its resistance to E.
B. cereus isolates were sensitive to OTC, CIP, ENR, F, and SP, but they resisted E. This result was compatible with that done by Chandra et al. [31] except for OTC. Weber et al. [47] also reported the sensitivity of B. cereus to chloramphenicol, CIP, and tetracycline. The mentioned difference in antibiotic susceptibility profile of S. epidermidis and B. cereus isolates could be attributed to the presence of certain antibiotic resistance genes.
OTC is currently available and approved by the U.S. Food and Drug Administration for use as a chemotherapeutic agent in food fish [48]. Intraperitoneal administration of long-acting OTC at a dose of 100 mg/kg body weight was very effective in termination of the present infection as the mortality rate decreases from 40% in infected non-treated group to 10% in infected treated group, together with disappearance of hemorrhage and congestion followed by regenerative changes as ulcer healing, regrowth of eroded fins, and tail. Only one fish died in the treated group, which may have resulted from the presence of large ulcers on its body before treatment. It was severely affected by bacterial invasion and toxins or due to osmoregulation failure.
The successfulness of the treatment is not only reflecting the proper selection of antibiotic, but also the drug formulation has a role as long-acting formula administrated once (each fish handled once to decrease the stress and ulceration during manipulation), as well. Parenteral administration has many advantages over oral drug administration: Diseased fish became off-food, on the other hand, some interfering substances which may present in water as ions can inactivate, chelate, or decrease the concentration of the orally administrated drug.
Sensitivity test indicated the high susceptibility of S. epidermidis and B. cereus isolates to OTC, also, the low MIC accelerates the response to the treatment, although there were four S. epidermidis isolates having MIC of 16 and 32 µg/ml. That means these isolates resist OTC, but the treatment was successful, and this can be explained by increasing the serum concentration over 32 µg/ml, and at this point, OTC became effective.
Serum OTC level reaches 34.57±1.09 µg/ml at 24 h post-drug administration, and then, it remains over 5 µg/ml on the 7th day, which indicates the efficacy of single intraperitoneal inoculation in protection and treatment against susceptible pathogens for at least 7 consecutive days. Nearly similar result was recorded by Bowden [49], who found that OTC remains over 4 µg/ml after 168-h post-intramuscular or intraperitoneal administration in yellow perch (Perca flavescens), while Rigos et al. [16] determined the 24-h OTC plasma concentration as 18 µg/ml after intramuscular administration of 50 mg/kg in healthy grouper (Epinephelus marginatus) which differs from our findings, due to the difference in the used dose.
Conclusion
Single intraperitoneal injection of long-acting OTC at a dose of 100 mg/kg body weight was effective in termination of S. epidermidis and B. cereus infection in white sea bream (D. sargus) broodstock.
Authors’ Contributions
NGMA: designed the study, performed clinical and postmortem examination and bacterial isolation and identification, also performed the histopathological examination and wrote the manuscript. IMA: designed and conducted the treatment trial, sensitivity test, and HPLC analysis and helped in manuscript writing and preparations. HSE: fish sampling, rearing, and observation. All authors read and approved the final manuscript.
Acknowledgment
The authors thank Science and Technology Development Fund, Ministry of Higher Education and Scientific Research, Egypt, for supporting this research through project number 26275, entitled “Isolation and identification of viral, bacterial and parasitic etiological agents responsible for outbreaks affecting cultured marine fishes in Damietta governorate with establishing of control strategies.”
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Zhou X. An Overview of Recently Published Global Aquaculture Statistics, in FAO Aquaculture Newsletters, Proceedings No. 56. Switzerland: 2017. [Google Scholar]
- 2.OECD/FAO. OECD-FAO Agricultural Outlook 2016-2025. Paris: OECD Publishing; 2016. [Last accessed at 1/10/2018]. Available from: http://www.dx.doi.org/10.1787/agr_outlook-2016-en . [Google Scholar]
- 3.Golomazou E, Athanassopoulou F, Vagianou S, Sabatakou O, Tsantilas H, Rigos G, Kokkokiis L. Diseases of White Sea bream (Diplodus sargus L) Reared in experimental and commercial conditions in Greece. Turk. J. Vet. Anim. Sci. 2006;30(1):389–396. [Google Scholar]
- 4.Sa R, Pousao-Ferreira P, Oliva-Teles A. Effect of dietary starch source (normal versus waxy) and protein levels on the performance of white sea bream Diplodus sargus(Linnaeus) juveniles. Aquac. Res. 2008;39(10):1069–1076. [Google Scholar]
- 5.Pridgeon J.W, Klesius P.H. Major bacterial diseases in aquaculture and their vaccine development. CAB Rev. 2012;7(48):1–16. [Google Scholar]
- 6.Lafferty K.D, Harvell C.W, Conrad J.M, Friedman C.S, Kent M.L, Kuris A.K, Powell E.N, Rondeau D, Saksida S.M. Infectious diseases affect marine fisheries and aquaculture economics. Ann. Rev. Mar. Sci. 2015;7(2015):471–496. doi: 10.1146/annurev-marine-010814-015646. [DOI] [PubMed] [Google Scholar]
- 7.Aboyadak I.M, Sabry N.M, Ali N.G, El-Sayed H.S. Isolation of Staphylococcus epidermidis, Bacillus cereus and Pseudomonas stutzeri from diseased European sea bass (Dicentrarchus labrax) for the first time in Egypt. Egypt. J. Aquat. Biol. Fish. 2016;20(4):103–114. [Google Scholar]
- 8.Austin B, Newaj-Fyzul A. Diagnosis and Control of Diseases of Fish and Shellfish. Croydon: John Wiley and Sons Ltd, CPI Group (UK) Ltd; 2017. [Google Scholar]
- 9.Sudheesh P.S, Al-Ghabshi A, Al-Mazrooei N, Al-Habsi S. Comparative pathogenomics of bacteria causing infectious diseases in fish. Int. J. Evol. Biol. 2012;2012(1):457264. doi: 10.1155/2012/457264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al Bulushi I.M, Poole S.E, Barlow R, Deeth H.C, Dykes G.A. Speciation of Gram-positive bacteria in fresh and ambient-stored sub-tropical marine fish. Int. J. Food Microbiol. 2010;138:1–2. 32–38. doi: 10.1016/j.ijfoodmicro.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Abdelaziz M, Ibrahem M.D, Ibrahim M.A, Abu-Elala N.M, Abdel-Moneam D.A. Monitoring of different Vibrio species affecting marine fishes in Lake Qaroun and Gulf of Suez:Phenotypic and molecular characterization. Egypt. J. Aquat. Res. 2017;43(2):141–146. [Google Scholar]
- 12.Kalatzis P.G, Castillo D, Katharios P, Middelboe M. Bacteriophage Interactions with marine pathogenic Vibrio:Implications for phage therapy. Antibiotics. 2018;7(1):e15. doi: 10.3390/antibiotics7010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasreldin E. Clonal relationship among the Vibrio parahaemolyticus isolates from coastal water in Saudi Arabia. Egypt. J. Aquat. Res. 2018;44(2):131–137. [Google Scholar]
- 14.Shargel L, Yu A.B.C. Applied Biopharmaceutics and Pharmacokinetics. 7th ed. New York: McGraw-Hill Education; 2015. Introduction to biopharmaceutics and pharmacokinetics. [Google Scholar]
- 15.Buxton I.L.O, Benet L.Z. Pharmacokinetics:The dynamics of drug absorption, distribution, metabolism, and elimination. In: Brunton L.L, Chabner B.A, Knollmann B.C, editors. Goodman and Gilman's Pharmacological Basis of Therapeutics. 12th ed. New York: The McGraw-Hill Companies; 2011. [Google Scholar]
- 16.Rigos G, Katharios P, Papandroulakis N. Single intramuscular administration of long-acting oxytetracycline in grouper (Epinephelus marginatus) Turk. J. Vet. Anim. Sci. 2010;34(5):441–445. [Google Scholar]
- 17.NACLAR. National Advisory Committee for Laboratory Animals Research. 20 Biopolis Way #08-01 Centros Singapore 138668. 2004. [Last accessed at 10/10/2018]. Available from: http://www.research.ntu.edu.sg/guides/Documents/Ethics/NACLAR-guide%20Lines.pdf .
- 18.CCAC. Guidelines On:The Care and Use of Fish in Research, Teaching and Testing. Ottawa, Canada: Canadian Council on Animal Care, Guidelines Program; 2005. pp. 1510–130. [Google Scholar]
- 19.Stoskopf K.M. Fish Medicine. 2nd ed. North Carolina: ART Sciences LLC, 3512 Olive Chapel Road Extension Apex; 2010. pp. 125–151. [Google Scholar]
- 20.Aboyadak I.M, Ali N.G.M, Goda A.M.A, Saad W, Salam A.M.E. Non-Selectivity of R S Media for Aeromonas hydrophila and TCBS Media for Vibrio Species isolated from diseased Oreochromis niloticus. J. Aquac. Res. Dev. 2017;8(496):1–5. [Google Scholar]
- 21.Black J.G, Black L.J. Microbiology:Principles and Explorations. 9th ed. Hoboken: John Wiley and Sons, Inc; 2015. [Google Scholar]
- 22.Suvarna S.K, Layton C, Bancroft J.D. Bancroft's Theory and Practice of Histological Techniques. 8th ed. United Kingdom: Elsevier Limited; 2018. [Google Scholar]
- 23.CLSI. Performance Standards for Antimicrobial Susceptibility Testing;Twenty-Fifth Informational Supplement. CLSI document M100-S25. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 24.Hack D.M, Dressel D.C, Peterson L. Highly reproducible bactericidal activity test results by using a modified national committee for clinical laboratory standards broth macrodilution technique. J. Clin. Microbiol. 1999;37(6):1881–1884. doi: 10.1128/jcm.37.6.1881-1884.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei Z, Liu Q, Yang B, Ahmed S, Xiong J, Song T, Chen P, Cao J, He Q. Evaluation of bioequivalence of two long-acting 20% oxytetracycline formulations in pigs. Front. Vet. Sci. 2017;4(61):1–6. doi: 10.3389/fvets.2017.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toranzo A.E, Magari˜nos T.B, Romalde J.L. A review of the main bacterial fish diseases in mariculture systems. Aquaculture. 2005;246:1–4. 37–61. [Google Scholar]
- 27.Huang S.L, Chen W.C, Shei M.C, Liao I.C, Chen A.N. Studies on epizootiology and pathogenicity of Staphylococcus epidermidis in tilapia (Oreochromis Spp.) cultured in Taiwan. Zool. Stud. 1999;38(2):178–188. [Google Scholar]
- 28.Yiagnisis M, Athanassopoulou F, Aral F. Bacteria Isolated from Diseased Wild and Farmed Marine Fish in Greece, Recent Advances in Fish Farms. InTech Europe, University Campus STePRi, SlavkaKrautzeka 83/A, 51000 Rijeka, Croatia. 2011 [Google Scholar]
- 29.Buller N.B. Bacteria and Fungi from Fish and Other Aquatic Animals. 2nd ed. Wallingford, Oxfordshire: CABI, Nosworthy Way; 2014. [Google Scholar]
- 30.Baya A, Li T, Lupiani B, Hetrick F.M. Eastern Fish Health and American Fisheries Society FishHealth Section Workshop,16-19 June 1992. Auburn, Alabama: Auburn University; 1992. Bacillus cereus a Pathogen for Striped Bass; p. 67. [Google Scholar]
- 31.Chandra G, Bhattacharjee I, Chatterjee S. Bacillus cereus infection in stinging catfish Heteropneustes fossilis(Siluriformes, Heteropneustidae) and their recovery by Argemone mexicana seed extract. Iran. J. Fish. Sci. 2015;14(3):741–753. [Google Scholar]
- 32.Kubilay A, Uluköy G. First isolation of Staphylococcus epidermidis from cultured gilthead sea bream (Sparus aurata) in Turkey. Bull. Eur. Ass. Fish. Pathol. 2004;24(3):137–143. [Google Scholar]
- 33.Kusuda R, Sugiyama A. Studies on characters of Staphylococcus epidermidis isolated from diseased fishes, on the morphological, biological, and biochemical properties. Fish Pathol. 1981;16(1):15–24. [Google Scholar]
- 34.Austin B. Emerging bacterial fish pathogen. Bull. Eur. Ass. Fish. Pathol. 1999;19(6):231–234. [Google Scholar]
- 35.Namvar A.E, Bastarahang S, Abbasi N, Ghehi G.S, Farhadbakhtiarian S, Arezi P, Hosseini M, Baravati S.Z, Jokar Z, Chermahin S.G. Clinical characteristics of Staphylococcus epidermidis:A systematic review. GMS Hyg. Infect. Control. 2014;9(3):Doc23. doi: 10.3205/dgkh000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinheiro L, Brito C.I, Oliverra A.D, Martins P.Y.F, Pereira V.C, Cunha M.D. Staphylococcus epidermidis and Staphylococcus haemolyticus:Molecular detection of cytotoxic and enterotoxin genes. Toxins. 2015;7(9):3688–3699. doi: 10.3390/toxins7093688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Granum P.E, Lund T. Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Lett. 1997;157(2):223–228. doi: 10.1111/j.1574-6968.1997.tb12776.x. [DOI] [PubMed] [Google Scholar]
- 38.Prasad M.P. Molecular characterization of enterotoxigenic Bacillus cereus species isolated from tropical marine fishes using RAPD markers. Int. J. Pure. Appl. Biosci. 2014;2(4):189–195. [Google Scholar]
- 39.Visiello R, Colombo S, Carretto E. Bacillus cereus hemolysins and other virulence factors. In: Savini V, editor. The Diverse Faces of Bacillus cereus. London Wall, London: Academic Press is an Imprint of Elsevier; 2016. pp. 35–44. [Google Scholar]
- 40.Steen M.K, Bruno-Murtha L.A, Chaux G, Lazar H, Bernard S, Sulis C. Bacillus cereus endocarditis:Report of a case and review. Clin. Infect. Dis. 1992;14(4):945–946. doi: 10.1093/clinids/14.4.945. [DOI] [PubMed] [Google Scholar]
- 41.Callegan M.C, Booth M.C, Jett B.D, Gilmore M.S. Pathogenesis of gram-positive bacterial endophthalmitis. Infect. Immun. 1999;67(7):3348–3356. doi: 10.1128/iai.67.7.3348-3356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darbar A, Harris I.A, Gosbell I.B. Necrotizing infection due to Bacillus cereus mimicking gas gangrene following penetrating trauma. J. Orthop. Trauma. 2005;19(5):353–355. [PubMed] [Google Scholar]
- 43.Auger S, Ramarao N, Faille C, Fouet A, Aymerich S, Gohar M. Biofilm formation and cell surface properties among pathogenic and nonpathogenic strains of the Bacillus cereus group. Appl. Environ. Microbiol. 2009;75(20):6616–6618. doi: 10.1128/AEM.00155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lebessi E, Dellagrammaticas H.D, Antonaki G, Foustoukou M, Iacovidou N. Bacillus cereus meningitis in a term neonate. J Matern. Fetal Neonatal. Med. 2009;22(5):458–461. doi: 10.1080/14767050802610336. [DOI] [PubMed] [Google Scholar]
- 45.Bottone E.J. Bacillus cereus a volatile human pathogen. Clin. Microbiol. Rev. 2010;23(2):382–398. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sergelidis D, Abrahim A, Papadopoulos T, Soultos N, Martziou E, Koulourida A, Govaris A, Pexara A, Zdragas A, Papa A. Isolation of methicillin-resistant Staphylococcus spp. From ready-to-eat fish products. Lett. Appl. Microbiol. 2014;59(5):500–506. doi: 10.1111/lam.12304. [DOI] [PubMed] [Google Scholar]
- 47.Weber D.J, Saviteer S.M, Rutala W.A, Tomann C.A. In vitro susceptibility of Bacillus spp. To selected antimicrobial agents. Antimicrob. Agents Chemother. 1988;32(5):642–645. doi: 10.1128/aac.32.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller R.A. Ph. D. Thesis. USA: University of Maryland, College Park; 2007. Development of Standardized Antimicrobial Susceptibility Testing Methods and Aeromonas salmonicida Epidemiologic Cutoff Values for Antimicrobial Agents Used in Aquaculture; p. 3. [Google Scholar]
- 49.Bowden B.C. Pharmacokinetic Profiles of Oxytetracycline in Yellow Perch (Perca flavescens) as Determined by Plasma Concentration Following Different Routes of Administration. M. V. Sc. Thesis, Department of Biomedical Sciences and Pathobiology, Virginia-Maryland Regional College of Veterinary Medicine, USA. 2001 [Google Scholar]


