Short abstract
Heart failure affects 1–2% of the population worldwide, and it is characterized by episodes of decompensation often requiring hospitalization. Although targeted treatment has reduced the prevalence of rehospitalizations to 30–50%, mortality rates remain high. A complex blend of structural and functional alterations accounts for the genesis and progression of heart failure, but the exact underlying pathophysiology remains poorly understood. The aim of this review is to summarize endothelial dysfunction and its role in the pathogenesis and progression of heart failure. Moreover, it sums up all the appropriate methods of assessing endothelial dysfunction emphasizing on flow-mediated dilation and introduces endothelium as a potential target for new therapeutic development and research in the wide spectrum of the syndrome called heart failure.
Keywords: Heart failure, cardiology, endothelial dysfunction
Introduction
Heart failure (HF) is a complicated syndrome affecting 1–2% of the population in the developed world. The prevalence of this chronic illness rises up to 10% and more in population over 70 years old. It is characterized by episodes of decompensation often requiring hospitalization. Lately, effective treatment has reduced the prevalence of rehospitalizations to 30–50%, but mortality rates remain high. About 60–70% of the new diagnosed and hospitalized HF patients will have died within 5 years.1 A complex blend of structural and functional alterations accounts for the genesis and progression of HF, but the exact mechanisms underlying this disease remain poorly defined. Many advances and new therapeutic targets have arisen in the past few years, although successful translation of breakthroughs to meaningful clinical benefit requires a deeper understanding of the relevant pathophysiology.
All these years, mysterious endothelial dysfunction and its role in the pathogenesis and progression of HF have been thoroughly investigated. There has been a lot of discussion about endothelium as a potential target for new therapeutic development and research in the wide spectrum of the syndrome called HF.
Endothelium: Role properties
Endothelium is a mono-layer of cells covering the inner surface of blood vessels and acts as a functional and structural barrier between blood and vessel wall, preventing platelet and leukocyte adhesion and aggregation, controlling permeability to plasma components and modulating blood flow. It provides antiproliferative and anti-inflammatory actions and protects against oxidative stress. It also balances the cellular proliferation and death.2 Moreover, it regulates fibrinolysis as well as the coagulation cascade through a balanced production of anticoagulant and procoagulant factors, which maintain the haemostatic properties of blood vessels. It is also responsible for the vascular tone regulation by balancing the production of vasodilators and vasoconstrictors in response to a variety of stimuli. It generates a great number of bioactive molecules such as nitric oxide (NO), prostaglandins and cytokines, which play a crucial role in the physiological adaptation or pathophysiological dysfunction that regulates and redistributes regional blood flow. The endothelium regulates blood vessel diameter via the release of NO in response to stimulation with agonists (acetylocholine, bradykinin, thrombin, diphosphate and serotonin), mechanical stimuli such as changes in shear stress, as well as ischemia and temperature change, that lead to smooth muscle dilation and myofibrillar relaxation.3 NO is synthesized from l-arginine and oxygen by NO synthase (NOS). There are three main isoforms of NOS: constitutive endothelial NOS (eNOS or NOS3), neuronal NOS (or NOS1) and inducible NOS (iNOS) that are differently coexpressed in NO-producing cells and are also induced by immunological stimuli. All of the three synthases produce NO that regulates normal physiology, but sometimes iNOS is overexpressed and releases great amounts of NO which may have cytotoxic effects and inhibit myocardial contractility. Normally, in the intact endothelium, hormonal and physical stimuli cause the constitutively expressed eNOS to generate NO, which then diffuses into smooth muscle cells and stimulates soluble guanylate cyclase to produce cyclic guanine monophosphate. The last causes smooth muscle relaxation and has also antiproliferative effects. As mentioned before, NO can act as an endocrine vasoregulator, modulating blood flow in the microcirculation when vehiculated by S-nitrosohaemoglobin, which transports and releases NO to areas of tissue hypoxia or increases oxygen extraction. Disruption of NO delivery to the microcirculation contributes to vasoconstriction leading to adverse events for the organism.4
The role of the endothelium in chronic HF
Chronic HF is a clinical syndrome characterized by abnormalities of left ventricular function and neurohormonal regulation, which are accompanied by effort intolerance, fluid retention and decreased longevity. Many pathophysiologic factors contribute to the increased peripheral vascular resistance in chronic HF. These mechanisms include increased water and sodium content of the vasculature, increased neurohormonal activation (i.e. activation of the sympathetic nervous system, the renin–angiotensin–aldosterone system) and intrinsic abnormalities of the vasculature. Recent findings suggest a more central and significant contributory role of the vascular endothelium.5
Chronic HF patients appear with excessive systemic vasoconstriction and reduced peripheral tissue perfusion. Endothelial dysfunction seems to play an important role in this phenomenon, because it exacerbates the already existing vasoconstriction, augmenting myocardial damage. It increases afterload due to systemic and pulmonary vascular constriction. Moreover, it underlies the regional vasomotor dysregulation in the renal and coronary circulation. Decreased coronary endothelium-dependent vasodilation impairs myocardial perfusion, reduces coronary flow and worsens ventricular function. This decrease in cardiac output seen in HF patients culminates endothelial shear stress which stimulates eNOS expression. In HF, once eNOS expression is down-regulated, less NO is produced and consequently flow-mediated vasodilation (FMD) is diminished, giving its place to concomitant vasoconstriction. Moreover, production of vasoconstrictors, mainly endothelin 1, deteriorates vascular resistance, smooth muscle cell growth and matrix production, resulting to vascular remodelling, endothelial dysfunction and HF progression.
It is known that endothelium-derived NO plays a significant role in blood flow redistribution during exercise. That means that endothelial dysfunction in HF patients results in impaired exercise capacity due to alterations in the exercise-induced hyperemic blood flow response in this group. In other words, the sensitivity to shear stress of the skeletal muscle arterioles during the adaptation of the peripheral circulation in response to exercise plays an important role.
Lack of NO in HF patients affects cell migration, cardiac hypertrophy and athelosclerotic plaque stability. Reduced NO affects endothelial progenitor cells (EPCs), disabling endothelial repair and regeneration.
HF is associated to an altered redox state and production of reactive oxygen species, and there has been evidence that the syndrome worsens partially due to imbalances between NO bioavailability and oxidative stress. In HF, neurohormonal activation, release of inflammatory messengers such as prostaglandins, catecholamines and altered local shear stress due to low cardiac output, modulate gene expression and promote atherogenesis, increasing oxidative stress. Oxygen-derived free radicals (OFR), such as superoxide anion, hydrogen peroxide and hydroxyl radical, are produced in great amounts in the state of HF. These OFR react with NO, and a powerful oxidant called peroxynitrite anion is formed. The result is a reduction of NO bioavailability and a consequent deterioration of the clinical state of the patient (progression of HF from asymptomatic to symptomatic, decreased functional capacity, etc.). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase has been shown to be the major source of reactive ROS in both vascular smooth muscle and the endothelium. Angiotensin II and TNF-α (both increased in chronic HF) can significantly stimulate the activity and expression of this oxidase. This explains the recent studies that tend to interrupt this NADPH stimulation by using therapies such as ACE, ARBs and spironolactone with beneficial results in improvement of endothelial function, exercise tolerance and overall morbidity and mortality in HF patients.
It is known that patients suffering from HF have affected antioxidant defense. Healthy subjects have several antioxidant defense systems such as superoxide dismutase, catalase, glutathione peroxidase, antioxidant vitamins C, E, etc., which balance free radical formation. This lack in HF patients leads to enhanced biodegradation of NO by the superoxide anion, contributing to the impaired endothelial function. That urged researchers to use substances with antioxidant properties for treatment in HF patients (carvedilol b-blocker, vitamin C) with promising results.
Acute HF: Has endothelium anything to do with its etiology and progression?
NO-dependent regulation of ventricular function and vascular tone also determines haemodynamic status in acute HF.
Decreased NO bioavailability induces vasoconstriction and increased vascular stiffness in the systemic and pulmonary circulation, resulting in augmented left ventricular and right ventricular systolic workload. These high ventricular filling pressures negatively impact cardiac function by causing subendocardial ischemia, left ventricular remodeling, impairment of cardiac venous drainage from coronary veins and a lower threshold for arrhythmias.6 The resulting decrease in cardiac output may further impair renal perfusion and function, thereby causing additional fluid retention.
‘Vasomotor nephropathy’ is a transient renal dysfunction resulting from alterations in the perfusion of the renal arteries due to vascular, haemodynamic, neural and humoral mechanisms. From a vascular aspect, as mentioned before, low NO leads to decreased cardiac output and therefore decreased renal blood flow and diminished sodium excretion. As for haemodynamics, both arterial and venous pressures regulate sodium excretion. Once there is a problem in the filling of the arterial circulation, vascular and neurohumoral mechanisms (sympathetic nervous system activated, catecholamine release, RAAS stimulation) are triggered, leading to fluid retention.7 Oxidative stress is increased in acute HF. A great amount of reactive oxygen species is produced, which trap NO by reacting with it and thus reducing its bioavailability in the organism. All the aforementioned are important to the vicious cycle of acute HF syndrome.
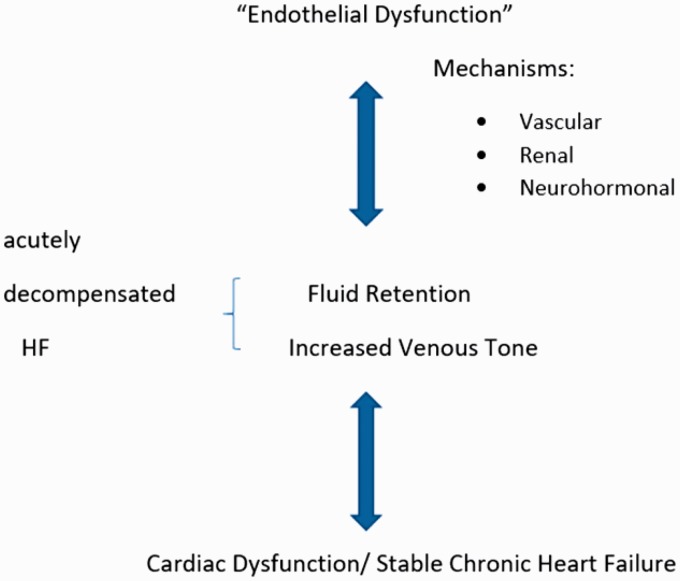
There has been a theory that acute HF should be the effect result of as-called acute endothelitis.8 According to this theory, an inflammatory triggering factor (infection, noncompliance to medication, etc.) could activate systemic endothelitis which is defined as endothelial oxidative stress and activation. This procedure leads through vascular, renal and neurohormonal mechanisms to fluid retention. Moreover, congestion and activation of the stretched endothelium worsen the existing systemic endothelitis. This results in constriction of capacitance veins, which leads to blood centralization. 9 This worsens the cardiac function and leads to another vicious cycle as described before. The ongoing fluid retention leads to clinical decompensation. Figure 1 summarizes all the aforementioned mechanisms.
Figure 1.
Endothelial dysfunction in the pathophysiology of HF. Endothelial dysfunction through vascular, renal and neurohormonal mechanisms leads to fluid overload. It also causes vasoconstriction of the capacitance veins leading to centralisation of blood volume. Moreover, congestion itself, through the same mechanisms, leads to more fluid retention and consequently to the vicious cycle of heart failure. An insult combined with the progressive fluid overload may lead to decompensated HF.
Methods to assess endothelial function – Emphasis on FMD of the brachial artery
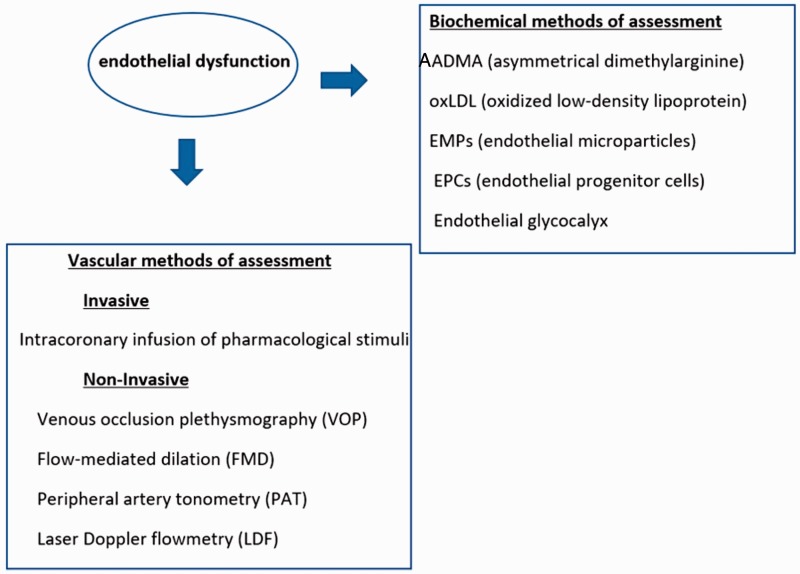
There are biochemical or vascular methods to assess endothelial dysfunction.10
Biochemical methods give the opportunity to measure NO metabolites and pro-inflammatory and vasoconstricting factors released by the endothelium. The most popular biochemical markers are asymmetrical dimethylarginine, oxidized low density lipoprotein, endothelial microparticles, EPCs and endothelial glycocalyx.11
Vascular methods are divided in two categories: invasive and noninvasive.
In the invasive way, the researchers evaluate the endothelial function by infusing intracoronary endothelium-dependent vasodilators. Moreover, they measure the endothelium-independent function by measuring dose response to increasing concentrations of vasodilators that donate only NO.
The non-invasive way provides more methods to the researchers to choose from and includes venous occlusion digital plethysmography, flow-mediated dilation of the brachial artery and peripheral artery tonometry. There are also optical techniques using laser that can be coupled with provocation tests. Each of the above methodologies has its own advantages and limitations. All methods are summarized in Figure 2.
Figure 2.
Methods of assessing endothelial dysfunction.
This review contains studies that have investigated the endothelium – its function and effect on the pathophysiology of both acute and chronic HF – assessed by the FMD method. FMD as mentioned above stands for flow-mediated dilation of the brachial artery. This method is based on the release of NO, which results in vasodilation that can be quantitated as an index of vasomotor function. This method is popular because it allows repetition of measurements over time, and it is mainly noninvasive. Although it has some limitations (quiet, temperature-controlled environment, well-trained operators), investigators were motivated and wrote guidelines for the assessment of endothelium-dependent FMD of the brachial artery.12 Briefly, the methodology of assessing FMD includes measurements in the right brachial artery. A blood pressure cuff is placed 1–2 cm below the antecubital fossa and inflated to 250 mmHg for a total of 4 min. Images are acquired at baseline and every 30 s, from 1 to 3 min after deflation of the wrist cuff, and FMD is calculated as the maximum percent increase in arterial diameter during the first 3 min of hyperemia compared with the diameter at rest.13
Studies – Existing bibliography
Many studies have been designed and carried out over the past years, paying extra attention on the role of the endothelial dysfunction in the syndrome of HF and its vicious cycle. The investigators have emphasized on the general idea of oxidative stress in HF leading to dysfunctional endothelium and negative effects on the organism which are destructive for the already failing heart. The questions that have mainly concerned are whether endothelial dysfunction is of different importance and origin among the different types, causes and duration of HF, whether it alters the prognosis of these patients, and whether interventions to normalize endothelial function could possibly act therapeutically.
So, for our convenience, we have divided the studies concerning endothelium and HF in three main categories:
Studies that intended to investigate possible differences in endothelial dysfunction among a variety of types of patients with HF or to find correlations between FMD and other factors in patients with HF .
Studies that targeted the possible connection between level of endothelial dysfunction and prognosis among HF patients.
Studies that investigated possible alterations or improvements in endothelial dysfunction after pharmaceutical intervention.
Endothelial dysfunction in different types of HF
Several studies conducted have revealed that patients with HF of ischaemic etiology suffer from impaired FMD and appear with lower levels of FMD in different measurements. Shah et al.14 found that abnormalities of endothelium-mediated dilatation existed only in patients with HF of nonischaemic etiology. Endothelium-dependent and -independent vasodilation was found more attenuated in ischaemic than in non-ischaemic HF patients in another study conducted by Klonsinska et al.15 Another research found that FMD was lower in ischaemic HF patients, a parameter that did not improve even after heart transplantation.16 Among other factors that may affect FMD, researchers have found that diabetes, worse functional status (higher NYHA class, worse LVEF: left ventricle ejection fraction) and worse renal function have strong correlation with lower FMD regardless etiology of HF.17 Kishimoto et al.18 proved that endothelial dysfunction measured by FMD was significantly smaller in patients with HFpEF (HF with preserved ejection fraction) than in patients without HF. The aforementioned studies are summarized in Table 1.
Table 1.
Studies assessing endothelial dysfunction in different types of heart failure.
| Study, Journal, Year of publication | Study population | Conclusion |
|---|---|---|
| Shah et al. J Card Fail, 201014 | • 11 patients with heart failure and IHD (ischaemic heart disease) | Heart failure of nonischaemic etiology is not associated with abnormalities of endothelium-mediated dilatation or of arterial compliance. |
| • 12 patients with heart failure from angiographically verified idiopathic nonischaemic dilated cardiomyopathy | ||
| Klosinska M et al, Eur J Heart Fail, 200915 | • 57 patients with systolic CHF (chronic heart failure) | Endothelium-dependent vasodilation was markedly reduced in patients with ischaemic CHF compared with those with non-ischaemic aetiology of CHF |
| • 34 of ischaemic aetiology | ||
| • 23 of non-ischaemic aetiology | ||
| Patel AR et al. J Am Coll Cardiol, 200116 | • 10 patients of nonischaemic cardiomyopathy | Peripheral vascular endothelial function is normal in cardiac transplant recipients with antecedent nonischaemic cardiomyopathy but remains impaired in those with prior ischaemic cardiomyopathy. Endothelial function is uniformly abnormal for patients with heart failure, regardless of etiology. |
| • 7 patients of ischaemic cardiomyopathy | ||
| • 10 cardiac transplant recipients with prior nonischaemic cardiomyopathy | ||
| • 10 cardiac transplant recipients without prior ischaemic cardiomyopathy | ||
| • 10 normal controls | ||
| Kishimoto S. Int J Cardiol, 201718 | • 41 patients with HFpEF | FMD was significantly smaller in patients with HFpEF than in patients without HF |
| • 165 patients without HF |
Endothelial dysfunction and prognosis
Although many studies have proven that acute and chronic HF patients have different degrees of endothelial dysfunction, maybe due to different pathophysiological etiology, most researchers have measured FMD in patients with chronic HF. Their aim was to investigate any significant correlation between FMD and patients’ prognosis. Shechter et al.19 found that FMD predicted mortality risk in patients with chronic end stage ischaemic HF patients. Endothelial dysfunction in chronic HF – assessed by FMD – is associated with an increased mortality risk in subjects of both ischaemic and nonischaemic origin. Moreover, it has been found that FMD represents an independent predictor of cardiac death and hospitalization in HF patients.20–22 On the other hand, Paine et al.23 did not associate FMD with clinical outcomes in HF patients.
Alterations in endothelial function after medical intervention
There is a number of pharmacological agents that have been used in order to examine the possibility that they play a role in alteration of endothelial function, which is estimated by flow-mediated dilation of the brachial artery. It was proved that rosuvastatin improves endothelial function in patients with congestive HF.24 Angiotensin-converting enzyme and phosphodiesterase type 5 were found to acutely improve FMD in chronic HF patients, and their combination showed additive benefits in FMD as well.25 Freimark et al.26 compared short-term intermittent intravenous low-dose dobutamine therapy in patients with severe chronic HF and ischaemic cardiomyopathy NYHA IV to control subjects and found that it significantly improved vascular endothelial function. Beneficial effects on endothelial function were recorded with levosimendan in advanced HF.27 Last but not least, allopurinol was studied and showed improvement in endothelial function of HF patients, but researchers did not use flow-mediated dilation method but the standard forearm venous occlusion plethysmography.28 Balmain et al.29 studied the effect of folic acid supplementation on endothelial function (measured by FMD) in HF patients versus healthy controls and found that FMD improved by 2.1 ± 1.3% in HF patients (P < 0.01), but no change was observed in healthy population postintervention (P = 0.20).29
Conclusion – Discussion
It is already known that endothelial function is an important factor in the pathogenesis of atherosclerosis, hypertension and HF. FMD of the brachial artery as a depiction of the endothelial function, apart from its acceptable limitations, is an easy, reproducible, repeatable and noninvasive method for the assessment of endothelial function. Studies on HF have revealed a diminished FMD in HF patients, although the type of HF that is most affected is not yet determined. We have to note that, in some studies, FMD managed to correlate with prognosis in patients with chronic HF, but there is a lack of evidence in patients with acute HF syndromes. FMD is used in some interventional studies and has proven beneficial actions of some drugs on the endothelial function of HF patients. In conclusion, more studies have to be designed in order to investigate FMD’s real role as an indicator of endothelial function, especially in groups hospitalized for acute HF.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1.Ponikowski P, Voors AA and Anker SDet al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18(8): 891-975. [DOI] [PubMed]
- 2.Esper RJ, Nordaby RA, Vilariño JO, et al. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol 2006; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lüscher TF, Barton M. Biology of the endothelium. Clin Cardiol 1997; 20: II–310. [PubMed] [Google Scholar]
- 4.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev 1991; 43: 109–142. [PubMed] [Google Scholar]
- 5.Remme WJ. Congestive heart failure – pathophysiology and medical treatment. J Cardiovasc Pharmacol 1986; 8: S36–S52. [PubMed] [Google Scholar]
- 6.Gheorghiade M, De Luca L, Fonarow GC, et al. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol 2005; 19: 96. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Zannad F, Sopko G, et al. Acute heart failure syndromes: current state and framework for future research. Circulation 2005; 112: 3958–3968. [DOI] [PubMed] [Google Scholar]
- 8.Colombo PC, Onat D, Sabbah HN. Acute heart failure as “acute endothelitis” – interaction of fluid overload and endothelial dysfunction. Eur J Heart Fail 2008; 10: 170–175. [DOI] [PubMed] [Google Scholar]
- 9.Colombo PC, Banchs JE, Celaj S, et al. Endothelial cell activation in patients with decompensated heart failure. Circulation 2005; 111: 58–62. [DOI] [PubMed] [Google Scholar]
- 10.Puissant C, Abraham P, Durand S, et al. Endothelial function: role, assessment and limits. J Mal Vasc 2014; 39: 47–56. [DOI] [PubMed] [Google Scholar]
- 11.Lekakis J, Abraham P, Balbarini A, et al. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil 2011; 18: 775–2. [DOI] [PubMed] [Google Scholar]
- 12.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation (FMD) of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 39: 257–265. [DOI] [PubMed] [Google Scholar]
- 13.Naka KK, Papathanassiou K, Bechlioulis A. Determinants of vascular function in patients with type 2 diabetes. Cardiovasc Diabetol 2012; 11: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah A, Gkaliagkousi E, Ritter JM, et al. Endothelial function and arterial compliance are not impaired in subjects with heart failure of non-ischemic origin. J Card Fail 2010; 16: 114–120. [DOI] [PubMed] [Google Scholar]
- 15.Klosinska M, Rudzinski T, Grzelak P, et al. Endothelium-dependent and -independent vasodilation is more attenuated in ischaemic than in non-ischaemic heart failure. Eur J Heart Fail 2009; 11: 765–770. [DOI] [PubMed] [Google Scholar]
- 16.Patel AR, Kuvin JT, Pandian NG, et al. Heart failure etiology affects peripheral vascular endothelial function after cardiac transplantation. J Am Coll Cardiol 2001; 37: 195–200. [DOI] [PubMed] [Google Scholar]
- 17.Ciccone MM, Iacoviello M, Puzzovivo A, et al. Clinical correlates of endothelial function in chronic heart failure. Clin Res Cardiol 2011; 100: 515–521. [DOI] [PubMed] [Google Scholar]
- 18.Kishimoto S, Kajikawa M, Maruhashi T, et al. Endothelial dysfunction and abnormal vascular structure are simultaneously present in patients with heart failure with preserved ejection fraction. Int J Cardiol 2017; 231: 181–187. [DOI] [PubMed] [Google Scholar]
- 19.Shechter M, Matetzky S, Arad M, et al. Vascular endothelial function predicts mortality risk in patients with advanced ischaemic chronic heart failure. Eur J Heart Fail 2009; 11: 588–593. [DOI] [PubMed] [Google Scholar]
- 20.Meyer B, Mörtl D, Strecker K, et al. Flow-mediated vasodilation predicts outcome in patients with chronic heart failure: comparison with B-type natriuretic peptide. J Am Coll Cardiol 2005; 46: 1011–1018. [DOI] [PubMed] [Google Scholar]
- 21.Katz SD, Hryniewicz K, Hriljac I, et al. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation 2005; 111: 310. [DOI] [PubMed] [Google Scholar]
- 22.Fischer D, Rossa S, Landmesser U, et al. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J 2005; 26: 65–69. [DOI] [PubMed] [Google Scholar]
- 23.Paine NJ, Hinderliter AL, Blumenthal JA, et al. Reactive hyperemia is associated with adverse clinical outcomes in heart failure. Am Heart J 2016; 178: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gounari P, Tousoulis D, Antoniades C, et al. Rosuvastatin but not ezetimibe improves endothelial function in patients with heart failure, by mechanisms independent of lipid lowering. Int J Cardiol 2010; 142: 87–91. [DOI] [PubMed] [Google Scholar]
- 25.Hryniewicz K, Dimayuga C, Hudaihed A, et al. Inhibition of angiotensin-converting enzyme and phosphodiesterase type 5 improves endothelial function in heart failure. Clin Sci (Sci) 2005; 108: 331–338. [DOI] [PubMed] [Google Scholar]
- 26.Freimark D, Feinberg MS, Matezky S, et al. Impact of short-term intermittent intravenous dobutamine therapy on endothelial function in patients with severe chronic heart failure. Am Heart J 2004; 148: 878–882. [DOI] [PubMed] [Google Scholar]
- 27.Parissis JT, Karavidas A, Bistola V, et al. Effects of levosimendan on flow-mediated vasodilation and soluble adhesion molecules in patients with advanced chronic heart failure. Atherosclerosis 2008; 197: 278–282. [DOI] [PubMed] [Google Scholar]
- 28.Farquharson CA, Butler R, Hill A, et al. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation 2002; 106: 221–226. [DOI] [PubMed] [Google Scholar]
- 29.Balmain BN, Jay O, Morris NR. Folic acid supplementation improves vascular endothelial function, yet not skin blood flow during exercise in the heat, in patients with heart failure. Am J Physiol Regul Integr Comp Physiol 2018; 315(4): R810–R819. [DOI] [PubMed] [Google Scholar]