Abstract
Objective(s):
To assess the effect of pulmonary hypertension on NICU mortality and hospital readmission through one year corrected age in infants with severe bronchopulmonary dysplasia with a large, multicenter cohort.
Study Design:
This was a multicenter, retrospective cohort study of 1677 infants born <32 weeks’ gestation with severe bronchopulmonary dysplasia (positive pressure ventilation, >2 L/min flow by nasal cannula, or fraction of inspired oxygen (FiO2) >0.3 at 36 weeks’ postmenstrual age) enrolled in the Children’s Hospital Neonatal Consortium with records linked to the Pediatric Health Information System.
Results:
Pulmonary hypertension occurred in 370/1677 (22%) infants. During the neonatal admission, pulmonary hypertension was associated with mortality (OR= 3.15, 95%CI 2.10–4.73, p<0.001), ventilator support at 36 weeks’ post-menstrual age (60% vs 40%, p<0.001), duration of ventilation (72 IQR 30–124 vs 41 IQR 17–74 days, p<0.001), and higher respiratory severity score (3.6 IQR 0.4–7.0 vs 0.8 IQR 0.3–3.3, p<0.001). At discharge, pulmonary hypertension was associated with tracheostomy (27% vs 9%, p<0.001), supplemental oxygen use (84% vs 61%, p<0.001), and tube feeds (80% vs 46%, p<0.001). Through one year corrected age, pulmonary hypertension was associated with increased frequency of readmission (IRR=1.38, 95%CI 1.18–1.63, p<0.001).
Conclusion:
Infants with severe bronchopulmonary dysplasia-associated pulmonary hypertension have increased morbidity and mortality through one year corrected age. This highlights the urgent need for improved diagnostic practices and prospective studies evaluating treatments for this high-risk population.
Background
Severe bronchopulmonary dysplasia (sBPD) occurs in more than 20% of extremely-low-birth-weight infants. It is a major driver of mortality, prolonged hospitalization, and healthcare utilization after discharge from the neonatal intensive care unit (NICU).(1–4) Pulmonary hypertension (PH) is a serious comorbidity of sBPD, thought to result from a combination of intrinsic, secondary, and acquired abnormalities in the pulmonary vasculature.(5–8) Although diagnostic criteria vary, PH is found in 10–20% of infants with sBPD(2, 9–11) and has been linked with increased risk of death or tracheostomy.(8, 10, 12)
The understanding of PH has been limited by the small numbers of neonates reported in single-center studies, varied timing and diagnostic criteria of echocardiography, and logistic limitations of cardiac catheterization.(11, 13–15) Regardless of how or when the diagnosis of PH is established, an understanding of outcomes following a diagnosis of PH could help categorize illness severity, plan clinical studies, and tailor bedside management by identifying effective therapies.
The overall goal of this study was to describe the NICU and post-NICU outcomes of PH in a large, multi-center cohort of infants with sBPD referred to regional NICUs. Our primary objective was to determine whether PH is associated with NICU mortality and hospital readmission through one year corrected age. We hypothesized that infants with sBPD and PH would have increased mortality and hospital readmission than those with sBPD alone.
Methods/Study Design
We conducted a retrospective cohort study of infants in the Children’s Hospitals Neonatal Database (CHND) between January 1, 2010 and June 30, 2015. CHND prospectively captures clinical data of all infants admitted to participating referral-based NICUs.(16) Chart review is conducted by trained staff at each site and has been described previously.(17) Readmission data from neonatal discharge through one year corrected age were obtained by linking CHND data to the Pediatric Health Information System (PHIS), which holds administrative data about hospital encounters over time for all but three hospitals participating in CHND. Patient records in CHND and PHIS were linked using masked billing and medical record numbers. After linkage, multiple encounters of care (consisting of inpatient admissions, outpatient surgery, emergency department and observation visits) were observed for each patient through one year corrected age. Prior studies have demonstrated >94% linkage between CHND and PHIS.(18, 19)
Inclusion and exclusion criteria
We included all infants admitted to a CHND NICU who were born at <32 weeks’ gestation and had sBPD, defined as positive pressure ventilation, >2 L/min flow by nasal cannula, or fraction of inspired oxygen (FiO2) >0.3 at 36 weeks’ postmenstrual age or at the time of referral to the CHND NICU, whichever came later.(20, 21) We excluded those who died prior to 36 weeks’ postmenstrual age, were hospitalized in a CHND NICU for < 3 days, or were admitted to centers with <20 CHND-PHIS-linked records. We also excluded infants with major congenital anomalies. Infants with patent ductus arteriosus, atrial septal defects, or ventricular septal defects were retained in the study unless other major cardiac malformations were also present.
Outcomes and covariates
The primary outcome was mortality after 36 weeks’ postmenstrual age during the neonatal admission. The CHND records data throughout the entire hospital stay from neonatal admission through discharge, even if the infant is transferred to another unit within the same hospital. Thus, we defined the “neonatal admission” as including the CHND NICU stay plus any transfer to another unit within the same institution. Secondary outcomes during the neonatal admission included the length of stay from 36 weeks’ postmenstrual age to discharge to adjust for differences due to prematurity, receipt of gastrostomy and/or fundoplication, receipt of tracheostomy, and neonatal admission continuing beyond one year corrected age.
For infants who survived to discharge home from the CHND hospital and had PHIS-linked data available, a second primary outcome was hospital readmission between NICU discharge and one year corrected age. Readmission was defined as any inpatient-type episode of care occurring in PHIS between discharge from the neonatal admission and one year corrected age. Readmission to an ICU and the use of mechanical ventilation during an inpatient readmission were also identified in the PHIS. Emergency department, observation-only encounters, and outpatient surgical procedures in the PHIS were evaluated, but not considered as readmissions.
The main exposure was PH, as reported in the medical record and recorded in CHND. The diagnosis of PH was based on local clinical practices and standards. We did not define explicit criteria for clinical diagnosis of PH for this study, as this was a retrospective review of multiple centers over a five-year period. Only infants with an echocardiogram performed at ≥34 weeks’ postmenstrual age were included in our primary analysis. A sensitivity analysis was performed for all infants regardless of having undergone echocardiography. We recorded the number of infants at each center undergoing cardiac catheterization, however, the low frequency (n=66) of this procedure precluded further inquiries.
Additional covariates were obtained from the CHND. Gestational age was defined by obstetric estimate,(22) and small for gestational age was defined as <10th sex- and gestational age- specific birth weight percentiles.(23) Age at admission to the CHND NICU was rounded to the nearest week. Sex, maternal racial and ethnic status, insurance status, maternal chorioamnionitis, maternal diabetes, multiple gestation, antenatal steroids, surgical ligation of a patent ductus arteriosus, blood stream infection, and surgical necrotizing enterocolitis (characteristic systemic signs with pneumatosis intestinalis, portal venous gas, and/or intraperitoneal gas and receipt of surgical procedure) were abstracted from the CHND. Receipt of mechanical ventilation was assessed at 36 weeks’ postmenstrual age or time of admission to the CHND hospital, whichever occurred later. Respiratory severity score was defined for mechanically ventilated patients at 36 weeks’ postmenstrual age as the product of mean airway pressure and fraction of inspired oxygen.(24) Ventilator days were counted from the time of admission to the CHND NICU through discharge. Postnatal systemic steroids were defined as receipt of any oral or intravenous, but not inhaled, corticosteroid during the CHND NICU admission. Weight-gain velocity was calculated from birth to 36 weeks’ postmenstrual age and reported in g/kg/day.(25)
Data analyses
We used descriptive statistics to compare illness characteristics for all infants stratified by PH. We assessed the correlation between each center’s prevalence of diagnosis of PH and use of echocardiography at ≥34 weeks’ postmenstrual age, as well as the correlation between center diagnosis of PH and center use of inhaled nitric oxide and sildenafil.
Next, we determined the relationship between PH and mortality and inpatient readmissions through one year corrected age. Unadjusted associations were compared using chi-squared or Wilcoxon rank-sum tests. To estimate independent associations, we performed multivariable regression with mixed effects. We included covariates which were associated with PH at p<0.1 in bivariate analyses and potential confounders of an association between PH and outcomes; covariates were removed by backwards elimination if they were not associated with the outcome (p>0.1). Center was used as a random intercept. Mortality was modeled using mixed logistic regression. Frequency of hospital readmission from NICU discharge through one year corrected age was modeled using mixed Poisson regression. For each model, the area under the receiver operating characteristic (ROC) curve was calculated and goodness-of-fit was assessed using the Hosmer-Lemeshow test. As a sensitivity analysis, we repeated both mortality and readmission models for all infants, regardless of receipt of an echocardiogram at ≥34 weeks’ postmenstrual age.
Statistical significance was defined as p <0.05. Analyses were performed using SAS version 9 (Cary, NC). The CHND was approved by the Institutional Review Board of Stanley Manne Research Institute affiliated with the Ann & Robert H. Lurie Children’s Hospital of Chicago (#2011–14673), and all participating centers.
Results
There were 95,954 database records in the CHND; of those, 17,053 were from unique infants born <32 weeks’ gestation. We excluded infants who died prior to 36 weeks’ postmenstrual age (n=1659), who were hospitalized for <3 days (n=1059), or who had congenital anomalies (n=2114). We also excluded infants without sBPD (n=9242) and who came from centers with <20 CHND-PHIS linked cases (n=173).
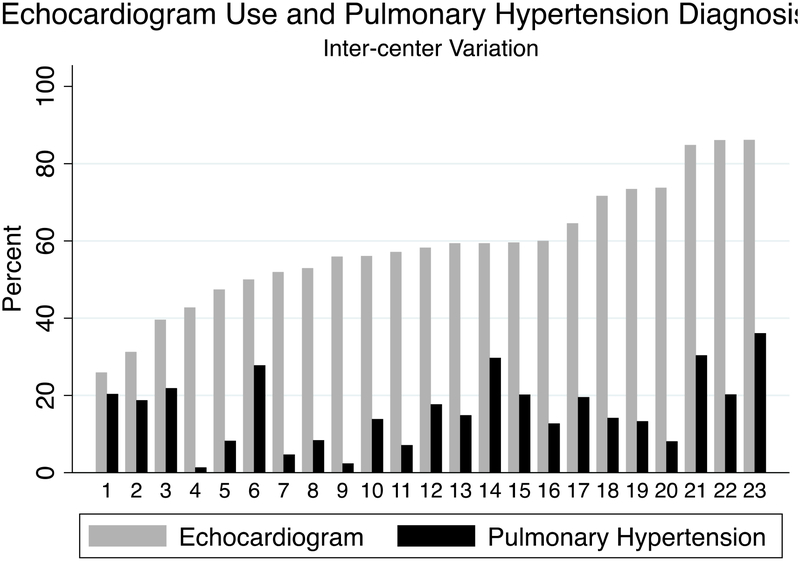
Of the 23 centers included in this analysis, the median center prevalence of PH was 14% (IQR 8–20%), which varied significantly across centers (p<0.001). Echocardiography at ≥34 weeks’ postmenstrual age was performed in 1677; the median center prevalence of echocardiography was 60% (IQR 51–68%) which was also different across centers (p<0.001) (Figure 1). There was no correlation between center prevalence of echocardiography and PH (R2 = 0.09). Infants with PH were frequently treated with inhaled nitric oxide [128/401 (32%)] during their CHND hospital stay, with a center median of 33% (IQR 20–42). Infants with PH were also often treated with sildenafil (222/401 (55%)), with a center median of 60% (IQR 49–78). The use of sildenafil (p<0.001) and inhaled nitric oxide (p<0.001) in patients with PH varied significantly by center (Figure 2, online only). However, there was no correlation between center prevalence of PH and use of inhaled nitric oxide (R2 = 0.0009) or sildenafil (R2 = 0.07).
Figure 1. Inter-center variation in prevalence of sBPD-associated PH and use of echocardiogram.
Numbers on the x axis indicate individual centers participating in CHND. There was no correlation between center prevalence of sBPD-associated PH and use of echocardiogram at ≥ 34 weeks’ corrected age, with a linear trend equation of 0.51x+0.5 and R2 of 0.09. PH = pulmonary hypertension.
There were 1677 infants with sBPD with at least one echocardiogram who were included for our primary analysis. PH was diagnosed in 370 (22%) patients. Infants with PH were more likely to be born at a lower birth weight, nearly twice as likely to be small for gestational age, and be born to mothers of black race. Although maternal chorioamnionitis was less common in PH, maternal hypertension was more common. Infants with PH were admitted seven weeks later by postmenstrual age to a CHND center and had more severe respiratory illness than infants without PH. Those with PH had longer duration of mechanical ventilation than those without PH, and were more likely to require mechanical ventilation at 36 weeks’ postmenstrual age or at admission. Among infants requiring mechanical ventilation at 36 weeks’ postmenstrual age, PH was associated with a more than four-fold increase in respiratory severity score. Two-thirds of infants with PH received postnatal systemic steroids, which was almost twice as common as infants without PH (Table I). When analyzing all infants with sBPD regardless of echocardiography at ≥34 weeks postmenstrual age (n=2806), the comparison of clinical characteristics between infants with and without PH was essentially unchanged (Table IV, online only).
Table I.
Demographic and illness characteristics of infants by sBPD-associated PH with at least one echocardiogram
| Variable | All | No PH | PH | p |
|---|---|---|---|---|
| N | 1677 | 1307 | 370 | |
| Gestational age (weeks), median [IQR] | 25 [24, 27] | 25 [24, 27] | 25 [24, 26] | 0.03 |
| Admission PMA (weeks), median [IQR] | 30 [26, 36] | 29 [26, 34] | 36.5 [28, 45] | <0.001 |
| Median birth weight (g) | 715 [600, 885] | 735 [620, 910] | 650 [530, 800] | <0.001 |
| SGA (≤10th percentile) | 403 (24.0) | 262 (20.0) | 141 (38.1) | <0.001 |
| Maternal Hispanic ethnicity | 196 (11.7) | 160 (12.2) | 36 (9.7) | 0.06 |
| Maternal race | 0.03 | |||
| White | 825 (49.2) | 660 (50.5) | 165 (44.6) | — |
| Black | 621 (37.0) | 462 (35.34) | 159 (43.0) | — |
| Other | 191 (11.4) | 156 (11.9) | 35 (9.5) | — |
| Insurance Status | 0.62 | |||
| Public | 1046 (62.4) | 824 (63.0) | 222 (60.0) | — |
| Private | 393 (23.4) | 297 (22.7) | 96 (26.0) | — |
| Not insured or self-pay | 10 (0.6) | 8 (0.6) | 2 (0.5) | — |
| Combined Private/Public | 228 (13.6) | 178 (13.6) | 50 (13.5) | — |
| Chorioamnionitis | 179 (10.7) | 150 (11.5) | 29 (7.8) | 0.05 |
| Maternal hypertension | 437 (26.1) | 314 (24.0) | 123 (33.2) | <0.001 |
| Multiple births | 407 (24.3) | 330 (25.2) | 77 (20.8) | 0.08 |
| Ventilator days in CHND hospital, median [IQR] | 46 [19, 84] | 41 [17, 74] | 72.5 [30, 124] | <0.001 |
| Mechanical ventilation at 36 weeks’ PMA or admission | 749 (44.7) | 524 (40.1) | 225 (60.8) | <0.001 |
| Respiratory severity score for ventilated patients, median [IQR] | 1 [0.3, 3.9] | 0.8 [0.3, 3.3] | 3.6 [0.4, 7] | <0.001 |
| Postnatal systemic steroids | 756 (45.1) | 506 (38.7) | 250 (67.6) | <0.001 |
| PDA ligation | 372 (22.2) | 303 (23.2) | 69 (18.6) | 0.07 |
| PMA at PDA ligation, weeks, median [IQR] | 30.3 [28, 34.6] | 29.9 [28, 32.9] | 31.9 [28.9, 42.7] | <0.001 |
| Median growth velocity, birth to 36 weeks’ PMA (g/kg/d) | 13.2 [11.6, 14.6] | 13.1 [11.6, 14.5] | 13.5 [12.2, 15.1] | 0.02 |
| Blood stream infection | 423 (25.2) | 317 (24.2) | 106 (28.6) | 0.09 |
| Atrial Septal Defect | 219 (13.1) | 146 (11.2) | 73 (19.7) | <0.001 |
| Ventricular Septal Defect | 78 (4.65) | 57 (4.36) | 21 (5.68) | 0.33 |
Results are displayed in n (%) unless indicated otherwise. p values indicate chi squared tests or Wilcoxon rank-sum test as appropriate. SGA = small for gestational age; IQR = inter-quartile range; PH= pulmonary hypertension; Respiratory severity score = mean airway pressure times fraction of inhaled oxygen; PDA = patent ductus arteriosus; NEC = necrotizing enterocolitis, PMA = postmenstrual age.
Infants with PH had an unadjusted 4-fold higher prevalence of mortality during the neonatal admission (21% vs 5%, p<0.001), and a higher likelihood of remaining hospitalized through one year corrected age (5% vs 1%, p<0.001). Among survivors to NICU discharge, PHIS linkage was available in 1145/1222 (94%) infants. Those with PH had higher prevalence of tracheostomy (27% vs 9%, p<0.001) and gastrostomy (80% vs 46%, p<0.001). At one year corrected age, surviving infants with sBPD had 2925 total inpatient encounters including inpatient, emergency room, observation and ambulatory surgery. Infants with PH were more likely to have inpatient readmissions, intensive care unit readmissions, and readmissions requiring mechanical ventilation. Mortality during readmission was infrequent and was similar among infants with and without PH (Table II). When comparing outcomes for infants regardless of receiving echocardiography at ≥ 34 weeks’ postmenstrual age, the associations between PH and outcomes were similar in direction and magnitude (Table V, online only).
Table II.
Outcomes at NICU discharge and one year corrected age by sBPD-associated PH in infants with at least one echocardiogram
| All | No PH | PH | p | |
|---|---|---|---|---|
| N | 1677 | 1307 | 370 | |
| DISCHARGE STATUS SUMMARYa | ||||
| Died before discharge of initial hospitalization | 145 (8.6) | 68 (5.2) | 77 (20.8) | <0.001 |
| Transferred | 277 (16.5) | 205 (15.7) | 72 (19.5) | 0.66 |
| Still in CHND hospital at 1 year corrected age | 33 (2.0) | 13 (1.0) | 20 (5.4) | <0.001 |
| Survived to discharge home from CHND hospital | 1222 (72.9) | 1021 (78.1) | 201 (54.3) | <0.001 |
| SURVIVING INFANTS OUTCOME SUMMARYb | ||||
| PHIS linkage available | 1145 (93.7) | 961 (94.1) | 184 (91.5) | 0.18 |
| At NICU Discharge | ||||
| Supplemental oxygen, n (%) | 817 (48.7) | 658 (68.5) | 159 (86.4) | <0.001 |
| Tracheostomy with home mechanical ventilation, n (%) | 132 (7.9) | 83 (8.6) | 49 (26.6) | <0.001 |
| Feeding tube, n (%) | 590 (35.2) | 442 (46.0) | 148 (80.4) | <0.001 |
| PMA at discharge from birth hospitalization | ||||
| Discharged from NICU, weeks, median [IQR] | 46.4 [42.6, 53] | 45.6 [42.1, 51.1] | 53.6 [48.1, 61.9] | <0.001 |
| Discharged after intra-hospital transfer, weeks, median [IQR] | 62.1 [54.3, 71] | 58.6 [50.3, 66.6] | 68.6 [62.1, 75.3] | <0.001 |
| At 1 year corrected age | ||||
| Total encounters between discharge and 1 year corrected age (n) | 2925 | 2380 | 546 | |
| Inpatient | 1096 | 870 | 226 | |
| Emergency room | 1328 | 1110 | 218 | |
| Observation | 233 | 196 | 37 | |
| Ambulatory surgery | 268 | 203 | 65 | |
| Patients with at least 1 inpatient readmission | 490 (42.8) | 396 (41.2) | 94 (51.1) | 0.01 |
| ICU readmission | 212 (18.5) | 156 (16.2) | 56 (30.4) | <0.001 |
| Mechanical ventilation during inpatient admission | 197 (17.2) | 142 (14.7) | 55 (29.9) | <0.001 |
| Died during inpatient readmission | 11 (0.9) | 9 (0.9) | 2 (1.1) | 0.80 |
| Total length of stay of all readmissions, days, median [IQR] | 5 [2,13] | 5 [2,13] | 8 [3,19] | 0.004 |
Displays discharge status for the 1677 infants in the study. Data are reported as n (%) unless otherwise stated.
Outcomes for infants who survived to discharge from the CHND hospital, with available linked PHIS data, as indicated in the “PHIS linkage available” row. Subsequent outcome rows are calculated as percentage of that row as a denominator.
CHND = Children’s Hospitals Neonatal Database; PH= pulmonary hypertension; PHIS = Pediatric Hospitals Information System; IQR = inter-quartile range; PMA = postmenstrual age.
In multivariable regressions, PH was independently associated with both mortality and readmissions. Infants with PH had a greater than 3-fold increased odds of mortality during the neonatal admission (p<0.001); respiratory failure or multi-system organ failure were the cause of death in 83% of infants. Mortality was also related to later postmenstrual age at admission to a CHND hospital, mechanical ventilation at 36 weeks’ postmenstrual age, postnatal steroids, and atrial septal defects (Table III). Similarly, PH was associated with a 38% increased frequency of inpatient readmissions (p<0.001) between NICU discharge and one year corrected age. Mechanical ventilation at 36 weeks’ postmenstrual age, postnatal steroids, blood stream infections, and airway comorbidities such as laryngotracheobronchomalacia were also associated with increased frequency of readmission. While infants born to black mothers were more likely to be readmitted following the initial NICU discharge, those with private insurance were less likely to be readmitted (Table III). The association of pulmonary hypertension with mortality and hospital readmission persisted in the sensitivity analysis including all infants regardless of receiving echocardiography at ≥ 34 weeks’ postmenstrual age (Table VI, online only).
Table III.
Multivariable regression models for relationship of sBPD-associated PH with mortality and hospital readmission in infants with at least one echocardiogram
| Mortality (N=1677) | OR | 95% CI | p |
| PH | 3.15 | 2.10–4.73 | <0.001 |
| Ventilator at 36 weeks’ PMA | 4.29 | 2.72–6.78 | <0.001 |
| Postnatal Steroids | 2.06 | 1.33–3.18 | 0.002 |
| Atrial Septal Defect | 1.74 | 1.04–2.92 | 0.04 |
| Hospital Readmission (N=1145) | IRR | 95% CI | p |
| Pulmonary Hypertension | 1.38 | 1.18–1.63 | <0.001 |
| Small for Gestational Age | 1.20 | 1.04–1.39 | 0.01 |
| Maternal Race | |||
| White | 1.0 | - | - |
| Black | 1.19 | 1.04–1.37 | 0.01 |
| Other | 0.88 | 0.69–1.13 | 0.31 |
| Unknown | 0.48 | 0.27–0.88 | 0.02 |
| Ventilator at 36 weeks’ PMA | 1.27 | 1.11–1.44 | <0.001 |
| Postnatal Steroids | 1.57 | 1.37–1.80 | <0.001 |
| Blood Stream Infection | 1.32 | 1.15–1.52 | <0.001 |
| Insurance Status | |||
| Public | 1.0 | - | - |
| Combined Public/Private | 1.12 | 0.93–1.36 | 0.24 |
| Not Insured | 0.84 | 0.36–1.97 | 0.69 |
| Private | 0.82 | 0.69–0.96 | 0.01 |
| Laryngotracheobronchomalacia | 1.61 | 1.32–1.96 | <0.001 |
Discussion
This study compares outcomes of a large, contemporary, multicenter cohort of infants with sBPD by the presence of PH. We found that PH was associated with a broad range of adverse outcomes including mortality, gastrostomy, tracheostomy, home oxygen, and readmission before one year corrected age.
Many investigators have focused their efforts on risk factors for PH in infants with BPD.(8, 10, 11, 26, 27) Most of these studies evaluated clinical characteristics, placental architecture, or biomarkers of infants in a single center during the initial NICU hospitalization. Mourani et al described the relationship between early echocardiographic signs of pulmonary vascular disease and the later development of BPD and PH in two centers.(28) A strength of our study is that we evaluated neonatal morbidity and mortality in a large, multicenter cohort and followed infants through one year corrected age. To our knowledge, this study is the largest multi-center cohort that has evaluated outcomes for infants with sBPD-associated PH.
We identified associations between PH and both mortality as well as frequency of inpatient readmissions between NICU discharge and one year corrected age. As 5% of neonates with PH were not discharged by one year corrected age, our findings may underestimate the association between PH and hospital readmission. Ventilator use at 36 weeks’ postmenstrual age and use of postnatal steroids were also associated with adverse outcomes, likely reflecting increased underlying disease severity. Furthermore, we identified a racial disparity with infants born to black mothers having greater rates of hospital readmission, even after accounting for insurance status at NICU discharge as a surrogate for socioeconomic status. While prior reports have linked race to respiratory disease in premature infants (3, 29), to our knowledge, this is the first report linking race specifically to hospital readmission in neonates with sBPD.
A majority of infants diagnosed with PH were treated with sildenafil, inhaled nitric oxide or both despite the paucity of strong evidence for these therapies in sBPD-associated PH.(13–15, 30, 31) In the absence of a standardized illness severity definition, we explicitly chose not to compare outcomes of individual infants treated versus not treated with PH-targeted medications. As severity of PH could not be directly assessed, patients not treated with pulmonary vasodilators could have mild disease; thus, our results may underestimate the impact of more severe PH and the potential benefits of treatment. The 2015 guidelines from the American Heart Association recognize that PH-targeted therapy could be useful for infants with BPD and symptomatic PH; further research on therapeutic interventions will be essential to improve outcomes in this population.(32)
We found wide variation in prevalence of PH and use of echocardiography across centers. Interestingly, centers that used echocardiography more often did not diagnose more patients with PH; this may be due to the lack of standardized guidelines for evaluation of PH in infants with sBPD during the study period. Cardiac catheterization was used rarely in this cohort. Prior studies have found that echocardiographic measures of PH are variably present and have limited reproducibility and predictive value, such that relying solely on echocardiography is also problematic.(8–10, 33) Standardized, practical, and validated definitions will be essential to future advances in the understanding of this disease and were recently suggested by Krishnan et al.(13)
This study has important limitations. The lack of standardized criteria to establish a diagnosis of PH is a significant limitation, both for this study and for the field in general. One of our main goals in conducting this study despite the absence of a standardized definition of PH was to assess outcomes for use in future study design. If infants without an echocardiogram were misclassified as not having PH, this could have caused us to underestimate the true impact of PH. Conversely, if sicker infants received more frequent screening or were more likely to be labeled as having PH, it could falsely over-estimate the impact of PH on outcomes. However, outcomes were similar in the sensitivity analysis, suggesting that misclassification and ascertainment bias had minimal impact on our findings. Respiratory or multi-system organ failure were the causes of death in most infants, but we do not know exactly how PH may or may not have been linked to the infants’ demise.
Because CHND sites are largely referral-based children’s hospitals, echocardiographic data collected from referring centers may not be available. Although data abstractors are rigorously trained at each site, there is inter-center variability in reporting in the medical records. We attempted to minimize systematic bias by regular training for abstractors and requiring thresholds for inter-rater agreement at each site, so we believe that we have reduced this chance of error as much as possible.(17) While the the CHND allows for adjustment for many confounders, unmeasured confounders could have modified the associations.
With regards to readmissions, another important limitation is that infants may have been readmitted to institutions outside of participating hospitals in CHND and PHIS or readmitted for non-pulmonary causes. Readmission to a hospital outside the CHND and PHIS might be more common among less sick infants without PH; however, the readmission rates appear similar to other reports of readmissions for infants with sBPD (21, 34). Also, these results may not generalizeable to institutions that do not participate in CHND and PHIS. Nevertheless, we believe our findings reinforce the fact that lung disease severity including PH is a likely contributor to mortality and healthcare utilization after NICU discharge.(34, 35)
In conclusion, this study identified outcomes associated with PH in a multi-center cohort of infants with sBPD in regional NICUs. Despite variation in diagnosis across centers, PH is associated with a wide range of adverse outcomes including tracheostomy, home oxygen use, gastrostomy tubes, hospital readmissions, and death. These data highlight the urgent need for improved diagnostic standards, better understanding of the specific cause of adverse outcomes, and prospective studies to evaluate treatments for PH in this high-risk population.
Supplementary Material
Figure 2. Inter-center variation in use of inhaled nitric oxide and sildenafil. Numbers on the x axis indicate individual centers participating in CHND. iNO = inhaled nitric oxide.
Abreviations
- CHND
Children’s Hospital Neonatal Database
- NICU
Neonatal Intensive Care Unit
- PH
Pulmonary Hypertension
- PHIS
Pediatric Health Information System
- PMA
Postmenstrual Age
- sBPD
Severe bronchopulmonary dysplasia
Footnotes
None
References
- 1.Kuint J, Lerner-Geva L, Chodick G, Boyko V, Shalev V, Reichman B, et al. Rehospitalization Through Childhood and Adolescence: Association with Neonatal Morbidities in Infants of Very Low Birth Weight. The Journal of pediatrics. 2017. [DOI] [PubMed] [Google Scholar]
- 2.Padula MA, Grover TR, Brozanski B, Zaniletti I, Nelin LD, Asselin JM, et al. Therapeutic interventions and short-term outcomes for infants with severe bronchopulmonary dysplasia born at <32 weeks’ gestation. J Perinatol. 2013;33(11):877–81. [DOI] [PubMed] [Google Scholar]
- 3.Keller RL, Feng R, DeMauro SB, Ferkol T, Hardie W, Rogers EE, et al. Bronchopulmonary Dysplasia and Perinatal Characteristics Predict 1-Year Respiratory Outcomes in Newborns Born at Extremely Low Gestational Age: A Prospective Cohort Study. The Journal of pediatrics. 2017;187:89–97 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lapcharoensap W, Gage SC, Kan P, Profit J, Shaw GM, Gould JB, et al. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA Pediatr. 2015;169(2):e143676. [DOI] [PubMed] [Google Scholar]
- 5.Gorenflo M, Vogel M, Obladen M. Pulmonary vascular changes in bronchopulmonary dysplasia: a clinicopathologic correlation in short- and long-term survivors. Pediatr Pathol. 1991;11(6):851–66. [DOI] [PubMed] [Google Scholar]
- 6.Jobe AJ. The new BPD: an arrest of lung development. Pediatric research. 1999;46(6):641–3. [DOI] [PubMed] [Google Scholar]
- 7.Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, et al. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1981–7. [DOI] [PubMed] [Google Scholar]
- 8.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120(6):1260–9. [DOI] [PubMed] [Google Scholar]
- 9.Mourani PM, Abman SH. Pulmonary Hypertension and Vascular Abnormalities in Bronchopulmonary Dysplasia. Clin Perinatol. 2015;42(4):839–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics. 2012;129(3):e682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weismann CG, Asnes JD, Bazzy-Asaad A, Tolomeo C, Ehrenkranz RA, Bizzarro MJ. Pulmonary hypertension in preterm infants: results of a prospective screening program. J Perinatol. 2017;37(5):572–7. [DOI] [PubMed] [Google Scholar]
- 12.Murthy K, Savani RC, Lagatta JM, Zaniletti I, Wadhawan R, Truog W, et al. Predicting death or tracheostomy placement in infants with severe bronchopulmonary dysplasia. J Perinatol. 2014;34(7):543–8. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan U, Feinstein JA, Adatia I, Austin ED, Mullen MP, Hopper RK, et al. Evaluation and Management of Pulmonary Hypertension in Children with Bronchopulmonary Dysplasia. The Journal of pediatrics. 2017. [DOI] [PubMed] [Google Scholar]
- 14.Trottier-Boucher MN, Lapointe A, Malo J, Fournier A, Raboisson MJ, Martin B, et al. Sildenafil for the Treatment of Pulmonary Arterial Hypertension in Infants with Bronchopulmonary Dysplasia. Pediatr Cardiol. 2015;36(6):1255–60. [DOI] [PubMed] [Google Scholar]
- 15.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. The Journal of pediatrics. 2009;154(3):379–84, 84 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Academy of Pediatrics Committee on F, Newborn. Levels of neonatal care. Pediatrics. 2012;130(3):587–97. [DOI] [PubMed] [Google Scholar]
- 17.Murthy K, Dykes FD, Padula MA, Pallotto EK, Reber KM, Durand DJ, et al. The Children’s Hospitals Neonatal Database: an overview of patient complexity, outcomes and variation in care. J Perinatol. 2014;34(8):582–6. [DOI] [PubMed] [Google Scholar]
- 18.Massaro AN, Murthy K, Zaniletti I, Cook N, DiGeronimo R, Dizon ML, et al. Intercenter Cost Variation for Perinatal Hypoxic-Ischemic Encephalopathy in the Era of Therapeutic Hypothermia. The Journal of pediatrics. 2016. [DOI] [PubMed] [Google Scholar]
- 19.Murthy K, Pallotto EK, Gien J, Brozanski BS, Porta NF, Zaniletti I, et al. Predicting death or extended length of stay in infants with congenital diaphragmatic hernia. Journal of perinatology: official journal of the California Perinatal Association. 2016. [DOI] [PubMed] [Google Scholar]
- 20.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–9. [DOI] [PubMed] [Google Scholar]
- 21.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–60. [DOI] [PubMed] [Google Scholar]
- 22.Engle WA, American Academy of Pediatrics Committee on F, Newborn. Age terminology during the perinatal period. Pediatrics. 2004;114(5):1362–4. [DOI] [PubMed] [Google Scholar]
- 23.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214–24. [DOI] [PubMed] [Google Scholar]
- 24.Malkar MB, Gardner WP, Mandy GT, Stenger MR, Nelin LD, Shepherd EG, et al. Respiratory severity score on day of life 30 is predictive of mortality and the length of mechanical ventilation in premature infants with protracted ventilation. Pediatric pulmonology. 2015;50(4):363–9. [DOI] [PubMed] [Google Scholar]
- 25.Patel AL, Engstrom JL, Meier PP, Jegier BJ, Kimura RE. Calculating postnatal growth velocity in very low birth weight (VLBW) premature infants. J Perinatol. 2009;29(9):618–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeVries LB, Heyne RJ, Ramaciotti C, Brown LS, Jaleel MA, Kapadia VS, et al. Mortality among infants with evolving bronchopulmonary dysplasia increases with major surgery and with pulmonary hypertension. J Perinatol. 2017. [DOI] [PubMed] [Google Scholar]
- 27.Mestan KK, Check J, Minturn L, Yallapragada S, Farrow KN, Liu X, et al. Placental pathologic changes of maternal vascular underperfusion in bronchopulmonary dysplasia and pulmonary hypertension. Placenta. 2014;35(8):570–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;191(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrow LA, Wagner BD, Ingram DA, Poindexter BB, Schibler K, Cotten CM, et al. Antenatal Determinants of Bronchopulmonary Dysplasia and Late Respiratory Disease in Preterm Infants. Am J Respir Crit Care Med. 2017;196(3):364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrington KJ, Finer N, Pennaforte T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev. 2017;1:CD000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadmon G, Schiller O, Dagan T, Bruckheimer E, Birk E, Schonfeld T. Pulmonary hypertension specific treatment in infants with bronchopulmonary dysplasia. Pediatric pulmonology. 2017;52(1):77–83. [DOI] [PubMed] [Google Scholar]
- 32.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. Pediatric Pulmonary Hypertension. Guidelines From the American Heart Association and American Thoracic Society. 2015. [DOI] [PubMed] [Google Scholar]
- 33.Benatar A, Clarke J, Silverman M. Pulmonary hypertension in infants with chronic lung disease: non-invasive evaluation and short term effect of oxygen treatment. Arch Dis Child Fetal Neonatal Ed. 1995;72(1):F14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ, et al. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. The Journal of pediatrics. 2004;144(6):799–803. [DOI] [PubMed] [Google Scholar]
- 35.Mourani PM, Kinsella JP, Clermont G, Kong L, Perkins AM, Weissfeld L, et al. Intensive care unit readmission during childhood after preterm birth with respiratory failure. The Journal of pediatrics. 2014;164(4):749–55 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 2. Inter-center variation in use of inhaled nitric oxide and sildenafil. Numbers on the x axis indicate individual centers participating in CHND. iNO = inhaled nitric oxide.