Abstract
Background:
Prenatal and childhood exposure to polybrominated diphenyl ether (PBDE) flame retardants has been inversely associated with cognitive performance, however, few studies have measured PBDE concentrations in samples collected during both prenatal and postnatal periods.
Methods:
We examined prenatal (cord) and childhood (ages 2, 3, 5, 7 and 9 years) plasma PBDE concentrations in relation to memory outcomes assessed between the ages of 9 and 14 years. The study sample includes a subset (n=212) of the African American and Dominican children enrolled in the Columbia Center for Children’s Environmental Health Mothers and Newborns birth cohort. We used multivariable linear regression to examine associations between continuous log10-transformed PBDE concentrations and performance on tests of visual, verbal and working memory in age-stratified models. We additionally used latent class growth analysis to estimate trajectories of exposure across early life, which we analyzed as a categorical variable in relation to memory outcomes. We examined interactions between PBDE exposure and sex using cross-product terms.
Results.
Associations between prenatal exposure and working memory significantly varied by sex (p-interaction= 0.02), with inverse relations observed only among girls (i.e. βBDE-47 = −7.55, 95% CI: −13.84, −1.24). Children with sustained high concentrations of BDEs-47, 99 or 100 across childhood scored approximately 5–8 standard score points lower on tests of visual memory. Children with PBDE plasma concentrations that peaked during toddler years performed better on verbal domains, however, these associations were not statistically significant.
Conclusions.
Exposure to PBDEs during both prenatal and postnatal periods may disrupt memory domains in early adolescence. These findings contribute to a substantial body of evidence supporting the developmental neurotoxicity of PBDEs and underscore the need to reduce exposure among pregnant women and children.
Keywords: PBDE, flame retardant, prenatal, trajectory, childhood, memory
1.1. Introduction
Polybrominated diphenyl ethers (PBDEs) are a class of organohalogenated flame retardant chemicals that were used extensively in furniture and furnishings to meet United States fire safety standards until their phase-out between 2004 and 2013 1. Exposure to PBDEs occurs primarily through incidental ingestion of dust 2 and owing to their lipophilicity, PBDEs readily cross the placenta 3 and partition into breastmilk 4.
Mounting evidence supports an association between prenatal exposure to PBDEs and reduced cognitive abilities in children 5,6. Importantly, the brain continues to develop postnatally, remaining vulnerable to insult by environmental toxicants throughout childhood 7. Additionally, research indicates PBDE exposure may peak during childhood due to breastfeeding, as well as increased ingestion of dust from close proximity to the floor and frequent hand to mouth behavior 8. Despite these factors, limited research has examined health effects associated with PBDE concentrations measured during both prenatal and postnatal periods 9–11.
In the present study, we examined associations between plasma PBDE concentrations measured repeatedly throughout the early lifecourse (birth through age 9 years) in relation to several subdomains of memory measured during early adolescence. We selected memory outcomes based on the results of animal research demonstrating inverse associations between PBDEs and performance on tests of memory and learning 12–15, as well as findings from human studies demonstrating inverse associations between PBDEs and cognitive performance 9,16.
2. Methods
2.1. Study design and participants
The Columbia Center for Children’s Environmental Health (CCCEH) Mothers and Newborns study is a longitudinal birth cohort of African American and Dominican mother-child pairs in Northern Manhattan and the South Bronx. Additional details describing the study design and participant recruitment have been previously published 17. Eligible (healthy, 18–35 year old women free of tobacco and illicit drug use who initiated prenatal care by the 20th week of gestation) participants were recruited between 1998 and 2006 from two prenatal clinics. At the time of delivery, 727 mother-child pairs remained eligible and were fully enrolled into the cohort.
Bilingual research staff conducted structured participant interviews during the prenatal period, after delivery, and repeatedly during childhood (3 months, 6 months, 1, 2, 3, 5, 7, 9, 11, and 14 years) to collect information on demographic and lifestyle factors. As previously described 18, prenatal exposure to environmental tobacco smoke was assessed using a combination of questions about smokers in the home and validated by blood cotinine concentrations. Maternal distress was evaluated using the Psychiatric Epidemiology Research Instrument- Demoralization (PERI-D) scale 19 and material hardship was indexed using a series of questions about access to basic needs (food, housing and clothing) 20. At the 3-year follow-up visit, maternal non-verbal intelligence was assessed using the Test of Nonverbal Intelligence, 2rd Edition (TONI-II) 21 and the child’s living environment was evaluated using the Home Observation for Measurement of the Environment (HOME) Early Childhood Inventory, which was completed during a visit to the family’s home 22. The HOME inventory is designed to evaluate stimulation and support available to children within their family surroundings and includes 8 areas of emphasis, including academic stimulation. Information on birthweight and gestational age was abstracted from medical records.
Before each visit, mothers signed a letter of informed consent and children ≥7 years additionally signed a letter of informed assent. Study procedures were approved by the Institutional Review Boards of Columbia University Medical Center and the New York State Psychiatric Institute. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects’ research.
2.2. Memory Assessment
Trained research staff administered the Children’s Memory Scale (CMS) to children between the ages of 9 and 14 years (mean±SD: 11.1±1.1); all tests were administered in English. The CMS is designed to measure memory and learning across three domains (attention-concentration, visual/non-verbal, and auditory/verbal ) in children and adolescents 23. We examined scores from the Attention-Concentration, Immediate (i.e. short-term recall) Visual Memory and Immediate Verbal Memory indices, which are age-scaled and standardized against a normative sample to reflect a mean±SD of 100±15 and range from 50 to 150. The visual (‘dot locations’ and ‘faces’) and verbal (‘stories’ and ‘word pairs’) indices are each comprised of two subtests which require the child to recall previously seen or heard information and reflect the ability to process, organize and hold material in short term visual and verbal memory. The Attention-Concentation Index is comprised of scores on two core subtests (‘numbers’ and ‘sequences’), which require the participant to recall, manipulate and repeat sequences of numbers, letters, or categories, and thus places a heavy demand on auditory working memory 23. Additional information describing each subtest is provided in the supplemental material (Table S1). Sixteen children were excluded from models examining the Attention-Concentration Index scale because they were not administered one of the two core subtests due to factors unrelated to the child. At the time of CMS testing, child self-report of anxiety was ascertained using the Revised Children’s Manifest Anxiety Scale (RCMAS), which is a brief self-report inventory designed to measure the degree and nature of anxiety 24.
2.3. PBDE exposure assessment
We measured PBDE concentrations in 903 stored plasma samples collected from 334 children between birth and age 9 years (Ncord=327, N2-years=56, N3-years=115, N5-years=42, N7-years=203, and N9-years=160). Details pertaining to sample collection and analysis of PBDE concentrations in this cohort have been previously published 25. Briefly, hospital staff collected umbilical cord blood at the child’s delivery and a pediatric phlebotomist collected child venous blood at 2, 3, 5, 7 and 9-year follow-up visits. All samples were immediately transported to the CCCEH laboratory, processed and stored in multiple aliquots at −70C.
Plasma PBDE concentrations were measured by the CDC using gas chromatography isotope dilution high-resolution mass spectrometry on a DFS instrument (ThermoFisher, Bremen, Germany) 26,27. Before final analytic determinations were made, samples were fortified with internal standards and extracted using a Gilson 215 liquid handler (Gilson Inc., Middleton, WI). Blanks (n=3) were processed with every 30 samples and the median blank value was subtracted from the final result. Lipids were co-extracted using a Rapid Trace modular SPE work station (Biotage, Uppsala, Sweden) and total cholesterol and triglycerides were measured using commercially available test kits (Roche Diagnostics, Indianapolis, IN). We estimated total cord blood lipids using a recently developed cord blood-specific formula [total cord blood lipids = 2.66 × total cord blood cholesterol + cord blood triglycerides + 0.268, in g lipids/L plasma] (Sjodin A, unpublished data) and child blood lipids using the short formula described by Philips et al. 1989 28.
The limits of detection (LODs) for BDE-47 ranged from 0.69 to 11.59 ng/g lipid for cord plasma samples and 1.10 to 20.20 for child plasma samples. For the other three congeners investigated (BDEs-99, 100 and 153), LODs ranged from 0.29 to 5.46 ng/g lipid for cord plasma and 0.45 to 6.40 ng/g lipid for child plasma. As previously described 25, we replaced concentrations less than the LOD with values drawn at random from a sample-specific normal probability distribution with the same mean and variance of the natural-log transformed detected concentrations with an equal or lower LOD. To incorporate uncertainty introduced by imputation, we repeated this procedure 10 times. Given variation in PBDE detection frequencies for cord (BDE-47: 80%, BDE-99: 51%, BDE-100: 42%, BDE-153: 38%) versus child (across ages 2–9 years: BDE-47: 100%, BDE-99: 80–98%, BDE-100: 90–100%, BDE-153: 90–98%) plasma samples, we performed multiple imputation on cord plasma and child plasma samples separately (i.e. only cord plasma samples were used to impute non-detectable cord plasma concentrations and only child plasma samples were used to impute non-detectable child plasma concentrations).
We additionally measured several biomarkers of other developmental neurotoxicants with dietary or indoor (polychlorinated biphenyls (PCBs),lead, chlorpyrifos) exposure sources. Cord plasma PCB concentrations were measured simultaneous to PBDE measurement, cord plasma chlorpyrifos concentrations were measured by gas chromatography isotope dilution high-resolution mass spectrometry as previously described 29, and cord blood lead was measured by Zeeman graphite furnace atomic absorption spectrometry with a phosphate/Triton X-100/nitric acid matrix modifier.
2.4. Statistical analysis
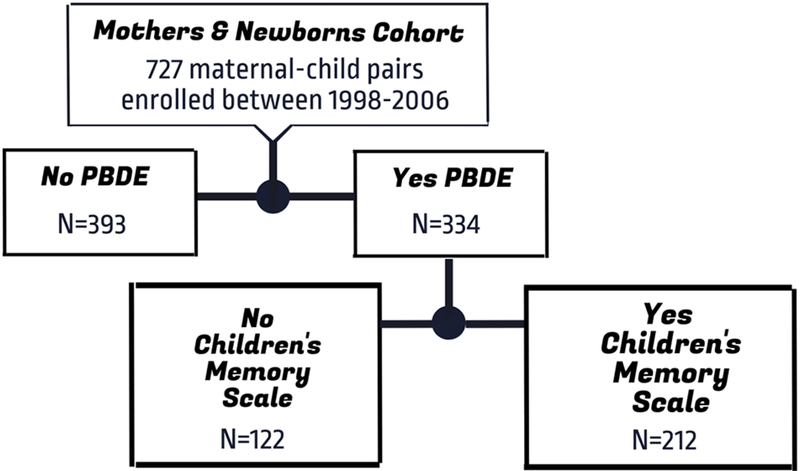
As illustrated by Figure 1, of the 334 children who had at least one measure of PBDEs, 212 also completed neurodevelopmental testing and were included in the analysis. We examined log10-transformed, lipid-standardized PBDE concentrations (ng PBDE/g lipid) measured in plasma collected at the prenatal (umbilical cord n=208), 3-year (n=70), 7-year (n=158), and 9-year (n=128) follow-up visits in relation to scores on the CMS Attention-Concentration, Visual Memory, and Verbal Memory indices in separate models (n=12 models). We did not examine PBDE concentrations at the 2=year (n=41) or 5-year (n=35) follow-up visits given the small sample sizes at these ages. We performed regression analyses on each of the 10 datasets with non-detectable concentrations replaced with imputed values and pooled resulting parameter estimates following Rubin’s rules 30.
Figure 1.
Diagram of study enrollment and follow-up.
To better understand the neurodevelopmental impacts of cumulative exposure across childhood, we examined CMS scores in relation to trajectories of PBDE change across age, which we estimated using latent class growth analysis (LCGA) 31. Briefly, LCGA is a discrete mixture method for clustering individuals with similar patterns of a characteristic of interest over time 32,33. We fit trajectories using log10-transformed PBDE concentrations (ng/g lipid) and selected the best fitting model for each congener using the Bayesian Information Criterion, as well as the probability of correct trajectory membership. Before fitting trajectories, we replaced non-detectable PBDE concentrations with the sample-specific mean concentration from the 10 multiply imputed datasets.
As previously described31, we found that across the four congeners, one group of children was characterized by low exposure at all ages (“persistent low”) and a second group was characterized by low prenatal exposure that increased during toddler years and remained high throughout childhood (“sustained childhood high”). For BDEs 47, 99 and 100, concentrations among a third group of children increased between birth and toddler years, but subsequently decreased (“early postnatal peak”) and concentrations among a fourth group were highest during the prenatal period (“prenatal high”). Finally, for BDE-153, we did not detect an “early postnatal peak” trajectory, but rather a group of children with “sustained moderate” concentrations throughout childhood (see Supplemental Material, Figure S2 and S3, which plot PBDE trajectories and present sample sizes). We used multivariable linear regression to examine associations between trajectory membership, treated as a categorical variable, and continuous index scores on the CMS. In all models, the ‘persistent low’ trajectory served as the reference group; beta coefficients are interpreted as the unit-change in memory score among children assigned to a given trajectory versus the ‘persistent low’ trajectory.
We used directed acyclic graph (DAG)-theory to select the set of minimally sufficient adjustment variables to allow identification of an unconfounded effect of PBDE exposure patterns on memory outcomes. First, we a priori diagramed our understanding of the relationships and dependencies among variables expected to be associated with PBDEs or memory outcomes based on substantive knowledge and previously published research. We conceptualized potential covariates as belonging to one of several categories, including: indicators of socioeconomic status (maternal education, maternal employment, material hardship), demographic and cultural factors (year of birth, ethnicity, language spoken in the home, maternal demoralization, maternal relationship status), physical characteristics (maternal age, gestational age, birthweight, breastfeeding history, child age at testing), and variables that span these categories (maternal intelligence, parity, ETS, HOME total and academic score, child anxiety at the time of testing). Second, when possible (data available and distributional assumptions met), we evaluated the consistency of the proposed DAG by statistically testing the encoded processes. Specifically, we used the ‘localTests’ function within the R package ‘Daggity’ to apply d-separation criterion and enumerate the implied conditional independencies, which we evaluated using tests of zero (partial) correlation. Variables included in the minimally sufficient adjustment set included ethnicity (African American/Dominican), exact age at CMS testing (continuous, in years), maternal nonverbal intelligence (continuous TONI-II score), maternal education during pregnancy (< high school/ ≥ high school degree or equivalent), maternal employment during pregnancy (employed/unemployed), maternal demoralization during pregnancy (continuous PERI-D score), parity (multiparous/nulliparous), birthweight (continuous, in grams), and breastfeeding history of the study child (continuous, in weeks). We additionally a priori adjusted models for date of birth (continuous, in days) to account for differences in exposure among children born earlier versus later during the enrollment period, which spanned the phase-out of pentaBDEs in the United States. Given sexually dimorphic patterns of brain development, we examined interactions between sex and PBDE concentration using cross product terms and stratified analyses34.
We visually inspected residual plots to confirm normality of residuals and examined the impact of outlying CMS scores by examining models excluding observations with a value more than 1.5 times the interquartile range below or above the first or third quartile. In models examining PBDE trajectories, we evaluated the impact of potential trajectory misclassification by modeling associations subset on children with a high posterior probability of correct trajectory assignment (>60%). Finally, we evaluated correlations between cord plasma PBDE concentrations (ng/g lipid) and cord blood concentrations of PCBs, lead and chlorpyrifos. If significant correlations were observed, we examined models adjusting for the relevant co-exposure.
We constructed DAGs using DAGitty v2.334 and conducted regression analyses using SAS v9.4 (SAS Institute Inc., Cary, North Carolina). We performed LCGA model estimation using the SAS Proc Traj macro 35.
3. Results
3.1. Study participants
Children included in final models were African American (43%) or Dominican (57%). At delivery, 38% of mothers had less than a high school education and 51% were first time mothers. Over the first five years of life, 32% of mothers breastfed the study child for 12 weeks or longer. Mean Early Childhood HOME total (mean±standard deviation (SD) 39.3±6.5) and academic (4.3±1.0) scores were similar to those of the normative sample (total: 37.5±10.34, academic: 3.3±1.3)22. No cut-off threshholds indicating clinical demoralization have been established for the PERI-D, however, the mean±SD in our sample (1.2±0.6) is equal to a previously proposed demoralization score based on a large community sample, 36 suggesting mothers in the present sample may experience greater demoralization than the general population.
Additional characteristics of study participants are presented in Table 1. CMS scores were normally distributed with means± SD of 94±16, 99±13 and 95±16 for the Attention-Concentration, Visual Memory and Verbal Memory indices, respectively. Scores were not significantly different between boys and girls for any of these indices. Compared to children without a measure of PBDEs or CMS testing (n=515), children included in final models (n=212) weighed more at birth (mean: 145 grams) and were more frequently a firstborn child (51% versus 42%). We detected no other differences between children included in the analysis and those excluded (see Supplemental Material, Table S4). Likewise, age-specific PBDE concentrations did not significantly differ between the 334 children with a measure of PBDEs and the 212 children included in the analysis for any congener.
Table 1.
Characteristics of participants with plasma PBDE concentrations and CMS scores (n=212)
| Maternal characteristicsa | Mean±SD or n (%) |
|---|---|
| Age | 25.2±5.1 |
| <High school education | 80 (38) |
| Employed | 120 (56) |
| Stable relationship | 49 (23) |
| Nulliparous | 108 (51) |
| Nonverbal intelligence | 84.1±13.1 |
| Demoralization | 1.2±0.6 |
| Child characteristics | |
| Birth weight (kg) | 3.5±0.5 |
| Gestational age (weeks) | 39.4±1.3 |
| African American | 92 (43) |
| Dominican | 120 (57) |
| Girl | 119 (56) |
| Breastfed ≥ 12 weeks | 67 (32) |
| Anxiety score at CMS testingb | 9.5±6.2 |
| Age at CMS testing (years) | 11.1±1.1 |
| Household characteristics | |
| Material hardship at deliveryb | 85 (41) |
| Material hardship at CMS testingb | 81 (39) |
| Primarily Spanish-speaking home (age 3) | 88 (42) |
| HOME academic scoreb (age 3) | 4.3 (1.0) |
| HOME total scoreb (age 3) | 39.3 (6.5) |
| Environmental tobacco smoke | 77 (36) |
HOME: Home Observation for Measurement of the Environment; RCMAS: Revised Children’s Manifest Anxiety Scale.
Measured prenatally unless otherwise noted.
Missing (n): material hardship at delivery (5) and at CMS testing (2), HOME score (6), RCMAS Total Anxiety score (4).
3.2. PBDE exposure
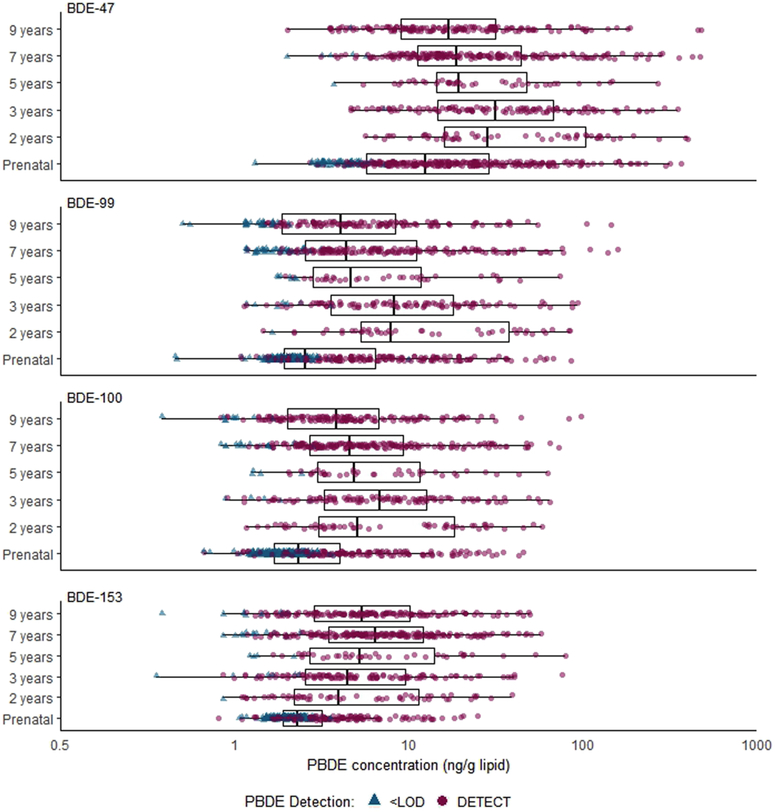
BDEs-47, 99, 100 and 153 were the most frequently detected congeners. At all ages BDE-47 concentrations predominated samples; concentrations were lowest in cord blood (geometric mean±geometric standard deviation: 14.1±0.9) and highest during toddler years (age 2: 37.8±5.8, age 3: 32.1±3.1). As illustrated by Figure 2, across congeners, PBDE concentrations and detection frequencies were consistently higher in child (range across congeners and postnatal visits: 80–100%) compared to cord plasma (range across congeners: 38–80%). Within a congener, concentrations across postnatal ages were moderately to highly correlated (Spearman’s ρ: BDE-47 = 0.61–0.80, BDE-99 = 0.49–0.83, BDE-100: 0.72–0.89, BDE-153: 0.71–0.93), however, concentrations between cord plasma and age 2 plasma were poorly correlated (BDE-47: −0.03, BDE-99: 0.04, BDE-100: 0.21, BDE-153: 0.26). Across congeners, BDEs-47, −99 and −100 were moderately to highly correlated at each time point (Spearman’s ρ: birth = 0.76–0.83, age 2= 0.92–0.94, age 3 = 0.91–0.96, age 5 = 0.81–0.92, age 7 = 0.90–0.93, age 9 = 0.89–0.94). BDE-153 was highly correlated with BDE-100 at each age (Spearman’s ρ: birth = 0.66, age 2= 0.89, age 3 = 0.90, age 5 = 0.82, age 7 = 0.67, age 9 = 0.65), however, correlations with BDEs-47 and 99 were lower (Spearman’s ρ: birth = 0.47–0.50, age 2= 0.71–0.75, age 3 = 0.73–0.76, age 5 = 0.55–0.58, age 7 = 0.49–0.51, age 9 = 0.43–0.46), Additional details describing PBDE detection frequencies and concentrations at each age, as well as specific correlations between congeners within age periods and within congeners over age periods in this cohort have been previously described 31 (see reference Supplemental Tables S1 and S2).
Figure 2. Distribution of plasma PBDE concentrations (ng/g lipid) by study visit (n=903 data points from 334 children).
PBDE concentrations with a value less than the limit of detection (<LOD) were replaced with the sample-specific mean concentration across 10 imputed datasets.
3.3. Associations between PBDEs and memory scores
3.3.1. Attention-Concentration Index (ACI)
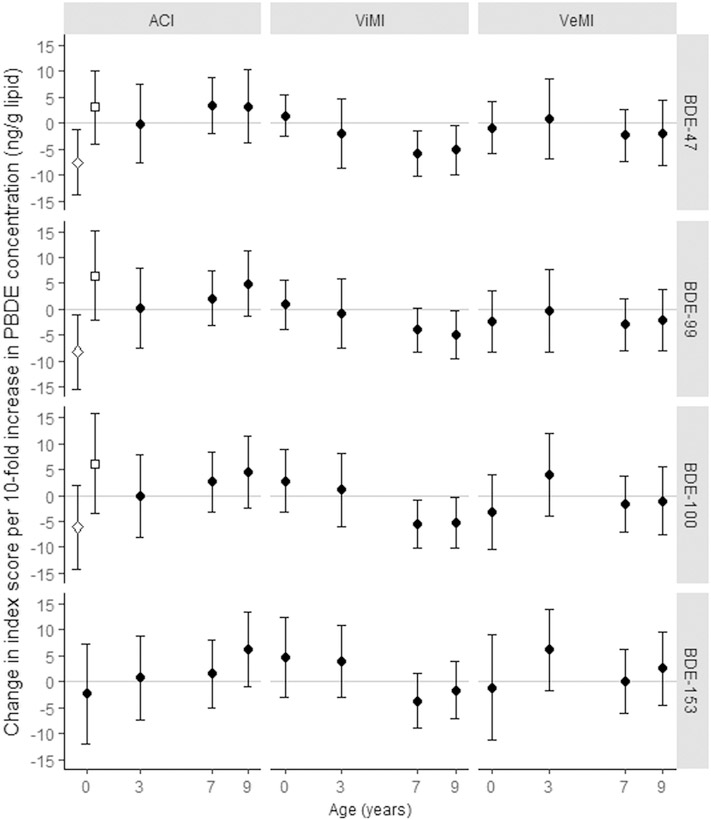
In models examining attention-concentration scores, we detected a significant interaction between sex and log10-transformed, continuous cord plasma BDE-47 (p-value for interaction: 0.02) and BDE-99 (p-value for interaction: 0.02) concentrations (ng/g lipid) measured in cord plasma, but not child venous plasma (modeled separately at ages 3, 7, or 9 years). In sex-stratified models, higher exposure was associated with lower scores among girls (βBDE-47: −7.55, 95% CI: −13.87, −1.24, βBDE-99: −8.14, 95% CI: −15.34, −0.93), but not boys (Figure 3). We did not observe statistically significant inverse associations between postnatal PBDE concentrations and attention-concentration scores, however, BDE-153 measured at the 9-year visit was marginally (p<0.10) associated with better performance (βBDE-153: 6.22, 95% CI: −1.10, 13.53). Likewise, we did not detect significant sex interactions or main effects when examining PBDE trajectories as the exposure variable (Figure 4).Notably, we were unable to investigate sex × ‘prenatal high’ trajectory interactions given the small proportion of children assigned to this group (BDE-47: 19%, BDE-99: 13%).
Figure 3. Point estimates (ß) and 95% CIs from adjusted models examining continuous, log10-transformed plasma PBDE concentrations (ng/g lipid) in relation to Attention Concentration Index (ACI), Visual Memory Index (ViMI), and Verbal Memory Index (VeMI) scores.
Hollow diamonds indicate girls, hollow squares indicate boys, solid circles indicate boys and girls. PBDEs measured at ages 2 and 5 years were not included due to small sample sizes at these ages. Sample size for ViMI and VeMI models: prenatal (n=208), age 3-years (n=70), age 7-years (n=158), age 9-years (n=128). Sample size for ACI models: prenatal (n=194), age 3-years (n=63), age 7-years (n=144), age 9-years (n=116).
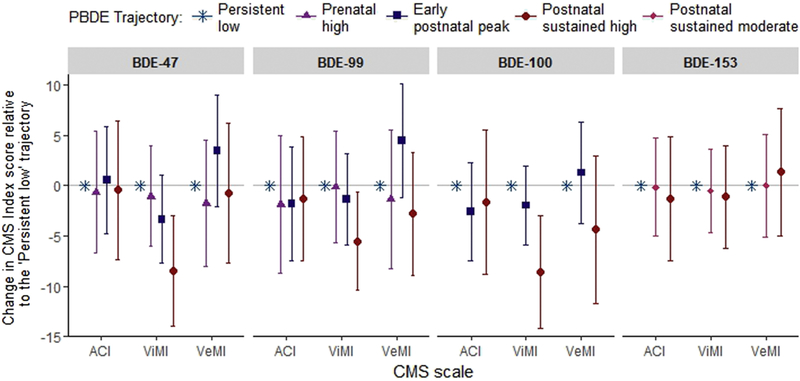
Figure 4. Point estimates (ß) and 95% CIs from adjusted models examining trajectories of plasma PBDE concentrations in relation to Attention Concentration Index (ACI), Visual Memory Index (ViMI), and Verbal Memory Index (VeMI) scores (n=212).
See Supplemental Material, Figure S2, which plots PBDE trajectories.
3.3.2. Visual Memory Index (ViMI)
We did not detect significant associations between cord or 3-year plasma BDE-47, −99, −100, or −153 concentrations and Visual Memory Index scores, however, we found BDEs-47, −99, and 100 measured at ages 7 and 9 years were inversely associated with this domain (i.e. age 9: βBDE-47: −5.18, 95% CI: −9.95, −0.42; βBDE-99: −4.90, 95% CI: −9.47, −0.33; βBDE-100: −5.24, 95% CI: −10.23, −0.25). We did not find a corresponding significant association with BDE-153 measured at 7 and 9 years, however, the direction of the observed association corresponded with the other three congeners (Figure 3). Consistent with finding from models treating PBDEs as a continuous variable, children assigned to the ‘sustained postnatal high’ BDE-47, −99 and −100 trajectoriesscored 5–8 standard score points lower on the Visual Memory Index compared to children assigned to the ‘persistent low’ trajectory (βBDE-47 = −8.44, 95% CI: −13.95, −2.93; βBDE-99 = −5.51, 95% CI: −10.41, −0.61; βBDE-100 = −8.57, 95% CI: −14.19, −2.96) (Figure 4). We did not find a corresponding association for BDE-153. We detected no statistically significant sex × PBDE interactions for visual memory.
3.3.3. Verbal Memory Index (VeMI)
In contrast to attention concentration and visual memory domains, we did not detect significant inverse associations between any PBDE congener, modeled as a continuous (Fig 3) or trajectory variable (Fig 4), and Verbal Memory Index scores. Likewise, we detected no statistically significant sex × PBDE interactions for verbal memory. In models examining PBDE trajectories in relation to verbal memory, we found positive, albeit not statistically significant, associations between the ‘early postnatal peak’ BDE-47 (β = 3.47, 95% CI: −2.02–8.97) and BDE-99 (β = 4.46, 95% CI: −1.18, 10.10) trajectories, however, these positive trends were not observed when examining age 3-year continuous BDE-47 (β = 0.85, 95% CI: −6.82, 8.52) or BDE-99 (β = −0.20, 95% CI: −8.12, 7.72) models.
3.4. Supplemental and sensitivity analyses
Results from models 1) excluding influential observations, or 2) subset on children with a high probability of correct trajectory assignment were marginally strengthened or unchanged compared to our main findings (see Supplemental Material, Table S5a-c), except that the positive association between continuous BDE-153 concentrations measured at age 9 years and ACI scores was attenuated when outlying values (n=3) were excluded (βBDE-153: 4.25, 95% CI: −2.96, 11.47).
BDE-47 was notcorrelated (all Spearman) with cord blood lead (r=0.02, p=0.78, n=192) or ΣPCB118, 153, 138–158, 180 (r=0.06, p=0.33, n=288), but was weakly correlated with chlorpyrifos (r=0.17, p=0.01, n=261).Correlations between each of these chemicals and BDEs-99, −100 and −153 were similar or smaller. Given the small, but significant correlation with chlorpyrifos, a pesticide that we previously demonstrated to be associated with working memory deficits among children enrolled in this cohort 29, we further investigated whether its inclusion as a covariate altered our findings. Despite the reduced sample size, inclusion of chlorpyrifos (>4.15 pg/g vs. ≤4.15 pg/g) did not substantially change the magnitude or direction of associations between continuous cord plasma BDE-47 or BDE-99 concentrations with Attention Concentration Index scores among girls (βBDE-47=−8.81, 95% CI: −15.28, −2.35; βBDE-99: −9.20, 95% CI: −17.02, −1.38, n=90), suggesting that the observed associations are not driven by concurrent exposure to chlorpyrifos.
4. Discussion
We detected significant inverse associations between cord plasma PBDE concentrations (BDE-47 and BDE-99) and performance on the CMS Attention-Concentration Index, an indicator of auditory working memory, among girls but not boys. We did not detect corresponding associations between exposure during childhood, suggesting that working memory may be most sensitive to disruption by PBDEs during gestation, when rapid anatomical and functional development 38, including normal differentiation of sexually dimorphic regions 39 occurs. Animal research has demonstrated associations between gestational exposure to PBDEs and changes in sexually dimorphic brain regions 40, with effects on sexual maturation that persist into adolescence 41In contrast, while Eskenazi et al. found prenatal exposure to PBDEs was associated with decrements on the WISC-IV Working Memory Index among 7-year old Mexican-American children enrolled in a California-based cohort (β per 10-fold increase in ΣBDEs-47, −99, −100, −153 = −2.4, 95% CI: −7.2, 2.3, n= 231), they did not detect a significant sex interaction at the level of p<0.10 9. This inconsistency may relate to differences in the study sample (geography, age at assessment), exposure metrics examined (ΣPBDE versus ‘prenatal high’ trajectory), tests administered (WISC-IV versus CMS) or other variables that differ between cohorts. Importantly, the sex-specific associations we detected were imprecisely estimated given the relatively small sample size of PBDE trajectories in sex-stratified models.
We found visual memory, which develops rapidly between toddler years and adolescence 43, may be impaired by sustained exposure to PBDEs during childhood. Unlike verbal memory, few human studies have investigated PBDEs in relation to visual domains, however, our findings are consistent with animal research demonstrating adult male mice exposed to PBDEs during the neonatal period performed worse on tests of spatial learning and memory (i.e. swim maze) compared to vehicle-exposed controls44,45. In contrast, the only other epidemiologic study to examine visual domains found that PBDE concentrations measured prenatally and at several ages postnatally (1–8 years) were generally associated with improved visual spatial abilities (memory retention and visual learning) at age 8-years as assessed by performance on the Virtual Morris Water Maze 11. Given the virtual nature of this test, the authors suggest their findings may reflect uncontrolled confounding by video game proficiency, which may be associated with higher PBDE exposure due to increased time spent indoors. Notably, the authors did not adjust for breastfeeding, which may have masked potential inverse associations between PBDEs and scores on visual outcomes.
While the rapid brain development that occurs during the prenatal period enhances fetal vulnerability to exogenous insults, exposure to environmental chemicals is reduced, although not eliminated, by the placental barrier. In contrast, during postnatal life children directly interact with their environment, leading to increased exposure to environmental chemicals. Notably, young children may be at increased risk for exposure to indoor contaminants, such as PBDEs, which often have hand-to-mouth exposure pathways. Indeed, we found that plasma concentrations were, on average, twice as high during early childhood than at birth. Despite the enhanced vulnerability often ascribed to the rapidly developing fetal brain, the significant inverse associations we detected between postnatal exposure and visual memory may reflect the higher exposure levels characteristic of childhood. Additonally, our finding of inverse associations with visual memory among children in the ‘sustained postnatal high’ trajectory, but not the ‘early postnatal peak’ trajectory, suggests duration of exposure may be an important factor in PBDE toxicity.
Several previous epidemiological studies have detected inverse or null associations between PBDE concentrations measured during prenatal or childhood periods and performance on tests of verbal comprehension 9,10,16,46. In contrast, we found children assigned to the ‘early postnatal peak’ trajectory generally performed better on the Verbal Memory Index. Notably, we did not observe corresponding positive associations when examining associations with continuous PBDE concentrations measured at age 3-years. We previously found that children assigned to the ‘early postnatal peak’ trajectory were significantly more likely to be breastfed for 12 weeks or more, suggesting that the positive associations we observed between this trajectory and verbal memory may relate to the positive effects of breastmilk or breastfeeding behavior on cognitive development37. The potential positive influence of breastfeeding is further supported by Adgent et al, who found that verbal domains (expressive and receptive language scores) assessed by the Mullen Scales of Early Learning were generally positively associated with PBDE concentrations measured in breast milk 48 Alternatively, the discrepancy between our finding and those of previous studies may relate to differences in the specific verbal domains assessed (i.e. comprehension versus memory) or variation in the age at assessment (approximately 4–7 years versus approximately 11 years).
As with all cohort studies, some participants were lost to follow-up between birth and neurodevelopmental testing during early adolescence, which may have introduced selection bias if attrition was related to PBDE exposure and/or CMS scores. While it is not possible to compare the CMS scores among those who were and were not lost to follow-up, we compared PBDE exposure between these two groups and noted no differences, indicating that selection bias related to differential exposure is unlikely. Additionally, the relatively small sample of children with both PBDE measures and CMS scores limited our ability to examine sex-specific effects in models examining the ‘prenatal high’ exposure trajectory, however, the high resolution of repeated samples enabled us to examine memory outcomes in relation to changes in PBDE plasma concentrations over time. We additionally collected extensive information on sociodemographic and lifestyle factors, enabling us to explore the impact of many potential confounders and effect measure modifiers. Unfortunately, while we were able to examine correlations between PBDE concentrations and several other developmental neurotoxicants, given the limited number of children with overlapping exposure measures, we were insufficiently powered to investigate interactions between co-exposures.
5. Conclusion
We found that children with low prenatal, but high postnatal plasma PBDE concentrations throughout childhood performed significantly worse on tests of visual memory during early adolescence compared to children with persistent low plasma PBDE concentrations. In addition, girls with high cord plasma PBDE concentrations performed significantly worse on tests of auditory working memory during early adolescence. Taken together, these results suggest that the developing brain remains vulnerable to insult by PBDEs from gestation through childhood and that the effects of PBDEs on memory, and potentially other neurocognitive domains, may vary by the duration and developmental period(s) during which exposure occurs. Our findings contribute to a substantial body of evidence 5 demonstrating the developmental neurotoxicity of PBDEs and underscore the need to reduce exposure among pregnant women and children.
Supplementary Material
Highlights.
We examined prenatal (cord) and childhood (ages 2, 3, 5, 7 and 9 years) plasma PBDE concentrations in relation to memory outcomes assessed between the ages of 9 and 14 years.
We detected significant inverse associations between cord plasma PBDE concentrations (BDE-47 and BDE-99) and performance on the CMS Attention-Concentration Index, an indicator of auditory working memory, among girls but not boys.
Children with sustained high concentrations of BDEs-47, 99 or 100 across childhood scored approximately 5–8 standard score points lower on tests of visual memory.
Exposure to PBDEs during both prenatal and postnatal periods may disrupt memory domains in early adolescence.
Acknowledgements:
This research was supported by: NIH R01 ES021806 and NIH R01DA027100. During preparation of this manuscript, WJC was supported by NIH T32 ES023772, NIH T32 ES007322 and EPA FP-91779001.
Footnotes
Competing financial interests: The authors declare they have no actual or potential competing financial interests.
Disclaimer: The findings and conclusions in this publication are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services. This publication was developed under STAR Fellowship Assistance Agreement no. FP-91779001 awarded by the U.S. Environmental Protection Agency (EPA). It has not been formally reviewed by the EPA. The views expressed in this publication are solely those of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbasi G, Buser AM, Soehl A, Murray MW, Diamond ML. Stocks and flows of PBDEs in products from use to waste in the U.S. and Canada from 1970 to 2020. Environmental science & technology. 2015;49(3):1521–1528. [DOI] [PubMed] [Google Scholar]
- 2.EPA. An Exposure Assessment of Polybrominated Diphenyl Ethers. National Center for Environmental Assessment. Washington, DC: Environmental Protection Agency; 2010. [Google Scholar]
- 3.Leonetti C, Butt CM, Hoffman K, Miranda ML, Stapleton HM. Concentrations of polybrominated diphenyl ethers (PBDEs) and 2,4,6-tribromophenol in human placental tissues. Environment international. 2016;88:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrizo D, Grimalt JO, Ribas-Fito N, Sunyer J, Torrent M. Influence of breastfeeding in the accumulation of polybromodiphenyl ethers during the first years of child growth. Environmental science & technology. 2007;41(14):4907–4912. [DOI] [PubMed] [Google Scholar]
- 5.Lam J, Lanphear BP, Bellinger DC, et al. Developmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environmental health perspectives. 2017;125(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth N, Wilks MF. Neurodevelopmental and neurobehavioural effects of polybrominated and perfluorinated chemicals: a systematic review of the epidemiological literature using a quality assessment scheme. Toxicology letters. 2014;230(2):271–281. [DOI] [PubMed] [Google Scholar]
- 7.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fromme H, Becher G, Hilger B, Volkel W. Brominated flame retardants - Exposure and risk assessment for the general population. Int J Hyg Environ Health. 2016;219(1):1–23. [DOI] [PubMed] [Google Scholar]
- 9.Eskenazi B, Chevrier J, Rauch SA, et al. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environmental health perspectives. 2013;121(2):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gascon M, Vrijheid M, Martinez D, et al. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environment international. 2011;37(3):605–611. [DOI] [PubMed] [Google Scholar]
- 11.Vuong AM, Braun JM, Yolton K, et al. Prenatal and postnatal polybrominated diphenyl ether exposure and visual spatial abilities in children. Environmental research. 2017;153:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driscoll LL, Gibson AM, Hieb A. Chronic postnatal DE-71 exposure: effects on learning, attention and thyroxine levels. Neurotoxicology and teratology. 2009;31(2):76–84. [DOI] [PubMed] [Google Scholar]
- 13.Dufault C, Poles G, Driscoll LL. Brief postnatal PBDE exposure alters learning and the cholinergic modulation of attention in rats. Toxicological sciences : an official journal of the Society of Toxicology. 2005;88(1):172–180. [DOI] [PubMed] [Google Scholar]
- 14.Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol Appl Pharmacol. 2003;192(2):95–106. [DOI] [PubMed] [Google Scholar]
- 15.Viberg H, Johansson N, Fredriksson A, Eriksson J, Marsh G, Eriksson P. Neonatal exposure to higher brominated diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions of adult mice. Toxicological sciences : an official journal of the Society of Toxicology. 2006;92(1):211–218. [DOI] [PubMed] [Google Scholar]
- 16.Herbstman JB, Sjodin A, Kurzon M, et al. Prenatal exposure to PBDEs and neurodevelopment. Environmental health perspectives. 2010;118(5):712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perera FP, Rauh V, Whyatt RM, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environmental health perspectives. 2006;114(8):1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicology and teratology. 2004;26(3):373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dohrenwend BP, Dohrenwend BS, Warheit GJ, et al. Stress in the community: a report to the President’s Commission on the Accident at Three Mile Island. Annals of the New York Academy of Sciences. 1981;365:159–174. [DOI] [PubMed] [Google Scholar]
- 20.Mayer S, Jencks C. Poverty and the distribition of material hardship. J Hum Resour. 1988:88–112. [Google Scholar]
- 21.Brown L, Sherbenou R, Johnsen S. Test of Nonverbal Intelligence, Third Edition Examiner’s Manual; Austin, TX: Pro-Ed; 1997. [Google Scholar]
- 22.Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment: Administration Manual. Tempe, AZ: Family & Human Dynamics Resarch Institute, Arizona University; 2003. [Google Scholar]
- 23.Cohen M Children’s Memory Scale (CMS) Manual. San Antonio, Texas: Pearson; 1997. [Google Scholar]
- 24.Reynolds C, Richond B. Revised Children’s Manifest Anxiety Scale Manual. Los Angeles: Western Psychological Services; 1985. [Google Scholar]
- 25.Cowell WJ, Sjodin A, Jones R, Wang Y, Wang S, Herbstman JB. Determinants of prenatal exposure to polybrominated diphenyl ethers (PBDEs) among urban, minority infants born between 1998–2006. Environmental Pollution. 2018;223(Feb):774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones R, Edenfield E, Anderson S, Zhang Y, Sjodin A. Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum. Organohalogen Comp. 2012;74:97–98. [Google Scholar]
- 27.Sjodin A, Jones RS, Lapeza CR, Focant JF, McGahee EE, 3rd, Patterson DG, Jr. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Analytical chemistry. 2004;76(7):1921–1927. [DOI] [PubMed] [Google Scholar]
- 28.Phillips DL, Pirkle JL, Burse VW, Bernert JT Jr., Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18(4):495–500. [DOI] [PubMed] [Google Scholar]
- 29.Rauh VA, Garfinkel R, Perera FP, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118(6):e1845–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons; 1987. [Google Scholar]
- 31.Cowell WJ, Sjodin A, Jones R, Wang Y, Wang S, Herbstman JB. Temporal trends and developmental patterns of plasma polybrominated diphenyl ether concentrations over a 15-year period between 1998 and 2013 J Expo Sci Environ Epidemiol. 2018;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagin D Group-based modeling of development. Cambridge, Massachusetts,: Harvard University Press; 2005. [Google Scholar]
- 33.Nagin DS. Group-based trajectory modeling: an overview. Ann Nutr Metab. 2014;65(2–3):205–210. [DOI] [PubMed] [Google Scholar]
- 34.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745. [DOI] [PubMed] [Google Scholar]
- 35.Jones B, Nagin D, KA R. SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methodology Research. 2001;29:374–393. [Google Scholar]
- 36.Roberts RE, Vernon SW. Usefulness of the PERI demoralization scale screen for psychiatric disorder in a community sample. Psychiatry Res. 1981;5(2):183–193. [DOI] [PubMed] [Google Scholar]
- 37.Fergusson DM, Beautrais AL, Silva PA. Breast-feeding and cognitive development in the first seven years of life. Soc Sci Med. 1982;16(19):1705–1708. [DOI] [PubMed] [Google Scholar]
- 38.Antonelli MC, Pallares ME, Ceccatelli S, Spulber S. Long-term consequences of prenatal stress and neurotoxicants exposure on neurodevelopment. Prog Neurobiol. 2017;155:21–35. [DOI] [PubMed] [Google Scholar]
- 39.Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16(4):248–266. [DOI] [PubMed] [Google Scholar]
- 40.Faass O, Ceccatelli R, Schlumpf M, Lichtensteiger W. Developmental effects of perinatal exposure to PBDE and PCB on gene expression in sexually dimorphic rat brain regions and female sexual behavior. Gen Comp Endocrinol. 2013;188:232–241. [DOI] [PubMed] [Google Scholar]
- 41.Lilienthal H, Hack A, Roth-Harer A, Grande SW, Talsness CE. Effects of developmental exposure to 2,2,4,4,5-pentabromodiphenyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environmental health perspectives. 2006;114(2):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ribas-Fito N, Torrent M, Carrizo D, et al. In utero exposure to background concentrations of DDT and cognitive functioning among preschoolers. American journal of epidemiology. 2006;164(10):955–962. [DOI] [PubMed] [Google Scholar]
- 43.Bauer PJ, Fivush R. The Wiley handbook on the development of children’s memory. John Wiley & Sons, Ltd.; 2014. [Google Scholar]
- 44.Eriksson P, Jakobsson E, Fredriksson A. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environmental health perspectives. 2001;109(9):903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He P, Wang A, Niu Q, Guo L, Xia T, Chen X. Toxic effect of PBDE-47 on thyroid development, learning, and memory, and the interaction between PBDE-47 and PCB153 that enhances toxicity in rats. Toxicol Ind Health. 2011;27(3):279–288. [DOI] [PubMed] [Google Scholar]
- 46.Sagiv SK, Kogut K, Gaspar FW, et al. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12 years of age. Neurotoxicology and teratology. 2015;52(Pt B):151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins,; 2008: http://www.columbia.edu/cgi-bin/cul/resolve?clio8363805. [Google Scholar]
- 48.Adgent MA, Hoffman K, Goldman BD, Sjodin A, Daniels JL. Brominated flame retardants in breast milk and behavioural and cognitive development at 36 months. Paediatric and perinatal epidemiology. 2014;28(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walfisch A, Sermer C, Cressman A, Koren G. Breast milk and cognitive development--the role of confounders: a systematic review. BMJ Open. 2013;3(8):e003259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.APA. American Academy of Pediatrics policy statement. Breastfeeding and the use of human milk. Pediatrics. 1997;100(6):5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.