Abstract
Background and Aims:
Aprepitant, a Neurokinin-1 receptor antagonist, has been evaluated in abdominal and neurosurgeries, but its effect is less clear in breast and thyroid surgeries, which are also known to be high risk for post-operative nausea and vomiting (PONV). This study was done to compare the antiemetic efficacy of ondansetron and aprepitant in women undergoing mastectomy and thyroidectomy.
Methods:
One hundred and twenty-five ASA I and II, female patients, aged between 18 and 65 years were randomly assigned into Group I (ondansetron group, n = 62) or Group II (aprepitant group, n = 63), by computer-generated random sequencing. Per protocol analysis was done to assess the incidence and severity of PONV, use of rescue antiemetics, and patient satisfaction with PONV control between the two groups, till 24 h post-surgery.
Results:
In the immediate postoperative period, 79.7% of patients in Group I and 85.2% in Group II were free of emesis (P value: 0.49). In Group I, the first episode of vomiting occurred within a median duration 90 min (IQR 2575: 45-147) postoperatively, whereas the median duration in Group II was 160 min (IQR 25-75: 26-490), with request for rescue antiemetic at 60 min in Group I (IQR 25-75: 27-360) and 147 min in Group II (IQR 25-75: 11-457).
Conclusion:
A single dose of oral aprepitant has comparable effects to injection ondansetron administered eighth hourly in preventing PONV, the severity of nausea, number of rescue antiemetics, and the time to first emetic episode in the 24-h postoperative period.
CTRI Reg No:
REF/2017/06/014637.
Key words: Mastectomy, NK-1 receptor antagonist, post-operative nausea and vomiting, thyroidectomy
INTRODUCTION
Postoperative nausea and vomiting (PONV) is the second commonest reported side effect of general anaesthesia. It is a complex problem, caused by interplay of patient factors, anaesthetic techniques, and surgical factors. The risk of PONV is higher in certain patient groups and after certain types of surgeries such as gynaecological surgeries, endocrine surgeries, and ophthalmic surgeries.[1] Despite advances in anaesthetic practices, the incidence of PONV continues to be as high as 20-30%.[2] The most favoured drug for the prophylaxis of PONV in anaesthetic practice is the prototypical 5HT3 receptor antagonist, ondansetron.[3] Despite its widespread use, PONV is still very prevalent, especially after mastectomy or thyroidectomy.[4] PONV is often cited as the most upsetting concern in the postoperative period, sometimes even more distressing than pain. The growing awareness to improve patient satisfaction has prompted anaesthesiologists to strive for a postoperative period free of nausea and vomiting.
Among the many signalling mechanisms involved in PONV is the tachykinin (NK-1, NK-2, substance P) system. The Neurokinin-1 (NK1) receptors in vagal afferent and central nervous system vomiting reflex pathway are activated by Substance P, leading to nausea and vomiting. Aprepitant is an NK1 receptor/substanceP antagonist that was developed as a therapy for chemotherapy and opioid-induced emesis. It is of added interest because of its mechanism of action via substance P, which outside of nausea and vomiting is also involved in pain, anxiety, and depression, thereby adding to its potential applications. It has shown to be more effective than ondansetron after open abdominal surgical procedures and craniotomies.[5] However, there is not much literature on its efficacy in thyroid and breast surgeries, which are among the surgeries known to be high risk for PONV. In this project, we aimed to study the antiemetic efficacy of aprepitant as compared against ondansetron, in women undergoing mastectomy or thyroidectomy.
METHODS
This study was a double-blinded, randomised clinical control trial. It was carried out in the department of Anesthesiology of a tertiary care hospital. Approval by the ethics and research committee of our institution was obtained before the study was carried out.
All female American Society of Anesthesiologists physical status 1 or 2 patients, between the age of 18 and 65 years, scheduled for thyroid or breast surgeries were recruited for the study. Exclusion criteria were any patient who was already on treatment with anti-emetics, steroid medication, or any other drug known to cause emesis currently or in the immediate past, patients with known hypersensitivity to ondansetron or aprepitant, pregnant or nursing mothers.
All patients were included in the study after obtaining informed consent.
Computer-generated random sequencing was done by an independent biostatistician for the purpose of randomisation. This was forwarded directly to a pharmacist who was also not involved with the study. All drugs for administration, including matching placebos for both arms, were prepared by the pharmacist in the pharmacy special preparation lab in our institution. Sealed envelopes with randomisation codes were made for purpose of patient allocation into groups.
On the day of surgery, patients were randomised into two groups by opening sealed envelopes with randomisation codes inside [Table 1].
Table 1.
Randomisation groups
| Group I | Group II |
|---|---|
| Cap. Placebo 1 h preoperatively | Cap. Aprepitant 1 h preoperatively |
| Inj. Ondansetron 4 ml (8 mg), 1st dose at the end of surgery, and repeated 8th hourly for 24 h (total 3 doses) | Inj. Placebo 4 ml, 1st dose at end of surgery and repeated 8th hourly for 24 h (total 3 doses) |
Anaesthesia technique included optimal premedication and standard anaesthetic agents. None of the patients received any form of regional anaesthesia. Intraoperative analgesia was provided using paracetamol and fentanyl upto 5 mcg/kg. Postoperatively, patients were monitored for an hour in post anaesthesia care unit, before shifting to the ward. Prophylactic antiemetic beyond the scope of study protocol was prohibited for 24 h after surgery. However, rescue therapy was offered on patient request, presence of persistent nausea, or emetic episode. The type of rescue medication was left to the discretion of the postoperative care provider. The duration of anaesthesia and timing of all the emetic episodes and rescue medications given postoperatively were recorded. Data was collected by an independent investigator unaware of the patient's randomisation.
The primary outcomes of the study were the incidence of postoperative vomiting. Secondary outcomes were the number of emetic episodes, severity of postoperative nausea, timing of the first vomiting episode, and use of rescue antiemetics, and patient satisfaction rating. Using a 11-point verbal rating scale, patients graded nausea from 0 (“no nausea”) to 10 (“nausea as bad as it could be”) at 0-2, 2-12 and 12-24 h after the operation.[6] Verbal rating score of postoperative nausea between 1 and 3 was rated mild, between 4 and 7 as moderate, and more that 8 was considered severe on a scale of 0 to 10. Nausea was defined as an uncomfortable feeling that leads to a tendency to vomit. Retching was defined as an effort to vomit which is not under voluntary control and that does not cause expulsion of stomach contents. Vomiting was defined as an expulsion of stomach contents. An emetic episode was described as a single retch or vomit or any number of continuous vomits or retches. The time of the first emetic episode and the request for rescue antiemetic was noted in the postoperative period. At 24 h, patients were asked about their satisfaction with the control of nausea and vomiting using a 5 point scale ranging from 1--very dissatisfied, to 5--very satisfied.[7]
The required sample size to show a difference in the proportion of post operative vomiting between aprepitant and ondansetron was found to be 60 in each arm with 80% power and at 5% level of significance with an anticipated post operative nausea of 14% and 36% in the aprepitant and ondansetron respectively. Normally distributed variables are presented with mean (SD) and skewed variables with median (IQR). The categorical variables are presented with number and percentage. Chi-square test, Fisher's exact test, and Yate's continuity correction was used to find the association between categorical variables and two groups. The other parameters like the duration of anaesthesia and the timing of the first vomiting episode were compared between the two groups using nonparametric Mann-Whitney test. All tests was two sided at α = 0.05 level of significance. All statistical analysis was done using SAS package (SAS® Institute Inc., USA, version 9.2).
RESULTS
A total of 125 patients were randomised for the study into two groups, with 62 patients in Group 1 and 63 patients in Group 2. Of these, 3 patients were excluded in Group 1, and 2 in Group 2, respectively, after randomisation, since they required unanticipated intensive care or high dependency unit admissions or required intraoperative steroids which would influence the assessment of efficacy of the antiemetic drugs under study [Figure 1].
Figure 1.
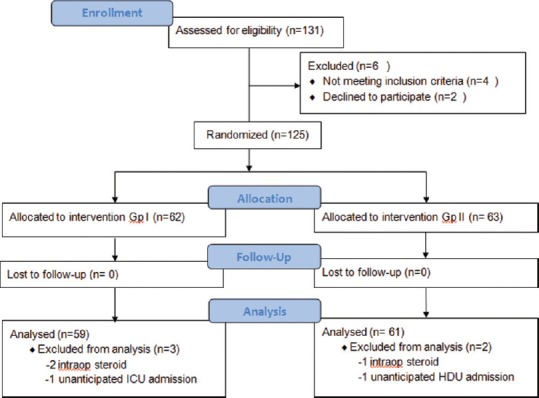
CONSORT diagram
Participants in both groups were matched in terms of demographic details [Table 2]. The distribution of diagnoses in both groups were similar, carcinoma breast being the commonest followed by various thyroid diseases. The commonest surgery was modified radical mastectomy, 72 cases followed by total thyroidectomy, 38 cases, and these were evenly distributed in both groups. All the 120 patients included in the study had an Apfel's simplified risk score of 2-3.
Table 2.
Summary of demographic and other baseline characteristics
| Demographics | Group I n (%) | Group II n (%) |
|---|---|---|
| Age (yrs) [Mean (SD)] | 42.5 (11.5) | 45.4 (11.1) |
| BMI (kg/m2) [Mean (SD)] | 25.6 (4.8) | 25.6 (4.7) |
| ASA | ||
| I | 33 (55.9) | 25 (41.0) |
| II | 26 (44.0) | 36 (59.0) |
| Risk factors | ||
| Female | 59 (100.0) | 61 (100.0) |
| Non-smoking | 59 (100.0) | 61 (100.0) |
| PONV/MS history | 2 (3.4) | 2 (3.3) |
| Opiods post-op | 59 (100.0) | 61 (100.0) |
| Surgery | ||
| Simple Mastectomy | 37 (62.7) | 35 (57.4) |
| MR Mastectomy | 16 (27.1) | 22 (36.2) |
| Total thyroidectomy | 5 (8.5) | 4 (6.6) |
| Hemithyroidectomy | 1 (1.7) | 0 |
| Anaesthesia time (min) [median (IQR)] | 120 (90-140) | 110 (100-142) |
BMI – Body Mass Index; PONV – Post-operative Nausea Vomiting; MS – Motion Sickness; MR – Modified Radical
The overall incidence of vomiting in our study was 30%. 68.5% patients were emesis free in Group I (ondansetron group), whereas 69.5% of patients were emesis free in Group II (aprepitant group), over a period of 24 h postsurgery.
In the immediate postoperative period, i.e., within the first 2 h, 79.7% of patients (n = 47) in Group I (Ondansetron group), whereas 85.2% (n = 52) in Group II (Aprepitant group) were free of emesis. Although a smaller percent, 18.7% and 14.7%, respectively, in Group I and Group II, had one emetic episode, no patient in aprepitant group had more than two episodes of vomiting. However, a P value of 0.49 in this group indicates that both ondansetron and aprepitant are equally effective in the immediate postoperative period. In the 2-12 h period, both the groups displayed similar statistics; 86.4% (n = 51) in the ondansetron group and 85.2% (n = 52) in the aprepitant group were emesis free. When comparing the number of vomiting episodes, both groups had similar number of patients; Group I had 6 patients with one episode of vomiting, whereas Group II had 7, and both groups had 2 patients who vomited more than twice. A P value of 0.97 implies that this result is not significant. After 12 h, the ondansetron group did better. Although all patients in Group I were free of vomiting, 3 patients in the aprepitant group had 1-2 episodes of vomiting. The P value of 0.23 is not significant, indicating that both the drugs were equally effective in the first postoperative day [Table 3].
Table 3.
Emetic episodes and nausea VRS in post-operative period
| Post- op h | 0-2 (h) |
2-12 (h) |
12-24 (h) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group I n (%) | Group II n (%) | P | Group I n (%) | Group II n (%) | P | Group I n (%) | Group II n (%) | P | |||
| Emetic episodes | 0 | 47 (79.7%) | 52 (85.2%) | 0.49* | 51 (86.4%) | 52 (85.2%) | 0.97# | 59 (100%) | 58 (95.0%) | 0.23* | |
| 1-2 | 11 (18.7%) | 9 (14.7%) | 6 (10.1%) | 7 (11.4%) | 0 | 3 (4.9%) | |||||
| >2 | 1 (1.6%) | 0 | 2 (3.3%) | 2 (3.2%) | 0 | 0 | |||||
| Nausea VRS | 0 | 47 (79.6%) | 51 (83.6%) | 0.84# | 48 (81.3%) | 46 (75.4%) | 0.73# | 57 (96.6%) | 57 (93.4%) | 0.62# | |
| 1-2 | 2 (3.3%) | 2 (3.2%) | 3 (5.0%) | 4 (6.5%) | 1 (1.6%) | 1 (1.6%) | |||||
| >2 | 18 (30.5%) | 8 (13.1%) | 8 (13.5%) | 11 (18.0%) | 1 (1.6%) | 3 (4.9%) | |||||
VRS – Verbal Rating Score, *P is obtained from Yate’s continuity correction; #P is obtained from Fisher’s exact test
In the first 2 h after surgery, both the groups had similar verbal rating score for nausea. 79.7% (n = 47) in ondansetron group and 83.6% (n = 51) in aprepitant group were free of nausea. Although 2 patients in both the groups had a lower VRS score (of 1-2), 10 patients in Group I (54.5%) had a score of greater than 2, as opposed to 8 (45.5%) in Group II. In 2-12 h of postoperative period, 81.4% in ondansetron group and 75.4% in aprepitant group did not experience nausea, and a similar number of patients in both the groups (n = 3 in Group I and n = 4 in Group II) had nausea score of 1-2. After 12 h, the ondansetron group did better. Most patients in both groups were free of vomiting and 3 patients in the aprepitant group had 1-2 episodes of vomiting. The P value for both groups were not significant for nausea in the first postoperative day, with the ondansetron group doing slightly better than the aprepitant group.
Of the 120 patients, 21 had mild nausea and 23 had moderate nausea indicating that 38.3% of total number of cases experienced mild to moderate nausea. In Group I, the first episode of vomiting occurred within a median duration 90 min (IQR 25-75:45-147) postoperatively, whereas the median duration in Group II was 160 min (IQR 25-75: 26-490). The average time to ask for rescue antiemetic was 60 min in Group I (IQR 25-75: 27-360) and 147 min in Group II (IQR 25-75: 11-457). Although these values showed delayed onset of nausea and vomiting in patients who received aprepitant, the P value of 0.46 reveals that both drugs were comparable in their antiemetic effects [Table 4].
Table 4.
Analysis of association between group and secondary outcomes
| Variables | Group I n=59, (%) | Group II n=61, (%) | P |
|---|---|---|---|
| Peak nausea score | |||
| Mild | 7 (11.8%) | 14 (22.9%) | 0.46 |
| Moderate | 13 (22.0%) | 10 (16.3%) | |
| Request for first rescue anti-emetic (min) Median (IQR; 25-75) | 60 (27-360 ) | 147 (11-457) | 0.80 |
| Time of first emetic episode (min) Median (IQR ; 25-75) | 90 (45-147) | 160 (26-490) | 0.20 |
| Satisfaction rating¶ | |||
| 1 | 1 (1.6%) | 1 (1.6%) | 0.67 |
| 2 | 3 (5.0%) | 2 (3.2%) | |
| 3 | 4 (6.7%) | 4 (6.5%) | |
| 4 | 9 (15.25%) | 16 (26.2%) | |
| 5 | 42 (71.1%) | 38 (62.2%) | |
| IQR (25th percentile - 75th percentile) |
¶Satisfaction rating: 5 – very satisfied, 4 – Somewhat satisfied, 3 – neither satisfied or dissatisfied, 2 – somewhat dissatisfied, 1 – very dissatisfied
One-hundred and five patients (87.5%) inclusive of both groups were satisfied with the intervention for PONV. 71.2% of patients (42 out of 59) in Group I were very satisfied (satisfaction rating: 5) with their outcome, whereas 77% of patients (47 out of 61) in Group II said they were very satisfied. An equal number of 4 patients (3.3%) in both groups were noncommittal in their opinion. A small number comprising of 7 patients (5.8%) were dissatisfied with the PONV management. Both the groups displayed good PONV management; hence, the P value of 0.676 is insignificant.
DISCUSSION
This double-blinded randomised clinical control trial was designed to assess efficacy of the NK1 antagonist, aprepitant, in preventing PONV in the subset of patients undergoing breast and thyroid surgeries. We chose to compare aprepitant with ondansetron, a 5HT3 antagonist, as the latter was the standard antiemetic in our practice.[8] In order to make the “pharmacological” drugs comparable, aprepitant 40 mg was given as a single oral dose and injection ondansetron 8 mg was given in 3 doses, 8 h apart, on the first postoperative day.[9] Both these drugs have dissimilar half-lives; aprepitant has an elimination half-life of 9-12 h and hence is administered once daily as compared with 5-7 hourly administration for ondansetron.[10]
In this study, the overall incidence of PONV was around 30%, which is comparable to the existing literature on the incidence of PONV.[11,12] All the 120 patients included in the study had an Apfel's simplified risk score of 2-3, indicating high risk score and also received volatile anaesthetics, increasing the risk of PONV incidence to 60-80%. Earlier studies also show similar incidences in high risk patients.[13,14,15,16,17] However, our patient subset exhibited lower incidences of PONV, probably because of the anaesthesia protocol in place in our institution.
This study found that the antiemetic efficacy of ondansetron and aprepitant was comparable in preventing PONV in patients undergoing thyroidectomy and mastectomy. We found both ondansetron and aprepitant were equally efficacious in preventing emetic episodes, reducing the incidence of nausea and delaying the time to request of a rescue antiemetic. Although not statistically significant, the aprepitant group had a higher incidence of vomiting in the 12-24 h period. However, this group took longer to develop the first episode of vomiting and also to receive the first dose of rescue antiemetic, when compared with ondansetron group. Although the ondansetron group had less vomiting after 12 h, there was a higher incidence of nausea (both being statistically insignificant). So, overall there was no statistically significant difference in the incidence of PONV in both the groups.
Our findings differ from earlier studies. Diemunsch et al. studied 922 individuals who had open abdominal surgeries and found that one oral dose of aprepitant either 40 mg or 125 mg were more effective than a single dose of ondansetron 4 mg I.V in preventing vomiting at 24 and 48 h after surgery.[5] This was supported by Gan et al. who also studied similar doses of both the drugs and concluded that aprepitant was better than ondansetron in preventing vomiting in the first 24-48 h.[6] However, we agree with both the authors above who concluded that ondansetron was not inferior to aprepitant in preventing nausea, timing of first vomiting episode, and in the use of rescue antiemetics. Aprepitant has been studied in other groups of patients and with other drug combinations.[18] The efficacy of aprepitant has also been studied in neurosurgical patients, along with dexamethasone and promethasone.[19] However, our study protocol did not include either of these drugs as we believed them to have an impact on the results. Kakuta et al. studied higher doses of aprepitant (80 mg) and observed a lesser incidence of PONV.[20] A recent study by Jung et al. on postoperative analgesia with fentanyl-based PCA after gynaecological laparoscopy, quoted that oral aprepitant 80 mg was more efficacious in lowering the incidence of PONV in the first 48 h after surgery.[21] This study also showed a trend towards a more complete response in patients who received aprepitant 125 mg group, although the difference was not statistically significant. A meta-analysis of the available literature shows that aprepitant has been beneficial in preventing acute and more so, delayed emesis caused by chemotherapeutic agents, in doses of 125 mg on the first day and 80 mg each for the next 2 days.[10] The role of doses higher than 40 mg in PONV management should be considered especially in high risk patients. We were limited to the use of oral aprepitant 40 mg as it is the approved dose by the Drug Controller General of India for PONV.
Neurotransmitter receptor systems involved in transmission of impulses causing nausea and vomiting include cholinergic (muscarinic), dopaminergic (D2), serotonergic (5-HT3), histaminergic (H1), and NK1 systems. Hence, targeting one particular receptor may not confer complete protection against PONV. NK-1RAs may be combined with antiemetics from other classes for optimal efficacy.[22] Thus inclusion of aprepitant to multimodal PONV therapy may have positive attributes of long half-life, lack of sedation, innocuous effects on QTc prolongation, and effective prevention of PONV. Although aprepitant is more expensive than the commonly used alternatives, the traditional antiemetics are limited in their antiemetic efficacy and by their side effects. On the other hand, the routine use of aprepitant to prevent PONV may be expensive.[23] Therefore, it might be worthwhile to limit its use to patients at high risk of PONV such as high risk surgeries, risk of severe complications of PONV, hyper-reaction to opioids or anaesthetics, unsuccessful treatment with low cost antiemetics, or a past history of severe PONV despite multimodal antiemetic therapy.
CONCLUSION
In conclusion, a single dose of oral aprepitant has comparable effects to injection ondansetron administered eighth hourly in preventing PONV, the severity of nausea, number of rescue antiemetics, and the time to first emetic episode in the 24 h postoperative period. However, further research is warranted to determine the optimal dose of aprepitant in PONV prophylaxis and treatment, rescue schemes to be put in place, its interaction with other anti-emetics, as well as its cost-effectiveness.
Financial support and sponsorship
The study was supported by the Fluid Research Grant, Christian Medical College, Vellore, India, and partly by Dr Reddy's laboratories, who sponsored the drug Aprepitant. However, the funding agencies had no role in the data collection, analysis or write-up of the study.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Apfel CC, Heidrich FM, Jukar-Rao S, Jalota L, Hornuss C, Whelan RP, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109:742–53. doi: 10.1093/bja/aes276. [DOI] [PubMed] [Google Scholar]
- 2.Maddali MM, Mathew J, Fahr J, Zarroug AW. A prospective study of incidence of postoperative nausea and vomiting in a tertiary care hospital in Oman. Middle East J Anaesthesiol. 2003;17:131–41. [PubMed] [Google Scholar]
- 3.Kranke P, Eberhart LH. Possibilities and limitations in the pharmacological management of postoperative nausea and vomiting. Eur J Anaesthesiol. 2011;28:758–65. doi: 10.1097/EJA.0b013e32834a4e1e. [DOI] [PubMed] [Google Scholar]
- 4.Park SH, Lee HG, Jeong CY, Jeong SW, Lee SH, Kim HJ. Postoperative nausea and vomiting after total thyroidectomy: Sevoflurane combined with prophylactic ramosetron vs. propofol-based total intravenous anesthesia. Korean J Anesthesiol. 2014;66:216–21. doi: 10.4097/kjae.2014.66.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diemunsch P, Gan TJ, Philip BK, Girao MJ, Eberhart L, Irwin MG, et al. Single-dose aprepitant vs ondansetron for the prevention of postoperative nausea and vomiting: A randomized, double-blind phase III trial in patients undergoing open abdominal surgery. Br J Anaesth. 2007;99:202–11. doi: 10.1093/bja/aem133. [DOI] [PubMed] [Google Scholar]
- 6.Gan TJ, Apfel CC, Kovac A, Philip BK, Singla N, Minkowitz H, et al. A randomized, double-blind comparison of the NK1 antagonist, aprepitant, versus ondansetron for the prevention of postoperative nausea and vomiting. Anesth Analg. 2007;104:1082–9. doi: 10.1213/01.ane.0000263277.35140.a3. [DOI] [PubMed] [Google Scholar]
- 7.Habib AS, Keifer JC, Borel CO, White WD, Gan TJ. A comparison of the combination of aprepitant and dexamethasone versus the combination of ondansetron and dexamethasone for the prevention of postoperative nausea and vomiting in patients undergoing craniotomy. Anesth Analg. 2011;112:813–8. doi: 10.1213/ANE.0b013e3181ff47e2. [DOI] [PubMed] [Google Scholar]
- 8.Dzwonczyk R, Weaver TE, Puente EG, Bergese SD. Postoperative nausea and vomiting prophylaxis from an economic point of view. Am J Ther. 2012;19:11–5. doi: 10.1097/MJT.0b013e3181e7a512. [DOI] [PubMed] [Google Scholar]
- 9.Chrisp P. Aprepitant: The evidence for its place in the prevention of chemotherapy-induced nausea and vomiting. Core Evidence. 2007;2:15–30. [PMC free article] [PubMed] [Google Scholar]
- 10.Singh PM, Borle A, Rewari V, Makkar JK, Trikha A, Sinha AC, et al. Aprepitant for postoperative nausea and vomiting: A systematic review and meta-analysis. Postgrad Med J. 2016;92:87–98. doi: 10.1136/postgradmedj-2015-133515. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann M, Monte K, Barach P, Kindler CH. Postoperative patient complaints: A prospective interview study of 12,276 patients. J Clin Anesth. 2010;22:13–21. doi: 10.1016/j.jclinane.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000;59:213–43. doi: 10.2165/00003495-200059020-00005. [DOI] [PubMed] [Google Scholar]
- 13.Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006;102:1884–98. doi: 10.1213/01.ANE.0000219597.16143.4D. [DOI] [PubMed] [Google Scholar]
- 14.Camu F, Lauwers MH, Verbessem D. Incidence and aetiology of postoperative nausea and vomiting. Eur JAnaesthesiol Suppl. 1992;6:25–31. [PubMed] [Google Scholar]
- 15.Sonner JM, Hynson JM, Clark O, Katz JA. Nausea and vomiting following thyroid and parathyroid surgery. J Clin Anesth. 1997;9:398–402. doi: 10.1016/s0952-8180(97)00069-x. [DOI] [PubMed] [Google Scholar]
- 16.Fujii Y. Prophylaxis of postoperative nausea and vomiting in patients scheduled for breast surgery. Clin Drug Investig. 2006;26:427–37. doi: 10.2165/00044011-200626080-00001. [DOI] [PubMed] [Google Scholar]
- 17.Bakshi SG, Jibhkate B, Sareen R, Badwe R. Nausea and vomiting after breast cancer surgery, and relationship with tumor receptor status. J Anesth. 2012;26:187–95. doi: 10.1007/s00540-011-1274-5. [DOI] [PubMed] [Google Scholar]
- 18.Vallejo MC, Phelps AL, Ibinson JW, Barnes LR, Milord PJ, Romeo RC, et al. Aprepitant plus ondansetron compared with ondansetron alone in reducing postoperative nausea and vomiting in ambulatory patients undergoing plastic surgery. Plast Reconstr Surg. 2012;129:519–26. doi: 10.1097/PRS.0b013e31822b6932. [DOI] [PubMed] [Google Scholar]
- 19.Bergese S, Viloria A, Uribe A, Antor A, Fernandez S. Aprepitant versus ondansetron in preoperative triple-therapy treatment of nausea and vomiting in neurosurgery patients: Study protocol for a randomized controlled trial. Trials. 2012;13:130. doi: 10.1186/1745-6215-13-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakuta N, Tsutsumi YM, Horikawa YT, Kawano H, Kinoshita M, Tanaka K, et al. Neurokinin-1 receptor antagonism, aprepitant, effectively diminishes post-operative nausea and vomiting while increasing analgesic tolerance in laparoscopic gynecological procedures. J Med Invest. 2011;58:246–51. doi: 10.2152/jmi.58.246. [DOI] [PubMed] [Google Scholar]
- 21.Jung WS, Kim YB, Park HY, Choi WJ, Yang HS. Oral administration of aprepitant to prevent postoperative nausea in highly susceptible patients after gynecological laparoscopy. J Anesth. 2013;27:396–401. doi: 10.1007/s00540-012-1529-9. [DOI] [PubMed] [Google Scholar]
- 22.Okafor D, Kaye A, Kaye R, Urman R. The role of neurokinin-1 (substance P) antagonists in the prevention of postoperative nausea and vomiting. J Anaesthesiol Clin Pharmacol. 2017;33:441–5. doi: 10.4103/0970-9185.222511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierre S, Whelan R. Nausea and vomiting after surgery. Contin Educ Anaesth Crit Care Pain. 2013;13:28–32. [Google Scholar]


