Abstract
Purpose
The beneficial, neuroprotective effects of curcumin against ischemia-reperfusion injury have been demonstrated. In the present study, whether curcumin exerts neuroprotective effects associated with the inhibition of autophagy and hypoxia inducible factor-1α (HIF-1α) was investigated.
Materials and methods
PC12 cellular model of oxygen glucose deprivation/reperfusion (OGD/R) has been developed to mimic cerebral ischemia-reperfusion injury. Cell viability was evaluated using the CellTiter 96® AQueous One Solution Cell Proliferation Assay. Apoptosis was assessed using flow cytometry. The expression levels of HIF-1α and autophagy-associated proteins, LC3 and P62, were examined using Western blot. The autophagy flux was quantitatively estimated based on the number of autophagic compartments using fluorescence microscopy. In addition, 3-methyladenine (3-MA) was administered to PC12 cells to investigate how autophagy affects HIF-1α. Moreover, the inhibitory effects of HIF-1α on autophagy activation level were examined.
Results
In this study, curcumin decreased the death and apoptosis of cells, and inhibited autophagy and HIF-1α under OGD/R conditions, consistent with 3-MA treatment or HIF-1α downregulation. Moreover, inhibition of autophagy caused a decrease in HIF-1α, and the attenuation of HIF-1α induced autophagy suppression under OGD/R conditions.
Conclusion
The results of this study showed that curcumin exerts neuroprotective effects against ischemia-reperfusion, which is associated with the regulation of the reciprocal function between autophagy and HIF-1α.
Keywords: curcumin, cerebral ischemia-reperfusion, autophagy, hypoxia inducible factor-1α
Introduction
Cerebral ischemia-reperfusion injury is one of the most common diseases of the central nervous system. Additional serious pathological damage can be caused by inappropriate secondary blood flow after ischemic stroke, with high morbidity and fatality rates.1 However, effective treatments beyond 6 hours after stroke onset are not currently available.2 Therefore, finding effective therapeutic methods to protect neurons from ischemia-reperfusion injury is essential. Curcumin is a liposoluble phenolic pigment, isolated from Curcuma longa, with anti-inflammatory and antioxidative pharmacological functions.3,4 In several studies, curcumin was shown to alleviate the damage caused by oxidative stress on organs.5 In addition, curcumin has been found to improve neurological function score, maintain the integrity of the blood–brain barrier, reduce the infarct volume of the cerebral cortex, and decrease mortality as well as apoptosis of neurons in animal models.6–8 However, the potential neuroprotective mechanisms of curcumin against cerebral ischemia-reperfusion remain undefined.
Autophagy is a complex process for degradation of cytoplasmic components and is upregulated to counteract nutrient deprivation during the starvation period.9 In ischemia-reperfusion conditions, autophagy involves numerous proteins and signaling pathways for sequestration and degradation.10 Reportedly, autophagy of neurons can be activated by ischemia-reperfusion injury, and high levels of autophagy are closely related to ischemic neuronal death and apoptosis.11,12 However, the modulation of curcumin on autophagy in the pathological injury of cerebral ischemia-reperfusion remains controversial.
Hypoxia inducible factor-1α (HIF-1α) has been considered as an important regulator of cerebral ischemia-reperfusion and is responsible for controlling many pathological processes of neurons, such as apoptosis, energy metabolism, and gene transcription during inflammatory reactions.13 Ischemic stroke results in high HIF-1α expression levels in damaged brain tissues.14,15 Increased HIF-1α levels appear to induce autophagy activation in certain ischemic organs.16 The relationship between autophagy and HIF-1α in cerebral ischemia-reperfusion under curcumin treatment conditions has not been reported.
In our study, the relationship between the neuroprotective effects of curcumin by inhibiting autophagy and HIF-1α, and the reciprocal mechanism of autophagy in regulating HIF-1α was investigated.
Materials and methods
Cell culture
PC12 cell line is derived from a rat adrenal pheochromocytoma and expresses the nerve growth factor receptor.17 The cells with a passage range of 9–13 maintain invariable cell viability and provide sufficient accuracy to determine neuroprotective effects of compounds under serum deprivation conditions.18 PC12 cells at passage 5 were obtained from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and were cultured to 9-11 passage for the following research. We cultured PC12 cells in high glucose DMEM medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific) and 7.5% horse serum (Thermo Fisher Scientific). Cells were incubated at 37°C and 5% CO2 in a humidified incubator. Nerve growth factor (10 nM 7S; Sigma-Aldrich Co., St Louis, MO, USA) was added to the cell culture medium to induce differentiation of PC12 cells into neuronal PC12 cells.
OGD/R and chemicals
In our previous study, the oxygen glucose deprivation/reperfusion (OGD/R) model of PC12 cells was successfully established.19 The original high glucose DMEM medium was replaced with glucose-free DMEM medium and the cells were exposed to a gas mixture of 95% N2 and 5% CO2 in a hypoxia chamber at 37°C for 8 hours. Following OGD, the glucose-free DMEM medium was changed to the original high glucose DMEM medium and cells were cultured for 24 hours for reoxygenation. Curcumin (Sigma-Aldrich Co.) was dissolved in dimethyl sulfoxide (Sigma-Aldrich Co.) and diluted to 1.25–20 μM with medium before use. The curcumin treatment groups were established by treating PC12 cells with curcumin at various concentrations. The cells in the normal control group were cultured in the original high glucose DMEM medium under normal conditions, and the culture time was the same as in the experimental treatment groups. The 3-methyladenine (3-MA; Sigma-Aldrich Co.) groups were established by pretreating PC12 cells with 5 mM of 3-MA for 1 hour.
Cell viability
PC12 cells (2×104 cells/mL/well) were incubated in 96-well plates. Following the different treatments and 24 hours of reperfusion, 20 μL of CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS; Promega, Madison, WI, USA) reagent was added to each well and then incubated for 2 hours at 37°C. The absorbance at 490 nm was analyzed using a microplate reader (Synergy H1; Bio Tek, Winooski, VT, USA).
Flow cytometry
PC12 cells (2×105 cells/mL/well) were cultured in six-well plates. After the different treatments and reperfusions, cells were collected into flow tubes. Apoptosis in each group was detected using the FITC Annexin V Apoptosis detection kit (Becton, Dickinson and Company, Mountain View, CA, USA) following manufacturer’s instructions. Cells were stained with Annexin V-FITC and propidium iodide for 15 minutes in the dark at room temperature. Each flow tube was placed on ice to stop cell staining. Apoptosis was detected using flow cytometry (FACScalibur; Becton, Dickinson and Company).
RNA interference
PC12 cells were transfected with siRNA against HIF-1α (GenePharma, Shanghai, China) or negative control siRNA transiently (GenePharma) using Lipofectamine 2000 reagent (Thermo Fisher Scientific). Sense sequence of HIF-1α siRNA was 5′-CCAUGUGACCAUGAGGAAATT-3′ and antisense sequence was 5′-UUUCCUCAUGGUCACAUGGTT-3′. Cells (4×105 cells/mL/well) were seeded in six-well plates. Following normal culture for 24 hours, the original medium was changed to 1.5 mL of high glucose DMEM solution containing siRNA/Lipo 2000 mixture (mixed with 5 μL siRNA, 5 μL Lipo2000, and 400 μL high glucose DMEM solution), and cells were cultured for 24 hours under starvation conditions in a humidified incubator. Transfection efficiency was assessed using Western blot analysis.
Western blotting
Cells (2×105 cells/mL/well) were seeded in 60 mm plates. The total proteins of various groups were extracted using a lysis buffer (Beyotime, Shanghai, China). The concentration of protein in each group was quantified and separated using 10% SDS-PAGE. Proteins were transferred onto polyvinylidene fluoride membranes (Thermo Fisher Scientific) and then incubated at 4°C overnight with the following antibodies: HIF-1α (1:1,000; Cell Signaling Technology, Danvers, MA, USA), GAPDH (1:2,000; Cell Signaling Technology), P62 (1:1,000; Cell Signaling Technology), and LC3A/B (1:1,000; Cell Signaling Technology). The proteins were then incubated with anti-rabbit secondary antibodies (1:2,000; Zhongshan Jinqiao Biological Technology, Beijing, China) at room temperature for 2 hours. A chemiluminescence imaging analysis system was used for detecting antibody activity (Amersham Imager 600; GE, Chicago, IL, USA). The results were analyzed using the Gel-Pro ANALYZER (version 4.0; Media Cybernetics, Silver Spring, MD, USA).
Fluorescence microscopy
PC12 cells were incubated in 24-well plates with 4×104 cells/well. The autophagy flux in each group was quantitatively estimated based on the number of autophagic compartments (including amphisomes and autolysosomes) using CYTO-ID® Autophagy Detection Kits (Enzo Life Sciences, Farmingdale, NY, USA). In brief, the cells were washed with PBS (containing 5% FBS) and mixed with the reagents Green Detection Reagent (diluted to 1:500) and Hoechst 33342 Nuclear Stain (diluted to 1:1,000), and incubated at 37°C for 30 minutes in the dark. After washing the cells twice with PBS, quantitative detection of fluorescence intensity was performed using a fluorescence microscope (Nikon Corporation, Tokyo, Japan).
Statistical analysis
All experiments in our study were performed at least three times. The measurement data are presented as mean ± SD and obeyed normal distribution. One-way ANOVA followed by least significant difference test was utilized for multiple comparisons. A P-value <0.05 was considered statistically significant.
Results
Curcumin protects PC12 cells against OGD/R injury
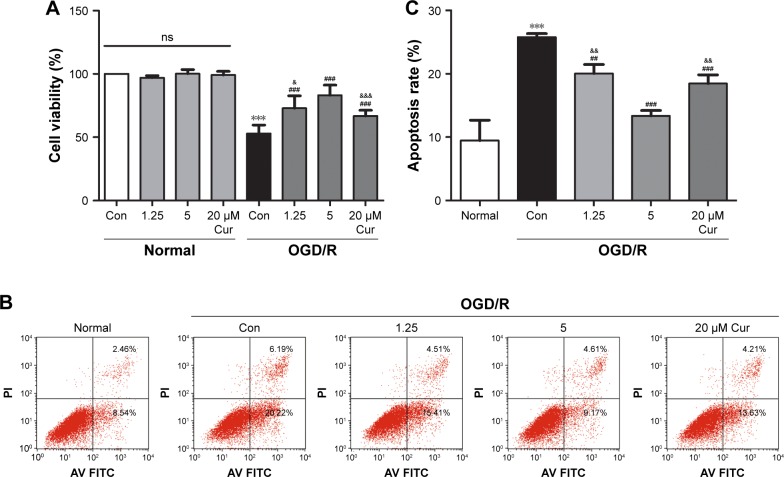
PC12 cells were exposed to OGD for 8 hours and reperfusion for 24 hours. The cell viability under OGD/R conditions was decreased to ~53% compared with normal cells (P<0.001; Figure 1A). Curcumin increased cell viability to protect against OGD/R injury: the cell viability in the 1.25 μM curcumin treatment group was 73% (P<0.001, Figure 1A), the cell viability in the 20 μM curcumin treatment group was ~66% (P<0.001, Figure 1A), and the maximal cell viability in the 5 μM curcumin treatment group waŝ83% (P<0.001, Figure 1A). The protective effects of curcumin were dose-dependent (Figure 1A); however, cellular survival and death were not affected by curcumin under normal conditions.
Figure 1.
Curcumin protects PC12 cells against OGD/R injury.
Notes: (A) PC12 cells were treated with different concentrations of curcumin under either normal or OGD/R conditions, and cell viability was analyzed using MTS. Data were represented as the mean ± SD, n=3; ***P<0.001 vs normal control group, ###P<0.001 vs OGD/R control group, &P<0.05 and &&&P<0.001 vs OGD/R 5 μM Cur group. (B, C) PC12 cells were treated with different concentrations of curcumin under OGD/R conditions, and cell apoptotic rate was analyzed using flow cytometry. Data were represented as the mean ± SD, n=3; ***P<0.001 vs normal group; ##P<0.01 and ###P<0.001 vs OGD/R control group; &&P<0.01 vs OGD/R 5 μM Cur group.
Abbreviations: Con, control; OGD/R, oxygen glucose deprivation/reperfusion; MTS, CellTiter 96® AQueous One Solution Cell Proliferation Assay; Cur, curcumin; ns, not significant; Con, control; PI, propidium iodide.
We also investigated the effects of curcumin on apoptosis in PC12 cell models. Flow cytometry results showed that cell apoptosis in the OGD/R control group was significantly increased compared with the normal group (P<0.001, Figure 1B and C). The high level of apoptosis under OGD/R conditions was inhibited by curcumin; 5 μM curcumin treatment showed the best effect on apoptosis (P<0.001, Figure 1B and C). Therefore, 5 μM curcumin was chosen as the optimal therapeutic concentration for subsequent studies.
Curcumin inhibits autophagy and HIF-1α in OGD/R model
To examine the autophagy and HIF-1α levels of PC12 cells under OGD/R conditions, Western blotting and fluorescence microscopy were performed. Significantly increased LC3 II (P=0.002, Figure 2A and B) and decreased P62 (P=0.012, Figure 2A and C) were observed in the OGD/R control group compared with the normal group. Curcumin decreased the expression of LC3 II (P=0.006, Figure 2A and B) and increased P62 level (P=0.005, Figure 2A and C) in the OGD/R group. Moreover, the HIF-1α expression was significantly increased after OGD/R injury (P=0.024 vs normal group, Figure 2A and D); however, this effect could be reversed by curcumin (P=0.045, Figure 2A and D). This result was also confirmed by the decrease of autophagic compartments after curcumin treat ment under OGD/R conditions (Figure 2E). These results illustrated that overexpression of HIF-1α and autophagy-associated proteins caused by OGD/R injury could be abolished by curcumin.
Figure 2.
Curcumin inhibits autophagy and HIF-1α in the OGD/R model.
Notes: (A) PC12 cells were treated with curcumin under OGD/R conditions. LC3 II, LC3 I, P62, and HIF-1α expressions were detected using Western blot. (B–D) Figures showing LC3 II/LC3 I (B), P62/GAPDH (C), HIF-1α/GAPDH (D) in each group. Data were represented as the mean ± SD, n=3; *P<0.05 and **P<0.01 vs normal group; #P<0.05 and ##P<0.01 vs OGD/R control group. (E) Autophagic compartments of PC12 cells under OGD/R and curcumin treatment conditions detected using fluorescence microscopy. Scale bar =50 μm, magnification 400×. The above-mentioned column shows Hoechst 33342 Nuclear Stain (blue), the middle shows autophagic compartment stain (green), and the bottom shows merged images. (F) PC12 cells were transfected with HIF-1α siRNA under either normal or OGD/R conditions. HIF-1α expression level was detected using Western blot. Data were represented as the mean ± SD, n=3; *P<0.05 and **P<0.01 vs normal control group; ###P<0.001 vs OGD/R control group.
Abbreviations: Con, control; Cur, curcumin; OGD/R, oxygen glucose deprivation/reperfusion; ncRNA, negative control siRNA; HIF-1α siRNA, hypoxia inducible factor-1α siRNA; ns, not significant; LC3 II, light chain 3-II; LC3 I, light chain 3-I.
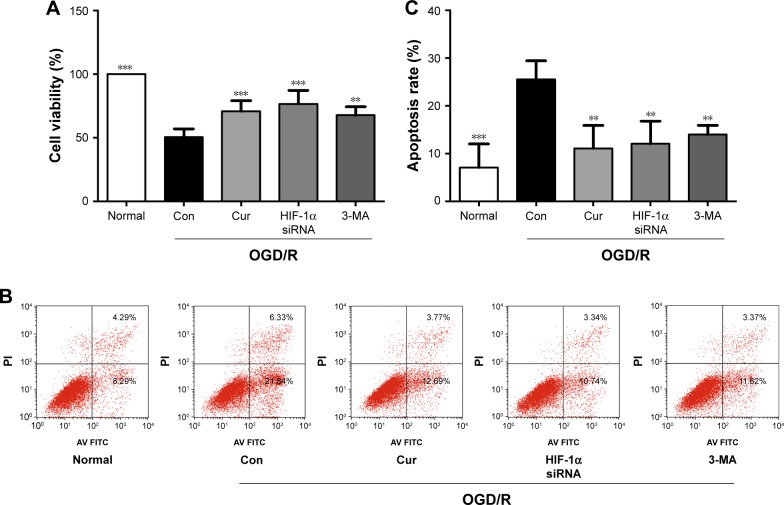
To explore the role of autophagy and HIF-1α in PC12 cells following OGD/R injury, PC12 cells were treated with 3-MA or HIF-1α siRNA. We have successfully transfected PC12 cells with HIF-1α siRNA (Figure 2F). The PC12 cells exposed to 3-MA (P=0.001 vs OGD/R control group, Figure 3A) or transfected with HIF-1α siRNA (P<0.001 vs OGD/R control group, Figure 3A) exhibited a significant increase in cell viability under OGD/R conditions. Moreover, the apoptosis induced by OGD/R decreased with 3-MA treatment (P=0.007 vs OGD/R control group, Figure 3B and C) or HIF-1α transfection (P=0.003, vs OGD/R control group, Figure 3B and C). The above-mentioned results indicated that excessive activation of HIF-1α and autophagy could aggravate the damaging effects of OGD/R on PC12 cells, whereas these effects were alleviated by curcumin.
Figure 3.
Autophagy inhibition or HIF-1α siRNA transfection protects PC12 cells against OGD/R injury.
Notes: (A) The effects of curcumin, 3-MA, and HIF-1α siRNA on cell viability under OGD/R conditions were analyzed using MTS. Data were represented as the mean ± SD. n=3, **P<0.01 and ***P<0.001 vs OGD/R control group. (B, C) The effects of curcumin, 3-MA, and HIF-1α siRNA on cell apoptosis under OGD/R conditions were analyzed using flow cytometry. Data were represented as the mean ± SD. n=3, **P<0.01 and ***P<0.001 vs OGD/R control group.
Abbreviations: Con, control; Cur, curcumin; HIF-1α siRNA, hypoxia inducible factor-1α siRNA; 3-MA, 3-methyladenine; OGD/R, oxygen glucose deprivation/reperfusion; MTS, CellTiter 96® AQueous One Solution Cell Proliferation Assay; PI, propidium iodide.
Autophagy modulates HIF-1α in a reciprocal manner
Next, the relationship between autophagy and HIF-1α interaction in the OGD/R model was further investigated. 3-MA significantly inhibited the activation of autophagy in PC12 cells under OGD/R condition (Figure 4B and C). Compared with the OGD/R control group, treatment with 3-MA reduced expression levels of HIF-1α (P=0.002, Figure 4A and D). The PC12 cells transfected with HIF-1α siRNA exhibited a decrease in LC3 II (P=0.004 vs OGD/R control group, Figure 4E and F) and an increase in P62 expression under OGD/R conditions (P=0.010 vs OGD/R control group, Figure 4E and G). The PC12 cells transfected with HIF-1α siRNA also showed a obvious decrease in HIF-1α under OGD/R condition (Figure 4H). These results were also confirmed using fluorescence microscopy. The number of autophagic compartments was significantly reduced after transfecting cells under OGD/R conditions with HIF-1α siRNA compared with the OGD/R control group (Figure 5). Interestingly, curcumin treatment exerted a similar effect as 3-MA administration or HIF-1α siRNA transfection (Figure 4). In conclusion, these observations indicate that autophagy functions in a reciprocal manner to modulate HIF-1α, and the effect of this reciprocal action may be regulated by curcumin under OGD/R conditions.
Figure 4.
Autophagy functions in a reciprocal manner to modulate HIF-1α.
Notes: (A) PC12 cells were treated with curcumin or 3-MA under OGD/R conditions. LC3 II, LC3 I, P62, and HIF-1α expressions were detected using Western blot. (B–D) Figures showing LC3 II/LC3 I (B), P62/GAPDH (C), and HIF-1α/GAPDH (D) expression in each group. Data were represented as the mean ± SD, n=3; *P<0.05, **P<0.01, and ***P<0.001 vs OGD/R control group. (E) PC12 cells were treated with curcumin or HIF-1α siRNA under OGD/R conditions. LC3 II, LC3 I, P62, and HIF-1α expressions were detected using Western blot. (F–H) Figures showing LC3 II/LC3 I (F), P62/GAPDH (G), and HIF-1α/GAPDH (H) expression in each group. Data were represented as the mean ± SD, n=3; *P<0.05, **P<0.01, and ***P<0.001 vs OGD/R control group.
Abbreviations: Con, control; Cur, curcumin; 3-MA, 3-methyladenine; OGD/R, oxygen glucose deprivation/reperfusion; LC3 II, light chain 3-II; LC3 I, light chain 3-I; P62, sequestome 1; HIF-1α siRNA, hypoxia inducible factor-1α siRNA.
Figure 5.
Fluorescence images of autophagic compartments in PC12 cells treated with curcumin, HIF-1α siRNA, or 3-MA (scale bar =50 μm, magnification 400×). The top row shows Hoechst 33342 Nuclear Stain (blue), the middle row shows autophagic compartment stain (green), and the bottom row shows merged images.
Abbreviations: Con, control; Cur, curcumin; HIF-1α siRNA, hypoxia inducible factor-1α siRNA; 3-MA, 3-methyladenine; OGD/R, oxygen glucose deprivation/reperfusion.
Discussion
In the present study, curcumin protected PC12 cells against death and apoptosis induced by OGD/R injury. Furthermore, the neuroprotective effects of curcumin were associated with the inhibition of HIF-1α and autophagy. In addition, this is the first report of the reciprocal regulation of function between autophagy and HIF-1α after OGD/R injury in PC12 cells.
The OGD/R is a classic model for ischemic-reperfusion injury. In the present study, the OGD/R model was applied to PC12 cells to investigate the effects of curcumin on cerebral ischemic-reperfusion. The results showed that curcumin inhibited cell death and apoptosis during OGD/R. Moreover, the protective function of curcumin was dose-dependent under OGD/R conditions, while normal cells were not affected. However, the protective mechanism of curcumin remains unclear.
Autophagy is a critical intracellular homeostatic pathway for the degradation of harmful materials through the lysosomal system.20 Upon induction of autophagy, LC3 I undergoes ubiquitination and conjugation through different autophagy-related genes to generate LC3 II.21 The P62 protein binds to polyubiquitinated proteins through a ubiquitin-associated domain and combines with LC3 II through its LC3-interaction region domain for attachment to the autophagosomes, which is finally degraded in lysosomes.22,23 In the process of autophagy, P62 is continuously degraded, whereas LC3 II is ultimately delipidated and recycled. Therefore, LC3 and P62 can be used to monitor autophagic activity. PI3K/Akt/mTOR pathway is regarded as the main signaling pathway of autophagy, and 3-MA is widely used as an autophagy inhibitor to inhibit the activity of PI3K.24 Curcumin has protective effects against cerebral ischemia-reperfusion injury in animal models, and autophagy was considered as an effective therapeutic target of ischemic stroke.25 Increasing evidence shows that autophagy participates in the pathological process of cerebral ischemia as a double-edged sword.26 Modulation of autophagy can have a neuroprotective or deteriorative effect based on the stage which is targeted. Shi et al observed that inhibiting autophagy-aggravated neuronal injury in the early stage of OGD/R, whereas the autophagy inhibitor significantly protected neurons from dying after a shorter time of reperfusion.27 Therefore, the effects of autophagy on neurons change dynamically in the progression of ischemia-reperfusion injury. Autophagy can be induced to alleviate cells energy crisis in the early stage of ischemia. Moreover, induction in autophagy has a beneficial effect against neurodegeneration,28 which is associated with clearance of abnormal and misfolded proteins.29,30 However, autophagy will be further activated by the persistent ischemia or reperfusion, which may aggravate the damage to autophagic flux and eventually result in cerebral injury.31 Many clinical patients with ischemic stroke often are not treated in a timely manner. Excessive number of autophagosomes will be formed in this process, leading to more serious cerebral reperfusion injury. In the present study, autophagy was significantly activated upon completion of ischemia-reperfusion injury and was inhibited by curcumin. To further clarify the relationship between curcumin and autophagy, PC12 cells were treated with curcumin or 3-MA under OGD/R conditions. Curcumin exerted a similar effect as 3-MA, decreasing the death and apoptosis of cells. This result indicates that the neuroprotective effects of curcumin on ischemia-reperfusion injury are associated with the inhibition of autophagy. Curcumin can also reduce cerebral infarction volume and neurological deficit by inhibiting autophagy in a rat model of cerebral ischemia-reperfusion injury.32 However, autophagy is mildly activated in the early stage of ischemia and often manifests as a protective effect.33 The effects of curcumin on autophagy in the early stage of cerebral ischemia need to be further studied. HIF-1α was shown to participate in the pathological process of hypoxia in some organs and is considered an important target for the treatment of ischemic diseases.34,35 Under hypoxic conditions, the HIF-1α expression in neurons was increased due to inhibited degradation.36 The accumulated HIF-1α translocated to the nucleus and bound to the hypoxia response element sequence of the target gene promoter, ultimately destroying the integrity of the blood–brain barrier and causing neuronal death.37,38 Therefore, the effects of curcumin on HIF-1α was investigated in the present study. PC12 cells were transfected with HIF-1α siRNA to inhibit the HIF-1α expression induced by OGD/R. HIF-1α siRNA transfection was consistent with the curcumin treatment, which significantly improved cell survival rate and decreased neuronal apoptosis in OGD/R. In many studies, inhibition or knockdown of the HIF-1α gene protected cortical neurons from ischemia-reperfusion.39,40 In addition, the increased HIF-1α may aggravate the range of cerebral infarction in the ischemic model.41 In the present study, curcumin showed a similar neuroprotective effect as inhibition of HIF-1α, indicating that the neuroprotec-tive effects of curcumin were closely related to inhibition of HIF-1α.
Both HIF-1α and autophagy were increased significantly following OGD/R compared with normal conditions and could be reversed by curcumin. Because the levels of HIF-1α and autophagy were simultaneously increased by OGD/R or inhibited by curcumin, the interaction between HIF-1α and autophagy remains unclear. Reportedly, HIF-1α has a close relationship to the activation of autophagy.42 The tolerance of renal cells to ischemia-reperfusion injury is dose-dependently associated with autophagy in HIF-1α; the activation level of autophagy increases with the secretion of HIF-1α.16 However, activation of autophagy was significantly inhibited following the upregulation of HIF-1α in the mouse ischemia model.43 The regulatory relationship between HIF-1α and autophagy in neuronal ischemia-reperfusion injury requires further investigation. In the present study, the activation level of autophagy was significantly decreased after inhibition of HIF-1α under OGD/R conditions, and the HIF-1α expression was inhibited by 3-MA. These results indicate autophagy functions in a reciprocal manner to regulate HIF-1α. Curcumin has been reported to inhibit PI3K in glioblastoma and lung cancer to exert anticancer effects.44,45 In our present study, curcumin exhibited a similar effect as PI3K inhibitor, 3-MA, and inhibited autophagy and HIF-1α under OGD/R conditions. Therefore, the neuroprotective effects of curcumin are associated with the inhibition of the reciprocal function between autophagy and HIF-1α in cerebral ischemia-reperfusion injury.
Conclusion
In conclusion, the results from the present study showed that neuronal ischemia-reperfusion injury can be alleviated by inhibiting HIF-1α and autophagy. Moreover, this is the first report showing that autophagy functions in a reciprocal manner to regulate HIF-1α after OGD/R injury in PC12 cells. The protective effects of curcumin on cerebral ischemia-reperfusion injury is closely related to the inhibition of HIF-1α and autophagy. The results show a neuroprotective mechanism of curcumin, indicating that curcumin has great potential for clinical applications in the future.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No 81771271), the Second Batch Basic Clinical Tight Integration Platform Project (No S208), and the project titled “Tanshinone IIA Regulates CD4+ Cell Differentiation in the Treatment of Multiple Sclerosis Diseases and Related Mechanisms” (No D255).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Park HR, Lee H, Lee JJ, Yim NH, Gu MJ, Ma JY. Protective effects of spatholobi caulis extract on neuronal damage and focal ischemic stroke/reperfusion injury. Mol Neurobiol. 2018;55(6):4650–4666. doi: 10.1007/s12035-017-0652-x. [DOI] [PubMed] [Google Scholar]
- 2.Lu Z, Liu Y, Shi Y, et al. Curcumin protects cortical neurons against oxygen and glucose deprivation/reoxygenation injury through flotillin-1 and extracellular signal-regulated kinase1/2 pathway. Biochem Biophys Res Commun. 2018;496(2):515–522. doi: 10.1016/j.bbrc.2018.01.089. [DOI] [PubMed] [Google Scholar]
- 3.Fan Y, Chen H, Peng H, Huang F, Zhong J, Zhou J. Molecular mechanisms of curcumin renoprotection in experimental acute renal injury. Front Pharmacol. 2017;8:912. doi: 10.3389/fphar.2017.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunnumakkara AB, Bordoloi D, Padmavathi G, et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol. 2017;174(11):1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kheradpezhouh E, Barritt GJ, Rychkov GY. Curcumin inhibits activation of TRPM2 channels in rat hepatocytes. Redox Biol. 2016;7:1–7. doi: 10.1016/j.redox.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia G, Tan B, Ma J, Zhang L, Jin X, Li C. Prdx6 upregulation by curcumin attenuates ischemic oxidative damage via SP1 in rats after stroke. Biomed Res Int. 2017;2017:6597401. doi: 10.1155/2017/6597401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W, Suwanwela NC, Patumraj S. Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc Res. 2016;106:117–127. doi: 10.1016/j.mvr.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Li J, Li S, et al. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol Appl Pharmacol. 2015;286(1):53–63. doi: 10.1016/j.taap.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Ferrucci M, Biagioni F, Ryskalin L, et al. Ambiguous effects of autophagy activation following hypoperfusion/ischemia. Int J Mol Sci. 2018;19(9):2756. doi: 10.3390/ijms19092756.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaushal GP, Shah SV. Autophagy in acute kidney injury. Kidney Int. 2016;89(4):779–791. doi: 10.1016/j.kint.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao J, Shen C, Sun Y, Chen W, Yan G. Neuroprotective effects of pinocembrin on ischemia/reperfusion-induced brain injury by inhibiting autophagy. Biomed Pharmacother. 2018;106:1003–1010. doi: 10.1016/j.biopha.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Hua R, Han S, Zhang N, Dai Q, Liu T, Li J. cPKCγ-modulated sequential reactivation of mTOR inhibited autophagic flux in neurons exposed to oxygen glucose deprivation/reperfusion. Int J Mol Sci. 2018;19(5):1380. doi: 10.3390/ijms19051380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barteczek P, Li L, Ernst AS, Böhler LI, Marti HH, Kunze R. Neuronal HIF-1α and HIF-2α deficiency improves neuronal survival and sensorimotor function in the early acute phase after ischemic stroke. J Cereb Blood Flow Metab. 2017;37(1):291–306. doi: 10.1177/0271678X15624933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma DK, Rothe M, Zheng S, et al. Cytochrome P450 drives a HIF-regulated behavioral response to reoxygenation by C. elegans. Science. 2013;341(6145):554–558. doi: 10.1126/science.1235753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng YL, Park JS, Manzanero S, et al. Evidence that collaboration between HIF-1α and Notch-1 promotes neuronal cell death in ischemic stroke. Neurobiol Dis. 2014;62:286–295. doi: 10.1016/j.nbd.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Y, Jiang D, Xiao J, et al. Ischemic preconditioning attenuates ischemia/reperfusion-induced kidney injury by activating autophagy via the SGK1 signaling pathway. Cell Death Dis. 2018;9(3):338. doi: 10.1038/s41419-018-1111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinarivala N, Shah K, Abbruscato TJ, Trippier PC. Passage variation of PC12 cells results in inconsistent susceptibility to externally induced apoptosis. ACS Chem Neurosci. 2017;8(1):82–88. doi: 10.1021/acschemneuro.6b00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Han D, Sun M, Feng J. Cerebral ischemic post-conditioning induces autophagy inhibition and a HMGB1 secretion attenuation feedback loop to protect against ischemia reperfusion injury in an oxygen glucose deprivation cellular model. Mol Med Rep. 2016;14(5):4162–4172. doi: 10.3892/mmr.2016.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wurzer B, Zaffagnini G, Fracchiolla D, et al. Oligomerization of p62 allows for selection of ubiquitinated cargo and isolation membrane during selective autophagy. Elife. 2015;4:e08941. doi: 10.7554/eLife.06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ricci V. Relationship between VacA toxin and host cell autophagy in helicobacter pylori infection of the human stomach: a few answers, many questions. Toxins (Basel) 2016;8(7):203. doi: 10.3390/toxins8070203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islam MA, Sooro MA, Zhang P. Autophagic regulation of p62 is critical for cancer therapy. Int J Mol Sci. 2018;19(5):1405. doi: 10.3390/ijms19051405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pankiv S, Clausen TH, Lamark T, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282(33):24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 24.Cao Y, Luo Y, Zou J, et al. Autophagy and its role in gastric cancer. Clin Chim Acta. 2019;489:10–20. doi: 10.1016/j.cca.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Fang M, Sun Y, et al. Curcumin attenuates cerebral ischemia injury in Sprague-Dawley rats and PC12 cells by suppressing overactivated autophagy. J Photochem Photobiol B. 2018;184:1–6. doi: 10.1016/j.jphotobiol.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Sun Y, Liu K, Sun X. Autophagy: a double-edged sword for neuronal survival after cerebral ischemia. Neural Regen Res. 2014;9(12):1210–1216. doi: 10.4103/1673-5374.135329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J. Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther. 2012;18(3):250–260. doi: 10.1111/j.1755-5949.2012.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chauhan S, Ahmed Z, Bradfute SB, et al. Pharmaceutical screen identifies novel target processes for activation of autophagy with a broad translational potential. Nat Commun. 2015;6:8620. doi: 10.1038/ncomms9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinarivala N, Patel R, Boustany RM, Al-Ahmad A, Trippier PC. Discovery of aromatic carbamates that confer neuroprotective activity by enhancing autophagy and inducing the anti-apoptotic protein B-Cell lymphoma 2 (Bcl-2) J Med Chem. 2017;60(23):9739–9756. doi: 10.1021/acs.jmedchem.7b01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menzies FM, Fleming A, Caricasole A, et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron. 2017;93(5):1015–1034. doi: 10.1016/j.neuron.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Wu J, Shen H, et al. Autophagy in hemorrhagic stroke: mechanisms and clinical implications. Prog Neurobiol. 2018:163–164. 79–97. doi: 10.1016/j.pneurobio.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Huang L, Chen C, Zhang X, et al. Neuroprotective effect of curcumin against cerebral ischemia-reperfusion via mediating autophagy and inflammation. J Mol Neurosci. 2018;64(1):129–139. doi: 10.1007/s12031-017-1006-x. [DOI] [PubMed] [Google Scholar]
- 33.Wang P, Shao BZ, Deng Z, Chen S, Yue Z, Miao CY. Autophagy in ischemic stroke. Prog Neurobiol. 2018:163–164. 98–117. doi: 10.1016/j.pneurobio.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Sun P, Lu YX, Cheng D, et al. Monocyte chemoattractant protein-induced protein 1 targets hypoxia-inducible factor 1α to protect against hepatic ischemia/reperfusion injury. Hepatology. 2018 doi: 10.1002/hep.30086. [DOI] [PubMed] [Google Scholar]
- 35.Bishop T, Ratcliffe PJ. HIF hydroxylase pathways in cardiovascular physiology and medicine. Circ Res. 2015;117(1):65–79. doi: 10.1161/CIRCRESAHA.117.305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirayama Y, Koizumi S. Hypoxia-independent mechanisms of HIF-1α expression in astrocytes after ischemic preconditioning. Glia. 2017;65(3):523–530. doi: 10.1002/glia.23109. [DOI] [PubMed] [Google Scholar]
- 37.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41(4):518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang F, Zhou L, Wang D, Wang Z, Huang QY. Minocycline ameliorates hypoxia-induced blood-brain barrier damage by inhibition of HIF-1α through SIRT-3/PHD-2 degradation pathway. Neuroscience. 2015;304:250–259. doi: 10.1016/j.neuroscience.2015.07.051. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y, Chen X, Zhang X, et al. Corrigendum: β2-adrenergic receptor-mediated HIF-1α upregulation mediates blood brain barrier damage in acute cerebral ischemia. Front Mol Neurosci. 2017;10:392. doi: 10.3389/fnmol.2017.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh CH, Lin YJ, Chen WL, et al. HIF-1α triggers long-lasting glutamate excitotoxicity via system xc- in cerebral ischaemia-reperfusion. J Pathol. 2017;241(3):337–349. doi: 10.1002/path.4838. [DOI] [PubMed] [Google Scholar]
- 41.Koh HS, Chang CY, Jeon SB, et al. The HIF-1/glial TIM-3 axis controls inflammation-associated brain damage under hypoxia. Nat Commun. 2015;6:6340. doi: 10.1038/ncomms7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang IK, Sun KT, Tsai TH, et al. MiR-20a-5p mediates hypoxia-induced autophagy by targeting ATG16L1 in ischemic kidney injury. Life Sci. 2015;136:133–141. doi: 10.1016/j.lfs.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Luo C, Ouyang MW, Fang YY, et al. Dexmedetomidine protects mouse brain from ischemia-reperfusion injury via inhibiting neuronal autophagy through up-regulating HIF-1α. Front Cell Neurosci. 2017;11:197. doi: 10.3389/fncel.2017.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanotto-Filho A, Braganhol E, Edelweiss MI, et al. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J Nutr Biochem. 2012;23(6):591–601. doi: 10.1016/j.jnutbio.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 45.Man S, Zhang L, Cui J, Yang L, Ma L, Gao W. Curcumin enhances the anti-cancer effects of Paris Saponin II in lung cancer cells. Cell Prolif. 2018;51(4):e12458. doi: 10.1111/cpr.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]