Abstract
Background:
Regional anesthesia may mitigate the risk of persistent postoperative pain (PPP). This Cochrane review, published originally in 2012, was updated in 2017.
Methods:
We updated our search of Cochrane CENTRAL, PubMed, EMBASE and CINAHL to December 2017. Only RCTs investigating local anesthetics (by any route) or regional anesthesia versus any combination of systemic (opiod or non-opioid) analgesia in adults or children, reporting anypain outcomes beyond three months were included.
Data were extracted independently by at least two authors, who also appraised methodological quality with Cochrane ‘Risk of bias’ assessment and pooled data in surgical subgroups. We pooled studies across different follow-up intervals. As summary statistic, we reported the odds ratio (OR) with 95% confidence intervals and calculated the number needed to benefit (NNTB). We considered classical, Bayesian alternatives to our evidence synthesis. We explored heterogeneity and methodological bias.
Results:
40 new and seven ongoing studies, identified in this update, brought the total included RCTs to 63. We were only able to synthesize data from 39 studies enrolling 3027 participants in a balanced design.
Evidence synthesis favored regional anesthesia for thoracotomy (OR 0.52 [0.32 to 0.84], moderate-quality evidence), breast cancer surgery (OR 0.43 [0.28 to 0.68], low-quality evidence), and cesarean section (OR 0.46, [0.28 to 0.78], moderate-quality evidence). Evidence synthesis favored continuous infusion of local anesthetic after breast cancer surgery (OR 0.24 [0.08 to 0.69], moderate-quality evidence), but was inconclusive after iliac crest bone graft harvesting (OR 0.20, [0.04 to 1.09], low-quality evidence).
Conclusions:
Regional anesthesia reduces the risk of PPP. Small study size, performance, null, and attrition bias considerably weakened our conclusions. We cannot extrapolate to other interventions or to children.
Keywords: Chronic Pain/prevention & control; Anesthesia, Conduction; Meta-Analysis
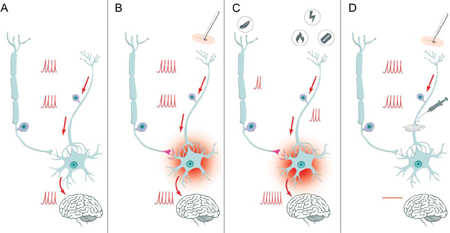
Graphical Abstract: Regional anesthesia prevents central sensitization
This graphical abstract explains how regional anesthesia prevents central sensitization[3]. Panel A depicts the normal pain transmission from the primary nociceptor via the synapsis in the posterior horn of the spinal column to the brain, modulated and altered by low threshold mechanoceptors as described by Woolf[23]. The barrage of perioperative pain leads to persistent sensitization of the synapsis, as shown in Panel B. As a consequence, mild pain is augmented in the sensitized synapsis and transmitted as severe pain (hyperalgesia), even touch can be transmitted as painful (allodynia), as explicated in Panel C. This process termed central sensitization, can be mitigated or prevented by blocking the barrage of pain signals with local anesthetics, preventing the development of persistent pain after surgery, as demonstrated in Panel D.
INTRODUCTION
Paradigm change focuses on long term benefits of regional anesthesia
Decreased anesthesia related perioperative morbidity and mortality and the shift to bundled capitated payments resulted in a paradigm shift:[1] to justify the inherent resource utilization, we are increasingly asked to demonstrate that regional anesthesia affords improved long-term benefits, beyond the superior pain control immediately after surgery.[2,3] Pain persisting beyond three months after surgery is the prime example of a frequent, devastating long-term harm resulting from many surgical interventions, which may be mitigated by optimal perioperative anti-nociception, primarily regional anesthesia.[4–6] Gender, genetics and phenotype predispose to persistent postoperative pain (PPP).[4,5,7]
Persistent postoperative pain is devastating, hence prevention is paramount
PPP is frequent.[5,6,8–10] One in three to five patients undergoing thoracotomy, cardiac surgery, limb amputation, or breast surgery will experience chronic pain lasting months beyond the surgical intervention.[4,11–14] PPP has been shown to affect quality of life, even when mild.[8,15] PPP treatment modalities are sparse and frustrating.[16,17] The individual and societal burden of PPP is immense, afflicting one in five patients after surgery[18] and may contribute to the current opioid epidemic.[19] Coley et al. estimated costs per patient follow-up visit for PPP in the order of $2000.[20] Therefore, it is imperative to develop effective approaches to reduce the risk of PPP.[3,13]
We hypothesize that regional anesthesia may prevent the central sensitization leading to persistent postoperative pain.[5,10,21] Woolf et al explained the transition from acute to chronic pain after surgery with central sensitization (Graphical Abstract).[3,22,23] Many have since contributed to elucidate the precise molecular mechanisms.[24,25] Anti-nociception with regional anesthesia decreases the barrage of painful stimuli that otherwise would trigger the augmentation of synaptic strength in the dorsal horn between the primary and secondary nociceptive neuron.[3,5,25]
Our previous systematic review and meta-analysis for the Cochrane Collaboration investigated regional anesthesia for the prevention of persistent postoperative pain.[26,27] Evidence synthesis suggested that regional anesthesia reduces the risk of PPP six months after breast surgery and thoracotomy. Over 40 new randomized controlled trials investigating regional anesthesia for mitigation of PPP have since been conducted and an update of our outdated search and evidence synthesis was overdue.[26,27] To overcome the diversity of reporting which hampered evidence synthesis for our first review,[28] we chose to synthesize the data across different follow up intervals within each surgical subgroup as a novel approach in this update[29]. This manuscript is a co-publication1 of our recently updated Cochrane review to reach a broader audience.[29]
Objective
To synthesize outcome data across different follow up intervals in our updated systematic review and meta-analysis for the Cochrane Collaboration comparing local and regional anesthesia versus conventional analgesia for the prevention of persistent postoperative pain beyond three months in adults and children undergoing elective surgery.
METHODS
Search and selection
Our a priori protocol, methods and search were described in our Cochrane Review in detail[21,26,27,29] and follow the PRISMA Statement.[30] Briefly, PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials were searched again from inception through December 2017. We combined controlled vocabulary with free-text search and employed a highly sensitive search strategy to limit our results to randomized clinical trials (RCT).[31,32] Manuscripts published in any language were included without a restriction of publication status. Our handsearch included the reference lists of included studies and conference abstracts of the International Anesthesia Research Society (IARS), and the European Society of Regional Anaesthesia (ESRA) for 2005 through to 2007. The systematic review registry PROSPERO was searched for related systematic reviews.
Study inclusion criteria
Participants:
Trials investigating adults and/or children undergoing elective surgery were included, regardless of the surgical approach (e.g. laparoscopic versus open), but excluding trauma, orthopedic and emergency surgery.
Interventions:
Studies comparing a local or regional anesthesia intervention against a conventional analgesia approach were included, regardless of the route of delivery of the local anesthetic, the timing of the nociceptive blockade, or the co-administration of adjuvants. We did not include comparisons of one local/regional technique versus another and excluded studies focused on the effect of timing.
Comparators:
Any conventional analgesic modality was acceptable as comparator, including any combination of nonsteroidal anti-inflammatory drugs with adjuvants and/or opioids as defined and detailed in the appendix of our Cochrane Review.[29]
Outcomes:
We included studies assessing persistent pain beyond three months after surgery, as a dichotomous outcome (as reported/defined in the primary studies) or by a continuous pain instrument.
Study Design:
Only RCTs were included. As patients and providers can easily discern the effects of regional anesthesia, masking of only the outcome assessor was acceptable for inclusion.
Data extraction
If a study qualified for inclusion based upon the aforementioned principles, data were extracted independently by two authors, and entered in a templated form on the online systematic review software, DistillerSR,[33] and subsequently transferred into RevMan 5.1, the Cochrane Review Manager.[34] We contacted the primary study authors for clarification of the methods or to acquire additional data as needed. An overview of study characteristics and populations is presented in Table 1 Table of surgeries, interventions, timing and outcomes by subgroup of pooled studies and in the Suppl. Table 1 Participants of pooled studies by follow up interval, respectively. Study level details on population, intervention, control, outcomes investigated and design are tabulated in the Suppl. Table 2 Characteristics of included studies.
Table 1. Table of surgeries, interventions, timing and outcomes by subgroup of pooled studies.
An overview of the surgeries, interventions employed, the timing and the outcomes observed is provided by surgical subgroup in this table. We were able to pool study data for the subgroups of thoracotomy, breast cancer surgery, hysterectomy, ICBG, cesarean section, and prostatectomy. The majority of studies investigated epidural analgesia for thoracotomy, but for breast surgery the regional anesthesia interventions were more varied, with four studies investigating paravertebral blocks and several studies using local infiltration and even including intravenous infusions. Three of the studies on ICBG used wound instillation and intravenous infusions, while for cesarean section transverse abdominal plain block was the most frequently employed technique. (Footnotes VAS: Visual Analogue Scale, VRS: Verbal Rating Scale, DN4: DN4 questionnaire, NRS: Numerical Rating Scale, SF-36: Short Form 36)
| STUDY ID | REGIONAL TECHNIQUE | TIMING OF INTERVENTION | ADJUVANTS | OUTCOME | CONTINUOUS | FOLLOW-UP |
|---|---|---|---|---|---|---|
| BREAST CANCER SURGERY | ||||||
| ALBI- FELDZER 2013 | Infiltration and intervertebral block | Postincision, single shot vs placebo | None | Pain/no pain | Brief Pain Index | 3, 6 and 12 months |
| BAUDRY 2008 | Local Infiltration | Single shot, postincision vs control | None | Pain/no pain | McGill results not reported | 18 months |
| BESIC 2014 | Local Infiltration | Postincision, continuous post-op vs control | None | Pain/no pain | None | 3 months |
| FASSOULAKI 2000 | Topical application | Preincision, continuous post-op vs placebo | Propoxyphene | Pain/no pain | Verbal Intensity Scale | 3 months |
| FASSOULAKI 2001 | Brachial plexus block | Postincision, single shot vs placebo | Mexiletine, propoxyphene | Pain/no pain | VAS | 3 months |
| FASSOULAKI 2005 | Topical application | Postincision, continuous postop vs control | Gabapentin | Pain/no pain | Analgesic consumption | 6 months |
| GACIO 2016 | Paravertebral block | Single shot, preincision vs control | Parecoxib, opioid and adrenaline | Pain/no pain | None | 6 months |
| GRIGORAS 2012 | IV lidocaine | Preincision, continuous intra-op vs placebo | None | Pain/no pain | Short-form McGill Pain Questionnaire | 3 months |
| IBARRA 2011 | Single shot, paravertebral block | Single shot, preincision vs control | None | Phantom or neuropathic pain | None | 3 and 5 months |
| KAIRALUOMA 2006 | Single shot, paravertebral block | Single shot, preincision vs control | None | NRS > 3 | Analgesic consumption | 12 months |
| KARMAKAR 2014 | Thoracic paravertebral block | Single shot, pre vs post, continuous vs control | Epinephrine | Pain/no pain | VRS | 3 and 6 months |
| LAM 2015 | Paravertebral block | Not specified | None | Pain/no pain | None | 6 months |
| LEE 2013 | Paravertebral block | Preincision, continuous intra-op and post-op vs control | Pregabalin | Pain/no pain | Short-form McGill Pain Questionnaire | 3 months |
| MICHA 2012 | Local infiltration with brachial plexus | Postincision, single shot vs placebo | None | DN4 | None | 6 months |
| STRAZISAR 2012 | Local infiltration | Postincision, continuous post-op vs control | None | Pain/no pain | None | 3 months |
| STRAZISAR 2014 | Local infiltration | Postincision, continuous post-op vs control | None | Pain/no pain | None | 3 months |
| TECIRLI 2014 | Intercostal nerve block | Postincision, single shot vs control | None | DN4 | VAS | 3 months |
| TERKAWI 2015B | IV lidocaine | Preincision, continuous intra-op and post-op vs placebo | None | Pain/no pain | VAS | 6 months |
| CESAREAN SECTION | ||||||
| BOLLAG 2012 | Transversus abdominis plane block | Single shot, post-op vs placebo | Clonidine | None | Short form McGill Pain Questionnaire | 3, 6 and 12 months |
| LAVAND’HOM ME 2007 | Wound irrigation | Preincision, continuous post-op vs control | None | Pain/no pain | Analgesic consumption | 6 months |
| LOANE 2012 | Transversus abdominis plane block | Postincision, single shot vs placebo | None | Pain/no pain | None | 3 months |
| MCKEEN 2014 | Transversus abdominis plane block | Postincision, single shot vs placebo | None | None | SF-36 | 6 months |
| SHAHIN 2010 | Peritoneal instillation | Postincision, single shot vs placebo | None | Pain/no pain | NRS | 8 months |
| SINGH 2013 | Transversus abdominis plane block | Postincision, single shot vs placebo | None | None | NRS | 3 months |
| ILIAC CREST BONE GRAFT | ||||||
| BARKHUYSEN 2010 | Local Infiltration | Postincision, single shot vs control | Epinephrine | Pain/no pain | None | 1 Year |
| GUNDES 2000 | Wound Instillation | Postincision, single shot vs placebo | None | Pain and dysesthesia vs none | None | 3 months |
| SINGH 2007 | Wound Irrigation | Postincision, continuous post-op vs control | None | Pain/no pain | VAS, activity, Satisfaction | 4.7 years |
| PROSTATECTOMY | ||||||
| BROWN 2004 | Spinal | Preincision, continuous intra-op vs placebo | Clonidine | Pain/no pain | Numerical Pain Scale, SF-36 | 3 months |
| GUPTA 2006 | Epidural | Continuous, post-op vs placebo | Adrenaline | None | SF-36 | 3 months |
| THORACATOMY | ||||||
| CAN 2013 | Epidural | Single shot, pre vs postincision, continuous vs control | None | Pain/no pain | VAS, patient Satisfaction | 6 months |
| COMEZ 2015 | Epidural | Preincision, continuous intra-op vs control | Dexketoprofe, morphine, and fentanyl | Pain/no pain | VAS | 3 and 6 months |
| JU 2008 | Epidural | Preincision and post-op vs control | None | Pain/no pain | Allodynia | 12 months |
| KATZ 1996 | Intercostal nerve block | Single shot, postincision vs control | None | Pain/no pain | VRS, analgesic Consumption | 18 months |
| LIU 2015 | Wound Irrigation | Postincision, continuous post-op vs control | Fentanyl | Pain/no pain | None | 3 months |
| LU 2008 | Epidural | Preincision vs post-op vs control | None | Pain/no pain | None | 6 months |
| SENTURK 2002 | Epidural | Preincision vs post-op vs control | None | Pain/no pain | NRS, pain affecting living | 6 months |
| VAGINAL HYSTERECTOMY | ||||||
| PURWAR 2015 | Spinal | Single shot, preincision vs control | Fentanyl | None | VAS, SF-36 | 3 months |
| SPRUNG 2006 | Spinal | Single shot, preincision vs control | Clonidine | None | NRS, SF-36 | 3 months |
| ABDOMINAL HYSTERECTOMY | ||||||
| WODLIN 2011 | Spinal | Single shot, preincision vs control | None | None | SF-36 | 6 months |
Assessment of risk of bias
Following guidance from the Cochrane Handbook, in addition to extracting data in duplicate, two authors independently evaluated the methodological quality of included studies based upon randomization, allocation concealment, observer and participant blinding, selective reporting and funding.[35] Each category and study was graded based upon likelihood of bias (low, high, or unclear), with reasons for the authors’ judgement presented in Suppl. Table 2 Characteristics of included studies. Authors of included trials were also contacted for this purpose to clarify when needed. Otherwise, consensus was reached by having a third author review the study. Attrition and follow-up interval could influence effect size. We explored this graphically, plotting attrition versus effects size in Supplemental Figure 3 Attrition effect size graph.[29,36]
Data synthesis
Responder analysis and summary statistic
Responder analysis considers the number of subjects reporting an above threshold outcome, in our case more than three out of ten pain on numerical rating scale or the equivalent.[16,37] Responder analysis informed also this evidence synthesis, pooling the number of study participants with a favorable outcome (no pain versus pain above threshold beyond three months after surgery). For this dichotomous outcome, we choose the odds ratio as our summary statistic.[38] Despite the different scales and instruments used by the primary study authors, we again accepted their thresholds and definitions for the presence of absence of pain.[26] Our data imputation of missing data used a similar responder analysis concept.[39] The standard mean difference is reported for studies whose pain outcomes instruments were primarily continuous. Confidence intervals were calculated for any statistical measure to precision of our estimates and to make inferences. We calculated and reported the number needed to benefit (NNTB),[40] using the statistical software package R[41] and summarized our results in Summary of Findings Tables, published in the Cochrane Library.[29]
Diversity of Design and Heterogeneity
Diversity of design and outcome reporting remains a major challenge for evidence synthesis of function and pain after surgery.[42] Heterogeneity between studies can be categorized as statistical, methodological, or clinical.[43] Heterogeneity is particularly pronounced among long-term studies. Anticipating challenges posed by the disparate and variable reporting, we had defined our approach a priori.
Clinical Heterogeneity: Stratifying by Surgical Intervention
The first challenge (clinical heterogeneity) is to explain and integrate differences in clinical effects observed between trials at the study or population level. It is well known that effects are contingent on populations, interventions or settings, that clinical differences across individual studies all can induce puzzling variance in effect estimates.[44] We therefore a priori decided to stratify, by grouping the studies according to surgical intervention.[21,29] We followed the paradigm of procedure specific pain control in stratifying our comparisons hierarchically according to surgical procedure in broad groups (breast surgery, thoracotomy, cesarean section, etc.).[40] The diversity in natural histories of PPP after different procedures and significant dissimilarities between populations undergoing different surgeries informed our group choices.
Methodological Heterogeneity
The second challenge (methodological heterogeneity) is to synthesize effect estimates despite differences in design, assessment instruments, follow-up intervals and outcome reporting. For example, we may want to pool dichotomous outcomes (pain versus no pain) with continuous outcomes (numerical Rating Scale 1–10) or to pool studies reporting data at repeated but variable follow-ups without counting any single patient twice. We referenced all manuscripts reporting on included studies, but counted each study only once [28].
POOLING ACROSS VARIABLE FOLLOW-UP INTERVALS
Studies observed participants’ pain outcomes at variable follow-up intervals. We pooled studies across different follow-ups, our primary inclusive analysis approach. [28]. For studies reporting on more than one follow up interval, we used only the latest follow-up, which we considered the most conservative (considering attrition bias in Supplemental Figure 3 Attrition versus effect size graph)[36] and the most impactful, because it investigated the longest lasting sequelae.[29,36] We pooled the data using the inverse variance approach to weight studies adjusted by the variation among their estimates of intervention effects.[41] We chose a priori the random effects method for our meta-analyses, which leads to more cautious effect estimates, to allow for the expected clinical between-study heterogeneity.[42] As a sensitivity analysis, we pooled only studies with similar follow up intervals, with similar inferences, as detailed in our Cochrane Review.[28]
POOLING DICHOTOMOUS WITH CONTINUOUS OUTCOMES
We had a priori planned evidence synthesis to pool dichotomous with continuous outcomes for this review update using a Bayesian approach for the surgical subgroup of Iliac Crest Bone Harvesting. Bayesian statistics is an alternative statistical approach suitable for evidence synthesis.[45] Bayesian hierarchical evidence synthesis can pool effects assessed by different instruments at variable intervals.[16,39,46]
Statistical Heterogeneity
As customary, we explored between-study heterogeneity and reporting bias with classical methods graphically, with funnel plots, the χ2, the I2 statistic[47] and Egger’s test.[48,49] We did so at the subgroup level of our comparisons. Given our between study-heterogeneity, we did not consider Duval and Tweedie’s trim and fill analysis to adjust for publication bias[49,50]. Following the thresholds suggested in the Cochrane Handbook for Systematic Reviews of Interventions, we abstained from pooling studies, if between study statistical heterogeneity seemed excessive.[51]
RESULTS
Results of the search and description of studies
Figure 1 provides a diagrammatic schema of our search update which lead to the identification of 40 new RCTs included in this updated review.[29] In short, searches were conducted from September 2014 to January 2015, April 2015, and updated in December 2016. An additional search was performed in December 2017 with the results added to Studies awaiting classification to be incorporated into the next update of this review.
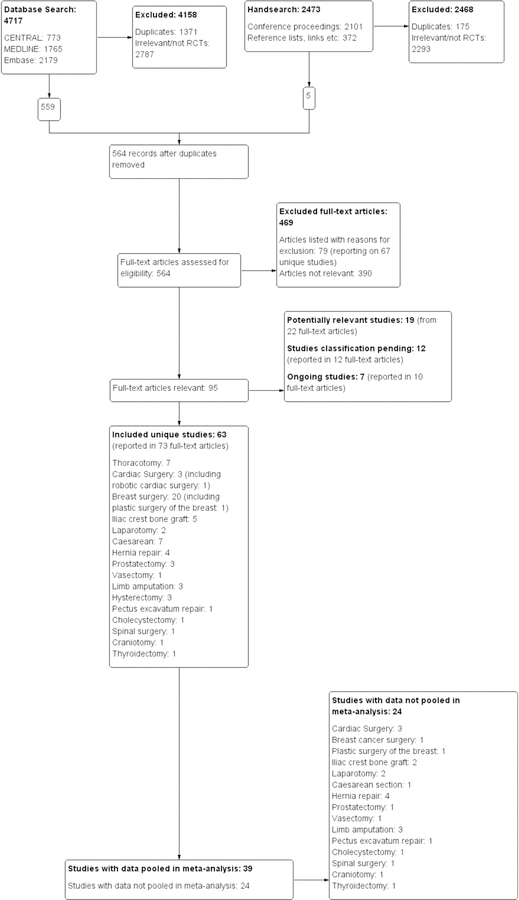
Figure 1: Quorum flow diagram.
The process of reference search and selection is detailed in this Quorum flow diagram, depicting the study flow. Among the 469 articles evaluated in full text, 79 were excluded and listed in the Supple Table 3 of Characteristics of Excluded Studies with details as to why they were excluded. Of the 63 included randomized trials, we were able to include 39 in our inclusive analysis. For the remaining 24 trials, only a single study was found for the surgical intervention investigated, study data were unavailable, or data could not be pooled for other reasons (reported in our Cochrane Review)[29]. We enumerate every single included study for which the data could not be pooled in a meta-analysis in Suppl. Table 4: Study data not included in meta-analysis.
The electronic searches collectively yielded 4717 references, 1765 in MEDLINE, 2179 in EMBASE, and 773 in CENTRAL. Of these, 1371 were determined to be duplicates. Of the remaining references, 2787 were excluded for irrelevance or not being randomized controlled trials. 12 study reports from the search conducted in December 2017 were added to Studies awaiting classification.
This left 564 studies for full text review, of which 63 unique studies were selected for inclusion, among them 40 newly identified RCTs not described in our previous review.[26,27] Additionally, seven ongoing studies, reported in 10 full-text articles, were identified and will be assessed upon completion.
Included Studies
63 studies comparing standard methods to the use of regional or local anesthesia for risk reduction of PPP are included in our review, (among them 40 newly identified RCTs). Study data for 39 trials were pooled in our inclusive meta-analysis. Table 1 provides an overview of the type and timing of the regional or local anesthesia intervention, outcomes, and follow up for the pooled studies. Exhaustive details about each included study, pooled and not, are provided as an online supplement (Suppl. Table 1 Participants of pooled studies by follow up interval Suppl. Table 2 Characteristics of included studies, Suppl. Table 3 Characteristics of excluded studies,) and in the Cochrane Library.[29] For each study not included in a meta-analysis, despite meeting inclusion criteria, we explain why the data were not included in our evidence synthesis in Supplemental Table 4. For some surgical subgroups, the I2 statistics suggested clinical heterogeneity was too large to justify pooling of clinical diverse studies. For some studies, data were not available. No study meeting the inclusion criteria was excluded for methodological shortcomings alone.
Excluded Studies
From the 564 articles selected for full text review, 79 articles, reporting on 67 unique studies, were excluded for reasons other than not being pertinent, with reasons for their exclusion tabulated in the online supplement (Suppl. Table 2 Characteristics of excluded studies).[29] 11 additional studies were excluded for what we determined to be insufficient randomization. 24 included studies were not pooled (Figure 1 Quorum Flow Diagram). The reasons for not pooling them were detailed in the supplement (Suppl. Table 4 Study data not included in meta-analysis) and in the Table of Characteristics of Included Studies published in the Cochrane Review.[29]
Regional Techniques and Surgical Interventions
Included studies were categorized by surgical subgroup. An overview of the surgeries and regional interventions investigated is provided in Table 1. The number of participants enrolled in the pooled studies, broken down by follow-up interval is rendered in the Suppl. Table 1. More comprehensive details, tabulating all study characteristics including methods and risk of bias, are provided online as a supplement (Suppl. Table 2 Characteristics of included studies, Suppl. Table 3 Characteristics of excluded studies, Suppl. Table 4 Study data not included in meta-analysis) are/or published in our Cochrane Review.[29] The method of regional anesthesia application varied typically by surgical subgroup (Table 1). The cesarean section group, for example, employed predominantly Transversus Abdominis Plane blocks, while the thoracotomy group largely utilized epidurals.
Methodological quality and risk of bias of included studies
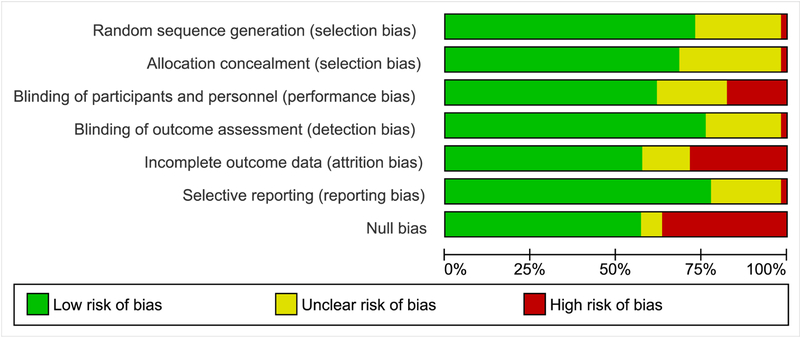
Figure 2 Risk of bias graph presents an overview of the risk of bias for the included studies, Suppl. Figure 1 summarizes the risk of bias for each of the 63 included studies. More detailed tables with explanations and support for the authors’ assignment of the risk of bias are available online as a supplement (Suppl. Table 2 Characteristics of included studies, Suppl. Table 3 Characteristics of excluded studies) and/or in our Cochrane Review.[29]
Figure 2. Risk of bias graph.
Figure 3 summarizes the risk of bias graphically across all included studies based on the review authors’ judgements about selection, performance, detection and attrition bias, as well as selective reporting and Null bias. A comprehensive risk of bias tables, published in our Cochrane Review, provides detail at the study level and support for the judgement in tabular form[29].
Randomization
The method of sequence generation (randomization) was not well described in 11 studies. Further, three studies were excluded for presumed pseudo-randomization.[52–54]
Allocation Concealment
Concealment of allocation via use of sealed opaque envelopes or a similar mode was sufficient in most included studies, but not detailed in 16 studies (Figure 2, Suppl. Figure 1)
Blinding
Only blinding of outcome assessors was a requirement for study inclusion. Because of the evident effects of regional anesthesia, blinding anesthesia providers or participants effectively is difficult and no study was excluded for a lack thereof.
Incomplete Outcome Data
Data for incomplete outcomes was more likely to be reported in newer studies. When data was reported, loss to follow up was significant in many studies. This allows for the possibility of attrition bias[36], explored in Supplemental Figure 3[29,36]. We enumerate every single included study for which the data could not be pooled in a meta-analysis in Suppl. Table 4: Study data not included in meta-analysis.
Selective Reporting
The description of adverse effects among study participants was concerningly sparse. Often, adverse effects were not reported and when they were, details were lacking. Therefore, significant potential for reporting bias of unintended consequences exists.
Effects of Interventions
Thoracotomy
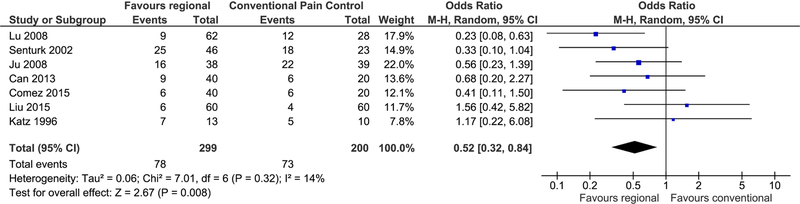
Overall, regional anesthesia was favored over standard analgesia with an OR of 0.52 (95% CI 0.32–0.84, p=0.008) (Figure 3 Forest plot thoracotomy). This results in slightly moderating our previous estimate of 0.34 (95% CI 0.19–0.60). Moderation of effect estimates is typically with the inclusion of more data, now seven studies and a total of 499 participants. We determined there was little heterogeneity among pooled studies (I2=14%).
Figure 3. Forest plot thoracotomy.
In this forest plot, each of the seven randomized trials investigating regional anesthesia for the prevention of prevention of persistent postoperative pain after thoracotomy is depicted as a small blue square. Their sizes correspond to the number of study participants with bars on either side indicating the confidence in the effect estimate. The midline indicates no effect, with studies on the left favoring regional anesthesia. The diamond below reflects the pooled estimate favoring regional anesthesia with an odds ratio of 0.52 and a 95% confidence interval ranging from 0.32 to 0.84. The use of epidural anesthesia may mitigate the risk of persistent pain after thoracotomy in one patient out of every six treated.
Breast Surgery
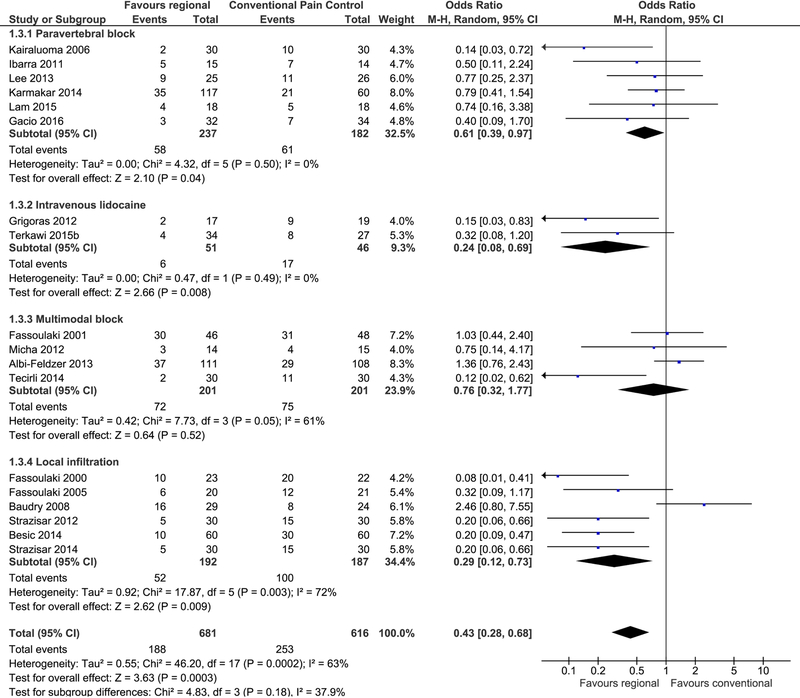
Regional anesthesia was also favored for PPP risk reduction after breast surgery. Pooling 18 studies and 1297 participants reaffirmed an OR of 0.43 and improved our confidence in the estimate (95% CI 0.28 to 0.6, p = 0.0003) (Figure 4 Forest plot breast surgery) compared to our last evidence synthesis of only 4 studies.[29] This evidence synthesis pooled six studies investigating paravertebral block,[55–60] four investigating a multimodal block,[61–64] six investigating local infiltration,[65–70] and two studies investigating intravenous local anesthetics.[71,72] A sub-analysis of the six studies[55–60] employing only paravertebral block still favored regional anesthesia over conventional methods (OR of 0.61, 95% CI 0.39 to 0.97) while reducing the heterogeneity from 63% to 0%. Another study examining plastic surgery of the breast was not included in either analysis because of the difference in surgical technique and participant comorbidity.[73] Nota bene, evidence synthesis of two trials[71,72] with 97 participants showed a statistically meaningful benefit of intravenous local anesthetics for PPP after breast cancer surgery (OR of 0.24; 95% CI 0.08 to 0.69), with a NNTB of 4 (Figure 4 Forrest Plot breast surgery).
Figure 4. Forest plot breast surgery.
18 studies investigating the effect of regional anesthesia for the prevention of persistent pain after breast surgery are grouped by intervention and shown on this forest plot. Each study is shown by a small blue square. The number of study participants and the confidence in the effect estimate are reflected in the size of the square and the lateral bars, respectively. Studies favoring regional anesthesia fall on the left of the midline of no effect. The pooled effect estimates are shown for each subgroup and for all studies as black diamonds. Pooling all studies results favors regional anesthesia (odds ratio 0.43; 95% CI [0.28, 0.68]). The number needed to benefit for paravertebral block for breast cancer surgery is about seven.
Cesarean Section
Chronic postoperative pain was markedly reduced following cesarean section when using regional methods compared to control (Suppl. Figure 2 Forest plot cesarean section), a novel finding. An OR of 0.46 (95% CI 0.28 to 0.78, p = 0.0004) was calculated from pooling four studies[74,75] (551 participants). Heterogeneity was determined to be minimal with an I2=0%. Two additional studies[76,77] reporting continuous outcomes were incorporated in an inclusive analysis but the results were inconclusive.
Iliac Crest Bone Graft Harvesting
Three studies[78–80] with 123 participants analyzing persistent postoperative pain after iliac crest bone graft harvesting (IBGH) were pooled. Though an overall favorable effect was expected with an OR of 0.20, the results were inconclusive as the p value exceeded 0.05. Employing an alternative method, four studies[78–81] and 159 participants were pooled in a Bayesian analysis.[39] Results favored use of regional anesthesia with an OR equal to 0.1 (95% Bayesian credible interval ranging from 0.01 to 0.59). We were unable to include one study observing zero PPP events at 6 months.[82]
Limb Amputation
The timing of intervention studies examining the use of epidural anesthesia to reduce the risk of phantom limb pain after amputation varied, some beginning analgesia 24hrs before surgery. The data from two RCTs[83,84] were not pooled due to this clinical heterogeneity and for others reasons, detailed the supplement to this manuscript (Suppl. Table 4 Study data not included in meta-analysis).[29]
Prostatectomy
Continuous outcome data after prostatectomy was pooled from two studies[85,86] (150 participants). The standard mean difference of 0.06 (95% CI −0.26 to 0.38) was inconclusive.
Hysterectomy
Data of 297 participants of three studies[87–89] investigating the use of regional anesthesia for avoidance of chronic postoperative pain was pooled. The measured outcome was continuous (Short Form Health Survey 36) and the calculated standard mean difference was inconclusive (SMD 1.70, 95% CI −1.06 to 4.46).
Other Surgeries
Two surgical subgroups, vasectomy[90] and pectus excavatum repair,[91] each contained only one study and thus could not be included in the meta-analysis. Clinical heterogeneity was the reason we did not perform evidence synthesis for some surgical subgroups including laparotomy, hernia repair, and cardiac surgery (Supple. Table 4).
DISCUSSION
This review and search update identified 40 new randomized controlled trials investigating the use of regional anesthesia to reduce the risk of PPP three or more months following surgery (Figure 1) and employed a new approach to synthesize the evidence across different follow up intervals within surgical subgroups[29].
Regional anesthesia implemented during thoracotomy, breast surgery, and cesarean section demonstrated a marked reduction in the risk to develop persistent postoperative pain compared to standard analgesia (Figure 3 Forest plot thoracotomy, Figure 4 Forest plot thoracotomy, Suppl. Figure 2 Forest plot cesarean section). In our current reproducibility crisis, this affirmation of our previous evidence synthesis, improving the confidence in our estimates with data from many additional studies is important. The number of about six to seven needed to benefit for thoracotomy (6.3, 95% CI 3.9 to 23) and breast surgery (6.9, 95% CI 5.2 to 13) were slightly adjusted compared to our previous evidence synthesis[29] (Figure 3 and Figure 4).
We tabulated the total 63 trials included (Table 1, Figure 1) in tables and graphs with detailed study level information and methodological quality available online as a supplement (Suppl. Table 1 Participants of pooled studies by follow up interval, Suppl. Table 2 Characteristics of included studies, Figure 2 Risk of bias graph, Suppl. Figure 1 methodological quality summary), and in the Cochrane Library.[29]
The available evidence markedly increased compared to our previous Cochrane review search [which had reached only up to May 2012].[29] Even recent reviews on prevention of PPP failed to cite most of the studies we included.[4,92] The evidence favoring regional anesthesia to reduce the risk of post-mastectomy pain is now supported by 18 studies including 1297 participants, a significant increase in data over our previous review. Our inference that regional anesthesia reduces PPP after cesarean section is novel (Suppl. Figure 2). The number needed to benefit from use of regional anesthesia for cesarean section is 19 (95% CI 14 to 49) (Suppl. Figure 2). In the original protocol, in the first review published as well in this update, we included studies investigating intravenous administration of local anesthetics a priori, because we hypothesize that the mechanism of action of regional anesthesia interventions may not be through locally mediated nociceptive blockade, but through systemically mediated effects.[21,26,27,93] Our evidence synthesis suggested furthermore that intravenous administration of local anesthetics may be equally protective against PPP as regional anesthesia, a remarkable new finding that questions the paradigm of how regional anesthesia works through prevention of central sensitization.[23,93] Data for many studies in the iliac crest bone graft, prostatectomy, and hysterectomy surgical subgroups initially appeared to also favor the use of regional anesthesia. However, results were deemed inconclusive as the confidence interval included the null value. Excessive heterogeneity limited our ability to pool RCTs studying the use of regional anesthesia in laparotomy, hysterectomy, and cardiac surgery. Conclusions could not be drawn from studies investigating limb amputation as the timing of the applied interventions was variable. Another Cochrane review addresses the effect of adjuvant pharmacotherapy on the prevention of PPP.[94]
Limitations
Methodological shortcomings of included studies-in particular small study size, attrition and data loss, high risk of performance bias due to incomplete participant blinding, and high risk of selection bias due to lack of allocation concealment-markedly weaken our conclusions (Figure 2, Suppl. Figure 3 Attrition versus effect size graph). Supporting details with study-level risk of bias tables are available online as a supplement (Suppl. Table 2 Characteristics of included studies, Suppl. Figure 1 Methodological quality summary, Suppl. Table 4: Study data not included in meta-analysis), and published in the Cochrane Library.[29]
Influence of attrition and follow-up interval on effect size
We pooled studies eliciting pain outcomes with different instruments and at variable follow-up intervals to increase our power (Table 1 Overview of surgeries, timing and oud outcomes by subgroup and Suppl. Table 1 Participants pooled by follow up period). Concerns remain about attrition biasing estimates of treatment effects. These may be biased in unforeseeable ways, if outcomes, interventions, or effect mediation are correlated with loss to or duration of follow-up. Consider that participants with persistent pain symptoms may be more likely to be retained in the study, because their symptoms give them reason to continue to seek care. We may hence observe PPP more frequently in the experimental or control group, given differential retention, leading (spurious) effect estimates. Time, healing all wounds, may also mitigate PPP. Reducing signals in both the experimental and the control group, dilution could bias or obliterate effects of regional anesthesia on PPP. We explored this unforeseeable effect of time and attrition on effect estimates graphically in a novel attrition effect size plot (Suppl. Figure 3 Attrition versus effect size graph).[36] We are unaware of a similar graphical test in the context of meta-analysis to investigate the correlation between study effect size estimates and their different follow-up interval or attrition.[29] The graphical exploration is without any apparent trend (Suppl. Figure 3Attrition versus effect size graph) reassuring us about our decision to pool observations across different follow up intervals. Still, the clinical heterogeneity in some subgroups, e.g. breast surgery, and our choice to pool studies across variable follow-up intervals, paired with high risk of bias from lack of participant blinding, may induce skepticism among readers. Small study size alone may explain the variability of effect estimates between studies and constitutes a risk of bias in its own right.[95]
Published aggregate study data did not provide the granularity to discriminate mild PPP from severe disabling PPP.[8] While this is an important distinction,[5] we argue that pain even when not severe, impacts quality of life and function.[6,8] The prevention or mitigation of even mild persistent pain after surgery is an important goal, especially after elective interventions like cesarean section, breast lumpectomy, vasectomy, or after harvesting iliac bone grafts Iliac.
The funnel plot (Suppl. Figure 4 Funnel plot) shown for the subgroup of breast surgery is inconclusive and the small number of included studies precluded a formal analysis of publication bias for the other surgical subgroups. We acknowledge the possible publication bias, given that not all study data were accessible for evidence synthesis (Figure 1, Suppl. Table 4), e.g. due to excessive disparity in design or reporting.[29]
Future Studies
The focus on long-term benefits of regional anesthesia is relatively new, but very promising.[4][3] Evidence is lacking for several surgical interventions. Though limited by technical difficulty and availability of resources, more methodologically sound, randomized controlled trials investigating the use of regional anesthesia, especially in pediatric patients, are desirable. Adaptive trial designs[96,97] and focusing on high risk patients,[5]especially patients with a pain phenotype predisposing them to persistent pain after surgery[98] could increase the yield of interventions and trials, but may render evidence synthesis more difficult. Studies should include validated instruments for chronic pain,[8] and study authors should make individual patient data freely accessible for meta-analysis.[99] Additionally, a direct comparison of the effects of regional techniques versus intravenous infusion of local anesthetics is warranted.[71,72,93] The potential synergy of adjuvant medications with regional anesthesia remains unclear.[94] The definition and nomenclature of PPP is shifting over time and currently varies from 2 to 3 months. [4,5,25,92] We had committed to a cutoff of 3 months for this update. Studies with shorter follow-up are enumerated in Suppl. Table 3 Characteristics of excluded studies and will likely be considered in the subsequent review update.
CONCLUSIONS
The evidence favoring regional anesthesia to reduce the risk of developing persistent pain after surgery increased, with 40 newly identified randomized trials. Data pooled on 3027 participants enrolled in 39 randomized trials (Table 1, Suppl. Table 1, Suppl. Table 2) suggest that regional anesthesia can markedly reduce the risk for persistent postoperative pain beyond three months after many surgical procedures.[29] The evidence is strongest and most homogenous regarding epidurals for thoracotomy (OR of 0.52; 95% CI 0.32–0.84, p=0.008) (Figure 3 Forest plot thoracotomy) and paravertebral blocks for breast surgery (OR of 0.61; 95% CI 0.39 to 0.97, p=0.04) (Figure 4 Forest plot breast surgery). Regional anesthesia may prevent PPP in approximately one out of every six to seven patients undergoing thoracotomy or breast surgery. Surprisingly, two RCTs suggest that continuous intravenous local anesthetic infusion after breast cancer surgery may be at least equally effective (Figure 4 Forest plot breast surgery), a striking new finding that questions the utility and mechanism of regional anesthesia for the reduction of PPP risk altogether. Our results are robust to our modelling choices. However, shortcomings in allocation concealment, performance bias, incomplete outcome data and considerable attrition considerably weaken the confidence in our inferences (Suppl. Table 2 Characteristics of included studies, Figure 2, Suppl. Figure 1). More research is needed in additional surgical subgroups, especially in children and to compare regional versus intravenous administration of local anesthetics. We cannot extrapolate to other regional anesthesia or surgical interventions or to children.
Supplementary Material
The methodological quality of the included studies is graphically summarized in this figure, with details for the judgement substantiated in the Supplemental Table 2 Characteristics of included studies, available online.
Four randomized trials investigating regional anesthesia for the prevention of persistent postoperative pain three to eight months after cesarean section are shown in this forest plot, (with bars on the sides, indicating the confidence interval of the individual study effect estimate). The black diamond indicates the pooled effect estimate, favoring regional anesthesia with an odds ratio of 0.46 [0.28, 0.78].
In this graph, we explore the potential effect of time to follow-up and attrition on effect size in the included randomized trials investigating the benefit of regional anesthesia for the prevention of persistent pain after surgery. Estimates of effect are shown for each study, with attrition in percent of the study population lost to follow up and effect size [log odds ratio] in the y axis. Studies are colored by surgical intervention group. The symbols indicate time to follow up. The symbols decrease in size with attrition. Consecutive follow-up observations for one study are linked with lines. The random pattern observed provides no support against our Null-hypothesis of no association between attrition/follow-up and study effect estimates.
In a graphical analysis of publication[48], we plotted effect estimates versus precision for all outcomes at any follow-up interval for all breast surgery studies, with odds ratio in the x-axis and standard error in the y-axis. For most surgical subgroups, not enough study data was available. Despite a lack of evidence for publication bias in this funnel plot, we acknowledge the risk of publication bias in our review given that not all study outcome data were available for evidence synthesis as detailed in our Cochrane Review[29].
This table of participants by follow-up interval provides a detailed census of the 3027 participants enrolled in those 39 studies we pooled in our inclusive evidence synthesis. Surgical subgroups are listed in the first and the total number of participants for each subgroup in the second column. In the remaining columns, participants are tabulated by follow-up interval for each surgical subgroup. The bulk of outcome data were observed at three and six months postoperatively. Studies reporting outcomes at several follow-up intervals were counted only once, using the final observation reported for this study, to avoid unit of analysis issues.
The supplemental table of characteristics of included studies provides study level details on participants, the regional technique and control, outcomes and study design and substantiates the authors’ judgement of the study’s risk of bias in the domains of selection, performance, detection, attrition, reporting and Null bias. (Footnotes AKA: above-the-knee amputation; BKA: below-the-knee amputation; GA: general anesthesia, VAS: Visual Analogue Scale, VRS: Verbal Rating Scale, NRS: Numerical Rating Scale, SF-36: Short Form 36)
The supplemental table of characteristics of excluded studies list important excluded studies with reasons for their exclusion. (Footnotes ICBG: iliac crest bone graft; IV: intravenous; NMDA : N-methyl-D-aspartate receptor; PCA: patient controlled analgesia; RCT: randomized controlled trial; VAS: visual analogue scale LA: local anesthetic, TAP, Transabdominal plane block, RCT: Randomized controlled trial, SA: Spinal anesthesia)
For each study data not included in a meta-analysis, despite the study meeting inclusion criteria, we explain why the data were not included in our evidence synthesis. For some surgical subgroups, the I2 statistics suggested clinical heterogeneity was too large to justify pooling of clinically diverse studies (Hernia repair, Cardiac surgery, Laparotomy), according to the thresholds suggested in the Cochrane Handbook for Systematic Reviews of Interventions.[51] For some studies, data were not reported in the manuscript or could not be extracted, despite our best efforts to contact the authors (Data NA). Some studies were the only study within their surgical subgroup (Single Study), rendering evidence synthesis pointless. The design of studies investigating limb amputation differed too much to allow evidence synthesis. Additional detail on reasons why study data were not available for inclusion in the evidence synthesis can be found in the extensive Table of Characteristics of Included Studies, published in the full Cochrane Review.[29]
Key Points:
Question: [Can local anesthetics or regional anesthesia & analgesia mitigate the risk of persistent pain after elective surgery in adults and children?]
Findings: [Data from 39 studies enrolling 3027 participants favored epidural anesthesia, regional & intravenous local anesthesia and local infiltration, for thoracotomy, breast cancer surgery, and cesarean section, respectively.
Meaning: [Local anesthetics and regional anesthesia reduce the risk of persistent pain after surgery, but small study size, performance, and attrition bias considerably weakened our conclusions.]
Highlights:
Persistent pain after surgery is frequent, debilitating, and prevention is paramount.
39 RCTs enrolling 3027 participants favored epidural anesthesia, regional & intravenous local anesthesia and local infiltration, for the prevention of persistent pain after thoracotomy, breast cancer surgery, and cesarean section.
Local anesthetics and regional anesthesia reduce the risk of persistent pain after surgery, but small study size, performance, and attrition bias considerably weakened the strength of the evidence.
ACKNOWLEDGEMENTS
Funding:
This publication was supported in part by the CTSA Grant 1 UL1 TR001073-01, 1 TL1 TR001072-01, 5KL2TR001071-03 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH).
Author Contribution:
Jacob L. Levene: This author helped to screen the search results, screen retrieved papers against inclusion criteria, appraise paper quality, abstract data from papers, obtain and screen data on unpublished studies, write to authors of papers for additional information, organize retrieval of papers, manage the data for the review, enter data into Review manager software, check data with double entry, interpret data and make statistical inferences, write this manuscript, and write the Cochrane review this manuscript is the co-publication of.
Erica J. Weinstein: This author helped to screen the search results, screen retrieved papers against inclusion criteria, organize retrieval of papers, appraise paper quality, abstract data from papers, obtain and screen data on unpublished studies, write to authors of papers for additional information, manage the data for the review, enter data into Review manager software, check data with double entry, interpret data and make statistical inferences, and write the Cochrane review this manuscript is the co-publication of.
Marc S. Cohen: This author helped to screen the search results, screen retrieved papers against inclusion criteria, appraise paper quality, abstract data from papers, enter data into Review manager software, revise the review manuscripts and discuss the interpretation of the statistical inferences.
Doerthe A. Andreae: This author helped to perform previous work that was the foundation of the present study, screen the search results, organize retrieval of papers, screen retrieved papers against inclusion criteria, appraise paper quality, abstract data from papers, enter data into Review manager software, interpret the data, and revise the review manuscripts.
Jerry Y. Chao: This author helped to revise the review protocol with regards to inclusion of children, screen the search results, screen retrieved papers against inclusion criteria, appraise paper quality, abstract data from papers, revise the review manuscripts and discuss the interpretation of the data.
Matthew Johnson: This author helped to perform statistical analysis not using RevMan (Bayesian models for iliac crest bone graft harvesting) and to interpret the data and draw conclusions.
Charles B. Hall: This author helped to perform statistical analysis not using RevMan, interpret data and make statistical inferences, conceive and design graphical interpretation of attrition, writing and reviewing the Cochrane manuscript this manuscript is a cop-publication of.
Michael H. Andreae: This author helped to conceive and coordinate the review, design the original protocol and the revised protocol for this review, screen the search results, screen retrieved papers against inclusion criteria, organize retrieval of papers, appraise paper quality, abstract data from papers, obtain and screen data on unpublished studies, write to authors of papers for additional information, manage the data for the review, enter data into Review manager software, to check data with double entry, interpret data and make statistical inferences, conceive and design graphical interpretation of attrition, perform statistical analysis not using RevMan, secure funding for this review, write this manuscript, and write the Cochrane review this manuscript is the co-publication of.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Interim data from this work were presented at the 2017 Annual Meeting of the American Society Anesthesiology in Boston, October 21 to October 25, 2017.
Conflicts of Interest:
The authors declare no conflicts of interest.
This review is an abridged version of a Cochrane Review previously published in the Cochrane Database of Systematic Reviews 2018, Issue 6, DOI: 10.1002/14651858.CD007105.pub4 (see www.thecochranelibrary.com for information). Cochrane Reviews are regularly updated as new evidence emerge sand in response to feedback, and Cochrane Database of Systematic Reviews should be consulted for the most recent version of the review.
REFERENCES
- [1].Vetter TR, Boudreaux AM, Jones KA, Hunter JM Jr, Pittet J-F. The perioperative surgical home: how anesthesiology can collaboratively achieve and leverage the triple aim in health care. Anesth Analg 2014;118:1131–6. doi: 10.1213/ANE.0000000000000228. [DOI] [PubMed] [Google Scholar]
- [2].Mariano ER. Making It Work: Setting up a Regional Anesthesia Program that Provides Value. Anesthesiol Clin 2008;26:681–92. doi: 10.1016/j.anclin.2008.07.006. [DOI] [PubMed] [Google Scholar]
- [3].Atchabahian A, Andreae M. Long-term functional outcomes after regional anesthesia: a summary of the published evidence and a recent Cochrane Review. Refresh Courses Anesthesiol 2015;43:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Richebé P, Capdevila X, Rivat C. Persistent Postsurgical Pain. Anesthesiology 2018:1. doi: 10.1097/ALN.0000000000002238. [DOI] [PubMed] [Google Scholar]
- [5].Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- [6].MacRae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth 2008;101:77–86. [DOI] [PubMed] [Google Scholar]
- [7].Edwards RR, Mensing G, Cahalan C, Greenbaum S, Narang S, Belfer I, et al. Alteration in Pain Modulation in Women With Persistent Pain After Lumpectomy: Influence of Catastrophizing. J Pain Symptom Manage 2013;46:30–42. doi: 10.1016/j.jpainsymman.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gewandter JS, Dworkin RH, Turk DC, Farrar JT, Fillingim RB, Gilron I, et al. Research design considerations for chronic pain prevention clinical trials: IMMPACT recommendations. Pain 2015;7:1184–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis. J Pain 2014;15:887–97. [DOI] [PubMed] [Google Scholar]
- [10].Perkins FM, Kehlet H. Chronic pain after surgery: a review of predictive factors. Anesthesiology 2000;93:1123–33. [DOI] [PubMed] [Google Scholar]
- [11].Sng BL, Sia AT, Quek K, Woo D, Lim Y. Incidence and risk factors for chronic pain after caesarean section under spinal anaesthesia. Anaesth Intensive Care 2009;37:748–52. [DOI] [PubMed] [Google Scholar]
- [12].Gottschalk A, Cohen SP, Yang S, Ochroch EA. Preventing and treating pain after thoracic surgery. Anesthesiology 2006;104:594–600. [DOI] [PubMed] [Google Scholar]
- [13].McCartney CJL, Tremblay S. Chronic Pain After Surgery. Essentials Pain Med, Elsevier; 2018, p. 147–154.e2. doi: 10.1016/B978-0-323-40196-8.00018-8. [DOI] [Google Scholar]
- [14].Guimarães-Pereira L, Reis P, Abelha F, Azevedo LF, Castro-Lopes JM. Persistent postoperative pain after cardiac surgery. Pain 2017;158:1869–85. doi: 10.1097/j.pain.0000000000000997. [DOI] [PubMed] [Google Scholar]
- [15].Niraj G, Rowbotham DJ. Persistent postoperative pain: where are we now? Br J Anaesth 2011;107:25–9. doi: 10.1093/bja/aer116. [DOI] [PubMed] [Google Scholar]
- [16].Andreae MH, Carter GM, Shaparin N, Suslov K, Ellis RJ, Ware MA, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain 2015;16:1221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet 2011;377:2226–35. doi: 10.1016/S0140-6736(11)60402-9. [DOI] [PubMed] [Google Scholar]
- [18].Kalso E Persistent post-surgery pain: research agenda for mechanisms, prevention, and treatment. Br J Anaesth 2013;111:9–12. doi: 10.1093/bja/aet211. [DOI] [PubMed] [Google Scholar]
- [19].Hah JM, Bateman BT, Ratliff J, Curtin C, Sun E. Chronic Opioid Use After Surgery. Anesth Analg 2017;125:1733–40. doi: 10.1213/ANE.0000000000002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Coley KC, Williams BA, DaPos SV, Chen C, Smith RB. Retrospective evaluation of unanticipated admissions and readmissions after same day surgery and associated costs. J Clin Anesth 2002;14:349–53. [DOI] [PubMed] [Google Scholar]
- [21].Andreae MH, Andreae DA, Motschall E, Rücker G, Timmer A. Local anaesthetics and regional anaesthesia for preventing chronic pain after surgery. Cochrane Database Syst Rev 2008. [DOI] [PMC free article] [PubMed]
- [22].Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science (80- ) 2000;288:1765–9. [DOI] [PubMed] [Google Scholar]
- [23].Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011;152:S2–15. doi: 10.1016/J.PAIN.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Duarte AM, Pospisilova E, Reilly E, Mujenda F, Hamaya Y, Strichartz GR. Reduction of postincisional allodynia by subcutaneous bupivacaine: findings with a new model in the hairy skin of the rat. Anesthesiology 2005;103:113–25. [DOI] [PubMed] [Google Scholar]
- [25].Chapman CR, Vierck CJ. The Transition of Acute Postoperative Pain to Chronic Pain: An Integrative Overview of Research on Mechanisms. J Pain 2017;18:359.e1–359.e38. doi: 10.1016/j.jpain.2016.11.004. [DOI] [PubMed] [Google Scholar]
- [26].Andreae MH, Andreae DA. Local anaesthetics and regional anaesthesia for preventing chronic pain after surgery. Cochrane Database Syst Rev 2012. [DOI] [PMC free article] [PubMed]
- [27].Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta-analysis. Br J Anaesth 2013;111:711–20. doi: 10.1093/bja/aet213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: A Cochrane systematic review and meta-analysis. Br J Anaesth 2013;111. doi: 10.1093/bja/aet213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weinstein EJ, Levene JL, Cohen MS, Andreae DA, Chao JY, Johnson M, et al. Local anaesthetics and regional anaesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database Syst Rev 2018;6:CD007105. doi: 10.1002/14651858.CD007105.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [31].Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011. Available from HandbookCochraneOrg n.d. [Google Scholar]
- [32].Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. J Med Libr Assoc 2006;94:130–6. [PMC free article] [PubMed] [Google Scholar]
- [33].Evidence Partners. DestillerSR 2018.
- [34].The Nordic Center. Review Manager 5 (RevMan 5) 2014.
- [35].Higgins JPT, Altman DG, Sterne JAC (.e.d.i.t.o.r.s.). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011. Available from HandbookCochraneOrg n.d. [Google Scholar]
- [36].Levene J, Weinstein E, Cohen M, Hall C, Johnson M, Andreae M. The impact of attrition on effect size in meta-analysis: a graphical test WwwAsaabstractsCom/n.d:A2092–A2092.
- [37].Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146:238–44. [DOI] [PubMed] [Google Scholar]
- [38].Bland JM, Altman DG. Statistics notes. The odds ratio. BMJ 2000;320:1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Andreae MH, Johnson M, Sacks H. Bayesian responder meta-analysis of regional anaesthesia to prevent chronic pain after iliac crest bone graft harvesting. Reg Anesth Pain Med 2013;38:A1–A1. [Google Scholar]
- [40].Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011. Available from HandbookCochraneOrg n.d. [Google Scholar]
- [41].R Development Core Team. R: a language and environment for statistical computing [2.12.2] 2011.
- [42].Atchabahian A, Schwartz G, Hall CB, Lajam CM, Andreae MH. Regional analgesia for improvement of long-term functional outcome after elective large joint replacement. Cochrane Database Syst Rev 2015. [DOI] [PMC free article] [PubMed]
- [43].Melsen WG, Bootsma MCJ, Rovers MM, Bonten MJM. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect 2014;20:123–9. doi: 10.1111/1469-0691.12494. [DOI] [PubMed] [Google Scholar]
- [44].Bailey KR. Inter-study differences: how should they influence the interpretation and analysis of results? Stat Med n.d;6:351–60. [DOI] [PubMed] [Google Scholar]
- [45].Gelman A Carlin JB, Stern HS, Rubin DBBT-BDA. Bayesian data analysis Abdindon, OXON, UK: Taylor & Francis; 2014. [Google Scholar]
- [46].Carter GM, Indyk D, Johnson M, Andreae M, Suslov K, Busani S, et al. Micronutrients in HIV: a Bayesian meta-analysis. PLoS One 2015;10:e0120113–e0120113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;15:1539–58. [DOI] [PubMed] [Google Scholar]
- [48].Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sterne JAC, Egger M, D M. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011. [Google Scholar]
- [50].Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [51].Deeks JJ, Higgins JPT, DG A. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011. [Google Scholar]
- [52].Bach S, Noreng MF, Tjéllden NU. Phantom limb pain in amputees during the first 12 months following limb amputation, after preoperative lumbar epidural blockade. Pain 1988;33:297–301. [DOI] [PubMed] [Google Scholar]
- [53].da Costa VV, de Oliveira SB, Fernandes Mdo C, Saraiva RA. Incidence of regional pain syndrome after carpal tunnel release. Is there a correlation with the anesthetic technique? Rev Bras Anestesiol 2011;61:425–33. [DOI] [PubMed] [Google Scholar]
- [54].Nikolajsen L, Ilkjaer S, Christensen JH, Kroner K, Jensen TS. Randomised trial of epidural bupivacaine and morphine in prevention of stump and phantom pain in lower-limb amputation. Lancet 1997;350:1353–7. [DOI] [PubMed] [Google Scholar]
- [55].Kairaluoma PM, Bachmann MS, Rosenberg PH, Pere PJ. Preincisional paravertebral block reduces the prevalence of chronic pain after breast surgery. Anesth Analg 2006;103:703–8. doi: 10.1213/01.ane.0000230603.92574.4e. [DOI] [PubMed] [Google Scholar]
- [56].Ibarra MML S-Carralero G-CM, Vicente GU, Cuartero del Pozo A, López Rincón R, Fajardo del Castillo MJ. [Chronic postoperative pain after general anesthesia with or without a single-dose preincisional paravertebral nerve block in radical breast cancer surgery]. Rev Esp Anestesiol Reanim 2011;58:290–4. [DOI] [PubMed] [Google Scholar]
- [57].Lee P, McAuliffe N, Dunlop C, Palanisamy M, Shorten G. A comparison of the effects of two analgesic regimens on the development of persistent post-surgical pain (PPSP) after breast surgery. Jurnalul Rom Anestezie Ter Intensiva/Romanian J Anaesth Intensive Care 2013;20:83–93. [Google Scholar]
- [58].Karmakar MK, Samy W, Li JW, Lee A, Chan WC, Chen PP, et al. Thoracic Paravertebral Block and Its Effects on Chronic Pain and Health-Related Quality of Life After Modified Radical Mastectomy. Reg Anesth Pain Med 2014;39:289–98. doi: 10.1097/AAP.0000000000000113. [DOI] [PubMed] [Google Scholar]
- [59].Lam D, Green J, Henschke S, Cameron J, Hamilton S, Van Wiingaarden-Stephens M, et al. Paravertebral block vs. sham in the setting of a multimodal analgesia regimen and total intravenous anesthesia for mastectomy: a prospective, randomized, controlled trial. 40th Annu. Reg. Anesthesiol. Acute Pain Med. Meet, 2015.
- [60].Gacio MF, Lousame AMA, Pereira S, Castro C, Santos J. Paravertebral block for management of acute postoperative pain and intercostobrachial neuralgia in major breast surgery. Brazilian J Anesthesiol (English Ed 2016;66:475–84. doi: 10.1016/j.bjane.2015.02.007. [DOI] [PubMed] [Google Scholar]
- [61].Fassoulaki A, Sarantopoulos C, Melemeni A, Hogan Q. Regional block and mexiletine: the effect on pain after cancer breast surgery. Reg Anesth Pain Med 2001;26:223–8. doi: 10.1053/rapm.2001.23205. [DOI] [PubMed] [Google Scholar]
- [62].Micha G, Vassi A, Balta M, Panagiotidou O, El Saleh M, Chondreli S. The effect of local infiltration of ropivacaine on the incidence of chronic neuropathic pain after modified radical mastectomy. Eur J Anaesthesiol 2012;29:199. [Google Scholar]
- [63].Albi-Feldzer A, Mouret-Fourme EE, Hamouda S, Motamed C, Dubois P-Y, Jouanneau L, et al. A double-blind randomized trial of wound and intercostal space infiltration with ropivacaine during breast cancer surgery: effects on chronic postoperative pain. Anesthesiology 2013;118:318–26. doi: 10.1097/ALN.0b013e31827d88d8. [DOI] [PubMed] [Google Scholar]
- [64].Tecirli AT, Inan N, Inan G, Kurukahveci O, Kuruoz S. The effects of intercostobrachial nerve block on acute and chronic pain after unilateral mastectomy and axillary lymph node dissection surgery. Pain Pract 2014;14:63. doi: 10.1111/papr.12201. [DOI] [Google Scholar]
- [65].Fassoulaki A, Sarantopoulos C, Melemeni A, Hogan Q. EMLA reduces acute and chronic pain after breast surgery for cancer. Reg Anesth Pain Med 2000;25:350–5. doi: 10.1053/rapm.2000.7812. [DOI] [PubMed] [Google Scholar]
- [66].Fassoulaki A, Triga A, Melemeni A, Sarantopoulos C. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesth Analg 2005;101:1427–32. doi: 10.1213/01.ANE.0000180200.11626.8E. [DOI] [PubMed] [Google Scholar]
- [67].Baudry G, Steghens A, Laplaza D, Koeberle P, Bachour K, Bettinger G, et al. Ropivacaine infiltration during breast cancer surgery: postoperative acute and chronic pain effect. Ann Fr Anesth Reanim 2008;27:979–86. [DOI] [PubMed] [Google Scholar]
- [68].Strazisar B, Besic N. Comparison of continuous local anesthetic and systemic pain treatment after axillary lymphadenectomy in breast carcinoma patients - A prospective randomized study - Final results. Reg Anesth Pain Med 2012;37:E218. doi: 10.1097/AAP.0b013e31826a8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Besic N, Strazisar B. Incidence of chronic pain after continuous local anesthetic in comparison to standard systemic pain treatment after axillary lymphadenectomy or primary reconstruction with a tissue expander in breast carcinoma patients: A prospective randomized study. Ann Surg Oncol 2014;21:S47–8. doi: 10.1245/s10434-013-3474-8. [DOI] [Google Scholar]
- [70].Strazisar B, Besic N. Continuous infusion of local anesthetic into surgical wound after breast cancer operations efficiently reduces pain. Reg Anesth Pain Med 2014;39:e219–e219. [Google Scholar]
- [71].Grigoras A, Lee P, Sattar F, Shorten G. Perioperative Intravenous Lidocaine Decreases the Incidence of Persistent Pain After Breast Surgery. Clin J Pain 2012;28:567–72. doi: 10.1097/AJP.0b013e31823b9cc8. [DOI] [PubMed] [Google Scholar]
- [72].Terkawi AS, Sharma S, Durieux ME, Thammishetti S, Brenin D, Tiouririne M. Perioperative lidocaine infusion reduces the incidence of post-mastectomy chronic pain: a double-blind, placebo-controlled randomized trial. Pain Physician n.d;18:E139–46. [PubMed] [Google Scholar]
- [73].Bell RF, Sivertsen A, Mowinkel P, Vindenes H. A bilateral clinical model for the study of acute and chronic pain after breast-reduction surgery. Acta Anaesthesiol Scand 2001;45:576–82. [DOI] [PubMed] [Google Scholar]
- [74].Bollag L, Richebe P, Siaulys M, Ortner CM, Gofeld M, Landau R. Effect of transversus abdominis plane block with and without clonidine on post-cesarean delivery wound hyperalgesia and pain. Reg Anesth Pain Med 2012;37:508–14. doi: 10.1097/AAP.0b013e318259ce35. [DOI] [PubMed] [Google Scholar]
- [75].Lavand’homme PM, Roelants F, Waterloos H, De Kock MF. Postoperative analgesic effects of continuous wound infiltration with diclofenac after elective cesarean delivery. Anesthesiology 2007;106:1220–5. doi: 10.1097/01.anes.0000267606.17387.1d. [DOI] [PubMed] [Google Scholar]
- [76].McKeen DM, George RB, Boyd JC, Allen VM, Pink A. Transversus abdominis plane block does not improve early or late pain outcomes after Cesarean delivery: a randomized controlled trial. Can J Anesth Can d’anesthésie 2014;61:631–40. doi: 10.1007/s12630-014-0162-5. [DOI] [PubMed] [Google Scholar]
- [77].Singh S, Dhir S, Marmai K, Rehou S, Silva M, Bradbury C. Efficacy of ultrasound-guided transversus abdominis plane blocks for post-cesarean delivery analgesia: A double-blind, dose-comparison, placebo-controlled randomized trial. Int J Obstet Anesth 2013;22:188–93. doi: 10.1016/j.ijoa.2013.03.003. [DOI] [PubMed] [Google Scholar]
- [78].Barkhuysen R, Meijer GJ, Soehardi A, Merkx MAW, Borstlap WA, Bergé SJ, et al. The effect of a single dose of bupivacaine on donor site pain after anterior iliac crest bone harvesting. Int J Oral Maxillofac Surg 2010;39:260–5. doi: 10.1016/j.ijom.2009.10.015. [DOI] [PubMed] [Google Scholar]
- [79].Gundes H, Kilickan L, Gurkan Y, Sarlak A, Toker K. Short- and long-term effects of regional application of morphine and bupivacaine on the iliac crest donor site. Acta Orthop Belg 2000;66:341–4. [PubMed] [Google Scholar]
- [80].Singh K, Phillips FM, Kuo E, Campbell M. A prospective, randomized, double-blind study of the efficacy of postoperative continuous local anesthetic infusion at the iliac crest bone graft site after posterior spinal arthrodesis: a minimum of 4-year follow-up. Spine (Phila Pa 1976) 2007;32:2790–6. doi: 10.1097/BRS.0b013e31815b7650. [DOI] [PubMed] [Google Scholar]
- [81].Blumenthal S, Dullenkopf A, Rentsch K, Borgeat A. Continuous infusion of ropivacaine for pain relief after iliac crest bone grafting for shoulder surgery. Anesthesiology 2005;102:392–7. [DOI] [PubMed] [Google Scholar]
- [82].O’Neill KR, Lockney DT, Bible JE, Crosby CG, Devin CJ. Bupivacaine for pain reduction after iliac crest bone graft harvest. Orthopedics 2014;37:e428–34. [DOI] [PubMed] [Google Scholar]
- [83].Karanikolas M, Aretha D, Monantera G, TsolakisI, Swarm RA, Filos KS. Rigorous perioperative analgesia decreases phantom pain frequency and intensity after lower limb amputation. A prospective, randomized, double-blind clinical trial XXV Annu Congr Eur Soc Reg Anaesthesia, Monte Carlo, Monaco: 2006. [Google Scholar]
- [84].Katsuly-Liapis I, Georgakis P, Tierry C. Preemptive extradural analgesia reduces the incidence of phantom pain in lower limb amputees. Br J Anaesth 1996;76 Suppl 2:125: A410–125: A410. [Google Scholar]
- [85].Brown DR, Hofer RE, Patterson DE, Fronapfel PJ, Maxson PM, Narr BJ, et al. Intrathecal anesthesia and recovery from radical prostatectomy: a prospective, randomized, controlled trial. Anesthesiology 2004;100:926–34. [DOI] [PubMed] [Google Scholar]
- [86].Gupta A, Fant F, Axelsson K, Sandblom D, Rykowski J, Johansson J-E, et al. Postoperative analgesia after radical retropubic prostatectomy: a double-blind comparison between low thoracic epidural and patient-controlled intravenous analgesia. Anesthesiology 2006;105:784–93. [DOI] [PubMed] [Google Scholar]
- [87].Purwar B, Ismail KM, Turner N, Farrell A, Verzune M, Annappa M, et al. General or Spinal Anaesthetic for Vaginal Surgery in Pelvic Floor Disorders (GOSSIP): a feasibility randomised controlled trial. Int Urogynecol J 2015;26:1171–8. doi: 10.1007/s00192-015-2670-4. [DOI] [PubMed] [Google Scholar]
- [88].Sprung J, Sanders MS, Warner ME, Gebhart JB, Stanhope CR, Jankowski CJ, et al. Pain relief and functional status after vaginal hysterectomy: intrathecal versus general anesthesia. Can J Anaesth 2006;53:690–700. doi: 10.1007/BF03021628. [DOI] [PubMed] [Google Scholar]
- [89].Wodlin NB, Nilsson L, Arestedt K, Kjolhede P. Mode of anesthesia and postoperative symptoms following abdominal hysterectomy in a fast-track setting. Acta Obstet Gynecol Scand 2011;90:369–79. [DOI] [PubMed] [Google Scholar]
- [90].Paxton LD, Huss BK, Loughlin V, Mirakhur RK. Intra-vas deferens bupivacaine for prevention of acute pain and chronic discomfort after vasectomy. Br J Anaesth 1995;74:612–3. [DOI] [PubMed] [Google Scholar]
- [91].Weber T, Matzl J, Rokitansky A, Klimscha W, Neumann K, Deusch E. Superior postoperative pain relief with thoracic epidural analgesia versus intravenous patient-controlled analgesia after minimally invasive pectus excavatum repair. J Thorac Cardiovasc Surg 2007;134:865–70. [DOI] [PubMed] [Google Scholar]
- [92].Anwar S, O’Brien B. The role of intraoperative interventions to minimise chronic postsurgical pain. Br J Pain 2017;11:186–91. doi: 10.1177/2049463717720640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Strichartz GR. Novel ideas of local anaesthetic actions on various ion channels to ameliorate postoperative pain. Br J Anaesth 2008;101:45–7. [DOI] [PubMed] [Google Scholar]
- [94].Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev 2013. [DOI] [PMC free article] [PubMed]
- [95].Moore RA, Derry S, Wiffen PJ. Challenges in design and interpretation of chronic pain trials. Br J Anaesth 2013;111:38–45. [DOI] [PubMed] [Google Scholar]
- [96].Pallmann P, Bedding AW, Choodari-Oskooei B, Dimairo M, Flight L, Hampson LV., et al. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med 2018;16:29. doi: 10.1186/s12916-018-1017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Park JJ, Thorlund K, Mills EJ. Critical concepts in adaptive clinical trials. Clin Epidemiol 2018;10:343–51. doi: 10.2147/CLEP.S156708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Schreiber KL, Kehlet H, Belfer I, Edwards RR. Predicting, preventing and managing persistent pain after breast cancer surgery: the importance of psychosocial factors. Pain Manag 2014;4:445–59. doi: 10.2217/pmt.14.33. [DOI] [PubMed] [Google Scholar]
- [99].Simmonds MC, Higginsa JPT, Stewartb LA, Tierneyb JF, Clarke MJ, Thompson SG. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials J Soc Clin Trials 2005;2:209–17. doi: 10.1191/1740774505cn087oa. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The methodological quality of the included studies is graphically summarized in this figure, with details for the judgement substantiated in the Supplemental Table 2 Characteristics of included studies, available online.
Four randomized trials investigating regional anesthesia for the prevention of persistent postoperative pain three to eight months after cesarean section are shown in this forest plot, (with bars on the sides, indicating the confidence interval of the individual study effect estimate). The black diamond indicates the pooled effect estimate, favoring regional anesthesia with an odds ratio of 0.46 [0.28, 0.78].
In this graph, we explore the potential effect of time to follow-up and attrition on effect size in the included randomized trials investigating the benefit of regional anesthesia for the prevention of persistent pain after surgery. Estimates of effect are shown for each study, with attrition in percent of the study population lost to follow up and effect size [log odds ratio] in the y axis. Studies are colored by surgical intervention group. The symbols indicate time to follow up. The symbols decrease in size with attrition. Consecutive follow-up observations for one study are linked with lines. The random pattern observed provides no support against our Null-hypothesis of no association between attrition/follow-up and study effect estimates.
In a graphical analysis of publication[48], we plotted effect estimates versus precision for all outcomes at any follow-up interval for all breast surgery studies, with odds ratio in the x-axis and standard error in the y-axis. For most surgical subgroups, not enough study data was available. Despite a lack of evidence for publication bias in this funnel plot, we acknowledge the risk of publication bias in our review given that not all study outcome data were available for evidence synthesis as detailed in our Cochrane Review[29].
This table of participants by follow-up interval provides a detailed census of the 3027 participants enrolled in those 39 studies we pooled in our inclusive evidence synthesis. Surgical subgroups are listed in the first and the total number of participants for each subgroup in the second column. In the remaining columns, participants are tabulated by follow-up interval for each surgical subgroup. The bulk of outcome data were observed at three and six months postoperatively. Studies reporting outcomes at several follow-up intervals were counted only once, using the final observation reported for this study, to avoid unit of analysis issues.
The supplemental table of characteristics of included studies provides study level details on participants, the regional technique and control, outcomes and study design and substantiates the authors’ judgement of the study’s risk of bias in the domains of selection, performance, detection, attrition, reporting and Null bias. (Footnotes AKA: above-the-knee amputation; BKA: below-the-knee amputation; GA: general anesthesia, VAS: Visual Analogue Scale, VRS: Verbal Rating Scale, NRS: Numerical Rating Scale, SF-36: Short Form 36)
The supplemental table of characteristics of excluded studies list important excluded studies with reasons for their exclusion. (Footnotes ICBG: iliac crest bone graft; IV: intravenous; NMDA : N-methyl-D-aspartate receptor; PCA: patient controlled analgesia; RCT: randomized controlled trial; VAS: visual analogue scale LA: local anesthetic, TAP, Transabdominal plane block, RCT: Randomized controlled trial, SA: Spinal anesthesia)
For each study data not included in a meta-analysis, despite the study meeting inclusion criteria, we explain why the data were not included in our evidence synthesis. For some surgical subgroups, the I2 statistics suggested clinical heterogeneity was too large to justify pooling of clinically diverse studies (Hernia repair, Cardiac surgery, Laparotomy), according to the thresholds suggested in the Cochrane Handbook for Systematic Reviews of Interventions.[51] For some studies, data were not reported in the manuscript or could not be extracted, despite our best efforts to contact the authors (Data NA). Some studies were the only study within their surgical subgroup (Single Study), rendering evidence synthesis pointless. The design of studies investigating limb amputation differed too much to allow evidence synthesis. Additional detail on reasons why study data were not available for inclusion in the evidence synthesis can be found in the extensive Table of Characteristics of Included Studies, published in the full Cochrane Review.[29]