Abstract
Objectives: Ginsenosides Rb1 (Rb1) could form micelles in aqueous solutions. Self-assembled Rb1 micelles could potentially be utilized as ocular drug delivery system, and it was postulated that the encapsulation of a medicine within Rb1 micelles might strengthen the drug’s therapeutic action and reduce side effects.
Methods: Diclofenac-loaded Rb1 micelles (Rb1-Dic micelles) were formulated, optimized, and then further evaluated for in vitro cytotoxicity/in vivo ocular irritation, in vivo corneal permeation, and in vivo anti-inflammatory efficacy.
Results: Rb1 self-assembled into micelles with ultra-small particle size (<8 nm) in a homogeneous distribution state (polydispersity index [PDI] < 0.3). Diclofenac was highly encapsulated into the micelles according to the weight ratios of Rb1 to diclofenac. The ophthalmic solution of Rb1-Dic micelle was simple to prepare. Rb1 had good cellular tolerance, and it also improved the cellular tolerance of the encapsulated diclofenac. Rb1-Dic micelles also showed non-irritants to the rabbit eyes. The use of Rb1 micelles significantly improved the in vivo corneal permeation as well as the anti-inflammatory efficacy of diclofenac when compared to commercial diclofenac eye drops.
Conclusion: Rb1 micelle formulations have great potential as a novel ocular drug delivery system to improve the bioavailability of drugs such as diclofenac.
Keywords: Ginsenoside Rb1, diclofenac, micelle, ocular drug delivery
Introduction
Most ophthalmic medications are formulated as eye drops intended for topical delivery to the eye (Jiao, 2008; Di Trani et al., 2019). However, the bioavailability of eye drops is typically less than 5%, despite frequent instillations. Attempts to increase the bioavailability of the ocular topical formulation have focused on the use of novel formulations, and especially nanomaterial or nanocarrier based drug delivery systems, to address the challenges imposed by conventional eye drops. However, a major challenge with these nanomaterial-based drug delivery systems is that the nanomaterials have unknown or objectionable toxicity profiles and are not approved by regulatory authorities (Suresh & Sah, 2014; Song et al., 2018).
Several in vivo studies have shown that some nanomaterials do not induce any signs of irritation or inflammatory responses (Wadhwa et al., 2009; Li et al., 2012); however, the long-term effects of these nanomaterials in both the ocular region and the systemic circulation require rigorous analysis. This is especially the case for non-biodegradable polymers, but the fate of polymers after ocular application remains poorly studied.
Because of the potential risks arising from the use of nanomaterials in the eye, green chemistry and green preparation are welcomed in the design of ocular drug delivery systems (Vaccaro et al., 2014). Delivery systems prepared with natural nanomaterials are of particular interests for applications in food, pharmaceutics, and biomedicine (Luo et al., 2015; Song et al., 2018). Natural nanomaterials, especially those small molecules that are available in large quantities from renewable sources, are viewed as safe for use in drug delivery systems, and pharmaceutical formulations based on natural small molecules are considered to represent nontoxic materials (Song et al., 2018).
Over the past few decades, the use of naturally sourced materials for ocular drug delivery has attracted considerable attention (Wadhwa et al., 2009). Rebaudioside A, a kind of steviol glycoside extracted from the natural herb Stevia rebaudiana Bertoni, was designed as a potential ocular drug-delivery system based on its self-assembling into micelles (Song et al., 2018). However, the aqueous solubility of rebaudioside A limited its application in ocular drug-delivery system. A concentration less than 20 mg/ml of rebaudioside A had to be kept in mind as the ophthalmic solution formulation based on higher concentration was observed with precipitation of rebaudioside A (Song et al., 2018). Another natural small molecule of note is ginsenoside Rb1 (Rb1), one of the principle bioactive ingredients in the roots of Panax ginseng. Rb1 is of considerable medicinal interest due to its wide-ranging biological functions that include antioxidative, anti-inflammatory, and various neuroprotective effects. All these Rb1 biological functions would be useful in the treatment of eye diseases such as diabetic keratopathy, making Rb1 also attractive for ophthalmological purposes. In addition, ginsenosides (including Rb1, the major (20S)-protopanaxadiol type) have both hydrophobic triterpenes or steroid aglycones and hydrophilic sugar side chain(s) in their structures, which allows them to form micelles in aqueous solutions (Xiong et al., 2008; Zhang et al., 2012; Dai et al., 2013). We, therefore, considered that self-assembled micelles of Rb1 could potentially be utilized to be as a drug delivery system for the eye. We also postulated that the encapsulation of a medicine within Rb1 micelles might strengthen the drug’s therapeutic action and reduce side effects. The use of Rb1 as a carrier could also overcome some of the drawbacks of many active medicinal molecules, such as poor aqueous solubility, instability, and poor bioavailability.
In the present study, we investigated the use of Rb1 micelles as an effective strategy to improve the ocular bioavailability of poorly water-soluble drugs and as a valid therapeutic approach for the treatment of ocular inflammation. We considered that this new formulation could have a therapeutic effect and might also attenuate the side effects of eye medicines, such as the eye irritation of eye drops.
Materials and animals
Chemical reagents and animals
Details of the materials are described in the supporting information (SI) Materials and Methods. New Zealand white rabbits were obtained from Qingdao Kangda Foodstuffs Co., Ltd. (Qingdao, China). The animal care and procedures were conducted according to the Principles of Laboratory Animal Care. All animals were healthy and free from clinically observable ocular abnormalities. The use of animals in this study adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the animal study was approved by the Qingdao University of Science and Technology Ethics Committee for Animal Experimentation, Qingdao, China (Approval document No 2017-1, approved on Feb 6, 2017).
Preparation of the Rb1 micelles
Blank Rb1 micelles or diclofenac-loaded Rb1 micelles (Rb1-Dic micelles) were prepared with thin film hydration technique (Di Tommaso et al., 2011; Guo et al., 2015). In brief, diclofenac (10 mg) and Rb1 – predetermined weights – were accurately weighed and dissolved in ethanol (10 ml) in a one-hundred-milliliter round-bottom flask. The solvent was slowly evaporated at 40 °C under reduced pressure using a rotary evaporator (Yarong Shanghai, China) revolving at 120 rpm until a thin dry film was formed on the inner wall of the flask, and this procedure was usually about 10 minutes. The dried thin film was hydrated with 9 ml PBS by rotating the flask in water bath at 30 °C using rotary evaporator at 120 rpm for about 10 minutes under normal pressure to obtain micelle dispersions. The micelles were filtered through a 0.22 μm to discard the unencapsulated diclofenac (Di Tommaso et al., 2011). The optimized formulation (weight ratios of Rb1 to diclofenac = 30:1, with pH 6.6–6.8) was diluted with PBS to obtain a diclofenac concentration of 1.0 mg/ml, then another filtration with 0.22 μm sterile filter was performed to obtain sterile formulation for further use.
Determination of critical micelle concentration (CMC)
Detailed methods describing the determination of CMC are described in the SI Materials and Methods.
Characterization of the Rb1 micelles
Detailed methods describing the characterization of the Rb1 micelles are described in the SI Materials and Methods.
In vitro cytotoxicity assay
The cytotoxicity of various concentration of Rb1 and the Rb1-Dic micelles on Human corneal epithelial cells (HCECs; ATCC CRL-11135) was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, as described in detail elsewhere (Wu et al., 2010). In experiment one, the Rb1 solution was diluted to 31.3, 62.5, 125, 250, 500, 1000, 2000, 5000, 7500, 10 000, and 15 000 μg/ml with culture medium and the cells were incubated for 24, 48, and 72 h. In experiment two, the Rb1-Dic micelles solution was diluted to 1.56, 3.13, 6.25, 12.50, 50.00, and 100.00 μg/ml with culture medium and the cells were incubated for 24, 48, and 72 h. In experiment three, one-hour incubation of the original Rb1-Dic micelle solution was performed to test the formulation cytotoxicity (Di Tommaso et al., 2011). The controls consisted of benzalkonium chloride (0.05 and 0.1 mg/ml).
Animal experiments
Eye irritancy test
Eye irritancy was tested as previously reported, using the Rb1-Dic micelle solution (with diclofenac 1 mg/ml, and an Rb1/diclofenac weight ratio of 30:1), Rb1 (30 mg/ml) in phosphate buffer solution (PBS), commercial diclofenac eye drops, marketed sodium hyaluronate eyedrops (10 ml: 10 mg, HYCOSAN), 0.1 mg/ml benzalkonium chloride in PBS, and PBS (Guo et al., 2015).
Corneal penetration test
In vivo permeation testing were performed using rabbits. Rabbits were randomly divided into two groups. Group one received the commercial diclofenac eye drops, and group two received eye drops of the Rb1-Dic micelle formulation. Three animals (six eyes) were used for each time point in each group. For single instillation test, one drop (50 μl) of the eye drops was administered to both eyes of the animals. For four instillations test, formulations were administered to the eye at the rate of one drop (50 μl) into each eye 4 times in 10 min intervals. At 30, 60, and 120 min following the last administration of the formulation, their aqueous humor was aspirated, and the corneas were removed after sacrificing the animals with a sodium pentobarbital overdose. Aqueous humor samples were analyzed by mixing 100 µl aqueous humor with 100 µl of methanol, vortexing for 2 min, and centrifuging at 7378×g for 10 min. The corneas were assayed by weighing and homogenizing 50 mg tissue/1.0 ml methanol and centrifuging at 7378×g for 10 min. Aliquots of the supernatants of all samples were filtered through 0.45 μm membranes and then analyzed by HPLC as described above.
Anti-inflammatory efficacy
To assess inflammation prevention, Rb1-Dic micelle solution (containing diclofenac 1 mg/ml, and an Rb1/diclofenac weight ratio of 30:1) were tested in rabbit eyes. The 30 mg/ml Rb1 in PBS, commercial diclofenac (1 mg/ml) eye drops, and blank PBS eye drops were also tested and their anti-inflammatory efficacies were compared. A single dose of 50 μl of each formulation was dropped in the conjunctival sac of the right eye of the rabbits. The contralateral eye was used as the untreated control. Each formulation was tested in 6 rabbits. After 30 min, 0.05 ml of 5 mg/ml sodium arachidonate solution (SAS) in PBS (pH 7.4) was dropped in the right eye (Bucolo et al., 2002; Alvarado et al., 2015). Inflammation was quantified at 30, 60, 90, 120, 180, 240, and 360 min after the instillation of SAS. Ocular changes were scored as described previously (Bucolo et al., 2002; Alvarado et al., 2015).
Statistical analysis
Comparisons of cytotoxicity results between groups were made using one-way ANOVA. The comparisons of clinical scores for anti-inflammatory effects were subjected to a nonparametric analysis of variance (Kruskal–Wallis test) followed by post hoc Mann-Whitney U tests to compare the individual groups. Statistically significant difference was set at p < .05. SPSS software, version 11.5(SPSS, Chicago, IL, USA) was used to carry out the analysis.
Results
CMC Of Rb1
The CMC values of Rb1 at 34 °C were 0.3728 ± 0.0271, 0.3593 ± 0.0119, and 0.3516 ± 0.0154 mg/ml in artificial tears, PBS, and water, respectively, suggesting that Rb1 has a great tendency to form micelles (Figure S2).
Preparation and characterization of Rb1-Dic micelles
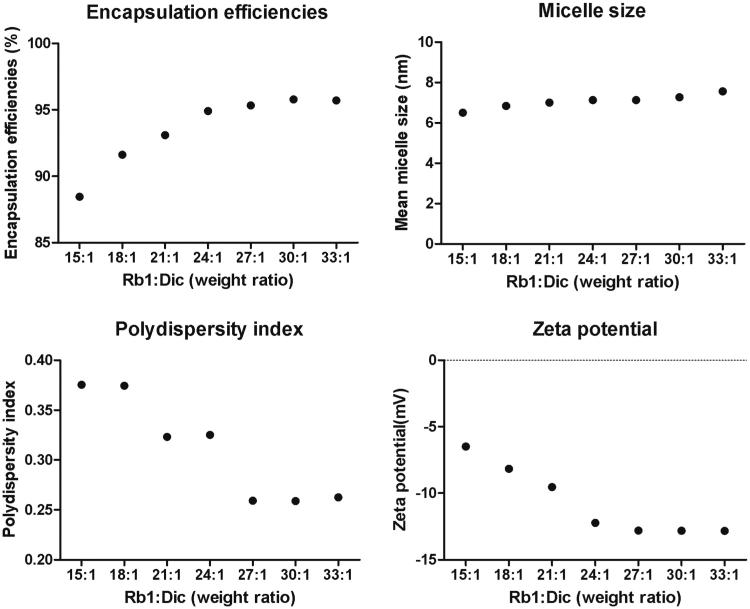
The Rb1 could self-assemble into micelles with an ultra-small micelle size, and the micelles could encapsulate diclofenac, but the characteristics of the micelles varied with the Rb1/diclofenac weight ratios. Some characteristics of Rb1-Dic micelles with different Rb1/diclofenac weight ratios are shown in Figure 1. Micelles prepared with a Rb1/diclofenac weight ratio of 15:1 during the encapsulation process showed a final load efficiency for diclofenac of 88.45%, and this increased to about 95.70% when the weight ratio was increased to 27:1, 30:1, or 33:1. The mean micelle size maintained a stable size range of 6.5–7.5 nm, although a slight increase was observed as the Rb1/diclofenac weight ratios increased. By contrast, the polydispersity index (PDI) decreased from 0.375 to 0.262 when the weight ratio increased from 15:1 to 33:1. The zeta potential also changed with the Rb1/diclofenac weight ratios, decreasing from −6.45 mV to −12.23 mV when the weight ratio increased from 15:1 to 24:1, but no further decreases were observed with increases in the weight ratio from 24:1 to 33:1.
Figure 1.
Changes in Rb1 micelle characteristics. Encapsulation efficiencies, micelle size, polydispersity index, and zeta potential were tested as functions of different weight ratios of Rb1 to diclofenac.
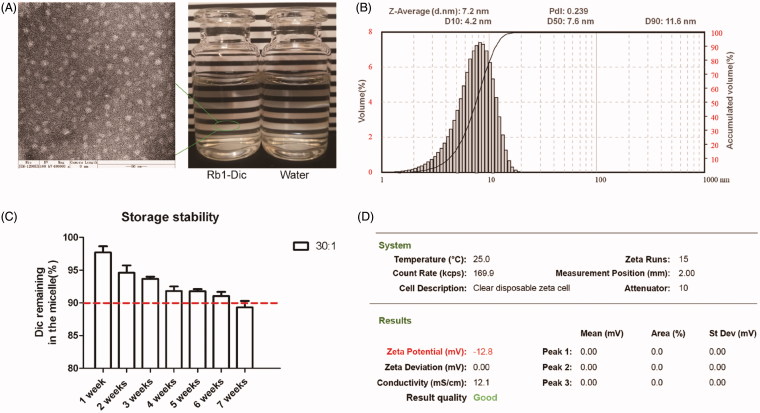
The micelles prepared with weight ratios of 30:1 and 33:1 showed similar loading efficiencies, micelle size, PDI, and zeta potential, so the micelles prepared with an Rb1/diclofenac weight ratio of 30:1 were chosen for further investigation. As shown in Figure 2, the Rb1-Dic micelle solution appeared as colorless and transparent as water to the naked eye. The mean diameter of the Rb1-Dic micelle was (7.27 ± 0.12) nm with a small PDI (0.259 ± 0.012). The particle size of Rb1-Dic micelle visualized by transmission electron microscopy (TEM) was consistent with the size obtained from the photon-correlation spectroscopy. TEM observation revealed that the Rb1 micelles were spherical in shape and lacked any signs of aggregation. The mean zeta potential of Rb1-Dic micelle was – (12.82 ± 0.37) mV. The encapsulation efficiency was (95.78 ± 1.44)%. The values for the blank Rb1 micelles were mean diameter (7.59 ± 0.41) nm, PDI 0.217 ± 0.008, and mean zeta potential –(12.85 ± 0.36) mV. No differences between the Rb1-Dic micelles and the blank Rb1 micelles in terms of morphology were also confirmed by the TEM observation.
Figure 2.
Characterization of the Rb1-Dic micelle solution. (A) TEM morphology of Rb1-Dic micelles (×400k magnification, bar = 50 nm) and the appearance of the Rb1-Dic micelle solution; (B) Storage stability at 25 °C and protected from light; (C) Particle size distribution; and (D) Zeta potential characterization of the Rb1-Dic micelle ophthalmic solution (Rb1/diclofenac weight ratio of 30:1).
The storage characteristics were also evaluated. A slow and continuous leakage of diclofenac from the Rb1 micelle solution could be observed at a storage condition of 25 °C and protected from light. Only 89.33 ± 0.97% of the original loaded drug still remained after 7 weeks of storage.
Infrared (IR) absorption spectrophotometry, differential scanning calorimetry (DSC), and X-Ray diffraction (XRD) test revealed amorphous diclofenac in the Rb1 micelle formulation, while the original diclofenac was a crystalline state. Rb1 showed no obvious changes in its physicochemical characterization (Figure S3, Figure S4, and Figure S5).
In vitro cytotoxicity evaluations
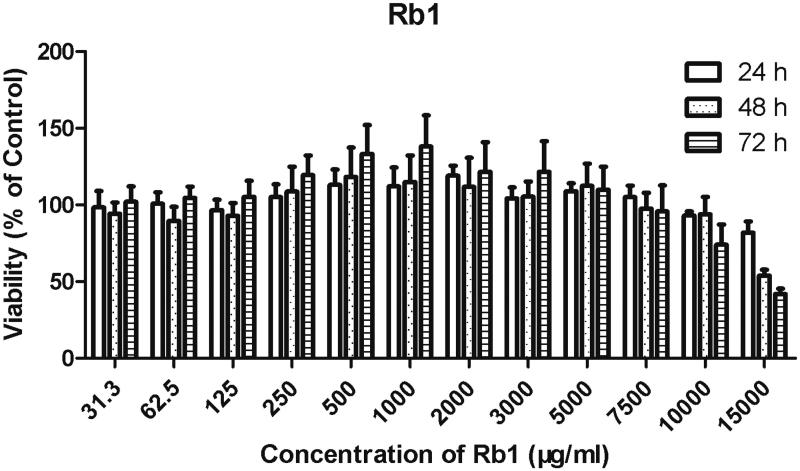
The in vitro cytotoxic effects of Rb1 in HCECs are presented in Figure 3. The CMC of Rb1 was approximately 350 μg/ml, so a concentration range from 31.3 μg/ml to 500 μg/ml was evaluated for cytotoxicity, and the toxicity of the non-assembled Rb1 molecules was also assessed at this concentration range. After a 72 h incubation, cell viability was high (>90% when compared to the control) for these five concentrations, indicating no cytotoxicity detected for the concentrations around the CMC of Rb1. Concentrations above the CMC were also evaluated, and no cytotoxicity was observed at concentrations no higher than 7.5 mg/ml after a 72 h incubation. But a slight cytotoxicity was noted when the concentration of Rb1 climbed to 10 mg/ml with a 72 h incubation (74.08 ± 13.12% cell survival) and 20 mg/ml with 48 and 72 h incubations (53.83 ± 3.85% and 41.96 ± 3.42% cell survival, respectively).
Figure 3.
Cytotoxicity evaluation of Rb1 (n = 3). The cytotoxicity of Rb1 and the Rb1-Dic micelle was tested on Human corneal epithelial cells (HCECs; ATCC CRL-11135) using standard MTT testing. HCECs were incubated with the indicated concentrations of ginsenoside Rb1 for 24, 48, and 72 h, followed by 4 hours incubation with MTT. The MTT transformed crystals were dissolved in DMSO and absorbance at 490 nm was measured. Absorbances were normalized to the untreated control cultures, which represented 100% viability.
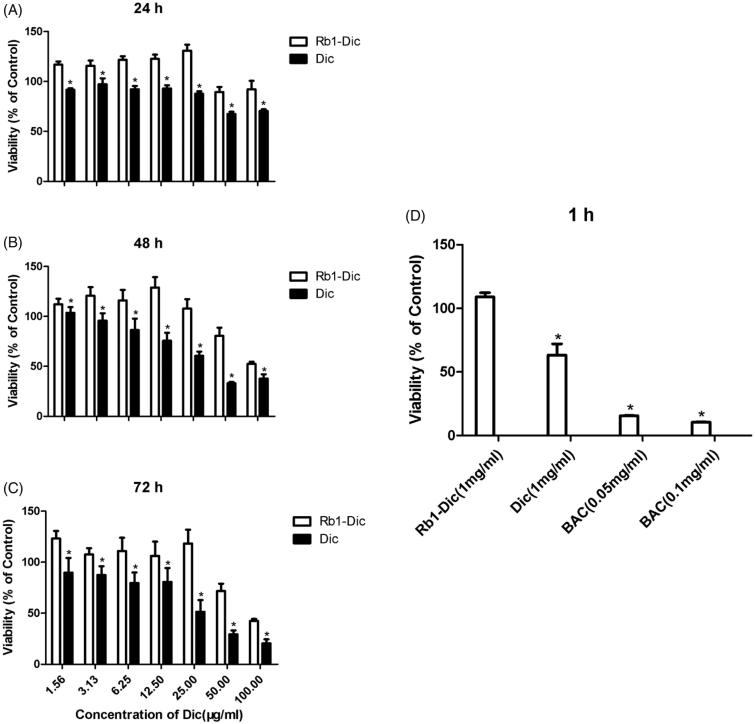
The long-term and short-term cytotoxicities of the Rb1-Dic micelle solution and 1 mg/ml commercial diclofenac eye drops were also evaluated, and the results of cytotoxicity observation after treatment are shown in Figure 4(A–C). Cytotoxicity could be found to be time- and concentration-dependent. The Rb1-Dic micelle solution showed no obvious cytotoxicity at concentrations below 25 μg/ml, even after 72 h of incubation. Increasing the concentration to 50 μg/ml led to a slight cytotoxicity (60.62 ± 8.04% and 41.77 ± 7.16% cell survival after a 48 and 72 h incubation, respectively). By contrast, the same concentration of diclofenac caused a significantly greater cytotoxicity at 25 μg/ml, even after only a 24 h incubation (67.55 ± 2.19% cell survival).
Figure 4.
Cytotoxicity evaluation of diclofenac-loaded ginsenoside Rb1 (Rb1-Dic) micelles (n = 3). An MTT assay was performed on Human ATCC CRL-11135 corneal epithelial cells (HCECs). HCECs were incubated with Rb1-Dic micelles for (A) 24 h, (B) 48 h, and (C) 72 h; (D) HCECs incubated for 1 h with Rb1-Dic micelle formulations, commercial diclofenac (Dic) eye drops, and benzalkonium chloride (BAC) as a positive control. MTT transformed crystals were dissolved in DMSO and absorbance at 490 nm was measured. Absorbances were normalized to the untreated control cultures, which represented 100% viability. (*p < .05 when compared to the Rb1-Dic micelle group).
The results for cytotoxicities to the Rb1-Dic micelle ophthalmic solution are shown in Figure 4(D); no cytotoxicities were noted after 1 h of incubation. By contrast, significant cytotoxicity was noted to the commercial diclofenac eye drops. Benzalkonium chloride also showed significant cytotoxicity at concentrations of 0.05 and 0.1 mg/ml, which are the concentrations commonly used in eyedrops.
In vivo corneal permeation of diclofenac from diclofenac-loaded ginsenoside Rb1 (Rb1-Dic) micelles
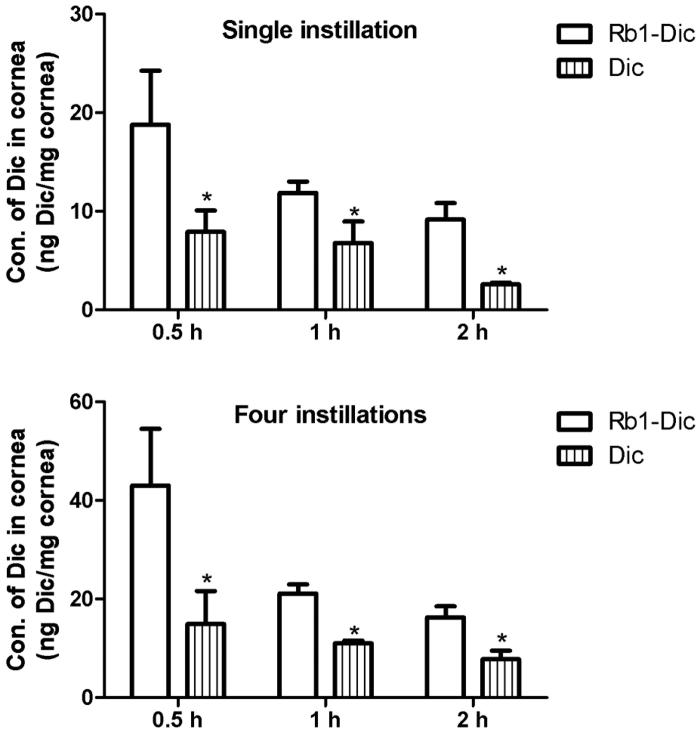
After topical administration of Rb1-Dic micelle solution and the commercial 1 mg/ml diclofenac eye drops, the concentrations of diclofenac in the rabbit corneas are shown in Figure 5. After a single instillation, the diclofenac levels were 137.54%, 74.93%, and 255.43% higher in the Rb1-Dic micelle solution group than in the commercial diclofenac eye drop group at the 0.5, 1, and 2 h time points, respectively. After four instillations, the diclofenac levels were 187.95%, 91.91%, and 108.60% higher in the Rb1-Dic micelle solution group than in the commercial diclofenac eye drop group at the 0.5, 1, and 2 h time points, respectively. The differences between the Rb1–Dic micelle solution group and the commercial diclofenac eye drop group were statistically significant (p < .05) for both the single and four instillations at all three-time points.
Figure 5.
In vivo corneal permeation of diclofenac from diclofenac-loaded ginsenoside Rb1 (Rb1-Dic) micelles. (A) Diclofenac concentration in rabbit corneas after a single instillation (50 μl) of either an ophthalmologic preparation of diclofenac-loaded ginsenoside Rb1 (Rb1-Dic) micelles or commercial diclofenac eye drops (*p < .05 compared to the Rb1-Dic micelle ophthalmic solution, n = 6). (B) Diclofenac concentration in rabbit corneas after four instillations (50 μl/instillation at 10 min intervals) (*p < .05 compared to the Rb1-Dic micelle ophthalmic solution group, n = 6).
Ocular tolerance of diclofenac-loaded ginsenoside Rb1 (Rb1-Dic) micelles
No significant eye irritation in the PBS group or the 1 mg/ml sodium hyaluronate eyedrops group (this eyedrop is widely used as artificial tears for dry eye and is commonly considered as non-irritating) was revealed according to the modified Draize test (Wadhwa et al., 2009; Li et al., 2012), as the clinical scores were all <3, indicating no irritation. Eyes treated with Rb1 solution at a concentration of 30 mg/ml also showed no irritation (all clinical scores <3). The eyes treated with 0.1 mg/ml benzalkonium chloride solution showed only slight irritation at the 3 h observation time point, and the commercial 1 mg/ml diclofenac eye drops also caused only slight irritation at the 3 h observation time point, whereas the Rb1-Dic micelle solution caused no irritation (clinical scores <3) (Table S1).
Anti-inflammatory efficacy of diclofenac-loaded ginsenoside Rb1 (Rb1-Dic) micelles
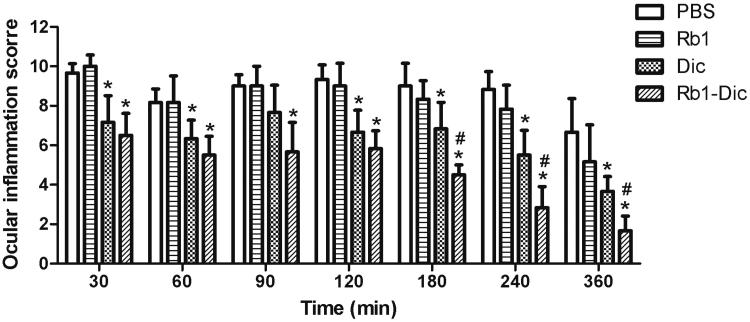
A mild to moderate ocular inflammation in rabbit eyes was produced after topical SAS application. As shown in Figure 6, the commercial diclofenac eye drops (1 mg/ml) had an obvious anti-inflammatory effect when compared to PBS group (p < .05), except at the 30 min time point (p > .05). The Rb1-Dic micelle solution (1 mg/ml) showed significant anti-inflammatory efficacy during the entire observation period when compared to PBS group (p < .05), and was significantly more effective when compared to the commercial diclofenac eye drops at the 180, 240, and 360 min time points (p < .05). Topical application of Rb1 itself had no obvious anti-inflammatory effect at any time point when compared to PBS group (p > .05).
Figure 6.
Anti-inflammatory efficacy of diclofenac-loaded ginsenoside Rb1 (Rb1-Dic) micelles. Anti-inflammatory efficacy of an ophthalmologic preparation of diclofenac-loaded ginsenoside Rb1 (Rb1-Dic) micelles, commercial diclofenac eye drops, and Rb1 solution after sodium arachidonate solution (SAS)-induced inflammation in rabbit eyes (Mean ± SD, n = 6, *p < .05 compared to the PBS control group, #p < .05 compared to the commercial diclofenac eye drop group).
Discussion
The genus Panax (ginseng) has a long history of clinical use in Chinese traditional medicine, and it is also widely used as a food and a food additive worldwide. In fact, ginseng may be one of the most popular and most prescribed natural remedies in folk medicine and its long history of use points to its safety. Parallel findings reported in several systematic reviews investigating the efficacy and safety of ginseng have led to the conclusion that while its efficacy remains questionable, its use appears to be generally safe (Shishtar et al., 2014). The pharmacologically active components of ginseng are the ginsenosides. Among more than 40 different ginsenosides isolated from ginseng, Rb1 is recognized as the major active component responsible for many pharmaceutical actions of ginseng (Betts et al., 2012; Liu et al., 2013). Rb1 also shows many bioactivities in ophthalmology, including antioxidative, anti-inflammatory, anti-angiogenesis, and retinal cell protective functions.
From a structural view, Rb1 falls into the class of steroid glycosides and triterpene saponins, and it can self-assemble into micelles to enhance the solubility or absorption of pharmacologically active substances or drugs in pharmaceutical preparations. Dai L et al. reported that Rb1 could self-assemble in a precipitation method with anticancer drugs to form stable hybrid nanoparticles that show greater in vitro and in vivo anticancer effects than the free drugs (Dai et al., 2016). Interestingly, Rb1 was also found to self-assemble into ultra-small nanoscale micelles with a thin-film dispersion method, a widely used method in preparing micelle formulation. These novel ultra-small nanoscale micelles were explored as an ocular drug delivery system, but to our best knowledge, micelles based on Rb1 have rarely been explored as drug delivery systems.
The CMC determination results revealed that Rb1 had a CMC value about 0.35 mg/ml (0.316 mM) at 34 °C, and the variation in this value was minor when it was determined in different solutions, such as artificial tears, PBS, and water. The CMC data found here agreed with some previously reported values. For example, the CMC of Panax notoginseng saponins in deionized water was 0.339 mg/ml, and ginsenosides Rg1 and Rb1 were extracted from Panax notoginseng saponins (Xiong et al., 2008). The CMC value of Rb1 indicates little advantage over many synthetic amphiphilic polymers that are widely used in drug delivery systems (e.g. 8 μg/ml for polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer [PVCL-PVA-PEG]), but the CMC for Rb1 was much lower than that reported for many other saponins. For example, the CMC for rebaudioside A was 4.5 mM and 5 mM at pH 3 and 6.7 respectively (Mudgal et al., 2016), and the CMC for glycyrrhizic acid was 2.9 mM and 5.3 mM at pH 5 and 6, respectively (Matsuoka et al., 2016). Then its much lower CMC value suggested that Rb1 had a much greater tendency to form micelles, encapsulate drugs, and remain stable in aqueous solutions.
Rb1 formed ultra-small micelles in PBS solution. The blank Rb1 micelles showed a particle size less of than 8 nm, and the Pdl was less than 0.3, indicating a narrow size distribution. The particle size of the blank Rb1 micelles was similar to that reported in the literature (Xiong et al., 2008). Rb1 exhibited a low CMC and ultra-small micelle size in a homogeneous distribution state; however, the encapsulation efficiency showed obvious variations when different hydrophobic drugs were tested. The encapsulation was 50.66 ± 0.96% for coumarin 6 (a fluorescence marker [Miao et al., 2015; Li et al., 2017]), when the Rb1/coumarin 6 weight ratios were increased to 1800:1 (90 mg/ml Rb1 per 0.05 mg/ml coumarin 6), and no higher encapsulation could be obtained with further increases in the Rb1/coumarin 6 weight ratios (unpublished data). By contrast, the encapsulation of curcumin, a hydrophobic polyphenol, was 95.42 ± 1.48% when the Rb1/curcumin weight ratios were only 15:1(15 mg/ml Rb1 per 1.0 mg/ml curcumin, unpublished data). The diclofenac used in this study was successfully encapsulated in the Rb1 micelles with an appropriate choice of Rb1/diclofenac weight ratios. The detailed story behind the encapsulation differences for various hydrophobic molecules needs further investigation, but the fact that both diclofenac and curcumin are amphipathic drugs might explain their high encapsulation. Rb1 and diclofenac (or curcumin) might interpenetrate and self-assemble into hybrid micelles (Sun et al., 2008) so that this potential drug-Rb1 interaction could improve encapsulation and stability.
Polymeric micelles have many attractive advantages and are viewed as promising drug delivery systems, but one drawback is their physical instability. Therefore, the stability of the Rb1-Dic micelle ophthalmic solution prepared in this research was also a concern. In this test, a slow and time-dependent leakage of diclofenac was observed from the Rb1-Dic micelle in ophthalmic solution (Figure 2(B)) and, in fact, only 6 weeks of storage meets the current requirements of the U.S. Pharmacopeia (the proportion of the initial concentration remaining no less than 90%). This physical stability was better than reported in many studies (Nagai et al., 2015; Yu et al., 2018), but other technical strategies, such as lyophilization, should be further explored to extend its physical stability.
Rb1 and other ginsenosides have been wildly investigated in ophthalmology, but the ocular safety of Rb1 is still in question. The in vitro cytotoxicity assays and in vivo ocular irritation tests performed here confirmed the ocular safety of Rb1 and its diclofenac-loaded micelle formulation. But the Rb1-Dic micelle formulation showed a compromised cellular tolerance. For example, a concentration of 3000 μg/ml Rb1 was well tolerated even after 72 h incubation, whereas a Rb1-Dic micelle formulation containing 3000 μg/ml Rb1 and 100 μg/ml diclofenac showed only 52.51 ± 2.17% cell survival after a 48 h incubation. However, the compromised cellular tolerance might originate from the diclofenac, as the same concentration of diclofenac showed higher cytotoxicity when compared to the Rb1-Dic micelle formulation. In other words, Rb1 alleviated some of the cytotoxicity of diclofenac in the cytotoxicity tests. This was also confirmed by the lack of cytotoxicity of the formulation of Rb1-Dic micelles and the significant cytotoxicity of diclofenac after 1 h of incubation. In another test, treatment with 30 mg/ml Rb1 in PBS resulted in 99.35 ± 6.98% cell survival after a 4 h incubation (unpublished data). All these in vitro cytotoxicity results were further confirmed by the in vivo ocular irritation tests, which supported the safety of Rb1 in ophthalmic solution.
Particle size is widely regarded as affecting cellular uptake and biodistribution in vivo. Small nanodrugs of 10–30 nm diameter have recently been proven effective against tumor metastasis (Yue et al., 2013; Li et al., 2014; Wei et al., 2015). Another report also revealed that small-sized nanoparticles (10 nm) favored deeper penetration into inner tumor regions and concurrently prolonged the overall circulation time for beneficial accumulation of drugs in tumor tissues via the enhanced permeability and retention (EPR) effect (Wei et al., 2015). However, to the best of our knowledge, rare studies have touched the efficacy of ultra-small (<8 nm) nanoparticles for ocular drug delivery in cells or animal models, even though the development of an ultra-small nanocarrier is desperately needed for ocular drug delivery. As Rb1 has no autofluorescence, and it had low encapsulation efficiency with some hydrophobic fluorescent dyes, such a coumarin 6, we used an in vivo animal test to characterize the corneal permeation of the Rb1-Dic micelles. The animal tests showed higher concentrations of diclofenac in the corneas of rabbits in the Rb1-Dic micelle solution group than in the commercial diclofenac eye drop group, confirming that the Rb1 micelles had an excellent capacity for corneal permeation. However, the concentration of diclofenac in the aqueous humor of rabbits was at an undetectable level in both the Rb1-Dic micelle solution group and the commercial diclofenac eye drop group. The Rb1-Dic micelle ophthalmic solution showed no additional viscosity above that of water, so rapidly drained away from the ocular surface due to blinking tear flow and lachrymal nasal drainage of the eye might compromise the concentration in the aqueous humor.
The results from anti-inflammatory efficacy tests also confirmed that the Rb1 micelles had excellent corneal penetration. Notably, Rb1 (30 mg/ml) did not decrease the ocular inflammation induced by topical drop of SAS in rabbit eyes in this experiment. Many reports have indicated that Rb1 has anti-inflammatory effects (Lee et al., 2013; Liu et al., 2013; Wu et al., 2014), but its anti-inflammatory activity has not been fully explored in ophthalmology. The potential anti-inflammatory activity and the dose-effect relationship of Rb1 in the cornea needs further study. Since Rb1 showed no anti-inflammatory activity in the present study, one conclusion might be that the anti-inflammatory activity of the Rb1-Dic micelle solution resulted simply from the nano-medicinal efficacy of the Rb1 micelles. Nevertheless, Rb1 micelles still offer a promising alternative for the treatment of ocular surface diseases, even though they may not be promising in delivering therapeutic concentrations of drugs to the aqueous humor of eyes with the integrity of the corneal epithelium using the formulation explored in this text.
The production of an Rb1 micelle ophthalmic solution requires no hazardous substances and the preparation protocol is easily adaptable to large-scale preparation if desired. The formulation can readily meet with the sterile requirements needed for an ophthalmic solution, so the Rb1 micelle ophthalmic solution meets the criteria for green preparation. Therefore, Rb1 micelles might be a promising green drug delivery system for those ocular surface disorders.
Although some of the results presented here for Rb1 micelles were promising, further investigations are needed to develop hybrid formulations of the Rb1 micelle ophthalmic solutions with more stability to promote their clinical application, though the instability and leakage of entrapped drug from micelles an inherent obstacle to micelle formulation. It also needs further studies on the mechanism of corneal penetration to this ultra-small Rb1 micelle.
Conclusion
Novel ultra-small micelles based on Rb1 were successfully prepared, and its physicochemical properties were characterized in this study. In vitro cytotoxicity and in vivo ocular irritation tests confirmed the Rb1 solution and the Rb1 micelles had well tolerance as ocular topical drug delivery system. In vivo corneal permeation study clearly demonstrated the ability of the Rb1 micelle delivering a high concentration of diclofenac into the cornea, offering promise for local treatment of inflammatory-mediated corneal diseases. Overall, Rb1 could self-assemble into ultra-small micelles, and these Rb1 micelles could represent a promising green therapeutic tool for the treatment of ocular surface disorders.
Supplementary Material
Funding Statement
This research was supported by the National Natural Science Foundation of China (Project nos. 81770895), the Project of Medical and Health Technology Development Program in Shandong Province, China (project no. 2017WS633), and the QUST Innovation and Entrepreneurship Training Programs for Undergraduate Students (X Lu and Z Zhuang as project leaders).
Acknowledgements
We are particularly grateful to Prof Jiandu Lei (Beijing Key Laboratory of Lignocellulosic Chemistry, Beijing Forestry University), for his donating Rb1 in our preliminary experiment.
Disclosure statement
The authors report no conflict of interest in this work.
References
- Alvarado HL, Abrego G, Garduno-Ramirez ML, et al. (2015). Design and optimization of oleanolic/ursolic acid-loaded nanoplatforms for ocular anti-inflammatory applications. Nanomedicine 11:521–30. [DOI] [PubMed] [Google Scholar]
- Betts BS, Parvathaneni K Yendluri BB, et al. (2012). Ginsenoside-Rb1 Induces ARPE-19 Proliferation and Reduces VEGF Release. ISRN Ophthalmol 2011:184295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucolo C, Maltese A, Puglisi G, et al. (2002). Enhanced ocular anti-inflammatory activity of ibuprofen carried by an Eudragit RS100 nanoparticle suspension. Ophthalmic Res 34:319–23. [DOI] [PubMed] [Google Scholar]
- Dai L, Liu KF, Si CL, et al. (2016). Ginsenoside nanoparticle: a new green drug delivery system. J Mater Chem B 4:529–38. [DOI] [PubMed] [Google Scholar]
- Dai XX, Shi XY, Yin QQ, et al. (2013). Multiscale study on the interaction mechanism between ginsenoside biosurfactant and saikosaponin a. J Colloid Interface Sci 396:165–72. [DOI] [PubMed] [Google Scholar]
- Di Tommaso C, Torriglia A, Furrer P, et al. (2011). Ocular biocompatibility of novel Cyclosporin A formulations based on methoxy poly(ethylene glycol)-hexylsubstituted poly(lactide) micelle carriers. Int J Pharm 416:515–24. [DOI] [PubMed] [Google Scholar]
- Di Trani N, Jain P, Chua CYX, et al. (2019). Nanofluidic microsystem for sustained intraocular delivery of therapeutics. Nanomedicine 16:1–9. [DOI] [PubMed] [Google Scholar]
- Guo C, Zhang Y, Yang Z, et al. (2015). Nanomicelle formulation for topical delivery of cyclosporine A into the cornea: in vitro mechanism and in vivo permeation evaluation. Sci Rep 5:12968. [Google Scholar]
- Jiao J. (2008). Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv Drug Deliv Rev 60:1663–73. [DOI] [PubMed] [Google Scholar]
- Lee JS, Song JH, Sohn NW, et al. (2013). Inhibitory effects of ginsenoside Rb1 on neuroinflammation following systemic lipopolysaccharide treatment in mice. Phytother Res 27:1270–6. [DOI] [PubMed] [Google Scholar]
- Li JY, Tan GX, Cheng BC, et al. (2017). Transport mechanism of chitosan-N-acetylcysteine, chitosan oligosaccharides or carboxymethyl chitosan decorated coumarin-6 loaded nanostructured lipid carriers across the rabbit ocular. Eur J Pharm Biopharm 120:89–97. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Z, Li J, et al. (2012). Diclofenac/biodegradable polymer micelles for ocular applications. Nanoscale 4:4667–73. [DOI] [PubMed] [Google Scholar]
- Li YF, Jin MJ, Shao SA, et al. (2014). Small-sized polymeric micelles incorporating docetaxel suppress distant metastases in the clinically-relevant 4T1 mouse breast cancer model. Bmc Cancer 14:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZC, Chen JY, Huang WD, et al. (2013). Ginsenoside Rb1 protects rat retinal ganglion cells against hypoxia and oxidative stress. Mol Med Rep 8:1397–403. [DOI] [PubMed] [Google Scholar]
- Luo Y, Pan K, Zhong Q (2015). Casein/pectin nanocomplexes as potential oral delivery vehicles. Int J Pharm 486:59–68. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Miyajima R, Ishida Y (2016). Aggregate formation of glycyrrhizic acid. Coll Surf a-Physicochem Engineer Aspects 500:112–7. [Google Scholar]
- Miao XQ, Li Y, Wyman I, et al. (2015). Enhanced in vitro and in vivo uptake of a hydrophobic model drug coumarin-6 in the presence of cucurbit[7]uril. Med Chem Commun 6:1370–4. [Google Scholar]
- Mudgal S, Keresztes I, Feigenson GW, et al. (2016). Controlling the taste receptor accessible structure of rebaudioside A via binding to bovine serum albumin. Food Chem 197:84–91. [DOI] [PubMed] [Google Scholar]
- Nagai N, Yoshioka C, Mano Y, et al. (2015). A nanoparticle formulation of disulfiram prolongs corneal residence time of the drug and reduces intraocular pressure. Exp Eye Res 132:115–23. [DOI] [PubMed] [Google Scholar]
- Shishtar E, Sievenpiper JL, Djedovic V, et al. (2014). The effect of ginseng (the genus panax) on glycemic control: a systematic review and meta-analysis of randomized controlled clinical trials. Plos One 9:e107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Xin M, Yu H, et al. (2018). Novel ultra-small micelles based on rebaudioside A: a potential nanoplatform for ocular drug delivery. Int J Pharm 552:265–76. [DOI] [PubMed] [Google Scholar]
- Sun Y, Lee CC, Hung WC, et al. (2008). The bound states of amphipathic drugs in lipid bilayers: Study of curcumin. Biophys J 95:2318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh PK, Sah AK (2014). Nanocarriers for ocular delivery for possible benefits in the treatment of anterior uveitis: focus on current paradigms and future directions. Exper Opin Drug Deliv 11:1747–68. [DOI] [PubMed] [Google Scholar]
- Vaccaro L, Lanari D, Marrocchi A, Strappaveccia G (2014). Flow approaches towards sustainability. Green Chem 16:3680–704. [Google Scholar]
- Wadhwa S, Paliwal R, Paliwal SR, Vyas SP (2009). Chitosan and its role in ocular therapeutics. Mini Rev Med Chem 9:1639–47. [DOI] [PubMed] [Google Scholar]
- Wei T, Chen C, Liu J, et al. (2015). Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc Natl Acad Sci USA 112:2978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XG, Xin M, Chen H, et al. (2010). Novel mucoadhesive polysaccharide isolated from Bletilla striata improves the intraocular penetration and efficacy of levofloxacin in the topical treatment of experimental bacterial keratitis. J Pharm Pharmacol 62:1152–7. [DOI] [PubMed] [Google Scholar]
- Wu YZ, Yu YH, Szabo A, et al. (2014). Central inflammation and leptin resistance are attenuated by ginsenoside Rb1 treatment in obese mice fed a high-fat diet. Plos One 9:e92618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Guo JX, Huang LS, et al. (2008). Self-micelle formation and the incorporation of lipid in the formulation affect the intestinal absorption of Panax notoginseng. Int J Pharm 360:191–6. [DOI] [PubMed] [Google Scholar]
- Yu YL, Chen DQ, Li YN, et al. (2018). Improving the topical ocular pharmacokinetics of lyophilized cyclosporine A-loaded micelles: formulation, in vitro and in vivo studies. Drug Deliv 25:888–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J, Liu S, Xie ZG, et al. (2013). Size-dependent biodistribution and antitumor efficacy of polymer micelle drug delivery systems. J Mater Chem B 1:4273–80. [DOI] [PubMed] [Google Scholar]
- Zhang J, Han XZ, Li X, et al. (2012). Core-shell hybrid liposomal vesicles loaded with panax notoginsenoside: preparation, characterization and protective effects on global cerebral ischemia/reperfusion injury and acute myocardial ischemia in rats. Int J Nanomed 7:4299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.