Abstract
Fibrillin microfibrils are extensible polymers that endow connective tissues with long-range elasticity and have widespread distributions in both elastic and non-elastic tissues. They act as a template for elastin deposition during elastic fibre formation and are essential for maintaining the integrity of tissues such as blood vessels, lung, skin and ocular ligaments. A reduction in fibrillin is seen in tissues in vascular ageing, chronic obstructive pulmonary disease, skin ageing and UV induced skin damage, and age-related vision deterioration. Most mutations in fibrillin cause Marfan syndrome, a genetic disease characterised by overgrowth of the long bones and other skeletal abnormalities with cardiovascular and eye defects. However, mutations in fibrillin and fibrillin-binding proteins can also cause short-stature pathologies. All of these diseases have been linked to dysregulated growth factor signalling which forms a major functional role for fibrillin.
Keywords: Fibrillin, Elastin, Fibulin, LTBP, ADAMTS
1. Introduction
Fibrillin, an extracellular matrix glycoprotein, assembles into microfibrils, a component of many connective tissues, where they form the template for elastic fibre formation. Fibrillin is also found in tissues devoid of elastin such as the ciliary zonules of the eye. In elastic fibre assembly, it is understood that elastin globules are deposited directly onto a microfibril template and the elastic fibre is comprised of an amorphous elastin central core surrounded by a fibrillin microfibril sheath [1]. A wide array of fibrillin binding proteins are known to facilitate the assembly of elastic fibres and contribute to their functionality, and these will be discussed herein.
2. Fibrillin microfibrils
Fibrillin microfibrils, which have a beads-on-a-string appearance, are a major component of elastic fibres, conferring long range extensibility and contributing to the elastic deformation of tissues. Microfibrils also play a key role in tissue homeostasis through their interaction with growth factors such as transforming growth factor-β (TGFβ) and bone morphogenetic proteins (BMPs) and through interaction with cell surface receptors such as the integrins [2,3] and syndecans [4]. The importance of fibrillin-1 in the function of tissues is further highlighted by fibrillin-1 mutations that cause a number of heritable connective tissue disorders termed fibrillinopathies, such as Marfan syndrome (MFS) [5], Weill Marchesani syndrome (WMS) [6] and geleophysic dysplasia (GD) [7].
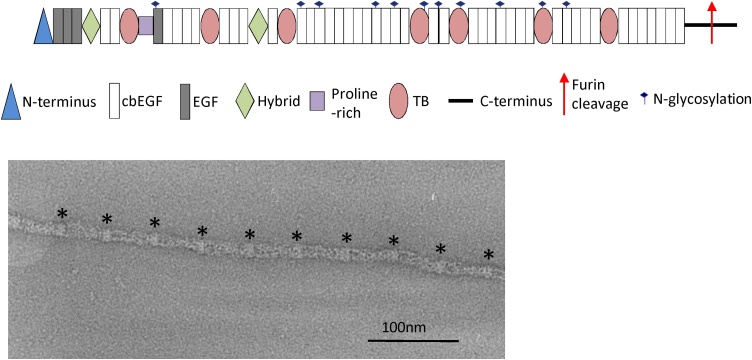
Though three fibrillin isoforms have been identified in humans, fibrillin-1 (FBN1) is most abundant in adult tissues. Fibrillin-1 is a glycoprotein composed of 2871 amino acids with a molecular mass of ∼320 kDa [1]. It consists of 59 domains including an N-terminal region, epidermal growth factor-like repeats (EGF), most of which are calcium binding (cb), TB (TGFβ-binding like) domains and hybrid domains which bear homology to both cbEGF domains and TB domains (Fig. 1). It has been suggested that arrays of domains have a linear, rod-like structure, modelled from high resolution structures of domain pairs and triplets [8,9]. However, X-ray scattering data show that, in solution, longer domain arrays from fibrillin and the homologous latent TGFβ binding protein (LTBP)-1 are flexible and adopt non-linear conformations [10,11]. Fibrillin assembles into microfibrils, but despite knowing the structures of some domains, how individual fibrillin monomers are arranged in the microfibril is still not understood. Fibrillin microfibrils imaged in tissues have a diameter of ∼10-12 nm [12] with a 57 nm periodic beaded structure [13] and in cross-section they appear hollow with a ring of eight filaments [14,15]. They have a mass per repeat ranging from ∼1400 kDa, in some early foetal tissues and cell culture, to ∼2500 kDa in adult tissues [16]. This mass is consistent with up to eight fibrillin monomers per repeat [13].
Fig. 1.
The domain organisation of fibrillin-1 is shown. Fibrillin assembles into microfibrils with a beads on a string appearance. A microfibril is imaged by negative stain TEM and the beaded structure is highlighted by asterisks.
3. Fibrillin-binding proteins
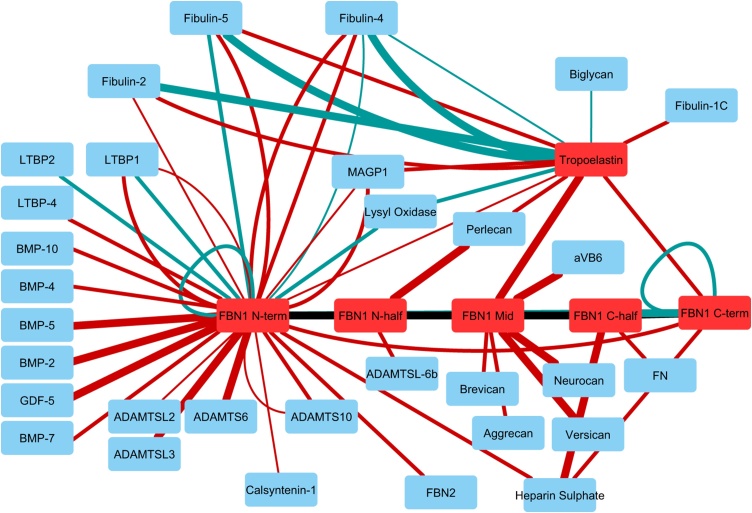
A large number of molecules have been shown to colocalise or interact with fibrillin. Here we have focussed on key binding proteins that have been shown to bind directly with fibrillin or tropoelastin, the soluble elastin precursor, and have roles in microfibril assembly or growth factor regulation. Table 1 shows the direct molecular interactions between fibrillin and tropoelastin that have been quantified and these are also presented in Fig. 2.
Table 1.
Affinity between fibrillin-1 or tropoelastin and their binding proteins with the protein interaction analysis approach (SPR = surface plasmon resonance, IP = immunoprecipitation, Solid Phase = solid phase binding assay) and dissociation constant (Kd).
| Protein A | Protein B | Reference | Approach | Kd (nM) |
|---|---|---|---|---|
| FBN1 N-ter | ADAMTS10 | [17,18,19] | SPR | 11-35; 245 |
| FBN1 N-ter | ADAMTS6 | [18] | SPR | 1-7 |
| FBN1 N-ter | ADAMTSL2 | [19] | SPR | 200 |
| FBN1 N-ter | ADAMTSL3 | [19] | SPR | 6 |
| FBN1 N-half | ADAMTSL5 | [20] | IP | N/A |
| FBN1 N-half | ADAMTSL6β | [21] | SPR | 80 |
| FBN1 N-half | Aggrecan | [22] | SPR | 49 and 42 |
| FBN1 cbEGF22-TB4-cbEGF23 | Integrin αVβ6 | [3] | SPR | 1 |
| FBN1 N-ter | BMP-2, 4, 5, 7, 10, GDF5 | [23,24] | SPR | 6 - 34 |
| FBN1 N-ter | Brevican, Neurocan, Versican | [22] | SPR | 20, 2, 7.1 |
| FBN1 N-ter | Calsyntenin-1 | [25] | SPR | 240 |
| FBN1 N- and C-ter | FBN1 | [26] | Solid Phase | 5-11 |
| FBN1 N-ter | FBN1 C-ter | [27] | SPR | 3-25 |
| FBN1 N-ter | FBN2 C-ter | [27] | SPR | 10-74 |
| FBN1 N-ter | Fibulin-2 | [28] | SPR | 160 |
| FBN1 N-ter | Fibulin-4 | [28,29] | SPR | 74; 54 |
| FBN1 N-ter | Fibulin-5 | [28,29] | Solid Phase, SPR | 63; 23 |
| FBN1 C-half | Fibronectin | [30] | SPR | 55 |
| FBN1 N- and C-ter | Heparan Sulphate | [30,31] | SPR | 27, 93; 16 |
| FBN1 N-ter | LTBP1 | [28] | SPR | 21 |
| FBN1 N-ter | LTBP2 | [32] | Solid Phase | 22 |
| FBN1 N-ter | LTBP-4 | [28] | SPR | 24 |
| FBN1 N-ter | Lysyl Oxidase | [29] | Solid Phase | 26 |
| FBN1 N-ter | MAGP1 | [31,33] | SPR, Solid Phase |
140-240; 36.5 |
| FBN1 N-half | Perlecan | [34] | SPR | 6-9 |
| FBN1 N-ter | MFAP4 | [35] | SPR | N.D. |
| Tropoelastin | Biglycan | [36] | Solid Phase | 195 |
| Tropoelastin | FBN1 N-ter, Mid, C-ter | [31,33] | SPR | 280, 5; 27 |
| Tropoelastin | Fibulin-1C | [37] | SPR | 18 |
| Tropoelastin | Fibulin-2 | [38] | Solid Phase | 1-2 |
| Tropoelastin | Fibulin-2 | [37] | SPR | 18 |
| Tropoelastin | Fibulin-4 | [29,38] | Solid Phase | 8, 131 |
| Tropoelastin | Fibulin-5 | [29,38] | Solid Phase; SPR | 2; 64 |
| Tropoelastin | Lysyl Oxidase | [29] | Solid Phase | 49 |
| Tropoelastin | MAGP1 | [33] | SPR | 22 |
| Tropoelastin | Perlecan | [37] | SPR | 21 |
Fig. 2.
An interaction network showing the fibrillin and tropoelastin interactions with their binding proteins listed in Table 1. The line width increases with increasing interaction strength (from >100, 10–100 to 1–10 nM Kd) and the line colour indicates either SPR (red) or solid phase binding (blue).
3.1. Latent TGFβ binding proteins
Latent TGFβ binding proteins (LTBPs) 1–4 are extracellular glycoproteins with structural similarities to fibrillins, with long or short splice forms (L and S). LTBPs -1, -3 and -4 can covalently bind to the latency-associated peptide of TGFβ, producing the large latent complex (LLC) [39,40]. The LLC becomes sequestered within the matrix, and can regulate TGFβ bioavailability [[39], [40], [41]]. Both LTBP-1 L knockout and complete null mice (i.e LTBP-1S and L) die at birth from severe aortopathy [42,43]; the phenotype produced is similar to that observed in TGFβ-knockout aortic smooth muscle cells [44], supporting the requirement of LTBP-1 L for TGFβ signalling. LTBP-2 binds to the matrix via fibrillin [45,46] and, through binding to fibulin-5, negatively regulates elastic fibre assembly [47,48]. Mutations cause ectopia lentis (EL) [49,50] (a manifestation of MFS) and WMS and WMS-like syndrome [51] and loss of LTBP-2 in patients leads to congenital glaucoma [52,53]. LTBP-2 has also been implicated in wound healing [54]. LTBP-3 deletion in humans produces similar phenotypes to those of LTBP-3 null mice, including short stature, spinal curvature, craniofacial abnormalities and increased bone mass [[55], [56], [57]]. Aberrant LTBP-3-TGFβ complexes may be responsible for aortic dilation and dissection in MFS as MFS mice lacking LTBP-3 had fewer aneurysms and less fragmented elastic fibres [58]. LTBP-4 is required for proper elastogenesis, involving fibulin-4 and -5 incorporation into the matrix [[59], [60], [61], [62]]. Humans with inactive LTBP-4 develop autosomal recessive cutis laxa type 1C (ARCL1C), characterised by severe craniofacial, developmental and potentially fatal pulmonary defects [63]. Knockout studies using both LTBP-4S-/- and/or LTBP-4L-/- mice have uncovered discrete roles for these isoforms. Whereas mice that express only LTBP-4 L normally survive to adulthood [64], complete null mice develop an ARCL1C-like phenotype where mice die perinatally with highly impaired elastogenesis [60], likely due to lack of fibulin-4 in the matrix [61]. LTBP-4 has also been identified as a modifier of muscular dystrophy (MD) [[65], [66], [67], [68]]. MD mice with an LTBP-4 allele that contains an insertion in the hinge region had a less severe phenotype, including increased muscle mass and reduced fibrosis [65,68], coinciding with lower levels of extracellular TGFβ.
3.2. Fibulins
Fibulin-4 and -5 are extracellular glycoproteins that have roles in elastogenesis [38,69]. They bind to both tropoelastin and lysyl oxidase (LOX) or LOX-like 1 (LOXL1) [29,70,71] as well as to fibrillin-1 [29], thereby facilitating elastin cross-linking by LOX/LOXL1 and deposition onto microfibrils. Mutations in fibulin-4 (EFEMP2) cause ARCL1B, characterised by severe cardiovascular abnormalities, joint laxity and arachnodactyly [72]. LTBP-4S-/- mice with additional fibulin-4 deficiency have more perturbed elastogenesis [60,61], and fibulin-4 -/- mice develop significant lung and vascular defects and die perinatally [69], potentially due to altered LOX-mediated cross-linking and matrix interactions [73]. However, this dependency on fibulin-4 may vary with vessel type [74]. Mice with a human fibulin-4 mutation (E57 K) have disrupted elastic fibres and developed ascending aortic aneurysms [74,75], whereas muscular arteries and resistance vessels, such as those in the kidney and mesentery, have normal elastic fibres and cross-linking is unaffected [74]. Upregulation of fibulin-4 (and colocalisation with LOX1) has been observed in exfoliation syndrome, an elastic fibre disorder that leads to glaucoma [76] and bronchopulmonary dysplasia (BPD) [77]. Fibulin-5 depletion disrupts elastic fibre assembly to a lesser extent than fibulin-4 [78,79]. Patients with fibulin-5 (DANCE) mutations develop ARCL1A, characterised by loose skin, emphysema and arterial tortuosity. These mutations produce conformational changes that lower fibulin-5 secretion and affinity for tropoelastin [[80], [81], [82], [83], [84]].
3.3. Microfibril associated glycoproteins
Microfibril associated glycoproteins (MAGPs)-1 and -2 (also known as microfibrillar associated proteins (MFAPs)-2 and -5, respectively) copurify and colocalise with microfibrils [[85], [86], [87], [88], [89]]. Although neither is required for elastic fibre assembly [[90], [91], [92]], both may promote elastin deposition onto microfibrils [33,87,93]. However, MAGP-1 has been shown to influence wound healing, ciliary zonule formation, ageing, bone remodelling and thrombus formation [90,91,[94], [95], [96], [97]]. Despite the apparent lack of cardiovascular effects observed in either MAGP-1 or MAGP-2 knockout mice, depletion of both genes resulted in aortic dilation in older mice, suggesting a possible compensation mechanism [92]. Additionally, Barbier et al discovered an association between MAGP-2 mutations and thoracic aortic aneurysms and dissection (TAAD) [98]. Although little is known about the role of MFAP-4 in elastogenesis, Pilecki et al identified tropoelastin, fibrillin-1 and -2 and desmosine as MFAP-4 interaction partners in vitro. MFAP-4 also enhanced elastin assembly and colocalised with fibrillin-1 microfibrils in vivo [35].
3.4. ADAMTS and ADAMTSL proteins
A Disintegrin And Metalloprotease with Thrombospondin type-1 repeats (ADAMTS) and ADAMTS-Like (ADAMTSL) are matrix glycoproteins with many biological functions including morphogenesis, development, angiogenesis, inflammation and coagulation, as well as maintaining the structural integrity of tissues [99]. Recently, a subset of ADAMTS and ADAMTSL proteins have been implicated in microfibril assembly, adhesion and matrix stability [100]. Mutations in ADAMTS6 and -10 give rise to WMS [101]; gene disruption of ADAMTS17 leads to WMS-like syndrome [102]; and mutations in ADAMTSL2 and –L4 result in GD [103] and EL, respectively [104]. The association of ADAMTSs and ADAMTSLs with fibrillin-1 related pathologies suggests that they modulate fibrillin-1 function. Indeed, perturbations in the expression of ADAMTSs and ADAMTSLs disrupt the deposition of fibrillin microfibrils and TGFβ regulation [17,102,103]. Mouse models either lacking ADAMTSL2 or bearing a nonsense mutation in ADAMTSL4 mimic phenotypic characteristics observed in patients with GD or EL, further corroborating the involvement of ADAMTSs and ADAMTSLs in these fibrillinopathies [[105], [106], [107]].
ADAMTS10 is involved in the biogenesis and maintenance of fibrillin-1 microfibrils, whereas ADAMTS6 inhibits microfibril deposition [17,18]. ADAMTS10 binds the N- and C-terminal regions of fibrillin-1 [17,19], while ADAMTS6 also interacts with the N-terminus of fibrillin-1 and the C-terminus of LTBP1 [18]. ADAMTS10 is required for and can enhance the formation of focal adhesion complexes through interactions with fibronectin and heparan sulphate (HS), whereas ADAMTS6 depletes HS and hence focal adhesions [18]. ADAMTS10 has a negative effect on ADAMTS6 expression, whereas ADAMTS6 shows synergistic effects on ADAMTS10 expression [18]. ADAMTS10 colocalises with fibrillin and addition of exogenous ADAMTS10 enhances microfibril deposition [17]. Mutations in ADAMTS17 also result in the dislocation of the ocular lens due to progressive degradation of the ciliary zonules in WMS-like patients [102], implying ADAMTS17 is also an accessory to fibrillin microfibril biogenesis and regulation.
ADAMTSL2 binds to the N- and C-terminus of fibrillin-1 [7,19], and the N-terminal binding site overlaps with a three domain fibrillin-1 deletion that causes WMS [19]. Increased levels of fibrillin-2 but not fibrillin-1 were observed in ADAMTSL2-deficient mouse lung [107]. This study also showed enhanced staining of LTBP-1 in bronchial tissues, increased levels of active TGFβ, as well as substantial epithelial dysplasia. ADAMTSL2 interacts with different regions of LTBP-1 suggesting that ADAMTSL2 may also play a role in regulating TGFβ availability in the matrix [19,103]. Found in ocular tissue, ADAMTSL4 is deposited in the matrix in a fibrillar arrangement co-localised with fibrillin microfibrils and addition of ADAMTSL4 to cultured fibroblasts enhances microfibril deposition [108,109]. Furthermore, analysis of ciliary zonules in mutant mice bearing a nonsense mutation in ADAMTSL4 revealed disorganised arrangements of fibrillin microfibrils [106] supporting a role for ADAMTSL4 in microfibril deposition.
3.5. Potential new associated proteins
Mass spectrometry has proven useful for identifying new fibrillin microfibril-associated proteins. MMP3 and annexins V and II co-purified with fibrillin microfibrils purified from human ciliary zonules [86]. Molecular fishing identified elastic fibre-associated proteins including fibronectin, perlecan, LOX, fibrillin-2, and TGFβ2 [25]. Again, the annexins were detected along with other candidate proteins, such as vimentin, βig-H3, thrombospondin-1, S100-A7, plasminogen activator inhibitor 1 (PAI-1) and IGF-binding proteins (IGFBP)-3 and -7 [25]. More recent characterisation of potential associated proteins from purified human ciliary body and skin fibrillin microfibrils identified MFAP5, versican and fibrillin-2 in both eye and skin-derived samples, whereas perlecan was identified solely in eye-derived samples and elastin, EMILIN-2 and fibulin-2 and -1 were identified solely in skin-derived samples [110]. Concordant with the molecular fishing study, annexins V and II, vimentin and βig-H3 all co-purified with eye and skin fibrillin microfibrils, whereas IGFBP7 and PAI-1 co-purified with only those derived from eyes. Interestingly the chaperones, protein disulphide isomerase and calreticulin, which play a role in intracellular fibrillin assembly [111], were also identified in both tissues (Table 2).
Table 2.
Fibrillin-associated candidate proteins co-identification by molecular fishing and native tissue co-purification [25,110].
| New Associated Protein Candidates |
Known extracellular matrix interactions |
|---|---|
| Annexins V, II | Ca2+ channels, major components of matrix vesicles with activity stimulated by matrix binding e.g. collagens II and X [112]. |
| Vimentin | Intracellular intermediate filaments interact with matrix indirectly via vimentin-associated matrix adhesions (VAMs) [113]. |
| βig-H3 | Matrix molecule with versatile roles in tissue homeostasis; interacts with numerous matrix components [114]. |
| IGFBP3, -7 | Modulate IGF in tissue which can be affected by their direct interaction with fibronectin [115]. |
| PAI-1 | Protease inhibitor mediates the degradation of matrix [116]. |
4. Functional modifiers of elastic fibres
4.1. Transglutaminase
Transglutaminases regulate matrix remodelling and are associated with numerous pathologies including cancer, inflammation and fibrosis [117]. Tissue transglutaminase is known to have a significant role in elastic fibre assembly, both in the cross-linking of fibrillin microfibrils [118] and between fibrillin-1 and tropoelastin [33,119]. The LLC and LTBP-1 N-terminus are also transglutaminase substrates [120], and LTBP-1 forms multimers (both N-N and N-C) that may be cross-linked [11], enhancing its incorporation into the matrix [121] and consequently, regulation of TGFβ signalling. Since proper incorporation of the latent complex is required for normal TGFβ regulation, this finding has implications for fibrillinopathies such as MFS, where these processes may become dysregulated when aberrant complexes are formed. Abnormal transglutaminase activity is also associated with BPD in premature infants and impaired mitral valve development, both attributed to a dysregulated matrix [122,123].
4.2. Lysyl oxidases
Lysyl oxidases LOX and LOX-like1 promote the cross-linking of elastin and its subsequent deposition onto microfibril scaffolds to form elastic fibres [124]. Consequently, LOX is essential for cardiovascular development and function, indeed null mice have defective arterial wall structure with fragmented elastic fibres and die perinatally from aortic aneurysms [125]. Lysyl oxidases may protect against aortic aneurysm formation in MFS, as both LOX and LOXL1 were expressed more highly in a MFS mouse model and in aortic tissue from MFS patients. In the mouse model, LOX inhibition prevented collagen deposition and exacerbated elastic fibre defects, leading to aggressive aortic dilation and death [126]. Guo et al recently identified the LOX mutation that causes familial TAAD: the Ser280Arg mutation produced a less active variant that led to disorganised elastic fibres and increased collagen in patients’ aortas [127]. Several reports have described an association between LOXL1 and glaucoma, specifically in the early stages of the elastic fibre disorder exfoliation syndrome (XFS) [76,[128], [129], [130], [131]]. LOXL1 colocalised with elastin, fibrillin-1 and fibulin-4, which were also upregulated, and its expression was enhanced by TGFβ1 and other XFS-associated stimuli in Tenon’s capsule fibroblasts [76].
5. Extracellular regulation of growth factor signalling
5.1. Transforming growth factor (TGF)β
As mentioned, dysregulated TGFβ signalling is a defining characteristic of MFS and other fibrillinopathies. Several studies have reported a direct association between elevated TGFβ signalling and MFS pathology in mouse models, where injection of a TGFβ-neutralising antibody restored alveolarisation and atrioventricular valve integrity and prevented aortic aneurysms, the most severe clinical feature of MFS [[132], [133], [134]]. However, there are a number of compelling reports that mitigate TGFβ’s role in disease pathology [135,136], while some point to angiotensin II as the major factor [[137], [138], [139]]. Some also argue a protective role for TGFβ [140,141]. Whereas TGFβ neutralisation reduced the incidence of aortic aneurysms in the study by Habashi et al [134], recent data suggests otherwise and, in some cases, show enhanced aneurysm formation upon TGFβ antagonism [140,141]. Neutralising TGFβ in smooth muscle cells increased aortic aneurysms in an AngII-induced model, but not when the antibody was administered systemically [136]. In order to directly address whether excess TGFβ signalling is responsible for the aortopathy observed in MFS, the same group deleted the type II TGFβ receptor in the smooth muscle cells of fibrillin-1 deficient mice. Aortopathy was exacerbated upon loss of TGFβ signalling, and was still observed even without changes to signalling in young mice [135].
A number of clinical trials support this emerging hypothesis. A trial using Losartan to block or diminish the effect of excess TGFβ appeared successful in reducing aortic dilation in MFS patients [142]. However, the same group later reported that Losartan efficacy depended on the patients’ fibrillin-1 status: patients with haploinsufficiency (i.e. a lower quantity of normal fibrillin-1) responded to Losartan better than those with dominant negative fibrillin-1 mutations (i.e. defective fibrillin-1) [137]. In a separate study, the authors determined that patient responsiveness to Losartan correlated with higher baseline levels of TGFβ and a higher aortic dilation rate; they concluded that TGFβ could be considered a biomarker for MFS status (as did Radonic et al [143]), but that angiotensin II triggers initial aortic dilation and increases TGFβ levels [138]. In support of these data, Losartan was no more effective in trials comparing it with other MFS therapies. For example, in the largest study of its kind, aortic dilation was not significantly different with Losartan than standard therapy with beta-blockers [139,144].
5.2. Bone morphogenetic proteins (BMP) and growth and differentiation factors (GDF)
MFS patients and fibrillin-1 insufficient mice have reduced bone mass, suggesting perturbed BMP signalling. Fibrillin-2 null mice have reduced bone formation and impaired osteoblast maturation, implicating a direct role for microfibrils in bone formation [145]. Moreover, abnormal activation of BMP signalling causes myopathy in fibrillin-2 null mice but deletion of a single allele of BMP7 rescues the muscle phenotype [146]. A direct interaction between BMP and the related GDF growth factors and fibrillin-1 was shown; BMP7 is secreted with its bound prodomain which binds fibrillin-1 [147]. Furthermore, GDF5, BMP2, BMP4 and BMP10 but not GDF8 prodomains bind fibrillin-1 via the N-terminal region showing widespread interaction of BMP/GDF family members with fibrillin-1 [23]. Indeed binding to fibrillin-1 was shown to induce latency for proBMP7 where a conformational change from the activatable open form to the closed latent form was observed upon fibrillin-1 binding [148]. A role for BMP antagonists in modulating extracellular regulation of BMPs via fibrillin was shown for gremlin-1. Fibrillin-2 is upregulated in mesothelioma and localises gremlin-1 to the tumour microenvironment where it binds fibrillin-1 and -2 and colocalises with microfibrils in cells and mesothelioma tumours [149].
6. Summary and future directions
Fibrillin microfibrils are periodic, multi-component assemblies with a widespread tissue distribution. In most organs they associate with elastin to form elastic fibres and hence make key contributions to tissue mechanics and structure function. Although there are three fibrillin isoforms, in adults fibrillin-1 is most abundant. In tissues it forms calcium-stabilised microfibrils which are thought to be composed of eight monomers per repeat. In addition to elastin, fibrillin microfibrils interact and/or co-localise with proteins from the ADAMTS/L, LTBP and fibulin families and with proteoglycans and enzymes. The LTBPs and fibulins both share considerable structural homology with the fibrillins as all three families are characterised by multiple cbEGF domains. These proteins play key roles in elastogenesis and mutations in LTBP and fibulin genes, in common with Marfan syndrome causing fibrillin-1 mutations, can manifest as pathologies in multiple organ systems. The pathological mechanisms conferred by LTBP mutations are driven by dysregulation of TGFβ signalling whilst fibulin mutations may induce aberrant tissue remodelling as a consequence of impaired LOX interactions. In contrast the role of MAGPs in elastogenesis is less well defined but knockout experiments and MAGP genetic mutations are clearly associated with pathologies in elastin-rich tissues such as the aorta. Recently the importance of the ADAMTS and ADAMTS-like glycoproteins in mediating pathological changes (which may be phenotypically indistinguishable from some mutations in the FBN1 gene) has been recognised. Members of this large family of proteins exhibit diverse and complex biological functions including both the inhibition and promotion of microfibril deposition and the mediation of focal adhesion formations. Finally it is clear that elastic fibre formation and function may be influenced enzymatically by the expression of transglutaminase, LOX and LOXL and that elastic fibres can exert considerable influence over tissue homeostasis via regulation of TGFβ and the BMP/GDF families of cytokines.
Fibrillin microfibril and elastic fibre biology is highly complex. This complexity presents the research community with a difficult technical challenge in unravelling the multiple molecular and cellular interactions. However, understanding the multiplicity of elastic fibre protein interactions also presents a clear future opportunity to intervene in, and to recognise, disease processes and mechanisms of ageing and to control tissue development for tissue engineering applications.
Acknowledgements
The Wellcome Trust Centre for Cell-Matrix Research is supported by funding from the Wellcome Trust (088785/Z/09/Z). C.B gratefully acknowledges BBSRC (Ref: BB/N015398/1 and BB/R008221/1) and MRC funding (Ref: MR/L016540/1) and A.E. and M.J.S. Walgreens Boots Alliance for programme grant funding.
References
- 1.Kielty C.M., Sherratt M.J., Marson A., Baldock C. Fibrillin microfibrils. Adv. Protein Chem. 2005;70:405–436. doi: 10.1016/S0065-3233(05)70012-7. [DOI] [PubMed] [Google Scholar]
- 2.Bax D.V., Bernard S.E., Lomas A., Morgan A., Humphries J., Shuttleworth C.A., Humphries M.J., Kielty C.M. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha 5 beta 1 and alpha v beta 3 integrins. J. Biol. Chem. 2003;278:34605–34616. doi: 10.1074/jbc.M303159200. [DOI] [PubMed] [Google Scholar]
- 3.Jovanovic J., Takagi J., Choulier L., Abrescia N.G., Stuart D.I., van der Merwe P.A., Mardon H.J., Handford P.A. alphaVbeta6 is a novel receptor for human fibrillin-1. Comparative studies of molecular determinants underlying integrin-rgd affinity and specificity. J. Biol. Chem. 2007;282:6743–6751. doi: 10.1074/jbc.M607008200. [DOI] [PubMed] [Google Scholar]
- 4.Bax D.V., Mahalingam Y., Cain S., Mellody K., Freeman L., Younger K., Shuttleworth C.A., Humphries M.J., Couchman J.R., Kielty C.M. Cell adhesion to fibrillin-1: identification of an Arg-Gly-Asp-dependent synergy region and a heparin-binding site that regulates focal adhesion formation. J. Cell Sci. 2007;120:1383–1392. doi: 10.1242/jcs.003954. [DOI] [PubMed] [Google Scholar]
- 5.Robinson P.N., Arteaga-Solis E., Baldock C., Collod-Beroud G., Booms P., De Paepe A., Dietz H.C., Guo G., Handford P.A., Judge D.P. The molecular genetics of Marfan syndrome and related disorders. J. Med. Genet. 2006;43:769–787. doi: 10.1136/jmg.2005.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faivre L., Gorlin R.J., Wirtz M.K., Godfrey M., Dagoneau N., Samples J.R., Le Merrer M., Collod-Beroud G., Boileau C., Munnich A. In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J. Med. Genet. 2003;40:34–36. doi: 10.1136/jmg.40.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Goff C., Mahaut C., Wang L.W., Allali S., Abhyankar A., Jensen S., Zylberberg L., Collod-Beroud G., Bonnet D., Alanay Y. Mutations in the TGFbeta binding-protein-like domain 5 of FBN1 are responsible for acromicric and geleophysic dysplasias. Am. J. Hum. Genet. 2011;89:7–14. doi: 10.1016/j.ajhg.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downing A.K., Knott V., Werner J.M., Cardy C.M., Campbell I.D., Handford P.A. Solution structure of a pair of calcium-binding epidermal growth factor-like domains: implications for the Marfan syndrome and other genetic disorders. Cell. 1996;85:597–605. doi: 10.1016/s0092-8674(00)81259-3. [DOI] [PubMed] [Google Scholar]
- 9.Jensen S.A., Iqbal S., Lowe E.D., Redfield C., Handford P.A. Structure and interdomain interactions of a hybrid domain: a disulphide-rich module of the fibrillin/LTBP superfamily of matrix proteins. Structure. 2009;17:759–768. doi: 10.1016/j.str.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldock C., Siegler V., Bax D.V., Cain S.A., Mellody K.T., Marson A., Haston J.L., Berry R., Wang M.C., Grossmann J.G. Nanostructure of fibrillin-1 reveals compact conformation of EGF arrays and mechanism for extensibility. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11922–11927. doi: 10.1073/pnas.0601609103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troilo H., Steer R., Collins R.F., Kielty C.M., Baldock C. Independent multimerization of latent TGFbeta binding Protein-1 stabilized by cross-linking and enhanced by heparan sulfate. Sci. Rep. 2016;6:34347. doi: 10.1038/srep34347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakai L.Y., Keene D.R., Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J. Cell Biol. 1986;103:2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldock C., Koster A.J., Ziese U., Rock M.J., Sherratt M.J., Kadler K.E., Shuttleworth C.A., Kielty C.M. The supramolecular organization of fibrillin-rich microfibrils. J. Cell Biol. 2001;152:1045–1056. doi: 10.1083/jcb.152.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis E.C., Roth R.A., Heuser J.E., Mecham R.P. Ultrastructural properties of ciliary zonule microfibrils. J. Struct. Biol. 2002;139:65–75. doi: 10.1016/s1047-8477(02)00559-2. [DOI] [PubMed] [Google Scholar]
- 15.Wang M.C., Lu Y., Baldock C. Fibrillin microfibrils: a key role for the interbead region in elasticity. J. Mol. Biol. 2009;388:168–179. doi: 10.1016/j.jmb.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 16.Sherratt M.J., Bayley C.P., Reilly S.M., Gibbs N.K., Griffiths C.E., Watson R.E. Low-dose ultraviolet radiation selectively degrades chromophore-rich extracellular matrix components. J. Pathol. 2010;222:32–40. doi: 10.1002/path.2730. [DOI] [PubMed] [Google Scholar]
- 17.Kutz W.E., Wang L.W., Bader H.L., Majors A.K., Iwata K., Traboulsi E.I., Sakai L.Y., Keene D.R., Apte S.S. ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts. J. Biol. Chem. 2011;286:17156–17167. doi: 10.1074/jbc.M111.231571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cain S.A., Mularczyk E.J., Singh M., Massam-Wu T., Kielty C.M. ADAMTS-10 and -6 differentially regulate cell-cell junctions and focal adhesions. Sci. Rep. 2016;6:35956. doi: 10.1038/srep35956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sengle G., Tsutsui K., Keene D.R., Tufa S.F., Carlson E.J., Charbonneau N.L., Ono R.N., Sasaki T., Wirtz M.K., Samples J.R. Microenvironmental regulation by fibrillin-1. PLos Genet. 2012;8:e1002425. doi: 10.1371/journal.pgen.1002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bader H.L., Wang L.W., Ho J.C., Tran T., Holden P., Fitzgerald J., Atit R.P., Reinhardt D.P., Apte S.S. A disintegrin-like and metalloprotease domain containing thrombospondin type 1 motif-like 5 (ADAMTSL5) is a novel fibrillin-1-, fibrillin-2-, and heparin-binding member of the ADAMTS superfamily containing a netrin-like module. Matrix Biol. 2012;31:398–411. doi: 10.1016/j.matbio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsutsui K., Manabe R., Yamada T., Nakano I., Oguri Y., Keene D.R., Sengle G., Sakai L.Y., Sekiguchi K. ADAMTSL-6 is a novel extracellular matrix protein that binds to fibrillin-1 and promotes fibrillin-1 fibril formation. J. Biol. Chem. 2010;285:4870–4882. doi: 10.1074/jbc.M109.076919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isogai Z., Aspberg A., Keene D.R., Ono R.N., Reinhardt D.P., Sakai L.Y. Versican interacts with fibrillin-1 and links extracellular microfibrils to other connective tissue networks. J. Biol. Chem. 2002;277:4565–4572. doi: 10.1074/jbc.M110583200. [DOI] [PubMed] [Google Scholar]
- 23.Sengle G., Charbonneau N.L., Ono R.N., Sasaki T., Alvarez J., Keene D.R., Bachinger H.P., Sakai L.Y. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J. Biol. Chem. 2008;283:13874–13888. doi: 10.1074/jbc.M707820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sengle G., Ono R.N., Sasaki T., Sakai L.Y. Prodomains of transforming growth factor beta (TGFbeta) superfamily members specify different functions: extracellular matrix interactions and growth factor bioavailability. J. Biol. Chem. 2011;286:5087–5099. doi: 10.1074/jbc.M110.188615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cain S.A., McGovern A., Small E., Ward L.J., Baldock C., Shuttleworth A., Kielty C.M. Defining elastic fiber interactions by molecular fishing: an affinity purification and mass spectrometry approach. Mol. Cell. Proteomics. 2009;8:2715–2732. doi: 10.1074/mcp.M900008-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marson A., Rock M.J., Cain S.A., Freeman L.J., Morgan A., Mellody K., Shuttleworth C.A., Baldock C., Kielty C.M. Homotypic fibrillin-1 interactions in microfibril assembly. J. Biol. Chem. 2005;280:5013–5021. doi: 10.1074/jbc.M409029200. [DOI] [PubMed] [Google Scholar]
- 27.Lin G., Tiedemann K., Vollbrandt T., Peters H., Batge B., Brinckmann J., Reinhardt D.P. Homo- and heterotypic fibrillin-1 and -2 interactions constitute the basis for the assembly of microfibrils. J. Biol. Chem. 2002;277:50795–50804. doi: 10.1074/jbc.M210611200. [DOI] [PubMed] [Google Scholar]
- 28.Ono R.N., Sengle G., Charbonneau N.L., Carlberg V., Bachinger H.P., Sasaki T., Lee-Arteaga S., Zilberberg L., Rifkin D.B., Ramirez F. Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. J. Biol. Chem. 2009;284:16872–16881. doi: 10.1074/jbc.M809348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhury R., McGovern A., Ridley C., Cain S.A., Baldwin A., Wang M.C., Guo C., Mironov A., Jr, Drymoussi Z., Trump D. Differential regulation of elastic fiber formation by fibulin-4 and -5. J. Biol. Chem. 2009;284:24553–24567. doi: 10.1074/jbc.M109.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabatier L., Djokic J., Hubmacher D., Dzafik D., Nelea V., Reinhardt D.P. Heparin/heparan sulfate controls fibrillin-1, -2 and -3 self-interactions in microfibril assembly. FEBS Lett. 2014 doi: 10.1016/j.febslet.2014.06.061. [DOI] [PubMed] [Google Scholar]
- 31.Cain S.A., Baldwin A.K., Mahalingam Y., Raynal B., Jowitt T.A., Shuttleworth C.A., Couchman J.R., Kielty C.M. Heparan sulfate regulates fibrillin-1 N- and C-terminal interactions. J. Biol. Chem. 2008;283:27017–27027. doi: 10.1074/jbc.M803373200. [DOI] [PubMed] [Google Scholar]
- 32.Hirani R., Hanssen E., Gibson M.A. LTBP-2 specifically interacts with the amino-terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biol. 2007;26:213–223. doi: 10.1016/j.matbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Rock M.J., Cain S.A., Freeman L.J., Morgan A., Mellody K., Marson A., Shuttleworth C.A., Weiss A.S., Kielty C.M. Molecular basis of elastic fiber formation. Critical interactions and a tropoelastin-fibrillin-1 cross-link. J. Biol. Chem. 2004;279:23748–23758. doi: 10.1074/jbc.M400212200. [DOI] [PubMed] [Google Scholar]
- 34.Tiedemann K., Sasaki T., Gustafsson E., Gohring W., Batge B., Notbohm H., Timpl R., Wedel T., Schlotzer-Schrehardt U., Reinhardt D.P. Microfibrils at basement membrane zones interact with perlecan via fibrillin-1. J. Biol. Chem. 2005;280:11404–11412. doi: 10.1074/jbc.M409882200. [DOI] [PubMed] [Google Scholar]
- 35.Pilecki B., Holm A.T., Schlosser A., Moeller J.B., Wohl A.P., Zuk A.V., Heumuller S.E., Wallis R., Moestrup S.K., Sengle G. Characterization of Microfibrillar-associated Protein 4 (MFAP4) as a Tropoelastin- and fibrillin-binding protein involved in elastic fiber formation. J. Biol. Chem. 2016;291:1103–1114. doi: 10.1074/jbc.M115.681775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reinboth B., Hanssen E., Cleary E.G., Gibson M.A. Molecular interactions of biglycan and decorin with elastic fiber components: biglycan forms a ternary complex with tropoelastin and microfibril-associated glycoprotein 1. J. Biol. Chem. 2002;277:3950–3957. doi: 10.1074/jbc.M109540200. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki T., Gohring W., Miosge N., Abrams W.R., Rosenbloom J., Timpl R. Tropoelastin binding to fibulins, nidogen-2 and other extracellular matrix proteins. FEBS Lett. 1999;460:280–284. doi: 10.1016/s0014-5793(99)01362-9. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi N., Kostka G., Garbe J.H., Keene D.R., Bachinger H.P., Hanisch F.G., Markova D., Tsuda T., Timpl R., Chu M.L. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. J. Biol. Chem. 2007;282:11805–11816. doi: 10.1074/jbc.M611029200. [DOI] [PubMed] [Google Scholar]
- 39.Gleizes P.E., Beavis R.C., Mazzieri R., Shen B., Rifkin D.B. Identification and characterization of an eight-cysteine repeat of the latent transforming growth factor-beta binding protein-1 that mediates bonding to the latent transforming growth factor-beta1. J. Biol. Chem. 1996;271:29891–29896. doi: 10.1074/jbc.271.47.29891. [DOI] [PubMed] [Google Scholar]
- 40.Saharinen J., Taipale J., Keski-Oja J. Association of the small latent transforming growth factor-beta with an eight cysteine repeat of its binding protein LTBP-1. EMBO J. 1996;15:245–253. [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y., Ali T., Todorovic V., O’Leary J.M., Kristina Downing A., Rifkin D.B. Amino acid requirements for formation of the TGF-beta-latent TGF-beta binding protein complexes. J. Mol. Biol. 2005;345:175–186. doi: 10.1016/j.jmb.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 42.Todorovic V., Frendewey D., Gutstein D.E., Chen Y., Freyer L., Finnegan E., Liu F., Murphy A., Valenzuela D., Yancopoulos G. Long form of latent TGF-beta binding protein 1 (Ltbp1L) is essential for cardiac outflow tract septation and remodeling. Development. 2007;134:3723–3732. doi: 10.1242/dev.008599. [DOI] [PubMed] [Google Scholar]
- 43.Horiguchi M., Todorovic V., Hadjiolova K., Weiskirchen R., Rifkin D.B. Abrogation of both short and long forms of latent transforming growth factor-beta binding protein-1 causes defective cardiovascular development and is perinatally lethal. Matrix Biol. 2015;43:61–70. doi: 10.1016/j.matbio.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choudhary B., Ito Y., Makita T., Sasaki T., Chai Y., Sucov H.M. Cardiovascular malformations with normal smooth muscle differentiation in neural crest-specific type II TGFbeta receptor (Tgfbr2) mutant mice. Dev. Biol. 2006;289:420–429. doi: 10.1016/j.ydbio.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Hyytiainen M., Taipale J., Heldin C.H., Keski-Oja J. Recombinant latent transforming growth factor beta-binding protein 2 assembles to fibroblast extracellular matrix and is susceptible to proteolytic processing and release. J. Biol. Chem. 1998;273:20669–20676. doi: 10.1074/jbc.273.32.20669. [DOI] [PubMed] [Google Scholar]
- 46.Vehvilainen P., Hyytiainen M., Keski-Oja J. Matrix association of latent TGF-beta binding protein-2 (LTBP-2) is dependent on fibrillin-1. J. Cell. Physiol. 2009;221:586–593. doi: 10.1002/jcp.21888. [DOI] [PubMed] [Google Scholar]
- 47.Hirai M., Horiguchi M., Ohbayashi T., Kita T., Chien K.R., Nakamura T. Latent TGF-beta-binding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly. EMBO J. 2007;26:3283–3295. doi: 10.1038/sj.emboj.7601768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sideek M.A., Menz C., Parsi M.K., Gibson M.A. LTBP-2 competes with tropoelastin for binding to fibulin-5 and heparin, and is a negative modulator of elastinogenesis. Matrix Biol. 2014;34:114–123. doi: 10.1016/j.matbio.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Desir J., Sznajer Y., Depasse F., Roulez F., Schrooyen M., Meire F., Abramowicz M. LTBP2 null mutations in an autosomal recessive ocular syndrome with megalocornea, spherophakia, and secondary glaucoma. Eur. J. Hum. Genet. 2010;18:761–767. doi: 10.1038/ejhg.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan A.O., Aldahmesh M.A., Alkuraya F.S. Congenital megalocornea with zonular weakness and childhood lens-related secondary glaucoma - a distinct phenotype caused by recessive LTBP2 mutations. Mol. Vis. 2011;17:2570–2579. [PMC free article] [PubMed] [Google Scholar]
- 51.Haji-Seyed-Javadi R., Jelodari-Mamaghani S., Paylakhi S.H., Yazdani S., Nilforushan N., Fan J.B., Klotzle B., Mahmoudi M.J., Ebrahimian M.J., Chelich N. LTBP2 mutations cause Weill-Marchesani and Weill-Marchesani-like syndrome and affect disruptions in the extracellular matrix. Hum. Mutat. 2012;33:1182–1187. doi: 10.1002/humu.22105. [DOI] [PubMed] [Google Scholar]
- 52.Narooie-Nejad M., Paylakhi S.H., Shojaee S., Fazlali Z., Rezaei Kanavi M., Nilforushan N., Yazdani S., Babrzadeh F., Suri F., Ronaghi M. Loss of function mutations in the gene encoding latent transforming growth factor beta binding protein 2, LTBP2, cause primary congenital glaucoma. Hum Mol Genet. 2009;18:3969–3977. doi: 10.1093/hmg/ddp338. [DOI] [PubMed] [Google Scholar]
- 53.Ali M., McKibbin M., Booth A., Parry D.A., Jain P., Riazuddin S.A., Hejtmancik J.F., Khan S.N., Firasat S., Shires M. Null mutations in LTBP2 cause primary congenital glaucoma. Am. J. Hum. Genet. 2009;84:664–671. doi: 10.1016/j.ajhg.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menz C., Parsi M.K., Adams J.R., Sideek M.A., Kopecki Z., Cowin A.J., Gibson M.A. LTBP-2 Has a single High-affinity binding site for FGF-2 and blocks FGF-2-induced cell proliferation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morkmued S., Hemmerle J., Mathieu E., Laugel-Haushalter V., Dabovic B., Rifkin D.B., Dolle P., Niederreither K., Bloch-Zupan A. Enamel and dental anomalies in latent-transforming growth factor beta-binding protein 3 mutant mice. Eur. J. Oral Sci. 2017;125:8–17. doi: 10.1111/eos.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dabovic B., Chen Y., Colarossi C., Zambuto L., Obata H., Rifkin D.B. Bone defects in latent TGF-beta binding protein (Ltbp)-3 null mice; A role for Ltbp in TGF-beta presentation. J. Endocrinol. 2002;175:129–141. doi: 10.1677/joe.0.1750129. [DOI] [PubMed] [Google Scholar]
- 57.Dabovic B., Levasseur R., Zambuto L., Chen Y., Karsenty G., Rifkin D.B. Osteopetrosis-like phenotype in latent TGF-beta binding protein 3 deficient mice. Bone. 2005;37:25–31. doi: 10.1016/j.bone.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 58.Zilberberg L., Phoon C.K., Robertson I., Dabovic B., Ramirez F., Rifkin D.B. Genetic analysis of the contribution of LTBP-3 to thoracic aneurysm in Marfan syndrome. Proc. Natl. Acad. Sci. U. S. A. 2015;112:14012–14017. doi: 10.1073/pnas.1507652112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aya R., Ishiko T., Noda K., Yamawaki S., Sakamoto Y., Tomihata K., Katayama Y., Yoshikawa K., Kubota H., Nakamura T. Regeneration of elastic fibers by three-dimensional culture on a collagen scaffold and the addition of latent TGF-beta binding protein 4 to improve elastic matrix deposition. Biomaterials. 2015;72:29–37. doi: 10.1016/j.biomaterials.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 60.Bultmann-Mellin I., Conradi A., Maul A.C., Dinger K., Wempe F., Wohl A.P., Imhof T., Wunderlich F.T., Bunck A.C., Nakamura T. Modeling autosomal recessive cutis laxa type 1C in mice reveals distinct functions for Ltbp-4 isoforms. Dis Model Mech. 2015;8:403–415. doi: 10.1242/dmm.018960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bultmann-Mellin I., Essers J., van Heijingen P.M., von Melchner H., Sengle G., Sterner-Kock A. Function of Ltbp-4L and fibulin-4 in survival and elastogenesis in mice. Dis Model Mech. 2016;9:1367–1374. doi: 10.1242/dmm.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noda K., Dabovic B., Takagi K., Inoue T., Horiguchi M., Hirai M., Fujikawa Y., Akama T.O., Kusumoto K., Zilberberg L. Latent TGF-beta binding protein 4 promotes elastic fiber assembly by interacting with fibulin-5. Proc. Natl. Acad. Sci. U. S. A. 2013;110:2852–2857. doi: 10.1073/pnas.1215779110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urban Z., Hucthagowder V., Schurmann N., Todorovic V., Zilberberg L., Choi J., Sens C., Brown C.W., Clark R.D., Holland K.E. Mutations in LTBP4 cause a syndrome of impaired pulmonary, gastrointestinal, genitourinary, musculoskeletal, and dermal development. Am. J. Hum. Genet. 2009;85:593–605. doi: 10.1016/j.ajhg.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sterner-Kock A., Thorey I.S., Koli K., Wempe F., Otte J., Bangsow T., Kuhlmeier K., Kirchner T., Jin S., Keski-Oja J. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev. 2002;16:2264–2273. doi: 10.1101/gad.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heydemann A., Ceco E., Lim J.E., Hadhazy M., Ryder P., Moran J.L., Beier D.R., Palmer A.A., McNally E.M. Latent TGF-beta-binding protein 4 modifies muscular dystrophy in mice. J. Clin. Invest. 2009;119:3703–3712. doi: 10.1172/JCI39845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flanigan K.M., Ceco E., Lamar K.M., Kaminoh Y., Dunn D.M., Mendell J.R., King W.M., Pestronk A., Florence J.M., Mathews K.D. LTBP4 genotype predicts age of ambulatory loss in Duchenne muscular dystrophy. Ann. Neurol. 2013;73:481–488. doi: 10.1002/ana.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ceco E., Bogdanovich S., Gardner B., Miller T., DeJesus A., Earley J.U., Hadhazy M., Smith L.R., Barton E.R., Molkentin J.D. Targeting latent TGFbeta release in muscular dystrophy. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lamar K.M., Bogdanovich S., Gardner B.B., Gao Q.Q., Miller T., Earley J.U., Hadhazy M., Vo A.H., Wren L., Molkentin J.D. Overexpression of latent TGFbeta binding Protein 4 in muscle ameliorates muscular dystrophy through Myostatin and TGFbeta. PLos Genet. 2016;12 doi: 10.1371/journal.pgen.1006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McLaughlin P.J., Chen Q., Horiguchi M., Starcher B.C., Stanton J.B., Broekelmann T.J., Marmorstein A.D., McKay B., Mecham R., Nakamura T. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol. Cell. Biol. 2006;26:1700–1709. doi: 10.1128/MCB.26.5.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horiguchi M., Inoue T., Ohbayashi T., Hirai M., Noda K., Marmorstein L.Y., Yabe D., Takagi K., Akama T.O., Kita T. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc. Natl. Acad. Sci. U. S. A. 2009;106:19029–19034. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirai M., Ohbayashi T., Horiguchi M., Okawa K., Hagiwara A., Chien K.R., Kita T., Nakamura T. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J. Cell Biol. 2007;176:1061–1071. doi: 10.1083/jcb.200611026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papke C.L., Yanagisawa H. Fibulin-4 and fibulin-5 in elastogenesis and beyond: insights from mouse and human studies. Matrix Biol. 2014;37:142–149. doi: 10.1016/j.matbio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sasaki T., Hanisch F.G., Deutzmann R., Sakai L.Y., Sakuma T., Miyamoto T., Yamamoto T., Hannappel E., Chu M.L., Lanig H. Functional consequence of fibulin-4 missense mutations associated with vascular and skeletal abnormalities and cutis laxa. Matrix Biol. 2016;56:132–149. doi: 10.1016/j.matbio.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Halabi C.M., Broekelmann T.J., Lin M., Lee V.S., Chu M.L., Mecham R.P. Fibulin-4 is essential for maintaining arterial wall integrity in conduit but not muscular arteries. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1602532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Igoucheva O., Alexeev V., Halabi C.M., Adams S.M., Stoilov I., Sasaki T., Arita M., Donahue A., Mecham R.P., Birk D.E. Fibulin-4 E57K knock-in mice recapitulate cutaneous, vascular and skeletal defects of recessive cutis Laxa 1B with both elastic fiber and collagen fibril abnormalities. J. Biol. Chem. 2015;290:21443–21459. doi: 10.1074/jbc.M115.640425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zenkel M., Schlotzer-Schrehardt U. Expression and regulation of LOXL1 and elastin-related genes in eyes with exfoliation syndrome. J. Glaucoma. 2014;23:S48–50. doi: 10.1097/IJG.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 77.Han W., Guo C., Liu Q., Yu B., Liu Z., Yang J., Deng C. Aberrant elastin remodeling in the lungs of O(2)-exposed newborn mice; Primarily results from perturbed interaction between integrins and elastin. Cell Tissue Res. 2015;359:589–603. doi: 10.1007/s00441-014-2035-1. [DOI] [PubMed] [Google Scholar]
- 78.Yanagisawa H., Davis E.C., Starcher B.C., Ouchi T., Yanagisawa M., Richardson J.A., Olson E.N. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 79.Nakamura T., Lozano P.R., Ikeda Y., Iwanaga Y., Hinek A., Minamisawa S., Cheng C.F., Kobuke K., Dalton N., Takada Y. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 80.Loeys B., Van Maldergem L., Mortier G., Coucke P., Gerniers S., Naeyaert J.M., De Paepe A. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum Mol Genet. 2002;11:2113–2118. doi: 10.1093/hmg/11.18.2113. [DOI] [PubMed] [Google Scholar]
- 81.Hu Q., Loeys B.L., Coucke P.J., De Paepe A., Mecham R.P., Choi J., Davis E.C., Urban Z. Fibulin-5 mutations: mechanisms of impaired elastic fiber formation in recessive cutis laxa. Hum Mol Genet. 2006;15:3379–3386. doi: 10.1093/hmg/ddl414. [DOI] [PubMed] [Google Scholar]
- 82.Elahi E., Kalhor R., Banihosseini S.S., Torabi N., Pour-Jafari H., Houshmand M., Amini S.S., Ramezani A., Loeys B. Homozygous missense mutation in fibulin-5 in an Iranian autosomal recessive cutis laxa pedigree and associated haplotype. J. Invest. Dermatol. 2006;126:1506–1509. doi: 10.1038/sj.jid.5700247. [DOI] [PubMed] [Google Scholar]
- 83.Claus S., Fischer J., Megarbane H., Megarbane A., Jobard F., Debret R., Peyrol S., Saker S., Devillers M., Sommer P. A p.C217R mutation in fibulin-5 from cutis laxa patients is associated with incomplete extracellular matrix formation in a skin equivalent model. J. Invest. Dermatol. 2008;128:1442–1450. doi: 10.1038/sj.jid.5701211. [DOI] [PubMed] [Google Scholar]
- 84.Callewaert B., Su C.T., Van Damme T., Vlummens P., Malfait F., Vanakker O., Schulz B., Mac Neal M., Davis E.C., Lee J.G. Comprehensive clinical and molecular analysis of 12 families with type 1 recessive cutis laxa. Hum. Mutat. 2013;34:111–121. doi: 10.1002/humu.22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gibson M.A., Kumaratilake J.S., Cleary E.G. The protein components of the 12-nanometer microfibrils of elastic and nonelastic tissues. J. Biol. Chem. 1989;264:4590–4598. [PubMed] [Google Scholar]
- 86.Cain S.A., Morgan A., Sherratt M.J., Ball S.G., Shuttleworth C.A., Kielty C.M. Proteomic analysis of fibrillin-rich microfibrils. Proteomics. 2006;6:111–122. doi: 10.1002/pmic.200401340. [DOI] [PubMed] [Google Scholar]
- 87.Lemaire R., Bayle J., Mecham R.P., Lafyatis R. Microfibril-associated MAGP-2 stimulates elastic fiber assembly. J. Biol. Chem. 2007;282:800–808. doi: 10.1074/jbc.M609692200. [DOI] [PubMed] [Google Scholar]
- 88.Gibson M.A., Finnis M.L., Kumaratilake J.S., Cleary E.G. Microfibril-associated glycoprotein-2 (MAGP-2) is specifically associated with fibrillin-containing microfibrils but exhibits more restricted patterns of tissue localization and developmental expression than its structural relative MAGP-1. J. Histochem. Cytochem. 1998;46:871–886. doi: 10.1177/002215549804600802. [DOI] [PubMed] [Google Scholar]
- 89.Hanssen E., Hew F.H., Moore E., Gibson M.A. MAGP-2 has multiple binding regions on fibrillins and has covalent periodic association with fibrillin-containing microfibrils. J. Biol. Chem. 2004;279:29185–29194. doi: 10.1074/jbc.M313672200. [DOI] [PubMed] [Google Scholar]
- 90.Weinbaum J.S., Broekelmann T.J., Pierce R.A., Werneck C.C., Segade F., Craft C.S., Knutsen R.H., Mecham R.P. Deficiency in microfibril-associated glycoprotein-1 leads to complex phenotypes in multiple organ systems. J. Biol. Chem. 2008;283:25533–25543. doi: 10.1074/jbc.M709962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Craft C.S., Zou W., Watkins M., Grimston S., Brodt M.D., Broekelmann T.J., Weinbaum J.S., Teitelbaum S.L., Pierce R.A., Civitelli R. Microfibril-associated glycoprotein-1, an extracellular matrix regulator of bone remodeling. J. Biol. Chem. 2010;285:23858–23867. doi: 10.1074/jbc.M110.113019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Combs M.D., Knutsen R.H., Broekelmann T.J., Toennies H.M., Brett T.J., Miller C.A., Kober D.L., Craft C.S., Atkinson J.J., Shipley J.M. Microfibril-associated glycoprotein 2 (MAGP2) loss of function has pleiotropic effects in vivo. J. Biol. Chem. 2013;288:28869–28880. doi: 10.1074/jbc.M113.497727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jensen S.A., Reinhardt D.P., Gibson M.A., Weiss A.S. Protein interaction studies of MAGP-1 with tropoelastin and fibrillin-1. J. Biol. Chem. 2001;276:39661–39666. doi: 10.1074/jbc.M104533200. [DOI] [PubMed] [Google Scholar]
- 94.Fujita T., Tsuruga E., Yamanouchi K., Sawa Y., Ishikawa H. Microfibril-associated glycoprotein-1 controls human ciliary zonule development in vitro. Acta Histochem. Cytochem. 2014;47:11–17. doi: 10.1267/ahc.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zheng Q., Chen S., Chen Y., Lyga J., Wyborski R., Santhanam U. Investigation of age-related decline of microfibril-associated glycoprotein-1 in human skin through immunohistochemistry study. Clin Cosmet Investig Dermatol. 2013;6:317–323. doi: 10.2147/CCID.S51958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Werneck C.C., Vicente C.P., Weinberg J.S., Shifren A., Pierce R.A., Broekelmann T.J., Tollefsen D.M., Mecham R.P. Mice lacking the extracellular matrix protein MAGP1 display delayed thrombotic occlusion following vessel injury. Blood. 2008;111:4137–4144. doi: 10.1182/blood-2007-07-101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vassequi-Silva T., Pereira D.S., Nery Diez A.C.C., Braga G.G., Godoy J.A., Mendes C.B., Dos Santos L., Krieger J.E., Antunes E., Costa F.T.M. Losartan and captopril treatment rescue normal thrombus formation in microfibril associated glycoprotein-1 (MAGP1) deficient mice. Thromb. Res. 2016;138:7–15. doi: 10.1016/j.thromres.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Barbier M., Gross M.S., Aubart M., Hanna N., Kessler K., Guo D.C., Tosolini L., Ho-Tin-Noe B., Regalado E., Varret M. MFAP5 loss-of-function mutations underscore the involvement of matrix alteration in the pathogenesis of familial thoracic aortic aneurysms and dissections. Am. J. Hum. Genet. 2014;95:736–743. doi: 10.1016/j.ajhg.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Apte S.S. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J. Biol. Chem. 2009;284:31493–31497. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hubmacher D., Apte S.S. ADAMTS proteins as modulators of microfibril formation and function. Matrix Biol. 2015;47:34–43. doi: 10.1016/j.matbio.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dagoneau N., Benoist-Lasselin C., Huber C., Faivre L., Megarbane A., Alswaid A., Dollfus H., Alembik Y., Munnich A., Legeai-Mallet L. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am. J. Hum. Genet. 2004;75:801–806. doi: 10.1086/425231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morales J., Al-Sharif L., Khalil D.S., Shinwari J.M., Bavi P., Al-Mahrouqi R.A., Al-Rajhi A., Alkuraya F.S., Meyer B.F., Al Tassan N. Homozygous mutations in ADAMTS10 and ADAMTS17 cause lenticular myopia, ectopia lentis, glaucoma, spherophakia, and short stature. Am. J. Hum. Genet. 2009;85:558–568. doi: 10.1016/j.ajhg.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Le Goff C., Morice-Picard F., Dagoneau N., Wang L.W., Perrot C., Crow Y.J., Bauer F., Flori E., Prost-Squarcioni C., Krakow D. ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-beta bioavailability regulation. Nat. Genet. 2008;40:1119–1123. doi: 10.1038/ng.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ahram D., Sato T.S., Kohilan A., Tayeh M., Chen S., Leal S., Al-Salem M., El-Shanti H. A homozygous mutation in ADAMTSL4 causes autosomal-recessive isolated ectopia lentis. Am. J. Hum. Genet. 2009;84:274–278. doi: 10.1016/j.ajhg.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chandra A., Aragon-Martin J.A., Hughes K., Gati S., Reddy M.A., Deshpande C., Cormack G., Child A.H., Charteris D.G., Arno G. A genotype-phenotype comparison of ADAMTSL4 and FBN1 in isolated ectopia lentis. Invest. Ophthalmol. Vis. Sci. 2012;53:4889–4896. doi: 10.1167/iovs.12-9874. [DOI] [PubMed] [Google Scholar]
- 106.Collin G.B., Hubmacher D., Charette J.R., Hicks W.L., Stone L., Yu M., Naggert J.K., Krebs M.P., Peachey N.S., Apte S.S. Disruption of murine Adamtsl4 results in zonular fiber detachment from the lens and in retinal pigment epithelium dedifferentiation. Hum. Mol. Genet. 2015;24:6958–6974. doi: 10.1093/hmg/ddv399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hubmacher D., Wang L.W., Mecham R.P., Reinhardt D.P., Apte S.S. Adamtsl2 deletion results in bronchial fibrillin microfibril accumulation and bronchial epithelial dysplasia – a novel mouse model providing insights into geleophysic dysplasia. Disease Models & Mechanisms. 2015;8:487–499. doi: 10.1242/dmm.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gabriel L.A., Wang L.W., Bader H., Ho J.C., Majors A.K., Hollyfield J.G., Traboulsi E.I., Apte S.S. ADAMTSL4, a secreted glycoprotein widely distributed in the eye, binds fibrillin-1 microfibrils and accelerates microfibril biogenesis. Invest. Ophthalmol. Vis. Sci. 2012;53:461–469. doi: 10.1167/iovs.10-5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chandra A., Jones M., Cottrill P., Eastlake K., Limb G.A., Charteris D.G. Gene expression and protein distribution of ADAMTSL-4 in human iris, choroid and retina. Br. J. Ophthalmol. 2013;97:1208–1212. doi: 10.1136/bjophthalmol-2013-303353. [DOI] [PubMed] [Google Scholar]
- 110.Eckersley A., Mellody K.T., Pilkington S.M., Griffiths C.E., Watson R.E.B., O’Cualain R., Baldock C., Knight D., Sherratt M.J. Structural and compositional diversity of fibrillin microfibrils in human tissues. J. Biol. Chem. 2018 doi: 10.1074/jbc.RA117.001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ashworth J.L., Kelly V., Wilson R., Shuttleworth C.A., Kielty C.M. Fibrillin assembly: dimer formation mediated by amino-terminal sequences. J. Cell Sci. 1999;112(Pt 20):3549–3558. doi: 10.1242/jcs.112.20.3549. [DOI] [PubMed] [Google Scholar]
- 112.Kirsch T., Harrison G., Golub E.E., Nah H.D. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J. Biol. Chem. 2000;275:35577–35583. doi: 10.1074/jbc.M005648200. [DOI] [PubMed] [Google Scholar]
- 113.Ivaska J., Pallari H.M., Nevo J., Eriksson J.E. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell. Res. 2007;313:2050–2062. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 114.Hanssen E., Reinboth B., Gibson M.A. Covalent and non-covalent interactions of betaig-h3 with collagen VI. Beta ig-h3 is covalently attached to the amino-terminal region of collagen VI in tissue microfibrils. J. Biol. Chem. 2003;278:24334–24341. doi: 10.1074/jbc.M303455200. [DOI] [PubMed] [Google Scholar]
- 115.McIntosh J., Dennison G., Holly J.M., Jarrett C., Frankow A., Foulstone E.J., Winters Z.E., Perks C.M. IGFBP-3 can either inhibit or enhance EGF-mediated growth of breast epithelial cells dependent upon the presence of fibronectin. J. Biol. Chem. 2010;285:38788–38800. doi: 10.1074/jbc.M110.177311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cajot J.F., Bamat J., Bergonzelli G.E., Kruithof E.K., Medcalf R.L., Testuz J., Sordat B. Plasminogen-activator inhibitor type 1 is a potent natural inhibitor of extracellular matrix degradation by fibrosarcoma and colon carcinoma cells. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6939–6943. doi: 10.1073/pnas.87.18.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Collighan R.J., Griffin M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids. 2009;36:659–670. doi: 10.1007/s00726-008-0190-y. [DOI] [PubMed] [Google Scholar]
- 118.Qian R.Q., Glanville R.W. Alignment of fibrillin molecules in elastic microfibrils is defined by transglutaminase-derived cross-links. Biochemistry. 1997;36:15841–15847. doi: 10.1021/bi971036f. [DOI] [PubMed] [Google Scholar]
- 119.Clarke A.W., Wise S.G., Cain S.A., Kielty C.M., Weiss A.S. Coacervation is promoted by molecular interactions between the PF2 segment of fibrillin-1 and the domain 4 region of tropoelastin. Biochemistry. 2005;44:10271–10281. doi: 10.1021/bi050530d. [DOI] [PubMed] [Google Scholar]
- 120.Nunes I., Gleizes P.E., Metz C.N., Rifkin D.B. Latent transforming growth factor-beta binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor-beta. J. Cell Biol. 1997;136:1151–1163. doi: 10.1083/jcb.136.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Verderio E., Gaudry C., Gross S., Smith C., Downes S., Griffin M. Regulation of cell surface tissue transglutaminase: effects on matrix storage of latent transforming growth factor-beta binding protein-1. J. Histochem. Cytochem. 1999;47:1417–1432. doi: 10.1177/002215549904701108. [DOI] [PubMed] [Google Scholar]
- 122.Witsch T.J., Niess G., Sakkas E., Likhoshvay T., Becker S., Herold S., Mayer K., Vadasz I., Roberts J.D., Jr, Seeger W. Transglutaminase 2: a new player in bronchopulmonary dysplasia? Eur. Respir. J. 2014;44:109–121. doi: 10.1183/09031936.00075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pang K.L., Parnall M., Loughna S. Effect of altered haemodynamics on the developing mitral valve in chick embryonic heart. J. Mol. Cell. Cardiol. 2017;108:114–126. doi: 10.1016/j.yjmcc.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Molnar J., Fong K.S., He Q.P., Hayashi K., Kim Y., Fong S.F., Fogelgren B., Szauter K.M., Mink M., Csiszar K. Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim. Biophys. Acta. 2003;1647:220–224. doi: 10.1016/s1570-9639(03)00053-0. [DOI] [PubMed] [Google Scholar]
- 125.Maki J.M., Rasanen J., Tikkanen H., Sormunen R., Makikallio K., Kivirikko K.I., Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 126.Busnadiego O., Gorbenko Del Blanco D., Gonzalez-Santamaria J., Habashi J.P., Calderon J.F., Sandoval P., Bedja D., Guinea-Viniegra J., Lopez-Cabrera M., Rosell-Garcia T. Elevated expression levels of lysyl oxidases protect against aortic aneurysm progression in Marfan syndrome. J. Mol. Cell. Cardiol. 2015;85:48–57. doi: 10.1016/j.yjmcc.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 127.Guo D.C., Regalado E.S., Gong L., Duan X., Santos-Cortez R.L., Arnaud P., Ren Z., Cai B., Hostetler E.M., Moran R. LOX mutations predispose to thoracic aortic aneurysms and dissections. Circ. Res. 2016;118:928–934. doi: 10.1161/CIRCRESAHA.115.307130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vranka J.A., Kelley M.J., Acott T.S., Keller K.E. Extracellular matrix in the trabecular meshwork: intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 2015;133:112–125. doi: 10.1016/j.exer.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Laczko R., Szauter K.M., Csiszar K. LOXL1-associated candidate epithelial pathomechanisms in exfoliation glaucoma. J. Glaucoma. 2014;23:S43–7. doi: 10.1097/IJG.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vazquez L.E., Lee R.K. Genomic and proteomic pathophysiology of pseudoexfoliation glaucoma. Int. Ophthalmol. Clin. 2014;54:1–13. doi: 10.1097/IIO.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schlotzer-Schrehardt U., Pasutto F., Sommer P., Hornstra I., Kruse F.E., Naumann G.O., Reis A., Zenkel M. Genotype-correlated expression of lysyl oxidase-like 1 in ocular tissues of patients with pseudoexfoliation syndrome/glaucoma and normal patients. Am. J. Pathol. 2008;173:1724–1735. doi: 10.2353/ajpath.2008.080535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Neptune E.R., Frischmeyer P.A., Arking D.E., Myers L., Bunton T.E., Gayraud B., Ramirez F., Sakai L.Y., Dietz H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 133.Ng C.M., Cheng A., Myers L.A., Martinez-Murillo F., Jie C., Bedja D., Gabrielson K.L., Hausladen J.M., Mecham R.P., Judge D.P. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J. Clin. Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Habashi J.P., Judge D.P., Holm T.M., Cohn R.D., Loeys B.L., Cooper T.K., Myers L., Klein E.C., Liu G., Calvi C. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wei H., Hu J.H., Angelov S.N., Fox K., Yan J., Enstrom R., Smith A., Dichek D.A. Aortopathy in a mouse model of Marfan syndrome Is not mediated by altered transforming growth factor beta signaling. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Angelov S.N., Hu J.H., Wei H., Airhart N., Shi M., Dichek D.A. TGF-beta (transforming growth factor-beta) signaling protects the thoracic and abdominal aorta from angiotensin II-induced pathology by distinct mechanisms. Arterioscler. Thromb. Vasc. Biol. 2017;37:2102–2113. doi: 10.1161/ATVBAHA.117.309401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Franken R., den Hartog A.W., Radonic T., Micha D., Maugeri A., van Dijk F.S., Meijers-Heijboer H.E., Timmermans J., Scholte A.J., van den Berg M.P. Beneficial outcome of losartan therapy depends on type of FBN1 mutation in Marfan syndrome. Circ Cardiovasc Genet. 2015;8:383–388. doi: 10.1161/CIRCGENETICS.114.000950. [DOI] [PubMed] [Google Scholar]
- 138.Franken R., Radonic T., den Hartog A.W., Groenink M., Pals G., van Eijk M., Lutter R., Mulder B.J., Zwinderman A.H., de Waard V. The revised role of TGF-beta in aortic aneurysms in Marfan syndrome. Neth Heart J. 2015;23:116–121. doi: 10.1007/s12471-014-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Milleron O., Arnoult F., Ropers J., Aegerter P., Detaint D., Delorme G., Attias D., Tubach F., Dupuis-Girod S., Plauchu H. Marfan Sartan: a randomized, double-blind, placebo-controlled trial. Eur. Heart J. 2015;36:2160–2166. doi: 10.1093/eurheartj/ehv151. [DOI] [PubMed] [Google Scholar]
- 140.Chen X., Rateri D.L., Howatt D.A., Balakrishnan A., Moorleghen J.J., Cassis L.A., Daugherty A. TGF-beta neutralization enhances AngII-induced aortic rupture and aneurysm in both thoracic and abdominal regions. PLoS One. 2016;11:e0153811. doi: 10.1371/journal.pone.0153811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cook J.R., Clayton N.P., Carta L., Galatioto J., Chiu E., Smaldone S., Nelson C.A., Cheng S.H., Wentworth B.M., Ramirez F. Dimorphic effects of transforming growth factor-beta signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 2015;35:911–917. doi: 10.1161/ATVBAHA.114.305150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Groenink M., den Hartog A.W., Franken R., Radonic T., de Waard V., Timmermans J., Scholte A.J., van den Berg M.P., Spijkerboer A.M., Marquering H.A. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur. Heart J. 2013;34:3491–3500. doi: 10.1093/eurheartj/eht334. [DOI] [PubMed] [Google Scholar]
- 143.Radonic T., de Witte P., Groenink M., de Waard V., Lutter R., van Eijk M., Jansen M., Timmermans J., Kempers M., Scholte A.J. Inflammation aggravates disease severity in Marfan syndrome patients. PLoS One. 2012;7:e32963. doi: 10.1371/journal.pone.0032963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lacro R.V., Dietz H.C., Sleeper L.A., Yetman A.T., Bradley T.J., Colan S.D., Pearson G.D., Selamet Tierney E.S., Levine J.C., Atz A.M. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N. Engl. J. Med. 2014;371:2061–2071. doi: 10.1056/NEJMoa1404731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nistala H., Lee-Arteaga S., Smaldone S., Siciliano G., Carta L., Ono R.N., Sengle G., Arteaga-Solis E., Levasseur R., Ducy P. Fibrillin-1 and -2 differentially modulate endogenous TGF-beta and BMP bioavailability during bone formation. J. Cell Biol. 2010;190:1107–1121. doi: 10.1083/jcb.201003089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sengle G., Carlberg V., Tufa S.F., Charbonneau N.L., Smaldone S., Carlson E.J., Ramirez F., Keene D.R., Sakai L.Y. Abnormal activation of BMP signaling causes myopathy in Fbn2 null mice. PLos Genet. 2015;11:e1005340. doi: 10.1371/journal.pgen.1005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gregory K.E., Ono R.N., Charbonneau N.L., Kuo C.L., Keene D.R., Bachinger H.P., Sakai L.Y. The prodomain of BMP-7 targets the BMP-7 complex to the extracellular matrix. J. Biol. Chem. 2005;280:27970–27980. doi: 10.1074/jbc.M504270200. [DOI] [PubMed] [Google Scholar]
- 148.Wohl A.P., Troilo H., Collins R.F., Baldock C., Sengle G. Extracellular regulation of bone morphogenetic protein activity by the microfibril component fibrillin-1. J. Biol. Chem. 2016;291:12732–12746. doi: 10.1074/jbc.M115.704734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tamminen J.A., Parviainen V., Ronty M., Wohl A.P., Murray L., Joenvaara S., Varjosalo M., Lepparanta O., Ritvos O., Sengle G. Gremlin-1 associates with fibrillin microfibrils in vivo and regulates mesothelioma cell survival through transcription factor slug. Oncogenesis. 2013;2:e66. doi: 10.1038/oncsis.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]