Abstract
Background
Ventilator‐associated pneumonia (VAP) is a significant cause of morbidity and mortality, complicating the medical course of approximately 10% of mechanically‐ventilated patients, with an estimated attributable mortality of 13%. To treat VAP empirically, the American Thoracic Society currently recommends antibiotic therapy based on the patients' risk of colonisation by an organism with multidrug resistance. The selection of initial antibiotic therapy in VAP is important, as inappropriate initial antimicrobial treatment is associated with higher mortality and longer hospital stay in intensive care unit (ICU) patients.
While guidelines exist for the antibiotic treatment of hospital‐acquired pneumonia (HAP) from the American Thoracic Society and the British Society for Antimicrobial Chemotherapy, there are many limitations in the quality of available evidence. This systematic review aimed to summarise the results of all randomised controlled trials (RCTs) that compare empirical antibiotic regimens for VAP.
Objectives
The primary objective of this review was to assess the effect of different empirical antimicrobial therapies on the survival and clinical cure of adult patients with ventilator‐associated pneumonia (VAP). Secondary objectives included reporting the incidence of adverse events, new superinfections, length of hospital stay, and length of intensive care unit (ICU) stay associated with these therapies.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, LILACS, CINAHL and Web of Science to December 2015; we searched ClinicalTrials.gov to September 2016.
Selection criteria
Two review authors independently assessed RCTs comparing empirical antibiotic treatments of VAP in adult patients, where VAP was defined as new‐onset pneumonia that developed more than 48 hours after endotracheal intubation. Physicians and researchers were not required to be blinded for inclusion in this review.
Data collection and analysis
Two review authors independently extracted study data. We pooled studies and analysed them in two ways. We examined monotherapy, or a single experimental antimicrobial drug, versus combination therapy, or multiple experimental antimicrobial drugs. We also examined carbapenem therapy versus non‐carbapenem therapy.
Main results
We included 12 studies with 3571 participants. All included studies examined the empiric use of one antimicrobial regimen versus another for the treatment of adults with VAP, but the particular drug regimens examined by each study varied. There was potential for bias because some studies did not report outcomes for all participants. All but one study reported sources of funding or author affiliations with pharmaceutical companies.
We found no statistical difference in all‐cause mortality between monotherapy and combination therapy (N = 4; odds ratio (OR) monotherapy versus combination 0.97, 95% confidence interval (CI) 0.73 to 1.30), clinical cure (N = 2; OR monotherapy versus combination 0.88, 95% CI 0.56 to 1.36), length of stay in ICU (mean difference (MD) 0.65, 95% CI 0.07 to 1.23) or adverse events (N = 2; OR monotherapy versus combination 0.93, 95% CI 0.68 to 1.26). We downgraded the quality of evidence for all‐cause mortality, adverse events, and length of ICU stay to moderate for this comparison. We determined clinical cure for this comparison to be of very low‐quality evidence.
For our second comparison of combination therapy with optional adjunctives only one meta‐analysis could be performed due to a lack of trials comparing the same antibiotic regimens. Two studies compared tigecycline versus imipenem‐cilastatin for clinical cure in the clinically evaluable population and there was a statistically significant increase in clinical cure for imipenem‐cilastatin (N = 2; OR tigecycline versus imipenem‐cilastatin 0.44, 95% CI 0.23 to 0.84). Of importance, this effect was due to a single study.
We found no statistical difference in all‐cause mortality between carbapenem and non‐carbapenem therapies (N = 1; OR carbapenem versus non‐carbapenem 0.59, 95% CI 0.30 to 1.19) or adverse events (N = 3; OR carbapenem versus non‐carbapenem 0.78, 95% CI 0.56 to 1.09), but we found that carbapenems are associated with a statistically significant increase in the clinical cure (N = 3; OR carbapenem versus non‐carbapenem 1.53, 95% CI 1.11 to 2.12 for intention‐to‐treat (ITT) analysis and N = 2; OR carbapenem versus non‐carbapenem 2.29, 95% CI 1.19 to 4.43 for clinically evaluable patients analysis). For this comparison we downgraded the quality of evidence for mortality, and clinical cure (ITT and clinically evaluable populations) to moderate. We determined the quality of evidence for adverse events to be low.
Authors' conclusions
We did not find a difference between monotherapy and combination therapy for the treatment of people with VAP. Since studies did not identify patients with increased risk for multidrug‐resistant bacteria, these data may not be generalisable to all patient groups. However, this is the largest meta‐analysis comparing monotherapy to multiple antibiotic therapies for VAP and contributes further evidence to the safety of using effective monotherapy for the empiric treatment of VAP.
Due to lack of studies, we could not evaluate the best antibiotic choice for VAP, but carbapenems as a class may result in better clinical cure than other tested antibiotics.
Keywords: Adult; Humans; Anti‐Bacterial Agents; Anti‐Bacterial Agents/adverse effects; Anti‐Bacterial Agents/therapeutic use; Carbapenems; Carbapenems/therapeutic use; Cause of Death; Drug Therapy, Combination; Drug Therapy, Combination/mortality; Empirical Research; Pneumonia, Ventilator‐Associated; Pneumonia, Ventilator‐Associated/drug therapy; Pneumonia, Ventilator‐Associated/mortality; Randomized Controlled Trials as Topic
Plain language summary
Antibiotic treatment for ventilated patients with pneumonia
Background Ventilators are machines that breathe for patients. The ventilator tube goes into the mouth and through the windpipe. Sometimes there are bacteria on the ventilator tube that infect the patient's lungs, leading to a disease called ventilator‐associated pneumonia. Ventilator‐associated pneumonia can cause significant harmful effects, and can sometimes lead to death. When treating people with ventilator‐associated pneumonia, doctors must decide which antibiotic therapy to prescribe, usually without knowing the particular type of bacterial infection. This decision is important because inappropriate initial treatment may increase risk of harmful effects and longer hospital stays.
Search date We searched for studies to December 2015.
Study characteristics We looked at studies involving adults aged over 18 years who were treated in intensive care units for ventilator‐associated pneumonia and needed antibiotic treatment. We analysed 12 studies with 3571 participants.
Key results All included studies looked at the use of one antibiotic treatment plan versus another, but these varied among studies. There was potential for bias because some studies did not report outcomes for all participants, and funding for many was provided by pharmaceutical companies and study authors were affiliated with these companies.
We used statistical techniques to evaluate our results. For single versus multiple antibiotics, we found no difference in rates of death or cure, or adverse events. For our comparison of combination therapies with optional adjunctives we were only able to analyse clinical cure for one the antibiotics Tigecycline and imipenem‐cilastatin for which imipenem‐cilastatin was found to have higher clinica cure. We also looked at carbapenem (antibiotics used to treat infections caused by multidrug‐resistant bacteria) versus non‐carbapenem treatment; we found no difference in death rate or adverse effects, but we found that carbapenems are associated with an increase in clinical cure.
Quality of evidenceWe assessed evidence quality as moderate for most outcomes, and very low for clinical cure when single‐antibiotic treatment was compared with multiple antibiotic therapy. We also found that evidence quality was low for adverse events when carbapenem was compared with non‐carbapenem treatment.
Conclusions We did not find differences between single and combination therapy, lending support to use of a single‐antibiotic treatment plan for people with ventilator‐associated pneumonia. This may not be applicable to all patients because studies did not identify patients who are at risk of exposure to harmful types of bacteria.
We could not evaluate the best single‐antibiotic choice to treat people with ventilator‐associated pneumonia because there were too few studies, but carbapenems may achieve better cure rates than other tested antibiotics.
Summary of findings
Summary of findings for the main comparison. Monotherapy compared to combination therapy for ventilator‐associated pneumonia.
| Monotherapy compared to combination therapy for ventilator‐associated pneumonia | ||||||
| Patient or population: Ventilator‐associated pneumonia Setting: ICUs Intervention: Monotherapy Comparison: Combination therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with combination therapy | Risk with Monotherapy | |||||
| All‐cause mortality; follow‐up: range 28 days to 30 days | Study population | OR 0.97 (0.73 to 1.30) | 1163 (4 RCTs) | ⊕⊕⊕⊝ Moderate¹ | ||
| 201 per 1000 | 196 per 1000 (155 to 247) | |||||
| Moderate | ||||||
| 195 per 1000 | 191 per 1000 (151 to 240) | |||||
| Clinical cure (ITT) assessed with: clinical assessment and chest radiograph; follow‐up: range 7 days to 14 days | Study population | OR 0.88 (0.56 to 1.36) | 350 (2 RCTs) | ⊕⊝⊝⊝ Very low¹ ² | ITT analysis. OR of 0.88 means monotherapy is less likely to achieve clinical cure. Follow‐up 7 to 14 days after completing treatment. |
|
| 441 per 1000 | 409 per 1000 (306 to 517) | |||||
| Moderate | ||||||
| 459 per 1000 | 427 per 1000 (322 to 535) | |||||
| Clinical cure (CE) assessed with: clinical assessment and chest radiograph; follow‐up: range 7 days to 14 days | Study population | OR 0.97 (0.56 to 1.68) | 228 (2 RCTs) | ⊕⊝⊝⊝ Very low¹ ³ | CE patient analysis. OR of 0.97 means monotherapy is less likely to achieve clinical cure. Follow‐up 7 to 14 days after completing treatment. |
|
| 610 per 1000 | 603 per 1000 (467 to 724) | |||||
| Moderate | ||||||
| 610 per 1000 | 603 per 1000 (467 to 725) | |||||
| Adverse events assessed with: attributable adverse events or events leading to discontinuation | Study population | OR 0.93 (0.68 to 1.26) | 921 (2 RCTs) | ⊕⊕⊕⊝ Moderate¹ | ||
| 239 per 1000 | 226 per 1000 (176 to 283) | |||||
| Moderate | ||||||
| 210 per 1000 | 198 per 1000 (153 to 251) | |||||
| Length of ICU stay | MD 0.65 higher (0.07 higher to 1.23 higher) | ‐ | 813 (2 RCTs) | ⊕⊕⊕⊝ Moderate¹ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CE: clinically evaluable; CI: confidence interval; ICU: intensive care unit; ITT: intention‐to‐treat; MD: mean difference; OR: Odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High‐quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low‐quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
¹ Downgraded one level due to serious imprecision (wide confidence interval). ² Downgraded two levels due to very serious inconsistency (I² = 85%). ³ Downgraded two levels due to very serious inconsistency (I² = 87%).
Summary of findings 2. Carbapenems compared to non‐carbapenems for ventilator‐associated pneumonia.
| Carbapenems compared to non‐carbapenems for ventilator‐associated pneumonia | ||||||
| Patient or population: Ventilator‐associated pneumonia Setting: ICUs Intervention: Carbapenems Comparison: Non‐carbapenems | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with non‐carbapenem | Risk with Carbapenem | |||||
| All‐cause mortality; follow‐up: 28 days | Study population | OR 0.59 (0.30 to 1.19) | 253 (1 RCT) | ⊕⊕⊕⊝ Moderate¹ | Only one study contributed to this outcome for this comparison. | |
| 191 per 1000 | 122 per 1000 (66 to 219) | |||||
| Moderate | ||||||
| 191 per 1000 | 122 per 1000 (66 to 219) | |||||
| Clinical cure (ITT) assessed with: clinical response; follow‐up: range 10 days to 21 days | Study population | OR 1.53 (1.11 to 2.12) | 598 (3 RCTs) | ⊕⊕⊕⊝ Moderate² | ITT analysis. OR of 1.53 means carbapenems are more likely to achieve clinical cure. Follow‐up 10‐21 days after completing treatment. |
|
| 495 per 1000 | 600 per 1000 (521 to 675) | |||||
| Moderate | ||||||
| 466 per 1000 | 571 per 1000 (492 to 649) | |||||
| Clinical cure (CE) assessed with: clinical response; follow‐up: range 10 days to 21 days | Study population | OR 2.29 (1.19 to 4.43) | 163 (2 RCTs) | ⊕⊕⊕⊝ Moderate³ | CE patient analysis. OR of 2.29 means carbapenems are more likely to achieve clinical cure. Follow up 10‐21 days after completing treatment. |
|
| 529 per 1000 | 720 per 1000 (572 to 833) | |||||
| Moderate | ||||||
| 633 per 1000 | 798 per 1000 (672 to 884) | |||||
| Adverse events assessed with: attributable adverse events or events leading to discontinuation | Study population | OR 0.78 (0.56 to 1.09) | 1510 (3 RCTs) | ⊕⊕⊝⊝ Low¹ ² | ||
| 118 per 1000 | 94 per 1000 (69 to 127) | |||||
| Moderate | ||||||
| 109 per 1000 | 87 per 1000 (64 to 118) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CE: clinically evaluable; CI: confidence interval; ICU: intensive care unit; ITT: intention‐to‐treat; MD: mean difference; OR: Odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High‐quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low‐quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
¹ Downgraded one level due to serious imprecision (wide confidence interval). ² Downgraded one level due to serious risk of bias. Unclear or risk of bias for randomisation, concealment, and outcome assessment. High risk of bias for attrition in one study and selective reporting in another study. ³ Downgraded one level due to serious imprecision (wide confidence interval).
Background
Description of the condition
Despite advances in antimicrobial therapy, improved supportive care modalities, and the use of preventive measures, ventilator‐associated pneumonia (VAP) remains an important cause of morbidity and mortality, complicating the course of approximately 10% of patients receiving mechanical ventilation, with an estimated attributable mortality rate of 13% (Meslen 2013; West 2003).
VAP is defined as pneumonia that develops more than 48 hours after endotracheal intubation. The definition of VAP has remained unchanged in the 2005 and 2016 Clinical Practice Guidelines developed by the Infectious Diseases Society of America and the American Thoracic Society (ATS 2005; ATS 2016). VAP diagnosis is usually based on three components: clinical signs of infection (fever, leukocytosis (increased white blood cell counts), or purulent tracheobronchial secretions); new or worsening infiltrates seen on the chest X‐ray; and bacteriologic evidence of pulmonary parenchymal infection (Chastre 2002). Unfortunately, unlike community‐acquired pneumonia, the clinical and radiological signs are non‐specific in hospitalised ventilated patients. The systemic signs of infection (fever, tachycardia and leukocytosis) are non‐specific findings and can be caused by any condition that releases cytokines. Furthermore, the plain chest X‐ray is most helpful when it is normal and rules out pneumonia; when infiltrates are evident, the particular pattern is of limited value for differentiating among cardiogenic pulmonary oedema, non‐cardiogenic pulmonary oedema, pulmonary contusion, atelectasis (or collapse), and pneumonia.
The aetiologic diagnosis generally requires a lower respiratory tract culture (although it is occasionally made from blood or pleural fluid cultures). Respiratory tract cultures can include endotracheal aspirates, broncheo‐alveolar lavage, or protected specimen brush specimens (ATS 2005). It is preferable to use non‐invasive respiratory sampling with endotracheal aspirates and semiquantitative cultures (ATS 2016). Although an aetiologic diagnosis can be made from an upper respiratory tract culture, colonisation of the trachea precedes development of pneumonia in almost all people with VAP, so a positive culture cannot always distinguish a pathogen from a colonising organism (ATS 2005). Despite this, an attempt to establish a microbiological diagnosis is desirable in every patient with suspected VAP, because detection of causative organisms enables the initial empiric antibiotic regimen to be adjusted (Torres 2001).
Time of pneumonia onset is an important epidemiologic variable and risk factor for specific pathogens and outcomes in patients with VAP (ATS 2016). Early‐onset VAP, occurring during the first four days (96 hours) of mechanical ventilation, is likely to be caused by pathogens which originate in the oropharyngeal cavity Staphylococcus aureus (S aureus),Streptococcus pneumoniae (S pneumoniae), andHaemophilus influenzae (H influenzae). Late‐onset VAP, which develops five or more days after starting mechanical ventilation, is more likely to be caused by Gram‐negative bacilli, S aureus, including methicillin‐resistant, Pseudomonas aeruginosa (P aeruginosa), and Acinetobacter spp (ATS 2005). These organisms are more likely to be multi‐resistant to antimicrobials and therefore difficult to treat. However, the most recent American Thoracic Society guidelines state that the key decision in initial empiric therapy is whether the patient has risk factors for multidrug‐resistant organisms rather than time of onset of VAP. Risk factors for multidrug‐resistant pathogens include the following (ATS 2016).
-
Risk factors for multidrug‐resistant VAP:
prior intravenous antibiotic use within 90 days;
septic shock at time of VAP;
acute respiratory distress syndrome preceding VAP;
five or more days of hospitalisation prior to the occurrence of VAP; and
acute renal replacement therapy prior to VAP onset.
-
Risk factors for multidrug‐resistant hospital‐acquired pneumonia (HAP):
prior intravenous antibiotic use within 90 days.
-
Risk factors for methicillin‐resistant S aureus (MRSA) VAP/HAP:
prior intravenous antibiotic use within 90 days.
-
Risk factors for multidrug‐resistant P aeruginosa VAP/HAP:
prior intravenous antibiotic use within 90 days.
Description of the intervention
Empiric therapy is defined as the use of antibiotics before a bacteriologic diagnosis of infection is proven (Kim 1989). This differs from directed therapy that is targeted at a specific known pathogen, and prophylactic therapy given to prevent the development of infection (Kim 1989). According to the American Thoracic Society guidelines, empirical antibiotic selection for each patient should be based on the risk for multidrug‐resistant pathogens (ATS 2016).
Inappropriate initial antimicrobial treatment is associated with higher mortality and longer hospital stays in intensive care unit (ICU) patients (Rello 2007). Delays in the administration of appropriate therapy are associated with increased hospital mortality from HAP, and furthermore, changing antimicrobial therapy once culture results are available may not reduce the increased risk of hospital mortality associated with inappropriate initial antibiotic therapy. Therefore, selection of initial appropriate therapy (that is, getting the antibiotic treatment right the first time) is an important aspect of care for hospitalised patients with serious infections (ATS 2016).
The pathogens commonly associated with inappropriate initial empiric antimicrobial therapy include P aeruginosa, Acinetobacter spp, Klebsiella pneumoniae (K pneumoniae), Enterobacter species, and MRSA (ATS 2005). Patients at risk of infection with these pathogens should initially receive a combination of agents that can provide a broad‐spectrum of coverage to minimise the potential for inappropriate antibiotic treatment (ATS 2005). The choice of agents should be based on local patterns of antimicrobial susceptibility, and anticipated side effects, and should also take into account which therapies patients have recently received (within the past two weeks), striving not to repeat the same antimicrobial class, if possible. The initial antimicrobial therapy regimen needs to take into account local bacteriologic patterns, and each hospital and ICU should ideally have their own antibiogram (recording the antimicrobial susceptibility of the locally identified micro‐organisms), which is updated as often as possible.
Finally, broad‐spectrum, empiric antibiotic therapy should be accompanied by a commitment to de‐escalate antibiotics, on the basis of serial clinical and microbiological data, to limit the emergence of resistance in the hospital (ATS 2016).
How the intervention might work
Because appropriate antimicrobial treatment of patients with VAP significantly improves outcomes, more rapid identification of infection and accurate selection of antimicrobial agents are important clinical goals (Chastre 2002). Conversely, studies using multivariate analysis have clearly demonstrated that delayed and/or inappropriate initial antibiotic therapy is strongly associated with fatality in critically ill patients with infections, including VAP (Fagon 2006).
Why it is important to do this review
A previous Cochrane Review found that a combination of topical and systemic antibiotics reduces the occurrence of respiratory tract infections and overall mortality in patients in ICUs (Liberati 2009). A systematic review of the evidence for the treatment of HAP in ICU patients has not been published. Guidelines are available for the antibiotic treatment of HAP from the American Thoracic Society/Infectious Diseases Society of America (ATS 2016), and the British Society for Antimicrobial Chemotherapy (Masterton 2008). However, there are many limitations in the quality of the available evidence to assist in selecting the most appropriate antimicrobial regimen for people with VAP. Few studies have compared more than two therapeutic options and very few studies had sufficient power to demonstrate the superiority of one regimen over another (Masterton 2008). We aimed to summarise the results of all RCTs that compare empirical antibiotic regimens for people with VAP.
Objectives
The primary objective of this review was to assess the effect of different empirical antimicrobial therapies on the survival and clinical cure of adult patients with ventilator‐associated pneumonia (VAP). Secondary objectives included reporting the incidence of adverse events, new superinfections, length of hospital stay, and length of intensive care unit (ICU) stay associated with these therapies.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing antibiotic treatment regimens for patients with ventilator‐associated pneumonia (VAP). We excluded studies that examined the treatment of specific pathogens, because the primary focus of this review was the empiric treatment of VAP.
Types of participants
We included data from adult (aged ≥ 18 years) ICU patients with suspected VAP who developed new or progressive infiltrates after 48 hours or more of mechanical ventilation. Patients must have had two of the following signs and symptoms of a pneumonia infection: fever or hypothermia; leukocytosis or leukopenia; and/or purulent respiratory secretions. We excluded studies of patients who had not undergone mechanical ventilation for more than 48 hours before enrolment.
Types of interventions
We included studies comparing one antibiotic regimen with a placebo or another antibiotic regimen. We also included trials evaluating monotherapy versus combination therapy. We classified antibiotic groups as follows.
Penicillins
Cephalosporins
Carbapenems
Aminoglycosides
Quinolones
Clindamycin
Vancomycin
Linezoli
Quinupristin/dalfopristin
Aztreonam
Tigecycline.
We included trials in which patients were receiving other concurrent medications, such as antipyretics, bronchodilators, or mucolytics, if patients in both arms of the trial had equal access to such medications.
Types of outcome measures
Primary outcomes
All‐cause mortality (28‐day).
Clinical cure. Resolution of pneumonia may be assessed subjectively by the clinician but must also be accompanied by a more objective indicator of improvement, for example, disappearance of infiltrates, repeat negative cultures, or decrease in the white blood cell count.
Secondary outcomes
Attributable adverse events and/or any events requiring discontinuation of the trial antibiotic. We included adverse events from data on hospital‐acquired pneumonia (HAP) and ventilator‐associated pneumonia (VAP) combined groups in studies that do not have data specific for the VAP group.
Superinfections. Any new, persistent or worsening signs or symptoms of infection associated with the isolation of a new pathogen (or similar pathogen with a new site of infection or different antibiotic susceptibility profile).
Length of hospital stay.
Length of ICU stay.
Search methods for identification of studies
Electronic searches
We searched:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 11) in the Cochrane Library (searched 7 December 2015); which contains the Cochrane Acute Respiratory Infections Group's Specialised Register;
MEDLINE (1946 to December week 1, 2015);
Embase (2010 to December 2015);
LILACS (1982 to December 2015);
CINAHL (1981 to December 2015); and
Web of Science (1955 to December 2015).
We used the search strategy described in Appendix 1 to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy for Embase (Appendix 2), LILACS (Appendix 3), CINAHL, (Appendix 4) and Web of Science (Appendix 5).
Searching other resources
We consulted ClinicalTrials.gov (clinicaltrials.gov; 10 September 2016), using the search terms 'ventilator associated pneumonia' and 'antibiotics'. We also searched references and eligible trials from the reference lists of identified trials. We planned to contact experts in the field and pharmaceutical companies for additional published or unpublished trials. We also planned to contact corresponding authors of included trials to identify other published and unpublished studies. We did not apply any language or publication restrictions.
Data collection and analysis
Selection of studies
Two review authors (LA, RK) independently assessed the results of the electronic searches in order to identify eligible articles for inclusion. If one of the review authors felt that the trial might possibly fulfil the criteria, we obtained the full paper for further study. Two review authors (MVD, LS) reviewed the list of included studies to ensure all relevant studies were included. The selection process is reported in the PRISMA flowchart (Figure 1).
1.
Study flow diagram.
Data extraction and management
Two review authors (LA, RK) independently assessed the quality of the included studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The same two review authors independently extracted data using preset data entry forms. We resolved discrepancies by consensus or with a third review author (MVD). The review authors were not blinded to the studies. We sought clarification from the trial author if published data provided inadequate information for the review.
We extracted the following information from each trial.
Methods: randomisation procedure, allocation, blinding (people administering treatment, outcome assessors), duration of study, design, analysis (intention‐to‐treat (ITT)).
Participants: number, age, diagnostic criteria, in‐ and exclusion criteria, baseline characteristics.
Interventions (description of intervention and control therapies): antibiotic, dose, route, timing, duration; comparison group.
Outcomes: outcomes as specified above (all‐cause mortality, clinical resolution, superinfections or persistence of infection, microbiological confirmation of infection, serious adverse events, and length of hospital stay), any other outcomes assessed, other events, length of follow‐up.
Results: for outcomes and times of assessment. We recorded outcomes from both the intention‐to‐treat (ITT) and the efficacy (on treatment) analysis.
Other: source of funding, aim of the study and conflicts of interest.
Assessment of risk of bias in included studies
We assessed risk of bias related to randomisation, concealment of allocation, blinding (if relevant), and follow‐up of participants using the Cochrane Risk of Bias tool (Higgins 2011).
Selection bias: we assessed random sequence generation and allocation concealment. If a study was reported as 'randomised', but the method of randomisation or concealment was not reported, then we assigned the study as 'unclear risk'.
Blinding of participants and personnel (performance bias): participants were on mechanical ventilation and hence under deep sedation and unaware of their assigned treatment group. Therefore only blinding of personnel was assessed for this criterion. If the study was reported as blinded but no details of personnel blinding were provided, we assigned the study as 'unclear risk'.
Blinding of outcome assessment (detection bias): we assigned studies with blinded evaluation committees that reviewed the clinical and microbiological data relevant to the outcome as 'low risk'. We assigned studies in which all outcomes are objective and the outcome assessor was blinded as 'low risk'. We assigned studies that did not report blinding of outcome assessment as 'unclear risk'. We assigned studies that assessed outcomes in an open way without independent and blinded review committees as 'high risk'.
Incomplete outcome data (attrition bias): if no participants were lost or the reasons for exclusion and number or participants lost are the same for both arms, we assigned the study as 'low risk'. If reasons for exclusions were not reported, we assigned the study as 'high risk'.
Selective reporting (reporting bias): if studies did not report all outcomes stated in their methods section, we assigned them as 'high risk'.
Measures of treatment effect
We calculated the proportions of dichotomous outcome variables (such as the primary outcome mortality) with 95% confidence interval (CI). We used the weighted means and the standard deviation (SD) of the means for continuous variables. In case medians and percentile points of the effect estimate were reported, we used the formula developed by Hozo 2005 to calculate the medians and SDs.
Unit of analysis issues
Individual study participants were the unit of analysis. We did not include any cluster RCTs.
Dealing with missing data
We followed recommendations from the Cochrane Handbook for Systematic Reviews of Interventions regarding strategies for dealing with missing data (Higgins 2011).
Assessment of heterogeneity
We assessed heterogeneity among studies in two ways. First, we assessed heterogeneity at face value: heterogeneity of population, interventions, or outcomes. Second, we employed a Chi² test (P < 0.1 was considered to be consistent with statistical heterogeneity) and the I² statistic to assess presence of statistical heterogeneity. We interpreted I² per the guide given in the Cochrane Handbook for Systematic Reviews of Interventions where 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% is considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
We planned to create funnel plots if we found sufficient numbers (more than 10) of studies for inclusion.
Data synthesis
We included results from studies that met the inclusion criteria and reported any of the selected outcomes in the meta‐analysis. We calculated the summary weighted odds ratio (OR) and 95% CI for dichotomous secondary outcomes using the inverse variance method for weighting each study (RevMan 2014). We calculated the number needed to benefit (NNTB) using the summary OR and the average control event rate described in the relevant studies. We used a fixed‐effect model for pooling data. The difference in effect estimates is presented in the Discussion, where relevant.
Grade and 'Summary of findings' tables
We created two 'Summary of findings' tables for the two comparisons of monotherapy versus combination therapy (Table 1), and carbapenem versus non‐carbapenem (Table 2), with the prespecified primary outcomes of all‐cause mortality and clinical cure (ITT and clinically evaluable) and the secondary outcome, adverse events (plus length of ICU stay for the second comparison only). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to down‐ or upgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
We did not create a 'Summary of findings' table for results comparing combination therapy with optional adjunctives. In this comparison both arms received a combination therapy with mostly different antibiotics. The adjunctives in each of the studies are specifically aimed at other pathogens that can be dependent on local prevalences and resistance patterns. The comparison would then encompass combination therapy versus combination therapy which would not make much clinical sense.
Subgroup analysis and investigation of heterogeneity
We did not identify any studies that differentiated late versus early VAP, therefore we did not perform any subgroup analysis.
Sensitivity analysis
We performed a sensitivity analysis of the impact of high risk of bias on the outcome of the meta‐analysis. We included open‐label studies and performed sensitivity analyses.
Results
Description of studies
Results of the search
We identified a total of 2121 records through electronic searches of MEDLINE, Embase, LILACS, CINAHL, and Web of Science on 7 December 2015. We excluded 2098 records, either because they were duplicates or they did not meet predefined inclusion criteria. We retrieved 23 full‐text papers for assessment. Of these, eight failed to meet our inclusion criteria and were excluded. Two of these studies did not evaluate the correct intervention (Amonova 2011; Giamerellos‐Bourboulis 2008). Amonova 2011 compared two different doses of a single‐antibiotic and not different antibiotic regimens. Giamerellos‐Bourboulis 2008 studied the non‐antibiotic effects of clarithromycin. We excluded four studies for evaluating the wrong population (Barriere 2014; Chastre 2008; Iakovlev 2006; Polk Jr 1997). The study population for Barriere 2014 was only patients with Gram‐positive VAP, Chastre 2008 defined VAP as > 24 hours, Iakovlev 2006 studied nosocomial infections and did not have VAP‐specific data, and Polk Jr 1997 studied pneumonia in mechanically‐ventilated trauma patients, not VAP. We excluded two studies for not being RCTs (Bassetti 2007; Klapdor 2014). Details for exclusion can be seen in Figure 1 and Characteristics of excluded studies. We identified one ongoing study from the search of ClinicalTrials.gov (NCT01808092). We identified 12 studies (15 reports) that met our inclusion criteria. Of these, we meta‐analysed four studies (Alvarez Lerma 2001; Awad 2014; Damas 2006; Heyland 2008) for monotherapy versus combination therapy and another four studies (Freire 2010; Ramirez 2013; Rea‐Neto 2008; Shorr 2005) for carbapenem versus non‐carbapenem. Two of the studies (Freire 2010; Ramirez 2013) in the carbapenem versus non‐carbapenem comparison were also meta‐analysed in the combination therapy with optional adjunctives comparison. The remaining four included studies were part of the combination therapy with optional adjunctives comparison and are not meta‐analysed but are described narratively.
Included studies
See Characteristics of included studies table.
Populations
The 12 included studies (15 reports) enrolled 3571 participants with VAP. Of these participants, 3405 were randomised and reported. The included studies were published between 1998 and 2014, and included between 23 and 1144 participants. Four studies investigated the broader topic of HAP, not specifically VAP; however, we performed a subgroup analysis on participants with VAP (Awad 2014; Freire 2010; Ramirez 2013; Rea‐Neto 2008). Two studies were secondary analyses of VAP subgroups of larger RCTs examining the broader topic of HAP (Kollef 2004; Shorr 2005). Three studies were the original papers of studies included in our review. Rubinstein 2001 and Wunderink 2003 describe the results of the full cohort of Kollef 2004, which reports on the VAP subgroup of the study. West 2003 is the original report of the VAP subgroup analysis included as Shorr 2005.
Interventions
Most included studies (10/12) compared two different antibiotic regimens; two studies compared three regimens (Damas 2006; Ramirez 2013). Some drug classes were commonly investigated in at least one of the tested antibiotic regimens: seven studies evaluated the carbapenems (Alvarez Lerma 2001; Freire 2010; Heyland 2008; Kollef 2012; Ramirez 2013; Rea‐Neto 2008; Shorr 2005); five studies evaluated a cephalosporin (Alvarez Lerma 2001; Awad 2014; Beaucaire 1999; Brun‐Buisson 1998; Damas 2006); three studies evaluated quinolones (Damas 2006; Heyland 2008; Shorr 2005); and three studies evaluated the aminoglycosides as an adjunct therapy (Alvarez Lerma 2001; Beaucaire 1999; Damas 2006). One study compared low‐dose with high‐dose tigecycline (Ramirez 2013).
Most included studies (8/12) evaluated the effects of different single‐antibiotic regimens with adjunctive antibiotics to cover multidrug‐resistant pathogens available to participants in both study arms (Beaucaire 1999; Brun‐Buisson 1998; Freire 2010; Kollef 2004; Kollef 2012; Ramirez 2013; Rea‐Neto 2008; Shorr 2005).
Four included studies compared a single‐antibiotic regimen with a multiple‐antibiotic regimen (Alvarez Lerma 2001; Awad 2014; Damas 2006; Heyland 2008). Three studies evaluated a combination of a cephalosporin with another drug, and one compared a carbapenem with another drug (Heyland 2008). One study evaluated three separate cephalosporin regimens: cephalosporin only, cephalosporin with an aminoglycoside, and cephalosporin with a quinolone (Damas 2006).
Funding source
Most included studies (9/12) were funded at least in part by pharmaceutical companies (Alvarez Lerma 2001; Awad 2014; Brun‐Buisson 1998; Freire 2010; Heyland 2008; Kollef 2004; Kollef 2012; Ramirez 2013; Rea‐Neto 2008). Three studies did not report funding sources (Beaucaire 1999; Damas 2006; Shorr 2005).
Outcomes
Nine included studies reported all‐cause mortality (Alvarez Lerma 2001; Awad 2014; Beaucaire 1999; Brun‐Buisson 1998; Damas 2006; Freire 2010; Heyland 2008; Kollef 2004; Kollef 2012). Clinical cure was reported in all but three of the 12 included studies (Damas 2006; Heyland 2008; Kollef 2012). Ramirez 2013 reported clinical cure only for the clinically evaluable patients and not the ITT group. Seven studies reported adverse events that were attributable to the treatment and/or required discontinuation of the experimental medication (Alvarez Lerma 2001; Awad 2014; Beaucaire 1999; Brun‐Buisson 1998; Freire 2010; Rea‐Neto 2008; Shorr 2005). Two reported superinfections (Alvarez Lerma 2001; Rea‐Neto 2008). One reported length of hospital stay (Heyland 2008), and three reported length of ICU stay (Damas 2006; Freire 2010; Heyland 2008).
Author contact
We attempted to contact authors to clarify if patients were ventilated for longer than 48 hours as part of the definition of VAP (Alvarez Lerma 2001; Kollef 2004; Rea‐Neto 2008). Kollef 2004 confirmed 48‐hour cut‐off, but we did not receive responses from Alvarez Lerma 2001 or Rea‐Neto 2008.
Excluded studies
See Characteristics of excluded studies.
We excluded eight studies following full‐text assessment. We excluded Amonova 2011 because it compared different dosing of a single‐antibiotic; we excluded two studies because they tested the antibiotic treatment effect against a specific, known microbial organism (our aim was to investigate empiric treatment for VAP) (Barriere 2014; Bassetti 2007); we excluded Giamerellos‐Bourboulis 2008 because it investigated the effects of clarithromycin versus a placebo for non‐antibiotic effects. We excluded Chastre 2008 because investigators defined VAP as greater than 24 hours of ventilation. We excluded Polk Jr 1997 because the study report did not clearly distinguish VAP participants from people with HAP. Iakovlev 2006 studied hospital‐acquired infections and we excluded it because it did not report specifically on patients with VAP. We excluded Klapdor 2014 because it was not a RCT.
Risk of bias in included studies
Figure 2; Figure 3; Characteristics of included studies.
2.
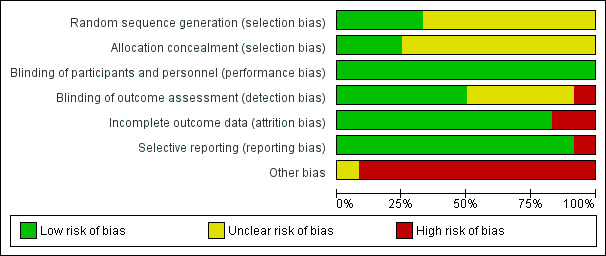
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
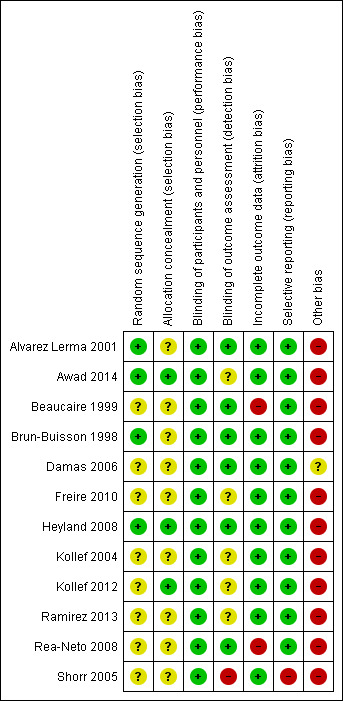
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was explained in four studies and we considered them to be at low risk of bias (Alvarez Lerma 2001; Awad 2014; Brun‐Buisson 1998; Heyland 2008). The other studies did not explicitly detail how the randomisation process occurred and we therefore considered them to be at unclear risk of bias.
Allocation concealment was discussed in three studies that we assessed to be at low risk of bias (Awad 2014; Heyland 2008; Kollef 2012). The remainder did not explicitly discuss concealment and we considered them to be unclear risk of bias.
Blinding
We considered the risk associated with unblinded patients to be low in all studies because eligible participants were ventilated and sedated.
Six studies clearly described the blinding process for outcome assessors and we considered them to be at low risk of bias (Alvarez Lerma 2001; Beaucaire 1999; Brun‐Buisson 1998; Damas 2006; Heyland 2008; Rea‐Neto 2008). One study discussed why assessors were unblinded and we considered this study to be at high risk of bias (Shorr 2005). All other studies did not clearly describe the outcome assessors blinding process and we considered them to be at unclear risk of bias.
Incomplete outcome data
Two studies had very high attrition rates and we therefore considered them to be at high risk of attrition bias (Beaucaire 1999; Rea‐Neto 2008).
Selective reporting
One study did not report data used to achieve their conclusion that 28‐day mortality was similar among test groups, and we therefore considered this study to be at high risk of reporting bias (Shorr 2005). All other studies reported outcomes with supporting data and we considered them to be at low risk of bias.
Other potential sources of bias
All included studies (except Damas 2006) were funded by pharmaceutical companies and/or included authors affiliated with pharmaceutical companies. In seven included studies, at least one author was an employee of the pharmaceutical company funding the study during the time the study was conducted (Awad 2014; Beaucaire 1999; Brun‐Buisson 1998; Freire 2010; Ramirez 2013; Rea‐Neto 2008; Shorr 2005). We assessed these studies at high risk of bias for this domain. In three studies, the authors acknowledged they received some form of financial compensation from the pharmaceutical company funding the study, but the authors did not report a specific conflict of interest (Alvarez Lerma 2001; Kollef 2004; Kollef 2012). We also assessed these studies at high risk of bias for this domain. Heyland 2008 reported independent funding sources and an unrestricted grant from a pharmaceutical company, but several authors declared ties with relevant companies; we also assessed this study at high risk of bias for this domain. We assessed Damas 2006 at unclear risk of bias for this domain because it did not report a funding source, but reported no authors' conflicts of interest.
Effects of interventions
Comparison 1: Monotherapy versus combination therapy
Four studies compared a single‐antibiotic regimen with a multiple‐antibiotic regimen and enrolled a total of 1163 participants (Alvarez Lerma 2001; Awad 2014; Damas 2006; Heyland 2008). Awad 2014 did not have a true monotherapy arm since they allowed open‐label use of adjunctive fluoroquinolones or aminoglycosides in both study arms for patients at high risk of pseudomonal infection. For meta‐analysis we include this study in the monotherapy versus combination therapy group because it was set up to compare a monotherapy and combination therapy with additional adjunctives available to participants in both arms.
Primary outcomes
1.1 All‐cause mortality (28‐day)
All‐cause mortality was reported as ITT analysis in all four studies, involving 1163 participants. In Alvarez Lerma 2001 meropenem was compared to the combination of ceftazidime and amikacin, the mortality rate in this study was 5% lower in the monotherapy group (all‐cause mortality: 23.2% for monotherapy and 28.2% for combination therapy). Awad 2014 compared treatment with ceftobiprole to combination treatment with ceftazidime plus linezolid and additional open‐label treatment with fluoroquinolone or aminoglycoside available to participants in both arms. This study showed an increase of 7.1% in mortality rate for monotherapy (all‐cause mortality: 26.9% for monotherapy and 19.8% for combination therapy). Treatment with cefepime alone was compared to the combination of cefepime and either amikacin or levofloxacin in Damas 2006. This study showed a decrease in the mortality rate by 7.7% for monotherapy (all‐cause mortality: 8.3% for monotherapy and 16% for combination therapy). Heyland 2008 compared meropenem alone to meropenem plus ciprofloxacin and showed a 1.1% decrease in mortality rate for the monotherapy group (all‐cause mortality: 18.1% for monotherapy and 19.2% for combination therapy) (Analysis 1.1).
1.1. Analysis.
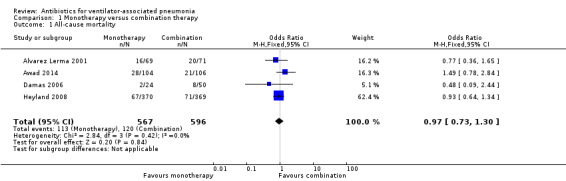
Comparison 1 Monotherapy versus combination therapy, Outcome 1 All‐cause mortality.
Pooled studies
Meta‐analysis of dichotomous outcomes using a fixed‐effect model showed no statistical difference at 95% confidence interval (CI) between single‐antibiotic regimen and multiple‐antibiotic regimen for all‐cause mortality (odds ratio (OR) for monotherapy versus combination therapy 0.97, 95% CI 0.73 to 1.30; 1163 participants; I² statistic = 0%). Using GRADE assessment, we downgraded this outcome one level from high to moderate‐quality for serious imprecision due to a wide confidence interval (Table 1).
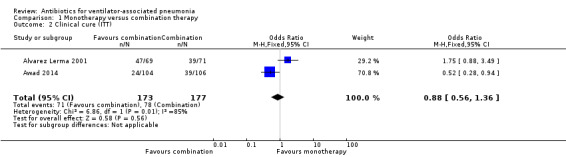
1.2 Clinical cure (ITT) and 1.3 Clinical cure (clinically evaluable patients)
Clinical cure was reported as both an ITT analysis and a clinically evaluable patients analysis. ITT analysis was used in two studies for a total of 350 participants (Alvarez Lerma 2001; Awad 2014). Alvarez Lerma 2001 showed a 13.2% higher clinical cure for monotherapy (clinical cure: 68.1% for monotherapy and 54.9% for combination therapy). Awad 2014 showed a 13.7% lower clinical cure for monotherapy (clinical cure: 23.1% for monotherapy and 36.8% for combination therapy) (Analysis 1.2).
1.2. Analysis.
Comparison 1 Monotherapy versus combination therapy, Outcome 2 Clinical cure (ITT).
Pooled studies
Meta‐analysis of dichotomous outcomes using a fixed‐effect model showed no statistical difference at 95% CI between single‐antibiotic regimen or multiple‐antibiotic regimen for clinical cure in the ITT population (OR for monotherapy versus combination therapy 0.88, 95% CI 0.56 to 1.36; participants = 350) showing monotherapy is less likely to produce clinical cure, although not statistically significant. There was substantial/considerable heterogeneity (I² statistic = 85%). Using GRADE assessment, we downgraded this outcome one level for serious imprecision due to a wide confidence interval and another two levels for very serious inconsistency due to heterogeneity, giving an overall rating of very low‐quality (Table 1).
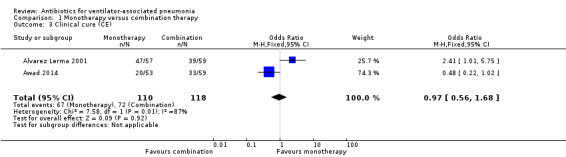
Alvarez Lerma 2001 and Awad 2014 also reported clinical cure as a clinically evaluable analysis for a total of 228 participants. In Alvarez Lerma 2001 the clinically evaluable population was defined as patients who could be assessed on the basis of clinical cure, excluding those for whom the protocol was not followed, where the patient died less than 72 hours after initiation of therapy, or where the pathogens were resistant to any of the antibiotics studied or were non‐bacterial pathogens. The clinical cure in this clinically evaluable group was 16.4% higher in the monotherapy group (clinical cure: 82.5% for monotherapy and 66.1% for combination therapy). The clinically evaluable population was defined as those patients who received at least one dose of study medication and were clinically evaluable at the test‐of‐cure visit for Awad 2014. The clinical cure for the clinically evaluable group was 18.2% lower in the monotherapy group compared to the combination therapy group (clinical cure: 37.7% for monotherapy and 55.9% for combination therapy) (Analysis 1.3).
1.3. Analysis.
Comparison 1 Monotherapy versus combination therapy, Outcome 3 Clinical cure (CE).
Meta‐analysis of dichotomous outcomes using a fixed‐effect model showed no statistical difference at 95% CI between single‐antibiotic regimen or multiple‐antibiotic regimen for clinical cure in the clinically evaluable population (OR for monotherapy versus combination therapy 0.97, 95% CI 0.56 to 1.68; participants = 228) showing monotherapy is less likely to produce clinical cure, although not statistically significant. There was substantial/considerable heterogeneity (I² statistic = 87%). Using GRADE assessment, we downgraded this outcome one level for serious imprecision due to a wide confidence interval and another two levels for very serious inconsistency, giving an overall rating of very low‐quality (Table 1).
Secondary outcomes
1.4 Adverse events
Two studies reported adverse events that were attributable to the treatment and/or required discontinuation of the study medication as an ITT analysis for a total of 921 participants (Alvarez Lerma 2001; Awad 2014). Alvarez Lerma 2001 reported adverse events that were possibly or probably related to the study medication as 5.3% lower in the monotherapy group (treatment‐related adverse events: 11.6% for monotherapy and 16.9% for combination therapy). Adverse events that required discontinuation of the trial antibiotic were also reported and were shown to be 1.4% lower in the monotherapy group (adverse events requiring discontinuation: 4.3% for monotherapy and 5.6% for combination therapy). Awad 2014 is a study of hospital‐acquired pneumonia (HAP) with subgroup analysis of ventilator‐associated pneumonia (VAP) patients. The adverse event data used from this study is for the entire population of HAP patients since subgroup data were not provided for this outcome. Treatment‐related adverse events were 0.5% lower in the monotherapy group (treatment‐related adverse events: 24.9% for monotherapy and 25.4% for combination therapy). Treatment‐related serious adverse events were also reported and were 0.8% higher in the monotherapy group (treatment‐related serious adverse events: 3.9% for monotherapy and 3.1% for combination therapy). The rates of adverse events defined as treatment‐related in Awad 2014 and probably or possible treatment‐related in Alvarez Lerma 2001 were used for the meta‐analysis.
Pooled studies
Meta‐analysis of dichotomous outcomes using a fixed‐effect model showed no statistical difference at 95% CI between single‐antibiotic regimen or multiple‐antibiotic regimen for treatment‐related adverse events (OR for monotherapy versus combination therapy 0.93, 95% CI 0.68 to 1.26; participants = 921; I² statistic = 0%). Using GRADE assessment, we downgraded this outcome one level from high to moderate‐quality for serious imprecision due to a wide confidence interval (Table 1).
1.5 Superinfections
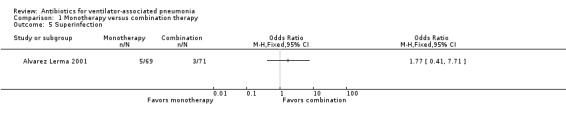
Alvarez Lerma 2001 reported rates of superinfection. Superinfection was defined as appearance of a new pathogen, different from the original causative agent during the period of treatment or during the immediate post‐treatment period, accompanied by clinical manifestations of sepsis, septic syndrome or septic shock. Rates of superinfection were performed as an ITT analysis for a population of 140 participants. There was a 3% higher rate in monotherapy (superinfection: 7.2% in monotherapy and 4.2% in combination therapy) (Analysis 1.5).
1.5. Analysis.
Comparison 1 Monotherapy versus combination therapy, Outcome 5 Superinfection.
We could not perform meta‐analysis as only one study reported rates of superinfection. The results of Alvarez Lerma 2001 do not show statistical significance at 95% CI between single‐antibiotic regimen or multiple‐antibiotic regimen for rates of superinfection (OR monotherapy versus combination therapy 1.77, 95% CI 0.41 to 7.71; participants = 140)
1.6 Length of hospital stay
Heyland 2008 reported no difference between combination and monotherapy groups in the median (IQR) time from randomisation to discharge from the hospital alive: 45.8 days (24.0 and 316.8) versus 39.1 days (19.7 and undefined), P = 0.49.
1.7 Length of ICU stay
Pooled studies
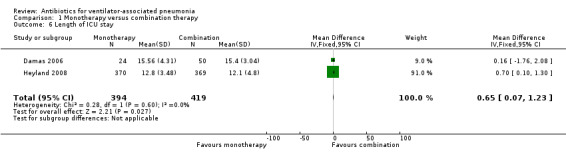
Length of ICU stay was reported in two studies (Damas 2006; Heyland 2008). Damas 2006 reported length of stay in the ICU as medians with 25th to 75th percentile ranges: the median length of stay was 15 days (7.5 and 24.75) in the cefepime group, 16 days (9 and 21) in the cefepime‐amikacin group and 14 days (9.5 and 21.5) in the cefepime‐levofloxacin group. Heyland 2008 reported no difference between the combination and monotherapy groups in the median (IQR) time from randomisation to discharge from the ICU alive: median length of stay in the ICU 12.1 days (6.4 and 35.2) in the combination group versus 12.8 days (6.1 and 27.0) in the monotherapy group (P = 0.79).
Meta‐analysis of data from two studies (Damas 2006; Heyland 2008), for the length of stay in the ICU showed no difference between those receiving monotherapy and those receiving a combination therapy (MD 0.65 days, 95% CI 0.07 to 1.23, studies = 2, participants = 813; I² statistic = 0%) (Analysis 1.6). Using GRADE assessment, we downgraded this outcome one level from high to moderate‐quality for serious imprecision due to a wide confidence interval (Table 1).
1.6. Analysis.
Comparison 1 Monotherapy versus combination therapy, Outcome 6 Length of ICU stay.
Comparison 2: Combination therapy with optional adjunctives
Eight studies compared two different antibiotic therapies with optional adjunctives to cover for methicillin‐resistant S aureus (MRSA) and P aeruginosa available to both treatment arms for treatment of VAP (Beaucaire 1999; Brun‐Buisson 1998; Freire 2010; Kollef 2004; Kollef 2012; Ramirez 2013; Rea‐Neto 2008; Shorr 2005). After constructing a matrix comparing the different antibiotic regimens used, we found there were only two studies that looked at the same antibiotic regimens; both compared tigecycline to imipenem‐cilastatin (Freire 2010; Ramirez 2013). We constructed another matrix comparing antibiotic regimens based on class of antibiotics and found no additional overlapping studies (Table 3). Studies that could not be pooled are described separately.
1. Matrix of interventions based on antibiotic class for studies in comparison 2.
| Cephalosporins | Carbapenems | Quinolones | Linezolid | Tigecycline | |
| Penicillins | Brun‐Buisson 1998 | Rea‐Neto 2008 | |||
| Cephalosporins | Beaucaire 1999 | ||||
| Carbapenems | Kollef 2012 | Shorr 2005 |
Freire 2010 Ramirez 2013 |
||
| Vancomycin | Kollef 2004 |
Primary outcomes
2.1 All‐cause mortality (28‐day)
Five studies reported all‐cause mortality (Beaucaire 1999; Brun‐Buisson 1998; Freire 2010; Kollef 2004; Kollef 2012) (Analysis 2.1).
2.1. Analysis.
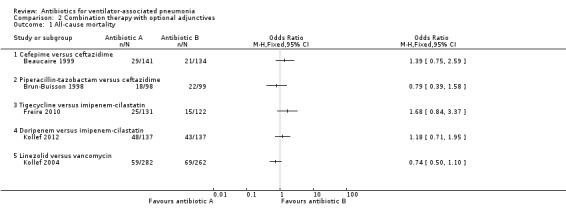
Comparison 2 Combination therapy with optional adjunctives, Outcome 1 All‐cause mortality.
2.1.1 Cefepime versus ceftazidime with amikacin available to both arms
Beaucaire 1999 compared the empirical antibiotic treatment of cefepime to ceftazidime as an ITT analysis for a total of 275 participants. The all‐cause mortality rate was 4.9% higher in the cefepime group (all‐cause mortality: 20.6% for cefepime and 15.7% for ceftazidime). This difference in mortality rate did not reach statistical significance at a 95% CI (OR cefepime versus ceftazidime 1.39, 95% CI 0.75 to 2.59). We could not perform meta‐analysis because there was only one study presenting this antibiotic comparison.
2.1.2 Piperacillin‐tazobactam versus ceftazidime with amikacin available to both arms
Brun‐Buisson 1998 compared the empirical antibiotic treatment of piperacillin‐tazobactam to ceftazidime as an ITT analysis for a total of 197 participants. The all‐cause mortality rate was 3.8% lower in the piperacillin‐tazobactam group (all‐cause mortality: 18.4% for piperacillin‐tazobactam and 22.2% for ceftazidime). This difference in mortality rate did not reach statistical significance at a 95% CI (OR piperacillin‐tazobactam versus ceftazidime 0.79, 95% CI 0.39 to 1.58). We could not perform meta‐analysis because there was only one study presenting this antibiotic comparison.
2.1.3 Tigecycline with optional ceftazidime versus imipenem‐cilastatin with optional vancomycin
Freire 2010 compared the empirical antibiotic treatment of tigecycline to imipenem‐cilastatin as a modified‐ITT (m‐ITT) analysis for a total of 253 participants. The m‐ITT group is defined as a randomised (ITT) patient who received any study drug. This study was on HAP with a subgroup analysis for VAP. Only the m‐ITT group was reported for the ventilator subgroup and it was not possible to determine the original ITT groups, we used m‐ITT for our analysis. The all‐cause mortality rate for tigecycline was 6.8% higher than for imipenem‐cilastatin (all‐cause mortality: 19.1% for tigecycline and 12.3% for imipenem‐cilastatin). This difference in mortality rate did not reach statistical significance at a 95% CI (OR ceftazidime versus imipenem‐cilastatin 1.68, 95% CI 0.84 to 3.37). We could not perform meta‐analysis for all‐cause mortality because the only other study that compared tigecycline and imipenem‐cilastatin did not report VAP data for all‐cause mortality (Ramirez 2013).
2.1.4 Doripenem versus imipenem‐cilastatin with optional vancomycin or linezolid and amikacin available to both arms
Kollef 2012 compared the empirical antibiotic treatment of doripenem to imipenem‐cilastatin as an ITT analysis for a total of 274 participants. The all‐cause mortality rate was 3.6% higher in the doripenem group (all‐cause mortality: 35.0% for doripenem and 31.4% for imipenem‐cilastatin). This difference in mortality rate did not reach statistical significance at a 95% CI (OR doripenem versus imipenem‐cilastatin 1.18, 95% CI 0.71 to 1.95). We could not perform meta‐analysis because only one study presented this antibiotic comparison.
2.1.5 Linezolid versus vancomycin with aztreonam available to both arms
Kollef 2004 compared the empirical antibiotic treatment of linezolid to vancomycin as an ITT analysis for a total of 544 participants. The all‐cause mortality rate was 5.4% lower in the linezolid group (all‐cause mortality: 20.9% for linezolid and 26.3% for vancomycin). This difference in mortality rate did not reach statistical significance at a 95% CI (OR linezolid versus vancomycin 0.74, 95% CI 0.50 to 1.10). We could not perform meta‐analysis because there was only one study presenting this antibiotic comparison.
2.2 Clinical Cure (ITT) and 2.3 Clinical cure (clinically evaluable patients)
Seven studies reported clinical cure (Beaucaire 1999; Brun‐Buisson 1998; Freire 2010; Kollef 2004; Ramirez 2013; Rea‐Neto 2008; Shorr 2005). Both ITT and clinically evaluable analysis were reported in three of the studies (Beaucaire 1999; Freire 2010; Kollef 2004). One study only reported clinically evaluable analysis (Ramirez 2013). (See Analysis 2.2 for ITT analysis and Analysis 2.3 for clinically evaluable analysis).
2.2. Analysis.
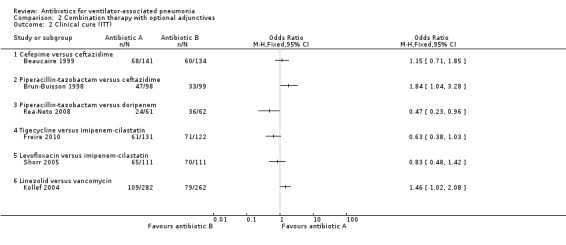
Comparison 2 Combination therapy with optional adjunctives, Outcome 2 Clinical cure (ITT).
2.3. Analysis.
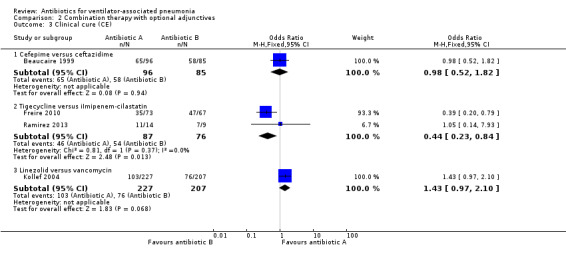
Comparison 2 Combination therapy with optional adjunctives, Outcome 3 Clinical cure (CE).
2.2.1 and 2.3.1 Cefepime versus ceftazidime with amikacin available to both arms
2.2.1: Beaucaire 1999 reported an ITT analysis of clinical cure for a total of 275 participants. The clinical cure was 3.4% higher in the cefepime group (clinical cure: 48.3% for cefepime and 44.8% for ceftazidime). This difference in clinical cure did not reach statistical significance at a 95% CI (OR cefepime versus ceftazidime 1.15, 95% CI 0.71 to 1.85), with cefepime being more likely to achieve clinical cure. We could not perform meta‐analysis as there is only one study with this antibiotic comparison.
2.3.1: A second analysis of clinical cure was performed with the clinically evaluable population for a total of 181 participants. The clinically evaluable population in this study excluded patients who were resistant to the treatment medications, had major deviations from the protocol, and those who had a duration of treatment less than five days. There was a 0.2% difference in clinical cure between the groups (clinical cure clinically evaluable: 68% for cefepime and 68.2% for ceftazidime). This difference in clinical cure for the clinically evaluable group did not reach statistical significance at 95% CI (OR cefepime versus ceftazidime 0.98, 95% CI 0.52 to 1. 82), with cefepime being less likely to achieve clinical cure. We could not perform meta‐analysis because only one study presented this antibiotic comparison.
2.2.2 Piperacillin‐tazobactam versus ceftazidime with amikacin available to both arms
Brun‐Buisson 1998 reported an ITT analysis of clinical cure for a total of 197 participants. The clinical cure was 14.7% higher in the piperacillin‐tazobactam group (clinical cure: 48.0% for piperacillin‐tazobactam and 33.3% for ceftazidime). This difference in clinical cure reached statistical significance at a 95% CI (OR piperacillin‐tazobactam versus ceftazidime 1.84, 95% CI 1.04 to 3.28) with piperacillin‐tazobactam being more likely to achieve clinical cure. We could not perform meta‐analysis because only one study presented this antibiotic comparison.
2.2.3 Piperacillin‐tazobactam versus doripenem with vancomycin and amikacin available to both arms
Rea‐Neto 2008 reported a clinically modified‐ITT (cm‐ITT) analysis for a total of 123 participants. They defined their cm‐ITT group as participants who met the clinical definition of pneumonia and received at least one dose of the study drug. This is a study on HAP with VAP as a subgroup analysis and the ITT information on the ventilator subgroup was not supplied, therefore we conducted our analysis on the cm‐ITT group. The clinical cure was 18.8% lower in the piperacillin‐tazobactam group (clinical cure: 39.3% for piperacillin‐tazobactam and 58.1% for doripenem). This difference in clinical cure reached statistical significance at a 95% CI (OR piperacillin‐tazobactam versus doripenem 0.47, 95% CI 0.23 to 0.96) with piperacillin‐tazobactam being less likely to achieve clinical cure. We could not perform meta‐analysis because only one study presented this antibiotic comparison.
2.2.4 and 2.3.2 Tigecycline versus imipenem‐cilastatin plus optional adjunctives
2.2.4: Freire 2010 reported a cm‐ITT analysis for a total of 243 participants for clinical cure. They defined cm‐ITT as participants who received any study drug and met minimum disease requirements. VAP was a subgroup analysis of a larger HAP group in this study so we were unable to determine what the ITT group was for the VAP participants. The m‐ITT group, all patients who received the study drug, was reported for all‐cause mortality so all patients missing from this group were considered a failure so that our analysis could be based on the m‐ITT group in an attempt to be as close to ITT as possible. This resulted in a m‐ITT group of 253 participants for our analysis of clinical cure. The clinical cure was 11.6% lower in the tigecycline group (clinical cure: 46.6% for tigecycline and 58.2% for imipenem‐cilastatin). This difference in clinical cure did not reach statistical significance at a 95% CI (OR tigecycline versus imipenem‐cilastatin 0.63, 95% CI 0.38 to 1.03) with tigecycline being less likely to achieve clinical cure. We could not perform meta‐analysis because only one study presented this antibiotic comparison.
2.3.2Freire 2010 and Ramirez 2013 both reported clinical cure in the clinically evaluable population for a total of 163 participants. These studies differed in their available adjunctives with Freire 2010 comparing tigecycline with optional ceftazidime versus imipenem‐cilastatin with optional vancomycin and Ramirez 2013 comparing tigecycline with optional ceftazidime and tobramycin or amikacin versus imipenem‐cilastatin with optional vancomycin and tobramycin or amikacin. Freire 2010 defined their clinically evaluable population as all participants who received any study drug, met minimum disease requirements, they could not have received confounding doses of prior or concomitant antibiotics, had to have received sufficient doses of the study drug, and had a test‐of‐cure efficacy assessment per protocol. The clinical cure was 22.2% lower in the tigecycline group (clinical cure: 47.9% for tigecycline and 70.1% for imipenem‐cilastatin). Ramirez 2013 define their clinically evaluable population as those participants who met inclusion and exclusion criteria at randomisation, did not receive any potentially effective concomitant systemic or aerosolised antibacterial treatment other than the study medication, received less than 24 hours of antibiotic therapy for the infection before enrolment, and had an evaluation test‐of‐cure assessment. The clinical cure was 0.8% higher in the tigecycline group (clinical cure: 78.6% for tigecycline and 77.8% for imipenem‐cilastatin). We focused on different antibiotics, not dosages, but it should be noted that Ramirez 2013 used lower dosages of tigecycline than Freire 2010. Dosages of imipenem‐cilastatin were comparable between studies.
Pooled studies
Meta‐analysis of dichotomous outcomes for this clinically evaluable clinical cure group showed a statistically significant higher cure rate at 95% CI for tigecycline over imipenem‐cilastatin (OR tigecycline versus imipenem‐cilastatin 0.44, 95% CI 0.23 to 0.84) with tigecycline being less likely to achieve clinical cure (I² statistic = 0%).
2.2.5 Levofloxacin with optional ceftazidime or other non‐carbapenem beta lactam versus imipenem‐cilastatin with optional amikacin or other aminoglycoside, vancomycin was also available to both arms
Shorr 2005 reported an ITT analysis of clinical cure for a total of 140 participants. The clinical cure was 4.5% lower in the levofloxacin group (clinical cure: 58.6% for levofloxacin and 63.1% for imipenem‐cilastatin). This difference in clinical cure did not reach statistical significance at a 95% CI (OR levofloxacin versus imipenem‐cilastatin 0.83, 95% CI 0.48 to 1.42) with levofloxacin being less likely to achieve clinical cure. We could not perform meta‐analysis because only one study presented this antibiotic comparison.
2.2.6 and 2.3.3 Linezolid versus vancomycin with aztreonam available to both arms
2.2.6: Kollef 2004 reported an ITT analysis of clinical cure for a total of 544 participants. The clinical cure was 8.5% higher in the linezolid group (clinical cure: 38.7% for linezolid and 30.2% for vancomycin). This difference in clinical cure reached statistical significance at a 95% CI (OR linezolid versus vancomycin 1.46, 95% CI 1.02 to 2.08) with linezolid being more likely to achieve clinical cure. We could not perform meta‐analysis because only one study presented this antibiotic comparison.
2.3.3: A second analysis of clinical cure was performed with the clinically evaluable population for a total of 434 participants. The clinically evaluable population in this study excluded all outcomes that were missing or indeterminate. The clinical cure was 8.7% higher in the linezolid group (clinical cure: 45.4% for linezolid and 36.7% for vancomycin). This difference in clinical cure did not reach statistical significance at a 95% CI (OR linezolid versus vancomycin 1.43, 95% CI 0.97 to 2.10) with linezolid being more likely to achieve clinical cure. We could not perform meta‐analysis because only one study presented this antibiotic comparison.
Secondary outcomes
2.4 Adverse events
Five studies reported adverse events that were attributable to treatment and/or required discontinuation of the study medication (Beaucaire 1999; Brun‐Buisson 1998; Freire 2010; Rea‐Neto 2008; Shorr 2005) (Analysis 2.4).
2.4. Analysis.
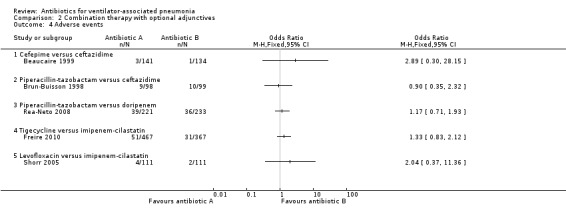
Comparison 2 Combination therapy with optional adjunctives, Outcome 4 Adverse events.
2.4.1 Cefepime versus ceftazidime with amikacin available to both arms
Beaucaire 1999 reported an ITT analysis of adverse events for a total of 275 participants. The rate of adverse events that were judged as being caused by the test drug was found to be 1.4% higher in the cefepime group (adverse events related to test drug: 2.1% for cefepime and 0.7% for ceftazidime). This difference in adverse event rate did not reach statistical significance at a 95% CI (OR cefepime versus ceftazidime 2.89, 95% CI 0.30 to 28.15). We could not perform meta‐analysis because only one study presented this antibiotic comparison.
2.4.2 Piperacillin‐tazobactam versus ceftazidime with amikacin available to both arms
Brun‐Buisson 1998 reported an ITT analysis of adverse events for a total of 197 participants. The rate of adverse events that were judged as being definitely, possibly, or probably related to the test drug was 0.9% lower in the piperacillin‐tazobactam group (adverse events related to test drug: 9.2% for piperacillin‐tazobactam and 10.1% for ceftazidime). This difference in adverse event rate did not reach statistical significance at a 95% CI (OR piperacillin‐tazobactam versus ceftazidime 0.90, 95% CI 0.35 to 2.32). We could not perform meta‐analysis because only one study presented this antibiotic comparison.
2.4.3 Piperacillin‐tazobactam versus doripenem with vancomycin and amikacin available to both arms
Rea‐Neto 2008 reported an ITT analysis of adverse events for a total of 444 participants. This is a study of HAP with subgroup analysis of VAP patients. The adverse event data used from this study is for the entire population of hospital‐associated pneumonia patients since subgroup data were not provided for this outcome. Adverse events related to the study drug were found to be 1.5% higher in the piperacillin‐tazobactam (adverse events related to test drug: 17.6% for piperacillin‐tazobactam and 16.1% for doripenem). This difference in adverse event rate did not reach statistical significance at a 95% CI (OR piperacillin‐tazobactam versus doripenem 1.17, 95% CI 0.71 to 1.93). We could not perform meta‐analysis because only one study presented this antibiotic comparison.
2.4.4 Tigecycline with optional ceftazidime versus imipenem‐cilastatin with optional vancomycin
Freire 2010 reported a m‐ITT analysis of adverse events for a total of 934 participants with the m‐ITT group being defined as any randomised patient who received any study drug. This is a study of HAP with subgroup analysis of VAP patients. The adverse event data used from this study is for the entire population of HAP patients since subgroup data were not provided for this outcome. They reported all adverse events and those causing discontinuation of the study drugs. The discontinuation rate of study drugs due to adverse events was 4.3% higher in the tigecycline group (adverse events leading to discontinuation: 10.9% for tigecycline and 6.6% for imipenem‐cilastatin). This difference in adverse event rate was statistically significance at a 95% CI (OR tigecycline versus imipenem‐cilastatin 1.33, 95% CI 0.83 to 2.12). We could not perform meta‐analysis because only one study presented this antibiotic comparison.
2.4.5 Levofloxacin with optional ceftazidime or other non‐carbapenem beta lactam versus imipenem‐cilastatin with optional amikacin or other aminoglycoside, vancomycin was also available to both arms
Shorr 2005 reported an ITT analysis of adverse events for a total of 222 participants. They reported all serious adverse events and serious adverse events leading to discontinuation of the antibiotics. The rate of adverse events requiring discontinuation of study drugs was 1.8% higher in the levofloxacin group (adverse events leading to discontinuation: 3.6% for levofloxacin and 1.8% for imipenem‐cilastatin). This difference in adverse event rate did not reach statistical significance at a 95% CI (OR levofloxacin versus imipenem‐cilastatin 2.04, 95% CI 0.37 to 11.36). We could not perform meta‐analysis because only one study presented this antibiotic comparison.
2.5 Superinfections
Rea‐Neto 2008 reported superinfection rates.
2.5.1 Piperacillin‐tazobactam versus doripenem with vancomycin and amikacin available to both arms
Rea‐Neto 2008 reported a cm‐ITT analysis of superinfections for a total of 123 participants. They defined their cm‐ITT group as participants who met the clinical definition of pneumonia and received at least one dose of the study drug. This is a study of HAP with VAP as a subgroup analysis and the ITT information on the ventilator subgroup was not supplied, therefore our analysis was conducted on the cm‐ITT group. The rate of superinfections was 3.7% higher in the piperacillin‐tazobactam group (superinfection: 10% for piperacillin‐tazobactam and 6.3% for doripenem). This difference in superinfection rate did not reach statistical significance at a 95% CI (OR piperacillin‐tazobactam versus doripenem 1.64, 95% CI 0.44 to 6.12) (Analysis 2.5). We could not perform meta‐analysis because only one study presented this antibiotic comparison.
2.5. Analysis.
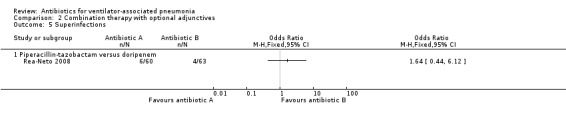
Comparison 2 Combination therapy with optional adjunctives, Outcome 5 Superinfections.
2.6 Length of hospital stay
No studies in this comparison reported on length of hospital stay.
2.7 Length of ICU stay
Freire 2010 reported that there was no significant difference between treatment groups (tigecycline versus imipenem‐cilastatin) in ICU length of stay (P = 0.937). The length of stay for each group was not reported.
Comparison 3: Carbapenems versus non‐carbapenems
Four studies compared use of a carbapenem to a non‐carbapenem antibiotic (Freire 2010; Ramirez 2013; Rea‐Neto 2008; Shorr 2005). The adjunctive antibiotics that were made available in these studies were ceftazidime, vancomycin, tobramycin, and amikacin.
Primary outcomes
3.1 All‐cause mortality (28‐day)
All‐cause mortality was reported as a m‐ITT analysis for a total of 253 participants. The m‐ITT group participant is defined as a randomised (ITT) patient who received any study drug. This study was on HAP with a subgroup analysis for VAP. Only the m‐ITT group was reported for the ventilator subgroup and it was not possible to determine the original ITT groups; therefore, m‐ITT was used for our analysis. Freire 2010 compared imipenem‐cilastatin to tigecycline. The mortality rate was 6.8% lower in the carbapenem group (mortality rate: 12.3% for carbapenem and 19.1% for non‐carbapenem). This difference in mortality rate did not reach statistical significance (OR carbapenem versus non‐carbapenem 0.59, 95% CI 0.30 to 1.19) (Analysis 3.1). We could not perform meta‐analysis because there was only one study with this reported outcome for the carbapenem versus non‐carbapenem comparison. Using GRADE assessment, we downgraded this outcome one level from high to moderate‐quality for serious imprecision due to a wide confidence interval (Table 2).
3.1. Analysis.
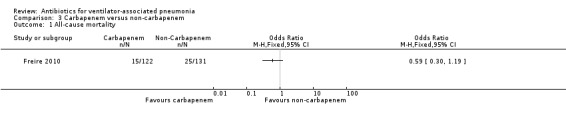
Comparison 3 Carbapenem versus non‐carbapenem, Outcome 1 All‐cause mortality.
3.2 Clinical cure (ITT) and 3.3 Clinical cure (clinically evaluable patients)
Clinical cure was reported as ITT analysis in Shorr 2005; as cm‐ITT in Rea‐Neto 2008; and as m‐ITT in Freire 2010. Two studies reported clinically evaluable analyses (Freire 2010; Ramirez 2013).
Pooled studies
3.2: ITT groups could not be used for Freire 2010 and Rea‐Neto 2008 due to both being studies on HAP with VAP as a subgroup analysis and ITT information on the ventilator subgroup was not supplied. Rea‐Neto 2008 defined the cm‐ITT group as participants who met the clinical definition of pneumonia and received at least one dose of the study drug. Freire 2010 defined the m‐ITT group as a randomised (ITT) patient who received any study drug.
The combined ITT, cm‐ITT, and m‐ITT analysis included a total of 598 participants. Freire 2010 and Shorr 2005 both used imipenem‐cilastatin and Rea‐Neto 2008 used doripenem. The non‐carbapenems used were tigecycline in Freire 2010, levofloxacin in Shorr 2005, and piperacillin‐tazobactam in Rea‐Neto 2008. In Freire 2010 the clinical cure was 11.6% higher in the carbapenem group (clinical cure: 58.2% for carbapenems and 46.6% for non‐carbapenems). In Rea‐Neto 2008 the clinical cure was 18.8% higher in the carbapenem group (clinical cure: 58.1% for carbapenems and 39.3% for non‐carbapenems). In Shorr 2005 the clinical cure was 4.5% higher in the carbapenem group (clinical cure: 63.1% for carbapenems and 58.6% for non‐carbapenems) (Analysis 3.2).
3.2. Analysis.
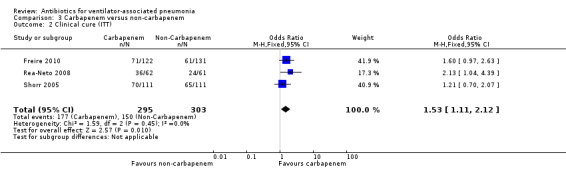
Comparison 3 Carbapenem versus non‐carbapenem, Outcome 2 Clinical cure (ITT).
Meta‐analysis of dichotomous outcomes using a fixed‐effect model for the ITT analysis showed a statistically significant better clinical cure at 95% CI for carbapenem over non‐carbapenem antibiotics (OR carbapenem versus non‐carbapenem 1.53, 95% CI 1.11 to 2.12; I² statistic = 0%) with carbapenems being more likely to achieve clinical cure (Table 2). Using GRADE assessment, we downgraded this outcome one level from high to moderate‐quality for risk of bias. All three studies had unclear risk of bias for both random sequence generation (selection bias) and allocation concealment (selection bias) (Freire 2010; Rea‐Neto 2008; Shorr 2005). Rea‐Neto 2008 additionally had high risk of bias for incomplete outcome data (attrition bias) due to seven patients that were missing from the outcome data. Shorr 2005 had high risk of bias for blinding of outcome assessment (detection bias) and selective reporting (reporting bias) due to there being no mortality data reported (Table 2).
3.3: The clinically evaluable analysis included 163 participants. Freire 2010 and Ramirez 2013 both looked at the same comparison of imipenem‐cilastatin versus tigecycline. In Freire 2010 the clinical cure for the clinically evaluable population was 22.2% higher in the carbapenem group (clinical cure: 70.1% for carbapenems and 47.9% for non‐carbapenems). In Ramirez 2013 the clinical cure for the clinically evaluable population was 0.8% lower in the carbapenem group (clinical cure: 77.8% for carbapenems and 78.6% for non‐carbapenems) (Analysis 3.3).
3.3. Analysis.
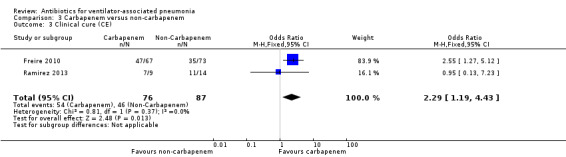
Comparison 3 Carbapenem versus non‐carbapenem, Outcome 3 Clinical cure (CE).
Meta‐analysis of dichotomous outcomes using a fixed‐effect model for the clinically evaluable analysis showed a statistically significant difference in clinical cure at 95% CI between carbapenem and non‐carbapenem antibiotics (OR carbapenem versus non‐carbapenem 2.29, 95% CI 1.19 to 4.43; I² statistic = 0%) with carbapenems being more likely to achieve clinical cure. Using GRADE assessment, we downgraded this outcome one level from high to moderate‐quality for imprecision due to a wide confidence interval (Table 2).
Secondary outcomes
3.4 Adverse events
Three studies reported adverse events that were attributable to the treatment and/or required discontinuation of the study medication as an ITT analysis (Freire 2010; Rea‐Neto 2008; Shorr 2005), and one study reported a m‐ITT analysis (Freire 2010), for a total of 1510 participants. Freire 2010 and Rea‐Neto 2008 were studies of HAP with subgroup analysis of VAP patients. The adverse event data used from these studies is for the entire population of HAP patients, since subgroup data were not provided for this outcome. In Freire 2010 the discontinuation rate of study drugs due to adverse events was 4.3% lower in the carbapenem group (adverse events leading to discontinuation: 6.6% for carbapenem and 10.9% for non‐carbapenem). In Rea‐Neto 2008 adverse events related to the study drug were found to be 1.5% lower in the carbapenem group (adverse events related to test drug: 16.1% for carbapenem and 17.6% for non‐carbapenem). In Shorr 2005 the rate of adverse events requiring discontinuation of study drugs was 1.8% lower in the carbapenem group (adverse events leading to discontinuation: 1.8% for carbapenem and 3.6% for non‐carbapenem) (Analysis 3.4).
3.4. Analysis.
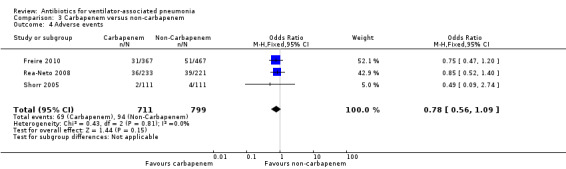
Comparison 3 Carbapenem versus non‐carbapenem, Outcome 4 Adverse events.
Pooled studies
Meta‐analysis of dichotomous outcomes using a fixed‐effect model for the ITT/m‐ITT analysis showed no statistical significance in the difference in clinical cure at 95% CI between carbapenem and non‐carbapenem antibiotics (OR carbapenem versus non‐carbapenem 0.78, 95% CI 0.56 to 1.09; I² statistic = 0%). Using GRADE assessment, we downgraded this outcome two levels from high to low‐quality for imprecision due to a wide confidence interval and serious risk of bias (Table 2).
3.5 Superinfections
Rea‐Neto 2008 reported superinfection rates. They reported a cm‐ITT analysis of superinfections for a total of 123 participants. They defined the cm‐ITT group as participants who met the clinical definition of pneumonia and received at least one dose of the study drug. This is a study on HAP with VAP as a subgroup analysis and the ITT information on the ventilator subgroup was not supplied, therefore our analysis was done on the cm‐ITT group. The rate of superinfections was 3.7% lower in the carbapenem group (superinfection: 6.3% for carbapenem and 10% for non‐carbapenem). This difference in superinfection rate did not reach statistical significance at a 95% Cl (OR carbapenem versus non‐carbapenem 0.61, 95% CI 0.16 to 2.28) (Analysis 3.5). We could not perform meta‐analysis because only one study presented this antibiotic comparison.
3.5. Analysis.
Comparison 3 Carbapenem versus non‐carbapenem, Outcome 5 Superinfections.
3.6 Length of hospital stay
None of the studies in this comparison reported on length of hospital stay.
3.7 Length of ICU stay
Freire 2010 reported that there was no significant difference between treatment groups (carbapenem versus non‐carbapenem) in ICU length of stay (P = 0.937). The length of stay for each group was not reported.
Discussion
Summary of main results
Monotherapy versus combination therapy
We found four studies that compared monotherapy to combination therapy (Alvarez Lerma 2001; Awad 2014; Damas 2006; Heyland 2008). Three studies had true monotherapy arms (Alvarez Lerma 2001; Damas 2006; Heyland 2008), and Awad 2014 had the option of adding adjunctive fluoroquinolones or aminoglycosides. Our analysis demonstrated no significant difference in our primary end point of all‐cause mortality between monotherapy and combination therapy. The studies we reviewed did not distinguish between early‐ or late‐onset ventilator‐associated pneumonia (VAP).
We also evaluated differences in clinical cure on an intention‐to‐treat (ITT) basis and for clinically evaluable patients based on data from two studies (Alvarez Lerma 2001; Awad 2014). The results of the meta‐analysis revealed no significant difference between monotherapy versus combination therapy with regard to clinical cure.
Two studies reported adverse events (Alvarez Lerma 2001; Awad 2014). Our analysis did not reveal any significant differences in adverse events between monotherapy and combination therapy. The rate of superinfections was reported in only one study and we were therefore unable to pool data (Alvarez Lerma 2001). Of note, is that no increase in superinfections was found in the monotherapy group in the only study reporting this outcome.
Our meta‐analysis data did not show a difference between monotherapy and combination therapy for VAP. This would support the use of a single‐antibiotic regimen, with the understanding that resistance patterns vary depending on the local environment. However, epidemiologic data that relies on culture positive data only does not discriminate between colonisation and true infections. This is especially true for VAP where tracheal aspirates may reflect upper respiratory colonisation rather than a true cause of VAP.
Combination therapy with optional adjunctives
We identified eight studies that compared different antibiotics controlling for adjuncts (Beaucaire 1999; Brun‐Buisson 1998; Freire 2010; Kollef 2004; Kollef 2012; Ramirez 2013; Rea‐Neto 2008; Shorr 2005). The included adjuncts were amikacin, vancomycin, linezolid, aztreonam, ceftazidime, and tobramycin. Unfortunately, only two studies evaluated the same antibiotics (tigecycline versus imipenem‐cilastin) (Freire 2010; Ramirez 2013). These studies both reported clinical cure for the clinically evaluable population. Our meta‐analysis demonstrated an improved clinical cure for imipenem‐cilastatin (odds ratio (OR) tigecycline versus imipenem‐cilastatin 0.44, 95% confidence interval (CI) 0.23 to 0.84).
Five studies reported all‐cause mortality (Beaucaire 1999; Brun‐Buisson 1998; Freire 2010; Kollef 2004; Kollef 2012). No antibiotic combination was significantly better than another. With regard to clinical cure, three studies reported a significant improvement: in Brun‐Buisson 1998 piperacillin‐tazobactam demonstrated improved clinical cure over ceftazidime (OR piperacillin‐tazobactam versus ceftazidime 1.84, 95% CI 1.04 to 3.28); Rea‐Neto 2008 favoured doripenem over piperacillin‐tazobactam (OR piperacillin‐tazobactam versus doripenem 0.47, 95% CI 0.23 to 0.96); Kollef 2004 favoured linezolid over vancomycin (OR linezolid versus vancomycin 1.46, 95% CI 1.02 to 2.08).
Five studies reported adverse events (Beaucaire 1999; Brun‐Buisson 1998; Freire 2010; Rea‐Neto 2008; Shorr 2005). Only Freire 2010 reached statistical significance, revealing an increase in adverse events for tigecycline when compared to imipenem‐cilastin (OR tigecycline vs imipenem‐cilastatin 1.33, 95% CI 0.83 to 2.12). Due to lack of data we were unable to evaluate if a particular empiric antibiotic therapy led to improved outcomes.
Carbapenems versus non‐carbapenems
Carbapenems are potent broad‐spectrum antibiotics with coverage of extended spectrum beta‐lactamases (ESBLs). We found four studies that compared carbapenems with other antibiotics (Freire 2010; Ramirez 2013; Rea‐Neto 2008; Shorr 2005). Freire 2010 was the only study to report all‐cause mortality, but there was no significant difference. Three studies reported clinical cure on an ITT basis (Freire 2010; Rea‐Neto 2008; Shorr 2005). Treatment with carbapenems had significantly higher clinical cure (OR carbapenem versus non‐carbapenem 1.53, 95% CI 1.11 to 2.12) when compared to non‐carbapenems including tigecycline, levofloxacin, and piperacillin‐tazobactam. Three studies reported adverse events which did not reveal any significant difference (Freire 2010; Rea‐Neto 2008; Shorr 2005).
Carbapenems are broader spectrum antibiotics than the comparative antibiotics in the three studies. Specifically, all carbapenems cover Pseudomonas species (except ertapenem) and ESBLs. Studies have been consistent in demonstrating worse outcomes with ineffective empiric antibiotics (Alvarez‐Lerma 1996; Garnacho‐Montero 2007; Iregui 2002; Rello 2007). These data may represent a high rate of multidrug‐resistant bacteria pathogens in intensive care unit (ICU) settings of these studies and may not be generalisable.
Overall completeness and applicability of evidence
We used a comprehensive search strategy to identify all clinical trials evaluating antibiotic regimens for VAP. We also attempted to contact trial authors for other references. Our searches identified non‐English language papers, indicating that we covered a wide range of references. However, it is possible that we missed some trials not published in mainstream journals, but it is unlikely that other small trials would change our conclusions. We will nevertheless monitor and continue searching for references to include in future review updates.
A major difficulty we encountered was the lack of studies examining the same antibiotics. This meant we were unable to perform meta‐analysis for many comparisons. Since many included studies were small, meta‐analysis would enable us to increase the power to levels sufficient to assess if there was superiority of one regimen.
We did not create a 'Summary of findings' table for results comparing combination therapy with optional adjunctives; in this comparison both arms received a combination therapy with mostly different antibiotics. The adjunctives in each of the studies are specifically aimed at other pathogens that can be dependent on local prevalences and resistance patterns. The comparison would then encompass combination therapy versus combination therapy which would not make much clinical sense.
We were unable to identify sufficient data for the outcomes of superinfections, length of hospital stay, and length of ICU stay.
Lastly, studies did not differentiate early‐ versus late‐onset VAP and did not select patients at risk of multidrug‐resistant bacteria. Since antibiotic resistance patterns are regionally defined, these data may not be generalisable.
Quality of the evidence
We assessed quality of evidence using GRADE analysis.
For monotherapy versus combination therapy we assessed the quality of evidence for five comparisons: mortality rate, clinical cure in the ITT population, clinical cure in the clinically evaluable population, adverse events, and length of ICU stay. We downgraded the quality of evidence for all‐cause mortality, adverse events, and length of ICU stay to moderate because of serious imprecision due to wide confidence intervals. For clinical cure in the ITT and clinically evaluable populations, we downgraded the quality of evidence from high to very low due to inconsistency (substantial heterogeneity) and imprecision (wide CI).
For carbapenems versus non‐carbapenems we also assessed the quality of evidence for four outcomes: mortality rate, clinical cure in the ITT population, clinical cure in the clinically evaluable population, and adverse events. We downgraded the quality of evidence for the outcome, mortality rate, to moderate because of serious imprecision due to a wide confidence interval. Only Freire 2010 reported mortality as an outcome. We downgraded the quality of evidence for the outcome, clinical cure in the ITT population, from high to moderate due to high risk of bias. We downgraded the quality of evidence for the clinically evaluable population from high to moderate for failure to meet optimal information size. We downgraded the quality of evidence for adverse events from high to low for serious imprecision due to a wide confidence interval and serious risk of bias.
Potential biases in the review process
To minimise risk of bias in the review process two review authors independently assessed the results of the searches. Likewise, two review authors independently assessed risk of bias. All review authors discussed discrepancies and contributed to the GRADE assignment process and interpretation of the findings. We conducted a comprehensive search in multiple databases, but it is still possible that studies were missed. We will continue to scrutinise relevant publications in search of other studies that could be included in an update of this review.
Agreements and disagreements with other studies or reviews
Empiric broad‐spectrum antibiotic therapy is a cornerstone for treating VAP. American Thoracic Society/Infectious Diseases Society of America guidelines recommend broad‐spectrum monotherapy to cover both methicillin‐resistant S aureus (MRSA) and Pseudomonas in patients with risk factors for multidrug‐resistant pathogens receiving treatment in units with low prevalence rates of MRSA and resistant Gram‐negatives. The American Thoracic Society/Infectious Diseases Society of America guidelines also recommend broad‐spectrum combination therapy with an agent active against MRSA and at least two agents active against Gram‐negative organisms in patients with risk factors for multidrug‐resistant pathogens and in units with high prevalence rates of MRSA and Pseudomonas (ATS 2016). These guidelines are supported by epidemiologic data from the USA which revealed a high prevalence of resistant S aureus and Pseudomonas species in patients with culture positive VAP (Kollef 2005). In addition, studies have demonstrated worse outcomes when appropriate antibiotic therapy is delayed (Alvarez‐Lerma 1996; Garnacho‐Montero 2007; Iregui 2002; Rello 2007). A meta‐analysis by Kuti 2008 supports the use of early broad‐spectrum antibiotics by reporting increased mortality in patients receiving inappropriate empiric therapy. On the other hand, overuse of broad‐spectrum antibiotics may lead to increased costs and increased resistance. There are few data comparing empiric monotherapy with combination therapy in VAP or evaluating the best empiric therapy.
In an observational cohort study, Garnacho‐Montero 2007 evaluated monotherapy versus combination therapy for Pseudomonas VAP in Spain. They reported that inappropriate therapy was associated with increased mortality. However, a Cox proportional hazard regression analysis found effective monotherapy was not associated with increased mortality when controlling for disease severity. This supports the use of effective monotherapy in VAP. Heyland 2008 studied empiric monotherapy including meropenem with combination therapy of meropenem and ciprofloxacin for late‐onset VAP in Canada and the USA. The authors reported no difference in 28‐day mortality, ICU length of stay, or emergence of resistance.
Authors' conclusions
Implications for practice.
The 2005 American Thoracic Society/Infectious Diseases Society of America guidelines promoted a combination of three different classes of antibiotics to treat patients with ventilator‐associated pneumonia (VAP) at risk for multidrug‐resistant bacteria. Those guidelines and the classification of health care associated‐pneumonia (HCAP) was supported by a retrospective cohort study characterising the epidemiology of patients with culture positive community‐acquired pneumonia (CAP), HCAP, hospital‐acquired pneumonia (HAP), and VAP in the first five days of hospital admission (Kollef 2005). Patients with culture positive VAP grew S aureus 42.5% of the time; methicillin‐resistant S aureus (MRSA) grew 14.6%; S pneumoniae grew 8.6%: Pseudomonas species grew 21.2%; Haemophilus species grew 12.2%. However, cultures in VAP do not necessarily discriminate between pathologic infections versus colonisation, leading to a significant amount of false positives. Schreiber 2010 found the HCAP criteria to have poor sensitivity (78%) and specificity (56%) for predicting pneumonia due to a resistant organism. In addition, they may promote over‐prescribing, resulting in antibiotic resistance. Indeed, Magill 2014 noted an increased use of broad‐spectrum antibiotics in hospitals across the USA since the promotion of guidelines in healthcare delivery. This concept of HCAP introduced in the 2005 American Thoracic Society/Infectious Diseases Society of America guidelines was removed from the 2016 HAP/VAP guidelines due to evidence from numerous subsequent studies demonstrating that patients defined as having HCAP are not at high risk of multidrug‐resistant pathogens (ATS 2016).
These same guidelines recommend monotherapy for patients with VAP and those at low risk for multidrug‐resistant bacteria. This may lead to clinical treatment failures and worse outcomes. This Cochrane Review and meta‐analysis did not find a difference between monotherapy and combination therapy for treatment. Combination therapy can be associated with increased risk of resistance and cost. Without evidence for improvement in clinical cure with the use of combination therapy, this review lends support to the use of effective monotherapy for the treatment of VAP. Since the studies did not identify patients with increased risk for multidrug‐resistant bacteria, these data may not be generalisable to all patient groups. Additionally, the quality of the evidence was low and the number and size of trials were small, which limits the meaningful clinical application. However, this is the largest meta‐analysis comparing monotherapy to multiple antibiotic therapies for VAP, and contributes further evidence to the safety of using effective monotherapy for the empiric treatment of VAP.
We were unable to evaluate which antibiotic is the best choice for the treatment of people with VAP. However, carbapenems as a class may result in better clinical cure than piperacillin‐tazobactam, tigecycline, or levofloxacin in certain populations.
Implications for research.
Further studies are needed to develop more effective clinical predictors of VAP due to multidrug‐resistant antimicrobials to help guide optimal empiric therapy. Since no current antibiotic covers MRSA and multidrug‐resistant Gram‐negative bacillus, predictors of multidrug‐resistant pathogens will help determine which patients would benefit from combination therapy. Prospective studies should evaluate the efficacy of monotherapy for Gram‐negative bacillus that tend to develop multidrug‐resistance, for example, Serratia species, Pseudomonas species, Acinetobacter species, Citrobacter species, and Enterobacter species. In particular, carbapenems should be evaluated in prospective randomised studies to determine if, as a class, they are preferable to more restrictive antibiotics or combination therapy.
History
Protocol first published: Issue 2, 2003 Review first published: Issue 10, 2016
| Date | Event | Description |
|---|---|---|
| 2 September 2009 | Amended | Previously withdrawn protocol has been updated by a new team of authors. |
| 15 February 2009 | Amended | Withdrawn, Issue 2, 2009 |
| 15 March 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to acknowledge the contribution of MA Aarts, JN Hancock, DK Heyland, and JC Marshall who registered the title for this review and wrote the initial Cochrane Review protocol, and also Adrian Selim, George Balalis, and Balu Bhaskar who wrote and published the updated version of the Cochrane Review protocol. We also thank Lars Eriksson, Librarian at the University of Queensland, who assisted us with the searches for this review. Finally, we thank the following people for commenting on the draft of this review: Julie Gildie, Rodrigo Cavallazzi, Neill Adhikari, Elaine Beller, and Matthew Thompson.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1 exp Pneumonia/ (79194) 2 pneumon*.tw. (147249) 3 or/1‐2 (177880) 4 exp Respiration, Artificial/ (63971) 5 exp Ventilators, Mechanical/ (8453) 6 ventilat*.tw. (131877) 7 or/4‐6 (160698) 8 3 and 7 (12523) 9 Pneumonia, Ventilator‐Associated/ (2341) 10 (pneumon* adj5 ventilator*).tw. (4156) 11 vap.tw. (2946) 12 or/8‐11 (13815) 13 exp Anti‐Bacterial Agents/ (585417) 14 antibiot*.tw,nm. (275353) 15 antimicrob*.tw,nm. (111218) 16 (empiric* adj3 therap*).tw. (7200) 17 exp Penicillins/ (72679) 18 exp Cephalosporins/ (38352) 19 exp Carbapenems/ (8121) 20 exp Aminoglycosides/ (135580) 21 exp Quinolones/ (39334) 22 Clindamycin/ (5180) 23 Vancomycin/ (11363) 24 Aztreonam/ (1320) 25 (penicillin* or cephalosporin* or carbapenem* or aminoglycoside* or quinolone* or clindamycin* or vancomycin* or linezolid* or quinupristin* or dalfopristin* or aztreonam*).tw. (113197) 26 or/13‐25 (845860) 27 12 and 26 (3580)
Appendix 2. Embase (Elsevier) search strategy
No. Query Results Results Date #24. 'pneumonia'/exp OR (pneumon*:ab,ti AND [embase]/lim) AND ('mechanical ventilator'/de AND [embase]/lim OR ('artificial ventilation'/exp AND [embase]/lim) OR (ventilator*:ab,ti AND [embase]/lim)) OR ('ventilator associated pneumonia'/de AND [embase]/lim) OR ((pneumon* NEAR/5 ventilator*):ab,ti AND [embase]/lim) OR (vap:ab,ti AND [embase]/lim) AND ('antibiotic agent'/exp AND [embase]/lim OR (antibiotic*:ab,ti AND [embase]/lim) OR (antimicrob*:ab,ti AND [embase]/lim) OR ((empiric* NEAR/3 therap*):ab,ti AND [embase]/lim) OR ('quinoline derived antiinfective agent'/exp AND [embase]/lim) OR (penicillin*:ab,ti OR cephalosporin*:ab,ti OR carbapenem*:ab,ti OR aminoglycoside*:ab,ti OR quinolone*:ab,ti OR clindamycin*:ab,ti OR vancomycin*:ab,ti OR linezolid*:ab,ti OR quinupristin*:ab,ti OR dalfopristin*:ab,ti OR aztreonam*:ab,ti AND [embase]/lim)) AND ('randomised controlled trial'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'single blind procedure'/de OR random*:ab,ti OR placebo*:ab,ti OR allocat*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti OR trial:ti) NOT ('animal'/exp OR 'invertebrate'/exp OR 'animal experiment'/de OR 'animal model'/exp OR 'animal cell'/de OR 'animal tissue'/de OR 'nonhuman'/de NOT ('animal'/exp OR 'invertebrate'/exp OR 'animal experiment'/de OR 'animal model'/exp OR 'animal cell'/de OR 'animal tissue'/de OR 'nonhuman'/de AND ('human'/exp OR 'human cell'/de))) 749 07 Dec 2015 #23. 'randomised controlled trial'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'single blind procedure'/de OR random*:ab,ti OR placebo*:ab,ti OR allocat*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti OR trial:ti NOT ('animal'/exp OR 'invertebrate'/exp OR 'animal experiment'/de OR 'animal model'/exp OR 'animal cell'/de OR 'animal tissue'/de OR 'nonhuman'/de NOT ('animal'/exp OR 'invertebrate'/exp OR 'animal experiment'/de OR 'animal model'/exp OR 'animal cell'/de OR 'animal tissue'/de OR 'nonhuman'/de AND ('human'/exp OR 'human cell'/de))) 1,293,445 07 Dec 2015 #22. 'animal'/exp OR 'invertebrate'/exp OR 'animal experiment'/de OR 'animal model'/exp OR 'animal cell'/de OR 'animal tissue'/de OR 'nonhuman'/de NOT ('animal'/exp OR 'invertebrate'/exp OR 'animal experiment'/de OR 'animal model'/exp OR 'animal cell'/de OR 'animal tissue'/de OR 'nonhuman'/de AND ('human'/exp OR 'human cell'/de)) 5,834,564 07 Dec 2015 #21. 'randomised controlled trial'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'single blind procedure'/de OR random*:ab,ti OR placebo*:ab,ti OR allocat*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti OR trial:ti 1,459,838 07 Dec 20154 #20. 'pneumonia'/exp OR (pneumon*:ab,ti AND [embase]/lim) AND ('mechanical ventilator'/de AND [embase]/lim OR ('artificial ventilation'/exp AND [embase]/lim) OR (ventilator*:ab,ti AND [embase]/lim)) OR ('ventilator associated pneumonia'/de AND [embase]/lim) OR ((pneumon* NEAR/5 ventilator*):ab,ti AND [embase]/lim) OR (vap:ab,ti AND [embase]/lim) AND ('antibiotic agent'/exp AND [embase]/lim OR (antibiotic*:ab,ti AND [embase]/lim) OR (antimicrob*:ab,ti AND [embase]/lim) OR ((empiric* NEAR/3 therap*):ab,ti AND [embase]/lim) OR ('quinoline derived antiinfective agent'/exp AND [embase]/lim) OR (penicillin*:ab,ti OR cephalosporin*:ab,ti OR carbapenem*:ab,ti OR aminoglycoside*:ab,ti OR quinolone*:ab,ti OR clindamycin*:ab,ti OR vancomycin*:ab,ti OR linezolid*:ab,ti OR quinupristin*:ab,ti OR dalfopristin*:ab,ti OR aztreonam*:ab,ti AND [embase]/lim)) 8,955 07 Dec 2015 #19. 'antibiotic agent'/exp AND [embase]/lim OR (antibiotic*:ab,ti AND [embase]/lim) OR (antimicrob*:ab,ti AND [embase]/lim) OR ((empiric* NEAR/3 therap*):ab,ti AND [embase]/lim) OR ('quinoline derived antiinfective agent'/exp AND [embase]/lim) OR (penicillin*:ab,ti OR cephalosporin*:ab,ti OR carbapenem*:ab,ti OR aminoglycoside*:ab,ti OR quinolone*:ab,ti OR clindamycin*:ab,ti OR vancomycin*:ab,ti OR linezolid*:ab,ti OR quinupristin*:ab,ti OR dalfopristin*:ab,ti OR aztreonam*:ab,ti AND [embase]/lim) 1,281,559 07 Dec 2015 #18. penicillin*:ab,ti OR cephalosporin*:ab,ti OR carbapenem*:ab,ti OR aminoglycoside*:ab,ti OR quinolone*:ab,ti OR clindamycin*:ab,ti OR vancomycin*:ab,ti OR linezolid*:ab,ti OR quinupristin*:ab,ti OR dalfopristin*:ab,ti OR aztreonam*:ab,ti AND [embase]/lim 134,980 07 Dec 2015 #17. 'quinoline derived antiinfective agent'/exp AND [embase]/lim 139,536 07 Dec 2015 #16. (empiric* NEAR/3 therap*):ab,ti AND [embase]/lim 9,955 07 Dec 2015 #15. antimicrob*:ab,ti AND [embase]/lim 140,462 07 Dec 2015 #14. antibiotic*:ab,ti AND [embase]/lim 321,257 07 Dec 2015 #13. 'antibiotic agent'/exp AND [embase]/lim 1,077,882 07 Dec 2015 #12. 'pneumonia'/exp OR (pneumon*:ab,ti AND [embase]/lim) AND ('mechanical ventilator'/de AND [embase]/lim OR ('artificial ventilation'/exp AND [embase]/lim) OR (ventilator*:ab,ti AND [embase]/lim)) OR ('ventilator associated pneumonia'/de AND [embase]/lim) OR ((pneumon* NEAR/5 ventilator*):ab,ti AND [embase]/lim) OR (vap:ab,ti AND [embase]/lim) 24,855 07 Dec 2015 #11. vap:ab,ti AND [embase]/lim 4,560 07 Dec 2015 #10. (pneumon* NEAR/5 ventilator*):ab,ti AND [embase]/lim 9,030 07 Dec 2015 #9. 'ventilator associated pneumonia'/de AND [embase]/lim 6,915 07 Dec 2015 #8. 'pneumonia'/exp OR (pneumon*:ab,ti AND [embase]/lim) AND ('mechanical ventilator'/de AND [embase]/lim OR ('artificial ventilation'/exp AND [embase]/lim) OR (ventilator*:ab,ti AND [embase]/lim)) 21,666 07 Dec 2015 #7. 'mechanical ventilator'/de AND [embase]/lim OR ('artificial ventilation'/exp AND [embase]/lim) OR (ventilator*:ab,ti AND [embase]/lim) 176,305 07 Dec 2015 #6. ventilator*:ab,ti AND [embase]/lim 58,628 07 Dec 2015 #5. 'artificial ventilation'/exp AND [embase]/lim 141,062 07 Dec 2015 #4. 'mechanical ventilator'/de AND [embase]/lim 1,131 07 Dec 2015 #3. 'pneumonia'/exp OR (pneumon*:ab,ti AND 305,165 07 Dec 2015 #2. pneumon*:ab,ti AND [embase]/lim 193,007 07 Dec 2015 #1. 'pneumonia'/exp 222,653 07 Dec 2015
Appendix 3. LILACS (BIREME) search strategy
(((mh:pneumonia OR pneumon* OR neumonía OR pulmonía OR mh:c08.381.677* OR mh:c08.730.610*) AND (mh:"Respiration, Artificial" OR "Respiración Artificial" OR "Respiração Artificial" OR mh:e02.041.625* OR mh:e02.365.647.729* OR mh:e02.880.820* OR mh:"Ventilators, Mechanical" OR "Ventiladores Mecánicos" OR mh:e07.950* OR "Ventiladores Pulmonares" OR respiradores OR "artificial respiration" OR "mechanical ventilation" OR ventilator*)) OR mh:"Pneumonia, Ventilator‐Associated" OR "Neumonia Asociada al Ventilador" OR "Pneumonia Associada à Ventilação Mecânica" OR "Ventilator‐Associated Pneumonia" OR "Neumonía del Ventilador" OR vap) AND (mh:"Anti‐Bacterial Agents" OR antibiot* OR antibacterianos OR mh:d27.505.954.122.085* OR antibacter* OR antimicrob* OR "empiric therapy" OR "empirical therapy" OR "empiric therapies" OR "empirical therapies" OR mh:penicillins OR penicilinas OR mh:d02.065.589.099.750* OR mh:d02.886.108.750* OR mh:d03.438.260.825* OR mh:d03.605.084.737* OR mh:d04.075.080.875.099.221.750* OR penicil* OR mh:cephalosporins OR cefalosporinas OR cefalosporinas OR cephalosporin* OR mh:d02.065.589.099.249* OR mh:d02.886.665.074* OR mh:d04.075.080.875.099.221.249* OR mh:carbapenems OR carbapenem* OR carbapenémicos OR mh:d02.065.589.099.124* OR mh:d04.075.080.875.099.221.124* OR mh:aminoglycosides OR aminoglicósidos OR aminoglicosídeos OR mh:d09.408.051* OR aminoglycoside* OR mh:quinolones OR quinolone* OR quinolonas OR mh:d03.438.810.835* OR mh:clindamycin OR clindamycin* OR mh:vancomycin OR vancomycin* OR mh:aztreonam OR aztreonam OR linezolid* OR quinupristin* OR dalfopristin*) AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("clinical_trials"))
Appendix 4. CINAHL (EBSCO) search strategy
| S30 | S19 AND S29 | 140 |
| S29 | S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 | 236,015 |
| S28 | (MH "Quantitative Studies") | 11,269 |
| S27 | TI placebo* OR AB placebo* | 34,795 |
| S26 | (MH "Placebos") | 7,502 |
| S25 | (MH "Random Assignment") | 32,825 |
| S24 | TI random* OR AB random* | 129,513 |
| S23 | TI ( (singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*) ) OR AB ( (singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*) ) | 2,937 |
| S22 | TI clinic* trial* OR AB clinic* trial* | 40,833 |
| S21 | PT clinical trial | 52,728 |
| S20 | (MH "Clinical Trials+") | 132,800 |
| S19 | S12 AND S18 | 648 |
| S18 | S13 OR S14 OR S15 OR S16 OR S17 | 45,820 |
| S17 | TI empiric* N3 therap* OR AB empiric* N3 therap* | 1,072 |
| S16 | (MH "Antiinfective Agents, Quinolone+") | 2,463 |
| S15 | TI ( penicillin* or cephalosporin* or carbapenem* or aminoglycoside* or quinolone* or clindamycin* or vancomycin* or linezolid* or quinupristin* or dalfopristin* or aztreonam* ) OR AB ( penicillin* or cephalosporin* or carbapenem* or aminoglycoside* or quinolone* or clindamycin* or vancomycin* or linezolid* or quinupristin* or dalfopristin* or aztreonam* ) | 4,758 |
| S14 | TI ( antibiot* or antimicrob* ) OR AB ( antibiot* or antimicrob* ) | 23,254 |
| S13 | (MH "Antibiotics+") | 30,805 |
| S12 | S8 OR S9 OR S10 OR S11 | 2,862 |
| S11 | TI vap OR AB vap | 805 |
| S10 | TI ventilator* N5 pneumon* OR AB ventilator* N5 pneumon* | 1,641 |
| S9 | (MH "Pneumonia, Ventilator‐Associated") | 1,867 |
| S8 | S6 AND S7 | 1,582 |
| S7 | S3 OR S4 OR S5 | 20,651 |
| S6 | S1 OR S2 | 9,440 |
| S5 | TI ventilator* OR AB ventilator* | 8,093 |
| S4 | (MH "Ventilators, Mechanical") | 1,463 |
| S3 | (MH "Respiration, Artificial+") | 15,672 |
| S2 | TI pneumon* OR AB pneumon* | 6,560 |
| S1 | (MH "Pneumonia+") | 5,358 |
Appendix 5. Web of Science (Thomson Reuters) search strategy
| # 7 | 1,008 | #6 AND #5 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years |
| # 6 | 1,806,910 |
TOPIC: (random* or placebo* or crossover* or "cross over" or allocat* or ((singl* or doubl*) NEAR/1 blind*)) ORTITLE: (trial) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years |
| # 5 | 4,080 | #4 AND #3 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years |
| # 4 | 420,521 | TS=(antibiotic* or antimicrob* or (empiric* NEAR/3 therap*) or penicillin* or cephalosporin* or carbapenem* or aminoglycoside* or quinolone* or clindamycin* or vancomycin* or linezolid* or quinupristin* or dalfopristin* or aztreonam*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years |
| # 3 | 12,967 | #2 OR #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years |
| # 2 | 3,129 |
TOPIC: (vap) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years |
| # 1 | 11,483 |
TOPIC: ((ventilator* or respirator*) NEAR/3 pneumon*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years |
Data and analyses
Comparison 1. Monotherapy versus combination therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 4 | 1163 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.30] |
| 2 Clinical cure (ITT) | 2 | 350 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.56, 1.36] |
| 3 Clinical cure (CE) | 2 | 228 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.56, 1.68] |
| 4 Adverse events | 2 | 921 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.68, 1.26] |
| 5 Superinfection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Length of ICU stay | 2 | 813 | Mean Difference (IV, Fixed, 95% CI) | 0.65 [0.07, 1.23] |
1.4. Analysis.
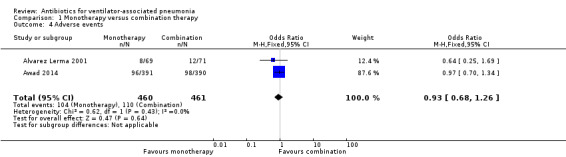
Comparison 1 Monotherapy versus combination therapy, Outcome 4 Adverse events.
Comparison 2. Combination therapy with optional adjunctives.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Cefepime versus ceftazidime | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Piperacillin‐tazobactam versus ceftazidime | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Tigecycline versus imipenem‐cilastatin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Doripenem versus imipenem‐cilastatin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Linezolid versus vancomycin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Clinical cure (ITT) | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Cefepime versus ceftazidime | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Piperacillin‐tazobactam versus ceftazidime | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Piperacillin‐tazobactam versus doripenem | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Tigecycline versus imipenem‐cilastatin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Levofloxacin versus imipenem‐cilastatin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Linezolid versus vancomycin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Clinical cure (CE) | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Cefepime versus ceftazidime | 1 | 181 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.52, 1.82] |
| 3.2 Tigecycline versus iImipenem‐cilastatin | 2 | 163 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.23, 0.84] |
| 3.3 Linezolid versus vancomycin | 1 | 434 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.97, 2.10] |
| 4 Adverse events | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Cefepime versus ceftazidime | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Piperacillin‐tazobactam versus ceftazidime | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Piperacillin‐tazobactam versus doripenem | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Tigecycline versus imipenem‐cilastatin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Levofloxacin versus imipenem‐cilastatin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Superinfections | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Piperacillin‐tazobactam versus doripenem | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Carbapenem versus non‐carbapenem.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical cure (ITT) | 3 | 598 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.11, 2.12] |
| 3 Clinical cure (CE) | 2 | 163 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.29 [1.19, 4.43] |
| 4 Adverse events | 3 | 1510 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.56, 1.09] |
| 5 Superinfections | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alvarez Lerma 2001.
| Methods | Parallel RCT Randomisation ratio: 1:1 Equivalence design: (2‐sided CI) Open‐label |
|
| Participants | N recruited = 140 N randomised = 140 (69 intervention A, 71 intervention B) N reported outcomes ITT = 140 N reported outcomes CE = 116 (57 intervention A, 59 intervention B) N reported outcomes microbiologically evaluable = 93 (49 intervention A, 44 intervention B) Inclusion criteria: "Patients were eligible for admission to the study if they were over 18 years of age, receiving mechanical ventilation and diagnosed with pneumonia in accordance with the following criteria: appearance of new and persistent radiological infiltrates or spread of previous infiltrates, unrelated to any other diagnosis, associated with the presence of one or more of the following signs and symptoms: presence of purulent secretions; leukocytosis (> 10,000 leukocytes/mm³) or leukopenia (< 5000 leukocytes/mm³); fever (temperature above 38.2°C) or hypothermia (below 36.0°C). In all cases, written consent to participate in the study was obtained from the patient or their legal representative." Exclusion criteria: "Patients were excluded from the study if they had received antibacterial treatment active against the pathogens responsible for the pneumonia in the 72 hours prior to the initial administration of the study medication, except where the infectious process had developed to the detriment of the patient, or if concomitant antimicrobial treatment active against the pathogens responsible for the pneumonia was necessary. Patients were also excluded if they had known hypersensitivity to either the study medication or to any other beta‐lactam. Other exclusion criteria included renal insufficiency, impaired hepatic function, leukopenia (< 500 cells/mm³) and pregnancy or lactation. In addition, patients were excluded if they had a life expectancy of less than one month, or a “do not resuscitate” order existed in case of cardiac arrest." Microbiological diagnosis: "Deep respiratory samples were obtained before commencing the study. One of the following three techniques was considered acceptable for obtaining respiratory samples: simple tracheal aspiration by endotracheal tube; protected bronchial brush; or bronchoalveolar lavage. All techniques were performed “blind” or directed by fibreoptic bronchoscopy. The etiology of the pneumonia was confirmed when the cultures from at least one of these techniques produced bacteria. A concentration of at least 10⁵ colony forming units (cfu)/mL was required for the samples obtained by endotracheal aspiration, at least 10³ cfu/mL for those obtained by bronchial brush and at least 10⁴ cfu/mL in those obtained by bronchoalveolar lavage." Diagnostic criteria: "Appearance of new and persistent radiological infiltrates or spread of previous infiltrates, unrelated to any other diagnosis, associated with the presence of one or more of the following signs and symptoms: presence of purulent secretions; leukocytosis (> 10,000 leukocytes/mm³) or leukopenia (< 5000 leukocytes/mm³); fever (temperature above 38.2°C) or hypothermia (below 36.0°C)." VAP definition: Not stated |
|
| Interventions | Number of study centres: "performed in 14 Spanish intensive care units" Intervention A: Study group “1 G meropenem i.v. every 8 hours” Intervention B: Control group “2 G ceftazidime i.v. every 8 hours, in combination with 15 mg/kg/day amikacin i.v., administered as two equal daily doses in those patients with normal renal function.” "The dose of amikacin in patients with impaired renal function was adjusted in proportion to creatinine clearance or plasma levels." All interventions: "The recommended treatment duration was 10 days. For inclusion in the analysis of evaluable patients, treatment duration had to exceed 72 hours and be less than 28 days." |
|
| Outcomes |
Primary outcome: Clincal cure at 24 to 72 hours after completion of treatment
Secondary outcomes: A) Clinical cure in 2 weeks following treatment "Clinical response in the 2 weeks following treatment was evaluated in 56 patients and assessed as satisfactory in 28/31 patients treated with meropenem (90.3%) and in 21/25 patients treated with ceftazidime/amikacin (84.0%) (P = 0.688)." B) Bacteriological response "Bacteriological response was assessed as satisfactory (eradication or assumed eradication) in 74.5% and 53.3% of the two treatment groups (OR 1.40; 95% CI 1.02, 1.92; P = 0.030)." C) Superinfection "Superinfection was detected in 5/57 evaluable patients treated with meropenem (8.8%) and in 3/59 patients treated with ceftazidime/amikacin (5.1%)." D) Safety
E) Mortality
|
|
| Notes |
Commercial funding/non‐commercial funding/other funding: “This study was supported by a grant from Zeneca Farma S.A., Spain.” Stated aim for study: “The study hypothesis was that meropenem given as a monotherapy is equivalent to combination therapy with ceftazidime and amikacin in terms of clinical and microbiological efficacy and safety in the treatment of nosocomial pneumonia occurring during mechanical ventilation in intensive care units.” Conflict of interest: None stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised by computer in a ratio of 1:1 in blocks of six patients, with a randomisation list being generated for each hospital." |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients ventilated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Case report forms of all randomised patients were reviewed by an independent Clinical Evaluation Committee to confirm the presence of the criteria for admission, as well as the clinical and microbiological data that enabled the cases studied to be classified. The Committee carried out this function without knowledge of the treatment group to which patients belonged." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Twelve patients (17.4%) in the meropenem group and 12 patients (16.9%) in the control group were excluded from clinical and microbiological evaluation for the following reasons: initial presence of bacteria resistant to the trial antibiotics (n = 6); violation of the pro‐ tocol (n = 8); early death (less than 72 hours from commencement of treatment) (n = 6); and adverse reactions (n = 4)." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | Supported by a grant from Zeneca Farma S.A., Spain. |
Awad 2014.
| Methods | Parallel RCT Randomisation ratio: 1:1 Non‐Inferiority design: (2‐sided CI) |
|
| Participants | N recruited = 795 (includes VAP and HAP) N randomised = 781 (391 intervention A, 390 intervention B) N reported outcomes = 781 N VAP randomised: 210 (104 intervention A, 106 intervention B) N VAP reported outcomes: ITT = 210 CE = 112 (53 intervention A, 59 intervention B) MITT = 176 (90 intervention A, 86 intervention B) Microbiological CE = 96 (46 intervention A, 50 intervention B) Inclusion criteria: “Men and women aged 18 years or older were eligible for enrolment if they had a clinical diagnosis of pneumonia after at least 72 hours of hospitalisation or stay in a chronic care facility, clinical signs or symptoms of pneumonia (at least 2 of purulent respiratory secretion, tachypnoea, or hypoxaemia), fever or leukocytosis/leukopenia, new or persistent radiographic infiltrates, and an Acute Physiology and Chronic Health Evaluation II (APACHE II) score ≥ 8 and ≤ 25. Ventilator‐associated pneumonia (VAP) was defined as pneumonia developing >48 hours after onset of mechanical ventilation. Of the 781 HAP patients enrolled, 210 had VAP.” Exclusion criteria: ”The main exclusion criteria included severe renal impairment (calculated creatinine clearance rate < 30 mL/minute or oliguria < 20 mL/hour unresponsive to fluid challenge) or hepatic dysfunction (at least 3 times the upper limit of normal for total bilirubin, alanine aminotransferase or aspartate aminotransferase), evidence of infection with ceftazidime‐ or ceftobiprole‐resistant pathogens, and clinical conditions that would interfere with efficacy assessment, such as sustained shock, active tuberculosis, lung abscess, or post‐obstructive pneumonia. With predefined exceptions, participants must not have had systemic antibiotic treatment for > 24 hours in the 48 hours before enrolment.” Diagnostic criteria: "Clinical signs or symptoms of pneumonia (at least 2 of purulent respiratory secretion, tachypnoea, or hypoxaemia), fever or leukocytosis/leukopenia, new or persistent radiographic infiltrates, and an Acute Physiology and Chronic Health Evaluation II (APACHE II) score ≥ 8 and ≤ 25" VAP definition: "Ventilator‐associated pneumonia (VAP) was defined as pneumonia developing > 48 hours after onset of mechanical ventilation." |
|
| Interventions | Number of study centres: “57 sites in Europe, North and South America, and the Asia‐Pacific region.” Intervention A: “Ceftobiprole 500 mg every 8 hours as a 120‐minute intravenous infusion, plus placebo every 12 hours as a 60‐minute intravenous infusion.” Intervention B: “Ceftazidime 2 G every 8 hours as a 120‐minute intravenous infusion plus linezolid 600 mg every 12 hours as a 60‐minute intravenous infusion. For blinding reasons, the 120‐minute infusion time was longer than the recommended infusion time in the ceftazidime label.” All interventions: “Planned treatment duration was 7 days, to a maximum of 14 days.” “Additional open‐label treatment with a fluoroquinolone or an aminoglycoside was allowed for patients at risk of pseudomonal infection.” |
|
| Outcomes | TOC visit = 7 to 14 days following the end of treatment. Primary outcome: “Clinical cure at the test‐of‐cure visit.”
Secondary outcomes: A) Microbiological eradication for HAP excluding VAP "Microbiological eradication rates at TOC for HAP (excluding VAP) patients in the microbiologically evaluable analysis set were 62.9% for ceftobiprole and 67.5% for ceftazidime/linezolid (difference, −4.6% [95% CI, ‐16.7 to 7.6])" B) Thirty‐day all‐cause mortality and 30‐day pneumonia‐specific mortality for HAP excluding VAP "For HAP (excluding VAP) patients in the ITT analysis set, 30‐day all‐cause mortality was 16.7% for ceftobiprole and 18.0% for ceftazidime/linezolid (difference, −1.2 [95% CI, −7.4 to 5.0]), and pneumonia‐specific mortality was 5.9% and 5.6%, respectively (difference, 0.3 [95% CI, −3.5 to 4.1])." C) Safety and Tolerability "Treatment‐related AEs were reported for 96 ceftobiprole patients (24.9%) and 98 ceftazidime/linezolid patients (25.4%) (Table 7). Ceftobiprole patients had fewer treatment‐related events of diarrhoea than patients treated with ceftazidime/linezolid (3.1% and 6.5%, respectively), whereas hyponatraemia was observed more frequently with ceftobiprole than with ceftazidime/linezolid (4.4% and 2.6%, respectively). Dysgeusia occurred only in patients treated with ceftobiprole (1.3%), as ceftobiprole medocaril is known to arouse a caramel taste. There were 15 treatment‐related serious AEs reported for ceftobiprole (3.9%), and 12 (3.1%) for ceftazidime/linezolid. No clinically relevant differences in other laboratory values, vital signs, physical examinations, or electrocardiograms were observed between treatment groups." VAP subgroup analyses A) “Clinical cure at the test‐of‐cure visit”
B) Microbiological eradication rates at TOC
C)Mortality‐“Thirty‐day all‐cause mortality and 30‐day pneumonia‐specific mortality” “For VAP patients, 30‐day all‐cause mortality was 26.9% for ceftobiprole and 19.8% for ceftazidime/linezolid (difference, 7.1 [95% CI, −4.3 to 18.5]), and pneumonia‐specific mortality was 8.7% and 7.5%, respectively (difference, 1.1 [95% CI, −6.3 to 8.5]).” |
|
| Notes |
Commercial funding/non‐commercial funding/other funding: “This work was supported by Basilea Pharmaceutica International Ltd, Basel, Switzerland.
” Stated aim for study: “demonstrate that ceftobiprole is non inferior to ceftazidime plus linezolid for clinical cure.” Conflict of interest: “M. E. is a full‐time employee of Basilea Pharmaceutica International Ltd. M. S. is a full‐time employee of Aptiv Solutions, providing biostatistical and data management services to Basilea Pharmaceutica International Ltd. A. H. R. has received honoraria for participating in speakers’ bureaus from MSD, Pfizer, Novartis, Thermo Fisher, Astellas, and Gilead Sciences. T. W. L. S. reports receiving compensation for costs of recruiting patients that was paid to University Hospital Rostock. G. R. has a National Institutes of Health grant pending, and has received a research grant from Basilea Pharmaceutica. All other authors report no potential conflicts." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...based on a computer‐generated randomisation schedule." |
| Allocation concealment (selection bias) | Low risk | "Participants were randomly assigned to treatment via a central interactive voice response system..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The study was conducted in a double‐blind fashion.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcomes not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Of the 247 patients who discontinued the study, 126 patients (32%) were from the ceftobiprole group, and 121 (31%) were from the ceftazidime/linezolid group. The most common reasons for discontinuation were death (77 ceftobiprole [20%] and 74 ceftazidime/linezolid [19%]), and AEs (14 ceftobiprole [4%], and 6 ceftazidime/linezolid [2%]).” |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | Supported by Basilea Pharmaceutica International Ltd, Basel, Switzerland. One author is employee of company funding the study. |
Beaucaire 1999.
| Methods | Parallel randomised open controlled clinical trial (RCT) Randomisation ratio: 1:1 Non‐Inferiority design: (2‐sided CI) |
|
| Participants | N recruited = 275 N randomised = 275 (141 intervention A, 134 intervention B) N reported outcomes = 181 (96 intervention A, 85 intervention B) ITT analysis = 275 CE = 181 (96 intervention A, 85 intervention B) MITT = 189 (105 intervention A, 84 intervention B) Microbiological CE = 96 (46 intervention A, 50 intervention B) Inclusion criteria: Men and women aged 18 years or older who had provided written informed consent either themselves or through a close relative. All patients were mechanically‐ventilated and diagnosed with nosocomial pneumonia. See definition of nosocomial pneumonia below. Exclusion criteria:
Diagnostic criteria: Nosocomial pneumonia defined as: a) chest radiograph showing either pulmonary consolidation with an air bronchogram, or pulmonary infiltrates, or worsening of a pre‐existing radiographic image; b) more than 1 degree Celsius increase of body temperature compared top previous assessment, with a temperature equal or higher than 38.3 degrees Celsius or hypothermia (=/< 36.5); c) one of the following symptoms: mucupurulent or purulent bronchial secretions (> 25 leucocytes and <10 squamous epithelial cells per field), hyper‐leucocytosis (=/> 12,000/mm3, leucopenia (=/<5,000/mm3). The "Indice de gravité simplifié (IGS I)" (Simplified Acute Physiology Score) was =/> 7 at time of inclusion in the study. VAP definition: See above |
|
| Interventions | Number of study centres: 62 sites in France Intervention A: Cefepime + amikacin Cefepime 2 G every 12 hours, administered per IV infusion over 30 minutes Amikacin dose 15 mg/kg/day, every 12 hours, administered IV over 30 minutes, adjusted according to serum levels Intervention B: Ceftazidime + amikacin Ceftazidime 2 G every 8 hours, administered per IV infusion over 30 minutes Amikacin dose 15 mg/kg/day, every 12 hours, administered IV over 30 minutes, adjusted according to serum levels All interventions: Planned treatment duration was 14 consecutive days (not less than 5 days or more than 28 days) for cefepime and ceftazidime. For amikacin the maximum duration of treatment was 10 days. For all treatments the dose was adjusted in case of renal impairment. In case of a documented MRSA infection, glycopeptides were authorised as an adjunct treatment from day 2 to 3. Also from day 2 to 3 addition of imidazoles, antifungals or antivirals was permitted. In the context of decontamination of the digestive tract, only the following oral treatments were permitted: colimycin, polymyxin, tobramycin, gentamicin. |
|
| Outcomes | Outcomes were assessed at the end of treatment. In case results of a culture reveal that the patient carries a bacteria that is resistant to the allocated cephalosporin, the patient was considered a primary treatment failure in the ITT analysis. Primary outcome:Clinical cure Clinical cure was defined as complete remission of local and systemic signs and symptoms of pneumonia, without addition at days 2‐3 of other antibiotics and without relapse. Secondary outcomes:
|
|
| Notes | No specific timeframe for onset of the VAP was defined, however it was reported that 57 patients in the cefepime group and 55 in the ceftazidime group had been ventilated less than 4 days prior to enrolling in the study. Study was funded by Bristol‐Myers Squibb, 92044 Paris La Defense, France. One of the authors was affiliated with Bristol‐Myers Squibb. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients were unblinded (ventilated). Doctors were unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All patient charts were reviewed by a committee that was blinded to the allocated treatments. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rate (94/275, 34%). Reasons for attrition reported: 54/275 drug resistant bacteria; 16/275 major protocol violation; 24/275 treatment less than 5 days. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | Study was funded by Bristol‐Myers Squibb. One of the authors was affiliated with Bristol‐Myers Squibb. |
Brun‐Buisson 1998.
| Methods | Parallel randomised controlled clinical trial (RCT) Randomisation ratio: 1:1 Equivalence design: (2‐sided CI) |
|
| Participants | N recruited = 204 N randomised = 197 (98 intervention A, 99 intervention B) N reported outcomes = 127 (58 intervention A, 69 intervention B) N micro‐confirmed VAP = 127 (58 intervention A, 69 intervention B) N per‐protocol (after secondary exclusion) = 115 (51 intervention A, 64 intervention B) Inclusion criteria: “Patients hospitalised for ≥ 72 hours and having undergone mechanical ventilation for at least 48 hours were eligible for inclusion in the study when clinically suspected of having VAP.” “The protocol required that one or several specific sampling techniques, followed by quantitative cultures, be used before inclusion of a patient in the study. Any one of the following three techniques was considered acceptable for obtaining respiratory tract secretions: bronchoalveolar lavage [23], protected specimen brush sampling via bronchoscopy [24], or protected telescoping catheter sampling performed blindly or via fibreoptic bronchoscopy [25].” Exclusion criteria: “Patients were not eligible if they were diagnosed as having AIDS, a hematologic malignancy, or severe neutropenia (< 500 polymorphonuclear cells/mm³) or had a history of documented allergy to β‐lactam antibiotics. Likewise, patients were not eligible ( l ) if death was expected within 7 days of inclusion or a do‐not‐resuscitate order had been written or (2) if they had a severity score (simplified acute physiology [SAPS 11] score) [20] on inclusion higher than 50 and three or more organ failures [21] or a rapidly fatal underlying disease [22]. In addition, patients with suspected or documented tuberculosis, suspected or documented infection due to MRSA only, or a concomitant infection requiring other antimicrobial therapy (or that had necessitated the recent [<48 hours previously] introduction of antibiotics) were not eligible.” Secondary exclusion criteria: “Although therapy was often initiated because of a clinical suspicion of VAP , only patients with microbiologically confirmed VAP were subsequently retained in the primary efficacy analysis.” “Patients whose samples yielded MRSA only were also secondarily excluded. However, patients having infection caused by both MRSA and other organisms susceptible to the assigned study drug regimen were given vancomycin in addition and were retained in the efficacy analysis.” Diagnostic criteria: “The criteria for clinical suspicion of VAP included all of the following: clinical signs of sepsis (new fever, increase in temperature over 38.2°C, or decrease below 36.5°C; and increase in WBC count to > 10,000/mm³); purulent tracheal aspirates; and a new infiltrate or otherwise unexplained persistence or worsening of preexisting infiltrates on chest radiographs.” VAP definition: "Mechanical ventilation for at least 48 hours." |
|
| Interventions | Number of study centres: “27 intensive care units in France." Intervention A: “Fixed combination of piperacillin and tazobactam (4 G of piperacillin and 500 mg of tazobactam q.i.d.).” “The β‐lactam drug was expected to be administered for 15 days, or up to 21 days for patients with difficult‐to‐treat organisms.” Intervention B: “ceftazidime (l G q.i.d.)” All interventions: “both in combination with amikacin ( 15 mg/[kg · d] in two divided doses for patients with normal renal function).” “Amikacin dosage was adapted to renal function according to nomograms and trough serum levels. Amikacin was expected to be given for at least l0 days to patients with infection involving P. aeruginosa and for at least 5 days to other patients.” |
|
| Outcomes |
Primary outcome:"The primary endpoint was clinical cure at 6‐ 8 days after the end of therapy" A) Clinical outcome: all patients with VAP “the overall success rate was 48% in the TAZ group and 33% in the CAZ group (OR, 2.14; 95% CI, 0.5%‐29.5%).” B) Clinical outcome: per‐protocol analysis “26 TAZ recipients (51%) and 23 CAZ recipients (36%) had a successful clinical and bacteriologic outcome, as assessed by the CEC; the difference in efficacy rate was 15% (OR, 1.85; 95% CI, ‐0.2%‐ 30.2%), favouring TAZ recipients. Since the difference in efficacy rate did not exceed 15%, the two regimens were found to be of equivalent clinical efficacy, according to the hypothesis tested.” Secondary outcomes: A) Treatment failures
B) Mortality
C) Safety
|
|
| Notes |
Commercial funding/non‐commercial funding/other funding: “Grant support: Wyeth‐Lederle, France.
” Stated aim for study: compare “clinical efficacy and safety of piperacillin‐tazobactam (TAZ; 4 G/0.5 G q.i.d.) and of ceftazidime (CAZ; 1 G q.i.d.), both combined with amikacin (7.5 mg/kg b.i.d.), as therapy for ventilator‐associated pneumonia (VAP; acquired after ≥ 48 hours of mechanical ventilation).” (q.i.d. = four times a day; b.i.d. = two times a day) Conflict of interest: None stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomised by centre in blocks of four, according to a computer‐generated randomisation list.” |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants ventilated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “All case report forms from all patients randomised were reviewed by a Clinical Evaluation Committee (CEC), which examined the adequacy of criteria for inclusion and diagnosis of VAP and the clinical and microbiological data relevant to outcome; the CEC members were blind to the treatment group assignment.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Of 204 patients randomised in the study, 197 received at least one dose of either drug regimen tested according to the protocol and were evaluable with regard to tolerance (98 TAZ and 99 CAZ recipients); 127 patients (64.5%) had microbiologically confirmed VAP (58 TAZ and 69 CAZ recipients). From this group, 12 patients were excluded because of infection caused by MRSA only (n = 5) or because of a major protocol violation (n = 7), i.e., use of concomitant antimicrobial therapy not allowed by the protocol. Thus, 115 patients (51 TAZ and 64 CAZ recipients) with confirmed VAP were evaluable as per‐protocol, according to the CEC.” |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | Study funded by Wyeth‐Lederle, France. Authors affiliated with Wyeth‐Lederle. |
Damas 2006.
| Methods | Factorial randomised controlled clinical trial (RCT) Randomisation ratio: 1:1:1 Equivalence design: (2‐sided CI) |
|
| Participants | N recruited = 74 N randomised = 74 (24 intervention A, 26 intervention B, 24 intervention C) N VAP confirmed= 59 (20 intervention A, 19 intervention B, 20 intervention C) N reported outcomes = 59 Inclusion criteria: “Patients were eligible for this study if they were older than 18 years, were mechanically‐ventilated for more than 48 hours and developed clinical evidence of VAP as defined by new and persistent radiographic infiltrate for at least 48 hours with at least three of the following: body temperature > 38°C or < 36°C; white blood cells > 10,000 mm³ or < 4,000 mmª; macroscopically purulent tracheal aspirate; increase in CRP level of at least 50 mg/l within the last 24 hours. The PaO2/FiO2 ratio was also obtained in order to calculate a modified version of the CPIS [15], with a score superior to 6 considered as a high probability of VAP (Table 1). In addition, VAP had to be confirmed by culture of pathogens from the tracheal aspirate. Quantitative bacteriology was not required but, when obtained, growth of ≥ 10⁵ in tracheal aspirate or 10⁴ in bronchoalveolar lavage confirmed VAP. ” Exclusion criteria: “Exclusion criteria included: patients already treated for another infection or having received antibiotic treatment during the last 15 days; patients with organ transplantation or suffering from hematological malignancy; and patients with a life expectancy of less than two days.” Diagnostic criteria: "Clinical evidence of VAP as defined by new and persistent radiographic infiltrate for at least 48 hours with at least three of the following: body temperature > 38°C or < 36°C; white blood cells > 10,000 mmª or < 4000 mm³; macroscopically purulent tracheal aspirate; increase in CRP level of at least 50 mg/L within the last 24 hours." VAP definition: "Mechanically ventilated for more than 48 hours." |
|
| Interventions | Number of study centres: Single centre ‐ “University Hospital of Liege Sart‐Tilman, Belgium.” Intervention A: group C “Cefepime only (2 G every 8 hours) for 8 to 10 days; this dose was reduced if necessary according to the clearance of creatinine.” Intervention B: group C‐A “Cefepime combined with amikacin (20 mg/kg, once daily) for 5 days, with adaptation to the level of the clearance of creatinine by increasing the delay between doses.” Intervention C: group C‐L “Cefepime associated with levofloxacin (750 mg once daily) for 8 to 10 days.” All interventions: “Cefepime could be changed to a narrower spectrum beta‐lactamine in the case of susceptible agent or to imipenem in the case of resistance; amikacin or levofloxacin were kept during the entire course except if the pathogen was found to be resistant. The attending physician could overrule the protocol in the case of multidrug resistance.” |
|
| Outcomes |
"The efficacy of treatments was evaluated during treatment by the evolution in inflammatory parameters: PaO2/FiO2, temperature, leukocytosis and CRP level were measured each day for eight days. The improvement or worsening of the patient was also assessed by the change in SOFA score [16]. Eradication from or persistence of bacteria in tracheal aspirate was documented." Primary outcome:“The efficacy of treatments was evaluated during treatment by the evolution in inflammatory parameters” "There were no significant differences in the evolution of PaO2/ FiO2, temperature, leukocytosis and CRP level between the three groups." Secondary outcomes: A) “Eradication from or persistence of bacteria in tracheal aspirate” “Within 3 to 5 days after the start of therapy, new endotracheal samples were obtained from 70% of group C, 89% of group C‐A and 85% of group C‐L: the same bacteria as those found on day 1 were still present in the sputum of 8 patients in group C, 4 in group C‐A and 12 in group C‐L. After 7 to 10 days, persistence was documented in 4 patients out of 16 in group C, 5 out of 18 in group C‐A, and 3 out of 13 in group C‐L. New bacteria strains requiring new treatment were found in one patient in group C (one P. aeruginosa), three patients in group C‐A (two P. aeruginosa and one Enterobacter aerogenes with extended spectrum beta‐lactamase) and in three patients in group C‐L (one Proteus mirabilis, one Serratia marcescens and one methicillin‐resistant S. aureus).” A) Length of ICU stay “The length of ICU stay after the occurrence of infection was not different between the three groups: the medians (and 25th to 75th percentile in parentheses) were 15 (7.5 to 24.75), 16 (9 to 21) and 14 days (9.5 to 21.5) for groups C, C‐A and C‐ L, respectively.” B) VFDs “There was also no difference between VFDs within 28 days after infection: the number of VFDs for each group was 16.1 ± 8.3 VFDs after C treatment, 12.6 ± 8.1 VFD after C‐A treatment and 12.6 ± 10.4 VFD after C‐L treatment (P > 0.05).” C) Mortality at 28 days “Ten patients died within 28 days, 2 in the C group (10%), 4 in the C‐A group (21%) and 4 in the C‐L group (20%).” "Among the 15 patients with no microbiologically confirmed VAP, there was 1 other death within 28 days, in the C‐A group." |
|
| Notes |
Commercial funding/non‐commercial funding/other funding: Not stated. Stated aim for study: “The aim of the present study was to compare the clinical outcome and the course of biological variables in patients treated for a VAP, using a monotherapy with a beta‐lactam versus a combination therapy.” Conflict of interest: “The authors declare that they have no competing interests.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients ventilated. “Weaknesses of this study include a lack of blinding of administration to therapy.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding not reported but outcomes are objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all participants with confirmed VAP. "Seventy‐four patients fulfilling the clinical VAP criteria were randomised into three groups. Of these, 24 patients received cefepime only (group C), 26 received cefepime with amikacin (group C‐A) and 24 received cefepime with levofloxacin (group C‐L). Pneumonia was not microbiologically confirmed in 15 of these patients: 4 in the C group, 7 in the C‐A group and 4 in the C‐ L group." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Funding not reported but authors state no conflict of interest. |
Freire 2010.
| Methods | Parallel randomised controlled clinical trial (RCT) Randomisation ratio: 1:1 Noninferiority: (2‐sided CI) |
|
| Participants | N recruited = 979 (includes HAP and VAP) N randomised = 945 (474 intervention A, 471 intervention B) N reported outcomes modified ITT (received at least 1 dose)= 934 (467 intervention A, 467 intervention B) N cm‐ITT = 869 (440 intervention A, 429 intervention B) N CE = 511 (268 intervention A, 243 intervention B) N MITT = 531 (263 intervention A, 268 intervention B) N Microbiologically evaluable = 383 (194 intervention A, 189 intervention B) N Modified ITT VAP = 253 (131 intervention A, 122 intervention B) N Clinical modified ITT VAP = 243 (127 intervention A, 116 intervention B) N CE VAP = 140 (73 intervention A, 67 intervention B) Inclusion criteria: “Inclusion requirements included age > 18 years, onset of symptoms > 48 h after admission or < 7 days after discharge (if initial hospitalisation was > 3 days), and a new or evolving infiltrate on chest X‐ray. Minimum disease requirements included fever or leukocytosis/leukopenia along with respiratory failure requiring mechanical ventilation or at least 2 of the following: cough, dyspnea or tachypnoea, pleuritic chest pain, auscultatory findings of rales or evidence of consolidation, hypoxemia, and purulent sputum or change in sputum character.” Exclusion criteria: “Exclusion criteria included antibacterial drugs administered for > 24 h to treat the current episode of suspected HAP unless a repeat respiratory culture showed that a pathogen was resistant to that agent and/or the patient had worsening or no improvement in clinical signs and symptoms of pneumonia, HIV positive, on immunosuppressive therapy, APACHE II score > 30, cystic fibrosis, pulmonary malignancy, postobstructive pneumonia, bronchiectasis, sarcoidosis, pulmonary abscess, empyema, active tuberculosis, and infections known to be caused by Legionella, Pneumocystis, or mycobacteria. Additional exclusions included absolute neutrophil count < 1 × 109/L, aspartate aminotransferase or alanine aminotransferase > 10× upper limit of normal (ULN) or bilirubin or alkaline phosphatase > 3× ULN, creatinine clearance (CL) < 41 mL/min per 1.73 m², or hypersensitivity to any of the agents that could be used in the trial.” Diagnostic criteria: “A new or evolving infiltrate on chest X‐ray. Minimum disease requirements included fever or leukocytosis/leukopenia along with respiratory failure requiring mechanical ventilation or at least 2 of the following: cough, dyspnea or tachypnoea, pleuritic chest pain, auscultatory findings of rales or evidence of consolidation, hypoxemia, and purulent sputum or change in sputum character.” VAP definition: Not Stated. |
|
| Interventions | Number of study centres: This trial was conducted in 138 sites in 31 countries. Intervention A: “An initial tigecycline dose of 100 mg intravenously (iv) followed by 50 mg every 12 h and optional adjunctive therapy with ceftazidime 2 G iv every 8 h for P. aeruginosa coverage.” Intervention B: “An initial tigecycline dose of 100 mg intravenously (iv) followed by … imipenem/cilastatin 500 mg to 1 G iv every 8h, and optional adjunctive therapy with vancomycin 1 G iv every 12 h for MRSA coverage.” All interventions: "7 to 14 days of therapy." “Doses for imipenem/cilastatin and adjunctive therapies were in accordance with local labelling and standard practices. Total daily dosages of imipenem were at the discretion of the investigator dependent upon the severity of the infection and the presence of known or suspected organisms, and the doses of imipenem and the adjunctive therapies could be adjusted based upon weight and/or the calculated creatinine CL. If needed for double coverage of P. aeruginosa, an aminoglycoside could be added to either regimen.” “Placebo treatments were administered at appropriate time points to ensure blinding of the treatment regimens. For adjunctive therapies, again to maintain blinded conditions, vancomycin or placebo was given in 12‐h multiples, based on renal function. Similarly, ceftazidime or placebo was administered every 8 h or every 12 h. Because an aminoglycoside was allowed for patients randomised to tigecycline or imipenem/cilastatin, blinding of that agent was not required.” |
|
| Outcomes |
Primary outcome:Clinical cure at the TOC assessment
Secondary outcomes: A) Adverse events
B) Serious AEs
C) Mortality
D) Discontinuations "A total of 82 (8.8%) patients discontinued study drug because of an AE. Overall discontinuation rates due to an AE were significantly (P = 0.028) higher in the tigecycline (10.9%) compared with the imipenem/cilastatin treatment group (6.6%). This difference generally relates to cure/ failure rates and progression/complications of underlying diseases. Median time to discontinuation was 2.0 days for both treatment groups. AEs leading to discontinuation of treatment in 3 or more patients in the tigecycline group were pneumonia (5 patients, 1.1%), respiratory failure (4 patients, 0.9%), and peritonitis, vomiting, septic shock, and heart arrest (3 patients each, 0.6%). In the imipenem/cilastatin group, 3 patients (0.6%) discontinued due to rash. There were no significant differences between treatment groups for any single AE in the number of patients discontinuing treatment because of an AE." E) "Clinical laboratory, vital signs, and ECG evaluations"
F) Pharmacokinetic/pharmacodynamic evaluation "Pharmacokinetic evaluation was possible in 202 patients, including 71 VAP patients." "The mean AUC observed in VAP patients was 15% lower (P = 0.041) than the value observed in non‐VAP patients, although there was a large overlap (Table 10). A total of 60 patients, including 22 VAP patients, also had isolates for which MIC values could be determined. The median fAUC0–24/MIC observed in VAP patients was 60% lower (P = 0.002) than the value observed in non‐VAP patients (Table 10)." VAP‐only data A) VAP patient cure rates in CE and cm‐ITT groups
B) Length of stay in ICU "For the VAP population, there were no significant differences between the 2 treatment groups in the length of stay in the intensive care unit (ICU) (P = 0.937) during the primary hospitalisation." |
|
| Notes |
Commercial funding/non‐commercial funding/other funding: “This study was sponsored and funded by Wyeth Research, Collegeville, PA, which was acquired by Pfizer Inc in October 2009.” Stated aim for study: “The primary objective of this study was to compare the efficacy and safety of a tigecycline ± ceftazidime ± aminoglycoside regimen with that of an imipenem/cilastatin ± vancomycin ± aminoglycoside regimen to treat patients with HAP.” Conflict of interest: "Phil Vinall, a former Wyeth employee, assisted in the preparation of the preliminary draft of this article. Additional editorial support was provided by Upside Endeavors, LLC (Sanatoga)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Study drugs were prepared by an unblinded third party, and blinding was maintained at all times during the course of patient treatment.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Bliniding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Of the 979 patients screened, 34 were screen failures. A total of 945 patients comprised the randomised (ITT) population. A total of 934 patients (467 in each treatment group) were randomly assigned and received at least 1 dose of assigned treatment, comprising the modified intent‐to‐ treat (mITT or safety) population. Of these, the CE subset of patients included 268 patients treated with tigecycline and 243 patients treated with imipenem/cilastatin (Fig. 1). The most common reasons for exclusion from the CE population (n = 358) were that patients received more than 24 h of antibiotics before the first dose of study medication without having failed that regimen (23.6%) and that patients did not have a clinical response evaluation at the TOC visit (10.1%)." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | Study was sponsored and funded by Wyeth Research. One author was employee of Wyeth Research. |
Heyland 2008.
| Methods | Factorial RCT Randomisation ratio: 1:1 Superiority: (2‐sided CI) |
|
| Participants | N recruited = 1144 N randomised = 740 (369 intervention A, 371 intervention B) N reported outcomes = 739 Inclusion criteria: “Adult patients who were mechanically ventilated in ICU for ≥ 96 hrs were potentially eligible if they developed suspected pneumonia while they were intubated and ventilated. Suspected pneumonia was defined by the presence of new or persistent radiographic features suggestive of pneumonia without another obvious cause and any two of the following: fever > 38°C, leukocytosis (> 11.0 x 10⁹/L) or neutropenia (< 3.5 x 10⁹/L), purulent endotracheal aspirate secretions, recent isolation of pathogenic bacteria from the endotracheal aspirates, and increasing oxygen requirements.” Exclusion criteria: “We excluded patients who were immunocompromised; considered to be unsuitable for bronchoscopy by the attending physician; allergic to penicillins, cephalosporins, carbapenems, or ciprofloxacin; infected or colonised with pseudomonas species or methicillin‐resistant Staphylococcus aureus; recent recipients of study drugs (ciprofloxacin within 24 hours and meropenem within 7 days before enrolment); expected to die or undergo withdrawal of treatment within 72 hours after enrolment; unlikely to leave the ICU within 3 weeks; pregnant or lactating; or previously enrolled in this or another interventional trial.” Diagnostic criteria: "Pneumonia was defined by the presence of new or persistent radiographic features suggestive of pneumonia without another obvious cause and any two of the following: fever > 38°C, leukocytosis (> 11.0 x 10⁹/L) or neutropenia (< 3.5 x 10⁹/L), purulent endotracheal aspirate secretions, recent isolation of pathogenic bacteria from the endotracheal aspirates, and increasing oxygen requirements.” VAP definition: "Mechanically ventilated in ICU for ≥ 96 hrs." |
|
| Interventions | Number of study centres: “Twenty‐eight intensive care units in Canada and the United States.” Intervention A: “Meropenem (AstraZeneca) 1 G every 8 hrs and ciprofloxacin (Bayer) 400 mg every 12 hrs.” Intervention B: “Meropenem alone.” All interventions: “We protocolised the mandatory review of culture results and adjustment of antibiotics; physicians were requested to adjust antibiotic therapy according to these results (targeted therapy) as soon as possible. In both groups, if patients had a positive culture result, physicians were recommended to prescribe a single‐antibiotic with the narrowest spectrum that had activity against the infecting organism. As this was a trial of empirical therapy, we did not specify the choice, dose, or duration of subsequent antibiotics.” |
|
| Outcomes |
Primary outcome:28‐day all‐cause mortality “Overall mortality at 28 days was 18.7% (95% confidence intervals 15.9% to 21.7%). The relative risk of 28‐day mortality in the combination group versus monotherapy group was 1.05 (0.78–1.42, P = .74; Table 4) after stratification for APACHE II and diagnostic technique. There was no evidence that the effect of the treatment was different between the two diagnostic groups (test of interaction P=.37), and there was no effect of bronchoscopy or endotracheal aspirates on mortality.” Secondary outcomes: A) Duration of ventilation, length of ICU stay, length of hospital stay “There were no differences between the combination and monotherapy groups in the median (IQR) time from randomisation to discontinuation of mechanical ventilation alive (8.7 [3.8 –24.8] versus 9.3 [3.8 –21.6] days, P = .79), discharge from ICU alive (12.1 [6.4 – 35.2] versus 12.8 [6.1–27.0] days, P = .84), or discharge from hospital alive (45.8 [24.0 – 316.8] versus 39.1 [19.7 to undefined] days, P = .49).” B) Adequacy of initial treatment “The proportion of patients who received adequate initial antibiotics was significantly greater in the combination group than in the monotherapy group (93.1% versus 85.1%, P = .01).” C)Emergence of resistant organisms “Of the 412 patients who had positive enrolment cultures, 38 (9.2%) acquired resistance to a single‐antibiotic class during the study (9.1% of patients in combination group and 9.3% in the monotherapy group, P = .99). Rates of colonisation of sputum with Pseudomonas species, MRSA, Acinetobacter species, vancomycin‐resistant enterococci, or any multidrug‐resistant organisms (resistant to two or more drug classes) and yeast were not significantly different between groups.” D) Rates of infection due to Clostridium difficile “C. difficile toxin was isolated from stool in 5.4% of patients receiving combination therapy and 7.6% of patients in the monotherapy group during the study period (P = .65)." Subgroup analysis “Fifty‐six patients who had at least one Pseudomonas, Acinetobacter, or another multidrug‐resistant Gram‐negative organism present in the enrolment cultures.” A) Duration of ventilation, ICU stay, Hospital mortality “In this subgroup of 56 patients with multidrug‐resistant Gram‐negative bacilli at enrolment, we also observed trends toward greater rate of eradication of infecting microorganisms, a shorter duration of mechanical ventilation and ICU stay, and lower ICU and hospital mortality in the combination therapy group.” B) Adequacy of initial treatment “In this subgroup, we observed a significant difference in the rate of adequacy of empirical antibiotic therapy favouring combination therapy over monotherapy (84.2% versus 18.8%, P = .001).” C) Emergence of resistant organisms “Among the 56 patients in the group who grew multidrug‐resistant Gram‐negative bacilli at enrolment, cultures from 30 of 33 (90.9%) patients, whose specimens were tested for susceptibility to meropenem, were susceptible in the combination group compared with 13 of 15 (86.7%) patients in the monotherapy group (P = .64).” |
|
| Notes |
Commercial funding/non‐commercial funding/other funding: “Supported, in part, by grants from the Canadian Institutes of Health Research and Physicians Services Inc. of Ontario and unrestricted grants from AstraZeneca Inc. and Bayer Inc., Ontario, Canada. The sponsors had no role in the conception, design, data collection, analysis, or interpretation of the results.” Stated aim for study: “To compare a strategy of combination therapy with a strategy of monotherapy with broad‐spectrum antibiotics for suspected late ventilator‐associated pneumonia.” Conflict of interest: “Dr. Heyland has received less than $10,000 in lecture fees from AstraZeneca. Dr. Muscedere has received honoraria from AstraZeneca. Drs. Heyland and Muscedere have received research grants for $50,000 from AstraZeneca and $80,000 from Bayer Pharmaceuticals. The remaining authors have not disclosed any potential conflicts of interest.” Heyland 2008 describes their study as factorial design but it is unclear to us how this is a factorial design study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomised using a central telephone system with a variable undisclosed block size.” |
| Allocation concealment (selection bias) | Low risk | “The strengths of our trial include the use of concealed randomisation.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants ventilated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “One important limitation is that the trial was necessarily unblinded; however, we minimised bias by protocolising patient management and outcome ascertainment using standardised definitions.” Outcomes are objective measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “One patient withdrew consent 2 days after randomisation and was excluded from all analyses.” |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | Independent funding but authors linked to pharmaceutical companies. |
Kollef 2004.
| Methods | Secondary analysis of 2 randomised controlled clinical trials (RCT) Randomisation ratio: 1:1 "Combining data from the two studies is appropriate because the protocols were identical, approximately one‐ half of the investigators were the same, and no baseline differences between the two study populations were found." |
|
| Participants | N recruited = 1030 (includes HAP and VAP) N randomised = 1030 N reported outcomes = 1019 (524 intervention A, 496 intervention B)‐ 11 patients did not receive study drugs N VAP ITT = 544 (282 intervention A, 262 intervention B) N VAP ITT Gram‐positive = 264 (134 intervention A, 130 intervention B) N VAP ITT S aureus = 221 (110 intervention A, 111 intervention B) N VAP ITT MRSA = 91 (44 intervention A, 47 intervention B) Inclusion criteria: "Adult men and women with pneumonia acquired after 48 h in an inpatient facility were eligible for enrolment. Patients had to have at least two of the following: cough; purulent sputum; auscultatory findings of pneumonia; dyspnea, tachypnoea, or hypoxemia; and isolation of a respiratory pathogen from respiratory or blood cultures. Patients also had to have at least two of the following: fever or hypothermia, respiratory rate higher than 30 breaths/min, systolic blood pressure less than 90 mmHg, pulse rate 120 beats/min or higher, altered mental status, need for mechanical ventilation, total peripheral white blood cell count greater than 10,000/ mm³ or less than 4,500/mm³, and more than 15% immature neutrophils. Patients had to have radiographic findings of pneumonia (new or progressive infiltrates, consolidation, or pleural effusion), adequate respiratory and sputum specimens for Gram’s stain and culture, and life expectancy of at least 7 days." "Acceptable culture methods included endotracheal suction specimen, and blood cultures as well as “invasive methods” such as protected specimen brush, bronchoalveolar lavage, and thoracentesis. Blood cultures and thoracentesis with an identified Gram‐positive pathogen (e.g., MRSA) and bronchoalveolar lavage or protected specimen brush cultures yielding a quantitative culture of 10³ and 10⁴ cfu/ml, respectively, were employed to establish the presence of infection." Exclusion criteria: "Exclusion criteria included infecting Gram‐positive organism resistant to either study medication." From Rubinstein 2001 and Wunderink 2003: "Exclusion criteria were infection with pathogens resistant to study medication; meningitis, endocarditis, or osteomyelitis; CD4 cell count ˜200 cells/mm³ secondary to HIV infection; previous antibiotic treatment for > 24 hours, unless documented treatment failure or pathogen resistance to previous non study antibiotic therapy was present; liver disease and total bilirubin > 5 times the upper limit of normal; and severe neutropenia (< 500 cells/mm³)." Diagnostic criteria: "Patients had to have at least two of the following: cough; purulent sputum; auscultatory findings of pneumonia; dyspnea, tachypnoea, or hypoxemia; and isolation of a respiratory pathogen from respiratory or blood cultures. Patients also had to have at least two of the following: fever or hypothermia, respiratory rate higher than 30 breaths/min, systolic blood pressure less than 90 mmHg, pulse rate 120 beats/min or higher, altered mental status, need for mechanical ventilation, total peripheral white blood cell count greater than 10,000/ mm³ or less than 4500/mm³, and more than 15% immature neutrophils. Patients had to have radiographic findings of pneumonia (new or progressive infiltrates, consolidation, or pleural effusion)." VAP definition: Greater than 48 hours. |
|
| Interventions | Number of study centres: "Multinational study with 134 sites." Intervention A: "600 mg linezolid... administered by intravenous infusion every 12 h for 7–21 consecutive days." Intervention B "1 G vancomycin administered by intravenous infusion every 12 h for 7–21 consecutive days." "Vancomycin dosage adjustments were required for patients with renal impairment and were permitted for other patients according to the local standard of care. If drug monitoring for vancomycin was performed, trough serum values were to be obtained not more than 1 h before the next dose, and peak serum levels were to be obtained 1–2 h after completion of the intravenous dose. A trough target of 5–10 μg/ml was recommended, and a peak target of 25–40 μg/mL was recommended." All interventions: "All patients received concurrent aztreonam 1–2 G every 8 h for possible Gram‐negative infection; aztreonam therapy could be discontinued if no Gram‐negative pathogens were identified. If only Gram‐negative pathogens were identified, the patient was dropped from the study." |
|
| Outcomes |
Primary outcome:Clinical Cure
Secondary outcomes: A) Bacterial eradication
B) Survival
|
|
| Notes |
Commercial funding/non‐commercial funding/other funding: “This study was supported by a grant from Pharmacia Corporation, Peapack, N.J., USA.” Stated aim for study: “To assess the effect of baseline variables, including treatment, on clinical cure and survival rates in patients with Gram‐positive, ventilator‐associated pneumonia (VAP).” Conflict of interest: None Stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐bind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not stated. "The physicians and investigators caring for patients and making clinical assessments were completely blinded to vancomycin serum levels and dosing changes." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Clinical outcome was missing at follow‐up in 41 linezolid and 46 vancomycin recipients for the following reasons: death (n =14 and n =24), loss to follow‐up and other administrative reasons (n= 18 and n= 11), isolation of Gram‐negative pathogens only (n =6 and n =4), and adverse events (n =3 and n =7). Clinical outcome was indeterminate at follow‐up in 11 linezolid and 12 vancomycin recipients; these patients were assessed as cured or improved at their EOT visit." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | Study supported by a grant from Pharmacia Corporation, Peapack, NJ, USA. |
Kollef 2012.
| Methods | Parallel randomised controlled clinical trial (RCT) Randomisation ratio: 1:1 Non‐inferiority design: (2‐sided CI) |
|
| Participants | N recruited = 274 N randomised = 274 (137 intervention A, 137 intervention B) N reported outcomes = 274 N ITT = 227 (115 intervention A, 112 intervention B) N MITT = 167 (79 intervention A, 88 intervention B) (Gram‐negative only) Inclusion criteria: “Male and nonpregnant female patients aged ≥ 18 years were eligible for enrolment if they had pneumonia acquired after at least 48 hours of mechanical ventilation, had been hospitalised or been in a chronic care facility for a total of 5 or more days within the last 90 days, and had a baseline Clinical Pulmonary Infection Score (CPIS) ≥ 6 and an Acute Physiology and Chronic Health Evaluation (APACHE) II score > 8 and < 35. Patients were required to have new or worsening radiographic infiltrates consistent with VAP and at least one of the following: fever (in the absence of fever‐reducing agents) defined as a rectal temperature greater than 39°C or an increase in core temperature of greater than 1°C; hypothermia, defined as a rectal/core body temperature of less than 35°C; or white blood cell count > 10,000 cells/mm³.” Exclusion criteria: “Patients were excluded if they had received antibiotics for the current episode of VAP for >24 hours before study drug was assigned, had known presence at baseline of only methicillin‐resistantStaphylococcus aureus (MRSA) or Stenotrophomonas maltophilia infection, or acute respiratory distress syndrome. Patients were also excluded if they had any of the following conditions that could interfere with the assessment or interpretation of the diagnosis of VAP or response to therapy: chest trauma with severe lung bruising or loss of stability of the thoracic cage following a fracture of the sternum, ribs, or both, pleural effusion or empyema requiring drainage, lung cancer within the last 2 years, chronic bronchitis with an increase in disease severity within the last 30 days, chronic enlargement of the bronchi or bronchioles related to inflammatory disease or obstruction, lung abscess(s), anatomical bronchial obstruction, respiratory tuberculosis on treatment, suspected atypical pneumonia, chemical pneumonitis (e.g., aspiration of gastric contents, inhalation injury), cystic fibrosis, congestive heart failure, active seizure disorder within the last 2 years or brain injury such that imipenem‐cilastatin would not be administered to the patient in usual practice, severe burns to greater than 15% of the body, evidence of severe and chronic liver disease indicating cirrhosis in the opinion of the investigator, and a history of hypersensitivity reactions to carbapenems, penicillins, other beta‐lactam antibiotics, or beta‐lactamase inhibitors.” MITT exclusion criteria: “A sample of fluid from each patient had to be obtained for Gram stain and culture by bronchoalveolar lavage (BAL) or mini‐BAL prior to administration of study drug. Patients were started on empiric study drug therapy prior to the results of the culture being known. Patients whose culture results from the BAL/mini‐BAL yielded at least one bacterial pathogen that grew at a density of ≥ 10⁴ colony forming units/mL and with an imipenem‐cilastatin minimum inhibitory concentration (MIC) ≤ 8 μg/mL based on local MIC testing (or missing imipenem‐cilastatin MIC) were allowed to continue receiving study drug therapy. Patients whose BAL/min‐BAL culture results did not yield at least one bacterial pathogen meeting these criteria were to be discontinued from study drug therapy but were to be continued in the study and followed for safety.” Diagnostic criteria: "Patients were required to have new or worsening radiographic infiltrates consistent with VAP and at least one of the following: fever (in the absence of fever‐reducing agents) defined as a rectal temperature greater than 39°C or an increase in core temperature of greater than 1°C; hypothermia, defined as a rectal/core body temperature of less than 35°C; or white blood cell count > 10,000 cells/mm³." VAP definition: " At least 48 hours of mechanical ventilation." |
|
| Interventions | Number of study centres: “Patients were enrolled from 56 sites in 19 countries.” Intervention A: “Fixed 7‐day course of doripenem one gram as a four‐hour infusion every eight hours.” Intervention B: “Fixed 10‐day course of imipenem‐cilastatin one gram as a one‐hour infusion every eight hours.” All interventions: “Patients randomised to doripenem treatment received in parallel 7 days of active therapy and 10 days of placebo. Patients randomised to imipenem‐cilastatin treatment received in parallel 10 days of active therapy and 7 days of placebo. All patients received active study drug and placebo infusions on Days 1 through 7. Patients randomised to imipenem‐cilastatin continued to receive active study drug on Days 8, 9 and 10 and patients randomised to doripenem received placebo.” “Adjunctive therapy was allowed at the discretion of the treating physician with vancomycin (1 gram every 12 hours) or linezolid (600 mg every 12 hours) directed at methicillin‐resistant Staphylococcus aureus (MRSA) and amikacin (15 mg/kg once daily) for patients at risk for infection with a carbapenem‐resistant Gram‐negative pathogen.” |
|
| Outcomes |
Primary outcome:Clinical cure at EOT (Day 10) in the MITT population
Secondary outcomes: A) Safety
B) Clinical cure in subgroups
C) All‐cause 28‐day mortality in MITT group “All‐cause 28‐day mortality in the MITT group was numerically greater for patients in the doripenem arm compared to the imipenem‐cilastatin arm (21.5% versus 14.8%; 95% CI, ‐5.0 to 18.5) and for patients with P. aeruginosa VAP (35.3% versus 0.0%; 95% CI, 12.6 to 58.0).” |
|
| Notes |
Commercial funding/non‐commercial funding/other funding: “This study was funded by Janssen Pharmaceutical Research and Development.” Stated aim for study: “The aim of this study was to compare a 7‐day course of doripenem to a 10‐day course of imipenem‐cilastatin for ventilator‐associated pneumonia (VAP) due to Gram‐negative bacteria.” Conflict of interest: “Dr. Kollef’s effort was supported by the Barnes‐Jewish Hospital Foundation and Dr. Kollef has received consulting fees from Janssen. Dr. Restrepo’s time is partially protected by Award Number K23HL096054 from the National Heart, Lung, and Blood Institute.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Treatment was randomised..." |
| Allocation concealment (selection bias) | Low risk | “Treatment was randomised with use of a central interactive phone system.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients ventilated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “In addition to the 41 patients from the good clinical practice non‐compliant sites, 7 patients were excluded who never received the study drug (1 patient was excluded for meeting both of these criteria).” |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | Study was funded by Janssen Pharmaceutical Research and Development. |
Ramirez 2013.
| Methods | Factorial randomised controlled clinical trial (RCT) Randomisation ratio: 1:1:1 Non‐inferiority design: (2‐sided CI) |
|
| Participants | N recruited = 108 N randomised = 108 (37 intervention A, 36 intervention B, 35 intervention C) N Safety m‐ITT(received drug) = 105 (36 intervention A, 35 intervention B, 34 intervention C) N cm‐ITT = 105 (36 intervention A, 35 intervention B, 34 intervention C) N MITT = 65 (25 intervention A, 19 intervention B, 21 intervention C) N CE = 67 (23 intervention A, 20 intervention B, 24 intervention C) N Microbiologically evaluable = 38 (13 intervention A, 10 intervention B, 15 intervention C) Inclusion criteria: Referenced to be the same as Freire 2010 "Inclusion requirements included age > 18 years, onset of symptoms > 48 h after admission or < 7 days after discharge (if initial hospitalisation was > 3 days), and a new or evolving infiltrate on chest X‐ray. Minimum disease requirements included fever or leukocytosis/leukopenia along with respiratory failure requiring mechanical ventilation or at least 2 of the following: cough, dyspnea or tachypnoea, pleuritic chest pain, auscultatory findings of rales or evidence of consolidation, hypoxemia, and purulent sputum or change in sputum character." Exclusion criteria: Referenced to be the same as Freire 2010 "Exclusion criteria included antibacterial drugs administered for >24 h to treat the current episode of suspected HAP unless a repeat respiratory culture showed that a pathogen was resistant to that agent and/or the patient had worsening or no improvement in clinical signs and symptoms of pneumonia, HIV positive, on immunosuppressive therapy, APACHE II score > 30, cystic fibrosis, pulmonary malignancy, postobstructive pneumonia, bronchiectasis, sarcoidosis, pulmonary abscess, empyema, active tuberculosis, and infections known to be caused by Legionella, Pneumocystis, or mycobacteria. Additional exclusions included absolute neutrophil count < 1 × 10⁹/L, aspartate aminotransferase or alanine aminotransferase >10× upper limit of normal (ULN) or bilirubin or alkaline phosphatase > 3 × ULN, creatinine clearance (CL) <41 mL/min per 1.73 m², or hypersensitivity to any of the agents that could be used in the trial." From Ramirez 2013: "those with known Pseudomonas aeruginosa infection were excluded" Diagnostic criteria: "new or evolving infiltrate on chest X‐ray. Minimum disease requirements included fever or leukocytosis/leukopenia along with respiratory failure requiring mechanical ventilation or at least 2 of the following: cough, dyspnea or tachypnoea, pleuritic chest pain, auscultatory findings of rales or evidence of consolidation, hypoxemia, and purulent sputum or change in sputum character." VAP definition: "VAP was defined as the onset of pneumonia 48 h or more after endotracheal intubation." |
|
| Interventions | Number of study centres: “75 sites in Europe, Asia, Latin America, the United States, Canada, and Australia.” Intervention A: I.V. tigecycline 150mg followed by 75 mg every 12h Intervention B: I.V. tigecycline 200 mg followed by 100 mg every 12h Intervention C: Control “imipenem/cilastatin was dosed at 1 G i.v. every 8 h” All interventions: “Patients randomised to tigecycline also received adjunctive i.v. therapy (ceftazidime 2 G every 8 h and tobramycin 7 mg/kg of body weight daily or amikacin 20 mg/kg daily and vancomycin placebo) at the start of therapy unless there was no concern regarding P. aeruginosa or methicillin‐resistant Staphylococcus aureus infection. Adjunctive i.v. therapy was given to patients randomised to receive imipenem/cilastatin (vancomycin 15 mg/kg and tobramycin or amikacin dosed as described above, plus ceftazidime placebo). Adjunctive therapies were discontinued based on available cultures; subjects with P. aeruginosa isolated from the baseline culture were withdrawn from the study.” Patients received "dosing for up to 14 consecutive days; the exact duration of treatment was at the discretion of the investigator." |
|
| Outcomes |
Primary outcome:Clinical cure at TOC assessment “The clinical response at TOC was numerically higher with the tigecycline 100 mg regimen (17/20, 85.0%) than with the tigecycline 75 mg regimen (16/23, 69.6%) and the imipenem/ cilastatin regimen (18/24, 75.0%).” Secondary outcomes: A) Subgroup clinical cure
B) Safety
C) PK/PD results "PK/PD assessment of clinical or microbiological outcome could not be made because MIC data were available for only the 25 subjects for whom PK data were available. The mean AUC/MIC ratios were 24.3 +/‐ 20.4 for subjects with a clinical cure (n = 17) and 22.8 +/‐ 9.59 for subjects with treatment failure or an indeterminate outcome (n = 8)." |
|
| Notes |
Commercial funding/non‐commercial funding/other funding: “This study was sponsored by Wyeth Research, which was acquired by Pfiser Inc. in October 2009.” Stated aim for study: “This phase 2 study compared the safety and efficacy of two higher doses of tigecycline with imipenem/cilastatin in subjects with hospital‐acquired pneumonia.” Conflict of interest: “Julio Ramirez has received research grants, consulting fees, and speaker fees from Pfiser Inc. Nathalie Dartois, Jean Li Yan, Joan Korth‐Bradley, and Paul C. McGovern are employees of Pfiser Inc. Hassan Gandjini was an employee of Pfiser Inc. at the time that this study was conducted.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The unblinded dispenser (pharmacy or nursing staff) covered infusion bags and tubing to maintain blinding.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “A total of 105 subjects received study medication and constituted the safety population. Since no subjects were excluded, the safety and c‐mITT populations are the same.” |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | Study was sponsored by Wyeth Research. Authors are employees of Pfizer Inc. |
Rea‐Neto 2008.
| Methods | Parallel randomised controlled clinical trial (RCT) Randomisation ratio: 1:1 Non‐inferiority design: (2‐sided CI) Open‐label |
|
| Participants | N recruited = 448 (including HAP and VAP) N randomised = 448 (225 intervention A, 223 intervention B) N reported outcomes ITT = 444 (223 intervention A, 221 intervention B)‐ 4 patients did not receive study drugs N cm‐ITT = 429 (217 intervention A, 212 intervention B) N CE = 253 (134 intervention A, 119 intervention B) N MITT = 285 (141 intervention A, 144 intervention B) N microbiologically evaluable = 167 (84 intervention A, 83 intervention B) N VAP clinical modified ITT = 123 (62 intervention A, 61 intervention B) Inclusion criteria: “Patients aged 18 years or older with signs and symptoms of NP, including nonventilated patients and those with early‐onset VAP (< 5 days of ventilation), were eligible if they had been hospitalised for at least 48 h or had been discharged within the past 7 days after being hospitalised for 48h or longer. Residents of chronic care facilities were also eligible if admitted to the hospital with pneumonia. Eligible patients had a new or progressive infiltrate on chest radiograph; either fever, hypothermia, or changes in peripheral white blood cell (WBC) count attributable to infection (i.e. ≥ 10 000/mm³, > 15% immature forms regardless of WBC count, or leukopenia); and if intubated, a clinical pulmonary infection score (CPIS) ≥ 5 (where the maximum score was 11). In addition, patients had either respiratory failure requiring mechanical ventilation or at least two of the following signs and symptoms: cough; new‐onset production of purulent sputum or other respiratory secretions, or a change in the character of sputum; auscultatory findings of rales or evidence of pulmonary consolidation; dyspnea, tachypnoea, or respiratory rate ≥ 30/min; and hypoxemia with a partial oxygen pressure < 60 mm Hg while breathing room air. All patients or their legally acceptable representatives provided written informed consent.” Exclusion criteria: “Patients were excluded from entry into the study if the NP was known (prior to the study) to be caused by pathogens resistant to either meropenem (used as a surrogate for doripenem) or piperacillin/tazobactam (other than methicillin‐resistant S. aureus [MRSA]). Patients were also excluded if they required concomitant systemic antimicrobial therapy (other than vancomycin or amikacin) in addition to study drug, or had received systemic antibiotic therapy for ≥ 24 h in the 72‐h period before randomisation to study drug (unless they failed prior therapy for NP or developed symptoms of pneumonia with a new pulmonary infiltrate while receiving the prior antibiotic regimen). Other exclusion criteria were: Acute Physiology and Chronic Health Evaluation II (APACHE II) scores 28 < 8 or > 25, mechanical ventilation for ≥5 days, presence of known bronchial obstruction or history of postobstructive pneumonia (other than chronic obstructive pulmonary disease), cavitary lung disease, primary lung cancer or another malignancy with lung metastases, adult respiratory distress syndrome, cystic fibrosis, Pneumocystis jiroveci (carinii) pneumonia, Legionella infection, active tuberculosis, immunocompromising illness, need for dialysis, and any rapidly progressive disease or immediately life‐threatening illness. Patients with significant liver function abnormalities, neutropenia, or thrombocytopenia were excluded, as were those with a history of moderate or severe hypersensitivity to β‐lactam antibiotics or β‐lactamase inhibitors. Treatment with >1 dose of piperacillin/tazobactam or a carbapenem for the current infection, or treatment with an investigational drug or device within the previous 30‐day period, was prohibited.” Diagnostic criteria: "Eligible patients had a new or progressive infiltrate on chest radiograph; either fever, hypothermia, or changes in peripheral white blood cell (WBC) count attributable to infection (i.e. ≥ 10 000/mm3, >15% immature forms regardless of WBC count, or leukopenia); and if intubated, a clinical pulmonary infection score (CPIS) ≥ 5 (where the maximum score was 11). In addition, patients had either respiratory failure requiring mechanical ventilation or at least two of the following signs and symptoms: cough; new‐onset production of purulent sputum or other respiratory secretions, or a change in the character of sputum; auscultatory findings of rales or evidence of pulmonary consolidation; dyspnea, tachypnoea, or respiratory rate ≥ 30/min; and hypoxemia with a partial oxygen pressure < 60 mm Hg while breathing room air." VAP definition: "Early‐onset VAP (< 5 days of ventilation)." Minimum ventilation time not reported. |
|
| Interventions | Number of study centres: “24 centres in North America, 18 in South America, and 26 in Europe.” Intervention A: “Doripenem 500 mg every 8 h was administered as a 60‐min IV infusion.” “The dosage of doripenem was adjusted to 250mg every 8h or every 12h for patients with a calculated CrCL of 30–50 mL/min or 10–29 mL/min, respectively.” Intervention B: “piperacillin/tazobactam 4.5g every 6h was given as a 30‐min IV infusion” “the dosage of piperacillin/ tazobactam was adjusted to 3.375 g or 2.25 g every 6 h for patients with CrCL of 20–40 mL/min or 520 mL/min, respectively” All interventions: “The IV study drug was administered for at least 72h (i.e., nine doses of doripenem or 12 doses of piperacillin/tazobactam), and then patients could be switched to oral levofloxacin 750 mg once daily if they met all four of the following criteria: afebrile for at least 24h (without need for aspirin, acetaminophen, nonsteroidal anti‐inflammatory drugs, or corticosteroids); WBC count ≤ 15 000 cells/mm2 or a decrease in WBC count by 25% from the peak; absence or improvement of signs and symptoms of pneumonia compared with predosing; and improvement or lack of progression of chest X‐ray findings. An alternative oral antibiotic could be selected based on the susceptibility of the isolated pathogen or patient intolerance.” “Vancomycin could be added at the discretion of the investigator if MRSA was suspected but was to be discontinued if MRSA was not confirmed by culture results. Because the addition of an aminoglycoside is recommended with piperacillin/tazobactam therapy in patients at risk for P. aeruginosa infection (according to specific country labeling or guidelines), amikacin was recommended in both treatment arms to ensure balance. If P. aeruginosa was not confirmed by culture, amikacin was to be discontinued. However, if P. aeruginosa was isolated, amikacin was continued for approximately 5 days in patients assigned to the piperacillin/tazobactam arm. It could be discontinued, however, in the doripenem arm if the patient improved clinically and if the P. aeruginosa isolate was susceptible to meropenem.” |
|
| Outcomes |
Primary outcomes:Clinical Cure
A)VAP population clinical cure
Secondary outcomes: A) Clinical relapse
B) Clinical and Microbiological cure rates in microbiologically evaluable patients “The clinical cure rates in microbiologically evaluable patients at the TOC visit were 82.1% (69/84) and 78.3% (65/83) (difference, 3.8%; 95% CI, ‐9.4 to 17.1) in the doripenem and piperacillin/tazobactam arms, respectively. In the mMITT population, clinical cure rates were 67.6% (94 of 139) and 67.4% (97 of 144), respectively (difference, 0.3%; 95% CI, ‐11.4 to 11.9).” C) Emergent infections
D) All‐cause mortality “The all‐cause mortality at day 28 in the cMITT population was 13.8% (30/217) with doripenem and 14.6% (31/212) with piperacillin/tazobactam (difference, 0.8%; 95% CI, ‐7.9 to 6.3%). A Kaplan‐Meier analysis (not shown) found no difference in cumulative mortality rate between the two treatment arms.” E) Safety
|
|
| Notes |
Commercial funding/non‐commercial funding/other funding: “This study was funded by Johnson & Johnson Pharmaceutical, Raritan, NJ, USA.” Stated aim for study: “This prospective, randomised, open‐label, multicenter study was designed to establish whether doripenem was non inferior to piperacillin/ tazobactam in NP.” Conflict of interest: “ML, KK, PP, and IF were employees of Johnson & Johnson during the term of this study. AR‐N, SML, and ES were lead investigators in this study. MN served as a consultant for this study. The authors have no financial interests to declare.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients ventilated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Although this was an open‐label study, in‐house blinding procedures were implemented by the sponsor to ensure that the data were assessed objectively post hoc. In addition, an external blinded expert evaluation committee was convened to review the case report records of all treated patients. The committee evaluated whether the diagnosis of pneumonia had been adequately established and whether they concurred with the clinical outcome determined by the unblinded investigator. The primary analyses in this study were based on the final decision of the Committee.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “A total of 448 patients were randomised to study treatment, including 225 patients (50.2%) to doripenem and 223 patients (49.8%) to piperacillin/tazobactam (Figure 1). Of the 444 patients treated, 382 (86%) completed IV study drug therapy, including 195 (87.4%) in the doripenem arm and 187 (84.6%) in the piperacillin/tazobactam arm; 177 of 444 (39.9%) were switched to oral antibiotic therapy. A total of 195 patients in the ITT population were excluded from the clinically evaluable population (Table 1), principally because of a missing or indeterminate clinical outcome assessment at the TOC visit, use of concomitant antibiotic therapy, and isolation of only resistant pathogens at baseline. The reasons for excluding patients from the evaluable populations and for discontinuations of IV study drug therapy were similar between the two treatment arms.” 7 patients are not accounted for in m‐ITT clinical cure statistics. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | Study was funded by Johnson & Johnson Pharmaceutical. Authors employees of study Johnson & Johnson Pharmaceutical. |
Shorr 2005.
| Methods | Secondary analysis of parallel randomised controlled clinical trial (RCT) (West 2003) Randomisation ratio: 1:1 Open‐label |
|
| Participants | N recruited = 438 N recruited VAP= 222 (111 intervention A, 111 intervention B) N randomised VAP= 222 (111 intervention A, 111 intervention B) N reported outcomes ITT = 222 Inclusion criteria: “Patients > 18 years old with signs and symptoms of NP were eligible for the original trial. Patients had to have been hospitalised for > 72 h, and radiographic evidence of a new infiltrate was required. The presence of either abnormal body temperature (≥ 38°C or ≤ 35°c) or an abnormal peripheral leukocyte count (≥ 12,000 cells/mm³, > 10% immature forms, or ≤ 3500 cells mm³) was further required for enrolment in the study.” Exclusion criteria: “Patients with neutropenia (≤ 500 neutrophils/mm³) were excluded.” From West 2003: "Patients who had received a minimum of 72 hours of antibacterial therapy for pneumonia could enter the study provided they had a positive respiratory culture at the time of enrolment and worsening signs and symptoms of nosocomial pneumonia, indicating therapeutic failure. Patients were excluded from the study if the infecting organisms were known to be resistant to study therapy They were also excluded if they were receiving additional antibacterial therapy (except in the case of therapeutic failure, as described in the previous paragraph). Other exclusion criteria were a score >35 on the Acute Physiology and Chronic Health Evaluation II (APACHE II) scale (higher score indicates more severe disease and greater risk of mortality),11 terminal illness, pregnancy, and creatinine clearance <20 mL/min. Burn patients with >15% total body burn or significant third‐degree burns were excluded, as were immunosuppressed patients. Patients with structural lung disease, empyema, or pulmonary infection with organisms other than bacteria were also excluded." Diagnostic criteria: “Radiographic evidence of a new infiltrate was required. The presence of either abnormal body temperature (≥38°C or ≤35°c) or an abnormal peripheral leukocyte count (≥12,000 cells/mm³, >10% immature forms, or ≤3500 cells mm³) was further required for enrolment in the study.” VAP definition: "We defined "VAP," the focus for the present report, as the development of pneumonia in a patient who had been receiving MV for at least 48 h before the development of a new infiltrate, accompanied by the evolution of other signs and symptoms of pneumonia." |
|
| Interventions | Number of study centres: From West 2003: "67 centres in the United States and Canada." Intervention A: “levofloxacin (750mg iv q24h) Intervention B: “imipenem‐cilastatin 500‐1000 mg iv q6‐8h” All interventions: “Dosing was adjusted for renal impairment. Combination therapy was administered for cases of suspected infection with Pseudomonas aeruginosa. For patients receiving levofloxacin, the additional agent was ceftazidime (2 G iv q8h) or another noncarbapenem β‐lactam; for patients receiving imipenem‐cilastatin, the additional agent was amikacin (7.5 mg/kg iv ql2h) or an alternative aminoglycoside. In suspected or documented cases of infection with methicillin‐resistant Staphylococcus aureus, investigators used vancomycin, irrespective of study treatment.” |
|
| Outcomes |
Primary outcome:“clinical success in the ITT population”
Secondary outcomes: A) Pseudomonas species subgroup clinical success “In those patients infected with P. aeruginosa, clinical success rates were also comparable (87.5% for patients receiving levofloxacin versus 61.1 % for patients receiving imipenem‐cilastatin; P, not significant). Most patients (> 85%) survived. Patients receiving levofloxacin were 30% less likely to die than were patients receiving imipenem‐cilastatin (OR, 0.70), but this difference was not statistically significant (95% Cl, 0.33‐1.48; P = .37).” B) Superinfection with Pseudomonas “Superinfection with Pseudomonas species was more likely to occur in patients treated with imipenem‐cilastatin (3 patients receiving levofloxacin versus 10 patients receiving imipenem‐cilastatin; P=.045).” C) Safety
D) 28‐day mortality rates “28‐day mortality rates were also comparable.” |
|
| Notes |
Commercial funding/non‐commercial funding/other funding: Not reported Stated aim for study: “We conducted a secondary analysis of a multicenter, prospective, randomised trial comparing levofloxacin (750 mg iv q24h) with imipenem‐cilastatin (500‐1000 mg iv q6‐8h) for treatment of nosocomial pneumonia and focused on the subgroup of patients with VAP.” Conflict of interest: “N.Z., S.‐C.W., and A.M.T. were employees of Ortho‐McNeil Pharmaceutical at the time the present study was conducted, but no extramural funding was provided for the preparation of this analysis. A.ES. and M.H.K. have received research support from Ortho‐McNeil Pharmaceutical, but no extra funding was provided for the preparation of this analysis. W.L.J. and A.S.R.: no conflicts.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients ventilated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Blinding was not included in the study design because of the great differences in dosing schedules between the various study agents, because imipenem/cilastatin is not available in an oral form, and because of the variable timing of the switch from IV to oral antibacterial therapy. Study investigators also felt that it would be undesirable to blind evaluators to treatment assignment in this critically ill patient population." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised participants |
| Selective reporting (reporting bias) | High risk | No numbers for secondary end point of 28‐day mortality. "28‐day mortality rates were also comparable." |
| Other bias | High risk | Three authors were employees of Ortho‐McNeil Pharmaceutical. Study seems to have been supported by Ortho‐McNeil Pharmaceutical. |
AEs: adverse events; APACHE II: acute physiology and chronic healthy evaluation II; AUC: area under the serum concentration time curve; BAL: bronchoalveolar lavage; CAZ: ceftazidime; CE: clinically evaluable; CEC: clinical evaluation committee; CI: confidence interval; CL or CrCL: creatinine clearance; cm‐ITT: clinically modified‐ITT; CPIS: clinical pulmonary infection score; CRP: c‐reactive protein; EOT: end of therapy/treatment; HAP: hospital‐acquired pneumonia; ICU: intensive care unit; IDMC: independent data monitoring committee; IQR: interquartile range; ITT: intention‐to‐treat; IV or i.v. or iv: intravenous; m‐ITT: modified‐ITT; MITT: microbiological intention‐to‐treat; MIC: minimum inhibitory concentration; MRSA: methicillin‐resistant Staphylococcus aureus N: number; NP: nosocomial pneumonia; PD: pharmacodynamics; PK: pharmacokinetics; RCT: randomised controlled trial; RR: risk ratio; SAEs: serious adverse events; SAPs: simplified acute physiology score; SOPA: sequential organ failure assessment; TAZ: piperacillin‐tazobactam; TOC: test‐of‐cure; ULN: upper limit of normal; VAP: ventilator‐associated pneumonia; VFDs: ventilator‐free days; WBC: white blood cell
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amonova 2011 | Wrong intervention: Compared different doses of a single type of antibiotic and not different antibiotic regimens. |
| Barriere 2014 | Wrong population: Provided data for Gram‐positive organisms only; mainly focuses on MRSA. Therefore does not fit our criteria of being empiric treatment. |
| Bassetti 2007 | Not RCT: "This was a prospective, open‐label, non‐comparative pilot trial." |
| Chastre 2008 | Wrong population: VAP defined as > 24 hours ventilation; did not meet our protocol requirement of > 48 hours of ventilation. |
| Giamerellos‐Bourboulis 2008 | Wrong intervention: Clarithromycin versus placebo for non‐antibiotic effects. |
| Iakovlev 2006 | Wrong population: Nosocomial infections; VAP patients not specifically reported. |
| Klapdor 2014 | Not RCT: This is a review. |
| Polk Jr 1997 | Wrong population: Treatment of pneumonia in mechanically‐ventilated trauma patients, not VAP. |
MRSA: methicillin‐resistant staphylococcus aureus RCT: randomised control trial VAP: ventilator‐associated pneumonia
Characteristics of ongoing studies [ordered by study ID]
NCT01808092.
| Trial name or title | A study comparing ceftazidime‐avibactam versus meropenem in hospitalized adults with nosocomial pneumonia |
| Methods | A phase III, randomised, multicentre, double‐blind, double‐dummy, parallel‐group comparative study |
| Participants |
|
| Interventions | Intervention: 2000 mg ceftazidime plus 500 mg avibactam Control: 1000 mg meropenem |
| Outcomes | Primary outcome: The proportion of patients with clinical cure in the clinically modified intent‐to‐treat and clinically evaluable analysis sets (co‐primary analyses) (Time Frame: up to 25 days from randomisation) (Designated as safety issue: No) 21st ‐ 25th day from randomisation |
| Starting date | April 2013 ‐ completed January 2016 |
| Contact information | Joseph Chow, MD, FIDSA |
| Notes | AstraZeneca |
Differences between protocol and review
The author team changed between publishing of the protocol and writing the review.
We changed our inclusion criteria from double‐blind randomised control trials (RCTs) to all RCTs because double‐blinding was not common in papers studying treatment of people with ventilator‐associated pneumonia (VAP). We determined the lack of blinding of patients to be low risk since all patients would be sedated while ventilated. We did not classify the antibiotics into classes since this did not increase the overlap in studies for meta‐analysis. We also did not perform any subgroup analysis for late versus early VAP since we did not identify any studies providing late VAP data.
A study we identified compared tigecycline to imipenem, therefore tigecycline was added to Types of interventions in the Methods section.
The protocol specified only all‐cause mortality as the primary outcome (Selim 2010). The current author team considered clinical cure to be equally relevant and this was added as a second primary outcome.
We also added length of intensive care unit (ICU) stay as a secondary outcome because this is a relevant economic outcome. We recorded this outcome using continuous variables, weighted means and standard deviation (SD) of means. Where medians and percentile points of the effect estimate were reported, we used the formula developed by Hozo 2005 to calculate medians and SDs.
We performed GRADE analysis and created SoF tables that were not mentioned in the protocol.
Contributions of authors
All review authors Lauren Arthur (LA), Russell Kizor (RK), Leonardo Seoane (LS), Adrian Selim (AS), and Mieke L van Driel (MvD) contributed to conducting the review and approved the final draft.
Sources of support
Internal sources
No internal support received, Other.
External sources
No external support received, Other.
Declarations of interest
Lauren E Arthur: none known. Russell S Kizor: none known. Adrian G Selim: none known. Mieke L van Driel: none known. Leonardo Seoane: none known.
New
References
References to studies included in this review
Alvarez Lerma 2001 {published data only (unpublished sought but not used)}
- Alvarez Lerma F, Serious Infections Study Group. Efficacy of meropenem as monotherapy in the treatment of ventilator‐associated pneumonia. Journal of Chemotherapy 2001;13(1):70‐81. [DOI] [PubMed] [Google Scholar]
Awad 2014 {published data only}
- Awad SS, Rodriguez AH, Chuang YC, Marjanek Z, Pareigis AJ, Reis G, et al. A phase 3 randomized double‐blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital‐acquired pneumonia. Clinical Infectious Diseases 2014;59(1):51‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beaucaire 1999 {published data only}
- Beaucaire G, Nicolas MH, Martin C, Offenstadt G, Philippon A, Holzapfel L, et al. Comparative study of the association cefepime‐amikacin versus the association ceftazidime‐amikacin in the treatment of nosocomial pneumonias in ventilated patients [Etude comparative de l'association céfépime‐amikacine versus ceftazidime en association avec l'amikacine dans le traitement des pneumonies nosocomiales chez les patients ventilés]. Annales Françaises d'Anesthésie et de Réanimation 1999;18:186‐95. [PubMed] [Google Scholar]
Brun‐Buisson 1998 {published data only}
- Brun‐Buisson C, Sollet JP, Schweich H, Brière S, Petit C. Treatment of ventilator‐associated pneumonia with piperacillin‐tazobactam/amikacin versus ceftazidime/amikacin: a multicenter, randomized controlled trial. Clinical Infectious Diseases 1998;26:346‐54. [DOI] [PubMed] [Google Scholar]
Damas 2006 {published data only}
- Damas P, Garweg C, Monchi M, Nys M, Canivet JL, Ledoux D, et al. Combination therapy versus monotherapy: a randomised pilot study on the evolution of inflammatory parameters after ventilator associated pneumonia [ISRCTN31976779]. Critical Care 2006;10(2):R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freire 2010 {published data only}
- Freire AT, Melnyk V, Kim MJ, Datsenko O, Dzyublik O, Glumcher F, et al. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital‐acquired pneumonia. Diagnostic Microbiology and Infectious Disease 2010;68(2):140‐51. [DOI] [PubMed] [Google Scholar]
Heyland 2008 {published data only}
- Heyland DK, Dodek P, Muscedere J, Day A, Cook D, Canadian Critical Care Trials Group. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator‐associated pneumonia. Critical Care Medicine 2008;36(3):737‐44. [DOI] [PubMed] [Google Scholar]
Kollef 2004 {published and unpublished data}
- Kollef MH, Rello J, Cammarata SK, Croos‐Dabrera RV, Wunderink RG. Clinical cure and survival in Gram‐positive ventilator‐associated pneumonia: retrospective analysis of two double‐blind studies comparing linezolid with vancomycin. Intensive Care Medicine 2004;30(3):388‐94. [DOI] [PubMed] [Google Scholar]
- Rubinstein E, Cammarata S, Oliphant T, Wunderink R, Linezolid Nosocomial Pneumonia Study Group. Linezolid (PNU‐100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double‐blind, multicenter study. Clinical Infectious Diseases 2001;32(3):402‐12. [DOI] [PubMed] [Google Scholar]
- Wunderink RG, Cammarata SK, Oliphant TH, Kollef MH, Linezolid Nosocomial Pneumonia Study Group. Continuation of a randomized, double‐blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia. Clinical Therapeutics 2003;25(3):980‐92. [DOI] [PubMed] [Google Scholar]
Kollef 2012 {published data only}
- Kollef MH, Chastre J, Clavel M, Restrepo MI, Michiels B, Kaniga K, et al. A randomized trial of 7‐day doripenem versus 10‐day imipenem‐cilastatin for ventilator‐associated pneumonia. Critical Care 2012;16(6):R218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramirez 2013 {published data only}
- Ramirez J, Dartois N, Gandjini H, Yan JL, Korth‐Bradley J, McGovern PC. Randomized phase 2 trial to evaluate the clinical efficacy of two high‐dosage tigecycline regimens versus imipenem‐cilastatin for treatment of hospital‐acquired pneumonia. Antimicrobial Agents and Chemotherapy 2013;57(4):1756‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rea‐Neto 2008 {published data only (unpublished sought but not used)}
- Réa‐Neto A, Niederman M, Lobo SM, Schroeder E, Lee M, Kaniga K, et al. Efficacy and safety of doripenem versus piperacillin/tazobactam in nosocomial pneumonia: a randomized, open‐label, multicenter study. Current Medical Research and Opinion 2008;24(7):2113‐26. [DOI] [PubMed] [Google Scholar]
Shorr 2005 {published data only}
- Shorr AF, Zadeikis N, Jackson WL, Ramage AS, Wu SC, Tennenberg AM, et al. Levofloxacin for treatment of ventilator‐associated pneumonia: a subgroup analysis from a randomized trial. Clinical Infectious Diseases 2005;40(Suppl):123‐9. [DOI] [PubMed] [Google Scholar]
- West M, Boulanger BR, Fogarty C, Tennenberg A, Wiesinger B, Oross M, et al. Levofloxacin compared with imipenem/cilastatin followed by ciprofloxacin in adult patients with nosocomial pneumonia: a multicenter, prospective, randomized, open‐label study. Clinical Therapeutics 2003;25(2):485‐506. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amonova 2011 {published data only}
- Amonova DS, Ibadova DN. Clinical and bacteriological efficiency of two modes of ceftazidim and aminkacin dosing in patients with ventilator‐associated pneumonia. Likars'ka Sprava / Ministerstvo Okhorony Zdorov'ia Ukrainy 2011;1‐2:110‐7. [PubMed] [Google Scholar]
Barriere 2014 {published data only}
- Barriere SL. The ATTAIN trials: efficacy and safety of telavancin compared with vancomycin for the treatment of hospital‐acquired and ventilator‐associated bacterial pneumonia. Future Microbiology 2014;9(3):281‐9. [DOI] [PubMed] [Google Scholar]
Bassetti 2007 {published data only}
- Bassetti M, Righi E, Fasce R, Molinari MP, Rosso R, Biagio A, et al. Efficacy of ertapenem in the treatment of early ventilator‐associated pneumonia caused by extended‐spectrum beta‐lactamase‐producing organisms in an intensive care unit. Journal of Antimicrobial Chemotherapy 2007;60(2):433‐5. [DOI] [PubMed] [Google Scholar]
Chastre 2008 {published data only}
- Chastre J, Wunderink R, Prokocimer P, Lee M, Kaniga K, Friedland I. Efficacy and safety of intravenous infusion of doripenem versus imipenem in ventilator‐associated pneumonia: a multicenter, randomized study. Critical Care Medicine 2008;36(4):1089‐96. [DOI] [PubMed] [Google Scholar]
Giamerellos‐Bourboulis 2008 {published data only}
- Giamerellos‐Bourboulis E, Pechere J, Routsi C, Plachouras D, Kollias S, Raftogiannis M, et al. Effect of clarithromycin in patients with sepsis and ventilator‐associated pneumonia. Clinical Infectious Diseases 2005;46(8):1157‐64. [DOI] [PubMed] [Google Scholar]
Iakovlev 2006 {published data only}
- Iakovlev SV, Beloborodov VB, Sidorenko SV, Iakovlev VP, Grigor'ev KB, Eliseeva EV, et al. Multicentre study of comparative efficacy of meropenem and combined regimens for empirical antibacterial therapy of severe nosocomial infections: results of clinical and pharmacoeconomic analysis. Antibiotiki i Khimioterapiia 2006;51(7):15‐27. [PubMed] [Google Scholar]
Klapdor 2014 {published data only}
- Klapdor B, Ewig S. Ventilator associated pneumonia [Ventilator‐assoziierte Pneumonie]. Deutsche Medizinische Wochenschrift 2014;139:251‐4. [DOI] [PubMed] [Google Scholar]
Polk Jr 1997 {published data only}
- Polk HC Jr, Livingston DH, Fry DE, Malangoni MA, Fabian T, Trachtenberg LS, et al. Treatment of pneumonia in mechanically ventilated trauma patients. Results of a prospective trial. Archives of Surgery 1997;132(10):1086‐92. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01808092 {unpublished data only}
- NCT01808092. A study comparing ceftazidime‐avibactam versus meropenem in hospitalized adults with nosocomial pneumonia [A phase III, randomized, multicentre, double‐blind, double‐dummy, parallel‐group comparative study to determine the efficacy, safety and tolerability of ceftazidime‐avibactam versus meropenem in the treatment of nosocomial pneumonia including ventilator‐associated pneumonia in hospitalized adults]. clinicaltrials.gov/ct2/show/NCT01808092 (first received 28 February 2013).
Additional references
Alvarez‐Lerma 1996
- Alvarez‐Lerma F. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU‐Acquired Pneumonia Study Group. Intensive Care Medicine 1996;22(5):387‐94. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
ATS 2005
- American Thoracic Society. Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. American Journal of Respiratory and Critical Care Medicine 2005;171(4):388‐416. [DOI] [PubMed] [Google Scholar]
ATS 2016
- Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital‐acquired and ventilator‐associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Disease Society of America and the American Thoracic Society. Clinical Infectious Diseases 2016;63(5):e61‐e111. [DOI: 10.1093/cdi/ciw353] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chastre 2002
- Chastre J, Fagon JY. Ventilator‐associated pneumonia. American Journal of Respiratory and Critical Care Medicine 2002;165(7):867‐903. [DOI] [PubMed] [Google Scholar]
Fagon 2006
- Fagon JY, Rello J. Targeted antibiotic management of ventilator‐associated pneumonia. Clinical Microbiology and Infection 2006;12(Suppl 9):17‐22. [Google Scholar]
Garnacho‐Montero 2007
- Garnacho‐Montero J, Sa‐Borges M, Sole‐Violan J, Barcenilla F, Escoresca‐Ortega A, Ochoa M, et al. Optimal management therapy for Pseudomonas aeruginosa ventilator‐associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Critical Care Medicine 2007;35(8):1888‐95. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed prior to 7 October 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Chichester: Wiley‐Blackwell.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [http://vassarstats.net/median_range.html] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iregui 2002
- Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator‐associated pneumonia. Chest 2002;122(1):262‐8. [DOI] [PubMed] [Google Scholar]
Kim 1989
- Kim JH, Gallis HA. Observations on spiraling empiricism: its causes, allure, and perils, with particular reference to antibiotic therapy. American Journal of Medicine 1989;87(2):201‐6. [DOI] [PubMed] [Google Scholar]
Kollef 2005
- Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health‐care associated pneumonia: results from a large US database of culture‐positive pneumonia. Chest 2005;128(6):3854‐62. [DOI] [PubMed] [Google Scholar]
Kuti 2008
- Kuti EL, Patel AA, Coleman CI. Impact of inappropriate antibiotic therapy on mortality in patients with ventilator‐associated pneumonia and blood stream infection: a meta‐analysis. Journal of Critcal Care 2008;23(1):91‐100. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Chichester: Wiley‐Blackwell.
Liberati 2009
- Liberati A, D'Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD000022.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Magill 2014
- Magill SS, Edwards JR, Beldavs ZG, Dumyati G, Janelle SJ, Kainer MA, et al. Prevalence of antimicrobial use in US acute care hospitals, May‐September 2011. JAMA 2014;312(14):1438‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Masterton 2008
- Masterton RG, Galloway A, French G, Street M, Armstrong J, Brown E, et al. Guidelines for the management of hospital‐acquired pneumonia in the UK: Report of the Working Party on Hospital‐Acquired Pneumonia of the British Society for Antimicrobial Chemotherapy. Journal of Antimicrobial Chemotherapy 2008;62(1):5‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meslen 2013
- Meslen WG, Rovers MM, Groenwold R, Bergmans D, Camus C, Bauer TT, et al. Attributable mortality of ventilator‐associated pneumonia: a meta‐analysis of individual patient data from randomised prevention studies. Lancet Infectious Disease 2013;13:665‐71. [DOI] [PubMed] [Google Scholar]
Rello 2007
- Rello J. Importance of appropriate initial antibiotic therapy and de‐escalation in the treatment of nosocomial pneumonia. European Respiratory Review 2007;16:33‐9. [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rubinstein 2001
- Rubinstein E, Cammarata S, Oliphant T, Wunderink R. Linezolid (PNU‐100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double‐blind, multicenter study. Clinical Infectious Diseases 2001;32(3):402‐12. [DOI] [PubMed] [Google Scholar]
Schreiber 2010
- Schreiber MP, Chan CM, Shorr AF. Resistant pathogens in non‐nosocomial pneumonia and respiratory failure: is it time to refine the definition of health‐care‐associated pneumonia?. Chest 2010;137(6):1283‐8. [DOI] [PubMed] [Google Scholar]
Torres 2001
- Torres A, Carlet J, European Task Force on ventilator‐associated pneumonia. Ventilator‐associated pneumonia. European Respiratory Journal 2001;17(5):1034‐45. [DOI] [PubMed] [Google Scholar]
West 2003
- West M, Boulanger B, Fogarty C, Tennenberg A, Wiesinger B, Oross M, et al. Levofloxacin compared with imipenem/cilastatin followed by ciprofloxacin in adult patients with nosocomial pneumonia: a multicenter, prospective, randomized, open‐label study. Clinical Therapeutics 2003;25(2):485‐506. [DOI] [PubMed] [Google Scholar]
Wunderink 2003
- Wunderink RG, Cammarata S, Oliphant T, Kollef M. Continuation of a randomized, double‐blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia. Clinical Therapeutics 2003;25(3):980‐92. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Aarts 2003
- Aarts MW, Hancock JN, Heyland DK, Marshall JC. Antibiotics for ventilator‐associated pneumonia. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004267] [DOI] [Google Scholar]
Selim 2010
- Selim AG, Balalis G, Bhaskar B, Driel ML. Antibiotics for ventilator‐associated pneumonia. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD004267.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


