Abstract
Kallikrein is the key contact system mediator responsible for the conversion of high-molecular-weight kininogen into the inflammatory vasodilator peptide bradykinin, a process regulated by C1-esterase inhibitor (C1-INH). In hereditary angioedema (HAE), genetic mutations result in deficient or dysfunctional C1-INH and dysregulation of the contact system leading to recurrent, sometimes fatal, angioedema attacks. IONIS-PKKRx is a second-generation 2′-O-(2-methoxyethyl)-modified chimeric antisense oligonucleotide, designed to bind and selectively reduce prekallikrein (PKK) mRNA in the liver. IONIS-PKKRx demonstrated dose-dependent reduction of human prekallikrein hepatic mRNA and plasma protein in transgenic mice and dose- and time-dependent reductions of plasma PKK in Cynomolgus monkeys. Similar dose-dependent reductions of plasma PKK levels were observed in healthy human volunteers accompanied by decreases in bradykinin generation capacity with an acceptable safety and tolerability profile. These results highlight a novel and specific approach to target PKK for the treatment of HAE and other diseases involving contact system activation and overproduction of bradykinin.
Keywords: plasma prekallikrein, hereditary angioedema, antisense oligonucleotide
Introduction
Hereditary angioedema (HAE) is an autosomal dominant disorder caused by a quantitative (Type I) or qualitative (Type II) deficiency in functional C1 esterase inhibitor (C1-INH), and is characterized by recurrent episodes of edema and swelling involving the extremities, trunk, gastrointestinal tract, genitalia, face, tongue, or larynx [1]. The prevalence is estimated to be ∼1 per 50,000 individuals in the United States [2] with no known differences between ethnic groups [3]. Episodes affecting the abdomen or oropharynx can be associated with significant morbidity and mortality, and combined with the unpredictable occurrence and severity of attacks, HAE results in a substantial physical, emotional, and economic burden [4].
The contact system is a protease cascade, initiated by Factor XII (FXII), which activates the intrinsic coagulation pathway and the kallikrein–kinin system [5]. Kallikrein is the key mediator responsible for the conversion of high-molecular-weight kininogen into the proinflammatory vasodilator peptide bradykinin [6], a process negatively regulated by C1-INH [7]. Human-derived C1-INH protein replacement therapies are currently approved for acute (Berinert®; and recombinant Ruconest®) and prophylactic (Cinryze® and Haegarda®) treatment of HAE attacks (all current treatments reviewed in [8,9]). Both prophylactic and acute C1-INH replacement therapies have the disadvantages of thromboembolic events, hypersensitivity reactions, and rare cases of anaphylaxis.
Other therapeutic approaches for HAE focus on reducing bradykinin production through direct kallikrein inhibition. Lanadelumab (Takhzyro™), a recently approved monoclonal antibody (mAb) for HAE prophylaxis, demonstrated significant reduction of HAE attacks when administered subcutaneously (SC) every 2 weeks [10,11]. Measures of kallikrein activity showed a maximum reduction of 60% at the approved dose with reemergence of HAE attacks when kallikrein inhibition dipped below 40%. Another inhibitor of plasma kallikrein, the small molecule BCX7353, is currently being evaluated as an oral treatment, administered three times a day, for HAE prophylaxis. This inhibitor significantly lowered the HAE attack rate in the lower 125 mg dose group with maximum kallikrein inhibition of 60%, but has been associated with toxicities, including gastrointestinal side effects [12]. While these approaches present a significant improvement over current standard of care for HAE patients, a therapy with a novel mechanism of action and an acceptable safety and tolerability profile, which also results in less-frequent attacks, presents a clinically meaningful opportunity [8].
Antisense oligonucleotides (ASOs) are short synthetic nucleotide polymers that selectively bind to a target RNA through Watson–Crick base pairing and, based on the nucleic acid chemistries employed, can be designed to (1) recruit the cellular enzyme RNase H1 leading to the catalytic destruction of the target RNA, or (2) alter the processing (eg, splicing) of their RNA targets [13,14]. ASOs of similar structure and chemistry share common chemical and biological properties. The second-generation 2′-O-(2-methoxyethyl) (2′MOE)-modified full phosphorothioate ASOs are the most advanced [15–17]. This class of ASOs has been tested clinically for multiple disease indications, including diabetes, hyperlipidemias, cardiovascular diseases, neurodegenerative diseases, and cancer [13,14]. Those presently approved for therapeutic use include inotersen (Tegsedi™; hereditary transthyretin-mediated amyloidosis) and mipomersen (Kynamro®; familial hypercholesterolemia) by systemic administration, and nusinersen (Spinraza™; spinal muscular atrophy) by intrathecal administration.
Selective inhibition of prekallikrein (PKK; the zymogen of the serine protease kallikrein) expression with ASO technology presents an attractive and novel therapeutic approach for HAE. Genetic deficiencies of PKK [18] or FXII [19] are not associated with an increased risk of bleeding in humans and ASO-mediated inhibition of PKK or FXII did not identify an increased risk of bleeding in preclinical models [20], altogether supporting an ASO-mediated PKK depletion approach as a potentially safe therapeutic strategy to inhibit bradykinin generation. In this study, we describe the identification, preclinical characterization, and initial clinical investigations of a second-generation 2′MOE-modified chimeric ASO targeting human PKK mRNA.
Materials and Methods
ASO design
IONIS-PKKRx is a second-generation ASO, 20 nucleotides in length, connected sequentially by phosphorothioate internucleoside linkages. The five nucleotides at both the 5′ and 3′ ends are composed of 2′MOE-modified ribonucleotides, which confer an increased affinity to the target mRNA [21,22] and increased resistance to exo- and endonucleases within the cells [23]. The central portion of IONIS-PKKRx is composed of 10 deoxynucleotides, enabling RNase H1 to recognize and cleave the target mRNA in the ASO:RNA duplex. The ASO binds to PKK mRNA through Watson–Crick base pairing, and is fully complementary to a 20 nucleotide sequence within exon 9 of the transcript (NM_000892.3; nucleotides 1019–1038).
Preclinical studies
Cell culture assays
Human terminally differentiated HepaRG (Sigma-Aldrich, St. Louis, MO) and HepG2 (Sigma-Aldrich) human hepatocellular carcinoma (HCC) cells were cultured in Williams Media E media with Maintenance Supplement (Sigma-Aldrich). Cells were harvested from tissue culture vessel, electroporated using ECM 830 System (BTX, Holliston, MA) in media containing different concentrations of IONIS-PKKRx or control ASO, and plated in growth media. Cells were harvested 24 h later for human PKK mRNA reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis.
Transgenic mouse generation
Human PKK transgenic (hPKK-Tg) mice were generated by Ionis Pharmaceuticals (Carlsbad, CA). The genomic region of the human PKK gene was excised from the appropriate fosmid, purified, and microinjected into fertilized oocytes. Oocytes were transferred to a pseudopregnant female, and pups were born. The pups were genotyped, and transgene-positive pups were checked for the expression of plasma hPKK protein. One animal was selected as a founder of the transgenic line, and was transferred to Taconic Biosciences, Inc. (Oxnard, CA) for breeding.
Transgenic mouse study
The hPKK-Tg mouse study was performed at Ionis Pharmaceuticals in accordance with the guidelines established by the internal Institutional Animal Care and Use Committee (IACUC) (Protocol No. P-0223). Mice were housed in individual ventilated cages under conditions controlled for temperature (19°C–23°C), humidity (55% ± 10%), photoperiod (12-h light/12-h dark), and air exchange, with food and water provided ad libitum. Male and female hPKK-Tg mice 8 weeks of age were used in the study. Blood was collected from all animals before the study to measure baseline hPKK protein levels in circulation. The mice were treated with a SC injection of vehicle (phosphate-buffered saline; PBS) or IONIS-PKKRx at 2.5, 5, 10, and 20 mg/kg/week for 3 weeks. Mice were humanely sacrificed 48 h after the last dose, and blood was collected for analysis. Liver fragments were frozen for subsequent RNA extraction and RT-qPCR analysis of hPKK mRNA expression.
Monkey study
The monkey study was conducted according to Good Laboratory Practices (GLP) guidelines at the Korea Institute of Toxicology, Daejeon, South Korea IACUC (Study No. 1305-0135). Cynomolgus monkeys were housed individually in stainless steel cages as specified in the Guide for the Care and Use of Laboratory Animals [24]. Conditions were controlled for temperature (20°C–29°C), humidity (45%–70%), photoperiod (12-h light/12-h dark), and air exchange (10–20 changes/h), with water provided ad libitum and food provided twice daily. Male and female Cynomolgus monkeys were 2 to 4 years of age at the start of treatment. Blood was collected from all animals before the study to measure circulating PKK protein levels at baseline. Vehicle (PBS) or IONIS-PKKRx was administered SC at doses of 4, 8, 12, or 40 mg/kg on days 1, 4, and 7 of the study, and then weekly thereafter for a total of 16 weeks. Monkeys were humanely sacrificed 48 h after the last dose, and blood was collected for analysis. Liver fragments were frozen for subsequent RNA extraction and RT-qPCR analysis of PKK mRNA expression. Monkey blood samples were collected through venipuncture into sample tubes coated with EDTA. Blood was centrifuged at 4,000 g for 15 min and platelet-poor plasma was collected and stored at −80°C before analysis. Plasma samples were analyzed by PKK ELISA.
RNA isolation and RT-qPCR analysis
HepaRG cells were directly lysed in RLT buffer (QIAGEN) containing 1% 2-mercaptoethanol. Livers from monkeys or hPKK-Tg mice were homogenized in RLT buffer (QIAGEN) containing 1% 2-mercaptoethanol. Total mRNA was prepared using the PureLink™ Pro 96 RNA Total RNA Isolation Kit (Invitrogen, Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. The amount of specific mRNA was analyzed using a StepOne™ Real-Time PCR System (Applied Biosystems, Life Technologies, Carlsbad, CA). In mice, hPKK mRNA expression was normalized to the total RNA levels measured by RiboGreen (Invitrogen, Life Technologies), and in monkey, PKK mRNA expression was normalized to the housekeeping gene Cyclophilin A. The sequences of primer probe sets (PPS) for RT-qPCR analysis were as follows: Human/Monkey PKK PPS CCTGTGTGGAGGGTCACTCA (forward), CCACTATAGATGCGCCAAACATC (reverse), CCCACTGCTTTGATGGGCTTCCC (probe);
Monkey Cyclophilin A PPS CGACGGCGAGCCTTTG (forward), TCTGCTGTCTTTGGAACCTTGTC (reverse), CGCGTCTCCTTCGAGCTGTTTGC (probe).
Quantification of plasma PKK levels
Levels of mouse plasma hPKK and monkey plasma PKK protein were measured using Ionis in-house human/monkey-specific PKK ELISA. Briefly, 5 μL of EDTA anticoagulated plasma was diluted with Diluent 4 (Meso Scale Diagnostics, Rockville, MD) and added to the 96-well MSD PKK ELISA plates (Meso Scale Diagnostics, custom order) precoated with human PKK-specific antibody (LifeSpan Biosciences, Seattle, WA). After washes, monkey or hPKK was detected with biotinylated human/monkey-specific PKK antibody (LifeSpan Biosciences) and streptavidin-conjugated SULFO-TAG (Meso Scale Diagnostics). Electrochemiluminescence was measured on SECTOR Imager (Meso Scale Diagnostics). Relative monkey or human (in hPKK-Tg mouse) plasma PKK protein levels were calculated from serial dilutions of monkey plasma in Diluent 2 (Meso Scale Discovery) or hPKK transgenic mouse plasma in normal mouse plasma, respectively.
Preclinical statistical analyses
Animal studies used ANOVA for statistical analyses as shown in text and figures. P values of less than 0.05 were considered statistically significant.
Phase 1 clinical study
Study design
IONIS-PKKRx was evaluated in a Phase 1, double-blind, randomized, placebo-controlled, dose-escalation study in healthy volunteers at a single site in Canada (BioPharma Services, Inc., Toronto, Canada) from May 2014 to January 2015. The clinical trial protocol (ISIS 546254-CS1) was approved by an Institutional Review Board (Institutional Review Board Services, Ontario, Canada) and complied with the guidelines of the 2002 Declaration of Helsinki and the International Conference on Harmonization Guidelines on Good Clinical Practice. Written informed consent was obtained from all participants before participation in the study.
Adults, 18 to 65 years of age, with a body mass index ≤32.0 kg/m2 and in good health were eligible for this study. Subjects were randomly assigned 3:1 to receive IONIS-PKKRx or placebo, by cohort. In the single ascending dose (SAD) cohort, subjects received a single SC injection of IONIS-PKKRx (50, 100, 200, or 400 mg) or placebo. Dose escalation of SAD cohorts proceeded only after all subjects in the preceding cohort had completed dosing with an acceptable clinical and laboratory safety profile on day 4. Enrollment in the first multiple ascending dose (MAD) cohort proceeded only after subjects in the 200 mg single-dose cohort completed dosing and with acceptable clinical and laboratory safety profile on day 4. In the MAD cohorts, subjects received six SC injections of IONIS-PKKRx (50, 100, 200, or 400 mg) or placebo on day 1, 3, 5, 8, 15, and 22. Enrollment in the subsequent cohorts proceeded only after at least four subjects in the preceding MAD cohort completed all doses and day 29 clinical and laboratory safety evaluations were acceptable. A schematic of the study schedules is included as Supplementary Fig. S1.
Pharmacokinetics
Plasma samples were collected at prespecified intervals for pharmacokinetic and pharmacodynamic analyses at central laboratories. Pharmacokinetic assessments of human plasma and urine were analyzed using a validated hybridization-ELISA assay [25] by Pharmaceutical Product Development Laboratories (Richmond, VA). Pharmacokinetic analysis was completed on all subjects who received at least one dose of IONIS-PKKRx. Noncompartmental analysis of data was performed using Phoenix™WinNonlin®, Version 6.3 or higher (Pharsight Corp., Mountain View, VA).
Pharmacodynamics
Pharmacodynamic measurements of PKK (MedPace Reference Laboratories, Cincinnati OH), contact activation (KininX SAS, Grenoble France), and coagulation (MedPace Reference Laboratories, Cincinnati OH; Hemostasis Reference Laboratory, Ontario Canada) were performed on blood samples collected throughout the study. Blood samples collected for coagulation measurements were drawn directly through a cleared venipuncture needle into a vacutainer tube that contained sodium citrate for inactivation of enzyme activity by chelation of calcium.
Levels of PKK/kallikrein protein were measured using the Prekallikrein and Kallikrein Human ELISA Kit (Abcam, Eugene, OR). Briefly, 50 μL of plasma was added in a 96-well polypropylene microplate precoated with PKK/kallikrein antibody. After a 2-h incubation, the wells were washed, and 50 μL of PKK/kallikrein crossreacting, biotinylated detector antibody was added. streptavidin/peroxidase conjugate was added for 30 min and unbound conjugates were washed away. Chromogen substrate TMB (3,3′,5,′-tetramethylbenzidine) was then used to visualize the streptavidin/peroxidase enzymatic reaction at 405 nm and compared with standard curves.
Bradykinin generation assay
The plasma proenzyme assay is a standard surrogate measure for bradykinin generation [26]. Plasma was cooled to 0°C to neutralize C1-INH while leaving serine proteases of the contact system active. The contact system was activated with dextran sulfate, and the enzymatic cleavage of a HWMK-like chromogenic substrate (H-D-Pro-Phe-Arg-pNA) was measured by absorbance (405 nm) at 30°C for 45 min.
FXII-FXIIa conversion assay
FXIIa activity was determined through the FXII to FXIIa conversion assay. The contact system was activated in platelet-poor plasma by cold preincubation with dextran sulfate. Kallistop™ (kallikrein inhibitor) was then added to a final concentration of 100 μM, and enzyme activity was assessed using a chromogenic substrate (H-D-Pro-Phe-Arg-pNA) that was measured by absorbance (405 nm) at 30°C for 45 min.
Optimized activated partial thromboplastin time assay
The activated partial thromboplastin time (aPTT) assay was optimized by Hemostasis Reference Laboratory (Ontario Canada) to account for pharmacological reduction of plasma PKK. Multiple aPTT reagents are available with varying sensitivity to deficiencies in contact activators, including PKK and FXII [27]. Therefore, multiple reagents and incubation times were tested to identify those that would be the most sensitive to decreases in plasma PKK. The final optimized assay used the APTT-A reagent from Stago (Parsippany, NJ) with an incubation time of 60 s and was analyzed with a STA-R Evolution by Diagnostica Stago (Parsippany, NJ).
Safety assessments
Safety and tolerability were evaluated by incidence and severity of adverse events, and by changes in clinical laboratory parameters, electrocardiograms (ECGs) and vital signs. Laboratory evaluations included standard laboratory tests for blood chemistry, coagulation, complement, and hematology parameters (Medpace Reference Laboratories).
Phase 1 statistical analysis
Pharmacodynamics and safety were analyzed for all subjects who received at least one dose of study drug. Placebo subjects from each dose cohort were pooled. Baseline was defined as the last value before the first dose, except where otherwise noted.
Change and percent change from baseline in PKK, and the bradykinin generation assay values were compared between IONIS-PKKRX treatment and pooled placebo using the Exact Wilcoxon Rank-Sum test. Ad hoc analyses of bradykinin generation assay, aPTT, and FXII-FXIIa conversion were performed. All statistical significance testing was two tailed using α = 0.05. Data summary and analyses were performed with SAS® 9.4 or higher.
Results
Design, selection, and in vitro evaluation of IONIS-PKKRX
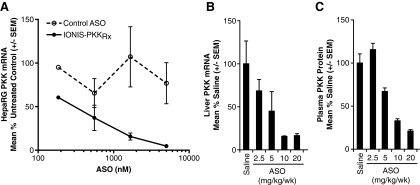
The human PKK gene is 49.5 kb in length and is located on chromosome 4. The identification of highly optimized ASO sequences targeting PKK mRNA involved an extensive screening process as described previously [28–30]. In brief, a total of 1,313 ASOs were designed to bind various sites along human PKK RNA and were evaluated for the ability to deplete PKK mRNA levels in terminally differentiated HCC cells in vitro. Representative data from an initial screen, including control oligonucleotides is shown in Supplementary Fig. S2). IONIS-PKKRx was selected for further evaluation in vivo, based on its high potency inhibition of PKK RNA in vitro, target mRNA specificity confirmed by in silico and in vitro analysis (showing it was not predicted to target any other known human gene transcript), and lack of known overlapping single nucleotide polymorphisms in the human PKK gene. In vitro reduction of PKK was confirmed by exposure of HepaRG cells to multiple concentrations of IONIS-PKKRx, which produced dose-dependent reductions in PKK mRNA levels with an IC50 < 0.25 μM (Fig. 1A). Exposure to a control ASO did not affect PKK mRNA expression.
FIG. 1.
IONIS-PKKRx reduces expression of human mRNA in HepaRG human hepatoma cells and transgenic mice. (A) IONIS-PKKRx inhibits PKK mRNA expression in HepaRG human hepatoma cells. Data are presented as mean ± SEM (N = 3). Control ASO sequence: 5′-CCTTCCCTGAAGGTTCCTCC-3′; underline denotes 2′MOE-modified nucleotides. (B) Liver hPKK mRNA expression in hPKK-Tg mice after 3 weeks of IONIS-PKKRx treatment. (C) Plasma hPKK levels in hPKK-Tg mice after 3 weeks of IONIS-PKKRx treatment. ASO, antisense oligonucleotides; hPKK-Tg, human PKK transgenic; 2′MOE, 2′-O-(2-methoxyethyl).
Evaluation of IONIS-PKKRx in hPKK-Tg mice
The in vivo activity of IONIS-PKKRx was first evaluated in a transgenic mouse system designed to express the human PKK gene (hPKK-Tg). Systemic delivery of 2.5, 5, 10, or 20 mg/kg/week of IONIS-PKKRx by SC injection to hPKK-Tg mice produced dose-dependent reductions in liver hPKK mRNA expression and corresponding decreases of plasma hPKK protein (Fig. 1B, C). The ED50 values for hPKK mRNA and plasma protein reductions were consistent at 5 and 7.5 mg/kg/week, respectively.
Evaluation of IONIS-PKKRx in nonhuman primates
The pharmacokinetics and pharmacodynamics of IONIS-PKKRx was then evaluated in cynomolgus monkeys. The sequence targeted by IONIS-PKKRx has 100% complementarity to the human PKK gene, but contains a 1 base mismatch within the binding site in the monkey PKK mRNA (Table 1) [28,31]. As part of our standard toxicology studies [15,32,33], IONIS-PKKRx was administered SC at doses of 4, 8, 12, or 40 mg/kg on days 1, 4, and 7 of the study, and then weekly thereafter for a total of 16 weeks. Treatment with IONIS-PKKRx was well tolerated at all doses and no deleterious effects were observed based upon blood chemistry, complete blood count, and organ histopathology.
Table 1.
Sequence of IONIS-PKKRx-Binding Sites Across Different Species
| IONIS-PKKRx | 5′ TGCAAGTCTCTTGGCAAACA 3′ |
| Human RNA | 3′ ACGTTCAGAGAACCGTTTGT 5′ |
| Cynomolgus RNA | 3′ ATGTTCAGAGAACCGTTTGT 5′ |
| Rhesus RNA | 3′ ATGTTCAGAGAACCGTTTGT 5′ |
| Baboon RNA | 3′ ATGTTCAGAGAACCGTTTGT 5′ |
Underline denotes 2′MOE-modified nucleotides; all cytosines are methylated at the 5-position. Nucleotide in italics denotes mismatch in Monkey mRNA.
2′MOE, 2′-O-(2-methoxyethyl).
Plasma and tissue concentrations of IONIS-PKKRx decreased over time in a generally monophasic pattern. The estimated plasma terminal half-life was 25.0 ± 8.72 and 20.2 ± 2.80 days in the 12 and 40 mg/kg-dosed animals, respectively. The mean liver tissue half-life was 20.5 to 27.7 days.
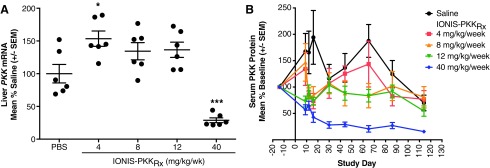
Despite the potential for suboptimal mRNA binding [28], IONIS-PKKRx was active in monkeys, and liver PKK mRNA was reduced up to 70% compared with vehicle control after 16 weeks of treatment in the 40 mg/kg/week group (Fig. 2A). The decrease in liver PKK mRNA was associated with a time-dependent decrease in plasma PKK protein. After 1 week at 40 mg/kg dose, there was an ∼45% reduction (compared with baseline) in plasma PKK protein, which progressed to an ∼75% reduction by week 4, and 85% reduction by week 16 (Fig. 2B).
FIG. 2.
IONIS-PKKRx reduces hepatic PKK mRNA and serum PKK protein levels in Cynomolgus monkeys. (A) Reduction of hepatic PKK mRNA expression in Cynomolgus monkeys after 16 weeks of treatment with IONIS-PKKRx as a percent of saline treated. Data points represent individual animals with mean ± SEM. Significant difference from PBS by one-way ANOVA with Dunnett's post-test (*P < 0.05, ***P < 0.001). (B) Time- and dose-dependent reduction of plasma PKK protein levels in Cynomolgus monkeys over 16 weeks of treatment with IONIS-PKKRx. Data are presented as mean ± SEM. PBS, phosphate-buffered saline.
There were no lasting effects on aPTT associated with the pharmacologic reduction of PKK. Acute and transient prolongation of aPTT is a known class effect of 2′MOE ASOs and is directly related to the ASO plasma concentration [33]. The aPTT was prolonged slightly (by 2.5 s compared with baseline) at 12 mg/kg/week and by ∼13 s at 40 mg/kg/week (that is approximately eightfold higher than the highest dose tested in the phase 1 study). The aPTT peaked at 4 to 8 h postdose then returned to baseline by 24 h postdose. This temporal increase is the typical pattern for 2′MOE ASOs and indicates that the observed effects on aPTT were not related to the pharmacologic reduction of PKK.
Phase I study results
Based on the encouraging preclinical activity and tolerability of IONIS-PKKRx, a phase 1 clinical study in healthy volunteers was initiated to evaluate the pharmacokinetics, safety, and pharmacodynamic activity of this novel PKK inhibitor.
Study population
A total of 49 eligible subjects were randomized with 16 assigned to the SAD cohorts (12 IONIS-PKKRx; 4 placebo) and 33 assigned to the MAD cohorts (25 IONIS-PKKRx; 8 placebo). Thirty-one (94%) subjects completed treatment in the MAD cohort. One subject in the placebo group discontinued dosing after receiving three of six doses due to an adverse event (rectal hemorrhage); and a second subject in the 200 mg dose group withdrew consent on day 1 after receiving the first dose. The flow of subjects through the study is shown in Supplementary Fig. S3. The baseline characteristics of the SAD and MAD cohorts are shown in Supplementary Tables S1 and S2, respectively.
Pharmacokinetics
Following SC administration, IONIS-PKKRx was absorbed rapidly into the systemic circulation, with Tmax values ranging from 3 to 8 h in all evaluated cohorts (Supplementary Table S2). Mean Cmax and AUC values were dose-dependent, with no increased levels after six repeated doses compared with the single-dose regimen, indicating little to no plasma accumulation.
The primary routes of elimination of ASOs as a chemical class are exo- and endonuclease metabolism and excretion of the chain-shortened metabolites in urine [34]. Urinary excretion of intact IONIS-PKKRx was a small fraction of the administered dose, with ≤0.4% excreted after a single dose (on day 1), and ≤3.4% on day 22 (Supplementary Table S3). The low renal clearance over the first 24 h suggests that initial plasma clearance is related to distribution into the tissues, and not excretion. The mean terminal plasma elimination half-life (t1/2λz), consistent with nonhuman primates, was independent of dose, and ranged from 19.0 to 31.4 days (Supplementary Table S2).
Pharmacodynamics
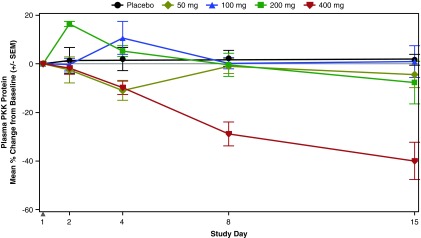
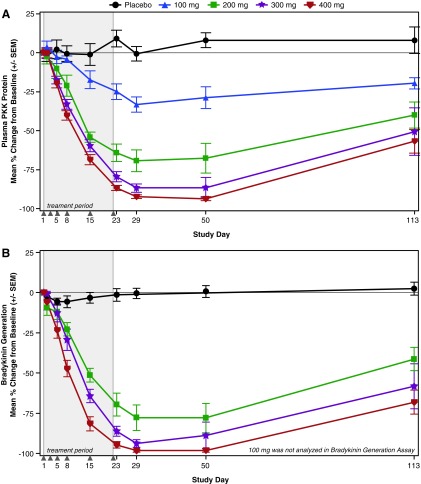
There was no statistically significant reduction of plasma PKK protein following single doses of IONIS-PKKRx, but reductions were observed at the 400 mg dose on day 8 (28.9% reduction) and day 15 (40.0% reduction) (Fig. 3). In the MAD cohorts, dose-dependent reductions of plasma PKK protein levels were statistically significant compared with placebo as early as day 5 for the 300 and 400 mg doses (Fig. 4A; P < 0.05, P < 0.01) and at all doses by day 23 (P ≤ 0.001). The 400 mg dose group achieved a 92% and 94% reduction in PKK on days 29 and 50 (respectively) that remained at ∼60% below baseline 3 months postfinal dose on day 113.
FIG. 3.
Mean percent change from baseline in plasma PKK levels in the SAD cohorts. Solid gray triangle indicates dosing day. SAD, single ascending dose.
FIG. 4.
Mean percent change from baseline in (A) plasma PKK levels and (B) the bradykinin generation assay in the MAD cohorts. Shaded region represents treatment period and solid gray triangles indicate dosing days. MAD, multiple ascending dose.
Table 2.
Baseline Characteristics of the Participants in the Multiple Ascending Dose Cohorts
| Characteristic | Placebo (N = 8) | IONIS-PKKRx | |||
|---|---|---|---|---|---|
| 100 mg (n = 6) | 200 mg (n = 7) | 300 mg (n = 6) | 400 mg (n = 6) | ||
| Age, mean (range), years | 53.8 (40–62) | 55.5 (43–65) | 49.6 (19–64) | 48.5 (33–63) | 51.5 (39–59) |
| Sex (M:F) | 6:2 | 3:3 | 5:2 | 4:2 | 2:4 |
| White | 5 (62.5) | 4 (66.7) | 6 (85.7) | 3 (50.0) | 4 (66.7) |
| Black | 3 (37.5) | 1 (16.7) | 0 (0.0) | 1 (16.7) | 1 (16.7) |
| Asian | 0 (0.0) | 1 (16.7) | 1 (14.3) | 1 (16.7) | 1 (16.7) |
| American Indian or Alaskan Native | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| BMI, mean ± SD, kg/m2 | 25.1 ± 3.2 | 27.0 ± 5.2 | 25.8 ± 3.1 | 25.4 ± 2.1 | 26.6 ± 2.7 |
| Prekallikreina,b, mean ± SD, μg/mL | 128.0 ± 33.5 | 140.2 ± 38.1 | 144.3 ± 22.4 | 116.5 ± 13.6 | 113.7 ± 20.4 |
Baseline is defined as the average of all pretreatment assessments, including day 1 predose assessments where applicable.
Pharmacodynamic analysis set used.
Ad hoc analyses were performed to assess bradykinin generation capacity, FXII-to-FXIIa conversion, and aPTT in the 200, 300, and 400 mg MAD dose cohorts. At day 29, treatment with IONIS-PKKRx produced a mean reduction from baseline of bradykinin generation capacity of 77% to 98% across the three dose groups (Fig. 4B; P < 0.01 vs. placebo) that was sustained through day 113 at 41% to 68% (P < 0.05 vs. placebo). Consistent with the role of PKK/kallikrein in the feedback activation of FXII, statistically significant, dose-dependent reductions in FXII-FXIIa conversion were observed. The mean reduction from baseline ranged from 75% to 97% on day 29 (P < 0.01, vs. placebo) and was sustained at 36% to 70% on day 113 (Supplementary Fig. S4; P < 0.05). Lastly, dose-dependent increases in the optimized aPTT were observed in the 300 and 400 mg cohort on days 23 and 50 (Supplementary Fig. S5; P < 0.05 vs. placebo).
Safety and tolerability
There were no serious or severe nonserious adverse events observed in the study. The most common treatment-emergent adverse event in both cohorts was mild injection site reactions [6 (50.0%) subjects in SAD cohort and 16 (64.0%) subjects in MAD cohort experienced at least one, all receiving IONIS-PKKRx]. In the SAD cohorts, urinary tract infection (UTI) was the only other systemic treatment-emergent adverse event that occurred in two or more IONIS-PKKRx-treated subjects. In the MAD cohorts, UTIs, contusion, headache, vessel puncture site bruise and catheter site bruise occurred in two or more IONIS-PKKRx-treated subjects (Supplementary Table S4B). UTI, vessel puncture site bruises, and catheter bruises also occurred at similar incidence rates in placebo subjects. Contusions were reported in three IONIS-PKKRx-treated subjects from the lower dose groups (n = 2, 100 mg; n = 1, 200 mg), but were mild in severity and considered not related to the study drug by the investigator. Similarly, bruising at the vessel puncture and catheter site were mild in severity and resolved without sequelae. No other bleeding events were reported.
IONIS-PKKRx was well tolerated and no subjects discontinued dosing due to adverse events at the injection site and no flu-like symptoms were reported. The mean and median percent of SC injections leading to a local cutaneous reaction at the injection site (LCRIS; started on the day of injection and lasted for at least 2 days) was 21.3% and 16.7%, respectively (Supplementary Table S5) for all subjects that received IONIS-PKKRx.
No clinically significant changes or patterns were identified at any dose in the SAD or MAD cohorts for the serum chemistry (including liver transaminases), hematology, urinalysis ECG parameters, or vital signs, including blood pressure. As expected, there were no effects on prothrombin time in the MAD cohort (PT; Supplementary Fig. S5), demonstrating that PKK inhibition has a specific effect on the intrinsic coagulation pathway without affecting the extrinsic pathway. The mean values from the standard aPTT test remained below the upper limit of normal on treatment and during posttreatment follow-up (Supplementary Fig. S6).
Discussion
In this report, preclinical and clinical studies demonstrate that IONIS-PKKRx effectively reduces plasma PKK and bradykinin generation, without pharmacologically driven safety, or toxicity concerns. Preclinical studies of mice expressing the human PKK transgene (hPKK-Tg) showed that a 3-week administration of IONIS-PKKRx resulted in dose-dependent reductions in liver hPKK mRNA and plasma hPKK protein levels. In nonhuman primates, a 16-week treatment with IONIS-PKKRx resulted in dose-dependent decreases in hepatic PKK mRNA expression and dose- and time-dependent reductions of plasma PKK protein levels up to 85% in the highest dose group (40 mg/kg/week). The pharmacokinetic data for IONIS-PKKRx in nonhuman primates demonstrated that the drug had a prolonged tissue half-life of 20.5 to 27.7 days, which is consistent with other 2′MOE ASOs, and supported weekly dosing in humans.
The pharmacodynamic effects in healthy human volunteers were consistent with the preclinical results. IONIS-PKKRx produced significant dose-dependent reductions in plasma PKK protein levels and bradykinin generation assay values at day 29 (1 week after the last dose) in the 300 and 400 mg cohorts, with significant reductions in plasma PKK levels and bradykinin generation assay values sustained through day 113 (3 months after the last dose). Analysis of FXII-FXIIa conversion showed highly statistically significant, dose-dependent, and sustained reduction in all MAD cohorts through day 113, which is consistent with PKK/kallikrein's role as a feedback activator of FXII.
Both PKK and FXII are integral components of the intrinsic coagulation pathway. Mouse models and humans with genetic deficiencies in PKK or FXII display increased aPTT, but do not display the associated increased bleeding risk [18–20]. Additionally, ASO-mediated inhibition of the contact system proteases in animal models has not been associated with bleeding risk or disruption of hemostasis [20]. Altogether, these data support the hypothesis that PKK and FXII proteins are not required for hemostasis [35,36]. In the current Phase 1 study in healthy volunteers, we observed increased aPTT accompanying the pharmacological reductions of PKK and FXIIa activation by using an optimized aPTT assay sensitive to deficiencies in PKK. In the standard laboratory assays for aPTT and prothrombin time, slight, transient mean increases in aPTT were not clinically significant, and prothrombin time was normal. These data support the hypothesis that ASO-mediated inhibition of PKK will not interfere with hemostasis. Additionally, no safety concerns, including bleeding events, were observed in any of the treated healthy volunteers and the drug was well tolerated in the Phase 1 study.
The overall profile of IONIS-PKKRx, established in preclinical studies and confirmed by the Phase 1 clinical trial results, supports the use of an ASO therapeutic approach in the chronic prophylactic setting for HAE, and potentially in other clinical settings that involve the activation of contact system. Targeting the zymogen, PKK, IONIS-PKKRx demonstrated a mechanistically differentiated means to reduce bradykinin production compared with direct kallikrein inhibition or C1-INH replacement. Other therapeutic modalities have decreased the occurrence of HAE attacks with 60% reduction in kallikrein activity. IONIS-PKKRx has the potential to further decrease attack rate, by reducing the total available prekallikrein, only a fraction of which is converted to active kallikrein, rather than inhibiting kallikrein on a stoichiometric basis. Antisense inhibition of PKK also has the potential to provide benefit for several other diseases where bradykinin or the contact system plays a mechanistic role in the disease pathogenesis, such as coagulopathies, inflammatory diseases, and diseases with significant pain components.
Results from this phase 1 study of IONIS-PKKRx provide a proof-of-principle for an ASO-mediated therapeutic approach for HAE prophylactic treatment. Since the identification of IONIS-PKKRx and initiation of this study, advances in the field have led to a new class of ASOs designed for targeted delivery to hepatocytes through the asialoglycoprotein receptor (ASGR1) and its ligand, triantennary N-acetyl galactosamine (GalNAc3) [37,38]. No chemical class effects were identified in a recent integrated assessment of phase 1 data from healthy volunteers treated with GalNAc3-conjugated 2′MOE ASOs, supporting that increased potency by targeted delivery to the liver results in an increased safety margin. As PKK is predominantly expressed in hepatocytes, and IONIS-PKKRx displayed favorable reduction of PKK and bradykinin generation capacity, it was selected as a candidate for GalNAc3 conjugation. Initial testing in healthy volunteers has demonstrated a marked reduction in plasma PKK levels at lower monthly doses [38] and clinical analyses of safety and tolerability are underway.
Supplementary Material
Acknowledgments
The first draft medical writing support was provided by Michael G. Baker, PhD, Samorn Biosciences, Inc. Graphics support was provided by Tracy Reigle, and technical support was provided by Lisa Hannan, PhD and Ekaette Mbong, PhD, Ionis Pharmaceuticals, Inc.
Author Disclosure Statement
At the time that these studies were performed, all authors were employees and shareholders of Ionis Pharmaceuticals, Inc.
Supplementary Material
References
- 1. Zuraw BL. (2008). Clinical practice. Hereditary angioedema. N Engl J Med 359:1027–1036 [DOI] [PubMed] [Google Scholar]
- 2. Lumry WR. (2013). Overview of epidemiology, pathophysiology, and disease progression in hereditary angioedema. Am J Manag Care 19:s103–s110 [PubMed] [Google Scholar]
- 3. Nzeako UC, Frigas E. and Tremaine WJ. (2001). Hereditary angioedema: a broad review for clinicians. Arch Intern Med 161:2417–2429 [DOI] [PubMed] [Google Scholar]
- 4. Lumry WR, Castaldo AJ, Vernon MK, Blaustein MB, Wilson DA. and Horn PT. (2010). The humanistic burden of hereditary angioedema: impact on health-related quality of life, productivity, and depression. Allergy Asthma Proc 31:407–414 [DOI] [PubMed] [Google Scholar]
- 5. Long AT, Kenne E, Jung R, Fuchs TA. and Renné T. (2016). Contact system revisited: an interface between inflammation, coagulation, and innate immunity. J Thromb Haemost 14:427–437 [DOI] [PubMed] [Google Scholar]
- 6. Cicardi M. and Zuraw BL. (2018). Angioedema Due to Bradykinin Dysregulation. J Allergy Clin Immunol Pract 6:1132–1141 [DOI] [PubMed] [Google Scholar]
- 7. Davis AE, Mejia P. and Lu F. (2008). Biological activities of C1 inhibitor. Mol Immunol 45:4057–4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen M. and Riedl MA. (2017). Emerging therapies in hereditary angioedema. Immunol Allergy Clin North Am 37:585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lumry WR. (2018). Current and emerging therapies to prevent hereditary angioedema attacks. Am J Manag Care 24:S299–S307 [PubMed] [Google Scholar]
- 10. Banerji A, Busse P, Shennak M, Lumry W, Davis-Lorton M, Wedner HJ, Jacobs J, Baker J, Bernstein JA, et al. (2017). Inhibiting plasma kallikrein for hereditary angioedema prophylaxis. N Engl J Med 376:717–728 [DOI] [PubMed] [Google Scholar]
- 11. Banerji A, Riedl MA, Bernstein JA, Cicardi M, Longhurst HJ, Zuraw BL, Busse PJ, Anderson J, Magerl M, et al. (2018). Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA 320:2108–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aygören-Pürsün E, Bygum A, Grivcheva-Panovska V, Magerl M, Graff J, Steiner UC, Fain O, Huissoon A, Kinaciyan T, et al. (2018). Oral plasma kallikrein inhibitor for prophylaxis in hereditary angioedema. N Engl J Med 379:352–362 [DOI] [PubMed] [Google Scholar]
- 13. Bennett CF, Baker BF, Pham N, Swayze E. and Geary RS. (2017). Pharmacology of antisense drugs. Annu Rev Pharmacol Toxicol 57:81–105 [DOI] [PubMed] [Google Scholar]
- 14. Crooke ST, Witztum JL, Bennett CF. and Baker BF. (2018). RNA-targeted therapeutics. Cell Metab 27:714–739 [DOI] [PubMed] [Google Scholar]
- 15. Crooke ST, Baker BF, Kwoh TJ, Cheng W, Schulz DJ, Xia S, Salgado N, Bui H-HH, Hart CE, et al. (2016). Integrated safety assessment of 2′-O-methoxyethyl chimeric antisense oligonucleotides in nonhuman primates and healthy human volunteers. Mol Ther 24:1771–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crooke ST, Baker BF, Pham NC, Hughes SG, Kwoh TJ, Cai D, Tsimikas S, Geary RS. and Bhanot S. (2018). The effects of 2′-O-methoxyethyl oligonucleotides on renal function in humans. Nucleic Acid Ther 28:10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crooke ST, Baker BF, Witztum JL, Kwoh TJ, Pham NC, Salgado N, McEvoy BW, Cheng W, Hughes SG, Bhanot S. and Geary RS. (2017). The effects of 2′-O-methoxyethyl containing antisense oligonucleotides on platelets in human clinical trials. Nucleic Acid Ther 27:121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Girolami A, Allemand E, Bertozzi I, Candeo N, Marun S. and Girolami B. (2010). Thrombotic events in patients with congenital prekallikrein deficiency: a critical evaluation of all reported cases. Acta Haematol 123:210–214 [DOI] [PubMed] [Google Scholar]
- 19. Ratnoff OD. and Colopy JE. (1955). A familial hemorrhagic trait associated with a deficiency of a clot-promoting fraction of plasma. J Clin Invest 34:602–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Revenko AS, Gao D, Crosby JR, Bhattacharjee G, Zhao C, May C, Gailani D, Monia BP. and MacLeod AR. (2011). Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood 118:5302–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Altmann KH, Dean NH, Fabbro D, Freier SM, Geiger T, Harner R, Husken D, Martin P, Monia BP, et al. (1996). Second generation antisense oligonucleotides: from nuclease resistance to biological efficacy in animals. CHIMIA 50:168–176 [Google Scholar]
- 22. McKay RA, Miraglia LJ, Cummins LL, Owens SR, Sasmor H. and Dean NM. (1999). Characterization of a potent and specific class of antisense oligonucleotide inhibitor of human protein kinase C-alpha expression. J Biol Chem 274:1715–1722 [DOI] [PubMed] [Google Scholar]
- 23. Geary RS, Yu RZ, Watanabe T, Henry SP, Hardee GE, Chappell A, Matson J, Sasmor H, Cummins L. and Levin AA. (2003). Pharmacokinetics of a tumor necrosis factor-alpha phosphorothioate 2′-O-(2-methoxyethyl) modified antisense oligonucleotide: comparison across species. Drug Metab Dispos 31:1419–1428 [DOI] [PubMed] [Google Scholar]
- 24. Clark JD, Gebhart GF, Gonder JC, Keeling ME. and Kohn DF. (1997). Special report: the 1996 guide for the care and use of laboratory animals. ILAR J 38:41–48 [DOI] [PubMed] [Google Scholar]
- 25. Yu RZ, Baker B, Chappell A, Geary RS, Cheung E. and Levin AA. (2002). Development of an ultrasensitive noncompetitive hybridization-ligation enzyme-linked immunosorbent assay for the determination of phosphorothioate oligodeoxynucleotide in plasma. Anal Biochem 304:19–25 [DOI] [PubMed] [Google Scholar]
- 26. Defendi F, Charignon D, Ghannam A, Baroso R, Csopaki F, Allegret-Cadet M, Ponard D, Favier B, Cichon S, et al. (2013). Enzymatic assays for the diagnosis of bradykinin-dependent angioedema. PLoS One 8:e70140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bowyer A, Kitchen S. and Makris M. (2011). The responsiveness of different APTT reagents to mild factor VIII, IX and XI deficiencies. Int J Lab Hematol 33:154–158 [DOI] [PubMed] [Google Scholar]
- 28. Freier SM. and Watt AT. (2008). Basic principles of antisense drug discovery. In: Antisense Drug Technology: Principles, Strategies, and Applications, 2nd ed. Crooke ST, ed. CRC Press, Boca Raton, FL, pp 117–141 [Google Scholar]
- 29. Crooke ST, Vickers T, Lima W. and Wu H. (2008). Mechanisms of antisense drug action, an introduction. In: Antisense Drug Technology: Principles, Strategies, and Applications, 2nd ed. Crooke ST, ed. CRC Press, Boca Raton, FL, pp 3–46 [Google Scholar]
- 30. Crooke RM. and Graham MJ. (2013). Modulation of lipoprotein metabolism by antisense technology: preclinical drug discovery methodology. Methods Mol Biol 1027:309–324 [DOI] [PubMed] [Google Scholar]
- 31. Cioffi CL, Garay M, Johnston JF, McGraw K, Boggs RT, Hreniuk D. and Monia BP. (1997). Selective inhibition of A-Raf and C-Raf mRNA expression by antisense oligodeoxynucleotides in rat vascular smooth muscle cells: role of A-Raf and C-Raf in serum-induced proliferation. Mol Pharmacol 51:383–389 [PubMed] [Google Scholar]
- 32. Henry SP, Narayanan P, Shen L, Bhanot S, Younis HS. and Burel SA. (2017). Assessment of the effects of 2′-methoxyethyl antisense oligonucleotides on platelet count in cynomolgus nonhuman primates. Nucleic Acid Ther 27:197–208 [DOI] [PubMed] [Google Scholar]
- 33. Henry SP, Kim T-W, Kramer-Stickland K, Zanardi TA, Fey RA. and Levin AA. (2008). Toxicologic properties of 2′-O-methoxyethyl chimeric antisense inhibitors in animals and man. In: Antisense Drug Technology: Principles, Strategies, and Applications, 2nd ed. Crooke ST, ed. CRC Press, Boca Raton, FL, pp 327–363 [Google Scholar]
- 34. Geary RS, Wancewicz E, Matson J, Pearce M, Siwkowski A, Swayze E. and Bennett F. (2009). Effect of dose and plasma concentration on liver uptake and pharmacologic activity of a 2′-methoxyethyl modified chimeric antisense oligonucleotide targeting PTEN. Biochem Pharmacol 78:284–291 [DOI] [PubMed] [Google Scholar]
- 35. Roller RE, Korninger C. and Binder BR. (1997). Assessment of hemostasis. In: Diagnostics of Vascular Diseases: Principles and Technology. Lanzer P, Lipton M, eds. Springer, Berlin, Germany, pp 157–169 [Google Scholar]
- 36. Schmaier AH. (2016). The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost 14:28–39 [DOI] [PubMed] [Google Scholar]
- 37. Nair JK, Willoughby JLS, Chan A, Charisse K, Alam MR, Wang Q, Hoekstra M, Kandasamy P, Kel'in AV, et al. (2014). Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc 136:16958–16961 [DOI] [PubMed] [Google Scholar]
- 38. Crooke ST, Baker BF, Xia S, Yu RZ, Viney NJ, Wang Y, Tsimikas S. and Geary RS. (2018). Integrated assessment of the clinical performance of GalNAc3-conjugated 2′-O-methoxyethyl chimeric antisense oligonucleotides: I. Human Volunteer Experience. Nucleic Acid Ther 27:121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.