Abstract
Background
Resin‐based composite (RBC) is currently accepted as a viable material for the restoration of caries for posterior permanent teeth requiring surgical treatment. Despite the fact that the thermal conductivity of the RBC restorative material closely approximates that of natural tooth structure, postoperative hypersensitivity is sometimes still an issue. Dental cavity liners have historically been used to protect the pulp from the toxic effects of some dental restorative materials and to prevent the pain of thermal conductivity by placing an insulating layer between restorative material and the remaining tooth structure.
Objectives
The objective of this review was to assess the effects of using dental cavity liners in the placement of Class I and Class II resin‐based composite posterior restorations in permanent teeth in children and adults.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (to 25 May 2016), the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4) in the Cochrane Library (searched 25 May 2016), MEDLINE Ovid (1946 to 25 May 2016), Embase Ovid (1980 to 25 May 2016) and LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; 1982 to 25 May 2016). We searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform for ongoing trials. No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria
We included randomized controlled trials assessing the effects of the use of liners under Class I and Class II posterior resin‐based composite restorations in permanent teeth (in both adults and children). We included both parallel and split‐mouth designs.
Data collection and analysis
We utilized standard methodological procedures prescribed by Cochrane for data collection and analysis. Two review authors screened the search results and assessed the eligibility of studies for inclusion against the review inclusion criteria. We conducted risk of bias assessments and data extraction independently and in duplicate. Where information was unclear we contacted study authors for clarification.
Main results
Eight studies, recruiting over 700 participants, compared the use of dental cavity liners to no liners for Class I and Class II resin‐based composite restorations.
Seven studies evaluated postoperative hypersensitivity measured by various methods. All studies were at unclear or high risk of bias. There was inconsistent evidence regarding postoperative hypersensitivity (either measured using cold response or patient‐reported), with a benefit shown at some, but not all, time points (low‐quality evidence).
Four trials measured restoration longevity. Two of the studies were judged to be at high risk and two at unclear risk of bias. No difference in restoration failure rates were shown at one year follow‐up, with no failures reported in either group for three of the four studies; the fourth study had a risk ratio (RR) 1.00 (95% confidence interval (CI) 0.07 to 15.00) (low‐quality evidence). Three studies evaluated restoration longevity at two years follow‐up and, again, no failures were shown in either group.
No adverse events were reported in any of the included studies.
Authors' conclusions
There is inconsistent, low‐quality evidence regarding the difference in postoperative hypersensitivity subsequent to placing a dental cavity liner under Class I and Class II posterior resin‐based composite restorations in permanent posterior teeth in adults or children 15 years or older. Furthermore, no evidence was found to demonstrate a difference in the longevity of restorations placed with or without dental cavity liners.
Keywords: Adolescent; Adult; Humans; Composite Resins; Dental Restoration, Permanent; Dental Restoration, Permanent/adverse effects; Dental Restoration, Permanent/classification; Thermal Conductivity; Dental Caries; Dental Caries/classification; Dental Caries/surgery; Dental Cavity Lining; Dental Cavity Lining/instrumentation; Dentin Sensitivity; Dentin Sensitivity/etiology; Dentin Sensitivity/prevention & control; Pain, Postoperative; Pain, Postoperative/prevention & control; Randomized Controlled Trials as Topic
Dental cavity liners under tooth‐colored resin fillings placed into permanent teeth in the back of the mouth
Review question
This review was conducted to assess the effects of using liners under tooth‐colored resin fillings in cavities on the biting surface (Class I) and the biting surface and side(s) (Class II) of permanent teeth in the back of the mouth in children and adults.
Background
Tooth decay is the most common disease affecting children and adults worldwide. If left untreated, acid produced by bacteria in the dental plaque or biofilm forms cavities or holes in the teeth. A number of techniques and a variety of materials can be used to restore or fill teeth affected by decay. One of these materials is tooth‐colored, resin‐based composite or RBC. This material is increasingly used as an alternative to amalgam (a mixture of mercury and metal alloy particles).
Since the 19th century liners have often been placed in cavities in the teeth under the filling material. The liners are thought to protect the living pulp of the tooth from filling materials themselves and also from their potential to allow more heat or cold through than the natural tooth would. Although RBC filling materials are thought to be similar to the natural material of teeth in terms of how they conduct heat, sensitivity to temperature change is sometimes still an issue for people after treatment.
Study characteristics
The evidence in this review, carried out by authors from Cochrane Oral Health, is up to date as of 25th May 2016.
Eight studies, with over 700 participants, were included. Two studies were conducted in the USA, two in Thailand, two in Germany and one each in Saudi Arabia and Turkey. The studies compared the use of liners under tooth‐colored resin fillings (RBC) in permanent teeth at the back of the mouth to no liners for Class I and Class II fillings. One of the two studies in the USA took place in dental practices, the others in university‐based dental schools. All participants were over 15 years of age.
Key results
Very little evidence was found to show that a liner under Class I and II RBC fillings in permanent teeth in the back of the mouth reduced sensitivity in adults or children 15 years or older. No evidence was found to show that there was any difference in the length of time fillings lasted when placed with or without a cavity liner.
Quality of evidence
The body of evidence identified in this review does not allow for robust conclusions about the effects of dental cavity liners. The quality of the evidence identified in this review is low and there is a lack of confidence in the effect estimates. Furthermore, no evidence was found to demonstrate a difference in how long restorations last when placed with or without dental cavity liners.
Summary of findings
Summary of findings for the main comparison.
| Liner versus no liner for Class I and Class II resin‐based composite restorations | ||||||
|
Patient or population: Patients requiring Class I or Class II resin‐based composite restorations Settings: General practice Intervention: Liner Comparison: No liner | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of restorations (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk* | Corresponding risk | |||||
| No liner | Liner | |||||
|
Postoperative hypersensitivity (POH) (Patient‐reported Y/N) Follow‐up: 1 week |
100 per 1000 | 56 per 1000 (26 to 117) | RR 0.56 (0.26 to 1.17) | 299 (3 studies) | ⊕⊕⊝⊝1 low | POH was also measured at 24 hours (1 trial at high risk of bias) and 1 month (3 trials at high/unclear risk of bias). A benefit in favour of liners was shown at 24 hours; this difference was not maintained at any other time point 1 additional high risk of bias study measured patient‐reported POH using VAS. A benefit was shown in favour of liners at 1 week and 1 month follow‐up |
|
Postoperative cold response measurement (CRM) (time it took in seconds for patient to feel cold sensation) Follow‐up: 1 week |
The mean postoperative CRM at 1 week (time it took in seconds for patient to feel cold sensation) was 16 seconds | MD 6 seconds more (1.36 more to 10.64 more) | 88 (1 study) | ⊕⊕⊝⊝2 low | CRM was also measured at 24 hours (1 trial at high risk of bias) and 1 month (1 trial at high risk of bias). No difference was shown between the use of liners and no liners at either time point Other methods of measuring CRM (using VAS or Y/N response) showed no difference between liners and no liners at any time point |
|
|
Restoration failure Follow‐up: 1 year |
7 per 1000 | 7 per 1000 (0 to 104) | RR 1.00 (0.07 to 15.00) | 281 (4 studies)3 | ⊕⊕⊝⊝1 low | Restoration failure at 2‐year follow‐up also showed no difference between the use of liners or not |
| Adverse events | None reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; MD: mean difference; RR: risk ratio; VAS: visual analog scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
*Assumed risk based on control group risk. 1Downgraded due to high risk of bias and imprecision. 2Downgraded due to single study at high risk of bias. 34 studies reported on restoration failure at 1 year. However, no failures were reported in either group for 3 of the 4 studies; these studies do not inform the RR presented.
Background
Description of the condition
Dental caries is a condition in which a tooth has been subjected to a demineralization process that can lead to a carious lesion and eventually to a cavity in the tooth. Demineralization is due to an acidic environment created by the metabolic by‐products of certain bacteria (Fejerskov 2003). Dental caries is currently the most prevalent disease in the world, affecting 60% to 90% of the school‐aged population in low‐income, middle‐income and high‐income countries and almost all of the adults in most countries (Petersen 2003; Petersen 2005). Caries prevalence varies significantly from country to country with some of the low‐income countries having the lowest caries rates (Edelstein 2006). This is thought to be due to the maintenance of a traditional diet with lower sugar consumption and lower levels of urbanization in the poorest, low‐income countries (Diehnelt 2001). As these poorest countries begin to develop, urbanization and sugar consumption increase and a rise in caries prevalence is seen (Diehnelt 2001). Caries prevalence also varies significantly within individual low‐income, middle‐income and high‐income countries with people having the lowest education levels, the lowest socioeconomic status and those living in poverty having the highest prevalence (Selwitz 2007).
Dental caries can be classified by location and extent of the lesions produced by the demineralization. The most common classification system is the one created by GV Black that assigns a classification to the lesion based on its location on the tooth. In this system, a lesion located in the pits and fissures (grooves) of the occlusal (biting) surface of a tooth is considered a Class I lesion, and a lesion located on a proximal (in between) surface of a posterior tooth is considered a Class II lesion (Black 1924). Once a carious lesion has developed to the point where it must be restored, the traditional method of restoring the lesion is to surgically remove the caries using a dental drill and filling the resulting cavity with a restorative material. The most common materials currently in use for the permanent restoration of carious lesions in posterior teeth are dental amalgam and resin‐based composite.
Description of the intervention
Resin‐based composite (RBC) is currently accepted as a viable material for the restoration of caries for posterior permanent teeth requiring surgical treatment (Demarco 2012). These materials are formulated to be placed into the prepared tooth cavity in a soft, viscous state, and then made to harden through a process known as polymerization. Polymerization can be initiated by one of two methods. In the first method, a catalyst is mixed with a base, and chemical activation hardens the material. In the second method, the material is formulated to harden via light activation. The light‐activated materials have the advantages of setting more quickly, of not having to be mixed and of giving the operator control over when the material will harden. Since the 19th century, dental materials have been developed and used to protect the pulp by being placed between the tooth structure and the restorative material (Harris 1863). Liners are purported to protect the pulp from the toxic effects of some dental restorative materials and to prevent the pain of thermal conductivity by placing an insulating layer between restorative material and the remaining tooth structure (Roberson 2006). Like liners, sealers are sometimes advocated to reduce thermal sensitivity under metallic restorations. However, while it is possible to place a resin‐based composite without a liner, sealers are an integral part of the technique of placing an RBC restoration, since the sealer is used to bond the material to the tooth structure. Both liners and sealers can also be light cured or chemically cured.
Despite the fact that the thermal conductivity of the RBC restorative material closely approximates that of natural tooth structure, postoperative thermal sensitivity is sometimes still an issue (Briso 2007). The liners most commonly used in restorative dentistry include calcium hydroxide and glass ionomer cements, both of which are available in either chemical or light‐cured formulations. Current evidence indicates that posterior composite restorative dental materials are likely to be very well tolerated by the pulp, and that significant adverse reactions are most likely the result of the presence of bacteria and their by‐products (Summitt 2006). Even when the placement of liners is limited to the deeper restorations, their clinical benefits may not live up to their theoretical value (Unemori 2007).
How the intervention might work
Current theories regarding postoperative tooth sensitivity following the placement of RBC restorations are based on microleakage as the cause either directly by hydrodynamic flow of fluid through the dentinal tubules or from bacterial by‐products reaching the pulp through the tubules (Summitt 2006). Liners are advocated to provide a better seal of the tubules in order to reduce or eliminate postoperative sequelae (Murray 2001). Liners are also sometimes advocated to stimulate favorable pulpal reactions underneath restorations in close proximity to the pulpal tissue (Murray 2002). However, overall there is little clinical evidence linking the use of liners to a reduction in postoperative sensitivity (Wegehaupt 2009). Liners placed for the purpose of pulpal protection are thought to medicate the pulpal tissue, causing sedation and hopefully stimulation of reparative dentin formation (Roberson 2006). Calcium hydroxide liners are most frequently advocated for the deepest restorations due to their high pH, which stimulates the formation of reparative dentin (Murray 2002a). Zinc oxide eugenol liners are most frequently advocated due to their sedative effect on pulpal tissue but are not commonly used under RBCs (Murray 2001). Liners placed for the purpose of reducing postoperative sensitivity are thought to better seal the dentinal tubules than bonding the RBC restoration directly to tooth structure. The improved seal would reduce microleakage and prevent or reduce the hydrodynamic flow of fluid through the tubules, subsequently preventing or reducing the by‐products of bacterial activity from reaching the pulp (Summitt 2006).
Why it is important to do this review
Dentists frequently choose the materials and the techniques they use in practice based on the education and clinical experiences they receive while in school (Lynch 2006). However, survey results show that there is significant variation in what is being taught in dental schools, both within and among different countries across the globe, regarding the placement of liners underneath RBC restorations (Castillo‐de Oyagüe 2012; Gordan 2000; Hayashi 2009; Liew 2011; Lynch 2006; Lynch 2006a; Lynch 2006b; Lynch 2007; Lynch 2007a; Lynch 2011; Sadeghi 2009; Wilson 2000). All of the surveys reviewed asked similar questions, and responses were obtained for preparations that were shallow (outer one‐third of dentin), moderate (middle one‐third of dentin), and deep (inner one‐third of dentin). The surveys revealed that for shallow preparations dental school faculty members do not typically recommend a liner. Approximately half of the respondents advocated the placement of liners for moderate preparations. The majority of respondents did advocate the placement of liners for deep preparations, but some controversy remains. In fact, some dental educators contend that the placement of liners (as opposed to the direct bonding of the RBC to the tooth) is not beneficial, and may be detrimental, even in the restoration of deep caries (Castillo‐de Oyagüe 2012; Gordan 2000; Hayashi 2009; Lynch 2006; Lynch 2006a; Lynch 2006b; Lynch 2007; Lynch 2007a; Lynch 2011; Sadeghi 2009; Wilson 2000). There is some evidence that the placement of a liner underneath an RBC restoration shortens the life expectancy of that restoration significantly (Demarco 2012). This may be due to the fact that the lining material does not bond to tooth structure or does not bond well to RBC allowing greater microleakage. The liners reported on in these surveys were exclusively calcium hydroxide and glass ionomer cement. In 2002 Deliperi and Bardwell suggested the use of flowable composite as a cavity liner in order to "reduce marginal discoloration, recurrent caries and postoperative sensitivity, and potentially improve longevity of these Class I and Class II RBC restorations" (Deliperi 2002). The surveys revealed that no dental school curricula incorporated the use of flowable composite as a liner. One of the few clinical studies conducted on the subject showed no improvement in restoration performance by placing a flowable composite liner under a Class II RBC (Efes 2006).
Whenever possible, the most biocompatible, longest lasting restorations should be utilized in the restoration of defective or missing tooth structure. While much time, effort and expense is spent researching, developing, manufacturing, testing, marketing, and placing dental cavity liners, little evidence exists as to whether or not these materials are effective. This review has compiled the evidence regarding the effectiveness of cavity liners for the translation into practice, thus assisting in the creation of an evidence‐based rationale for the use of cavity liners.
Objectives
To assess the effects of using dental cavity liners in the placement of Class I and Class II resin‐based composite posterior restorations in permanent teeth in children and adults.
Methods
Criteria for considering studies for this review
Types of studies
All studies included were randomised controlled clinical trials comparing the use of liners under Class I and Class II posterior resin‐based composite restorations in permanent teeth. We included both parallel and split‐mouth designs.
We excluded studies examining:
bases;
amalgam or any other metallic restorations;
any indirect restorations;
anterior restorations;
liners in vitro.
Types of participants
Adults or children with at least one restoration in a posterior permanent tooth/teeth undergoing a Class I or Class II resin‐based composite restoration(s).
Types of interventions
Any type of dental cavity liner placed under a Class I or Class II resin‐based composite restoration on a posterior tooth was considered, including but not limited to calcium hydroxide, glass ionomer, resin‐modified glass ionomer, flowable composite, zinc phosphate cement, zinc and eugenol cement . The comparison group in included trials received Class I or Class II resin‐based composite restoration on a posterior tooth directly bonded to the tooth without the use of a dental cavity liner.
Types of outcome measures
Primary outcomes
Postoperative hypersensitivity to hot, cold, biting, chewing, and/or sweets experienced by the patient within one month following the intervention. Postoperative hypersensitivity could be measured by a visual analog scale (VAS) or by hypersensitivity present or absent as tested by dentist or patient self‐report.
Restoration failure. Survival time of the resin‐based composite restoration (in months) from the time of placement with a minimum follow‐up of one year.
Secondary outcomes
Cost of materials.
Adverse events: pulpal involvement, tooth fracture, hypersensitivity reactions to the materials, etc. or any other adverse event described in any of the studies.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions:
Cochrane Oral Health's Trials Register (searched 25 May 2016) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4) in the Cochrane Library (searched 25 May 2016) (Appendix 2);
MEDLINE Ovid (1946 to 25 May 2016) (Appendix 3);
Embase Ovid (1980 to 25 May 2016) (Appendix 4);
LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; 1982 to 25 May 2016) (Appendix 5).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011).
Searching other resources
We searched the following trial registries for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 25 May 2016) (Appendix 6);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 25 May 2016) (Appendix 6).
The reference lists of relevant articles were checked and we contacted known experts in the field.
We did not perform a separate search for adverse effects of interventions used. We considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors (Andrew Schenkel (AS) reviewed all and Ivy Peltz (IP)) and Analia Veitz‐Keenan (AVK) each reviewed some) screened titles and abstracts from the electronic searches to identify potentially eligible studies, which required further evaluation to determine whether they met the inclusion criteria for this review. No language restrictions were imposed. The third review author moderated any disagreement as appropriate (either IP or AVK). Full‐text copies of all eligible and potentially eligible studies were obtained and these were further evaluated in detail by two review authors (AS reviewed all and IP or AVK reviewed some) to identify those studies which actually met all the inclusion criteria. The third review author moderated any disagreement (AVK or IP as appropriate). From this group, we recorded those studies not meeting the inclusion criteria in the excluded studies section of the review and the reasons for exclusion were noted in the 'Characteristics of excluded studies' table. A PRISMA flow chart was created to summarize this process.
Data extraction and management
A form was created for data extraction. The form included the author, the date, the journal, the type of trial, the type of randomization (sample size, allocation concealment, masking, and dropouts), the type of intervention, the comparison, outcomes reported, duration of the trial, and funding details. Two review authors extracted the data independently from each study (AS from all and IP or AVK from some). The third review author (AVK or IP as appropriate) moderated any disagreements.
The form also included the following categories.
Conducted in: (country).
Number of centers.
Setting.
Number of participants recruited.
Recruitment period.
Inclusion criteria.
Exclusion criteria.
Number of participants randomized.
Number of patients evaluated.
-
Study design.
Parallel‐group.
Split‐mouth study.
Type(s) of treatment(s) and control intervention(s).
Type of liner(s).
Treatment and control interventions.
Mode of administration of intervention(s) and control(s).
When were outcomes measured.
Duration of follow‐up.
Were groups comparable at baseline.
Were there any co‐interventions.
Any other issues.
Assessment of risk of bias in included studies
We followed the assessment of risk of bias suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and utilised the two‐part tool, addressing the seven specific key domains (sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and 'other bias') as described in the Cochrane Handbook for Systematic Reviews of Interventions. For each domain in the tool we included one or more specific entry in a 'Risk of bias' table. Within each entry, the first part of the tool described what was reported to have happened in the study in sufficient detail to support a judgment about the risk of bias. The second part of the tool assigned a judgment of 'low risk' of bias, 'high risk' of bias, or 'unclear risk' of bias regarding the risk of bias for that domain.
The domains of sequence generation, allocation concealment, selective outcome reporting and 'other bias' were each addressed in the tool by a single entry for each study. For blinding of participants and personnel, blinding of outcome assessment and for incomplete outcome data, two or more entries could be used because assessments generally need to be made separately for different outcomes (or for the same outcome at different time points). We made an overall judgment of 'low risk' of bias for a study when any plausible bias across all seven domains was unlikely to have altered the results. We made an overall judgment of 'unclear risk' of bias for a study when any plausible bias across one or more of the key domains raises some doubt that it may have altered the results. We made an overall judgment of 'high risk' of bias for a study when any plausible bias across one or more of the key domains seriously weakened our confidence in the results reported in that study.
Two review authors conducted the assessment of risk of bias independently and in duplicate (AS for all studies and IP or AVK for some studies). The third review author (AVK or IP as appropriate) moderated any disagreements. For each included study we presented a 'Risk of bias' table as described in the Cochrane Handbook for Systematic Reviews of Interventions. We also included a 'Risk of bias summary' graph as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
For dichotomous outcomes, we presented the estimate of treatment effect of an intervention as a risk ratio (sensitivity present/not present) together with the 95% confidence interval. For continuous outcomes (such as mean VAS scores), we used mean differences and standard deviations to summarize the data for each trial. We considered each category of sensitivity separately if there were enough data from included studies or pooled together as one category if there were not enough separate data. We standardised VAS scales of different lengths as a result.
Unit of analysis issues
Where the unit of randomization was a tooth, a trial participant was permitted to contribute more than one tooth to the study. This clustering of teeth within an individual was accounted for in the analysis of the outcomes in order to avoid unit of analysis errors. If it had been unclear from the reports of included trials whether clustering had been considered, we would have contacted authors to clarify how this dependence had been accounted for in the analysis.
Where repeated measures were made (e.g. sensitivity measurements over weeks), we considered time points of up to 30 days after restoration placement likely to provide the most clinically meaningful data for postoperative hypersensitivity.
Dealing with missing data
In cases of missing or incomplete data, we attempted to contact the study authors.
Assessment of heterogeneity
We assessed heterogeneity by inspection of the point estimates and confidence intervals on the forest plots. We assessed the variation in treatment effects by means of Cochran's test for heterogeneity and the I2 statistic. We considered heterogeneity statistically significant if the P value was < 0.1. A rough guide to the interpretation of the I2 statistic given in the Cochrane Handbook for Systematic Reviews of Interventions is: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% may represent considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
If there had been more than 10 studies in one outcome we would have constructed a funnel plot in order to look for evidence of publication bias.
Data synthesis
Where studies of similar comparisons reporting the same outcome measures were included, we combined these in a meta‐analysis. We combined risk ratios for dichotomous data, and mean differences for continuous data, using random‐effects models, provided there were more than three studies in the meta‐analysis.
Treatment effects from split‐mouth trials were combined with those from parallel‐group trials where appropriate using the generic inverse variance method incorporated in Review Manager (RevMan) (RevMan 2014). Where this was not appropriate we have presented a narrative synthesis.
Subgroup analysis and investigation of heterogeneity
The following subgroups would have been investigated, if data had allowed.
Different types of liners.
Different depths of caries.
Sensitivity analysis
Had sufficient trials been identified, we would have conducted sensitivity analysis including only those trials at low risk of bias.
Presentation of main results
We developed a 'Summary of findings' table for the primary outcomes of this review following GRADE methods (GRADE 2004) and using GRADEproGDT software (GRADEproGDT 2014). The quality of the body of evidence was assessed with reference to the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates, the risk of publication bias, and the magnitude of the effect. We categorised the quality of the body of evidence for each of the primary outcomes as high, moderate, low or very low.
Results
Description of studies
See 'Characteristics of included studies' and 'Characteristics of excluded studies' tables.
Results of the search
The electronic searches identified a total of 1300 references of which 453 proved to be duplicates. Other sources identified three additional references to make a total of 850 records that were screened. Two review authors independently screened these titles and abstracts (where available). From these, we identified 27 reports of trials as potentially eligible according to the defined inclusion criteria for this review with regard to study design, participants, and interventions. We obtained full‐text copies of these 27 reports, and after further evaluation, we excluded 18 of these studies. We recorded the reasons for exclusion of these 18 studies in the 'Characteristics of excluded studies' table. Eight studies (nine reports) (Akpata 2001; Banomyong 2013; Boeckler 2012; Browning 2006; Burrow 2009; Efes 2006; Strober 2013; Wegehaupt 2009) met the inclusion criteria for this review. This process is presented as a flow chart in Figure 1.
Figure 1.
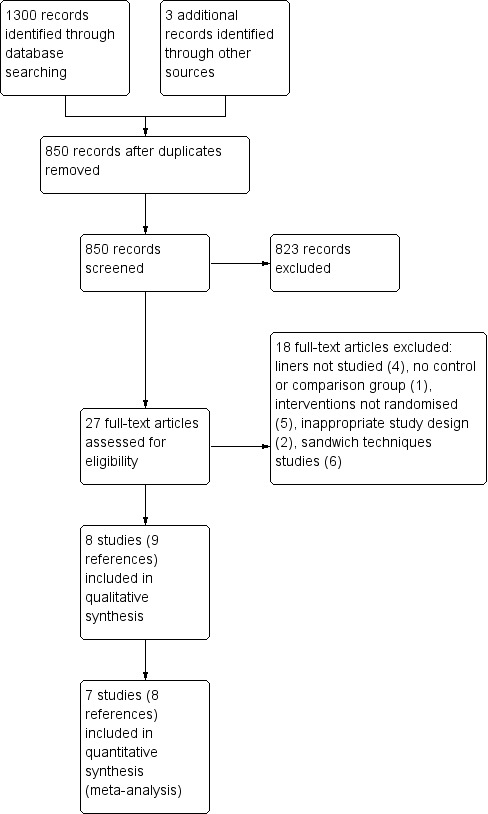
Study flow diagram.
Included studies
Characteristics of the trial settings and investigators
Three of the eight included studies were designed as split‐mouth studies (Akpata 2001; Boeckler 2012; Efes 2006). The remaining five were parallel‐group studies (Banomyong 2013; Browning 2006; Burrow 2009; Strober 2013; Wegehaupt 2009). Of the eight included studies, two were conducted in the USA (Browning 2006; Strober 2013), two in Thailand (Banomyong 2013; Burrow 2009), two in Germany (Boeckler 2012; Wegehaupt 2009), and one each in Saudi Arabia (Akpata 2001) and Turkey (Efes 2006).
Only two studies provided funding information (Boeckler 2012; Strober 2013). Boeckler 2012 was conducted in a university‐based dental school setting and funding was provided by Ivoclar Vivadent. Strober 2013 was conducted in 28 private dental practices that were part of a practice‐based research network in the USA, and funding was provided by grant U01‐DE016755, which was awarded to the College of Dentistry, New York University, New York City, by the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, USA. The other six studies (Akpata 2001; Banomyong 2013; Browning 2006; Burrow 2009; Efes 2006; Wegehaupt 2009) were conducted in university‐based dental school settings. It is possible that these studies may have received institutional funding.
Sample size calculations were reported in only one of the included trials (Strober 2013).
Characteristics of the participants
All included trials were conducted in patients with solely adult dentition. Trials recruited between 44 and 351 participants, with a mean of 99 participants per trial. However, the largest study (Strober 2013) considerably skews this mean. Removing the largest study from this calculation yields a mean of 64 participants for the remaining seven studies.
The participants all needed restorations placed due to primary or secondary carious lesions in one or more permanent teeth. Six studies specified moderate to large sized lesions (Banomyong 2013; Browning 2006; Burrow 2009; Efes 2006; Strober 2013; Wegehaupt 2009), one study included small or moderately large (bucco‐lingual dimension up to half the inter‐cuspal width) sized lesions (Akpata 2001) and one study included lesions of any size without limitations (Boeckler 2012). One study (Akpata 2001) included only male participants while all other studies including both males and females.
Characteristics of outcome measures
Primary outcomes
Seven of the included trials evaluated postoperative hypersensitivity (POH) measured by various methods (Akpata 2001; Banomyong 2013; Boeckler 2012; Burrow 2009; Efes 2006; Strober 2013; Wegehaupt 2009). Five studies measured POH via a yes/no patient report (Akpata 2001; Banomyong 2013; Burrow 2009; Efes 2006; Wegehaupt 2009). Two studies measured POH via a cold response measurement (CRM) on a visual analog scale (VAS) (Burrow 2009; Strober 2013). One study (Akpata 2001) measured POH via CRM in time (seconds) and one study (Burrow 2009) measured POH via a yes/no CRM. One study (Boeckler 2012) measured POH via CRM using subjective descriptive patient response criteria at baseline, six months, one and two years. This study found two restorations in the intervention group (liner) and two restorations in the control group (no liner) exhibiting significant POH at baseline but no subjects reported any POH at six months, one year or two years. No discussion of these results was included in this study. These data were not included in any analyses in this review since this review is limited to POH measured up to one month postoperatively.
Four of the included trials measured restoration longevity (Banomyong 2013; Boeckler 2012; Browning 2006; Efes 2006).
Secondary outcomes
We listed cost of materials and adverse events (pulpal involvement, tooth fracture, hypersensitivity reactions to the materials, etc. or any other adverse event described in any of the studies) as secondary outcomes that we would include in this review. No adverse events were reported in any of the included studies. Authors of one study indicated that they would report adverse events but no adverse events were reported (Strober 2013). Strober 2013 stated that an adverse event was considered to be "lingering pain upon removal of the stimulus". None of the other included studies made any mention of any adverse events. Only one study (Strober 2013) included cost. (See 'Effects of interventions' section for their analysis.)
Excluded studies
See 'Characteristics of excluded studies' table for information on each excluded study.
We obtained full‐text copies of the following 18 studies, which appeared from their titles and abstracts to be eligible for inclusion. Evaluation of these trials resulted in their exclusion from this review for the following reasons.
No control or comparison group included in the trial (one report: Huth 2003).
Inappropriate study design in the trial (two reports: Rasmusson 1998a; Whitworth 2005b).
Liners were not studied in the trial (four reports: Akpata 2006; Fagundes 2009; Loguercio 2001; Shi 2010).
Not actually a randomized controlled trial (five reports: Ernst 2002; Ernst 2003; Kaurani 2007; Noro 1983; Unemori 20011).
Restorative 'sandwich' techniquec utilized in the trial (six reports: Andersson‐Wenckert 2002; Andersson‐Wenckert 2004; Grogono 1990; Knibbs 1992; van Dijken 1999; Vilkinis 2000).
aThis study compared one brand of resin‐based composite placed without a liner to a second brand of resin‐based composite placed with a flowable composite as a liner. bThe decision of which restoration to place (composite or amalgam) was left to the discretion of the operator, and information regarding how this decision was made was not provided. cThe placement of a restoration utilizing the 'sandwich' technique has many similarities to the placement of a restoration utilizing a cavity liner under a resin‐based composite material. The techniques differ significantly, however, in that the resin‐modified glass ionomer (RMGI) placed under the resin composite in the 'sandwich' technique is much thicker and extends out to the cavo‐surface margin at the gingival margin of the Class II restoration ('open sandwich' technique). This requires the RMGI material to perform the same function as the resin‐based composite in this area. This is a much different function from RMGI liners (or any liners) placed entirely beneath a resin‐based composite restoration and not exposed to the oral cavity. Even in the 'closed sandwich' technique, where the RMGI is not brought out to the cavo‐surface margin, the extra thickness of RMGI alone does not qualify it to be considered a liner.
Risk of bias in included studies
We assessed five studies as being at overall high risk of bias (Akpata 2001; Banomyong 2013; Browning 2006; Burrow 2009; Strober 2013). The remaining three studies were at unclear risk of bias (Boeckler 2012; Efes 2006; Wegehaupt 2009) (Figure 2).
Figure 2.
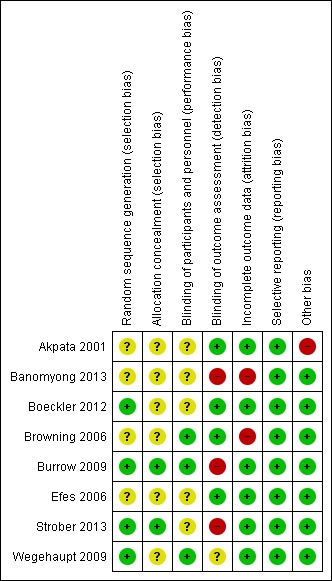
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We deemed four studies to have adequate sequence generation and therefore we classified them as being at low risk of bias for this domain (Boeckler 2012; Burrow 2009; Strober 2013; Wegehaupt 2009). We judged the remaining four studies as being at unclear risk of bias for this domain because they gave no information other than that they were 'randomized' (Akpata 2001; Banomyong 2013; Browning 2006; Efes 2006).
Allocation concealment
Two studies employed adequate methods of allocation concealment. We therefore classified them as being at low risk of bias for this domain (Burrow 2009; Strober 2013). The remaining six studies did not mention allocation concealment, so we classified them as being at unclear risk of bias for this domain (Akpata 2001; Banomyong 2013; Boeckler 2012; Browning 2006; Efes 2006; Wegehaupt 2009).
Blinding
Performance bias ‐ Blinding of participants and personnel
Four studies were deemed to have adequate blinding of participants (Banomyong 2013; Browning 2006; Burrow 2009; Wegehaupt 2009). The remaining four studies gave no information regarding blinding of the participants (Akpata 2001; Boeckler 2012; Efes 2006; Strober 2013).
It is important to note that blinding of personnel was not possible for the types of trials included in this review. In all cases, the operator placing the restoration was aware of whether or not a liner had been placed under the restoration. The impact of this lack of blinding was felt to be unclear in all studies. In those studies in which the operators and the outcome assessors were the same persons, the risk of bias was considered to be high for detection bias, but not necessarily for performance bias.
Detection bias ‐ Blinding of outcome assessors
We deemed four studies to have adequate blinding of outcome assessors and therefore we classified them as being at low risk of bias for this domain (Akpata 2001; Boeckler 2012; Browning 2006; Efes 2006). Three studies were judged as being at high risk of bias for this domain because the outcome assessor and the operator placing the restoration were the same person (Banomyong 2013; Burrow 2009; Strober 2013). As previously stated, there is no way to blind the operator in this type of study. Since the operator and the outcome assessor were the same person and the operator could not be blinded, the outcome assessor also could not have been adequately blinded since she or he might have remembered which restoration had the liner and which restoration did not. The remaining study did not provide any information regarding blinding of outcome assessors so we classified it as being at unclear risk of bias for this domain (Wegehaupt 2009). Additionally, we judged Strober 2013 to be at high risk of bias for this domain because we question the protocol of the study regarding data collection and recording. Practice‐based network studies are typically conducted in an actual dental office setting as opposed to an artificially controlled clinical setting such as a dental school. Often, a single dentist in the practice serves as the 'practitioner‐investigator' (P‐I). The P‐I is frequently the only dentists in the practice and must perform all the tasks required for the study, providing no opportunity for blinding of the outcome assessor. In these cases, the P‐I would examine and evaluate each subject and his/her carious lesions for exclusion or inclusion and would also place the restorations, with or without the liner, and evaluate all the restorations at all intervals and record all data. In Strober 2013 it was specifically reported that this was the case. This protocol may affect the risk of bias for this and all practice‐based network studies.
Incomplete outcome data
We deemed six studies to have adequate outcome data and therefore we classified them as being at low risk of bias for this domain (Akpata 2001; Boeckler 2012; Burrow 2009; Efes 2006; Strober 2013; Wegehaupt 2009). We judged the remaining two studies as being at high risk of bias for this domain, one because of a high number of dropouts (Banomyong 2013) and the other because the authors gave no information regarding how the missing data were treated (Browning 2006).
Selective reporting
All eight studies were deemed to have adequate outcome data reported and therefore we classified all eight studies as being at low risk of bias for this domain.
Other potential sources of bias
We deemed seven studies to have no other potential sources of bias and therefore classified them as being at low risk of bias for this domain (Banomyong 2013; Boeckler 2012; Browning 2006; Burrow 2009; Efes 2006; Strober 2013; Wegehaupt 2009). We judged one study as being at high risk of bias for this domain because that study did not utilize a validated instrument to measure patient‐reported sensitivity (Akpata 2001).
Effects of interventions
See: Table 1
Liner versus no liner
Postoperative hypersensitivity (POH) ‐ Patient‐reported
Five studies, three of which were at high risk of bias, measured POH using a dichotomous yes/no patient report (Akpata 2001; Banomyong 2013; Burrow 2009; Efes 2006; Wegehaupt 2009).
In one study (n = 88; high risk of bias) patient‐reported dichotomous data on POH at 24 hours showed a reduced risk of hypersensitivity in the liner group (risk ratio (RR) 0.26; 95% confidence interval (CI) 0.11 to 0.64) (Analysis 1.1). This difference was not shown at one week or one month follow‐up (Analysis 1.1) (Akpata 2001).
Analysis 1.1.
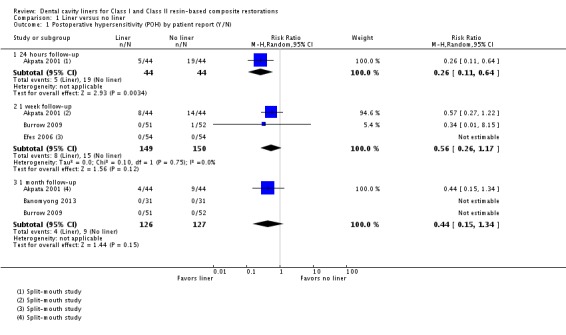
Comparison 1 Liner versus no liner, Outcome 1 Postoperative hypersensitivity (POH) by patient report (Y/N).
One study (n = 344), at high risk of bias, presented visual analog scale (VAS) results for patient‐reported POH at one week and one month (Strober 2013). A lower mean VAS score was shown in favour of the liner group at both time points (mean difference (MD) ‐0.33; 95% CI ‐0.43 to ‐0.23 and MD ‐0.20; 95% CI ‐0.31 to ‐0.09 respectively) (Analysis 1.2).
Analysis 1.2.
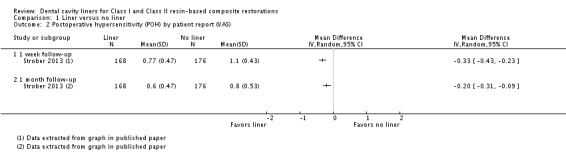
Comparison 1 Liner versus no liner, Outcome 2 Postoperative hypersensitivity (POH) by patient report (VAS).
An additional study (Wegehaupt 2009) instructed patients to record "whether any hypersensitivity, pain, or discomfort occurred following treatment." Nine of 75 patients in the liner group and 12 of 48 patients that did not receive a liner responded "yes" when asked if any hypersensitivity, pain, or discomfort occurred after the restoration was placed. Based on these data they concluded that the occurrence of pain or hypersensitivity does not depend on the remaining dentin thickness, calcium hydroxide lining, or the restorative system used. There was no information regarding when this POH occurred.
Postoperative hypersensitivity (POH) ‐ Cold response measurement (CRM)
One study (n = 88; high risk of bias) measured POH via CRM in time (seconds) (Akpata 2001). While a beneficial effect was seen in favor of cavities prepared using a liner at one week assessment (MD 6.00; 95% CI 1.36 to 10.64), this difference was not seen at any other time point (24 hours or one month) (Analysis 1.3).
Analysis 1.3.
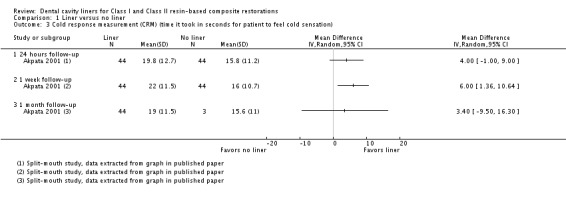
Comparison 1 Liner versus no liner, Outcome 3 Cold response measurement (CRM) (time it took in seconds for patient to feel cold sensation).
Two studies (n = 447; high risk of bias) measured POH via a cold response measurement on a VAS (Burrow 2009; Strober 2013). No difference between cavities prepared with and without liners were shown at either one week (MD ‐0.20; 95% CI ‐0.67 to 0.26) or one month follow‐up (MD ‐0.33; 95% CI ‐0.76 to 0.11) (Analysis 1.4).
Analysis 1.4.
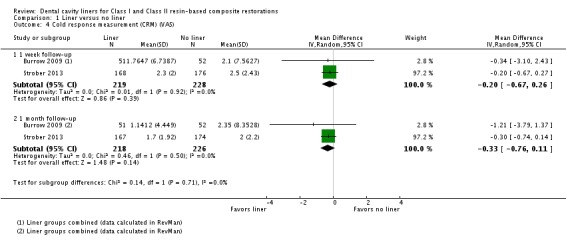
Comparison 1 Liner versus no liner, Outcome 4 Cold response measurement (CRM) (VAS).
Burrow 2009 also measured POH via a yes/no CRM. Again, no difference between cavities prepared with and without liners were shown at either one week (Analysis 1.5).
Analysis 1.5.
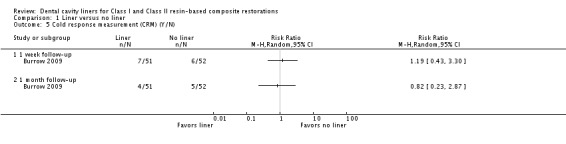
Comparison 1 Liner versus no liner, Outcome 5 Cold response measurement (CRM) (Y/N).
An additional study (Boeckler 2012) measured POH via CRM using subjective descriptive patient‐response criteria at baseline, six months, one and two years. This study found two restorations in the intervention group (liner) and two restorations in the control group (no liner) exhibiting significant POH at baseline but no subjects reported any POH at six months, one or two years. No data for POH measured up to one month postoperatively were reported.
Restoration failure
Four of the included trials measured restoration longevity (Banomyong 2013; Boeckler 2012; Browning 2006; Efes 2006). Two of the studies were judged to be at high risk and two at unclear risk of bias. No difference in restoration failure rates was shown at one year follow‐up, with no failures reported in either group for three of the four studies; the fourth study had a RR 1.00 (95% CI 0.07 to 15.00). Three studies evaluated restoration longevity at two years follow‐up and, again, no failures were shown in either group (Analysis 1.6).
Analysis 1.6.
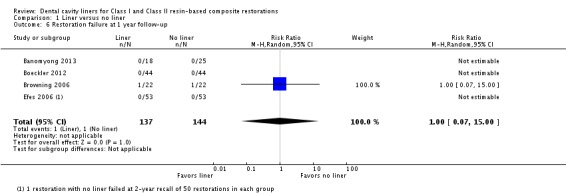
Comparison 1 Liner versus no liner, Outcome 6 Restoration failure at 1 year follow‐up.
Cost
Only one study (Strober 2013) included cost. Strober 2013 concluded that dentists in the United States could save approximately USD 4.50 per filling in materials and office overhead costs by eliminating a resin‐modified glass ionomer lining under resin composite restorations. The authors calculated that this would result in a saving of approximately USD 2000 per dentist per year for an annual saving of approximately USD 82.8 million in the United States.
Adverse events
No adverse events were reported in any of the included studies. Only one study indicated that adverse events would be reported; however, no such report was included in the findings (Strober 2013). Strober 2013 stated that they considered an adverse event to be "lingering pain upon removal of the stimulus." None of the other included studies made any mention of any adverse events.
Discussion
Summary of main results
Eight studies, recruiting over 700 participants compared the use of dental cavity liners to no liners for Class I and Class II resin‐based composite restorations. All studies were at unclear or high risk of bias. There was inconsistent evidence regarding postoperative hypersensitivity (either measured using cold response or patient‐reported), with a benefit shown at some, but not all, time points (low‐quality evidence).
Four trials measured restoration longevity. Two of the studies were judged to be at high risk and two at unclear risk of bias. No difference in restoration failure rates was shown at one year follow‐up, with no failures reported in either group for three of the four studies; the fourth study had a risk ratio (RR) 1.00 (95% confidence interval (CI) 0.07 to 15.00) (low‐quality evidence). Three studies evaluated restoration longevity at two years follow‐up and, again, no failures were shown in either group.
No adverse events were reported in any of the included studies.
Overall completeness and applicability of evidence
There is limited available evidence on the effects of using a dental cavity liner beneath Class I and Class II resin‐based composite restorations. The evidence identified is applicable when placing composite‐based restorations in posterior teeth of adult patients. None of the trials evaluated the effects of using a dental cavity liner in the permanent teeth of children under the age of 15. Thus, it may not be appropriate to apply this evidence to permanent teeth in younger children.
Quality of the evidence
The body of evidence identified in this review does not allow for robust conclusions about the effects of dental cavity liners. The quality of the evidence for each outcome was considered to be of low quality due to only single studies reporting certain outcomes/time points, a high/unclear risk of bias in the individual studies and imprecision in the pooled estimate. A GRADE rating of low‐quality evidence can be interpreted as meaning that there is a lack of confidence in the effect estimates. Further research is very likely to change these estimates, and our confidence in them.
Potential biases in the review process
Searching of multiple databases, with no language or date restrictions, was intended to limit bias by including all relevant studies. Some studies did not have usable data, and this introduces bias into the review as it distorts our overall view of the effects of dental cavity liners.
Agreements and disagreements with other studies or reviews
To the best knowledge of these review authors, no studies have been conducted showing any significant benefit to the placement of any dental cavity liner under Class I and Class II resin‐based composite posterior restorations in permanent teeth in children and adults either in terms of postoperative hypersensitivity reduction, restoration longevity or any other benefit. To our knowledge, no other systematic reviews have been published on this topic. Our findings are similar to a recently published systematic review investigating the effects of a dental cavity liner under Class I and Class II resin‐based composite posterior restorations in primary teeth in children (Schwendicke 2015).
Authors' conclusions
There is inconsistent evidence regarding the difference between resin‐based composite restorations placed with liners and those placed without liners when considering postoperative hypersensitivity. There is no evidence of a difference between the use of liners or not with regard to restoration failure. Despite the low quality of the evidence, we feel that this evidence is applicable when placing routine composite‐based restorations in adult posterior teeth and that placing a liner is an unnecessary step. Any cost savings can be passed along to the public. Even without any cost savings, the evidence does not currently support including the unnecessary step of placing any lining material underneath routine composite‐based restorations in adult posterior teeth.
If new liner materials are developed then future clinical trials should be undertaken to determine if the new liner materials are of any benefit in terms of postoperative hypersensitivity and restoration failure. Any additional research on calcium hydroxide or resin‐modified glass ionomer liners should focus on their use as pulp capping materials rather than on their use as dental cavity liners under routine composite‐based restorations.
Future trials should be well‐designed randomized controlled trials (with adequate sequence generation and allocation concealment methods, blinding of participants and outcome assessors) reported according to the Consolidated Standards of Reporting Trials (CONSORT) Statement (www.consort‐statement.org).
The trials included in the current review used a variety of methods for assessing postoperative hypersensitivity that precluded pooling in some instances. It would be helpful if future studies use agreed, standardized outcome assessment methods, as recommended by the Core Outcome Measures in Effectiveness Trials (COMET) Initiative (www.comet‐initiative.org), to allow for greater comparison of results across studies. Better reporting of adverse events is required and the planning and conducting of an economic analysis alongside future clinical trials would also be beneficial.
Acknowledgements
The authors would like to acknowledge the assistance provided by Cochrane Oral Health and in particular, the assistance of our Contact Editor Anne‐Marie Glenny without whose help this review would not have been possible.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
#1 (((dental or cavity) AND (liner* or lining*)):ti,ab) AND (INREGISTER) #2 (("cavity lining varnish*" or "cavity varnish*"):ti,ab) AND (INREGISTER) #3 (((liner* or lining* or base*) AND ("zinc‐oxide‐eugenol" or "zinc phosphate*" or polycarboxylate or "glass ionomer" or glassionomer or glass‐ionomer or "calcium hydroxide")):ti,ab) AND (INREGISTER) #4 (#1 or #2 or #3) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 [mh ^"Dental cavity liners"] #2 ((dental near/3 liner*) or (dental near/3 lining) or (cavit* near/3 liner*) or (cavit* near/3 lining)) #3 ("cavity lining varnish*" or "cavity varnish*") #4 ((liner* or lining* or base*) near/3 ("zinc‐oxide‐eugenol" or "zinc oxide eugenol" or "zinc phosphate" or polycarboxylate or "glass ionomer" or glassionomer or glass‐ionomer or "calcium hydroxide")) #5 {or #1‐#4}
Appendix 3. MEDLINE Ovid search strategy
1. Dental cavity liners/ 2. ((dental or cavit$) adj3 (liner$ or lining$)).ti,ab. 3. ("cavity lining varnish$" or "cavity varnish$").ti,ab. 4. ((liner$ or lining$ or base$) adj3 ("zinc oxide‐eugenol" or "zinc oxide eugenol" or "zinc phosphate" or polycarboxylate or "glass ionomer" or glassionomer or glass‐ionomer or "calcium hydroxide")).ti,ab. 5. or/1‐4
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomized trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011) (Lefebvre 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. ((dental or cavit$) adj3 (liner$ or lining$)).ti,ab. 2. ("cavity lining varnish$" or "cavity varnish$").ti,ab. 3. ((liner$ or lining$ or base$) adj3 ("zinc oxide‐eugenol" or "zinc oxide eugenol" or "zinc phosphate" or polycarboxylate or "glass ionomer" or glassionomer or glass‐ionomer or "calcium hydroxide")).ti,ab. 4. or/1‐3
The above subject search was linked to Cochrane Oral Health's filter for identifying RCTs in Embase via Ovid:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 16. 14 NOT 15
Appendix 5. LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database) search strategy
(Mh "Dental cavity lining" or "cavity lining*" or "cavity liner*" or "cavity varnish" or "lining varnish" or "Recubrimiento de la Cavidad Dental" or "Forramento da Cavidade Dentária") [Words]
The above subject search was linked to the Brazilian Cochrane Center filter for identifying RCTs in LILACs:
((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) [Words]
Appendix 6. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and World Health Organization International Clinical Trials Registry Platform search strategy
dental cavity liners
Data and analyses
Comparison 1.
Liner versus no liner
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative hypersensitivity (POH) by patient report (Y/N) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 24 hours follow‐up | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.11, 0.64] |
| 1.2 1 week follow‐up | 3 | 299 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.26, 1.17] |
| 1.3 1 month follow‐up | 3 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.15, 1.34] |
| 2 Postoperative hypersensitivity (POH) by patient report (VAS) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 1 week follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 1 month follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Cold response measurement (CRM) (time it took in seconds for patient to feel cold sensation) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 24 hours follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 1 week follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 1 month follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Cold response measurement (CRM) (VAS) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 1 week follow‐up | 2 | 447 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.67, 0.26] |
| 4.2 1 month follow‐up | 2 | 444 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.76, 0.11] |
| 5 Cold response measurement (CRM) (Y/N) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 1 week follow‐up | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 1 month follow‐up | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Restoration failure at 1 year follow‐up | 4 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 15.00] |
Differences between protocol and review
This review differs from the protocol in several aspects. In the 'Selection of studies' section the protocol states "Full‐text copies of all eligible and potentially eligible studies will be obtained and these will be further evaluated in detail by two review authors (AS and IP) to identify those studies which actually meet all the inclusion criteria. A third review author will moderate any disagreement (AVK)." Due to time constraints and other personal obligations author Ivy Peltz (IP) was unable to evaluate in detail the full‐texts of the eligible and potentially eligible studies. This role was taken on by author Analia Veitz‐Keenan (AVK) and author IP served to moderate any disagreement between authors Andrew Schenkel (AS) and AVK.
Additionally, for developing the 'Summary of findings' table for the 'Presentation of main results' section, two review authors (AS and IP) planned to extract the findings from each of the included studies and the third review author was expected to moderate any disagreement (AVK). However, this was not explicitly stated in the protocol. Nevertheless, due to time constraints and other personal obligations author IP was unable to complete the extraction of the findings from each of the included studies. This aspect of the data extraction was taken on by author AVK and author IP was available to moderate any disagreement between authors AS and AVK.
Also, this review was intended to be limited to postoperative hypersensitivity (POH) after two weeks; however, no studies reported any data for this time frame (recall interval). Therefore this review included one month POH data which was the time frame most often reported.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Trial design: Split‐mouth Location: Dental school, Saudi Arabia Funding source: None mentioned |
|
| Participants | Inclusion criteria: Occlusal caries on contralateral posterior teeth with small or moderately large carious lesions ‐ the bucco‐lingual dimensions of each cavity were less than half the intercuspal width Age: Males 16‐52 years Exclusion criteria: Orofacial pain, including toothache, percussion tenderness, periapical radiolucency Number of randomised individuals: n/a Number of randomised teeth: 88 Number of individuals evaluated: 44 Dropouts: None |
|
| Interventions | RMGI liner under RBC restoration (no bonding agent used) compared to no liner (bonding agent only) under RBC restoration | |
| Outcomes | Postoperative hypersensitivity as measured by CRM in time (seconds) and patient reporting | |
| Notes | Based on these data the study authors concluded that the liner group had less sensitivity but it seems that both groups had no clinically significant sensitivity after 30 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "There was randomization in the selection of the right and left teeth for the adhesive or glass‐ionomer lining" Comment: No other additional information was provided ‐ it is unclear how the randomization was performed and how easy it would have been for the operators to deviate from the randomization prescribed |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants may or may not have been blinded – no information provided. Operator was not blinded – knew which tooth received liner |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The measurement of CRM at the recall visits was by another dentist who was unaware of the lining that the experimental teeth had received" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants dropped out |
| Selective reporting (reporting bias) | Low risk | All data reported |
| Other bias | High risk | A validated instrument to measure patient‐reported sensitivity was not used |
| Methods | Trial design: Parallel‐group Location: Dental school postgraduate clinic Bangkok, Thailand Funding source: None mentioned |
|
| Participants | Inclusion criteria: At least 1 deep primary occlusal caries without other defects in a first or second permanent molar, at least 1 opposing tooth, periodontal tissues healthy or only mildly inflamed, no previous signs and symptoms of pulpal and periapical disease, preoperative sensitivity relieved immediately after removal of stimulus, and no spontaneous pain Age: 18‐30 years Exclusion criteria: Medical problems (unspecified), orofacial pain, other defects or restorations on the tooth, cavity depth less than 3 mm, pulpal exposure, no opposing tooth, periodontal disease, signs or symptoms of periapical or pulpal disease Number of randomised individuals: n/a Number of randomised teeth: 62 Number of individuals evaluated: 34 Dropouts: 19 |
|
| Interventions | RMGI liner under RBC restoration compared to no liner under RBC restoration | |
| Outcomes | Postoperative hypersensitivity as measured by patient reporting Restoration longevity |
|
| Notes | 2 different bonding agents were used, with no explanation of how the distribution was determined. A further study was identified (Banomyong 2011); authors confirmed overlap in participants between the 2013 and 2011 papers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "One of the two restorative procedures was randomly allocated. Each participant was unaware of the restoration" Comment: No other additional information provided ‐ it is unclear how the randomization was performed and how easy it was for the operators to deviate from the randomization prescribed |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were unaware of the intervention, however, the operator and evaluator were the same person, "the operator (DB)" "all restorations were examined by one evaluator (DB)" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Operator and evaluator were the same person, "the operator (DB)" (page 3) "all restorations were examined by one evaluator (DB)" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13/31 teeth from experimental group and 6/31 teeth from control group were not included in evaluation |
| Selective reporting (reporting bias) | Low risk | All data reported |
| Other bias | Low risk | None detected |
| Methods | Trial design: Split‐mouth Location: Department of Operative Dentistry and Periodontolgy, Germany Funding source: Ivoclar Vivadent |
|
| Participants | Inclusion criteria: Adults with 2 comparable Class I or II cavities to be restored with a dental composite; positive sensitivity and existing antagonist and neighboring teeth Age: Not specified Exclusion criteria: Underage, systemic diseases, allergies to 1 of the substances of content, gravidity, lactation, teeth that needed direct pulp capping, and endodontically treated teeth Number of randomised individuals: 50 Number of randomised teeth: 100 Number of individuals evaluated: 44 (87 teeth) Dropouts: 6 |
|
| Interventions | Flowable composite Tetric EvoFlow under Tetric EvoCeram compared to Tetric EvoCeram only (both groups used adhesive system AdheSE One) | |
| Outcomes | Postoperative hypersensitivity as measured by CRM using subjective descriptive patient‐response criteria Modified Ryge criteria categories evaluated (color match, marginal discoloration, filing integrity, marginal adaptation, surface, secondary caries, proximal contact, and hypersensitivity) Restoration longevity |
|
| Notes | "The sample size was determined by a statistician ....for 5% level of significance and a power of 90%" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants may or may not have been blinded – no information provided. Operator was not blinded – knew which tooth received liner |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Two blinded, calibrated clinicians not involved with the treatment procedures evaluated each restoration" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants evaluated at 6 and 12 months. 6 participants were lost for the 2‐year evaluation due to address changes; unlikely to influence results |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Low risk | None detected |
| Methods | Trial design: Parallel‐group Location: Dental school, USA Funding source: None mentioned |
|
| Participants | Inclusion criteria: Adults in need of only 1 moderate to large Class II or complex Class I restoration on a molar; occlusally the final margin had to extend more than halfway from the central groove to the cusp tip; interproximally, the final facial and/or lingual margin of the proximal box had to extend at least halfway between minimal clearance and the line angle; no contraindications to routine dental treatment; participant had to be likely to remain in the area for the length of the study Age: Adults (specific age not reported) Exclusion criteria: Removal of caries resulting in exposure of dental pulp Number of randomised individuals: 50 Number of randomised teeth: 25 teeth in each group Number of individuals evaluated: 44 Dropouts: 6 total ‐ 3 from each group |
|
| Interventions | Flowable liner under 1 brand RBC restoration compared to no flowable liner under another brand of RBC restoration | |
| Outcomes | Restoration longevity | |
| Notes | Results for marginal staining reported in Table 1 for only 43 of the 44 restorations evaluated. (1) "restoration experienced a bulk fracture and loss of restorative material substantial enough to expose the dentin. The loss of restorative material created a situation where it was not possible to rate this restoration for any of the other categories" (page 365). Additionally, half of the restorations in each group also received surface sealer postplacement and two subjects were not treated due to depth of caries (pulp exposures anticipated). It should also be noted that although the authors listed postoperative sensitivity among the criteria to be evaluated they did not mention how this would be measured and they did not include any data for this criteria or provide any information in the results nor discussion regarding postoperative sensitivity. Therefore, we included this study only in the longevity portion of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "At the operative appointment, eligible participants were randomly assigned to 1 of 4 groups. While the operators were aware of this assignment, the evaluators and the participants were not. Thus the study design was a randomized, double‐blind clinical trial" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded but operators were not |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "..the evaluators and the participants were not aware of the assignment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There is no mention of how missing data due to dropouts were treated |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Low risk | None detected |
| Methods | Trial design: Parallel‐group Location: Dental school postgraduate clinic Bangkok, Thailand Funding source: None mentioned |
|
| Participants | Inclusion criteria: At least 1 moderate to deep primary occlusal caries (at least 2 mm deep after caries removal) in a first or second permanent molar without caries on other surfaces; at least 1 opposing tooth; periodontal tissues healthy or mildly inflamed without gingival recession/alveolar bone loss; no previous signs and symptoms of pulpal and periapical disease, preoperative sensitivy relieved immediately after removal of stimulus, and no spontaneous pain; at least 1 antagonist tooth with occlusal contact more than 50% of the occlusal surface Age: 18‐40 years Exclusion criteria: Either the cavity depth after caries removal was less than 2 mm or a pulp exposure or near pulp exposure, in which a calcium hydroxide agent was placed; psychological disorders; neurological diseases; TMD; pregnancy or lactation; patients taking any analgesic or anti‐inflammatory drugs regularly; allergies to materials used in the trial; teeth with previous restoration(s), tooth surface loss (attrition, erosion, abrasion or abfraction); teeth diagnosed with cracked tooth syndrome; teeth that had received orthodontic treatment in past 3 months Number of randomised individuals: 72 Number of randomised teeth: 106 Number of individuals evaluated: 70 Dropouts: 2 |
|
| Interventions | RMGI liner under RBC restoration using 2 different bonding agents compared to no liner under RBC restoration using 2 different bonding agents | |
| Outcomes | Postoperative hypersensitivity as measured CRM on a VAS, yes/no criteria, and also by patient reporting | |
| Notes | Some participants had multiple restorations in different quadrants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...blocking randomization list" |
| Allocation concealment (selection bias) | Low risk | "...sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded but operators were not |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "One to four restorations were randomly allocated in each patient by a single operator (DB) according to a blocking randomization list." "At recall, the evaluator (DB) was blinded to the restoration that was being evaluated" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two patients (three restorations) were lost during recall and were excluded before data analysis (from telephone interviewing, these patients reported no postoperative tooth sensitivity in daily function)" |
| Selective reporting (reporting bias) | Low risk | "…five patients (five restorations) missed the one‐week recall; however, these patients were still included in the data analysis. All patients attended the one‐month recall" |
| Other bias | Low risk | None detected |
| Methods | Trial design: Split‐mouth Location: Dental school faculty practice Istanbul, Turkey Funding source: None mentioned |
|
| Participants | Inclusion criteria: Patients with 2 primary occlusal caries in molars in occlusion that were not mobile. Lesions diagnosed by visual inspection and radiographically Age: 18 to 48 years Exclusion criteria: Poor oral hygiene Number of randomised individuals: 54 Number of randomised teeth: 27 in each group Number of individuals evaluated: 50 Dropouts: 4 |
|
| Interventions | Flowable liner under 2 brands RBC restoration compared to no liner under same 2 brands RBC restoration | |
| Outcomes | Postoperative hypersensitivity as measured by patient reporting Restoration longevity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "…materials were allocated randomly" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The examiners were not involved in the filling placements" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All data reported |
| Other bias | Low risk | None detected |
| Methods | Trial design: Parallel‐group Location: Private dental offices, USA Funding source: NIDCR |
|
| Participants | Inclusion criteria: Moderate to deep primary occlusal caries (at least 2 mm deep after caries removal) in a first or second permanent molar without caries on other surfaces; at least 1 opposing tooth; periodontal tissues healthy or mildly inflamed without gingival recession/alveolar bone loss; no previous signs and symptoms of pulpal and periapical disease, preoperative sensitivity relieved immediately after removal of stimulus, and no spontaneous pain; at least 1 antagonist tooth with occlusal contact more than 50% of the occlusal surface Age: 15‐60 years Exclusion criteria: Either the cavity depth after caries removal was less than 2 mm or a pulp exposure or near pulp exposure, in which a calcium hydroxide agent was placed; psychological disorders; neurological diseases; TMD; pregnancy or lactation; patients taking any analgesic or anti‐inflammatory drugs regularly; allergies to materials used in the trial; teeth with previous restoration(s), tooth surface loss (attrition, erosion, abrasion or abfraction); teeth diagnosed with cracked tooth syndrome; teeth that had received orthodontic treatment in past 3 months Number of randomised individuals: 341 Number of randomised teeth: 347 Number of individuals evaluated: 333 Dropouts: 8 |
|
| Interventions | RMGI liner under RBC restoration compared to no liner under RBC restoration | |
| Outcomes | Postoperative hypersensitivity as measured by CRM on a VAS | |
| Notes | Caries classification and dentin caries activity for each lesion, sleep bruxism and caries risk for each patient were also assessed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "On enrolment, eligible participants were randomly assigned one to one (blocking within practice, using random block sizes) between the following treatment arms..." |
| Allocation concealment (selection bias) | Low risk | Randomization done centrally ‐ not in each practice |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants may or may not have been blinded – no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Operator and evaluator were the same person "P‐Is completed restorations according to the treatment arm assigned and liner (if used)" and "P‐Is saw participants for evaluation at one and four weeks after treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All data reported |
| Other bias | Low risk | None detected |
| Methods | Trial design: Parallel‐group Location: Göttingen, Germany Funding source: University of Göttingen |
|
| Participants | Inclusion criteria: Caries media or caries profunda (according to bitewing radiographs), insufficient fillings, positive reaction to a vitality test (cold test), no signs of pulp inflammation, no spontaneous pain attacks before treatment, only premolars and molars, only 1 filling per tooth, and a minimum extension of the cavity of 1 mm in width Age: 18 years or older Exclusion criteria: Patients under 18 years, pregnancy, breastfeeding, immunosuppressed or addicted patients Number of randomised individuals: 123 Number of randomised teeth: 123 Number of individuals evaluated: 123 Dropouts: None |
|
| Interventions | Calcium hydroxide (CaOH) liner under 2 brands RBC restoration compared to no liner under 2 brands RBC restoration | |
| Outcomes | Postoperative hypersensitivity as measured by patient reporting | |
| Notes | Maximum age of participants not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The decision to use a calcium hydroxide liner or not was made by tossing a coin" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. "The patients were not told to which cavity‐depth group their tooth was allocated and if calcium hydroxide was used or not." Operators were not blinded but risk of bias still low even though operator not blinded as it is not possible to blind the operator |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All data reported |
| Other bias | Low risk | None detected |
CRM: cold response measurement; RBC: resin‐based composite; RMGI: resin‐modified glass ionomer; TMD: temporomandibular disorder; VAS: visual analog scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akpata 2006 | Liners not studied |
| Andersson‐Wenckert 2002 | Sandwich technique study |
| Andersson‐Wenckert 2004 | Sandwich technique study |
| Ernst 2002 | Interventions not randomized |
| Ernst 2003 | Interventions not randomized |
| Fagundes 2009 | Liners not studied |
| Grogono 1990 | Sandwich technique study |
| Huth 2003 | No control or comparison group |
| Kaurani 2007 | Interventions not randomized |
| Knibbs 1992 | Sandwich technique study |
| Loguercio 2001 | Liners not studied |
| Noro 1983 | Interventions not randomized, restorative material no longer available (UV light‐cured RBC) |
| Rasmusson 1998 | Inappropriate study design ‐ the study compared 1 brand of RBC placed without a liner to a second brand of RBC placed with a flowable composite as a liner |
| Shi 2010 | Liners not studied |
| Unemori 2001 | Interventions not randomized |
| van Dijken 1999 | Sandwich technique study |
| Vilkinis 2000 | Sandwich technique study |
| Whitworth 2005 | Inappropriate study design ‐ the decision of which restoration to place (composite or amalgam) was left to the discretion of the operator and information regarding the decisions was not provided |
RBC: resin‐based composite; UV: ultra violet.
Contributions of authors
Conceiving and designing the review: Andrew B Schenkel conceived and designed the review with significant contributions from Ivy Peltz and Analia Veitz‐Keenan. Co‐ordinating the review: Andrew B Schenkel. Screening the search results and retrieving the papers: Andrew B Schenkel and Ivy Peltz with Analia Veitz‐Keenan moderating any disagreements. Data extraction and risk of bias assessment: Andrew B Schenkel, Analia Veitz‐Keena and Ivy Peltz (as stated in the review). Analysing the data and interpreting the results: Andrew B Schenkel and Analia Veitz‐Keenan. Writing the results, discussion, conclusions and abstract: Andrew B Schenkel wrote the protocol and main text with significant contributions from Ivy Peltz and Analia Veitz‐Keenan.
Sources of support
Internal sources
New York University College of Dentistry, USA.
External sources
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the British Association for the Study of Community Dentistry, UK; British Society of Paediatric Dentistry, UK; Canadian Dental Hygienists Association, Canada; Centre for Dental Education and Research at All India Institute of Medical Sciences, India; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and NHS Education for Scotland, UK.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Andrew B Schenkel: no interests to declare. Ivy Peltz: no interests to declare. Analia Veitz‐Keenan participated in the Strober study as a dental practitioner investigator for the PEARL (Practitioners Engaged in Applied Research and Learning) Network. She did not however, have access to any final collected data and she did not participate in the data extraction and risk of bias analysis for the Stober study in this systematic review.
New
References
References to studies included in this review
- Akpata ES, Sadiq W. Post‐operative sensitivity in glass‐ionomer versus adhesive resin‐lined posterior restorations. American Journal of Dentistry 2001;14(1):34‐8. [] [PubMed] [Google Scholar]; 4439242
- Banomyong D, Harnirattisai C, Burrow MF. Posterior resin composite restorations with or without resin‐modified, glass‐ionomer cement lining: a 1‐year randomised, clinical trial. Journal of Investigative and Clinical Dentistry 2011;2(1):63‐9. [] [DOI] [PubMed] [Google Scholar]; Banomyong D, Messer, H. Two‐year clinical study on postoperative pulpal complications arising from the absence of a glass‐ionomer lining in deep occlusal resin‐composite restoration. Journal of Investigative and Clinical Dentistry 2013;4(4):265‐70. [] [DOI] [PubMed] [Google Scholar]; 4439244
- Boeckler A, Schaller H‐G, Gernhardt CR. A prospective, double‐blind, randomized clinical trial of a one‐step, self‐etch adhesive with and without an intermediary layer of a flowable composite: a 2‐year evaluation. Quintessence International 2012;43(4):279‐86. [] [PubMed] [Google Scholar]; 4439247
- Browning WD, Myers ML, Chan DC, Downey MC, Pohjola RM, Frazier KB. Performance of 2 packable composites at 12 months. Quintessence International 2006;37(5):361‐8. [] [PubMed] [Google Scholar]; 4439249
- Burrow MF, Banomyong D, Harnirattisai C, Messer HH. Effect of glass‐ionomer cement lining on postoperative sensitivity in occlusal cavities restored with resin composite ‐ a randomized clinical trial. Operative Dentistry 2009;34(6):648‐55. [] [DOI] [PubMed] [Google Scholar]; 4439251
- Efes BG, Dorter C, Gomec Y, Koray F. Two‐year clinical evaluation of ormocer and nanofill composite with and without a flowable liner. Journal of Adhesive Dentistry 2006;8(2):119‐26. [] [PubMed] [Google Scholar]; 4439253
- Strober B, Veitz‐Keenan A, Barna JA, Matthews AG, Vena D, Craig RG, et al. Effectiveness of a resin‐modified glass ionomer liner in reducing hypersensitivity in posterior restorations: A study from the Practitioners Engaged in Applied Research and Learning Network. Journal of the American Dental Association 2013;144(8):886‐97. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 4439255
- Wegehaupt F, Betke H, Solloch N, Musch U, Wiegand A, Attin T. Influence of cavity lining and remaining dentin thickness on the occurrence of postoperative hypersensitivity of composite restorations. Journal of Adhesive Dentistry 2009;11(2):137‐41. [] [PubMed] [Google Scholar]; 4439257
References to studies excluded from this review
- Akpata ES, Behbehani J. Effect of bonding systems on post‐operative sensitivity from posterior composites. American Journal of Dentistry 2006;19(3):151‐4. [] [PubMed] [Google Scholar]; 4439259
- Andersson‐Wenckert IE, Dijken JW, Horstedt P. Modified Class II open sandwich restorations: evaluation of interfacial adaptation and influence of different restorative techniques. European Journal of Oral Sciences 2002;110(3):270‐5. [] [DOI] [PubMed] [Google Scholar]; 4439261
- Andersson‐Wenckert IE, Dijken JW, Kieri C. Durability of extensive Class II open‐sandwich restorations with a resin‐modified glass ionomer cement after 6 years. American Journal of Dentistry 2004;17:43‐50. [] [PubMed] [Google Scholar]; 4439263
- Ernst CP, Buhtz C, Rissing C, Willershausen B. Clinical performance of resin composite restorations after 2 years. Compendium of Continuing Education in Dentistry 2002;23(8):711‐4. [] [PubMed] [Google Scholar]; 4439265
- Ernst CP, Canbek K, Aksogan K, Willershausen B. Two‐year clinical performance of a packable posterior composite with and without a flowable composite liner. Clinical Oral Investigations 2003;7(3):129‐34. [] [DOI] [PubMed] [Google Scholar]; 4439267
- Fagundes TC, Barata TJ, Carvalho CA, Franco EB, Dijken JW, Navarro MF. Clinical evaluation of two packable posterior composites: a five‐year follow‐up. Journal of the American Dental Association 2009;140(4):447‐54. [] [DOI] [PubMed] [Google Scholar]; 4439269
- Grogono AL, McInnes PM, Zinck JH, Weinberg R. Posterior composite and glass ionomer/composite laminate restorations: 2‐year clinical results. American Journal of Dentistry 1990;3(4):147‐52. [] [PubMed] [Google Scholar]; 4439271
- Huth KC, Manhard J, Hickel R, Kunzelmann KH. Three‐year clinical performance of a compomer in stress‐bearing restorations in permanent posterior teeth. American Journal of Dentistry 2003;16(4):255‐9. [] [PubMed] [Google Scholar]; 4439273
- Kaurani M, Bhagwat SV. Clinical evaluation of postoperative sensitivity in composite resin restorations using various liners. New York State Dental Journal 2007;73(2):23‐9. [] [PubMed] [Google Scholar]; 4439275
- Knibbs PJ. The clinical performance of a glass polyalkenoate (glass ionomer) cement used in a 'sandwich' technique with a composite resin to restore Class II cavities. British Dental Journal 1992;172(3):103‐7. [] [DOI] [PubMed] [Google Scholar]; 4439277
- Loguercio AD, Reis A, Rodrigues Filho LE, Busato AL. One‐year clinical evaluation of posterior packable resin composite restorations. Operative Dentistry 2001;26:427‐34. [] [PubMed] [Google Scholar]; 4439279
- Noro A, Ishikawa T. A clinico‐pathological study of pulp response of a restoration system with ultraviolet‐light polymerised resin and the effectiveness of several lining materials. Bulletin of the Tokyo Dental College 1983;24(2):61‐77. [] [Google Scholar]; 4439281
- Rasmusson CG, Kohler B, Odman P. A 3‐year clinical evaluation of two composite resins in class‐II cavities. Acta Odontologica Scandinavica 1998;56(2):70‐5. [] [DOI] [PubMed] [Google Scholar]; 4439283
- Shi L, Wang X, Zhao Q, Zhang Y, Zhang L, Ren Y, et al. Evaluation of packable and conventional hybrid resin composites in Class I restorations: three‐year results of a randomized, double‐blind and controlled clinical trial. Operative Dentistry 2010;35(1):11‐9. [] [DOI] [PubMed] [Google Scholar]; 4439285
- Unemori M, Matsuya Y, Akashi A, Goto Y, Akamine A. Composite resin restoration and postoperative sensitivity: clinical follow‐up in an undergraduate program. Journal of Dentistry 2001;29(1):7‐13. [] [DOI] [PubMed] [Google Scholar]; 4439287
- Dijken JW, Kieri C, Carlen M. Longevity of extensive Class II open‐sandwich restorations with a resin‐ modified glass‐ionomer cement. Journal of Dental Research 1999;78:1319‐25. [] [DOI] [PubMed] [Google Scholar]; 4439289
- Vilkinis V, Horsted‐Bindslev P, Baelum V. Two‐year evaluation of class II resin‐modified glass ionomer cement/composite open sandwich and composite restorations. Clinical Oral Investigations 2000;4:133‐9. [] [DOI] [PubMed] [Google Scholar]; 4439291
- Whitworth JM, Myers PM, Smith J, Walls AW, McCabe JF. Endodontic complications after plastic restorations in general practice. International Endodontic Journal 2005;38(6):409‐16. [] [DOI] [PubMed] [Google Scholar]; 4439293
Additional references
- Banomyong D, Harnirattisai C, Burrow MF. Posterior resin composite restorations with or without resin‐modified, glass‐ionomer cement lining: a 1‐year randomised, clinical trial. Journal of Investigative and Clinical Dentistry 2011;2(1):63‐9. [DOI] [PubMed] [Google Scholar]
- Black GV, Black AD. The Pathology of the Hard Tissues of the Teeth. Vol. 1, Chicago: Medico‐Dental Publishing Company, 1924. [Google Scholar]
- Briso AL, Mestrener SR, Delício G, Sundfeld RH, Bedran‐Russo AK, Alexandre RS, et al. Clinical assessment of postoperative sensitivity in posterior composite restorations. Operative Dentistry 2007;32(5):421‐6. [DOI] [PubMed] [Google Scholar]
- Castillo‐de Oyagüe R, Lynch C, McConnell R, Wilson N. Teaching the placement of posterior resin‐based composite restorations in Spanish dental schools. Medicina Oral, Patología Oral, y Cirugía Bucal 2012;17(4):e661‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliperi S, Bardwell DN. An alternative method to reduce polymerization shrinkage in direct posterior composite restorations. Journal of the American Dental Association 2002;133(10):1387‐98. [DOI] [PubMed] [Google Scholar]
- Demarco FF, Corrêa MB, Cenci MS, Moraes RR, Opdam NJ. Longevity of posterior composite restorations: not only a matter of materials. Dental Materials 2012;28(1):87‐101. [DOI] [PubMed] [Google Scholar]
- Diehnelt DE, Kiyak AH. Socioeconomic factors that affect international caries levels. Community Dentistry and Oral Epidemiology 2001;29(3):226‐33. [DOI] [PubMed] [Google Scholar]
- Edelstein BL. The dental caries pandemic and disparities problem. BMC Oral Health 2006;6 Suppl 1:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejerskov O, Kidd EAM, editor(s). Dental Caries: the Disease and its Clinical Management. Copenhagen: Blackwell Monksgaard, 2003. [Google Scholar]
- Gordan VV, Mjör IA, Veiga Filho LC, Ritter AV. Teaching of posterior resin‐based composite restorations in Brazilian dental schools. Quintessence International 2000;31(10):735‐40. [PubMed] [Google Scholar]
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRADE Working Group, McMaster University. GRADEproGDT. Version accessed 25 May 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
- Harris CA. Principles and Practice of Dental Surgery. 8th Edition. Philadelphia: Lindsay and Blankiston, 1863. [Google Scholar]
- Hayashi M, Seow LL, Lynch CD, Wilson NH. Teaching of posterior composites in dental schools in Japan. Journal of Oral Rehabilitation 2009;36(4):292‐8. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Liew Z, Nguyen E, Stella R, Thong I, Yip N, Zhang F, et al. Survey on the teaching and use in dental schools of resin‐based materials for restoring posterior teeth. International Dental Journal 2011;61(1):12‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CD, McConnell RJ, Wilson NH. Teaching the placement of posterior resin‐based composite restorations in US dental schools. Journal of the American Dental Association 2006;137(5):619‐25. [DOI] [PubMed] [Google Scholar]
- Lynch CD, McConnell RJ, Hannigan A, Wilson NH. Teaching the use of resin composites in Canadian dental schools: how do current educational practices compare with North American trends?. Journal of the Canadian Dental Association 2006;74(4):321. [PubMed] [Google Scholar]
- Lynch CD, McConnell RJ, Wilson NH. Teaching of posterior composite resin restorations in undergraduate dental schools in Ireland and the United Kingdom. European Journal of Dental Education 2006;10(1):38‐43. [DOI] [PubMed] [Google Scholar]
- Lynch CD, Shortall AC, Stewardson D, Tomson PL, Burke FJ. Teaching posterior composite resin restorations in the United Kingdom and Ireland: consensus views of teachers. British Dental Journal 2007;203(4):183‐7. [DOI] [PubMed] [Google Scholar]
- Lynch CD, McConnell RJ, Wilson NH. Trends in the placement of posterior composites in dental schools. Journal of Dental Education 2007;71(3):430‐4. [PubMed] [Google Scholar]
- Lynch CD, Frazier KB, McConnell RJ, Blum IR, Wilson NH. Minimally invasive management of dental caries: contemporary teaching of posterior resin‐based composite placement in US and Canadian dental schools. Journal of the American Dental Association 2011;142(6):612‐20. [DOI] [PubMed] [Google Scholar]
- Murray PE, About I, Franquin JC, Remusat M, Smith AJ. Restorative pulpal and repair responses. Journal of the American Dental Association 2001;132(4):482‐91. [DOI] [PubMed] [Google Scholar]
- Murray PE, Windsor LJ, Smyth TW, Hafez AA, Cox CF. Analysis of pulpal reactions to restorative procedures, materials, pulp capping, and future therapies. Critical Reviews in Oral Biology and Medicine 2002;13(6):509‐20. [DOI] [PubMed] [Google Scholar]
- Murray PE, About I, Lumley PJ, Franquin JC, Remusat R, Smith AJ. Cavity remaining dentin thickness and pulpal activity. American Journal of Dentistry 2002;15(1):41‐6. [PubMed] [Google Scholar]
- Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century‐ the approach of the WHO Global Oral Health Programme. Community Dentistry and Oral Epidemiology 2003;31 Suppl 1:3‐23. [DOI] [PubMed] [Google Scholar]
- Petersen PE, Bougeois D, Ogawa H, Estupinan‐Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bulletin of the World Health Organization 2005;83(9):661‐9. [PMC free article] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Roberson TM, Heymann HO, Swift EJ. Sturdevant's Art and Science of Operative Dentistry. 5th Edition. St. Louis: Mosby Elsevier Health Science, 2006. [Google Scholar]
- Sadeghi M, Lynch CD, Wilson NH. Trends in dental education in the Persian Gulf ‐an example from Iran: contemporary placement of posterior composites. European Journal of Prosthodontics and Restorative Dentistry 2009;17(4):182‐7. [PubMed] [Google Scholar]
- Schwendicke F, Gostemeyer G, Gluud C. Cavity lining after excavating caries lesions: Meta‐analysis and trial sequential analysis of randomized clinical trials. Journal of Dentistry 2015;43(11):1291‐7. [DOI] [PubMed] [Google Scholar]
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007;369(9555):51–9. [DOI] [PubMed] [Google Scholar]
- Summitt JB, William Robbins J, Hilton TJ, Schwartz RS, Dos Santos J Jr. Fundamentals of Operative Dentistry: A Contemporary Approach. 3rd Edition. Hanover Park: Quintessence Publishing, 2006. [Google Scholar]
- Unemori M, Matsuya Y, Hyakutake H, Matsuya S, Goto Y, Akamine A. Long‐term follow‐up of composite resin restorations with self‐etching adhesives. Journal of Dentistry 2007;35(6):535‐40. [DOI] [PubMed] [Google Scholar]
- Wilson NH, Mjör IA. The teaching of Class I and Class II direct composite restorations in European dental schools. Journal of Dentistry 2000;28(1):15‐21. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Schenkel AB, Peltz I, Veitz‐Keenan A. Dental cavity liners for Class I and Class II resin‐based composite restorations. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD010526] [DOI] [PMC free article] [PubMed] [Google Scholar]


