Abstract
Background
This is an update of a Cochrane Review first published in 1999. Corticosteroids are widely used in inflammatory conditions as an immunosuppressive agent. Bone loss is a serious side effect of this therapy. Several studies have examined the use of bisphosphonates in the prevention and treatment of glucocorticosteroid‐induced osteoporosis (GIOP) and have reported varying magnitudes of effect.
Objectives
To assess the benefits and harms of bisphosphonates for the prevention and treatment of GIOP in adults.
Search methods
We searched CENTRAL, MEDLINE and Embase up to April 2016 and International Pharmaceutical Abstracts (IPA) via OVID up to January 2012 for relevant articles and conference proceedings with no language restrictions. We searched two clinical trial registries for ongoing and recently completed studies (ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal). We also reviewed reference lists of relevant review articles.
Selection criteria
We included randomised controlled trials (RCTs) satisfying the following criteria: 1) prevention or treatment of GIOP; 2) adults taking a mean steroid dose of 5.0 mg/day or more; 3) active treatment including bisphosphonates of any type alone or in combination with calcium or vitamin D; 4) comparator treatment including a control of calcium or vitamin D, or both, alone or with placebo; and 4) reporting relevant outcomes. We excluded trials that included people with transplant‐associated steroid use.
Data collection and analysis
At least two review authors independently selected trials for inclusion, extracted data, performed ‘risk of bias’ assessment and evaluated the certainty of evidence using the GRADE approach. Major outcomes of interest were the incidence of vertebral and nonvertebral fractures after 12 to 24 months; the change in bone mineral density (BMD) at the lumbar spine and femoral neck after 12 months; serious adverse events; withdrawals due to adverse events; and quality of life. We used standard Cochrane methodological procedures.
Main results
We included a total of 27 RCTs with 3075 participants in the review. Pooled analysis for incident vertebral fractures included 12 trials (1343 participants) with high‐certainty evidence and low risk of bias. In this analysis 46/597 (or 77 per 1000) people experienced new vertebral fractures in the control group compared with 31/746 (or 44 per 1000; range 27 to 70) in the bisphosphonate group; relative improvement of 43% (9% to 65% better) with bisphosphonates; absolute increased benefit of 2% fewer people sustaining fractures with bisphosphonates (5% fewer to 1% more); number needed to treat for an additional beneficial outcome (NNTB) was 31 (20 to 145) meaning that approximately 31 people would need to be treated with bisphosphonates to prevent new vertebral fractures in one person.
Pooled analysis for incident nonvertebral fractures included nine trials with 1245 participants with low‐certainty evidence (downgraded for imprecision and serious risk of bias as a patient‐reported outcome). In this analysis 30/546 (or 55 per 1000) people experienced new nonvertebral fracture in the control group compared with 29/699 (or 42 per 1000; range 25 to 69) in the bisphosphonate group; relative improvement of 21% with bisphosphonates (33% worse to 53% better); absolute increased benefit of 1% fewer people with fractures with bisphosphonates (4% fewer to 1% more).
Pooled analysis on BMD change at the lumbar spine after 12 months included 23 trials with 2042 patients. Eighteen trials with 1665 participants were included in the pooled analysis on BMD at the femoral neck after 12 months. Evidence for both outcomes was moderate‐certainty (downgraded for indirectness as a surrogate marker for osteoporosis) with low risk of bias. Overall, the bisphosphonate groups reported stabilisation or increase in BMD, while the control groups showed decreased BMD over the study period. At the lumbar spine, there was an absolute increase in BMD of 3.5% with bisphosphonates (2.90% to 4.10% higher) with a relative improvement of 1.10% with bisphosphonates (0.91% to 1.29%); NNTB 3 (2 to 3). At the femoral neck, the absolute difference in BMD was 2.06% higher in the bisphosphonate group compared to the control group (1.45% to 2.68% higher) with a relative improvement of 1.29% (0.91% to 1.69%); NNTB 5 (4 to 7).
Pooled analysis on serious adverse events included 15 trials (1703 participants) with low‐certainty evidence (downgraded for imprecision and risk of bias). In this analysis 131/811 (or 162 per 1000) people experienced serious adverse events in the control group compared to 136/892 (or 147 per 1000; range 120 to 181) in the bisphosphonate group; absolute increased harm of 0% more serious adverse events (2% fewer to 2% more); a relative per cent change with 9% improvement (12% worse to 26% better).
Pooled analysis for withdrawals due to adverse events included 15 trials (1790 patients) with low‐certainty evidence (downgraded for imprecision and risk of bias). In this analysis 63/866 (or 73 per 1000) people withdrew in the control group compared to 76/924 (or 77 per 1000; range 56 to 107) in the bisphosphonate group; an absolute increased harm of 1% more withdrawals with bisphosphonates (95% CI 1% fewer to 3% more); a relative per cent change 6% worse (95% CI 47% worse to 23% better).
Quality of life was not assessed in any of the trials.
Authors' conclusions
There was high‐certainty evidence that bisphosphonates are beneficial in reducing the risk of vertebral fractures with data extending to 24 months of use. There was low‐certainty evidence that bisphosphonates may make little or no difference in preventing nonvertebral fractures. There was moderate‐certainty evidence that bisphosphonates are beneficial in preventing and treating corticosteroid‐induced bone loss at both the lumbar spine and femoral neck. Regarding harm, there was low‐certainty evidence that bisphosphonates may make little or no difference in the occurrence of serious adverse events or withdrawals due to adverse events. We are cautious in interpreting these data as markers for harm and tolerability due to the potential for bias.
Overall, our review supports the use of bisphosphonates to reduce the risk of vertebral fractures and the prevention and treatment of steroid‐induced bone loss.
Plain language summary
Bisphosphonates for treating osteoporosis caused by the use of steroids
Background
Steroids (glucocorticosteroids) are widely used to treat inflammation. Bone loss (osteoporosis) and spinal fractures are serious side effects of this therapy. Bisphosphonates are considered a first‐line treatment for osteoporosis and have been used since the 1990s.
Methods
We examined the research published up to April 2016 and found a total of 27 eligible trials, which included 3075 adults with inflammatory diseases that required steroid treatment for at least one year. People were randomly assigned to receive either bisphosphonate treatment (alone or with calcium or vitamin D, or both) or 'no treatment' (given calcium or vitamin D or a placebo). Our objective was to determine the benefits and harms of bisphosphonates for adults on long‐term steroid therapy.
Main Results
New spinal fractures (12 to 24 months)
There were 12 trials with 1343 people for this analysis. We found that 77 per 1000 people with no treatment experienced new spinal fracture compared to 44 per 1000 (range 27 to 70) people taking bisphosphonates; an absolute benefit of 2% fewer people (5% fewer to 1% more) sustaining spinal fractures when taking bisphosphonates.
Approximately 31 people (range 20 to 145) would need to be treated with bisphosphonates to prevent spinal fractures in one person.
New non‐spinal fractures (12 to 24 months)
There were nine trials with 1245 people for this analysis. We found that 55 per 1000 people with no treatment experienced new non‐spinal fractures compared to 42 per 1000 (range 25 to 69) people taking bisphosphonates; an absolute benefit of 1% fewer people (4% fewer to 1% more) sustaining non‐spinal fractures when taking bisphosphonates.
Lumbar spine bone mineral density (BMD) at 12 months
There were 23 trials with 2042 people for this outcome. We found that the BMD of the lumbar spine of people taking bisphosphonates was 3.50% higher (2.90% to 4.10% higher) than in people who had no treatment.
Approximately three people (range 2 to 3) would need to be treated with bisphosphonates for 12 months for one person to see a minimally important difference in BMD at the lumbar spine.
Femoral neck (top of thigh bone) BMD at 12 months
There were 18 trials with 1665 people for this outcome. We found that the BMD of the femoral neck was 2.06% higher in the bisphosphonate group (1.45% to 2.68% more) than in people with no treatment.
Approximately five people (range 4 to 7) would need to be treated with bisphosphonates for 12 months for one person to see a minimally important difference in BMD at the femoral neck.
Serious adverse events (requiring hospitalisations, life threatening or fatal)
There were 15 trials with 1703 people for this outcome. We found that 162 per 1000 people with no treatment experienced serious adverse events compared to 147 per 1000 (range 120 to 181) taking bisphosphonates; an absolute increased harm of 0% more serious adverse events (2% fewer to 2% more) with bisphosphonates.
Withdrawals due to adverse events
There were 15 trials with 1790 people for this outcome. We found that 73 per 1000 people with no treatment withdrew compared to 77 per 1000 (range 56 to 107) people taking bisphosphonates; an absolute increased harm of 1% more withdrawals due to adverse events (1% fewer to 3% more) with bisphosphonates.
Authors' conclusions
Based on moderate‐ to high‐certainty evidence, we found that bisphosphonates are beneficial in preventing new spinal fractures and preventing and treating steroid‐induced bone loss at the lumbar spine and femoral neck. For preventing non‐spinal fractures, we found that there was little or no difference whether patients used bisphosphonates or not, although this evidence was low‐certainty because the methods used to assess non‐spinal fractures were subject to bias.
We found that there was little or no difference in the number of serious adverse events or withdrawals due to adverse events when comparing bisphosphonates to no treatment. The evidence for these outcomes was of low certainty and we are cautious in making firm conclusions about the harm of bisphosphonates based only on these measures.
Overall, our review supports the use of bisphosphonates to reduce the risk of spinal fractures and in the prevention and treatment of steroid‐induced bone loss.
Summary of findings
Summary of findings for the main comparison. Bisphosphonates versus control for adults with GIOP.
| Bisphosphonates (alone or with calcium and/or vitamin D) compared with control (calcium and/or vitamin D and/or placebo) for adults with GIOP | ||||||
|
Patient or population: adults with GIOP Settings: ambulatory Intervention: bisphosphonates (alone or with calcium and/or vitamin D) Comparison: control (calcium and/or vitamin D and/or placebo) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (calcium and/or vitamin D and/or placebo) | Bisphosphonates (alone or with calcium and/or vitamin D) | |||||
|
Incident vertebral fractures Radiographic follow‐up: 12‐24 months |
77 per 1000 | 44 per 1000 (27 to 70) |
RR 0.57 (0.35 to 0.91) RD ‐0.02 (‐0.05 to 0.01) |
1343 (12 RCTs) | ⊕⊕⊕⊕ high1 | Absolute increased benefit 2% fewer people with fractures using bisphosphonates (95% CI 5.00% fewer to 1.00% more) Relative per cent change 43% improvement with bisphosphonates (95% CI 9.00% to 65.00% better) NNTB = 31 (95% CI 20 to 145) |
|
Incident nonvertebral fractures Radiographic follow‐up: 12‐24 months |
55 per 1000 | 42 per 1000 (25 to 69) |
RR 0.79 (0.47 to 1.33) RD ‐0.01 (‐0.04 to 0.01) |
1245 (9 RCTs) | ⊕⊕⊝⊝
low2,3 due to risk of bias and imprecision |
Absolute increased benefit 1% fewer people with fractures using bisphosphonates (95% CI 4.00% fewer to 1.00% more) Relative per cent change 21% improvement with bisphosphonates (95% CI 33.00% worse to 53.00% better) NNTB = n/a4 |
|
Lumbar spine BMD DEXA follow‐up: 12 months |
Mean per cent change in BMD across control groups was ‐3.19% (‐8.08% to 1.70%) from baseline5 | Mean per cent change in BMD from baseline in bisphosphonate groups was 3.50% higher than control groups (2.90% to 4.10% higher) | ‐ | 2042 (23 RCTs) | ⊕⊕⊕⊝
moderate6,7,8 due to indirectness |
Absolute increased benefit 3.50% with bisphosphonates (95% CI 2.90 to 4.10) Relative per cent change 1.10% (95% CI 0.91 to 1.29) with bisphosphonates NNTB = 3 (95% CI 2 to 3) |
|
Femoral neck BMD DEXA follow‐up: 12 months |
Mean per cent change in BMD across control groups was ‐1.59% (‐10.49% to 7.31%) from baseline 5 | Mean per cent change in BMD from baseline in bisphosphonate groups was 2.06% higher than control groups (1.45% to 2.68% higher) | ‐ | 1665 (18 RCTs) | ⊕⊕⊕⊝
moderate7,8 due to indirectness |
Absolute increased benefit 2.06% with bisphosphonates (95% CI 1.45 to 2.68) Relative per cent change 1.29% with bisphosphonates (95% CI 0.91 to 1.69) NNTB = 5 (95% CI 4 to 7) |
|
Serious adverse events follow‐up: 12‐24 months |
162 per 1000 | 147 per 1000 (120 to 181) |
RR 0.91 (0.74 to 1.12) RD 0.00 (‐0.02, 0.02) |
1703 (15 RCTs) | ⊕⊕⊕⊝
low3,9 due to risk of bias and imprecision |
Absolute increased harm 0% more adverse events with bisphosphonates (95% CI 2.00% fewer to 2.00% more) Relative per cent change 9% improvement with bisphosphonates (95% CI 12.00% worse to 26.00% better) NNTH = n/a4 |
|
Withdrawals due to adverse events follow‐up: 12‐24 months |
73 per 1000 | 77 per 1000 (56 to 107) |
RR 1.06 (0.77 to 1.47) RD 0.01 (‐0.01 to 0.03) |
1790 (15 RCTs) | ⊕⊕⊕⊝
low3,9 due to risk of bias and imprecision |
Absolute increased harm 1% more withdrawals with bisphosphonates (95% CI 1.00% fewer to 3.00% more) Relative per cent change 6% worsening with bisphosphonates (95% CI 47.00% worse to 23.00% better) NNTH = n/a4 |
| Quality of life | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | (0 studies) | This outcome was not assessed by any of the trials | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; RD: Risk Difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Vertebral fractures meet calculated OIS threshold of 1174 (calculation not shown ‐ Brant 2014)
2Downgraded for risk of bias: nonvertebral fractures were a patient‐reported, subjective outcome
3Downgraded for imprecision: total sample size is below calculated optimal information size (OIS) (calculations not shown ‐ Brant 2014) and the 95% confidence interval around the pooled estimate of effect includes both the possibility of no effect and appreciable benefit or harm
4Number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) is not applicable when result is not statistically significant
5We calculated mean baseline risk for the control group in RevMan using generic inverse variance (calculations not shown)
6Most heterogeneity explained through sensitivity analyses
7Downgraded for indirectness: bone density is a surrogate marker for fracture risk
8Clinically relevant change in BMD: the natural history of participants starting steroid therapy based on control arms in our prevention trials is to see a 1%‐6% decrease in lumbar spine BMD and 1%‐4% decrease in femoral neck BMD in the first year of treatment. We have used an SMD of 0.5 as an estimate of the minimal clinically important difference for BMD change to calculate the NNTB (Schünemann 2011b)
9Downgraded for risk of bias: the protocols for the collection of harm data in a large number of trials were unclear
Background
Description of the condition
Corticosteroids are widely used in inflammatory conditions as an immunosuppressive agent. Diseases treated with corticosteroids include connective tissue diseases, respiratory diseases, haematological diseases, inflammatory bowel disease and organ transplantation. Bone loss is a serious side effect of this therapy, commonly referred to as glucocorticoid‐induced osteoporosis (GIOP), and is likely mediated through a variety of mechanisms.
The most widely accepted mechanisms in the pathogenesis of GIOP are the direct inhibition of bone formation and increase in bone resorption. Inhibition of bone formation is mediated by a decrease in osteoblast differentiation, impaired maturation and function, and premature osteoblast apoptotic death, as evidenced by decreased serum osteocalcin levels (Canalis 2007; Saag 2003). Glucocorticoids enhance osteoclast‐mediated bone resorption by suppressing osteoprotegerin; stimulating RANK/RANKL; and decreasing apoptosis, all of which result in increased levels of osteoclasts. There is also evidence of decreased calcium absorption, increased calcium excretion and decreased serum concentration of sex hormones (Canalis 2007; Saag 2003). In addition to their effect on bone density, steroids are known to affect bone architecture and quality (Kanis 2007; Saag 2003; Van Staa 2002). These two factors likely contribute to a lower BMD threshold for fracture in people with GIOP.
There is controversy in the literature regarding the minimum dose and duration of corticosteroids required to produce bone loss and fractures, with reports of doses as low as 2.5 to 7.5 mg/day leading to statistically significant bone loss and a 2.5 fold increase in vertebral fractures (Canalis 2007; Steinbuch 2004; Van Staa 2002). Fracture risk may be confounded by the underlying inflammatory disease processes themselves, which may independently lead to bone loss and fractures (Saag 2003).
Description of the intervention
Bisphosphonates have been used to treat osteoporosis since the 1990s and are considered to be first line treatment when pharmacological therapy is recommended. Various types of bisphosphonates exist, most commonly in oral pill form, although intravenous bisphosphonates are also available and in North America are reserved traditionally for individuals who are unable to tolerate oral bisphosphonates. Oral regimes include daily or weekly administration, whereas intravenous bisphosphonates are administered every few months or on a yearly basis (National Osteoporosis Foundation 2014).
How the intervention might work
Bisphosphonates reduce bone loss through various mechanisms of actions that are not fully understood. In general, bisphosphonates are shown to reduce the rate of bone turnover through a strong affinity for bone mineral, which translates to a decrease in bone loss. They also have an inhibitory effect that decreases the number and activity of osteoclasts, which dissolve bone as part of the normal bone turnover process (Russell 2007). Bisphosphonate efficacy, measured as per cent change in BMD over one year, ranges from ‐3% to +12% in bisphosphonate studies.
Why it is important to do this review
A consequence of low bone mass is the development of vertebral and nonvertebral fractures. GIOP often manifests as clinically silent until the development of a fracture. BMD is commonly used as an intermediate outcome to extrapolate fracture risk, however it should be noted that the correlation between BMD and fracture risk that exists in post‐menopausal osteoporosis has not been established in corticosteroid‐induced osteoporosis. Therefore it is important to have direct evidence of the benefit of bisphosphonates in reducing fracture risk in GIOP.
The routine use of prophylactic therapy to prevent bone loss and fractures is suboptimal despite recommendations from American College of Rheumatology (ACR) practice guidelines (Grossman 2010). Recent North American prescription patterns for GIOP prevention demonstrate a prevalence of anti‐resorptive co‐prescription of only 15% to 37% in people receiving long‐term steroid treatment (Curtis 2005; Feldstein 2005; Mckeown 2012).
A Cochrane Systematic Review and meta‐analysis is useful to determine the benefits and harms of bisphosphonates in the prevention and treatment of GIOP and fractures in order to justify and encourage their routine use.
Objectives
To assess the benefits and harms of bisphosphonates for the prevention and treatment of GIOP in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCT).
Types of participants
Participants were men or women over the age of 18 with underlying inflammatory disorders, initiating treatment or currently being treated with systemic corticosteroids, and who had not received bisphosphonates in the six months prior to the start of the study. We defined prevention studies by bisphosphonate treatment starting within three months of initiating corticosteroids, while treatment (secondary prevention) studies included those that initiated bisphosphonate treatment beyond three months of starting corticosteroid therapy. These definitions are widely used in GIOP literature and are based on the notion that rapid bone loss is seen within the first three to six months of corticosteroid use (Canalis 2007; Van Staa 2002).
Due to controversy in the literature regarding low‐dose steroids and the risk of osteoporosis and fracture, we used only those trials where the mean corticosteroid dose was 5 mg/day or higher. Participants had to be continuing corticosteroid treatment throughout the entire course of the study. We excluded trials that included people with transplant‐associated steroid use from the review.
Types of interventions
We included trials that evaluated any bisphosphonate alone or in combination with calcium or vitamin D, or both, as the active treatment group. The control groups were taking calcium or vitamin D, or both, alone or with placebo.
Types of outcome measures
Benefits
Major outcomes
Number of participants with incident radiographic vertebral fractures.
Number of participants with incident radiographic nonvertebral fractures.
Per cent change in BMD of the lumbar spine and femoral neck measured by dual energy X‐ray absorptiometry (DEXA).
Quality of life using any measurement tool.
Minor outcomes
Per cent change in BMD of the lumbar spine using low‐dose versus standard‐dose bisphosphonates.
Per cent change in BMD of the femoral neck using low‐dose versus standard‐dose bisphosphonates.
Harms
Major outcomes
Serious adverse events (requiring hospitalisation, life threatening or fatal).
Withdrawals due to adverse events.
Timing of outcome assessment
We extracted incident fracture data, serious adverse events and withdrawals due to adverse events for any time points where available.
We extracted data for our BMD outcomes at 12 months and at 18 to 24 months for use in separate analyses.
We extracted data on low‐dose versus standard‐dose bisphosphonates at 12 months.
If data existed at multiple time points within the above periods, we only extracted data at the latest possible time point of each period.
Search methods for identification of studies
Electronic searches
The original review searched MEDLINE and Embase from inception to 1997 (Appendix 1). For this review update we searched the Cochrane Central Register of Controlled Trials (CENTRAL Issue 5, 2015); MEDLINE, Embase, and the International Pharmaceutical Abstracts (IPA) via OVID for relevant articles and conference proceedings. We also searched two clinical trials registries (ClinicalTrials.gov and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal) for ongoing and recently completed studies.
CENTRAL from inception to 1 April 2016 (Appendix 2)
MEDLINE from January 1997 to 25 January 2010 (Appendix 3) and updated searches from January 2010 to 3 April 2013 and January 2013 to 1 April 2016 (Appendix 4)
Embase from January 1997 to 27 January 2010 (Appendix 5) and updated searches from January 2010 to 3 April 2013 and January 2013 to 1 April 2016 Appendix 6)
IPA from 1970 to 27 January 2012 (Appendix 7)
clinicaltrials.gov/ from inception to March 15 2016 (search terms were (diphosphonates OR biphosphonates) AND osteoporosis)
WHO ICTRP from inception to 15 March 2016 (search terms were bisphosphonates AND osteoporosis)
Differences in search strategy keywords reflect changes in database indexing over time. All languages were included in the search and retrieved.
Searching other resources
We reviewed the reference lists of relevant review articles and the existing Cochrane Review by the same author (Homik 1999) to identify any other potentially relevant trials.
Data collection and analysis
Selection of studies
We included RCTs that satisfied the following initial criteria:
prevention or treatment of GIOP;
included adults taking a mean steroid dose of 5 mg/day or more;
active treatment included any bisphosphonate alone or in combination with calcium or vitamin D;
comparator included calcium and/or vitamin D alone or with placebo; and
reported relevant outcomes (see Types of outcome measures).
We excluded trials including people with transplant‐associated steroid use.
After fulfilling the above initial criteria, we looked for the following in order for a study to be included in the review:
adequate description of the intervention medications in terms of administration route and schedule;
use of standard doses of bisphosphonates (National Osteoporosis Foundation 2014) in at least one treatment group; and
for incident vertebral fractures, radiographic screening was performed routinely, not just in the presence of symptoms, and the criteria used to assess incident fractures were clearly outlined.
Two review authors (JH and JY or JH and CA) independently performed the primary screen of abstracts and full‐text reviews of the eligible reports. Any disagreement on the inclusion of an article was resolved through discussion between the two authors.
Data extraction and management
Two review authors (JH, CA) independently extracted data from the included trials. In cases of discrepancies in extracted data, the two authors would refer back to the original articles and reach a consensus. For each included trial we recorded the following:
type of trial (method; prevention versus treatment);
participant characteristics (age, sex, prevalent vertebral fractures and underlying diseases);
mean steroid dose;
intervention characteristics for each treatment group;
outcome data;
fracture assessment criteria.
Assessment of risk of bias in included studies
Two review authors (JH, JY) independently assessed the methodological quality of the trials included in the primary and secondary analyses using the Cochrane tool for assessing risk of bias (Higgins 2011a). We assessed the following domains:
sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective reporting; and
other bias (i.e. source of funding).
We judged the above criteria using 'Yes': low risk of bias; 'No': high risk of bias; and 'Unclear': lack of information or uncertainty over the potential for bias. The review authors (JH, JY, CA) resolved any disagreements through discussion.
Measures of treatment effect
We analysed the results of the trials using Review Manager (RevMan) 5.3 statistical software (RevMan 2014). We conducted pooled analyses for dichotomous variables (incident vertebral and nonvertebral fractures, withdrawals due to adverse events and serious adverse events) using the Mantel‐Haenszel risk ratio (RR) with 95% confidence intervals (CI) (Deeks 2011).
We analysed continuous data (BMD outcomes) as the mean difference (MD) in BMD between the two treatment groups and the corresponding standard deviation. That is, the per cent change in treatment group BMD minus the per cent change in placebo group BMD. We conducted analysis separately for bone loss at the femoral and lumbar sites, because of the differential effects of corticosteroids on cortical and trabecular bone mass (Rickers 1984). Each trial was weighted taking into account sample size and variance in the outcome variable.
To enhance the interpretability of our outcomes we also calculated relative per cent changes; absolute risk differences; and for outcomes with statistically significant differences between intervention groups, the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH). These calculations are described below under the heading ‘Summary of findings' table.
Unit of analysis issues
The unit of analysis for each outcome was the participant. For studies containing more than two intervention groups, multiple pair‐wise comparisons were performed so that the same group of participants was included only once in the meta‐analysis.
Dealing with missing data
We worked with a biostatistician to compute missing or incomplete data from other available statistics. When studies reported the median change in BMD instead of the mean, we used the median as the best estimate for the mean.
When studies did not report standard deviation (SD), we calculated it using either the standard error of the mean (SEM), an exact P value, 95% CI, range, or interquartile range (IQR):
when SEM was reported, we calculated SD as the product of the SEM and the square root of n, where n is the number of subjects in the group;
from an exact P value, we calculated SEM as the mean difference between treatment and control groups divided by the z‐stat, where z‐stat was calculated in Microsoft Excel as “=normsinv(1‐p/2)” and then converted to SD using the above formula;
from a 95% CI, SEM was calculated as the difference between the upper confidence bound and the lower confidence bound divided by 3.92 and then converted to SD;
if using range, we calculated SEM as range divided by the corresponding divisor based on sample size as per Wiebe 2006;
if given IQR, we calculated SD as IQR divided by 1.35;
where no numerical data were provided, we measured SD from the error bars of a graph;
where no error measurement was reported either numerically or graphically, we estimated SD using the mean coefficient of variation of the other trials, weighted by the sample size of each study;
where number of participants completing was not reported, the number of participants randomised was used as n.
Where data were imputed or calculated as described above, we reported this in the ‘Characteristics of included studies’ tables.
Assessment of heterogeneity
We assessed heterogeneity of the data by visual inspection of forest plots and using the I2 statistic (Higgins 2003), for which we interpreted a value greater than 50% as evidence of substantial heterogeneity (Schünemann 2011a). Where substantial heterogeneity was found, we explored the data further using subgroup analyses in an attempt to explore the causes for heterogeneity.
'Summary of findings' table
Our major outcomes (incident vertebral and nonvertebral fractures, BMD change after 12 months at the lumbar spine and femoral neck, serious adverse events, withdrawals due to adverse events, and quality of life) are presented in the Table 1 produced using GRADEpro software (GRADEpro GDT 2015). This provides information on the certainty of evidence, the magnitude of intervention effect, and the summary of data available for each outcome. The overall certainty of evidence for each outcome was graded (high, moderate, low and very low) using the GRADE approach (Schünemann 2013). For dichotomous outcomes with low event rates, we used an optimal information size (OIS) calculator (Brant 2014) to assess the precision of data.
We included the absolute risk difference and the relative per cent change for each outcome. For statistically significant differences, we also calculated NNTB or NNTH.
For dichotomous outcomes we calculated the absolute risk difference using the risk difference (RD) statistic in RevMan 5.3 (RevMan 2014) expressed as a percentage; we calculated the relative per cent change as risk ratio (RR) minus one and expressed it as a percentage; we calculated the NNTB/NNTH from the control group event rate and the risk ratio using the Visual RX NNT calculator (Cates 2015).
For continuous outcomes, we calculated absolute risk difference as the mean difference between intervention and control group. We calculated the relative difference as the mean difference divided by the mean baseline risk of the control group, calculated in RevMan 5.3 (RevMan 2014) using generic inverse variance. We calculated NNTB/NNTH using the Wells calculator software available at Cochrane Musculoskeletal editorial office. There are no published or agreed upon minimal clinically important differences (MCID) for BMD results that we are aware of. In cases where this occurs, the Cochrane Handbook for Systematic Reviews of Interventions recommends using a standardised mean difference (SMD) of 0.5 as an estimate of the minimal clinically important difference (MCID) for each outcome, a rule of thumb representing a moderate effect (Schünemann 2011b). It is difficult to ascribe a minimal clinically important difference in terms of per cent change in BMD as a predictable correlation between BMD and fracture risk has not been established in the GIOP setting. BMD changes only account for a small increase in fracture risk (Kanis 2007; Saag 2003; Van Staa 2002). The clinical relevance of BMD outcomes is further discussed in 'Summary of main results.'
Data synthesis
We analysed both dichotomous and continuous data using a random‐effects model to provide a conservative estimate of effect.
Subgroup analysis and investigation of heterogeneity
A subgroup analysis planned a priori compared the treatment effect in prevention trials (bisphosphonates starting within three months of initiating steroids) and in treatment trials (bisphosphonates starting beyond three months of steroid therapy).
Where sufficient data existed, we considered post‐hoc subgroup analyses with regard to:
gender and menopausal status;
mean steroid dose;
prevalent fractures (whether incident fractures occurred in participants with prevalent fractures).
Sensitivity analysis
We planned exploratory sensitivity analyses a priori to evaluate the effect of:
study quality, based on the exclusion of studies that were not blinded; and
route of administration of bisphosphonate (oral and parenteral).
Results
Description of studies
Results of the search
Details of the study selection are presented in Figure 1. Our initial search of the databases, clinical trials registries and handsearching provided 3934 records after the results were merged and duplicate records removed. A primary screen of the abstracts or protocols resulted in the exclusion of 3347 records. We retrieved the full‐texts of the remaining 587 records and assessed them for eligibility. Based on the full‐text review; 534 were excluded, eight were identified as 'awaiting classification' and six were ongoing trials. The remaining 39 trials underwent data extraction. Another 12 were found to be ineligible and were thus excluded, leaving 27 trials to be included in the review (25 studies from the updated search and two studies from the existing Cochrane Review). Altogether, a total of 27 trials were included in the review, which reported on 3075 participants.
1.
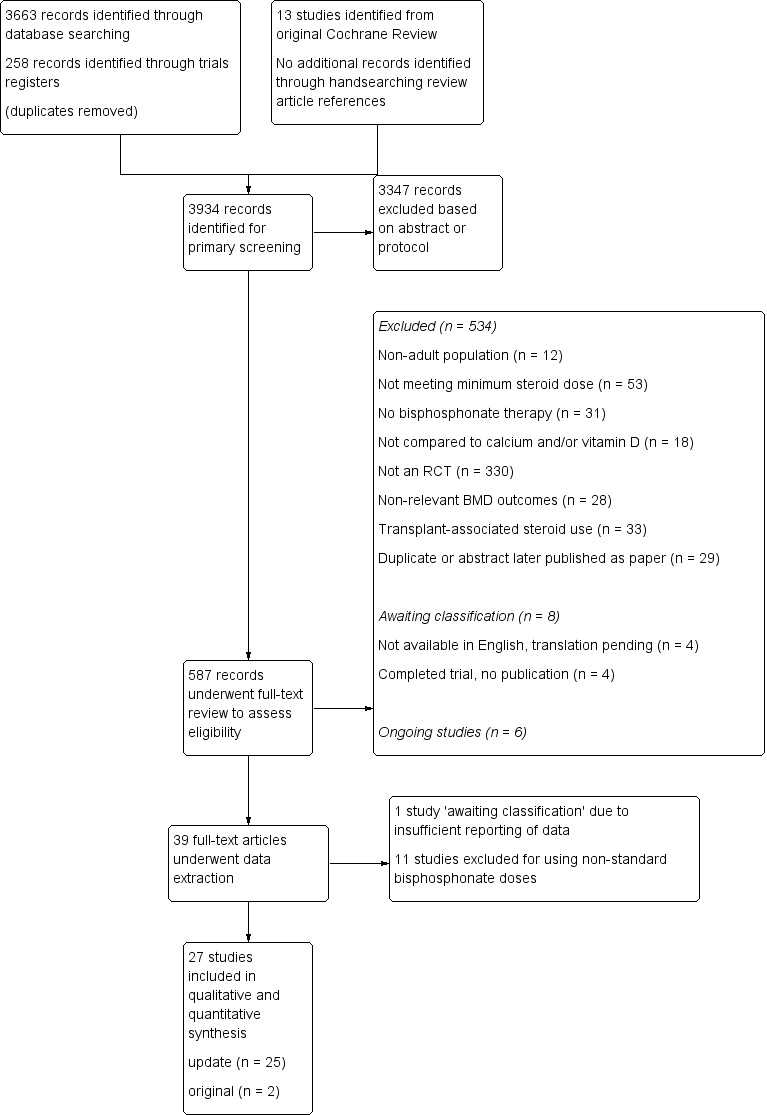
Study flow diagram.
Included studies
Key characteristics of the included trials are contained in the Characteristics of included studies tables.
Interventions
Most trials used alendronate (n = 9) or cyclic etidronate (n = 8). Other bisphosphonates used include risedronate (n = 2), pamidronate (n = 3), clodronate (n = 3), and ibandronate (n = 2).
Underlying diseases
Underlying conditions that required steroid treatment included rheumatological, respiratory, nephrological, gastrointestinal, haematological, dermatological and neurological systemic inflammatory diseases. Most trials (n = 19) included participants with a variety of diseases. Two trials included only participants with rheumatoid arthritis (Lems 2006; Van Offel 2001); two trials included only participants with systemic lupus erythematosus (Li 2010; Yeap 2008); Wolfhagen 1997 reported only on participants with primary biliary cirrhosis; Herrala 1998 included only participants with chronic obstructive pulmonary disease (COPD) and asthma; Abitbol 2007 included only participants with inflammatory bowel disease; and Tee 2012 included only participants with immunobullous skin diseases.
Mean steroid dose
Although a minimum mean steroid dose of 5 mg/day was reported in the protocols of our included trials, we found that there was much variability between trials in the actual mean steroid doses used throughout the study period. This was not surprising given the variation in underlying inflammatory diseases and considering that steroid regimes are tapered to fit individual clinical context.
Three trials reported a mean steroid dose of 5 to 7.5 mg/day (Abitbol 2007; Geusens 1998; Hakala 2012). Ten trials reported a mean steroid dose of approximately 7.5 mg/day (Cortet 1999; Frediani 2003; Herrala 1998; Jenkins 1999; Lems 2006; Pitt 1998; Saag 1998; Sambrook 2003; Skingle 1997; Van Offel 2001). Eleven trials reported a mean steroid dose of 10 to 15 mg/day (Adachi 1997; Adachi 2001; Boutsen 1997; Boutsen 2001; De Nijs 2006; Reid 2000; Roux 1998; Stoch 2009; Tee 2012; Wolfhagen 1997; Yeap 2008). Two trials reported a mean steroid dose of greater than 20 mg/day (Cohen 1999; Saadati 2008). Li 2010 was unclear in describing the mean steroid dose used throughout the study period and reported a range of steroid doses that varied between under 7.5 mg/day and 40 mg/day.
Prevalent vertebral fractures
Twelve trials had participants with vertebral fractures at baseline (Abitbol 2007; Adachi 1997; Adachi 2001; Cohen 1999; De Nijs 2006; Frediani 2003; Geusens 1998; Lems 2006; Reid 2000; Saag 1998; Sambrook 2003; Skingle 1997). Hakala 2012 included prevalent vertebral fractures but excluded participants with symptomatic or two or more radiographic vertebral fractures. Three trials reported no prevalent vertebral fractures in their participants (Li 2010; Pitt 1998; Yeap 2008), whereas Boutsen 2001; Tee 2012 and Wolfhagen 1997 excluded participants with prevalent vertebral fractures. The remaining eight trials did not explicitly state whether or not there were prevalent vertebral fractures (Boutsen 1997; Cortet 1999; Herrala 1998; Jenkins 1999; Roux 1998; Saadati 2008; Stoch 2009; Van Offel 2001).
Multiple treatment groups
Six of the included trials reported on multiple treatment groups. The standard‐dose arms and control groups were included in the major analyses (Boutsen 2001; Cohen 1999; Herrala 1998; Lems 2006; Reid 2000; Saag 1998). If a low‐dose arm was reported, we included the studies in a minor outcome analysis of standard‐dose versus low‐dose bisphosphonates (Boutsen 2001; Cohen 1999; Lems 2006; Reid 2000; Saag 1998). One of the multi‐group trials using clodronate reported on groups with dosages of 1600 mg and 2400 mg daily, which were both higher than the current standard and therefore not eligible for the standard‐dose versus low‐dose meta‐analysis (Herrala 1998).
Another trial used two distinct pair‐wise comparisons with two independent treatment groups and two independent placebo groups (Sambrook 2003). In this study, one pair‐wise comparison involved steroid use of less than six months and the other comparison involved steroid use for longer than six months. Data were entered as two separate trials, as there was no overlap in participants.
Prevention versus treatment of GIOP
Thirteen trials involved the prevention of GIOP and 14 trials involved the treatment of GIOP. For trials that had mixed prevention and treatment individuals, we categorised the trial based on whether the majority of participants were considered 'prevention' or 'treatment' as per our criteria described in Types of participants. Saadati 2008 was unclear in describing the type of study so we categorised it as a treatment trial.
Outcomes
Incident radiographic vertebral fractures
Twenty‐one trials reported this outcome, 12 trials were included and nine trials excluded from meta‐analysis.
Two trials combined fracture data from their 5 mg and 10 mg alendronate treatment arms (Lems 2006; Saag 1998) and although 5 mg daily alendronate was not considered a standard dose, we included these data in the analysis. Of the 12 included studies, seven were prevention trials (Abitbol 2007; Adachi 1997; Boutsen 1997; Boutsen 2001; Cohen 1999; De Nijs 2006; Tee 2012) and five were treatment trials (Lems 2006; Pitt 1998; Reid 2000; Saag 1998; Sambrook 2003).
Since fractures occur at a variable length of time after the onset of osteoporosis, we included trials in which the follow‐up for new fractures occurred between 12 to 24 months, to better ascertain the benefits of bisphosphonates in fracture prevention. We excluded one trial that assessed incident fractures after four years (Frediani 2003) as the timeline differed too greatly from all other trials. Four trials were excluded because they only reported radiographically confirmed symptomatic vertebral fractures and not all participants were screened for vertebral fractures (Roux 1998; Cortet 1999; Geusens 1998; Stoch 2009). We excluded Jenkins 1999 because vertebral radiographs were reported on less than half of completing participants, despite a protocol stating routine radiographic screening at baseline and 52 weeks. Saadati 2008 did not outline their assessment criteria for fractures and was therefore excluded. One trial reported the number of vertebral fractures rather than the number of participants who experienced fractures and could not be included in the analysis (Skingle 1997). Fracture data from Adachi 2001 were not included as this study was a partial cohort from Saag 1998.
Fracture Assessment Criteria
Methods for assessing incident vertebral fractures included quantitative morphometry, semiquantitative grading and a spinal deformity index. Data were analysed regardless of which of the three methods of fracture determination was used.
Saag 1998 and its extension study Adachi 2001 assessed fractures using both semiquantitative and quantitative methods. For these two trials we included the semiquantitative data as more trials reported incident fractures using this method.
Two trials (Cohen 1999; Reid 2000) used the quantitative morphometric criteria of Kiel 1995 and Melton 1993 in which incident fractures were defined as either a reduction in vertebral height of 15% or more (for intact vertebrae at baseline) or 4 mm or more (for fractured vertebrae at baseline). Two other trials (Abitbol 2007; Lems 2006) defined incident fractures by quantitative morphometry as a reduction in vertebral height of 20% or 4 mm or more, or both, based on criteria outlined by Black 1996 and Genant 1996.
Six trials used semiquantitative grading with a minimum reduction in vertebral height of 20% as criteria for incident vertebral fractures (Adachi 1997; Adachi 2001; Pitt 1998; Saag 1998; Sambrook 2003; Tee 2012) as per Genant 1993 and Van Kujik 1995 and one trial used or a reduction in height of 15% or more (De Nijs 2006) according to Kleerekoper 1984.
Two trials used the Minne 1988 spinal deformity index (Boutsen 1997; Boutsen 2001), which determines the extent of vertebral compression by comparison of the actual vertebral body height to the presumable original height.
Incident radiographic nonvertebral fractures
Thirteen trials reported this outcome, nine trials were included and four trials excluded from meta‐analysis.
Fracture sites included but were not limited to the hip, wrist, forearm, and midfoot. No atypical femur fractures were reported in any of the included trials. We reported all nonvertebral fracture data together as the majority of trials did not include complete information on specific fracture sites. Three trials reported nonvertebral fractures but provided the total number of fractures rather than the number of participants suffering from fractures and were therefore not included in the analysis (Adachi 1997; Roux 1998; Stoch 2009). Another trial reported nonvertebral fractures occurring after four years and was not included in the analysis (Frediani 2003). Protocols for assessing nonvertebral fractures were typically not stated in study procedures. We assumed all nonvertebral fractures were self‐reported symptomatic ones.
BMD data
12 months (lumbar spine)
Twenty‐six trials reported this outcome, 23 trials were included and three trials excluded from meta‐analysis.
Two trials (Saadati 2008; Sambrook 2003) reported data that were insufficient for inclusion in the lumbar spine analysis. Tee 2012 reported BMD using T scores and was therefore not included in the analysis.
12 months (femoral neck)
Twenty‐three trials reported this outcome, 18 trials were included and five trials excluded from meta‐analysis.
Reported data from Saadati 2008; Sambrook 2003 and Skingle 1997 were insufficient for inclusion in the femoral neck analysis. Van Offel 2001 reported “no change” in femoral neck BMD without providing any numerical data and was therefore not included in the femoral neck analysis. Tee 2012 reported BMD using T scores and was therefore not included in the femoral neck analysis.
18 to 24 months
Nine trials reported BMD outcomes at 18 to 24 months at both the lumbar spine and femoral neck and were included in separate analyses. One multi‐arm trial used two distinct pair‐wise comparisons with two independent treatment groups and two independent placebo groups (Sambrook 2003). In this study, one pair‐wise comparison involved steroid‐use of less than six months and the other comparison involved steroid‐use greater than six months. The data were entered as two separate trials, therefore each analysis has 10 pair‐wise comparisons.
Low‐dose versus standard‐dose bisphosphonates
Five trials included multi‐group trials with head‐to‐head comparisons of standard‐dose versus low‐dose bisphosphonates. All five trials reported on lumbar spine BMD and were included in this meta‐analysis. Four trials reported on femoral neck BMD and were all included in the analysis on femoral neck BMD.
Serious adverse events
Nineteen trials reported this outcome, 15 trials were included and four were excluded from the meta‐analysis.
Serious adverse events were most commonly defined as any event requiring hospitalisation, that was life‐threatening or fatal. One trial that we included defined serious adverse events as any event that rendered a patient incapable of performing normal activities (Reid 2000). Few included trials provided details on the types of serious adverse events that occurred (see Characteristics of included studies for further details). Five trials only reported deaths and did not specify the occurrence of other forms of serious adverse events (Adachi 1997; De Nijs 2006; Geusens 1998; Herrala 1998; Jenkins 1999) though we still included these trials in the analysis. The protocol for assessing adverse events was not clearly stated in seven of the trials included in this analysis (Adachi 1997; Geusens 1998; Jenkins 1999; Lems 2006; Pitt 1998; Saag 1998; Tee 2012).
Deaths
From the included trials, nine deaths were reported in the experimental groups (Adachi 1997; De Nijs 2006; Geusens 1998; Hakala 2012; Herrala 1998; Pitt 1998; Stoch 2009; Tee 2012) and five deaths were reported in the control groups (Boutsen 1997; De Nijs 2006; Herrala 1998; Jenkins 1999; Pitt 1998). In none of these cases did the study authors consider the deaths to be related to the study or placebo drugs. See Characteristics of included studies for further details.
Data from Adachi 2001 were not included as it was an extension trial of Saag 1998, which provided more complete data. Cortet 1999; Sambrook 2003 and Yeap 2008 each reported deaths but did not specify from which treatment group they occurred and were therefore not included in the analysis.
Withdrawals due to adverse events
Twenty‐one trials reported this outcome, 15 trials were included and six trials were excluded from meta‐analysis.
For this analysis we included withdrawals due to all adverse events regardless of their association to either the control or drug of study. We did not include death as a withdrawal due to an adverse event. See Characteristics of included studies for further details on types of adverse events leading to study withdrawal.
Four trials did not specify which treatment groups the withdrawals came from (Herrala 1998; Lems 2006; Sambrook 2003; Yeap 2008) and were therefore excluded from the analysis. Another trial did not provide numerical data on the withdrawals (Cortet 1999) and could not be included. Data from Adachi 2001 were not included as it was an extension trial of Saag 1998.
Quality of life
This outcome was not assessed by any of the trials.
Excluded studies
From our search of the databases, clinical trials registries and handsearching, we excluded 534 of the 587 articles that underwent full‐text review. The main reason for exclusion was not being an RCT (n = 330). We excluded 33 trials as they involved transplant‐associated steroid use. See Figure 1 for complete details. During data extraction from 39 studies, we excluded 11 for using only non‐standard bisphosphonate doses (Benucci 2009; Fujii 2006; Jinnouchi 2000; Kikuchi 2006; Kitazaki 2008; Nakayamada 2004; Okada 2008; Sato 2003; Takeda 2008; Takei 2010; Toukap 2005), and we categorised Ozoran 2007 as 'awaiting classification' pending data clarification from the study authors due to insufficient reporting of relevant outcome data. We have included the references of the 12 trials that we excluded during data extraction as they did meet our initial criteria for inclusion and may still be of relevance (details in Characteristics of excluded studies). In addition, eight trials were deemed to be 'awaiting classification' (details in Characteristics of studies awaiting classification). Of these, four trials identified from trials registers were completed but with no publications available and four trials were published as articles in Japanese with no English versions available (Imanishi 2006; Nakamura 2002; Okazaki 2015; Suzuki 2015). We are currently awaiting the translation of these four trials and will update the review once information is available. Finally, we identified six ongoing trials that may be of relevance to this review (details in Characteristics of ongoing studies).
The existing Cochrane Review included 13 trials and of these, only two trials were included in this review (Adachi 1997; Wolfhagen 1997). Five trials were conference abstracts that have been subsequently published and the full publications were included (Jenkins 1999; Pitt 1998; Roux 1998; Saag 1998; Skingle 1997). We excluded four trials because they were not RCTs. We excluded one trial because it measured BMD by computerised tomography (CT) instead of DEXA. Another trial from the original review reported outcome measurements at six months only. We decided that this time point was not clinically significant and not analysed in this review update, therefore we excluded this trial.
Risk of bias in included studies
Detailed results of this assessment are found in a table attached to the Characteristics of included studies table and are summarised below in Figure 2 and Figure 3.
2.
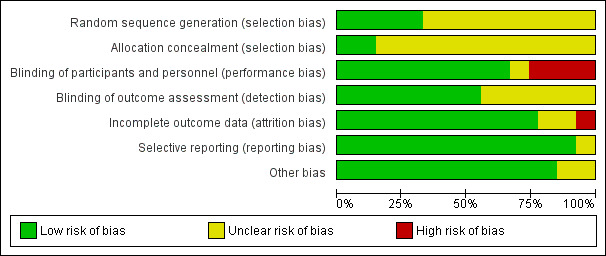
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
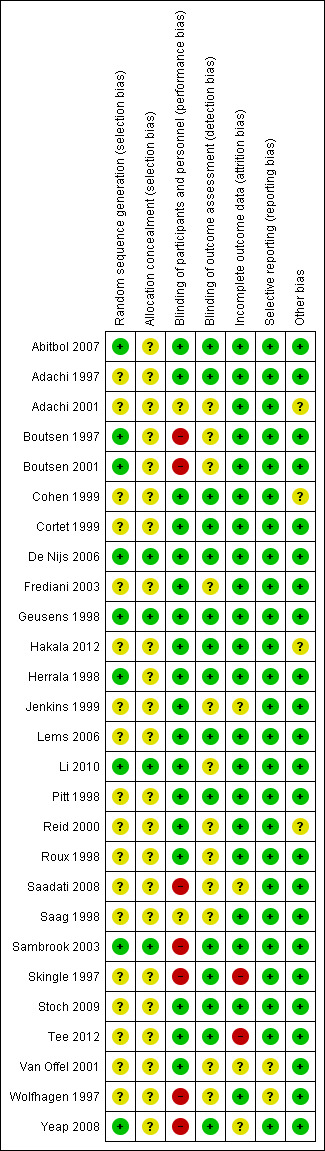
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four studies clearly described random sequence generation methods and allocation concealment and were at low risk for selection bias (De Nijs 2006; Geusens 1998; Li 2010; Sambrook 2003).
Eighteen studies did not provide details on random sequence generation methods or allocation concealment and so were at unclear risk for selection bias (Adachi 1997; Adachi 2001; Cohen 1999; Cortet 1999; Frediani 2003; Hakala 2012; Jenkins 1999; Lems 2006; Pitt 1998; Reid 2000; Roux 1998; Saadati 2008; Saag 1998; Skingle 1997; Stoch 2009; Tee 2012; Van Offel 2001; Wolfhagen 1997).
Five studies were at low risk of bias for randomisation as they provided clear descriptions of random sequence generation but were considered to have unclear risk of bias for allocation concealment, as no allocation details were provided (Abitbol 2007; Boutsen 1997; Boutsen 2001; Herrala 1998; Yeap 2008).
Blinding
Twelve studies provided adequate detail on blinding of participants, personnel and outcome assessment, so were at low risk for performance and detection bias (Abitbol 2007; Adachi 1997; Cohen 1999; Cortet 1999; De Nijs 2006; Geusens 1998; Hakala 2012; Herrala 1998; Lems 2006; Pitt 1998; Stoch 2009; Tee 2012)
Two studies did not provide details on blinding of participants, personnel or outcome assessors, so were at unclear risk for performance and detection bias (Adachi 2001; Saag 1998).
Six studies provided adequate detail on blinding of participants/personnel but did not mention blinding of outcome assessment so were at low risk for performance bias and unclear risk for detection bias (Frediani 2003; Jenkins 1999; Li 2010; Reid 2000; Roux 1998; Van Offel 2001).
Three studies had explicit and appropriate outcome assessment blinding, but clearly stated that participants/personnel were not blinded or were not placebo‐controlled, so were at high risk for performance bias and low risk for detection bias (Sambrook 2003; Skingle 1997; Yeap 2008).
Four studies had insufficient participant/personnel blinding and did not provide clear details on outcome assessment blinding, so were at high risk for performance bias and unclear risk for detection bias (Boutsen 1997; Boutsen 2001; Saadati 2008; Wolfhagen 1997).
Incomplete outcome data
Twenty‐one studies had sufficiently complete outcome data, adequately addressed reasons for dropout, with dropout similar between both groups, so were at low risk for attrition bias (Abitbol 2007; Adachi 1997; Adachi 2001; Boutsen 1997; Boutsen 2001; Cohen 1999; Cortet 1999; De Nijs 2006; Frediani 2003; Geusens 1998; Hakala 2012; Herrala 1998; Lems 2006; Li 2010; Pitt 1998; Reid 2000; Roux 1998; Saag 1998; Sambrook 2003; Stoch 2009; Wolfhagen 1997).
Three studies did not clearly address the reasons for participant dropout, so were at unclear risk for attrition bias (Saadati 2008; Van Offel 2001; Yeap 2008). Jenkins 1999 had low dropout and addressed reasons for dropout but only screened vertebral fractures radiographically in 13 of 28 completing participants. No explanation was provided for the low yield of vertebral radiographs so this study was at unclear risk for attrition bias.
Skingle 1997 had 31% of participants that did not complete the first year and only 23 of 38 completing participants were screened for radiographic vertebral fractures. Tee 2012 had 30% of participants that did not complete the study with the main reason being that they were unavailable for follow‐up. Both these studies were at high risk for attrition bias.
Selective reporting
Twenty‐five studies reported all outcomes that were listed in the methods section and were therefore considered to be at low risk for reporting bias (Abitbol 2007; Adachi 1997; Adachi 2001; Boutsen 1997; Boutsen 2001; Cohen 1999; Cortet 1999; De Nijs 2006; Frediani 2003; Geusens 1998; Hakala 2012; Herrala 1998; Jenkins 1999; Lems 2006; Li 2010; Pitt 1998; Reid 2000; Roux 1998; Saadati 2008; Saag 1998; Sambrook 2003; Skingle 1997; Stoch 2009; Tee 2012; Yeap 2008).
Van Offel 2001 had no mention of adverse events. Wolfhagen 1997 took spinal radiographs to validate DEXA measurements only, not as an outcome. Both these studies were at unclear risk for reporting bias.
Other potential sources of bias
Eighteen studies were judged to be at low risk as no other sources of bias were apparent (Abitbol 2007; Boutsen 1997; Boutsen 2001; Cortet 1999; Frediani 2003; Geusens 1998; Herrala 1998; Jenkins 1999; Lems 2006; Li 2010; Pitt 1998; Roux 1998; Saadati 2008; Skingle 1997; Stoch 2009; Tee 2012; Van Offel 2001; Wolfhagen 1997). Five studies were also rated as low risk and had pharmaceutical industry contribution that was limited to supplying the study drug or providing grants, with no industry authorship (Adachi 1997; De Nijs 2006; Saag 1998; Sambrook 2003; Yeap 2008).
Four studies were rated as having an unclear risk of bias due to other sources. Two studies had industry authorship (Cohen 1999; Hakala 2012); and Reid 2000 reported industry involvement in the design, implementation and analysis of the trial. We rated Adachi 2001 as unclear because it was an extension study, which has the potential risk of unblinding.
Effects of interventions
See: Table 1
Benefits
Incident radiographic vertebral fractures
Twelve trials (1343 participants) reported the number of participants with new vertebral fractures. We combined symptomatic and asymptomatic fractures. In this analysis 46/597 (or 77 per 1000) people experienced new vertebral fractures in the control group compared with 31/746 (or 44 per 1000; range 27 to 70) in the bisphosphonate group. The resulting RR was statistically significant at 0.57 (95% CI 0.35 to 0.91) (Analysis 1.1) signifying a relative per cent improvement of 43% (95% CI 9% to 65% better) with bisphosphonates; an absolute increased benefit of 2% fewer people experiencing fractures (95% CI 5% fewer to 1% more); and NNTB of 31 (95% CI 20 to 145) meaning that approximately 31 people would need to be treated with bisphosphonates to prevent new vertebral fractures in one person (Table 1). There was no statistical heterogeneity in the incident vertebral fracture analysis (I2 = 0%). Overall, there was high‐certainty evidence for a reduction in new vertebral fractures with bisphosphonates.
1.1. Analysis.
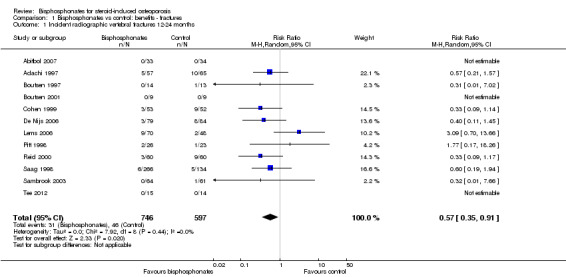
Comparison 1 Bisphosphonates vs control: benefits ‐ fractures, Outcome 1 Incident radiographic vertebral fractures 12‐24 months.
Incident radiographic nonvertebral fractures
In the analysis of nine trials (1245 participants), 30/546 (or 55 per 1000) people experienced new nonvertebral fractures in the control group compared with 29/699 (or 42 per 1000; range 25 to 69) in the bisphosphonate group. The resulting RR was 0.79 (95% CI 0.47 to 1.33) although this was not statistically significant (Analysis 1.2). There was a relative per cent improvement of 21% (95% CI 33% worse to 53% better) with bisphosphonates; an absolute increased benefit of 1% fewer people experiencing fractures (95% CI 4% fewer to 1% more); NNTB not applicable as results were not statistically significant (Table 1). There was no statistical heterogeneity in the nonvertebral fracture analysis (I2 = 0%). Overall, there was low‐certainty evidence that bisphosphonates may make little or no difference in the reduction of new nonvertebral fractures (downgraded for risk of bias and imprecision).
1.2. Analysis.
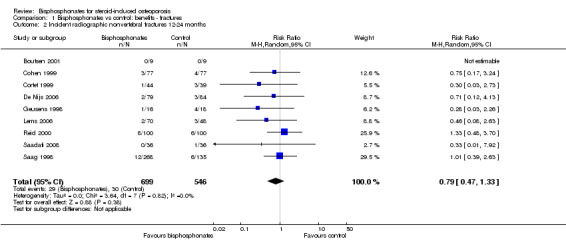
Comparison 1 Bisphosphonates vs control: benefits ‐ fractures, Outcome 2 Incident radiographic nonvertebral fractures 12‐24 months.
Per cent change in lumbar spine BMD
Bisphosphonate treatment up to 12 months
In the analysis of 23 trials (2042 participants), bisphosphonate use resulted in a statistically significant MD of 3.50% (95% CI 2.90% to 4.10%) (Analysis 2.1) representing an absolute benefit with BMD 3.5% higher with bisphosphonates as compared to calcium or vitamin D alone (95% CI 2.90% to 4.10% higher); a relative per cent improvement of 1.1% (95% CI 0.91% to 1.29% better); NNTB 3 (95% CI 2 to 3) meaning that approximately three people would need to be treated with bisphosphonates over 12 months in order to see a minimally important difference in lumbar spine BMD in one person (Table 1). There was substantial heterogeneity among these trials (I2 = 70%) that we found was adequately explained through subgroup and sensitivity analyses shown below. Overall, there was moderate‐certainty evidence of a clinically important increase in lumbar spine BMD with bisphosphonates (downgraded for indirectness).
2.1. Analysis.
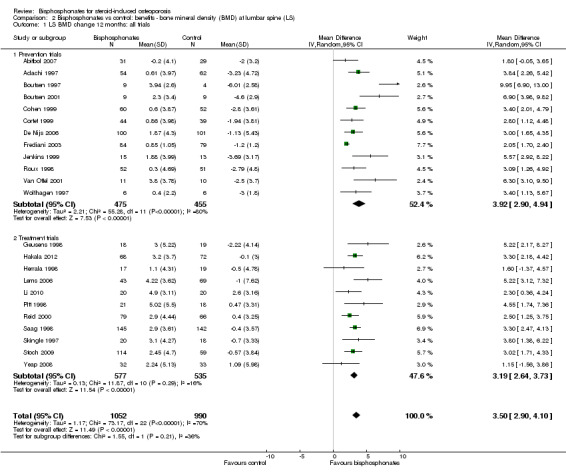
Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 1 LS BMD change 12 months: all trials.
Bisphosphonate treatment 18 to 24 months
In the analysis of nine trials (10 treatment groups) reporting on 802 participants, bisphosphonate use resulted in a statistically significant 5.49% (95% CI 3.47% to 7.51%) increase in BMD as compared to treatment with calcium or vitamin D alone (Analysis 2.5). There was substantial heterogeneity in this analysis (I2 = 91%). We removed one trial that differed from the others by using IM bisphosphonates in a female‐only population, which reduced the I2 statistic to 41% (result not shown).
2.5. Analysis.
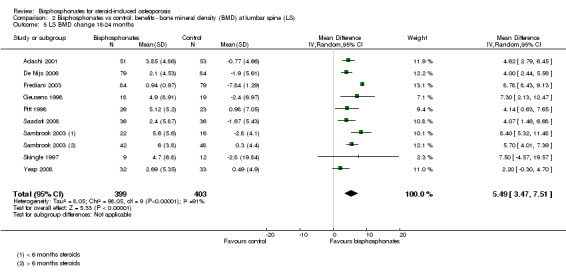
Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 5 LS BMD change 18‐24 months.
Per cent change in femoral neck BMD
Bisphosphonate treatment up to 12 months
Results from 18 trials (1665 participants) showed that bisphosphonate use resulted in a statistically significant MD of 2.06% (95% CI 1.45 to 2.68) (Analysis 3.1) representing an absolute benefit with BMD 2.06% higher in the bisphosphonate group as compared to treatment with calcium or vitamin D alone (95% CI 1.45% to 2.68% higher); a relative per cent improvement of 1.29% (95% CI 0.91% to 1.69% better); NNTB 5 (95% CI 4 to 7) meaning that approximately five people would need to be treated with bisphosphonates over 12 months in order to see a minimally important difference in femoral neck BMD in one person (Table 1). The heterogeneity among these trials was not substantial (I2 = 34%). Overall, there was moderate‐certainty evidence of a clinically important increase in femoral neck BMD with bisphosphonates (downgraded for indirectness) .
3.1. Analysis.
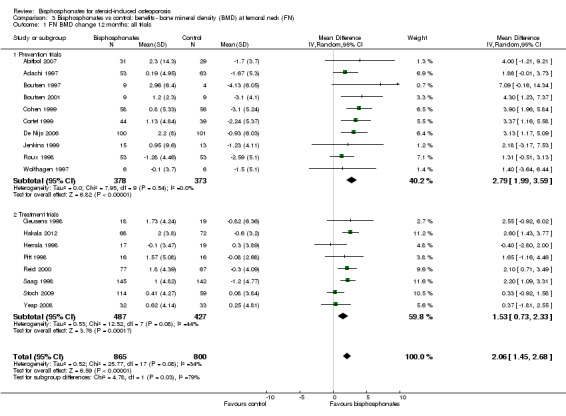
Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 1 FN BMD change 12 months: all trials.
Bisphosphonate treatment 18 to 24 months
Analysis of nine trials (10 treatment groups) reporting on 802 participants showed that bisphosphonate use resulted in a statistically significant 3.28% (95% CI 1.70% to 4.87%) increase in BMD as compared to treatment with calcium or vitamin D alone (Analysis 3.5). There was substantial heterogeneity among the trials in this analysis (I2 = 83%). One trial differed by using intramuscular (IM) bisphosphonates and another trial had a lower mean participant age, however removing single trials did not appreciably alter the heterogeneity.
3.5. Analysis.
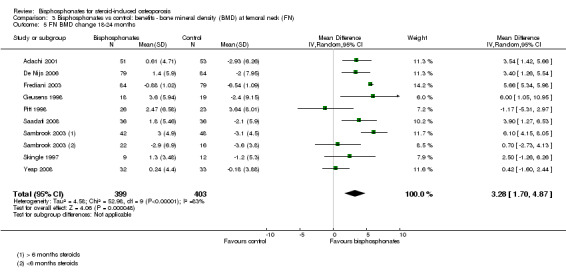
Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 5 FN BMD change 18‐24 months.
Quality of life
Quality of life was not assessed by any of the trials.
Per cent change in BMD at 12 months with low‐dose versus standard‐dose bisphosphonates
Lumbar spine
Head‐to‐head analysis of five trials (642 participants) resulted in a MD of 0.95% (95% CI 0.37% to 1.53%) that was statistically significant (Analysis 2.4). There was no statistical heterogeneity in this analysis (I2 = 0%)
2.4. Analysis.
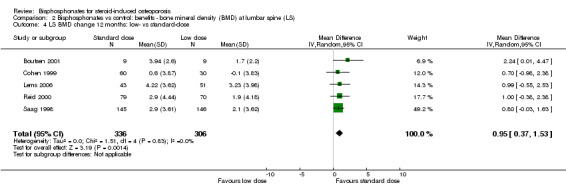
Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 4 LS BMD change 12 months: low‐ vs standard‐dose.
Femoral neck
Head‐to‐head analysis of four trials (542 participants) showed a MD of 0.74% (95% CI ‐0.42% to 1.90%) that did not reach statistical significance with substantial heterogeneity among trials (I2 = 54%) (Analysis 3.4).
3.4. Analysis.
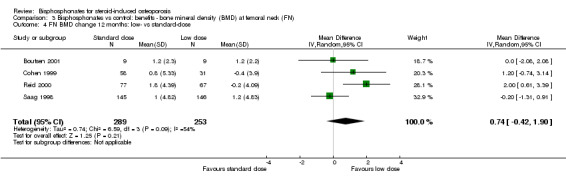
Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 4 FN BMD change 12 months: low‐ vs standard‐dose.
Harms
Serious adverse events
Fifteen trials (1703 participants) reported on serious adverse events with 131/811 (or 162 per 1000) people experiencing serious adverse events in the control group compared to 136/892 (or 147 per 1000; range 120 to 181) in the bisphosphonate group. The RR for serious adverse events in the bisphosphonate group was 0.91 (95% CI 0.74 to 1.12) (Analysis 4.1); an absolute increased harm of 0% more serious adverse events (95% CI 2.00% fewer to 2.00% more); a relative per cent change 9% improvement (95% CI 12% worse to 26% better); NNTH not applicable as there was no statistically significant difference (Table 1). There was no statistical heterogeneity in the analysis on serious adverse events (I2 = 0%). Overall, there was low‐certainty evidence (downgraded for imprecision and risk of bias) that bisphosphonates may make little or no difference in the number of serious adverse events.
4.1. Analysis.
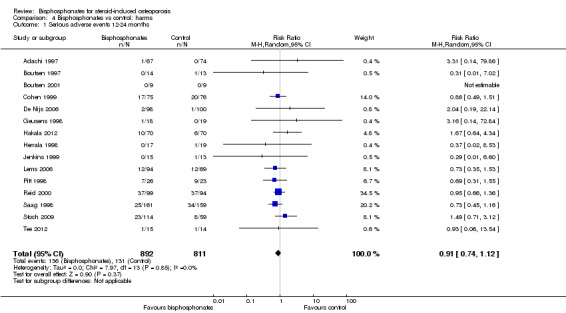
Comparison 4 Bisphosphonates vs control: harms, Outcome 1 Serious adverse events 12‐24 months.
Withdrawals due to adverse events
Fifteen trials (1790 participants) reported withdrawals due to adverse events. Not all adverse events were listed, but in those trials that did have information, the most common adverse events were upper gastrointestinal symptoms and musculoskeletal pain. In this analysis, 63/866 (or 73 per 1000) people withdrew in the control group compared to 76/924 (or 77 per 1000; range 56 to 107) in the bisphosphonate group. The RR for withdrawals due to adverse events in the bisphosphonate group was not statistically significant at 1.06 (95% CI 0.77 to 1.47) (Analysis 4.2); an absolute increased harm of 1% more withdrawals with bisphosphonates (95% CI 1% fewer to 3% more); a relative per cent change 6% worse (95% CI 47% worse to 23% better); NNTH not applicable as there was no statistically significant difference between groups (Table 1). There was no substantial heterogeneity in the withdrawals due to adverse events analysis (I2 = 2%). Overall, there was low‐certainty evidence (downgraded for imprecision and risk of bias) that bisphosphonates may make little or no difference in the number of withdrawals due to adverse events.
4.2. Analysis.
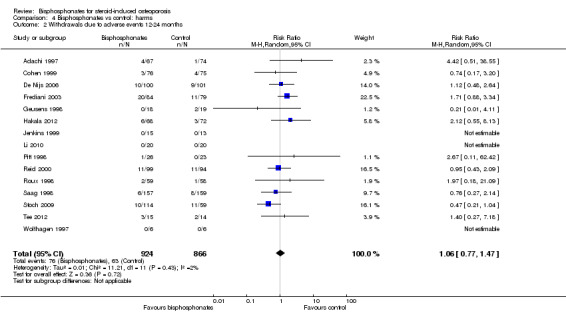
Comparison 4 Bisphosphonates vs control: harms, Outcome 2 Withdrawals due to adverse events 12‐24 months.
Subgroup analyses
Prevention and treatment studies
A pre‐specified subgroup analysis was used to analyse separately prevention and treatment effects in trials reporting on BMD at the lumbar spine and femoral neck at 12 months.
Lumbar spine
In the prevention analysis (12 trials, 930 participants) bisphosphonate use resulted in an increase in BMD of 3.92% (95% CI 2.90% to 4.94%) as compared to treatment with calcium or vitamin D alone (Analysis 2.1). In the treatment analysis (11 trials, 1112 participants), bisphosphonate use resulted in an increase in BMD of 3.19% (95% CI 2.64% to 3.73%) as compared to treatment with calcium or vitamin D alone (Analysis 2.1). Both were statistically significant. There was substantial heterogeneity among the studies in the prevention analysis (I2 = 80%) but not the treatment analysis (I2 = 16%). A sub‐subgroup analysis was done for the prevention analysis to separate trials using parenteral and oral bisphosphonates (Analysis 2.6). This appears to explain the significant heterogeneity in the prevention analysis. There were no parenteral bisphosphonates used in treatment trials.
2.6. Analysis.
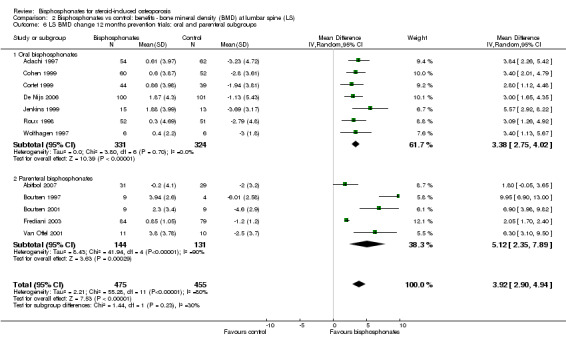
Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 6 LS BMD change 12 months prevention trials: oral and parenteral subgroups.
Femoral neck
In the prevention analysis (10 trials, 751 participants), bisphosphonate use resulted in a statistically significant increase in BMD of 2.79% (95% CI 1.99% to 3.59%) as compared to treatment with calcium or vitamin D alone (Analysis 3.1). In the treatment analysis (eight trials, 914 participants), the increase in BMD was 1.53% (95% CI 0.73% to 2.33%) (Analysis 3.1). Both were statistically significant. There was no substantial heterogeneity in either the prevention or treatment analyses (I2 = 0% and 44%, respectively).
Gender and menopausal status
Fracture data were not broken down by gender and menopausal status in the individual trials included in our analysis and therefore post‐hoc subgroup analyses were not possible for fracture outcomes. Sufficient data on BMD at the lumbar spine and femoral neck at 12 months were available to analyse subgroups of gender and menopausal status (men, premenopausal women, postmenopausal women). Please see Analysis 2.7 and Analysis 3.6 for full details on results.
2.7. Analysis.
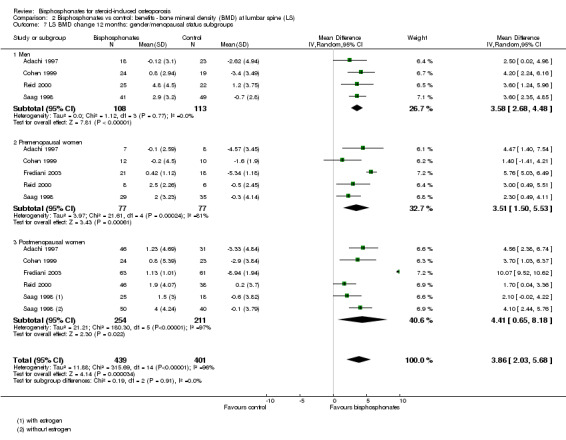
Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 7 LS BMD change 12 months: gender/menopausal status subgroups.
3.6. Analysis.
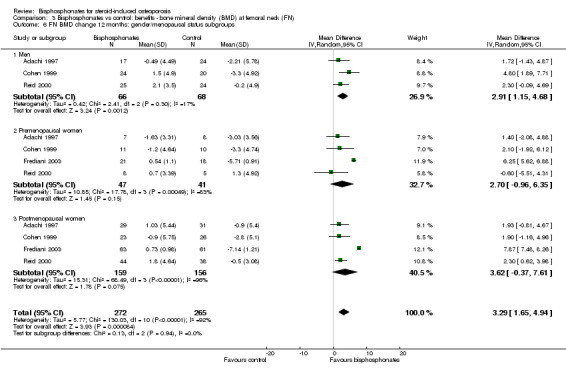
Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 6 FN BMD change 12 months: gender/menopausal status subgroups.
Mean steroid dose
In the vertebral fracture, nonvertebral fracture and 12 months BMD outcomes, Cohen 1999 was the only trial to use high‐dose steroids (greater or equal to 20 mg/day).
Prevalent fractures
No trials reported outcome data separately for those participants with and without a prevalent fracture.
Sensitivity analyses
Risk of bias
To analyse the effect of study quality, based on the exclusion of non‐blinded trials (high risk for performance or detection bias) we performed a sensitivity analysis. Sensitivity analyses excluding high‐risk trials for the outcomes of incident vertebral fractures; incident nonvertebral fractures; BMD at the lumbar spine and femoral neck after 12 months and 18 to 24 months; serious adverse events; withdrawals due to adverse events; BMD using low‐ versus standard‐dose bisphosphonates did not appreciably change the effect sizes and did not resolve heterogeneity among trials (results not shown).
Route of administration
We performed a sensitivity analysis based on route of administration. We analysed trials using oral bisphosphonates separately from those using parenteral bisphosphonates. The trials reported BMD data at the lumbar spine and femoral neck at 12 months.
Lumbar spine
At the lumbar spine, pooled analysis of 18 trials (1767 participants) using oral treatments showed a statistically significant result with MD 3.25% (95% CI 2.88% to 3.63%) (Analysis 2.2).
2.2. Analysis.
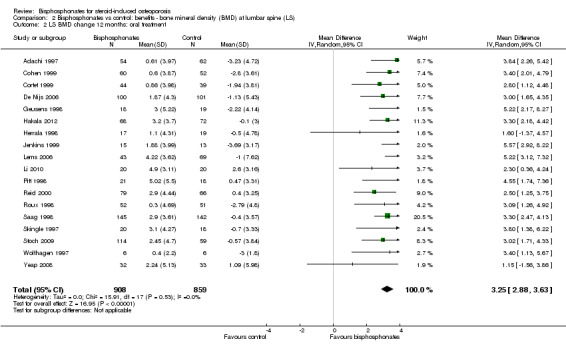
Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 2 LS BMD change 12 months: oral treatment.
Analysis of five trials (275 participants) using parenteral treatments had a statistically significant MD 5.12% (95% CI 2.35% to 7.89%) at the lumbar spine (Analysis 2.3). There was no statistical heterogeneity in the oral analysis (I2 = 0%) but heterogeneity was substantial in the lumbar spine parenteral treatment analysis (I2 = 90%).
2.3. Analysis.
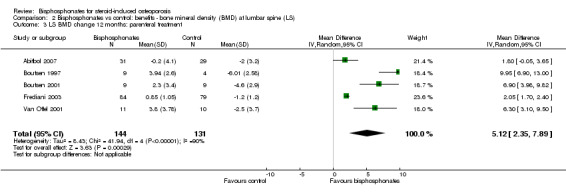
Comparison 2 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS), Outcome 3 LS BMD change 12 months: parenteral treatment.
Femoral neck
At the femoral neck, analysis of 15 trials (1574 participants) using oral administration had a statistically significant MD 1.92% (95% CI 1.31% to 2.53%) (Analysis 3.2).
3.2. Analysis.
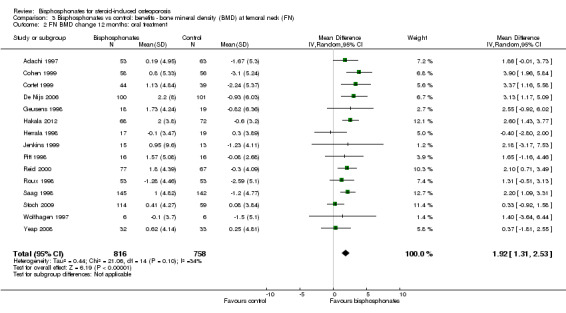
Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 2 FN BMD change 12 months: oral treatment.
Analysis of three trials (91 participants) using parenteral therapy was also statistically significant with MD 4.56% (95% CI 2.07% to 7.05%) (Analysis 3.3). There was no substantial heterogeneity in either the oral or parenteral treatment analyses at the femoral neck (I2 = 34% and 0%, respectively).
3.3. Analysis.
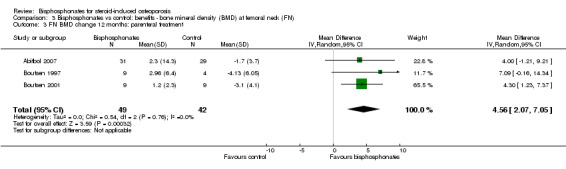
Comparison 3 Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN), Outcome 3 FN BMD change 12 months: parenteral treatment.
Discussion
Summary of main results
We carried out this review to evaluate the benefits and harms of bisphosphonates in GIOP. We examine benefits in terms of fracture outcomes and BMD change; and include subgroup analyses of primary prevention versus treatment. The review provides new data on oral and parenteral bisphosphonates and a direct comparison of low and standard bisphosphonate doses. Harm data include serious adverse events and withdrawals due to adverse events.
Fracture Data
The most clinically relevant outcome is the effect of bisphosphonates on fracture prevention. Results from our analyses show that bisphosphonates reduce the total number of incident vertebral fractures by approximately 40% with a NNTB of 31. There was no statistically significant reduction in fractures at nonvertebral sites, including the hip.
Harm Data
There were no statistically significant differences in either the incidence of serious adverse events or withdrawals due to adverse events between active drug and control groups. The most frequently reported adverse events in our review were musculoskeletal (myalgias and arthralgias) and upper gastrointestinal; though typically mild in nature and seen in both the bisphosphonate and control groups. Gastrointestinal side effects, or even fear of experiencing them, are cited as a primary reason for decreased compliance or discontinuation of treatment (Pazianas 2011). Other common associated side effects cited in the literature include transient asymptomatic hypercalcaemia and a transient acute phase response (two to three days of flu‐like symptoms most often associated with the initiation of intravenous treatment). These were seen sporadically in the trials included in our review.
Although high‐certainty epidemiological evidence is limited, osteonecrosis of the jaw is a rare but serious adverse event that is associated with bisphosphonate use; specifically with long‐standing use and frequent intravenous dosing (Pazianas 2011).
Bisphosphonates are associated with an increased risk of atypical femur fractures (subtrochanteric or diaphyseal). Estimated incidences vary from 12.5 to 31 of 10,000 people/year with approximately 10 years of alendronate exposure (Abrahamsen 2010; Dell 2012). Current data are conflicting, however most studies agree that the absolute risk of atypical femur fracture with bisphosphonates is low. A recent meta‐analysis pooled nine observational trials and one RCT (n = 658,497) and found a statistically significant increased risk of atypical femur fractures with bisphosphonates; adjusted OR 1.99 (95% CI 1.28 to 3.10) (Lee 2015). Although the clinical implication is uncertain, both long‐term steroid use and systemic inflammatory disease processes are considered to be risk factors associated with atypical femur fractures (Lee 2015).
Neither osteonecrosis of the jaw nor atypical femur fractures were reported in any of the trials included in our review.
Experts still recommend caution in using bisphosphonates in women of childbearing age until further systematic research has been performed (Djokanovic 2008; Losada 2010).
BMD Data
Our major BMD outcomes show statistically significant and clinically meaningful increases in bone density at the lumbar spine and femoral neck after 12 months, with MD of 3.50% and 2.06%, respectively. The NNTB at the lumbar spine after 12 months is 3, and the NNTB at the femoral neck after 12 months is 5. It is generally believed that steroid‐induced bone loss is not as prominent in cortical bone (Rickers 1984) and that changes in BMD are not as dramatic at the femoral neck, as it takes longer for cortical bone to turn over. Our results support these beliefs and data from the placebo arms of the trials show a smaller magnitude of bone loss at the femoral neck than the lumbar spine.
Our NNTBs for the change in BMD at the lumbar spine and femoral neck are based on an SMD of 0.5 as an estimate of the minimal clinically important difference for BMD change (Schünemann 2011b). It is difficult to express our findings in terms of a minimally clinically relevant change in BMD as that value has not been established for GIOP. Fractures in GIOP often occur at a higher BMD than seen in primary osteoporosis and, unlike primary osteoporosis, a predictable correlation between BMD and fracture risk has not been established in the GIOP setting. Furthermore, improvement in BMD only accounts for a small part of the reduction in vertebral fracture risk observed with antiresorptive therapy (Cummings 2002; Kanis 2007; Saag 2003; Van Staa 2002).
A previous meta‐analysis evaluating BMD improvements and vertebral fracture risk reduction in postmenopausal women and elderly men suggests that each 1% improvement in spine BMD during anti‐resorptive therapy is associated with a 0.03 decrease in relative risk of vertebral fracture (Cummings 2002). Although the meta‐analysis is neither specific to GIOP nor to bisphosphonates; it does provide a rough clinical context to consider our findings of a 3.5% difference between treatment and control group lumbar spine BMD after 12 months of bisphosphonate therapy.
The natural history of patients starting steroid therapy based on control arms in our prevention trials is to see a 1% to 6% decrease in lumbar spine BMD and 1% to 4% decrease in femoral neck BMD in the first year of treatment.
We were interested in analysing the prevention and treatment trials separately as the two clinical scenarios are distinct. In general, the prevention trials showed greater bone loss in the control arm with maintenance or small bone accrual in the treatment arm. In contrast, the treatment trials showed a greater degree of accrual in the treatment arm with less dramatic bone loss in the control arm. This supports the belief that bone loss is more prominent in the early stages of corticosteroid therapy, with a slower rate of loss as therapy continues. As such, prophylactic therapy demonstrates an ability to reduce bone loss, whereas bisphosphonate treatment provides an opportunity to build bone mass in chronic steroid‐using people.
We included post‐hoc subgroup analyses based on gender and menopausal status (men, premenopausal women, postmenopausal women) however we are cautious in interpreting these results because subgroup comparisons are observational in nature (Deeks 2011). We found that there remains a similar magnitude of effect in the subgroups as compared to the whole group analyses except for a wider spread of variability, perhaps due to fewer trials in each analysis. Results for the female groups at the femoral neck were not statistically significant. There was no significant heterogeneity in the male analyses but significant heterogeneity in the female plots. Frediani 2003 was a visible outlier in the female plots and differed from the other trials by its use of parenteral bisphosphonates, which may explain such a difference. Our a priori sensitivity analyses that separated oral and enteral bisphosphonates revealed that heterogeneity was likely in part due to this factor. Removal of Frediani 2003 reduced the I2 statistic in all female analyses (I2= 0% for premenopausal lumbar spine BMD analysis; I2= 43% for postmenopausal lumbar spine BMD analysis; I2= 0% for premenopausal and postmenopausal femoral neck BMD analyses; results not shown). These post‐hoc analyses included mixed data from prevention and treatment trials. Sub‐subgroup analyses, broken down by gender and prevention or treatment, could not be performed as data were not presented in the trials at that level of detail.
Given the poor absorption of oral bisphosphonates (Gertz 1995; Russell 2007) there is perceived differential efficacy based on route of administration. Our analysis showed that the effect size of BMD at lumbar spine differed between parenteral bisphosphonate trials and oral bisphosphonate trials (5.12% and 3.25%, respectively). This difference in effect size between the parenteral analysis and oral analysis was also seen at the femoral neck (4.56% and 1.92%, respectively). In the lumbar spine BMD analysis all parenteral bisphosphonates were prevention studies which likely contributed to the significant heterogeneity in the lumbar spine BMD prevention subgroup analysis. A sub‐subgroup analysis of parenteral and oral bisphosphonates subsequently eliminated the heterogeneity in the lumbar spine BMD prevention subgroup.
There are few head‐to‐head trials of oral versus parenteral bisphosphonates in GIOP. A 12‐month RCT including 265 men, divided into prevention and treatment subgroups, compared a single 5 mg infusion of zoledronic acid to 5 mg daily oral risedronate. The authors found a statistically significant increase in lumbar spine BMD of 2.7% (95% CI 0.99% to 4.43%) with zoledronic acid over risedronate in their prevention subgroup. The treatment difference at the femoral neck was not statistically significant in the prevention subgroup at 1.38% (95% CI ‐0.18% to 2.95%) (Sambrook 2012). Another RCT (n = 771) found statistically significant improvements in BMD at 12 months with zoledronic acid over risedronate at both the lumbar spine and femoral neck in the prevention subgroups; 1.96% (95% CI 1.04 to 2.88) and 1.33% (95% CI 0.41% to 2.25%) (Reid 2009). Overall, parenteral regimes were vastly preferred over oral regimes in these trials. Route of administration is a factor to be considered in the treatment of GIOP.
We analysed head‐to‐head comparisons of the low‐ and standard‐dose bisphosphonate treatment groups in five studies (Boutsen 2001; Cohen 1999; Lems 2006; Reid 2000; Saag 1998). The standard‐dose bisphosphonate groups showed a small increase in benefit at the lumbar spine (0.95%, 95% CI 0.37% to 1.53%). The data suggest that even low‐dose bisphosphonates can be beneficial in the treatment of GIOP.
It was interesting to see that an ad‐hoc pooled analysis of low‐dose bisphosphonates (Boutsen 2001; Cohen 1999; Lems 2006; Reid 2000; Saag 1998) compared to treatment with calcium or vitamin D alone resulted in a mean difference of 3.15% (95% CI 1.87% to 4.44%) at the lumbar spine. This effect size is not out of the range of our pooled estimate for all studies using standard‐dose bisphosphonates supporting the suggestion that low‐dose bisphosphonates may be beneficial (results not shown).
There is a perception that newer bisphosphonates are more effective than etidronate in treating osteoporosis. In both the vertebral fracture analyses and both the BMD analyses of oral bisphosphonates there was no heterogeneity among the studies, which included three different types of bisphosphonates. We did not perform a post‐hoc analysis on newer bisphosphonates.
One critique of bisphosphonate studies is the lack of long‐term follow‐up. For this updated review we decided to include the analysis of BMD at the lumbar spine and femoral neck after 18 to 24 months. Results from these analyses show increases in bone density at the lumbar spine and femoral neck with mean differences of 5.49% and 3.28%, respectively. This suggests ongoing efficacy up to two years.
Overall completeness and applicability of evidence
This updated review examined the evidence from 27 RCTs for the use of bisphosphonates in the prevention and treatment of GIOP. We included 25 new studies for this update, and, due to more rigorous inclusion criteria, only two of the 13 studies from the existing review, as there were numerous higher quality studies published since the original review. A variety of outcomes measured both the benefits and the harms of bisphosphonates. Benefits included decreased bone loss or bone accrual at both the lumbar spine and femoral neck and a reduced risk of vertebral fractures. Although fracture outcome data may have more direct clinical relevance than BMD outcome measurements, most GIOP studies reported BMD as the primary outcome. Vertebral fracture data were sufficient to address the objectives of our review, however data on nonvertebral fractures often lacked detail and we were unable to examine the incidence of hip and wrist fractures separately. None of the trials reported on quality of life so we were unable to include this as an outcome measure in our review.
This updated review provides confirmatory evidence that bisphosphonates, alone or in combination with calcium or vitamin D, or both, are more beneficial than calcium or vitamin D, or both, alone or with placebo, for both the prevention and treatment of GIOP at the hip and spine. The trials used in this review used similar inclusion criteria, however the participant groups differed between trials in terms of the prior steroid usage, baseline BMD measurements and prevalent fractures. This updated review provides new data on the benefits of bisphosphonates in reducing the risk of vertebral fractures and the prevention versus treatment of GIOP. We provide new data on the different effect sizes seen in oral and parenteral bisphosphonate clinical trials, and the potential benefit of low‐dose bisphosphonates.
Regarding bisphosphonate harms, we could not find any statistically significant differences in the occurrence of serious adverse events or withdrawals due to adverse events between the bisphosphonate and control groups. In nine of the trials analysed, the protocols for the collection of harm data were unclear and may have resulted in biased results. Some of the included studies based their power calculations solely on benefit outcomes and may not have been sufficiently powered to adequately assess harm outcomes. Although findings on serious adverse events and withdrawals due to adverse events are important to consider in evaluating bisphosphonate therapy, we are cautious in interpreting these data as markers for harm and tolerability due to the potential for bias (Higgins 2011a).
Quality of the evidence
The Table 1 shows the overall certainty and importance of the body of evidence using the GRADE Working Group Approach (Schünemann 2013). We rated the certainty of the evidence for vertebral fractures as high, which indicates that further research is very unlikely to change our confidence in the estimate of effect. We downgraded the nonvertebral fracture outcome to low‐certainty evidence for imprecision due to a total sample size below the calculated optimal information size, and risk of bias for being a patient‐reported subjective outcome. We rated the certainty of remaining outcomes as moderate, which indicates that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. BMD outcomes were downgraded for indirectness as surrogate markers for osteoporosis. Serious adverse events and withdrawals due to adverse events were downgraded for imprecision due to small total sample size and 95% CI including the possibility of no effect and appreciable harm. We assessed all outcomes as important in terms of their impact on decisions regarding optimal management.
Potential biases in the review process
Our methods and reporting are based on the Cochrane Handbook for Systematic Reviews of Interventions recommendations (Higgins 2011). We devised a thorough search strategy with no language restrictions and believe that we identified all relevant studies. Two review authors in various combinations independently assessed the trials for inclusion in the review, assessed risk of bias and extracted data for analysis. The biggest limitations of the review process were the heterogeneity between the trials, likely related to different patient characteristics (differences in underlying inflammatory conditions, steroid doses, and prevalent fractures) and that some trials reported outcomes but did not provide data in a form that could be extracted for meta‐analysis.
Agreements and disagreements with other studies or reviews
Our findings are similar to those reported in the 1999 Cochrane Review evaluating the efficacy of bisphosphonates in steroid‐induced osteoporosis (Homik 1999). As there were very few high‐quality papers included in the original review, this updated review includes data from only two of the 13 studies that were included in the original version. Therefore, the effect estimates have changed. While the original review was not able to establish bisphosphonate benefit beyond one year or against spinal fractures, the meta‐analyses in this updated review have provided conclusive data on the above measures of benefit.
A subsequent systematic review by the Health Technology Assessment programme of the NHS by Kanis 2007 assessed the clinical effectiveness of numerous active and inactive treatments for GIOP. Outcome measures included incident vertebral fractures, incident nonvertebral fractures, associated effects, compliance and continuance. Kanis 2007 included 23 bisphosphonate studies, of which only eight overlap with the 27 studies in our updated review. Many of the studies in Kanis 2007 were not eligible for inclusion in our review for using active comparators or including participants with transplant‐associated steroid use. Kanis 2007 analysed different bisphosphonate types individually and found that, in the non‐transplant population, only risedronate 5 mg/day showed a statistically significant reduction in vertebral fractures as compared to placebo or no treatment. No intervention was shown to be beneficial in preventing nonvertebral fractures. In our analysis of vertebral fractures nine of the 10 studies used alendronate and showed a statistically significant reduction in vertebral fractures.
There are some other differences between our updated review and that of Kanis 2007. Our review includes 14 bisphosphonate trials that were not included in Kanis 2007 because fracture was not the primary outcome. Our fracture analyses include only studies with standardised radiographic screening of vertebral fractures in order to ensure complete capture of fracture incidence, whereas many of the studies in Kanis 2007 report only symptomatic fractures. It is known that up to 65% of vertebral fractures may be asymptomatic (Cooper 1992; Kanis 2007). Combining all types of bisphosphonate in our pooled analyses allows for a stronger effect size estimate. As their report shows, most studies only achieved statistically significant findings in pooled analyses.
Current American College of Rheumatology clinical practice guidelines for post‐menopausal women and men 50 years old or more recommend bisphosphonate therapy for GIOP based on FRAX risk assessment (Grossman 2010). Bisphosphonate therapy is recommended in low‐risk patients (FRAX < 10% for 10‐year major osteoporotic fracture) on corticosteroid treatment for greater or equal to 7.5 mg/day for three or more months' duration; moderate‐risk patients (FRAX 10% to 20%) on doses less than 7.5 mg/day for three or more months' duration; and high‐risk patients (FRAX > 20%) on any dose and duration of corticosteroid (Grossman 2010). In comparison, the International Osteoporosis Foundation and European Calcified Tissue Society framework for GIOP management of post‐menopausal women and men 50 years old or more recommend treatment to be considered in patients on corticosteroid treatment greater or equal to 7.5 mg/day for three or more months' duration with a previous fracture or that are greater than 70 years of age (Lekamwasam 2012). For those with no previous fracture, younger than 70 years, or on corticosteroid doses less than 7.5 mg/day, FRAX assessment with or without BMD assessment is recommended to further guide decision‐making (Lekamwasam 2012). Our study supports the use of bisphosphonates in people who are either starting corticosteroids or on established corticosteroid therapy.
Authors' conclusions
Implications for practice.
There is high‐certainty evidence that bisphosphonates are beneficial in reducing the risk of vertebral fractures in people on corticosteroids with data extending to 24 months of use. There is moderate‐certainty evidence that bisphosphonates are beneficial in preventing and treating corticosteroid‐induced bone loss at both the lumbar spine and femoral neck. Though the magnitude of effect on BMD differs between sites and whether used for treatment or prophylaxis, in all groups there are statistically and clinically relevant findings. There was low‐certainty evidence that bisphosphonates may make little or no difference in preventing nonvertebral fractures, although the low number of events reported in all studies makes it difficult to show a benefit without a larger sample size.
Prophylactic therapy demonstrates an ability to reduce bone loss, whereas treatment of GIOP provides an opportunity to build bone mass in chronic steroid‐using patients. There is a trend toward greater benefit with the use of IV bisphosphonates although this review was not set up as a comparative effectiveness analysis. Notions of increased efficacy in IV therapy as compared to oral have yet to be firmly established in the setting of GIOP. It was interesting to see that low‐dose bisphosphonate regimes had only a slightly lower benefit as compared to standard doses given the poor absorption of oral bisphosphonates.
Bisphosphonates are generally well tolerated with the number of reported side effects being similar between treatment and placebo groups. Upper gastrointestinal symptoms and musculoskeletal pain are the most common side effects reported and are typically mild in nature. There was low‐certainty evidence that bisphosphonates may make little or no difference in serious adverse events or withdrawals due to adverse events.
We conclude that the outcomes assessed in this review are important in terms of their impact on decisions regarding optimal GIOP management. Based on the currently available moderate‐ to high‐certainty evidence, our review supports the use of bisphosphonates to reduce the risk of vertebral fractures and the prevention and treatment of steroid‐induced bone loss and is consistent with current guidelines.
Implications for research.
Further high‐quality research on vertebral fractures is unlikely to substantially change the conclusions of this review. The benefits of bisphosphonates in the prevention and treatment of GIOP is well established when BMD data are used as surrogate markers for fracture risk. More research needs to be conducted into long‐term nonvertebral fracture prevention in this patient population. Recommendations regarding the routine use of these medications in people on corticosteroids require further research to answer questions regarding cost‐effectiveness. Further head‐to‐head trials comparing parenteral to oral bisphosphonates with fracture outcome data are needed to investigate the potential increased efficacy of parenteral bisphosphonates in GIOP. Finally, confirmation regarding the efficacy of low‐dose bisphosphonates in the GIOP population is required.
What's new
| Date | Event | Description |
|---|---|---|
| 14 October 2016 | Amended | Minor correction in the plain language summary |
History
Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 16 September 2016 | New search has been performed | New search has been performed: 25 new studies added to this update. 11 studies from original review excluded. Background section revised to provide current information. Methods updated to reflect current clinical relevance and availability of higher quality studies since original review, in accordance with current Cochrane Muskuloskeletal Group methods: 'Summary of findings' tables added. Details in ‘differences between protocol and review’ section. |
| 1 June 2016 | New citation required and conclusions have changed | Change in conclusions on update: bisphosphonates beneficial in reducing risk of vertebral fractures. |
| 19 September 2008 | Amended | Converted to new review format. C012‐R |
Acknowledgements
The authors would like to thank the members of Cochrane Musculoskeletal for their guidance; Trish Chatterley and Marlene Dorgan, University of Alberta Health Sciences Librarians, for their assistance with literature searches. We also wish to acknowledge the work of Ann Cranney, Beverley Shea, Peter Tugwell, George Wells, Jonathan Adachi and Maria Suarez‐Almazor as authors of the original review.
Appendices
Appendix 1. Original Review search strategies
MEDLINE (1966‐1997) 1. exp "osteoporosis"/ 2. exp "adrenal cortex hormones"/ 3. exp "anabolic steroids"/ 4. exp "bone density"/ 5. exp "anti‐inflammatory agents, steroidal"/ 6. 1 or 4 7. 2 or 3 or 5 8. 6 and 7 9. exp "diphosphonates"/ 10. 9 and 6 11. exp "osteoporosis"/ci 12. 8 or 10 or 11 13. limit 12 to human 14. limit 13 to English language 15. exp osteoporosis/dt 16. exp bone diseases/ 17. 16 and 7 18. limit 17 to human 19. limit 18 to English language 20. 14 or 15 or 19 Embase (1988‐1997) 1. exp bone demineralization/ 2. exp bone density/ 3. exp bone disease/ 4. bone demineralization/ 5. osteopenia/ 6. osteoporosis/ 7. postmenopause osteoporosis/ 8. posttraumatic osteoporosis/ 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp corticosteroid/ 11. exp antirheumatic agent/ 12. antiinflammatory agent/ 13. exp antiinflammatory agent/ 14. exp nonsteroid antiinflammatory agent/ 15. 13 not 14 16. 10 or 11 or 12 or 15 17. exp bisphosphonic acid derivative/ 18. 9 and 17 19. 9 and 16 20. exp bone demineralization/si 21. exp osteopenia/si 22. exp bone demineralization/dt 23. 18 or 19 or 20 or 21 or 22
Appendix 2. CENTRAL search strategy 2015
Database: Cochrane Central Register of Controlled Trials (CENTRAL)
Date Searched: 07 May 2015
1. exp Osteoporosis/
2. (osteoporo* or osteopeni*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
3. Bone Density/
4. exp Fractures, Bone/
5. (bone* adj fragil*).mp.
6. exp Bone Resorption/
7. bone loss.mp.
8. (bmd or bone mineral densit* bone deminerali*).mp.
9. or/1‐8
10. exp Steroids/
11. exp Adrenal Cortex Hormones/
12. (steroid* or corticosteroid* or glucocorticoid*).mp.
13. or/10‐12
14. 9 and 13
15. Bone Diseases, Metabolic/ci [Chemically Induced]
16. 14 or 15
17. exp Diphosphonates/
18. (diphosphonate* or bisphosphonate*).mp.
19. etidronate.mp.
20. alendronate.mp.
21. pamidronate.mp.
22. clodronate.mp.
23. tiludronate.mp.
24. olpadronate.mp.
25. incadronate.mp.
26. zolendronate.mp.
27. risedronate.mp.
28. zolendronic acid.mp.
29. ibandronate.mp.
30. medronate.mp.
31. minodronate.mp.
32. neridronate.mp.
33. oxidronate.mp.
34. or/17‐33
35. 16 and 34
36. randomized controlled trial.pt.
37. controlled clinical trial.pt.
38. clinical trial.pt.
39. randomi?ed.ti,ab.
40. placebo.ti,ab.
41. dt.fs.
42. randomly.ti,ab.
43. trial.ti,ab.
44. groups.ti,ab.
45. or/36‐44
46. animals/
47. humans/
48. 46 not (46 and 47)
49. 45 not 48
50. 35 and 49
51. limit 50 to yr="2010 ‐Current"
Appendix 3. MEDLINE search strategy 1997‐2010
Database: Medline via Ovid <1946 to Present>
Date Searched: 25 January 2010
| 1. exp Osteoporosis/ 2. osteoporo$.tw. 3. osteopeni$.tw. 4. exp Bone Density/ 5. exp Fractures, Bone/ 6. (bone$ adj fragil$).tw. 7. (bone adj loss).tw. 8. bmd.tw. 9. (bone adj2 densit$).tw. |
| 10. or/1‐9 |
| 11. exp Adrenal Cortex Hormones/ 12. exp Steroids/ 13. exp Glucocorticoids/ 14. (corticosteroid$ or $corticoid$).tw. 15. (adrenal adj cortex adj hormone$).tw. 16. steroid$.tw. |
| 17. or/11‐16 |
| 18. exp Diphosphonates/ 19. risedronate.tw. 20. pamidronate.tw. 21. zolendronate$.tw. 22. (bisphosphonate$ or biphosphonate$ or diphosphonate).tw. 23. tiludronate.tw. 24. etidronate.tw. 25. alendronate.tw. 26. olpadronate.tw. 27. incadronate.tw. 28. exp Clodronic Acid/ 29. clodronate.tw. |
| 30. or/18‐29 |
| 31. and/10,17,30 |
| 32. randomized controlled trial.pt. 33. controlled trial.pt. 34. randomized.ab. 35. placebo.ab. 36. drug therapy.fs. 37. randomly.ab. 38. trial.ab. 39. groups.ab. |
| 40. or/32‐39 |
| 41. (animals not (humans and animals)).sh. 42. 40 not 41 |
| 43. 31 and 42 44. limit 43 to yr="1997 ‐ current" |
Appendix 4. MEDLINE search strategy 2010‐2015
Database: Medline via Ovid <1946 to Present>
Date Searched: 03 April 2013; 07 May 2015
1. exp Osteoporosis/
2. (osteoporo* or osteopeni*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
3. Bone Density/
4. exp Fractures, Bone/
5. (bone* adj fragil*).mp.
6. exp Bone Resorption/
7. bone loss.mp.
8. (bmd or bone mineral densit* bone deminerali*).mp.
9. or/1‐8
10. exp Steroids/
11. exp Adrenal Cortex Hormones/
12. (steroid* or corticosteroid* or glucocorticoid*).mp.
13. or/10‐12
14. 9 and 13
15. Bone Diseases, Metabolic/ci [Chemically Induced]
16. 14 or 15
17. exp Diphosphonates/
18. (diphosphonate* or bisphosphonate*).mp.
19. etidronate.mp.
20. alendronate.mp.
21. pamidronate.mp.
22. clodronate.mp.
23. tiludronate.mp.
24. olpadronate.mp.
25. incadronate.mp.
26. zolendronate.mp.
27. risedronate.mp.
28. zolendronic acid.mp.
29. ibandronate.mp.
30. medronate.mp.
31. minodronate.mp.
32. neridronate.mp.
33. oxidronate.mp.
34. or/17‐33
35. 16 and 34
36. randomized controlled trial.pt.
37. controlled clinical trial.pt.
38. clinical trial.pt.
39. randomi?ed.ti,ab.
40. placebo.ti,ab.
41. dt.fs.
42. randomly.ti,ab.
43. trial.ti,ab.
44. groups.ti,ab.
45. or/36‐44
46. animals/
47. humans/
48. 46 not (46 and 47)
49. 45 not 48
50. 35 and 49
51. limit 50 to yr="2010 ‐Current"
Appendix 5. Embase search strategy 1997‐2010
Database: Embase via Ovid <1980 to present>
Date Searched: 25 January 2010
| 1. exp osteoporosis/ 2. osteoporo$.tw. 3. osteopeni$.tw. 4. exp bone density/ 5. exp fracture/ 6. (bone$ adj fragil$).tw. 7. (bone adj loss).tw. 8. bmd.tw. 9. (bone adj2 densit$).tw. |
| 10. or/1‐9 |
| 11. exp steroid/ 12. steroid$.tw. 13. exp corticosteroid/ 14. exp glucocorticoid/ 15. (corticosteroid$ or $corticoid$).tw. |
| 16. or/11‐15 |
| 17. 10 and 16 |
| 18. exp corticosteroid induced osteoporosis/ |
| 19. 17 or 18 |
| 20. exp bisphosphonic acid derivative/ 21. (diphosphonate$ or biphosphonate$ or bisphosphonate$).tw. 22. etidronate.tw. 23. alendronate.tw. 24. tiludronate.tw. 25. olpadronate.tw. 26. incadronate.tw. 27. risendronate$.tw. 28. pamidronate$.tw. 29. clodronate$.tw. 30. zolendronate$.tw. 31. exp clodronic acid/ |
| 32. or/20‐31 |
| 33. 19 and 32 |
| 34. (random$ or placebo$).ti,ab. 35. ((single$ or double$ or triple$ or treble$) and (blind$ or mask$)).ti,ab. 36. (controlled adj clinical adj trial$).ti,ab. 37. RETRACTED ARTICLE/ |
| 38. or/34‐37 |
| 39. (animal$ not human$).sh,hw. 40. 38 not 39 |
| 41. 33 and 40 42. limit 41 to yr="1997 ‐ current" |
Appendix 6. Embase search strategy 2010‐2015
Database: Embase via Ovid <1974 to present>
Date Searched: 03 April 2013; 07 May 2015
1. exp osteoporosis/
2. (osteoporo* or osteopeni*).mp.
3. bone demineralization/
4. bone density/
5. exp fracture/
6. (bone* adj fragil*).mp.
7. osteolysis/
8. bone loss.mp.
9. (bmd or bone mineral densit* bone deminerali*).mp.
10. or/1‐9
11. exp steroid/
12. (steroid* or corticosteroid* or glucocorticoid*).mp.
13. 11 or 12
14. 10 and 13
15. corticosteroid induced osteoporosis/
16. 14 or 15
17. exp bisphosphonic acid derivative/
18. (diphosphonate* or bisphosphonate*).mp.
19. etidronate.mp.
20. alendronate.mp.
21. pamidronate.mp.
22. clodronate.mp.
23. tiludronate.mp.
24. olpadronate.mp.
25. incadronate.mp.
26. zolendronate.mp.
27. risedronate.mp.
28. zolendronic acid.mp.
29. ibandronate.mp.
30. medronate.mp.
31. minodronate.mp.
32. neridronate.mp.
33. oxidronate.mp.
34. or/17‐33
35. 16 and 34
36. controlled clinical trial/ or clinical trial/ or controlled study/ or randomized controlled trial/ or major clinical study/
37. randomi?ed.ti,ab.
38. placebo.ti,ab.
39. randomly.ti,ab.
40. trial.ti.
41. or/36‐40
42. (exp vertebrate/ or animal/ or exp experimental animal/ or nonhuman/ or animal.hw.) not (exp human/ or human experiment/)
43. (rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. not (exp human/ or human experiment/)
44. 42 or 43
45. 41 not 44
46. 35 and 45
47. limit 46 to yr="2010 ‐ current"
Appendix 7. IPA search strategy 1970‐2012
Database: International Pharmaceutical Abstracts via Ovid <1970 to present>
Date Searched: 27 January 2012
| 1. osteoporosis.hw,sh. 2. bone density.hw,sh. 3. fracture$.hw,sh. 4. osteoporo$.tw. 5. osteopeni$.tw. 6. (bone adj2 density).tw. 7. bmd.tw. 8. (bone$ adj fragil$).tw. |
| 9. or/1‐8 |
| 10. Adrenal cortex hormones.hw,sh. 11. steroid$.hw,sh,tw. 12. (adrenal adj cortex adj hormone$).tw. 13. anabolic agents.hw,sh. 14. (anabolic adj agent$).tw. 15. glucocorticoids.hw,sh. 16. corticosteroid.hw,sh. 17. (corticosteroid$ or $corticoid$).tw. |
| 18. or/10‐17 |
| 19. bisphosphonates.hw,sh. 20. (diphosphonate$ or bisphosphonate$ or biphosphonate$).tw. 21. risedronate.hw,sh,tw. 22. pamidronate.hw,sh,tw. 23. clodronic acid.hw,sh,tw. 24. clodronate.hw,sh,tw. 25. etidronate.hw,sh,tw. 26. alendronate.hw,sh,tw. 27. tiludronate.hw,sh,tw. 28. olpadronate.hw,sh,tw. 29. incadronate.hw,sh,tw. |
| 30. or/19‐29 |
| 31. random$.hw,sh,tw. 32. ((double or single) and procedure$).mp. 33. (clin$ adj25 trial$).mp. 34. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp. 35. placebo$.mp. 36. (comparative adj25 stud$).mp. 37. evaluation$.mp. 38. (control$ or prospectiv$ or volunteer$).mp. |
| 39. or/31‐38 |
| 40. (animals not (humans and animals)).sh,hw. 41. 39 not 40 |
| 42. and/9,18,30 43. 41 and 42 |
Data and analyses
Comparison 1. Bisphosphonates vs control: benefits ‐ fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident radiographic vertebral fractures 12‐24 months | 12 | 1343 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.35, 0.91] |
| 2 Incident radiographic nonvertebral fractures 12‐24 months | 9 | 1245 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.47, 1.33] |
Comparison 2. Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at lumbar spine (LS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LS BMD change 12 months: all trials | 23 | 2042 | Mean Difference (IV, Random, 95% CI) | 3.50 [2.90, 4.10] |
| 1.1 Prevention trials | 12 | 930 | Mean Difference (IV, Random, 95% CI) | 3.92 [2.90, 4.94] |
| 1.2 Treatment trials | 11 | 1112 | Mean Difference (IV, Random, 95% CI) | 3.19 [2.64, 3.73] |
| 2 LS BMD change 12 months: oral treatment | 18 | 1767 | Mean Difference (IV, Random, 95% CI) | 3.25 [2.88, 3.63] |
| 3 LS BMD change 12 months: parenteral treatment | 5 | 275 | Mean Difference (IV, Random, 95% CI) | 5.12 [2.35, 7.89] |
| 4 LS BMD change 12 months: low‐ vs standard‐dose | 5 | 642 | Mean Difference (IV, Random, 95% CI) | 0.95 [0.37, 1.53] |
| 5 LS BMD change 18‐24 months | 9 | 802 | Mean Difference (IV, Random, 95% CI) | 5.49 [3.47, 7.51] |
| 6 LS BMD change 12 months prevention trials: oral and parenteral subgroups | 12 | 930 | Mean Difference (IV, Random, 95% CI) | 3.92 [2.90, 4.94] |
| 6.1 Oral bisphosphonates | 7 | 655 | Mean Difference (IV, Random, 95% CI) | 3.38 [2.75, 4.02] |
| 6.2 Parenteral bisphosphonates | 5 | 275 | Mean Difference (IV, Random, 95% CI) | 5.12 [2.35, 7.89] |
| 7 LS BMD change 12 months: gender/menopausal status subgroups | 5 | 840 | Mean Difference (IV, Random, 95% CI) | 3.86 [2.03, 5.68] |
| 7.1 Men | 4 | 221 | Mean Difference (IV, Random, 95% CI) | 3.58 [2.68, 4.48] |
| 7.2 Premenopausal women | 5 | 154 | Mean Difference (IV, Random, 95% CI) | 3.51 [1.50, 5.53] |
| 7.3 Postmenopausal women | 5 | 465 | Mean Difference (IV, Random, 95% CI) | 4.41 [0.65, 8.18] |
Comparison 3. Bisphosphonates vs control: benefits ‐ bone mineral density (BMD) at femoral neck (FN).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FN BMD change 12 months: all trials | 18 | 1665 | Mean Difference (IV, Random, 95% CI) | 2.06 [1.45, 2.68] |
| 1.1 Prevention trials | 10 | 751 | Mean Difference (IV, Random, 95% CI) | 2.79 [1.99, 3.59] |
| 1.2 Treatment trials | 8 | 914 | Mean Difference (IV, Random, 95% CI) | 1.53 [0.73, 2.33] |
| 2 FN BMD change 12 months: oral treatment | 15 | 1574 | Mean Difference (IV, Random, 95% CI) | 1.92 [1.31, 2.53] |
| 3 FN BMD change 12 months: parenteral treatment | 3 | 91 | Mean Difference (IV, Random, 95% CI) | 4.56 [2.07, 7.05] |
| 4 FN BMD change 12 months: low‐ vs standard‐dose | 4 | 542 | Mean Difference (IV, Random, 95% CI) | 0.74 [‐0.42, 1.90] |
| 5 FN BMD change 18‐24 months | 9 | 802 | Mean Difference (IV, Random, 95% CI) | 3.28 [1.70, 4.87] |
| 6 FN BMD change 12 months: gender/menopausal status subgroups | 4 | 537 | Mean Difference (IV, Random, 95% CI) | 3.29 [1.65, 4.94] |
| 6.1 Men | 3 | 134 | Mean Difference (IV, Random, 95% CI) | 2.91 [1.15, 4.68] |
| 6.2 Premenopausal women | 4 | 88 | Mean Difference (IV, Random, 95% CI) | 2.70 [‐0.96, 6.35] |
| 6.3 Postmenopausal women | 4 | 315 | Mean Difference (IV, Random, 95% CI) | 3.62 [‐0.37, 7.61] |
Comparison 4. Bisphosphonates vs control: harms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events 12‐24 months | 15 | 1703 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.74, 1.12] |
| 2 Withdrawals due to adverse events 12‐24 months | 15 | 1790 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.77, 1.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abitbol 2007.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 67 participants; men (45%) and premenopausal women (55%) Conditions: inflammatory bowel disease Mean age (range) Intervention: 30 (19‐50) Comparator: 30 (21‐51) Baseline vertebral fractures: yes Serious adverse events: not reported Withdrawals due to adverse events: not reported |
|
| Interventions |
Active group: clodronate 900 mg IV every 3 months, daily elemental calcium/vitamin D Comparator: placebo IV every 3 months, daily elemental calcium/vitamin D |
|
| Outcomes |
|
|
| Types of studies | Prevention study | |
| Incident vertebral fractures | Assessment criteria: quantitative morphometry1 | |
| Mean steroid dose | 5‐7.5 mg/day | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States “randomised in blocks of four” |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double blinded, intravenous placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | DEXA results interpreted by central blinded outcome assessor. No mention of how radiographs were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/67 dropouts all accounted for, none due to adverse events. No outcome data to carry forward so not an intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
Adachi 1997.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 141 participants; men (38%), premenopausal women (12%) and postmenopausal women (50%) Comparison: rheumatoid arthritis and polymyalgia rheumatica Mean age (range) Intervention: 62 (31‐83) Comparator: 60 (19‐87) Baseline vertebral fractures: yes Serious adverse events: one death in bisphosphonate group (pneumonia) Withdrawals due to adverse events: details incomplete, one withdrawal from intervention group due to increased serum creatinine |
|
| Interventions |
Active group: cyclic etidronate 400 mg orally and elemental calcium Comparator: cyclic placebo and elemental calcium |
|
| Outcomes |
|
|
| Types of studies | Prevention study | |
| Incident vertebral fractures | Assessment Criteria: semiquantitative2 | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified then randomised, no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No mention of blinding investigators, used placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All radiographs interpreted by central blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, 24/141 participants did not complete the study, reasons given, numbers given for those who withdrew for adverse events |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | Supported by grant from drug manufacturer, no industry authorship |
Adachi 2001.
| Methods | RCT; study duration 12 months (extension trial from 12‐24 months) | |
| Participants |
N: 116 participants; men (29%), premenopausal women (27%) and postmenopausal women (44%) Conditions: rheumatoid arthritis, polymyalgia rheumatica, systemic lupus erythematosus, pemphigus, asthma, inflammatory myopathy, inflammatory bowel disease, giant cell arteritis, sarcoidosis, myasthenia gravis, COPD, and nephrotic syndrome Mean age (range) Intervention: 53 (21‐78) Comparator: 54 (23‐76) Baseline vertebral fractures: yes Serious adverse events: see Saag 1998 Withdrawals due to adverse events: see Saag 1998 |
|
| Interventions |
Active group: alendronate 10 mg/day orally, daily elemental calcium/vitamin D Comparator: daily elemental calcium/vitamin D |
|
| Outcomes |
|
|
| Types of studies | *Treatment study | |
| Incident vertebral fractures | Assessment Criteria: semiquantitative2 | |
| Mean steroid dose | 10‐5 mg/day | |
| Notes | Extension trial of Saag 1998 Fracture/harm data not included ‐ partial cohort of Saag 1998 *Majority of participants had previous steroid use > 3 months Other treatment groups of 5 mg and 2.5/10 mg not included |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | as per Saag 1998 |
| Allocation concealment (selection bias) | Unclear risk | as per Saag 1998 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | as per Saag 1998 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | as per Saag 1998 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | as per Saag 1998 |
| Selective reporting (reporting bias) | Low risk | as per Saag 1998 |
| Other bias | Unclear risk | extension trial ‐ risk of unblinding |
Boutsen 1997.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 27 participants; men (19%), premenopausal women (11%) and postmenopausal women (70%) Conditions: polymyalgia rheumatica, temporal arteritis, rheumatoid arthritis, haemolytic anaemia, inflammatory bowel disease, asthma, uveitis, sarcoidosis, reactive arthritis Mean age (SD) Intervention: 60 (16) Comparator: 61 (12) Baseline vertebral fractures: not explicitly stated Serious adverse events: one death due to severe pulmonary infection in control group Withdrawals due to adverse events: not reported |
|
| Interventions |
Active group: pamidronate 90 mg loading dose IV then 30 mg every 3 months IV, daily elemental calcium Comparator: daily elemental calcium |
|
| Outcomes |
|
|
| Types of studies | Prevention study | |
| Incident vertebral fractures | Assessment Criteria: spinal deformity index3 | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer software, including minimisation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 death, 4 dropouts all in control group, 1 protocol violation |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
Boutsen 2001.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 27 participants; men (44%), premenopausal women (12%) and postmenopausal women (44%) Conditions: polymyalgia rheumatica, temporal arteritis, rheumatoid arthritis, inflammatory bowel disease, reactive arthritis, asthma Mean age (SD) Intervention: 55 (17) Comparator: 57 (18) Low dose: 59 (21) Baseline vertebral fractures: none, exclusion criteria Serious adverse events: none occurred Withdrawals due to adverse events: not reported |
|
| Interventions |
Active group: pamidronate 90 mg IV loading dose then 30 mg IV every 3 months, daily elemental calcium Comparator: daily elemental calcium Low dose: pamidronate IV 90 mg single infusion, daily elemental calcium |
|
| Outcomes |
|
|
| Types of studies | Prevention study | |
| Incident vertebral fractures | Assessment criteria: spinal deformity index3 | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly matched, 3 x 3, taking into account starting dose of steroid, sex and menopausal status |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only participants who were matched in the other 2 groups were analysed. 30 matched ‐ 1 dropped out, only 27 analysed |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
Cohen 1999.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 228 participants; men (34%), premenopausal women (20%) and postmenopausal women (46%) Conditions: rheumatoid arthritis, polymyalgia rheumatica, systemic lupus erythematosus, giant cell arteritis, vasculitis, asthma, chronic interstitial lung disease, polymyositis, dermatomyositis Mean age (SD) Intervention: 61.9 (14.3) Comparator: 57.2 (14.7) Low dose: 59.5 (14.0) Baseline vertebral fractures: yes Serious adverse events: details on type of serious adverse events not provided Withdrawals due to adverse events: details on adverse events leading to withdrawal not provided |
|
| Interventions |
Active group: risedronate 5 mg/day orally, daily elemental calcium Comparator: placebo, daily elemental calcium Low dose: risedronate 2.5 mg/day orally, daily elemental calcium |
|
| Outcomes |
|
|
| Types of studies | Prevention study | |
| Incident vertebral fractures | Assessment criteria: quantitative morphometry4 | |
| Mean steroid dose | > 20 mg/day | |
| Notes | Missing data: SD calculated from SE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were stratified then randomised, no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated double‐blinded, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | X‐ray data reviewed by single observer blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 77/224 participants dropped out. Note 2.5 mg risedronate group stopped halfway through study |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Unclear risk | One author and study sponsorship from Proctor & Gamble |
Cortet 1999.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 83 participants; men (34%), premenopausal women (11%) and postmenopausal women (55%) Conditions: rheumatoid arthritis, polymyalgia rheumatica, giant cell arteritis Mean age (SD) Intervention: 61.4 (12.5) Comparator: 63.3 (11.5) Baseline vertebral fractures: not explicitly stated Serious adverse events: reported 2 deaths but did not specify in which treatment group they occurred Withdrawals due to adverse events: 1 withdrawal due to myocardial infarction, 1 due to heart failure, 1 due to lung cancer. Did not specify in which treatment group they occurred |
|
| Interventions |
Active group: cyclic etidronate 400 mg orally and elemental calcium Comparator: cyclic placebo and elemental calcium Daily vitamin D permitted in all participants (set maximum dose) |
|
| Outcomes |
|
|
| Types of studies | Prevention study | |
| Incident vertebral fractures | Incomplete data: only screened symptomatic, not included in analysis | |
| Mean steroid dose | ˜ 7.5 mg/day | |
| Notes | Missing data: we assumed authors incorrectly reported SE as SD. This is justified by the P value as per our biostatistician. We have corrected for this error in our data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double blind, placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | DEXA and biochemical results interpreted by central blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/87 participants did not complete the study, reasons given. No adverse events |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
De Nijs 2006.
| Methods | RCT; study duration 18 months | |
| Participants |
N: 200 participants; men (38%), premenopausal women (9%) and postmenopausal women (53%) Conditions: polymyalgia rheumatica, rheumatoid arthritis or other rheumatic disease Mean age (SD) Intervention: 60 (14) Comparator: 62 (15) Baseline vertebral fractures: yes Serious adverse events: only deaths reported Intervention: 1 death due to diverticulitis with perforation, 1 death due to non‐Hodgkin's lymphoma Comparator: 1 death due to cerebrovascular accident Withdrawals due to adverse events: Intervention: 5 due to gastrointestinal side effects, 2 due to cancer, 3 due to "other conditions" Comparator: 5 due to gastrointestinal side effects, 1 due to cancer, 6 due to "other conditions" |
|
| Interventions |
Active group: alendronate 10 mg/day orally, daily placebo (vitamin D look‐alike) Comparator: placebo, daily vitamin D Any participant with dietary intake below set threshold received daily calcium Any participant with serum levels below set threshold received daily vitamin D |
|
| Outcomes |
|
|
| Types of studies | Prevention study | |
| Incident vertebral fractures | Assessment criteria: semiquantitative5 | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | Missing data: SD measured from graph | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed computer‐generated randomisations |
| Allocation concealment (selection bias) | Low risk | Pharmacist did allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated double blinded, placebo tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Radiographs assessed by blinded individuals |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 163/201 completed study. See Figure 1 and text page 678, right column 3rd paragraph for details |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | No industry sponsorship or authorship although industry supplied drug |
Frediani 2003.
| Methods | RCT; study duration 48 months | |
| Participants |
N: 163 participants; premenopausal (24%) and postmenopausal women (68%) Conditions: rheumatoid arthritis and psoriatic arthritis Mean age (SD) Intervention: 61.1 (12.2) Comparator: 62.4 (13.4) Baseline vertebral fractures: yes Serious adverse events: not reported Withdrawals due to adverse events: Intervention: due to gastralgia and/or local pain at injection site Comparator: due to gastralgia |
|
| Interventions |
Active group: clodronate 100 mg/week IM, daily elemental calcium/vitamin D Comparator: placebo, daily elemental calcium/vitamin D |
|
| Outcomes |
|
|
| Types of studies | Prevention study | |
| Incident vertebral fractures | Incomplete data: measured at 4 years, not included in analysis | |
| Mean steroid dose | ˜7.5 mg/day | |
| Notes | Missing data: SD measured from graph | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" but no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No mention of double blind or blinding of personnel, placebo given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 31/163 participants dropped out for "gastralgia" or "GI intolerance". Twice as many in the clodronate group |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
Geusens 1998.
| Methods | RCT; study duration 24 months | |
| Participants |
N: 37 participants; all postmenopausal women Conditions: rheumatoid arthritis, polymyalgia rheumatica, osteoarthritis, chronic bronchitis, inflammatory bowel disease, idiopathic eosinophilia, sarcoidosis Mean age (SD) Intervention: 61.1 (12.2) Comparator: 62.4 (13.4) Baseline vertebral fractures: yes Serious adverse events: 1 death due to ruptured aortic aneurysm in bisphosphonate group Withdrawals due to adverse events: Intervention: none Comparator: 1 due to anaphylaxis, 1 due to shoulder fracture |
|
| Interventions |
Active group: cyclic etidronate 400 mg orally and elemental calcium Comparator: cyclic placebo and elemental calcium |
|
| Outcomes |
|
|
| Types of studies | Treatment study | |
| Incident vertebral fractures | Incomplete data: only screened symptomatic, not included in analysis | |
| Mean steroid dose | 5‐7.5mg/day | |
| Notes | Missing data: 12 months BMD mean and SD measured from graph | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned in blocks of two |
| Allocation concealment (selection bias) | Low risk | Code located in sponsor's office and only broken after full statistical analysis |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Explicit "blinding was successful among participants, doctors, data managers," identical placebo given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Explicitly stated as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11/37 dropouts. Stated they used an intention‐to‐treat population for their ANCOVA analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
Hakala 2012.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 140 participants; all postmenopausal women Conditions: rheumatoid arthritis, polymyalgia rheumatica and other rheumatic diseases Mean age (SD) Intervention: 64 (8) Comparator: 63 (7) Baseline vertebral fractures: yes, but those with symptomatic or 2 or more radiographic vertebral fractures were excluded from the study Serious adverse events: Intervention: gastrointestinal bleeding, transient ischaemic attack, acute pancreatitis, death (agranulocytosis and sepsis), acute pyelonephritis, concussion, poisoning, follicle center lymphoma, malignant tongue neoplasm, deep vein thrombosis, pulmonary embolism Comparator: erysipelas, pneumonia, radius fracture, hip fracture, headache Withdrawals due to adverse events: reasons for withdrawal included anaemia, palpitations, reflux oesophagitis, stomach discomfort, pyrexia, arthralgia, back pain associated with a prestudy operation, myalgia, dizziness, headache, tremor, and cough. Did not specify in which treatment groups these occurred |
|
| Interventions |
Active group: ibandronate 150 mg/month orally, daily elemental calcium/vitamin D Comparator: placebo, daily elemental calcium/vitamin D |
|
| Outcomes |
|
|
| Types of studies | Treatment study* | |
| Incident vertebral fractures | Not reported as outcome | |
| Mean steroid dose | 5‐7.5 mg/day | |
| Notes | *Majority of participants had previous steroid use > 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States investigators and co‐ordinators were blinded to BMD results, placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data processing of BMD was done centrally |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 124/140 completed, intention‐to‐treat analysis included any participant with one follow‐up data point |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Unclear risk | One author worked for Roche |
Herrala 1998.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 74 participants; men (45%) and postmenopausal women (55%) Conditions: COPD and asthma Mean age (range) Intervention: 56.1 (43‐71) Comparator: 57.3 (39‐73) Baseline vertebral fractures: not explicitly stated Serious adverse events: Intervention: 1 death due to end‐stage COPD (clodronate 2400 mg/day group) Comparator: 1 death due to asthma attack Withdrawals due to adverse events: 7 withdrawals due to gastrointestinal reasons but did not specify in which treatment groups these occurred |
|
| Interventions |
Active group: clodronate 800 mg/day orally Comparator: placebo |
|
| Outcomes |
|
|
| Types of studies | Treatment study | |
| Incident vertebral fractures | Not reported as outcome | |
| Mean steroid dose | ˜7.5 mg/day | |
| Notes | Other treatment arms (1600 mg/day and 2400 mg/day) not included Missing data: SD calculated from 95% CI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by using table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Refer to study as double blinded, identical number of tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | BMD data analysed at end of study by one technician blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 61/74 completed study. 68 participants had BMD data from 2 visits and were analysed, 7 dropouts due to GI adverse events |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
Jenkins 1999.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 28 participants; men (39%) and women (61%) Conditions: rheumatoid arthritis and polymyalgia rheumatica Mean age (SD) Intervention: 68.7 (10.9) Comparator: 65.9 (9.7) Baseline vertebral fractures: not explicitly stated Serious adverse events: 1 death in control group, other types of serious events not reported Withdrawals due to adverse events: none occurred |
|
| Interventions |
Active group: cyclic etidronate 400 mg orally and elemental calcium Comparator: cyclic placebo and elemental calcium |
|
| Outcomes |
|
|
| Types of studies | Prevention study | |
| Incident vertebral fractures | Incomplete data: screened < 50% participants, not included in analysis | |
| Mean steroid dose | ˜7.5 mg/day | |
| Notes | Update of Jenkins 1997 from original review Missing data: SD calculated from SE |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of allocation method |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Paper states that study is a double blind placebo controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 25/28 completed study and dropouts accounted for. Vertebral radiographs available on only 13/28 participants despite protocol stating screening of all participants |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
Lems 2006.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 163 participants*; men (44%), premenopausal women (9%) and postmenopausal women (47%) Conditions: rheumatoid arthritis Mean age (SD) Intervention + low dose: 61.7 (11.0) Comparator: 61.6 (11.3) Baseline vertebral fractures: yes Serious adverse events: details on type of serious events not provided Withdrawals due to adverse events: did not specify in which treatment groups these occurred nor provide details on adverse events leading to withdrawal |
|
| Interventions |
Active group: alendronate 10 mg/day orally Comparator: placebo Low dose: alendronate 5 mg/day orally Any participant with self‐reported low dietary intake received daily calcium/vitamin D |
|
| Outcomes |
|
|
| Types of studies | Treatment study | |
| Incident vertebral fractures | Assessment criteria: quantitative morphometry1 | |
| Mean steroid dose | ˜7.5mg/day | |
| Notes | *Active group = postmenopausal women; comparator group = pre/postmenopausal women and men; low‐dose group = premenopausal women and men Did not provide data on withdrawals from each group: not included in meta‐analysis Missing data: SD calculated from P value |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised," no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blind" but not sure if this refers to all personnel or just outcome assessors, identical placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Radiographs assessed by blinded individuals |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Accounted for dropouts. Intention‐to‐treat analysis on any participant with a follow‐up BMD, 144/163 |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
Li 2010.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 40 participants; all Chinese premenopausal (40%) and postmenopausal women (60%) Conditions: systemic lupus erythematosus Median age (IQR) Intervention: 47 (33.5, 50) Comparator: 45.5 (0.5, 49) Baseline vertebral fractures: no Serious adverse events: not reported Withdrawals due to adverse events: none occurred |
|
| Interventions |
Active group: ibandronate 150 mg/month orally, daily elemental calcium/vitamin D Comparator: placebo, daily elemental calcium/vitamin D |
|
| Outcomes |
|
|
| Types of studies | Treatment study | |
| Incident vertebral fractures | Not reported as outcome | |
| Mean steroid dose | Unclear: began with ˜ 25‐30 mg/day | |
| Notes | Missing data: median used, SD calculated from IQR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomizations conducted with a computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Project co‐ordinator and investigators blinded to group assignment, the method of concealed random allocation was used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Project co‐ordinators and study investigators blinded, placebo tablets used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study |
| Selective reporting (reporting bias) | Low risk | The authors published the planned outcomes in a trial protocol and provided results for each planned outcome |
| Other bias | Low risk | None apparent |
Pitt 1998.
| Methods | RCT; study duration 104 weeks | |
| Participants |
N: 49 participants; men (39%), premenopausal and postmenopausal women (61%) Conditions: asthma, polymyalgia rheumatica, systemic lupus erythematosus, emphysema, fasciitis, giant cell arteritis, polyarteritis nodosa, bronchiectasis, fibrosing alveolitis, and scleroderma Mean age (SD) Intervention: 58.9 (13.7) Comparator: 59.2 (10.8) Baseline vertebral fractures: not explicitly stated Serious adverse events: details on types of serious events other than death not provided Intervention: 1 death due to respiratory failure, 1 death due to adenocarcinoma of lung Comparator: 1 death due to perforated bowel Withdrawals due to adverse events: Intervention: 1 withdrawal due to myocardial infarction Comparator: none occurred |
|
| Interventions |
Active group: cyclic etidronate 400 mg orally and elemental calcium/vitamin D Comparator: cyclic placebo and elemental calcium/vitamin D |
|
| Outcomes |
|
|
| Types of studies | Treatment study | |
| Incident vertebral fractures | Assessment criteria: semiquantitative3 | |
| Mean steroid dose | ˜ 7.5 mg/day | |
| Notes | Update of Pitt 1997 from original review Missing data: SD calculated from SE |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double‐blind, placebo given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | X‐rays were assessed centrally by a blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 41/49 participants completed, dropouts were explained, intention‐to‐treat analysis included any participant who took 1 dose of drug |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
Reid 2000.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 290 participants; men (37%), premenopausal women (9%) and postmenopausal women (54%) Conditions: rheumatoid arthritis, asthma, systemic lupus erythematosus, temporal arteritis, vasculitis, COPD, polymyositis, chronic interstitial lung disease and other Mean age (SD) Intervention: 58 (12) Comparator: 59 (12) Low dose: 59 (14) Baseline vertebral fractures: yes Serious adverse events: details on type of serious events not provided Withdrawals due to adverse events: details on adverse events leading to withdrawal not provided |
|
| Interventions |
Active group: risedronate 5 mg/day orally, daily elemental calcium/vitamin D Comparator: placebo, daily elemental calcium/vitamin D Low dose: risedronate 2.5 mg/day orally, daily elemental calcium/vitamin D |
|
| Outcomes |
|
|
| Types of studies | Treatment study | |
| Incident vertebral fractures | Assessment criteria: quantitative morphometry1 | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | Missing data: SD calculated from SE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomised," no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blind", placebo given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22% dropouts ‐ adverse events only in text ‐ "no difference between groups" |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Unclear risk | Clear articulation regarding the role of the funding pharmaceutical company in design, implementation and analysis of study. It indicates a potential for bias |
Roux 1998.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 117 participants; men (36%), premenopausal women (15%) and postmenopausal women (49%) Conditions: vasculitis, rheumatoid arthritis, polymyalgia rheumatica, temporal arteritis, systemic lupus erythematosus, asthma, chronic interstitial lung disease, polymyositis, dermatomyositis, and skin disease Mean age (SD) Intervention: 58.5 (13.9) Comparator: 59.0 (13.6) Baseline vertebral fractures: not explicitly stated Serious adverse events: not reported Withdrawals due to adverse events: details on adverse events leading to withdrawal not provided |
|
| Interventions |
Active group: cyclic etidronate 400 mg orally and daily elemental calcium Comparator: cyclic placebo and daily elemental calcium Daily vitamin D permitted in all participants (set maximum dose) |
|
| Outcomes |
|
|
| Types of studies | Prevention study | |
| Incident vertebral fractures | Incomplete data: only screened symptomatic, not included in analysis | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | Update of Roux 1997 from original review Missing data: total number of participants provided with BMD results so we estimated as equal per group; SD calculated from SE |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double blinded, placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | X‐rays were assessed using qualitative scale, no mention of blinding. BMD interpreted centrally, no mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 107/117 participants completed, dropouts were explained, intention‐to‐treat analysis included any participant who was randomised |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
Saadati 2008.
| Methods | RCT; study duration 18 months | |
| Participants |
N: 72 participants; men (10%) and premenopausal women (90%) Conditions: systemic lupus erythematosus, polymyositis, dermatomyositis etc. Mean age: 36.6 Baseline vertebral fractures: not explicitly stated Serious adverse events: not reported Withdrawals due to adverse events: not reported |
|
| Interventions |
Active group: alendronate 10 mg/day orally, daily elemental calcium, twice weekly vitamin D Comparator: daily elemental calcium, twice weekly vitamin D |
|
| Outcomes |
|
|
| Types of studies | Treatment study* | |
| Incident vertebral fractures | Assessment criteria: not specified, not included in analysis | |
| Mean steroid dose | > 20 mg/day | |
| Notes | * Type of study unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised into two groups," no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding, no placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 100 participants consented: 28 did not follow treatment and were excluded, 72 did follow treatment (calcium + vitamin D or calcium + vitamin D + alendronate) |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
Saag 1998.
| Methods | RCT; study duration 48 weeks | |
| Participants |
N: 477 participants; men (29%), premenopausal women (22%) and postmenopausal women (49%) Conditions: rheumatoid arthritis, polymyalgia rheumatica, systemic lupus erythematosus, pemphigus, asthma, inflammatory myopathy, inflammatory bowel disease, giant cell arteritis, sarcoidosis, myasthenia gravis, COPD, and nephrotic syndrome Mean age (SD) Intervention: 55 (15) Comparator: 54 (15) Low dose: 56 (15) Baseline vertebral fractures: yes Serious adverse events: details on type of serious adverse events incomplete Intervention: serious gastro‐intestinal events in 2 participants (alendronate 10 mg/day group) Comparator: serious gastro‐intestinal events in 2 participants Withdrawals due to adverse events: details on adverse events leading to withdrawal not provided |
|
| Interventions |
Active group: alendronate 10 mg/day orally, daily elemental calcium/vitamin D Comparator: placebo, daily elemental calcium/vitamin D Low dose: alendronate 5 mg/day orally, daily elemental calcium/vitamin D |
|
| Outcomes |
|
|
| Types of studies | Treatment study* | |
| Incident vertebral fractures | Assessment criteria: semiquantitative2 | |
| Mean steroid dose | ˜7.5 mg/day | |
| Notes | Update of Saag 1997 from original review Aledronate 5 mg and 10 mg are combined in analyses of fractures *Majority of participants with > 3 months steroid use Missing data: SD calculated from SE |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding, USA arm had matching placebo. No mention of placebo in Europe arm |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | X‐ray analysis read centrally but not mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used an intention‐to‐treat analysis with last observation carried forward (12 week result). All dropouts accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | Supported by a grant from Merck |
Sambrook 2003.
| Methods | RCT; study duration 24 months | |
| Participants |
N: 195 participants; men (31%), premenopausal women (14%) and postmenopausal women (55%) Conditions: polymyalgia rheumatica/giant cell arteritis, rheumatoid arthritis, systemic lupus erythematosus, polymyositis, inflammatory bowel disease, respiratory disease, neurologic disease, and other Mean age (SD) Intervention: 62.4 (13.5) Comparator: 57.9 (13.0) Baseline vertebral fractures: yes Serious adverse events: reported 2 deaths but did not specify in which treatment group they occurred Withdrawals due to adverse events: details on adverse events leading to withdrawal not provided and did not specify in which treatment groups these occurred |
|
| Interventions |
Active group: alendronate 10 mg/day orally, daily elemental calcium Comparator: ergocalciferol 0.25 mg orally 3 times weekly, daily elemental calcium |
|
| Outcomes |
|
|
| Types of studies | Treatment study* | |
| Incident vertebral fractures | Assessment criteria: semiquantitative2 | |
| Mean steroid dose | ˜7.5 mg/day | |
| Notes | 12 months BMD data insufficient for analysis: not included in meta‐analysis Calcitriol treatment arm not included Did not report which groups withdrawals came from: not included in meta‐analysis *Majority of participants had prior steroid therapy > 3 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisations by CRO using adaptive assignment |
| Allocation concealment (selection bias) | Low risk | Allocation performed centrally |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | X‐ray analysis read centrally by blinded individual and densitometry technician was blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 177/195 completed 1 year, authors stated no apparent differences between groups, dropouts fully accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | Supported by a grant from Merck |
Skingle 1997.
| Methods | RCT; study duration 24 months | |
| Participants |
N: 38 participants; men (24%), premenopausal women (8%) and postmenopausal women (68%) Conditions: polymyalgia rheumatica, temporal arteritis and COPD Mean age Intervention: 65 Comparator: 64 Baseline vertebral fractures: yes Serious adverse events: not reported Withdrawals due to adverse events: not reported |
|
| Interventions |
Active group: cyclic etidronate 400 mg orally, daily elemental calcium Comparator: daily elemental calcium |
|
| Outcomes |
|
|
| Types of studies | Treatment study | |
| Incident vertebral fractures | Incomplete data: reported total number of fractures and not participants with fractures, not included in analysis | |
| Mean steroid dose | ˜7.5 mg/day | |
| Notes | Update of Skingle 1994 from original review Missing data: median used; SD calculated from P value |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study, no placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Spinal X‐rays interpreted by single blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17/55 did not complete first year of study. 7 because prednisone dose too low, 10 for non compliance, lost to follow‐up. Completer analysis. Only 23 participants out of 38 completers had X‐rays |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
Stoch 2009.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 173 participants; men (42%), premenopausal women (31%) and postmenopausal women (27%) Conditions: rheumatoid arthritis, Still's disease, connective tissue disorder, arthritis, osteoarthritis, polymyalgia, polymyalgia rheumatica, polymyositis, psoriatic arthritis, scleroderma, and systemic lupus erythematosus Mean age (SD) Intervention: 51.9 (14.4) Comparator: 54.6 (14.8) Baseline vertebral fractures: not explicitly stated Serious adverse events: details on types of serious events other than death not provided Intervention: one death due to cardiac arrest Comparator: no deaths Withdrawals due to adverse events: details on adverse events leading to withdrawal not provided |
|
| Interventions |
Active group: alendronate 70 mg/week orally, daily elemental calcium/vitamin D Comparator: placebo, daily elemental calcium/vitamin D |
|
| Outcomes |
|
|
| Types of studies | Treatment study* | |
| Incident vertebral fractures | Incomplete data: only screened symptomatic, not included in analysis | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | *Majority of participants had prior steroid therapy > 3 months Missing data: SD measured from graph |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only mentioned “randomised in a 2:1 ratio," no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States all study personnel blinded, placebo used and same administration instructions given to both |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States central lab and DEXA personnel blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified intention‐to‐treat analysis (as long as 1 dose of medication and 1 follow‐up outcome measured), used last observation carried forward, provided detailed patient flow diagram |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
Tee 2012.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 44 participants; men (57%), premenopausal women (11%) and postmenopausal women (32%) Conditions: immunobullous skin diseases Mean age (SD) Intervention: 56.8 (16.2) Comparator: 61.5 (15.2) Baseline vertebral fractures: none, exclusion criteria Serious adverse events: Intervention: 1 death, cause not reported Comparator: 1 participant suffered myocardial infarction Withdrawals due to adverse events: Intervention: 1 due to nausea and vomiting, 1 due to drug‐related rash, 1 due to leukopenia Comparator: 1 due to abdominal pain, 1 due to leukopenia |
|
| Interventions |
Active group: alendronate 10 mg/day orally, daily elemental calcium/vitamin D Comparator: placebo, daily elemental calcium/vitamin D |
|
| Outcomes |
|
|
| Types of studies | Prevention study | |
| Incident vertebral fractures | Assessment criteria: semiquantitative2 | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | BMD data reported as change in T‐score, unable to include in meta‐analysis as per our biostatistician | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants stratified and randomly assigned in blocks of 6 |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double‐blind, placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States X‐ray assessment blinded and performed independently by two assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30% dropout rate, stated main reason due to unavailability for follow‐up, no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | None apparent |
Van Offel 2001.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 20 participants; men (30%) and premenopausal and postmenopausal women (70%) Conditions: rheumatoid arthritis Mean age (range) Intervention: 56 (35‐77) Comparator: 62 (41‐77) Baseline vertebral fractures: not explicitly stated Serious adverse events: not reported Withdrawals due to adverse events: not reported |
|
| Interventions |
Active group: pamidronate 60 mg IV every 3 months, daily elemental calcium Comparator: placebo IV every 3 months, daily elemental calcium Vitamin D provided at baseline to any participant with level below set minimum threshold |
|
| Outcomes |
|
|
| Types of studies | Prevention study | |
| Incident vertebral fractures | Not reported as outcome | |
| Mean steroid dose | ˜7.5 mg/day | |
| Notes | Did not provide numerical data for BMD at femoral neck Missing data: median used, SD calculated from range |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no details of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double blind, placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinded outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of dropouts or adverse events |
| Selective reporting (reporting bias) | Unclear risk | No mention of adverse events |
| Other bias | Low risk | None apparent |
Wolfhagen 1997.
| Methods | RCT; study duration 12 months | |
| Participants |
N: 12 participants; men (25%) and women (75%) Conditions: primary biliary cirrhosis Mean age (SD) Intervention: 57 (11) Comparator: 49 (6) Baseline vertebral fractures: no, exclusion criteria Serious adverse events: not reported Withdrawals due to adverse events: none occurred |
|
| Interventions |
Active group: cyclic etidronate 400 mg orally and elemental calcium Comparator: daily elemental calcium |
|
| Outcomes |
|
|
| Types of studies | Prevention study | |
| Incident vertebral fractures | Not reported as outcome | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | Missing data: SD calculated from SE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified then randomised, no mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding, no placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of a blinded outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed |
| Selective reporting (reporting bias) | Unclear risk | X‐rays of the spine were done to look for fractures, but only to validate DEXA measurement, not as an outcome. Not reported |
| Other bias | Low risk | None apparent |
Yeap 2008.
| Methods | RCT; study duration 24 months | |
| Participants |
N: 98 participants; all premenopausal women Conditions: systemic lupus erythematosus Mean age (SD) Intervention: 31.13 (8.44) Comparator: 28.09 (6.49) Baseline vertebral fractures: no Serious adverse events: 3 deaths due to infective complications of lupus but did not specify in which treatment group these occurred Withdrawals due to adverse events: 3 with renal impairment, 1 with fractured tibia and fibula, 1 avascular necrosis of hip (control group), and 1 severe thrombocytopenia. Did not specify in which treatment groups these occurred |
|
| Interventions |
Active group: alendronate 70 mg/week orally, daily elemental calcium Comparator group: daily elemental calcium |
|
| Outcomes |
|
|
| Types of studies | Treatment study | |
| Incident vertebral fractures | Not reported as outcome | |
| Mean steroid dose | 10‐15 mg/day | |
| Notes | Calcitriol treatment group not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisations used |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Specifically stated not blinded, no placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | BMD assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 77/98 participants completed the study, adverse events were detailed, no intention‐to‐treat analysis done |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results |
| Other bias | Low risk | Supported by grant from two pharmaceutical companies |
ANCOVA: analysis of covariance BMD: bone mineral density DEXA: dual energy X‐ray absorptiometry GIOP: glucocorticoid‐induced osteoporosis IM: intramuscular IV: intravenous RCT: randomised controlled trial
1Black 1996 and Genant 1996; 2Genant 1993 and Van Kujik 1995; 3Minne 1988; 4Kiel 1995 and Melton 1993; 5Kleerekoper 1984
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Benucci 2009 | Low‐dose bisphosphonates: neridronate 25 mg/day IM |
| Fujii 2006 | Low‐dose bisphosphonates: risedronate 2.5 mg/day orally |
| Jinnouchi 2000 | Low‐dose bisphosphonates: cyclic etidronate 200 mg orally |
| Kikuchi 2006 | Low‐dose bisphosphonates: risedronate 2.5 mg/day orally |
| Kitazaki 2008 | Low‐dose bisphosphonates: alendronate 5 mg/day orally |
| Nakayamada 2004 | Low‐dose bisphosphonates: cyclic etidronate 200 mg orally |
| Okada 2008 | Low‐dose bisphosphonates: alendronate 5 mg/day orally |
| Sato 2003 | Low‐dose bisphosphonates: cyclic etidronate 200 mg orally |
| Takeda 2008 | Low‐dose bisphosphonates: alendronate 5 mg/day orally |
| Takei 2010 | Low‐dose bisphosphonates: risedronate 2.5 mg/day orally |
| Toukap 2005 | Non‐standard‐dose bisphosphonates: pamidronate 100 mg/week orally |
Characteristics of studies awaiting assessment [ordered by study ID]
Imanishi 2006.
| Methods | Not yet known |
| Participants | Not yet known |
| Interventions | Not yet known |
| Outcomes | Not yet known |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not yet known |
| Mean steroid dose | Not yet known |
| Notes | Article in Japanese with abstract in English, awaiting translation |
Nakamura 2002.
| Methods | Not yet known |
| Participants |
N: 34 participants; all women Conditions: not yet known Mean age (SD) not yet known Baseline vertebral fractures: not yet known |
| Interventions |
Active group: Cyclic etidronate 400 mg orally Comparator group: Not yet known Low dose group: Cyclic etidronate 200 mg orally |
| Outcomes | Not yet known |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not yet known |
| Mean steroid dose | Not yet known |
| Notes | Article in Japanese with abstract in English, awaiting translation |
NCT00097825.
| Methods | RCT; completed trial, no publication |
| Participants | Men, ages 25‐85 |
| Interventions | zoledronic acid vs alendronate (unsure if vitamin D or calcium control) |
| Outcomes | Per cent change in BMD at the lumbar spine and femoral neck at 24 months |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not included |
| Mean steroid dose | Unsure if GIOP population included |
| Notes | Title: Efficacy and safety of zoledronic acid for the treatment of osteoporosis in men |
NCT00372372.
| Methods | RCT; completed trial, no publication |
| Participants | Men and women, ages 18‐75 |
| Interventions | Risedronate vs placebo |
| Outcomes | BMD, incident vertebral fractures, adverse events |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not yet known |
| Mean steroid dose | Pulse methylprednisolone or oral prednisolone (> = 0.8mg/kg/day) or equivalent for at least 6 weeks |
| Notes | Title: The efficacy of risedronate in prevention of bone loss in patients receiving high‐dose corticosteroid treatment |
NCT01215890.
| Methods | RCT; completed trial, no publication |
| Participants | Men and women with Crohn's disease |
| Interventions | Risedronate vs placebo |
| Outcomes | Per cent change in BMD at the lumbar spine and hip at 12 months |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not yet known |
| Mean steroid dose | Unsure if GIOP population included |
| Notes | Title: A randomized, data collection program to determine the efficacy and safety of risedronate (Actonel) therapy plus calcium and vitamin D supplementation versus placebo plus calcium and vitamin D supplementation in the treatment of low bone mineral density in Crohn's disease patients |
NCT01287533.
| Methods | RCT; completed trial, no publication |
| Participants | Women with rheumatoid arthritis |
| Interventions | Ibandronate vs placebo |
| Outcomes | Per cent change in BMD at the lumbar spine and femoral neck at 12 months Incident vertebral fractures |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not yet known |
| Mean steroid dose | Minimum 5 mg/day prednisolone for 3 months |
| Notes | Title: Efficacy of monthly ibandronate in women with rheumatoid arthritis and reduced bone mineral density receiving long‐term glucocorticoids |
Okazaki 2015.
| Methods | Not yet known |
| Participants | Not yet known |
| Interventions | Not yet known |
| Outcomes | Not yet known |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not yet known |
| Mean steroid dose | Not yet known |
| Notes | Article in Japanese, awaiting translation |
Ozoran 2007.
| Methods | RCT, study duration 12 months |
| Participants |
N: 50 participants; men (14%) and women (86%) Conditions: rheumatoid arthritis Mean age (SD) Intervention: 49.9 (11.6) Comparator: 47.3 (13.6) Baseline vertebral fractures: not explicitly stated |
| Interventions |
Active group: alendronate 70 mg/week orally, daily calcium/vitamin D Comparator group: daily calcium/vitamin D |
| Outcomes | Insufficient reporting of BMD data, pending clarification from authors |
| Types of studies | Treatment study |
| Incident vertebral fractures | Not reported as outcome |
| Mean steroid dose | ˜7.5 mg/day |
| Notes | Calcitriol and alendronate + calcitriol groups not included |
Suzuki 2015.
| Methods | Not yet known |
| Participants | Not yet known |
| Interventions | Not yet known |
| Outcomes | Not yet known |
| Types of studies | Not yet known |
| Incident vertebral fractures | Not yet known |
| Mean steroid dose | Not yet known |
| Notes | Article in Japanese, awaiting translation |
Characteristics of ongoing studies [ordered by study ID]
NCT00058188.
| Trial name or title | A phase III randomized study of zoledronate bisphosphonate therapy for the prevention of bone loss in men with prostate cancer receiving long‐term androgen deprivation |
| Methods | RCT; study ongoing |
| Participants | Men, ages 18 and older with stage III and IV prostate cancer |
| Interventions | Zolendronate IV vs calcium gluconate and cholecalciferol |
| Outcomes | BMD changes |
| Starting date | March 2003 |
| Contact information | Study Chair: Charles L. Bennett, MD, PhD, Robert H. Lurie Cancer Center |
| Notes | Steroids allowed, unsure if meet minimum 5 mg/day dose |
NCT02589600.
| Trial name or title | ZEST II for osteoporotic fracture prevention |
| Methods | RCT; recruiting participants |
| Participants | Women in LTC facilities with osteoporosis, ages 65 and older |
| Interventions | zoledronic acid vs placebo |
| Outcomes | Clinical vertebral and nonvertebral fractures |
| Starting date | January 2016 |
| Contact information | Principal Investigator: Susan L Greenspan, MD, University of Pittsburgh |
| Notes | Unsure if GIOP population included |
UMIN000009222.
| Trial name or title | Drug therapy for the prevention of glucocorticoid induced osteoporosis in elderly patients: teriparatide or bisphosphonates? |
| Methods | RCT |
| Participants | Women and men, ages 65 and older with collagen vascular disorders on steroid therapy |
| Interventions | Teriparative vs alendronate or risedronate |
| Outcomes | Vertebral and nonvertebral fractures, BMD changes |
| Starting date | 2012/12/01 |
| Contact information | Principle Investigator: Koichi Amano, Saitama Medical University |
| Notes | Unsure if placebo and/or vitamin D and calcium control |
UMIN000013305.
| Trial name or title | Efficacy of once every four week oral minodronate in patients with glucocorticoid‐induced osteoporosis after switching from weekly oral bisphosphonate |
| Methods | RCT |
| Participants | Women and men, ages 20 and older with rheumatic diseases on steroid therapy |
| Interventions | Minodronate vs alendronate or risedronate |
| Outcomes | BMD changes |
| Starting date | 2013/10/25 |
| Contact information | Principle Investigator: Taio Naniwa, Nagoya City University Hospital |
| Notes | Unsure if placebo and/or vitamin D and calcium control |
UMIN000013659.
| Trial name or title | Efficacy of a human anti‐RANKL antibody (Denosumab) on prevention of steroid‐induced osteoporosis in patients with autoimmune hepatitis (AIH) |
| Methods | RCT |
| Participants | Women and men, ages 20‐75 with autoimmune hepatitis on steroid therapy |
| Interventions | Denosumab vs bisphosphonate |
| Outcomes | BMD changes |
| Starting date | 2014/04/08 |
| Contact information | Principle Investigator: Kenichi Ikejima, Juntendo University School of Medicine |
| Notes | unsure if placebo and/or vitamin D and calcium control |
UMIN000014341.
| Trial name or title | Glucocorticoid‐induced osteoporosis treated with bisphosphonate and denosumab |
| Methods | RCT |
| Participants | Women and men, ages 18 and older on steroid therapy |
| Interventions | Bisphosphonate vs denosumab |
| Outcomes | Incident vertebral fractures, BMD changes |
| Starting date | 2014/06/24 |
| Contact information | Principle Investigator: Hisaji Oshima, Tokyo Medical Center, National Hospital Organization |
| Notes | Unsure if placebo and/or vitamin D and calcium control |
Differences between protocol and review
We have updated the methods in the review since the original review in accordance with current Cochrane and Cochrane Musculoskeletal recommendations. Due to the increased number of high‐quality studies that have been published since the original review, we have made our selection criteria more rigorous to include only RCTs that measured BMD by DEXA (and not quantitative CT). We have added four more outcomes: number of participants with new radiographic non‐vertebral fractures; quality of life; serious adverse events; and direct comparison of lumbar spine and femoral neck BMD change using low‐dose versus standard‐dose bisphosphonates.
We have changed the minimum steroid dose of 7.5 mg/day from the original review to include a minimum of 5 mg/day to reflect current literature on the effect of low‐dose steroids.
We have altered our time points for BMD analysis to reflect clinical relevance. Specifically, we have removed the six‐month time point and changed our two‐year time point to include data between 18 to 24 months.
In the original review, sensitivity analyses were performed for methodological quality, BMD technique and study duration. The sensitivity analyses in our updated review were performed to evaluate the effect of risk of bias in included studies, based on the exclusion of non‐blinded studies, and route of administration (oral and parenteral). This change reflects differences in the certainty of evidence and advances in therapy since the original review.
Contributions of authors
JH and CA wrote the review; JH, JY and CA screened initial search results and identified studies that fulfilled inclusion criteria; JH, CA and BV were involved in data extraction and management; JH, JY and CA performed ‘risk of bias’ assessment; JH and CA performed GRADE evaluation. All authors reviewed and approved the final draft prior to submission.
Sources of support
Internal sources
University of Alberta, Edmonton, Canada.
University of Ottawa, Ontario, Canada.
External sources
No sources of support supplied
Declarations of interest
Claire S Allen: none known James HS Yeung: none known Ben Vandermeer: none known Joanne Homik: none known
Edited (no change to conclusions)
References
References to studies included in this review
Abitbol 2007 {published data only}
- Abitbol V, Briot K, Roux C, Roy C, Seksik P, Charachon A, et al. A double‐blind placebo‐controlled study of intravenous clodronate for prevention of steroid‐induced bone loss in inflammatory bowel disease. Clinical Gastroenterology & Hepatology 2007;5:1184‐9. [DOI] [PubMed] [Google Scholar]
Adachi 1997 {published data only}
- Adachi JD, Bensen WG, Brown J, Hanley D, Hodsman A, Josse R, et al. Intermittent etidronate therapy to prevent corticosteroid‐induced osteoporosis. New England Journal of Medicine 1997;337(6):382‐7. [DOI] [PubMed] [Google Scholar]
Adachi 2001 {published data only}
- Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, et al. Two‐year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double‐blind, placebo‐controlled extension trial. Arthritis & Rheumatism 2001;44:202‐11. [DOI] [PubMed] [Google Scholar]
Boutsen 1997 {published data only}
- Boutsen Y, Jamart J, Esselinckx W, Stoffel M, Devogelaer JP. Primary prevention of glucocorticoid‐induced osteoporosis with intermittent intravenous pamidronate: a randomized trial. Calcified Tissue International 1997;61:266‐71. [DOI] [PubMed] [Google Scholar]
Boutsen 2001 {published data only}
- Boutsen Y, Jamart J, Esselinckx W, Devogelaer JP. Primary prevention of glucocorticoid‐induced osteoporosis with intravenous pamidronate and calcium: a prospective controlled 1‐year study comparing a single infusion, an infusion given once every 3 months, and calcium alone. Journal of Bone & Mineral Research 2001;16:104‐12. [DOI] [PubMed] [Google Scholar]
Cohen 1999 {published data only}
- Cohen S, Levy RM, Keller M, Boling E, Emkey RD, Greenwald M, et al. Risedronate therapy prevents corticosteroid‐induced bone loss: a twelve‐month, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study. Arthritis & Rheumatism 1999;42:2309‐18. [DOI] [PubMed] [Google Scholar]
Cortet 1999 {published data only}
- Cortet B, Hachulla E, Barton I, Bonvoisin B, Roux C. Evaluation of the efficacy of etidronate therapy in preventing glucocorticoid‐induced bone loss in patients with inflammatory rheumatic diseases. A randomized study. Revue du Rhumatisme (English Edition) 1999;66:214‐9. [PubMed] [Google Scholar]
De Nijs 2006 {published data only}
- Nijs RN, Jacobs JW, Lems WF, Laan RF, Algra A, Huisman AM, et al. Alendronate or alfacalcidol in glucocorticoid‐induced osteoporosis.[Reprint in Ned Tijdschr Geneeskd. 2007 May 26;151(21):1178‐85; PMID: 17557758]. New England Journal of Medicine 2006;355:675‐84. [Google Scholar]
Frediani 2003 {published data only}
- Frediani B, Falsetti P, Baldi F, Acciai C, Filippou G, Marcolongo R. Effects of 4‐year treatment with once‐weekly clodronate on prevention of corticosteroid‐induced bone loss and fractures in patients with arthritis: evaluation with dual‐energy X‐ray absorptiometry and quantitative ultrasound. Bone 2003;33:575‐81. [DOI] [PubMed] [Google Scholar]
Geusens 1998 {published data only}
- Geusens P, Dequeker J, Vanhoof J, Stalmans R, Boonen S, Joly J, et al. Cyclical etidronate increases bone density in the spine and hip of postmenopausal women receiving long term corticosteroid treatment. A double blind, randomised placebo controlled study. Annals of the Rheumatic Diseases 1998;57:724‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hakala 2012 {published data only}
- Hakala M, Kröger H, Valleala H, Hienonen‐Kempas T, Lehtonen‐Veromaa M, Heikkinen J, et al. Once‐monthly oral ibandronate provides significant improvement in bone mineral density in postmenopausal women treated with glucocorticoids for inflammatory rheumatic diseases: a 12‐month, randomized, double‐blind, placebo‐controlled trial. Scandinavian Journal of Rheumatology 2012;41(4):260‐6. [DOI] [PubMed] [Google Scholar]
Herrala 1998 {published data only}
- Herrala J, Puolijoki H, Liippo K, Raitio M, Impivaara O, Tala E, et al. Clodronate is effective in preventing corticosteroid‐induced bone loss among asthmatic patients. Bone 1998;22:577‐82. [DOI] [PubMed] [Google Scholar]
Jenkins 1999 {published data only}
- Jenkins EA, Walker‐Bone KE, Wood A, McCrae FC, Cooper C, Cawley MI. The prevention of corticosteroid‐induced bone loss with intermittent cyclical etidronate. Scandinavian Journal of Rheumatology 1999;28:152‐6. [DOI] [PubMed] [Google Scholar]
Lems 2006 {published data only}
- Lems WF, Lodder MC, Lips P, Bijlsma JW, Geusens P, Schrameijer N, et al. Positive effect of alendronate on bone mineral density and markers of bone turnover in patients with rheumatoid arthritis on chronic treatment with low‐dose prednisone: a randomized, double‐blind, placebo‐controlled trial. Osteoporosis International 2006;17:716‐23. [DOI] [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li EK, Zhu TY, Hung VY, Kwok AW, Lee VW, Lee KK, et al. Ibandronate increases cortical bone density in patients with systemic lupus erythematosus on long‐term glucocorticoid. Arthritis Research & Therapy 2010;12(5):R198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pitt 1998 {published data only}
- Pitt P, Li F, Todd P, Webber D, Pack S, Moniz C. A double blind placebo controlled study to determine the effects of intermittent cyclical etidronate on bone mineral density in patients on long‐term oral corticosteroid treatment. Thorax 1998;53:351‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 2000 {published data only}
- Reid DM, Hughes RA, Laan RF, Sacco‐Gibson NA, Wenderoth DH, Adami S, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid‐induced osteoporosis in men and women: a randomized trial. European Corticosteroid‐Induced Osteoporosis Treatment Study. Journal of Bone & Mineral Research 2000;15:1006‐13. [DOI] [PubMed] [Google Scholar]
Roux 1998 {published data only}
- Roux C, Oriente P, Laan R, Hughes RA, Ittner J, Goemaere S, et al. Randomized trial of effect of cyclical etidronate in the prevention of corticosteroid‐induced bone loss. Ciblos Study Group. Journal of Clinical Endocrinology & Metabolism 1998;83:1128‐33. [DOI] [PubMed] [Google Scholar]
Saadati 2008 {published data only}
- Saadati N, Rajabian R. The effect of bisphosphonate on prevention of glucocorticoid‐induced osteoporosis. Iranian Red Crescent Medical Journal 2008;10(1):8‐11. [Google Scholar]
Saag 1998 {published data only}
- Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, et al. Alendronate for the prevention and treatment of glucocorticoid‐induced osteoporosis. New England Journal of Medicine 1998;339:292‐9. [DOI] [PubMed] [Google Scholar]
Sambrook 2003 {published data only}
- Sambrook PN, Kotowicz M, Nash P, Styles CB, Naganathan V, Henderson‐Briffa KN, et al. Prevention and treatment of glucocorticoid‐induced osteoporosis: a comparison of calcitriol, vitamin D plus calcium, and alendronate plus calcium. Journal of Bone & Mineral Research 2003;18:919‐24. [DOI] [PubMed] [Google Scholar]
Skingle 1997 {published data only}
- Skingle SJ, Moore DJ, Crisp AJ. Cyclical etidronate increases lumbar spine bone density in patients on long‐term glucocorticosteroid therapy. International journal of clinical practice 1997;51:364‐7. [PubMed] [Google Scholar]
Stoch 2009 {published data only}
- Stoch SA, Saag KG, Greenwald M, Sebba AI, Cohen S, Verbruggen N, et al. Once‐weekly oral alendronate 70 mg in patients with glucocorticoid‐induced bone loss: a 12‐month randomized, placebo‐controlled clinical trial. Journal of Rheumatology 2009;36:1705‐14. [DOI] [PubMed] [Google Scholar]
Tee 2012 {published data only}
- Tee SI, Yosipovitch G, Chan YC, Chua SH, Koh ET, Chan YH, et al. Prevention of glucocorticoid‐induced osteoporosis in immunobullous diseases with alendronate: a randomized, double‐blind, placebo‐controlled study. Archives of Dermatology 2012;148(3):307‐14. [DOI] [PubMed] [Google Scholar]
Van Offel 2001 {published data only}
- Offel JF, Schuerwegh AJ, Bridts CH, Bracke PG, Stevens WJ, Clerck LS. Influence of cyclic intravenous pamidronate on proinflammatory monocytic cytokine profiles and bone density in rheumatoid arthritis treated with low dose prednisolone and methotrexate. Clinical & Experimental Rheumatology 2001;19:13‐20. [PubMed] [Google Scholar]
Wolfhagen 1997 {published data only}
- Wolfhagen FH, Buuren HR, Ouden JW, Hop WC, Leeuwen JP, Schalm SW, et al. Cyclical etidronate in the prevention of bone loss in corticosteroid‐treated primary biliary cirrhosis. A prospective, controlled pilot study. Journal of Hepatology 1991;26:325‐30. [DOI] [PubMed] [Google Scholar]
Yeap 2008 {published data only}
- Yeap SS, Fauzi AR, Kong NC, Halim AG, Soehardy Z, Rahimah I, et al. A comparison of calcium, calcitriol, and alendronate in corticosteroid‐treated premenopausal patients with systemic lupus erythematosus. Journal of Rheumatology 2008;35:2344‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Benucci 2009 {published data only}
- Benucci M, Saviola G, Baiardi P, Abdi‐Ali L, Povino MR, Dolenti S, et al. Effects of monthly intramuscular neridronate in rheumatic patients in chronic treatment with low‐dose glucocorticoids. Clinical and Experimental Rheumatology 2009;27(4):567‐73. [PubMed] [Google Scholar]
Fujii 2006 {published data only}
- Fujii N, Hamano T, Mikami S, Nagasawa Y, Isaka Y, Moriyama T, et al. Risedronate, an effective treatment for glucocorticoid‐induced bone loss in CKD patients with or without concomitant active vitamin D (PRIUS‐CKD). Nephrology Dialysis Transplantation 2007;22:1601‐7. [DOI] [PubMed] [Google Scholar]
Jinnouchi 2000 {published data only}
- Jinnouchi Y. Efficacy of intermittent etidronate therapy for corticosteroid‐induced osteoporosis in patients with diffuse connective tissue disease. Kurume Medical Journal 2000;47:219‐24. [DOI] [PubMed] [Google Scholar]
Kikuchi 2006 {published data only}
- Kikuchi Y, Imakiire T, Yamada M, Saigusa T, Hyodo T, Kushiyama T, et al. Effect of risedronate on high‐dose corticosteroid‐induced bone loss in patients with glomerular disease. Nephrology Dialysis Transplantation 2007;22:1593‐600. [DOI] [PubMed] [Google Scholar]
Kitazaki 2008 {published data only}
- Kitazaki S, Mitsuyama K, Masuda J, Harada K, Yamasaki H, Kuwaki K, et al. Clinical trial: comparison of alendronate and alfacalcidol in glucocorticoid‐associated osteoporosis in patients with ulcerative colitis. Alimentary Pharmacology & Therapeutics 2009;29(4):424‐30. [DOI] [PubMed] [Google Scholar]
Nakayamada 2004 {published data only}
- Nakayamada S, Okada Y, Saito K, Tanaka Y. Etidronate prevents high dose glucocorticoid induced bone loss in premenopausal individuals with systemic autoimmune diseases. Journal of Rheumatology 2004;31:163‐6. [PubMed] [Google Scholar]
Okada 2008 {published data only}
- Okada Y, Nawata M, Nakayamada S, Saito K, Tanaka Y. Alendronate protects premenopausal women from bone loss and fracture associated with high‐dose glucocorticoid therapy. Journal of Rheumatology 2008;35:2249‐54. [DOI] [PubMed] [Google Scholar]
Sato 2003 {published data only}
- Sato S, Ohosone Y, Suwa A, Yasuoka H, Nojima T, Fujii T, et al. Effect of intermittent cyclical etidronate therapy on corticosteroid induced osteoporosis in Japanese patients with connective tissue disease: 3 year follow up. Journal of Rheumatology 2003;30:2673‐9. [PubMed] [Google Scholar]
Takeda 2008 {published data only}
- Takeda S, Kaneoka H, Saito T. Effect of alendronate on glucocorticoid‐induced osteoporosis in Japanese women with systemic autoimmune diseases: versus alfacalcidol. Modern Rheumatology 2008;18(3):271‐6. [DOI] [PubMed] [Google Scholar]
Takei 2010 {published data only}
- Takei T, Itabashi M, Tsukada M, Sugiura H, Moriyama T, Kojima C, et al. Risedronate therapy for the prevention of steroid‐induced osteoporosis in patients with minimal‐change nephrotic syndrome. Internal Medicine 2010;49(19):2065‐70. [DOI] [PubMed] [Google Scholar]
Toukap 2005 {published data only}
- Toukap AN, Depresseux G, Devogelaer JP, Houssiau FA. Oral pamidronate prevents high‐dose glucocorticoid‐induced lumbar spine bone loss in premenopausal connective tissue disease (mainly lupus) patients. Lupus 2005;14:517‐20. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Imanishi 2006 {published data only}
- Imanishi Y, Nishizawa Y. Activate form vitamin D3 or bisphosphonate in glucocorticoid‐induced osteoporosis. Clinical Calcium 2006;16(11):1844‐50. [PubMed] [Google Scholar]
Nakamura 2002 {published data only}
- Nakamura T, Maekawa S, Morinobu S, Morinobu A, Koshiba M, Yamauchi M, et al. The clinical benefits to bone mineral density were shown by cyclical oral etidronate administration in steroid induced osteoporosis. Ryumachi 2002;42(4):666‐75. [PubMed] [Google Scholar]
NCT00097825 {unpublished data only}
NCT00372372 {unpublished data only}
NCT01215890 {unpublished data only}
NCT01287533 {unpublished data only}
Okazaki 2015 {published data only}
- Okazaki, R. Pharmacological treatment of other types of secondary osteoporosis. Nippon Rinsho ‐ Japanese Journal of Clinical Medicine 2015;73(10):1740‐5. [PubMed] [Google Scholar]
Ozoran 2007 {published data only}
- Ozoran K, Yildirim M, Önder M, Sivas F, Inanir A. The bone mineral density effects of calcitonin and alendronate combined therapy in patients with rheumatoid arthritis. Asia Pacific League of Associations for Rheumatology Journal of Rheumatology 2007;10:17‐22. [Google Scholar]
Suzuki 2015 {published data only}
- Suzuki Y. Glucocorticoid‐induced osteoporosis. Nippon Rinsho ‐ Japanese Journal of Clinical Medicine 2015;73(10):1733‐39. [PubMed] [Google Scholar]
References to ongoing studies
NCT00058188 {unpublished data only}
- A phase III randomized study of zoledronate bisphosphonate therapy for the prevention of bone loss in men with prostate cancer receiving long‐term androgen deprivation. Ongoing study March 2003.
NCT02589600 {unpublished data only}
- ZEST II for osteoporotic fracture prevention. Ongoing study January 2016.
UMIN000009222 {unpublished data only}
- Drug therapy for the prevention of glucocorticoid induced osteoporosis in elderly patients: teriparatide or bisphosphonates?. Ongoing study 2012/12/01.
UMIN000013305 {unpublished data only}
- Efficacy of once every four week oral minodronate in patients with glucocorticoid‐induced osteoporosis after switching from weekly oral bisphosphonate. Ongoing study 2013/10/25.
UMIN000013659 {unpublished data only}
- Efficacy of a human anti‐RANKL antibody (Denosumab) on prevention of steroid‐induced osteoporosis in patients with autoimmune hepatitis (AIH). Ongoing study 2014/04/08.
UMIN000014341 {unpublished data only}
- Glucocorticoid‐induced osteoporosis treated with bisphosphonate and denosumab. Ongoing study 2014/06/24.
Additional references
Abrahamsen 2010
- Abrahamsen B, Eiken P, Eastell R. Cumulative alendronate dose and the long‐term absolute risk of subtrochanteric and diaphyseal femur fractures: a register‐based national cohort analysis. Journal of Clinical Endocrinology & Metabolism 2010;95(12):5258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 1996
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt M C, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996;348:1535‐41. [DOI] [PubMed] [Google Scholar]
Brant 2014 [Computer program]
- Brant, R. Inference for Proportions: Comparing Two Independent Samples. Accessed June 12 2015: stat.ubc.ca/˜rollin/stats/ssize/b2.html, 2014.
Canalis 2007
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid‐induced osteoporosis: pathophysiology and therapy. Osteoporosis International 2007;18:1319‐28. [DOI] [PubMed] [Google Scholar]
Cates 2015 [Computer program]
- Cates C. Visual Rx Software. 2008. Version Version 1.0 ‐ 3.0. Available from nntonline.net/visualrx/, accessed June 12, 2015.
Cooper 1992
- Cooper C, Atkinson EJ, O'Fallon WM, Melton JK 3rd. Incidence of clinically diagnosed vertebral fractures: a population‐based study in Rochester, Minnesota, 1985‐1989. Journal of Bone and Mineral Research 1992;7:221‐7. [DOI] [PubMed] [Google Scholar]
Cummings 2002
- Cummings, S. R, Karpf, D. B, Harris, F, Genant, H. K, Ensrud, K, LaCroix, A. Z, et al. Improvement in Spine Bone Density and Reduction in Risk of Vertebral Fractures during Treatment with Antiresorptive Drugs. American Journal of Medicine 2002;112:281‐9. [DOI] [PubMed] [Google Scholar]
Curtis 2005
- Curtis JR, Westfall AO, Allison JJ, Becker A, Casebeer L, Freeman A, et al. Longitudinal patterns in the prevention of osteoporosis in glucocorticoid‐treated patients. Arthritis & Rheumatism 2005;52(8):2485‐94. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dell 2012
- Dell R, Greene D, Ott SM, Silverman S, Eisemon E, Funahashi T, et al. A retrospective analysis of all atypical femur fractures seen in a large California HMO from the years 2007 to 2009. Journal of Bone and Mineral Research 2012;27(12):2544–50. [DOI] [PubMed] [Google Scholar]
Djokanovic 2008
- Djokanovic N, Klieger‐Grossmann C, Koren G. Does treatment with bisphosphonates endanger the human pregnancy?. Journal of Obstetrics and Gynaecology Canada 2008;30(12):1146‐8. [DOI] [PubMed] [Google Scholar]
Feldstein 2005
- Feldstein AC, Elmer PJ, Nichols GA, Herson M. Practice patterns in patients at risk for glucocorticoid‐induced osteoporosis. Osteoporosis International 2005;16(12):2168‐74. [DOI] [PubMed] [Google Scholar]
Genant 1993
- Genant HK, Wu CY, Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. Journal of Bone and Mineral Research 1993;8(9):1137‐48. [DOI] [PubMed] [Google Scholar]
Genant 1996
- Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis The Study of Osteoporotic Fractures Research Group. Journal of Bone and Mineral Research 1996;11(7):984‐96. [DOI] [PubMed] [Google Scholar]
Gertz 1995
- Gertz BJ, Holland SD, Kline WF, Matuszewski BK, Freeman A, Quan H, et al. Studies on the oral availablility of alendronate. Clinical Pharmacology & Therapeutics 1995;58(3):288‐98. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 12 June 2015. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Grossman 2010
- Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid‐induced osteoporosis. Arthritis Care and Research 2010;62(11):1515‐26. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kanis 2007
- Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd‐Jones M. Glucocorticoid‐induced osteoporosis: a systematic review and cost–utility analysis. Health Technology Assessment 2007;11:7. [DOI] [PubMed] [Google Scholar]
Kiel 1995
- Kiel, D. Assessing vertebral fractures: National Osteoporosis Working Group on Vertebral Fractures. Journal of Bone and Mineral Research 1995;10:518–23. [DOI] [PubMed] [Google Scholar]
Kleerekoper 1984
- Kleerekoper M, Parfitt AM, Ellis BI. Measurements of vertebral fracture rates in osteoporosis. Proceedings of the Copenhagen International Symposium on Osteoporosis. Copenhagen: AalbergStiftsbogtrykkeri, June 3‐8, 1984:103‐109.
Lee 2015
- Lee S, Yin RV, Hirpara H, Lee NC, Lee A, Llanos S, et al. Increased risk for atypical fractures associated with bisphosphonate use. Family Practice 2015;32(3):276–81. [DOI] [PubMed] [Google Scholar]
Lekamwasam 2012
- Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S, Borgström F, et al. A framework for the development of guidelines for the management of glucocorticoid‐induced osteoporosis. Osteoporosis International 2012;23(9):2257‐76. [DOI] [PubMed] [Google Scholar]
Losada 2010
- Losada I, Sartori L, Gianantonio E, Zen M, Clementi M, Doria A. Bisphosphonates in patients with autoimmune rheumatic diseases: can they be used in women of childbearing age?. Autoimmunity Reviews 2010;9(8):547‐52. [DOI] [PubMed] [Google Scholar]
Mckeown 2012
- McKeown E, Vivian P, Bykerk VP, Leon F, Bonner A, Thorne C, et al. Quality assurance study of the use of preventative therapies in glucocorticoid‐induced osteoporosis in early inflammatory arthritis: results from the CATCH cohort. Rheumatology 2012;51:1662‐9. [DOI] [PubMed] [Google Scholar]
Melton 1993
- Melton LJ 3rd, Lane AW, Eastell R, O’Fallon WM, Riggs BL. Prevalence and incidence of vertebral deformities. Osteoporosis International 1993;3:113‐9. [DOI] [PubMed] [Google Scholar]
Minne 1988
- Minne HW, Leidig G, Wuster C, Siromachkostov L, Baldauf G, Bickel R, et al. A newly developed spine deformity index (SDI) to quantitate vertebral crush fractures in patients with osteoporosis. Bone Mineral 1988;3:335–49. [PubMed] [Google Scholar]
National Osteoporosis Foundation 2014
- National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2014. [DOI] [PMC free article] [PubMed]
Pazianas 2011
- Pazianas M, Abrahamsen B. Safety of Bisphosphonates. Bone 2011;49:103–10. [DOI] [PubMed] [Google Scholar]
Reid 2009
- Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid‐induced osteoporosis (HORIZON): a multicentre, double‐blind, double‐dummy, randomised controlled trial. Lancet 2009;373:1253–63. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rickers 1984
- Rickers H, Deding A, Christiansen C, Rodbro P. Mineral loss in cortical and trabecular bone during high‐dose prednisone treatment. Calcified Tissue International 1984;36(3):269–73. [DOI] [PubMed] [Google Scholar]
Russell 2007
- Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, et al. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Annals of the New York Academy of Sciences 2007;1117:209‐57. [DOI] [PubMed] [Google Scholar]
Saag 2003
- Saag KG. Glucocorticoid‐induced osteoporosis. Endocrinology and Metabolism Clinics of North America 2003;32:135‐57. [DOI] [PubMed] [Google Scholar]
Sambrook 2012
- Sambrook PN, Roux C, Devogelaer JP, Saag K, Lau CS, Reginster JY, et al. Bisphosphonates and glucocorticoid osteoporosis in men: results of a randomized controlled trial comparing zoledronic acid with risedronate. Bone 2012;50:289–95. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. www.handbook.cochrane.org.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, (editors). GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from guidelinedevelopment.org/handbook.
Steinbuch 2004
- Steinbuch M, Thomas E, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporosis International 2004;15:323‐8. [DOI] [PubMed] [Google Scholar]
Van Kujik 1995
- Kuijk C, Genant HK. Radiological aspects. In: Riggs BL, Melton LJ III editor(s). Osteoporosis: etiology, diagnosis, and management. 2nd Edition. Philadelphia: Lippincott‐Raven, 1995:249‐73. [Google Scholar]
Van Staa 2002
- Staa TP, Leufkens HGM, Cooper C. The Epidemiology of Corticosteroid‐Induced Osteoporosis: a Meta‐analysis. Osteoporosis International 2002;13:777‐87. [DOI] [PubMed] [Google Scholar]
Wiebe 2006
- Wiebe N, Vandermeer B, Platt RW, Klassen TP, Moher D, Barrowman NJ. A systematic review identifies a lack of standardization in methods for handling missing variance data. Journal of Clinical Epidemiology 2006;59:342‐53. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Homik 1999
- Homik J, Cranney A, Shea B, Tugwell P, Wells GA, Adachi J, et al. Bisphosphonates for steroid induced osteoporosis. Cochrane Database of Systematic Reviews 1999, Issue 1 Art. No.: CD001347. [DOI: 10.1002/14651858.CD001347] [DOI] [PubMed] [Google Scholar]
Homik 1999a
- Homik JE, Cranney A, Shea B, Tugwell P, Wells G, Adachi JD, et al. A meta‐analysis on the use of bisphosphonates in corticosteroid induced osteoporosis. Journal of Rheumatology 1999;26(5):1148‐57. [PubMed] [Google Scholar]


