Abstract
Background
Many treatments for the common cold exist and are sold over‐the‐counter. Nevertheless, evidence on the effectiveness and safety of nasal decongestants is limited.
Objectives
To assess the efficacy, and short‐ and long‐term safety, of nasal decongestants used in monotherapy to alleviate symptoms of the common cold in adults and children.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 6, June 2016), which contains the Cochrane Acute Respiratory Infections (ARI) Specialised Register, MEDLINE (1946 to July 2016), Embase (2010 to 15 July 2016), CINAHL (1981 to 15 July 2016), LILACS (1982 to July 2016), Web of Science (1955 to July 2016) and clinical trials registers.
Selection criteria
Randomised controlled trials (RCTs) and cluster‐RCTs investigating the effectiveness and adverse effects of nasal decongestants compared with placebo for treating the common cold in adults and children. We excluded quasi‐RCTs.
Data collection and analysis
Three review authors independently extracted and summarised data on subjective measures of nasal congestion, overall patient well‐being score, objective measures of nasal airway resistance, adverse effects and general recovery. One review author acted as arbiter in cases of disagreement. We categorised trials as single and multi‐dose and analysed data both separately and together. We also analysed studies using an oral or topical nasal decongestant separately and together.
Main results
We included 15 trials with 1838 participants. Fourteen studies included adult participants only (aged 18 years and over). In six studies the intervention was a single dose and in nine studies multiple doses were used. Nine studies used pseudoephedrine and three studies used oxymetazoline. Other decongestants included phenylpropanolamine, norephedrine and xylometazoline. Phenylpropanolamine (or norephedrine) is no longer available on the market therefore we did not include the results of these studies in the meta‐analyses. Eleven studies used oral decongestants; four studies used topical decongestants.
Participants were included after contracting the common cold. The duration of symptoms differed among studies; in 10 studies participants had symptoms for less than three days, in three studies symptoms were present for less than five days, one study counted the number of colds over one year, and one study experimentally induced the common cold. In the single‐dose studies, the effectiveness of a nasal decongestant was measured on the same day, whereas the follow‐up in multi‐dose studies ranged between one and 10 days.
Most studies were conducted in university settings (N = eight), six at a specific university common cold centre. Three studies were conducted at a university in collaboration with a hospital and two in a hospital only setting. In two studies the setting was unclear.
There were large differences in the reporting of outcomes and the reporting of methods in most studies was limited. Therefore, we judged most studies to be at low or unclear risk of bias. Pooling was possible for a limited number of studies only; measures of effect are expressed as standardised mean differences (SMDs). A positive SMD represents an improvement in congestion. There is no defined minimal clinically important difference for measures of subjective improvement in nasal congestion, therefore we used the SMDs as a guide to assess whether an effect was small (0.2 to 0.49), moderate (0.5 to 0.79) or large (≥ 0.8).
Single‐dose decongestant versus placebo: 10 studies compared a single dose of nasal decongestant with placebo and their effectiveness was tested between 15 minutes and 10 hours after dosing. Seven of 10 studies reported subjective symptom scores for nasal congestion; none reported overall patient well‐being. However, pooling was not possible due to the large diversity in the measurement and reporting of symptoms of congestion. Two studies recorded adverse events. Both studies used an oral decongestant and each of them showed that there was no statistical difference between the number of adverse events in the treatment group versus the placebo group.
Multi‐dose decongestant versus placebo: nine studies compared multiple doses of nasal decongestants with placebo, but only five reported on the primary outcome, subjective symptom scores for nasal congestion. Only one study used a topical decongestant; none reported overall patient well‐being. Subjective measures of congestion were significantly better for the treatment group compared with placebo approximately three hours after the last dose (SMD 0.49, 95% confidence interval (CI) 0.07 to 0.92; P = 0.02; GRADE: low‐quality evidence). However, the SMD of 0.49 only indicates a small clinical effect. Pooling was based on two studies, one oral and one topical, therefore we were unable to assess the effects of oral and topical decongestants separately. Seven studies reported adverse events (six oral and one topical decongestant); meta‐analysis showed that there was no statistical difference between the number of adverse events in the treatment group (125 per 1000) compared to the placebo group (126 per 1000). The odds ratio (OR) for adverse events in the treatment group was 0.98 (95% CI 0.68 to 1.40; P = 0.90; GRADE: low‐quality evidence). The results remained the same when we only considered studies using an oral decongestant (OR 0.95, 95% CI 0.65 to 1.39; P = 0.80; GRADE: low‐quality evidence).
Authors' conclusions
We were unable to draw conclusions on the effectiveness of single‐dose nasal decongestants due to the limited evidence available. For multiple doses of nasal decongestants, the current evidence suggests that these may have a small positive effect on subjective measures of nasal congestion in adults with the common cold. However, the clinical relevance of this small effect is unknown and there is insufficient good‐quality evidence to draw any firm conclusions. Due to the small number of studies that used a topical nasal decongestant, we were also unable to draw conclusions on the effectiveness of oral versus topical decongestants. Nasal decongestants do not seem to increase the risk of adverse events in adults in the short term. The effectiveness and safety of nasal decongestants in children and the clinical relevance of their small effect in adults is yet to be determined.
Plain language summary
Do nasal decongestants used alone relieve cold symptoms?
Review question
We wanted to find out if nasal decongestants used alone can ease nasal congestion symptoms in people with colds.
Background
Colds, although not serious, are common illnesses responsible for many visits to family doctors and days lost from work and school. Cold symptoms include runny nose, sore throat and sneezing, and they can last up to two weeks. There is no cure for colds; treatments only ease the symptoms. Many people use over‐the‐counter medicines such as nasal decongestants to treat cold symptoms. However, there is little evidence that nasal decongestants actually work. We wanted to find out if nasal decongestants help ease congestion caused by colds.
We considered studies that used a nasal decongestant as the only treatment for colds. We looked at subjective symptoms of congestion ‐ this means that symptoms and overall well‐being were self‐rated by patients.
Search date
We searched for studies in July 2016.
Study characteristics
We included 15 studies with 1838 participants; 14 included only adults aged 18 years or over. Six studies used a single‐dose nasal decongestant and measured the effects on the day it was administered. Nine studies used multiple doses and the effects were measured between one and 10 days after first administration. Eleven studies used tablets or syrup and four studies used nasal sprays. Eight studies were conducted at universities, three at universities in collaboration with hospitals and two in hospitals. The setting was unclear in two studies.
Study funding sources
Nine studies were funded by drug manufacturers or agencies with commercial interests in the study results. Funding sources were unclear in six studies.
Key results
We were unable to draw conclusions about single‐dose nasal decongestants. We found a small benefit in the relief of nasal congestion from multiple doses, but it was unclear if this was beneficial for patients. No studies reported overall patient well‐being. There was no difference in the numbers of adverse events between people who used a nasal decongestant and those who did not. We could not determine if there was a difference in effects between decongestant tablets and nasal sprays. The results relate to adults; there was no evidence on the effectiveness or safety of nasal decongestants for children.
Quality of the evidence
We assessed the quality of the evidence for subjective cold symptoms as low for the multi‐dose studies ‐ there were few data and reporting was unclear. We also assessed the quality of the evidence for adverse events as low because of unclear reporting and because the estimates were not precise (there were wide confidence intervals ‐ a measure of statistical uncertainty).
Summary of findings
Summary of findings for the main comparison. Summary of findings for single‐dose nasal decongestant compared to placebo in adults with the common cold.
| Should a single dose of decongestant in monotherapy be used for the common cold in adults? | ||||||
|
Patient or population: adult patients with the common cold Settings: common cold centres, universities and hospitals Intervention: single‐dose decongestant in monotherapy, oral and topical decongestants combined Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with single‐dose decongestant | |||||
| Primary outcome: subjective symptom score ‐ 3 hours after dosing |
— | — | — | 540 (4 RCTs, oral) | — | Insufficient data to pool results Pseudoephedrine (Eccles 2005; Latte 2007; Taverner 1999); Phenylpropanolamine (Cohen 1978) |
| Primary outcome: overall patient well‐being ‐ 3 hours after dosing |
— | — | — | (0 studies) | — | Not reported |
| Secondary outcome: all adverse events | Two single‐dose trials reported adverse events (Gronborg 1983; Taverner 1999). Both used an oral decongestant. Taverner 1999 (pseudoephedrine versus placebo) reported no adverse events in either the treatment or the placebo group; we did not include Gronborg 1983 (norephedrine versus placebo) results in the meta‐analysis because a cross‐over study design was used and several events per patient were reported | — | 82 (2 RCTs, oral) | — | Insufficient data to pool results Norephedrine (Gronborg 1983); Pseudoephedrine (Taverner 1999) |
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NAR: nasal airway resistance; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
Summary of findings 2. Summary of findings for multi‐dose nasal decongestant compared to placebo in adults with the common cold.
| Should multiple doses of decongestant in monotherapy be used for the common cold in adults? | ||||||
|
Patient or population: adult patients with the common cold Settings: common cold centres, universities and hospitals Intervention: multi‐dose decongestant in monotherapy, oral and topical decongestants combined Comparison: placebo Measure of effect: we transformed results from all studies to ensure that higher scores represent better functioning. We standardised results using the standardised mean differences (SMD). As such differences are expressed in standardised units. As a rough guide, a SMD of 0.2 to 0.49 represents a small, 0.5 to 0.79 a moderate and ≥ 0.8 a large clinical effect. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with multi‐dose decongestant | |||||
| Primary outcome: subjective symptom score (mean) ‐ 3 hours after dosing | The unstandardised mean subjective symptom score ranged from ‐7 to ‐35.79 | Subjective nasal congestion was 0.49 standard units better in the treatment group (95% CI 0.07 to 0.92; P value 0.02) | — | 94 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Xylometazoline (Eccles 2008); pseudoephedrine (Sperber 1989) |
| Primary outcome: subjective symptom score (mean) ‐ 3 hours after dosing Oral | — | — | — | 33 (1 RCT) | — | Insufficient data to pool results Pseudoephedrine (Sperber 1989) |
| Primary outcome: subjective symptom score (mean) ‐ 3 hours after dosing Topical | — | — | — | 61 (1 RCT) | — | Insufficient data to pool results Xylometazoline (Eccles 2008) |
| Primary outcome: overall patient well‐being | — | — | — | (0 studies) | — | Not reported |
| Secondary outcome: all adverse events | Study population | OR 0.98 (0.68 to 1.40) | 1195 (7 RCTs) | ⊕⊕⊝⊝ LOW 3 | Pseudoephedrine (Bye 1980; Eccles 2005; Eccles 2006; Eccles 2014; Sperber 1989); triprolidine (Bye 1980); xylometazoline (Eccles 2008) | |
| 126 per 1000 | 124 per 1000 (89 to 168) | |||||
| Secondary outcome: all adverse events Oral | Study population | OR 0.95 (0.65 to 1.39) | 1134 (6 RCTs) | ⊕⊕⊝⊝ LOW 4 | Pseudoephedrine (Bye 1980; Eccles 2005; Eccles 2006; Eccles 2014; Sperber 1989); triprolidine (Bye 1980) | |
| 121 per 1000 | 115 per 1000 (82 to 160) | |||||
| Secondary outcome: all adverse events Topical | Study population | — | 61 (1 RCT) | — | Insufficient data to pool results Xylometazoline (Eccles 2008) |
|
| — | — | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded because of possible risk of bias; random sequence generation and allocation concealment was not clear in both studies.
2Downgraded because data came from only two studies.
3Downgraded because three studies had unclear risk of bias on five out of seven domains (Eccles 2008; Latte 2007; Sperber 1989), and the estimate had a wide confidence interval (imprecision).
4Downgraded because two studies had unclear risk of bias on five out of seven domains (Latte 2007; Sperber 1989), and the estimate had a wide confidence interval (imprecision).
Summary of findings 3. Summary of findings for all doses of nasal decongestant compared to placebo in adults with the common cold.
| Should a decongestant (any dose) in monotherapy be used for the common cold in adults? | ||||||
|
Patient or population: adult patients with the common cold Settings: common cold centres, universities and hospitals Intervention: single‐dose or multi‐dose decongestant in monotherapy, oral and topical decongestants combined Comparison: placebo Measure of effect: we transformed results from all studies to ensure that higher scores represent better functioning. We standardised results using the standardised mean differences (SMD). As such differences are expressed in standardised units. As a rough guide, a SMD of 0.2 to 0.49 represents a small, 0.5 to 0.79 a moderate and ≥ 0.8 a large clinical effect. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with all doses of decongestants | |||||
| Primary outcome: subjective symptom score (mean) ‐ 3 hours after dosing | The unstandardised mean subjective symptom score ranged from ‐2.54 to ‐35.79 | Subjective nasal congestion was 0.44 standard units better in the treatment group (95% CI 0.11 to 0.78; P value 0.01) | — | 146 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Pseudoephedrine (Sperber 1989; Taverner 1999); xylometazoline (Eccles 2008) |
| Primary outcome: subjective symptom score (mean) ‐ 3 hours after dosing Oral | The unstandardised mean subjective symptom score ranged from ‐2.54 to ‐7 | Subjective nasal congestion was 0.33 standard units better in the treatment group (95% CI ‐0.11 to 0.77; P value 0.14) | — | 85 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | Pseudoephedrine (Sperber 1989; Taverner 1999) |
| Primary outcome: subjective symptom score (mean) ‐ 3 hours after dosing Topical |
— | — | — | 61 (1 RCT) | — | Insufficient data to pool results Xylometazoline (Eccles 2008) |
| Primary outcome: subjective symptom score (AUC) ‐ 3 hours after dosing Oral | The unstandardised mean AUC for the subjective symptom score ranged from 22 to ‐77.45 | Subjective nasal congestion was 0.11 standard units better in the treatment group (95% CI ‐0.14 to 0.35; P value 0.39) | — | 260 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | None of the included studies that used a topical decongestant reported the AUC for subjective symptoms of congestion Pseudoephedrine (Latte 2004; Latte 2007) |
| Primary outcome: overall patient well‐being |
— | — | — | (0 studies) | — | Not reported |
| Secondary outcome: All adverse events | Only 2 single‐dose studies reported adverse events. One study reported no events (Taverner 1999, pseudoephedrine) and the other was excluded from meta‐analyses as this was a cross‐over study (Gronborg 1983, norephedrine). Combining single‐dose and multi‐dose studies would not change the results of the multi‐dose analyses. | — | — | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; AUC: area under the curve; NAR: nasal airway resistance; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
¹Downgraded because two studies had unclear risk of bias on five out of seven domains (Eccles 2008; Sperber 1989).
²Downgraded because one study had unclear risk of bias on five out of seven domains (Sperber 1989).
³Downgraded because data came from only two studies.
⁴Downgraded because one study had unclear risk of bias on five out of seven domains (Latte 2007).
Background
Description of the condition
The common cold is viral in nature, afflicts individuals of all ages and often necessitates utilisation of over‐the‐counter and prescription medications, and complementary interventions (Simasek 2007). Often caused by the rhinovirus, people typically experience rhinorrhoea, sneezing, headache, nasal congestion, cough, fatigue and pharyngitis (Eccles 2000).
Despite the common cold not being a serious condition, it has a substantial impact in terms of time lost from work and school, as well as money spent on both prescription and over‐the‐counter medications (Heikkinnenn 2003). In the USA, the common cold contributes to 22 million missed days from school and 20 million absences from work annually, including days missed due to caring for ill children (Pappas 2015). In Australia, upper respiratory tract infections, nasal congestion, pharyngitis and cough constitute 11% of all consultations in general practice (Fry 1993). In the USA, there are 25 million visits to the family physician annually due to the common cold and the total economic impact of the common cold reached around USD 40 billion annually (Fendrick 2003).
Description of the intervention
Nasal congestion is one of the most uncomfortable symptoms experienced with the common cold (Fry 1993). There is no cure for the common cold, therefore symptomatic therapy is the only treatment option. Nasal decongestants are widely utilised for symptomatic relief in both adults and children and can be administered in oral or topical form (Del Mar 2003).
Decongestants may contain pseudoephedrine, phenylephrine, oxymetazoline or xylometazoline. Nasal decongestants are available as tablets or nasal sprays or drops. They are mostly available over‐the‐counter without restrictions (Eccles 2009). It is recommended that they should not be given to children under the age of six years (NPS Medicinewise 2012). Due to the risk of rebound congestion after stopping use of decongestants it is advised that people should not use a decongestant for longer than five days. Nasal decongestants mainly act locally but there may be systemic effects, such as hypertension. Other common side effects include headache, nausea, insomnia and dizziness (NPS Medicinewise 2012).
People taking topical nasal or ophthalmic decongestants quickly develop tachyphylaxis (a rapid decrease in the response to a drug after repeated doses over a short period of time). Long‐term use is therefore not recommended, since the agents lose effectiveness after a few days.
Previous reviews have considered the safety and efficacy of therapies for indications including seasonal and perennial allergic rhinitis, chronic rhinitis, common cold and influenza (Dolansky 2008). Many marketed treatments for the common cold exist and they may consist of multiple active agents with claimed decongestant, anti‐secretory and anti‐cough actions.
Heated, humidified air is one type of treatment intervention. The mechanism of action includes the liquefying of mucus if it is dry, thereby allowing it to be cleared more effectively. It also works by the heat of the steam killing the cold virus that may be present in the mucus. However, it is not routinely recommended as there is insufficient evidence for its use (Singh 2013).
Corticosteroids are also used for the treatment of the common cold and have been recently reviewed (Hayward 2015). Intranasal ipratropium bromide has been reviewed and was found to be effective in reducing rhinorrhoea but ineffective in reducing nasal congestion (AlBalawi 2013).
A Cochrane Review of saline nasal irrigation has reported limited evidence of its efficacy in relieving symptoms of nasal secretion and nasal congestion in upper respiratory tract infections (King 2015).
Combination medications have also been studied. For overall recovery it has been reported that combinations of antihistamines, decongestants and analgesics have proven to be more effective compared to placebo (De Sutter 2012). There was only a modest effect of oral antihistamine‐decongestant combinations, oral decongestant‐analgesic combinations and oral antihistamine‐decongestant‐analgesic combinations on nasal congestion. Only oral analgesic combinations seemed to have no effect on the symptoms of nasal congestion.
Since these medications for the common cold have already been previously researched, this review will focus on nasal decongestants.
How the intervention might work
Nasal decongestants are sympathomimetic amines that stimulate the alpha‐adrenergic receptors leading to vasoconstriction in the blood vessels supplying the upper respiratory tract structures (NPS Medicinewise 2012; Wicker 2009). This results in a net reduction in oedema and nasal secretions and hence easier breathing.
Why it is important to do this review
This systematic review studied the efficacy and safety of nasal decongestants in people with the common cold. This review will provide evidence‐based guidance to clinicians and people with the common cold.
Objectives
To assess the efficacy, and short‐ and long‐term safety, of nasal decongestants used in monotherapy to alleviate symptoms of the common cold in adults and children.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and cluster‐RCTs or randomised cross‐over studies comparing nasal decongestants with placebo. We excluded quasi‐RCTs.
Types of participants
Adults and children of all ages and either gender with the common cold, characterised by defined symptoms of an upper respiratory tract infection (URTI), were eligible for inclusion. We included participants who had symptoms for no more than seven days prior to the start of the study. We excluded studies where another upper respiratory condition (such as influenza, sinusitis or rhinitis) had been diagnosed.
Types of interventions
Oral or topical nasal decongestants versus placebo (oral or spray, as appropriate).
We included trials using topical and oral nasal decongestants administered as aqueous spray, drops, dry powder, tablets or capsules. We focused on nasal decongestants only, which work by stimulating the alpha‐adrenergic receptors in upper respiratory tract blood vessels, leading to vasoconstriction (Wicker 2009). We excluded studies reporting combined interventions such as warm humidified air, steam, aromatic vapours, inhaled corticosteroids and interventions using menthol.
Types of outcome measures
Primary outcomes
Subjective symptom scores for nasal congestion (self‐reported scores of congestion).
Overall patient well‐being score (self‐reported).
Secondary outcomes
Objective measures of nasal airway resistance (NAR).
Adverse events (for example, dry mucous membranes, rebound congestion).
Complications (for example, sinusitis, otitis media, lower respiratory tract infections).
Time to full recovery.
Time to return to school or work.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 6 June, 2016), which contains the Cochrane Acute Respiratory Infections (ARI) Specialised Register, MEDLINE (1946 to July 15 July 2016), Embase (2010 to 15 July 2016), CINAHL (1981 to 15 July 2016), LILACS (1982 to 15 July 2016) and Web of Science (1955 to 15 July 2016).
We used the search strategy as outlined in Appendix 1 to search MEDLINE and CENTRAL. We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE (Lefebvre 2011; Appendix 1). We adapted the search strategy to search Embase (Appendix 2), CINAHL (Appendix 3), LILACS (Appendix 4) and Web of Science (Appendix 5). There were no language or publication restrictions.
Searching other resources
We searched www.clinicaltrials.gov and www.anzctr.org.au to identify completed and ongoing trials (July 2016). We reviewed reference lists and contacted researchers in the field to identify further relevant studies. We contacted manufacturers of nasal decongestants for unpublished studies.
Data collection and analysis
This review is based on our published protocol (Ta'i 2012).
Selection of studies
Two review authors (LD, NM) independently reviewed and applied the inclusion and exclusion criteria to the titles and abstracts identified by the search. We retrieved the full text if there was insufficient information in the titles or abstracts to exclude a study. Three review authors (LD, NM, LG) reviewed full‐text articles, ensuring that two review authors independently judged each article. We consulted a fourth review author (MLvD) if there was any discrepancy between the two authors and the issue was resolved by discussion. The review authors were not blinded to information about the article, such as the journal title, the authors of the articles or the results.
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1; Moher 2009) and Characteristics of excluded studies table. We did not impose any language restrictions.
1.
Study flow diagram
Data extraction and management
Three review authors (LD, NM, LG) independently extracted data from all included articles using pre‐designed data extraction forms. A fourth author (MLvD) assisted in reaching a consensus if data entries differed. We extracted the following data:
First author, publication year, journal.
Number, age and gender distribution of the patients included in the trial.
Case definitions (symptoms and measurements).
Type, dosage, duration and route of administration of nasal decongestant.
Results (primary and secondary outcomes).
If a paper did not provide sufficient information about either study details or results, we contacted the authors where possible.
Assessment of risk of bias in included studies
Three review authors (LD, NM, LG) independently assessed risk of bias. We resolved disagreements by discussion with an arbiter (MLvD). We assessed:
random sequence generation;
allocation concealment;
blinding of participants and personnel (if relevant);
blinding of outcome assessors;
incomplete outcome data;
dropout/selective outcome reporting; and
other potential sources of bias.
We judged each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in 'Risk of bias' tables. We summarised the risk of bias judgements across different studies for each of the domains listed. We reported the risk of bias using the 'Risk of bias' tool from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
We reported continuous data as the standardised mean difference (SMD) because subjective and objective measures of congestion were measured on different scales. The SMD adjusts for the differences in measurement scales and enables data from different scoring systems to be pooled; it is the absolute mean difference divided by the standard deviation (SD). Dichotomous outcomes were reported as odds ratios (ORs). SMDs and ORs were generated by RevMan software (RevMan 2014). We calculated 95% confidence intervals (CIs) for each estimate.
Unit of analysis issues
We analysed the outcomes of the individual participants of each trial. If the unit of randomisation was not the same as the level of analysis (i.e. the individual participants), such as in cluster‐RCTs, we planned to make adjustments by taking into account the impact of clustering as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
If a trial included more than one treatment arm that was similar (such as different doses of the same nasal decongestant), we combined data from the treatment arms that were similar and compared this group to the control group, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, section 7.7.3.8 and Table 7.7a (Higgins 2011).
For studies using a cross‐over design, we reported results separately and did not include them in the meta‐analysis.
Dealing with missing data
We were unable to obtain additional data from study authors (many studies were quite old and authors could not be contacted). Therefore, where possible, we compared studies that used an intention‐to‐treat (ITT) analysis (assuming that all missing data represented unsuccessful outcomes) to those not reporting ITT analysis (on‐treatment analysis) in a sensitivity analysis to assess the potential impact of missing data on the overall effect of treatment.
Assessment of heterogeneity
We assessed heterogeneity in two ways. First, we explored the presence of heterogeneity at face value by comparing population groups, interventions or outcomes across studies. In the case of clear face value heterogeneity we reported the outcomes of the studies as in a systematic review but we did not pool the results. If there was no obvious heterogeneity we used statistical tests such as the Cochrane Chi² (Q) test and the I² statistic to determine the presence and level of statistical heterogeneity for each outcome (Higgins 2003). We considered an I² statistic of 60% or more to represent important heterogeneity. Where possible we explored the causes of statistical heterogeneity using subgroup and sensitivity analyses. We specified a priori that we would not carry out a meta‐analysis if heterogeneity was greater than 90% and there was too much variation in the results, particularly inconsistency in the direction of the effect.
Assessment of reporting biases
We did not identify more than 10 studies for any of the outcome measures. Therefore, it was not possible to assess reporting bias using funnel plots as described in section 10.4.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011).
Data synthesis
We analysed single‐dose and multi‐dose studies separately as well as combined. Clinically, we expect that a single or multi‐dose of nasal decongestant would have a similar effect, although it may not be as long lasting in the case of a single dose. Therefore, we combined measurements of single and multi‐dose studies for up to three hours after dosing.
We included results from studies that met the inclusion criteria and reported the selected outcomes in the meta‐analysis. We calculated the summary weighted OR and 95% CI for dichotomous secondary outcomes using the inverse of the variance of each study result for weighting. We standardised the results of the studies to a uniform scale when looking at continuous outcomes. In this case, we used the SMD to express the size of the intervention effect in each study relative to the variability observed in that study. We planned to calculate the number needed to treat to benefit (NNTB) for an additional beneficial outcome using the summary OR and the average control event rate described in the relevant studies. However, this was not possible because all studies assessed improvement in nasal congestion on a continuous scale. We performed fixed‐effect meta‐analyses and random‐effects meta‐analyses and compared the two models. We reported any differences between the models, but throughout all analyses we used a random‐effects model for final reporting.
There is no defined minimal clinically important difference for measures of subjective improvement in nasal congestion, therefore we used the SMDs as a guide. However, SMDs are difficult to interpret and several options are available for re‐expressing SMDs: every method has its benefits and pitfalls. For this review we decided to use rules of thumb for effect sizes as a guide: 0.2 to 0.49 represents a small effect, 0.5 to 0.79 a moderate effect and ≥ 0.8 a large effect, as described in section 12.6.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011).
GRADE and 'Summary of findings' tables
We created 'Summary of findings' tables using the following outcomes: subjective symptom scores for nasal congestion, overall patient well‐being and adverse events. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it relates to the studies that contributed data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We included the following comparisons: single‐dose decongestant versus placebo, multi‐dose decongestant versus placebo and all doses versus placebo. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using the GRADEproGDT software (GRADEproGDT 2015). We justified all decisions to downgrade or upgrade the quality of studies using footnotes, and we made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We decided a priori that, if sufficient data were available, we would conduct the following subgroup analyses to explore differential treatment effects.
Children (aged up to 12 years) versus others (aged over 12 years).
Topical versus oral nasal decongestants.
Sensitivity analysis
We decided a priori to perform sensitivity analyses to assess the impact of heterogeneity on the overall outcome (pooled estimate) of the meta‐analysis. We did this by gradually removing single trials to investigate the extent to which they contributed to heterogeneity. We also used sensitivity analyses to assess the impact of risk of bias on the overall pooled estimate by first pooling the studies with low risk of bias and then gradually adding the studies assessed as having a high risk of bias.
Results
Description of studies
All results are based on published data only. More information about the studies is presented in Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification and Characteristics of ongoing studies.
Results of the search
We retrieved 888 records with duplicates removed from the searches of the electronic databases (CENTRAL 285, MEDLINE 364, Embase 427, CINAHL 70, LILACS 3 and Web of Science 216). Based on screening of titles and abstracts, we excluded 847 records; we assessed the full text of the remaining 41 articles for eligibility (Figure 1). We excluded 25 studies based on the full text. Two studies have not yet been classified (NCT00452270; NCT01062360), and two studies are ongoing (EUCTR2006‐006690‐25‐GB; NCT01744106). The reasons for the exclusion of the 25 excluded studies are shown in Characteristics of excluded studies table.
Included studies
We included 16 references to 15 RCTs (Eccles 2008 was published as a full paper as well as an abstract). The interventions consisted of single doses (N = 6) (Akerlund 1989; Cohen 1978; Ferguson 1997; Gronborg 1983; Latte 2004; Taverner 1999), and multiple doses (N = 9) of nasal decongestants (Bye 1980; Eccles 2005; Eccles 2006; Eccles 2008; Eccles 2014; Jawad 1998; Latte 2007; Reinecke 2005; Sperber 1989). Some interventions included a treatment arm with combination therapy (e.g. pseudoephedrine plus paracetamol). However, in this review we focused on the effectiveness of nasal decongestants only. Therefore, we did not include the treatment arms that considered combination therapy.
Design
The included trials were randomised and placebo‐controlled. With the exception of one (Jawad 1998), all trials were double‐blinded. Fourteen of the RCTs were parallel‐group studies, and only one cross‐over design trial was included (Gronborg 1983).
Sample sizes
The included trials involved 2596 participants, including all treatment groups, as well as those receiving combination therapy. When participants receiving combination therapy or other drugs (e.g. paracetamol only) were excluded, the total number of participants was 1838.
Setting
The studies were conducted in the United States (Cohen 1978; Sperber 1989), United Kingdom (Bye 1980; Eccles 2005; Eccles 2006; Eccles 2008; Eccles 2014; Ferguson 1997; Jawad 1998), Sweden (Akerlund 1989), Denmark (Gronborg 1983), Germany (Reinecke 2005) and Australia (Latte 2004; Latte 2007; Taverner 1999). Six studies from the UK were conducted at the Common Cold Centre of Cardiff University (Eccles 2005; Eccles 2006; Eccles 2008; Eccles 2014; Ferguson 1997; Jawad 1998), and the three Australian studies were conducted at the University of Adelaide and the Royal Adelaide Hospital (Latte 2004; Latte 2007; Taverner 1999). The remaining studies were conducted in a university (Gronborg 1983; Sperber 1989), or hospital setting (Akerlund 1989; Cohen 1978). The setting was unclear in two studies (Cohen 1978; Reinecke 2005).
Participants
The participants of six trials were recruited from the community via poster advertisements (Eccles 2005; Eccles 2006; Gronborg 1983; Jawad 1998; Latte 2007; Taverner 1999), one of which only advertised in a students’ magazine (Gronborg 1983). Two trials recruited males undergoing military training (Akerlund 1989), and staff from a charity foundation (Bye 1980). Recruitment procedures were unclear in seven trials (Cohen 1978; Eccles 2008; Eccles 2014; Ferguson 1997; Latte 2004; Reinecke 2005; Sperber 1989).
With one exception, all studies included adult participants only (Reinecke 2005). The cut‐off in 10 studies was 18 years of age (Akerlund 1989; Cohen 1978; Eccles 2005; Eccles 2006; Eccles 2008; Gronborg 1983; Jawad 1998; Latte 2004; Latte 2007; Taverner 1999); five studies did not provide an age range (Bye 1980; Cohen 1978; Eccles 2014; Ferguson 1997; Sperber 1989). In most trials the mean age was under 25 years (Akerlund 1989; Eccles 2005; Ferguson 1997; Gronborg 1983; Jawad 1998; Latte 2004; Sperber 1989); mean ages were 26 years and 30 years respectively in Taverner 1999 and Bye 1980. Six trials did not provide the mean age (Cohen 1978; Eccles 2006; Eccles 2008; Eccles 2014; Latte 2007; Reinecke 2005). Reinecke 2005 was the only study to include younger people (participants had to be older than 12 years; however, the mean age of included participants was not provided.
Thirteen studies clearly defined cut‐offs for the time since onset of the common cold (Akerlund 1989; Cohen 1978; Eccles 2005; Eccles 2006; Eccles 2008; Eccles 2014; Ferguson 1997; Gronborg 1983; Jawad 1998; Latte 2004; Latte 2007; Reinecke 2005; Taverner 1999). Ten studies used cut‐off durations of less than three days (Akerlund 1989; Cohen 1978; Eccles 2005; Eccles 2006; Eccles 2008; Eccles 2014; Gronborg 1983; Latte 2004; Latte 2007; Reinecke 2005); three studies used cut‐offs of less than five days (Ferguson 1997; Jawad 1998; Taverner 1999). One study counted the total number of colds over the period of a year and did not specify the duration between onset of symptoms and enrolment in the study (Bye 1980); another experimentally induced the common cold via intranasal rhinovirus challenge (Sperber 1989). The duration of follow‐up varied from one to 10 days. All six single‐dose studies measured the effectiveness of a nasal decongestant on the same day and, thus, had a follow‐up of one day (Akerlund 1989; Cohen 1978; Ferguson 1997; Gronborg 1983; Latte 2004; Taverner 1999). The remaining multi‐dose studies had follow‐up of one (Jawad 1998), three (Eccles 2005; Eccles 2006; Eccles 2014), four (Latte 2007; Sperber 1989), or 10 days (Bye 1980; Eccles 2008; Reinecke 2005).
Ten included RCTs clearly defined the inclusion criteria for cold symptoms (Akerlund 1989; Eccles 2005; Eccles 2006; Eccles 2008; Eccles 2014; Ferguson 1997; Gronborg 1983; Jawad 1998; Latte 2004; Taverner 1999). Six studies used an objective criterion (e.g. nasal obstruction as measured by posterior rhinomanometry) (Akerlund 1989; Eccles 2005; Eccles 2006; Eccles 2014; Ferguson 1997; Taverner 1999), whereas four trials used a certain number of symptoms or a subjective measure as cut‐off (Eccles 2008; Gronborg 1983; Jawad 1998; Latte 2004). Ferguson 1997, Bye 1980, Latte 2007 and Reinecke 2005 did not clearly describe a diagnostic criterion.
Interventions
Nine trials used pseudoephedrine (Bye 1980; Eccles 2005; Eccles 2006; Eccles 2014; Jawad 1998; Latte 2004; Latte 2007; Sperber 1989; Taverner 1999), and three investigated oxymetazoline (Akerlund 1989; Ferguson 1997; Reinecke 2005). Others used xylometazoline (Eccles 2008), phenylpropanolamine (Cohen 1978), or norephedrine (Gronborg 1983). However, in 2000, the US Food and Drugs Administration (FDA) issued a public health advisory recommending that phenylpropanolamine (also known as norephedrine) should not be considered safe for over‐the‐counter use and asked the drug manufacturers to voluntarily discontinue marketing products containing phenylpropanolamine (FDA 2000). As a consequence, phenylpropanolamine is no longer available as a decongestant in most countries. Therefore, we excluded Cohen 1978 and Gronborg 1983 from the meta‐analyses.
Seven studies that used pseudoephedrine generally administered multiple doses (Bye 1980; Eccles 2005; Eccles 2006; Eccles 2014; Jawad 1998; Latte 2007; Sperber 1989), and all used oral tablets, with the exception of Eccles 2014 and Sperber 1989 who used granule sachets and oral capsules respectively. Of the remaining six studies that did not use pseudoephedrine, four used a single dose of medication (Akerlund 1989; Cohen 1978; Ferguson 1997; Gronborg 1983). Only four studies used a topical decongestant (Akerlund 1989; Eccles 2008; Ferguson 1997; Reinecke 2005). See Table 4 for an overview.
1. Study characteristics.
| Reference | Single/multi‐dose study | Decongestant | Mode of administration | Follow‐up | Comments |
| Akerlund 1989 | Single | Oxymetazoline | Topical | 1 day | — |
| Ferguson 1997 | Single | Oxymetazoline | Topical | 1 day | Excluded from meta‐analyses because insufficient details were provided to standardise the results |
| Gronborg 1983 | Single | Norephedrine | Oral | 1 day | Excluded from meta‐analyses because a cross‐over design was used and because norephedrine (phenylpropanolamine) is no longer available on the market |
| Cohen 1978 | Single | Phenylpropanolamine | Oral | 1 day | Excluded from meta‐analyses because phenylpropanolamine is no longer available on the market |
| Taverner 1999 | Single | Pseudoephedrine | Oral | 1 day | — |
| Latte 2004 | Single | Pseudoephedrine | Oral | 1 day | — |
| Eccles 2008 | Multiple | Xylometazoline | Topical | Max 10 days | — |
| Eccles 2005 | Multiple | Pseudoephedrine | Oral | 3 days | — |
| Eccles 2006 | Multiple | Pseudoephedrine | Oral | 3 days | — |
| Eccles 2014 | Multiple | Pseudoephedrine | Oral | 3 days | — |
| Sperber 1989 | Multiple | Pseudoephedrine | Oral | 4 days | — |
| Bye 1980 | Multiple | Pseudoephedrine | Oral | 10 days | — |
| Jawad 1998 | Multiple | Pseudoephedrine | Oral | 1 day | — |
| Reinecke 2005 | Multiple | Oxymetazoline | Topical | 10 days | — |
| Latte 2007 | Multiple | Pseudoephedrine | Oral | 4 days | — |
Outcomes
Seven RCTs reported nasal airway resistance (NAR) as the primary outcome (Akerlund 1989; Cohen 1978; Eccles 2005; Eccles 2008; Gronborg 1983; Latte 2007; Taverner 1999). Two studies reported nasal airway conductance (NAC) as the primary outcome, which is the inverse of NAR (Eccles 2006; Eccles 2014). The benefit of NAC over NAR is that with NAC it is possible to collect data from participants with total nasal obstruction (= zero conductance), whereas resistance would tend towards infinity. Other primary outcome measures were severity of subjective symptoms (Bye 1980; Sperber 1989), nasal nitric oxide levels (Ferguson 1997), nasal volume (Latte 2004), minimum and maximum airflow (Jawad 1998), and numbers of days until full recovery (Reinecke 2005). NAR was measured by a rhinomanometry test, which assesses nasal airflow obstructions by measuring pressure and flow during normal inspiration and expiration. Most studies used posterior rhinomanometry, where both nostrils are measured simultaneously (Cohen 1978; Eccles 2005; Eccles 2006; Eccles 2008; Eccles 2014; Ferguson 1997; Gronborg 1983; Latte 2007). Only one study used anterior rhinomanometry only, which measures one nostril at a time (Akerlund 1989). Latte 2004 and Taverner 1999 used both posterior and anterior rhinomanometry, and Jawad 1998 used posterior rhinomanometry, but each nostril was assessed separately by alternately occluding each nostril with surgical tape.
Subjective symptom scores for congestion were often reported as secondary outcome measurements (Akerlund 1989; Cohen 1978). In total, 12 of 15 studies reported subjective symptom scores for congestion (Akerlund 1989; Bye 1980; Cohen 1978; Eccles 2005; Eccles 2008; Eccles 2014; Gronborg 1983; Jawad 1998; Latte 2004; Latte 2007; Sperber 1989; Taverner 1999). Subjective symptom scores were either reported on a Likert scale of severity (ranging from 4 to 7 points) (Akerlund 1989; Bye 1980; Cohen 1978; Eccles 2014; Gronborg 1983; Jawad 1998; Sperber 1989; Taverner 1999), or on a 100 mm visual analogue scale (VAS) where 0 mm represented complete nasal patency and 100 mm represented complete nasal blockage (Eccles 2005; Eccles 2008; Latte 2004; Latte 2007).
Two studies also measured the time to onset of subjective relief (Eccles 2008; Reinecke 2005), but other preselected outcomes such as overall well‐being, complications, time to full recovery and time to return to school or work were not reported. Most included studies also reported the frequency of adverse effects (Bye 1980; Eccles 2005; Eccles 2006; Eccles 2008; Eccles 2014; Gronborg 1983; Latte 2007; Sperber 1989; Taverner 1999). Reinecke 2005 stated that adverse events were measured but these were not reported.
Funding
Nine RCTs clearly stated funding sources. These were usually commercial entities. Funding involved pharmaceutical companies such as Pfizer Consumer HealthCare group (Eccles 2005; Latte 2007), Procter and Gamble Company (Ferguson 1997; Taverner 1999), Bayer HealthCare LLC (Eccles 2014), GlaxoSmithKline (Eccles 2006), Novartis (Eccles 2008), H. Lundbeck and Co (Gronborg 1983), Richardson‐Vicks and the Aspirin Foundation of America (Sperber 1989). Five trials did not indicate sources of funding (Akerlund 1989; Bye 1980; Cohen 1978; Jawad 1998; Reinecke 2005); the source of funding for Latte 2004 was not clear, but the treatment medication was provided by Pfizer Consumer HealthCare Group.
Excluded studies
We excluded 25 trials. Ten trials were excluded because the study participants’ symptoms of nasal congestion or obstruction were for reasons other than the common cold (e.g. allergic rhinitis) (Akerlund 1989; Ashe 1968; Bailey 1969; Bende 1984; Bende 1985; Castellano 2002; Connell 1969; Pritchard 2014; Tzachev 2002; Zumpft 1975). We excluded five studies due to lack of randomisation (Anderson 1956; Anonymous 1975; Katrana 1956; McElhenney 1966; Smith 1999), three due to lack of a placebo control group (Dorn 2003; Fox 1967; Meurman 1975), and four because only combination therapy was used or reported (Cohen 1977; De Paula Neves 1966; Rumiantsev 1993; Weisberg 1966). Other reasons included symptom duration of more than six months (Broms 1982), and not measuring any of the predefined outcomes (Hummel 1998; Winther 1983).
Ongoing studies
We identified two ongoing trials. Both are commercially funded, double‐blind RCTs involving participants with the common cold and use of nasal decongestants. EUCTR2006‐006690‐25‐GB is a parallel‐group study entered into the European clinical trials register in 2007. This study investigates the changes in nasal conductance in participants aged over 18 years with the decongestant xylometazoline. No information on the expected end date of this study was provided. NCT01744106 is a multicentre study that began in November 2012 involving the response of nasal congestion severity in children between the ages of six and 11 years to the decongestant pseudoephedrine. The expected completion date was April 2015, however, this was changed to May 2016.
Studies awaiting classification
Two studies are awaiting classification (NCT00452270; NCT01062360). Both are commercially funded, double‐blind RCTs involving the response of nasal congestion in participants aged over 18 years with the common cold to nasal decongestants (xylometazoline and pseudoephedrine). Although both studies were completed before 2011, we were unable to find their published results.
Risk of bias in included studies
We assessed all included trials using the six specific domains detailed in the Cochrane Handbook of Systematic Review of Interventions 'Risk of bias' tool (Higgins 2011). The results of this assessment are shown for each study in Figure 2 and summarised in Figure 3. Details of the included studies are presented in Characteristics of included studies. Overall, most judgements were unclear due to lack of detail provided in the trials reports. For example, clear and detailed descriptions of the methods of sequence generation, allocation concealment and blinding were often missing. Many studies also had pharmaceutical company funding of unknown significance. We deemed only one study to be high risk in any of the six domains (Jawad 1998); this was because the study was not blinded and therefore assessed as high risk in both blinding domains.
2.
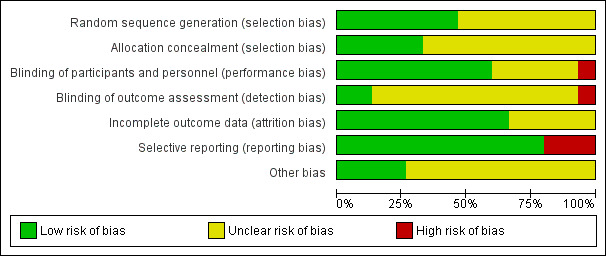
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages for all included studies
3.
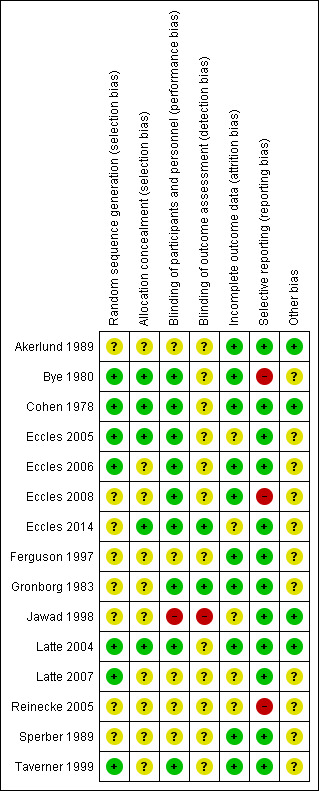
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation
We assessed seven studies as low risk in this domain, as the authors referenced a randomisation schedule or the method of sequence generation was described in detail (Bye 1980; Cohen 1978;Eccles 2005;Eccles 2006;Latte 2004;Latte 2007;Taverner 1999). For example, "Treatment randomisation was from a random numbers table, in blocks of four" (Latte 2004).
We assessed the remaining eight studies as unclear risk for this domain because methods of random sequence generation were not described (Akerlund 1989;Eccles 2008; Eccles 2014;Ferguson 1997;Gronborg 1983;Jawad 1998;Reinecke 2005; Sperber 1989).
Allocation concealment
The method of allocation concealment was poorly described, or insufficient detail was provided to enable definitive judgement on how concealment was achieved. We assessed five studies to be low risk in the allocation concealment domain (Bye 1980; Cohen 1978;Eccles 2005; Eccles 2014;Latte 2004). For example, "it was not possible to distinguish between combination product, monotherapies, and placebo granules…they had the same appearance, taste, and no noticeable smell" (Eccles 2014). We assessed the remaining 10 studies as unclear risk because none provided the methods of allocation concealment in the text (Akerlund 1989; Eccles 2006; Eccles 2008; Ferguson 1997; Gronborg 1983; Jawad 1998; Latte 2007; Reinecke 2005; Sperber 1989; Taverner 1999).
Blinding
Blinding of participants and personnel
We assessed nine studies to be low risk in this domain as blinding of participants and key study personnel was ensured and it was unlikely that the blinding could have been broken (Bye 1980; Cohen 1978; Eccles 2005; Eccles 2006; Eccles 2008; Eccles 2014; Gronborg 1983; Latte 2004; Taverner 1999). For example, "the randomisation code was not broken until all data, including delayed adverse events, had been allocated" (Taverner 1999). We assessed five studies as unclear risk because insufficient information was provided to permit a judgement of risk (Akerlund 1989; Ferguson 1997; Latte 2007; Reinecke 2005; Sperber 1989). We assessed the remaining study as high risk because the it was not blinded (Jawad 1998).
Blinding of outcome assessment
We assessed two studies to be low risk in this domain as both had stated methods of blinding of outcome assessment (Eccles 2014; Gronborg 1983). We assessed 12 studies as unclear risk, because there was insufficient information on the methods of blinding outcome assessment to permit judgement (Akerlund 1989; Bye 1980; Cohen 1978; Eccles 2005; Eccles 2006; Eccles 2008; Ferguson 1997; Latte 2007; Latte 2004; Reinecke 2005; Sperber 1989; Taverner 1999). We assessed the remaining study as high risk because it was not blinded (Jawad 1998).
Incomplete outcome data
We assessed 10 studies to be low risk for this domain. All participants, including those who discontinued, were accounted for in the text and the authors clearly indicated the numbers of remaining participants per treatment group (Akerlund 1989; Bye 1980; Cohen 1978; Eccles 2006; Eccles 2008; Ferguson 1997; Gronborg 1983; Latte 2004; Sperber 1989; Taverner 1999). We assessed five studies as unclear risk (Eccles 2005; Eccles 2014; Jawad 1998; Latte 2007; Reinecke 2005). Numbers of remaining participants in each treatment group could not be ascertained.
Selective reporting
We assessed 12 studies as low risk for selective reporting; these studies reported all intended outcomes (Akerlund 1989; Cohen 1978; Eccles 2005; Eccles 2006; Eccles 2014; Ferguson 1997; Gronborg 1983; Jawad 1998; Latte 2004; Latte 2007; Sperber 1989; Taverner 1999). We assessed three studies to be at high risk of selective reporting bias (Bye 1980; Eccles 2008; Reinecke 2005). Bye 1980 reported only significant results in detail; Eccles 2008 reported NAC rather than NAR (Eccles 2008).
Other potential sources of bias
We assessed four studies to be at low risk of other potential sources of bias; there was no evidence of pharmaceutical company funding or any other sources of bias identified in these studies (Akerlund 1989; Cohen 1978; Jawad 1998; Latte 2004). We assessed 11 studies to be unclear risk; nine reported receiving pharmaceutical company (Eccles 2005; Eccles 2006; Eccles 2008; Eccles 2014; Gronborg 1983; Latte 2007; Sperber 1989) or commercial funding (Ferguson 1997; Taverner 1999). Reinecke 2005 was described very briefly and there was not enough detail to exclude the possibility of other bias. In Bye 1980, participants were monitored for a six‐month period and had between one and four colds in this time. It is not clear from the text if participants with multiple colds were re‐randomised to a treatment group or continued on their original assigned treatment.
Effects of interventions
See: Table 1; Table 2; Table 3
See Table 1, Table 2 and Table 3 for the primary outcome, subjective symptom scores for nasal congestion, and our main comparisons: single‐dose nasal decongestant versus placebo, multi‐dose nasal decongestant versus placebo and all doses of nasal decongestant versus placebo.
There was considerable variability in the way outcomes were reported. The primary outcome, subjective nasal congestion, was measured on Likert or visual analogue scales (VAS) with different levels. Similarly, the secondary outcome, objective measurement of nasal congestion, was reported as the mean nasal airway resistance (NAR) (Akerlund 1989; Eccles 2005), the mean difference of NAR (Cohen 1978; Taverner 1999), the area under the curve (AUC) for the different NAR measurements from baseline to a certain follow‐up (Latte 2004; Latte 2007), the mean nasal airway conductance (NAC) (Eccles 2006; Jawad 1998), and the least square mean of NAC (Eccles 2008). NAC is the inverse of NAR, however the methods of calculating the NAC were not described, making it impossible to recalculate the NAR or vice versa. Given these differences, we were unable to combine different statistical representations (e.g. mean and mean difference) as described in section 9.4.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011). Therefore, results are presented for each outcome type separately and only SMDs are presented for all analyses to enable straightforward comparison of the effect size. In some studies a positive score indicated better nasal patency, whereas in other studies a negative score reflected better functioning. We transformed the negative scores (e.g. by multiplying by ‐1) so that for all comparisons a higher score reflected better functioning and studies could be combined. One study included more than one treatment arm (four different doses of the same nasal decongestant); we combined data from treatment arms that were similar and compared this group to the control group (Akerlund 1989).
Heterogeneity was not greater than 90% for any analyses. We tested and reported differences between using fixed‐effect and random‐effects models, but we applied the random‐effects model as the final model for all analyses. The random‐effects model generates wider confidence intervals (CIs) than the fixed‐effect model (Higgins 2011).
We present results for single and multi‐dose studies separately as well as all dosages combined for each outcome. We evaluated the effectiveness of a nasal decongestant compared to placebo approximately three hours after the last dose. We chose the timeframe of three hours because clinically we expect that a single or multi‐dose of nasal decongestant would have a similar effect, although it may not be as long lasting in the case of a single dose. Furthermore, most multi‐dose studies measured nasal decongestant effectiveness approximately three hours after the last dose. We discuss the results for this comparison only where both single and multi‐dose studies were available for the same outcome. Otherwise, we refer to the results for single or multi‐dose comparisons separately. Furthermore, some multi‐dose studies also reported outcomes after a single dose. In this comparison only the results after multiple doses are included otherwise the study would be counted twice. If possible, we also present results for studies that used an oral or topical decongestant separately and combined.
Primary outcomes
1. Subjective symptom scores for nasal congestion (self‐reported scores of congestion)
1.1. Single‐dose decongestant versus placebo
Six trials were single‐dose studies (Akerlund 1989; Cohen 1978; Ferguson 1997; Gronborg 1983; Latte 2004; Taverner 1999), two used a topical decongestant (Akerlund 1989; Ferguson 1997), and four used an oral decongestant (Cohen 1978; Gronborg 1983; Latte 2004; Taverner 1999). Four of the nine multi‐dose studies also reported results after a single dose of nasal decongestant (Eccles 2005; Eccles 2006; Eccles 2008; Latte 2007). Of these multi‐dose studies, only Eccles 2008 used a topical decongestant. As such, 10 studies compared a single dose of nasal decongestant with placebo, three of which used a topical decongestant. We have differentiated between single‐dose studies and studies reporting after a single dose. The effectiveness of the nasal decongestant was tested between 15 minutes and 10 hours after dosing. Given the large diversity in time points and methods we were unable to pool results. Results for all time points are reported in more detail for each study separately in the following sections.
1.1.1 10 or 15 minutes after dosing
Two studies measured the immediate effect of a nasal decongestant versus placebo 10 minutes (Akerlund 1989, topical decongestant) or 15 minutes (Cohen 1978, oral decongestant) after a single administration. We were unable to pool results because Cohen 1978 was excluded from all meta‐analyses. For both studies, the estimated standardised mean difference (SMD) between treatment and placebo was statistically significant and in favour of the treatment group (SMD 0.88, 95% CI 0.23 to 1.53; 40 participants; Cohen 1978, oral decongestant) (SMD 0.51, 95% CI 0.03 to 0.99; 106 participants; Akerlund 1989, topical decongestant). The SMDs corresponded to a large and moderate effect respectively.
1.1.2 30 minutes after dosing
Two studies assessed the effectiveness of treatment after 30 minutes (Cohen 1978; Taverner 1999). Both studies used an oral decongestant. We were unable to pool results because Cohen 1978 was excluded from all meta‐analyses. Only for Cohen 1978 was the estimated SMD between treatment and placebo statistically significant and in favour of the treatment group (SMD 0.88, 95% CI 0.23 to 1.53; 40 participants; Cohen 1978, oral decongestant) (SMD 0.46, 95% CI ‐0.09 to 1.01; 52 participants; Taverner 1999, oral decongestant). SMDs corresponded to a large and small effect respectively.
1.1.3 One hour after dosing
Three studies measured the effectiveness of a nasal decongestant subjectively one hour after dosing (Akerlund 1989; Cohen 1978; Taverner 1999). However, we were unable to pool results. Akerlund 1989 used a topical decongestant and showed a small clinical effect that was not significantly different between treatment and placebo (SMD 0.22, 95% CI ‐0.25 to 0.70; 106 participants; Akerlund 1989, topical decongestant). Cohen 1978 and Taverner 1999 used an oral decongestant and showed a large and moderate clinical effect that was statistically significant (SMD 0.88, 95% CI 0.23 to 1.54; 40 participants; Cohen 1978, oral decongestant) (SMD 0.72, 95% CI 0.15 to 1.28; 52 participants; Taverner 1999, oral decongestant).
1.1.4 Two hours after dosing
Three studies reported the effectiveness of a nasal decongestant compared to placebo two hours after treatment (Cohen 1978; Latte 2004; Taverner 1999). All three studies used an oral decongestant. However, we were unable to pool results. In Latte 2004 and Taverner 1999, the estimated SMD between treatment and placebo was not significant (SMD ‐0.10, 95% CI ‐0.66 to 0.47; 48 participants; Latte 2004, oral decongestant) (SMD 0.53 95% CI ‐0.03 to 1.08; 52 participants; Taverner 1999, oral decongestant). Only Cohen 1978 showed a large clinical effect that was statistically significant (SMD 0.88, 95% CI 0.23 to 1.54; 40 participants; Cohen 1978, oral decongestant).
1.1.5 Three hours after dosing
Four studies reported on the effectiveness of a nasal decongestant compared to placebo three hours after dosing (Cohen 1978; Eccles 2005; Latte 2007; Taverner 1999). All four studies used an oral decongestant. Again, we were unable to pool results. Eccles 2005 did not provide baseline values and there was insufficient information to standardise the results for comparison with the other studies. Eccles 2005 found that the AUC of the VAS between 0 and 3 hours was significantly lower for the treatment group compared to placebo participants after a single dose (P = 0.029; difference in VAS AUC ‐8.33, 95%CI ‐15.80 to ‐0.85; 236 participants; Eccles 2005, oral decongestant). However, Latte 2007 showed that the AUC of subjective congestion was not significantly different for the treatment group compared to the placebo group (SMD 0.22, 95% CI ‐0.05 to 0.49; 212 participants; Latte 2007, oral decongestant). In Cohen 1978 and Taverner 1999, the estimated SMD between treatment and placebo was not statistically significant (SMD 0.31, 95% CI ‐0.31 to 0.94; 40 participants; Cohen 1978, oral decongestant) (SMD 0.36, 95% CI ‐0.19 to 0.91; 52 participants; Taverner 1999, oral decongestant).
1.1.6 Four hours after dosing
Akerlund 1989 and Cohen 1978 also reported on the effectiveness of a nasal decongestant compared to placebo four hours after dosing. Akerlund 1989 used a topical decongestant and Cohen 1978 used an oral decongestant. We were unable to pool results because we excluded Cohen 1978 from all meta‐analyses and both studies reported different outcome measures (mean and mean difference (MD)). Neither Akerlund 1989 (SMD 0.31, 95% CI ‐0.17 to 0.79; 106 participants; topical decongestant), nor Cohen 1978 (SMD 0.40, 95% CI ‐0.23 to 1.02; 40 participants; oral decongestant) showed a statistically significant difference between the treatment and placebo groups. Furthermore, for both studies the SMD corresponded to a small clinical effect.
1.1.7 Other
Akerlund 1989, who used a topical decongestant, also reported on the effectiveness of a single dose of a nasal decongestant seven hours after dosing. The difference between treatment and placebo group participants was not statistically significant (SMD 0.10, 95% CI ‐0.37 to 0.58; 106 participants; Akerlund 1989, topical decongestant).
Gronborg 1983 is a single‐dose study that used an oral decongestant. Gronborg 1983 used a cross‐over design and, therefore, these results were not included in the meta‐analyses. Gronborg 1983 reported that during the two to 10 hours observation period after dosing the mean score for subjective nasal congestion was better for the treatment group compared to placebo (P < 0.01; 30 participants).
Results reported by Ferguson 1997 (oral decongestant) were not included in the meta‐analyses because insufficient details were provided to standardise the results, and it was not clear when NAR was measured. Ferguson 1997 reported that the NAR was improved in the treatment group (P < 0.0001) but not in the control group (P = 0.98). This study was based on 82 participants.
1.2. Multi‐dose decongestant versus placebo
1.2.1 Oral and topical decongestants combined
Subjective symptom scores were reported by five multi‐dose studies (Bye 1980; Eccles 2005; Eccles 2008; Latte 2007; Sperber 1989). Only Eccles 2008 used a topical decongestant. Latte 2007 and Eccles 2005 reported the effectiveness about three hours after the last dose. Time since the last dose was not clear in Bye 1980, Eccles 2008 and Sperber 1989. We assumed that overall subjective symptom scores were measured about three hours after the last dose because other multi‐dose studies also used this timeframe.
We were able to pool results for Eccles 2008 and Sperber 1989 (topical and oral decongestant respectively). These studies provided a mean score for subjective nasal congestion and the pooled SMD was statistically significant and in favour of the treatment group (SMD 0.49, 95% CI 0.07 to 0.92; 94 participants; two studies; Analysis 1.1). However, a SMD of 0.49 corresponds to a small clinical effect.
1.1. Analysis.
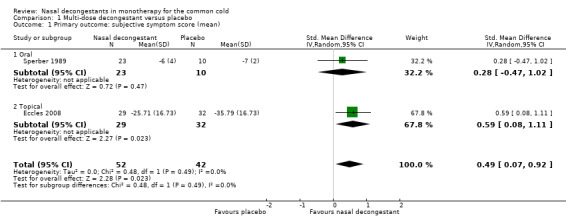
Comparison 1 Multi‐dose decongestant versus placebo, Outcome 1 Primary outcome: subjective symptom score (mean).
The difference between studies that used oral or topical decongestants was not significant (P = 0.49).
There was no major statistical heterogeneity as confirmed by an I² statistic of 0%. Nevertheless, we used a random‐effects model and this did not change our results. We judged the level of evidence to be of low quality because the pooled result was based on only two studies (imprecision) and there was possible risk of bias due to unclear reporting.
1.2.2 Oral decongestants
Sperber 1989 was the only study that reported a mean score and Latte 2007 was the only study that reported the MD. Therefore we were unable to pool these results. In both studies, the difference between subjective congestion in the treatment versus placebo groups was not statistically significant (SMD 0.28, 95% CI ‐0.47 to 1.02; 33 participants; Sperber 1989, oral decongestant) (SMD 0.15, 95% CI ‐0.12 to 0.42; 212 participants; Latte 2007, oral decongestant).
Bye 1980 and Eccles 2005 were not included in the meta‐analyses because insufficient details were provided to standardise results. Bye 1980 (140 participants) reported that there was a statistically significant improvement for subjective nasal congestion in the treatment group at the end of day one. However, no results for subjective nasal congestion were provided on days two and three and it was unclear if subjective nasal congestion in the treatment group was compared to the control group. Eccles 2005 (238 participants) reported no statistically significant difference between treatment and placebo for the AUC of the VAS between 0 and 3 hours (P = 0.79) and between 0 and 4 hours (P = 0.75) after the last dose. Only over the three‐day period was there a statistically significant improvement for the mean difference in nasal congestion score for treatment compared to placebo.
1.2.3 Topical decongestants
Only Eccles 2008 used a topical decongestant. In this study the difference between treatment and placebo was statistically significant and the SMD of 0.59 corresponded to a moderate clinical effect (SMD 0.59, 95% CI 0.08 to 1.11; 61 participants; Eccles 2008, topical decongestant).
1.3. All doses of decongestants versus placebo
1.3.1 Oral and topical decongestants combined
Four single‐dose studies (Cohen 1978; Eccles 2005; Latte 2004; Taverner 1999) and five multi‐dose studies (Bye 1980; Eccles 2005; Eccles 2008; Latte 2007;Sperber 1989) reported on subjective symptoms scores. All studies used an oral decongestant except Eccles 2008, which used a topical decongestant. However, pooling was possible for three studies only (Eccles 2008; Sperber 1989; Taverner 1999).
These three studies reported a decline in the mean subjective symptom score for nasal congestion, and the pooled effect was statistically significant (SMD 0.44, 95% CI 0.11 to 0.78; 146 participants; three studies; Analysis 2.1). However, a SMD of 0.44 corresponds to a small effect.
2.1. Analysis.
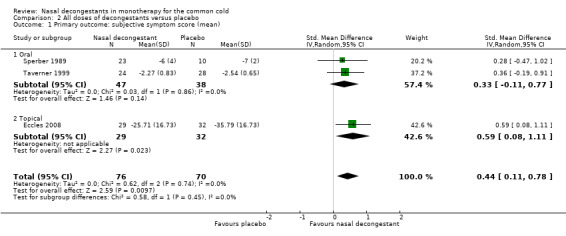
Comparison 2 All doses of decongestants versus placebo, Outcome 1 Primary outcome: subjective symptom score (mean).
Only Eccles 2008 used a topical decongestant; Sperber 1989 and Taverner 1999 used oral decongestants. The difference between studies that used oral or topical decongestants was not significant (P = 0.49).
There was no major statistical heterogeneity as confirmed by an I² statistic of 0%. As such, using a random‐effects model did not change our results. We judged the evidence to be of moderate quality due to possible risk of bias.
1.3.2 Oral decongestants
When only studies that used an oral decongestant were considered (Sperber 1989; Taverner 1999), the difference between treatment and placebo was no longer statistically significant (SMD 0.33, 95% CI ‐0.11 to 0.77; 85 participants; two studies; Analysis 2.1).
Latte 2004 and Latte 2007 reported the AUC for subjective nasal congestion; the pooled effect was very small and not statistically significant (SMD 0.11, 95% CI ‐0.14 to 0.35; 260 participants; two studies; Analysis 2.1).
There was no major statistical heterogeneity as confirmed by an I² statistic of 0% for both the mean nasal congestion and the AUC. Using a random‐effects model did not change our results. We judged the evidence to be of low quality because of possible risk of bias due to unclear reporting and imprecision.
None of the studies included in the meta‐analyses for the primary outcome measure reported intention‐to‐treat (ITT) analyses (Eccles 2008; Latte 2004; Latte 2007; Sperber 1989; Taverner 1999). Only in Eccles 2008 was the mean subjective score for nasal congestion significantly better for the treatment group compared to placebo. Eccles 2008 randomised 61 participants but five people were not dosed or analysed; reasons for exclusion were not provided. In the other studies, participants were excluded from analysis because they were unable to perform the rhinomanometry (Latte 2004), had incomplete data (Latte 2007), were infected with a wild type rhinovirus, withdrew for personal reasons (Sperber 1989), or were unable to complete the study (Taverner 1999).
1.3.3 Topical decongestants
Only Eccles 2008 used a topical decongestant. The difference between treatment and placebo was statistically significant and the SMD of 0.59 corresponded to a moderate clinical effect (SMD 0.59, 95% CI 0.08 to 1.11; 61 participants).
2. Overall patient well‐being score (self‐reported)
The included trials did not report this outcome.
Secondary outcomes
1. Objective measures of nasal airway resistance (NAR)
1.1. Single‐dose decongestant versus placebo
Objective measures of NAR were tested between 15 minutes and 10 hours after dosing. Similar to subjective measures of NAR, we were unable to pool results. Therefore, we report results for all time points in more detail for each study.
1.1.1 15 minutes after dosing
Only Cohen 1978 objectively measured the immediate effect of a nasal decongestant versus placebo 15 minutes after administration. This was assessed by the mean difference in NAR. The estimated SMD was small and the difference between the treatment and placebo groups was not statistically significant (SMD 0.42, 95% CI ‐0.21 to 1.04; 40 participants). This study used an oral decongestant.
1.1.2 30 minutes after dosing
Two studies objectively assessed the effectiveness of oral decongestant treatment after 30 minutes (Cohen 1978; Taverner 1999). In both studies, the estimated SMD between treatment and placebo groups was not statistically significant (SMD 0.21 95% CI ‐0.41 to 0.83; 40 participants; Cohen 1978), (SMD 0.08 95% CI ‐0.49 to 0.64; 48 participants; Taverner 1999).
1.1.3 One hour after dosing
Five studies objectively measured the effectiveness of a nasal decongestant one hour after dosing (Akerlund 1989; Cohen 1978; Eccles 2006; Eccles 2008; Taverner 1999). Of these, two used a topical decongestant (Akerlund 1989; Eccles 2008), and three used an oral decongestant (Cohen 1978; Eccles 2006; Eccles 2008; Taverner 1999). The reported outcome measurements varied considerably; mean scores and mean differences of NAR as well as mean scores and least square mean scores of NAC were reported. Therefore, we were unable to pool results. Akerlund 1989, Eccles 2006 and Eccles 2008 showed that the difference between treatment and placebo was statistically significant and corresponded to a moderate to large effect (SMD 0.65, 95% CI 0.14 to 1.15; 102 participants; Akerlund 1989, topical decongestant), (SMD 0.58, 95% CI 0.26 to 0.90; 153 participants; Eccles 2006, oral decongestant), (SMD 1.06, 95% CI 0.52 to 1.59; 61 participants; Eccles 2008, topical decongestant). In contrast, the estimated SMD between treatment and placebo was not statistically significant in Cohen 1978 and Taverner 1999 (SMD 0.54 95% CI ‐0.10 to 1.17; 40 participants; Cohen 1978, oral decongestant), (SMD 0.43 95% CI ‐0.14 to 1.00; 48 participants; Taverner 1999, oral decongestant).
1.1.4 Two hours after dosing
Three studies reported the effectiveness of oral nasal decongestant compared to placebo two hours after treatment (Cohen 1978; Latte 2004; Taverner 1999). Again, we were unable to pool results. Latte 2004 showed a large and statistically significant difference between treatment and placebo groups for the AUC from baseline to two hours (SMD 0.88, 95% CI 0.28 to 1.47; 48 participants; Latte 2004, oral decongestant). However, the estimated SMD between treatment and placebo was not statistically significant in Cohen 1978 and Taverner 1999 (SMD 0.27, 95% CI ‐0.35 to 0.89; 40 participants; Cohen 1978, oral decongestant), (SMD 0.17, 95% CI ‐0.40 to 0.74; 48 participants; Taverner 1999, oral decongestant).
1.1.5 Three hours after dosing
Four studies reported on the effectiveness of a nasal decongestant compared to placebo three hours after dosing (Akerlund 1989; Cohen 1978; Latte 2007; Taverner 1999). Only Akerlund 1989 used a topical decongestant and showed a large and statistically significant difference for mean NAR between treatment and placebo, in favour of the treatment group (SMD 0.74, 95% CI 0.23 to 1.25; 102 participants). None of the other studies showed a statistically significant difference between treatment and placebo (SMD 0.28, 95% CI ‐0.34 to 0.90; 40 participants; Cohen 1978, oral decongestant), (SMD 0.20, 95% CI ‐0.07 to 0.47; 212 participants; Latte 2007, oral decongestant), (SMD ‐0.30 95% CI ‐0.87 to 0.27; 48 participants; Taverner 1999, oral decongestant).
1.1.6 Four hours after dosing
Three studies reported on the effectiveness of an oral nasal decongestant compared to placebo four hours after dosing (Cohen 1978; Eccles 2005; Eccles 2006). Cohen 1978 was excluded from all meta‐analyses; all three studies reported different outcome measures (mean, MD and AUC) so we were unable to pool results. Only Eccles 2006 showed that the AUC of the NAC measurements between baseline and four hours was significantly better for the treatment group compared to the placebo group (SMD 0.54, 95% CI 0.21 to 0.86; 153 participants). The SMD corresponded to a moderate clinical effect. Cohen 1978 (SMD 0.40, 95% CI ‐0.23 to 1.02; 40 participants) and Eccles 2005 (SMD 0.19, 95% CI ‐0.06 to 0.45; 236 participants) did not report a statistically significant difference between the treatment and placebo group.
1.1.6 Other
Only Akerlund 1989 reported the effectiveness of a single dose of a nasal decongestant up to seven hours after dosing. This study used a topical decongestant and showed a small difference between treatment and placebo that was not statistically significant (SMD 0.36, 95% CI ‐0.14 to 0.86; 102 participants).
Gronborg 1983, a single‐dose oral decongestant study that used a cross‐over design, involved 30 participants. It was not included in the meta‐analyses. Gronborg 1983 found that NAR worsened in the placebo group whereas it improved in the treatment group; this difference was statistically significant (P < 0.02).
1.2. Multi‐dose decongestant versus placebo
1.2.1 Oral and topical decongestants combined
Four multi‐dose studies reported objective measurements of nasal congestion (Eccles 2005; Eccles 2008; Jawad 1998; Latte 2007). All studies except Eccles 2008 used an oral decongestant. Objective measurements of nasal congestion were represented as mean NAR (Eccles 2005), the AUC for NAR (Latte 2007), the mean NAC (Jawad 1998), and the least square mean of NAC (Eccles 2008). As all four studies reported different outcome measures, we were unable to pool results. Jawad 1998 and Latte 2007 reported the effectiveness of a nasal decongestant three hours after the last dose, and Eccles 2005 measured this four hours after the last dose. Timing since last dose was not clear in Eccles 2008. Therefore, we report the effectiveness of multi‐dose decongestants assessed approximately three hours after the last dose.
1.2.2 Oral decongestants
In Eccles 2005 (SMD 0.11, 95% CI ‐0.14 to 0.37; 230 participants), Jawad 1998 (SMD 0.23, 95% CI ‐0.39 to 0.86; 40 participants) and Latte 2007 (SMD 0.10, 95% CI ‐0.17 to 0.37; 212 participants) the effect of multiple doses of oral decongestants on objective measurements of nasal congestion was not statistically significant.
1.2.3 Topical decongestants
Of the four studies that reported objective measurements of nasal congestion, Eccles 2008 was the only study that used a topical decongestant and showed a statistically significant effect of multiple doses of nasal decongestant on objective measurements of nasal congestion: in this study expressed as the least square mean of NAC (SMD 0.89, 95% CI 0.36 to 1.41; 61 participants). The SMD of 0.89 corresponded to a significant clinical effect.
1.3. All doses of decongestants versus placebo
1.3.1 Oral and topical decongestants combined
In total, four single‐dose (Akerlund 1989; Cohen 1978; Latte 2004; Taverner 1999) and four multi‐dose studies (Eccles 2005; Eccles 2008; Jawad 1998; Latte 2007) reported on objective measures of NAR. Only one single‐dose study (Akerlund 1989) and one multi‐dose study (Eccles 2008) used a topical decongestant. Pooling was possible for four studies only (Analysis 2.3): two single‐dose studies (Akerlund 1989; Latte 2004) and two multi‐dose studies (Eccles 2005; Latte 2007).
2.3. Analysis.
Comparison 2 All doses of decongestants versus placebo, Outcome 3 Secondary outcome: objective NAR (mean).
Two studies reported a reduced mean NAR compared with placebo (Akerlund 1989; Eccles 2005), but the reduction was small and not statistically significant (SMD 0.39, 95% CI ‐0.22 to 0.99; 332 participants; two studies; Analysis 2.3). Akerlund 1989 used a topical decongestant and Eccles 2005 used an oral decongestant. The difference between studies that used an oral or topical decongestants was statistically significant (P = 0.03); the effect of treatment was significant in Akerlund 1989 (topical decongestant) and not significant in Eccles 2005 (oral decongestant). However, each subgroup only included one study.
There seemed to be considerable heterogeneity as shown by an I² statistic of 78%. The different measurement instruments probably explain the high heterogeneity; Akerlund 1989 measured NAR with anterior rhinomanometry in the left nostril whereas Eccles 2005 measured NAR with posterior rhinomanometry. We applied a random‐effects model because this changed the interpretation of the results from statistically significant to not statistically significant.
1.3.2. Oral decongestants
Latte 2004 and Latte 2007 used an oral decongestant and reported the AUC for NAR. Their pooled effect was not statistically significant (SMD 0.44, 95% CI ‐0.32 to 1.20; 260 participants; two studies; Analysis 2.3).
For the pooled effect of the AUC for NAR, there seemed to be considerable heterogeneity as shown by an I² statistic of 82%. Interestingly, Latte 2004 and Latte 2007 were conducted at the same institution with the same research team. Both assessed the effectiveness of pseudoephedrine in a similar population. The only differences between the studies were the dosage and the measurement instrument; Latte 2004 was a single‐dose study and NAR was measured with acoustic rhinomanometry, whereas Latte 2007 was a multi‐dose study, with NAR being measured by posterior rhinomanometry. We applied a random‐effects model because this changed the interpretation of the results from statistically significant to not statistically significant.
1.3.3 Topical decongestant
Only Akerlund 1989 and Eccles 2008 used a topical decongestant. Akerlund 1989 reported the mean NAR whereas Eccles 2008 reported the least square mean of NAC. Therefore, we were unable to pool results. Both studies showed a large and statistically significant difference between treatment and placebo, in favour of the treatment group (SMD 0.74, 95% CI 0.23 to 1.25; 102 participants; Akerlund 1989), (SMD 0.89, 95% CI 0.36 to 1.41; 61 participants; Eccles 2008).
2. Adverse events
2.1. Single‐dose decongestant versus placebo
Two single‐dose trials reported adverse events (Gronborg 1983; Taverner 1999); both used an oral decongestant. Taverner 1999 reported no adverse events in either the treatment or the placebo group. Results from Gronborg 1983 were not included in the meta‐analysis because this study used a cross‐over study design and several events per patient were reported. Overall, 32 and 21 events were reported in the treatment and placebo groups respectively; this was not statistically significant.
2.2. Multi‐dose decongestant versus placebo
2.2.1 Oral and topical decongestants combined
Adverse events were reported by seven multi‐dose studies (Analysis 1.2) (Bye 1980; Eccles 2005; Eccles 2006; Eccles 2008; Eccles 2014; Latte 2007; Sperber 1989). All studies except Eccles 2008 used an oral decongestant. With the exception of Eccles 2014, all studies reported specific adverse events. However, the adverse events differed among studies and ranged from vomiting and dry mouth, to lethargy, dizziness, pain and mouth ulcers. In Latte 2007, adverse events were not clearly described in the original paper, but these were included in the Cochrane Review by the same authors (Taverner 2007); therefore, we used these numbers in our review as well.
1.2. Analysis.
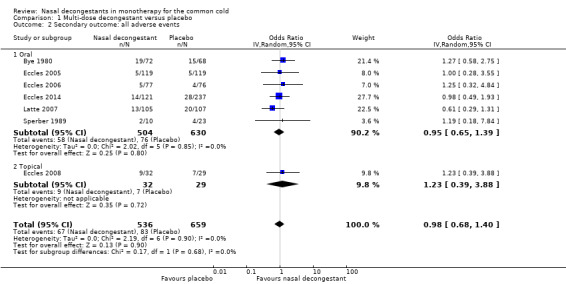
Comparison 1 Multi‐dose decongestant versus placebo, Outcome 2 Secondary outcome: all adverse events.
We only reported the specific type of adverse event if this was reported by more than one study.
All adverse events
Seven studies reported the total number of participants with adverse events (Bye 1980; Eccles 2005; Eccles 2006; Eccles 2008; Eccles 2014; Latte 2007; Sperber 1989). In both treatment and placebo arms, 13% of participants reported an adverse event. The chance of having an adverse event was slightly lower for treatment group participants. However, the difference with the placebo was not statistically significant (OR 0.98, 95% CI 0.68 to 1.40; 1195 participants; seven studies; Analysis 1.2).
The difference between studies that used an oral or topical decongestant was not significant (P = 0.68).
For the pooled effect of all adverse events there was no clear heterogeneity given the I² statistic of 0%. We used a random‐effects model throughout all analyses for adverse events and this did not change the interpretation of the results.
We judged the evidence for all adverse events to be of low quality because of possible risk of bias due to unclear reporting and lack of precision (wide confidence interval).
Insomnia or difficulty sleeping
Four studies reported the incidence of insomnia or difficulty sleeping (Bye 1980; Eccles 2005; Latte 2007; Sperber 1989); all used an oral decongestant. These results are reported in 2.2.2 Oral decongestants.
Headache
Headache was reported by three studies (Eccles 2005; Eccles 2008; Latte 2007). Neither individual studies nor the pooled estimate showed a statistically significant difference between treatment and placebo group participants (OR 0.89, 95% CI 0.23 to 3.37; 511 participants; three studies; Analysis 1.4). Only Eccles 2008 used a topical decongestant.
1.4. Analysis.
Comparison 1 Multi‐dose decongestant versus placebo, Outcome 4 Secondary outcome: adverse events ‐ headache.
The difference between studies that used an oral or topical decongestant was not significant (P = 0.14).
There seemed to be moderate heterogeneity given the I² statistic of 34%. We used a random‐effects model and this did not change the interpretation or the results.
2.2.2 Oral decongestants
All adverse events
Six studies that used an oral decongestant reported total numbers of participants with adverse events (Bye 1980; Eccles 2005; Eccles 2006; Eccles 2014; Latte 2007; Sperber 1989). The difference with placebo was not statistically significant (OR 0.95, 95% CI 0.65 to 1.39; 1134 participants; six studies; Analysis 1.2).
For the pooled effect of all adverse events there was no clear heterogeneity given the I² statistic of 0%. We used a random‐effects model for all analyses of adverse events; this did not change the interpretation or the results. We judged the evidence for all adverse events to be of moderate quality because of possible risk of bias due to unclear reporting.
Insomnia or difficulty sleeping
Four studies reported the incidence of insomnia or difficulty sleeping (Bye 1980; Eccles 2005; Latte 2007; Sperber 1989); all used an oral decongestant. Two studies found a significantly lower risk in the treatment group compared to placebo (Bye 1980; Latte 2007), but the pooled estimate was not statistically significant (OR 0.39, 95% CI 0.09 to 1.62; 623 participants; four studies; Analysis 1.3).
1.3. Analysis.
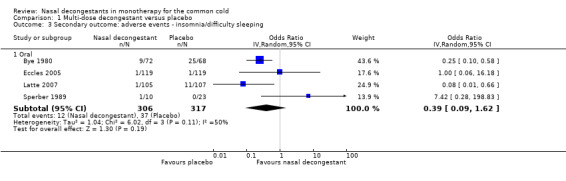
Comparison 1 Multi‐dose decongestant versus placebo, Outcome 3 Secondary outcome: adverse events ‐ insomnia/difficulty sleeping.
There seemed to be moderate heterogeneity given the I² statistic of 50%. We used a random‐effects model and this changed the effect from statistically significant to not significant.
Headache
Headache was reported by two studies that used an oral decongestant (Eccles 2005; Latte 2007). Neither the individual studies nor the pooled estimate showed a statistically significant difference between treatment and placebo groups (OR 0.58, 95% CI 0.19 to 1.84; 450 participants; two studies; Analysis 1.4).
There was no clear heterogeneity given the I² statistic of 0%. We used a random‐effects model and this did not change the interpretation or the results.
2.2.3 Topical decongestants
All adverse events
Only Eccles 2008 used a topical decongestant and reported adverse events. In this study the difference between treatment and placebo was not statistically significant (OR 1.23, 95% CI 0.39 to 3.88; 61 participants).
Insomnia or difficulty sleeping
None of the studies that used a topical decongestant reported insomnia or difficulty sleeping.
Headache
Only Eccles 2008 used a topical decongestant and reported headache as an adverse event. In this study the difference between treatment and placebo groups was not statistically significant (OR 4.00, 95% CI 0.42 to 38.07; 61 participants).
2.3. All doses of decongestants versus placebo
Two single‐dose studies reported adverse events associated with oral decongestants (Gronborg 1983; Taverner 1999). Taverner 1999 reported no events and Gronborg 1983 was excluded from meta‐analyses because it was a cross‐over study. Therefore, combining single‐dose and multi‐dose studies resulted in the same results for multi‐dose adverse events (Analysis 1.2).
3. Complications
None of the included studies reported this outcome.
4. Time to full recovery
4.1. Single‐dose decongestant versus placebo
The included single‐dose trials did not report this outcome.
4.2. Multi‐dose decongestant versus placebo
Only one multi‐dose study reported the time to full recovery (Reinecke 2005, 247 participants). Reinecke 2005 used a topical decongestant and showed that the mean time until full recovery was better for the treatment group (four days) compared to the placebo group (six days; P = 0.001).
5. Time to return to school/work
The included trials did not report this outcome.
Subgroup analyses
1. Single‐dose decongestant versus placebo
1.1 Children versus adults
We planned to compare studies in children aged up to 12 years compared with those aged over 12 years. However, none of the single‐dose studies included children so we were unable to perform this subgroup analysis.
1.2 Oral versus topical
The other subgroup analysis, as specified a priori, was to compare oral versus nasal decongestants. Since we were unable to pool studies we could not perform this subgroup analysis. However, for each comparison in this review we report whether an oral or topical decongestant was used.
2. Multi‐dose decongestant versus placebo
2.1 Children versus adults
We planned to compare studies in children aged up to 12 years compared with those aged over 12 years. However, none of the multi‐dose studies included children. Reinecke 2005 included people aged 12 years and above so excluded children.
2.2 Oral versus topical
For the multi‐dose studies, we were only able to pool results for the primary outcome, subjective symptom scores for nasal congestion (three studies), and adverse events (seven studies). Therefore, assessing the difference between oral versus topical nasal decongestants on subjective symptom scores for nasal congestion involved two studies that used an oral decongestant (Sperber 1989; Taverner 1999), and one that used a topical decongestant (Eccles 2008). The difference between studies that used an oral decongestant and the study that used a topical decongestant was not statistically significant (P = 0.45). Also for adverse events, there was only one study that used a topical decongestant (Eccles 2008); all others used an oral decongestant (Bye 1980; Eccles 2005; Eccles 2006; Eccles 2014; Latte 2007; Sperber 1989). The difference between the pooled result for the oral decongestant versus the only study on the topical decongestant was not statistically significant (P = 0.68).
3. All doses of decongestants versus placebo
3.1 Children versus adults
We planned to compare studies in children aged up to 12 years compared with those aged over 12 years. However, none of the studies included children.
3.2 Oral versus topical
We were able to assess the impact of oral versus topical decongestants only for outcomes where we could pool results and there were both single and multi‐dose studies available. This was only possible for the primary outcome, subjective symptom scores, and the secondary outcome, objective measures of NAR.
For the primary outcome of subjective symptom scores, three studies reported the mean score (Eccles 2008; Sperber 1989; Taverner 1999), and two studies reported an AUC (Latte 2004; Latte 2007). Since pooling of these two outcome measures was not appropriate we discuss both separately. For studies that reported the mean subjective symptom score, we compared the pooled effect of the two studies that used an oral decongestant (Sperber 1989; Taverner 1999) with the results of one study that used a topical decongestant (Eccles 2008). The difference between the two was not statistically significant (P = 0.45). For the two studies that used AUC as an outcome we were unable to assess differences between oral and topical decongestants because both used an oral decongestant.
Similar to the subjective symptom scores, objective measures of NAR were reported as the mean (Akerlund 1989; Eccles 2005), and the AUC (Latte 2004; Latte 2007). Since Latte 2004 and Latte 2007 both used an oral decongestant we were only able to assess the difference between Akerlund 1989 and Eccles 2005, which used topical and oral decongestants respectively. The difference between oral and topical decongestants was statistically significant (P = 0.03). However, each subgroup only included one study.
As for multi‐dose decongestants we indicated if results were based on studies using an oral or topical decongestant. These results are reported in more detail above.
Discussion
Summary of main results
We compared the effectiveness of nasal decongestants administered as single and multiple doses to treat nasal congestion in people with the common cold. We included 15 trials and analyses were based on a total of 1838 participants. Nine studies used pseudoephedrine and three used oxymetazoline. Other decongestants included phenylpropanolamine, norephedrine and xylometazoline. The studies that used phenylpropanolamine or norephedrine were excluded from meta‐analyses because this drug is no longer available in most countries (FDA 2000). There were large differences in reported outcomes. Reporting of study methods was limited in most studies. Therefore, pooling was only possible for a few studies. All studies, except one, included adult participants only (aged 18 years and over). Reinecke 2005 included younger people (aged up to 12 years) but did not provide details on the mean age of participants. As such, the results of this review are applicable to adults only.
We were unable to draw conclusions on the effectiveness of single‐dose decongestants because pooling was not possible due to the large diversity in measuring and reporting symptoms of congestion. For other outcome measures, such as overall patient well‐being and time to recovery, either no data or insufficient data were available.
For multiple doses of decongestant, meta‐analysis was possible for subjective measures of congestion only (measured approximately three hours after the last dose). Subjective measures of congestion were significantly better for treatment group participants compared to placebo group participants. However, the clinical effect was small, and this result was based on two studies only (one topical and one oral decongestant) and involved a total of 88 participants.
Hence, for multiple doses of a nasal decongestant it seems that there is a small beneficial effect on nasal congestion when measured subjectively. Although subjective measures of nasal congestion are probably the most relevant outcomes, since nasal decongestants are used for symptomatic relief only, it is not clear if this small effect is clinically relevant and sufficient to justify widespread use of decongestants (Taverner 2007).
There were insufficient data to estimate differences in the efficacy of oral versus topical decongestants. Adverse events were reported in nine studies and the risk of adverse events in treatment group participants was not significantly different from people in the placebo group. It seems that short‐term use of nasal decongestants in adults with the common cold can be considered safe.
Overall completeness and applicability of evidence
We recognise that there were few included studies. In addition, because these studies were very heterogeneous in their approach to outcome measures, the number of studies that could be pooled for each outcome was even smaller, with most subgroup analyses only containing two studies. Hence, the generalisability of our results is very limited.
With respect to adverse events it was surprising to note that none of the included multi‐dose studies investigated the problem of tachyphylaxis or rhinitis medicamentosa. This could be due to the limited follow‐up in most studies (between one and 10 days). Furthermore, the side effects profile of oral versus topical decongestants might differ; one might expect more systemic and delayed side effects with oral preparations. We were unable to assess this aspect because only one study that used a topical decongestant reported adverse effects.
Twelve studies used subjective measures as outcomes, which are probably the most relevant outcomes to clinical applicability, because nasal decongestants are used for symptomatic relief. In addition, all studies, except one, included participants with community‐acquired common cold.
The results are only applicable to an adult population because none of the studies included children aged 12 years or younger.
Another concern about the applicability of the current evidence relates to the use of pseudoephedrine. Nine studies tested the effectiveness of pseudoephedrine; however, this has not been available since 2005 as an over‐the‐counter decongestant because it can be used to make methamphetamine (FDA 2005). Currently, pseudoephedrine is being replaced by phenylephrine as a way to control methamphetamine abuse. Despite phenylephrine being a common decongestant that is available over‐the‐counter, none of the studies in this review evaluated its effectiveness.
Quality of the evidence
For most outcomes we downgraded the quality of evidence to moderate or low due to the limited data available or poor 'Risk of bias' assessments. We included 15 studies, with a total of 1838 participants, but pooling was often not possible. When pooling was possible, results were often only based on two studies. All studies except one included adult participants and so the findings of our review cannot be generalised to children. We recognise that over half of the included studies were published before 2000, and that many of the studies do not provide sufficient methodological information to exclude risk of bias. Hence, for most studies, risk of bias is unclear.
Potential biases in the review process
Given the small number of included studies, it is possible that we did not find all relevant trials. Hence, publication bias cannot be definitively ruled out, which may lead to overestimation of the treatment effect. For example, we found four studies that were relevant for this review, but no results were available. Two are completed trials with no published results (NCT00452270; NCT01062360), and a third, although originally registered in 2007, is stated to be ongoing (EUCTR2006‐006690‐25‐GB). The fourth study is also ongoing; it started in 2012, but the estimated completion date recently changed from April 2015 to May 2016 (NCT01744106).
This review was conducted as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We searched several databases and three authors (LD, LG, NM) independently selected studies and extracted data, thus minimising the risk of introducing bias during the review process.
Agreements and disagreements with other studies or reviews
Our results are in line with those of other systematic reviews and meta‐analyses. In a similar, but older, Cochrane Review on nasal decongestants for the common cold, Taverner 2007 concluded that nasal decongestants are modestly effective for the short‐term relief of congestion in adults. Two hours after dosing, a significant decrease in patient‐reported symptoms for treatment versus placebo was described. Similar to our findings, the effects were small and although statistically significant, it is not clear if the reduction in symptoms is clinically relevant and sufficient to justify widespread use of decongestants (Taverner 2007).
Several other reviews on nasal decongestants for the common cold have also been published (e.g. Allan 2014; Arroll 2005; Arroll 2011; Meltzer 2010). Generally, their conclusions were based on the Cochrane Reviews Taverner 2004 and Taverner 2007, or more recent studies that were included in this review. Accordingly, the results and conclusions are similar and in line with this review.
Kollar 2007 investigated the effectiveness of a single 10 mg dose of phenylephrine compared to placebo. Kollar 2007 showed that a single oral dose of phenylephrine significantly improved acute nasal congestion. However, it must be noted that the meta‐analyses in this review were almost completely based on small unpublished studies conducted between 1968 and 1975, which were included in a monograph by the US Food and Drug Administration (FDA). According to Eccles 2007, the studies included in the FDA report were in‐house studies provided by representatives of pharmaceutical companies (Eccles 2007).
Another review on xylometazoline (alone and in combination) concluded that xylometazoline provides fast and effective relief from nasal congestion (Eccles 2010). However, this review included only four studies and did not include a meta‐analysis. We included only one study that used xylometazoline (Eccles 2008). Hence, we were unable to formulate firm conclusions about the effectiveness of this medication. Furthermore, it was beyond the scope of our review to evaluate the effectiveness of different nasal decongestants individually.
The Cochrane Review on combination drugs for the common cold (oral antihistamine‐decongestant‐analgesic combinations) found that the effect on individual symptoms is small, "probably too small to be clinically relevant" (De Sutter 2012). The lack of evidence in children was also confirmed by other studies on decongestants alone and combination products (Allan 2014; Arroll 2005; De Sutter 2012).
In terms of the risk of side effects our results were in agreement with other reviews and studies; the incidence of side effects with short‐term use of decongestants is low and side effects are mostly mild to moderate (e.g. insomnia and headache) (Allan 2014; Eccles 2010; Taverner 2007). Although we did not identify data on the safety of nasal decongestants in children, there are reports of adverse events after single use of nasal decongestant. For example, the FDA published a warning in 2012 about serious adverse events after ingestion of over‐the‐counter nasal sprays containing oxymetazoline by children under the age of six years (FDA 2012).
Authors' conclusions
Implications for practice.
The effectiveness of nasal decongestants in monotherapy is uncertain. There may be a small benefit on the subjective experience of nasal congestion after multiple doses of a nasal decongestant (low‐quality evidence). However, it is unclear if the small effect is clinically relevant. Due to the small number of studies that used a topical nasal decongestant, we were unable to draw conclusions on the effectiveness of oral versus topical decongestants. The incidence of mostly mild‐to‐moderate side effects was low. These results are applicable to adults only. Despite common colds being equally common in children, there is no evidence available on either effectiveness or safety in children.
Implications for research.
Given the high consumption of nasal decongestants it was surprising that so little evidence is available. Questions about the effectiveness and safety in children remain unanswered. However, given the reports of serious adverse events in young children, further research in this group does not appear to be warranted or feasible (FDA 2012). Some questions regarding the effectiveness and safety of nasal decongestants in adults also remain unanswered. More research is need to investigate the long‐term effects of multiple doses of nasal decongestants on tachyphylaxis or rhinitis medicamentosa and the differences between oral and topical decongestants need to be explored.
We were unable to compare the effectiveness of different decongestants. For example, pseudoephedrine is often replaced by phenylephrine as a way to control methamphetamine abuse. However, as a decongestant, phenylephrine may not be as effective (Eccles 2007). Despite phenylephrine being a common decongestant that is available over‐the‐counter, none of the included studies evaluated its effectiveness. Hence, more large‐scale, good‐quality randomised controlled trials evaluating the effectiveness of several nasal decongestants are needed.
Furthermore, future research should seek to define a minimal clinically important difference for measures of subjective improvement in nasal congestion. As this was not available, we used a statistical approach to interpret our results. For example, a we regarded a standardised mean difference between 0.2 and 0.49 as a small effect. However, it remains unclear how this small statistical effect corresponds to clinical effect.
Acknowledgements
We wish to thank Sarah Thorning for her assistance with the searches and Liz Dooley for her support with the review process. We also wish to thank Laura Alder for her assistance in the initial selection of studies. We thank Ann Fonfa, Dee Shneiderman, Mark Jones, Tim Kenealy and Bruce Arroll for their helpful comments on drafts of this review.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1 Common Cold/ 2 common cold*.tw. 3 head cold*.tw. 4 coryza.tw. 5 upper respiratory infection*.tw. 6 upper respiratory tract infection*.tw. 7 (infection* adj3 upper respiratory).tw. 8 (nasopharyngit* or rhinopharyngit*).tw. 9 nasosinusit*.tw. 10 (acute adj2 (rhinit* or rhinosinusit*)).tw. 11 (rhinorrhoea or rhinorrhoea).tw. 12 Nasal Obstruction/ 13 ((nasal or nose*) adj3 (block* or obstruct* or congest* or discharge* or runny or running or stuffy or stuffed)).tw. 14 Rhinovirus/ 15 rhinovir*.tw. 16 Coronavirus Infections/ 17 coronavirus/ or coronavirus 229e, human/ or coronavirus oc43, human/ 18 coronavir*.tw. 19 adenoviridae/ or adenoviruses, human/ 20 Adenovirus Infections, Human/ 21 adenovir*.tw. 22 or/1‐21 23 exp Nasal Decongestants/ 24 decongestant*.tw,nm. 25 oxymetazoline.tw,nm. 26 norepinephrine.tw,nm. 27 pseudoephedrine.tw,nm. 28 phenylephrine.tw,nm. 29 xylometazoline.tw,nm. 30 tramazoline.tw. 31 Ephedrine/ 32 ephedrin*.tw,nm. 33 or/23‐33 34 22 and 34
Appendix 2. Embase (Elsevier) search strategy
#36 #24 AND #27 AND #35 #35 #30 NOT #34 #34 #31 NOT #33 #33 #31 AND #32 #32 'human'/de #31 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de #30 #28 OR #29 #29 random*:ab,ti OR placebo*:ab,ti OR allocat*:ab,ti OR trial:ti OR crossover*:ab,ti OR 'cross over':ab,ti OR (doubl* NEXT/1 blind*):ab,ti #28 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #27 #25 OR #26 #26 oxymetazoline:ab,ti OR norepinephrine:ab,ti OR pseudoephedrine:ab,ti OR phenylephrine:ab,ti AND xylometazoline:ab,ti OR tramazoline:ab,ti OR ephedrin*:ab,ti OR (intranasal NEAR/2 corticosteroid*):ab,ti #25 'decongestive agent'/exp AND [embase]/lim79259 #24 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #2384658 #23 adenovir*:ab,ti AND [embase]/lim35904 #22 'human adenovirus'/exp OR 'human adenovirus infection'/de #21 coronavir*:ab,ti #20 'coronavirus'/de OR 'human coronavirus nl63'/de #19 rhinovir*:ab,ti #18 'human rhinovirus'/de OR 'rhinovirus infection'/de #17 ((nasal OR nose*) NEAR/3 (block* OR congest* OR obstruct* OR discharg* OR runny OR running OR stuffy OR stuffed)):ab,ti #16 'nose congestion'/de OR 'nose infection'/de #15 rhinorrhoea:ab,ti OR rhinorrhea:ab,ti #14 'rhinorrhea'/de #13 sneez*:ab,ti #12 'sneezing'/de #11 'common cold symptom'/de #10 (acute NEAR/2 (rhinit* OR rhinosinusit*)):ab,ti #9 'rhinosinusitis'/de #8 rhinopharyngit*:ab,ti OR nasopharyngit*:ab,ti #7 'rhinopharyngitis'/de #6 (infection* NEAR/3 'upper respiratory'):ab,ti #5 'upper respiratory tract infection':ab,ti OR 'upper respiratory tract infections':ab,ti OR urti:ab,ti #4 'upper respiratory tract infection'/de OR 'viral upper respiratory tract infection'/de #3 'head cold':ab,ti OR 'head colds':ab,ti OR coryza:ab,ti #2 'common cold':ab,ti OR 'common colds':ab,ti #1 'common cold'/de
Appendix 3. CINAHL (Ebsco) search strategy
S34 S23 and S33 S33 S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 S32 (MH "Quantitative Studies") S31 TI placebo* OR AB placebo* S30 (MH "Placebos") S29 TI random* OR AB random* S28 (MH "Random Assignment") S27 TI ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*) ) OR AB ( (singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) S26 TI clinic* W1 trial* OR AB clinic* W1 trial* S25 PT clinical trial S24 (MH "Clinical Trials+") S23 S17 and S22 S22 S18 or S19 or S20 or S21 S21 TI intranasal N2 corticosteroid* OR AB intranasal N2 corticosteroid* S20 TI (oxymetazoline or norepinephrine or pseudoephedrine or phenylephrine or xylometazoline or tramazoline or ephedrin* ) OR AB ( oxymetazoline or norepinephrine or pseudoephedrine or phenylephrine or xylometazoline or tramazoline or ephedrin*) S19 TI decongestant* OR AB decongestant* S18 (MH "Vasoconstrictor Agents, Nasal+") OR (MH "Ephedrine") OR (MH "Phenylephrine") S17 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 S16 TI (rhinovir* or adenovir*) OR AB (rhinovir* or adenovir*) S15 TI coronavir* OR AB coronavir* S14 (MH "Coronavirus Infections") S13 (MH "Coronavirus") S12 TI ((nasal or nose*) N3 (block* or obstruct* or congest* or discharg* or runny or running or stuffy or stuffed)) OR AB ((nasal or nose*) N3 (block* or obstruct* or congest* or discharg* or runny or running or stuffy or stuffed)) S11 (MH "Nasal Obstruction") S10 TI (sneez* or rhinorrhea* or rhinorrhoea*) OR AB (sneez* or rhinorrhea* or rhinorrhoea*) S9 (MH "Sneezing") S8 TI (nasopharyngit* or rhinopharyngit*) OR AB (nasopharyngit* or rhinopharyngit*) S7 TI (acute N2 (rhinit* or rhinosinusit* or nasosinusit*)) OR AB (acute N2 (rhinit* or rhinosinusit* or nasosinusit*)) S6 (MH "Rhinosinusitis") S5 TX (upper respiratory infection* or upper respiratory tract infection* or urti) OR AB (upper respiratory infection* or upper respiratory tract infection* or urti) S4 TI coryza OR AB coryza S3 TI head cold* OR AB head cold* S2 TI common cold* OR AB common cold* S1 (MH "Common Cold")
Appendix 4. LILACS (BIREME) search strategy
(MH:"Common Cold" OR "Resfriado Común" OR "Resfriado Comum" OR "Coriza Aguda" OR Catarro OR coryza OR "upper respiratory tract infection" OR "upper respiratory tract infections" OR "upper respiratory infection" OR "upper respiratory infections" OR "Infecciones del Tracto Respiratorio Superior" OR "Infecciones de las Vías Respiratorias Superiores" OR "Infecções do Trato Respiratório Superior" OR "Infecções do Sistema Respiratório Superior" OR MH:Nasopharyngitis OR Nasofaringitis OR Nasofaringite OR nasophayrngit$ or rhinopharyngit$ OR nasosinusit$ OR rhinosinusit$ OR rhinit$ OR rinit$ OR MH:sneezing OR Estornudo OR Espirro OR rhinorrhea OR rhinorrhoea OR Rinorrea OR Rinorréia OR "blocked nose" OR "nasal obstruction" OR "runny nose" OR "running nose" OR "nasal congestion" OR "nasal discharge" OR "stuffy nose" OR "stuffed nose" OR "stuffy nose" OR MH:rhinovirus OR rhinovir$ OR MH:"Coronavirus Infections" OR MH:Coronavirus OR MH:"Coronavirus 229E, Human" OR MH:"Coronavirus OC43, Human" OR MH:"Coronavirus NL63, Human" OR MH:"Adenovirus Infections, Human" OR MH:"Adenoviruses, Human" OR adenovir$) AND (MH:"Nasal Decongestants" OR MH:D27.505.954.411.793.610$ OR MH:D27.505.954.796.560$ OR "Descongestionantes Nasales" OR "Descongestionantes Nasais" OR Descongestionantes OR "Vasoconstrictores Nasales" OR descongestionantes OR "Vasoconstritores Nasais" OR decongestant$ OR oxymetazolin$ OR Oximetazolina OR norepinephrine OR Norepinefrina OR pseudoephedrine OR Seudoefedrina OR Pseudoefedrina OR Isoephedrine OR xylometazoline OR MH:Ephedrine OR efedrina OR "intranasal corticosteroid" OR "intranasal corticosteroids")
Appendix 5. Web of Science (Thomson Reuters) search strategy
| #16 | #15 AND #12 DocType=All document types; Language=All languages; |
| #15 | #14 OR #13 DocType=All document types; Language=All languages; |
| #14 | Topic=((single or double) NEAR/1 blind*) OR Title=(trial) DocType=All document types; Language=All languages; |
| #13 | Topic=(random* or placebo* or "clinical trial$" or allocat*) OR Title=(trial) DocType=All document types; Language=All languages; |
| #12 | #11 AND #7 DocType=All document types; Language=All languages; |
| #11 | #10 OR #9 OR #8 DocType=All document types; Language=All languages; |
| #10 | Topic=(intranasal NEAR/2 corticosteroid$) DocType=All document types; Language=All languages; |
| #9 | Topic=(oxymetazoline or norepinephrine or pseudoephedrine or phenylephrine or xylometazoline or tramazoline or ephedrin*) DocType=All document types; Language=All languages; |
| #8 | Topic=(nasal NEAR/2 decongestant$) DocType=All document types; Language=All languages; |
| #7 | #6 OR #5 OR #4 OR #3 OR #2 OR #1 DocType=All document types; Language=All languages; |
| #6 | Topic=(rhinovir* or coronavir* or adenovir*) DocType=All document types; Language=All languages; |
| #5 | Topic=((nasal or nose$) NEAR/3 (block* or congest* or discharg* or runny or running or stuffy or stuffed)) DocType=All document types; Language=All languages; |
| #4 | Topic=(acute NEAR/2 rhinit*) DocType=All document types; Language=All languages; |
| #3 | Topic=(nasopharyngit* or rhinopharyngit* or nasosinusit* or rhinosinusit* or sneez* or rhinorrhea or rhinorrhoea) DocType=All document types; Language=All languages; |
| #2 | Topic=(infection$ NEAR/3 "upper respiratory") DocType=All document types; Language=All languages; |
| #1 | Topic=("common cold" or "common colds" or "head cold$" or coryza) DocType=All document types; Language=All languages; |
Data and analyses
Comparison 1. Multi‐dose decongestant versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: subjective symptom score (mean) | 2 | 94 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [0.07, 0.92] |
| 1.1 Oral | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.47, 1.02] |
| 1.2 Topical | 1 | 61 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.08, 1.11] |
| 2 Secondary outcome: all adverse events | 7 | 1195 | Odds Ratio (IV, Random, 95% CI) | 0.98 [0.68, 1.40] |
| 2.1 Oral | 6 | 1134 | Odds Ratio (IV, Random, 95% CI) | 0.95 [0.65, 1.39] |
| 2.2 Topical | 1 | 61 | Odds Ratio (IV, Random, 95% CI) | 1.23 [0.39, 3.88] |
| 3 Secondary outcome: adverse events ‐ insomnia/difficulty sleeping | 4 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Oral | 4 | 623 | Odds Ratio (IV, Random, 95% CI) | 0.39 [0.09, 1.62] |
| 4 Secondary outcome: adverse events ‐ headache | 3 | 511 | Odds Ratio (IV, Random, 95% CI) | 0.89 [0.23, 3.37] |
| 4.1 Oral | 2 | 450 | Odds Ratio (IV, Random, 95% CI) | 0.58 [0.19, 1.84] |
| 4.2 Topical | 1 | 61 | Odds Ratio (IV, Random, 95% CI) | 4.0 [0.42, 38.07] |
Comparison 2. All doses of decongestants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: subjective symptom score (mean) | 3 | 146 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.11, 0.78] |
| 1.1 Oral | 2 | 85 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.11, 0.77] |
| 1.2 Topical | 1 | 61 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.08, 1.11] |
| 2 Primary outcome: subjective symptom score (AUC) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Oral | 2 | 260 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.14, 0.35] |
| 3 Secondary outcome: objective NAR (mean) | 2 | 332 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.22, 0.99] |
| 3.1 Oral | 1 | 230 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.14, 0.37] |
| 3.2 Topical | 1 | 102 | Std. Mean Difference (IV, Random, 95% CI) | 0.74 [0.23, 1.25] |
| 4 Secondary outcome: objective NAR (AUC) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Oral | 2 | 260 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.32, 1.20] |
2.2. Analysis.
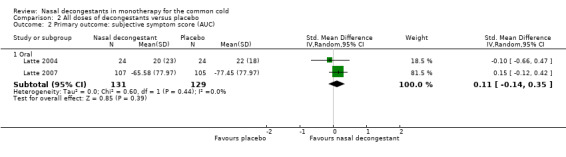
Comparison 2 All doses of decongestants versus placebo, Outcome 2 Primary outcome: subjective symptom score (AUC).
2.4. Analysis.
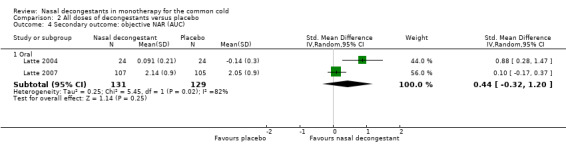
Comparison 2 All doses of decongestants versus placebo, Outcome 4 Secondary outcome: objective NAR (AUC).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akerlund 1989.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants |
N recruited: 106 males undergoing military training N analysed: 106 ‐ Oxymetazoline (4 groups with different dosages) N = 85 ‐ Placebo N = 21 Age: between 18 and 28 years, mean age 20.1 years Country: Sweden Inclusion criteria: Patients with the common cold Not more than 2 days duration of symptoms Exclusion criteria: Patients with nasal polyps or a pronounced septal deviation Patients with a history of frequent nasal congestion, sinusitis or allergy Diagnostic criteria: only patients with objectively obstructed noses, defined as V2 > 35° and a reproducible initial value (difference in V2 ≤ 6° in 2 consecutive measurements) was required |
|
| Interventions |
Treatment before study: no medication possibly influencing nasal congestion was allowed Nasal decongestant dose: ‐ Group 1: 0.1 mg/mL oxymetazoline, in 0.1 mL dose pipettes, N = 22 ‐ Group 2: 0.25 mg/mL oxymetazoline, in 0.1 mL dose pipettes, N = 21 ‐ Group 3: 0.25 mg/mL oxymetazoline, in 0.2 mL dose pipettes, N = 21 ‐ Group 4: 0.5 mg/mL oxymetazoline, in 0.1 mL dose pipettes, N = 21 ‐ Placebo: 0 mg/mL in 0.1 mL dose pipettes, N = 21 Administration: single dose Nasal spray: the drug was administered once into each nostril using single‐dose pipettes with the patient's head inclined backwards Follow‐up: 1 day Measurements: Before and 10 min after administration of the drug and then every hour for 7 hours Changes in nasal airway resistance were measured by anterior rhinomanometry, in the left nostril Subjective symptom scores were the patient's experience of the symptoms of nasal blockage, secretion, itching, sneezing and coughing, on a 4‐point score scale (0 to 3) from none to severe symptoms just before every nasal airway resistance measurement |
|
| Outcomes |
Primary outcome: changes in nasal airway resistance Secondary outcomes: subjective symptom scores of nasal blockage, secretion, itching, sneezing, coughing and taste sensation |
|
| Notes |
Funding: source of funding not indicated Declarations of interest: not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods of random sequence generation not discussed |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants not discussed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data were reported: "102 of the 106 patients had valid nasal airway resistance measurements" In the table the remaining number of participants in each treatment group was provided |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No evidence of pharmaceutical company funding or any other sources of bias |
Bye 1980.
| Methods | Randomised, double‐blind, placebo‐controlled, 4‐arm, parallel‐group study | |
| Participants |
N recruited: 466 healthy adults, 199 participants reported a total of 243 colds over a 6‐month period N participants analysed:
Age: mean age 30.9 ± 10 years Country: UK Inclusion criteria: having a cold Remark: homogeneity was checked for age, sex, usual number of colds each winter, absence of allergic disorder, smoking habits, duration of symptoms and signs of fever Exclusion criteria: taking medicines that could interfere with the study |
|
| Interventions |
Nasal decongestant dose: Pseudoephedrine hydrochloride 60 mg Triprolidine hydrochloride 2.5 mg Pseudoephedrine 60 mg and triprolidine 2.5 mg Administration: multiple doses Oral tablets: bottles with 20 tablets; 1 tablet 3 times daily for as long as participant felt necessary Follow‐up: 10 days Measurements: Subjective symptom score reported on a 4‐point scale for: ‐ 12 symptoms relating to common cold (cold in the head, running nose, sneezing, blocked nose, sore throat, headache, cough, feeling ill, phlegm, hoarseness, ache in the back or limb and feeling feverish) ‐ 4 possible unwanted effects of treatment (palpitations, sleepiness, drowsiness and dry mouth) ‐ 3 unlikely effects (not specified) |
|
| Outcomes |
Primary outcome: severity of 12 subjective symptoms
Secondary outcomes:
Severity of unwanted effects of treatment or served as an index of suggestibility Overall impression while taking tablets |
|
| Notes |
Funding: source of funding not indicated Declarations of interest: not provided Note: unit of analysis is number of colds and not patients (some patients have more than 1 cold) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation lists were used: "Drugs were allocated from separate randomisation lists for men and women aged below and above 40 years and at each centre (16 lists in all). Balance in numbers was arranged after every eight person in each list" |
| Allocation concealment (selection bias) | Low risk | Drugs were issued in coded bottles: "Drugs were issued to patients in coded bottles. All tablets were identical in appearance. All were specially made and differed in appearance from marketed preparations" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The appearance of the tablets was identical: "Drugs were issued to patients in coded bottles. All tablets were identical in appearance. All were specially made and differed in appearance from marketed preparations" However, patients were also asked 'do you think the trial tablets you took were placebo'; about 75% of the patients on placebo indicated that they thought they were on placebo compared to 45% in the treatment group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Methods of outcome assessment blinding not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data are described and in the tables the number of remaining participants per treatment group are provided: "Three stopped taking tablets (and completing the diary) because of unwanted side effects; two because of excessive drowsiness while taking triprolidine and one because urticaria developed during treatment with pseudoephedrine. No reasons were obtained from the other volunteers who did not complete diaries" |
| Selective reporting (reporting bias) | High risk | Separate results for the 12 primary symptoms were not provided. Only the significant difference for sneezing was discussed |
| Other bias | Unclear risk | Funding organisation is not described Some patients had more than 1 cold over the 6‐month period. It is not clear if these participants received the same treatment or were randomised again |
Cohen 1978.
| Methods | Randomised, double‐blind, placebo‐controlled, 4‐arm, parallel‐group study | |
| Participants |
N recruited: 80 male and female N analysed: all 80 participants As we focus on monotherapy in this review, only results for phenylpropanolamine‐only and placebo groups were extracted. Randomisation ratio was not clearly stated, we assumed equal numbers in the different treatment groups:
Age: not stated Country: USA Inclusion criteria: Acute nasal congestion due to common cold Symptoms for less than 48 hours Exclusion criteria: No anatomical nasal obstruction No significant metabolic, cardiovascular or bronchopulmonary disease |
|
| Interventions |
Nasal decongestant dose: Phenylpropanolamine, 37.5 mg Placebo Administration: single dose Oral syrup: the study preparations were given as identically appearing cherry‐flavoured hydroalcoholic syrups Follow‐up: 1 day Measurements: nasal airway resistance was measured by electronic posterior rhinometry All resistance readings were made at a reference flow rate of 0.5 L/s and were the means of 3 successive determinations of inspiratory and expiratory flow Total nasal resistance was calculated as the sum of mean inspiratory and expiratory resistances Participants were asked to grade their nasal congestion using a 6‐ranked scale ranging from 1 (no congestion) to 6 (very severe congestion) After treatment, measurements were recorded 15, 30, 60, 120, 180 and 240 min from baseline No smoking or meals before and during the trial |
|
| Outcomes |
Primary outcome: nasal airway resistance Secondary outcome: subjective rating of nasal obstruction |
|
| Notes |
Funding: source of funding not indicated Declarations of interest: not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation code was used: "The four study preparations were given as identically‐appearing cherry flavoured hydroalcoholic syrups in double‐blind fashion according to the randomisation code". "The four formulations and the coded randomised allocation were prepared under the supervision of Mr. Robert Kirpitch, Senior Clinical Scientist, Warner‐Lambert Research Institute, Morris Plains, N.J" |
| Allocation concealment (selection bias) | Low risk | The treatment and placebo preparations looked identical: "The four study preparations were given as identically‐appearing cherry flavoured hydroalcoholic syrups in double‐blind fashion according to the randomisation code" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was stated that treatment was allocated in a double‐blind fashion under the supervision of a senior scientist: "The four study preparations were given as identically‐appearing cherry flavoured hydroalcoholic syrups in double‐blind fashion according to the randomisation code" "The four formulations and the coded randomised allocation were prepared under the supervision of Mr. Robert Kirpitch, Senior Clinical Scientist, Warner‐Lambert Research Institute, Morris Plains, N.J" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Methods of blinding outcome assessment were not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the results it was reported that all participants were able to co‐ordinate with the method and completed their protocol assignments |
| Selective reporting (reporting bias) | Low risk | All scores for all outcome measures were reported |
| Other bias | Low risk | No evidence of pharmaceutical company funding or any other sources of bias |
Eccles 2005.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group | |
| Participants |
N recruited: 238 N analysed: 238 ‐ Placebo: N = 119 ‐ Pseudoephedrine: N = 119 Participants that were protocol valid: 236 for day 1 and 230 for day 3; not clear how many were placebo or pseudoephedrine We assume equal numbers in each group Age: between 18 and 65 years, mean age 20 years Country: UK Inclusion criteria:
Exclusion criteria:
Diagnostic criteria:
|
|
| Interventions |
Nasal decongestant dose: Sudafed tablets containing 60 mg of pseudoephedrine hydrochloride Matched placebo tablets Administration: multiple doses Oral tablets: patients were instructed to take the tablet 4 times a day (every 4 to 6 hour) for 3 days Follow‐up: 3 days Measurements: Total nasal airway resistance was measured with posterior rhinomanometry at a sample pressure of 75 Pa at baseline and every 60 min on day 1 and day 3 up until 4 hours after dosing Subjective nasal congestion: measured with a 100 mm visual analogue scale: 0 = nose completely clear, 100 = nose completely blocked. Symptoms of nasal congestion/stuffiness and nasal discharge/runny nose were scored on a 7‐point ordinal scale at baseline and at 6 PM on days 1 and 2 |
|
| Outcomes |
Primary outcome: AUC of the NAR from 0 to 3 hours after the first dose on day 1 Secondary outcomes:
|
|
| Notes |
Funding: Pfizer Consumer HealthCare Declarations of interest: not provided, but one author was employed by Pfizer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation code was used: "The medication and randomisation code were prepared by Pfizer Consumer HealthCare" |
| Allocation concealment (selection bias) | Low risk | A concealed disclosure envelope was used and placebo matched treatment: "The identity of each treatment kit was concealed in a disclosure envelope only to be opened in case of emergency" "The allocation of medication was stratified according to baseline nasal airway resistance (low nasal airway resistance 0.2 to 0.4 and high nasal airway resistance ≥ 0.41 Pa/cm³/sec)" "Study medication consisted of 60 mg pseudoephedrine or matched placebo tablets" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients received matched placebo tablets ensuring that participants/personnel had no knowledge of the intervention they were allocated to "Study medication consisted of 60 mg pseudoephedrine or matched placebo tablets" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Methods of outcome assessment blinding not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of missing values is described. However, it is not clear how many persons remained in each group: "238 participants were recruited, 236 were protocol‐valid for day 1 and 230 were protocol‐valid for day 3" "Missing pre‐dose assessments were not imputed and the corresponding AUC was set to missing. Missing data for AUC were only imputed if either a 3‐ or 4‐hour reading was missing" |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Unclear risk | Study was funded by Pfizer |
Eccles 2006.
| Methods | Randomised, double‐blind, placebo‐controlled, 4‐arm, parallel‐group study | |
| Participants |
N recruited: 384 patients were screened, 305 were randomised N analysed: unclear from text, presumably 305: ‐ 76 used paracetamol and pseudoephedrine ‐ 76 paracetamol only ‐ 76 pseudoephedrine only ‐ 77 placebo As we only focus on monotherapy in this review, only the results for the treatment group on pseudoephedrine only and placebo were extracted Age: 18 or older Country: UK Inclusion criteria: patients had to be in general good health and at least 18 years old Exclusion criteria: nasal resistance within normal range at screening (≤ 0.25 Pa/cm³/s), history of allergic rhinitis, chronic respiratory disease, anatomical nasal obstruction or deformity, the presence of nasal polyps, or a disease which contra‐indicated the use of either paracetamol or pseudoephedrine. Patients who had taken certain medications within a given timescale of study entry were also excluded; astemizole (30 days), monoamine oxidase inhibitors (14 days), antibiotics (7 days), antihistamines (72 hours), analgesics/antipyretics (24 hours), nasal decongestants (12 hours), antitussives, medicated lozenge or throat spray (8 hours), alcohol (6 hours) and menthol products (2 hours). Patients using metoclopramide, domperidone, cholestyramine or anticoagulation therapy were also excluded from the study Diagnostic criteria: symptomatic upper respiratory tract infections of up to 3 days duration, nasal congestion (i.e. total nasal airflow resistance of > 0.25 Pa/cm³/s determined by posterior rhinomanometry) and pain of at least moderate intensity at baseline |
|
| Interventions |
Nasal decongestant dose: 1000 mg of paracetamol combined with 60 mg of pseudoephedrine 1000 mg of paracetamol alone 60 mg of pseudoephedrine alone Placebo Administration: multiple doses Oral medication: 1 single dose given at clinic; patients were then instructed to dose as required up to 3 times per day with minimum dosing interval of 4 hours for 3 days Follow‐up: 3 days Measurements: Nasal airflow conductance was calculated from measurements of nasal resistance to airflow (Pa/cm³/s) at a fixed sample nasal pressure of 75 Pa using posterior rhinomanometry Pain relief of cold and flu‐like symptoms (composite of sore throat, headache, body aches and pains) was assessed using a 5‐point verbal rating scale ("0 = none, 1 = a little, 2 = some, 3 = a lot, 4 = complete relief") Pain intensity (composite of sore throat, headache, body aches and pains) and nasal congestion (4‐point scale of "0 = none, 1 = mild, 2 = moderate, 3 = severe") Global assessment of pain relief and of nasal congestion relief at the follow‐up visit (5‐point scale of "0 = poor, 1 = fair, 2 = good, 3 = very good, 4 = excellent") Adverse events were recorded Assessments of nasal airflow resistance and pain relief and intensity were made at 1, 2, 3 and 4 hours after dosing. Patients assessed nasal congestion and pain intensity each evening |
|
| Outcomes |
Primary outcomes: nasal airflow conductance, pain relief Secondary outcome: adverse events |
|
| Notes |
Funding: GlaxoSmithKline Declarations of interest: "This study was funded by GlaxoSmithKline PLC. DR, MN, EJ and IB are employees of GlaxoSmithKline PLC. RE has acted as a consultant to GlaxoSmithKline PLC" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation schedule was used: "Eligible patients were then assigned to 1 of 4 treatment regimens (the combination, paracetamol alone pseudoephedrine alone or placebo), in equal ratio, according to a parallel group randomisation schedule" |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment were not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study states that it was double‐blinded and that the double‐dummy method used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study states that it was double‐blinded, but there is no mention of the method of blinding outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of discontinuations per treatment group is described: "No patients withdrew due to an adverse event during the single dose phase of the study. In the multiple dose phase 2 patients (dry mouth ‐ combination treatment and vomiting ‐ pseudoephedrine treatment) discontinued dosing due to adverse events" |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Other bias | Unclear risk | Study was funded by GlaxoSmithKline PLC |
Eccles 2008.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants |
N recruited: 78 were screened, 12 did not meet the inclusion criteria 66 were randomised 5 were not dosed N analysed: 61 ‐ Xylometazoline: N = 29 ‐ Placebo: N = 32 Age: 18 years and over, median age 20 Country: UK Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: Minimum nasal congestion score of 2 (rated on a 4‐point scale; 0 = not present; 1 = mild; 2 = moderate; 3 = severe) Minimum 2 common cold symptoms (runny nose, blocked nose, sore throat, cough) |
|
| Interventions |
Nasal decongestant dose: Xylometazoline 0.1%, 1.0 mg/mL of F2 metered‐dose nasal spray Placebo: saline solution Administration: multiple doses Nasal spray: 1 spray (0.14 g) in each nostril 3 times per day until the total common cold symptom score was recorded to be 0 or for a maximum of 10 days Follow‐up: not clear, maximum 10 days Measurements: Nasal airway resistance was measured using active posterior rhinomanometry at baseline, 30 min and 1, 6, 7, 8, 9, 10, 11 and 12 hours after dosing Subjective symptom scores: a visual analogue scale (0 = nose completely clear, 100 = nose completely blocked); this was measured every 5 min over a 30 min period Runny nose, blocked nose, sore throat, cough, sneezing and ear ache scores recorded every day on a 4‐point scale (0 = not present, 1 = mild, 2 = moderate, 3 = severe) Other measures: Subjective measures of sleep, tiredness, daily activities, general well‐being, smell Adverse events were recorded |
|
| Outcomes |
Primary outcomes: total nasal airway resistance Secondary outcomes: Time to onset of subjective relief of nasal congestion The peak subjective relief of nasal congestion (lowest score) Symptoms of common cold: runny nose, blocked nose, sore throat, cough, sneezing and ear ache scores recorded on a 4‐point scale |
|
| Notes |
Funding: Novartis Declarations of interest: "M. Eriksson, S. Graffera, and S.C. Chen are employees of Novartis, the clinical trial was sponsored by Novartis" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not clearly described: "Eligible patients were randomised in a 1:1 ratio and treated double‐blind" |
| Allocation concealment (selection bias) | Unclear risk | It is not clear if participants could not foresee treatment allocation since they were stratified according to severity of nasal congestion: "Patients were stratified according to severity of nasal congestion as measured by posterior rhinomanometry during screening on the first study visit (nasal airway resistance, 0.2 to 0.4 and > 0.41 Pa/cm³ per second)" "The nasal spray devices were identical and delivered 0.14 G/actuation" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is stated that treatment was administered double‐blind and "The nasal spray devices were identical and delivered 0.14 G/actuation" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Methods of blinding outcome assessment were not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data were described and the number of remaining participants per treatment group is provided in the tables: "66 patients were randomised to the study but 5 were randomised not dosed because they either did not return on day 1 of treatment (N = 4) or on returning were unable to reproduce the technique required for measurement of nasal airway resistance (N = 1)." All 61 patients were included in the intention‐to‐treat efficacy and safety analysis) |
| Selective reporting (reporting bias) | High risk | Not all outcomes were reported: total nasal airway resistance was mentioned as the primary outcome, but total nasal airway conductance was reported. In the baseline table investigators differentiated between nasal resistance and nasal conductance; further in the results only nasal conductance is reported |
| Other bias | Unclear risk | Funded by Novartis |
Eccles 2014.
| Methods | Randomised, double‐blind, placebo‐controlled, 4‐arm parallel‐group study | |
| Participants |
N recruited: 833 N analysed: 829 (4 withdrew consent) As we only focus on monotherapy in this review, only the results for treatment group on pseudoephedrine only and placebo were extracted The number of patients per treatment group was not provided; from the percentage of adverse events we calculated the following numbers:
Age: not stated Country: UK Inclusion criteria: nasal congestion and pain associated with upper respiratory tract infections for no more than 3 days Exclusion criteria: Allergic rhinitis, chronic respiratory disease, hyperthyroidism, cardiovascular disease, severe hypertension, peptic ulcer and hypersensitivity to acetylsalicylic acid, aspirin or pseudoephedrine Some medications were not allowed prior to the study entry: monoamine‐oxidase inhibitors (30 days), antihistamines and antibiotics (7 days), analgesics and antipyretics (24 hours), nasal decongestants (12 hours), lozenges and throat sprays (6 hours), menthol containing products (6 hours) Patients under anti‐coagulation therapy and pregnant or lactating females were also excluded from the study Diagnostic criteria: Pain score of at least moderate intensity (2 on a 4‐point scale) Total nasal airway resistance > 0.25 Pa/cm³ as determined by posterior rhinomanometry |
|
| Interventions |
Nasal decongestant dose:
Administration: multiple doses Sachets (small disposable bags) with granules to be dissolved in water and taken orally 3 doses per day during 3 days, with a minimum dosing interval of 4 hours Follow‐up: 3 days Measurements: Pain symptom score (composite score for sore throat and/or headache) recorded on a 4‐point categorical scale consisting of no pain = 0, mild pain = 1, moderate pain = 2 and severe pain = 3 Nasal obstruction with a total nasal air flow resistance of > 0.25 Pa/cm³/s as determined by posterior rhinomanometry; this was measured 1, 2, 3 and 4 hours after dosing At the evening of every day patients were asked to assess pain intensity, pain relief, nasal congestion intensity and nasal congestion relief and at day 3 they were asked for a global assessment of pain relief and global assessment of nasal congestion relief Adverse events were recorded during the whole study period |
|
| Outcomes |
Primary outcome: Reduction of nasal congestion (nasal airflow conductance) Secondary outcomes: Total subjective nasal congestion relief Global assessment of nasal congestion relief Total pain relief |
|
| Notes |
Funding: Bayer HealthCare LLC Declarations of interest: "This study was sponsored by Bayer HealthCare LLC, Morristown, NJ, USA. R.E. acted as a consultant for Bayer HealthCare LLC. M.V. is an employee of Bayer HealthCare. The authors acknowledge the participation of the staff at the Common Cold Centre, Cardiff University in managing and conducting the study (Dr. M. Jawad, Miss S. Jawad, Mr. B. Pope, and Miss H. Crowdy). R.E. and staff are employees of Cardiff University and they received no payment above normal university salary for conducting this clinical study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods of randomisation sequence generation not discussed |
| Allocation concealment (selection bias) | Low risk | Participants could not foresee treatment allocation since the different treatments had the same appearance, taste and smell: "Double blinding was guaranteed since it was not possible to distinguish between combination product, monotherapies, and placebo granules. All treatments were dispensed as sachets containing white granules for dissolving in water, and they had the same appearance, taste, and no noticeable smell. Neither the investigators nor the patients were aware of the nature of the treatments and both were therefore blinded for any assessments. The placebo contained all the flavouring and excipients that were present in the other medications, which were sucrose, hypromellose binder, orange flavour, and citric acid" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The investigators state that double‐blinding was guaranteed: "Double blinding was guaranteed since it was not possible to distinguish between combination product, monotherapies, and placebo granules. All treatments were dispensed as sachets containing white granules for dissolving in water, and they had the same appearance, taste, and no noticeable smell. Neither the investigators nor the patients were aware of the nature of the treatments and both were therefore blinded for any assessments. The placebo contained all the flavouring and excipients that were present in the other medications, which were sucrose, hypromellose binder, orange flavour, and citric acid" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Neither the investigators nor the patients were aware of the nature of the treatments and both were therefore blinded for any assessments" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The missing data are described. However, it is not clear how many patients remained in each treatment group: "There were 833 patients randomised 833 patients randomised to the study. All of them were treated. 4 participants withdrew consent, but all other participants were included in the analyses" |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcome measures were reported |
| Other bias | Unclear risk | Study sponsored by Bayer HealthCare LLC The number of participants in each group was not provided. Only in the methods is a sample size calculation provided, which yielded a total sample size of 875 (“approximately 250 patients were to be randomised into each of the aspirin/ pseudoephedrine combination, aspirin alone, and pseudoephedrine alone groups and 125 patients were to be randomised into the placebo group”), whereas 833 were eventually randomised |
Ferguson 1997.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants |
N recruited: 97 recruited and 80 received treatment
‐ 67 oxymetazoline ‐ 13 placebo N analysed: 82: 15 failed to return 4 to 6 weeks later Country: UK Age: mean age 22 years Inclusion criteria: upper respiratory tract infection symptoms between 12 and 120 hours Exclusion criteria: any prescribed medication other than the contraceptive pill, respiratory or cardiovascular disease, secondary bacterial infection, menthol exposure in previous 12 hours, sinusitis symptoms Diagnostic criteria: total nasal airway resistance > 0.3 Pa/cm/s |
|
| Interventions |
Nasal decongestant dose: 50 µg oxymetazoline hydrochloride Placebo: spray containing only vehicle Administration: single dose Nasal spray: 2 sprays in each nostril Follow‐up: 1 day Measurements: 60 min after treatment Total nasal airway resistance measured by posterior rhinomanometry at a sample pressure of 75 Pa. Nasal nitric oxide levels measured using a chemiluminescence gas analyser |
|
| Outcomes |
Primary outcome: nasal nitric oxide levels Secondary outcome: total nasal airway resistance |
|
| Notes |
Funding: Procter and Gamble Company Declarations of interest: not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not discussed |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Methods of blinding not clearly stated, only that both treatment and placebo used the same vehicle; a spray |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Methods of blinding not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 participants failed to return, but for all results the number of participants is indicated |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Unclear risk | Commercial funding |
Gronborg 1983.
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over design | |
| Participants |
N recruited: 34 N analysed: 30 ("Four subjects were excluded as it became evident on the second day that their cold symptoms had disappeared") Country: Denmark Age: range was 18 to 32 years, mean age was 23.0 years Inclusion criteria: a nose blowing of at least 0.1 mL could be provided in the observation period Exclusion criteria: none stated. "Four subjects were excluded as it became evident on the second day that their cold symptoms had disappeared" Diagnostic criteria: sudden occurrence of sneezing, nasal discharge and blockage, or at least of 2 of these symptoms. Nasal symptoms lasting 12 to 48 hours. The student felt sure that he/she caught a cold. The investigator observed signs of a cold (nasal voice, sneezing, nose blowing) during the 10 to 15 min observation period |
|
| Interventions |
Nasal decongestant dose: single dose of 100 mg norephedrine in a sustained release form Administration: single dose Oral tablets (cross‐over design: each participant received treatment on 1 day and placebo on another day) Follow‐up: 1 day Measurements: nasal airway resistance measured by posterior rhinomanometry and nasal peak flow measured immediately after rhinomanometry, using a Write Peak Flow minimeter. This was measured before and 2 hours after treatment Self‐assessment test for nasal blockage (scale from 0 "completely free" to 5 "complete blockage") and recording of numbers of sneezes and nose blowing per hour. This was measured hourly (2 to 10 hours after treatment) Side effects: a questionnaire about new symptoms |
|
| Outcomes | Study does not explicitly state which of the measured outcomes were primary or secondary We assumed the following: Primary outcomes: nasal airway resistance, nasal peak flow, self‐assessment of nasal blockage Secondary outcomes: numbers of sneezes and nose blowing per hours, side effects |
|
| Notes |
Funding: H. Lundbeck and Co. funded the study and provided the medication Declarations of interest: not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described, only that "each student got a single dose of 100 mg norephedrine in sustained release form and placebo in randomised order" |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment described but due to cross‐over design, all patients enrolled in the study received both treatment and placebo |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study reports that it is double‐blind: "the tablets were supplied in coded vials by H. Lundbeck and Co., Copenhagen, Denmark" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study reports that it is double‐blind. For rhinomanometry, because "the reading of the V2 value depends to some degree upon the investigator’s interpretation of the curve", "in order to eliminate this as a potential source of bias, [the authors] have added a computer to the set‐up, which digitally displays the means V2 value of five consecutive respiration curves." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 of the original 34 students who fulfilled the inclusion criteria "were excluded as it became evident on the second day that their cold symptoms had disappeared". The remaining 30 students completed the trial |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported |
| Other bias | Unclear risk | H. Lundbeck and Co. funded the study and provided the medication |
Jawad 1998.
| Methods | Open, randomised, parallel‐group, placebo‐controlled study | |
| Participants |
N recruited: 40 N analysed: 40 20 pseudoephedrine 20 placebo Age: range 18 to 49 years, mean 23 years Country: UK Inclusion criteria: patients with nasal congestion associated with a history of common cold for less than 96 hours Exclusion criteria: anatomical nasal obstruction or gross anatomical deformity, including moderate or severely deviated septum or the presence of nasal polyps; taken menthol lozenges or a menthol containing product in the past hour; taken any nasal decongestant in the past 12 hours; taken any antihistamine in the last 72 hours or astemizole in the last 30 days; taken any analgesic in the last 24 hours; taken any prescribed medication within the last 30 days (with the exception of the contraceptive pill); a history of hyperthyroidism, diabetes mellitus, heart disease prostatic hypertrophy or hypertension Diagnostic criteria: subjective score of 2 (moderate) for blocked nose, and at least 1 (mild) for any other cold symptoms. Patients were screened by the physician and a medical history was taken; blood pressure and pulse were measured |
|
| Interventions |
Nasal decongestant dose: Pseudoephedrine 60 mg tablet Placebo (Sanatogen multi‐vitamin tablet) Administration: multiple doses Oral tablets (2 doses on the same day with a 4‐hour interval) Follow‐up: 1 day Measurements: Unilateral nasal airflow was measured using posterior rhinomanometry at an inspiratory reference pressure of 75 Pa with an oral cannula to sense posterior nasal pressure. Measured 1 hour after the first dose of medication and then every hour over a 7‐hour period In this study nasal patency has been expressed in terms of nasal airflow at a reference pressure of 75 Pa, rather than as nasal resistance because nasal resistance tends towards infinity with nasal obstruction whereas nasal airflow tends towards zero. We assume this is nasal airway conductance Patients were asked to score their common cold symptoms of cough, runny nose, blocked nose and sore throat on a 5‐point box scale with symptoms labelled 0 = not present, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe |
|
| Outcomes | No distinction between primary and secondary outcomes was made Outcomes: Minimum and maximum unilateral airflow during the 7‐hour period of study Total nasal airflow |
|
| Notes |
Funding: not reported Declarations of interest: not provided Note: the study was not blinded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not clearly described: "Treatments […] were allocated according to a randomisation list. Those patients with a total nasal airflow of 175 cm³/sec or less were randomised to a low treatment group and those with a total nasal airflow of 176 cm³/sec or greater were randomised to a high treatment group" |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of patients involved in study is stated once in the results, with no mention of discontinuations or exclusions throughout the study |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Other bias | Low risk | No pharmaceutical funding or other sources of bias identified |
Latte 2004.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants |
N recruited: 50 N analysed: 48 ‐ 24 pseudoephedrine ‐ 24 placebo Age: range 18 to 52 years, mean age of 23 years Country: Australia Inclusion criteria: 18 years of age or older, healthy Exclusion criteria: nasal deformity, chronic obstruction, participants on vasoactive or nasally active drugs, or a drug with a potential interaction with pseudoephedrine, history of severe (greater than 2 weeks duration each year) allergic rhinitis Diagnostic criteria: symptoms of coryza for less than 48 hours, including at least ‘moderate’ symptoms of nasal congestion and a ‘mild’ sore throat or runny nose (on self‐assessed scales of none/mild/moderate/moderate severe/severe) at the time of the study |
|
| Interventions |
Treatment before study: none Nasal decongestant dose: Pseudoephedrine tablet 60 mg Placebo Administration: single dose Oral tablet Follow‐up: 1 day Measurements: Acoustic rhinometry and active posterior rhinomanometry at 75 Pa Subjective ratings of nasal congestion, measured on a 100 mm visual analogue scale anchored by the descriptors "nose completely clear" (0 mm) and "nose completely blocked" (100 mm) Baseline measurements were conducted at 20 min intervals for 1 hour, followed by dosing with the study treatment. Post‐treatment, 2 hours of serial measurements were undertaken at 20 min intervals |
|
| Outcomes |
Primary outcomes: total volume and total minimum cross‐sectional area inside nasal cavity Secondary outcomes: nasal airway resistance, visual analogue scale for perception of congestion |
|
| Notes |
Funding: source of funding was not provided, but the treatment medication was 60 mg SudafedTM tablet; Pfizer Consumer HealthCare Group (Caringbah, NSW, Australia) Declarations of interest: not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment randomisation was from a random numbers table, in blocks of four" |
| Allocation concealment (selection bias) | Low risk | "To conceal treatment allocation, the pseudoephedrine and placebo were placed in identical opaque gelatine capsules" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The pseudoephedrine and placebo were placed in identical opaque gelatine capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of method of blinding outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were accounted for |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported |
| Other bias | Low risk | No (clear) pharmaceutical funding or other sources of bias identified |
Latte 2007.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants |
N recruited: 216 N analysed: ‐ Placebo: N = 105 ‐ Pseudoephedrine: N = 107 212 had complete data on visit 1 210 had complete data on visit 2 211 completed treatment on day 3 It is not indicated how many participants of the treatment group or placebo group had complete data on day 2 and 3 Age: 18 to 65 years Country: Australia Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: episode of common cold no more than 48 hours duration before visit 1 |
|
| Interventions |
Nasal decongestant dose: Pseudoephedrine 60 mg Placebo Administration: multiple doses Oral tablets; 1 tablet 4 times a day for 4 days Follow‐up: 4 days Measurements: Total nasal airways resistance was measured using the posterior rhinomanometry technique Total nasal volume and total minimum cross‐sectional area were measured using acoustic rhinometry A 100 mm visual analogue scale from 0 mm to 100 mm was used to assess symptoms of nasal congestion Subjective measurements on a 7‐point categorical scale for the previous 24 hours (0 none to 6 incapacitating) ‐ worst levels of congestion ‐ worst levels of nasal discharge ‐ worst levels of sneezing Measurements were performed hourly (for 4 hours) after the first dose on day 1 and after the last dose on day 4 |
|
| Outcomes |
Primary outcome: the area under the logarithm‐transformed total nasal airways resistance curve from 0.5 to 3 hours after the first dose of study medication on day 1 Secondary outcomes: The area under the curve for the total minimum cross‐sectional area of the combined left and right nasal cavities from 0 to 3 hours on day 1 and 3 The area under the curve for the total nasal volume of the combined left and right nasal cavities from 0 to 3 hours on day 1 and 3 The area under the curve for the nasal congestion visual analogue scale from 0 to 3 hours on day 1 and 3 |
|
| Notes |
Funding: Pfizer Consumer Health Care Declarations of interest: not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation schedule was used: "Medication was allocated blindly according to a centrally generated randomisation code" |
| Allocation concealment (selection bias) | Unclear risk | Study reports that "medication was allocated blindly", but method of allocation concealment not clearly stated. Only that "subjects were given treatments consisting of either active medication […] or matching placebo". However, it is not clear if participants could not foresee assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study reports that it was double‐blind, but methods of blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study reports that it was double‐blind, but there is no mention of method of blinding outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There are 6 dropouts throughout the study, but only 1 discontinuation is explained in the text. The number of remaining participants in each treatment group is not clear |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Other bias | Unclear risk | Study supported by Pfizer Consumer Health Care |
Reinecke 2005.
| Methods | Randomised, double‐blind, placebo‐controlled, multi‐centre, parallel‐group study | |
| Participants |
N recruited: 247 N analysed: 247 Number of participants per treatment group not reported but we assume equal numbers (1 extra in the treatment group) ‐ Oxymetazoline N = 124 ‐ Placebo N = 123 Age: older than 12 Country: Germany Inclusion criteria: Patients with acute rhinitis, clinically healthy Duration of symptoms: not more than 48 hours Exclusion criteria: Patients could not use other nasal decongestants that work by stimulating the alpha‐adrenergic receptors, antihistamines, corticosteroids and other medication against the common cold including analgesics Diagnostic criteria: none documented |
|
| Interventions |
Treatment before study: not clear if the medication that was listed as an exclusion was not allowed during the first 48 hours before inclusion Nasal decongestant dose: ‐ Oxymetazoline: dose not described ‐ Saline nose spray Administration: multiple doses Nasal spray: patients were allowed to administer the treatment/placebo 3 times a day until the symptoms disappeared (maximum of 10 days) Follow‐up: 10 days Measurements: patient reports |
|
| Outcomes |
Primary outcome: number of days until full recovery Secondary outcomes: seconds until some improvement was perceived after a single dose, global satisfaction with treatment, reported symptoms, safety (i.e. satisfaction as rated by patients and doctors, blood pressure, heart rate and adverse events) |
|
| Notes |
Funding: source of funding not reported Declarations of interest: not provided Note: only a summary of the study was available; the authors refer to a full version of the study results, however we were not able to find this extensive version |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomised in each centre but methods of allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants not discussed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 247 patients were included, but data for only 214 are reported for the primary outcome. The reason for excluding 33 participants from analysis is not reported |
| Selective reporting (reporting bias) | High risk | Not all outcomes that were described in the methods were reported in the results (only a summary of the study was available; the authors refer to a full version of the study results, however we were not able to find this extensive version) |
| Other bias | Unclear risk | Not enough detail in the paper to exclude the possibility of other bias (e.g. funding and recruitment strategies not described) |
Sperber 1989.
| Methods | Randomised, double‐blind, placebo‐controlled, 3‐arm, parallel‐group study | |
| Participants |
N recruited: 58 N analysed: 56 All participants were included in the evaluation of tolerance, whereas analysis of efficacy was based only on infected participants As we only focus on monotherapy in this review, only the results for treatment group on pseudoephedrine only and placebo were extracted ‐ Pseudoephedrine: N = 23 ‐ Pseudoephedrine and ibuprofen: N = 23 ‐ Placebo: N = 10 Age: mean age 21 Country: USA Inclusion criteria: serum neutralising antibody titter of ≤ 1:2 to the challenge rhinovirus Exclusion criteria: Upper respiratory symptoms or fever within 1 week prior to initiation of the study History of active or chronic sinusitis, asthma or recent hay fever Required use of antihistamines, systemic or topical nasal decongestants, aspirin or other nonsteroidal anti‐inflammatory drugs, monoamine oxidase inhibitors or phenothiazines Had a history of hypersensitivity to aspirin or other anti‐inflammatory drugs, pseudoephedrine or other sympathomimetics Were pregnant or lactating Would be smoking during the study period Diagnostic criteria: none as patients were inoculated with virus at the beginning of the study |
|
| Interventions |
Treatment before study: experimentally induced rhinovirus colds: intranasal rhinovirus challenge was administered in 2 inocula over a 15‐min period by a calibrated pipette (50 µL per nostril) with the participant supine Nasal decongestant dose: Pseudoephedrine 60 mg Pseudoephedrine 60 mg and ibuprofen 200 mg Placebo Administration: multiple doses Oral capsules. 2 doses were given the first day. On the subsequent 4 days, the drug was administered 4 times daily for a total of 18 doses Follow‐up: 4 days Measurements: Infection: nasal washings were collected prior to virus inoculation and each morning on days 2 through 6 after challenge. Infection was defined as seroconversion and/or recovery of the challenge virus from nasal washings on at least 1 day Illness: twice daily recording of the volunteers' symptoms: nasal (discharge, obstruction, sneezing), throat (sore throat, hoarseness, cough) and systemic (headache, chills, feverishness, malaise) on a 4‐point scale (0 to 3, absent to severe). The higher of the 2 daily ratings was used as the score for that day The need for concomitant medications dispensed for cold symptoms Participants were questioned twice daily concerning the presence of any unusual symptoms potentially referable to drug toxicity Objective measures of illness severity included morning and evening oral temperatures; daily collection of nasal tissues for tissue counts and determination of nasal secretion weights Blood pressure was measured 3 times and pulse rate once daily in all participants during the study period |
|
| Outcomes | There is no distinction between primary and secondary outcomes in the text Outcomes: infection and virus shedding rates post‐infection, symptom scores, mucus weight, nasal tissue count, rates and indication for use of acetaminophen in infected patients, nasal patency, adverse events |
|
| Notes |
Funding: funded by Richardson‐Vick’s Research Center Shleton, CT, and by a grant of the National Institute of Allergy and Infectious Diseases Declarations of interest: not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of sequence generation reported |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study reports that it is double‐blinded, but no method reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study reports that it is double‐blinded, but no method reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients are accounted for |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Other bias | Unclear risk | Funded by Richardson‐Vick’s Research Center and also supported by The Aspirin Foundation of America |
Taverner 1999.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants |
N recruited: 99 participants were screened 54 satisfied the inclusion and exclusion criteria N analysed: 52 completed the study ‐ 25 pseudoephedrine ‐ 27 placebo Age: range 18 to 55 years, mean age 26 (± 9 years) Country: Australia Inclusion criteria: Symptoms of an acute common cold (defined as acute nasal congestion, combined with rhinorrhoea and/or a sore throat) for less than 5 days, acute viral upper respiratory tract infections (defined by presence of pharyngeal erythema and moderate‐severe nasal obstruction as examined by anterior rhinoscopy) Exclusion criteria: Hay fever, broncho‐pulmonary disease, anatomical nasal obstruction, hypertension, prior ingestion of vasoactive drugs, caffeine and alcohol Diagnostic criteria: Symptoms of an acute common cold (defined as acute nasal congestion, combined with rhinorrhoea and/or a sore throat) for less than 5 days, acute viral upper respiratory tract infections (defined by presence of pharyngeal erythema and moderate‐severe nasal obstruction as examined by anterior rhinoscopy) |
|
| Interventions |
Nasal decongestant dose: 60 mg pseudoephedrine Administration: single dose Oral tablets, dose administered within 10 min of baseline Follow‐up: 1 day Measurements: Acoustic rhinometry: total minimal cross‐sectional area and total nasal volume Active posterior rhinomanometry: total nasal airway resistance Subjective congestion: a 5‐point categorical score: 0 = no congestion; 4 = severe congestion Measurements were performed at 30‐min intervals until 180 min after dosing |
|
| Outcomes |
Primary outcome: changes in nasal congestion measured by total minimal cross‐sectional area, total nasal volume and total nasal airway resistance Secondary outcomes: subjective symptoms of nasal congestion and adverse effects |
|
| Notes |
Funding: Procter & Gamble Technical Centres Ltd. Declarations of interest: not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation scheme was used: "Eligible subjects were allocated in a double‐blind randomisation schedule which assigned subjects to 1 of the 2 rhinoanemometers/acoustic rhinometers used in this study and to the pseudoephedrine or placebo group in equal numbers" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The randomisation code was not broken until all data, including delayed adverse events had been allocated" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data are discussed and the number of remaining participants for each treatment group is provided: "Of the 54 subjects who were entered randomisation, 2 failed to complete the study" |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Unclear risk | Commercial funding: Procter & Gamble Technical Centres Ltd |
AUC: area under the curve NAR: nasal airway resistance
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ackerhans 1994 | Participants had acute rhinitis |
| Anderson 1956 | Not a RCT |
| Anonymous 1975 | Not a RCT |
| Ashe 1968 | Participants had nasal obstruction due to various reasons including allergy |
| Bailey 1969 | Participants had nasal obstruction due to various reasons including allergy |
| Bende 1984 | Participants had nasal obstruction, not due to common cold |
| Bende 1985 | Nasal stuffiness was the only inclusion criterion, hence it is not clear for how long participants experienced nasal congestion and whether this was due to the common cold |
| Broms 1982 | Participants suffered from nasal obstruction for more than 6 months |
| Castellano 2002 | Participants had reduced airflow, which was not necessarily due to the common cold |
| Cohen 1977 | Combination therapy |
| Connell 1969 | Some of the patients had allergic rhinitis |
| De Paula Neves 1966 | Combination therapy |
| Dorn 2003 | Trial was not placebo‐controlled |
| Fox 1967 | Trial was not placebo‐controlled |
| Hummel 1998 | None of pre‐specified outcome measures were reported |
| Katrana 1956 | Not a RCT |
| McElhenney 1966 | Not a RCT |
| Meurman 1975 | Trial was not placebo‐controlled |
| Pritchard 2014 | Participants with hay fever were also included |
| Rumiantsev 1993 | Combination therapy |
| Smith 1999 | Not an RCT |
| Tzachev 2002 | Participants had allergic rhinitis |
| Weisberg 1966 | Combination therapy |
| Winther 1983 | Nasal airway resistance was not measured; the weight of nasal discharge was measured |
| Zumpft 1975 | Participants had allergic rhinitis |
RCT: Randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00452270.
| Methods | Double‐blind, randomised, placebo‐controlled trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | The decongestant effect of xylometazoline in participants with common cold compared to placebo |
| Outcomes |
Primary outcome: Rhinomanometry over a period of 12 hours Secondary outcome: The peak subjective effect, time to onset of subjective relief of nasal obstruction and duration of relief of nasal obstruction |
| Notes | Commercial funding: study sponsored by Novartis This study was completed in April 2007, however we could not find published results for this study |
NCT01062360.
| Methods | Double‐blind, randomised, placebo‐controlled trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | Compare efficacy and tolerability of a fixed combination, containing 500 mg acetylsalicylic acid and 30 mg pseudoephedrine, in comparison to its single components and placebo |
| Outcomes |
Primary outcome: AUC calculated for baseline adjusted nasal congestion score for the initial 2 hours post‐dosing Secondary outcome: Nasal congestion score: 15, 30, 60, 90, 120, 240 and 360 min Nasal congestion relief score: 15, 30, 60, 90, 120, 240 and 360 min Symptoms of common cold at 120 min post‐dose |
| Notes | Commercial funding: study sponsored by Bayer The protocol was first received February 2010 and the study has been completed, however we could not find published results for this study |
ASA: acetyl salicylic acid AUC: area under the curve PSE: pseudoephedrine URTI: upper respiratory tract infection
Characteristics of ongoing studies [ordered by study ID]
EUCTR2006‐006690‐25‐GB.
| Trial name or title | A study to evaluate the decongestant effect of Otrivin F2 |
| Methods | A double‐blind, randomised, parallel‐group, placebo‐controlled study |
| Participants |
Inclusion criteria:
Exclusion criteria
|
| Interventions | The decongestant effect of xylometazoline hydrochloride (Otrivin® F2) in participants with common cold compared to placebo treatment |
| Outcomes |
Primary outcome: The upper airway conductance at 1 hour |
| Starting date | Not known |
| Contact information | Not known |
| Notes | Commercial funding: sponsored by Novartis Consumer Health SA This study record was entered in EuraCT in 2007, however it is indicated as still ongoing |
NCT01744106.
| Trial name or title | A multicentre study of pseudoephedrine for the temporary relief of nasal congestion in children with the common cold |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled study |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | The temporary relief of nasal congestion due pseudoephedrine versus placebo |
| Outcomes |
Primary outcome: Nasal congestion severity (instantaneous) scores ‐ Day 1 Weighted sum of change from baseline in nasal congestion severity (instantaneous) scores over the first 8 hours of treatment on Day 1 Secondary outcomes: Change from baseline in nasal congestion severity (instantaneous) scores from 0 to 4 hours ‐ Day 1 Sum of change from baseline in nasal congestion severity (instantaneous) scores from 0 to 4 hours after the first dose on Day 1 Change from baseline in nasal congestion severity (instantaneous) scores from 6, 7, and 8 hours ‐ Day 1 Weighted sum of change from baseline in nasal congestion severity scores (instantaneous) from 6, 7 and 8 hours after the first dose on Day 1 Nasal congestion relief reflective scores at 4 hours and 8 hours ‐ Day 1 Sum of nasal congestion relief (reflective) scores at 4 hours and 8 hours on Day 1 Nasal congestion severity scores (instantaneous) score at each time point from 0 to 8 hours ‐ Day 1 Nasal congestion severity scores (instantaneous) score at each time point from 0 to 8 hours after the first dose on Day 1 Nasal congestion relief (reflective) scores at 6 hours and 12 hours ‐ Day 2 Sum of nasal congestion relief (reflective) scores at 6 hours and 12 hours on Day 2 Nasal congestion relief (reflective) scores at 6 and 12 hours ‐ Day 2 Nasal congestion relief (reflective) scores at 6 and 12 hours on Day 2 |
| Starting date | November 2012 |
| Contact information | Only contact information of participating centres is available: e.g. United States, California Emmaus Research Center Anaheim, California, United States, 92804 Contact: Filipinas Vitug 714‐826‐8800 |
| Notes |
Commercial funding: sponsored by Perrigo Company in collaboration with McNeil Consumer Healthcare Division of McNEIL‐PPC, Inc. Pfizer, and Novartis Pharmaceuticals Estimated completion date: was initially April 2015 and recently updated to May 2016 |
Differences between protocol and review
The review differs from the protocol (Ta'i 2012) in the following sections.
In Methods, Data collection and analysis and Unit of analysis issues we specified how we handled data from trials that included more than one treatment arm. If the treatment arm was similar (e.g. different doses of the same nasal decongestant), we combined data from these treatment arms and compared this group to the control group, as recommended in section 7.7.3.8 and Table 7.7a of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We added randomised cross‐over trials to the inclusion criteria because cross‐over studies with adequate randomisation can be regarded as RCTs. We also specified how we handled data from trials using cross‐over designs; results of these studies were not included in the meta‐analysis, but were reported narratively.
In Methods, Data collection and analysis and Data synthesis we specified the rule‐of‐thumb for effect sizes to facilitate interpretation of the SMD as described in section 12.6.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In the same section, we also added a description of how we assessed the overall quality of the evidence. We used the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach, which gives an indication of the confidence that can be placed in the estimate of treatment effect. The effect estimates and GRADE ratings were summarised in the 'Summary of findings' tables. We only summarised results for which more than one study was available. We used the GRADEprofiler tool (GRADEpro) and followed the advice from the GRADE Handbook (Schünemann 2009).
We also analysed single and multi‐dose studies together, whereas in the protocol it was stated that we would analyse them separately (Ta'i 2012). Clinically it could be argued that a single or multiple‐dose nasal decongestant would have a similar effect, although it may not be as long lasting from a single dose. We expect that up to three hours after dosing, the effect of a single dose is clinically not expected to be inferior to multiple doses. Therefore we combined single and multiple doses up to three hours after dosing.
Based on feedback from the statistical editor we decided to use a random‐effects model for all meta‐analyses. Nevertheless, we reported whether using a fixed‐effect or random‐effects model affected the results (see Methods; Data collection and analysis; Data synthesis).
Contributions of authors
Laura Deckx (LD) contributed to the data extraction, performed the data analyses and wrote the first draft of the review. An De Sutter (ADS) and Mieke van Driel (MLvD) commented on the draft and contributed to the final version. Linda Guo (LG) and Nabiel Mir (NM) contributed to the data extraction, 'Risk of bias' assessment and drafting of the manuscript under the supervision of LD and MLvD.
Sources of support
Internal sources
Discipline of General Practice, Australia.
External sources
No sources of support supplied
Declarations of interest
Laura Deckx: none known An IM De Sutter: none known Linda Guo: none known Nabiel A Mir: none known Mieke L van Driel: none known
New
References
References to studies included in this review
Akerlund 1989 {published data only}
- Akerlund A, Klint T, Olen L, Rundcrantz H. Nasal decongestant effect of oxymetazoline in the common cold: an objective dose‐response study in 106 patients. Journal of Laryngology & Otology 1989;103(8):743‐6. [DOI] [PubMed] [Google Scholar]
Bye 1980 {published data only}
- Bye CE, Cooper J, Empey DW, Fowle AS, Hughes DT, Letley E, et al. Effects of pseudoephedrine and triprolidine, alone and in combination, on symptoms of the common cold. BMJ 1980;281(6234):189‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1978 {published data only}
- Cohen BM. Clinical correlants of changes in nasal flow/resistance (Rn) measurements. Allergologia et Immunopathologia 1978;6(3):217‐23. [PubMed] [Google Scholar]
Eccles 2005 {published data only}
- Eccles R, Jawad MSM, Jawad SSM, Angello JT, Druce HM. Efficacy and safety of single and multiple doses of pseudoephedrine in the treatment of nasal congestion associated with common cold. American Journal of Rhinology 2005;19(1):25‐31. [PubMed] [Google Scholar]
Eccles 2006 {published data only}
- Eccles R, Jawad M, Jawad S, Ridge D, North M, Jones E, et al. Efficacy of a paracetamol‐pseudoephedrine combination for treatment of nasal congestion and pain‐related symptoms in upper respiratory tract infection. Current Medical Research and Opinion 2006;22(12):2411‐8. [DOI] [PubMed] [Google Scholar]
Eccles 2008 {published data only}
- Eccles R, Eriksson M, Garreffa S, Chen SC. The nasal decongestant effect of xylometazoline in the common cold. American Journal of Rhinology 2008;22(5):491‐6. [DOI] [PubMed] [Google Scholar]
- Eccles R, Martensson K, Stephen G, Chen S. A double‐blind, randomised, parallel group, placebo‐controlled study evaluating the nasal decongestant effect of xylometazoline in common cold [Abstract]. Primary Care Respiratory Journal 2008;17(2):120. [Google Scholar]
Eccles 2014 {published data only}
- Eccles R, Voelker M. Analgesic and decongestant efficacy of the combination of aspirin with pseudoephedrine in patients with symptoms of upper respiratory tract infection. Clinical Pharmacology in Drug Development 2014;3(2):118‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferguson 1997 {published data only}
- Ferguson EA, Eccles R. Changes in nasal nitric oxide concentration associated with symptoms of common cold and treatment with a topical nasal decongestant. Acta Oto‐Laryngologica 1997;117(4):614‐7. [DOI] [PubMed] [Google Scholar]
Gronborg 1983 {published data only}
- Gronborg H, Winther B, Brofeldt S, Borum P, Mygind N. Effects of oral norephedrine on common cold symptoms. Rhinology 1983;21(1):3‐12. [PubMed] [Google Scholar]
Jawad 1998 {published data only}
- Jawad SS, Eccles R. Effect of pseudoephedrine on nasal airflow in patients with nasal congestion associated with common cold. Rhinology 1998;36(2):73‐6. [PubMed] [Google Scholar]
Latte 2004 {published data only}
- Latte J, Taverner D, Slobodian P, Shakib S. A randomized, double‐blind, placebo‐controlled trial of pseudoephedrine in coryza. Clinical and Experimental Pharmacology & Physiology 2004;31(7):429‐32. [DOI] [PubMed] [Google Scholar]
Latte 2007 {published data only}
- Latte J, Taverner D. Clinical trial of 3 days of treatment with oral pseudoephedrine for the common cold in the southern hemisphere. American Journal of Rhinology 2007;21(4):452‐5. [DOI] [PubMed] [Google Scholar]
Reinecke 2005 {published data only}
- Reinecke S, Tschaikin M. Investigation of the effect of oxymetazoline on the duration of rhinitis [Alpha‐Sympathomimetikum bessert nicht nur die nasale Obstruktion: Schnupfendauer wird verkürzt]. MMW Fortschritte der Medizin 2005;147(41):46. [PubMed] [Google Scholar]
Sperber 1989 {published data only}
- Sperber SJ, Sorrentino JV, Riker DK, Hayden FG. Evaluation of an alpha agonist alone and in combination with a nonsteroidal antiinflammatory agent in the treatment of experimental rhinovirus colds. Bulletin of the New York Academy of Medicine 1989;65(1):145‐60. [PMC free article] [PubMed] [Google Scholar]
Taverner 1999 {published data only}
- Taverner D, Danz C, Economos D. The effects of oral pseudoephedrine on nasal patency in the common cold: a double‐blind single‐dose placebo‐controlled trial. Clinical Otolaryngology and Allied Sciences 1999;24(1):47‐51. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ackerhans 1994 {published data only}
- Ackerhans M, Jannert M, Tonnesson M. Effects of a new administration form of oxymetazoline on maxillary ostial patency in healthy individuals and patients with acute rhinitis. Acta Oto‐Laryngologica Supplement 1994;515:49‐52. [DOI] [PubMed] [Google Scholar]
Anderson 1956 {published data only}
- Anderson HA. Clinical trial of tetrahydrozoline pediatric nasal decongestant. Antibiotic Medicine & Clinical Therapy 1956;3(3):199‐201. [PubMed] [Google Scholar]
Anonymous 1975 {published data only}
- Anonymous. Evaluation of a new oxymetazoline preparation in the treatment of nasal congestion. A multicentre trial. Practitioner 1975;214(1283):685‐8. [PubMed] [Google Scholar]
Ashe 1968 {published data only}
- Ashe GJ. Oral medications in nasal decongestion. A study among industrial workers. Industrial Medicine and Surgery 1968;37(3):212‐4. [PubMed] [Google Scholar]
Bailey 1969 {published data only}
- Bailey BJ. Clinical evaluation of a topical nasal decongestant ‐ oxymetazoline. Eye, Ear, Nose & Throat Monthly 1969;48(9):512‐5. [PubMed] [Google Scholar]
Bende 1984 {published data only}
- Bende M, Andersson KE, Johansson CJ, Sjögren C, Svensson G. Dose‐response relationship of a topical nasal decongestant: phenylpropanolamine. Acta Oto‐Laryngologica 1984;98(5‐6):543‐7. [DOI] [PubMed] [Google Scholar]
Bende 1985 {published data only}
- Bende M. Contralateral effects of unilateral nasal decongestion. Acta Oto‐Laryngologica 1985;99(5‐6):637‐40. [DOI] [PubMed] [Google Scholar]
Broms 1982 {published data only}
- Broms P, Malm L. Oral vasoconstrictors in perennial non‐allergic rhinitis. Allergy 1982;37(2):67‐74. [DOI] [PubMed] [Google Scholar]
Castellano 2002 {published data only}
- Castellano F, Mautone G. Decongestant activity of a new formulation of xylometazoline nasal spray: a double‐blind, randomized versus placebo and reference drugs controlled, dose‐effect study. Drugs Under Experimental & Clinical Research 2002;28(1):27‐35. [PubMed] [Google Scholar]
Cohen 1977 {published data only}
- Cohen BM. Physiologic and subjective comparisons of 2 oral sustained‐release nasal decongestant combinations and placebo in patients with common colds. Current Therapeutic Research 1977;22(4):522‐8. [Google Scholar]
Connell 1969 {published data only}
- Connell JT. Effectiveness of topical nasal decongestants. Annals of Allergy 1969;27(11):541‐6. [PubMed] [Google Scholar]
De Paula Neves 1966 {published data only}
- Paula Neves F. Clinical trial of a drug combination (JJ‐4482) used for the treatment of catarrhal inflammations of the upper respiratory tract [Ensaio clinico com uma associacao medicamentosa (JJ‐4482) destinada ao tratamento das inflamacoes catarrais das vias aereas superiores]. Revista Brasileira de Medicina 1966;23(12):878‐80. [PubMed] [Google Scholar]
Dorn 2003 {published data only}
- Dorn M, Hofmann W, Knick E. Tolerance and effectiveness of oxymetazoline and xylometazoline in treatment of acute rhinitis [Verträglichkeit und Wirksamkeit von Oxymetazolin* und Xylometazolin bei der Behandlung der akuten Rhinitis]. HNO 2003;51(10):794‐9. [DOI] [PubMed] [Google Scholar]
Fox 1967 {published data only}
- Fox SL. The use of oral decongestants in the management of diverse types of nasal congestion. Current Therapeutic Research 1967;9(5):247‐52. [PubMed] [Google Scholar]
Hummel 1998 {published data only}
- Hummel T, Rothbauer C, Pauli E, Kobal G. Effects of the nasal decongestant oxymetazoline on human olfactory and intranasal trigeminal function in acute rhinitis. European Journal of Clinical Pharmacology 1998;54(7):521‐8. [DOI] [PubMed] [Google Scholar]
Katrana 1956 {published data only}
- Katrana NJ. Clinical trial of tyzine, a new nasal decongestant. Illinois Medical Journal 1956;110(1):19‐21. [PubMed] [Google Scholar]
McElhenney 1966 {published data only}
- McElhenney TR, Grater WC, Pfeiffer GO, Sanders SH, McGovern JP, Weisberg J. A comparative evaluation of four oral nasal decongestants. Current Therapeutic Research 1966;8(6):291‐8. [PubMed] [Google Scholar]
Meurman 1975 {published data only}
- Meurman OH. A controlled clinical comparison of nasal decongestants in acute rhinitis. Journal of International Medical Research 1975;3(5):356‐62. [Google Scholar]
Pritchard 2014 {published data only}
- Pritchard S, Glover M, Guthrie G, Brum J, Ramsey D, Kappler G, et al. Effectiveness of 0.05% oxymetazoline (Vicks Sinex Micromist®) nasal spray in the treatment of objective nasal congestion demonstrated to 12 h post‐administration by magnetic resonance imaging. Pulmonary Pharmacology and Therapeutics 2014;27(1):121‐6. [DOI] [PubMed] [Google Scholar]
Rumiantsev 1993 {published data only}
- Rumiantsev AG, Aldonina VV, Il'ina TP, Snitarenko VI, Biriuchinskaia IG, Shevaldina TV. A trial of the use of the prolonged‐action preparation Koldakt from the Natko firm for treating upper respiratory tract diseases in polyclinics [Opyt primeneniia preparata prolongirovannogo deistviia "Koldakt" firmy "Natko" dlia lecheniia zabolevanii verkhnikh dykhatel'nykh putei v poliklinicheskikh usloviiakh]. Terapevticheskii Arkhiv 1993;65(11):70, 72. [PubMed] [Google Scholar]
Smith 1999 {published data only}
- Smith A, Sturgess W, Rich N, Brice C, Collison C, Bailey J, et al. The effects of idazoxan on reaction times, eye movements and the mood of healthy volunteers and patients with upper respiratory tract illnesses. Journal of Psychopharmacology 1999;13(2):148‐51. [DOI] [PubMed] [Google Scholar]
Tzachev 2002 {published data only}
- Tzachev CT, Mandajieva M, Minkov EH, Popov TA. Comparison of the clinical efficacy of standard and mucoadhesive‐based nasal decongestants. British Journal of Clinical Pharmacology 2002;53(1):107‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weisberg 1966 {published data only}
- Weisberg J, Breslow I. The evaluation of a nasal decongestant preparation. Archives of Otolaryngology 1966;83(5):477‐9. [DOI] [PubMed] [Google Scholar]
Winther 1983 {published data only}
- Winther B, Brofeldt S, Borum P, Pedersen M, Mygind N. Lack of effect on nasal discharge from a vasoconstrictor spray in common cold. European Journal of Respiratory Diseases Supplement 1983;128(Pt 2):447‐8. [PubMed] [Google Scholar]
Zumpft 1975 {published data only}
- Zumpft CW. Double‐blind comparison of metizoline hydrochloride (Ellsyl) and phenylephrine in allergic and vasomotor rhinitis. Current Therapeutic Research 1975;17(1):40‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00452270 {unpublished data only}
- NCT00452270. A double‐blind, randomized, parallel group, placebo controlled study, evaluating the decongestant effect, time to onset, duration of effect and impact on sleep and general well‐being of xylometazoline in subjects with a common cold. https://clinicaltrials.gov/ct2/show/NCT00452270 (accessed 6 October 2015).
NCT01062360 {unpublished data only}
- NCT01062360. A pivotal, placebo controlled, phase III study to compare efficacy and tolerability of a fixed combination, containing 500 mg ASA and 30 mg pseudoephedrine, in comparison to its single components in patients with sore throat and nasal congestion. https://clinicaltrials.gov/ct2/show/NCT01062360 (accessed 6 October 2015).
References to ongoing studies
EUCTR2006‐006690‐25‐GB {unpublished data only}
- EUCTR2006‐006690‐25‐GB. A double‐blind, randomized, parallel group, placebo controlled study, evaluating the decongestant effect, time to onset, duration of effect and impact on sleep and general well‐being of otrivin F2 in subjects with a common cold ‐ a study to evaluate the decongestant effect of Otrivin F2. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2006‐006690‐25/GB (accessed 6 October 2015).
NCT01744106 {unpublished data only}
- NCT01744106. A multicenter study of pseudoephedrine for the temporary relief of nasal congestion in children with the common cold. https://clinicaltrials.gov/ct2/show/NCT01744106 (accessed 6 October 2015). [DOI] [PMC free article] [PubMed]
Additional references
AlBalawi 2013
- AlBalawi ZH, Othman SS, AlFaleh K. Intranasal ipratropium bromide for the common cold. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD008231.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allan 2014
- Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. Canadian Medical Association Journal 2014;186(3):190‐9. [DOI: 10.1503/cmaj.121442] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arroll 2005
- Arroll B. Non‐antibiotic treatments for upper‐respiratory tract infections (common cold). Respiratory Medicine 2005;99:1477‐84. [DOI] [PubMed] [Google Scholar]
Arroll 2011
- Arroll B. Common cold. BMJ Clinical Evidence 2011. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3275147/pdf/2011‐1510.pdf (accessed 3 November 2015).
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Sutter 2012
- Sutter AIM, Driel ML, Kumar AA, Lesslar O, Skrt A. Oral antihistamine‐decongestant‐analgesic combinations for the common cold. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD004976.pub3] [DOI] [PubMed] [Google Scholar]
Del Mar 2003
- Mar C, Glasziou P. Upper respiratory tract infection. BMJ Clinical Evidence 2003;10:1747‐56. [PubMed] [Google Scholar]
Dolansky 2008
- Dolansky G, Rieder M. What is the evidence for the safety and efficacy of over‐the‐counter cough and cold preparations for children younger than six years of age?. Journal of Paediatrics and Child Health 2008;13(2):125‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eccles 2000
- Eccles R. Pathophysiology of nasal symptoms. American Journal of Rhinology 2000;14(5):335‐8. [DOI] [PubMed] [Google Scholar]
Eccles 2007
- Eccles R. Substitution of phenylephrine for pseudoephedrine as a nasal decongestant. An illogical way to control methamphetamine abuse. British Journal of Clinical Pharmacology 2007;63(1):10‐4. [DOI: 10.1111/j.1365-2125.2006.02833.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eccles 2009
- Eccles R. Over the counter medicines for colds. In: Eccles R, Webber O editor(s). Common Cold. Basel: Birkhauser Verlag, 2009:23‐45. [Google Scholar]
Eccles 2010
- Eccles R, Martensson K, Chen SC. Effects of intranasal xylometazoline, alone or in combination with ipratropium, in patients with common cold. Current Medical Research & Opinion 2010;26:889‐99. [DOI] [PubMed] [Google Scholar]
FDA 2000
- FDA Public Health Advisory: Safety of Phenylpropanolamine. Federal Register 2012. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm052236.htm (accessed 22 April 2016).
FDA 2005
- Information by drug class: Legal Requirements for the Sale and Purchase of Drug Products Containing Pseudoephedrine, Ephedrine, and Phenylpropanolamine. Federal Register 2005. http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm072423.htm (accessed 22 April 2016).
FDA 2012
- FDA Drug Safety Communication: Serious adverse events from accidental ingestion by children of over‐the‐counter eye drops and nasal sprays. Federal Register 2012. http://www.fda.gov/Drugs/DrugSafety/ucm325257.htm (accessed 3 November 2015).
Fendrick 2003
- Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non‐influenza‐related viral respiratory tract infection in the United States. Archives of Internal Medicine 2003;163(4):487–94. [DOI] [PubMed] [Google Scholar]
Fry 1993
- Fry J, Sandler G. Common Diseases. Their Nature, Prevalence and Care. 5th Edition. Dordrecht: Kluwer Academic, 1993. [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEproGDT: GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
Hayward 2015
- Hayward G, Thompson MJ, Perera R, Mar CB, Glasziou PP, Heneghan CJ. Corticosteroids for the common cold. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD008116.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heikkinnenn 2003
- Heikkinnenn T, Jarvinen A. The common cold. Lancet 2003;361(9351):51‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
King 2015
- King D, Mitchell B, Williams CP, Spurling GKP. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD006821.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kollar 2007
- Kollar C, Schneider H, Waksman J, Krusinska E. Meta‐analysis of the efficacy of a single dose of phenylephrine 10 mg compared with placebo in adults with acute nasal congestion due to the common cold. Clinical Therapeutics 2007;29:1057‐70. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J (editors). Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Meltzer 2010
- Meltzer EO, Caballero F, Fromer LM, Krouse JH, Scadding G. Treatment of congestion in upper respiratory diseases. International Journal of General Medicine 2010;3:69‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
NPS Medicinewise 2012
- NPS Medicinewise. Nasal decongestants. National Prescribing Service (http://www.nps.org.au/medicines/ear‐nose‐mouth‐and‐throat/nasal‐decongestants) (accessed 9 July 2016) 2012. [http://www.nps.org.au/medicines/ear‐nose‐mouth‐and‐throat/nasal‐decongestants]
Pappas 2015
- Pappas D. The common cold in children. Available from www.uptodate.com 2015.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2009
- Schünemann H, Brozek J, Oxman A (editors). GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2 [updated March 2009]. The GRADE Working Group. Available from http://www.cc‐ims.net/gradepro 2009.
Simasek 2007
- Simasek M, Blandino D. Treatment of the common cold. American Family Physician 2007;75(4):515‐20. [PubMed] [Google Scholar]
Singh 2013
- Singh M, Singh M. Heated, humidified air for the common cold. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD001728.pub5] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Wicker 2009
- Wicker A, Labruzzo B. Recommendations for the use of OTC cough and cold medications in children: decongestants. US Pharmacist 2009;34(3):33‐6. [Google Scholar]
References to other published versions of this review
Ta'i 2012
- Ta'i SH, Ferguson KAM, Singh HK, Sharma AN, Kumar S, Driel ML, et al. Nasal decongestants for the common cold. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009612] [DOI] [Google Scholar]
Taverner 2004
- Taverner D, Latte J, Draper M. Nasal decongestants for the common cold. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD001953.pub2] [DOI] [PubMed] [Google Scholar]
Taverner 2007
- Taverner D, Latte J. Nasal decongestants for the common cold. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001953.pub3] [DOI] [PubMed] [Google Scholar]


