Abstract
Background
Essential tremor is one of the most common movement disorders. Treatment primarily consists of pharmacological agents. While primidone and propranolol are well‐established treatments in clinical practice, they may be ineffective in 25% to 55% of patients and can produce serious adverse events in a large percentage of them. For these reasons, it is worth evaluating the treatment alternatives for essential tremor. Some specialists have suggested that pregabalin could be a potentially useful agent, but there is uncertainty about its efficacy and safety.
Objectives
To assess the effects of pregabalin versus placebo or other treatment for essential tremor in adults.
Search methods
We performed a systematic search without language restrictions to identify all relevant trials up to December 2015. We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, NICE, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform (ICTRP). We handsearched grey literature and examined the reference lists of identified studies and reviews.
Selection criteria
We included all randomised controlled trials (RCTs) of pregabalin versus placebo or any other treatments. We included studies in which the diagnosis of ET was made according to accepted and validated diagnostic criteria. We excluded studies conducted in patients presenting secondary forms of tremor or reporting only neurophysiological parameters to assess outcomes.
Data collection and analysis
Two reviewers independently collected and extracted data using a data collection form. We assessed the risk of bias of the body of evidence, and we used inverse variance methods to analyse continuous outcomes and measurement scales. We compared the mean difference between treatment groups, and we combined results for dichotomous outcomes using Mantel‐Haenszel methods and risk differences We used Review Manager software for data management and analysis.
Main results
We only found one study eligible for this review (22 participants). We assessed the risk of bias for most domains as unclear. We graded the overall quality of evidence as very low. Compared to placebo, patients treated with pregabalin showed no significant improvement of motor tasks on the 36‐point subscale of the Fahn‐Tolosa‐Marin Tremor Rating Scale (TRS) (MD −2.15 points; 95% CI −9.16 to 4.86) or on the 32‐point functional abilities subscale of the TRS (MD −0.66 points; 95% CI −2.90 to 1.58).The limited evidence showed no difference in study withdrawal (Mantel‐Haenszel RD −0.09; 95% CI −0.48 to 0.30) and presentation of adverse events between pregabalin and placebo (Mantel‐Haenszel RD 0.18; 95% CI −0.13 to 0.50).
Authors' conclusions
The effects of pregabalin for treating essential tremor are uncertain because the quality of the evidence is very low. One small study did not highlight any effect of this treatment; however, the high risk of bias and the lack of other studies on this topic limit further conclusion.
Plain language summary
Use of pregabalin for the treatment of essential tremor
Review question
The authors of this review tried to assess the effectiveness and safety of pregabalin in people with essential tremor.
Backgound
Essential tremor is the most common movement disorder. Although benign in terms of its effect on life expectancy, it is typically progressive and potentially disabling. Treatment consists primarily of pharmacological agents (propranolol and primidone as first‐line therapy), which could be ineffective for 25% to 55% of patients. Some specialists have suggested that pregabalin could be a potentially useful drug for treating the condition.
Study characteristics
We found one study comparing pregabalin versus placebo, involving 22 randomised participants with essential tremor.
Key results
The impact of pregabalin on functional abilities and adverse effects is uncertain because the quality of the evidence is very low.
Quality of the evidence
The lack of studies and the significant limitations in the one included trial preclude firm conclusions about the risk‐benefit profile of this treatment.
Summary of findings
Summary of findings 1. Pregabalin for essential tremor.
| Pregabalin versus placebo for essential tremor | |||||
|
Patient or population: patients with essential tremor
Settings: outpatients
Intervention: pregabalin Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Pregabalin | ||||
|
Functional abilities Change in TRS part B score (motor tasks).36 points (0 is better) Change in TRS part C score (functional disability), 32 points (0 is better) Follow‐up: 42 days |
The mean change in the control group was 1.63 points in TRS part B score and 0.90 points in TRS part C score at the end of follow‐up, compared to baseline. | The mean change in the intervention group was 2.15 points higher (9.16 points lower to 4.86 points higher) in TRS part B score and 0.66 points higher (2.90 points lower to 1.58 points higher) in TRS part C score, compared to control. | — | 22 (1 study) | ⊕⊝⊝⊝ Very lowa,b |
|
Study withdrawal Number of patients withdrawn from the study Follow‐up: 42 days |
Study population | RD −0.09 (−0.48 to 0.30) | 22 (1 study) | ⊕⊝⊝⊝ Very lowa,b | |
| 4 per 11 patients | 3 per 11 patients | ||||
|
Adverse events Number of adverse events Follow‐up: 42 days |
Study population | RD 0.18 (−0.13 to 0.50) | 22 (1 study) | ⊕⊝⊝⊝ Very lowa,b | |
| 1 adverse events per 11 patients | 3 adverse events per 11 patients | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). adverse events: adverse events; CI: Confidence interval; OR: Odds ratio; TRS: tremor rating scale. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded one level due to very serious risk of bias: allocation not described (selection bias) and incomplete outcome reporting (attrition bias). bDowngraded two levels due to very serious imprecision: uncertainty of clinical relevance of the results reported and of the effects measured; small sample size.
Background
Description of the condition
Essential tremor is one of the most common movement disorders, presenting an overall estimated prevalence ranging from 0.9% to 2.2%, with a higher rate among people over 65 years of age (4.6%) (Louis 2010). It is characterised by postural and kinetic tremor involving the arms, and less commonly the head, lower limbs and voice, frequently accompanied by a family history of a similar tremor (Louis 2005). However, essential tremor is a heterogeneous disorder, and there is little agreement among neurologists regarding either clinical definition or diagnostic criteria (Jankovic 2002). Although benign in term of its effect on life expectancy, it often causes embarrassment and, in a small percentage of patients, also serious disability (Koller 1986; Busenbark 1991). Moreover, symptoms are typically progressive and potentially disabling, often forcing patients to change job or seek early retirement (Deuschl 2000).Treatment consists primarily of pharmacological agents, although surgical intervention may be an option in the most disabling cases. Pharmacotherapy may be used to improve function or reduce the embarrassment associated with the disorder, but treatment should be tailored to the patient's level of disability. Although primidone and propranolol are well‐established therapeutic agents for the disorder, additional medications may be useful in reducing tremor (Sullivan 2004). In fact, although studies report that both propranolol and primidone improve tremor in about two‐thirds of patients (Koller 1989; Wasielewski 1998), these agents tend to lose efficacy over time (Louis 2001a). In addition, their use is limited, particularly among people over 70 years old because of the interactions with other commonly used medications (e.g. digoxin, calcium channel blockers and antiarrhythmics) (Hansten 2004; Zesiewicz 2002).
Although the exact pathophysiology of essential tremor is unknown, there is evidence that the neurotransmitter gamma‐aminobutyric acid (GABA) may be involved (Kralic 2005), and studies have suggested that (usually well‐tolerated) anticonvulsants that enhance GABAergic neurotransmission, such as gabapentin and topiramate, could potentially be useful (Pahwa 1998; Ondo 2000; Ondo 2006).
Description of the intervention
Pregabalin (S‐(+)‐3‐isobutyl GABA) is an anticonvulsant and a structural analogue of GABA. It binds in a very potent fashion to the alpha‐2‐delta protein subunit of the voltage‐gated calcium channel, reducing calcium influx and consequently reducing neurotransmitters' release. This mechanism of action probably explains its strong analgesic and anxiolytic effect. Pregabalin is rapidly absorbed orally with a bioavailability of over 90%, reaching peak levels within one hour. Its plasma half‐life is approximately six hours. It is not metabolised in humans, and 98% of the drug can be recovered unchanged in the urine (Shorvon 2000).
How the intervention might work
Essential tremor may be caused by a deficiency in the alpha‐1‐subunit of the gamma‐aminobutyric acid (GABA) A receptor, as demonstrated in a knockout model in mice (Kralic 2005). This mechanism suggests that the GABAergic system could be a potential target for pharmacotherapy, and that GABA‐A receptor agonists may be an effective treatment (Pahwa 2003; Kralic 2005). In fact, considering their mechanisms of action, this could be true of all anticonvulsants that enhance GABAergic neurotransmission. Gabapentin, which may facilitate GABAergic function, demonstrated efficacy in essential tremor (Ondo 2000). Although pregabalin has structural similarities to gabapentin, its potency in animal models of epilepsy, pain and anxiety is significantly greater. As an isomer of GABA (French 2003), pregabalin could reduce essential tremor. Moreover, its well‐known anxiolytic effect might be helpful for patients with anxiety, which worsens the symptoms of essential tremor.
Why it is important to do this review
In 2005, the American Academy of Neurology published their practice parameter for essential tremor (Zesiewicz 2005), basing the recommendation on an arbitrary four‐tiered level of evidence scheme and concluding that propranolol and primidone should be used as first‐line therapy. The review update examined studies considering pregabalin (Zesiewicz 2011), showing insufficient evidence to support or refute pregabalin treatment for essential tremor. Another recent study based on the use of GRADE system for grading the quality of evidence and the strength of recommendations, assigned to pregabalin a weak recommendation, with very low quality of evidence, concluding that physicians should not prescribe the drug for essential tremor because it is probably ineffective (Zappia 2013). As primidone or propranolol administration may be limited due to the risk of serious adverse events, and as these agents could lose their efficacy in long‐term therapies, it may be worth evaluating treatment alternatives for essential tremor. As there is uncertainty about the efficacy of pregabalin, a systematic review evaluating whether this agent is an effective therapy may generate clinically useful information.
Objectives
To assess the effects of pregabalin versus placebo or other treatment for essential tremor in adults. Primary outcomes are functional abilities and safety, and secondary outcomes are tremor severity and quality of life.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with both parallel group and cross‐over design.
Types of participants
Adults (aged 16 years or older) with essential tremor diagnosed according to the criteria proposed by the Tremor Investigation Group (Bain 2000a), the Consensus Statement of the Movement Disorder Society on Tremor (Deuschl 1998), or previous accepted and validated clinical criteria (Rajput 1984; Snow 1989; Haerer 1992; Salemi 1994; Chouinard 1997; Louis 1998).
We excluded participants with secondary forms of tremor (e.g. thyroid disease) from our review.
Types of interventions
Pregabalin for essential tremor versus any other pharmacological treatment or placebo.
We did not exclude trials on the basis of dose or route of administration.
Types of outcome measures
We excluded studies that reported only neurophysiological parameters (e.g. electromyographic recordings, accelerometry, spirography, digitising tablets) to assess outcomes. These instrumental tests have important limitations since their accuracy and reproducibility are not well established. Moreover, neurophysiological measures can lead to a fallacious assessment of the benefit of treatment, as cross‐sectional studies show a weak correlation between those measures and patients' functional abilities (Bain 1997; Bain 2000b).
Primary outcomes
Change in the functional abilities component related to tremor, measured by the Fahn‐Tolosa‐Marin Tremor Rating Scale (TRS) subscales B and C (Fahn 1988) between baseline and end of follow‐up. The TRS assesses rest, postural and action tremor. The total score is derived from three TRS subscales.
Subscale A: examiner‐reported upper limb postural and action tremor severity (amplitude), four elements.
Subscale B: examiner reported ability to perform specific motor tasks (writing, drawing and pouring with dominant and non‐dominant hands), nine elements.
Subscale C: patient‐reported functional disabilities due to tremor (eating, speaking, drinking, hygiene, dressing, writing, working and social activities), eight elements.
Each subscale element is rated from 0 to 4 (none to severe tremor) giving a maximum score of 16, 36 and 32 for each subscale. The overall TRS score is the sum of individual elements calculated as a fraction of the subscale's maximum score and converted to a 100‐point scale (0 to 100).
We also considered other validated scales to assess and measure tremor severity, such as the Unified Tremor Rating Scale (UTRA) (Findley 1995; Jankovic 1996), the Bain scale (Bain 1998) and the Washington Heights‐Inwood Genetic Study of Essential Tremor (WHIGET) rating scale (Louis 2001b).
Safety outcomes: withdrawal, defined in a standard manner, and number of adverse events associated with treatment.
Secondary outcomes
Change in tremor severity, measured by the Fahn‐Tolosa‐Marin TRS subscale A, between baseline and end of follow‐up.
Change in quality of life, between baseline and end of follow‐up, measured by:
a validated quality of life scale or questionnaire, such as the Short Form (36) Health Survey (SF‐36) or the EuroQoL five‐dimensional scale (EQ‐5D);
a patient self‐rated severity score, such as the patient global impression (PGI); or
a clinician‐rated global score, such as the clinical global impression (CGI), a seven‐point rating scale.
Search methods for identification of studies
We carried out a systematic search without language restrictions to identify all relevant published and unpublished RCTs.
Electronic searches
We searched the following databases for relevant trials.
The Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 9) in the Cochrane Library (searched 30 September 2019).
MEDLINE (1966 to 30 September 2019).
Embase (1988 to 30 September 2019).
NICE (National Institute for Health and Care Excellence; from 1999 to 30 September 2019).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; search 30 September 2019).
WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch; searched 30 September 2019).
We additionally searched BIOSIS Citation Index (2000 to320 September 2019) for conference proceedings.
We based the search strategies for each database on the strategy developed for MEDLINE, revising it appropriately for each database to take into account the differences in controlled vocabulary and syntax rules. See Appendix 1 and Appendix 2.
Searching other resources
In addition to the electronic searches above, we:
screened reference lists of all available review articles and primary studies;
handsearched the references quoted in the recent abstract books of the European Federation of Neurological Societies (2005 to 2019), the American Academy of Neurology (2003 to 2019), the American Neurological Association (2006 to 2019) and the Movement Disorder Society (2003 to 2019);
contacted the corresponding authors of relevant trials;
contacted drug manufacturers for information on ongoing trials.
Data collection and analysis
Two reviewers (EB and GQ) independently assessed the titles and abstracts of all the studies identified by the electronic searching or handsearching. We obtained the full text of potentially relevant trials.
Selection of studies
After reading the abstracts, EB and GQ independently selected the eligible articles and independently scrutinised the full texts of the selected studies and decided which trials met the inclusion criteria considered for this review. We resolved any disagreements concerning inclusion and exclusion of trials by discussion.
Data extraction and management
EB and GQ extracted the following data independently with a data collection form.
Trial design.
Randomisation methods.
Allocation concealment.
Blinding of treatments and assessments.
Comparability of treatment groups in terms of demographic and clinical characteristics.
Inclusion and exclusion criteria.
Duration of treatment.
Length of follow‐up.
Outcome measures (use of validated scales).
Number of withdrawals and respective causes.
Description of adverse events.
We resolved disagreements on extracted data by discussion.
Assessment of risk of bias in included studies
The review authors independently judged trial quality according to the methods set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We considered seven specific domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective reporting.
Other sources of bias.
Two review authors (EB, GQ) independently assessed risk of bias in each of these domains for all included studies, resolving any disagreement by discussion to reach consensus. The overall 'Risk of bias' assessment was based on recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If we assessed one or more domains as being at high risk of bias, we rated the overall score as high. If we rated all domains as being at low risk of bias, we considered the overall score to be low. We rated studies with all the other combinations as being at unclear risk of bias overall.
We took into account the risk of bias in included studies in the interpretation of primary outcomes results using the GRADE approach, also examining consistency, directness and precision to grade the quality of evidence (GRADEpro GDT). We rated overall quality of evidence as 'high', 'moderate', 'low' or 'very low'. The GRADE approach assigns RCTs an initially high rating that investigators may lower based on their judgment of study limitations, inconsistency of the results, indirectness of the evidence, imprecision of data and presence of publication bias. The primary outcomes considered were functional abilities, withdrawals and number of adverse events. Two review authors (EB, GQ) independently graded the body of evidence using GRADE guidance and resolved discrepancies through discussion aimed at achieving consensus. We reported and summarised the results of this assessment using a 'Summary of findings' table.
Measures of treatment effect
We analysed measurement scales to assess essential tremor as continuous variables. We calculated and expressed the intervention effect as mean differences (MD) and standard deviations (SDs) for individual studies and for pooled estimates when studies used the same scale of measurement. We used change from baseline for all continuous variables.
We expressed categorical variables (number of withdrawals and number of adverse events) as frequencies and percentages.
Unit of analysis issues
To avoid the carry‐over effect that can induce alteration of the response to subsequent treatment (Sibbald 1998), we used only data from the first treatment phase after randomisation for cross‐over studies.
Dealing with missing data
In order to estimate the effect of participant withdrawals or loss to follow‐up on primary outcomes, we extracted available information about incomplete data and about the intention‐to‐treat analysis performed. We only included data for participants whose results were available. We calculated the frequency of withdrawals for each treatment group for individual studies and pooled analyses. We considered the impact of missing data during the 'Risk of bias' assessment.
Assessment of reporting biases
We assessed reporting biases concerning both primary and secondary outcomes, comparing outcomes reported in the Results section with the outcomes planned in the trial protocol published on ClinicalTrials.gov.
Data synthesis
We checked data distribution patterns for normality. The check involved calculating the observed mean minus the lowest possible value of the outcome scale and dividing this by the SD (Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions,Higgins 2011). A ratio less than 2 suggests skew. If the ratio is less than 1, there is strong evidence of a skewed distribution. Since this rough check may not be appropriate for change‐from‐baseline measures, we have applied this method only for the means measured at baseline and at the end of the follow‐up and reported in the included study. Within the different comparisons, we calculated mean differences (MDs) and SDs to assess efficacy. We calculated frequencies and percentages for withdrawals and adverse events. Provided that at least two included studies reported an outcome of interest for each comparison, we planned to combine data in a meta‐analysis without any restrictions based on risk of bias. In the presence of between‐trial homogeneity, we planned to use a fixed‐effect model. In case of heterogeneity, we planned to combine data using a random‐effects model. We planned to use inverse variance methods for continuous outcomes and measurement scales. We compared differences between treatment groups as mean difference (MD). We combined results for dichotomous outcomes (withdrawals, adverse events) using Mantel‐Haenszel methods and obtained risk differences (RDs) to compare treatment groups. We used Review Manager software for data management and analysis (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
We planned to address the following different comparisons: pregabalin versus placebo; pregabalin versus other anticonvulsant; pregabalin versus other pharmacological treatment. We planned to investigate potential positive or negative interactions between pregabalin and other anti‐tremor medications on primary outcomes, performing a subgroup analysis of trials in which only the experimental anti‐tremor medication was allowed (pregabalin or placebo) and of trials including participants using other anti‐tremor medications during the study period. For trials reporting treatment effects for more than one dose, we planned to investigate the effect of the different doses reported separately. We assessed heterogeneity using the I2 statistic (Higgins 2003).
Sensitivity analysis
We did not perform any sensitivity analyses.
Results
Description of studies
Results of the search
The search of electronic databases yielded a total of 13 records, 4 of which we excluded because they were published as review articles and 5 of which we excluded because they were duplicate references. We obtained the full text of four studies evaluating pregabalin treatment for essential tremor and finally selected one for inclusion. A flowchart describes the results of the search in Figure 1. We did not identify any additional records from searching other resources.
1.
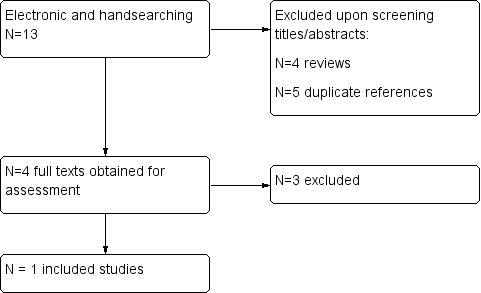
Flowchart of the literature search on pregabalin and essential tremor
Included studies
We considered one study comparing pregabalin with placebo to be eligible for this review (Zesiewicz 2007a).
Trial design
This study was a double‐blind RCT with a duration of 42 days.
Participants
Zesiewicz 2007a included 22 people with a defined upper‐limb essential tremor according to criteria proposed by the Tremor Investigator Group (Bain 2000a). Moreover, the study included 13 participants (60%) who had been receiving a stable anti‐tremor medication (including alprazolam, propranolol, primidone, topiramate, clonazepam, sotalol and metoprolol) for at least 14 days before randomisation. These participants maintained the co‐therapy throughout the study period. Baseline TRS total score (from 0 to 100) was 43.18 (SD 22.04) for the pregabalin group and 35.91 (SD 20.50) for the placebo group, with a disease duration of 17.58 (SD 19.86) years for the pregabalin group and 18.33 (SD 14.07) for the placebo group. Mean age was of 57 (SD 14) years. The trial excluded people presenting concomitant systemic or neurological diseases, psychiatric disorders, or history of alcohol or drugs addiction. Likewise, people who underwent botulinum toxin treatment for upper limb tremor, deep brain stimulation, other brain surgery or pregabalin treatment 30 days prior the study entry were not eligible.
Intervention
Participants were randomly assigned to receive either pregabalin or placebo over a period of 42 days. The therapeutic scheme ranged from 50 mg to 600 mg per day, with a gradual titration of 75 mg increased every four days. Physicians halted titration if participants experienced resolution of tremor or adverse events. The mean dose reached was 286.76 mg/day (SD 100.05).
Outcome measures
The primary efficacy parameter was change in TRS scores. Authors reported TRS total score and TRS subscales A, B and C both at baseline and at study endpoint. CGI and a self‐report scale for assessing pain were reported at study endpoint only.
Adverse events
The study reported the number of participants experiencing adverse events and the number of those who were withdrawn/dropped out because of adverse events.
Excluded studies
We excluded three studies after reading the full texts. Zesiewicz 2012 and Ferrara 2009 were randomised, double‐blind, placebo‐controlled cross‐over trials. Authors did not report data from the first treatment phase after randomisation in either of these studies, and we did not manage to obtain the data through contacting the study authors. Zesiewicz 2007b was a case report of two essential tremor patients treated with pregabalin.
Risk of bias in included studies
We present the results of the 'Risk of bias' assessment in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Investigators performed sequence generation through a computer‐generated randomisation schedule. In small trials simple randomisation can be a source of bias, resulting in an unequal distribution of participants and covariables, thus we considered it to be at unclear risk of bias. Block randomisation and stratification may have been used to ensure balance between groups in size and patient characteristics.
Blinding
While investigators reported the study as being double‐blind, they did not describe the methods for blinding personnel, participants and outcome assessors in enough detail. Specifically, they did not report information about methods to avoid unmasking (including centralised preparation of similar tablets, dosage modification techniques, participation of outcome assessors not involved in treatment administration and assessment of clinical exam through video, etc.). We judged the risk of bias to be unclear.
Incomplete outcome data
Three of the 11 randomised participants in the pregabalin group and 4 of the 11 participants in the placebo group dropped out before study completion. Although the number of withdrawals was balanced between pregabalin and placebo groups, reasons for missing data were different, since more participants withdrew for adverse events in the pregabalin group. We used a last observation carried forward (LOCF) procedure to impute missing data (seven patients who withdrew prior to endpoint), and this may lead to serious bias (Higgins 2011). We judged the to be at high risk of attrition bias because it did not report when investigators actually measured the outcomes in participants for whom observations were carried forward.
Selective reporting
Comparison between the study protocol (published on clinicaltrial.gov) and the final study publication demonstrated no difference, and we considered the study to be free from reporting bias.
Other potential sources of bias
The study appeared to be free of other sources of bias.
Effects of interventions
See: Table 1
See: Table 1 reporting the comparison 'pregabalin versus placebo' and the GRADE assessment.
The included study compared pregabalin versus placebo in 22 participants (11 pregabalin and 11 placebo). We rated the overall risk of bias to be unclear. We considered the overall quality of evidence to be very low.
We checked data for normality, obtaining results between 1.0 and 2.0, which suggests that data were probably skewed. In light of this non‐normal distribution for the analysed data, readers should interpret the findings with caution.
Primary outcomes
Zesiewicz 2007a reported the functional abilities assessment and the number of adverse events and withdrawals.
At the study endpoint (42 days), examiner‐reported specific motor tasks function mproved by 3.78 (SD 9.62) points (on a 36‐point scale) for pregabalin and by 1.63 (SD 6.96) points for placebo (MD −2.15 points, 95% CI −9.16 to 4.86; Analysis 1.1). Participant‐reported functional abilities improved by 1.56 (SD 3.68) points (on a 32‐point scale) for pregabalin and by 0.90 (SD 0.93) for placebo (MD −0.66 points, 95% CI −2.90 to 1.58; Analysis 1.2). However, as the confidence intervals cross the line of no effect, these results do not provide strong evidence of a true difference in efficacy between pregabalin and placebo.
1.1. Analysis.

Comparison 1: Pregabalin versus placebo: efficacy, Outcome 1: Change in TRS part B score (motor function) between baseline and end of follow‐up
1.2. Analysis.

Comparison 1: Pregabalin versus placebo: efficacy, Outcome 2: Change in TRS part C score (functional abilities) between baseline and end of follow‐up
Three patients in the pregabalin group (27.3%) and four patients in the placebo group (36.4%) discontinued the treatment and dropped out of the study. Authors reported a statistically non‐significant reduced risk of withdrawal for pregabalin, with a Mantel‐Haenszel RD of −0.09 (95% CI −0.48 to 0.30; Analysis 2.1). The occurrence of adverse events was the only reason for pregabalin discontinuation, whilst investigators cited unspecified 'personal reasons' for dropouts in the placebo group (see Analysis 2.1).
2.1. Analysis.

Comparison 2: Pregabalin versus placebo: safety, Outcome 1: Withdrawals
Considering adverse events, the trial reported their occurrence without specifying severity. Three patients in pregabalin group (27.3%) and one patient in the placebo group (9.1%) developed adverse events, with a Mantel‐Haenszel RD of 0.18 (95% CI −0.13 to 0.50) between the two groups (Analysis 2.2). The most common adverse events experienced with pregabalin treatment were dizziness and malaise. However, it is uncertain if there is any difference between the two groups due to the very low quality of the evidence.
2.2. Analysis.

Comparison 2: Pregabalin versus placebo: safety, Outcome 2: Adverse events
We did not perform meta‐analysis since there was only one included study. Likewise, we did not perform any subgroup analyses to assess differences on efficacy and safety due to the interaction between combined anti‐tremor treatments because there were not enough trials included.
Secondary outcomes
At the study endpoint (42 days), authors reported a mean improvement from baseline of the overall TRS score of 14.89 (SD 16.25) points (on a 0 to 100 scale) for the pregabalin group and of 7.38 (SD 12.65) points for the placebo group. Examiner‐reported upper limb tremor severity improved by 8.89 (SD 11.29) points (on a 16‐point scale) for pregabalin and by 5.25 (SD 10.08) for placebo (MD −3.64, 95% CI −12.58 to 5.30; Analysis 1.3). This data analysis showed no statistically significant difference between pregabalin and placebo in terms of efficacy.
1.3. Analysis.

Comparison 1: Pregabalin versus placebo: efficacy, Outcome 3: Change in TRS part A score (tremor severity) between baseline and end of follow‐up
At the study endpoint, the CGI assessment indicated that six patients (67%) taking pregabalin considered their tremor 'improved' compared to baseline, while two patients (20%) reported a 'minimal improvement' of their tremor in the placebo group. Investigators reported no change in the pain scale for either group.
Discussion
Summary of main results
We included one RCT comparing pregabalin versus placebo for the treatment of essential tremor in this review (Zesiewicz 2007a). Twenty‐two participants were enrolled and randomised. After a follow‐up of 42 days, investigators reported no significant improvements in motor functions or abilities in people treated with pregabalin versus those receiving a placebo. Moreover, we found a non‐significant high risk of discontinuation due to adverse events in participants treated with pregabalin. Nevertheless, readers should interpret these data cautiously, as they arose from a single trial at unclear risk of bias that provided very low quality evidence. Moreover, the data may have had a skewed distribution, which could have influenced the validity of the results obtained, further limiting the possibility to draw firm conclusions.
Overall completeness and applicability of evidence
Important factors limited the validity of the results reported. The study population comprised participants with tremor that was both long‐standing (about 20 years) and probably pharmacoresistant (a large proportion was on other medications), recruited from a tertiary referral centre for movement disorders. Thus, information concerning the clinical benefit of pregabalin in people with less severe and previously untreated essential tremor is unknown. The presence of a large proportion of patients (64% in the pregabalin group, 54% in the placebo group) receiving other anti‐tremor medications during the study period hinders the evaluation of pregabalin as first‐line or add‐on therapy. Safety and tolerability is an issue of great importance when treating chronic diseases. However, investigators did not collect adverse events data on standardised questionnaires, so there may have been unreported symptoms. Furthermore, authors did not report the effect of treatment on patients' quality of life, limiting the overall completeness of the assessment. Finally, it is possible that a skewed distribution of data influenced the analysis performed in the present review.
Quality of the evidence
We assessed the overall quality of the evidence to be very low and insufficient to provide adequate evidence regarding the efficacy of this treatment on essential tremor. Among the factors influencing the quality of the evidence are the very small sample size and the high proportion of dropouts.
Potential biases in the review process
To minimise biases, we performed a comprehensive systematic review searching different databases, without language restrictions, to identify all relevant studies. Two authors performed data management.
Agreements and disagreements with other studies or reviews
Two literature reviews have analysed pregabalin treatment for essential tremor (Zesiewicz 2011; Zappia 2013). The practice parameter for essential tremor gave a level U recommendation to pregabalin, meaning uncertain efficacy, due to the inconclusive results of the studies identified (Zesiewicz 2011). The systematic review of evidence and recommendations from the Italian Movement Disorders Association (DISMOV‐SIN) assigned a weak recommendation with very low quality of evidence (2D), discouraging the use of this agent for essential tremor patients (Zappia 2013).
Authors' conclusions
Implications for practice.
The effects of pregabalin in the treatment of essential tremor are uncertain because the quality of the evidence is very low. One small study did not highlight any effect of this treatment; however, the high risk of bias and the lack of other studies on this topic limit further conclusion.
Implications for research.
Essential tremor represents one of the most prevalent movement disorders. Nevertheless, its management still remains a challenge for many people who are often refractory to or intolerant of conventional therapies. This systematic review highlighted a paucity of well‐designed studies aimed at investigating the efficacy and safety of potential new drugs, such as pregabalin, as additional treatment options for essential tremor. Researchers should perform RCTs with adequate methodology and larger samples of participants with essential tremor, assessing long‐term efficacy with appropriate duration of follow‐up. Investigators should better control the inclusion of patients using other concomitant anti‐tremor treatment by stratifying this variable at randomisation and by performing adequate pre‐specified subgroup analysis. Moreover, considering the substantial impact of essential tremor on patients' everyday life, studies should consider adequate quality of life measures as important outcomes to be assessed in trials.
What's new
| Date | Event | Description |
|---|---|---|
| 21 February 2020 | Amended | Conflict of interest amended. Literature serach updated |
History
Protocol first published: Issue 3, 2012 Review first published: Issue 10, 2016
| Date | Event | Description |
|---|---|---|
| 23 August 2016 | Amended | amended according to CEU report |
| 1 March 2016 | Amended | amended |
| 9 October 2015 | Amended | amended |
| 28 October 2014 | Amended | amended according to the reviewer's comments |
| 7 July 2013 | Amended | Review updated and completed |
Acknowledgements
The authors would like to express their gratitude to Ricardo M Fernandes for the valuable comments and guidance provided during the development of the final version of this review.
Appendices
Appendix 1. MEDLINE search strategy
1 exp Essential Tremor/ (1183)
2 (essential adj3 tremor*).ab,ti. (2473)
3 (familia* adj3 tremor*).ab,ti. (132)
4 1 or 2 or 3 (2654)
5 pregabalin.ab,ti. (1604)
6 lyrica.ab,ti. (64)
7 5 or 6 (1612)
8 randomized controlled trial.pt. (367656)
9 controlled clinical trial.pt. (87895)
10 randomized.ab. (287683)
11 placebo.ab. (151722)
12 drug therapy.fs. (1677138)
13 randomly.ab. (208754)
14 trial.ab. (298006)
15 groups.ab. (1332158)
16 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 (3287589)
17 exp animals/ not humans.sh. (3903063)
18 16 not 17 (2818660)
19 4 and 7 and 18 (7)
Appendix 2. CENTRAL search strategy
1 MeSH descriptor: [Essential Tremor] explode all trees (62)
2 essential tremor*:ti,ab,kw (Word variations have been searched) (202)
3 familia* tremor:ti,ab,kw (Word variations have been searched) (7)
4 1 or 2 or 3 (208)
5 pregabalin:ti,ab,kw (Word variations have been searched) (449)
6 "Lyrica":ti,ab,kw (Word variations have been searched) (6)
7 5 or 6 (450)
8 4 and 7 (4)
Data and analyses
Comparison 1. Pregabalin versus placebo: efficacy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Change in TRS part B score (motor function) between baseline and end of follow‐up | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐2.15 [‐9.16, 4.86] |
| 1.2 Change in TRS part C score (functional abilities) between baseline and end of follow‐up | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐2.90, 1.58] |
| 1.3 Change in TRS part A score (tremor severity) between baseline and end of follow‐up | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐3.64 [‐12.58, 5.30] |
Comparison 2. Pregabalin versus placebo: safety.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Withdrawals | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Number of withdrawals | 1 | 22 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.09 [‐0.48, 0.30] |
| 2.1.2 Withdrawal due to adverse events | 1 | 22 | Risk Difference (M‐H, Fixed, 95% CI) | 0.18 [‐0.13, 0.50] |
| 2.1.3 Withdrawals for other reasons | 1 | 22 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.27 [‐0.55, 0.01] |
| 2.2 Adverse events | 1 | 22 | Risk Difference (M‐H, Fixed, 95% CI) | 0.18 [‐0.13, 0.50] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Zesiewicz 2007a.
| Study characteristics | ||
| Methods | Double‐blind, placebo controlled, parallel study | |
| Participants | 22 patients randomised (11 to pregabalin, 11 to placebo) Mean age 57 years (SD 13) Male 40% Baseline TRS 39 (SD 20) |
|
| Interventions | Group 1: pregabalin 50 mg‐600 mg (titration 75 mg every 4 days) Group 2: placebo Follow‐up: 42 days |
|
| Outcomes | TRS total score (0 to 100) and subscales: severity (16 points), motor tasks (36 points) and functional disability (32 points), CGI (seven‐point scale) | |
| Notes | Trial setting: out‐patients (Parkinson’s Disease and Movement Disorders Center) Country: University of South Florida, Tampa, Florida, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Computer generated randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "PGB and placebo were supplied in identical containers"; "both patients and raters were blind to randomization" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Tremor measurements were conducted by a blinded rater" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Last observation carried forward was used for analysis for patients who prematurely withdrew the study" |
| Selective reporting (reporting bias) | Low risk | Free |
| Other bias | Low risk | Free |
CGI: clinical global impression;PGB: pregabalin; RCT: randomised controlled trial; TRS: Fahn‐Tolosa‐Marin Tremor Rating Scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ferrara 2009 | Cross‐over design. Data from the first phase not reported |
| Zesiewicz 2007b | Case report |
| Zesiewicz 2012 | Cross‐over design. Data from the first phase not reported |
Differences between protocol and review
Studies widely report tremor severity, and we initially selected this measure as a primary outcome. However, during the review process, we came to recognise tremor severity as a poor clinimetric tool with uncertain significance for both clinicians and patients, and we changed it from a primary to a secondary outcome. Conversely, we judged the functional abilities outcome to be more relevant, and we prioritised it among the primary outcomes.
In an attempt to provide a standardised and reliable assessment of the quality of the evidence of the study outcomes, we decided to use the GRADE evidence profile, a systematic and explicit system for grading the evidence into four quality categories. We reported the results obtained through this approach in a 'Summary of findings' table.
Methods for future updates
We did not perform two pre‐planned analyses due to insufficient data, but if possible, we will eventually implement these in future updates of the review.
Regarding the methods for analysing continuous data, most studies use continuous scales to assess tremor. In future updates, we will transform ordinal scales with enough categories to continuous scales by assigning a score to each grade so that we can express the intervention effect as a difference in means or as a standardised mean difference (SMD). In the case of an ordinal scale with few categories, we will combine data from adjacent categories into two categories and use methods for binary data as odds ratios (ORs) or risk differences (RDs) to evaluate the intervention effect.
In addition, we will undertake sensitivity analyses to assess the robustness of results to fixed‐effect versus random‐effects assumptions and to the inclusion or exclusion of studies at high risk of bias (i.e. inadequate allocation concealment and lack of blinded outcome assessor). We will use best‐ and worst‐case scenarios for taking into account missing data.
Contributions of authors
EB: protocol and review editing, literature searching, study selection, quality assessment, data extraction.
AN: protocol and review editing.
GQ: literature searching, quality assessment, data extraction.
CC: protocol editing, quality assessment, study selection.
GF: protocol editing, editing and revising the review.
MZ: protocol editing, revising review.
Declarations of interest
The original review was not compliant with Cochrane Commercial Sponsorship policy for the following reasons:
CC received financial support from Merz (manufacturer of Botulinum toxin), Teva (manufacturer of propranolol) and other pharma companies. AN received financial support from Lundbeck (manufacturer of benzodiazepine clobazam) and UCB (manufacturer of levetiracetam). MZ received financial support from Novartis (manufacturer of propranolol [Sandoz]), UCB, Lundbeck and other pharma companies.
However, the current update have a majority of authors and lead author free of conflicts as the lead author and all the other authors have not received payments from manufacturers or marketers of the interventions of interest or potential comparators within the 3 years of the decision to update and none of the authors are/were employed by a company who has a real or potential financial interest in the findings of the review and/or have a relevant patent.
Edited (no change to conclusions)
References
References to studies included in this review
Zesiewicz 2007a {published data only}
- Zesiewicz TA, Ward CL, Hauser RA, Salemi JL, Siraj S, Wilson MC, Sullivan KL. A pilot, double-blind, placebo controlled trial of pregabalin (Lyrica) in the treatment of essential tremor. Movement Disorders 2007;22(11):1660-3. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ferrara 2009 {published data only}
- Ferrara JM, Kenney C, Davidson AL, Shinawi L, Kissel AM, Jankovic J. Efficacy and tolerability of pregabalin in essential tremor: a randomized, double blind, placebo controlled, crossover trial. Journal of the Neurological Sciences 2009;285(1-2):195-7. [DOI] [PubMed] [Google Scholar]
Zesiewicz 2007b {published data only}
- Zesiewicz TA, Ward CL, Hauser RA, Paese Campbell JA, Sullivan KL. Pregabalin (Lyrica) in the treatment of essential tremor. Movement Disorders 2007;22(1):139-41. [DOI] [PubMed] [Google Scholar]
Zesiewicz 2012 {published data only}
- Zesiewicz TA, Sullivan KL, Hinson V, Stover NP, Fang J, Jahan I, et al. Multisite, double-blind, randomized, controlled study of pregabalin for essential tremor. Movement Disorder 2013;28(2):249-50. [DOI] [PubMed] [Google Scholar]
Additional references
Bain 1997
- Bain PG. The effectiveness of treatments for essential tremor. Neurologist 1997;3:305-21. [Google Scholar]
Bain 1998
- Bain PG. Clinical measurement of tremor. Movement Disorders 1998;13(Suppl 3):77-80. [DOI] [PubMed] [Google Scholar]
Bain 2000a
- Bain P, Brin M, Deuschl G, Elble R, Jankovic J, Findley L, et al. Criteria for the diagnosis of essential tremor. Neurology 2000;54(Suppl 4):S7. [PubMed] [Google Scholar]
Bain 2000b
- Bain PG. Tremor assessment and quality of life measurements. Neurology 2000;54(Suppl 4):S26-S29. [PubMed] [Google Scholar]
Busenbark 1991
- Busenbark KL, Nash J, Nash S, Hubble JP, Koller WC. Is essential tremor benign? Neurology 1991;41(12):1982-3. [DOI] [PubMed] [Google Scholar]
Chouinard 1997
- Chouinard S, Luois ED, Fahn S. Agreement among Movement Disorder Specialists on the clinical diagnosis of Essential Tremor. Movement Disorders 1997;12(6):973-6. [DOI] [PubMed] [Google Scholar]
Deuschl 1998
- Deuschl G, Bain P, Brin M. Consensus Statement of the Movement Disorder Society on Tremor. Movement Disorders 1998;13(Suppl 3):S2-23. [DOI] [PubMed] [Google Scholar]
Deuschl 2000
- Deuschl G, Koller WC. Essential tremor. Neurology 2000;54(Suppl 4):S1. [PubMed] [Google Scholar]
Fahn 1988
- Fahn S, Tolosa E, Marin C. Clinical Rating Scale for Tremor. In: Jankovic J, Tolosa E, editors(s). Parkinson's Disease and Movement Disorders. 2nd edition. Baltimore, MD: Williams & Wilkins, 1988:225-34. [Google Scholar]
Findley 1995
- Findley LJ, Koller W. Definitions and behavioural classifications. In: Findley LG, Koller W, editors(s). Handbook of Tremor Disorders. New York: Dekker, 1995:1-5. [Google Scholar]
French 2003
- French JA, Kugler AR, Robbins JL, Knapp LE, Garofalo EA. Dose-response trial of pregabalin adjunctive therapy in patients with partial seizures. Neurology 2003;60(10):1631-7. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University GRADEpro GDT. Version accessed 20 August 2015. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Haerer 1992
- Haerer AF, Anderson DW, Schoenberg BS. Prevalence of essential tremor: results from the Copiah county study. Archives of Neurology 1992;39(12):750-1. [DOI] [PubMed] [Google Scholar]
Hansten 2004
- Hansten PD, Horn JR. Managing clinically important drug interactions. 4th edition. St. Louis: Facts & Comparisons, 2004. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Jankovic 1996
- Jankovic J, Schwartz K, Clemence J. A randomized, double-blind, placebo controlled study to evaluate botulinum toxin type A in essential tremor. Movement Disorders 1996;11(3):250-6. [DOI] [PubMed] [Google Scholar]
Jankovic 2002
- Jankovic J. Essential tremor: a heterogeneous disorder. Movement Disorders 2002;17(4):638-44. [DOI] [PubMed] [Google Scholar]
Koller 1986
- Koller W, Biary N, Cone S. Disability in essential tremor: effect of treatment. Neurology 1986;36(7):1001-4. [DOI] [PubMed] [Google Scholar]
Koller 1989
- Koller WC, Vetere-Overfield B. Acute and chronic effects of propranolol and primidone in essential tremor. Neurology 1989;39(12):1587-8. [DOI] [PubMed] [Google Scholar]
Kralic 2005
- Kralic JE, Criswell HE, Osterman JL, O'Buckley TK, Wilkie ME, Matthews DB, et al. Genetic essential tremor in gamma-aminobutyric acid A receptor alpha1 subunit knockout mice. The Journal of Clinical Investigation 2005;115(3):774-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Louis 1998
- Louis ED, Ford B, Lee H. Diagnostic criteria for Essential Tremor. Archives of Neurology 1998;55(6):823-8. [DOI] [PubMed] [Google Scholar]
Louis 2001a
- Louis ED. Clinical practice, essential tremor. New England Medical Journal 2001;342(12):887-91. [DOI] [PubMed] [Google Scholar]
Louis 2001b
- Louis ED, Barnes L, Wendt KJ, Ford B, Sangiorgio M, Tabbal S, et al. A teaching videotape for the assessment of essential tremor. Movement Disorders 2001;16(1):89-93. [DOI] [PubMed] [Google Scholar]
Louis 2005
- Louis ED. Essential tremor. Lancet Neurology 2005;4(2):100-10. [DOI] [PubMed] [Google Scholar]
Louis 2010
- Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Movement Disorders 2010;25(5):534-41. [DOI] [PubMed] [Google Scholar]
Ondo 2000
- Ondo W, Hunter C, Vuong KD, Schwartz K, Jankovic J. Gabapentin for essential tremor: a multiple-dose, double-blind, placebo-controlled trial. Movement Disorders 2000;15(4):678-82. [DOI] [PubMed] [Google Scholar]
Ondo 2006
- Ondo WG, Jankovic J, Connor JS, Pahwa R, Elble R, Stacy MA, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology 2006;66(5):672-7. [DOI] [PubMed] [Google Scholar]
Pahwa 1998
- Pahwa R, Lyons K, Hubble JP, Busenbark K, Rienerth JD, Pahwa A, et al. Double-blind placebo-controlled study of gabapentin in essential tremor. Movement Disorders 1998;13(3):465-7. [DOI] [PubMed] [Google Scholar]
Pahwa 2003
- Pahwa R, Lyons KE. Essential tremor: differential diagnosis for the development of novel therapeutics. American Journal of Medicine 2003;115:134-42. [DOI] [PubMed] [Google Scholar]
Rajput 1984
- Rajput AH, Offord KP, Beard CM, Kurland LT. Essential tremor in Rochester, Minnesota: a 45-year study. Journal of Neurology, Neurosurgery and Psychiatry 1984;47(5):466-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salemi 1994
- Salemi G, Savettieri G, Rocca WA, Meneghini F, Saporito V, Morgante L, et al. Prevalence of essential tremor: a door to-door survey in Terrasini, Sicily. Neurology 1994;44(1):61-4. [DOI] [PubMed] [Google Scholar]
Shorvon 2000
- Shorvon SD. Handbook of Epilepsy Treatment. 2nd edition. Oxford: Blackwell, 2000. [Google Scholar]
Sibbald 1998
- Sibbald B, Roberts C. Understanding controlled trials: Crossover trials. BMJ 1998;316(7146):1719–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Snow 1989
- Snow B, Wiens M, Hertzman C, Calne D. A community survey of Parkinson's disease. Canadian Medical Association Journal 1989;141(5):418-24. [PMC free article] [PubMed] [Google Scholar]
Sullivan 2004
- Sullivan KL, Hauser RA, Zesiewicz TA. Essential Tremor Epidemiology, Diagnosis, and Treatment. Neurologist 2004;10(5):250-8. [DOI] [PubMed] [Google Scholar]
Wasielewski 1998
- Wasielewski PG, Burns JM, Koller WC. Pharmacologic treatment of tremor. Movement Disorders 1998;13(Suppl. 3):90-100. [DOI] [PubMed] [Google Scholar]
Zappia 2013
- Zappia M, Albanese A, Bruno E, Colosimo C, Filippini G, Martinelli P, et al. Treatment of essential tremor: a systematic review of evidence and recommendations from the Italian Movement Disorders Association. Journal of Neurology 2013;260(3):714-40. [DOI] [PubMed] [Google Scholar]
Zesiewicz 2002
- Zesiewicz TA, Encarnacion E, Hauser RA. Management of Essential Tremor. Current Neurology and Neuorscience Reports 2002;2(4):324-30. [DOI] [PubMed] [Google Scholar]
Zesiewicz 2005
- Zesiewicz TA, Elble R, Louis ED, Hauser RA, Sullivan KL, Dewey RB Jr, et al. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2005;64(12):2008-20. [DOI] [PubMed] [Google Scholar]
Zesiewicz 2011
- Zesiewicz TA, Elble RJ, Louis ED, Gronseth GS, Ondo WG, Dewey RB Jr, et al. Evidence-based guideline update: treatment of essential tremor: report of the Quality Standards subcommittee of the American Academy of Neurology. Neurology 2011;77(19):1752-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Bruno 2012
- Bruno E, Nicoletti A, Quattrocchi G, Colosimo C, Filippini G, Zappia M. Pregabalin for essential tremor. Cochrane Database of Systematic Reviews 14 March 2012, Issue 3. Art. No: CD009682. [DOI: 10.1002/14651858.CD009682] [DOI] [PMC free article] [PubMed] [Google Scholar]


