Abstract
Background
Herpes simplex labialis (HSL), also known as cold sores, is a common disease of the lips caused by the herpes simplex virus, which is found throughout the world. It presents as a painful vesicular eruption, forming unsightly crusts, which cause cosmetic disfigurement and psychosocial distress. There is no cure available, and it recurs periodically.
Objectives
To assess the effects of interventions for the prevention of HSL in people of all ages.
Search methods
We searched the following databases up to 19 May 2015: the Cochrane Skin Group Specialised Register, the Oral Health Group Specialised Register, CENTRAL in the Cochrane Library (Issue 4, 2015), MEDLINE (from 1946), EMBASE (from 1974), LILACS (from 1982), the China National Knowledge Infrastructure (CNKI) database, Airiti Library, and 5 trial registers. To identify further references to relevant randomised controlled trials, we scanned the bibliographies of included studies and published reviews, and we also contacted the original researchers of our included studies.
Selection criteria
Randomised controlled trials (RCTs) of interventions for preventing HSL in immunocompetent people.
Data collection and analysis
Two authors independently selected trials, extracted data, and assessed the risk of bias. A third author was available for resolving differences of opinion.
Main results
This review included 32 RCTs, with a total of 2640 immunocompetent participants, covering 19 treatments. The quality of the body of evidence was low to moderate for most outcomes, but was very low for a few outcomes. Our primary outcomes were 'Incidence of HSL' and 'Adverse effects during use of the preventative intervention'.
The evidence for short‐term (≤ 1 month) use of oral aciclovir in preventing recurrent HSL was inconsistent across the doses used in the studies: 2 RCTs showed low quality evidence for a reduced recurrence of HSL with aciclovir 400 mg twice daily (risk ratio (RR) 0.26, 95% confidence interval (CI) 0.13 to 0.51; n = 177), while 1 RCT testing aciclovir 800 mg twice daily and 2 RCTs testing 200 mg 5 times daily found no similar preventive effects (RR 1.08, 95% CI 0.62 to 1.87; n = 237; moderate quality evidence and RR 0.46, 95% CI 0.20 to 1.07; n = 66; low quality evidence, respectively). The direction of intervention effect was unrelated to the risk of bias. The evidence from 1 RCT for the effect of short‐term use of valaciclovir in reducing recurrence of HSL by clinical evaluation was uncertain (RR 0.55, 95% CI 0.23 to 1.28; n = 125; moderate quality evidence), as was the evidence from 1 RCT testing short‐term use of famciclovir.
Long‐term (> 1 month) use of oral antiviral agents reduced the recurrence of HSL. There was low quality evidence from 1 RCT that long‐term use of oral aciclovir reduced clinical recurrences (1.80 versus 0.85 episodes per participant per a 4‐month period, P = 0.009) and virological recurrence (1.40 versus 0.40 episodes per participant per a 4‐month period, P = 0.003). One RCT found long‐term use of valaciclovir effective in reducing the incidence of HSL (with a decrease of 0.09 episodes per participant per month; n = 95). One RCT found that a long‐term suppressive regimen of valaciclovir had a lower incidence of HSL than an episodic regimen of valciclovir (difference in means (MD) ‐0.10 episodes per participant per month, 95% CI ‐0.16 to ‐0.05; n = 120).
These trials found no increase in adverse events associated with the use of oral antiviral agents (moderate quality evidence).
There was no evidence to show that short‐term use of topical antiviral agents prevented recurrent HSL. There was moderate quality evidence from 2 RCTs that topical aciclovir 5% cream probably has little effect on preventing recurrence of HSL (pooled RR 0.91, 95% CI 0.48 to 1.72; n = 271). There was moderate quality evidence from a single RCT that topical foscarnet 3% cream has little effect in preventing HSL (RR 1.08, 95% CI 0.82 to 1.40; n = 295).
The efficacy of long‐term use of topical aciclovir cream was uncertain. One RCT found significantly fewer research‐diagnosed recurrences of HSL when on aciclovir cream treatment than on placebo (P < 0.05), but found no significant differences in the mean number of participant‐reported recurrences between the 2 groups (P ≥ 0.05). One RCT found no preventive effect of topical application of 1,5‐pentanediol gel for 26 weeks (P > 0.05). Another RCT found that the group who used 2‐hydroxypropyl‐β‐cyclo dextrin 20% gel for 6 months had significantly more recurrences than the placebo group (P = 0.003).
These studies found no increase in adverse events related to the use of topical antiviral agents.
Two RCTs found that the application of sunscreen significantly prevented recurrent HSL induced by experimental ultraviolet light (pooled RR 0.07, 95% CI 0.01 to 0.33; n = 111), but another RCT found that sunscreen did not prevent HSL induced by sunlight (RR 1.13, 95% CI 0.25 to 5.06; n = 51). These RCTs did not report adverse events.
There were very few data suggesting that thymopentin, low‐level laser therapy, and hypnotherapy are effective in preventing recurrent HSL, with one to two RCTs for each intervention. We failed to find any evidence of efficacy for lysine, LongoVital® supplementation, gamma globulin, herpes simplex virus (HSV) type I subunit vaccine, and yellow fever vaccine in preventing HSL. There were no consistent data supporting the efficacy of levamisole and interferon, which were also associated with an increased risk of adverse effects such as fever.
Authors' conclusions
The current evidence demonstrates that long‐term use of oral antiviral agents can prevent HSL, but the clinical benefit is small. We did not find evidence of an increased risk of adverse events. On the other hand, the evidence on topical antiviral agents and other interventions either showed no efficacy or could not confirm their efficacy in preventing HSL.
Plain language summary
Measures for preventing cold sores
Review question
What measures are effective in preventing recurrence of cold sores?
Background
A cold sore is an irritating recurrent viral infection with no proven cure. It gives rise to painful vesicles on the lips that form unsightly crusts, causing an unpleasant look and mental distress. We aimed to examine the effects of available measures for preventing recurrence of cold sores in people with normal immunity.
Study characteristics
We examined the research published up to 19 May 2015. We wanted to include studies only if receiving one preventative measure or another was decided by chance. This research method, termed randomised controlled trial (RCT), is the best way to test that a preventive effect is caused by the measure being tested. We found 32 RCTs that included 2640 people and examined 19 preventative measures. The drug manufacturer funded a total of 18 out of 32 studies, non‐profit organisations funded 4, and we do not know how the other 10 were funded.
Key results
Long‐term use of antiviral drugs taken by mouth prevented cold sores, though with a very small decrease of 0.09 episodes per person per month. The preventative effect of long‐term use of aciclovir cream applied to the lips was uncertain. Long‐term use of 1,5‐pentanediol gel and 2‐hydroxypropyl‐β‐cyclo dextrin 20% gel applied to the lips did not prevent cold sores.
Short‐term use of either antiviral drugs or creams did not prevent cold sores. Neither short‐term nor long‐term use of these antiviral drugs or creams appeared to cause side‐effects.
The preventative effects of sunscreen were uncertain. Application of sunscreen prevented cold sores induced by experimental ultraviolet light, but did not prevent cold sores induced by sunlight.
We found very little evidence about the preventative effects of thymopentin, low‐energy laser, and hypnotherapy for cold sores. The available evidence found no preventative effects of lysine, LongoVital® supplementation, gamma globulin, herpes virus vaccine, and yellow fever vaccine. There were no consistent data to confirm that levamisole and interferon do prevent cold sores.
These studies found no increase in adverse events related to the use of topical antiviral agents.
Quality of the evidence
The quality of the evidence was low to moderate for most outcomes, but was very low for some outcomes.
Summary of findings
Background
Description of the condition
A virus that resides in the skin of the lips causes herpes simplex labialis (HSL) (Higgins 1993). Its manifestation on the skin is also known as a 'cold sore' or 'fever blister'. The initial infection with the virus, which is called herpes simplex virus (HSV), is by direct contact between the mucous membranes or abraded skin of the lips or mouth and the saliva or other secretions of a person with active primary or recurrent infection (Higgins 1993). Primary infection with HSV typically occurs in early childhood, often with no symptoms, but primary HSV infection may also present as herpetic gingivostomatitis, which is characterised by oral and perioral vesicles (tiny blisters) and ulcers (Higgins 1993). It has been reported that when clinical disease is not present, the virus spreads through respiratory droplets or through interaction with the mucocutaneous releases of an asymptomatic person shedding the virus (Fatahzadeh 2007). Following the primary infection, the virus resides in the sensory ganglia (nerve endings) in a latent form (Higgins 1993). After reactivation, HSV migrates from these sensory ganglia to the outer layer of the skin of the lips or mouth to cause recurrent HSL (Fatahzadeh 2007). Herpes simplex virus type 1 (HSV‐1) causes recurrent HSL. Although herpes simplex virus type 2 (HSV‐2) may occasionally cause primary oral infection, it rarely causes recurrent HSL (Fatahzadeh 2007).
Herpes simplex labialis affects the lips, with the outer third of the lower lip being most frequently affected (Marques 2003). In up to 60% of affected people, HSL is preceded by warning signs, which are known as 'prodromal symptoms'; these are feelings of pain, burning, itching, or tingling at the site of subsequent vesicle development. Headache may also occur in the prodromal stage (Joseph 1985). Within 24 hours of the prodrome, multiple grouped vesicles appear and then weep until they finally form crusts (Fatahzadeh 2007). Such crusts can often bleed quite easily, forming unsightly blackish crusts due to dried blood, which can bleed again when the skin is stretched, e.g., when smiling (Fatahzadeh 2007). These usually heal without scarring within 5 to 15 days (Marques 2003). Herpes simplex labialis may cause pain, discomfort, inconvenience, and some amount of psychological and social distress as a result of cosmetic disfigurement (Fatahzadeh 2007).
Herpes simplex labialis occurs worldwide and is a very common disease (Higgins 1993). The lifetime prevalence of recurrent herpes labialis is 20% to 52.5% (Celik 2013; Higgins 1993). It has been estimated that there are 98 million cases of HSL each year in the US alone (Higgins 1993). Most people with recurrent HSL have fewer than 2 episodes per year, but 5% to 10% of affected people have a minimum of 6 recurrences per annum (Celik 2013; Rooney 1993). Recurrences of HSL seem to be precipitated by a number of factors, including ultraviolet light (UVL); illness; stress; premenstrual tension; severe drug eruptions; and surgical procedures, such as dental surgery, neural surgery, and dermabrasion (a cosmetic procedure used to smooth scars) (Celik 2013; Higgins 1993; Shiohara 2013). People with atopic dermatitis who carry filaggrin mutations are prone to recurrent HSL, which may be attributed to their deficient antiviral immune response (Leung 2014; Rystedt 1986).
Description of the intervention
To date, there has been no proven way of eradicating HSV from the body completely. A number of interventions have been proposed for the prevention of recurrent HSL, including oral antivirals, topical antivirals, and sunscreens (Worrall 2009).
Antiviral agents, including aciclovir, famciclovir, penciclovir, and valaciclovir, inhibit DNA polymerase and viral replications. Before converting to the active antiviral triphosphate form, these drugs need to be phosphorylated by enzymes, such as viral thymidine kinase (TK) or host cellular kinases. Compared with aciclovir, famciclovir and valaciclovir have greater bioavailability and need less frequent dosing. Foscarnet inhibits viral DNA polymerase independent of phosphorylation and is thus used in aciclovir‐resistant HSV infections (Fatahzadeh 2007).
The active ingredients of sunscreens are generally classified into inorganic and organic UVL filters. Inorganic filters, such as titanium oxide, reflect or scatter UVL, while organic filters absorb UVL and convert the energy into heat. The most frequently‐used efficacy index of sunscreen in preventing sunburns is the sun protection factor (SPF), which is measured after application of 2 mg/cm² of product (Kullavanijaya 2005).
How the intervention might work
Long‐term prophylactic administration of oral antivirals (e.g., aciclovir, famciclovir, and valaciclovir) is expected to prevent reactivation of HSV (Worrall 2009). However, continuous daily intake of antivirals is not only costly but also requires the person to adhere to such a programme consistently (Fatahzadeh 2007). Therefore, it is important to design an optimal regimen, balancing known effectiveness of any preventative intervention with the inconvenience and possible side‐effects of continuous medication.
When topical aciclovir cream is used as a treatment for HSL, the frequency of application is five times daily (four hours apart except for sleep) (GSK 2008). However, the efficacy and frequency of application when used as a preventative intervention is unclear.
Based on the fact that ultraviolet light induces the recurrence of HSL (Higgins 1993), sunscreens, theoretically, can prevent recurrence of HSL. However, commercially available sunscreens vary greatly in their active ingredients and the effectiveness of their photoprotection. The effectiveness of photoprotection also depends on the appropriate application of sunscreens; frequency of re‐application after sweating or water sports (Kullavanijaya 2005); and in the case of lips, eating or drinking (Rooney 1991). In actual use, most people apply less than the amounts used in testing SPF, which compromises the efficacy of the sunscreen (Kullavanijaya 2005). Photoprotective lipscreens often contain less UVL‐absorbing ingredients than skin sunscreens (Wahie 2007).
Why it is important to do this review
There has been a Cochrane review on the effects of systemic aciclovir for primary herpetic gingivostomatitis (Nasser 2008) and another on the interventions for the prevention and treatment of HSV in people being treated for cancer (Glenny 2009). However, a systematic review on interventions for preventing HSL in those who are immunocompetent is lacking. We aimed to conduct such a review in order to find out the best evidence on the effects of those interventions currently available for the prevention of recurrent HSL.
The plans for this review were published as a protocol 'Interventions for prevention of herpes simplex labialis (cold sores on the lips)' (Chi 2012).
Objectives
To assess the effects of interventions for the prevention of HSL in people of all ages.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of systemic, topical, and physical interventions for the prevention of herpes simplex labialis (HSL).
Types of participants
Anyone who was immunocompetent and had been initially diagnosed with recurrent HSL by a healthcare professional or trained researcher.
Types of interventions
Any systemic, topical, or physical intervention used for the prevention of HSL. The interventions could be either a single intervention or a combination of interventions. When there were different lengths of use of the intervention, we regarded those of ≤ 1 month as short‐term use and those of > 1 month as long‐term use. The controls might be a placebo, no intervention, or another active intervention.
Types of outcome measures
Primary outcomes
Incidence of HSL during use of the preventative intervention. We accepted both researcher‐diagnosed and participant‐reported recurrences.
Adverse effects during use of the preventative intervention.
Secondary outcomes
Duration of attack of recurrent HSL during use of the preventative intervention.
Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention.
Viral load in saliva.
Rate of adherence to the regimen of the preventative intervention.
Incidence of HSL after use of the preventative intervention. We accepted both researcher‐diagnosed and participant‐reported recurrences.
Duration of attack of recurrent HSL after use of the preventative intervention.
Severity (lesion area, stage, pain) of attack of recurrent HSL after use of the preventative intervention.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 19 May 2015:
the Cochrane Skin Group Specialised Register using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (Issue 4, 2015) using the strategy in Appendix 2;
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3;
EMBASE via Ovid (from 1974) using the strategy in Appendix 4; and
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 5.
We searched the Cochrane Oral Health Group Specialised Register using the search strategy in Appendix 1 up to 19 May 2015.
On 22 May 2015, we searched the China National Knowledge Infrastructure (CKNI) database (from 1994) using the strategy in Appendix 6 and Airiti Library (publications and theses from Taiwan, from 1991) using the strategy in Appendix 7.
Trials registers
We searched the following trials databases on 25 May 2015 using the strategy in Appendix 8.
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov).
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
The World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch).
The EU Clinical Trials Register (www.clinicaltrialsregister.eu).
We searched the metaRegister of Controlled Trials (www.controlled‐trials.com) on 13 June 2014, but this was closed and under review when we updated our search on 25 May 2015.
Searching other resources
Reference lists
We scanned the bibliographies of the included studies and published reviews for further references to relevant trials.
Unpublished literature
We tried to identify further unpublished trials through correspondence with the original researchers of the included studies.
Adverse effects
We did not run separate searches for adverse effects of the target interventions. However, we did extract relevant data from the included trials that we identified.
Data collection and analysis
Some parts of this section uses text that was originally published in another Cochrane review (Chi 2011). We included 'Summary of findings' tables where we used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of the evidence for the primary outcomes for the treatment comparisons.
Selection of studies
Two authors (CC and SW) independently checked titles and abstracts identified from the searches. The authors were not blinded to the names of the original researchers, journals, or institutions. If it was clear from the abstract that the study did not refer to a RCT on interventions for prevention of HSL, we excluded it. The same two authors independently assessed the full text version of each remaining study to determine whether it met the predefined selection criteria. We resolved any disagreement by discussion with referral to a third author (FW), if necessary. We listed the studies that we could only exclude after reading the full text and reasons for exclusion in the 'Characteristics of excluded studies' tables.
Data extraction and management
Two authors (CC and SW) independently extracted the data using a specialised data extraction form. We resolved discrepancies by discussion with a third author (FW). One author (CC) entered the data into Review Manager (RevMan) (Review Manager 2014).
Assessment of risk of bias in included studies
We evaluated the following components since there is some evidence that these are associated with biased estimates of intervention effect (Higgins 2011):
random sequence generation ‐ adequacy of the method of random sequence generation to produce comparable groups in every aspect except for the intervention;
allocation concealment ‐ adequacy of the method used to conceal the allocation sequence to prevent anyone foreseeing the allocation sequence in advance of, or during, enrolment;
blinding of participants and personnel ‐ adequacy of blinding study participants and researchers from knowledge of the allocated interventions;
blinding of outcome assessment ‐ adequacy of blinding outcome assessors from knowledge of the allocated interventions;
incomplete outcome data ‐ the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis, whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions where reported, and any re‐inclusions in our analyses;
selective reporting ‐ whether all prespecified outcomes were reported when the trial protocol was available; and
other sources of bias ‐ any other important concerns about bias.
Measures of treatment effect
For dichotomous outcomes, we expressed the results as risk ratios (RR) with 95% confidence intervals (CI) and where appropriate as number needed to treat to benefit (NNTB) with 95% CI and the baseline risk to which it applies. For continuous outcomes, we expressed the results as difference in means (MD) with 95% CI or where different outcome scales were pooled as standardised mean differences (SMD) with 95% CI. For time‐to‐event outcomes, we expressed the results as hazard ratios (HRs). If Kaplan‐Meier curves were presented, we would have extracted the data from the graphs and calculated HRs according to the methods given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, time‐to‐event outcomes were treated as continuous data in a few included trials. We therefore could only present the original data reported.
With regard to our primary outcome 'Adverse effects during use of the preventative intervention', we measured this by assessing the proportion of participants who experienced adverse events.
With regard to our secondary outcome 'Rate of adherence to the regimen of the preventative intervention', we measured this by assessing either the proportion of participants who adhered to the interventions or the mean proportion of interventions participants received.
Unit of analysis issues
All randomised participants in the control and intervention groups were the unit of analysis. We did not pool the following types of studies with studies of other designs.
Cluster‐randomised trials
For cluster‐randomised trials, we would have used appropriate techniques described in section 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Cross‐over trials
For cross‐over trials, we used appropriate techniques described in section 16.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Studies with multiple treatment groups
Where there were multiple intervention groups within a trial, we made pair‐wise comparisons of an intervention versus no intervention, placebo, or another active intervention.
Dealing with missing data
We contacted the original researchers of studies less than 15 years old for missing data (Table 21). When the missing data were not available, we initially assumed those data were missing at random. If the missing data were caused by participants' dropout, we conducted intention‐to‐treat analyses. For dichotomous outcomes, we would have regarded participants with missing outcome data as treatment failures and included them in the analyses. For continuous outcomes, we would have carried forward the last recorded value for participants with missing outcome data. Where high levels of missing data were seen within the analyses, we would have conducted sensitivity analyses to assess the robustness of the results from the approach described above by comparing the results with those that exclude the missing data from the analyses. However, we failed to conduct the planned analyses because of lacking adequate data, for example, the respective number of randomised participants and those who were lost to follow up in each group.
1. Trialists contacted for missing or unpublished data.
| Study | Enquiries | Reply |
| Baker 2003 | We sent the following request on 13 February 2015: (1) How did you randomise the participants? (2) Did you do any measures for allocation concealment? (3) Could you please offer the details of how you achieved double blindness? (4) Did you use a person other than the physician to assess the outcomes? |
No reply |
| de Carvalho 2010 | We sent the following request on 23 June 2014: (1) How did you randomise the participants? (2) Did you do any measures for allocation concealment? (3) Did you use a person other than the physician to assess the outcomes? (4) The number of dropouts or withdrawals in this trial (5) Did you assess any outcomes regarding adverse events? If you did, what were the results? |
4 August 2014 (1) Randomisation was down through sortition (2) No (3) No (4) 01 (5) Adverse events were evaluated, but there were no adverse events detected |
| Gilbert 2007 | We sent the following request on 13 February 2015: (1) How did you randomise the participants? (2) Did you do any measures for allocation concealment? |
No reply |
| Pfitzer 2005 | We sent the following request on 23 June 2014: (1) How did you randomise the participants? (2) Did you do any measures for allocation concealment? (3) The number of dropouts or withdrawals in this trial (4) Did you assess any outcomes regarding adverse events? If you did, what were the results? |
No reply |
| Senti 2013 | This trial was identified from searching trial registers (NCT00914745). We sent the following request on 27 December 2013: "Dear Prof Kündig, I am conducting a Cochrane review on interventions for prevention of herpes simplex labialis (see http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010095/abstract). I have noticed that you have completed a trial (http://clinicaltrials.gov/show/NCT00914745) that assessed a topical ointment for prevention of herpes simplex labialis, and was wondering if you would like to share your results with us, thus we could include your trial in our review. Your assistance would be appreciated" |
The trialists provided us with the full published article |
| ISRCTN03397663 | We sent the following request on 27 December 2013: "Dear Dr Cheras, I am conducting a Cochrane review on interventions for prevention of herpes simplex labialis (see http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010095/abstract). I have noticed that you completed a trial that used Sheabutter extract BSP110 for prevention of herpes simplex labialis (http://www.controlled‐trials.com/ISRCTN03397663#?close=1). I was wondering if you would like to share your results with us. Thus, we could include your trial in our review. Your assistance would be appreciated" |
No reply |
| NCT01225341 | We sent the following request on 27 December 2013: "Dear Dr Dayan, I am conducting a Cochrane review on interventions for prevention of herpes simplex labialis (see http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010095/abstract). I have noticed that you are conducting a trial that uses botulinum toxin A injections for prevention of herpes simplex labialis (http://clinicaltrials.gov/show/NCT01225341). I was wondering if you have completed the trial and would like to share your results with us. Thus, we could include your trial in our review. Your assistance would be appreciated" |
No reply |
| NCT01971385 | We sent the following request on 19 January 2014: "Dear Dr Kimball, I am conducting a Cochrane review on interventions for prevention of herpes simplex labialis (see http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010095/abstract). I have noticed that you are doing a trial (http://www.clinicaltrials.gov/ct2/show/NCT01971385) that assessed a topical ointment for prevention of herpes simplex labialis, and was wondering if you would like to share your results with us if you have completed the trial, thus we could include your trial in our review. Your assistance would be greatly appreciated" |
No reply |
Assessment of heterogeneity
We assessed clinical heterogeneity inherent in the study design, interventions, participants, and outcome measures to determine whether a meta‐analysis was appropriate. The anticipated clinical heterogeneity included various lengths and regimens of the same intervention, presence of atopic dermatitis, and induction by UVL. We also determined the I² statistic to assess the statistical heterogeneity. When there was clinical heterogeneity or the I² statistic was greater than 80%, we did not perform a meta‐analysis.
Assessment of reporting biases
We would have tested publication bias for primary outcomes by using a funnel plot when at least 10 trials on an intervention were available. However, the limited number of trials for each intervention meant it was impossible to do this test.
Data synthesis
For trials on a particular intervention, we conducted a meta‐analysis using a random‐effects model (DerSimonian and Laird model) to calculate a weighted intervention effect across trials when the I² statistic was 80% or less with reasonable clinical homogeneity. We decided clinical homogeneity based on similar participants and intervention regimens. Where it was inappropriate or impossible to perform a meta‐analysis, we summarised the data narratively for each trial.
Subgroup analysis and investigation of heterogeneity
We discussed similarities and differences of included RCTs in terms of the study design, interventions, participants, and outcome measures. We would have conducted subgroup analyses of the following if adequate data were available:
participants with atopic dermatitis: we found no data relevant to atopic dermatitis and thus did not conduct a subgroup analysis; and
participants with UVL‐induced HSL: for sunscreen where relevant data were available, we conducted a subgroup analysis on HSL induced by natural and experimental UVL separately.
Sensitivity analysis
We would have performed a sensitivity analysis to examine the intervention effects after excluding those studies with lower methodological quality if appropriate. However, we did not do so because of a very limited number of trials for the same intervention.
Other
We involved a consumer coauthor (FD) throughout the review process to help improve the relevance and readability of the final review.
Results
Description of studies
Results of the search
As shown in Figure 1, our search identified 1387 citations. After removing duplicates, we assessed 1329 citations. We excluded 1252 citations because the title, abstract, or both did not meet our inclusion criteria. We sought the full texts of the remaining 77 citations. We excluded 38 citations, mostly because these were either non‐randomised studies or randomised controlled trials (RCTs) on interventions for treatments of herpes simplex labialis (HSL). Of the remaining 39 citations, we transferred 4 studies to the section 'Ongoing studies' as they were not yet completed. We included the remaining 35 citations, reporting 32 relevant trials, in this review. One included citation reported four trials, of which three met our inclusion criteria (Spruance 1991a; Spruance 1991b; Spruance 1991c). Five included trials, Miller 2004; Pazin 1979; Pedersen 2001; Russell 1978; Schindl 1999, had two citations.
1.

Study flow diagram.
Included studies
This review included 32 trials, with a total of 2640 participants, covering 19 treatments. We describe the details of the included studies in the 'Characteristics of included studies' tables.
Design
All of the 32 included studies were RCTs, with 5 being cross‐over RCTs (Gibson 1986; Gilbert 2007; Rooney 1991; Rooney 1993; Thein 1984).
Sample sizes
The number of participants in the included studies ranged from 19 to 310. Seven of the included trials had a small sample size of less than 30 participants (Duteil 1998; Gibson 1986; Møller 1997; Pfitzer 2005; Rooney 1993; Thein 1984).
Setting
The setting was multicentre in 13 trials (Altmeyer 1991; Bernstein 1994; Bernstein 1997; Bolla 1985; Busch 2009; Gibson 1986; Mills 1987; Raborn 1997; Raborn 1998; Rooney 1991; Spruance 1988; Spruance 1991c; Spruance 1999) and single‐centre in 19 trials (Baker 2003; de Carvalho 2010; Duteil 1998; Gilbert 2007; Ho 1984; Miller 2004; Møller 1997; Pazin 1979; Pedersen 2001; Pfitzer 2005; Redman 1986; Rooney 1993; Russell 1978; Schädelin 1988; Schindl 1999; Senti 2013; Spruance 1991a; Spruance 1991b; Thein 1984). All of the included trials were conducted either in Europe or North America.
Participants
All of the included trials included adults aged 18 years or older, with 2 trials extending to persons aged 16 years or older, Bolla 1985; Gibson 1986, and 1 trial extending to persons aged at least 12 years (Miller 2004). Two trials, Russell 1978; Thein 1984, did not state the age limit of inclusion criteria but included participants aged seven and eight years, respectively.
Interventions
The included trials assessed the effects of 19 interventions for preventing HSL, including 6 oral treatments (aciclovir (Raborn 1998; Rooney 1993; Schädelin 1988; Spruance 1988; Spruance 1991a; Spruance 1991b), valaciclovir (Baker 2003; Gilbert 2007; Miller 2004), famciclovir (Spruance 1999), levamisole (Russell 1978), lysine (Thein 1984), and LongoVital® (a vitamin and herbs supplement) (Pedersen 2001)), 5 topical treatments (aciclovir cream (Gibson 1986; Raborn 1997; Spruance 1991c), aciclovir plus 348U87 cream (Bernstein 1994), topical foscarnet 3% (Bernstein 1997), 1,5‐pentanediol (a low‐toxicity molecule with an antiviral activity) gel (Busch 2009), 2‐hydroxypropyl‐β‐cyclo dextrin gel (Senti 2013)), sunscreens (Duteil 1998; Mills 1987; Rooney 1991), 3 immunomodulating treatments given by injection (interferon (Ho 1984; Pazin 1979), intradermal gamma globulin (Redman 1986), and thymopentin (Bolla 1985)), 2 vaccines (herpes simplex virus (HSV) type I subunit vaccine (Altmeyer 1991) and yellow fever vaccination (Møller 1997)), low‐intensity lasers (de Carvalho 2010; Schindl 1999), and hypnotherapy (Pfitzer 2005).
Outcomes
Of the 32 included trials, all reported either the incidence or frequency of HSL during use of the preventative intervention, and 17 trials (53%) reported adverse events. There were 12 and 20 trials reporting the duration and severity of recurrent HSL, respectively. Only one trial, Miller 2004, measured the shedding of HSV in the saliva, and only two trials, Rooney 1993; Spruance 1999, assessed participants' adherence to study medications.
Funding source
Of the included 32 trials, industry supported 18, and non‐profit organisations (such as government or academic institutions) supported 4; the other 10 trials did not report the funding source.
Excluded studies
We excluded 38 citations after examining the full text. We list the reasons for exclusion in the 'Characteristics of excluded studies' tables.
Ongoing Studies
We identified 4 ongoing trials that were on a sheabutter extract (BSP110), botulinum toxin A injection, an experimental drug (BTL‐TML‐HSV), and squaric acid dibutylester, respectively (ISRCTN03397663; NCT01225341; NCT01902303; NCT01971385). We contacted the four trialists, but none of them replied. We present the details of these trials in the 'Characteristics of ongoing studies' tables.
Risk of bias in included studies
We summarise our judgements about each 'Risk of bias' item presented as percentages across all of the included trials in Figure 2, and we summarise our judgements about each 'Risk of bias' item for each included trial in Figure 3. We present further details in the 'Risk of bias' tables in the 'Characteristics of included studies' section. The risk of bias of the included trials varied from low to high.
2.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Nine trials used an adequate method of generation of the randomisation sequence (Bernstein 1994; Busch 2009; de Carvalho 2010; Miller 2004; Mills 1987; Møller 1997; Pazin 1979; Rooney 1991; Schädelin 1988), but all the other 23 trials did not describe the process of randomisation.
Allocation could not be foreseen in 5 trials (Busch 2009; Miller 2004; Møller 1997; Schädelin 1988; Spruance 1999), while it was unclear if allocation was concealed in the other 27 trials.
Blinding
Twenty‐six trials blinded both the investigators and participants (Altmeyer 1991; Baker 2003; Bernstein 1994; Bernstein 1997; Bolla 1985; Busch 2009; Gibson 1986; Ho 1984; Miller 2004; Mills 1987; Møller 1997; Pazin 1979; Pedersen 2001; Raborn 1997; Raborn 1998; Redman 1986; Rooney 1993; Russell 1978; Schädelin 1988; Senti 2013; Spruance 1988; Spruance 1991a; Spruance 1991b; Spruance 1991c; Spruance 1999; Thein 1984), while 5 trials did not blind them (de Carvalho 2010; Gilbert 2007; Pfitzer 2005; Rooney 1991; Schindl 1999). The de Carvalho 2010 trial compared laser treatments with no interventions. The Schindl 1999 trial performed the placebo irradiation in the same manner as in the laser group except that the laser was not turned on. However, laser irradiation might produce the sensation of sound and heat that could have been sensed by the participants. The Gilbert 2007 trial compared episodic and suppressive valaciclovir regimens. The Pfitzer 2005 trial compared hypnotherapy with no hypnotherapy. The Rooney 1991 trial compared a sunscreen with placebo solution, but the placebo recipients had sunburn while none of the sunscreen recipients had sunburn. Thus, the participants and researchers might have known the assigned treatments. It was unclear if the investigators and participants were blinded in the Duteil 1998 trial.
Outcome assessment was blinded in 27 trials (Altmeyer 1991; Baker 2003; Bernstein 1994; Bernstein 1997; Bolla 1985; Busch 2009; Gibson 1986; Ho 1984; Miller 2004; Mills 1987; Møller 1997; Pazin 1979; Pedersen 2001; Raborn 1997; Raborn 1998; Redman 1986; Rooney 1993; Russell 1978; Schädelin 1988; Schindl 1999; Senti 2013; Spruance 1988; Spruance 1991a; Spruance 1991b; Spruance 1991c; Spruance 1999; Thein 1984) and unblinded in 4 trials (de Carvalho 2010; Gilbert 2007; Pfitzer 2005; Rooney 1991). It was unclear if the outcome assessors were blinded in the other trial (Duteil 1998).
Incomplete outcome data
The risk of attrition bias was low in 17 trials because of a low or null dropout rate (Baker 2003; Busch 2009; de Carvalho 2010; Miller 2004; Mills 1987; Møller 1997; Pazin 1979; Pedersen 2001; Raborn 1997; Raborn 1998; Rooney 1991; Rooney 1993; Schindl 1999; Schädelin 1988; Senti 2013; Spruance 1988; Spruance 1999). On the other hand, the risk of attrition bias was high in two trials because of a high dropout rate (Gilbert 2007; Russell 1978). No dropouts or withdrawals were mentioned in the other 13 trials.
Selective reporting
A total of 18 trials reported both the prespecified primary efficacy and adverse outcomes (Altmeyer 1991; Baker 2003; Bernstein 1997; Bolla 1985; Busch 2009; de Carvalho 2010; Gibson 1986; Gilbert 2007; Ho 1984; Miller 2004; Møller 1997; Pazin 1979; Pfitzer 2005; Raborn 1998; Russell 1978; Schädelin 1988; Spruance 1988; Spruance 1999). We judged these 18 trials to be at a low risk of reporting bias.
The Schindl 1999 trial reported the median recurrence‐free interval, which was not a prespecified outcome in our review protocol. The study protocol of the Senti 2013 trial is available on the US National Institutes of Health ongoing trials register (identifier: NCT00914745). The prespecified primary outcome (the number of herpes labialis relapse) has been reported. However, the exact numerical data were not provided; the authors only provided the data in plots. We therefore judged the two trials to be at an unclear risk of bias.
A total of 10 trials did not report adverse events (Bernstein 1994; Duteil 1998; Mills 1987; Redman 1986; Rooney 1991; Rooney 1993; Spruance 1991a; Spruance 1991b; Spruance 1991c; Thein 1984). The Pedersen 2001 and Raborn 1997 trials did not fully report the details of outcome data. All of these 12 trials were marked as high risk of bias for this domain.
Other potential sources of bias
A total of nine trials had a high risk of other potential bias for various reasons including early termination (Bernstein 1994), no washout period (Gibson 1986; Gilbert 2007; Rooney 1993; Thein 1984), different baseline frequency of recurrence of HSL (Pedersen 2001; Russell 1978), lack of standardised follow‐up plan (Schindl 1999), and a low percentage of participants having a history of HSL (Schädelin 1988). We judged Spruance 1988 at a low risk of other potential bias because of the trialists' advice to participants on frequent use of a standard sunscreen and no relation between the occurrence of herpes labialis and the potential confounding factors.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18; Table 19; Table 20
Summary of findings for the main comparison. Oral aciclovir (short‐term) compared with placebo for prevention of herpes simplex labialis.
| Oral aciclovir (short‐term) compared with placebo for prevention of herpes simplex labialis | ||||||
| Patient or population: participants with recurrent herpes simplex labialis (cold sores on the lips) Settings: ski sites and university hospitals Intervention: oral aciclovir (short‐term) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Oral aciclovir (short‐term) | |||||
| Incidence of herpes labialis during use of the preventative intervention (by clinical evaluation) ‐ aciclovir 800 mg twice daily | Study population | RR 1.08 (0.62 to 1.87) | 237 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 171 per 1000 | 184 per 1000 (106 to 319) | |||||
| Moderate | ||||||
| 171 per 1000 | 185 per 1000 (106 to 320) | |||||
| Incidence of herpes labialis during use of the preventative intervention (by clinical evaluation) ‐ aciclovir 400 mg twice daily | Study population | RR 0.26 (0.13 to 0.51) | 177 (2 studies) | ⊕⊕⊝⊝ Low² | ‐ | |
| 364 per 1000 | 95 per 1000 (47 to 185) | |||||
| Moderate | ||||||
| 538 per 1000 | 140 per 1000 (70 to 274) | |||||
| Incidence of herpes labialis during use of the preventative intervention (by clinical evaluation) ‐ aciclovir 200 mg 5 times/day | Study population | RR 0.46 (0.2 to 1.07) | 66 (1 study) | ⊕⊕⊝⊝ Low³ | ‐ | |
| 394 per 1000 | 181 per 1000 (79 to 422) | |||||
| Moderate | ||||||
| 394 per 1000 | 181 per 1000 (79 to 422) | |||||
| Incidence of herpes labialis during use of the preventative intervention (by culture) ‐ aciclovir 400 mg twice daily | Study population | RR 0.05 (0 to 0.7) | 30 (1 study) | ⊕⊕⊝⊝ Low³ | ‐ | |
| 750 per 1000 | 38 per 1000 (0 to 525) | |||||
| Moderate | ||||||
| 750 per 1000 | 38 per 1000 (0 to 525) | |||||
| Adverse effects during use of the preventative intervention ‐ aciclovir 800 mg twice daily | Study population | RR 0.98 (0.7 to 1.38) | 239 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 363 per 1000 | 356 per 1000 (254 to 501) | |||||
| Moderate | ||||||
| 363 per 1000 | 356 per 1000 (254 to 501) | |||||
| Adverse effects during use of the preventative intervention ‐ aciclovir 400 mg twice daily | Study population | RR 2.3 (0.62 to 8.58) | 183 (2 studies) | ⊕⊕⊝⊝ Low² | ‐ | |
| 33 per 1000 | 75 per 1000 (20 to 280) | |||||
| Moderate | ||||||
| 20 per 1000 | 46 per 1000 (12 to 172) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded one level due to imprecision: the available evidence is limited to one single randomised trial. ²Downgraded two levels due to risk of bias and imprecision: the available evidence is limited to two randomised trials, with one having a high risk of other biases. ³Downgraded two levels due to risk of bias and imprecision: the available evidence is limited to one single randomised trial with a high risk of reporting bias.
Summary of findings 2. Oral aciclovir (long‐term) compared with placebo for prevention of herpes simplex labialis.
| Oral aciclovir (long‐term) compared with placebo for prevention of herpes simplex labialis | ||||||
| Patient or population: participants with recurrent herpes simplex labialis Settings: a medical centre Intervention: oral aciclovir (long‐term) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | Oral aciclovir (long‐term) | |||||
| Incidence of herpes labialis during use of the preventative intervention (by culture) | 1.40 episodes per participant per a 4‐month period | 0.40 episodes per participant per a 4‐month period | Not estimable | 40 (1 study) | ⊕⊕⊝⊝ Low¹ | ‐ |
| Incidence of herpes labialis during use of the preventative intervention (by clinical evaluation) | 1.80 episodes per participant per a 4‐month period | 0.85 episodes per participant per a 4‐month period | Not estimable | 40 (1 study) | ⊕⊕⊝⊝ Low¹ | ‐ |
| Duration of attack of herpes labialis during use of the preventative intervention | ‐ | The mean duration of attack of herpes labialis during use of the preventative intervention in the intervention groups was 3.6 lower (7.2 lower to 0 higher) | ‐ | 40 (1 study) | ⊕⊕⊝⊝ Low¹ | ‐ |
| Rate of adherence to the regimen of the preventative intervention | 99% of the prescribed study medication | 99% of the prescribed study medication | ‐ | 40 (1 study) | ⊕⊕⊝⊝ Low¹ | ‐ |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded two levels due to risk of bias and imprecision: the available evidence is limited to one single randomised trial with a high risk of reporting bias.
Summary of findings 3. Valaciclovir (short‐term) compared with placebo for prevention of herpes simplex labialis.
| Valaciclovir (short‐term) compared with placebo for prevention of herpes simplex labialis | ||||||
| Patient or population: participants with recurrent herpes simplex labialis Settings: a university hospital Intervention: valaciclovir (short‐term) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Valaciclovir (short‐term) | |||||
| Incidence of HSL during use of the preventative intervention (by clinical evaluation) | Study population | RR 0.55 (0.23 to 1.28) | 125 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 206 per 1000 | 113 per 1000 (47 to 264) | |||||
| Moderate | ||||||
| 206 per 1000 | 113 per 1000 (47 to 264) | |||||
| Incidence of HSL during use of the preventative intervention (by culture) | Study population | RR 0.47 (0.21 to 1.08) | 125 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 238 per 1000 | 112 per 1000 (50 to 257) | |||||
| Moderate | ||||||
| 238 per 1000 | 112 per 1000 (50 to 257) | |||||
| Adverse effects during use of the preventative intervention | Study population | RR 1.33 (0.71 to 2.5) | 125 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 206 per 1000 | 274 per 1000 (147 to 516) | |||||
| Moderate | ||||||
| 206 per 1000 | 274 per 1000 (146 to 515) | |||||
| Viral load (shedding) in saliva | Study population | RR 0.16 (0.02 to 1.26) | 120 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 103 per 1000 | 17 per 1000 (2 to 130) | |||||
| Moderate | ||||||
| 103 per 1000 | 16 per 1000 (2 to 130) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; HSL: herpes simplex labialis; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded one level due to imprecision: the available evidence is limited to one single randomised trial.
Summary of findings 4. Valaciclovir (long‐term) compared with placebo for prevention of herpes labialis.
| Valaciclovir (long‐term) compared with placebo for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: a university hospital Intervention: valaciclovir (long‐term) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Valaciclovir (long‐term) | |||||
| Incidence of herpes labialis during use of the preventative intervention | 0.21 episodes per participant per month | 0.12 episodes per participant per month | Not estimable | 95 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ |
| Adverse effects during use of the preventative intervention | Study population | RR 0.86 (0.51 to 1.46) | 95 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 396 per 1000 | 340 per 1000 (202 to 578) | |||||
| Moderate | ||||||
| 396 per 1000 | 341 per 1000 (202 to 578) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded one level due to imprecision: the available evidence is limited to one single randomised trial.
Summary of findings 5. Valaciclovir (suppressive regimen compared with episodic regimen) for prevention of herpes labialis.
| Valaciclovir (suppressive regimen compared with episodic regimen) for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: a university hospital Intervention: suppressive regimen Comparison: episodic regimen | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Episodic regimen | Suppressive regimen | |||||
| Incidence of herpes labialis during use of the preventative intervention (number of recurrences per participant per month) | 0.1775 ± 0.1975 | 0.075 ± 0.1025 | The mean incidence of herpes labialis during use of the preventative intervention in the intervention groups was 0.1 lower (0.16 to 0.05 lower) | 120 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ |
| Adverse effects during use of the preventative intervention | Study population | RR 1.21 (0.78 to 1.87) | 152 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ | |
| 316 per 1000 | 382 per 1000 (246 to 591) | |||||
| Moderate | ||||||
| 316 per 1000 | 382 per 1000 (246 to 591) | |||||
| Duration of attack of recurrent herpes labialis during use of the preventative intervention | 2.86 ± 3.10 days | 1.78 ± 2.92 days | The mean duration of attack of recurrent herpes labialis during use of the preventative intervention in the intervention groups was 1.08 days shorter (2.16 lower to 0 higher) | 120 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ |
| Severity (pain) of attack of recurrent herpes labialis during use of the preventative intervention | 0.23 ± 0.32 | 0.14 ± 0.27 | The mean severity (pain) of attack of recurrent herpes labialis during use of the preventative intervention in the intervention groups was 0.09 lower (0.2 lower to 0.02 higher) | 120 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ |
| Severity (maximum total lesion area) of attack of recurrent herpes labialis during use of the preventative intervention | 10.52 ± 19.45 mm² | 5.14 ± 9.98 mm² | The mean severity (maximum total lesion area) of attack of recurrent herpes labialis during use of the preventative intervention in the intervention groups was 5.38 smaller (10.91 lower to 0.15 higher) | 120 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded three levels due to imprecision and multiple risk of biases in performance, detection, attrition, and other sources: the available evidence is limited to one single randomised trial with a high risk of biases.
Summary of findings 6. Famciclovir compared with placebo for prevention of herpes labialis.
| Famciclovir compared with placebo for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: multicentre Intervention: famciclovir Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Famciclovir | |||||
| Incidence of herpes labialis during use of the preventative intervention (by clinical evaluation) ‐ famciclovir 125 mg | Study population | RR 0.74 (0.5 to 1.11) | 120 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 517 per 1000 | 382 per 1000 (258 to 574) | |||||
| Moderate | ||||||
| 517 per 1000 | 383 per 1000 (259 to 574) | |||||
| Incidence of herpes labialis during use of the preventative intervention (by clinical evaluation) ‐ famciclovir 250 mg | Study population | RR 0.69 (0.45 to 1.04) | 122 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 517 per 1000 | 357 per 1000 (232 to 537) | |||||
| Moderate | ||||||
| 517 per 1000 | 357 per 1000 (233 to 538) | |||||
| Incidence of herpes labialis during use of the preventative intervention (by clinical evaluation) ‐ famciclovir 500 mg | Study population | RR 0.82 (0.56 to 1.21) | 121 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 517 per 1000 | 424 per 1000 (289 to 625) | |||||
| Moderate | ||||||
| 517 per 1000 | 424 per 1000 (290 to 626) | |||||
| Duration of attack of recurrent herpes labialis during use of the preventative intervention ‐ famciclovir 125 mg | Study population | HR 1.63 (0.84 to 3.15) | 47 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| See comment² | See comment² | |||||
| Duration of attack of recurrent herpes labialis during use of the preventative intervention ‐ famciclovir 250 mg | Study population | HR 1.59 (0.79 to 3.2) | 45 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| See comment² | See comment² | |||||
| Duration of attack of recurrent herpes labialis during use of the preventative intervention ‐ famciclovir 500 mg | Study population | HR 2.39 (1.23 to 4.63) | 51 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| See comment² | Shortened by 2.8 days | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; HR: hazard ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded one level due to imprecision: the available evidence is limited to one single randomised trial. ²Data unavailable.
Summary of findings 7. Levamisole compared with placebo for prevention of herpes labialis.
| Levamisole compared with placebo for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: a university hospital Intervention: levamisole Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Levamisole | |||||
| Incidence of herpes labialis during use of the preventative intervention | 2.7 ± 2.3 recurrences during a 6‐month period | The mean incidence of herpes labialis during use of the preventative intervention in the intervention groups was 2 lower (2.24 to 1.76 lower) during a 6‐month period | ‐ | 72 (1 study) | ⊕⊝⊝⊝ Very low¹ | Of the 99 participants randomised, 27 (27.2%) did not complete the trial and were excluded from the analysis, with 19 (39.6%) in the levamisole group and 8 (15.7%) in the placebo group |
| Adverse effects during use of the preventative intervention (leading to withdrawal) | Study population | See comment | 99 (1 study) | ⊕⊝⊝⊝ Very low¹ | Risks were calculated from pooled risk differences | |
| 157 per 1000 | 395 per 1000 (227 to 566) | |||||
| Moderate | ||||||
| 157 per 1000 | 396 per 1000 (228 to 567) | |||||
| Duration of attack of recurrent herpes labialis during use of the preventative intervention | 8.2 ± 2.8 days | The mean duration of attack of recurrent herpes labialis during use of the preventative intervention in the intervention groups was 0.7 days longer (0.22 to 1.18 longer) | ‐ | 72 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded three levels due to imprecision and attrition and other biases: the available evidence is limited to a single study with a high risk of attrition and other biases.
Summary of findings 8. Lysine compared with placebo for prevention of herpes labialis.
| Lysine compared with placebo for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes simplex labialis (cold sores on the lips) Settings: a university hospital Intervention: lysine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Lysine | |||||
| Incidence of herpes labialis during use of the preventative intervention (number of recurrences per participant per month) | ‐ | The mean incidence of herpes labialis during use of the preventative intervention in the intervention groups was 0.04 lower (0.37 lower to 0.29 higher) | ‐ | 26 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded three levels due to imprecision and reporting and other biases: the available evidence is limited to a single study with a high risk of reporting and other biases.
Summary of findings 9. Topical aciclovir (short‐term) compared with placebo for prevention of herpes labialis.
| Topical aciclovir (short‐term) compared with placebo for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: ski sites and university hospitals Intervention: topical aciclovir (short‐term) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Topical aciclovir (short‐term) | |||||
| Incidence of herpes labialis during use of the preventative intervention | Study population | RR 0.91 (0.48 to 1.72) | 271 (2 studies) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 304 per 1000 | 276 per 1000 (146 to 522) | |||||
| Moderate | ||||||
| 328 per 1000 | 298 per 1000 (157 to 564) | |||||
| Adverse effects during use of the preventative intervention | Study population | RR 1.17 (0.59 to 2.32) | 191 (1 study) | ⊕⊕⊝⊝ Low² | ‐ | |
| 135 per 1000 | 158 per 1000 (80 to 314) | |||||
| Moderate | ||||||
| 135 per 1000 | 158 per 1000 (80 to 313) | |||||
| Severity (aborted lesions) of attack of recurrent herpes labialis during use of the preventative intervention | Study population | RR 1.02 (0.19 to 5.57) | 52 (1 study) | ⊕⊕⊝⊝ Low² | ‐ | |
| 95 per 1000 | 97 per 1000 (18 to 530) | |||||
| Moderate | ||||||
| 95 per 1000 | 97 per 1000 (18 to 529) | |||||
| Incidence of herpes labialis after use of the preventative intervention | Study population | RR 0.35 (0.13 to 0.94) | 181 (1 study) | ⊕⊕⊝⊝ Low² | ‐ | |
| 156 per 1000 | 54 per 1000 (20 to 146) | |||||
| Moderate | ||||||
| 156 per 1000 | 55 per 1000 (20 to 147) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded one level due to risk of bias: the evidence is from two trials with a high risk of reporting bias. ²Downgraded two levels due to risk of bias and imprecision: the evidence is from a single trial with a high risk of bias.
Summary of findings 10. Topical aciclovir and 348U87 cream (short‐term) compared with placebo for prevention of herpes labialis.
| Topical aciclovir and 348U87 cream (short‐term) compared with placebo for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: research institutes Intervention: topical aciclovir and 348U87 cream (short‐term) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Topical aciclovir and 348U87 cream (short‐term) | |||||
| Incidence of herpes labialis during use of the preventative intervention (by culture) | Study population | RR 0.78 (0.19 to 3.14) | 51 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ | |
| 154 per 1000 | 120 per 1000 (29 to 483) | |||||
| Moderate | ||||||
| 154 per 1000 | 120 per 1000 (29 to 484) | |||||
| Incidence of herpes labialis during use of the preventative intervention (by clinical evaluation) | Study population | RR 1.46 (0.53 to 3.99) | 51 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ | |
| 192 per 1000 | 281 per 1000 (102 to 767) | |||||
| Moderate | ||||||
| 192 per 1000 | 280 per 1000 (102 to 766) | |||||
| Duration of attack of recurrent herpes labialis during use of the preventative intervention | ‐ | The mean duration of attack of recurrent herpes labialis during use of the preventative intervention in the intervention groups was 2.5 days longer (1.39 shorter to 6.39 longer) | ‐ | 9 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ |
| Severity of attack of recurrent herpes labialis during use of the preventative intervention (maximum lesion area) | ‐ | The mean severity of attack of recurrent herpes labialis during use of the preventative intervention (maximum lesion area) in the intervention groups was 73 larger (42.22 smaller to 188.22 larger) | ‐ | 9 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded three levels due to imprecision and reporting and other biases: the available evidence is from a single trial with a high risk of reporting and other biases.
Summary of findings 11. Topical foscarnet compared with placebo for prevention of herpes labialis.
| Topical foscarnet compared with placebo for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: medical centres Intervention: topical foscarnet Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Topical foscarnet | |||||
| Incidence of herpes labialis during use of the preventative intervention | Study population | RR 1.08 (0.82 to 1.4) | 295 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 408 per 1000 | 441 per 1000 (335 to 571) | |||||
| Moderate | ||||||
| 408 per 1000 | 441 per 1000 (335 to 571) | |||||
| Adverse effects during use of the preventative intervention (leading to discontinuation) | Study population | RR 2.96 (0.12 to 72.11) | 302 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Adverse effects during use of the preventative intervention (application site reactions) | Study population | RR 2.47 (0.79 to 7.69) | 302 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 27 per 1000 | 66 per 1000 (21 to 205) | |||||
| Moderate | ||||||
| 27 per 1000 | 67 per 1000 (21 to 208) | |||||
| Duration of attack of recurrent herpes labialis during use of the preventative intervention (healing time) | ‐ | The mean duration of attack of recurrent herpes labialis during use of the preventative intervention (healing time) in the intervention groups was 0.21 days shorter (1.68 shorter to 1.26 longer) | ‐ | 125 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ |
| Severity of attack of recurrent herpes labialis during use of the preventative intervention (mean lesion area) | ‐ | The mean severity of attack of recurrent herpes labialis during use of the preventative intervention (mean lesion area) in the intervention groups was 16 lower (38.96 lower to 6.96 higher) | ‐ | 124 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ |
| Severity of attack of recurrent herpes labialis during use of the preventative intervention (maximum lesion area) | ‐ | The mean severity of attack of recurrent herpes labialis during use of the preventative intervention (maximum lesion area) in the intervention groups was 30 lower (72.64 lower to 12.64 higher) | ‐ | 124 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ |
| Severity of attack of recurrent herpes labialis during use of the preventative intervention (duration of pain) | ‐ | The mean severity of attack of recurrent herpes labialis during use of the preventative intervention (duration of pain) in the intervention groups was 0.1 higher (1.11 lower to 1.31 higher) | ‐ | 113 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded one level due to imprecision: the available evidence is from a single trial.
Summary of findings 12. Topical 1,5‐pentanediol compared with placebo for prevention of herpes labialis.
| Topical 1,5‐pentanediol compared with placebo for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: study centres Intervention: topical 1,5‐pentanediol Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Topical 1,5‐pentanediol | |||||
| Incidence of herpes labialis during use of the preventative intervention | Study population | Not estimable | 102 (1 study) | ⊕⊕⊕⊝ Moderate¹ | P > 0.05 calculated using the Mann‐Whitney test by the trialists | |
| 109 episodes out of 50 | 120 episodes out of 52 | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| Adverse effects during use of the preventative intervention | Study population | Not estimable | 102 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| See comment | See comment | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| Severity (blistering, swelling, or pain) of recurrence | Study population | RR 1.05 (0.91 to 1.2) | 224 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ | |
| 756 per 1000 | 794 per 1000 (688 to 908) | |||||
| Moderate | ||||||
| 756 per 1000 | 794 per 1000 (688 to 907) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded one level due to imprecision: the available evidence is from a single study.
Summary of findings 13. Sunscreen compared with placebo for prevention of herpes labialis.
| Sunscreen compared with placebo for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: single centre and multicentre Intervention: sunscreen Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Sunscreen | |||||
| Incidence of herpes labialis during use of the preventative intervention (by clinical evaluation) ‐ solar radiation | Study population | RR 1.12 (0.25 to 5.06) | 51 (1 study) | ⊕⊕⊝⊝ Low¹ | ‐ | |
| 111 per 1000 | 124 per 1000 (28 to 562) | |||||
| Moderate | ||||||
| 111 per 1000 | 124 per 1000 (28 to 562) | |||||
| Incidence of herpes labialis during use of the preventative intervention (by clinical evaluation) ‐ experimental ultraviolet light | Study population | RR 0.07 (0.01 to 0.33) | 111 (2 studies) | ⊕⊝⊝⊝ Very low² | ‐ | |
| 456 per 1000 | 32 per 1000 (5 to 151) | |||||
| Moderate | ||||||
| 487 per 1000 | 34 per 1000 (5 to 161) | |||||
| Incidence of herpes labialis during use of the preventative intervention (by culture) | Study population | See comment | 73 (1 study) | ⊕⊝⊝⊝ Very low³ | Risks were calculated from pooled risk differences | |
| 658 per 1000 | 26 per 1000 (0 to 191) | |||||
| Moderate | ||||||
| 658 per 1000 | 26 per 1000 (0 to 191) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded two levels due to risk of bias and imprecision: the available evidence is limited to a single study with a high risk of reporting bias. ²Downgraded three levels due to imprecision and multiple risk of bias in performance, detection, and reporting. The available evidence is from two trials with a high risk of biases. ³Downgraded three levels due to imprecision and multiple risk of bias in performance, detection, and reporting: the available evidence is from a single trial with a high risk of biases.
Summary of findings 14. Interferon compared with placebo for prevention of herpes labialis.
| Interferon compared with placebo for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: hospitals Intervention: interferon Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Interferon | |||||
| Incidence of herpes labialis during use of the preventative intervention ‐ presurgical | Study population | RR 1.59 (1.05 to 2.41) | 32 (1 study) | ⊕⊕⊝⊝ Low¹, ² | ‐ | |
| 571 per 1000 | 909 per 1000 (600 to 1000) | |||||
| Moderate | ||||||
| 571 per 1000 | 908 per 1000 (600 to 1000) | |||||
| Incidence of herpes labialis during use of the preventative intervention ‐ postsurgical | Study population | RR 0.99 (0.59 to 1.66) | 44 (1 study) | ⊕⊕⊝⊝ Low¹, ² | ‐ | |
| 571 per 1000 | 566 per 1000 (337 to 949) | |||||
| Moderate | ||||||
| 571 per 1000 | 565 per 1000 (337 to 948) | |||||
| Incidence of herpes labialis during use of the preventative intervention ‐ pre‐ and postsurgical | Study population | RR 0.57 (0.34 to 0.95) | 37 (1 study) | ⊕⊕⊝⊝ Low¹, ² | ‐ | |
| 833 per 1000 | 475 per 1000 (283 to 792) | |||||
| Moderate | ||||||
| 833 per 1000 | 475 per 1000 (283 to 791) | |||||
| Adverse effects during use of the preventative intervention (fever) ‐ presurgical | Study population | RR 2.45 (1.26 to 4.78) | 32 (1 study) | ⊕⊕⊕⊝ Moderate² | ‐ | |
| 333 per 1000 | 817 per 1000 (420 to 1000) | |||||
| Moderate | ||||||
| 333 per 1000 | 816 per 1000 (420 to 1000) | |||||
| Adverse effects during use of the preventative intervention (fever) ‐ postsurgical | Study population | RR 1.96 (1 to 3.84) | 44 (1 study) | ⊕⊕⊕⊝ Moderate² | ‐ | |
| 333 per 1000 | 653 per 1000 (333 to 1000) | |||||
| Moderate | ||||||
| 333 per 1000 | 653 per 1000 (333 to 1000) | |||||
| Adverse effects during use of the preventative intervention (fever) ‐ pre‐ and postsurgical | Study population | RR 11.76 (0.71 to 195.11) | 38 (1 study) | ⊕⊕⊕⊝ Moderate² | ‐ | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded one level due to inconsistency: the effects of presurgical, postsurgical, and continuous pre‐ and postsurgical administration of interferon were inconsistent. ²Downgraded one level due to imprecision: the available evidence is from a single trial.
Summary of findings 15. Gamma globulin compared with histamine (control) for prevention of herpes labialis.
| Gamma globulin compared with histamine (control) for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: single centre Intervention: gamma globulin Comparison: histamine (control) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Histamine (control) | Gamma globulin | |||||
| Duration of attack of recurrent herpes labialis during use of the preventative intervention | ‐ | The mean duration of attack of recurrent herpes labialis during use of the preventative intervention in the intervention groups was 0.7 higher (0.55 lower to 1.95 higher) | ‐ | 72 (1 study) | ⊕⊕⊝⊝ Low¹ | ‐ |
| Severity of attack of recurrent herpes labialis during use of the preventative intervention (less severe recurrences than usual) | Study population | RR 0.97 (0.74 to 1.28) | 73 (1 study) | ⊕⊕⊝⊝ Low¹ | ‐ | |
| 750 per 1000 | 728 per 1000 (555 to 960) | |||||
| Moderate | ||||||
| 750 per 1000 | 728 per 1000 (555 to 960) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded two levels due to risk of bias and imprecision: the available evidence is from a single trial with a high risk of reporting bias.
Summary of findings 16. Thymopentin compared with placebo for prevention of herpes labialis.
| Thymopentin compared with placebo for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: medical centres Intervention: thymopentin Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Thymopentin | |||||
| Incidence of herpes labialis during use of the preventative intervention | 0.9 (range 0.1 to 2.0) | Median 0.2 (range 0.0 to 2.7) | ‐ | 36 (1 study) | ⊕⊕⊕⊝ Moderate¹ | P = 0.0027 using the Mann‐Whitney test by the trialists |
| Adverse effects during use of the preventative intervention | 111 per 1000 | 222 per 1000 (47 to 1000) | RR 2 (0.42 to 9.58) | 36 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded one level due to imprecision: the available evidence is from a single trial.
Summary of findings 17. HSV vaccination compared with placebo for prevention of herpes labialis.
| HSV vaccination compared with placebo for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: university hospitals Intervention: HSV vaccination Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | HSV vaccination | |||||
| Incidence of herpes labialis during use of the preventative intervention | 1.3 recurrences in a 4‐month period | 1.6 recurrences in a 4‐month period | P = 0.10 calculated by the trialists | 64 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ |
| Adverse effects during use of the preventative intervention | 13 adverse events per 100 injections | 22 adverse events per 100 injections | RR 0.33 (0.01 to 7.45) | 64 (1 study) | ⊕⊕⊕⊝ Moderate¹ | Several adverse events might have occurred in the same participant; no statistical tests were conducted by the trialists |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; HSV: herpes simplex virus; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded one level due to imprecision: the available evidence is from a single trial.
Summary of findings 18. Yellow fever vaccination compared with placebo for prevention of herpes labialis.
| Yellow fever vaccination compared with placebo for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: hospital Intervention: yellow fever vaccination Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Yellow fever vaccination | |||||
| Incidence of herpes labialis during use of the preventative intervention | See comment | See comment | ‐ | 1 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ |
| Adverse effects during use of the preventative intervention | 83 per 1000 | 28 per 1000 (1 to 621) | RR 0.33 (0.01 to 7.45) | 24 (1 study) | ⊕⊕⊕⊝ Moderate¹ | ‐ |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded one level due to imprecision: the available evidence is from a single trial.
Summary of findings 19. Laser compared with no interventions for prevention of herpes labialis.
| Laser compared with no interventions for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: university hospitals Intervention: laser Comparison: no interventions | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No interventions | Laser | |||||
| Incidence of herpes labialis during use of the preventative intervention | 0.116 recurrences per month | 0.076 recurrences per month | Not estimable | 71 (1 study) | ⊕⊝⊝⊝ Very low¹ | P = 0.076, calculated using the Mann‐Whitney U test by the trialists |
| Adverse effects during use of the preventative intervention | 0 | 0 | Not estimable | 119 (2 studies) | ⊕⊕⊝⊝ Low² | No adverse events were observed in either group |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded three levels due to imprecision: the evidence is from a single trial with a high risk of performance and detection biases. ²Downgraded two levels due to risk of bias and imprecision: the evidence is from two trials with a high risk of biases.
Summary of findings 20. Hypnotherapy compared with control for prevention of herpes labialis.
| Hypnotherapy compared with control for prevention of herpes labialis | ||||||
| Patient or population: participants with recurrent herpes labialis Settings: psychological institute Intervention: hypnotherapy Comparison: control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Hypnotherapy | |||||
| Incidence of herpes labialis during use of the preventative intervention (change in frequency of recurrence) | ‐ | The mean incidence of herpes labialis during use of the preventative intervention (change in frequency of recurrence) in the intervention groups was 6.5 lower (8.76 to 4.24 lower) | ‐ | 21 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ |
| Severity of attack of recurrent herpes labialis during use of the preventative intervention (change in intensity) | ‐ | The mean severity of attack of recurrent herpes labialis during use of the preventative intervention (change in intensity) in the intervention groups was 9.7 lower (12.46 to 6.94 lower) | ‐ | 21 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ |
| Change in severity (pain) of herpes labialis | ‐ | The mean change in severity (pain) of herpes labialis in the intervention groups was 2.2 lower (3.14 to 1.26 lower) | ‐ | 21 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ |
| Change in severity (impairment of appearance) of herpes labialis | ‐ | The mean change in severity (impairment of appearance) of herpes labialis in the intervention groups was 1.6 lower (2.5 to 0.7 lower) | ‐ | 21 (1 study) | ⊕⊝⊝⊝ Very low¹ | ‐ |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded three levels due to imprecision (the evidence is from a single trial) with a high risk of performance and detection biases.
Our prespecified outcomes were as follows:
Primary outcomes
Incidence of HSL during use of the preventative intervention. We accepted both researcher‐diagnosed and participant‐reported recurrences.
Adverse effects during use of the preventative intervention.
Secondary outcomes
Duration of attack of recurrent HSL during use of the preventative intervention.
Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention.
Viral load in saliva.
Rate of adherence to the regimen of the preventative intervention.
Incidence of HSL after use of the preventative intervention. We accepted both researcher‐diagnosed and participant‐reported recurrences.
Duration of attack of recurrent HSL after use of the preventative intervention.
Severity (lesion area, stage, pain) of attack of recurrent HSL after use of the preventative intervention.
Not all of the included studies addressed our prespecified outcomes. In which case, we indicated this at the bottom of the section for the specific comparison.
We only provided short‐term and long‐term subheadings when both short‐ and long‐term data were available. If only one kind of data were available, we described the length of trial in the text.
In general, the quality of the body of evidence is low to moderate, but very low for some outcomes of few interventions. We present the respective judgement of the quality of evidence for each intervention in the 'Summary of findings' tables.
Oral interventions
Oral aciclovir
Short‐term (≤ 1 month) use
A total of five trials tested the efficacy of short‐term use of oral aciclovir in preventing HSL (Raborn 1998; Schädelin 1988; Spruance 1988; Spruance 1991a; Spruance 1991b). Please see Table 1 where we judged the quality of the evidence for this comparison as low to moderate for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
One trial on aciclovir 800 mg twice daily beginning 12 to 24 hours before sun exposure and continuing for the entire sun‐exposure period (3 to 7 days), Raborn 1998, found no significant evidence for the prevention of HSL (risk ratio (RR) 1.08, 95% confidence interval (CI) 0.62 to 1.87; n = 237; see Analysis 1.1). Two trials tested the efficacy of 200 mg 5 times daily beginning immediately after, or 7 days before, ultraviolet radiation exposure and continuing for 7 days following the exposure (Spruance 1991a; Spruance 1991b). The trialists pooled the data from the two trials because of similar results. No significant effects in preventing HSL were found (RR 0.46, 95% CI 0.20 to 1.07; n = 66; see Analysis 1.1). However, aciclovir 400 mg twice daily (starting on the evening prior to surgery or 12 hours prior to the first anticipated sun exposure and continued for 5 to 7 days) significantly reduced the occurrence of HSL either by clinical evaluation (RR 0.26, 95% CI 0.13 to 0.51; n = 177; 2 trials (Schädelin 1988; Spruance 1988); see Analysis 1.1) or culture (RR 0.05, 95% CI 0.00 to 0.70; n = 30; 1 trial (Schädelin 1988); see Analysis 1.2).
1.1. Analysis.

Comparison 1 Oral aciclovir (short‐term) versus placebo, Outcome 1 Incidence of HSL during use of the preventative intervention (by clinical evaluation).
1.2. Analysis.

Comparison 1 Oral aciclovir (short‐term) versus placebo, Outcome 2 Incidence of HSL during use of the preventative intervention (by culture).
Primary outcome 2. Adverse effects during use of the preventative intervention
Three trials, Raborn 1998; Schädelin 1988; Spruance 1988, found no significant differences in adverse events between placebo and aciclovir 800 mg or 400 mg twice daily (aciclovir 800 mg twice daily: RR 0.98, 95% CI 0.70 to 1.38; n = 239; 1 trial; aciclovir 400 mg twice daily: RR 2.30, 95% CI 0.62 to 8.58; n = 183; 2 trials) (see Analysis 1.3).
1.3. Analysis.

Comparison 1 Oral aciclovir (short‐term) versus placebo, Outcome 3 Adverse effects during use of the preventative intervention.
Secondary outcome 2. Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention
The Raborn 1998 trial found a shorter length and width of the lesion in the placebo group when compared with the aciclovir 800 mg group (see Analysis 1.4), but found no differences in disease stage between the aciclovir 800 mg and placebo groups (see Analysis 1.5). The Spruance 1988 trial found no differences in lesional size and pain between the aciclovir 400 mg and placebo groups (see Analysis 1.4 and Analysis 1.6).
1.4. Analysis.

Comparison 1 Oral aciclovir (short‐term) versus placebo, Outcome 4 Severity (lesion size) of attack of herpes labialis during use of the preventative intervention.
1.5. Analysis.

Comparison 1 Oral aciclovir (short‐term) versus placebo, Outcome 5 Severity (stage) of attack of recurrent HSL during use of the preventative intervention.
1.6. Analysis.

Comparison 1 Oral aciclovir (short‐term) versus placebo, Outcome 6 Severity (pain) of attack of recurrent HSL during use of the preventative intervention.
Secondary outcome 5. Incidence of HSL after use of the preventative intervention
The Spruance 1988 trial followed up the participants for 4 weeks after treatment and found no significant difference in the recurrence of HSL after use of the preventative intervention (RR 1.23, 95% CI 0.49 to 3.14; n = 147; see Analysis 1.7).
1.7. Analysis.

Comparison 1 Oral aciclovir (short‐term) versus placebo, Outcome 7 Incidence of HSL after use of the preventative intervention.
There were no relevant data for this intervention for our other outcomes.
Long‐term (> 1 month) use
Only one cross‐over trial assessed the efficacy of 4‐month use of oral aciclovir in preventing HSL (Rooney 1993). Please see Table 2 where we judged the quality of the evidence for this comparison as low for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
Aciclovir therapy when compared with placebo resulted in a reduced mean of clinically documented recurrences (0.85 versus 1.80 episodes per participant per a 4‐month period, P = 0.009) and culture‐positive recurrence (0.40 versus 1.40 episodes per participant per a 4‐month period, P = 0.003).
When comparing with placebo, Rooney 1993 also found a longer time to first recurrence (which was not a prespecified outcome in this review) during aciclovir treatment (clinically determined recurrence: 46 versus 118 days, P = 0.05; culture‐positive recurrence: > 118 versus 46 days, P = 0.002).
Secondary outcome 1. Duration of attack of recurrent HSL during use of the preventative intervention
The Rooney 1993 trial did not prespecify an analysis on the duration of recurrent HSL but did a posthoc comparison and found a marginally shorter duration of recurrent HSL during aciclovir treatment when compared with placebo (difference in means (MD) ‐3.60, 95% CI ‐7.20 to 0; n = 40; see Analysis 2.1).
2.1. Analysis.

Comparison 2 Oral aciclovir (long‐term) versus placebo, Outcome 1 Duration of attack of herpes labialis during use of the preventative intervention.
Secondary outcome 4. Rate of adherence to the regimen of the preventative intervention
The rate of adherence to the preventative intervention was very high; the participants took 99% of the prescribed study medication during both aciclovir and placebo treatments.
There were no relevant data for this intervention for our other outcomes.
Valaciclovir
Short‐term (≤ 1 month) use
Only one trial, Miller 2004, investigated the effects of a two‐day valaciclovir treatment (on the day of dental procedure and the following day) in preventing recurrence of HSL during a one‐week observation period. Please see Table 3 where we judged the quality of the evidence for this comparison as moderate for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
There was no reduction in the recurrence of HSL either by clinical evaluation (RR 0.55, 95% CI 0.23 to 1.28; n = 125; see Analysis 3.1) or culture confirmation (RR 0.47, 95% CI 0.21 to 1.08; n = 125; see Analysis 3.2).
3.1. Analysis.

Comparison 3 Valaciclovir (short‐term) versus placebo, Outcome 1 Incidence of herpes labialis during use of the preventative intervention (by clinical evaluation).
3.2. Analysis.

Comparison 3 Valaciclovir (short‐term) versus placebo, Outcome 2 Incidence of herpes labialis during use of the preventative intervention (by culture).
Primary outcome 2. Adverse effects during use of the preventative intervention
There were no significant differences in adverse events found between the valaciclovir and placebo groups (RR 1.33, 95% CI 0.71 to 2.50; n = 125; see Analysis 3.3).
3.3. Analysis.

Comparison 3 Valaciclovir (short‐term) versus placebo, Outcome 3 Adverse effects during use of the preventative intervention.
Secondary outcome 1. Duration of attack of recurrent HSL during use of the preventative intervention
The Miller 2004 trial found that valaciclovir treatment was associated with a significantly shorter time to cessation of pain in comparison with placebo (3.2 versus 6.2 days; P = 0.006; n = 125).
Secondary outcome 2. Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention
There were no significant differences in the clinical severity (1.7 versus 1.9; P = non‐significant; n = 125) between the valaciclovir and placebo groups.
Secondary outcome 3. Viral load in saliva
There were no significant differences in the viral load, i.e., HSV‐1 shedding in the saliva (RR 0.16, 95% CI 0.02 to 1.26; n = 120; see Analysis 3.4), between the valaciclovir and placebo groups.
3.4. Analysis.

Comparison 3 Valaciclovir (short‐term) versus placebo, Outcome 4 Viral load (shedding) in saliva.
There were no relevant data for this intervention for our other outcomes.
Long‐term (> 1 month) use
Please see Table 4 where we judged the quality of the evidence for this comparison as moderate for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
Only 1 placebo‐controlled trial, Baker 2003, assessed the effects of valaciclovir 500 mg once daily for 16 weeks in preventing HSL and found a significantly lower incidence of HSL in the valaciclovir group (0.12 versus 0.21 episodes per participant per month; P = 0.042; n = 95).
Primary outcome 2. Adverse effects during use of the preventative intervention
No differences in adverse events existed between the 2 groups (RR 0.86, 95% CI 0.51 to 1.46; n = 95; see Analysis 4.1).
4.1. Analysis.
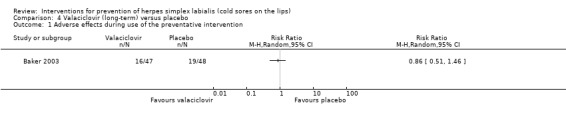
Comparison 4 Valaciclovir (long‐term) versus placebo, Outcome 1 Adverse effects during use of the preventative intervention.
There were no relevant data for this comparison for our secondary outcomes.
Suppressive regimen versus episodic regimen
A cross‐over trial, Gilbert 2007, compared an 'episodic regimen' (two 2 gm doses of valaciclovir separated by 12 hours at the first sign of prodrome) and 'suppressive regimen' (valaciclovir 1 gm once daily) for 6 months, respectively. Please see Table 5 where we judged the quality of the evidence for this comparison as very low for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
Compared with the episodic regimen, the suppressive regimen had a significantly lower incidence of HSL (MD ‐0.10 episodes per participant per month, 95% CI ‐0.16 to ‐0.05; n = 120; see Analysis 5.1).
5.1. Analysis.

Comparison 5 Valaciclovir (suppressive regimen versus episodic regimen), Outcome 1 Incidence of herpes labialis during use of the preventative intervention (number of recurrences per participant per month).
Primary outcome 2. Adverse effects during use of the preventative intervention
There were no significant differences in adverse events between the 2 regimens (RR 1.21, 95% CI 0.78 to 1.87; n = 152; see Analysis 5.2).
5.2. Analysis.

Comparison 5 Valaciclovir (suppressive regimen versus episodic regimen), Outcome 2 Adverse effects during use of the preventative intervention.
Secondary outcome 1. Duration of attack of recurrent HSL during use of the preventative intervention
There were no significant differences in the duration of attack (MD ‐1.08, 95% CI ‐2.16 to 0.00; n = 120; see Analysis 5.3) between the 2 regimens.
5.3. Analysis.

Comparison 5 Valaciclovir (suppressive regimen versus episodic regimen), Outcome 3 Duration of attack of recurrent HSL during use of the preventative intervention.
Secondary outcome 2. Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention
There were no significant differences in the pain (MD ‐0.09, 95% CI ‐0.20 to 0.02; n = 120; see Analysis 5.4) and maximal total lesion area (MD ‐5.38, 95% CI ‐10.91 to 0.15; n = 120; see Analysis 5.5) between the 2 regimens.
5.4. Analysis.

Comparison 5 Valaciclovir (suppressive regimen versus episodic regimen), Outcome 4 Severity (pain) of attack of recurrent HSL during use of the preventative intervention.
5.5. Analysis.

Comparison 5 Valaciclovir (suppressive regimen versus episodic regimen), Outcome 5 Severity (maximum total lesion area) of attack of recurrent HSL during use of the preventative intervention.
There were no relevant data for this intervention for our other outcomes.
Famciclovir
A placebo‐controlled trial, Spruance 1999, assessed the effects of various dosages of famciclovir (125 mg, 250 mg, and 500 mg) 3 times daily for 5 days, beginning 48 hours after ultraviolet radiation exposure in preventing HSL. Please see Table 6 where we judged the quality of the evidence for this comparison as moderate for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
The Spruance 1999 trial found no differences in recurrence of HSL between 3 different doses of famciclovir and placebo (n = 243; see Analysis 6.1).
6.1. Analysis.

Comparison 6 Famciclovir versus placebo, Outcome 1 Incidence of HSL during use of the preventative intervention (by clinical evaluation).
Primary outcome 2. Adverse effects during use of the preventative intervention
No significant differences in adverse events were found between three different doses of famciclovir and placebo. (The trialists did not provide exact numerical data.)
Secondary outcome 1. Duration of attack of recurrent HSL during use of the preventative intervention
The difference in time to healing compared with the placebo group was significantly shorter in the famciclovir 500 mg group (by 2.8 days: hazard ratio (HR) 2.39; 95% CI 1.23 to 4.63; P = 0.010), but not for the other 2 groups (n = 243; see Analysis 6.2).
6.2. Analysis.

Comparison 6 Famciclovir versus placebo, Outcome 2 Duration of attack of recurrent HSL during use of the preventative intervention.
Secondary outcome 2. Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention
There were no differences in pain between the 3 famciclovir groups and the placebo groups (RR 1.0, 95% CI 0.90 to 1.16; RR 0.92, 95% CI 0.76 to 1.12; and RR 0.90, 95% CI 0.75 to 1.09 for the famciclovir 125 mg, 250 mg, and 500 mg groups, respectively, when compared with the placebo group; n = 102; see Analysis 6.3).
6.3. Analysis.

Comparison 6 Famciclovir versus placebo, Outcome 3 Severity (pain) of attack of recurrent HSL during use of the preventative intervention.
Secondary outcome 4. Rate of adherence to the regimen of the preventative intervention
The rate of adherence was very high: 100% of the participants in all 3 famciclovir groups (n = 183) and 95% of those in the placebo group (n = 60) took all of the prescribed study medication.
There were no relevant data for this intervention for our other outcomes.
Levamisole
Only 1 trial with a high withdrawal rate (27.2%), Russell 1978, evaluated the effects of levamisole in preventing HSL. Please see Table 7 where we judged the quality of the evidence for this comparison as very low for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
Among the 72 participants who completed the trial, both the levamisole group and placebo group showed a reduction in the frequency of HSL, but there were no significant differences between the 2 groups (2.1 ± 1.2 versus 2.7 ± 2.3 episodes during a 6‐month period). When taking into account the different baseline frequency of HSL (4.8 ± 2.7 and 3.4 ± 1.8 episodes during a 6‐month period for the levamisole and placebo group, respectively), levamisole was associated with a greater reduction in the frequency of HSL (MD ‐2.00, 95% CI ‐2.24 to ‐1.76; n = 72; see Analysis 7.1).
7.1. Analysis.

Comparison 7 Levamisole versus placebo, Outcome 1 Incidence of HSL during use of the preventative intervention.
Primary outcome 2. Adverse effects during use of the preventative intervention
Of the 99 randomised participants, 27 (27.2%) did not complete the trial because of either adverse events (such as nausea and fever) or lack of efficacy: the trialists' analysis excluded 19 (39.6%) in the levamisole group and 8 (15.7%) in the placebo group. The levamisole group had a significantly higher withdrawal rate than the placebo group (risk difference (RD) 0.24, 95% CI 0.07 to 0.41; n = 99; see Analysis 7.2).
7.2. Analysis.

Comparison 7 Levamisole versus placebo, Outcome 2 Adverse effects during use of the preventative intervention (leading to withdrawal).
Secondary outcome 1. Duration of attack of recurrent HSL during use of the preventative intervention
Compared with the placebo group, the levamisole group was associated with a lesser reduction in the duration of attack of HSL (MD 0.70, 95% CI 0.22 to 1.18; n = 72; see Analysis 7.3).
7.3. Analysis.

Comparison 7 Levamisole versus placebo, Outcome 3 Duration of attack of recurrent HSL during use of the preventative intervention.
There were no relevant data for this intervention for our other outcomes.
Lysine
A placebo‐controlled cross‐over trial, Thein 1984, investigated the effects of L‐lysine monolysine monohydrochloride 1000 mg per day for 6 months in preventing recurrent HSL. Please see Table 8 where we judged the quality of the evidence for this comparison as very low for the following outcome.
Primary outcomes 1. Incidence of HSL during use of the preventative intervention
Because the Thein 1984 trial lacked a washout period, we used only the data from the first period before cross‐over for analysis and found no significant difference in the incidence of recurrent HSL between lysine and placebo treatment (MD ‐0.04, 95% CI ‐0.37 to 0.29; n = 26; see Analysis 8.1).
8.1. Analysis.

Comparison 8 Lysine versus placebo, Outcome 1 Incidence of HSL during use of the preventative intervention (number of recurrences per participant per month).
There were no relevant data for this intervention for our other outcomes.
LongoVital®
A placebo‐controlled trial, Pedersen 2001, evaluated the effects of daily intake of LongoVital® (a vitamin and herbs supplement) in preventing recurrence of HSL. The treatment period was four months, and the participants were followed up for another four months after stopping the study medications.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
During the treatment period, there were no significant differences in the number of recurrent HSL episodes found between the LongoVital® (LV) and placebo groups (the median being 1.2 and 1.6 during the period 'days 0 to 60' and 0.7 and 1.0 during the period 'days 61 to 120' for the LV and placebo groups, respectively; n = 52).
Secondary outcome 1. Duration of attack of recurrent HSL during use of the preventative intervention
There were no significant differences in the median duration of recurrent HSL episodes between the LongoVital® and placebo groups (the median being 5.0 days and 4.3 days during the period 'days 0 to 60' and 3.0 days and 4.2 days during the period 'days 61 to 120', respectively; n = 52).
Secondary outcome 2. Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention
The maximal size of recurrent HSL lesions did not significantly differ between the LongoVital® and placebo groups (the median being 5.1 mm and 5.0 mm during the period 'days 0 to 60' and 2.5 mm and 5.2 mm during the period 'days 61 to 120', respectively; n = 52).
Secondary outcome 5. Incidence of HSL after use of the preventative intervention
During the post‐treatment follow‐up period, there were no significant differences in the number of recurrent HSL episodes between the LongoVital® and placebo groups (the median being 1.1 and 1.4 during the period 'days 121 to 180' and 0.9 and 0.8 during the period 'days 181 to 240', respectively; n = 52).
Secondary outcome 6. Duration of attack of recurrent HSL after use of the preventative intervention
During the post‐treatment follow‐up period, there were no significant differences in the median duration of recurrent HSL episodes between the LongoVital® and placebo groups (the median being 4.0 and 4.0 days during the period 'days 121 to 180' and 6.3 and 4.0 days during the period 'days 181 to 240', respectively; n = not reported).
Secondary outcome 7. Severity (lesion area, stage, pain) of attack of recurrent HSL after use of the preventative intervention
During the post‐treatment follow‐up period, there were no significant differences in the maximal size of recurrent HSL lesions between the LongoVital® and placebo groups (median = 2.9 and 5.0 mm during the period 'days 121 to 180' and 4.3 and 2.0 mm during the period 'days 181 to 240', respectively; n = not reported).
There were no relevant data for this intervention for our other outcomes.
Topical interventions
Topical aciclovir
Short‐term (≤ 1 month) use
Two trials assessed the effects of short‐term use of topical aciclovir 5% cream in preventing recurrence of HSL induced by sunlight or ultraviolet light (UVL) (Raborn 1997; Spruance 1991c). The Raborn 1997 trial assessed the effects of short‐term use of topical aciclovir 5% cream starting 12 hours before sunlight exposure and continuing for 72 to 168 hours in preventing recurrence of HSL. The Spruance 1991c trial assessed the effects of short‐term use of topical aciclovir 5% cream, beginning 5 minutes following experimental UVL exposure for 7 days. Please see Table 9 where we judged the quality of the evidence for this comparison as low to moderate for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
Neither of the 2 placebo‐controlled trials found significant differences in the recurrence of HSL between the aciclovir and placebo groups nor did the meta‐analysis of the 2 trials (pooled RR 0.91, 95% CI 0.48 to 1.72; n = 271; I² statistic = 66%; 2 trials; see Analysis 9.1).
9.1. Analysis.

Comparison 9 Topical aciclovir (short‐term) versus placebo, Outcome 1 Incidence of HSL during use of the preventative intervention.
Primary outcome 2. Adverse effects during use of the preventative intervention
Only the Raborn 1997 trial assessed the adverse events and found no differences between the 2 groups (RR 1.17, 95% CI 0.59 to 2.32; n = 191; see Analysis 9.2).
9.2. Analysis.

Comparison 9 Topical aciclovir (short‐term) versus placebo, Outcome 2 Adverse effects during use of the preventative intervention.
Secondary outcome 1. Duration of attack of recurrent HSL during use of the preventative intervention
Only the Spruance 1991c trial assessed this outcome and found no differences in the mean healing time to normal skin (6.8 days versus 7.4 days; P = 0.70; n = 52).
Secondary outcome 2. Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention
Only the Spruance 1991c trial assessed the severity of recurrent HSL and found no differences in aborted lesions (RR 1.02, 95% CI 0.19 to 5.57; n = 52; see Analysis 9.3), mean maximal lesion area (110 mm² versus 72 mm²; P = 0.88; n = 52), and mean duration of pain (3.7 days versus 3.6 days; P > 0.10; n = 52).
9.3. Analysis.

Comparison 9 Topical aciclovir (short‐term) versus placebo, Outcome 3 Severity (aborted lesions) of attack of recurrent HSL during use of the preventative intervention.
Secondary outcome 5. Incidence of HSL after use of the preventative intervention
The Raborn 1997 trial also assessed the recurrences of HSL in a 4‐day post‐treatment follow‐up period and found fewer recurrences of HSL in the aciclovir group (RR 0.35, 95% CI 0.13 to 0.94; n = 181; Analysis 9.4).
9.4. Analysis.

Comparison 9 Topical aciclovir (short‐term) versus placebo, Outcome 4 Incidence of HSL after use of the preventative intervention.
There were no relevant data for this intervention for our other outcomes.
Topical aciclovir 5% plus 348U87 3%
Short‐term (≤ 1 month) use
A placebo‐controlled trial evaluated the effects of short‐term use of topical aciclovir 5% plus 348U87 3% (a ribonucleotide reductase inhibitor) cream, starting immediately after UVL exposure and continuing for 7 days (Bernstein 1994). Please see Table 10 where we judged the quality of the evidence for this comparison as very low for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
There were no significant differences in the development of HSV(+) lesions (RR 0.78, 95% CI 0.19 to 3.14; n = 51; Analysis 10.1) and development of lesions consistent with HSL (RR 1.46, 95% CI 0.53 to 3.99; n = 51; see Analysis 10.2) between the 2 groups.
10.1. Analysis.

Comparison 10 Topical aciclovir and 348U87 cream (short‐term) versus placebo, Outcome 1 Incidence of HSL during use of the preventative intervention (by culture).
10.2. Analysis.

Comparison 10 Topical aciclovir and 348U87 cream (short‐term) versus placebo, Outcome 2 Incidence of HSL during use of the preventative intervention (by clinical evaluation).
Secondary outcome 1. Duration of attack of recurrent HSL during use of the preventative intervention
There were no significant differences in the healing time (MD 2.50 days, 95% CI ‐1.39 to 6.39; n = 9; see Analysis 10.3) between the 2 groups.
10.3. Analysis.

Comparison 10 Topical aciclovir and 348U87 cream (short‐term) versus placebo, Outcome 3 Duration of attack of recurrent HSL during use of the preventative intervention.
Secondary outcome 2. Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention
There were no significant differences in the maximal lesion size (MD 73.00 cm², 95% CI ‐42.22 to 188.22; n = 9; see Analysis 10.4) between the 2 groups.
10.4. Analysis.

Comparison 10 Topical aciclovir and 348U87 cream (short‐term) versus placebo, Outcome 4 Severity of attack of recurrent HSL during use of the preventative intervention (maximum lesion area).
There were no relevant data for the comparison of these interventions for our other outcomes.
Long‐term (> 1 month) use
A placebo‐controlled cross‐over trial, Gibson 1986, evaluated the efficacy of aciclovir cream applied to all previously affected areas 4 times per day for 16 weeks.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
The trial found significantly fewer research‐diagnosed recurrences of HSL during a 16‐week period when on aciclovir cream treatment than on placebo (the mean being 0.5 and 1.1, respectively; standard deviation (SD) not reported; P < 0.05 calculated by trialists; n = 23). However, no significant differences existed in the mean number of participant‐reported recurrences between aciclovir cream treatment and placebo (the mean being 1.6 and 2.4, respectively; SD not reported; P ≥ 0.05 calculated by trialists; n = 23).
Primary outcome 2. Adverse effects during use of the preventative intervention
There were no significant adverse events while on either aciclovir cream or placebo (n = 23).
Secondary outcome 1. Duration of attack of recurrent HSL during use of the preventative intervention
The trial found significantly fewer mean days with HSL present when on aciclovir cream treatment than on placebo (the mean being 9.5 and 12.4 days, respectively; SD not reported; P < 0.01 calculated by trialists). Also, the trial found significantly fewer mean days with any symptom or sign of HSL present when on aciclovir cream treatment than on placebo (the mean being 12.2 and 17.4 days, respectively; SD not reported; P < 0.001 calculated by trialists).
There were no relevant data for this intervention for our other outcomes.
Foscarnet
A placebo‐controlled trial, Bernstein 1997, examined the effects of topical application of foscarnet 3% cream 8 times daily (at least every 2 hours while awake) for 7 days in preventing experimental UVL‐induced HSL. Please see Table 11 where we judged the quality of the evidence for this comparison as moderate for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
There were no significant differences in the researcher‐diagnosed recurrence of HSL (RR 1.08, 95% CI 0.82 to 1.40; n = 295; see Analysis 11.1) between the foscarnet and placebo groups.
11.1. Analysis.

Comparison 11 Topical foscarnet versus placebo, Outcome 1 Incidence of HSL during use of the preventative intervention.
Primary outcome 2. Adverse effects during use of the preventative intervention
No significant differences were found in adverse events either leading to withdrawals (RR 2.96, 95% CI 0.12 to 72.11; n = 302; see Analysis 11.2) or application site reactions (RR 2.47, 95% CI 0.79 to 7.69; n = 302; see Analysis 11.3) between the foscarnet and placebo groups.
11.2. Analysis.

Comparison 11 Topical foscarnet versus placebo, Outcome 2 Adverse effects during use of the preventative intervention (leading to discontinuation).
11.3. Analysis.

Comparison 11 Topical foscarnet versus placebo, Outcome 3 Adverse effects during use of the preventative intervention (application site reactions).
Secondary outcome 1. Duration of attack of recurrent HSL during use of the preventative intervention
The healing time did not significantly differ between the foscarnet and placebo groups (MD ‐0.21 days, 95% CI ‐1.68 to 1.26; n = 125; see Analysis 11.4).
11.4. Analysis.

Comparison 11 Topical foscarnet versus placebo, Outcome 4 Duration of attack of recurrent HSL during use of the preventative intervention (healing time).
Secondary outcome 2. Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention
There were no significant differences in the mean lesion area (MD ‐16.00, 95% CI ‐38.96 to 6.96; n = 124; see Analysis 11.5), maximum lesion area (MD ‐30.00, 95% CI ‐72.64 to 12.64; n = 124; see Analysis 11.6), and duration of pain (MD 0.10, 95% CI ‐1.11 to 1.31; n = 113; see Analysis 11.7) between the foscarnet and placebo groups.
11.5. Analysis.

Comparison 11 Topical foscarnet versus placebo, Outcome 5 Severity of attack of recurrent HSL during use of the preventative intervention (mean lesion area).
11.6. Analysis.

Comparison 11 Topical foscarnet versus placebo, Outcome 6 Severity of attack of recurrent HSL during use of the preventative intervention (maximum lesion area).
11.7. Analysis.

Comparison 11 Topical foscarnet versus placebo, Outcome 7 Severity of attack of recurrent HSL during use of the preventative intervention (duration of pain).
There were no relevant data for this intervention for our other outcomes.
1,5‐pentanediol
A placebo‐controlled trial evaluated the effects of twice daily application of topical 1,5‐pentanediol (PD) gel for 26 weeks in preventing HSL (Busch 2009). Please see Table 12 where we judged the quality of the evidence for this comparison as moderate to low for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
The trial found no significant differences in the number of recurrences between the PD and placebo groups (109 episodes out of 50 participants versus 120 episodes out of 52 participants; P > 0.05 calculated using the Mann‐Whitney test by trialists; n = 102).
Primary outcome 2. Adverse effects during use of the preventative intervention
No adverse events leading to discontinuation were observed in either group (n = 102).
Secondary outcome 2. Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention
There were no significant differences in the severity of attack of recurrent HSL between the 2 groups (RR 1.05, 95% CI 0.91 to 1.20; episodes = 224; see Analysis 12.1).
12.1. Analysis.

Comparison 12 Topical 1,5‐pentanediol versus placebo, Outcome 1 Severity (blistering, swelling, or pain) of recurrence.
There were no relevant data for this intervention for our other outcomes.
2‐hydroxypropyl‐β‐cyclo dextrin
A placebo‐controlled trial, Senti 2013, examined the effects of twice daily application of topical 2‐hydroxypropyl‐β‐cyclo dextrin (2‐HPβCD) 20% gel for 6 months in preventing HSL.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
The trialists did not provide the exact numerical data on recurrences but presented them in plots. The 2‐HPβCD group had significantly more recurrences than the placebo group (P = 0.003 calculated using the Mann‐Whitney test by the trialists; n = 33). Both groups had significantly fewer recurrences during than before the study (P < 0.001 calculated by the trialists; n = 33).
Secondary outcome 1. Duration of attack of recurrent HSL during use of the preventative intervention
There were no differences in the duration of the relapses between the 2‐HPβCD and placebo groups.
Secondary outcome 2. Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention
There were no differences in the maximal size of the relapses between the 2‐HPβCD and placebo groups. Although the 2‐HPβCD group experienced less pain than the placebo group, the cumulative burden of pain assessed using the area‐under‐curve (AUC) of the daily pain visual analogue scale level was not significantly different between the 2 groups (P = 0.101). However, the symptoms were more severe in the placebo than in the 2‐HPβCD group: the symptom scores were significantly higher in the former group for tingling (P = 0.040), burning (P = 0.028), and total symptoms (P = 0.048), but not for tension (P = 0.156), hypersensitivity (P = 0.119), and itching (P = 0.283).
There were no relevant data for this intervention for our other outcomes.
Sunscreen
A total of three placebo‐controlled trials assessed the efficacy of sunscreen in preventing HSL, with one parallel trial using solar radiation, Mills 1987, and two cross‐over trials using experimental UVL (Duteil 1998; Rooney 1991). Please see Table 13 where we judged the quality of the evidence for this comparison as low to very low for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
As shown in Analysis 13.1, application of sunscreen did not reduce the recurrences of HSL induced by sunlight (RR 1.13, 95% CI 0.25 to 5.06; n = 51; 1 trial), but significantly reduced the clinically diagnosed recurrences induced by experimental UVL (pooled RR 0.07, 95% CI 0.01 to 0.33; n = 111; I² statistic = 0%; 2 trials; number needed to treat to benefit (NNTB) = 3; 95% CI 2 to 4). The Rooney 1991 trial found sunscreen use significantly reduced virologically confirmed recurrences of HSL (RR 0.04, 95% CI 0.01 to 0.30; n = 73; 1 trial; NNTB = 2; 95% CI 2 to 3; see Analysis 13.2).
13.1. Analysis.

Comparison 13 Sunscreen versus placebo, Outcome 1 Incidence of HSL during use of the preventative intervention (by clinical evaluation).
13.2. Analysis.

Comparison 13 Sunscreen versus placebo, Outcome 2 Incidence of HSL during use of the preventative intervention (by culture).
There were no relevant data for this intervention for our other outcomes.
Interventions given by injection
Three immunomodulating treatments were given by injection (interferon (Ho 1984; Pazin 1979), intradermal gamma globulin (Redman 1986), and thymopentin (Bolla 1985)).
Interferon
A placebo‐controlled trial, Ho 1984, investigated whether either presurgical or postsurgical intramuscular administration of interferon (3 and 7 doses of 3.5 x 10⁴ units/kg of body weight, respectively) could reduce recurrences of HSL in participants receiving microvascular decompression for trigeminal neuralgia. Another placebo‐controlled trial, Pazin 1979, evaluated the effects of interferon administered intramuscularly for 5 days (10 doses of 3.5 x 10⁴ units/kg of body weight), beginning on the day before receiving the same surgical procedure. Please see Table 14 where we judged the quality of the evidence for this comparison as very low to moderate for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
When assessing recurrences of HSL defined by the presence of clinical lesions, isolation of virus, or both (Analysis 14.1), the presurgical group was associated with a significant increase in recurrences (RR 1.59, 95% CI 1.05 to 2.41; n = 32), but no significant differences were found between the postsurgical and placebo groups (RR 0.99, 95% CI 0.59 to 1.66; n = 44). On the other hand, continuous pre‐ and postsurgical administration of interferon was associated with a significant decrease in the recurrences of HSL (RR 0.57, 95% CI 0.34 to 0.95; n = 37).
14.1. Analysis.

Comparison 14 Interferon versus placebo, Outcome 1 Incidence of HSL during use of the preventative intervention.
Primary outcome 2. Adverse effects during use of the preventative intervention
A significant increase in adverse events presenting as fever was found across the 3 interferon groups when compared with placebo (pooled RR 2.30, 95% CI 1.44 to 3.67; I² statistic = 0%; n = 114; 3 trials; see Analysis 14.2). One trial, Pazin 1979, found no significant differences in other adverse events including pain and tenderness at injection site (RR 0.95, 95% CI 0.06 to 14.04), malaise, nausea, or vomiting (RR 1.74, 95% CI 0.81 to 3.70) between the interferon and placebo groups (n = 37; see Analysis 14.3).
14.2. Analysis.

Comparison 14 Interferon versus placebo, Outcome 2 Adverse effects during use of the preventative intervention (fever).
14.3. Analysis.

Comparison 14 Interferon versus placebo, Outcome 3 Adverse effects during use of the preventative intervention (other).
Secondary outcome 2. Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention
In the Ho 1984 trial, the mean lesion area was 26, 135, and 30 mm² for the presurgical, postsurgical, and placebo groups, respectively (the trials did not report the SDs but stated no differences between them). The Pazin 1979 trial found no significant difference in the mean lesion area between the interferon and placebo groups (0.7 and 4.0 cm², respectively; SD not reported; P > 0.05 calculated by trialists).
There were no relevant data for this intervention for our other outcomes.
Gamma globulin
The Redman 1986 trial assessed the efficacy of intradermal administration of gamma globulin in preventing recurrence of HSL in a six‐month follow‐up period. Please see Table 15 where we judged the quality of the evidence for this comparison as low for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
The gamma globulin and control groups did not significantly differ in the mean number of herpes lesions (2.65 and 2.76 days, respectively; SD not reported; no significant differences calculated by the trialists; n = 84).
Secondary outcome 1. Duration of attack of recurrent HSL during use of the preventative intervention
The gamma globulin and control groups did not significantly differ in the mean number of days to vesicle healing (MD 0.70 days, 95% CI ‐0.55 to 1.95; n = 72; see Analysis 15.1).
15.1. Analysis.

Comparison 15 Gamma globulin versus histamine (control), Outcome 1 Duration of attack of recurrent HSL during use of the preventative intervention.
Secondary outcome 2. Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention
The gamma globulin and control groups did not significantly differ when 'less severe recurrences than usual' were measured (RR 0.97, 95% CI 0.74 to 1.28; n = 73; see Analysis 15.2).
15.2. Analysis.

Comparison 15 Gamma globulin versus histamine (control), Outcome 2 Severity of attack of recurrent HSL during use of the preventative intervention (less severe recurrences than usual).
There were no relevant data for this intervention for our other outcomes.
Thymopentin
A placebo‐controlled trial, Bolla 1985, evaluated the effects of 6 weeks of treatment with subcutaneous administration of thymopentin in preventing recurrence of HSL in a 18‐week follow‐up period. Please see Table 16 where we judged the quality of the evidence for this comparison as moderate for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
During the follow‐up period, the incidence of recurrent HSL was lower in the thymopentin group than the placebo group (median = 0.2 (range = 0.0 to 2.7) and 0.9 (range = 0.1 to 2.0) relapses/month, respectively; P = 0.0027 using the Mann‐Whitney test by trialists; n = 36).
Primary outcome 2. Adverse effects during use of the preventative intervention
The 2 groups did not significantly differ in adverse events (RR 2.00, 95% CI 0.42 to 9.58; n = 36; see Analysis 16.1).
16.1. Analysis.

Comparison 16 Thymopentin versus placebo, Outcome 1 Adverse effects during use of the preventative intervention.
There were no relevant data for this intervention for our secondary outcomes.
Interventions given by vaccination
HSV vaccine
A placebo‐controlled trial, Altmeyer 1991, tested the efficacy of a HSV type I subunit vaccine in preventing recurrences of HSL. Please see Table 17 where we judged the quality of the evidence for this comparison as moderate for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
The vaccine and placebo groups did not differ in the mean number of recurrences (1.6 versus 1.3 recurrences in a 4‐month period; P = 0.10 calculated by trialists; n = 58). Both groups had a significantly fewer number of recurrences when compared with baseline (vaccine group: from 2.2 to 1.6, P < 0.01 calculated by trialists; placebo group: from 2.6 to 1.3, P < 0.001 calculated by the trialists; n = 58).
Primary outcome 2. Adverse effects during use of the preventative intervention
The vaccine and placebo groups had 22 and 13 adverse events per 100 injections. (Several adverse events might have occurred in the same participant; the trialists conducted no statistical tests.)
There were no relevant data for this intervention for our secondary outcomes.
Yellow fever vaccination
A placebo‐controlled trial, Møller 1997, examined the efficacy of yellow fever vaccination in preventing recurrences of HSL in a 12‐month follow‐up period. Please see Table 18 where we judged the quality of the evidence for this comparison as moderate for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
The vaccine and placebo groups did not significantly differ in the mean number of recurrences (5 and 7, respectively; SD and P values not reported; n = 24) and the median number of recurrences (both being 5.5; P values not reported; n = 24).
Primary outcome 2. Adverse effects during use of the preventative intervention
The vaccine and placebo groups did not differ significantly in the number of participants with adverse events (RR 0.33, 95% CI 0.01 to 7.45; n = 24; see Analysis 17.1).
17.1. Analysis.

Comparison 17 Yellow fever vaccination versus placebo, Outcome 1 Adverse effects during use of the preventative intervention.
There were no relevant data for this intervention for our secondary outcomes.
Laser
Please see Table 19 where we judged the quality of the evidence for these comparisons as low to very low for the following outcomes.
Low‐energy gallium‐aluminium‐arsenide laser
The de Carvalho 2010 trial evaluated the efficacy of a 10‐week low‐energy gallium‐aluminium‐arsenide laser phototherapy (3 to 4.5 J/cm²) in preventing recurrence of HSL during a 16‐month follow‐up period.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
The number of recurrences per month did not differ significantly between the laser and control groups (0.076 and 0.116, respectively; P = 0.076 calculated using the Mann‐Whitney U test by the trialists; n = 71).
Primary outcome 2. Adverse effects during use of the preventative intervention
No adverse events were observed in either group (n = 71).
Secondary outcome 2. Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention
The monthly average lesion size was significantly smaller in the laser group than in the control group (0.122 and 0.223 mm, respectively; P = 0.013 calculated using the Mann‐Whitney U test by the trialists; n = 71). The inflammatory oedema was significantly less in the laser group than in the control group (the monthly mean being 0.015 and 0.00196, respectively; P = 0.031 calculated using the Mann‐Whitney U test by the trialists; n = 71). There were no significant differences in the pain levels between the 2 groups (the monthly mean being 0.113 and 0.184; P = 0.051 calculated using the Mann‐Whitney U test by the trialists; n = 71).
There were no relevant data for this intervention for our other outcomes.
Low‐intensity diode laser therapy
The Schindl 1999 trial tested the effects of a 2‐week low‐intensity diode laser therapy (48 J/cm²) in preventing recurrence of HSL during a 52‐week follow‐up period. A significantly longer median recurrence‐free interval was found in the laser group (37.5 weeks; range = 2 to 52 weeks) than in the control group (3 weeks; range = 1 to 20 weeks) (P < 0.0001 calculated using the Wilcoxon rank‐sum test by trialists; MD 30.00, 95% CI 21.42 to 38.58; n = 48; see Analysis 18.1), although this measure was not a prespecified outcome in our protocol.
18.1. Analysis.

Comparison 18 Laser versus no interventions, Outcome 1 Time to first recurrence.
Primary outcome 2. Adverse effects during use of the preventative intervention
No adverse events were observed in either group (n = 48).
There were no relevant data for this intervention for our other outcomes.
Hypnotherapy
The Pfitzer 2005 trial assessed the efficacy of five weekly hypnotherapy sessions in preventing recurrence of HSL during a follow‐up period of six months in comparison with no hypnotherapy (control). Please see Table 20 where we judged the quality of the evidence for this comparison as very low for the following outcomes.
Primary outcome 1. Incidence of HSL during use of the preventative intervention
The frequency of recurrences significantly decreased in the hypnotherapy group (from 10.4 ± 7.6 to 5.2 ± 3.3; MD ‐5.20, 95% CI ‐10.34 to ‐0.06), but did not change in the control group (from 7.2 ± 5.7 to 8.5 ± 6.8; MD 1.30, 95% CI ‐3.94 to 6.54) (mean change in frequency of recurrences: MD ‐6.50, 95% CI ‐8.76 to ‐4.24; n = 21; see Analysis 19.1).
19.1. Analysis.

Comparison 19 Hypnotherapy versus control, Outcome 1 Incidence of HSL during use of the preventative intervention (change in frequency of recurrence).
Secondary outcome 2. Severity (lesion area, stage, pain) of attack of recurrent HSL during use of the preventative intervention
The intensity of symptoms significantly diminished in the hypnotherapy group (from 26.0 ± 10.3 to 15.0 ± 7.0; MD ‐11.00, 95% CI ‐18.72 to ‐3.28), while that of the control group did not change significantly (from 24.4 ± 6.1 to 23.1 ± 3.8; MD ‐1.30, 95% CI ‐5.55 to 2.95) (mean change in the intensity of symptoms: MD ‐9.70, 95% CI ‐12.46 to ‐6.94; n = 21; see Analysis 19.2). The levels of pain did not change significantly in either the hypnotherapy group (MD ‐2.10, 95% CI ‐4.46 to 0.26) or the control group (MD 0.10, 95% CI ‐1.78 to 1.98). However, the levels of pain decreased significantly greater in the hypnotherapy group than in the control group (mean change in pain: MD ‐2.20, 95% CI ‐3.14 to ‐1.26; n = 21; see Analysis 19.2). The subjective impairment of appearance also improved significantly greater in the hypnotherapy group than in the control group (mean change in subjective impairment of appearance: MD ‐1.60, 95% CI ‐2.50 to ‐0.70; n = 21; see Analysis 19.2).
19.2. Analysis.

Comparison 19 Hypnotherapy versus control, Outcome 2 Severity of attack of recurrent HSL during use of the preventative intervention.
There were no relevant data for this intervention for our other outcomes.
Discussion
Summary of main results
The evidence does not support the efficacy of short‐term use of oral antiviral agents in preventing recurrence of herpes simplex labialis (HSL). The efficacy of short‐term use of oral aciclovir in preventing recurrent HSL was inconsistent and lacked a dose‐response relationship: 2 trials testing aciclovir 400 mg twice daily showed a reduced risk of recurrence of HSL (Schädelin 1988; Spruance 1988), while 1 trial testing aciclovir 800 mg twice daily, Raborn 1998, and 2 trials testing 200 mg 5 times daily, Spruance 1991a; Spruance 1991b, found no similar preventative effects. The direction of intervention effect was unrelated to the risk of bias of the studies. One trial, Miller 2004, found no preventative effect of short‐term use of valaciclovir in reducing recurrence of HSL nor did a trial testing short‐term use of famciclovir (Spruance 1999). On the other hand, long‐term use of oral antiviral agents reduced the recurrence of HSL, but the clinical benefit was small. One trial found long‐term use of oral aciclovir resulted in a small but significant reduction in either clinical or virological recurrence (by one episode per participant over a four‐month period) (Rooney 1993). One trial found long‐term use of valaciclovir effective in reducing the incidence of HSL (Baker 2003), but the clinical significance of the difference was very small, with a decrease of 0.09 episodes per participant per month. One trial, Gilbert 2007, found that when compared with an episodic regimen, a long‐term suppressive regimen of valaciclovir had a lower incidence of HSL, but the difference was also very small, with a reduction of 0.10 episodes per participant per month.
One trial, Russell 1978, with a very high withdrawal rate (39.6% in the levamisole group and 15.7% in the placebo group) showed a reduced frequency of HSL in both the levamisole and placebo groups among those who completed the trial, but there were no significant differences between the 2 groups. Although the levamisole group was associated with a greater reduction in the frequency of HSL after taking into account the different baseline frequency of HSL (difference in means (MD) ‐2.00, 95% CI ‐2.24 to ‐1.76; see Analysis 7.1), the placebo group was associated with a greater reduction in the duration of attack of HSL (MD 0.70, 95% CI 0.22 to 1.18; see Analysis 7.3). Thus, there was no consistent evidence supporting the efficacy of levamisole in preventing HSL. Two other oral interventions, lysine and LongoVital® supplementation, did not prevent recurrence of HSL (Thein 1984; Pedersen 2001).
Similar to that for oral antiviral agents, the evidence shows no efficacy of short‐term use of topical antiviral agents in preventing recurrent HSL. Two trials found no effects of short‐term use of topical aciclovir 5% cream in preventing recurrence of HSL, Raborn 1997; Spruance 1991c, nor did another trial testing topical aciclovir 5% plus 348U87 3% cream (Bernstein 1994). One trial found no effects of short‐term use of topical foscarnet 3% cream in preventing recurrent HSL (Bernstein 1997). The efficacy of long‐term use of topical antiviral agents is uncertain. One trial, Gibson 1986, found long‐term use of aciclovir cream significantly reduced research‐diagnosed recurrences of HSL, but not participant‐reported recurrences. Another trial found no effects of long‐term use of topical 1,5‐pentanediol gel in preventing HSL (Busch 2009). One study, Senti 2013, found participants who applied topical 2‐hydroxypropyl‐β‐cyclo dextrin 20% gel had more recurrences than the placebo group, and the placebo group had milder symptoms of tingling and burning.
As shown in Analysis 13.1, application of sunscreen significantly prevented recurrent HSL induced by experimental ultraviolet light (UVL) (Duteil 1998; Rooney 1991), but did not reduce the recurrence of HSL induced by sunlight (Mills 1987). The efficacy of sunscreen under natural sunlight has not been confirmed.
The was a lack of consistent evidence supporting the efficacy of interferon in preventing recurrent HSL. Data from two trials, Ho 1984; Pazin 1979, showed an increased recurrence of HSL after presurgical administration of interferon, no difference in recurrence with postsurgical administration of interferon, but a decreased recurrence in those receiving continuous pre‐ and postsurgical administration of interferon. A trial, Redman 1986, found no efficacy of gamma globulin in preventing recurrent HSL, while another, Bolla 1985, found fewer incidences of recurrent HSL after six weeks of treatment with subcutaneous administration of thymopentin.
Both a HSV type I subunit vaccine and a yellow fever vaccine did not show a higher efficacy than placebo in preventing HSL (Altmeyer 1991; Møller 1997).
Two trials investigated the effects of low‐level laser therapy in preventing recurrent HSL. One trial, de Carvalho 2010, found no difference in the number of recurrences and pain, but found a significantly smaller average lesion size (with a very small difference of 0.1 mm) and a significantly lower monthly average inflammatory oedema (with a tiny difference of 0.0046 on a '0 to 3' oedema score) in the laser group. Although the latter two measures were statistically significant, the differences between the laser and control groups did not appear to be clinically significant. The other trial, Schindl 1999, found a significantly longer median recurrence‐free interval in the laser group (37.5 weeks versus 3 weeks in the control group), which was not a prespecified outcome in the present review.
One trial, Pfitzer 2005, found that hypnotherapy significantly reduced the frequency (MD ‐5.20, 95% CI ‐10.34 to ‐0.06 during a 6‐month follow‐up) and intensity of symptoms of HSL recurrences.
Overall completeness and applicability of evidence
The effects of oral and topical antiviral agents in preventing recurrent HSL have been extensively investigated. The body of evidence regarding oral and topical antiviral agents is adequate for us to conclude that long‐term use of oral aciclovir and valaciclovir are effective in preventing recurrent HSL, while short‐term use of either oral or topical antiviral agents is ineffective. On the other hand, there is a lack of evidence supporting the efficacy of long‐term use of topical antiviral agents in preventing recurrent HSL.
The available body of evidence regarding other interventions is scanty, with only one or two trials for each intervention. There is no consistent evidence supporting the efficacy of levamisole and interferon in preventing HSL. The current limited evidence found no efficacy of lysine, LongoVital® supplementation, gamma globulin, HSV type I subunit vaccine, and yellow fever vaccine in preventing HSL. There is very limited evidence suggesting that thymopentin, low‐level laser therapy, and hypnotherapy are effective in preventing HSL.
Quality of the evidence
Based on the following limitations, we rated the quality of the body of evidence low to moderate for most outcomes and very low for a few outcomes.
Limitations in the design and implementation of available studies suggesting high likelihood of bias
The risk of bias of the included trials varied from low to high (Figure 3). As shown in Figure 2, the high risk of bias most often appeared in the 'selective reporting' domain (12 (34%) out of 32 trials), followed by the 'other bias' domain (9 (28%) trials). The cause for a high risk of bias in 'selective reporting' was either a lack of data on adverse events or details on efficacy outcomes. The causes for a high risk of 'other bias' included early stopping of the trial (Bernstein 1994), no washout period in cross‐over trials (Gibson 1986; Gilbert 2007; Rooney 1993; Thein 1984), different baseline frequencies of HSL recurrences between the experimental and control groups (Pedersen 2001; Russell 1978), a low percentage of participants having a history of HSL (Schädelin 1988), and a lack of scheduled follow‐ups (Schindl 1999).
Over half of the included trials (17/32) were published before 1996 when the reporting guidelines for randomised controlled trials (RCTs), the CONsolidated Standards Of Reporting Trials (CONSORT) Statement, was first proposed. These trials often did not provide detailed reports on the methods of random sequence generation, allocation concealment, blinding, and withdrawal or dropout.
In five included trials (Altmeyer 1991; Bolla 1985; Russell 1978; Senti 2013; Thein 1984), the incidence of HSL decreased in both the experimental and placebo groups, which may be attributed to either the placebo effect or an overestimation of the baseline incidence of HSL.
Indirectness of evidence (indirect population, intervention, control, outcomes)
Direct evaluation under natural sunlight exposure in the de Carvalho 2010 trial did not confirm the indirect evidence of the preventative efficacy of sunscreen use under experimental UVL in the Schindl 1999 trial.
Unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses)
As stated previously (Analysis 1.1), the preventative efficacy of short‐term administration of oral aciclovir was inconsistent and lacked a dose‐response relationship (see Table 1). Also, the efficacy of levamisole and interferon was inconsistent (see Table 7; Table 14). For other interventions, the direction of intervention effect was consistent.
Imprecision of results (wide confidence intervals)
For most interventions, there were only one or two relevant trials of limited sample size. We therefore downgraded the quality of evidence for imprecision.
High probability of publication bias
We were unable to detect publication bias because of the limited number of trials for each intervention.
Potential biases in the review process
We planned to conduct an intention‐to‐treat analysis by considering those with missing binary outcomes as treatment failures and carrying out a 'last observation carried forward' analysis for those with missing continuous or ordinal outcomes. However, many included trials did not report details of withdrawals or dropouts nor provided a participant flow chart (Bernstein 1994; Bolla 1985; de Carvalho 2010; Duteil 1998; Gibson 1986; Pfitzer 2005; Spruance 1991a; Spruance 1991b; Spruance 1991c; Thein 1984). We failed to conduct the planned analysis for missing data, and it is thus unclear whether the intervention effects were overestimated in these trials.
Agreements and disagreements with other studies or reviews
Three reviews, Opstelten 2008; Worrall 2009; Rahimi 2012, were published before we conducted this review, with Rahimi 2012 limited to antiviral agents and having a four‐year gap between the year of literature search and publication. They included RCTs from searching various databases up to April 2008, February 2009, and 2008, respectively. Two reviews, Opstelten 2008; Worrall 2009, found in line with our review that long‐term use of oral antiviral agents are effective in preventing HSL and found mixed results regarding the preventative efficacy of sunscreens.
The Opstelten 2008 review regarded short‐term use of topical antiviral agents effective in preventing HSL and interpreted Raborn 1997 as showing the efficacy of topical aciclovir cream in preventing HSL. However, in the Raborn 1997 trial, the proportion of participants presenting with recurrent HSL did not significantly differ between the aciclovir and placebo groups (15/91 versus 23/90). Only in the 'treatment period plus four days' follow‐up period' did the proportion of participants having recurrent HSL differ significantly between the two groups. The abstract of the Opstelten 2008 review stated short‐term use of oral antiviral agents would provide some protection against recurrent HSL, although its main text reported the inconsistent results from the Raborn 1998; Spruance 1988; Spruance 1991a; and Spruance 1991b trials.
The Worrall 2009 review could not conclude whether topical antiviral agents are effective in preventing HSL based on results from 2 trials on aciclovir 5% cream (Raborn 1997; Spruance 1991c). Our review included 2 more trials on aciclovir 5% plus 348U87 3% cream, Bernstein 1994, and foscarnet 3% cream, Bernstein 1997, and found no effects of short‐term use of topical antiviral agents in preventing recurrent HSL.
The Rahimi 2012 review was in agreement with us that topical aciclovir cream did not appear effective in preventing HSL. The Rahimi 2012 review examined the effects of various antivirals and found oral aciclovir and valaciclovir, but not famciclovir, effective in preventing HSL. However, the Rahimi 2012 review did not take into consideration the length of antiviral use.
Authors' conclusions
Implications for practice.
The evidence indicates that long‐term use of oral antiviral agents reduces the recurrence of herpes simplex labialis (HSL). There is very limited evidence suggesting that thymopentin, low‐level laser therapy, and hypnotherapy are effective in preventing recurrent HSL. The efficacy of long‐term use of topical aciclovir cream is uncertain. The preventative efficacy of sunscreen under realistic natural sunlight conditions has not been confirmed.
On the other hand, the current evidence found no preventative effects of short‐term use of oral or topical antiviral agents, lysine, LongoVital® supplementation, gamma globulin, HSV type I subunit vaccine, and yellow fever vaccine. Also, there is no consistent evidence supporting the efficacy of levamisole and interferon in preventing HSL.
Implications for research.
Although the Rooney 1993 trial found long‐term use of oral aciclovir 400 mg twice daily effective in preventing HSL, the long‐term safety was unclear. It is also unknown if long‐term use of a smaller dosage of oral aciclovir is effective in preventing recurrent HSL. The current evidence regarding long‐term use of topical antiviral agents, thymopentin, low‐level laser therapy, and hypnotherapy is very limited. Further trials on these interventions are required to fill in the gap in knowledge. There is only one small randomised controlled trial (RCT) examining the effects of sunscreens in preventing HSL induced by sunlight. Thus, there is a call for large RCTs of adequate use of high‐SPF (sun protection factor) sunscreens for preventing HSL under realistic natural sunlight conditions.
Furthermore, we found that measured outcomes varied widely across the included trials, which resulted in difficulty in completing the present review. It is desirable to define a set of core outcomes for studies on the interventions for prevention of HSL, and all future trials should measure and report these core outcomes. Before such a set of core outcomes is defined, we suggest trialists measure and report the outcomes of interest in the present review (see Types of outcome measures).
What's new
| Date | Event | Description |
|---|---|---|
| 19 October 2016 | Amended | A search of MEDLINE, PubMed, and Embase in October 2016 found only one relevant study of a new intervention, which our Co‐ordinating Editor and the lead author decided did not merit an update at this time. Thus, an update of this review has been postponed. Our Information Specialist will run a new search in October 2017 to re‐assess whether an update is needed. |
Notes
A search of MEDLINE, PubMed, and Embase in October 2016 found only one relevant study of a new intervention, which our Co‐ordinating Editor and the lead author decided did not merit an update at this time. Thus, an update of this review has been postponed. Our Information Specialist will run a new search in October 2017 to re‐assess whether an update is needed.
Acknowledgements
The Cochrane Skin Group editorial base wishes to thank Hywel Williams who was the Dermatology Editor for this review; Ben Carter and Esther van Zuuren who were the Statistical and Methods Editors, respectively; the clinical referees, Olivier Chosidow and Richard Bohay; and the consumer referee, Jo Foster.
Appendices
Appendix 1. Skin & Oral Health Groups' Specialised Registers' search strategy
("cold sore*" or "herpes labialis" or (herpe* and (stomatiti* or gingivostomatiti*)) or "fever blister*") or (("herpes simplex" or herpesvirus or simplexvirus or "hsv‐1" or herpes or herpetic or herpesvir* or herpetiform) and (mouth or lip* or labial or orolabial or perioral or extraoral or intraoral or intra‐oral or extra‐oral or peri‐oral or oro‐labial or gingiva* or gingivo*))
Appendix 2. CENTRAL (the Cochrane Library) search strategy
#1 MeSH descriptor: [Herpes Labialis] explode all trees #2 MeSH descriptor: [Stomatitis, Herpetic] explode all trees #3 "herpes labialis" #4 (herpe* near/3 (stomatitis or gingivostomatitis*)) #5 "cold sore*" OR "fever blister*" #6 #1 or #2 or #3 or #4 or #5 #7 MeSH descriptor: [Herpes Simplex] explode all trees #8 MeSH descriptor: [Herpesvirus 1, Human] explode all trees #9 MeSH descriptor: [Simplexvirus] explode all trees #10 "herpes simplex" and simplexvirus and "hsv‐1" and herpes or herpetic or herpesvir* or herpetiform* #11 #7 or #8 or #9 or #10 #12 MeSH descriptor: [Mouth] explode all trees #13 MeSH descriptor: [Mouth Diseases] explode all trees #14 MeSH descriptor: [Lip] explode all trees #15 MeSH descriptor: [Lip Diseases] explode all trees #16 mouth or lip*1 #17 labial or orolabial or perioral or extraoral or intraoral #18 "intra‐oral" or "extra‐oral" or "peri‐oral" or "oro‐labial" #19 gingiva* or gingivo* #20 #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 #21 #11 and #20 #22 #6 or #21
Appendix 3. MEDLINE (Ovid) search strategy
1. Herpes Labialis.mp. or exp Herpes Labialis/ 2. exp Stomatitis, Herpetic/ 3. (herpe: adj3 (stomatiti: or gingivostomatiti:)).mp. 4. cold sore$.mp. 5. fever blister$.mp. 6. 1 or 2 or 3 or 4 or 5 7. herpes simplex.mp. or exp Herpes Simplex/ 8. exp Herpesvirus 1, Human/ 9. simplexvirus.mp. or exp Simplexvirus/ 10. "hsv‐1".mp. 11. (herpes or herpetic or herpesvir$ or herpetiform$).mp. 12. 7 or 8 or 9 or 10 or 11 13. exp Mouth/ or exp Mouth Diseases/ or mouth.mp. 14. exp Lip/ or exp Lip Diseases/ 15. lip$1.mp. 16. (labial or orolabial or perioral or extraoral or intraoral).mp. 17. (intra‐oral or extra‐oral or peri‐oral or oro‐labial).mp. 18. (gingiva: or gingivo:).mp. 19. 13 or 14 or 15 or 16 or 17 or 18 20. 12 and 19 21. 6 or 20 22. randomized controlled trial.pt. 23. controlled clinical trial.pt. 24. randomized.ab. 25. placebo.ab. 26. clinical trials as topic.sh. 27. randomly.ab. 28. trial.ti. 29. 22 or 23 or 24 or 25 or 26 or 27 or 28 30. exp animals/ not humans.sh. 31. 29 not 30 32. 21 and 31
Appendix 4. EMBASE (Ovid) search strategy
1. herpes labialis.mp. or exp herpes labialis/ 2. exp herpetic stomatitis/ 3. (herpe$ adj3 (stomatiti$ or gingivostomatiti$)).mp. 4. cold sore$.mp. 5. fever blister$.mp. 6. 1 or 2 or 3 or 4 or 5 7. herpes simplex.mp. or exp herpes simplex/ 8. exp Herpes simplex virus 1/ 9. simplexvirus.mp. or exp Simplexvirus/ 10. "hsv‐1".mp. 11. (herpes or herpetic or herpesvir$ or herpetiform$).mp. 12. 7 or 8 or 9 or 10 or 11 13. exp mouth/ or mouth.mp. or exp mouth disease/ 14. exp lip disease/ or exp lip/ 15. lip$1.mp. 16. (labial or orolabial or perioral or extraoral or intraoral).mp. 17. (intra‐oral or extra‐oral or peri‐oral or oro‐labial).mp. 18. (gingiva$ or gingivo$).mp. 19. 13 or 14 or 15 or 16 or 17 or 18 20. 12 and 19 21. 6 or 20 22. crossover procedure.sh. 23. double‐blind procedure.sh. 24. single‐blind procedure.sh. 25. (crossover$ or cross over$).tw. 26. placebo$.tw. 27. (doubl$ adj blind$).tw. 28. allocat$.tw. 29. trial.ti. 30. randomized controlled trial.sh. 31. random$.tw. 32. or/22‐31 33. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 34. human/ or normal human/ 35. 33 and 34 36. 33 not 35 37. 32 not 36 38. 21 and 37
Appendix 5. LILACS search strategy
(cold sore$) or (fever blister$) or calenturas Or (herpe$ and (stomatiti$ or gingivostomatiti$ or labial$ or simple$ or febril))
Appendix 6. CNKI search strategy
(篇名 = 皰疹) AND (摘要 = 唇) AND (摘要 = 隨機)
Appendix 7. Airiti search strategy
(皰疹) = 篇名關鍵字摘要 AND (唇) = 篇名關鍵字摘要
Appendix 8. Trial register search strategy
herpes labialis
Data and analyses
Comparison 1. Oral aciclovir (short‐term) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of HSL during use of the preventative intervention (by clinical evaluation) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Aciclovir 800 mg twice daily | 1 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.62, 1.87] |
| 1.2 Aciclovir 400 mg twice daily | 2 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.13, 0.51] |
| 1.3 Aciclovir 200 mg 5 times/day | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.20, 1.07] |
| 2 Incidence of HSL during use of the preventative intervention (by culture) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse effects during use of the preventative intervention | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Aciclovir 800 mg twice daily | 1 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.38] |
| 3.2 Aciclovir 400 mg twice daily | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.62, 8.58] |
| 4 Severity (lesion size) of attack of herpes labialis during use of the preventative intervention | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Length | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Width | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Area | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Severity (stage) of attack of recurrent HSL during use of the preventative intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Prodrome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Erythema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Papule | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Severity (pain) of attack of recurrent HSL during use of the preventative intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Incidence of HSL after use of the preventative intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Oral aciclovir (long‐term) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of attack of herpes labialis during use of the preventative intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Valaciclovir (short‐term) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of herpes labialis during use of the preventative intervention (by clinical evaluation) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Incidence of herpes labialis during use of the preventative intervention (by culture) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse effects during use of the preventative intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Viral load (shedding) in saliva | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. Valaciclovir (long‐term) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects during use of the preventative intervention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. Valaciclovir (suppressive regimen versus episodic regimen).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of herpes labialis during use of the preventative intervention (number of recurrences per participant per month) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Adverse effects during use of the preventative intervention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Duration of attack of recurrent HSL during use of the preventative intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Severity (pain) of attack of recurrent HSL during use of the preventative intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Severity (maximum total lesion area) of attack of recurrent HSL during use of the preventative intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 6. Famciclovir versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of HSL during use of the preventative intervention (by clinical evaluation) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Famciclovir 125 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Famciclovir 250 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Famciclovir 500 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Duration of attack of recurrent HSL during use of the preventative intervention | 1 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 2.1 Famciclovir 125 mg | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Famciclovir 250 mg | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Famciclovir 500 mg | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Severity (pain) of attack of recurrent HSL during use of the preventative intervention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Famciclovir 125 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Famciclovir 250 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Famciclovir 500 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Levamisole versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of HSL during use of the preventative intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Adverse effects during use of the preventative intervention (leading to withdrawal) | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Duration of attack of recurrent HSL during use of the preventative intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 8. Lysine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of HSL during use of the preventative intervention (number of recurrences per participant per month) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 9. Topical aciclovir (short‐term) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of HSL during use of the preventative intervention | 2 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.48, 1.72] |
| 2 Adverse effects during use of the preventative intervention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Severity (aborted lesions) of attack of recurrent HSL during use of the preventative intervention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Incidence of HSL after use of the preventative intervention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 10. Topical aciclovir and 348U87 cream (short‐term) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of HSL during use of the preventative intervention (by culture) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Incidence of HSL during use of the preventative intervention (by clinical evaluation) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Duration of attack of recurrent HSL during use of the preventative intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Severity of attack of recurrent HSL during use of the preventative intervention (maximum lesion area) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 11. Topical foscarnet versus placebo.
Comparison 12. Topical 1,5‐pentanediol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severity (blistering, swelling, or pain) of recurrence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 13. Sunscreen versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of HSL during use of the preventative intervention (by clinical evaluation) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Solar radiation | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.25, 5.06] |
| 1.2 Experimental ultraviolet light | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.33] |
| 2 Incidence of HSL during use of the preventative intervention (by culture) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 14. Interferon versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of HSL during use of the preventative intervention | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Presurgical | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Postsurgical | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Pre‐ & postsurgical | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse effects during use of the preventative intervention (fever) | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.44, 3.67] |
| 2.1 Presurgical | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 2.45 [1.26, 4.78] |
| 2.2 Postsurgical | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.00, 3.84] |
| 2.3 Pre‐ & postsurgical | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 11.76 [0.71, 195.11] |
| 3 Adverse effects during use of the preventative intervention (other) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Pain & tenderness at injection site | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Malaise, nausea or vomiting | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. Gamma globulin versus histamine (control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of attack of recurrent HSL during use of the preventative intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Severity of attack of recurrent HSL during use of the preventative intervention (less severe recurrences than usual) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 16. Thymopentin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects during use of the preventative intervention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 17. Yellow fever vaccination versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects during use of the preventative intervention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 18. Laser versus no interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to first recurrence | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 19. Hypnotherapy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of HSL during use of the preventative intervention (change in frequency of recurrence) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Severity of attack of recurrent HSL during use of the preventative intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Intensity | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Pain | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Impairment of appearance | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Altmeyer 1991.
| Methods | This was a multicentre, randomised, double‐blind study | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 64 participants were randomised, with 35 in the vaccine group and 29 in the placebo group |
|
| Interventions |
The participants were followed up for a 4‐month 'pilot phase' without treatments. Then in the first main phase of 4 months' duration, the assigned treatment was given weekly for 3 times on days 120, 127, and 134. In the second main phase of another 4 months' duration, the assigned treatment was repeated for another 3 times on days 240, 247, and 254 |
|
| Outcomes |
|
|
| Notes | Setting: university hospitals Country: Germany Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The methods of random sequence generation were not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The vaccine and placebo preparations were identical in appearance and labelling except for the consecutive number of the labelling |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The vaccine and placebo preparations were identical in appearance and labelling except for the consecutive number of the labelling |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant dropped out immediately after allocation to the vaccine group. 1 participant in the vaccine group and 1 in the placebo group withdrew before treatments started. 1 in the vaccine group withdrew after completing the vaccination due to an adverse event. 1 in the vaccine group and 1 in the placebo group withdrew in the second main phase. Thus, a total of 4 (11.4%) and 2 (6.9%) participants in the vaccine and placebo group, respectively, did not complete the study |
| Selective reporting (reporting bias) | Low risk | Both efficacy and adverse outcomes were reported |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Baker 2003.
| Methods | This was a pooled analysis of 2 randomised, double‐blind, placebo‐controlled, single‐centre studies | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 98 participants were randomised, with 49 in each group |
|
| Interventions |
If there was clinical evidence of recurrent herpes labialis, participants received open‐label oral valaciclovir 500 mg twice daily for 5 days. Participants resumed their assigned study medication at the end of the 5‐day open‐label regimen |
|
| Outcomes |
|
|
| Notes | Setting: a university hospital Country: US Funding source: supported in part by GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The methods of random sequence generation were not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients randomized to treatment who attended at least 1 of the monthly clinic visits were included in the efficacy analyses." "Two patients (1 in the valaciclovir group and 1 in the placebo group) who were lost to follow‐up and 1 patient in the valaciclovir group who withdrew prior to the first clinic visit were not included in the efficacy analyses" Comment: only 3 (3.1%) out of 98 participants were lost to follow up |
| Selective reporting (reporting bias) | Low risk | The number of participants with recurrences, number of recurrences per participant per month, and incidence of adverse events during the treatment period were reported |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Bernstein 1994.
| Methods | This was a double‐blind, randomised, placebo‐controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 51 participants were randomised, with 25 and 26 in the aciclovir and 348U87 group and placebo group, respectively |
|
| Interventions |
Immediately after UV exposure, participants began treatment by application of the study medication to the UV‐exposed quadrant. The cream was applied every 2 hours while awake (maximum 8 applications/day), for 7 days. If herpetic lesions developed, treatment was continued until the lesions healed up to a maximum of 5 additional days |
|
| Outcomes |
|
|
| Notes | Setting: research institutes (James N. Gamble Institute of Medical Research and Hilltop Research) Country: US Funding source: Burroughs Wellcome & Co. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned according to a code supplied by the sponsor" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A subsequent double blind evaluation was performed...on 51 subjects to assess the effects of a combination of topical aciclovir and 348U87 compared to placebo on lesion development and severity" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A subsequent double blind evaluation was performed...on 51 subjects to assess the effects of a combination of topical aciclovir and 348U87 compared to placebo on lesion development and severity" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or withdrawals were reported |
| Selective reporting (reporting bias) | High risk | The adverse effects during use of the preventative intervention were not reported |
| Other bias | High risk | Quote: "The sample size for the drug evaluation was estimated to be 50 patients per group to achieve a power of 80% and a significance level of 0.05 if the drug decreased recurrences by 60%. An interim analysis after 50 subjects was planned." "Because there was no trend for the benefit of the drug treatment the study was discontinued after the interim analyses" Comment: this was an early‐stopped trial. Therefore, a small benefit of the study drug could not be ruled out |
Bernstein 1997.
| Methods | This was a randomised, double‐blind, multicentre trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 310 participants were enrolled at the 4 centres, but 8 did not receive the study drug. Of the 302 treated participants, 152 received foscarnet 3% and 150 received placebo cream. 7 participants (4 for foscarnet and 3 for placebo) were excluded from the efficacy analysis because of major predefined protocol violations |
|
| Interventions |
Beginning immediately after ultraviolet radiation (UVR) exposure of the lips, participants applied the cream on the UVR‐exposed area and surrounding skin 8 times daily (at least every 2 hours while awake) for 7 days, but if a herpetic lesion developed, dosing was extended as necessary to treat the lesion for at least 4 days. The time of each application was recorded in a participant diary |
|
| Outcomes |
|
|
| Notes | Setting: 4 medical centres Country: US Funding source: Astra Arcus AB, Sweden |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The methods of randomisation were not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This was a randomized, double‐blind investigation" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "This was a randomized, double‐blind investigation" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "A total of 310 subjects were enrolled at the four centers, but 8 did not receive the study drug." "Seven subjects (four for foscarnet and three for placebo) were excluded from the efficacy analysis because of major predefined protocol violations" Comment: there were 15 (4.8%) dropouts/withdrawals out of 310 enrolled participants |
| Selective reporting (reporting bias) | Low risk | The efficacy outcomes and adverse effects were reported |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Bolla 1985.
| Methods | This was a placebo‐controlled, double‐blind, randomised, multicentre study | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 36 participants older than 16 years and suffering from frequent recurrences (≥ 12 relapses/year) of herpes labialis infections, with 18 in each group, entered this study |
|
| Interventions |
Thymopentin was provided in a concentration of 100 mg/ml. A 6‐week treatment with 0.5 ml of the test drug (50 mg thymopentin or placebo), administered by the subcutaneous route 3 times weekly, was performed |
|
| Outcomes | After the double‐blind course of treatment, a follow‐up period of 18 weeks without any treatment was proposed. After 6 weeks' treatment and at the end of the follow‐up period, the outcomes below were assessed:
|
|
| Notes | Setting: 14 medical centres Country: Europe Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The methods of randomisation were not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Thymopentin and placebo (vehicle) were supplied in coded, unidentifiable 5‐ml multidose vials" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or withdrawals were reported |
| Selective reporting (reporting bias) | Low risk | Both the efficacy outcomes and adverse effects were reported |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Busch 2009.
| Methods | This was a randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 105 participants were randomised to either 1,5‐pentanediol (PD) (53 participants) or placebo (52 participants) |
|
| Interventions |
The clinical trial consisted of a prophylactic period of 26 weeks, during which at least 2 examinations (at the start of the trial and after 25 to 27 weeks) were performed. During the prophylactic phase, the participants applied PD or placebo gel twice daily to both lips. Upon occurrence of a herpes episode, the participant started immediately with the therapy phase and presented herself/himself promptly to the participating investigator for confirmation of the herpes symptoms. During the 5‐day therapy phase, the gel was applied 8 times daily. On day 6 after the start of the therapy, the participant visited the investigator again for examination and evaluation of the healing process and started prophylactic treatment twice daily again until the next herpes episode |
|
| Outcomes |
|
|
| Notes | Setting: 4 study centres in Berlin Country: Germany Funding source: Natumin Pharma AB |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization for this clinical trial was undertaken with the help of randomization plan NP/RL/060407/132" |
| Allocation concealment (selection bias) | Low risk | Quote: "The gel supplied to the patients had a consecutive random number on the label. This number was previously assigned to the PD gel and to the placebo gel externally. Neither the investigator nor the patients had any knowledge of what kind of treatment was given" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The PD and the placebo gel were packed in 4 g tubes and labelled for the clinical trial. The gels were not distinguishable by color or smell." "Neither the investigator nor the patients had any knowledge of what kind of treatment was given" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The gel supplied to the patients had a consecutive random number on the label. This number was previously assigned to the PD gel and to the placebo gel externally. Neither the investigator nor the patients had any knowledge of what kind of treatment was given." "The randomization code remained with the study sponsor until the final closure of the database" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 105 participants recruited, 3 (2.9%) participants who had been assigned to the PD group dropped out of the study |
| Selective reporting (reporting bias) | Low risk | The efficacy outcomes and adverse events were reported |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
de Carvalho 2010.
| Methods | This was a randomised controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 71 participants were randomly allocated to the experimental (laser) group (41 participants) and the control group (30 participants) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Setting: a university hospital Country: Brazil Funding source: non‐profit organisations (Fundação de Amparo à Pesquisa do Estado de São Paulo, the Conselho Nacional de Desenvolvimento Científico e Tecnológico, and the Center of Research, Teaching and Clinics of Laser in Dentistry, School of Dentistry, University of São Paulo, Brazil) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author's reply to our request: "Randomization was down [sic] through sortition" |
| Allocation concealment (selection bias) | Unclear risk | The authors replied to our request to say that no measures for allocation concealment were arranged |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was impossible because laser therapy was used in the experimental group without a corresponding sham treatment in the placebo group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The authors replied to our request to say that the outcome assessors were the participant physicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors replied to our request to say that there was only 1 dropout |
| Selective reporting (reporting bias) | Low risk | Efficacy data were reported in the article. The authors replied to our request to say that they evaluated adverse events, but there were none detected |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Duteil 1998.
| Methods | This was a randomised cross‐over trial on preventing ultraviolet light‐induced herpes labialis | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 19 participants were randomised to intervention A (9) and B (10) |
|
| Interventions |
The very high protection sunblock stick (UVA and UVB) contained a photostable combination of Parsol® 1789, Eusolex® 6300, and Mexoryl™ SX (Laboratoires Galderma) The test product was applied (2 mg/cm²) to the lips of the participant. 10 minutes after application of the product, half of the test zone (left or right, depending on where the last recurrence of herpes had occurred) was exposed to 4 times the participant's minimal erythema dose. Linen towels protected the remainder of the face and the neck. There was a 4‐week washout period between the 2 phases |
|
| Outcomes |
|
|
| Notes | Setting: a university hospital Country: France Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The methods of randomisation were not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or withdrawals were mentioned |
| Selective reporting (reporting bias) | High risk | Only an efficacy outcome was reported |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Gibson 1986.
| Methods | This was a randomised, double‐blind, placebo‐controlled, cross‐over study | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 23 participants completed the trial |
|
| Interventions |
The cream was applied to all previously affected areas 4 times per day. There was no washout period. The participants were subsequently observed for a further 16 weeks with no treatments |
|
| Outcomes |
|
|
| Notes | Setting: 3 hospitals (London Hospital, Basildon Hospital, and Sant'Orsola Hospital) Country: UK and Italy Funding source: Wellcome Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The methods of randomisation were not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or withdrawals were mentioned |
| Selective reporting (reporting bias) | Low risk | Efficacy outcomes and adverse events were reported |
| Other bias | High risk | There was no washout period. The outcome data of the first phase were unavailable |
Gilbert 2007.
| Methods | This was a randomised, open‐label, cross‐over trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 76 participants were randomised, received at least 1 dose of valaciclovir, and had at least 1 postbaseline evaluation (termed 'intention‐to‐treat exposed population' by the trialists). Of them, 60 participants had at least 1 postbaseline evaluation during each treatment period (termed 'cross‐over population' by the trialists) |
|
| Interventions |
Recurrences of HSL during the suppressive treatment were treated with episodic therapy |
|
| Outcomes |
|
|
| Notes | Setting: a university hospital Country: US Funding source: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The methods of randomisation were not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was impossible because of different regimens |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding was impossible because of different regimens |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16 (21%) out of 76 participants did not complete the study |
| Selective reporting (reporting bias) | Low risk | Efficacy and adverse outcomes were reported |
| Other bias | High risk | There was no washout period |
Ho 1984.
| Methods | This was a randomised trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 55 participants were analysed |
|
| Interventions |
On days when the treatment groups did not receive IFN, they received placebo injections so that all 3 groups received 2 injections per day for 5 days |
|
| Outcomes |
|
|
| Notes | Setting: a university hospital Country: US Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The methods of randomisation were not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind"; "all three groups received two injections per day for 5 days" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind"; "after data were collected on a total of 55 patients, the code was broken again and the results were analyzed" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A total of 55 (85%) participants completed the study and were analysed. 10 other participants were enrolled and randomised but not evaluated: 5 were shedding HSV in the oropharynx before surgery, 2 refused treatment, and surgery was cancelled or postponed for 3 participants |
| Selective reporting (reporting bias) | Low risk | Both efficacy and adverse outcomes were reported |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Miller 2004.
| Methods | This was a randomised, double‐blind, placebo‐controlled study | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 150 participants were enrolled in the trial. 23 participants who failed to return to the clinic and 2 participants who were HSV‐seronegative were excluded from analysis. 63 participants in the placebo group and 62 participants in the valaciclovir group who had evaluable efficacy data were analysed. There were no data on the number of originally randomised participants in each group |
|
| Interventions |
The trialists determined compliance via oral confirmation by the participant that all medication had been taken according to the prescribed schedule and with the return of the empty pill bottle |
|
| Outcomes |
|
|
| Notes | Setting: a university hospital Country: US Funding source: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We assigned patients sequentially to the study medication, which was numbered according to a computer‐generated randomized code. Three randomization codes were used per treatment group based on lesion frequency categories. Category 1 was composed of patients with a history of one lesion per year; category 2, patients with a history of two to four lesions per year; and category 3, patients who had a history of more than four lesions per year" |
| Allocation concealment (selection bias) | Low risk | Quote: "We assigned patients sequentially to the study medication, which was numbered according to a computer‐generated randomized code" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "For the double‐blinded study medications, we packaged 12 pills per identical white bottle" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The treatment blind was maintained throughout the trial and was not broken for any subject" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 23 (15.5%) of 148 eligible participants were lost to follow up |
| Selective reporting (reporting bias) | Low risk | Both efficacy and adverse outcomes were reported |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Mills 1987.
| Methods | This was a randomised trial | |
| Participants | Volunteers were recruited for this study from physicians, medical scientists, medical personnel, and their spouses attending week‐long conferences at 3 United States ski resorts Inclusion criteria
Exclusion criteria
For the purposes of randomisation, participants were stratified into those with a self‐perceived risk of developing herpes labialis after 3 days of skiing of greater than 75%, 50% to 75%, or less than 50%. A total of 51 participants were enrolled: 29 at conference 1, 14 at conference 2, and 8 at conference 3. 24 participants received sunscreen, and 27 received placebo |
|
| Interventions |
The study medication was supplied both in lipstick form and as a lotion. The participants applied the study medication (both lipstick and lotion) hourly, immediately before and during skiing each day for the 6 days of the study |
|
| Outcomes |
|
|
| Notes | Setting: conferences held at ski resorts Country: US Funding source: Herbert Laboratories and Dorsey Laboratories |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...according to a blocked and stratified randomization scheme" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither subjects nor investigators knew the identity of the study medications" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The code was not broken until after the data had been analyzed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | Only efficacy data on the number of participants having a recurrence were reported. The trials did not provide respective data on the mean lesion size of the 2 groups |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Møller 1997.
| Methods | This was a double‐blind randomised trial | |
| Participants |
Inclusion criteria
Exclusion criteria
24 persons with culture‐proven herpes labialis, with 12 in each group, were included in the study |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Setting: hospital Country: Denmark Funding source: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by drawing lots |
| Allocation concealment (selection bias) | Low risk | Randomisation was done by drawing lots |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (translated): "double‐blind"; "the vaccinating physician had not participated in patient selection, and he was not at the subsequent follow‐up of the patients" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The blinded participant returned a mail every other month, reporting the number of attacks during the previous 2 months |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (translated): "All 24 patients completed the study, including the 12‐month follow‐up" |
| Selective reporting (reporting bias) | Low risk | Both efficacy and adverse outcomes were reported |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Pazin 1979.
| Methods | This was a double‐blind randomised trial | |
| Participants |
Inclusion criteria
Exclusion criteria
42 persons were enrolled, but 3 in the placebo group and 2 in the interferon group had to be dropped from the study or omitted from the analysis. The causes were deferral of operation (2 persons), asymptomatic excretion of HSV on the day before operation (2), and change to a different operation (1). 19 were treated with interferon and 18, with placebo |
|
| Interventions |
Both groups received high‐dose corticosteroid therapy before and after operation. Dexamethasone 10 mg was administered at the same time as the initial interferon or placebo injection, and approximately 90 mg of dexamethasone was administered over the ensuing 90 hours |
|
| Outcomes |
|
|
| Notes | Setting: hospital Country: US Funding source: US National Health Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A paired randomisation schedule was used |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was double blinded" Comment: interferon and equivalent volumes of albumin were administered, respectively |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study was double blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 (12%) of 42 enrolled participants did not complete the trial because of a cause unrelated to efficacy, with 3 (14%) in the placebo group and 2 (10%) in the interferon group |
| Selective reporting (reporting bias) | Low risk | Both efficacy and adverse outcomes were reported |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Pedersen 2001.
| Methods | This was a double‐blind, randomised, placebo‐controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 52 persons with an estimated average number of recurrent herpes labialis in the preceding year of 10.3 (range 4 to 45) were enrolled, with 27 in the LongoVital® (LV) group and 25 in the placebo group. 3 persons withdrew before the end of the study for reasons unrelated to the medication |
|
| Interventions |
Both groups were followed up without study medications for another 4 months |
|
| Outcomes |
In the previous studies with LV, it has taken 2 months before any benefit was demonstrated. Therefore, the various statistics were evaluated in periods of 2 months during both the treatment and post‐treatment follow‐up periods in this study, i.e., days 0 to 60, days 61 to 120, days 121 to 180, and days 181 to 240 |
|
| Notes | Setting: Oral Medicine Clinic Country: Denmark Funding source: Paramedical A/S |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The methods of randomisation were not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...double‐blind" Quote: "The LV tablets were coated to make them indistinguishable from the inert lactose, placebo tablets" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcomes were assessed and reported by the blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 (6%) of 52 participants withdrew before the end of the study for reasons unrelated to the medication |
| Selective reporting (reporting bias) | High risk | Pain was measured by visual analogue scale, but the results were not reported. In the follow‐up period, the duration of herpes labialis episodes and maximal size of herpetic lesions in the LongVital group were greater than the placebo group, but the authors did not report or make statistical comparisons |
| Other bias | High risk | The estimated number of recurrent herpes labialis episodes the year before the study tended to be higher in the placebo group (P = 0.09) |
Pfitzer 2005.
| Methods | This was a randomised trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 21 participants were classified according to the frequency of occurrence in 3 different categories (I = 5 times per year, II = 6 to 12 times/year, and III = > 12 times/year). Within these categories, they were randomised to an experimental (n = 10) and a control group (n = 11) |
|
| Interventions |
|
|
| Outcomes |
The final assessment took place 6 months after treatment |
|
| Notes | Setting: Psychological Institute of the University of Tübingen Country: Germany Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The methods of randomisation were not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was impossible as hypnotherapy was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The unblinded participants assessed the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or withdrawals were mentioned |
| Selective reporting (reporting bias) | Low risk | All of the outcomes specified in the Methods were reported |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Raborn 1997.
| Methods | This was a double‐blind, randomised, placebo‐controlled, multicentre trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 196 participants were enrolled. 5 enrolled participants who did not receive medication were excluded from the analysis. The remaining 191 participants (95 treated with aciclovir, 96 given the placebo) constituted the intent‐to‐treat group and were included in the safety and efficacy analysis. Of these 191 participants, 10 were excluded from the efficacy subset for various protocol violations that ranged from using lipstick while skiing to applying the study medication less than 12 hours before sun exposure. A separate efficacy analysis was conducted for the remaining 181 participants (91 aciclovir, 90 placebo) |
|
| Interventions |
The participants were given the study drug to apply 12 hours before intensive sun exposure (in other words, during the evening preceding the day they would start to ski). The study drug was applied 5 times per day: at bedtime, on waking, and 3 times during the course of the day at 4‐hour intervals. This treatment continued for a period of at least 72 hours, to a maximum of 168 hours |
|
| Outcomes |
Each participant was contacted daily and examined within 24 hours by a dentist, physician, physician assistant, or nurse if signs or symptoms of recurrent disease appeared. Each participant was contacted either by mail or by phone 7 to 10 days after completing the study to determine whether there were any problems and to note any formation of lesions since discontinuation of the study drug |
|
| Notes | Setting: 7 ski sites Country: Canada and the US Funding source: Burroughs Wellcome, Canada |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The methods of randomisation were not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The subjects and all of the study personnel were blinded as to which treatment was being applied to which person" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The subjects and all of the study personnel were blinded as to which treatment was being applied to which person" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 (7.7%) of 196 enrolled participants did not complete the trial or violated the protocol |
| Selective reporting (reporting bias) | High risk | The authors assessed pain and found no significant differences in the amount of pain between the aciclovir and placebo groups. However, the authors did not report the statistics |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Raborn 1998.
| Methods | This was a randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
239 persons were enrolled, but 2 who did not receive the test drug were excluded from analysis by the trialists. 114 received aciclovir, and 123 received placebo |
|
| Interventions |
Participants (all of whom were at least 18 years of age) were given 800 mg of the study drug twice daily (1600 mg daily) beginning 12 to 24 hours before sun exposure, with the same dosage continuing for the entire sun‐exposure period (minimum: 3 days; maximum: 7 days). They were required to complete at least 3 hours of outdoor activity (downhill or cross‐country skiing) for at least 3 days, allowed to use acetaminophen as an analgesic, and provided with and encouraged to use a standard sunscreen (in lipstick form) with a sun prevention factor of 15 |
|
| Outcomes |
|
|
| Notes | Setting: 3 centres Country: Canada Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...double‐blind, placebo‐controlled" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...double‐blind, placebo‐controlled" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 (0.8%) of 239 enrolled persons did not receive the test drug and were excluded from analysis by the trialists |
| Selective reporting (reporting bias) | Low risk | Efficacy and safety outcomes were reported in detail |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Redman 1986.
| Methods | This was a double‐blind, placebo‐controlled, randomised trial | |
| Participants |
Inclusion criteria
Exclusion criteria
100 healthy adults participated in this trial; 84 participants returned their report forms, giving a response rate of 84% |
|
| Interventions |
The participants were given a single 0.2 ml intradermal injection of the study drug in the anterior midforearm |
|
| Outcomes |
The participants were given a report form to record the above data for 6 months following the treatment and were asked to post the form back to the researcher |
|
| Notes | Setting: a family practice Country: US Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...placebo‐controlled double‐blind" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The blinded participants assessed the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16 (16%) out of 100 participants did not return their report form |
| Selective reporting (reporting bias) | High risk | Only efficacy outcomes were assessed and reported. The SDs of the frequency of recurrences before and after treatment were not provided |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Rooney 1991.
| Methods | This was a double‐blind, placebo‐controlled, cross‐over trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 38 participants were enrolled |
|
| Interventions |
Each participant received 1 exposure with sunscreen and 1 with placebo, the order of administration being randomised (by Efron's biased coin method) and double blind. A solution of sunscreen or placebo was applied to the exposure site and was allowed to dry before exposure to UV light. The minimum time between UV exposures or between previous HSV recurrence and UV exposure was 3 weeks |
|
| Outcomes |
|
|
| Notes | Setting: 2 medical centres (the Clinical Centre of the National Institutes of Health and the University of California Los Angeles Hospital) Country: US Funding source: not mentioned, but Eclipse Laboratories provided the placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by Efron's biased coin method |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "double blind" Quote: "The study code was broken only after all UV exposures were completed" Quote: "As a control for the blinding investigators and patients were asked to guess which treatment was given 3 days after UV exposure" Quote: "In the assessment of the blinding, both investigators and patients could correctly identify placebo in over 80% of cases" Comment: although the trialists made efforts in blinding, placebo recipients had sunburn while none of the sunscreen recipients had sunburn. Participants and researchers might thus have known the assigned treatments |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "double blind" Quote: "The study code was broken only after all UV exposures were completed" Quote: "In the assessment of the blinding, both investigators and patients could correctly identify placebo in over 80% of cases" Comments: participants and researchers might have known the assigned treatments because of the presence of sunburn |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants (5%) out of 38 enrolled participants withdrew from the study after 1 exposure with placebo ‐ 1 because of pregnancy and 1 because of relocation for a new job. Another 1 was excluded from the analysis because of violation of the protocol. Thus, a total of 38 placebo and 35 sunscreen exposures were analysed |
| Selective reporting (reporting bias) | High risk | Only efficacy outcomes were assessed and reported |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Rooney 1993.
| Methods | This was a randomised, placebo‐controlled, cross‐over trial | |
| Participants |
Inclusion criteria
Exclusion criteria
56 people entered a pretreatment 4‐month observation phase. 22 participants who had 2 or more recurrences of herpes labialis were randomised |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Setting: a medical centre (the Clinical Centre of the National Institutes of Health) Country: US Funding source: Partly from Burroughs Wellcome Company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind" Comments: matched placebo provided by the pharmaceutical company was administered in the same regimen |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 (9.1%) out of 22 participants who received aciclovir in the first phase did not complete the study and were excluded from analysis |
| Selective reporting (reporting bias) | High risk | Only efficacy outcomes were reported |
| Other bias | High risk | There was no washout period between the 2 phases of the study. The trialists excluded recurrences that occurred during the first week of each treatment phase, but the duration might have been too short |
Russell 1978.
| Methods | This was a double‐blind placebo‐controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 99 participants were randomised, with 48 in the levamisole group and 51 in the placebo group. 27 participants did not complete the trial (19 in the levamisole group and 8 in the placebo group) |
|
| Interventions |
The treatment drugs were taken on 2 consecutive days each week for 6 months |
|
| Outcomes |
|
|
| Notes | Setting: a university hospital Country: Canada Funding source: supported in part by a grant from the Medical Research Council of Canada |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "a double‐blind, controlled trial" Quote: "The placebo and active drug were structurally identical and taken on two consecutive days each week" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcomes were assessed and reported by the participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 99 participants, 27 (27.2%) did not complete the trial and were excluded from the analysis, with 19 (39.6%) in the levamisole group and 8 (15.7%) in the placebo group |
| Selective reporting (reporting bias) | Low risk | Both efficacy and adverse outcomes were reported |
| Other bias | High risk | The baseline frequency of HSL in the levamisole group was higher than that in the placebo group (4.8 ± 2.7 versus 3.4 ± 1.8 during a 6‐month period before treatment) |
Schindl 1999.
| Methods | This was a randomised placebo‐controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 50 participants were enrolled, but 2 did not complete the study (1 each in the laser and placebo group). 48 participants completed the study, with 24 in each group |
|
| Interventions | All participants in both groups were treated by the same physician
|
|
| Outcomes |
|
|
| Notes | Setting: a university hospital Country: Austria Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All participants in both groups were treated by the same physician. Participants in both groups wore non‐transparent protection glasses during the procedure |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The evaluator was not aware of the study protocol" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two of 50 enrolled subjects did not complete the study: one patient of the placebo group discontinued because of time problems and one patient of the laser group had to undergo an appendectomy" |
| Selective reporting (reporting bias) | Unclear risk | Efficacy and adverse outcomes were reported |
| Other bias | High risk | No scheduled follow‐ups were planned, but the participants were told to return to the clinic at the time of recurrence |
Schädelin 1988.
| Methods | This was a randomised placebo‐controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 30 participants entered and completed the study, including 14 assigned to the aciclovir group and 16 to the placebo group |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Setting: a university hospital Country: Switzerland Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was used for assigning the participants |
| Allocation concealment (selection bias) | Low risk | The randomisation list was not revealed to the investigators until submission of the results |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The investigators and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled 30 participants completed the study |
| Selective reporting (reporting bias) | Low risk | Efficacy and adverse outcomes were reported |
| Other bias | High risk | Only 13 (43%) of the 30 participants had a history of herpes labialis |
Senti 2013.
| Methods | This was a randomised placebo‐controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 40 participants were randomised, including 20 assigned to the 2‐hydroxypropyl‐β‐cyclo dextrin (2‐HPβCD) group and 20 to the placebo group. Of them, 2 (10%) in the 2‐HPβCD group and 4 (20%) in the placebo group did not complete the study |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Setting: Clinical Trials Center Zurich Country: Switzerland Funding source: Devirex AG |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The investigators and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study remained blinded until after the database was unlocked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
|
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available on ClinicalTrials.gov (identifier: NCT00914745). The prespecified primary outcome (the number of herpes labialis relapse) was reported. However, the exact numerical data were not provided; the authors only provided the data in plots |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Spruance 1988.
| Methods | This was a randomised placebo‐controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
Study participants were stratified prospectively according to their self‐perceived risk of herpes labialis while skiing (> 50% or < 50% chance). 153 participants were enrolled, but 6 did not return for clinical evaluation. A total of 101 high‐risk participants (52 in the aciclovir group and 49 in the placebo group) and 46 low‐risk participants (23 in each group, respectively) completed the study and were analysed |
|
| Interventions |
Participants were instructed to take 2 200 mg capsules of the study medication twice a day, beginning 12 hours prior to their first anticipated sun exposure. Therapy was continued throughout the period of skiing, up to a maximum of 7 days. A standard sunscreen of sun protection factor 15 was provided to all participants, and frequent use was advised |
|
| Outcomes |
|
|
| Notes | Setting: 2 ski resorts Country: US Funding source: Burroughs Wellcome & Co. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Burroughs Wellcome & Co. provided 200 mg capsules of aciclovir (Zovirax®) and identical placebo capsules that were randomised among serially numbered bottles |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators were unaware of the assigned treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 out of 153 (3.9%) enrolled participants did not return for evaluation and were excluded from the analysis |
| Selective reporting (reporting bias) | Low risk | Efficacy and adverse outcomes were reported |
| Other bias | Low risk | A standard sunscreen of sun protection factor 15 was provided to all participants, and frequent use was advised. The trialists found no relation between the development of herpes and the potential confounding factors including skin type, pre‐existing tan, pre‐existing burn, facial hair, history of recent heavy sun exposure, history of skiing on the date of enrolment, frequency of herpes labialis, susceptibility to sun‐induced recurrences, hours of sun exposure during the treatment period, number of sunscreen applications during the treatment period, and degree of sunburn |
Spruance 1991a.
| Methods | An article reported 3 randomised trials included in this review, including 2 on oral aciclovir and 1 on topical aciclovir for prophylaxis of ultraviolet radiation (UVR)‐induced herpes labialis. We labelled the 3 included trials as Spruance 1991a, Spruance 1991b, and Spruance 1991c, respectively. The article also reported 1 trial on early oral aciclovir treatment begun 48 hours after UVR exposure, but we excluded the trial from this review as aciclovir was used as treatment | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 30 participants were enrolled in this trial, with an equal number of participants randomised to the active treatment and placebo groups, respectively |
|
| Interventions | Peroral study 1:
|
|
| Outcomes | The outcome data of the 2 peroral studies, Spruance 1991a and Spruance 1991b, were combined because of similar results
|
|
| Notes | Setting: university hospital (the University of Utah School of Medicine) Country: US Funding source: Burroughs Wellcome & Co. and National Institutes of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Gelatin capsules (Eli Lilly, Indianapolis) were filled with 200 mg of ACV from commercially available capsules (Burroughs Wellcome) or lactose placebo compound and randomly allocated to serially numbered bottles. The drug code for topical and peroral clinical trial materials was concealed from both patients and investigators until the end of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As mentioned above, the evaluating investigators were blinded to the treatments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or withdrawals were mentioned |
| Selective reporting (reporting bias) | High risk | Only efficacy outcomes were reported. The severity data were not reported |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Spruance 1991b.
| Methods | This was a randomised trial on oral aciclovir for prophylaxis of ultraviolet radiation (UVR)‐induced herpes labialis, which was reported along with Spruance 1991a and Spruance 1991c in the same article | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 36 participants were enrolled, with an equal number of participants randomised to the active treatment and placebo groups, respectively |
|
| Interventions | Peroral study 2:
|
|
| Outcomes | The outcome data of the 2 peroral studies, Spruance 1991a and Spruance 1991b, were combined because of similar results
|
|
| Notes | Setting: university hospital (the University of Utah School of Medicine) Country: US Funding source: Burroughs Wellcome & Co. and National Institutes of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Acyclovir 5% cream and placebo cream were provided in identically appearing 15‐g tubes by Burroughs Wellcome (Research Triangle Park, NC)... The drug code for topical and peroral clinical trial materials was concealed from both patients and investigators until the end of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As mentioned above, the evaluating investigators were blinded to the treatments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or withdrawals were mentioned |
| Selective reporting (reporting bias) | High risk | Only efficacy outcomes were reported. The severity data were not reported |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Spruance 1991c.
| Methods | This was a randomised trial on topical aciclovir for prophylaxis of ultraviolet radiation (UVR)‐induced herpes labialis, which was reported along with Spruance 1991a and Spruance 1991b in the same article | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 90 participants were enrolled, with an equal number of participants randomised to the active treatment and placebo groups, respectively |
|
| Interventions | Topical study:
|
|
| Outcomes | The outcome data of the 2 peroral studies were combined because of similar results
|
|
| Notes | Setting: university hospitals (the University of Utah School of Medicine, the University of Michigan School of Dentistry, and the Graduate School of Public Health, University of Pittsburg) Country: US Funding source: Burroughs Wellcome & Co. and National Institutes of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Acyclovir 5% cream and placebo cream were provided in identically appearing 15‐g tubes by Burroughs Wellcome (Research Triangle Park, NC)... The drug code for topical and peroral clinical trial materials was concealed from both patients and investigators until the end of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As mentioned above, the evaluating investigators were blinded to the treatments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or withdrawals were mentioned |
| Selective reporting (reporting bias) | High risk | Only efficacy outcomes were reported |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Spruance 1999.
| Methods | This was a double‐blind, dose‐ranging, placebo‐controlled, multicentre trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 243 participants who were randomised (60 in the famciclovir 125 mg group, 62 in the famciclovir 250 mg group, 61 in the famciclovir 500 mg group, and 60 in the placebo group) and took study medication comprised the intention‐to‐treat population |
|
| Interventions |
The participants received the study medication 3 times daily for 5 days beginning 48 hours after ultraviolet radiation exposure |
|
| Outcomes | The primary efficacy variables were:
The secondary variables included:
Adherence to the medication and adverse reactions were also assessed |
|
| Notes | Setting: 5 academic medical centres Country: US and Canada Funding source: SmithKline Beecham Pharmaceuticals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized in a double‐blind fashion to receive 1 of 3 doses of famciclovir or placebo" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study medication was provided as white film‐coated tablets containing 125, 250, or 500 mg of famciclovir or matching placebo. All tablets were identical in shape, weight, and color" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above, the evaluating investigators were blinded to the treatments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Compliance with study medication was excellent" Quote: "One placebo‐treated patient was withdrawn from the study due to adverse experiences (diarrhea, nausea) that occurred on therapy and were considered related to study medication" Comments: adherence to study medication was 100% in the 3 famciclovir groups and 95% in the placebo group |
| Selective reporting (reporting bias) | Low risk | Both efficacy and adverse outcomes were reported |
| Other bias | Unclear risk | There was insufficient information to permit judgement |
Thein 1984.
| Methods | This was a randomised, placebo‐controlled, cross‐over trial | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 26 participants were enrolled, with 15 in group A and 11 in group B (see below) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Setting: a university hospital Country: US Funding source: Baylor College of Dentistry research grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The blinded participants were the outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or withdrawals were mentioned |
| Selective reporting (reporting bias) | High risk | Only efficacy outcomes were reported |
| Other bias | High risk | There was no washout period between the 2 6‐month periods |
2‐HPβCD: 2‐hydroxypropyl‐β‐cyclo dextrin. ACV: aciclovir. GaAlAs: gallium‐aluminium‐arsenide. HIV: human immunodeficiency virus. HSL: herpes simplex labialis. HSV: herpes simplex virus. IFN: interferon. LV: LongoVital®. PABA: para‐aminobenzoic acid. PD: pentanediol. PEGs: polyethylene glycols. SDs: standard deviations. SPF: sun protection factor. UV: ultraviolet. UVA: ultraviolet A. UVB: ultraviolet B. UVR: ultraviolet radiation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alster 1999 | Only 19 out of the 99 participants had a history of HSL |
| Altorfer 1996 | This was a commentary, not a randomised trial |
| Armstrong | This was a review of 2 trials on the efficacy of interferon in preventing herpes simplex in renal transplant participants, not a randomised trial |
| Bernstein 2005 | Imiquimod cream was used as a treatment rather than a preventative intervention |
| Blough 1983 | This was a commentary, not a randomised trial |
| DeMaubeuge 1985 | This was an uncontrolled open trial, not a randomised trial |
| DiGiovanna 1984 | This was a trial on the therapeutic and preventive efficacy of lysine on recurrent herpes simplex infection. Only 4 out of the 20 participants had a history of herpes labialis |
| Donatini 2010 | The participants had the option to switch therapy each month according to their satisfaction during the 6‐month experiment |
| Dundarov 1994 | This was not a randomised trial |
| El‐Farrash 2003 | This was not a randomised trial |
| Fawcett 1983 | 4 (25%) out of the 16 participants had had herpes simplex infection in the genital area or buttocks |
| Hellgren 1983 | This was an open trial on the preventive efficacy of tromantadine in genital herpes, not a randomised trial |
| Jose 1980 | 21 (64%) of the 33 participants did not have HSL, but had genital herpes |
| Kalimo 1983 | 14 (24%) of the 58 participants did not have HSL, but had genital herpes or herpes involving other locations. The data on HSL could not be separated out from the 24% with genital herpes |
| Lacour 2000 | This was a commentary on an included trial (Schindl 1999) |
| Lamey 2000 | This was a trial on the therapeutic efficacy but not on the preventative efficacy of aciclovir cream |
| Lamura 1997 | This was not a randomised trial |
| Likar 1968 | A trial on the therapeutic efficacy but not on the preventative efficacy of 5‐carboxymethyl‐3‐p‐tolyl‐thiazolidine‐2,4‐dione‐2‐acetophenonehydrazone |
| Milman 1980 | This was a quasi‐randomised cross‐over trial, not a randomised trial. The participants were alternatively assigned to lysine or placebo for 12 weeks, then switched to the other treatment for another 12 weeks without a washout period |
| Mindel 1985 | This was not a randomised trial |
| Munoz 2012 | This was a RCT on the therapeutic efficacy of low‐level laser therapy. Recurrence was assessed as a follow‐up study |
| Myers 1975 | This was a RCT on the therapeutic efficacy of photodynamic therapy on herpes simplex infection including genital herpes and herpes on other non‐labial sites of the skin. Recurrence was assessed as a follow‐up study |
| NCT00913692 | The trial was terminated because of slow recruitment |
| Pedrazini 2007 | This was a case‐series study, not a randomised trial |
| Qadripur 1976 | This was a small controlled study with 36 out of 41 participants having a history of herpes labialis. No subgroup data on those with herpes labialis were provided, and whether randomisation was applied was unknown |
| Queiroz Carvalho 1976 | This was a case‐series study, not a randomised trial |
| Rosenthal 1992 | This was a commentary on an included trial (Rooney 1991) |
| Rowe 1978 | This was a trial on the therapeutic efficacy but not on the preventative efficacy of topically applied vidarabine 3% |
| Rowe 1980 | This was a trial on the therapeutic efficacy but not on the preventative efficacy of topically applied vidarabine 3% and 10% |
| Schmitt 1987 | This was a trial on the therapeutic efficacy but not on the preventative efficacy of topical alpha interferon |
| Siegel 1990 | This was a trial on the therapeutic efficacy but not on the preventative efficacy of a lip balm |
| Simon 1985 | This was a randomised trial on the efficacy of lysine in preventing recurrent herpes simplex labialis or genitalis in 31 participants. However, the authors did not report how many of the 31 participants had herpes labialis |
| Spruance 1979 | This was a trial on the therapeutic efficacy but not on the preventative efficacy of topical adenine arabinoside 5'‐monophosphate |
| Strand 2003 | This was a subgroup analysis on the occurrence of herpes labialis using the participants attending a randomised trial on valaciclovir in prevention of genital herpes transmission |
| Thomas 1985 | In this trial, not all of the 11 participants had herpes labialis: 2 had herpes labialis, 7 had herpes labialis and herpes involving 1 or more other sites (ear, cheek, nose, finger, or thigh), and 2 had herpes simplex on the finger |
| Viza 1985 | This was a case series including participants with labial and genital herpes |
| Weitgasser 1977 | This was not a randomised trial |
| Worrall 1996 | This was a commentary, not a randomised trial |
HSL: herpes simplex labialis. RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ISRCTN03397663.
| Trial name or title | Evaluation of the efficacy and safety of a sheabutter extract on cold sores (herpes simplex labialis) |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | 1 February 2005 |
| Contact information | Dr Phillip Cheras, Mater Health Services, 2nd Floor, Community Health Services Building, 39 Annerley Rd, South Brisbane, 4101, Australia E‐mail: philcheras@yahoo.com.au |
| Notes | www.controlled‐trials.com/ISRCTN03397663 |
NCT01225341.
| Trial name or title | A double‐blind, randomised, placebo controlled, cross‐over study to assess the safety and efficacy of botulinum toxin A injections as a preventative measure for herpes labialis |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | August 2010 |
| Contact information | Steven H Dayan, MD, Medical Director, DeNova Research, Water Tower Place, 845 N Michigan Avenue, Suite 923 E, Chicago, IL 60611, US E‐mail: selika@drdayan.com |
| Notes | www.clinicaltrials.gov/show/NCT01225341 |
NCT01902303.
| Trial name or title | Evaluation of cold sore treatments on UV‐induced cold sores |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | July 2013 |
| Contact information | Elsie Kohoot, Hill Top Research, Incorporated, US E‐mail: ekohoot@hill‐top.com |
| Notes | www.clinicaltrials.gov/ct2/show/NCT01902303 |
NCT01971385.
| Trial name or title | Safety and efficacy of squaric acid dibutylester for the treatment of herpes labialis (Squarex) |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | October 2013 |
| Contact information | Lynne Hermosilla, Massachusetts General Hospital, Boston, Massachusetts, US, 02114 E‐mail: harvardskinstudies@partners.org |
| Notes | www.clinicaltrials.gov/ct2/show/NCT01971385 |
CHF: chronic heart failure. CVA: cerebrovascular accident. EF: ejection fraction. HIV: human immunodeficiency virus. HSV: herpes simplex virus. IUD: intrauterine device. MD: Doctor of Medicine. MI: myocardial infarction. PI: principal investigator. SADBE: squaric acid dibutylester. TIA: transient ischemic attack. UV: ultraviolet.
Differences between protocol and review
We failed to conduct some analyses that we planned in the protocol as follows. Future updates may be different.
Because of lacking relevant data, we were unable to implement some methods planned in our protocol, including analysis of time‐to‐event outcomes and the unit of analysis issue for cluster‐randomised trials.
We failed to conduct the planned analyses dealing with missing data because of lacking adequate data, for example, the respective number of randomised participants and those who were lost to follow up in each group.
We did not assess reporting biases by using a funnel plot because of the limited number of trials for each intervention.
We did not perform the planned subgroup analysis and sensitivity analyses because of the lack of relevant data.
The following edits were made in response to comments from the referees.
We made some changes to the Background section.
We more clearly defined short‐ and long‐term use of interventions.
We clarified how our primary outcome of adverse effects and our secondary outcome of adherence was measured and added sentences to the Measures of treatment effect section.
In the Unit of analysis issues section, we revised the use of the term 'internally‐controlled' and that cross‐over and cluster‐randomised trials were eligible for inclusion.
We added 'Summary of findings' tables for our primary outcomes for each of our comparisons, which we did not originally plan at the time we wrote our protocol.
Contributions of authors
MC and PK conceived the review. CC was the contact person with the editorial base. CC co‐ordinated contributions from the co‐authors and wrote the final draft of the review. CC and SW screened papers against eligibility criteria, with FW available for arbitration. CC obtained data on ongoing and unpublished studies. CC and SW appraised the quality of papers. CC and SW extracted data for the review and sought additional information about papers. CC entered data into RevMan. CC and SW analysed and interpreted data. CC worked on the methods sections. CC drafted the clinical sections of the background and responded to the clinical comments of the referees. CC responded to the methodology and statistics comments of the referees. FD was the consumer co‐author and checked the review for readability and clarity, as well as ensuring that outcomes were relevant to consumers. CC is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
-
Chang Gung Memorial Hospital, Chiayi, Taiwan.
Provided funding (Chang Gung Research Project (CMRPG6B0551))
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group
Declarations of interest
Ching‐Chi Chi: nothing to declare. Shu‐Hui Wang: nothing to declare. Finola M Delamere: nothing to declare. Fenella Wojnarowska: nothing to declare. Mathilde C Peters: nothing to declare. Preetha P Kanjirath: nothing to declare.
Oliver Chosidow, who refereed this protocol, has acted as a consultant for BioAlliance Pharma, a company developing a long‐acting aciclovir formulation in the management of episodic therapy of cold sores.
Edited (no change to conclusions)
References
References to studies included in this review
Altmeyer 1991 {published data only}
- Altmeyer P, Wehrenberg O, Holzmann H, Paul E, Kimmig WK, Wassilew SW, et al. Treatment of recurrent herpes labialis using a herpes simplex Type‐I subunit vaccine. A prospective randomized double‐blind multicenter study (German). Hautarzt 1991;42(12):759‐63. [MEDLINE: ] [PubMed] [Google Scholar]
Baker 2003 {published data only}
- Baker D, Eisen D. Valacyclovir for prevention of recurrent herpes labialis: 2 double‐blind, placebo‐controlled studies. Cutis 2003;71(3):239‐42. [MEDLINE: ] [PubMed] [Google Scholar]
Bernstein 1994 {published data only}
- Bernstein DI, Rheins LA. Solar simulator‐induced herpes simplex labialis: Use in evaluating treatment with acyclovir plus 348U87. Antiviral Research 1994;23(3‐4):225‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bernstein 1997 {published data only}
- Bernstein DI, Schleupner CJ, Evans TG, Blumberg DA, Bryson Y, Grafford K, et al. Effect of foscarnet cream on experimental UV radiation‐induced herpes labialis. Antimicrobial Agents & Chemotherapy 1997;41(9):1961‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolla 1985 {published data only}
- Bolla K, Djawari D, Kokoschka EM, Petres J, Lidén S, Gonseth R, et al. Prevention of recurrences in frequently relapsing herpes labialis with thymopentin. A randomized double‐blind placebo‐controlled multicenter study. Survey of Immunologic Research 1985;4(Suppl 1):37‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Busch 2009 {published data only}
- Busch R, Graubaum HJ, Gruenwald J, Faergemann J. Therapeutic effect of 1,5‐pentanediol for herpes simplex labialis: a randomized, double‐blind, placebo‐controlled clinical trial. Advances in Therapy 2009;26(7):719‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Carvalho 2010 {published data only}
- Carvalho RR, Paula Eduardo F, Ramalho KM, Antunes JL, Bezinelli LM, Magalhães MH, et al. Effect of laser phototherapy on recurring herpes labialis prevention: an in vivo study. Lasers in Medical Science 2010;25(3):397‐402. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Duteil 1998 {published data only}
- Duteil L, Queille‐Roussel C, Loesche C, Verschoore M. Assessment of the effect of a sunblock stick in the prevention of solar‐simulating ultraviolet light‐induced herpes labialis. Journal of Dermatological Treatment 1998;9(1):11‐4. [EMBASE: 1998118784] [Google Scholar]
Gibson 1986 {published data only}
- Gibson JR, Klaber MR, Harvey SG, Tosti A, Jones D, Yeo JM. Prophylaxis against herpes labialis with acyclovir cream‐‐a placebo‐controlled study. Dermatologica 1986;172(2):104‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gilbert 2007 {published data only}
- Gilbert SC. Suppressive therapy versus episodic therapy with oral valacyclovir for recurrent herpes labialis: efficacy and tolerability in an open‐label, crossover study. Journal of Drugs in Dermatology 2007;6(4):400‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Ho 1984 {published data only}
- Ho M, Pazin GJ, Armstrong JA, Haverkos HS, Dummer JS, Jannetta PJ. Paradoxical effects of interferon on reactivation of oral infection with herpes simplex virus after microvascular decompression for trigeminal neuralgia. Journal of Infectious Diseases 1984;150(6):867‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miller 2004 {published data only}
- Miller CS, Avdiushko SA, Kryscio RJ, Danaher RJ, Jacob RJ. Effect of prophylactic valacyclovir on the presence of human herpesvirus DNA in saliva of healthy individuals after dental treatment. Journal of Clinical Microbiology 2005;43(5):2173‐80. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CS, Cunningham LL, Lindroth JE, Avdiushko SA. The efficacy of valacyclovir in preventing recurrent herpes simplex virus infections associated with dental procedures. Journal of the American Dental Association 2004;135(9):1311‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mills 1987 {published data only}
- Mills J, Hauer L, Gottlieb A, Dromgoole S, Spruance S. Recurrent herpes labialis in skiers. Clinical observations and effect of sunscreen. American Journal of Sports Medicine 1987;15(1):76‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Møller 1997 {published data only}
- Møller A, Andersen PL, Korsager B, Black FT. Yellow fever vaccination as prophylaxis of herpes labialis [Gul feber‐vaccination som profylakse ved herpes labialis]. Ugeskrift for Laeger 1997;159(15):2228‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Pazin 1979 {published data only}
- Haverkos HW, Pazin GJ, Armstrong JA, Ho M. Follow‐up of interferon treatment of herpes simplex. New England Journal of Medicine 1980;303(12):699‐700. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pazin GJ, Armstrong JA, Lam MT, Tarr GC, Jannetta PJ, Ho M. Prevention of reactivated herpes simplex infection by human leukocyte interferon after operation on the trigeminal root. New England Journal of Medicine 1979;301(5):225‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pedersen 2001 {published data only}
- Pedersen A. LongoVital® and herpes labialis: a randomised, double‐blind, placebo‐controlled study. Oral Diseases 2001;7(4):221‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Pedersen A. Longovital® and herpes labialis: A randomized, double‐blind, placebo‐controlled study (Abstract). Joint Meeting of the Academy of Oral Medicine and the European Academy of Oral Medicine. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, & Endodontics 2001;91(4):428. [CENTRAL: CN‐00396378] [Google Scholar]
Pfitzer 2005 {published data only}
- Pfitzer BE, Clark K, Revenstorf D. Medical hypnosis in cases of herpes labialis improves propensity for recurrence. A pilot study [Hypnotherapie bei Herpes labialis verbessert Rezidivneigung]. Hautarzt 2005;56(6):562‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raborn 1997 {published data only}
- Raborn GW, Martel AY, Grace MG, McGaw WT. Herpes labialis in skiers: randomized clinical trial of acyclovir cream versus placebo. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, & Endodontics 1997;84(6):641‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raborn 1998 {published data only}
- Raborn GW, Martel AY, Grace MG, McGaw WT. Oral acyclovir in prevention of herpes labialis. A randomized, double‐blind, multi‐centered clinical trial. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 1998;85(1):55‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Redman 1986 {published data only}
- Redman JC, Davis LE, McLaren LC, Skipper BJ. Intradermal gamma globulin for herpes labialis? Results of a double‐blind study. Postgraduate Medicine 1986;79(8):315‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rooney 1991 {published data only}
- Rooney JF, Bryson Y, Mannix ML, Dillon M, Wohlenberg CR, Banks S, et al. Prevention of ultraviolet‐light‐induced herpes labialis by sunscreen. Lancet 1991;338(8780):1419‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rooney 1993 {published data only}
- Rooney JF, Straus SE, Mannix ML, Wohlenberg CR, Alling DW, Dumois JA, et al. Oral acyclovir to suppress frequently recurrent herpes labialis. A double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1993;118(4):268‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Russell 1978 {published data only}
- Russell AS, Brisson E, Grace M. A double‐blind, controlled trial of levamisole in the treatment of recurrent herpes labialis. Journal of Infectious Diseases 1978;137(5):597‐600. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Russell AS, Brisson E, Miller C, Grace M. A double blind controlled trial of levamisole in subjects with recurrent cold sores: a clinical and immunological evaluation. Clinical Research 1977;25(5):697A. [EMBASE: 0978248515] [Google Scholar]
Schädelin 1988 {published data only}
- Schädelin J, Schilt HU, Rohner M. Preventive therapy of herpes labialis associated with trigeminal surgery. American Journal of Medicine 1988;85(2A):46‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Schindl 1999 {published data only}
- Rallis TM. Low‐intensity laser therapy for recurrent herpes labialis. Journal of Investigative Dermatology 2000;115(1):131‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schindl A, Neumann R. Low‐intensity laser therapy is an effective treatment for recurrent herpes simplex infection. Results from a randomized double‐blind placebo‐controlled study. Journal of Investigative Dermatology 1999;113(2):221‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Senti 2013 {published data only}
- Senti G, Iannaccone R, Graf N, Felder M, Tay F, Kündig T. A randomized, double‐blind, placebo‐controlled study to treat the efficacy of topical 2‐hydroxypropyl‐Beta‐cyclodextrin in the prophylaxis of recurrent herpes labialis. Dermatology 2013;226(3):247–252. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Spruance 1988 {published data only}
- Spruance SL, Hamill ML, Hoge WS, Davis LG, Mills J. Acyclovir prevents reactivation of herpes simplex labialis in skiers. JAMA 1988;260(11):1597‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Spruance 1991a {published data only}
- Spruance SL, Freeman DJ, Stewart JC, McKeough MB, Wenerstrom LG, Krueger GG, et al. The natural history of ultraviolet radiation‐induced herpes simplex labialis and response to therapy with peroral and topical formulations of acyclovir. Journal of Infectious Diseases 1991;163(4):728‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Spruance 1991b {published data only}
- Spruance SL, Freeman DJ, Stewart JC, McKeough MB, Wenerstrom LG, Krueger GG, et al. The natural history of ultraviolet radiation‐induced herpes simplex labialis and response to therapy with peroral and topical formulations of acyclovir. Journal of Infectious Diseases 1991;163(4):728‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Spruance 1991c {published data only}
- Spruance SL, Freeman DJ, Stewart JC, McKeough MB, Wenerstrom LG, Krueger GG, et al. The natural history of ultraviolet radiation‐induced herpes simplex labialis and response to therapy with peroral and topical formulations of acyclovir. Journal of Infectious Diseases 1991;163(4):728‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Spruance 1999 {published data only}
- Spruance SL, Rowe NH, Raborn GW, Thibodeau EA, D'Ambrosio JA, Bernstein DI. Peroral famciclovir in the treatment of experimental ultraviolet radiation‐induced herpes simplex labialis: A double‐blind, dose‐ranging, placebo‐controlled, multicenter trial. Journal of Infectious Diseases 1999;179(2):303‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thein 1984 {published data only}
- Thein DJ, Hurt WC. Lysine as a prophylactic agent in the treatment of recurrent herpes simplex labialis. Oral Surgery, Oral Medicine, Oral Pathology 1984;58(6):659‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alster 1999 {published data only}
- Alster TS, Nanni CA. Famciclovir prophylaxis of herpes simplex virus reactivation after laser skin resurfacing. Dermatologic Surgery 1999;25(3):242‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Altorfer 1996 {published data only}
- Altorfer R. Herpes labialis ‐ An annoying detail? Double‐blind study with penciclovir [Herpes labialis ‐ Eine lastige Bagatelle? Doppelblindstudie mit Penciclovir]. Ars Medici 1996;86(15):918‐9. [EMBASE: 1996302882] [Google Scholar]
Armstrong {published data only}
- Armstrong JA. The effect of interferon in herpes simplex infections in man. Texas Reports on Biology & Medicine 1981‐1982;41:571‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Bernstein 2005 {published data only}
- Bernstein DI, Spruance SL, Arora SS, Schroeder JL, Meng TC. Evaluation of imiquimod 5% cream to modify the natural history of herpes labialis: a pilot study. Clinical Infectious Diseases 2005;41(6):808‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blough 1983 {published data only}
- Blough HA. Topical 2‐deoxy‐D‐glucose for herpes simplex. Journal of the American Academy of Dermatology 1983;8(3):422‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DeMaubeuge 1985 {published data only}
- DeMaubeuge J, Haneke E, Djawari D, Wolff K, Stingl G, Molin L, et al. Thymopentin treatment of herpes simplex infections. An open, monitored, multicenter study. Survey of Immunologic Research 1985;4(Suppl 1):30‐6. [MEDLINE: ] [PubMed] [Google Scholar]
DiGiovanna 1984 {published data only}
- DiGiovanna JJ, Blank H. Failure of lysine in frequently recurrent herpes simplex infection. Treatment and prophylaxis. Archives of Dermatology 1984;120(1):48‐51. [MEDLINE: ] [PubMed] [Google Scholar]
Donatini 2010 {published data only}
- Donatini B. Prevention of recurrence of herpes virus infection with Ganoderma lucidum plus Coriolus versicolor [Prevention des recurrences d'herpes par l'association Ganoderma lucidum + Coriolus versicolor]. Phytotherapie 2010;8(4):259‐60. [EMBASE: 2010481726] [Google Scholar]
Dundarov 1994 {published data only}
- Dundarov S, Andonov P. Seventeen years of application of herpes vaccines in Bulgaria. Acta Virologica 1994;38(4):205‐8. [MEDLINE: ] [PubMed] [Google Scholar]
El‐Farrash 2003 {published data only}
- El‐Farrash MA, Youssef JM, El‐Mongy SE. Allopurinol as a potential therapeutic agent for recurrent herpes labialis. Journal of Medical & Dental Sciences 2003;50(2):147‐54. [MEDLINE: ] [PubMed] [Google Scholar]
Fawcett 1983 {published data only}
- Fawcett HA, Wansbrough‐Jones MH, Clark AE, Leigh IM. Prophylactic topical acyclovir for frequent recurrent herpes simplex infection with and without erythema multiforme. British Medical Journal 1983;287(6395):798‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hellgren 1983 {published data only}
- Hellgren L Hermann LS. Tromantadine hydrochloride in the treatment of herpes simplex. Results of a double‐blind therapeutic trial and an open prophylactic investigation. Dermatologica 1983;167(5):267‐72. [MEDLINE: ] [PubMed] [Google Scholar]
Jose 1980 {published data only}
- Jose DG, Minty CC. Levamisole in patients with recurrent herpes infection. Medical Journal of Australia 1980;2(7):390‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kalimo 1983 {published data only}
- Kalimo KO, Joronen IA, Havu VK. Failure of oral inosiplex treatment of recurrent herpes simplex virus infections. Archives of Dermatology 1983;119(6):463‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Lacour 2000 {published data only}
- Lacour J. Low‐power laser and recurrent labial herpes [Laser de basse intensite et herpes labial recurrent]. Annales de Dermatologie et de Venereologie 2000;127(6‐7):652‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Lamey 2000 {published data only}
- Lamey PJ, Biagioni PA. Evaluation of Zovirax 5% cream in subjects with recurrent herpes labialis (Abstract no: 2690). IADR abstracts. Journal of Dental Research 2000;79(Spec Iss):480. [CENTRAL: CN‐00793708] [Google Scholar]
Lamura 1997 {published data only}
- Lamura A, Castro A, Lamura G, Garriga EE, Castro YK. Effects of sunscreens on an emplient base used in 100 cases of recurrent herpes labialis. Journal of Dental Research 1997;76(1 Suppl):236. [Google Scholar]
Likar 1968 {published data only}
- Likar M, Schauer P, Tišler M. An effective antiherpetic drug?. Lancet 1968;2(7571):776. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Milman 1980 {published data only}
- Milman N, Scheibel J, Jessen O. Lysine prevention in recurrent labial herpes simplex [Lysinprofylakse af recidiverende herpes simplex labialis]. Ugeskrift for Laeger 1980;142(19):1202‐3. [MEDLINE: ] [PubMed] [Google Scholar]
- Milman N, Scheibel J, Jessen O. Lysine prophylaxis in recurrent herpes simplex labialis: a double‐blind, controlled crossover study. Acta Dermato‐Venereologica 1980;60(1):85‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Mindel 1985 {published data only}
- Mindel A. Inosine pranobex for mucocutaneous herpes. Lancet 1985;1(8429):631‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Munoz 2012 {published data only}
- Muñoz Sanchez PJ, Capote Femenías JL, Díaz Tejeda A, Tuner J. The effect of 670‐nm low laser therapy on herpes simplex type 1. Photomedicine & Laser Surgery 2012;30(1):37‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Myers 1975 {published data only}
- Myers MG, Oxman MN, Clark JE, Arndt KA. Failure of neutral‐red photodynamic inactivation in recurrent herpes simplex virus infections. New England Journal of Medicine 1975;293(19):945‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT00913692 {unpublished data only}
- NCT00913692. A randomized double‐blind control‐comparison crossover trial of oral glutamine to suppress frequently recurrent herpes labialis. clinicaltrials.gov/show/NCT00913692 (accessed 8 October 2014).
Pedrazini 2007 {published data only}
- Pedrazini MC, Cury PR, Araujo VC, Wassall T. The effect of lysine in the incidence and period of healing of recurrent herpes labialis [Efeito da lisina na incidência e duração das lesões de herpes labial recorrente]. Revista Gaúcha de Odontologia 2007;55(1):7‐10. [Google Scholar]
Qadripur 1976 {published data only}
- Qadripur SA. Controlled clinical trial of the herpes antigen 'lupidon' against placebo [ERGEBNISSE EINER KONTROLLIERTEN KLINISCHEN STUDIE MIT DEM HERPES ANTIGEN LUPIDON GEGEN PLACEBO]. Aktuelle Dermatologie 1976;2(3):131‐6. [EMBASE: 0977155159] [Google Scholar]
Queiroz Carvalho 1976 {published data only}
- Queiroz Carvalho CA, Kinue Otuki T, Elizabeth Poli M, Nogueira JL, Guerrero J. Recurrent herpes infections and levamisole. Revista Latinoamericana de Microbiologia 1976;18(4):225‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Rosenthal 1992 {published data only}
- Rosenthal D. Prevention of UV‐light‐induced herpes labialis by sunscreen. Annals of Internal Medicine 1992;116(Suppl 3):82. [EMBASE: 1993034838] [Google Scholar]
Rowe 1978 {published data only}
- Rowe NH, Brooks SL, Young SK. A clinical trial of topically applied 3% vidarabine against recurrent herpes labialis. Journal of Dental Research 1978;57(spec. A):No. 482. [EMBASE: 0978331762] [DOI] [PubMed] [Google Scholar]
Rowe 1980 {published data only}
- Rowe NH, Brookes SL, Spencer J. A clinical trial of topically applied 3 and 10 percent vidarabine against recurrent herpes labialis (Abstract 236). Journal of Dental Research 1980;59(Spec Issue B):946. [CENTRAL: CN‐00453643] [Google Scholar]
Schmitt 1987 {published data only}
- Schmitt J, Rees T. Use of topical alpha interferon on recurrent herpetic infections (Abstract 380). Journal of Dental Research 1987;66(1 Suppl):154. [Google Scholar]
Siegel 1990 {published data only}
- Siegel MA, Kutcher M, Romberg E, Wilson M. Lip balm efficacy in the treatment of recurrent herpes labialis (Abstract 1127). Journal of Dental Research 1990;69(1 Suppl):249. [CENTRAL: CN‐00432855] [Google Scholar]
Simon 1985 {published data only}
- Simon CA, Melle GD, Ramelet AA. Failure of lysine in frequently recurrent herpes simplex infection. Archives of Dermatology 1985;121(2):167‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Spruance 1979 {published data only}
- Spruance SL, Crumpacker CS, Haines H, Bader C, Mehr K, MacCalman J, et al. Ineffectiveness of topical adenine arabinoside 5'‐monophosphate in the treatment of recurrent herpes simplex labialis. New England Journal of Medicine 1979;300(21):1180‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strand 2003 {published data only}
- Strand A. Once‐daily valaciclovir for the prevention of recurrent herpes labialis (cold sores) (Abstract FC2‐4). The 12th Congress of the European Academy of Dermatology and Venereology. Barcelona, Spain 15‐18th October 2003. Journal of the European Academy of Dermatology & Venereology 2003;17(Suppl 3):105‐6. [Google Scholar]
Thomas 1985 {published data only}
- Meyrick Thomas RH, Dodd HJ, Yeo JM, Kirby JD. Oral acyclovir in the suppression of recurrent non‐genital herpes simplex virus infection. British Journal of Dermatology 1985;113(6):731‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Viza 1985 {published data only}
- Viza D, Vich JM, Phillips J, Rosenfeld F. Orally administered specific transfer factor for the treatment of herpes infections. Lymphokine Research 1985;4(1):27‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Weitgasser 1977 {published data only}
- Weitgasser H. Controlled clinical trial of the proprietary herpes antigens LUPIDON H and LUPIDON G [Kontrollierte klinische Studie mit den Herpes‐Antigenen Lupidon H und Lupidon G]. Zeitschrift für Hautkrankheiten 1977;52(11):625‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Worrall 1996 {published data only}
- Worrall G. Acyclovir in recurrent herpes labialis. BMJ 1996;312(7022):6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN03397663 {unpublished data only}
- ISRCTN03397663. Evaluation of the efficacy and safety of a Sheabutter extract on cold sores (herpes simplex labialis). www.controlled‐trials.com/ISRCTN03397663 (accessed 8 October 2014).
NCT01225341 {unpublished data only}
- NCT01225341. Botulinum toxin A for herpes labialis. clinicaltrials.gov/show/NCT01225341 (accessed 8 October 2014).
NCT01902303 {unpublished data only}
- NCT01902303. Evaluation of cold sore treatments on UV induced cold sores. clinicaltrials.gov/show/NCT01902303 (accessed 8 October 2014).
NCT01971385 {unpublished data only}
- NCT01971385. Safety and efficacy of squaric acid dibutyl ester for the treatment of herpes labialis (Squarex). clinicaltrials.gov/show/NCT01971385 (accessed 8 October 2014).
Additional references
Celik 2013
- Celik M, Sucakli MH, Kirecci E, Ucmak H, Ekerbicer HC, Ozturk P. Recurrent herpes labialis among health school students in Kahramanmaraş, Turkey: A cross‐sectional survey. Dermatologica Sinica 2013;31(2):64‐67. [EMBASE: 2013334713] [Google Scholar]
Chi 2011
- Chi CC, Kirtschig G, Baldo M, Brackenbury F, Lewis F, Wojnarowska F. Topical interventions for genital lichen sclerosus. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008240.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chi 2012
- Chi CC, Wang SH, Peters MC, Kanjirath PP, Delamere FM, Wojnarowska F. Interventions for prevention of herpes simplex labialis (cold sores on the lips). Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fatahzadeh 2007
- Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. Journal of the American Academy of Dermatology 2007;57(5):737‐63. [EMBASE: 2007495455] [DOI] [PubMed] [Google Scholar]
Glenny 2009
- Glenny AM, Fernandez Mauleffinch LM, Pavitt S, Walsh T. Interventions for the prevention and treatment of herpes simplex virus in patients being treated for cancer. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006706.pub2] [DOI] [PubMed] [Google Scholar]
GSK 2008
- GlaxoSmithKline. Zovirax cream patient information leaflet. Patient Information Leaflet 2008.
Higgins 1993
- Higgins CR, Schofield JK, Tatnall FM, Leigh IM. Natural history, management and complications of herpes labialis. Journal of Medical Virology 1993;41(Suppl 1):22‐26. [EMBASE: 1993290493] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration. Available from www.cochrane‐handbook.org..
Joseph 1985
- Joseph R, Rose FC. Cluster headache and herpes simplex: an association?. British Medical Journal 1985;290(6482):1625‐6. [EMBASE: 1985162634] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kullavanijaya 2005
- Kullavanijaya P, Lim HW. Photoprotection. Journal of the American Academy of Dermatology 2005;52(6):937‐58. [EMBASE: 2005237865] [DOI] [PubMed] [Google Scholar]
Leung 2014
- Leung DY, Guttman‐Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. Journal of Allergy and Clinical Immunology 2014;134(4):769‐79. [PUBMED: 25282559] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marques 2003
- Marques AR, Straus SE. Herpes simplex. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI editor(s). Fitzpatrick's Dermatology in General Medicine. 6th Edition. Vol. II, New York: McGraw‐Hill, 2003:2059‐70. [Google Scholar]
Nasser 2008
- Nasser M, Fedorowicz Z, Khoshnevisan MH, Shahiri Tabarestani M. Acyclovir for treating primary herpetic gingivostomatitis. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006700.pub2] [DOI] [PubMed] [Google Scholar]
Opstelten 2008
- Opstelten W, Neven AK, Eekhof J. Treatment and prevention of herpes labialis. Canadian Family Physician 2008;54(12):1683‐7. [EMBASE: 2009002597] [PMC free article] [PubMed] [Google Scholar]
Rahimi 2012
- Rahimi H, Mara T, Costella J, Speechley M, Bohay R. Effectiveness of antiviral agents for the prevention of recurrent herpes labialis: a systematic review and meta‐analysis. Oral Surgery, Oral Medicine, Oral Pathology & Oral Radiology 2012;113(5):618‐27. [PUBMED: 22668620] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rystedt 1986
- Rystedt I, Strannegard IL, Strannegard O. Recurrent viral infections in patients with past or present atopic dermatitis. British Journal of Dermatology 1986;114(5):575‐82. [EMBASE: 1986149942] [DOI] [PubMed] [Google Scholar]
Shiohara 2013
- Shiohara T. The role of viral infection in the development of severe drug eruptions. Dermatologica Sinica 2013;31(4):205‐10. [DOI: 10.1016/j.dsi.2013.09.003] [DOI] [Google Scholar]
Wahie 2007
- Wahie S, Lloyd JJ, Farr PM. Sunscreen ingredients and labelling: a survey of products available in the UK. Clinical & Experimental Dermatology 2007;32(4):359‐64. [PUBMED: 17376206] [DOI] [PubMed] [Google Scholar]
Worrall 2009
- Worrall G. Herpes labialis 23 September 2009 (based on February 2009 search) BMJ Clinical Evidence. clinicalevidence.bmj.com/x/systematic‐review/1704/overview.html (accessed 8 October 2014):1704.


