Abstract
Background
Randomised controlled trials (RCTs) show that breastfeeding newborn infants during painful procedures reduces pain. Mechanisms are considered to be multifactorial and include sucking, skin‐to‐skin contact, warmth, rocking, sound and smell of the mother, and possibly endogenous opiates present in the breast milk.
Objectives
To determine the effect of breastfeeding on procedural pain in infants beyond the neonatal period (first 28 days of life) up to one year of age compared to no intervention, placebo, parental holding, skin‐to‐skin contact, expressed breast milk, formula milk, bottle feeding, sweet‐tasting solutions (e.g. sucrose or glucose), distraction, or other interventions.
Search methods
We searched the following databases to 18 February 2016: the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library), MEDLINE including In‐Process & Other Non‐Indexed Citations (OVID), Embase (OVID), PsycINFO (OVID), and CINAHL (EBSCO); the metaRegister of Controlled Trials (mRCT), ClinicalTrials.gov (clinicaltrials.gov), and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/) for ongoing trials.
Selection criteria
We included RCTs and quasi‐RCTs involving infants aged 28 days postnatal to 12 months and receiving breastfeeding while undergoing a painful procedure. Comparators included, but were not limited to, oral administration of water, sweet‐tasting solutions, expressed breast or formula milk, no intervention, use of pacifiers, positioning, cuddling, distraction, topical anaesthetics, and skin‐to‐skin care. Procedures included, but were not limited to: subcutaneous or intramuscular injection, venipuncture, intravenous line insertion, heel lance, and finger lance. We applied no language restrictions.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently considered trials for inclusion in the review, assessed risk of bias, and extracted data. The main outcome measures were behavioural or physiological indicators and composite pain scores, as well as other clinically important outcomes reported by the authors of included studies. We pooled data for the most comparable outcomes and where data from at least two studies could be included. We used mean difference (MD) with 95% confidence interval (CI), employing a random‐effects model for continuous outcomes measured on the same scales. For continuous outcomes measured on different scales, we pooled standardised mean differences (SMDs) and associated 95% CIs. For dichotomous outcomes, we planned to pool events between groups across studies using risk ratios (RRs) and 95% CIs. However, as insufficient studies reported dichotomous outcomes, we did not pool such events. We assessed the evidence using GRADE and created a 'Summary of findings' table.
Main results
We included 10 studies with a total of 1066 infants. All studies were conducted during early childhood immunisation. As the breastfeeding intervention cannot be blinded, we rated all studies as being at high risk of bias for blinding of participants and personnel. We assessed nine studies as being at low risk of bias for incomplete outcome data. In addition, we rated nine studies as high risk for blinding of outcome assessment. We scored risk of bias related to random sequence generation, allocation concealment, and selective reporting as unclear for the majority of the studies due to lack of information.
Our primary outcome was pain. Breastfeeding reduced behavioural pain responses (cry time and pain scores) during vaccination compared to no treatment, oral water, and other interventions such as cuddling, oral glucose, topical anaesthetic, massage, and vapocoolant. Breastfeeding did not consistently reduce changes in physiological indicators, such as heart rate. We pooled data for duration of cry from six studies (n = 547 infants). Breastfeeding compared to water or no treatment resulted in a 38‐second reduction in cry time (MD ‐38, 95% CI ‐50 to ‐26; P < 0.00001). The quality of the evidence according to GRADE for this outcome was moderate, as most infants were 6 months or younger, and outcomes may be different for infants during their 12‐month immunisation. We pooled data for pain scores from five studies (n = 310 infants). Breastfeeding was associated with a 1.7‐point reduction in standardised pain scores (SMD ‐1.7, 95% CI ‐2.2 to ‐1.3); we considered this evidence to be of moderate quality as data were primarily from infants younger than 6 months of age. We could pool heart rate data following injections for only two studies (n = 186); we considered this evidence to be of low quality due to insufficient data. There were no differences between breastfeeding and control (MD ‐3.6, ‐23 to 16).
Four of the 10 studies had more than two study arms. Breastfeeding was more effective in reducing crying duration or pain scores during vaccination compared to: 25% dextrose and topical anaesthetic cream (EMLA), vapocoolant, maternal cuddling, and massage.
No included studies reported adverse events.
Authors' conclusions
We conclude, based on the 10 studies included in this review, that breastfeeding may help reduce pain during vaccination for infants beyond the neonatal period. Breastfeeding consistently reduced behavioural responses of cry duration and composite pain scores during and following vaccinations. However, there was no evidence that breastfeeding had an effect on physiological responses. No studies included in this review involved populations of hospitalised infants undergoing other skin‐breaking procedures. Although it may be possible to extrapolate the review results to this population, further studies of efficacy, feasibility, and acceptability in this population are warranted.
Plain language summary
Does breastfeeding reduce vaccination pain in babies aged 1 to 12 months?
Bottom line
We found that breastfeeding before and during vaccination injections helped to reduce pain in most babies up to the age of one year.
Background
Needles are used for babies' early childhood vaccinations and medical care during childhood illnesses. These are essential, but painful. They cause distress for the babies and often their parents/caregivers, and can result in future anxiety and fear about needles. Breastfeeding during blood tests in newborn babies reduces pain. Breastfeeding when possible and feasible may also help to comfort babies and reduce their pain beyond the newborn period and throughout infancy.
Study characteristics
In February 2016 we searched the medical literature for studies examining the effectiveness of breastfeeding babies 1 to 12 months old during the use of needles. We compared effectiveness of breastfeeding in reducing pain (as scored by crying time and pain scores), to holding, babies lying flat, or the giving of water or sweet solutions. We found 10 studies with a total of 1066 infants. All studies examined if breastfeeding reduced pain during vaccinations.
Key results
Breastfeeding reduced crying in young babies having vaccinations. On average, breastfed babies cried for 38 seconds less than babies who were not breastfed (6 studies; 547 infants; moderate‐quality evidence), and pain scores were significantly lower (5 studies; 310 infants; moderate‐quality evidence).
No studies reported on any harm (very low‐quality evidence). We could draw no conclusions on risk of harm while breastfeeding healthy babies during vaccination.
Going forward: if mothers are breastfeeding, it could be considered when possible for babies during vaccinations. More evidence is needed to learn if breastfeeding helps older babies and babies in hospital during blood work or procedures such as insertion of drips.
Quality of the evidence
The quality of the evidence was moderate for crying time and pain scores. Most studies included younger infants aged 1 to 6 months. Further research including older infants up to 12 months of age may change our conclusions. In addition, the studies evaluated the effects of breastfeeding during vaccination. We do not know whether breastfeeding helps sick babies aged 1 to 12 months in hospital during blood sampling or drip insertion.
Summary of findings
Summary of findings 1. Breastfeeding compared with other interventions, oral water, or no treatment for pain during vaccination in infants 1 to 12 months.
| Breastfeeding compared with other interventions, oral water, or no treatment for pain during vaccination in infants 1 to 12 months | ||||
|
Patient or population: Infants 1 to 12 months during vaccination Settings: Diverse community settings Intervention: Breastfeeding Comparison: Other interventions, such as cuddling, sweet solutions, or placebo (oral water) or no treatment | ||||
| Outcomes | No of Participants (studies) | Effect Estimates and 95% CI | Quality of the evidence (GRADE) | Comments |
|
Cry duration Seconds cry time during procedure or proportion of crying during procedure. Crying was measured during and up to 3 minutes following completion of vaccination. |
547 (6 studies) | MD (seconds) ‐38 ( ‐49.84 to 26.35) |
Moderate | Not further downgraded: Breastfeeding consistently resulted in a reduction of crying time. However, as most trials included infants aged 1 to 6 months, further research including older infants up to 12 months of age may have an important impact on our confidence in the estimate of effect and may change the estimate. |
|
All pain scores during injection Due to range of pain scores used, data analysed using SMDs. Pain scores were measured during and up to 3 minutes following completion of vaccination. |
310 (5 studies) | SMD ‐1.7 ( ‐2.2 to ‐1.3) |
Moderate | Downgraded once: Breastfeeding consistently resulted in a reduction of pain scores. However, only 5 studies and 310 infants were included, and most of the trials included the younger infants aged 1 to 6 months. Further research including older infants up to 12 months of age and larger sample sizes may have an important impact on our confidence in the estimate of effect. |
|
NIPS score during injection The NIPS was measured during and up to 3 minutes following completion of vaccination. |
174 (3 studies) | MD ‐1.89 (‐2.55 to ‐1.24) | Low | Not further downgraded: Grade of evidence already taken into account. Only 3 studies and 174 infants were included. Further studies using this pain assessment score may lead to more certainty and have an important impact on our confidence in the estimate of effect. |
|
Heart rate after injection Heart rate in the period following completion of the injections was measured in 2 studies. |
186 (2 studies) | MD (beats per minute) ‐3.6 ( ‐23 to 16) |
Low | Not further downgraded: Grade of evidence already taken into account. Breastfeeding did not have an effect on heart rate change from baseline. As only 2 studies were included in this outcome, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. |
| Adverse events | No studies reported on adverse events. | ‐ | Very low | No studies reported on outcomes such as coughing or gagging. |
| CI: confidence interval; MD: mean difference; NIPS: Neonatal Infant Pain Scale; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
Background
Infants and children require needle‐related painful procedures for scheduled childhood immunisations (PHA Canada 2014), as well as medical procedures performed for diagnostic and treatment purposes during the course of childhood illnesses (Johnston 2011). Such procedures are known to be painful, causing distress at the time of the procedure and for many children, anxiety and fear during subsequent needle‐related procedures (Schechter 2007; Taddio 2007; Taddio 2009; Wright 2009), and altered pain responses later in life (Taddio 2005). Fear of the pain associated with immunisations, with subsequent fear of needles, has been shown to be one of the reasons why parents do not complete their infants' recommended immunisation schedule (Mills 2005; Schechter 2007; Taddio 2007; Taddio 2009; Wright 2009). It is therefore imperative that effective pain management strategies be consistently used for infants and children in diverse settings where needle‐related painful procedures are performed.
Recently conducted systematic reviews of pain management strategies in the newborn period demonstrated that breastfeeding, in Shah 2012, and sweet solutions of sucrose, in Stevens 2013, and glucose, in Bueno 2013, reduced behavioural responses and composite pain scores during painful procedures. In addition, systematic reviews of sweet‐tasting solutions beyond the neonatal period up to one year of age demonstrated analgesic effects during needle‐related painful procedures when compared to water or no treatment (Harrison 2010a; Kassab 2012). There is a need for this evidence relating to analgesic effects of breastfeeding beyond the newborn period to be systematically reviewed on an ongoing basis to critically evaluate the effectiveness of this intervention in infants up to one year of age.
Description of the condition
Studies of pain management strategies used during commonly performed needle‐related procedures show inconsistent use of recommended interventions (Harrison 2013; Johnston 2011; Stevens 2011; Taddio 2007). However, it is known that untreated or poorly treated procedural pain has negative effects, including infant and parental distress at the time of the procedure, with the risk of longer‐term fear of needles (Schechter 2007; Taddio 1995; Taddio 2007; Taddio 2009; Wright 2009).
Description of the intervention
Breastfeeding commenced prior to the painful procedure, and continuing throughout the procedure until completion. High‐quality evidence from randomised controlled trials (RCTs) and systematic reviews supports the role of breastfeeding in reducing procedural pain during the neonatal period (first 28 days of life). Shah and colleagues conducted a systematic review of 20 RCTs or quasi‐RCTs evaluating breastfeeding (10 studies) or supplemental breast milk placed on the tongue or given through gastric tube (10 studies) during heel lance or venipuncture in newborn infants (Shah 2012). The authors concluded that breastfeeding effectively reduced behavioural responses, including crying duration and total crying time, facial expressions and pain scores, as well the physiological response of heart rate, compared to positioning (swaddled and nursed in a crib), holding by the mother, placebo, or no intervention.
How the intervention might work
Several elements are postulated to contribute to the analgesic effects of breastfeeding. These include skin‐to‐skin contact, sight, sound and smell of the mother, sucking, distraction, pleasant and slightly sweet taste, and intake of naturally occurring endorphins that are present in breast milk (Blass 1995; Blass 1997; Shah 2012). As detailed in Shah 2012, breast milk also contains higher concentrations of tryptophan compared to formula milk (Heine 1999). Tryptophan is a precursor to melatonin, which in animal studies has been shown to increase concentrations of beta‐endorphin (Barrett 2000), a naturally occurring endorphin that is assumed to be one of the mechanisms responsible for the analgesic effects of breast milk. However, small volumes of expressed breast milk do not result in analgesia. Expressed breast milk given in small quantities failed to consistently reduce physiological or behavioural pain indicators or composite pain scores (Shah 2012). This discrepancy may be due to the contribution of multiple factors influencing analgesia during breastfeeding other than taste alone, including maternal contact, skin‐to‐skin contact, familiar smell, heart rate and body movement, sucking, and intake of naturally occurring endorphins present in the breast milk (Harrison 2010b; Zanardo 2001). As breast milk contains around 7% lactose, which is the least sweet of the sugars (sucrose > fructose > glucose > lactose) (Blass 1992), the mildly sweet taste most likely contributes little to analgesia in isolation (e.g. delivered by oral syringe or via pacifier). Bottle feeding larger volumes of either breast milk or formula milk also removes the multiple analgesic factors associated with being held skin‐to‐skin. Evidence of the analgesic effects of breastfeeding in newborn infants is demonstrated in a publicly accessible YouTube video (Harrison 2016).
Why it is important to do this review
Although a systematic review of breastfeeding newborn infants during painful procedures was shown to effectively reduce pain (Shah 2012), and a systematic review of multiple strategies to reduce immunisation pain, which included breastfeeding, also demonstrated analgesic effects of breastfeeding (Shah 2009), to date there is no published systematic review that exclusively focuses on the effectiveness of breastfeeding for pain management during painful procedures beyond the neonatal period. This review is therefore important to further establish the effectiveness of breastfeeding in infants up to one year of age. This strategy has universal applicability for breastfeeding mothers, as it requires no additional cost, no special equipment, and no special preparation or storage.
Objectives
We sought to determine the effect of breastfeeding on procedural pain in infants beyond the neonatal period (first 28 days of life) up to one year of age compared to no intervention, placebo, parental holding, skin‐to‐skin contact, expressed breast milk, formula milk, bottle feeding, sweet‐tasting solutions (e.g. sucrose or glucose), distraction, or other interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi‐RCTs as defined by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Types of participants
Infants undergoing a painful procedure who are 28 days postnatal age (corrected for prematurity, 37 weeks plus 28 days) up to aged 12 months, including infants receiving their 12‐month vaccination. The procedures included, but were not limited to: subcutaneous or intramuscular injection, venipuncture, intravenous line insertion, heel lance, and finger lance.
Types of interventions
Breastfeeding during a painful procedure, with or without additional interventions such as topical anaesthetic agents or sweet solutions. We did not classify expressed breast milk delivered by methods such as a dropper, syringe, spoon, or bottle as breastfeeding, but did include such delivery methods as comparators in control or other treatment arms.
Comparators included oral administration of: water, sweet‐tasting solutions (such as sucrose or glucose), expressed breast or formula milk, no intervention, use of pacifiers, positioning, cuddling, distraction, topical anaesthetics, and skin‐to‐skin care, also referred to as kangaroo care.
We included all settings where breastfeeding was evaluated for pain reduction during painful procedures. These included inpatient hospital units, emergency departments, and outpatient or community settings.
Types of outcome measures
Primary outcomes
The primary outcome was pain, as assessed by at least one of the following.
-
Behavioural indicators such as:
cry variables (duration of crying, expressed in total seconds of crying or proportion of duration of crying during painful procedure or following completion of painful procedure (recovery period));
facial expressions (grimace); or
body posture and movements.
Physiological responses such as heart rate, heart rate variability, respiratory rate, transcutaneous oxygen (TcPO2), transcutaneous carbon dioxide (TcPCO2), oxygen saturation (SpO2), or other measures such as skin conductance or biomedical markers (e.g. serum, salivary or urinary cortisol).
Composite pain measures: unidimensional or multidimensional (including a combination of behavioural, physiological, and contextual indicators).
Secondary outcomes
Other clinically important outcomes reported by authors of included studies (not prespecified).
Any adverse effects reported by authors (e.g. choking, gagging, spitting, coughing).
Timing of measurements and aggregation of data will vary from study to study, but common times of measurement include:
baseline prior to delivery of intervention/control;
upon commencement of procedure;
15, 30, 60, 120, 180 seconds following commencement of procedure;
throughout entire duration of procedure; and
up to 10 minutes following completion of procedure.
Search methods for identification of studies
Electronic searches
We searched the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Library (Issue 1 of 12, 2016).
MEDLINE including In‐Process & Other Non‐Indexed Citations (OVID) (1946 to 18 February 2016).
Embase (OVID) (1947 to 18 February 2016).
PsycINFO (OVID) (1806 to 18 February 2016).
CINAHL (EBSCO) (searched 18 February 2016).
Our librarian (MS), who is highly qualified in systematic review searching, developed the MEDLINE search strategy, which another librarian peer reviewed using the Peer Review of Electronic Search Strategies (PRESS) standard (McGowan 2010). We adapted the MEDLINE search strategy for the other databases (see Appendix 1, Appendix 2, Appendix 3, Appendix 4, Appendix 5). We limited study design to controlled trials only in MEDLINE and Embase. We applied no language restrictions. We included studies irrespective of their publication status. In addition, we reviewed eligible studies for cited references.
Searching other resources
As per Cochrane Pain, Palliative and Supportive Care (PaPaS) Review Group recommendations, we also searched the metaRegister of controlled trials (mRCT), ClinicalTrials.gov (clinicaltrials.gov), and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/) for ongoing trials. In addition, we checked reference lists of reviews and retrieved articles; relevant recent neonatal, paediatric, and pain journals; and paediatric pain conference proceedings.
Data collection and analysis
Selection of studies
Two review authors (JR and MB) independently screened abstracts to identify potentially eligible studies found via electronic searching. Any conflicts were resolved through a consensus process, with a third review author (DH), if required. We retrieved full‐text articles of all potentially relevant abstracts, which two review authors (JR and MB) independently assessed for inclusion. We resolved all discrepancies over the full texts through a consensus process or by consulting a third review author (DH) if required. We included a PRISMA study flow diagram to document the screening process (Liberati 2009), as recommended in Part 2, Section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Data extraction and management
We extracted the following data from each study: study design, setting, age of infants, overall sample size, sample size per group, number of groups, painful procedure, and outcomes and adverse events per groups as reported by the authors. Two review authors (JR and MB or JR and CL) independently extracted data from the studies using a standardised data extraction form. Differences were resolved through a consensus process or by consulting a third review author (DH) when necessary.
Assessment of risk of bias in included studies
We used standard methods of Cochrane to assess the potential risk of bias using the Cochrane 'Risk of bias' classification tool (Higgins 2011b).
Two review authors (JR and MB) independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion or by consulting a third review author (DH), if necessary (Higgins 2011a). The review authors were not blinded to authors or institutions. We assessed the following for each study.
Selection bias (random sequence generation and allocation concealment)
Random sequence generation
For each included study, we categorised the risk of selection bias as:
low risk: adequate (any truly random process, e.g. random number table, computer random number generator);
high risk: inadequate (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); or
unclear risk: no or unclear information provided.
Allocation concealment
The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. For each included study, we categorised the risk of bias regarding allocation concealment as:
low risk: adequate (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
high risk: inadequate (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth); or
unclear risk: no or unclear information provided.
Blinding of participants, personnel, and outcome assessors (performance and detection bias)
Blinding of participants and personnel (performance bias)
Due to the nature of the intervention of breastfeeding, blinding of participants and personnel was not possible. Nevertheless, for each included study, we categorised the methods used to blind study participants and personnel to knowledge of which intervention a participant received as:
low risk: adequate for personnel (a placebo that could not be distinguished from the active solution was used in the control group);
high risk: inadequate (participants or personnel were aware of group assignment); or
unclear risk: no or unclear information provided.
Blinding of outcome assessment (detection bias)
For each included study, we categorised the methods used to blind outcome assessors to knowledge of which intervention a participant received (if the study population consisted of young children unable to verbalise, they were considered to pose no risk of revealing group assignment). We assessed blinding separately for different outcomes or classes of outcomes. We categorised the methods used with regards to detection bias as:
low risk: adequate (study states that pain assessment from audio data or physiological data download was blinded and describes the method used to achieve blinding);
high risk: inadequate (assessors at follow‐up were aware of group assignment); or
unclear risk: no or unclear information provided.
Incomplete outcome data
Incomplete outcome data (attrition bias)
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), whether reasons for attrition or exclusion were reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we planned to re‐include missing data in the analyses. We categorised the methods with respect to attrition bias as:
low risk: adequate (less than 10% missing data);
high risk: inadequate (more than 10% missing data); or
unclear risk: no or unclear information provided.
Selective outcome reporting
Selective outcome reporting (reporting bias)
For each included study, we described how we investigated the risk of selective outcome reporting bias and what we found. We assessed the methods as:
low risk: adequate (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported, as per the study protocol);
high risk: inadequate (where not all of the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported, as per the study protocol); or
unclear risk: no or unclear information provided (the study protocol was not available).
Other bias
Other potential sources of bias
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk (no concerns of other bias raised);
high risk (concerns raised about multiple examinations of the data before completion of data collection; differences in the number of participants enrolled between the abstract and final publications of the paper); or
unclear (concerns raised about potential sources of bias that could not be verified by contacting the authors).
Size
Size ‐ based on number of participants in each study arm
According to the Cochrane PaPaS Review Group guidance on sample size, for each included study we evaluated risk of bias based on number of participants included in the study as:
low risk (≥ 200 participants per arm);
high risk (< 50 participants per arm); or
unclear risk (50 to 199 participants per arm).
We integrated risk of bias results into the analyses wherever possible in the results of this review.
Measures of treatment effect
We used the statistical package Review Manager 5 provided by Cochrane (RevMan 2014). For continuous outcomes, we extracted mean scores and their standard deviations in the treatment and control groups. For dichotomous outcomes, we collected the number of events per comparison group.
We pooled data for the most comparable outcomes and where data from at least two studies could be included. This included data for cry duration, standardised mean pain scores, Neonatal Infant Pain Scale (NIPS) pain scores, and heart rate following the procedure.
Unit of analysis issues
The unit of analysis was the infant receiving breastfeeding or control. When multiple intervention groups were included, the primary comparison was breastfeeding compared to the control group, or group receiving 'no intervention'.
For cross‐over trials, we used the data from the first intervention infants received and treated the study as a RCT.
Dealing with missing data
We presented the data in narrative form only if the data were not in a format suitable for use in Review Manager 5. We attempted to contact study authors if data were missing or required clarification, requesting data in a format that could be used for inclusion in meta‐analyses, and obtained the necessary information to complete a 'Risk of bias' assessment.
Assessment of heterogeneity
We explored heterogeneity using the I2 statistic (Higgins 2011a), and visually inspected forest plots for heterogenous estimates of effect. We graded the degree of heterogeneity as none, low, moderate, and high for values of < 25%, 25% to 49%, 50% to 74%, and ≥ 75%, respectively (GRADEpro 2015). We explored possible causes of heterogeneity using sensitivity analysis if applicable.
We used the I2 statistic for between‐study heterogeneity to assess the appropriateness of pooling data from studies for meta‐analyses, employing random‐effects models. We used GRADEpro to present a summary of findings table in this review (Table 1; GRADEpro 2015).
Assessment of reporting biases
We planned to use Review Manager 5 funnel plots to investigate small‐study effects. However, no single meta‐analysis included at least 10 trials (Higgins 2011b; Sterne 2011).
Data synthesis
We pooled data for the most comparable outcomes and where data from at least two studies could be included using mean difference (MD) with 95% confidence interval (CI), employing a random‐effects model for continuous outcomes measured on the same scales. For continuous outcomes measured on different scales, we pooled standardised mean differences (SMDs) and associated 95% CIs.
For dichotomous outcomes, we had planned to pool events between groups across studies using risk ratios (RRs) and 95% CIs, risk differences (RDs), and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH). However, there were no data reported as dichotomous outcomes that we were able to pool.
Quality of the evidence
Two review authors (MB and JR) independently rated the quality of all outcomes (behavioural responses (crying time, pain scores) and physiological outcomes of heart rate and oxygen saturation). The first review author (DH) reviewed and finalised all decisions with MB and JR. We used the GRADE system to rank the quality of the evidence employing GRADEpro GDT software and the guidelines provided in Section 12.2 of the CochraneHandbook for Systematic Reviews of Interventions (GRADEPro GDT 2015).
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for each outcome. The GRADE system uses the following criteria for assigning grade of evidence.
High: further research is very unlikely to change our confidence in the estimate of effect.
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: any estimate of effect is very uncertain.
Summary of findings table
We included a summary of findings table table to present the main findings in a transparent and simple tabular format. In particular, we included key information concerning the quality of the evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes of crying time, pain scores, and heart rate.
Subgroup analysis and investigation of heterogeneity
If, as we expected, the majority of eligible trials were related to the procedure of vaccination, we planned to report data by age group, and perform subgroup analyses according to the early‐childhood immunisation schedules of 2, 4, 6, and 12 months' age groups, if possible. For trials using breastfeeding plus another intervention, we subgrouped these into breastfeeding alone and breastfeeding plus another intervention. We performed subgroup analyses on different comparisons (i.e. no treatment, water, sucrose).
Sensitivity analysis
We included all studies regardless of their risk of bias, and presented a narrative discussion of the potential influence of the risk of bias.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Our initial search of the literature retrieved 756 records after duplicates were removed. Two review authors (JR and MB) screened the 756 titles and abstracts and identified 62 potentially eligible studies. We excluded 52 studies, primarily because the paper was a commentary, editorial, or conference abstract only. We finally included a total of 10 studies with 1066 infants (Figure 1). There were no disagreements between the two review authors that required a third review author to reach a consensus.
1.
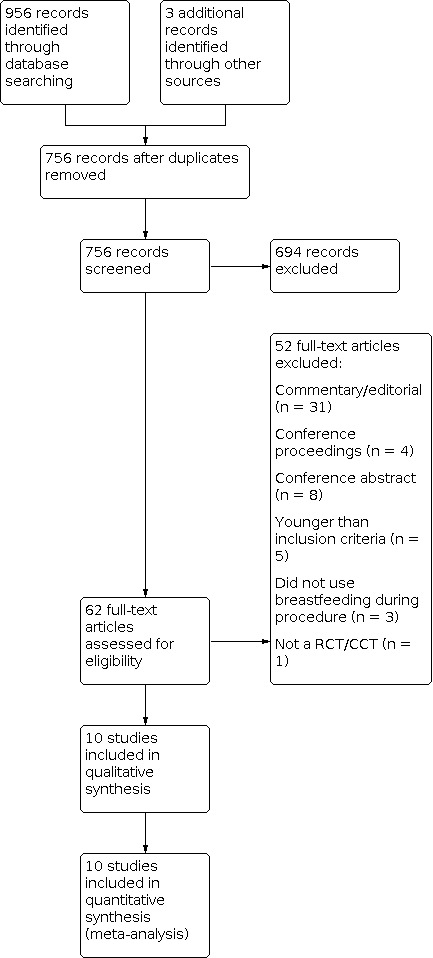
Study flow diagram.
Included studies
Details of the included studies are summarised in Characteristics of included studies.
All 10 included studies evaluated breastfeeding during vaccination injections. Nine studies evaluated breastfeeding during a single vaccination episode; one evaluated breastfeeding during two episodes of vaccination, at 2 and again at 4 months (Barr unpublished). Of the 10 included studies, eight were RCTs (Barr unpublished; Boroumandfar 2013; Dilli 2009; Efe 2007; Esfahani 2013; Goswami 2013; Gupta 2013; Taavoni 2009a), and two were quasi‐RCTs (Razek 2009; Thomas 2011). All 10 studies involved intramuscular injections including HepB (hepatitis B) (Boroumandfar 2013; Dilli 2009; Esfahani 2013), DPT (diphtheria, tetanus, pertussis) (Barr unpublished; Boroumandfar 2013; Dilli 2009; Efe 2007; Esfahani 2013; Goswami 2013; Gupta 2013; Taavoni 2009a; Thomas 2011), DTaP‐IPV (diptheria, tetanus, pertussis, polio) (Dilli 2009), Haemophilus influenzae type b (Dilli 2009), MMR (measles, mumps, rubella) (Esfahani 2013), bacillus Calmette‐Guérin (Dilli 2009), and one study did not identify the type of vaccination (Razek 2009).
The studies included infants aged 0 to 12 months. For the one study that included infants under the age of one month (Dilli 2009), we obtained study data from the first author and only included data for infants over the age of one month in this review. Study sample sizes, based on the number of participants included in this review, ranged from 40 to 144 in total. Four studies reported a sample size calculation (Barr unpublished; Goswami 2013; Gupta 2013; Taavoni 2009a). Barr unpublished estimated their sample size using cry data from previous immunisation studies (effect size of 0.40, type I and type II error rates of 5% and 10%); Goswami 2013 based their sample size on their primary outcome of cry duration (90% power and significance of 0.05); Gupta 2013 based their sample size on a previously conducted pilot study (90% power and significance of 0.05); Taavoni 2009a based their sample size on a 95% confidence interval and 90% power.
All studies involved an intervention group with the mother initiating breastfeeding prior to the immunisation procedure and continuing breastfeeding during the immunisation procedure. The breastfeeding group in one study also received 1 g of topical anaesthetic cream (EMLA Cream), applied topically on the injection site 60 minutes before the immunisation (Gupta 2013). All studies included a comparison group where the infant received no pain treatment. Four studies included other comparator groups: 2 mL of 25% dextrose (Goswami 2013); 1 g EMLA Cream plus 2 mL oral distilled water (Gupta 2013); massage therapy (Esfahani 2013); and topical vapocoolant spray (Boroumandfar 2013).
Six studies measured cry duration (Barr unpublished; Dilli 2009; Efe 2007; Goswami 2013; Gupta 2013; Razek 2009), and two studies measured latency of onset of cry (Goswami 2013; Gupta 2013). Eight studies included at least one validated pain scale as an outcome measurement, including the Neonatal Infant Pain Scale (NIPS) (Boroumandfar 2013; Dilli 2009; Esfahani 2013; Razek 2009; Thomas 2011), Neonatal Facial Coding System (NFCS) (Barr unpublished), Modified Facial Coding System (MFCS) (Goswami 2013; Gupta 2013), Modified Behavioral Pain Scale (MBPS) (Taavoni 2009a), and Wong‐Baker FACES Pain Rating Scale (Razek 2009). Three studies measured physiological responses (Barr unpublished; Efe 2007; Razek 2009). Razek 2009 reported heart rate before and after the injection. Efe 2007 reported heart rate and oxygen saturation during and after the injection (Efe 2007). One study also measured facial flushing before, during, and after the injection and salivary cortisol level before and after the injection (Barr unpublished).
Excluded studies
Of the 62 titles/abstracts identified as potentially eligible for inclusion, we excluded 52. Thirty‐one of these were commentaries, newsletters, or editorials, and four were conference proceedings with no relevant abstracts identified. Of the remaining 17 excluded studies, eight were reported as conference abstracts only (Barr 2001; Gradin 2003; Singal 2004; Taavoni 2009b; Taavoni 2010; Taavoni 2011; Taavoni 2012; Taavoni 2013), five studied populations younger than our inclusion criteria (Gray 2002; Hashemi 2016; Modarres 2014; Shendurnikar 2005; Uga 2008), three did not use breastfeeding during the painful procedure (Achema 2011; Jebreili 2015; Sahebihag 2011), and one was not a RCT or quasi‐RCT (Otero López 2014).
Risk of bias in included studies
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We rated five studies as at low risk of bias for random sequence generation that used methods such as shuffling envelopes (Efe 2007), use of a random number table (Esfahani 2013; Taavoni 2009a), or computer‐generated random numbers (Goswami 2013; Gupta 2013). We assessed one study as being at high risk of bias since it was a quasi‐experimental study and thus did not involve randomisation of participants (Razek 2009). We rated four studies in which the specific randomisation method was not described as unclear (Barr unpublished; Boroumandfar 2013; Dilli 2009; Thomas 2011).
Allocation concealment
We rated two studies as being at low risk of bias for allocation concealment as they both used the SNOSE method (serially numbered, opaque, sealed envelopes) (Goswami 2013; Gupta 2013). We rated the remaining eight studies as unclear, as not enough information was provided to assess how allocation was concealed (Barr unpublished; Boroumandfar 2013; Dilli 2009; Efe 2007; Esfahani 2013; Razek 2009; Taavoni 2009a; Thomas 2011).
Blinding
Blinding of participants and personnel
We rated all studies as at high risk for blinding of participants and personnel due to the nature of using breastfeeding as a pain management intervention; there is no practical way for the mothers, healthcare professionals performing the vaccinations, and research staff present during the procedure to be blinded to whether the infant is breastfeeding or not.
Blinding of outcome assessment
We rated only one study as at low risk of bias for blinding of outcome assessment; the study used audio recording only, thus ensuring that the research personnel coding the cry time were not able to see if the infant was being breastfed or not (Efe 2007). The remaining nine studies either completed the outcome assessment at the time of the procedure (Boroumandfar 2013; Dilli 2009; Esfahani 2013; Razek 2009; Taavoni 2009a; Thomas 2011), or used video recording (Barr unpublished; Goswami 2013; Gupta 2013), and were therefore assessed as being at high risk of bias due to the inability to blind outcome assessors to breastfeeding.
Incomplete outcome data
We rated nine studies as at low risk of bias for incomplete outcome data, as they either reported that all randomised infants were included in the results (Efe 2007; Goswami 2013; Gupta 2013; Razek 2009; Taavoni 2009a; Thomas 2011), or more than 90% of participants completed the study (Barr unpublished; Dilli 2009; Esfahani 2013). In one study, there was no information on how many infants were randomised and completed the study per group, and was thus rated as unclear (Boroumandfar 2013).
Selective reporting
We rated one study as at high risk of bias that presented the outcome data in a way that was inconsistent with the recommended use of the validated pain scales being used (Razek 2009). We rated eight studies as being at unclear risk of bias related to selective reporting: seven studies did not have a study protocol registered in the trial registries searched (metaRegister of Controlled Trials, ClinicalTrials.gov (clinicaltrials.gov), and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/)), therefore there was insufficient information to judge (Barr unpublished; Boroumandfar 2013; Dilli 2009; Efe 2007; Esfahani 2013; Goswami 2013; Taavoni 2009a; Thomas 2011), and the remaining study had a protocol available, but it was registered retrospectively (Gupta 2013).
Other potential sources of bias
Sample size
We rated nine studies as at high risk of bias due to fewer than 50 infants being enrolled in each arm (Barr unpublished; Boroumandfar 2013; Dilli 2009; Efe 2007; Esfahani 2013; Goswami 2013; Gupta 2013; Taavoni 2009a; Thomas 2011). One study had between 50 and 199 infants enrolled in each arm and was thus rated as at unclear risk of bias (Razek 2009). It should be noted that although Barr unpublished, Goswami 2013, Gupta 2013, and Taavoni 2009a were rated as at high risk of bias for sample size based on the Cochrane PaPaS guidance on sample size, these four studies reported performing sample size calculations. It should also be noted that Dilli 2009 included 162 infants between the ages of 0 and 6 months in their breastfeeding study (77 in breastfeeding group, 85 in control group), but because we only included the data for infants between the aged of 1 and 6 months, the reduced number per group led us to rate the study as at high risk of bias for sample size.
Other
We rated most studies as at low risk for other sources of bias (Barr unpublished; Boroumandfar 2013; Efe 2007; Esfahani 2013; Goswami 2013; Gupta 2013; Taavoni 2009a; Thomas 2011). One study, Razek 2009, included use of a pain assessment tool (Wong‐Baker FACES), which is considered as self report (Wong 1999), yet was reported by nursing staff. This tool is not validated for the infant population and was therefore rated as at high risk of bias. We rated one study as at unclear risk of bias due to demographic data (including information on number of injections and type of injection) being presented for the whole group only, rather than by intervention and control group (Dilli 2009).
Effects of interventions
See: Table 1
Breastfeeding versus control: cry duration
Six studies comparing breastfeeding (547 infants in total) to control conditions reported cry duration during immunisation. Three studies reported the duration of crying up to a maximum of 3 minutes (Efe 2007; Goswami 2013; Gupta 2013), and two studies reported the period until all crying ceased (Dilli 2009; Razek 2009). One study reported the percentage of time the infant cried during three time periods (Barr unpublished): 60 seconds before the injection; from time of injection to 10 seconds after the injection; and from 11 seconds to 60 seconds after the injection. We obtained the raw data from the study authors. To make the data more consistent with other data in this meta‐analysis, we converted the raw data from Barr unpublished from percentage of time crying to cry time in seconds between time of injection and 60 seconds after the injection. Barr unpublished collected data from the same infants at two months and again at four months. We assumed independence and entered both age groups separately into this meta‐analysis.
Goswami 2013 included three groups (breastfeeding, 25% dextrose, distilled water). For the purpose of meta‐analysis, to ensure the groups were as comparable as possible, we included data for breastfeeding compared to distilled water in Analysis 1.1. Gupta 2013 also included three groups: topical EMLA combined with breastfeeding; topical EMLA combined with water; and topical placebo cream combined with water. For the purpose of meta‐analysis, we included data for topical EMLA combined with breastfeeding and topical EMLA combined with water in Analysis 1.1 to ensure the groups were as comparable as possible.
1.1. Analysis.

Comparison 1: Breastfeeding versus control, Outcome 1: Cry duration
We pooled data for cry time (in seconds) from these six studies. Analysis 1.1 and Figure 4 show the results of the meta‐analysis. There was a significant reduction in cry time in seconds in the breastfeeding groups compared to the control groups (MD ‐38, 95% CI ‐50 to ‐26; P < 0.00001). However, there was high between‐study heterogeneity (I2 = 85%).
4.

Forest plot of comparison: 1 Breastfeeding versus control, outcome: 1.1 Cry duration.
We judged the quality of the evidence according to the GRADE criteria, for breastfeeding during vaccinations, on the outcome of cry duration, to be moderate. Most of the included infants were younger than 6 months of age, and outcomes may be different for older infants during their 12‐month immunisation. We did not further downgrade the quality of evidence, but consider that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate (Table 1).
Breastfeeding versus control: all pain scores
We pooled data for pain scores following immunisation from 5 studies and 310 infants (NIPS ‐ Dilli 2009, Esfahani 2013, Thomas 2011; MFCS ‐ Gupta 2013; MBPS ‐ Taavoni 2009a). Pain scores were collected at more than one time point for Gupta 2013, Taavoni 2009a, and Thomas 2011. The data used in this meta‐analysis was for the closest time point following completion of the procedure. We conducted the meta‐analysis using standardised mean differences (SMD). Results showed a significant reduction in pain scores in the breastfeeding group compared to the control (SMD ‐1.7, 95% CI ‐2.2 to ‐1.3; P < 0.00001) and moderate between‐study heterogeneity (I2 = 69%) (Analysis 1.2; Figure 5).
1.2. Analysis.

Comparison 1: Breastfeeding versus control, Outcome 2: All pain scores during injection
5.

Forest plot of comparison: 1 Breastfeeding versus control, outcome: 1.2 All pain scores during injection.
We could not include MFCS scores from Goswami 2013 (for breastfeeding and control groups) in this composite pain score meta‐analysis, as data were presented in graphical form only. We contacted the authors requesting raw data but did not receive a response.
We judged the quality of the evidence according to the GRADE criteria, for breastfeeding during vaccinations, on the outcome of combined pain scores, to be moderate. Most of the included infants were younger than 6 months of age, and outcomes may be different for older infants during their 12‐month immunisation.
Breastfeeding versus control: NIPS
Five studies reported NIPS scores during immunisation (Boroumandfar 2013; Dilli 2009; Esfahani 2013; Razek 2009; Thomas 2011). Dilli 2009 included infants between the ages of 0 and 6 months, however they provided us with raw data, and we excluded infants under the age of 1 month from the analysis.
Boroumandfar 2013 presented the data in dichotomous form only (pain = NIPS score greater than 3, or no pain = NIPS score 3 or less), which precluded inclusion in meta‐analyses. We requested data from the authors but did not receive a response; we have therefore reported the data in narrative form only. The authors reported statistically significant differences in frequency of pain between the three groups (breastfeeding, vapocoolant, and control), with the breastfeeding group having significantly lower NIPS scores during the vaccination procedure (P < 0.001).
Razek 2009 presented the frequency of infants with NIPS scores of 0, 1, 2, 3, 4, and "hurts worst". The inclusion of the "hurts worst" category is inconsistent with the NIPS tool, which is a score ranging from 0 to 7. We contacted the authors for clarification and to request raw data but did not receive a response, and therefore excluded this data from our meta‐analysis. Although the published paper did not state whether there was a statistically significant difference in frequency of each NIPS score between the breastfeeding and control groups, 6 of the 60 infants (10%) in the breastfeeding group scored the highest NIPS scores of 5 to 7, whereas 12 of the 60 infants (20%) in the control group scored the highest NIPS scores.
We pooled NIPS scores during immunisation for meta‐analysis from three studies enrolling 174 infants (Dilli 2009; Esfahani 2013; Thomas 2011). Esfahani 2013 included three groups (breastfeeding, massage, and control), but we included only breastfeeding and control in the meta‐analysis. Meta‐analysis results showed a significant reduction in mean NIPS scores in the breastfeeding group compared to control (MD ‐1.9, 95% CI ‐2.5 to ‐1.2; P = 0.04). There was moderate between‐study heterogeneity (I2 = 69%) (Analysis 1.3; Figure 6).
1.3. Analysis.

Comparison 1: Breastfeeding versus control, Outcome 3: NIPS
6.

Forest plot of comparison: 1 Breastfeeding versus control, outcome: 1.3 NIPS.
We judged the quality of the evidence according to the GRADE criteria, for breastfeeding during vaccinations, on the outcome of the behavioural pain score NIPS, to be moderate. Most of the included infants were younger than 6 months of age, and outcomes may be different for older infants during their 12‐month immunisation.
Breastfeeding versus control: heart rate
One study measured heart rate during immunisation (Efe 2007). Based on 66 infants, there was no statistically significant difference in mean heart rate. Heart rate following completion of the injections was measured by Efe 2007 (N = 66) and Razek 2009 (N = 120). We pooled data for heart rate following the procedure and found no statistically significant differences (MD ‐4, 95% CI ‐23 to 16; P = 0.03) (Analysis 1.4). There was high heterogeneity (I2 = 78%).
1.4. Analysis.

Comparison 1: Breastfeeding versus control, Outcome 4: Heart rate after injection
As we could pool results for only two studies, we judged the quality of the evidence according to the GRADE criteria, for breastfeeding during vaccinations, on the outcome of heart rate differences, to be low.
Breastfeeding versus control: oxygen saturation
One study measured oxygen saturation during and after immunisation (Efe 2007). There was no statistically significant difference in mean oxygen saturation during or following completion of the injection.
As only one study measured oxygen saturation, we judged the quality of the evidence to be very low.
Breastfeeding versus control: MBPS
One study of 76 infants reported MBPS scores 5 seconds before the injection and 15 seconds after the injection (Taavoni 2009a). There was no significant difference in MBPS scores for the breastfeeding group 5 seconds before the injection, however breastfeeding resulted in a statistically significant mean reduction of MBPS of 3.8 in the 15 seconds following the injection procedure.
As only one study measured the MBPS, we judged the quality of the evidence to be very low.
Comparisons of breastfeeding with other interventions
Four studies had more than two study arms (Boroumandfar 2013; Esfahani 2013; Goswami 2013; Gupta 2013). Our summaries of the results for these additional arms are as follows.
Breastfeeding versus 25% dextrose versus water: MFCS and cry duration
Goswami 2013 included three study arms: breastfeeding, water, and 25% dextrose. MFCS data were presented in graphical form only and therefore could not be extracted. The authors reported a significant reduction in MFCS for infants who were breastfed or given 25% dextrose compared to water at 1 minute and 3 minutes after needle insertion. Breastfeeding resulted in a significantly reduced cry time compared to 25% dextrose and compared to water.
Breastfeeding with EMLA versus control with EMLA versus no intervention: MFCS and cry duration
Gupta 2013 reported MFCS scores and crying characteristics for three groups: EMLA combined with breastfeeding, EMLA combined with water, and placebo cream combined with water. The combination of EMLA and breastfeeding resulted in a statistically significant reduction in MFCS pain scores at 1 minute and 3 minutes following the vaccinations compared to EMLA and water. In addition, the combination of breastfeeding and EMLA resulted in significantly shorter crying duration compared to both EMLA and water, and placebo cream and water.
Breastfeeding versus vapocoolant versus water: NIPS scores > 3
Boroumandfar 2013 included three study arms: breastfeeding, water and vapocoolant. They classified NIPS pain scores of greater than 3 as "pain". The number and proportion of babies allocated a score of "pain" were lowest in the breastfeeding group compared to vapocoolant and water (P < 0.001): breastfeeding: n = 17/48 (35%); vapocoolant: n = 36/48 (75%); and control (water): n = 48/48 (100%).
Breastfeeding versus massage with maternal hugging versus maternal hugging only (control): NIPS scores
Esfahani 2013 reported that the mean (standard deviation) of NIPS in the breastfeeding, massage combined with maternal hugging, and maternal hugging only (control) groups were 3.4 (0.83), 3.9 (1.0), and 4.8 (1.1), respectively. The difference between breastfeeding and massage combined with maternal hugging was statistically significant (P = 0.04), and the differences between both interventions compared to the control were statistically significant (breastfeeding compared to control: P < 0.001; massage with maternal hugging compared to control: P = 0.002).
Secondary outcomes and subgroup analyses
None of the included studies reported adverse effects such as choking, gagging, spitting, coughing, aspiration, or cyanosis. Only Dilli 2009 reported the number of infants coming off the breast during the procedure, stating that 4 of the 158 infants (2.5%) failed to complete the study as they did not resume feeding.
We were unable to conduct planned subgroup analyses based on age of the infants at the time of study as data were not reported separately for the different ages. Importantly, however, only two studies included infants up to 12 months of age. Esfahani 2013 included infants at 6 and 12 months of age, and Razek 2009 included infants from 1 month to 12 months of age.
Discussion
Summary of main results
This systematic review of RCTs or quasi‐RCTs evaluating breastfeeding for procedural‐pain reduction in infants aged 1 to 12 months included 10 trials. All trials evaluated breastfeeding during early childhood vaccination, with nine of the 10 evaluating breastfeeding during a single injection. Results showed that breastfeeding was associated with statistically significant reductions in cry duration and composite pain scores (NIPS, NFCS, MFCS, MBPS, Wong‐Baker FACES Pain Rating Scale) compared to no treatment or water. Breastfeeding was not associated with a statistically significant reduction in physiological responses of heart rate or oxygen saturation levels. Breastfeeding was more effective in reducing pain than other interventions including massage combined with maternal hugging, maternal hugging alone, topical vapocoolant, topical anaesthetic cream (EMLA), and 25% dextrose.
Outcome measures differed across the studies, however we were able to pool results for meta‐analysis for crying duration in seconds for six studies. Results showed a statistically significant reduction in cry duration of 38 seconds associated with breastfeeding compared to control. We were also able to conduct a meta‐analysis using standardised mean pain scores, pooled from five studies. Based on a pain score range of 0 to 10, with 10 being the maximum pain score, breastfeeding was associated with a statistically significant reduction in pain scores of ‐1.73.
Only two studies reported physiological responses of heart rate following the vaccination procedure (Efe 2007; Razek 2009). A meta‐analysis of these pooled data for 186 infants showed a non‐significant reduction of 3.6 beats/min, with a wide 95% CI of ‐23.17 to 16.05 beats/min. Only Efe 2007 reported oxygen saturation changes, and showed no differences between study groups.
No study reported any adverse outcomes. In addition, no study reported on acceptability of breastfeeding, from the perspectives of mothers or healthcare providers, and no studies reported on the logistics of facilitating breastfeeding in the clinical settings. Only one study reported on infants failing to continue breastfeeding after the vaccination Dilli 2009, and this applied to only a small number of infants.
Eight of the 10 trials included infants aged 1 to 6 months, with only 2 studies including older infants up to 1 year of age (Esfahani 2013; Razek 2009). We could not conduct any subgroup analyses by age, as the study outcomes were not reported in sufficient detail to be extracted for pooling, or data differed. For example, Razek 2009 reported means and standard deviations of cry duration, and Esfahani 2013 reported means and standard deviations of NIPS scores. However, individual results of these two studies were consistent with the overall results, showing a statistically significant reduction in behavioural pain responses.
Breastfeeding effectively reduces needle‐pain during vaccinations, based on reduced crying and pain scores. If mothers are breastfeeding, then breastfeeding is free during vaccinations. Its use is therefore recommended during early childhood vaccinations. Breastfeeding mothers are encouraged to advocate for their infants and request to breastfeed before and during early childhood vaccinations.
Overall completeness and applicability of evidence
All 10 studies in this systematic review reported that breastfeeding reduced behavioural responses to pain during vaccination injections in infants beyond the newborn period compared to no treatment or control conditions. These consistent findings across diverse settings globally (Iran (n = 3); India (n = 2); Turkey (n = 2), Jordan (n = 1); Canada (n = 1)) highlights the applicability of the evidence and the generalisability of the findings.
Eight of the 10 studies in this review assessed the analgesic effects of breastfeeding prior to and during early childhood vaccination in infants aged 1 to 12 months during single‐vaccination procedures; one study evaluated breastfeeding during multiple injections in a single consultation (Dilli 2009), and one study evaluated effects at two time points, a two‐month and a four‐month vaccination (Barr 2001). We can conclude that the results of this review are applicable to large populations of infants requiring early childhood immunisation. If the mother is breastfeeding, then breastfeeding infants during vaccinations and other non‐urgent painful procedures should be promoted and supported where feasible. Potential benefits of breastfeeding during painful procedures, in addition to pain reduction for the infant, include the possibility that this may further encourage mothers to continue to breastfeed, and empower the mother through her knowledge that she can effectively reduce her baby's distress during a painful procedure. However, if the mother did not establish breastfeeding, or had ceased to breastfeed by the time of early childhood vaccinations, or breastfeeding during vaccinations is simply not possible, sucrose or glucose with or without a pacifier can be recommended for pain treatment (Harrison 2010a; Harrison 2010c).
Quality of the evidence
All studies scored high risk of bias for blinding of participants and personnel, and all studies except for one scored high risk of bias for blinding of outcome assessment, highlighting the challenges in conducting research evaluating breastfeeding for procedural‐pain treatment. Based on the Cochrane PaPaS guidance on sample size, we scored nine of the 10 included studies as high risk of bias, as there were fewer than 50 participants per arm. However, three of these nine studies that included fewer than 50 participants per arm reported their a priori power calculation to inform their sample size.
Most studies scored low risk of bias for incomplete outcome data and other bias, while results were variable for random sequence generation and allocation concealment. We were unable to identify completed protocols for most studies, therefore we predominantly gave a risk of bias rating of unclear for selective reporting bias.
For behavioural responses of crying time and standardised pain scores, the overall evidence based on GRADE was moderate. As most studies (8 out of 10) included infants in the younger age spectrum, of 1 to 6 months, further research including older infants up to 12 months of age may have an important impact on our confidence in the estimate of effect and may change the estimate. As we could only pool heart rate data for two studies, evidence for physiological outcomes based on GRADE was considered to be low quality, and further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Potential biases in the review process
There were no known potential biases in the review process. We performed extensive searches of the literature with no language restrictions, and attempted to contact authors for additional information where required.
Agreements and disagreements with other studies or reviews
In terms of effects of breastfeeding on behavioural responses to pain, these review findings agree with the systematic review of breastfeeding for procedural pain in newborn infants (Shah 2012), and a recently published systematic review of interventions to reduce vaccine pain that included infants and children (Shah 2015). In all three systematic reviews, breastfeeding resulted in a statistically significant reduction in behavioural parameters and composite pain scores. However, in this current systematic review, there were no consistent effects on physiological parameters of heart rate and oxygen saturation levels. This finding disagrees with the Cochrane systematic review of breastfeeding for procedural pain in neonates (Shah 2012), in which breastfeeding reduced heart rate change from baseline compared to being held by the mother, sucking on a pacifier, sucrose, or simply positioned supine or prone. Both Shah 2012 and this current review included one study that compared breastfeeding with small volumes of sweet solutions. Results of both reviews showed that breastfeeding was associated with reduced behavioural responses to pain compared to sweet solutions. In pain studies, a difference of at least 13% is considered by parents of newborn infants to be clinically important, and a difference of 19% is considered by nurses to be important (Shah 2004). The 1.75 difference in pain scores out of a possible standardised score of 10 is 17.5%, therefore well within the range of what parents consider to be important, and just under what nurses in general consider to be important.
Our findings in this review are also consistent with that of the systematic review of skin‐to‐skin care for procedural pain in neonates (Johnston 2014); both reviews demonstrated that maternal care resulted in a statistically significant difference in composite pain scores immediately and within 90 seconds after completion of the painful procedures compared to no treatment, yet there were inconsistencies in physiological responses of heart rate and oxygen saturation levels. To our knowledge, there are no studies evaluating skin‐to‐skin care in infants beyond the neonatal period, and there are only three studies that included full‐term newborn infants in the systematic review of skin‐to‐skin care for procedural pain in neonates (Johnston 2014). We are therefore unable to make a direct comparison of analgesic effects of breastfeeding and skin‐to‐skin care in the age group of 1 to 12 months.
Adverse outcomes were not reported in any of the trials included in this review, or in the systematic review of breastfeeding for procedural pain in neonates (Shah 2012). This suggests that there are no adverse outcomes such as airway compromise including coughing, choking, gagging, or aspiration. However, the inclusion of adverse events as an outcome measure and clear reporting of the same in future studies of breastfeeding all populations of infants and young children during painful procedures is recommended.
In this review, we identified no studies comparing formula feeding or bottle feeding expressed breast milk with breastfeeding. Shah 2012 included one such study in their systematic review of breastfeeding for procedural pain in neonates (Weissman 2009), which showed that formula feeding was as effective as breastfeeding in reducing behavioural and physiological responses during heel lance.
Breastfeeding is now recommended by the World Health Organization (WHO) for pain management for infants (World Health Organization 2015). Although previous studies have shown that breastfeeding is infrequently used for vaccination pain management (Lisi 2013; Russell 2015; Taddio 2009), the global recommendations made by WHO, alongside the additional evidence of pain management effectiveness provided in this systematic review, may lead to more consistent adoption of this strategy in diverse settings where vaccinations take place.
Authors' conclusions
Implications for practice.
We included 10 studies enrolling 1066 infants in this review, all of which examined pain associated with early childhood vaccinations. Breastfeeding consistently reduced behavioural responses of cry duration and composite pain scores during and following vaccinations compared to no treatment, oral water, oral dextrose, maternal cuddling, massage, vapocoolant spray, and topical anaesthetic (EMLA). We saw no additional benefit of adjunct EMLA or vapocoolant. However, there was evidence that breastfeeding did not impact on physiological responses.
No studies included populations of hospitalised infants undergoing other skin‐breaking procedures. Further studies of efficacy, feasibility, and acceptability in this population are warranted. No adverse effects of breastfeeding for procedural pain have been reported in this current review.
Implications for research.
General
Infants' distress during early childhood vaccinations is common and can lead to long‐term fear of needles and parents' reluctance to adhere to recommended immunisation schedules. The available evidence suggests that breastfeeding may help reduce pain in healthy infants beyond the newborn period, especially up to six months of age, during early childhood immunisation. Additional research gaps include evaluating the effectiveness of breastfeeding throughout multiple injections during a vaccination episode, including the rate of success of re‐attaching the infant to the breast following repositioning for subsequent vaccinations.
Only two studies in this review included infants up to one year of age, however the overall results of these studies showed analgesic effects of breastfeeding during vaccination. Further research in this upper age limit of infancy and into early childhood is therefore warranted.
Design
The design and implementation of studies could be improved, although breastfeeding as an intervention cannot be blinded.
No studies in this review included hospitalised infants. It is therefore uncertain if we can extrapolate the results to sick hospitalised infants undergoing painful procedures. Pilot randomised controlled trials or observational studies to establish feasibility, acceptability, and safety for hospitalised infants who are medically stable, able to breastfeed, and undergoing blood work or insertion of intravenous lines are warranted to inform research and practice.
None of the included trials reported on acceptability of breastfeeding from the perspective of mothers or healthcare providers, and ways to facilitate breastfeeding in the clinical settings. Inclusion of these factors in future research is recommended.
Measurement (endpoints)
Further trials and observational studies should include adverse events as an outcome measure, and clearly report the results. If no adverse events are observed, this should be stated.
In addition, observational and longitudinal studies are warranted that follow up babies who have consistently received recommended pain management during vaccinations.
Other
Quality improvement research aimed at increasing the use of breastfeeding for healthy and medically stable hospitalised infants during non‐urgent painful procedures in diverse settings is warranted. Similarly, knowledge translation research aimed at exploring the most effective ways to improve implementation of effective pain treatment for infants is recommended.
What's new
| Date | Event | Description |
|---|---|---|
| 25 August 2020 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 8, 2014 Review first published: Issue 10, 2016
| Date | Event | Description |
|---|---|---|
| 5 June 2018 | Review declared as stable | See Published notes |
Notes
Assessed for updating in 2018
A restricted search in June 2018 did not identify any potentially relevant studies likely to change the conclusions. Therefore this review will be reassessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Assessed for updating in 2020
At July 2020 we are aware of new potentially relevant studies, but none are likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be reassessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We thank Jo Abbott, Information Specialist, Cochrane Pain, Palliative and Supportive Care Review Group, for peer review of the MEDLINE search strategy.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: The views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy
1. (procedur* adj3 pain*).tw.
2. Needles/
3. exp Immunization/
4. exp Injections/
5. exp Punctures/
6. exp Biopsy/
7. exp Vascular Access Devices/
8. exp Catheterization/
9. exp Paracentesis/
10. thoracocent*.mp.
11. exp Blood Specimen Collection/
12. exp Administration, Intravenous/
13. exp Infusions, parenteral/
14. needle*.tw.
15. (blood sampl* or immuni* or inoculat* or vaccin* or inject*).tw.
16. (finger prick* or heel prick* or heel lanc* or heel punctur* or heel stick* or sutur* or (laceration* adj3 repair*)).tw.
17. (lumbar punctur* or spinal tap*).tw.
18. (bone marrow adj4 (aspiration* or biops*)).tw.
19. (intravenous or intra venous or venepuncture* or venipuncture* or venous cannulation* or (arterial blood gas* adj4 cannul*)).tw.
20. (catheter* or port‐a‐cath* or portacath*).tw.
21. (central line adj6 (insert* or remov*)).tw.
22. (central venous catheter* adj6 insert*).tw.
23. (local analges* or local anaesthe* or local anesthe*).tw.
24. (arter* adj6 punctur*).tw.
25. arterial line*.tw.
26. (thoracocentesis or paracentesis).tw.
27. or/1‐26
28. Breast Feeding/
29. (breastfeed* or breastfed or (breast adj2 feed*) or (breast adj2 fed)).mp.
30. 28 or 29
31. (Infan* or newborn* or new‐born* or perinat* or neonat* or baby or baby* or babies or toddler* or prematur* or preterm*).mp.
32. 27 and 30
33. limit 32 to "all infant (birth to 23 months)"
34. 32 and (Infan* or newborn* or new‐born* or perinat* or neonat* or baby or baby* or babies or toddler* or prematur* or preterm*).mp.
35. 33 or 34
36. (35 and ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti. or quasi*.it,ab.)) not (exp animals/ not humans.sh.)
37. remove duplicates from 36
Appendix 2. Embase search strategy
1. (procedur* adj3 pain*).tw. 2. exp Needle/ 3. exp Immunization/ 4. exp Injection/ 5. exp "Aspiration, puncture and suction"/ 6. exp Biopsy/ 7. exp Catheters/ 8. exp Catheterization/ 9. Blood sampling/ 10. Vascular access/ 11. exp Paracentesis/ 12. Intravenous administration/ 13. Infusion/ 14. needle*.tw. 15. (blood sampl* or immuni* or inoculat* or vaccin* or inject*).tw. 16. (finger prick* or heel prick* or heel lanc* or heel punctur* or heel stick* or sutur* or (laceration* adj3 repair*)).tw. 17. (lumbar punctur* or spinal tap*).tw. 18. (bone marrow adj4 (aspiration* or biops*)).tw. 19. (intravenous or intra venous or venepuncture* or venipuncture* or venous cannulation* or (arterial blood gas* adj4 cannul*)).tw. 20. (catheter* or port‐a‐cath* or portacath*).tw. 21. (central line adj6 (insert* or remov*)).tw. 22. (central venous catheter* adj6 insert*).tw. 23. (local analges* or local anaesthe* or local anesthe*).tw. 24. (arter* adj6 punctur*).tw. 25. arterial line*.tw. 26. (thoracocentesis or paracentesis).tw. 27. or/1‐26 28. Breast Feeding/ 29. (breastfeed* or breastfed or (breast adj2 feed*) or (breast adj2 fed)).mp. 30. 28 or 29 31. (Infan* or newborn* or new‐born* or perinat* or neonat* or baby or baby* or babies or toddler* or prematur* or preterm*).mp. 32. 27 and 30 33. limit 32 to infant 34. 32 and (Infan* or newborn* or new‐born* or perinat* or neonat* or baby or baby* or babies or toddler* or prematur* or preterm*).mp. 35. 33 or 34 36. Randomized Controlled Trial/ or Single‐blind Procedure/ or Crossover Procedure/ or Double‐blind Procedure/ or random$.tw. or factorial$.tw. or crossover$.tw. or cross over$.tw. or cross‐over$.tw. or placebo$.tw. or (doubl$ adj blind$).tw. or (singl$ adj blind$).tw. or assign$.tw. or allocat$.tw. or volunteer$.tw. 37. 35 and 36 38. limit 37 to embase
Appendix 3. CENTRAL search strategy
1. (procedur* adj3 pain*).tw. 2. needle*.tw. 3. (blood sampl* or immuni* or inoculat* or vaccin* or inject*).tw. 4. (finger prick* or heel prick* or heel lanc* or heel punctur* or heel stick* or sutur* or (laceration* adj3 repair*)).tw. 5. (lumbar punctur* or spinal tap*).tw. 6. (bone marrow adj4 (aspiration* or biops*)).tw. 7. (intravenous or intra venous or venepuncture* or venipuncture* or venous cannulation* or (arterial blood gas* adj4 cannul*)).tw. 8. (catheter* or port‐a‐cath* or portacath*).tw. 9. (central line adj6 (insert* or remov*)).tw. 10. (central venous catheter* adj6 insert*).tw. 11. (local analges* or local anaesthe* or local anesthe*).tw. 12. (arter* adj6 punctur*).tw. 13. arterial line*.tw. 14. (thoracocentesis or paracentesis).tw. 15. (breastfeed* or breastfed or (breast adj2 feed*) or (breast adj2 fed)).mp. 16. (Infan* or newborn* or new‐born* or perinat* or neonat* or baby or baby* or babies or toddler* or prematur* or preterm*).mp. 17. or/1‐14 18. 17 and 15 19. 18 and 16 20. limit 19 to (medline records or embase records) 21. 19 not 20 22. 1 and 15 and 16 23. 21 or 22
Appendix 4. CINAHL search strategy
S36(S32 and S33) or S34 Limiters ‐ Exclude MEDLINE records S35(S32 and S33) or S34 S34S28 AND S31 Limiters ‐ Age Groups: Infant, Newborn: birth‐1 month, Infant: 1‐23 months S33Infan* or newborn* or new‐born* or perinat* or neonat* or baby or baby* or babies or toddler* or prematur* or preterm* S32S28 AND S31 S31S29 OR S30 S30breastfeed* or breastfed or (breast w2 feed*) or (breast w2 fed) S29MH Breast Feeding or MH Breast Feeding Positions S28S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 S27arterial blood gas* w4 cannul* S26(bone marrow w4 aspiration*) or (bone marrow w4 biops*) S25thoracocentesis or paracentesis S24arterial line* S23arter* w6 punctur* S22local analges* or local anaesthe* or local anesthe* S21(central venous catheter* w6 insert*) S20(central line w6 insert*) or (central line w6 remov*) S19catheter* or port‐a‐cath* or portacath* S18intravenous or intra venous or venepuncture* or venipuncture* or venous cannulation* S17lumbar punctur* or spinal tap* S16finger prick* or heel prick* or heel lanc* or heel punctur* or heel stick* or sutur* or (laceration* w3 repair*) S15blood sampl* or immuni* or inoculat* or vaccin* or inject* S14needle* S13MH Biopsy+ S12MH Infusions, Parenteral+ S11MH Administration, Intravenous+ S10MH Blood Specimen Collection+ S9MH Paracentesis+ S8MH Catheterization+ S7MH Vascular Access Devices+ S6MH Punctures+ S5MH Injections+ S4MH Immunization+ S3MH Needles S2MH Treatment Related Pain S1procedur* w3 pain*
Appendix 5. PsycINFO search strategy
1. (procedur* adj3 pain*).tw. 2. Immunization/ 3. exp Injections/ 4. Biopsy/ 5. Catheterization/ 6. thoracocent*.mp. 7. needle*.tw. 8. (blood sampl* or immuni* or inoculat* or vaccin* or inject*).tw. 9. (finger prick* or heel prick* or heel lanc* or heel punctur* or heel stick* or sutur* or (laceration* adj3 repair*)).tw. 10. (lumbar punctur* or spinal tap*).tw. 11. (bone marrow adj4 (aspiration* or biops*)).tw. 12. (intravenous or intra venous or venepuncture* or venipuncture* or venous cannulation* or (arterial blood gas* adj4 cannul*)).tw. 13. (catheter* or port‐a‐cath* or portacath*).tw. 14. (central line adj6 (insert* or remov*)).tw. 15. (central venous catheter* adj6 insert*).tw. 16. (local analges* or local anaesthe* or local anesthe*).tw. 17. (arter* adj6 punctur*).tw. 18. arterial line*.tw. 19. (thoracocentesis or paracentesis).tw. 20. or/1‐19 21. Breast Feeding/ 22. (breastfeed* or breastfed or (breast adj2 feed*) or (breast adj2 fed)).mp. 23. 21 or 22 24. 20 and 23 25. limit 24 to (120 neonatal or 140 infancy ) 26. 24 and (Infan* or newborn* or new‐born* or perinat* or neonat* or baby or baby* or babies or toddler* or prematur* or preterm*).mp. 27. 25 or 26 28. 27 and (random* or trial* or control group* or methods).mp.
Data and analyses
Comparison 1. Breastfeeding versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Cry duration | 6 | 547 | Mean Difference (IV, Random, 95% CI) | ‐38.09 [‐49.84, ‐26.35] |
| 1.2 All pain scores during injection | 5 | 310 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐2.20, ‐1.25] |
| 1.3 NIPS | 3 | 174 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐2.55, ‐1.24] |
| 1.4 Heart rate after injection | 2 | 186 | Mean Difference (IV, Random, 95% CI) | ‐3.56 [‐23.17, 16.05] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barr unpublished.
| Study characteristics | ||
| Methods | Randomised controlled trial at 2 community health clinics in Quebec, Canada | |
| Participants | Longitudinal study ‐ enrolling infants at 2 & 4 months vaccination. 96 infants age 2 months, randomised to intervention or control. 64 infants remained in study at 4 months and remained in the intervention or control group to which they were initially assigned. 2 additional infants were included at 4 months, and randomised. Painful procedure: DPT vaccination Study period: Not specified |
|
| Interventions | Intervention: Breastfeeding before, during, and after vaccination Control: Maternal holding during vaccination |
|
| Outcomes | Percentage of time crying at baseline, during vaccination, and after vaccination NFCS at baseline, during vaccination, and after vaccination Facial flushing at baseline, during vaccination, and after vaccination Salivary cortisol at baseline and 25 minutes after vaccination |
|
| Notes | Power calculation: Yes (p. 9) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "96 infants were randomised at 2 months of age, in blocks of eight to 'breastfeeding' (intervention) or 'non‐breastfeeding' (control) groups." (p. 9) Specific randomisation method not described. |
| Allocation concealment (selection bias) | Unclear risk | "96 infants were randomised in blocks of eight to 'breastfeeding' (intervention) or 'non‐breastfeeding' (control) groups." (p. 9) Not enough information to assess how allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Mothers whose infants were assigned to the breastfeeding condition were then asked to start breastfeeding 3 minutes before the injection and to continue breast feeding during and after the injection." (p. 10) Personnel could not be blinded to whether infant was breastfeeding or not. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Audiotaping and videotaping started 3 minutes before the injection and continued for 3 minutes after the injection." (p. 10) The researcher completing the NFCS scores could not be blinded to whether the infant was breastfeeding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant flow chart presented (p. 23). Data analysed for 89 out of 96 infants at 2 months and 61 out of 66 infants at 4 months (due to technical difficulties, physical illness, no demographic information, not breastfeeding, or refusal). Less than 10% missing data. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not registered in trial registries searched. Study has not been published, however unpublished raw data were shared. |
| Sample size | High risk | Fewer than 50 infants per arm. |
| Other bias | Low risk | Appears free of other bias. |
Boroumandfar 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial at a single site (healthcare centre) in Iran | |
| Participants | 144 infants ages 2 to 6 months Painful procedure: Hepatitis B and DPT vaccination Study period: 2009 |
|
| Interventions | Intervention 1: Breastfeeding before and during vaccination Intervention 2: Vapocoolant before vaccination Control: No intervention |
|
| Outcomes | NIPS score during vaccination (reported as pain if NIPS >3 and painless if NIPS =< 3) | |
| Notes | Power calculation: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They were randomly divided into three groups of vapocoolant, breastfeeding, and control (48 subjects in each group) through systematic random sampling." (p. 3) Specific randomisation method not described. |
| Allocation concealment (selection bias) | Unclear risk | "They were randomly divided into three groups of vapocoolant, breastfeeding, and control (48 subjects in each group) through systematic random sampling." (p. 3) Not enough information to assess how allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "If the infant was in breastfeeding group, vaccination was administrated after the researcher had observed continuous active sucking (infants’ intraoral form, raised cheeks, and active jaw movements). If the infant was in vapocoolant group, the injection site was sprayed from a distance of 15 cm for 13 s, and after evaporation (10 s later), the injection site was disinfected by alcohol and the vaccination administered. In the control group, injection was administered with no pain control intervention." (p. 4) Personnel could not be blinded to whether infant was breastfeeding, had received vapocoolant, or no intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "NIPS was filled out during vaccination by a researcher in all three groups." (p. 4) The researcher completing the NIPS scores could not be blinded to whether the infant was breastfeeding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Does not state how many participants were randomised. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not registered in trial registries searched. |
| Sample size | High risk | Fewer than 50 infants per arm. |
| Other bias | Low risk | Appears free of other bias. |
Dilli 2009.
| Study characteristics | ||
| Methods | Randomised controlled trial at a single site (well‐child unit) in Turkey | |
| Participants | 158 infants ages 0 to 6 months, of which data for 70 infants aged 1 to 6 months were included in this review. Painful procedure: Multiple injections for vaccination (intramuscular or subcutaneous): Hepatitis B, bacillus Calmette‐Guérin, diphtheria‐tetanus‐pertussis and inactive polio, Haemophilus influenzae type b vaccination Study period: Not specified |
|
| Interventions | Intervention: Breastfeeding before and during vaccination Control: No intervention |
|
| Outcomes | Cry duration from time of needle insertion until all crying activity stopped NIPS score during vaccination |
|
| Notes | Power calculation: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A consecutive sample of 243 infants and children age 0 to 48 months receiving routine vaccinations were randomly assigned by the first assistant to 1 of the study groups, stratified by age using sealed envelopes." (p. 386) Specific randomisation method not described. |
| Allocation concealment (selection bias) | Unclear risk | "A consecutive sample of 243 infants and children age 0
to 48 months receiving routine vaccinations were randomly assigned by the first assistant to 1 of the study groups, stratified by age using sealed envelopes." (p. 386) Sealed envelopes used but does not specify whether envelopes were opaque and sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The mother was asked to continue breast‐feeding the infant during the procedure." (p. 386) Personnel could not be blinded to whether infant was breastfeeding or not. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The paediatrician responsible for recording the crying time and pain score was not present during the interventions and was blinded to participant’s allocation except to the breast‐feeding group." (p. 386) The researcher completing the cry time and NIPS scores could not be blinded to whether the infant was breastfeeding or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Four infants failed to complete the study because they did not resume feeding." (p. 386) Outcome data reported on all remaining infants. Less than 10% missing data. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not registered in trial registries searched. |
| Sample size | High risk | In the subgroup of infants aged 1 to 6 months included in this review, there were fewer than 50 infants per arm. |
| Other bias | Unclear risk | Children receiving subcutaneous and intramuscular injections as well as single and multiple injections included (p. 388). Unclear as to how these are broken down by intervention and control group in breastfeeding trial (only data on whole group provided). |
Efe 2007.
| Study characteristics | ||
| Methods | Randomised controlled trial at a single site (health child clinic) in Turkey | |
| Participants | 66 infants ages 2 to 4 months Painful procedure: DPT vaccination Study period: June 2001 to July 2002 |
|
| Interventions | Intervention: Breastfeeding before, during, and after vaccination Control: Baby swaddled with only leg to be injected exposed, and positioned on soft mattress on examination table. Mothers encouraged to soothe infant vocally during and after the injection. The infant was cuddled by mother after vaccination. |
|
| Outcomes | Cry duration from time of needle insertion until all crying activity stopped, up to 3 minutes Heart rate during the injection and after needle was removed Oxygen saturation during the injection and after needle was removed |
|
| Notes | Power calculation: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sixty‐six envelopes designating group assignments, 33 for breast‐feeding and 33 for control, were mixed and shuffled. After we obtained informed consent from the participants, we opened the top envelope from the stack to identify each infant’s group assignment." (p. 11) Infants were randomised by shuffling envelopes. |
| Allocation concealment (selection bias) | Unclear risk | "Sixty‐six envelopes designating group assignments, 33 for breast‐feeding and 33 for control, were mixed and shuffled." (p. 11) Envelopes were used but does not specify if they were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "At the end of the third minute of breast‐feeding, while the infants were still sucking, the immunization injections were performed. The nurse practitioner gave her standard care, which included giving advice, preparing and administering the immunization solutions, and supervising the soothing techniques." (p. 12) Personnel could not be blinded to whether infant was breastfeeding or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All infants were audiotaped (Sony M‐529V microcassette recorder)." (p. 12) The researcher completing the duration of cry time was blinded to intervention group since only audio recordings were used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data reported on all 66 randomised infants. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not registered in trial registries searched. |
| Sample size | High risk | Fewer than 50 infants per arm. |
| Other bias | Low risk | Appears free of other bias. |
Esfahani 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial at a single site (healthcare centre) in Iran | |
| Participants | 96 infants ages 6 to 12 months Painful procedure: DPT and MMR vaccination Study period: April to July 2011 |
|
| Interventions | Intervention 1: Breastfeeding before and during vaccination Intervention 2: Massage on infant's palm or sole 1 minute before injection Control: Maternal hugging only |
|
| Outcomes | NIPS score during vaccination | |
| Notes | Power calculation: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The type of vaccine to be investigated was randomly selected from 96 envelopes marked as A and B, which the researcher had already made by random number table (zero was ignored; numbers 1, 2, 3 were assigned to the massage group; 4, 5, 6, to the breastfeeding group; and 7, 8, 9 to the control group)." (p. 4) Sequence generated using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | "The envelope was selected and opened based on the age of the qualified subjects who had referred for vaccination, and according to an already recorded method, necessary interventions were conducted." (pp. 4‐5) Envelopes were used but does not specify if they were sequentially numbered, sealed, and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "In the mother's breast feeding group, the mother started breast feeding the infant and vaccination was conducted during active and constant sucking. In the massage therapy group, the researcher massaged the first knuckle of the middle or ring finger of the infants’ palm or sole of the injection side for 60 sec and finally vaccinated the subject." (p. 4) Personnel could not be blinded to whether infant was breastfeeding, receiving massage, or no intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Next, neonatal infant pain scale (NIPS) checklist, which is a standard tool to measure pain with its validity and reliability having been confirmed, [1,21] was ticked in all groups by a researcher through observing the subjects during the procedure of vaccination." (p. 4) The researcher completing the NIPS scores could not be blinded to whether the infant was breastfeeding or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "A total of 99 infants entered the study. Three infants were left out due to restlessness and cry before injection. Data analysis was conducted for 96 subjects." (p. 5) Outcome data reported on all remaining infants. Less than 10% missing data. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not registered in trial registries searched. |
| Sample size | High risk | Fewer than 50 infants per arm. |
| Other bias | Low risk | Appears free of other bias. |
Goswami 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial at a single site (immunisation clinic) in India | |
| Participants | 120 infants ages 6 weeks to 3 months Painful procedure: wDPT vaccination Study period: Not specified |
|
| Interventions | Intervention 1: Breastfeeding before and during vaccination Intervention 2: 2 mL of 25% dextrose given orally 2 minutes prior to vaccination Control: 2 mL of distilled water given orally 2 minutes prior to vaccination |
|
| Outcomes | Cry duration from time of needle insertion until a period of silence of more than 5 seconds, up to a maximum of 3 minutes (presented as median and IQR but converted to mean and standard deviation for meta‐analysis) Latency of onset of cry (the time from needle insertion to onset of vocalisation of cry) MFCS at time of needle insertion, 1 minute after needle insertion, and 3 minutes after needle insertion (presented in graphical form only) |
|
| Notes | Power calculation: Yes (p. 650) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The subjects were randomised into three groups of 40 each through computers generated random numbers and put in serially numbered opaque sealed envelopes (SNOSE method)." (p. 649) Sequence generated using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | "The subjects were randomised into three groups of 40 each through computers generated random numbers and put in serially numbered opaque sealed envelopes (SNOSE method)." (p. 649) Serially numbered, opaque, sealed envelopes used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Babies in breastfed group were breastfed throughout the intervention, starting 2 minutes prior to the vaccination; 25% dextrose group: 2 ml of 25% dextrose was given orally by a sterile syringe 2 minutes prior to intramuscular vaccination; Placebo group: 2 ml distilled water was given orally by a sterile syringe 2 minutes prior to intramuscular vaccination." (p. 65) Personnel could not be blinded to whether or not infant was breastfeeding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "All events were recorded by the investigator on a digital video camera (model Sony CCDTRV238E) for total duration of three minutes from the removal of the needle." (p. 650) The researcher completing the cry duration and MFCS could not be blinded to whether the infant was breastfeeding or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "120 babies were randomised into 3 groups of 40 babies each." (p. 650) Outcome data reported for all 120 randomised infants. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not registered in trial registries searched. |
| Sample size | High risk | Fewer than 50 infants per arm. |
| Other bias | Low risk | Appears free of other bias. |
Gupta 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial at a single site (immunisation clinic) in India | |
| Participants | 120 infants under the age of 3 months Painful procedure: wDPT vaccination Study period: October 2009 to September 2010 |
|
| Interventions | Intervention 1: Breastfeeding before and during vaccination + 1 g topical EMLA Cream applied to injection site 60 minutes before vaccination Intervention 2: 2 mL of distilled water given orally 2 minutes prior to vaccination + 1 g topical EMLA Cream applied to injection site 60 minutes before vaccination Control: 2 mL of distilled water given orally 2 minutes prior to vaccination + 1 g topical placebo cream applied to injection site 60 minutes before vaccination |
|
| Outcomes | Cry duration from time of needle insertion until a period of silence of more than 5 seconds, up to a maximum of 3 minutes Latency of onset of cry (the time from needle insertion to onset of vocalisation of cry) MFCS at time of needle insertion, 1 minute after needle insertion, and 3 minutes after needle insertion |
|
| Notes | Power calculation: Yes (p. 1529) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The subjects were randomised into three groups of 30 infants each through computer‐generated random numbers." (p. 1528) Sequence generated using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | "The numbers were written on paper slips, and these slips were put in serially numbered opaque sealed envelopes (SNOSE method)." (p. 1528) Serially numbered, opaque, sealed envelopes used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Breastfeeding started 2 min prior to vaccination and continued throughout the procedure." (p. 1528) Personnel could not be blinded to whether or not infant was breastfeeding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "All events were recorded by JK on a digital video camera (model Sony CCD‐TRV238E) for a total duration of 3 min from just before needle insertion." (p. 1528) The researcher completing the cry duration and MFCS could not be blinded to whether the infant was breastfeeding or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "A total of 90 babies were randomised in three groups of 30 each." (p. 1529) Outcome data reported for all 90 randomised infants. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol available (CTRI ‐ CTRI/2011/06/001783), but trial registered retrospectively. |
| Sample size | High risk | Fewer than 50 infants per arm. |
| Other bias | Low risk | Appears free of other bias. |
Razek 2009.
| Study characteristics | ||
| Methods | Quasi‐RCT study at 2 sites (maternal and child health centres) in Jordan | |
| Participants | 120 infants ages 1 to 12 months Painful procedure: Vaccination (type not specified) Study period: Not specified |
|
| Interventions | Intervention: Breastfeeding before, during, and after vaccination Control: No intervention |
|
| Outcomes | Cry duration from time of needle insertion until all crying activity stopped NIPS score during vaccination Wong‐Baker FACES Pain Rating Scale score during vaccination Heart rate before and after injection |
|
| Notes | Power calculation: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "All infants included in the study were divided into two equivalent groups (of 60 infants)." (p. 100) No random sequence generation. *Quasi‐RCT study |
| Allocation concealment (selection bias) | Unclear risk | "All infants included in the study were divided into two equivalent groups (of 60 infants)." (p. 100) Not enough information to assess how allocation was concealed. *Quasi‐RCT study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The mothers cradled their infants during breast‐feeding to maintain full‐body skin‐to‐skin contact during immunization injections." (p. 101) Personnel could not be blinded to whether or not infant was breastfeeding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The mothers cradled their infants during breast‐feeding to maintain full‐body skin‐to‐skin contact during immunization injections." (p. 101) The researcher completing the cry duration and pain scores (NIPS, Wong‐Baker) could not be blinded to whether the infant was breastfeeding or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The subjects of this study were 120 infants chosen according to the following inclusion criteria: full‐term, age between 1 and 12 months old, breast‐feed, no concurrent illness." (p. 100) Outcome data reported for all 120 infants. |
| Selective reporting (reporting bias) | High risk | In Table 7 NIPS data are not presented consistent with the tool ‐ data is presented with scores from 0 to 4, rather than 0 to 7, and includes language from Wong‐Baker FACES Pain Rating Scale. |
| Sample size | Unclear risk | Between 50 and 199 infants per arm. |
| Other bias | High risk | Study used the Wong‐Baker FACES Pain Rating Scale, which is meant to be used for self assessment only. |
Taavoni 2009a.
| Study characteristics | ||
| Methods | Randomised controlled trial at 2 sites (health clinics) in Iran | |
| Participants | 76 infants ages 2 to 4 months Painful procedure: DPT vaccination Study period: February 2008 to April 2008 |
|
| Interventions | Intervention: Breastfeeding before, during, and after vaccination Control: No intervention |
|
| Outcomes | MBPS 5 seconds before injection and 15 seconds after injection | |
| Notes | Power calculation: Yes (p. 35) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used. |
| Allocation concealment (selection bias) | Unclear risk | Not enough information provided to assess. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel could not be blinded to whether or not infant was breastfeeding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The researcher completing the MBPS scores could not be blinded to whether the infant was breastfeeding or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not registered in trial registries searched. |
| Sample size | High risk | Fewer than 50 infants per arm. |
| Other bias | Low risk | Appears free of other bias. |
Thomas 2011.
| Study characteristics | ||
| Methods | Quasi‐RCT | |
| Participants | Infants aged 5 to 15 weeks and receiving their 1st, 2nd, or 3rd doses of DPT immunisation | |
| Interventions | Intervention: Breastfeeding 2 minutes before and during injection Control: No intervention. Position of infant not specified |
|
| Outcomes | Modified NIPS at 1 and 5 minutes following injection. No description given of modifications made to the NIPS. | |
| Notes | Power calculation: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors did not specify how infants were allocated to each group. They stated "... 20 were assigned to the experimental group and 20 to the control group" (p. 184). They also stated: "Non‐probability purposive sampling technique was found more appropriate to make the study more feasible". It is unclear what this means. |
| Allocation concealment (selection bias) | Unclear risk | The following statement by the authors provided insufficient information to assess this category: "... Who possess their immunisation card were selected, out of which 20 were assigned to the experimental group and 20 to the control group" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of breastfeeding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment done at the time of vaccine, therefore blinding of breastfeeding not possible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40 infants were included, and data were reported for 40 infants. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not registered in trial registries searched. |
| Sample size | High risk | Fewer than 50 infants per arm. |
| Other bias | Low risk | Appears free of other bias. |
DPT: diphtheria‐pertussis‐tetanus IQR: interquartile range MBPS: Modified Behavioral Pain Scale MFCS: Modified Facial Coding System MMR: measles, mumps, rubella NFCS: Neonatal Facial Coding System NIPS: Neonatal Infant Pain Scale RCT: randomised controlled trial wDPT: whole cell diphtheria‐pertussis‐tetanus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Achema 2011 | Breastfeeding was not used during the painful procedure. |
| Barr 2001 | Conference abstract |
| Gradin 2003 | Conference abstract |
| Gray 2002 | Infants younger than inclusion criteria. |
| Hashemi 2016 | Infants younger than inclusion criteria. |
| Jebreili 2015 | Breastfeeding was not used during the painful procedure. |
| Modarres 2014 | Infants younger than inclusion criteria. |
| Otero López 2014 | Not a randomised controlled trial (review) |
| Sahebihag 2011 | Breastfeeding was not used during the painful procedure. |
| Shendurnikar 2005 | Infants younger than inclusion criteria. |
| Singal 2004 | Conference abstract |
| Taavoni 2009b | Conference abstract; appeared to be same population as Taavoni 2010, Taavoni 2011, and Taavoni 2012; presented information found in published study included in this review (Taavoni 2009a). |
| Taavoni 2010 | Conference abstract; appeared to be same population as excluded studies Taavoni 2009b, Taavoni 2011, and Taavoni 2012; presented information found in published study included in this review (Taavoni 2009a). |
| Taavoni 2011 | Conference abstract; appeared to be same population as excluded studies Taavoni 2009b, Taavoni 2010, and Taavoni 2012; presented information found in published study included in this review (Taavoni 2009a). |
| Taavoni 2012 | Conference abstract; appeared to be same population as excluded studies Taavoni 2009b, Taavoni 2010, and Taavoni 2011; presented information found in published study included in this review (Taavoni 2009a). |
| Taavoni 2013 | Conference abstract |
| Uga 2008 | Infants younger than inclusion criteria. |
Characteristics of ongoing studies [ordered by study ID]
Mohan 2014.
| Study name | Comparison of breastfeeding and 25% oral dextrose for pain relief during immunization of infants: a randomized controlled trial |
| Methods | Randomised controlled trial at a single site (immunisation clinic) in India |
| Participants | Infants ages 1 to 6 months Painful procedure: Pentavalent vaccination |
| Interventions | Intervention 1: Breastfeeding before and during vaccination Intervention 2: 2 mL of 25% dextrose given orally 2 minutes prior to vaccination |
| Outcomes | FLACC score at time of needle insertion, 1 minute after needle insertion, and 3 minutes after needle insertion |
| Starting date | 15 May 2014 (first infant enrolled) |
| Contact information | Dr. Anna Mathew MOSC Medical College, Department of Pharmacology Ernakulam, Kerala, PIN 682311, India 9442221950 350south@gmail.com |
| Notes | Study registered on Clinical Trials Registry ‐ India (CTRI #CTRI/2014/07/004724) |
FLACC: Face, Legs, Activity, Cry, Consolability
Differences between protocol and review
We had stated in our protocol that we would exclude quasi‐randomised controlled trials using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number). However, we revised this exclusion, and included all such studies.
In addition, in our protocol we stated that we would use the Oxford Quality Score as the basis for inclusion (Jadad 1996), limiting inclusion to studies that were randomised and double‐blind as a minimum. We did not use this, as the blinding of breastfeeding is not possible.
Contributions of authors
Denise Harrison (DH) oversaw the entire process of conducting the systematic review. In addition, DH arbitrated if required when there were disagreements relating to data collection, data extraction, and 'Risk of bias' ratings. All authors assisted in the conduct of the systematic review. Jessica Reszel (JR) and Mariana Bueno (MB) were primarily responsible for reviewing and rating articles. Catherine Larocque (CL) supported all aspects of organizing and entering data, supporting data analysis and reviewing and editing draft versions. Lucy Turner (LT) served as the methodological expert. Margaret Sampson (MS) developed the search strategies. Vibhuti Shah (VSS) and Anna Taddio (AT) provided clinical, methodological and editing expertise throughout the entire process.
Sources of support
Internal sources
-
CHEO, CHEO Foundation and University of Ottawa, Canada
Personnel support for Harrison and Reszel
External sources
-
CHEO Research Institute Summer Studentship (Catherine Larocque), Canada
Personnel support for student research internship
Declarations of interest
DH: none known. DH is a registered nurse and midwife, and specialist in neonatal and infant pain research.
JR: none known.
MB: none known. MB is a registered nurse and specialist in neonatal and infant pain research.
MS: none known.
VSS: none known. VSS is a specialist physician and manages patients in the neonatal intensive care unit.
AT: none known. AT is a specialist pharmacist and researches knowledge translation about immunisation pain.
CL: none known.
LT: none known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Barr unpublished {published data only}
- Barr RG, Holsti L, Young SN, Paterson JA, MacMartin LM. Breastfeeding effects on biobehavioral responses to diptheria-pertussis-tetanus immunizations in infants at two and four months. 3rd Annual Canadian Child Health Clinician Scientist National Symposium.
Boroumandfar 2013 {published data only (unpublished sought but not used)}
- Boroumandfar K, Khodaei F, Abdeyazdan Z, Maroufi M. Comparison of vaccination-related pain in infants who receive vapocoolant spray and breastfeeding during injection. Iranian Journal of Nursing and Midwifery Research 2013;18(1):33-7. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Dilli 2009 {published and unpublished data}
- Dilli D, Küçük I, Dallar Y. Interventions to reduce pain during vaccination in infancy. Journal of Pediatrics 2009;154(3):385-90. [DOI: 10.1016/j.jpeds.2008.08.037] [DOI] [PubMed] [Google Scholar]
Efe 2007 {published data only}
- Efe E, Ozer Z. The use of breast-feeding for pain relief during neonatal immunization injections. Applied Nursing Research 2007;20(1):10-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Esfahani 2013 {published data only}
- Esfahani MS, Sheykhi S, Abdeyazdan Z, Jodakee M, Boroumandfar K. A comparative study on vaccination pain in the methods of massage therapy and mothers' breast feeding during injection of infants referring to Navabsafavi Health Care Center in Isfahan. Iranian Journal of Nursing and Midwifery Research 2013;18(6):494-8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Goswami 2013 {published data only (unpublished sought but not used)}
- Goswami G, Upadhyay A, Gupta NK, Chaudhry R, Chawla D, Sreenivas V. Comparison of analgesic effect of direct breastfeeding, oral 25% dextrose solution and placebo during 1st DPT vaccination in healthy term infants: a randomized, placebo controlled trial. Indian Pediatrics 2013;50(7):649-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gupta 2013 {published data only}
- Gupta NK, Upadhyay A, Agarwal A, Goswami G, Kumar J, Sreenivas. Randomized controlled trial of topical EMLA and breastfeeding for reducing pain during wDPT vaccination. European Journal of Pediatrics 2013;172:1527-33. [DOI: 10.1007/s00431-013-2076-6] [DOI] [PubMed] [Google Scholar]
Razek 2009 {published data only (unpublished sought but not used)}
- Razek A, El-Dein NAZ. Effect of breast-feeding on pain relief during infant immunization injections. International Journal of Nursing Practice 2009;15(2):99-104. [PMID: ] [DOI] [PubMed] [Google Scholar]
Taavoni 2009a {published data only}
- Taavoni S, ShahAli S, Haghani H, Neisani Samani L. Comparison the effect of breast feeding with routine clinical procedure on pain relieving during immunization injection. Journal of Arak University of Medical Sciences 2009;11(4):33-40. [Google Scholar]
Thomas 2011 {published data only}
- Thomas T, Shetty AP, Bagali PV. Role of breastfeeding in pain response during injectable immunisation among infants. Nursing Journal of India 2011;102(8):184-6. [PubMed] [Google Scholar]
References to studies excluded from this review
Achema 2011 {published data only}
- Achema G, Oyeleye B, Olaogun AAE. Pain relief strategies for infants taking DPT immunization in health centres. African Journal of Midwifery & Women's Health 2011;5(3):123-7. [Google Scholar]
Barr 2001 {published data only}
- Barr RG, Paterson JA, MacMartin LM, Lessard J, Calinoiu N, Young SN. Breastfeeding analgesia for procedural pain in 2-month-old infants. Pediatric Research 2001;49(4):313A. [Google Scholar]
Gradin 2003 {published data only}
- Gradin M, Finnstrom O, Schollin J. Comparison of oral glucose and breast-feeding on neonatal pain response to venipuncture. Pediatric Research 2003;53:122. [Google Scholar]
Gray 2002 {published data only}
- Gray L, Miller LW, Philipp BL, Blass EM. Breastfeeding is analgesic in healthy newborns. Pediatrics 2002;109(4):590-3. [DOI] [PubMed] [Google Scholar]
Hashemi 2016 {published data only}
- Hashemi F, Taheri L, Ghodsbin F, Pishva N, Vossoughi M. Comparing the effect of swaddling and breastfeeding and their combined effect on the pain induced by BCG vaccination in infants referring to Motahari Hospital, Jahrom, 2010–2011. Applied Nursing Research 2016;29:217-21. [DOI] [PubMed] [Google Scholar]
Jebreili 2015 {published data only}
- Jebreili M, Neshat H, Seyyedrasouli A, Ghojazade M, Hosseini MB, Hamishehkar H. Comparison of breastmilk odor and vanilla odor on mitigating premature infants’ response to pain during and after venipuncture. Breastfeeding Medicine 2015;10(7):362-5. [DOI: 10.1089/bfm.2015.0060] [DOI] [PubMed] [Google Scholar]
Modarres 2014 {published data only}
- Modarres M, Jazayeri A, Rahnama P, Montazeri A. Breastfeeding and pain relief in full-term neonates during immunization injections: a clinical randomized trial. MIDIRS Midwifery Digest 2014;13(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
Otero López 2014 {published data only}
- Otero López MC, Gago López MM, Bouzada Rodriguez AL, Ballesteros Mantecón M, Garcia Álvarez MM, González Centeno MJ. Effective interventions in the handling of the pain in children submitted to procedures with needles [Intervenciones efectivas en el manejo del dolor en niños sometidos a procedimientos con agujas]. Nure Investigación 2014;72:1-17. [Google Scholar]
Sahebihag 2011 {published data only}
- Sahebihag MH, Hosseinzadeh M, Mohammadpourasi A, Kosha A. The effect of breastfeeding, oral sucrose and combination of oral sucrose and breastfeeding in infant’s pain relief during the vaccination. Iranian Journal of Nursing and Midwifery Research 2011;16(1):9-15. [Google Scholar]
Shendurnikar 2005 {published data only}
- Shendurnikar N, Gandhi K. Analgesic effects of breastfeeding on heel lancing. Indian Pediatrics 2005;42(7):730-2. [PubMed] [Google Scholar]
Singal 2004 {published data only}
- Singal A, Upadhyay A, Aggarwal R, Kaushal M, Upadhyay M, Agarwal R, et al. Analgesic effect of breast feeding prior to venepuncture in term neonates: a randomized double blind, controlled trial [abstract]. In: 13th Congress of the Federation of Asia and Oceania Perinatal Societies. 2004.
Taavoni 2009b {published data only}
- Taavoni S, Shahali S, Haghani H, Neisani SL. Effect of breast feeding on pain relief during immunization injection. International Journal of Gynecology & Obstetrics 2009;107(S2):S683. [Google Scholar]
Taavoni 2010 {published data only}
- Taavoni S, Shahali S, Haghani H. Pain relief during immunization injection: Comparison between the effects of breast feeding with routine procedure. Journal of Paediatrics and Child Health 2010;World Congress of Internal Medicine, WCIM 2010 in Conjunction with Physicians Week Melbourne, VIC Australia:21-2. [Google Scholar]
Taavoni 2011 {published data only}
- Taavoni S, Shahali S Haghani H. Vaccination pain management: Effect of breast feeding on infant's behavioral pain scale, a randomized control trial study. European Journal of Pain Supplements 2011;5:159. [Google Scholar]
Taavoni 2012 {published data only}
- Taavoni S, Shahali S, Hagahni H, Neisani SL. Effect of breast feeding on infants behavioral pain scale during DPT vaccination. European Psychiatry 2012;27(S1):1. [DOI: ] [Google Scholar]
Taavoni 2013 {published data only}
- Taavoni S, Shahali S, Haghani H. Infants' vaccination pain management and effect of breast feeding, sucking the pacifier and mother's hug: a randomized control trial. European Psychiatry 2013;28(S1):1. [Google Scholar]
Uga 2008 {published data only}
- Uga E, Candriella M, Perino A, Alloni V, Angilella G, Trada M. Heel lance in newborn during breastfeeding: an evaluation of analgesic effect of this procedure. Italian Journal of Pediatrics 2008;34(3):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Mohan 2014 {unpublished data only}
- Comparison of breastfeeding and 25% oral dextrose for pain relief during immunization of infants: a randomized controlled trial. Ongoing study. 15 May 2014 (first infant enrolled). Contact author for more information.
Additional references
Barrett 2000
- Barrett T, Kent S, Voudoris N. Does melatonin modulate beta-endorphin, corticosterone, and pain threshold? Life Sciences 2000;66(6):467-76. [DOI] [PubMed] [Google Scholar]
Blass 1992
- Blass EM, Smith BA. Differential effects of sucrose, fructose, glucose, and lactose on crying in 1- to 3-day-old human infants: qualitative and quantitative considerations. Developmental Psychology 1992;28(5):804-10. [Google Scholar]
Blass 1995
- Blass EM, Shide DJ, Zaw-Mon C, Sorrentino J. Mother as shield: differential effects of contact and nursing on pain responsivity in infant rats - evidence for nonopioid mediation. Behavioral Neuroscience 1995;109(2):342-53. [DOI] [PubMed] [Google Scholar]
Blass 1997
- Blass EM. Milk-induced hypoalgesia in human newborns. Pediatrics 1997;99(6):825-9. [DOI] [PubMed] [Google Scholar]
Bueno 2013
- Bueno M, Yamada J, Harrison D, Kahn S, Ohlsson A, Adams-Webber T, et al. A systematic review and meta-analyses of non-sucrose sweet solutions for pain relief in neonates. Pain Research and Management 2013;18(3):153-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- GRADE Working Group, McMaster University GRADEpro (www.gradepro.org) (developed by Evidence Prime, Inc.). Version accessed 12 October, 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Harrison 2010a
- Harrison D, Bueno M, Yamada J, Adams-Webber T, Stevens B. Analgesic effects of sweet tasting solutions in infants: Do we have equipoise yet? Pediatrics 2010;126(5):894-902. [DOI] [PubMed] [Google Scholar]
Harrison 2010b
- Harrison D, Yamada J, Stevens B. Strategies for the prevention and management of neonatal and infant pain. Current Pain and Headache Reports 2010;14(2):113-23. [DOI] [PubMed] [Google Scholar]
Harrison 2010c
- Harrison D, Stevens B, Bueno M, Yamada J, Adams-Webber T, Beyene J, et al. Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review. Archives of Disease in Childhood 2010;95(6):406-13. [DOI: 10.1136/406 adc.2009.174227] [DOI] [PubMed] [Google Scholar]
Harrison 2013
- Harrison D, Elia S, Royle J, Manias E. Pain management strategies used during early childhood immunisation in Victoria. Journal of Paediatrics and Child Health 2013;49(4):313-8. [DOI] [PubMed] [Google Scholar]
Harrison 2016
- Harrison D, Children's Hospital of Eastern Ontario (CHEO). Reduce your infant's pain during newborn blood tests (updated). YouTube video (https://www.youtube.com/watch?v=HmJGQJ8ayL8&feature=youtu.be) 2016.
Heine 1999
- Heine WE. The significance of tryptophan in infant nutrition. Advances in Experimental Medicine and Biology 1999;467:705-10. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:889-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2011
- Johnston C, Barrington KJ, Taddio A, Carbajal R, Filion F. Pain in Canadian NICUs: Have we improved over the past 12 years? Clinical Journal of Pain 2011;27(3):225-32. [DOI] [PubMed] [Google Scholar]
Johnston 2014
- Johnston C, Campbell-Yeo M, Fernandes A, Inglis D, Streiner D, Zee R. Skin-to-skin care for procedural pain in neonates. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD008435. [DOI: 10.1002/14651858.CD008435.pub2] [DOI] [PubMed] [Google Scholar]
Kassab 2012
- Kassab M, Foster JP, Foureur M, Fowler C. Sweet-tasting solutions for needle-related procedural pain in infants one month to one year of age. Cochrane Database of Systematic Reviews 2013, Issue 2. Art. No: CD008411. [DOI: 10.1002/14651858.CD008411.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidiset JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of Internal Medicine 2009;151(4):65-94. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PubMed] [Google Scholar]
Lisi 2013
- Lisi D, Campbell L, Pillai Riddell R, Garfield H, Greenberg S. Naturalistic parental pain management during immunizations during the first year of life: observational norms from the OUCH cohort. Pain 2013;154:1245-53. [DOI: 10.1016/j.pain.2013.03.036] [DOI] [PubMed] [Google Scholar]
McGowan 2010
- McGowan J, Sampson M, Lefebvre C. An evidence based checklist for the peer review of electronic search strategies. Evidence Based Library and Information Practice 2010;5(1):149-54. [Google Scholar]
Mills 2005
- Mills E, Jadad AR, Ross C, Wilson K. Systematic review of qualitative studies exploring parental beliefs and attitudes toward childhood vaccination identifies common barriers to vaccination. Journal of Clinical Epidemiology 2005;58(11):1081-8. [DOI] [PubMed] [Google Scholar]
PHA Canada 2014
- Public Health Agency of Canada. Immunization programs in Canada - routine schedule for infants and children including special programs and catch-up programs (as of January 2014). www.phac-aspc.gc.ca/im/iyc-vve/is-cv-eng.php (accessed 30 July 2014).
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Russell 2015
- Russell K, Harrison D. Managing pain in early childhood immunisation. Kai Tiaki Nursing New Zealand 2015;21(2):22-4. [PMID: ] [PubMed] [Google Scholar]
Schechter 2007
- Schechter NL, Zempsky WT, Cohen LL, McGrath PJ, McMurtry CM, Bright NS. Pain reduction during pediatric immunizations: evidence-based review and recommendations. Pediatrics 2007;119(5):e1184-98. [DOI] [PubMed] [Google Scholar]
Shah 2004
- Shah V, Ipp M, Sam J, Einarson TR, Taddio A. Eliciting the minimal clinically important difference in the pain response from parents of newborn infants and nurses. Paediatrics & Child Health 2004;9(Suppl A):Abstract 82. [Google Scholar]
Shah 2009
- Shah V, Taddio A, Rieder MJ. Effectiveness and tolerability of pharmacologic and combined interventions for reducing injection pain during routine childhood immunizations: systematic review and meta-analyses. Clinical Therapeutics 2009;31(Suppl B):S104-51. [DOI] [PubMed] [Google Scholar]
Shah 2012
- Shah PS, Herbozo C, Aliwalas LI, Shah VS. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD004950. [DOI: 10.1002/14651858.CD004950.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2015
- Shah V, Taddio A, Mcurtry CM, Halperin S, Noel M, Pillai Riddell R, et al. Pharmacological and combined interventions to reduce vaccine injection pain in children and adults. Clinical Journal of Pain 2015;31(10):S38-63. [DOI: 10.1097/AJP.0000000000000281] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Sutton AJ, Ioanidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Stevens 2011
- Stevens B, Abbott L, Yamada J, Harrison D, Stinson J, Taddio A, et al, CIHR Team in Children's Pain. Epidemiology and management of painful procedures in hospitalized children across Canada. Canadian Medical Association Journal 2011;183(7):E403-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2013
- Stevens B, Yamada J, Lee GY, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD001069. [DOI: 10.1002/14651858.CD001069.pub4] [DOI] [PubMed] [Google Scholar]
Taddio 1995
- Taddio A, Goldbach M, Ipp M, Stevens B, Koren G. Effect of neonatal circumcision on pain responses during vaccination in boys. Lancet 1995;345(8945):291-2. [DOI] [PubMed] [Google Scholar]
Taddio 2005
- Taddio A, Katz J. The effects of early pain experience in neonates on pain responses in infancy and childhood. Pediatric Drugs 2005;7(4):245-57. [DOI] [PubMed] [Google Scholar]
Taddio 2007
- Taddio A, Manley J, Potash L, Ipp M, Sgro M, Shah V. Routine immunization practices: use of topical anesthetics and oral analgesics. Pediatrics 2007;120(3):e637-43. [DOI] [PubMed] [Google Scholar]
Taddio 2009
- Taddio A, Chambers CT, Halperin SA, Ipp M, Lockett D, Rieder MJ, et al. Inadequate pain management during routine childhood immunizations: the nerve of it. Clinical Therapeutics 2009;31(Suppl 2):S152-67. [DOI] [PubMed] [Google Scholar]
Weissman 2009
- Weissman A, Aranovitch M, Blazer S, Zimmer ET. Heel-lancing in newborns: Behavioural and spectral analysis assessment of pain control methods. Pediatrics 2009;124:e9216. [DOI: 10.1542/peds.2009-0598] [DOI] [PubMed] [Google Scholar]
Wong 1999
- Wong D, Hockenberry-Eaton M, Wilson D, Winkelstein M, Ahlman E, Devito-Thomas M. Nursing Care of Infants and Children. 6th edition. St. Louis (MO): Mosby, 1999. [Google Scholar]
World Health Organization 2015
- World Health Organization. Reducing pain at the time of vaccination: WHO position paper - September 2015. Weekly Epidemiological Record September 2015;90:505-16. [Google Scholar]
Wright 2009
- Wright S, Yelland M, Heathcote K, Ng SK, Wright G. Fear of needles - nature and prevalence in general practice. Australian Family Physician 2009;38(3):172-6. [PubMed] [Google Scholar]
Zanardo 2001
- Zanardo V, Nicolussi S, Carlo G, Marzari F, Faggian D, Favaro F, et al. Beta endorphin concentrations in human milk. Journal of Pediatric Gastroenterology and Nutrition 2001;33(2):160-4. [DOI] [PubMed] [Google Scholar]


