Abstract
Background
Progesterone prepares the endometrium for pregnancy by stimulating proliferation in response to human chorionic gonadotropin (hCG) produced by the corpus luteum in the luteal phase of the menstrual cycle. In assisted reproduction techniques (ART), progesterone and/or hCG levels are low, so the luteal phase is supported with progesterone, hCG or gonadotropin‐releasing hormone (GnRH) agonists to improve implantation and pregnancy rates.
Objectives
To determine the relative effectiveness and safety of methods of luteal phase support provided to subfertile women undergoing assisted reproduction.
Search methods
We searched databases including the Cochrane Menstrual Disorders and Subfertility Group (MDSG) Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO and trial registers up to November 2014. Further searches were undertaken in August 2015.
Selection criteria
Randomised controlled trials (RCTs) of luteal phase support using progesterone, hCG or GnRH agonist supplementation in ART cycles.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Our primary outcome was live birth or ongoing pregnancy. The overall quality of the evidence was assessed using GRADE methods.
Main results
Ninety‐four RCTs (26,198 women) were included. Most studies had unclear or high risk of bias in most domains. The main limitations in the evidence were poor reporting of study methods and imprecision due to small sample sizes.
1. hCG vs placebo/no treatment (five RCTs, 746 women)
Findings suggested benefit for the hCG group in live birth or ongoing pregnancy rates when data were analysed with a fixed‐effect model (OR 1.76, 95% CI 1.08 to 2.86, three RCTs, 527 women, I2 = 24%, very low‐quality evidence) but there was no clear evidence of a difference using a random‐effects model (OR 1.67, 95% CI 0.90 to 3.12). hCG may increase ovarian hyperstimulation syndrome (OHSS) rates (OR 4.28, 95% CI 1.91 to 9.6, one RCT, 387 women, low‐quality evidence).
2. Progesterone vs placebo/no treatment (eight RCTs, 875 women)
Findings suggested benefit for the progesterone group in live birth or ongoing pregnancy rates when data were analysed with a fixed‐effect model (OR 1.77, 95% CI 1.09 to 2.86, five RCTs, 642 women, I2 = 35%, very low‐quality evidence) but there was no clear evidence of a difference using a random‐effects model (OR 1.77, 95% CI 0.96 to 3.26). OHSS was not reported.
3. Progesterone vs hCG regimens (16 RCTs, 2162 women)
hCG regimens included hCG alone and hCG with progesterone. There was no evidence of a difference between progesterone and hCG regimens in live birth or ongoing pregnancy rates (OR 0.95, 95% CI 0.65 to 1.38, five RCTs, 833 women, I2 = 0%, low‐quality evidence). Progesterone was associated with lower OHSS rates than hCG regimens (OR 0.46, 95% CI 0.30 to 0.71, 5 RCTs, 1293 women , I2=48%).
4. Progesterone vs progesterone with oestrogen (16 RCTs, 2577 women)
There was no evidence of a difference between the groups in rates of live birth or ongoing pregnancy (OR 1.12, 95% CI 0.91 to 1.38, nine RCTs, 1651 women, I2 = 0%, low‐quality evidence) or OHSS (OR 0.56, 95% CI 0.2 to 1.63, two RCTs, 461 women, I2 = 0%, low‐quality evidence).
5. Progesterone vs progesterone + GnRH agonist (seven RCTs, 1708 women)
Live birth or ongoing pregnancy rates were lower in the progesterone‐only group than the progesterone plus GnRH agonist group (OR 0.62, 95% CI 0.48 to 0.81, nine RCTs, 2861 women, I2 = 55%, random effects, low‐quality evidence). Statistical heterogeneity was high but the direction of effect was consistent across studies. OHSS was reported in one study only; there was no evidence of a difference between the groups (OR 1.00, 95% CI 0.33 to 3.01, one RCT, 300 women, very low quality evidence).
6. Progesterone regimens (45 RCTs, 13,814 women)
There were nine different comparisons between progesterone regimens. Findings for live birth or ongoing pregnancy were as follows: intramuscular (IM) versus oral: OR 0.71, 95% CI 0.14 to 3.66 (one RCT, 40 women, very low‐quality evidence); IM versus vaginal/rectal: OR 1.37, 95% CI 0.94 to 1.99 (seven RCTs, 2309 women, I2 = 71%, random effects, very low‐quality evidence); vaginal/rectal versus oral: OR 1.19, 95% CI 0.83 to 1.69 (four RCTs, 857 women, I2 = 32%, low‐quality evidence); low‐dose versus high‐dose vaginal: OR 0.97, 95% CI 0.84 to 1.11 (five RCTs, 3720 women, I2 = 0%, moderate‐quality evidence); short versus long protocol: OR 1.04, 95% CI 0.79 to 1.36 (five RCTs, 1205 women, I2 = 0%, low‐quality evidence); micronised versus synthetic: OR 0.9, 95% CI 0.53 to 1.55 (two RCTs, 470 women, I2 = 0%, low‐quality evidence); vaginal ring versus gel: OR 1.09, 95% CI 0.88 to 1.36 (one RCT, 1271 women, low‐quality evidence); subcutaneous versus vaginal gel: OR 0.92, 95% CI 0.74 to 1.14 (two RCTs, 1465 women, I2 = 0%, low‐quality evidence); vaginal versus rectal: OR 1.28, 95% CI 0.64 to 2.54 (one RCT, 147 women, very low‐quality evidence). OHSS rates were reported for only two comparisons: IM versus oral, and low versus high‐dose vaginal; there was no evidence of a difference between the groups.
7. Progesterone and oestrogen regimens (two RCTs, 1195 women)
The included studies compared two different oestrogen protocols. There was no evidence of a difference in live birth or ongoing pregnancy rates between a short or long protocol (OR 1.08, 95% CI 0.81 to 1.43, one RCT, 910 women, low‐quality evidence) or between a low or high dose of oestrogen (OR 0.65, 95% CI 0.37 to 1.13, one RCT, 285 women, very low‐quality evidence). Neither study reported OHSS.
Authors' conclusions
hCG or progesterone given during the luteal phase may be associated with higher rates of live birth or ongoing pregnancy than placebo or no treatment, but the evidence is not conclusive. The addition of GnRHa to progesterone appears to improve outcomes. hCG may increase the risk of OHSS compared to placebo. Moreover hCG, with or without progesterone, is associated with higher rates of OHSS than progesterone alone. Neither the addition of oestrogen nor the route of progesterone administration appears to be associated with an improvement in outcomes.
Plain language summary
Luteal phase support for assisted reproduction
Review question
Many different interventions, dosages and administration routes of luteal phase support have been investigated. We made seven different comparisons to prepare a complete overview of this topic.
Background
After ovulation, the luteal phase of the menstrual cycle starts, and continues until the next menstruation. Remnants of the ovulated egg in the ovary are known as 'corpus luteum', or yellow body. The yellow body produces hormones, including progesterone. Progesterone stimulates proliferation of the lining of the uterus to prepare for implantation.
During assisted reproduction, the woman's pituitary gland is desensitised with medications so that the ovaries can be stimulated in a controlled manner. This results in more mature eggs, which can be harvested and fertilised outside the woman's body. Hyperstimulation of the ovaries causes a luteal phase defect, as the corpus luteum is unable to produce sufficient progesterone.
As a low progesterone level may lower the chance of implantation, the luteal phase needs to be supported. This may involve oral, vaginal or intramuscular progesterone, human chorionic gonadotropin (hCG) (which stimulates progesterone production) or gonadotropin‐releasing hormone (GnRH) agonists. GnRH agonists stimulate the production of GnRH, a hormone responsible for follicle‐stimulating hormone (FSH), and luteinising hormone (LH), which triggers ovulation and develops the yellow body. GnRH agonists are thought to restore LH levels and support the luteal phase naturally.
Study characteristics
We found 94 randomised controlled trials comparing different luteal phase support regimens in a total of 26,198 women. Our primary outcome was live birth or ongoing pregnancy. Other outcomes were clinical pregnancy, ovarian hyperstimulation syndrome (OHSS), miscarriage and multiple pregnancy. The evidence is current to August 2015.
Key results
hCG or progesterone given during the luteal phase may be associated with higher rates of live birth or ongoing pregnancy than placebo or no treatment, but the evidence is not conclusive. The addition of GnRHa to progesterone appears to improve outcomes. hCG may increase the risk of OHSS compared to placebo. Moreover hCG, with or without progesterone, is associated with higher rates of OHSS than progesterone alone. Neither the addition of oestrogen nor the route of progesterone administration appears to be associated with an improvement in outcomes.
Quality of the evidence
Evidence for most comparisons was of low or very low quality. The main limitations in the evidence were poor reporting of study methods and imprecision due to small sample sizes.
Summary of findings
Summary of findings for the main comparison. hCG compared with placebo/no treatment for assisted reproduction cycles.
| hCG compared with placebo/no treatment for assisted reproduction cycles | ||||||
| Population: subfertile women Setting: assisted reproduction Intervention: hCG Comparison: placebo/no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/No treatment | hCG | |||||
| Live birth or ongoing pregnancy | 120 per 1000 | 194 per 1000 (128 to 281) | OR 1.76 (1.08 to 2.86) | 527 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,f | |
| Clinical pregnancy | 155 per 1000 | 192 per 1000 (141 to 256) | OR 1.3 (0.9 to 1.88) | 746 (5 RCTs) | ⊕⊕⊝⊝ Very lowa,b,c,d | |
| OHSS | 41 per 1000 | 155 per 1000 (76 to 292) | OR 4.28 (1.91 to 9.6) | 387 (1 RCT) | ⊕⊕⊝⊝ Lowa,c,e | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aSerious risk of bias due to inadequate reporting of study methods. Risk of bias unclear in most domains of most studies.
bSerious imprecision with low event rate.
cNumber of studies was not sufficient for assessment of publication bias.
dFindings compatible with meaningful benefit for hCG group, or with no effect.
eSerious imprecision; single study with low event rate.
fFindings not statistically significant when random‐effects model was used (OR 1.67, 95% CI 0.90 to 3.12), or when analysis was restricted to studies reporting live birth.
Summary of findings 2. Progesterone compared with placebo/no treatment for assisted reproduction cycles.
| Progesterone compared with placebo/no treatment for assisted reproduction cycles | ||||||
| Population: subfertile women Setting: assisted reproduction Intervention: progesterone Comparison: placebo/no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no treatment | Progesterone | |||||
| Live birth or ongoing pregnancy | 39 per 1000 | 66 per 1000 (42 to 103) | OR 1.77 (1.09 to 2.86) | 642 (5 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,d | |
| Clinical pregnancy | 100 per 1000 | 174 per 1000 (126 to 234) | OR 1.89 (1.3 to 2.75) | 841 (7 RCTs) | ⊕⊕⊝⊝ Lowa,b,c | |
| OHSS | Not reported in any included studies | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aSerious risk of bias due to inadequate reporting of study methods. Risk of bias unclear in most domains of most studies.
bSerious imprecision with low event rate.
cNumber of studies was not sufficient for assessment of publication bias.
d Findings not statistically significant when random‐effects model was used (OR 1.77, 95% CI 0.96 to 3.26), or when analysis was restricted to studies reporting live birth.
Summary of findings 3. Progesterone compared with hCG regimens for assisted reproduction cycles.
| Progesterone compared with hCG regimens for assisted reproduction cycles | ||||||
| Population: subfertile women Setting: assisted reproduction Intervention: progesterone Comparison: hCG (alone or with progesterone) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| hCG (alone or with progesterone) | Progesterone | |||||
| Live birth or ongoing pregnancy | 198 per 1000 | 190 per 1000 (138 to 254) | OR 0.95 (0.65 to 1.38) | 833 (5 RCTs) | ⊕⊕⊝⊝ Lowa,b,c,d | |
| Clinical pregnancy | 284 per 1000 | 300 per 1000 (263 to 340) | OR 1.08 (0.9 to 1.3) | 2355 (16 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| OHSS | 118 per 1000 | 58 per 1000 (39 to 87) |
OR 0.46 (0.30 to 0.71) |
1293 (5 studies) |
⊕⊕⊝⊝ Lowa,c,e | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aSerious risk of bias due to inadequate reporting of study methods. Risk of bias unclear in most domains of most studies.
bSerious imprecision with low event rate.
cNumber of studies was not sufficient for assessment of publication bias.
dFindings compatible with meaningful benefit for either group, or with no effect
e Some inconsistency: I2=48% overall, I2=60% in progesterone vs hCG subgroup
Summary of findings 4. Progesterone compared with progesterone + oestrogen for assisted reproduction cycles.
| Progesterone compared with progesterone + oestrogen for assisted reproduction cycles | ||||||
| Population: subfertile women Setting: assisted reproduction Intervention: progesterone Comparison: progesterone + oestrogen (route of oestrogen: oral, transdermal, vaginal or oral + transdermal) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Progesterone + oestrogen | Progesterone | |||||
| Live birth or ongoing pregnancy | 367 per 1000 | 393 per 1000 (345 to 444) | OR 1.12 (0.91 to 1.38) | 1651 (9 RCTs) | ⊕⊕⊝⊝ Lowa, b, c | |
| Clinical pregnancy | 433 per 1000 | 397 per 1000 (355 to 443) | OR 0.86 (0.72 to 1.04) | 2169 (14 RCTs) | ⊕⊕⊝⊝ Lowa, d, f | |
| OHSS | 51 per 1000 | 30 per 1000 (11 to 82) | OR 0.58 (0.2 to 1.68) | 461 (2 RCTs) | ⊕⊕⊝⊝ Lowb, c, e | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aSerious risk of bias due to inadequate reporting of study methods. Risk of bias unclear in most domains of most studies.
bSerious imprecision with low event rate.
cNumber of studies was not sufficient for assessment of publication bias.
dSerious inconsistency with substantial statistical heterogeneity (I2 = 56%). Limiting analysis to the 9 studies using oral oestrogen yielded OR of 1.01 (95% CI 0.80 to 1.27) and reduced heterogeneity (I2 = 16%).
eSerious risk of bias due to inadequate reporting of study methods. Risk of bias both 'high risk' and 'low risk'
fTwo studies with an outlying result.
Summary of findings 5. Progesterone compared with progesterone + GnRH agonist for assisted reproduction cycles.
| Progesterone compared with progesterone + GnRH agonist for assisted reproduction cycles | ||||||
| Population: women who had undergone IVF/ICSI Setting: clinic Intervention: progesterone luteal support Comparison: progesterone + GnRH agonist | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Progesterone + GnRH agonist | Progesterone luteal support | |||||
| Live birth or ongoing pregnancy | 356 per 1000 | 255 per 1000 (209 to 309) | OR 0.62 (0.48 to 0.81) | 2861 (9 RCTs) | ⊕⊝⊝⊝ Very lowa,b | |
| Clinical pregnancy | 405 per 1000 | 310 per 1000 (258 to 367) | OR 0.66 (0.51 to 0.85) | 2435 (8 RCTs) | ⊕⊕⊝⊝ Lowc,d | |
| OHSS | 50 per 1000 | 50 per 1000 (17 to 137) | OR 1.00 (0.33 to 3.01) |
300 (1 study) | ⊕⊝⊝⊝ Very lowe,f, |
|
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a Evidence of significant heterogeneity I2 = 69%
b Only three of the studies reported on live birth as an outcome
c Evidence of heterogeneity I2=47%
d Some studies used multiple doses and some used single doses. We have used subgroup analysis to explore this further
e Lack of detail to make a judgement of risk of bias
f Evidence based on a single trial
Summary of findings 6. Progesterone regimens for assisted reproduction cycles.
| Progesterone regimens for assisted reproduction cycles | ||||||
| Population: subfertile women Setting: assisted reproduction Comparisons of progesterone regimens | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Comparison | Intervention | |||||
| Live birth or ongoing pregnancy IM vs oral |
200 per 1000 | 151 per 1000 (34 to 478) | OR 0.71 (0.14 to 3.66) | 40 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Live birth or ongoing pregnancy IM vs vaginal/rectal |
266 per 1000 | 310 per 1000 (272 to 353) | OR 1.24 (1.03 to 1.5) | 2309 (7 RCTs) | ⊕⊝⊝⊝ Very lowa,c,d | |
| Live birth or ongoing pregnancy Vaginal/rectal vs oral |
205 per 1000 | 235 per 1000 (176 to 303) | OR 1.19 (0.83 to 1.69) | 857 (4 RCTs) | ⊕⊕⊝⊝ Lowa,c,e | |
| Live birth or ongoing pregnancy Low dose vaginal vs high dose vaginal |
301 per 1000 | 295 per 1000 (266 to 324) | OR 0.97 (0.84 to 1.11) | 3720 (5 RCTs) | ⊕⊕⊕⊝ Moderatea,c | |
| Live birth or ongoing pregnancy Short protocol vs long protocol |
664 per 1000 | 672 per 1000 (609 to 728) | OR 1.04 (0.79 to 1.36) | 1205 (5 RCTs) | ⊕⊕⊕⊝ Lowa,c,e | |
| Live birth or ongoing pregnancy Micronised vs synthetic |
220 per 1000 | 203 per 1000 (130 to 305) | OR 0.9 (0.53 to 1.55) | 470 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c,e | |
| Live birth or ongoing pregnancy Vaginal ring vs vaginal gel |
441 per 1000 | 462 per 1000 (409 to 517) | OR 1.09 (0.88 to 1.36) | 1271 (1 RCT) | ⊕⊕⊝⊝ Lowc,f,g | |
| Live birth or ongoing pregnancy Subcutaneous vs vaginal gel |
358 per 1000 | 339 per 1000 (292 to 388) | OR 0.92 (0.74 to 1.14) | 1465 (2 RCTs) | ⊕⊕⊝⊝ Lowc,g,h | |
| Live birth or ongoing pregnancy Vaginal vs rectal |
306 per 1000 | 360 per 1000 (220 to 528) |
OR 1.28 (0.64 to 2.54) |
147 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b,c | |
| OHSS IM vs oral |
50 per 1000 | 50 per 1000 (3 to 475) |
OR 1.00 (0.06 to 17.18) |
40 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b,c | |
| OHSS Low dose vaginal vs high dose vaginal |
60 per 1000 | 55 per 1000 (35 to 86) |
OR 0.91 (0.57 to 1.46) | 1251 (2 RCTs) |
⊕⊕⊕⊝ Lowb,c,g | |
| OHSS rates not reported for other comparisons. | ||||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aSerious risk of bias due to inadequate reporting of study methods. Risk of bias unclear in most study domains.
bVery serious imprecision with low event rate; findings compatible with meaningful benefit in either arm or with no effect.
cNumber of studies was not sufficient for assessment of publication bias.
dVery serious inconsistency with varying directions of effect (I2 = 71%). Findings not statistically significant when random‐effects model was used, or when analysis was restricted to studies reporting live births.
eSerious imprecision with low event rate; findings compatible with meaningful benefit in the oral arm or with no effect.
fSerious imprecision; findings compatible with meaningful benefit in the gel arm or with no effect.
gSerious risk of bias due to inadequate reporting of study methods in 1 or more studies.
hSerious imprecision; findings compatible with meaningful benefit in the subcutaneous arm or with no effect.
Summary of findings 7. Progesterone + oestrogen regimens for assisted reproduction cycles.
| Progesterone + oestrogen regimens for assisted reproduction cycles | ||||||
| Population: subfertile women Setting: assisted reproduction Comparisons of progesterone and oestrogen regimens | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Comparison | Progesterone regimens | |||||
| Live birth/ongoing pregnancy ‐ short vs long protocol | 293 per 1000 | 309 per 1000 (251 to 372) | OR 1.08 (0.81 to 1.43) |
910 (1 RCT) |
⊕⊕⊝⊝ Lowa,b,c | |
| Live birth/ongoing pregnancy ‐ low vs high dose protocol | 342 per 1000 | 253 per 1000 (161 to 370) | OR 0.65 (0.37 to 1.13) |
285 (1 RCT) |
⊕⊝⊝⊝ Very lowa,c,d | |
| OHSS ‐ short vs long protocol | Not reported in any studies | |||||
| OHSS ‐ low vs high dose protocol | Not reported in any studies | |||||
| *The basis for the assumed risk is the risk in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aSerious risk of bias due to inadequate reporting of study methods. Risk of bias unclear in most study domains.
bSerious imprecision; findings compatible with meaningful benefit in either arm or with no effect.
cNumber of studies was not sufficient for assessment of publication bias.
dVery serious imprecision with low event rate; findings compatible with meaningful benefit in either arm or with no effect.
Background
Description of the condition
Assisted reproductive technology (ART), such as in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI), is used increasingly to assist couples to have a family. In cases of fertility treatment during which one or more embryos were transferred, less than one‐third of cases resulted in a live birth (CDC 2009; de Mouzon 2010; Macaldowie 2014). These figures suggest that implantation failure is an important limiting factor in the outcomes of ART.
The endometrium, which lines the uterus, prepares for implantation of the embryo. This process starts in the proliferative phase (from menstruation to ovulation) and extends throughout the luteal phase (from ovulation until menstruation). The luteal phase begins on the day of the luteinising hormone (LH) surge, which causes ovulation. The luteal phase ends at the onset of the next menstruation and usually lasts 12 to 16 days. During the luteal phase, the corpus luteum undergoes morphological and biochemical changes known as 'luteinisation'. Under the influence of LH, specific cells called granulosa cells produce progesterone. This in turn induces the secretory transformation of the endometrium, preparing it for implantation by thickening and increasing vascularisation to facilitate implantation (Farquhar 2010). Implantation occurs six days after fertilisation in natural cycles.
After implantation, trophoblastic tissue of the placenta secretes human chorionic gonadotropin (hCG), which acts on the ovary. hCG maintains and stimulates the corpus luteum, the remnant of the follicle, to produce oestradiol and progesterone (Pabuccu 2005). This is important in maintaining the pregnancy until the placenta takes over steroid hormone production at approximately seven weeks.
From the early phase of assisted reproduction, it has been clear that the luteal phase in ART is not sufficient, although the underlying mechanism is unclear (Edwards 1980). Several theories have been proposed to explain the deficient luteal phase in ART. In ART cycles, the corpus luteum is formed from the remnants of aspirated follicles under the influence of LH and produces progesterone and oestradiol (Messinis 2009). It was first thought that oocyte retrieval caused a luteal phase defect and, in particular, steroid secretion, but this theory was rejected when Kerin (Kerin 1981) demonstrated that aspiration of a single follicle did not lead to impaired steroid function. Another theory was that gonadotropin‐releasing hormone (GnRH) agonist co‐treatment caused prolonged pituitary recovery, which resulted in lack of LH; thus the corpus luteum did not develop fully (Smitz 1992a). Lack of LH was thought to be caused by a short‐loop negative feedback mechanism after hCG administration for oocyte maturation. This theory was also rejected, as long‐loop negative feedback by ovarian oestrogens has a greater effect on LH levels (Miyake 1979), and hCG does not lower LH secretion in non‐stimulated, normal ovulating women (Tavaniotou 2003). Currently it is thought that LH levels are lowered by high steroid levels (Fatemi 2009). Steroid levels are high because of the multiple corpora lutea, which produce more steroids than are produced in a natural cycle. This causes negative feedback on the pituitary gland and lowers LH levels. In this way, the luteal phase is shortened (known as premature luteolysis), and chances of pregnancy are reduced. In summary, premature luteolysis results from high concentrations of steroids caused by higher numbers of corpora lutea (secondary to controlled ovarian stimulation) during the early luteal phase, which in turn inhibit LH release directly by negative feedback.
In 2005, GnRH was introduced as a new means of providing luteal phase support. GnRH blocks the LH surge, and it was assumed that GnRH agonists might maintain their stimulatory effect throughout the luteal phase and restore LH levels ‐ a process that would support the luteal phase (Pirard 2006a). In 2004, Tesarik reported on the use of GnRH agonists six days after ICSI amongst oocyte donors. This study showed that single‐dose agonist administration increased the implantation rate without affecting miscarriage and abortion rates, resulting in an improved birth rate. However the multiple pregnancy rate was also increased (Tesarik 2004).
Adequate luteal phase support is therefore essential during IVF and ICSI for improving implantation and pregnancy rates. This can be achieved by substituting deficient LH with GnRH agonists or hCG, which has a longer half‐life, or directly by using progesterone with or without oestrogen. The ideal method of luteal phase supplementation remains a matter of debate and is the focus of this review.
Description of the intervention
The following agents can be used during the luteal phase.
-
Progesterone (including micronised progesterone or synthetic progestogens such as dydrogesterone, which have higher bioavailability (Schindler 2009)), administered by the following routes.
Intramuscular (IM).
Oral.
Vaginal ‐ an oral progesterone supplement administered by the vaginal route can lead to higher serum progesterone concentrations (Choavaratana 2004). Progesterone can also be administered vaginally by a gel or cream, which can generate high concentrations by bypassing the first‐pass effect through the liver (Geber 2007a).
Rectal.
-
Human chorionic gonadotropin (hCG) is similar to LH in its mode of action and physiological effects. Molecular structure is also similar. However hCG differs from LH in that elevated sialic acid residues are responsible for the longer serum half‐life and potency (Balasch 2004). Two types of hCG have been used: human derived and recombinant (Mochtar 2007). hCG is administered by the following routes.
Intramuscular (IM).
Subcutaneous (SC). It has been suggested that the bioavailability of hCG is lower after SC injections than after IM injections, but this remains unclear (Chan 2003;Mannaerts 1998; Saal 1991; Wikland 1995).
Oestrogen: oral, transdermal or vaginal administration in combination with progesterone.
-
GnRH agonists.
Intranasal.
Intramuscular (IM).
Subcutaneous (SC).
How the intervention might work
In ART, levels of progesterone in the luteal phase are insufficient (see above); therefore the levels of progesterone need to be increased. The progesterone level can be increased directly by giving progesterone, or progesterone and oestrogen in combination, or indirectly by giving hCG, which in turn stimulates progesterone secretion. Addition of a GnRH agonist is thought to restore LH levels during the luteal phase.
Why it is important to do this review
Less than one‐third of all cases involving an embryo result in a live birth. Luteal phase support has a positive effect on the outcome of ART compared with no treatment (van der Linden 2011). Many randomised trials have compared different methods of administration and different preparations to identify the best method of providing luteal phase support. This updated Cochrane review examines all currently available evidence on hCG, progesterone, oestrogen and GnRH analogues as agents for luteal phase support in ART.
Objectives
To determine the relative effectiveness and safety of methods of luteal phase support provided to subfertile women undergoing assisted reproduction.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing any of the agents used for luteal phase support during the luteal phase of an ART cycle. We included cross‐over trials in the review for completeness but used only first phase data in the analysis. We did not include quasi‐RCTs. We excluded studies investigating luteal phase support involving intrauterine insemination (IUI).
Types of participants
We included all subfertile women undergoing treatment with ART, including IVF or ICSI. We did not take the cause of subfertility into account. We excluded studies including women who had cycles of gamete intrafallopian transfer (GIFT) or zygote intrafallopian transfer (ZIFT), unless these treatments took place in less than 20% of cycles, as pregnancy outcomes with GIFT and ZIFT are less than with IVF. This 20% threshold was arbitrary.
Types of interventions
We included trials if they investigated or included:
any type, dose or route of progesterone, provided at least five doses were given during the luteal phase, to ensure the inclusion of true luteal phase support studies;
any type, dose or route of hCG, provided at least two doses were given during the luteal phase, to ensure the inclusion of true luteal phase support studies;
progesterone combined with oestrogen;
progesterone combined with hCG; or
GnRH agonist during the luteal phase.
We considered all ovarian stimulation protocols.
We excluded trials if they investigated or included:
luteal phase support after frozen embryo transfer;
luteal phase support after embryo transfer from donated oocytes;
luteal phase support after embryo transfer from frozen oocytes or frozen ovarian tissue;
luteal phase support after in vitro maturation (IVM) cycles; or
luteal phase support after intrauterine insemination (IUI) cycles.
Types of outcome measures
Primary outcomes
1. Live birth rate (LBR) or ongoing pregnancy per woman ('live birth' defined as the delivery of one or more living infants; 'ongoing pregnancy' defined as a pregnancy beyond 12 weeks' gestation).
Secondary outcomes
2. Clinical pregnancy rate (CPR) per woman (defined as the presence of a gestational sac, with or without a foetal heartbeat, on ultrasonography).
3. Miscarriage rate (MR) per woman.
4. Ovarian hyperstimulation syndrome (OHSS) per woman.
5. Multiple pregnancy rate per woman (counted as one).
Search methods for identification of studies
This review used information provided in the Cochrane Menstrual Disorder and Subfertility Group (MDSG) module regarding search strategies (www.mrw.interscience.wiley.com/cochrane/clabout/articles/MENSTR/frame.html). We sought all published and unpublished RCTs that described progesterone or hCG, or both, for luteal support in women undergoing ART. We used indexed and free‐text terms. We designed search strategies in consultation with the MDSG Trials Search Co‐ordinator. All searches were run from inception until 05.08.15
Electronic searches
We searched the following databases.
MDSG Specialised Register (see Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL) (see Appendix 2).
MEDLINE (see Appendix 3).
EMBASE (see Appendix 4).
PsycINFO (see Appendix 5).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (see Appendix 6).
Database of Abstracts of Reviews of Effects (DARE) (see Appendix 7).
The MDSG Specialised Register has been prepared through handsearching.
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in the Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0, Chapter 6, 6.4.11 (Higgins 2011).
We combined the EMBASE search with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/mehodology/filters.html#random).
We imposed no language restrictions on the searches.
Searching other resources
We searched the following.
ClinicalTrials.gov (http://clinicaltrials.gov/ct2/home) for ongoing and registered trials (Appendix 8)
The World Health Organization International Trials Registry Platform (www.who.int/trialsearch/Default.aspx) for ongoing and registered trials (Appendix 8)
Conference abstracts on the Web of Science (http://wokinfo.com) (see Appendix 9).
OpenSigle for grey literature from Europe (http://opensigle.inist.fr), using the search string "((chorionic gonadotropin) OR (progesterone)) AND (luteal phase)".
Latin American Caribbean Health Sciences Literature (LILACS) (http://regional.bvsalud.org/php/index.php?lang=en), using the keywords "luteal phase support".
Data collection and analysis
Selection of studies
Two review authors (MvdL, MM) independently screened titles and abstracts to exclude studies that were clearly irrelevant. We retrieved the full texts of potentially eligible studies for further independent scrutiny by two review authors (MvdL, MM) and checked compliance with the inclusion criteria by using the study eligibility form (see Appendix 10). We provided reasons for exclusion in the 'Characteristics of excluded trials' table. When it was unclear whether a study was eligible, we contacted the original study authors. We resolved disagreements through consultation with a third review author (CF).
Data extraction and management
We extracted data using a data extraction form (see Appendix 11) that was designed and pilot‐tested by the review authors. In the case of multiple publications, we referenced studies by their main trial report and linked the references. We contacted the original study authors if further information was required. Three review authors (MvdL, MM, KB) independently extracted data and resolved disagreements through consultation with the other review authors.
Assessment of risk of bias in included studies
We assessed risk of bias with regard to sequence generation, allocation, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective reporting and other potential sources of bias. We summarised conclusions in a 'Risk of bias' table (see Appendix 12). Review authors judged all six domains as at 'low risk', 'high risk' or 'unclear risk' of bias and described in the table the methods used. When information was missing, we contacted study authors.
-
Random sequence generation (selection bias).
Proper methods included use of a computer random number generator, coin tossing, dice throwing and shuffling of cards or envelopes.
Allocation by judgement of clinician, preference of participant, lab tests, date of birth, record number and inadequate sequence generation such as day of the week was not sufficient.
-
Allocation concealment (selection bias).
Proper methods required sequentially numbered drug containers of identical appearance, numbered opaque sealed envelopes or secure third party randomisation such as by telephone or computer allocation.
Prior knowledge of the allocation because of an open random allocation schedule or alternation, rotation, etc, was not sufficient.
-
Blinding of participants and personnel (performance bias).
Review authors assigned low risk of bias when blinding of clinicians and participants (when possible) was ensured, or when incomplete blinding had no effect on the outcome measurement.
When no blinding was provided and this had an influence on the outcome measurement, review authors identified the study as having risk of bias.
-
Blinding of outcome assessment (detection bias).
Review authors assigned low risk of bias when blinding of researchers (when possible) was ensured, or when incomplete blinding had no effect on the outcome measurement.
When no blinding was provided and this had an influence on the outcome measurement, review authors identified the study as having risk of bias.
-
Incomplete outcome data (attrition bias).
Review authors assigned low risk of bias when missing outcome data were unlikely to be related to true outcomes, or when all outcome data were complete.
High risk of bias indicated that missing outcome data were likely to be related to true outcomes, or that the proportion of missing outcome results compared with observed event risk was sufficient to induce clinically relevant bias in observed effect size.
-
Selective reporting (reporting bias).
Review authors assigned low risk of bias when all prespecified outcomes that were of interest or described in the protocol were reported.
High risk of bias indicated that not all prespecified outcomes were mentioned, reported outcomes were not prespecified or a key outcome that would be expected was not reported.
-
Free of other bias.
Risk of other bias (e.g. embryo transfer policies different in different arms of the study) showed extreme baseline imbalance.
When risk of bias tables had been completed, we generated a risk of bias summary figure (Higgins 2011).
Measures of treatment effect
We retrieved only dichotomous data for this review; thus we calculated Peto odds ratios (ORs) with 95% confidence intervals (CIs).
Unit of analysis issues
The primary analysis was per woman randomly assigned. We counted multiple live births as one live birth and included cross‐over data from the first phase of the study. When information were missing, we contacted the study authors.
Dealing with missing data
To obtain complete data, as much as possible, we contacted the original study authors. In case data could not be obtained, we undertook imputation for the primary outcome and assumed that no live birth occurred when this was not reported. When data for secondary outcomes were missing, we analysed only available data.
Assessment of heterogeneity
We assessed heterogeneity by examining a forest plot and the I2 statistic according to guidelines set forth in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If we detected substantial heterogeneity, that is, I2 ≥ 50%, we performed a sensitivity analysis to explore possible explanations.
Assessment of reporting biases
We assessed publication bias by examining a funnel plot if more than 10 studies were included. An asymmetrical funnel plot indicates possible publication bias, although the asymmetry may have other causes. We assessed within‐study reporting bias if study protocols were available, and if we noted differences between outcomes in the protocol and in the subsequent publication.
Data synthesis
We combined the data from primary studies by using a fixed‐effect model in the following comparisons.
hCG versus placebo or no treatment.
Progesterone versus placebo or no treatment.
-
Progesterone versus hCG regimens.
Progesterone versus hCG.
Progesterone versus progesterone and hCG.
-
Progesterone versus progesterone and oestrogen.
Oral oestrogen.
Transdermal oestrogen.
Vaginal oestrogen.
Oral and transdermal oestrogen.
-
Progesterone versus progesterone and GnRH agonist.
Single dose.
Multiple dose.
-
Progesterone regimens.
IM progesterone versus oral progesterone.
IM progesterone versus vaginal or rectal progesterone.
Vaginal or rectal progesterone versus oral progesterone.
Low‐dose vaginal progesterone (≤ 100 mg) versus high‐dose vaginal progesterone (> 100 mg).
Short protocol versus long protocol.
Micronised progesterone versus synthetic progesterone.
Vaginal ring versus vaginal gel.
Subcutaneous versus vaginal gel.
Vaginal progesterone versus rectal progesterone.
-
Progesterone + oestrogen regimens.
Short protocol versus long protocol.
Low‐dose oestrogen (≤ 2 mg) versus high‐dose oestrogen (> 2 mg).
When studies contributed to more than one comparison in a pooled analysis, we split as equally as possible comparisons data from the group that appeared in both comparisons. When data were split in this way, we provided details in a footnote in the forest plot.
Subgroup analysis and investigation of heterogeneity
We analysed data in the following subgroups as well.
-
Ovarian stimulation protocols including:
clomiphene citrate alone without GnRH agonists;
human gonadotropins with clomiphene citrate without GnRH agonists;
human gonadotropins with or without GnRH agonists; and
human gonadotropins with or without GnRH antagonists.
-
Participants with previously failed cycles.
≤ 2 failed ART cycles.
> 2 failed ART cycles.
-
Duration of progesterone.
Stop at day of positive pregnancy test.
Given up to 12 weeks for women who conceive.
-
Number of embryos transferred.
Single embryo transfer.
> one embryo transferred.
Sensitivity analysis
We performed a sensitivity analysis for the primary outcome to determine differences in results caused by:
eligibility restricted to studies without high risk of bias;
alternative imputation strategies that had been adopted;
use of risk ratio rather than odds ratio as the effect estimate; or
use of a random‐effects rather than a fixed‐effect analysis.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared 'Summary of findings' tables using GRADEPRO software. These tables evaluate the overall quality of the body of evidence for the primary review outcomes, using GRADE (Grades of Recommendation, Assessment, Development and Evaluation) criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). We have justified, documented and incorporated into reporting of results for each outcome judgements about evidence quality (high, moderate or low).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We conducted our searches on 05 August 2015 (using the strings reported in the appendices (Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 1; Appendix 6; Appendix 7; Appendix 8; Appendix 9), and in Open System for Information on Grey Literature in Europe (OpenSigle) and Latin American Caribbean Health Sciences Literature (LILACS). We identified 2441 studies and found six studies by using other methods such as handsearching.
On the website ClinicalTrials.gov in November 2014, we found 10 ongoing studies after using the keywords "luteal phase support". One study did not provide sufficient contact details. Five study authors did not reply (NCT01178931, NCT00828191, NCT00656201, NCT00708539, NCT01850030). One study author replied, and this study turned out to be already published (NCT00827983 as Baker 2014). Three studies were already published (NCT01147770 as Kyrou 2011, NCT01367912 as Tonguc 2011 and NCT01177904 as Kohls 2012). The World Health Organization International Trials Registry Platform (ICTRP), when searched with the keywords "luteal phase support", brought up eight new studies. Three studies did not provide sufficient contact details. Three studies were already published (ISRCTN88722916 as Aboulghar 2008, ICTR2013050713265N1 as Salehpour 2013, and ICTR138807192568N1 as Aghsa 2012). One study author did reply but had no data ready (EUCTR2013‐001105‐81‐HU). One study author did not reply (NCT01237535). From both these sites there were 11 ongoing studies.
Further database searches on 4 August 2015 identified two new studies eligible for inclusion and we have incorporated their data in the abstract, results and discussion sections of the review. Two studies requiring additional information before we could assess eligibility await classification for inclusion or exclusion at the next update of this review.
A further search of the ICTRP and clinicaltrials.gov (Appendix 8) sites on 4 August 2015 found another 17 ongoing studies. After de‐duplication a total of 22 ongoing studies remained. For full details of all ongoing studies see Characteristics of ongoing studies.
After duplicates were removed, 578 studies were left for screening. We excluded 352 clearly irrelevant studies. We obtained and fully reviewed the full‐text articles for the other 226 studies. We excluded all quasi‐randomised trials, together with articles that did not meet our inclusion criteria. We excluded another 31 articles from the review and included in the meta‐analysis a total of 94 studies (see Figure 1 for a study flow diagram). Three trials are awaiting assessment (Pirard 2015; Tomic 2015; Zafardoust 2015) .
1.
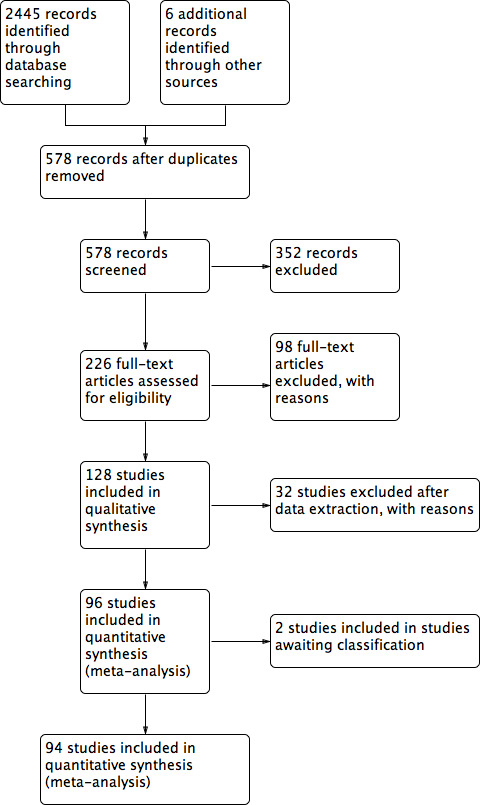
Study flow diagram.
Included studies
Study design
We included 94 studies, all of which were randomised controlled trials. We found no cross‐over trials for inclusion. In total, included studies consisted of 25,471 women with a mean age of 32.4 years. Inclusion and exclusion criteria varied among studies. Some studies included women with polycystic ovarian syndrome (PCOS), but this was an exclusion criterion in other studies. A few studies included women undergoing their first cycle, but most studies included women who had already undergone ART. Overall a mean of 2.43 embryos per woman were transferred, most with a maximum of three or four embryos.
For 24 studies, only the abstract was published (Albert 1991; Ata 2010; Beltsos 2011; Brigante 2013; Caligara 2007; Colakoglu 2011; Dunstone 1999; Erdem 2013; Geber 2007; Geusa 2001; Kably Ambe 2005; Loh 1996; Macrolin 1993; Miller 2010; Nallapeta 2013; Porcu 2003; Rodriguez‐Pezino 2004; Salehpour 2013; Saucedo 2000; Saucedo 2003; Serour 2012; Strehler 1999; Sumita 2003; Ugur 2001); the other studies were full‐text journal publications. Only 13 were multi‐centre studies (Baker 2014; Belaisch‐Allart 1990; Beltsos 2011; Bergh 2012; Doody 2009; Elgindy 2010; Kleinstein 2005; Lockwood 2014; Miller 2010; Nyboe Andersen 2002; Pouly 1996; Stadtmauer 2013; Zegers‐Hochschild 2000).
Thirteen of our included studies were carried out in the United States of America (Albert 1991; Baker 2014; Beltsos 2011; Doody 2009; Engmann 2008; Goudge 2010; Hurd 1996; Licciardi 1999; Miller 2010; Propst 2001; Stadtmauer 2013; Williams 2001; Yanushpolsky 2010). Ten studies were reported from Turkey (Ata 2008; Ata 2010; Ceyhan 2008; Colakoglu 2011; Erdem 2013; Gorkemli 2004; Isik 2009; Isikoglu 2007; Tonguc 2011; Ugur 2001; Yildiz 2014) and eight from Italy (Abate 1999; Abate 1999a; Artini 1995; Brigante 2013; Dal Prato 2008; Geusa 2001; Perino 1997; Porcu 2003). Twenty‐seven studies were conducted in other European countries: Austria (Feichtinger 2011), Belgium (Fatemi 2006), Denmark (Humaidan 2006; Nyboe Andersen 2002), Finland (Vimpeli 2001), France (Belaisch‐Allart 1987; Belaisch‐Allart 1990; Macrolin 1993; Pouly 1996), Germany (Kleinstein 2005; Ludwig 2001; Ludwig 2002; Strehler 1999), Greece (Drakakis 2007; Kyrou 2011), the Netherlands (Beckers 2000; Mochtar 2006), Spain (Caligara 2007; Kably Ambe 2005; Kohls 2012; Martinez 2000; Serna 2008; Tesarik 2006) and the UK (Dunstone 1999; Nallapeta 2013; Tay 2005). Thirteen were carried out in Asia: China (Lam 2008; Lin 2013; Ng 2003; Ng 2007; Wong 1990), India (Chakravarty 2005; Ganesh 2011; Inamdar 2012; Patki 2007; Sumita 2003), Japan (Fujimoto 2002; Iwase 2008) and Singapore (Loh 1996). We also found studies from Australia (Torode 1987), Brasil (Geber 2007; Geber 2007a), Canada (Colwell 1991), Egypt (Aboulghar 2008; Elgindy 2010; Serour 2012; Aboulghar 2015), Israel (Friedler 1999; Golan 1993; Kupferminc 1990; Lewin 1994), Iran (Aghahosseini 2011; Aghsa 2012; Moini 2011; Salehpour 2013), Jordan (Qublan 2008) and Mexico (Rodriguez‐Pezino 2004; Saucedo 2000; Saucedo 2003), and we found three multi‐centre, multi‐national studies: one from Chile, Colombia and Brazil (Zegers‐Hochschild 2000), one from Denmark and Sweden (Bergh 2012) and one from Hungary, Germany, Italy, Switzerland and the UK (Lockwood 2014).
Participants
Participants were women undergoing ART for a large variety of indications, including (low‐grade) endometriosis, polycystic ovarian syndrome or an unknown or unspecified cause of infertility.
Interventions
Thirteen studies investigated down‐regulation using GnRH antagonists (Baker 2014; Ceyhan 2008; Engmann 2008; Fatemi 2006; Geber 2007; Humaidan 2006; Isik 2009; Kohls 2012; Kyrou 2011; Nyboe Andersen 2002; Rodriguez‐Pezino 2004; Serna 2008; Tesarik 2006), and six studies did not use down‐regulation with GnRH analogues (Colwell 1991; Hurd 1996; Kupferminc 1990; Lewin 1994; Torode 1987; Wong 1990); clomiphene citrate, human menopausal gonadotropin (hMG) or both were used in most of those studies. Fifty‐three studies investigated GnRH agonists, and two studies investigated both GnRH agonists and antagonists (Kably Ambe 2005; Lockwood 2014). The other studies did not define the down‐regulation protocol used.
Outcomes
Live birth was reported in only 28 studies (Abate 1999; Abate 1999a; Ata 2010; Baker 2014; Beckers 2000; Bergh 2012; Chakravarty 2005; Dal Prato 2008; Doody 2009; Golan 1993; Goudge 2010; Isik 2009; Isikoglu 2007; Iwase 2008; Lewin 1994; Lin 2013; Lockwood 2014; Ludwig 2001; Mochtar 2006; Nyboe Andersen 2002; Pouly 1996; Propst 2001; Qublan 2008; Stadtmauer 2013; Tay 2005; Tesarik 2006; Yanushpolsky 2010; Zegers‐Hochschild 2000).
Fifty‐five studies reported ongoing pregnancy (Abate 1999a; Aghahosseini 2011; Aghsa 2012; Ata 2010; Aboulghar 2015Baker 2014; Beckers 2000; Belaisch‐Allart 1987; Belaisch‐Allart 1990; Beltsos 2011; Bergh 2012; Brigante 2013; Ceyhan 2008; Chakravarty 2005; Colwell 1991; Dal Prato 2008; Doody 2009; Engmann 2008; Fatemi 2006; Feichtinger 2011; Friedler 1999; Ganesh 2011; Golan 1993; Gorkemli 2004; Goudge 2010; Hurd 1996; Inamdar 2012; Isik 2009; Isikoglu 2007; Iwase 2008; Kleinstein 2005; Kohls 2012; Kupferminc 1990; Kyrou 2011; Lewin 1994; Lin 2013; Lockwood 2014; Ludwig 2001; Ludwig 2002; Macrolin 1993; Miller 2010; Mochtar 2006; Ng 2007; Nyboe Andersen 2002; Perino 1997; Pouly 1996; Propst 2001; Qublan 2008; Salehpour 2013; Serna 2008; Stadtmauer 2013; Tay 2005; Tesarik 2006; Tonguc 2011; Yanushpolsky 2010; Yildiz 2014Zegers‐Hochschild 2000).
All studies reported (clinical) pregnancy, except for seven studies, which used miscarriage rate (Nallapeta 2013) or ongoing pregnancy as the main outcome (Beltsos 2011; Colwell 1991; Fatemi 2006; Feichtinger 2011; Serna 2008; Tay 2005).
Miscarriage is reported in 45 studies (Aghahosseini 2011; Aghsa 2012; Ata 2008; Baker 2014; Beckers 2000; Belaisch‐Allart 1987; Bergh 2012; Chakravarty 2005; Colwell 1991; Dal Prato 2008; Drakakis 2007; Elgindy 2010; Engmann 2008; Fatemi 2006; Friedler 1999; Ganesh 2011; Geber 2007a; Golan 1993; Iwase 2008; Kably Ambe 2005; Kleinstein 2005; Kohls 2012; Kupferminc 1990; Kyrou 2011; Lam 2008; Licciardi 1999; Lin 2013; Lockwood 2014; Ludwig 2001; Ludwig 2002; Martinez 2000; Miller 2010; Nallapeta 2013; Ng 2007; Nyboe Andersen 2002; Perino 1997; Pouly 1996; Qublan 2008; Rodriguez‐Pezino 2004; Salehpour 2013; Saucedo 2000; Serna 2008; Strehler 1999; Tonguc 2011; Yanushpolsky 2010), OHSS in 10 studies (Albert 1991; Belaisch‐Allart 1990; Ceyhan 2008; Doody 2009; Iwase 2008; Lin 2013; Ludwig 2001; Macrolin 1993; Martinez 2000; Ugur 2001) and multiple pregnancy in 20 studies (Aghsa 2012; Ata 2008; Bergh 2012; Colwell 1991; Geber 2007a; Goudge 2010; Inamdar 2012; Isik 2009; Iwase 2008; Kleinstein 2005; Kohls 2012; Kyrou 2011; Licciardi 1999; Ludwig 2001; Ng 2007; Nyboe Andersen 2002; Pouly 1996; Strehler 1999; Tonguc 2011; Zegers‐Hochschild 2000).
Excluded studies
We excluded from the review 129 studies that did not meet our inclusion criteria. In accordance with the guidelines of the MDSG, we excluded all quasi‐randomised trials (Anserini 2001; Anthony 1993; Buvat 1988; Buvat 1990; Herman 1990; Herman 1996; Leeton 1985; Mahadevan 1985; McBain 1987; Polson 1992; Smith 1989; Smitz 1993; Yovich 1984; Yovich 1985; Yovich 1991), which had been included in an older version of this review (Daya 2004). We excluded all studies that included GIFT or ZIFT in more than 20% of cycles, or that did not mention the percentage of GIFT or ZIFT cycles used (Allen 2004; Araujo 1994; Araujo Filho 1996; Smitz 1988; Smitz 1992; van Steirteghem 1988).
Risk of bias in included studies
See the 'Summary of findings' tables for an overall assessment of the quality of evidence for each comparison. We prepared a table for each comparison (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7). See also the risk of bias graph (see Figure 2) and the risk of bias summary (see Figure 3) for an overview.
2.
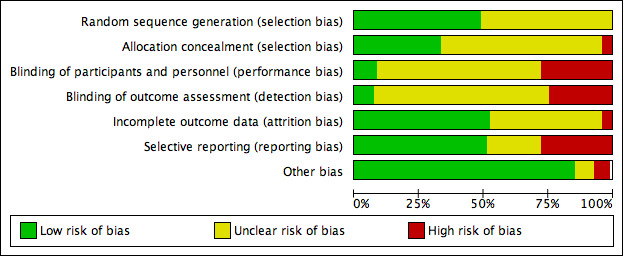
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
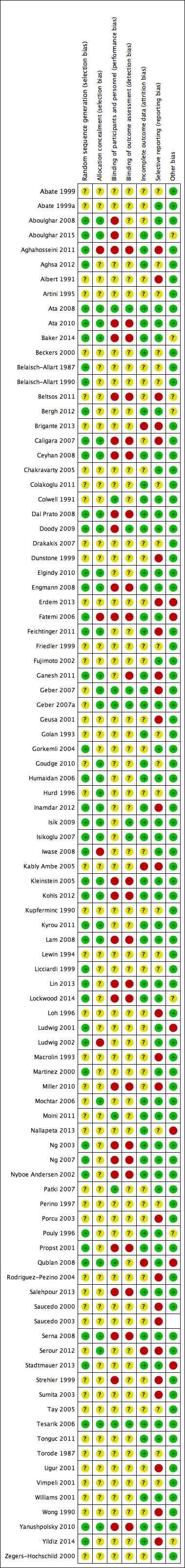
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Forty eight studies did not report the method of randomisation used. Most of those that did report the randomisation method used computerised randomisation (Aghahosseini 2011; Aghsa 2012; Ata 2008; Ata 2010; Aboulghar 2015; Baker 2014; Bergh 2012; Caligara 2007; Ceyhan 2008; Engmann 2008; Fatemi 2006; Feichtinger 2011; Gorkemli 2004; Humaidan 2006; Inamdar 2012; Isik 2009; Isikoglu 2007; Iwase 2008; Kleinstein 2005; Kohls 2012; Kyrou 2011; Lam 2008; Lin 2013; Lockwood 2014; Ludwig 2002; Martinez 2000; Ng 2003; Ng 2007; Pouly 1996; Serna 2008; Tesarik 2006; Yanushpolsky 2010; Yildiz 2014; Zegers‐Hochschild 2000). Randomisation lists or tables were often used (Belaisch‐Allart 1987; Belaisch‐Allart 1990; Ludwig 2001; Qublan 2008), as was a third party or study investigator (Aboulghar 2008; Dal Prato 2008; Ganesh 2011; Stadtmauer 2013). Doody 2009 used a telephone‐based electronic interactive voice response system, and Elgindy 2010 and Propst 2001 used permuted block randomisation.
Fifty studies did not report the method of allocation concealment used. Numbered, sealed envelopes were used most of the time (Aboulghar 2008; Ata 2008; Baker 2014; Beckers 2000; Dal Prato 2008; Elgindy 2010; Engmann 2008; Ganesh 2011; Geber 2007; Geber 2007a; Goudge 2010; Humaidan 2006; Hurd 1996; Kleinstein 2005; Kohls 2012; Kyrou 2011; Lam 2008; Lockwood 2014; Mochtar 2006; Ng 2003; Ng 2007; Nyboe Andersen 2002; Propst 2001; Qublan 2008; Salehpour 2013; Serna 2008; Serour 2012; Tesarik 2006; Tonguc 2011; Williams 2001). Caligara 2007 used a phone call to an unrelated department, Ceyhan 2008 central consultation, Doody 2009 a telephone‐based electronic interactive voice response system and Feichtinger 2011; Inamdar 2012 and Isik 2009 a third party nurse. Ata 2010, Isikoglu 2007, Lam 2008 and Yanushpolsky 2010 concealed allocation via an onsite computer system by utilising locked files.
Fatemi 2006 and Ludwig 2002 were the only studies that reported using a non‐concealed randomisation list.
Blinding
Fourteen studies mentioned that they used blinding (Aghsa 2012; Ata 2008; Belaisch‐Allart 1990; Bergh 2012; Colwell 1991; Doody 2009; Ganesh 2011; Geber 2007; Geber 2007a; Inamdar 2012; Isik 2009; Isikoglu 2007; Tesarik 2006; Tonguc 2011). The other studies did not blind personnel, researchers or participants (Aboulghar 2008; Aghahosseini 2011; Ata 2010; Caligara 2007; Ceyhan 2008; Dal Prato 2008; Doody 2009; Engmann 2008; Fatemi 2006; Ganesh 2011; Kleinstein 2005; Kohls 2012; Lam 2008; Lin 2013; Lockwood 2014; Miller 2010; Ng 2003; Ng 2007; Nyboe Andersen 2002; Propst 2001; Salehpour 2013; Serna 2008; Yanushpolsky 2010) or did not mention blinding. The studies of Moini 2011, Patki 2007 and Qublan 2008 were placebo controlled but did not specify the use of blinding. The main reason reported (in the paper or after contact with the original authors) for not blinding was that the study authors believed blinding would be difficult because of the different routes of administration used. We believe it is possible to use proper blinding with a double‐dummy design.
Incomplete outcome data
Fifty‐two studies reported the numbers of and reasons for withdrawal, or reported no drop‐outs. Qublan 2008 reported that more participants were recruited than analysed but did not report the reasons, and Brigante 2013 reported more outcomes than included patients.
Selective reporting
As stated before, only an abstract was available for 14 studies, which suggested high risk of selective reporting. Most studies reported planned outcomes, except for 18 (Abate 1999; Artini 1995; Beckers 2000; Belaisch‐Allart 1987; Belaisch‐Allart 1990; Drakakis 2007; Feichtinger 2011; Friedler 1999; Golan 1993; Goudge 2010; Hurd 1996; Kupferminc 1990; Lewin 1994; Licciardi 1999; Perino 1997; Tay 2005; Torode 1987; Vimpeli 2001). Aghahosseini 2011, Aghsa 2012, Ganesh 2011 and Wong 1990 reported outcomes in the Results section that were different from those reported in the Methods section.
Other potential sources of bias
Eight studies were supported by the pharmaceutical companies that had supplied the investigated interventions (Baker 2014; Beltsos 2011; Bergh 2012; Doody 2009; Lockwood 2014; Miller 2010; Propst 2001; Stadtmauer 2013). Two were supported by a grant from a pharmaceutical company (Ludwig 2002; Vimpeli 2001), and Kleinstein 2005 was supported by a pharmaceutical company, but this company does not supply the investigated products. One study (Ludwig 2001) reported a relatively large number of miscarriages, which were not consistent with reported rates of live birth, clinical pregnancy and ongoing pregnancy. This study was rated as having high risk of bias in this domain.
Assessment for publication bias
We looked at the following comparisons: 3.2 Progesterone versus hCG regimens, outcome clinical pregnancy rate (CPR); 4.2 Progesterone versus progesterone + oestrogen (CPR); 6.2.2 Progesterone regimens, outcome CPR: IM progesterone versus vaginal or rectal progesterone; and 6.2.4 Progesterone regimens, outcome CPR: low‐dose vaginal progesterone versus high‐dose vaginal progesterone for publication bias, as these four comparisons involved more than 10 included studies. We did this by making three funnel plots, combining comparisons 6.2.2 Progesterone regimens, outcome CPR: IM progesterone versus vaginal or rectal progesterone; and 6.2.4 Progesterone regimens, outcome CPR: low‐dose vaginal progesterone versus high‐dose vaginal progesterone (see Figure 4; Figure 5; Figure 6). Figure 4 shows most of the studies around the pooled estimate, suggesting that different sizes of studies were included. Although one study (Golan 1993) seemed to be out of the expected pattern, we did not see asymmetry; therefore this funnel plot indicated a small risk of publication bias. Figure 5 shows most of the studies around the pooled estimate with the studies reasonably equally divided on both sides. A large space at the lower side of the graph means that small studies may not be published. Overall the funnel plot revealed a small risk of publication bias. Figure 6 shows most of the studies around the pooled estimate with all studies reasonably equally divided on both sides. This funnel plot also showed that small studies may not be published. Overall it indicated a small risk of publication bias.
4.
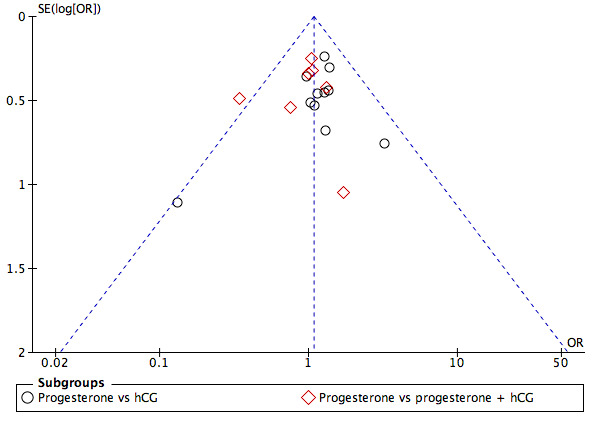
Funnel plot of comparison: 2 Progesterone vs hCG, outcome: 2.2 Clinical pregnancy rate.
5.
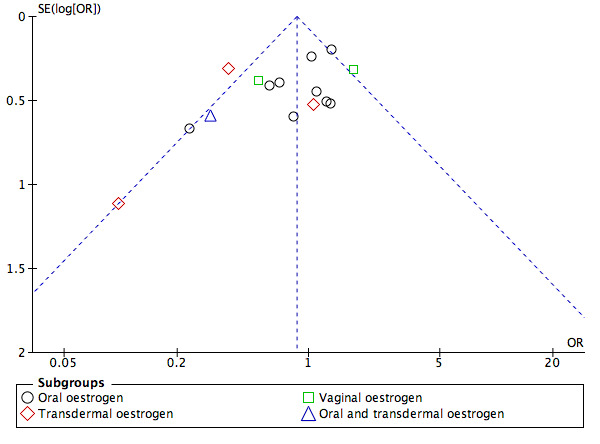
Funnel plot of comparison: 4 [NEW] Progesterone vs progesterone + oestrogen, outcome: 4.2 Clinical pregnancy rate.
6.
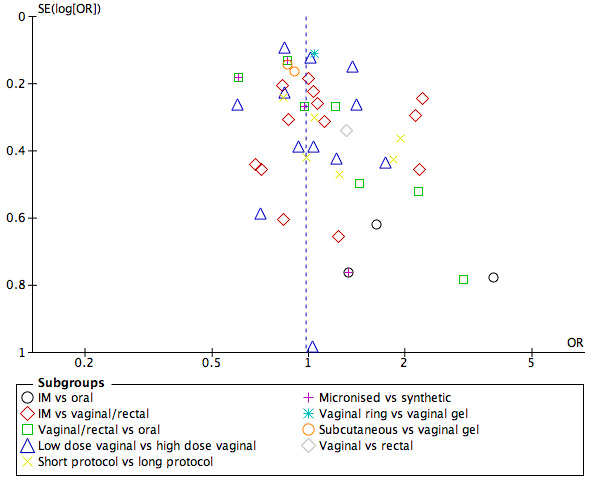
Funnel plot of comparison: 6 [NEW] Progesterone regimens, outcome: 6.2 Clinical pregnancy rate.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
1. hCG versus placebo or no treatment
Primary outcome
1.1 Live birth/ongoing pregnancy rate
Three studies reported live birth (Beckers 2000) or ongoing pregnancy (Belaisch‐Allart 1990; Kupferminc 1990). See Figure 7 for details of this comparison.
7.
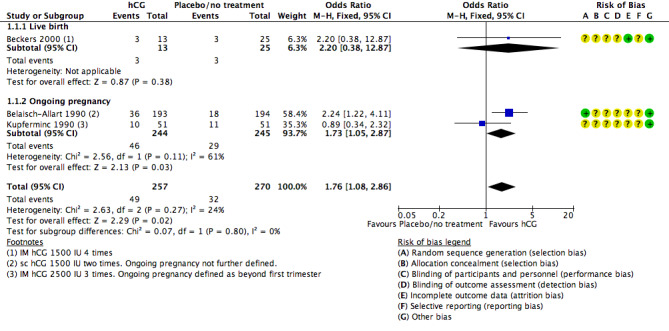
Forest plot of comparison: 1 Human chorionic gonadotropin (hCG) vs placebo or no treatment, outcome: 1.1 Live birth/ongoing pregnancy rate.
Live birth and pregnancy rates were higher in the hCG group (OR 1.76, 95% CI 1.08 to 2.86, three RCTs, 527 women, I2 = 24%, very low‐quality evidence).
However this findings was sensitive to choice of statistical model, and when a random‐effects model was used there was no longer evidence of a difference between the groups (OR 1.67, 95% CI 0.90 to 3.12).
When the analysis was restricted to live birth, only 38 women were included and again there was no evidence of a difference between the groups (OR 2.20, 95% CI 0.38 to 12.87).
Secondary outcomes
1.2 Clinical pregnancy rate (CPR)
Five studies (Artini 1995; Beckers 2000; Belaisch‐Allart 1990; Kupferminc 1990; Torode 1987) reported this outcome. Evidence suggested no differences between groups (OR 1.30, 95% CI 0.90 to 1.88, five RCTs, 746 women, I2 = 0%, very low quality evidence). See Analysis 1.2 for details of this comparison.
1.2. Analysis.
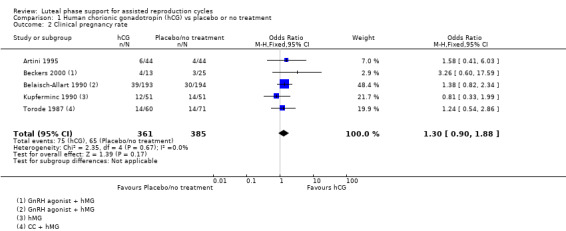
Comparison 1 Human chorionic gonadotropin (hCG) vs placebo or no treatment, Outcome 2 Clinical pregnancy rate.
Subgroup analysesfor clinical pregnancy rate
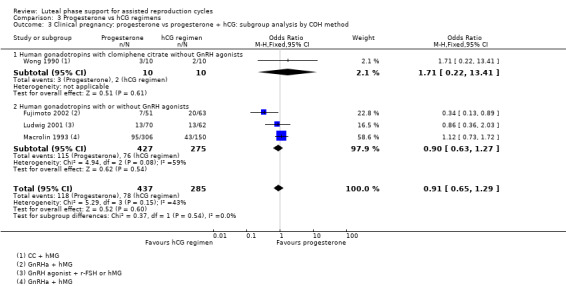
1.2.1 Ovarian stimulation protocol
Five studies were included in subgroups. Researchers utilised hCG with clomiphene citrate without GnRH agonists (Torode 1987), hCG with or without GnRH agonists (Artini 1995; Beckers 2000; Belaisch‐Allart 1990) or hCG with or without GnRH agonists (Kupferminc 1990). Evidence suggested no substantial differences from the main analysis in any of the subgroups. See Analysis 1.3 for details.
1.3. Analysis.
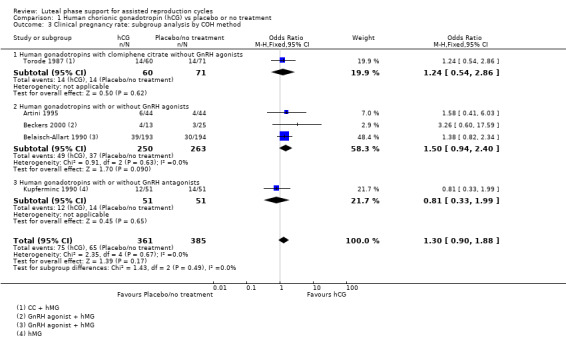
Comparison 1 Human chorionic gonadotropin (hCG) vs placebo or no treatment, Outcome 3 Clinical pregnancy rate: subgroup analysis by COH method.
1.2.2 Women with previously failed cycles
No data were available for this subgroup analysis.
1.2.3 Duration of treatment
Not applicable.
1.2.4Number of embryos transferred
No data were available for this subgroup analysis.
1.3 Miscarriage rate
Two studies (Beckers 2000; Kupferminc 1990) reported this outcome. Evidence suggested no differences between groups (OR 1.51, 95% CI 0.37 to 6.21, two RCTs, 140 women, I2 = 0%). See Analysis 1.4 for details of this comparison.
1.4. Analysis.
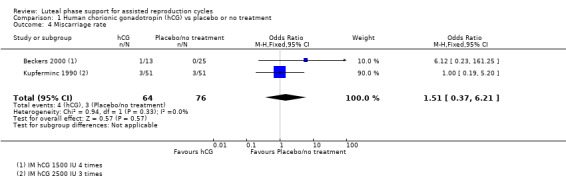
Comparison 1 Human chorionic gonadotropin (hCG) vs placebo or no treatment, Outcome 4 Miscarriage rate.
1.4 Ovarian hyperstimulation syndrome (OHSS)
One study (Belaisch‐Allart 1990) reported this outcome. This result showed benefit for the placebo group (OR 4.28, 95% CI 1.91 to 9.60, one RCT, 387 women, low quality evidence). As this result was based on a single study, it should be interpreted with caution. See Analysis 1.5 for details of this comparison.
1.5. Analysis.
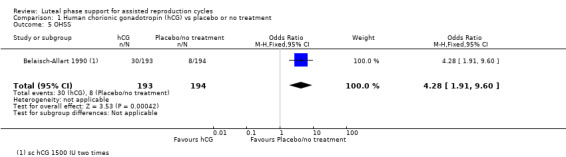
Comparison 1 Human chorionic gonadotropin (hCG) vs placebo or no treatment, Outcome 5 OHSS.
1.5 Multiple pregnancy
No studies reported this outcome.
2. Progesterone versus placebo or no treatment
Primary outcome
2.1 Live birth/ongoing pregnancy rate
Five studies reported live birth (Abate 1999a) or ongoing pregnancy (Belaisch‐Allart 1987; Colwell 1991; Hurd 1996; Kupferminc 1990).
Rates of live birth or ongoing pregnancy were higher in the progesterone group (OR 1.77, 95% CI 1.09 to 2.86, five RCTs, 642 women, I2 = 19%, very low quality evidence).
Findings require cautious interpretation, as when the analysis was restricted to live birth, evidence suggested no differences between groups (OR 4.21, 95% CI 0.93 to 19.18, one RCT, 156 women). Heterogeneity was high in the studies of ongoing pregnancy (I2 = 68%).
See Figure 8 for details of this comparison.
8.
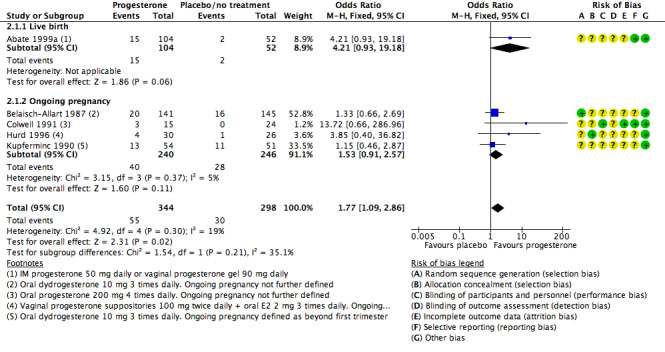
Forest plot of comparison: 2 Progesterone vs placebo or no treatment, outcome: 2.1 Live birth/ongoing pregnancy rate.
Sensitivity analyses
Pooled findings for live birth/ongoing pregnancy were no longer statistically significant when a random‐effects model was used (OR 1.77, 95% CI 0.96 to 3.26); this underlines the need for caution in interpreting these findings. Other sensitivity analyses did not materially affect the findings.
Secondary outcomes
2.2 Clinical pregnancy rate
Seven studies (Abate 1999; Abate 1999a; Artini 1995; Belaisch‐Allart 1987; Hurd 1996; Kupferminc 1990; Wong 1990) reported this outcome. Pregnancy rates were higher in the progesterone group (OR 1.89, 95% CI 1.30 to 2.75, seven RCTs, 841 women, I2 = 0%, low quality evidence). See Analysis 2.2 for details of this comparison.
2.2. Analysis.
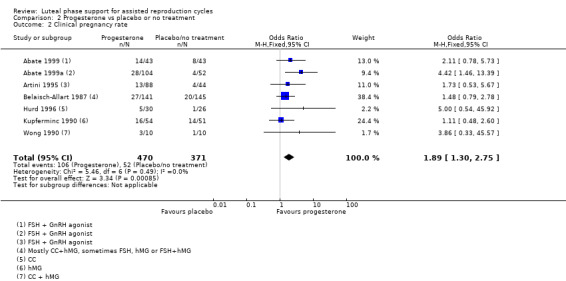
Comparison 2 Progesterone vs placebo or no treatment, Outcome 2 Clinical pregnancy rate.
Subgroup analysesfor clinical pregnancy rate
2.2.1 Ovarian stimulation protocol
Findings of the subgroup of four studies (Abate 1999; Abate 1999a; Artini 1995; Kupferminc 1990) that administered human gonadotropins with or without GnRH agonists were consistent with the main findings, showing benefit for the progesterone group. Benefit was stronger when the study without GnRH agonists was excluded. Studies that administered clomiphene citrate alone without GnRH agonists (Hurd 1996) or human gonadotropins with clomiphene citrate without GnRH agonists (Belaisch‐Allart 1987; Wong 1990) did not clearly show benefit for the progesterone group. However results of the test for subgroup differences were not statistically significant.
See Analysis 2.3 for details.
2.3. Analysis.
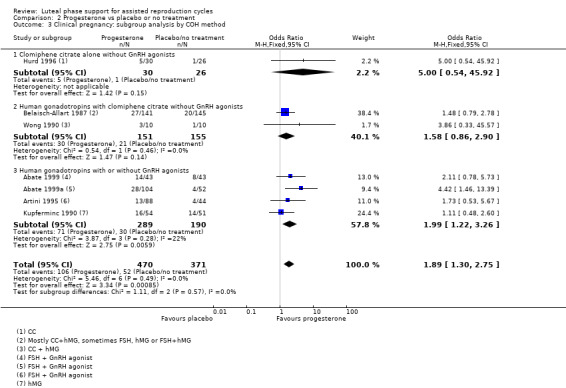
Comparison 2 Progesterone vs placebo or no treatment, Outcome 3 Clinical pregnancy: subgroup analysis by COH method.
2.2.2 Women with previously failed cycles
No data were available for this subgroup analysis.
2.2.3 Duration of progesterone
Findings of the subgroup of four studies (Abate 1999; Abate 1999a; Belaisch‐Allart 1987; Hurd 1996) that administered progesterone for up to 12 weeks were consistent with the main findings, showing benefit for the progesterone group. The subgroup of three studies (Artini 1995; Kupferminc 1990; Wong 1990) that stopped progesterone at the time of the pregnancy test did not clearly show benefit for the progesterone group. However results of the test for subgroup differences were not statistically significant.
See Analysis 2.4 for details.
2.4. Analysis.
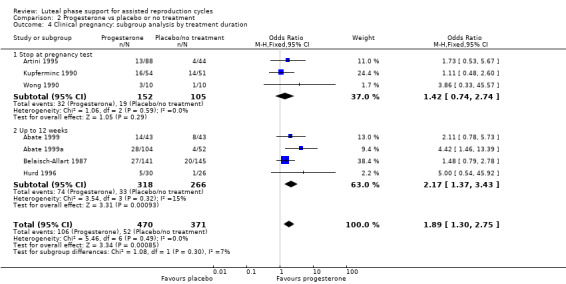
Comparison 2 Progesterone vs placebo or no treatment, Outcome 4 Clinical pregnancy: subgroup analysis by treatment duration.
2.2.4 Number of embryos transferred
No data were available for this subgroup analysis.
2.3 Miscarriage rate
Three studies (Belaisch‐Allart 1987; Colwell 1991; Kupferminc 1990) reported this outcome. No evidence suggested differences between groups (OR 1.22, 95% CI 0.49 to 3.03, three RCTs, 425 women, I2 = 0%). See Analysis 2.5 for details of this comparison.
2.5. Analysis.
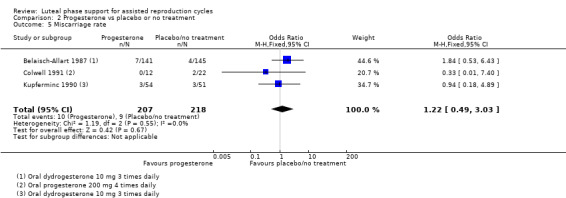
Comparison 2 Progesterone vs placebo or no treatment, Outcome 5 Miscarriage rate.
2.4 Ovarian hyperstimulation syndrome (OHSS)
No studies reported this outcome.
2.5 Multiple pregnancy
One study (Colwell 1991) reported this outcome. Evidence suggested no differences between groups (OR 5.87, 95% CI 0.22 to 155.76, one RCT, 34 women). See Analysis 2.6 for details of this comparison.
2.6. Analysis.
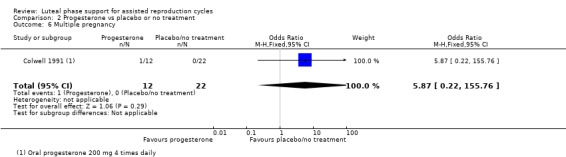
Comparison 2 Progesterone vs placebo or no treatment, Outcome 6 Multiple pregnancy.
3. Progesterone versus hCG regimens
Primary outcome
3.1 Live birth/ongoing pregnancy rate
Five studies reported live birth (Golan 1993; Ludwig 2001) or ongoing pregnancy (Kupferminc 1990; Macrolin 1993; Tay 2005). Researchers compared progesterone versus hCG (four RCTs, 434 women) or versus progesterone plus hCG (two RCTs, 399 women).
Evidence suggested no differences between groups in rates of live birth or ongoing pregnancy (OR 0.95, 95% CI 0.65 to 1.38, five RCTs, 833 women, I2 = 0%, low quality evidence).
Findings were similar, regardless of whether the comparison group received hCG only or hCG plus progesterone. See Figure 9 for details of this comparison.
9.
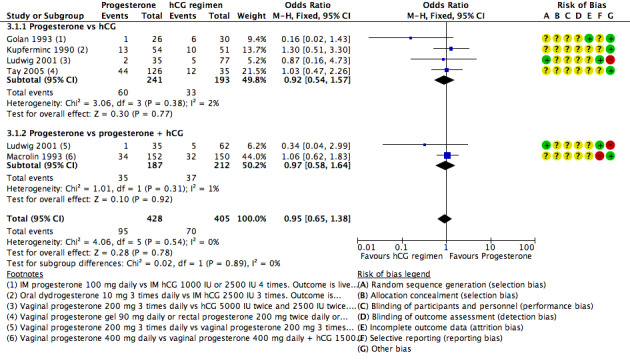
Forest plot of comparison: 3 Progesterone vs hCG regimens, outcome: 3.1 Live birth or ongoing pregnancy rate.
Restriction of the analysis to studies reporting live birth also showed no evidence of differences between groups.
Secondary outcomes
3.2 Clinical pregnancy rate
Eighteen studies reported this outcome. Researchers compared progesterone versus hCG (11 RCTs, 1378 women) or versus progesterone plus hCG (seven RCTs, 977 women).
Evidence suggested no differences between groups in rates of clinical pregnancy (OR 1.08, 95% CI 0.90 to 1.30, 16 RCTs, 2355 women, I2 = 0%, moderate quality evidence).
Findings did not differ substantially, regardless of whether the comparison group received hCG only or hCG plus progesterone. See Analysis 3.2 for details.
3.2. Analysis.
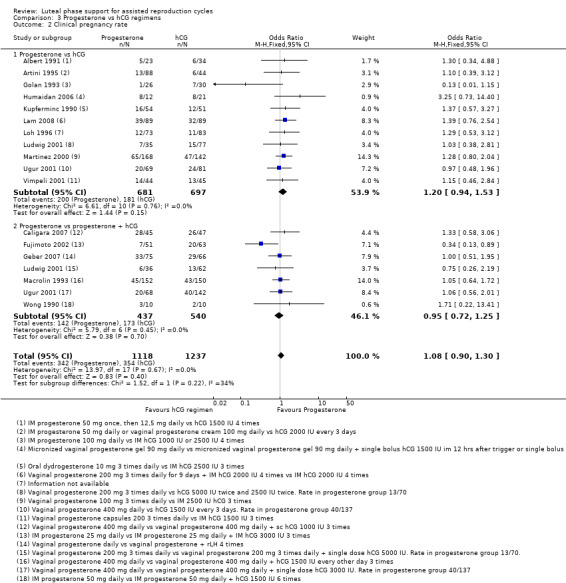
Comparison 3 Progesterone vs hCG regimens, Outcome 2 Clinical pregnancy rate.
Because this comparison included more than 10 studies, we prepared a funnel plot to determine the risk of publication bias (see Figure 4), which is assessed in the section Selective reporting (reporting bias). We concluded that this study showed a small risk of publication bias.
Subgroup analyses for clinical pregnancy rate
3.2.1 Ovarian stimulation method
Four studies of progesterone versus progesterone plus hCG were subgrouped by method of ovarian stimulation. One (Wong 1990) utilised hCG with clomiphene citrate without GnRH agonists, and three utilised hCG with or without GnRH agonists (Fujimoto 2002; Ludwig 2001; Macrolin 1993). Findings did not differ substantially from those of the main analysis in either subgroup (see Analysis 3.3). No studies of progesterone versus hCG alone were available for this subgroup analysis.
3.3. Analysis.
Comparison 3 Progesterone vs hCG regimens, Outcome 3 Clinical pregnancy: progesterone vs progesterone + hCG: subgroup analysis by COH method.
3.2.2 Women with previously failed cycles
No data were available for this subgroup analysis.
3.2.3 Duration of progesterone
Seven studies of progesterone versus progesterone plus hCG were subgrouped by duration of progesterone treatment. Six stopped treatment at the pregnancy test (Artini 1995; Golan 1993; Humaidan 2006; Kupferminc 1990; Ludwig 2001; Martinez 2000), and one administered progesterone for up to 12 weeks when pregnant (Vimpeli 2001). Findings did not differ substantially from those of the main analysis in either subgroup. No studies of progesterone versus hCG alone were available for this subgroup analysis. See Analysis 3.4 for details of this comparison.
3.4. Analysis.
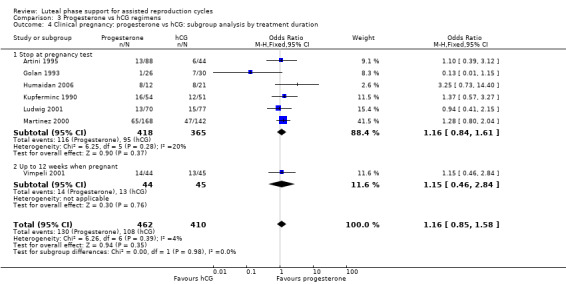
Comparison 3 Progesterone vs hCG regimens, Outcome 4 Clinical pregnancy: progesterone vs hCG: subgroup analysis by treatment duration.
3.2.4 Number of embryos transferred
No data were available for this subgroup analysis.
3.3 Miscarriage rate
Five studies reported this outcome. Researchers compared progesterone versus hCG (five RCTs, 735 women) or versus progesterone plus hCG (one RCT, 97 women).
Evidence suggested no differences between groups in rates of miscarriage (OR 1.24, 95% CI 0.66 to 12.31, five RCTs, 832 women, I2 = 0%). Findings did not differ substantially, regardless of whether the hCG group received progesterone as well. See Analysis 3.6 for details.
3.6. Analysis.
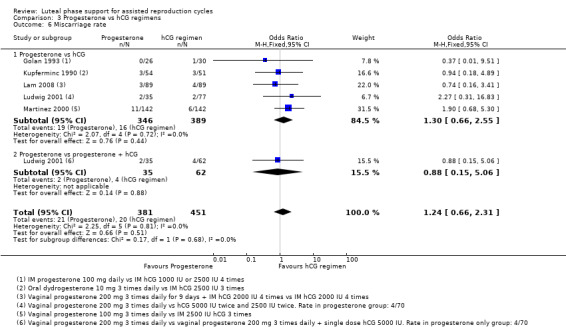
Comparison 3 Progesterone vs hCG regimens, Outcome 6 Miscarriage rate.
3.4 Ovarian hyperstimulation syndrome (OHSS)
Five studies reported this outcome. They compared progesterone versus hCG (four RCTs, 671 women) or versus progesterone plus hCG (three RCTs, 678 women). Figure 10
10.
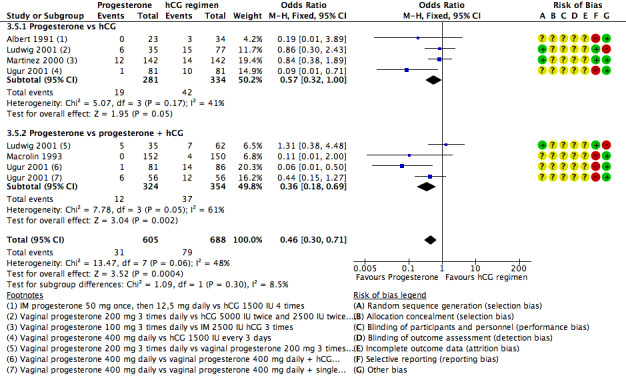
Forest plot of comparison: 3 Progesterone vs hCG regimens, outcome: 3.5 OHSS.
Progesterone was associated with lower rates of OHSS rates than hCG with or without progesterone (OR 0.46, 95% CI 0.30 to 0.71, 5 RCTs, 1293 women , I2=48%) .
Findings differed according to whether the hCG group received progesterone as well, though the statistical test for subgroup differences suggested no significant difference between the groups (p=0.30). When progesterone was compared with hCG alone, there was no clear evidence of a difference between the groups (OR 0.57, 95% CI 0.32 to 1.00, 4 studies I2=41%). When progesterone alone was compared with hCG plus progesterone, rates were lower in the progesterone alone group, though with substantial statistical heterogeneity (OR 0.36, 95% CI 0.18 to 0.69, 3 RCTs, 678 women ,l2=61%).
3.5 Multiple pregnancy
One study reported this outcome. Researchers compared progesterone versus hCG (112 women) or versus progesterone plus hCG (97 women).
Evidence suggested no differences between groups for this outcome (OR 0.44, 95% CI 0.07 to 2.65, one RCT, 209 women). See Analysis 3.7 for details of this comparison.
3.7. Analysis.
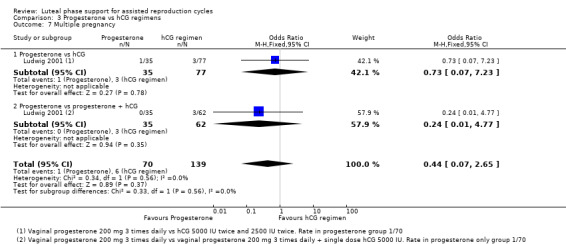
Comparison 3 Progesterone vs hCG regimens, Outcome 7 Multiple pregnancy.
4. Progesterone versus progesterone + oestrogen
After data extraction, we found that different routes of oestrogen administration were used as well as different dosages of oestrogen. Therefore we decided to stratify the analysis on the basis of route of administration.
Primary outcome
4.1 Live birth/ongoing pregnancy rate
Nine studies reported live birth (Ata 2010; Lewin 1994; Lin 2013) or ongoing pregnancy (Aghahosseini 2011; Ceyhan 2008; Engmann 2008; Fatemi 2006; Serna 2008; Yanushpolsky 2010). Routes of oestrogen administration were oral (six RCTs, 1266 women), transdermal (two RCTs, 219 women) and vaginal (one RCT, 166 women). See Figure 11 for details of this comparison.
11.
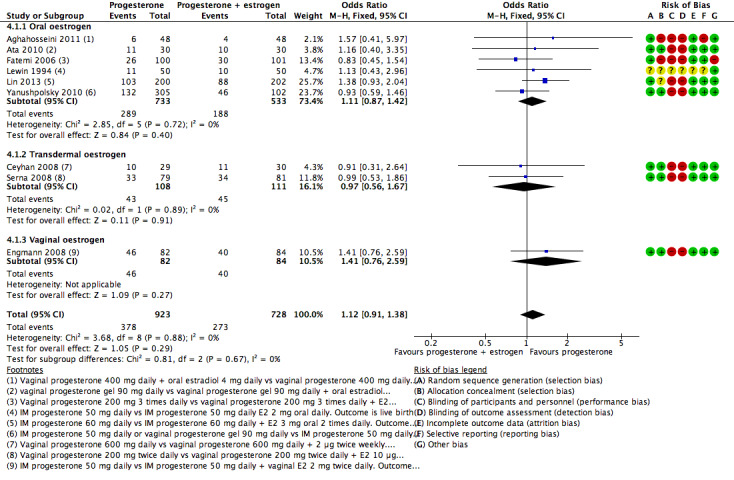
Forest plot of comparison: 4 Progesterone vs progesterone + oestrogen, outcome: 4.1 Live birth/ongoing pregnancy rate.
Evidence suggested no differences between groups in rates of live birth or ongoing pregnancy (OR 1.12, 95% CI 0.91 to 1.38, nine RCTs, 1651 women, I2 = 0%, low quality evidence).
Findings were similar when the analysis was restricted to studies reporting live birth (OR 1.32, 95% CI 0.93 to 1.86, three RCTs, 562 women, I2 = 0%).
When data were considered by route of oestrogen administration, findings did not differ substantially from those of the main analysis.
Secondary outcomes
4.2 Clinical pregnancy rate
Fourteen studies reported this outcome (Aghahosseini 2011; Ata 2010; Ceyhan 2008; Colakoglu 2011; Drakakis 2007; Elgindy 2010; Engmann 2008; Erdem 2013; Gorkemli 2004; Kably Ambe 2005; Lewin 1994; Lin 2013; Moini 2011; Yanushpolsky 2010). Routes of oestrogen administration were oral (nine RCTs, 1427 women), transdermal (three RCTs, 364 women), vaginal (two RCTs, 301 women) and oral/transdermal (one RCT, 77 women). Elgindy 2010 was a three‐arm study comparing progesterone, progesterone + oral oestrogen and progesterone + vaginal oestrogen. To make sure we did not duplicate data, we divided data from the progesterone‐only arm by two, so half of the progesterone‐only events and participants were reported under the subgroup 'oral', and the other half of the progesterone‐only events and participants were reported under the subgroup 'vaginal'.
When studies were pooled, no evidence suggested differences between groups (OR 0.86, 95% CI 0.72 to 1.04, 14 RCTs, 4169 women, I2 = 56%, low quality evidence). However substantial heterogeneity was noted for this analysis (56%), and results of the test for subgroup differences were statistically significant (P value = 0.004).
When the data were considered by route of oestrogen administration, heterogeneity in the comparison using oral oestrogen was relatively low (I2 = 16%), and evidence suggested no differences between groups. However heterogeneity in comparisons using other routes of administration was high (I2 = 56% to 82%), and in studies using transdermal oestrogen, a higher pregnancy rate was reported in the progesterone + oestrogen groups. These findings should be regarded with caution because of the inconsistency observed between studies and the small quantity of data provided.
Because this comparison included more than 10 studies, we prepared a funnel plot to determine the risk of publication bias (see Figure 5), which is discussed in the section Selective reporting (reporting bias). We concluded that the study showed a small risk of publication bias.
Subgroup analysesfor clinical pregnancy rate
4.2.1 Ovarian stimulation protocol
Eight studies were subgrouped by method of ovarian stimulation. Seven utilised hCG with or without GnRH agonists (Aghahosseini 2011; Drakakis 2007; Elgindy 2010; Engmann 2008; Lewin 1994; Moini 2011; Yanushpolsky 2010), and two utilised hCG with or without GnRH antagonists (Ceyhan 2008; Engmann 2008). Findings did not differ substantially from those of the main analysis in either subgroup. See Analysis 4.3.
4.3. Analysis.
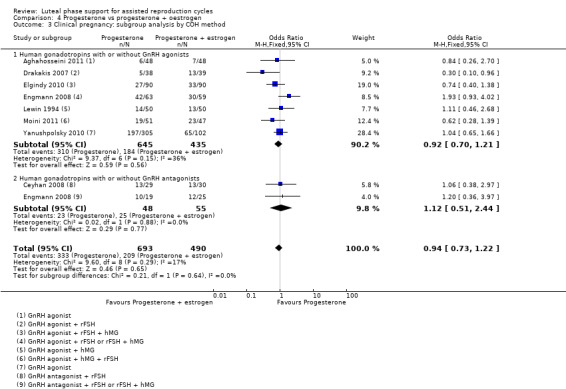
Comparison 4 Progesterone vs progesterone + oestrogen, Outcome 3 Clinical pregnancy: subgroup analysis by COH method.
4.2.2 Women with previously failed cycles
No data were available for this subgroup analysis.
4.2.3 Duration of progesterone
Ten studies were subgrouped by duration of progesterone. Two stopped treatment at the pregnancy test (Drakakis 2007; Lewin 1994), and eight administered progesterone for up to 12 weeks when pregnant (Aghahosseini 2011; Ceyhan 2008; Elgindy 2010; Engmann 2008; Gorkemli 2004; Lin 2013; Moini 2011; Yanushpolsky 2010). Studies in the subgroups were not pooled because of marked heterogeneity (I2 = 63% to 67%), possibly related to the differing methods of oestrogen administration described within each subgroup. See Analysis 4.4.
4.4. Analysis.
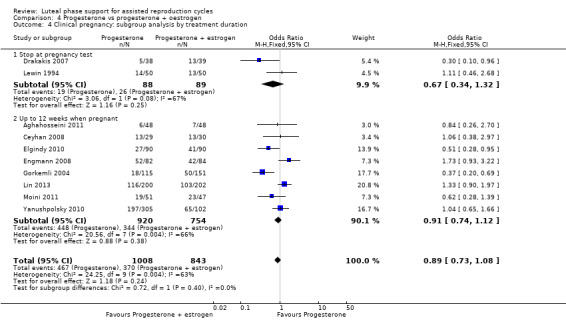
Comparison 4 Progesterone vs progesterone + oestrogen, Outcome 4 Clinical pregnancy: subgroup analysis by treatment duration.
4.2.4 Number of embryos transferred
No data were available for this subgroup analysis.
4.3 Miscarriage rate
Ten studies reported this outcome (Aghahosseini 2011; Ata 2008; Drakakis 2007; Elgindy 2010; Engmann 2008; Fatemi 2006; Kably Ambe 2005; Lin 2013; Serna 2008; Yanushpolsky 2010). Routes of oestrogen administration were oral (seven RCTs, 1370 women), transdermal (one RCT, 160 women), vaginal (two RCTs, 301 women) and oral/transdermal (one RCT, 77 women).
When studies were pooled, no evidence suggested differences between groups (OR 0.98, 95% CI 0.72 to 1.35, 10 RCTs, 1908 women, I2 = 15%).
When data were considered by route of oestrogen administration, findings did not differ substantially from those of the main analysis. However heterogeneity was high in the comparison using vaginal oestrogen (I2 = 59%).
4.4 Ovarian hyperstimulation syndrome (OHSS)
Two studies reported this outcome (Ceyhan 2008;Lin 2013). Routes of oestrogen administration were oral (one RCT, 461 women) and transdermal (one RCT, 59 women).
When these studies were pooled, evidence suggested no differences between groups (OR 0.58, 95% CI 0.20 to 1.68, two RCTs, 461 women, I2 = 0%, low quality evidence).
When the data were considered by route of oestrogen administration, findings did not differ substantially. See Analysis 4.6 for details of this comparison.
4.6. Analysis.
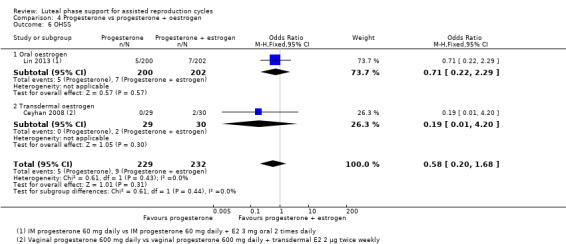
Comparison 4 Progesterone vs progesterone + oestrogen, Outcome 6 OHSS.
4.5 Multiple pregnancy
No studies reported this outcome.
5. Progesterone versus progesterone + GnRH agonist
Primary outcome
5.1 Live birth/ongoing pregnancy rate
Nine studies reported live birth (Isik 2009; Isikoglu 2007; Qublan 2008) or ongoing pregnancy (Aboulghar 2015; Ata 2008; Brigante 2013; Inamdar 2012; Tesarik 2006; Yildiz 2014). Researchers administered the GnRH agonist as a single dose (five RCTs, 1536 women) or in multiple doses (five RCTs, 1325 women). See Figure 12 for details of this comparison.
12.
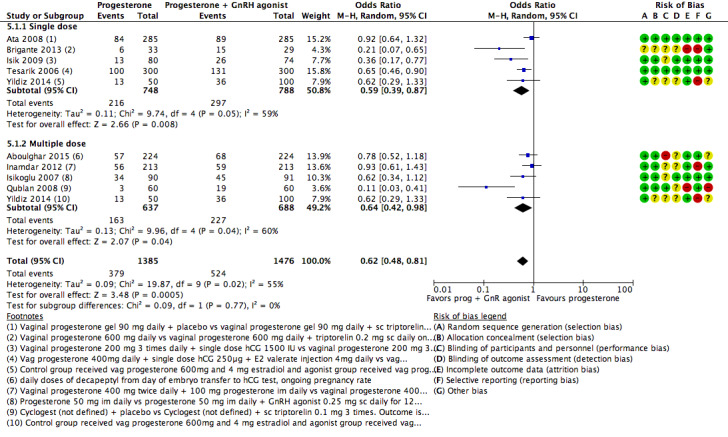
Forest plot of comparison: 5 Progesterone vs progesterone + GnRH agonist, outcome: 5.1 Live birth or ongoing pregnancy rate.
The live birth/ongoing pregnancy rate was lower in the progesterone‐only group than the progesterone + GnRHa group (OR 0.62, 95% CI 0.48 to 0.81, nine RCTs, 2861 women, I2 = 55%, random effects, low quality evidence). The high statistical heterogeneity in this analysis was due to wide variation between studies in size of the effect, although the direction of the effect was consistent.
Findings were similar when the analysis was restricted to studies reporting live birth only (OR 0.34, 95% CI 0.15 to 0.59, three RCTs, 455 women, I2 = 66%).
When the data were considered by number of doses of GnRH agonist, findings did not differ substantially. See Analysis 5.1 for details of this comparison.
5.1. Analysis.
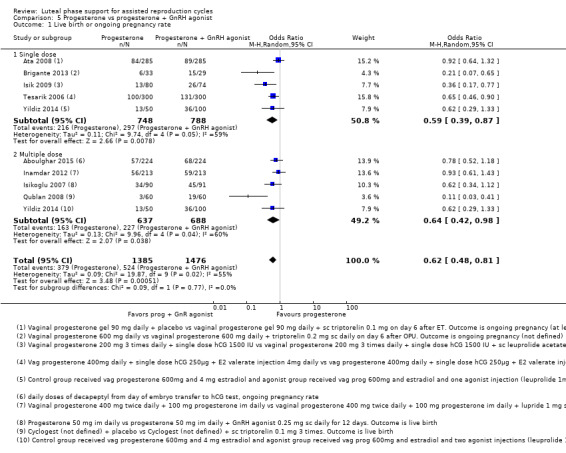
Comparison 5 Progesterone vs progesterone + GnRH agonist, Outcome 1 Live birth or ongoing pregnancy rate.
Secondary outcomes
5.2 Clinical pregnancy rate
Eight studies reported this outcome (Ata 2008; Aboulghar 2015; Brigante 2013; Isik 2009; Isikoglu 2007; Qublan 2008; Tesarik 2006; Yildiz 2014). Researchers administered the GnRH agonist as a single dose (five RCTs, 1536 women) or in multiple doses (four RCTs, 899 women); One trial had two intervention arms of single and multiple doses (Yildiz 2014).
Pregnancy rates were lower in the progesterone‐only group (OR 0.66, 95% CI 0.51 to 0.85, eight RCTs, 2435 women, I2 = 47%, random effects, low quality evidence). High statistical heterogeneity in this analysis appeared to be largely due to wide variation between studies in the size of the effect. See Analysis 5.2 for details.
5.2. Analysis.
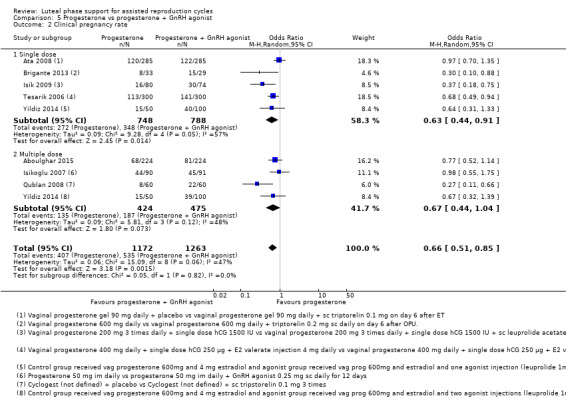
Comparison 5 Progesterone vs progesterone + GnRH agonist, Outcome 2 Clinical pregnancy rate.
When data were considered by number of doses of GnRH agonist, findings were consistent with those of the analysis of live birth/ongoing pregnancy in the single‐dose progesterone group but were not statistically significant in the multiple‐dose group. Results of the statistical test for subgroup differences were not significant. See Analysis 5.1 for details.
Subgroup analyses for clinical pregnancy rate
5.2.1 Ovarian stimulation protocol
In subgroup analyses, findings were consistent with those of the main analysis in the subgroup that received hCG with or without GnRH antagonists (Isik 2009; Porcu 2003; Tesarik 2006;Yildiz 2014) and the subgroup that received hCG with or without GnRH agonists (Aboulghar 2015Ata 2008; Isikoglu 2007; Qublan 2008; Tesarik 2006). Results of the statistical test for subgroup differences were not significant. See Analysis 5.3 for details.
5.3. Analysis.
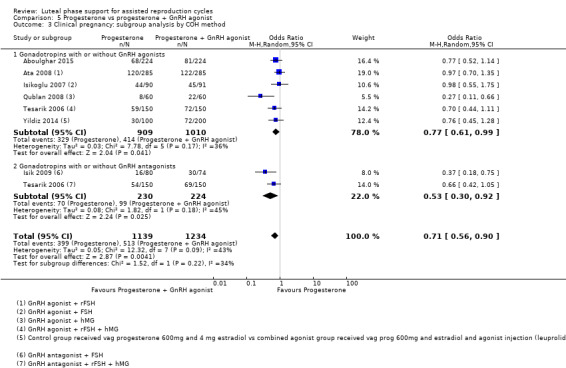
Comparison 5 Progesterone vs progesterone + GnRH agonist, Outcome 3 Clinical pregnancy: subgroup analysis by COH method.
5.2.2 Women with previously failed cycles
No data were available for this subgroup analysis.
5.2.3 Duration of progesterone (clinical pregnancy rate)
In subgroup analyses, findings were consistent with those of the main analysis in the subgroup that stopped treatment at the time of the pregnancy test (Aboulghar 2015; Isik 2009; Isikoglu 2007; Tesarik 2006; Yildiz 2014). Random effects model. Evidence suggested no differences between groups in the subgroup of women who were treated for up to 12 weeks when pregnant (Ata 2008). However results of the statistical test for subgroup differences were not significant. See Analysis 5.4 for details of this comparison.
5.4. Analysis.
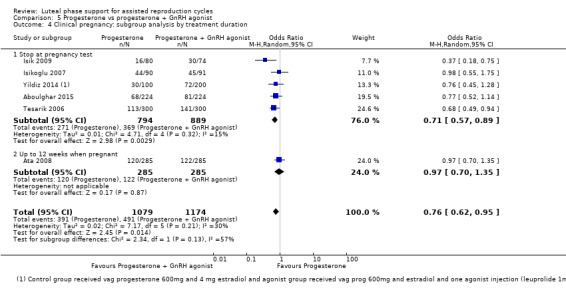
Comparison 5 Progesterone vs progesterone + GnRH agonist, Outcome 4 Clinical pregnancy: subgroup analysis by treatment duration.
5.2.4 Number of embryos transferred (clinical pregnancy rate)
No data were available for this subgroup analysis.
5.3 Miscarriage rate
Two studies reported this outcome (Qublan 2008;Yildiz 2014). The GnRH agonist was mostly administered in multiple doses. Evidence suggested no differences between groups (OR 1.37, 95% CI 0.53 to 3.52, 2 RCTs, 420 women). See Analysis 5.5 for details.
5.5. Analysis.
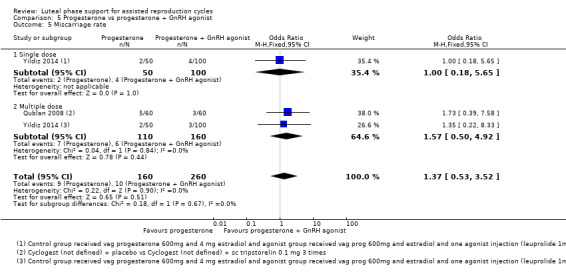
Comparison 5 Progesterone vs progesterone + GnRH agonist, Outcome 5 Miscarriage rate.
5.4 Multiple pregnancy
Four studies reported this outcome (Ata 2008; Inamdar 2012; Isik 2009;Yildiz 2014). Researchers administered the GnRH agonist as a single dose (two RCTs, 724 women) or in multiple doses (one RCT, 426 women) and one study both single and multiple doses. Evidence suggested no differences between groups. When the data were considered by number of doses of GnRH agonist, findings did not differ substantially. See Analysis 5.6 for details.
5.6. Analysis.
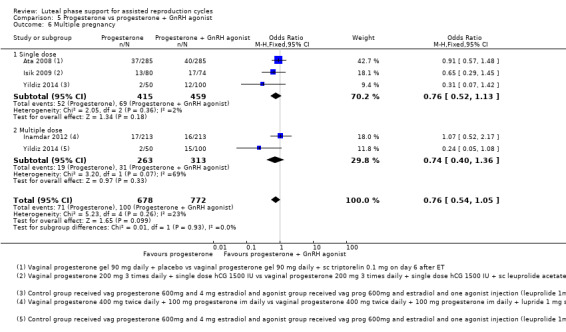
Comparison 5 Progesterone vs progesterone + GnRH agonist, Outcome 6 Multiple pregnancy.
5.5 Ovarian hyperstimulation syndrome (OHSS)
One study reported OHSS and showed no evidence of a difference between the groups (OR 1.00, 95% CI 0.33 to 3.01, 1 RCT, 300 women, very low quality evidence). See Analysis 5.7 for details.
5.7. Analysis.
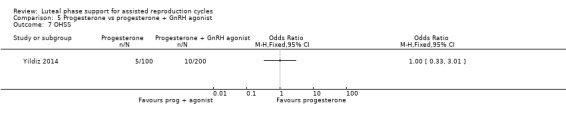
Comparison 5 Progesterone vs progesterone + GnRH agonist, Outcome 7 OHSS.
6. Progesterone regimens
Primary outcome
6.1 Live birth/ongoing pregnancy rate
Twenty‐five studies compared progesterone regimens and reported live birth (Abate 1999a; Baker 2014; Bergh 2012; Chakravarty 2005; Dal Prato 2008; Doody 2009; Goudge 2010; Iwase 2008; Lockwood 2014; Mochtar 2006; Nyboe Andersen 2002; Pouly 1996; Propst 2001; Stadtmauer 2013; Zegers‐Hochschild 2000) or ongoing pregnancy (Aghsa 2012; Beltsos 2011; Friedler 1999; Kohls 2012; Kyrou 2011; Ludwig 2002; Perino 1997; Salehpour 2013; Tay 2005; Yanushpolsky 2010).
See Figure 13 for details of this comparison.
13.
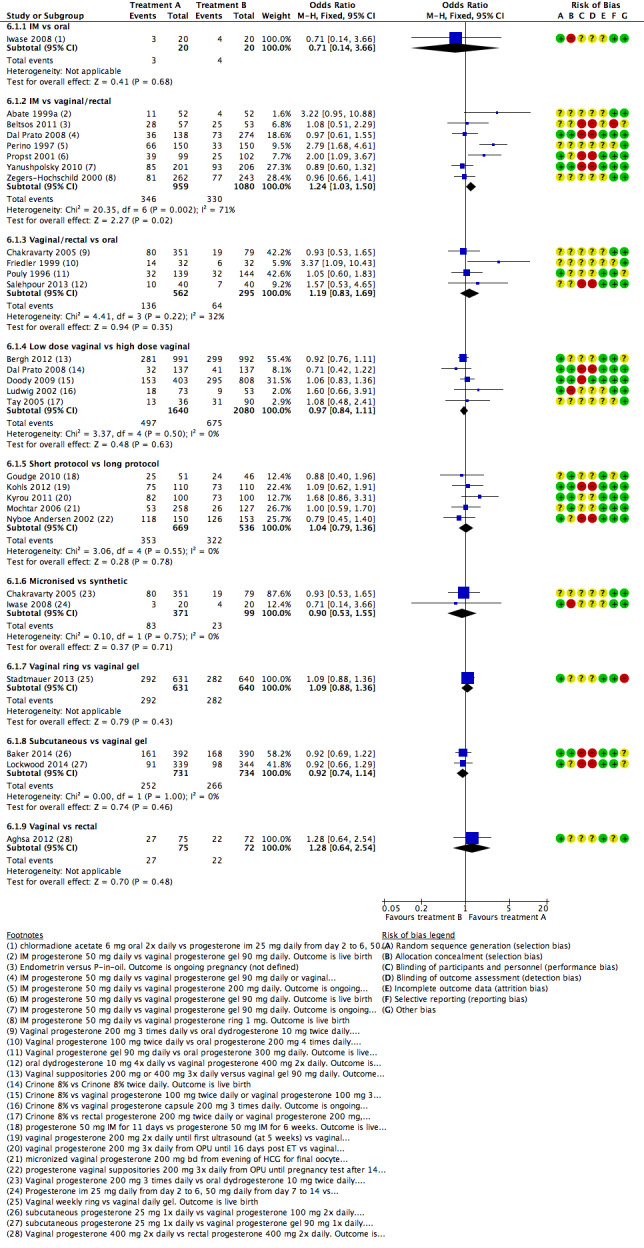
Forest plot of comparison: 6 Progesterone regimens, outcome: 6.1 Live birth or ongoing pregnancy rate.
6.1.1 Intramuscular (IM) vs oral
One study made this comparison and reported live birth (Iwase 2008). No evidence suggested differences between groups (OR 0.71, 95% CI 0.14 to 3.66, one RCT, 40 women, very low quality evidence).
6.1.2 IM vs vaginal or rectal
Seven studies made this comparison and reported live birth (Abate 1999a; Dal Prato 2008; Propst 2001; Zegers‐Hochschild 2000) or ongoing pregnancy (Beltsos 2011; Perino 1997; Yanushpolsky 2010;). Dal Prato 2008 was a three‐arm study investigating IM progesterone versus vaginal gel 90 mg daily versus vaginal gel 90 mg twice daily. We combined both vaginal arms and compared them with the IM arm.
Live birth and ongoing pregnancy rates were higher in the vaginal/rectal group (OR 1.24, 95% CI 1.03 to 1.50, seven RCTs, 2039 women, I2 = 71%, very low quality evidence). However, statistical heterogeneity was high, and when a random‐effects model was used, no evidence suggested differences between groups (OR 1.37, 95% CI 0.94 to 1.99).
When analysis was restricted to studies reporting live birth, no evidence suggested differences between groups (OR 1.31, 95% CI 0.84 to 2.05, four RCTs, 1222 women, I2 = 59%, random‐effects model).
6.1.3 Vaginal or rectal vs oral
Four studies made this comparison and reported live birth (Chakravarty 2005; Pouly 1996) or ongoing pregnancy (Friedler 1999; Salehpour 2013). Evidence suggested no differences between groups (OR 1.19, 95% CI 0.83 to 1.69, four RCTs, 857 women, I2 = 32%, low quality evidence).
Findings did not differ substantially when analysis was restricted to studies reporting live birth.
6.1.4 Low dose vaginal (≤ 100 mg) vs high dose vaginal (> 100 mg)
Five studies made this comparison and reported live birth (Bergh 2012; Dal Prato 2008; Doody 2009) or ongoing pregnancy (Ludwig 2002; Tay 2005). Doody 2009 was a three‐arm study comparing micronised progesterone vaginal gel 90 mg versus vaginal progesterone 100 mg twice daily versus vaginal progesterone 100 mg three times daily. We combined the two high‐dose arms in this comparison.
Evidence suggested no differences between groups (OR 0.97, 95% CI 0.84 to 1.11, five RCTs, 3720 women, I2 = 0%, moderate quality evidence).
Findings did not differ substantially when analysis was restricted to studies reporting live birth.
6.1.5 Short protocol vs long protocol
FIve studies made this comparison and reported live birth (Goudge 2010; Mochtar 2006; Nyboe Andersen 2002) or ongoing pregnancy (Kohls 2012; Kyrou 2011). Mochtar 2006 was a three‐arm study comparing micronised vaginal progesterone 200 mg twice daily starting at the evening of hCG administration for final oocyte maturation versus starting at the evening after oocyte retrieval versus starting at the evening after ET. We combined the first two arms.
No evidence suggested differences between groups (OR 1.04, 95% CI 0.79 to 1.36, five RCTs, 1205 women, I2 = 0%, low quality evidence).
Findings did not differ substantially when analysis was restricted to studies reporting live birth.
6.1.6 Micronised progesterone vs synthetic progesterone
Two studies made this comparison (Chakravarty 2005; Iwase 2008). Both reported live birth. Evidence suggested no differences between groups (OR 0.90, 95% CI 0.53 to 1.55, two RCTs, 470 women, I2 = 0%, low quality evidence).
6.1.7 Vaginal ring vs vaginal gel
One study made this comparison (Stadtmauer 2013) and reported live birth.
Evidence suggested no differences between groups (OR 1.09, 95% CI 0.88 to 1.36, one RCT, 1271 women, low quality evidence).
6.1.8 Subcutaneous vs vaginal gel
Two studies made this comparison (Baker 2014; Lockwood 2014). Both reported live births.
Evidence suggested no differences between groups (OR 0.92, 95% CI 0.74 to 1.14, two RCTs, 1465 women, I2 = 0%, low quality evidence).
6.1.9 Vaginal vs rectal
One study made this comparison (Aghsa 2012) and reported ongoing pregnancy.
Evidence suggested no differences between groups (OR 1.28, 95% CI 0.64 to 2.54, one RCT, 147 women, very low quality evidence).
Secondary outcomes
6.2 Clinical pregnancy rate
Forty‐one studies compared progesterone regimens and reported clinical pregnancy. See Analysis 6.2 for details of this comparison.
6.2. Analysis.
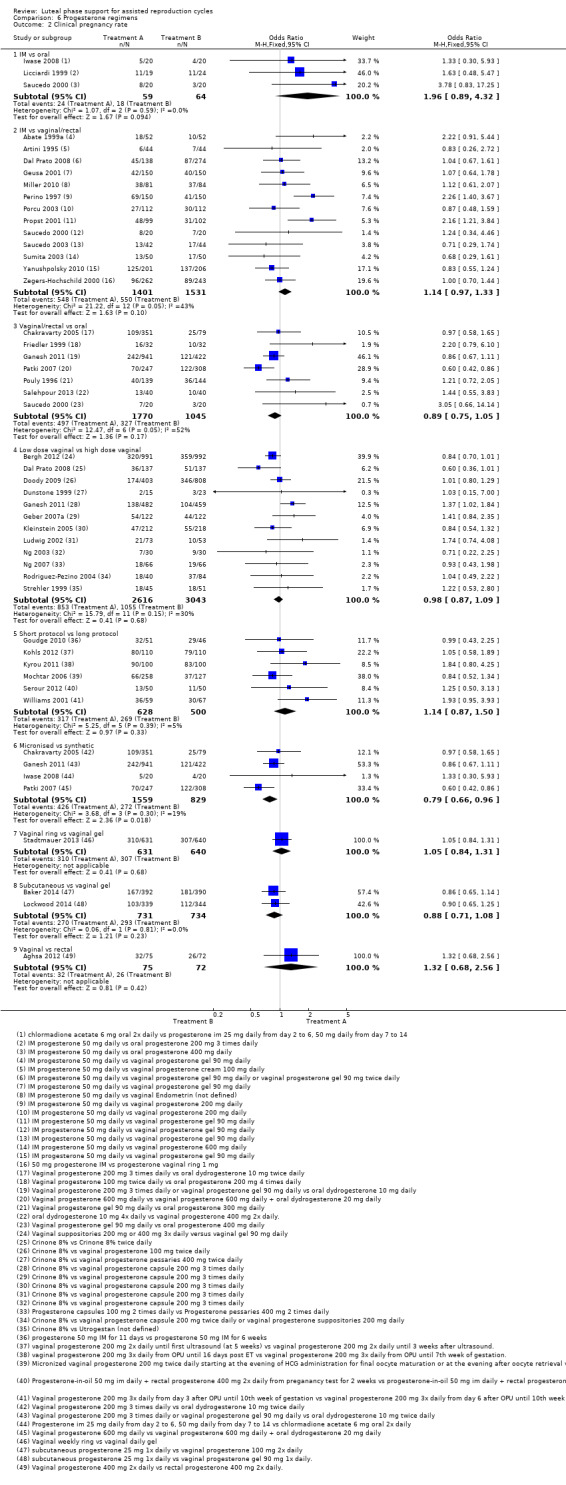
Comparison 6 Progesterone regimens, Outcome 2 Clinical pregnancy rate.
6.2.1 IM vs oral
Three studies made this comparison (Iwase 2008; Licciardi 1999; Saucedo 2000). Evidence suggested no differences between groups (OR 1.96, 95% CI 0.89 to 4.32, three RCTs, 123 women, I2 = 0%).
Subgroup analyses for clinical pregnancy rate
No data were available for subgroup analyses.
6.2.2 IM vs vaginalor rectal
Thirteen studies made this comparison (Abate 1999a; Artini 1995; Dal Prato 2008; Geusa 2001; Miller 2010; Perino 1997; Porcu 2003; Propst 2001; Saucedo 2000; Saucedo 2003; Sumita 2003; Yanushpolsky 2010; Zegers‐Hochschild 2000). Dal Prato 2008 was a three‐arm study investigating IM progesterone versus vaginal gel 90 mg daily versus vaginal gel 90 mg twice daily. We combined both vaginal arms and compared them with the IM arm. Evidence suggested no differences between groups (OR 1.14, 95% CI 0.97 to 1.33, 13 RCTs, 2932 women, I2 = 43%).
Because this comparison included more than 10 studies, we prepared a funnel plot to determine the risk of publication bias (see Figure 5). This was discussed in the section Selective reporting (reporting bias). We concluded that the study showed a small risk of publication bias.
Subgroup analyses for clinical pregnancy rate
6.2.2.1 Ovarian stimulation protocol
Eleven studies were subgrouped by method of ovarian stimulation. Ten utilised hCG with or without GnRH agonists (Abate 1999a; Artini 1995; Dal Prato 2008; Geusa 2001; Perino 1997; Porcu 2003; Saucedo 2003; Sumita 2003; Yanushpolsky 2010; Zegers‐Hochschild 2000). Studies in this subgroup were not pooled because of marked heterogeneity (I2 = 65%). A single study utilised hCG with or without GnRH agonists (Miller 2010) and reported findings similar to those of the main analysis. See Analysis 6.6 for details.
6.6. Analysis.
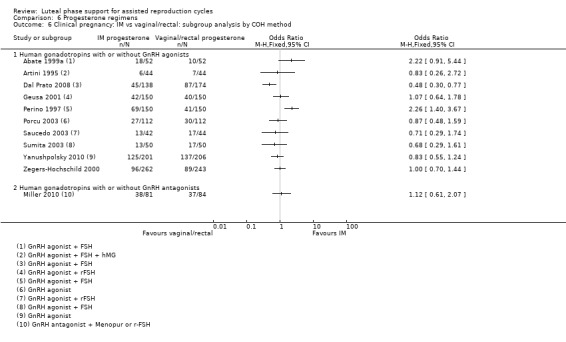
Comparison 6 Progesterone regimens, Outcome 6 Clinical pregnancy: IM vs vaginal/rectal: subgroup analysis by COH method.
6.2.2.2 Women with previously failed cycles
No data were available for this subgroup analysis.
6.2.2.3 Duration of progesterone
Seven studies were subgrouped by duration of progesterone. Two stopped treatment at the pregnancy test (Artini 1995;Perino 1997), and five administered progesterone for up to 12 weeks when pregnant (Abate 1999a; Dal Prato 2008; Propst 2001; Sumita 2003; Yanushpolsky 2010). Studies in these subgroups were not pooled because of statistical heterogeneity (I2 = 68%), and inconsistency was noted in the directions of effect. See Analysis 6.7 for details of this comparison.
6.7. Analysis.
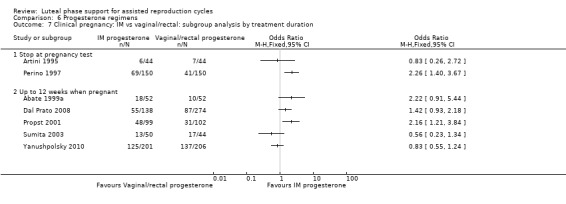
Comparison 6 Progesterone regimens, Outcome 7 Clinical pregnancy: IM vs vaginal/rectal: subgroup analysis by treatment duration.
6.2.2.4 Number of embryos transferred
No data were available for this subgroup analysis.
6.2.3 Vaginal or rectal vs oral
Seven studies made this comparison (Chakravarty 2005; Friedler 1999; Ganesh 2011; Patki 2007; Pouly 1996; Salehpour 2013; Saucedo 2000).
Evidence suggested no differences between groups (OR 0.89, 95% CI 0.75 to 1.05, seven RCTs, 2815 women, I2 = 52%).
Heterogeneity was substantial, in part because of the findings of Patki 2007. This study compared vaginal progesterone versus vaginal progesterone + oral progesterone, and the two different routes may have played a role in creating heterogeneity. When this study was omitted from the analysis, the I2 value was reduced to 25%.
Subgroup analyses for clinical pregnancy rate
6.2.3.1 Ovarian stimulation protocol
No data were available for this subgroup analysis.
6.2.3.2 Women with previously failed cycles
No data were available for this subgroup analysis.
6.2.3.3 Duration of progesterone
Five studies were subgrouped by duration of progesterone treatment. Two studies stopped treatment at the pregnancy test (Friedler 1999; Patki 2007), and four administered progesterone for up to 12 weeks when pregnant (Chakravarty 2005; Ganesh 2011; Pouly 1996; Salehpour 2013). Findings in the subgroup that stopped at the pregnancy test suggested benefit for the oral group, but inconsistency and very high heterogeneity were noted for the subgroup (I2 = 82%), which indicates that this finding should be regarded very cautiously. Findings in the group that administered progesterone for up to 12 weeks did not differ substantially from those of the main analysis. Results of the test for subgroup differences were not statistically significant. See Analysis 6.8 for details of this comparison.
6.8. Analysis.
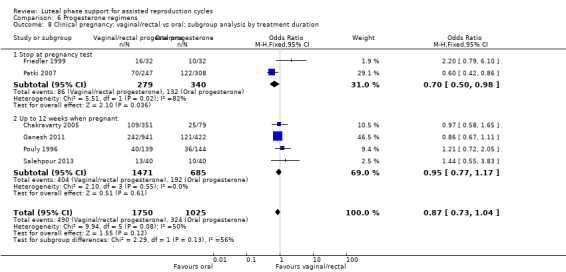
Comparison 6 Progesterone regimens, Outcome 8 Clinical pregnancy: vaginal/rectal vs oral: subgroup analysis by treatment duration.
6.2.3.4 Number of embryos transferred
No data were available for this subgroup analysis.
6.2.4 Low dose vaginal (≤ 100 mg) vs high dose vaginal (> 100 mg)
Twelve studies made this comparison (Bergh 2012; Dal Prato 2008; Doody 2009; Dunstone 1999; Ganesh 2011; Geber 2007a; Kleinstein 2005; Ludwig 2002; Ng 2003; Ng 2007; Rodriguez‐Pezino 2004; Strehler 1999). Doody 2009 was a three‐arm study comparing micronised progesterone vaginal gel 90 mg versus vaginal progesterone 100 mg twice daily versus vaginal progesterone 100 mg three times daily. We combined the two high‐dose arms in this comparison.
Evidence suggested no differences between groups (OR 0.98, 95% CI 0.87 to 1.09, 12 RCTs, 5659 women, I2 = 30%).
Because this comparison included more than 10 studies, we prepared a funnel plot to determine the risk of publication bias (see Figure 5). This was discussed in the section Selective reporting (reporting bias); we concluded that the study showed a small risk of publication bias.
Subgroup analyses for clinical pregnancy rate
6.2.4.1 Ovarian stimulation protocol
Nine studies were subgrouped by method of ovarian stimulation. Eight utilised hCG with or without GnRH agonists (Dal Prato 2008; Doody 2009; Ganesh 2011; Geber 2007a; Kleinstein 2005; Ng 2003; Ng 2007; Strehler 1999), and one utilised hCG with or without GnRH agonists (Rodriguez‐Pezino 2004). For both subgroups, findings were similar to those of the main analysis. See Analysis 6.9 for details.
6.9. Analysis.
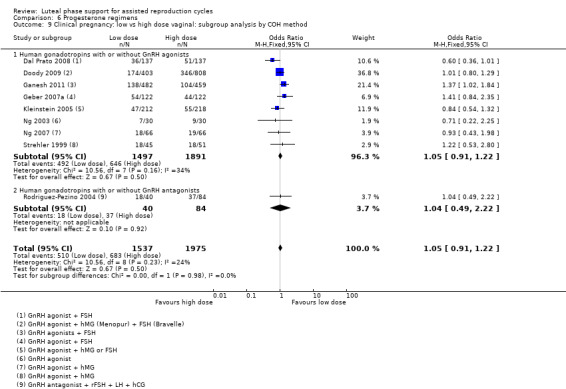
Comparison 6 Progesterone regimens, Outcome 9 Clinical pregnancy: low vs high dose vaginal: subgroup analysis by COH method.
6.2.4.2 Women with previously failed cycles
No data were available for this subgroup analysis.
6.2.4.3 Duration of progesterone
Nine studies were subgrouped by duration of progesterone. Three stopped treatment at the pregnancy test (Ludwig 2002; Ng 2003; Ng 2007), and six administered progesterone for up to 12 weeks when pregnant ((Dal Prato 2008; Doody 2009; Ganesh 2011; Geber 2007a; Kleinstein 2005; Strehler 1999). In both subgroups, findings were similar to those of the main analysis. See Analysis 6.10 for details.
6.10. Analysis.
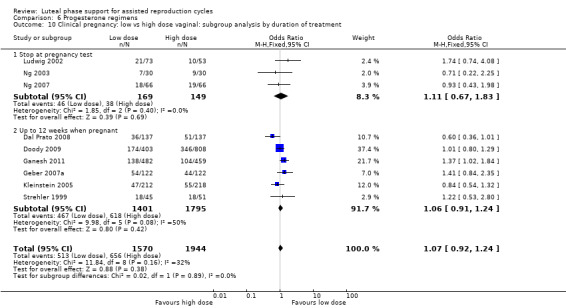
Comparison 6 Progesterone regimens, Outcome 10 Clinical pregnancy: low vs high dose vaginal: subgroup analysis by duration of treatment.
6.2.4.4 Number of embryos transferred
No data were available for this subgroup analysis.
6.2.5 Short protocol vs long protocol
Six studies made this comparison (Goudge 2010; Kohls 2012; Kyrou 2011; Mochtar 2006; Serour 2012; Williams 2001). Mochtar 2006 was a three‐arm study comparing micronised vaginal progesterone 200 mg twice daily starting at the evening of hCG administration for final oocyte maturation versus starting at the evening after oocyte retrieval versus starting at the evening after embryo transfer (ET). We combined the first two arms. Evidence suggested no differences between groups (OR 1.14, 95% CI 0.87 to 1.50, six RCTs, 1128 women, I2 = 5%).
Subgroup analyses for clinical pregnancy rate
6.2.5.1 Ovarian stimulation protocol
Four studies were subgrouped by method of ovarian stimulation. Two utilised hCG with or without GnRH agonists (Goudge 2010; Mochtar 2006), and two utilised hCG with or without GnRH antagonists (Kohls 2012; Kyrou 2011). In both subgroups, findings were similar to those of the main analysis. See Analysis 6.11 for details.
6.11. Analysis.
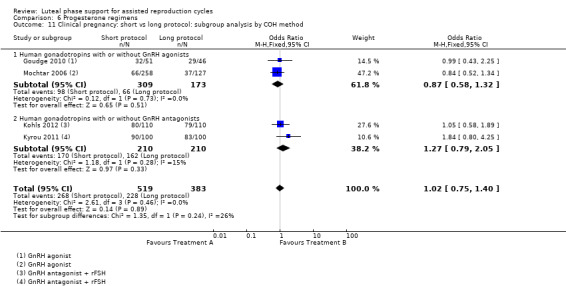
Comparison 6 Progesterone regimens, Outcome 11 Clinical pregnancy: short vs long protocol: subgroup analysis by COH method.
6.2.5.2 Women with previously failed cycles
No data were available for this subgroup analysis.
6.2.5.3 Duration of progesterone
No data were available for this subgroup analysis.
6.2.5.4 Number of embryos transferred
No data were available for this subgroup analysis.
6.2.6 Micronised progesterone vs synthetic progesterone
Four studies made this comparison (Chakravarty 2005; Ganesh 2011; Iwase 2008; Patki 2007).
Clinical pregnancy rates were lower in the micronised progesterone group (OR 0.79, 95% CI 0.66 to 0.96, four RCTs, 2388 women, I2 = 19%), suggesting benefit for the synthetic progesterone group.
Subgroup analyses for clinical pregnancy rate
No data were available for subgroup analyses.
6.2.7 Vaginal ring vs vaginal gel
One study made this comparison (Stadtmauer 2013).
Evidence suggested no differences between groups (OR 1.05, 95% CI 0.84 to 1.31, one RCT, 1271 women).
6.2.8 Subcutaneous vs vaginal gel
Two studies made this comparison (Baker 2014; Lockwood 2014). Evidence suggested no differences between groups (OR 0.88, 95% CI 0.71 to 1.08, two RCTs, 1465 women, I2 = 0%).
Subgroup analyses for clinical pregnancy rate
No data were available for subgroup analyses.
6.2.9 Vaginal vs rectal
One study made this comparison (Aghsa 2012). Evidence suggested no differences between groups (OR 1.32, 95% CI 0.68 to 2.56, one RCT, 147 women).
6.3 Miscarriage rate
Twenty‐six studies compared progesterone regimens and reported miscarriage. See Analysis 6.3 for details of this comparison.
6.3. Analysis.
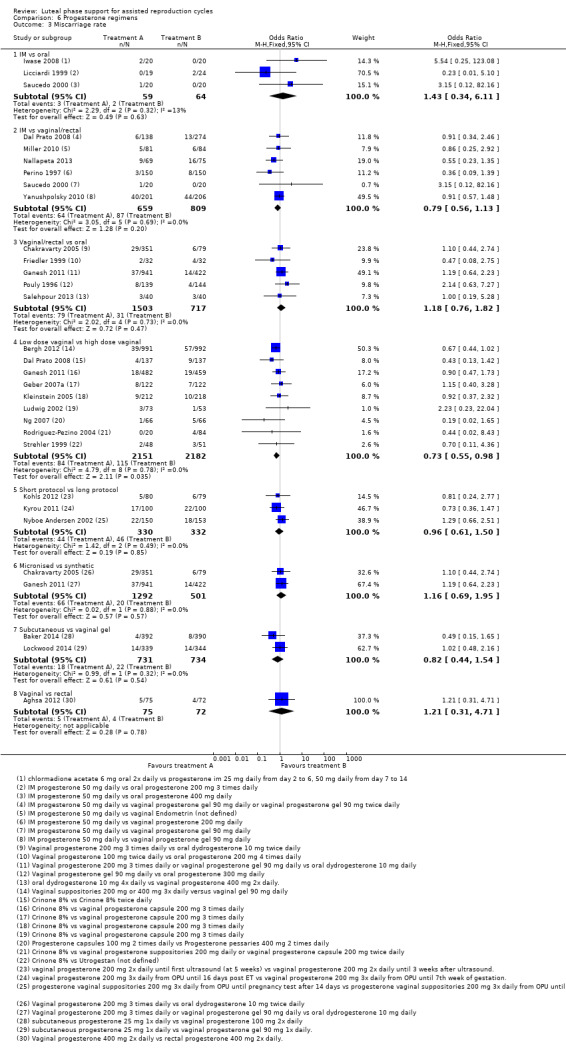
Comparison 6 Progesterone regimens, Outcome 3 Miscarriage rate.
6.3.1 IM vs oral
Three studies made this comparison (Iwase 2008; Licciardi 1999; Saucedo 2000). Evidence suggested no differences between groups (OR 1.43, 95% CI 0.34 to 6.11, three RCTs, 123 women, I2 = 13%).
6.3.2 IM vs vaginal or rectal
Six studies made this comparison (Dal Prato 2008; Miller 2010; Nallapeta 2013; Perino 1997; Saucedo 2000; Yanushpolsky 2010). Evidence suggested no differences between groups (OR 0.79, 95% CI 0.56 to 1.13, six RCTs, 1468 women, I2 = 0%).
6.3.3 Vaginalor rectal vs oral
Five studies made this comparison (Chakravarty 2005; Friedler 1999; Ganesh 2011; Pouly 1996; Salehpour 2013). Dal Prato 2008 was a three‐arm study investigating IM progesterone versus vaginal gel 90 mg daily versus vaginal gel 90 mg twice daily. We combined both vaginal arms and compared them with the IM arm. Evidence suggested no differences between groups (OR 1.18, 95% CI 0.76 to 1.82, five RCTs, 2220 women, I2 = 0%).
6.3.4 Low dose vaginal (≤ 100 mg) vs high dose vaginal (> 100 mg)
Nine studies made this comparison (Bergh 2012; Dal Prato 2008; Ganesh 2011; Geber 2007a; Kleinstein 2005; Ludwig 2002; Ng 2007; Rodriguez‐Pezino 2004; Strehler 1999).
Miscarriage rates were lower in the low‐dose group (OR 0.73, 95% CI 0.55 to 0.98, nine RCTs, 4333 women, I2 = 0%), suggesting benefit for this group.
6.3.5 Short protocol vs long protocol
Three studies made this comparison (Kohls 2012; Kyrou 2011; Nyboe Andersen 2002). Evidence suggested no differences between groups (OR 0.96, 95% CI 0.61 to 1.50, three RCTs, 662 women, I2 = 0%).
6.3.6 Micronised progesterone vs synthetic progesterone
Two studies made this comparison (Chakravarty 2005; Ganesh 2011). Evidence suggested no differences between groups (OR 1.16, 95% CI 0.69 to 1.95, two RCTs, 1793 women, I2 = 0%).
6.3.7 Vaginal ring vs vaginal gel
No studies reported this outcome.
6.3.8 Subcutaneous vs vaginal gel
Two studies made this comparison (Baker 2014; Lockwood 2014). Evidence suggested no differences between groups (OR 0.82, 95% CI 0.44 to 1.54, two RCTs, 1465 women, I2 = 0%).
6.3.9 Vaginal vs rectal
One study made this comparison (Aghsa 2012). Evidence suggested no differences between groups (OR 1.21, 95% CI 0.31 to 4.71, one RCT, 147 women).
6.4 Ovarian hyperstimulation syndrome (OHSS)
Two studies compared progesterone regimens and reported OHSS. See Analysis 6.4 for details of this comparison.
6.4. Analysis.
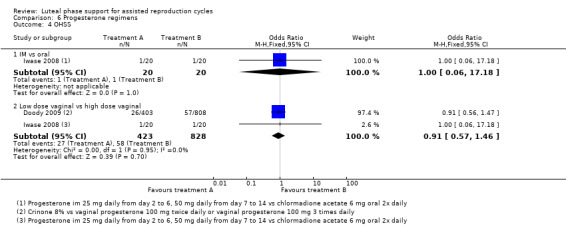
Comparison 6 Progesterone regimens, Outcome 4 OHSS.
6.4.1 IM vs oral
One study made this comparison (Iwase 2008).
Evidence suggested no differences between groups (OR 1.00, 95% CI 0.06 to 17.18, one RCT, 40 women, very low quality evidence).
6.4.2 IM vs vaginal or rectal
No studies made this comparison.
6.4.3 Vaginal or rectal vs oral
No studies made this comparison.
6.4.4 Low dose vaginal (≤ 100 mg) vs high dose vaginal (> 100 mg)
Two studies made this comparison (Doody 2009; Iwase 2008). Doody 2009 was a three‐arm study comparing micronised progesterone vaginal gel 90 mg versus vaginal progesterone 100 mg twice daily versus vaginal progesterone 100 mg three times daily. We combined the two high‐dose arms in this comparison. Evidence suggested no differences between groups (OR 0.91, 95% CI 0.57 to 1.46, two RCTs, 1251 women, I2 = 0%, low quality evidence).
6.4.5 Short protocol vs long protocol
No studies made this comparison.
6.4.6 Micronised progesterone vs synthetic progesterone
No studies made this comparison.
6.4.7 Vaginal ring vs vaginal gel
No studies made this comparison.
6.4.8 Subcutaneous vs vaginal gel
No studies made this comparison.
6.4.9 Vaginal vs rectal
No studies made this comparison.
6.5 Multiple pregnancy
Fourteen studies compared progesterone regimens and reported multiple pregnancy. See Analysis 6.5 for details of this comparison.
6.5. Analysis.
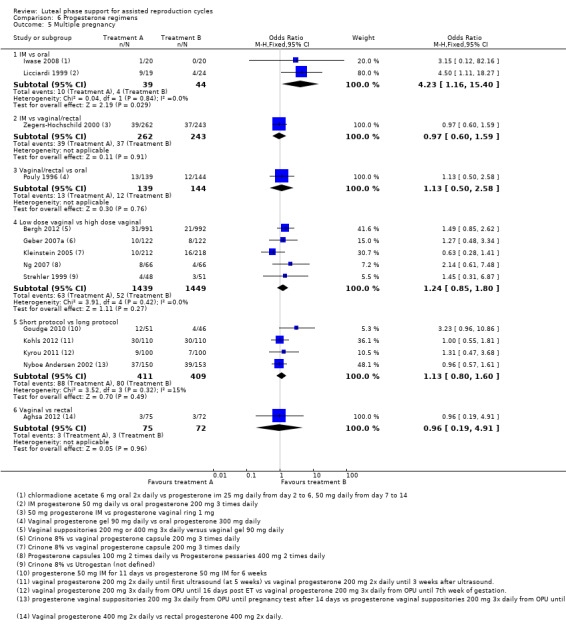
Comparison 6 Progesterone regimens, Outcome 5 Multiple pregnancy.
6.5.1 IM vs oral
Two studies made this comparison (Iwase 2008; Licciardi 1999).
More multiple pregnancies were reported in the IM arm (OR 4.23, 95% CI 1.16 to 15.40, two RCTs, 83 women, I2 = 0%), suggesting benefit for the oral arm. This analysis included only 14 events and 80 women, so this finding should be interpreted with caution.
6.5.2 IM vs vaginalor rectal
One study made this comparison (Zegers‐Hochschild 2000). Evidence suggested no differences between groups (OR 0.97, 95% CI 0.60 to 1.59, one RCT, 505 women).
6.5.3 Vaginalor rectal vs oral
One study made this comparison (Pouly 1996). Evidence suggested no differences between groups (OR 1.13, 95% CI 0.50 to 2.58, one RCT, 283 women).
6.5.4 Low dose vaginal (≤ 100 mg) vs high dose vaginal (> 100 mg)
Five studies made this comparison (Bergh 2012; Geber 2007a; Kleinstein 2005; Ng 2007; Strehler 1999). Evidence suggested no differences between groups (OR 1.24, 95% CI 0.85 to 1.80, five RCTs, 2888 women, I2 = 0%).
6.5.5 Short protocol vs long protocol
Four studies made this comparison (Goudge 2010; Kohls 2012; Kyrou 2011; Nyboe Andersen 2002). Evidence suggested no differences between groups (OR 1.13, 95% CI 0.80 to 1.60, four RCTs, 820 women, I2 = 15%).
6.5.6 Micronised progesterone vs synthetic progesterone
No studies reported this outcome.
6.5.7 Vaginal ring vs vaginal gel
No studies reported this outcome.
6.5.8 Subcutaneous vs vaginal gel
No studies reported this outcome.
6.5.9 Vaginal vs rectal
One study made this comparison (Aghsa 2012).
Evidence suggested no differences between groups (OR 0.96, 95% CI 0.19 to 4.91, one RCT, 147 women).
7. Progesterone + oestrogen regimens
Primary outcome
7.1 Live birth/ongoing pregnancy rate
Two studies comparing progesterone and oestrogen regimens reported ongoing pregnancy. One compared short versus long protocol (Feichtinger 2011), and one compared low versus high dosage (Tonguc 2011).
Tonguc 2011 was a three‐arm study comparing 2 mg oestradiol versus 4 mg oestradiol versus 6 mg oestradiol. We combined the arms with 4 mg and 6 mg oestradiol supplementation.
7.1.1 Short protocol vs long protocol
No studies making this comparison reported live birth.
Evidence suggested no differences between groups (OR 1.08, 95 CI 0.81 to 1.43, one RCT, 910 women, low quality evidence).
7.1.2 Low dosage vs high dosage
Evidence suggested no differences between groups (OR 0.65, 95% CI 0.37 to 1.13, one RCT, 285 women, very low quality evidence).
Secondary outcomes
7.2 Clinical pregnancy rate
7.2.1 Short protocol vs long protocol
No reported studies made this comparison.
7.2.2 Low dosage vs high dosage
One study (Tonguc 2011) made this comparison.
Evidence suggested no differences between groups (OR 0.81, 95% CI 0.48 to 1.37, one RCT, 285 women). See Analysis 7.2 for details.
7.2. Analysis.
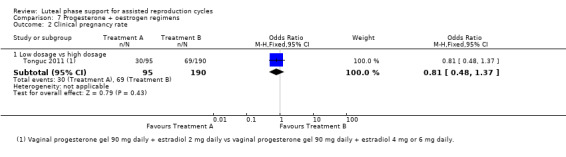
Comparison 7 Progesterone + oestrogen regimens, Outcome 2 Clinical pregnancy rate.
7.3 Miscarriage rate
7.3.1 Short protocol vs long protocol
No studies reported this outcome.
7.3.2 Low dosage vs high dosage
One study made this comparison (Tonguc 2011) .
Evidence suggested no differences between groups (OR 3.13, 95% CI 0.86 to 11.39, one RCT, 285 women).
See Analysis 7.3 for details of this comparison.
7.3. Analysis.
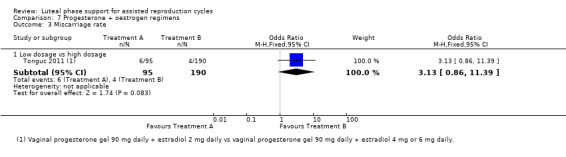
Comparison 7 Progesterone + oestrogen regimens, Outcome 3 Miscarriage rate.
7.4 OHSS
No studies reported this outcome.
7.5 Multiple pregnancy rates
7.5.1 Short protocol vs long protocol
No studies reported this outcome.
7.5.2 Low dosage vs high dosage
One study made this comparison (Tonguc 2011).
Evidence suggested no differences between groups (OR 0.25, 95% CI 0.06 to 1.12, one RCT, 285 women). See Analysis 7.4 for details,
7.4. Analysis.
Comparison 7 Progesterone + oestrogen regimens, Outcome 4 Multiple pregnancy.
8. Funnel plots
For comparisons with more than 10 included studies, we constructed a funnel plot. None of the funnel plots (Figure 4; Figure 5; Figure 6) suggested publication bias.
Discussion
Summary of main results
This systematic review of all randomised controlled trials (RCTs) of luteal phase support from 1987 to 2015 had a broad range of subjects. We had seven comparisons to ensure we included as many studies as possible. Because we did not include quasi‐RCTs, we had to exclude quite a few older studies; therefore we have sparse evidence regarding current standard luteal phase protocols. For an overview of the main findings, see the 'Summary of findings' tables (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7).
We can summarise the following results based on our review.
Human chorionic gonadotropin (hCG) for luteal phase support
hCG given during the luteal phase may be associated with higher rates of live birth or ongoing pregnancy than placebo or no treatment, but the evidence is not conclusive. hCG increased the risk of ovarian hyperstimulation syndrome (OHSS) (1 RCT only).
Progesterone for luteal phase support
Progesterone given during the luteal phase may be associated with higher rates of live birth or ongoing pregnancy than placebo or no treatment, but the evidence is not conclusive. Progesterone was associated with lower rates of OHSS rates than hCG with or without progesterone.
Progesterone and oestrogen for luteal phase support
We found no conclusive evidence of differences between groups for any outcome. Supplementation of progesterone with oral oestrogen did not appear to influence live birth and ongoing pregnancy rates, but benefit from transdermal or oral + transdermal oestrogen supplementation is suggested. Findings for supplementation of progesterone with vaginal oestrogen were inconsistent.
Progesterone and GnRH agonists for luteal phase support
A relatively new method of luteal phase support involves use of GnRH agonists, which appeared to increase rates of live birth/ongoing pregnancy and clinical pregnancy compared with progesterone alone. There was no evidence of a difference between the groups for other outcomes, though OHSS was reported in only one study.
Different progesterone regimens
When we compared routes of progesterone administration, we gathered no conclusive findings. Vaginal progesterone is the most commonly used formulation in Europe according to a survey of 21 European centres (Aboulghar 2008). Sixteen centres used vaginal progesterone, three used IM progesterone, one used oral progesterone and one hCG.
We found several studies investigating Crinone 8% vaginal micronised progesterone gel. Therefore we conducted a comparison to investigate low‐dose (≤ 100 mg) versus high‐dose (> 100 mg) vaginal progesterone. The analysis for the miscarriage rate suggested benefit derived from low‐dose vaginal progesterone. No evidence revealed differences between low‐dose and high‐dose groups for the other outcomes. A new method consists of a weekly progesterone ring, for which we also conducted a comparison. No evidence favoured the vaginal ring or gel.
Comparisons of long versus short duration of progesterone administration showed no evidence of differences between groups in live birth and ongoing pregnancy rates. Findings for clinical pregnancy were inconsistent.
Comparisons of synthetic versus micronised progesterone yielded no evidence of differences between groups in live birth and ongoing pregnancy rates. However, evidence suggested that synthetic progesterone was associated with a higher clinical pregnancy rate than micronised progesterone. The only synthetic progesterone used was oral dydrogesterone.
With regard to multiple pregnancy, the only evidence of differences between groups was seen for the comparison of IM progesterone with oral progesterone, which suggested that IM progesterone is associated with multiple pregnancies to a greater extent than oral progesterone.
Overall completeness and applicability of evidence
Most studies provided 'implantation rate' as an outcome. For clinicians, this outcome is not useful, as they would rather know the pregnancy rate or the live birth rate.
For women the most important outcome is live birth, which was reported in only 31 studies. For the 2015 update of this review, we pooled live birth and ongoing pregnancy rates to increase the power of the analysis. Sensitivity analyses limited to studies reporting live birth yielded findings very similar to those obtained for the combined outcome, suggesting that ongoing pregnancy was a reasonable surrogate for live birth in this review. The outcome clinical pregnancy was reported in most studies and did provide a few significant results. To investigate the safety of luteal phase support, we also looked at the negative side effects, OHSS and multiple pregnancy. These outcomes were not reported in all studies; therefore this review might not give an accurate representation of these important factors in luteal phase support.
Adverse effects were reported poorly in most of the included studies.
Some of the studies that we found investigated procedures or interventions influencing the luteal phase but did not investigate an intervention used as luteal phase support. All of these studies are reported in the Characteristics of excluded studies.
We included only first cycle data, and four studies reported more cycles than included women (Erman Akar 2005; Lukaszuk 2005; Unfer 2004; Unfer 2004a). We contacted the authors but have not received a reply.
In our protocol, we stated that we would include two other comparisons: urinary hCG versus recombinant hCG and single‐dose GnRH agonist versus placebo. We found no studies that were conducted to investigate these comparisons. It is unlikely that these comparisons will be made in the future, as hCG is an older method of luteal phase support and is known for its high risk of OHSS. We do not expect new trials to investigate the differences between urinary and recombinant hCG. Nowadays, progesterone is an accepted method for luteal phase support, and it is considered unethical to not provide any form of luteal phase support. Therefore we do not expect any new trials to investigate the effects of GnRH agonists for luteal phase support versus placebo. For these reasons, we chose to remove these comparisons.
To make sure we were as thorough as possible, we came up with some additional comparisons: low‐dose vaginal progesterone versus high‐dose vaginal progesterone, short protocol progesterone versus long protocol progesterone, vaginal ring versus vaginal gel, subcutaneous versus vaginal gel, vaginal versus rectal progesterone and progesterone versus progesterone + multiple‐dose GnRH agonist.
The included studies used differing inclusion and exclusion criteria, but this was not a limitation for inclusion in our review.
In conclusion, we included all first cycles of randomised trials of luteal phase support. We changed our comparisons after we completed our search to ensure that we covered as much as possible on this topic.
Quality of the evidence
This review has a huge number of included studies ‐ 94 studies ‐ that investigated many different interventions for luteal phase support. The total number of included participants was 25,471, but because of the variety of included studies per comparison, the total sample size per comparison ranged from 30 to well over 5000 participants.
Only 25 studies (27%) reported methods of randomisation and allocation concealment in sufficient detail, and only half (48/93) were rated as having low risk of attrition bias. More than half were rated as having high risk of bias in one or more domains. Although sensitivity analyses limited to studies with clear description of allocation concealment did not substantially affect any of our findings, we rated down the quality of evidence for all comparisons in our 'Summary of findings' tables because of the high proportions of "unclear" and "high" risk of bias ratings in most studies (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7).
Heterogeneity was low for most comparisons. For most cases in which substantial heterogeneity was detected, we were not able to find a clear reason, and for these analyses, our findings should be interpreted with caution.
The overall quality of the evidence ranged from very low to moderate. The main limitations were poor reporting of study methods and imprecision.
Potential biases in the review process
Two review authors extracted all data. MvdL extracted data from all studies, and MM and KB divided all studies between them; thus each extracted data from half of the studies. MvdL, who wrote the review, compared all results. In cases of disagreement, CF acted as a third review author and determined the final verdict. This usually happened after consultation with MvdL. This means that MvdL had a big influence on these decisions, and this might have introduced bias.
As a result of the large number of topics within the review, we might not have discussed all topics in depth.
We are quite sure that we found all relevant studies, but some studies might not have been published at the time of our search and are published now, at the time of publication. As discussed above, four studies did meet our inclusion criteria but reported more cycles than included women (Erman Akar 2005; Lukaszuk 2005; Unfer 2004; Unfer 2004a). We contacted these study authors but received no reply, so we did not include these studies.
In the 2015 update, we have made several changes and additions to the original protocol (see Differences between protocol and review). We believe that these changes are likely to have reduced rather than increased the potential for bias in the review, by ensuring that we consider all relevant comparisons and use the latest recommended Cochrane standards.
Agreements and disagreements with other studies or reviews
Upon comparing this review with a previous version (Daya 2004), we found a lot of similarities regarding the quality of included studies. Although this previous review included quasi‐randomised trials, the overall quality of the present review is still poor. As stated in the implications for research section of the previous versions of this review (Daya 2004, van der Linden 2011), improvement of the quality of studies, especially in terms of blinding, is important for further research. Among the more recent (after 2004) studies that we have included, the quality of studies does indeed seem to be better, although most of these researchers did not use blinding. In Daya 2004, it was discussed that live birth was not often reported as an outcome. This seems to be improved but in most cases, it still is not the main outcome.
We have noted some agreements and some disagreements between the findings of Daya 2004 and the results of this review. As no new studies have investigated hCG versus placebo, we found no new results. In all comparisons investigating hCG, we found that hCG involves higher risk of OHSS. This was significant for hCG versus placebo and progesterone versus progesterone + hCG.
In the previous versions of this review (Daya 2004 and van der Linden 2011), no evidence favoured a particular route of administration. Both versions of the review reported no differences in effect between different doses of vaginal progesterone.
Both reviews found higher pregnancy rates for progesterone in GnRH agonist‐stimulated cycles.
Authors' conclusions
Implications for practice.
hCG or progesterone given during the luteal phase may be associated with higher rates of live birth or ongoing pregnancy than placebo or no treatment, but the evidence is not conclusive. The addition of GnRHa to progesterone appears to improve outcomes. hCG may increase the risk of OHSS compared to placebo. Moreover hCG, with or without progesterone, is associated with higher rates of OHSS than progesterone alone. Neither the addition of oestrogen nor the route of progesterone administration appears to be associated with an improvement in outcomes.
Implications for research.
Of the 94 included studies, only 10 used blinding. The method of blinding used and specification of who was blinded were poorly reported. If high‐quality evidence is to be gained, studies should be properly blinded and should use a double‐dummy design.
Only 31 studies reported live birth as an outcome. We believe this outcome is more valuable than short‐term outcomes such as clinical pregnancy rate. Therefore researchers should use live birth as the main outcome of their study.
Early studies were placebo controlled. As research has shown that luteal phase support has a positive effect on pregnancy outcomes, it is unethical to not use luteal phase support. Therefore we support the trend in more recent studies to compare different kinds of support, doses or routes of administration. GnRH agonist is relatively new as a method of providing luteal phase support, but it shows promising results. Therefore, high‐quality randomised double‐blind controlled trials should be conducted to compare GnRH agonist support versus progesterone.
What's new
| Date | Event | Description |
|---|---|---|
| 20 October 2016 | Amended | Conclusions reworded to clarify that the evidence for hCG is very similar to the evidence for progesterone, with respect to their effect on live birth and pregnancy. |
History
Protocol first published: Issue 6, 2011 Review first published: Issue 10, 2011
| Date | Event | Description |
|---|---|---|
| 28 October 2015 | Amended | Response to feedback, opportunity taken to add two new studies (Aboulghar 2015;Yildiz 2014). Corrected analyses 1.1 and 3.5, corrected median values in summary of findings table. |
| 29 April 2015 | New citation required but conclusions have not changed | The addition of 24 new studies has not led to a change in the conclusions of the review |
| 29 April 2015 | New search has been performed | This is an update of a previously published review (van der Linden 2011). We have included 24 new studies in the review (Aghahosseini 2011; Aghsa 2012; Ata 2010; Baker 2014; Beltsos 2011; Bergh 2012; Brigante 2013; Colakoglu 2011; Erdem 2013; Feichtinger 2011; Humaidan 2006; Inamdar 2012; Kably Ambe 2005; Kyrou 2011; Lin 2013; Lockwood 2014; Mochtar 2006; Moini 2011; Nallapeta 2013; Nyboe Andersen 2002; Salehpour 2013; Serour 2012; Tonguc 2011; Williams 2001). Kohls 2012 and Stadtmauer 2013 replace the abstracts previously published in 2010 New comparisons added: progesterone + oestrogen regimens, vaginal suppositories vs vaginal gel, vaginal progesterone vs rectal progesterone, subcutaneous progesterone vs vaginal gel, vaginal ring vs vaginal gel Correction to analyses: all now set to record "event" rather than "non‐event" Primary outcome: changed from live birth to live birth or ongoing pregnancy No major change to conclusions (although advantage for synthetic progesterone is no longer evident) |
| 4 June 2012 | Amended | Correction to summary of main results: progesterone and oestrogen for luteal phase support |
| 11 May 2012 | Amended | Correction of erroneous data for Elgindy 2010 (Analyses 4.2.1 and 4.2.3) |
| 16 March 2011 | New search has been performed | This is an update of a previously published review with the same title and has been prepared by a new review author team |
Acknowledgements
This review is an of an update of a previously withdrawn Cochrane review (Daya 2004), which was withdrawn in 2008 because of changes in the methodology of Cochrane systematic reviews. We would like to thank the previous review authors.
MDSG, especially Jane Marjoribanks, for all her editorial work; Marian Showell, Trial‐Search Co‐ordinator for MDSG, for writing and running the search strings; Vanessa Jordan, NZ Cochrane Fellow, for help with statistics and heterogeneity; Jane Clarke and Helen Nagels, Managing Editors of MDSG, and Julie Brown, systematic reviewer, for answering our questions; and the Obstetrics & Gynaecology Department of the University of Auckland for the support provided.
Dr Luiz Eduardo Albuquerque and Dr Mario Tristán for help in translating articles.
Prof Eman Elgindy for constructive advice.
All original authors of study papers who took the time to reply to our queries.
Appendices
Appendix 1. Gynaecology and Fertility search strategy
From inception until 05.08.15
Keywords CONTAINS "luteal phase" or "luteal phase support" or "luteal phase support timing" or "luteal phase supprt" or "luteal support" or Title CONTAINS "luteal phase" or "luteal phase support" or "luteal phase support timing" or "luteal phase supprt" or "luteal support"
AND
Keywords CONTAINS "Progesterone" or "progesterone capsule" or "progesterone gel" or "progesterone, micronized" or "progesterone receptor agonist" or "HCG" or "human chorionic gonadotrophin" or "human chorionic gonadotropin" or "dydrogesterone" or "dydrogestrone" or "utrogestan" or "vaginal micronised progesterone" or "vaginal micronized progesterone capsules" or "vaginal micronized progesterone gel" or "vaginal progesterone" or "17‐alpha hydroxyprogesterone" or"GnRH a"or "GnRH agonist"or "Gonadotrophin releasing agonist"or "gonadotropin releasing hormone agonist"or "triptorelin"or"leuprolide"or"leuprolide acetate"or"leuprolide depot"or "Goserelin"or "Zoladex"or "nafarelin"or"buserelin"or"Buserelin Acetate"or "crinone"or "Crinone 8"or Title CONTAINS "Progesterone" or "progesterone capsule" or "progesterone gel" or "progesterone, micronized" or "progesterone receptor agonist" or "HCG" or "human chorionic gonadotrophin" or "human chorionic gonadotropin" or "dydrogesterone" or "dydrogestrone" or "utrogestan" or "vaginal micronised progesterone" or "vaginal micronized progesterone capsules" or "vaginal micronized progesterone gel" or "vaginal progesterone" or "17‐alpha hydroxyprogesterone" or "GnRH a"or "GnRH agonist"or "Gonadotrophin releasing agonist"or "gonadotropin releasing hormone agonist"or "triptorelin"or"leuprolide"or"leuprolide acetate"or"leuprolide depot"or "Goserelin"or "Zoladex"or "nafarelin"or"buserelin"or"Buserelin Acetate"or "crinone"or "Crinone 8" (361 hits)
Appendix 2. CENTRAL search strategy
From inception until 05.08.15
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (1756) 2 embryo transfer$.tw. (1200) 3 in vitro fertili?ation.tw. (1610) 4 ivf‐et.tw. (324) 5 (ivf or et).tw. (13581) 6 icsi.tw. (992) 7 intracytoplasmic sperm injection$.tw. (538) 8 (blastocyst adj2 transfer$).tw. (130) 9 exp Insemination, Artificial/ (296) 10 Insemination$.tw. (820) 11 iui.tw. (398) 12 or/1‐11 (15755) 13 exp Luteal Phase/ (455) 14 (luteal adj5 support$).tw. (285) 15 (luteal adj5 phase).tw. (1041) 16 (ischemic adj5 phase).tw. (158) 17 post ovulat$.tw. (20) 18 (post adj5 transfer$).tw. (76) 19 (after adj5 transfer$).tw. (8297) 20 (post adj5 trigger$).tw. (19) 21 (after adj5 trigger$).tw. (3284) 22 or/13‐21 (12631) 23 12 and 22 (2186) 24 exp Progesterone/ (2262) 25 Progesterone$.tw. (2894) 26 dydrogesterone.tw. (167) 27 utrogest.tw. (7) 28 17 alpha‐hydroxyprogesterone.tw. (93) 29 Prontogest.tw. (5) 30 exp chorionic gonadotropin/ or exp chorionic gonadotropin, beta subunit, human/ (632) 31 HCG.tw. (1313) 32 crinone.tw. (46) 33 chorionic gonadotropin$.tw. (515) 34 chorionic gonadotrophin$.tw. (287) 35 exp Gonadotropin‐Releasing Hormone/ (1892) 36 gnrha.tw. (246) 37 gnrh agonist$.tw. (821) 38 gnrh a.tw. (1441) 39 Gonadotrop?in‐Releasing Hormone agonist$.tw. (514) 40 buserelin/ or goserelin/ or leuprolide/ or nafarelin/ or triptorelin pamoate/ (1268) 41 leuprolide.tw. (460) 42 triptorelin.tw. (205) 43 (goserelin or Zoladex).tw. (550) 44 (nafarelin or buserelin).tw. (360) 45 or/24‐44 (8084) 46 23 and 45 (925)
Appendix 3. MEDLINE search strategy
From inception until 05.08.15
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (34795) 2 embryo transfer$.tw. (8997) 3 in vitro fertili?ation.tw. (18351) 4 ivf‐et.tw. (1956) 5 (ivf or et).tw. (199930) 6 icsi.tw. (6126) 7 intracytoplasmic sperm injection$.tw. (5452) 8 (blastocyst adj2 transfer$).tw. (634) 9 exp Insemination, Artificial/ (10415) 10 Insemination$.tw. (13455) 11 iui.tw. (1330) 12 or/1‐11 (239597) 13 exp Luteal Phase/ (4707) 14 (luteal adj5 support$).tw. (609) 15 (luteal adj5 phase).tw. (9066) 16 (ischemic adj5 phase).tw. (1010) 17 post ovulat$.tw. (722) 18 (post adj5 transfer$).tw. (1196) 19 (after adj5 transfer$).tw. (18597) 20 (post adj5 trigger$).tw. (423) 21 (after adj5 trigger$).tw. (2718) 22 or/13‐21 (34992) 23 12 and 22 (4736) 24 exp Progesterone/ (65145) 25 Progesterone$.tw. (71879) 26 dydrogesterone.tw. (386) 27 utrogest.tw. (4) 28 17 alpha‐hydroxyprogesterone.tw. (1233) 29 Prontogest.tw. (5) 30 exp chorionic gonadotropin/ or exp chorionic gonadotropin, beta subunit, human/ (29789) 31 HCG.tw. (21949) 32 crinone.tw. (55) 33 chorionic gonadotropin$.tw. (14208) 34 chorionic gonadotrophin$.tw. (4428) 35 exp Gonadotropin‐Releasing Hormone/ (29797) 36 gnrha.tw. (1216) 37 gnrh agonist$.tw. (3674) 38 gnrh a.tw. (962) 39 Gonadotrop?in‐Releasing Hormone agonist$.tw. (2279) 40 buserelin/ or goserelin/ or leuprolide/ or nafarelin/ or triptorelin pamoate/ (7400) 41 leuprolide.tw. (1648) 42 triptorelin.tw. (591) 43 (goserelin or Zoladex).tw. (1060) 44 (nafarelin or buserelin).tw. (1508) 45 or/24‐44 (158445) 46 23 and 45 (1880) 47 randomized controlled trial.pt. (415276) 48 controlled clinical trial.pt. (92000) 49 randomized.ab. (337080) 50 randomised.ab. (68774) 51 placebo.tw. (173943) 52 clinical trials as topic.sh. (179581) 53 randomly.ab. (243225) 54 trial.ti. (148605) 55 (crossover or cross‐over or cross over).tw. (66344) 56 or/47‐55 (1052850) 57 exp animals/ not humans.sh. (4138378) 58 56 not 57 (970697) 59 46 and 58 (413)
Appendix 4. EMBASE search strategy
From inception until 05.08.15
1 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (58478) 2 embryo$ transfer$.tw. (14723) 3 in vitro fertili?ation.tw. (22782) 4 ivf‐et.tw. (2613) 5 icsi.tw. (11295) 6 intracytoplasmic sperm injection$.tw. (7090) 7 (blastocyst adj2 transfer$).tw. (1405) 8 (ivf or et).tw. (562334) 9 exp artificial insemination/ (13046) 10 Insemination$.tw. (14515) 11 iui.tw. (2291) 12 or/1‐11 (613465) 13 exp luteal phase/ (8055) 14 (luteal adj5 support$).tw. (935) 15 (luteal adj5 phase).tw. (9877) 16 (ischemic adj5 phase).tw. (1407) 17 post ovulat$.tw. (724) 18 (post adj5 transfer$).tw. (1828) 19 (after adj5 transfer$).tw. (21870) 20 (post adj5 trigger$).tw. (617) 21 (after adj5 trigger$).tw. (3522) 22 or/13‐21 (41810) 23 12 and 22 (7785) 24 exp PROGESTERONE/ (74004) 25 Progesterone$.tw. (76866) 26 dydrogesterone.tw. (481) 27 utrogest.tw. (39) 28 17 alpha‐hydroxyprogesterone.tw. (544) 29 Prontogest.tw. (69) 30 exp chorionic gonadotropin/ (39901) 31 HCG.tw. (26131) 32 crinone.tw. (342) 33 chorionic gonadotropin$.tw. (14449) 34 chorionic gonadotrophin$.tw. (4514) 35 exp gonadorelin/ (29277) 36 gnrha.tw. (1660) 37 gonadorelin.tw. (265) 38 gnrh agonist$.tw. (5201) 39 gnrh a.tw. (1168) 40 Gonadotrop?in‐Releasing Hormone agonist$.tw. (2638) 41 exp triptorelin/ (4310) 42 exp leuprorelin/ (9237) 43 (leuprolide or leuprorelin).tw. (2810) 44 (triptorelin or nafarelin).tw. (1207) 45 nafarelin acetate/ or nafarelin/ (1355) 46 exp goserelin/ (6081) 47 buserelin acetate/ or buserelin/ (4709) 48 (goserelin or Zoladex).tw. (2780) 49 (nafarelin or buserelin).tw. (1792) 50 or/24‐49 (187454) 51 23 and 50 (3417) 52 Clinical Trial/ (852394) 53 Randomized Controlled Trial/ (387382) 54 exp randomization/ (68604) 55 Single Blind Procedure/ (21212) 56 Double Blind Procedure/ (124532) 57 Crossover Procedure/ (44903) 58 Placebo/ (265208) 59 Randomi?ed controlled trial$.tw. (125918) 60 Rct.tw. (18621) 61 random allocation.tw. (1464) 62 randomly.tw. (303665) 63 randomly allocated.tw. (23537) 64 allocated randomly.tw. (2071) 65 (allocated adj2 random).tw. (741) 66 Single blind$.tw. (16522) 67 Double blind$.tw. (155916) 68 ((treble or triple) adj blind$).tw. (497) 69 placebo$.tw. (222683) 70 prospective study/ (312235) 71 or/52‐70 (1692110) 72 case study/ (34359) 73 case report.tw. (293151) 74 abstract report/ or letter/ (942602) 75 or/72‐74 (1263530) 76 71 not 75 (1651662) 77 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5399612) 78 76 not 77 (1535561) 79 51 and 78 (837)
Appendix 5. PsycINFO search strategy
From inception until 05.08.15 1 exp Reproductive Technology/ (1519) 2 embryo transfer$.tw. (103) 3 in vitro fertili?ation.tw. (603) 4 ivf‐et.tw. (17) 5 (ivf or et).tw. (105955) 6 icsi.tw. (58) 7 intracytoplasmic sperm injection$.tw. (44) 8 (blastocyst adj2 transfer$).tw. (4) 9 Insemination$.tw. (644) 10 iui.tw. (27) 11 or/1‐10 (107656) 12 (luteal adj5 support$).tw. (1) 13 (luteal adj5 phase).tw. (901) 14 (ischemic adj5 phase).tw. (69) 15 post ovulat$.tw. (16) 16 (post adj5 transfer$).tw. (170) 17 (after adj5 transfer$).tw. (1133) 18 (post adj5 trigger$).tw. (46) 19 (after adj5 trigger$).tw. (224) 20 or/12‐19 (2539) 21 11 and 20 (88) 22 exp Progesterone/ (1933) 23 Progesterone$.tw. (3631) 24 dydrogesterone.tw. (9) 25 utrogest.tw. (0) 26 17 alpha‐hydroxyprogesterone.tw. (6) 27 Prontogest.tw. (0) 28 exp Gonadotropic Hormones/ (3880) 29 HCG.tw. (81) 30 crinone.tw. (0) 31 chorionic gonadotropin$.tw. (87) 32 chorionic gonadotrophin$.tw. (12) 33 exp Gonadotropic Hormones/ (3880) 34 gnrha.tw. (29) 35 gnrh agonist$.tw. (58) 36 gnrh a.tw. (9) 37 Gonadotrop?in‐Releasing Hormone agonist$.tw. (61) 38 (leuprolide or leuprorelin).tw. (85) 39 (triptorelin or nafarelin).tw. (24) 40 gonadorelin.tw. (3) 41 (goserelin or Zoladex).tw. (28) 42 buserelin.tw. (6) 43 or/22‐42 (7576) 44 21 and 43 (4)
Appendix 6. CINAHL search strategy
| From 1982 to 05.08.15 |
| # | Query | Results |
| S29 | S20 AND S28 | 58 |
| S28 | S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 | 856 |
| S27 | TX after N5 trigger* | 214 |
| S26 | TX post N3 transfer* | 81 |
| S25 | TX post ovulat* | 11 |
| S24 | TX ischemic N5 phase | 105 |
| S23 | TX luteal N5 phase | 446 |
| S22 | TX luteal N5 support* | 29 |
| S21 | (MM "Luteal Phase") | 54 |
| S20 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 | 5,745 |
| S19 | TX intra‐uterine insemination | 9 |
| S18 | TX (ovari* N2 induction) | 12 |
| S17 | TX COH | 61 |
| S16 | TX ovarian hyperstimulation | 317 |
| S15 | TX superovulat* | 22 |
| S14 | TX intrauterine insemination | 142 |
| S13 | TX IUI | 75 |
| S12 | TX artificial insemination | 443 |
| S11 | TX assisted reproduct* | 1,250 |
| S10 | (MM "Insemination, Artificial") | 236 |
| S9 | (MM "Reproduction Techniques+") | 3,791 |
| S8 | TX intracytoplasmic sperm injection* | 223 |
| S7 | TX embryo* N3 transfer* | 729 |
| S6 | TX ovar* N3 hyperstimulat* | 319 |
| S5 | TX ovari* N3 stimulat* | 236 |
| S4 | TX IVF or TX ICSI | 1,196 |
| S3 | (MM "Fertilization in Vitro") | 1,388 |
| S2 | TX vitro fertilization | 2,750 |
| S1 | TX vitro fertilisation | 259 |
Appendix 7. The Cochrane Library ‐ DARE search strategy
| ID | Search |
| #1 | utrogestan in Other Reviews |
| #2 | vaginal micronised progesterone in Other Reviews |
| #3 | dydrogestrone in Other Reviews |
| #4 | Progesterone in Other Reviews |
| #5 | human chorionic gonadotrophin in Other Reviews |
| #6 | human chorionic gonadotropin in Other Reviews |
| #7 | luteal phase in Other Reviews |
| #8 | luteal phase support in Other Reviews |
| #9 | luteal phase support in Other Reviews |
| #10 | (( #1 OR #2 OR #3 OR #4 OR #5 OR #6 ) AND ( #7 OR #8 OR #9 )) |
Appendix 8. WHO ICTRP and clinicaltrials.gov
Searched from inception until 05.08.15
ICTRP
"Agonist and luteal phase" (39 hits)
"Progesterone and luteal phase support" (14 hits)
"Estradiol and luteal phase support" (4 hits)
"Estrogen and luteal phase support" (1 hit)
"Progesterone and luteal phase" (10 hits)
" HCG and luteal phase" (14 hits)
Clinicaltrials.gov
"Agonist and luteal phase" (55 hits)
"GNRH and luteal phase" (67 hits)
"Progesterone and luteal phase support" (60)
"Estrogen and luteal phase support" (16 hits)
Appendix 9. Web of Science search strategy
| Set | Results | |
| #7 | 323 | #5 AND #6 Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All Years |
| #6 | 55,927 | TS=(embryo transfer) OR TS=(ivf) OR TS=(in vitro fertilisation) OR TS=(in vitro infertilization) OR TS=(iui) OR TS=(icsi) Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All Years |
| #5 | 740 | #1 AND #4 Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All Years |
| #4 | 4,153 | #3 AND #2 Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All Years |
| #3 | 28,229 | TS=(hcg) OR TS=(chorionic gonadotropin) OR TS=(chorionic gonadotrophin) OR TS=(human chorionic gonadotropin) OR TS=(human chorionic gonadotrophin) Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All Years |
| #2 | 74,620 | TS=(progesterone) OR TS=(progesteron) OR TS=(dydrogesterone) OR TS=(utrogest) OR TS=(prontogest) OR TS=(17 alpha‐hydroxyprogesterone) Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All Years |
| #1 | 9,045 | TS=(Luteal phase) OR TS=(luteal support) OR TS=(Luteal phase support) Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All Years |
Appendix 10. Study eligibility form
| Study ID | |
| Report ID | |
| ID review author | |
| Date form completed | |
| Report authors | |
| Complete reference | |
| Publication type | |
| Report author contact details |
| Study characteristics | Review inclusion criteria | Assessment | Quotation | ||
| Yes | No | Unclear | |||
| Type of study | RCT or cross‐over? | ||||
| Participants | Women undergoing ART? | ||||
| When used: GIFT or ZIFT < 20%? | |||||
| Interventions | No frozen ET? | ||||
| No other substances than progesterone/hCG/oestrogen? | |||||
| No ET from donated oocytes? | |||||
| No ET from frozen oocytes/frozen ovarian tissue? | |||||
| No in vivo maturation (IVM)? | |||||
| Include progesterone administration (any route/type/duration) and/or hCG administration (any route/type/duration)? | |||||
| Progesterone administration for at least 5 days in luteal phase? | |||||
| At least 2 times hCG administration in luteal phase? | |||||
| Final decision |
| o Include (if all ‘yes’) |
| o Exclude (if any ‘no’) |
|
Reason for exclusion: If Unclear, action undertaken: |
Appendix 11. Data extraction form
General information
| Study ID | |
| ID review author | |
| Date form completed | |
| Complete reference | |
| Published? | o Yes o No |
| Publication type | Journal/Abstract/Other (specify) |
| Report author contact details | |
| Notes: |
| Study eligibility | |
|
Confirm eligibility |
o Included |
| o Excluded Reason for exclusion: | |
| o Unclear Reason: Action undertaken: | |
| Study details | ||
| Study intention | Description as stated in report | Reference |
| Aim of study | |
|
| Setting | o Multi‐centre o Single centre o Unclear |
|
| Type of study | o RCT o Cross‐over |
|
| Country | ||
| Power calculation done | o Yes o No o Unclear |
| Methods | Description as stated in report | Reference |
| Inclusion/Exclusion criteria for participation in study | ||
| Total number of intervention groups (specify) | ||
| Allocation concealment? | ||
| Moment of randomisation | ||
| Method randomisation sequence | ||
| Blinding | Clinician o Yes o No o Unclear Researcher o Yes o No o Unclear Participant o Yes o No o Unclear |
|
| Method of blinding | ||
| Reporting bias |
| Participants | Description | Reference |
| Total number randomly assigned | ||
| Total number analysed | ||
| Reason why not analysed | ||
| Number of cycles per woman | ||
| Number allocated to each intervention group | ||
| Numbers and reasons for exclusion for each intervention group | ||
| Age (median, mean, range, if available) | ||
| Number of IVF | ||
| Number of ICSI | ||
| Number of previous cycles | ||
| Number of transferred embryo’s |
| Intervention group | ||
| Group name | ||
| Intervention | Description | Reference |
| Type | ||
| Dosage | ||
| Number of doses | ||
| Route | ||
| Duration | ||
| Duration of follow‐up | ||
| Protocol for ovulation induction | ||
| Scheme for trigger | ||
| GnRH | o Agonist o Antagonist |
|
| GnRH scheme | o Duration o Dose o Route of administration |
|
| Comparison group | ||
| Group name | ||
| Comparison | Description | Reference |
| Type | ||
| Dosage | ||
| Number of doses | ||
| Route | ||
| Duration | ||
| Duration of follow‐up | ||
| Protocol for ovulation induction | ||
| Scheme for trigger | ||
| GnRH | o Agonist o Antagonist |
|
| GnRH scheme | o Duration o Dose o Route of administration |
|
| Outcomes | Yes | No | Definition? | References |
| LBR | ||||
| CPR | ||||
| OPR | ||||
| MR | ||||
| OHSS | ||||
| MPR | ||||
| Other (specify) |
| Results | Copy table for each comparison. | |||
| Comparison | ||||
| ITT? | ||||
| Results | Intervention |
Comparison |
||
| Events | Number of participants | Events | Number of participants | |
| ET | |
|||
| CPR | ||||
| OPR |
||||
| LBR | ||||
| OHSS | ||||
| MR | ||||
| MultiP | ||||
| Number of missing participants | ||||
| Reasons for missing participants | |
|||
| Any other results reported | |
|||
Other relevant information
| Information | Description | References |
| Funding source and possible conflict of interest | ||
| Notes review author | ||
| Correspondence required (specify) | o No o Yes o E‐mail sent on o Letter sent on o Fax sent on Reaction received: Yes/No USE NEW FORM WITH COMPLETE INFO |
Appendix 12. Risk of Bias
| Entry | Judgement | Description | Reference |
| Adequate sequence generation? | High risk Unclear risk Low risk |
Method used to produce comparable groups | |
| Adequate allocation concealment? | High risk Unclear risk Low risk |
Method used in detail | |
| Adequate blinding? | High risk Unclear risk Low risk |
All measures used | |
| Incomplete outcome data addressed? | High risk Unclear risk Low risk |
Completeness of data primary outcome (LBR) incl attrition and exclusions from analysis | |
| Free of selective reporting? | High risk Unclear risk Low risk |
State how possibility of selective outcome reporting is examined | |
| Free of other bias? | High risk Unclear risk Low risk |
State any important concerns |
Data and analyses
Comparison 1. Human chorionic gonadotropin (hCG) vs placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth/ongoing pregnancy rate | 3 | 527 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.08, 2.86] |
| 1.1 Live birth | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.2 [0.38, 12.87] |
| 1.2 Ongoing pregnancy | 2 | 489 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.05, 2.87] |
| 2 Clinical pregnancy rate | 5 | 746 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.90, 1.88] |
| 3 Clinical pregnancy rate: subgroup analysis by COH method | 5 | 746 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.90, 1.88] |
| 3.1 Human gonadotropins with clomiphene citrate without GnRH agonists | 1 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.54, 2.86] |
| 3.2 Human gonadotropins with or without GnRH agonists | 3 | 513 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.94, 2.40] |
| 3.3 Human gonadotropins with or without GnRH antagonists | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.33, 1.99] |
| 4 Miscarriage rate | 2 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.37, 6.21] |
| 5 OHSS | 1 | 387 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.28 [1.91, 9.60] |
1.1. Analysis.
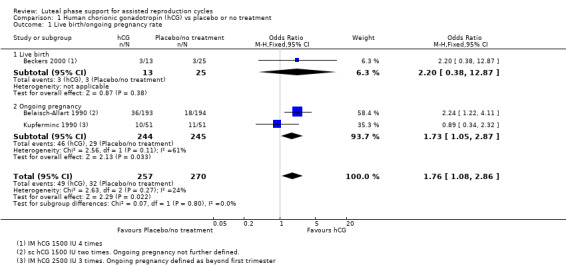
Comparison 1 Human chorionic gonadotropin (hCG) vs placebo or no treatment, Outcome 1 Live birth/ongoing pregnancy rate.
Comparison 2. Progesterone vs placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth/ongoing pregnancy rate | 5 | 642 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.09, 2.86] |
| 1.1 Live birth | 1 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.21 [0.93, 19.18] |
| 1.2 Ongoing pregnancy | 4 | 486 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.91, 2.57] |
| 2 Clinical pregnancy rate | 7 | 841 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.30, 2.75] |
| 3 Clinical pregnancy: subgroup analysis by COH method | 7 | 841 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.30, 2.75] |
| 3.1 Clomiphene citrate alone without GnRH agonists | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.54, 45.92] |
| 3.2 Human gonadotropins with clomiphene citrate without GnRH agonists | 2 | 306 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.86, 2.90] |
| 3.3 Human gonadotropins with or without GnRH agonists | 4 | 479 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.22, 3.26] |
| 4 Clinical pregnancy: subgroup analysis by treatment duration | 7 | 841 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.30, 2.75] |
| 4.1 Stop at pregnancy test | 3 | 257 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.74, 2.74] |
| 4.2 Up to 12 weeks | 4 | 584 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.37, 3.43] |
| 5 Miscarriage rate | 3 | 425 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.49, 3.03] |
| 6 Multiple pregnancy | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.87 [0.22, 155.76] |
2.1. Analysis.
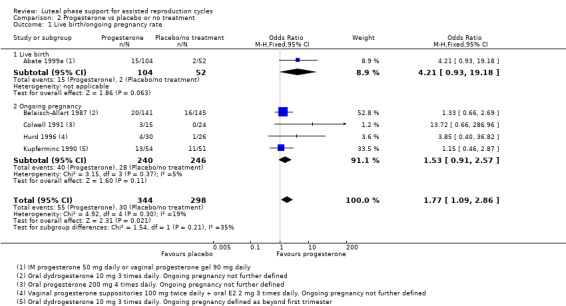
Comparison 2 Progesterone vs placebo or no treatment, Outcome 1 Live birth/ongoing pregnancy rate.
Comparison 3. Progesterone vs hCG regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth or ongoing pregnancy rate | 5 | 833 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.65, 1.38] |
| 1.1 Progesterone vs hCG | 4 | 434 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.54, 1.57] |
| 1.2 Progesterone vs progesterone + hCG | 2 | 399 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.58, 1.64] |
| 2 Clinical pregnancy rate | 16 | 2355 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.30] |
| 2.1 Progesterone vs hCG | 11 | 1378 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.94, 1.53] |
| 2.2 Progesterone vs progesterone + hCG | 7 | 977 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.25] |
| 3 Clinical pregnancy: progesterone vs progesterone + hCG: subgroup analysis by COH method | 4 | 722 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.65, 1.29] |
| 3.1 Human gonadotropins with clomiphene citrate without GnRH agonists | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.22, 13.41] |
| 3.2 Human gonadotropins with or without GnRH agonists | 3 | 702 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.63, 1.27] |
| 4 Clinical pregnancy: progesterone vs hCG: subgroup analysis by treatment duration | 7 | 872 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.85, 1.58] |
| 4.1 Stop at pregnancy test | 6 | 783 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.84, 1.61] |
| 4.2 Up to 12 weeks when pregnant | 1 | 89 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.46, 2.84] |
| 5 OHSS | 5 | 1293 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.30, 0.71] |
| 5.1 Progesterone vs hCG | 4 | 615 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.32, 1.00] |
| 5.2 Progesterone vs progesterone + hCG | 3 | 678 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.18, 0.69] |
| 6 Miscarriage rate | 5 | 832 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.66, 2.31] |
| 6.1 Progesterone vs hCG | 5 | 735 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.66, 2.55] |
| 6.2 Progesterone vs progesterone + hCG | 1 | 97 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.15, 5.06] |
| 7 Multiple pregnancy | 1 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.07, 2.65] |
| 7.1 Progesterone vs hCG | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.07, 7.23] |
| 7.2 Progesterone vs progesterone + hCG | 1 | 97 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 4.77] |
3.1. Analysis.
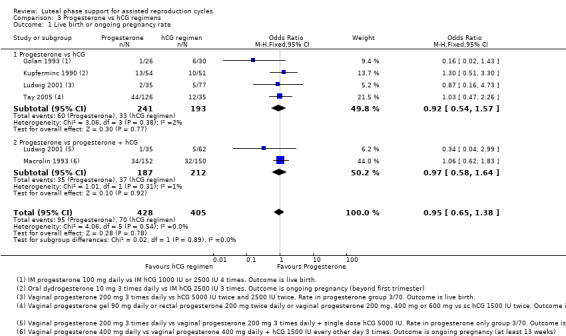
Comparison 3 Progesterone vs hCG regimens, Outcome 1 Live birth or ongoing pregnancy rate.
3.5. Analysis.
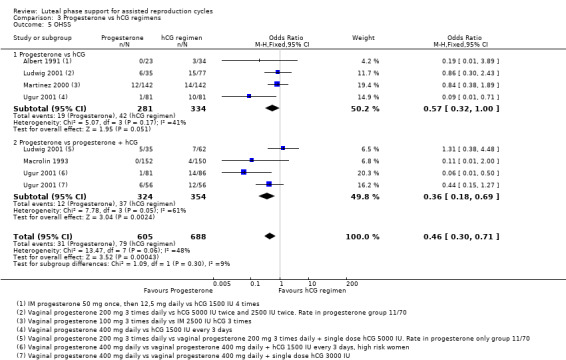
Comparison 3 Progesterone vs hCG regimens, Outcome 5 OHSS.
Comparison 4. Progesterone vs progesterone + oestrogen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth/ongoing pregnancy rate | 9 | 1651 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.91, 1.38] |
| 1.1 Oral oestrogen | 6 | 1266 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.87, 1.42] |
| 1.2 Transdermal oestrogen | 2 | 219 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.56, 1.67] |
| 1.3 Vaginal oestrogen | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.76, 2.59] |
| 2 Clinical pregnancy rate | 14 | 2169 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.04] |
| 2.1 Oral oestrogen | 9 | 1427 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.80, 1.27] |
| 2.2 Transdermal oestrogen | 3 | 364 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.26, 0.70] |
| 2.3 Vaginal oestrogen | 2 | 301 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.67, 1.71] |
| 2.4 Oral and transdermal oestrogen | 1 | 77 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.10, 0.96] |
| 3 Clinical pregnancy: subgroup analysis by COH method | 8 | 1183 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.22] |
| 3.1 Human gonadotropins with or without GnRH agonists | 7 | 1080 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.21] |
| 3.2 Human gonadotropins with or without GnRH antagonists | 2 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.51, 2.44] |
| 4 Clinical pregnancy: subgroup analysis by treatment duration | 10 | 1851 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.08] |
| 4.1 Stop at pregnancy test | 2 | 177 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.32] |
| 4.2 Up to 12 weeks when pregnant | 8 | 1674 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.12] |
| 5 Miscarriage rate | 10 | 1908 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.03, 0.03] |
| 5.1 Oral oestrogen | 7 | 1370 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.02, 0.04] |
| 5.2 Transdermal oestrogen | 1 | 160 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.09, 0.07] |
| 5.3 Vaginal oestrogen | 2 | 301 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.08, 0.07] |
| 5.4 Oral and transdermal oestrogen | 1 | 77 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.10 [‐0.22, 0.01] |
| 6 OHSS | 2 | 461 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.20, 1.68] |
| 6.1 Oral oestrogen | 1 | 402 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.22, 2.29] |
| 6.2 Transdermal oestrogen | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.20] |
4.1. Analysis.
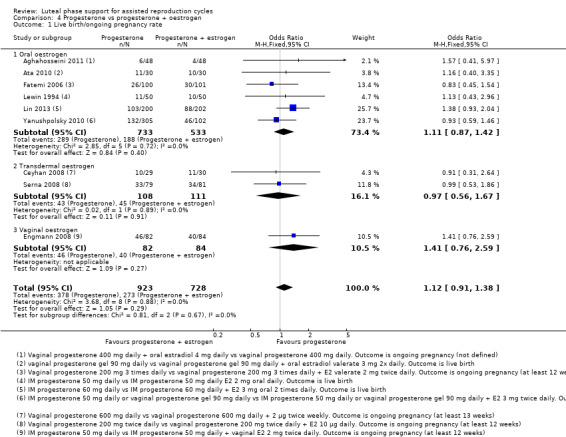
Comparison 4 Progesterone vs progesterone + oestrogen, Outcome 1 Live birth/ongoing pregnancy rate.
4.2. Analysis.
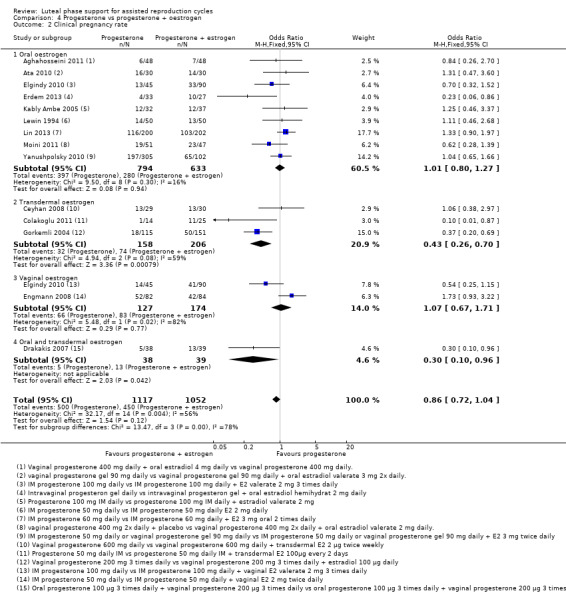
Comparison 4 Progesterone vs progesterone + oestrogen, Outcome 2 Clinical pregnancy rate.
4.5. Analysis.
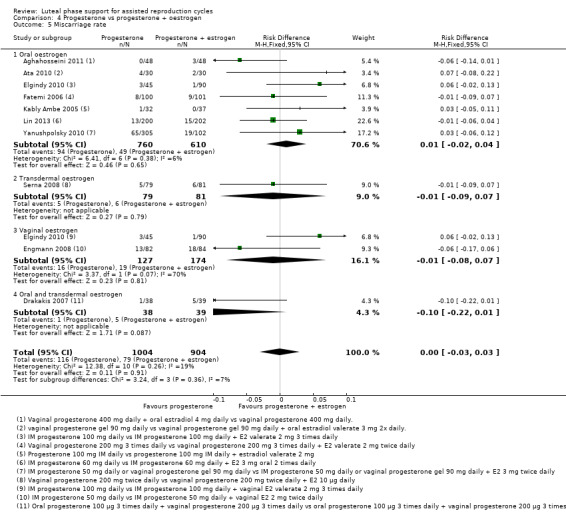
Comparison 4 Progesterone vs progesterone + oestrogen, Outcome 5 Miscarriage rate.
Comparison 5. Progesterone vs progesterone + GnRH agonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth or ongoing pregnancy rate | 9 | 2861 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.48, 0.81] |
| 1.1 Single dose | 5 | 1536 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.39, 0.87] |
| 1.2 Multiple dose | 5 | 1325 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.42, 0.98] |
| 2 Clinical pregnancy rate | 8 | 2435 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.51, 0.85] |
| 2.1 Single dose | 5 | 1536 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.44, 0.91] |
| 2.2 Multiple dose | 4 | 899 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.44, 1.04] |
| 3 Clinical pregnancy: subgroup analysis by COH method | 7 | 2373 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.56, 0.90] |
| 3.1 Gonadotropins with or without GnRH agonists | 6 | 1919 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.99] |
| 3.2 Gonadotropins with or without GnRH antagonists | 2 | 454 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.92] |
| 4 Clinical pregnancy: subgroup analysis by treatment duration | 6 | 2253 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.62, 0.95] |
| 4.1 Stop at pregnancy test | 5 | 1683 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.57, 0.89] |
| 4.2 Up to 12 weeks when pregnant | 1 | 570 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.70, 1.35] |
| 5 Miscarriage rate | 2 | 420 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.53, 3.52] |
| 5.1 Single dose | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.18, 5.65] |
| 5.2 Multiple dose | 2 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.50, 4.92] |
| 6 Multiple pregnancy | 4 | 1450 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.54, 1.05] |
| 6.1 Single dose | 3 | 874 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.52, 1.13] |
| 6.2 Multiple dose | 2 | 576 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.40, 1.36] |
| 7 OHSS | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. Progesterone regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth or ongoing pregnancy rate | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 IM vs oral | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.66] |
| 1.2 IM vs vaginal/rectal | 7 | 2039 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.03, 1.50] |
| 1.3 Vaginal/rectal vs oral | 4 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.83, 1.69] |
| 1.4 Low dose vaginal vs high dose vaginal | 5 | 3720 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.11] |
| 1.5 Short protocol vs long protocol | 5 | 1205 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.79, 1.36] |
| 1.6 Micronised vs synthetic | 2 | 470 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.53, 1.55] |
| 1.7 Vaginal ring vs vaginal gel | 1 | 1271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.88, 1.36] |
| 1.8 Subcutaneous vs vaginal gel | 2 | 1465 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.74, 1.14] |
| 1.9 Vaginal vs rectal | 1 | 147 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.64, 2.54] |
| 2 Clinical pregnancy rate | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 IM vs oral | 3 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.89, 4.32] |
| 2.2 IM vs vaginal/rectal | 13 | 2932 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.97, 1.33] |
| 2.3 Vaginal/rectal vs oral | 7 | 2815 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.05] |
| 2.4 Low dose vaginal vs high dose vaginal | 12 | 5659 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.87, 1.09] |
| 2.5 Short protocol vs long protocol | 6 | 1128 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.87, 1.50] |
| 2.6 Micronised vs synthetic | 4 | 2388 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.66, 0.96] |
| 2.7 Vaginal ring vs vaginal gel | 1 | 1271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.84, 1.31] |
| 2.8 Subcutaneous vs vaginal gel | 2 | 1465 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.71, 1.08] |
| 2.9 Vaginal vs rectal | 1 | 147 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.68, 2.56] |
| 3 Miscarriage rate | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 IM vs oral | 3 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.34, 6.11] |
| 3.2 IM vs vaginal/rectal | 6 | 1468 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.56, 1.13] |
| 3.3 Vaginal/rectal vs oral | 5 | 2220 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.76, 1.82] |
| 3.4 Low dose vaginal vs high dose vaginal | 9 | 4333 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.55, 0.98] |
| 3.5 Short protocol vs long protocol | 3 | 662 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.61, 1.50] |
| 3.6 Micronised vs synthetic | 2 | 1793 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.69, 1.95] |
| 3.7 Subcutaneous vs vaginal gel | 2 | 1465 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.44, 1.54] |
| 3.8 Vaginal vs rectal | 1 | 147 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.31, 4.71] |
| 4 OHSS | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 IM vs oral | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 17.18] |
| 4.2 Low dose vaginal vs high dose vaginal | 2 | 1251 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.57, 1.46] |
| 5 Multiple pregnancy | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 IM vs oral | 2 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.23 [1.16, 15.40] |
| 5.2 IM vs vaginal/rectal | 1 | 505 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.60, 1.59] |
| 5.3 Vaginal/rectal vs oral | 1 | 283 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.50, 2.58] |
| 5.4 Low dose vaginal vs high dose vaginal | 5 | 2888 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.85, 1.80] |
| 5.5 Short protocol vs long protocol | 4 | 820 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.80, 1.60] |
| 5.6 Vaginal vs rectal | 1 | 147 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.19, 4.91] |
| 6 Clinical pregnancy: IM vs vaginal/rectal: subgroup analysis by COH method | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Human gonadotropins with or without GnRH agonists | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Human gonadotropins with or without GnRH antagonists | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Clinical pregnancy: IM vs vaginal/rectal: subgroup analysis by treatment duration | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Stop at pregnancy test | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Up to 12 weeks when pregnant | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Clinical pregnancy: vaginal/rectal vs oral: subgroup analysis by treatment duration | 6 | 2775 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.73, 1.04] |
| 8.1 Stop at pregnancy test | 2 | 619 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.50, 0.98] |
| 8.2 Up to 12 weeks when pregnant | 4 | 2156 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.17] |
| 9 Clinical pregnancy: low vs high dose vaginal: subgroup analysis by COH method | 9 | 3512 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.91, 1.22] |
| 9.1 Human gonadotropins with or without GnRH agonists | 8 | 3388 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.91, 1.22] |
| 9.2 Human gonadotropins with or without GnRH antagonists | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.49, 2.22] |
| 10 Clinical pregnancy: low vs high dose vaginal: subgroup analysis by duration of treatment | 9 | 3514 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.24] |
| 10.1 Stop at pregnancy test | 3 | 318 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.67, 1.83] |
| 10.2 Up to 12 weeks when pregnant | 6 | 3196 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.91, 1.24] |
| 11 Clinical pregnancy: short vs long protocol: subgroup analysis by COH method | 4 | 902 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.75, 1.40] |
| 11.1 Human gonadotropins with or without GnRH agonists | 2 | 482 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.58, 1.32] |
| 11.2 Human gonadotropins with or without GnRH antagonists | 2 | 420 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.79, 2.05] |
6.1. Analysis.
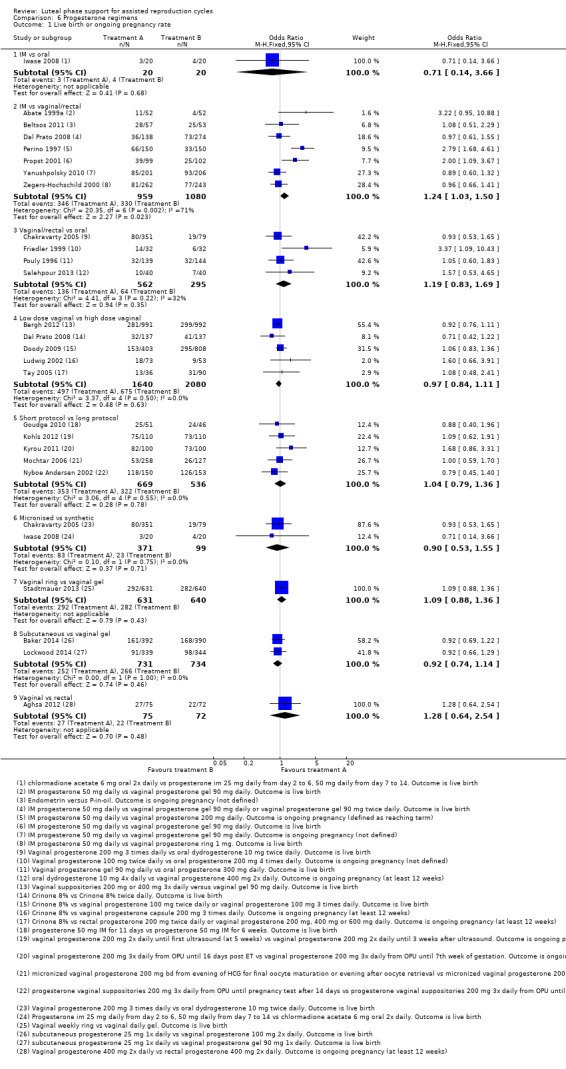
Comparison 6 Progesterone regimens, Outcome 1 Live birth or ongoing pregnancy rate.
Comparison 7. Progesterone + oestrogen regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth/ongoing pregnancy rate | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Short protocol vs long protocol | 1 | 910 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.81, 1.43] |
| 1.2 Low dosage vs high dosage | 1 | 285 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.37, 1.13] |
| 2 Clinical pregnancy rate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Low dosage vs high dosage | 1 | 285 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.48, 1.37] |
| 3 Miscarriage rate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Low dosage vs high dosage | 1 | 285 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.86, 11.39] |
| 4 Multiple pregnancy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Low dosage vs high dosage | 1 | 285 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.12] |
7.1. Analysis.
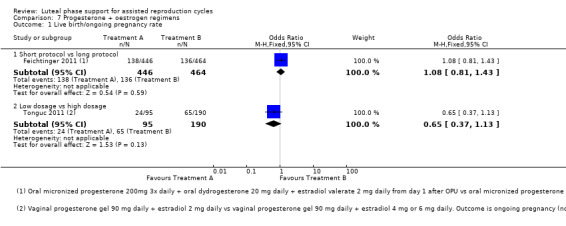
Comparison 7 Progesterone + oestrogen regimens, Outcome 1 Live birth/ongoing pregnancy rate.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abate 1999.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Women undergoing first‐time IVF/ET for tubal factor infertility, age < 38 (n = 86) | |
| Interventions | Pituitary desensitisation (PD) and controlled ovarian hyperstimulation (COH): GnRH agonist, 400 µg SC twice daily, and FSH ET: day +2, max 4 embryos transferred LPS: 17 alpha‐hydroxyprogesterone 341 mg IM every 3 days vs saline IM every 3 days. From day before ET until pregnancy test (day +14) |
|
| Outcomes | Pregnancy (not defined) | |
| Notes | No reply from author in 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients [...] were randomly allocated" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Abate 1999a.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Women undergoing IVF/ET for tubal occlusion, age 25 to 35 years (n = 156) | |
| Interventions | PD/COH: GnRH agonist IM and FSH ET: day +2, max 4 embryos transferred LPS: progesterone 50 mg IM daily vs progesterone 90 mg vaginal gel daily vs saline solution every 3 days. All from day before ET (+1) until hCG test (+16) |
|
| Outcomes | Biochemical pregnancy (small transitory increase in β‐hCG levels, followed by a decrease within a week), clinical pregnancy (gestational sac or serum hCG ≥1400 mIU), ongoing pregnancy (20 weeks' gestation), live birth | |
| Notes | No reply from study author in 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They were randomly treated" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Aboulghar 2008.
| Methods | Randomised controlled trial | |
| Participants | Women who have a clinical pregnancy after ICSI with IM progesterone or vaginal progesterone as luteal phase support, mean age 30 (n = 257) | |
| Interventions | PD/COH: GnRH agonist LPS: vaginal progesterone 600 mg or progesterone 50 mg IM until first US vs vaginal progesterone 600 mg or progesterone 50 mg IM until 3 weeks after first US |
|
| Outcomes | Miscarriage (up to 20 weeks' gestation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Dark, sealed envelopes contained the intervention (continuation or stoppage of LPS) were created by a third party not involved in the allocation process. Randomization was performed by picking one envelope for each patients from sequentially numbered envelopes" |
| Allocation concealment (selection bias) | Low risk | Dark, sealed envelopes created by third party |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Patient was informed about the allocated arm" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Aboulghar 2015.
| Methods | Randomsed controlled trial | |
| Participants | Women who were having IVF‐ICSI were randomised on the day of embryo transfer, "all received standard long GnRHa protocol) | |
| Interventions | LPS both groups: vaginal progesterone suppositories daily (total dose prontogest 600mg) Gp 1 (224 women): vaginal progesterone daily vaginal progesterone suppositories plus daily sub cutaneous 0.1 decapeptyl (agonist) until day of beta‐hCG detection Gp 2 (222 women): vaginal progesterone daily vaginal progesterone suppositories, GnRHa stopped on day of hCG injection |
|
| Outcomes | clinical and ongoing pregnancy rate | |
| Notes | ISRCTN13123887 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomisation table 1:1" |
| Allocation concealment (selection bias) | Low risk | "a nurse not involved in the study picked one envelope for each patient from sequentially numbered envelopes on the day of embryo transfer and informed patient about their allocated arm. Allocation concealment was ensured by the use of dark, sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients were "informed about their allocation" on the day of randomisation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | no stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were analysed. see Figure 1 in paper |
| Selective reporting (reporting bias) | Low risk | ongoing pregnancy reported |
| Other bias | Unclear risk | nil |
Aghahosseini 2011.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF, mean age 35 (n = 108) | |
| Interventions | PD/COH: GnRH agonist 500 mg/d SC from day 21 to 3 ET: mean 2 embryos transferred LPS: vaginal progesterone 400 mg daily + oral estradiol 4 mg daily vs vaginal progesterone 400 mg daily. Both until 12th week of gestation |
|
| Outcomes | Clinical pregnancy (heartbeat on ultrasound at 12 weeks), ongoing pregnancy (not defined), miscarriage rate (not defined), multiple pregnancy rate (not defined) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | High risk | None used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal reported with reasons |
| Selective reporting (reporting bias) | High risk | Planned outcomes reported but not analysed (MPR not analysed) |
| Other bias | Low risk | No specific source of other potential bias identified |
Aghsa 2012.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing ICSI, mean age 31 (n = 145) | |
| Interventions | PD/COH: GnRH antagonist 0.25 mg SC daily from day 8 until trigger + FSH ET: mean 2 embryos transferred, max 3 LPS: vaginal progesterone 400 mg 2× daily vs rectal progesterone 400 mg 2× daily. Both from ET until 8th week of gestation |
|
| Outcomes | Clinical pregnancy (foetal heart rate on ultrasound at 8 weeks' gestation), ongoing pregnancy (being pregnant after 12th week of gestation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal reported with reasons |
| Selective reporting (reporting bias) | Unclear risk | More outcomes reported than stated in protocol |
| Other bias | Low risk | No specific source of other potential bias identified |
Albert 1991.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET (n = 57) | |
| Interventions | PD/COH: GnRH agonist and hMG LPS: hCG 2500 IU 4× vs progesterone 50 mg IM at day of ET, then 12.5 mg IM daily |
|
| Outcomes | Clinical pregnancy (not defined), OHSS | |
| Notes | Only abstract available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were treated in a prospective, randomized fashion" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Only abstract available Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Artini 1995.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET for tubal factor, oligospermia or unexplained infertility, mean age 33 (n = 176) | |
| Interventions | COH: GnRH agonist IM and FSH ET: day +2 LPS: progesterone 50 mg IM daily vs progesterone 100 mg daily in vaginal cream vs hCG 2000 IU IM every 3 days vs no supplementation |
|
| Outcomes | Viable pregnancy (not defined) | |
| Notes | No reply from study author in 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly divided" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Ata 2008.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing ART with at least 1 embryo available for transfer, mean age 31 (n = 570) | |
| Interventions | COH: GnRH agonist 0.1 mg SC from 21st day of preceding cycle + rFSH ET: day +3, max 3 embryos transferred LPS: progesterone 90 mg vaginal gel daily + 0.1 mg GnRH agonist (triptorelin) SC at day +9 vs progesterone 90 mg vaginal gel daily + saline SC at day +9 |
|
| Outcomes | Clinical pregnancy (foetus with heartbeat at 6 weeks' gestation), ongoing pregnancy (beyond 20th week of gestation), multiple pregnancy (gestation with more than 1 foetus) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women were randomized according to a computer generated randomization list prepared by the chief investigator. Study subjects were randomized in blocks of 10. Opaque envelopes, which were numbered and sealed, containing the allocation information were given to the hospital pharmacy" |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed, opaque envelopes were given to hospital pharmacy "The allocation code was broken upon completion of the 20th gestational week of the last pregnant subject" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both the nurse injecting the study medication and the women receiving injections were blinded for allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Outcome assessors who performed the pregnancy tests and ultrasonographic examinations to determine if the patient was pregnant were also blinded for allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Ata 2010.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing ICSI after long GnRH agonist protocol, mean age 32.3 (n = 60) | |
| Interventions | PD: GnRH agonist LPS: vaginal progesterone gel 90 mg daily vs vaginal progesterone gel 90 mg daily + oral oestradiol valerate 3 mg 2× daily. Both until 10th week of gestation |
|
| Outcomes | Live birth rate, clinical pregnancy rate, miscarriage rate | |
| Notes | Abstract only Study author contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Computerised allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal reported with reasons |
| Selective reporting (reporting bias) | Low risk | Study author contacted |
| Other bias | Low risk | No specific source of other potential bias identified |
Baker 2014.
| Methods | Multi‐centre, randomised, open‐label trial | |
| Participants | Women undergoing IVF/ICSI, mean age 34.3 (n = 800) | |
| Interventions | PD/COH: local protocol, including GnRH agonists, GnRH antagonist or both ET: days 2 to 7, mean 2.2 embryos transferred LPS: subcutaneous progesterone 25 mg 1× daily vs vaginal progesterone 100 mg 2× daily. Both from OPU until 12th week of gestation |
|
| Outcomes | Clinical pregnancy (not defined), ongoing pregnancy (12 weeks' gestation), live birth rate | |
| Notes | Study author contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially numbered, identical envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal reported with reasons |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Unclear risk | Supported by developer of subcutaneous progesterone |
Beckers 2000.
| Methods | Randomised controlled trial, 3 different pituitary desensitisation protocols whether combined with LPS | |
| Participants | Women undergoing IVF for tubal or male factor, age < 39, mean age 32 (n = 38). Women with ovarian hyperresponse (oestradiol > 8000 pmol/L) were excluded from analysis | |
| Interventions | PD/COH: GnRH agonist 0.1 mg SC from cycle day 1 until trigger vs GnRH agonist 0.1 mg SC from cycle day 1 until 3rd day of hMG stimulation vs GnRH agonist 0.1 mg SC from cycle day 1 until hCG trigger. Followed by hMG for COH ET: day 4, max 2 embryos transferred LPS: hCG 1500 IU IM on day of oocyte retrieval, +2, +4, +6 vs no treatment vs no treatment |
|
| Outcomes | Pregnancy (positive urine test), ongoing pregnancy, live birth and miscarriage. Multiple pregnancy rate is mentioned but is not defined per group | |
| Notes | Study author contacted in 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized on the same day (i.e. day 1 of the treatment cycle) by means of sealed envelopes for one of the three treatment groups A, B or C (20 patients each)" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Belaisch‐Allart 1987.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Women undergoing IVF (87% for tubal factor), mean age 33 (n = 286) | |
| Interventions | PD/COH: clomiphene + hMG or pure FSH or FSH + hMG or oral contraceptive pill + clomiphene‐hMG ET: mean 2.2 embryos transferred LPS: oral dydrogesterone 10 mg 3× daily vs oral placebo 3× daily. Both from oocyte retrieval for 21 days |
|
| Outcomes | Pregnancy (not defined), ongoing pregnancy, miscarriage | |
| Notes | Study author contacted in 2004, unable to provide information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The treatment was allocated according to a double‐blind randomized list" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind randomized list" Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind randomized list" Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Belaisch‐Allart 1990.
| Methods | Multi‐centre (12) randomised placebo‐controlled trial | |
| Participants | Women undergoing IVF for tubal sterility (50%), serum oestradiol on day of ET < 2500 pg/mL, mean age 33 (n = 387) | |
| Interventions | PD/COH: GnRH agonist in long (67%) or short protocol + hMG ET: mean 3 embryos transferred, up to > 4 LPS: hCG 1500 IU vs placebo. Both on day of ET and 4 days after ET |
|
| Outcomes | Pregnancy (not defined), ongoing pregnancy rate, OHSS | |
| Notes | 2 study authors employed by Organon Study author contacted in 2004, unable to provide information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A double‐blind, randomized list in each centre" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind, not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Beltsos 2011.
| Methods | Multi‐centre randomised controlled trial | |
| Participants | PCOS patients undergoing IVF, mean age 31 (n = 110) | |
| Interventions | LPS: vaginal progesterone vs progesterone IM | |
| Outcomes | Ongoing pregnancy (not defined) | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawal reported |
| Selective reporting (reporting bias) | High risk | Abstract only |
| Other bias | Unclear risk | Supported by Ferring Pharmaceuticals Inc |
Bergh 2012.
| Methods | Multi‐centre randomised controlled trial | |
| Participants | Women undergoing IVF (n = 1983) | |
| Interventions | PD/COH: GnRH agonist 400 to 600 mg daily nasally + FSH ET: 1 embryo transferred LPS: progesterone vaginal gel 90 mg daily vs vaginal progesterone suppositories 200 mg or 400 mg 3× daily. Both for 19 days |
|
| Outcomes | Ongoing pregnancy (sonographically verified intrauterine pregnancy, positive heartbeat 5 weeks after ET), clinical pregnancy (not defined), miscarriage rate (not defined), multiple pregnancy rate (not defined) | |
| Notes | As the result of data entry errors in the date of birth of 2 participants, the distribution of participants by age was very unbalanced. Both participants were in the vaginal progesterone gel arm of the study. Therefore, subsequent participants tended to be allocated to that arm if they were younger than average, and to the vaginal micronised progesterone tablet arm if they were older. Study authors contacted 2 well‐recognised and independent statisticians. Both statisticians came to the same conclusion: The results would be correct provided that a stratified analysis with regard to age as a continuous variable was performed. Investigators followed this advice, and results are presented accordingly in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomisation programme |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinicians blinded, participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Researchers blinded, method not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal reported with reasons |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Unclear risk | Statistical errors reported and handled appropriately Financial support provided by Merck Serono |
Brigante 2013.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ICSI (n = 61) | |
| Interventions | LPS: vaginal progesterone 600 mg daily from OPU vs vaginal progesterone 600 mg daily from OPU + triptorelin 0.2 mg SC daily on day 6 after OPU | |
| Outcomes | Clinical pregnancy (not defined), ongoing pregnancy (not defined) | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes of 62 women reported; 61 were randomly assigned |
| Selective reporting (reporting bias) | High risk | Abstract only |
| Other bias | Low risk | No specific source of other potential bias identified |
Caligara 2007.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF with 10 or fewer oocytes retrieved and a max oestradiol level of 2500 pg/mL at hCG trigger | |
| Interventions | LPS: vaginal progesterone 200 mg 2× daily vs vaginal progesterone 200 mg 2× daily plus hCG 1000 IU SC on days +4, +7 and +10. Progesterone from day after oocyte retrieval | |
| Outcomes | Pregnancy rate (not defined) | |
| Notes | Only abstract available Study author contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | By phone call to unrelated department |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Only abstract available Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Ceyhan 2008.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF, excluding endometriosis, polycystic ovarian syndrome and severe male factor, age < 36, mean 31 (n = 60) | |
| Interventions | PD/COH: GnRH antagonist 0.25 mg daily from day 6 until day of trigger + rFSH ET: day 3 or 5, mean 2 embryos transferred LPS: vaginal progesterone 600 mg daily vs vaginal progesterone 600 mg daily + oestrogen transdermal 100 μg/d estradiol release, twice weekly. Both from day of oocyte retrieval until 8 weeks' gestation when pregnant | |
| Outcomes | Pregnancy rate (serum β‐hCG > 10 mIU/mL), clinical pregnancy (intrauterine gestational sac), ongoing pregnancy (intrauterine gestational sac and foetal heartbeat after 13th week amenorrhoea), OHSS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sample randomization performed by a computer" |
| Allocation concealment (selection bias) | Low risk | "Central consultation was used for allocation of patients" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "No blinding was used during follow‐up" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "No blinding was used during follow‐up" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Chakravarty 2005.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ICSI‐ET, excluding women with PCOS, advanced endometriosis, dense pelvic adhesions, genital tuberculosis or previous failed IVF/ICSI cycles, age 25 to 42 (n = 430) | |
| Interventions | PD/COH: GnRH agonist 1 mg SC + rFSH 150 to 200 IU SC ET: day 2, average of 3 embryos transferred LPS: micronised vaginal progesterone 200 mg 3× daily vs oral dydrogesterone 10 mg twice daily. Both from day of ET until β‐hCG test or up to 12 weeks when pregnant |
|
| Outcomes | Clinical pregnancy (not defined), miscarriage and viable delivery rate | |
| Notes | No reply from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomly selected" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Colakoglu 2011.
| Methods | Randomised controlled trial | |
| Participants | Women with PCOS undergoing IVF (n = 39) | |
| Interventions | LPS: progesterone 50 mg daily IM vs progesterone 50 mg daily IM + transdermal E2 100 μg every 2 days | |
| Outcomes | Clinical pregnancy rate (not defined) | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "These patients were divided randomly into 2 groups." Methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal reported |
| Selective reporting (reporting bias) | Unclear risk | Abstract only Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Colwell 1991.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF, excluding women with luteal phase < 12 days in previous cycles, mean age 33 (n = 39) | |
| Interventions | PD/COH: clomiphene citrate 100 mg oral from day 5 until 9+ hMG ET: day +2, mean 2.6 embryos transferred, ET in only 55% of women LPS: progesterone 200 mg oral 4× daily vs no supplementation |
|
| Outcomes | Ongoing pregnancy (not defined), multiple pregnancy | |
| Notes | No reply from study author in 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomly assigned" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All RIAs were performed by personnel blinded to the group assignment of each subject" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Dal Prato 2008.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF for idiopathic, tubal or male factor, grade I to II endometriosis and no more than 3 previous cycles, mean age 33 (n = 412) | |
| Interventions | PD/COH: long protocol GnRH agonist + FSH IVF/ET: age < 35: 2 embryos transferred; age > 35: 3 embryos transferred LPS: progesterone 50 mg IM daily vs vaginal progesterone gel 90 mg once daily vs vaginal progesterone gel 90 mg twice daily. All from oocyte retrieval for 15 days or until first US when pregnant |
|
| Outcomes | Live birth (1 or more live babies), clinical pregnancy (1 or more gestational sacs), ongoing pregnancy, miscarriage (pregnancy loss after US confirmation of embryo implantation and before 12 weeks). | |
| Notes | Study author contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization list was provided by an external statistician and the treatment sequence given to the investigator using sealed envelopes containing the name of one of the three medications" |
| Allocation concealment (selection bias) | Low risk | "Dark envelopes were used, so their content could not be seen against bright light. Each envelope and allocation was sequentially numbered to prevent patients from being randomized out of sequence. Envelopes were not allowed to be opened in advance and were opened only by a nurse not involved in the trial" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "No blinding procedure was planned for this study due to the complex management of the blinding procedures with two different routes of administration" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "No blinding procedure was planned for this study due to the complex management of the blinding procedures with two different routes of administration" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Doody 2009.
| Methods | Multi‐centre (25) randomised controlled trial | |
| Participants | Women undergoing IVF, excluding women who had a history of recurrent (≥ 3 spontaneous abortions) pregnancy loss, abnormal uterine bleeding of undetermined origin or a history of poor response to gonadotropin or 2 previously cancelled cycles, mean age 33 (n = 1211) | |
| Interventions | PD/COH: long protocol GnRH agonist + hMG (Menopur) + FSH (Bravelle) ET: max 3, mean 2.4 embryos transferred LPS: progesterone vaginal capsules 100 mg 2× daily vs progesterone vaginal capsules 100 mg 3× daily vs progesterone vaginal gel. |
|
| Outcomes | Ongoing pregnancy (foetal heart movement at 6 weeks), clinical pregnancy (gestational sac), live birth | |
| Notes | 2 study authors are employees of Ferring Pharmaceuticals; 1 author receives grant support from Ferring Pharmaceuticals; acts as a consultant for Ferring Pharmaceuticals, Ethicon Endo Surgery, Ethicon Women’s Health and Urology, Smith & Nephew, Galil Medical and Boston Scientific; and serves on speakers bureaus for Boston Scientific, Ferring Pharmaceuticals, Ethicon Endo Surgery and Ethicon Women’s Health and Urology | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocation to treatment group was performed by a telephone‐based electronic interactive voice response system, which ensured an equal number of patients per treatment group across the study centers and stratification factors" |
| Allocation concealment (selection bias) | Low risk | Telephone‐based electronic interactive voice response system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Study drug was administered on an open‐label basis" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The study was assessor‐blinded; the person who performed the transvaginal ultrasound examinations to confirm clinical and ongoing pregnancy was blinded to the patient’s treatment group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Drakakis 2007.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ICSI for tubal factor, male infertility, anovulation, endometriosis and unexplained infertility, mean age 35 (n = 76) | |
| Interventions | PD/COH: GnRH agonist 100 µg intranasal 5× daily from day 21 preceding cycle for 15 to 24 days + rFSH LPS: progesterone 100 µg oral 3× daily + vaginal progesterone capsules 200 mg 3× daily until pregnancy test + oestradiol valerate oral 2 mg + 0.5 mg norgestrel 3× daily for 15 days + oestradiol hemihydrate 50 µg transdermal patch every 4 days vs progesterone 100 µg oral 3× daily + vaginal progesterone capsules 200 mg 3× daily until pregnancy test |
|
| Outcomes | Clinical pregnancy, miscarriage | |
| Notes | No reply from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were divided randomly into two groups according to the protocol used" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Dunstone 1999.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET (n = 38) | |
| Interventions | LPS: progesterone 400 mg vaginal pessaries twice daily vs progesterone 90 mg vaginal gel daily. Both from night before oocyte retrieval until pregnancy test | |
| Outcomes | Clinical pregnancy (foetal heartbeat at ultrasound) | |
| Notes | No reply from study author in 2004 Only abstract available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Women undergoing IVF‐ET treatment were randomly assigned" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Preliminary results are available for 38 women of a planned total of 100" Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Only abstract available Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Elgindy 2010.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing their first ICSI cycle for male factor infertility, mean age 29 (n = 270) | |
| Interventions | PD/COH: GnRH agonist 0.1 mg SC from midluteal phase of pretreatment cycle + rFSH + hMG ET: day 2, mean 3 embryos transferred LPS: progesterone 100 mg IM daily vs progesterone 100 mg IM daily + E2 valerate 2 mg orally 3× daily vs progesterone 100 mg IM daily + E2 valerate 2 mg vaginally 3× daily |
|
| Outcomes | Clinical pregnancy (not defined) | |
| Notes | No reply from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Study participants were randomized into three groups, 90 women each, using the block randomization technique" |
| Allocation concealment (selection bias) | Low risk | "Two hundred seventy identical sealed envelopes were prepared by one of the investigators (M.I.M.) and kept in the unit pharmacy. When the woman was eligible and agreed to participate, she was instructed to select only one envelope only once to determine the group to which she was assigned. The randomization key was kept with the pharmacy director and was not opened until after statistical analysis" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Engmann 2008.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing first cycle of IVF, excluding women with high risk of OHSS, mean age 35 (n = 166) | |
| Interventions | PD/COH: GnRH agonist or antagonist or microdose GnRH agonist and rFSH or FSH + u‐hMG
ET: day 3, mean 2.5 embryos transferred LPS: progesterone 50 mg IM daily vs progesterone 50 mg IM daily + oestradiol 2 mg vaginally 2× daily. Both from oocyte retrieval until pregnancy test or foetal heartbeat when pregnant |
|
| Outcomes | Clinical pregnancy (gestational sac and positive heartbeat), ongoing pregnancy (beyond 12 weeks), miscarriage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "They were randomly assigned to either group in a ratio of 1:1 by means of computer‐generated random numbers on the day of ET. To ensure similar distribution of patients with low peak serum E2 concentration in the two groups, separate randomization schedules were drawn up for women with peak E2 levels on the day of hCG administration of %1200 pg/mL and for those with levels > 1200 pg/mL by the use of stratified randomized blocks" |
| Allocation concealment (selection bias) | Low risk | "Selection into the groups was performed by a research nurse using a series of consecutively numbered sealed opaque envelopes (one for each category of peak serum E2 level), so the sequence of allocation was concealed" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The study was not blinded because the patients as well as the clinicians were aware of the treatment group" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The study was not blinded because the patients as well as the clinicians were aware of the treatment group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Erdem 2013.
| Methods | Randomised controlled trial | |
| Participants | Women with poor ovarian response undergoing IVF (n = 95) | |
| Interventions | LPS: intravaginal progesterone gel daily vs intravaginal progesterone gel + oral oestradiol hemihydrate 2 mg daily vs intravaginal progesterone gel + oral oestradiol hemihydrate 6 mg daily | |
| Outcomes | Clinical pregnancy rate (not defined) | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawal reported |
| Selective reporting (reporting bias) | High risk | Only outcomes of groups 1 and 2 are reported |
| Other bias | High risk | Abstract only |
Fatemi 2006.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF or ICSI/ET, excluding women with PCO, endometriosis > grade 2, TESE and need for pre‐implantation genetic diagnosis; mean age 32 (n = 201) | |
| Interventions | PD/COH: GnRH antagonist 0.25 mg daily from day 6+ rFSH ICSI or IVF/ET: day 3, 1 or 2 embryos transferred LPS: natural micronised progesterone vaginal capsules 200 mg 3× daily vs natural micronised progesterone vaginal capsules 200 mg 3× daily + oral E2 valerate 2 mg twice daily. From day after oocyte retrieval until 7 weeks' gestation |
|
| Outcomes | Ongoing pregnancy (beyond 12 weeks), early pregnancy loss (initially positive hCG test, failed to develop beyond 12 weeks) | |
| Notes | Study author contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "According to a computer‐generated not concealed randomization list prior to initiation of stimulation" |
| Allocation concealment (selection bias) | High risk | No concealed randomisation list |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | High risk | Only abstract available |
Feichtinger 2011.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF (n = 1053) | |
| Interventions | ET: mean 1.9 embryos transferred LPS: oral micronised progesterone 200 mg 3× daily + oral dydrogesterone 20 mg daily + oestradiol valerate 2 mg daily from day 1 after OPU vs oral micronised progesterone 200 mg 3× daily + oral dydrogesterone 20 mg daily + oestradiol valerate 2 mg daily from day 4 after OPU |
|
| Outcomes | Ongoing pregnancy rate (not defined) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Third party |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinician and researcher blinded, method unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | High risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Friedler 1999.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing ICSI/ET for male factor infertility with > 1 embryo available and serum oestradiol > 2500 pg/mL on day of hCG, mean age 31 (n = 64) | |
| Interventions | PD/COH: GnRH agonist + hMG ICSI/ET: day 2, max 3 embryos transferred except in older women (> 38 years) or in cases of recurrent failure of implantation LPS: micronised progesterone 200 mg oral 4× daily vs micronised progesterone 100 mg vaginal 2× daily. Both from day +1 after ET until serum test (+14) |
|
| Outcomes | Pregnancy (not defined), ongoing pregnancy, miscarriage | |
| Notes | No reply from study author in 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients included in this study were prospectively randomized by order of embryo transfer" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Fujimoto 2002.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET, including only women with low midluteal serum oestradiol in a previous cycle, mean age 35 (n = 114) | |
| Interventions | PD/COH: long protocol GnRH agonist 300 µg intranasal 3× daily + hMG ET: day 2 or 3, max 3 embryos transferred LPS: progesterone 25 mg injection once daily from day after oocyte retrieval vs progesterone 25 mg injection once daily from day after oocyte retrieval + hCG 3000 IU IM on days 1, 4, 7 after ET |
|
| Outcomes | Pregnancy (gestational sac) | |
| Notes | Study investigates progesterone as luteal phase support in 436 women. Women who fail to conceive (n = 114) are included in a second cycle, in which they are randomly assigned to receive progesterone or progesterone + hCG. Only women undergoing the second cycle are included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They were randomly treated" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Ganesh 2011.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ICSI, excluding women with baseline FSH > 12 IU and adenomyosis, mean age 32 (n = 1363) | |
| Interventions | PD/COH: GnRH agonist 500 µg SC daily + rFSH ET: day 2, average of 3 embryos transferred LPS: dydrogesterone 10 mg oral daily vs micronised progesterone vaginal gel 90 mg daily vs micronized progesterone vaginal capsules 200 mg 3× daily. All from ET until 12 weeks' gestation |
|
| Outcomes | Clinical pregnancy (viable foetus on US), ongoing pregnancy (viable foetus at 12 weeks' gestation), miscarriage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sequentially numbered sealed envelopes were prepared and provided by the study coordinator, according to random‐number tables" |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Single‐blinding was achieved by keeping the person enrolling participants, study investigators, ultrasound technicians, and clinicians unaware of the type of protocol used" Method of blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Only the statisticians had access to the unblinded data. A double‐blind study protocol was not possible because the drug delivery method in the three groups was different" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal reported |
| Selective reporting (reporting bias) | High risk | Ongoing pregnancy results not reported, but they are reported in the protocol |
| Other bias | Low risk | No specific source of other potential bias identified |
Geber 2007.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing ART (n = 150) | |
| Interventions | PD/COH: GnRH agonist or antagonist + rFSH ET: mean 3 embryos transferred LPS: vaginal progesterone daily (dose not reported) + rLH on day 5, 8, 11 and 14 vs vaginal progesterone daily (dose not reported) |
|
| Outcomes | Clinical pregnancy (not defined) | |
| Notes | Only abstract available Study author contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated on the day of embryo transfer" By sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinicians blinded, a non‐participant (nurse) gave the medicine |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | High risk | Only abstract available Planned outcomes not reported Dose of progesterone not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Geber 2007a.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ICSI‐ET with serum FSH concentrations < 15 IU/L on day 3 of menstrual cycle, mean age 35 (n = 122) | |
| Interventions | PD/COH: GnRH agonist 3.6 mg SC + rFSH SC IVF/ICSI‐ET: day 3 or 5, 1 to 4, mean 3.4 embryos transferred LPS: micronised progesterone vaginal capsules 200 mg 3× daily vs micronised progesterone vaginal gel 90 mg daily. Both from day after oocyte retrieval for 13 days or 12 weeks when pregnant |
|
| Outcomes | Pregnancy (foetal heartbeat), miscarriage, multiple pregnancy | |
| Notes | Study author contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated (sealed envelopes) into two groups" |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinicians blinded, a non‐participant (nurse) gave the medicine |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "A total of 122 patients were allocated to each group and all completed the study" |
| Selective reporting (reporting bias) | Low risk | Planned outcome data reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Geusa 2001.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET, excluding women with systemic or endocrine pathologies, age < 42 years (n = 300) | |
| Interventions | PD/COH: GnRH agonist + rFSH LPS: progesterone 90 mg vaginal gel daily vs progesterone 50 mg IM daily. Both starting at oocyte retrieval |
|
| Outcomes | Clinical pregnancy (not defined) | |
| Notes | Only abstract available No reply from study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "All 318 patients were randomized" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Only abstract available Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Golan 1993.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET for male factor infertility, mechanical or unexplained infertility, mean age 33 (n = 56) | |
| Interventions | PD/COH: GnRH agonist + hMG IVF/ET: max 4 embryos transferred LPS: hCG 1000 or 2500 IU IM every 3 days, 4× vs progesterone 100 mg IM daily. Both from day of ET |
|
| Outcomes | Clinical pregnancy (gestational sac), miscarriage, OHSS | |
| Notes | Study author contacted in 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were prospectively randomized" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Gorkemli 2004.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ICSI (n = 266) | |
| Interventions | PD/COH: GnRH agonist 1 mg/mL SC from day 21 from menstruation + rFSH or rFSH/hMG ET: day 2 or 3, mean 3.5 embryos transferred LPS: progesterone vaginal capsules 200 mg 3× daily vs progesterone vaginal capsules 200 mg 3× daily + oestradiol transdermal 100 µg daily. Both from oocyte retrieval for 14/15 days, when pregnant progesterone until 10 weeks' gestation |
|
| Outcomes | Clinical pregnancy (foetal heart), ongoing pregnancy, miscarriage | |
| Notes | First cycle data obtained from study author (only clinical pregnancy) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomization" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Goudge 2010.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing their first IVF/ET cycle for any indication, mean age 32 (n = 97) | |
| Interventions | PD/COH: GnRH agonist 0.5 mg SC daily + oral contraceptive IVF/ET: mean 2 embryos transferred LPS: progesterone‐in‐oil 50 mg IM daily from oocyte retrieval until US at 5 or 6 weeks vs progesterone‐in‐oil 50 mg IM daily from oocyte retrieval until 11 days after ET |
|
| Outcomes | Live birth, clinical pregnancy, ongoing pregnancy, multiple pregnancy (all not defined) | |
| Notes | No reply from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was accomplished using sequentially numbered, opaque, sealed envelopes" Method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Humaidan 2006.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ICSI‐ET with baseline FSH and LH < 12 IU/L, menstrual cycles between 25 and 34 days, body mass index (BMI) > 18 and < 30, both ovaries present and absence of uterine abnormalities, aged 25 to 40 (n = 45) | |
| Interventions | PD/COH: single bolus of 10.000 hCG SC or GnRH antagonist 0.25 mg SC + rFSH 150 to 200 IU SC ET: day 2 or 3, 2 embryos transferred LPS: micronised vaginal progesterone gel 90 mg daily vs micronised vaginal progesterone gel 90 mg daily + single bolus hCG 1500 IU IM 12 hours after trigger vs micronised vaginal progesterone gel 90 mg daily + single bolus hCG 1500 IU IM 35 hours after trigger. Progesterone from day after OPU until β‐hCG test |
|
| Outcomes | Clinical pregnancy (intrauterine gestational sac with a heartbeat 3 weeks after a positive hCG test) | |
| Notes | Participants were randomly assigned for ovulation induction protocol (hCG vs GnRH antagonist). Participants randomly assigned for GnrH antagonist were randomly assigned again for time of single‐bolus hCG during LPS. All participants received vaginal progesterone for LPS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Third party, sealed and unlabelled envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal reported with reasons |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Hurd 1996.
| Methods | Randomised cross‐over study | |
| Participants | Women undergoing IVF‐ET, excluding women with a history of anovulation, unresponsive to CC or with ovaries not accessible for vaginal retrieval of oocytes, mean age 34 (n = 56) | |
| Interventions | PD/COH: CC 100 mg oral ET: day 2, mean 2.2 embryos transferred LPS: none vs vaginal progesterone suppositories 100 mg 2× daily from embryo transfer + E2 2 mg oral 3× daily from oocyte retrieval. Both until pregnancy test or until 8th week when pregnant |
|
| Outcomes | Clinical pregnancy (gestational sac), multiple pregnancy, miscarriage, ongoing pregnancy, OHSS | |
| Notes | Contacted in 2004, only first cycle data used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "..., she was randomized" Method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | "using a sealed opaque envelope technique with blocked allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Inamdar 2012.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ICSI, mean age 31 (n = 426) | |
| Interventions | PD/COH: GnRH agonist from 21st day until rhCG trigger 0.5 mg daily, from start menses 0.25 mg + rFSH ET: day 2, max 3 embryos transferred LPS: vaginal progesterone 400 mg twice daily + 100 mg progesterone IM daily vs vaginal progesterone 400 mg twice daily + 100 mg progesterone IM daily + lupiride 1 mg SC on days 6, 7 and 8 after oocyte retrieval |
|
| Outcomes | CPR (pregnancy diagnosed by ultrasonographic visualisation of 1 or more gestational sacs or definitive clinical signs of pregnancy), OPR (pregnancy proceeding beyond the 20th gestational week) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Low risk | Third party |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Method of blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal reported |
| Selective reporting (reporting bias) | High risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Isik 2009.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing ICSI/ET, excluding donor, freeze/thaw and/or TESA cycles, mean age 35 (n = 154) | |
| Interventions | PD/COH: GnRH antagonist + FSH LPS: micronised progesterone 600 mg 3× daily vaginal capsules from oocyte retrieval for 17 days + single‐dose hCG 1500 IU SC on day +8 + single dose GnRH agonist 0.5 mg SC on day +6 vs micronised progesterone 600 mg 3× daily vaginal capsules from oocyte retrieval for 17 days + single‐dose hCG 1500 IU SC on day +8 |
|
| Outcomes | Live birth, clinical pregnancy (foetal heartbeat), multiple pregnancy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated random table was used for randomization and performed on the day of embryo transfer by a nurse to assign participants to their groups" |
| Allocation concealment (selection bias) | Low risk | By a nurse |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The clinicians and the laboratory staff were blinded to groups" Participant blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The clinicians and the laboratory staff were blinded to groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Isikoglu 2007.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing ICSI, mean age 30 (n = 181) | |
| Interventions | PD/COH: GnRH agonist 0.5 mg SC daily from 21st day of preceding cycle + FSH ET: max 4, mean 2.8 embryos transferred LPS: progesterone 50 mg IM daily vs progesterone 50 mg IM daily + GnRH agonist 0.25 mg SC daily for 12 days |
|
| Outcomes | Live birth, clinical pregnancy (foetal cardiac activity) | |
| Notes | Study author contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized at initiation of stimulation by a computer‐generated list" |
| Allocation concealment (selection bias) | Low risk | Via onsite computer system utilising locked files |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The embryologists were blind to this randomization process" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Iwase 2008.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ICSI for tubal factor, male factor or unexplained infertility, mean age 33 (n = 40) | |
| Interventions | PD/COH: long or short protocol GnRH agonist + hMG ET: day 2, max 3 embryos transferred LPS: chlormadione acetate 6 mg oral 2× daily vs progesterone IM 25 mg daily from day 2 to 6, 50 mg daily from day 7 to 14. Both until pregnancy test, when pregnant 125 mg hydroxyprogesterone caproate weekly until 6 or 7 weeks' gestation |
|
| Outcomes | Live birth, clinical pregnancy (foetal heart activity) and OHSS | |
| Notes | No reply from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "According to a randomization table generated using computer software into two groups of 20 patients each" |
| Allocation concealment (selection bias) | High risk | "The random allocation sequence was concealed until the interventions were assigned" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Kably Ambe 2005.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ICSI (n = 69). | |
| Interventions | PD/COH: GnRH analogues + rFSH LPS: progesterone 100 mg IM daily vs progesterone 100 mg IM daily + estradiol valerate 2 mg |
|
| Outcomes | Clinical pregnancy rate (not defined), miscarriage (not defined) | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Abstract only |
| Other bias | Low risk | No specific source of other potential bias identified |
Kleinstein 2005.
| Methods | Multi‐centre (17) randomised controlled trial | |
| Participants | Women undergoing first IVF/ICSI cycle, successful transfer of 2 or 3 embryos, normal smear in past 12 months, age ≥ 18 and ≤ 35, mean age 30 (n = 430) | |
| Interventions | PD/COH: long GnRH agonist protocol + hMG or FSH ET: 2 (74.4%) or 3 embryos transferred LPS: progesterone vaginal capsules 200 mg 3× daily vs progesterone vaginal gel 90 mg daily. Both from ET until pregnancy or 12 weeks' gestation when pregnant |
|
| Outcomes | Clinical pregnancy (amniotic sac), ongoing pregnancy (12 weeks' gestation, with foetal heart activity) | |
| Notes | Supported by Dr Kade, Besins Pharma GmbH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomly assigned to one of the treatments with the aid of a randomization code. The randomization code (Blocking‐Factor 10) was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | "The trial investigators received consecutively numbered envelopes corresponding to the envisaged number to be recruited. An envelope was allowed to be opened in chronological sequence to assign treatment group only after successful transfer" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open, phase 3 RCT |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open, phase 3 RCT |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Kohls 2012.
| Methods | Randomised controlled trial | |
| Participants | Women with gestational sac at first ultrasound, mean age 35 (n = 220) | |
| Interventions | PD/COH: GnRH antagonist 0.25 µg SC daily from day 5 or 6 + rFSH 200 to 225 IU ET: mean 2 embryos transferred LPS: vaginal progesterone 200 mg 2× daily until first ultrasound (at 5 weeks) vs vaginal progesterone 200 mg 2× daily until 3 weeks after ultrasound |
|
| Outcomes | Clinical pregnancy (gestational sac and heartbeat at 6 weeks), ongoing pregnancy (gestation > 12 weeks), miscarriage rate (in singleton pregnancies only) and multiple pregnancy rate | |
| Notes | Study author contacted in 2010 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Opaque consecutively numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | Results directly obtained from study author in 2010, complemented study published in 2012 |
Kupferminc 1990.
| Methods | Randomised placebo‐controlled trial, allocation computer generated, using sealed envelopes, partially blinded, power calculation done | |
| Participants | Women undergoing IVF/ET for mechanical, male factor or unexplained infertility, mean age 33 (n = 156) | |
| Interventions | PD/COH: hMG from day 3 of menses ET: day 2, mean 2.8 embryos transferred LPS: dydrogesterone 10 mg oral 3× daily from ET for 14 days vs oral placebo vs hCG 2500 IU IM on days 3, 6 and 10 |
|
| Outcomes | Clinical pregnancy (gestational sac), ongoing pregnancy (beyond first trimester) and miscarriage | |
| Notes | Study author contacted in 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomized into one of three treatment groups" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The current prospective blind study..." Method of blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The current prospective blind study..." Method of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Kyrou 2011.
| Methods | Randomised controlled trial | |
| Participants | Women with a positive b‐hCG test after a fixed recombinant FSH/GnRH antagonist protocol for IVF/ICSI and a day 3 fresh embryo transfer, mean age 31 (n = 200) | |
| Interventions | PD/COH: GnRH antagonist 0.25 mg SC daily from day 6 + rFSH 150 to 200 IU ET: day 3, mean 1.5 embryos transferred LPS: vaginal progesterone 200 mg 3× daily from OPU until 16 days post ET vs vaginal progesterone 200 mg 3× daily from OPU until 7th week of gestation |
|
| Outcomes | Pregnancies (> 7 weeks' gestation), ongoing pregnancies (> 12 weeks' gestation), miscarriage rate (not defined), multiple pregnancy rates | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated by third party |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Lam 2008.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET with a normal uterine cavity, excluding women using vaginal progesterone for LPS, age < 40, mean age 34 (n = 197) | |
| Interventions | PD/COH: long GnRH agonist protocol 600 µg intranasal for at least 14 days + hMG or rFSH IVF/ET: day 3, mean 2.2 embryos transferred LPS: micronised progesterone 200 mg 3× daily vaginal capsules from oocyte retrieval until ET + hCG 2000 IU on day of oocyte retrieval, +3, +6 and +9 vs hCG 2000 IU IM on day of oocyte retrieval, +3, +6 and +9 |
|
| Outcomes | Pregnancy (positive urine pregnancy test), miscarriage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was performed by a computer‐generated program" |
| Allocation concealment (selection bias) | Low risk | "Sealed opaque envelopes were used for allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Both investigators and the participants were not blinded of the intervention groups" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Both investigators and the participants were not blinded of the intervention groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Lewin 1994.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET for mechanical infertility, mean age 33 (n = 100) | |
| Interventions | PD/COH: 3 ampoules hMG a day and GnRH agonist 0.5 mg/d SC ET: max 4 embryos transferred LPS: progesterone 50 mg IM from ET vs progesterone 50 mg IM + oestradiol valerate 2 mg oral daily |
|
| Outcomes | Clinical pregnancy (not defined), live birth | |
| Notes | No reply from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated" Method of allocation not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Licciardi 1999.
| Methods | Randomised controlled trial, allocation by randomisation table | |
| Participants | Women undergoing IVF/ET, age < 40 years, mean 35 years (n = 43) | |
| Interventions | PD/COH: GnRH agonist and FSH IM or hCG or a combination of both ET: day 3, mean 3.4 embryos transferred LPS: progesterone 50 mg IM daily vs micronised progesterone 200 mg 3× daily. Both from day after oocyte retrieval |
|
| Outcomes | Clinical pregnancy (gestational sac), multiple pregnancy, miscarriage | |
| Notes | No reply from study author in 2004 Study terminated early for ethical reasons: differences in implantation rates highly statistically significant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned to receive either IM or oral progesterone supplementation according to a randomization table" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Lin 2013.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing GnRG agonist long protocol IVF/ICSI cycles, mean age 31 (n = 402) | |
| Interventions | PD/COH: GnRH agonist ET: day 2 or 3, mean 2.2 embryos transferred LPS: progesterone 60 mg IM 1× daily + oral oestradiol valerate 3 mg 2× daily from OPU for 17 days vs progesterone 60 mg IM 1× daily from OPU for 17 days |
|
| Outcomes | Live birth rate, clinical pregnancy rate (positive b‐hCG test and gestational sac with heartbeat on ultrasound), miscarriage rate (clinical pregnancy failed to develop > 12 weeks' gestation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal reported with reasons |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Lockwood 2014.
| Methods | Open‐label, multi‐centre randomised controlled trial | |
| Participants | Women undergoing ART, mean age 34 (n = 683) | |
| Interventions | PD/COH: any kind of LH suppression and any gonadotropin stimulation regimen LPS: subcutaneous progesterone 25 mg 1× daily vs vaginal progesterone gel 90 mg 1× daily. Both from OPU until 8th week of pregnancy |
|
| Outcomes | Live birth (delivery of 1 or more live babies), clinical pregnancy (presence of 1 or more gestational sacs detected on ultrasound scan performed 4 weeks after embryo transfer), ongoing pregnancy (pregnancy after 10 weeks' treatment), miscarriage (pregnancy loss after ultrasound confirmation of embryo implantation and before 12 weeks) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Sequentially numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal reported with reasons |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Unclear risk | Supported by developer of subcutaneous progesterone |
Loh 1996.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET (n = 156); 8% of randomised cycles did not result in ET (numbers by group not provided) | |
| Interventions | PD/COH: "standard GnRH agonist" protocol LPS: IM progesterone vs hCG |
|
| Outcomes | Pregnancy (not defined) | |
| Notes | Only abstract available No reply from study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized at recruitment" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Only abstract available Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Ludwig 2001.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF or ICSI, excluding women with abdominal discomfort on day of ET and oestradiol levels > 5000 pg/mL, mean age 32 (n = 413) | |
| Interventions | PD/COH: long protocol GnRH agonist + rFSH or hMG ET: mean 2.7 embryos transferred LPS: low risk category (< 12 oocytes retrieved and oestradiol on day of ovulation induction < 2.500 pg/mL); hCG 5000 IU on day of ET and day +3, 2500 IU on day +6 vs hCG 5000 IU on day of ET + progesterone vaginal capsules 200 mg 3× daily vs progesterone vaginal capsules 200 mg 3× daily High risk category (≥ 12 oocytes retrieved and oestradiol on day of ovulation induction ≥ 2.500 pg/mL); hCG 5000 IU on day of ET + progesterone vaginal capsules 200 mg 3× daily vs progesterone vaginal capsules 200 mg 3× daily |
|
| Outcomes | Clinical pregnancy (positive foetal heartbeat), ongoing pregnancy (delivery of live born or stillborn baby > 500 g or delivery of live born baby < 500 g), miscarriage | |
| Notes | Because the high risk category is quasi‐randomised, data for these arms are not included in the meta‐analysis Study author contacted in 2004 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subsequently randomized according to a randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | High risk | This study had a relatively high rate of miscarriage, which was not consistent with reported rates of live birth, clinical pregnancy and ongoing pregnancy |
Ludwig 2002.
| Methods | Randomised controlled trial, allocation by computer‐generated open list | |
| Participants | Women undergoing IVF or ICSI/ET, age < 40, mean age 31 (n = 126). Patients with oestradiol levels < 2000 pg/mL on day of hCG trigger were not selected | |
| Interventions | PD/COH: long protocol GnRH agonist or multiple dose antagonist + FSH or hMG ET: mean 2.8 embryos transferred LPS: progesterone in capsules 200 mg 3× daily vaginally vs progesterone in gel 90 mg daily. Both from evening before ET until menses or pregnancy test |
|
| Outcomes | Clinical pregnancy (positive foetal heartbeat on US), ongoing pregnancy (> 12 weeks), miscarriage | |
| Notes | Additional information obtained in 2004 from handout provided at poster presentation Funded by an unconditional grant from Wyeth Pharma GmbH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized on an individual basis by use of an open computerized randomization list" |
| Allocation concealment (selection bias) | High risk | Open randomisation list |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Macrolin 1993.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET, excluding women with OHSS, repeated implantation failure or oestradiol > 2700 pg/mL, age < 38 years (n = 302) | |
| Interventions | PD/COH: GnRHa in long or short protocol + hMG ET: max 3 (41%) or 2 embryos transferred LPS: vaginal micronised progesterone 400 mg daily from the day after oocyte retrieval vs vaginal micronised progesterone 400 mg daily from the day after oocyte retrieval + hCG 1500 IU every other day 3× from ET |
|
| Outcomes | Clinical pregnancy, OHSS, ongoing pregnancy (13 weeks) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomisée' Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Only abstract available |
| Other bias | Low risk | No specific source of other potential bias identified |
Martinez 2000.
| Methods | Randomised controlled trial, sample size calculation based on OHSS rates | |
| Participants | Women undergoing IVF/ICSI with normal ovarian response, mean age 33, BMI between 21 and 27, no history of OHSS (n = 310) | |
| Interventions | PD/COH: GnRH agonist 0.2 mL SC and FSH or hMG ET: day 2, when possible at least 3 embryos transferred LPS: progesterone 100 mg 3× daily vaginally for 10 days from ET vs hCG 2500 IU IM on days +2, +4 and +6 after oocyte retrieval | |
| Outcomes | Clinical pregnancy (gestational sac), miscarriage, multiple pregnancy, OHSS | |
| Notes | Study author contacted in 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly allocated (according to a computer‐generated random assignment table)" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Miller 2010.
| Methods | Multi‐centre randomised controlled trial, number of centres not reported | |
| Participants | Women undergoing IVF with GnRH antagonist down‐regulation, mean age 33 (n = 165) | |
| Interventions | PD/COH: GnRH antagonist + Menopur or rFSH ET: mean 2.3 embryos transferred LPS: progesterone vaginal capsules (Endometrin) vs progesterone IM |
|
| Outcomes | Ongoing pregnancy, miscarriage | |
| Notes | Only abstract available No reply from study author Support from Ferring Pharmaceuticals Inc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "With randomization prior to stimulation" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "A multicenter, randomized, open‐label exploratory study" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "A multicenter, randomized, open‐label exploratory study" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Only abstract available |
| Other bias | Low risk | No specific source of other potential bias identified |
Mochtar 2006.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing their first IVF cycle, mean age 34 (n = 355) | |
| Interventions | PD/COH: GnRH agonist LPS: micronised vaginal progesterone 200 mg twice daily starting at the evening of hCG administration for final oocyte maturation vs micronised vaginal progesterone 200 mg twice daily starting at the evening after oocyte retrieval vs micronised vaginal progesterone 200 mg twice daily starting at the evening after ET |
|
| Outcomes | Biochemical pregnancies (serum hCG > 2 IU/mL or a positive pregnancy test at the 18th day after oocyte retrieval), clinical pregnancies (gestational sac seen by transvaginal ultrasound at day 35 after oocyte retrieval), ongoing pregnancies (positive foetal heartbeat by transvaginal ultrasound 10 weeks after oocyte retrieval), live births | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Envelopes prepared by main investigator, method of preparation and randomisation list not reported |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal reported with reasons |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Moini 2011.
| Methods | Randomised, placebo‐controlled trial | |
| Participants | Women under 35 undergoing IVF/ICSI, mean age 30 (n = 98) | |
| Interventions | PD/COH: GnRH agonist SC from 21st day until complete suppression + hMG or rFSH ET: day 2 or 3, mean 2.8 embryos transferred LPS: vaginal progesterone 400 mg 2× daily + placebo vs vaginal progesterone 400 mg 2× daily + oral oestradiol valerate 2 mg daily. Both from OPU until 10th week |
|
| Outcomes | Clinical pregnancy (presence of at least 1 gestational sac with detectable foetal heartbeat) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Nallapeta 2013.
| Methods | Randomised controlled trial | |
| Participants | Women age 18 to 39 undergoing IVF/ICSI (n = 309) | |
| Interventions | LPS: progesterone 100 mg 1× daily IM vs vaginal progesterone 400 mg 1× daily. Both from OPU until 10th week | |
| Outcomes | PR (not defined) and OHSS | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | High risk | Abstract only |
Ng 2003.
| Methods | Randomised controlled trial, sample size calculation based on rate of perineal irritation (primary outcome of study) | |
| Participants | Women undergoing ICVF/ICSI with high risk of OHSS because of E2 level on day of hCG administration > 10,000 pmol/L or > 15 oocytes obtained (n = 60) | |
| Interventions | PD/COH: GnRH agonist long protocol ET: max 3 embryos transferred LPS: progesterone suppositories (Cyclogest) 400 mg 2× daily vaginally vs progesterone gel (Crinone 8%) 90 mg once daily vaginally. Both for 14 days from day of ET |
|
| Outcomes | Clinical pregnancy (not defined) | |
| Notes | Study author contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "They were randomized according to a computer‐generated randomization list in sealed envelopes" |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Ng 2007.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ICSI with long protocol GnRH agonist, mean age 35 (n = 132) | |
| Interventions | PD/COH: GnRH agonist 150 µg intranasal 4× daily from midluteal phase preceding cycle + hMG ET: max 3 embryos, most often 2 embryos transferred LPS: progesterone vaginal suppositories 400 mg 2× daily vs progesterone vaginal capsules 100 mg 2× daily. Both from ET for 14 days |
|
| Outcomes | Clinical pregnancy (1 or more gestational sacs), ongoing pregnancy (beyond 10 to 12 weeks' gestation), miscarriage, multiple pregnancy | |
| Notes | Study author contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "They were randomized according to a computer‐generated randomization list in sealed envelopes" |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Nyboe Andersen 2002.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ICSI with long protocol GnRH agonist, mean age 32 (n = 303) | |
| Interventions | PD/COH: long protocol with nafarelin 600 µg/d or buserelin 0.5 mg/d for at least 14 days + rFSH ET: max 3 embryos transferred, mean 2 embryos LPS: progesterone vaginal suppositories 200 mg 3× daily from OPU until pregnancy test after 14 days vs progesterone vaginal suppositories 200 mg 3× daily from OPU until 3 weeks after pregnancy test |
|
| Outcomes | Live birth rate, ongoing pregnancy (> 7 weeks' gestational age), multiple pregnancies | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Patki 2007.
| Methods | Randomised placebo‐controlled trial, "dose‐finding study" consisting of 2 phases. Phase 1 investigates 20 mg dydrogesterone vs placebo, phase 2 investigates 30 mg dydrogesterone vs placebo | |
| Participants | Women undergoing ART divided into groups with low or high risk of OHSS, down‐regulation by long protocol GnRH agonist, excluding all other protocols (phase 1: n = 404; phase 2: n = 555) | |
| Interventions |
Phase 1 PD/COH: long protocol GnRH agonist LPS: micronised progesterone vaginal capsules 600 mg daily + dydrogesterone 20 mg daily oral vs micronised progesterone vaginal capsules 600 mg daily from day of oocyte retrieval + placebo Phase 2 PD/COH: long protocol GnRH agonist LPS: micronised progesterone vaginal capsules 600 mg daily from day of oocyte retrieval + dydrogesterone 30 mg daily oral vs micronized progesterone vaginal capsules 600 mg daily from day of oocyte retrieval + placebo All progesterone from day of oocyte retrieval, dydrogesterone or placebo from day of ET until pregnancy test or continued when pregnant |
|
| Outcomes | Pregnancy (intrauterine viable pregnancy) | |
| Notes | Phase 1 investigates vaginal progesterone + oral dydrogesterone vs vaginal progesterone + placebo. This does not fit into any of our comparisons; therefore phase 1 is excluded
Both phases included an extra group; both examined participants in a donor oocyte programme and therefore were not included in our data analysis Study author contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant receives intervention or placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Perino 1997.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET for the first time for tubal factor infertility, age < 38, mean age 31 (n = 300) | |
| Interventions | PD/COH: GnRH agonist + FSH ET: day 2, max 4 embryos transferred LPS: micronised progesterone 50 mg IM daily vs natural progesterone 200 mg vaginally daily. Both from day before ET until pregnancy test | |
| Outcomes | Clinical pregnancy (not defined), ongoing pregnancy (term), miscarriage (not defined) | |
| Notes | No reply from study author in 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Porcu 2003.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET (n = 224) | |
| Interventions | PD/COH: GnRH agonist LPS: natural progesterone 50 mg IM daily vs micronised progesterone 200 mg vaginally daily |
|
| Outcomes | Pregnancy per transfer (not defined) | |
| Notes | No reply from study author in 2004 Only abstract available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Only abstract available Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Pouly 1996.
| Methods | Multi‐centre (6) randomised controlled trial | |
| Participants | Women undergoing IVF/ET for tubal, idiopathic or endometriosis‐related infertility, age < 38, mean age 32 (n = 283) | |
| Interventions | PD/COH: GnRH agonist + hMG ET: mean 3 embryos transferred LPS: progesterone 90 mg vaginal gel daily vs micronised progesterone 100 mg oral, 1 in morning, 2 in evening. Both from day after ET for 14 days or 30 days in case of pregnancy |
|
| Outcomes | Clinical pregnancy (gestational sac or β‐hCG > 1000 IU), miscarriage, multiple pregnancy, ongoing pregnancy (13 weeks) | |
| Notes | Study author contacted in 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated random assignment schedule for each centre" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Unclear risk | No specific source of other potential bias identified |
Propst 2001.
| Methods | Randomised controlled trial, sample size calculation based on LBR | |
| Participants | Women undergoing IVF or ICSI/ET, no cryopreserved ET or donor recipients were included, mean age 35 (n = 201) | |
| Interventions | PD/COH: GnRHa in 76%, rest had different protocols IVF (64%), ICSI (36%)/ET: 79% on day 3, mean 3.5 embryos transferred, 21% on day 5, 2 embryos transferred LPS: progesterone gel 90 mg vaginally once daily vs progesterone 50 mg IM daily. Both from day after oocyte retrieval until pregnancy test +10 weeks in case of pregnancy | |
| Outcomes | Clinical pregnancy (gestational sac), miscarriage (loss of clinical pregnancy before 20 weeks' gestation), live birth | |
| Notes | Recruitment terminated after interim results showed high rate of early bleeding in Crinone group Crinone 8% was provided by Serono Laboratories, Inc., Randolph, Massachusetts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized by permuted blocks of four in sealed envelopes" |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Open‐label study" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Open‐label study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Qublan 2008.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Women undergoing IVF/ICSI‐ET, excluding PCO, endometriosis, hydrosalpinx thrombophilia, abnormal uterine cavity, women receiving any other form of hormonal treatment and women with ≥ 3 previous cycles. Age between 19 and 36, mean age 29 (n = 120) | |
| Interventions | PD/COH: long protocol GnRH agonist + hMG IVF/ICSI‐ET: day 3, 1 to 3 embryos transferred LPS: progesterone pessaries (Cyclogest) + GnRH agonist triptorelin 0.1 mg SC on day of oocyte retrieval, day of ET and day +3 vs progesterone pessaries (Cyclogest) + placebo (solvent) on day of oocyte retrieval, day of ET and day +3 |
|
| Outcomes | Clinical pregnancy (positive foetal heartbeat), miscarriage, live birth rate | |
| Notes | Dosage/frequency of Cyclogest usage not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was accomplished by using a selection from a table of random numbers available in a standard statistics textbook" |
| Allocation concealment (selection bias) | Low risk | "Allocation to the groups was concealed from both researchers and patients. The randomization sequence was placed into sealed, numbered opaque envelopes that were only opened once the consent form was signed" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded by using placebo in control group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 234 participants recruited, 120 analysed, no reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | High risk | Dosage/frequency of Cyclogest usage not mentioned |
Rodriguez‐Pezino 2004.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF (n = 124) | |
| Interventions | PD/COH: GnRH antagonist 0.25 mg SC + rFSH + LH + hCG ET: day 3 LPS: vaginal progesterone gel 90 mg daily vs vaginal progesterone capsules (Utrogestan) 200 mg twice daily vs vaginal progesterone suppositories 200 mg daily. All from oocyte retrieval |
|
| Outcomes | Pregnancy (not defined), miscarriage | |
| Notes | Only abstract available No reply from study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Were randomised" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Only abstract available Objective states same outcome as reported outcome |
| Other bias | Low risk | No specific source of other potential bias identified |
Salehpour 2013.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF because of male factor infertility, mean age 30 (n = 80) | |
| Interventions | PD/COH: GnRH agonist 500 µg SC 1× daily + rFSH or FSH highly purified ET: day 2 or 3, mean 3 embryos transferred LPS: oral dydrogesterone 10 mg 4× daily vs vaginal progesterone 400 mg 2× daily. Both from OPU until 12 weeks of pregnancy |
|
| Outcomes | Clinical pregnancy (viable foetus on ultrasound 6 weeks after ET), miscarriage (loss of a fetus before the 20th week of pregnancy), ongoing pregnancy (at least 1 viable foetus at 12 weeks' gestation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly divided" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Saucedo 2000.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing ART (n = 60) | |
| Interventions | ET: day 3, average of 3 embryos transferred LPS: progesterone 400 mg oral daily vs progesterone vaginal gel 90 mg daily vs progesterone 50 mg IM daily |
|
| Outcomes | Clinical pregnancy (not defined) | |
| Notes | Only abstract available No reply from study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prospectively randomized" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Only abstract available Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Saucedo 2003.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET, mean age 35 (n = 86) | |
| Interventions | PD/COH: GnRH agonist + rFSH ET: day 3 LPS: progesterone 50 mg IM daily vs vaginal progesterone gel 90 mg daily. Both from day of oocyte retrieval |
|
| Outcomes | Pregnancy (not defined) | |
| Notes | Only abstract available No reply from study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Only abstract available |
Serna 2008.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ICSI‐ET with at least 2 good quality embryos available for ET, age < 42, excluding women with FSH > 12 IU/L, liver or renal disease, alcoholism, drug abuse, abnormal thyroid function tests or hyperprolactinaemia, mean age 34 (n = 160) | |
| Interventions | PD/COH: long protocol GnRH agonist or GnRH antagonist + rFSH IVF/ICSI‐ET: 2 embryos transferred LPS: vaginal progesterone 200 mg 2× daily + transdermal E2 10 µg daily vs vaginal progesterone 200 mg 2× daily. Progesterone from oocyte retrieval until 10th week of gestation, E2 from ET until 10th week of gestation |
|
| Outcomes | Ongoing pregnancy (> 12 weeks' gestation), miscarriage (positive test, failed to develop > 12 weeks) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated random number list was created, and patients were included consecutively" |
| Allocation concealment (selection bias) | Low risk | "Sequence was concealed—opaque consecutively numbered envelopes—until intervention was assigned; a study nurse generated the allocation sequence, enrolled the participants, and assigned participants to their group" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Serour 2012.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing ICSI (n = 147) | |
| Interventions | LPS: progesterone‐in‐oil 50 mg IM daily vs progesterone‐in‐oil 50 mg IM daily + rectal progesterone 400 mg 2× daily from pregnancy test for 2 weeks vs progesterone‐in‐oil 50 mg IM daily + rectal progesterone 400 mg 2× daily from pregnancy test until 12 weeks' gestation. Progesterone‐in‐oil in all groups from ET until pregnancy test | |
| Outcomes | Clinical pregnancy (not defined), miscarriage rate (not defined) | |
| Notes | Abstract only No reply from study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Dark sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal and reasons not reported |
| Selective reporting (reporting bias) | High risk | Abstract only Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Stadtmauer 2013.
| Methods | Multi‐centre randomised controlled trial | |
| Participants | Women undergoing ART, mean age 31 (n = 1297) | |
| Interventions | PD/COH: long down‐regulation protocol GnRH agonist + FSH 75 to 450 IU daily + LH 75 to 150 IU daily ET: day 3 or 5 LPS: progesterone weekly vaginal ring vs progesterone vaginal gel 90 mg daily. Birth from day after OPU for 10 weeks |
|
| Outcomes | Live birth rate, clinical pregnancy (gestational sac and foetal heartbeat), ongoing pregnancy (intrauterine gestation with foetal heartbeat at 12 weeks' gestation), miscarriage rate (not defined) | |
| Notes | Supported by Teva Pharmaceuticals T&D | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After 1:1 randomisation by third party |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported, participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | High risk | Supported by Teva Pharmaceuticals T&D |
Strehler 1999.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF, mean age 32 (n = 99) | |
| Interventions | PD/COH: short GnRH agonist protocol + hMG IVF/ET: day 2, mean 2.8 embryos transferred LPS: progesterone vaginal gel 90 mg daily vs progesterone vaginal suppositories 200 mg 3× daily. Both from oocyte retrieval until eighth week of pregnancy |
|
| Outcomes | Clinical pregnancy (foetal sac), miscarriage and multiple pregnancy | |
| Notes | Only abstract available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were prospectively randomized" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Correct blinding was used for researchers" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Only abstract available Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Sumita 2003.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET, mean age 34 (n = 100) | |
| Interventions | PD/COH: GnRH agonist + FSH IVF/ET: 2 embryos transferred LPS: progesterone 50 mg IM daily vs vaginal micronised progesterone 600 mg daily, both from day of oocyte retrieval until 12th week of pregnancy |
|
| Outcomes | Clinical pregnancy (not defined) | |
| Notes | Additional information obtained in 2004 from poster presentation Only abstract available No reply from study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A randomized prospective trial" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Only abstract available Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Tay 2005.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF for tubal disease, male factor, ovulatory dysfunction, endometriosis or unexplained infertility, excluding women with pre‐ovulatory oestradiol concentration ≥ 15,000 pmol/L and/or total oocyte number ≥ 15. Age between 21 and 41, mean 32.4 (n = 168) | |
| Interventions | PD/COH: long protocol stimulated IVF regimens IVF/ET: mean 2.3 embryos transferred LPS Group 1: natural progesterone 200 mg rectally twice daily vs Group 2: natural progesterone vaginal gel 90 mg daily vs Group 3: natural progesterone vaginal capsules, 200 mg once, twice or 3× daily vs Group 4: hCG 1500 IU SC on days 4 and 7 after oocyte retrieval All progesterone supplements were administered from day 4 until 14 days after oocyte retrieval |
|
| Outcomes | Expected live birth rate (> 14 weeks' gestation) | |
| Notes | 5 egg donor cycles and 5 natural cycle frozen embryo replacement cycles were recruited as controls. None of them conceived and none were given any form of luteal support. These are not included in our data analysis No reply from study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomised on the day of embryo transfer" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Tesarik 2006.
| Methods | Randomised placebo‐controlled trial, including 2 separate participant groups: participants undergoing a GnRH agonist protocol and participants undergoing a GnRH antagonist protocol; this is subjectively decided depending on clinical context | |
| Participants | Women undergoing ICSI/ET excluding women with age > 40 and non‐obstructive azoospermia requiring testicular sperm retrieval, mean age in agonist group 35, in antagonist group 31 (agonist: n = 283; antagonist: n = 289) | |
| Interventions |
Agonist PD/COH: GnRH agonist, triptorelin 0.1 mg SC daily starting in luteal phase of preceding cycle, reduced to 0.05 mg after first bleeding + rFSH and hMG ET: day 3, mean 2.2 embryos transferred LPS: single‐dose GnRH agonist 0.1 mg 6 days after ICSI (3 days after ET) vs placebo Antagonist PD/COH: rFSH + hMG from day 2 of menstrual bleeding, followed by withdrawal of a contraceptive pill. GnRH antagonist 0.25 mg SC daily from started on day 5 until trigger ET: day 3, mean 2.3 embryos transferred LPS: single‐dose GnRH agonist 0.1 mg 6 days after ICSI (3 days after ET) vs placebo All women received vaginal micronised progesterone 400 mg and E2 valerate 4 mg daily from oocyte retrieval for 17 days and an injection of 250 µg human rhCG on day of embryo transfer |
|
| Outcomes | Live birth, clinical pregnancy (not defined), ongoing pregnancy (not defined) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done with the use of a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes with treatment allocation instructions were opened on the day of embryo transfer by a nurse who assigned participants to their groups" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The doctor and the biological team performing the ART were blinded to group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The doctor and the biological team performing the ART were blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Tonguc 2011.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF treatment with a long GnRH agonist protocol, excluding women thought to be at risk for the development of OHSS and patients with endometriosis. Mean age 30 (n = 285) | |
| Interventions | PD/COH: long GnRH agonist protocol ET: mean 2.6 embryos transferred LPS: vaginal progesterone gel 90 mg daily + oestradiol 2 mg daily vs vaginal progesterone gel 90 mg daily + oestradiol 4 mg daily vs vaginal progesterone gel 90 mg daily + oestradiol 6 mg daily |
|
| Outcomes | Clinical pregnancy rate (positive serum b‐hCG result with ultrasound evidence of a gestational sac and foetal heartbeat), miscarriage rate (proportion of participants with initially positive hCG or ultrasound evidence of a gestational sac with or without a foetal pole in whom pregnancy failed to develop before 12 weeks' gestation) and multiple pregnancy rate (not defined) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Third party", method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Identical sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Personnel blinded, participant blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Torode 1987.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Women undergoing IVF (n = 131) | |
| Interventions | PD/COH: clomiphene citrate + hMG ET: day 2 LPS: hCG 1500 IU every other day vs placebo |
|
| Outcomes | Pregnancy (not defined) | |
| Notes | No reply from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Ugur 2001.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF with high and normal risk of developing OHSS (n = 375) | |
| Interventions |
High risk PD/COH: GnRH agonist LPS: vaginal micronised progesterone 400 mg daily vs vaginal micronised progesterone 400 mg daily + hCG 3000 IU on day 7 Low risk PD/COH: GnRH agonist LPS: vaginal micronised progesterone 400 mg daily vs hCG 1500 IU every 3 days vs vaginal micronised progesterone 400 mg daily + hCG 1500 IU every 3 days |
|
| Outcomes | Clinical pregnancy | |
| Notes | Only abstract available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Only abstract available Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Vimpeli 2001.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF, excluding women with PCO, previous case of OHSS or > 20 oocytes (n = 89) | |
| Interventions | PD/COH: GnRH agonist + hMG LPS: hCG 1500 IU IM on days 3, 6 and 9 after oocyte retrieval vs vaginal micronised natural progesterone 200 mg 3× daily. from day of oocyte retrieval for 2 weeks, or 4 when pregnant |
|
| Outcomes | Pregnancy (not defined) | |
| Notes | No reply in 2004 Supported by a grant from Organon, the Netherlands |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomly assigned" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes not reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Williams 2001.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF, mean age 34.5 (n = 126) | |
| Interventions | PD/COH: individual protocol per participant including GnRH agonist protocol or microdose GnRH agonist flare protocol or no GnRH protocol ET: day 3, mean 3 embryos transferred LPS: vaginal progesterone 200 mg 3× daily from day 3 after OPU until 10th week of gestation vs vaginal progesterone 200 mg 3× daily from day 6 after OPU until 10th week of gestation |
|
| Outcomes | Clinical pregnancy rate (presence of a gestational sac by ultrasound with appropriately rising b‐hCG levels) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization occurred using a sealed‐envelope technique" |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Wong 1990.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF/ET for tubal factor (n = 30) | |
| Interventions | PD/COH: clomiphene citrate + hMG ET: day 2 LPS: progesterone 50 mg IM daily from day 2 until day 11 vs progesterone 50 mg IM daily from day 2 until day 11 + hCG 1500 IU alternate days from day 5 to day 15 vs no luteal support |
|
| Outcomes | Pregnancy (not defined) | |
| Notes | No reply from study author in 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated" Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Given outcome (pregnancy) not stated in Methods section |
| Other bias | Low risk | No specific source of other potential bias identified |
Yanushpolsky 2010.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing IVF with fewer than 3 prior unsuccessful cycles, mean age 34 (n = 407) | |
| Interventions | ET: mean 2.1 embryos transferred LPS: progesterone 50 mg IM daily from day after oocyte retrieval vs progesterone vaginal gel 90 mg daily from 48 hours after oocyte retrieval. In both arms, 51 women received E2 3 mg oral daily | |
| Outcomes | Pregnancy (not defined), failed pregnancy (chemical pregnancy + spontaneous abortion + ectopic pregnancy) | |
| Notes | Study author contacted; the article, published in Fertility & Sterility (2011) describes a retrospective analysis of women receiving LPS with E2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized with equal probability to receive either [...]" Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Via onsite computer system utilising locked files |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
Yildiz 2014.
| Methods | Randomised study of infertile women having ICSI | |
| Participants | infertile women having ICSI | |
| Interventions | COS: long agonist protocol LPS: all women had 600 mg/day vaginal micronized progesterone plus 4 mg 17beta estradiol for LPS starting from the day of oocyte retrieval until the pregnancy test was performed at day 12 after embryo transfer Group A (n=100) received leuprolide acetate 1 mg s.c. injection 3 days after ET in addition to routine LPS. Group B (n=100) received two sequential doses of leuprolide acetate 1 mg s.c. injections 3 and 6 days after ET in addition to routine LPS. Control group (n=100) received only the routine LPS. RESULTS: A total of 279 patients completed the study. |
|
| Outcomes | Clinical pregnancy rate, ongoing pregnancy rate, multiple pregnancy, OHSS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomisation model" |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | some losses to follow up |
| Selective reporting (reporting bias) | High risk | did not report live birth data |
| Other bias | Unclear risk | nil |
Zegers‐Hochschild 2000.
| Methods | Multi‐centre (3) randomised controlled trial, including 2 different studies: IVF‐embryo transfer trial and oocyte donation trial. Only the IVF‐ET trial is included in the review | |
| Participants | Women undergoing ICSI/IVF‐ET (n = 505) | |
| Interventions | PD/COH: GnRH agonist + hMG ET: day 2 or 3, mean 3.7 embryos transferred LPS: 1 gram progesterone vaginal ring vs 50 mg progesterone IM daily |
|
| Outcomes | Clinical pregnancy (gestational sac), multiple gestation (2 or more gestational sacs visualised 5 weeks after embryo transfer), live birth | |
| Notes | Laboratorios Silesia S.A. provided the vaginal rings | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "On day of oocyte retrieval patient were randomly allocated..." Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No specific source of other potential bias identified |
ART: assisted reproduction techniques.
BMI: body mass index.
CC: clomifene citrate.
COH: controlled ovarian hyperstimulation.
CPR: clinical pregnancy rate, pregnancy diagnosed by ultrasonographic visualisation of 1 or more gestational sacs or definitive clinical signs of pregnancy.
ET: embryo transfer.
FSH: follicle‐stimulating hormone.
GnRH: gonadotropin‐releasing hormone.
hCG: human chorionic gonadotropin.
hMG: human menopausal gonadotropin.
ICSI: intracytoplasmic sperm injection.
IVF: in vitro fertilisation.
LH: luteinising hormone.
LS: luteal support.
OHSS: ovarian hyperstimulation syndrome.
OPR: ongoing pregnancy rate, pregnancy proceeding beyond 20th gestational week.
OPU: ovum pick up.
PCO: polycystic ovary.
PCOS: polycystic ovarian syndrome.
PD: pituitary desensitisation.
RCT: randomised controlled trial.
rFSH: recombinant follicle stimulating hormone
TESA: testicular sperm aspiration.
TESE: testicular sperm extraction.
US: ultrasound.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abu‐Musa 2008 | This RCT investigated the role of 17a‐hydroxyprogesterone caproate given before embryo transfer to decrease uterine contractions and thereby improve implantation rates |
| Abu‐Musa 2008a | This RCT investigated the role of 17a‐hydroxyprogesterone caproate given before embryo transfer to decrease uterine contractions and thereby improve implantation rates |
| Aleyasin 2012 | This RCT investigated methods of final oocyte maturation |
| Allahbadia 2004 | This comparative study investigated pregnancy outcomes with IM progesterone (n = 94) vs oral dydrogesterone (n = 30) for luteal phase support in cycles using donated eggs |
| Allen 2004 | This RCT included ZIFT cycles only (n = 99) |
| Alsanie 2005 | This retrospective case control study compared serum hCG levels when progesterone and oestrogen were used (n = 15) vs progesterone alone (n = 15) for luteal phase support in IVF‐ET cycles |
| Andersen 2014 | Not a primary study; a literature review |
| Anserini 2001 | This is a quasi‐RCT |
| Anthony 1993 | This is a quasi‐RCT |
| Araujo 1994 | This RCT investigated IVF and ZIFT cycles but did not describe the distribution of these interventions Study authors previously contacted (in 2004) |
| Araujo Filho 1996 | Study did not report the percentage of ZIFT cycles |
| Baber 1988 | This study was excluded from the previous version of this review, as in this RCT, allocation to hCG or no treatment included only women with a positive pregnancy test; thus treatment did not truly consist of luteal phase support |
| Beckers 2006 | This RCT investigated high doses of steroids administered after the LH surge in normo‐ovulatory volunteers to investigate whether this would give rise to endocrine changes and shortening of the luteal phase |
| Belaisch‐Allart 1988 | This was an interim analysis of 295 cases in a total of 525 women. Data included 451 transfers Study author contacted in 2004 but not able to provide any information |
| Ben‐Nun 1990 | This study was excluded from the previous version of this review, as it was not a randomised trial ‐ compared IM progesterone vs historical controls receiving no progesterone. Treatment was given for only 6 days around the time of oocyte retrieval |
| Berjis 2008 | This study included only rapid‐ZIFT procedures |
| Bjuresten 2011 | This RCT investigated the effects of luteal phase support in frozen embryo transfers only (n = 435) |
| Blake 2010 | This pharmacokinetic study did not include patients undergoing ART |
| Buvat 1988 | This was a quasi‐RCT |
| Buvat 1990 | This was a quasi‐RCT |
| Casini 2003 | This study was excluded from the previous version of this review because in this RCT, some women contributed more than 1 cycle to the study (n = 201 women, 436 cycles) Study author was unable to provide first cycle data |
| Chakravarty 2012 | Abstract only, no data reported No reply from study author |
| Chang 2008 | Not a randomised trial; review about intramuscular progesterone for luteal phase support in IVF |
| Chang 2009 | Not a randomised trial; retrospective analysis of outcomes of IVF cycles with GnRH antagonist administration on ovulation triggering day |
| Chantilis 1999 | This study was excluded from the previous version of this review, as it was not a randomised trial ‐ compared vaginal progesterone vs historical controls using IM progesterone |
| Check 2010 | This RCT investigated the dosage of progesterone supplementation in frozen embryo transfers only (n = 408) |
| Check 2012 | Not a primary study; literature review |
| Check 2013 | Not a randomised controlled trial; retrospective cohort study |
| Claman 1992 | This RCT included IVF/ETcycles (n = 121) rather than women (n = unknown) Study author contacted in 2004 and was not able to provide first cycle data |
| Costabile 2001 | This RCT was excluded because it included more cycles (n = 300) than women (n = 220) No reply from study author |
| Daya 2009 | Not a randomised trial; review about progestogens for luteal support |
| Demir 2013 | This article pertains to a subset of women with a thin endometrium; therefore the results cannot be generalised |
| Demirel 2003 | This RCT was excluded, as the abstract does not provide details on the number of participants allocated to each intervention group No reply from study author |
| Ding 2005 | This RCT was excluded because it included more cycles (n = 114) than women (n = 95) No reply from study author |
| Ellenbogen 2011 | This study investigated in vitro maturation of oocytes |
| Erman Akar 2005 | This RCT was excluded because it included more cycles (n = 115) than women (n = 95) No reply from study author |
| Escriba 2006 | This RCT investigated initiation of progesterone supplementation in donated oocyte transfers only (n = 300) |
| Farhi 2000 | This study was excluded from the previous version of this review because in this RCT, some women contributed more than 1 cycle to the study (n = 271 women, 285 cycles) |
| Farrag 2008 | This RCT investigated the use of recombinant hCG to induce final oocyte maturation in ICSI cycles |
| FeiYang 2013 | Abstract of an RCT investigating different LH and luteal phase protocols. Limited information on outcomes reported, no contact details available |
| Feliciani 2004 | This RCT compared the effects of intravaginal (n = 14) and IM progesterone (n = 14) in frozen/thawed embryo transfers only |
| Gallardo 2004 | This study was excluded as only the abstract is available, and it provides no details on participants allocated to each intervention group and no contact details for study authors |
| Garcia‐Velasco 2009 | This RCT investigated the effects of letrozole administered during the luteal phase after oocyte retrieval in oocyte donors only |
| Gazvani 2012 | This is a study protocol only |
| Germond 2002 | Not a randomised trial. This cohort study investigated 2 types of micronised progesterone as luteal phase support |
| Ghanem 2009 | This study was quasi‐randomised, as randomisation was performed using a sequential allocation method |
| Gibbons 1998 | This study was excluded from the previous version of this review, as this RCT compared vaginal and IM progesterone only in women receiving donated oocytes (n = 72) |
| Griesinger 2006 | Not a randomised trial; review |
| Herman 1990 | This was a quasi‐RCT |
| Herman 1996 | This was a quasi‐RCT |
| Ho 2008 | Not a randomised trial; retrospective case control study |
| Hokenstad 2013 | This RCT included frozen embryo transfers only (n = 71) |
| Humaidan 2010 | This RCT was excluded, as it investigated only 1 dose of hCG as a trigger; not a luteal phase support study |
| Humaidan 2013 | This study investigated risk of OHSS |
| Hutchinson‐Williams 1990 | This study was excluded from the previous version of this review, as it was not a randomised trial ‐ the treatment group was "randomly" selected, but the control group was retrospectively selected and was age‐matched to the treatment group |
| Iliodromiti 2013 | Not a randomised trial; retrospective study on the effects of GnRH agonist trigger and modified intensive luteal phase support on pregnancy outcomes and risk of OHSS |
| Jee 2010 | Not a randomised trial; meta‐analysis |
| Johnson 1999 | This study was excluded from the previous version of this review, as this RCT compared hCG vs no treatment, with primary objective of measuring relaxin levels during the luteal phase Complete pregnancy outcomes by groups were not reported |
| Jung 2010 | Randomisation unclear No reply from study author |
| Kahraman 2010 | This was a quasi‐RCT |
| Kaser 2012 | This study investigated intramuscular progesterone vs Crinone 8% in cryopreserved embryos (n = 738) |
| Kol 2011 | This proof‐of‐concept study investigated an hCG‐based, progesterone‐free luteal phase |
| Koper 2008 | This randomised trial investigated the dose‐response relationship of corifollitropin alfa to initiation of multi‐follicular development for the first 7 days of controlled ovarian stimulation |
| Krause 2006 | This RCT investigated the efficiency and safety of different luteal support regimens in non‐IVF cycles (n = 36) |
| Krischker 1998 | This study was excluded from the previous version of this review, as this RCT compared progesterone IM, 2 types of oral progesterone and hCG, using long GnRHa (n = 30) or ultrashort GnRHa (n = 273). Pregnancy rates by group were provided, but numbers of transfers in each group were not provided. Attempts to contact study authors were unsuccessful |
| Kwon 2012 | This randomised study investigated the effects of intravenous immunoglobulin treatment on pregnancy outcomes |
| Kyrou 2011a | Not a randomised trial; meta‐analysis on addition of GnRH agonist for luteal phase support |
| Lainas 2012 | Not a randomised trial; observational cohort study on outpatient management of severe early OHSS |
| Lam 2003 | This study was excluded from the previous version of this review, as this RCT compared hCG plus vaginal progesterone administered only between oocyte retrieval and embryo transfer vs hCG alone (n = 102). This was the only identified study that made this comparison |
| Lan 2007 | This RCT compared the efficacy and tolerability of 2 formulations of vaginal progesterone ‐ Crinone 8% (n = 100) and Utrogestan (n = 100) ‐ in frozen embryo transfers only |
| Lee 2013 | Not a randomised trial; retrospective analysis on frozen‐thawed cycles |
| Lee 2013a | This retrospective study investigated the effects of additional hCG with vaginal progesterone in luteal phase support |
| Leeton 1985 | This was a quasi‐RCT |
| Lightman 1999 | This was a quasi‐RCT |
| Lin 2013a | This report investigated the effects of delayed initiation of gonadotropin in luteal long protocol on outcomes of in vitro fertilisation |
| Liu 2012 | Not a randomised trial; meta‐analysis about duration of luteal phase support |
| Lukaszuk 2005 | This RCT was excluded because it included more cycles (n = 231) than women (n = 166) No reply from study author |
| Mahadevan 1985 | This was a quasi‐RCT |
| Marianowski 2000 | This study was excluded from the previous version of this review, as it was not a randomised trial ‐ compared IM and vaginal progesterone vs allocation by the woman's preference (n = 79) |
| Martins 2010 | Not a randomised trial; review about luteal phase support |
| McBain 1987 | This was a quasi‐RCT |
| Michnova 2011 | Not a primary study; literature review |
| Miller 2013 | Abstract only; no details on number of participants randomly assigned to each intervention group No reply from study author |
| Mochtar 1996 | This study was excluded from the previous version of this review because in this RCT, some women contributed more than 1 cycle to the study (n = 98 women, 176 cycles) An attempt was made to contact study authors, but no reply was received |
| Moraloglu 2008 | This study compared the effects of GnRH agonist (n = 48) and GnRH antagonist (n = 45) use in 2 matched groups of women undergoing IVF/ICSI |
| Munoz 2013 | Not a primary study; literature review |
| Nader 1988 | This study was excluded from the previous version of this review, as this RCT compared progesterone vs hCG but was excluded because some women contributed more than 1 cycle to the study (n = 17 women, 20 cycles) Study author was unable to provide first cycle data |
| NCT01007851 2006 |
NCT01007851 https://ClinicalTrials.gov/show/NCT01007851 Study terminated for lower than anticipated recruitment. |
| Nikkanen 1992 | This was a quasi‐RCT |
| Nyboe Andersen 2012 | This was not a primary study ‐ data from 2 other studies were reported |
| Osmanagaoglu 2013 | This randomised study investigated differences in the numbers of metaphase 2 oocytes after triggering with hCG vs triggering with the combination of hCG and GnRH agonist |
| Ozcimen 2004 | This cross‐over study investigated the effects of luteal phase support on non‐IVF gonadotropin induction of ovulation |
| Papanikolaou 2010 | This RCT compared recombinant hCG (n = 59) vs urinary hCG (n = 60) as a final oocyte maturation trigger |
| Papanikolaou 2011 | This was a proof‐of‐concept study on use of luteal phase support as a final oocyte maturation trigger |
| Paredes 2004 | Abstract only available ‐ does not provide details on outcomes No reply from study author |
| Pirard 2005 | This randomised trial included IUI only |
| Pirard 2006 | Comparison did not meet inclusion criteria |
| Polson 1992 | This was a quasi‐RCT |
| Priyadharshini 2013 | Not a randomised trial; observational study |
| Propst 2012 | This study investigated intrauterine insemination |
| Santibanez 2014 | This randomised study investigated the effects of human chorionic gonadotropin on clinical pregnancy before embryo transfer |
| Satir 2013 | This retrospective study investigated intramuscular progesterone vs vaginal progesterone gel |
| Schwarzler 2003 | This study was excluded from the previous version of this review because in this RCT, some women contributed more than 1 cycle to the study (n = 603 women, 945 cycles) |
| Shamma 1992 | Abstract only ‐ no contact details for study authors |
| Silverberg 2010 | Not a true randomised trial; study investigating vaginal progesterone vs intramuscular progesterone Study author contacted |
| Simunic 2007 | Not a randomised trial; cohort study investigating the efficacy and tolerability of Crinone 8% gel vs Utrogestan capsules |
| Singh 2010 | This randomised trial investigated supplementation of GnRH agonists during the luteal phase in IUI only |
| Smith 1989 | This was a quasi‐RCT |
| Smitz 1988 | Study did not report the percentage of GIFT cycles |
| Smitz 1992 | Study included > 20% GIFT/ZIFT cycles |
| Smitz 1993 | This was a quasi‐RCT |
| Sordal 1993 | This study was excluded from the previous version of this review, as this RCT compared progesterone IM, 2 doses of vaginal progesterone and no treatment (n = 40) but did not provide pregnancy rates by group Attempts to contact study author were unsuccessful |
| Stadtmauer 2009 | This randomised trial compared the effects of progesterone in a vaginal ring (n = 10) vs progesterone vaginal gel (n = 10) in donor oocytes |
| Stovall 1998 | Not a randomised trial ‐ this study investigated selective early elimination of luteal phase support |
| Tay 2003 | This study divided study population into 2 groups; group A underwent GnRH‐a/rFSH ovarian stimulation followed by IVF, and group B underwent CC/rFSH ovarian stimulation and IUI After ET or insemination, participants were randomly assigned to 2 different luteal phase support protocols No reply from study author in 2004 |
| Tomic 2011 | Not a randomised trial; case control study investigating oral micronised progesterone combined with vaginal progesterone |
| Trounson 1986 | This study was excluded from the previous version of this review, as this RCT assessed luteal support with progesterone IM or hCG given only around the time of oocyte retrieval (n = 42) |
| Unfer 2004 | This RCT was excluded because it included more cycles (n = 284) than women (n = 213) No reply from study author |
| Unfer 2004a | This RCT was excluded because it included more cycles (n = 734) than women (n = 320) No reply from study author |
| Vaisbuch 2012 | This was a World Wide Web‐based survey |
| Valentino 2004 | This study was excluded from the previous version of this review, as this RCT compared vaginal and IM progesterone (n = 40) but did not provide pregnancy rates (main outcome measures were side effects and convenience) Attempts to contact study author were unsuccessful |
| van Steirteghem 1988 | Study did not report percentage of GIFT procedures |
| Var 2011 | This was a quasi‐RCT ‐ allocation was based on application number |
| Wang 2009 | Not a randomised trial; cohort study comparing Crinone 8% gel vs Utrogestan capsules |
| Wilcox 2001 | This study was excluded from the previous version of this review, as this RCT compared luteal support with progesterone vaginal gel alone or in combination vs IM progesterone in frozen embryo transfer cycles (n = 97) |
| Yazici 2014 | This randomised study investigated the role of luteal phase support in ovulation induction and intrauterine insemination |
| Ye 2009 | This RCT investigated luteal oestradiol pretreatment before the GnRH antagonist protocol and the GnRH agonist protocol |
| Yigit 2002 | This study was excluded from the previous version of this review, as it was not a randomised trial According to information received from study author, this was a retrospective study comparing vaginal gel vs IM progesterone |
| Yovich 1984 | This was a quasi‐RCT with allocation based on study number Study author contacted in 2004 |
| Yovich 1985 | This was a quasi‐RCT |
| Yovich 1991 | This RCT included ZIFT cycles only |
ART: assisted reproduction techniques.
CC: clomifene citrate.
ET: embryo transfer.
GIFT: gamete intrafallopian transfer.
GnRH: gonadotropin‐releasing hormone.
hCG: human chorionic gonadotropin.
ICSI: intracytoplasmic sperm injection.
IVF: in vitro fertilisation.
LH: luteinising hormone.
OHSS: ovarian hyperstimulation syndrome.
RCT: randomised controlled trial.
rFSH: recombinant follicle stimulating hormone.
ZIFT: zygote intrafallopian transfer.
Characteristics of studies awaiting assessment [ordered by study ID]
Pirard 2015.
| Methods | Computer‐generated randomization was applied (2/1; group A/B). Treatment allocation instructions were placed in individually sealed envelopes to be opened at the center in chronological order on the day of signing the informed consent form. |
| Participants | Women undergoing IVF/ICSI after stimulation of multiple follicular development with human menopausal gonadotropin (hMG). Inclusion criteria were the age between 18 and 39 and BMI ≥ 18 but ≤35, while exclusion criteria were a history of poor response, systemic disease (diabetes, severe migraine, hepatic, renal, or cardiovascular disease, and corticodependent asthma), and ovarian cysts ≥11 mm. |
| Interventions | In study group A, GnRH agonist (buserelin) was administered IN to trigger final follicular maturation and support the luteal phase. In control group B, hCG was administered to trigger final follicular maturation and vaginal progesterone to support the luteal phase. |
| Outcomes | The primary end‐point was the comparison of pregnancy rates between the two groups. Pregnancy was diagnosed by measuring serum hCG levels on day 14 of the luteal phase (day of first hCG/buserelin administration = D0). Clinical pregnancy was defined as the presence of an intrauterine gestational sac with a positive heartbeat visual‐ized by vaginal ultrasound. |
| Notes | Single‐center, prospective, randomised, open, parallel group study. |
Tomic 2015.
| Methods | Patients were randomly assigned at the day of oocyte retrieval following computerized random number generator in procedure, to study or control group. Random allocation concealment with intervention drug was ensured by sequentially numbered, sealed, opaque envelopes. Patients were aware of the allocated arm since the treatment drugs have different route of administration, but investigators and outcome assessor were kept blinded to the allocation. |
| Participants | Eligible participants were all women undergoing controlled ovarian stimulation for IVF/ICSI treatment who met the following inclusion criteria: aged 18–45 years, a body mass index (BMI) < 35 kg/m2 , applied routine short ovulation induction protocol with GnRH agonist, with less than three prior IVF cycles and at least one aspirated oocyte. Exclusion criteria included: a history of dysfunctional uterine bleeding, recurrent miscarriage (defined as three or more spontaneous miscarriage), acute urogenital disease, transfer of frozen embryos and previous allergic reactions to progesterone products. |
| Interventions | Study group: recieved 2 10 mg of oral dydrogesterone (Duphaston1, Abbot Biologicals B.V., Olst, Netherlands) from the day of oocyte retrieval until a pregnancy test or in the case of pregnancy until week 10. Control group: recieved 1 90 mg of vaginal progesterone gel (Crinone 8%, Fleet Laboratories Ltd., Watford, UK) in the same fashion i.e. from the day of oocyte retrieval until pregnancy test or in the case of pregnancy until week 10. |
| Outcomes | The primary outcome was ongoing pregnancy rate, defined by the presence of gestational sac(s) with viable fetal heart beats at 12 weeks’ gestation by transvaginal ultrasound. Secondary outcome measures were satisfaction score, determinate on the 5‐point level scale (with 1 being ‘‘absolutely unsatisfied’’ and 5 being ‘‘absolutely dissatisfied’’) and tolerability accessed by questionnaire with different side effects that the supplements could cause. |
| Notes | The prospective, randomized, double‐blinded clinical trial was conducted from October 2010 to October 2013 in a tertiary infertility unit at University Hospital Center ‘‘Sisters of Mercy’’, Zagreb, Croatia. Corresponding author at: DZ Zagreb Centar, Department of Gynecology and Obstetrics, Runjaninova 4, 10 000 Zagreb, Croatia. Fax: +385 1 37 68 272. E‐mail address: tomic.vlatka@gmail.com (V. Tomic) |
Zafardoust 2015.
| Methods | Computer‐generated randomization list was used for randomization. Selection was performed on the day of OCP admin‐istration for GnRH antagonist cycle. |
| Participants | 100 infertile couples with history of 2 or more previous IVF‐ET or ICSI‐ET failures treated by GnRH antagonist protocol for ICSI. Inclusions: Women with history of 2 or more previous IVF‐ET or ICSI‐ET failures; women were under 42 years old and had FSH levels <12 mIU/ml on 2nd or 3rd day of menstrual bleeding with normal thyroid and prolactin levels and the couples had at least one embryo available for transfer. Exclusions: Women with hydrosalpinx or anatomical uterine disorders or those with thrombophilia disorders; couples suffering from azoospermia who required testicular sperm retrieval; those who had undergone Preimplantation Genetic Diagnosis (PGD) 100 couples; 17 dropouts, 83 analysed ‐ 43 in intervention group and 40 in control. |
| Interventions | There were two groups. Intervention group received Decapep‐til (Ferring, Germany) 0.1 mg S.C., 6 days after oocyte retrieval and control group did not receive Decapeptil. All women received routine luteal phase support with 800 mg vaginal progesterone daily. |
| Outcomes | Pregnancy was tested by measuring serum beta‐hCG levels 14 days after ET. The implantation rate was calculated as the ratio of the number of embryonic sacs detected by ultrasonography to the total number of embryos transferred. Clinical pregnancy was defined as the presence of a fetus with a heart beat by vaginal ultrasonography at 6 weeks of pregnancy. Multiple pregnancies were defined by presence of more than one fetus in vaginal ultrasonography. |
| Notes | This study was conducted between February 2013 and January 2014 in Avicenna infertility Clinic affiliated to Avicenna Research institute, Tehran, Iran. This study was approved by the Ethical Commit‐tee of Avicenna Research Institute and informed consent was obtained from all participants. |
Characteristics of ongoing studies [ordered by study ID]
EUCTR2012‐002215‐26‐BE 2013.
| Trial name or title | A Multicenter Study Comparing the Efficacy, Safety and Tolerability of Oral Dydrogesterone 30 mg Daily Versus Intravaginal Micronized Progesterone Capsules 600 mg Daily for Luteal Support in In‐Vitro Fertilization (Lotus I) |
| Methods | A Double‐Blind, Double‐Dummy, Randomized, Two‐arm, Multicenter Study |
| Participants | Infertile women undergoing IVF |
| Interventions | Oral dydrogesterone 10 mg TID versus micronized progesterone vaginal capsules 200 mg TID |
| Outcomes | Primary Outcome: Pregnancy Rate, defined as the presence of fetal heart beats at 12 weeks gestation determined by transvaginal ultrasound Secondary Outcome: Positive Pregnancy test rate, defined as positive biochemical pregnancy test on Day 14 after embryo transfer, Rate of successful completion of pregnancy, Incidence of live births and healthy newborns, Adverse Events, Status newborn. The gender, APGAR score, height, weight and head circumference, physical examination and any malformations of the newborn(s) will be recorded, Adverse Events At Study Completion (about 10 months after IVF) |
| Starting date | 2013 |
| Contact information | Simone Schicker Email: simone.schicker@quintiles.com Contact telephone: +496102 296 213 |
| Notes | Sponsorship:Quintiles GmbH and Abbott Laboratories GmbH https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2012‐002215‐26 NB: This trial has a second registration NCT01850030 https://clinicaltrials.gov/ct2/show/NCT01850030 |
EUCTR2013‐001105‐81‐2013.
| Trial name or title | Randomized Clinical Trial to Compare the Pregnancy Rates of Vaginally Applied Cyclogest® Pessary and Crinone® 8% Gel After In‐vitro Fertilization |
| Methods | Randomized clinical parallel group trial |
| Participants | Women having IVF, age 18‐40 |
| Interventions | Cyclogest® Pessary or Crinone® 8% Gel for luteal phase support after IVF |
| Outcomes | Clinical pregnancy rate (Clinical pregnancy rate achieved after 38 days of luteal phase support (primary), Clinical pregnancy rate achieved after 70±3 days (10 weeks) of luteal phase support (secondary), Clinical pregnancy rate achieved after 70 ±3 days (10 weeks) of luteal phase support (fetal heart movement measured by TVUS), Clinical implantation rates per number of embryos transferred after 38 days of luteal phase support (fetal heart movement measured by TVUS), Biochemical pregnancy rate at Day 18 and 38 after OR The patient's evaluation of treatment convenience, The patient's evaluation of bleeding and leakage (diary), Incidence of adverse events. |
| Starting date | 31 July 2013 |
| Contact information | Email: iklingmann@pharmaplex.be |
| Notes | Sponsorship: Actavis Group PTC ehf. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=2013‐001105‐81 http://adisinsight.springer.com/trials/700235403 |
IRCT201402191141N18 2015.
| Trial name or title | Subcutaneous progesterone (Prolutex) versus vaginal (Cyclogest) for luteal phase support in IVF/ICSI cycles: a randomized controlled clinical trial study phase 3 |
| Methods | RCT |
| Participants | Infertile women undergoing IVF |
| Interventions | Intervention: Luteal phase support during ART treatment with subcutaneous injections of progesterone (Prolutex): since ovum pick up day, a daily subcutaneous injection of progesterone (25 mg) (Prolutex®; IBSA Institut, SA Biochimique) will be used and if pregnancy is occurred it continues until 10 weeks of pregnancy. Control group : Luteal phase support during ART treatment using a vaginal suppository (Cyclogest) : Since ovum pick up day, one vaginal suppository every 12 hours will be used (Cyclogest ®; Actavis, Barnstaple, UK), If pregnancy is occurred it continues until 10 weeks of pregnancy. |
| Outcomes | Clinical pregnancy rate: evidence of pregnancy by clinical (fetal heartbeat) or ultrasound parameters (ultrasound visualization of a gestational sac, embryonic pole with heartbeat) after 7‐6 weeks after embryos transfer. Early miscarriage rate. |
| Starting date | 2015 |
| Contact information | Dr Ashraf Moini Email: a_moini@royaninstitute.org Contact telephone: 00982123562640 |
| Notes | Sponsorship: Royan Institute and Shafayab gostar pharmaceutical company http://www.irct.ir/searchresult.php?keyword=&id=1141&number=18&prt=6166&total=10&m=1 |
IRCT2014030916912N1 2014.
| Trial name or title | Comparison administration single dose GNRH agonist (Triptrolin) with placebo in the luteal phase on clinical pregnancy rate in ART cycle in the infertile women. |
| Methods | RCT |
| Participants | Infertile women undergoing IVF |
| Interventions | Intervention: Three days after embryo transfer, 0.1 mg (1ml) triptrolin subcutaneous injected Control: 1ml normal saline subcutaneous injection three days after embryo transfer |
| Outcomes | Clinical pregnancy, 8 week after intervention. Implantation rate, 10 weeks after intervention. |
| Starting date | 2014 |
| Contact information | Saeedeh Gharahjeh
Tehran University of Medical Sciences Email: s‐gharahgeh@razi.ac.ir, Dr.gharahgeh_1388@yahoo.com Contact telephone: 00982184902421 |
| Notes | http://www.irct.ir/searchresult.php?keyword=stimulatio&id=16912&field=&number=1&prt=171&total=10&m=1 |
IRCT2014071212494N2 2014.
| Trial name or title | Comparison of oral progesterone with vaginal and subcutaneous progesterone for luteal phase support on pregnancy rate of infertile patients underwent intracytoplasmic sperm injection ‐ Embryo transfer cycles |
| Methods | RCT |
| Participants | Infertile women undergoing IVF |
| Interventions | Intervention 1: Duphaston(Oral Didrogesterone 10mg, Abbott, Netherland) 20mg , Twice daily until 12 weeks Intervention 2: Subcutaneous progesterone (Prolutex, 25mg, IBSA company, Switzerland) daily injection until 12 weeks Control: Vaginal suppository cyclogest, (A kind of vaginal progesterone, 400 mg, actover company, Britain) 400mg twice daily until 12 weeks. |
| Outcomes | Clinical pregnancy rate, five weeks after start of intervention by transvaginal ultrasonography.
Miscarriage rate, until 24 weeks after start of intervention. Patients acceptance, until 12 weeks by questionnaire |
| Starting date | 2014 |
| Contact information | Nasrin Saharkhiz Reproductive Health Research Centre ‐ Shahid Beheshti of Medical Science Email: saharkhiz1377@yahoo.com; www.irhrc.sbmu.ac.ir Contact telephone: 00982122432558 |
| Notes | Sponsorship: Vice chancellor for research, Shahid Beheshti University of Medical Science; Shafayab Gostar company http://www.irct.ir/searchresult.php?keyword=&id=12494&number=2&prt=7064&total=10&m=1 |
NCT00490308 2007.
| Trial name or title | Blinded Randomised Trial About the Influence of Estradiol Supplementation During the Luteal in Patients Undergoing in Vitro Fertilization (IVF) Treatment |
| Methods | RCT |
| Participants | Inclusion Criteria: Women treated for infertility with controlled ovarian hyperstimulation using daily GnRH agonist Exclusion Criteria: Women younger then 18 or older then 40, Women with systemic disease, Women with a family or personal history of thromboembolic event |
| Interventions | Treatment with estradiol valerate |
| Outcomes | Secondary Outcome Measures: E2 and progesterone levels |
| Starting date | 2007 |
| Contact information | Ran Svirsky, MD Assaf‐Harofeh Medical Center Email: rsvirs@gmail.com Contact telephone: +972‐0523‐859521 |
| Notes | https://ClinicalTrials.gov/show/NCT00490308 |
NCT01081652.
| Trial name or title | A Study Using Micronised Progesterone (Crinone® 8%) in the Luteal Phase Support of Women Undergoing in Vitro Fertilisation (IVF) and Embryo Transfer (ET) |
| Methods | RCT |
| Participants | Infertile women undergoing IVF |
| Interventions | Intervention: Micronised progesterone administered intravaginally once daily from the day of ET. If pregnancy was confirmed on day 14 of progesterone administration, progesterone was continued for another 45 days. Comparison: Progesterone 60 mg administered intramuscular once daily from the day of ET. If pregnancy was confirmed on day 14 of progesterone administration, progesterone was continued for another 45 days. |
| Outcomes | The difference in hCG positive rate in the two arms 14 days after embryo transfer. The difference in pregnancy rates in the two arms 30 and 60 days after embryo transfer. The difference in implantation rate in the two arms 30 days after embryo transfer. |
| Starting date | 2014 |
| Contact information | Huafei Li Serono Pharmaceutical Limited |
| Notes | https://ClinicalTrials.gov/show/NCT01081652 Completed with no results available. |
NCT01237535.
| Trial name or title | Luteal Phase Support With Progesterone Versus Estrogen and Progesterone on Pregnancy Rates |
| Methods | RCT |
| Participants | Infertile women undergoing IUI, age 20‐40 years |
| Interventions | Intervention 1: Luteal support with progesterone only (they will received vaginal P gel (Crinone 8% vaginal gel; Serono, Israel) Intervention 2: Luteal support with estrogen + progesterone [(Crinone 8% vaginal gel; Serono, Israel) and Estrofem 4mg] Control: No luteal support |
| Outcomes | Clinical Pregnancy, a pregnancy test will be performed 2 weeks after insemination (Serum hCG) an intrauterine pregnancy will be confirmed using a transvaginal ultrasound 2 weeks after a positive pregnancy test |
| Starting date | 2010 |
| Contact information | Dr. Galia Oron Rabin Medical Center, Petach‐Tikva, Israel Email: orong@clalit.org.il Contact telephone: 972‐3‐9377492 |
| Notes | https://ClinicalTrials.gov/show/NCT01237535 |
NCT01504139 2012.
| Trial name or title | The Luteal Phase After GnRHa Trigger ‐ a Proof of Concept Study |
| Methods | RCT |
| Participants | Women undergoing IVF, age 25‐40 years |
| Interventions | Intervention 1: hCG in the late follicular phase + luteal phase, when the follicles are over 12 mm FSH is replaced by hCG Intervention 2: hCGi n the follicular phase + luteal phase, hCG is given together with FSH from the beginning of the FSH stimulation. Intervention 3: LH in the luteal phase, LH replaces progesterone and estradiol in the luteal phase. Control: vaginal progesterone and estradiol in the luteal phase.The usual dose of vaginal progesterone and estradiol is given in the luteal phase. |
| Outcomes | Levels of progesterone in the mid‐luteal phase |
| Starting date | 2012 |
| Contact information | Helen Olesen Elbaek The Fertility Clinic, Skive Regional Hospital, Denmark |
| Notes | Sponsor: Regionshospitalet Viborg, Skive https://ClinicalTrials.gov/show/NCT01504139 |
NCT01638026 2012.
| Trial name or title | Final Oocyte Maturation Via Administration of GnRH Agonists Followed By Luteal Support With hCG |
| Methods | RCT |
| Participants | Inclusion Criteria: patients who are eligible for in vitro fertilization using an antagonist protocol Exclusion Criteria: patients diagnosed with hypogonadotrophic hypogonadism, sensitivity to any of the drugs used in the study A patient enrolled in the study who, as a result of ovarian stimulation, responds in a way that puts her in risk of developing ovarian hyperstimulation, will be ultimately excluded from the study. |
| Interventions | In the study group women will receive GnRH agonist (decapeptyl 0.2 mg) for oocyte maturation, followed by ovum pick‐up which will be performed 35 hours later. Embryo transfer will be performed 48‐72 hours after ovum pick‐up. Luteal support will include HCG 1500 IU. |
| Outcomes | Primary Outcome Measures: fertilization rate Secondary Outcome Measures: satisfaction, no. of oocyte, pregnancy rate, no. of embryos, quality of embryos |
| Starting date | 2012 |
| Contact information | Ronit Beck Fruchter, MD HaEmek Medical Center, Israel Contact telephone: 0097246494475 Email: beck_r@clalit.org.il |
| Notes | https://ClinicalTrials.gov/show/NCT01638026 |
NCT01790282 2013.
| Trial name or title | Is Adding E2 to P4 Luteal Support In High Responder Long Gn‐RH Agonist ICSI Cycles Detrimental to Outcome? |
| Methods | RCT |
| Participants | Inclusion criteria: age<40 years, first ICSI cycle, third day FSH< 10 mIU/mL, serum E2 level on day of hCG administration <4,000 pg/mL, number of ova obtained >15 Exclusion Criteria: age 40 years or more, basal FSH 10 mIU/mL or more, eggs retrieved 15 or less, E2 level on day of hCG administration 4000 or more pg/ mL or more, repeat ICSI , need for PGD, presence of myoma, hydrosalpinx (unless disconnected) |
| Interventions | Estradiole ‐ progesterone arm: estradile valaerate 2mg plus progesterone 100 mg/day support arm :E2 valerate 2mg three times /day are given to the arm cases plus P4 100 IM/day for 14 days starting on day of ovum pickup and single IM injection of 0.1 mg decapeptyl on day of ET Progesterone only arm: Starting on day of ovum pickup ICSI cases are given prontogest 100 mg IM /day plus single dose dose of treptorline 0.1mg is given sc on day of embryo transfer |
| Outcomes | Primary Outcome Measures: cycle pregnancy rate, pregnancy rate per started cycle Secondary Outcome Measures: implantation rate, multiple pregnancy rate, ongoing pregnancy rate ,live birth rate, implantation rate, multiple pregnancy rate, abortion rate |
| Starting date | 2013 |
| Contact information | Mohamad E GHanem, MD Mansoura Integrated Fertility Center Email: meghanem87@gmail.com Contact telephone: 00201223366955 |
| Notes | http://clinicaltrials.gov/show/NCT01790282 |
NCT01850030.
| Trial name or title | A Double‐Blind, Double‐Dummy, Randomized, Two‐arm, Multicenter Study Comparing the Efficacy, Safety and Tolerability of Oral Dydrogesterone 30 mg Daily Versus Intravaginal Micronized Progesterone Capsules 600 mg Daily for Luteal Support in In‐Vitro Fertilization (Lotus I) |
| Methods | RCT |
| Participants | Infertile women undergoing IVF |
| Interventions | Intervention 1: Oral Dydrogesterone 10 mg tablets tid, Placebo intravaginal micronized progesterone 200 mg capsules tid Intervention 2: Intravaginal micronized progesterone 200 mg capsules tid, placebo oral dydrogesterone 10 mg tablets tid |
| Outcomes | Primary Outcome Measures: Pregnancy Rate Secondary Outcome Measures: Positive Pregnancy test rate, Rate of successful completion of pregnancy, Adverse Events, Status newborn ‐ The gender, APGAR score, height, weight and head circumference, physical examination and any malformations of the newborn(s) will be recorded, Adverse Events At Study Completion (about 10 months after IVF) |
| Starting date | 2015 |
| Contact information | Darline Cheatham‐Seitz, MD, PhD Abbott |
| Notes | Sponsors: Abbott, Quintiles https://ClinicalTrials.gov/show/NCT01850030 |
NCT01863680 2013.
| Trial name or title | Open‐label, Single‐arm, Multicenter Phase III Trial to Evaluate the Efficacy and Safety of COL‐1620 8% Vaginal Progesterone Gel for Luteal Phase Support in In‐vitro Fertilization and Embryo Transfer (IVF/ET) Cycles in Japanese Women |
| Methods | RCT |
| Participants | Infertile women undergoing IVF |
| Interventions | COL‐1620 vaginal progesterone gel (1.125 grams of progesterone gel containing 90 milligram that is 8% gel) will be administered by the vaginal route once daily, from the day of ovum pick‐up (OPU) until Week 12, or until the confirmation of miscarriage or extra‐uterine pregnancy. |
| Outcomes | Primary Outcome Measures: Percentage of subjects with Clinical pregnancy per Embryo Transfer Secondary Outcome Measures: Percentage of subjects with Biochemical pregnancy per Embryo Transfer, Serum progesterone level |
| Starting date | 2013 |
| Contact information | Unknown |
| Notes | Sponsorship: Merck KGaA Based in Japan http://clinicaltrials.gov/show/NCT01863680 |
NCT01980680 2013.
| Trial name or title | The Exogenous Progesterone Free Luteal Phase After GnRHa Trigger ‐ a Randomized Controlled Pilot Study in Normo‐responder IVF Patients |
| Methods | RCT |
| Participants | Inclusion Criteria: Age between 20 and 40, Normal menstrual cycles: 25‐34 days Oligomenorrhea/amenorrhea or polycystic syndrome (defined according to the Rotterdam criteria 2004), BMI >18 and <35 kg/m2 Exclusion Criteria: Patients with >14 follicles on day of trigger, Previous hyperresponse with OHSS development, Previous low response (less than 3 oocytes on a high dose of FSH stimulation), Endocrine disorders |
| Interventions | Intervention: Agonist trigger Buserelin 0,5 mg and Pregnyl (hCG) Control: hCG trigger Pregnyl (hCG) and Progesterone and Estradiol |
| Outcomes | Primary Outcome Measures: Ongoing pregnancy rate per patient |
| Starting date | 2013 |
| Contact information | Peter S Humaidan, MD Email: peter.humaidan@sygehusviborg.dk Contact telephone: +45 89 27 40 13 |
| Notes | http://clinicaltrials.gov/show/NCT01980680 |
NCT02053779 2014.
| Trial name or title | The Impact of a Single Dose of GnRH Agonist (Triptorelin 0,1 mg) at the Time of Implantation on the Reproductive Outcome in IVF Cycles Triggered by a GnRH Agonist Followed by a Small Bolus of HCG the Day of Oocyte Retrieval |
| Methods | RCT |
| Participants | Inclusion Criteria: Female age < 40 years, Baseline FSH and LH < 12 IU/l, Body Mass Index > 18 and < 35 kg/m2, No uterine (fibroids, mullerian malformations), ovarian ( endometrioma) or adnexa (hydrosalpinx) abnormalities, Patients with at least one embryo at transfer time Exclusion Criteria: Very high risk of OHSS (> 30 follicles > 12 mm the day of ovulation triggering), Reduced ovarian reserve, Fertilization failure, Severe endocrinopathy, Azoospermia |
| Interventions | Intervention: Triptorelin 0.1 mg administered subcutaneously 6 days after ovum pick‐up (OPU) in IVF/ICSI cycles triggered by triptorelin 0.2 mg followed by hCG 1500 iu the day of OPU. Control: Placebo (1 ml Nacl 0.9% solution) administered subcutaneously 6 days after ovum pick‐up (OPU) in IVF/ICSI cycles triggered by triptorelin 0.2 mg followed by hCG 1500 iu the day of OPU. |
| Outcomes | Primary Outcome Measures: implantation rate, number of gestational sacs per number of embryos transferred Secondary Outcome Measures: chemical pregnancy, confirmed by beta‐hCG 14 days post embryo transfer, clinical pregnancy, appearance of yolk sac with foetal heart beat at 7 weeks of gestation, live birth, birth of baby beyond 28 weeks of gestation Other Outcome Measures: ovarian hyperstimulation syndrome OHSS |
| Starting date | 2014 |
| Contact information | Dr Abdelhamid benmachiche
Ibn roch infertility centre, cité boussouf, Constantine Algeria Email: benmachiche@gmail.com Contact telepgone: 00213773112786 |
| Notes | http://clinicaltrials.gov/show/NCT02053779 |
NCT02114645 2014.
| Trial name or title | To Evaluate the Effect of GnRH Agonist Administered in the Luteal Phase on ART Cycle Outcomes in Both GnRH Agonist and GnRH Antagonist Treated Ovarian Stimulation Protocols |
| Methods | RCT crossover |
| Participants | Inclusion Criteria: Couples undergoing ART with their own gametes, Couples having at least one good embryo available for transfer, Normoresponder, Infertility etiology is unexplained, ovulation triggered by intramuscular injection of 10000 IU of HCG Exclusion Criteria: Patients older than 38 years old, High and poor responder patients |
| Interventions | Intervention 1: Long GnRH agonist protocol, Luteal Phase Support: Vaginal progesterone+oral estradiol valerate subcutaneous 0.5mg leuprolide acetate fifth and tenth day after embryo transfer Control 1: Long GnRH agonist protocol, Luteal Phase Support: Vaginal progesterone + 4mg oral estradiol valerate Intervention 2: GnRH antagonist protocol, Luteal Phase Support: Vaginal progesterone + 4mg oral estradiol valerate + subcutaneous 0.5mg leuprolide acetate fifth and tenth day after embryo transfer Control 2: GnRH antagonist protocol, Luteal Phase Support: Vaginal progesterone + 4mg oral estradiol valerate |
| Outcomes | Primary Outcome Measures: Live Birth Rate Secondary Outcome Measures: Ongoing pregnancy, miscarriage, OHSS |
| Starting date | 2014 |
| Contact information | Nagihan Cengaver, MD Zekai Tahir Burak Women's Health Research and Education Hospital Email: nagihancengaver@gmail.com Contact telephone: +905556309298 |
| Notes | http://clinicaltrials.gov/show/NCT02114645 |
NCT02262416 2014.
| Trial name or title | A Prospective Randomised Controlled Trial of GnRH Agonist and Progesterone Versus Progesterone Only for Luteal Phase Support in Antagonist Cycles |
| Methods | RCT |
| Participants | Inclusion Criteria: Single embryo transfer, Antagonist cycle with HCG trigger, Use of progesterone as luteal phase support (crinone or progesterone pessary), Women undergoing their first IVF cycle with TFC, Age 18‐42 inclusive Exclusion Criteria: No or frozen embryo transfer planned, Use of other luteal support, Known contraindication to the use of GnRH analogue |
| Interventions | Intervention: 0.5mg Leuprolide acetate injection Control: Normal saline of equivalent volume |
| Outcomes | Primary Outcome Measures: live birth, ongoing pregnancy Secondary Outcome Measures: pregnancy, ovarian hyperstimulation syndrome |
| Starting date | 2014 |
| Contact information | Queensland Fertility Group, Brisbane, Queensland, Australia, 4000 |
| Notes | http://clinicaltrials.gov/show/NCT02262416 |
NCT02312076 2014.
| Trial name or title | Gonadotropin Releasing Hormone Agonist for Luteal Phase Support in Long Gonadotropin Releasing Hormone Agonist Protocol Cycles |
| Methods | RCT |
| Participants | Inclusion Criteria: Women subjected to ICSI through controlled ovarian hyperstimulation (COH) with pituitary downregulation by GnRHa. Exclusion Criteria: Moderate or severe endometriosis, Hydrosalpinx, Uterine abnormalities, Myoma, Previous uterine surgery. |
| Interventions | Intervention: Luteal phase support will be continued by the same regimen started on the day of oocytes retrieval until 2 weeks after embryo transfer (ET) with subcutaneous administration of a single dose (0.2 mg) of GnRHa (Triptorelin) 6 days after oocyte retrieval Control: No GnRHa administration in luteal phase |
| Outcomes | Primary Outcome Measures: Clinical pregnancy rate, Number of clinical pregnancies Secondary Outcome Measures: Implantation rate, Miscarriage rate |
| Starting date | 2014 |
| Contact information | Dr Mohamed S Abdelhafez Mansoura University Email: msabdelhafez@gmail.com Contact telephone: +201124442800 |
| Notes | http://clinicaltrials.gov/show/NCT02312076 |
NCT02312089 2014.
| Trial name or title | Gonadotropin Releasing Hormone Agonist for Luteal Phase Support in Gonadotropin Releasing Hormone Antagonist Protocol Cycles |
| Methods | RCT |
| Participants | Inclusion Criteria: Women subjected to ICSI through controlled ovarian hyperstimulation (COH) with pituitary downregulation by GnRH antagonist. Exclusion Criteria: Moderate or severe endometriosis, Hydrosalpinx, Uterine abnormalities, Myoma, Previous uterine surgery. |
| Interventions | Intervention: Luteal phase support will be continued by the same regimen started on the day of oocytes retrieval until 2 weeks after embryo transfer (ET) with subcutaneous administration of a single dose (0.2 mg) of GnRHa (Triptorelin) 6 days after oocyte retrieval Control: No GnRHa administration in luteal phase |
| Outcomes | Primary Outcome Measures: Clinical pregnancy rate, Number of clinical pregnancies Secondary Outcome Measures: Implantation rate, Miscarriage rate |
| Starting date | 2014 |
| Contact information | Dr Mohamed S Abdelhafez Mansoura University Email: msabdelhafez@gmail.com Contact telephone: +201124442800 |
| Notes | http://clinicaltrials.gov/show/NCT02312089 |
NCT02316626 2014.
| Trial name or title | Subcutaneous Progesterone Versus Vaginal Progesterone Gel for Luteal Phase Support in Gonadotropin Ovarian Stimulation for Intrauterine Insemination: a Pilot Randomized Controlled Study |
| Methods | RCT |
| Participants | Inclusion Criteria: <38 years of age with either primary or secondary infertility for at least 1 years; body mass index between 19 and 30 kg/m2; Day 2 serum FSH <15 IU/ml; normal serum prolactin level; normal uterine cavity on hysterosalpingography or hysteroscopy. Exclusion Criteria: female partners with previous ovarian surgery, one ovary, polycystic ovaries on ultrasound examination, other endocrine abnormalities (i.e., polycystic ovarian syndrome, thyroid disorders, hyperprolactinemia, hypogonadotropic hypogonadism), diminished ovarian reserve (basal FSH level >15 IU/mL), or age of >38 years |
| Interventions | Intervention: Luteal phase support cycles will involve once‐daily administration of 25 mg of SC P from the day after insemination for 14 days. Control: Luteal phase support cycles will involve once‐daily administration of 90 mg vaginal gel from the day after insemination for 14 days. |
| Outcomes | Primary Outcome Measures: Clinical pregnancy Secondary Outcome Measures: Side effects |
| Starting date | 2014 |
| Contact information | Fulvio Zullo, MD, PhD Magna Graecia University of Catanzaro; Email: zullo@unicz.it Contact telephone: 00390961883234 |
| Notes | http://clinicaltrials.gov/show/NCT02316626 |
NCT02357654 2015.
| Trial name or title | GnRH for Luteal Support in IVF/ICSI/FET Cycles |
| Methods | RCT |
| Participants | Inclusion Criteria: women undergoing IVF/ICSI or frozen embryo transfers (FET) that less than 40 years old. Exclusion Criteria: day 3 transfers |
| Interventions | Intervention: GnRH agonist Control: placebo |
| Outcomes | Primary Outcome Measure: Live birth per transfer Secondary Outcome Measure: Implantation rates, clinical pregnancy, rates of OHSS |
| Starting date | 2015 |
| Contact information | Peter G McGovern, MD University Reproductive Associates Email: mcgovepg@gmail.com Contact telephone: 201‐288‐6330 |
| Notes | https://ClinicalTrials.gov/show/NCT02357654 |
NCT02491437.
| Trial name or title | A Randomized, Open‐label, Two‐arm, Multicenter Study Comparing the Efficacy, Safety and Tolerability of Oral Dydrogesterone 30 mg Daily Versus Crinone 8% Intravaginal Progesterone Gel 90 mg Daily for Luteal Support in In‐Vitro Fertilization (LOTUS II) |
| Methods | RCT |
| Participants | Infertile women undergoing IVF |
| Interventions | Intervention: Dydrogesterone tablets 3x10 mg Control: Crinone 8% intravaginal progesterone gel 90 mg |
| Outcomes | Primary Outcome Measures: Pregnancy rate Secondary Outcome Measures:Positive Pregnancy test rate, Rate of successful completion of pregnancy, Incidence of live births and healthy newborns Adverse Events, physical examination newborn |
| Starting date | 2015 |
| Contact information | Erik van Leeuwen, MSc The First Affiliated Hospital of Nanjing Medical University Email: erik.vanleeuwen@abbott.com Contact telephone: +31294479241 |
| Notes | Sponsorship: Abbott, PRA Health Sciences, Datamap https://ClinicalTrials.gov/show/NCT02491437 |
Differences between protocol and review
1. Objective.
We changed the objective from "To determine the effectiveness and safety of luteal phase support in subfertile women undergoing assisted reproductive technology" to "To determine the relative effectiveness and safety of methods of luteal phase support provided to subfertile women undergoing assisted reproduction". We made this change because we investigated not only the use of luteal phase support but also the different ways by which luteal phase support is delivered.
2. Inclusion criteria.
In the protocol, we stated that we would exclude studies using any other substance in the luteal phase than progesterone, hCG or GnRH agonists. We found one study investigating LH instead of hCG (Geber 2007). Because LH is very similar to hCG, we decided to include this study in the comparison of progesterone versus progesterone + hCG. We also decided to delete the exclusion criterion "use of other substances for luteal phase support than progesterone, hCG or oestrogen". This means that in the future we will be able to include new agents.
3. Exclusion criteria.
In the 2015 update, we have added luteal phase support after intrauterine insemination cycles as an exclusion criterion, as we believe this is based on a different physiological process.
4. Effect estimate.
In the 2015 update, we used Mantel‐Haenszel odds ratios rather than Peto odds ratios for the main analysis, as this is recommended (in the Cochrane Handbook for Systematic Reviews of Interventions) as an option for default unless events are very rare.
5. Outcomes.
In the 2015 update, we combined live birth and ongoing pregnancy as our primary outcomes to improve the power of this analysis. We conducted a sensitivity analysis that included only studies that reported live birth to determine how use of a combined outcome influenced review findings. Sensitivity analyses limited to studies reporting live birth yielded findings very similar to the combined outcome, suggesting that ongoing pregnancy was a reasonable surrogate for live birth in this review.
6. Comparisons.
We stated 10 comparisons in the protocol, namely:
progesterone versus placebo or no treatment;
progesterone versus hCG;
progesterone versus progesterone and hCG;
progesterone versus progesterone and oestrogen;
progesterone versus progesterone and GnRH agonist;
different methods of administration of progesterone: IM versus vaginal versus rectal versus oral;
micronised versus synthetic progesterone;
hCG versus placebo or no treatment;
urinary versus recombinant hCG; and
single‐dose GnRH agonist versus placebo.
We changed these to:
hCG versus placebo or no treatment;
progesterone versus placebo or no treatment;
-
progesterone versus hCG regimens:
Progesterone versus hCG.
Progesterone versus progesterone and hCG.
-
progesterone versus progesterone and oestrogen.
Oral oestrogen.
Transdermal oestrogen.
Vaginal oestrogen.
Oral and transdermal oestrogen.
-
progesterone versus progesterone and GnRH agonist.
Single dose.
Multiple doses.
-
progesterone regimens.
IM progesterone versus oral progesterone.
IM progesterone versus vaginal or rectal progesterone.
Vaginal or rectal progesterone versus oral progesterone.
Low‐dose vaginal progesterone (≤ 100 mg) versus high‐dose vaginal progesterone (> 100 mg).
Short protocol versus long protocol.
Micronised progesterone versus synthetic progesterone.
Vaginal ring versus vaginal gel.
Subcutaneous versus vaginal gel.
Vaginal progesterone versus rectal progesterone.
-
progesterone + oestrogen regimens.
Short protocol versus long protocol.
Low dose oestrogen (≤ 2 mg) versus high dose oestrogen (> 2 mg).
To keep things clear, we split comparison six in the protocol into three different subgroups but combined vaginal and rectal administration of progesterone. After our search, we found a large number of studies that researched different types and dosages of vaginal progesterone administration. Therefore we added comparison 6d: low‐dose vaginal progesterone versus high‐dose vaginal progesterone. We also found some studies that compared different durations of progesterone administration, which we included in comparison 6e: short protocol progesterone versus long protocol progesterone.
In the update of van der Linden 2011, we found studies comparing a new vaginal progesterone ring versus vaginal gel, subcutaneous progesterone versus vaginal gel and vaginal versus rectal progesterone. So we added comparisons 6g, 6h and 6i.
We found no studies comparing urinary hCG and recombinant hCG, and no studies comparing only single‐dose GnRH agonist versus placebo, but we did come across some studies that used multiple doses of a GnRH agonist. Therefore we included these in comparison five, changing 'single‐dose GnRH agonist' to 'GnRH agonist'. It is unlikely that comparisons for urinary hCG versus recombinant hCG and GnRH agonist versus placebo will be made in the future, as hCG is an older method of providing luteal phase support and is known for its high risk of OHSS; we do not expect new trials will be conducted to investigate differences between urinary and recombinant hCG. Nowadays, progesterone is an accepted method of providing luteal phase support, and it is considered unethical to not provide any form of luteal phase support. Therefore we do not expect that new trials will investigate the effects of GnRH agonists in providing luteal phase support versus placebo. For these reasons, we chose to remove these comparisons.
We believe that these changes in the comparisons enabled us to present an overview of luteal phase support in assisted reproduction cycles that is as complete as possible.
7. Sensitivity analyses.
In the 2015 update, we added sensitivity analyses for choice of effect estimate and statistical model to determine whether these choices influenced our findings. We discontinued the sensitivity analysis that excluded outliers, as this is a data‐driven approach that is not recommended best practice.
Contributions of authors
MvdL, MM and KB extracted data. MvdL entered data and wrote the review and the update. CF acted as a third review author in cases of disagreement, helped draft the review, acted as a clinical expert and commented on the review and the update. JK acted as a clinical expert and commented on the review and the update.
Sources of support
Internal sources
MDSG, Other.
External sources
-
Stichting Nijmeegs Universiteitsfonds, Netherlands.
Scholarship to support students from the Radboud University Nijmegen to study, do an internship or conduct research abroad.
-
Commissie Voorzieningen Studenten Budget (CVSB), Netherlands.
Grant to subsidise activities of (medical) student organisation and foreign internships of individual students from the medical faculty of the Radboud University Nijmegen.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Abate 1999 {published data only}
- Abate A, Brigandi A, Abate FG, Manti F, Unfer V, Perino M. Luteal phase support with 17alpha‐hydroxyprogesterone versus unsupported cycles in in vitro fertilization: a comparative randomized study. Gynaecologic and Obstetric Investigation 1999;48(2):78‐80. [DOI] [PubMed] [Google Scholar]
- Abate A, Brigandi A, Costabile L, Abate FG, Balzano E, Perino M. 17‐alpha‐Hydroxyprogesterone caproate and natural progesterone in assisted reproduction: a comparative study. Clinical and Experimental Obstetrics & Gynecology 1997;24(4):190‐2. [PubMed] [Google Scholar]
Abate 1999a {published data only}
- Abate A, Perino M, Abate FG, Brigandi A, Costabile L, Manti F. Intramuscular versus vaginal administration of progesterone for luteal phase support after in vitro fertilization and embryo transfer. A comparative randomized study. Clinical and Experimental Obstetrics and Gynecology 1999;26:203‐6. [PubMed] [Google Scholar]
Aboulghar 2008 {published data only}
- Aboulghar MA, Amin YM, Al‐Inany HG, Aboulghar MM, Mourad LM, Serour GI, et al. Prospective randomized study comparing luteal phase support for ICSI patients up to the first ultrasound compared with an additional three weeks. Human Reproduction 2008;23(4):857‐62. [DOI] [PubMed] [Google Scholar]
Aboulghar 2015 {published data only}
- Aboulghar, Mohamed A, Marie, Heba, Amin, Yahia M, Aboulghar, Mona M, Nasr, Ahmed, Serour, Gamal I, Mansour, Ragaa T. GnRH agonist plus vaginal progesterone for luteal phase support in ICSI cycles: a randomized study.. Reproductive Biomedicine Online 2015;30:52‐56. [DOI] [PubMed] [Google Scholar]
Aghahosseini 2011 {published data only}
- Aghahosseini M, Aleyassin A, Khodaverdi S, Esfahani F, Mohammadbeigi R, Movahedi S, et al. Estradiol supplementation during the luteal phase in poor responder patients undergoing in vitro fertilization: a randomized clinical trial. Journal of Assisted Reproduction and Genetics 2011;28:785‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aghsa 2012 {published data only}
- Aghsa MM, Rahmanpour H, Bagheri M, Davari‐Tanha F, Nasr R. A randomized comparison of the efficacy, side effects and patient convenience between vaginal and rectal administration of Cyclogest when used for luteal phase support in ICSI treatment. Archives of Gynecology and Obstetrics 2012;286:1049‐54. [DOI] [PubMed] [Google Scholar]
Albert 1991 {published data only}
- Albert J, Pfeifer S. Luteal phase hormone levels after in vitro fertilization and embryo transfer (IVF‐ET): a prospective randomized trial of human chorionic gonadotropin (hCG) vs. intramuscular (im) progesterone (P) for luteal phase support following stimulation with gonadotropin‐releasing hormone agonist (GnRH‐a) and human menopausal gonadotropins (hMG) [abstract]. Fertility and Sterility. 1991:S18 (Abs # O‐041).
Artini 1995 {published data only}
- Artini PG, Volpe A, Angioni S, Galassi MC, Battaglia C, Genazzani AR. A comparative, randomized study of three different progesterone support of the luteal phase following IVF/ET program. Journal of Endocrinological Investigation 1995;18:51‐6. [DOI] [PubMed] [Google Scholar]
Ata 2008 {published data only}
- Ata B, Yakin K, Balaban B, Urman B. GnRH agonist protocol administration in the luteal phase in ICSI‐ET cycles stimulated with the long GnRH agonist protocol: a randomized, controlled double blind study. Human Reproduction 2008;23(3):668‐73. [DOI] [PubMed] [Google Scholar]
- Urman B. Single dose gonadotropin‐releasing hormone (GnRH) agonist administration in the luteal phase of GnRH antagonist stimulated ICSI‐ET cycles. Directly obtained from author, 17 March 2011. [Clinicaltrials.gov: NCT01007851]
Ata 2010 {published and unpublished data}
- Ata B, Kucuk M, Seyhan A, Urman B. Effect of high‐dose estrogen in luteal phase support on live birth rates after assisted reproduction treatment cycles. Journal of Reproductive Medicine for the Obstetrician and Gynecologist 2010;55:485‐90. [PubMed] [Google Scholar]
Baker 2014 {published and unpublished data}
- Baker VL, Jones CA, Doody K, Foulk R, Yee B, Adamson GD, et al. A randomized, controlled trial comparing the efficacy and safety of aqueous subcutaneous progesterone with vaginal progesterone for luteal phase support of in vitro fertilization. Human Reproduction 2014;29(10):12‐20. [DOI: 10.1093/humrep/deu194] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beckers 2000 {published data only}
- Beckers NGM, Laven JSE, Eijkemans MJC, Fauser BCJM. Follicular and luteal phase characteristics following early cessation of gonadotrophin‐releasing hormone agonist during ovarian stimulation for in‐vitro fertilization. Human Reproduction 2000;15(1):43‐9. [DOI] [PubMed] [Google Scholar]
Belaisch‐Allart 1987 {published data only}
- Belaisch‐Allart J, Testart J, Fries N, Forman R G, Frydman R. The effect of dydrogesterone supplementation in an IVF programme. Human Reproduction 1987;2:183‐5. [DOI] [PubMed] [Google Scholar]
- Belaisch‐Allart J, Testart J, Fries N, Forman R, Hazout A, Declerc l, et al. The effect of dydrogesterone and hCG supplementation in an IVF program [abstract]. 5th World Congress on In Vitro Fertilization and Embryo Transfer Abstract Book. 1987:41 (Abs #PP‐35).
Belaisch‐Allart 1990 {published data only}
- Belaisch‐Allart J, Mouzon J, Lapousterle C, Mayer M. The effect of HCG supplementation after combined GnRH agonist/HMG treatment in an IVF programme. Human Reproduction 1990;5:163‐6. [DOI] [PubMed] [Google Scholar]
Beltsos 2011 {published data only}
- Beltsos A, Sanchez M, Doody K, Bush M, Scobey J. Efficacy of vaginal progesterone inserts (Endometrin®) compared to intramuscular progesterone in oil for luteal support in PCOS patients. Fertility and Sterility 2011;96(Suppl):S130 (Abs # P‐71). [Google Scholar]
Bergh 2012 {published data only}
- Bergh C, Lindenberg S. A prospective randomized multicentre study comparing vaginal progesterone gel and vaginal micronized progesterone tablets for luteal support after in vitro fertilization/intracytoplasmic sperm injection. Human Reproduction 2012;27(12):3467‐73. [DOI] [PubMed] [Google Scholar]
Brigante 2013 {published data only}
- Brigante CMM, Mignini Renzini M, Dal Canto M, Coticchio G, Comi R, Fadini R. Efficacy of luteal phase support with GnRH agonists: a preliminary comparative study. Fertility and Sterility 2013;100(Suppl):S299 (Abs # P‐521). [Google Scholar]
Caligara 2007 {published data only}
- Caligara C, Carranza F, Ramos J, Rodriguez I, Gonzalez A, Fernandez‐Sanchez M. Luteal phase support in IVF patients at low risk for OHSS: progesterone vs. progesterone plus HCG. A prospective randomized study. Fertility and Sterility 2007;88:S163 (Abs#P‐166). [Google Scholar]
Ceyhan 2008 {published data only}
- Ceyhan ST, Basaran M, Kemal Duru N, Yilmaz A, Goktolga U, Baser I. Use of luteal estrogen supplementation in normal responder patients treated with fixed multidose GnRH antagonist: a prospective randomized controlled study. Fertility and Sterility 2008;89(6):1827‐30. [DOI] [PubMed] [Google Scholar]
Chakravarty 2005 {published data only}
- Chakravarty BN, Shirazee HH, Dam P, Chattopadhyay R, Ghosh S, Dam M, Goswami SK. A randomized prospective study comparing dydrogesterone and intravaginal micronised progesterone as luteal phase support in ART cycle [abstract]. Human Reproduction. 2004; Vol. 19:i126 (Abs #P‐364).
- Chakravarty BN, Shirazee HH, Dam P, Goswami SK, Chatterjee R, Ghosh S. Oral dydrogesterone versus intravaginal micronised progesterone as luteal phase support in assisted reproductive technology (ART) cycles: results of a randomised study. Journal of Steroid Biochemistry and Molecular Biology 2005;97(5):416‐20. [DOI] [PubMed] [Google Scholar]
Colakoglu 2011 {published data only}
- Colakoglu M, Toy H, Icen MS, Vural M, Mahmoud AS, Yazici F. The impact of estrogen supplementation on IVF outcome in patients with polycystic ovary syndrome. Human Reproduction 2011;26(Suppl 1):i296 (Abs # P‐450). [Google Scholar]
Colwell 1991 {published data only}
- Colwell KA, Tummon IS. Elevation of serum progesterone with oral micronized progesterone after in vitro fertilization. Journal of Reproductive Medicine 1991;36:170‐2. [PubMed] [Google Scholar]
Dal Prato 2008 {published data only}
- Dal Prato L, Bianchi L, Cattoli M, Tarozzi N, Flamigni C, Borini A. Vaginal gel versus intramuscular progesterone for luteal phase supplementation: a prospective randomized trial. Reproductive BioMedicine Online 2008;16(3):361‐7. [DOI] [PubMed] [Google Scholar]
- Dal Prato L, Borini A, Bonu MA, Maccolini A, Cattoli M, Flamigni C. Luteal support in IVF: i.m. versus intravaginal progesterone. Human Reproduction. 2004 Suppl 1; Vol. 19:i69‐70 (Abs #O‐198).
Doody 2009 {published data only}
- Doody KJ, Schnell VL, Foulk RA, Miller CE, Kolb BA, Blake EJ, et al. Endometrin for luteal phase support in a randomized, controlled, open‐label, prospective in‐vitro fertilization trial using a combination of Menopur and Bravelle for controlled ovarian hyperstimulation. Fertility and Sterility 2009;91(4):1012‐7. [DOI] [PubMed] [Google Scholar]
Drakakis 2007 {published data only}
- Drakakis P, Loutradis D, Vomvolaki E, Stefanidis K, Kiapekou E, Anagnostou E, et al. Luteal estrogen supplementation in stimulated cycles may improve the pregnancy rate in patients undergoing in vitro fertilization/intracytoplasmic sperm injection‐embryo transfer. Gynecological Endocrinology 2007;23(11):645‐52. [DOI] [PubMed] [Google Scholar]
Dunstone 1999 {published data only}
- Dunstone T, Zosmer A, Hussain S, Tozer A, Paney N, Wilson C, et al. A comparison between Cyclogest pessaries and Crinone gel as luteal support in IVF‐ET cycles [abstract]. British Fertility Society Annual Meeting Abstract Book. 1999:62 (Abs # FC21).
Elgindy 2010 {published data only}
- Elgindy EA, El‐Haieg DO. Does luteal estradiol supplementation have a role in long agonist cycles? Does luteal estradiol supplementation have a role in long agonist cycles? [abstract]. Fertility and Sterility. 2010; Vol. 88, issue Suppl 1:164 (Abs #168). [DOI] [PubMed]
- Elgindy EA, El‐Haieg DO, Mostafa MI, Shafiek M. Does luteal estradiol supplementation have a role in long agonist cycles?. Fertility and Sterility 2010;93(7):2182‐8. [DOI] [PubMed] [Google Scholar]
Engmann 2008 {published data only}
- Engmann L, DiLuigi A, Schmidt D, Benadiva C, Maier D, Nulsen J. The effect of luteal phase vaginal estradiol supplementation on the success of in vitro fertilization treatment: a prospective randomized study. Fertility and Sterility 2008;89(3):554‐61. [DOI] [PubMed] [Google Scholar]
Erdem 2013 {published data only}
- Erdem M, Kutlusoy F, Erdem A, Guler I, Mesut O, Biberoglu K. Luteal phase support with estrogen in addition to progesterone in patients with poor response to gonadotropins undergoing IVF. Fertility and Sterility 2013;100(Suppl 1):S300‐1 (Abs # P‐526). [Google Scholar]
Fatemi 2006 {published data only}
- Fatemi HM, Kolibianakis EM, Camus M, Tournaye H, Donoso P, Papanikolaou E, et al. Addition of estradiol to progesterone for luteal supplementation in patients stimulated with GnRH antagonist/rFSH for IVF: a randomized controlled trial. Human Reproduction 2006;21(10):2628‐32. [DOI] [PubMed] [Google Scholar]
- Fatemi HM, Kolibianakis EM, Camus M, Tournaye H, Steirteghem A, Devroey P. Progesterone versus progesterone combined with estradiol as luteal support in cycles stimulated with GnRH antagonist/rec‐FSH for IVF: a randomized clinical trial [abstract]. Fertility and Sterility. 2005; Vol. 84, issue Suppl 1:s322 (Abs #P‐475).
Feichtinger 2011 {published data only}
- Feichtinger M, Hejek J, Kemter P, Feichtinger W. Effect of luteal phase support comparing early (day 1) and late (day 4) initiation with pregnancy rates. Journal of Reproductive Medicine and Endocrinology 2011;8(4):288‐90. [Google Scholar]
Friedler 1999 {published data only}
- Friedler S, Raziel A, Schachter M, Cohen O, Yaron M, Tartakovsky L, et al. Characteristics of conceptional and non‐conceptional cycles after IVF using micronized progesterone for luteal support: a comparative study of vaginal or oral administration [abstract]. Abstract Book 1. 1998; Vol. 13, issue Abstract Book 1:161 (Abs # P‐063).
- Friedler S, Raziel A, Schachter M, Strassburger D, Bukovsky I, Ron‐El R. Luteal support with micronized progesterone following in‐vitro fertilization using a down‐regulation protocol with gonadotrophin‐releasing hormone agonist: a comparative study between vaginal and oral administration. Human Reproduction 1999;14(8):1944‐8. [DOI] [PubMed] [Google Scholar]
Fujimoto 2002 {published data only}
- Fujimoto A, Osuga Y, Fujiwara T, Yano T, Tsutsumi O, Momoeda M, et al. Human chorionic gonadotropin combined with progesterone for luteal support improves pregnancy rate in patients with low late‐midluteal estradiol levels in IVF cycles. Journal of Assisted Reproduction and Genetics 2002;19(12):550‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto A, Osuga Y, Ooi N, Fujiwara T, Yano T, Taketani Y. Addition of hCG to progesterone as a luteal support improves pregnancy rates for patients with low mid‐luteal oestradiol levels in IVF and ICSI [abstract]. Human Reproduction. 2001; Vol. 16, issue Suppl 1:143 (Abs #P‐143).
Ganesh 2011 {published data only}
- Ganesh A, Chakravorty N, Mukherjee R, Goswami S, Chaudhury K, Chakravarty B. Comparison of oral dydrogesterone with progesterone gel and micronized progesterone for luteal support in 1,373 women undergoing in vitro fertilization: a randomized clinical study. Fertility and Sterility 2011;95(6):1961‐5. [DOI: 10.1016/j.fertnstert.2011.01.148] [DOI] [PubMed] [Google Scholar]
Geber 2007 {published data only}
- Geber S, Maia L, Lauar I, Valle MP, Sampaio AC. Does recombinant LH combined to progesterone for luteal phase interfere in the outcome of assisted reproduction technique cycles? [abstract]. Fertility and Sterility 2007;88 Suppl 1:25 (Abs #66). [Google Scholar]
Geber 2007a {published data only}
- Geber S, Moreira ACF, Paula SOC, Sampaio M. Comparison of two different vaginal progesterone for luteal phase support in cycles of assisted reproduction. Jornal Brasileiro de Reproducao Assistida 2006;10(1):17‐21. [Google Scholar]
- Geber S, Moreira ACF, Paula SOC, Sampaio M. Comparison between two forms of vaginally administered progesterone for luteal phase support in assisted reproduction cycles. Reproductive BioMedicine Online 2007;14(2):155‐8. [DOI] [PubMed] [Google Scholar]
Geusa 2001 {published data only}
- Geusa S, Causio F, Marinaccio M, Stanziano A, Sarcina E. Luteal phase support with progesterone in IVF/ET cycles: a prospective, randomized study comparing vaginal and intramuscular administration [abstract]. Human Reproduction 2001;16 Suppl 1:145 (Abs # P‐111). [Google Scholar]
Golan 1993 {published data only}
- Golan A, Herman A, Soffer Y, Bukovsky I, Caspi E, Ron‐El R. Human chorionic gonadotrophin is a better luteal support than progesterone in ultrashort gonadotrophin‐releasing hormone agonist/menotrophin in‐vitro fertilization cycles. Human Reproduction 1993;8:1372‐5. [DOI] [PubMed] [Google Scholar]
Gorkemli 2004 {published data only}
- Gorkemli H, Ak D, Akyurek C, Aktan M, Duman S. Comparison of pregnancy outcomes of progesterone or progesterone plus estradiol for luteal phase support in ICSI‐ET cycles. Gynecologic and Obstetric Investigation 2004;58(3):140‐4. [DOI] [PubMed] [Google Scholar]
Goudge 2010 {published data only}
- Goudge CS, Nagel TC, Damario MA. Duration of progesterone‐in‐oil support after in vitro fertilization and embryo transfer: a randomized, controlled trial. Fertility and Sterility 2010;94(3):946‐51. [DOI] [PubMed] [Google Scholar]
Humaidan 2006 {published data only}
- Humaidan P, Bungum L, Bungum M, Andersen CY. Rescue of corpus luteum function with peri‐ovulatory HCG supplementation in IVF/ICSI GnRH antagonist cycles in which ovulation was triggered with a GnRH agonist: a pilot study. Reproductive BioMedicine Online 2006;13(2):173‐8. [DOI] [PubMed] [Google Scholar]
Hurd 1996 {published data only}
- Hurd WW, Randolph JF Jr, Christman GM, Ansbacher R, Menge AC, Gell JS. Luteal support with both estrogen and progesterone after clomiphene citrate stimulation for in vitro fertilization. Fertility and Sterility 1996;66:587‐92. [DOI] [PubMed] [Google Scholar]
Inamdar 2012 {published data only}
- Inamdar DB, Majumdar A. Evaluation of the impact of gonadotropin‐releasing hormone agonist as an adjuvant in luteal‐phase support on IVF outcome. Journal of Human Reproductive Sciences 2012;5(3):279‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Isik 2009 {published data only}
- Isik AZ, Caglar GS, Sozen E, Akarsu C, Tuncay G, Ozbicer T, et al. Single‐dose GnRH agonist administration in the luteal phase of GnRH antagonist cycles: a prospective randomized study. Reproductive BioMedicine Online 2009;19(4):472‐7. [DOI] [PubMed] [Google Scholar]
Isikoglu 2007 {published data only}
- Isikoglu M, Ozgur K, Oehninger S. Extension of GnRH agonist through the luteal phase to improve the outcome of intracytoplasmic sperm injection. Journal of Reproductive Medicine 2007;52(7):639‐44. [PubMed] [Google Scholar]
Iwase 2008 {published data only}
- Iwase A, Ando H, Toda S, Ishimatsu S, Harata T, Kurotsuchi S, et al. Oral progestogen versus intramuscular progesterone for luteal support after assisted reproductive technology treatment: a prospective randomized study. Archives of Gynecology and Obstetrics 2008;277(4):319‐24. [DOI] [PubMed] [Google Scholar]
Kably Ambe 2005 {published data only}
- Kably Ambe A, Ruiz Anguas J, Walters Arballo F, García Benitez CQ, Karchmer Krivitsky S. Results' analysis of estradiol and progesterone supplementation in luteal phase vs progesterone alone in an assisted reproduction program. Ginecologia y Obstetricia de Mexico 2005;73:173‐82. [PubMed] [Google Scholar]
Kleinstein 2005 {published data only}
- Kleinstein J. Efficacy and tolerability of vaginal progesterone capsules (Utrogest 200) compared with progesterone gel (Crinone 8%) for luteal phase support during assisted reproduction. Fertility and Sterility 2005;83(6):1641‐9. [DOI] [PubMed] [Google Scholar]
- Kleinstein, J. Efficacy of Utrogest 200 and Crinone 8% for luteal phase support during ART: a comparative, multicenter study [Abstract]. Human Reproduction. 2004; Vol. 19:i123 (Abs #P‐357).
Kohls 2012 {published data only}
- Kohls G, Ruiz F, Martinez M, Hauzman E, Fuente G, Pellicer A, Garcia‐Velasco JA. Early progesterone cessation after in vitro fertilization/intracytoplasmic sperm injection: a randomized, controlled trial. Fertility and Sterility 2012;98:858‐62. [DOI] [PubMed] [Google Scholar]
- Kohls G, Ruiz FJ, Fuente G, Toribio M, Martinez M, Pellicer A, et al. Early progesterone cessation after in vitro fertilization. Human Reproduction 2010;25 Suppl 1(6):i249 Abstract no. P‐344. [Google Scholar]
Kupferminc 1990 {published data only}
- Kupferminc MJ, Lessing JB, Amit A, Yovel I, David MP, Peyser MR. A prospective randomized trial of human chorionic gonadotrophin or dydrogesterone support following in‐vitro fertilization and embryo transfer. Human Reproduction 1990;5(3):271‐3. [DOI] [PubMed] [Google Scholar]
Kyrou 2011 {published data only}
- Kyrou D, Fatemi HM, Zepiridis L, Riva A, Papanikolaou EG, Tarlatzis BC, Devroey P. Does cessation of progesterone supplementation during early pregnancy in patients treated with recFSH/GnRH antagonist affect ongoing pregnancy rates? A randomized controlled trial. Human Reproduction 2011;26(5):1020‐4. [DOI] [PubMed] [Google Scholar]
Lam 2008 {published data only}
- Lam PM, Cheung MC, Cheung LP, Lok HI, Haines CJ. Effects of early luteal‐phase vaginal progesterone supplementation on the outcome of in vitro fertilization and embryo transfer. Gynecological Endocrinology 2008;24(12):674‐80. [DOI] [PubMed] [Google Scholar]
Lewin 1994 {published data only}
- Lewin A, Benshushan A, Mezker E, Yanai N, Schenker JG, Goshen R. The role of estrogen support during the luteal phase of in vitro fertilization‐embryo transplant cycles: a comparative study between progesterone alone and estrogen and progesterone support. Fertility and Sterility 1994;62:121‐5. [DOI] [PubMed] [Google Scholar]
- Lewin A, Pisov G, Turgeman R, Fatum M, Shufaro Y, Simon A, et al. Simplified artificial endometrial preparation, using oral estradiol and novel vaginal progesterone tablets: a prospective randomized study. Gynecological Endocrinology 2002;16(2):131‐6. [PubMed] [Google Scholar]
Licciardi 1999 {published data only}
- Licciardi F, Kwiatkowski A, Noyes N, Berkeley AS, Krey LL, Grifo JA. Oral versus intramuscular progesterone for in vitro fertilization: a prospective randomized study. Fertility and Sterility 1999;71:614‐8. [DOI] [PubMed] [Google Scholar]
Lin 2013 {published data only}
- Lin H, Li Y, Li L, Wang W, Zhang Q, Chen X, Yang D. Oral oestradiol supplementation as luteal support in IVF/ICSI cycles: a prospective, randomized controlled study. European Journal of Obstetrics Gynecology and Reproductive Biology 2013;167:171‐5. [DOI] [PubMed] [Google Scholar]
Lockwood 2014 {published data only}
- Lockwood G, Griesinger G, Cometti B. Subcutaneous progesterone versus vaginal progesterone gel for luteal phase support in in vitro fertilization: a noninferiority randomized controlled study. Fertility and Sterility 2014;101:112‐9. [DOI] [PubMed] [Google Scholar]
Loh 1996 {published data only}
- Loh SKE, Leong NKY. Luteal phase support in IVF‐cycles ‐ is intramuscular progesterone the therapy of choice? [abstract]. Fertility Society of Australia XV Annual Meeting Abstract Book (Abs #O24). 1996.
Ludwig 2001 {published data only}
- Ludwig M, Finas A, Bals‐Pratsch M, Felberbaum RE, Schopper B, Al‐Hasani S, et al. Prospective, randomized study to evaluate the pregnancy rate using HCG, vaginal progesterone (Utrogest), or a combination of both for luteal‐phase support: preliminary results [abstract]. Human Reproduction. 1999; Vol. 14 Suppl 1:2‐3 (Abs # O‐004).
- Ludwig M, Finas A, Katalinic A, Strik D, Kowalcek I, Schwartz P, et al. Prospective, randomized study to evaluate the success rates using hCG, vaginal progesterone or a combination of both for luteal phase support. Acta Obstetricia et Gynecologica Scandinavica 2001;80:574‐82. [PubMed] [Google Scholar]
Ludwig 2002 {published data only}
- Ludwig M, Schwartz P, Babahan B, Katalinic A, Bals‐Pratsch M, Diedrich K. Progesterone gel (Crinone 8%) is more comfortable than progesterone suppositories (Utrogest) for luteal phase support and results in comparable pregnancy rates: results of a prospective, randomized study [abstract]. Fertility and Sterility. 2000; Vol. 74:S210 (Abs #P‐S210).
- Ludwig M, Schwartz P, Babahan B, Katalinic A, Weiss JM, Felberbaum R, et al. Luteal phase support using either Crinone 8% or Utrogest: results of a prospective, randomized study. European Journal of Obstetrics and Gynaecology 2002;103:48‐52. [DOI] [PubMed] [Google Scholar]
- Schwartz P, Ludwig M, Babahan B, Katalinic A, Bals‐Pratsch M, Felberbaum R, et al. Luteal phase support using either progesterone gel (Crinone 8%) or progesterone suppositories (Utrogest): results of a prospective, randomized study [abstract]. Human Reproduction 2000;15(Abstract Book 1):43‐4 (Abs #O‐111). [Google Scholar]
Macrolin 1993 {published data only}
- Macrolin G, Buvat J, Guittard C, Herbaut JC, Louvet AL, Dehaene JL. [Fécondation in vitro après agoniste de la LHRH: comparison randomisée de soutiens lutéaux par progestérone vaginale seule ou associee a la gonadotrophine chorionique]. Contraception Fertilité Sexualité 1993;21(5):434. [Google Scholar]
Martinez 2000 {published data only}
- Martinez F, Coroleu B, Parera N, Alvarez M, Traver JM, Boada M, et al. Human chorionic gonadotropin and intravaginal natural progesterone are equally effective for luteal phase support in IVF. Gynaecological Endocrinology 2000;14:316‐20. [DOI] [PubMed] [Google Scholar]
Miller 2010 {published data only}
- Miller CE, Doody KJ, Zbella E, Webster B, Bush M, Scobey J. Efficacy of vaginal progesterone inserts (Endometrin) compared to intramuscular progesterone in oil for luteal support in IVF patients. Fertility and Sterility 2010;94 Suppl 1(4):20‐1 Abstract no. O‐68. [Google Scholar]
Mochtar 2006 {published data only}
- Mochtar MH, Wely M, Veen F. Timing luteal phase support in GnRH agonist down‐regulated IVF/embryo transfer cycles. Human Reproduction 2006;21(4):905‐8. [DOI] [PubMed] [Google Scholar]
Moini 2011 {published data only}
- Moini A, Zadeh Modarress S, Amirchaghmaghi E, Mirghavam N, Khafri S, Reza Akhoond M, Salman Yazdi R. The effect of adding oral oestradiol to progesterone as luteal phase support in ART cycles ‐ a randomized controlled study. Archives of Medical Science 2011;7:112‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nallapeta 2013 {published data only}
- Nallapeta S, Sharma V. Intra‐muscular progesterone as a luteal phase support increases live birth rate as compared to vaginal route. Fertility and Sterility 2013;100(Supp):S8 (Abs #O‐25). [Google Scholar]
- Nallapeta S, Sharma V. Relationship of different progesterone preparations on ovarian volumes in women undergoing IVF/ICSI treatment. Fertility and Sterility 2013;100(Suppl):S60 (Abs # O‐196). [Google Scholar]
Ng 2003 {published data only}
- Ng EHY, Miao B, Cheung W, Ho PC. A randomised comparison of side effects and patient inconvenience of two vaginal progesterone formulations used for luteal support in in vitro fertilisation cycles. European Journal of Obstetrics Gynecology and Reproductive Biology 2003;111:50‐4. [DOI] [PubMed] [Google Scholar]
Ng 2007 {published data only}
- Ng EHY, Chan CCW, Tang OS, Ho PC. A randomized comparison of side effects and patient convenience between Cyclogest suppositories and Endometrin tablets used for luteal phase support in IVF treatment. European Journal of Obstetrics Gynecology and Reproductive Biology 2007;131(2):182‐8. [DOI] [PubMed] [Google Scholar]
Nyboe Andersen 2002 {published data only}
- Nyboe Andersen A, Popovic‐Todorovic B, Schmidt KT, Loft A, Lindhard A, Hojgaard A, et al. Progesterone supplementation during early gestations after IVF or ICSI has no effect on the delivery rates: a randomized controlled trial. Human Reproduction 2002;17:357‐61. [DOI] [PubMed] [Google Scholar]
Patki 2007 {published data only}
- Patki A, Pawar VC. Modulating fertility outcome in assisted reproductive technologies by the use of dydrogesterone. Gynecological Endocrinology 2007;23 Suppl 1:68‐72. [DOI] [PubMed] [Google Scholar]
Perino 1997 {published data only}
- Perino M, Brigandi A, Abate FG, Costabile L, Balzano E, Abate A. Intramuscular versus vaginal progesterone in assisted reproduction: a comparative study. Clinical and Experimental Obstetrics and Gynecology 1997;24:228‐31. [PubMed] [Google Scholar]
Porcu 2003 {published data only}
- Porcu E. Intramuscular versus vaginal progesterone in assisted reproduction [abstract]. Fertility and Sterility. 2003; Vol. 80:S131 (Abs # P‐32).
Pouly 1996 {published data only}
- Pouly JL, Bassil S, Frydman R, Hedon B, Nicollet B, Prada Y, et al. Luteal phase support after vaginal progesterone: comparative study with micronized oral progesterone. Contraception, Fertilité, Sexualité 1997;25:596‐601. [PubMed] [Google Scholar]
- Pouly JL, Bassil S, Frydman R, Hedon B, Nicollet B, Prada Y, et al. Luteal support after in‐vitro fertilization: Crinone 8%, a sustained release vaginal progesterone gel, versus Utrogestan, an oral micronized progesterone. Human Reproduction 1996;11:2085‐9. [DOI] [PubMed] [Google Scholar]
Propst 2001 {published data only}
- Propst AM, Hill JA, Ginsburg ES, Hurwitz S, Politch J, Yanushpolsky EH. A randomized study comparing Crinone 8% and intramuscular progesterone supplementation in in vitro fertilization‐embryo transfer cycles. Fertility and Sterility 2001;76:1144‐9. [DOI] [PubMed] [Google Scholar]
- Propst AM, Hill JA, Politch J, Yanushpolsky EH. A prospective, randomized study comparing Crinone and intramuscular progesterone supplementation in IVF/ET cycles [abstract]. Fertility and Sterility. 2000; Vol. 74:s30‐1 (Abs #O‐084). [DOI] [PubMed]
Qublan 2008 {published data only}
- Qublan H, Amarin Z, Al‐Quda M, Diab F, Nawasreh M, Malkawi S, et al. Luteal phase support with GnRH‐a improves implantation and pregnancy rates in IVF cycles with endometrium of [less‐than or equal to]7 mm on day of egg retrieval. Human Fertility 2008;11(1):43‐7. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Pezino 2004 {published data only}
- Rodriguez‐Pezino J, Saucedo‐de la Llata E, Batiza‐Resendiz V, Galache‐Vega P, Santos‐Haliscak R, Hernandez‐Ayup S, et al. Vaginal progesterone in assisted reproduction. Human Reproduction. Berlin, Germany, 2004; Vol. 19 Suppl 1:i51.
Salehpour 2013 {published data only}
- Salehpour S, Tamimi M, Saharkhiz N. Comparison of oral dydrogesterone with suppository vaginal progesterone for luteal‐phase support in in vitro fertilization (IVF): a randomized clinical trial. Iranian Journal of Reproductive Medicine 2013;11(11):913‐8. [PMC free article] [PubMed] [Google Scholar]
Saucedo 2000 {published data only}
- Saucedo‐de la Llata E, Galache VP, Hernandez AS, Santos HR, Arenas ML, Patrizio P. Randomized trial of three different forms of progesterone supplementation in ART: preliminary results [abstract]. Fertility and Sterility 2000;74 Suppl 1:S150 (Abs # P‐175). [Google Scholar]
Saucedo 2003 {published data only}
- Saucedo‐de la Llata E, Batiza V, Arenas L, Santos R, Galache P, Hernandez‐Ayup S, et al. Progesterone for luteal support: randomized, prospective trial comparing vaginal and i.m. administration [abstract]. Fertility and Sterility 2003;18 Suppl 1:130 (Abs # P‐382). [Google Scholar]
Serna 2008 {published data only}
- Serna J, Cholquevilque JL, Cela V, Martinez‐Salazar J, Requena A, Garcia‐Velasco JA. Estradiol supplementation during the luteal phase of IVF‐ICSI patients: a randomized, controlled trial. Fertility and Sterility 2008;90(6):2190‐5. [DOI] [PubMed] [Google Scholar]
Serour 2012 {published data only}
- Serour AG. Luteal phase support in fresh IVF/ICSI cycles. International Journal of Gynecology and Obstetrics 2012;119:S533 (Abs# M007). [Google Scholar]
Stadtmauer 2013 {published data only}
- Howard B, Weiss H, Doody K. Efficacy of a progesterone vaginal ring compared to a vaginal gel for luteal phase supplementation in patients with and without risk factors for poor ovarian response. Human Reproduction 2012;27:Abs# P‐338. [Google Scholar]
- Perloe M, Weiss H, Howard B. Impact of luteal supplementation with a weekly progesterone vaginal ring during in vitro fertilization (IVF) by day of embryo transfer (ET). Fertility and Sterility 2012;98(S1):S4‐5 (Abs # O‐14). [Google Scholar]
- Schnell V, Howard B, Weiss H. Number of embryos transferred and multiple pregnancy rates in a randomized study of progesterone vaginal ring versus gel for luteal support following in vitro fertilization. Fertility and Sterility 2013;99(3):S36‐S37. [DOI] [PubMed] [Google Scholar]
- Silverberg KM, Reape KZ, Howard BK. Efficacy of a progesterone vaginal ring versus progesterone gel for luteal phases supplementation by body mass index (BMI). Fertility and Sterility 2011;96 Suppl:S279 (Abs# P‐538). [Google Scholar]
- Stadtmauer L, Silverberg KM, Ginsburg ES, Weiss H, Howard B. Progesterone vaginal ring versus vaginal gel for luteal support with in vitro fertilization: a randomized comparative study. Fertility and Sterility 2013;99:1543‐9. [DOI] [PubMed] [Google Scholar]
- Stadtmauer LA, Reape KZ, Shu H. Luteal supplementation with a weekly progesterone vaginal ring in infertile women undergoing in vitro fertilization (IVF). Fertility and Sterility. Denver, CO United States, 2010; Vol. 94 Suppl 1:244.
Strehler 1999 {published data only}
- Strehler E, Abt M, El‐Danasouri I, Sterzik K. Transvaginal administration of micronized progesterone does not differ to progesterone gel application in the efficacy of luteal phase support in IVF cycles [abstract]. 11th World Congress of In Vitro Fertilization and Human Reproductive Genetics Abstract Book. 1999:287 (Abs # P‐243).
Sumita 2003 {published data only}
- Sumita S, Sofat S Sr. Intramuscular versus intra vaginal progesterone as luteal phase and early pregnancy support in patients undergoing IVF‐ET [abstract]. Fertility and Sterility 2003;80 Suppl 3:134‐5 (Abs # P‐44). [Google Scholar]
Tay 2005 {published data only}
- Tay PYS, Lenton EA. The impact of luteal supplement on pregnancy outcome following stimulated IVF cycles. Medical Journal of Malaysia 2005;60(2):151‐7. [PubMed] [Google Scholar]
Tesarik 2006 {published data only}
- Tesarik J, Hazout A, Mendoza‐Tesarik R, Mendoza N, Mendoza C. Beneficial effect of luteal‐phase GnRH agonist administration on embryo implantation after ICSI in both GnRH agonist‐ and antagonist‐treated ovarian stimulation cycles. Human Reproduction 2006;21(10):2572‐9. [DOI] [PubMed] [Google Scholar]
Tonguc 2011 {published data only}
- Esra T, Var T, Citil A, Dogan M, Cicek N. Estradiol supplementation in luteal phase: how much matter?. Human Reproduction. Rome, Italy, 2010; Vol. 25 Suppl 1:i307‐8.
- Tonguc E, Var T, Ozyer S, Citil A, Dogan M. Estradiol supplementation during the luteal phase of in vitro fertilization cycles: a prospective randomised study. European Journal of Obstetrics Gynecology and Reproductive Biology 2011;154:172‐6. [DOI] [PubMed] [Google Scholar]
Torode 1987 {published data only}
- Torode HW, Porter RN, Vaughan JI, Saunders DM. Luteal phase support after in vitro fertilisation: a trial and rationale for selective use. Clinical Reproduction and Fertility 1987;5:255‐61. [Google Scholar]
Ugur 2001 {published data only}
- Ugur M, Yenicesu O, Ozcan S, Keles G, Gokmen O. A prospective randomized study comparing hCG, vaginal micronized progesterone and a combination regimen for luteal phase support in an in‐vitro fertilization programme [abstract]. Fertility and Sterility 2001;76 Suppl 1:118 (Abs # P‐19). [Google Scholar]
Vimpeli 2001 {published data only}
- Vimpeli T, Tinkanen H, Huhtala H, Ronnberg L, Kujansuu E. Salivary and serum progesterone concentrations during two luteal support regimens used in in vitro fertilization treatment. Fertility and Sterility 2001;76:847‐8. [DOI] [PubMed] [Google Scholar]
Williams 2001 {published data only}
- Williams SC, Oehninger S, Gibbons WE, Cleave WC, Muasher SJ. Delaying the initiation of progesterone supplementation results in decreased pregnancy rates after in vitro fertilization: a randomized, prospective study. Fertility and Sterility 2001;76:1140‐3. [DOI] [PubMed] [Google Scholar]
Wong 1990 {published data only}
- Wong YF, Loong EPL, Mao KR, Tam PPL, Panesar NS, Neale E, et al. Salivary oestradiol and progesterone after in vitro fertilization and embryo transfer using different luteal support regimens. Reproduction Fertility and Development 1990;2:351‐8. [DOI] [PubMed] [Google Scholar]
Yanushpolsky 2010 {published data only}
- Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Compared to Crinone, intramuscular progesterone (IMP) delays menstrual bleeding but does not improve pregnancy rates or outcomes in IVF/ET cycles. Fertility and Sterility. 2009; Vol. 92 Suppl 1:243.
- Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Crinone vaginal gel is equally effective and better tolerated than intramuscular progesterone for luteal phase support in in vitro fertilization‐embryo transfer cycles: a prospective randomized study. Fertility and Sterility 2010;94(7):2596‐9. [DOI] [PubMed] [Google Scholar]
- Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Patterns of luteal phase bleeding in in vitro fertilization cycles supplemented with Crinone vaginal gel and with intramuscular progesterone ‐ Impact of luteal estrogen: prospective, randomized study and post hoc analysis. Fertility and Sterility 2011;95(2):617‐20. [DOI] [PubMed] [Google Scholar]
- Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein MD. Comparison of Crinone 8% intravaginal gel and intramuscular progesterone supplementation for in vitro fertilization/embryo transfer in women under age 40: interim analysis of a prospective randomized trial. Fertility and Sterility 2008;89(2):485‐7. [DOI] [PubMed] [Google Scholar]
Yildiz 2014 {published data only}
- Yildiz, Gulsah Aynaoglu, Sukur, Yavuz Emre, Ates, Can, Aytac, Rusen. The addition of gonadotrophin releasing hormone agonist to routine luteal phase support in intracytoplasmic sperm injection and embryo transfer cycles: a randomized clinical trial.. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2014;182:66‐70. [DOI] [PubMed] [Google Scholar]
Zegers‐Hochschild 2000 {published data only}
- Zegers‐Hochschild F, Balmaceda JP, Fabres C, Alam V, Mackenna A, Fernandez E, et al. Efficacy and acceptability of a vaginal ring releasing progesterone for in‐vitro fertilization and oocyte donation [abstract]. Human Reproduction 1998;13(Abstract Book 1):118‐9 (Abs #O‐231). [DOI] [PubMed] [Google Scholar]
- Zegers‐Hochschild F, Balmaceda JP, Fabres C, Alam V, Mackenna A, Fernández E, et al. Prospective randomized trial to evaluate the efficacy of a vaginal ring releasing progesterone for IVF and oocyte donation. Human Reproduction 2000;15(10):2093‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abu‐Musa 2008 {published data only}
- Abu‐Musa A, Usta I, Nassar A, Hajami F, Hannoun A. Effect of 17alpha‐hydroxyprogesterone caproate before embryo transfer on the outcome of in vitro fertilization and embryo transfer: a randomized trial. Fertility and Sterility 2008;89(5):1098‐102. [DOI] [PubMed] [Google Scholar]
Abu‐Musa 2008a {published data only}
- Abu‐Musa A, Usta I, Nassar A, Hajami F, Hannoun A. Effect of 17alpha‐hydroxyprogesterone caproate before embryo transfer on the outcome of in vitro fertilization and embryo transfer: a randomized trial. Fertility and Sterility 2008;89(5):1098‐102. [DOI] [PubMed] [Google Scholar]
Aleyasin 2012 {published data only}
- Aleyasin A, Mahdavi A, Agha Hosseini M, Safdarian L, Fallahi P, Bahmaee F. Comparison of two doses of recombinant human chorionic gonadotropin and urinary human chorionic gonadotropin during intracytoplasmic sperm injection cycles. Human Reproduction 2012;27:P‐324. [Google Scholar]
Allahbadia 2004 {published data only}
- Allahbadia GN, Kaur K, Kadam K, Virk S, Gandhi G, Gosrani S. The comparison of pregnancy outcomes of intramuscular progesterone versus oral dydrogesterone for luteal phase support in donor egg IVF recipient cycles. Fertility and Sterility 2004;82 Suppl 2:194. [Google Scholar]
Allen 2004 {published data only}
- Allen C, Harrison RF. Luteal support progesterone vaginal gel v pessary: clinical/endocrine outcome. Human Reproduction 2004;19 Suppl:i125‐6. [Google Scholar]
Alsanie 2005 {published data only}
- Alsanie A, Kadoch I, Phillips S, Lapensee L, Hemmings R, Bissonnette F. Adding estrogen to progesterone in luteal phase support in vitro fertilization‐embryo transfer (IVF‐ET) cycles produces pregnancies with higher quantitative beta human chorionic gonadotropins (beta hCG). Fertility and Sterility 2005;84 Suppl:155. [Google Scholar]
Andersen 2014 {published data only}
- Andersen CY, Andersen KV. Improving the luteal phase after ovarian stimulation: reviewing new options. Reproductive Biomedicine Online 2014;28(5):552‐9. [DOI] [PubMed] [Google Scholar]
Anserini 2001 {published data only}
- Anserini P, Costa M, Remorgida V, Sarli R, Guglielminetti E, Ragni N. Luteal phase support in assisted reproductive cycles using either vaginal (Crinone 8) or systemic (Prontogest) progesterone: results of a prospective randomized study. Minerva Ginecologica 2001;53:297‐301. [PubMed] [Google Scholar]
Anthony 1993 {published data only}
- Anthony FW, Smith EM, Gadd SC, Masson GM, Chard T, Perry L. Placental protein 14 secretion during in vitro fertilization cycles with and without human chorionic gonadotropin for luteal support. Fertility and Sterility 1993;59:187‐91. [DOI] [PubMed] [Google Scholar]
Araujo 1994 {published data only}
- Araujo E Jr, Bernardini L, Frederick JL, Asch RH, Balmaceda JP. Prospective randomized comparison of human chorionic gonadotropin versus intramuscular progesterone for luteal‐phase support in assisted reproduction. Journal of Assisted Reproduction and Genetics 1994;11(2):74‐8. [DOI] [PubMed] [Google Scholar]
Araujo Filho 1996 {published data only}
- Araujo Filho E, Asch RH, Araujo E, Luz OA, Balmaceda JP. Prospective and randomized trial comparing human chorionic gonadotropin and intramuscular progesterone for luteal phase support in assisted fertilization [Estudo prospectivo e randomizado comparando gonadotrofina coriônica humana e progesterona intramuscular para suporte da fase lútea em reproduçao assistida]. Revista Brasileira de Ginecologia e Obstetrica 1996;18(2):131‐7. [Google Scholar]
Baber 1988 {published data only}
- Baber RJ, Kuan R, Porter RN, Saunders DM. Early pregnancy support in an in vitro fertilization program: does human chorionic gonadotropin reduce the miscarriage rate?. Asia‐Oceania Journal of Obstetrics and Gynaecology 1988;14:453‐5. [DOI] [PubMed] [Google Scholar]
Beckers 2006 {published data only}
- Beckers NGM, Platteau P, Eijkemans MJ, Macklon NS, Jong FH, Devroey P, et al. The early luteal phase administration of estrogen and progesterone does not induce premature luteolysis in normo‐ovulatory women. European Journal of Endocrinology 2006;155(2):355‐63. [DOI] [PubMed] [Google Scholar]
Belaisch‐Allart 1988 {published data only}
- Belaisch‐Allart J, Mouzon J. Effect of luteal phase supplementation in an IVF programme after ovarian stimulation by LH‐RH analogs. Multicentric analysis [Effet de la supplementation de la phase luteale dans un programme de fecondation in vitro apres stimulation de l'ovulation par les agonistes du LHRH. Etude multicentrique]. Contraception, Fertilite, Sexualite 1988;16(7):654‐6. [Google Scholar]
Ben‐Nun 1990 {published data only}
- Ben‐Nun I, Ghetler Y, Jaffe R, Siegal A, Kaneti H, Fejgin M. Effect of preovulatory progesterone administration on the endometrial maturation and implantation rate after in vitro fertilization and embryo transfer. Fertility and Sterility 1990;53:276‐81. [DOI] [PubMed] [Google Scholar]
Berjis 2008 {published data only}
- Berjis K, Sarem A, Moaya M, Mohamad Alayha N. The comparative assessment of intramuscular progesterone and intravaginal progesterone to support luteal phase in IVF cycle [Farsi]. Medical Sciences Journal of the Islamic Azad University of Tehran Medical Unit 2008;18(1):9. [Google Scholar]
Bjuresten 2011 {published data only}
- Bjuresten K, Landgren BM, Hovatta O, Stavreus‐Evers A. Luteal phase progesterone increases live birth rate after frozen embryo transfer. Fertility and Sterility 2011;95:534‐7. [DOI] [PubMed] [Google Scholar]
Blake 2010 {published data only}
- Blake EJ, Norris PM, Dorfman SF, Longstreth J, Yankov VI. Single and multidose pharmacokinetic study of a vaginal micronized progesterone insert (Endometrin) compared with vaginal gel in healthy reproductive‐aged female subjects. Fertility and Sterility 2010;94(4):1296‐301. [DOI] [PubMed] [Google Scholar]
Buvat 1988 {published data only}
- Buvat J, Marcolin G, Herbaut JC, Dehaene JL, Verbecq P, Fourlinnie JC. A randomized trial of human chorionic gonadotropin support following in vitro fertilization and embryo transfer. Fertility and Sterility 1988;49:458‐61. [DOI] [PubMed] [Google Scholar]
- Macrolin G, Buvat J, Herbaut JC, Louvet AL, Dehaene JL, Renouard O. Luteal phase support with HCG ‐ can it be of any benefit following in vitro fecundation (IVF)? A controlled randomized study covering 116 cycles [Le soutien de la phase lutéale par HCG a‐t‐il de l'intérêt après fécondation in vitro?]. Gynecologie 1988;39:163‐6. [Google Scholar]
Buvat 1990 {published data only}
- Buvat J, Marcolin G, Guittard C, Dehaene JL, Herbaut JC, Louvet AL. Luteal support after administration of an LHRH analog for in vitro fertilization. Superiority of vaginal progesterone in comparison with oral progesterone [Soutien lutéal après analogue de la gonadoréline pour fécondation in vitro. Supériorité de la progestérone vaginale sur la progestérone orale]. La Presse Médicale 1990;19:527. [PubMed] [Google Scholar]
- Buvat J, Marcolin G, Guittard C, Herbaut JC, Louvet AL, Dehaene JL. Luteal phase support after LHRH‐agonist for in vitro fertilization (IVF): vaginal progesterone is superior to oral progesterone and as much effective as human chorionic gonadotropin (hCG) [Soutien lutéal après LHRH‐agonistes pour fécondation in vitro: la progestérone vaginale est supérieure à la progestérone orale, et aussi efficace que la gonadotrophine chorionique (hCG)]. Contraception Fertilite Sexualite 1990;18:616‐7. [Google Scholar]
- Buvat J, Marcolin G, Guittard C, Herbaut JC, Louvet AL, Dehaene JL. Luteal support after luteinizing hormone‐releasing hormone agonist for in vitro fertilization: superiority of human chorionic gonadotropin over oral progesterone. Fertility and Sterility 1990;53:490‐4. [DOI] [PubMed] [Google Scholar]
- Buvat J, Mercolin G, Guittard C, Dehaene JL, Verbecq P, Renouard O, et al. Chorionic gonadotropin support of the luteal phase following in vitro fertilization and embryo transfer. Randomized comparison wih oral progesteronein protocols using triptoreline [Soutien de la phase lutéal par la gonadotrophine chorionique après fécondation in vitro et transfert d'embryon. Comparaison randomisée à la progestérone per os dans les protocoles utilitsant la triptoréline]. La Presse Médicinale 1989;18:539. [PubMed] [Google Scholar]
Casini 2003 {published data only}
- Casini ML, Unfer V, Costabile L, Gerli S, Agostini R, Renzo GC. Oral versus i.m. progesterone supplementation in IVF‐embryo transfer cycles: a randomized study [abstract]. Human Reproduction 2003;18 Suppl 1:106 (Abs # P‐307). [Google Scholar]
Chakravarty 2012 {published and unpublished data}
- Chakravarty A, Sharma Palchaudhuri S, Chakraborty P, Goswami SK, Chattopadhyay R, Chakravarty B. Role of estrogen as luteal phase support (LPS) in normal and expected poor responders in long agonist in vitro fertilization (IVF)/inta‐cytoplasmic sperm injection (ICSI) cycles. Fertility and Sterility 2012;98(Suppl 1):S257 (Abs # P‐491). [Google Scholar]
Chang 2008 {published data only}
- Chang S‐P. Comparison of Crinone 8% intravaginal gel and intramuscular progesterone for luteal support in in vitro fertilization. Journal of the Chinese Medical Association 2008;71(8):381‐5. [DOI] [PubMed] [Google Scholar]
Chang 2009 {published data only}
- Chang HJ, Lee JR, Jee BC, Suh CS, Kim SH. Cessation of gonadotropin‐releasing hormone antagonist on triggering day: an alternative method for flexible multiple‐dose protocol. Journal of Korean Medical Science 2009;24(2):262‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chantilis 1999 {published data only}
- Chantilis SJ, Zeitoun KM, Patel SI, Johns DA, Madziar VA, McIntire DD. Use of Crinone vaginal progesterone gel for luteal support in in vitro fertilization cycles. Fertility and Sterility 1999;72:823‐9. [DOI] [PubMed] [Google Scholar]
Check 2010 {published data only}
- Check J H, Dietterich C, Cohen R, Choe J K, Amui J, Brasile D. Increasing the dosage of progesterone (P) supplemention from the mid‐luteal phase in women not attaining a mid‐luteal homogeneous hyperechogenic (HH) pattern with sonography improves pregnancy rates (PRS) following frozen embryo transfer (ET). Clinical and Experimental Obstetrics and Gynecology 2010;37(1):13‐4. [PubMed] [Google Scholar]
Check 2012 {published data only}
- Check JH. Luteal phase support for in vitro fertilization‐embryo transfer ‐ present and future methods to improve successful implantation. Clinical and Experimental Obstetrics & Gynecology 2012;39(4):422‐8. [PubMed] [Google Scholar]
Check 2013 {published data only}
- Check JH, Wilson C, Levine K, Cohen R, Corley D. Improved implantation and live delivered pregnancy rates following transfer of embryos derived from donor oocytes by single injection of leuprolide in mid‐luteal phase. Fertility and Sterility 2013;100(3 Suppl):S301. [PubMed] [Google Scholar]
Claman 1992 {published data only}
- Claman P, Domingo M, Leader A. Luteal phase support in in‐vitro fertilization using gonadotrophin releasing hormone analogue before ovarian stimulation: a prospective randomized study of human chorionic gonadotrophin versus intramuscular progesterone. Human Reproduction 1992;7(4):487‐9. [DOI] [PubMed] [Google Scholar]
- Claman P, Domingo M, Leader A, Gauthier C. Preliminary results suggest an advantage to using human chorionic gondotropin over progesterone treatment to support the luteal phase after treatment with leuprolide acetate and human menopausal gonadotrophin for superovulation in IVF‐ET. Fertility & Sterility 1991;54(116). [Google Scholar]
Costabile 2001 {published data only (unpublished sought but not used)}
- Costabile L, Gerli S, Manna C, Rossetti D, Renzo GC, Unfer V. A prospective randomized study comparing intramuscular progesterone and 17alpha‐hydroxyprogesteronecaproate in patients undergoing in vitro fertilization‐embryo transfer cycles. Fertility and Sterility 2001;76(2):964‐6. [DOI] [PubMed] [Google Scholar]
Daya 2009 {published data only}
- Daya S. Luteal support: progestogens for pregnancy protection. Maturitas 2009;65 Suppl 1:29‐34. [DOI] [PubMed] [Google Scholar]
Demir 2013 {published data only}
- Demir B, Dilbaz S, Cinar O, Ozdegirmenci O, Dede S, Dundar B, Goktolga U. Estradiol supplementation in intracytoplasmic sperm injection cycles with thin endometrium. Gynecological Endocrinology 2013;29(1):42‐5. [DOI] [PubMed] [Google Scholar]
Demirel 2003 {published data only}
- Demirel LC, Baltaci V, Aydos K, Aytaç R, Satiroglu H, Ünlü C. A randomized comparison of the timing for initiation of luteal phase support. Human Reproduction 2003;18(Suppl 1):130 (Abs # P‐382). [Google Scholar]
Ding 2005 {published data only}
- Ding J, Rana N, Dmowski W. Comparative effectiveness of two luteal phase support protocols for IVF. Fertility and Sterility 2005;84 Suppl 1:349. [Google Scholar]
Ellenbogen 2011 {published data only}
- Ellenbogen A, Atamny R, Fainaru O, Meidan E, Rotfarb N, Michaeli M. In vitro maturation of oocytes: a novel method of treatment of patients with polycystic ovarian syndrome undergoing in vitro fertilization. Harefuah 2011;150(11):833‐76. [PubMed] [Google Scholar]
Erman Akar 2005 {published data only}
- Erman Akar M, Kursun S, Taskin O, Simsek M, Kaba M, Uner M. Intravaginal progesterone gel vs 17oe hydroxyprogesterone caproate in ICSI embryo transfer cycles: a prospective randomized study. Fertility and Sterility 2005;84 Suppl 1:320. [Google Scholar]
Escriba 2006 {published data only}
- Escriba MJ, Bellver J, Bosch E, Sanchez M, Pellicer A, Remohi J. Delaying the initiation of progesterone supplementation until the day of fertilization does not compromise cycle outcome in patients receiving donated oocytes: a randomized study. Fertility and Sterility 2006;86(1):92‐7. [DOI] [PubMed] [Google Scholar]
Farhi 2000 {published data only}
- Farhi J, Nahum H, Steinfeld Z, Shorer M, Zakut H. Do adequate E2 and progesterone levels during luteal phase promote or merely reflect implantation in IVF cycles. Fertility & Sterility 1998;70(3):Abs # S‐429. [Google Scholar]
- Farhi J, Weissman A, Steinfeld Z, Shorer M, Nahum H, Levran D. Estradiol supplementation during the luteal phase may improve the pregnancy rate in patients undergoing in vitro fertilization‐embryo transfer cycles. Fertility and Sterility 2000;73:761‐6. [DOI] [PubMed] [Google Scholar]
Farrag 2008 {published data only}
- Farrag A, Costantini A, Manna C, Grimaldi G. Recombinant HCG for triggering ovulation increases the rate of mature oocytes in women treated for ICSI. Journal of Assisted Reproduction and Genetics 2008;25(9‐10):461‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
FeiYang 2013 {published data only (unpublished sought but not used)}
- Fei Yang D, Jia Yin L. Different LH add‐back and luteal phase supplement influence clinical outcome in GnRH antagonist protocol ‐ a prospective RCT study in fresh and frozen transfer cycles. Fertility and Sterility 2013;100(Suppl):S270 (Abs # P‐424). [Google Scholar]
Feliciani 2004 {published data only}
- Feliciani E, Ferraretti AP, Balicchia B, Grieco N, Magli MC, Gianaroli A. A prospective randomised study comparing the effect of intravaginal progesterone and intramuscular progesterone in frozen/thawed embryo transfer (FET) cycles. Human Reproduction 2004;82:i51. [Google Scholar]
Gallardo 2004 {published data only}
- Gallardo LE, Ayón P, Neuspiller F. [Estudio de dos vías diferentes de administración de progesterona micronizada en reproducción asistida]. Ginecología y Obstetricia de México 2004;72(8):407‐10. [PubMed] [Google Scholar]
Garcia‐Velasco 2009 {published data only}
- Garcia‐Velasco J, Motta L, Lopez A, Mayoral M, Cerrillo M, Pellicer A, Pacheco A. Estradiol/progesterone vs low dose hCG luteal phase support in GnRH agonist triggered ART cycles: a pilot study. Human Reproduction 2010;25 Suppl 1:i86 (Abs #O‐224). [Google Scholar]
- Garcia‐Velasco JA, Motta L, Lopez A, Mayoral M, Cerrillo M, Pacheco A. Low‐dose human chorionic gonadotropin versus estradiol/progesterone luteal phase support in gonadotropin‐releasing hormone agonist‐triggered assisted reproductive technique cycles: understanding a new approach. Fertility and Sterility 2010;94:2820‐3. [DOI] [PubMed] [Google Scholar]
- Garcia‐Velasco JA, Quea G, Piro M, Mayoral M, Ruiz M, Toribio M, et al. Letrozole administration during the luteal phase after ovarian stimulation impacts corpus luteum function: a randomized, placebo‐controlled trial. Fertility and Sterility 2009;92(1):222‐5. [DOI] [PubMed] [Google Scholar]
Gazvani 2012 {published data only}
- Gazvani R, Russell R, Sajjad Y, Alfirevic Z. Duration of luteal support (DOLS) with progesterone pessaries to improve the success rates in assisted conception: study protocol for a randomized controlled trial. Trials 2012;13:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Germond 2002 {published data only}
- Germond M, Capelli P, Bruno G, Vesnaver S, Senn A, Rouge N, Biollaz J. Comparison of the efficacy and safety of two formulations of micronized progesterone (Ellios and Utrogestan) used as luteal phase support after in vitro fertilization. Fertility and Sterility 2002;77(2):313‐7. [DOI] [PubMed] [Google Scholar]
Ghanem 2009 {published data only}
- Ghanem M, Sadek E, Helal A, Gamal A, Eldiasty E, Bakre NI, Houssen M. The effect of luteal phase support protocol on luteal phase serum estradiol and progesterone and cycle outcome in ICSI cycles: a randomized trial [Abstract]. Human Reproduction. 2008; Vol. 23, issue Suppl 1:i124 (Abs #P‐307).
- Ghanem ME, Sadek EE, Elboghdady LA, Helal AS, Gamal A, Eldiasty A, et al. The effect of luteal phase support protocol on cycle outcome and luteal phase hormone profile in long agonist protocol intracytoplasmic sperm injection cycles: a randomized clinical trial. Fertility and Sterility 2009;92(2):486‐93. [DOI] [PubMed] [Google Scholar]
Gibbons 1998 {published data only}
- Gibbons W E, Toner J P, Hamacher P, Kolm P. Experience with a novel vaginal progesterone preparation in a donor oocyte program. Fertility and Sterility 1998;69:96‐101. [DOI] [PubMed] [Google Scholar]
Griesinger 2006 {published data only}
- Griesinger G, Diedrich K. Vaginal progesterone for luteal phase support in assisted reproduction [Die vaginale Anwendung von natürlichem Progesteron als Lutealphasenunterstützung nach IVF und Embryotransfer]. Geburtsh Frauenheilk 2006;66:655‐64. [Google Scholar]
Herman 1990 {published data only}
- Herman A, Ron‐El R, Golan A, Raziel A, Soffer Y, Caspi E. Pregnancy rate and ovarian hyperstimulation after luteal human chorionic gonadotropin in in vitro fertilization stimulated with gonadotropin‐releasing hormone analog and menotropins. Fertility and Sterility 1990;53:92‐6. [DOI] [PubMed] [Google Scholar]
Herman 1996 {published data only}
- Herman A, Raziel A, Nachum H, Strassburger D, Soffer Y, Bukovsky Y, et al. The benefits of midluteal addition of human chorionic gonadotrophin in IVF using a down‐regulation protocol and luteal support with progesterone [abstract]. Human Reproduction 1995;10(Abstract Book 2):63 (Abs #127). [Google Scholar]
- Herman A, Raziel A, Strassburger D, Soffer Y, Bukovsky I, Ron‐El R. The benefits of mid‐luteal addition of human chorionic gonadotrophin in in‐vitro fertilization using a down‐regulation protocol and luteal support with progesterone. Human Reproduction 1996;11:1552‐7. [DOI] [PubMed] [Google Scholar]
Ho 2008 {published data only}
- Ho CH, Chen SU, Peng FS, Chang CY, Yang YS. Luteal support for IVF/ICSI cycles with Crinone 8% (90 mg) twice daily results in higher pregnancy rates than with intramuscular progesterone. Journal of the Chinese Medical Association 2008;71(8):386‐91. [DOI] [PubMed] [Google Scholar]
Hokenstad 2013 {published data only}
- Hokenstad AN, Leonard PH, Morbeck DE, Khan Z, Asante A, Coddington CC. Route of luteal phase support for frozen embryo transfers: a randomized control trial. Reproductive Sciences 2013;20(S3):233A (Abs# F‐141). [Google Scholar]
Humaidan 2010 {published data only}
- Humaidan P, Ejdrup Bredkjaer H, Westergaard LG, Yding Andersen C. 1,500 IU human chorionic gonadotropin administered at oocyte retrieval rescues the luteal phase when gonadotropin‐releasing hormone agonist is used for ovulation induction: a prospective, randomized, controlled study. Fertility and Sterility 2010;93(3):847‐54. [DOI] [PubMed] [Google Scholar]
Humaidan 2013 {published data only}
- Humaidan P, Polyzos NP, Alsbjerg B, Erb K, Mikkelsen AL, Elbaek HO, et al. GnRHa trigger and individualized luteal phase hCG support according to ovarian response to stimulation: two prospective randomized controlled multi‐centre studies in IVF patients. Human Reproduction 2013;28(9):2511‐21. [DOI] [PubMed] [Google Scholar]
Hutchinson‐Williams 1990 {published data only}
- Hutchinson‐Williams KA, DeCherney AH, Lavy G, Diamond MP, Naftolin F, Lunenfeld B. Luteal rescue in in vitro fertilization‐embryo transfer. Fertility and Sterility 1990;53:495‐9. [PubMed] [Google Scholar]
Iliodromiti 2013 {published data only}
- Iliodromiti S, Blockeel C, Tremellen KP, Fleming R, Tournaye H, Humaidan P, Nelson SM. Consistent high clinical pregnancy rates and low ovarian hyperstimulation syndrome rates in high‐risk patients after GnRH agonist triggering and modified luteal support: a retrospective multicentre study. Human Reproduction 2013;28:2529‐36. [DOI] [PubMed] [Google Scholar]
Jee 2010 {published data only}
- Jee BC, Suh CS, Kim SK, Kim YB, Moon SY. Effects of estradiol supplementation during the luteal phase of in vitro fertilization cycles: a meta‐analysis. Fertility and Sterility 2010;93(2):428‐36. [DOI] [PubMed] [Google Scholar]
Johnson 1999 {published data only}
- Johnson MR, Okokon E, Collins WP, Sharma V, Lightman SL. The effect of human chorionic gonadotropin and pregnancy on the circulating level of relaxin. Journal of Clinical Endocrinology and Metabolism 1999;72:1042‐7. [DOI] [PubMed] [Google Scholar]
Jung 2010 {published data only}
- Jung YH, Kim YY, Kim MH, Cho JD. The best luteal phase support protocol for patients who had E2 levels <1500 pg/ml on the hCG day in a long GnRH agonist cycles. Fertility and Sterility 2010;94 Suppl 1(4):177. [Google Scholar]
Kahraman 2010 {published data only}
- Kahraman S, Karagozoglu SH, Karlikaya G. The efficiency of progesterone vaginal gel versus intramuscular progesterone for luteal phase supplementation in gonadotropin‐releasing hormone antagonist cycles: a prospective clinical trial. Fertility and Sterility 2010;94(2):761‐3. [DOI] [PubMed] [Google Scholar]
- Karagozoglu H, Kahraman S, Karlikaya G, Kavrut M, Ersahin A. The efficiency of vaginal gel vs intramuscular progesterone for luteal phase support in GnRH antagonist cycles: a prospective, randomized trial. Human Reproduction. 2009; Vol. 24 Suppl 1:i109‐10 (Abs #O‐273).
Kaser 2012 {published data only}
- Kaser D, Ginsburg E, Missmer S, Correia K, Racowsky C. Intramuscular progesterone versus Crinone 8% vaginal gel for luteal phase replacement in day 3 cryopreserved embryo transfer. Human Reproduction 2012;27:P‐243. [DOI] [PubMed] [Google Scholar]
- Kaser DJ, Ginsburg ES, Missmer SA, Correia KF, Racowsky C. Intramuscular progesterone versus 8% Crinone vaginal gel for luteal phase support for day 3 cryopreserved embryo transfer. Fertility and Sterility 2012;98:1464‐9. [DOI] [PubMed] [Google Scholar]
Kol 2011 {published data only}
- Kol S, Humaidan P, Itskovitz‐Eldor J. GnRH agonist ovulation trigger and hCG‐based, progesterone‐free luteal support: a proof of concept study. Human Reproduction 2011;26:2874‐7. [DOI] [PubMed] [Google Scholar]
Koper 2008 {published data only}
- The Corifollitropin Alfa Dose‐finding Study Group. A randomized dose‐response trial of a single injection of corifollitropin alfa to sustain multifollicular growth during controlled ovarian stimulation. Human Reproduction 2008;23(11):2484‐92. [DOI] [PubMed] [Google Scholar]
Krause 2006 {published data only}
- Krause BT, Ohlinger R. Safety and efficacy of low dose hCG for luteal support after triggering ovulation with a GnRH agonist in cases of polyfollicular development. European Journal of Obstetrics Gynecology and Reproductive Biology 2006;126(1):87‐92. [DOI] [PubMed] [Google Scholar]
Krischker 1998 {published data only}
- Krischker U, Poehl M, Bichler K, Feichtinger W. Different methods of luteal phase support in an in vitro fertilization (IVF) program [abstract]. Fertility and Sterility 1998;70 Suppl 1:327 (Abs # P‐639). [Google Scholar]
Kwon 2012 {published data only}
- Kwon SK, Kim CH, Ahn JW, Lee KH, Chae HD, Kang BM. Effect of intravenous immunoglobulin on pregnancy outcome following IVF/ICSI in infertile patients with endometriosis. Fertility and Sterility 2012;100:S263 (Abs# P‐511). [Google Scholar]
Kyrou 2011a {published data only}
- Kyrou D, Kolibianakis EM, Fatemi HM, Tarlatzi TB, Devroey P, Tarlatzis BC. Increased live birth rates with GnRH agonist addition for luteal support in ICSI/IVF cycles: a systematic review and meta‐analysis. Human Reproduction Update 2011;17:734‐40. [DOI] [PubMed] [Google Scholar]
Lainas 2012 {published data only}
- Lainas GT, Kolibianakis EM, Sfontouris IA, Zorzovilis IZ, Petsas GK, Tarlatzi TB, et al. Outpatient management of severe early OHSS by administration of GnRH antagonist in the luteal phase: an observational cohort study. Reproductive Biology and Endocrinology 2012;10:69‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lam 2003 {published data only}
- Lam PM, Cheung LP, Haines CJ. Early luteal phase progesterone supplementation and IVF‐ET outcome [abstract]. Reproduction 2003;Abstract Series #30:51 (Abs # P2). [Google Scholar]
Lan 2007 {published data only}
- Lan VTN, Tuan P, Canh L, Tuong H, Howles CM. Comparison of the efficacy and tolerability of two formulations of vaginal progesterone for luteal phase support in frozen embryo transfer cycles. Fertility and Sterility 2007;88 Suppl 1:164 (Abs #169). [Google Scholar]
Lee 2013 {published data only}
- Lee VC, Li RH, Ng EH, Yeung WS, Ho PC. Luteal phase support does not improve the clinical pregnancy rate of natural cycle frozen‐thawed embryo transfer: a retrospective analysis. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2013;169:50‐3. [DOI] [PubMed] [Google Scholar]
Lee 2013a {published data only}
- Lee JH, Kim SG, Kim YY, Kim HJ, Lee KH, Park IH, Sun HG. The effect of additional low dose hCG with vaginal progesterone gel in luteal phase of IVF cycles. Human Reproduction 2013;28:323. [Google Scholar]
Leeton 1985 {published data only}
- Leeton J, Trounson A, Jessup D. Support of the luteal phase in in vitro fertilization programs: results of a controlled trial with intramuscular proluton. Journal of In Vitro Fertilization and Embryo Transfer 1985;2:166‐9. [DOI] [PubMed] [Google Scholar]
Lightman 1999 {published data only}
- Lightman A, Kol S, Itskovitz‐Eldor J. A prospective randomized study comparing intramuscular with intravaginal natural progesterone in programmed thaw cycles. Human Reproduction 1999;14:2596‐9. [DOI] [PubMed] [Google Scholar]
Lin 2013a {published data only}
- Lin H, Li Y, Li L, Zhang Q, Wang W, Chen X, Yang D. Effect of delayed initiation of gonadotropin in luteal long protocol on in vitro fertilization. Gynecological Endocrinology 2013;29:846‐50. [DOI] [PubMed] [Google Scholar]
Liu 2012 {published data only}
- Liu XR, Mu HQ, Shi Q, Xiao XQ, Qi HB. The optimal duration of progesterone supplementation in pregnant women after IVF/ICSI: a meta‐analysis. Reproductive Biology and Endocrinology 2013;10:107‐14. [DOI: 10.1186/1477-7827-10-107] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lukaszuk 2005 {published data only}
- Lukaszuk K, Liss J, Lukaszuk M, Maj B. Optimization of estradiol supplementation during the luteal phase improves the pregnancy rate in women undergoing in vitro fertilization‐embryo transfer cycles. Fertility and Sterility 2005;83(5):1372‐6. [DOI] [PubMed] [Google Scholar]
Mahadevan 1985 {published data only}
- Mahadevan MM, Leader A, Taylor PJ. Effects of low‐dose human chorionic gonadotropin on corpus luteum function after embryo transfer. Journal of In Vitro Fertilization and Embryo Transfer 1985;2:190‐4. [DOI] [PubMed] [Google Scholar]
Marianowski 2000 {published data only}
- Marianowski P, Radwanska E. Intramuscular vs vaginal progesterone for luteal support in cycles of in vitro fertilization. Ginekologia Polska 2000;71:1064‐70. [PubMed] [Google Scholar]
Martins 2010 {published data only}
- Martins WdP. Suporte da fase lutea. Femina 2010;38(5):271. [Google Scholar]
McBain 1987 {published data only}
- McBain J C, Clarke G A, Molloy D, Yeates J, Johnston W I H, McKenna M. A randomized trial of progesterone support following ovarian stimulation with clomiphene hMG for IVF and GIFT [abstract]. 5th World Congress on In Vitro Fertilization and Embryo Transfer Abstract Book. 1987:75 (Abs # PP‐126).
Michnova 2011 {published data only}
- Michnova L, Rumpikova T, Dostal J. Luteal support in the IVF/ET programme. Ceska Gynekologie / Ceska Lekarska Spolecnost J. Ev. Purkyne 2011;76(2):104‐7. [PubMed] [Google Scholar]
Miller 2013 {published data only}
- Miller CE, Zbella E, Webster BW, Doody KJ, Bush MR, Collins MG. Clinical comparison of ovarian stimulation and luteal support agents in patients undergoing GnRH antagonist IVF cycles. Journal of Reproductive Medicine 2013;58:153‐60. [PubMed] [Google Scholar]
Mochtar 1996 {published data only}
- Mochtar MH, Hogerzeil HV, Mol BWJ. Progesterone alone versus progesterone combined with HCG as luteal support in GnRHa/HMG induced IVF cycles: a randomized clinical trial. Human Reproduction 1996;11:1602‐5. [DOI] [PubMed] [Google Scholar]
Moraloglu 2008 {published data only}
- Moraloglu O, Kilic S, Karayalcin R, Yuksel B, Tasdemir N, Isik A, et al. Comparison of GnRH agonists and antagonists in normoresponder IVF/ICSI in Turkish female patients. Advances in Therapy 2008;25(3):266‐73. [DOI] [PubMed] [Google Scholar]
Munoz 2013 {published data only}
- Munoz E, Taboas E, Portela S, Aguilar J, Fernandez I, Munoz L, et al. Treatment of luteal phase defects in assisted reproduction. Current Drug Targets 2013;14(8):832‐42. [DOI] [PubMed] [Google Scholar]
Nader 1988 {published data only}
- Nader S, Berkowitz AS, Ochs D, Held B, Winkel CA. Luteal‐phase support in stimulated cycles in an in vitro fertilization/embryo transfer program: progesterone versus human chorionic gonadotropin. Journal of In Vitro Fertilization and Embryo Transfer 1988;5:81‐4. [DOI] [PubMed] [Google Scholar]
NCT01007851 2006 {published data only}
- NCT01007851. Single Dose Gonadotropin‐releasing Hormone (GnRH) Agonist Administration in the Luteal Phase of GnRH Antagonist Stimulated ICSI‐ET Cycles. https://clinicaltrials.gov/ct2/show/NCT01007851 2006. [MEDLINE: ]
Nikkanen 1992 {published data only}
- Nikkanen V, Kresanov I, Makinen J, Vuorento T. The effect of luteal support with human chorionic gonadotrophin or progesterone on the daily progesterone profile after different types of ovarian stimulation. Human Reproduction 1992;7:333‐6. [DOI] [PubMed] [Google Scholar]
Nyboe Andersen 2012 {published data only}
- Nyboe Andersen A, Lauritsen MP, Thuesen LL. Withdrawal of progesterone support on the day of positive hCG after IVF/ICSI has no effect on miscarriage rates. Evidence from two large prospective trials. Human Reproduction 2012;27:Abs# P‐486. [Google Scholar]
Osmanagaoglu 2013 {published data only}
- Osmanagaoglu K, Decleer W, Seynhave B, Kolibianakis E, Tarlatzis B, Devroey P. Prospective randomized controlled trial in gnrh antagonist stimulated cycles comparing HCG triggering alone versus HCG triggering associated with gnrh agonist. Fertility and Sterility 2013;100:S1‐S2. [Google Scholar]
Ozcimen 2004 {published data only}
- Ozcimen EE, Ugur M, Ozcimen N, Yilmaz Z. Is luteal phase support with hCG or vaginal micronised progesterone beneficial in non‐IVF gonadotropin induction of ovulation?. Fertility and Sterility 2004;82 Suppl 2:142. [Google Scholar]
Papanikolaou 2010 {published data only}
- Papanikolaou E, Verpoest W, Fatemi H, Polyzos N, Humaidan P, Tarlatzis B, et al. Recombinant LH as luteal supplementation method after agonist triggering in IVF. A proof of concept study [abstract]. Human Reproduction. 2010; Vol. 25:i167‐8 (Abs #P‐134).
- Papanikolaou EG, Fatemi H, Kyrou D, Polyzos NP, Humaidan P, Tarlatzis B, et al. Higher birth rate after recombinant hCG triggering compared with urinary‐derived hCG in single‐blastocyst IVF antagonist cycles: a randomized controlled trial. Fertility and Sterility 2010;94(7):2902‐4. [DOI] [PubMed] [Google Scholar]
Papanikolaou 2011 {published data only}
- Papanikolaou EG, Verpoest W, Fatemi H, Tarlatzis B, Devroey P, Tournaye H. A novel method of luteal supplementation with recombinant luteinizing hormone when a gonadotropin‐releasing hormone agonist is used instead of human chorionic gonadotropin for ovulation triggering: a randomized prospective proof of concept study. Fertility and Sterility 2011;95:1174‐7. [DOI] [PubMed] [Google Scholar]
Paredes 2004 {published data only}
- Paredes Chavez FC, Barros Delgadillo JC, Ochoa Rueda SS, Barroso Villa G, et al. [Papel de los estrógenos en el soporte de la fase lútea en ciclos de fertilización in vitro con transferencia de embriones]. Ginecología y Obstetricia de México 2004;72(12):645‐55. [PubMed] [Google Scholar]
Pirard 2005 {published data only}
- Pirard C, Donnez J, Loumaye E. GnRH agonist as novel luteal support: results of a randomized, parallel group, feasibility study using intranasal administration of buserelin. Human Reproduction 2005;20(7):1798‐804. [DOI] [PubMed] [Google Scholar]
Pirard 2006 {published data only}
- Pirard C, Donnez J, Loumaye E. GnRH agonist as luteal phase support in assisted reproduction technique cycles: results of a pilot study. Human Reproduction 2006;21(7):1894‐900. [DOI] [PubMed] [Google Scholar]
Polson 1992 {published data only}
- Polson DW, Rogers PAW, Krapez JA, Leeton JF. Vaginal progesterone as luteal phase support in an IVF/GIFT programme. European Journal of Obstetrics Gynecology and Reproductive Biology 1992;46(1):35‐8. [DOI] [PubMed] [Google Scholar]
Priyadharshini 2013 {published data only}
- Priyadharshini M, Sathya B, Varma T. Dydrogesterone is not inferior to natural progesterone for luteal support in ART (IVF/ICSI) cycles. BJOG: An International Journal of Obstetrics and Gynaecology 2013;120:214. [Google Scholar]
Propst 2012 {published data only}
- Propst AM, Thoppil JJ, Groll JM, Frattarelli JL, Robinson RD, Retzloff MG. A single pre‐ovulatory IUI at 12 hours after hCG trigger is comparable to a traditional IUI at 36 hours. Fertility and Sterility 2012;98(Suppl 1):S85‐S86 (Abs# O‐288). [Google Scholar]
Santibanez 2014 {published data only}
- Santibanez A, Garcia J, Pashkova O, Colin O, Castellanos G, Sanchez AP, Jara JF. Effect of intrauterine injection of human chorionic gonadotropin before embryo transfer on clinical pregnancy rates from in vitro fertilisation cycles: a prospective study. Reproductive Biology and Endocrinology 2014;12:9‐13. [DOI: 10.1186/1477-7827-12-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Satir 2013 {published data only}
- Satir F, Toptas T, Inel M, Erman‐Akar M, Taskin O. Comparison of intravaginal progesterone gel and intramuscular 17‐alpha‐hydroxyprogesterone caproate in luteal phase support. Experimental and Therapeutic Medicine 2013;5(6):1740‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwarzler 2003 {published data only}
- Schwarzler P, Abendstein BJ, Klingler A, Kreuzer E, Rjosk HK. Prevention of severe ovarian hyperstimulation syndrome (OHSS) in IVF patients by steroidal ovarian suppression ‐ A prospective randomized study. Human Fertility 2003;6(3):125‐9. [DOI] [PubMed] [Google Scholar]
Shamma 1992 {published data only}
- Shamma F, Haj‐Hassan L, Penzias A, Gutmann J, Leykin L, Jones E. Luteal phase support in in vitro fertilization embryo transfer (IVF‐ET) ‐ a prospective randomized trial [abstract]. American Fertility Society 48th Annual Meeting Abstract Book. 1992:S140‐1 (Abs # P‐074).
Silverberg 2010 {published data only}
Simunic 2007 {published data only}
- Simunic V, Tomic V, Tomic J, Nizic D. Comparative study of the efficacy and tolerability of two vaginal progesterone formulations, Crinone 8% gel and Utrogestan capsules, used for luteal support. Fertility and Sterility 2007;87(1):83‐7. [DOI] [PubMed] [Google Scholar]
Singh 2010 {published data only}
- Singh T, Majumdar A. Supplementation of gonadotrophin releasing hormone (GnRH) agonist during the luteal phase improves the pregnancy outcome in intrauterine insemination (IUI) cycles, when compared with human chorionic gonadotrophin (hCG). Journal fur Reproduktionsmedizin und Endokrinologie. Munich, Germany, 2010:277 (Abs 15‐7).
Smith 1989 {published data only}
- Smith EM, Anthony FW, Gadd SC, Masson GM. Trial of support treatment with human chorionic gonadotrophin in the luteal phase after treatment with buserelin and human menopausal gonadotrophin in women taking part in an in vitro fertilisation programme. BMJ 1989;298:1483‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smitz 1988 {published data only}
- Smitz J, Devroey P, Camus M, Deschacht J, Khan I, Staessen C, et al. The luteal phase and early pregnancy after combined GnRH‐agonist/HMG treatment for superovulation in IVF or GIFT. Human Reproduction 1988;3(5):585‐90. [DOI] [PubMed] [Google Scholar]
Smitz 1992 {published data only}
- Smitz J, Devroey P, Faguer B, Bourgain C, Camus M, Steirteghem A. A prospective randomized comparison of intramuscular or intravaginal natural progesterone as a luteal phase and early pregnancy supplement. Human Reproduction 1992;7:168‐75. [DOI] [PubMed] [Google Scholar]
- Smitz J, Devroey P, Faguer B, Bourgain C, Camus M, Steirteghem AC. A randomized prospective study comparing supplementation of the luteal phase and early pregnancy by natural progesterone administered by intramuscular or vaginal route [Etude prospective randomisee comparant la supplementation de la phase luteale et de la grossesse debutante par la progesterone naturelle administree par voie intra‐musculaire ou vaginale]. Revue Francaise de Gynecologie et d Obstetrique 1992;87(10):507‐16. [PubMed] [Google Scholar]
Smitz 1993 {published data only}
- Smitz J, Bourgain C, Waesgerghe L, Camus M, Devroey P, Steirteghem AC. A prospective randomized study on oestradiol valerate supplementation in addition to intravaginal micronized progesterone in buserelin and HMG induced superovulation. Human Reproduction 1993;8:40‐5. [DOI] [PubMed] [Google Scholar]
Sordal 1993 {published data only}
- Sordal T, Kahn J A, Sunde A, Düring V, Molne K. A prospective randomized study comparing natural progesterone administered intramuscularly and vaginal micronized progesterone for luteal support [abstract]. Human Reproduction 1993;8 Suppl 1:39 (Abs # 99). [Google Scholar]
Stadtmauer 2009 {published data only}
- Stadtmauer L, Harrison DD, Boyd J, Bocca S, Oehninger S. Pilot study evaluating a progesterone vaginal ring for luteal‐phase replacement in donor oocyte recipients. Fertility and Sterility 2009;92(5):1600‐5. [DOI] [PubMed] [Google Scholar]
Stovall 1998 {published data only}
- Stovall DW, Voorhis BJ, Sparks AE, Adams LM, Syrop CH. Selective early elimination of luteal support in assisted reproduction cycles using a gonadotropin‐releasing hormone agonist during ovarian stimulation. Fertility & Sterility 1998;70(6):1056‐62. [DOI] [PubMed] [Google Scholar]
Tay 2003 {published data only}
- Tay PY, Lenton EA. Inhibition of progesterone secretion by oestradiol administered in the luteal phase of assisted conception cycles. Medical Journal of Malaysia 2003;58(2):187‐95. [PubMed] [Google Scholar]
Tomic 2011 {published data only}
- Tomic V, Tomic J, Klaic DZ. Oral micronized progesterone combined with vaginal progesterone gel for luteal support. Gynecological Endocrinology 2011;27:1010‐3. [DOI] [PubMed] [Google Scholar]
Trounson 1986 {published data only}
- Trounson A, Howlett D, Rogers P, Hoppen HO. The effect of progesterone supplementation around the time of oocyte recovery in patients superovulated for in vitro fertilization. Fertility and Sterility 1986;45:532‐5. [DOI] [PubMed] [Google Scholar]
Unfer 2004 {published data only}
- Unfer V, Casini M, Costabile L, Gerli S, Baldini D, Renzo GC. 17 alpha‐hydroxyprogesterone caproate versus intravaginal progesterone in IVF‐embryo transfer cycles: a prospective randomized study. Reproductive BioMedicine Online 2004;9(1):17‐21. [DOI] [PubMed] [Google Scholar]
Unfer 2004a {published data only}
- Unfer V, Casini ML, Gerli S, Costabile L, Mignosa M, Renzo GC. Phytoestrogens may improve the pregnancy rate in in vitro fertilization‐embryo transfer cycles: a prospective, controlled, randomized trial. Fertility and Sterility 2004;82(6):1509‐13. [DOI] [PubMed] [Google Scholar]
Vaisbuch 2012 {published data only}
- Vaisbuch E, Leong M, Shoham Z. Progesterone support in IVF: is evidence‐based medicine translated to clinical practice? A worldwide web‐based survey. Reproductive Biomedicine Online 2012;25(2):139‐45. [DOI] [PubMed] [Google Scholar]
Valentino 2004 {published data only}
- Valentino V, Artini PG, Ruggiero M, Parisen Toldin MR, Cristello F, et al. A randomised comparison of effects and patient inconvenience of two progesterone supplementation used in in vitro fertilisation cycles. Gynaecological Endocrinology 2004;18 Suppl 1:358 (Abs # P‐170). [Google Scholar]
van Steirteghem 1988 {published data only}
- Steirteghem AC, Smitz J, Camus M, Waesberghe L, Deschacht J, Khan I, et al. The luteal phase after in‐vitro fertilization and related procedures. Human Reproduction 1988;3:161‐4. [DOI] [PubMed] [Google Scholar]
Var 2011 {published data only}
- Var T, Aysin Tonguc E, Doganay M, Gulerman C, Gungor T, Mollamahmutoglu L. A comparison of the effects of three different luteal phase support protocols on in vitro fertilization outcomes: a randomized clinical trial. Fertility and Sterility 2011;95:985‐9. [DOI] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang LJ, Huang FJ, Kung FT, Lin PY, Chang SY, Lan KC. Comparison of the efficacy of two vaginal progesterone formulations, Crinone 8% gel and Utrogestan capsules, used for luteal support in blastocyst stage embryo transfers. Taiwanese Journal of Obstetrics & Gynecology 2009;48(4):375‐9. [DOI] [PubMed] [Google Scholar]
Wilcox 2001 {published data only}
- Wilcox J, Nelson JR, Potter D, Frederick J, Feinman M, Batzofin J. Comparison of different luteal phase support protocols for frozen embryo transfer (FET) [abstract]. Fertility and Sterility 2001;76 Suppl 1:124 (Abs # P‐37). [Google Scholar]
Yazici 2014 {published data only}
- Yazici G, Savas A, Tasdelen B, Dilek S. Role of luteal phase support on gonadotropin ovulation induction cycles in patients with polycystic ovary syndrome. Journal of Reproductive Medicine 2014;59:25‐30. [PubMed] [Google Scholar]
Ye 2009 {published data only}
- Ye H, Huang GN, Zeng PH, Pei L. IVF/ICSI outcomes between cycles with luteal estradiol (E2) pre‐treatment before GnRH antagonist protocol and standard long GnRH agonist protocol: a prospective and randomized study. Journal of Assisted Reproduction and Genetics 2009;26(2‐3):105‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yigit 2002 {published data only}
- Yigit N, Halicigil C, Basaran M, Aksu T, Yarali H. Crinone and i.m. progesterone yield comparable pregnancy rates following ICSI and embryo transfer [title only]. Human Reproduction 2002;17(Abstract Book 1):201 (Abs # R‐624). [Google Scholar]
Yovich 1984 {published data only}
- Yovich JL, Stanger JD, Yovich JM, Tuvik AI. Assessment and hormonal treatment of the luteal phase of in vitro fertilization cycles. Australian & New Zealand Journal of Obstetrics & Gynaecology 1984;24:125‐30. [DOI] [PubMed] [Google Scholar]
Yovich 1985 {published data only}
- Yovich JL, McColm SC, Yovich JM, Matson PL. Early luteal serum progesterone concentrations are higher in pregnancy cycles. Fertility and Sterility 1985;44:185‐9. [DOI] [PubMed] [Google Scholar]
Yovich 1991 {published data only}
- Yovich JL, Rohini Edirisinghe W, Cummins JM. Evaluation of luteal support therapy in a randomized controlled study within a gamete intrafallopian transfer program. Fertility and Sterility 1991;55:131‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Pirard 2015 {published data only}
- Pirard C, Loumaye E, Laurent P, Wyns C. Contribution to More Patient‐Friendly ART Treatment: Efficacy of Continuous Low‐Dose GnRH Agonist as the Only Luteal Support‐Results of a Prospective, Randomized, Comparative Study. International Journal of Endocrinology 2015;Apr 05:727569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomic 2015 {published data only}
- Tomic V, Tomic J, Klaic DZ, Kasum M, Kuna K. Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: randomized controlled trial. European journal of obstetrics, gynecology, and reproductive biology 2015;186:49‐53. [PUBMED: 25622239] [DOI] [PubMed] [Google Scholar]
Zafardoust 2015 {published data only}
- Zafardoust S, et al. Effect of administration of single dose GnRH agonist in luteal phase on outcome of ICSI‐ET cycles in women with previous history of IVF/ICSI failure: A randomized controlled trial.. Journal of Reproduction and Infertility 2015;16(2):116‐120. [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
EUCTR2012‐002215‐26‐BE 2013 {published data only}
- EUCTR 2012‐002215‐26‐BE. A study to compare if 30mg of oral Dydrogesterone is as good, tolerable and safe as 600mg of intravaginal capsules for luteal support in IVF pregnancies. This study will be conducted at several study sites and neither the patient or the doctor will know which of the two different treatments a patient will receive. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=2012‐002215‐26 2013.
EUCTR2013‐001105‐81‐2013 {published data only}
- EUCTR2013‐001105‐81‐2013. Randomised clinical trial comparing highly purified FSH formulation (Fostimon®) and recombinant FSH (Gonal‐F®) in GnRH‐antagonist controlled ovarian hyperstimulation cycles. EU Clinical Trials Registry.
IRCT201402191141N18 2015 {published data only}
- IRCT201402191141N18. Subcutaneous progesterone versus vaginal suppository for luteal phase support in assisted reproductive technology cycles in patients referred to Royan Institute. https://translate.google.co.nz/translate?hl=en&sl=fa&u=http://www.irct.ir/fa/searchresult.php%3Fkeyword%3D%25D8%25A7%25D8%25AB%25D8%25B1%25D8%25A8%25D8%25AE%25D8%25B4%25DB%258C%26id%3D1141%26field%3D%26number%3D18%26prt%3D80%26total%3D10%26m%3D1&prev=search 2015.
IRCT2014030916912N1 2014 {published data only}
- IRCT2014030916912N1. Effect of administration GNRH agonist(Triptrolin) on clinical pregnancy in ART. https://translate.google.co.nz/translate?hl=en&sl=fa&u=http://www.irct.ir/fa/searchfa.php%3Fkeyword%3Dstimulatio%26page%3D17&prev=search 2014.
IRCT2014071212494N2 2014 {published data only}
- IRCT2014071212494N2. Effect of progesterone on pregnancy rate of assisted reproduction. http://www.irct.ir/searchtotal.php?&page=706 2014.
NCT00490308 2007 {published data only}
- NCT00490308. The Influence of Estradiol Supplementation During the Luteal in Patients Undergoing IVF Treatment. https://clinicaltrials.gov/ct2/show/NCT00490308 2007. [MEDLINE: ]
NCT01081652 {published data only}
- NCT01081652. A Study Using Micronised Progesterone (Crinone® 8%) in the Luteal Phase Support of Women Undergoing in Vitro Fertilisation (IVF) and Embryo Transfer (ET). https://www.clinicaltrials.gov/ct2/show/NCT01081652 2014.
NCT01237535 {published data only}
- NCT01237535. Luteal Phase Support With Progesterone Versus Estrogen and Progesterone on Pregnancy Rates. https://clinicaltrials.gov/ct2/show/NCT01237535 2010.
NCT01504139 2012 {published data only}
- NCT01504139. The Luteal Phase After GnRHa Trigger ‐ a Proof of Concept Study. http://www.ncbi.nlm.nih.gov/pubmed/26209535 2012. [MEDLINE: ]
NCT01638026 2012 {published data only}
- NCT01638026. Final Oocyte Maturation Via Administration of GnRH Agonists Followed By Luteal Support With hCG. https://clinicaltrials.gov/ct2/show/NCT01638026 2012. [MEDLINE: ]
NCT01790282 2013 {published data only}
- NCT01790282. Is Adding E2 to P4 Luteal Support In High Responder Long Gn‐RH Agonist ICSI Cycles Detrimental to Outcome? RCT. https://clinicaltrials.gov/ct2/show/NCT01790282 2013.
NCT01850030 {published data only}
- NCT01850030. A Multicenter Study Comparing the Efficacy, Safety and Tolerability of Oral Dydrogesterone 30 mg Daily Versus Intravaginal Micronized Progesterone Capsules 600 mg Daily for Luteal Support in In‐Vitro Fertilization. https://clinicaltrials.gov/ct2/show/NCT01850030 2015.
NCT01863680 2013 {published data only}
- NCT01863680. Phase 3 Trial to Evaluate the Efficacy and Safety of COL‐1620 Vaginal Progesterone Gel. https://clinicaltrials.gov/ct2/show/NCT01863680 2013.
NCT01980680 2013 {published data only}
- NCT01980680. The Exogenous Progesterone Free Luteal Phase After GnRHa Trigger ‐ a Pilot Study in Normo‐responder IVF Patients. https://clinicaltrials.gov/ct2/show/NCT01980680 2013.
NCT02053779 2014 {published data only}
- NCT02053779. Luteal Phase Plus GnRH‐agonist After GnRH‐agonist Triggering Combined With Low Dose HCG in IVF (LPGGTHI). https://clinicaltrials.gov/ct2/show/NCT02053779 2014.
NCT02114645 2014 {published data only}
- NCT02114645. The Effect of GnRH Agonist Administered in the Luteal Phase on ART Cycle Outcomes. https://clinicaltrials.gov/ct2/show/NCT02114645 2014.
NCT02262416 2014 {published data only}
- NCT02262416. GnRH Agonist and Progesterone Versus Progesterone Only for Luteal Phase Support in Antagonist Cycles. https://clinicaltrials.gov/ct2/show/NCT02262416 2014.
NCT02312076 2014 {published data only}
- NCT02312076. GnRHa for Luteal Phase Support in Long GnRHa Protocol Cycles. https://clinicaltrials.gov/ct2/show/NCT02312076 2014.
NCT02312089 2014 {published data only}
- NCT02312089. GnRHa for Luteal Phase Support in GnRH Antagonist Protocol Cycles. https://clinicaltrials.gov/ct2/show/NCT02312089 2014.
NCT02316626 2014 {published data only}
- NCT02316626. Subcutaneous Progesterone Versus Vaginal Progesterone Gel for Luteal Phase Support. https://clinicaltrials.gov/ct2/show/NCT02316626 2014. [DOI] [PubMed]
NCT02357654 2015 {published data only}
- NCT02357654. GnRH for Luteal Support in IVF/ICSI/FET Cycles. https://clinicaltrials.gov/ct2/show/NCT02357654 2015. [MEDLINE: ]
NCT02491437 {published data only}
- NCT02491437. A Randomized, Open‐label, Two‐arm, Multicenter Study Comparing the Efficacy, Safety and Tolerability of Oral Dydrogesterone 30 mg Daily Versus Crinone 8% Intravaginal Progesterone Gel 90 mg Daily for Luteal Support in In‐Vitro Fertilization (LOTUS II). https://clinicaltrials.gov/ct2/show/NCT02491437 2015.
Additional references
Balasch 2004
- Balasch J. The role of FSH and LH in ovulation induction: current concepts and the contribution of recombinant gonadotropins. In: Gardner DK, Weissman A, Howles and Shoham V editor(s). Textbook of Assisted Reproductive Techniques. Laboratory and Clinical Perspectives. 2nd Edition. London and New York: Taylor & Francis, 2004:541‐65. [Google Scholar]
CDC 2009
- Centers for Disease Control and Prevention ASfRM, Society for Assisted Reproductive Technology 2007. Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2009.
Chan 2003
- Chan CC, Ng EH, Chan MM, Tang OS, Lau EY, Yeung WS, et al. Bioavailability of hCG after intramuscular or subcutaneous injection in obese and non‐obese women. Human Reproduction 2003;18(11):2294‐7. [DOI] [PubMed] [Google Scholar]
Choavaratana 2004
- Choavaratana R, Manoch D. Efficacy of oral micronized progesterone when applied via vaginal route. Journal of the Medical Association of Thailand 2004;87(5):455‐8. [PubMed] [Google Scholar]
de Mouzon 2010
- Mouzon J, Goossens V, Bhattacharya S, Castilla JA, Ferraretti AP, Korsak V, et al. Assisted reproductive technology in Europe, 2006: results generated from European registers by ESHRE. Human Reproduction 2010;25(8):1851‐62. [DOI] [PubMed] [Google Scholar]
Edwards 1980
- Edwards RG, Steptoe PC, Purdy JM. Establishing full‐term human pregnancies using cleaving embryos grown in vitro. British Journal of Obstetrics and Gynaecology 1980;87(9):737‐56. [DOI] [PubMed] [Google Scholar]
Farquhar 2010
- Farquhar C, Roberts H. Introduction to Obstetrics and Gynaecology. Third Edition. Auckland: Department of Obstetrics & Gynaecology, The University of Auckland, 2010. [Google Scholar]
Fatemi 2009
- Fatemi HM. The luteal phase after 3 decades of IVF: what do we know?. Reproductive Biomedicine Online 2009;19(4):4331. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Kerin 1981
- Kerin JF, Broom TJ, Ralph MM, Edmonds DK, Warnes GM, Jeffrey R, et al. Human luteal phase function following oocyte aspiration from the immediately preovular graafian follicle of spontaneous ovular cycles. British Journal of Obstetrics and Gynaecology 1981;88(10):1021‐8. [DOI] [PubMed] [Google Scholar]
Macaldowie 2014
- Macaldowie A, Wang YA, Chughtai AA, Chambers GM. Assisted reproductive technology in Australia and New Zealand 2012. Sydney: National Perinatal Epidemiology and Statistics Unit, the University of New South Wales. 2014.
Mannaerts 1998
- Mannaerts BM, Geurts TB, Odink J. A randomized three‐way cross‐over study in healthy pituitary‐suppressed women to compare the bioavailability of human chorionic gonadotrophin (Pregnyl) after intramuscular and subcutaneous administration. Human Reproduction 1998;13(6):1461‐4. [DOI] [PubMed] [Google Scholar]
Messinis 2009
- Messinis IE, Messini CI, Dafopoulos K. Luteal‐phase endocrinology. Reproductive Biomedicine Online 2009;19(4):4314. [PubMed] [Google Scholar]
Miyake 1979
- Miyake A, Aono T, Kinugasa T, Tanizawa O, Kurachi K. Suppression of serum levels of luteinizing hormone by short‐ and long‐loop negative feedback in ovariectomized women. Journal of Endocrinology 1979;80(3):353‐6. [DOI] [PubMed] [Google Scholar]
Mochtar 2007
- Mochtar MH, Veen F, Ziech M, Wely M, Musters A. Recombinant luteinizing hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005070] [DOI] [PubMed] [Google Scholar]
Pabuccu 2005
- Pabuccu R, Akar ME. Luteal phase support in assisted reproductive technology. Current Opinion in Obstetrics and Gynecology 2005;17(3):277‐81. [DOI] [PubMed] [Google Scholar]
Saal 1991
- Saal W, Glowania HJ, Hengst W, Happ J. Pharmacodynamics and pharmacokinetics after subcutaneous and intramuscular injection of human chorionic gonadotropin. Fertility and Sterility 1991;56(2):225‐9. [PubMed] [Google Scholar]
Schindler 2009
- Schindler AE. Progestational effects of dydrogesterone in vitro, in vivo and on the human endometrium. Maturitas 2009;65(1):S3‐11. [DOI] [PubMed] [Google Scholar]
Smitz 1992a
- Smitz J, Erard P, Camus M, Devroey P, Tournaye H, Wisanto A, et al. Pituitary gonadotrophin secretory capacity during the luteal phase in superovulation using GnRH‐agonists and HMG in a desensitization or flare‐up protocol. Human Reproduction 1992;7(9):1225‐9. [DOI] [PubMed] [Google Scholar]
Tavaniotou 2003
- Tavaniotou A, Devroey P. Effect of human chorionic gonadotropin on luteal luteinizing hormone concentrations in natural cycles. Fertility and Sterility 2003;80(3):654‐5. [DOI] [PubMed] [Google Scholar]
Tesarik 2004
- Tesarik J, Hazout A, Mendoza C. Enhancement of embryo developmental potential by a single administration of GnRH agonist at the time of implantation. Human Reproduction 2004;19(5):1176‐80. [DOI] [PubMed] [Google Scholar]
Wikland 1995
- Wikland M, Borg J, Forsberg AS, Jakobsson AH, Svalander P, Waldenstrom U. Human chorionic‐gonadotropin self‐administered by the subcutaneous route to induce oocyte maturation in an in‐vitro fertilization and embryo‐transfer program. Human Reproduction 1995;10(7):1667‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Daya 2004
- Daya S, Gunby J. Luteal phase support in assisted reproduction cycles. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004830.pub2] [DOI] [PubMed] [Google Scholar]
van der Linden 2011
- Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD009154.pub2] [DOI] [PubMed] [Google Scholar]


