Abstract
Background
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are one of the most frequently prescribed drugs for the treatment of sciatica. A previous Cochrane review on the efficacy of NSAIDs summarised findings for acute and chronic low back pain (LBP) and sciatica. This is an update of the original review (2008) focusing on people suffering from sciatica.
Objectives
To determine the efficacy of NSAIDs in pain reduction, overall improvement, and reported side effects in people with sciatica.
Search methods
We performed electronic searches up to 24 June 2015 in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PubMed, and two trials registers. We searched reference lists of included studies and relevant reviews on the topics for additional trials.
Selection criteria
We included randomised controlled trials (double‐blind, single‐blind, and open‐label) that assessed the efficacy of NSAIDs in sciatica. We included all trials that compared NSAIDs to placebo, to other NSAIDs, or to other medication. Additional interventions were allowed if there was a clear contrast for the treatment with NSAIDs in the trial.
Data collection and analysis
Three review authors independently assessed the risk of bias and extracted the data. Where feasible we calculated pooled results using Review Manager 5.3. We reported pain relief outcomes using mean difference (MD) with 95% confidence intervals (95% CI). We used risk ratios (RR) with 95% CI to report global improvement of treatment, adverse effects, and additional medication. We performed a meta‐analysis if possible. We assessed level of evidence using the GRADE approach. We used standard methodological procedures recommended by The Cochrane Collaboration.
Main results
We included 10 trials reported in 9 publications (N = 1651). Only one trial out of 10 was assessed at low risk of bias. Five trials used the currently recommended daily dose for the drug, and two trials used lower daily doses available over the counter. Three trials investigated NSAIDs no longer approved for human use. The follow‐up duration was short in all studies but one.
Three trials (n = 918) compared the effects of NSAIDs to those of placebo on pain reduction. The pooled mean difference showed comparable pain reduction (visual analogue scale, 0 to 100) in the NSAIDs and placebo groups (MD ‐4.56, 95% CI ‐11.11 to 1.99). Heterogeneity was high (I2 = 82%), and the quality of the evidence was very low. When we excluded one trial with a short follow‐up of eight hours, the mean difference further decreased (MD ‐0.09, 95% CI ‐9.89 to 9.71). Three trials (n = 753) compared NSAIDs to placebo regarding global improvement. We found low‐quality evidence that NSAIDs are more effective than placebo with a risk ratio of 1.14 (95% CI 1.03 to 1.27). One trial (n = 214) studied the effect of NSAIDs on disability, finding very low‐quality evidence that NSAIDs are no more effective than placebo on disability. Four trials (n = 967) comparing NSAIDs to placebo reported adverse effects, with low‐quality evidence that the risk for adverse effects is higher in the NSAID group than in the placebo group (RR 1.40, 95% CI 1.02 to 1.93). The adverse effects reported in this review are consistent with those previously reported in the literature.
Authors' conclusions
This updated systematic review including 10 trials evaluating the efficacy of NSAIDs versus placebo or other drugs in people with sciatica reports low‐ to very low‐level evidence using the GRADE criteria. The efficacy of NSAIDs for pain reduction was not significant. NSAIDs showed a better global improvement compared to placebo. These findings must be interpreted with caution, as the level of evidence according to the GRADE classification was very low for the outcome pain reduction and low for global improvement due to small study samples, inconsistent results, imprecision, and a high risk of bias in the included trials. While the trials included in the analysis were not powered to detect potential rare side effects, we found an increased risk for side effects in the short‐term NSAIDs use. As NSAIDs are frequently prescribed, the risk‐benefit ratio of prescribing the drug needs to be considered.
Keywords: Humans; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/therapeutic use; Cyclooxygenase Inhibitors; Cyclooxygenase Inhibitors/therapeutic use; Randomized Controlled Trials as Topic; Sciatica; Sciatica/drug therapy
Plain language summary
Non‐steroidal anti‐inflammatory drugs for low back pain with sciatica
Review question
We reviewed the evidence regarding the effect of non‐steroidal anti‐inflammatory drugs (NSAIDs) among people with sciatica on pain reduction, overall improvement and side effects. NSAIDs were compared to placebo, other NSAIDs, or other drugs.
Background
NSAIDs are the most frequently prescribed medication worldwide and are commonly used to treat low back pain with or without pain radiating to the leg (sciatica).
Study characteristics
We searched for both published and unpublished trials up to 24 June 2015. We included 10 trials (reported in 9 publications) with 1651 participants that compared NSAIDs with placebo or other drugs. Participants in the trials were 16 to 75 years of age and reported sciatica. The trials followed the participants for a short time, up to three weeks.
Key results
NSAIDs are no more effective in reducing pain in sciatica than placebo or other drugs. NSAIDs are more effective in overall improvement compared to placebo or other drugs, but this finding should be interpreted with caution as the methodological quality of included trials is low. There is an increased risk of side effects when using NSAIDs compared to placebo. In light of the potentially serious adverse effects associated with NSAIDs, and as this drug is frequently prescribed, high‐quality trials in different patient populations are warranted to address the short‐ and long‐term benefits and long‐term risks of NSAIDs.
Quality of the results
The quality of the evidence in this review ranged from very low to low that NSAIDs are more effective than placebo, therefore the results of this review should be interpreted with caution.
Summary of findings
Summary of findings for the main comparison. NSAIDs compared with placebo for low back pain with sciatica.
| NSAIDs compared with placebo for low back pain with sciatica | ||||||
|
Patient or population: people with low back pain with sciatica Settings: mainly outpatient treatment Intervention: NSAID Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | NSAID | |||||
|
Change in pain intensity from baseline on VAS (0 to 100) short‐term follow‐up of immediate pain reduction up to 3 weeks |
The mean decrease in pain ranged across control groups from ‐13.7 to ‐40 (SD 13.4 to 26.8) on VAS (0 to 100) | The mean decrease in the intervention groups was from ‐11 to ‐45 (SD 13.5 to 26.1) |
Pooled MD ‐4.56 (95% CI ‐11.11 to 1.99; random‐effects model; I2 = 82%). The mean difference ranged between 5.0 (95% CI ‐0.44, 10.44) (Weber 1993) and ‐10.3 (95% CI ‐10.38, ‐4.22) (Herrmann 2009). In a sensitivity analysis excluding Herrmann 2009 (short follow‐up of 8 hours), the effect was ‐0.09 (95% CI ‐9.89 to 9.71) |
N = 918 (3 trials; 1 trial with 2 arms) |
⊕⊝⊝⊝ very low | We downgraded 2 levels due to high risk of bias1 and 1 level due to inconsistency2 |
| Change in disability from baseline to follow‐up | The decrease in the modified RMDQ score was 5.8 points (no SD reported) | The decrease in the modified RMDQ was 5.8 points (no SD reported) | No pooled estimate, no difference between the groups (1 trial, Weber 1993) |
N = 214 (1 trial) |
⊕⊝⊝⊝ very low | We downgraded 2 levels due to high risk of bias3, 1 level due to inconsistency (only 1 trial) and imprecision (only 1 trial)4 |
|
Global Improvement short‐term follow‐up of immediate pain reduction up to 3 weeks |
All participants |
Pooled RR 1.14 (95% CI 1.03 to 1.27; fixed‐effect model; I2 = 0%). The NNTB was 12 participants based on an absolute risk difference of 0.09 (95% CI 0.02, 0.16) |
N = 753 (3 trials, 1 trial with 2 arms) |
⊕⊕⊝⊝ low | We downgraded 2 levels due to high risk of bias5 |
|
| 622 per 1000 | 729 per 1000 (280 to 770) | |||||
|
Side effects short‐term follow‐up of immediate pain reduction up to 3 weeks |
All participants |
Pooled RR 1.40 (95% CI 1.02 to 1.93; fixed‐effect model; I2 = 0%). The NNTH was 20 participants based on an absolute risk difference of 0.05 (95% CI 0.00, 0.10) |
N = 967 (4 trials, 1 trial with 2 arms) | ⊕⊕⊝⊝ low | We downgraded 2 levels due to high risk of bias5 |
|
| 129 per 1000 | 175 per 1000 (122 to 183) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; NSAID: non‐steroidal anti‐inflammatory drug; RMDQ: Roland Morris Disability Questionnaire; RR: risk ratio; SD: standard deviation; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence (Atkins 2004) High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1We assessed two of the included trials with a high risk of bias (with greater than four biases estimated as high or unclear risk) (Dreiser 2001a; Weber 1993). These two trials had an unclear risk of selection bias, unclear risk of detection bias, unclear risk of influence of co‐intervention, and unclear risk of bias regarding funding. In addition, Herrmann 2009 had an unclear risk of bias regarding funding and unclear risk of bias regarding compliance. 2The heterogeneity of the included trials was high (I2 = 82%). 3Weber 1993 had an unclear or a high risk of bias for more than five biases investigated. 4The trial reported on more than 300 events; only a single trial for this outcome. 5We considered only one of the studies to have a low risk of bias (Herrmann 2009). The other studies had selection bias, detection bias, and lack of clarity regarding compliance. All studies had either a high risk of bias or an unclear risk of bias regarding industry funding.
Background
Description of the condition
Low back pain (LBP) is one of the most common medical disorders in the world (Hoy 2014; Vos 2012), affecting functional capacity and work absence and resulting in personal suffering and huge socioeconomic costs (Balague 2012; Hoy 2014). The trajectory of LBP is described by remissions and exacerbations over the lifetime and may be considered as a life‐course pattern instead of leading to a full recovery (Axen 2013; van Tulder 2002). Those who suffer from more disabling and chronic back pain contribute to the majority of the indirect and direct costs associated with LBP (Airaksinen 2006; van Tulder 2006). For effective treatment interventions, factors that influence the prevalence and trajectory of LBP, including the presence of sciatica, lifestyle, social factors, and occupational and psychosocial aspects, need to be taken into account (Manchikanti 2014).
Sciatica is an important subgroup in the heterogeneous group of LBP. The term 'sciatica' describes a symptom and not a specific diagnosis (Koes 2010; Konstantinou 2013; Lewis 2011; Valat 2010; van Tulder 2003). The prevalence of sciatica varies depending on the time period studied: lifetime prevalence is reported as between 12.2% and 43%, period prevalence between 2.2% and 34%, and point prevalence between 1.5% and 13.4% (Koes 2007). The prognosis is considered to be worse and more disabling than common LBP (Koes 2007; Koes 2010; Valat 2010). However, the clinical course of acute sciatica is in general considered to be favourable, and most pain and related disabilities resolve within two weeks (Valat 2010). Several clinical symptoms are associated with sciatica, with the most important symptom being leg pain radiating below the knee and into the foot and toes. Other clinical findings are muscle weakness, sensory changes such as pins and needles or numbness following the dermal pattern, impaired reflexes, or the presence of a positive straight leg raising test (Koes 2007; Koes 2010; Stafford 2007; Valat 2010). If a patient reports the typical radiating pain in one leg combined with a positive result on one or more neurological tests indicating nerve root tension or neurological deficit, the diagnosis of sciatica appears justified (Valat 2010). Sciatica is usually attributed to herniated intervertebral disc, lumbar spinal stenosis, spondylolisthesis, tumours, or cysts. The nerve root can be symptomatic with the presence of both inflammation and compression of the nerve (Koes 2010; Valat 2010). Sciatica or pain radiating to the leg is not always due to an inflammation of the nerve root; nociceptive muscle or joint pain may mimic sciatica symptoms (Cannon 2007; Swezey 2003; Visser 2013).
Description of the intervention
Non‐steroidal anti‐inflammatory drugs (NSAIDs) are one of the most frequently prescribed pain drugs worldwide for treating LBP and sciatica (Roelofs 2008). NSAIDs have been prescribed for pain and inflammation for more than 100 years. In the late 1870s, salicylic acid and phenazone were produced in a synthetic process (Brune 2004). By the end of the 19th century, three prototype substances were available for the treatment of pain, fever, and inflammation: salicylic acid, phenazone, and phenacetin (Brune 2004). After World War II, the discovery of phenylbutazone, a more effective anti‐inflammatory drug, prompted the development of new NSAIDs (Brune 2004). To date, many different NSAIDs exist based on six major chemical structures that differ in their dose, drug interactions, and side effects. NSAIDs aim to provide anti‐inflammatory, antipyretic, and analgesic effects in acute and chronic conditions of pain and inflammation (Dwivedi 2015).
Several trials have examined the effectiveness of various treatments for sciatica such as surgery and conservative treatment. Medication plays an important role in the management of both common LBP and sciatica. The first‐line drug for common back pain is paracetamol (acetaminophen). However, it was recently shown that paracetamol is no more effective than placebo for reducing pain in acute LBP, even if the efficacy does not differ from other drugs (Williams 2014). Guidelines recommend NSAIDs as a treatment option when paracetamol is ineffective (Airaksinen 2006; Koes 2010; van Tulder 2006). The American College of Physicians/American Pain Society consensus guidelines recommend opioid analgesics for the short‐term management of severe and disabling LBP or sciatica in those with no response to paracetamol or NSAIDs (Chou 2007; Kreiner 2014). However, an updated Cochrane review on the efficacy of opioids concluded that there is no difference in efficacy between opioids and NSAIDs on pain reduction in the treatment of sciatica (Chaparro 2013). Various drugs are recommended for the treatment of sciatica: NSAIDs, opioid analgesics, muscle relaxants, antidepressants, systemic corticosteroids, and anticonvulsants (Cherkin 1998; Kreiner 2014).
To date, there is low evidence to support the efficacy of analgesics in sciatica (Luijsterburg 2007; Roelofs 2008; Valat 2010). A previous Cochrane review on the efficacy of NSAIDs in acute and chronic LBP and in a sciatica population concluded that when compared to placebo NSAIDs do not have any effect in sciatica (Roelofs 2008). This is also in accordance with two previous reviews (Pinto 2012; Wong 2015).
How the intervention might work
The main anti‐inflammatory, antipyretic, and analgesic effect of NSAIDs is based on the suppression of the cyclooxygenase (COX)‐1 and COX‐2 enzymes. By blocking the COX enzymes, vasodilation is reduced and inflammation relieved. Further, the synthesis of prostaglandins is blocked, leading to reduced pain (AOOS 2009; Dwivedi 2015). The NSAIDs block the prostaglandin synthesis similar to steroids but without the side effects observed in steroids. Conventional NSAIDs block COX‐1 and COX‐2 enzymes unselectively. Conventional NSAIDs include aspirin, ibuprofen, diclofenac, indomethacin, naproxen, and piroxicam (Rao 2008). Selective COX‐2 inhibiting NSAIDs are available, which inhibit the COX‐2 enzyme with a 5‐50 fold selectivity. Examples of selective COX‐2 inhibiting NSAIDs are celecoxib, etodolac, meloxicam, and nimesulide (Rao 2008). Rofecoxib inhibits the COX‐2 enzyme with a > 50 fold selectivity (Rao 2008). Rofecoxib was withdrawn from the market due to an increased risk of cardiovascular events.
NSAIDs are responsible for various side effects; gastrointestinal, cardiovascular, renal, and hepatotoxic side effects are described (Brune 2015). The well‐known gastrointestinal side effects of NSAIDs are caused by the blocking of the COX‐1 enzyme, which leads to a reduction in mucosal prostaglandin synthesis and its protective effects. NSAIDs are therefore associated with an increased risk for early gastrointestinal complications (for example ulcer, bleeding).
Why it is important to do this review
This Cochrane review is an update of a previous Cochrane review on the effect of NSAIDs on acute and chronic LBP and sciatica (Roelofs 2008). This review focuses on the effect of NSAIDs and sciatica. NSAIDs are commonly prescribed for sciatica, and the response to NSAIDs may differ in sciatica compared to common LBP. Although NSAIDs are commonly recommended and prescribed, only a few studies have assessed their efficacy. Given the potential adverse effects of NSAIDs, it is important to analyse the evidence on the efficacy of these drugs.
Objectives
This review aims to determine the efficacy of NSAIDs in pain reduction, overall improvement, and reported side effects in people with sciatica.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) (double‐blind, single‐blind, and open‐label). We used no language restriction.
Types of participants
Inclusion criteria
We included people aged 16 years or older with acute, subacute (less than 12 weeks), and chronic (more than 12 weeks) sciatica.
We defined sciatica as pain radiating to one or both legs below the knee with some of the following signs:
positive straight leg raising test, or Lasègue sign;
positive leg raising test presenting with numbness, pins or needles in a dermatomal distribution;
muscle weakness or reflex changes or both in a myotome distribution.
Exclusion criteria
We excluded people with sciatica caused by specific pathological entities such as infection, neoplasm, metastasis, osteoporosis, rheumatoid arthritis, or fractures.
Types of interventions
We included RCTs that investigated one or more types of NSAIDs. Additional interventions were allowed if there was a contrast for the treatment with NSAIDs in the trial. We considered trials with the following comparisons:
NSAIDs compared to placebo;
NSAIDs compared to NSAIDs;
NSAIDs compared to other pharmacological agents, alone or in combination (e.g. corticosteroids, muscle relaxants, antidepressants).
We excluded trials that compared NSAIDs given in combination with other pharmacological agents (for example NSAIDs, muscle relaxants, antidepressants) or non‐pharmacological treatments compared to another intervention (that is without a contrast for NSAIDs). We excluded trials that compared NSAIDs to non‐drug treatments.
NSAIDs were categorised according to the World Health Organization and Anatomical Therapeutic Chemical (ATC) classification system into the following six groups: butylpyrazolidines (e.g. phenylbutazone), acetic acid derivatives and related substances (e.g. indomethacin, diclofenac), oxicams (e.g. piroxicam, meloxicam, lornoxicam), propionic acid derivatives (e.g. ibuprofen, naproxen), fenamates (e.g. mefenamic acid), coxibs (e.g. celecoxib, rofecoxib), others (e.g. nimesulide).
Types of outcome measures
Primary outcomes
Change in pain intensity (e.g. visual analogue scale (VAS) or numerical rating scale (NRS))
Change in disability or functional status (reported on e.g. Oswestry Disability Questionnaire or Roland Morris Disability Questionnaire (RMDQ))
Global measures (e.g. overall improvement)
Adverse effects (proportions of participants experiencing adverse effects of NSAIDs) graded according to the standardised definitions published by the National Cancer Institute of the National Institutes of Health into mild (grade 1), moderate (grade 2), severe (grade 3), or life‐threatening (grade 4).
Secondary outcomes
Return to work status or productivity
Additional use of pain medication
We grouped all outcome measures based on follow‐up duration, that is short term (up to three weeks) and studies analysing long‐term outcome.
Search methods for identification of studies
Electronic searches
We searched the following databases, without language restrictions, to 24June 2015 for RCTs meeting the inclusion criteria.
Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library, Issue 5, 2015; includes the Cochrane Back and Neck (CBN) Review Group's Trials Register)
MEDLINE (OvidSP, 1946 to June Week 2 2015)
MEDLINE In‐Process & Other Non‐Indexed Citations (OvidSP, 23 June 2015)
EMBASE (OvidSP, 1980 to 2015 Week 25)
ClinicalTrials.gov
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP)
PubMed
The Information Specialist of the CBN conducted the searches annually between 2012 and 2015. We added ClinicalTrials.gov and WHO ICTRP to the search in 2013, MEDLINE In‐Process & Other Non‐Indexed Citations in 2014, and PubMed in 2015 in order to identify studies not in MEDLINE using the strategy recommended by Duffy 2014.
The complete search strategy is presented in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5. We developed strategies in accordance with the CBN method guidelines and the Cochrane Handbook for Systematic Reviews of Interventions (Furlan 2015; Higgins 2011).
Searching other resources
We screened the reference lists of all included trials. We screened previous reviews regarding sciatica for additional trials not retrieved by the search. We have included all trials on NSAIDs and sciatica that were included in the original version of the review (Roelofs 2008)
Data collection and analysis
Selection of studies
Several review authors (ERB, MW, WG, PR, BK) independently screened titles, abstracts, and keywords to identify trials that met the inclusion criteria. We obtained full texts of trials if either the study seemed to meet the inclusion criteria or if inclusion was uncertain. Disagreements were solved by consensus of the review authors or third‐party arbitration (UH).
Two review authors (ERB, MW) independently read in full text all potentially eligible trials identified by the title and abstract screening for inclusion. We used no language restriction. Alternative researchers with specific language proficiencies were used for non‐English language references (Dutch, German, French, and Polish).
Data extraction and management
Two review authors (ERB, MW) extracted the data from the trials, and another review author (WG) checked the data extraction. We extracted data on characteristics of participants, interventions, primary and secondary outcomes, adverse effects, and industry sponsorship of the trial. Three review authors (ERB, MW, UH) extracted the mean difference scores, standard deviations, and sample size using a data extraction form. We resolved all disagreements through discussion.
In the case of potentially relevant missing information in the papers, we contacted the corresponding authors. If we could not obtain the additional information, we referred to the Cochrane Handbook for Systematic Reviews of Interventions for further handling of missing variables. When there was no information available for calculating a corresponding standard deviation to a mean change, we used the standard deviation from the most similar trial, as recommended by the Cochrane Handbook (Higgins 2011).
Assessment of risk of bias in included studies
Three review authors (ERB, MW, WG) independently assessed the risk of bias of the included studies based on criteria described in the CBN's tool for assessing risk of bias (Furlan 2015). We rated each criterion as low risk, high risk, or unclear (Table 2; Table 3).
1. Sources of risk of bias.
| Bias domain | Source of bias | Possible answers |
| Selection | (1) Was the method of randomisation adequate? | Yes/No/Unsure |
| Selection | (2) Was the treatment allocation concealed? | Yes/No/Unsure |
| Performance | (3) Was the patient blinded to the intervention? | Yes/No/Unsure |
| Performance | (4) Was the care provider blinded to the intervention? | Yes/No/Unsure |
| Detection | (5) Was the outcome assessor blinded to the intervention? | Yes/No/Unsure |
| Attrition | (6) Was the drop‐out rate described and acceptable? | Yes/No/Unsure |
| Attrition | (7) Were all randomised participants analysed in the group to which they were allocated? | Yes/No/Unsure |
| Reporting | (8) Are reports of the study free of suggestion of selective outcome reporting? | Yes/No/Unsure |
| Selection | (9) Were the groups similar at baseline regarding the most important prognostic indicators? | Yes/No/Unsure |
| Performance | (10) Were co‐interventions avoided or similar? | Yes/No/Unsure |
| Performance | (11) Was the compliance acceptable in all groups? | Yes/No/Unsure |
| Detection | (12) Was the timing of the outcome assessment similar in all groups? | Yes/No/Unsure |
| Other | (13) Are other sources of potential bias unlikely? | Yes/No/Unsure |
2. Criteria for a judgement of "yes" for the sources of risk of bias.
| 1 | A random (unpredictable) assignment sequence. Examples of adequate methods are coin toss (for studies with 2 groups), rolling a dice (for studies with 2 or more groups), drawing of balls of different colours, drawing of ballots with the study group labels from a dark bag, computer‐generated random sequence, preordered sealed envelopes, sequentially‐ordered vials, telephone call to a central office, and preordered list of treatment assignments. Examples of inadequate methods are: alternation, birth date, social insurance/security number, date in which they are invited to participate in the study, and hospital registration number. |
| 2 | Assignment generated by an independent person not responsible for determining the eligibility of the patients. This person has no information about the persons included in the trial and has no influence on the assignment sequence or on the decision about eligibility of the patient. |
| 3 | Index and control groups are indistinguishable for the patients or if the success of blinding was tested among the patients and it was successful. |
| 4 | Index and control groups are indistinguishable for the care providers or if the success of blinding was tested among the care providers and it was successful. |
| 5 | Adequacy of blinding should be assessed for each primary outcome separately. This item should be scored "yes" if the success of blinding was tested among the outcome assessors and it was successful or:
|
| 6 | The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and dropouts does not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and does not lead to substantial bias, a ‘‘yes’’ is scored. (N.B. these percentages are arbitrary and not supported by literature) |
| 7 | All randomised patients are reported/analysed in the group they were allocated to by randomisation for the most important moments of effect measurement (minus missing values) irrespective of noncompliance and co‐interventions |
| 8 | All the results from all prespecified outcomes have been adequately reported in the published report of the trial. This information is either obtained by comparing the protocol and the report, or in the absence of a protocol, by assessing that the published report includes enough information to make this judgement |
| 9 | Groups have to be similar at baseline regarding demographic factors, duration and severity of complaints, percentage of patients with neurological symptoms, and value of main outcome measure(s) |
| 10 | If there were no co‐interventions or if they were similar between the index and control groups |
| 11 | The reviewer determines if the compliance with the interventions is acceptable, based on the reported intensity, duration, number and frequency of sessions for both the index intervention and control intervention(s). For example, physiotherapy treatment is usually administered for several sessions; therefore it is necessary to assess how many sessions each patient attended. For single‐session interventions (e.g. surgery), this item is irrelevant |
| 12 | Timing of outcome assessment should be identical for all intervention groups and for all primary outcome measures |
| 13 | Other types of biases. For example:
|
We assessed the following factors for other sources of potential bias: funding and other biases such as (low) sample size and how the data was presented (Furlan 2015).
We did not downgrade the evidence when all trials were judged as at low risk of bias for all five categories. We downgraded the evidence by one level when more than three categories had a high or unclear risk. We downgraded the evidence by two levels when four or more categories had a high or unclear risk (Appendix 6).
Measures of treatment effect
The primary outcome pain intensity was measured on a VAS from 0 to 100 or a NRS from 0 to 10. Global improvement was measured by the proportion of participants who expressed an improvement or recovery. Disability was measured by self reported validated scales (for example the RMDQ). Adverse events were measured by the proportion of participants experiencing any adverse event.
Unit of analysis issues
Some of the included studies had more than two study arms. In order to avoid unit of analysis error, we followed the recommendation in the Cochrane Handbook and either split the placebo group or combined treatment arms with different dosages (Higgins 2011). Specifically, the placebo group was divided into two subgroups by dividing the number of events and number of cases by two for dichotomous outcomes, or by dividing the sample size by two, and to assume mean and standard deviations reported for continuous outcomes (for Herrmann 2009). In studies with treatment arms that used the same drug in different dosage, we used an alternative approach in which treatment arms were combined. For dichotomous outcomes we added number of participants and events. For continuous outcomes we calculated weighted mean and pooled standard deviation (for Dreiser 2001a; Dreiser 2001b).
Dealing with missing data
If data were missing, we emailed the author. If data were only presented in graphs, we collected data from the graphs. Where needed, we recalculated the data in order to provide standard deviations. We performed all calculations in accordance with the Cochrane Handbook (Higgins 2011).
Assessment of heterogeneity
We reported the between‐study variance in a random‐effects meta‐analysis with tau‐squared. Regarding choice of fixed‐effect or random‐effects model, we used the I2 statistic as a measure of heterogeneity. Inconsistency refers to an unexplained heterogeneity of results. Heterogeneity and variability in results across trials suggest true differences in underlying treatment effect. Inconsistency can arise from differences in populations, interventions, or outcomes.
Assessment of reporting biases
Publication bias refers to a systematic under‐ or overestimate of the underlying beneficial or harmful effect due to the selective publication of trials. Before pooling results we assessed for each outcome the potential risk of reporting bias by inspection of the corresponding funnel plot. We visually assessed the potential influence of the year of publication on the forest plots. Furthermore, we studied the difference between the currently recommended treatment dose and the actual dose used in the studies, the treatment duration and the sample size calculations. If the funnel plots suggested publication bias, we downgraded the quality of the evidence.
Data synthesis
We followed recommendations in the Cochrane Handbook (Higgins 2011). We analysed dichotomous outcomes by calculating the risk ratio (RR). We analysed continuous outcomes by calculating the mean difference (MD) when the same instrument was used to measure outcomes. Uncertainty was expressed with 95% confidence intervals (CI). We considered a P value of less than 0.05 to be statistically significant. We considered pooling study results if two or more studies investigated comparable outcome measures.
For the meta‐analyses we considered only studies that used medications currently on the market. We started using a fixed‐effect approach. When the heterogeneity measure I2 was 25% or more, we used both the fixed‐ and the random‐effects approach and presented the more conservative estimate with respect to the 95% CI. Furthermore, we calculated and reported the number needed to treat for an additional beneficial outcome (NNTB) and number needed to treat for an additional harmful outcome (NNTH) based on absolute risk difference.
We assessed the quality of the evidence for all outcomes regardless of whether there were sufficient data available to use quantitative analyses to summarise the data. We rated the quality of the evidence according to the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach (Atkins 2004), recommended in the Cochrane Handbook (Higgins 2011), and adapted in the updated CBN guidelines (Furlan 2015) (Appendix 6). We graded the evidence of the included trials on specific domains recommended by the Cochrane CBN tool for assessing risk of bias: risk of bias (Table 2; Table 3), inconsistency of results, indirectness (not generalisable), imprecision (sparse data), and other factors (for example publication bias) (Furlan 2015). We used the statistical software Review Manager (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
We aimed to analyse short‐ and long‐term follow‐up. Only one trial reported a follow‐up duration of four weeks and more (Weber 1993). No subgroup analysis was conducted.
Sensitivity analysis
We aimed to conduct a sensitivity analysis for the consistency of results within subgroups. However, the available data was insufficient to conduct a sensitivity analysis.
We performed a sensitivity analysis concerning NSAIDs and placebo for pain reduction, excluding a trial with a treatment arm with a very short follow‐up (eight hours) (Herrmann 2009).
We performed a sensitivity analysis concerning NSAIDs and placebo for adverse effects, excluding a trial with a high risk of bias (Weber 1993).
Results
Description of studies
The Characteristics of included studies and Characteristics of excluded studies tables summarise information on the specific characteristics of included and excluded studies.
Results of the search
The results of the annual searches conducted between May 2012 and June 2015 are summarised in the study flow diagram (Figure 1). The most recent search in June 2015 identified no additional studies.
1.
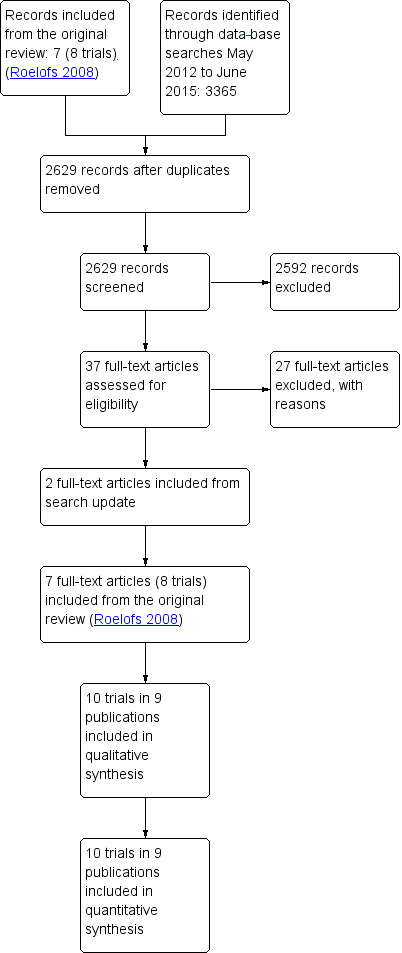
Study flow diagram.
We identified a total of 3365 studies through database searches; after duplicates were removed this was reduced to 2629 studies: 1247 references in May 2012, 277 references in April 2013, 639 references in April 2014, and 466 references in June 2015. We screened a total of 2629 references. Two review authors (BK, PR) screened the results of the 2012 and 2013 searches, and two review authors (ERB, MW) screened the results of the 2014 and 2015 searches.
Four review authors (ERB, MW, PR, BK) independently read and assessed 37 full‐text articles, including 8 trials published in 7 publications from the original review (Roelofs 2008) (Figure 1). We included the 8 trials from the original review (Braun 1982; Dreiser 2001a; Dreiser 2001b; Goldie 1968a; Grevsten 1975; Radin 1968; Weber 1980; Weber 1993). We identified two additional trials from the updated searches (Herrmann 2009; Kanayama 2005). In total, 9 publications reporting on 10 trials met the inclusion criteria and were included in the present review. One of the included studies reported on two separate trials, presented as Dreiser 2001a and Dreiser 2001b in the analyses. All of the included studies are in the English language, except for one German study (Braun 1982)
Included studies
We included 10 trials with a total of 1651 participants (ranging between 23 and 532) aged 16 to 70 years (Braun 1982; Dreiser 2001a; Dreiser 2001b; Goldie 1968a; Grevsten 1975; Herrmann 2009; Kanayama 2005; Radin 1968; Weber 1980; Weber 1993). All trials included approximately the same number of men as women, and the mean age of the study populations ranged between 33 and 48. Included trials are described in more detail in the Characteristics of included studies table.
Most trials included smaller samples, ranging from 25 to 59 participants, while Dreiser 2001a, Dreiser 2001b, Herrmann 2009, and Weber 1993 included larger samples (171 to 532 participants). In six trials the participants sought primary care (Dreiser 2001a; Dreiser 2001b; Grevsten 1975; Herrmann 2009; Kanayama 2005; Weber 1993), while in three trials participants were included in secondary care (Goldie 1968a; Radin 1968; Weber 1980). Radin 1968 did not specify if the participants sought primary or secondary care. Four trials were multicentre trials, while the rest were single‐centre trials (Dreiser 2001a; Dreiser 2001b; Herrmann 2009; Weber 1993). One trial was conducted in the USA (Radin 1968), one in Japan (Kanayama 2005), two in Germany (Braun 1982; Herrmann 2009), two in Sweden (Goldie 1968a; Grevsten 1975), and two in Norway (Weber 1980; Weber 1993). Two trials were conducted as multicentre trials in several countries in Europe, Canada, and South America (Dreiser 2001a; Dreiser 2001b).
Most trials included participants seeking care for acute sciatica of less than three weeks' duration. One trial included 40 participants with a disease duration of less than four weeks to more than three months (n = 14, less than 4 weeks; n = 18, 1 to 3 months; n = 8, more than 3 months) (Kanayama 2005). Radin 1968 did not report pain duration.
Table 4 provides a summary of the daily dose and treatment duration of the NSAIDs that were studied. Five trials used the currently recommended daily dose of NSAIDs (Braun 1982; Dreiser 2001a; Dreiser 2001b; Herrmann 2009; Weber 1993), and two used lower doses (Goldie 1968a; Kanayama 2005). Three trials investigated NSAIDs no longer approved for human use (Grevsten 1975; Radin 1968; Weber 1980). The following substances (following the ATC classification system) were studied:
3. Summary of treatments.
| ATC group, trial | Substance | Daily dose | Maximum recommended dose | Treatment duration | Sample size calculation | Participants per group (n) |
| Butylpyrazolidin | ||||||
| Grevsten 1975 | Phenylbutazone (Butazolidin) IM day 1; phenylbutazone (Butazolidin Alka) orally day 2 to 4 | 0.6 g IM day 1, 0.6 g day 2 to 4 by mouth, 0.3 g day 5 to 14 | No longer approved for human use | 14 days | No | 36 |
| Weber 1980 | Phenylbutazone (Butazolidin Alka) | 600 mg day 1 to 3, 300 mg day 4 to 5 | No longer approved for human use | 5 days | No | 59 |
| Radin 1968 | Phenylbutazone | 600 mg day 1 to 2, 300 to 800 mg day 3 to 8 | No longer approved for human use | 8 days | No | 25 |
| Acetic acid derivatives | ||||||
| Goldie 1968a | Indomethacin | 75 mg | 225 mg | 14 days | No | 25 |
| Herrmann 2009 | Diclofenac | 100 mg day 1 and 5; 150 mg day 2 to 4 | 150 to 200 mg | 4 days | 50 per group | 57 |
| Dreiser 2001b | Diclofenac | 150 mg | 150 to 200 mg | 14 days | 150 per group | 162 |
| Kanayama 2005 | Diclofenac vs active treatment | 75 mg | 150 to 200 mg | 14 days | 20 per group | 20 |
| Oxicams | ||||||
| Dreiser 2001a (placebo‐controlled trial) | Meloxicam 7.5/15 mg | 7.5/15 mg | 15 mg | 7 days | 150 per group | 171/181 |
|
Dreiser 2001b (diclofenac‐controlled trial) |
Meloxicam 7.5/15 mg | 7.5/15 mg | 15 mg | 14 days | 150 per group | 164/163 |
| Weber 1993 | Piroxicam | 100 mg day 1 to 2, 20 mg day 3 to 14 | 20 mg | 14 days | No | 120 |
| Herrmann 2009 | Lornoxicam | 24 mg day 1; 16 mg day 2 to 4; 8 mg day 5 | 16 mg | 5 days | 50 per group | 57 |
| Propionic acid derivative | ||||||
| Braun 1982 | Ketoprofen vs active treatment | 200 mg IM day 1 to 3, 300 mg orally + supp day 4 to 8 | 200 (max 300) mg | 9 days | No | 17 |
| Fenamates, coxibs, or others | ||||||
| No studies | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
ATC: Anatomical Therapeutic Chemical IM: intramuscular
butylpyrazolidin (no longer approved for human use): three trials investigated phenylbutazone (Grevsten 1975; Radin 1968; Weber 1980);
acetic acid derivatives: four trials used indomethacin, in Goldie 1968a, or diclofenac, in Dreiser 2001b, Herrmann 2009, and Kanayama 2005;
oxicams: four trials used meloxicam, in Dreiser 2001a and Dreiser 2001b, piroxicam, in Weber 1993, or lornoxicam, in Herrmann 2009;
propionic acid derivative: one trial used a ketoprofen substance (Braun 1982).
No trial used fenamates, coxibs, or other substances.
The follow‐up duration varied from three to eight hours to one year. Three trials reported very short follow‐up results (three to eight hours) (Dreiser 2001a; Dreiser 2001b; Herrmann 2009). Only one trial followed participants for more than four weeks (Weber 1993).
NSAIDs versus placebo
Four trials reported on pain relief using a visual analogue scale (VAS) (0 to 100) (Braun 1982; Dreiser 2001a; Kanayama 2005; Weber 1993). One trial reported on functional outcomes using the Roland Morris Disability Questionnaire (RMDQ) for outcome at 14 days and 4 weeks (Weber 1993). Four trials reported on an overall improvement using a global measure: a scale with different steps of improvement (Dreiser 2001a; Grevsten 1975; Herrmann 2009; Radin 1968; Weber 1980). One trial did not report how global improvement was measured and was therefore not included in the analysis of this outcome (Radin 1968). All trials except two, Kanayama 2005 and Radin 1968, reported on side effects, that is the proportions of participants reporting adverse effects due to the NSAIDs. These were mostly gastrointestinal problems. In all trials except one, Kanayama 2005, the use of additional medication was allowed, such as paracetamol with or without codeine, promethazine (Weber 1980), and levomepromazine (Weber 1993).
NSAIDs versus NSAIDs
Two trials compared NSAIDs to NSAIDs (Dreiser 2001b; Herrmann 2009). Herrmann 2009 compared lornoxicam to diclofenac, and Dreiser 2001b compared meloxicam 7.5 mg and 15 mg to diclofenac 150 mg.
NSAID versus other drugs
Two trials compared NSAIDs to other drugs (Braun 1982; Kanayama 2005). Braun 1982 compared ketoprofen orally to a combination of steroids and phenylbutazone (first intramuscular followed by oral form). Kanayama 2005 compared diclofenac (75 mg/day) to a serotonin or 5‐hydroxytryptamine (5‐HT) inhibitor (sarpogrelate 300 mg/day).
Excluded studies
We excluded 27 studies during full‐text review (Figure 1). The main reason for exclusion was that included participants did not suffer from sciatica or the study design was not an RCT. Reasons for exclusion of studies are presented in the Characteristics of excluded studies table.
Risk of bias in included studies
We have presented 'Risk of bias' assessment in Figure 2. Only one of the included trials, Herrmann 2009, was assessed as at low risk of bias; criteria assessed as unclear were selective reporting and compliance with intervention.
2.
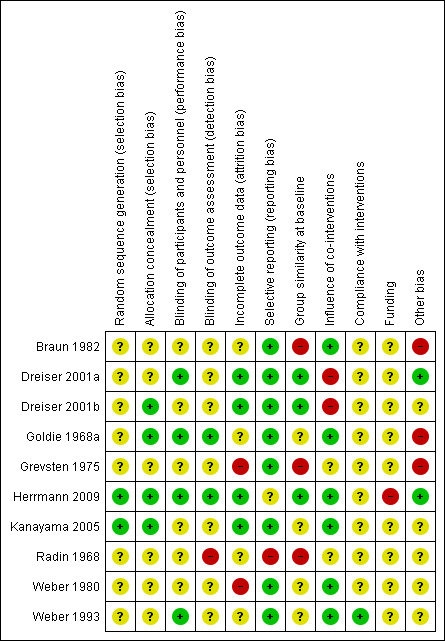
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed five trials with a low risk of bias reporting on randomisation process (Dreiser 2001a; Dreiser 2001b; Goldie 1968a; Herrmann 2009; Kanayama 2005), and five trials with an unclear risk of bias due to insufficient information on randomisation or allocation (Braun 1982; Grevsten 1975; Radin 1968; Weber 1980; Weber 1993).
Blinding
We assessed four trials with a low risk of bias for describing blinding of participants and personnel (Dreiser 2001a; Goldie 1968a; Herrmann 2009; Kanayama 2005). These trials reported on NSAIDs versus placebo, in Dreiser 2001a, Goldie 1968a, and Herrmann 2009, or NSAID versus 5‐HT2A inhibitor (Kanayama 2005). We assessed the rest of the trials with an unclear risk of bias due to insufficient information on blinding or because the trial did not address the outcome at all (Braun 1982; Dreiser 2001b; Grevsten 1975; Radin 1968; Weber 1980; Weber 1993).
Incomplete outcome data
We assessed four trials with a low risk of bias regarding incomplete outcome data (Dreiser 2001a; Dreiser 2001b; Herrmann 2009; Kanayama 2005). We assessed two trials with a high risk of bias (Grevsten 1975; Weber 1980). We assessed the remaining trials with an unclear risk of bias (Braun 1982; Goldie 1968a; Radin 1968; Weber 1993).
Selective reporting
We assessed all trials except two, Herrmann 2009 and Radin 1968, with a low risk of bias regarding selective reporting. These trials reported on NSAIDs versus placebo, in Braun 1982, Dreiser 2001a, Dreiser 2001b, Goldie 1968a, Grevsten 1975, Weber 1980, and Weber 1993, or NSAIDs versus 5‐HT2A inhibitor (Kanayama 2005). We assessed one study, Herrmann 2009, with an unclear risk of bias and one study, Radin 1968, with a high risk of bias due to outcomes that were not prespecified.
Other potential sources of bias
For group similarities at baseline, we assessed three trials with a low risk of bias (Dreiser 2001a; Dreiser 2001b; Herrmann 2009), and two trials with a high risk of bias (Grevsten 1975; Radin 1968). We considered five trials to have an unclear risk of bias due to not enough information about baseline similarities (Braun 1982; Goldie 1968a; Kanayama 2005; Weber 1980; Weber 1993). Regarding influence of co‐interventions, we assessed six trials with a low risk of bias (Braun 1982; Goldie 1968a; Herrmann 2009; Kanayama 2005; Weber 1980; Weber 1993), and two trials with a high risk of bias (Grevsten 1975; Radin 1968). Moreover, we assessed two trials with an unclear risk (Dreiser 2001a; Dreiser 2001b). For compliance, we assessed all included trials with an unclear risk of bias due to insufficient information.
All but one trial had an unclear risk of bias regarding funding, as we considered it to be unclear how and if these trials were supported by a pharmaceutical company. We assessed the trial by Herrmann 2009 with a high risk bias due to explicit reporting of funding from a pharmaceutical company.
We considered other risks of bias to be small sample sizes and poor data presentation, that is presentation of averages without confidence intervals or standard deviations. All trials except Dreiser 2001a, Dreiser 2001b, Herrmann 2009, and Kanayama 2005 reported on small study samples, that is data available from fewer than 60 participants.
We constructed funnel plots, but we could not detect any evidence of publication bias.
Effects of interventions
See: Table 1
Primary outcome, short‐term follow‐up
A summary of the primary outcomes is reported in the Table 1.
Change in pain intensity
NSAIDs versus placebo
Three trials (four treatment arms) with a total of 918 participants reported on pain reduction (on a VAS, 0 to 100). We considered one of the trials to have a low risk of bias (Herrmann 2009) while the two of the trials were assessed with high risk of bias (Dreiser 2001a; Weber 1993). The pooled mean difference using a random‐effects model demonstrated no difference between NSAIDs and placebo (MD ‐4.56, 95% CI ‐11.11 to 1.99) (Analysis 1.1;Analysis 1.2) (Figure 3) (Dreiser 2001a; Herrmann 2009; Weber 1993). Using GRADE criteria, we downgraded the quality of the evidence to very low quality due to the high risk of bias and inconsistency. There was a considerable amount of heterogeneity (I2 = 82%).
1.1. Analysis.
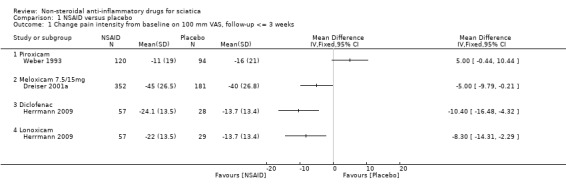
Comparison 1 NSAID versus placebo, Outcome 1 Change pain intensity from baseline on 100 mm VAS, follow‐up <= 3 weeks.
1.2. Analysis.
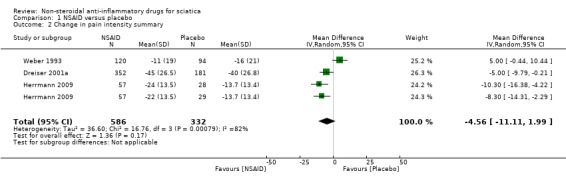
Comparison 1 NSAID versus placebo, Outcome 2 Change in pain intensity summary.
3.
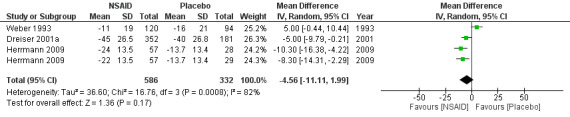
Forest plot of comparison: 1 NSAID versus placebo, outcome: 1.2 Change in pain intensity summary.
Herrmann 2009 investigated in two treatment arms the effect of lornoxicam or diclofenac versus placebo. Pain reduction assessed eight hours following administering of the medication was similar for lornoxicam and diclofenac and superior compared to placebo (Herrmann 2009). Weber 1993 found piroxicam to be no more effective than placebo. After seven days of NSAIDs (meloxicam), Dreiser 2001a found meloxicam 7.5 mg was superior to placebo, but 15 mg did not increase the effect (Dreiser 2001a). When excluding Herrmann 2009 from the meta‐analysis, the pooled mean difference of the remaining two trials, Dreiser 2001a and Weber 1993, was ‐0.09 (95% CI ‐9.89 to 9.71), random‐effects model, I2 = 86%.
We downgraded the evidence two levels due to high risk of bias and one level due to inconsistency (Table 1)
NSAIDs versus NSAID
Two trials compared the effect of two types of NSAIDs (Dreiser 2001b; Herrmann 2009). There was no difference in mean pain reduction between lornoxicam, in Herrmann 2009, and meloxicam (7.5 mg or 15 mg), in Dreiser 2001b, compared to diclofenac treatment.
NSAIDs versus other drugs
Two trials compared NSAIDs to other drugs (Braun 1982; Kanayama 2005). Braun 1982 compared ketoprofen intramuscular injection followed by ketaprofen oral to corticosteroids plus phenylbutazone intramuscular followed by oral corticosteroid plus phenylbutazone. Kanayama 2005 compared a serotonin or 5‐hydroxytryptamine (5‐HT) inhibitor (sarpogrelate 300 mg/day) to diclofenac (75 mg/day). No difference in pain reduction was found between the treatments in either of the trials.
Change in disability
NSAIDs compared to placebo
Only one trial investigated the efficacy of NSAID compared to placebo for change in disability. Weber 1993 (N = 214) reported on functional improvement measured by a modified RMDQ with 17 questions. The improvement in disability was measured at 14 days and 4 weeks, and the effect was comparable between the NSAID (piroxicam) group and the placebo group. There was very low‐quality evidence that NSAIDs are no better than placebo for change in disability, due to high risk of bias and imprecision.
Global improvement
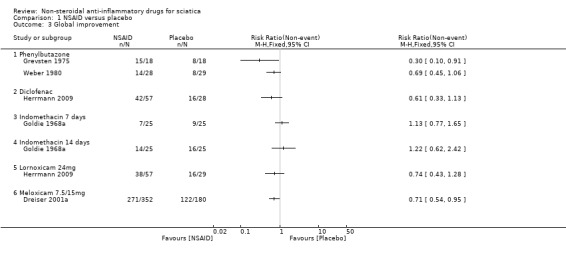
NSAID versus placebo
Five trials with a total of 846 participants reported on global improvement, two with a high risk of bias using medications no longer on the market (phenylbutazone) (Grevsten 1975; Weber 1980), two with a high risk (Dreiser 2001a; Goldie 1968a), and one with a low risk of bias (Herrmann 2009).Three trials were small‐sample trials including 36 to 59 participants (Goldie 1968a; Grevsten 1975; Weber 1980). The funnel plot inspection of these trials indicated no clear sign for publication bias, although the number of trials is probably too low for a valid assessment (Figure 4). The pooled analyses (Figure 5) (4 comparisons, N = 753) showed low‐quality evidence that NSAIDs are more effective than placebo for global improvement (RR 1.14, 95% CI 1.03 to 1.27; fixed‐effect model) (Analysis 1.3; Analysis 1.4). Heterogeneity was low (I2 = 0%). We downgraded the evidence two levels due to high risk of bias (Table 1). For the pooled analysis, we excluded Grevsten 1975 and Weber 1980, as they used NSAIDs no longer on the market. The corresponding NNTB was 12 participants based on the absolute risk difference of 0.09 (95% CI 0.02 to 0.16). Fifteen milligrams of meloxicam was no more effective than 7.5 mg (Dreiser 2001a).
4.
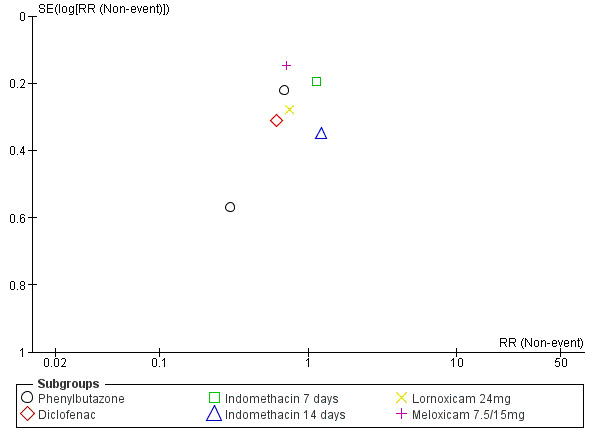
Funnel plot of comparison: 1 NSAID versus placebo, outcome: 1.2 Global improvement.
5.
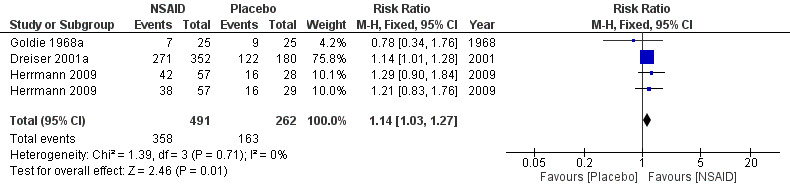
Forest plot of comparison: 1 NSAID versus placebo, outcome: 1.6 Global improvement summary.
1.3. Analysis.
Comparison 1 NSAID versus placebo, Outcome 3 Global improvement.
1.4. Analysis.
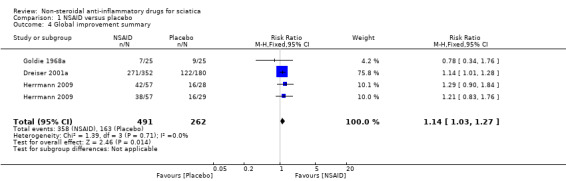
Comparison 1 NSAID versus placebo, Outcome 4 Global improvement summary.
NSAID versus NSAID
Two trials showed no difference in global improvement when lornoxicam, in Herrmann 2009, and meloxicam, in Dreiser 2001b, were compared to diclofenac treatment.
NSAID versus other drugs
Two trials compared NSAIDs to other drugs (Braun 1982; Kanayama 2005). Braun 1982 compared ketoprofen intramuscular injection followed by ketaprofen oral to corticosteroids plus phenylbutazone intramuscular followed by oral corticosteroid plus phenylbutazone. The effects of both groups were comparable. Kanayama 2005 found no group difference in effect between serotonin or 5‐hydroxytryptamine (5‐HT) inhibitor (sarpogrelate 300 mg/day) and diclofenac (75 mg/day).
Adverse effects
NSAID versus placebo
All trials but Braun 1982 and Kanayama 2005 reported on side effects . Weber 1980 reported that there were no side effects. A funnel plot for side effects showed no clear signs of publication bias. The pooled analyses of four trials (N = 967) showed low‐quality evidence for all side effects of NSAIDs compared to placebo (RR 1.40, 95% CI 1.02 to 1.93) (Analysis 1.5;Analysis 1.6) (Figure 6). We downgraded the evidence two levels due to high risk of bias (Table 1). The meta‐analysis included only those trials that used NSAIDs currently on the market (Goldie 1968a; Herrmann 2009; Weber 1993). The corresponding NNTH was 20 participants for one adverse effect based on an absolute risk difference of 0.05 (95% CI 0.00 to 0.10). When excluding one trial assessed with a high risk of bias (Weber 1993), the summary estimate was similar (RR 1.42, 95% CI 0.98 to 2.07).
1.5. Analysis.
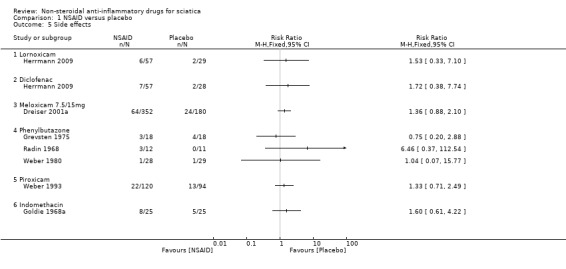
Comparison 1 NSAID versus placebo, Outcome 5 Side effects.
1.6. Analysis.
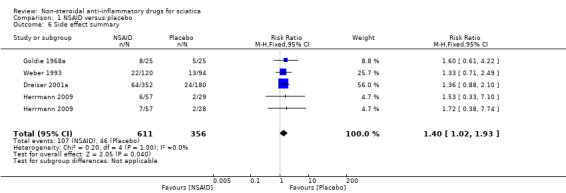
Comparison 1 NSAID versus placebo, Outcome 6 Side effect summary.
6.
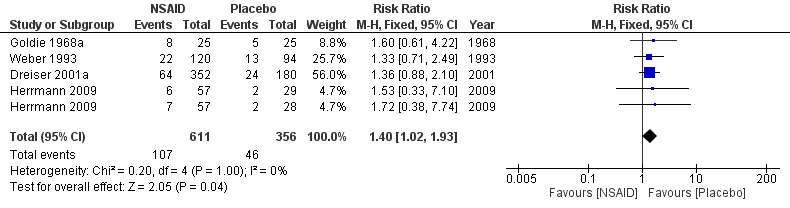
Forest plot of comparison: 1 NSAID versus placebo, outcome: 1.6 Side effect summary.
Most side effects were reported to be mild and comprised gastrointestinal (GI) problems described as nausea, dyspepsia, epigastric burning, abdominal pain, and in addition headache and dizziness. Herrmann 2009 reported that six participants treated with lornoxicam, four with placebo, and seven with diclofenac had perceived side effects. All events except for two were mild or moderate, and the investigators considered only the GI events to be related to treatment. The two severe events (abdominal pain and nausea) were both experienced by a single participant in the diclofenac group who discontinued study treatment as a result. Two participants in the lornoxicam group also discontinued study treatment because of nausea and dyspepsia.
Dreiser 2001a reported one adverse event in 29 participants (17%) on meloxicam 7.5 mg, 35 participants (19%) on meloxicam 15 mg, and 24 participants (13%) on placebo. These adverse events were judged to be treatment‐related in 13 participants (8%) receiving meloxicam 7.5 mg, 12 participants (7%) receiving meloxicam 15 mg, and 10 participants (6%) receiving placebo. The incidence of overall and treatment‐related adverse events was similar for the three treatment groups. Nausea, dyspepsia, and abdominal pain were the most common treatment‐related adverse events. No perforation, ulceration, or bleeding of the upper gastrointestinal tract was reported. The number of withdrawals due to adverse events was similar in the three groups. One life‐threatening adverse event occurred: an anaphylactic shock requiring steroid therapy in the meloxicam 7.5 mg group (treatment related), and there was one serious adverse event with deterioration of back pain in the placebo group (not treatment related). Both participants recovered.
Weber 1993 reported that the adverse effects in the two groups were mild and moderate, with a few exceptions. However, nearly twice as many participants (22 versus 13) reported adverse effects in the piroxicam group compared to the placebo group. Grevsten 1975 reported on seven participants with adverse effects; six of these, of whom four were in the placebo group, experienced mild adverse effects. One participant in the phenylbutazone (Butazolidin) group discontinued treatment after four days because of symptoms of gastritis. Kanayama 2005, which compared the effects of NSAID with 5‐hydroxytryptamine (5‐HT) inhibitor, did not report side effects.
NSAID versus NSAID
Two trials comparing NSAIDs versus NSAIDs reported no difference in side effects (Dreiser 2001b; Herrmann 2009).
NSAID versus other drugs
Braun 1982 did not report on adverse effects. Kanayama 2005 reported no side effects for the current trial.
Primary outcome, long‐term follow‐up
Only one trial followed participants longer than three weeks (Weber 1993). A reduction in pain (VAS, 0 to 100) at 14 days and four weeks from baseline was observed, however the reduction was not significant. No results for the treatment groups were reported for the follow‐up duration of 12 months.
Secondary outcomes
Return to work status or productivity
One trial reported on return‐to‐work status (Weber 1993). At 4 weeks, 60% of the sample had returned to work, and at 12 months only 7.5% of the total sample was still on sick‐leave. The mean duration of the sick‐leave was 27.9 days. No specific group differences were reported.
Additional use of pain medication
All but three trials, Goldie 1968a, Herrmann 2009, and Kanayama 2005, did not permit additional pain medication during the intervention (Analysis 1.7). Additional analgesics used in the included trials were paracetamol with or without codeine (Braun 1982; Weber 1980; Weber 1993), promethazine (Weber 1980), levomepromazine (Weber 1993), and non‐specified day and night analgesics (Grevsten 1975). In one trial (Dreiser 2001a), participants who were treated with meloxicam 7.5 mg used less pain medication than the placebo group. An increase in the dose of meloxicam to 15 mg did not decrease the need of additional pain medication. Two trials found no difference between NSAIDs and placebo with regard to the use of additional pain medication (Weber 1980; Weber 1993). Braun 1982 reported no difference in paracetamol use in the NSAID group compared to the control group. Grevsten 1975 reported that participants were allowed to use additional analgesic if needed, but reported no outcome. Radin 1968 did not report on additional analgesics.
1.7. Analysis.
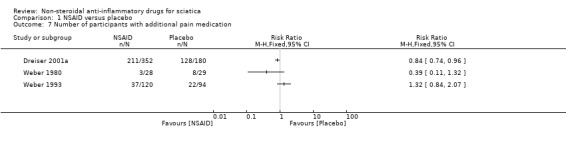
Comparison 1 NSAID versus placebo, Outcome 7 Number of participants with additional pain medication.
Subgroup analyses
We were not able to conduct analyses on subgroups due to paucity of data. Only one trial reported a follow‐up duration of four weeks and more (Weber 1993). No subgroup analysis was conducted.
Sensitivity analyses
For the analysis of NSAIDs compared to placebo on pain reduction, we conducted a sensitivity analysis excluding one treatment arm that investigated a short‐term outcome of eight hours pain reduction (Herrmann 2009). The effect further decreased (‐0.09, 95% CI ‐9.89 to 9.71) compared to the analysis including all trials (‐4.56, 95% CI ‐11.11 to 1.99).
For global improvement, no studies with a high risk of bias were included in the meta‐analysis. We did not conduct a sensitivity analysis for this outcome.
For the analyses of adverse effects, we conducted a sensitivity analysis excluding one trial assessed with a high risk of bias (Weber 1993). The summary estimate (1.42, 95% CI 0.98 to 2.07) was similar to the primary analysis including all trials (RR 1.40, 95% CI 1.02 to 1.93).
Discussion
Summary of main results
In this updated Cochrane review, we included 10 trials reported in 9 publications that assessed the efficacy of NSAIDs in sciatica. We included two additional trials, Herrmann 2009 and Kanayama 2005, to those included in the original review (Roelofs 2008). For all trials, the follow‐up duration was short, with only one trial reporting a follow‐up of more than three weeks (Weber 1993). We assessed a single trial with a low risk of bias (Herrmann 2009), while the rest of the trials showed a high risk. Three trials compared NSAIDs to placebo (n = 918) on pain reduction. The pooled mean difference showed comparable pain reduction (VAS, 0 to 100) in the NSAIDs and the placebo groups (MD ‐4.56, 95% CI ‐11.11 to 1.99; random‐effects model; I2 = 82%). Three trials (n = 753) compared NSAIDs to placebo for the outcome global improvement. We found low‐quality evidence that NSAIDs are more effective than placebo, with a risk ratio of 1.14 (95% CI 1.03 to 1.27). Our findings must be interpreted with caution, as the level of evidence according to the GRADE classification was very low for the outcome pain reduction and low for the global improvement due to small study samples, inconsistent results, imprecision, and a high risk of bias in included trials.
Four trials (n = 967) comparing NSAIDs to placebo reported adverse effects, with low‐quality evidence of a higher risk for adverse effects in the NSAID group than in the placebo group (RR 1.40, 95% CI 1.02 to 1.93). Only one trial (Weber 1993) (n = 214) studied the effect of NSAID on disability, with very low‐quality evidence that NSAIDs are no more effective than placebo.
Overall completeness and applicability of evidence
For this updated Cochrane review we only included trials reporting on the efficacy of NSAIDs in sciatica, thus we excluded other trials including people suffering from acute or chronic LBP. We updated the search until June 2015 and included only two additional trials, Herrmann 2009 and Kanayama 2005, in this review compared to the original review (Roelofs 2008). We did a thorough search of databases and clinical trials registries to find all possible trials investigating the efficacy of NSAIDs on sciatica. We searched the reference lists of other reviews and other studies to avoid publication bias and to gain a complete identification of studies. Even so, we might have missed trials, such as those poorly indexed in the databases. This may especially refer to non‐English trials. However, a strength of this review is that we set no restriction concerning language, although we identified only one non‐English language trial (Braun 1982), which was also included in the original review (Roelofs 2008). Four researchers independently performed the inclusion process and the study quality assessment. In addition to the treatment efficacy of NSAIDs, adverse effects were assessed. Based on our thorough literature search, and as it seems that our results are in line with the original review on the efficacy of NSAIDs on sciatica (Roelofs 2008), and other recently published reviews in the same area (Pinto 2012; Wong 2015), we find the evidence applicable.
The present review included trials reporting on people with acute sciatica of less than three weeks' duration. Only one trial included patients with different duration of sciatica (Kanayama 2005), and two trials provided no information on duration of sciatica (Radin 1968; Weber 1993). The external validity of our review thus only extends to those suffering from sciatica for less than three weeks. In addition, only one trial reported on the effect of NSAIDs on disability (Weber 1993), with very low‐quality evidence that NSAIDs are no more effective than placebo on disability. The same trial was the only trial to report on return to work (Weber 1993).
The risk for adverse effects of NSAIDs is well documented in the literature (Kowalski 2015; Trelle 2011). All but two trials, Braun 1982 and Kanayama 2005, reported on the risk of adverse effects for NSAIDs compared to placebo. Weber 1980 reported that there were no side effects. In the current analysis, the risk of adverse effects was higher in the NSAID group compared to the placebo group (RR 1.40, 95% CI 1.02 to 1.93), and the corresponding NNTH was 20 participants. When excluding Weber 1993, which we assessed with a high risk of bias, the summary estimate was similar (RR 1.42, 95% CI 0.98 to 2.07).
Our finding of an increased risk for adverse effects was graded as low‐quality evidence, and in addition the included trials did not have enough power to detect rare adverse effects. Thus, based on our findings we cannot draw any conclusion on long‐term effects of NSAIDs in sciatica. While the GRADE quality of evidence was low due to the small study sample and a high risk of bias, the findings of risk for adverse effects in the present review are consistent with the literature. The very low methodological quality of most trials may also be associated with low reporting of adverse events. Furthermore, we cannot exclude the possibility that rare adverse events were not detected due to small study sizes and short follow‐up duration.
Quality of the evidence
Given the high risk of bias in all but one trial, Herrmann 2009, there is low‐ to very low‐quality evidence of the effect of NSAIDs compared to placebo or other drugs in the treatment of sciatica. Small study samples, incomplete outcome reporting, and inconsistency affected the grading of the quality of the evidence. Even if more participants with NSAIDs experienced global improvement, the grading of the evidence of the pooled analyses was low. Thus, the findings must be interpreted with caution as several of the included trials were assessed with a high risk of bias. More participants in the NSAIDs group experienced adverse effects. While the quality of the evidence for adverse effects was graded low, the findings from the present review are consistent with the literature. Our results align with the previous Cochrane review of the efficacy of NSAIDS in LBP with or without sciatica (Roelofs 2008), as well as with other reviews (Pinto 2012; Wong 2015).
Potential biases in the review process
We strived to grade the evidence as recommended by the GRADE group (Atkins 2004), the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and according to Cochrane Back and Neck (CBN) (Furlan 2015). We cannot exclude the possibility that we may have missed some trials on the topic, even if the search was thorough. The present review only included two additional trials, Herrmann 2009 and Kanayama 2005, to the original review (Roelofs 2008). We performed an updated search until June 2015 without finding additional trials to include in the review. It thus seems that recent publications on the effects of NSAIDs in the treatment of sciatica are sparse.
Agreements and disagreements with other studies or reviews
To the authors’ knowledge this is the first review to only include trials that consider the complaint of sciatica and the efficacy of NSAIDs. Other reviews have included studies reporting on the efficacy of NSAIDs in acute and chronic LBP and with or without sciatica (Pinto 2012; Roelofs 2008; Wong 2015). The role of NSAIDs in the treatment of common LBP and sciatica is less well defined. A recent review of reviews on the efficacy of NSAIDs in neck and low back pain concluded that there was inconsistent evidence for the treatment of recent‐onset LBP with radiculopathy (Wong 2015). However, Wong 2015 included people with both neck and back pain, and therefore the results may not be generalisable to people suffering from sciatica.
A recent systematic review, Pinto 2012, included five of the trials included in our review and concluded that the graded evidence for the efficacy of NSAIDs was low due to limitations of study design and inconsistency. While we chose to report on all trials for full transparency on NSAIDs and sciatica, Pinto 2012 excluded three trials included in the present review due to unclear randomisation (Braun 1982; Radin 1968; Weber 1980). We excluded Radin 1968 and Weber 1980 in the meta‐analysis, as these trials used NSAIDs no longer on the market. In addition, we excluded Weber 1993 to conduct a sensitivity analysis for the outcome adverse effects. However, the finding of the sensitivity analysis did not differ from the one including Weber 1993. In addition, our findings are consistent with the findings of Pinto 2012 for the effect of overall pain reduction.
Our review expands the current evidence on the treatment efficacy of NSAIDs in sciatica with regard to several aspects. In addition to pain reduction, we assessed the effect on global improvement, finding that NSAIDs are more effective than placebo (pooled RR 1.14, 95% CI 1.03 to 1.27; random‐effects model; I2 = 0%), with a corresponding NNTB of 12 participants. However, this finding must be treated with caution, even if indicating that NSAIDs are more effective compared to placebo. In addition, as some of the trials allowed the use of additional pain medications (Goldie 1968a; Herrmann 2009; Kanayama 2005), and showed inconsistent results, the findings are very unsure. While three trials found no difference between NSAIDs and placebo with regard to the use of additional pain medication (Braun 1982; Weber 1980; Weber 1993), one trial found less use of pain medication in the NSAID group (Dreiser 2001a). Moreover, the level of evidence according to the GRADE classification in these trials was very low for the outcomes pain and global improvement due to small study samples, incomplete outcome reporting, inconsistent results, and a high risk of bias of the included trials.
Limitations
The main limitations of the current review are the number of trials available, the moderate to high risk of bias, and the small sample size of included trials. Moreover, only five of the included trials reported on a power calculation (Dreiser 2001a; Dreiser 2001b; Goldie 1968a; Herrmann 2009; Kanayama 2005). Another limitation is that we were not able to perform meta‐analyses for all outcomes. CBN recommends that "the results from studies should only be combined when they are judged to be sufficiently clinically similar to yield meaningful results" (Furlan 2015). For the outcome pain, heterogeneity of more than 80% between trials indicated that there was a wide range in treatment responses, thus no meta‐analysis was performed. To be able to detect if subgroups of participants with sciatica benefit from NSAIDs, additional analyses may be conducted which in the present review were not feasible due to insufficient trials and specific treatment responses. A further limitation is that only five trials assessed the treatment efficacy of currently available drugs in the recommended daily dose (Braun 1982; Dreiser 2001a; Dreiser 2001b; Herrmann 2009; Weber 1993) (Table 4). Moreover, two trials used lower doses of NSAIDs (over the counter), which might explain less efficacy in those trials (Goldie 1968a; Kanayama 2005).
Due to the low number of included trials (n = 10) in the present review, we decided to include all eligible trials in the analyses even if assessed with a high risk of bias, using various doses, or reporting different short treatment outcome. For the meta‐analyses, we decided to exclude those trials that used NSAIDs no longer on the market. For the analyses of adverse effects, we included three trials, of which Weber 1993, assessed with a high risk of bias, was one. In addition to our analysis of these trials, we therefore conducted a sensitivity analysis in which we excluded Weber 1993, giving a similar result. A limitation for the outcome of adverse effects is that for the individual studies there was clearly not enough power to detect rare adverse events, which means that we cannot fully exclude that potential rare events may occur.
For the analyses of pain reduction compared to placebo, one study arm reported a short follow‐up of eight hours (Herrmann 2009). We conducted a sensitivity analysis excluding the short‐term treatment study, finding that the effect on pain reduction further decreased.
Authors' conclusions
Implications for practice.
We found that NSAIDS are no more effective than placebo in short‐term pain reduction (very low‐quality evidence). NSAIDs were associated with more global improvement for sciatica at short‐term follow‐up (low‐quality evidence). Only one trial assessed disability, and found no difference in effects between placebo and NSAIDs (very low‐quality evidence). When prescribing NSAIDs in people with sciatica, the increased risk for adverse effects (low‐quality evidence), also in short treatment duration, needs to be taken into account in the treatment decision.
Implications for research.
We found only two additional trials for this updated review assessing the effect of NSAIDs compared to placebo or other drugs in sciatica, compared with the original review published in 2008. Most trials were assessed with a high risk of bias and included small sample sizes. For future studies on the efficacy of NSAIDs in sciatica, it might be important to investigate defined subgroups of participants in methodologically sound RCTs.
What's new
| Date | Event | Description |
|---|---|---|
| 6 February 2017 | Amended | Citation correction |
History
Review first published: Issue 10, 2016
| Date | Event | Description |
|---|---|---|
| 1 July 2016 | New citation required and conclusions have changed | We included two trials in addition to the eight trials included in the original review (Roelofs 2008). Conclusion is unchanged that NSAIDs are no more effective in reducing pain than placebo or other drugs in sciatica. Conclusion is changed that NSAIDs are more effective in overall improvement compared to placebo or other drugs, but this should be interpreted with caution as the methodological quality of the included trials is low. Conclusion is unchanged that there is an increased risk of side effects when using NSAIDs compared to placebo. |
| 24 June 2015 | New search has been performed | We added the following databases to the search strategy: ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (2013), MEDLINE In‐Process & Other Non‐Indexed Citations (2014), and PubMed
(2015). New update search by 24 June 2015; no further trials were included. |
Acknowledgements
We thank Rachel Couban and Shireen Harbin RN, BScN of Cochrane Back and Neck for their assistance. Rickard A Deyo and Rob JPM Sholten, co‐authors of the previous version of this Cochrane review, are acknowledged for their work on the original review (Roelofs 2008).
Appendices
Appendix 1. CENTRAL strategy
Last searched June 24, 2015. Line 34 was added and line 42 was revised.
#1 MeSH descriptor: [Back Pain] explode all trees
#2 dorsalgia
#3 backache
#4 lumbar next pain or coccyx or coccydynia or spondylosis
#5 MeSH descriptor: [Spine] explode all trees
#6 MeSH descriptor: [Spinal Diseases] explode all trees
#7 lumbago and discitis and disc near herniation
#8 spinal fusion
#9 spinal neoplasms
#10 facet near joints
#11 MeSH descriptor: [Intervertebral Disk] explode all trees
#12 postlaminectomy
#13 arachnoiditis
#14 failed near back
#15 MeSH descriptor: [Cauda Equina] explode all trees
#16 lumbar near vertebra*
#17 spinal near stenosis
#18 slipped near (disc* or disk*)
#19 degenerat* near (disc* or disk*)
#20 stenosis near (spine or root or spinal)
#21 displace* near (disc* or disk*)
#22 prolap* near (disc* or disk*)
#23 MeSH descriptor: [Sciatic Neuropathy] explode all trees
#24 sciatic*
#25 back disorder*
#26 back near pain
#27 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26
#28 nsaid*
#29 MeSH descriptor: [Anti‐Inflammatory Agents, Non‐Steroidal] explode all trees
#30 MeSH descriptor: [Cyclooxygenase Inhibitors] explode all trees
#31 MeSH descriptor: [Cyclooxygenase 2 Inhibitors] explode all trees
#32 non‐steroidal anti inflammat*
#33 non‐steroidal anti‐inflammat*
#34 (cyclooxygenase or cyclo‐oxygenase) next/3 inhibitor*
#35 aspirin
#36 acetylsalicyl*
#37 carbasalate calcium
#38 diflunisal
#39 aceclofenac
#40 alclofenac
#41 diclofenac
#42 indometacin or indomethacin
#43 sulindac
#44 meloxicam
#45 piroxicam
#46 dexibuprofen
#47 dexketoprofen
#48 fenoprofen
#49 flurbiprofen
#50 ibuprofen
#51 ketoprofen
#52 naproxen
#53 tiapro*
#54 metamizol
#55 phenylbutazone
#56 phenazone
#57 propyphenazone
#58 celecoxib
#59 etoricoxib
#60 nabumeton
#61 parecoxib
#62 rofecoxib
#63 celecoxib
#64 valdecoxib
#65 lumiracoxib
#66 parecoxib
#67 vioxx
#68 celebrex
#69 bextra
#70 prexige
#71 arcoxia
#72 etodolac
#73 floctafenine
#74 meclofenam*
#75 meloxicam
#76 oxaprozin
#77 piroxicam
#78 tenoxicam
#79 tolmetin
#80 #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79
#81 #27 and #80
#82 #81 in Trials
May 2012 strategy. In 2015, Line 77 and 66 were removed (duplicate with line 52 and 59), disc degeneration and prolapse were removed from line 8 (captured in line 20 and 23), and sciatica was removed from line 5 (captured in line 25).
#1 MeSH descriptor Back Pain explode all trees
#2 dorsalgia
#3 backache
#4 MeSH descriptor Low Back Pain explode all trees
#5 (lumbar next pain) or (coccyx) or (coccydynia) or (sciatica) or (spondylosis)
#6 MeSH descriptor Spine explode all trees
#7 MeSH descriptor Spinal Diseases explode all trees
#8 (lumbago) or (discitis) or (disc near degeneration) or (disc near prolapse) or (disc near herniation)
#9 spinal fusion
#10 spinal neoplasms
#11 facet near joints
#12 MeSH descriptor Intervertebral Disk explode all trees
#13 postlaminectomy
#14 arachnoiditis 36
#15 failed near back
#16 MeSH descriptor Cauda Equina explode all trees
#17 lumbar near vertebra*
#18 spinal near stenosis
#19 slipped near (disc* or disk*)
#20 degenerat* near (disc* or disk*)
#21 stenosis near (spine or root or spinal)
#22 displace* near (disc* or disk*)
#23 prolap* near (disc* or disk*)
#24 MeSH descriptor Sciatic Neuropathy explode all trees
#25 sciatic*
#26 back disorder*
#27 back near pain
#28 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27)
#29 nsaid*
#30 MeSH descriptor Anti‐Inflammatory Agents, Non‐Steroidal explode all trees
#31 MeSH descriptor Cyclooxygenase Inhibitors explode all trees
#32 MeSH descriptor Cyclooxygenase 2 Inhibitors explode all trees
#33 non‐steroidal anti inflammat*
#34 non‐steroidal anti‐inflammat*
#35 aspirin
#36 acetylsalicyl*
#37 carbasalate calcium
#38 diflunisal
#39 aceclofenac
#40 alclofenac
#41 diclofenac
#42 indometacin
#43 sulindac
#44 meloxicam
#45 piroxicam
#46 dexibuprofen
#47 dexketoprofen
#48 fenoprofen
#49 flurbiprofen
#50 ibuprofen
#51 ketoprofen
#52 naproxen
#53 tiapro*
#54 metamizol
#55 phenylbutazone
#56 phenazone
#57 propyphenazone
#58 celecoxib
#59 etoricoxib
#60 nabumeton
#61 parecoxib
#62 rofecoxib
#63 celecoxib
#64 valdecoxib
#65 lumiracoxib
#66 etoricoxib
#67 parecoxib
#68 vioxx
#69 celebrex
#70 bextra
#71 prexige
#72 arcoxia
#73 etodolac
#74 floctafenine
#75 meclofenam*
#76 meloxicam
#77 naproxen
#78 oxaprozin
#79 piroxicam
#80 tenoxicam
#81 tolmetin
#82 (#29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81)
#83 (#28 AND #82), from 2007 to 2012
Appendix 2. MEDLINE strategy
Last searched June 24, 2015. Line 3 and 61 were added and line 6, 22, 29, and 39 were revised.
randomized controlled trial.pt.
controlled clinical trial.pt.
pragmatic clinical trial.pt.
comparative study.pt.
clinical trial.pt.
randomi#ed.ab.
placebo.ab,ti.
drug therapy.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐11
(animals not (humans and animals)).sh.
12 not 13
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
exp sciatic neuropathy/
spondylosis.ti,ab.
lumbago.ti,ab.
back disorder$.ti,ab.
or/15‐25
exp Anti‐Inflammatory Agents, Non‐Steroidal/
nsaids.mp.
non‐steroidal antiinflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
non‐steroidal anti‐inflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
aspirin.mp. or exp Aspirin/
acetylsalicyl$.mp.
exp Salicylic Acid/
carbasalate calcium.mp.
diflunisal.mp. or exp Diflunisal/
aceclofenac.mp.
alclofenac.mp.
diclofenac.mp. or exp Diclofenac/
(indometacin or indomethacin).mp. or exp Indomethacin/
sulindac.mp. or exp Sulindac/
meloxicam.mp.
piroxicam.mp. or exp Piroxicam/
dexibuprofen.mp.
dexketoprofen.mp.
fenoprofen.mp. or exp Fenoprofen/
flurbiprofen.mp. or exp Flurbiprofen/
ibuprofen.mp. or exp Ibuprofen/
ketoprofen.mp. or exp Ketoprofen/
naproxen.mp. or exp Naproxen/
tiapro$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
metamizol.mp. or exp Dipyrone/
phenylbutazone.mp. or exp Phenylbutazone/
phenazone.mp. or exp Antipyrine/
propyphenazone.mp.
celecoxib.mp.
etoricoxib.mp.
nabumeton.mp.
parecoxib.mp.
or/27‐58
exp cyclooxygenase inhibitors/ or exp cyclooxygenase 2 inhibitors/
((cyclooxygenase or cyclo‐oxygenase) adj3 inhibitor*).mp.
rofecoxib.mp.
celecoxib.mp.
valdecoxib.mp.
lumiracoxib.mp.
etoricoxib.mp.
parecoxib.mp.
vioxx.mp.
celebrex.mp.
bextra.mp.
prexige.mp.
arcoxia.mp.
etodolac.mp. or exp Etodolac/
floctafenine.mp.
exp Meclofenamic Acid/
meclofenamate.mp.
meloxicam.mp.
oxaprozin.mp.
piroxicam.mp. or exp Piroxicam/
tenoxicam.mp.
tolmetin.mp. or exp Tolmetin/
or/60‐81
59 or 82
14 and 26 and 83
limit 84 to yr=2014‐2015
limit 84 to ed=20140410‐20150624
85 or 86
May 2012 strategy. Line 77 was removed in 2015 (duplicate with line 49).
randomized controlled trial.pt.
controlled clinical trial.pt.
comparative study.pt.
clinical trial.pt.
randomized.ab.
placebo.ab,ti.
drug therapy.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐10
(animals not (humans and animals)).sh.
11 not 12
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
exp Low Back Pain/
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatic neuropathy/
spondylosis.ti,ab.
lumbago.ti,ab.
back disorder$.ti,ab.
or/14‐25 33294
exp Anti‐Inflammatory Agents, Non‐Steroidal/
nsaids.mp.
non‐steroidal anti inflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
non‐steroidal anti‐inflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
aspirin.mp. or exp Aspirin/
acetylsalicyl$.mp.
exp Salicylic Acid/
carbasalate calcium.mp.
diflunisal.mp. or exp Diflunisal/
aceclofenac.mp.
alclofenac.mp.
diclofenac.mp. or exp Diclofenac/
indometacin.mp. or exp Indomethacin/
sulindac.mp. or exp Sulindac/
meloxicam.mp.
piroxicam.mp. or exp Piroxicam/
dexibuprofen.mp.
dexketoprofen.mp.
fenoprofen.mp. or exp Fenoprofen/
flurbiprofen.mp. or exp Flurbiprofen/
ibuprofen.mp. or exp Ibuprofen/
ketoprofen.mp. or exp Ketoprofen/
naproxen.mp. or exp Naproxen/
tiapro$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
metamizol.mp. or exp Dipyrone/
phenylbutazone.mp. or exp Phenylbutazone/
phenazone.mp. or exp Antipyrine/
propyphenazone.mp.
celecoxib.mp.
etoricoxib.mp.
nabumeton.mp.
parecoxib.mp.
or/27‐58
exp cyclooxygenase inhibitors/ or exp cyclooxygenase 2 inhibitors/
rofecoxib.mp.
celecoxib.mp.
valdecoxib.mp.
lumiracoxib.mp.
etoricoxib.mp.
parecoxib.mp.
vioxx.mp.
celebrex.mp.
bextra.mp.
prexige.mp.
arcoxia.mp.
etodolac.mp. or exp Etodolac/
floctafenine.mp.
exp Meclofenamic Acid/
meclofenamate.mp.
meloxicam.mp.
naproxen.mp. or exp Naproxen/
oxaprozin.mp.
piroxicam.mp. or exp Piroxicam/
tenoxicam.mp.
tolmetin.mp. or exp Tolmetin/
or/60‐81
59 or 82
13 and 26 and 83
limit 84 to yr="2007 ‐ 2012"
limit 84 to ed=20070601‐20120524
85 or 86
Appendix 3. MEDLINE In‐Process & Other Non‐Indexed Citations strategy
Last searched June 24, 2015. Line 3 was added, line 6, 27, 37, and 58 were revised.
randomized controlled trial.ti,ab.
controlled clinical trial.ti,ab.
pragmatic.ti,ab.
comparative study.ti,ab.
clinical trial.ti,ab.
randomi#ed.ab.
placebo.ab,ti.
drug therapy.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐11
dorsalgia.ti,ab.
Back Pain.ti,ab.
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatic neuropathy.ti,ab.
spondylosis.ti,ab.
lumbago.ti,ab.
back disorder$.ti,ab.
or/13‐23
Anti‐Inflammatory Agents, Non‐Steroidal.mp.
nsaids.mp.
non‐steroidal antiinflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
non‐steroidal anti‐inflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
aspirin.mp.
acetylsalicyl$.mp.
Salicylic Acid.mp.
carbasalate calcium.mp.
diflunisal.mp.
aceclofenac.mp.
alclofenac.mp.
diclofenac.mp.
(indomethacin or indometacin).mp.
sulindac.mp.
meloxicam.mp.
piroxicam.mp.
dexibuprofen.mp.
dexketoprofen.mp.
fenoprofen.mp.
flurbiprofen.mp.
ibuprofen.mp.
ketoprofen.mp.
naproxen.mp.
tiapro$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
metamizol.mp.
phenylbutazone.mp.
phenazone.mp.
propyphenazone.mp.
celecoxib.mp.
etoricoxib.mp.
nabumeton.mp.
parecoxib.mp.
or/25‐56
((cyclooxygenase or cyclo‐oxygenase) adj3 inhibitor*).mp.
rofecoxib.mp.
celecoxib.mp.
valdecoxib.mp.
lumiracoxib.mp.
etoricoxib.mp.
parecoxib.mp.
vioxx.mp.
celebrex.mp.
bextra.mp.
prexige.mp.
arcoxia.mp.
etodolac.mp.
floctafenine.mp.
Meclofenamic Acid.mp.
meclofenamate.mp.
meloxicam.mp.
oxaprozin.mp.
piroxicam.mp.
tenoxicam.mp.
tolmetin.mp.
or/58‐78
57 or 79
12 and 24 and 80
limit 81 to yr=2014‐2015
limit 81 to ed=20140410‐20150624
82 or 83
April 2014 strategy
randomized controlled trial.ti,ab.
controlled clinical trial.ti,ab.
comparative study.ti,ab.
clinical trial.ti,ab.
randomized.ab.
placebo.ab,ti.
drug therapy.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐10
dorsalgia.ti,ab.
Back Pain.ti,ab.
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatic neuropathy.ti,ab.
spondylosis.ti,ab.
lumbago.ti,ab.
back disorder$.ti,ab.
or/12‐22
Anti‐Inflammatory Agents, Non‐Steroidal.mp.
nsaids.mp.
non‐steroidal anti inflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
non‐steroidal anti‐inflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
aspirin.mp.
acetylsalicyl$.mp.
Salicylic Acid.mp.
carbasalate calcium.mp.
diflunisal.mp.
aceclofenac.mp.
alclofenac.mp.
diclofenac.mp.
indomethacin.mp.
sulindac.mp.
meloxicam.mp.
piroxicam.mp.
dexibuprofen.mp.
dexketoprofen.mp.
fenoprofen.mp.
flurbiprofen.mp.
ibuprofen.mp.
ketoprofen.mp.
naproxen.mp.
tiapro$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
metamizol.mp.
phenylbutazone.mp.
phenazone.mp.
propyphenazone.mp.
celecoxib.mp.
etoricoxib.mp.
nabumeton.mp.
parecoxib.mp.
or/24‐55
(cyclooxygenase inhibitors or cyclooxygenase 2 inhibitors).mp.
rofecoxib.mp.
celecoxib.mp.
valdecoxib.mp.
lumiracoxib.mp.
etoricoxib.mp.
parecoxib.mp.
vioxx.mp.
celebrex.mp.
bextra.mp.
prexige.mp.
arcoxia.mp.
etodolac.mp.
floctafenine.mp.
Meclofenamic Acid.mp.
meclofenamate.mp.
meloxicam.mp.
oxaprozin.mp.
piroxicam.mp.
tenoxicam.mp.
tolmetin.mp.
or/57‐77
56 or 78
11 and 23 and 79
Appendix 4. EMBASE strategy
Last searched June 24, 2015. The study design filter, line 38, and line 46 were revised and line 68 was added.
Randomized Controlled Trial/
exp Controlled Clinical Trial/
Controlled Study/
Double Blind Procedure/
Single Blind Procedure/
crossover procedure/
placebo/
allocat$.mp.
assign$.mp.
blind$.mp.
((control$ or compar$ or prospectiv$ or clinical) adj25 (trial or study)).mp.
(crossover or cross‐over).mp.
factorial$.mp.
(followup or follow‐up).mp.
placebo$.mp.
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
volunteer$.mp.
or/1‐18
exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
human/ or normal human/ or human cell/
20 and 21
20 not 22
19 not 23
dorsalgia.mp.
back pain.mp.
exp BACKACHE/
(lumbar adj pain).mp.
coccyx.mp.
coccydynia.mp.
sciatica.mp.
exp ISCHIALGIA/
spondylosis.mp.
lumbago.mp.
or/25‐34
exp Nonsteroid Antiinflammatory Agent/
nsaids.mp.
non‐steroidal anti‐inflammator$.mp.
exp Acetylsalicylic Acid/
acetylsalicyl$.mp.
carbasalate calcium.mp. or exp Carbasalate Calcium/
diflunisal.mp. or exp DIFLUNISAL/
aceclofenac.mp. or exp ACECLOFENAC/
alclofenac.mp. or exp ALCLOFENAC/
diclofenac.mp. or exp DICLOFENAC/
exp INDOMETACIN/ or (indometacin or indomethacin).mp.
sulindac.mp. or exp SULINDAC/
meloxicam.mp. or exp MELOXICAM/
exp PIROXICAM/ or piroxicam.mp.
dexibuprofen.mp. or exp DEXIBUPROFEN/
dexketoprofen.mp. or exp DEXKETOPROFEN/
exp FENOPROFEN/ or fenoprofen.mp.
flurbiprofen.mp. or exp FLURBIPROFEN/
ibuprofen.mp. or exp IBUPROFEN/
ketoprofen.mp. or exp KETOPROFEN/
naproxen.mp. or exp NAPROXEN/
tiapro$.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
metamizol.mp. or exp Dipyrone/
phenylbutazone.mp. or exp PHENYLBUTAZONE/
phenazone.mp. or exp PHENAZONE/
exp PROPYPHENAZONE/ or propyphenazone.mp.
celecoxib.mp. or exp CELECOXIB/
etoricoxib.mp. or exp ETORICOXIB/
exp Nabumetone/ or nabumeton.mp.
parecoxib.mp. or exp PARECOXIB/
or/36‐65
exp Cyclooxygenase 2 Inhibitor/
((cyclooxygenase or cyclo‐oxygenase) adj3 inhibitor*).mp.
rofecoxib.mp. or exp ROFECOXIB/
valdecoxib.mp. or exp VALDECOXIB/
lumiracoxib.mp. or exp LUMIRACOXIB/
etoricoxib.mp. or exp ETORICOXIB/
parecoxib.mp. or exp PARECOXIB/
vioxx.mp.
celebrex.mp.
bextra.mp.
prexige.mp.
arcoxia.mp.
etodolac.mp. or exp ETODOLAC/
floctafenine.mp. or exp FLOCTAFENINE/
exp Meclofenamic Acid/
meclofenam$.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
oxaprozin.mp. or exp OXAPROZIN/
exp PIROXICAM/ or piroxicam.mp.
tenoxicam.mp. or exp TENOXICAM/
tolmetin.mp. or exp TOLMETIN/
or/67‐86
66 or 87
24 and 35 and 88
limit 89 to yr="2014 ‐ 2015"
limit 89 to em=201414‐201525
90 or 91
Study design and animal filter used in the April 2014 search. The animal filter was revised in 2013 and line 31 was revised in 2014.
1 Clinical Article/
2 exp Clinical Study/
3 Clinical Trial/
4 Controlled Study/
5 Randomized Controlled Trial/
6 Major Clinical Study/
7 Double Blind Procedure/
8 Multicenter Study/
9 Single Blind Procedure/
10 Phase 3 Clinical Trial/
11 Phase 4 Clinical Trial/
12 crossover procedure/
13 placebo/
14 or/1‐13
15 allocat$.mp.
16 assign$.mp.
17 blind$.mp.
18 (clinic$ adj25 (study or trial)).mp.
19 compar$.mp.
20 control$.mp.
21 cross?over.mp.
22 factorial$.mp.
23 follow?up.mp.
24 placebo$.mp.
25 prospectiv$.mp.
26 random$.mp.
27 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
28 trial.mp.
29 (versus or vs).mp.
30 or/15‐29
31 14 or 30
32 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
33 human/ or normal human/ or human cell/
34 32 and 33
35 32 not 34
36 31 not 35
May 2012 search
Clinical Article/
exp Clinical Study/
Clinical Trial/
Controlled Study/
Randomized Controlled Trial/
Major Clinical Study/
Double Blind Procedure/
Multicenter Study/
Single Blind Procedure/
Phase 3 Clinical Trial/
Phase 4 Clinical Trial/
crossover procedure/
placebo/
or/1‐13
allocat$.mp.
assign$.mp.
blind$.mp.
(clinic$ adj25 (study or trial)).mp.
compar$.mp.
control$.mp.
cross?over.mp.
factorial$.mp.
follow?up.mp.
placebo$.mp.
prospectiv$.mp.
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
trial.mp.
(versus or vs).mp.
or/15‐29
14 and 30
human/
Nonhuman/
exp ANIMAL/
Animal Experiment/
33 or 34 or 35
32 not 36
31 not 36
37 and 38
38 or 39
dorsalgia.mp.
back pain.mp.
exp BACKACHE/
(lumbar adj pain).mp.
coccyx.mp.
coccydynia.mp.
sciatica.mp.
exp ISCHIALGIA/
spondylosis.mp.
lumbago.mp.
exp Low Back Pain/
or/41‐51
exp Nonsteroid Antiinflammatory Agent/
nsaids.mp.
non‐steroidal anti‐inflammatory.mp.
exp Acetylsalicylic Acid/
acetylsalicyl$.mp.
carbasalate calcium.mp. or exp Carbasalate Calcium/
diflunisal.mp. or exp DIFLUNISAL/
aceclofenac.mp. or exp ACECLOFENAC/
alclofenac.mp. or exp ALCLOFENAC/
diclofenac.mp. or exp DICLOFENAC/
exp INDOMETACIN/ or indometacin.mp.
sulindac.mp. or exp SULINDAC/
meloxicam.mp. or exp MELOXICAM/
exp PIROXICAM/ or piroxicam.mp.
dexibuprofen.mp. or exp DEXIBUPROFEN/
dexketoprofen.mp. or exp DEXKETOPROFEN/
exp FENOPROFEN/ or fenoprofen.mp.
flurbiprofen.mp. or exp FLURBIPROFEN/
ibuprofen.mp. or exp IBUPROFEN/
ketoprofen.mp. or exp KETOPROFEN/
naproxen.mp. or exp NAPROXEN/
tiapro$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
metamizol.mp. or exp Dipyrone/
phenylbutazone.mp. or exp PHENYLBUTAZONE/
phenazone.mp. or exp PHENAZONE/
exp PROPYPHENAZONE/ or propyphenazone.mp.
celecoxib.mp. or exp CELECOXIB/
etoricoxib.mp. or exp ETORICOXIB/
exp Nabumetone/ or nabumeton.mp.
parecoxib.mp. or exp PARECOXIB/
or/53‐82
exp Cyclooxygenase 2 Inhibitor/
rofecoxib.mp. or exp ROFECOXIB/
valdecoxib.mp. or exp VALDECOXIB/
lumiracoxib.mp. or exp LUMIRACOXIB/
etoricoxib.mp. or exp ETORICOXIB/
parecoxib.mp. or exp PARECOXIB/
vioxx.mp.
celebrex.mp.
bextra.mp.
prexige.mp.
arcoxia.mp.
etodolac.mp. or exp ETODOLAC/
floctafenine.mp. or exp FLOCTAFENINE/
exp Meclofenamic Acid/
meclofenam$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
oxaprozin.mp. or exp OXAPROZIN/
exp PIROXICAM/ or piroxicam.mp.
tenoxicam.mp. or exp TENOXICAM/
tolmetin.mp. or exp TOLMETIN/
or/84‐102
83 or 103
40 and 52 and 104
limit 105 to yr="2007 ‐ 2012"
limit 105 to em=200712‐201220 1071
106 or 107
Appendix 5. Clinical trials registry and PubMed strategies
ClinicalTrials.gov
Last searched June 24, 2015
Basic search: “back pain” and NSAIDS, received from 04/10/2014 to 06/24/2015
WHO ICTRP
Last searched June 24, 2015. Results from 2014 to 2015 were reviewed.
Basic search: back pain and NSAIDS
PubMed
Last searched June 24, 2015
((nsaids OR non‐steroidal anti‐inflammator* OR non‐steroidal antiinflammator* OR aspirin OR acetylsalicyl* OR salicylic acid OR carbasalate calcium OR diflunisal OR aceclofenac OR alclofenac OR diclofenac OR indomethacin OR indometacin OR sulindac OR meloxicam OR piroxicam OR dexibuprofen OR dexketoprofen OR fenoprofen OR flurbiprofen OR ibuprofen OR ketoprofen OR naproxen OR tiapro* OR metamizol OR phenylbutazone OR phenazone OR propyphenazone OR celecoxib OR etoricoxib OR nabumeton OR parecoxib OR cyclooxygenase inhibitor* OR cyclo‐oxygenase inhibitor* OR rofecoxib OR celecoxib OR valdecoxib OR lumiracoxib OR etoricoxib OR parecoxib OR vioxx OR celebrex OR bextra OR prexige OR arcoxia OR etodolac OR floctafenine OR Meclofenamic Acid OR meclofenamate OR meloxicam OR oxaprozin OR piroxicam OR tenoxicam OR tolmetin) AND (back pain OR sciatica OR lumbar pain OR lumbago OR dorsalgia OR backache OR back disorder*) AND (pubstatusaheadofprint OR publisher[sb] or pubmednotmedline[sb]))
Appendix 6. The GRADE approach to evidence synthesis
The quality of evidence will be categorised as follows:
High (⊙⊙⊙⊙): further research is very unlikely to change the confidence in the estimate of effect.
Moderate (⊙⊙⊙○): further research is likely to have an important impact in the confidence in the estimate of effect.
Low (⊙⊙○○): further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low (⊙○○○): any estimate of effect is very uncertain.
The evidence available to answer each sub‐question will be graded on the domains in the following manner:
1. Study design
Only randomised controlled trials (double‐blind, single‐blind, and open‐label) were included.
2. Risk of bias
The risk of bias of included trials was assessed independently by the authors, based on criteria described in Cochrane Back and Neck's tool for assessing risk of bias (Furlan 2015). Each criterion was rated as low risk,high risk, orunclear (Table 2; Table 3).
To assess for other sources of potential bias the following factors were assessed: funding and other biases such as (low) sample size and how the data were presented (Furlan 2015).
The evidence was not downgraded when all trials were judged as low risk of bias for all five categories. We downgraded the evidence by one level when more than three categories had a high or unclear risk. We downgraded the evidence by two levels when four or more categories had a high or unclear risk.
3. Inconsistency
The evidence was downgraded when heterogeneity or variability was large and there was in addition inconsistency arising from populations, interventions, or outcomes (Atkins 2004; Furlan 2015).
The quality of evidence was downgraded by one level when the heterogeneity or variability in results was large (for example I2 > 80%). The evidence was downgraded by two levels when heterogeneity or variability was large and there was inconsistency arising from populations, interventions, or outcomes.
4. Indirectness
Indirectness refers to the extent to which the people, interventions and outcomes in the trials are not representative of those defined in the inclusion criteria of the review. The evidence was downgraded one level when there was an uncertainty about generalizability of the results in one area and two levels when there was indirectness in two or more areas. (Atkins 2004; Furlan 2015).
5. Imprecision
Results are imprecise when trials include relatively few patients and few events and thus have wide CIs around the estimate of the effect. The evidence was downgraded one level if the results were considered imprecise when trials included relatively few participants and few events or had wide confidence intervals around the estimate of the effect and when there was only one trial and when there was more than one trial and the total number of events was lower than 300 for dichotomous data and 400 for continuous data (Atkins 2004; Furlan 2015; Mueller 2007). We downgraded the evidence by two levels if there were both few events, few patients and wide confidence intervals.
6. Publication bias
The quality of evidence was downgraded by one level if the funnel plots suggested publication bias.
Data and analyses
Comparison 1. NSAID versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change pain intensity from baseline on 100 mm VAS, follow‐up <= 3 weeks | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Piroxicam | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Meloxicam 7.5/15mg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Diclofenac | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Lonoxicam | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in pain intensity summary | 3 | 918 | Mean Difference (IV, Random, 95% CI) | ‐4.56 [‐11.11, 1.99] |
| 3 Global improvement | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Phenylbutazone | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Diclofenac | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Indomethacin 7 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Indomethacin 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Lornoxicam 24mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Meloxicam 7.5/15mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Global improvement summary | 3 | 753 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.03, 1.27] |
| 5 Side effects | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Lornoxicam | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Diclofenac | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Meloxicam 7.5/15mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Phenylbutazone | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Piroxicam | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Indomethacin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Side effect summary | 4 | 967 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.02, 1.93] |
| 7 Number of participants with additional pain medication | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. NSAID versus NSAID.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in LBP pain intensity from baseline on 100 mm VAS, follow‐up <= 3 weeks | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Meloxicam | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Lornoxicam | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Global improvement | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Diclofenac | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Meloxicam 7.5/15mg vs. Diclofenac 150mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Lornoxicam | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of participants with additional pain medication | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Meloxicam 7.5/15mg vs. Diclofenac | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
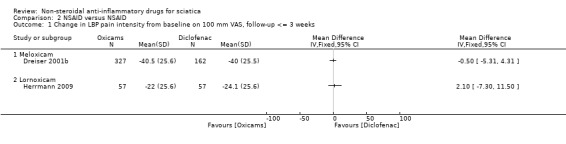
Comparison 2 NSAID versus NSAID, Outcome 1 Change in LBP pain intensity from baseline on 100 mm VAS, follow‐up <= 3 weeks.
2.2. Analysis.
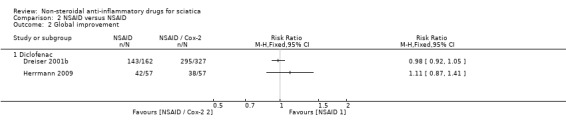
Comparison 2 NSAID versus NSAID, Outcome 2 Global improvement.
2.3. Analysis.
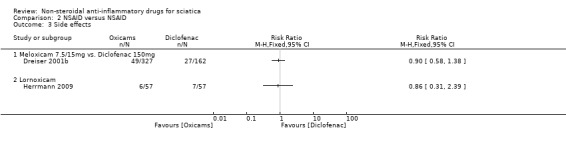
Comparison 2 NSAID versus NSAID, Outcome 3 Side effects.
2.4. Analysis.
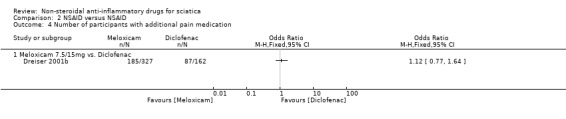
Comparison 2 NSAID versus NSAID, Outcome 4 Number of participants with additional pain medication.
Comparison 3. NSAID versus other drug.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change pain intensity from baseline in 100 mm VAS, follow‐up <= 3 weeks | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Diclofenac | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Ketoprofen vs. Phenylbutazone + Dexamethasone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
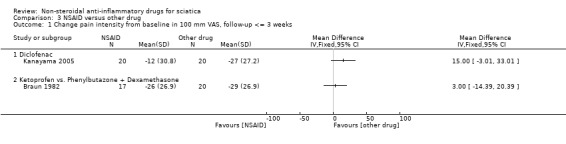
Comparison 3 NSAID versus other drug, Outcome 1 Change pain intensity from baseline in 100 mm VAS, follow‐up <= 3 weeks.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Braun 1982.
| Methods | RCT; double‐blind | |
| Participants | 37 patients aged > 18 years with acute lumbo‐ischialgia (not further specified) | |
| Interventions | (i) NSAID group: ketoprofen day 1 to 3 intramuscular (IM) 200 mg twice a day, day 4 to 8, 200 mg (4 x 50 mg capsules) and 1 rectal suppository 100 mg per/day (ii) Comparison group: IM injections with a corticosteroid (dexamethasone) in combination with phenylbutazone‐natrium, carbamoyl phenoxy acid, lidocaine, and cyanocobalamin on day 1 to 3, in tablets on day 4 to 8, 3 times a day, and 1 rectal suppository at bedtime |
|
| Outcomes | Pain: The follow‐up duration was 9 days. No significant difference in spontaneous pain (VAS 0 to 10) was observed between the groups at day 4 and 9. In both groups the pain decreased. Disability: Not assessed. Clinical findings: There was no significant difference between the groups in the straight leg raise (Lasègue test) and fingertip‐to floor distance. Global improvement: No information. Additional drug use: The average additional use of pain medication (paracetamol) was 6 tablets in the NSAID group and 7 tablets in the control group on day 0 to 4, and 8 tablets in the NSAID group and 10 tablets in the control group on day 4 to 9. Other outcomes: No information. Adverse effects: Not reported |
|
| Notes | No information on funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not addressed |
| Allocation concealment (selection bias) | Unclear risk | Not addressed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The results were blinded from the personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Low risk | All outcomes defined in the methods section were reported |
| Group similarity at baseline | High risk | Not addressed |
| Influence of co‐interventions | Low risk | Reported mean use of paracetamol tablets per group |
| Compliance with interventions | Unclear risk | Not addressed |
| Funding | Unclear risk | No information |
| Other bias | High risk | Small trial, no confidence interval or standard deviation for the outcome reported. No adverse effects reported |
Dreiser 2001a.
| Methods | RCT; multicentre (79 centres), double‐blind, double‐dummy, placebo‐controlled trial. No description of the randomisation procedure | |
| Participants | 532 outpatients (male 44%, female 56%); age older than 18 years. Inclusion criteria (the same for the placebo‐controlled and the diclofenac‐controlled trials): common sciatica with at least 5 out of 8 criteria: radiculalgia with LBP; sudden onset during exertion or wrong movement; mechanical pain; absence of progressive aggravation; history of LBP; antalgic spine deviation or stiffness; sciatica pain exacerbated by pressure of segments L4‐5, L5‐S1; sciatica pain exacerbated by coughing/defecation. Other inclusion criteria were: onset of pain within 3 days; pain intensity of > 50 on VAS; positive straight leg raise ≦ 60°, and a requirement of NSAIDs. Exclusion criteria: treatment with any NSAID within 3 days; adverse effects of NSAIDs; hypersensitive to analgesics, antipyretics, or NSAIDs; other NSAIDs or analgesic agents; previous or active peptic ulcer; former lumbar surgery or symptomatic sciatica during the previous 6 months; cauda equina syndrome; paralysing sciatica; sciatica requiring surgery; hyperalgic sciatica; bilateral swing sciatica; truncular sciatica; sciatica due to tumour; spondylolisthesis or known lumbar narrowing |
|
| Interventions | Placebo‐controlled trial: treatment duration and follow‐up duration 7 days (i) NSAID group 1: meloxicam 7.5 mg once a day (n = 171) (ii) NSAID group 2: meloxicam 15 mg once a day (n = 181) (iii) Control group: placebo once a day (n = 180) |
|
| Outcomes | Pain: All groups improved significantly in spontaneous pain at 3 hours, 6 hours, 3 days, and 8 days compared to baseline. At day 7 pain decrease in the placebo group was ‐40 (±26.8), in group 1 ‐46 (±26.1), and in group 2 ‐45 (±26.9) on a 100 mm VAS scale. Disability: Not investigated. No differences in clinical findings between the groups, including Schober's test, fingers‐to‐floor test, straight leg raise test. Global improvement: Global efficacy was assessed at the end of treatment (day 7), by participant and investigator, using a 4‐point verbal rating scale (good, satisfactory, not satisfactory, bad), and the number of withdrawals due to lack of efficacy was monitored. The percentage of participants reporting a good or satisfactory response was 78% in group 1 and 76% in group 2. Additional drug use: Paracetamol daily use was lower for meloxicam 15 mg compared with placebo (P = 0.0320; mean (SD) daily use in the meloxicam 7.5 mg group was 939 (974) mg; meloxicam 15 mg group 869 (929) mg; placebo group 1110 (1022) mg). The number of participants who took paracetamol was lower in the meloxicam 15 mg group (105 participants; 58%) compared with the placebo group (128 participants; 71%; P = 0.033). Other outcomes: No difference in the daily standardised bed rest in hours (SD) between the treatment groups: meloxicam 7.5 mg 2.1 (2.1) hours, meloxicam 15 mg 2.2 (2.6) hours, and placebo group 2.5 (2.6) hours. Adverse effects: At least 1 adverse event occurred in 29 participants (17%) in the meloxicam 7.5 mg group , 35 participants (19%) in the meloxicam 15 mg group, and in 24 participants (13%) in the placebo group. The difference in the overall and treatment‐related adverse events was not statistically significant between the 3 treatment groups. Nausea, dyspepsia, and abdominal pain were the most common treatment‐related adverse events |
|
| Notes | Withdrawal 6%, no difference between groups. Unclear whether the trial was funded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not addressed |
| Allocation concealment (selection bias) | Unclear risk | Not addressed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed, even if the design is double blinded, double dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rate similar across groups (6%), reasons for drop‐out given |
| Selective reporting (reporting bias) | Low risk | No previous protocol, but reported all prespecified outcomes |
| Group similarity at baseline | Low risk | Comparable groups at baseline |
| Influence of co‐interventions | High risk | Not reported |
| Compliance with interventions | Unclear risk | Not addressed |
| Funding | Unclear risk | Unclear whether the trial was funded |
| Other bias | Low risk | 2 studies within 1 publication. Certain study‐specific information not reported; compliance not reported, additional interventions not reported |
Dreiser 2001b.
| Methods | RCT; multicentre, double‐blind, double‐dummy, placebo‐controlled trial. No randomisation procedures described | |
| Participants | 489 outpatients (male 53%, female 47%); age older than 18 years. Inclusion criteria (the same for the placebo‐controlled and the diclofenac‐controlled trials): common sciatica with at least 5 out of 8 criteria: radiculalgia with LBP; sudden onset during exertion or wrong movement; mechanical pain; absence of progressive aggravation; history of LBP; antalgic spine deviation or stiffness; sciatica pain exacerbated by pressure of segments L4‐5, L5‐S1; sciatica pain exacerbated by coughing/defecation. Other inclusion criteria were: onset of pain within 3 days; pain intensity of > 50 on VAS; positive straight leg raise ≦ 60°, and a requirement of NSAIDs. Exclusion criteria: treatment with any NSAID within 3 days; adverse effects of NSAIDs; hypersensitive to analgesics, antipyretics or NSAIDs; other NSAIDs or analgesic agents; previous or active peptic ulcer; former lumbar surgery or symptomatic sciatica during the previous 6 months; cauda equina syndrome; paralysing sciatica; sciatica requiring surgery; hyperalgic sciatica; bilateral swing sciatica; truncular sciatica; sciatica due to tumour; spondylolisthesis or known lumbar narrowing |
|
| Interventions | Diclofenac‐controlled trial, treatment duration and follow‐up 14 days: (i) Meloxicam 7.5 mg once a day (n = 489) (ii) Meloxicam 15 mg once a day (n = 163) (iii) Diclofenac 50 mg 3 times a day (n = 162) |
|
| Outcomes | Pain: In all groups significant decrease in pain (VAS, 0 to 100) from baseline to follow‐up (at 3 hours, 6 hours, 7 and 14 days) with no between‐group differences. Disability: Not investigated. No differences in clinical findings between the groups, including Schober's test, fingers‐to‐floor test, straight leg raise test. Global improvement: No significant difference in proportion of participants experiencing pain relief between the groups. Additional drug use: The mean daily paracetamol consumption and number of participants who took paracetamol was comparable between the groups. The mean (SD) daily paracetamol use was 751 (890) mg, 748 (869) mg, and 727 (871) mg for participants on meloxicam 7.5 mg, meloxicam 15 mg, and diclofenac 150 mg, respectively. Other outcomes: Daily standardised bed rest hours (SD were similar in each group, 2.6 (2.5) for meloxicam 7.5 mg, 2.8 (2.8) for meloxicam 15 mg, and 2.7 (2.8) for diclofenac 150 mg. Adverse effects: No significant difference between groups ((i) 13%; (ii) 17%; (iii) 17%) for the overall or treatment‐related side effects (nausea, dyspepsia, and abdominal pain most common side effects) |
|
| Notes | Withdrawal 12%, no significant difference between groups. Unclear whether the trial was funded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not addressed |
| Allocation concealment (selection bias) | Low risk | Double‐blind, double‐dummy trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rate similar across groups (12%), reasons given |
| Selective reporting (reporting bias) | Low risk | No previous protocol, but reported all prespecified outcomes |
| Group similarity at baseline | Low risk | Comparable groups at baseline |
| Influence of co‐interventions | High risk | Not reported |
| Compliance with interventions | Unclear risk | Not addressed |
| Funding | Unclear risk | Unclear whether the trial was funded |
| Other bias | Unclear risk | 2 studies within 1 publication. Additional interventions not reported |
Goldie 1968a.
| Methods | RCT; double‐blind, placebo‐controlled trial | |
| Participants | 50 inpatients and 5 outpatients with true sciatica, low back pain with irradiation down either leg were included. Duration of symptoms did not exceed 3 weeks | |
| Interventions | Treatment duration 16 days, follow‐up duration 7 and 14 days (i) NSAID group: indomethacin 25 mg 3 times daily (n = 25) (ii) Control group: placebo 3 times daily in identical capsules (n = 25) No other drugs and no physical therapy allowed during the treatment duration |
|
| Outcomes | Pain: Daily registration of pain, results not reported. Disability: Not assessed. Clinical tests including straight leg raise test were performed daily. No difference between the groups at 7 and 15 days. Global improvement: Relief in pain intensity using a scoring system: no recovery from initial pain (3 points), lessened pain (= fair relief, 2 points), no pain (= complete pain relief, 1 point). At day 7, 15 out of 25 participants in the NSAID group and 18 out of 25 in the placebo group reported a fair or complete relief of pain. After 14 days, 15 in the NSAID group and 18 in the placebo group reported fair or complete relief. Additional drug use: No additional drug use allowed. Other outcomes: None. Adverse effects: Adverse effects were reported in 13/50 participants: headache (NSAID group n = 4, placebo n = 2), nausea (NSAID group n = 4, placebo n = 2), dizziness (NSAID group n = 0, placebo n = 1) |
|
| Notes | Did not report on funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given on how the random sequence was generated |
| Allocation concealment (selection bias) | Low risk | Only the manufacturer of the indomethacin and the identical placebo capsules knew the code |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Doctors, nurses, and participants were unaware of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Doctors, nurses, and participants were unaware of the treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reporting on missing data and ITT analysis |
| Selective reporting (reporting bias) | Low risk | The outcomes defined in the methods section were reported in the results. No protocol published |
| Group similarity at baseline | Unclear risk | Only limited information on baseline characteristics reported |
| Influence of co‐interventions | Low risk | No additional treatment during the intervention |
| Compliance with interventions | Unclear risk | Not addressed |
| Funding | Unclear risk | Not reported |
| Other bias | High risk | Small trial. The scale used for the outcome only allowed answers for the following responses: lessened pain, no pain, pain relief, therefore no deterioration could be reported |
Grevsten 1975.
| Methods | RCT; randomised in pairs (pairs with similar symptoms and comparable clinical findings); double blind | |
| Participants | 36 outpatients (17 men, 19 women), aged between 23 and 62 years with acute lumbago‐ischias without paresis. Some participants had sciatica for the first time, while others had an acute exacerbation of previous symptoms | |
| Interventions | Treatment day 1 intramuscular (IM), day 2 to 4 oral treatment 3 times daily. Treatment duration 14 days, follow‐up duration 14 days. (i) NSAID group: day 1, 0.6 g phenylbutazone (Butazolidin) IM; day 2 to 4, Butazolidin Alka 0.2 g 3 times daily, day 5 to 14, 0.1 g 3 times daily. (ii) Control group: placebo (distilled water) IM followed by oral placebo treatment in the same manner as group (i) |
|
| Outcomes | Outcome assessment by the orthopaedic surgeon during a follow‐up evaluation. Participants' subjective symptoms were graded on a 4‐point scale. Pain: Not reported. Disability: Not reported. Global improvement: Improved vs not improved. At 14 days, 15 out of 18 participants in the NSAID group had improved. In the placebo group, 8 out of 18 participants had improved. Additional drug use: Not assessed, analgesics as needed were allowed but not recorded. Other outcomes: None Adverse effects: In 7 participants (6 in the placebo group, 1 in the NSAID group). The participant in the NSAID group discontinued treatment after 4 days due to symptoms of gastritis. Most of these participants experienced mild, transient nausea (n = 6) |
|
| Notes | Funding was not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not addressed |
| Allocation concealment (selection bias) | Unclear risk | Pairs of patients with similar symptoms and comparable clinical findings were selected and given in randomised order |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not addressed. 36 participants included, 1 outcome is presented for 34 participants |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Group similarity at baseline | High risk | No table of baseline characteristics given |
| Influence of co‐interventions | Unclear risk | Day and night analgesic in conventional doses, no specifics about how many medications were used |
| Compliance with interventions | Unclear risk | Not addressed |
| Funding | Unclear risk | Not reported |
| Other bias | High risk | Small trial, no standard deviation for results reported |
Herrmann 2009.
| Methods | Prospective, double‐blind, randomised, multicentre, placebo‐ and active‐controlled, parallel‐group trial | |
| Participants | 171 outpatients (men 44%, women 56%), aged 18 to 70 years. Inclusion criteria: acute sciatica/lumbago‐sciatica with onset < 72 hrs; pain < 70 (VAS); previous episode > 3 months; pain radiating along the sciatic nerve (include below the knee); worsening with straight leg raise test (< 60 degrees). Lumbo‐sciatica defined as sciatica associated with paravertebral pain (superior spina iliaca and gluteal fold). Exclusion criteria: neurological symptoms of herniated disc (paraesthesia; muscular weakness; paralysis); cauda equina syndrome; ankylosing spondylitis; rheumatoid arthritis; significant disease, alcohol abuse; history of hospitalisation or bed rest; physiotherapy; hypersensitive of NSAIDs; use of other NSAIDs during last week; use of corticosteroids within 4 weeks; no narcotic analgesics within 12 hrs; anxiolytics, antidepressants and/or muscle relaxants, topical treatment with NSAIDs, anticoagulants, immunosuppressants; known adverse drug reaction to oxicam |
|
| Interventions | Treatment duration: 5 days, follow‐up duration/time of measurement: 3 and 8 hours, 2 and 3 days. (i) NSAID group 1: lornoxicam (LNX) day 1, 8 mg TID; day 2 to 4, 8 mg BID; day 5, 8 mg OD (ii) NSAID group 2: diclofenac day 1 and 5, 50 mg BID; day 2 to 4, 50 mg TID (iii) Placebo administrated as LNX |
|
| Outcomes | Pain: Pain reduction (VAS 0 to 100) significantly higher for LNX vs placebo from 3 to 8 hrs. No significant differences were seen for days 2 and 3 between the groups, no values for pain reduction reported. Disability: Not assessed. Global improvement: Overall efficacy rated as very good or good by the participants at day 2 to 4: 65% in the lornoxicam group, 72% in the diclofenac group, 56% in the placebo group. Additional drug use: Addditional analgesics were not permitted during study. Other outcomes: Not assessed Adverse effects: Mild to moderate adverse symptoms were reported by (i) n = 6; (ii) n = 7; (iii) n = 4. 2 participants reported severe nausea, abdominal pain, and dyspepsia |
|
| Notes | Withdrawal: 7 participants (4%) withdrew: due to early success (n = 1); due to insufficient efficacy (n = 3); result of adverse events (n = 3). Adherence: On day 5 47% of participants took capsules: (i) n = 20; (ii) n = 27; (iii) n = 33. Funding was reported. Nycomed Pharma Austria supplied the medication, and at least 1 author was employed at Nycomed Pharma |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to treatment groups in blocks |
| Allocation concealment (selection bias) | Low risk | Randomisation performed centrally |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo capsules were added to the LNX and diclofenac blister packs provided by the investigator to ensure blinding and correct dosage |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomisation was concealed until trial was completed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat (LOCF) |
| Selective reporting (reporting bias) | Unclear risk | Reported all prespecified outcomes; no protocol |
| Group similarity at baseline | Low risk | Similar baseline characteristics |
| Influence of co‐interventions | Low risk | No rescue medication allowed, short follow‐up duration (5 days) |
| Compliance with interventions | Unclear risk | Yes, 6 participants discontinued treatment in the placebo group due to insufficient efficacy, and 1 discontinued treatment in the diclofenac group due to side effects |
| Funding | High risk | Nycomed Pharma Austria supplied the medication, and at least 1 author was employed at Nycomed Pharma |
| Other bias | Low risk | Medium‐size trial. Well presented |
Kanayama 2005.
| Methods | Single‐centre RCT | |
| Participants | 40 outpatients (50% men), mean age 32.7 years Inclusion criteria: patients with LBP and sciatic symptoms, associated with an L4L5 or L5S1 herniated disc, seeking treatment at the orthopaedic department of a hospital. Pain duration: 14 patients < 1 months, 18 patients 1 to 3 months, and 8 patients > 3 months. Exclusion criteria: patients referred for surgical treatment with indicators as presence of cauda equina syndrome or drop foot |
|
| Interventions | Treatment duration 14 days, follow‐up duration 14 days (i) Control group: sarpogrelate, a 5‐HT2A inhibitor; orally 300 mg OD (ii) NSAID group: diclofenac orally 75 mg OD |
|
| Outcomes | Pain: In both groups significant decrease of pain (VAS 0 to 100) from baseline to day 14. No significant difference between the groups. No significant difference between the groups regarding back pain, leg pain, or leg numbness. Disability: Not assessed. Global improvement: Improvement rate (%) = (baseline VAS score ‐ postintervention VAS score)/baseline VAS score x 100%: 33% (i) and 46% (ii) for low back pain, 32% (i) and 32% (ii) for leg pain, and 35% (i) and 32% (ii) for leg numbness, respectively. Additional drug use: Supplemental NSAID needed (i) n = 11, (ii) n = 6. Other outcomes: No Adverse effects: The trial did not report information on side effects. The pharmaceutical company reported the following information from unpublished sources: overall side effects: 2% in 5‐HT2A inhibitor group (out of 4807 participants) and 8% in NSAID group (out of 35,653 participants). Gastrointestinal side effects: 7% in 5‐HT2A inhibitor group and 1% in NSAID group). |
|
| Notes | No withdrawals. 4 participants (20%) who received 5‐HT2A inhibitor treatment and 6 participants (30%) who received NSAIDs eventually underwent surgery for unremitting sciatic symptoms or muscle weakness. Funding: Mitsubishi Pharma Corp is mentioned in the trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 40 envelopes, 20 for each treatment group |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used, but whether or not they are opaque is not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed for the personnel; for the participants randomisation was used by creating 40 envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐out |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported, no study protocol |
| Group similarity at baseline | Unclear risk | Limited baseline characteristics presented (age, gender, level of herniation) |
| Influence of co‐interventions | Low risk | Only allocated medication for 14 days, after that participants were free to choose |
| Compliance with interventions | Unclear risk | Not addressed |
| Funding | Unclear risk | Mitsubishi Pharma Corp mentioned |
| Other bias | Unclear risk | Small trial |
Radin 1968.
| Methods | RCT; double blind | |
| Participants | 23 inpatients, aged 18 to 53 years Inclusion criteria: pain from lower back or buttock, radiating down the back of the leg to at least the heel; pain on crossed straight leg raise test referred to the contralateral thigh or back; muscular weakness; or sciatic nerve tenderness. Exclusion criteria: X‐ray evidence of disease of lumbo sacral spine; history of prior back surgery or upper gastrointestinal disease |
|
| Interventions | Treatment duration 8 days. Follow‐up duration unclear (i) NSAID group: phenylbutazone day 1 to 2, 100 mg 6 times daily; day 3to 8 TID (n = 12). (ii) Placebo group: not reported how the placebo formula was administered (n = 11). No results are reported for groups |
|
| Outcomes | Pain: Not reported. Disability: Not assessed. Global improvement: Global rating of discomfort and sciatic‐at‐rest as absent, slight, moderate, and severe; (i) showed significant improvement in muscle weakness and in painful straight leg raise test (P < 0.05). Otherwise no significant differences between the groups (P < 0.05). Additional drug use: Other outcomes: 12 underwent laminectomy (n = 6 in the drug‐treated group, n = 6 in the placebo group) Adverse effects: 3 participants with side effects in the NSAID group: mild to moderate in 2 participants (epigastric burning); serious in 1 participant (transient melena) |
|
| Notes | Withdrawal 12%: 2 in the NSAID group and 1 in the placebo group. Funded: Geigy Pharmaceutical Corp NY |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, the authors made all of the assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No methods section where outcomes were defined |
| Selective reporting (reporting bias) | High risk | No predefined outcomes |
| Group similarity at baseline | High risk | Not reported |
| Influence of co‐interventions | Unclear risk | Usual conservative methods for patients |
| Compliance with interventions | Unclear risk | Not reported |
| Funding | Unclear risk | Geigy Pharmaceutical Corp NY |
| Other bias | Unclear risk | Small trial, no standard deviation or confidence intervals provided |
Weber 1980.
| Methods | RCT; double blind | |
| Participants | 59 inpatients (32 male, 27 female), aged 16 to 69 years Inclusion criteria: acute and subacute sciatica, admitted consecutively to a hospital. Clinical symptoms and signs due to fifth lumbar and/or first sacral root lesion. Exclusion: Patients with indication for immediate surgical intervention; contraindication to NSAIDs, previous history of dyspepsia or tuberculosis |
|
| Interventions | Treatment started on day 2 of admission. Treatment duration 5 days. Follow‐up duration 5 days (?) (i) NSAID group: Butazolidin Alka 200 mg TID for 3 days, 100 mg TID for 2 days (ii) Placebo group: administered as in group (i) |
|
| Outcomes | Pain: Not reported. Disability: Not reported. Global improvement: Global effect as definite positive effect or indefinite at day 5; immediate result showed a trend in pain relief in favour of the NSAID group, but not significantly: 14/27 and 8/30 scored a definite positive effect. No difference in straight leg raise test following treatment. Additional drug use: Additional drugs were given on request: paracetamol, codeine, and promethazine. The use of additional drugs was not recorded. Other outcomes: None. Adverse effects: No side effects reported. |
|
| Notes | Withdrawal: 2 participants were excluded from the trial (1 male, 1 female) because they withheld information on contraindications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome not reported for all participants (reported for 30 and 27 out of 59 included participants) |
| Selective reporting (reporting bias) | Low risk | All outcomes defined in the methods section were reported |
| Group similarity at baseline | Unclear risk | Limited information on baseline characteristics presented |
| Influence of co‐interventions | Low risk | Use of additional analgesics reported |
| Compliance with interventions | Unclear risk | Not addressed |
| Funding | Unclear risk | Not reported |
| Other bias | Unclear risk | Small trial, numbers without standard deviation or confidence intervals reported |
Weber 1993.
| Methods | RCT; double‐blinded | |
| Participants | 214 outpatients, mean age 48 years (range 18 to 75 years) Inclusion criteria: radiating pain corresponding to L5/S1 root syndrome with/without sensory and/or motor deficits; positive straight leg raise test; radiating pain provoked by finger pressure; free from sciatica the previous 6 months. Exclusion criteria: cauda equina syndrome; "acute back"; progressive paresis; suspected tumour or local inflammation; ankylosing spondylitis; rheumatoid arthritis; history of peptic ulcer or severe dyspepsia; hypersensitivity to aspirin or other NSAIDs; any other known hematologic, hepatic, renal, pulmonary, cardiac, or systemic disease. Patients with severe psychiatric disease, drug addiction, or alcoholism were excluded |
|
| Interventions | Treatment duration 14 days. Follow‐up duration 2 and 4 weeks, 12 months. No results reported for the respective treatment groups at 12 months. (i) NSAID group: piroxicam 20 mg day 1 to 2, 2 tablets OD; day 3 to 14, 1 tablet OD (ii) Placebo group: administered as in group (i) |
|
| Outcomes | Pain: Marked reduction in pain (VAS 0 to 100) at 14 days and 4 weeks compared to baseline in both groups, but no significant difference between groups. Disability: Marked improved function (modified Roland Morris Disability Questionnaire 0 to 17) at 14 days and 4 weeks compared to baseline in both groups, but no significant difference between groups. Global improvement: No. Additional drug use: Additional analgesics as needed included paracetamol with or without codeine and/or levomepromazine. No other opioids were allowed. Other outcomes: At 4 weeks return to work 60%, at 12 months 92.5%. The mean duration of sick leave was 27.9 days. 62 participants were not sick‐listed at all. Adverse effects: 35 participants reported mainly mild to moderate side effects (22 in the piroxicam group, 13 in the placebo group) |
|
| Notes | Withdrawal: 6 participants were dropped out at 4 weeks Funding: Pfizer A/S Norway provided piroxicam and placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported, design was double blind |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo capsules of identical appearance were administered in the same manner as the active substance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported how blinding was maintained throughout the trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reporting on drop‐out or ITT analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes defined in the methods section were reported |
| Group similarity at baseline | Unclear risk | Limited information on baseline characteristics reported |
| Influence of co‐interventions | Low risk | Additional analgesic use did not differ between the groups |
| Compliance with interventions | Low risk | Compliance 95% (piroxicam) and 98% (placebo) up until week 2 |
| Funding | Unclear risk | Pfizer A/S Norway provided piroxicam and placebo |
| Other bias | Unclear risk | Good sample size, did not report standard deviation and confidence interval |
BID: 2 times a day ITT: intention to treat LBP: low back pain LOCF: last observation carried forward NSAID: non‐steroidal anti‐inflammatory drug OD: once a day RCT: randomised controlled trial SD: standard deviation TID: 3 times a day VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adorable 2013 | Participants had mechanical low back pain without sciatica |
| Auvinet 1995 | No clear contrast between treatments |
| Borghi 2013 | Not an RCT; clinical trial without randomisation and control group |
| Brizzi 2004 | Participants had chronic low back pain, not sciatica |
| Cervera‐Irimia 2013 | Mixed patients with back pain with and without leg pain |
| Costantino 2011 | Participants with acute pain, no sciatica |
| Day 2013 | Review; not an RCT |
| Dincer 2007 | Not an RCT; no clear contrast to treatment with NSAIDs |
| Dyszkiewicz 2006 | Participants with low back pain, no sciatica; contrast to NSAIDs by herbal medicine |
| Eken 2014 | Participants with acute pain, no sciatica |
| Ferreira 2013 | Not an RCT, no participants with sciatica |
| Goertz 2013 | No sciatica; comparison of chiropractic treatment versus standard medication |
| Goldie 1968 | Not an RCT |
| Hyup 2013 | No sciatica |
| Kivitz 2013 | No sciatica, mixed patient sample |
| Liedgens 2013 | Not an RCT; letter to the editor |
| Lurie 2014 | Standard care; physical therapy + NSAID |
| Onda 2013 | Participants with spinal stenosis, no sciatica |
| Peng 2014 | Chronic non‐specific low back pain, no sciatica |
| Riou 2014 | Participants with trauma‐ or rheumatic disease‐related conditions, no sciatica |
| Shell 2012 | No sciatica |
| Skljarevski 2012 | No sciatica |
| Staal 2013 | Not an RCT; review |
| Tavafian 2014 | No NSAID, no sciatica |
| Von Heymann 2013 | No sciatica; non‐specific low back pain |
| Wallis 2013 | No sciatica |
| Wetzel 2014 | No sciatica; chronic low back pain |
NSAID: non‐steroidal anti‐inflammatory drug RCT: randomised controlled trial
Differences between protocol and review
We followed the new method guidelines as recommended by CBN (Furlan 2015).
Contributions of authors
Eva Rasmussen‐Barr, Maria M Wertli, Pepijn DDM Roelofs, and Bart W Koes screened the results of the updated database searches (2012, 2013 PR, BK; 2014, 2015 ERB, MW).
Eva Rasmussen‐Barr and Maria M Wertli read all studies included in the full‐text analysis, assessed risk of bias, extracted data, performed the analyses, and wrote the manuscript.
Wilhelmus JA Grooten assessed the risk of bias, extracted the data, checked the results, and contributed to the manuscript.
Ulrike Held extracted the data, performed the analysis, interpreted the results, and contributed to the final manuscripts.
Bart W Koes, Pepijn DDM Roelofs, and Maurits W van Tulder wrote the previous version of this Cochrane review, wrote the protocol of the present review, interpreted the results, and contributed to the final manuscript.
Lisa Winer copy edited the updated review.
Sources of support
Internal sources
-
None, Other.
No sources of support
External sources
-
None, Other.
No sources of support
Declarations of interest
Eva Rasmussen‐Barr has no known conflicts of interest.
Ulrike Held has no known conflicts of interest.
Wilhelmus JA Grooten has no known conflicts of interest.
Pepijn DDM Roelofs has no known conflicts of interest.
Bart W Koes has no known conflicts of interest.
Maurits W van Tulder has no known conflicts of interest.
Maria M Wertli has no known conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Braun 1982 {published data only}
- Braun H, Huberty R. Therapy of lumbar sciatica. A comparative clinical study of a corticoid‐free monosubstance and a corticoid‐containing combination drug. Die Medizinische Welt 1982;33:490‐1. [PubMed] [Google Scholar]
Dreiser 2001a {published data only}
- Dreiser R L, Parc J M, Velicitat P, Lleu P L. Oral meloxicam is effective in acute sciatica: two randomised, double‐blind trials versus placebo or diclofenac. Inflammation Research 2001;50 Suppl 1:S17‐23. [DOI] [PubMed] [Google Scholar]
Dreiser 2001b {published data only}
- Dreiser R L, Parc J M, Velicitat P, Lleu P L. Oral meloxicam is effective in acute sciatica: two randomised, double‐blind trials versus placebo or diclofenac. Inflammation Research 2001;50(Suppl 1):17‐23. [DOI] [PubMed] [Google Scholar]
Goldie 1968a {published data only}
- Goldie I. A clinical trial with indomethacin (indomee (R)) in low back pain and sciatica. Acta Orthopaedica Scandinavica 1968;39:117‐28. [DOI] [PubMed] [Google Scholar]
Grevsten 1975 {published data only}
- Grevsten S, Johansson H. Phenylbutazone in treatment of acute lumbago‐sciatica [Zur Behandlung des akuten Lumbago‐Ischialgie mit Phenylbutazon]. Zeitschrift fur Rheumatologie 1975;34(11/12):444‐7. [PubMed] [Google Scholar]
Herrmann 2009 {published data only}
- Herrmann W A, Geertsen M S. Efficacy and safety of lornoxicam compared with placebo and diclofenac in acute sciatica/lumbo‐sciatica: an analysis from a randomised, double‐blind, multicentre, parallel‐group study. International Journal of Clinical Practice 2009;63:1613‐21. [DOI] [PubMed] [Google Scholar]
Kanayama 2005 {published data only}
- Kanayama M, Hashimoto T, Shigenobu K, Oha F, Yamane S. New treatment of lumbar disc herniation involving 5‐hydroxytryptamine2A receptor inhibitor: a randomized controlled trial. Journal of Neurosurgery. Spine 2005;2:441‐6. [DOI] [PubMed] [Google Scholar]
Radin 1968 {published data only}
- Radin E L, Bryan R S. Phenylbutazone for prolapsed discs?. The Lancet 1968;2:736. [DOI] [PubMed] [Google Scholar]
Weber 1980 {published data only}
- Weber H, Aasand G. The effect of phenylbutazone on patients with acute lumbago‐sciatica. A double blind trial. Journal of Oslo City Hospitals 1980;30:69‐72. [PubMed] [Google Scholar]
Weber 1993 {published data only}
- Weber H, Holme I, Amlie E. The natural course of acute sciatica with nerve root symptoms in a double‐blind placebo‐controlled trial evaluating the effect of piroxicam. Spine (Phila Pa 1976) 1993;18:1433‐8. [PubMed] [Google Scholar]
References to studies excluded from this review
Adorable 2013 {published data only}
- Adorable L V, Uy R V, Tangarorang J Y, Caputolan JC. The effect of phonophoresis using nimesulide gel on mechanical low back pain. Conference: 2013 Annual Assembly of the American Academy of Physical Medicine and Rehabilitation National Harbor, MD United States. Conference Publication. 2013; Vol. (9 SUPPL.1).
Auvinet 1995 {published data only}
- Auvinet B, Ziller R, Appelboom T, Velicitat P. Comparison of the onset and intensity of action of intramuscular meloxicam and oral meloxicam in patients with acute sciatica. Clin Ther 1995;17:1078‐98. [DOI] [PubMed] [Google Scholar]
Borghi 2013 {published data only}
- Borghi B, Aurini L, White P F, Mordenti A, Lolli F, Borghi R, et al. Long‐lasting beneficial effects of periradicular injection of meloxicam for treating chronic low back pain and sciatica. Minerva Anestesiologica 2013;79:370‐8. [PubMed] [Google Scholar]
Brizzi 2004 {published data only}
- Brizzi A, Giusti A, Giacchetti P, Stefanelli S, Provinciali L, Ceravolo M G. A randomised controlled trial on the efficacy of hydroelectrophoresis in acute recurrences in chronic low back pain patients. Europa Medicophysica 2004;40:303‐9. [PubMed] [Google Scholar]
Cervera‐Irimia 2013 {published data only}
- Cervera‐Irimia J, Tome‐Bermejo F. Caudal epidural steroid injection in the treatment of chronic discogenic low back pain. Comparative, prospective and randomized study [Spanish]. Revista Espanola de Cirugia Ortopedica y Traumatologia 2013;57:324‐32. [DOI] [PubMed] [Google Scholar]
Costantino 2011 {published data only}
- Costantino C, Marangio E, Coruzzi G. Mesotherapy versus systemic therapy in the treatment of acute low back pain: a randomized trial. Evidence‐Based Complementary and Alternative Medicine: eCAM 2011;2011:Article ID 317183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Day 2013 {published data only}
- Day R O, Graham G G. Non‐steroidal anti‐inflammatory drugs (NSAIDs).[Erratum appears in BMJ. 2013;347:f4310], [Reprint in Br J Sports Med. 2013 Nov;47(17):1127; PMID: 24159094]. BMJ 2013;195:346:f3. [DOI] [PubMed] [Google Scholar]
Dincer 2007 {published data only}
- Dincer U, Kiralp M Z, Cakar E, Yasar E, Dursan H. Caudal epidural injection versus non‐steroidal anti‐inflammatory drugs in the treatment of low back pain accompanied with radicular pain. Joint Bone Spine 2007;74:467‐71. [DOI] [PubMed] [Google Scholar]
Dyszkiewicz 2006 {published data only}
- Dyszkiewicz A, Opara J. Monitoring the treatment of low back pain using non‐steroid anti‐inflammatory drugs and aromatic oil components. Ortopedia Traumatologia Rehabilitacja 2006;8:210‐8. [PubMed] [Google Scholar]
Eken 2014 {published data only}
- Eken C, Serinken M, Elicabuk H, Uyanik E, Erdal M. Intravenous paracetamol versus dexketoprofen versus morphine in acute mechanical low back pain in the emergency department: a randomised double‐blind controlled trial. Emergency Medicine Journal 2014;31:177‐81. [DOI] [PubMed] [Google Scholar]
Ferreira 2013 {published data only}
- Ferreira M L, Herbert R D, Ferreira P H, Latimer J, Ostelo R W, Grotle M, et al. The smallest worthwhile effect of nonsteroidal anti‐inflammatory drugs and physiotherapy for chronic low back pain: a benefit‐harm trade‐off study. Journal of Clinical Epidemiology 2013;66:1397‐404. [DOI] [PubMed] [Google Scholar]
Goertz 2013 {published data only}
- Goertz C M, Long C R, Hondras M A, Petri R, Delgado R, Lawrence D J, et al. Adding chiropractic manipulative therapy to standard medical care for patients with acute low back pain: Results of a pragmatic randomized comparative effectiveness study. Spine 2013;38(8):627‐34. [DOI] [PubMed] [Google Scholar]
Goldie 1968 {published data only}
- Goldie I, Peterhoff V. Epidural anaesthesia in low‐back pain and sciatica. Acta Orthop Scand 1968;39:261‐9. [DOI] [PubMed] [Google Scholar]
Hyup 2013 {published data only}
- Hyup Lee J, Lee C S, Ultracet ER Study Group. A randomized, double‐blind, placebo‐controlled, parallel‐group study to evaluate the efficacy and safety of the extended‐release tramadol hydrochloride/acetaminophen fixed‐dose combination tablet for the treatment of chronic low back pain. Clinical Therapeutics 2013;35:1830‐40. [DOI] [PubMed] [Google Scholar]
Kivitz 2013 {published data only}
- Kivitz A J, Gimbel J S, Bramson C, Nemeth M A, Keller D S, Brown M T, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain 2013;154:1009‐21. [DOI] [PubMed] [Google Scholar]
Liedgens 2013 {published data only}
- Liedgens H, Henske R. The cost‐effectiveness of duloxetine in chronic low back pain: A US private payer perspective. Value in Health 2013;16:1172. [DOI] [PubMed] [Google Scholar]
Lurie 2014 {published data only}
- Lurie J D, Tosteson T D, Tosteson A N A, Zhao W, Morgan T S, Abdu W A, et al. Surgical versus nonoperative treatment for lumbar disc herniation: Eight‐year results for the spine patient outcomes research trial. Spine 2014;39(1):3‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Onda 2013 {published data only}
- Onda A, Kikuchi S, Yabuki S, Otani K, Nikaido T, Watanabe K, et al. Limaprost alfadex and nonsteroidal anti‐inflammatory drugs for sciatica due to lumbar spinal stenosis. European Spine Journal 2013;22:794‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peng 2014 {published data only}
- Peng Y L, Wu Q M, Li Y F, Mu C Y, Hu A H, Zhang Y. Temperature‐controlled self‐heated pain relief plaster for chronic nonspecific lower back pain: A prospective randomized controlled trial. Chinese Journal of Evidence‐Based Medicine 2014;14(2):147‐51. [Google Scholar]
Riou 2014 {published data only}
- Riou B, Plaisance P, Lecomte F, Soulat L, Orcel P, Mazoit J X. Comparison of two doses of ketoprofen to treat pain: A double‐blind, randomized, noninferiority trial. Fundamental and Clinical Pharmacology 2014;28(1):20‐8. [DOI] [PubMed] [Google Scholar]
Shell 2012 {published data only}
- Shell W E, Charuvastra E H, DeWood M A, May L A, Bullias D H, Silver D S. A double‐blind controlled trial of a single dose naproxen and an amino acid medical food theramine for the treatment of low back pain. Am J Ther 2012;19:108‐14. [DOI] [PubMed] [Google Scholar]
Skljarevski 2012 {published data only}
- Skljarevski V, Liu P, Zhang S, Ahl J, Martinez J M. Efficacy and safety of duloxetine in patients with chronic low back pain who used versus did not use concomitant nonsteroidal anti‐inflammatory drugs or acetaminophen: a post hoc pooled analysis of 2 randomized, placebo‐controlled trials. Pain Research and Treatment 2012;2012:296710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Staal 2013 {published data only}
- Staal J B, Nelemans P J, Bie R A. Spinal injection therapy for low back pain. JAMA 2013;309:2439‐40. [DOI] [PubMed] [Google Scholar]
Tavafian 2014 {published data only}
- Tavafian S S, Jamshidi A R, Mohammad K. Treatment of low back pain: Randomized clinical trial comparing a multidisciplinary group‐based rehabilitation program with oral drug treatment up to 12 months. International Journal of Rheumatic Diseases 2014;17(2):159‐64. [DOI] [PubMed] [Google Scholar]
Von Heymann 2013 {published data only}
- Heymann W J, Schloemer P, Timm J, Muehlbauer B. Spinal high‐velocity low amplitude manipulation in acute nonspecific low back pain: a double‐blinded randomized controlled trial in comparison with diclofenac and placebo. Spine 2013;38:540‐8. [DOI] [PubMed] [Google Scholar]
Wallis 2013 {published data only}
- Wallis D, O'Shea F D, Salonen D, Haroon N, Anton A, Passalent L A, et al. Mechanical back pain demonstrates better response to celecoxib than acetaminophen despite lack of MRI‐defined inflammatory changes in the spine. Arthritis and Rheumatism. Conference: American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting, ACR/ARHP 2013 San Diego, CA United States.. 2013:S903‐4.
Wetzel 2014 {published data only}
- Wetzel L, Zadrazil M, Paternostro‐Sluga T, Authried G, Kozek‐Langenecker S, Scharbert G. Intravenous nonopioid analgesic drugs in chronic low back pain patients on chronic opioid treatment: a crossover, randomised, double‐blinded, placebo‐controlled study. European Journal of Anaesthesiology 2014;31:35‐40. [DOI] [PubMed] [Google Scholar]
Additional references
Airaksinen 2006
- Airaksinen O, Brox J I, Cedraschi C, Hildebrandt J, Klaber‐Moffett J, Kovacs F, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. European Spine Journal 2006;15 Suppl 2:S192‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
AOOS 2009
- American Academy of Orthopaedic Surgeons (AOOS). What are NSAIDs?. orthoinfo.aaos.org/topic.cfm?topic=a00284 2009 (accessed 01 July 2016).
Atkins 2004
- Atkins D, Eccles M, Flottorp S, Guyatt G H, Henry D, Hill S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res 2004; Vol. 4, issue 1:38. [DOI] [PMC free article] [PubMed]
Axen 2013
- Axen I, Leboeuf‐Yde C. Trajectories of low back pain. Best practice & research. Clinical rheumatology 2013;27:601‐12. [DOI] [PubMed] [Google Scholar]
Balague 2012
- Balague F, Mannion A F, Pellise F, Cedraschi C. Non‐specific low back pain. Lancet 2012;379:482‐91. [DOI] [PubMed] [Google Scholar]
Brune 2004
- Brune K, Hinz B. The discovery and development of antiinflammatory drugs. Arthritis and rheumatism 2004;50(8):2391‐9. [DOI] [PubMed] [Google Scholar]
Brune 2015
- Brune K, Patrignani P. New insights into the use of currently available non‐steroidal anti‐inflammatory drugs. Journal of Pain Research 2015;8:105‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cannon 2007
- Cannon D E, Dillingham T R, Miao H, Andary M T, Pezzin L E. Musculoskeletal disorders in referrals for suspected lumbosacral radiculopathy. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists 2007;86(12):957‐61. [DOI] [PubMed] [Google Scholar]
Chaparro 2013
- Chaparro L E, Furlan A D, Deshpande A, Mailis‐Gagnon A, Atlas S, Turk D C. Opioids compared to placebo or other treatments for chronic low‐back pain. The Cochrane database of systematic reviews 2013;8:CD004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherkin 1998
- Cherkin D C, Wheeler K J, Barlow W, Deyo R A. Medication use for low back pain in primary care. Spine (Phila Pa 1976) 1998;23(5):607‐14. [DOI] [PubMed] [Google Scholar]
Chou 2007
- Chou R, Huffman L H. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Annals of internal medicine 2007;147(7):505‐14. [DOI] [PubMed] [Google Scholar]
Duffy 2014
- Duffy S, Misso K, Noake C, Ross J, Stirk L. Supplementary searches of PubMed to improve currency of MEDLINE and MEDLINE In‐Process searches via OvidSP. Kleijnen Systematic Reviews Ltd, York. Poster presented at the UK InterTASC Information Specialists' Sub‐Group (ISSG) Workshop; 9 July 2014; Exeter: UK (2014). medicine.exeter.ac.uk/media/universityofexeter/medicalschool/research/pentag/documents/Steven_Duffy_ISSG_Exeter_2014_poster_1.pdf 1 January 2014.
Dwivedi 2015
- Dwivedi A K, Gurjar V, Kumar S, Singh N. Molecular basis for nonspecificity of nonsteroidal anti‐inflammatory drugs (NSAIDs). Drug discovery today 2015;20(7):863‐73. [DOI: 10.1016/j.drudis.2015.03.004] [DOI] [PubMed] [Google Scholar]
Furlan 2015
- Furlan A D, Malmivaara A, Chou R, Maher C G, Deyo R A, Schoene M, et al. 2015 Updated Method Guideline for Systematic Reviews in the Cochrane Back and Neck Group. Spine (Phila Pa 1976) 2015;40(21):1660‐73. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins J P T, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoy 2014
- Hoy D G, Smith E, Cross M, Sanchez‐Riera L, Buchbinder R, Blyth F M, et al. The global burden of musculoskeletal conditions for 2010: An overview of methods. Annals of the rheumatic diseases 2014;73(6):982‐9. [DOI] [PubMed] [Google Scholar]
Koes 2007
- Koes B W, Tulder M W, Peul W C. Diagnosis and treatment of sciatica. BMJ (Clinical research ed.) 2007;334:1313‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koes 2010
- Koes B W, Tulder M, Lin C W, Macedo L G, McAuley J, Maher C. An updated overview of clinical guidelines for the management of non‐specific low back pain in primary care. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 2010;19(12):2075‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Konstantinou 2013
- Konstantinou K, Hider S L, Jordan J L, Lewis M, Dunn K M, Hay E M. The impact of low back‐related leg pain on outcomes as compared with low back pain alone: a systematic review of the literature. The Clinical journal of pain 2013;29(7):644‐54. [DOI] [PubMed] [Google Scholar]
Kowalski 2015
- Kowalski M L, Makowska J S. Seven steps to the diagnosis of NSAIDs hypersensitivity: how to apply a new classification in real practice?. Allergy, asthma & immunology research 2015;7(4):312‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kreiner 2014
Lewis 2011
- Lewis R, Williams N, Matar H E, Din N, Fitzsimmons D, Phillips C, et al. The clinical effectiveness and cost‐effectiveness of management strategies for sciatica: systematic review and economic model. HealthHealth technology assessment (Winchester, England) 2011;15(39):1‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luijsterburg 2007
- Luijsterburg P A, Verhagen A P, Ostelo R W, Os T A, Peul W C, Koes B W. Effectiveness of conservative treatments for the lumbosacral radicular syndrome: a systematic review. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 2007;16(7):881‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manchikanti 2014
- Manchikanti L, Singh V, Falco F J, Benyamin R M, Hirsch J A. Epidemiology of low back pain in adults. Neuromodulation : journal of the International Neuromodulation Society 2014;17(Suppl 2):3‐10. [DOI] [PubMed] [Google Scholar]
Mueller 2007
- Mueller P S, Montori V M, Bassler D, Koenig B A, Guyatt G H. Ethical issues in stopping randomized trials early because of apparent benefit. Annals of internal medicine 2007;146(12):878‐81. [DOI] [PubMed] [Google Scholar]
Pinto 2012
- Pinto R Z, Maher C G, Ferreira M L, Ferreira P H, Hancock M, Oliveira V C, et al. Drugs for relief of pain in patients with sciatica: systematic review and meta‐analysis. BMJ (Clinical research ed.) 2012;344(doi: 10.1136/bmj.e497.):e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2008
- Rao P, Knaus E E. Evolution of nonsteroidal anti‐inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. ournal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques 2008;11(2):81s‐110s. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roelofs 2008
- Roelofs P D, Deyo R A, Koes B W, Scholten R J, Tulder M W. Non‐steroidal anti‐inflammatory drugs for low back pain. Cochrane Database of Systematic Reviews 2008, Issue 1. DOI: 10.1002/14651858.CD000396.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stafford 2007
- Stafford M A, Peng P, Hill D A. Sciatica: a review of history, epidemiology, pathogenesis, and the role of epidural steroid injection in management. British journal of anaesthesia 2007;99(4):461‐73. [DOI] [PubMed] [Google Scholar]
Swezey 2003
- Swezey R L. Overdiagnosed sciatica and stenosis, underdiagnosed hip arthritis. Orthopedics 2003;26(2):173‐4; discussion 174. [DOI] [PubMed] [Google Scholar]
Trelle 2011
- Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger P M, et al. Cardiovascular safety of non‐steroidal anti‐inflammatory drugs: network meta‐analysis. BMJ 2011; Vol. 342:c7086. [DOI] [PMC free article] [PubMed]
Valat 2010
- Valat J P, Genevay S, Marty M, Rozenberg S, Koes B. Sciatica. Best practice & research. Clinical rheumatology 2010;24(2):241‐52. [DOI] [PubMed] [Google Scholar]
van Tulder 2002
- Tulder M, Koes B, Bombardier C. Low back pain. Best practice & research. Clinical rheumatology 2002;16(5):761‐75. [DOI] [PubMed] [Google Scholar]
van Tulder 2003
- Tulder M, Koes B. Low back pain and sciatica (chronic). Clinical evidence 2003;19(10):1359‐76. [PubMed] [Google Scholar]
van Tulder 2006
- Tulder M W, Koes B, Seitsalo S, Malmivaara A. Outcome of invasive treatment modalities on back pain and sciatica: an evidence‐based review. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 2006;15 Suppl 1:S82‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Visser 2013
- Visser L H, Nijssen P G, Tijssen C C, Middendorp J J, Schieving J. Sciatica‐like symptoms and the sacroiliac joint: clinical features and differential diagnosis. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 2013;22(7):1657‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; Vol. 380:2163‐96. [DOI] [PMC free article] [PubMed]
Williams 2014
- Williams C M, Maher C G, Latimer J, McLachlan A J, Hancock M J, Day R O, et al. Efficacy of paracetamol for acute low‐back pain: a double‐blind, randomised controlled trial. Lancet 2014;384:1586‐96. [DOI] [PubMed] [Google Scholar]
Wong 2015
- Wong J J, Cote P, Ameis A, Varatharajan S, Varatharajan T, Shearer H M, et al. Are non‐steroidal anti‐inflammatory drugs effective for the management of neck pain and associated disorders, whiplash‐associated disorders, or non‐specific low back pain? A systematic review of systematic reviews by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 2016;25(1):34‐61. [DOI] [PubMed] [Google Scholar]


