Abstract
Management of aortic dissections (AD) is still challenging, with no universally approved guideline among possible surgical, endovascular, or medical therapies. Approximately 25% of patients with AD suffer postintervention malperfusion syndrome or hemodynamic instability, with the risk of sudden death if left untreated. Part of the issue is that vascular implants may themselves induce flow disturbances that critically impact vital organs. A multilayer mesh construct might obviate the induced flow disturbances, and it is this concept we investigated. We used preintervention and post-multilayer flow modulator implantation (PM) geometries from clinical cases of type B AD. In-house semiautomatic segmentation routines were applied to computed tomography images to reconstruct the lumen. The device was numerically reconstructed and adapted to the PM geometry concentrically fit to the true lumen centerline. We also numerically designed a pseudohealthy case, where the geometry of the aorta was extracted interpolating geometric features of preintervention, postimplantation, and published representative healthy volunteers. Computational fluid dynamics methods were used to study the time-dependent flow patterns, shear stress metrics, and perfusion to vital organs. A three-element Windkessel lumped parameter module was coupled to a finite-volume solver to assign dynamic outlet boundary conditions. Multilayer flow modulator not only significantly reduced false lumen blood flow, eliminated local flow disturbances, and globally regulated wall shear stress distribution but also maintained physiological perfusion to peripheral vital organs. We propose further investigation to focus the management of AD on both modulation of blood flow and restoration of physiologic end-organ perfusion rather than mere restoration of vascular lamina morphology.
NEW & NOTEWORTHY The majority of aortic dissection modeling efforts have focused on the maintenance of physiological flow using minimally invasive placed grafts. The multilayer flow modulator is a complex mesh construct of wires, designed to eliminate flow disruptions in the lumen, regulate the physiological wall stresses, and enhance endothelial function and offering the promise of improved perfusion of vital organs. This has never been fully proved or modeled, and these issues we confirmed using a dynamic framework of time-varying arterial waveforms.
Keywords: aortic dissection, flow rate, malperfusion, multilayer flow modulator
INTRODUCTION
Aortic dissection (AD) is a rapidly growing, often catastrophic condition (53) characterized by separation of the aortic wall layers enabling blood to flow into normally apposed fascial planes (52). Type B AD, which accounts for one-third of all AD, initiates its tear after the proximal greater arteries forming a pressurized hematoma (2, 52); the tear is propagated toward the iliac bifurcations by hemodynamic forces, which allow blood to penetrate the intima layer and flow into the media (52, 73). Two parallel lumenae allow blood to flow through its normal course, the true lumen (TL), and a channel created by communicating TL tears into a false lumen (FL). Most often the TL is the anatomophysiological channel that supplies blood to vital peripheral organs (43). As flow and volume rise in the FL, the TL is severely compressed, compromising distal flow and escalating exacerbation of flow disruption (73, 77). Ultimately, as arterial wave propagation in the TL is disturbed, forward flow to the peripheral organs is profoundly compromised resulting in end-organ malperfusion (18, 21, 47).
Early interventions to treat type B AD include pharmacological control of flow, pressure, and shear stress (71), which may obviate long-term complications. Full surgical repair is avoided, and minimally invasive interventions like thoracic endovascular aortic repair (TEVAR) with covered grafts are increasingly used (19, 44). In theory, stent grafts reduce the FL, improve blood flow to the TL, and restore intramural stresses to pre-AD levels (44, 50). TEVAR-covered grafts are placed to scaffold the AD and seal the reentries to the FL but also increase the probability of thrombosis and subsequent aneurysms in the FL (44, 50). TEVAR approaches complete primary procedural success but possess poor long-term prognosis (60–80% survival within 5 yr) (78). Approximately 25% of all patients post-TEVAR suffer from malperfusion syndrome or hemodynamic instability, increasing the risk of sudden death if left untreated (24, 76), demanding alternate treatments for type B AD (28, 30, 50).
Existing grafts can seal connections between the FL and TL and/or reduce the FL and increase the TL dimensions. What they do not appear to do is restore normal blood flow patterns and, in particular, flow through the great and distal vessels. This issue we sought to investigate and, for this reason, used a multilayer flow modulator (MFM) to understand the impact of concentrating first on managing the lumen and then on flow. The MFM is a complex mesh construct of cobalt alloy wires designed to reestablish the flow in TL, eliminate the FL, and mitigate hemodynamic disruptions (31). The MFM is a self-expanding tubular stent that is both flexible and fatigue resistant (65). It is designed to laminarize the flow as it permeates through its wires, in contrast to covered endoluminal grafts that totally exclude blood flow. The meshed structure of MFM is claimed to allow proper perfusion to side branches while simultaneously reducing flow to the FL (31, 64). The reduced wall stress, as a result of modulated blood flow through a porous device, promotes endothelialization and thrombosis of aneurysms (67). In contrast, aneurysm sac exclusion by contemporary covered grafts increases the risk of rupture and malperfusion of the spinal artery because of adverse effects on wall stress, pressure distribution, and compliance of aortae based on their relatively stiff structure (29, 70, 74). Structurally speaking, more compliant MFMs would experience smaller drag forces and induce less elevated pulse wave pressure in comparison to covered grafts (34, 48). The functionality and biocompatibility of the MFM device have been previously tested in porcine models, and its role in treating complex aortic aneurysms has been well documented (67a, 68). Computational studies are invaluable tools with a great potential to delineate the biomechanical environment and, as a result, improve management of aortic diseases (12, 36, 41, 59). The MFM device has been shown to reduce FL forward flow in computational models of type B AD and holds the promise of restoring end-organ perfusion (64), but this work used a steady-state model, wherein a constant arterial flow waveform and fixed-boundary conditions at the outlets (and thus frozen flow) were assumed. This approach overlooks the dynamic nature of arterial flow and precludes robust analysis of peripheral perfusion. Other studies have used a time-varying arterial waveform (5, 13) and three-element Windkessel (WK) circuit boundary conditions at the outlets (5) to study the time-averaged shear metrics, FL perfusion, and proximal artery perfusion pre- and postintervention (4, 13, 33). However, to our knowledge, no study has dynamically quantified peripheral end-organ perfusion in type B AD pre- and post-MFM implantation.
Here, we present a computational fluid dynamics (CFD) framework with a time-varying arterial waveform and dynamic three-element WK boundary conditions at the outlets to study the role of the MFM device in restoring peripheral end-organ perfusion in type B AD as a more advanced metric of therapeutic effect. A CFD simulation with ordinary preset outlet pressure condition, for example, can miss the benefits of state-of-the-art endovascular implants in maintaining the physiological flow in terms of vital organ perfusion. The dynamic outlet boundary condition, in contrast, enables computational tools to assess the device performance beyond directing the flow into the TL and shrinkage of the FL. We study the time-averaged wall shear stress (TAWSS), forward flow through the FL, endothelial oscillatory shear index (OSI), and time-varying perfusion to greater and peripheral arteries to quantify end-organ perfusion. The presented framework was applied to a patient with type B AD pre- and post-MFM implantation, and the results were compared with pre-AD to show the efficacy of a device that concentrates on restoring flow patterns rather than lumen dimensions in maintaining end-organ perfusion.
METHODS
Geometry
We examined MFM end-organ perfusion effects by comparing flow in three-dimensional (3-D) arterial models for one patient at pseudohealthy (PH), preintervention (PI), and post-MFM placement (PM) states.
The cases for PI and PM were segmented from computed tomography (CT) scans (Siemens SOMATOM Dual Source CT Scanner) acquired before and 3 days after device implantation. Medical images were anonymously saved in Digital Imaging and Communications in Medicine format. The patient had provided informed consent under supervision and approval of the local ethics committee. A semiautomatic approach was employed for segmentation, combining an in-house code (Matlab, MathWorks, Natick, MA) (7, 8), VMTKlab software (VMTKlab OROBIX, v.1.5.1), and an open-source segmentation platform (3D Slicer v.4.0). Only major outlets were reconstructed and the geometries stored in STL (STereoLithography) format (Fig. 1). As AD is a chronic disease, a healthy, intact arterial geometry does not exist. Therefore, we artificially created a mock physiological healthy artery (PH) interpolating between PI and PM cases with prior knowledge of a generic healthy aorta (63) using Geomagic Wrap (3D SYSTEMS). The PH case, then, actually represents a model of normal flow and stress condition, should the MFM be able to return the diseased case to its intact form.
Fig. 1.
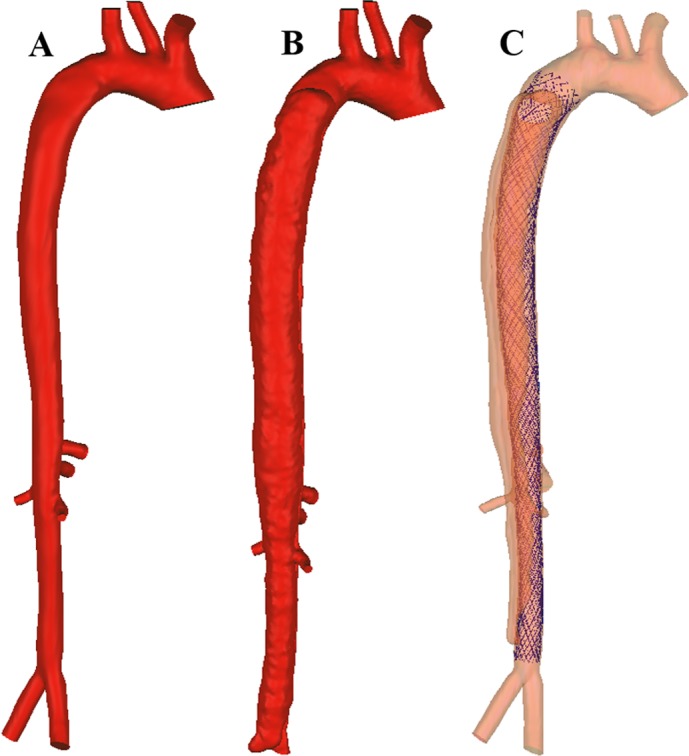
Reconstructed geometry of a pseudohealthy artery (A), preintervention case (B), and post-multilayer flow modulator (MFM) implantation case (C).
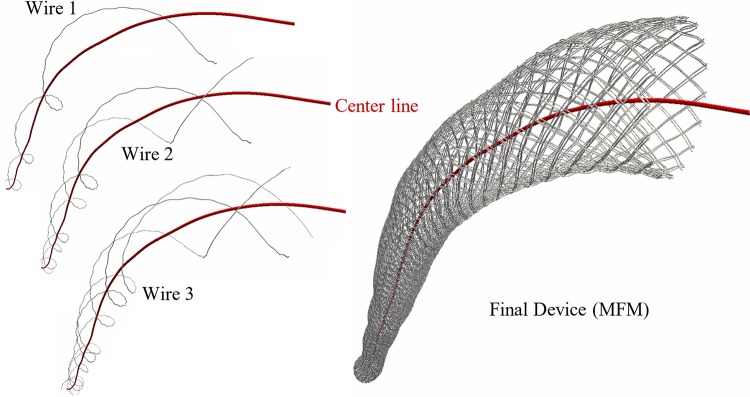
It was not also possible to reconstruct the device segmenting clinical CT images because of the small size of metallic wires and their blooming artifact. Geometric methods, thus, were used to reconstruct the device and concentrically fit it within the TL centerline obtained from the PM case. The proximal and distal edges of the device were marked in PM CT scans and in the 3-D model; the TL was isolated in those locations, and its centerline was extracted. The MFM design is a helicoid of multiple wires on a multilayer formation. Thus, we used Frenet-Serret formulas to create centerline points of each wire along the TL centerline. The Frenet-Serret frame (TNB frame) is composed of the tangent (T), normal (N), and binormal (B) unit vectors forming an orthonormal basis spanning the 3-D space. T was defined by the distance of the TL surface to the TL centerline, whereas N and B were described by the design of the MFM. Once the wire centerline was constructed, octagons were placed perpendicular to it, forming the skeleton of wire that then became a surface using the triangulation algorithm. The entire procedure for device reconstruction and fit used an in-house Matlab code (R2017a, MathWorks; Fig. 2).
Fig. 2.
Numerical wire-by-wire generation of multilayer flow modulator (MFM) helixes concentrically along the true lumen centerline.
In the diseased case, the AD started from early descending aortae and extended to the proximal iliac bifurcation. The PI case exhibited major TL restriction with minimum area at zone 5 with most distal reentry located at the abdominal aorta, zone 9 (refer to Ref. 25 for aortic zone classification).
Computational Model
Transient simulations incorporated the dynamics of blood flow under realistic cardiac cycling to show how the oscillatory nature of blood ejected from the heart impacts the natural history and interventional response of AD. Transient blood flow simulation also included determination of the time-averaged metrics of wall shear as predictive means of adverse clinical responses and quantified the variability of perfusion to vital organs as a result of disease and intervention.
Grid generation.
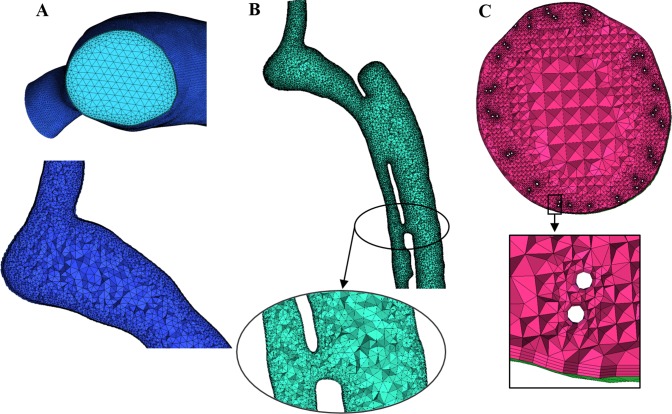
The computational mesh inside the fluid domain was generated using ANSYS ICEM CFD (ANSYS v.16.0, Canonsburg, PA), and the lumen was divided into unstructured tetrahedral elements (Fig. 3). The computational grid was refined near the arterial wall, MFM wires, and in the vicinity of considerable geometric feature change and coarsened toward the lumen center to minimize the computational cost. PH and PI cases included 3,866,399 and 7,486,446 tetrahedral elements, respectively. Considerably more computational elements were used in the PM state to resolve the device accurately (91,303,852 tetrahedral elements). Grid independency was scrutinized with consecutive refinement of the computational mesh to reduce the relative error of velocity in selected cross-sections of the aorta to below 1%.
Fig. 3.
Cross-sectional view of constructed volume mesh in pseudohealthy (A), preintervention (B), and post-multilayer flow modulator (MFM) implantation (C) cases with magnifying insets.
Governing equations and modeling assumptions.
Navier-Stokes equations for transient incompressible blood flow were solved using a fully coupled finite volume ANSYS CFX solver (ANSYS, Canonsburg, PA). At integral points of a colocated grid, the Rhie-Chow interpolation algorithm was applied to calculate pressure redistribution up to third-order accuracy. Blood was modeled as a non-Newtonian fluid with density of 1,060 kg/m3 and shear-dependent dynamic viscosity according to the Carreau model (15, 58) to account for reduced shear strains around a device of small strut size and with small gaps between the MFM and arterial wall, as follows (Eq. 1):
| (1) |
where μ0 (equal to 0.25 Pa·s) and μ∞ (equal to 0.0035 Pa·s) are the blood viscosities at zero and infinite shear rates, λ (equal to 25 s) is the relaxation time constant, is the scalar shear rate, and n (equal to 0.25) is the power law index, respectively (Eq. 1).
The convergence of variables was examined up to 10−6 of the initial values, and mass convergence was externally monitored. Postprocessing of simulations to extract velocity contours, perfusion charts, and wall shear parameters used CFD-Post (ANSYS v.16.0).
Boundary conditions.
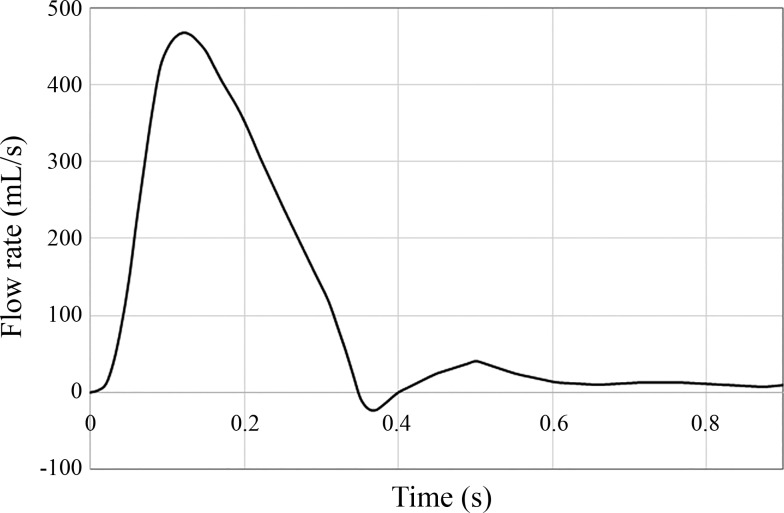
Inlet flow immediately after the aortic sinus was extracted from the literature (3) to study time-dependent hemodynamics over a cardiac cycle at systole and diastole (Fig. 4). Both the MFM device (in the post-MFM case) and vessel were assumed as no-slip walls.
Fig. 4.
Blood flow waveform over a cardiac cycle measured by phase-contrast magnetic resonance imaging (PC-MRI) at ascending aorta distal to valvular ostium (3).
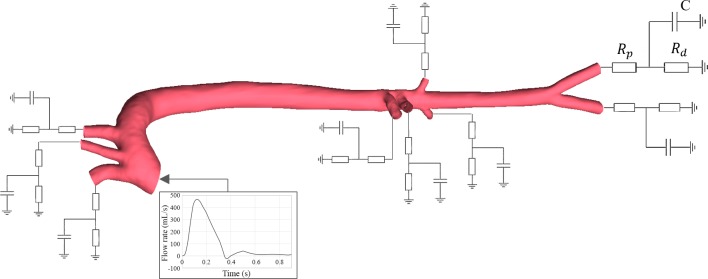
A critical aspect of this work is that we rejected prespecifying fixed conditions on the outlets to allow mutable flow patterns to emerge. Instead, we applied a modified approach where a lumped parameter model dynamically updated the outlet pressure based on peripheral resistances, proximal and distal to the outlet, and compliance in addition to vascular hemodynamics. This classic lumped model embraces an electrical analogy of hemodynamic circulation with inductive (L), resistive (R), or capacitance (C) elements representing effects of inertia, fluid friction, and vascular distension and fluid storage, respectively. The outlet boundary was modeled as a modified circuit with characteristic and distal resistive and capacitive elements alone, sufficient to represent the peripheral vessels and with values drawn from the literature (Fig. 5) (3, 75).
Fig. 5.
Coupling the three-element lumped-parameter model with the computational three-dimensional study. Rd, distal resistance; Rp, proximal resistance; C, distal vasculature compliance.
Coupling the lumped parameter model with a 3-D CFD simulation enables use of the simpler three-element WK model using parameters from the literature (3, 75). Calculation of patient-specific lumped parameter coefficients is still a great challenge and not the scope of this research. Moreover, although pathological changes alter the hemodynamic pattern within diseased arteries, peripheral resistances based on which some parameters are calculated are left intact. The differential equation for this circuit is given by Kirchoff’s laws as follows (10, 22):
| (2) |
where p is pressure, t is time, Q is the volumetric flow rate, C is the compliance of the distal vasculature, and Rp and Rd are proximal and distal resistances, respectively. The analytic solution was as follows:
| (3) |
Studies using a four-element WK model provided identical results to the three-element case and did not justify the additional computational complexity (61). We first provided simple initial conditions for flow and pressure and then the above solution (Eq. 3) iteratively updated the pressure at the boundary based on the flow information it received from the CFD solution within the computational domain.
RESULTS
The primary aim and final results of the transient simulation were to use a dynamic CFD framework to study end-organ perfusion in patients with type B AD at PH, PI, and PM time points. We quantified the local time-varying hemodynamic effects of type B AD, assessed the global effect of it on end-organ blood perfusion, and studied the potential of the MFM device to alleviate local hemodynamic stresses and restore the vital organ perfusion. All simulations were time dependent, and the results were saved in predefined time intervals.
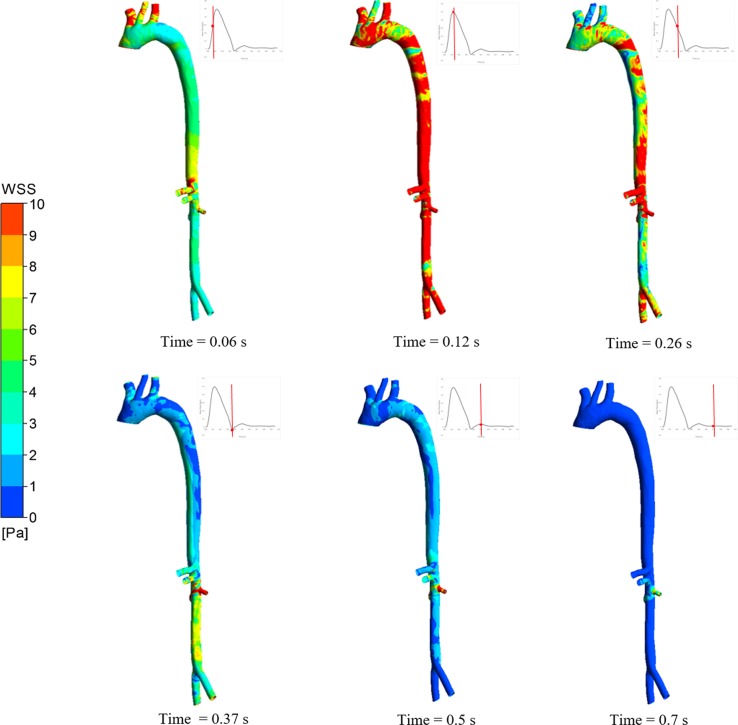
Nonphysiological, i.e., low, high, and oscillatory, wall shear stress (WSS) is centered in specific locations (aortic arch, branching ostia, and bifurcations, for instance) modulating endothelial function, inflammatory/oxidative processes, atherosclerosis susceptibility, and wall degradation/remodeling (11, 20). Different aortic pathophysiological processes directly depend on mechanical stressors, including gene expression, apoptosis pathways, calcification, transmural transport, and neoangeogenesis (9). Marked flow disruption increases risk of inflammation, tissue integrity loss, and vascular weakening (60). For instance, low WSS, attributed to flow separation, may increase monocyte adhesion, and recirculating flow may increase their delivery to the vessel wall (54). Components of inflammatory cells are found in aortic pathologies, which in part are attributed to biomechanical factors (9, 32). Thus, it is critical to assess biomechanical metrics when aortic pathophysiology is studied. Our simulation results showed time-varying hemodynamic patterns and areas exposed to nonphysiological metrics of WSS, which colocate with aortic pathologies observed in clinics. The example of six different time points at the systolic and diastolic phases shown in Fig. 6 demonstrates how instantaneously WSS changes over the cardiac cycle. This way, the areas exposed to low/high/oscillatory WSS are more prone to adverse clinical outcomes.
Fig. 6.
Transient wall shear stress map at different time points of systole and diastole in a pseudohealthy artery.
Because of the time-varying nature of WSS, however, it is a common practice and a fruitful approach to study the time-averaged metrics of WSS in different cases as predictors of adverse clinical outcomes (39, 57). We first present here the TAWSS on the arterial tree (c), calculated as follows:
| (4) |
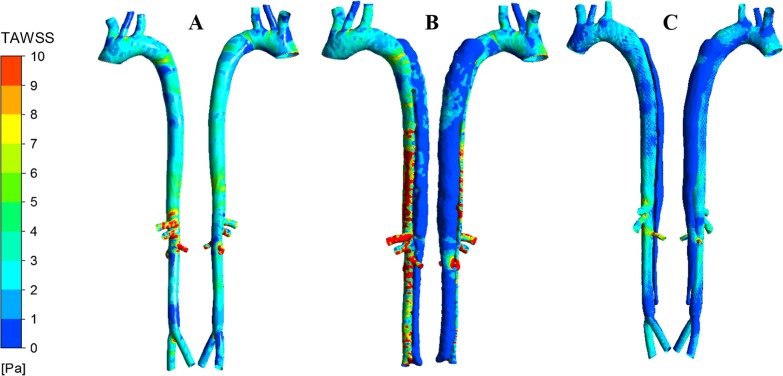
where is the wall shear vector. For the PH case, as expected, we observed moderate TAWSS distribution with low values near the bifurcations and inner wall of abruptly curved regions (Fig. 7A). For the PI case, with blood flow channeling into TL and FL, on average the WSS was much lower in the FL compared with the TL (Fig. 7B). In general, TAWSS was higher at TL and FL connections and near outlets as the blood flow accelerates because of cross-sectional area change. In this case, where the outlet to right renal artery is in the vicinity of reentry, TAWSS was considerably high. The proximal and distal ends of the FL are exposed to very low TAWSS as there is considerable flow recirculation and significant reduction in near-wall velocity with no passage for blood to escape. All these nonphysiological WSS patterns will further disrupt endothelial function and accelerate aortic pathology. The PM case, compared with the diseased artery, showed a significant change in the local WSS pattern, wherein TAWSS was reduced even further in the FL as a result of higher perfusion of the TL (Fig. 7C). Local high WSS areas were not observed in the TL, which is more physiological and less prone to adverse clinical events. Thus, reestablishing physiological hemodynamics and stresses in the TL by MFM will successfully restore normal endothelial function, physiological state of the aortic wall, and efficient reendothelialization of the device.
Fig. 7.
Comparison of the time-averaged wall shear stress (TAWSS) in pseudohealthy (PH; A), preintervention (PI; B), and post-multilayer flow modulator (MFM) implantation (PM; C) cases in anterior (left) and posterior (right) views. The TAWSS pattern in the PI case was dramatically different than that of the PH case, with increased local shear stress in the true lumen, which was alleviated in the PM case maintaining the physiological flow.
The oscillatory behavior of the flow was explained by a quantitative index called OSI, wherein a higher amount of OSI points to regions of considerable oscillation, as follows:
| (5) |
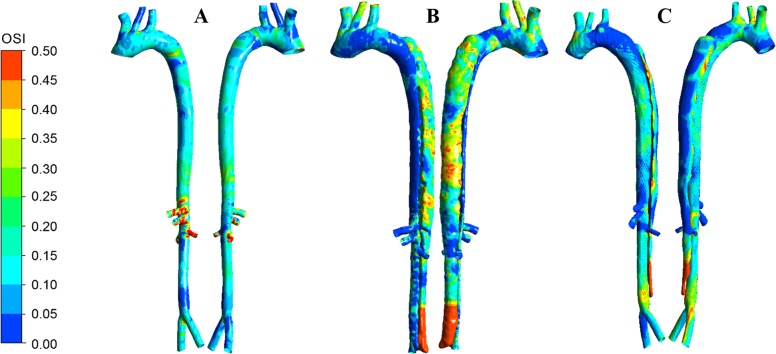
Similar to low TAWSS, we observed that areas near the bifurcation and curved artery were also exposed to high OSI, which attracts adverse biological outcomes (Fig. 8). In the PI case, a higher amount of OSI in the FL represents a larger amount of oscillation compared with the TL (Fig. 8B). Especially near peripheral outlets, the OSI significantly decreased, which shows how the blood flows more uniformly near the outlets. There was also a pattern of increased oscillatory flow and recirculation distal to where the TL and FL were connected. The largest area exposed to high OSI was at the distal end of the FL, which, similar to the TAWSS contour, correctly depicted a large amount of recirculation. The suppression of flow disruption and blood recirculation as a result of MFM implantation, primarily in the descending aorta, reduced the oscillatory behavior of blood flow in the FL (Fig. 8C) and increased the physiological spiral behavior of blood flow in the TL, similar to the PH case (Fig. 8A). In addition, the distal extremity of the FL where the blood flow is trapped was still exposed to high OSI, with the value and area lower than the PI model as the MFM had reduced flow to the FL in general.
Fig. 8.
Comparison of the oscillatory shear index (OSI) in pseudohealthy (PH; A), preintervention (PI; B), and post-multilayer flow modulator (MFM) implantation (PM; C) cases in anterior (left) and posterior (right) views. The increased OSI in both the true and false lumen of the PI model was considerably reduced in the PM model, with a pattern very similar to the PH model.
Perfusion was defined as the mass flow rate exiting each outlet and was compared in different cases to assess the portion of blood flow exiting aorta toward cerebral limb or peripheral vital organs decided by the hemodynamic pattern. It is recommended to compare the perfusion of proximal, mid, and distal outlets separately to study the effect of dissection on end-organ malperfusion better.
In more detail, we start with perfusion in aortic arch branches (Fig. 9). There was an increase of blood flow toward the cerebral limb in the PI case compared with the PH case. This is because of the higher resistance distal to aortic arch, which prevents larger portions of blood mass to flow distally. Comparing all three cases, it was clear that the mass flow to every single artery in the PM case was very similar to PH and less to the PI case. This is very promising in the sense that MFM implantation recovers the physiological flow to different organs with a pattern comparable to a healthy artery.
Fig. 9.
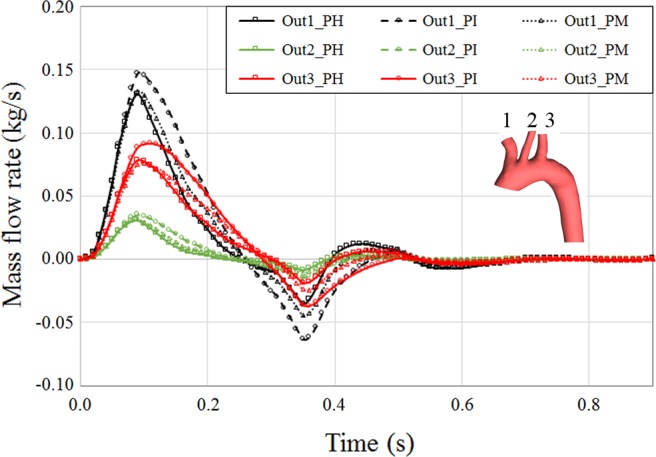
Perfusion of proximal aortic outlets over cardiac cycle in pseudohealthy (PH), preintervention (PI), and post-multilayer flow modulator (MFM) implantation (PM) cases. Outlets 1, 2, and 3 are brachiocephalic, left common carotid, and left subclavian arteries, respectively. Implantation of the MFM reduced the perfusion to proximal arteries with a pattern similar to the PH case.
We did not observe significant change in perfusion of midaorta outlets except the right renal artery where the flow exchange with FL increased the outlet mass flow (Fig. 10). Moreover, channeling of flow to the FL slightly decreased perfusion to the celiac artery. However, in the PI case, the larger amount of mass flow to renal arteries as a result of TL and FL connection increased the blood mass exiting the superior mesenteric artery most probably because of the pressure drop. A similar pattern of perfusion recovery in PM case was observed in midaortic outlets, where the mass flow to the branches in the PM case was very similar to the PH artery.
Fig. 10.
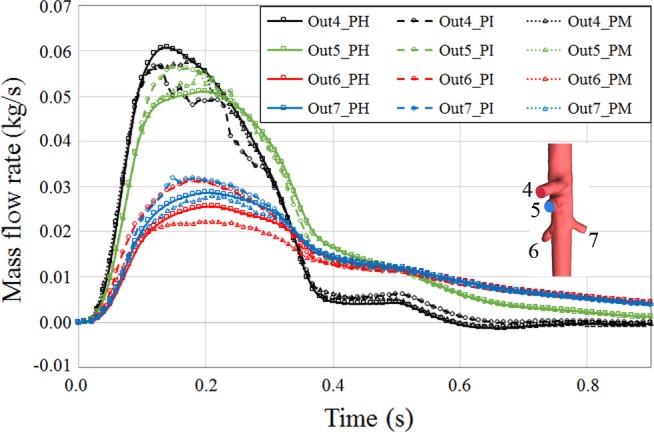
Perfusion of midaortic outlets over cardiac cycle in pseudohealthy (PH), preintervention (PI), and post-multilayer flow modulator (MFM) implantation (PM) cases. Outlets 4, 5, 6, and 7 are celiac, superior mesenteric, right renal, and left renal arteries, respectively. Perfusion in the PM case was similar to the PH case, whereas depending on geometric configuration, perfusion of the PI outlets was comparably higher or lower.
Comparing the PH case with the PI case, there was a considerable amount of perfusion drop in iliac arteries, which is mainly the amount of perfusion increased to the cerebral limb as a result of resistance rise in descending aortae (Fig. 11). Another important factor was the asymmetry seen in the left and right iliac artery perfusion that was mainly because of geometric change due to dissection. The left iliac artery was smaller than the right iliac artery in the diseased case. The perfusion to iliac arteries was also better for the PM case. Although the bifurcation geometry and cross-sectional area deviated from the PH case and became less symmetric for both the PI and PM cases, the PM case still maintained better perfusion to peripherals as a result of maintaining more physiological flow.
Fig. 11.
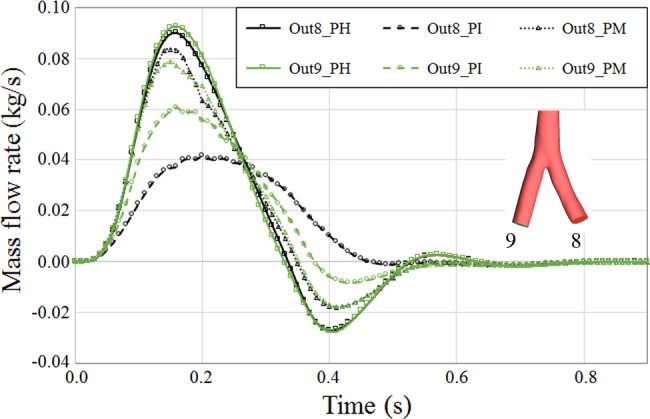
Perfusion of iliac arteries over the cardiac cycle in pseudohealthy (PH), preintervention (PI), and post-multilayer flow modulator (MFM) implantation (PM) cases. Outlets 8 and 9 are left and right iliac arteries, respectively. Because of specific geometric changes near the right iliac artery, a significant change of perfusion pattern was observed for this artery in the PI case, whereas the perfusion pattern for both iliac arteries was almost identical because of symmetric geometrical configuration in the PH case.
Ultimately, if we compare the summation of cerebral artery perfusion to the peripherals, we observe improved perfusion to distal vital organs with a pattern notably similar to the PH artery (Fig. 12). As a result, this performance of MFM should be highlighted in combination with the laminarization of blood flow streamlines and suppression of blood recirculation, which is underlined recruiting updated setup of computational study.
Fig. 12.
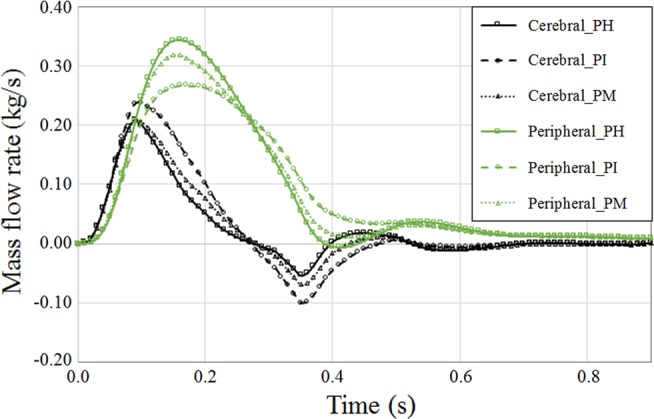
Perfusion of cerebral versus peripheral arteries in pseudohealthy (PH), preintervention (PI), and post-multilayer flow modulator (MFM) implantation (PM) cases obtained from summation of outlet flow rates in corresponding arteries. A considerable increase in perfusion of the proximal arteries at the diastolic phase was observed for the diseased case.
We are excited to see that using dynamic boundary conditions in computational tools enabled us to present the MFM performance not only in maintaining the physiological hemodynamics and suppressing the local recirculations but also in retaining the perfusion to vital organs.
DISCUSSION
Although short-term outcomes are good (70) and survival 3 yr after intervention/surgery is higher for TEVAR compared with open repair (35), there was greater reintervention for the former (22% vs. 14%) (55). We await the results of long-term followup especially in more complex pathologies of thoracoabdominal aortic aneurysm and dissection. In these cases, covered grafts or even fenestrated and/or branched endovascular aortic repair (EVAR) have failed to address malperfusion or rupture properly (17, 56), leaving those patients with the option of open surgery or no choice. This causes a real dilemma for elderly patients with heavy comorbidities. Only time and further trials will validate MFM performance in the long term (51), and our work, therefore, is intended to provide the community with an engineering set of tools by which to make these determinations more precisely, advance understanding of the nature of the disease processes at hand, and create more advanced metrics of therapeutic effect.
Malperfusion syndromes after AD correction result from restriction of the TL and loss of mass of perfusing blood. Although easy to embrace, such a paradigm does not explain the clinical presentation that often is accompanied by reduced flow to selective arterial beds. Accordingly, we seek a more global picture of how different configurations of a side-by-side parallel construct of the FL and TL affect vital organ perfusion taking into account reentries, remodeling of the TL, and the tear itself, focusing on type B AD. Malperfusion exists in cases of AD and even postsurgery, which is highly dependent on geometric configuration and blood exchange between the TL and FL (5, 13). The phenomenon is more intense when the TL is negatively remodeled and flow is considerably reduced. The FL, then, becomes pressurized, compromising the outlets coming out of the TL. Therefore, the realistic hemodynamics of how blood flow is redistributed is of great importance, and the exact share of flow inside the TL and FL must be calculated by numeric simulations. To this end, updated outlet boundary conditions are needed to avoid preset pressure conditions. The majority of published research thus far has focused on the immediate outcome of the MFM device in increasing the blood flow in the TL and shrinkage of the FL. The preset boundary condition in those studies prevents them from evaluating the performance of MFM beyond vasculature scaffolding and flow division, thus ending up reporting wall shear patterns and first-hand hemodynamic features, including pressure and velocity. However, using dynamic boundary conditions enables researchers to study the performance of MFM in modulation of flow to avoid malperfusion of vital organs, in addition to accurate modeling of flow patterns. We will further extend this work in the future to study the effect of important geometrical features, e.g., shape and location of reentries, numerically to show how without them, perfusion is even more compromised.
Geometric features of dissected artery and flow exchange between the TL and FL are paramount determining factors of local fluid dynamics (5, 13). It has been shown that the size and height of proximal entry may serve as a prognosis tool to estimate the volume of blood circulating in FL (59), with the former being positively correlated with FL flow rate and therefore clinical events. Thus elaborate morphological analysis, including preliminary sizing of TL and FL area and geometric features of dissection in combination with computational hemodynamics, may accurately determine clinical risks and provide clinicians with insights to select appropriate treatment procedures.
The division of flow between the FL and TL and recirculation zones generated by abnormal flow in these cases considerably change the shear stress metrics (Fig. 7). Aortic pathophysiology is universally attributed to aberrant stress (9). With a direct impact of WSS on endothelial cell function, disrupted flow degrades the aortic wall and accelerates propagation of intimal flap (41, 60). Together, altered flow and stress contribute to the generation, progression, and even rupture of aortic aneurysms (26, 27).
The resultant flow pattern and WSS distribution in the PI case are in agreement with predictions made by published literature (14, 49, 64) in terms of spatial distribution of low/high WSS, recirculation in the FL, and acceleration of blood flow in the squeezed TL. Time-averaged metrics of shear stress also profoundly affect the expansion of the aorta, modulating endothelial function and remodeling (62). Also, pressure differences as a result of disrupted flow may result in high intramural stress and thus promote aortic expansion (41). Geometric features of a dissected artery and flow exchange with regard to midaortic outlets, as previously reported similar to our study (13), determine the oscillatory behavior of flow and wall shear patterns (Figs. 7 and 8). Acceleration and deceleration of blood flow in the immediate vicinity of outlets and distribution of blood between the TL and FL deviate the physiological patterns of shear stress, in agreement with published studies that reported higher velocity (thus higher WSS) in the TL and increased flow in the FL (5, 13, 64). However, implantion of a MFM considerably increases blood flow into the TL and alleviates shear stress in the artery postoperatively (Fig. 7), in agreement with a previous reported study considering similar devices (66). This is critically important for the long-term outcome of intervention from a thrombogenesis perspective. Partial FL thrombosis is associated with complications such as aneurismal dilatation and rupture (14, 49, 70) or even death (72). Computational studies have predicted the location and rate of thrombus formation based on the AD geometric configuration and modulated hemodynamic features. It is shown that generated vortical structures, over time, trigger platelet activation (46, 49). Partial FL thrombosis in patients with acute type B AD increases mortality as it may occlude the distal reentry tears and increase the diastolic pressure and rupture risk. As the TL and FL are competing over their share of blood flow based on the resistances they induce, implantation of a MFM directs the bloodstream to flow further in the TL and, not fully blocking the FL, balances the pressure within the system. Thus, FL thrombosis occurs without compromising visceral or spinal cord perfusion decreasing the risk of paraplegia and mesenteric ischemia (69).
Although WSS is reduced as a result of device implantation in general, the overall map of shear stress in the TL is not significantly different compared with the physiological condition, and flow is considerably blocked from flowing into the FL. This successful channeling of blood flow into the TL results in the suppression of localized shear stress ramp, as observed in the PI case. As a result, laminarization of blood flow by MFM leads to a more physiological pattern of blood flow and subsequently a more physiological pattern of shear stress metrics. This often disregarded outcome is of dire importance from a biological perspective of endovascular implant performance in restoring (or at least stabilizing) a normal vasculature state. The endothelium has a profound effect on vascular hemostasis and regulation of cell signaling, arterial healing, and smooth muscle cell function (16). Mechanical stressors affect endothelial proliferation, apoptosis, permeability, migration, remodeling, and platelet adhesion (42, 45). Preclinical and clinical studies of MFM have reported not only a promotion of rapid reendothelialization compared with conventional stents (65) but also full integration and endothelialization of the device itself without interference in side branch perfusion (40). The device, then, is embedded within the aortic wall much earlier, which significantly reduces the adverse effects associated with peak wall stress. Our results predict that maintained hemodynamic and stress patterns, which enhance endothelial function, significantly reduce WSS and its share in exacerbating pathology progression (wall degradation and inflammatory processes), and speed endothelialization of the implanted graft.
Dissection of aortae, in general, increases the peripheral resistance opposing the blood flow, which considerably increases the perfusion to proximal organs leaving peripheral vital organs in risk of malperfusion (Figs. 9 and 11). Implantion of the MFM device channels the majority of blood flow into the TL, and by decreasing the distal resistance maintains the physiological perfusion to proximal outlets of the aortic arc. However, as a result of amplified resistance induced by the device itself, proximal perfusion was still slightly higher in the PM case compared with the PH case.
Perfusion to midaortic arteries was observed to depend considerably on local geometric features. Blood exchange between the TL and FL and the blood flow pattern dictated by it are principal factors affecting perfusion to the organs in the vicinity and progression of disease (37). Implantation of the MFM decreases blood flow to the FL, accelerates FL thrombosis (46, 49), diminishes risks of disease progression and aneurysmal degeneration (62) associated with WSS, and reduces the role that flow exchange between lumens plays in perfusion patterns. However, the device does not block blood flow to vital organs, and the share of blood perfusion is similar to the physiological condition (Fig. 10).
A significant perfusion pattern change can be observed in iliac arteries in terms of magnitude and symmetry of blood perfusion to the left and right branches (Fig. 11). The remodeling of the dissected artery and the position of the FL connection to the TL, specifically when it exists near iliac bifurcation, considerably change the pattern of perfusion to these arteries. MFM implantation increases the blood flow to iliac arteries and maintains a more physiological flow despite geometric differences of the postoperative arterial tree compared with the PH case. This physiological pattern of flow promises healthy remodeling of the artery in this area toward a physiological pattern of flow in peripherals.
In general, by comparing the overall perfusion of upper body outlets to peripheral organs (Fig. 12), we observed recovery of a physiological flow after implantation of the MFM devices. The complex design of the MFM, despite a slight increase in systemic resistance opposing the blood flow, does not block exit ways to aortic outlets. The laminarization of blood flow and focusing the stream into the TL are the principal performance factors for the MFM resulting in a flow pattern and stress distribution comparable to the PH case and well distinguished from a diseased artery.
Antihypertension medications are often all that a clinician can offer to some patients with AD. Although accurate assessment of hemodynamic alteration via pressure control is only possible taking into account the arterial remodeling, we did an abridged experiment on current geometries ignoring this critical mechanism. Pressure control on PI cases, as universally perceived, primarily reduced the pressure forces on the arterial tree and, as a result, slightly improved the shear stress aberration, which abates the impetus of further dissection, to some extent. However, the perfusion pattern was not significantly changed as a result of this remedy. We observed far more physiological perfusion pattern recovery deploying endovascular implants within the dissected artery than merely from reducing the pressure.
Although our numeric research has achieved simplified yet interesting and clinically relevant results, it bears some limitations toward realistic clinical scenarios. First and foremost, as AD is a chronic disease, we have had limited access to the information of the healthy status of patients and needed to construct the healthy state hypothetically. Access to patient-specific data for modeling, in terms of inlet flow and circulation system parameters for lumped model, was therefore limited. This limitation might be also the case for pre- and postoperative models wherein invasive/noninvasive measurements for inlet flow profile might not be available. The number of cases considered here was limited. We, however, frequently compared our results with the published literature and confirmed the outcomes to compensate for this limitation. Access to higher numbers of patients to conduct the current research with higher statistical power would be of great value and is actually the scope of our future work. Another limitation of this study is minimal consideration of aortic wall motion, which might affect the disease progression (6, 38). Major difficulties to model wall motion, which are pervasive in this field, are the characterization, localization, and modeling of diseased tissue in a dissected artery. Fluid-structure interaction modeling can be complied if patient-tailored intervention tools or disease progression are to be included. Here, however, we studied the postintervention effect of MFM implantation on vital organ perfusion and as from one side, the metallic device adds to the stiffness of the artery and from the other preintervention progress of disease is not the focus, it is acceptable to model the cases as rigid walls.
Conclusions
Computational study of perfusion to aortic outlets in different states of a dissected artery focusing on the performance of flow modulator devices could serve as an effective and useful tool to assess the performance of endovascular devices. The perfusion of vital organs is significantly disrupted by the dissection of aortae, as is the physiological hemodynamic patterns. The MFM device maintains the physiological flow in dissected arteries, directing the aortic flow into the TL. However, the complex design of the device does not fade its performance in maintaining the physiologic perfusion. Although the systematic resistance of peripheral aortae is slightly increased by the device, the overall perfusion to cerebral limb and peripheral vital organs is comparably similar to the physiological condition. Numerical simulations fed by patient-specific input may serve as a method of choice to assess the performance of the device to prevent the previously reported postoperative malperfusion and to optimize the design of future endovascular implants. Despite the promising results in the short term, future followups and systematic procedural and clinical data are required to delineate further the precise indication of MFM in the long term.
GRANTS
E. Edelman and F. Rikhtegar Nezami were funded in part by National Institutes of Health Grant R01-GM-49039.
DISCLOSURES
Cardiatis provided access to data and partial funding for L. Athanasiou.
AUTHOR CONTRIBUTIONS
F.R.N., L.S.A., and E.R.E. conceived and designed research; F.R.N., L.S.A., and J.M.A. performed experiments; F.R.N., L.S.A., and J.M.A. analyzed data; F.R.N. and E.R.E. interpreted results of experiments; F.R.N. prepared figures; F.R.N. and J.M.A. drafted manuscript; F.R.N., L.S.A., J.M.A., and E.R.E. edited and revised manuscript; F.R.N., L.S.A., J.M.A., and E.R.E. approved final version of manuscript.
REFERENCES
- 1.Abraham F, Behr M, Heinkenschloss M. Shape optimization in steady blood flow: a numerical study of non-Newtonian effects. Comput Methods Biomech Biomed Engin 8: 127–137, 2005. doi: 10.1080/10255840500180799. [DOI] [PubMed] [Google Scholar]
- 2.Afifi RO, Sandhu HK, Leake SS, Boutrous ML, Kumar V III, Azizzadeh A, Charlton-Ouw KM, Saqib NU, Nguyen TC, Miller CC III, Safi HJ, Estrera AL. Outcomes of patients with acute type b (DeBakey III) Aortic dissection: a 13-year, single-center experience. Circulation 132: 748–754, 2015. doi: 10.1161/CIRCULATIONAHA.115.015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alastruey J, Xiao N, Fok H, Schaeffter T, Figueroa CA. On the impact of modelling assumptions in multi-scale, subject-specific models of aortic haemodynamics. J R Soc Interface 13: 20160073, 2016. doi: 10.1098/rsif.2016.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alimohammadi M, Agu O, Balabani S, Díaz-Zuccarini V. Development of a patient-specific simulation tool to analyse aortic dissections: assessment of mixed patient-specific flow and pressure boundary conditions. Med Eng Phys 36: 275–284, 2014. doi: 10.1016/j.medengphy.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Alimohammadi M, Bhattacharya-Ghosh B, Seshadhri S, Penrose J, Agu O, Balabani S, Díaz-Zuccarini V. Evaluation of the hemodynamic effectiveness of aortic dissection treatments via virtual stenting. Int J Artif Organs 37: 753–762, 2014. doi: 10.5301/ijao.5000310. [DOI] [PubMed] [Google Scholar]
- 6.Alimohammadi M, Sherwood JM, Karimpour M, Agu O, Balabani S, Díaz-Zuccarini V. Aortic dissection simulation models for clinical support: fluid-structure interaction vs. rigid wall models. Biomed Eng Online 14: 34, 2015. doi: 10.1186/s12938-015-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Athanasiou L, Rikhtegar Nezami F, Zanotti Galon M, Lopes AC, Lemos PA, de la Torre Hernandez JM, Ben-Assa E, Edelman ER. Optimized computer-aided segmentation and 3D reconstruction using intracoronary optical coherence tomography. IEEE J Biomed Health Inform, 22: 1168–176, 2018. doi: 10.1109/JBHI.2017.2762520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Athanasiou LS, Fotiadis DI, Michalis LK. Atherosclerotic Plaque Characterization Methods Based on Coronary Imaging. London: Elsevier Science, 2017, p. 115–129. doi: 10.1016/B978-0-12-804734-7.00006-3. [DOI] [Google Scholar]
- 9.Bäck M, Gasser TC, Michel JB, Caligiuri G. Biomechanical factors in the biology of aortic wall and aortic valve diseases. Cardiovasc Res 99: 232–241, 2013. doi: 10.1093/cvr/cvt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonfanti M, Balabani S, Greenwood JP, Puppala S, Homer-Vanniasinkam S, Díaz-Zuccarini V. Computational tools for clinical support: a multi-scale compliant model for haemodynamic simulations in an aortic dissection based on multi-modal imaging data. J R Soc Interface 14: 20170632, 2017. doi: 10.1098/rsif.2017.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 49: 2379–2393, 2007. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 12.Chen D, Müller-Eschner M, Kotelis D, Böckler D, Ventikos Y, von Tengg-Kobligk H. A longitudinal study of Type-B aortic dissection and endovascular repair scenarios: computational analyses. Med Eng Phys 35: 1321–1330, 2013. doi: 10.1016/j.medengphy.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Chen D, Müller-Eschner M, von Tengg-Kobligk H, Barber D, Böckler D, Hose R, Ventikos Y. A patient-specific study of type-B aortic dissection: evaluation of true-false lumen blood exchange. Biomed Eng Online 12: 65, 2013. doi: 10.1186/1475-925X-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Z, Riga C, Chan J, Hamady M, Wood NB, Cheshire NJW, Xu Y, Gibbs RGJ. Initial findings and potential applicability of computational simulation of the aorta in acute type B dissection. J Vasc Surg 57, Suppl: 35S–43S, 2013. doi: 10.1016/j.jvs.2012.07.061. [DOI] [PubMed] [Google Scholar]
- 15.Chien S, Usami S, Taylor HM, Lundberg JL, Gregersen MI. Effects of hematocrit and plasma proteins on human blood rheology at low shear rates. J Appl Physiol 21: 81–87, 1966. doi: 10.1152/jappl.1966.21.1.81. [DOI] [PubMed] [Google Scholar]
- 16.Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol 31: 2391–2396, 2011. doi: 10.1161/ATVBAHA.111.231548. [DOI] [PubMed] [Google Scholar]
- 17.Costache V, Hulpus R, Costache A, Voican A, Matei C. The use of multilayer flow modulators in the endovascular treatment of complex aortic aneurysms and aortic dissections. J Indian Coll Cardiol 6: 44–51, 2016. doi: 10.1016/j.jicc.2015.10.028. [DOI] [Google Scholar]
- 18.Czerny M, Schoenhoff F, Etz C, Englberger L, Khaladj N, Zierer A, Weigang E, Hoffmann I, Blettner M, Carrel TP. The impact of pre-operative malperfusion on outcome in acute type A aortic dissection: results from the GERAADA registry. J Am Coll Cardiol 65: 2628–2635, 2015. doi: 10.1016/j.jacc.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 19.Dake MD, Kato N, Mitchell RS, Semba CP, Razavi MK, Shimono T, Hirano T, Takeda K, Yada I, Miller DC. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 340: 1546–1552, 1999. doi: 10.1056/NEJM199905203402004. [DOI] [PubMed] [Google Scholar]
- 20.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deeb GM, Patel HJ, Williams DM. Treatment for malperfusion syndrome in acute type A and B aortic dissection: a long-term analysis. J Thorac Cardiovasc Surg 140, Suppl: S98–S100, 2010. doi: 10.1016/j.jtcvs.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 22.Dillon-Murphy D, Noorani A, Nordsletten D, Figueroa CA. Multi-modality image-based computational analysis of haemodynamics in aortic dissection. Biomech Model Mechanobiol 15: 857–876, 2016. doi: 10.1007/s10237-015-0729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fattori R, Montgomery D, Lovato L, Kische S, Di Eusanio M, Ince H, Eagle KA, Isselbacher EM, Nienaber CA. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD). JACC Cardiovasc Interv 6: 876–882, 2013. doi: 10.1016/j.jcin.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL; Society for Vascular Surgery Ad Hoc Committee on TEVAR Reporting Standards . Reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg 52: 1022–1033.e5, 2010. doi: 10.1016/j.jvs.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Fillinger MF, Marra SP, Raghavan ML, Kennedy FE. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J Vasc Surg 37: 724–732, 2003. doi: 10.1067/mva.2003.213. [DOI] [PubMed] [Google Scholar]
- 27.Fillinger MF, Raghavan ML, Marra SP, Cronenwett JL, Kennedy FE. In vivo analysis of mechanical wall stress and abdominal aortic aneurysm rupture risk. J Vasc Surg 36: 589–597, 2002. doi: 10.1067/mva.2002.125478. [DOI] [PubMed] [Google Scholar]
- 28.Gargiulo M, Bianchini Massoni C, Gallitto E, Freyrie A, Trimarchi S, Faggioli G, Stella A. Lower limb malperfusion in type B aortic dissection: a systematic review. Ann Cardiothorac Surg 3: 351–367, 2014. doi: 10.3978/j.issn.2225-319X.2014.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Georgakarakos E, Ioannou CV, Papaharilaou Y, Kostas T, Katsamouris AN. Computational evaluation of aortic aneurysm rupture risk: what have we learned so far? J Endovasc Ther 18: 214–225, 2011. doi: 10.1583/10-3244.1. [DOI] [PubMed] [Google Scholar]
- 30.Hahtapornsawan S, Bisdas T, Torsello G, Criado FJ, Austermann M, Donas KP. Importance of early aortic surveillance after endovascular treatment of type b aortic dissection with malperfusion syndrome. Ann Vasc Surg 36: 106–111, 2016. doi: 10.1016/j.avsg.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Henry M, Benjelloun A, Henry I, Wheatley G. The multilayer flow modulator stent for the treatment of arterial aneurysms. J Cardiovasc Surg (Torino) 54: 763–783, 2013. doi: 10.1016/j.jacc.2012.08.174. [DOI] [PubMed] [Google Scholar]
- 32.Houard X, Ollivier V, Louedec L, Michel J-B, Bäck M. Differential inflammatory activity across human abdominal aortic aneurysms reveals neutrophil-derived leukotriene B4 as a major chemotactic factor released from the intraluminal thrombus. FASEB J 23: 1376–1383, 2009. doi: 10.1096/fj.08-116202. [DOI] [PubMed] [Google Scholar]
- 33.Long Ko JK, Liu RW, Ma D, Shi L, Ho Yu SC, Wang D. Pulsatile hemodynamics in patient-specific thoracic aortic dissection models constructed from computed tomography angiography. J Xray Sci Technol 25: 233–245, 2017. doi: 10.3233/XST-17256. [DOI] [PubMed] [Google Scholar]
- 34.Kadoglou NPE, Moulakakis KG, Papadakis I, Ikonomidis I, Alepaki M, Lekakis J, Liapis CD. Changes in aortic pulse wave velocity of patients undergoing endovascular repair of abdominal aortic aneurysms. J Endovasc Ther 19: 661–666, 2012. doi: 10.1583/JEVT-12-3916MR.1. [DOI] [PubMed] [Google Scholar]
- 35.Kang WC, Greenberg RK, Mastracci TM, Eagleton MJ, Hernandez AV, Pujara AC, Roselli EE. Endovascular repair of complicated chronic distal aortic dissections: intermediate outcomes and complications. J Thorac Cardiovasc Surg 142: 1074–1083, 2011. doi: 10.1016/j.jtcvs.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Karmonik C, Bismuth J, Shah DJ, Davies MG, Purdy D, Lumsden AB. Computational study of haemodynamic effects of entry- and exit-tear coverage in a DeBakey type III aortic dissection: technical report. Eur J Vasc Endovasc Surg 42: 172–177, 2011. doi: 10.1016/j.ejvs.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Karmonik C, Partovi S, Müller-Eschner M, Bismuth J, Davies MG, Shah DJ, Loebe M, Böckler D, Lumsden AB, von Tengg-Kobligk H. Longitudinal computational fluid dynamics study of aneurysmal dilatation in a chronic DeBakey type III aortic dissection. J Vasc Surg 56: 260–263.e1, 2012. doi: 10.1016/j.jvs.2012.02.064. [DOI] [PubMed] [Google Scholar]
- 38.Khanafer K, Berguer R. Fluid-structure interaction analysis of turbulent pulsatile flow within a layered aortic wall as related to aortic dissection. J Biomech 42: 2642–2648, 2009. doi: 10.1016/j.jbiomech.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Kolandaivelu K, Rikhtegar F. The systems biocompatibility of coronary stenting. Interv Cardiol Clin 5: 295–306, 2016. doi: 10.1016/j.iccl.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Lazaris AM, Maheras AN, Vasdekis SN. A multilayer stent in the aorta may not seal the aneurysm, thereby leading to rupture. J Vasc Surg 56: 829–831, 2012. doi: 10.1016/j.jvs.2012.03.252. [DOI] [PubMed] [Google Scholar]
- 41.Lee JJ, D’Ancona G, Amaducci A, Follis F, Pilato M, Pasta S. Role of computational modeling in thoracic aortic pathology: a review. J Card Surg 29: 653–662, 2014. doi: 10.1111/jocs.12413. [DOI] [PubMed] [Google Scholar]
- 42.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med 259: 381–392, 2006. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 43.LePage MA, Quint LE, Sonnad SS, Deeb GM, Williams DM. Aortic dissection: CT features that distinguish true lumen from false lumen. AJR Am J Roentgenol 177: 207–211, 2001. doi: 10.2214/ajr.177.1.1770207. [DOI] [PubMed] [Google Scholar]
- 44.Li DL, Zhang HK, Chen XD, Tian L, Jin W, Li M. Thoracic endovascular aortic repair for type b aortic dissection: analysis among acute, subacute, and chronic patients. J Am Coll Cardiol 67: 1255–1257, 2016. doi: 10.1016/j.jacc.2015.12.044. [DOI] [PubMed] [Google Scholar]
- 45.Li Y-SJ, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 38: 1949–1971, 2005. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 46.Menichini C, Cheng Z, Gibbs RGJ, Xu XY. Predicting false lumen thrombosis in patient-specific models of aortic dissection. J R Soc Interface 13: 20160759, 2016. doi: 10.1098/rsif.2016.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Midulla M, Fattori R, Beregi J-P, Dake M, Rousseau H. Aortic dissection and malperfusion syndrome: a when, what and how-to guide. Radiol Med (Torino) 118: 74–88, 2013. doi: 10.1007/s11547-012-0815-9. [DOI] [PubMed] [Google Scholar]
- 48.Molony DS, Kavanagh EG, Madhavan P, Walsh MT, McGloughlin TM. A computational study of the magnitude and direction of migration forces in patient-specific abdominal aortic aneurysm stent-grafts. Eur J Vasc Endovasc Surg 40: 332–339, 2010. doi: 10.1016/j.ejvs.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Naim WNWA, Ganesan PB, Sun Z, Liew YM, Qian Y, Lee CJ, Jansen S, Hashim SA, Lim E. Prediction of thrombus formation using vortical structures presentation in Stanford type B aortic dissection: a preliminary study using CFD approach. Appl Math Model 40: 3115–3127, 2016. doi: 10.1016/j.apm.2015.09.096. [DOI] [Google Scholar]
- 50.Nauta FJH, Lau KD, Arthurs CJ, Eagle KA, Williams DM, Trimarchi S, Patel HJ, Figueroa CA. Computational fluid dynamics and aortic thrombus formation following thoracic endovascular aortic repair. Ann Thorac Surg 103: 1914–1921, 2017. doi: 10.1016/j.athoracsur.2016.09.067. [DOI] [PubMed] [Google Scholar]
- 51.Oderich GS. Evidence of use of multilayer flow modulator stents in treatment of thoracoabdominal aortic aneurysms and dissections. J Vasc Surg 65: 935–937, 2017. doi: 10.1016/j.jvs.2016.12.092. [DOI] [PubMed] [Google Scholar]
- 52.Pape LA, Awais M, Woznicki EM, Suzuki T, Trimarchi S, Evangelista A, Myrmel T, Larsen M, Harris KM, Greason K, Di Eusanio M, Bossone E, Montgomery DG, Eagle KA, Nienaber CA, Isselbacher EM, O’Gara P. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. J Am Coll Cardiol 66: 350–358, 2015. doi: 10.1016/j.jacc.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 53.Patel AY, Eagle KA, Vaishnava P. Acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection. Ann Cardiothorac Surg 3: 368–374, 2014. doi: 10.3978/j.issn.2225-319X.2014.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pritchard WF, Davies PF, Derafshi Z, Polacek DC, Tsao R, Dull RO, Jones SA, Giddens DP. Effects of wall shear stress and fluid recirculation on the localization of circulating monocytes in a three-dimensional flow model. J Biomech 28: 1459–1469, 1995. doi: 10.1016/0021-9290(95)00094-1. [DOI] [PubMed] [Google Scholar]
- 55.Pujara AC, Roselli EE, Hernandez AV, Vargas Abello LM, Burke JM, Svensson LG, Greenberg RK. Open repair of chronic distal aortic dissection in the endovascular era: Implications for disease management. J Thorac Cardiovasc Surg 144: 866–873, 2012. doi: 10.1016/j.jtcvs.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 56.Raux M, Patel VI, Cochennec F, Mukhopadhyay S, Desgranges P, Cambria RP, Becquemin J-P, LaMuraglia GM. A propensity-matched comparison of outcomes for fenestrated endovascular aneurysm repair and open surgical repair of complex abdominal aortic aneurysms. J Vasc Surg 60: 858–864, 2014. doi: 10.1016/j.jvs.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Rikhtegar F, Knight JA, Olgac U, Saur SC, Poulikakos D, Marshall W JR, Cattin PC, Alkadhi H, Kurtcuoglu V. Choosing the optimal wall shear parameter for the prediction of plaque location-A patient-specific computational study in human left coronary arteries. Atherosclerosis 221: 432–437, 2012. doi: 10.1016/j.atherosclerosis.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 58.Rikhtegar F, Wyss C, Stok KS, Poulikakos D, Müller R, Kurtcuoglu V. Hemodynamics in coronary arteries with overlapping stents. J Biomech 47: 505–511, 2014. doi: 10.1016/j.jbiomech.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 59.Rinaudo A, D’Ancona G, Lee JJ, Pilato G, Amaducci A, Baglini R, Follis F, Pilato M, Pasta S. Predicting outcome of aortic dissection with patent false lumen by computational flow analysis. Cardiovasc Eng Technol 5: 176–188, 2014. doi: 10.1007/s13239-014-0182-x. [DOI] [Google Scholar]
- 60.Sakamoto N, Saito N, Han X, Ohashi T, Sato M. Effect of spatial gradient in fluid shear stress on morphological changes in endothelial cells in response to flow. Biochem Biophys Res Commun 395: 264–269, 2010. doi: 10.1016/j.bbrc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 61.Segers P, Rietzschel ER, De Buyzere ML, Stergiopulos N, Westerhof N, Van Bortel LM, Gillebert T, Verdonck PR. Three- and four-element Windkessel models: assessment of their fitting performance in a large cohort of healthy middle-aged individuals. Proc Inst Mech Eng H 222: 417–428, 2008. doi: 10.1243/09544119JEIM287. [DOI] [PubMed] [Google Scholar]
- 62.Shang EK, Nathan DP, Fairman RM, Bavaria JE, Gorman RC, Gorman JH III, Jackson BM. Use of computational fluid dynamics studies in predicting aneurysmal degeneration of acute type B aortic dissections. J Vasc Surg 62: 279–284, 2015. doi: 10.1016/j.jvs.2015.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin I-Y, Chung Y-G, Shin W-H, Im SB, Hwang SC, Kim BT. A morphometric study on cadaveric aortic arch and its major branches in 25 Korean adults: the perspective of endovascular surgery. J Korean Neurosurg Soc 44: 78–83, 2008. doi: 10.3340/jkns.2008.44.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stefanov F, Sultan S, Morris L, Elhelali A, Kavanagh EP, Lundon V, Sultan M, Hynes N. Computational fluid analysis of symptomatic chronic type B aortic dissections managed with the Streamliner Multilayer Flow Modulator. J Vasc Surg 65: 951–963, 2017. doi: 10.1016/j.jvs.2016.07.135. [DOI] [PubMed] [Google Scholar]
- 65.Sultan S, Hynes N. Multilayer flow modulator stent technology: a treatment revolution for US patients? Expert Rev Med Devices 12: 217–221, 2015. doi: 10.1586/17434440.2015.1030339. [DOI] [PubMed] [Google Scholar]
- 66.Sultan S, Hynes N, Kavanagh EP, Diethrich EB. How does the multilayer flow modulator work? The science behind the technical innovation. J Endovasc Ther 21: 814–821, 2014. doi: 10.1583/14-4858.1. [DOI] [PubMed] [Google Scholar]
- 67.Sultan S, Kavanagh E, Stefanov F, Sultan M, Costache V, Elhelali A, London V, Diethrich E, Hynes N. Streamliner multilayer flow modulator stents as a therapeutic option in the management of complex thoraco-abdominal aortic pathology report from Global SMFM Registry. J Indian Coll Cardiol 6: 77–84, 2016. doi: 10.1016/j.jicc.2015.10.022. [DOI] [Google Scholar]
- 67a.Sultan S, Kavanagh EP, Bonneau M, Kang C, Alves A, Hynes N. Assessment of biocompatibility of the multilayer flow modulator with differing thread designs. J Vasc Med Surg 2: 167, 2014. doi: 10.4172/2329-6925.1000167. [DOI] [Google Scholar]
- 68.Sultan S, Kavanagh EP, Hynes N, Diethrich EB. Evaluation of functionality and biological response of the multilayer flow modulator in porcine animal models. Int Angiol 35: 31–39, 2016. [PubMed] [Google Scholar]
- 69.Sultan S, Kavanagh EP, Stefanov F, Sultan M, Elhelali A, Costache V, Diethrich E, Hynes N; Global MFM Collaborators . Endovascular management of chronic symptomatic aortic dissection with the streamliner multilayer flow modulator: twelve-month outcomes from the global registry. J Vasc Surg 65: 940–950, 2017. doi: 10.1016/j.jvs.2016.09.059. [DOI] [PubMed] [Google Scholar]
- 70.Sultan S, Sultan M, Hynes N. Early mid-term results of the first 103 cases of multilayer flow modulator stent done under indication for use in the management of thoracoabdominal aortic pathology from the independent global MFM registry. J Cardiovasc Surg (Torino) 55: 21–32, 2014. doi: 10.1016/j.jvs.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki T, Eagle KA, Bossone E, Ballotta A, Froehlich JB, Isselbacher EM. Medical management in type B aortic dissection. Ann Cardiothorac Surg 3: 413–417, 2014. doi: 10.3978/j.issn.2225-319X.2014.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trimarchi S, Eagle KA. Thoracic endovascular aortic repair in acute and chronic type b aortic dissection. JACC Cardiovasc Interv 9: 192–194, 2016. doi: 10.1016/j.jcin.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 73.Tsai TT, Evangelista A, Nienaber CA, Myrmel T, Meinhardt G, Cooper JV, Smith DE, Suzuki T, Fattori R, Llovet A, Froehlich J, Hutchison S, Distante A, Sundt T, Beckman J, Januzzi JL JR, Isselbacher EM, Eagle KA; International Registry of Acute Aortic Dissection . Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 357: 349–359, 2007. doi: 10.1056/NEJMoa063232. [DOI] [PubMed] [Google Scholar]
- 74.Wang X, Li X. Fluid-structure interaction based study on the physiological factors affecting the behaviors of stented and non-stented thoracic aortic aneurysms. J Biomech 44: 2177–2184, 2011. doi: 10.1016/j.jbiomech.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 75.Xiao N, Alastruey J, Alberto Figueroa C. A systematic comparison between 1-D and 3-D hemodynamics in compliant arterial models. Int J Numer Methods Biomed Eng 30: 204–231, 2014. doi: 10.1002/cnm.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiong J, Zhang M, Guo W, Liu X, Yin T, Jia X, Zhang H, Xu Y, Wang L. Early malperfusion, ischemia reperfusion injury, and respiratory failure in acute complicated type B aortic dissection after thoracic endovascular repair. J Cardiothorac Surg 8: 17, 2013. doi: 10.1186/1749-8090-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamamoto H, Yamamoto F, Izumoto H, Shiroto K, Tanaka F, Yamaura G, Motokawa M, Ishibashi K. Acute aortic occlusion due to false-lumen expansion after repair of abdominal aortic rupture in type B acute aortic dissection. Ann Vasc Surg 24: 951.e1–951.e6, 2010. doi: 10.1016/j.avsg.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 78.Zhang M-H, Du X, Guo W, Liu X-P, Jia X, Ge Y-Y. Early and midterm outcomes of thoracic endovascular aortic repair (TEVAR) for acute and chronic complicated type B aortic dissection. Medicine (Baltimore) 96: e7183, 2017. doi: 10.1097/MD.0000000000007183. [DOI] [PMC free article] [PubMed] [Google Scholar]