Abstract
Digital pills, gelatin capsules with radiofrequency transmitters activated by stomach chloride ions, directly measure antiretroviral therapy adherence. In individuals with substance use disorders and HIV, real-time nonadherence detected by digital pills creates a platform to deliver substance use and adherence interventions. In this study, we determined the bioequivalence of tenofovir (TFV), administered as tenofovir disoproxil fumarate (TDF) in healthy human volunteers administered a commercial drug product and a digital pill formulation. We adhered generally to the US FDA Analytical Procedures and Methods for Validation for Drugs and Biologics guidelines. Ten HIV-uninfected adults without reported allergy to TFV, emtricitabine, or rilpivirine were enrolled. Participants ingested a digital pill containing TDF/emtricitabine/rilpivirine. Peripheral venous blood samples were collected at 0.5, 1, 2, 4, 8, and 24 h postingestion. After a 14-day washout period, the same participants ingested Complera™. Serial venous blood samples were collected using the same protocol as the digital pill. Liquid chromatography/mass spectrometry was used to determine a maximum concentration (Cmax), area under curve from time zero to last measured concentration (AUCo-t), and area under curve from time zero to infinity (AUCoo) of TFV. Ten participants with an average age of 27 and body mass index of 25.4 successfully completed the study. Predose TFV was undetectable before the second administration of Complera confirming adequate washout period after ingestion of the digital pill. The geometric means of AUCo-t, AUCoo, and Cmax for test and reference products were within the 95% confidence intervals and, therefore, bioequivalent. TFV overencapsulated in digital pills are bioequivalent to TFV in commercial formulations.
Keywords: medication adherence, digital pills, PrEP, HIV prevention
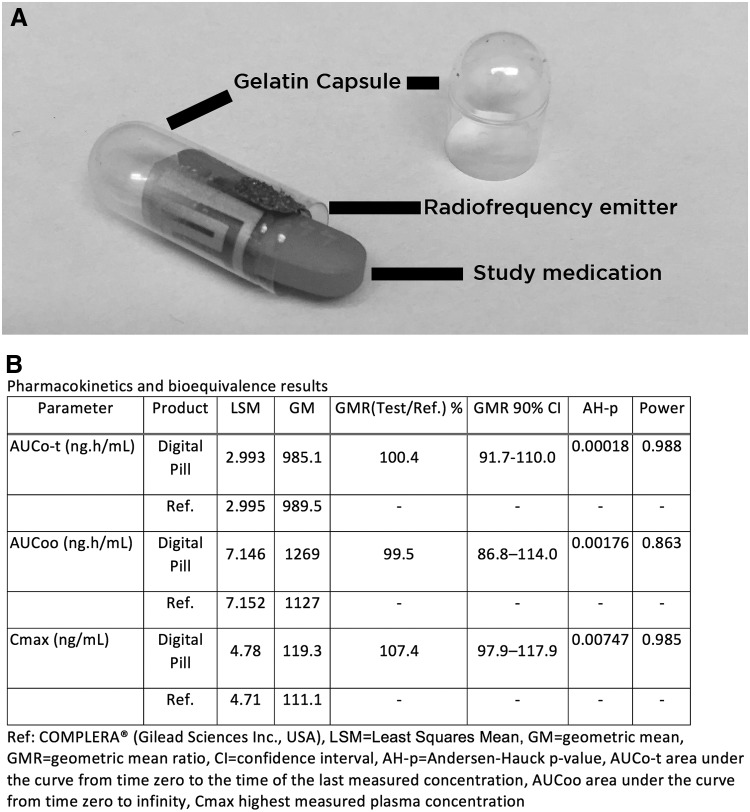
The ID Cap system (eTectRx, Newberry, FL) is a digital pill comprising a commercial gelatin capsule overencapsulating a tiny battery, radiofrequency emitter, and antiretroviral therapy (ART) medication (Fig. 1). Ingestion of the ID Cap activates the integrated transmitter, producing a signal that is captured by a wearable device (e.g., the reader). The reader then stores medication ingestion data while simultaneously transmitting it to the cloud, allowing patients and clinicians to view and respond to real-time adherence data.1 Direct monitoring of antiretroviral pre-exposure prophylaxis (PrEP) allows for real-time detection of nonadherence and enables interventions to support adherence in individuals at risk for HIV.2,3 Recent investigations have demonstrated bioequivalence of tenofovir (TFV) and emtricitabine overencapsulated by other digital pill systems.4 In this study, we studied in healthy human volunteers, the bioequivalence of TFV using Complera (TFV/emtricitabine/rilpivirine) with and without digital pills.
FIG. 1.
(A) The ID Cap digital pill comprising a gelatin capsule with integrated radio frequency emitter and circuit that over encapsulates medication (sample pill sized to dimensions of Complera™ pictured). (B) Bioequivalence data of tenofovir.
This longitudinal crossover design generally adhered to the United States Food and Drug Administration (US FDA) Analytical Procedures and Methods for Validation for Drugs and Biologics guidelines.5 We enrolled participants >18 years, nonpregnant, HIV seronegative, and without reported allergy to TFV, emtricitabine, or rilpivirine. We excluded individuals on medications known to interact with TFV. Participants fasted overnight, then received a meal of at least 400 calories and 10 g fat, and ingested a digital pill containing TFV/emtricitabine/rilpivirine (Complera). Peripheral venous blood samples were collected at 0.5, 1, 2, 4, 8, and 24 h postingestion. After a 14 days washout period, the same 10 participants repeated the procedure with Complera alone (i.e., without the digital pill). Serial venous blood samples were collected using the identical protocol to that used with the digital pill.
A tandem liquid chromatography mass spectrometry (MS) was validated according to FDA guidance on Good Laboratory Practices (GLP).5 The ultrahigh-performance liquid chromatography separation (UPLC) was carried out on an Acquity H-Class System (Waters Co., Milford, MA) equipped with an Acquity UPLC BEH C18 column (1.7 μm 2.1 × 100 mm). The mobile phase consisted of a gradient of MilliQ water with 0.1% formic acid (component A) and acetonitrile (component B). From 0.3 to 2.5 min the gradient changed from 10% A:90% B to 90% A:10% B. This mixture was held until minute 4.5, after which it reverted to 10% A –90% B until the end of the run at 5.5 min. The flow rate was a constant 0.3 mL/min under a column temperature of 35°C. The sample vials were thermostatic at 4°C and the injection volume was 3 μL.
Detection by MS was performed on a tandem Xevo TQ system (Waters Co, Milford MA) running multiple reaction monitoring in electrospray ionization (ESI) positive mode. The lower limit of quantitation of this assay was 1 ng/mL and the precision and accuracy were 5.3% and 99.3%, respectively.
Plasma was separated from blood samples and 100 μL of either spiked or study plasma was transferred to a 1.5 mL tube, and added 10 μL of a 1 μg/mL stock solution of lamivudine as internal standard. Next, 600 μL acetonitrile and 10 μL of 5% trichloroacetic acid were added to each vial and vortexed. We centrifuged all vials at 10,000 rpm for 10 min to complete the extraction. About 700 μL of each sample were then transferred to a new tube and evaporated to dryness and reconstituted with 100 μL aqueous 0.1% formic acid:acetonitrile (95:5). These concentrates were transferred into a Waters amber screw cap conic vial and loaded onto the autosampler tray for injection. At least three injections were made from each vial to generate triplicate results.
A noncompartmental analysis of the concentration versus time data was conducted to estimate parameters such as the maximum observed concentration (Cmax), the area under the curve from time zero to time of the last measured concentration (AUCo-t), the AUC from time zero extrapolated to infinity (AUCoo), the time to Cmax (Tmax), the apparent first-order elimination rate constant (ke), and the apparent elimination half-life (t½). According to FDA guidance, in the absence of documented estimates of intrasubject variability, an adaptive two-stage design is advisable.6 We conducted an interim analysis after a first-stage study conducted with a number of subjects based on a true geometric mean ratio of 0.95 and V = 0.05. Based on observed intrasubject variability, a new power calculation is made, which if <90% triggers a second stage of bioequivalence assessment now with V = 0.0294. If at least 90% then the study is concluded after the first stage.
Bioequivalence criteria were based on log-transformed AUCoo and Cmax data from which a geometric mean ratio is calculated. The 90% confidence intervals for its point estimate must be within 0.80 and 1.25 to validate bioequivalence between the formulations.
We enrolled a total of 10 participants who successfully completed the study. Mean age was 27, 20% (N = 2) men, and 80% (N = 8) women. Participants had a mean body mass index of 25.4.
At the interim analysis of the data collected from 10 subjects sequentially administered with both test and reference formulations, as per FDA guidance, the calculated power for AUCo-t, AUCoo, and Cmax ratios was 98.8%, 86.3%, and 98.5%, respectively. Since the calculated power was >90% for both AUC0-t and Cmax, we determined that a second stage was not deemed necessary and concluded the bioequivalence study. Analysis of standardized residuals identified no outliers. Predose concentrations were not observed upon the second administration to each subject, which validated the washout period established.
The geometric means of AUCo-t, AUCoo, and Cmax for test and reference products were 985.1 and 989.5 ng.h/mL; 1269 and 1127 ng.h/mL; and 119.3 and 111.1 ng/mL, respectively. Their ratios were 1.00, 0.995, and 1.07, respectively, all falling within 90% confidence intervals of 0.8–1.25 (Fig. 1).
We confirmed that digital pills have comparable Cmax of TFV, used for PrEP and incorporated into multiple regimens for ART. Our results support the findings of a recent study demonstrating that digital pills have no effect on the dissolution of Complera.4 The significance of our findings is that digital pills containing TFV can be used to measure adherence without concern for altered drug levels.
The efficacy of ART and PrEP is closely tied to adherence, yet “adherence” is often determined as the number of missed doses for a specified time period, often weeks or even months.7,8 Existing methods to measure adherence include electronic adherence monitoring (EAM) and dried blood spots detecting specific drug concentrations. These methods measure adherence in aggregate but may miss nascent periods of nonadherence. Digital pills such as the ID Cap System offer the ability to detect suboptimal adherence and deliver interventions that may serve as corrective feedback and return individuals to adherence, a significant difference from other EAMs such as Wisepill that only indirectly infers medication ingestion. Similar to other EAMs, digital pills may suffer from technological shortcomings (e.g., reader out of range or out of battery), and their real-world acceptance remains to be described.
Limitations are few. First, we utilized a small sample size. Second, our study design enrolled a sample of HIV-uninfected individuals. We were unable to enroll HIV-infected individuals because it would have been unethical to ask participants who were HIV-infected to stop taking their ARV for a 2-week washout.
Acknowledgments
The authors thank Jung Lee and the Brigham and Women's Hospital Center for Clinical Investigation for the support of this study. Funding: The study was funded by K24DA037109 (Boyer) and Dr. Chai is supported by K23DA044874.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Chai PR, Castillo-Mancilla J, Buffkin E, et al. : Utilizing an ingestible biosensor to assess real-time medication adherence. J. Med. Toxicol. 2015;11:439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mayer KH, Ramjee G: The current status of the use of oral medication to prevent HIV transmission. Curr Opin HIV AIDS 2015;10:226–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chai P, Rosen RK, Boyer EW: Ingestible Biosensors for Real-Time Medication Adherence Monitoring: MyTMed. IEEE & Computer Society Press, Hawaii, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ibrahim ME, Brooks KM, Castillo-Mancilla JR, et al. : Bioequivalence of tenofovir and emtricitabine after coencapsulation with the proteus ingestible sensor. AIDS Res Hum Retroviruses 2018;34:835–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Food and Drug Administration: Analytical Procedures and Methods Validation for Drugs and Biologics. Center for Drug Evaluation and Research, Food and Drug Administration. Silver Spring, MD; 2015, pp. 1–18 [Google Scholar]
- 6. Food and Drug Administration: Guidance for Industry: Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA Center for Drug Evaluation and Research, Food and Drug Administration. Silver Spring, MD 2013, pp. 1–24 [Google Scholar]
- 7. Nance RM, Delaney JAC, Golin CE, et al. : Co-calibration of two self-reported measures of adherence to antiretroviral therapy. AIDS Care 2017;29:464–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robbins RN, Spector AY, Mellins CA, Remien RH: Optimizing ART adherence: update for HIV treatment and prevention. Curr HIV/AIDS Rep 2014;11:423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]